Highlights
-
•
First internationally agreed minimum set of outcomes deemed essential to be measured in all future studies evaluating interventions to improve decisions about participating in an randomized controlled trial.
-
•
Broad stakeholder involvement, including; potential trial participants (e.g., patients or others who could provide a lay perspective), trialists, research nurses, social scientists, clinicians, bioethicists, and research ethics committee members.
-
•
Represents outcomes that are of core importance to multiple stakeholders and, if adopted, will improve the relevance of future trials in this field.
Keywords: informed consent, decision making, clinical trials, core outcome sets, methodology, research waste
Abstract
Objective
To develop a core outcome set for the evaluation of interventions that aim to improve how people make decisions about whether to participate in randomized controlled trials (of healthcare interventions), the ELICIT Study.
Study Design
International mixed-method study involving a systematic review of existing outcomes, semi-structured interviews, an online Delphi survey, and a face-to-face consensus meeting.
Results
The literature review and stakeholder interviews (n = 25) initially identified 1045 reported outcomes that were grouped into 40 individually distinct outcomes. These 40 outcomes were scored for importance in two rounds of an online Delphi survey (n = 79), with 18 people attending the consensus meeting. Consensus was reached on 12 core outcomes: therapeutic misconception; comfort with decision; authenticity of decision; communication about the trial; empowerment; sense of altruism; equipoise; knowledge; salience of questions; understanding, how helpful the process was for decision making; and trial attrition.
Conclusion
The ELICIT core outcome set is the first internationally agreed minimum set of outcomes deemed essential to be measured in all future studies evaluating interventions to improve decisions about participating in an randomized controlled trial. Use of the ELICIT core set will ensure that results from these trials are comparable and relevant to all stakeholders.
Registration
COMET database - http://www.comet-initiative.org/Studies/Details/595.
1. Introduction
Informed consent is central to participation in clinical research. The requirement of informed consent for participation in randomized controlled trials (RCTs) was established to allow people to recognize and consider potential risks or benefits of participating, and to respect their autonomous choices [1]. The adequacy of these processes has, however, been called into question [2,3]. Concern stems from evidence that trial participants often lack understanding (so cannot be considered "informed”) about the trial rationale and the risks of interventions [4]. Questions have also been raised about the absence, from informed consent processes, of features of “good” decision-making, including attention to the influence of context on preferences, ability to predict future well-being, and integration of information with personal values and goals [5].
Suggestions for improving consent processes have included interventions targeting aspects of information provision, usually the content (including length of information, readability, etc) or mode of delivery (e.g., paper, multimedia) [6], [7], [8]. Systematic reviews of interventions to improve participants’ understanding in informed consent for research are inconclusive about their effectiveness [6], [7], [8]. The range of interventions is one reason for this, but outcome assessment heterogeneity has also hampered evaluation.
A raft of outcomes has been proposed in evaluations of interventions to improve the invitation and recruitment process in RCTs (now referred to, for short, as the RCT decision process) [6], [7], [8], [9]. Some have focused on how well-informed potential participants are, or whether they are recruited into the trial. However, very few studies have considered what outcomes are important to potential participants or attempted to assess their experiences of the process. The lack of consistency in outcome measurement and reporting hampers synthesis across the studies, reducing the value of this research literature.
One way to help prioritize and standardize the selection and reporting of outcomes is to develop a core outcome set (COS) - a minimum set of outcomes that should be considered essential to be measured in all evaluations and reporting of specific interventions or conditions to improve the informed consent process [10].
The ELICIT study aimed to develop such a COS for the evaluation of interventions that aim to improve how people make decisions about whether to participate in RCTs of healthcare interventions (i.e., those which are delivered to individuals in healthcare settings and/or by health professionals).
2. Methods
2.1. Study overview
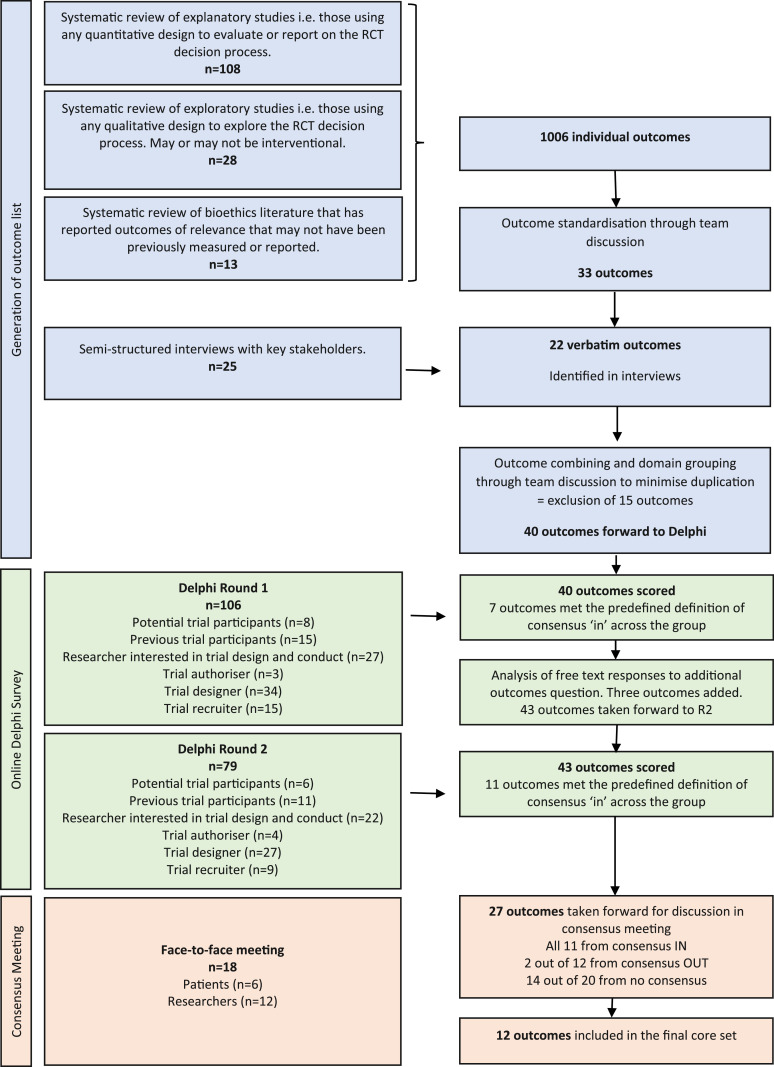
The development of the COS followed best practice and involved three sequential stages [11]. The methods for each stage are outlined below and in Figure 1. The study protocol is published and the COS was prospectively registered on the COMET database [12].
Fig. 1.
Core outcome set development overview.
The scope was restricted to interventions that target the decisions of adults deemed to have adequate mental capacity and who are deciding, prospectively, about their own participation in a randomized trial evaluating the effectiveness of healthcare interventions. The ELICIT COS is focused on outcomes relevant to potential trial participants.
Stage 1 - Generation of outcome list
To generate the initial long list of outcomes, we first conducted a systematic review of studies that had reported or proposed outcome measures associated with decisions to participate in trials. Detailed search strategies and a PRISMA diagram are provided in supplementary information files S1 and S2. The screening of titles, abstracts, and potentially eligible studies was done by KG, with HG reviewing a random 25%. Data extraction was conducted by KG and HG independently for all included studies to extract all relevant outcomes reported (or proposed), authors rationale, definition, and operationalization of measurement. Data on general characteristics of the included studies (e.g., study type, setting, sample size, and where appropriate, intervention being tested) were also extracted. In addition, validated tools measuring informed consent for clinical trials were reviewed and the specific domains extracted. The review of tools is published elsewhere [13].
The review was supplemented with interviews with national and international key stakeholders (i.e., potential trial participants (e.g., patients or others who could provide a lay perspective), trialists, research nurses, social scientists, clinicians, bioethicists, and research ethics committee members) to identify any additional outcomes of importance. Participants were recruited from several sources, namely: patients through the Scottish Health Research Register (SHARE); Research Nurses through the Scottish Research Nurse and Coordinators Network; Social Scientists through the Society of Social Medicine; Research Ethics Committee Members through the Health Research Authority; and Ethicists through the Feminist Approaches to Bioethics network. In addition, invites were sent through known professional networks to key experts in the field, targeting each of the groups mentioned above and targeting experienced clinical trialists. All participants gave written consent to participate in a telephone interview. Interviews were supported by a topic guide to ensure coverage of key issues. Interviewees described their experiences of what a “good” consent process for clinical trials might look like (See S3 file). All interviews were conducted by KG, audio-recorded, and transcribed verbatim. A thematic content analysis of the transcripts was conducted: following familiarization with the transcripts, a priori and emergent themes were identified, discussed and agreed by the research team [14]. One author (K.G.) reviewed the transcripts and documented major themes with 10% reviewed independently (V.E.). A thematic framework was generated and agreed through discussion by the team. The framework detailed codes for labelling textual data related to the major themes and subthemes. KG applied it systematically to all transcripts using text management software (NVivo V.10).
Outcomes were extracted verbatim from the information sources (systematic review and interviews), then grouped into broad outcome domains determined through discussion with the project team (supplementary information –S4 file).
Stage 2 - International Delphi survey
An international Delphi survey was used to seek agreement on the relative importance of outcomes identified from Stage 1.
Each outcome generated from the systematic review and interviews was listed together with a plain language definition on the online DelphiManager platform [15] – supplementary information S5 Table. Individuals from the key stakeholder groups listed previously were invited using national and international organizations (supplementary information – S6 file). We did not preclude participants who had been involved in the interviews, but did not invite interview participants directly to participate in the Delphi survey. Whilst there is no standard sample size for the “expert” panel in Delphi surveys, a minimum of 10-18 participants per expert group has been suggested [16,17].
Two rounds of the Delphi survey (R1 and R2) were completed. The ELICIT protocol envisaged three rounds of voting, as R2 originally planned a randomized evaluation of different approaches to stakeholder feedback. However recent evidence emerged to support feedback by each stakeholder group (i.e., patients = 5, researchers = 6, clinicians = 5, etc) so this approach was instead adopted [18]. In R1, the outcomes were presented and participants asked to score their importance. A 9-point Likert scale was used, with 1-3 being not important and 7-9 being essential. This scale has been recommended for consensus processes that require a final validation (3-point scales may be preferred for determining final consensus) [19]. R1 participants were also invited to submit any additional outcomes, which were reviewed by the study team and considered for inclusion in R2. No outcomes were removed between rounds.
Only participants who completed R1 were invited to participate in R2. During R2 each outcome was presented to participants with their own R1 score and an anonymized distribution of scores from each stakeholder group. Participants were asked to score the outcome again, taking into account the scores of others.
2.2. Consensus definition
Our original definition of consensus was based on previous COS studies and required that 70% or more of the entire group had to agree as important (or not) and less than 15% scoring in the opposite direction [20,21]. This rule was applied successfully for determining "consensus in,” however, the "consensus out” definition was subsequently amended (blinded to identity of outcomes) to 50% or fewer participants scoring the outcome as important. The reason for this change was that no outcomes met the previously defined consensus "out” criterion but a large number of outcomes were considered ”no consensus” (32 out of 43, 74%). This amendment has been used in several recent COS studies [22] and allowed more explicit demarcation of those outcomes which displayed the least consensus.
Stage 3 - Face-to-face consensus meeting
In order to ratify the outcomes identified in the Delphi survey, and provide an opportunity for discussion to clarify any misunderstanding, a face-to-face consensus meeting was held in Aberdeen, UK. Participants who had completed both rounds of the Delphi survey were invited. Additional invitations were sent through social media to target patients and researchers. Participants were sent a summary of the study in advance and a consensus matrix detailing the R2 summary scores for all outcomes– see supplementary information S7 file. Participants who took part in the Delphi were also given a reminder of their R2 scores.
As with Delphi surveys, there is no standard for sample sizes for consensus meetings. Reported numbers range from two to 14, and some authors recommend a maximum of seven (for Nominal Group Technique) [23]. We planned to recruit at least 5 participants from each stakeholder group. These groups were reduced to ‘potential trial participants’ and ‘researchers’ for the purposes of the consensus meeting.
The consensus meeting was chaired by a trials methodologist from the study team (MC) with expertise on COS development methodology and consensus facilitation. Outcomes that had reached consensus "in” across the whole group from R2 were presented first, followed by outcomes that reached consensus "out” across the whole group from R2. Participants were asked to confirm they agreed (or not) with the inclusion or exclusion of these outcomes in the COS. Outcomes scored as ‘no consensus’ were split into two groups: 1. more than 50% of both groups scored the outcome as 7-9 would be discussed and voted on again; and 2. those with 50% or less of one group scoring 7-9 would not be voted on again. See supplementary information file S8 Table for a summary of these re-scoring rules. These "rules” for progression of outcomes for discussion were agreed by the meeting participants before presentation and discussion of the outcomes.
Views for and against inclusion of the outcomes for which there was “no consensus” after R2 were sought by the meeting chair. Discussion was supplemented with the following questions: Is this outcome a surrogate for something already in the COS?; Does the group think it is really important to add?; Should it replace an outcome that is already in (i.e., is there an outcome in the COS that is a surrogate for this)? . Following discussion, participants were invited to vote on each outcome anonymously on the PollEverywhere [https://www.polleverywhere.com/] website through WiFi enabled devices. Following voting, the whole group results were presented to participants. The consensus definition used in the Delphi survey (see above) was applied to determine consensus “in” or “out.”
2.3. Research ethics
The study was approved by the London-Chelsea Research Ethics Committee (REC reference 15/LO/0375). All participants gave informed consent before taking part.
2.4. Patient and public involvement
Patients and the public were involved in developing the Delphi survey, specifically through guidance on layout, comprehensibility of items and scoring, and overall objective.
3. Results
3.1. Generation of outcome list
The systematic review identified 7767 titles and abstracts for screening. Of those, 205 full text articles were assessed for eligibility and 149 studies included in the final analysis. These 149 studies included three broad study types (studies using quantitative methods = 108, studies using qualitative methods = 28, and bioethics literature = 13 (see S2 file)). They yielded 1006 individually characterized outcomes which were reduced to 33 distinct outcome concepts (e.g., recruitment, understanding, decision conflict, coercion).
A total of 25 interviews were conducted across the stakeholder groups. Table 1 details full sample characteristics. A total of 39 outcomes were identified in the interviews, of which 22 were "new” outcomes.
Table 1.
Interview sample demographics
| Patients (n = 4) | Researchers (n = 21) | |||
|---|---|---|---|---|
| Sub-group(s) | Previous trial participants | =4 | Researcher interested in trial design and conduct | = 9 |
| Trial authorizer | = 2 | |||
| Trial designer | = 4 | |||
| Trial recruiter | = 6 | |||
| Gender | 75% female | 38% female | ||
| Experience of working on trials (yr) | n/a | Less than 1 | = 1 | |
| 1-5 | = 4 | |||
| 6-10 | = 4 | |||
| More than 10 | = 12 | |||
| Country | UK | Australia | = 1 | |
| Canada | = 1 | |||
| Ireland | = 1 | |||
| Norway | = 2 | |||
| UK | = 12 | |||
| USA | = 4 |
The 33 outcomes from the literature review and the 22 additional outcomes from the interviews were combined and reviewed by the study team. Fifteen outcomes were excluded - some overlapped (e.g., comprehension was subsumed into understanding) and others related to health systems or healthcare professionals rather than potential trial participants. The remaining 40 outcomes were mapped across four broad domains: 1. What decisions people make and/or the quality of those decisions (n = 8); 2. Experiences of decision making (n = 15); 3. How people make decisions (n = 14); 4. Potential participants’ abilities relevant to decision making that influence the decision (n = 3). These were taken forward to the Delphi.
3.2. International Delphi survey
Round one of the Delphi survey was completed by 106 participants, and R2 by 79 (75% of those in R1) from 9 countries. The R2 sample comprised potential trial participants (n = 6); previous trial participants (n = 11); researchers interested in trial design and conduct (n = 22); trial authorizers (n = 4); trial designers (n = 27); and trial recruiters (n = 9) (Table 2).
Table 2.
Delphi sample demographics
| Patients (n = 17) | Researchers (n = 62) | |||
|---|---|---|---|---|
| Sub-group | Potential trial participants | = 6 | Researcher interested in trial design and conduct | = 22 |
| Previous trial participants | = 11 | Trial authorizer | = 4 | |
| Trial designer | = 27 | |||
| Trial recruiter | = 9 | |||
| Gender | 53% female | 59% female | ||
| Experience of working on trials (yr) | Never involved | = 5 | Never involved | = 0 |
| Less than 1 | = 1 | Less than 1 | = 0 | |
| 1-3 | = 1 | 1-3 | = 6 | |
| 3-5 | = 2 | 3-5 | = 7 | |
| 5-10 | = 5 | 5-10 | = 16 | |
| More than 10 | = 3 | More than 10 | = 33 | |
| Country | Canada | = 1 | Australia | = 1 |
| Ireland | = 1 | Brazil | = 1 | |
| UK | = 15 | Canada | = 1 | |
| India | = 1 | |||
| Ireland | = 2 | |||
| Malaysia | = 1 | |||
| Switzerland | = 1 | |||
| UK | = 51 | |||
| USA | = 3 |
At the end of R1, seven outcomes met the predefined criteria for consensus at whole group level. Twenty-one “new” outcomes were suggested for consideration and the study team took 3 of these forwards for scoring in R2: cost of intervention (development and implementation); the individual had the support they needed from others to reach their decision; and salience of informed consent questions to individual). The other suggested outcomes were excluded due to being out of scope (i.e., not relevant for informed consent for adults with capacity to consent for themselves in non-emergency settings) or duplicates.
Following completion of R2, 11 outcomes achieved consensus for inclusion in the COS at whole group level and 12 for exclusion. Twenty outcomes were classified "no consensus.” All were taken forward to the consensus meeting.
3.3. Face-to-face consensus meeting
The consensus meeting was attended by 18 participants (6 patients and 12 researchers) from across the UK (see Table 3). The group were first presented with the consensus in (n-11) and the consensus out outcomes (n-12) and asked if there were any they thought should be discussed. The group decided that two outcomes ruled “out” at Delphi R2 should included in the voting and rescored. For the no consensus outcomes, the rules for re-scoring (i.e., more than 50% of both groups scored 7-9) were agreed and this resulted in 13 outcomes being taken forward for scoring. The group also asked for one further outcome from the no consensus group (n = 7) to be rescored, so 14 outcomes from the no consensus group were taken forward. Sixteen outcomes in total (14 "no consensus” and 2 "consensus out”) were taken forward for full discussion and re-voting. Supplementary Information Table S9 shows meeting participants’ scores on the 16 outcomes that were re-voted.
Table 3.
Consensus meeting demographics
| Group | Patients | =6 |
| Researchers | =12 | |
| Gender | 55% female | |
| Experience of working on trials (yr) | Never involved | = 1 |
| 1-3 | = 2 | |
| 3-5 | = 4 | |
| 5-10 | = 5 | |
| More than 10 | = 6 | |
| Country | UK |
There were discussions on nearly all of the outcomes presented, and focused discussions on the 16 taken forward for re-scoring. We focus here on three outcomes that received considerable attention: trust, understanding and recruitment. Trust and understanding had scored consensus “out” in the Delphi but been elevated for discussion and re-scoring in the meeting. The decision to re-score trust was based on it being the only consensus “out” outcome with a marked difference in scoring between stakeholder groups (94% of patients and 11% of researchers scored it 7-9). Meeting attendees argued that trust could be covered through other outcomes already included e.g., feeling comfortable with the decisions, and on re-scoring trust was agreed as consensus “out.” Discussion about understanding centered around how it was, or was not, linked to knowledge and communication, both outcomes that were already considered consensus “in.” Some patient participants felt that having only knowledge as an outcome was not appropriate as the symptoms of some diseases or side effects of relevant treatments can impact on an individual's ability to recall information (which was equated with knowledge). They thought it important to include an additional measure of "informedness.” Others considered that the three outcomes of knowledge, understanding and communication exist in a causal chain and by excluding one of these outcomes it would not allow investigations of this model. When the group re-scored after discussion, understanding was scored consensus "in.”
Recruitment was the only consensus "out” or "no consensus” outcome from the Delphi (n = 32) that more than 70% of researchers (74%) and less than 70% of patients (47%) had scored important (i.e., 7-9). Discussion of recruitment as an outcome was extensive and opinion divided. Some participants (both researchers and patients) felt strongly that recruitment should not be regarded as a marker of, or proxy for, the "quality” of informed consent processes. There was concern that trial teams might treat a negative impact on recruitment as indicative of “harm” and argue that even if all other aspects of the informed consent process were deemed "good,” it should rule out use of the intervention. However, other participants (again both researchers and patients) considered it important to assess any detriment to recruitment, and argued in practical terms that, for example, if an intervention halved the recruitment rate but had a positive impact on decision making, this information could be used for recruitment planning. At re-scoring, it was considered “no consensus.”
3.4. The core outcome set
The final COS includes 12 outcomes grouped across 3 domains (Table 4). These were: therapeutic misconception; comfort with decision; authenticity of decision; communication about the trial; empowerment; sense of altruism; equipoise; knowledge; salience of questions; understanding, how helpful the process was for decision making; and attrition from the trial.
Table 4.
Outcomes included in the ELICIT core outcome set
| Outcome (n = 12) | Domain |
|---|---|
| Whether a potential participant is comfortable (feels happy and relaxed) with the decision.a | Experiences of decision-making in this context. |
| How helpful a potential participant finds the consent process to make a decision. | |
| The authenticity of a potential participant's decision, i.e., how genuine their decision was.a | |
| The communication (both written and verbal) about the trial.a | |
| Feelings of empowerment (the process of becoming stronger and more confident, especially in controlling one's life and claiming one's right) from the perspective of a potential participant.a | |
| Salience of informed consent questions to individual.b | |
| A potential participant's sense of altruism (selfless concern for the well-being of others). | How people make decisions. |
| Uncertainty about the comparative therapeutic merits of each treatment being tested in a trial (equipoise). | |
| A potential participant's knowledge, i.e., the recall of facts, information or skills. | |
| A potential participant's understanding, i.e., the ability to comprehend something without necessarily knowing how it relates to or affects other things. | |
| A potential participant's understands that the treatment they receive will not be individualized and the overall purpose of the research is to advance scientific knowledge rather than benefiting them personally (therapeutic misconception). | What decisions people make and/or the quality of those decisions. |
| The proportion of trial participants who do not complete the trial (attrition). |
Outcome identified during stakeholder interviews.
Outcome identified during Delphi R1 survey.
4. Discussion
This study developed the first internationally agreed COS for evaluating interventions to improve decisions about participating in an RCT. It accounts for the views of all key stakeholders.
During the Delphi, patients generally rated more items as critically important for inclusion in a COS than researchers. In R2, based on patients’ scores alone, 30 outcomes would have been considered in compared to 10 for researchers. One comment from the meeting was that it became easier to be more discriminating when a proposed core set (based on the R2 result) was presented as a standard to consider against. When considering each outcome in isolation, assessment of importance may have been cognitively harder. In addition, the face-to-face meeting provided opportunities for clarification of the overall purpose of the ELICIT COS and further discussion of what outcomes might mean.
It is important to consider where the outcomes that feature in the ELICIT COS entered the COS development process. Four (33%) of the 12 were identified only through interviews with stakeholders. In other words, they had not been previously measured or reported in studies investigating decision processes for RCTs. In addition, one outcome (the salience of informed consent questions) was suggested for the first time in the Delphi (the remaining 7 were identified from the systematic review phase). This provides reassurance that the ELICIT process enabled the contributions of key stakeholders to be heard and represented in the final output. It is also important to highlight that the decision of the Delphi survey was maintained through the consensus meeting with all 11 Delphi “in” outcomes being retained in the final core set and the addition of one further outcome from the “no consensus” Delphi group.
An important finding is the exclusion of trial recruitment rate as a core outcome for these interventions. Within the systematic review, trial recruitment was the third most frequently reported outcome (after understanding and knowledge), cited in 54 (44%) of the 122 studies. It was the only outcome about which consensus meeting participants’ opinions were polarized (and across both patients and researchers). In contrast to recruitment, attrition was included in the ELICIT COS with consensus meeting participants commenting that it would provide information about longer term impact of the quality of the decision. However, this needs to be considered with caution as attrition may be strongly influenced by various factors other than interventions to support decision-making about trial participation.
Some interpretive caution will be needed for other COS elements too, as they vary in proximity to (different aspects of) interventions intended to improve informed consent processes and may be variously influenced or moderated, including by each other. As was evident in the consensus meeting, outcome descriptors can be variously understood, and ideas about causal and other relationships between different COS elements can vary depending on how they are defined and operationalized.
The ELICIT study identified "what”outcomes needs to be measured, but not "how.” Measurement tools exist for some of the outcomes listed in the COS (e.g., therapeutic misconception [24], understanding [25] have been assessed in RCT settings and other outcomes in non-RCT settings [26,27]). It will be important to determine how fit these are for purpose, whether new tools are needed, and (more fundamentally) how measurable some outcomes are, especially across different trial contexts. Measurement issues will be key when moving this work forward. Specific attention to ensuring construct validity through the use of, for example, cognitive interviewing, will be key to robust measurement. Given the COS contains 12 outcomes, considering the data collection burden for each of these outcomes is also important for future work e.g., can some of these outcomes be combined, could they be measured with tools containing 1 item, etc.
Further methodological work linked to ELICIT is ongoing. This includes a COS for evaluation of interventions to improve the informed consent process for elective surgical procedures [27] and a COS for interventions to improve informed consent to trials for adults who lack capacity [28]. The growth of COS work in methodological areas highlights the problem of outcome measurement and reporting that extends beyond clinical studies. It reinforces the critical importance of these COS to contribute to efforts to reduce research waste and improve patient care.
4.1. Strengths and limitations
A strength of the process was the inclusion of patient partners at each stage of development from generation of the long list through to ratification of the final COS in the consensus meeting. Their involvement ensured a COS that considers what matters most to potential trial participants about the informed consent process.
Although outcomes of international relevance were well represented in the systematic review and 43% of interview participants were non-UK, only 18% of Delphi participants were from outside the UK, and only 3% of those from low- or middle-income countries. Nearly all of the patients involved in the ELICIT Study were UK based and were fluent English speakers. This raises considerations about the relevance of the COS in countries dissimilar to the UK. For example, healthcare funding arrangements and cultural differences in relational dynamics could affect patients’ decisions about trial participation and raise important questions for further research. An additional limitation is the potential attrition bias associated with the two round Delphi survey. Whilst attempts were made to reengage with non-responders, 25% did not provide data for the final analysis. It cannot be ruled out that participants who did not complete Round 2 had views which differed.
5. Conclusions
The ELICIT COS provides an internationally agreed minimum set of outcomes that should be measured in all future studies evaluating interventions to improve how adults with capacity prospectively, make decisions about participating in an RCT. It represents outcomes that are of core importance to multiple stakeholders and, if adopted, will improve the relevance of future trials in this field. Uptake of the ELICIT COS will also impact directly on the potential for cross-learning and synthesis of results from across different trial settings, thus maximizing efficiency and reducing research waste.
Acknowledgments
The ELICIT Study team would like to thank Cynthia Fraser for help with developing and running the search strategies for the literature review, the DelphiManager team for all their support and guidance on the use of the Delphi platform, Health Services Research Unit Patient Involvement Group critical review of the Delphi questionnaire before dissemination, Heather Bagley for further comments on the Delphi questionnaire and also for dissemination of the survey information and link to a range of patient facing organizations, and to Beverley Smith for her assistance in organizing the consensus meeting. We would also like to thank all participants of the study (including interview participants, Delphi respondents, and consensus meeting members) and organizations who disseminated the survey.
Data Sharing
Data from the systematic review and Delphi survey phases of work are available from the corresponding author on request.
Transparency statement
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned have been explained.
Funding
KG was supported by an MRC Methodology Research Fellowship (MR/L01193X/1). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the MRC. PRW was funded by the MRC North West Hub for Trials Methodology Research (MR/K025635/1) and the MRC/NIHR Trials Methodology Research Partnership (MR/S014357/1). The Health Services Research Unit is core-funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorates (CZU/3/3).
Footnotes
Conflict of Interest: None.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jclinepi.2021.02.020.
Appendix. Supplementary materials
References
- 1.World Medical Association (WMA) Ferney-Voltaire: WMA; 2008. WMA declaration of helsinki: ethical principles for medical research involving human subjects.http://www.wma.net/en/30publications/10policies/b3/index.html Available at: Accessed April 04, 2021. [Google Scholar]
- 2.Doshi P, Hur P, Jones M, Albarmawi H, Jefferson T, Morgan D. Informed consent to study purpose in randomized clinical trials of antibiotics, 1991 Through 2011. JAMA Intern Med. 2017;177:1452–1459. doi: 10.1001/jamainternmed.2017.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olver I, Buehanan L, Laidlaw C, Poulton G. The adequacy of consent forms for informing patients entering oncological clinical trials. Ann Oncol. 1995;6:867–870. doi: 10.1093/oxfordjournals.annonc.a059352. [DOI] [PubMed] [Google Scholar]
- 4.Canvin K, Jacoby A. Duty, desire or indifference? A qualitative study of patient decisions about recruitment to an epilepsy treatment trial. Trials. 2006;7 doi: 10.1186/1745-6215-7-32. 32-101186/1745-6215-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bekker HL, Thornton J, Airey C, Connelly J, Hewison J, Robinson M. Informed decision making: an annotated bibliography and systematic review. Health Technol. Assess. 1999;3:1–159. [PubMed] [Google Scholar]
- 6.Flory J, Emanuel E. Interventions to improve research participants’ understanding in informed consent for research: a systematic review. JAMA. 2000;6:1593–1601. doi: 10.1001/jama.292.13.1593. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura A, Carey J, Erwin PJ, Tilburt JC, Murad MH, McCormick JB. Improving understanding in the research informed consent process: a systematic review of 54 interventions tested in randomized control trials. BMC Med Ethics. 2013;14:28. doi: 10.1186/1472-6939-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sand K, Kaasa S, Havard Loge J. The understanding of informed consent information—definitions and measurements in empirical Studies. AJOB Prim Res. 2010;1:4–24. [Google Scholar]
- 9.Synnot A, Ryan R, Prictor M, Fetherstonhaugh D, Parker B. Audio-visual presentation of information for informed consent for participation in clinical trials. Cochrane Database Syst Rev. 2014;5 doi: 10.1002/14651858.CD003717.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E. Developing core outcome sets for clinical trials: issues to consider. Trials. 2012;13:132. doi: 10.1186/1745-6215-13-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirkham JJ, Davis K, Altman DG, Blazeby JM, Clarke M, Tunis S. Core outcome set-standards for development: the COS-STAD recommendations. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002447. http://www.comet-initiative.org/studies/details/595?result=true Available at: Accessed January 09, 2020. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillies K, Duthie A, Cotton S, Campbell MK. Patient reported measures of informed consent for clinical trials: a systematic review. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0199775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritchie J, Spencer L. In: Analysing qualitative data. Bryman A, Burgess R, editors. Routledge; London: 1993. Qualitative data analysis for applied policy research; pp. 173–194. [Google Scholar]
- 14.COMET Initiative. DelphiManager. Available at: http://www.comet-initiative.org/delphimanager/ Last accessed 14/04/21
- 15.Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J. Consensus development methods, and their use in clinical guideline development. Health Technol Assess. 1998;2:88. [PubMed] [Google Scholar]
- 16.Okoli C, Pawlowski SD. The Delphi method as a research tool: an example, design considerations and applications. Inform Manag. 2004;42:15–29. Return. [Google Scholar]
- 17.Brookes ST, Macefield RC, Williamson PR. Three nested randomized controlled trials of peer-only or multiple stakeholder group feedback within Delphi surveys during core outcome and information set development. Trials. 2016;17:409. doi: 10.1186/s13063-016-1479-x. Published 2016 Aug 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Meyer D, Kottner J, Beele H. Delphi procedure in core outcome set development: rating scale and consensus criteria determined outcome selection. J Clin Epidemiol. 2019;111:23–31. doi: 10.1016/j.jclinepi.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Harman NL, Bruce IA, Callery P. MOMENT–management of otitis media with effusion in cleft palate: protocol for a systematic review of the literature and identification of a core outcome set using a Delphi survey. Trials. 2013;14:70. doi: 10.1186/1745-6215-14-70. Published 2013 Mar 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macefield R., Blencowe N., Brookes S., Jacobs M, Sprangers M, Williamson P. Core outcome set development: the effect of Delphi panel composition and feedback on prioritisation of outcomes. Trials. 2013;14 doi: 10.1186/1745-6215-14-S1-P77. P77. [DOI] [Google Scholar]
- 21.Srikandarajah N, Noble A, Clark S, Wilby M, Freeman BJC, Fehlings MG. Cauda Equina Syndrome Core Outcome Set (CESCOS): an international patient and healthcare professional consensus for research studies. PLoS One. 2020;15(1):e0225907.. doi: 10.1371/journal.pone.0225907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm. 2016;38:655–662. doi: 10.1007/s11096-016-0257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appelbaum PS, Anatchkova M, Albert K, Dunn LB, Lidz CW. Therapeutic misconception in research subjects: development and validation of a measure. Clin Trials. 2012;9:748–761. doi: 10.1177/1740774512456455. pmid:22942217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent: a new measure of understanding among research subjects. J Natl Cancer Inst. 2001;93:139–147. doi: 10.1093/jnci/93.2.139. pmid:11208884. [DOI] [PubMed] [Google Scholar]
- 25.O'Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 26.Müller E, Zill JM, Dirmaier J, Härter M, Scholl I. Assessment of trust in physician: a systematic review of measures. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0106844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.http://www.comet-initiative.org/Studies/Details/1024 Accessed January 9, 2020, 2021.
- 28.https://www.comet-initiative.org/Studies/Details/1409
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.