Significance
The Siberian Arctic has witnessed numerous societal changes since the first known appearance of dogs in the region ∼10,000 years ago. These changes include the introduction of ironworking ∼2,000 years ago and the emergence of reindeer pastoralism ∼800 years ago. The analysis of 49 ancient dog genomes reveals that the ancestry of Arctic Siberia dogs shifted over the last 2,000 years due to an influx of dogs from the Eurasian Steppe and Europe. Combined with genomic data from humans and archaeological evidence, our results suggest that though the ancestry of human populations in Arctic Siberia did not change over this period, people there participated in trade with distant communities that involved both dogs and material culture.
Keywords: dogs, palaeogenomics, Arctic, population genetics
Abstract
Dogs have been essential to life in the Siberian Arctic for over 9,500 y, and this tight link between people and dogs continues in Siberian communities. Although Arctic Siberian groups such as the Nenets received limited gene flow from neighboring groups, archaeological evidence suggests that metallurgy and new subsistence strategies emerged in Northwest Siberia around 2,000 y ago. It is unclear if the Siberian Arctic dog population was as continuous as the people of the region or if instead admixture occurred, possibly in relation to the influx of material culture from other parts of Eurasia. To address this question, we sequenced and analyzed the genomes of 20 ancient and historical Siberian and Eurasian Steppe dogs. Our analyses indicate that while Siberian dogs were genetically homogenous between 9,500 to 7,000 y ago, later introduction of dogs from the Eurasian Steppe and Europe led to substantial admixture. This is clearly the case in the Iamal-Nenets region (Northwestern Siberia) where dogs from the Iron Age period (∼2,000 y ago) possess substantially less ancestry related to European and Steppe dogs than dogs from the medieval period (∼1,000 y ago). Combined with findings of nonlocal materials recovered from these archaeological sites, including glass beads and metal items, these results indicate that Northwest Siberian communities were connected to a larger trade network through which they acquired genetically distinctive dogs from other regions. These exchanges were part of a series of major societal changes, including the rise of large-scale reindeer pastoralism ∼800 y ago.
Early archaeological and genomic evidence from Zhokhov Island in Arctic Siberia indicates that dogs belonging to a distinct lineage were an essential component of life in the Arctic for over 9,500 y (1, 2). This tight link between people and dogs continues in Siberian communities such as the Koryaks, Itel’mens, Chukchi, and Nenets, where dogs continued to be used for hunting, herding, and sledding among other activities (3–5). Recent genomic data obtained from Samoyedic-speaking communities such as Nenets and Selkups suggest that during the Holocene they received limited gene flow from neighboring groups, including Steppe pastoralists (6, 7). Given that humans and their dogs often migrate and interact in parallel (8), it is possible that Siberian dogs also experienced limited gene flow from other populations.
In contrast to the human genomic evidence, linguistic and ethnographic data suggest more dynamic processes. Specifically, these data suggest that Samoyedic-speaking peoples of Northwest Siberia migrated from southern Siberia, or neighboring regions of southeast Europe, to the Arctic as recently as ∼3,000 to 4,000 y ago (9–12). In addition, archaeological sites such as Ust’-Polui in Northwest Siberia show evidence of iron and bronze metallurgy and isolated finds such as glass beads that were likely introduced from the Steppe, Black Sea, or the Near East (13–15). The presence of this material culture suggests that these communities participated in broad-ranging trade networks (13–15). The proposed migrations and exchanges of materials and practices potentially also involved dogs, which could have led to admixture, improvement, and ultimately to the establishment of modern Siberian dog lineages such as the modern Samoyed breed.
To assess whether the Northwest Siberian Arctic dogs population was continuous, or was instead marked by admixture (possibly in relation to the influx of material culture from other parts of Eurasia), we sequenced 20 ancient and historical Siberian and Eurasian Steppe dogs ranging in age from 11,000 to 60 y ago and in genomic coverage between 0.1× and 11.1× (Dataset S1). We then analyzed these genomes alongside publicly available ancient (n = 29) and modern (n = 120) canids (Fig. 1A and Dataset S2).
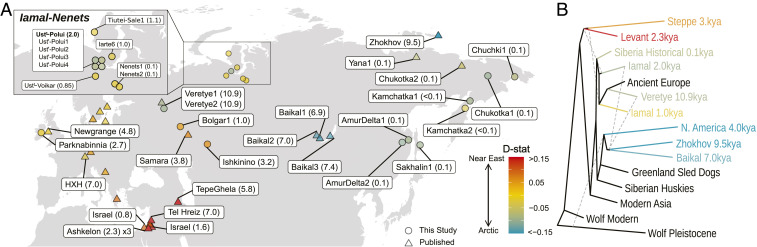
Fig. 1.
(A) Map of ancient dogs included in the study with sample name and age (kya) with an Inset map of the Iamal-Nenets region of Northwest Siberia. Data from samples represented by circles were generated in this study with a mean genome coverage between 0.1 and 19.9×; triangles represent publicly available ancient dogs. The colors of the data points represent the D-statistic value of the form D (black jackal, sample; Zhokhov, ASHQ01). Red-shifted colors show a closer affinity to ASHQ01 (ancient Near Eastern dog), and blue-shifted colors indicate a closer affinity to Zhokhov (ancient Arctic dog). (B) A TreeMix phylogeny with five migration edges that are indicated by gray dotted lines. Each population contains between one and three individuals (SI Appendix, Table S1). The color of the branches correspond to average D-statistic value in A. Complete models with the outgroup and edge weights, as well as models with additional edges can be found in SI Appendix, Fig. S3.
Results
Evaluating Broad Ancestry Patterns in Siberian Dogs.
We first assessed whether Siberian dogs possessed similar ancestry using both principal component analyses (PCA; SI Appendix, Fig. S1) and phylogenetic analyses (Fig. 1B and SI Appendix, Fig. S2). These analyses recapitulate findings of previous studies indicating that modern and ancient dogs can be broadly classified into three major groups: West Eurasian, East Asian, and Arctic/Americas (8, 16). The West Eurasian lineage includes ancient Near Eastern and Levantine, modern African, ancient and modern European, and newly sequenced Bronze Age Steppe dogs (8, 16). The East Asian lineage consists of modern dogs from China, Vietnam, Island Southeast Asia, Dingoes, and New Guinea Singing Dogs. The Arctic/Americas lineage includes modern Arctic breeds such as Greenland Sled Dogs and Siberian Huskies, ancient American dogs, mid-Holocene dogs from Lake Baikal, a 9,500-y-old dog from Zhokhov Island, dogs from the Iamal-Nenets region, and historical dogs from across Siberia sequenced for this study (Fig. 1B). This indicates that the newly sequenced Siberian dogs in this study possess genetic ancestry that was continuous for at least 9,500 y.
We then made use of TreeMix to assess admixture between these lineages. These analyses, based on 21 ancient and 14 modern dog genomes, broadly recapitulate our neighbor-joining tree (Fig. 1B and SI Appendix, Figs. S2 and S3 A and B) and support the results of previous studies (8, 17) showing that the ancestry of European dogs resulted from admixture between Near Eastern and Arctic dog lineages as early as 10.9 thousand years ago (kya). Our expanded dataset indicated that the 9,500-y-old Zhokhov Island sample is closest to a 6,000-y-old sample from Lake Baikal, rather than to ancient North American dogs (18) (SI Appendix, Figs. S2 and S4A). This result is consistent with the split between ancient American and Siberian Arctic dogs and between divergent Siberian lineages being older than the calibrated radiocarbon date of the Zhokhov Island sample (∼9.5 kya) and with an end-Pleistocene or very early Holocene introduction (and subsequent long-term isolation) of dogs into the Americas (18, 19).
Analyses of a published and a newly sequenced genome from the Mesolithic site of Veretye I (∼10.9 kya; Fig. 1) in Northeast Europe recapitulate previous findings showing that these dogs possessed ancestry related to both Arctic (66% and 71%) and Western Eurasian (34% and 29%) lineages (8, 17) (Figs. 1 and 2, SI Appendix, Figs. S3 A and B and S4 A and D, and Dataset S3). This result suggests that akin to all other modern and ancient (post-Mesolithic) European dogs sequenced to date, Mesolithic dogs in Europe already possessed both Arctic and Western Eurasian ancestry. The fact that the ancient Siberian dog from Zhokhov Island, dated to ∼1,000 y after the Veretye dogs, possesses no Western Eurasian ancestry, however, indicates that Western dog ancestry had not reached the Siberian Arctic by 9.5 kya.
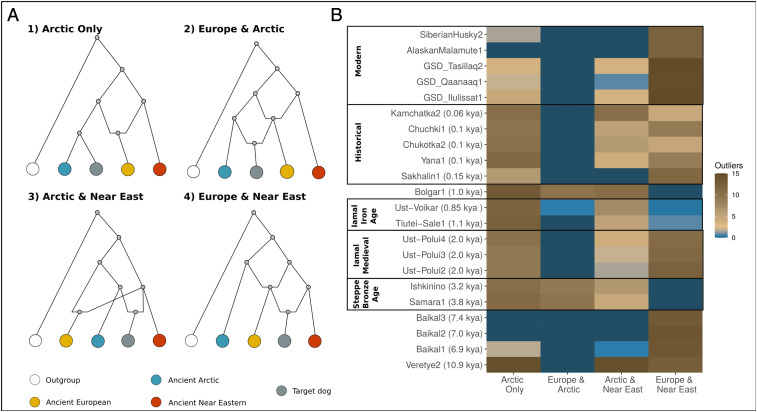
Fig. 2.
(A) Schematic representation of predefined models fitted using Admixturegraph to assess the ancestry of Siberian/Steppe dogs. (B) Heat map based on the number of D-stats outliers under each model. Best fitting models left no outliers (dark blue). The values are averaged across four different backbones each including the black backed jackal (outgroup), an Ancient Arctic (Zhokhov dog), an ancient European lineage (HXH or Newgrange), and an ancient Near Eastern lineage (TepeGhela or ASHQ01).
Establishing the Ancestry of Bronze Age Steppe Dogs.
The lack of Western Eurasian ancestry in the Zhokhov dog does not preclude the possibility of later influx of dogs into the Siberian Arctic from neighboring regions such as Europe, the Near East, or the Steppe. To test this hypothesis, we first established the ancestry of Steppe dogs from the Bronze Age [5 to 3 kya (20)], since their ancestry has not been characterized, and they represent a potential source of admixture in Siberian dogs. Analyses of one publicly available (Samara1, 0.7×) and one newly sequenced Bronze Age Steppe dog (Ishkinino, 1.4×) revealed that they form a genetically homogenous group that is closest to West Eurasian dogs (Fig. 1A and SI Appendix, Figs. S1 and S5). In fact, TreeMix analyses indicated that these dogs show strong affinities to ancient Near Eastern dogs (SI Appendix, Fig. S3 A and B), and admixture graph modeling suggested that Steppe dogs possessed additional Near Eastern ancestry greater than that found in ancient European dogs (Fig. 2). In addition, D-statistics detected no evidence of admixture from other populations (including from Arctic dogs) in either of the two Steppe dogs since their most recent common ancestor (>3.8 kya) (SI Appendix, Fig. S5).
Additional modeling using F4 ratios suggested that the ancestry of Steppe dogs can be modeled as ∼40% Arctic (represented by the Zhokhov Island dog) and ∼60% Near East (represented by Iranian and Levantine ancient dogs), reference SI Appendix. Previous studies of ancient human genomes indicated that a population related to Chalcolithic Iranians contributed ∼40% of the ancestry of Bronze Age Steppe populations likely as a result of expansion of farming from the Near East into the Steppe (21). Although we cannot rule out the hypothesis that Steppe dogs were introduced from Europe, our analyses suggest that, as in Europe (8, 22), the Neolithic expansion of farming from the Near East into the Steppe also involved the dispersal of dogs. The analyses of a more recent (0.7 kya) Steppe dog genome (Bolgar1) from the medieval city of Bolgar in Tatarstan (Russia) indicates that although European ancestry increased over time (Fig. 1A and SI Appendix, Figs. S2 and S4 A and D), the Near Eastern ancestry likely remained in dogs from the Steppe at least until the Middle Ages (Fig. 2 and SI Appendix, Fig. S6 B and C).
Testing for Admixture in Siberian Dogs.
In contrast to Bronze Age Steppe dogs, our analyses found that there are varying degrees of affinities (as revealed by D-statistics) among ancient and historical Siberian dogs to Near Eastern (Western Eurasian) and ancient Arctic ancestry (Fig. 1 and Datasets S1 and S4). In fact, our PCA indicated that all Siberian dogs sequenced in this study fall along a cline between West Eurasian and Arctic dogs (SI Appendix, Fig. S1), suggesting that Siberian dogs possessed different levels of West Eurasian ancestry. This influx of non-Arctic ancestry into Siberian dogs could be the result of the introduction of dogs with either Near Eastern/Steppe ancestry, European ancestry, or both. Testing these scenarios is difficult, since previous studies have shown that “European” ancestry itself resulted from admixture between Arctic and Western Eurasian (Near Eastern) ancestries (8).
In order to ascertain the different ancestry components of the dogs that admixed with the Siberian dogs dated between 10.9 and 0.06 kya, we defined six separate models (four of which are displayed in Fig. 2A) using the Admixture Graph R package (23) in which we used representatives of Near Eastern, European, and Arctic dogs, and iteratively tested how well each of the models fit the genomes of 22 Siberian dogs. We did not include East Asian dogs in this analysis for two reasons. First, archaeological evidence suggests that Northwestern Siberian communities primarily interacted with communities from the Steppe, Black Sea, and the Near East (13–15). Second, the results of our own analyses (SI Appendix, Figs. S3 A and B, S7, and S8 and Dataset S4) and previous studies (8) indicate that although Arctic dogs (represented by the ancient Zhokhov dog) may have contributed to East Asian dog ancestry, there is little evidence of gene flow in the opposite direction (SI Appendix).
To represent each of the three ancestral lineages in the models, we chose two representatives from the Near East (TepeGhela, 5.8 kya; ASHQ01, 2.3 kya), two representatives from Europe (Newgrange, 4.8 kya; HXH, 7.0 kya), and one representative from the Arctic (Zhokhov, 9.5 kya). We then defined a set of models to characterize the ancestry of each Siberian dog as having derived from either of the following: l) solely the Arctic dog lineage, 2) both Arctic and European dog lineages, 3) both Arctic and Near Eastern lineages, and 4) exclusively European and Near Eastern dogs only (Fig. 2). In all models, European ancestry was modeled as a mixture of Near East and Arctic (8). We then applied all four possible combinations of representative genomes and tested the goodness of fit with each Siberian/Steppe dog.
Ancient Lake Baikal Dogs (∼7 kya) Possess Limited Western Eurasian Ancestry.
We first applied this method to assess whether the three Lake Baikal dogs (7.4, 7.0, and 6.9 kya) possessed any non-Arctic ancestry. Our analysis indicates that three of our four models fit the data equally well for the two oldest, lower coverage (Baikal2 and Baikal3; ∼0.3×) dogs (Fig. 2). In the case of the youngest and higher coverage (Baikal1; 2.2×) Lake Baikal sample, however, we found that models involving admixture from Europe did fit slightly better (Fig. 2). Though it is tempting to interpret these results as evidence for the arrival of dogs possessing non-Arctic ancestry between 7.4 and 6.9 ka, this finding could also result from the lower coverage of the two older genomes. To further query whether these dogs possessed a history of gene flow at all, we performed an exhaustive model search using qpBrute (17), in which all three Lake Baikal dogs were analyzed as a single population (SI Appendix). The results suggest that only ∼3% of more than 20,000 tested scenarios fit the data, and of these, the majority (∼60%) involved no admixture from a Western Eurasian source into any of the Lake Baikal dogs since their split with Zhokhov.
Although more complex models with admixture from a Western source postdating the common ancestor of Lake Baikal and Zhokhov Island dogs did fit the data (also evident when considering only the youngest dog [6.9 kya]; Fig. 2), F4 ratio tests suggested that this contribution was marginal (∼9% of the ancestry in the Lake Baikal dogs). In addition, D-statistics analyses based on alignments to a red fox (Vulpes vulpes) reference genome (VulVul2.2) indicated a slight reference bias when compared to the canFam3.1 assembly (European Boxer dog) (SI Appendix, Fig. S10 and Dataset S4). However, regardless of the reference genome, D-statistics for Baikal1 showed statistically significant signals for gene flow from West Eurasian dogs, but this signal was not seen in the older Baikal dogs (Datasets S3 and S4). Combined, our results suggest that Lake Baikal dogs potentially received a limited influx of ancestry, around ∼6.9 kya from Western Eurasian dogs. Greater depth of coverage from these samples is required to more confidently assess this scenario. If correct, however, this would suggest that the spread of dogs from West and Southern regions, into the Siberian Arctic, began between 9.5 kya (the age of the Zhokhov dog) and 6.9 kya.
Increasing Levels of Western Eurasian Ancestry in Siberian Dogs over the Last 2 kya.
We then applied our admixture graph–based method to assess admixture from Western Eurasian sources into later Siberian dogs from contexts dating between 2.0 kya to the present day, following a period of an apparent absence of directly dated dog remains in Siberia between ∼6.0 kya to 4.3 kya (24). In contrast to the earlier Lake Baikal dogs, all of the best fitting models for these dogs involved admixture from a Western Eurasian source (Fig. 2), which suggests additional influx of European or Near Eastern–like ancestry into Siberia after ∼6.9 kya (the youngest Lake Baikal sample). Although in most cases models involving admixture from a European source fit best, some Iron Age (∼2.0 kya) and medieval (1.1 to 0.85 kya) dogs obtained from the Iamal-Nenets region in Northwest Siberia (Fig. 1A) potentially also possessed additional ancestry related to that of Near Eastern dogs (Fig. 2 and SI Appendix, Fig. S8). The signal for an additional contribution from the Near East to the medieval Iamal dogs was slightly weaker when using alignments to the red fox genome instead of the dog reference but is still present (compare SI Appendix, Fig. S6 B and C).
We further explored this scenario through an exhaustive model search using qpBrute (17) in which the Iron Age and medieval Iamal dogs were treated as two populations (SI Appendix, Fig. S11). Although the number and the topology of the fitting models varied slightly across the different runs (SI Appendix, Table S3 and Fig. S11), all models showed different independent admixture from European and/or Near Eastern dogs into medieval and Iron Age Iamal dogs. In line with nonlocal materials including glass beads and various metal items (13, 15) recovered from the same archaeological sites, our results indicate that the communities living in Iamal during the Iron Age and medieval periods were connected to a larger trade network through which they acquired dogs with non-Arctic ancestries (SI Appendix, Fig. S8).
The Emergence of the Modern Siberian Dog Lineages.
We then assessed whether the genome of two dogs sampled ∼100 y ago (1927 CE) from a Nenets community on the Iamal peninsula was related to Iamal dogs from medieval and Iron Age periods. Identity-by-descent pairwise distances indicated that the closest ancient Arctic dog relative of these two historical Nenets dogs was a medieval Iamal dog from Ust’-Voikar (SI Appendix, Fig. S4 B and D) suggesting a degree of population continuity from the medieval period through to the early 20th century. Interestingly, this analysis indicated that these two 100-y-old Nenets dogs were also closely related to Samoyed dogs, a modern spitz breed introduced to the United Kingdom in the nineteenth century from Siberia and which became popular among polar explorers such as Scott and Shackleton (25). This result indicates that although there have been multiple admixture episodes of Northwest Siberian dogs, their Arctic ancestry component survives in the modern Samoyed breed. Similarly, we found that modern Siberian Huskies share an affinity with historical East Siberian dogs and ancient Lake Baikal dogs (25) (Fig. 1 and SI Appendix, Figs. S4 C and F and S7). Together, these results indicate that several popular modern Arctic breeds maintained significant levels of ancestry from a lineage established prior to 9.5 kya in Siberia. Importantly, non-Siberian ancestry in Siberian dog breeds is not a modern phenomenon but has instead been an ongoing process for at least 2,000 y.
Conclusions
Our results indicate that Arctic dogs likely evolved in near isolation from other dog populations until at least the mid-Holocene (∼7 kya). These ancient Arctic dogs likely inhabited a large region of Siberia from the New Siberian Islands to Lake Baikal. Over the last 7,000 y, however, the evolutionary history of Siberian dogs has been influenced by multiple introductions of dogs from the Eurasian Steppe and Western Eurasia and little to no gene flow from East Asian dogs. Some of these introductions coincide with periods of major transformations within Northwest Siberian societies, including the introduction of metallurgy to the Arctic (15), the advent of the use of reindeer for transportation ∼2 kya, and the rise of reindeer pastoralism ∼800 y ago (26). Altogether, this suggests that these profound transformations in Northwest Siberia were linked with the importation of material culture (including dogs) from neighboring regions through the establishment of large-scale trade networks. This influx of genetic variability associated with dog lineages adapted to farming (Europe) and pastoralism (Steppe) potentially resulted in behavioral and morphological changes in Arctic dogs that may have facilitated the transition from foraging to pastoralism in the Siberian Arctic. The generation, analysis, and interpretation of additional ancient dog genomes within their archaeological context will help to address questions related to the role that dogs have played in the long-term human occupation of the Arctic, including the advent of novel survival strategies more generally, and more specifically, reindeer domestication and the transition to large-scale reindeer pastoralism.
Materials and Methods
Samples used for this study were obtained from archaeological contexts (n = 19) or ethnographic collections held at museums (n = 10). Detailed descriptions of the samples used for this study, including site information and context information, are presented in SI Appendix. DNA was extracted from each sample in laboratories dedicated to work on ancient or degraded DNA materials at the University of Copenhagen, Swedish Museum of Natural History, and Trinity College Dublin. All PCR reactions were conducted in separate facilities to prepare shotgun sequencing libraries; screening to determine endogenous DNA content for each sample was performed with Illumina sequencing technology, accordingly 20 of the 29 samples were selected for deep sequencing on Illumina and BGISeq platforms. Each sample was aligned to the CanFam3.1 reference dog genome (27) using the Burrows-Wheeler Alignment Backtrack algorithm (BWA aln) (28, 29), subsequently pseudohaploid calling was performed on the samples and a panel of publicly available canid samples with ANGSD (30) to be used for downstream analyses. Complete details of methods used for sampling, laboratory work, and computational analyses in this study are provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank B. Grønnow, F. Racimo, B. Sacks, and E. Ostrander for input and comments in the conceptualization and early drafts of this study. This research used both the University of Oxford’s Advanced Research Computing and Queen Mary's Apocrita High Performance Computing facility. We would like to acknowledge support from Science for Life Laboratory, the Swedish National Genomics Infrastructure for providing assistance in DNA sequencing. The following institutions are acknowledged for providing additional access to collections and logical support: museum of Institute of Plant and Animal Ecology of the Ural Branch of the Russian Academy of Sciences (Ekaterinburg) and Arctic Research Center (Salekhard). T.R.F. was supported by the European Union’s EU framework programme for research and innovation Horizon 2020 under Grant Agreement 676154. T.R.F. also received funding for analysis through the Qimmeq Project that came from the Velux Foundations and the Aage og Johanne Louis-Hansens Fond. M.-H.S.S. was supported by the Independent Research Fund Denmark (8028-00005B). L.A.F. and G.L. were supported by European Research Council grants (ERC-2013-StG-337574-UNDEAD and ERC-2019-StG-853272-PALAEOFARM) and Natural Environmental Research Council grants (NE/K005243/1, NE/K003259/1, NE/S007067/1, and NE/S00078X/1). L.A.F. and A.C. were supported by the Wellcome Trust (210119/Z/18/Z). R.J.L. and T.N. were supported by a Social Sciences and Humanities Research Council Grant IG 435-2014-0075. O.S. was supported by the European Union’s Horizon 2020 program (H2020-MSCA-IF-2015, project “EpiCDomestic,” Grant 704254). Collection of specimens was performed under the State Contract of the IPAE UB RAS (No. AAAA-A19-119031890086-0).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2100338118/-/DCSupplemental.
Data Availability
The raw fastq files for the DNA Sequencing data have been deposited on the European Nucleotide Archive (https://www.ebi.ac.uk/ena/browser/home) under project ID PRJEB42261 which is publically available for download (31).
References
- 1.Pitul’ko V. V., Kasparov A. K., Ancient Arctic hunters: Material culture and survival strategy. Arctic Anthropol. 33, 1–36 (1996). [Google Scholar]
- 2.Pitul’ko V. V., Kasparov A. K., Archaeological dogs from the Early Holocene Zhokhov site in the Eastern Siberian Arctic. J. Archaeol. Sci. Rep. 13, 491–515 (2017). [Google Scholar]
- 3.Adaev V. D., Reindeer herding laika among tundra Nenets: Specifics of exterior and performance of tasks. Scientific Vestnik of IaNAO 1, 25–33 (2014). [Google Scholar]
- 4.Batianova E. P., Turaev V. A., Peoples of the Northeastern Siberia (Nauka, 2010). [Google Scholar]
- 5.Taksami C. M., Ethnic Culture of Nivkhi–Aboriginals of the Pacific North and Far East (Nauka, Novosibirsk, 2007). [Google Scholar]
- 6.Wong E. H. M., et al., Reconstructing genetic history of Siberian and Northeastern European populations. Genome Res. 27, 1–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tambets K., et al., Genes reveal traces of common recent demographic history for most of the Uralic-speaking populations. Genome Biol. 19, 139 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergström A., et al., Origins and genetic legacy of prehistoric dogs. Science 370, 557–564 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prokof’ev G. N., Ethnogony of the ethnic groups in the Ob–Yenisei basin. Sov. Etnogr. 3, 67–76 (1940). [Google Scholar]
- 10.Hajdú P., The Samoyed Peoples and Languages (Indiana University, 1963). [Google Scholar]
- 11.Helimski E. A., The external connections and early contacts of the Uralic languages. Problems of Uralistics 1, 19–43 (1990). [Google Scholar]
- 12.Janhunen J., “Proto-Uralic—What, where, and when” in The Quasquicentennial of the Finno-Ugrian Society, Ylikoski J., Ed. (Suomalais-Ugrilainen Seura., Helsinki, 2009), pp. 57–78. [Google Scholar]
- 13.Fedorova N. V., Gusev A. V., The north of Western Siberia and the cultural worlds of Eurasia at the turn of the eras, Vestnik of St. Petersburg University. History (Lond.) 64, 740–761 (2019). [Google Scholar]
- 14.Pugach I., et al., The complex admixture history and recent southern origins of Siberian populations. Mol. Biol. Evol. 33, 1777–1795 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vodyasov E. V., Gusev A. V., Asochakova E. M., Ust-Polui: The earliest evidence of iron metallurgy in the Arctic region. Siberian Hist. Res. 3, 113–132 (2017). [Google Scholar]
- 16.Frantz L. A. F., et al., Genomic and archaeological evidence suggest a dual origin of domestic dogs. Science 352, 1228–1231 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Ní Leathlobhair M., et al., The evolutionary history of dogs in the Americas. Science 361, 81–85 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinding M. S., et al., Arctic-adapted dogs emerged at the Pleistocene-Holocene transition. Science 368, 1495–1499 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ameen C., et al., Specialized sledge dogs accompanied Inuit dispersal across the North American Arctic. Proc. Biol. Sci. 286, 20191929 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allentoft M. E., et al., Population genomics of Bronze Age Eurasia. Nature 522, 167–172 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Lazaridis I., et al., Genomic insights into the origin of farming in the ancient Near East. Nature 536, 419–424 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ollivier M., et al., Dogs accompanied humans during the Neolithic expansion into Europe. Biol. Lett. 14, 14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leppälä K., Nielsen S. V., Mailund T., admixturegraph: An R package for admixture graph manipulation and fitting. Bioinformatics 33, 1738–1740 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Losey R. J., et al., Dog body size in Siberia and the Russian Far East and its implications. Quat. Sci. Rev. 241, 106430 (2020). [Google Scholar]
- 25.Larson G., et al., Rethinking dog domestication by integrating genetics, archeology, and biogeography. Proc. Natl. Acad. Sci. U.S.A. 109, 8878–8883 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Røed K. H., et al., Temporal and structural genetic variation in reindeer (Rangifer tarandus) associated with the pastoral transition in Northwestern Siberia. Ecol. Evol. 10, 9060–9072 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindblad-Toh K., et al., Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 438, 803–819 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Li H., Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv [Preprint] (2013). arXiv:1303.3997v2 (Accessed 1 September 2019).
- 29.Li H., Durbin R., Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korneliussen T. S., Albrechtsen A., Nielsen R., ANGSD: Analysis of next generation sequencing data. BMC Bioinformatics 15, 356 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feuerborn T., Project: PRJEB422611, European Nucleotide Archive. https://www.ebi.ac.uk/ena/browser/view/PRJEB42261. Deposited 9 February 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw fastq files for the DNA Sequencing data have been deposited on the European Nucleotide Archive (https://www.ebi.ac.uk/ena/browser/home) under project ID PRJEB42261 which is publically available for download (31).