ABSTRACT
Background
Systemic lupus erythematosus (SLE) affects African-American (AA) women disproportionately. The few prospective studies assessing dietary intake in relation to risk of SLE have been conducted in predominantly white populations and have been null.
Objectives
The present study assessed associations of macronutrients and dietary patterns with risk of SLE in AA women.
Methods
Data from the Black Women's Health Study was collected prospectively via biennial questionnaires starting in 1995. Participants completed a self-administered 68-item FFQ in 1995. Self-reported SLE was verified through medical record review. We used multivariable (MV) Cox regression models to estimate HRs and 95% CIs for macronutrients, carbohydrates, proteins, total fats, PUFAs, ω-3 fatty acids, ω-6 fatty acids, MUFAs, saturated fats, trans fatty acids, Alternative Healthy Eating Index score, vegetable/fruit and meat/fried food dietary patterns, and a reduced rank regression (RRR)-derived dietary pattern in relation to SLE risk.
Results
We confirmed a total of 114 incident cases of SLE among 51,934 women during 1995–2015. MVHRs and 95% CIs for the highest quintile of intake versus the lowest were HR: 1.96, 95% CI: 1.02, 3.67 for carbohydrates; HR: 0.66, 95% CI: 0.37, 1.18 for protein; and HR: 0.54, 95% CI: 0.28, 1.01 for total fats. MUFAs, saturated fatty acids, and trans fatty acids were significantly associated with a lower risk of SLE. An RRR-derived factor, rich in fruits and sugar-sweetened drinks and low in margarines and butter, red and processed meats, fried chicken, poultry, and eggs, which explained 53.4% of the total variation of macronutrients, was the only food pattern associated with increased SLE risk (HR: 1.88, 95% CI: 1.06, 3.35).
Conclusion
These analyses suggest that a diet high in carbohydrates and low in fats is associated with increased SLE risk in AA women.
Keywords: macronutrients, carbohydrates, fats, proteins, diet, reduced rank regression, systemic lupus erythematosus, African American, Black Women's Health Study
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease. Genetic and environmental factors have been associated with the development and pathogenesis of the disease through biological mechanisms such as oxidative stress, changes in proinflammatory biomarkers, and changes in immune signaling (1). Women of childbearing age are disproportionately affected, and African-American (AA) women have an incidence of SLE ∼1.6 times that of white women (2).
Dietary intake differences may contribute in part to the higher incidence of SLE among AA women compared with white women. Fructose consumption, which may contribute to biochemical alterations observed in inflammatory diseases (3), is higher in AAs than in other US groups (4). In addition, AA women have higher consumption of sugar-sweetened beverages than other population groups, including white women, in the USA (5), and AAs are more likely to consume foods prepared away from home (i.e. fast foods) (6, 7).
Case-control studies suggest differential intake of macronutrients among SLE cases and controls. Elkan et al. (8) found that 114 SLE patients reported slightly higher energy consumption from carbohydrates (49.8% of total caloric intake) relative to 122 population-based controls (47.3%). In a study conducted in Spain (9) examining nutritional status, there was significantly lower intake of fat and proteins in 92 SLE patients (85 females) compared with an all-female reference sample (n = 1734). The cross-sectional nature of these studies, which measured dietary intake after SLE diagnosis, makes it difficult to determine whether dietary components such as macronutrients are risk factors for SLE.
To our knowledge, the only prospective studies addressing the effect of diet on incidence of SLE are from the two Nurses’ Health Study (NHS) cohorts (NHSI & NHSII)consisting primarily of white women. In pooled analyses of the NHS cohorts, Western and Prudent dietary patterns (10) and 4 different dietary indices (the Alternative Healthy Eating Index-2010 [AHEI-2010], the Alternative Mediterranean Diet Score [aMed], Dietary Approach to Hypertension [DASH], and the Empirical Inflammatory Pattern [EDIP]) (11) were not associated with risk of SLE. The results for Western and Prudent patterns were based on analyses of 180 SLE cases, whereas results for the other patterns were based on 194 SLE cases. One component of the AHEI-2010 score (11), nuts and legumes, was inversely associated with risk of SLE (HR: 0.59, 95% CI: 0.40, 0.87). In other analyses from the NHS, there was no association of intake of vitamin D during adolescence or adulthood with incidence of either SLE or rheumatoid arthritis in adulthood (12, 13). Antioxidant vitamin intake in diet and supplements was also not associated with risk of SLE (14).
How diet relates to risk of incident SLE has not, to our knowledge, been studied in AA populations. In the present work, we assessed whether intake of macronutrients (e.g. carbohydrates, fats) was associated with risk of incident SLE in AA women and then identified which foods best explained the variation in macronutrient intake. All analyses were conducted in the Black Women's Health Study (BWHS), a large prospective cohort of AA women.
Methods
Study population
The BWHS is a follow-up study of AA women. Invitations to participate accompanied by a questionnaire were sent in 1995 largely to subscribers to Essence magazine, a magazine targeted to AA women (15, 16). The questionnaire collected information on demographics, anthropometrics, lifestyle, medical history, and diet through a modified National Cancer Institute (NCI)-Block FFQ (17, 18). A total of 59,000 AA women, aged 21–69 y and living in the continental USA, completed the questionnaire in 1995 and have been followed by mailed and web-based questionnaires every 2 y. Participants self-identified as “black” on the 1997 follow-up questionnaire. The Boston University medical campus Institutional Review Board approved the protocol.
SLE cases
Every biennial questionnaire asked about the diagnosis and year of diagnosis of different conditions, including “lupus (systemic lupus erythematosus).” Among women who reported SLE, the medical records related to SLE of those who consented were obtained or their physicians were asked to complete a checklist about the presence of SLE using the 1997 Updated American College of Rheumatology (ACR) SLE Classification Criteria (19). Study rheumatologists (KHC and MB) reviewed all records and checklists, with high interrater agreement (κ: 0.96, 95% CI: 0.89, 1.00) (20). Cases were classified as confirmed SLE if they had ≥4 of the 11 symptoms of the ACR SLE Classification Criteria.
Dietary assessment
In 1995, participants were asked to complete a 68-item FFQ, which was a modified version of the NCI-Block short form FFQ (18). Frequencies for each response ranged from never or <1/mo to ≥2/d for foods and from never or <1/mo to ≥6/d for beverages. Sizes were categorized as small, medium, and large, where small was ½ of medium size and large was 1.5 times medium size. Nutrients were calculated using the Diet*Calc software from NCI (version 1.4.1).
A validation study of the 1995 FFQ was conducted among 408 BWHS participants who were interviewed by telephone over the course of a year for 3 nonconsecutive 24-h diet recalls (17). The women also provided 1 3-d food record. Energy-adjusted and deattenuated Pearson correlation coefficients between FFQ-derived nutrients and recall/record-derived averages ranged from 0.5 to 0.8. In particular, the correlation coefficient was 0.5 for the intake of carbohydrates, 0.8 for the intake of proteins, and 0.5 for the intake of total fat (17). The dietary information assessed in the present study was from the 1995 FFQ.
Dietary patterns
In the present analysis, we calculated dietary patterns using reduced rank regression (RRR) (21). Other data-reduction techniques such as principal component analysis (PCA) calculate dietary patterns by maximizing explained variation of food intake. However, PCA-derived dietary patterns may not be related to the outcome of interest because they do not consider variation of disease-related nutrients. On the other hand, RRR-derived dietary patterns take into account potential mediators (e.g. macronutrients) of the relation of food intake and disease. RRR then aims to create linear combinations of predictors to explain most of the variation of response variables (22–24). As in a previous dietary analysis in the BWHS, we first created 35 food groups based on nutrient content similarities of the individual foods (25). We then used these food groups as predictors and 6 macronutrients (carbohydrates, proteins, PUFAs, MUFAs, saturated fats, and trans fatty acids) as response variables. These factors are independent and can be used in regression models without confounding each other (21). Correlations between each food group and factor are shown as food loadings. Larger food loadings represent larger contributions of the food group to the factor. A positive food loading represents a positive correlation of the food loading with the factor. Inverse correlations are represented by negative food loadings. Conventionally, a loading of 20% or above is considered important.
We also examined 2 major dietary patterns in relation to incidence of SLE. These 2 patterns (vegetable/fruit pattern and meat/fried food pattern) were found to explain 10% and 6%, respectively, of the total variance of food intake in a previous BWHS analysis (25). The vegetable/fruit pattern consists of a high intake of vegetables, fruits, legumes, fish, and whole grains. The meat/fried food pattern includes the high consumption of red meat, processed meat, French fries, fried chicken, and added fats (25).
Finally, we assessed the AHEI-2010 diet score (26) in relation to SLE incidence. The AHEI-2010 score is a diet quality score based on 11 food and nutrient components. High values of the AHEI-2010 score have been associated with a lower risk of cardiovascular disease outcomes in both men and women (26). The AHEI-2010 score has also been associated with rheumatoid arthritis risk (27).
Covariates
All covariates for the present analysis were collected at baseline in 1995. Participants provided information on current height (inches) and weight (pounds), weight (pounds) at age 18 y, age at menarche, cigarette smoking, alcohol consumption, physical activity, use of oral contraceptives, menopausal status, and years of education. BMI was calculated as weight (in kilograms) divided by height squared (in square meters). Finally, a neighborhood socioeconomic status (SES) score was derived from socioeconomic data obtained by linking the women's addresses to US Census block data on wealth, income, and education; lower scores denote lower SES (28).
Study cohort and statistical analysis
For the current analysis, we excluded 757 women who reported SLE at baseline and 6309 women with energy intake <500 kcal/d or ≥3800 kcal/d, >10 missing items on the FFQ, or those with macronutrient intake above the 99th percentile (Figure 1). We followed the remaining women until SLE diagnosis, loss to follow-up, death, or end of follow-up (31 December, 2015) whichever came first. Self-reported SLE cases whose symptoms did not meet the ACR criteria for SLE contributed person time until the year of the self-reported diagnosis, at which point they were censored. The analytical cohort included 51,934 participants.
FIGURE 1.
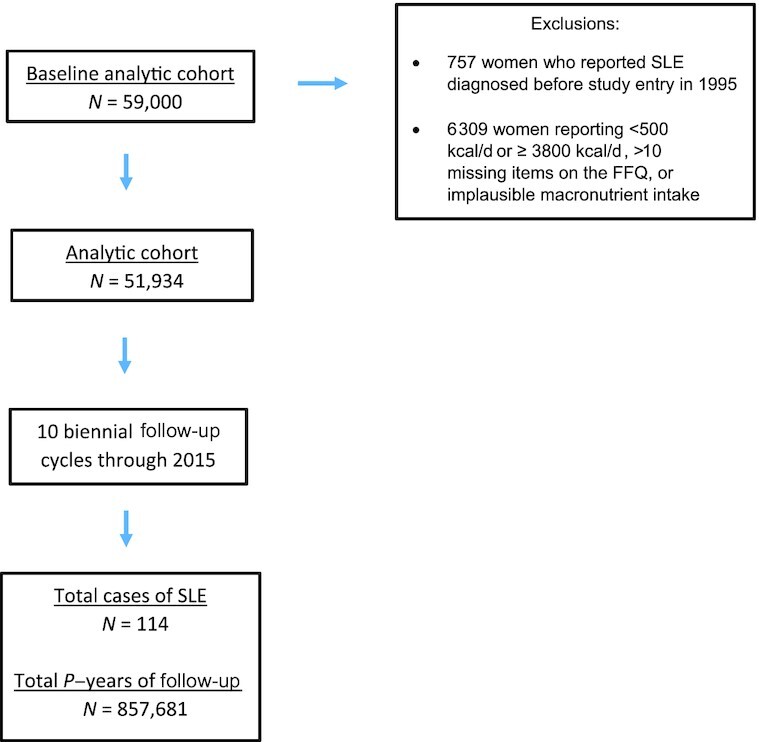
Black Women's Health Study follow up flow chart among 51,934 participants in the Black Women's Health Study, 1995–2015. p-years, Person-years; SLE, systemic lupus erythematosus.
Cox proportional hazards regression was used to estimate HRs and 95% CIs for the association of individual macronutrient intake (quintiles) and dietary patterns (quintiles) with incidence of SLE. The following macronutrients were examined: carbohydrates, proteins, total fats, PUFAs, ω-3 fatty acids (20:5 [n–3], 22:5 [n–3], and 22:6 [n–3]), ω-6 fatty acids (18:2[n–6], 20:4 [n–6]), MUFAs, saturated fats, and trans fatty acids. Our “base” model was adjusted for age, questionnaire period, and energy intake (continuous). We adjusted the final multivariable (MV) models for age, questionnaire cycle (calendar time), energy intake (continuous), BMI (<20, 20–24.9, 25–29.9, ≥30 kg/m2), neighborhood SES (quintiles) (29), years of education (≤12, 13–15, 16, ≥17), vigorous physical activity (none, <5, ≥5 h/wk), alcohol intake (current, past, never), and smoking (current, past, never). Alcohol consumption and smoking are associated inversely and positively, respectively, with risk of SLE in the BWHS (20), as in other studies (30–32). Other potential confounders assessed but not ultimately included as they did not affect risk estimates were BMI at age 18 y (<20, 20–24.9, 25–29.9, ≥30), age at menarche (<11, 11, 12–14, ≥15 y), oral contraceptive use (current/never, past), and menopausal status (premenopausal, postmenopausal). We tested for linear trend by using a continuous variable that included the median value for each category or quintile of the variable. We estimated associations with SLE by substituting 10 g/d of 1 macronutrient at the expense of another macronutrient using continuous variables (substitution analyses) (33).
All macronutrients and food groups were energy adjusted using the residual method (34). The residuals were standardized by the mean energy intake of the study cohort. Mean energy intake was 1460 kcal/d.
Finally, in a sensitivity analysis we lagged the analysis by excluding the first 2 y of follow-up and cases diagnosed before 1999 to account for the possibility that symptoms of SLE before diagnosis had affected dietary intake. All statistical analyses were performed using SAS version 9.4.
Results
During 857,681 person-years of follow-up (1995 through to 2015) of 51,934 BWHS participants, 114 cases of SLE were confirmed.
Table 1 shows the baseline characteristics of the lowest and highest quintiles of intake of carbohydrates, proteins, and total fats. Consumption of these macronutrients was unrelated to age, BMI, BMI at age 18 y, premenopausal status, and current oral contraceptive use. Women in the highest quintile of intake of carbohydrates and proteins and the lowest quintile of intake of total fats more often had a college education, lived in higher SES neighborhoods, and were more physically active. Women in the highest quintile of intake of carbohydrates and lowest quintile of intake of fats were less likely to smoke or drink alcohol.
TABLE 1.
Baseline characteristics in 1995 by lowest and highest quintiles of intake of carbohydrates, proteins, and total fats among 51,934 participants in the Black Women's Health Study1
| Quintiles of intake of carbohydrates2 | Quintiles of intake of proteins2 | Quintiles of intake of total fat2 | ||||
|---|---|---|---|---|---|---|
| Q1 | Q5 | Q1 | Q5 | Q1 | Q5 | |
| Number of participants | 10,386 | 10,387 | 10,386 | 10,387 | 10,386 | 10,387 |
| Mean age, mean ± SD | 38.6 ± 10.2 | 39.2 ± 11.1 | 38.8 ± 10.6 | 39.5 ± 10.7 | 39.3 ± 11.1 | 38.9 ± 10.3 |
| BMI, kg/m2, mean ± SD | 28.8 ± 7.2 | 27.3 ± 6.2 | 27.6 ± 6.5 | 28.5 ± 6.9 | 27.3 ± 6.1 | 28.6 ± 7.1 |
| BMI at age 18, kg/m2, mean ± SD | 21.7 ± 4.3 | 21.3 ± 3.9 | 21.2 ± 3.9 | 21.9 ± 4.3 | 21.4 ± 4.0 | 21.6 ± 4.2 |
| Education ≥16 y | 42 | 47 | 42 | 48 | 50 | 41 |
| Neighborhood SES, quintiles | ||||||
| Q1 (lowest SES) | 21 | 18 | 20 | 19 | 17 | 21 |
| Q5 (highest SES) | 16 | 19 | 17 | 19 | 21 | 16 |
| Current smoking | 21 | 14 | 17 | 16 | 13 | 21 |
| Current alcohol consumption | 33 | 19 | 21 | 29 | 20 | 31 |
| Age at menarche ≤11 y | 27 | 29 | 28 | 29 | 29 | 28 |
| Premenopausal | 76 | 76 | 76 | 77 | 76 | 77 |
| Current OC use | 26 | 25 | 25 | 25 | 26 | 26 |
| Vigorous exercise ≥ 5 h/wk | 10 | 17 | 13 | 16 | 19 | 9 |
Values are means ± SD or percentages and are standardized to the age distribution of the study population. OC, oral contraceptive; Q, quintile; SES, socioeconomic status.
All macronutrients were assessed as g/d and were adjusted for energy using the residual method.
As shown in Table 2, carbohydrate consumption was significantly associated with a higher risk of SLE, MVHR: 1.96, 95% CI: 1.02, 3.67 for the highest carbohydrate quintile compared with the lowest quintile, P trend = 0.02. In contrast, intake of proteins and total fats were inversely associated with SLE: for the highest quintile compared with the lowest quintile, MVHR for proteins: 0.66, 95% CI: 0.37, 1.18, P trend = 0.07; MVHR for fats: 0.54, 95% CI: 0.28, 1.01, P trend = 0.02. Among the fats, MUFAs, saturated fats, and trans fatty acids were associated with a lower risk of SLE (all P trends <0.05) whereas there were no significant trends for intake of PUFAs, ω-3 fatty acids, and ω-6 fatty acids.
TABLE 2.
Macronutrients in relation to risk of systemic lupus erythematosus among 51,934 participants in the Black Women's Health Study, 1995–20151
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P trend5 | |
|---|---|---|---|---|---|---|
| Macronutrients2 | ||||||
| Carbohydrates | ||||||
| Cases/person years | 15/169,675 | 19/172,869 | 25/172,802 | 25/172,546 | 30/169,790 | |
| Age-adj. HR (95% CI)3 | 1.00 Ref | 1.24 (0.63, 2.45) | 1.64 (0.86, 3.11) | 1.67 (0.88, 3.18) | 2.04 (1.10, 3.79) | __ |
| MVHR (95% CI)4 | 1.00 Ref | 1.23 (0.62, 2.42) | 1.61 (0.84, 3.06) | 1.63 (0.86, 3.11) | 1.96 (1.02, 3.67) | 0.02 |
| Proteins | ||||||
| Cases/person years | 29/170,031 | 26/171,787 | 22/172,856 | 18/172,299 | 19/170,708 | |
| Age-adj. HR (95% CI) | 1.00 Ref | 0.88 (0.52, 1.50) | 0.75 (0.43, 1.31) | 0.61 (0.34, 1.10) | 0.66 (0.37, 1.18) | __ |
| MVHR (95% CI) | 1.00 Ref | 0.90 (0.53, 1.53) | 0.76 (0.44, 1.33) | 0.61 (0.34, 1.11) | 0.66 (0.37, 1.18) | 0.07 |
| Total fat | ||||||
| Cases/person years | 29/170,219 | 27/172,724 | 27/172,430 | 16/172,587 | 15/169,721 | |
| Age-adj. HR (95% CI) | 1.00 Ref | 0.91 (0.54, 1.53) | 0.90 (0.53, 1.52) | 0.53 (0.29, 0.98) | 0.52 (0.28, 0.96) | __ |
| MVHR (95% CI) | 1.00 Ref | 0.91 (0.54, 1.53) | 0.92 (0.54, 1.56) | 0.54 (0.30, 1.00) | 0.54 (0.28, 1.01) | 0.02 |
| Types of fat | ||||||
| PUFAs | ||||||
| Cases/person years | 31/169,756 | 19/172,209 | 26/172,312 | 19/171,460 | 19/171,943 | |
| Age-adj. HR (95% CI) | 1.00 Ref | 0.61 (0.34, 1.08) | 0.84 (0.50, 1.41) | 0.62 (0.35, 1.09) | 0.65 (0.36, 1.15) | __ |
| MVHR (95% CI) | 1.00 Ref | 0.61 (0.34, 1.08) | 0.84 (0.50, 1.42) | 0.63 (0.35, 1.12) | 0.66 (0.37, 1.17) | 0.18 |
| ω-3 fatty acids | ||||||
| Cases/person years | 32/167,779 | 19/168,647 | 15/168,906 | 25/168,961 | 21/168,522 | __ |
| Age-adj. HR (95% CI) | 1.00 Ref | 0.59 (0.34, 1.05) | 0.48 (0.26, 0.88) | 0.80 (0.47, 1.35) | 0.68 (0.39, 1.19) | __ |
| MVHR (95% CI) | 1.00 Ref | 0.59 (0.33, 1.04) | 0.47 (0.26, 0.88) | 0.79 (0.46, 1.34) | 0.66 (0.38, 1.16) | 0.47 |
| ω-6 fatty acids | ||||||
| Cases/person years | 29/169,888 | 21/172,002 | 26/172,305 | 20/171,581 | 18/171,905 | |
| Age-adj. HR (95% CI) | 1.00 Ref | 0.72 (0.41, 1.26) | 0.90 (0.53, 1.52) | 0.69 (0.39, 1.23) | 0.66 (0.36, 1.19) | __ |
| MVHR (95% CI) | 1.00 Ref | 0.72 (0.41, 1.26) | 0.90 (0.53, 1.53) | 0.71 (0.40, 1.25) | 0.66 (0.37, 1.20) | 0.19 |
| MUFAs | ||||||
| Cases/person years | 32/170,292 | 22/172,515 | 28/172,754 | 15/172,055 | 17/170,064 | |
| Age-adj. HR (95% CI) | 1.00 Ref | 0.67 (0.39, 1.15) | 0.84 (0.51, 1.40) | 0.45 (0.24, 0.84) | 0.53 (0.29, 0.95) | __ |
| MVHR (95% CI) | 1.00 Ref | 0.67 (0.39, 1.16) | 0.86 (0.51, 1.43) | 0.47 (0.25, 0.87) | 0.55 (0.30, 1.00) | 0.02 |
| Saturated fats | ||||||
| Cases/person years | 29/170,633 | 28/172,915 | 23/172,362 | 17/171,797 | 17/169,974 | |
| Age-adj. HR (95% CI) | 1.00 Ref | 0.93 (0.55, 1.56) | 0.75 (0.43, 1.30) | 0.54 (0.30, 0.99) | 0.55 (0.30, 1.00) | __ |
| MVHR (95% CI) | 1.00 Ref | 0.95 (0.56, 1.60) | 0.77 (0.44, 1.34) | 0.57 (0.31, 1.04) | 0.57 (0.31, 1.06) | 0.02 |
| Trans fatty acids | ||||||
| Cases/person years | 30/169,432 | 31/171,655 | 15/173,349 | 20/171,574 | 18/171,671 | |
| Age-adj. HR (95% CI) | 1.00 Ref | 1.02 (0.61, 1.68) | 0.49 (0.26, 0.90) | 0.66 (0.37, 1.16) | 0.60 (0.34, 1.08) | __ |
| MVHR (95% CI) | 1.00 Ref | 1.03 (0.62, 1.70) | 0.49 (0.26, 0.92) | 0.66 (0.38, 1.17) | 0.61 (0.34, 1.10) | 0.03 |
Analysis was done using Cox proportional regression. MVHR, multivariable HR.
All macronutrients were assessed as g/d and were adjusted for energy using the residual method. Each model includes only the respective energy-adjusted macronutrient. For example, the carbohydrate model is comparing carbohydrate intake compared with all other macronutrients.
Age-adjusted (Age-adj.) HRs were adjusted for age, period, and energy intake (continuous).
MVHRs were adjusted for age, period, energy intake (continuous), alcohol intake status (current, past, never), smoking status (current, past, never), BMI (<20, 20–24.9, 25–29.9, ≥30 kg/m2), quintiles of neighborhood socioeconomic status, years of education (≤ 12, 13–15, 16, ≥ 17), vigorous physical activity (none, <5, ≥5 h/wk).
Tests of linear trend assessed the median of each quintile as scores, adjusted for age, period, energy intake (continuous), alcohol intake status (current, past, never), smoking status (current, past, never), BMI (<20, 20–24.9, 25–29.9, ≥30 kg/m2), quintiles of neighborhood socioeconomic status, years of education (≤ 12, 13–15, 16, ≥ 17), vigorous physical activity (none, <5, ≥5 h/wk).
In substitution analyses (Table 3), substituting 10 g/d of carbohydrates with the energy equivalent of total fats was associated with a 20% reduction in risk of SLE (MVHR: 0.80, 95% CI: 0.65, 0.97, P trend = 0.03) and substituting 10 g/d of total fat with the equivalent of carbohydrates increased the risk of SLE by 10% (MVHR: 1.10, 95% CI: 1.01, 1.20, P trend = 0.02). There were no significant associations for substituting proteins or individual types of fat (PUFAs, MUFAs, saturated fat, trans fatty acids).
TABLE 3.
HRs from substitution models of macronutrients and risk of systemic lupus erythematosus among 51,934 participants in the Black Women's Health Study, 1995–20151
| MVHR (95% CI)5 | P trend6 | ||
|---|---|---|---|
| Substituting carbohydrates2 | |||
| Proteins | 0.95 | (0.80, 1.12) | 0.51 |
| Total fat | 0.80 | (0.65, 0.97) | 0.03 |
| PUFAs | 1.05 | (0.41, 2.67) | 0.92 |
| MUFAs | 0.99 | (0.28, 3.47) | 0.99 |
| Saturated fats | 0.61 | (0.24, 1.54) | 0.29 |
| Trans fatty acids | 0.89 | (0.68, 1.16) | 0.38 |
| Substituting proteins3 | |||
| Carbohydrates | 1.06 | (0.91, 1.23) | 0.48 |
| Total fat | 0.91 | (0.55, 1.38) | 0.69 |
| PUFAs | 1.16 | (0.44, 3.04) | 0.77 |
| MUFAs | 1.13 | (0.28, 4.51) | 0.86 |
| Saturated fats | 0.67 | (0.25, 1.84) | 0.44 |
| Trans fatty acids | 0.89 | (0.68, 1.16) | 0.38 |
| Substituting total fat4 | |||
| Carbohydrates | 1.10 | (1.01, 1.20) | 0.02 |
| Proteins | 1.05 | (0.85, 1.30) | 0.67 |
Analysis was done using Cox proportional regression. MVHR, multivariable HR.
Substitution of 10 g/d of carbohydrates with the equivalent energy of proteins, total fat, PUFAs, MUFAS, saturated fat, or trans fat.
Substitution of 10 g/d of proteins with the equivalent energy of carbohydrates, total fat, PUFAs, MUFAS, saturated fat, or trans fat.
Substitution of 10 g/d of total fat with the equivalent energy of carbohydrates or proteins.
MVHR (and 95% CIs) adjusted for age, period, energy intake (continuous), alcohol intake status (current, past, never), and smoking status (current, past, never), BMI (<20, 20–24.9, 25–29.9, ≥30 kg/m2), quintiles of neighborhood socioeconomic status, years of education (≤12, 13–15, 16, ≥17), vigorous physical activity (none, <5, ≥5 h/wk).
P trend was adjusted for age, period, energy intake (continuous), alcohol intake status (current, past, never), and smoking status (current, past, never), BMI (<20, 20–24.9, 25–29.9, ≥30 kg/m2), quintiles of neighborhood socioeconomic status, years of education (≤12, 13–15, 16, ≥17), vigorous physical activity (none, <5, ≥5 h/wk).
With use of RRR, we identified 6 dietary patterns that explained 81.8% of the consumption variation in macronutrients and 23.5% of the variation in food groups (Supplementary Table 1). The first factor explained 53.4% of the total variation in macronutrients and was positively correlated with carbohydrates (r = 0.935, P < 0.0001) and inversely correlated with proteins, PUFAs, MUFAs, saturated fat, and trans fatty acids (r = –0.536, r = –0.615, r = –0.880, r = –0.747, r = –0.577, respectively; P < 0.0001 for all correlation coefficients). The AHEI-2010 score had a weak positive correlation with carbohydrates (r = 0.118) and a weak inverse correlation with intake of total fat (r = –0.163). Correlations of the vegetable/fruit pattern were even weaker (r = –0.080 for carbohydrates and r = 0.021 for total fats). Conversely, the meat/fried food pattern, which was inversely correlated with the RRR-derived factor 1 (r = –0.667), was inversely correlated with carbohydrates (r = –0.578) and positively correlated with total fats (r = 0.690) (data not shown).
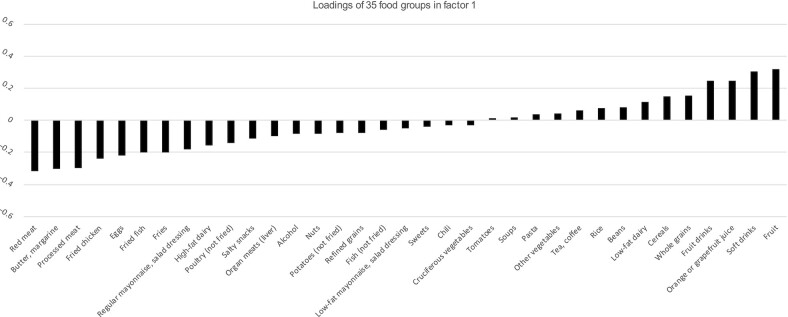
Supplementary Table 2 displays the food loadings of the 6 factors. Figure 2 shows factor loadings of the different food groups of the first factor. Intake of red meat, butter and margarines, processed meat, fried chicken, eggs, fried fish, and fries were inversely correlated (loadings ≤ –0.2) with factor 1. Consumption of fruit drinks, orange or grapefruit juice, soft drinks, and fruits were positively correlated (loadings ≥0.2) with factor 1.
FIGURE 2.
Factor loadings of 35 food groups with the RRR-derived dietary pattern (factor 1) among 51,934 participants in the Black Women's Health Study, 1995–2015. Positive loadings indicate positive correlation of food group with factor 1. Negative correlation indicates inverse correlation of the food group with factor 1. Red meat, butter and margarines, processed meat, fried chicken, eggs, fried fish, and fries had factor loadings ≤ –0.2 and fruit drinks, orange or grapefruit juice, soft drinks, and fruits had a factor loading ≥0.2. RRR, reduced rank regression.
Table 4 shows the relation of various dietary patterns to risk of SLE. The MVHR associated with quintiles of the meat/fried food dietary pattern was 0.57 (95% CI: 0.30, 1.09) for the highest quintile compared with the lowest quintile, P trend = 0.06. The vegetable fruit/dietary pattern and the AHEI-2010 diet were not significantly associated with risk of SLE. From the 11 components of the AHEI-2010 diet, only the top tertile of AHEI-fruit component relative to the lowest was associated with a higher risk of SLE (P trend = 0.02). The MVHR for the association of the first factor with SLE was 1.88 (95% CI: 1.06, 3.35) for the highest quintile compared with the lowest quintile; P trend = 0.02. Because the meat/fried food and the factor 1 dietary patterns are inversely correlated, we ran a single model including both patterns as continuous variables. The meat/fried food pattern was not associated with SLE risk in this joint model: MVHR: 1.02, 95% CI: 0.78, 1.31, P trend = 0.91. On the other hand, the factor 1 diet pattern remained associated with SLE risk in the joint model: MVHR: 1.37, 95% CI: 1.07, 1.75, P trend = 0.01. For the other factors, there was weaker evidence of association with SLE. In a sensitivity analysis that lagged exposure to baseline macronutrients and included 84 SLE cases, results were unchanged (Supplementary Table 3). Intake of carbohydrates was significantly associated with a higher risk of SLE (MVHR: 2.44, 95% CI: 1.12, 5.32, P trend = 0.01) for the highest carbohydrate quintile compared with the lowest quintile. We observed a significant trend of higher intake of total fat associated with lower SLE risk (P trend = 0.01) (data not shown).
TABLE 4.
Dietary patterns in relation to risk of systemic lupus erythematosus among 51,934 participants in the Black Women's Health Study, 1995–20151
| Dietary pattern | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile5 | P trend3 |
|---|---|---|---|---|---|---|
| AHEI diet | ||||||
| Cases/person years | 26/170,100 | 24/170,431 | 16/170,649 | 28/170,051 | 19/171,136 | |
| MVHR (95% CI)2 | 1.00 Ref | 0.99 (0.56, 1.74) | 0.68 (0.36, 1.29) | 1.22 (0.69, 2.15) | 0.83 (0.44, 1.57) | 0.82 |
| Meat/fried food | ||||||
| Cases/person years | 26/172,747 | 25/172,354 | 29/172,470 | 19/171,264 | 15/168,846 | |
| MVHR (95% CI) | 1.00 Ref | 0.94 (0.54, 1.64) | 1.08 (0.63, 1.86) | 0.71 (0.39, 1.30) | 0.57 (0.30, 1.09) | 0.06 |
| Vegetable/fruit | ||||||
| Cases/person years | 28/170,515 | 25/172,238 | 19/172,513 | 21/171,367 | 21/171,048 | |
| MVHR (95% CI) | 1.00 Ref | 0.92 (0.53, 1.58) | 0.72 (0.40, 1.30) | 0.81 (0.45, 1.45) | 0.83 (0.45, 1.53) | 0.49 |
| RRR-dietary pattern | ||||||
| Factor 1 | ||||||
| Cases/person years | 18/169,784 | 22/172,421 | 15/173,235 | 25/172,814 | 34/169,428 | |
| MVHR (95% CI) | 1.00 Ref | 1.20 (0.64, 2.24) | 0.82 (0.41, 1.62) | 1.37 (0.74, 2.52) | 1.88 (1.06, 3.35) | 0.02 |
| Factor 2 | ||||||
| Cases/person years | 22/171,027 | 17/170,692 | 28/172,812 | 27/170,040 | 20/172,110 | |
| MVHR (95% CI) | 1.00 Ref | 0.79 (0.42, 1.48) | 1.31 (0.75, 2.30) | 1.27 (0.72, 2.24) | 0.95 (0.52, 1.75) | 0.65 |
| Factor 3 | ||||||
| Cases/person years | 25/170,148 | 18/170,967 | 19/172,235 | 27/172,155 | 25/172,175 | |
| MVHR (95% CI) | 1.00 Ref | 0.71 (0.39, 1.31) | 0.78 (0.42, 1.41) | 1.12 (0.64, 1.95) | 1.06 (0.60, 1.88) | 0.45 |
| Factor 4 | ||||||
| Cases/person years | 27/169,095 | 27/171,194 | 16/173,135 | 18/172,717 | 26/171,540 | |
| MVHR (95% CI) | 1.00 Ref | 0.98 (0.58, 1.67) | 0.58 (0.31, 1.07) | 0.65 (0.36, 1.18) | 0.94 (0.55, 1.62) | 0.47 |
| Factor 5 | ||||||
| Cases/person years | 26/168,944 | 23/171,136 | 27/172,536 | 26/173,245 | 12/171,820 | |
| MVHR (95% CI) | 1.00 Ref | 0.87 (0.50, 1.54) | 1.02 (0.59, 1.76) | 0.98 (0.57, 1.70) | 0.45 (0.23, 0.89) | 0.06 |
| Factor 6 | ||||||
| Cases/person years | 23/169,658 | 33/172,008 | 21/173,107 | 12/172,524 | 25/170,277 | |
| MVHR (95% CI) | 1.00 Ref | 1.45 (0.85, 2.48) | 0.95 (0.52, 1.72) | 0.56 (0.28, 1.14) | 1.26 (0.70, 2.28) | 0.77 |
Analysis was done using Cox proportional regression. AHEI-2010, Alternate Healthy Eating Index-2010; MVHR, multivariable HR; RRR, reduced rank regression.
Multivariable HR adjusted for age, period, energy intake (continuous), smoking status (current, past, never), BMI (<20, 20–24.9, 25–29.9, ≥30 kg/m2), quintiles of neighborhood socioeconomic status, years of education (≤12, 13–15, 16, ≥17), vigorous physical activity (none, <5, ≥5 h/wk).
Tests linear trend were calculated by assigning the median of each quintile as scores; adjusted for age, period, energy intake (continuous), smoking status (current, past, never), BMI (<20, 20–24.9, 25–29.9, ≥30 kg/m2), quintiles of neighborhood socioeconomic status, years of education (≤12, 13–15, 16, ≥17), vigorous physical activity (none, <5, ≥5 h/wk).
Discussion
In this prospective study of AA women, we found that the higher intake of carbohydrates and lower intake of total fat were associated with increased risk of SLE. Our analysis of RRR-derived dietary patterns confirmed these results, as the RRR-derived factor 1, which was associated with high carbohydrate intake and low fat and protein consumption, was also associated with a higher risk of SLE. It is noteworthy that this RRR-derived factor alone explained more than half (53.4%) of the variation of macronutrient intake in this population. In contrast, the AHEI-2010, the vegetable/fruit, and the meat/fried food patterns were not associated with risk of SLE.
The lack of association of the vegetable/fruit dietary pattern and AHEI-2010 diet score with risk of SLE are consistent with recent results from the NHS cohorts (10, 11). Barbhaiya et al. found an inverse association of the nut/legume component of the AHEI-2010 diet with SLE (11), possibly due to the high concentration of polyunsaturated fats in those foods. In the current study, we did not find evidence of the association of PUFAs alone with risk of SLE. The positive association of the AHEI-fruit component with risk of SLE is consistent with our RRR-derived factor 1 results as fruits are 1 of the major components of the factor 1 diet. Contributions of nuts and beans to the RRR-derived factor 1 are very small. Thus, results for RRR-derived factor 1 may indicate that overall dietary patterns are more important than individual foods in determining the risk of SLE.
We were not able to separate the effect of carbohydrates from fats. Increasing the consumption of 1 macronutrient might well be associated with a decrease in the consumption of the other. We did not observe a specific type of fat, including PUFAs, to be positively or inversely associated with SLE. We are unaware of a biological mechanism that would explain how the low intake of fat could lead to the development of SLE. Evidence is scarce. One small clinical trial found that a eucaloric diet consisting of 15% fat and 67% carbohydrates was associated with worsening of several inflammatory biomarkers (35). Regarding carbohydrates, however, published evidence suggests potential mechanisms for a harmful effect on SLE risk (36). The high consumption of carbohydrates may lead to upregulated glycosylation of proteins to form advanced glycation end-products (37). These are products of the nonenzymatic glycosylation of sugars with protein, creating toxic, irreversible compounds that accumulate in tissue through time and are associated with pathogenesis of inflammatory diseases. Abnormal glycosylation of T-cell proteins could contribute to the pathogenesis of SLE by suppressing the effects of Gal-1 in the apoptosis of activated T-cells (38). Fructose, in contrast to glucose, has higher reactivity and could represent an excellent source of endogenous advanced glycation end-products and excessive glycosylation. Good sources of fructose are sugar-sweetened fruit drinks and juices and sugar-sweetened soft drinks. The RRR-derived factor 1, which was associated with risk of SLE, is high in fruits, orange juice, other fruit juices, and sugar-sweetened soft drinks. Interestingly, the increased consumption of sugar-sweetened beverages, including fruit drinks, was associated with an elevated risk of developing a related autoimmune disease, rheumatoid arthritis, among the women in the NHS cohort (39). If carbohydrates are indeed involved in SLE development, these data suggest a potential mechanism.
Our study is the first prospective study examining the role of macronutrients and dietary patterns in relation to incident risk of SLE in an AA population. A strength of the present study is the assessment of an isoenergetic diet by adjusting macronutrients and food items by energy intake. Variation observed was due to the composition of the nutrients in a diet and not the amount of nutrients, reducing confounding by energy intake (34). Other strengths include the prospective design, in that dietary data were collected prospectively relative to the outcome, and validation of SLE cases by rheumatologists to determine if ACR criteria were met. The results were robust in a lagged analysis. Limitations of the study include the relatively small number of SLE cases and the reliance on FFQs to obtain the dietary data; however, other possibly more accurate, but labor-intensive, methods (e.g. long-term food diaries) are not feasible in very large studies. Nevertheless, our validation study demonstrated that the short NCI FFQ was effective in describing intakes for many nutrients examined in the BWHS (17). Although we controlled for different confounders, we cannot rule out the possibility of residual confounding. Our work was focused on macronutrients and food items. We do not know whether micronutrients affect risk of SLE. Finally, the RRR method that we used is limited by the understanding of previous biologic mechanisms or hypothesis (40). We applied RRR under the hypothesis that macronutrient intake was associated with SLE risk. Further studies are needed to replicate our findings in other population groups.
In summary, we found that the high intake of carbohydrates and low intake of total fats were associated with higher risk of SLE. These results were confirmed using substitution and RRR analyses. In particular, we identified a dietary pattern high in fruits, orange juice, and sweetened soft and fruit drinks and low in margarines and butter, red and processed meats, fried chicken, poultry, and eggs to be associated with risk of SLE. The present findings emerged from multiple analyses exploring dietary patterns, rather than being based on a specific hypothesis that we sought to test. Thus, the present findings could be due to chance and require confirmation in other studies.
Supplementary Material
Acknowledgments
The authors’ contributions were as follows—NCW and LR: designed and conducted the research; NCW: analyzed the data and drafted the manuscript; ERN: advised on the analysis; and all authors: contributed to the interpretation of the data, critically revised the manuscript, and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Research reported in this publication was supported by the National Cancer Institute R01- CA058420, U01-CA164974; National Institute of Arthritis and Musculoskeletal and Skin Diseases R01 AR0573727, K24 AR 066109; MB is supported by the Rheumatology Research Foundation Scientist Development Award.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AA, African American; ACR, American College of Rheumatology; AHEI, Alternative Healthy Eating Index; BWHS, Black Women's Health Study; MVHR, multivariable HR; NCI, National Cancer Institute; NHS, Nurses’ Health Study; PCA, principal component analysis; RRR, reduced rank regression; SES, socioeconomic status; SLE, systemic lupus erythematosus.
Contributor Information
Nelsy Castro-Webb, Slone Epidemiology Center at Boston University, Boston, MA, USA.
Yvette C Cozier, Slone Epidemiology Center at Boston University, Boston, MA, USA.
Medha Barbhaiya, Division of Rheumatology, Department of Medicine, Hospital for Special Surgery and Weill Cornell Medicine, New York, NY, USA.
Edward A Ruiz-Narváez, Department of Nutritional Services, University of Michigan School of Public Health, Ann Arbor, MI, USA.
Shanshan Li, Slone Epidemiology Center at Boston University, Boston, MA, USA.
Karen H Costenbader, Division of Rheumatology, Inflammation and Immunity, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Lynn Rosenberg, Slone Epidemiology Center at Boston University, Boston, MA, USA.
Data Availability
The data used in this analysis will not be shared as the study participants did not consent to sharing their data in a public repository.
References
- 1.Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet North Am Ed. 2014;384(9957):1878–88. [DOI] [PubMed] [Google Scholar]
- 2.Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcon GS, Winkelmayer WC, Costenbader KH. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Arthritis Rheum. 2013;65(3):753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gugliucci A. Formation of fructose-mediated advanced glycation end products and their roles in metabolic and inflammatory diseases. Adv Nutr. 2017;8(1):54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med. 2008;10(7):160. [PMC free article] [PubMed] [Google Scholar]
- 5.Rosinger A, Herrick K, Gahche J, Park S. Sugar-sweetened beverage consumption among U.S. adults, 2011–2014. NCHS Data Brief. 2017;(270):1–8. [PubMed] [Google Scholar]
- 6.Larson N, Neumark-Sztainer D, Laska MN, Story M. Young adults and eating away from home: associations with dietary intake patterns and weight status differ by choice of restaurant. J Am Diet Assoc. 2011;111(11):1696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowman SA, Vinyard BT. Fast food consumption of U.S. adults: impact on energy and nutrient intakes and overweight status. J Am Coll Nutr. 2004;23(2):163–8. [DOI] [PubMed] [Google Scholar]
- 8.Elkan AC, Anania C, Gustafsson T, Jogestrand T, Hafström I, Frostegård J. Diet and fatty acid pattern among patients with SLE: associations with disease activity, blood lipids and atherosclerosis. Lupus. 2012;21(13):1405–11. [DOI] [PubMed] [Google Scholar]
- 9.Pocovi-Gerardino G, Correa-Rodríguez M, Callejas-Rubio JL, Ríos-Fernández R, Ortego-Centeno N, Rueda-Medina B. Dietary intake and nutritional status in patients with systemic lupus erythematosus. Endocrinol Diabetes Nutr. 2018;65(9):533–9. [DOI] [PubMed] [Google Scholar]
- 10.Tedeschi SK, Barbhaiya M, Sparks JA, Karlson EW, Kubzansky LD, Roberts AL, Willett WC, Lu B, Costenbader KH. Dietary patterns and risk of systemic lupus erythematosus in women. Lupus. 2020;29(1):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbhaiya M, Tedeschi S, Sparks JA, Leatherwood C, Karlson EW, Willett WC, Lu B, Costenbader KH. Association of dietary quality with risk of incident systemic lupus erythematosus in the Nurses' Health Studies. Arthritis Care Res. 2020. doi: 10.1002/acr.24443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiraki LT, Munger KL, Costenbader KH, Karlson EW. Dietary intake of vitamin D during adolescence and risk of adult-onset systemic lupus erythematosus and rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64(12):1829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costenbader KH, Feskanich D, Holmes M, Karlson EW, Benito-Garcia E. Vitamin D intake and risks of systemic lupus erythematosus and rheumatoid arthritis in women. Ann Rheum Dis. 2008;67(4):530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costenbader KH, Kang JH, Karlson EW. Antioxidant intake and risks of rheumatoid arthritis and systemic lupus erythematosus in women. Am J Epidemiol. 2010;172(2):205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg L, Adams-Campbell L, Palmer JR. The Black Women's Health Study: a follow-up study for causes and preventions of illness. J Am Med Womens Assoc (1972). 1995;50(2):56–8. [PubMed] [Google Scholar]
- 16.Russell C, Palmer JR, Adams-Campbell LL, Rosenberg L. Follow-up of a large cohort of black women. Am J Epidemiol. 2001;154(9):845–53. [DOI] [PubMed] [Google Scholar]
- 17.Kumanyika SK, Mauger D, Mitchell DC, Phillips B, Smiciklas-Wright H, Palmer JR. Relative validity of food frequency questionnaire nutrient estimates in the Black Women's Health Study. Ann Epidemiol. 2003;13(2):111–8. [DOI] [PubMed] [Google Scholar]
- 18.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1(1):58–64. [DOI] [PubMed] [Google Scholar]
- 19.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. [DOI] [PubMed] [Google Scholar]
- 20.Cozier YC, Barbhaiya M, Castro-Webb N, Conte C, Tedeschi SK, Leatherwood C, Costenbader KH, Rosenberg L. Relationship of cigarette smoking and alcohol consumption to incidence of systemic lupus erythematosus in a prospective cohort study of black women. Arthritis Care Res (Hoboken). 2019;71(5):671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann K, Schulze MB, Schienkiewitz A, Nöthlings U, Boeing H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am J Epidemiol. 2004;159(10):935–44. [DOI] [PubMed] [Google Scholar]
- 22.Sherafat-Kazemzadeh R, Egtesadi S, Mirmiran P, Gohari M, Farahani SJ, Esfahani FH, Vafa MR, Hedayati M, Azizi F. Dietary patterns by reduced rank regression predicting changes in obesity indices in a cohort study: Tehran Lipid and Glucose Study. Asia Pac J Clin Nutr. 2010;19(1):22–32. [PubMed] [Google Scholar]
- 23.Jacobs S, Kroeger J, Schulze MB, Frank LK, Franke AA, Cheng I, Monroe KR, Haiman CA, Kolonel LN, Wilkens LRet al. Dietary patterns derived by reduced rank regression are inversely associated with type 2 diabetes risk across 5 ethnic groups in the multiethnic cohort. Curr Dev Nutr. 2017;1(5):e000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner S, Lioret S, Girerd N, Duarte K, Lamiral Z, Bozec E, Van den Berghe L, Hoge A, Donneau AF, Boivin JMet al. Association of dietary patterns derived using reduced-rank regression with subclinical cardiovascular damage according to generation and sex in the STANISLAS Cohort. J Am Heart Assoc. 2020;9(7):e013836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boggs DA, Palmer JR, Spiegelman D, Stampfer MJ, Adams-Campbell LL, Rosenberg L. Dietary patterns and 14-y weight gain in African American women. Am J Clin Nutr. 2011;94(1):86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu Y, Sparks JA, Malspeis S, Costenbader KH, Hu FB, Karlson EW, Lu B. Long-term dietary quality and risk of developing rheumatoid arthritis in women. Ann Rheum Dis. 2017;76(8):1357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnan S, Cozier YC, Rosenberg L, Palmer JR. Socioeconomic status and incidence of type 2 diabetes: results from the Black Women's Health Study. Am J Epidemiol. 2010;171(5):564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coogan PF, Cozier YC, Krishnan S, Wise LA, Adams-Campbell LL, Rosenberg L, Palmer JR. Neighborhood socioeconomic status in relation to 10-year weight gain in the Black Women's Health Study. Obesity. 2010;18(10):2064–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbhaiya M, Lu B, Sparks JA, Malspeis S, Chang SC, Karlson EW, Costenbader KH. Influence of alcohol consumption on the risk of systemic lupus erythematosus among women in the Nurses' Health Study Cohorts. Arthritis Care & Research. 2017;69(3):384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbhaiya M, Tedeschi SK, Lu B, Malspeis S, Kreps D, Sparks JA, Karlson EW, Costenbader KH. Cigarette smoking and the risk of systemic lupus erythematosus, overall and by anti-double stranded DNA antibody subtype, in the Nurses' Health Study cohorts. Ann Rheum Dis. 2018;77(2):196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang F, Li S, Jia C. Smoking and the risk of systemic lupus erythematosus: an updated systematic review and cumulative meta-analysis. Clin Rheumatol. 2015;34(11):1885–92. [DOI] [PubMed] [Google Scholar]
- 33.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–40. [DOI] [PubMed] [Google Scholar]
- 34.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4):1220S–8S.; discussion 9S–31S. [DOI] [PubMed] [Google Scholar]
- 35.Kasim-Karakas SE, Tsodikov A, Singh U, Jialal I. Responses of inflammatory markers to a low-fat, high-carbohydrate diet: effects of energy intake. Am J Clin Nutr. 2006;83(4):774–9. [DOI] [PubMed] [Google Scholar]
- 36.Navarro Quiroz E, Chavez-Estrada V, Macias-Ochoa K, Ayala-Navarro MF, Flores-Aguilar AS, Morales-Navarrete F, de la Cruz Lopez F, Gomez Escorcia L, Musso CG, Aroca Martinez Get al. Epigenetic mechanisms and posttranslational modifications in systemic lupus erythematosus. Int J Mol Sci. 2019;20(22):5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aragno M, Mastrocola R. Dietary sugars and endogenous formation of advanced glycation endproducts: emerging mechanisms of disease. Nutrients. 2017;9(4):385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szabó E, Hornung Á, Monostori É, Bocskai M, Czibula Á, Kovács L. Altered cell surface n-glycosylation of resting and activated T cells in systemic lupus erythematosus. Int J Mol Sci. 2019;20(18):4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu Y, Costenbader KH, Gao X, Al-Daabil M, Sparks JA, Solomon DH, Hu FB, Karlson EW, Lu B. Sugar-sweetened soda consumption and risk of developing rheumatoid arthritis in women. Am J Clin Nutr. 2014;100(3):959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kroke A. Re: “Application of a new statistical method to derive dietary patterns in nutritional epidemiology.”. Am J Epidemiol. 2004;160(11):1132; author reply 1132-3. doi: 10.1093/aje/kwh329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this analysis will not be shared as the study participants did not consent to sharing their data in a public repository.