Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a new type of coronavirus causing coronavirus 2019 (COVID-19) that was first observed in Wuhan, China, in Dec. 2019. An inflammatory immune response targeting children appeared during the pandemic, which was associated with COVID-19 named multisystem inflammatory syndrome in children (MIS-C). Characteristics of MIS-C include the classic inflammation findings, multi-organ dysfunction, and fever as the cardinal feature. Up to now, no specific therapy has been identified for MIS-C. Currently, considerable progress has been obtained in the MIS-C treatment by cell therapy, specially Mesenchymal stem cells (MSCs). Unique properties have been reported for MSCs, such as various resources for purification of cell, high proliferation, self-renewal, non-invasive procedure, tissue regenerator, multidirectional differentiation, and immunosuppression. As indicated by a recent clinical research, MSCs have the ability of reducing disease inflammation and severity in children with MIS-C. In the present review study, the benefits and characteristics of MSCs and exosomes are discussed for treating patients with MIS-C.
Keywords: Coronavirus disease 2019, MIS-C, Mesenchymal stem cell, Exosomes
Abbreviations: COVID-19, Coronavirus disease 2019; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; MSC, Mesenchymal stem cell; ACE2, Angiotensin-converting enzyme 2; KD, Kawasaki disease; PCR, Polymerase chain reaction; TFH, T-follicular helper cells; SCF, Stem cell factor; ADA, Adenosine deaminase; IVIG, Intravenous immunoglobulin; TRALI, Transfusion-related acute lung injury; PGE2, Prostaglandin E2; IDO, Indoleamine 2,3-dioxygenase; ATP, Adenosine triphosphate; ADP, Adenosine diphosphate; AMP, Adenosine monophosphate; NK cells, Natural killer; DCs, Dendritic cells; ASC, Apoptosis-associated Speck-like Protein; ARDS, Acute respiratory distress syndrome; TSG-6, TNF stimulated gene-6; UK, United Kingdom; AKI, Acute kidney injury
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a new type of coronavirus causing coronavirus 2019 (COVID-19) that was first observed in Wuhan, China, in Dec. 2019, and the World Health Organization (WHO) reported it as a pandemic in March 2020. This pandemic still continues in the world. This virus causes the respiratory illness that devastates adults. On the contrary, in general, mild symptoms or no symptoms of COVID-19 have been observed in children, although severe illness has developed similar to adults. An inflammatory immune response targeting children appeared during the pandemic that was associated with COVID-19. A unique hyperinflammatory shock was reported by Riphagren et al. in April 2020 that happened in eight children in the United Kingdom (UK) [1], [2], [3], [4]. Other reports were presented in other regions of the UK and in other countries, such as USA. This new inflammatory process was called multisystem inflammatory syndrome in children (MIS-C) [5], [6], [7]. Emergence of further reports of MIS-C broadened the syndrome’s clinical spectrum, studies gradually indicated its immune landscape that might be helpful in our knowledge of this problem [8], [9], [10], [11]. As shown by available data, characteristics of MIS-C include the classic inflammation findings, fever as the cardinal feature, and multi-organ dysfunction, which affects heart, skin, and mucous membranes, as well as the respiratory, neurologic, and gastrointestinal systems [8]. Allison Ross Eckard et al. in their recent clinical study showed improvement of functional outcomes by administration of human MSCs in two children with diagnosed MIS-C [12]. In the present review work, the remedial capability of MSCs and their exosomes as tissue regenerator and immunomodulatory in MIS-C patients are discussed. The sources for this study were extracted in June 2021 using relevant keywords of study from scientific databases, such as Google scholar and PubMed.
2. Definition and pathogenesis of MIS-C
In the first days of the COVID-19 pandemic, it was thought that healthy children have mild SARS- CoV-2 infections with satisfactory outcomes. In April 2020, COVID-19 studies gradually indicated children with toxic shock syndrome and Kawasaki disease (KD) features. This recently found entity had various names, and finally, it was named MIS-C by Centers for Disease Control and Prevention (CDC) and the WHO [1], [13]. There are many shared clinical characteristics between KD and MIS-C. KD is an acute febrile disease observed in children and infants with the potential of developing coronary problems. These common characteristics are exanthema, high fever, cardiovascular involvement, and conjunctivitis. Almost 40–50% of cases with MIS-C meet the KD or partial KD definition. MIS-C and KD are notably different in terms of their laboratory markers, epidemiology, clinical findings, and imaging that can be helpful to differentiate them (Table 1 ). Epidemiologically, KD shows prevalence in Asian children, while MIS-C mainly influences Hispanics and Blacks [13], [14], [15]. Based on definition of the CDC, MIS-C means patients younger than 21 with laboratory inflammation evidence, fever for above 24 h, severe illness requiring hospitalization, positive SARS-CoV-2 infection (serology, RT-PCR, or antigen test), involvement of above two organ systems, and exposure to a case with COVID-19 during four weeks before initiation of symptom. Common clinical manifestations in MIS-C cases are conjunctivitis, rash, shock, myocardial dysfunction, and gastrointestinal symptoms [16].
Table 1.
Comparison in laboratory and clinical findings between KD and MIS-C.
| KD | MIS-C | |
|---|---|---|
| Age of patients | <5 years [14] | <21 years [24] |
| Involvement of mucous membrane | Yes [117] | Yes/No [118] |
| Rash | Yes [119] | Yes [120] |
| Hypotension | No [121] | Yes [122] |
| Respiratory involvement | No [123] | Yes [27] |
| Vomiting, diarrhea, abdominal pain | Rare [124] | Yes [125] |
| Myalgias | Rare/No [126] | Yes [127] |
| WBC differential | Neutrophilia [128] | Lymphopenia, Neutrophilia [129] |
| PT/PTT | Normal [130] | Abnormal [131] |
| D-dimer | Normal [132] | Increase [133] |
| Creatinine | Normal [134] | Increase [135] |
| CRP | Increase [136] | Intense increase [137] |
| Platelets | Increase [138] | Decrease [139] |
| Ferritin | Normal [140] | Increase [141] |
| Troponin | Increase [142] (in myocardial involvement) | Increase [143] |
| AST and ALT | Normal or increase | Normal or increase [144] |
| Pro-BNP | Normal [145] | Increase [146] |
It has been indicated that SARS-CoV-2 is binding to angiotensin converting enzyme 2 (ACE2) due to its spike glycoproteins (S proteins) on the surface, infecting human cells. The function of ACE2 in various human cells is as a cell receptor, e.g. in gastrointestinal tract, lung, heart, and kidney cells. Although such extensive distribution of virus receptors in human cells can be an explanation for virus to invade multiple organs, the exact organ damage mechanism, such as that in MIS-C, is not still clear. Various mechanisms have been proposed for explaining the MIS-C pathogenesis. Nevertheless, since there is not sufficient data in this regard, it is not precisely explained yet. Considering that we urgently need to understand the action mechanism of the disease, it is required to conduct prompt molecular studies with a large number of patients [17], [18], [19]. Although Tan et al. reported direct viral invasion as the primary mechanism of the myocyte damage found in COVID-19, initial data provided by studies in different countries suggests higher prevalence of SARS-CoV-2 antibody positivity compared to SARS-CoV-2 polymerase chain reaction (PCR) positivity in MIS-C patients. Considering this immunological profile in the patients, a strong post-infectious etiology is proposed for the disease rather than a direct viral invasion. Additionally, it has been proved that a maladaptive immune response is induced by antigen-presenting cells, like dendritic cells and infected macrophages, immediately following entrance of the virus to human cells, which results in a considerable pro-inflammatory cytokine release. Vascular leakage of fluids and immune system cells is caused by this cytokine storm through activation of the coagulation and complement cascades and release of inflammatory kinins [20], [21], [22], [23]. Hence, it seems that the primary mechanism of the organ damage in MIS-C patients is an antigen–antibody–mediated cytokine storm. For example, Kaushik et al. found that most patients with SARS-CoV-2 antibody positive reported increased inflammatory markers, like IL-6 and IL-1, without strong evidence to support the virus cardiac tropism. Therefore, they reported that MIS-C might be developed chiefly due to an antibody-mediated cytokine storm [15], [24], [25]. Another mechanism of action of the virus resulting in multi-organ damage is endothelial dysfunction due to direct viral infection. Endotheliitis findings due to SARS-CoV-2 were demonstrated by Varga et al. in three adult patients with several comorbidities. Colmenero et al. recently studied the skin biopsies taken from 7 children with chilblains during the pandemic, and immunohistochemical evidence of endotheliitis and viroid particles of SARS-CoV-2 were shown [26], [27], [28].
KD is well-known as a differential diagnosis of MIS-C, that is prevalently observed in the Asian population, and the SARSCoV-2 pandemic originated from Far East Asia. However, reports of MIS-C cases were chiefly from children in western countries. Based on this asymmetric and paradoxical ethnic distribution, it is suggested that there is a distinct genetic predisposition to MIS-C. In agreement with this hypothesis, a new mutation at D839 was recently observed in a European SARS-CoV-2 strain, causing an elevated ability for binding the S antigen to T-cell receptors, and it can probably explain the disease’s geographical shift [29], [30].
Additionally, according to previous research works, vascular damage can be induced by SARS-CoV-2. Then, with exposure of the circulating platelets to endothelium and collagen, they are turned in the active platelet form. It causes releasing the essential factors from activated platelets, such as serotonin, adenosine diphosphate, prothrombin, and thromboxane A2, for more activation of platelets. Also, there is a need for 12 coagulation factors for starting the clotting process in the arteries. Shortly, the factor XII is activated, leading to conversion of prothrombin to thrombin. In the end, fibrinogen is converted to fibrin that makes a network of fibrins at the damaged sites for clotting blood. Hence, blood clots might be observed in some patients with MIS-C [31].
3. Immunological aspects of MIS-C
As shown by primary studies, there are some differences in the distributions of CD4+ T cells’ subpopulations that is described by the expression of CD27 and CD45RO, and the T-follicular helper cells (TFH) frequency expressing the chemokine receptor CXCR within the hyperinflammatory immune response. Children suffering from MIS-C show lower total T cell frequencies than healthy children. There are similar subset distributions in the CD4+ T cell compartment in patients with MIS-C and children with mild SARS-CoV-2 infection, which indicates that differences observed related to normal children might be attributed to the SARS-CoV2 infection itself. Furthermore, children with SARS-CoV-2 showed a reduction was observed in TFH, which play a significant role in germinal center reactions and support B cell responses. This was seen in children with and without MIS-C. CD57 is marker of terminally differentiated effector CD4+ T cells. It has been indicated that these are decreased in adult cases with acute respiratory distress syndrome and severe acute COVID-19. On the contrary, children with MIS-C and mild COVID-19 could show high level of these terminally differentiated cell. Also, children with MIS-C show a significantly lower count of CD8+ T cells in comparison with children with mild SARS-CoV-2 infection. CD8+ T cells play a pivotal role in the removal of virus-infected cells. Studies on immune cell populations prove lymphopenia in patients with COVID-19. Decreased peripheral blood lymphocytes occur following the migration of cells to a site infected with the virus, although dysfunction of lymphocytes during disease has been observed; and directly related to disease severity. According to these findings, it is emphasized that the hyperinflammation observed in adult cases of severe acute COVID-19 is different from one observed in MIS-C. As shown by complementary works, increased IL-17A, IL-6, CXCL10 had the highest contribution to the cytokine storm. Maeckeret al. interestingly found some other factors contributing to hyperinflammation and pathogenesis of MIS-C, such as stem cell factor (SCF), adenosine deaminase (ADA), and TWEAK as a cytokine regulating multiple cellular responses, like angiogenesis, pro-inflammatory activity, and cell proliferation [32], [33], [34], [35], [36], [37], [38].
Researchers have identified a vital role for macrophages in inducing macrophage activation syndrome (MAS) in patients with MIS-C [39]. There is an association between the acute phase of MAS and evidently increased levels of pro-inflammatory cytokines. As a result of this cytokine storm, a cascade of inflammatory pathways is triggered. If it is not treated, it damages tissues, and could cause death [40]. In this regard, there is a hypothesis suggesting production of cocktail of cytokines, mainly TNF and different interleukins (that is, IL-1β, IL-18, IL-6) by monocytes/ macrophages, triggering a cascade of inflammatory pathways and causing a cytokine storm. It has been indicated that the pro-inflammatory cytokine environment, especially IL-6, reduce cytolytic functioning of NK cell. Cell-to-cell interactions are prolonged and a pro-inflammatory cytokine cascade is amplified as a result of the inability of cytolytic CD8 T cells and NK cells in lysing infected and otherwise activated antigen presenting cells (APCs) [41], [42], [43].
It has been shown that autoantibodies have implications in MIS-C, and there are two research works looking for autoreactive antibodies in plasma or serum of patients with MIS-C. Gruber et al. and Consiglio et al. used panels of human antigens (protein arrays) for screening for autoantibodies, and they showed the existence of autoantibodies in patients with acute MIS-C. As reported by Consiglio et al., they observed an increase in anti-MAP2K2 (mitogen-activated protein kinase 2), proteins of anti-casein kinase family (activated in SARS-CoV-2-infected cells), anti-endoglin (expressed on endothelial cells), and antibodies that react against protein antigens, mapping to lymphocyte activation and heart development pathways. Also, anti-La and anti-Jo antibodies were highlighted by Gruber et al., which are observed in autoimmune diseases, and antibody reactivity against proteins engaged in immune regulation, gastrointestinal biology, and endothelial cell function. Despite attractiveness of induction of autoimmune responses by SARS-CoV-2 infection as a hypothesis for explaining inflammation and tissue damage in MIS-C, the effect of autoantibodies on pathogenesis is still uncertain [11], [33].
4. Genetic and MIS-C
Because MIS-C is a newfound disease, genetic association and susceptibility to MIS-C exists is still unknown [44], [45]; however, Cron R and et al. demonstrated in a study that DOCK8 (Dedicator of cytokinesis 8) is a novel gene linked with cytokine storm in patients with COVID-19 and MIS-C. Heterozygous mutations in DOCK8 lead to cell lytic dysfunction of NK cells and increased pro-inflammatory cytokine release. DOCK8 is a large protein (190 kDa) contributed to intracellular signaling networks [46], [47]. In addition, Janet Chou et al. indicated in another study that haploinsufficiency of suppressor of cytokine signaling 1 (SOCS1), a kind of negative regulator of interferons, and defects in X-linked inhibitor of apoptosis (XIAP) as well as CYBB (Cytochrome b-245, beta subunit) are genetic-associated risk factors for MIS-C [48].
5. Prevalent therapies for MIS-C
At the time of publication of this paper, there has not been identified any definitive treatment for MIS-C. Studies in this regard are ongoing, with emerging new evidence related to treatment of this disease and new professional recommendations that are proposed. Firstly, there should be adequate supportive care for mild to moderate MIS-C cases [49]. SARS-CoV-2 pathogenesis implies that heightened coagulation activation is potentially worrying in MIS-C. Hence, it is necessary to use anticoagulation agents for both prophylaxis and treatment [50]. The first-tier treatments for MIS-C include steroids and intravenous immunoglobulin (IVIG). It is thought that IVIG enhanced production of antibody and diminish the inflammatory response, while the immune system is suppressed by steroids [51]. Available data implies that there is an association between treatment by simultaneous use of methylprednisolone and IVIG, instead of using IVIG alone, and better fever resolution [14]. There are various clinical trials demonstrating effectiveness and well-tolerance of immunoglobulin. However, some works have reported various adverse effects. Most of these events, like headache, flushing, fever, malaise, fatigue, lethargy, and chills mild and transient. Nevertheless, there are some infrequent side effects, such as renal impairment, arrhythmia, thrombosis, aseptic meningitis, transfusion-related acute lung injury (TRALI), and hemolytic anemia that are serious [52]. IL antagonists and antibiotics are other treatments. Generally, since this disease has not any definitive treatment and due to the side effects of present treatments, it is inevitable to use novel therapeutic approaches [53], [54].
6. Mesenchymal stem cells, and characteristics
Nowadays, we witness considerable progress in the treatment of diseases using cell therapy, especially stem cells, providing a bright horizon for patients with incurable diseases [55]. Unique properties have been identified for mesenchymal stem cells (MSCs), such as various sources for cell purification, high proliferation, self-renewal, non-invasive procedure, multidirectional differentiation, and immunosuppression. It is possible to transform these cells into chondrocytes, adipocytes, and osteocytes in the induction medium [56]. Compared to other available treatments, applying MSC therapy brings about various benefits. Access to them is higher, and it is possible to separate and purify them from several tissues, like the menstrual blood, umbilical cord blood, bone marrow, buccal fat pad, adipose tissues, fetal liver, dental pulp, etc. [57], [58], [59]. Some studies have characterized cultured MSCs using cell surface antigens or through investigating the differentiation potential of the cells. Minimal criteria were presented by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy in 2006, by which human multipotent mesenchymal stromal cells can be defined (abbreviated as “MSCs”): (1) the requirement for these multipotent mesenchymal stromal cells to be plastic-adherent when kept under standard culture conditions and forming CFU-Fs; (2) the requirement for these cells to express CD73, CD90, and CD105, and not expressing CD34, CD45, CD14, CD79a, CD11b, or CD19, and human leukocyte antigen-antigen D-related surface molecules; and (3) the requirement for these cells to be differentiated into adipocytes, chondroblasts, and osteoblasts in vitro [60], [61]. However, as indicated by results of other studies, STRO1 or CD146 could be unique markers for MSCs [62]. Many microvesicles and immunomodulatory molecules are produced by MSCs, prostaglandin E2 (PGE2), like nitric oxide, indoleamine 2,3-dioxygenase (IDO), exosomes, IL-6, and other surface markers, like PD-L1, PD-L2, and FasL [63], [64], [65], [66]. According to other studies, chemokines’ receptors from the CC group are found on the MSCs surface obtained from bone marrow, including CCR3, CCR1, CCR7, CCR9, CCR10, and from the CXC group, like CXCR4, CXCR3, CXCR5, and CXCR6 [67], [68].
7. Immunomodulatory effect of MSCs
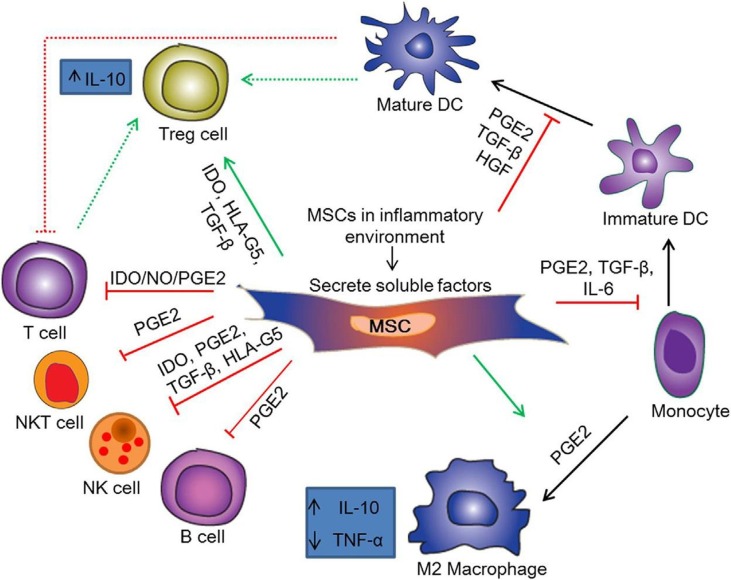
Immunomodulatory properties of MSCs are their significant functions. MSCs can regulate mechanisms of innate and adaptive immune response by modulating the secretion of inflammatory mediators and cellular responses (Fig. 1 ) [147] [69]. It has been indicated that there are receptors on MSCs surface obtained from bone marrow for complement components, like C5a and C3a, which are chemotactic factors for MSCs, increasing their resistance to oxidative stress and activating signal pathways in charge of anti-apoptotic and proliferation mechanisms. Also, it has been identified that there is protectin (CD59) expression on the MSCs surface, secreting factor H. The complement system is inhibited by both molecules that can partially provide protection for MSCs against damage by complement components. Moreover, it has been demonstrated that they have the ability of blocking complement-induced proliferation of peripheral blood mononuclear cells, as a mechanism of their anti-inflammatory function [70], [71].
Fig. 1.
Immunomodulatory mechanisms of MSCs. Abbreviations: HGF, Hepatocyte growth factor; NO, Nitric oxide; NKT cell, Natural killer T cell; HLA-G5, Human leukocyte antigen-G5. Figure is reused from Elsevier publisher.
There are many studies proving the ability of MSCs for regulating the activity of macrophages. MSCs interact with macrophages, promoting their polarization from the M1 pro-inflammatory phenotype to M2 anti-inflammatory phenotype cells, reducing the generation of pro-inflammatory cytokines, like IL-1, TNFα, IL-6, IFN-γ, IL12p70, and increasing release of anti-inflammatory cytokines, such as IL-12p40 and IL-10 [72], [73]. It is likely that overall suppressive impact of MSCs found in vivo is amplified by converting monocytes into M2 immunosuppressive macrophages. Monguio-Tortejada et al. confirmed these findings. In their work, it was indicated that umbilical cord-MSCs and human adipose tissue-MSCs cause promotion of polarization of monocytes toward regulatory M2 phenotype. They showed upregulation of expression of the CD73 and CD39 on monocytes in vitro by MSCs. The purinergic signaling is regulated by the CD73 and CD39 through the ATP/ADP hydrolysis to adenosine and AMP, respectively. Thus, the shifts are induced from the pro-inflammatory milieu caused by extracellular ATP into the anti-inflammatory environment controlled by adenosine [74].
Moreover, MSCs have the ability of modulating neutrophil activity. As shown by experiments, interleukin-6 generated by MSCs decreases the generation of reactive oxygen species by neutrophils, resulting in weakened unfavorable impacts of these cells [75]. Additionally, IDO is secreted by mesenchymal cells that is inhibitor of the stored a-denfensin production in secretory granules of neutrophils with pro-inflammatory properties. Microglia cells and macrophages are in turn simulated by prostaglandin-2 released by MSCs for the IL-10 production, which confines the neutrophils influx to damaged tissue. Also, secreted IL-10 works on endothelial cells, which reduces expression of E-selectin and inhibits migration of neutrophil to the injury area [76], [77].
According to previous studies, immunosuppressive effects have been identified for MSCs on natural killer (NK) cells. MSCs are inhibitors for the naturally cytotoxic cell proliferation through generating PGE-2 and IDO, which show synergistic activity. As indicated by other experiments, MSCs can release TGF-β as a factor for inhibition of NK cell proliferation. Moreover, production of IL-15 and IL-2 is limited by MSCs, resulting in decreased proliferation of NK cell and production of IFN-γ. Besides, MSCs can decrease the expression of the NK cell activating receptors, such as NKp44, NKG2D, and NKp30, contributing to the reduced cytotoxic activities of these cells and limiting the release of pro-inflammatory cytokines [66], [78].
The other immunomodulatory function of MSCs relates to dendritic cells (DCs). MSCs have interaction with DCs, which limits their functioning, like antigen presentation, maturation, and migration capacity. As confirmed by many experiments, MSCs decrease the expression of mature DCs markers, like MHC class II molecules, CD80, CD86, CD40, and have the ability of modulating the expression of “DCs deposition” markers in lymph nodes, such as CCR7 chemokines. As indicated by Li et al., cytokines generated by MSCs (e.g., IL-6) could mediate DC maturation regulation, it could be mediated by direct contact by the use of the Notch signaling pathway. Furthermore, under the MSCs influence, DCs increase the release of anti-inflammatory cytokines, like IL-10, reduce the generation of pro-inflammatory cytokines, like TNF-α and IL-12, and increase their phagocytic activity [79], [80], [81].
The activity of secondary immune response cells can be modulated by MSCs. Keeping a suitable balance between Th2 and Th1 phenotype CD4 T cells is one of the tasks of MSCs. As demonstrated by many in vivo and in vitro studies, MSCs has the ability of activating the alteration in CD4 T cells with a Th1 inflammatory phenotype, secreting IL-1β, IL-1α, IFN-γ, and TNF-α into Th2 anti-inflammatory phenotype cells generating IL-4, IL-3, IL-5, IL-10, and IL-13 [82], [83]. The TGF-β generated by MSCs has a crucial role to maintain a proper balance between the Th2, Th1, and Th17 helper lymphocytes and regulatory lymphocytes. Besides MSCs have an effect on B cells, which retain them in the G0/G1 phase and limit their chemotactic activities. According to previous studies, MSCs can decrease the differentiation, proliferation, and activity of B cells through the PD-1 signaling pathway [84], [85].
8. MSCs and tissue repair
Clinical findings indicate that multi-organ damage is one of the key and most important side effect of SARS-CoV-2 [86]. Nevertheless, tissue damage in COVID19-associated MIS-C cases have been confirmed by numerous clinical observations [87]. For example, BM-MSC mediate renal protection. With BM-MSC infusion in immune-deficient NOD/SCID mice with cisplatin-induced acute kidney injury (AKI), proximal tubular epithelial cell injury was decreased and impairment of renal function was reduced, resulting increased survival of the recipient. The intracarotid administration of MSC in an empirical model of reperfusion injury/renal ischemia significantly enhanced renal function, although MSC was present only momentarily in the renal vasculature, which confirms the MSC renoprotective action. Kidney's damaged cells of MSC-treated showed a reduction in gene expression of pro-inflammatory cytokines and an elevation in growth factors with pro-survival, mitogenic, and anti-apoptotic impacts [88], [89], [90]. In a study, high levels of complement system proteins, lymphocyte infiltration, and macrophage infiltration in tubulointerstitial sites were shown, where pro-inflammatory cytokines obtained from macrophages resulted in tubular injury, improving SARS-COV-2 cytotoxic effect [91]. MSCs can decrease TGF-β that plays a crucial role in fibrogenesis since it causes stimulation of ECM protein synthesis, stress fiber synthesis, and fibroblast proliferation, while enhances epithelial-mesenchymal transition of tubular cells and reduces matrix degradation. Moreover, tubulointerstitial fibrosis is improved by MSC. MSC also causes reduced expression of TGF-β, prevention of ZO-1 (a tight junction protein) degeneration in tubular epithelial cells, reduction of mesangial expansion, and suppression of excessive tubule dilatation in mice with diabetic nephropathy [92], [93], [94].
A cardiac pathophysiological mechanism has been suggested that is through direct myocardial injury because it seems that SARS-CoV-2 is able to infect pericytes, fibroblasts, and cardiomyocytes through the ACE2 pathway [95]. A cardioprotective role was found for MSCs in mice with Coxsackievirus B3-induced myocarditis. This role was imposed by inhibition of expressing Apoptosis-associated Speck-like Protein (ASC), caspase-1, NOD2, IL-18, IL-1β, and NLRP3 inflamassome in the left ventricle, recovering fibrosis and myocardial contractility. Also, they decreased oxidative stress, apoptosis, intracellular viral particles’ production, and expression of TNF-α mRNA, while activating the IFN-γ protective pathway and cardiac mononuclear cells [96], [97]. Besides, it has been shown that MSCs present useful effects in acute myocardial infarction models. MSCs had the ability of decreasing infarct size, activating resident cardiac stem cells, and preserving diastolic and systolic cardiac performance, which promote their migration, proliferation, and angiogenic capacity, leading to decreased fibrosis [98]. Clinical trials that involve the therapeutic application of MSCs in cardiac pathologies are in early stages yet. Employing mixed methodologies and source of MSCs, general safety has been shown. The most important challenges in this regard include low survival and low tissue retention following transplantation. Nevertheless, as suggested by preliminary findings, left ventricular function, questionnaire-evaluated symptoms and quality of life were improved by MSCs, despite the need for more robust surveys [99].
SARS-CoV-2 mostly affects the lungs and develops intra-alveolar fibrin with hyaline membrane, including interstitial fibroblasts and proliferative intra-alveolar, necrotizing bronchiolitis, and bronchopneumonia in some cases [100], [101]. As reported by numerous studies, lungs of COVID-19 patients showed diffuse alveolar damage with inflammatory cell infiltration in the alveolar cavity, such as monocytes, CD4+ T lymphocytes, macrophages, neutrophils, eosinophils, and alveolar wall thickening. Moreover, alveolar septum edema presented CD20 + B cells, CD68+ macrophages, type II pneumocyte hyperplasia, CD8+ T cells, and interstitial fibrosis, as well as SARS-CoV-2 inclusions in type II pneumocytes [102]. It has been demonstrated that MSCs have various mechanisms for improving resolution of Acute respiratory distress syndrome (ARDS) via their anti-apoptotic and anti-inflammatory impacts on host cells, which reduce lung alveolar epithelium permeability, enhance host mononuclear cell phagocytic activity, and increase alveolar fluid clearance. Alveolar edema and infiltration are among the vital mechanisms implicated in pathology of COVID-19, chiefly because of loss of epithelial selective permeability. Hence, recovering alveolar epithelial integrity with concomitant edema resolution is crucial [98], [103]. Ex vivo perfused human lungs have shown the capacity of MSCs for restoration of alveolar fluid clearance. Additionally, another mechanism of MSCs with the ability of enhancing epithelial regulation and integrity is through transfer of healthy mitochondria to epithelial cells, which reduce apoptosis and oxidative damage [104], [105]. There are some studies demonstrating TNF stimulated gene-6 (TSG-6) as another critical potent anti-inflammatory protein secreted by MSCs, which may have contribution to reducing cell counts and inflammatory cytokine in bronchoalveolar lavage fluid. It also can contribute to fibrosis resolution [106].
8.1. Tissue repair through MSCs-derived exosomes
MSCs have the ability of releasing small vesicles (with a dimension of 30 nm – 150 nm) named exosomes. Exosomes include nucleic acids
such as microRNA (miRNA) and mRNA and cellular proteins [107], [108], [109]. Since the discovery of exosome vesicles as a paracrine vesicle of the MSCs,
studies have investigated concerning their ability to find out the regenerative and mechanistic dimensions in treatment of diseases. Using MSC-derived exosomes as a cell-free therapy provides advantages over cell therapy including high stability low immunogenicity easy storage approaches and ability of crossing the blood–brain barrier [110]. One of the most serious risk factors for the COVID-19 is epithelial cell damage. Thus their generation and protection against unfavorable damage potentially through exosomes is necessary [111]. Bari et al. showed expression of Alpha-1-anti trypsin (AAT) by exosomes of mesenchymal cells on the surface. Neutrophil-derived proteolytic enzymes were inhibited by this structure and immune-regulating and anti-inflammatory effects were exhibited favored for the protection of lung epithelial cells [112]. Besides MSCs-derived exosomes are able to change pro-inflammatory macrophages phenotype to anti-inflammatory macrophages improving the unfavorable outcomes of the organ damage [113]. Thus various protocols have been considered by researchers for recovering the lungs pathogenicity related to COVID-19. As reported by these pre-clinical studies the lung tissue regeneration and maintenance are increased by the attendance of proteins and miR-145 in exosomes derived from MSCs. Moreover exosome vesicles can adjust the lung DCs function through overexpressing immune suppressing cytokines like IL10 and TGF-β. Hence the lungs are prohibited from the damaging macrophage cells and immune response associated with DCs [114], [115], [116]
9. Clinical study of MSCs in MIS-C: Case report
There is very limited evidence on the clinical application of MSCs in MIS-C. However, therapeutic effects of MSCs on two patients (10 and 4 years old) with MIS-C were studied by Allison Ross Eckard et al. Notably similar presentations were observed in two patients: both of them were healthy children before experiencing a disease with a duration of about 5 days, which consisted of gastrointestinal symptoms, generalized malaise, and high fever (39.0–40.6 °C). They suffered from severe clinical illness, which included hypotension, hemodynamic instability, shock requiring vasopressors, and acute kidney injury. Significant myocardial dysfunction was seen in both children, characterized mostly by reduced biventricular function. A marked elevation was observed in cardiac injury and/or congestion and/or coagulation biomarkers, and systemic inflammation. Both patients showed positive antibodies test against SARS-CoV-2 nucleocapsid and spike proteins. The children were treated with the existing standard of care MIS-C therapy during the first days of hospitalization, which included steroids, IVIG, anti-coagulants, and aspirin. Although the some improvement was observed in overall clinical status of the patients, many significant laboratory and clinical parameters stayed considerably abnormal. After treatment of patients with two intravenous doses (“two intravenous infusions were regarded and each injection contained 2 million cells per kg of body weight injected over 60 min”) of MSCs (derived from bone marrow), left ventricular ejection fraction was rapidly normalized, cardiac and systemic inflammation biomarkers were notably reduced, and clinical status was improved. The children did not experience adverse effects related to administration of MSCs. It appears that this treatment is a promising and new immunomodulatory cellular approach for children suffering from clinically significant cardiovascular manifestations of MIS-C [12].
10. Conclusion
Nowadays, COVID-19 pandemic has affected the world, and we can see it in the form of MIS-C as an emerging disease in children. Several organs could be damaged by this severe inflammatory disease, such as the heart, kidneys, and lungs, and if it is not treated, it can be lethal. Although it has been demonstrated that IVIG, anticoagulants, antibiotics, and supportive therapies can improve some of the symptoms, no definitive treatment is currently available for MIS-C. Cell-based therapy of MIS-C, particularly through derived exosomes and MSCs, has recently drawn the attention of many experts. Exosomes and MSCs are effective modulators of immune responses and repair of damaged tissues. Thus, they have critical role in improvement of the MIS-C symptoms. Nevertheless, further clinical studies are required for better understanding of its therapeutic facets.
Ethical approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
CRediT authorship contribution statement
Wanich Suksatan: Writing – original draft, Validation. Supat Chupradit: . Alexei Valerievich Yumashev: . Sahithya Ravali: . Mohammed Nader Shalaby: . Yasser Fakri Mustafa: . Anatoley Kurochkin: . Homayoon Siahmansouri: Conceptualization, Supervision, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. The Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dauletova M., Hafsan H., Mahhengam N., Zekiy A.O., Ahmadi M., Siahmansouri H. Mesenchymal stem cell alongside exosomes as a novel cell-based therapy for COVID-19: A review study. Clin. Immunol. 2021:108712. doi: 10.1016/j.clim.2021.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kadhom M., Al-Doori A.N., Ahmed D.S., Yousif E. Herbal medicine as an alternative method to treat and prevent COVID-19. Baghdad J. Biochem. Appl. Biol. Sci. 2021;2(01):1–20. [Google Scholar]
- 4.Al-Obaidi Z.M.J., Hussain Y.A., Ali A.A., Al-Rekabi M.D. The influence of vitamin-C intake on blood glucose measurements in COVID-19 pandemic. J. Infect. Dev. Countries. 2021;15(02):209–213. doi: 10.3855/jidc.13960. [DOI] [PubMed] [Google Scholar]
- 5.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F., et al. Multisystem inflammatory syndrome in US children and adolescents. N. Engl. J. Med. 2020;383(4):334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godfred-Cato S., Bryant B., Leung J., Oster M.E., Conklin L., Abrams J., et al. COVID-19–associated multisystem inflammatory syndrome in children—United States, March–July 2020. Morb. Mortal. Wkly Rep. 2020;69(32):1074. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonat B., Gorelik M., Boneparth A., Geneslaw A.S., Zachariah P., Shah A., et al. Multisystem inflammatory syndrome in children associated with coronavirus disease 2019 in a children’s hospital in New York city: patient characteristics and an institutional protocol for evaluation, management, and follow-up. Pediatr. Crit. Care Med. 2021;22(3) doi: 10.1097/PCC.0000000000002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abrams J.Y., Godfred-Cato S.E., Oster M.E., Chow E.J., Koumans E.H., Bryant B., et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J. Pediatr. 2020;226(45–54) doi: 10.1016/j.jpeds.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diorio C., Henrickson S.E., Vella L.A., McNerney K.O., Chase J., Burudpakdee C., et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS–CoV-2. J. Clin. Investig. 2020;130(11) doi: 10.1172/JCI140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dufort E.M., Koumans E.H., Chow E.J., Rosenthal E.M., Muse A., Rowlands J., et al. Multisystem inflammatory syndrome in children in New York State. N. Engl. J. Med. 2020;383(4):347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruber C.N., Patel R.S., Trachtman R., Lepow L., Amanat F., Krammer F., et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C) Cell. 2020;183(4) doi: 10.1016/j.cell.2020.09.034. 982–95.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckard A.R., Borow K.M., Mack E.H., Burke E., Atz A.M. Remestemcel-L Therapy for COVID-19–Associated Multisystem Inflammatory Syndrome in Children. Pediatrics. 2021;147(5) doi: 10.1542/peds.2020-046573. [DOI] [PubMed] [Google Scholar]
- 13.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. The Lancet. 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCrindle B.W., Rowley A.H., Newburger J.W., Burns J.C., Bolger A.F., Gewitz M., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 15.Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P., et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324(3):259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.E. Belay, E. Cheung, M. Oster, A. Tremoulet, Clinical management of multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19), 2020.
- 17.Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532(1–2):107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 18.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raheem R., Alsayed R., Yousif E., Hairunisa N. Coronavirus new variants: the mutations cause and the effect on the treatment and vaccination: Coronavirus new Variants: effect and treatments. Baghdad J. Biochem. Appl. Biol. Sci. 2021;2(02):70–78. [Google Scholar]
- 20.Cheung E.W., Zachariah P., Gorelik M., Boneparth A., Kernie S.G., Orange J.S., et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324(3):294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.T. Sakano, R. Rodrigues, A. Eisencraft, V. Carvalho, C. Schvartsman, R. AGADC, Multisystem inflammatory syndrome associated with COVID-19 from the pediatric emergency physician's point of view, 2021. [DOI] [PMC free article] [PubMed]
- 22.Tan W., Aboulhosn J. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. Int. J. Cardiol. 2020;309:70–77. doi: 10.1016/j.ijcard.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toubiana J., Poirault C., Corsia A., Bajolle F., Fourgeaud J., Angoulvant F., et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. bmj. 2020;369 doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaushik S., Aydin S.I., Derespina K.R., Bansal P.B., Kowalsky S., Trachtman R., et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection (MIS-C): a multi-institutional study from New York City. J. Pediatr. 2020;224:24–29. doi: 10.1016/j.jpeds.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waltuch T., Gill P., Zinns L.E., Whitney R., Tokarski J., Tsung J.W., et al. Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department. Am. J. Emerg. Med. 2020;38(10) doi: 10.1016/j.ajem.2020.05.058. 2246.e3–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colmenero I., Santonja C., Alonso-Riaño M., Noguera-Morel L., Hernández-Martín A., Andina D., et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br. J. Dermatol. 2020;183(4):729–737. doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riollano-Cruz M., Akkoyun E., Briceno-Brito E., Kowalsky S., Reed J., Posada R., et al. Multisystem inflammatory syndrome in children related to COVID-19: A New York City experience. J. Med. Virol. 2021;93(1):424–433. doi: 10.1002/jmv.26224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. The Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bittmann S., Weissenstein A., Luchter E., Moschüring-Alieva V.G., Villalon G. Multisystem inflammatory syndrome in children (MIS-C): The role of viral superantigens in COVID-19 disease. J. Allergy Infect. Dis. 2020;1(1):18–20. [Google Scholar]
- 30.Lin M.-T., Wu M.-H. The global epidemiology of Kawasaki disease: review and future perspectives. Global Cardiol. Sci. Pract. 2017;2017(3) doi: 10.21542/gcsp.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J., Hajizadeh N., Moore E.E., McIntyre R.C., Moore P.K., Veress L.A., et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J. Thromb. Haemost. 2020;18(7):1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anft M., Paniskaki K., Blazquez-Navarro A., Doevelaar A., Seibert F.S., Hoelzer B., et al. COVID-19 progression is potentially driven by T cell immunopathogenesis. medRxiv. 2020 [Google Scholar]
- 33.Consiglio C.R., Cotugno N., Sardh F., Pou C., Amodio D., Rodriguez L., et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183(4) doi: 10.1016/j.cell.2020.09.016. 968–81.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia S., Li C., Wang G., Yang J., Zu Y. The T helper type 17/regulatory T cell imbalance in patients with acute Kawasaki disease. Clin. Exp. Immunol. 2010;162(1):131–137. doi: 10.1111/j.1365-2249.2010.04236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maecker H., Varfolomeev E., Kischkel F., Lawrence D., LeBlanc H., Lee W., et al. TWEAK attenuates the transition from innate to adaptive immunity. Cell. 2005;123(5):931–944. doi: 10.1016/j.cell.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 36.Li T., Qiu Z., Zhang L., Han Y., He W., Liu Z., et al. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J. Infect. Dis. 2004;189(4):648–651. doi: 10.1086/381535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng H.-Y., Zhang M., Yang C.-X., Zhang N., Wang X.-C., Yang X.-P., et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sadeghi A., Tahmasebi S., Mahmood A., Kuznetsova M., Valizadeh H., Taghizadieh A., et al. Th17 and Treg cells function in SARS-CoV2 patients compared with healthy controls. J. Cell. Physiol. 2021;236(4):2829–2839. doi: 10.1002/jcp.30047. [DOI] [PubMed] [Google Scholar]
- 39.Poniecka A., Smolewska E. A fine line between macrophage activation syndrome and multisystem inflammatory syndrome in children–literature review based on two case reports. Reumatologia. 2021;59(1):47. doi: 10.5114/reum.2021.102871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canna S.W., Behrens E.M. Making sense of the cytokine storm: a conceptual framework for understanding, diagnosing, and treating hemophagocytic syndromes. Pediatric Clinics. 2012;59(2):329–344. doi: 10.1016/j.pcl.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kratochvill F., Neale G., Haverkamp J.M., Van de Velde L.-A., Smith A.M., Kawauchi D., et al. TNF counterbalances the emergence of M2 tumor macrophages. Cell Rep. 2015;12(11):1902–1914. doi: 10.1016/j.celrep.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peter J. Macrophage Polarization. Annu. Rev. Physiol. 2017;79(1):541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 43.Valizadeh H., Abdolmohammadi-Vahid S., Danshina S., Gencer M.Z., Ammari A., Sadeghi A., et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int. Immunopharmacol. 2020;89 doi: 10.1016/j.intimp.2020.107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen M.-R., Kuo H.-C., Lee Y.-J., Chi H., Li S.C., Lee H.-C., et al. Phenotype, susceptibility, autoimmunity, and immunotherapy between Kawasaki disease and coronavirus disease-19 associated multisystem inflammatory syndrome in children. Front. Immunol. 2021;12:276. doi: 10.3389/fimmu.2021.632890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esposito S., Principi N. Multisystem inflammatory syndrome in children related to SARS-CoV-2. Pediatric Drugs. 2021;23(2):119–129. doi: 10.1007/s40272-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cron R., Zhang M., Absher D., Bridges J., Schnell A., Bhatraju P., et al. BMJ Publishing Group Ltd; 2021. OP0314 DOCK8 Mutations IN COVID-19 AND MIS-C CYTOKINE STORM SYNDROME. [Google Scholar]
- 47.Su H.C. DOCK8 (Dedicator of cytokinesis 8) deficiency. Curr. Opin. Allergy Clin. Immunol. 2010;10(6):515. doi: 10.1097/ACI.0b013e32833fd718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chou J., Platt C.D., Habiballah S., Nguyen A.A., Elkins M., Weeks S., et al. Mechanisms underlying genetic susceptibility to multisystem inflammatory syndrome in children (MIS-C) J. Allergy Clin. Immunol. 2021;148(3) doi: 10.1016/j.jaci.2021.06.024. 732–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Philadelphia CsHo, Emergency Department, ICU, and inpatient clinical pathway for evaluation of possible multisystem inflammatory syndrome (MIS‐C), 2020.
- 50.Henderson L.A., Canna S.W., Friedman K.G., Gorelik M., Lapidus S.K., Bassiri H., et al. American college of rheumatology clinical guidance for pediatric patients with Multisystem Inflammatory Syndrome in Children (MIS-C) associated with SARS-CoV-2 and hyperinflammation in COVID-19. Version 1. Arthritis Rheumatol. (Hoboken Nj) 2020 doi: 10.1002/art.41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.AAo Pediatrics, Multisystem Inflammatory Syndrome in Children (MIS-C): Interim Guidance, 2020.
- 52.Guo Y., Tian X., Wang X., Xiao Z. Adverse effects of immunoglobulin therapy. Front. Immunol. 2018;9:1299. doi: 10.3389/fimmu.2018.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Belhadjer Z., Méot M., Bajolle F., Khraiche D., Legendre A., Abakka S., et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 54.Hennon T.R., Penque M.D., Abdul-Aziz R., Alibrahim O.S., McGreevy M.B., Prout A.J., et al. COVID-19 associated multisystem inflammatory syndrome in children (MIS-C) guidelines; a Western New York approach. Progr. Pediatr. Cardiol. 2020 doi: 10.1016/j.ppedcard.2020.101232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Golchin A., Farahany T.Z. Biological products: cellular therapy and FDA approved products. Stem Cell Rev. Rep. 2019;15(2):166–175. doi: 10.1007/s12015-018-9866-1. [DOI] [PubMed] [Google Scholar]
- 56.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 57.Fan X.-L., Zhang Y., Li X., Fu Q.-L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020;1–24 doi: 10.1007/s00018-020-03454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haider K.H., Aramini B. Mircrining the injured heart with stem cell-derived exosomes: an emerging strategy of cell-free therapy. Stem Cell Res. Ther. 2020;11(1):1–12. doi: 10.1186/s13287-019-1548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ullah M., Liu D., Thakor A. Mesenchymal stromal cell homing: mechanisms and strategies for improvement. iScience. 2019;15 doi: 10.1016/j.isci.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 61.Gharibi T., Ahmadi M., Seyfizadeh N., Jadidi-Niaragh F., Yousefi M. Immunomodulatory characteristics of mesenchymal stem cells and their role in the treatment of multiple sclerosis. Cell. Immunol. 2015;293(2):113–121. doi: 10.1016/j.cellimm.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 62.Gronthos S., Zannettino A.C., Hay S.J., Shi S., Graves S.E., Kortesidis A., et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J. Cell Sci. 2003;116(9):1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 63.Akiyama K., Chen C., Wang D., Xu X., Qu C., Yamaza T., et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10(5):544–555. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davies L.C., Heldring N., Kadri N., Le Blanc K. Mesenchymal stromal cell secretion of programmed death-1 ligands regulates T cell mediated immunosuppression. Stem Cells. 2017;35(3):766–776. doi: 10.1002/stem.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ren G., Zhao X., Zhang L., Zhang J., L'Huillier A., Ling W., et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J. Immunol. 2010;184(5):2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spaggiari G.M., Abdelrazik H., Becchetti F., Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood J. Am. Soc. Hematol. 2009;113(26):6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 67.Brooke G., Tong H., Levesque J.-P., Atkinson K. Molecular trafficking mechanisms of multipotent mesenchymal stem cells derived from human bone marrow and placenta. Stem Cells Dev. 2008;17(5):929–940. doi: 10.1089/scd.2007.0156. [DOI] [PubMed] [Google Scholar]
- 68.Honczarenko M.L.Y., Swierkowski M., Ghiran I., Glodek A.M., Silberstein L.E. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 69.English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol. Cell Biol. 2013;91(1):19–26. doi: 10.1038/icb.2012.56. [DOI] [PubMed] [Google Scholar]
- 70.Moll G., Jitschin R., Von Bahr L., Rasmusson-Duprez I., Sundberg B., Lönnies L., et al. Mesenchymal stromal cells engage complement and complement receptor bearing innate effector cells to modulate immune responses. PLoS ONE. 2011;6(7) doi: 10.1371/journal.pone.0021703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schraufstatter I.U., DiScipio R.G., Zhao M., Khaldoyanidi S.K. C3a and C5a are chemotactic factors for human mesenchymal stem cells, which cause prolonged ERK1/2 phosphorylation. J. Immunol. 2009;182(6):3827–3836. doi: 10.4049/jimmunol.0803055. [DOI] [PubMed] [Google Scholar]
- 72.François M., Romieu-Mourez R., Li M., Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2, 3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 2012;20(1):187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 73.Maggini J., Mirkin G., Bognanni I., Holmberg J., Piazzón I.M., Nepomnaschy I., et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS ONE. 2010;5(2) doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Monguió-Tortajada M., Roura S., Gálvez-Montón C., Franquesa M., Bayes-Genis A., Borràs F.E. Mesenchymal stem cells induce expression of CD73 in human monocytes in vitro and in a swine model of myocardial infarction in vivo. Front. Immunol. 2017;8:1577. doi: 10.3389/fimmu.2017.01577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raffaghello L., Bianchi G., Bertolotto M., Montecucco F., Busca A., Dallegri F., et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. 2008;26(1):151–162. doi: 10.1634/stemcells.2007-0416. [DOI] [PubMed] [Google Scholar]
- 76.Quinn K., Henriques M., Parker T., Slutsky A.S., Zhang H. Human neutrophil peptides: a novel potential mediator of inflammatory cardiovascular diseases. Am. J. Physiol.-Heart Circulat. Physiol. 2008;295(5):H1817–H1824. doi: 10.1152/ajpheart.00472.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van den Akker F., De Jager S., Sluijter J. Mesenchymal stem cell therapy for cardiac inflammation: immunomodulatory properties and the influence of toll-like receptors. Mediators Inflamm. 2013;2013 doi: 10.1155/2013/181020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao Z.-G., Cao Z., Xu W., Sun L., You Y., Li F., et al. Immune protection function of multipotent mesenchymal stromal cells: role of transforming growth factor-β1. Cancer Invest. 2012;30(9):646–656. doi: 10.3109/07357907.2012.721038. [DOI] [PubMed] [Google Scholar]
- 79.Djouad F., Charbonnier L.M., Bouffi C., Louis-Plence P., Bony C., Apparailly F., et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25(8):2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 80.Li Y.-P., Paczesny S., Lauret E., Poirault S., Bordigoni P., Mekhloufi F., et al. Human mesenchymal stem cells license adult CD34+ hemopoietic progenitor cells to differentiate into regulatory dendritic cells through activation of the Notch pathway. J. Immunol. 2008;180(3):1598–1608. doi: 10.4049/jimmunol.180.3.1598. [DOI] [PubMed] [Google Scholar]
- 81.Su W.R., Zhang Q.Z., Shi S.H., Nguyen A.L., Le A.D. Human gingiva-derived mesenchymal stromal cells attenuate contact hypersensitivity via prostaglandin E2-dependent mechanisms. Stem Cells. 2011;29(11):1849–1860. doi: 10.1002/stem.738. [DOI] [PubMed] [Google Scholar]
- 82.Bai L., Lennon D.P., Eaton V., Maier K., Caplan A.I., Miller S.D., et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57(11):1192–1203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Batten P., Sarathchandra P., Antoniw J.W., Tay S.S., Lowdell M.W., Taylor P.M., et al. Human mesenchymal stem cells induce T cell anergy and downregulate T cell allo-responses via the TH2 pathway: relevance to tissue engineering human heart valves. Tissue Eng. 2006;12(8):2263–2273. doi: 10.1089/ten.2006.12.2263. [DOI] [PubMed] [Google Scholar]
- 84.Peta K.T., Ambele M.A., Pepper M.S. Similarities between Tumour Immune Response and Chronic Wound Microenvironment: Influence of Mesenchymal Stromal/Stem Cells. J. Immunol. Res. 2021;2021 doi: 10.1155/2021/6649314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kong Q.-f., Sun B., Bai S.-s., Zhai D.-x., Wang G.-y., Liu Y.-m., et al. Administration of bone marrow stromal cells ameliorates experimental autoimmune myasthenia gravis by altering the balance of Th1/Th2/Th17/Treg cell subsets through the secretion of TGF-β. J. Neuroimmunol. 2009;207(1–2):83–91. doi: 10.1016/j.jneuroim.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 86.Tyagi S.C., Singh M. Multi-organ damage by covid-19: congestive (cardio-pulmonary) heart failure, and blood-heart barrier leakage. Mol. Cell. Biochem. 2021;476(4):1891–1895. doi: 10.1007/s11010-021-04054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Greene A.G., Saleh M., Roseman E., Sinert R. Toxic shock-like syndrome and COVID-19: Multisystem inflammatory syndrome in children (MIS-C) Am. J. Emerg. Med. 2020;38(11) doi: 10.1016/j.ajem.2020.05.117. 2492.e5–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morigi M., Introna M., Imberti B., Corna D., Abbate M., Rota C., et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26(8):2075–2082. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- 89.Tao J., Nie Y., Wu H., Cheng L., Qiu Y., Fu J., et al. Umbilical cord blood-derived mesenchymal stem cells in treating a critically ill COVID-19 patient. J. Infect. Dev. Countries. 2020;14(10) doi: 10.3855/jidc.13081. [DOI] [PubMed] [Google Scholar]
- 90.Togel F., Hu Z., Weiss K., Isaac J., Lange C., Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am. J. Physiol.-Renal Physiol. 2005;289(1):F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 91.Diao B., Wang C., Wang R., Feng Z., Zhang J., Yang H., et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat. Commun. 2021;12(1):1–9. doi: 10.1038/s41467-021-22781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gregorini M., Corradetti V., Rocca C., Pattonieri E.F., Valsania T., Milanesi S., et al. Mesenchymal stromal cells prevent renal fibrosis in a rat model of unilateral ureteral obstruction by suppressing the renin-angiotensin system via HuR. PLoS ONE. 2016;11(2) doi: 10.1371/journal.pone.0148542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kholia S., Herrera Sanchez M.B., Cedrino M., Papadimitriou E., Tapparo M., Deregibus M.C., et al. Mesenchymal stem cell derived extracellular vesicles ameliorate kidney injury in aristolochic acid nephropathy. Front. Cell Dev. Biol. 2020;8:188. doi: 10.3389/fcell.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nagaishi K., Mizue Y., Chikenji T., Otani M., Nakano M., Konari N., et al. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci. Rep. 2016;6(1):1–16. doi: 10.1038/srep34842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hendren N.S., Drazner M.H., Bozkurt B., Cooper L.T., Jr Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141(23):1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Au P., Tam J., Fukumura D., Jain R.K. Bone marrow–derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood J. Am. Soc. Hematol. 2008;111(9):4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miteva K., Pappritz K., Sosnowski M., El-Shafeey M., Müller I., Dong F., et al. Mesenchymal stromal cells inhibit NLRP3 inflammasome activation in a model of Coxsackievirus B3-induced inflammatory cardiomyopathy. Sci. Rep. 2018;8(1):1–16. doi: 10.1038/s41598-018-20686-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Walter J., Ware L.B., Matthay M.A. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respirat. Med. 2014;2(12):1016–1026. doi: 10.1016/S2213-2600(14)70217-6. [DOI] [PubMed] [Google Scholar]
- 99.Guo Y., Yu Y., Hu S., Chen Y., Shen Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020;11(5):1–10. doi: 10.1038/s41419-020-2542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sekulic M., Harper H., Nezami B.G., Shen D.L., Sekulic S.P., Koeth A.T., et al. Molecular detection of SARS-CoV-2 infection in FFPE samples and histopathologic findings in fatal SARS-CoV-2 cases. Am. J. Clin. Pathol. 2020;154(2):190–200. doi: 10.1093/ajcp/aqaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vasquez-Bonilla W.O., Orozco R., Argueta V., Sierra M., Zambrano L.I., Muñoz-Lara F., et al. A review of the main histopathological findings in the Coronavirus Disease 2019 (COVID-19) Hum. Pathol. 2020 doi: 10.1016/j.humpath.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang H., Zhou P., Wei Y., Yue H., Wang Y., Hu M., et al. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann. Intern. Med. 2020;172(9):629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Laffey J.G., Matthay M.A. Fifty years of research in ARDS. Cell-based therapy for acute respiratory distress syndrome. Biology and potential therapeutic value. Am. J. Respir. Crit. Care Med. 2017;196(3):266–273. doi: 10.1164/rccm.201701-0107CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ahmad T., Mukherjee S., Pattnaik B., Kumar M., Singh S., Kumar M., et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014;33(9):994–1010. doi: 10.1002/embj.201386030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee J.W., Krasnodembskaya A., McKenna D.H., Song Y., Abbott J., Matthay M.A. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am. J. Respir. Crit. Care Med. 2013;187(7):751–760. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee R.H., Pulin A.A., Seo M.J., Kota D.J., Ylostalo J., Larson B.L., et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang-Doran I., Zhang C.-Y., Vidal-Puig A. Extracellular vesicles: novel mediators of cell communication in metabolic disease. Trends Endocrinol. Metab. 2017;28(1):3–18. doi: 10.1016/j.tem.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 108.Alzahrani F.A., Saadeldin I.M., Ahmad A., Kumar D., Azhar E.I., Siddiqui A.J., et al. The potential use of mesenchymal stem cells and their derived exosomes as immunomodulatory agents for COVID-19 patients. Stem Cells Int. 2020;2020 doi: 10.1155/2020/8835986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Malekpour K., Hazrati A., Zahar M., Markov A., Zekiy A.O., Navashenaq J.G., et al. The potential use of mesenchymal stem cells and their derived exosomes for orthopedic diseases treatment. Stem Cell Rev. Rep. 2021:1–19. doi: 10.1007/s12015-021-10185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yin K., Wang S., Zhao R.C. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomarker Res. 2019;7(1):8. doi: 10.1186/s40364-019-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li J., Wei L., Han Z., Chen Z. Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. Eur. J. Pharmacol. 2019;852:68–76. doi: 10.1016/j.ejphar.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 112.Bari E., Ferrarotti I., Di Silvestre D., Grisoli P., Barzon V., Balderacchi A., et al. Adipose mesenchymal extracellular vesicles as Alpha-1-Antitrypsin physiological delivery systems for lung regeneration. Cells. 2019;8(9):965. doi: 10.3390/cells8090965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.He X., Dong Z., Cao Y., Wang H., Liu S., Liao L., et al. MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Int. 2019;2019 doi: 10.1155/2019/7132708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hao Q., Gudapati V., Monsel A., Park J.H., Hu S., Kato H., et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Decrease Lung Injury in Mice. J. Immunol. 2019;203(7):1961–1972. doi: 10.4049/jimmunol.1801534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim J., Yang Y.L., Jeong Y., Jang Y.-S. Middle East respiratory syndrome-coronavirus infection into established hDDP4-transgenic mice accelerates lung damage via activation of the pro-inflammatory response and pulmonary fibrosis. J. Microbiol. Biotechnol. 2020;30(3):427–438. doi: 10.4014/jmb.1910.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zeng S.L., Wang L.H., Li P., Wang W., Yang J. Mesenchymal stem cells abrogate experimental asthma by altering dendritic cell function. Mol. Med. Rep. 2015;12(2):2511–2520. doi: 10.3892/mmr.2015.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Labé P., Ly A., Sin C., Nasser M., Chapelon-Fromont E., Saïd P.B., et al. Erythema multiforme and Kawasaki disease associated with COVID-19 infection in children. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Simon H., Sakano T.M.S., Rodrigues R.M., Eisencraft A.P., Carvalho V.E.Ld., Schvartsman C., et al. Multisystem inflammatory syndrome associated with COVID-19 from the pediatric emergency physician's point of view☆. Jornal de pediatria. 2021;97:140–159. doi: 10.1016/j.jped.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sokolovsky S., Soni P., Hoffman T., Kahn P., Scheers-Masters J. COVID-19 associated Kawasaki-like multisystem inflammatory disease in an adult. Am. J. Emerg. Med. 2021;39 doi: 10.1016/j.ajem.2020.06.053. 253.e1–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sindhu C., Francis B., George S., Mariyath O.R., Peethambaran G., Shams S. Erythema multiforme-like rash as a manifestation of multisystem inflammatory syndrome in children. J. Skin Sex. Transmitted Dis. 2021:1–3. [Google Scholar]
- 121.Aung H.H., Nulman O., Nadroo I., Chhabra M. Case Series of Clinical Findings of Multi-System Inflammatory Syndrome in Children in Contrast to Kawasaki Disease. Cureus. 2021;13(7) doi: 10.7759/cureus.16446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Okarska-Napierała M., Mańdziuk J., Feleszko W., Stelmaszczyk-Emmel A., Panczyk M., Demkow U., et al. Recurrent assessment of lymphocyte subsets in 32 patients with multisystem inflammatory syndrome in children (MIS-C) Pediatr. Allergy Immunol. 2021 doi: 10.1111/pai.13611. [DOI] [PubMed] [Google Scholar]
- 123.Peng Y., Liu X., Duan Z., Cai S., Duan J., Zhou Y. Age-related differences in clinical characteristics of Kawasaki disease. Braz. J. Med. Biol. Res. 2021;54 doi: 10.1590/1414-431X202010281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.He T., Yang Z., Wang X., Yang J. Kawasaki disease associated pulmonary involvement in infants. Pediatr. Pulmonol. 2021 doi: 10.1002/ppul.25596. [DOI] [PubMed] [Google Scholar]
- 125.Matic K.M. SARS-CoV-2 and Multisystem Inflammatory Syndrome in Children (MIS-C) Curr. Problems Pediatr. Adolescent Health Care. 2021:101000. doi: 10.1016/j.cppeds.2021.101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marino A., Varisco T., Quattrocchi G., Amoroso A., Beltrami D., Venturiello S., et al. Children with Kawasaki disease or Kawasaki-like syndrome (MIS-C/PIMS) at the time of COVID-19: are they all the same? Case series and literature review. Reumatismo. 2021;73(1):48–53. doi: 10.4081/reumatismo.2021.1331. [DOI] [PubMed] [Google Scholar]
- 127.S. Öcal Demir, Ö. Tosun, K. Öztürk, M. Duyu, A. Bucak, G. Akkus, et al., SARS-CoV-2 associated multisystem inflammatory syndrome in children (MIS-C). A single center's experience, 2021. [DOI] [PubMed]
- 128.Nakada T. Useful predictors of Kawasaki disease without complications before initial acute-phase treatment. GSC Adv. Res. Rev. 2021;7(3):018–27. [Google Scholar]
- 129.C. Karagol, A.K. Tehci, A. Gungor, Z.E. Tekin, E. Çelikel, F. Aydın, et al. Delta Neutrophil Index: A Potential Diagnostic Marker of Multisystem Inflammatory Syndrome in Children (MIS-C), 2021. [DOI] [PMC free article] [PubMed]
- 130.Holstein B. Multisystem Inflammatory Syndrome in Children. J. Nurse Practition. 2021 doi: 10.1016/j.nurpra.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kashyap H., Kumar R.S., Gautam S., Gupta A., Gupta S., Tiwari P.K. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with COVID-19 Infection. Indian J. Pediatr. 2021;1 doi: 10.1007/s12098-021-03832-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Muto T., Nakamura N., Masuda Y., Numoto S., Kodama S., Miyamoto R., et al. Serum Free Carnitine Levels in Children with Kawasaki Disease. Pediatr. Int. 2021 doi: 10.1111/ped.14849. [DOI] [PubMed] [Google Scholar]
- 133.Al-Ghafry M., Vagrecha A., Malik M., Levine C., Uster E., Aygun B., et al. Multisystem inflammatory syndrome in children (MIS-C) and the prothrombotic state: Coagulation profiles and rotational thromboelastometry in a MIS-C cohort. J. Thromb. Haemost. 2021 doi: 10.1111/jth.15340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ashraf S., Abbasi F.S., Atiq M. Kawasaki Shock Syndrome and Covid-19. Platelets. 2021;13(74):22. doi: 10.29271/jcpsp.2021.Supp2.S135. [DOI] [PubMed] [Google Scholar]
- 135.C. Zhou, Y. Zhao, X. Wang, Y. Huang, X. Tang, L. Tang, Laboratory parameters between multisystem inflammatory syndrome in children and Kawasaki disease, 2021. [DOI] [PubMed]
- 136.Nakada T. Kawasaki disease with low C-reactive protein levels. World J. Adv. Res. Rev. 2021;10(2):241–245. [Google Scholar]
- 137.Zhao Y., Yin L., Patel J., Tang L., Huang Y. The inflammatory markers of multisystem inflammatory syndrome in children (MIS-C) and adolescents associated with COVID-19: A meta-analysis. J. Med. Virol. 2021;93(7):4358. doi: 10.1002/jmv.26951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Park J.H., Choi H.J. Clinical implications of thrombocytosis in acute phase Kawasaki disease. Eur. J. Pediatr. 2021;180(6):1841–1846. doi: 10.1007/s00431-021-03966-8. [DOI] [PubMed] [Google Scholar]
- 139.Kok E.Y., Srivaths L., Grimes A.B., Vogel T.P., Sexson Tejtel S.K., Muscal E. Immune thrombocytopenia following multisystem inflammatory syndrome in children (MIS-C)–a case series. Pediatr. Hematol. Oncol. 2021:1–8. doi: 10.1080/08880018.2021.1917737. [DOI] [PubMed] [Google Scholar]
- 140.Mizuta M., Shimizu M., Inoue N., Kasai K., Nakagishi Y., Takahara T., et al. Serum ferritin levels as a useful diagnostic marker for the distinction of systemic juvenile idiopathic arthritis and Kawasaki disease. Mod. Rheumatol. 2016;26(6):929–932. doi: 10.3109/14397595.2016.1159120. [DOI] [PubMed] [Google Scholar]
- 141.Nelson C., Ishimine P., Hayden S.R., Correia M., Wardi G. Multisystem inflammatory syndrome in children (MIS-C) in an adolescent that developed coronary aneurysms: a case report and review of the literature. J. Emerg. Med. 2020 doi: 10.1016/j.jemermed.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sakul F., Nathin D., Purewal J. Coronary artery aneurysms presenting as stemi in an adult patient with history of Kawasaki disease. J. Am. Coll. Cardiol. 2021;77(18_Supplement_1):2524. [Google Scholar]
- 143.Abrams J.Y., Oster M.E., Godfred-Cato S.E., Bryant B., Datta S.D., Campbell A.P., et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolescent Health. 2021;5(5):323–331. doi: 10.1016/S2352-4642(21)00050-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.X. Liu, Y.-H. Huang, Y.-C. Tsai, S.-F. Liu, H.-C. Kuo, Comparison laboratory data between children with Kawasaki Disease and COVID-19, 2021. [DOI] [PMC free article] [PubMed]
- 145.Menon J., Shanmugam N., Vasudevan A., Kumar N., Rammohan A., Rela M. Kawasaki disease in a pediatric liver transplant patient. Transpl. Immunol. 2021;67 doi: 10.1016/j.trim.2021.101416. [DOI] [PubMed] [Google Scholar]
- 146.Sinaei R., Hosseininasab A., Jafari M., Eslami S., Parvaresh S. The Multisystem Inflammatory Syndrome of Childhood (MIS-C) Indian J. Pediatr. 2021;1 doi: 10.1007/s12098-020-03617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Regmi S., Pathak S., Kim J.O., Yong C.S., Jeong J.-H. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: challenges, opportunities, and future perspectives. Eur. J. Cell Biol. 2019;98(5–8) doi: 10.1016/j.ejcb.2019.04.002. [DOI] [PubMed] [Google Scholar]