Abstract
Antibiotic resistance (ABR) has been identified as a critical threat to global health at the highest policy fora. A leading cause of ABR is the inappropriate use of antibiotics by both patients and healthcare providers. Although countries around the world have committed to developing and implementing national action plans to tackle ABR, there is a considerable gap in evidence about effective behaviour change interventions addressing inappropriate use of antibiotics in low- and middle-income countries (LMICs), where ABR is growing at an alarming rate. We conducted a systematic review to synthesize evidence about the effectiveness and cost-effectiveness of behaviour change interventions to reduce inappropriate use of antibiotics in LMICs. Three databases were searched using a set of predefined search terms and exclusion criteria. The search identified 43 relevant articles. A narrative synthesis of results was conducted using the Behaviour Change Wheel framework to categorize intervention components. The majority of the reviewed studies were set in lower-middle-income or low-income countries located in Sub-Saharan Africa or East Asia and the Pacific. Twenty-four articles evaluated multi-faceted interventions over a period of 12 months or less. Despite the widespread use of antibiotics in the community, interventions were primarily implemented in public health facilities, targeting health professionals such as doctors, nurses, and other allied medical staff. Although education for providers was the most widely used strategy for influencing antibiotic use, it was shown to be most effective when used in conjunction with training or other enabling and supportive measures to nudge behaviour. Six articles included an evaluation of costs of interventions and found a reduction in costs in inpatient and outpatient settings, and one article found a training and guidelines implementation-based intervention to be highly cost-effective. However, the small number of articles conducting an economic evaluation highlights the need for such analyses to be conducted more frequently to support priority setting in resource-constrained environments.
Keywords: Antibiotic use, behaviour change, systematic review, LMICs
KEY MESSAGES.
Behaviour change interventions that used education-based strategies either as a stand-alone intervention or as part of a multi-faceted intervention showed a positive impact on the use of antibiotics, compared to other strategies such as training, enablement or persuasion.
The majority of studies evaluated interventions that targeted the behaviour of healthcare providers in public health facilities, and only a few focused on patients and the wider community or pharmacy staff, particularly in the private sector.
The evidence base for effective interventions in low-income countries was weak and is likely to hinder the development of national action plans to curb antibiotic resistance.
There is a dearth of evidence on which interventions are cost-effective and affordable, which can limit the ability of a decision-maker to gauge the relative value of investment in interventions that have the potential to address antibiotic resistance.
Introduction
Antibiotic resistance (ABR) threatens our ability to cure common infectious diseases such as pneumonia, tuberculosis and gonorrhoea. It often results in prolonged illnesses as patients tend to remain infectious for longer periods of time (Neu, 1992), in turn increasing the risk of resistant infections spreading to other individuals (Mølbak, 2005; Holmes et al., 2016). ABR also leads to the use of alternative and often more expensive and lengthy treatment procedures that place a considerable economic strain on individuals, their families and communities (Holmberg et al., 1987; Paladino et al., 2002; Mølbak, 2005; Holmes et al., 2016) as well on resource-constrained healthcare systems (Okeke et al., 2005; Arnold and Straus, 2009; Espinoza Franco et al., 2009). Thus, it comes as no surprise that ABR has emerged as a growing threat to public health and societal well-being (Sumpradit et al., 2012; Llor and Bjerrum, 2014). ABR is also a political and financial priority (Khan et al., 2019) (Hernando-Amado et al., 2019), as reflected in the global health agenda of the United Nations General Assembly in September 2016 (World Health Organization (WHO), 2016a); and the Global Health Security Agenda and the International Health Regulations (World Bank, 2017). Along with this, an estimated US$40 billion has also been mobilized to fund strategies to address ABR (O’Neill, 2016).
The inappropriate prescription, dispensing, consumption and use of antibiotics (henceforth, antibiotic use) in clinical settings by providers (including primary care, hospitals and private drug sellers) and by patients has been identified as a key driver of ABR (Espinoza Franco et al., 2009; World Health Organization (WHO), 2016b). Inappropriate use of antibiotics includes, but is not limited to, treatment of conditions for which antibiotics are not clinically warranted, suboptimal dosage regimens, premature cessation of antibiotic treatment, lack of or poor quality consultation with healthcare providers, purchasing antibiotics without prescription and sharing antibiotics with others (Levy-Hara et al., 2011; Smith et al., 2018; Atif et al., 2019). A complex range of factors determine the inappropriate use of antibiotics in LMICs. Studies report on a variety of supply-side factors such as a lack of knowledge among prescribers or habitual prescribing that is not in line with best practice (Radyowijati and Haak, 2003; Espinoza Franco et al., 2009; Esmaily et al., 2010; Holloway, 2011; Ayukekbong et al., 2017; Suy et al., 2019), inadequate medical education, training and supervision (Wahlstrom et al., 2003; Holloway, 2011; Xiao et al., 2013; Liang et al., 2014); pharmaceutical promotion (Radyowijati and Haak, 2003; Holloway, 2011; Liang et al., 2014; Yip et al., 2014); inadequate interaction times between health workers and patients (Holloway, 2011; Llor and Bjerrum, 2014); inaccurate perceptions of patient needs and demands (Radyowijati and Haak, 2003; Holloway, 2011; Liu et al., 2016); limited availability of diagnostic support tools (Holloway, 2011; Llor and Bjerrum, 2014); and the inappropriate prescription of drugs (Radyowijati and Haak, 2003; Holloway, 2011; Aiken et al., 2013).
Studies also identify demand-side factors that relate to how individuals use or consume prescribed medicines. Commonly observed patient behaviour includes the overuse of antibiotics over unnecessarily long periods (Chan et al., 2012); non-adherence to appropriate or recommended treatment (Radyowijati and Haak, 2003; Ayukekbong et al., 2017) or the non-indicated use of antibiotics for uncomplicated viral infections such as upper respiratory tract infections (Owens et al., 2004; Chan et al., 2012). The reasons for these behaviours are varied and do not act independently of each other. They include high expectations or beliefs of how effective antibiotic treatment could be (Balabanova et al., 2009); poor availability of information and lack of knowledge about the appropriate use of drugs for different conditions (Holloway, 2011; Ayukekbong et al., 2017); the ability to easily access medicines over the counter without a prescription (Espinoza Franco et al., 2009); and a strong culture or norm of self-prescribing medicines (Balabanova et al., 2009). Geographical or economic barriers to accessing health facilities where prescription-based medicines may be obtained are also widely reported in the literature (Pavin et al., 2003; Suy et al., 2019).
Any strategy that aims to curb the spread of ABR must tackle these multi-dimensional supply- and demand-side behaviours in clinical care and community settings (World Health Organization (WHO), 2018) and extend to animal health and commercially driven agricultural settings, where the inappropriate use of antibiotics can further exacerbate ABR (Laxminarayan et al., 2013). This is likely to involve several stakeholders such as government and non-governmental organizations, civil society, the private sector and academic institutions working across public health, animal health and the environment (One Health Platform, no date). This presents a challenge to policy formulation due to the competing priorities and diverse solutions offered by these different stakeholders (Khan et al., 2019). Strategies to effectively address the public health threat posed by ABR would ideally need to achieve two goals: one, ensure access to effective treatment for common infections; and two, reduce the risk of emergence of ABR (Bloom et al., 2017).
Five systematic reviews have identified interventions designed to improve antibiotic and antimicrobial stewardship (Arnold and Straus, 2009; Charani et al., 2011; Davey et al., 2013, 2017; Cross et al., 2017; Wilkinson et al., 2018). Three of these reviews included a handful of interventions implemented in LMICs (Charani et al., 2011; Davey et al., 2017; Wilkinson et al., 2018). Davey et al.’s (2017) review focused on interventions to improve antibiotic use in inpatient settings and included five studies in LMICs. Charani et al. (2011), reviewed interventions to improve AB use among at clinicians and the general public but focused quite narrowly on mass media or campaign based and reviewed one study in an LMIC. The review by Wilkinson et al. (2018) focused on supply-side interventions in the public and private sector to improve antibiotic prescription in LMICs but did not include demand side interventions. No formal or established framework was used to categorize intervention strategy characteristics in this review, though some behaviour change interventions were identified. None of the five reviews analysed the cost and cost effectiveness of interventions, which is important as it provides policymakers with evidence on the relative value of investment in health intervention and aids efficient and equitable resource allocation (Drummond et al., 2005; Guinness and Wiseman, 2011; Vassall et al., 2017). This leaves a considerable knowledge gap for behaviour change with respect to the use of antibiotics in LMICs where ABR is growing rapidly and resources may be constrained (Yip et al., 2014; Ayukekbong et al., 2017; Wilkinson et al., 2018). This limited evidence is likely to inhibit progress on the development of effective national ABR mitigation strategies (World Health Organisation, 2018).
Our review aims to fill this evidence gap by summarizing and critically appraising the literature on the effectiveness and cost-effectiveness of behaviour change interventions implemented in LMICs to improve the use of antibiotics in the domain of human health. This will be achieved by:
Identifying behaviour change interventions for improved use of antibiotics in inpatient, outpatient and community settings in LMICs;
Synthesizing available evidence on the effectiveness and cost-effectiveness of behaviour change interventions, using a published framework for behaviour change;
Appraising the quality of the studies included in the review using the GRADE checklist (Atkins et al., 2004);
Discussing intervention types that are most strongly associated with effectiveness and cost-effectiveness; and
Identifying knowledge gaps that can help inform future research and policy agendas on ABR in LMICs.
Methods
Details of the methodology used for this review have been published in a review protocol (Batura et al., 2018). A summary of the review methods is presented below.
Search strategy
Two researchers independently conducted comprehensive searches for peer-reviewed articles using three research databases: Web of Science, PubMed and Google Scholar. This was followed by a hand search of the references included in the final set of papers to capture any additional papers that met the inclusion criteria (see below). The search terms are presented in Table 1.
Table 1.
Keywords for systematic review search
| Population—drugs | antibiotic*; antimicrobial*; ‘anti-bacterial agents’; antibacterial; anti-bacterial |
|---|---|
| Interventions | ‘behavioural intervention*’, ‘behavioral intervention*’, ‘behaviour intervention’, ‘behavior intervention’, ‘behaviour change’, ‘behavior change’, ‘behaviour modification’, ‘behavior modification’, ‘training’, ‘supervision’, ‘education’, ‘knowledge’, ‘feedback’, ‘audit’, ‘reminders’, ‘modelling’, ‘modeling’, ‘enablement’, ‘persuasion’, ‘incentivisation’, ‘incentivization’, ‘coercion’, ‘restriction’, ‘environmental restructuring’, ‘guidelines’, ‘stewardship’, ‘law enforcement’, ‘policy’, ‘governance’ |
| Outcomes | ‘use’, ‘rational use’, ‘irrational use’, ‘inappropriate use’, ‘appropriate use’, ‘appropriate treatment’, ‘treatment’, ‘prescription’, ‘adequate prescription’, ‘prescri*’, ‘knowledge’, ‘prophylactic use’, ‘prophilaxys’, ‘effectiveness’, ‘cost effectiveness’, ‘cost-effectiveness’, ‘economic evaluation’, ‘costs’, ‘costing’, ‘cost effectiveness analysis’, ‘cost-effectiveness analysis’, ‘cost benefit analysis’, ‘cost-benefit analysis’, ‘cost utility analysis’, ‘cost-utility analysis’, ‘utilization’, ‘utilisation’, ‘drug use’, ‘medicine use’, ‘essential medicine*’, ‘drug information’, ‘drug therapy’, ‘consumption’, ‘prescribing practices’, ‘prescribing behaviour’, ‘prescribing behavior’ |
| Countries | ‘low and middle income countr*’, ‘low income countr*’, ‘middle income countr*’, LMIC*, ‘developing countr*’, Afghanistan, Benin, Burkina Faso, Burundi, Central African Republic, Chad, Comoros, Democratic Republic of Congo, Eritrea, Ethiopia, The Gambia, Guinea, Guinea Bissau, Guinea-Bissau, Haiti, Democratic People's Republic of Korea,, Liberia, Madagascar, Malawi, Mali, Mozambique, Nepal, Niger, Republic of Yemen, Yemen, Rwanda, Senegal, Sierra Leone, Syria, Somalia, South Sudan,, Tajikistan, Tanzania, Togo, Uganda, Zimbabwe, Angola, Argentina, Bangladesh, Bhutan, Bolivia, Cabo Verde, Cambodia, Cameroon, Republic of Congo, Congo, Cote d'Ivoire, Djibouti, Arab Republic of Egypt, Egypt, El Salvador, Georgia, Ghana, Honduras, India, Indonesia, Kenya, Kiribati, Kosovo, Republic of Kyrgyz, Kyrgyz, Lao PDR, Lao, Lesotho, Mauritania, Federated States of Micronesia, Micronesia, Moldova, Mongolia, Morocco, Myanmar, Burma, Nicaragua, Nigeria, Pakistan, Papua New Guinea, Philippines, Sao Tome and Principe, Solomon Islands, Sri Lanka, Sudan, Swaziland, Arab Republic of Syria, Timor-Leste, Timor Leste, East Timor, Tunisia, Ukraine, Uzbekistan, Vanuatu, Vietnam, West Bank and Gaza, Zambia, Albania, Algeria, American Samoa, Armenia, Azerbaijan, Belarus, Belize, Bosnia and Herzegovina, Botswana, Brazil, Bulgaria, China, Colombia, Costa Rica, Cuba, Dominica, Dominican Republic, Equatorial Guinea, Guinea, Ecuador, Fiji, Gabon, Grenada, Guatemala, Guyana, Islamic Republic of Iran, Iran, Iraq, Jamaica, Jordan, Kazakhstan, Lebanon, Libya, Republic of Macedonia, Macedonia, Malaysia, Maldives, Marshall Islands, Mauritius, Mexico, Montenegro, Namibia, Nauru, Paraguay, Peru, Romania, Russian Federation, Russia, Samoa, Serbia, South Africa, St Lucia, St Vincent and the Grenadines, Suriname, Thailand, Tonga, Turkey, Turkmenistan, Tuvalu, Venezuela RB, Venezuela |
|
Terms within each row are separated by OR Terms across each row are separated by AND Limited to publications related to Humans Limited to publications since January 1990 to 2019 | |
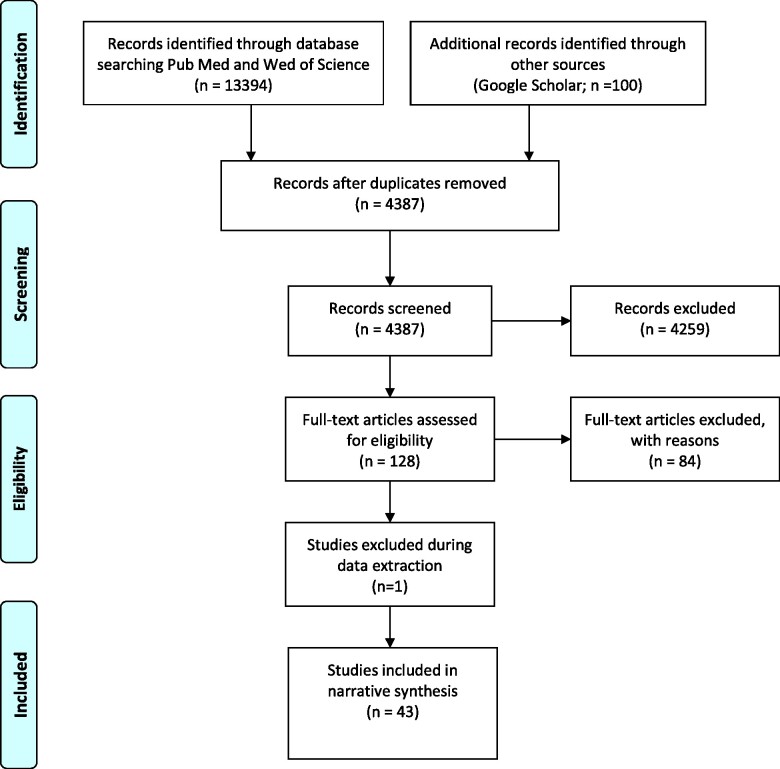
The search results were extracted into Mendeley 1.19.4 and checked for duplicates, which were subsequently removed. As a first step, all de-duplicated titles and abstracts retrieved from the literature searches were independently screened by A1 and A2. Any doubt around whether certain studies should be included was resolved by three other researchers on the team (A3, A4 and A5). Following this screening phase, two researchers reviewed the full text of the papers to ensure that all inclusion criteria were met (A2 and A1). The selection process is summarized in Figure 1.
Figure 1.
Search results and included studies.
Articles were eligible for inclusion in this review if they:
Were written in English, Spanish, French and Portuguese;
Were published in peer-reviewed journals between 1990 and 2019 (conference abstracts, trial protocols, systematic reviews and non-peer-reviewed publications were excluded);
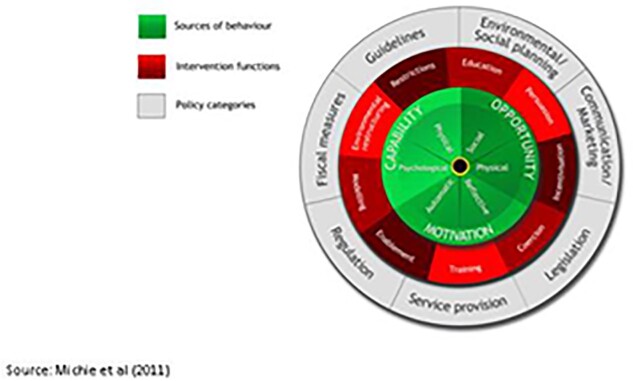
Included a behaviour change intervention defined using the Behaviour Change Wheel (BCW) developed by Michie et al. (2011) and explained in further detail below (Michie et al., 2011).
Included interventions evaluated within the framework of a randomized controlled trial (RCT), interrupted time series (ITS) analyses, controlled before-after (CBA) studies, or studies that had a quasi-experimental design that would allow the establishment of causal relationships;
Included primary and secondary outcomes that measured use of antibiotics, for example, the numbers of antibiotics prescribed by a provider, rate of antibiotic dispensing, rate of antibiotic use, etc; and
Were undertaken in countries classified as LMIC using the World Bank’s 2019 country classification (World Bank, 2020).
Intervention categorization
The BCW is a layered framework that allows situation analysis in a step-wise manner by (1) defining the problem; (2) specifying target behaviour(s); and (3) identifying changes needed (Michie et al., 2011). This can be linked to intervention functions such as training, enablement, education, etc. that might be necessary to change or shift behaviours in order to address the gaps identified. The framework then links the intervention functions to policy options that could support appropriate intervention implementation and delivery (Figure 2).
Figure 2.
Behaviour change wheel.
Using this framework, we categorized interventions as:
Education: Interventions such as face-to-face lectures; small-group discussion, workshops, or seminars; refresher courses; educational outreach and visual aids that focus on imparting knowledge and developing understanding.
Training: Interventions such as training sessions; and train-the-trainer sessions that lead to skill and capacity development.
Modelling: Interventions where imitation acts as a motivational tool, facilitated by peer-review committees or other monitoring/regulatory committees.
Enablement: Interventions such as feedback and audit; reminders and supervision that provide comprehensive support to trigger behaviour change by reducing barriers.
Persuasion or coercion: Interventions that use a stimulus such as public reporting; communication/information; leaflets; posters or waiting room videos to induce action driven by the expectation of punishment or cost, or positive or negative feelings towards something.
Incentivization: Interventions that create an expectation of a financial or non-financial reward conditional on engagement in an optimal behaviour.
Restriction: Interventions that use the implementation of rules such as law or guideline enforcement (e.g. antibiotic stewardship programmes and antibiotic prophylaxis policies); and changes in governance structure to improve the opportunity to engage in the targeted behaviour.
Data analysis and synthesis
Data were extracted into an Excel worksheet to capture details about the authors, country setting, type of intervention, target population, clinical or community setting and evaluation outcomes. As there was a high degree of heterogeneity in study outcomes, we conducted a narrative synthesis, whereby we collated the findings from the included studies to form a coherent description of study findings, along with differences in characteristics of the studies including context and quality (Popay et al., 2006; Petticrew et al., 2013; Campbell et al., 2018).
Quality appraisal
We conducted an appraisal of the quality of the included studies using the GRADE approach (Atkins et al., 2004; Guyatt et al., 2008), which specifies four levels of quality of evidence that range from very low to high (Table 2) (Guyatt et al., 2008). Evidence from RCT studies is rated as high quality while evidence from observational studies is rated with lower quality owing to the residual confounding in this type of study design (Shünemann et al., 2013).
Table 2.
GRADE quality ratings
| Quality | Meaning |
|---|---|
| Very low | True effect is probably markedly different from the estimated effect |
| Low | True effect might be markedly different from the estimated effect |
| Moderate | Authors believe that true effect is probably close to the estimated effect |
| High | Authors have a lot of confidence that the true effect is similar to the estimated effect |
Source:Guyatt et al. (2008).
We used the five criteria recommended by Ryan and Hill (2016) to assess quality: (1) study design, (2) overall risk of bias, (3) consistency in results, (4) precision of estimates and (5) whether studies evaluate interventions relevant to the research question (Ryan and Hill, 2016). The final GRADE quality ratings were based on the application of these criteria to the included studies. The quality appraisal was led by A3 and A1.
Results
Search results and included studies
The search generated 4387 possible articles as shown in Figure 1. Titles, key words and abstracts were reviewed as a first check, and 4259 (97.1%) articles were excluded on this basis. The full texts for all remaining articles were then reviewed, and 85 (1.9%) were excluded. This left 43 articles (1%), which are included in this review.
Geographical location of studies
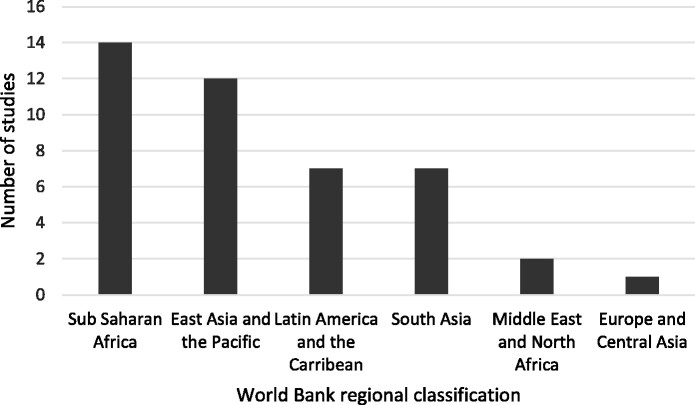
The majority of the included articles evaluated interventions in one country (95.3%); only two articles (4.7%) evaluated interventions in multiple settings (Chalker et al., 2005; Santa-Ana-Tellez et al., 2013). Twenty-three (53.5%) were from upper-middle-income countries; followed by lower-middle-income countries (n = 13; 30.2%), with very few from low-income countries (n = 7; 16.3%). Most of the included articles were from East Asia and the Pacific region (n = 14; 32.6%), followed by Sub-Saharan Africa (n = 12; 27.9%) (Figure 3).
Figure 3.
Study settings by World Bank regional classification.
Target population and study setting
Most of the articles evaluated interventions set in the public sector (n = 36; 83.7%). A total of 18 interventions (41.9%), were targeted at physicians (or doctors) only, six at pharmacy staff only (14%), two at nurses (4.7%), two at community health workers only (4.7%) and one at patients only (2.3%). Nine (20.9%) were targeted at multiple prescribers at health facilities such as physicians, medical officers, nursing staff but excluded all pharmacy staff, while one targeted multiple prescribers as well as pharmacy staff (2.3%). Four interventions targeted both physicians and patients (9.3%). The interventions were largely set in public health centres and clinics (Tables 3 and 4).
Table 3.
Summary of single component behaviour change interventions
| Author | Country | Intervention | Study design | Setting | Target population | Facillity ownership | Outcome(s) |
|---|---|---|---|---|---|---|---|
| Education | |||||||
| Angunawela et al. (1991) | Sri Lanka |
Two interventions arms:
|
RCT |
Outpatient; Hospital and other health facilities |
Multiple prescribers | Public |
|
| Bexell et al. (1996) | Zambia | Continuing education seminars to improve rational drug use and patient management | RCT | Outpatient; Health centre | Multiple prescribers | Public |
|
| Esmaily et al. (2010) | Iran |
Continuing medical education (CME):
|
RCT |
Outpatient and inpatient; Primary health care |
Physicians | Public |
|
| Fairall et al. (2005) | South Africa | Education outreach delivered to nurses on tuberculosis case detection and primary care of respiratory illness | RCT |
Outpatient; Primary health care |
Nurses | Public |
|
| Ngoh et al. (1997) | Cameroon | Visual aids to communicate prescription drug instructions and improve compliance | RCT |
Outpatient; Primary health care |
Patients | Public |
|
| Santoso et al. (1996) | Indonesia |
Two intervention arms:
|
RCT |
Outpatient; Primary heatlh care |
Multiple prescribers | Public |
|
| Obua et al. (2004) | Uganda | Educational seminar covering principles of rational drug use and National Standard Treatment Guidelines to improve prescribing practices for ARI, malaria, and non-dysenteric diarrhoea | Quasi- experimental |
Outpatient; Private clinics |
Physicians | Private |
|
| Akter et al. (2009) | Bangladesh | Education for all physicians on clinical topics to improve appropriate antimicrobial use | Quasi- experimental |
Inpatient; Hospital |
Physicians | Public |
|
| Training | |||||||
| Meyer et al. (2001) | South Africa | Training‐of‐trainers course followed by effective prescribing workshops to improve prescribing practices | RCT |
Outpatient; Primary health care |
Nurses | Public |
|
| Shrestha et al. (2006) | Nepal | Training healthworkers to implement the WHO clinical practice guidelines to improve the management of respiratory diseases in adults | RCT |
Outpatient; Primary health care |
Community health workers | Public |
|
| Babigumira et al. (2017) | Malawi | Combination of classroom-based (didactic) and field-based training under supervision to improve access to essential medicines | Quasi- experimental |
Outpatient; Health centre |
Pharmacy staff | Public |
|
| Aiken et al. (2013) | Kenya | Development and implementation of an AB pre- and post-operative prophylaxis policy to improve prescribing behaviour | Time series |
Inpatient; Hospital |
Multiple prescribers | Public |
|
| Restriction | |||||||
| Berild et al. (2008) | Russia | Development and implementation of treatment guidelines for gastrointestinal infections (GII) and respiratory tract infections (RTI) to improve AB use | Quasi- experimental |
Inpatient; Hospital |
Physicians | Public |
|
| Chandy et al. (2014) | India | Implementation of policy guidelines on hospital AB use | Time series |
Outpatient and inpatient; Hospital |
Multiple prescribers | Public |
|
| Liang et al. (2014) | China | Governance reform to disincentivise prescription of unnecessary and expensive medicines such as AB | Time series |
Outpatient; Primary health care |
Physicians | Public |
|
| Santa-Ana-Tellez et al. (2013) | Brazil and Mexico | Over-the-counter restrictions on AB consumption only to patients who present a prescription to improve AB consumption | Time series |
Community; Pharmacy |
Pharmacy staff | Private |
In Mexico:
In Brazil:
|
| Persuasion | |||||||
| Yang et al. (2014) | China | Public availability of ranking league tables of prescribing physicians and hospitals to consumers and health workers outpatient departments. To improve AB prescribing for URTIs. Ranking based on:
|
RCT |
Outpatient; Primary health care |
Physicians | Public |
|
| Liu et al. (2016) | China | Reports of percentage of prescriptions requiring AB, percentage of prescriptions requiring injections, and average expenditure of patients displayed at outpatient departments, sent monthly to physicians and made available to patient every 3 months to improve AB and injection prescription | RCT |
Outpatient; Primary health care |
Physicians | Public |
|
| Enablement | |||||||
| Magedanz et al. (2012) | Brazil | Prospective audit with feedback to prescriber, with and without the presence of a pharmacist to improve AB stewardship | Quasi- experimental |
Inpatient; Hospital |
Physicians | Public |
|
Table 4.
Summary of multi-faceted behaviour change interventions
| Author | Country | Intervention | Study design | Behaviour change components | Setting | Target Population | Facility ownership | Outcome(s) |
|---|---|---|---|---|---|---|---|---|
| Two-component interventions | ||||||||
| Podhipak et al. (1993) | Thailand |
Two intervention components
|
Quasi -experimental |
|
Outpatient; Pharmacy |
Pharmacy staff | Private |
|
| Tumwikirize et al. (2004) | Uganda | Two intervention components:
|
Quasi -experimental |
|
Outpatient; Pharmacy |
Pharmacy staff | Private |
|
| Ngasala et al. (2008) | Tanzania |
Two intervention arms:
|
RCT |
|
Outpatient; Primary health care |
Community health workers | Public |
|
| Pérez et al. (2003) | Colombia |
Two intervention components:
|
Time series |
|
Inpatient; Hospital |
Physicians | Public |
|
| Awad et al. (2006) | Sudan |
Three intervention arms:
|
RCT |
|
Outpatient; Health centre |
Multiple prescribers | Public |
One-month post intervention
Three months post intervention:
|
| Qidwai et al. (2006) | Pakistan | Implementation of diarrhoea treatment as per guidelines | Quasi experimental |
|
Outpatient; Pharmacy |
Pharmacy staff | Private |
|
| Hadi et al. (2008) | Indonesia |
Four intervention components:
|
Time series |
|
Inpatient; Hospital |
Physicians | Public |
|
| Zhang et al. (2018) | China |
|
RCT |
|
Outpatient; Hospital |
Physicians and patients | Public |
|
| Yip et al. (2014) | China |
Two intervention components:
|
RCT |
|
Outpatient; Primary health care |
Multiple prescribers | Public |
|
| Atchessi et al. (2013) | Burkina Faso |
Three intervention components:
|
Time series |
|
Outpatient; Primary health care |
Multiple prescribers | Public |
|
| Gutiérrez et al. (1994) | Mexico |
Two intervention components:
|
Quasi experimental |
|
Outpatient; Primary health care |
Physicians | Public |
|
| Chowdhury et al. (2007) | Bangladesh |
Two intervention arms:
|
RCT |
|
Outpatient; Health complex |
Physicians | Public |
|
| Rahbarimanesh et al. (2019) | Iran | Evaluation of patient’s electronic medical record, open communication with specialist physicians and feedback to providers on the use of AB to promote antimicrobial stewardship | Quasi experimental |
|
Inpatient; Hospital |
Physicians | Public |
|
| Wattal et al. (2015) | India | Audit and feedback of AB prescription rates to improve prescribing rates | RCT |
|
Inpatient; Hospital |
Physicians | Public |
|
| Three-component interventions | ||||||||
| Hoa et al. (2017) | Vietnam |
Sequential implementation of intervention activities:
|
RCT |
|
Outpatient and inpatient; Hospital and other health facilities |
Multiple prescribers and pharmacy staff |
Public/ Private |
|
| Shen et al. (2018) | China | Information on theory and evidence-based ingredients, that included operation guidelines, public commitment, and takeaway information, along with feedback component for participating doctors on performance scores and percentages of prescribed AB to improve knowledge of rational use and the use of AB | RCT |
|
Outpatient; Primary health care |
Physicians and patients | Public |
|
| Chalker et al. (2005) | Thailand and Vietnam |
Sequential implementation of three 3-month interventions:
|
RCT |
|
Outpatient; Pharmacy |
Pharmacy staff | Private |
|
| Perez-Cuevas et al. (1996) | Mexico | Educational intervention, training of instructors, peer-review committee and self-appraisal of clinical performance in two institutions to improve prescribing practices for rhinopharyngitis | Quasi-experimental |
|
Outpatient; Primary health care |
Physicians | Public |
|
| Zhen et al. (2018) | China | Audit of prescriptions against guidelines, along with educational workships and monthly meetings with feedback | Time series |
|
Outpatient; Primary health care |
Physicians | Public |
|
| Wahlstrom et al. (2003) | Lao PDR | Implementation of standard treatment guidelines and audit-feedback for improved case management of malaria, diarrhoea and pneumonia | RCT |
|
Inpatient; Hospital |
Multiple prescribers | Public |
|
| Four-component interventions | ||||||||
| Cundill et al. (2015) | Tanzania |
Two intervention arms:
|
RCT |
|
Outpatient; Primary health care |
Physicans and patients | Public |
|
| Reyes-Morales et al. (2009) | Mexico |
Three intervention components:
|
Quasi-experimental |
|
Outpatient; Primary health care |
Physicians | Public |
|
| Five-component interventions | ||||||||
| Guanche-Garcell et al. (2011) | Cuba | Six intervention components:
|
Time series |
|
Outpatient and inpatient; Hospital |
Physicians | Public |
|
| Wei et al. (2017) | China | Training on use of clinician guidelines, appropriate prescribing, monthly prescribing peer-review meetings, and caregiver education to improve AB prescription for upper respiratory tract infections (URTI) inc children | RCT |
|
Outpatient; Hospital |
Physicians and patients | Public |
|
Type of interventions
Nineteen articles evaluated interventions with a single component (44.2%) and 24 evaluated interventions with multiple components (55.8%). Interventions with a single component most commonly used education to influence behaviour (n = 8; 18.6%), followed by training (n = 4; 9.3%), restriction (n = 2; 4.7%) and persuasion (n = 2; 4.7%). Only one study used enablement as a behaviour change intervention strategy (2.3%). A summary of key characteristics of these studies is presented in Table 3.
Amongst the multi-faceted behaviour change interventions, 14 had two components (32.6%), 6 had three components (14%), 2 had four components (4.7%) and 2 had five components (4.7%) (see Table 4). Amongst these, the most common intervention component, combined with one or more components, was education (n = 16; 37.2%), followed by training (n = 12; 27.9%), enablement (n = 12; 27.9%) and restriction (n = 10; 23.3%). The most common combinations were education and training (n = 9; 20.9%) and education and enablement (n = 6; 14%). Table 4 presents a summary of key characteristics of these studies.
All interventions had varying participant follow-up periods ranging from 7 days (Ngasala et al., 2008) to 5 years (Santa-Ana-Tellez et al., 2013). The average follow-up period ∼12 months (361 days), and the median was 6 months (180 days).
The studies used various outcome indicators to measure change in antibiotic use, which can be categorized into three broad domains to aid synthesis. The vast majority of studies used outcome indicators that measured changes in antibiotic prescribing (n = 38; 88.4%). The interventions targeting antibiotic prescribing included: antibiotic prescription forms; face-to-face educational seminars or distribution of educational material; training workshops; and implementation of guidelines or antibiotic stewardship programmes either targeting at physicians, other prescribers such as nurses, medical officers or community health workers or pharmacy personnel. The other main outcome indicators were antibiotic use (Ngoh and Shepherd, 1997; Santa-Ana-Tellez et al., 2013) and antibiotic dispensing (Tumwikirize et al., 2004; Chalker et al., 2005; Babigumira et al., 2017). For studies focusing on antibiotic use, the target groups included patients, the community and pharmacy staff, and the behaviour change interventions implemented included education and restriction. For studies focusing on antibiotic dispensing, interventions most commonly included education, training, restriction and modelling that targeted private sector pharmacists or other pharmacy staff. Three articles had outcome measures that fell under two outcome categories. The article by Podhipak et al. (1993) had outcomes for both antibiotic prescription and use by patients. These interventions included education, training, restriction and enablement components targeted at health care providers, and pharmacists and drug sellers. The article by Hoa et al. (2017) considered outcomes of antibiotic prescription and dispensing and was targeted at health care providers and drug sellers through education, training and persuasion.
Evidence on the impact of the different interventions was mixed. Most of the interventions reported a positive impact (n = 30; 69.8%); 27 of these improved antibiotic prescriptions (62.8%) and the remaining three led to improvements in antibiotic use (n = 2; 4.7%) and antibiotics dispensed (n = 1; 2.3%). Eight studies (18.6%) had relatively smaller effect sizes and six studies (14%) had no statistically significant impact on antibiotic use or on antibiotic use. Amongst the single-faceted interventions (Table 3), all restriction-based interventions reported a positive impact (i.e. statistically significant improvement in antibiotic use). All but one of the eight education-based interventions reported a positive impact and the majority of the training interventions did not find a positive effect on the use of antibiotics (87.5%). Most multi-faceted interventions (Table 4) had a positive impact on the use of antibiotics (76.6%). Exceptions included an intervention combining education, training, enablement and persuasion that had a negative effect on antibiotic prescription (Cundill et al., 2015) and two education and training interventions that found a positive impact on antibiotic use for some clinical conditions but not others (Podhipak et al., 1993; Tumwikirize et al., 2004).
Costs and cost-effectiveness of interventions
Six articles conducted cost analyses along with the impact evaluation of the interventions (Tables 3 and 4). As a result, the methodology of the cost analyses and results were presented briefly in the articles, and thus, limited the reporting of results to descriptive outcomes. Three articles conducted cost analyses and found that behaviour change interventions reduced the costs of prescription and visits in outpatient settings (private practitioners, healthcare providers and community, patients and caregivers). The cost analysis by Obua (2004) nested within the evaluation of a quasi-experimental study found that an education-based intervention reduced the average cost of drugs prescribed by US$0.2. Two cost analyses were nested with an RCT framework. Yip et al. (2014) found that a two-component intervention based on training and enablement resulted in a decrease in total expenditure per visit by 6% at the village level, but not at larger administrative unit levels. Wei et al. (2017) found that a multifaceted intervention comprising education, training, persuasion, restriction and modelling components reduced the cost per antibiotic prescription by US$0.35 at 6 months after follow-up and by US$0.26 at the time of the 18 months of follow-up.
The remaining three articles that conducted cost analyses along with the impact evaluation of the interventions were done in the inpatient setting. These studies found that behaviour change interventions reduced costs due to a reduction in the number of prescribed antibiotics or other drugs, increases in the prescription of generic or essential drugs and reductions in the wastage of antibiotics. Shrestha et al. (2006) found that a training-based intervention led to a minor reduction in the cost per antibiotic prescription (<US$0.1). In their evaluation of a restriction-based intervention of different diseases, Berild et al. (2008) found that the average cost of antibiotics per patient decreased by 16% for patients with gastrointestinal infections and pneumonia patients but increased by 38% for patients with respiratory tract infections. Magedanz et al. (2012) estimated that an enablement-based intervention led to an overall decrease in the mean monthly cost of antibiotics from ∼US$31 000 at baseline to ∼US$10 000 post-intervention.
Only one article presented results from a full economic evaluation conducted alongside the evaluation of an RCT, comparing costs and consequences of a behaviour change intervention as a stand-alone analysis (Zhang et al., 2018). Zhang et al. (2018) found that a multifaceted training and persuasion programme when embedded into routine practice had an incremental cost of $0.03 per percentage point reduction in antibiotic prescribing and was highly cost‐effective from the provider perspective compared to the alternative scenario i.e. with no training and implementation of guidelines. The brevity in reporting costing methodology and results in six of the seven papers included in the review, and heterogeneity in the study design, scale and cost outcomes precludes us from making robust comparisons between studies.
Type of study and quality appraisal
The majority of the included articles used an RCT design (n = 22), which is considered high-quality as per the GRADE criteria (Table 2). Of the remaining 21 studies, 9 had an ITS study design and 12 had a quasi-experimental design (Tables 3 and 4). These are classified as a lower quality based on the GRADE criteria.
Discussion
Globally, several behaviour change interventions have been implemented, along with considerable investment to combat ABR in the last three decades. To date, most of the evidence on effectiveness has been from high-income settings and previous syntheses of the literature support this (Arnold and Straus, 2009; Charani et al., 2011; Davey et al., 2013, 2017; Cross et al., 2017). Only one review focused solely on interventions implemented in LMICs (Wilkinson et al., 2018) but did not explicitly focus on behaviour change or include demand-side interventions. These reviews did not include any evidence on the costs and cost-effectiveness of interventions to improve antibiotic use. To address this evidence gap, we synthesized and appraised 43 papers evaluating the effectiveness and/or cost-effectiveness of behaviour change interventions to improve the use of antibiotics in LMICs.
Overall, our findings indicate that multi-faceted interventions were more effective in improving antibiotic use than single-faceted behaviour change interventions; however, the degree of improvement in most interventions was <20%. This finding is consistent with previous systematic reviews from high-income countries (Arnold and Straus, 2009; Charani et al., 2011; Davey et al., 2017; Cross et al., 2017) and with the review by Wilkinson et al. (2018). Interventions based on education, restriction and training, either as a stand-alone intervention or as part of a multi-faceted intervention showed a positive impact on the use of antibiotics. Reflecting on these intervention functions and the policies that they link to in the BCW framework (Figure 2), it is likely policies that focus on developing and implementing guidelines, along with appropriate and context-relevant environmental and social planning can improve the use of antibiotics. The most common intervention type was education, closely followed by training. However, unlike education interventions, training interventions were more likely to succeed when combined with an education, restriction or enablement intervention component. Some have argued that this occurs because the motivational and capability development effects of education can bolster the impact of the training component (Michie et al., 2011), and thus, policies that focus on environmental/social planning alone may be less effective in improving the use of antibiotics.
Accommodating all studies evaluating behaviour change interventions to improve antibiotic use in LMIC, meant that comparisons of results were difficult due to variation in settings, target populations, study designs and outcome measures. Nonetheless, some methodological implications are noteworthy. We included studies with experimental, quasi-experimental and time-series designs. As these are analytic study designs (Peinemann et al., 2013), they allow us to infer causality of certain behaviour change strategies on the use of antibiotics to some degree. However, more than half of the included studies were classified as low-quality by the GRADE checklist as they did not employ an RCT design. On the one hand, non-RCT studies may not be able to account for confounders, consider adequate dose–response and/or the fact that all plausible biases could have an impact on the treatment effect (Goldet and Howick, 2013; Ryan and Hill, 2016), thereby reducing confidence in the accuracy of results. On the other hand, RCTs may provide exaggerated estimates of effect, may not be able to wholly eliminate bias (Jadad and Rennie, 1998), or have generalizable results (Hariton and Locascio, 2018). Strategies to curb ABR much tackle multi-dimensional behaviours in clinical care and community settings, highlighting the need for complex interventions. Limiting the evaluation design to RCTs may deter the implementation and assessment of public health interventions, especially when implemented at a large scale or in multiple sites (Sanson-Fisher et al., 2007).
In addition, we identified a large variation in how outcomes were measured and reported across different domains of antibiotic use i.e. antibiotic prescription, dispensing and consumption. Even within the same domain, different indicators or metrics were used. This prohibited a meta-analysis and subgroup analysis (Dwan, 2011). Some studies also reported multiple outcomes, which leads to a consideration of which outcome(s) should be considered when synthesizing evidence on the effectiveness and posing challenges to a straightforward interpretation of the evidence (Mayo-Wilson et al., 2017). Further, few evaluations measured the impact of behaviour change interventions over >12 months. While the majority of the studies had a short follow-up period (for example, Podiphak, 1993; Meyer 2001; Liang, 2014; Yang, 2014; Babigumira, 2017; Hoa, 2017; Wei, 2017; Zhang, 2018), some did consider the importance of longer-term benefit assessment by having an evaluation period of 18 months after the intervention (Gutierrez et al., 1994; Pérez-Cuevas et al., 1996). Some also recognized the importance of the benefits of program continuation beyond the project period for the yield of long-term benefit (Bexell, 1996; Chandy, 2014). It is widely recognized that the time-treatment interaction can lead to impacts that can either dissipate over time, or change direction (Cooper, 1989). Caution is therefore required when interpreting the results of current evaluations of behaviour change interventions that are based on short-term time horizons.
An evaluation of the sustainability of the interventions was challenged by the fact that the duration of the interventions and the assessment period within which the impact of the intervention was measured varied widely among studies. Some of the studies included in this review, however, discuss the importance of measuring the longer-term effect of the interventions, with one study (Berild et al., 2008) highlighting that the impact of the intervention level went back to the pre-intervention level after a 1-year period of assessment. Future studies might also consider how other factors such as staff turnover, health system settings and social and cultural context could have an impact on the sustainability of a positive study outcome.
Our review identified several key gaps in the existing evidence base. First, most included articles evaluated interventions implemented in middle-income countries and only a handful were set in low-income countries. Those that were implemented in low-income countries provided mixed evidence on the kinds of behaviour change interventions that can have a positive impact on antibiotic use. This presents a significant gap in the evidence base for these countries, many of which have been tasked with developing a national action plans to curb ABR (World Health Organization (WHO), 2015b). The lack of adequate evidence on how antibiotics and other essential medicines are used, different patterns of ABR amongst the population, and how these change over time, limits the development of effective policy strategies such as regulation, legislation, changes to service provision or implementation of fiscal measures to improve antibiotic use. This may be overcome by developing effective and reliable ABR surveillance systems that can integrate surveillance of ABR in human, animal and food-borne pathogens to provide comprehensive and dynamic situation analyses; information on overall mortality and morbidity; and capture the extent of the economic and social impacts of ABR (World Health Organization (WHO), 2014; Holloway et al., 2017). The importance of such surveillance and research has been globally recognized in the WHO’s global action plan to tackle antibacterial resistance as a way of generating knowledge and translation into policy action (World Health Organization (WHO), 2015a). However, it may be less feasible to do so in countries where health systems are too constrained to allow appropriate allocation of resources to improve the use of antibiotics.
Second, the majority of articles evaluated interventions targeting the behaviour of health care providers and only a few focused on patients and the wider community. The inappropriate use of antibiotics is highly influenced by human behaviour at many levels of society. Interventions targeting patients and the wider community, potentially using communication and environmental/social planning policies (Charani et al., 2011) are required to improve the use of antibiotics (Ayukekbong et al., 2017) and leaving out these key agents could hinder efforts to tackle ABR (Radyowijati and Haak, 2003; Holloway, 2011; Suy et al., 2019).
Third, only five studies in this review targeted healthcare providers outside the public sector. Although inappropriate use of antibiotics, owing at least partially to perceived demand from patients (Kotwani et al., 2010; Om et al., 2016), is known to occur at public and private health facilities, the relative lack of studies focusing on the private sector presents a considerable challenge to tackling ABR in LMICs where private drug sellers (including community pharmacies, drug shops and general stores) are the first primary contact point for outpatient services such as consultations, diagnoses, drug prescription and dispensing (Goel et al., 1996; Kwena et al., 2008). Antibiotics are typically purchased from these providers without a prescription and/or dispensed by personnel without adequate training (Lansang et al., 1990; Ayukekbong et al., 2017). The severity of this is illustrated through a recent systematic review, which found 62% of antibiotics were dispensed without a prescription in community pharmacies globally (Auta et al., 2019).
Fourth, the Medical Research Council’s framework for the evaluation of complex interventions recommends that in addition to looking at the effect of the intervention, studies should also conduct economic and process evaluations to support research translation (Craig et al., 2008). Process evaluations provide key evidence on the fidelity and the quality of implementation of interventions, clarification of causal pathways and means to identify any context-related factors that can lead to variations in outcomes (Craig et al., 2008). No studies in this review included a process evaluation. Without process evaluation results, policymakers lack adequate information on the barriers to or facilitators of success of behaviour change interventions to improve antibiotic use in a specific context; thus, reducing the likelihood of replication or uptake in another context (Moore et al., 2015).
Seven articles presented results from an economic evaluation of an intervention, of which six presented the results of a cost analysis conducted along with the main intervention evaluation. Only one article presented results from a full economic evaluation as a stand-alone analysis. The brief and descriptive nature of reporting economic evaluation methods and outcomes in the majority of studies and the heterogeneity in the scope of the economic evaluation, perspectives, scale and outcomes posed a challenge in making robust comparisons between interventions. While indicative of the relative value of investments in interventions that have the potential to address the public health problem that ABR poses, this evidence gap limits a decision-maker’s ability to compare between different programmes. Thus, evaluations of interventions should ideally be accompanied by a full economic evaluation that adheres to established guidelines and reports on the cost-effectiveness of the intervention for the trial duration, and for a long-term horizon would be beneficial for decision-makers. This would provide more rigorous evidence on the costs and benefits of such interventions as well as on the budget impact or affordability to aid decisions about the efficiency of intervention delivery, priority setting, financial planning and management and the formulation of resource requirements and budgets (Vassall et al., 2017). Thus, in our view, rigorous impact evaluations accompanied by process and economic evaluations that adhere to a published study protocol and provide transparency in the form of a declaration of conflict of interests amongst evaluators would allow policymakers to gauge whether a clinically effective intervention may be scalable and/or replicable within the same context or another, and aid a priority setting exercise that could lead to maximizing population health in the presence of health systems constraints.
Our review did not include any grey literature. This presents a possibility that we have excluded evidence on successful interventions implemented by government and/or non-government institutions. While including grey literature could potentially increase the comprehensiveness of the synthesized evidence, it also presents risks as studies may not always follow gold-standard or recommended guidelines for evaluation or may not be peer-reviewed (Adams et al., 2016).
Our research question is specific to whether behaviour change interventions can reduce antibiotic prescription, and therefore, our search terms are tailored to this objective. We included all studies that had antibiotic use and prescription as a primary, secondary or intermediate impact. We believe that the risk that papers where an improvement in antibiotic use through behaviour change interventions was a spill-over effect may have been captured but some publications may have been excluded because of titles, keywords and abstracts that did not explicitly match our research objective or search criteria.
Antibiotics remain a powerful and effective treatment for bacterial infections, but inappropriate use can pose a threat to health and well-being. Our review found that there are several effective behaviour strategies that can be implemented to improve antibiotic use in LMICs. However, the evidence base is heavily skewed towards healthcare providers with far less attention having been paid to improving antibiotic use amongst patients and the general public. Moreover, given the importance of private drug sellers in the provision of antibiotics in LMICs, it was surprising to see so few studies targeting these providers. From a design perspective, future studies in this field would also benefit from including longer time horizons for follow-up or more follow-up points to understand how the impact of interventions is sustained over time; process evaluations to understand the facilitators of and barriers to behaviour change; and full economic evaluations. Addressing these gaps will help to gain a clearer understanding of effective, sustainable and scalable approaches to tackle ABR, and in the long-term improve the health outcomes of individuals, and reduce resource burdens on household, families and health systems (Founou et al., 2017).
Conflict of interest statement. The authors have no conflicting interests.
Ethical approval. No ethical approval was required for this study.
Contributor Information
Carla Cuevas, Centre for Global Health Economics, Institute for Global Health, University College London, 30 Guilford Street, London WC1N 1EH, UK.
Neha Batura, Centre for Global Health Economics, Institute for Global Health, University College London, 30 Guilford Street, London WC1N 1EH, UK.
Luh Putu Lila Wulandari, The Kirby Institute, University of New South Wales, Level 6, Wallace Wurth BuildingHigh Street, UNSW Australia. Sydney, New South Wales, 2052, Australia; Department of Global Health and Development, Faculty of Public Health and Policy, London School of Hygiene and Tropical Medicine, 15-17 Tavistock Place, London, WC1H 9SH, UK.
Mishal Khan, Department of Global Health and Development, Faculty of Public Health and Policy, London School of Hygiene and Tropical Medicine, 15-17 Tavistock Place, London, WC1H 9SH, UK; Aga Khan University, National Stadium Road, Karachi, Pakistan.
Virginia Wiseman, The Kirby Institute, University of New South Wales, Level 6, Wallace Wurth BuildingHigh Street, UNSW Australia. Sydney, New South Wales, 2052, Australia; Department of Global Health and Development, Faculty of Public Health and Policy, London School of Hygiene and Tropical Medicine, 15-17 Tavistock Place, London, WC1H 9SH, UK.
References
- Adams J, Hillier-Brown FC, Moore HJ. et al. 2016. Searching and synthesising “grey literature” and “grey information” in public health: critical reflections on three case studies. Systematic Reviews 5: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken AM, Wanyoro AK, Mwangi J. et al. 2013. Changing use of surgical antibiotic prophylaxis in Thika Hospital, Kenya: a quality improvement intervention with an interrupted time series design. PLoS One 8: e78942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S, Straus S.. 2009. Interventions to improve antibiotic prescribing practices in ambulatory care (Review). Cochrane Database of Systematic Reviews 4. doi: 10.1002/14651858.CD003539.pub2.www.cochranelibrary.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atif M, Asghar S, Mushtaq I. et al. 2019. What drives inappropriate use of antibiotics? A mixed methods study from Bahawalpur, Pakistan. Infection and Drug Resistance 12: 687–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins D, Best D, Briss PA, GRADE Working Group et al. 2004. Grading quality of evidence and strength of recommendations. BMJ (Clinical Research ed.) 328: 1490–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auta A, Hadi MA, Oga E. et al. 2019. Global access to antibiotics without prescription in community pharmacies: a systematic review and meta-analysis. Journal of Infection 78: 8–18. [DOI] [PubMed] [Google Scholar]
- Ayukekbong JA, Ntemgwa M, Atabe AN.. 2017. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrobial Resistance & Infection Control 6: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babigumira JB, Lubinga SJ, Jenny AM. et al. 2017. Impact of pharmacy worker training and deployment on access to essential medicines for children under five in Malawi: a cluster quasi-experimental evaluation. Bmc Health Services Research 17: 638. doi: 10.1186/s12913-017-2530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanova Y, Drobniewski F, Nikolayevskyy V. et al. 2009. An integrated approach to rapid diagnosis of tuberculosis and multidrug resistance using liquid culture and molecular methods in Russia. PLoS One 4: e7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batura N, Cuevas C, Khan M. et al. 2018. How effective and cost-effective are behaviour change interventions in improving the prescription and use of antibiotics in low-income and middle-income countries? A protocol for a systematic review. BMJ Open 8: e021517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berild D, Abrahamsen TG, Andresen S. et al. 2008. A controlled intervention study to improve antibiotic use in a Russian paediatric hospital. International Journal of Antimicrobial Agents 31: 478–83. [DOI] [PubMed] [Google Scholar]
- Bloom G, Merrett GB, Wilkinson A. et al. 2017. Antimicrobial resistance and universal health coverage. BMJ Global Health 2: e000518–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M, Katikireddi SV, Sowden A. et al. 2018. Improving conduct and reporting of narrative synthesis of quantitative data (ICONS-Quant): protocol for a mixed methods study to develop a reporting guideline. BMJ Open 8: e020064–5. [Google Scholar]
- Chalker J, Ratanawijitrasin S, Chuc NTK. et al. 2005. Effectiveness of a multi-component intervention on dispensing practices at private pharmacies in Vietnam and Thailand—a randomized controlled trial. Social Science & Medicine 60: 131–41. [DOI] [PubMed] [Google Scholar]
- Chan Y-H, Fan MM, Fok C-M. et al. 2012. Antibiotics nonadherence and knowledge in a community with the world’s leading prevalence of antibiotics resistance: implications for public health intervention. American Journal of Infection Control 40: 113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charani E, Edwards R, Sevdalis N. et al. 2011. Behavior change strategies to influence antimicrobial prescribing in acute care: a systematic review. Clinical Infectious Diseases 53: 651–62. [DOI] [PubMed] [Google Scholar]
- Cooper H.1989. Integrating Research: A Guideline for Literature Reviews, 2nd edn.Newbury Park, London, New Delhi: Sage Publications. https://openlibrary.org/books/OL2057317M/Integrating_research, accessed 9 April 2020. [Google Scholar]
- Craig Pet al. 2008. Developing and evaluating complex interventions: the New Medical Research Council guidance. BMJ 337: 979–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross ELA, Tolfree R, Kipping R.. 2017. Systematic review of public-targeted communication interventions to improve antibiotic use. Journal of Antimicrobial Chemotherapy 72: 975–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundill B, Mbakilwa H, Chandler CI. et al. 2015. Prescriber and patient-oriented behavioural interventions to improve use of malaria rapid diagnostic tests in Tanzania: facility-based cluster randomised trial. BMC Medicine 13: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey Pet al. 2013. Interventions to improve antibiotic prescribing practices for hospital inpatients. In: Davey P (ed.) Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd. [DOI] [PubMed] [Google Scholar]
- Davey Pet al. 2017. Interventions to improve antibiotic prescribing practices for hospital inpatients (Review). Cochrane Database of Systematic Reviews 2, CD003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MFet al. 2005. Methods for the Economic Evaluation of Health Care Programmes, 3rd edn.Oxford University Press, Oxford. [Google Scholar]
- Dwan K.2011. Comparison of protocols and registry entries to published reports for randomised controlled trials. Journal of Evidence-Based Medicine 4: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaily HM, Silver I, Shiva S. et al. 2010. Can rational prescribing be improved by an outcome-based educational approach? A randomized trial completed in Iran. Journal of Continuing Education in the Health Professions 30: 11–18. [DOI] [PubMed] [Google Scholar]
- Espinoza Franco Bet al. 2009. The determinants of the antibiotic resistance process. Infection and Drug Resistance 2: 1–11. [PMC free article] [PubMed] [Google Scholar]
- Founou RC, Founou LL, Essack SY.. 2017. Clinical and economic impact of antibiotic resistance in developing countries: a systematic review and meta-analysis. PLoS One 12: e0189621–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel P, Ross-Degnan D, Berman P. et al. 1996. Retail pharmacies in developing countries: a behavior and intervention framework—ScienceDirect. Social Science & Medicine 42: 1155–1161. [DOI] [PubMed] [Google Scholar]
- Goldet G, Howick J.. 2013. Understanding GRADE: an introduction. Journal of Evidence-Based Medicine 6: 50–54. [DOI] [PubMed] [Google Scholar]
- Guinness L, Wiseman V.. 2011. Introduction to Health Economics, 2nd edn.Open University Press, McGraw-Hill Education. Maidenhead, Berkshire, England. [Google Scholar]
- Guyatt GH, Oxman AD, Vist GE, Kunz R. et al. 2008. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist GE, Knuz R. et al. 2008. What is “quality of evidence” and why is it important to clinicians. BMJ 336: 995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariton E, Locascio JJ.. 2018. Randomised controlled trials—the gold standard for effectiveness research. BJOG: An International Journal of Obstetrics & Gynaecology 125: 1716–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernando-Amado S, Coque TM, Baquero F. et al. 2019. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nature Microbiology 4: 1432–1442. [DOI] [PubMed] [Google Scholar]
- Holloway KA.2011. Combating inappropriate use of medicines. Expert Review of Clinical Pharmacology 4: 335–348. [DOI] [PubMed] [Google Scholar]
- Holloway KAet al. 2017. Antibiotic use in South East Asia and policies to promote appropriate use: reports from country situational analyses. BMJ (Clinical Research ed.) 358: j2291. doi: 10.1136/bmj.j2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg SD, Solomon SL, Blake PA.. 1987. Health and economic impacts of antimicrobial resistance. Clinical Infectious Diseases 9: 1065–78. [DOI] [PubMed] [Google Scholar]
- Holmes AH, Moore LSP, Sundsfjord A. et al. 2016. Understanding the mechanisms and drivers of antimicrobial resistance. The Lancet 387: 176–187. [DOI] [PubMed] [Google Scholar]
- Jadad AR, Rennie D.. 1998. The randomized controlled trial gets a middle-age checkup. Journal of the American Medical Association 279: 319–320. [DOI] [PubMed] [Google Scholar]
- Khan MS, Durrance-Bagale A, Legido-Quigley H. et al. 2019. ‘ LMICs as reservoirs of AMR’: a comparative analysis of policy discourse on antimicrobial resistance with reference to Pakistan. Health Policy and Planning 34: 178–187. [DOI] [PubMed] [Google Scholar]
- Kotwani A, Wattal C, Katewa S. et al. 2010. Factors influencing primary care physicians to prescribe antibiotics in Delhi India. Family Practice 27: 684–690. [DOI] [PubMed] [Google Scholar]
- Kwena Zet al. 2008. Provider characteristics among staff providing care to sexually transmitted infection self-medicating patients in retail pharmacies in Kibera slum, Nairobi, Kenya. Sexually Transmitted Diseases United States 35: 480–483. [DOI] [PubMed] [Google Scholar]
- Lansang MA, Lucas-Aquino R, Tupasi TE. et al. 1990. Purchase of antibiotics without prescription in Manila, The Philippines. Inappropriate choices and doses. Journal of Clinical Epidemiology 43: 61–67. [DOI] [PubMed] [Google Scholar]
- Laxminarayan R, Duse A, Wattal C. et al. 2013. Antibiotic resistance-the need for global solutions. The Lancet Infectious Diseases 13: 1057–1098. [DOI] [PubMed] [Google Scholar]
- Levy-Hara Get al. 2011. “Ten commandments” for the appropriate use of antibiotics by the practicing physician in an outpatient setting. Frontiers in Microbiology 2: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Xia T, Zhang X. et al. 2014. Governance structure reform and antibiotics prescription in community health centres in Shenzhen, China. Family Practice 31: 311–318. [DOI] [PubMed] [Google Scholar]
- Liu Cet al. 2016. ‘ Does public reporting influence antibiotic and injection prescribing to all patients? A cluster-randomized matched-pair trial in China. Medicine United States 95: e3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llor C, Bjerrum L.. 2014. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Therapeutic Advances in Drug Safety 5: 229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo-Wilson E, Fusco N, Li T. et al. 2017. Multiple outcomes and analyses in clinical trials create challenges for interpretation and research synthesis. Journal of Clinical Epidemiology 86: 39–50. [DOI] [PubMed] [Google Scholar]
- Michie S, van Stralen MM, West R. et al. 2011. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implementation Science 6: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mølbak K.2005. Human health consequences of antimicrobial drug—resistant salmonella and other foodborne pathogens. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 41: 1613–1620. [DOI] [PubMed] [Google Scholar]
- Moore GF, Audrey S, Barker M. et al. 2015. Process evaluation of complex interventions: medical Research Council guidance. BMJ 350: h1258–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu HC.1992. The crisis in antibiotic resistance. Science 257: 1064–1073. [DOI] [PubMed] [Google Scholar]
- Ngasala B, Mubi M, Warsame M. et al. 2008. Impact of training in clinical and microscopy diagnosis of childhood malaria on antimalarial drug prescription and health outcome at primary health care level in Tanzania: a randomized controlled trial. Malaria Journal 7: 199. doi: 10.1186/1475-2875-7-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngoh LN, Shepherd MD.. 1997. Design, development, and evaluation of visual aids for communicating prescription drug instructions to nonliterate patients in rural Cameroon. Patient Education and Counseling 30: 257–270. [DOI] [PubMed] [Google Scholar]
- O’Neill J.2016. Tackling drug-resistant infections globally: final report and recommendations. Review on Antimicrobial Resistance 110. doi: 10.4103/2045-080x.186181. [Google Scholar]
- Okeke IN, Laxminarayan R, Bhutta ZA. et al. 2005. Antimicrobial resistance in developing countries. Part I: Recent trends and current status. The Lancet Infectious Diseases 5: 481–493. [DOI] [PubMed] [Google Scholar]
- Om Cet al. 2016. “If it’s a broad spectrum, it can shoot better”: inappropriate antibiotic prescribing in Cambodia. Antimicrobial Resistance and Infection Control 5:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- One Health Agenda. 2020. One Health Platform (02-29-2020). https://onehealthplatform.com/sites/default/files/downloadables/OHP_agenda_0.pdf
- Owens RC, Fraser GL, Stogsdill P.. 2004. Antimicrobial stewardship programs as a means to optimize antimicrobial use. Pharmacotherapy 24: 896–908. [DOI] [PubMed] [Google Scholar]
- Paladino JA, Sunderlin JL, Price CS. et al. 2002. Economic consequences of antimicrobial resistance. Surgical Infections 3: 259–267. [DOI] [PubMed] [Google Scholar]
- Pavin M, Nurgozhin T, Hafner G. et al. 2003. Prescribing practices of rural primary health care physicians in Uzbekistan. Tropical Medicine and International Health 8: 182–190. [DOI] [PubMed] [Google Scholar]
- Peinemann F, Tushabe DA, Kleijnen J.. 2013. Using multiple types of studies in systematic reviews of health care interventions—a systematic review. PLoS One 8: e85035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petticrew M, Rehfuess E, Noyes J. et al. 2013. Synthesizing evidence on complex interventions: how meta-analytical, qualitative, and mixed-method approaches can contribute. Journal of Clinical Epidemiology 66: 1230–1243. [DOI] [PubMed] [Google Scholar]
- Podhipak A, Varavithya W, Punyaratabandhu P. et al. 1993. Impact of an educational program on the treatment practices of diarrheal diseases among pharmacists and drugsellers. The Southeast Asian Journal of Tropical Medicine and Public Health 24: 32–39. [PubMed] [Google Scholar]
- Popay Jet al. 2006. Guidance on the conduct of narrative synthesis in systematic reviews. A Product from the ESRC Methods Programme 211–219. doi: 10.1111/j.1523-536x.1995tb00261.x. [Google Scholar]
- Radyowijati A, Haak H.. 2003. Improving antibiotic use in low-income countries: an overview of evidence on determinants. Social Science & Medicine (1982)57: 733–744. [DOI] [PubMed] [Google Scholar]
- Ryan R, Hill S.. 2016. How to GRADE the quality of the evidence. Cochrane Consumers and Communication Group, Version 3. pp. 1–24. doi: 10.1021/acs.jpclett.8b02712. [Google Scholar]
- Sanson-Fisher RW, Bonevski B, Green LW. et al. 2007. Limitations of the randomized controlled trial in evaluating population-based health interventions. American Journal of Preventive Medicine 33: 155–161. [DOI] [PubMed] [Google Scholar]
- Santa-Ana-Tellez Y, Mantel-Teeuwisse AK, Dreser A. et al. 2013. Impact of over-the-counter restrictions on antibiotic consumption in Brazil and Mexico. PLoS One 8: e75550–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shünemann H.et al. (2013) GRADE handbook, Cochrane Collaboration. http://gdt.guidelinedevelopment.org/app/handbook/handbook.html, accessed 10 November 2019.
- Smith DRM, Dolk FCK, Pouwels KB. et al. 2018. Defining the appropriateness and inappropriateness of antibiotic prescribing in primary care. Journal of Antimicrobial Chemotherapy 73: ii11–ii18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpradit N, Chongtrakul P, Anuwong K. et al. 2012. Antibiotics smart use: a workable model for promoting the rational use of medicines in Thailand. Bulletin of the World Health Organization 90: 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suy S, Rego S, Bory S. et al. 2019. Invisible medicine sellers and their use of antibiotics: a qualitative study in Cambodia. BMJ Global Health 4: e001787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumwikirize WAet al. 2004. Impact of a face-to-face educational intervention on improving the management of acute respiratory infections in private pharmacies and drug shops in Uganda. East African Medical Journal. Kenya, 81: 2 Suppl S25–S32. [PubMed] [Google Scholar]
- Vassall A.et al. (2017) Reference Case for Estimating the Costs of Global Health Services and Interventions. https://www.ghcosting.org/pages/standards/reference_case, accessed 9 April 2020.
- Wahlstrom Ret al. 2003. Effectiveness of feedback for improving case management of malaria, diarrhoea and pneumonia–a randomized controlled trial at provincial hospitals in Lao PDR. Tropical Medicine & International Health : TM & IH 8: 901–909. [DOI] [PubMed] [Google Scholar]
- Wilkinson A, Ebata A, Macgregor H.. 2018. Interventions to reduce antibiotic prescribing in LMICs: a scoping review of evidence from human and animal health systems. Antibiotics 8: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank. 2017. Drug-Resistant Infections: A Threat to Our Economic Future, Publishing and Knowledge Division, The World Bank, 1818 H Street NW, Washington, DC 20433, USA. doi: 10.1007/s11947-009-0181-3.
- World Bank. 2020. World Bank Country and Lending Groups—World Bank Data Help Desk. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519, accessed 27 May 2020.
- World Health Organisation. 2018. Monitoring Global Progress on Addressing Antimicrobial Resistance. https://apps.who.int/iris/bitstream/handle/10665/273128/9789241514422-eng.pdf?ua=1.
- World Health Organization (WHO). 2014. Antimicrobial Resistance. Global Report on Surveillance. doi: 10.1016/j.giec.2020.06.004.
- World Health Organization (WHO). 2015a. Global Action Plan on Antimicrobial Resistance. doi: 10.1128/microbe.10.354.1. [DOI] [PubMed]
- World Health Organization (WHO). 2015b. WHO report finds systems to combat antibiotic resistance lacking. https://www.who.int/mediacentre/news/releases/2015/antibiotic-resistance-lacking/en/, accessed 29 February 2020.
- World Health Organization (WHO). 2016a. United Nations high-level meeting on antimicrobial resistance. https://www.who.int/antimicrobial-resistance/events/UNGA-meeting-amr-sept2016/en/, accessed 10 November 2019.
- World Health Organization (WHO). 2016b. ‘WHO | Antibiotic resistance’. World Health Organization. http://www.who.int/mediacentre/factsheets/antibiotic-resistance/en/#.WEXNQUSXazI.mendeley, accessed 5 December 2016.
- World Health Organization (WHO). 2018. Antibiotic resistance, Fact Sheets. https://www.who.int/en/news-room/fact-sheets/detail/antibiotic-resistance#.WEXNQUSXazI.mendeley, accessed 10 November 2019.
- Xiao Y, Zhang J, Zheng B. et al. 2013. Changes in Chinese policies to promote the rational use of antibiotics. PLoS Medicine 10: e1001556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip W, Powell-Jackson T, Chen W. et al. 2014. Capitation combined with pay-for-performance improves antibiotic prescribing practices in Rural China. Health Affairs 33: 502–510. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Dawkins B, Hicks JP. et al. 2018. Cost-effectiveness analysis of a multi-dimensional intervention to reduce inappropriate antibiotic prescribing for children with upper respiratory tract infections in China. Tropical Medicine & International Health 23: 1092–1100. [DOI] [PubMed] [Google Scholar]