Abstract
While the major virulence factors for Vibrio cholerae, the cause of the devastating diarrheal disease cholera, have been extensively studied, the initial intestinal colonization of the bacterium is not well understood because non-human adult animals are refractory to its colonization. Recent studies suggest the involvement of an interbacterial killing device known as the type VI secretion system (T6SS). Here, we tested the T6SS-dependent interaction of V. cholerae with a selection of human gut commensal isolates. We show that the pathogen efficiently depleted representative genera of the Proteobacteria in vitro, while members of the Enterobacter cloacae complex and several Klebsiella species remained unaffected. We demonstrate that this resistance against T6SS assaults was mediated by the production of superior T6SS machinery or a barrier exerted by group I capsules. Collectively, our data provide new insights into immunity protein-independent T6SS resistance employed by the human microbiota and colonization resistance in general.
Subject terms: Microbial communities, Microbial genetics
Here, the authors study the impact of Vibrio cholerae’s T6SS on human gut microbiota isolates and show that certain bacteria are protected from T6SS attacks in an immunity protein-independent manner. Specifically, protection occurred through superior T6SS weaponry in members of the Enterobacter cloacae complex and by molecular armors made of membrane-tethered capsular polysaccharides of diverse Klebsiella isolates.
Introduction
The human pathogen Vibrio cholerae is the causative agent of the severe diarrheal disease cholera, but its notoriously poor colonization ability of non-human adult animals makes it difficult to study. In fact, in one of the earliest studies on intestinal microbes, Metchnikoff suggested that adult experimental animals were refractory to the disease cholera due to the presence of their intestinal bacteria1. As a result, researchers have developed infant animal models (mice and rabbits) to study the pathogen’s virulence potential, since infant animals lack a mature microbiota1,2. We now know that the disease cholera progresses first through toxin-coregulated pilus (TCP)-induced self-aggregation and microcolony formation in the gut, followed by the secretion of cholera toxin, which induces profuse diarrhea3,4. While useful for pathogenesis studies, infant animal models do not undergo the first step of intestinal colonization, which consists of the interaction of ingested V. cholerae with the mature microbiota. Because numerous studies have also shown that commensal microbes are critical in providing colonization resistance against incoming bacteria5 and ultimately play a role in protecting humans from pathogens, it is important to study the interaction of V. cholerae with the human microbiota to better mimic the real-world infection conditions.
Intestinal pathogens can directly interact with the gut microbiota using strategies ranging from nutritional competition up to interbacterial warfare, with the latter encompassing the production of inhibitory molecules or contact-dependent inhibition/killing systems6,7. One example of a contact-dependent killing device is the type VI secretion system (T6SS), which was first described by Pukatzki and colleagues in 20068. The presence of T6SS is widespread, as it is encoded by 25% of all sequenced Gram-negative bacteria9, and more than 50% of ß- and γ-proteobacterial genomes harbor such a system10. The T6SS can be compared to an inverted contractile phage tail anchored to the cell envelope by a membrane complex11. A membrane complex-attached baseplate-like structure allows the polymerization of an internal tube made of Hcp protein rings, which is wrapped in a contractile sheath11. When the T6SS sheath contracts, the inner tube, the spike protein(s), and a cocktail of mostly tip-associated toxins is propelled into neighboring cells, causing growth inhibition or death12. The T6SS is therefore a well-suited nanomachine to drive interbacterial competition in the gut, as (i) the high bacterial density within this niche fosters direct contact between microbes, and (ii) the contact dependency of the T6SS limits collateral damage on non-neighboring bacteria.
There is evidence that the T6SS is important for colonization, as some intestinal pathogens, such as Salmonella enterica serovar Typhimurium, Shigella sonnei, and V. cholerae, are thought to utilize their T6SS to clear the resident microbiota and thereby promote their own colonization13–15. For instance, using the infant mouse cholera model, a recent study showed that V. cholerae outcompeted artificially pre-introduced mouse commensal Escherichia coli in a T6SS-dependent manner. T6SS-defective V. cholerae were therefore less abundant post-infection compared to their T6SS-positive parental strain15. This colonization defect was not observed when WT and T6SS-defective strains were co-administered, suggesting a global impact on niche clearing under the tested conditions.
Since the T6SS is an effective killing device, mechanisms need to exist to protect T6SS-positive bacteria and their siblings from (auto)intoxication. T6SS-positive bacteria therefore produce immunity proteins that directly interact with the cognate effector proteins and inhibit their toxic activity16,17. However, recent studies have also identified immunity protein-independent protection mechanisms18. For instance, the edited peptidoglycan of Acinetobacter baumannii provides protection from T6SS assaults19. Secreted exopolysaccharide (EPS), which is a primary component of bacterial biofilm matrices20, was also shown to confer partial protection against external T6SS attacks, especially in quorum-sensing-impaired and therefore EPS-overproducing V. cholerae21. Moreover, Hersch and colleagues recently demonstrated that T6SS intoxication can activate protective envelope stress response (ESR) pathways such as the ‘wall integrity gauge’ system (WigKR22) in V. cholerae or the ‘regulator of capsule synthesis’ (Rcs) system in E. coli K-1223. Notably, despite its name, the Rcs system does not trigger the production of a membrane-tethered capsule in E. coli K-12, as the bacterium lacks amongst others the gene encoding the outer membrane tethering protein Wzi24. As a result, the synthesized polysaccharide (colanic acid) is secreted into the extracellular milieu and forms a biofilm-like structure referred to as slime25. Hence, the role, if any, of bona fide membrane-attached capsules in T6SS defense has not been investigated yet.
Here, we studied the impact of V. cholerae’s T6SS on human gut commensal Proteobacteria isolates. Indeed, previous studies had suggested that the T6SS contributes to niche occupancy by intestinal pathogens such as S. enterica serovar Typhimurium, S. sonnei, and V. cholerae13–15. Interbacterial T6SS-mediated competition of these pathogens was mostly tested in vitro using well-characterized laboratory strains as prey (such as E. coli MG1655 or DH5α14) or mouse-derived bacterial isolates such as E. coli (e.g., JB213 and WZ1-1 & WZ2-115), E. cloacae KL1, K. oxytoca TS1, or K. variicola KL11 (the latter three strains were species-classified based on 16S rDNA sequencing13). In addition, these studies tested the impact of the T6SS-positive pathogens in infant or antibiotic-pretreated mice that had been pre-colonized with these strains. While highly informative, this previous work did not study human commensal isolates. We therefore wondered how V. cholerae would interact with members of the human microbiota and considered two hypotheses: (1) V. cholerae is able to compete with human gut commensals, given that it can infect human beings; and (2) human gut commensals at least partially protect against T6SS-mediated niche clearance by V. cholerae, which would be in line with the high infectious dose that was determined in healthy human volunteer studies26. We show that there is a large range in the efficiency of the contact-dependent killing of commensals, whereby certain members of the microbiota are protected from T6SS attacks in an immunity protein-independent manner. This protection occurred by a superior T6SS-mediated killing exerted by members of the Enterobacter cloacae complex and by molecular armors made of membrane-tethered capsular polysaccharides of diverse Klebsiella isolates. This study therefore contributes to a better understanding of the different mechanisms that underly T6SS-associated interbacterial competition and, accordingly, the maintenance of balanced bacterial communities.
Results and discussion
A subset of human gut commensals is protected from V. cholerae’s T6SS intoxication
To start addressing the opposing hypotheses related to V. cholerae’s ability/inability to compete with human gut commensal as stated above and to determine how different members of the human gut microbiota might react to V. cholerae’s T6SS assaults in vitro, we took advantage of bacterial samples from the Human Gastrointestinal Bacteria Culture Collection (HBC), which is composed of commensal bacteria that were isolated from the gut of healthy human volunteers27. We focused our attention on the Gram-negative Enterobacteriaceae (Supplementary Data file 1), as these bacteria are highly abundant in the small intestine28 where the primary colonization by pathogenic V. cholerae occurs29.
To test V. cholerae’s competitiveness against members of the human microbiota, we first needed to choose a strain that had an active T6SS. As members of the pandemic O1 El Tor clade of V. cholerae contain a tightly regulated T6SS that is silent under standard laboratory conditions8,30–32, we used the constitutive T6SS-active toxigenic strain ATCC 2587232,33 in this study (Supplementary Data file 1). This quorum-sensing-proficient strain is closely related to the O37 serogroup strain V52, which is routinely used in T6SS studies8,21,23,34. Notably, both of these O37 serogroup strains carry T6SS and effector/immunity modules identical to those of members of the pandemic O1 El Tor clade.
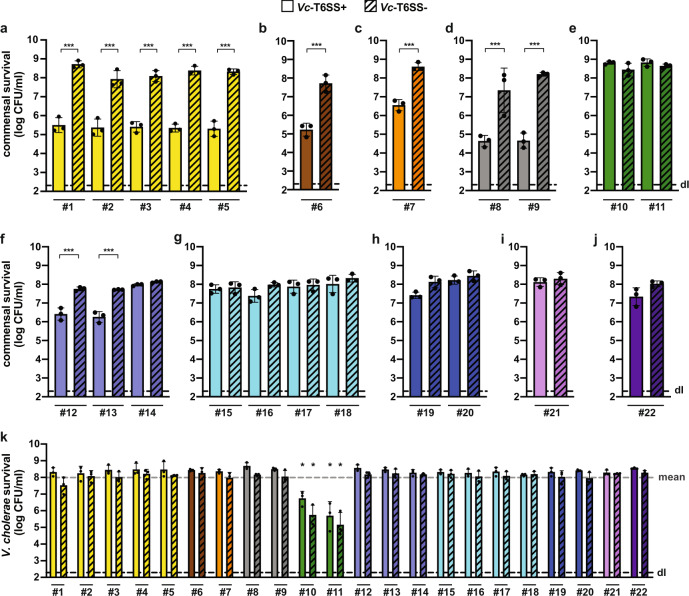
We first compared the killing ability of the T6SS-active wild-type strain (WT; ATCC 25872) to its T6SS-defective mutant, which lacks the sheath protein VipA-encoding gene, vipA. As shown in Fig. 1, the human commensal bacteria attacked by T6SS-positive V. cholerae displayed different levels of susceptibility, which correlated strongly with the phylogeny of the strains. Commensal Escherichia coli, Hafnia alvei, Citrobacter freundii, and Kluyvera cryocrescens strains were strongly depleted by T6SS-positive V. cholerae, while members of the Enterobacter cloacae complex (E. cloacae and E. ludwigii) and the Klebsiella genus (K. michiganensis, K. oxytoca, K. pneumoniae, K. variicola, and K. grimontii) were either resistant or only slightly impacted by the T6SS assaults under the tested conditions (Fig. 1). Given the large number of resistant strains and that V. cholerae cells secrete cocktails of several T6SS effectors into target cells, we concluded that these human commensals must possess immunity protein-independent T6SS-resistance mechanisms. Indeed, the likelihood is very low that all the resistant commensals produce all cognate immunity proteins.
Fig. 1. A subset of human gut commensals is resistant to V. cholerae’s T6SS attacks.
a–j Commensal Enterobacteriaceae show diverse sensitivity to T6SS assaults. Human commensals were tested for survival against toxigenic T6SS+ (WT; plain bars) or T6SS- (ΔvipA; stripped bars) V. cholerae. Isolates are grouped by taxa: a Escherichia coli; b Hafnia alvei; c Citrobacter freundii; d Kluyvera cryocrescens; e Enterobacter cloacae complex; f Klebsiella michiganensis; g Klebsiella oxytoca; h Klebsiella pneumoniae; i Klebsiella variicola; and j Klebsiella grimontii. The commensals’ survival is indicated on the Y-axis. Significant differences were determined using a two-sided Student’s t-test corrected for multiple comparisons. Only significant differences are indicated. ***p < 0.001. k V. cholerae is killed by commensal Enterobacter isolates. The survival of T6SS+ (WT; plain bars) or T6SS− (ΔvipA; stripped bars) V. cholerae when co-incubated with human commensals was scored. Color code and X-axis labels as in panels (a–j). *p < 0.05, indicating significant lower survival of V. cholerae (T6SS+ & T6SS−) when compared with the mean survival value of all the tested conditions (gray dashed line) as determined by two-sided Student’s t-tests. Values are derived from three independent experiments and the bars represent the mean (±SD, as defined by the error bars). dl, detection limit, as indicated by the dashed line. Source data underlying all panels are provided in the Source data file.
Strains of the Enterobacter cloacae complex kill V. cholerae in a T6SS-dependent manner
As previous work had shown that the colonic microbiota includes T6SS-positive microbes including those of the phylum Bacteroidetes35, we wondered if the commensals that we had tested for their intoxication by V. cholerae (Fig. 1) might also be T6SS-positive. We therefore tested V. cholerae survival upon co-incubation with these commensals and observed uniformly high recovery levels, with the exception of those that had encountered the Enterobacter strains (commensals #10 and #11) (Fig. 1k). A previous comparative genomics study revealed two T6SS gene clusters in the E. cloacae strain ATCC 1304736 and this E. cloacae-type strain was subsequently shown to constitutively produce its T6SS under in vitro conditions37. We therefore assessed whether the commensal Enterobacter strains (commensals #10 and #11; Supplementary Data files 1 and 2) also carried T6SS-encoding genes by screening their genomic sequences using the TXSScan program10. As a result, we identified a single T6SS cluster (referred to as T6SS-1) for Enterobacter strain #10, while commensal #11 possessed two T6SS gene clusters (T6SS-1 and T6SS-2) that differed in their genomic organization (Fig. S1).
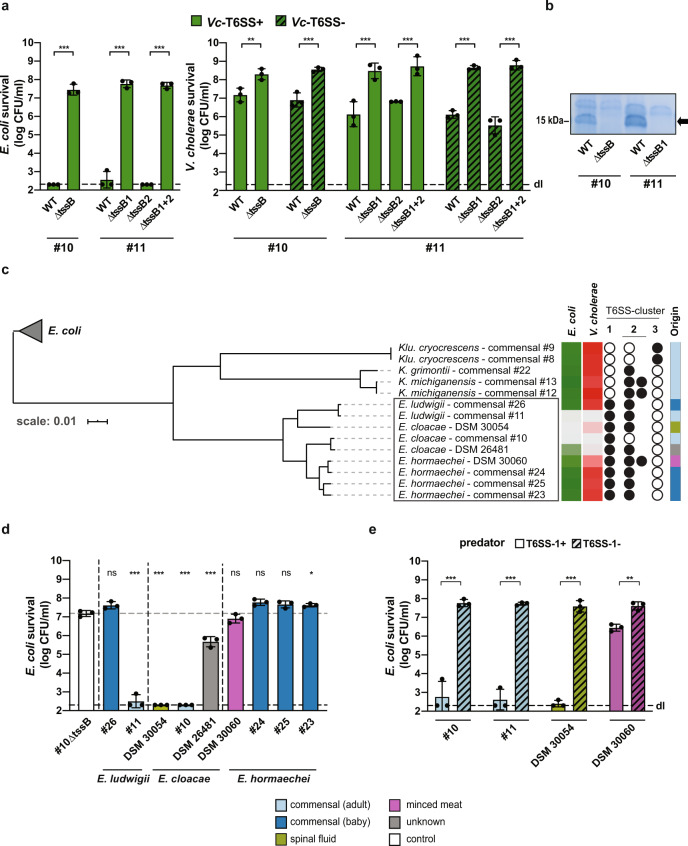
To experimentally demonstrate that the Enterobacter strains used their T6SS to kill V. cholerae, we generated T6SS-inactive mutants of both commensal strains by deleting the gene encoding the essential T6SS core component TssB (ΔtssB). TssB is one of the sheath building blocks and is homologous to VipA in V. cholerae. As shown in Fig. 2, WT Enterobacter strains #10 and #11 efficiently killed a laboratory strain of E. coli (i.e., strain TOP10), while the T6SS-1-impaired mutants (ΔtssB/ΔtssB1) displayed no predatory activity (Fig. 2a) and no Hcp secretion (Fig. 2b). The T6SS-2-impaired mutant (ΔtssB2) of Enterobacter strain #11 did not contribute to the antibacterial killing activity under the tested conditions (Fig. 2a). A similar killing pattern was observed when V. cholerae served as prey (Fig. 2a), excluding the possibility that the immunity against any putative T6SS-2 activity of commensal #11 was E. coli-specific. The lack of interbacterial killing of the T6SS-1 mutants could be complemented by providing tssB/tssB1 on a plasmid in trans (Fig. S2). Interestingly, the survival of T6SS-positive (Vc-T6SS+) and T6SS-negative (Vc-T6SS−; ΔvipA) V. cholerae was affected in a similar manner (Figs. 1 and 2a), suggesting that the Enterobacter T6SS killing activity was used in an offensive manner and not as a defensive weapon, as shown for the tit-for-tat strategy of Pseudomonas aeruginosa38. When we tested the genetically engineered strains of the commensal Enterobacter, we observed that V. cholerae impaired the survival of the T6SS-1-deficient mutant while the T6SS-2-deficient mutant of commensal #11 was still resistant to intoxication by the V. cholerae T6SS (Fig. S2d). Collectively, these data suggest that, at the population level, the commensal Enterobacter strains use their T6SS-1 to outcompete the V. cholerae population because of their superior killing abilities.
Fig. 2. A subset of Enterobacter strains kills E. coli and V. cholerae in a T6SS-1-dependent manner.
Survival of E. coli (a, d, e) or V. cholerae (a) was scored after co-incubation with wild-type (WT) or T6SS-1-/T6SS-2-negative (∆tssB or ∆tssB1 and/or ∆tssB2) Enterobacter commensals #10 and #11 (a, e) or a collection of E. cloacae complex strains (E. ludwigii, E. cloacae, and E. hormaechei) (d, e), as indicated on the Y-axis. Values are derived from three independent experiments and the bars represent the mean (±SD, as shown by the error bars). dl, detection limit, as indicated by the dashed line. Significant differences were determined using a two-sided Student’s t-test corrected for multiple comparisons (a, e) and a one-way ANOVA followed by Holm–Sidak’s multiple comparison test comparing each strain to the T6SS-deficient control commensal strain (#10∆tssB; value indicated by the dotted gray line) (d). *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant. b Absence of secreted Hcp protein in T6SS-1-negative Enterobacter mutants. The commensal Enterobacter strains #10 and #11 (WT) and their T6SS-1-deficient mutants (∆tssB/∆tssB1), were scored for secreted proteins, which were separated by SDS PAGE and stained using Coomassie blue. The arrow on the right indicates the migration position of the Hcp proteins (~17 kDa), compared to the 15 kDa ladder protein indicated on the left. Representative image (out of three independent experiments). c Core-genome-based phylogeny of E. cloacae complex strains and reclassified commensal isolates. The tree was rooted with the E. coli commensals #1, #2, and #5 as outgroup (gray triangle). The boxed Enterobacter strains were tested for interbacterial killing in panel (d). Details on the right of the tree (from left to right): First two columns: Summary heatmap of E. coli and V. cholerae survival, when challenged by the indicated strains as predators. Color scale from light (lowest survival) to dark (highest survival), according to the data provided in supplementary Fig. S5a and S5b. Middle column: Presence (closed circles) or absence (open circles) of the specific T6SS clusters. T6SS clusters were scored as present if at least 10 core T6SS genes were identified in the genome data (detailed information are provided in Supplementary Data file 5). Last column: Origin of isolates according to the legend at the bottom of the figure. Source data underlying all panels are provided in the Source data file.
Presence of T6SS cluster 1 is required but not sufficient for Enterobacter’s killing ability
Since both of the tested Enterobacter isolates (commensal #10, an E. cloacae species, and commensal #11, an E. ludwigii species) showed superior attacking behavior against V. cholerae by means of their T6SS-1 cluster, we wanted to verify whether this feature was common among other E. cloacae complex strains. This complex is composed of seven species: E. cloacae, E. asburiae, E. hormaechei, E. kobei, E. ludwigii, E. mori, and E. nimipressuralis. Of this set, E. cloacae and E. hormaechei are most frequently isolated from human clinical samples39. To investigate the broad T6SS-mediated killing abilities of this complex, we assembled a collection of E. cloacae complex strains composed of E. cloacae, E. hormaechei, and E. ludwigii isolates that we obtained from the HBC collection27, the Baby Biome Study (BBS) collection40, in which commensal bacteria were isolated from healthy full-term babies, and from the German Collection of Microorganisms and Cell Cultures (DSMZ) (Supplementary Data files 1 and 2). These latter strains were E. cloacae-type strain DSM 30054 (equal to ATCC 13047, used in previous studies36,37), E. cloacae strains DSM 16690 and DSM 26481, and E. hormaechei strains DSM 14563, and DSM 30060 (Supplementary Data files 1 and 2). The E. hormaechei strains were initially distributed by the DSMZ as E. cloacae species, but recently reclassified by the DSMZ (Supplementary Data file 2). As such reclassification of Enterobacter species after their whole-genome sequencing seemed to occur frequently, we first verified the identity of the Enterobacter commensal isolates based on the assembly of a core-genome-based phylogenetic tree (Supplementary Data file 3) for those isolates for which whole-genome sequencing data were available27,40 (Supplementary Data file 2). We also (re-)sequenced the Enterobacter commensal isolates #10 and #11 and three Enterobacter strains that we had obtained from the DSMZ (DSM 30060, DSM 30054, DSM 26481) using a PacBio-based long-read whole-genome sequencing approach (Supplementary Data file 4) and included these genomic data in the analysis.
The phylogenetic tree highlighted five commensal isolates (commensals #8, #9, #12, #13, and #22) that formed a distinctive clade within the tree (Fig. 2c). Based on their 16S rDNA sequence27, these HBC collection isolates were initially classified as Enterobacter species, while their whole-genome sequences reclassified them as Kluyvera cryocrescens (commensals #8 and #9), Klebsiella michiganensis (commensals #12 and #13), and Klebsiella grimontii (commensal #22) species (see “Methods” section). In addition, this core-genome-based phylogeny separated the different Enterobacter species into E. ludwigii, E. cloacae, and E. hormaechei (Fig. 2c). Next, we marked the absence or presence of the diverse T6SS cluster(s) (Supplementary Data files 5 to 7) next to the tree, which showed that all of the Enterobacter isolates carried a T6SS-1 while the non-Enterobacter isolates (#8, #9, #12, #13, and #22) did not (Fig. 2c). The T6SS-2 cluster was detected in all the strains with the exception of the gut commensal isolate #10 as well as the reclassified Kluyvera isolates (Fig. 2c). The latter commensals carried a third T6SS cluster (T6SS-3) instead with yet again a different gene order (Fig. S3). The categorization of the identified T6SS clusters into these three different classes (T6SS-1, T6SS-2, and T6SS-3) was further supported by the construction of a phylogenetic tree that was based on the sequences of the conserved T6SS sheaths proteins TssB and TssC encoded by each cluster (Fig. S4).
We next tested the killing capacity of our E. cloacae complex collection (including the reclassified Kluyvera and Klebsiella isolates) against E. coli or T6SS-inactivated V. cholerae (ΔvipA). These experiments revealed that all E. cloacae strains were able to kill both prey species, while the E. ludwigii and E. hormaechei isolates showed variable competition patterns (Fig. 2d and Fig. S5a, b). Next, we deleted the tssB1 gene in those predatory strains that were genetically tractable (which, in our hands, was not the case for strain DSM 26481), which abrogated their interbacterial killing capacity (Fig. 2e and Fig. S5c). Interestingly, we observed significant killing of E. coli by the WT E. hormaechei strain DSM 30060 when compared with its T6SS-1-deficient variant (Fig. 2e), while this was not the case when V. cholerae served as prey (Fig. S5c), suggesting a certain degree of target specificity.
The reclassified non-Enterobacter isolates displayed no prey killing activity, suggesting that neither the T6SS-2 nor the T6SS-3 could foster interbacterial competition under the tested conditions (Fig. S5a, b). These competition-related differences between the three T6SS systems could reflect variations in the underlying regulatory networks or inactivation of the non-T6SS-1 clusters, as, for instance, suggested by the absence of T6SS-2-mediated Hcp secretion in the tssB1 mutant of E. ludwigii commensal #11 (Fig. 2b). This idea is supported by two recent studies that were published while this work was under review. Briefly, Soria-Bustos and colleagues showed for E. cloacae strain ATCC 13047 that the T6SS-2 genes were highly expressed upon growth of the bacteria in Dulbecco’s modified Eagle’s medium (DMEM) while this was not the case in LB medium (the growth medium of our study). These authors also suggested that the T6SS-2 was implicated in biofilm formation and cell adherence and that it contributed to bacterial colonization of the mouse gut in vivo41. The finding on the system’s functionality should be taken with caution, however, as Donato and colleagues showed that the T6SS-2 of E. cloacae strain ATCC 13047 was defective due to a large deletion and the insertion of an IS903 element, which led to the pseudogenization of several T6SS-2 genes (clpV2, vgrG3, PAAR, and tssF2)42. Notably, the sequence of the T6SS-2 cluster in the here-described sequencing data of strain DSM 30054/ATCC 13047 was 100% identical to the one previously reported (accession number CP00191843), confirming these pseudogenes.
Interestingly, the T6SS-1 was also detected in most of the non-killing Enterobacter strains (Fig. 2c). While the sample number is low, it is interesting to consider the origin of these samples. Indeed, all four T6SS-1-carrying but non-killing Enterobacter isolates were from the microbiota collection of the Baby Biome Study (BBS40). It is therefore tempting to speculate that these baby-derived commensals had not yet adapted to the competitive intestinal community. Alternatively, they might depict a target specificity that is beneficial in the maturing phase of the microbiota but may not be functional for in vitro intoxication of E. coli or V. cholerae. Future studies are therefore required to test these strains’ T6SS-1 activity in vitro, their killing capacity against other prey bacteria, and to identify and characterize the strains’ effector repertoire.
The T6SS-1 of Enterobacter species is efficient against several human pathogens
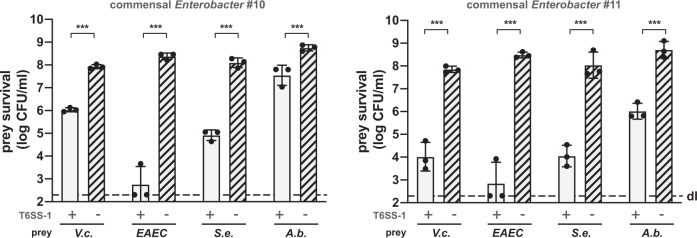
Because several of the T6SS-1-active Enterobacter strains were isolated from healthy adults whose microbiota supposedly provide colonization resistance against invading bacteria, we tested whether these commensals were also able to kill other human pathogens. We therefore incubated the Enterobacter isolates #10 and #11 with the enteric pathogens V. cholerae (strain ATCC 25872), enteroaggregative E. coli (EAEC; strain 17-2), S. enterica serovar Typhimurium (strain LT2), or A. baumannii (strain A118), which is a common colonizer of the gastrointestinal tract44. Because these strains also encode T6SSs, we used either T6SS-deficient mutants (for V.c. and A.b.) or in vitro conditions under which the T6SSs of these strains would not be produced (for EAEC and S.e.). As shown in Fig. 3, both commensal Enterobacter isolates were able to kill the tested pathogens in a T6SS-1-dependent manner. Notably and in contrast to what we observed for V. cholerae, T6SS-positive A. baumannii exerted superior killing against the commensal Enterobacter strains and remained unaffected by the commensals’ T6SS assaults (Fig. S6). Collectively, these data suggest that most Enterobacter cloacae and close relatives (e.g., commensal E. ludwigii isolate #11) have the capacity to kill selected pathogens using their T6SS-1 machinery. In addition, our work highlights a T6SS-mediated competition hierarchy under the tested conditions. Hence, some commensals and/or pathogens protect themselves from intoxication through the utilization of a superior T6SS, while loss of their T6SS activity makes them vulnerable to assaults from competitors. This finding is in line with a recent study by Perault and colleagues whereby the authors showed that P. aeruginosa strains isolated from young cystic fibrosis patients are protected from Burkholderia cepacia complex (Bcc) pathogens in a T6SS-dependent manner45. However, during adaption to the host, P. aeruginosa strains often acquire T6SS-abrogating mutations, which render them susceptible to T6SS assaults from Bcc strains45. The cause of the superiority of the T6SSs in the present study are not known. They could result from the strains’ effector repertoire12, the assembly and/or firing rate of the T6SS machinery, the precise targeting of prey38,46, the sheath length-dependent force generation, or a combination of all of these features. Apart from promoting their own survival and growth, T6SS-active commensals such as the two commensal Enterobacter strains described above, could therefore play a major role in the colonization resistance in human adults toward pathogens.
Fig. 3. E. cloacae complex isolates kill pathogenic bacteria in a T6SS-1-dependent manner.
Toxigenic V. cholerae (V.c.; T6SS-deficient), enteroaggregative E. coli (EAEC), S. enterica serovar Typhimurium (S.e.), or A. baumannii (A.b.; T6SS-deficient) were co-incubated with commensal Enterobacter isolates #10 and #11 and their survival was scored as indicated on the Y-axis. Values are derived from three independent experiments and the bars represent the mean (±SD, as defined by the error bars). Significant differences were determined using a two-sided Student’s t-test corrected for multiple comparisons. ***p < 0.001. Underlying source data are provided in the Source data file.
Resistance to T6SS assaults correlates with the presence of group I capsules
While our data unambiguously showed that, at the population level, members of the Enterobacter cloacae complex prevent their elimination by V. cholerae via T6SS-mediated superior killing, the non-killed Klebsiella species did not diminish the V. cholerae cell numbers (Fig. 1k). Indeed, most Klebsiella species are T6SS-silent under the tested conditions and require specific environmental cues to induce their T6SSs (e.g., change in temperature, oxygen tension, pH, osmolarity47). To attempt to explain the survival of Klebsiella in the presence of T6SS-positive V. cholerae, we looked to a hallmark of Klebsiella—its ability for the production of copious capsular polysaccharide (CPS)48. Indeed, CPS is known to confer protection from several external stresses such as phagocytosis, antimicrobial peptides, or components of the human complement48,49, and we hypothesized that CPS could be playing a role in protecting the tested Klebsiella species.
As most Enterobacteriaceae produce capsules belonging to several different capsule groups (such as group I, or Wzx/Wzy-dependent; group IV, or ABC-dependent50), we tested whether an association between a specific group and a T6SS-protective phenotype existed. To do so, we first scored the presence of the different capsule groups in all commensal bacteria using CapsuleFinder with the genome sequence of each strain as input50. To differentiate between group I capsules versus the secreted polysaccharide colanic acid, which share common biosynthetic pathways, we searched for the wzi gene within the identified biosynthetic gene clusters. Wzi is an outer membrane lectin that tethers the capsular polysaccharide to the cell surface51. We hypothesized that the capsule could form a physical barrier around the bacteria, and therefore the distinction between surface-tethered versus secreted polysaccharide seemed of prime importance, since common biofilm matrix polysaccharides are often also secreted. Indeed, secreted EPS is a loosely attached structure around the cell body20, while the membrane-tethered CPS forms a shield around the producing cell52. Therefore, for our analysis, wzi-carrying strains were considered bona fide group I capsule producers, while commensals that contained the biosynthetic gene cluster but lacked wzi were classified as colanic acid producers.
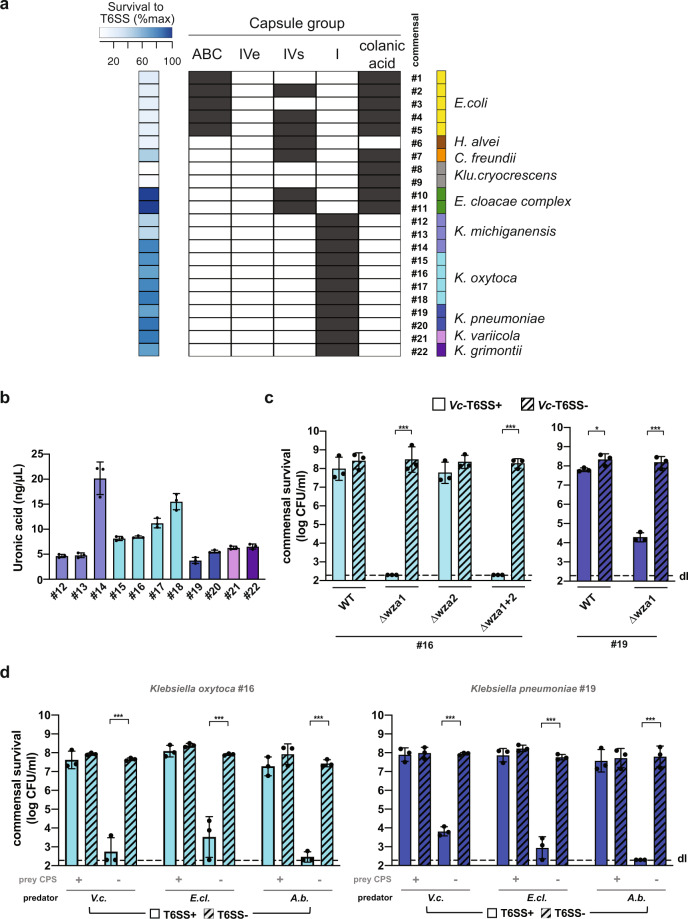
As shown in Fig. 4a, the CapsuleFinder program showed that all commensal strains carried genes encoding for at least one capsule group, while a few isolates, such as the E. coli strains (commensals #1–5), C. freundii (commensal #7), and the members of the Enterobacter cloacae complex (commensals #10–11), carried several capsular–group-encoding biosynthetic clusters. However, none of these strains harbored the wzi gene, which strongly suggests that they do not produce a membrane-anchored group I capsule. Klu. cryocrescens isolates (commensals #8–9) possessed solely colanic acid production genes, while H. alvei (commensal #6) was the only isolate in the tested collection that produced neither colanic acid nor a bona fide group I capsule. Interestingly, all the Klebsiella isolates (commensals #12–22) were in silico predicted to synthesize membrane-anchored group I capsules. Finally, none of the commensals carried enzymes to form a group IVe capsule type, which is commonly detected in pathogenic Enterobacteriaceae50.
Fig. 4. Group I capsule protect commensal Klebsiella isolates against V. cholerae’s T6SS attacks.
a In silico identification of capsular genes in the commensal collection. Enterobacteriaceae-specific capsule groups (ABC, IVe, IVs, I, colanic acid synthesis) are shown. The blue heatmap (on the left) shows the relative survival values of the T6SS-attacked commensals with 0 and 100 being defined as the lowest and highest log-transformed CFU/ml numbers, respectively (according to the data provided in Fig. 1a–j). Commensal strain numbers and the color code (on the right) are as defined in Fig. 1. b The production of group I capsules by the Klebsiella gut commensal isolates was assessed by quantification of the strains’ uronic acid content. c, d Deletion of capsule biosynthesis genes renders commensal Klebsiella sensitive to T6SS-mediated intoxication. Representative WT and wza-negative Klebsiella mutants (commensal K. oxytoca #16 and K. pneumoniae #19) were tested for survival in the presence of T6SS+ (WT; plain bars) or T6SS− (ΔvipA; stripped bars) V. cholerae (panel c) or against diverse T6SS-positive (plain bar) and T6SS-negative (stripped bar) pathogens (panel d; V. cholerae [V.c.], E. cloacae [E.cl.; commensal #10], or A. baumannii [A.b.; strain A118]). Values are derived from three independent experiments and the bars represent the mean (±SD, as defined by the error bars). dl, detection limit, as indicated by the dashed line. For panels c and d, significant differences between samples containing T6SS+ and T6SS− predators were determined using a two-sided Student’s t-test corrected for multiple comparisons. Only significant differences are indicated. *p < 0.05; ***p < 0.001. Source data underlying all panels are provided in the Source data file.
To determine whether a specific capsule group was associated with protection from T6SS attacks (Fig. 4a), we searched for associations between the presence of each capsule group and the commensals’ T6SS survival phenotype. We excluded E. cloacae, since we showed above the causes of its survival. Our analysis showed that survival was positively associated only with the group I capsule (P < 0.0002; Wilcoxon test; Supplementary Data file 8), and negatively associated with the colanic acid and ABC capsule. The bacteria with type I capsules do not encode other types of capsules, whereas the other types of capsules are often co-occurring in the same genomes. These results can thus be interpreted as either a positive association between type I capsules and survival that implies accessorily the anti-correlation for the other types, or vice versa. To disentangle this web of associations and pinpoint the most important correlation, we used a stepwise regression analysis to assess if one type of the capsule was sufficient to explain the association of the other types to the T6SS survival phenotype. Indeed, the stepwise regression of the effect on survival of the different capsule groups showed that the only significant variable was the presence or absence of the group I capsule. The integration of this variable in the regression was sufficient to explain most of the variance in the data (R2 = 0.862, P < 0.0001). This supports our hypothesis that this membrane-tethered polysaccharide serves as a protective shield against T6SS-mediated attacks.
Common Klebsiella strains are protected against V. cholerae’s T6SS assaults in a capsule-dependent manner
To test the hypothesis that the group I capsule specifically protects Klebsiella species from T6SS intoxication, we took advantage of several well-characterized Klebsiella isolates of clinical and environmental origin such as strain 342 (K. variicola) as well as strains BJ1-GA, SB617, NJST258-1, and NTUH K2044 (all K. pneumoniae; Supplementary Data files 1 and 9). First, we explored genetically engineered strains that were deleted for wza using a standard allelic exchange approach53,54. Wza is an integral outer membrane lipoprotein and essential for the export of group I capsular polysaccharide55. Consequently, wza- mutants are non-encapsulated56. We first confirmed the impaired capsule production by measuring their uronic acid content (Fig. S7a) followed by the imaging of these strains after staining with Indian ink (Fig. S7b). The former method is frequently used as a quantitative measurement for group I capsule production57. These experiments confirmed the loss of the group I capsule, so we next assessed the survival of these non-encapsulated mutants in the presence of T6SS attacks exerted by V. cholerae. As shown in Figure S7c, the encapsulated strains (CPS+) displayed a significantly higher survival rate compared to the CPS mutants (CPS−), while the survival of V. cholerae was indistinguishable after its co-incubation with either the encapsulated or the non-encapsulated Klebsiella strains (Fig. S7b). The use of a multivariate linear model accounting for the presence or absence of the capsule and the identity of the strains showed no effect of the capsule on Klebsiella survival in the absence of T6SS attacks (P = 0.56; t-test) and a strong positive effect in the presence of T6SS attacks (P < 0.0002). These data indicate a protective role of the Klebsiella group I capsule against T6SS-mediated killing by V. cholerae. An exception to the powerful capsule-mediated protection effect was observed for K. pneumoniae strain NJST258-1, where the survival of the CPS+ strain was also impaired upon T6SS attack. Nonetheless, the CPS− mutant was significantly more sensitive to T6SS assaults than the CPS+ strain (Fig. S7c), illustrating the protective role of the capsule even for this strain. One could speculate that the thickness or the compaction of the capsular material of this strain is lower, thereby providing less protection.
Encapsulated gut commensal Klebsiella are shielded from V. cholerae’s T6SS attacks
As we observed that a capsule-dependent T6SS protection occurred for these well-studied Klebsiella isolates, we aimed at testing whether this finding also applied to the Klebsiella commensals from the human gut. Hence, we scored their capsule production through uronic acid quantification (Fig. 4b), which supported the in silico predictions. To show causality between capsule production, uronic acid content, and, ultimately, T6SS shielding, we genetically engineered representative strain(s) from each of the commensal Klebsiella species by deleting the wza gene(s) from their genomes. While members belonging to the K. pneumoniae complex (e.g., K. pneumoniae and K. variicola) carried a single wza gene, the commensal K. oxytoca and K. michiganensis isolates each contained two wza copies, with the second copy potentially associated with the production of external structures unrelated to the group I capsule58. To determine if the protective activity was uniquely associated with the group I capsule-specific wza gene product, we generated single and double deletion strains of the respective wza copies. The uronic acid content decreased (Fig. S8a) in those wza mutants that lacked the wza gene located within the capsular gene cluster (Fig. S8b) and microscopy of the bacteria stained using Indian ink revealed that these mutants lost their cell-surrounding CPS material (Fig. S8c). Importantly, these wza mutants (CPS−) showed a strong sensitivity to T6SS attacks by V. cholerae, while their CPS+ parental strains (WT) mostly resisted against the T6SS assaults (Fig. 4c and Fig. S9a), as did representative wza-complemented strains (Fig. S9b) in which CPS was restored (Fig. S9c). Collectively, these data suggest that group I capsules confer a strong protection from V. cholerae’s T6SS attacks to commensal Klebsiella strains.
A horizontally acquired Klebsiella capsular polysaccharide gene cluster conveys T6SS protection to E. coli
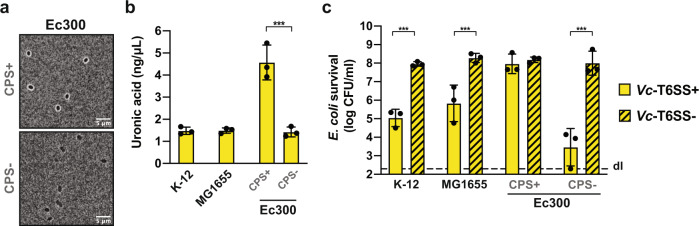
Since impairment of group I capsule production sensitized Klebsiella strains to intoxication by V. cholerae’s T6SS, we wondered if naturally non-encapsulated bacteria could be protected from T6SS attacks if the capsular material was genetically transferred to these bacteria. Fortunately, such a strain already exists in nature in the dog commensal E. coli isolate, strain Ec30059. It contains an extended galF-his region of about 40 kb (compared to ~16 kb in common K-12 E. coli strains), which carries a group I capsule biosynthetic gene cluster49 including wzi. It was hypothesized that this capsule-determinant gene cluster was horizontally acquired from Klebsiella strain 342 due to the very high sequence similarity between the loci49. We investigated this hybrid E. coli strain as described above for Klebsiella strain 342 (Fig. S7) by visualizing its capsule by microscopy using Indian ink staining (Fig. 5a) and quantifying the strain’s uronic acid content (Fig. 5b). The latter was significantly higher than the level of common laboratory E. coli strains (strain K-12 and its F-/λ-minus and rph-1 variant MG1655) or a CPS-negative mutant of Ec300 (ΔrfaH49) (Fig. 5b). Consistent with the encapsulation of Ec300, the WT (CPS+) was resistant to T6SS attacks, while the survival of the non-encapsulated mutant (CPS−) as well as the K-12 and MG1655 strains was significantly reduced by V. cholerae (Fig. 5c). Together, these experiments show that the group I capsule alone, independently of other genetic traits specific to the genus Klebsiella, can provide an efficient protection against T6SS attacks, even when expressed in a heterologous host. Interestingly, a recent study reported that 7% of E. coli isolates (with n = 1194) derived from Australian freshwater reservoirs had acquired diverse Klebsiella group I capsular gene clusters60. Based on the data we present in this study it is possible that those strains might have a fitness advantage when facing T6SS-positive competitors in aquatic environments.
Fig. 5. Group I capsule-mediated T6SS shielding is a broad protection mechanism that can be horizontally-transferred.
a–c Horizontally acquired capsule biogenesis gene cluster confers protections to E. coli against T6SS attacks. a, b Capsule visualization and uronic acid quantification of the dog commensal E. coli strain Ec300. a CPS+ (Ec300; WT) and CPS− (Ec300ΔrfaH) E. coli bacteria were imaged after India Ink staining. Representative images are shown. Scale bar, 5 μm. b The production of uronic acid was quantified in Ec300, its CPS-minus mutant (ΔrfaH), and two laboratory reference E. coli strains as controls. c Encapsulated E. coli is protected from T6SS assaults by V. cholerae. WT (CPS+) and the capsule-minus (CPS−) Ec300 bacteria as well as two reference E. coli strains were cocultured with T6SS+ (WT; plain bars) or T6SS− (ΔvipA; stripped bars) V. cholerae. Their survival is indicated on the Y-axis. b, c Values are derived from three independent experiments and the bars represent the mean (±SD, as defined by the error bars). dl, detection limit, as indicated by the dashed line. Significant differences were determined using a two-sided Student’s t-test corrected for multiple comparisons. Only significant differences are indicated. ***p < 0.001. Source data underlying all panels are provided in the Source data file.
Importantly, these experiments also highlight the difference between group I capsule-mediated protection against T6SS attacks compared to the Rcs pathway-regulated colonic acid production that has been previously described23. Indeed, our experiments showed that common laboratory strains of E. coli (e.g., K-12 or MG1655), despite their functional Rcs pathway, are insufficiently protected against T6SS attacks when V. cholerae secretes toxic effector cocktails instead of a single toxin (e.g., TseH in reference23). The T6SS-protective role of colanic acid described by Hersch and colleagues23 might therefore rely on a different mechanism than the physical shielding expected from a bona fide capsule. Recently, colanic acid was found to contribute to the maintenance of membrane potential during envelope stress, which may be a potential explanation for this protective phenotype61. In fact, Pando and colleagues showed that colanic acid-deficient S. enterica mutants were more susceptible to ß-lactam antibiotics, such as ampicillin61, which is consistent with the increased sensitivity of colanic acid-negative E. coli when confronted with the peptidoglycan-degrading T6SS toxin TseH of V. cholerae23.
Encapsulated Klebsiella are shielded from a variety of T6SS-producing bacteria
It is clear from our data that the group I capsules are protective. However, we wondered whether this molecular armor was specific to T6SS attacks of V. cholerae or whether this protection mechanism could be extended to other T6SS-active Gram-negative bacteria. To test this, we compared three T6SS-producing predators, namely toxigenic V. cholerae (strain ATCC 25872), E. cloacae (commensal #10; see above), and A. baumannii (strain A118; Supplementary Data file 1) for their competitiveness against either CPS+ or CPS− K. pneumoniae. As shown in Fig. 4d for two representative commensal Klebsiella strains and in Fig. S10 for two additional examples as well as for K. pneumoniae strains NTUH-K2044 or BJ1-GA as prey, the encapsulated Klebsiella strains (CPS+) survived at comparable levels in the presence of the T6SS-positive and T6SS-deficient competitors, while the non-encapsulated (CPS−) derivatives showed a highly significant survival defect when they encountered T6SS-positive attackers. This finding suggests that the barrier exerted by the group I capsular polysaccharide provides a general protective mechanism against T6SS assaults. Of note, while we favor and therefore propose a capsule-mediated physical barrier as protection mechanism, we cannot formally exclude a protective effect of the negative charge exerted by the acidic capsular polysaccharides, which is another commonality among all tested Klebsiella isolates and, in general, among group I capsular material58.
Collectively, in this study, we undertook the first study of V. cholerae’s T6SS-dependent interaction with selected human commensal gut Proteobacteria from healthy volunteers. Through our work, we were able to characterize two resistance mechanisms that human commensal gut microbes might use to defend themselves against enteric pathogens. First, we identified a conserved resistance mechanism, whereby commensal Enterobacter derived from adult guts carried their own specific and highly potent T6SS (T6SS-1) that prevented their elimination by V. cholerae’s T6SS assaults. Second, we identified a so-far undescribed role of the group I capsular polysaccharide of Klebsiella species that includes shielding the bacteria from T6SS intoxication. These immunity protein-independent T6SS-resistance mechanisms protect against a broad range of predators and therefore constitute a well-suited defense system. Taken together, our work provides insight into potential bacterial community behavior within the intestinal microbiota and sheds light on how T6SS defense systems might foster colonization resistance against invading pathogens. However, given the in vitro nature of our work, future studies are still needed to address such interactions in vivo, for instance in a humanized mouse model.
Importantly, the identified T6SS protection mechanisms may well extend beyond the gut to other colonization sites in which T6SS-mediated competition plays an important role, such as, for instance, the respiratory tract of cystic fibrosis patients45 or environmental reservoirs, as suggested above for the encapsulated E. coli isolates. A caveat of our study is, however, that the tested commensal isolates were derived from healthy humans, consistent with the state-of-the-art of past volunteer studies26 and the commonly used animal models2. Hence, these isolates might not well reflect the unique microbiome of predisposed people in cholera endemic areas where people are often malnourished and suffering from stunted childhood growth, from co-infections, or from environmental enteric dysfunction62,63. Unfortunately, microbiota data on such predisposed populations are still scarce and often lack healthy control groups due to ethical concerns64. Nonetheless, our work could serve as a starting point to rationally design T6SS-shielded probiotic strains that are able to restore defective colonization barriers or enhance the barriers’ efficiency.
Methods
Bacterial strains, plasmids, and growth conditions
The bacterial strains and plasmids used in this study are listed in Supplementary Data file 1. Unless otherwise stated, the strains were grown aerobically in Lysogeny broth medium (LB; 10 g/l of tryptone, 5 g/l of yeast extract, 10 g/l of sodium chloride; Carl Roth) or on LB agar plates at 37 °C. The following supplements were added at the given concentrations if required: diaminopimelic acid (DAP; 0.3 mM), kanamycin (75 μg/ml), ampicillin (100 μg/ml), carbenicillin (100 μg/ml), streptomycin (100 μg/ml), and chloramphenicol (25 μg/ml). DAP was added as an essential growth supplement for E. coli strain MFDpir65. S17‐1λpir66 and MFDpir65 were used for cloning purposes and/or served as donor in bacterial mating experiments. DAP-deficient medium was used to counter‐select strain MFDpir after bacterial mating.
Genetic engineering of strains and plasmids
DNA manipulations were performed according to standard molecular biology‐based protocols67. Primers used in this study are listed in Supplementary Data file 10. Enzymes were purchased from the indicated companies and were used as recommended by the manufacturer: Pwo polymerase (Roche), Q5 High fidelity polymerase (New England Biolabs), Expand High Fidelity polymerase (Roche), GoTaq polymerase (Promega), restriction enzymes (New England Biolabs), and T4 DNA ligase (New England Biolabs). Following initial screening by PCR (using bacterial cells as templates), genetically engineered strains and plasmids were verified by Sanger sequencing for their modified regions.
For Enterobacteriaceae, mutants were constructed by in-frame deletion of the target genes using standard allelic exchange approaches. Briefly, upstream and downstream sequences of the respective gene (>500 bp) were PCR amplified using oligonucleotides with 5′-encoded restriction sites and cloned into likewise digested suicide plasmids (pGP704‐Sac2868 or pGP704‐Sac-Kan69). Ligation was performed overnight at 16 °C with T4 DNA ligase (New England Biolabs). Competent E. coli S17‐1λpir cells were transformed with the ligated products and transformants were screened for correct insertions using PCR. Positively scored plasmids were verified by Sanger sequencing and then transferred into E. coli strain MFDpir65. E. coli MFDpir served as the donor for conjugation with the respective receptor strains. Bacterial mating was performed for at least 8 h at 37 °C. Single crossover transconjugants were selected on antibiotic-containing agar plates. Next, transconjugants were grown for 16 h at 37 °C and strains with excised plasmids were selected at room temperature on NaCl-free LB agar plates supplemented with 10% sucrose. To confirm the loss of the plasmid, colonies were tested for their antibiotic sensitivity. Deletion mutants were verified by PCR and Sanger sequencing.
Interbacterial killing assay
The interbacterial killing assay was performed following a previously established protocol with minor modifications30. Briefly, the defined prey and predator cells were harvested after overnight growth, washed, and concentrated to an optical density at 600 nm (OD600) of 10 in PBS. Predator and prey were mixed at a ratio of 1:1 and spotted onto membrane filters on pre‐warmed LB agar plates. After 4 h of incubation at 37 °C, bacteria were resuspended and serial dilutions were spotted onto selective media that were matched with the characteristics of the bacterial strains (e.g., different antibiotic resistance profiles or growth/no growth on selective media). For each killing assay, the recovery rate of both strains was scored. The Enterobacteriaceae mentioned in Supplementary Data files 1 and 2 were selected on the following selective plates if required: MacConkey agar plates (GMH081; Lucerna Chem AG, Switzerland), Simmons Citrate Agar plates (85463; Sigma-Aldrich) supplemented with 1% inositol (I5125; Sigma-Aldrich), and chloramphenicol-supplemented LB agar plates. Acinetobacter baumannii was selected on CHROMagar Acinetobacter medium (ACE092(B), CHROMagar, France). The different V. cholerae strains were counter-selected and enumerated after spotting on streptomycin-supplemented LB agar plates or Thiosulfate Citrate Bile Salts Sucrose (TCBS) agar plates (Sigma). Recovered colonies were counted to determine the colony-forming units (CFU) per ml. Each experiment was performed three independent times and mean values are shown in the bar graphs. Statistically significant differences were determined on log‐transformed data70. If no prey bacteria were recovered, the value was set to the detection limit to allow the calculation of the mean and the statistical analyses.
Hcp secretion assay
Bacteria were grown overnight under aerobic conditions in 25 ml LB medium at 37 °C. After 10 ml of the culture was harvested by centrifugation, 4.5 ml of the supernatants were sterile-filtered (0.2 μm filter; VWR) and the proteins precipitated using trichloroacetic acid (TCA). The precipitated proteins were washed with acetone before being resuspended in 50 μl of 2X Laemmli buffer. The samples were heated at 95 °C for 15 min before analysis. To visualize the secreted Hcp, the proteins were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis using precast Mini-PROTEAN TGX Stain-Free gels 8–16% (Bio-Rad). The gels were subsequently stained using InstantBlueTM Coomassie Protein Stain (Expedeon) according to the instructions provided by the manufacturer.
For complementation experiments, bacteria were grown aerobically at 37 °C in LB medium supplemented with kanamycin 75 μg/ml until they reached an optical density at 600 nm of ~0.5. At that point, the cultures were induced by the addition of 0.2% arabinose for 3 h before they were processed as described above.
Capsule extraction and uronic acid quantification
The bacterial capsular material was extracted as previously described71 and the uronic acid content quantified using the method reported by Blumenkrantz and colleagues57. Briefly, an overnight culture was diluted/concentrated to an OD600 value of 4. Five hundred microliters of this suspension was mixed with 100 µl of 1% Zwittergent 3–14 detergent (693017–5GM; Sigma; dissolved in 100 mM citric acid, pH 2.0) and heated at 56 °C for 20 min. After incubation, the mixture was centrifuged for 5 min at 20,817 × g and 300 μl of the supernatant was transferred to a new tube. Absolute ethanol was added to a final concentration of 80% and the samples were placed on ice for 20 min. After centrifugation, the pellet was washed with 70% ethanol, dried at 96 °C for 5 min before 250 µl of distilled water was added. The pellet was dissolved during 2 h at 56 °C. Polysaccharides in the isolated capsule material were subsequently quantified by measuring the amount of uronic acid. To do so, 1.2 ml of 0.0125 M tetraborate dissolved in concentrated H2SO4 was added to 200 μl of the respective sample. The mixture was vigorously vortexed, heated at 96 °C for 5 min, and allowed to cool down again before 20 μl of 0.15% 3-hydroxydiphenol (dissolved in 0.5% NaOH) was added. The tubes were vortexed before 1 ml of the sample was transferred to a cuvette for absorbance measurements at 520 nm. The uronic acid concentration of each sample was determined using a standard curve based on known concentrations of glucuronic acid.
Capsule visualization by Indian Ink staining
To visualize the capsule material, 5 μl samples from overnight grown bacterial cultures were mixed with 2 μl of India Ink reagent (BD #261194) on a microscope slide and covered with cover-slip. The bacteria were subsequently imaged in brightfield mode using a Plan-Apochromat 100x/1.4 Ph3 oil objective as part of a Zeiss Axio Imager M2 epifluorescence microscope with an attached AxioCam MRm camera, which was controlled by the ZEN BLUE 2.6 software from Zeiss. Images were analyzed and prepared for publication using ImageJ v2.0.0-rc-69/1.52p.
Genomic DNA preparation
Genomic DNA was isolated from 10 ml of LB-grown bacterial overnight cultures using a Qiagen genomic DNA buffer set combined with Qiagen 500/G Genomic-tips. The extraction was performed according to the manufacturer’s protocol.
Long-read whole-genome sequencing
High molecular weight DNA was sheared in a Covaris g-TUBE (Covaris, Woburn, MA, USA) to obtain an average fragment size of 10 kb. After shearing the DNA, size distribution was checked on a Fragment Analyzer (Advanced Analytical Technologies, Ames, IA, USA). Five hundred nanograms of the DNA was used to prepare a SMRTbell library with the PacBio SMRTbell Express Template Prep Kit 2.0 (Pacific Biosciences, Menlo Park, CA, USA) according to the manufacturer’s recommendations. No size selection was applied. The pooled bar-coded libraries were sequenced with v3.0/v3.0 chemistry and diffusion loading on a PacBio Sequel instrument (Pacific Biosciences, Menlo Park, CA, USA) at 600 min movie length, pre-extension time of 120 min, using one SMRT cell 1 M v3.
Genome assembly was performed using CANU 2.0 with the option ‘pacbio-raw’ and a defined expected genome size of 5 Mbp72. The circularization of the genomes was achieved using Circlator v.1.5.5 with default parameter settings73. Genes were predicted using Prokka v.1.14.674 but, during the submission of the sequenced genomes, reassigned through the NCBI Prokaryotic Genome Annotation Pipeline (PGAP, version 4.11). Sequencing details and NCBI accession numbers are summarized in Supplementary Data file 4.
Determination of the core genomes of selected strains
The core-genome reconstruction was done for the initially classified Enterobacter strains following a previously published approach75 using the software OPSCAN v.0.1 (https://bioinfo.mnhn.fr/abi/public/opscan/). Briefly, orthologs were identified as bidirectional best hits using an end-gap-free global alignment between a reference proteome from the group of interest and each of the other proteomes. Hits with <80% amino acid sequence similarity or more than 20% difference in protein length were discarded. As most tested genomes were solely draft assemblies, synteny was not used as a comparison criterium. The core-genome was defined as the shared group of orthologs genes that were identified in each of the comparison against one of the strains (commensal strain #9). The core-genome was judged to consist of 2018 protein-encoding gene sequences. The list of gene families included in the core-genome is in Supplementary Data file 3.
Phylogenetic reconstruction
The proteins encoded by the core-genome were individually aligned using the multi-sequence alignment program MAFFT version 7.45376 with the -linsi parameter. Non-informative regions of the alignment were trimmed using trimAl v1.4.rev1577 with the automated1 algorithm. The resulting alignments were then concatenated for each genome. The phylogenetic tree was inferred using IQ-TREE v.2.0.478 and the best model, LG + F + I + G4, was determined using the option TEST. The robustness of the topology was tested with 1000 rapid bootstrap experiments. The phylogenetic tree was visualized with iTol v5.5.179. Three E. coli genomes (ERR2221227, ERR2221250, ERR222139827) were used as outgroups to root the phylogenetic tree. Based on the phylogenetic tree, five commensals (#8, #9, #12, #13, #22) clustered outside the other Enterobacter strains. Consistent with this observation, we discovered a recent reclassification of these strains within the European Nucleotide Archive (ENA) repository, which were now no longer considered as Enterobacter strains (Supplementary Data file 2).
Species reclassification of the commensal isolates
The taxonomy of the commensal isolates was initially based on their 16S rDNA sequence27,40. Here, a whole-genome-based taxonomy approach was used. This taxonomy was either derived from the reclassification of several of the commensal isolates that was recently done by the ENA repository (accession numbers PRJEB23845 and PRJEB22252) or a refined classification using the program Kleborate v. 0.4.0b80, which was performed as part of this study for the Klebsiella strains (including the ENA-reclassified commensals #12, #13, and #22; Supplementary Data file 2). The input for Kleborate were fasta files of the draft/assembled genomes.
In silico detection of type VI secretion system gene cluster
Draft genomes of the gut commensal isolates27,40 were inspected for the presence of putative T6SS operons using the module TXSScan10 from MacSyFinder v.250 and then manually curated. TXSScan was used in ordered replicon mode with the default settings for HMMER options. The identified clusters were represented using the R package genoPlotR v. 0.8.9. The T6SS cluster types defined in this study (T6SS-1, T6SS-2, T6SS-3) are based on the gene organization within the clusters. This classification was further validated through construction of a phylogenetic tree based on the conserved T6SS sheath proteins TssB and TssC encoded in each T6SS cluster. The protein sequences corresponding to TssB and TssC were aligned separately using MAFFT76 (version 7.453; with -auto parameter) and the alignments were concatenated to infer the TssB-TssC phylogenetic tree with IQ-TREE v.2.0.478 using the best model LG + G4, determined using the option TEST. The robustness of the topology was tested with 1000 rapid bootstrap experiments. The phylogenetic tree was visualized with iTol v5.5.179.
In silico detection of capsules biosynthesis genes
Draft genomes (Illumina-based sequence data) of the gut commensal isolates27 were inspected for the presence of putative capsule biogenesis operons using CapsuleFinder v.150. This program identifies those capsule types that exist in Enterobacteria (e.g., ABC-dependent, group I or Wzx/Wzy-dependent [Wzy_stricte], group IV subgroups corresponding to the model organism E. coli [Group IV_e_stricte], or to the model organism Salmonella enterica [Group IV_s_stricte]). CapsuleFinder was used in diderm bacteria mode with the default settings for HMMER options. The absence of additional capsule operon(s) scattered across several contigs was verified manually by altering the contigs’ order, which did not result in the detection of additional capsule systems. For two of the Enterobacter strains (commensals #10 and #11), the analysis was repeated using their PacBio-sequenced genome assemblies as input; no additional capsule operon(s) were detected, supporting the notion that additional capsule operons were not missed in the incomplete Illumina-based assemblies. The results were further refined by separating the group I capsule types into two subcategories, namely bona fide wzi-dependent group I capsules versus wzi-lacking colanic acid producers (according to Whitfield58). The presence of the different capsule types in each isolate was represented using the R packages ggplot2 v.3.0.0, RColorBrewer v.1.1-2, and the function heatmap.2 from the package gplots v.3.0.1.1.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8.4.2 and 9.0.2 (GraphPad Software, Inc., CA, USA) using log-transformed data. Statistical significance was determined using unpaired two-tailed Student’s t-test, as indicated in the figure legends. In case of multiple comparisons, the statistical significance was corrected using the Holm–Sidak method. The significance level (α) was set to 0.05 in all cases. P values of all statistically significant results are listed in Supplementary Data file 11. In the graphs, the biologically independent replicates are indicated by circles, while the bars depict the mean of all experiments (±standard deviation, SD).
The association between the capsule types and protection from T6SS attacks was performed using a forward stepwise regression using JMP® 13.2.0 (SAS Institute Inc.), where the criterion of arrest was given by the BIC (similar results for AIC). The significance of the regression was evaluated using an F-test. The effect of each capsule type was tested through non-parametric Wilcoxon test (one-way test using the χ2 approximation).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
The authors thank Trevor Lawley and his team for provision of the strains from the Human Gastrointestinal Bacteria Culture Collection (HBC) and the Baby Biome Study (BBS) collection, Jean-Marc Ghigo for sharing E. coli strain Ec300 and its rfaH mutant, Eric Cascales for S. enterica and the EAEC strains, and Bärbel Stecher and Simone Herp for initial discussions on T6SS and microbiota. The authors acknowledge the staff of the Lausanne Genomic Technologies Facility at the University of Lausanne for sample processing and sequencing. This work was supported by a Consolidator Grant from the European Research Council (ERC; 724630-CholeraIndex), the Swiss National Science Foundation (NRP 72 grant 407240_167061), and a grant from the Novartis Foundation for medical-biological research (#18C178) to M.B. M.B. is a Howard Hughes Medical Institute (HHMI) International Research Scholar (#55008726). O.R. received funding from an Agence nationale de la recherche (ANR) JCJC grant [ANR 18 CE12 0001 01 ENCAPSULATION] and the work by E.R., O.R., and A.B. was supported by grants from the Laboratoire d’Excellence IBEID [ANR-10-LABX-62-IBEID] and the Fondation pour la Recherche Médicale [Equipe FRM: EQU201903007835].
Source data
Author contributions
M.B. secured funding, conceived the overall project, and oversaw its implementation; N.F., S.I., and M.B. designed the details of the study; N.F., S.I., L.F.L.R., S.S., C.S., N.V. and M.B. performed the wetlab experiments mostly overseen by N.F.; T.S. performed preliminary experiments; M.G.G. assembled the PacBio-based sequenced genomes; S.I., M.G.G. and E.P.C.R. performed the bioinformatic analyses; O.R., A.B. and E.P.C.R. contributed the CPS+ and CPS− Klebsiella clinical and environmental isolate strains; O.R. and E.P.C.R. provided advice on Klebsiella biology; S.I. and E.P.C.R. performed the statistical analyses; N.F., S.I. and M.B. analyzed the data; N.F., S.I. and M.B. wrote the manuscript with input from O.R. and E.P.C.R.; M.B. revised the manuscript; all authors approved the final version of the manuscript.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information and Supplementary Data files). Accession number for whole-genome sequencing data are provided in the text and in Supplementary Data files 2 and 4. The PacBio raw read data of the five whole-genome sequenced Enterobacter strains generated in this study have been deposited in the NCBI’s Sequence Read Archive (SRA) database under the Bioproject accession number PRJNA640151. Details on the SRA accession numbers, BioSample accession numbers, and individual genome accession numbers of the de-novo-assembled and circularized genomes are provided in Supplementary Data file 4. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review informationNature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nicolas Flaugnatti, Sandrine Isaac.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-26041-0.
References
- 1.Metchnikoff E. Recherches sur le choléra et les vibrions. Premier mémoire. Sur la propriété préventive du sang humain vis-à vis du vibrion de Koch. Ann. Inst. Pasteur. 1893;7:403–422. [Google Scholar]
- 2.Ritchie JM, Waldor MK. Vibrio cholerae interactions with the gastrointestinal tract: lessons from animal studies. Curr. Top. Microbiol. Immunol. 2009;337:37–59. doi: 10.1007/978-3-642-01846-6_2. [DOI] [PubMed] [Google Scholar]
- 3.Nelson EJ, Harris JB, Morris JGJ, Calderwood SB, Camilli A. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat. Rev. Microbiol. 2009;7:693–702. doi: 10.1038/nrmicro2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritchie JM, Rui H, Bronson RT, Waldor MK. Back to the future: studying cholera pathogenesis using infant rabbits. mBio. 2010;1:e00047–10. doi: 10.1128/mBio.00047-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Waaij D, Berghuis-de Vries JM, Lekkerkerk L-V. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J. Hyg. 1971;69:405–411. doi: 10.1017/S0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bäumler AJ, Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature. 2016;535:85–93. doi: 10.1038/nature18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chassaing B, Cascales E. Antibacterial weapons: targeted destruction in the microbiota. Trends Microbiol. 2018;26:329–338. doi: 10.1016/j.tim.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Pukatzki S, et al. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. USA. 2006;103:1528–1533. doi: 10.1073/pnas.0510322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bingle LE, Bailey CM, Pallen MJ. Type VI secretion: a beginner’s guide. Curr. Opin. Microbiol. 2008;11:3–8. doi: 10.1016/j.mib.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Abby SS, et al. Identification of protein secretion systems in bacterial genomes. Sci. Rep. 2016;6:23080. doi: 10.1038/srep23080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Brodmann M, Basler M. Assembly and subcellular localization of bacterial type VI secretion systems. Annu Rev. Microbiol. 2019;73:621–638. doi: 10.1146/annurev-micro-020518-115420. [DOI] [PubMed] [Google Scholar]
- 12.Russell AB, Peterson SB, Mougous JD. Type VI secretion system effectors: poisons with a purpose. Nat. Rev. Microbiol. 2014;12:137–148. doi: 10.1038/nrmicro3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sana TG, et al. Salmonella Typhimurium utilizes a T6SS-mediated antibacterial weapon to establish in the host gut. Proc. Natl. Acad. Sci. USA. 2016;113:E5044–E5051. doi: 10.1073/pnas.1608858113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson MC, Vonaesch P, Saffarian A, Marteyn BS, Sansonetti PJ. Shigella sonnei encodes a functional T6SS used for interbacterial competition and niche occupancy. Cell Host Microbe. 2017;21:769–776. doi: 10.1016/j.chom.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Zhao W, Caro F, Robins W, Mekalanos JJ. Antagonism toward the intestinal microbiota and its effect on Vibrio cholerae virulence. Science. 2018;359:210–213. doi: 10.1126/science.aap8775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacIntyre DL, Miyata ST, Kitaoka M, Pukatzki S. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc. Natl. Acad. Sci. USA. 2010;107:19520–19524. doi: 10.1073/pnas.1012931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell AB, et al. Type VI secretion delivers bacteriolytic effectors to target cells. Nature. 2011;475:343–347. doi: 10.1038/nature10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robitaille S, Trus E, Ross BD. Bacterial Defense against the Type VI Secretion System. Trends Microbiol. 2021;29:187–190. doi: 10.1016/j.tim.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Le N-H, et al. Peptidoglycan editing provides immunity to Acinetobacter baumannii during bacterial warfare. Sci. Adv. 2020;6:eabb5614. doi: 10.1126/sciadv.abb5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berk V, et al. Molecular architecture and assembly principles of Vibrio cholerae biofilms. Science. 2012;337:236–239. doi: 10.1126/science.1222981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toska J, Ho BT, Mekalanos JJ. Exopolysaccharide protects Vibrio cholerae from exogenous attacks by the type 6 secretion system. Proc. Natl. Acad. Sci. USA. 2018;115:7997–8002. doi: 10.1073/pnas.1808469115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dörr T, et al. A cell wall damage response mediated by a sensor kinase/response regulator pair enables beta-lactam tolerance. Proc. Natl. Acad. Sci. USA. 2016;113:404–409. doi: 10.1073/pnas.1520333113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hersch SJ, et al. Envelope stress responses defend against type six secretion system attacks independently of immunity proteins. Nat. Microbiol. 2020;5:706–714. doi: 10.1038/s41564-020-0672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahn A, Beis K, Naismith JH, Whitfield C. A novel outer membrane protein, Wzi, is involved in surface assembly of the Escherichia coli K30 group 1 capsule. J. Bacteriol. 2003;185:5882–5890. doi: 10.1128/JB.185.19.5882-5890.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant WD, Sutherland IW, Wilkinson JF. Exopolysaccharide colanic acid and its occurrence in the Enterobacteriaceae. J. Bacteriol. 1969;100:1187–1193. doi: 10.1128/jb.100.3.1187-1193.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cash RA, et al. Response of man to infection with Vibrio cholerae. I. Clinical, serologic, and bacteriologic responses to a known inoculum. J. Infect. Dis. 1974;129:45–52. doi: 10.1093/infdis/129.1.45. [DOI] [PubMed] [Google Scholar]
- 27.Forster SC, et al. A human gut bacterial genome and culture collection for improved metagenomic analyses. Nat. Biotechnol. 2019;37:186–192. doi: 10.1038/s41587-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016;14:20–32. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Millet YA, et al. Insights into Vibrio cholerae intestinal colonization from monitoring fluorescently labeled bacteria. PLoS Pathog. 2014;10:e1004405. doi: 10.1371/journal.ppat.1004405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borgeaud S, Metzger LC, Scrignari T, Blokesch M. The type VI secretion system of Vibrio cholerae fosters horizontal gene transfer. Science. 2015;347:63–67. doi: 10.1126/science.1260064. [DOI] [PubMed] [Google Scholar]
- 31.Metzger LC, et al. Independent regulation of type VI secretion in Vibrio cholerae by TfoX and TfoY. Cell Rep. 2016;15:951–958. doi: 10.1016/j.celrep.2016.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drebes Dörr NC, Blokesch M. Interbacterial competition and anti-predatory behavior of environmental Vibrio cholerae strains. Environ. Microbiol. 2020;22:4485–4504. doi: 10.1111/1462-2920.15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van der Henst C, et al. Molecular insights into Vibrio cholerae’s intra-amoebal host-pathogen interactions. Nat. Commun. 2018;9:3460. doi: 10.1038/s41467-018-05976-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basler M, Pilhofer M, Henderson GP, Jensen GJ, Mekalanos JJ. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature. 2012;483:182–186. doi: 10.1038/nature10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coyne, M. J. & Comstock, L. E. Type VI secretion systems and the gut microbiota. Microbiol. Spectr.7, PSIB-0009-2018 (2019). [DOI] [PMC free article] [PubMed]
- 36.Liu WY, Wong CF, Chung KM, Jiang JW, Leung FC. Comparative genome analysis of Enterobacter cloacae. PLoS ONE. 2013;8:e74487. doi: 10.1371/journal.pone.0074487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitney JC, et al. Genetically distinct pathways guide effector export through the type VI secretion system. Mol. Microbiol. 2014;92:529–542. doi: 10.1111/mmi.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basler M, Ho BT, Mekalanos JJ. Tit-for-tat: type VI secretion system counterattack during bacterial cell-cell interactions. Cell. 2013;152:884–894. doi: 10.1016/j.cell.2013.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davin-Regli A, Lavigne JP, Pages JM. Enterobacter spp.: update on taxonomy, clinical aspects, and emerging antimicrobial resistance. Clin. Microbiol. Rev. 2019;32:e00002–e00019. doi: 10.1128/CMR.00002-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao Y, et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. 2019;574:117–121. doi: 10.1038/s41586-019-1560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soria-Bustos J, et al. Two type VI secretion systems of Enterobacter cloacae are required for bacterial competition, cell adherence, and intestinal colonization. Front Microbiol. 2020;11:560488. doi: 10.3389/fmicb.2020.560488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donato SL, et al. The beta-encapsulation cage of rearrangement hotspot (Rhs) effectors is required for type VI secretion. Proc. Natl. Acad. Sci. USA. 2020;117:33540–33548. doi: 10.1073/pnas.1919350117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren Y, et al. Complete genome sequence of Enterobacter cloacae subsp. cloacae type strain ATCC 13047. J. Bacteriol. 2010;192:2463–2464. doi: 10.1128/JB.00067-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ketter PM, et al. Acinetobacter baumannii gastrointestinal colonization is facilitated by secretory IgA which is reductively dissociated by bacterial thioredoxin A. mBio. 2018;9:e01298–18. doi: 10.1128/mBio.01298-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perault AI, et al. Host adaptation predisposes Pseudomonas aeruginosa to type VI secretion system-mediated predation by the Burkholderia cepacia complex. Cell Host Microbe. 2020;28:534–547. doi: 10.1016/j.chom.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith WPJ, et al. The evolution of tit-for-tat in bacteria via the type VI secretion system. Nat. Commun. 2020;11:5395. doi: 10.1038/s41467-020-19017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Storey D, et al. Klebsiella pneumoniae type VI secretion system-mediated microbial competition is PhoPQ controlled and reactive oxygen species dependent. PLoS Pathog. 2020;16:e1007969. doi: 10.1371/journal.ppat.1007969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol. Mol. Biol. Rev. 2016;80:629–661. doi: 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rendueles O, et al. Screening of Escherichia coli species biodiversity reveals new biofilm-associated antiadhesion polysaccharides. mBio. 2011;2:e00043–11. doi: 10.1128/mBio.00043-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rendueles O, Garcia-Garcera M, Neron B, Touchon M, Rocha EPC. Abundance and co-occurrence of extracellular capsules increase environmental breadth: Implications for the emergence of pathogens. PLoS Pathog. 2017;13:e1006525. doi: 10.1371/journal.ppat.1006525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bushell SR, et al. Wzi is an outer membrane lectin that underpins group 1 capsule assembly in Escherichia coli. Structure. 2013;21:844–853. doi: 10.1016/j.str.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dorman MJ, Feltwell T, Goulding DA, Parkhill J, Short FL. The capsule regulatory network of Klebsiella pneumoniae defined by density-TraDISort. mBio. 2018;9:e01863–18. doi: 10.1128/mBio.01863-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Sousa JAM, Buffet A, Haudiquet M, Rocha EPC, Rendueles O. Modular prophage interactions driven by capsule serotype select for capsule loss under phage predation. ISME J. 2020;14:2980–2996. doi: 10.1038/s41396-020-0726-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buffet A, Rocha EPC, Rendueles O. Nutrient conditions are primary drivers of bacterial capsule maintenance in Klebsiella. Proc. R. Soc. B: Biol. Sci. 2021;288:20202876. doi: 10.1098/rspb.2020.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dong C, et al. Wza the translocon for E. coli capsular polysaccharides defines a new class of membrane protein. Nature. 2006;444:226–229. doi: 10.1038/nature05267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drummelsmith J, Whitfield C. Translocation of group 1 capsular polysaccharide to the surface of Escherichia coli requires a multimeric complex in the outer membrane. EMBO J. 2000;19:57–66. doi: 10.1093/emboj/19.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 58.Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu. Rev. Biochem. 2006;75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 59.Escobar-Paramo P, et al. Identification of forces shaping the commensal Escherichia coli genetic structure by comparing animal and human isolates. Environ. Microbiol. 2006;8:1975–1984. doi: 10.1111/j.1462-2920.2006.01077.x. [DOI] [PubMed] [Google Scholar]
- 60.Nanayakkara BS, O’Brien CL, Gordon DM. Diversity and distribution of Klebsiella capsules in Escherichia coli. Environ. Microbiol. Rep. 2019;11:107–117. doi: 10.1111/1758-2229.12710. [DOI] [PubMed] [Google Scholar]
- 61.Pando, J. M., Karlinsey, J. E., Lara, J. C., Libby, S. J. & Fang, F. C. The Rcs-regulated colanic acid capsule maintains membrane potential in Salmonella enterica serovar Typhimurium. mBio8, e00808–17 (2017). [DOI] [PMC free article] [PubMed]
- 62.Vonaesch P, et al. Stunted childhood growth is associated with decompartmentalization of the gastrointestinal tract and overgrowth of oropharyngeal taxa. Proc. Natl. Acad. Sci. USA. 2018;115:E8489–E8498. doi: 10.1073/pnas.1806573115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen RY, et al. Duodenal microbiota in stunted undernourished children with enteropathy. N. Engl. J. Med. 2020;383:321–333. doi: 10.1056/NEJMoa1916004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen RY, Ahmed T, Gordon JI. Duodenal microbiota in stunted undernourished children with enteropathy. Reply. N. Engl. J. Med. 2021;384:1778. doi: 10.1056/NEJMc2030388. [DOI] [PubMed] [Google Scholar]
- 65.Ferrières L, et al. Silent mischief: bacteriophage Mu insertions contaminate products of Escherichia coli random mutagenesis performed using suicidal transposon delivery plasmids mobilized by broad-host-range RP4 conjugative machinery. J. Bacteriol. 2010;192:6418–6427. doi: 10.1128/JB.00621-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Nat. Biotechnol. 1983;1:784–791. doi: 10.1038/nbt1183-784. [DOI] [Google Scholar]
- 67.Sambrook, J., Fritsch, E. F. & Maniatis, T. in Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory Press, 1982).
- 68.Meibom KL, et al. The Vibrio cholerae chitin utilization program. Proc. Natl. Acad. Sci. USA. 2004;101:2524–2529. doi: 10.1073/pnas.0308707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Metzger LC, Matthey N, Stoudmann C, Collas EJ, Blokesch M. Ecological implications of gene regulation by TfoX and TfoY among diverse Vibrio species. Environ. Microbiol. 2019;21:2231–2247. doi: 10.1111/1462-2920.14562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keene ON. The log transformation is special. Stat. Med. 1995;14:811–819. doi: 10.1002/sim.4780140810. [DOI] [PubMed] [Google Scholar]
- 71.Domenico P, Schwartz S, Cunha BA. Reduction of capsular polysaccharide production in Klebsiella pneumoniae by sodium salicylate. Infect. Immun. 1989;57:3778–3782. doi: 10.1128/iai.57.12.3778-3782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koren S, et al. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017;27:722–736. doi: 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hunt M, et al. Circlator: automated circularization of genome assemblies using long sequencing reads. Genome Biol. 2015;16:294. doi: 10.1186/s13059-015-0849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 75.Touchon M, et al. The genomic diversification of the whole Acinetobacter genus: origins, mechanisms, and consequences. Genome Biol. Evol. 2014;6:2866–2882. doi: 10.1093/gbe/evu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Capella-Gutierrez S, Silla-Martinez JM, Gabaldon T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Minh BQ, et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020;37:1530–1534. doi: 10.1093/molbev/msaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Letunic I, Bork P. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 2019;47:W256–W259. doi: 10.1093/nar/gkz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wyres KL, et al. Identification of Klebsiella capsule synthesis loci from whole genome data. Micro. Genom. 2016;2:e000102. doi: 10.1099/mgen.0.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information and Supplementary Data files). Accession number for whole-genome sequencing data are provided in the text and in Supplementary Data files 2 and 4. The PacBio raw read data of the five whole-genome sequenced Enterobacter strains generated in this study have been deposited in the NCBI’s Sequence Read Archive (SRA) database under the Bioproject accession number PRJNA640151. Details on the SRA accession numbers, BioSample accession numbers, and individual genome accession numbers of the de-novo-assembled and circularized genomes are provided in Supplementary Data file 4. Source data are provided with this paper.