Abstract
Objectives:
To better distinguish NOG-related-symphalangism spectrum disorder (NOG-SSD) from chromosomal 17q22 microdeletion syndromes and to inform surgical considerations in stapes surgery for patients with NOG-SSD.
Background:
Mutations in NOG cause a variety of skeletal syndromes that often include conductive hearing loss. Several microdeletions of chromosome 17q22 lead to severe syndromes with clinical characteristics that overlap NOG-SSD. Isolated deletion of NOG has not been described, and therefore the contribution of NOG deletion in these syndromes is unknown.
Methods:
Two families with autosomal dominant NOG-SSD exhibited stapes ankylosis, facial dysmorphisms, and skeletal and joint anomalies. In each family, NOG was evaluated by genomic sequencing and candidate mutations confirmed as damaging by in vitro assays. Temporal bone histology of a patient with NOG-SSD was compared to temporal bones of 40 patients diagnosed with otosclerosis.
Results:
Family 1 harbors a 555kb chromosomal deletion encompassing only NOG and ANKFN1. Family 2 harbors a missense mutation in NOG leading to absence of noggin protein. The incus-footplate distance of the temporal bone was significantly longer in a patient with NOG-SSD than in patients with otosclerosis.
Conclusion:
The chromosomal microdeletion of family 1 led to a phenotype comparable to that due to a NOG point mutation and much milder than the phenotypes due to other chromosome17q22 microdeletions. Severe clinical findings in other microdeletion cases are likely due to deletion of genes other than NOG. Based on temporal bone findings, we recommend that surgeons obtain longer stapes prosthetics prior to stapes surgery in individuals with NOG-SSD stapes ankylosis.
INTRODUCTION
Noggin is a secreted regulator of bone morphogenic proteins that is vital for the development of limb bones and joints of all vertebrates. Mutations in the noggin-encoding gene NOG are associated with multiple autosomal dominant syndromes including proximal symphalangism (SYM1, OMIM 185800)1, stapes ankylosis with broad thumbs and toes (SABTT, OMIM 184460)2,3, multiple synostoses syndrome (SYNS1, OMIM 186500)1, tarsal-carpal coalition syndrome (TCC, OMIM 186570)4,5, and brachydactyly type B2 (BDB2, OMIM 611377)6. Shared signs of these disorders include conductive hearing loss, hyperopia, and digital anomalies. The overlapping features of these syndromes, and their variable expression even within families, has led to their grouping under the term NOG-related-symphalangism spectrum disorder (NOG-SSD)7.
In our studies of families with inherited hearing loss, we discovered two novel mutations leading to NOG-SSD. In family 1, an Israeli kindred with a mild form of NOG-SSD, affected relatives are heterozygous for a 555kB microdeletion including only NOG and ANKFN1, the smallest 17q22 microdeletion described to date8-14. Examination of the clinical features of family 1 compared to other microdeletion cases could suggest which symptoms of NOG-SSD can be attributed to deletion of NOG versus other genes. In family 2, a child diagnosed with multiple synostoses syndrome (SYNS1) is heterozygous for NOG c.41T>C, p.L14P. Surgical reports from the two families together with previous literature suggest that exceptionally long stapes prostheses may be required during stapedectomy surgery for individuals with NOG-SSD. To test this possibility, we compared the incus-footplate distance in a temporal bone from an individual with NOG-SSD to this distance in 40 temporal bones with a diagnosis of otosclerosis.
MATERIALS AND METHODS
The project was approved by the human subjects committees of Seattle Children’s Hospital and the University of Washington (IRB# STUDY00001526) and of Tel Aviv University, and by the Israel National Helsinki Committee. Written informed consent was obtained from adults and assent from children. All included pictures are shared with permission from the families.
Genomic analyses.
DNA was extracted from whole blood. For sequencing using our deafness gene panel15, protein-coding genes and microRNAs related to hearing loss were captured using the SureSelect Target Enrichment system (Agilent). Molecular barcodes were assigned and the samples were multiplexed and sequenced in a single flow-cell of the HiSeq 2500 (Illumina) with 100bp paired-end reads. Whole exome sequencing, alignment, and interpretation of variants were performed as previously described16. Rare CNVs in exome data were identified by Conifer 0.11. Once the NOG microdeletion was found, all relatives in the family were genotyped using Taqman probes (NOG: Hs0059770, ANKFN1: Hs05501071; Thermo) for qPCR run on an ABI 7900HT Sequence Detection System (AB Biosciences)17. Whole genome sequencing was completed through MedGenome (Foster City CA). Sequencing reads were aligned to hg19 with BWA-MEM (0.7.12-r1039), split-reads and discordant-reads extracted using SAMBLASTER (0.1.22) and BAM files sorted and indexed using Sambamba (0.6.7).
Breakpoints of the genomic deletion were identified by using read-depth, split-reads and discordant-reads from BAM files using IGV (2.8.0).
Functional analyses.
Plasmid pCMV6-XL5 containing the human NOG open reading frame (Origene) was mutagenized using the Q5 site-directed mutagenesis kit (NEB) to create plasmids containing NOG missense mutations. Sequences were verified by Sanger sequencing. HEK 293T cells were transfected with each plasmid, or empty pCMV6 as control, using the Lipofectamine 2000 transfection kit (Invitrogen). Cells were plated in phenol red-free DMEM with reduced FBS (0.2%). After 48 hours, cells and media were collected separately. Cells were lysed and protein and mRNA were extracted. Media was concentrated 20x using Vivaspin 20 centrifugal concentrators (GE). Protein concentrations of lysate and media fractions were determined by Pierce BCA assay (Thermo). Identical quantities of protein for each sample were analyzed by western blot using a rabbit polyclonal anti-Noggin antibody (Abcam, ab16054). Lysate samples were normalized to a mouse monoclonal anti-actin antibody (Sigma, A2228). RT-qPCR of NOG was performed as described above to measure transcript levels and compare between conditions. For lactacystin experiments, 10μM of lactacystin (Sigma) in fresh media was added 10 hours prior to cell harvest.
Phenotypic analyses.
The incus-stapes footplate distance was measured in human temporal bone specimens collected as part of the NIDCD National Human Temporal Bone Pathology Resource Registry and processed at the Massachusetts Eye and Ear Otopathology Laboratory. Temporal bones were prepared for histologic analysis via standard techniques including fixation in formalin, decalcification in EDTA, dehydration in alcohols, and embedment in celloidin. Specimens were sectioned into 20 μm thick slices horizontally relative to the lateral semicircular canal. Every tenth section was stained with hematoxylin and eosin and mounted on a glass slide. From each case, the most inferior slide that included the stapes footplate was selected. The incus-stapes footplate distance was measured between the medial surface of the distal long process of the incus and the stapes footplate, with the measurement line being perpendicular to the footplate. All measurements were performed using ImageJ software18. As otosclerosis is by far the most common reason for stapedectomy surgery, temporal bones from 40 patients with otosclerosis were used as controls. These measurements were used to establish normative data for the incus-stapes footplate distance and to compare to the temporal bone measurement from the case of genetically confirmed NOG-SSD. The R Commander package of R was used for statistical analysis19.
RESULTS
Clinical Presentation
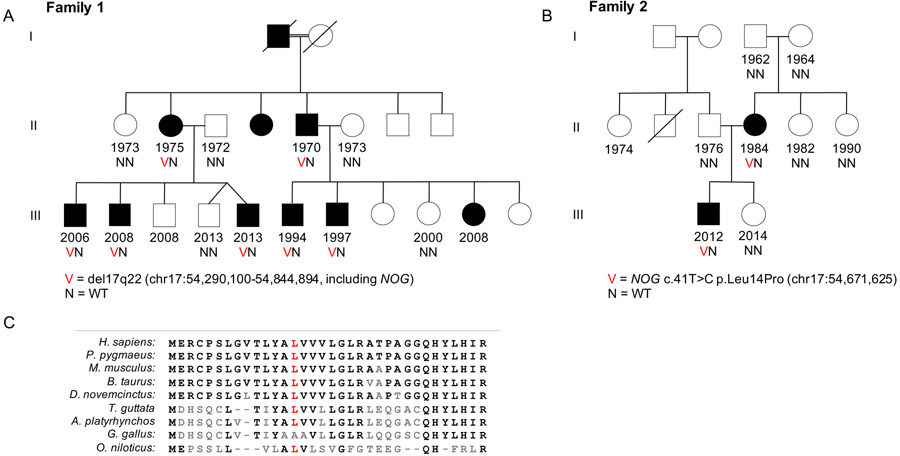
For both families, pedigrees are shown in Figure 1 and clinical features in Table 1. In family 1 (Fig. 1A), affected individuals exhibited congenital conductive hearing loss and hyperopia (Fig. 2A). For individuals II-2, II-4, II-5 and III-6, stapes ankylosis was confirmed and repaired surgically. Dysmorphic facial and skeletal features varied among family members (Fig. 3A; Table 1). None of the individuals in family 1 demonstrated vertebral or lower limb skeletal abnormalities, urogenital malformations, intellectual disability, or attention-deficit hyperactivity disorder.
Figure 1: Two families with mutations of NOG.
A) A 3-generation family with deletion of 555kb on chromosome 17q22 that includes NOG. B) Mother and son with point mutation in NOG. The mutation is de novo in the affected mother. All affected individuals in both families have conductive hearing loss and hyperopia among other symptoms (Table 1). C) Amino acid sequence at the N-terminus of noggin among selected species shows high conservation at the location of NOG p.L14P.
Table 1:
Affected members of each family share common features of NOG-SSD.
| Characteristic | II-2 | II-5 | III-1 | III-2 | III-5 | III-6 | II-4 | III-1 |
|---|---|---|---|---|---|---|---|---|
| Face | ||||||||
| Elongated Face | + | + | + | + | + | |||
| Maxillary hypoplasia | + | + | + | + | + | |||
| Short effaced philtrum | + | + | + | + | + | |||
| Thin upper lip | + | + | + | + | + | |||
| Micrognathia | + | + | + | + | + | |||
| Slightly upslanting palpebral fissures | + | + | + | + | ||||
| Blepharophimosis | + | + | ||||||
| Nose | ||||||||
| Long bulbous nose | + | + | + | + | + | |||
| Broad nasal base | + | + | + | + | + | |||
| Hypoplastic alae nasi | + | + | + | + | + | |||
| Low columella | + | + | + | + | + | |||
| Depressed nasal tip | + | + | ||||||
| Skeletal | ||||||||
| Broadened thumbs | + | + | ||||||
| Broadened halluces | + | |||||||
| Vertebral fusion | + | |||||||
| Fusion of bones in great toes | + | |||||||
| Protruding ribs | + | + | ||||||
| Unable to flex 5th PIP | + | + | + | |||||
| Unable to flex thumbs, no flexion creases | + | |||||||
| Unable to flex wrist | + | |||||||
| Other | ||||||||
| Confirmed stapes ankylosis | + | + | + | + | ||||
| Hyperopia | + | + | + | + | + | + | + | + |
| Abnormal outer ears | + |
Legend: The left section corresponds to family 1, and the right is for family 2.
Figure 2. Conductive hearing loss in families 1 and 2.
A) Audiograms from family 1 relatives aged 5 to 42 years. Individual II-5 was tested post right-sided stapedectomy. B) Audiograms from family 2 proband III-1 before and after bilateral stapedotomy.
Figure 3: Characteristic facial and skeletal features of NOG-SSD in families 1 and 2.
A) Facial features of family 1 relatives III-1 (top left), III-2 (top right), and III-5 (bottom left) showing characteristic signs of NOG-SSD. Radiographs (bottom right) of the right hand of individual III-5 show absence of digital abnormalities. The fracture that is present is the reason for the imaging but is unrelated to the syndrome. B) Facial features of family 2 individual III-1 (top), and radiographs of his hands (bottom left) without digital abnormalities, and of his spine (bottom right) demonstrating fusion of his C2 and C3 vertebrae.
The proband of family 2 (Fig. 1B, III-1) was diagnosed with moderate to severe bilateral conductive hearing loss at age 2.5y and was fitted with bilateral hearing aids at age 3y. The hearing loss progressed (Fig. 2B) and he underwent bilateral laser stapedotomies with consequent improvements in his hearing. He also has hyperopia (+8 in both eyes) and has worn eyeglasses since age 2.5y. Strabismus is present and corrected by eyeglasses. His facial features are similar to those of family 1 (Fig 3B; Table 1). He has slightly broadened thumbs bilaterally with a decreased range of flexion. He also has limitation of his neck motion due to C2-C3 vertebral fusion (Fig. 3B). He has no other joint restriction or laxity, body asymmetry, scoliosis, joint dislocation, or intellectual disability. The proband’s mother (II-4) reports unilateral conductive hearing loss. She also has hyperopia, bilateral broad thumbs, fusion of bones within her great toes, stiff hip joints, and is unable to flex her wrists. Her two adult sisters (II-5 and II-6) and parents (I-3 and I-4) have no symptoms of NOG-SSD.
Genetics of NOG-SSD
Family 1 relatives II-2, II-5, and III-6 had no mutations in any gene on the custom panel but shared a deletion at chromosome 17q22 detectable in whole exome sequence using our CNV analysis pipeline20. This deletion included both NOG and ANKFN1 and was confirmed by qPCR analysis to co-segregate perfectly with the phenotype in the family. Whole genome sequence of III-1 confirmed the breakpoints of the deletion as chr17:54,290,100-54,844,894 (GRCh37/hg19; SCV001448202.1).
For the proband of family 2, clinical suspicion for a diagnosis of SYNS1 led to gene-specific sequencing of NOG (Prevention Genetics, Marshfield, WI), yielding a single variant, NOG c.41T>C p.L14P at chr17:54,671,625 (GRCh37/hg19; NM_005450.6; SCV001451939). This mutation occurs at a residue (Fig. 1C) which is conserved as leucine in all vertebrates with high quality sequence (more than 100 species) with the exception of three bird species for whom this residue is valine, and appears private to this family (not present on gnomAD). The variant was also present in the proband’s affected mother (II-4) but not in his maternal grandfather or grandmother or maternal aunts (Fig. 1B), suggesting it occurred de novo in the mother.
Consequences of the mutations to protein secretion
We investigated the effects of the NOG p.L14P mutation on protein secretion using an in vitro secretion assay (Fig. 4A-B). NOG p.L14P resulted in complete absence of noggin from both cell lysates (mean 0.0 AU; p<0.0001) and supernatant (mean 0.0 AU; p<0.0001). Another NOG variant known to abolish noggin secretion, NOG p.P170L21, was included in the assay as well. It also led to significantly reduced protein in lysate (mean 0.47 AU; p<0.001) and supernatant (mean 0.14 AU; p<0.0001), although in contrast to the previous report21, some protein was still observed in the supernatant. NOG transcript levels were measured by RT-qPCR and confirmed to be high across all conditions (Fig. 4C).
Figure 4: NOG p.L14P leads to loss of cellular and secreted noggin.
A) Western blots showing intracellular (lysate) and extracellular (supernatant) noggin present in HEK 293T cells transfected with various NOG ORFs. NOG p.L14P is the missense mutation of family 2. NOG p.P170L was previously reported to cause conductive hearing loss and decrease noggin secretion21. B) Quantification of the data from part A shows complete absence of NOG p.L14P and reduced amounts of NOG p.P170L in both supernatant and lysate (*** p<0.001, **** p<0.0001). C) qPCR of cDNA from transfected cells shows NOG transcription from all plasmids, despite differences in noggin protein abundance (* p<0.05). Expression between transfected plasmids were not significantly different. D) Western blots as described in part A, but with the addition of 10μM lactacystin 10 hours prior to cell harvest. E) Quantification of the blots in part E shows an increase in intracellular NOG p.L14P in the presence of lactacystin, but still significantly lower than wild type (* p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001).
To investigate the role of proteasomal degradation in the absence of cellular NOG p.L14P protein, the secretion assay was repeated with the addition of lactacystin, a proteasome inhibitor (Fig. 4D-E). In the presence of lactacystin, NOG p.L14P noggin appeared in the lysate fraction at detectable levels (mean 0.12 AU; p<0.05 compared to empty condition), but remained absent from the supernatant (mean 0.0 AU; p<0.0001 compared to NOG wild type). NOG p.P170L protein was reduced compared to wild type in both lysate (mean 0.26 AU; p<0.01) and supernatant (mean 0.08 AU; p<0.001) fractions in the presence of lactacystin.
Incus-stapes footplate distance
To evaluate the possibility of increased incus-stapes footplate distance as a feature of NOG-SSD, this measurement was compared in the temporal bone of a previously described NOG-SSD patient22 versus 40 patients with otosclerosis (Fig. 5). In patients with otosclerosis, the mean incus-stapes footplate distance was 3.72mm with standard deviation ±0.38mm (range 3.01mm-4.53mm). Distances were normally distributed among otosclerosis cases (W=0.975; p=0.5, Shapiro Wilk test; Fig. 5C). The incus-stapes footplate distance of the NOG-SSD case was 4.62mm, more than 2.4 standard deviations above the mean for the otosclerosis controls, indicating that the NOG-SSD case is an outlier with an extremely long incus-footplate distance.
Figure 5. Footplate-incus distance of a NOG-SSD patient is significantly longer then otosclerosis controls.
Light microscopy at 1.25x of human temporal bone sections stained with hematoxylin and eosin. In both images, the double ended arrow indicates the incus-stapes footplate distance, as measured perpendicular to the stapes footplate in the most inferior section containing the footplate. A) In a patient with NOG-SSD, “+” indicates the area of footplate fixation due to ossified cartilage filling the stapedovestibular joint space. B) In a patient with otosclerosis, “*” indicates a focus of otosclerosis which fixes the stapes at its anterior footplate to the otic capsule. C) Distribution of incus-stapes (IS) distances in temporal bones of 40 patients with otosclerosis, and in the temporal bone of a patient with NOG-SSD (red arrow).
Treatment outcomes
For family 1, surgical reports of stapedectomies of II-2 at age 23y and III-6 at age 21y indicated that long prostheses were required for II-2 (4.5mm at initial surgery, 5.0mm at revision surgery) and for III-6 (5.0mm).
The proband of family 2 underwent laser-assisted stapedotomy of his left ear at age 7y. During the procedure the ossicular chain demonstrated good mobility at the level of the malleus, and incus, but there was rigid stapes fixation at the level of the footplate. A 4.75mm piston was noted to be too short and therefore a 5.5mm re-shaped malleus attachment piston was used. Following the operation, the patient demonstrated markedly improved audiologic testing in the treated ear (Fig. 2B). At age 8y, an identical surgery was performed on the proband’s right ear. Once again, the length between the footplate and the incus was measured and it was noted that the 4.75 length piston would be far too short, and so a 5.5 mm malleus piston prosthesis was used. Following placement, the prosthesis moved well but the malleus and/or incus were also noted to be fixed. The malleus/incus complex was partly mobilized by repeated pressure on the incus and malleus until mobility was improved but was unfortunately still not normal. Despite this finding, his post-operative audiogram showed notable improvement (Fig. 2B).
DISCUSSION
Noggin acts as an antagonist of bone morphogenetic proteins (BMPs)23,24. BMPs are key regulators of skeletal development25, responsible for recruiting mesenchymal cells into future skeletal anlagen, for promoting mesenchymal cell proliferation and differentiation into chondroblasts and osteoblasts, and for osteogenic transformation of stem cells26-28. The interaction between BMPs and noggin affects body patterning24,25, apoptosis induction in digital and interdigital regions29-31, and middle ear formation29,32. The role of noggin in mammalian skeletal development was confirmed by experiments in transgenic mice. Nog−/− mice do not survive past birth and display developmental abnormalities such as shorter bones, absence of multiple joints, and bony fusion of the appendicular skeleton30,33. These mice also display excess bone morphogenetic protein activity and cartilage formation30.
More than 60 mutations of NOG, including both missense and nonsense mutations, have been associated with autosomal dominant NOG-SSD7. Most pathogenic NOG point mutations lead to reduced secretion and/or altered dimerization of noggin34. Other NOG mutations alter binding of noggin to BMPs and to differentiation factor 5 and are associated with BDB235. Additionally, several microdeletions at chromosome 17q22 lead to phenotypes that include signs of NOG-SSD along with more severe developmental defects. The published deletions vary in size from 1.34Mb to 8.18Mb and encompass between 5 and 60 genes8-14. While deletion of NOG is present in the majority of cases, several microdeletions do not include NOG while still leading to severe syndromes. Because isolated deletion of NOG has not previously been observed in humans, the disorders and clinical characteristics that are attributable specifically to its loss have not been definitively determined.
In family 1, the 555kb deletion includes only NOG and ANKFN1 and is the smallest 17q22 deletion reported to date. It results in a mild phenotype of characteristic NOG-SSD features. Family 1 lacks the more severe features of other microdeletion cases, such as widespread skeletal dysmorphisms, urogenital malformations, and intellectual disability. The phenotypes of family 1 are similar to those resulting from point mutations of NOG, in particular those diagnosed with SABTT. The phenotypes of two published cases with NOG frameshift mutations leading to SABTT (NOG c.252insC p.E85fs*96 and c.304delG p.A102fs)3,36,37 are particularly similar to family 1, as expected given the consequences of the mutations to protein function. In all of these cases, affected individuals exhibit bilateral conductive hearing loss, hyperopia, distinct facial features, and bilateral broad thumbs. In contrast, the phenotype of family 1 differs from cases of chromosome 17q22 microdeletions that exclude NOG9,14. Neither of these latter cases exhibited conductive hearing loss, and both presented with severe syndromic features in organ systems other than skeletal and joint development such as neurological abnormalities and intellectual disability. These other features are likely due to the involvement of other genes. These findings suggest that nearly isolated deletion of NOG results in a more typical NOG-SSD phenotype, while microdeletions excluding NOG cause more severe and different syndromes.
Missense mutation NOG p.L14P of family 2 is located in the N-terminal signal peptide of the protein, a domain required for endoplasmic reticulum targeting and secretion. This location suggested that NOG p.L14P would lead to intracellular entrapment of noggin, but it instead served to eliminate the protein from the cell entirely. Transcriptional analysis confirmed high levels of message expression. Addition of the proteasome inhibitor lactacystin did lead to an increase in intracellular NOG p.L14P noggin, but not to match wild type levels. Notably, the protein that was produced was still not secreted. It is likely that this mutation’s effect is multifactorial, possibly leading to severe protein misfolding and/or to rapid degradation while also interfering with secretion. Regardless of the precise mechanism, NOG p.L14P decreases noggin secretion and consequently leads to the NOG-SSD phenotype.
The most common sign in NOG-SSD is conductive hearing loss related to congenital stapes fixation. This phenotype is of particular interest in otology, because NOG mutations represent one of the few established genetic causes for conductive hearing loss despite clear familial clustering of both conductive hearing loss and otosclerosis38. Increased risk of otosclerosis has been associated with variants in COL1A1 and in several genes of the TGF-ß superfamily39. Otosclerosis loci (OTSC1-10) have been identified by linkage analysis but the critical genes are not yet known40-49. We hypothesize that critical mutations for conductive hearing loss will be hypomorphic alleles of genes in which loss of function variants cause severe skeletal phenotypes. Mutations in NOG are consistent with this prediction, as conductive hearing loss is one of the most common symptoms of NOG-SSD, and possibly the only feature in the mildest cases, despite the fact that NOG is heavily involved in skeletal and joint development.
Malleus and/or incus fixation, as was seen in the right ear of the proband of Family 2, has not previously been reported in individuals with NOG-SSD. Prior histopathologic analysis of stapes fixation in an individual with NOG-SSD identified ossified cartilage across the stapedovestibular joints within an otherwise normal middle ear22. This report specifically states that no malleus or incus fixation was visible in the individual’s unoperated right ear. The possibility of malleus or incus fixation is an important surgical consideration for patients with NOG-SSD because the approach differs: If ankylosis involves ossicles beyond the stapes, then an atticotomy should be considered in order to perform more extensive middle ear repair. In addition, mobility of the malleus/incus complex should be assessed intraoperatively prior to stapedotomy. Future reports of middle ear exploration in NOG-SSD patients will be necessary to determine the frequency of malleus or incus fixation.
Fixation of the stapes may be treated by complete or partial stapedectomy which involves removal of all or a portion of the stapes50, or by stapedotomy which involves the use of a laser to make a small fenestra through the stapes footplate and removal of the stapes suprastructure only51,52. This is performed through the ear canal and the stapes is replaced with a prosthetic stapes piston. The length of the prosthetic must be fit properly for each individual, as a long prosthesis can lead to severe vertigo53 and a short prosthesis may not adequately conduct sound or can become displaced54,55. The vast majority of patients fit the same length prosthesis (e.g. either 4.25mm if a conventional prosthesis is selected or 4.5mm if a shape memory alloy prosthesis is used)56. It is crucial that the appropriately sized prosthetic be available for use during the surgery, and information about which size(s) may be necessary is critical prior to the operation as many hospitals stock only the common sizes.
We re-examined the genetically confirmed NOG-SSD temporal bone mentioned above22 in order to measure the incus-stapes footplate distance, comparing it to measurements from temporal bones of patients with otosclerosis. The footplate-incus distance is similar to the measurement that surgeons may take intraoperatively to estimate the appropriate prosthesis length. The incus-stapes footplate distance of the NOG-SSD case was 4.62mm, more than 2.4 standard deviations above the mean for specimens with otosclerosis and the largest distance out of the 41 measurements made. This distance likely would have required a 5.25mm incus attachment prosthesis which may not be routinely available in the operating room, as unusual sizes are often not stocked or may not even be manufactured for some prosthesis designs.
Many individuals with NOG-SSD, including those described here, have undergone stapedectomy or stapedotomy with improvement of their symptoms7,29,31,57,58. Our analysis of the incus-footplate distance for previously published patients, as well as surgical reports from the families in this study, indicate increased incus-stapes footplate distance, and for most cases, need for an unusually long prosthetic. If an extra-long incus-attachment prosthesis cannot be obtained, an alternative solution is to adapt a malleus-attachment prosthesis (which is typically available in longer lengths) for use as an incus-attachment prosthesis. For the proband of family 2, the required 5.5mm prosthetic is longer than the largest size that is available commercially, and a modified malleus attachment prosthesis was successfully used. Further work will be necessary to determine whether an unusually long incus-footplate distance is specific to certain clinical subtypes within NOG-SSD or is shared by all patients with NOG mutations. Regardless, the need for long or custom prostheses is an important surgical consideration in the treatment of individuals with NOG-SSD.
Acknowledgements:
We thank the families for their continued engagement and support, and Sarah Pierce, Hannah Kortbawi, and Silvia Casadei for technical and clinical advice.
Support: NIH 2R01DC011835 (KBA, MCK); NIH T32DC005361 (RJC); NIH T32DC000018 (RJC); NIH T32GM007266 (RJC); NIH U24DC013983 (AQ); ARCS Foundation (RJC); Ben B. Cheney Foundation (RJC); Virginia Merrill Bloedel Traveling Scholars Award (KBA)
REFERENCES
- 1.Gong Y, Krakow D, Marcelino J, et al. Heterozygous mutations in the gene encoding noggin affect human joint morphogenesis. Nat Genet 1999;21:302–304. [DOI] [PubMed] [Google Scholar]
- 2.Teunissen B, Cremer WR. An autosomal dominant inherited syndrome with congential stapes ankylosis. Laryngoscope 1990;100:380–384. [DOI] [PubMed] [Google Scholar]
- 3.Brown DJ, Kim TB, Petty EM, et al. Autosomal dominant stapes ankylosis with broad thumbs and toes, hyperopia, and skeletal anomalies is caused by heterozygous nonsense and frameshift mutations in NOG, the gene encoding noggin. Am J Hum Genet 2002;71:618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregersen HN, Petersen G. Congenital malformation of the feet with low body height: A new syndrome, caused by an autosomal dominant gene. Clin Genet 1977;12:255–262. [DOI] [PubMed] [Google Scholar]
- 5.Drawbert HP, Stevens DB, Cadle RG, et al. Tarsal and carpal coalition and symphlangism of the Fuhmann type: Report of a family. J Bone Jt Surg 1985;67:884–889. [PubMed] [Google Scholar]
- 6.Kjaer KW, Tiner M, Cingoz S, et al. A novel subtype of distal symphalangism affecting only the 4th finger. Am J Med Genet Part A 2009;149A:1571–1573. [DOI] [PubMed] [Google Scholar]
- 7.Potti TA, Petty EM, Lesperance MM. A comprehensive review of reported heritable noggin-associated syndromes and proposed clinical utility of one broadly inclusive diagnostic term: NOG-related-symphalangism spectrum disorder (NOG-SSD). Hum Mutat 2011;32(8):877–886. [DOI] [PubMed] [Google Scholar]
- 8.Laurell T, Lundin J, Anderlid B, et al. Molecular and clinical delineation of the 17q22 microdeletion phenotype. Eur J Med Genet 2013;21(10):1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khattab M, Xu F, Li P, et al. A de novo 3.54 mb deletion of 17q22-q23.1 associated with hydrocephalus: A case report and review of literature. Am J Med Genet Part A 2011;155(12):3082–3086. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Fernandez L, Fernandez-Toral J, Llano-Rivas I, et al. Delineation of the clinically recognizable 17q22 contiguous gene deletion syndrome in a patient carrying the smallest microdeletion known to date. Am J Med Genet Part A 2015;167(9):2034–2041. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu R, Mitsui N, Mori Y, et al. Cryptic 17q22 deletion in a boy with a t(10;17)(p15.3;q22) translocation, multiple synostosis syndrome 1, and hypogonadotropic hypogonadism. Am J Med Genet Part A 2008;146A(11):1458–1461. [DOI] [PubMed] [Google Scholar]
- 12.Pang X, Luo H, Chai Y, et al. A 1.6-Mb microdeletion in chromosome 17q22 leads to NOG-related symphalangism spectrum disorder without intellectual disability. PLoS One 2015;10(3):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puusepp H, Zilina O, Teek R, et al. 5.9 Mb microdeletion in chromosome band 17q22-q23.2 associated with tracheo-esophageal fistula and conductive hearing loss. Eur J Med Genet 2009;52:71–74. [DOI] [PubMed] [Google Scholar]
- 14.Nimmakayalu M, Major H, Sheffield V, et al. Microdeletion of 17q22q23.2 encompassing TBX2 and TBX4 in a patient with congenital microcephaly, thyroid duct cyst, sensorineural hearing loss, and pulmonary hypertension. Am J Med Genet 2011;155A(2):418–423. [DOI] [PubMed] [Google Scholar]
- 15.Abu Rayyan A, Kamal L, Casadei S, et al. Genomic analysis of inherited hearing loss in the Palestinian population. Proc Natl Acad Sci USA. 2020;117(33):20070–20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gulsuner S, Walsh T, Watts AC, et al. Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell. 2013;154(3):518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh T, Pierce SB, Lenz DR, et al. Genomic duplication and overexpression of TJP2/ZO-2 leads to altered expression of apoptosis genes in progressive nonsyndromic hearing loss DFNA51. Am J Hum Genet 2010;87(1):101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods 2012;9(7):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox J, Bouchet-Valat M. R Commander. 2018. [Google Scholar]
- 20.Nord AS, Lee M, King MC, et al. Accurate and exact CNV identification from targeted high-throughput sequence data. BMC Genomics 2011;12:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shu Y, Wang L, Cheng X, et al. The p.(Pro170Leu) variant in NOG impairs noggin secretion and causes autosomal dominant congenital conductive hearing loss due to stapes ankylosis. J Genet Genomics 2019;46(9):445–449. [DOI] [PubMed] [Google Scholar]
- 22.Quesnel AM, Nadol JB Jr, Nielsen GP, et al. Temporal bone histopathology in NOG-symphalangism spectrum disorder. Otol Neurotol 2015;36:1651–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the spemann organizer in xenopus embryos. Cell 1992;70:829–840. [DOI] [PubMed] [Google Scholar]
- 24.Zimmerman LB, De Jesu M, Harland RM. The spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell 1996;86:599–606. [DOI] [PubMed] [Google Scholar]
- 25.Hogan BLM. Bone morphogenetic proteins in development. Curr Opin Genet Dev 1996;6:432–438. [DOI] [PubMed] [Google Scholar]
- 26.Groeneveld EHJ, Burger EH. Bone morphogenetic proteins in human bone regeneration. Eur J Endocrinol 2000;142:9–21. [DOI] [PubMed] [Google Scholar]
- 27.Zou H, Niswander L. Requirement for BMP signaling in interdigital apoptosis and scale formation. Science 1996;272(15):1–7. [DOI] [PubMed] [Google Scholar]
- 28.Cheng H, Jiang W, Phillips FM, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Jt Surg 2003;85A:1544–1552. [DOI] [PubMed] [Google Scholar]
- 29.Declau F, Van Den Ende J, Baten E, et al. Stapes ankylosis in a family with a novel NOG mutation: Otologic features of the facioaudiosymphalangism syndrome. Otol Neurotol 2005;26(5):934–940. [DOI] [PubMed] [Google Scholar]
- 30.Brunet LJ, Mcmahon JA, Mcmahon AP, et al. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science 1998;280:1455–1458. [DOI] [PubMed] [Google Scholar]
- 31.Weekamp HH, Kremer H, Hoefsloot LH, et al. Teunissen-Cremers Syndrome: A clinical, surgical, and genetic report. Otol Neurotol 2005;26(1):38–51. [DOI] [PubMed] [Google Scholar]
- 32.Hwang C, Wu DK. Noggin heterozygous mice: an animal model for congenital conductive hearing loss in humans. Hum Mol Genet 2008;17(6):844–853. [DOI] [PubMed] [Google Scholar]
- 33.Mcmahon JA, Takada S, Zimmerman LB, et al. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev 1997;12:1438–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcelino J, Sciortino CM, Romero MF, et al. Human disease-causing NOG missense mutations: Effects on noggin secretion, dimer formation, and bone morphogenetic protein binding. Proc Natl Acad Sci USA 2001;98(20):11353–11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehmann K, Seemann P, Silan F, et al. A new subtype of brachydactyly type B caused by point mutations in the bone morphogenetic protein antagonist noggin. Am J Hum Genet 2007;81:388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milunsky J, Suntra C, Macdonald CB. Congenital stapes ankylosis, broad thumbs, and hyperopia: Report of a family and refinement of a syndrome. Am J Med Genet 1999;82A(5):404–408. [PubMed] [Google Scholar]
- 37.Thomeer HGXM, Admiraal RJC, Hoefsloot L, et al. Proximal symphalangism, hyperopia, conductive hearing impairment, and the NOG gene: 2 new mutations. Otol Neurotol 2011;32(4):632–638. [DOI] [PubMed] [Google Scholar]
- 38.Babcock TA. Otosclerosis from genetics to molecular biology. Otolaryngol Clin N Am 2019;51:305–318. [DOI] [PubMed] [Google Scholar]
- 39.Mckenna MJ, Nguyen-huynh AT, Kristiansen AG. Association of otosclerosis with Sp1 binding site polymorphism in COL1A1 gene: Evidence for a shared genetic etiology with osteoporosis. Otol Neurotol 2004;25(4):447–450. [DOI] [PubMed] [Google Scholar]
- 40.Tomek MS, Brown MR, Mani SR, et al. Localization of a gene for otosclerosis to chromosome 15q25–q26. Hum Mol Genet 1998;7(2):285–290. [DOI] [PubMed] [Google Scholar]
- 41.Van Den Bogaert K, Govaerts PJ, Schatteman I, et al. A second gene for otosclerosis, OTSC2, maps to chromosome 7q34-36. Am J Hum Genet 2001;68:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen W, Campbell CA, Green GE, et al. Linkage of otosclerosis to a third locus (OTSC3) on human chromosome 6p21.3-22.3. J Med Genet 2002;39:473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bel Hadj Ali I, Thys M, Beltaief N, et al. Clinical and genetic analysis of two Tunisian otosclerosis families. Am J Hum Genet 2006;221(3):212–221. [DOI] [PubMed] [Google Scholar]
- 44.Brownstein Z, Goldfarb A, Levi H, et al. Chromosomal mapping and phenotypic characterization of hereditary otosclerosis linked to the OTSC4 locus. Arch Otolaryngol Head Neck Surg 2019;132:416–424. [DOI] [PubMed] [Google Scholar]
- 45.Van Den Bogaert K, De Leenheer EMR, Chen W, et al. A fifth locus for otosclerosis, OTSC5, maps to chromosome 3q22-24. J Med Genet 2004;41(6):450–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iliadou V, Van Den Bogaert K, Eleftheriades N, et al. Monogenic nonsyndromic otosclerosis: Audiological and linkage analysis in a large Greek pedigree. Int J Pediatr Otorhinolaryngol 2006;70:631–637. [DOI] [PubMed] [Google Scholar]
- 47.Pauw RJ, Hygen PLM, Thys M, et al. Phenotype description of a Dutch otosclerosis family with suggestive linkage to OTSC7. Am J Hum Genet 2007;143A:1613–1622. [DOI] [PubMed] [Google Scholar]
- 48.Bel Hadj Ali I, Thys M, Beltaief N, et al. A new locus for otosclerosis, OTSC8, maps to the pericentromeric region of chromosome 9. Hum Genet 2008;123:267–272. [DOI] [PubMed] [Google Scholar]
- 49.Shrauwen I, Weegerink N, Fransen E, et al. A new locus for otosclerosis, OTSC10, maps to chromosome 1q41–44. Clin Genet 2011;79:495–497. [DOI] [PubMed] [Google Scholar]
- 50.Ishiyama A, Glasscock ME. Total stapedectomy. Oper Tech Otolaryngol - Head Neck Surg 1998;9(1):3–7. [Google Scholar]
- 51.De La Cruz A, Chandrasekhar SS. Mechanical small fenestra stapedotomy. Oper Tech Otolaryngol - Head Neck Surg 1998;9(1):33–37. [Google Scholar]
- 52.Young E, Mitchell-Innes A, Jindal M. Lasers in stapes surgery: A review. J Laryngol Otol 2015;129(7):627–633. [DOI] [PubMed] [Google Scholar]
- 53.Spandow O, Söderberg O, Bohlin L. Long-term results in otosclerotic patients operated by stapedectomy or stapedotomy. Scand Audiol 2000;29(3):186–190. [DOI] [PubMed] [Google Scholar]
- 54.Scierski W, Namysłowski G, Czerwińska G, et al. Postoperative vertigo caused by too long stapes prosthesis-radiological diagnostics. Otolaryngol Pol 2012;66(5):363–367. [DOI] [PubMed] [Google Scholar]
- 55.Portmann D, Alcantara M, Vianna M. The length of the piston in otosclerosis surgery. Rev Laryngol Otol Rhinol 2007;128(1-2):55–58. [PubMed] [Google Scholar]
- 56.Husain Q, Lin KF, Selesnick SH. Stapes prosthesis length and hearing outcomes. Laryngoscope 2018;128(3):722–726. [DOI] [PubMed] [Google Scholar]
- 57.Massey BL, Hillman TA, Shelton C. Stapedectomy in congenital stapes fixation: Are hearing outcomes poorer? Otolaryngol Neck Surg 2006;134:816–818. [DOI] [PubMed] [Google Scholar]
- 58.Ensink RJH, Sleeckx J-P, Cremers CWRJ. Proximal Symphalagism and Congenital Conductive Hearing Loss: Otologic Aspects. Am J Otol 1999;20:344–349. [PubMed] [Google Scholar]