Abstract
Purpose of Review
Childhood obesity, with persistent chronic inflammation, is a worldwide epidemic. Obesity causes dysregulation throughout the immune system, affecting the balance and levels of cytokines, adipokines and innate and adaptive immune cells. This review focuses on the impact of obesity on immune function in children: altering the baseline activation state of immune cells and affecting the ability of the host to combat pathogens and malignancy and respond appropriately to vaccination.
Recent Findings
Obesity causes dysregulation of the immune system. Single cell RNA-sequencing of adipose tissue and resident immune cells is quantifying the impact of obesity on the frequency of immune cell subsets and their states. The system-wide alterations in immune function in obesity are most evident upon perturbation, including the response to infection (e.g. increased risk of severe COVID-19 in the ongoing pandemic), vaccination and malignancy. However, mechanistic research in pediatric obesity is limited and this impacts our ability to care for these children.
Summary
We must better understand baseline and perturbed immune health in obese children to determine how to account for altered frequency and function of humoral and cellular immune components in acute infection, during vaccine design and when considering therapeutic options for this complex, medically vulnerable group.
Keywords: Obesity, immune health, immune dysregulation, human immunology
Introduction
We are experiencing a worldwide pediatric obesity epidemic, stretching from birth through adolescence and continuing through adulthood[1,2]. In the United States, there have been significant increases in childhood obesity over the last 40 years, with more than 13 million obese children and adolescents and with continued increases in the last decade in certain age groups[3]. Black and Hispanic children have increased prevalence of obesity compared to white and Asian American children[3,4]. Worldwide, more than 40 million children under five years of age were overweight or obese in 2016[1] and the continued increase in prevalence of childhood obesity continues, especially in resource limited settings [2]. Obese children experience early onset of obesity related comorbidities[5], the majority will go on to become obese adults (most likely in the most severely obese[6]), and they will experience early mortality[7]. It is not clear how obesity leads to these outcomes and here we will focus on immune dysregulation in the setting of obesity.
Immune health and the effect of childhood obesity
How can we define what is altered or dysfunctional in childhood obesity? We must first measure immune health, which is a challenge. While most organ systems have evidence-based monitoring tests and strategies to assess function (e.g. the EKG or echocardiogram in cardiology and the EEG in neurology), the immune system remains stubbornly challenging to encompass with a single or set of functional tests. The most basic immune evaluation of cellular and humoral immune function includes a complete blood count (CBC) and immunoglobulin levels and vaccine titers, but unless there are deficiencies in cells or antibody levels, this has limited utility. In clinical immunology we frequently enumerate rare immune cell subsets via flow cytometry and test immune cell function, but this has not yielded metrics for the healthy child or adult that synthesizes their “immune health”, simply tables of acceptable levels of each cell type (or function). Without this synthetic understanding, the quantitation of the impact of disease (on that baseline) is complicated.
It is also clear that there is significant variation among “healthy” participants[8] and that variation includes effects of age[9], gender, race and ethnicity and environment on immune cell subset frequency and immune function[9,10]. With regards to environment, early life events affect immune function. Before birth, maternal high fat diet in pregnancy has been linked to increased risk of infant obesity[11], altered infant microbiome[12] and altered cord blood immune components and function including reduced eosinophils and CD4T cells (especially CD4 naïve T cells) altered cytokine[13]. In addition, our diet and microbiome are deeply connected to our immune state. Connections among gut microbiota, serum metabolites and adiposity are the focus of intense research in mice and humans[14,15]. Historically, childhood obesity was linked to alterations in individual metabolites[16], and now across a wide breadth of metabolites [17]. For example, a recent study from our groups showed that gut microbiota produced tryptophan-pathway derivatives, leading to altered miR-181 expression and affecting white adipose tissue (WAT) metabolism in both mice and obese children[18]. This is reviewed in depth elsewhere [19].
Once a population is selected, choosing tissues and cells to measure and compare is also challenging. In mouse studies the routine collection of multiple tissues for deep immunoprofiling is routine, while in human studies we generally study peripheral blood and perhaps one target tissue (if easily accessible). It is important to note that given the challenges in pediatric translational research, there is limited data from healthy (or obese) children to support evidence-based analysis of pediatric immune components and function[20]. In the study of obesity, adipose tissue is frequently studied in adults or mice. There are multiple sources of adipose tissue, including subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT). However, these studies are challenging to control in humans as it is challenging to obtain adipose tissue from lean adults or to obtain longitudinal samples of human adipose tissue from the same patient. Studying adipose tissue in children is complex as there are limited programs for pediatric bariatric surgery, which limits both clinical care and research. Recent guidelines from the American Association of Pediatrics argue for increased bariatric surgery access [21].
We have used two strategies to synthesize our current understanding of immune function and dysregulation in childhood obesity. First, when discussing the quantity of immune cells and humoral factors, we have supplemented our knowledge from pediatric human studies with adult human and mouse studies (Table 1). Second, we included the outcomes of clinical perturbations on immune status to deepen our understanding of (limited) multimodal research data; using the response to severe infection, vaccination and malignancy (and immunotherapy), among others, to reveal the degree of dysfunction in childhood obesity (Figure 1).
Table 1: Impact of obesity on key human immune cell subsets.
Within AT, alterations are in VAT if not listed as SAT. Up arrows indicate enriched populations, down arrows indicate less prevalent populations and sideways arrow indicates no significant difference measured. AT = adipose tissue, PB = peripheral blood.
| Cell type | Adult | Child | ||
|---|---|---|---|---|
| AT | PB | AT | PB | |
| B cells | →VAT p = 0.055 ↑SAT [60] | →[23,48] ↑[59] | ↑Bnaive [53] →Bmemory [53] |
|
| Vaccine Responses | n/a | ↓Hepatitis B [98–102] →Influenza [103–105] ↑PPSV23 [106] |
n/a | ↓Hepatitis B [107] ↓Tetanus [108] →Influenza [109,110] |
| CD4 T cells | ↑[78] | ↑[59,77] ↓[23] →[48,51,52,58,60] |
→[72] | |
| Naïve CD4 T cells | ↑[77]↓[57] →[23] | |||
| Non-naïve CD4 T cells | ↑[75,76] TEM, ↓[23] TMEM, ↑[77] TN, TCM, TEMRA | |||
| Th1 cells | →[92] | →[77] | →↑[53,72] | |
| Th2 cells | →[92] | ↑[77] | →[72] | |
| Th17 cells | ↑[92] in insulin resistant ptx | |||
| Th22 cells | ↑[92] in insulin resistant ptx | |||
| Tregs | ↓ [84] | →[51,60] ↑[77] ↓[75] | →[53] | |
| CD8 T cells | ↑[78] | ↓[23] ↑[59] →[48] | ||
| Naïve CD8 T cells | →[77] | |||
| Non-naïve CD8 T cells | →[77] | |||
| NK cells | variable[69] | ↓[59,67,68] →[47,48,57] ↑[60] |
↓[71] | |
| NKT cells | ↓[52] | ↓[47,50,52] →[48,49] |
→[53] | |
| Monocytes | n/a | →[48,57,58] ↑[59] | →[53] ↑[26] | |
| Classical | n/a | ↑[60] | ↑[9] ↑[26] | |
| Non-classical | n/a | ↑[60] | ↑[35] | |
| Adipose Tissue Macrophages (ATM) | ↑[134] | n/a | ||
| Pro-inflammatory (M1) (v. Anti-inflammatory (M2) ATM | ↑[60,62] | |||
| CD9+ ATMs | ↑[64,65] | |||
| Eosinophil | ↑[41] in SAT | |||
| Neutrophil | ↑[59] | ↑[26] | ||
| Mast cell | ↑[38,39] |
|||
| MAIT cell | ↓[51,54] | ↓[51,54] →[49] |
↑[54] | |
Figure 1: Impact of childhood obesity on immune function.
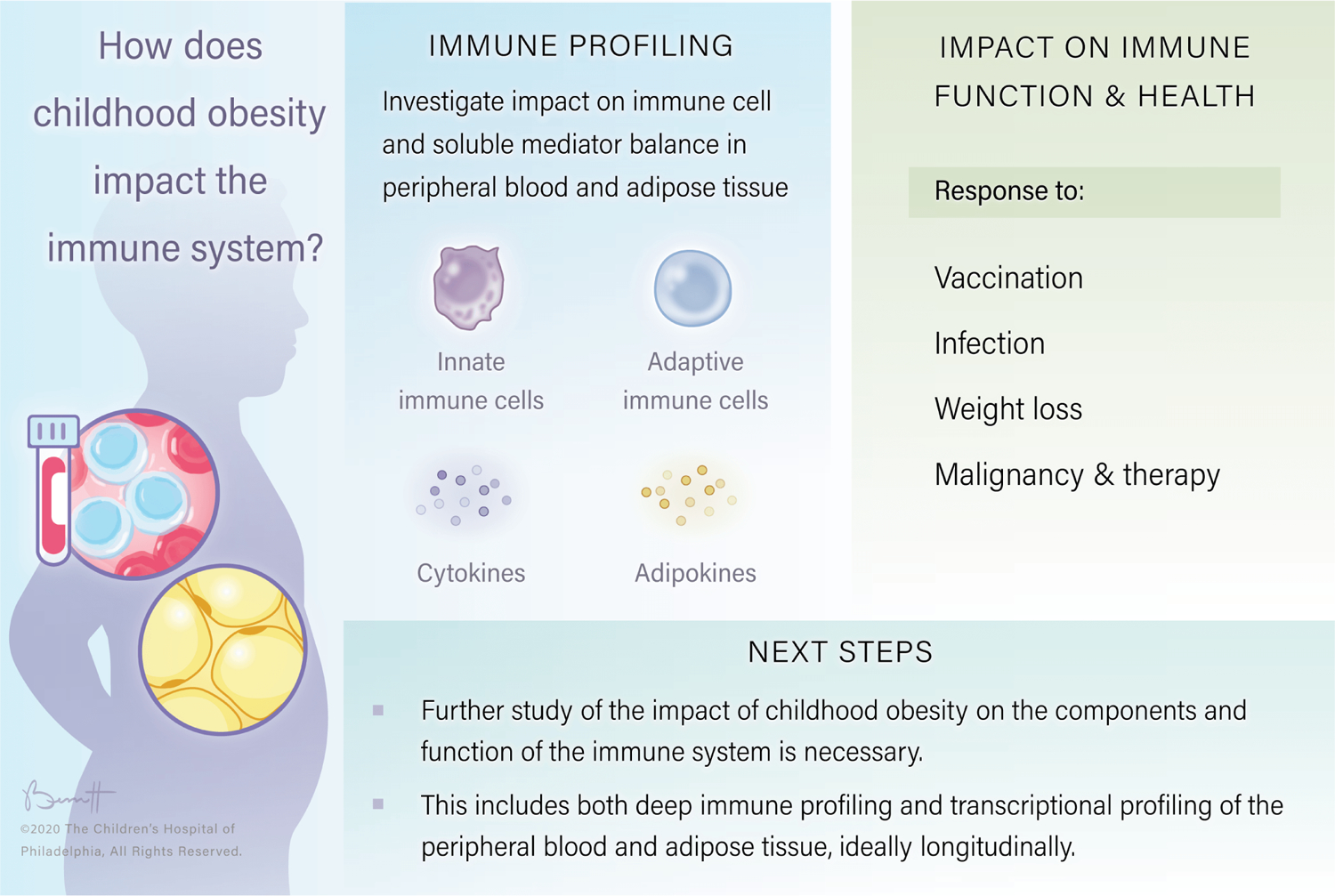
In order to measure the impact of childhood obesity on immune function it is necessary to start by quantifying levels of cytokines and adipokines as well as key immune cell subsets. To measure impacts on function we examine the effects of obesity on response to vaccination, acute infection, malignancy and oncologic therapy as well as weight loss. “©2020, The Children’s Hospital of Philadelphia, All Rights Reserved”
Immune Health: Quantity of Immune Cell Subsets and Humoral Factors
There are increases in pro-inflammatory cytokines and obese adipose tissue is infiltrated by increased numbers of adipose tissue macrophages (ATM), B cells, T cells and mast cells with decreased numbers of regulatory T cells (Tregs), MAIT cells, ILC2 and invariant NKT cells (iNKT) and accompanied by changes in immune cell frequencies in peripheral blood (Table 1). Of note, throughout our discussions of mouse models of obesity, we will focus on diet induced obesity (DIO) wherein wildtype mice receive a high fat diet (HFD).
Cytokines and Adipokines
In adults, both baseline TNF-α[22] and LPS stimulated TNF-α release are elevated[23], and improve with weight loss[22,23]. In obese children, a similar pattern was seen with elevated IL-6 and TNF-α [24–26].
Leptin is elevated in obesity and it has been shown to directly alter immune function [27,28]. Beyond key roles in hunger, leptin receptors are expressed on T cells and leptin increased IFN-γ secretion and decreased IL-4 secretion[29,30]. Leptin stimulated key cytokine pathways (e.g. JAK-STAT) and enhanced proliferation of PBMCs generally[27], and T cells specifically[29,30]. Leptin has been shown to be increased in obese children[31], correlated with adipose tissue mass and improving with physical training[32] or weight loss[33,34]. In addition to leptin, CRP is elevated and adiponectin is decreased [9,35–37]
Innate Immune Cells
The innate immune system, along with the physical barriers of our skin and mucous membranes, represents our front-line defenses against pathogen invasion and the site of our interactions with commensal microorganisms. This component of the immune response shows significant alterations in frequency of key cell subsets in adult obesity, including increased VAT mast cells and ATM and decreased VAT innate lymphocyte cells (ILC) type 2, invariant NKT cells (iNKT) and mucosal associated invariant T cells (MAIT). Circulating innate immune cells are complex in their alterations.
Mast Cells, Neutrophils and Eosinophils
In both mice[38] and adult obese humans[39] there is an increased frequency of mast cells in adipose tissue. There are decreased eosinophils in DIO mouse visceral adipose tissue (VAT) [40], while eosinophils are increased in obese adult human SAT[41]. Neutrophils are increased in childhood obesity, ANC and waist circumference more correlated in girls than in boys[9].
Type 2 Innate Lymphoid Cells (ILC2)
Obese adults have been shown to have decreased ILC2 in white adipose tissue (WAT)[42]. Depletion of ILC2 in T and B cell deficient (RAG deficient) DIO mice led to increased weight gain, all implying a role for ILC2 in obesity[43].
gdT cells
In obese adult humans there is a decreased frequency of circulating γδT cells and reduced secretion of IFNγ [44]. Mouse models of obesity demonstrate a decreased frequency and reduced function of circulating γδT cells[45], and suggest gdT in AT may provide significant fraction of total IL-17[46].
NKT cells
Levels of circulating NKT cells (CD3+ CD56+) are variable, with some studies showing decrease in adult obesity[47], with others showing no change[48,49]. Circulating invariant NKT (iNKT), identified by their canonical invariant TCR and activated by glycolipid antigens presented by CD1d, are capable of quickly secreting cytokines characteristic of both type 1 and type 2 CD4 T helper responses. Circulating iNKT are also decreased in adult obesity[50,51], but improved in frequency after bariatric surgery and with subsequent weight loss[50]. iNKT are enriched in lean human adult adipose tissue but reduced in frequency in obese adipose tissue[52]. In mouse DIO, NKT cells are reduced in frequency in WAT[40] and depletion of iNKT cells leads to increased weight gain and increased IL-6 and TNF-a. Increasing the frequency of iNKT cells protects DIO mice from gaining weight[50], and activation of iNKT with alpha-galactosylceramide leads to weight loss in DIO mice[50].
In a study of obese children there is no statistical difference in circulating NKT counts at baseline or after a lifestyle intervention [53].
MAIT cells
Circulating MAIT cells are generally decreased in obese human adults[51,54,55] (though not significant different in one study[49]), and they increase after bariatric surgery[51]. In adult obesity, there is a reduced frequency of VAT MAIT cells and obesity increases the relative likelihood of MAIT cells secreting IL-17[51] rather than IFN−γ[54]. The underlying mechanism has been recently clarified; glycolytic metabolism is dysfunctional in obese MAIT cells, in the setting of altered mTORC1 signaling which in turns impairs IFN-γ secretion[55]. There is new evidence in mice that MAIT may increase pro-inflammatory M1 macrophage differentiation as well as increasing the leakiness of the gut barrier[56].
In one study, childhood human obesity was associated with expanded circulating MAIT cells (rather than decreased as in adults), which were more likely to secrete IL-17 (consistent with adults)[54].
Monocytes
Monocytes are generally not significantly affected by obesity [48,57,58] though they were elevated in some studies[59,60] and in separate studies classical monocytes (CD14++ CD16-) and non-classical monocytes (CD14+ CD16++) are elevated in the blood of obese children respectively[9,35]. Stepping into the complexity of age and race and ethnicity on immune cell frequency, in participants over 12, hemoglobin A1c (an integrated measure of hyperglycemia) had a negative association with intermediate monocytes (CD14++ CD16+) and a positive association with elevated HDL levels[9]. In addition, race and ethnicity impact inflammatory cell subsets; Black children, who have an increased risk of obesity, lack the correlation of classical monocytes with fasting insulin seen in whites and “other races” (a diverse group in this study including Asian Americans and participants who identify with multiple races) highlighting the need to focusing investigations into mechanisms of immune dysregulation in this understudied (though common) disease and this highly affected population[9].
Adipose Tissue Macrophages
Adipose tissue macrophages (ATM) in mice and adult humans increase in frequency in obesity and are directly responsible for significant secretion of inflammatory cytokines (e.g. TNF-α and IL-6)[61]. Beyond their numbers, there are also effects on the nature of ATM in obesity. Historically, there was a dichotomy drawn between two phenotypes of macrophages: a lower frequency of pro-inflammatory M1 macrophages (e.g. secreting IL-6 and TNF-a) and a marked increase in anti-inflammatory (or ‘alternatively activated’) M2 macrophages[62], reliant in part on PPARγ signaling, in lean VAT[62]. While the source of IL-4 and IL-13 for the initiation of PPARγ signaling was initially attributed to Th2 cells, there is now evidence that eosinophils and iNKT may play a key role. Where eosinophils are reduced in VAT, this dysregulation of PPARγ signaling may impact macrophages[63]. More recently, there have been studies in mice demonstrating more nuanced strategies for dividing ATMs, one using three groups in mice: Ly6C+, CD9+ and CD9- Ly6C-[64], with evidence of both CD9+ and CD9- ATMs in human adipose tissue as well, with CD9+ ATMs increased in frequency in obese human VAT[64] and a key role for TREM2[65].
NK cells
NK cells bridge the innate and adaptive immune systems and can directly lyse infected and malignant cells. In adult obesity there is variability in peripheral NK cells with evidence of decreased [59,66,67] or increased[60] levels, and studies without statistically significant alterations[47,48,57]). In obese mice and humans there is also variable evidence of NK cell metabolic dysregulation and cytotoxic dysfunction[47,66]. It is important to note that some studies use different markers to define NK cells (CD56+ alone or CD56+ CD16+) and that the impact of obesity on NK cell subsets and function in blood and AT has also been deeply interrogated with variability in outcome[47,48,60,68]. This field is reviewed in detail in a recent paper[69]. NK cells have been shown to play a role in regulating Adipose Tissue Macrophages (ATM), including increasing insulin resistance [70].
In children, peripheral NK cells (CD56+ CD3-)[71,72] were decreased, with an reduction in CD56dim and increased CD56bright NK cells[71]. NK cells had increased levels of CD69 (an activation marker) at baseline and higher PD-1 after cytokine stimulation. PD-1 at later timepoints in an infection or malignancy is associated with exhaustion in T cells and with impaired NK function[73]. Functionally, NK cells from obese children had impaired proliferation and effector function, and metabolic derangement with increased rates of glycolysis[71].
Adaptive Immune Cells
Synergistic with pattern-based innate immune responses, T and B cells are antigen-specific lymphocytes which are fundamental to immune function, providing for the nature of the immune response (CD4 T cells) and direct cytotoxicity (primarily CD8 T cells) as well as antibody production (B cells), which are all affected by obesity (Table 1). VAT demonstrates increased infiltration by B cells and CD4 and CD8 T cells in obesity, and circulating cells are more complex in their alterations, with an intriguing finding that obesity yields increased expression of inhibitory receptors (e.g. PD-1) at baseline.
B cells
B cells play important roles in both humoral immunity, by generating antibodies, as well as serving as antigen presenting cells and thus contributing to T cell activation. Circulating B cell levels are variable in obese adults, with studies demonstrating stable levels[48,57,60] or elevated levels[59]. There may be increased infiltration by B cells of SAT in obese humans[60]. In DIO mice, B cells have been noted to infiltrate obese VAT in greater frequency than lean VAT where they contribute to the development of insulin resistance (IR). Depletion of B cells leads to improved metabolic state in DIO mice and, conversely, transferring IgG from a DIO mouse yields IR in recipients [74].
In obese children there was no difference in overall peripheral B cell frequency[72] and no significant increase in peripheral memory B cells, but increased naïve B cells (CD10- CD27-) and immature transitional B cells (CD10+ CD27+) [53].
CD3T cells
Obese adults show a wide range of peripheral total CD3T cells versus non-obese controls, from elevated[59], to not significantly different[57] and less frequent [23,48]. Obese children did not show significant change in peripheral CD3T cell counts versus lean children[72].
Peripheral CD4 T cells were generally stable in obese adults versus non-obese adults[48,51,52,58,60], though increased levels have also been seen[59]. Some studies showed a decrease in memory CD4T cells[23], one showed an increase in CD4T effector memory (TEM) [75,76], another with increased naïve T cell (TN), central memory T cell (TCM) and effector memory re-expressing CD45RA (TEMRA) subsets[77]. Obese children showed no significant change in total peripheral CD4 T cell frequency[72].
Peripheral CD8 T cells were generally stable in obese adults versus non-obese adults[48,51,52,57,58,60], though decreased [23] and increased [59] levels have also been seen. Obese children showed no significant change in peripheral CD8 T cell frequency[72].
In obese adults, SAT contained more CD4 and CD8 T cells[78].
In DIO mice, there was decreased level of naïve CD3T cells in subcutaneous adipose tissue with increase in effector memory T cells (Tem) in visceral adipose tissue (VAT) [78] with reduced TCR-Vβ diversity[78–80] . VAT is a niche for memory T cells which provide antigen specific protection to infection when transferred to naïve mice[81]. There is evidence in DIO mice that effector CD8T cells [28] infiltrate the VAT before macrophages[82] and that depletion of CD8 T cells reduced M1 macrophages and inflammatory cytokines[82]. In addition, STAT3 is elevated in DIO mice VAT T cells, with significant reductions in amount of AT in Stat3−/− mice and increased VAT CD4 TN and Th2 cells and reduction in Th17 and Th1, with improved frequency of M2 macrophages, partially correcting DIO immune dysregulation [83].
Altered balance of CD4 T cell subsets
CD4T cell subsets include those involved in tolerance (regulatory T cells, Tregs), helping B cells (T follicular helper cells, Tfh), combating helminths and contributing to allergy (T helper type 2, Th2), combating viral and intracellular pathogens (T helper type 1, Th1) and combating extracellular pathogens and contributing to autoimmunity (T helper type 17, Th17).
Regulatory T cells (Tregs) are variable in human adult obese blood including increased[77] and decreased levels[76], as well as settings with no detectable change[51,60]. In obese children, peripheral Treg levels were not significantly changed[53]. Tregs are decreased in obese adult VAT[84,85].
The balance between Th1 v. Th2, between anti-viral and anti-helminth/allergic immune tone, has been studied in adults and children. There is some evidence that CD4 T helper type 2 (Th2) were increased in adult obese peripheral blood[77], though they are not significantly altered in obese children[72]. Th1 cells were not significantly altered in obese adults, but were increased in obese children in one study (based on IFNg+)[72] and not significantly altered in another (based on CXCR3+ CD45RO+)[53]. In separate studies there no significant increase in frequency of Th1 between obese and non-obese children who are non-asthmatic [86,87]. In a study of VAT and SAT in adult obesity, there was significant Th1 and Th2 infiltration (but no comparison to healthy or post-surgical AT), simply enriched Th1 and Th17 in VAT v. SAT[88]. Of note, Th1 cells correlated with CRP and IL-6 and Th2 were inversely correlated CRP[88].
In obese adults increased[89] or not significantly altered circulating Th17[77] have been found. In childhood obesity, circulating Th17 cells were increased [90,91]. VAT from adult obese patients with insulin resistance VAT was enriched for Th17 and Th22 cells[92]. A mouse DIO study demonstrated that IL-17, which can also be secreted by γδ T cells, is an inhibitory factor in obesity[46].
Tfh directly assist B cells in activation and one study of pre/post bariatric surgery obese adults demonstrated more IL-10 and less pro-inflammatory cytokines from circulating Tfh post-bariatric surgery[93].
Altered states of CD8 T cells
In the presence of chronic antigen exposure and inflammation (e.g. malignancy and chronic infection) an immune process known as exhaustion takes place, which leads to upregulation of inhibitory receptors (IR), altered transcription and epigenetic state and poor function of the affected cells[94]. In DIO mice, non-human primate obesity and obese adults there is increased expression of PD-1 on CD8 T cells (a key IR in exhaustion) and evidence of reduced effector function in each species[95]. It remains unclear if there is truly exhaustion, and much work remains to be done. In addition, while the presence of inflammation is clear, it remains unclear what the antigenic stimulus is (or whether other pathways are active that remove this need).
Beyond altered circulating T cell subset and functional alterations, in DIO VAT the frequency of senescent T cells (CD153+ PD-1+ CD44hi CD4T, able to secrete osteopontin) is increased and those cells demonstrated poor IL-2 and IFN-γ secretion[96]. A recent strategy targeted those cells for depletion in DIO mice using a CD153 vaccine and improved insulin tolerance[97].
Immune Health: Quality of Immune function
Beyond enumerating altered immune components, there is clear evidence of the broad impact of obesity on the core mechanisms of immune function and here we will focus on evidence of impaired function in obesity and how this impacts the health of obese children (Figure 1).
Response to Vaccination
The effect of obesity on vaccine response is mixed as there are only a handful of studies which are generally small and focused on adult patients with minimal evidence in children. In adults, responses to Hepatitis B vaccine have been shown to be reduced in obesity[98–102]. There has been conflicting evidence of efficacy of influenza vaccine response, with higher initial influenza IgG antibody titers, followed by a more pronounced decline over 12 months[103] and another study without impact of obesity [104]. In addition, there was decreased CD8+ T cell activation with influenza vaccination and subsequent restimulation with influenza protein[103] and increased risk of influenza infection in vaccinated obese adults[105]. Most recently, obese adult recipients of the 23-valent pneumococcal vaccine demonstrated improved responses[106].
Decreased response to Hepatitis B vaccine has also been seen in adolescents[107]. Tetanus titers have been shown to be reduced in overweight adolescents compared to healthy weight children[108]. In children, there was no effect of BMI on influenza vaccine response after two doses (though there was not a 12 month assessment)[109]. While obese children who had received influenza infection missed more days of school, the influenza vaccine protected obese children from becoming infected with influenza given consistent frequency of influenza in vaccinated obese and non-obese children (of note, rates of influenza infection were also consistent between obese and non-obese unvaccinated children)[110].
Overall, the impact of obesity on vaccine response is unclear, especially in children, and requires further study.
Response to acute infection, including both the COVID-19 and H1N1 pandemics
The data in pediatric ICU outcomes is somewhat mixed, with some data showing increased mortality in PICU admissions [111], and some not[112]. In adults, NHANES data was assessed from 1971–2000 and found no evidence of excess deaths in obesity in the context of infections[113]. However, there is evidence in obese adults and children[114] of increased surgical site infections and evidence of increased morbidity from lower respiratory tract infections[115].
In obese adults there is clearly increased morbidity and mortality for certain respiratory infections including H1N1 influenza[116,117] and the current COVID-19 pandemic[2,118,119]. The mechanism for this increase is unclear, and an active area of study. Given how rare the cases of severely affected children and young adults are, there is active international collaboration to study whether there are novel or yet undiagnosed inborn errors of immunity in severely affected children and young adults with COVID-19[120]. Childhood obesity also may increase the risk of severe COVID-19[121]. Interestingly, we have learned from primary immune deficient patients who lack B cells (and have survived COVID-19) that B cells may be expendable[122], and the T cell immune dysregulation discussed above (e.g. increased PD-1 levels on CD8 T cells, altered CD4 T helper subsets, altered cytokines and adipokines, etc) may increase the risk of severe infection with viral infection.
Exhaustion in obesity with improved response to immune checkpoint inhibition
Childhood obesity increases the risk of cancer in early adulthood[123,124], much like the increased risk of malignancy in obese adults[125]. However, there is recent evidence of altered response to novel oncologic therapy in obesity [126]. This type of therapy, used for some forms of malignancy, is known as immune checkpoint inhibition and uses monoclonal antibodies to target elevated inhibitory receptors (e.g. PD-1) to reinvigorate stalled anti-tumor CD8 T cell immunity[94]. In obese adults there is evidence of both increased expression of PD-1 (a key inhibitory receptor and a target of immune checkpoint inhibition) on CD8 T cells in the tumor microenvironment (TME) and better outcomes with checkpoint inhibitor therapy (monoclonal antibodies directed towards inhibitory receptors)[95,127]. There is not yet published evidence on PD-1 in obese children and checkpoint inhibition in pediatric oncology has been less effective than in adult cancer[128–130] to this point, so there is no current evidence as to the impact of pediatric obesity on checkpoint inhibition response.
Effect of weight loss on immune function
Supporting the evidence of obesity in altering immune function, there is evidence across multiple studies of adult humans that weight reduction can improve immune function, as well as evidence of overall improvement in mortality. CD3T cells (specifically CD4 T cells and the CD4+ CD45RO+ memory T cell subset) were decreased in obese adults as was the T cell proliferative response to PHA [23], post-diet induced weight loss there was improvement in T and NK cell counts and response of T cells to PHA and concanavalin A[23]. In the same study, prior to weight loss, both baseline TNF-α and LPS stimulated TNF-α release were elevated[23], and the latter improved with weight loss. In obese adults there was improved cytokine production by T cells [131], as well as from bulk PBMCs stimulated with PHA (phytohemagglutinin)[132] after bariatric surgery. PBMC cytotoxicity was decreased in some studies of obese adults[132], with improvement in effector mechanisms after bariatric surgery [132]. Finally, in obese adolescents there was elevated IL-6 and leptin that improved with bariatric surgery, along with a resulting increase in adiponectin[133], with a similar reduction in leptin in adults post-surgically [132].
Conclusion
Childhood obesity is one of the most common non-communicable inflammatory diseases worldwide. However, while extremely common and with significant effects on the balance of the components of the immune response and concern for significant impact on immune function (given the alterations in response to some pathogens, concern for impact on vaccination responses and evidence of immune dysregulation), this is an understudied disease. More work must be done both to understand immune health at baseline in pediatrics, the alterations imposed by pediatric obesity, as well as response to perturbations (including infection, vaccination and weight loss, specifically bariatric surgery) to better care for this medically complex and fragile population.
Key points.
There are significant alterations in childhood and adult obesity in both the quantity and activation state of both peripheral and target tissue immune cells
Obese children and adults experience increased morbidity with some types of pathogens, including COVID-19, consistent with the concern for immune dysfunction based on altered immune cell components and function
There is a dearth of investigation into pediatric immune health generally and the impact of childhood obesity on immune function specifically
Acknowledgments:
Thanks to the Henrickson lab for helpful discussions around these issues.
Financial support: SEH: NIH K08AI135091 (SEH), the Burroughs Wellcome Fund Career Award for Medical Scientists, Chan Zuckerberg Initiative and CHOP Research Institute Developmental Awards. JH-M: NIH R21DK111755, R01HL136572, the PEW Biomedical Scholars award, Chan Zuckerberg Initiative and the Burroughs Wellcome Fund investigator in the pathogenesis of infectious diseases award.
Abbreviations:
- ATM
Adipose Tissue Macrophages
- DIO
Diet induced obesity
- HFD
high‐fat diet
- ILC2
Innate lymphoid cells, type 2
- NK
Natural Killer cells
- iNKT
invariant NK T cells
- MAIT
mucosal associated invariant T cells
- SAT
subcutaneous adipose tissue
- scRNA-seq
single cell RNA sequencing
- TCM
central memory T cells
- TEM
effector memory T cells
- TEMRA
effector memory T cells re-expressing CD45RA
- Texh
T cell exhaustion
- TMEM
memory T cells
- TN
naïve T cells
- TLR
Toll-like receptor
- Th2
type 2 CD4 T helper cells
- Th17
type 17 CD4 T helper cells
- VAT
visceral adipose tissue
Footnotes
Conflicts of interests: SEH has been on ad hoc advisory boards for Horizon Pharma.
References
- 1.Di Cesare M, Sorić M, Bovet P, Miranda JJ, Bhutta Z, Stevens GA, Laxmaiah A, Kengne AP, Bentham J: The epidemiological burden of obesity in childhood: A worldwide epidemic requiring urgent action. BMC Med 2019, 17:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bentham J, Di Cesare M, Bilano V, Bixby H, Zhou B, Stevens GA, Riley LM, Taddei C, Hajifathalian K, Lu Y, et al. : Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390:2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC: Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics 2018, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hales CM, Carroll MD, Fryar CD, Ogden CL: Prevalence of Obesity Among Adults and Youth: United States, 2015–2016.NCHS data brief, no 288 Hyattsville, MD: National Center for Health Statistics. NCHS data brief, no 288 Hyattsville, MD Natl Cent Heal Stat 2017, [Google Scholar]
- 5.Biro FM, Wien M: Childhood obesity and adult morbidities. Am J Clin Nutr 2010, 91:1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward ZJ, Long MW, Resch SC, Giles CM, Cradock AL, Gortmaker SL: Simulation of growth trajectories of childhood obesity into adulthood. N Engl J Med 2017, 377:2145–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lindberg L, Danielsson P, Persson M, Marcus C, Hagman E: Association of childhood obesity with risk of early all-cause and cause-specific mortality: A Swedish prospective cohort study. PLoS Med 2020, 17:e1003078. From a prospective cohort study tracing 41,359 Swedish individuals into young adulthood, researchers found out that the risk of mortality in early adulthood was higher for individuals with childhood obesity compared to those without childhood obesity. (*)
- 8.Alpert A, Pickman Y, Leipold M, Rosenberg-Hasson Y, Ji X, Gaujoux R, Rabani H, Starosvetsky E, Kveler K, Schaffert S, et al. : A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat Med 2019, 25:487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gállego-Suárez C, Bulan A, Hirschfeld E, Wachowiak P, Abrishami S, Griffin C, Sturza J, Tzau A, Hayes T, Woolford SJ, et al. : Enhanced Myeloid Leukocytes in Obese Children and Adolescents at Risk for Metabolic Impairment. Front Endocrinol (Lausanne) 2020, 11:1–12. Team defines peripheral circulating monocyte populations in childhood obesity and delves into the connections between inflammatory cell subsets and cytokines, adipokines, age, gender and race/ethnicity, uncovering discordant connections in different groups that highlight the need for more study of diverse participants in both heathy and obese cohorts. (**)
- 10.Bartlett JA, Goldklang AR, Schleifer SJ, Keller SE: Immune function in healthy inner-city children. Clin Diagn Lab Immunol 2001, 8:740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heslehurst N, Vieira R, Akhter Z, Bailey H, Slack E, Ngongalah L, Pemu A, Rankin J: The association between maternal body mass index and child obesity: A systematic review and meta-analysis. PLoS Med 2019, 16:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu DM, Antony KM, Ma J, Prince AL, Showalter L, Moller M, Aagaard KM: The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med 2016, 8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson RM, Marshall NE, Jeske DR, Purnell JQ, Thornburg K, Messaoudi I: Maternal obesity alters immune cell frequencies and responses in umbilical cord blood samples. Pediatr Allergy Immunol 2015, 26:344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ley RE, Turnbaugh PJ, Klein S, Gordon JI: Human gut microbes associated with obesity. Nature 2006, 444:1022–1023. [DOI] [PubMed] [Google Scholar]
- 15.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI: Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 2005, 102:11070–11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCormack SE, Shaham O, Mccarthy MA, Deik AA, Wang TJ, Gerszten RE, Clish CB, Mootha VK, Grinspoon SK, Fleischman A: Circulating Branched-chain Amino Acid Concentrations Are Associated with Obesity and Future Insulin Resistance in Children and Adolescents. Pediatr Obes 2013, 8:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butte NF, Liu Y, Zakeri IF, Mohney RP, Mehta N, Voruganti VS, Göring H, Cole SA, Comuzzie AG: Global metabolomic profiling targeting childhood obesity in the Hispanic population. Am J Clin Nutr 2015, 102:256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Virtue AT, McCright SJ, Wright JM, Jimenez MT, Mowel WK, Kotzin JJ, Joannas L, Basavappa MG, Spencer SP, Clark ML, et al. : The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci Transl Med 2019, 11:1–14.Gut microbiota regulated the expression of the miR-181 family in white adipocytes with tryptophan-derived metabolites in mice, and miR-181 family promoted diet induced obesity, fat mass gain, insulin resistance, and white adipose tissue inflammation. This was also examined in obese children. (*)
- 19.Canfora EE, Meex RCR, Venema K, Blaak EE: Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol 2019, 15:261–273. [DOI] [PubMed] [Google Scholar]
- 20.Lee HY, Lee EG, Hur J, Rhee CK, Kim YK, Lee SY, Kang JY: Pravastatin alleviates allergic airway inflammation in obesity-related asthma mouse model. Exp Lung Res 2019, 45:275–287. [DOI] [PubMed] [Google Scholar]
- 21. Armstrong SC, Bolling CF, Michalsky MP, Reichard KW: Pediatric metabolic and bariatric surgery: Evidence, barriers, and best practices. Pediatrics 2019, 144. Despite the generally successful outcomes of weight loss and comorbidity resolution after pediatric bariatric surgery, there are barriers preventing adolescents from receiving the surgery, including insurance authorization, providers’ concerns about the surgery, the preference for long-term lifestyle management and cost-effectiveness. Best practices for pediatric bariatric surgery are discussed. (*)
- 22.Dandona P, Weinstock R, Thusu K, Abdel-Rahman E, Aljada A, Wadden T: Tumor necrosis factor-α in sera of obese patients: Fall with weight loss. J Clin Endocrinol Metab 1998, 83:2907–2910. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka SI, Isoda F, Ishihara Y, Kimura M, Yamakawa T: T lymphopaenia in relation to body mass index and TNF-α in human obesity: Adequate weight reduction can be corrective. Clin Endocrinol (Oxf) 2001, 54:347–354. [PubMed] [Google Scholar]
- 24.Gallistl S, Sudi KM, Aigner R, Borkenstein M: Changes in serum interleukin-6 concentrations in obese children and adolescents during a weight reduction program. Int J Obes 2001, 25:1640–1643. [DOI] [PubMed] [Google Scholar]
- 25.Dixon D, Goldberg R, Schneiderman N, Delamater A: Gender differences in TNF-α levels among obese vs nonobese Latino children. Eur J Clin Nutr 2004, 58:696–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breslin WL, Johnston CA, Strohacker K, Carpenter KC, Davidson TR, Moreno JP, Foreyt JP, McFarlin BK: Obese Mexican American children have elevated MCP-1, TNF-α, monocyte concentration, and dyslipidemia. Pediatrics 2012, 129. [DOI] [PubMed] [Google Scholar]
- 27.Sánchez-Margalet V, Martín-Romero C, Santos-Alvarez J, Goberna R, Najib S, Gonzalez-Yanes C: Role of leptin as an immunomodulator of blood mononuclear cells: Mechanisms of action. Clin Exp Immunol 2003, 133:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rausch ME, Weisberg S, Vardhana P, Tortoriello D V.: Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes 2008, 32:451–463. [DOI] [PubMed] [Google Scholar]
- 29.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI: Leptin modulates the T-cell immune response and reverse starvation-induced immunosuppression. Nature 1998, 394:897–901. [DOI] [PubMed] [Google Scholar]
- 30.Martín-Romero C, Santos-Alvarez J, Goberna R, Sánchez-Margalet V: Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol 2000, 199:15–24. [DOI] [PubMed] [Google Scholar]
- 31.Hassink SG, Sheslow DV, De Lancey E, Opentanova I, Considine RV, Caro JF: Serum leptin in children with obesity: Relationship to gender and development. Pediatrics 1996, 98:201–203. [PubMed] [Google Scholar]
- 32.Gutin B, Ramsey L, Barbeau P, Cannady W, Ferguson M, Litaker M, Owens S : Plasma leptin concentrations in obese children: Changes during 4-mo periods with and without physical training. Am J Clin Nutr 1999, 69:388–394. [DOI] [PubMed] [Google Scholar]
- 33.Kumari M, Heeren J, Scheja L: Regulation of immunometabolism in adipose tissue. Semin Immunopathol 2018, 40:189–202. [DOI] [PubMed] [Google Scholar]
- 34.Holm JC, Gamborg M, Ward LC, Gammeltoft S, Kaas-Ibsen K, Heitmann BL, Sørensen TIA: Tracking of leptin, soluble leptin receptor, and the free leptin index during weight loss and regain in children. Obes Facts 2011, 4:461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattos RT, Medeiros NI, Menezes CA, Fares RCG, Franco EP, Dutra WO, Rios-Santos F, Correa-Oliveira R, Gomes JAS: Chronic low-grade inflammation in childhood obesity is associated with decreased il-10 expression by monocyte subsets. PLoS One 2016, 11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reyes M, Quintanilla C, Burrows R, Blanco E, Cifuentes M, Gahagan S: Obesity is associated with acute inflammation in a sample of adolescents. Pediatr Diabetes 2015, 16:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valle M, Martos R, Gascón F, Cañete R, Zafra MA, Morales R: Low-grade systemic inflammation, hypoadiponectinemia and a high concentration of leptin are present in very young obese children, and correlate with metabolic syndrome. Diabetes Metab 2005, 31:55–62. [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, et al. : Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med 2009, doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finlin BS, Confides AL, Zhu B, Boulanger MC, Memetimin H, Taylor KW, Johnson ZR, Westgate PM, Dupont-Versteegden EE, Kern PA: Adipose Tissue Mast Cells Promote Human Adipose Beiging in Response to Cold. Sci Rep 2019, 9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding X, Luo Y, Zhang X, Zheng H, Yang X, Yang X, Liu M: IL-33-driven ILC2/eosinophil axis in fat is induced by sympathetic tone and suppressed by obesity. J Endocrinol 2016, 231:35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moussa K, Gurung P, Adams-Huet B, Devaraj S, Jialal I: Increased eosinophils in adipose tissue of metabolic syndrome. J Diabetes Complications 2019, 33:535–538. [DOI] [PubMed] [Google Scholar]
- 42.Brestoff JR, Kim BS, Saenz S a, Stine RR, Monticelli L a, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, et al. : Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 2014, 519:242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hams E, Locksley RM, McKenzie ANJ, Fallon PG: Cutting Edge: IL-25 Elicits Innate Lymphoid Type 2 and Type II NKT Cells That Regulate Obesity in Mice. J Immunol 2013, 191:5349–5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costanzo AE, Taylor KR, Dutt S, Han PP, Fujioka K, Jameson JM: Obesity impairs γδ T cell homeostasis and antiviral function in humans. PLoS One 2015, 10:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor KR, Mills RE, Costanzo AE, Jameson JM: γδ T cells are reduced and rendered unresponsive by hyperglycemia and chronic TNFα in mouse models of obesity and metabolic disease. PLoS One 2010, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zúñiga LA, Shen W-J, Joyce-Shaikh B, Pyatnova EA, Richards AG, Thom C, Andrade SM, Cua DJ, Kraemer FB, Butcher EC: IL-17 Regulates Adipogenesis, Glucose Homeostasis, and Obesity. J Immunol 2010, 185:6947–6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laue T, Wrann CD, Hoffmann-Castendiek B, Pietsch D, Hübner L, Kielstein H: Altered NK cell function in obese healthy humans. BMC Obes 2015, 2:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bähr I, Jahn J, Zipprich A, Pahlow I, Spielmann J, Kielstein H: Impaired natural killer cell subset phenotypes in human obesity. Immunol Res 2018, 66:234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Woods K, Parry-Strong A, Anderson RJ, Capistrano C, Gestin A, Painter GF, Hermans IF, Krebs J, Gasser O: Distinct Dysfunctional States of Circulating Innate-Like T Cells in Metabolic Disease. Front Immunol 2020, 11:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, Balk SP, O’Shea D, O’Farrelly C, Exley MA: Adipose Tissue Invariant NKT Cells Protect against Diet-Induced Obesity and Metabolic Disorder through Regulatory Cytokine Production. Immunity 2012, 37:574–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magalhaes I, Pingris K, Poitou C, Bessoles S, Venteclef N, Kiaf B, Beaudoin L, Da Silva J, Allatif O, Rossjohn J, et al. : Mucosal-associated invariant T cell alterations in obese and type 2 diabetic patients. J Clin Invest 2015, 125:1752–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lynch L, O’Shea D, Winter DC, Geoghegan J, Doherty DG, O’Farrelly C: Invariant NKT cells and CD1d+ cells amass in human omentum and are depleted in patients with cancer and obesity. Eur J Immunol 2009, 39:1893–1901. [DOI] [PubMed] [Google Scholar]
- 53.Keustermans G, Van Der Heijden LB, Boer B, Scholman R, Nuboer R, Pasterkamp G, Prakken B, De Jager W, Kalkhoven E, Janse AJ, et al. : Differential adipokine receptor expression on circulating leukocyte subsets in lean and obese children. PLoS One 2017, 12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carolan E, Tobin LM, Mangan BA, Corrigan M, Gaoatswe G, Byrne G, Geoghegan J, Cody D, O’Connell J, Winter DC, et al. : Altered Distribution and Increased IL-17 Production by Mucosal-Associated Invariant T Cells in Adult and Childhood Obesity. J Immunol 2015, 194:5775–5780. [DOI] [PubMed] [Google Scholar]
- 55.O’Brien A, Loftus RM, Pisarska MM, Tobin LM, Bergin R, Wood NAW, Foley C, Mat A, Tinley FC, Bannan C, et al. : Obesity Reduces mTORC1 Activity in Mucosal-Associated Invariant T Cells, Driving Defective Metabolic and Functional Responses. J Immunol 2019, 202:3404–3411. [DOI] [PubMed] [Google Scholar]
- 56.Toubal A, Kiaf B, Beaudoin L, Cagninacci L, Rhimi M, Fruchet B, Silva J, Corbett A, Simoni Y, Lantz O, et al. : Mucosal-associated invariant T cells promote in fl ammation and intestinal dysbiosis leading to metabolic dysfunction during obesity. Nat Commun 2020, doi: 10.1038/s41467-020-17307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cottam DR, Schaefer PA, Shaftan GW, Angus LDG: Dysfunctional immune-privilege in morbid obesity: Implications and effect of gastric bypass surgery. Obes Surg 2003, 13:49–57. [DOI] [PubMed] [Google Scholar]
- 58.Kosaraju R, Guesdon W, Crouch MJ, Teague HL, Sullivan EM, Karlsson EA, Schultz-Cherry S, Gowdy K, Bridges LC, Reese LR, et al. : B Cell Activity Is Impaired in Human and Mouse Obesity and Is Responsive to an Essential Fatty Acid upon Murine Influenza Infection. J Immunol 2017, 198:4738–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ilavská S, Horváthová M, Szabová M, Nemessányi T, Jahnová E, Tulinská J, Líšková A, Wsolová L, Staruchová M, Volkovová K: Association between the human immune response and body mass index. Hum Immunol 2012, 73:480–485. [DOI] [PubMed] [Google Scholar]
- 60.Wouters K, Gaens K, Bijnen M, Verboven K, Jocken J, Wetzels S, Wijnands E, Hansen D, Van Greevenbroek M, Duijvestijn A, et al. : Circulating classical monocytes are associated with CD11c+ macrophages in human visceral adipose tissue. Sci Rep 2017, 7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weisberg SP, Leibel RL, Anthony W, Jr F, Weisberg SP, Mccann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW: Obesity is associated with macrophage accumulation in adipose tissue Find the latest version : Obesity is associated with. J Clin Invest 2003, 112:1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lumeng CN, Bodzin JL, Saltiel AR, Lumeng CN, Bodzin JL, Saltiel AR: Obesity induces a phenotypic switch in adipose tissue macrophage polarization Find the latest version : Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007, 117:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu D, Molofsky AB, Liang H-E, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM: Eosinophils Sustain Adipose Alternatively Activated Macrophages Associated with Glucose Homeostasis. Science (80- ) 2011, 332:243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hill DA, Lim HW, Kim YH, Ho WY, Foong YH, Nelson VL, Nguyen HCB, Chegireddy K, Kim J, Habertheuer A, et al. : Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc Natl Acad Sci U S A 2018, 115:E5096–E5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, Lundgren P, Bleriot C, Liu Z, Deczkowska A, et al. : Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell 2019, 178:686–698.e14. Researchers describe a Trem2+ lipid-associated macrophage(LAM) subset in mouse and human white adipose tissue. Lipid receptor Trem2 is a major driver of adipose tissue response during obesity, and is critical for LAM formation and assembly of crown-like structures. (*)
- 66.Michelet X, Dyck L, Hogan A, Loftus RM, Duquette D, Wei K, Beyaz S, Tavakkoli A, Foley C, Donnelly R, et al. : Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol 2018, doi: 10.1038/s41590-018-0251-7. [DOI] [PubMed] [Google Scholar]
- 67.Lynch LA, O’Connell JM, Kwasnik AK, Cawood TJ, O’Farrelly C, O’Shea DB: Are natural killer cells protecting the metabolically healthy obese patient? Obesity 2009, 17:601–605. [DOI] [PubMed] [Google Scholar]
- 68.Michelet X, Dyck L, Hogan A, Loftus RM, Duquette D, Wei K, Beyaz S, Tavakkoli A, Foley C, Donnelly R, et al. : Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol 2018, 19:1330–1340. [DOI] [PubMed] [Google Scholar]
- 69.Bähr I, Spielmann J, Quandt D, Kielstein H: Obesity-Associated Alterations of Natural Killer Cells and Immunosurveillance of Cancer. Front Immunol 2020, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee B, Kim M, Pae M, Yamamoto Y, Eberlé D, Shimada T, Kamei N, Park H, Woo JR, You J, et al. : Adipose natural killer cells regulate adipose tissue macrophages to promote insulin resistance in obesity. Cell Metab 2016, 23:685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tobin LM, Mavinkurve M, Carolan E, Kinlen D, O’Brien EC, Little MA, Finlay DK, Cody D, Hogan AE, O’Shea D: NK cells in childhood obesity are activated, metabolically stressed, and functionally deficient. JCI Insight 2017, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pacifico L, Di Renzo L, Anania C, Osborn JF, Ippoliti F, Schiavo E, Chiesa C: Increased T-helper interferon-γ-secreting cells in obese children. Eur J Endocrinol 2006, 154:691–697. [DOI] [PubMed] [Google Scholar]
- 73.Vari F, Arpon D, Keane C, Hertzberg MS, Talaulikar D, Jain S, Cui Q, Han E, Tobin J, Bird R, et al. : Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood 2018, 131:1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, et al. : B Lymphocytes Promote Insulin Resistance through Modulation of T Lymphocytes and Production of Pathogenic IgG Antibody. Nat Med 2011, 17:610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mauro C, Smith J, Cucchi D, Coe D, Fu H, Bonacina F, Baragetti A, Cermenati G, Caruso D, Mitro N, et al. : Obesity-Induced Metabolic Stress Leads to Biased Effector Memory CD4+ T Cell Differentiation via PI3K p110δ-Akt-Mediated Signals. Cell Metab 2017, 25:593–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Agabiti-Rosei C, Trapletti V, Piantoni S, Airo P, Tincani A, De Ciuceis C, Rossini C, Mittempergher F, Titi A, Portolani N, et al. : Decreased circulating t regulatory lymphocytes in obese patients undergoing bariatric surgery. PLoS One 2018, 13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van Der Weerd K, Dik WA, Schrijver B, Schweitzer DH, Langerak AW, Drexhage HA, Kiewiet RM, Van Aken MO, Van Huisstede A, Van Dongen JJM, et al. : Morbidly obese human subjects have increased peripheral blood CD4 + T cells with skewing toward a Treg- and Th2-dominated phenotype. Diabetes 2012, 61:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang H, Youm Y-H, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD: Obesity Increases the Production of Proinflammatory Mediators from Adipose Tissue T Cells and Compromises TCR Repertoire Diversity: Implications for Systemic Inflammation and Insulin Resistance. J Immunol 2010, 185:1836–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang H, Youm YH, Vandanmagsar B, Rood J, Kumar KG, Butler AA, Dixit VD: Obesity accelerates thymic aging. Blood 2009, 114:3803–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, et al. : Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 2009, 15:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han SJ, Glatman Zaretsky A, Andrade-Oliveira V, Collins N, Dzutsev A, Shaik J, Morais da Fonseca D, Harrison OJ, Tamoutounour S, Byrd AL, et al. : White Adipose Tissue Is a Reservoir for Memory T Cells and Promotes Protective Memory Responses to Infection. Immunity 2017, 47:1154–1168.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. : CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009, 15:914–920. [DOI] [PubMed] [Google Scholar]
- 83.Priceman SJ, Kujawski M, Shen S, Cherryholmes GA, Lee H, Zhang C, Kruper L, Mortimer J, Jove R, Riggs AD, et al. : Regulation of adipose tissue T cell subsets by Stat3 is crucial for diet-induced obesity and insulin resistance. Proc Natl Acad Sci U S A 2013, 110:13079–13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, et al. : Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009, 15:930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gyllenhammer LE, Lam J, Alderete TL, Allayee H, Akbari O, Katkhouda N, Goran MI: Lower omental t-regulatory cell count is associated with higher fasting glucose and lower β-cell function in adults with obesity. Obesity 2016, 24:1274–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rastogi D, Canfield SM, Andrade A, Isasi CR, Hall CB, Rubinstein A, Arens R: Obesity-associated asthma in children a distinct entity. Chest 2012, 141:895–905. [DOI] [PubMed] [Google Scholar]
- 87.Rastogi D, Fraser S, Oh J, Huber AM, Schulman Y, Bhagtani RH, Khan ZS, Tesfa L, Hall CB, Macian F: Inflammation, metabolic dysregulation, and pulmonary function among obese urban adolescents with asthma. Am J Respir Crit Care Med 2015, 191:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McLaughlin T, Liu LF, Lamendola C, Shen L, Morton J, Rivas H, Winer D, Tolentino L, Choi O, Zhang H, et al. : T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol 2014, 34:2632–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Winer S, Paltser G, Chan Y, Tsui H, Engleman E, Winer D, Dosch HM: Obesity predisposes to Th17 bias. Eur J Immunol 2009, 39:2629–2635. [DOI] [PubMed] [Google Scholar]
- 90.Schindler TI, Wagner JJ, Goedicke-Fritz S, Rogosch T, Coccejus V, Laudenbach V, Nikolaizik W, Härtel C, Maier RF, Kerzel S, et al. : TH17 cell frequency in peripheral blood is elevated in overweight children without chronic inflammatory diseases. Front Immunol 2017, 8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Łuczyński W, Grubczak K, Moniuszko M, Głowińska-Olszewska B, Bossowski A: Elevated levels of Th17 cells in children with central obesity. Scand J Clin Lab Invest 2015, 75:595–601. [DOI] [PubMed] [Google Scholar]
- 92.Fabbrini E, Cella M, McCartney SA, Fuchs A, Abrumad NA, Pietka TA, Chen Z, Finck BN, Han DH, Magkos F, et al. : Association Between Specific Adipose Tissue CD4+ T-Cell Populations and Insulin Resistance in Obese Individuals. Gastroenterology 2013, 145:366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhan J, Huang L, Ma H, Chen H, Yang Y, Tan S, Song W, Zhao W, Dai X: Reduced inflammatory responses of follicular helper T cell promote the development of regulatory B cells after Roux-en-Y gastric bypass. Clin Exp Pharmacol Physiol 2017, 44:556–565. [DOI] [PubMed] [Google Scholar]
- 94.McLane LM, Abdel-Hakeem MS, Wherry EJ: CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu Rev Immunol 2019, 37:457–495. [DOI] [PubMed] [Google Scholar]
- 95. Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, Mirsoian A, Minnar CM, Stoffel KM, Sturgill IR, et al. : Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med 2019, 25:141–151. Obese mice, non-human primates and adult humans demonstrated increased PD-1 expression. Obesity promoted tumor growth and increased PD-1 expression in tumor-infiltrating CD8 T cells, which was partly driven by leptin. Improved efficacy of PD-1 checkpoint inhibition was seen in diet induced obesity mice. In a large cohort of 152 human colorectal cancers, high BMI was significantly associated with better survival after anti PD-1 checkpoint inhibition. (**)
- 96.Shirakawa K, Minato N, Sano M, Shirakawa K, Yan X, Shinmura K, Endo J, Kataoka M, Katsumata Y: Obesity accelerates T cell senescence in murine visceral adipose tissue. J Clin Invest 2016, 126:4626–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yoshida S, Nakagami H, Hayashi H, Ikeda Y, Sun J, Tenma A, Tomioka H, Kawano T, Shimamura M, Morishita R, et al. : The CD153 vaccine is a senotherapeutic option for preventing the accumulation of senescent T cells in mice. Nat Commun 2020, 11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weber DJ, Rutala WA, Samsa GP, Santimaw JE, Lemon SM: Obesity as a Predictor of Poor Antibody Response to Hepatitis B Plasma Vaccine 2013, [PubMed] [Google Scholar]
- 99.Young KM, Gray CM, Bekker LG: Is obesity a risk factor for vaccine non-responsiveness? PLoS One 2013, 8:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roome AJ, Walsh SJ, Cartter ML, Hadler JL: Hepatitis B Vaccine Responsiveness in Connecticut Public Safety Personnel. JAMA J Am Med Assoc 1993, 270:2931–2934. [PubMed] [Google Scholar]
- 101.Wood RC, Macdonald KL, White KE, Hedberg CW, Hanson M, Osterholm MT: Risk Factors for Lack of Detectable Antibody Following Hepatitis B Vaccination of Minnesota Health Care Workers. JAMA J Am Med Assoc 1993, 270:2935–2939. [PubMed] [Google Scholar]
- 102.Averhoff F, Mahoney F, Coleman P, Schatz G, Hurwitz E, Margolis H: Immunogenicity of hepatitis B vaccines: Implications for persons at occupational risk of hepatitis B virus infection. Am J Prev Med 1998, 15:1–8. [DOI] [PubMed] [Google Scholar]
- 103.Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, Holland LA, Weir S, Noah TL, Beck MA: Obesity is associated with impaired immune response to influenza vaccination in humans. J Obes 2011, 36:1072–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Talbot HK, Coleman LA, Crimin K, Zhu Y, Rock MT, Meece J, Shay DK, Belongia EA, Griffin MR: Association between obesity and vulnerability and serologic response to influenza vaccination in older adults. Vaccine 2012, 30:3937–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Neidich SD, Green WD, Rebeles J, Karlsson EA, Schultz-Cherry S, Noah TL, Chakladar S, Hudgens MG, Weir SS, Beck MA: Increased risk of influenza among vaccinated adults who are obese. Int J Obes 2017, 41:1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sebastian M, Hsiao CJ, Futch HS, Eisinger RS, Dumeny L, Patel S, Gobena M, Katikaneni DS, Cohen J, Carpenter AM, et al. : Obesity and STING1 genotype associate with 23-valent pneumococcal vaccination efficacy. JCI Insight 2020, 5:1–10. This study evaluated the effect of obesity on humoral response to PPSV23 vaccine in relation to STING1 genotype, with a positive association between obesity and PPSV23 efficacy in participants with wildtype STING1. (*)
- 107.Miñana JS, Ganuza MG, Millán PF, Fernández MP: Hepatitis B vaccine immunoresponsiveness in adolescents: A revaccination proposal after primary vaccination. Vaccine 1996, 14:103–106. [DOI] [PubMed] [Google Scholar]
- 108.Eliakim A, Swindt C, Zaldivar F, Casali P, Cooper DM: Reduced tetanus antibody titers in overweight children. Autoimmunity 2006, 39:137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Callahan ST, Wolff M, Hill HR, Edwards KM, Keitel W, Atmar R, Patel S, El Sahly H, Munoz F, Glezen WP, et al. : Impact of body mass index on immunogenicity of pandemic H1N1 vaccine in children and adults. J Infect Dis 2014, 210:1270–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smit MA, Wang HL, Kim E, Barragan N, Aldrovandi GM, El Amin AN, Mascola L, Pannaraj PS: Influenza vaccine is protective against laboratory-confirmed influenza in obese children. Pediatr Infect Dis J 2016, 35:440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ross PA, Newth CJL, Leung D, Wetzel RC, Bs M, Khemani RG: Obesity and Mortality Risk in Critically Ill Children. Pediatrics 2016, 137:e20152035. [DOI] [PubMed] [Google Scholar]
- 112.Peterson LS, Suárez CG, Segaloff HE, Griffin C, Martin ET, Odetola FO, Singer K: Outcomes and Resource Use Among Overweight and Obese Children With Sepsis in the Pediatric Intensive Care Unit. J Intensive Care Med 2020 2020, 35:472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Flegal KM, Graubard BI, Williamson DF, Gail MH: Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA 2007, 298:2028–2037. [DOI] [PubMed] [Google Scholar]
- 114.Blackwood BP, Gause CD, Harris JC, Theodorou CM, Helenowski I, Lautz TB, Grabowski J, Hunter CJ: Overweight and Obese Pediatric Patients Have an Increased Risk of Developing a Surgical Site Infection. Surg Infect 2017, 18. [DOI] [PubMed] [Google Scholar]
- 115.Okubo Y, Nochioka K, Testa MA: The impact of pediatric obesity on hospitalized children with lower respiratory tract infections in the United States. Clin Respir J 2018, 12:1479–1484. [DOI] [PubMed] [Google Scholar]
- 116.Morgan OW, Bramley A, Fowlkes A, Freedman DS, Taylor TH, Gargiullo P, Belay B, Jain S, Cox C, Kamimoto L, et al. : Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS One 2010, 5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Honce R, Schultz-Cherry S: Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front Immunol 2019, 10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, Labreuche J, Mathieu D, Pattou F, Jourdain M, et al. : High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity 2020, 28:1195–1199. From a retrospective cohort study including 124 patients in intensive care for SARS-CoC-2 in a French center, researchers saw a high frequency of obesity among patients and disease severity increased with BMI. (*)
- 119.Cai Q, Chen F, Wang T, Luo F, Liu X, Wu Q, He Q, Wang Z, Liu Y, Liu L, et al. : Obesity and COVID-19 Severity in a Designated Hospital in Shenzhen, China. Diabetes Care 2020, 43:1392–1398. [DOI] [PubMed] [Google Scholar]
- 120.Casanova JL, Su HC, Abel L, Aiuti A, Almuhsen S, Arias AA, Bastard P, Biggs C, Bogunovic D, Boisson B, et al. : A Global Effort to Define the Human Genetics of Protective Immunity to SARS-CoV-2 Infection. Cell 2020, 181:1194–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zachariah P, Halabi KC, Ahn D, Sen AI, Fischer A, Banker SL, Giordano M, Manice CS, Diamond R, Sewell TB, et al. : Epidemiology, Clinical Features, and Disease Severity in Patients With Coronavirus Disease 2019 (COVID-19) in a Children’s Hospital in New York City, New York. JAMA Pediatr 2020, 2019:e202430. In a case series of 50 children and adolescents hospitalized with COVID-19 infection at the New York-Presbyterian Morgan Stanley Children’s Hospital, authors found that obesity was the most common comorbidity in their cohort, the requirement for mechanical ventilation was most significantly correlated with obesity and obese patients over the age of 2 were more likely to require ventilation (the study definition of severe COVID-19). (**)
- 122.Quinti I, Lougaris V, Milito C, Cinetto F, Pecoraro A, Mezzaroma I, Mastroianni CM, Turriziani O, Bondioni MP, Filippini M, et al. : A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol 2020, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Berger NA: Young Adult Cancer: Influence of the Obesity Pandemic. Obesity 2018, 26:641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weihrauch-Blüher S, Schwarz P, Klusmann JH: Childhood obesity: increased risk for cardiometabolic disease and cancer in adulthood. Metabolism 2019, 92:147–152. [DOI] [PubMed] [Google Scholar]
- 125.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M: Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371:569–578. [DOI] [PubMed] [Google Scholar]
- 126.Woodall MJ, Neumann S, Campbell K, Pattison ST, Young SL: The effects of obesity on anti-cancer immunity and cancer immunotherapy. Cancers (Basel) 2020, 12:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M, et al. : Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol 2018, 19:310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ihara K: Immune checkpoint inhibitor therapy for pediatric cancers: A mini review of endocrine adverse events. Clin Pediatr Endocrinol 2019, 28:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang C, Austin F, Richard H, Idowu M, Williamson V, Sabato F, Ferreira-Gonzalez A, Turner SA: Lynch syndrome-associated ultra-hypermutated pediatric glioblastoma mimicking a constitutional mismatch repair deficiency syndrome. Cold Spring Harb Mol Case Stud 2019, 5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Davis KL, Fox E, Merchant MS, Reid JM, Kudgus RA, Liu X, Minard CG, Voss S, Berg SL, Weigel BJ, et al. : Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): a multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol 2020, 21:541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, Garudathri J, Raymond D, Poynter ME, Bunn JY, et al. : Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol 2011, 128:508–515.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Moulin CM, Marguti I, Peron JPS, Halpern A, Rizzo LV: Bariatric surgery reverses natural killer (NK) cell activity and NK-related cytokine synthesis impairment induced by morbid obesity. Obes Surg 2011, 21:112–118. [DOI] [PubMed] [Google Scholar]
- 133.Kelly AS, Ryder JR, Marlatt KL, Rudser KD, Jenkins T, and Thomas H. Inge: Changes in Inflammation, Oxidative Stress, and Adipokines Following Bariatric Surgery among Adolescents with Severe Obesity. Int J Obes 2016, 40:275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW, et al. : T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 2007, 115:1029–1038. [DOI] [PubMed] [Google Scholar]


