Abstract
While ineffective erythropoiesis has long been recognized as a key contributor to anemia in thalassemia, its role in anemia of sickle cell disease (SCD) has not been critically explored. Using in vitro and in vivo derived human erythroblasts we assessed the extent of ineffective erythropoiesis in SCD. Modeling the bone marrow hypoxic environment, we found that hypoxia induces death of sickle erythroblasts starting at the polychromatic stage, positively selecting cells with high levels of fetal hemoglobin (HbF). Cell death was associated with cytoplasmic sequestration of heat shock protein 70 and was rescued by induction of HbF synthesis. Importantly, we document that in the bone marrow of SCD patients similar cell loss occurs during the final stages of terminal differentiation. Our study provides evidence for ineffective erythropoiesis in SCD and highlights an anti-apoptotic role for HbF during the terminal stages of erythroid differentiation. These findings imply that the beneficial effect on anemia of increased HbF levels is not only due to the increased life span of red cells but also a consequence of decreased ineffective erythropoiesis.
Introduction
Sickle cell disease (SCD) is an autosomal hereditary recessive disorder caused by a point mutation in the b-globin gene resulting in a Glu-to-Val substitution at the sixth position of the b-globin protein. The resulting abnormal hemoglobin (HbS) polymerizes under hypoxic conditions driving red blood cell (RBC) sickling.1 SCD is a multisystem disease characterized by hemolytic anemia, high susceptibility to infections, recurrent painful vaso-occlusive crises, strokes, acute chest syndrome and organ failure.2,3
While the pathobiology of circulating RBC has been extensively analyzed in SCD, erythropoiesis is surprisingly poorly documented. In b-thalassemia, ineffective erythropoiesis is characterized by high levels of apoptotic erythroblasts during the late stages of terminal differentiation, due to an accumulation of free α- globin chains.4-6 Ineffective erythropoiesis is the major cause of anemia in b-thalassemia patients. In contrast, a marked decrease in the life span of circulating red cells, a feature of sickle red cells, is considered to be the major determinant of chronic anemia in SCD. It is generally surmised that ineffective erythropoiesis contributes little to anemia. There have been, however, a number of sporadic reports that suggest defective terminal erythroid differentiation in SCD. For example, erythroblasts differentiated in vitro or isolated from bone marrow of SCD patients were shown to sickle under hypoxic conditions.7 Such sickling was also reported in the SAD mouse model, with altered morphology of late stage erythroid precursors within the bone marrow, as well as high levels of hemoglobin polymers and increased cell fragmentation occurring during medullary endothelial migration of reticulocytes.8 It was presumed that sickling of erythroblasts could lead to ineffective terminal erythroid differentiation. The study of Wu et al. showed for the first time evidence of ineffective erythropoiesis occurring in the bone marrow of transplanted SCD patients with the preferential survival of the donor erythroid cells in a small cohort of patients.9
In the present study, we performed a detailed characterization of terminal erythroid differentiation in non-transplanted SCD patients using both in vivo and in vitro assay systems to critically assess the extent of ineffective erythropoiesis. We documented in both our in vivo and in vitro studies, the occurrence of ineffective erythropoiesis at late stages of terminal erythroid differentiation reflected by high cell death rates between the polychromatic and the orthochromatic stages. We explored the potential mechanistic basis for ineffective erythropoiesis in SCD patients and showed that the molecular mechanism responsible for cell death is likely related to HbS polymerization and its interaction with chaperone protein HSP70 leading to its cytoplasmic sequestration. Importantly, we documented that increased expression of fetal hemoglobin (HbF) can rescue differentiating erythroblasts from cell death.
Methods
Biological samples
The study was conducted according to the declaration of Helsinki with approval from the Medical Ethics Committee (GR-Ex/CPP-DC2016-2618/CNILMR01). All SCD patients were of SS genotype. Blood bags from SCD patients enrolled in an exchange transfusion program, bone marrow aspirates from five SCD patients undergoing surgery and bone marrow tissues from five non-anemic donors undergoing hip/sternum surgery, were obtained after informed consent, from Necker-Enfants Malades Hospital (Paris, France) and the North Shore-LIJ Health System (New York, USA) under Institutional Review Board (IRB) approval. Control blood bags from healthy donors were obtained from the Etablissement Français du Sang (EFS).
In vitro differentiation of human erythroid progenitors
Peripheral blood mononuclear cells were isolated from blood samples after Pancoll fractionation (PAN Biotech). CD34+ cells were then isolated by a magnetic sorting system (Miltenyi Biotec CD34 Progenitor cell isolation kit) following the supplier protocol. CD34+ cells were placed in an in vitro two-phase liquid culture system, as previously described.10 For detailed protocols please refer to the Online Supplementary Appendix.
For cultures treated with pomalidomide, cells were incubated with 1 mM pomalidomide (Sigma) as previously described,11 starting from day 1 (D1) of phase II of culture. For γ-globin derepression experiments using CRISPR/Cas9, patient CD34+ cells were immunoselected and cultured for 48 hours (h) and then electroporated with ribonucleoprotein (RNP) complexes containing Cas9-GFP protein (4.5 mM) and the -197 guide RNA (gRNA) targeting both HBG1 and HBG2 γ-globin promoters (5’ ATTGAGATAGTGTGGGGAAGGGG 3’; protospacer adjacent motif in bold) or a gRNA targeting the Adeno-associated virus integration site 1 (AAVS1; 5’ GGGGCCACTAGGGACAGGATTGG 3’; protospacer adjacent motif in bold).12 Cleavage efficiency was evaluated in cells harvested 6 days after electroporation by Sanger sequencing followed by tracking of indels by decomposition (TIDE) analysis.13
Imaging flow cytometry analysis of human bone marrow samples
Bone marrow samples were processed as previously described.14 Detailed protocol are stated in the Online Supplemental Appendix.
Flow cytometry
Cells were analyzed using a BD FACScanto II flow cytometer and BD LSR Fortessa SORP flow cytometer (BD Biosciences) and acquired using the Diva software version 8 (BD Biosciences). Data was analyzed using FCS Express 6 software (DeNovo Software). Detailed protocols of cell staining are stated in the Online Supplemental Appendix.
Cell fractionation and western-blot
Cytoplasmic and nuclear protein fractions were extracted from erythroblasts at D7 of phase II of culture using the NE-PER nuclear and cytoplasmic kit (Pierce-Thermo Scientific). Ten μg of nuclear and cytoplasmic proteins were analyzed by SDS-PAGE, using 10% polyacrylamide gels, followed by immunoblotting. The antibodies used were rabbit anti-HSP70, mouse anti-actin and mouse anti-lamin A/C as a control for the nuclear extract. Proteins were revealed using electrochemiluminescence (ECL) clarity (Biorad) and the Chemidoc MP imaging system (Biorad). Analysis was performed using Image Lab (Biorad). Antibodies details are stated in the Online Supplemental Appendix.
Co-immunoprecipitation assays
Co-immunoprecipitation of HSP70 and hemoglobin was performed with lysates of 10 million RBC from SCD patients that were either exposed to hypoxia or not for one hour. HSP70 was immunoprecipitated by incubating the lysates with mouse anti- HSP70 antibody (Enzo Lifesciences) overnight at 4°C followed by a 45-minute incubation with protein-G sepharose beads (Cytiva-GE-Healthcare) at 4°C. Eluted proteins were analyzed by SDS-PAGE using a 4-12% polyacrylamide gel, followed by immunoblotting with mouse anti-α-globin (Santa Cruz Biotechnology) or rabbit anti-HSP70 (SANTA-CRUZ) antibodies. Proteins were revealed using ECL clarity and the Chemidoc MP imaging system.
Confocal microscopy and proximity ligation assay
Co-immunolocalization and proximity ligation assays (PLA) were performed with cells from D7 of phase II of culture. Acquisition was made on LSM700 Zeiss confocal microscope using Zen software. Analysis was performed using Fiji.15 Detailed protocols are stated in the Online Supplemental Appendix.
Statistical analysis
Statistical analyses were performed with GraphPad Prism (version 7). The data was analyzed using Mann-Whitney unpaired test and Wilcoxon paired test, as indicated in the figure legends.
Results
Hypoxia-induced cell death during in vitro terminal erythroid differentiation
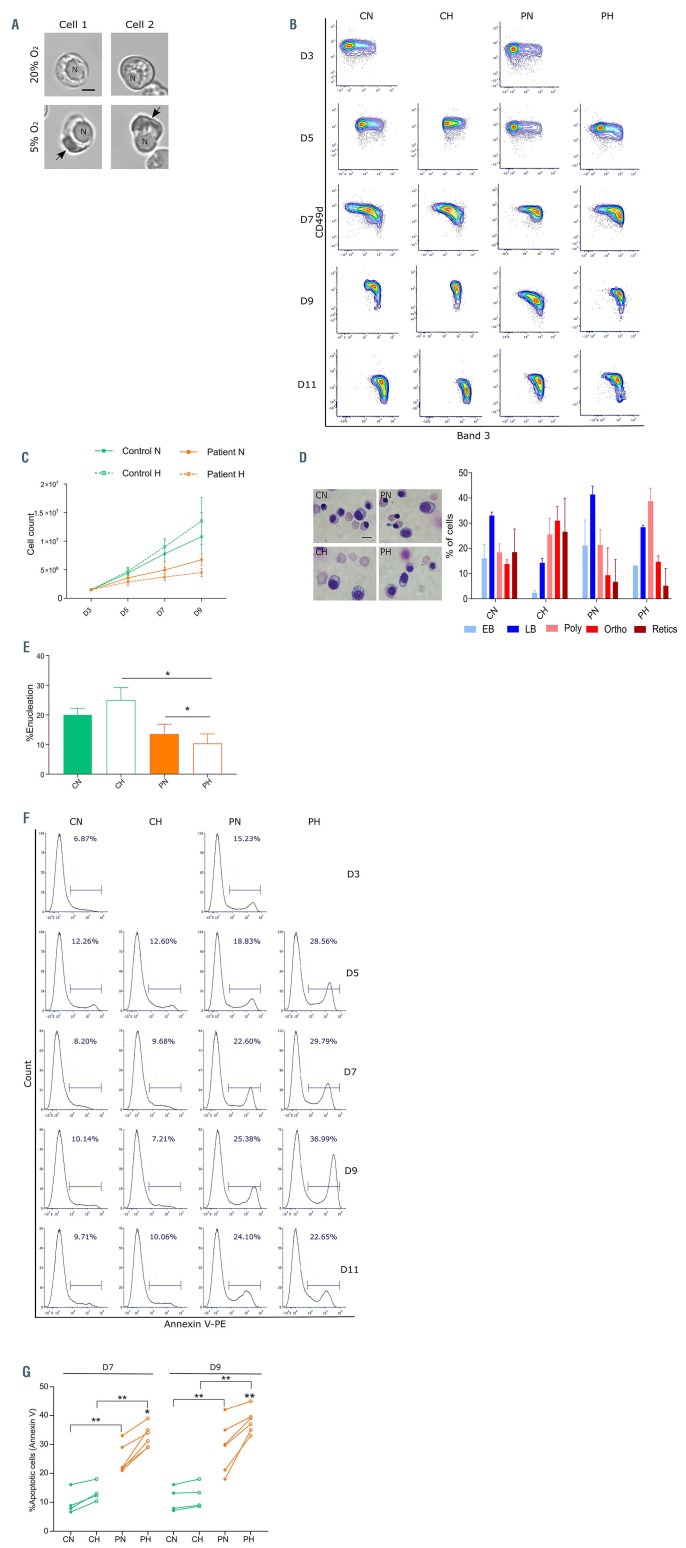
The bone marrow environment has been well documented to be hypoxic (0.1-6% O2).16-18 As hypoxia induces HbS polymerization, we hypothesized that cell death may occur in vivo because of HbS polymer formation in the late stages of differentiation characterized by high intracellular hemoglobin concentration. In order to test our hypothesis, we performed in vitro erythroid differentiation using CD34+ cells isolated from SCD patients and from healthy donors. A two-phase erythroid differentiation protocol was used and cultures were performed at two different oxygen conditions, i.e., normoxia and partial hypoxia (5% O2), starting at D3 of the second phase, at which time hemoglobin synthesis begins to increase markedly (Online Supplementary Figure S1). The choice of 5% O2 was made since it falls at the high end of the reported oxygen tension range of the hematopoietic niche (0.1-6% O2),17 and because it drives HbS polymerization and cell sickling, as we previously reported.19 First, we performed video microscopy experiments with nucleated SCD erythroblasts at D9 of culture and confirmed their ability to sickle at 5% O2 (Figure 1A; Online Supplementary Video S1). Differentiation of control erythroblasts showed no difference in the general waterfall pattern between normoxia and hypoxia (Figure 1B), although hypoxia translated into a consistently higher but not statistically significant increase in total cell count (Figure 1C). Under normoxia, SCD differentiation showed a mild deceleration till D9 as compared to control (Figure 1B), with a proliferation that was negatively impacted by hypoxia (Figure 1C). Under both oxygen conditions, cell proliferation was significantly higher in the control than in the SCD cultures, starting from D7 (Figure 1C). May Grünwald-Giemsa (MGG) staining confirmed the differentiation delay of SCD cells, especially under hypoxia where cells seemed to accumulate at the polychromatic stage when compared to control cells (Figure 1D). In addition, higher proportions of enucleated cells were found in control cells at D9 (Figure 1D) and D11 (Figure 1E). Enucleation was improved under hypoxia for control erythroblasts while it was significantly diminished for SCD cells (Figure 1E), indicating a negative impact of hypoxia at this critical maturation step in the context of SCD.
In order to assess if the decrease in proliferation in SCD was due to cell death, we measured the percentage of apoptotic cells in the cultures by staining the glycophorin A (GPA)-positive cells with annexin V (Figure 1F). The percentage of annexin V+ cells was higher in SCD than in control cultures under both oxygen conditions at D7 and D9, with a greater variability among SCD than in control cells (Figure 1G). Furthermore, the extent of apoptosis of SCD cells was higher under hypoxia than under normoxia at both time points while no difference was noted for control cells (Figure 1G). Altogether, these findings imply that even under a conservative choice of 5% O2 to mimic hypoxia in bone marrow there is increased cell death during the terminal differentiation stages in SCD cells only.
F-cells are enriched during sickle cell disease erythroid differentiation
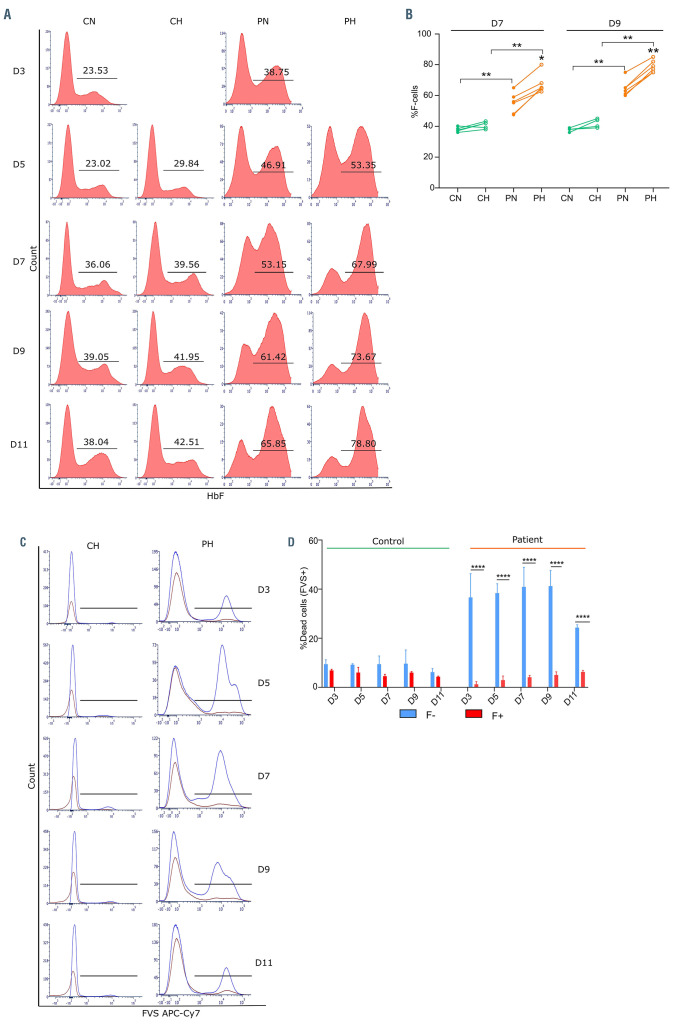
Using flow cytometry, we measured the percentage of cells expressing HbF (F-cells) during in vitro differentiation (Figure 2A). The percentage of F-cells (%F-cells) in control cultures fell within the reported range of 20-40%,20,21 while it was very variable for SCD cells reaching more than 70% at D9 (Figure 2B). Interestingly, there was no difference of %F-cells between normoxia and hypoxia for control cells, while for SCD, %F-cells was higher under hypoxia than under normoxia for all of the six independent primary cell samples (Figure 2B). Taken together with the apoptosis data, these findings imply that F-cells were positively selected under hypoxia in SCD. This inference was supported by flow cytometry data showing higher percentages of dead cells, based on the fixable viability stain (FVS), within the non-F-cell population as compared to the F-cell population for SCD cells (Figure 2C and D). In contrast, these percentages were similar between both cell populations in the control (Figure 2C and D), confirming preferential apoptosis of the cells with low/no HbF expression in the SCD context only.
HSP70 is sequestered in the cytoplasm of non-F-cells
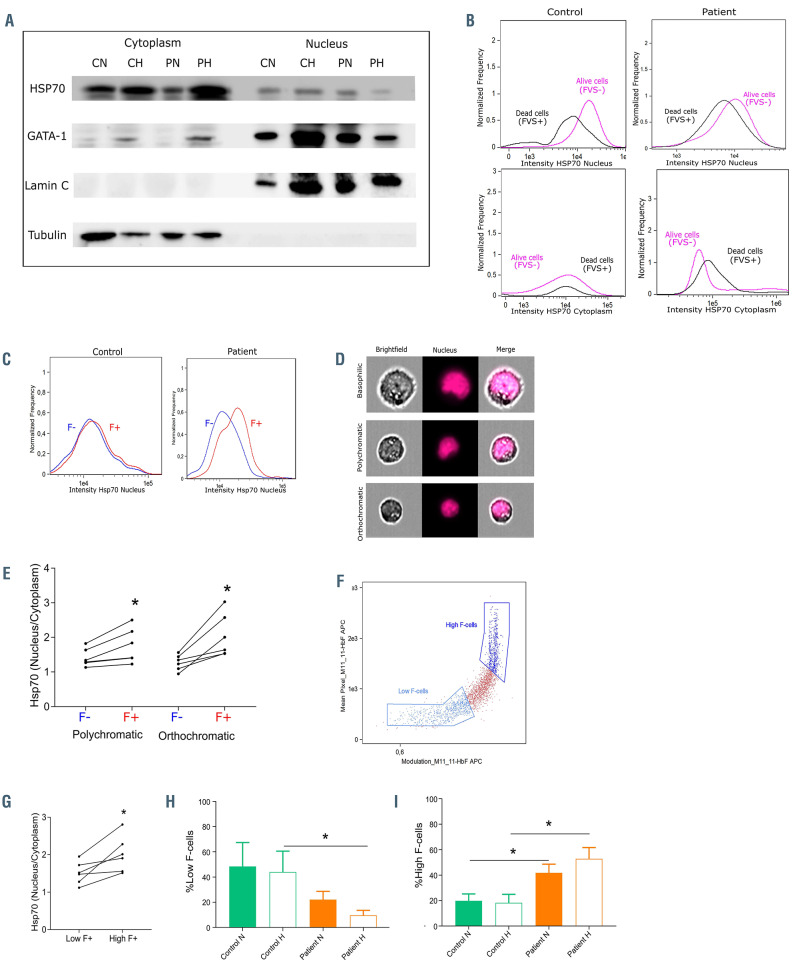
As cytoplasmic sequestration of the chaperone protein HSP70 by α-globin aggregates is associated with cell death during erythropoiesis in b-thalassemia major,5 we investigated if apoptosis of SCD erythroblasts might be due to cytoplasmic trapping of HSP70 by HbS polymers. We performed western blot analyses to quantify HSP70 in the cytoplasmic and nuclear extracts of erythroblasts at D7 of phase II of culture (Figure 3A). There was less HSP70 in the nucleus and more in the cytoplasm of SCD erythroblasts under hypoxia compared to normoxia (Figure 3A), indicating mislocalization of HSP70 in SCD cells under hypoxia. Importantly, these lower amounts of nuclear HSP70 were associated with lower amounts of GATA-1 (Figure 3A), suggesting that cell death under these conditions was likely due to altered protection of GATA-1 by HSP70.
The cells used in these assays were pools of live and apoptotic, F-cells and non-F-cells. In order to better address HSP70 localization in each of these subpopulations, we performed imaging flow cytometry experiments with cells stained for multiple markers. Only Hoechst-positive cells with high GPA expression were taken into consideration to exclude both reticulocytes and proerythroblasts. HSP70 fluorescence intensity was measured in the cytoplasm and the nucleus of live (FVS-) and dead (FVS+) cells at D7. HSP70 nuclear intensity was lower in dead than FVS- cells, for both control and SCD cells under hypoxia (Figure 3B). Moreover, SCD FVS+ cells showed higher HSP70 cytoplasmic levels than FVS- cells, while no difference was detected between both subpopulations for control cells (Figure 3B). These results suggest that hypoxia-induced cell death in the SCD context is likely due to HSP70 entrapment in the cytoplasm. Next, we measured HSP70 intensity in F-cells and non-F-cells. Under hypoxia at D7, HSP70 nuclear intensity was higher in SCD F-cells than non-F cells, while there was no difference between both cell types in control (Figure 3C; Online Supplementary Figure S2A). We then measured the nucleus/cytoplasm (N/C) ratio of HSP70 intensity in polychromatic and orthochromatic cells. The cell subtypes were identified based on morphological parameters of nuclear and cytoplasmic areas22 (Online Supplementary Figure S2B; Online Supplementary Table S1) that discriminate between the basophilic, polychromatic and orthochromatic erythroblasts (Figure 3D; Online Supplementary Figure S2C and D). HSP70 N/C ratio was significantly higher in SCD F than non-F-cells (Figure 3E) which was not the case for control cells (Online Supplementary Figure S2E), indicating that increased HSP70 cytoplasmic entrapment is a feature of SCD non-F-cells. Although there was no significant difference of HSP70 N/C ratio between SCD F-cells and control, this ratio showed a broader distribution in SCD F-cells (Online Supplementary Figure S2E). We hypothesized that this heterogeneity might be linked to the variable intracellular levels of HbF in these cells. Using an analysis mask that quantifies the amount of HbF per cell, we classified F-cells as either low or high F-expressing cells, depending on the intracellular expression level of HbF (Figure 3F). We found that HSP70 N/C ratio was higher in high F-cells compared to low Fcells (Figure 3G), implying that the amounts of HSP70 trapped in the cytoplasm were inversely related to the cytoplasmic content of HbF and suggesting that high amounts of HbF protect SCD erythroblasts against apoptosis. This inference was supported by our findings showing lower percentages of low F-cells in SCD cultures as compared to control, as well as lower percentages of SCD low F-cells under hypoxic than normoxic conditions (Figure 3H). On the other hand, the percentage of high F-cells was significantly higher in SCD erythroblasts as compared to control, with even higher percentages under hypoxia (Figure 3I). Of note, no difference in the percentage of low or high F-cells was observed between hypoxia and normoxia for control cells (Figure 3H and I), indicating that the differences observed for SCD cells are the result of differential cell death related to HbF expression levels.
Figure 1.
Cell proliferation and apoptosis during terminal erythroid differentiation in vitro under normoxia and partial hypoxia. (A) Microscopy images of two sickle cell disease (SCD) in vitro cultured erythroblasts incubated for 30 minutes at 20% (upper panel) and 5% (lower panel) oxygen. “N” represents the nucleus and the arrow points to the HbS polymers formed under 5% oxygen; scale bar: 5 mm. (B) A contour plot representing the distribution of glycophorin A (GPA)-positive cells with respect to the expression of Band 3 (x-axis) and CD49d (y-axis) at day (D) 3, D5, D7, D9 and D11 of phase II of culture in control erythroid precursors under normoxia (CN) or hypoxia (CH), and in patient erythroid precursors under normoxia (PN) or hypoxia (PH). (C) Cell count of erythroid precursors at D3, 5, 7 and 9 in control (n=4) and patient (n=6) under normoxia (N) and hypoxia (H) (means ± standard error of the mean [SEM]). (D) May Grunwald-Giemsa staining of erythroid precursors at D9 of phase II of culture (left panel; scale bar: 10 mm) and graph representing the cellular distribution as means ± SEM of early basophilic (EB), late basophilic (LB), polychromatic (Poly), orthochromatic (Ortho) and reticulocytes (Retics) at D9 of culture of control normoxia (CN), control hypoxia (CH), patient normoxia (PN) and patient hypoxia (PH). (E) Percentage of enucleation measured at D11 for CN, CH, PN and PH erythroblasts (means ± SEM; CN and CH: n=3; PN and PH: n=6). (F) Flow cytometry plots showing percentage of apoptotic cells (Annexin V-positive cells) measured at D3, D5, D7, D9 and D11. (G) Percentage of apoptotic cells in control (n=4) and patients (n=6) under normoxia (N) and hypoxia (H) at D7 and D9 of phase II of culture. *P<0.05, **P<0.01; Wilcoxon paired test and Mann-Whitney test (E and G).
HbS-HSP70 protein complex in sickle cell disease cells under hypoxia
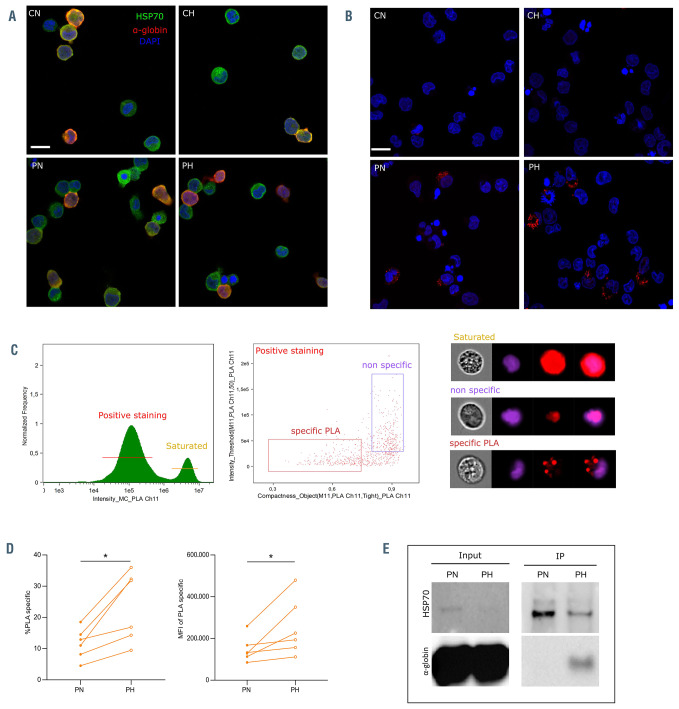
In order to explore the molecular mechanism of cytoplasmic trapping of HSP70, we performed immunofluorescence assays using confocal microscopy and found colocalization of HSP70 and hemoglobin in both control and SCD cells (Figure 4A). We used Pearson’s correlation coefficient23,24 to assess the degree of co-localization and found no difference between both cell types (data not shown), probably because of the high abundance of hemoglobin in the cytoplasm. In order to overcome this limitation, we performed PLA, that show fluorescent spots when two proteins are at a distance <40 nm.25 We detected SCD cells with fluorescent spots (PLA+) under both normoxic and hypoxic conditions (Figure 4B) that were further quantified by imaging flow cytometry (Figure 4C). The percentage of PLA+ cells was very low in control, with values close to background levels (1-1.5%) under both normoxia and hypoxia, while SCD cells showed higher values under normoxia (4.5-18.5%) with a systematic and significant increase under hypoxia (9.5-36%) (Figure 4D), indicating proximity between HSP70 and HbS but not HSP70 and HbA. Moreover, PLA+ SCD cells showed higher mean fluorescence intensity under hypoxia than under normoxia (Figure 4D), indicating that hypoxia induces the formation of more potent HbS-HSP70 complexes that could account for cytoplasmic retention of HSP70.
In order to explore the potential of HbS polymers and HSP70 interacting within the same protein complex, we performed co-immunoprecipitation assays. SCD RBC suspensions were placed at normoxia or hypoxia then lysed, and HSP70 was immunoprecipitated. Using an anti-α-globin antibody we found co-immunoprecipitation of HSP70 and α-globin under hypoxia but not under normoxia (Figure 4E) supporting the presence of HbS-HSP70 protein complex. Of note, despite using the same amounts of RBC for both conditions, there was less immunoprecipitated HSP70 under hypoxia than normoxia likely due to decreased solubility of HbS polymer fibers under hypoxia as evidenced by the color of the lysates under both conditions and the presence of a small red colored precipitate under hypoxia (Online Supplementary Figure S3).
Cell death during the terminal stages of erythroid differentiation in bone marrow of sickle cell disease patients
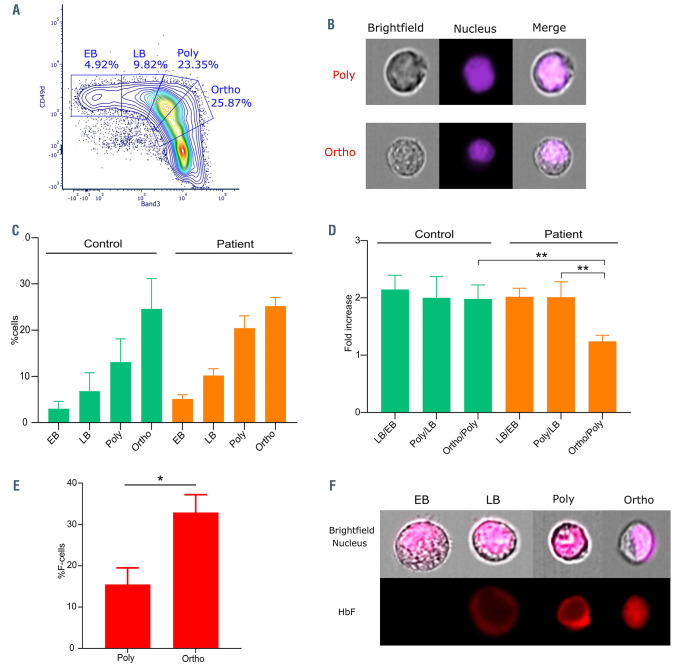
In order to validate the direct relevance of the in vitro findings to in vivo conditions, we analyzed terminal erythroid differentiation using unmanipulated marrow samples from SCD patients. Bone marrow aspirates were obtained from five SCD patients and cells were stained for surface markers GPA, CD49d and Band 3 and analyzed by flow cytometry. Differentiating erythroblasts were determined using the CD49d and Band 3 staining pattern within the GPA+ population (Figure 5A), as previously described.14 Imaging flow cytometry was used to confirm gating of all nucleated cells and cell homogeneity within each of the four gated populations by cellular features of size, morphology and nuclear size and polarization (Figure 5B). We quantified the cells at the early basophilic (EB), late basophilic (LB), polychromatic and orthochromatic stages. Considering the GPA positive population as 100%, the mean percentages of EB, LB, polychromatic and orthochromatic cells were 5.1%, 10.2%, 20.4% and 25.2% (Figure 5C), indicating loss of the expected cell doubling between the polychromatic and orthochromatic stages (1.24+/-0.1, P<0.01; Figure 5D). These results implied that cell death occurs between the polychromatic and orthochromatic stages in a significant proportion of erythroblasts, in accordance with our in vitro data. Similar analysis was performed with bone marrow aspirates of five controls (3% EB, 6.8% LB, 13% polychromatic cells and 24.6% orthochromatic cells, Figure 5C) that confirmed the expected doubling of cells with successive cell divisions without cell loss between development stages during normal erythropoiesis (Figure 5D). Next, we stained the cells with an anti-HbF antibody to measure the percentage of F-cells at the different stages. There was a significant increase in %F-cells between the polychromatic (16.4%±4) and orthochromatic stages (32.4%±4.79) (Figure 5E and F), concomitant with the cell loss observed between these stages (Figure 5C and D), indicating preferential survival of F-cells during late stages of erythroblast maturation in vivo and supporting our hypothesis for an anti-apoptotic role of HbF during in vivo erythropoiesis in SCD.
Induction of fetal hemoglobin protects against cell death
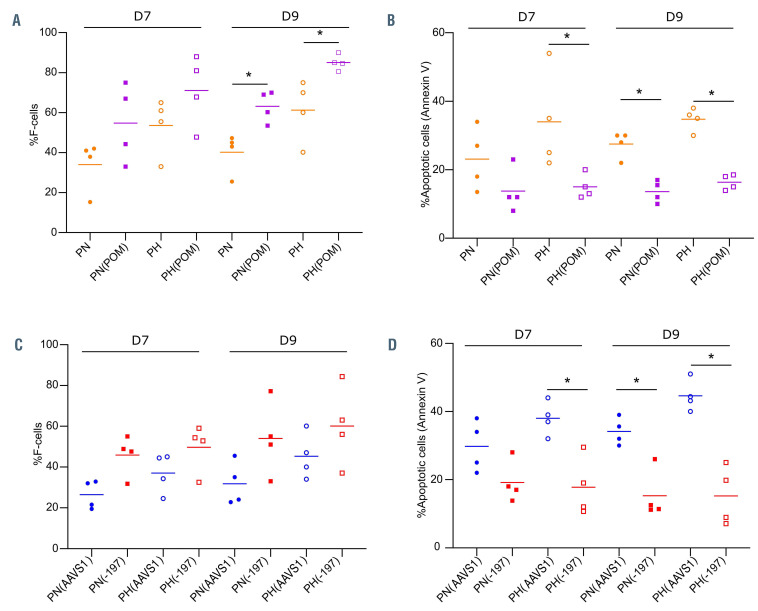
In order to confirm the anti-apoptotic role of HbF in SCD erythroblasts we induced its expression in vitro using pomalidomide (POM), an immunomodulatory drug previously shown to induce HbF expression during erythropoiesis,11,26 and determined if higher HbF levels could rescue the cells from apoptosis. As we were interested in monitoring the stages during which hemoglobin is synthesized, POM was added at D1 of phase II of culture. As expected, POMtreated SCD cultures showed higher percentages of F-cells than untreated cultures (Figure 6A; Online Supplementary Figure S4A). HbF induction by POM was associated with significantly lower levels of apoptosis compared to untreated cultures under both hypoxia and normoxia (Figure 6B; Online Supplementary Figure S4B). Importantly, there was no significant difference in the apoptosis levels between normoxic and hypoxic conditions of POM-treated cells indicating that the higher F-cell levels protected SCD cells from apoptosis under hypoxia.
In order to specifically address the role of HbF, we used a CRISPR-Cas9 approach that we have recently developed to mimic the effect of hereditary persistence of fetal hemoglobin (HPFH) by disrupting the binding site for HbFrepressor LRF in the fetal γ-globin promoters (HBG1 and HBG2)12 (Online Supplementary Figure S4C). CD34+ cells were transfected either with a gRNA targeting the LRF binding site (-197) or a gRNA targeting an unrelated locus (AAVS1). Genome editing efficiency at the γ-globin promoter ranged between 23.2% and 73.5%, as determined by Sanger sequencing at D6 of phase I of culture. Cells were grown and differentiated under normoxia or hypoxia. As expected, the disruption of the LRF binding site resulted in HbF induction as shown by higher %F-cells compared to AAVS1 control (Figure 6C; Online Supplementary Figure S4D). These higher levels of F-cells resulted in decreased apoptosis, under both normoxic and hypoxic conditions (Figure 6D; Online Supplementary Figure S4E), clearly demonstrating the positive and selective effect of HbF on SCD cell survival.
Figure 2.
Distribution of erythroid precursors expressing fetal hemoglobin in vitro. (A) Flow cytometry plots showing the percentage of cells expressing fetal hemoglobin (F-cells) at day (D) 3, D5, D7, D9 and D11 of phase II of culture in control erythroid precursors under normoxia (CN) or hypoxia (CH), and in patient erythroid precursors under normoxia (PN) or hypoxia (PH). (B) Flow cytometry plots showing dead cells, measured by fixable viability stain (FVS APC-Cy7), in non-F-cells (F-) (blue curve) and F-cells (F+) (red curve) of control (CH) and patient (PH) cells under hypoxia. (C) Percentage of F-cells measured at D7 and D9 of phase II of culture in control (n=4) and patient cells (n=6) under normoxia (N) and hypoxia (H). (D) Percentage of dead cells (means ± standard error of the mean) measured in the F- and F+ subpopulations for control and patient cells under hypoxia. *P<0.05, **P<0.01, ****P<0.0001; Wilcoxon paired test and Mann-Whitney test (C); Mann-Whitney test (D)
Discussion
Ineffective erythropoiesis has been previously suggested to be a feature of SCD7-9,27 but it has not been critically evaluated and documented. Our present findings provide direct evidence for ineffective erythropoiesis in SCD patients, with significant cell death occurring at the late stages of terminal erythroid differentiation in vivo.
Among previous reports, the study by Wu and collaborators has highlighted abnormalities during erythropoiesis in the bone marrow of transplanted SCD patients by showing progressive intramedullary loss of SCD erythroblasts and relative enrichment of donor erythroid precursors at the onset of hemoglobinization in a small cohort of patients.9 Our results further demonstrate significant cell death between the polychromatic and the orthochromatic stages, when cellular HbS concentration reaches sufficiently high levels to promote polymer formation under partial hypoxia. Our in vivo data unequivocally document the occurrence of ineffective erythropoiesis in non-transplanted SCD patients, in the absence of confounding factors like conditioning drugs and exogenous donor-related factors that can impact the hematopoietic niche and interfere with the survival of patient’s erythroblasts.
This study also reveals a new role for HbF in SCD by showing that it protects a subpopulation of differentiating erythroblasts from apoptosis, both in vivo and in vitro. HbF is a known modulator of disease severity in SCD as it inhibits HbS polymerization at the molecular level, preventing or attenuating RBC sickling and alleviating disease complications.28,29 In healthy adults, HbF accounts for less than 1% of total hemoglobin30 and is restricted to a small subset of RBC (2%) called F-cells.31-33 In SCD, the expression of HbF is higher than in healthy individuals and varies considerably among patients. Although the mechanisms underlying increased expression of HbF are not completely elucidated, stress erythropoiesis and preferential survival of F-cells in the circulation are suggested contributing factors.34-38 Our findings show that high HbF levels not only increase survival of circulating red cells but also play a role in the preferential survival of erythroblasts under physiologically relevant hypoxic conditions in the bone marrow.
Figure 3.
HSP70 cytoplasmic and nuclear distribution. (A) Western blot analysis of HSP70, GATA-1, lamin C and tubulin performed on cytoplasmic and nuclear extracts of erythroid precursors at day 7 (D7) of phase II of culture from control normoxia (CN), control hypoxia (CH), patient normoxia (PN) and patient hypoxia (PH). (B) Distribution of the nuclear intensity (upper panel) and cytoplasmic intensity (lower panel) of HSP70 at D7 of phase II of culture in dead cells (FVS+) and live cells (FVS-) of control and patient cells under hypoxia. (C) Distribution of the nuclear intensity of HSP70 at D7 of phase II of culture in the F- and F+ subpopulations of control (left) and patient (right) cells under hypoxia. (D) Images of basophilic, polychromatic and orthochromatic subpopulations obtained by imaging flow cytometry. (E) HSP70 nucleus/cytoplasm ratio in F- and F+ polychromatic and orthochromatic patient erythroblasts at D7 of phase II of culture (n=6). (F) Dot plot representing the modulation mask of fetal hemoglobin (HbF) (x-axis) and mean pixel HbF values (y-axis) used to discriminate between low and high F-cells. (G) HSP70 nucleus/cytoplasm ratio in low and high F-cells of patients’ orthochromatic erythroblasts at D7 of phase II of culture under hypoxia (n=6). Percentage of (H) Low Fcells and (I) High F-cells in control (n=3) and patient (n=6) cells at normoxia and hypoxia at D7 of phase II of culture (means ± standard error of the mean). *P<0.05. Wilcoxon paired test (E and G) and Mann-Whitney unpaired test (H and I). FVS: fixable viability stain; F-cells: cells expressing fetal hemoglobin.
Figure 4.
HSP70 and α-globin colocalization and co-immunoprecipitation. (A) Confocal microscopy images of control erythroid precursors under normoxia (CN) or hypoxia (CH), and of patient erythroid precursors under normoxia (PN) or hypoxia (PH) at day 7 (D7) of phase II of culture showing colocalization (in yellow) of HSP70 (green) and α-globin (red); nucleus is in blue (n=3); scale bar: 10 mm. (B) Proximity ligation assay (PLA) between HSP70 and α-globin at D7 of phase II of culture. Red spots indicate proximity (<40 nm) between both proteins. Spots were observed in PN and PH cultures, while no spots were seen in CN and CH. A representative image of each culture is shown (n=3); scale bar: 10 mm. (C) (Left) A histogram representing the intensity of APC signal generated by PLA, a gating of positive staining and saturated staining is indicated. (Middle) A dot plot representing an analysis mask using the compactness feature (x-axis) and intensity feature (y-axis) to discriminate between PLA-specific staining and non-specific staining. (Right) Representative images of each gate. (D) Percentage of cells (left) and mean fluorescence intensity (right) of PLA-specific staining in erythroid precursors of patient normoxia (PN) and patient hypoxia (PH) at D9 of phase II of culture (n=6). (E) Co-immunoprecipitation assay of HSP70 with α-globin using circulating sickle cell disease (SCD) red blood cells incubated under normoxia (PN) or hypoxia (PH) for 1 hour. HSP70 and α- globin bands are detected in the lysates (left panel) and after HSP70 immunoprecipitation (right panel). *P<0.05, Wilcoxon paired test (D).
Our findings show that in vitro induction of HbF by pomalidomide rescues SCD erythroblasts from cell death and improves their differentiation, in accordance with improved efficiency of erythropoiesis in vivo in treated SCD mice.39 Furthermore, results using genetically modified CD34+ cells strongly imply that overexpression of HbF and concomitant down-regulation of HbS12 in a specific and selective manner corrects the apoptosis observed during erythroid differentiation, which is therefore presumably a feature of HbS.
HSP70 is a chaperone protein that plays an important role during erythropoiesis by protecting GATA-1 from cleavage by caspase-3 in the presence of Epo,40 thus promoting normal terminal differentiation. From a mechanistic perspective, sequestration of HSP70 in the cytoplasm through interaction with HbS polymers is therefore a possible mechanism for ineffective erythropoiesis in SCD. Co-immunoprecipitation of HSP70 and HbS under hypoxia is indeed highly suggestive of such interactions between these proteins. Furthermore, specific HSP70 sequestration in the pool of SCD dead cells under hypoxia could account for the lower HSP70 nuclear content of these cells and the subsequent lower GATA-1 nuclear levels. As the molecular event that initiates HSP70 trapping in SCD, namely HbS polymerization, occurs in cells with high cellular concentration of HbS, required for polymer formation, our results show the absence of cell death in the early stages of differentiation where the intracellular concentration of HbS is likely insufficient to induce sickling. Likewise, low levels of HbF are less protective against cell death, since a minimal threshold of intracellular HbF is needed for a protective polymer-inhibiting effect.41 At physiological levels of hypoxia, there is however not an exclusive selection of F-cells, as significant amounts of cells with no/low HbF were found to complete erythroid differentiation. Further studies are needed to fully address the biological mechanisms underlying this observation and the commitment of erythroid progenitors to the F lineage in SCD.
Figure 5.
Analysis of human terminal erythroid differentiation in vivo. (A) A contour plot representing the distribution of glycophorin A (GPA)-positive cells with respect to Band 3 (x-axis) and CD49d (y-axis) from a bone marrow sample of a sickle cell disease (SCD) patient. (B) Images obtained using imaging flow cytometry from the gating of polychromatic (Poly) and orthochromatic (Ortho) erythroblasts. Nucleus was stained with Hoechst. (C) Percentage of cells at the early basophilic (EB), late basophilic (LB), polychromatic (Poly) and orthochromatic (Ortho) stages in five bone marrow samples of controls (green) and SCD patients (orange) (means ± standard error of the mean [SEM]). (D) The fold increase of cells between the EB and LB (LB/EB), LB and Poly (Poly/LB), Poly and Ortho (Ortho/Poly) stages in five controls and five SCD patients (means ± SEM). (E) Percentage of cells expressing fetal hemoglobin (F-cells) in vivo in the polychromatic (Poly) and orthochromatic (Ortho) subpopulations of the patient bone marrow samples (n=5) (means ± SEM). (F) Imaging flow cytometry images of early basophilic (EB), late basophilic (LB), polychromatic (Poly) and orthochromatic (Ortho) precursors. Upper images are a merge of brightfield and nucleus, lower images are for fetal hemoglobin (HbF) staining (red). *P<0.05, **P<0.01; Mann-Whitney test (D and E).
Finally, this study sheds light on the importance of applying partial hypoxia during erythroid differentiation in vitro in order to mimic in vivo conditions in SCD. In our view, this together with cell proliferation and apoptosis are important parameters to consider in assessing the beneficial impact of therapeutic approaches in SCD such as HbF induction,10,20,42 anti-sickling molecules such as voxelotor43,44 or gene therapy aiming at expressing a therapeutic b-globin.45 In addition, specific targeting of ineffective erythropoiesis should presumably have a major beneficial clinical impact.
In summary, our study shows that HbF has a dual beneficial effect in SCD by conferring a preferential survival of F-cells in the circulation and by decreasing ineffective erythropoiesis. These findings thus bring new insights into the role of HbF in modulating clinical severity of anemia in SCD by both regulating red cell production and red cell destruction.
Figure 6.
Effect of fetal hemoglobin induction by pomalidomide or CRISPR/Cas9 on terminal erythroid differentiation of sickle cell disease erythroblasts. (A) Percentage of cells expressing fetal hemoglobin (F-cells) at day (D) 7 and D9 of phase II of culture of culture in patient erythroblasts under normoxia (PN), hypoxia (PH), normoxia with POM [PN(POM)] and hypoxia with POM [PH(POM)] (n=4). (B) Percentage of F-cells at D7 and D9 of phase II of culture in patient erythroblasts under normoxia (PN) and hypoxia (PH) treated with guide RNA (gRNA) targeting the LRF binding site (-197) or an unrelated locus as control (AAVS1) (n=4). Genome editing efficiency was 56.1 ± 9.6% and 79.2± 2.8% for -197 and AAVS1 samples, respectively. (C) Percentage of apoptotic cells measured by flow cytometry in PN, PH, PN(POM) and PH(POM) at D7 and D9 of phase II of culture of culture (n=4). (D) Percentage of apoptotic cells at D7 and D9 of phase II of culture in patient erythroblasts under normoxia (PN) and hypoxia (PH) treated with gRNA targeting the LRF binding site (-197) or an unrelated locus as control (AAVS1) (n=4). Horizontal bars represent the mean of each group; *P<0.05, Mann-Whitney test (A, B and D).
Supplementary Material
Acknowledgments
We thank the patients and their families for accepting to be part of this study. We thank Dr. Jean-Philippe Semblat, Dr. Maria Alejandra Lizarralde-Iragorri and Ms Sandrine Genetet for technical support, Dr. Flavia Guillem, Dr. Thiago Trovati Maciel and Dr. Olivier Hermine for helpful discussions, Dr. Slimane Allali and the nursing staff of Hôpital de jour Pédiatrie Générale of Hôpital Necker Enfants Malades for patient management. We thank Dr. Lionel Blanc for providing non-anemic bone marrow samples.
Funding Statement
Funding: The work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM), Institut National de la Transfusion Sanguine, and grants from Laboratory of Excellence GR-Ex, reference ANR-11-LABX-0051, EUR G.E.N.E., reference ANR-17-EURE-0013, and NIH grant DK32094. The labex GR-Ex is funded by the IdEx program “Investissements d’avenir” of the French National Research Agency, reference ANR-18-IDEX-0001. PICT-IBiSA is part of the France- BioImaging infrastructure funded by ANR-10-INBS-04. SEH was funded by the Ministère de l’Enseignement Supérieur et de la Recherche (Ecole Doctorale BioSPC) and received financial support from, addmedica, the Club du Globule Rouge et du Fer and the Société Française d’Hématologie.
References
- 1.Pauling L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a molecular disease. Science. 1949;110(3):543-548. [DOI] [PubMed] [Google Scholar]
- 2.Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376(16):1561-1573. [DOI] [PubMed] [Google Scholar]
- 3.Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390(10091):311-323. [DOI] [PubMed] [Google Scholar]
- 4.Arlet JB, Dussiot M, Moura IC, Hermine O, Courtois G.Novel players in beta-thalassemia dyserythropoiesis and new therapeutic strategies. Curr Opin Hematol. 2016;23(3):181-188. [DOI] [PubMed] [Google Scholar]
- 5.Arlet JB, Ribeil JA, Guillem F, et al. HSP70 sequestration by free alpha-globin promotes ineffective erythropoiesis in beta-thalassaemia. Nature. 2014;514(7521):242-246. [DOI] [PubMed] [Google Scholar]
- 6.Rivella S. Ineffective erythropoiesis and thalassemias. Curr Opin Hematol. 2009;16(3): 187-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasegawa S, Rodgers GP, Dwyer N, et al. Sickling of nucleated erythroid precursors from patients with sickle cell anemia. Exp Hematol. 1998;26(4):314-319. [PubMed] [Google Scholar]
- 8.Blouin MJ, De Paepe ME, Trudel M.Altered hematopoiesis in murine sickle cell disease. Blood. 1999;94(4):1451-1459. [PubMed] [Google Scholar]
- 9.Wu CJ, Krishnamurti L, Kutok JL, et al. Evidence for ineffective erythropoiesis in severe sickle cell disease. Blood. 2005;106(10):3639-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McArthur JG, Svenstrup N, Chen C, et al. A novel, highly potent and selective phosphodiesterase- 9 inhibitor for the treatment of sickle cell disease. Haematologica. 2020;105(3):623-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dulmovits BM, Appiah-Kubi AO, Papoin J, et al. Pomalidomide reverses gamma-globin silencing through the transcriptional reprogramming of adult hematopoietic progenitors. Blood. 2016;127(11):1481-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber L, Frati G, Felix T, et al. Editing a gamma-globin repressor binding site restores fetal hemoglobin synthesis and corrects the sickle cell disease phenotype. Sci Adv. 2020;6(7):eaay9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinkman EK, Chen T, Amendola M, van Steensel B.Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 2014;42(22):e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu J, Liu J, Xue F, et al. Isolation and functional characterization of human erythroblasts at distinct stages: implications for understanding of normal and disordered erythropoiesis in vivo. Blood. 2013;121(16):3246-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological- image analysis. Nat Methods. 2012;9(7):676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantel CR, O'Leary HA, Chitteti BR, et al. Enhancing hematopoietic stem cell transplantation efficacy by mitigating oxygen shock. Cell. 2015;161(7):1553-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7(2):150-161. [DOI] [PubMed] [Google Scholar]
- 18.Yeo JH, Cosgriff MP, Fraser ST. Analyzing the formation, morphology, and integrity of erythroblastic islands. Methods Mol Biol. 2018;1698:133-152. [DOI] [PubMed] [Google Scholar]
- 19.Lizarralde Iragorri MA, El Hoss S, Brousse V, et al. A microfluidic approach to study the effect of mechanical stress on erythrocytes in sickle cell disease. Lab Chip. 2018;18(19):2975-2984. [DOI] [PubMed] [Google Scholar]
- 20.Antoniani C, Meneghini V, Lattanzi A, et al. Induction of fetal hemoglobin synthesis by CRISPR/Cas9-mediated editing of the human beta-globin locus. Blood. 2018;131(17):1960-1973. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Paikari A, Sumazin P, et al. Metformin induces FOXO3-dependent fetal hemoglobin production in human primary erythroid cells. Blood. 2018;132(3):321-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalfa T, McGrath KE. Analysis of erythropoiesis using imaging flow cytometry. Methods Mol Biol. 2018;1698:175-192. [DOI] [PubMed] [Google Scholar]
- 23.Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224(Pt 3):213-232. [DOI] [PubMed] [Google Scholar]
- 24.Manders EM, Stap J, Brakenhoff GJ, van Driel R, Aten JA. Dynamics of three-dimensional replication patterns during the Sphase, analysed by double labelling of DNA and confocal microscopy. J Cell Sci. 1992;103 (Pt 3):857-862. [DOI] [PubMed] [Google Scholar]
- 25.Schallmeiner E, Oksanen E, Ericsson O, et al. Sensitive protein detection via triple-binder proximity ligation assays. Nat Methods. 2007;4(2):135-137. [DOI] [PubMed] [Google Scholar]
- 26.Moutouh-de Parseval LA, Verhelle D, Glezer E, et al. Pomalidomide and lenalidomide regulate erythropoiesis and fetal hemoglobin production in human CD34+ cells. J Clin Invest. 2008;118(1):248-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finch CA, Lee MY, Leonard JM. Continuous RBC transfusions in a patient with sickle cell disease. Arch Intern Med. 1982;142(2):279-282. [PubMed] [Google Scholar]
- 28.Powars DR, Weiss JN, Chan LS, Schroeder WA. Is there a threshold level of fetal hemoglobin that ameliorates morbidity in sickle cell anemia? Blood. 1984;63(4):921-926. [PubMed] [Google Scholar]
- 29.Sewchand LS, Johnson CS, Meiselman HJ. The effect of fetal hemoglobin on the sickling dynamics of SS erythrocytes. Blood Cells. 1983;9(1):147-166. [PubMed] [Google Scholar]
- 30.Boyer SH, Margolet L, Boyer ML, et al. Inheritance of F cell frequency in heterocellular hereditary persistence of fetal hemoglobin: an example of allelic exclusion. Am J Hum Genet. 1977;29(3):256-271. [PMC free article] [PubMed] [Google Scholar]
- 31.Boyer SH, Belding TK, Margolet L, Noyes AN. Fetal hemoglobin restriction to a few erythrocytes (F cells) in normal human adults. Science. 1975;188(4186):361-363. [DOI] [PubMed] [Google Scholar]
- 32.Boyer SH, Belding TK, Margolte L, Noyes AN, Burke PJ, Bell WR. Variations in the frequency of fetal hemoglobin-bearing erythrocytes (F-cells) in well adults, pregnant women, and adult leukemics. Johns Hopkins Med J. 1975;137(3):105-115. [PubMed] [Google Scholar]
- 33.Wood WG, Stamatoyannopoulos G, Lim G, Nute PE. F-cells in the adult: normal values and levels in individuals with hereditary and acquired elevations of Hb F. Blood. 1975;46(5):671-682. [PubMed] [Google Scholar]
- 34.Stamatoyannopoulos G, Veith R, Galanello R, Papayannopoulou T.Hb F production in stressed erythropoiesis: observations and kinetic models. Ann N Y Acad Sci. 1985;445:188-197. [DOI] [PubMed] [Google Scholar]
- 35.Dover GJ, Boyer SH, Charache S, Heintzelman K.Individual variation in the production and survival of F cells in sicklecell disease. N Engl J Med. 1978;299(26): 1428-1435. [DOI] [PubMed] [Google Scholar]
- 36.Franco RS, Lohmann J, Silberstein EB, et al. Time-dependent changes in the density and hemoglobin F content of biotin-labeled sickle cells. J Clin Invest. 1998;101(12):2730-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franco RS, Yasin Z, Palascak MB, Ciraolo P, Joiner CH, Rucknagel DL. The effect of fetal hemoglobin on the survival characteristics of sickle cells. Blood. 2006;108(3):1073-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maier-Redelsperger M, Noguchi CT, de Montalembert M, et al. Variation in fetal hemoglobin parameters and predicted hemoglobin S polymerization in sickle cell children in the first two years of life: Parisian Prospective Study on Sickle Cell Disease. Blood. 1994;84(9):3182-3188. [PubMed] [Google Scholar]
- 39.Meiler SE, Wade M, Kutlar F, et al. Pomalidomide augments fetal hemoglobin production without the myelosuppressive effects of hydroxyurea in transgenic sickle cell mice. Blood. 2011;118(4):1109-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ribeil JA, Zermati Y, Vandekerckhove J, et al. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature. 2007;445(7123):102-105. [DOI] [PubMed] [Google Scholar]
- 41.Noguchi CT, Rodgers GP, Schechter AN. Intracellular polymerization. Disease severity and therapeutic predictions. Ann N Y Acad Sci. 1989;565:75-82. [DOI] [PubMed] [Google Scholar]
- 42.Paikari A, Sheehan VA. Fetal haemoglobin induction in sickle cell disease. Br J Haematol. 2018;180(2):189-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howard J, Hemmaway CJ, Telfer P, et al. A phase 1/2 ascending dose study and openlabel extension study of voxelotor in patients with sickle cell disease. Blood. 2019;133(17):1865-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vichinsky E, Hoppe CC, Ataga KI, et al. A phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med. 2019;381(6):509-519. [DOI] [PubMed] [Google Scholar]
- 45.Ribeil JA, Hacein-Bey-Abina S, Payen E, et al. Gene therapy in a patient with sickle cell disease. N Engl J Med. 2017;376(9): 848-855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.