Abstract
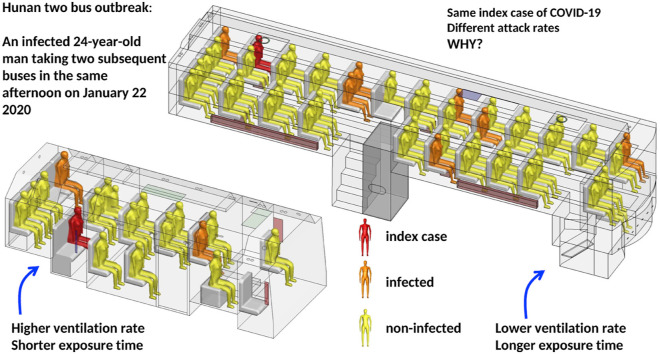
Uncertainty remains on the threshold of ventilation rate in airborne transmission of SARS-CoV-2. We analyzed a COVID-19 outbreak in January 2020 in Hunan Province, China, involving an infected 24-year-old man, Mr. X, taking two subsequent buses, B1 and B2, in the same afternoon. We investigated the possibility of airborne transmission and the ventilation conditions for its occurrence. The ventilation rates on the buses were measured using a tracer-concentration decay method with the original driver on the original route. We measured and calculated the spread of the exhaled virus-laden droplet tracer from the suspected index case. Ten additional passengers were found to be infected, with seven of them (including one asymptomatic) on B1 and two on B2 when Mr. X was present, and one passenger infected on the subsequent B1 trip. B1 and B2 had time-averaged ventilation rates of approximately 1.7 and 3.2 L/s per person, respectively. The difference in ventilation rates and exposure time could explain why B1 had a higher attack rate than B2. Airborne transmission due to poor ventilation below 3.2 L/s played a role in this two-bus outbreak of COVID-19.
Keywords: COVID-19, Public transport, SARS-CoV-2 infection, Airborne transmission, Ventilation requirement
Graphical abstract
1. Introduction
Human mobility significant impacts on the spatiotemporal transmission dynamics of infectious diseases, both globally and locally at city scale. The significance of human mobility and rapid global spread of infectious diseases had already been evidenced by the SARS epidemic in 2003 and the influenza A(H1N1) pandemic in 2009 before the current COVID-19 pandemic. Mass transportation can lead to rapid spread of emerging respiratory infections by either bringing infected individuals from the epicenter to a distant city [1] or becoming an infection venue where transmission takes place between the infected and the susceptible [2]. The enclosed and often crowded air cabins, metro trains or bus cabins increase human-to-human contact frequency and favor transmission of respiratory viruses and other infectious diseases that are spread by close contact. The role of air travel has been demonstrated using computer modelling in seeding global epidemics [3]; local traffic also has a role in local epidemics, for example by driving spatial transmission of influenza viruses [4]. The increasing importance of transportation in disease spread nowadays may be seen by the advances in transport systems and improvement in human transport infrastructure over the past century at the global and local scales. For example, more passengers than ever use rapid transit systems. In 2017, 168 million traveled daily via metro systems available in 182 cities, a growth of 70% in new metros since 2000 [5]. In China, there had been a rapid growth in passengers from 2012 to 2014 for cities, including the Beijing (+39%) and Shanghai (+25%) metro systems.
Despite the perceived or observed infection risk, it is not known what major environmental factors determines the infection risk after exposure. In 2003, some passengers who shared a flight from Hong Kong to Beijing with one symptomatic SARS patient were infected while others were not [6]. No passengers were infected in a Guangzhou-to-Huizhou China coach bus with a MERS (Middle East Respiratory Syndrome) patient in May 2015 [7]. In-flight transmission of SARS-CoV-2 has also been reported (e.g. Refs. [[8], [9], [10]]. In most of these reported outbreaks, lack of data on passenger contact and ventilation rate of the infection venue hindered the in-depth analyses of the factors that impact on infection risk.
The transmission routes of SARS-CoV-2 in transportation remain to be investigated. The possibility of airborne transmission has been recognized by major health authorities since October 2020 [[11], [12]]. According to the well-known Wells-Riley equation [13], the requirement for the minimum ventilation rate at an acceptable infection risk may be estimated if the quanta generation rate is known. However, significant uncertainty exists in estimating quanta generation rate and significant differences also exist in existing estimated values [[14], [15], [16]]. Alternatively, the ventilation rates can be measured in the infection venues involved in COVID-19 outbreaks. To the best of our knowledge, there is unfortunately a lack of a detailed measurement of ventilation rates in the infection venues in nearly all existing studies of COVID-19 outbreaks (e.g. Refs. [[16], [17], [18], [19], [20], [21], [22]].
We investigated the ventilation requirement by exploring possible airborne transmission in a COVID-19 outbreak in January 2020 in Hunan Province, China, involving two buses, bus B1 and minibus B2, with 10 non-associated infected passengers. We collected epidemiological data, bus itineraries, the seating plans of passengers, and the details of the ventilation systems and operation, and we performed detailed ventilation and dispersion measurements on the two buses with the original drivers on the original route.
2. Method
2.1. Epidemiological analysis
We obtained the dates of symptom onset and the seating arrangements on the two buses, and interviewed the drivers and passengers. All infected individuals were confirmed using throat swabs and analyzed by real-time polymerase chain reaction with reverse transcription (RT-PCR). The information collected also included the demographic data, travel history, and symptoms of the infected individuals. We also obtained details of the buses’ air conditioning and ventilation systems, and hourly weather data for January 22 in Changsha and city D. The closed-circuit television (CCTV) records of the two buses were reviewed by the co-authors from Hunan CDC in the early phase of the investigations, but unfortunately, the records were erased thereafter, however four screenshot images of B1, two screenshot images of B2, and the departure/arrival video clips of B2, were kept.
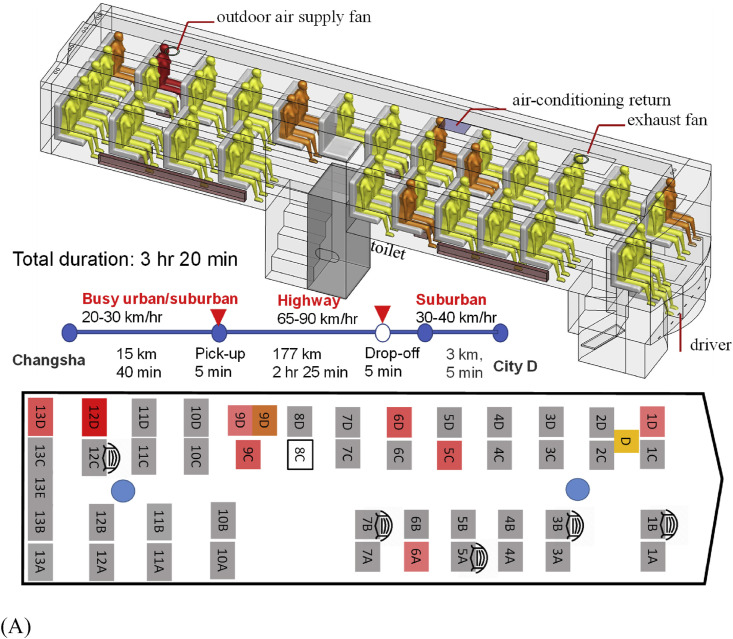
The 47-seat bus B1 is fully enclosed, with a luggage compartment and a toilet on the lower deck and the passenger cabin on the upper deck (Fig. 1 A). Its passenger cabin is 11.4 m long, 2.5 m wide, and 2 m high. The driver's seat is at a mid-level between the two decks. There were 46 passengers during Mr. X's journey. Seat 8C was empty. Forty-two passengers boarded using the front door at Changsha, two late passengers (seats 13B and 13E) at Changsha used the back door, and two other passengers (seats 13D and 9C) were picked up using the front door by the roadside 40 min after departing from Changsha. Four non-infected passengers were dropped off 16 km from City D station. The bus is equipped with an air conditioning system for cooling only, which was turned off during the trip. We also took the bus to a mechanic and repair station to examine its air conditioning and ventilation design. One ceiling-mounted outdoor air supply is provided at the rear and one ceiling exhaust at the front. The heating system includes floor-level convective radiators on both sides of the bus, although only the one at the driver side was functional. It was turned on.
Fig. 1.
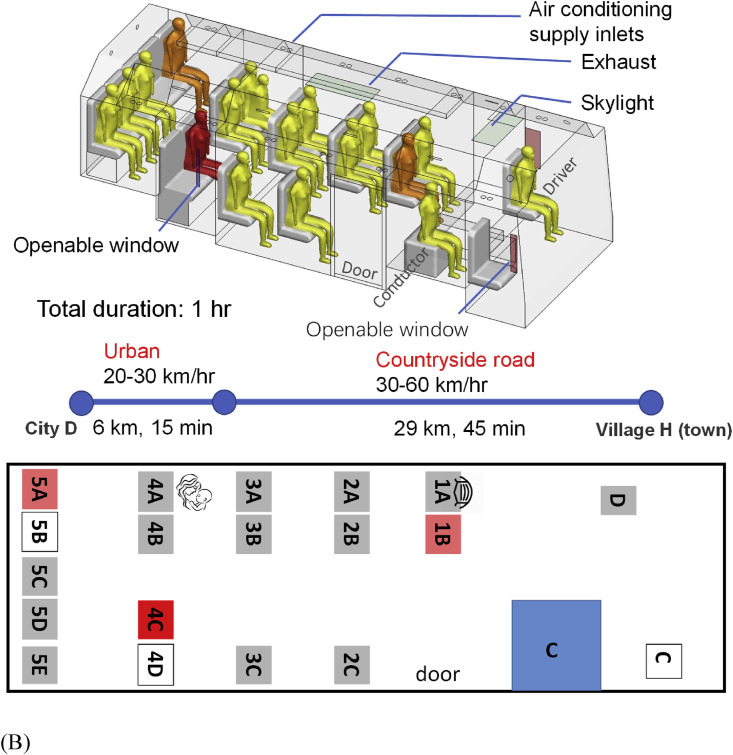
(Color Online) The two buses, seating plans, journeys, and distribution of confirmed cases of SARS-CoV-2 infection by seat numbers. (A) bus B1; (B) bus B2. The seat numbering has been assigned by the authors. Seat D is where the driver sat, and Seat C on B2 was reserved for the bus conductor, although she actually sat in the luggage area during the journey. The seat number in deep red on each seating plan indicates the possible location of the index case. Seat numbers in light red indicate where the infected individuals sat. The duration and journey length of each route are also shown. The passenger in seat 9D on B1 from Changsha to city D was infected, and the passenger in the same seat on the return trip from city D to Changsha also became infected (shown in brown with a separate seat symbol). Seats with a mask means the corresponding passengers wore a facial mask and seat 4A on bus B2 is where the passenger carrying a child sat on that day. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
B2 is a 17-seat air-conditioned minibus with both a driver and a ticket conductor. The cabin measures 5.5 m long, 2.5 m wide, and 2 m high. The windows were operable, and the driver recalled that his small window and two other windows were open occasionally. These opening-window areas are shown in brown or blue in Fig. 1B. The air conditioning system on the bus was not turned on at the time. A total of 16 passengers were on board, and all boarded the bus at the departing station or close to it. Two seats were empty, and a mother in Seat 4A held a 3-year-old child. Except for two passengers who got off 12 km before the final stop, including one infected passenger (Seat 5A), all others got off at or close to the final stop, including the index case. The whole journey of B2 took 60 min by the time the index case got off.
We studied the infection data on the two buses and explored the association between the attack rates and ventilation rates. On B1, we studied the infection data with regard to seating location and used a chi-square test to explore the relationship between a passenger's location (i.e., seat) and his/her probability of becoming infected. The seats were first grouped according to distance from the index case's seat, i.e., rear seats (rows 8–13) and front seats (rows 1–7). We also grouped the seats according to the aisle, i.e., the driver side and the opposite side.
2.2. Experimental tracer gas measurement and computer simulations
The tracer measurement was carried out on B2 on April 5–7 and on B1 on April 9–11. The weather on April 11 was similar to January 22, with low air temperature (5–11 °C). However, on the other measurement days, the temperatures were mostly 10 °C higher than on January 22 (11–22 °C on April 5–7 and 20–30 °C on April 9–10). We considered various combinations of air conditioning/heating and windows open/closed to simulate the airflow at the time of infection. Metallic sheet cylinders filled with hot water were used to reproduce the thermal plumes above the passengers. Between 9 and 11 members of our experimental group sat on selected seats while these warm metallic cylinders were placed on all other seats.
There were two parts to the tracer gas measurement. In part 1, we released ethane gas through a pipe of 8 mm inner diameter with a speed of 1.5 m/s at 32–34 °C, with the pipe outlet placed on an experimental worker's head. The release was designed to mimic the index case's exhalation. We monitored the tracer gas concentrations at nine seats or points, including those seats where some of the infected persons had sat; see Figure S2. We also detected the airflows at the outdoor-air supply fan and exhaust fan in B1 and at door/window openings in B2 during these tests. In part 2, the ventilation rate was measured using the tracer-concentration decay method. Unfortunately, the experiments in part 1 were performed on both buses with the wrong initial seating plans, i.e., with all seats occupied (Figure S4).
For CFD modeling [[23], [24], [25]], the widely used software package Ansys Fluent (Ansys, Canonsburg, PA, USA) was adopted, which is a three-dimensional, general-purpose CFD software package for modeling airflows. The virus-laden water droplets generated from the index case were assumed to rapidly evaporate after a few seconds in the air. We approximated the exhaled droplet nuclei as a passive scalar and neglected the deposition effect. Our computational fluid dynamics (CFD) modeling was first performed under the experimental conditions (Figure S3) for validation, and then with the correct seating plan on both buses.
3. Results
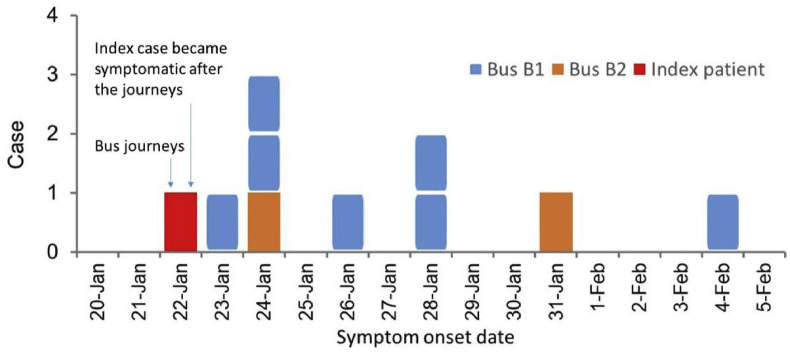
On 22 January 2020, the 47-seater bus B1 had 46 passengers during the 3 h 20 min journey from Changsha to City D, and the 17-seater bus B2 had 16 passengers during the 1 h journey from City D to a small town immediately after Mr. X's village. We identified nine secondary symptomatic infections, one asymptomatic secondary infection and two tertiary symptomatic cases. The epidemic curve for the nine symptomatic secondary infections is shown in Fig. 2 . Among the 10 confirmed secondary cases, 7 were on B1 from Changsha to City D, 2 on B2 from City D to the countryside, and 1 on B1 during its return to Changsha. The passenger infected in seat 9C on B1 was asymptomatic. Case 6A on B1 led to one tertiary infection. Case 4C on B2 led to another tertiary infection. Thus, 13 infected cases were associated with this cluster, including the index case.
Fig. 2.
(Color Online) The observed dates of symptom onset of the index case (represented by a red square), the six symptomatic passengers on bus B1 from Changsha to City D (represented by blue squares), one symptomatic passenger (with onset on January 24) on B1 from D to Changsha (represented by a blue square), and two symptomatic passengers on the minibus B2 (represented by yellow squares). The additional asymptomatic case on B1 is not shown here. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
These infected cases were identified by contact tracing after the index case Mr. X was confirmed as the first case in city D. A total of 243 close contacts and suspected cases were subsequently identified as associated with the index case. Nasal swabs were collected and tested with RT-PCR. Note that there was no confirmed case of COVID-19 in city D and only four in Changsha as of January 22, although it was later found that there were 9 cases with onset of symptoms in city D, and 23 in Changsha (Figure S1). The remaining 231 close contacts all tested negative and were released after 14 days of isolation. No other exposure history was identified. The outdoor air temperature in Changsha on January 22 was 6–9 °C with a 3-5-m/s northwesterly wind and rain, and in city D the temperature was 7–11 °C, with a 3-5-m/s northeasterly wind and light rain.
The index case was a 24-year-old man who worked in Changsha, and who had probably been exposed to SARS-CoV-2 on January 18 and 19 when he dined and worked with his colleagues there. He first developed symptoms on January 22 after the bus journeys. One of these colleagues had the onset of symptoms on January 16, and was confirmed later as a COVID-19 patient.
The infected seats and the seating plans of the two buses are shown in Fig. 1. The suspected index case sat in seat 12D on B1 and seat 4C on B2. On B1, the furthest infected case sat in the front row in seat 1D (9.46 m from the index case). Nearly all passengers, including the index case, got on the bus via the front door, except the two late passengers at the departing station, who used the back door. The remaining incomplete video record also shows that five non-infected B1 passengers wore masks, but one of them took off the mask for part of the time, and none of the infected cases wore masks. The suspected index case did not use the toilet during the journey.
Bus B1 stayed in city D for 30 min for simple cleaning only, without any disinfection at that time. On the return trip of B1 to Changsha, the passenger who sat in seat 9D (shown in brown in Fig. 1A) became infected, with onset on January 24. No contact or interaction between the index case and the new passenger in seat 9D was identified. By January 28 when the index case was confirmed, bus B1 had already been fully disinfected multiple times, making environmental sampling impossible. The passengers at the rear seats had a nearly identical infection probability to those at the front seats (Table 1 ). However, the infection risk was much higher for the passengers on the driver side than those on the opposite side.
Table 1.
The infected passengers on bus B1. The numbers of other passengers do not include the index case. Note that the division between the front and rear seat zones is arbitrary.
| Zones | No. of other passengers | No. of infected cases | Attack rate | P-value | |
|---|---|---|---|---|---|
| Distance from the index case | Rear seats (rows 8–13) | 19 | 3 | 15.8% | 1.000# |
| Front seats (rows 1–7) | 26 | 4 | 15.4% | ||
| Aisle* | Driver side | 24 | 6 | 25% | 0.164# |
| Opposite side | 20 | 1 | 5% | ||
* Passenger in seat 13E is ignored in the calculation.
# Chi-square test with continuity correction.
On B2, the distance between the seat where the index case sat and the farthest infected person (seat 1B) was 2.33 m. Hence, close-contact transmission might have occurred. After taking B2 and having arrived in his village, which is only 1 km from the final stop in a small town, Mr. X bought masks at a roadside pharmacy, donned one before returning home, and then undertook self-quarantine in his own room, and did not infect any of his family members.
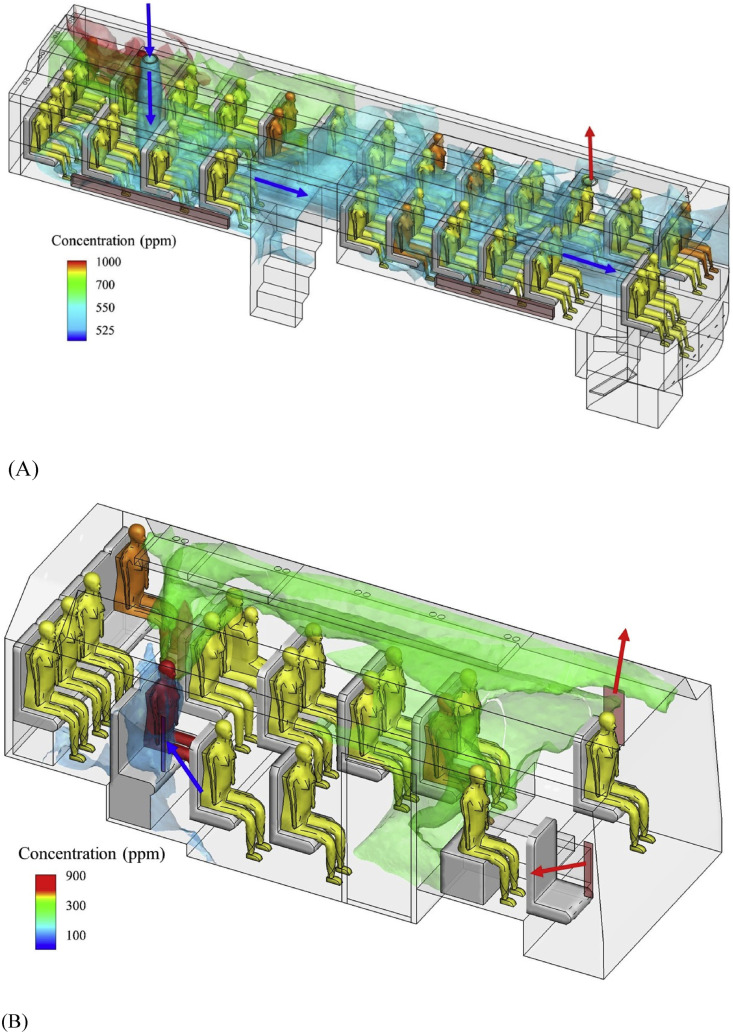
The time-averaged ventilation rate was 1.72 L/s per person on B1 and 3.22 L/s per person on B2. The ventilation rate of a bus depending on the driving speed [26,27] and extent of window opening. The measured ventilation rates are summarized in Table S1 and compared with the attack rates on the two buses in Table 2 . For both buses, the relatively large value of air change per hour (ACH) (Table S1) suggests that the steady-state assumption is valid [28]. The measured and predicted dispersion of the exhaled tracer gas from the index case is shown in Figure S3 and Figure S4. On both buses, the distribution of the exhaled tracer gas was rather uniform due to the airflow patterns (Fig. 3 ). This explains the relatively uniform distribution of the infected passengers on both buses. The mean airflow pattern on B1 was dominated by the thermal plumes above each person and their interaction with the supply outdoor-airflow at the rear of the bus. The supply of outdoor air established a cold air current that fell to the cabin floor, spread to the entire cabin as a cold “gravity current” [28] then rose following the body thermal plume of each individual passenger and eventually exhausted at the bus's front exhaust. It appears that the blockage of the floor-level gravity currents by the toilet in the passenger cabin explains why the contaminated supply air spread slightly more to the driver side than to the opposite side. Our simulation showed that the mean concentration in the driver-side half-volume was 24.5% greater than that of the half-volume on the opposite side. The mean airflow pattern on B2 was different from B1 in that the incoming wind flowing via the open back window was pushed upwards and circulated to the front along the ceiling. This probably explains how the passenger in seat 1B was infected. The departure and arrival video clips showed that the index case only passed the passenger at 1B while boarding, and that upon arrival, the passenger at 1B disembarked first. The overall rear-to-front airflow patterns on both buses were created by the wind pressure distribution.
Table 2.
The observed attack rates, measured ventilation rates, and exposure times in the two buses.
| Parameters | Bus B1 from Changsha to city D (12:10 p.m. to 3:30 p.m.) | Minibus B2 (15:43 p.m. to 16:43 p.m.) |
|---|---|---|
| Number of persons (other passengers + driver (conductor)) | 46 | 17 |
| Number of infected cases except index case | 7 | 2 |
| Attack rate (%) | 7/46, 15.2% | 2/17, 11.8% |
| Ventilation rate | 1.72 L/s per person | 3.22 L/s per person |
| Exposure time | 200 min | 60 min |
Fig. 3.
(Color Online) Predicted spread of the exhaled tracer gas in the buses. (A) B1; (B) B2. The ventilation inflow is shown by blue arrows, and outflow by red arrows. In bus B1 simulation using CFD, the tracer gas concentration at the mouth of index case is 31.83%, while that for bus B2, 43.77%. The predicted average concentration on bus B1 is 603.2 ppm, and on bus B2 is 251.7 ppm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
The most striking feature of the outbreak is the occurrence of infection in two subsequent buses due to the presence of the same suspected index case on the same afternoon of January 22 with a gap of just 10 min between the two trips. This provides an opportunity to explore the effect of bus ventilation rate on infection if airborne transmission dominates. The attack rate was slightly higher on B1 than on B2, although the same index case was present. Both buses were poorly ventilated, with measured time-averaged ventilation rates of 1.72 L/s per person on B1 and 3.22 L/s on B2. Both ventilation rates are much lower than the commonly recommended 8–10 L/s per person according to most authorities and professional societies (American Society of Heating, Refrigerating and Air-Conditioning Engineers Standard 62.1, 2019). The minibus B2 was better ventilated than B1, as its driver and passengers were able to open some windows for some of the time, even though the outdoor air was cold (6–9 °C) with light rain. Buses are known to be crowded environments. The area occupied by each person was 0.60 m2 on B1 and 0.72 m2 on B2. The exposure time on B2 was also shorter than on B1.
The index case already felt tired prior to taking the two buses. He remained asymptomatic on both journeys. On B1, he sat next to the window and close to the supply air jet, which carried some of his expired droplets or droplet nuclei to the floor level, where they spread to the front of the bus as a gravity current. The predicted blockage of this gravity current by the toilet would have prevented some spread of the expired droplets to the non-driver side of the passenger cabin, while a slightly higher concentration of expired droplets was seen on the driver side (Fig. 3A). This explains the higher attack rate on the driver side. Similarly, on B2, his window was faulty and thus permanently open, as found during our field study. The open window was the air supply for the entire bus. This provided a ventilation inflow that spread his expired droplets to the entire minibus cabin. Poor ventilation leads to infection, but the airflow actually spreads the infectious virus droplets.
Airborne transmission of SARS-CoV-2 had been debated [29,30] before US CDC (2020) and WHO [11] formally recognized it in October 2020 (US CDC, 2020; [11]. The possibility of airborne transmission may be supported by the detection of the virus in airborne aerosols and its survivability in fine aerosols. Sia et al. [31] demonstrated that SARS-CoV-2 can be transmitted efficiently by aerosols between hamsters. Positive air samples of SARS-CoV-2 have been detected in hospital wards and elsewhere (e.g., Ref. [32]. The new coronavirus can remain viable in aerosols for hours with a median half-life of approximately 1 h [33].
However, successful transmission is also determined by the room ventilation. To minimize airborne transmission, a minimum ventilation rate needs to be provided into an indoor space, and such a minimum ventilation requirement is probably a function of the quanta generation, number of infectors, exposure time of the susceptible and other physical and biological parameters. Our study here provides evidence for the probable airborne transmission of SARS-CoV-2 on two buses under a ventilation rate of 3.2 L/s per person or less. The importance of ventilation for SARS-CoV-2 intervention has been recently recognized in at least two major commentaries [34]; Li et al., 2021; [[35], [36], [37]].
The air flows on the two buses are approximately fully mixed as shown in Fig. 3. The volume of air is 60.42 m3 in Bus B1 and 21.69 m3 in Bus B2. The air change rate is 4.8 h−1 in B1 and 9.6 h−1 in B2. We adopted an aerosol deposition rate of 0.3 h−1 and virus deactivation rate of 0.63 h−1 [16]. The pulmonary flow rate for test and breathing is 0.49 h−1. As the effective air change rate is (4.8 + 0.93) h−1 on B1 and (9.6 + 0.93) h−1 on B1, the use of steady state Wells-Riley equation is justified, which gives an estimated quanta generation rate of 35.0 h−1 on B1 and 58.3 h−1 on B2, which are compared with 970 ± 390 quanta/h in the Skagit Valley Chorale outbreak [16], <1 quantum/h in resting conditions by Buonanno et al. [14], and 14–48 quanta/h using a sing a reproductive number-based fitting approach [15]. According to Sun et al. [38], “… infectiousness profile of a typical SARS-CoV-2 patient peaks just before symptom presentation”, which is also supported by other studies (e.g. Refs. [[39], [40], [41]]). Our estimated larger quanta generation rate on B2 than B1 for the pre-symptomatic index case is also in agreement with this theory of peak infectiousness just before the symptom presentation, as the index case only developed symptoms after the trip, and he did not infect others after the trip.
To ensure that there is less than one person to be infected on each bus, the estimated minimum ventilation rate becomes 15.4 L/s per person on B1 and 7.3 L/s per person on B2 using the estimated quanta values. These estimated minimum ventilation rates are in the same order of magnitude of the required minimum ventilation rate of 8–10 L/s per person in offices and other public spaces as required by international ventilation standards such as ASHRAE 62.1 (2019) [42].
Close contact between the index case and other passengers could also have occurred. The index case arrived at Changsha West Bus Station around 11:30 a.m. and picked up his bus ticket from a machine at the station. He waited in the lounge for approximately 20 min. Although many other passengers were waiting there, the index case was able to find a seat. He did not know anyone or speak with anyone. Thousands of buses are scheduled daily to depart from the station, and the fact that no passengers on any other buses were found to be infected suggests that the lounge room was probably not the infection venue. He was among the first few passengers to board the bus B1. Close contacts were also possible during boarding, de-boarding and luggage collection, but the probability of meeting all seven infected passengers during such a short encounter is low. At City D station, he was among the last passengers to get off B1, and then walked straight through the station's front square to the minibus stop in approximately 10 min. He did not know or speak with anyone at the station. He directly boarded the minibus as one of the first few passengers, took a seat and scanned the conductor's mobile phone to pay for his ticket, and did not request a paper ticket. We cannot find any evidence for the possibility of close contact transmission during his non-seated periods. Only one infected passenger on B1 was within 2 m of the index case, whereas both infected passengers on B2 were approximately within 2 m.
After stopping at city D station for 30 min, B1 began its journey back to Changsha. One of the new passengers who came aboard during this journey sat in seat 9D and became infected without encountering the index case. It appears that the most likely explanation is the touching of contaminated surfaces, such as the seat, i.e., the fomite route, but we do not have any environmental evidence for this. Note that the passenger in this seat was also infected during the same bus's previous trip. The bus was not disinfected at city D bus station. It is known that the virus can survive on surfaces for hours (e.g., Ref. [43]). However, the fomite route has been suspected to play a negligible role in SARS-CoV-2 infection (Mondelli et al., 2020). There is also a possibility that airborne aerosols were not completely removed after the index case left the bus. During idling, the ventilation rate on B1 was measured to be only 0.62 ACH (Table S1). Without considering the loss during the getting off period, such a low air change rate would lead to a reduction of fine aerosol concentration by only 26.7%.
We thus conclude that in addition to the possible close contact transmission, long-range airborne transmission caused some of the infections on B1 and also likely on B2. The outbreak occurred in crowded spaces with insufficient ventilation at 3.2 L/s per person or less. This reveals a clear need for providing sufficient ventilation in transportation and other indoor environments. To our knowledge, there is only one another study of a COVID-19 outbreak in which the ventilation rates were measured in the infection venue [44], in which an outbreak of COVID-19 in a restaurant was studied, where nine members from three non-associated families sitting in three separate tables were infected by an index patient. The three families did not know each other and had essentially no contact before, during or after the meal. The ventilation rate was measured to be only 1 L/s per person in the restaurant.
Although we have not been able to determine the minimum ventilation requirement for mitigating airborne transmission of COVID-19, however, it may be concluded that airborne transmission of COVID-19 outbreak in the indoor environment with passive or light activities was likely when the ventilation rate is less than 3 L/s per person, with a sufficient exposure period.
There are limitations in our study. First, we did not carry out virus sequencing to confirm that all infected individuals were infected from the index case. However, as of January 22, there were only 4 confirmed cases and 23 symptomatic cases of COVID-19 in Changsha, a city with a population of 8 million, and 0 confirmed cases and only 9 symptomatic cases in city D with a population of 1.3 million (Figure S1). Thus, the probability that the passengers on B1 and B2 were infected elsewhere in the community is presumably very low. Second, the seating plans of the infected passengers were identified wrongly at first due to the unusual seat numbering systems used on the buses, and our field dispersion tests were thus based on the wrong seating plans, as shown in Figure S3. However, the measured ventilation rates were not affected by this initial error. Additionally, the knowledge of asymptomatic infection was not sufficient at the time of investigating this outbreak, and it is uncertain if there were other asymptomatic cases on both buses.
Author contributions
Y. Li, S. Hu, C. Ou and K. Luo contributed to study design, hypothesis formulation, and coordination. C. Ou and J Hang led field environmental experiments and CFD modelling. K. Luo, Z. Hai, S. Xiao (Shanliang Xiao), X. Jing, Z. Xie and L. Gao contributed to epidemiological data collection and analyses, J Hang, C. Ou and H. Ling contributed to tracer gas experiments. H. Qian and L. Liu contributed to experimental design. S. Xiao (Shenglan Xiao) contributed to statistical analysis. Y. Li wrote the first draft of the manuscript. C. Ou, K. Luo, S. Hu contributed to major revision. All of the other authors contributed to revision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to Ms Xia Yang from Sun Yat-sen University for her initial effort in mesh generation for CFD simulation, to Dr Peizhi Yang, Ms Jing Li from Central South University, Mr Taihan Chen, Mr Yong Zhang, Ms Yujie Zhao, Ms Ying Zhu, Mr Haonan Pan, and Mr Mengrong Lu from Sun Yat-sen University for their participation in the field measurement.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.buildenv.2021.108414.
Funding
This work was supported by National Natural Science Foundation of China [grant numbers 51811530017, 41875015, 42005069]; Research Grants Council of Hong Kong’s Collaborative Research Fund [grant number C7025-16G]; COVID-19 Emergency Project of Hunan Province [grant numbers 2020SK3001, 2020SK3012]; and Basic Research Fund of the Chinese Academy of Medical Sciences [grant number 2020HY320003].
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mangili A., Gendreau M.A. Transmission of infectious diseases during commercial air travel. Lancet. 2005;365(9463):989–996. doi: 10.1016/S0140-6736(05)71089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balcan D., Colizza V., Goncalves B., Hu H., Ramasco J.J., Vespignani A. Multiscale mobility networks and the spatial spreading of infectious diseases. Proc. Natl. Acad. Sci. U. S. A. 2009;106:21484–21489. doi: 10.1073/pnas.0906910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charu V., Zeger S., Gog J., Bjornstad O.N., Kissler S., Simonsen L., Grenfell B.T., Viboud C. Human mobility and the spatial transmission of influenza in the United States. PLoS Comput. Biol. 2017;13 doi: 10.1371/journal.pcbi.1005382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UITP. WORLD METRO FIGURES 2018. International Association of Public Transport. https://cms.uitp.org/wp/wp-content/uploads/2020/06/Statistics-Brief-World-metro-figures-2018V3_WEB.pdf (Accessed 4 October 2021).
- 6.Olsen S.J., Chang H.L., Cheung T.Y., Tang A.F., Fisk T.L., Ooi S.P., Kuo H.W., Jiang D.D., Chen K.T., Lando J., Hsu K.H., Chen T.J., Dowell S.F. Transmission of the severe acute respiratory syndrome on aircraft. N. Engl. J. Med. 2003;349:2416–2422. doi: 10.1056/NEJMoa031349. [DOI] [PubMed] [Google Scholar]
- 7.Yang Xia, Ou Cuiyun, Yang Hongyu, et al. Transmission of pathogen-laden expiratory droplets in a coach bus. J. Hazard Mater. 2020;397:122609. doi: 10.1016/j.jhazmat.2020.122609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi E.M., Chu D., Cheng P., et al. In-flight transmission of SARS-CoV-2. Emerg. Inf. Disp. 2020;26(11):2713–2716. doi: 10.3201/eid2611.203254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Speake H.Phillips A., Chong T., et al. Flight-associated SARS-CoV-2 transmission from cruise ship passengers during a medium-haul Australian domestic flight supported by whole genome sequencing. Emerg. Infect. Dis. 2020;26(12):2872–2880. doi: 10.3201/eid2612.203910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swadi T., Geoghegan J.L., Devine T., McElnay C., Sherwood J., Shoemack P., et al. Genomic evidence of in-flight transmission of SARS-CoV-2 despite predeparture testing. Emerg. Infect. Dis. 2021;27(3):687–693. doi: 10.3201/eid2703.204714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization . 2020. Coronavirus Disease (COVID-19): How Is it Transmitted?https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-how-is-it-transmitted Accessed on. [Google Scholar]
- 12.US CDC Scientific Brief: SARS-CoV-2 and Potential Airborne Transmission. https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-sars-cov-2.html Accessed on 15 November 2020.
- 13.Riley E.C., Murphy G., Riley R.L. Airborne spread of measles in a suburban elementary school. Am. J. Epidemiol. 1978;107(5):421–432. doi: 10.1093/oxfordjournals.aje.a112560. [DOI] [PubMed] [Google Scholar]
- 14.Buonanno G., Stabile L., Morawska L. Estimation of airborne viral emission: quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ. Int. 2020:105794. doi: 10.1016/j.envint.2020.105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai H., Zhao B. Association of the infection probability of COVID-19 with ventilation rates in confined spaces. Build. Simul. 2020;13(6):1321–1327. doi: 10.1007/s12273-020-0703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller S.L., Nazaroff W.W., Jimenez J.L., et al. Transmission of SARS‐CoV‐2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air. 2020;31:314–323. doi: 10.1111/ina.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae S., Kim H., Jung T.Y., et al. Epidemiological characteristics of COVID-19 outbreak at Fitness centers in cheonan, Korea. J. Kor. Med. Sci. 2020;2020(31):35. doi: 10.3346/jkms.2020.35.e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baggett T.P., Keyes H., Sporn N., et al. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. Jama. 2020;323(21):2191–2192. doi: 10.1001/jama.2020.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlotte N. High rate of SARS-CoV-2 transmission due to choir practice in France at the beginning of the COVID-19 pandemic. J. Voice. 2020 doi: 10.1016/j.jvoice.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Günther T., Czech‐Sioli M., Indenbirken D., et al. SARS‐CoV‐2 outbreak investigation in a German meat processing plant. EMBO Mol. Med. 2020;12 doi: 10.15252/emmm.202013296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imbert E., Kinley P.M., Scarborough A., et al. Coronavirus disease 2019 (COVID-19) outbreak in a san Francisco homeless shelter. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tobolowsky F.A., Gonzales E., Self J.L., et al. COVID-19 outbreak among three affiliated homeless service sites—King County, Washington, 2020. MMWR (Morb. Mortal. Wkly. Rep.) 2020;69(17):523. doi: 10.15585/mmwr.mm6917e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y.G., Huang X., Yu I.T.S., et al. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air. 2005;15:83–95. doi: 10.1111/j.1600-0668.2004.00317.x. [DOI] [PubMed] [Google Scholar]
- 24.Wong T.W., Li C.K., Tam W., et al. Cluster of SARS among medical students exposed to single patient, Hong Kong. Emerg. Infect. Dis. 2004;10:269–276. doi: 10.3201/eid1002.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu I.T.S., Li Y.G., Wong T.W., et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N. Engl. J. Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 26.Zhu S., Demokritou P., Spengler J. Experimental and numerical investigation of micro-environmental conditions in public transportation buses. Build. Environ. 2010;45(10):2077–2088. doi: 10.1016/j.buildenv.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q., Fischer H.J., Weiss R.E., et al. Ultrafine particle concentrations in and around idling school buses. Atmos. Environ. 2013;69:65–75. doi: 10.1016/j.atmosenv.2012.12.015. [DOI] [Google Scholar]
- 28.Etheridge D.W., Sandberg M. John Wiley & Sons; Chichester, England: 1996. Building Ventilation: Theory and Measurement. [Google Scholar]
- 29.Lewis D. Is the coronavirus airborne? Experts can't agree. Nature. 2020;580:175. doi: 10.1038/d41586-020-00974-w. [DOI] [PubMed] [Google Scholar]
- 30.Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ. Int. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sia S.F., Yan L., Chin A.W.H., et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583(7818):834–838. doi: 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo Z.D., Wang Z.Y., Zhang S.F., et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg. Infect. Dis. 2020;26(7):1586–1591. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Doremalen N., Bushmaker T., Morris D.H., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blocken B., van Bruenen T., Ricci A., Kang L., van Hooff T., Qin P., Xia L., Alanis Ruiz C., Arts J.H., Diepens J.F.L., Maas G.A., Gillmeier S.G., Vos S.B., Brombacher A.C. Ventilation and air cleaning to limit aerosol particle concentrations in a gym during the COVID-19 pandemic. Build. Environ. 2021;193:107659. doi: 10.1016/j.buildenv.2021.107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morawska L., Allen J., Bahnfleth W., et al. A paradigm shift to combat indoor respiratory infection. Science. 2021;372(6543):689–691. doi: 10.1126/science.abg2025. [DOI] [PubMed] [Google Scholar]
- 36.Ren J., Wang Y., Liu Q., Liu Y. Numerical study of three ventilation strategies in a prefabricated COVID-19 inpatient ward. Build. Environ. 2021;188:107467. doi: 10.1016/j.buildenv.2020.107467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang J.W., Marr L.C., Li Y., Dancer S.J. Covid-19 has redefined airborne transmission. BMJ. 2021:373. doi: 10.1136/bmj.n913. [DOI] [PubMed] [Google Scholar]
- 38.Sun K., Wang W., Gao L., et al. Transmission heterogeneities, kinetics, and controllability of SARS-CoV-2. Science. 2021:371. doi: 10.1126/science.abe2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cevik M., Tate M., Lloyd O., et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2(1):e13–e22. doi: 10.2139/ssrn.3677918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He X., Lau E.H., Wu P., et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 41.Wei W.E., Li Z., Chiew C.J., et al. Presymptomatic transmission of SARS-CoV-2—Singapore, January 23–March 16, 2020. MMWR (Morb. Mortal. Wkly. Rep.) 2020;69(14):411. doi: 10.15585/mmwr.mm6914e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.American Society of Heating Refrigerating and Air-Conditioning Engineers (ASHRAE) ANSI/ASHRAE Standard 62.1-2019; Atlanta, USA: 2019. Ventilation for Acceptable Indoor Air Quality. [Google Scholar]
- 43.Mondelli M.U., Colaneri M., Seminari E.M., et al. Low risk of SARS-CoV-2 transmission by fomites in real-life conditions. Lancet Infect. Dis. 2021;21(5):e112. doi: 10.1016/S1473-3099(20)30678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y.G., Qian H., Hang J., et al. Probable evidence for aerosol transmission of SARS-CoV-2 in a poorly ventilated restaurant. Build. Environ. 2020;196:107788. doi: 10.1016/j.buildenv.2021.107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.