Abstract
STUDY QUESTION
What is the normal range of cervical mucus patterns and number of days with high or moderate day-specific probability of pregnancy (if intercourse occurs on a specific day) based on cervical mucus secretion, in women without known subfertility, and how are these patterns related to parity and age?
SUMMARY ANSWER
The mean days of peak type (estrogenic) mucus per cycle was 6.4, the mean number of potentially fertile days was 12.1; parous versus nulliparous, and younger nulliparous (<30 years) versus older nulliparous women had more days of peak type mucus, and more potentially fertile days in each cycle.
WHAT IS KNOWN ALREADY
The rise in estrogen prior to ovulation supports the secretion of increasing quantity and estrogenic quality of cervical mucus, and the subsequent rise in progesterone after ovulation causes an abrupt decrease in mucus secretion. Cervical mucus secretion on each day correlates highly with the probability of pregnancy if intercourse occurs on that day, and overall cervical mucus quality for the cycle correlates with cycle fecundability. No prior studies have described parity and age jointly in relation to cervical mucus patterns.
STUDY DESIGN, SIZE, DURATION
This study is a secondary data analysis, combining data from three cohorts of women: ‘Creighton Model MultiCenter Fecundability Study’ (CMFS: retrospective cohort, 1990–1996), ‘Time to Pregnancy in Normal Fertility’ (TTP: randomized trial, 2003–2006), and ‘Creighton Model Effectiveness, Intentions, and Behaviors Assessment’ (CEIBA: prospective cohort, 2009–2013). We evaluated cervical mucus patterns and estimated fertile window in 2488 ovulatory cycles of 528 women, followed for up to 1 year.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Participants were US or Canadian women age 18–40 years, not pregnant, and without any known subfertility. Women were trained to use a standardized protocol (the Creighton Model) for daily vulvar observation, description, and recording of cervical mucus. The mucus peak day (the last day of estrogenic quality mucus) was used as the estimated day of ovulation. We conducted dichotomous stratified analyses for cervical mucus patterns by age, parity, race, recent oral contraceptive use (within 60 days), partial breast feeding, alcohol, and smoking. Focusing on the clinical characteristics most correlated to cervical mucus patterns, linear mixed models were used to assess continuous cervical mucus parameters and generalized linear models using Poisson regression with robust variance were used to assess dichotomous outcomes, stratifying by women’s parity and age, while adjusting for recent oral contraceptive use and breast feeding.
MAIN RESULTS AND THE ROLE OF CHANCE
The majority of women were <30 years of age (75.4%) (median 27; IQR 24–29), non-Hispanic white (88.1%), with high socioeconomic indicators, and nulliparous (70.8%). The mean (SD) days of estrogenic (peak type) mucus per cycle (a conservative indicator of the fertile window) was 6.4 (4.2) days (median 6; IQR 4–8). The mean (SD) number of any potentially fertile days (a broader clinical indicator of the fertile window) was 12.1 (5.4) days (median 11; IQR 9–14). Taking into account recent oral contraceptive use and breastfeeding, nulliparous women age ≥30 years compared to nulliparous women age <30 years had fewer mean days of peak type mucus per cycle (5.3 versus 6.4 days, P = 0.02), and fewer potentially fertile days (11.8 versus 13.9 days, P < 0.01). Compared to nulliparous women age <30 years, the likelihood of cycles with peak type mucus ≤2 days, potentially fertile days ≤9, and cervical mucus cycle score (for estrogenic quality of mucus) ≤5.0 were significantly higher among nulliparous women age ≥30 years, 1.90 (95% confidence interval (CI) 1.18, 3.06); 1.46 (95% CI 1.12, 1.91); and 1.45 (95% CI 1.03, 2.05), respectively. Between parous women, there was little difference in mucus parameters by age. Thresholds set a priori for within-woman variability of cervical mucus parameters by cycle were examined as follows: most minus fewest days of peak type mucus >3 days (exceeded by 72% of women), most minus fewest days of non-peak type mucus >4 days (exceeded by 54% of women), greatest minus least cervical mucus cycle score >4.0 (exceeded by 73% of women), and most minus fewest potentially fertile days >8 days (found in 50% of women). Race did not have any association with cervical mucus parameters. Recent oral contraceptive use was associated with reduced cervical mucus cycle score and partial breast feeding was associated with a higher number of days of mucus (both peak type and non-peak type), consistent with prior research. Among the women for whom data were available (CEIBA and TTP), alcohol and tobacco use had minimal impact on cervical mucus parameters.
LIMITATIONS, REASONS FOR CAUTION
We did not have data on some factors that may impact ovulation, hormone levels, and mucus secretion, such as physical activity and body mass index. We cannot exclude the possibility that some women had unknown subfertility or undiagnosed gynecologic disorders. Only 27 women were age 35 or older. Our study participants were geographically dispersed but relatively homogeneous with regard to race, ethnicity, income, and educational level, which may limit the generalizability of the findings.
WIDER IMPLICATIONS OF THE FINDINGS
Patterns of cervical mucus secretion observed by women are an indicator of fecundity and the fertile window that are consistent with the known associations of age and parity with fecundity. The number of potentially fertile days (12 days) is likely greater than commonly assumed, while the number of days of highly estrogenic mucus (and higher probability of pregnancy) correlates with prior identifications of the fertile window (6 days). There may be substantial variability in fecundability between cycles for the same woman. Future work can use cervical mucus secretion as an indicator of fecundity and should investigate the distribution of similar cycle parameters in women with various reproductive or gynecologic pathologies.
STUDY FUNDING/COMPETING INTEREST(S)
Funding for the three cohorts analyzed was provided by the Robert Wood Johnson Foundation (CMFS), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (TTP), and the Office of Family Planning, Office of Population Affairs, Health and Human Services (CEIBA). The authors declare that they have no conflict of interest.
TRIAL REGISTRATION NUMBER
N/A
Keywords: menstrual cycle, peak day, cervical mucus, fertility, fertile window, fecundity
Introduction
One of the most practical and promising approaches to enable the discovery of biomarkers or pathophysiological mechanisms for disorders and diseases is the analysis of various human body fluids (Jozwik and Kaluzna-Czaplinska, 2016), including cervical mucus. Cervical mucus, a relatively viscous fluid produced by the secretory cells of the cervical crypt (Odeblad, 1997; Fernandez-Hermida et al., 2018), is a crucial element for human fertility, and is produced most prolifically in the days prior to ovulation (Billings et al., 1972; Moghissi, 1972; Ecochard et al., 2015). In response to highly coordinated series of hormonal fluctuations occurring within menstrual cycles (Ecochard et al., 2001; Jones and Lopez, 2006), the cervical mucus undergoes cyclic modifications throughout the cycle resulting in different biochemical and biophysical characteristics (Fernandez-Hermida et al., 2018), the latter readily observable by women through vulvar observations (Hilgers and Prebil, 1979; World Health Organization, 1981; Porucznik et al., 2014).
Previous studies that have used ultrasound, serum or urine hormones to assess cyclic changes in mucus quantity and quality have demonstrated the accuracy of observing changes in cervical mucus for identifying ovulation and the fertile window (fecund window), based on recognizing the appearance and disappearance of estrogenic quality mucus (World Health Organization, 1983b; Leader et al., 1985; Ecochard et al., 2001; Fehring, 2002; Stanford et al., 2003; Bigelow et al., 2004; Fehring et al., 2004; Alliende et al., 2005; Zinaman, 2006). In particular, the rise in estrogen before ovulation supports the secretion of estrogenic cervical mucus, which is observed by the woman as a discharge that is clear, stretchy, and or slippery (lubricative), known as peak type mucus (Billings et al., 1972; Hilgers and Prebil, 1979; Ecochard et al., 2015). The estrogenic quality of mucus is a strong indicator of the day-specific probability of pregnancy for intercourse on a specific day (Bigelow et al., 2004), and the overall mucus score for a menstrual cycle correlates strongly with cycle fecundability, for women without subfertility (Stanford et al., 2003). Clinically, women’s observations have been used successfully to aid in avoiding pregnancy (Peragallo Urrutia et al., 2018) or achieving pregnancy (Mu and Fehring, 2014; Stanford, 2015). However, there are limited data regarding the normal spectrum of cervical mucus patterns in the menstrual cycle across women of different reproductive characteristics (Colombo et al., 2006), and no prior studies have examined parity and age (strong correlates of fecundity) jointly in relation to cervical mucus patterns. There are also no published data on within woman cycle to cycle variability in cervical mucus secretion and the corresponding fertile window.
The objective of our study was to assess the normal spectrum (between women and within woman) of cervical mucus patterns, and cervical mucus indicators of the fertile window among women with no known subfertility and not currently taking exogenous hormonal treatments. We assessed the correlation of cervical mucus patterns with age, parity, race, recent oral contraceptive use (within 60 days), partial breast feeding, alcohol, and smoking. We further set out to understand whether cervical mucus patterns and the fertile window differed by parity and/or age after taking into account other factors that may impact fecundity, in particular, recent use of hormonal contraception or partial breast feeding. We also calculated cycle phase lengths for comparability with other studies of the menstrual cycle.
Materials and methods
Study design, data source and setting
This study is a pooled analysis of three cohorts of heterosexually active women who received instruction in the Creighton Model FertilityCare System (CrM) through CrM centers across USA and Canada, including ‘Creighton Model Effectiveness, Intentions, and Behaviors Assessment’ (CEIBA; 17 centers in 13 US states and Toronto, Canada; 2009–2013), a prospective cohort of women without known subfertility, aimed to evaluate and classify pregnancy rates and pregnancy intentions during use of the CrM (Stanford and Porucznik, 2017); ‘Creighton Model MultiCenter Fecundability Study’ (CMFS; 6 centers in 4 US states; 1990–1996), a retrospective cohort of presumably fertile and subfertile women using CrM, aimed to assess the relationship between vulvar mucus observations and the day and cycle–specific probabilities of conception (Stanford et al., 2003); and ‘Time to Pregnancy in Normal Fertility’ (TTP; single center in Utah; 2003–2006), a parallel-randomized trial, which aimed to assess the impact of CrM use on time to pregnancy in couples of proven fertility trying to conceive (Stanford et al., 2014). For CEIBA and CMFS, women were required at entry to the study to be seeking to avoid pregnancy; however, they were able at any point during the study follow-up to seek pregnancy (Stanford et al., 2003; Stanford and Porucznik, 2017).
Participants’ eligibility
From CEIBA, all participants; from CMFS, only data from the presumably fertile women; and from TTP, only participants in the CrM intervention group met the initial requirement for our study; i.e. women age 18–40 years, no history of subfecundity, and not breast feeding (CMFS and TTP), or if breast feeding, not doing so exclusively (CEIBA). All studies also required at least one normal menses since last use of hormonal contraception (Nassaralla et al., 2011; Girum and Wasie, 2018).
Eligible participants contributed a daily diary (CrM chart) for at least one full cycle, and up to one year (CMFS and CEIBA), or seven cycles (TTP). Earlier exit could occur due to pregnancy, withdrawal from study, loss to follow-up, or beginning hormonal contraception (Stanford et al., 2003; 2014; Stanford and Porucznik, 2017). The details of assembling the combined dataset have been published elsewhere (Najmabadi et al., 2020) (Supplementary Table SI).
CrM protocol
The CrM has a standardized protocol to teach women how to observe daily vaginal discharge from bleeding and cervical mucus at the vulva (not internally) during routine use of the bathroom. The CrM’s cervical mucus discharge recording system is based on mucus stretch, color, and sensation. Women record their observations for each of these mucus characteristics and bleeding each day in a daily diary (CrM chart). Women are also instructed how to use these observations to identify the estimated day of ovulation (EDO) and days when intercourse is likely to result in pregnancy (Hilgers et al., 1978; Nassaralla et al., 2011; Tham et al., 2012; Manhart et al., 2013; Stanford et al., 2014). For these three cohorts, the CrM charts were on paper and were collected by CrM teachers or study staff at least every month during the first three months, and at least every 3 months following (Hilgers and Prebil, 1979; Najmabadi et al., 2020).
Cycle parameters
Cycle phase lengths
The cycle length was defined as the number of days from the first day of menstrual bleeding, identified by the woman in the CrM chart, to the last day of the non-conception cycle before the start of the next menses (Reed and Carr, 2000; Mikolajczyk et al., 2010; Nassaralla et al., 2011; Najmabadi et al., 2020). We defined follicular (pre-ovulatory) phase length as the number of days from the first day of menstrual flow through the estimated day of ovulation (inclusive) in ovulatory cycles, irrespective of the occurrence of conception. Thus in our study, the estimated day of ovulation was counted as the last day of the follicular phase (Reed and Carr, 2000; Nassaralla et al., 2011; Najmabadi et al., 2020), and luteal (secretory) phase length included only days from the first day after the EDO through the last day of menstrual cycle, among ovulatory non-conception cycles.
Each day of each cycle was classified into one of three categories for mucus observation, except for days of moderate or heavy vaginal bleeding. The three categories were the presence of peak type mucus, the presence of non-peak type mucus (but no peak type mucus), and dry days without any mucus observed.
Days of peak type mucus
When a woman observed any mucus discharge that stretched over approximately 1 inch, was clear or partially clear in color, and/or had a sensation of lubrication, peak type mucus was considered to be present on that day. These qualities of mucus are associated with estrogen stimulation, in the absence of progesterone; i.e. estrogenic mucus (Hilgers and Prebil, 1979; Fehring, 2002; Bigelow et al., 2004; Ecochard et al., 2015).
Days of non-peak type mucus
When a woman observed any mucus discharge during the day, but never observed any of the three characteristics of peak type mucus, non-peak type mucus was considered to be present on that day.
Dry days
When a woman observed no mucus discharge, then the day was considered a dry day.
Estimated day of ovulation (mucus peak day)
According to the CrM protocol, a mucus peak day is the last day of any mucus discharge that is peak type, i.e. has estrogenic qualities: clear, stretchy, and/or slippery (lubricative). As the estrogen falls and/or the progesterone rises, these qualities disappear and the mucus ‘dries up’ (Billings et al., 1972). In some cycles, this pattern can repeat itself, resulting in two or more mucus peak days in a cycle (Brown, 2011). Also, some cycles have three or more days of non-peak mucus, without ever having peak type mucus, in which case the last day of non-peak mucus is considered a peak day which indicates the fall of estradiol (and possible rise of progesterone) (World Health Organization, 1983a). In cycles with ≥1 mucus peak day, woman usually identifies one of them as most likely to be the true cycle peak day or estimated day of ovulation. Prior research has established a high correlation of the peak day of cervical mucus in relation to the urinary surge of luteinizing hormone (Fehring, 2002), as well as ultrasound or other hormonal markers (Hilgers et al., 1978; Ecochard et al., 2001; Fehring, 2002; Porucznik et al., 2014; Ecochard et al., 2015; Stanford, 2015). We have also validated the expert-picked CrM mucus peak day in relation to urinary LH surge within one of the source cohorts for this study (TTP) (Stanford et al., 2020). In our study, all peak days were reviewed by at least two experts in the interpretation of CrM mucus records, including reference to information on the resulting luteal phase length and the consistency with other cycles from the same woman. Cycles without a plausible peak day of cervical mucus (3.5%) (Supplementary Table SI) were considered to be anovulatory and were excluded from this analysis (Najmabadi et al., 2020).
Mucus cycle score
To assess the quality of mucus objectively, we used the mucus score developed by Hilgers. Point values are assigned daily for consistency (four points for stretching on finger-test of about 1 inch or more, two points for a stretch less than that, zero points for no stretch), color (four points for clear, two for other color, zero for no color), sensation (four points for lubrication, zero for no lubrication), and change (four points for change to lubrication, two for change to nonlubrication, zero for change to dry). The daily score ranges from a minimum of 0 to a maximum of 16. The cycle mucus score is the arithmetic mean of the daily cumulative score over 6 days, beginning 5 days before the estimated day of ovulation (mucus peak day) and including the EDO (Hilgers, 1988). This score has been validated at the day level against microscopy (formation of channels for sperm transport and ferning) (Hilgers and Prebil, 1979), and has also been shown to correlate with day-specific probabilities of conception (Stanford et al., 2003). Further, when the score is averaged over the 6 days up to and including the day of ovulation, it correlates highly with cycle fecundability (Stanford et al., 2003). The score ranges from 0 to 16, with 16 indicating maximum fecundity (Hilgers, 1988; Stanford et al., 2003; Nassaralla et al., 2011).
Potentially fertile days
These are days identified to have a significant probability of pregnancy if intercourse were to occur on that day. They include most days with any mucus before the peak day (with some exceptions for prolonged unchanging mucus patterns), and the 3 days following any mucus peak day. They also include days of nonmenstrual bleeding or spotting (Hilgers and Prebil, 1979).
Non-fertile days
These are days identified to have a minimal probability of pregnancy if intercourse were to occur on that day. They include all dry days, except those that have nonmenstrual spotting or occur within 3 days after a mucus peak day, or within 3 days after nonmenstrual bleeding or spotting (Hilgers and Prebil, 1979).
Based on prior research and clinical experience, we designated clinically significant thresholds a priori for lower mucus production: cycles with ≤2 days of peak type mucus, ≤2 days of non-peak type mucus, a cervical mucus cycle score ≤5.0, or potentially fertile days ≤9 (Stanford et al., 2003; Nassaralla et al., 2011). Similarly, we designated thresholds for within woman cycle to cycle variability of >3 for days of peak type mucus, >4 days of non-peak type mucus, >8 days in potentially fertile days, and >4.0 difference in cervical mucus cycle score.
Statistical analysis
We used descriptive statistics to summarize women’s daily diary of cervical mucus observations and the related fertility status. We conducted stratified analyses of cervical mucus patterns with age, parity, race, recent oral contraceptive use (within 60 days), partial breast feeding, alcohol, and smoking (the latter two variables being available for only CEIBA and TTP). Linear mixed models were used to assess continuous cervical mucus parameters and generalized linear models using Poisson regression with robust variance were used to assess dichotomous outcomes, stratified simultaneously by the two covariates most well known to influence menstrual cycle function: parity (parous versus nulliparous) (Vollman, 1977; Jones and Lopez, 2006; Mishra et al., 2017), and age (≥30 years versus <30 years) (Meczekalski et al., 2016; Vollenhoven and Hunt, 2018). In a sensitivity analysis for age, we stratified women by a different age cut point (≥35 years versus <35 years). We chose random intercept models to account for variation in baseline cervical mucus characteristics in individual women and the correlation between cycles of the same woman. In our age and parity stratified assessments, we also adjusted for additional characteristics known to influence cycle function, also confirmed by our own bivariate analyses: partial breast feeding (Díaz et al., 1992), and use of oral contraceptives within the 60 days prior to the first day of the cycle (Gnoth et al., 2002; Nassaralla et al., 2011). For within woman cervical mucus variability, we subtracted the shortest from the longest value for each woman with at least two charted cycles.
We imputed missing mucus observations as dry days when the woman recorded the day as infertile, or the presence of light bleeding without explicitly noting the presence or absence of mucus. We also conducted a sensitivity analysis by repeating the analysis after dropping 741 cycles with imputed mucus type. There were also seven conception cycles (seven women) with unknown peak day. To impute these peak days, we used the peak day of the first previous cycle available. For one of these women with only one cycle, we used the population median peak day. We did not impute missing values for demographic and clinical factors examined in relation to cervical mucus parameters because of a very low proportion of missing values; in particular, our percent missing was as follows: age and breast feeding, zero missing; race and parity, <1% missing, oral contraceptives, <3% missing.
All statistical analyses were performed using SAS software (9.4—North Carolina).
Ethics approval
All studies were approved by the University of Utah Institutional Review Board (IRB), as well as local site IRBs.
Results
Analyzed data included 2488 ovulatory cycles among 528 women, followed for up to 1 year. Details of cycles excluded are shown in Supplementary Table SI, and the cycles used for each analysis are shown in Supplementary Table SII. The mean (SD) number of cycles contributed by each woman was 4.7 (3.5) cycles (median 4; range (R) 1–15; interquartile range (IQR) 2–7). One hundred one women (19.1%) contributed only 1 cycle. There were 158 (6.4%) conception cycles. In the adjusted models stratified by parity and age, a total of 18 women and 57 cycles were excluded because of missing parity (5 women, 32 cycles) or missing oral contraceptive history (13 women, 25 cycles).
Most women were non-Hispanic white (88.1%), <30 years of age (75.4%), with a mean (SD) age of 27.1 (4.1) years (median 27; IQR 24–29). Twenty-seven women were ≥35 years, 19 of them above 35 years. Most women were nulliparous (70.8%). Forty-four women (8.3%) had at least one prior obstetric or gynecologic surgery; most of these had a prior Caesarean section (29 women, 5.5%). The majority (78.6%) were employed, had a US college-level education (73.9%), or income >200% of the poverty level (76.3%). The demographic characteristics, and reproductive and medical history of participants are described further in Table I.
Table I.
Demographic and reproductive characteristics, and medical history of study population; 528 ovulatory women without known subfertility, 1990–2013.
| Total | No (%) 528 |
|---|---|
| Demographic characteristics | |
|
| |
|
Age (year) <30 • <20 • 20–24 • 25–29 ≥30 • 30–34 • ≥35 |
398 (75.4) 4 (0.8) 144 (27.3) 250 (47.3) 130 (24.6) 103 (19.5) 27 (5.1) Range: 18–40 IQR: 24–29 Median: 27 Mean (SD): 27.1 (4.1) Missing: 0 |
|
Race and ethnicity White (non-Hispanic) Hispanic/Latino Other Missing |
465 (88.1) 26 (4.9) 33 (6.3) 4 (0.8) |
|
Religion Catholic Protestant Latter-day Saint Other None Missing |
383 (72.5) 59 (11.2) 41 (7.8) 27 (5.1) 12 (2.3) 6 (1.1) |
|
Marital status Engaged Married Single/Other Missing |
154 (29.2) 320 (60.6) 50 (9.5) 4 (0.8) |
|
Completed education High school, vocational or technical school graduate or less Some college College graduate Missing |
39 (7.4) 93 (17.6) 390 (73.9) 6 (1.1) |
|
Employed Yes No Missing |
415 (78.6) 106 (20.1) 7 (1.3) |
|
Occupation Professional Clerical/Sales Homemaker Student Skilled and unskilled laborer/Other Missing |
268 (50.8) 58 (11.0) 73 (13.8) 71 (13.4) 50 (9.5) 8 (1.5) |
|
Income relative to US federal poverty level, adjusted by year <150% 150–200% >200% Missing |
44 (8.3) 38 (7.2) 403 (76.3) 43 (8.1) |
|
| |
| Reproductive history | |
|
| |
|
Age at first menstruation (year) ≤10 11–14 ≥15 Missing |
20 (3.8) 440 (83.3) 60 (11.4) 8 (1.5) |
|
Age at first pregnancy (year) Never pregnant ≤19 20–24 25–29 ≥30 Missing |
351 (66.5) 15 (2.8) 73 (13.8) 66 (12.5) 16 (3.0) 7 (1.3) |
|
Parity Nulliparous Parous • 1 • ≥2 Missing |
374 (70.8) 149 (28.3) 60 (11.4) 89 (16.9) 5 (0.9) |
|
Spontaneous abortion None At least one Missing |
477 (90.3) 43 (8.1) 8 (1.5) |
|
Breast feeding (partial) Yes No Missing |
23 (4.4) 505 (95.6) 0 (0.0) |
|
Recent use of oral contraceptives (OCs) ≤60 days prior to 1st day of 1st cycle in study >60 days prior to 1st day of 1st cycle in study or didn’t use OCs within past year Missing |
89 (16.9) 426 (80.7) 13 (2.5) |
|
| |
| Medical history | |
|
| |
|
Pelvic infection or sexually transmitted infection Yes No Missing |
26 (4.9) 500 (94.7) 2 (0.4) |
|
Vaginal infection (yeast infection) Yes No Missing |
204 (38.6) 323 (61.2) 1 (0.2) |
|
Cervical procedure, including cryotherapy, loop electrical excision, cauterization, colposcopy, biopsy Yes No Missing |
14 (2.7) 514 (97.3) 0 (0.0) |
|
Obstetrical/Gynecological surgery One or more procedures • Caesarean section • Dilation and curettage • Other, including laparoscopy No procedure Missing Current smokinga Yes No Missing Current alcohol consumptiona Yes No |
44 (8.3) 29 (5.5) 11 (2.1) 8 (1.5) 484 (91.7) 0 (0.0) 16 (5.8) 250 (90.2) 11 (4.0) 195 (70.4) 71 (25.6) |
| Missing | 11 (4.0) |
Not available for Creighton Model MultiCenter Fecundability Study (CMFS) 251 women (see Supplementary Table SI).
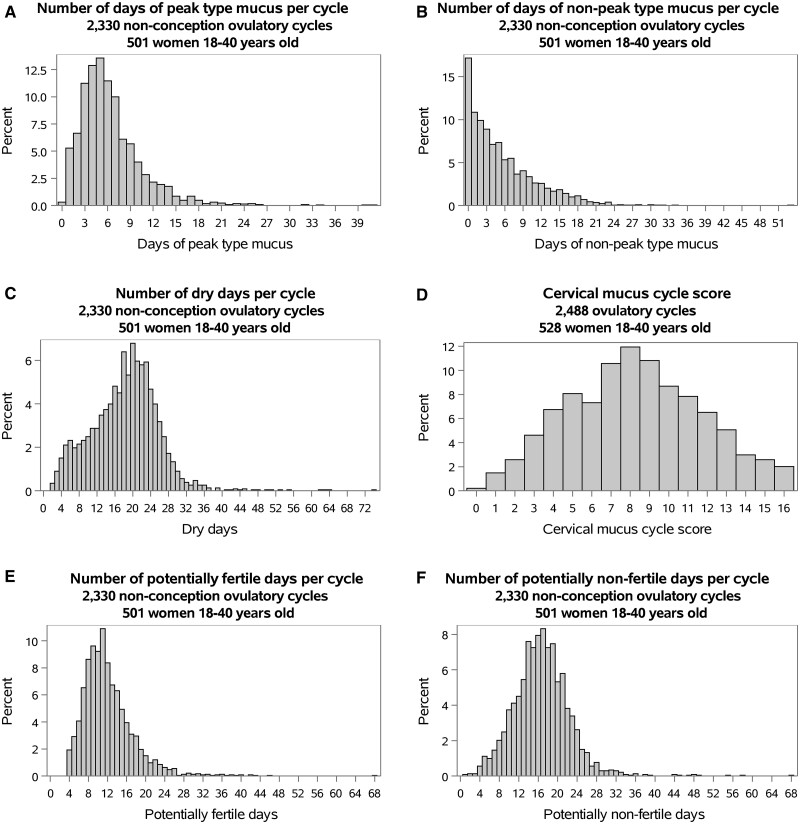
The overall distribution of all cycle parameters is displayed in Fig. 1. Mean, median, and percentiles of all cycle parameters are outlined in Supplementary Table SIII.
Figure 1.
Distribution of cervical mucus parameters.
Ovulation and cycle phase lengths
By selection, all study cycles were ovulatory. Only 8.6% of cycles had an estimated day of ovulation (peak day) on day 14; and 11.0% on day 15. The mean cycle length was 30.6 days (95% confidence interval (CI) 30.1, 31.1). The mean follicular phase was longer than the mean luteal phase, 18.8 days (95% CI 18.3, 19.3) versus 11.7 days (95% CI 11.5, 11.9). Women aged ≥30 had a shorter mean cycle length compared to women <30 years (29.4 versus 31.0 days, respectively), and a shorter follicular phase (17.8 versus 19.1 days, respectively; Supplementary Table SIV). In adjusted models, for nulliparous women aged ≥30 compared to nulliparous women <30, the follicular phase was shorter (17.4 versus 19.5 days, respectively). Among parous women, cycle lengths were similar for women <30 compared to ≥30 years. There were no differences in the luteal phase lengths by age or parity. Details are reported in Table II, stratified by age within parity, and for age and parity separately in Supplementary Table SIV.
Table II.
Adjusted cycle phase lengths and cervical mucus secretion patterns in 2488 ovulatory cycles of 528 women, by parity and agea.
| Self-reported cervical mucus characteristics (day) |
Unadjusted Total |
Adjusted
b
|
|||
|---|---|---|---|---|---|
| Nulliparous |
Parous |
||||
| Age <30 | Age ≥30 | Age <30 | Age ≥30 | ||
| Number of womenc | 528 | 311 | 63 | 83 | 66 |
| Number of cyclesc | 2488 | 1490 | 293 | 351 | 322 |
|
Mean (95% Confidence Interval) | |||||
| Length Characteristics | |||||
| Cycle length |
30.6 (30.1, 31.1) |
31.4 (30.6, 32.2) |
29.4* (27.9, 30.9) |
29.5 (27.8, 31.2) |
29.2 (27.4, 31.1) |
| Length of follicular phase |
18.8 (18.3, 19.3) |
19.5 (18.8, 20.3) |
17.4** (15.9, 18.9) |
17.9 (16.3, 19.5) |
18.0 (16.3, 19.8) |
| Length of luteal phase |
11.7 (11.5, 11.9) |
11.8 (11.4, 12.1) |
11.9 (11.3, 12.5) |
11.3 (10.6, 12.0) |
11.2 (10.4, 11.9) |
| Cervical Mucus Characteristics | |||||
| Days of peak type mucus |
6.4 (6.1, 6.8) |
6.4 (5.9, 6.9) |
5.3* (4.5, 6.2) |
8.7 (7.3, 10.1) |
8.1 (6.6, 9.6) |
| Days of non-peak type mucus |
5.4 (4.9, 5.8) |
5.7 (5.1, 6.3) |
4.1* (2.8, 5.3) |
5.4 (3.7, 7.0) |
6.6 (4.8, 8.4) |
| Dry days |
18.8 (18.2, 19.4) |
19.3 (18.4, 20.2) |
20.0 (18.2, 21.7) |
15.4 (13.5, 17.4) |
14.6 (12.5, 16.8) |
| Mucus peak days |
2.0 (1.9, 2.0) |
2.2 (2.1, 2.4) |
2.0 (1.7, 2.2) |
2.2 (1.9, 2.5) |
2.1 (1.8, 2.5) |
| Cervical mucus cycle score |
8.2 (7.9, 8.4) |
7.7 (7.3, 8.1) |
7.1 (6.3, 7.9) |
9.3 (8.4, 10.2) |
9.2 (8.2, 10.1) |
| Fertility Characteristics | |||||
| Potentially fertile days |
12.5 (12.1, 13.0) |
13.9 (13.3, 14.6) |
11.8** (10.6, 13.0) |
14.0 (12.4, 15.6) |
14.0 (12.3, 15.7) |
| Non-fertile days |
16.8 (16.4, 17.3) |
16.4 (15.7, 17.1) |
16.4 (15.0, 17.7) |
14.2 (12.7, 15.8) |
13.8 (12.1, 15.5) |
Linear mixed models were used to generate least square means.
Adjusted for partial breast feeding and recent use of oral contraceptives (see Table I). Five women (32 cycles) are excluded for missing parity and 13 women (25 cycles) are excluded for missing oral contraceptive history.
Number of cycles and women per variable is available in Supplementary Table SII.
0.01 < P < 0.05 for age comparison among nulliparous women.
P < 0.01 for age comparison among nulliparous women (There was no statistical significance within parous women.).
Cervical mucus type and quality score
Overall, cycles had more days identified as dry (no mucus) than days with peak type and non-peak type mucus, 18.8 days (95% CI 18.2, 19.4), 6.4 days (95% CI 6.1, 6.8), and 5.4 days (95% CI 4.9, 5.8), respectively. There were no differences by age alone (Supplementary Table SIV), but in adjusted models, nulliparous women age ≥30 years compared to nulliparous <30 had fewer mean number of days of peak type and non-peak type mucus: 5.3 days (95% CI 4.5, 6.2) versus 6.4 days (95% CI 5.9, 6.8), and 4.1 days (95% CI 2.8, 5.3) versus 5.7 days (95% CI 5.1, 6.3), respectively (Table II). Nulliparous women age ≥30 years compared to nulliparous <30 had 1.90 (95% CI 1.18, 3.06) higher prevalence ratio of a cycle with ≤2 days of peak type mucus, and 1.37 (95% CI 1.01, 1.87) prevalence ratio of cycles with ≤2 days of non-peak type mucus. There was no difference by age among parous women (Table III).
Table III.
Adjusted prevalence ratios for thresholds of cervical mucus parameters between and within women without known subfertility, stratified by parity and age.a
|
Self-reported
cervical mucus characteristics (day) |
Adjusted
b
|
|||||
|---|---|---|---|---|---|---|
|
Total proportion
|
Nulliparous
|
Parous
|
||||
| % (95% CI) | PR (95% CI) | PR (95% CI) | ||||
| Age < 30 (Reference) | Age ≥ 30 | Age < 30 (Reference) | Age ≥ 30 | |||
|
A: Between women
|
||||||
| Number of cycles c | 528 | 311 | 63 | 83 | 66 | |
| Number of women c | 2488 | 1490 | 293 | 351 | 322 | |
| Days of peak type mucus ≤2 | 12.2 (10.9, 13.6) | 1.90 (1.18, 3.06)** | 0.53 (0.19, 1.48) | |||
| Days of non-peak type mucus ≤2 | 37.9 (36.0, 39.9) | 1.37 (1.01, 1.87)* | 0.94 (0.60, 1.50) | |||
| Cervical mucus cycle score ≤5.0 | 20.4 (18.8, 22.0) | 1.45 (1.03, 2.05)* | 1.11 (0.56, 2.22) | |||
| Potentially fertile days ≤9 | 33.7 (31.8, 35.6) | 1.46 (1.12, 1.91)** | 0.89 (0.59, 1.34) | |||
|
B: Within woman
|
||||||
| Number of women c , d | 427 | 255 | 51 | 67 | 49 | |
| Most – fewest days of peak type mucus >3 days | 72.4 (68.1, 76.6) | 1.06 (0.90, 1.26) | 0.90 (0.69, 1.16) | |||
| Most – fewest days of non-peak type mucus >4 days | 54.1 (49.4, 58.8) | 0.78 (0.56, 1.09) | 1.18 (0.85, 1.65) | |||
| Greatest – least cervical mucus cycle score >4.0 | 73.3 (69.1, 77.5) | 1.02 (0.86, 1.21) | 1.01 (0.79, 1.28) | |||
| Most – fewest potentially fertile days >8 days | 49.7 (44.9, 54.4) | 1.09 (0.83, 1.44) | 1.05 (0.69, 1.61) | |||
Generalized linear models were used to generate prevalence ratios and 95% confidence interval (CI).
Adjusted for partial breast feeding and recent use of oral contraceptives (see Table I). Five women (32 cycles) are excluded for missing parity and 13 women (25 cycles) are excluded for missing oral contraceptive history.
Number of cycles and women per variable is available in Supplementary Table SII.
Among women who contributed minimum of two charted cycles.
0.01 < P < 0.05 for age comparison among nulliparous women.
P < 0.01 for age comparison among nulliparous women (There was no statistical significance within parous women.).
Supplementary Table SI.
Number of cycles and women excluded and included, by cohort: Creighton Model MultiCenter Fecundability Study (CMFS), Time to Pregnancy in Normal Fertility (TTP), Creighton Model Effectiveness, Intentions, and Behaviours Assessment (CEIBA).
|
CMFS (1990-1996)1 |
TTP (2003-2006)2 |
CEIBA (2009-2013)3 |
Total |
|
|---|---|---|---|---|
|
|
||||
| Number | ||||
|
Initial dataa Centers Women Cycles Days |
6 293 1827 56 076 |
1 50 169 5235 |
17 238 1328 40 671 |
23b 581 3324 101 982 |
|
| ||||
|
(Excluded cycles) |
||||
|
Anovulatory Women Cycles Days |
(1) (68) (1983) |
(0) (2) (119) |
(3) (45) (1651) |
(4) (115) (3753) |
|
Medications impacting cervical mucus Women Cycles Days |
(0) (12) (390) |
(4) (31) (952) |
(1) (5) (175) |
(5) (48) (1517) |
|
Streamlined data entry Women Cycles Days |
(41) (643) (19 898) |
(0) (1) (29) |
(3) (29) (933) |
(44) (673) (20 860) |
|
| ||||
|
Final data Centers Women Cycles Days |
6 251 1104 33 805 |
1 46 135 4135 |
17 231 1249 37 912 |
23b 528 2488c 75 852 |
Original cohorts data description has been published elsewhere.4
CMFS and CEIBA had one common center: St John’s Mercy Hospital—St Louis, Missouri.
Includes 158 (6.4%) conception cycles, dropped from some measurements (see Supplementary Table SII).
Stanford JB, Smith KR, Dunson DB. Vulvar mucus observations and the probability of pregnancy. Obstetrics and Gynecology 2003;101(6):1285-1293.
Stanford JB, Smith KR, Varner MW. Impact of instruction in the Creighton model fertilitycare system on time to pregnancy in couples of proven fecundity: results of a randomised trial. Paediatric and Perinatal Epidemiology 2014;28(5):391-399.
Stanford JB, Porucznik CA. Enrollment, Childbearing Motivations, and Intentions of Couples in the Creighton Model Effectiveness, Intentions, and Behaviors Assessment (CEIBA) Study. Frontiers in Medicine 2017;4:147.
Najmabadi S, Schliep KC, Simonsen SE, Porucznik CA, Egger MJ, Stanford JB. Menstrual bleeding, cycle length, and follicular and luteal phase lengths in women without known subfertility: A pooled analysis of three cohorts. Paediatric and Perinatal Epidemiology 2020;34(3):318-327.
The mean cervical mucus cycle score was 8.2 (95% CI 7.9, 8.4). For nulliparous women ≥30, it was 7.1 (95% CI 6.3, 7.9), while for parous women <30, it was 9.3 (95% CI 8.4, 10.2) (Table II). Nulliparous women age ≥30 years compared to nulliparous <30 had 1.45 (95% CI 1.03, 2.05) prevalence ratio of a cycle with cervical mucus score ≤5.0. There was no difference by age among parous women (Table III).
Potentially fertile and non-fertile days
The mean number of potentially fertile days in each cycle was 12.5 days (95% CI 12.1, 13.0). For nulliparous women ≥30, the number was 11.8 days (95% CI 10.6, 13.0), while for parous women <30, it was 14.0 days (95% CI 12.4, 15.6) (Table II). Nulliparous women age ≥30 years compared to nulliparous <30 had 1.46 (95% CI 1.12, 1.91) prevalence ratio of a cycle with the number of potentially fertile days ≤9. There was no difference by age among parous women (Table III).
Within woman variability
The proportion of women who displayed each of the a priori selected thresholds of variability for cervical mucus parameters was at least 50.0%. However, age and parity were not associated with within-woman variability for any of the cervical mucus parameters (Table III). Mean, median, and percentiles of within woman variability of cervical mucus parameters are outlined in Supplementary Table SV.
Recent oral contraceptive use, breast feeding
Cycles with recent oral contraceptive use, compared to cycles without recent oral contraceptive use, had a lower mucus cycle score (7.5 versus 8.3, respectively), but also slightly less clear mucus pattern, with more potentially fertile days (14.8 versus 12.4, respectively) and mucus peak days (2.4 versus 1.9 respectively; Supplementary Table SVI). Although not statistically significant, parous women who were partially breast feeding had more days of peak type mucus, compared to women not breast feeding (8.9 versus 7.2 days, respectively), as well as non-peak type mucus (6.7 versus 5.4 days) and more potentially fertile days (13.9 versus 12.8 days, respectively; Supplementary Table SVI).
Race, alcohol, smoking
Race did not have any association with cycle phase lengths or cervical mucus parameters (Supplementary Table SIV). Among the women for whom data were available (CEIBA and TTP), alcohol and tobacco use had minimal impact on cervical mucus parameters (Supplementary Table SVII).
Further analyses by age and parity
Cervical mucus parameters by more detailed age strata are reported in Supplementary Table SVIII. In addition, we found women aged ≥35 years compared to women <35 years had lower cervical mucus cycle score of 7.3 (95% CI 6.0, 8.6) versus 8.7 (95% CI 8.0, 9.4). They were also more likely to meet the a priori thresholds related to lower mucus production (Supplementary Table SIX). Finally, mutually adjusting for age, parity, recent OC use, and partial breast feeding, women ≥30 years versus women <30 had −1.32 days (95% CI −2.50, −0.15) shorter cycles, −1.19 days (95% CI −2.34, −0.05) shorter follicular phase, −0.86 (95% CI −1.64, −0.07) lower days of peak type mucus, and −1.28 days (95% CI −2.27, −0.30) lower potentially fertile days; while parous women versus nulliparous women had 1.31 days (95% CI 0.50, 2.12) more days of peak type mucus and a 1.00 point (95% CI 0.38, 1.63) higher cervical mucus score (Supplementary Table SX).
Sensitivity analysis for imputed mucus information
Dropping 741 imputed cycles for mucus type did not appreciably alter our findings (data not shown).
Discussion
While many studies have elucidated normal ranges of follicular, luteal, and total cycle lengths (Bull et al., 2019; Symul et al., 2019; Najmabadi et al., 2020), relatively few have investigated detailed clinical characteristics of cervical mucus, which reflects hormonal function and is a key clinical indicator of the fertile window (Moghissi, 1972; Colombo and Masarotto, 2000; Ecochard et al., 2001), day-specific probabilities of conception (Bigelow et al., 2004; Colombo et al., 2006), and cycle fecundability (Stanford et al., 2003). We assessed cervical mucus patterns of ovulatory cycles of women with no known subfertility, including between-women and within-woman variability of the parameters.
Fertile window
Ecochard et al. (2015) confirmed that the putative 6-day fertile window and 2-day ovulation window can be identified by women’s observations of cervical mucus. The fertile window has been commonly defined as the day of ovulation and preceding 5 days based on the seminal work of Wilcox and colleagues, but they also noted that they could not exclude a probability of conception of up to 12% on the day immediately before and the day immediately after this window (Wilcox et al., 1995). Other work has estimated the fertile window in different populations of as low as 1 day for a subgroup of subfertile couples, or up to 12 days in couples with higher levels of fecundity; these estimates also vary by the precision of the marker of ovulation, and the threshold set for a significant probability of conception (with higher sample sizes needed to detect marginal days with lower probabilities) (Colombo and Masarotto, 2000; Stanford et al., 2003; Keulers et al., 2007). In our analysis, the potentially fertile days (mean 12.1 days overall) were based on prior work identifying days with cervical mucus characteristics indicating any possibility of sperm transmission through the cervix (Hilgers and Prebil, 1979), which may overestimate the length of the ‘true’ fertile window. The days of peak type mucus (mean 6.4 days overall) are known to have the highest fertility (Bigelow et al., 2004; Ecochard et al., 2015), and give a narrower estimate of the fertile window, or the ‘most fertile’ days. Our current study adds greater insight into between-women variability of cervical mucus secretion, particularly by parity and age; as well as a normal variability of cervical mucus patterns between cycles of the same woman. Our analysis of the range of cervical mucus patterns among a relatively homogenous group of women without any conditions are known to impair reproduction can provide a normal baseline for future assessments in women with conditions impacting fertility and reproductive function.
Cycle phase lengths
In context, the cycle phase lengths in our study are consistent with prior studies. In the largest study to date of menstrual cycle phase lengths, the mean cycle length was 29.3 days; the study had a higher proportion of older women than our study (55% age 30 and older versus 25%), and also confirmed that older women had shorter mean cycle lengths (and shorter follicular phase lengths) than younger women (Bull et al., 2019). They also reported a mean follicular phase length 16.9 days (with the date of ovulation included in the follicular phase), and a mean luteal phase length 12.4 days, using basal body temperature as the ovulation marker, as compared to 18.8 and 11.7 days for the follicular and luteal phases in our study, respectively. The different ovulation markers contribute to the difference in phase lengths. The basal body temperature marker (last day before temperature rise) occurs mostly from 3 days before to 1 day after the day of ovulation by serial follicular ultrasound, while the cervical mucus peak day occurs mostly from 1 day before to 1 day after the ultrasound day of ovulation (Ecochard et al., 2001).
Variability in ovulatory cycles
Our results are consistent with prior research indicating that ovulatory cycles have a spectrum of ovulatory and hormonal function, so that ovulatory status is more accurately defined as a spectrum of function rather than a solely binary designation (Brown, 2011). This also leads to variability in the incidence of ovulation when it is identified by different biomarkers (Lynch et al., 2014). The variability of hormonal and reproductive function from cycle to cycle is a result of the perpetual interaction between the body and external environment affecting the ongoing function of the hypothalamic–pituitary–ovarian axis (Barron, 2007; Yavangi et al., 2013; Li et al., 2020). Our results suggest there is substantial within-woman variability in cycle fecundability, even in this group of women with high socioeconomic indicators.
Age and parity
Age is the single most important factor in determining a woman’s fertility potential (Meczekalski et al., 2016; Vollenhoven and Hunt, 2018). Peak fecundability in women occurs in the early to mid-20s and a steady decline is expected at older ages (Meczekalski et al., 2016; Wesselink et al., 2017). Consistent with this, we found reduced cervical mucus quantity and quality by age. However, our study had a limited number of women in the upper ages; only 27 women were age 35 or older.
Parity was the most consistent and impactful correlate of cervical mucus quantity and quality in our study. The most likely reason is that both parity and cervical mucus quality are both promoted by underlying fecundity. Our findings are consistent with previous studies showing that among nulliparous women, there is a stronger association between age and declining fecundability compared to parous women (Rothman et al., 2013; Wesselink et al., 2017). According to a recent pooled study of 51450 postmenopausal women from nine observational studies, nulliparity was associated with increased risk of premature menopause and early menopause (Jones and Lopez, 2006; Mishra et al., 2017).
Other clinical and demographic characteristics
We believe there is no reason to expect any biological impact of race on menstrual cycle function; however, race can sometimes indicate socioeconomic and generational environmental factors. We found no impact of race on cycle parameters, which may reflect the relatively high socioeconomic status of our study population. Although smoking is known to impact fecundity (Bolumar et al., 1996), we did not find an impact of smoking on cycle parameters. However, we had data on smoking available only for part of our study population, and the number of smokers was very low (only 16 women). In contrast, more women reported alcohol use (195 women), but we did not have detail on level and type of alcohol consumption that may be necessary to detect impacts on cycle function (Juhl et al., 2003). The impacts of recent oral contraceptive use (primarily lower cervical mucus quality) are consistent with previous work (Nassaralla et al., 2011). The higher number of days of all types of mucus for the 23 parous women who were breast feeding compared to parous women not breast feeding is consistent with other research indicating an increased number of days of mucus secretion during breast feeding (Kennedy et al., 1995; Bouchard et al., 2013).
Strengths and limitations
The cohort design with prospectively collected daily diaries using standardized notation is one of the major strengths of this study. By following women for up to a year, we were also able to assess the range of menstrual cycle parameter variability in the same woman across cycles, which is key to understanding normal reproductive and cycle function (Harlow and Ephross, 1995).
Our study is not without limitations. First, we cannot exclude the possibility that some women had unknown subfertility or undiagnosed gynecologic disorders. However, we believe the thorough inclusion/exclusion criteria of each of the studies minimize this potential misclassification bias. In addition, our study participants were geographically dispersed but relatively homogeneous with regard to race, ethnicity, income, and educational levels, which may limit the generalizability of the findings. We also could not evaluate the impact of diet or exercise on cycle parameters. Aging of the reproductive system may be impacted by health behaviors and life-style (Palomba et al., 2018).
Conclusion
In conclusion, women’s self-observed cervical mucus patterns can identify characteristics of the fertile window and reproductive function that vary by parity and age. There is also substantial within-woman intrinsic variability of the duration and quality of cervical mucus, consistent with normal within-woman variability in the hypothalamic–pituitary–ovarian axis. Assessing the patterns of cervical mucus provides additional insight into reproductive function beyond bleeding patterns alone. Future work should investigate the pattern of cervical mucus discharges among women with various reproductive or gynecologic pathologies.
Data availability
Data are available from authors upon reasonable request, subject to institutional and research ethics (IRB) approval.
Supplementary Material
Acknowledgements
The authors thank the Office of Cooperative Reproductive Health study teams and volunteers, in particular Becky Crockett, and the women and CrM teachers who participated in these three cohorts.
Authors’ roles
S.N. and J.B.S. conceived of the study and wrote the manuscript. S.N. conducted all analyses. K.C.S. and M.J.E. advised on statistical analyses. All authors revised the manuscript for important intellectual content.
Funding
Funding for the three cohorts analyzed was provided by the Robert Wood Johnson Foundation (CMFS), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (TTP), and the Office of Family Planning, Office of Population Affairs, Health and Human Services (CEIBA).
Conflict of interest
No competing interest declared.
References
- Alliende ME, Cabezon C, Figueroa H, Kottmann C.. Cervicovaginal fluid changes to detect ovulation accurately [Evaluation Studies Research Support, Non-U.S. Gov’t]. Am J Obstet Gynecol 2005;193:71–75. [DOI] [PubMed] [Google Scholar]
- Barron ML.Light exposure, melatonin secretion, and menstrual cycle parameters: an integrative review. Biol Res Nurs 2007;9:49–69. [DOI] [PubMed] [Google Scholar]
- Bigelow JL, Dunson DB, Stanford JB, Ecochard R, Gnoth C, Colombo B.. Mucus observations in the fertile window: a better predictor of conception than timing of intercourse. Hum Reprod 2004;19:889–892. [DOI] [PubMed] [Google Scholar]
- Billings EL, Brown JB, Billings JJ, Burger HG.. Symptoms and hormonal changes accompanying ovulation. Lancet 1972;1:282–284. [DOI] [PubMed] [Google Scholar]
- Bolumar F, Olsen J, Boldsen J, the European Study Group on nfertility Subfecundity. Smoking reduces fecundity: a European multicenter study on infertility and subfecundity. Am J Epidemiol 1996;143:578–587. [DOI] [PubMed] [Google Scholar]
- Bouchard T, Fehring RJ, Schneider M.. Efficacy of a new postpartum transition protocol for avoiding pregnancy. J Am Board Fam Med 2013;26:35–44. [DOI] [PubMed] [Google Scholar]
- Brown JB.Types of ovarian activity in women and their significance: the continuum (a reinterpretation of early findings). Hum Reprod Update 2011;17:141–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull JR, Rowland SP, Scherwitzl EB, Scherwitzl R, Danielsson KG, Harper J.. Real-world menstrual cycle characteristics of more than 600,000 menstrual cycles. NPJ Digit Med 2019;2:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo B, Masarotto G.. Daily fecundability: first results from a new data base. Demogr Res 2000;3:39. [PubMed] [Google Scholar]
- Colombo B, Mion A, Passarin K, Scarpa B.. Cervical mucus symptom and daily fecundability: first results from a new database. Stat Methods Med Res 2006;15:161–180. [DOI] [PubMed] [Google Scholar]
- Díaz S, Cárdenas H, Brandeis A, Miranda P, Salvatierra AM, Croxatto HB.. Relative contributions of anovulation and luteal phase defect to the reduced pregnancy rate of breastfeeding women. Fertil Steril 1992;58:498–503. [PubMed] [Google Scholar]
- Ecochard R, Boehringer H, Rabilloud M, Marret H.. Chronological aspects of ultrasonic, hormonal, and other indirect indices of ovulation. BJOG 2001;108:822–829. [DOI] [PubMed] [Google Scholar]
- Ecochard R, Duterque O, Leiva R, Bouchard T, Vigil P.. Self-identification of the clinical fertile window and the ovulation period. Fertil Steril 2015;103:1319–1325.e1313. [DOI] [PubMed] [Google Scholar]
- Fehring RJ.Accuracy of the peak day of cervical mucus as a biological marker of fertility. Contraception 2002;66:231–235. [DOI] [PubMed] [Google Scholar]
- Fehring RJ, Raviele K, Schneider M.. A comparison of the fertile phase as determined by the clearplan easy fertility monitor and self-assessment of cervical mucus. Contraception 2004;69:9–14. [DOI] [PubMed] [Google Scholar]
- Fernandez-Hermida Y, Grande G, Menarguez M, Astorri AL, Azagra R.. Proteomic markers in cervical mucus. Protein Pept Lett 2018;25:463–471. [DOI] [PubMed] [Google Scholar]
- Girum T, Wasie A.. Return of fertility after discontinuation of contraception: a systematic review and meta-analysis. Contracept Reprod Med 2018;3: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnoth C, Frank-Herrmann P, Schmoll A, Godehardt E, Freundl G.. Cycle characteristics after discontinuation of oral contraceptives. Gynecol Endocrinol 2002;16:307–317. [PubMed] [Google Scholar]
- Harlow SD, Ephross SA.. Epidemiology of menstruation and its relevance to women’s health. Epidemiol Rev 1995;17:265–286. [DOI] [PubMed] [Google Scholar]
- Hilgers TW.The objective assessment of the vulvar mucus cycle. Int Rev Nat Fam Plann 1988;12:250–256. [Google Scholar]
- Hilgers TW, Abraham GE, Cavanagh D.. Natural family planning. I. The peak symptom and estimated time of ovulation. Obstet Gynecol 1978;52:575–582. [PubMed] [Google Scholar]
- Hilgers TW, Prebil AM.. The ovulation method – vulvar observations as an index of fertility/infertility. Obstet Gynecol 1979;53:12–22. [PubMed] [Google Scholar]
- Jones RE, Lopez KH.. Human Reproductive Biology. 3rd edn. Cambridge, MA: Academic Press, 2006. [Google Scholar]
- Jozwik J, Kaluzna-Czaplinska J.. Current applications of chromatographic methods in the study of human body fluids for diagnosing disorders. Crit Rev Anal Chem 2016;46:1–14. [DOI] [PubMed] [Google Scholar]
- Juhl M, Olsen J, Andersen AM, Grønbaek M.. Intake of wine, beer and spirits and waiting time to pregnancy. Hum Reprod 2003;18:1967–1971. [DOI] [PubMed] [Google Scholar]
- Kennedy KI, Gross BA, Parenteau-Carreau S, Flynn AM, Brown JB, Visness CM.. Breastfeeding and the symptothermal method. Stud Fam Plann 1995;26:107–115. [PubMed] [Google Scholar]
- Keulers MJ, Hamilton CJ, Franx A, Evers JL, Bots RS.. The length of the fertile window is associated with the chance of spontaneously conceiving an ongoing pregnancy in subfertile couples. Hum Reprod 2007;22:1652–1656. [DOI] [PubMed] [Google Scholar]
- Leader A, Wiseman D, Taylor PJ.. The prediction of ovulation: a comparison of the basal body temperature graph, cervical mucus score, and real-time pelvic ultrasonography. Fertil Steril 1985;43:385–388. [DOI] [PubMed] [Google Scholar]
- Li K, Urteaga I, Wiggins CH, Druet A, Shea A, Vitzthum VJ, Elhadad N.. Characterizing physiological and symptomatic variation in menstrual cycles using self-tracked mobile-health data. NPJ Digit Med 2020;3:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch KE, Mumford SL, Schliep KC, Whitcomb BW, Zarek SM, Pollack AZ, Bertone-Johnson ER, Danaher M, Wactawski-Wende J, Gaskins AJ. et al. Assessment of anovulation in eumenorrheic women: comparison of ovulation detection algorithms. Fertil Steril 2014;102:511–518.e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manhart MD, Duane M, Lind A, Sinai I, Golden-Tevald J.. Fertility awareness-based methods of family planning: A review of effectiveness for avoiding pregnancy using SORT. Osteopath Fam Phys 2013;5:2–8. [Google Scholar]
- Meczekalski B, Czyzyk A, Kunicki M, Podfigurna-Stopa A, Plociennik L, Jakiel G, Maciejewska-Jeske M, Lukaszuk K.. Fertility in women of late reproductive age: the role of serum anti-Mullerian hormone (AMH) levels in its assessment. J Endocrinol Invest 2016;39:1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczyk RT, Louis GM, Cooney MA, Lynch CD, Sundaram R.. Characteristics of prospectively measured vaginal bleeding among women trying to conceive. Paediatr Perinat Epidemiol 2010;24:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra GD, Pandeya N, Dobson AJ, Chung HF, Anderson D, Kuh D, Sandin S, Giles GG, Bruinsma F, Hayashi K. et al. Early menarche, nulliparity and the risk for premature and early natural menopause. Hum Reprod 2017;32:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghissi KS.The function of the cervix in fertility. Fertil Steril 1972;23:295–306. [DOI] [PubMed] [Google Scholar]
- Mu Q, Fehring RJ.. Efficacy of achieving pregnancy with fertility-focused intercourse. MCN Am J Matern Child Nurs 2014;39:35–40. [DOI] [PubMed] [Google Scholar]
- Najmabadi S, Schliep KC, Simonsen SE, Porucznik CA, Egger MJ, Stanford JB.. Menstrual bleeding, cycle length, and follicular and luteal phase lengths in women without known subfertility: a pooled analysis of three cohorts. Paediatr Perinat Epidemiol 2020;34:318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassaralla CL, Stanford JB, Daly KD, Schneider M, Schliep KC, Fehring RJ.. Characteristics of the menstrual cycle after discontinuation of oral contraceptives. J Womens Health (Larchmt) 2011;20:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeblad E.Cervical mucus and their functions. Ir Coll Physicians Surg 26 1997;1:27–32. [Google Scholar]
- Palomba S, Daolio J, Romeo S, Battaglia FA, Marci R, La Sala GB.. Lifestyle and fertility: the influence of stress and quality of life on female fertility. Reprod Biol Endocrinol 2018;16:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peragallo Urrutia R, Polis CB, Jensen ET, Greene ME, Kennedy E, Stanford JB.. Effectiveness of fertility awareness-based methods for pregnancy prevention: a systematic review. Obstet Gynecol 2018;132:591–604. [DOI] [PubMed] [Google Scholar]
- Porucznik CA, Cox KJ, Schliep KC, Stanford JB.. Pilot test and validation of the peak day method of prospective determination of ovulation against a handheld urine hormone monitor. BMC Womens Health 2014;14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BG, Carr BR.The normal menstrual cycle and the control of ovulation.De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, et al. (eds.), Endotext. South Dartmouth, MA: MDText.com, Inc, 2000.
- Rothman KJ, Wise LA, Sørensen HT, Riis AH, Mikkelsen EM, Hatch EE.. Volitional determinants and age-related decline in fecundability: a general population prospective cohort study in Denmark. Fertil Steril 2013;99:1958–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford JB.Revisiting the fertile window. Fertil Steril 2015;103:1152–1153. [DOI] [PubMed] [Google Scholar]
- Stanford JB, Porucznik CA.. Enrollment, childbearing motivations, and intentions of couples in the Creighton Model Effectiveness, Intentions, and Behaviors Assessment (CEIBA) study. Front Med (Lausanne) 2017;4:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford JB, Schliep KC, Chang CP, O’Sullivan JP, Porucznik CA.. Comparison of woman-picked, expert-picked, and computer-picked Peak Day of cervical mucus with blinded urine luteinising hormone surge for concurrent identification of ovulation. Paediatr Perinat Epidemiol 2020;34:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford JB, Smith KR, Dunson DB.. Vulvar mucus observations and the probability of pregnancy. Obstet Gynecol 2003;101:1285–1293. [DOI] [PubMed] [Google Scholar]
- Stanford JB, Smith KR, Varner MW.. Impact of instruction in the Creighton model fertilitycare system on time to pregnancy in couples of proven fecundity: results of a randomised trial. Paediatr Perinat Epidemiol 2014;28:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symul L, Wac K, Hillard P, Salathe M.. Assessment of menstrual health status and evolution through mobile apps for fertility awareness. NPJ Digit Med 2019;2:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham E, Schliep K, Stanford J.. Natural procreative technology for infertility and recurrent miscarriage: outcomes in a Canadian family practice. Can Fam Physician 2012;58:e267–e274. [PMC free article] [PubMed] [Google Scholar]
- Vollenhoven B, Hunt S.. Ovarian ageing and the impact on female fertility. F1000Res 2018;7:1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollman RF.The menstrual cycle. Major Probl Obstet Gynecol 1977;7:1–193. [PubMed] [Google Scholar]
- Wesselink AK, Rothman KJ, Hatch EE, Mikkelsen EM, Sorensen HT, Wise LA.. Age and fecundability in a North American preconception cohort study. Am J Obstet Gynecol 2017;217:667 e661–667 e668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, Baird DD.. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med 1995;333:1517–1521. [DOI] [PubMed] [Google Scholar]
- World Health Organization. A prospective multicentre trial of the ovulation method of natural family planning. I. The teaching phase. Fertil Steril 1981;36:152–158. [PubMed] [Google Scholar]
- World Health Organization. A prospective multicentre trial of the ovulation method of natural family planning. III. Characteristics of the menstrual cycle and of the fertile phase. Fertil Steril 1983a;40:773–778. [PubMed] [Google Scholar]
- World Health Organization. Temporal relationships between indices of the fertile period. Fertil Steril. 1983b;39:647–655. [DOI] [PubMed] [Google Scholar]
- Yavangi M, Amirzargar MA, Amirzargar N, Dadashpour M.. Does Ramadan fasting has any effects on menstrual cycles? Iran J Reprod Med 2013;11:145–150. [PMC free article] [PubMed] [Google Scholar]
- Zinaman MJ.Using cervical mucus and other easily observed biomarkers to identify ovulation in prospective pregnancy trials. Paediatr Perinat Epidemiol 2006;20:26–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from authors upon reasonable request, subject to institutional and research ethics (IRB) approval.