Abstract
Background
To review and summarize all U.S. Food and Drug Administration (FDA) approvals of programmed death (PD)‐1 and PD‐ligand 1 blocking antibodies (collectively referred to as PD‐[L]1 inhibitors) over a 6‐year period and corresponding companion/complementary diagnostic assays.
Materials and Methods
To determine the indications and pivotal trials eligible for inclusion, approval letters and package inserts available on Drugs@FDA were evaluated for approved PD‐[L]1 inhibitors to identify all new indications granted from the first approval of a PD‐[L]1 inhibitor on September 4, 2014, through September 3, 2020. The corresponding FDA drug and device reviews from the marketing applications for the approved indications were identified through FDA internal records. Two reviewers independently extracted information for the endpoints, efficacy data, basis for approval, type of regulatory approval, and corresponding in vitro diagnostic device test. The results were organized by organ system and tumor type.
Results
Of 70 Biologic Licensing Application or supplement approvals that resulted in new indications, 32 (46%) were granted based on response rate (ORR) and durability of response, 26 (37%) on overall survival, 9 (13%) on progression‐free survival, 2 (3%) on recurrence‐free survival, and 1 (1%) on complete response rate. Most ORR‐based approvals were granted under the accelerated approval provisions and were supported with prolonged duration of response. Overall, 21% of approvals were granted with a companion diagnostic. Efficacy results according to tumor type are discussed.
Conclusion
PD‐[L]1 inhibitors are an effective anticancer therapy in a subset of patients. This class of drugs has provided new treatment options for patients with unmet need across a wide variety of cancer types. Yet, the modest response rates in several tumor types signal a lack of understanding of the biology of these diseases. Further preclinical and clinical investigation may be required to identify a more appropriate patient population, particularly as drug development continues and additional treatment alternatives become available.
Implications for Practice
The number of PD‐[L]1 inhibitors in drug development and the associated companion and complementary diagnostics have led to regulatory challenges and questions regarding generalizability of trial results. The interchangeability of PD‐L1 immunohistochemical assays between PD‐1/PD‐L1 drugs is unclear. Furthermore, robust responses in some patients with low levels of PD‐L1 expression have limited the use of PD‐L1 as a predictive biomarker across all cancers, particularly in the setting of diseases with few alternative treatment options. This review summarizes the biomarker thresholds and assays approved as complementary and companion diagnostics and provides regulatory perspective on the role of biomarkers in oncology drug development.
Keywords: Companion diagnostic, Checkpoint inhibitor, Regulatory science, Food and Drug Administration
Short abstract
This review provides a regulatory overview on the U.S. FDA approvals of PD‐1 and PD‐L1 inhibitors and highlights complementary and companion diagnostics within the context of each disease and indication.
Introduction
Immunotherapy is among the most active areas of oncology drug development, yet several uncertainties remain on how to realize the promise of maximizing immunotherapy benefits to patients with cancer, including how to better select patients who may benefit. This systematic review provides a regulatory overview on the U.S. Food and Drug Administration (FDA) approvals of programmed death‐1– and programmed death‐ligand 1–blocking antibodies (herein referred to collectively as PD‐[L]1 inhibitors), and highlights complementary and companion diagnostics within the context of each disease and indication. In regulatory use, the term complementary diagnostic denotes an in vitro diagnostic test that identifies a subgroup(s) of the indicated population that has a different benefit‐risk profile than the broader population for whom the corresponding therapeutic product is indicated, whereas a companion diagnostic is considered essential for the safe and effective use of the corresponding drug [1]. As the number of treatment options increases in each cancer setting, including the choice between frontline monotherapy and combination therapy, understanding the risk‐benefit ratio for each biomarker‐selected population has become increasingly important.
Materials and Methods
We reviewed FDA records to identify Biologic Licensing Applications (BLAs) for anti‐PD‐1– and anti‐PD‐L1–blocking antibodies approved during a 6‐year period from the first PD‐[L]1 inhibitor approval on September 4, 2014, through September 3, 2020. To determine the indications and pivotal trials eligible for inclusion, two physician reviewers independently abstracted all approved indications from the package inserts available on Drugs@FDA. The corresponding FDA reviews from the marketing applications for the approved indications were identified through FDA internal records. All studies that formed the primary basis for approval in the FDA review were selected for inclusion. The reviewers extracted the main efficacy data, type of regulatory approval (accelerated [2] vs. regular), and basis for the approval, including endpoints and biomarkers used, from FDA reviews and labels. We adhered to the principles provided by the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses report [3].
Results
During the 6‐year period, six PD‐[L]1 inhibitors were approved: nivolumab, pembrolizumab, and cemiplimab were the approved anti‐PD‐1 blocking antibodies; atezolizumab, avelumab, and durvalumab were the approved anti‐PD‐L1 blocking antibodies (see Fig. 1 for timeline). Of 70 BLA or supplement approvals that resulted in new indications, 32 (46%) were granted based on overall response rate (ORR), 26 (37%) were based on overall survival (OS), 9 (13%) were based on progression‐free survival (PFS), 2 (3%) were based on recurrence‐free survival, and 1 (1%) was based on complete response rate. Since the initial approvals in 2014, the pace of marketing applications for PD‐[L]1 inhibitors has not slowed (Fig. 1). Most ORR‐based approvals were accelerated, and all were supported by a prolonged duration of response (DOR), including 15 of 32 approvals demonstrating a 6‐month durability for greater than 75% of patients (Fig. 2). Overall, 15 (21%) of approvals were granted with a companion diagnostic (Fig. 1).
Figure 1.
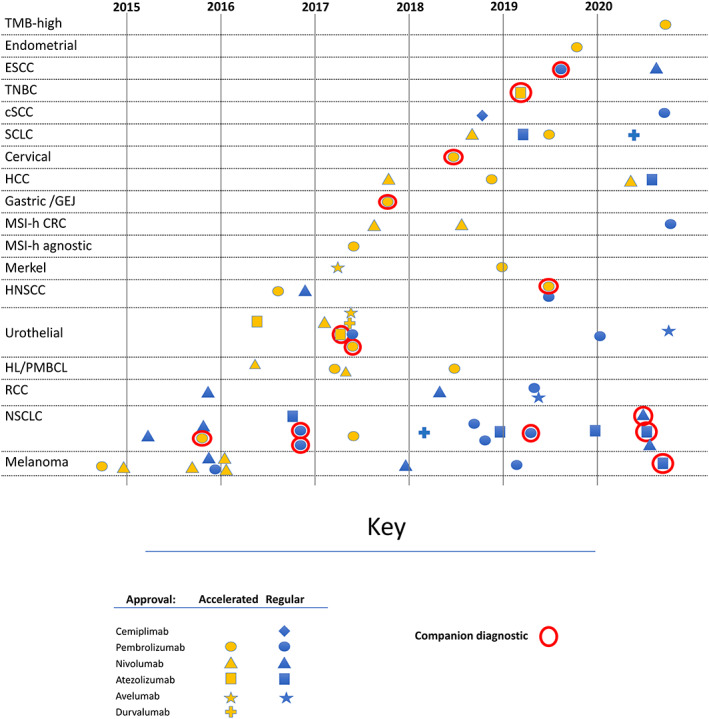
Timeline of PD‐[L]1 inhibitor approvals. Notes : approvals for new indications are shown in the figure. Conversions from accelerated to regular approval are not shown. Abbreviations: CRC, colorectal cancer; cutaneous squamous cell carcinoma; ESCC, esophageal squamous cell carcinoma; GEJ, gastroesophageal junction; HCC, hepatocellular carcinoma; HL, Hodgkin lymphoma; HNSCC, head and neck squamous cell carcinoma; MSI‐h, microsatellite instability high; NSCLC, non‐small cell lung cancer; PMBCL, primary mediastinal B cell lymphoma; RCC, renal cell carcinoma; SCLC, extensive‐stage small cell lung cancer; TMB, tumor mutation burden‐high; TNBC, triple‐negative breast cancer.
Figure 2.
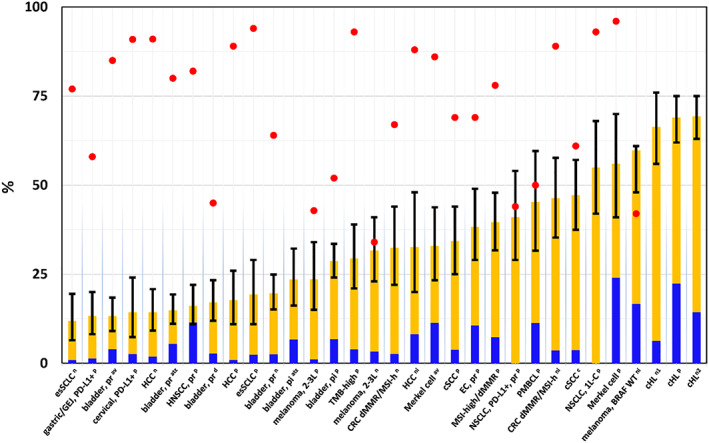
Response rate (RR) and duration of response in approvals based on RR. Red dots: proportion of responders with ≥6‐month duration of response. Note : data were not available for 4 indications (red dot is missing). Orange bars: partial responders; blue bars: complete responders; black bars: 95% confidence interval. Abbreviations: 1L‐C, first line combination; 2L, second line; av, avelumab; at, atezolizumab; classic Hodgkin lymphoma; c, cemiplimab; CRC, colorectal cancer; cSCC, cutaneous squamous cell carcinoma; d, durvalumab; dMMR/MSI‐h, deficient mismatch repair/microsatellite instability high; EC, endometrial carcinoma; esSCLC, extensive‐stage small cell lung cancer; GEJ, gastroesophageal junction; HCC, hepatocellular carcinoma; ICI, immune‐checkpoint inhibitor naive; n, nivolumab; ni, nivolumab‐ipilimumab combination; NSCLC, non‐small cell lung cancer; pi, cisplatin‐ineligible; pl, pembrolizumab‐lenvatinib combination; PMBCL, primary mediastinal B cell lymphoma; pr, platinum‐refractory; TMB, tumor mutation burden
Disease‐Specific Considerations
Dermatologic Malignancies
Melanoma
Melanoma, along with renal cell carcinoma, had been well known to be a tumor type responsive to immunotherapy [4]. Following the approval of the first immune checkpoint inhibitor targeting CTLA‐4, ipilimumab, the first approvals of PD‐[L]1 inhibitors were also granted in melanoma in 2014 (Fig. 1), with ORRs of 24 and 32% with pembrolizumab and nivolumab, respectively, in refractory melanoma (Fig. 2) [5, 6, 7]. Duration of response provided supportive evidence for approval: 86% and 87% of the responses were still ongoing at data cutoff in KEYNOTE‐001 and CheckMate 037, respectively, after most patients had at least 6 months of follow‐up at the time of submission [8, 9]. Both pembrolizumab and nivolumab were later confirmed to confer OS benefit in earlier line settings [10, 11, 12, 13].
In 2020, the first combination regimen of PD‐[L]1 inhibitor with targeted therapy was approved: atezolizumab in combination with vemurafenib and cobimetinib for patients with treatment‐naive melanoma positive for BRAF V600E/K mutation. This indication was approved based on the results of IMspire150 [14], which randomized patients to cobimetinib and vemurafenib for the first 28‐day cycle and then added atezolizumab versus placebo starting with the second cycle. The trial demonstrated an improvement in PFS, the primary endpoint: median 15.1 months versus 10.6 months (hazard ratio [HR], 0.78; 95% confidence interval [CI], 0.63–0.97) [15].
In addition to the success in the advanced metastatic setting, studies in melanoma demonstrated that PD‐[L]1 inhibitors also are effective in nonmetastatic disease settings [16, 17]. The adjuvant immune checkpoint inhibitor options are now ipilimumab, nivolumab, and pembrolizumab. Both nivolumab and pembrolizumab were studied in large randomized trials with 1 year as the duration of therapy per protocol, but the optimal duration has not been studied in a randomized setting.
Response criteria that best capture clinical benefit in patients treated with immunotherapy is an area of active research. Recent reports indicated that at least 7% [18, 19] of patients with melanoma receiving PD‐[L]1 inhibitors may have delayed response. Identifying patients with pseudoprogression or delayed response is challenging, even with novel and more specific response metrics such as immune RECIST (iRECIST). The RECIST working group published consensus guidelines in 2017 which still recommended the use of RECIST 1.1 as the primary criteria for response‐based endpoints for registrational trials; iRECIST could be used as an exploratory endpoint [20].
Other Cutaneous Malignancies
Metastatic Merkel cell carcinoma (mMCC) is an aggressive disease with a high mortality rate exceeding that of melanoma [21]. FDA approved both avelumab and pembrolizumab for mMCC on the basis of ORR with prolonged duration of responses [22, 23]. ORR in the avelumab trial in which patients who had received one or two prior lines of therapy was 33% (95% CI, 23.3–43.8). ORR in the pembrolizumab trial in which patients had not received prior systemic therapy was 56% (95% CI, 41–70). Merkel cell polyomavirus (MCPyV) can be detected in most MCCs, and, although MCPyV‐positive MCCs carry extremely low tumor mutation burden (TMB), MCPyV‐positive and MCPyV‐negative mMCCs were both reportedly associated with high response rates (62% and 44%, respectively, with pembrolizumab) [22] and durability (83% and 80% with ≥1 year response, respectively, with avelumab) [24]. Thus, MCC may serve as an example of a relatively unique situation where patients have a high probability of response despite low TMB.
Cemiplimab‐rwlc and pembrolizumab are approved for unresectable/metastatic cutaneous squamous cell carcinoma (cSCC) [22, 25]. Prior to September 2018, cytotoxic chemotherapy was used but no systemic therapies were FDA approved for locally advanced unresectable/metastatic cSCC [26]. In the trials that led to approval of cemiplimab‐rwlc and pembrolizumab (R2810‐ONC‐1423/‐1540 and KEYNOTE‐629), 34%–47% of patients with advanced cSCC experienced an objective responses, with a DOR of ≥6 months in 60%–69% of responders [26, 27]. The location of disease is important in considering the degree of clinical benefit for a response, and photographic evidence of improvement in disfiguring lesions was supportive in the FDA review of these indications.
Thoracic Malignancies
In contrast to melanoma and renal cell cancer (RCC), lung cancer was previously not considered an immunogenic cancer type after a history of failed immunotherapy clinical trials over decades. Between 2015 and 2017, four PD‐[L]1 inhibitors were approved for non‐small cell lung cancer (NSCLC) [15, 22, 28, 29], and drug development in this area has continued to be productive. Various combinations with vascular endothelial growth factor inhibitors and chemotherapy have been explored, and the first postchemoradiation consolidation therapy was approved [29, 30, 31, 32, 33, 34]. Patient selection to optimize risk‐benefit ratio continues to be an area in need of refinement. Until June 2020, the only biomarker approved for patient selection of therapy in NSCLC was PD‐L1. This reflects the designs and statistical analysis plans of the trials that led to approval in this setting. The statistical testing hierarchies generally did not include other biomarker‐defined populations beyond PD‐L1. FDA typically regards the results of comparisons that are not prespecified as exploratory [35]. Since approvals for first‐line combination (pembrolizumab with chemotherapy, atezolizumab with chemotherapy and bevacizumab, and nivolumab with ipilimumab and two cycles of platinum doublet) regimens for patients irrespective of PD‐L1 expression have been granted, another question has emerged: when would PD‐L1–expressing (≥1%), treatment‐naive patients achieve the optimal risk‐benefit ratio with PD–[L]1 inhibitor monotherapy, and in which situations would the additional potential benefit of a PD–[L]1 inhibitor combined with chemotherapy outweigh the risks? An ongoing SWOG trial (INSIGNA, NCT03793179) [36] may provide data to guide this decision.
Over time, the need for other predictive biomarkers to complement PD‐L1 expression has become apparent. Correlation between high levels of TMB and response to checkpoint inhibitor therapy have been observed [37, 38], but questions remain regarding the optimal thresholds to define low and high mutation burden, the incremental value of TMB to predict response over PD‐L1 immunohistochemistry (IHC), and how to address variation in TMB quantification across diagnostic platforms (see Regulatory Challenges: Diagnostics and Biomarkers) [39].
Small Cell Lung Cancer
In extensive‐stage small cell lung cancer (esSCLC) with disease progression on or after platinum‐based chemotherapy and at least one other line of therapy, both pembrolizumab and nivolumab were granted accelerated approval as monotherapy based on ORR [22, 28, 40]. Both indications have subsequently been withdrawn in consultation with FDA in late 2020 or early 2021 [41, 42, 43, 44, 45]. Atezolizumab was the first approval of a PD‐[L]1 inhibitor in the frontline setting. At the time of its approval, the standard of care for frontline therapy of esSCLC had not changed since the 1980s. The approval of atezolizumab in combination with carboplatin and etoposide was based on IMpower133 [46], which showed, at the planned interim analysis, a 2‐month median OS benefit (12.3 vs. 10.3 months) with HR of 0.70 (95% CI, 0.54–0.91) and 1‐month median PFS benefit (5.2 vs. 4.3 months) with HR of 0.77 (95% CI, 0.62–0.96). Durvalumab in combination with carboplatin or cisplatin and etoposide was also approved in the frontline; the CASPIAN trial showed a survival benefit in durvalumab with chemotherapy over chemotherapy alone: with OS median 13.0 versus 10.3 months and HR of 0.73 (95% CI, 0.59–0.91) [29].
Head and Neck Squamous Cell Carcinoma
Nivolumab was approved for platinum‐refractory or recurrent head and neck squamous cell carcinoma (HNSCC) in 2016. Pembrolizumab was initially granted accelerated approval for the same indication in 2016 as well, which was converted to regular approval with fulfillment of a postmarketing requirement in 2019. The ORRs in this setting have been consistently in the range of 13.3%–16% in clinical trials (KEYNOTE‐012, CheckMate 141) [22, 28, 47, 48]. Median DOR in the trial that led to accelerated approval of pembrolizumab based on ORR and DOR (KEYNOTE‐012) was not reached and 82% of 28 responding patients had median DOR >6 months (Fig. 2) [22], which was superior to median DORs with standard‐of‐care chemotherapy (e.g., 4.4 months in a single‐arm docetaxel trial [49]). ORRs were higher in human papillomavirus (HPV)‐positive patients (CheckMate 141), but HPV status was not predictive of survival benefit in another trial (KEYNOTE‐040 [50]). In both CheckMate 141 and KEYNOTE‐040, randomized trials of PD‐1 inhibitor compared with investigator's choice (methotrexate, docetaxel, or cetuximab), the survival benefit for the overall population was statistically significant, with HRs of 0.7–0.8.
Pembrolizumab was the first PD‐[L]1 inhibitor therapy to be approved as first‐line treatment of recurrent/metastatic HNSCC. KEYNOTE‐048 demonstrated OS benefit for pembrolizumab in two populations: (a) in combination with platinum and 5‐fluoropyrimidine (5‐FU) in a non–biomarker‐selected population; and (b) as monotherapy in patients with combined positive score (CPS; see biomarker section) ≥1. In the non–biomarker‐selected population, patients who received pembrolizumab in combination with platinum and 5‐FU, in comparison with combination triple‐therapy platinum, 5‐FU, and cetuximab, had a hazard ratio for death of 0.77; median OS was 13.0 months and 10.7 months, respectively. In the population with CPS ≥1, patients who received pembrolizumab monotherapy, in comparison to the control arm, had a hazard ratio for death of 0.78, median OS was 12.3 months and 10.3 months, respectively [22, 51].
Genitourinary Malignancies
Urothelial Carcinoma
PD‐[L]1 inhibitors have been approved for several different disease and treatment settings within urothelial carcinoma (UC). For platinum‐relapsed/refractory metastatic UC, five PD‐[L]1 inhibitors (atezolizumab, avelumab, durvalumab, nivolumab, and pembrolizumab) were initially approved. None of these approvals included a companion diagnostic; however, nivolumab and durvalumab were approved with complementary diagnostics (see Table 1 and supplemental online Table) [52, 53]. The durvalumab and atezolizumab indications were withdrawn in consultation with FDA in February and March 2021, respectively [44, 54, 55, 56]. The ORRs with the PD‐1 inhibitors (Checkmate‐275 and KEYNOTE‐045) for patients with cisplatin‐refractory disease were 20%–21% [57, 58], whereas the ORRs with PD‐L1 inhibitors were 13%–17% (atezolizumab [IMvigor210], durvalumab [NCT01693562], avelumab [JAVELIN]) [59, 60, 61].
Table 1.
Approved indications and diagnostic devices for PD‐[L]1 inhibitors
| Indications | PD‐1/PD‐L1 drugs approved (p denotes pediatric indication as well) | Companion diagnostics | Complementary diagnostics | Approved combinations |
|---|---|---|---|---|
| Dermatologic | ||||
| Advanced/metastatic melanoma | Atezolizumab (1st L‐C), nivolumab (1st L‐M; 1st L‐C), pembrolizumab (1st L‐M) | Nonea | None | Atezolizumab + vemurafenib + cobimetinib, 1st L if positive for BRAF V600E/K mutation; nivolumab + ipilimumab, 1st L |
| Adjuvant melanoma | Nivolumab (M), pembrolizumab (M) | None | None | None |
| Merkel cell, metastatic | Avelumab (1st L)p; pembrolizumab (1st L)p | None | None | None |
| Cutaneous SCC | Cemiplimab‐rwlc (M), pembrolizumab (M) | None | None | None |
| Thoracic | ||||
| Advanced/metastatic NSCLC | Atezolizumab (1st L‐M; 1st L‐C, 2nd L‐M)*, nivolumab (1st L‐C; 2nd L‐M)*, pembrolizumab (1st L‐M; 1st L‐C; 2nd L‐M)*, *2nd L: after platinum | Atezolizumab: 1st L‐M for patients with no EGFR or ALK aberrations AND either TC ≥50% or IC ≥10% (Ventana SP142); nivolumab: 1st L‐C for patients with no EGFR or ALK aberrations AND TC ≥1% (Dako 28–8); pembrolizumab: 1st L‐M for with no EGFR or ALK aberrations AND TPS ≥1% (Dako 22C3), 2nd L‐M for TPS ≥1% (Dako 22C3) | Nivolumab: TC ≥1%, 5%, 10% (Dako 28–8); atezolizumab: TC ≥1% or IC ≥1% (Ventana SP142) | Atezolizumab + bevacizumab + carboplatin + paclitaxel for nonsquamous, 1st L; nivolumab, 1st L: with ipilimumab (for patients with PD‐L1 TC ≥1%), with ipilimumab and 2 cycles platinum doublet (regardless of PD‐L1 expression); pembrolizumab, 1st L: with carboplatin and either paclitaxel or nab‐paclitaxel, for squamous, with pemetrexed and platinum, for nonsquamous, non‐EGFR/‐ALK mutated |
| Post‐chemoradiation NSCLC | Durvalumab (for stage III following platinum‐based chemoRT without progression, for ≤12 months) (M) | None | None | None |
| Small cell lung cancer | Atezolizumab (1st L‐C); durvalumab (1st L‐C); nivolumab (3rd L)*b; pembrolizumab (3rd L)*b; *3rd L: after platinum and at least 1 other line of therapy | None | None | Atezolizumab + carboplatin + etoposide, 1st L; durvalumab + [carbo/cis]platin + etoposide, 1st L |
| Head and neck squamous cell carcinoma | Nivolumab (2nd L‐M after platinum); pembrolizumab (1st L‐C; 1st L‐M; 2nd L‐M) | Pembrolizumab 1st L‐M for CPS ≥1 (Dako 22C3) | Nivolumab: TC ≥1%, 5%, 10% (Dako 28–8) | Pembrolizumab + platinum +5FU, 1st L |
| Genitourinary | ||||
|
Advanced urothelial |
Atezolizumab (1st L‐M, 2nd L‐M): 1st L platinum‐ineligible, 1st L cisplatin‐ineligible if PD‐L1+ IC ≥5%, 2nd L after platinumc; avelumab (maintenance, 2nd L‐M) maintenance following 1st L platinum, 2nd L after platinum; durvalumab (2nd L‐M)c; nivolumab (2nd L‐M); pembrolizumab (1st L‐M, 2nd L‐M) 1st L platinum‐ineligible; 1st L cisplatin‐ineligible PD‐L1 CPS ≥10% 2nd L (progressed ≤1 year after platinum) | Atezolizumab, cisplatin‐ineligible IC ≥5% (Ventana SP142); pembrolizumab, cisplatin‐ineligible CPS ≥10 (Dako 22C3) | Durvalumab: TC ≥25%; or ICP >1% with PD‐L1+ IC ≥25%; or ICP 1% with PD‐L1+ IC = 100% (Ventana SP 263); nivolumab: TC ≥1% (Dako 28–8) | None |
| Nonmuscle invasive bladder cancer | Pembrolizumab (BCG‐unresponsive carcinoma in situ with or without papillary tumors, cystectomy‐ineligible) (M) | None | None | None |
| RCC | Nivolumab intermediate‐/poor‐risk: 1st L‐C with ipi, 2nd L‐M after antiangiogenic therapy; pembrolizumab (1st L‐C); avelumab (1st L‐C) | None | None |
Nivolumab + ipilimumab, 1st L pembrolizumab + axitinib, 1st L avelumab + axitinib, 1st L |
| Gastrointestinal | ||||
| HCC | Atezolizumab (1st L‐C); nivolumab (2nd L‐C, 2nd L‐M after sorafenib); pembrolizumab (2nd L‐M after sorafenib) | None | None | Atezolizumab + bevacizumab, 1st L; nivolumab + ipilimumab, 2nd L |
| MSI‐H CRC | Nivolumab (3rd L‐M after 5FU, oxaliplatin, and irinotecan; or 3rd L‐C w/ ipilimumab)p; pembrolizumab (1st L‐M) | None | None | None |
| Gastric/GE junction | Pembrolizumab (after at least 5FU and platinum) (2nd L‐M) | Pembrolizumab CPS ≥1 (Dako 22C3) | None | None |
| Esophageal SCC | Nivolumab (2nd L‐M); pembrolizumab (2nd L‐M) | Pembrolizumab CPS ≥10 (Dako 22C3) | ||
| Gynecologic/breast | ||||
| Cervical SCC | Pembrolizumab (2nd L‐M) | Pembrolizumab CPS ≥1 (Dako 22C3) | ||
| Endometrial | Pembrolizumab (2nd L‐C) | None | None | Pembrolizumab + lenvatinib, 2nd L |
| TNBC | Atezolizumab (1st L‐C) | Atezolizumab IC ≥1 (Ventana SP142) | Atezolizumab + nab‐paclitaxel | |
| Hematologic | ||||
| Classic Hodgkin lymphoma | Nivolumab (relapsed/refractory to autoSCT and brentuximab, or 4th L‐M after autoSCT); pembrolizumab (4th L‐M)p | None | None | None |
| PMBCL | Pembrolizumab (3rd L‐M)p | None | None | None |
| MSI‐H tissue‐agnostic | Pembrolizumab (progressed following prior treatment and no satisfactory alternatives)p (M) | None; PMCs pending for MSI and MMR | None | None |
| TMB‐high tissue‐agnostic | Pembrolizumab (≥10 mutations/megabase, progressed following prior treatment and no satisfactory alternatives)p (M) | FoundationOneCDx assay | None | None |
Note: all indications are in advanced (unresectable or metastatic) disease unless otherwise noted.
BRAF kit (THxID) is used to select BRAF+ patients for the combination of atezolizumab with vemurafenib plus cobimetinib.
Nivolumab indication was withdrawn by the sponsor on December 29, 2020. Pembrolizumab indication was withdrawn by the sponsor on March 1, 2021.
Durvalumab indication was withdrawn by the sponsor on February 22, 2021. Atezolizumab indication was drawn by the sponsor on March 7, 2021.
Abbreviations: 5FU, fluoropyrimidine; autoSCT, autologous stem cell transplant; C, combination; chemoRT, concurrent chemotherapy and radiation therapy; CPS, combined proportion/positive score; IC, immune cell; ipi, ipilimumab; L, line (e.g., 2nd L, 2nd line); M, monotherapy (e.g., 3rd L‐M, 3rd‐line monotherapy); p, pediatric; PMC, postmarketing commitment; PMLBCL, primary mediastinal large B‐cell lymphoma; RFS, recurrence‐free survival; TMB, tumor mutation burden; TC, tumor cell; TPS, tumor proportion score; TNBC, triple‐negative breast cancer.
Many patients with UC are elderly or have had tobacco exposure. Comorbidities such as renal or cardiac dysfunction limit the safe use of cisplatin‐containing chemotherapy, the standard first‐line treatment [62]. Carboplatin was generally used for these patients in clinical practice prior to 2017. Therefore, PD‐[L]1 inhibitor therapies represented a new therapeutic option in 2017.
The accelerated approvals for the first‐line indications for cisplatin‐ineligible [63] patients in atezolizumab and pembrolizumab were based on data from IMvigor210 and KEYNOTE‐052, respectively, and were initially not restricted to any biomarker‐defined population [64, 65]. Durable responses across all subgroups based on PD‐L1 expression supported the approval for all patients regardless of PD‐L1 expression. However, in the ongoing confirmatory atezolizumab (IMvigor130) and pembrolizumab (KEYNOTE‐361) trials in the first‐line setting, both of which have a PD‐[L]1 inhibitor monotherapy arm and a combination PD‐[L]1 inhibitor with chemotherapy arm, early unplanned Data Monitoring Committee reviews showed that patients with PD‐L1–low expression status in the PD‐[L]1 inhibitor monotherapy arms of both trials had decreased survival compared with patients who received cisplatin‐ or carboplatin‐based chemotherapy [66]. In June 2018, the labels of both drugs were revised to limit the indications in cisplatin‐ineligible patients, to those with PD‐L1 immune cells (ICs) ≥5% (atezolizumab) or CPS ≥10 (pembrolizumab) [67]. No companion diagnostic is required for those patients who are both cisplatin‐ and carboplatin‐ineligible.
During 2020, two unique disease settings within UC were granted approval. The first was Bacillus Calmette‐Guerin (BCG)‐unresponsive high‐risk nonmuscle invasive bladder (NMIBC) with carcinoma in situ with or without papillary tumors in patients who are ineligible for or have elected not to undergo cystectomy. Among the 70 approvals within the study period, this was the only one granted for complete response (CR) rate and duration of response. This was based on KEYNOTE‐057, a single‐arm trial in which patients received pembrolizumab until disease progression, up until 24 months. The CR rate was 41% (95% CI, 31–51) among the 96 patients with high‐risk BCG‐unresponsive NMIBC, and median response duration was 16.2 months (0.0+, 30.4+). Almost half (46%) of responding patients experienced a CR lasting at least 12 months [22].
The other approval in 2020 was for the maintenance treatment of advanced UC that has not progressed with first‐line platinum‐containing chemotherapy, in response to the results of JAVELIN Bladder 100 [23, 68]. Within 4–10 weeks of completion of first‐line platinum‐containing chemotherapy, patients were randomized to avelumab plus best supportive care (BSC) or BSC alone. The primary endpoint of OS was improved in the avelumab arm (median 21.4 months) over the BSC alone arm (14.3 months), with HR 0.69 (95% CI, 0.40–0.79).
Renal Cell Cancer
The treatment landscape for RCC is evolving rapidly. Within the 6‐year study period, three combination regimens were approved for frontline therapy with a PD‐[L]1 inhibitor. The first approval, nivolumab with ipilimumab, was the first time the nivolumab 3 mg/kg until progression with ipilimumab 1 mg/kg for 4 weeks dosing regimen was approved, and it is the only combination regimen for RCC incorporating two checkpoint inhibitors. It is also the only combination regimen whose indication is limited to a specific International Metastatic RCC Database Consortium prognostic score risk group. The nivolumab‐ipilimumab combination regimen resulted in an improved OS with HR of 0.63 (99.8% CI, 0.44–0.89) over sunitinib for patients with poor‐/intermediate‐risk disease [69]. In 2019, KEYNOTE‐426 led to the approval of pembrolizumab/axitinib, also based on OS benefit with HR of 0.53 (95% CI, 0.38–0.74) [70]. JAVELIN Renal 100 led to the approval of avelumab/axitinib based on PFS benefit [71].
No biomarkers were approved as complementary or companion diagnostics for any of the RCC indications. PD‐L1 tumor cell (TC) expression has long been known to be a poor prognostic factor [72]. However, PD‐L1 TC expression has not been shown to be a strong predictive marker of PD‐[L]1 inhibitor response. Several factors have been proposed to explain the relative lack of predictive value of PD‐L1 staining, including intratumoral heterogeneity [73]; PD‐L1 expression discordance between the primary tumor (from where most biopsies are taken) and metastatic lesions [74]; and a tumor microenvironment that may be driven by other immune checkpoint receptors (B7‐H3, TIM‐3, LAG‐3, etc.) [75].
Gastrointestinal Malignancies
Hepatocellular Carcinoma, Gastric/Gastroesophageal Junction, and Esophageal SCC
Nivolumab and pembrolizumab monotherapy were both approved for hepatocellular carcinoma (HCC; in 2017 and 2018, respectively) based on single‐arm trials with relatively modest response rates (14.3%–17.0%) [22, 28]. However, results were in the context of available therapies such as tyrosine kinase inhibitors associated with single‐digit response rates with poor durability. Furthermore, half of anti‐PD‐[L]1 responding patients experienced a > 1‐year duration of response (Fig. 2) [75, 76, 77] for this subset of patients with few alternative options. In 2020, the combination of nivolumab and ipilimumab was approved for patients with HCC who have been previously treated with sorafenib based on patients in the combination therapy cohort of CheckMate 040. Patients received nivolumab 1 mg/kg in combination with ipilimumab 3 mg/kg every 3 weeks for four doses, followed by single‐agent nivolumab 240 mg every 2 weeks until disease progression or unacceptable toxicity. This trial demonstrated an ORR of 33% (n = 16; 95% CI, 20–48), with 31% of the responders having a response lasting at least 24 months.
Impacting first‐line standard‐of‐care therapy for HCC, atezolizumab in combination with bevacizumab was approved in May 2020 for patients with unresectable or metastatic HCC who have not received prior systemic therapy based on the IMbrave150 trial. IMbrave150 was a randomized trial in patients with locally advanced unresectable or metastatic HCC who had not received prior systemic therapy [78]. The primary endpoint was OS, which was significantly improved in patients who received atezolizumab plus bevacizumab (median not reached vs. 13.2 months; HR 0.58 [95% CI, 0.42–0.79]).
In gastric/gastroesophageal junction adenocarcinoma, pembrolizumab was approved for patients refractory to two lines of therapy and PD‐L1 CPS ≥10 after KEYNOTE‐059 demonstrated an ORR of 13% by blinded review, but duration of response was more than 6 months in the majority of responders [79, 80].
Pembrolizumab was also approved for esophageal squamous cell carcinoma (ESCC) with PD‐L1 CPS ≥10 in the second line [81]. This approval was based on KEYNOTE‐180 and KEYNOTE‐181. KEYNOTE‐180 was a single‐arm trial in third‐line whereas KEYNOTE‐181 was a randomized trial in the second line [82, 83]. In KEYNOTE‐181, patients were randomized to pembrolizumab monotherapy to physician's choice chemotherapy. The OS HR for patients with ESCC and PD‐L1 CPS ≥10 was 0.64 (95% CI, 0.46–0.90) [83]. Also, in patients with ESCC, ATTRACTION‐3 enrolled patients with unresectable advanced, recurrent, or metastatic ESCC that was refractory or intolerant to at least one fluoropyrimidine‐ and platinum‐based regimen. Patients were randomized to nivolumab versus investigator's choice of paclitaxel or docetaxel and demonstrated an improvement in OS, with medians of 10.9 months versus 8.4 months (HR, 0.77 [95% CI, 0.62–0.96]), regardless of tumor PD‐L1 expression level [28].
Microsatellite Instability High/Deficient Mismatch Repair‐Positive Colorectal Cancer
PD‐1 inhibitors were approved for both refractory and treatment‐naive deficient mismatch repair (dMMR) or microsatellite instability high (MSI‐H) colorectal cancer (CRC) [22, 28]. Following the tissue agnostic approval of pembrolizumab (see section below), in 2017, nivolumab was approved for dMMR/MSI‐H CRC that had progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan. This approval was based on a nivolumab monotherapy cohort in CheckMate 142 that demonstrated an ORR of 28%, with ≥6‐month DOR in 67% [28, 84]. Historically, response rates of other drugs approved in this setting (TAS‐102 or regorafenib) were less than 5% [85, 86].
In 2018, based on a combination therapy cohort of CheckMate 142, nivolumab and ipilimumab was approved for dMMR/MSI‐H CRC that had progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan. This approval was based on a 46% ORR, with ≥6‐month DOR in 89% of responders [28, 87]. Because of overlapping confidence intervals of the monotherapy and combination therapy cohorts, and limited ability to isolate contribute of effect without a randomized design, the postmarketing requirement is demonstration of PFS benefit in patients randomized to nivolumab + ipilimumab compared with nivolumab monotherapy in CheckMate 8HW. The dMMR/MSI‐H CRC indication was extended to pediatric patients aged ≥12 years for both nivolumab monotherapy and nivolumab combination with ipilimumab largely based on pharmacokinetic data and safety data from clinical trials involving pediatric patients receiving nivolumab and/or ipilimumab. In July 2020, based on the KEYNOTE‐177 trial, pembrolizumab was approved for the first‐line treatment of patients with unresectable or metastatic dMMR/MSI‐H colorectal cancer. In KEYNOTE‐177 [88], patients with previously untreated, unresectable or metastatic MSI‐H or dMMR CRC were randomized to pembrolizumab versus investigator's choice of mFOLFOX6 or FOLFIRI with or without bevacizumab or cetuximab. The primary endpoint of PFS was significantly improved in the pembrolizumab arm (median 16.5 months versus 8.2 months; HR, 0.60 [95% CI, 0.45–0.80]) [22].
Gynecologic and Breast
Cervical Cancer
The approval for pembrolizumab for refractory cervical cancer was granted based on ORR in a single‐arm trial. Although the ORR was in the range of single‐agent chemotherapy, 14% among patients with PD‐L1 CPS ≥1 (0 of 16 patients with PD‐L1 unknown or negative responded), almost half of responders had a DOR ≥15 months [22, 89]. This constituted an improvement in the context of the available therapies: generally single‐agent chemotherapies with high toxicity and single‐digit response rates with very short durations of response.
Endometrial Cancer
Patients with advanced endometrial carcinoma that is not MSI‐H or dMMR and with disease progression following prior systemic therapy and not candidates for curative surgery or radiation had no systemic therapy options prior to the approval of pembrolizumab in combination with lenvatinib in 2019. It was for this population that the pembrolizumab‐lenvatinib combination was granted accelerated approval after a collaborative review with the regulatory agencies of Australia and Canada of KEYNOTE‐146, based on the ORR of 38%, with median DOR not reached and 69% of responders achieving a ≥ 6‐month DOR [22, 81]. Data supporting contribution of effect from pembrolizumab and lenvatinib came from single‐arm monotherapy studies including Study 204 (lenvatinib) and KEYNOTE‐158 and KEYNOTE‐028 (pembrolizumab).
Triple‐Negative Breast Cancer
Atezolizumab was granted accelerated approval in 2019, after IMpassion130 [15, 90] demonstrated an improvement in PFS (median 7.5 months vs. 5.0 months; HR, 0.60 [95% CI, 0.48–0.77]) with the addition of atezolizumab to nab‐paclitaxel compared with nab‐paclitaxel alone for treatment‐naive patients with triple‐negative breast cancer (TNBC) and PD‐L1 IC ≥1%. This approval used the accelerated approval pathway, and therefore, continued approval may be contingent upon a confirmatory trial(s) [91]. In September 2020, FDA issued an alert regarding efficacy and potential safety concerns based on the results of IMpassion131 in which [92] the addition of atezolizumab to paclitaxel for treatment‐naive patients with TNBC did not improve PFS (the primary endpoint) over paclitaxel + placebo, even in patients with PD‐L1 IC ≥1%. Importantly, interim overall survival results favored paclitaxel + placebo. FDA's announcement served to warn providers against replacing nab‐paclitaxel with paclitaxel.
Hematologic Malignancies
Classic Hodgkin Lymphoma
Nivolumab was approved in 2016 and pembrolizumab in 2017 based on ORRs in Classic Hodgkin lymphoma (cHL) of 65%–69% [93, 94]. Some attribute the high response rates in cHL to the nearly universal genetic alterations in 9p24.1, resulting in constitutive expression of PD‐1 ligands. As in all cancer trials that led to approval of PD‐[L]1 inhibitors, no patients younger than age 18 participated in the clinical trials on which the approvals for cHL were based, but pembrolizumab was approved in pediatric populations with supportive safety data from KEYNOTE‐051, a phase I/II trial enrolling patients aged 6 months to <18 years. Efficacy data were extrapolated to pediatric patients based on the rationale that the biology of cHL in adolescents is comparable to the pathobiology in adults [95]. Additionally, the results of KEYNOTE‐051 will be submitted to the FDA as part of a pediatric postmarketing requirement. In the future, given that the age distribution of cHL is bimodal, and cHL is in fact the most common childhood cancer in the 15‐ to 19‐year‐old age group, consideration for lowering the age for eligibility for trials would likely provide a path to better understand safe and effective use of novel agents in adolescents and young adults and to study long‐term survivorship and effects of cancer therapy in this age group [96].
Primary Mediastinal B Cell Lymphoma
Because primary mediastinal B cell lymphoma (PMBCL) is a rare disease, the ORR of 45% with median DOR ≥6 months in a single‐arm trial of 53 patients (KEYNOTE‐170) [97] was considered sufficient evidence as the basis for accelerated approval of pembrolizumab. Given the similarities between PMBCL and Hodgkin lymphoma, the postmarketing requirement was to report the efficacy and safety data from KEYNOTE‐204, a phase III trial comparing pembrolizumab with brentuximab in refractory Classic Hodgkin lymphoma, which was fulfilled in October 2020 [98].
Tissue Site‐Agnostic: dMMR or MSI‐high; TMB‐high
Pembrolizumab is the only PD‐[L]1 inhibitor approved for a tumor site‐agnostic solid tumor indication [99]. The FDA approval processes for dMMR/MSI‐high and TMB‐high were propelled by a strong biological rationale. Microsatellite instability (MSI) is found in more than 20 cancer types and is often associated with histopathologic hallmarks such as somatic hypermutation and increased neoantigens and tumor infiltrating lymphocytes, suggesting that MSI is a generalized cancer phenotype [99, 100, 101]. Although the large majority of MSI‐high tumors will have high TMB, the overlap of either TMB or MSI with PD‐L1 is modest [102]. Alternative genetic mechanisms are also known to generate high TMB, such as POLE and POLD1 mutations, particularly in endometrial cancer [103, 104, 105].
The pembrolizumab dMMR/MSI‐high indication was defined by biomarker, but no companion diagnostics have yet been approved, which will be developed as a postmarketing commitment. The benefits of pembrolizumab were felt to outweigh the risks for patients who had already progressed on available therapy, considering the totality of the evidence with an ORR of 40%, durability of response, and no new safety signals [22].
The pembrolizumab TMB‐high indication with FoundationOne CDx was based on the results of a prospectively planned retrospective analysis of 10 cohorts of patients with previously treated unresectable or metastatic solid tumors of various primary sites with high TMB (KEYNOTE‐158). By the time that FDA was involved in discussions on this approach to a tumor site–agnostic TMB‐defined indication, studies across multiple tumor types had already reported improved ORR and survival in patients with high TMB [106]. The statistical analysis plan using KEYNOTE‐158 data prespecified ≥10 and ≥ 13 mutations per megabase (mut/Mb) as cutpoints to assess TMB, and ORR and DOR as the major efficacy outcomes. Of 790 patients with adequate tissue for TMB testing, 102 (13%) had TMB ≥10 mut/Mb, representing several tumor types: small cell lung cancer (n = 34), cervical cancer (n = 16), endometrial cancer (n = 15), anal cancer (n = 14), vulvar cancer (n = 12), neuroendocrine cancer (n = 5), salivary cancer (n = 3), thyroid cancer (n = 2), and mesothelioma (n = 1). Among these 102 patients, the ORR was 29% (95% CI, 21–39), including 4 patients with CR. Among the 30 responding patients, 57% had a duration of response ≥12 months and 50% had a response ≥24 months. In an exploratory analysis of the 32 patients whose cancers had TMB ≥10 mut/Mb and < 13 mut/Mb, the ORR was 13%, including two complete responses and two partial responses [22].
Regulatory Challenges
Endpoints
One challenge in regulatory science is identifying appropriate endpoints that may lead to marketing approval for each unique disease setting. Guidances have been published that describe general principles [107]. For example, time‐to‐event endpoints such as overall survival and progression‐free survival should be evaluated in randomized controlled studies. However, in rare diseases such as Merkel cell carcinoma, adequately powered randomized trials may not be feasible in the setting of unprecedented drug effects on durable ORR. Additionally, in tissue‐agnostic situations, a randomized design may not be practical as both the patient population and control arm treatments would be heterogeneous. For single‐arm trials, ORR can be attributed to the therapy under study, as tumors do not typically regress on their own, and this direct measure of treatment effect may support either traditional or accelerated approval, depending on the context. For example, ORR supported traditional approval for cSCC because of the direct observable impact on disfiguring cutaneous lesions, which was felt to represent additional direct clinical benefit.
Although ORR may be an imperfect endpoint, it remains the most important clinical endpoint in the single‐arm setting, and a large (durable) ORR result in a rare disease or context where tumor location is likely to cause significant morbidity or disfigurement may be considered an endpoint with its own clinical benefit in and of itself. In a randomized trial setting, overall survival remains an important endpoint as it encompasses both efficacy and safety information to inform benefit‐risk analyses. Indeed, effects of PD‐[L]1 inhibitors on overall survival have supported marketing approvals across multiple diseases based on improvements in OS and, rarely, labeling revisions related to safety (e.g., observation of an increased risk of mortality) in several clinical trials of PD‐[L]1 inhibitors when added to a thalidomide analog plus dexamethasone) [107, 108, 109, 110].
Diagnostics and Biomarkers
The three biomarkers that dominate clinical use are PD‐L1 immunohistochemistry, TMB, and mismatch repair/MSI. Multiplex biomarkers are now also under study. Although each individual biomarker can guide treatment in the appropriate setting, currently, no single unifying biomarker or predictive model has been validated across clinical contexts.
Many of the early PD‐1 inhibitor approvals, mostly for NSCLC, focused on tumor cell staining of PD‐L1, whereas the biomarkers in more recent years attempted to incorporate the presence of immune cell infiltration and PD‐L1 staining of immune cells. Indeed, all pembrolizumab approvals for non‐NSCLC indications use CPS (combined positive score = 100 * number of PD‐L1 staining cells [tumor cells, lymphocytes, macrophages]/number of viable tumor cells). Yet, scoring TC membranes is more routinely performed for PD‐L1 IHC, is more standardized, and represents a more transferable skill for pathologists experienced with other assays in the real world [111].
An ongoing question is whether the different PD‐L1 IHC assays are comparable. The Blueprint PD‐L1 IHC Assay Comparison Project [111] published results comparing the four PD‐L1 assays in 39 NSCLC tumors. Ventana SP142 was the only assay that exhibited fewer stained tumor cells compared with the other three, whose results were aligned. Ventana SP142 is also the only assay for NSCLC that includes IC staining as part of the scoring guideline.
Beyond inconsistencies in the analytical aspects of assays, the ability of PD‐L1 expression to predict which patients derive the most benefit from PD‐[L]1 inhibitors is limited. PD‐L1 expression appears to be correlated with improved response rate, progression‐free survival, and overall survival in many tumor types [4, 6, 35, 112, 113]. However, some patients with low PD‐L1 expression have robust or durable responses, whereas some patients with high PD‐L1 expression have little to no response [112]. In some settings, PD‐L1 expression appears to be more of a prognostic rather than predictive biomarker, as described in the RCC section earlier.
Additionally, the use of TMB as a patient selection tool for therapy in real‐world practice and clinical trial enrichment continues to be refined. Although 10 mut/Mb was selected based on the data submitted in the pembrolizumab application, because TMB is a continuum, the potential exists for responses to increase if the TMB is set higher and likewise decrease if the TMB is set lower. Additionally, there may be specific situations in which a patient may benefit with lower TMBs (e.g., certain viral mediated tumors such as Merkel cell carcinoma or when used in combination with chemotherapy). Multiple next‐generation sequencing platforms are available to measure TMB, and a harmonization effort is underway [39, 114]. The best way to measure TMB has not been defined: static (a single pretreatment tumor biopsy) versus dynamic (change in tumor burden during the course of therapy), and tumor biopsy versus circulating cell‐free tumor DNA (ctDNA) [115]. Tumor types with higher TMB generally have higher response rates [116], and individuals with higher TMB treated with checkpoint inhibitors tend to have favorable overall survival in most tumor types, with a few notable exceptions including RCC and Merkel cell carcinoma [115, 116, 117, 118]. Most studies have been based on pretreatment formalin‐fixed paraffin embedded tumor biopsy. Sensitivity and specificity improve with dynamic TMB compared with static, but multiple biopsies to evaluate TMB in a single patient would be invasive and burdensome [115]. Thus, the utility of ctDNA to quantify dynamic TMB and validate it as a predictor of clinical benefit is an area of active investigation [119].
Future
PD‐[L]1 inhibitors are an effective anticancer therapy in a subset of patients across an increasingly broad range of cancer types. Yet, the low response rates in several tumor types signal a lack of understanding of the immunobiology of these diseases and the mechanisms of response and resistance to guide optimal patient selection for treatment with PD‐[L]1 inhibitors. Further preclinical and clinical investigation may be required to identify a more appropriate patient population, particularly as drug development continues and the treatment landscape for several cancers becomes increasingly abound with new therapeutic options. However, biomarker‐driven prospective studies require codevelopment of in vitro diagnostic tests, which brings challenges. For example, the cutoffs (thresholds for positivity) should be prespecified prior to trial enrollment for hypothesis‐testing studies. The usefulness of a biomarker strategy for one tumor type may not translate to other tumor types. FDA encourages the discussion of development plans for each drug and biomarker, as unique risk‐benefit considerations must be carefully weighed for each situation.
Author Contributions
Conception/Design: Elaine Chang, Lorraine Pelosof, Gideon M. Blumenthal
Collection and/or assembly of data: Elaine Chang, Lorraine Pelosof, Janaki Veegharavan, Julia A. Beaver, Marc Theoret,
Data analysis and interpretation: Elaine Chang, Yutao Gong, Kirsten B. Goldberg, Janaki Veegharavan, Gideon M. Blumenthal, Marc Theoret.
Manuscript writing: Elaine Chang, Lorraine Pelosof, Gideon M. Blumenthal, Julia A. Beaver, Marc Theoret
Final approval of manuscript: Elaine Chang, Lorraine Pelosof, Steven Lemery, Yutao Gong, Kirsten B. Goldberg, Ann T. Farrell, Patricia Keegan, Janaki Veeraraghavan, Guo Wei, Gideon M. Blumenthal, Laleh Amiri‐Kordestani, Harpreet Singh, Lola Fashoyin‐Aje, Nicole Gormley, Paul G. Kluetz, Richard Pazdur, Julia A. Beaver, Marc R. Theoret
Disclosures
The authors indicated no financial relationships
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Table S1 Companion* and complementary# in vitro diagnostic (IVD) PD‐L1 assays
Acknowledgment
Dr. Gong, Dr. Keegan, and Dr. Blumenthal were employed at the FDA Oncology Center of Excellence when they worked on this manuscript.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1.In vitro companion diagnostic devices : Guidance for Industry and Food and Drug Administration Staff. U.S. Food and Drug Administration. 2014. Available at https://www.fda.gov/media/81309/download. Accessed March 17, 2021.
- 2.Accelerated approval program . 2020. U.S. Food and Drug Administration. Available at https://www.fda.gov/drugs/information-healthcare-professionals-drugs/accelerated-approval-program. Accessed March 17, 2021.
- 3.Shamseer L, Moher D, Clarke M et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015: Elaboration and explanation. BMJ 2015;349:g7647. [DOI] [PubMed] [Google Scholar]
- 4.Topalian SL, Hodi FS, Brahmer JR et al. Safety, activity, and immune correlates of anti–PD‐1 antibody in cancer. N Engl J Med 2012;366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Ribas A, Wolchok JD et al. Anti‐programmed‐death‐receptor‐1 treatment with pembrolizumab in ipilimumab‐refractory advanced melanoma: A randomised dose‐comparison cohort of a phase 1 trial. Lancet 2014;384:1109–1117. [DOI] [PubMed] [Google Scholar]
- 6.Weber JS, D'Angelo SP, Minor D et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti‐CTLA‐4 treatment (CheckMate 037): A randomised, controlled, open‐label, phase 3 trial. Lancet Oncol 2015;16:375–384. [DOI] [PubMed] [Google Scholar]
- 7.Pembrolizumab . US Prescribing Information. U.S. Food and Drug Administration. 2014. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125514lbl.pdf. Accessed March 17, 2021.
- 8.Hazarika M, Chuk MK, Theoret MR et al. U.S. FDA Approval Summary: Nivolumab for treatment of unresectable or metastatic melanoma following progression on ipilimumab. Clin Cancer Res 2017;23:3484–3488. [DOI] [PubMed] [Google Scholar]
- 9.125514Orig1s000: Medical review. 2014. Center for Drug Evaluation and Research. Available at https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125514Orig1s000MedR.pdf.
- 10.Wolchok JD, Chiarion‐Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:(14):1345–1356. 10.1056/nejmoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schachter J, Ribas A, Long GV et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open‐label phase 3 study (KEYNOTE‐006). Lancet 2017;390:1853–1862. [DOI] [PubMed] [Google Scholar]
- 12.Nivolumab . Prescribing information. U.S. Food and Drug Administration. 2019. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125554s073lbl.pdf. Accessed March 17, 2021.
- 13.Robert C, Long GV, Brady B et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–330. [DOI] [PubMed] [Google Scholar]
- 14.Gutzmer R, Stroyakovskiy D, Gogas H et al. Atezolizumab, vemurafenib, and cobimetinib as first‐line treatment for unresectable advanced BRAFV600 mutation‐positive melanoma (IMspire150): Primary analysis of the randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet 2020;395:1835–1844. [DOI] [PubMed] [Google Scholar]
- 15.Atezolizumab . Prescribing information. U.S. Food and Drug Administration. 2020. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761034s028lbl.pdf. Accessed March 17, 2021.
- 16.Weber J, Mandala M, Del Vecchio M et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377:1824–1835. [DOI] [PubMed] [Google Scholar]
- 17.Eggermont AMM, Blank CU, Mandala M et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 2018;378:1789–1801. [DOI] [PubMed] [Google Scholar]
- 18.Hodi FS, Hwu WJ, Kefford R et al. Evaluation of immune‐related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol 2016;34:1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beaver JA, Hazarika M, Mulkey F et al. Patients with melanoma treated with an anti‐PD‐1 antibody beyond RECIST progression: A US Food and Drug Administration pooled analysis. Lancet Oncol 2018;19:229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seymour L, Bogaerts J, Perrone A et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143–e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Merkel cell carcinoma. Version 1.2021. National Comprehensive Cancer Network. Available at https://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pembrolizumab . Prescribing information. U.S. Food and Drug Administration. 2020. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125514s084lbl.pdf. Accessed March 17, 2021.
- 23.Avelumab . Prescribing information. U.S. Food and Drug Administration. 2020. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761049s009lbl.pdf. Accessed March 17, 2021.
- 24.Kaufman HL, Russell JS, Hamid O et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow‐up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer 2018;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cemiplimab . Prescribing information. U.S. Food and Drug Administration. 2018. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761097s000lbl.pdf. Accessed March 17, 2021.
- 26.Jarkowski AI, Hare R, Loud P et al. Systemic therapy in advanced cutaneous squamous cell carcinoma (CSCC): The Roswell Park experience and a review of the literature. Am J Clin Oncol 2016;39:545–548. [DOI] [PubMed] [Google Scholar]
- 27.Migden MR, Rischin D, Schmults CD et al. PD‐1 blockade with cemiplimab in advanced cutaneous squamous‐cell carcinoma. N Engl J Med 2018;379:341–351. [DOI] [PubMed] [Google Scholar]
- 28.Nivolumab . Prescribing information. U.S. Food and Drug Administration. 2020. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125554s083lbl.pdf. Accessed March 17, 2021.
- 29.Durvalumab . Prescribing information. U.S. Food and Drug Administration. 2020. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761069s020lbl.pdf. Accessed March 17, 2021.
- 30.Antonia SJ, Villegas A, Daniel D et al. Durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med 2017;377:1919–1929. [DOI] [PubMed] [Google Scholar]
- 31.Gandhi L, Rodríguez‐Abreu D, Gadgeel S et al. Pembrolizumab plus chemotherapy in metastatic non–small‐cell lung cancer. N Engl J Med 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 32.Paz‐Ares L, Luft A, Vicente D et al. Pembrolizumab plus chemotherapy for squamous non–small‐cell lung cancer. N Engl J Med 2018;379:2040–2051. [DOI] [PubMed] [Google Scholar]
- 33.Socinski MA, Jotte RM, Cappuzzo F et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018;378:2288–2301. [DOI] [PubMed] [Google Scholar]
- 34.Spigel D, de Marinis F, Giaccone G et al. 6256: IMpower110: Interim overall survival (OS) analysis of a phase III study of atezolizumab (atezo) vs platinum‐based chemotherapy (chemo) as first‐line (1L) treatment (tx) in PD‐L1–selected NSCLC. Ann Oncol 2019;30:v851–v934. [Google Scholar]
- 35.Reck M, Rodríguez‐Abreu D, Robinson AG et al. Testing the timing of Pembrolizumab alone or with chemotherapy as first line treatment and maintenance in non‐small cell lung cancer. N Engl J Med 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 36.Firstline pembrolizumab alone or in combination with pemetrexed and carboplatin in induction/maintenance or postprogression in treating patients with stage IV non‐squamous non‐small cell lung cancer. ClinicalTrials.gov. Available at https://clinicaltrials.gov/ct2/show/NCT03793179. Accessed March 17, 2021.
- 37.Hellmann MD, Ciuleanu TE, Pluzanski A et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018;378:2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carbone DP, Reck M, Paz‐Ares L et al. First‐line nivolumab in stage IV or recurrent non‐small‐cell lung cancer. N Engl J Med 2017;376:2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fabrizio D, Chen SJ, Xie M et al. In silico assessment of variation in TMB quantification across diagnostic platforms: Phase 1 of the Friends of Cancer Research Harmonization Project. In Society for Immunotherapy of Cancer Annual Meeting, Washington, DC: November 7–11, 2018.
- 40.Antonia SJ, López‐Martin JA, Bendell J et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small‐cell lung cancer (CheckMate 032): A multicentre, open‐label, phase 1/2 trial. Lancet Oncol 2016;17:883–895. [DOI] [PubMed] [Google Scholar]
- 41.Merck provides update on KEYTRUDA® (pembrolizumab) indication in metastatic small cell lung cancer in the US. Merck. 2021. Available at https://www.merck.com/news/merck‐provides‐update‐on‐keytruda‐pembrolizumab‐indication‐in‐metastatic‐small‐cell‐lung‐cancer‐in‐the‐us/. Accessed March 17, 2021.
- 42.Owonikoko TK, Kim HR, Govindan R et al. Nivolumab (nivo) plus ipilimumab (ipi), nivo, or placebo (pbo) as maintenance therapy in patients (pts) with extensive disease small cell lung cancer (ED‐SCLC) after first‐line (1L) platinum‐based chemotherapy (chemo): Results from the double‐blind, randomized phase III CheckMate 451 study. Ann Oncol 2019;30(suppl 2):ii77. [Google Scholar]
- 43.Reck M, Vicente D, Ciuleanu T et al. Efficacy and safety of nivolumab (nivo) monotherapy versus chemotherapy (chemo) in recurrent small cell lung cancer (SCLC): Results from CheckMate 331. Ann Oncol 2018. (suppl 10);29:x43. [Google Scholar]
- 44.Bristol Myers Squibb statement on Opdivo (nivolumab) small cell lung cancer U.S. indication. 2020. Available at https://news.bms.com/news/details/2020/Bristol-Myers-Squibb-Statement-on-Opdivo-nivolumab-Small-Cell-Lung-Cancer-US-Indication/default.aspx. Accessed March 17, 2021.
- 45.Rudin CM, Awad MM, Navarro A et al. Pembrolizumab or placebo plus etoposide and platinum as first‐line therapy for extensive‐stage small‐cell lung cancer: Randomized, double‐blind, phase III KEYNOTE‐604 study. J Clin Oncol 2020;38:2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horn L, Mansfield AS, Szczęsna A et al. First‐line atezolizumab plus chemotherapy in extensive‐stage small‐cell lung cancer. N Engl J Med 2018;379:2220–2229. [DOI] [PubMed] [Google Scholar]
- 47.Ferris RL, Blumenschein G Jr., Fayette J et al. Nivolumab for recurrent squamous‐cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seiwert TY, Burtness B, Mehra R et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE‐012): An open‐label, multicentre, phase 1b trial. Lancet Oncol 2016;17:956–965. [DOI] [PubMed] [Google Scholar]
- 49.Cho BC, Keum KC, Shin SJ et al. Weekly docetaxel in patients with platinum‐refractory metastatic or recurrent squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol 2009;65:27–32. [DOI] [PubMed] [Google Scholar]
- 50.Cohen EEW, Soulières D, Le Tourneau C et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head‐and‐neck squamous cell carcinoma (KEYNOTE‐040): A randomised, open‐label, phase 3 study. Lancet 2019;393:156–167. [DOI] [PubMed] [Google Scholar]
- 51.Rischin D, Harrington KJ, Greil R et al. Protocol‐specified final analysis of the phase 3 KEYNOTE‐048 trial of pembrolizumab (pembro) as first‐line therapy for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J Clin Oncol 2019;37(suppl):6000a. [Google Scholar]
- 52.Premarket approval: PD‐L1 IHC 28‐8 pharmDx. U.S. Food and Drug Administration. Available at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P150025S003. Accessed March 17, 2021.
- 53.Premarket approval: VENTANA PD‐L1 SP263. U.S. Food and Drug Administration Available at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P160046. Accessed March 17, 2021
- 54.Voluntary withdrawal of Imfinzi indication in advanced bladder cancer in the US. AstraZeneca. 2021. Available at https://www.astrazeneca.com/media‐centre/press‐releases/2021/voluntary‐withdrawal‐imfinzi‐us‐bladder‐indication.html. Accessed March 17, 2021.
- 55.Powles T, van der Heijden MS, Castellano D et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): A randomised, open‐label, multicentre, phase 3 trial. Lancet Oncol 2020;21:1574–1588. [DOI] [PubMed] [Google Scholar]
- 56.Genentech provides update on Tecentriq U.S. indication in prior‐platinum treated metastatic bladder cancer. Genentech. 2021. Available at https://www.gene.com/media/press-releases/14898/2021–03–07/genentech-provides-update-on-tecentriq-u. Accessed March 17, 2021 [Google Scholar]
- 57.Powles T, Durán I, van der Heijden MS et al. Atezolizumab versus chemotherapy in patients with platinum‐treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open‐label, phase 3 randomised controlled trial. Lancet 2018;391:748–757. [DOI] [PubMed] [Google Scholar]
- 58.Sharma P, Retz M, Siefker‐Radtke A et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single‐arm, phase 2 trial. Lancet Oncol 2017;18:312–322. [DOI] [PubMed] [Google Scholar]
- 59.Bellmunt J, de Wit R, Vaughn DJ et al. Pembrolizumab as second‐line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Necchi A, Joseph RW, Loriot Y et al. Atezolizumab in platinum‐treated locally advanced or metastatic urothelial carcinoma: Post‐progression outcomes from the phase II IMvigor210 study. Ann Oncol 2017;28:3044–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Powles T, O'Donnell PH, Massard C et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open‐label study. JAMA Oncol 2017;3:e172411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel MR, Ellerton J, Infante JR et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): Pooled results from two expansion cohorts of an open‐label, phase 1 trial. Lancet Oncol 2018;19:51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galsky MD, Hahn NM, Rosenberg J et al. Treatment of patients with metastatic urothelial cancer "unfit" for cisplatin‐based chemotherapy. J Clin Oncol 2011;29:2432‐2438. [DOI] [PubMed] [Google Scholar]
- 64.Balar AV, Castellano D, O'Donnell PH et al. First‐line pembrolizumab in cisplatin‐ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE‐052): A multicentre, single‐arm, phase 2 study. Lancet Oncol 2017;18:1483–1492. [DOI] [PubMed] [Google Scholar]
- 65.Balar AV, Galsky MD, Rosenberg JE et al. Atezolizumab as first‐line treatment in cisplatin‐ineligible patients with locally advanced and metastatic urothelial carcinoma: a single‐arm, multicentre, phase 2 trial. Lancet 2017;389:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suzman DL, Agrawal S, Ning YM et al. FDA approval summary: Atezolizumab or pembrolizumab for the treatment of patients with advanced urothelial carcinoma ineligible for cisplatin‐containing chemotherapy. The Oncologist 2019;24:563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.FDA limits the use of Tecentriq and Keytruda for some urothelial cancer patients . U.S. Food and Drug Administration. 2018. Available at https://www.fda.gov/drugs/resources‐information‐approved‐drugs/fda‐limits‐use‐tecentriq‐and‐keytruda‐some‐urothelial‐cancer‐patients. Accessed March 17, 2021.
- 68.Powles T, Park SH, Voog E et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 2020;383:1218–1230. [DOI] [PubMed] [Google Scholar]
- 69.Motzer RJ, Tannir NM, McDermott DF et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal‐cell carcinoma. N Engl J Med 2018;378:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rini BI, Plimack ER, Stus V et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. 2019;380:1116–1127. [DOI] [PubMed] [Google Scholar]
- 71.Motzer RJ, Penkov K, Haanen J et al. Avelumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. 2019;380:1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thompson RH, Dong H, Kwon ED. Implications of B7‐H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res 2007;13:709s–715s. [DOI] [PubMed] [Google Scholar]
- 73.Gerlinger M, Rowan AJ, Horswell S et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366:883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Callea M, Albiges L, Gupta M et al. Differential expression of PD‐L1 between primary and metastatic sites in clear‐cell renal cell carcinoma. Cancer Immunol Res 2015;3:1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Signoretti S, Flaifel A, Chen YB, Reuter VE. Renal cell carcinoma in the era of precision medicine: From molecular pathology to tissue‐based biomarkers. J Clin Oncol 2018;36:JCO2018792259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.El‐Khoueiry AB, Sangro B, Yau T et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open‐label, non‐comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu AX, Finn RS, Edeline J et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE‐224): A non‐randomised, open‐label phase 2 trial. Lancet Oncol 2018;19:940–952. [DOI] [PubMed] [Google Scholar]
- 78.Finn RS, Qin S, Ikeda M et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894–1905. [DOI] [PubMed] [Google Scholar]
- 79.Fuchs CS, Doi T, Jang RW et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE‐059 Trial. JAMA Oncol 2018;4:e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fashoyin‐Aje L, Donoghue M, Chen H et al. FDA approval summary: Pembrolizumab for recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma expressing PD‐L1. The Oncologist 2019;24:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pembrolizumab . Prescribing information. U.S. Food and Drug Administration. 2019. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125514s065lbl.pdf. Accessed March 17, 2021.
- 82.Shah MA, Kojima T, Hochhauser D et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: The phase 2 KEYNOTE‐180 Study. JAMA Oncol 2019;5:546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kojima T, Muro K, Francois E et al. Pembrolizumab versus chemotherapy as second‐line therapy for advanced esophageal cancer: Phase III KEYNOTE‐181 study. 2019;37(suppl):2a. [Google Scholar]
- 84.Overman MJ, McDermott R, Leach JL et al. Nivolumab in patients with metastatic DNA mismatch repair‐deficient or microsatellite instability‐high colorectal cancer (CheckMate 142): An open‐label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grothey A, Cutsem EV, Sobrero A et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet 2013;381:303–312. [DOI] [PubMed] [Google Scholar]
- 86.Mayer RJ, Van Cutsem E, Falcone A et al. Randomized trial of TAS‐102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–1919. [DOI] [PubMed] [Google Scholar]
- 87.Overman MJ, Lonardi S, Wong KYM et al. Durable clinical benefit with nivolumab plus ipilimumab in dna mismatch repair–deficient/microsatellite instability–high metastatic colorectal cancer. J Clin Oncol 2018;36:773–779. [DOI] [PubMed] [Google Scholar]
- 88.Andre T, Shiu KK, Kim TW et al. Pembrolizumab versus chemotherapy for microsatellite instability‐high/mismatch repair deficient metastatic colorectal cancer: The phase 3 KEYNOTE‐177 Study. J Clin Oncol 2020;38(suppl):LBA4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chung HC, Ros W, Delord J‐P et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: Results from the phase II KEYNOTE‐158 study. 2019;37:1470–1478. [DOI] [PubMed] [Google Scholar]
- 90.Schmid P, Adams S, Rugo HS et al. Atezolizumab and nab‐paclitaxel in advanced triple‐negative breast cancer. 2018;379:2108–2121. [DOI] [PubMed] [Google Scholar]
- 91.Tecentriq® (atezolizumab) : Accelerated approval letter. U.S. Food and Drug Association. 2019. Available at https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2019/761034Orig1s018ltr.pdf. Accessed March 17, 2021.
- 92.FDA issues alert about efficacy and potential safety concerns with atezolizumab in combination with paclitaxel for treatment of breast cancer . U.S. Food and Drug Association. 2020. Available at https://www.fda.gov/drugs/resources‐information‐approved‐drugs/fda‐issues‐alert‐about‐efficacy‐and‐potential‐safety‐concerns‐atezolizumab‐combination‐paclitaxel. Accessed March 17, 2021.
- 93.Kasamon YL, de Claro RA, Wang Y et al. FDA approval summary: Nivolumab for the treatment of relapsed or progressive Classical Hodgkin lymphoma. The Oncologist 2017;22:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen R, Zinzani PL, Fanale MA et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory Classic Hodgkin lymphoma. J Clin Oncol 2017;35:2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.U.S. Food and Drug Administration . General Clinical Pharmacology Considerations for Pediatric Studies for Drugs and Biological Products: Guidance for Industry. Silver Spring, MD: U.S. Food and Drug Administration, 2014. Available at https://www.fda.gov/downloads/drugs/guidances/ucm425885.pdf. Accessed March 17, 2021. [Google Scholar]
- 96.Considerations for the inclusion of adolescent patients in adult oncology clinical trials: Guidance for industry . U.S. Food and Drug Administration. 2019. Available at https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM609513.pdf. Accessed March 17, 2021.
- 97.Zinzani P, Thieblemont C, Melnichenko V et al. Efficacy and safety of pembrolizumab in relapsed/refractory primary mediastinal large B‐cell lymphoma (rrPMBCL): Interim analysis of the KEYNOTE‐170 phase 2 trial. Hematol Oncol 2017;35:62–63. [Google Scholar]
- 98.BLA 125514/S‐030 accelerated approval letter . U.S. Food and Drug Administration. 2017. Available at https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2018/125514Orig1s030ltr.pdf. Accessed March 17, 2021.
- 99.Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: Pembrolizumab for the treatment of microsatellite instability‐high solid tumors. Clin Cancer Res 2019;25:3753–3758. [DOI] [PubMed] [Google Scholar]
- 100.Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site ‐ When a biomarker defines the indication. N Engl J Med 2017;377:1409–1412. [DOI] [PubMed] [Google Scholar]
- 101.Hause RJ, Pritchard CC, Shendure J et al. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med 2016;22:1342–1350. [DOI] [PubMed] [Google Scholar]
- 102.Vanderwalde A, Spetzler D, Xiao N et al. Microsatellite instability status determined by next‐generation sequencing and compared with PD‐L1 and tumor mutational burden in 11,348 patients. Cancer Med 2018;7:746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Howitt BE, Shukla SA, Sholl LM et al. Association of polymerase e‐mutated and microsatellite‐instable endometrial cancers with neoantigen load, number of tumor‐infiltrating lymphocytes, and expression of PD‐1 and PD‐L1. JAMA Oncol 2015;1:1319–1323. [DOI] [PubMed] [Google Scholar]
- 104.Briggs S, Tomlinson I. Germline and somatic polymerase ε and δ mutations define a new class of hypermutated colorectal and endometrial cancers. J Pathol 2013;230:148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang F, Zhao Q, Wang YN et al. Evaluation of POLE and POLD1 mutations as biomarkers for immunotherapy outcomes across multiple cancer types. JAMA Oncol 2019;5:1504–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goodman AM, Kato S, Bazhenova L et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 2017;16:2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.U.S. Food and Drug Administration . Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics: Guidance for Industry. Silver Spring, MD: U.S. Food and Drug Administration, 2018. Available at https://www.fda.gov/media/71195/download. Accessed April 13, 2021. [Google Scholar]
- 108.Mateos MV, Blacklock H, Schjesvold F et al. A phase 3 randomized study of pembrolizumab (Pembro) plus pomalidomide (Pom) and dexamethasone (Dex) for relapsed/refractory multiple myeloma (RRMM): KEYNOTE‐183. J Clin Oncol 2018;36:8021a. [Google Scholar]
- 109.Usmani SZ, Schjesvold F, Rocafiguera AO et al. A phase 3 randomized study of pembrolizumab (pembro) plus lenalidomide (len) and low‐dose dexamethasone (Rd) versus Rd for newly diagnosed and treatment‐naive multiple myeloma (MM): KEYNOTE‐185. J Clin Oncol 2018;36:8010a. [Google Scholar]
- 110.Gormley NJ, Pazdur R. Immunotherapy combinations in multiple myeloma — Known unknowns. N Engl J Med 2018;379:1791–1795. [DOI] [PubMed] [Google Scholar]
- 111.Hirsch FR, McElhinny A, Stanforth D et al. PD‐L1 immunohistochemistry assays for lung cancer: Results from phase 1 of the Blueprint PD‐L1 IHC Assay Comparison Project. J Thorac Oncol 2017;12:208–222. [DOI] [PubMed] [Google Scholar]
- 112.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor‐based immunotherapy. Lancet Oncol 2016;17:e542–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shen X, Zhao B. Efficacy of PD‐1 or PD‐L1 inhibitors and PD‐L1 expression status in cancer: Meta‐analysis. BMJ 2018;362:k3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.The future use of complex biomarkers . 2020. Friends of Cancer Research. Available at https://friendsofcancerresearch.org/events/future-use-complex-biomarkers.
- 115.Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 2019;19:133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD‐1 inhibition. N Engl J Med 2017;377:2500–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miao D, Margolis CA, Gao W et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 2018;359:801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McDermott DF, Huseni MA, Atkins MB et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 2018;24:749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nghiem PT, Bhatia S, Lipson EJ et al. PD‐1 blockade with pembrolizumab in advanced merkel‐cell carcinoma. N Engl J Med 2016;374:2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Table S1 Companion* and complementary# in vitro diagnostic (IVD) PD‐L1 assays


