Human leukocyte antigen (HLA)-haploidentical hematopoietic stem cell transplantation (haplo-HSCT) with post-transplant cyclophosphamide (pt-cy) is a valid alternative to HLA-identical HSCT,1 but many patients still suffer from viral infections, mostly cytomegalovirus (CMV) reactivation.2 CMV-specific T cells contribute to control of CMV reactivation post-transplant, but their evaluation has been limited to a handful of immunophenotypic parameters. In particular, the dynamics and quality of CMV-specific T cells in relation to immune reconstitution and control of CMV viremia following haplo- HSCT with pt-cy are poorly known. To these aims, we employed high-dimensional flow cytometry simultaneously investigating four effector functions and markers of T-cell differentiation, inhibitory molecules, and metabolic and activation markers, along with computational analysis of single-cell data.
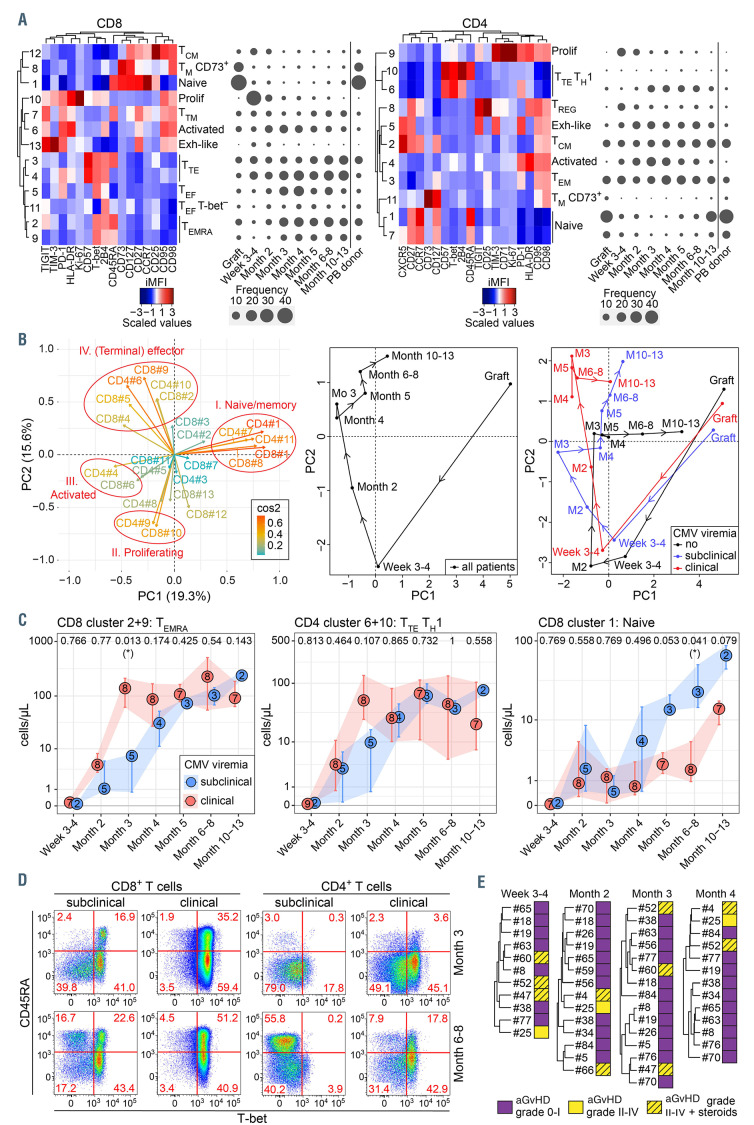
We performed longitudinal analysis of high-dimensional T-cell immunophenotypes in blood samples of 21 recipients of haplo-HSCT with pt-cy treated at our institution for hematological diseases (Online Supplementary Table S1). A median of seven samples per patient were analyzed, including graft (n=13) and peripheral blood (PB) samples ranging from day +21 to day +386 posttransplant (n=111). As control, we included PB samples from donors (n=9) and healthy individuals with detectable CMV pp65-specific T cells (n=17). The clustering tool PhenoGraph3 identified 13 CD8+ and 11 CD4+ T-cell clusters (Figure 1A). Principle component analysis (PCA) of cluster frequencies identified which of the 24 T-cell clusters were co-varying in a time-dependent manner. A variable plot of the first two principle components revealed four major groups of T-cell phenotypes (Figure 1B). Median PCA coordinates of all samples plotted at defined intervals indicated a clear pattern in T-cell dynamics: loss of naïve and CD73+ memory clusters and emergence of proliferating clusters at 3-4 weeks posttransplant, dominance of non-proliferating, activated clusters at month 2 and accumulation of effector and terminal effector (TTE) clusters from month 3 onwards (Figure 1B).
Pt-cy interferes with highly-proliferating alloreactive T cells, and alloreactivity resides preferentially in the naïve pool.4-6 Furthermore, naïve cells that escape pt-cy may initially acquire a stem cell memory phenotype to later give rise to effector cells.6,7 These processes may explain the rapid decline in naïve T cells early after transplant. By week 3-4, Ki-67+HLA–DR+ proliferating CD8+ cluster 10 and CD4+ cluster 9 expanded (Figure 1A), likely in response to a combination of exogenous or alloreactive antigens, homeostatic cytokines and inflammation.6,7 The frequency of proliferating cells declined by month 2 and was accompanied by an increase in activated CD8+ and CD4+ Ki-67–HLA-DR+ T cells (cluster 6 and 4, respectively) that persisted for several months. CD4+ cluster 8 of regulatory T cells (TREG) and CD8+ cluster 13 of TIM-3highPD-1highTIGIThigh cells resembling exhausted cells, displayed dynamics similar to that of proliferating cells (Figure 1A). Transient TREG expansion following haplo-HSCT with pt-cy corroborates previous findings6,8 and seems critical for the prevention of graft-versus-host disease (GvHD) by pt-cy.8,9 From month 3 onwards, the T-cell compartment became dominated by TTE or effector memory re-expressing CD45RA (TEMRA) clusters, expressing T-bet, 2B4, CD45RA and/or senescence marker CD57. One year after transplant, cluster distribution within the CD4+ compartment resembled that of the donor, including partial recovery of the naïve pool, while that within the CD8+ compartment showed a persistent defect in this regard (Figure 1A).
CMV infection is a major event following haplo-HSCT with pt-cy.2 In our cohort, 19 out of 21 patients experienced CMV viremia, with a median onset of 39 days post-transplant. In order to determine the effect of CMV viral load on T-cell reconstitution, we divided patients experiencing post-transplant CMV viremia into two groups using a viremia threshold above which antiviral therapy was given: subclinical CMV viremia (any viremia with peak ≤4,000 IU/mL; n=6) and clinical CMV viremia (peak viremia >4,000 IU/mL; n=13). PCA of T-cell immunophenotypes indicated an accelerated acquisition of T-bet+2B4+CD45RA+/-CD57+/- effector/terminal effector cells in patients with clinical CMV viremia (group IV of CD8 clusters 2, 4, 5, 9 and CD4 clusters 6, 10; Figure 1B). These cells originated from both the CD8+ and CD4+ compartment, reaching statistically significant differences for TEMRA cells in the former (Figure 1C and D). Furthermore, patients with subclinical viremia showed slightly improved recovery of naïve CD8+ T cells. Although limited to two individuals in our cohort, those patients who did not experience CMV viremia lacked TTE clusters and instead showed strong recovery of naïve subsets (Figure 1B). Accordingly, Suessmuth et al. found a decrease in naïve CD8+ T cells in CMV-reactivating patients receiving unmanipulated unrelated allografts, suggesting a link between CMV reactivation and a defect in thymopoiesis.10 Occurrence of clinically significant grade II-IV acute GvHD (aGvHD) and/or its treatment with corticosteroids could be a confounding factor and indeed tended to associate with worse CMV control in our cohort (Online Supplementary Figure S1A). However, hierarchical clustering indicated that patients developing aGvHD or receiving corticosteroids displayed overlapping T-cell cluster dynamics with aGvHD-negative patients (Figure 1E). These data suggest that CMV reactivation has a more prominent effect on T-cell reconstitution than does aGvHD, which is in line with findings at the clonal level in the HLA-matched setting.11 Collectively, our data suggest that high CMV viral load drives premature senescence of T cells and delays recovery of naïve T cells following haplo-HSCT with pt-cy.
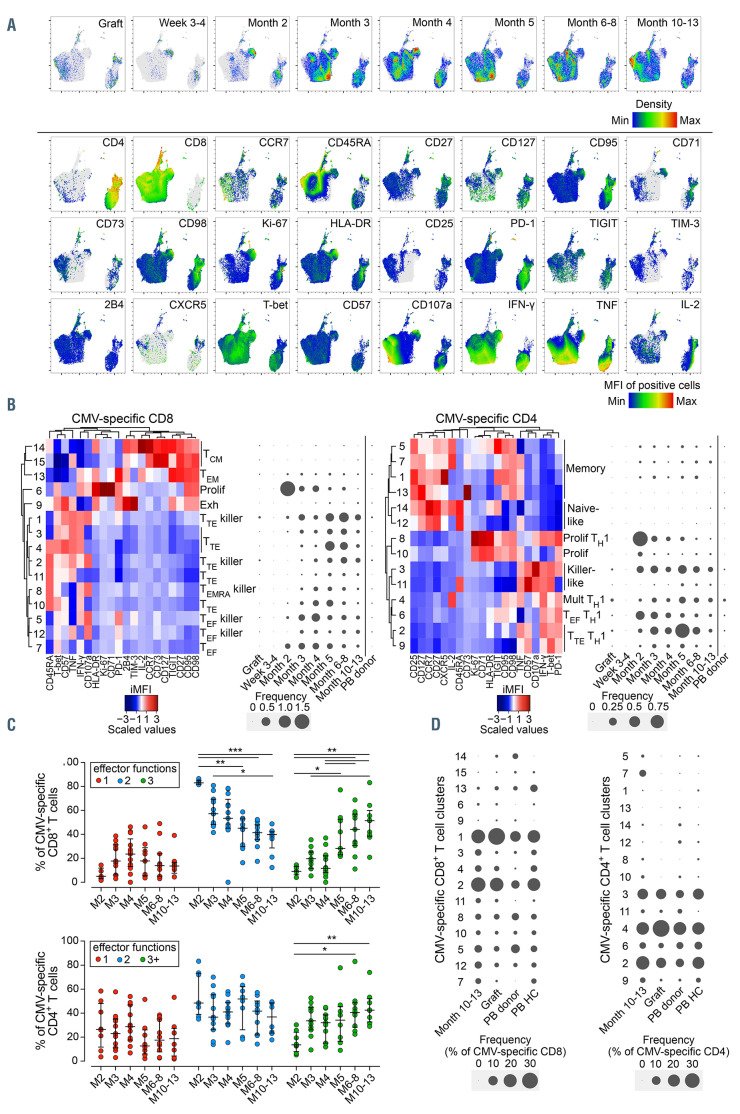
We next analyzed the functional and phenotypic profile of CMV-specific T-cells, identified through effector cytokines produced in response to CMV pp65 peptide library stimulation. Although likely underestimating the full extent of the CMV-directed T-cell response, which involves a broad range of antigens, pp65-specific T-cell responses are largely representative of the total response against CMV.12 Uniform manifold approximation and projection (UMAP) revealed dynamic changes in CMVspecific T-cell phenotypes during reconstitution (Figure 2A). PhenoGraph analysis of CMV-specific T cells generated 15 CD8+ and 14 CD4+ T-cell clusters (Figure 2B). At week 3-4 post-haplo-HSCT, CMV-specific T cells were undetectable in most patients. By month 2, in which CMV viremia emerged in the majority of patients, both CD8+ and CD4+ CMV-specific T cells expanded and displayed a proliferating phenotype, featuring high levels of Ki-67, HLA-DR, CD71, PD-1, CD95 and CD98 (Figure 2B; Online Supplementary Figures S1B and S2A). From month 3 onwards, these phenotypes were replaced by effector phenotypes expressing T-bet, often in conjunction with CD57 and/or CD45RA, indicative of terminal differentiation. Although CD8+ and CD4+ CMV-specific T-cell immunophenotypes displayed similar dynamics, we also observed lineage-specific differences, including identification of multifunctional CD4+ cluster 4 that highly expressed IL-2, IFN-γ, TNF and intermediate levels of CD107a. CMV-specific CD8+ T cells rarely expressed IL-2, rather, they commonly expressed IFN-γ and TNF. IFN-γ–TNF+ CD8+ T cells formed a minority, whereas IFN- γ+TNF+ T cells were commonly seen and dominated the overall response from month 5 onwards. Interestingly, we identified CD4+ clusters 3 and 11 expressing high levels of CD107a, T-bet, IFN-γ, TNF and CD57, reminiscent of killer-like cells otherwise identified in the CD8+ compartment. Overall, the CMV-specific T-cell response showed maturation of effector functions over time (acquisition of at least three functions simultaneously), most prominently among CD8+ T cells (Figure 2C). The frequency of both CD8+ and CD4+ CMV-specific T cells in the PB of haplo-HSCT patients was greatly increased compared to that of the graft and PB of CMV-seropositive donors or PB of unrelated healthy controls, but phenotype distribution at 1 year was remarkably similar, suggesting re-establishment of physiological homeostasis (Figure 2D).
Figure 1.
Cytomegalovirus reactivation impacts T-cell reconstitution following haploindentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide. (A) Integrated median fluorescence intensity (iMFI) values, considering both expression level and frequency of marker+ cells, of the CD8+ or CD4+ T-cell PhenoGraph clusters were visualized by heatmaps, while PhenoGraph cluster dynamics (median percentages of total CD8+ or CD4+ T cells) in haploidentical hematopoietic stem cell transplantatation (haplo-HSCT) patients were revealed by balloon plots. (B) Principle component analysis (PCA) of CD8+ and CD4+ T-cell cluster frequencies. The left panel depicts the clusters driving the PCA, the central and right panel show the median PCA coordinates of all patients, grouped together or according to cytomegalovirus (CMV) viremia (subclinical viremia, n=6; clinical viremia, n=13; no viremia, n=2), per time point in months after transplant. The central and right panel should be read in conjunction with the left panel; the relative position of data points in the central and right panel is indicative of dominance of T-cell clusters shown with a similar relative position in the left panel. (C) Dynamics of CD8+ TEMRA, CD4+ TTE TH1 and CD8+ naïve clusters in haplo-HSCT patients experiencing post-transplant CMV viremia. Medians with the number of patients per time point are shown and error bars represent interquartile range. Significance was determined by Kruskal-Wallis test and P-values are shown at the upper border of the plot for each time point (*P<0.05). (D) Flow cytometric analysis of CD45RA and T-bet expression in CD8+ and CD4+ T cells at month 3 and month 6-8 after transplantation. For both CMV groups a single representative patient is shown. (E) Hierarchical metaclustering using the Ward minimum variance method, of haplo-HSCT patients per month, based on the frequency of CD8+ and CD4+ T-cell PhenoGraph clusters. aGvHD: acute graft-versus-host disease; Exh: exhausted; PB: peripheral blood; Prolif: proliferating; TCM: central memory T cell; TEF: effector T cell; TEM: effector memory T cell; TEMRA: effector memory re-expressing CD45RA T cell; TM: memory T cell; TREG: regulatory T-cell T cell; TTE: terminal effector T cell; TM: transitional memory T cell.
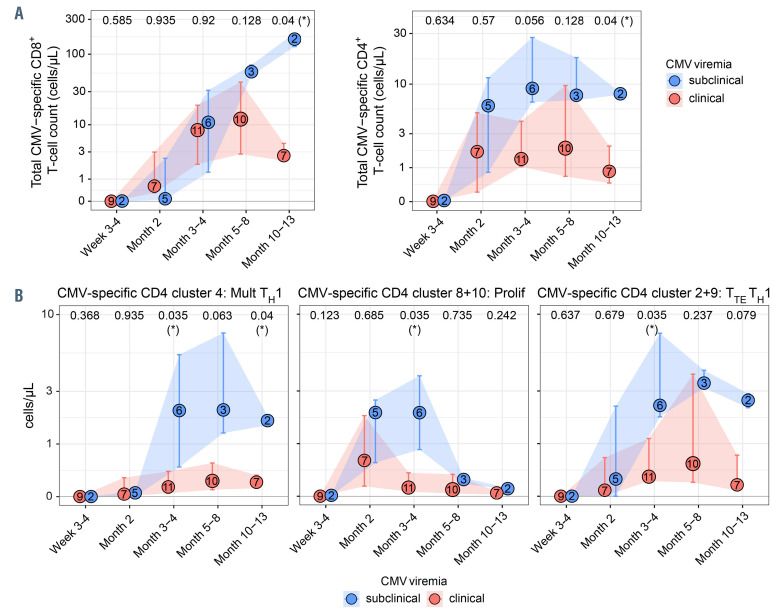
We next asked whether control of CMV viremia is associated with a greater abundance, or a specific functional or phenotypic profile, of CMV-specific T cells. Huntley et al. reported >1 and >1.2 counts/mL of IFN-γ+ CMV-specific CD8+ and CD4+ T cells, respectively, to protect against reactivation following haplo-HSCT with pt-cy,13 but other studies, predominantly on HLAmatched HSCT recipients, reported that multifunctional responses have a stronger predictive value.14,15 We did not detect a significant difference in the total count of CMV-specific CD8+ or CD4+ T cells in the first 6 months post-transplant between patients with subclinical versus clinical CMV viremia, although CMV-specific CD4+ T cells tended to be present in higher amounts among patients with subclinical CMV (Figure 3A). Significant differences may occur at later time points, but our analysis was limited by the low number of patient samples at these time points. Analyzing the dynamics of each T-cell cluster separately, we found lower counts of multifunctional (cluster 4), proliferating (sum of phenotypically similar clusters 8 and 10) and TTE TH1 (sum of phenotypically similar clusters 2 and 9) CD4+ T cells at month 3-4 for patients with clinical viremia (Figure 3B). Although the size of each given patient group is low, patients with repeated CMV episodes requiring multiple treatment cycles tended to develop even lower counts of these Tcell phenotypes (Online Supplementary Figure S2B). No such trends were seen in the CD8+ T-cell compartment (data not shown), thereby suggesting that control of CMV viremia post-haplo-HSCT mainly associates with the development of distinct antigen-specific CD4+ T-cell immunophenotypes.
In conclusion, CMV-specific T cells were primed early after haplo-HSCT with pt-cy and initially displayed a proliferating/activated phenotype, that was quickly replaced by a terminal effector phenotype. One year after transplant, CMV-specific T-cell profiles were similar to those of the CMV-seropositive donor, suggesting reestablishment of physiological homeostasis. Uncontrolled viral replication associated with lower abundance of distinct CMV-specific CD4+ T-cell immunophenotypes, hinting at a possible role of these cells in CMV control following haplo-HSCT with pt-cy. These data require additional, future investigations for confirmation.
Figure 2.
High-dimensional single-cell profiling reveals the dynamics of the cytomegalovirus-specific T-cell response following haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide. (A) Uniform manifold approximation and projection (UMAP) analysis of cytokine-positive CD8+ and CD4+ T cells from peripheral blood mononuclear cell (PBMC) samples stimulated overnight with cytomegalovirus (CMV) pp65 peptide mix. Graphs highlight events belonging to different time points or events positive for a given marker. (B) Heatmaps depict marker expression in normalized integrated median fluorescence intensity (iMFI) values of the antigen-specific CD8+ or CD4+ T-cell PhenoGraph clusters. Balloon plots show the median cluster frequencies as percentage of total CD8+ or CD4+ T cells in haploidentical hematopoietic stem cell transplantation (haplo-HSCT) patients experiencing post-transplant CMV viremia, after background correction was applied. (C) Polyfunctionality of the CMV-specific T-cell response over time in months post-transplant, as determined by assessment of expression of cytokines IFN-γ, TNF and IL-2, and degranulation marker CD107a by cell clusters identified in (B). Measurements containing <50 CMV-specific cells after background correction were discarded from analysis. Medians are depicted and error bars represent the interquartile range. Significance was determined by Kruskal-Wallis test with post-hoc Dunn’s test (*P<0.05, **P<0.01; ***P<0.001). (D) Balloon plots show the median cluster frequencies as percentage of CMV-specific CD8+ or CD4+ T-cells at month 10-13 compared to that found in the graft, periheral blood (PB) of the donor and PB of unrelated healthy controls, after background correction was applied. Measurements containing <50 CMV-specific cells after background correction were discarded from analysis. Exh: exhausted; HC: healthy control; Mult: multifunctional; Prolif: proliferating; TCM: central memory T cell; TEF: effector T cell; TEM: effector memory T cell; TEMRA: effector memory re-expressing CD45RA T cell; TTE: terminal effector T cell.
Figure 3.
Cytomegalovirus viremia control following haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide associates with the development of distinct CD4+ antigen-specific T-cell immunophenotypes. (A) Total cytomegalovirus (CMV)-specific CD8+ or CD4+ T-cell counts and (B) cluster-specific T-cell counts in the blood of haploidentical hematopoietic stem cell transplantation (haplo-HSCT) patients with subclinical (n=6) or clinical (n=13) CMV viremia during the first year post-transplant. Medians with the number of patients per time point are shown and error bars represent interquartile range. Significance was determined by Kruskal-Wallis test and P-values are shown at the upper border of the plot for each time point (*P<0.05). Mult: multifunctional; Prolif: proliferating; TTE: terminal effector.
Supplementary Material
Funding Statement
Funding: this work was funded by the European Research Council (ERC-StG-2014 PERSYST #640511 to EL). SP was supported by a fellowship from the Fondazione Italiana per la Ricerca sul Cancro- Associazione Italiana per la Ricerca sul Cancro (FIRC-AIRC). The purchase of a FACSymphony A5 was defrayed in part by a grant from the Italian Ministry of Health (agreement 82/2015).
References
- 1.Luznik L, O’Donnell P V, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crocchiolo R, Bramanti S, Vai A, et al. Infections after T-replete haploidentical transplantation and high-dose cyclophosphamide as graft-versus-host disease prophylaxis. Transpl Infect Dis. 2015; 17(2):242-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine JH, Simonds EF, Bendall SC, et al. Data-driven phenotypic dissection of AML reveals progenitor-like cells that correlate with prognosis. Cell. 2015;162(1):184-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson BE, McNiff J, Yan J, et al. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112(1):101-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen BJ, Deoliveira D, Cui X, et al. Inability of memory T cells to induce graft-versus-host disease is a result of an abortive alloresponse. Blood. 2007;109(7):3115-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberto A, Castagna L, Zanon V, et al. Role of naive-derived T memory stem cells in T-cell reconstitution following allogeneic transplantation. Blood. 2015;125(18):2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cieri N, Oliveira G, Greco R, et al. Generation of human memory stem T cells after haploidentical T-replete hematopoietic stem cell transplantation. Blood. 2015;125(18):2865-2874. [DOI] [PubMed] [Google Scholar]
- 8.Kanakry CG, Ganguly S, Zahurak M, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med. 2013;5(211):211ra157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wachsmuth LP, Patterson MT, Eckhaus MA, Venzon DJ, Gress RE, Kanakry CG. Posttransplantation cyclophosphamide prevents graftversus- host disease by inducing alloreactive T cell dysfunction and suppression. J Clin Invest. 2019;129(6):2357-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suessmuth Y, Mukherjee R, Watkins B, et al. CMV reactivation drives posttransplant T-cell reconstitution and results in defects in the underlying TCRb repertoire. Blood. 2015;125(25):3835-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanakry CG, Coffey DG, Towlerton AMH, et al. Origin and evolution of the T cell repertoire after posttransplantation cyclophosphamide. JCI Insight. 2016;1(5):e86252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wills MR, Carmichael AJ, Mynard K, et al. The human cytotoxic Tlymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70(11):7569-7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huntley D, Giménez E, Pascual MJ, et al. Reconstitution of cytomegalovirus-specific T-cell immunity following unmanipulated haploidentical allogeneic hematopoietic stem cell transplantation with posttransplant cyclophosphamide. Bone Marrow Transplant. 2020;55(7):1347-1356. [DOI] [PubMed] [Google Scholar]
- 14.Camargo JF, Wieder ED, Kimble E, et al. Deep functional immunophenotyping predicts risk of cytomegalovirus reactivation after hematopoietic cell transplantation. Blood. 2019;133(8):867-877. [DOI] [PubMed] [Google Scholar]
- 15.Pelák O, Stuchlý J, Król L, et al. Appearance of cytomegalovirus-specific T-cells predicts fast resolution of viremia post hematopoietic stem cell transplantation. Cytometry B Clin Cytom. 2017;92(5):380-388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.