Disappointing results in the humoral response after anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in kidney transplant recipients (KTRs) have emerged from the literature.1,2 In this context, exploring SARS-CoV-2-specific cellular response induced by vaccination is relevant.
We prospectively assessed the antibody (Ab) (with an immunoassay detecting Ab against the spike protein receptor-binding domain [RBD] [Elecsys anti-SARS-CoV-2, Roche Diagnostics GmbH, Mannheim, Germany—positive threshold >0.8 U/mL and upper limit of detection 250 U/mL]) and T-cell response (with a whole blood interferon-gamma [IFN-γ] release assay [IGRA] using the antigens of the SARS-CoV-2 spike protein to activate T cells, following manufacturer’s instructions [SARS-CoV-2 IGRA, Euroimmun, Lübeck, Germany—positive threshold >100 mIU/mL]) rates 1 mo after the second dose of the mRNA BNT162b2 vaccine (Pfizer-BioNTech), between April 17, 2021 and June 15, 2021, in a single-center cohort of KTRs. All patients signed informed consent and the study received institutional review board approval (B4032021000056).
Ninety KTRs were included (median age: 60 [range, 38–79] y, 48% female individual, and median time since transplantation: 102 [range, 9–440] mo). Fifty-one percent of patients were treated with an association of tacrolimus, mycophenolate, and steroids, and 24% received an antimetabolite-free regimen. Seven had a previous history of documented SARS-CoV-2 infection before vaccination.
One month after the second vaccine dose, 58 (64.4%) KTRs mounted a humoral response (mean [±SEM] anti-RBD Ab titer: 70.2 [±11.9] U/mL) and 29 (32.2%) displayed a cellular response (positive IGRA test) (mean [±SEM] IFN-γ value: 803.4 [±165.1] mUI/mL). Overall, 22 (24%) KTRs had both humoral and cellular responses, 7 (8%) had isolated cellular response, 36 (40%) had isolated humoral response, and 25 (28%) had no immune response.
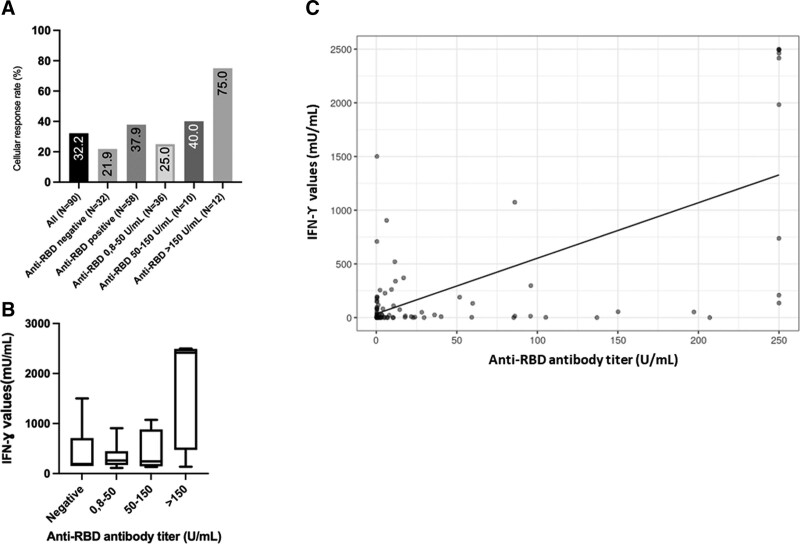
The cellular response rate and IFN-γ values were not significantly different between Ab responders and Ab nonresponders (Figure 1A). However, KTRs displaying high Ab response (anti-RBD Ab titer >150 U/mL) had significantly higher cellular response rate and IFN-γ values compared with KTRs with a weaker-or no-Ab response (Figure 1A and B). Correlation between IFN-γ and Ab values (Figure 1C) was statistically significant (r = 0.666, P < 0.001, Pearson correlation test).
FIGURE 1.
A, Cellular response rate in the cohort. The cellular response rate was not significantly different between patients with antibody response and without antibody response (P = 0.138, χ2 test). However, among patients with humoral response, those with high antibody response (anti-RBD titers >150 U/mL) showed higher rates of cellular response compared with others (P < 0.001, χ2 test). B, IFN-γ values (mIU/mL) and antibody titers. Patients with antibody titers >150 U/mL had higher IFN-γ values than others (P = 0.049, Kruskal-Wallis test). C, Correlation between anti-RBD antibody titers and IFN-γ values (r = 0.666, P < 0.001, Pearson correlation test). IFN-γ, interferon-gamma; RBD, receptor-binding domain.
Reports on the T-cell response rate after anti-SARS-CoV-2 vaccination in KTRs are limited. Bertrand et al3 found a 57.8% T-cell response rate in 26 KTRs after 2 doses of the BNT162b2 vaccine, using an ELISpot immunoassay. Cucchiari et al4 reported a 54.7% rate after the second dose of the mRNA-1273 vaccine in 117 SARS-CoV-2 naive transplant patients, also using an ELISpot immunoassay. We found a lower rate (32%) that might be explained by the differences in sensitivity of the ELISpot test compared with IGRA ELISA as reported for the detection of latent tuberculosis infection.5
Interestingly, we found that 20% of Ab nonresponders have a T-cell response and 72% of KTRs showed either IFN-γ or Ab response. Moreover, KTRs with higher Ab response are more likely to develop a T-cell response. In that context, a third vaccine dose might help boost both cellular and serological responses. Larger studies are needed to characterize the cellular response and its clinical relevance.
Footnotes
A.D., I.S.A., and H.G. are cofirst authors.
B.K. and N.K. are cosenior authors.
The authors declare no funding or conflicts of interest.
A.D., I.S.A., H.G., B.K., and N.K. participated in study design, data collection, and statistical analysis. A.D. and N.K. wrote the article. S.M., A.S., I.S.A., and B.K. participated in humoral and cellular tests. J.D.G. performed the statistical analysis. All authors took care of the patient and were involved in the organization of the vaccination of kidney transplant recipients. All authors discussed the results and approved the final version of the article.
REFERENCES
- 1.Georgery H, Devresse A, Yombi JC, et al. Disappointing immunization rate after two doses of the BNT162b2 vaccine in a Belgian cohort of kidney transplant recipients. Transplantation. [Epub ahead of print. June 24, 2021]. doi:10.1097/TP.0000000000003861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertrand D, Hamzaoui M, Lemée V, et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32:2147–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21:2727–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lange C, Mori T. Advances in the diagnosis of tuberculosis. Respirology. 2010;15:220–240. [DOI] [PubMed] [Google Scholar]