Abstract
Background
Hepatocellular carcinoma (HCC) recurrence rates following locoregional treatment are high. As multireceptor tyrosine kinase inhibitors targeting vascular endothelial growth factor receptors (VEGFRs) are effective in advanced HCC, we assessed the efficacy and safety of neoadjuvant systemic treatment with dovitinib in early‐ and intermediate‐stage HCC.
Materials and Methods
Twenty‐four patients with modified Child‐Pugh class A early‐ and intermediate‐stage HCC received neoadjuvant oral dovitinib 500 mg daily (5 days on/2 days off) for 4 weeks, followed by locoregional therapy. Primary endpoints were objective response rates and intratumoral blood flow changes. Secondary endpoints were safety, pharmacodynamical plasma markers of VEGFR‐blockade, time to progression (TTP), and overall survival (OS).
Results
Modified RECIST overall response rate was 48%, including 13% complete remission, and despite dose reduction/interruption in 83% of patients, intratumoral perfusion index decreased significantly. Grade 3–4 adverse events, most frequently (on‐target) hypertension (54%), fatigue (25%), and thrombocytopenia (21%), occurred in 88% of patients. Plasma VEGF‐A, VEGF‐D, and placental growth factor increased significantly, whereas sTie‐2 decreased, consistent with VEGFR‐blockade. Following neoadjuvant dovitinib, all patients could proceed to their original planned locoregional treatment. No delayed toxicity occurred. Seven patients (three early, four intermediate stage) underwent orthotopic liver transplant after median 11.4 months. Censoring at transplantation, median TTP and OS were 16.8 and 34.8 months respectively; median cancer‐specific survival was not reached.
Conclusion
Already after a short 4‐week dovitinib treatment period, intratumoral blood flow reduction and modest antitumor responses were observed. Although these results support use of systemic neoadjuvant strategies, the poor tolerability indicates that dovitinib dose adaptations are required in HCC.
Implications for Practice
Orthotopic liver transplantation may cure early and intermediate‐stage hepatocellular carcinoma. Considering the expected waiting time >6 months because of donor liver scarcity, there is an unmet need for effective neoadjuvant downsizing strategies. Angiogenesis inhibition by dovitinib does not negatively affect subsequent invasive procedures, is safe to administer immediately before locoregional therapy, and may provide a novel treatment approach to improve patient outcomes if tolerability in patients with hepatocellular carcinoma can be improved by therapeutic drug monitoring and personalized dosing.
Keywords: Biomarker, Inflammation, Multireceptor tyrosine kinase inhibitor, Perfusion computed tomography, Quality of life
Short abstract
Considering the scarcity of liver donors and expected waiting time to liver transplantation, there is an unmet need for effective neoadjuvant downsizing strategies. This article reports on the safety and efficacy of neoadjuvant dovitinib in patients with hepatocellular carcinoma eligible for locoregional treatment.
Introduction
Primary liver cancer is the sixth most commonly diagnosed cancer worldwide with 841,000 new cases and ~782,000 cancer‐related deaths annually [1]. Hepatocellular carcinoma (HCC) accounts for 75%–85% of liver cancers and predominantly arises from cirrhotic livers.
Orthotopic liver transplantation (OLT) is the preferred curative treatment option for HCC patients not amenable to resection and fulfilling the Milan criteria [2]. Patients with intermediate‐ and early‐stage Barcelona Clinic Liver Cancer (BCLC) with lesions >5 cm large may only become eligible for transplant after successful downsizing [3, 4], but the recurrence rate after locoregional treatment is high: 66% of patients relapse after radiofrequency ablation (RFA) of small HCC, 42% of whom became transplant ineligible [5]. To date, there is an unmet need for effective neoadjuvant downsizing strategies with durable responses to qualify for transplant and improve disease outcome.
Approved first‐line treatment options for advanced HCC are multireceptor tyrosine kinase inhibitors (TKIs) sorafenib and lenvatinib [2], which, among others, target vascular endothelial growth factor receptors (VEGFR)1–3. Dovitinib (CHIR‐258/TKI258), a potent oral inhibitor of multiple oncogenic and proangiogenic pathways implicated in tumor progression, metastasis, and poor prognosis [6], demonstrated similar time to progression (TTP) and overall survival (OS) as sorafenib in a phase II study of treatment‐naive patients with advanced HCC, but with more patients having tumor shrinkage as best response than sorafenib [7]. Frontline treatment with dovitinib has not been investigated in earlier stages of HCC and is of interest as the short half‐life of dovitinib compared with sorafenib may result in reduced occurrence of wound healing complications following subsequent invasive treatments [8, 9], whereas additional inhibition of fibroblast growth factor receptor (FGFR)/PI3K/Akt/mTOR and JAK/STAT3 signaling pathways may amplify antitumor effect [10, 11].
We therefore investigated the safety and efficacy of neoadjuvant dovitinib in patients with HCC eligible for locoregional treatment. In addition, the antiangiogenic properties of dovitinib were assessed by measuring pharmacodynamical biomarkers of VEGFR signaling blockade and intratumoral blood flow using perfusion computed tomography (CTP).
Patients, Materials, and Methods
Study Design
From May 1, 2012, to September 30, 2014, we screened 39 patients with HCC at the Leiden University Medical Center: 8 did not meet inclusion criteria and 6 refused study participation, resulting in 25 patients enrolled. All participants provided written informed consent. The study was approved by the institutional Medical Ethical Committee (MEC) and was registered as EU‐CTR 2011‐002445‐36 (www.clinicaltrialsregister.eu).
Eligible patients were ≥18 years of age, modified Child‐Pugh class A, and BCLC stages 0, A–B and had measurable disease (full inclusion and exclusion criteria in supplemental online Table 1). Patients received dovitinib 500 mg orally once daily, 5 days on/2 days off for 4 weeks followed by locoregional therapy between days 31 and 35. As toxicity data of dovitinib in neoadjuvant setting were lacking, the institutional MEC requested a short treatment period to avoid delay of subsequent locoregional treatments. Dose reduction was based on a prespecified dose modification protocol (supplemental online Table 2). In case of unacceptable toxicity or dose interruption >21 days, study treatment was halted.
Primary endpoints were objective response rates after 4 weeks dovitinib treatment and changes in intratumoral blood flow on CTP. Tumor response was assessed in accordance with the RECIST 1.1 and modified RECIST (mRECIST) criteria by two independent local reviewers [12, 13]. Secondary endpoints were safety and toxicity, changes in plasma angiogenic and inflammatory proteins, TTP, OS, radiological progression‐free survival (rPFS), and cancer‐specific survival (CSS). Survival was calculated from dovitinib administration day 1.
In this explorative study, no formal sample size calculation was performed because information on the effect size and variability is lacking. A minimum of 20 patients was considered sufficient to provide robust data.
Procedures
At study visits on days 0, 5, 12, 19, 26, and 30 days after locoregional therapy, patients were routinely assessed clinically and biochemically. Safety and toxicity were recorded throughout the study using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. Cardiotoxicity was evaluated by measurement of left ventricular ejection fraction (LVEF) at baseline and day 26 by multiple‐gated acquisition scan.
At baseline and prior to locoregional treatment, patients underwent staging by chest computed tomography (CT), contrast‐enhanced four‐phase abdominal CT, and dynamic volume sequence scans (Aquilion One 320‐MDCT, Canon Medical Systems, Japan) using Ultravist 370 contrast medium (Bayer Healthcare, Berlin, Germany). Recurrence after locoregional treatment was assessed three‐monthly by CT scan. Patients entered survival follow‐up at progression.
Three CTP scans (100 kV, 100 mA, rotation time 0.5 sec) were executed: sample times were 10–30 seconds, 33–51 seconds, 56–76 seconds; acquisition intervals were 2, 3, and 5. Arrival time in the aorta was established using a 10–15‐mL contrast test bolus. Dynamic volume sequence included 23 acquisitions of the liver (160 × 0.5 mm) and started 4 seconds before contrast entry in the aorta. Perfusion was analyzed using the Dual Input Maximum Slope algorithm. Regions of interest (ROIs) were positioned in the aorta, portal veins, normal hepatic tissue, and spleen. Color‐coded parametric maps and a set of arterial flow (AF), portal flow (PF), and perfusion index (PI) volumes corresponding to the tissue attenuation curves were plotted simultaneously. Finally, tumor AF, PF, and PI were determined by ROI placement in the tumor.
Health‐related quality of life (HRQoL) was registered prior to all study assessments using validated European Organization for the Research and Treatment of Cancer Quality of Life (EORTC QLQ)‐C30 version 3.0 and HCC18 questionnaires [14]. Items from global health, functional domains, and symptom scales were grouped and converted to scores ranging from 0 to 100 [15].
Histopathologic and Molecular Analysis
Tumor tissue was obtained by ultrasound‐guided 18 gauge needle biopsy before and after dovitinib treatment. To evaluate histopathological response (i.e., tumor necrosis, percentage vital tumor cells, lymphocyte infiltration, and microvessel density), formalin‐fixed paraffin‐embedded 3‐μm sections of paired biopsies were stained with H&E. Immunohistochemistry was performed routinely with Dako Omnis fully automatic stainer using anti‐β‐catenin clone 14 (BD Biosciences, San Jose, CA) and anti‐CD34 clone QBEnd10 (Dako, Glostrup, Denmark). Protein expression profiles were not determined because of tumor tissue exhaustion.
For molecular mutation profile assessment, genomic DNA was prepared from microdissected tumor specimens. Four samples were exhausted and two not suitable for targeted next generation sequencing (tNGS). From 18 of 24 (75%) patients, tumor samples were analyzed with the Ion Ampliseq Cancer Hotspot Panel v2 using Ion Torrent Personal Genome Machine (PGM)/Proton system chips (ThermoFisher Scientific, Waltham, MA). The tNGS kit included KRAS (exon 2–4), NRAS (exon 2–4), HRAS (exon 2–3), BRAF (exon 11, 15), EGFR (exon 3, 7, 15, 18–21), GNAQ (exon 5), GNAS (exon 8–9), IDH1 (exon 4), IDH2 (exon 4); KIT (exon 2, 9–18), PDGFRA (exon 12, 14, 15, 18, 23), PIK3CA (exon 2, 5, 6–10, 14, 18, 21); RET (exon 10–12, 15, 16); TP53 (exon 4–8, 11). In addition, hotspots were analyzed in ABL1, AKT1, ALK, APC, ATM, CDH1, CDKN2A, CSF1R, CTNNB1, ERBB2, ERBB4, EZH2, FBXW7, FGFR1, FGFR2, FGFR3, FLT3, GNA11, HNF1A, JAK2, JAK3, KDR, MET, MLH1, MPL, NOTCH1, NPM1, PTEN, PTPN11, RB1, SMAD4, SMARCB1, SMO, SRC, STK11, and VHL. Analysis was performed routinely according to local standards. Possible (class 3–4) or known (class 5) pathogenic variants with allele frequencies ≥0.1 and sequencing depth ≥ 100 reads were reported.
Plasma Biomarkers
Sodium heparin‐anticoagulated plasma was collected on days 0, 12, and 26 and stored at −20°C until analysis. Proteins involved in angiogenesis, inflammation, and vascular injury were quantitatively measured using the V‐PLEX Human Biomarker 40‐Plex kit (Meso Scale Diagnostics, Rockville, MD) in accordance with manufacturer's instructions and included soluble VEGFR1 (sVEGFR1), vascular endothelial growth factors A, C, and D, (VEGF‐A, VEGF‐C, VEGF‐D), placental growth factor (PlGF), soluble angiopoietin receptor 2 (sTie2), cytokines, and adhesion molecules, for example, interferon gamma (IFNγ), tumor necrosis factor alfa (TNF‐α), TNF‐β, granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), interleukin (IL)‐1α, IL‐2, IL‐4, IL‐5, IL‐6, IL‐7, IL‐8 (HA), IL‐10, IL‐12 (p70), IL‐13, IL‐15, IL‐16, IL‐17, eotaxin and eotaxin‐3, interferon gamma‐induced protein 10 (IP‐10), methyl‐accepting chemotaxis protein 1 and 4 (MCP1/MCP4), C‐C motif chemokine 22 (MDC), macrophage inflammatory proteins 1α/1β (MIP‐1α/MIP‐1β), thymus and activation regulated chemokine (TARC), C‐reactive protein (CRP), serum amyloid A (SAA), soluble intercellular adhesion molecule‐1 (sICAM‐1/CD54), and soluble vascular adhesion molecule‐1 (sVCAM‐1). Plasma of healthy subjects served as controls.
Statistical Analysis
Data analyses were conducted using Prism version 8.3.0 (GraphPad Software, San Diego, CA). The Wilcoxon matched‐pairs signed‐rank test was used to compare paired measurements at different time points. The Mann–Whitney U‐test was applied in case of nonparametric univariate analysis. Associations within categorical data were assessed with the Fisher's exact test, whereas linear and nonlinear correlations were evaluated using the Pearson (r) and Spearman's rho (r s) method respectively. Finally, Kaplan‐Meier estimates were used to determine TTP, rPFS, OS, and CSS. Statistical significance was set at p value <.05.
Results
Patient and Tumor Characteristics
Of 25 enrolled patients, 1 died before dovitinib dosing because of tumor biopsy‐related bleeding and was excluded from data analysis. Baseline characteristics and predetermined locoregional therapy of the remaining 24 patients are shown in Table 1. All patients (median age 63 years, 92% male) were treatment‐naive, had Child‐Pugh class A liver disease, and were free from cancer‐specific symptoms.
Table 1.
Study population at baseline (n = 24)
| Characteristics | Median (range) |
|---|---|
| Age, yr | 62.5 (46–81) |
| BMI, kg/m2 | 29.1 (20.2–50.0) |
| Male sex, n (%) | 22 (91.7) |
| Modified Child‐Pugh class A, n (%) | 24 (100) |
| ECOG performance score, n (%) | |
| 0 | 9 (37.5) |
| 1 | 15 (62.5) |
| Ethnicity, n (%) | |
| European | 18 (75.0) |
| Asian | 2 (8.3) |
| South American | 2 (8.3) |
| African | 2 (8.3) |
| Risk factors, n (%) | |
| Liver cirrhosis | 18 (75.0) |
| Alcohol abuse | 17 (70.8) |
| Smoking | 19 (79.2) |
| Hepatitis B | 6 (25.0) |
| Hepatitis C | 5 (20.8) |
| Diabetes | 12 (50.0) |
| Obesity | 10 (41.6) |
| Nonalcoholic steatohepatitis | 3 (12.5) |
| BCLC stage, n (%) | |
| 0 | 3 (12.5) |
| A | 10 (41.6) |
| B | 11 (45.8) |
| Histologic grade, n (%) | |
| Well differentiated | 6 (25.0) |
| Moderately differentiateda | 7 (29.2) |
| Poorly differentiated | 3 (12.5) |
| Not evaluable | 8 (33.3) |
| Serum AFP, n (%) | |
| <200 μg/L | 21 (87.5) |
| >200 μg/Lb | 3 (12.5) |
| Predetermined locoregional therapy, n (%) | |
| RFA or MWA | 12 (50.0) |
| TACE ± RFA | 7 (29.2) |
| Surgical resection | 3 (12.5) |
| Yttrium‐90 radioembolization | 1 (4.2) |
| Surgical treatment aborted | 1 (4.2) |
One patient had combined hepatocellular cholangiocarcinoma upon post‐treatment revision.
All three patients had large hepatocellular carcinoma (54–160 mm) with no signs of distant metastases radiographically.
Abbreviations: AFP, alfa‐fetoprotein; BCLC, Barcelona Clinic Liver Cancer; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; MWA, microwave ablation; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
HCC diagnosis was histologically confirmed in 22 and based on imaging criteria in 2 patients. Six tumors (25%) were well differentiated, seven (29%) were moderately differentiated, and three were poorly differentiated (13%). Differentiation grade was not evaluable in eight (33%) cases. All tumors showed high microvessel density and β‐catenin expression patterns consistent with CTNNB1 mutation status (Fig. 1). Tumor mutational profile was abnormal in 12 of 18 (67%) assessable HCC specimens. The most frequent somatic mutations were found in CTNNB1 (28%) and TP53 (22%). No pathogenic mutations were detected in FGFR1‐3 or KIT.
Figure 1.
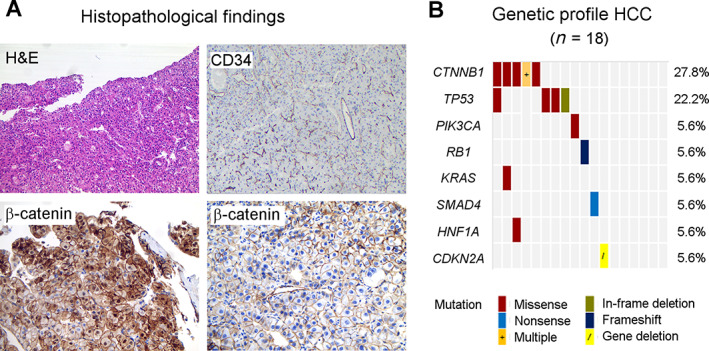
Tumor characteristics. (A): Representative HCC specimens, top panel: H&E and CD34 stains, respectively, show well differentiated HCC (×40 magnification) and typical high tumor vascular density (×25 magnification). Bottom panel from left to right: translocation of β‐catenin to the cytoplasm and nucleus in CTNNB1‐mutated HCC versus normal membrane β‐catenin expression in CTNNB1‐wildtype HCC (×100 magnification). (B): Molecular tumor profile by targeted next generation sequencing. Possible and known pathogenic gene mutations were most frequently found in CTNNB1 and TP53. CTNNB1 exon 3 variants: p.G34V, p.I35G38del, p.T41A, p.S45P, p.S45Y, and p.S45F mutations. TP53 exon 5 + 7 variants: p.T155K, p.C238R, p.G245C, and p.R249S mutations. Six tumors did not contain class 3–5 mutations in the evaluated genes or gene fragments. One patient with a CTNNB1 p.T41A and KRAS p.G12D mutation demonstrated aberrant nuclear β‐catenin staining at diagnosis, but wildtype CTNNB1 and membranous β‐catenin expression on the posttreatment sample. Abbreviation: HCC, hepatocellular carcinoma.
Tumor Response and Intratumoral Perfusion
Following dovitinib treatment, 92% patients had stable disease (SD), 8% had partial response (PR), and 0% had complete response (CR) according to RECIST1.1 (Fig. 2; see supplemental online Table 3 for response per tumor stage). Objective response rates based on mRECIST criteria, which measures viable tumor size only and predicts overall survival in contrast to classic RECIST 1.1 [13, 16], were much higher, showing 52% SD, 35% PR, and 13% CR.
Figure 2.
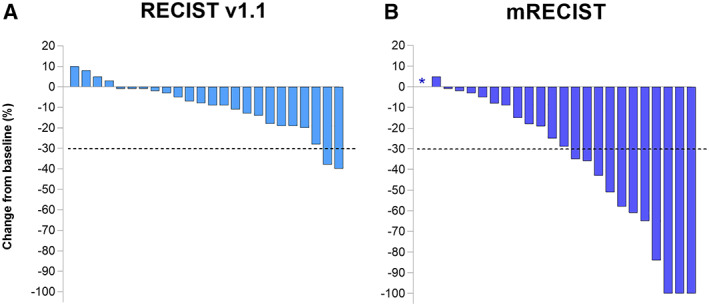
Radiologic response according to RECIST 1.1 (A) and mRECIST (B) criteria. No significant Pearson or Spearman correlation was found between mRECIST response and cumulative dovitinib dose, change in intratumoral arterial flow, or development of grade 3–4 on‐target hypertension. *One patient was not evaluable by mRECIST. Abbreviation: mRECIST, modified RECIST.
CTP confirmed changes in intratumoral blood flow: median AF decreased from 87 to 74 mL/minute (p = .052), median PF increased from 110 to 135 mL/minute (p = .073), median intratumoral PI decreased from 45% to 39% (p = .032). All four patients with >50% decrease in tumor AF demonstrated objective mRECIST responses. One patient even exhibited an acute decrease in intratumoral AF associated with massive tumor necrosis and rupture after 3 days dovitinib treatment (Fig. 3). Following emergency hemihepatectomy, this patient remained disease free for 14 months and survived ~35 months.
Figure 3.
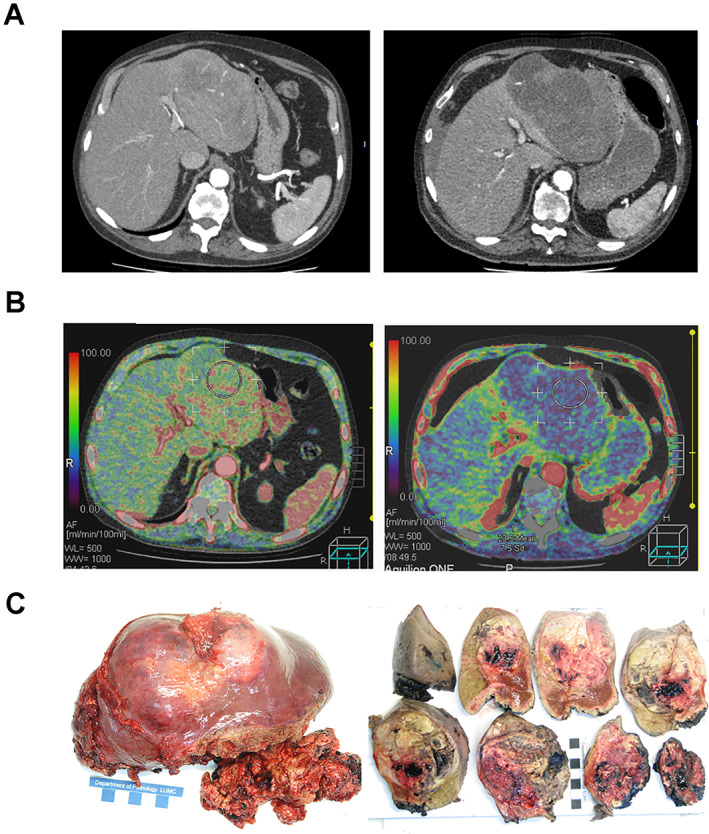
Changes in tumor density and intratumoral blood flow before (left panel) and after (right panel) dovitinib treatment. After 3 days of dovitinib treatment, one poorly differentiated patient with hepatocellular carcinoma demonstrated (A) massive necrosis on computed tomography (CT) scan and (B) 63% decrease in tumor arterial flow on perfusion CT. Even though 14% increase in tumor size was visible on CT scan, the lesion had become largely necrotic and ruptured. (C): At emergency hemihepatectomy, large fields of necrosis were macroscopically visible.
Safety and Quality of Life
Dovitinib tolerability varied greatly between individuals. Although 16 (67%) patients completed the 4‐week neoadjuvant dovitinib regimen (interquartile range [IQR], 14–28 days) and 7 (29%) patients experienced no physical side effects at all, dose modification and/or treatment interruption was required in 20 (83%) patients because of (laboratory) adverse events (AEs). The median relative dovitinib dose intensity was 64% (IQR, 40–87; range, 15–100) and dose reduction occurred more frequently in patients with liver cirrhosis (odds ratio, 25.5; 95% confidence interval, 2.198–341.2; p = .021). Ultimately, all 24 patients received their locoregional treatment as planned (prior to entering into the study). Most (63%) procedures were performed within 4–9 days after the last dovitinib dose; no bleeding or wound healing complications occurred.
Recorded treatment‐emergent AEs are shown in Figure 4 (AE frequencies ≥10%: supplemental online Table 4). On‐target arterial hypertension (79%), fatigue (75%), and diarrhea (63%) were the most common all grade AEs. Grade 3–4 AEs occurred in 21 (88%) patients and again, mostly consisted of hypertension (54%). Four patients stopped treatment already after 3–4 days following grade 3–4 confusion (n = 2; prior history of encephalopathy), grade 3 transaminase elevation (n = 1), and grade 3 ruptured HCC (n = 1). These four patients demonstrated 29%–65% reduction of viable tumor according to mRECIST (PR, n = 3). No cardiac events were reported except grade 2 atrial fibrillation (n = 1). Median post‐treatment LVEF was similar to baseline (67% vs. 69%; p = .739).
Figure 4.
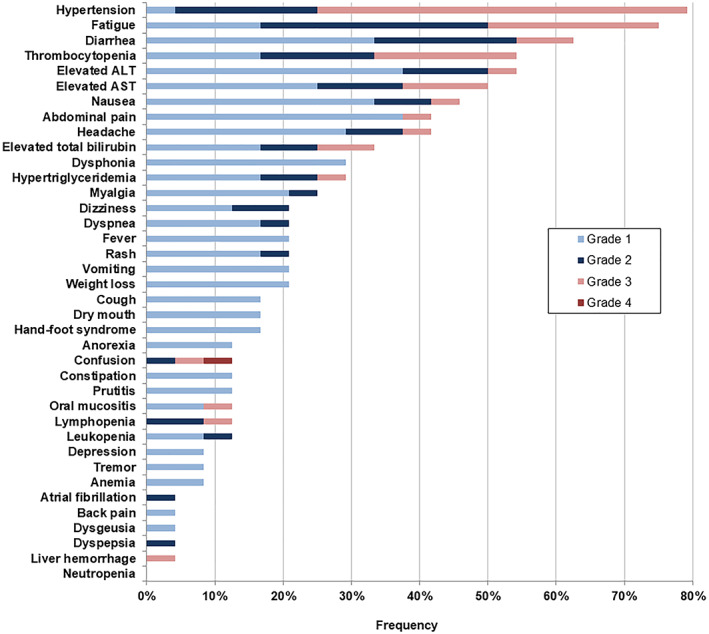
Treatment‐emergent adverse events from baseline to locoregional therapy. All 24 patients experienced at least one adverse event of any grade during dovitinib treatment. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Patient‐reported HRQoL decreased during dovitinib treatment (supplemental online Fig. 1): fatigue and physical and social functioning worsened on day 12 (p < .023) and showed some recovery at day 26 and follow‐up. A large interindividual variation was observed especially on day 12 following dovitinib initiation. Cognitive and emotional functioning scales were not affected by the treatment. At follow‐up (30 days after locoregional treatment), global health had deteriorated (p < .006). However, recovery of patients’ health status, as routinely assessed by their treating physicians in the outpatient HCC clinic, was observed in the following months.
Markers of Vascular Injury
Pretreatment plasma markers of angiogenesis and inflammation sVEGFR1, VEGF‐D, PlGF, sTie2, IL‐5, IL‐12, IL‐15, GM‐CSF, TNF‐β, Eotaxin, Eotaxin‐3, IP‐10, MDC, CRP, sICAM‐1, sVCAM‐1, and SAA levels were at least threefold higher in patients with HCC than in healthy controls (p < .01). TNFα and MIP‐1α were fivefold lower (p < .0001).
Following dovitinib administration, angiogenic markers VEGF‐A, VEGF‐D, and PlGF increased, whereas sTie2 levels decreased consistent with VEGFR pathway blockade (p < .04; Fig. 5). No significant changes were observed in levels of sVEGFR‐1 and VEGF‐C. Plasma levels of inflammatory and vascular injury markers TNF‐α, IL‐6, IL‐8, IL‐10, IL‐15, MCP1, TARC, sICAM‐1, CRP, and SAA increased significantly on day 12, whereas IL‐12 and MDC decreased (p < .04).
Figure 5.
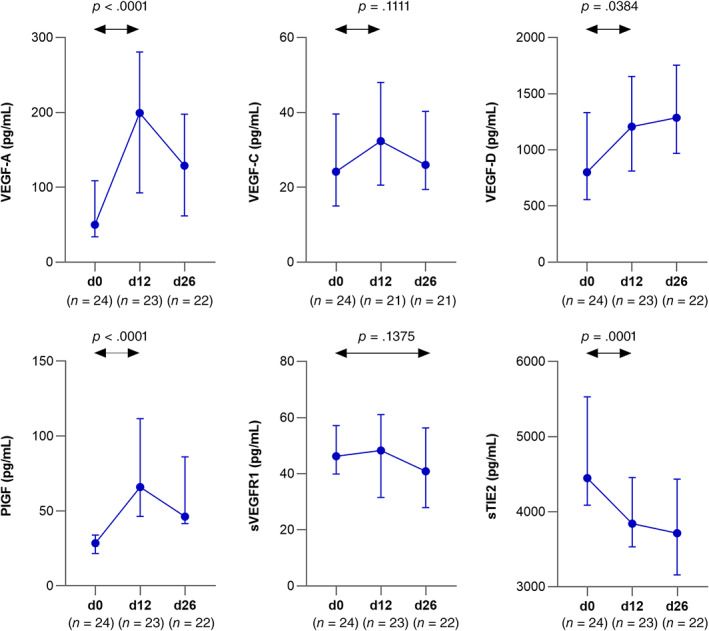
Plasma levels of pharmacodynamic biomarkers of VEGFR‐blockade. Median and interquartile range on days 0, 12, and 26 of dovitinib treatment are shown.
Abbreviations: PlGF, placental growth factor; sTIE2, soluble angiopoietin receptor 2; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Immunohistochemical assessment of the tumor microenvironment pre‐ and postdovitinib was feasible in only 11 patients because of small or fragmented biopsy specimens. No significant changes in percentage vital tumor cells, lymphocyte infiltration, or microvessel density were observed.
Orthotopic Liver Transplantation
Following neoadjuvant dovitinib and locoregional treatment, seven patients underwent OLT after a median of 11.4 months (range: 9.0–21.9). In line with the 2012 Zurich consensus conference recommendations [4], OLT was offered to patients with intermediate‐stage HCC with tumor regression following locoregional treatment (n = 4; 44%), who then met the Dutch selection criteria for liver transplantation. All except two patients with intermediate‐stage disease had intrahepatic tumor recurrence after neoadjuvant dovitinib and locoregional treatment (LRT), which was treated with RFA awaiting liver transplantation. Five (71%) of seven transplanted patients were alive without disease at data cut‐off, three of whom originally had intermediate‐stage HCC. Cause of death was transplant rejection in one patient with intermediate‐stage disease and cancer relapse in one patient with early‐stage disease.
Long‐Term (Secondary Endpoint) Outcomes
By February 1, 2019, at the end of survival follow‐up (median 5.5 years; IQR 5.1–6.0), 15 (63%) patients had died. Cause of death was cancer‐related (n = 9), liver failure (n = 5), or unknown (n = 1).
Three patients were excluded from TTP and survival analysis: one patient with mixed hepatocellular cholangiocarcinoma upon revision, one patient with advanced HCC retrospectively (extensive mesentery metastases at laparoscopy following tumor rupture prior to inclusion), and one patient with aspecific 1–3‐mm lung nodules, of which one in retrospect was a lung metastasis. Fourteen (67%) of 21 remaining patients relapsed. Twelve patients again received locoregional therapy, mostly RFA/microwave ablation. A few patients underwent transarterial chemoembolization (TACE) (n = 3), resection (n = 2), or Yttrium‐90 radioembolization (n = 1) during their disease course. Patients with distant metastasis (n = 9) were offered sorafenib or best supportive care. Censoring at time of liver transplantation, Kaplan‐Meier estimates of median TTP and OS were 16.8 and 34.8 months, respectively (Fig. 6); 5‐year rPFS and CSS were 11% and 51%. OS and TTP differed between BCLC stages 0, A, and B, reflecting the distinct disease states as well as the locoregional treatments given (supplemental online Fig. 2).
Figure 6.
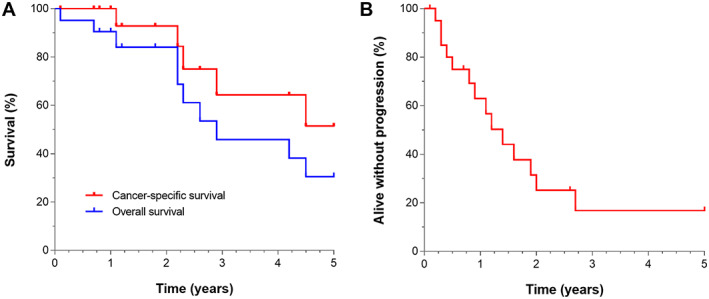
Survival outcomes of 21 evaluable patients with early to intermediate hepatocellular carcinoma. (A): Cancer‐specific and overall survival. (B): Time to progression. Patients were censored at liver transplant.
Discussion
This study is the first to examine the efficacy and tolerability of dovitinib as neoadjuvant treatment prior to locoregional therapy in patients with early‐ and intermediate‐stage HCC, while providing long‐term survival data. Overall response rate (ORR) based on mRECIST was 48%, including 13% CR. Intratumoral perfusion index decreased significantly, and all four patients with >50% decrease in CTP‐assessed tumor arterial flow demonstrated mRECIST responses. The rise of plasma VEGF‐A, VEGF‐D, and PlGF levels and decrease in shedding of Tie‐2 during treatment are consistent with literature and indicate that dovitinib effectively blocks angiogenesis via the angiopoietin/Tie‐2 and VEGFR signaling pathways [17, 18]. More than half of dovitinib‐treated patients developed grade 3–4 hypertension, which is an on‐target effect of VEGFR inhibition previously associated with treatment efficacy [19]. Despite the high rate of treatment‐emergent toxicity resulting in dose reduction and/or interruption, shrinkage of viable tumor mass occurred in a considerable number of patients. Remarkably, three patients stopped study treatment already after 3 days because of grade 3–4 toxicity yet still demonstrated a partial response. These findings support the notion that dovitinib is a highly potent drug capable of inducing tumor necrosis through an immediate effect on tumor vasculature.
Patients in this study were treated with the maximum tolerated dovitinib dose in the most optimal dosing schedule validated in patients with renal cell carcinoma [9], while taking into account the large interindividual variability in dovitinib exposure (coefficient of variation for area under the curve0–t and maximum concentration ~ 50%) [20]. No new safety concerns were identified although (on‐target) hypertension (79%) and fatigue (75%) occurred more often in our study population. The incidence of grade 3–4 AEs was high, but was similar to the 81% observed in 79 patients with dovitinib‐treated advanced‐stage HCC [7]. Dose reductions and interruptions due to AEs (83%) were required more frequently than in renal cell carcinoma (51%) and other cancer types but approximately as frequent as in advanced HCC (72%) [7, 21, 22, 23, 24]. In accordance with the observed AEs, assessment of QLQ‐C30/HCC18 scores showed a temporary decrease in our patients’ perceived quality of life. The large differences in tolerability compared with patients with other cancer types than HCC may be explained by the higher drug exposure in patients with impaired liver function [7, 18, 24]. Indeed, in our study there was a high prevalence of liver cirrhosis, and the patients with liver cirrhosis required dose reduction due to adverse events more often compared with those without. Tolerability may be improved with a 2‐week ramp‐up regimen taking into account that autoinduction of CYP1A1/A2 enzymes occurs after 2 weeks of treatment and results in an improved dovitinib metabolization (half‐life decrease to ~13 hours) in most patients [8, 20]. Reducing dovitinib dose intensity without therapeutic drug monitoring (TDM) may, however, compromise antitumor effect. Future studies in patients with HCC should therefore incorporate TDM in order to find the optimal balance between tolerability and tumor response for each patient, while taking into account the poor translatability of TKI dosing regimens established in other tumor types. Close monitoring and timely management of SAEs for individual patients remain crucial. In the future, individualized TDM‐based dosing schemes or alternative regimes (e.g., ramp‐up dosing) may improve tolerability of dovitinib. Execution of such pharmacokinetic studies in patients with Child‐Pugh B liver cirrhosis may, however, still remain challenging because of high rates of cirrhosis‐related events [25].
Considering the toxicity profile of the currently used dovitinib regimen, other VEGFR‐targeting agents, or combination therapies may be more attractive to investigate in neoadjuvant setting. Although several case reports have described extensive tumor necrosis and successful downsizing of large HCCs following sorafenib treatment [26, 27], it is not perceived as a sufficiently effective downstaging agent because of low response rates in pivotal phase III trials [28, 29]. Its use in adjuvant setting following local therapies, such as surgical resection or local ablation, has not resulted in improvement of outcome in a large placebo‐controlled trial in early‐stage HCC and is therefore not recommended [30]. In a randomized‐controlled study involving 50 systemic treatment‐naive patients with HCC waiting for OLT, no significant difference in median TTP between patients receiving TACE plus sorafenib versus TACE plus placebo (2.3 vs. 2.8 months, respectively) could be established [31]. Although cross‐study comparison is not possible, median TTP seemed longer in our dovitinib‐treated patients, intermediate‐ and early‐stage alike.
In a large, phase III advanced‐stage HCC study, first‐line treatment with the multireceptor tyrosine kinase inhibitor lenvatinib resulted in significantly better independent reviewer‐assessed mRECIST ORR (41% including 2% CR) than with sorafenib (12%) [32]. Another large, phase III advanced HCC study examining the efficacy of the combination of anti‐VEGF and anti‐programmed death ligand 1 antibodies demonstrated higher mRECIST ORR (43% including 10% CR) for the combination of atezolizumab‐bevacizumab than single agent sorafenib (13% including 2% CR) [33]. Although the results with atezolizumab‐bevacizumab seem similar to those obtained with dovitinib, safety and efficacy need to be confirmed in the neoadjuvant setting. Treatment‐emergent grade 3–4 hypertension, which arguably is an on‐target effect of VEGF(R) inhibition, occurred much more frequently (54%) in dovitinib‐treated patients than in lenvatinib‐, sorafenib‐, and atezolizumab‐bevacizumab–treated patients (23%, 14%, and 15% respectively). Whether combination of neoadjuvant dovitinib with checkpoint inhibitors, such as atezolizumab, and implementation of therapeutic drug monitoring further improves ORR as well as tolerability has to be evaluated in future studies.
Although the detailed clinicopathological characterization and assessment of vascular injury are an important strength of this study, the phase II design with a small number of patients precluded subgroup analysis and identification of prognostic factors. Therefore, the relevance of the marked changes in plasma concentration of a great number of cytokines following dovitinib remains uncertain. In our study, the observed pharmacodynamic angiogenesis inhibition and improvement of CTP‐assessed intratumoral blood flow were consistent with a decrease in mRECIST recorded tumor viability. However, firm conclusions with regard to intratumoral flow assessments cannot be drawn as CTP measurements lack standardization.
The frequencies of gene mutations in this study are in accordance with those reported in literature [34]. Because none of our patients had tumors with FGFR1‐3 hotspot mutations, the ability of dovitinib to inhibit FGFR1‐3 could not be studied. The most frequently mutated gene was CTNNB1, which regulates cell adhesion, growth, and differentiation. Pathologic mutations result in activation of the β‐catenin/Wnt signaling pathway, cytosolic accumulation of β‐catenin, and initiation of target gene transcription after translocation of β‐catenin to the nucleus. The post‐treatment normalization of β‐catenin expression patterns in one of our patients may possibly indicate that dovitinib can also modulate Wnt/β‐catenin signaling similar to what has been reported in xenograft models and cell lines [35, 36]. Tumor heterogeneity and clonal selection may also explain the observed differences.
Considering the risks of tumor biopsy in patients with HCC, tNGS of circulating tumor DNA may present a safer alternative to investigate the molecular genetic landscape in HCC and identify potential subgroups of patients benefiting from treatment with multireceptor tyrosine kinase inhibitors [37].
Finally, the study was limited by the lack of sufficient tumor tissue. Although pretreatment and post‐treatment specimens contained focal to dense immune infiltrates surrounding the tumor, the small and fragmented tumor biopsy specimens precluded immunohistochemical phenotyping of immune cells. Thus, the effect of dovitinib on the immune microenvironment remains unclear.
Conclusion
Neoadjuvant dovitinib treatment followed by locoregional therapy is a feasible strategy in patients with HCC and Child‐Pugh A liver disease. A significant proportion of patients obtained viable tumor reduction despite the short treatment period with frequent dose reductions and interruptions. Future studies should focus on therapeutic drug monitoring in order to facilitate individualized dosing and prolongation of the neoadjuvant drug treatment period. This may translate into better tolerability in patients with HCC and maximal tumor regression.
Author Contributions
Conception/design: Nir I. Weijl, Jacobus Burggraaf, Susanne Osanto
Provision of study material or patients: Nir Weijl, Mark C. Burgmans, Martin N.J.M. Wasser, Minneke J. Coenraad, Susanne Osanto
Collection and/or assembly of data: F.J. Sherida H. Woei‐A‐Jin, Mark C. Burgmans, Arantza Fariña Sarasqueta, J. Tom van Wezel, Martin N.J.M. Wasser, Jacobus Burggraaf, Susanne Osanto
Data analysis and interpretation: F.J. Sherida H. Woei‐A‐Jin, Mark C. Burgmans, Arantza Fariña Sarasqueta, J. Tom van Wezel, Minneke J. Coenraad, Jacobus Burggraaf, Susanne Osanto
Manuscript writing: F.J. Sherida H. Woei‐A‐Jin, Mark C. Burgmans, Susanne Osanto
Final approval of manuscript: F.J. Sherida H. Woei‐A‐Jin, Nir I. Weijl, Mark C. Burgmans, Arantza Fariña Sarasqueta, J. Tom van Wezel, Martin N.J.M. Wasser, Minneke J. Coenraad, Jacobus Burggraaf, Susanne Osanto
Disclosures
F.J. Sherida H. Woei‐A‐Jin: Takeda, Kyowa Kirin (RF); Susanne Osanto: EUSA Pharma (H), Astellas, BMS, Janssen, Pfizer (SAB). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Figures.
Appendix S2. Tables.
Acknowledgments
This investigator‐initiated study was supported by Novartis Pharmaceuticals Corporation, who had no role in study design, data collection, analysis, or decision to publish the manuscript. The authors thank Dr. Sahar Barjesteh van Waalwijk van Doorn‐Khosrovani for stimulating scientific discussions. F.J.S.H.W.‐A‐J. is currently affiliated with the Department of General Medical Oncology, University Hospitals Leuven, KU Leuven, Belgium. N.I.W. is currently affiliated with the Haaglanden Medical Center Antoniushove, Leidschendam, The Netherlands. A.F.S. is currently affiliated with the Department of Pathology, Amsterdam University Medical Centers, The Netherlands
Open access funding enabled and organized by Projekt DEAL.
Disclosures of potential conflicts of interest may be found at the end of this article.
Footnotes
For Further Reading: Dmitrii Shek, Scott A. Read, Adnan Nagrial et al. Immune‐Checkpoint Inhibitors for Advanced Hepatocellular Carcinoma: A Synopsis of Response Rates. The Oncologist 2021;26:e1216–e1225.
Implications for Practice: Immune‐checkpoint inhibitors (ICIs) can provide high objective response rates (ORR, estimated with RECIST 1.1. criteria) when used as first‐line treatment in advanced hepatocellular carcinoma, particularly pembrolizumab + lenvatinib (ORR 36%) or atezolizumab + bevacizumab (ORR 27.3%). In sorafenib‐experienced patients, nivolumab + ipilimumab (ORR 32%) provided the highest ORR among ICI‐based regimens. These findings emphasize high therapeutic potential of ICI‐based therapies in patients with advanced hepatocellular carcinoma, although further studies are required to further validate and define their role in this context.
References
- 1.Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Vogel A, Cervantes A, Chau I et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2018;29(suppl 4):iv238–iv255. [DOI] [PubMed] [Google Scholar]
- 3.Finkenstedt A, Vikoler A, Portenkirchner M et al. Excellent post‐transplant survival in patients with intermediate stage hepatocellular carcinoma responding to neoadjuvant therapy. Liver Int 2016;36:688–695. [DOI] [PubMed] [Google Scholar]
- 4.Clavien PA, Lesurtel M, Bossuyt PM et al. Recommendations for liver transplantation for hepatocellular carcinoma: An international consensus conference report. Lancet Oncol 2012;13:e11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doyle A, Gorgen A, Muaddi H et al. Outcomes of radiofrequency ablation as first‐line therapy for hepatocellular carcinoma less than 3cm in potentially transplantable patients. J Hepatol 2019;70:866–873. [DOI] [PubMed] [Google Scholar]
- 6.Karaman MW, Herrgard S, Treiber DK et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol 2008;26:127–132. [DOI] [PubMed] [Google Scholar]
- 7.Cheng AL, Thongprasert S, Lim HY et al. Randomized, open‐label phase 2 study comparing frontline dovitinib versus sorafenib in patients with advanced hepatocellular carcinoma. Hepatology 2016;64:774–784. [DOI] [PubMed] [Google Scholar]
- 8.Angevin E, Lopez‐Martin JA, Lin CC et al. Phase I study of dovitinib (TKI258), an oral FGFR, VEGFR, and PDGFR inhibitor, in advanced or metastatic renal cell carcinoma. Clin Cancer Res 2013;19:1257–1268. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Kay A, Anak O et al. Population pharmacokinetic/pharmacodynamic modeling to assist dosing schedule selection for dovitinib. J Clin Pharmacol 2013;53:14–20. [DOI] [PubMed] [Google Scholar]
- 10.Tai WT, Cheng AL, Shiau CW et al. Dovitinib induces apoptosis and overcomes sorafenib resistance in hepatocellular carcinoma through SHP‐1‐mediated inhibition of STAT3. Mol Cancer Ther 2012;11:452–463. [DOI] [PubMed] [Google Scholar]
- 11.Dey JH, Bianchi F, Voshol J et al. Targeting fibroblast growth factor receptors blocks PI3K/AKT signaling, induces apoptosis, and impairs mammary tumor outgrowth and metastasis. Cancer Res 2010;70:4151–4162. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 13.Lencioni R, Montal R, Torres F et al. Objective response by mRECIST as a predictor and potential surrogate end‐point of overall survival in advanced HCC. J Hepatol 2017;66:1166–1172. [DOI] [PubMed] [Google Scholar]
- 14.Chie WC, Blazeby JM, Hsiao CF et al. International cross‐cultural field validation of an European Organization for Research and Treatment of Cancer questionnaire module for patients with primary liver cancer, the European Organization for Research and Treatment of Cancer quality‐of‐life questionnaire HCC18. Hepatology 2012;55:1122–1129. [DOI] [PubMed] [Google Scholar]
- 15.Fayers PM, Aaronson NK, Bjordal K et al. The EORTC QLQ‐c30 scoring manual (3rd edition). Brussels: European Organisation for Research and Treatment of Cancer, 2001. [Google Scholar]
- 16.Ronot M, Bouattour M, Wassermann J et al. Alternative response criteria (Choi, European association for the study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. The Oncologist 2014;19:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llovet JM, Peña CE, Lathia CD et al. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res 2012;18:2290–2300. 10.1158/1078-0432.ccr-11-2175. [DOI] [PubMed] [Google Scholar]
- 18.Escudier B, Grünwald V, Ravaud A et al. Phase II results of dovitinib (TKI258) in patients with metastatic renal cell cancer. Clin Cancer Res 2014;20:3012–3022. 10.1158/1078-0432.ccr-13-3006. [DOI] [PubMed] [Google Scholar]
- 19.Versmissen J, Mirabito Colafella KM, Koolen SLW et al. Vascular cardio‐oncology: Vascular endothelial growth factor inhibitors and hypertension. Cardiovasc Res 2019;115:904–914. [DOI] [PubMed] [Google Scholar]
- 20.Kim KB, Chesney J, Robinson D et al. Phase I/II and pharmacodynamic study of dovitinib (TKI258), an inhibitor of fibroblast growth factor receptors and VEGF receptors, in patients with advanced melanoma. Clin Cancer Res 2011;17:7451–7461. [DOI] [PubMed] [Google Scholar]
- 21.Motzer RJ, Porta C, Vogelzang NJ et al. Dovitinib versus sorafenib for third‐line targeted treatment of patients with metastatic renal cell carcinoma: An open‐label, randomised phase 3 trial. Lancet Oncol 2014;15:286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi YJ, Kim HS, Park SH, et al. Phase II study of dovitinib in patients with castration‐resistant prostate cancer (KCSG‐GU11‐05). Cancer Res Treat 2018;50:1252–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joensuu H, Blay JY, Comandone A et al. Dovitinib in patients with gastrointestinal stromal tumour refractory and/or intolerant to imatinib. Br J Cancer 2017;117:1278–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang YK, Yoo C, Ryoo BY et al. Phase II study of dovitinib in patients with metastatic and/or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib. Br J Cancer 2013;109:2309–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labeur TA, Achterbergh R, Takkenberg B et al. Sorafenib for patients with hepatocellular carcinoma and Child‐Pugh B liver cirrhosis: Lessons learned from a terminated study. The Oncologist 2020;25:e1274–e1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbier L, Muscari F, Le Guellec S et al. Liver resection after downstaging hepatocellular carcinoma with sorafenib. Int J Hepatol 2011;2011:791013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irtan S, Chopin‐Laly X, Ronot M et al. Complete regression of locally advanced hepatocellular carcinoma induced by sorafenib allowing curative resection. Liver Int 2011;31:740–743. [DOI] [PubMed] [Google Scholar]
- 28.Llovet JM, Ricci S, Mazzaferro V et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–390. [DOI] [PubMed] [Google Scholar]
- 29.Cheng AL, Kang YK, Chen Z et al. Efficacy and safety of sorafenib in patients in the Asia‐Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double‐blind, placebo‐controlled trial. Lancet Oncol 2009;10:25–34. [DOI] [PubMed] [Google Scholar]
- 30.Bruix J, Takayama T, Mazzaferro V et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): A phase 3, randomised, double‐blind, placebo‐controlled trial. Lancet Oncol 2015;16:1344–1354. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann K, Ganten T, Gotthardtp D et al. Impact of neo‐adjuvant sorafenib treatment on liver transplantation in HCC patients ‐ A prospective, randomized, double‐blind, phase III trial. BMC Cancer 2015;15:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kudo M, Finn RS, Qin S et al. Lenvatinib versus sorafenib in first‐line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non‐inferiority trial. Lancet 2018;391:1163–1173. [DOI] [PubMed] [Google Scholar]
- 33.Finn RS, Qin S, Ikeda M et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894–1905. [DOI] [PubMed] [Google Scholar]
- 34.Khemlina G, Ikeda S, Kurzrock R. The biology of hepatocellular carcinoma: Implications for genomic and immune therapies. Mol Cancer 2017;16:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lachenmayer A, Alsinet C, Savic R et al. Wnt‐pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin Cancer Res 2012;18:4997–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeitlin BD, Ellis LM, Nör JE. Inhibition of vascular endothelial growth factor receptor‐1/wnt/β‐catenin crosstalk leads to tumor cell death. Clin Cancer Res 2009;15:7453–7455. 10.1158/1078-0432.ccr-09-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikeda S, Tsigelny IF, Skjevik AA et al. Next‐generation sequencing of circulating tumor DNA reveals frequent alterations in advanced hepatocellular carcinoma. The Oncologist 2018;23:586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Figures.
Appendix S2. Tables.


