Abstract
Objective
To investigate the efficacy and safety of antidepressants for back and osteoarthritis pain compared with placebo.
Design
Systematic review and meta-analysis.
Data sources
Medline, Embase, Cochrane Central Register of Controlled Trials, CINAHL, International Pharmaceutical Abstracts, ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform from inception to 15 November and updated on 12 May 2020.
Eligibility criteria for study selection
Randomised controlled trials comparing the efficacy or safety, or both of any antidepressant drug with placebo (active or inert) in participants with low back or neck pain, sciatica, or hip or knee osteoarthritis.
Data extraction and synthesis
Two independent reviewers extracted data. Pain and disability were primary outcomes. Pain and disability scores were converted to a scale of 0 (no pain or disability) to 100 (worst pain or disability). A random effects model was used to calculate weighted mean differences and 95% confidence intervals. Safety (any adverse event, serious adverse events, and proportion of participants who withdrew from trials owing to adverse events) was a secondary outcome. Risk of bias was assessed with the Cochrane Collaboration’s tool and certainty of evidence with the grading of recommendations assessment, development and evaluation (GRADE) framework.
Results
33 trials (5318 participants) were included. Moderate certainty evidence showed that serotonin-noradrenaline reuptake inhibitors (SNRIs) reduced back pain (mean difference −5.30, 95% confidence interval −7.31 to −3.30) at 3-13 weeks and low certainty evidence that SNRIs reduced osteoarthritis pain (−9.72, −12.75 to −6.69) at 3-13 weeks. Very low certainty evidence showed that SNRIs reduced sciatica at two weeks or less (−18.60, −31.87 to −5.33) but not at 3-13 weeks (−17.50, −42.90 to 7.89). Low to very low certainty evidence showed that tricyclic antidepressants (TCAs) did not reduce sciatica at two weeks or less (−7.55, −18.25 to 3.15) but did at 3-13 weeks (−15.95, −31.52 to −0.39) and 3-12 months (−27.0, −36.11 to −17.89). Moderate certainty evidence showed that SNRIs reduced disability from back pain at 3-13 weeks (−3.55, −5.22 to −1.88) and disability due to osteoarthritis at two weeks or less (−5.10, −7.31 to −2.89), with low certainty evidence at 3-13 weeks (−6.07, −8.13 to −4.02). TCAs and other antidepressants did not reduce pain or disability from back pain.
Conclusion
Moderate certainty evidence shows that the effect of SNRIs on pain and disability scores is small and not clinically important for back pain, but a clinically important effect cannot be excluded for osteoarthritis. TCAs and SNRIs might be effective for sciatica, but the certainty of evidence ranged from low to very low.
Systematic review registration
PROSPERO CRD42020158521.
Introduction
Back pain (low back or neck pain with or without radicular symptoms) and osteoarthritis are leading causes of disability worldwide.1 The prevalence of low back pain and neck pain is 7.3% and 5.0%, respectively. Osteoarthritis related hip and knee symptoms affect 12% of the global population.2 3 In 2016, back pain and osteoarthritis pain accounted for $214.5bn (£161.0bn; €177.0bn) in healthcare spending in the US, with the highest expenditure for back pain, among all health conditions.4
Prescriptions for antidepressants are increasing worldwide for a range of indications.5 Among drugs associated with dependence and withdrawal, antidepressants are the most commonly prescribed medicines in the UK, with more people prescribed antidepressants (7.2 million) than prescribed opioid analgesics (5.6 million).6 The use of antidepressants to treat pain, especially chronic pain, is also common—antidepressants are the fourth most prescribed medicine for low back pain in the US. More than one quarter of Americans with chronic low back pain are prescribed an antidepressant within three months of a first diagnosis.7 In Quebec, Canada, the tricyclic antidepressant (TCA) amitriptyline and its active metabolite nortriptyline are commonly prescribed for pain, representing 48.4% and 57.4% of all prescriptions for these antidepressants, respectively.8 In the UK, 16% of prescriptions for antidepressants in children and adolescents are for pain, and from 2003 to 2014 prescriptions for antidepressants in these age groups almost tripled.9 The widespread use of antidepressants for pain also occurs in middle income countries. Amitriptyline is widely used in primary care in South Africa, mostly for osteoarthritis. This accounts for just over a quarter of all amitriptyline prescribed.10
Antidepressants are endorsed by most (75%) clinical practice guidelines for low back pain11 and by two recently published osteoarthritis guidelines.12 13 The American College of Physicians, for example, recommends the serotonin-noradrenaline reuptake inhibitor (SNRI) duloxetine for low back pain.14 The UK National Institute for Health and Care Excellence recommends amitriptyline or duloxetine as the preferred treatment for people with different forms of neuropathic pain, including back pain with radicular symptoms.15 Osteoarthritis guidelines, such as those from the Osteoarthritis Research Society International (OARSI) and the American College of Rheumatology recommend duloxetine for pain management.12 13 In the OARSI guideline, the focus is on people with osteoarthritis who have concomitant depression or widespread pain, or both. Evidence supporting the use of antidepressants is, however, uncertain. Systematic reviews of antidepressants for back pain and osteoarthritis have either not included several published trials,16 17 considered only one type of antidepressant (eg, duloxetine),18 or failed to assess the certainty of evidence.18 None of the existing reviews included unpublished records from trial registries. Reliance on published data alone has been shown to overestimate the efficacy of drug interventions for pain.19 To close this gap in knowledge, we systematically investigated the efficacy and safety of antidepressants in people with back pain (including sciatica) or hip or knee osteoarthritis.
Methods
Data sources and searches
The review protocol was prospectively registered on PROSPERO (CRD42020158521) and our findings are reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.20 We searched Medline, Embase, Cochrane Central Register of Controlled Trials, CINAHL, International Pharmaceutical Abstracts, ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform from inception to 15 November and updated the searches on 12 May 2020 (supplemental file 1). Two authors (GEF, MOK) independently screened records by titles and abstracts, and two authors (GEF, JZ) read full texts of potentially eligible studies to determine eligibility (see supplemental file 2 for a list of excluded trials with reasons). Any disagreements were resolved by consensus.
Eligibility criteria
We included randomised controlled trials that compared any antidepressant drug with placebo in participants with back pain (neck or low back pain with or without radicular symptoms) or hip or knee osteoarthritis, or both. Symptoms of any duration were included. Trials including drug combinations were eligible if the treatment contrast between groups was antidepressant versus placebo. The placebo comparator could be active (a substance that has no known effect on pain but might mimic the adverse effects of antidepressants) or inert (a substance that is not thought to have a therapeutic or adverse effect). We included reports published in peer reviewed journals as well as unpublished data posted on trial registry platforms (ClinicalTrials.gov and WHO International Clinical Trials Registry Platform). Trials that reported data on either pain, disability, or adverse events were included. No restrictions were placed on language or publication date. We excluded studies that included participants with serious spinal conditions (eg, fractures, cancer) and rheumatic conditions (eg, rheumatoid arthritis), unless these studies also included participants with back pain or hip or knee osteoarthritis, or a combination of these, and their data were reported separately. We considered studies to be eligible when participants received previous back or osteoarthritis surgery but excluded studies that evaluated immediate postoperative pain management (ie, surgery within past month). Abstracts from conferences were also excluded.
Data extraction
Two authors (GEF, JZ) independently extracted data. Whenever possible, for each outcome we extracted post-treatment means, standard deviations, and number of participants in each group. When post-treatment scores were not reported, we extracted data according to the hierarchy of between group differences and corresponding 95% confidence intervals at follow-up and then pre-treatment to post-treatment within group change scores. When a study did not report standard deviations, we used estimation methods recommended by the Cochrane handbook for systematic reviews of interventions.21 Cochrane’s RevMan calculator was used to estimate standard deviations.22 These methods were used to estimate standard deviations for pre-treatment to post-treatment within group change scores, and to estimate standard deviations for between group differences. Briefly, we used the standard error and number of participants in each group to estimate standard deviations from pre-treatment to post-treatment within group change scores. To estimate standard deviations from between group differences, we used P values, number of participants, and between group mean differences to obtain standard errors and thus obtain the standard deviation. For crossover trials, we followed guidance from the Cochrane handbook21 and extracted data from the pre-crossover and post-crossover periods as if the trial were a parallel trial, as this is a conservative approach. When data were not available in the published manuscript, we sought and, when available, extracted data on safety from the trial registry.
Outcomes
The primary outcomes were pain intensity and disability. Adverse events were a secondary outcome. Adverse events included the number of participants who experienced any adverse event (as defined by each study), experienced any serious adverse event (as defined by each study), and withdrew because of adverse effects.
Risk of bias and certainty of evidence
Two reviewers (GEF, MOK) rated risk of bias of trials using the Cochrane Collaboration risk of bias tool.23 Disagreements were resolved by consensus. We assessed the certainty of evidence using the grading of recommendations assessment, development and evaluation (GRADE) framework.24 Certainty of evidence refers to the confidence that the true effect lies in a particular range.25 The certainty of evidence was downgraded by one level if a serious flaw was present in the domains of limitations in study design, inconsistency, imprecision, and small study bias. We did not downgrade for indirectness because patients, interventions, and comparators were similar across comparisons (see supplemental file 3 for a description of the GRADE framework used). The certainty of evidence was then classified as high, moderate, low, or very low. High certainty means we are confident that the true effect lies close to that of the estimate of the effect. Moderate certainty means we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty means our confidence in the effect estimate is limited; the true effect might be substantially different from the estimate of the effect. Very low certainty means we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.25
Data synthesis and analysis
We classified follow-up times into two weeks or less, 3-13 weeks, 3-12 months, and more than 12 months. In studies with multiple time points, we extracted data from the time point closest to two weeks and three, six and 12 months. When trials had multiple treatment groups, we divided the number of participants in the placebo group by the number of treatment groups. For dichotomous outcomes, both the number of events and the sample size were divided by the number of treatment groups.21
To facilitate the interpretation of our results, we converted pain and disability scores to a common 0-100 scale, with 0 denoting no pain or disability and 100 denoting the worst possible pain or disability. This was done because benchmarks for clinically important differences in pain and disability are expressed in points, usually on a 0-100 scale, and not in proportions of a standard deviation.26 27 Raw scores were thus expressed as a percentage of the maximum possible score on that scale, as done in previous systematic reviews.16 28 29 Supplemental files 4 and 5 present details on the pain and disability measures used by studies, and conversion procedures. Mean differences (95% confidence intervals) were calculated for continuous outcome measures, and risk ratios (95% confidence intervals) were calculated for dichotomous outcomes. Statistical heterogeneity in each meta-analysis was determined by means of the I2 test. A random effects model was used across all comparisons. We grouped antidepressants based on their drug class. A pooled between group mean difference of 10 points (on a 0-100 scale) was considered by us to be the threshold for the smallest worthwhile effect for pain and disability.30 Pooled mean differences between groups below this threshold were considered clinically unimportant. This threshold has been used in other reviews of drug treatments for back pain,17 31 32 and it is also the recommended threshold for pain and disability in osteoarthritis.33 34 We used funnel plots to test for small study effects when at least 10 trials were available within a comparison.21 The Egger’s test was used to investigate small study effects. For comparisons when a funnel plot was available, we downgraded the certainty of evidence when the Egger’s test result was significant (two tailed P<0.05). When a funnel plot was not available, we downgraded the certainty of evidence if more than 25% of the participants were from small studies (<100 participants in each arm).21 For continuous outcomes, we calculated the mean difference between groups and the respective standard error in Comprehensive Meta-analysis V3 and entered these data in RevMan version 5.3 using the Generic inverse variance method to obtain forest plots. For dichotomous outcomes, we used the Mantel-Haenszel method in RevMan version 5.3.
Exploratory meta-regression
We performed meta-regression to explore the moderator effects of risk of bias (high risk of bias if at least one domain was classified as high risk of bias or more than half of the domains were classified as unclear), industry sponsor (yes or no), small study effects (<100 participants in each arm), depression listed as inclusion criteria (yes or no), and dose of antidepressant on pain. For this analysis we grouped all antidepressant classes together.
Patient and public involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for the design of the study owing to lack of funding to include them as partners in this review. No patients were asked to advise on interpretation or writing up of results. Results of this review will be disseminated to the relevant patient organisations.
Results
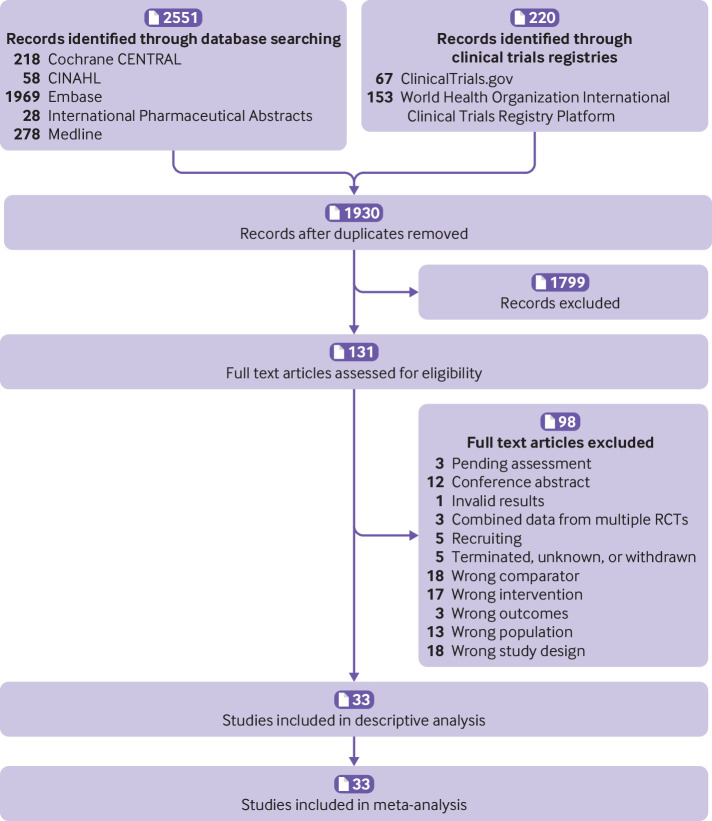
Overall, 2771 records were retrieved. Of these, 1930 records were screened after removal of duplicates and 1795 were excluded based on titles and abstracts. Of 131 potentially relevant trials screened for eligibility, 33 trials enrolling 5318 participants were included (fig 1). Most trials (n=28, 84.9%) used a parallel group design, whereas five (15.2%) used a crossover design with washout periods ranging from one week to two weeks.35 36 37 38 39 Fourteen trials were sponsored by industry,38 40 41 42 43 44 45 46 47 48 49 50 51 52 and sponsorship source was unclear in five trials.39 53 54 55 56 All but one trial reported data from participants with chronic pain.57 Only five trials restricted inclusion to participants with depression.45 53 54 55 58 In two other trials, between 40% and 50% of participants had depression.46 47 Others either excluded participants with depressive disorder or did not mention depression in the eligibility criteria. The median duration of the drug regimen was eight weeks. Six antidepressant drug classes were evaluated, including SNRIs (15 trials), TCAs (n=14 trials), serotonin reuptake inhibitors (SSRIs, n=3), noradrenaline-dopamine reuptake inhibitors (NDRIs, n=1), serotonin antagonist and reuptake inhibitors (SARIs, n=1), and tetracyclic antidepressants (n=1). Supplemental file 6 provides details of the included trials.
Fig 1.
Study flow diagram. RCTs=randomised controlled trials
For 26 of 33 trials, at least one domain was classified as high risk of bias (supplemental file 7). Twenty six trials were unclear in describing the methods used to conceal allocation and therefore were at unclear risk of selection bias. One trial was at high risk of performance bias owing to inadequate blinding of participants, and 13 were at unclear risk of performance bias. One trial was at high risk of detection bias owing to inadequate blinding of participants, and another 12 trials were at unclear risk of detection bias. Eighteen trials were at high risk of attrition bias. Five and 14 trials were at high and unclear risk of reporting bias, respectively.
Back pain
Nineteen trials (23 comparisons) determined the efficacy of antidepressants for back pain. Of these, 16 trials reported data for low back pain, one trial reported data for neck pain, and two trials reported data for low back and neck pain. Only one trial evaluated outcomes at 3-12 months. This trial investigated the efficacy of TCAs in participants with back pain. No trials evaluated outcomes at more than 12 months. Only pain outcomes were measured at two weeks or less of follow-up.
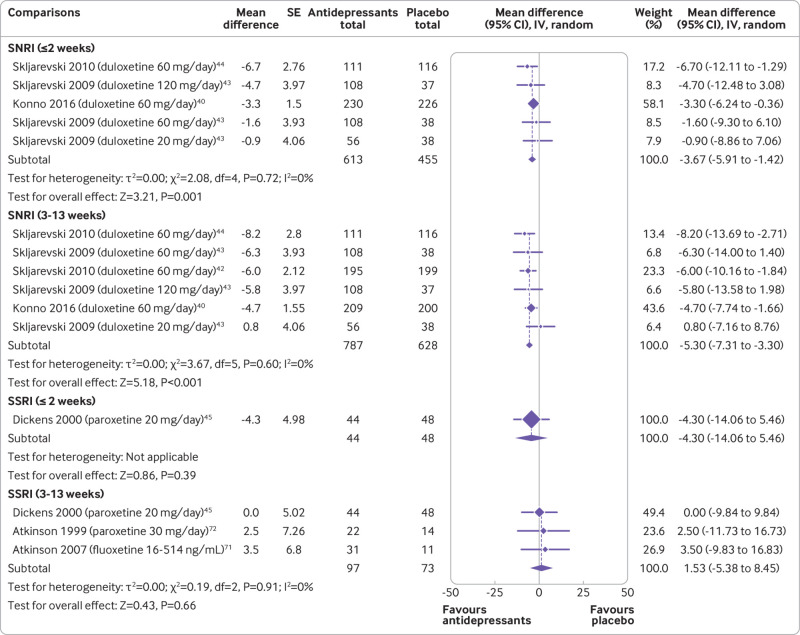
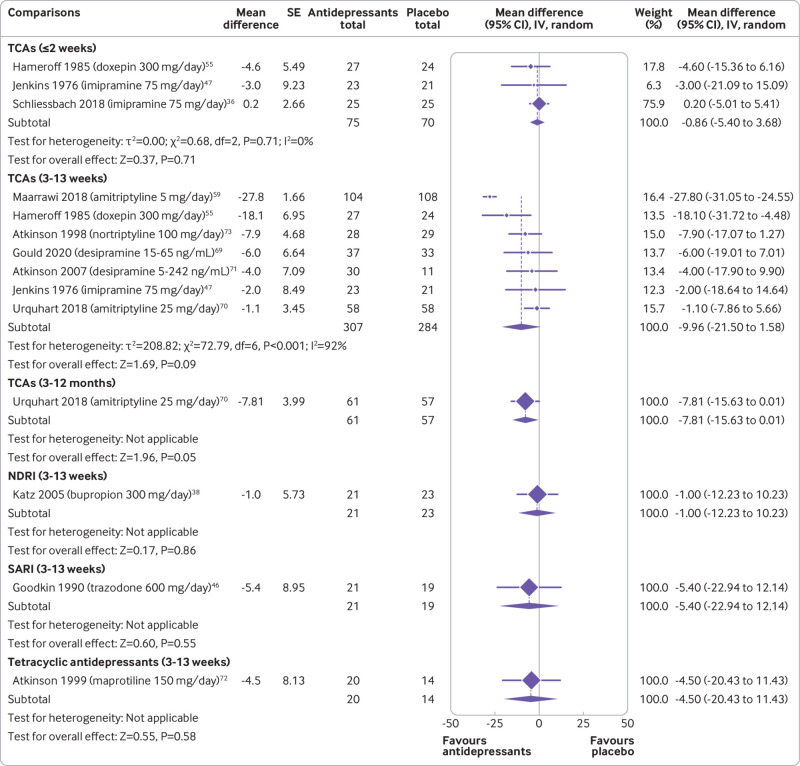
Moderate certainty evidence showed that SNRIs reduce pain at two weeks or less (mean difference −3.67, 95% confidence interval −5.91 to −1.42; three trials, 1068 participants) and 3-13 weeks (−5.30, −7.31 to −3.30; four trials, 1415 participants) (fig 2 and table 1). SNRIs were also shown to reduce disability at 3-13 weeks (−3.55, −5.22 to −1.88; four trials, 1423 participants; supplemental file 8 and table 2). The effect of SNRIs was small and below this review’s predetermined threshold of clinical importance. Low to very low certainty evidence showed that TCAs did not reduce pain at two weeks or less (−0.86, −5.40 to 3.68; three trials, 145 participants), 3-13 weeks (−9.96, −21.50 to 1.58; seven trials, 591 participants), and 3-12 months (−7.81, −15.63 to 0.01; one trial, 118 participants) (fig 3). Evidence ranging from low to very low certainty showed no benefit of a range of antidepressant classes, including SSRIs, tetracyclic antidepressants, SARIs, and NDRIs for pain and disability across follow-ups of two weeks or less, 3-13 weeks, and 3-12 months.
Fig 2.
Mean differences (95% confidence intervals) for pain in trials assessing the efficacy of antidepressants for back pain. Pain is expressed on a 0-100 scale. Studies are ordered by effect size. SE=standard error; IV=inverse variance; SNRI=serotonin-noradrenaline reuptake inhibitors; SSRI=selective serotonin reuptake inhibitors; TCA=tricyclic antidepressants; NDRI=noradrenaline-dopamine reuptake inhibitors; SARI=serotonin antagonist and reuptake inhibitors
Table 1.
Summary of findings and certainty of evidence for pain
| Summary of findings | Certainty of evidence | Certainty of evidence | ||||||
|---|---|---|---|---|---|---|---|---|
| No of participants (No of trials) | Mean difference (95% CI), 0-100 | Study design | Inconsistency | Imprecision | Small study effects | |||
| Back pain | ||||||||
| SNRI: | ||||||||
| ≤2 weeks | 1068 (3) | −3.67 (−5.91 to −1.42) | Downgraded* | Not downgraded | Not downgraded | Not downgraded | Moderate | |
| 3-13 weeks | 1415 (4) | −5.30 (−7.31 to −3.30) | Downgraded* | Not downgraded | Not downgraded | Not downgraded | Moderate | |
| SSRI: | ||||||||
| ≤2 weeks | 92 (1) | −4.30 (−14.06 to 5.46) | Downgraded* | Not downgraded | Not downgraded | Downgraded§ | Low | |
| 3-13 weeks | 170 (3) | 1.53 (−5.38 to 8.45) | Downgraded* | Not downgraded | Not downgraded | Downgraded§ | Low | |
| TCAs: | ||||||||
| ≤2 weeks | 145 (3) | −0.86 (−5.40 to 3.68) | Downgraded* | Not downgraded | Not downgraded | Downgraded§ | Low | |
| 3-13 weeks | 591 (7) | −9.96 (−21.50 to 1.58) | Downgraded* | Downgraded† | Not downgraded | Downgraded§ | Very low | |
| 3-12 months | 118 (1) | −7.81 (−15.63 to 0.01) | Downgraded* | Not downgraded | Not downgraded | Downgraded§ | Low | |
| NDRI: | ||||||||
| 3-13 weeks | 44 (1) | −1.0 (−12.23 to 10.23) | Downgraded* | Not downgraded | Not downgraded | Downgraded§ | Low | |
| SARI: | ||||||||
| 3-13 weeks | 40 (1) | −5.40 (−22.94 to 12.14) | Downgraded* | Not downgraded | Not downgraded | Downgraded§ | Low | |
| Tetracyclic antidepressants: | ||||||||
| 3-13 weeks | 34 (1) | −4.50 (−20.43 to 11.43) | Downgraded* | Not downgraded | Not downgraded | Downgraded§ | Low | |
| Sciatica | ||||||||
| SNRI: | ||||||||
| ≤2 weeks | 50 (1) | −18.60 (−31.87 to −5.33) | Downgraded* | Not downgraded | Downgraded‡ | Downgraded§ | Very low | |
| 3-13 weeks | 96 (3) | −17.50 (−42.90 to 7.89) | Downgraded* | Downgraded† | Downgraded‡ | Downgraded§ | Very low | |
| TCAs: | ||||||||
| ≤2 weeks | 94 (2) | −7.55 (−18.25 to 3.15) | Downgraded* | Not downgraded | Downgraded‡ | Downgraded§ | Very low | |
| 3-13 weeks | 114 (2) | −15.95 (−31.52 to −0.39) | Downgraded* | Downgraded† | Downgraded‡ | Downgraded§ | Very low | |
| 3-12 months | 60 (1) | −27.0 (−36.11 to −17.89) | Downgraded* | Not downgraded | Not downgraded | Downgraded§ | Low | |
| Osteoarthritis | ||||||||
| SNRI: | ||||||||
| ≤2 weeks | 1328 (4) | −4.66 (−6.28 to −3.04) | Downgraded* | Not downgraded | Not downgraded | Not downgraded | Moderate | |
| 3-13 weeks | 1941 (8) | −9.72 (−12.75 to −6.69) | Downgraded* | Downgraded† | Not downgraded | Not downgraded | Low | |
SNRI=serotonin-noradrenaline reuptake inhibitors; SSRI=selective serotonin reuptake inhibitors; TCA=tricyclic antidepressants; NDRI=noradrenaline-dopamine reuptake inhibitors.
Downgraded by one level because >25% of participants in this comparison were from studies at high risk of bias.
Downgraded by one level because heterogeneity (I2) >50%.
Downgraded by one level because the limits of the 95% confidence interval were 20 points different to smallest worthwhile effect.
Downgraded by one level owing to small study bias.
Table 2.
Summary of findings and certainty of evidence for disability
| Summary of findings | Certainty of evidence | Certainty of evidence | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of participants (No of trials) | Mean difference (95% CI), 0-100 |
Study design | Inconsistency | Imprecision | Small study effects | |||
| Back pain | ||||||||
| SNRI: | ||||||||
| 3-13 weeks | 1423 (4) | −3.55 (−5.22 to −1.88) | Downgraded* | Not downgraded | Not downgraded | Not downgraded | Moderate | |
| SSRI: | ||||||||
| 3-13 weeks | 1 (92) | −2.20 (−8.08 to 3.68) | Downgraded* | Not downgraded | Not downgraded | Downgraded§ | Low | |
| TCAs: | ||||||||
| 3-13 weeks | 439 (4) | −12.94 (−26.47 to 0.59) | Downgraded* | Downgraded† | Downgraded‡ | Downgraded§ | Very low | |
| 3-12 months | 118 (1) | −4.30 (−10.49 to 1.89) | Downgraded* | Not downgraded | Not downgraded | Downgraded§ | Low | |
| SARI: | ||||||||
| 3-13 weeks | 40 (1) | 2.60 (−6.79 to 11.99) | Downgraded* | Not downgraded | Not downgraded | Downgraded§ | Low | |
| Sciatica | ||||||||
| SNRI: | ||||||||
| 3-13 weeks | 8 (1) | −4.40 (−19.92 to 11.12) | Downgraded* | Not downgraded | Downgraded‡ | Downgraded§ | Very low | |
| TCAs: | ||||||||
| ≤2 weeks | 60 (1) | −5.00 (−12.23 to 2.23) | Downgraded* | Not downgraded | Not downgraded | Downgraded§ | Low | |
| 3-13 weeks | 116 (2) | −8.42 (18.18 to 1.35) | Downgraded* | Downgraded† | Not downgraded | Downgraded§ | Very low | |
| 3-12 months | 60 (1) | −20.0 (−27.74 to −12.26) | Downgraded* | Not downgraded | Downgraded‡ | Downgraded§ | Very low | |
| Osteoarthritis | ||||||||
| SNRI: | ||||||||
| ≤2 weeks | 353 (1) | −5.10 (−7.31 to −2.89) | Downgraded* | Not downgraded | Not downgraded | Not downgraded | Moderate | |
| 3-13 weeks | 1810 (7) | −6.07 (−8.13 to −4.02) | Downgraded* | Downgraded† | Not downgraded | Not downgraded | Low | |
SNRI=serotonin-noradrenaline reuptake inhibitors; SSRI=selective serotonin reuptake inhibitors; TCA=tricyclic antidepressants; NDRI=noradrenaline-dopamine reuptake inhibitors.
Downgraded by one level because >25% of participants in this comparison were from studies at high risk of bias.
Downgraded by one level because heterogeneity (I2) >50%.
Downgraded by one level because the limits of the 95% confidence interval were 20 points different to smallest worthwhile effect.
Downgraded by one level owing to small study bias.
Fig 3.
Mean differences (95% confidence intervals) for pain in trials assessing the efficacy of antidepressants for back pain. Pain is expressed on a 0-100 scale. Studies are ordered by effect size. SE=standard error; IV=inverse variance; TCA=tricyclic antidepressants; NDRI=noradrenaline-dopamine reuptake inhibitors; SARI=serotonin antagonist and reuptake inhibitors
A post hoc sensitivity analysis explored the effect of removing one trial from the pooled estimates for back pain.59 The participants in this trial had neck pain, received a dose of amitriptyline (5 mg/day) lower than the minimum dose recommended for pain relief (10-25 mg/day), and reported unexpectedly large improvements in pain and disability compared with other studies. That trial was also at high risk of attrition bias from lack of intention-to-treat analysis and a large proportion of participants lost to follow-up. The exclusion of this trial fully explained the heterogeneity for disability and heterogeneity was reduced from 92% to 7% for pain. After excluding this trial, TCAs were found to significantly reduce pain (−5.37, −9.93 to −0.80) and disability (−7.24, −11.25 to −3.22), but the effects were still small and below this review’s predetermined threshold for clinical importance.
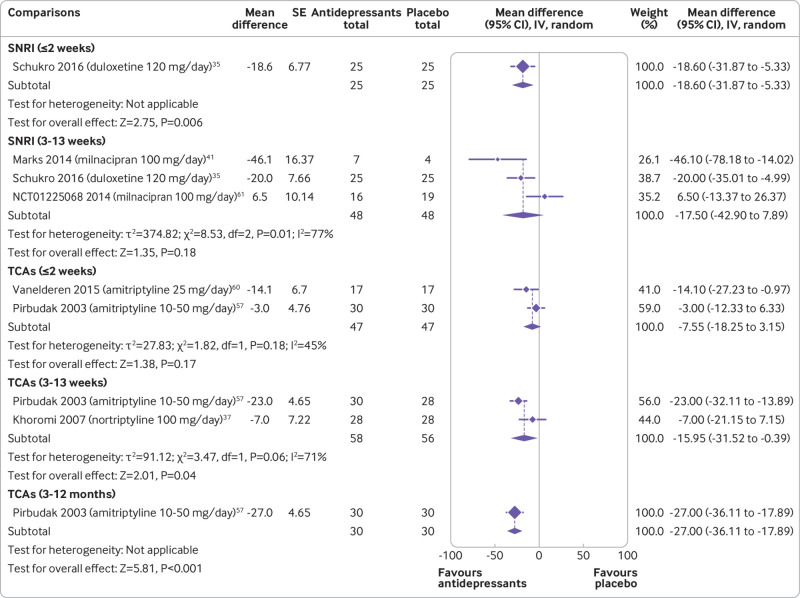
Sciatica
Six trials reported data for sciatica. No trials evaluated outcomes at more than 12 months. Very low certainty evidence showed that SNRIs reduce pain at two weeks or less (−18.60, −31.87 to −5.33; one trial, 50 participants) but not at 3-13 weeks (−17.50, −42.90 to 7.89; three trials, 96 participants). Low to very low certainty evidence showed that TCAs did not reduce pain at two weeks or less (−7.55, −18.25 to 3.15; two trials, 94 participants) but did at 3-13 weeks (−15.95, −31.52 to −0.39; two trials, 114 participants) and 3-12 months (−27.0, −36.11 to −17.89; one trial, 60 participants) follow-ups (fig 4 and table 1). Tricyclic antidepressants did not reduce disability at two weeks or less and at 3-13 weeks but did at 3-12 months (supplemental file 9 and table 2).
Fig 4.
Mean differences (95% confidence intervals) for pain in trials assessing the efficacy of antidepressants for sciatica. Pain is expressed on a 0-100 scale. Studies are ordered by effect size. SE=standard error; IV=inverse variance; SNRI=serotonin-noradrenaline reuptake inhibitors; SSRI=selective serotonin reuptake inhibitors; TCA=tricyclic antidepressants; NDRI=noradrenaline-dopamine reuptake inhibitors; SARI=serotonin antagonist and reuptake inhibitors
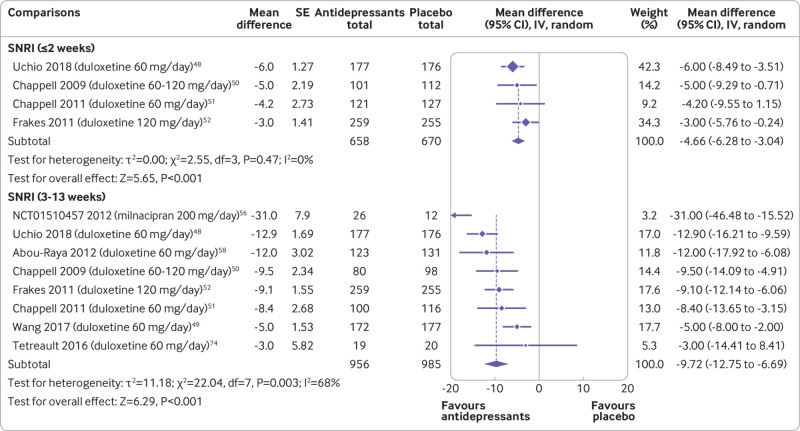
Osteoarthritis
Eight trials (eight comparisons) evaluated the efficacy of antidepressants in participants with knee osteoarthritis. All trials were of SNRIs. None of the osteoarthritis trials included participants with hip osteoarthritis or evaluated outcomes at 3-12 months or more than 12 months. Moderate certainty evidence showed that SNRIs reduce pain at two weeks or less (−4.66, −6.28 to −3.04; four trials, 1328 participants) and low certainty evidence that SNRIs reduce pain at 3-13 weeks (−9.72, −12.75 to −6.69; eight trials, 1941 participants) (fig 5 and table 1). Low certainty evidence showed that SNRIs reduce disability at two weeks or less (−5.10, −7.31 to −2.89; one trial, 353 participants) and 3-13 weeks (−6.07, −8.13 to −4.02; seven trials, 1810 participants) of follow-up (supplemental file 10 and table 2). The effect of SNRIs was small and below this review’s predetermined threshold of clinical importance—however, the lower limit of the confidence interval did contain clinically important effects for pain, but not for disability.
Fig 5.
Mean differences (95% confidence intervals) for pain in trials assessing the efficacy of antidepressants for osteoarthritis. Pain is expressed on a 0-100 scale. Studies are ordered by effect size. SE=standard error; IV=inverse variance; SNRI=serotonin-noradrenaline reuptake inhibitors; SSRI=selective serotonin reuptake inhibitors; TCA=tricyclic antidepressants; NDRI=noradrenaline-dopamine reuptake inhibitors; SARI=serotonin antagonist and reuptake inhibitors
Safety
Twenty one trials (25 comparisons) enrolling 4107 participants determined the safety of antidepressants for back pain and osteoarthritis. The type and reporting of adverse events varied noticeably across trials. Nausea was reported as the most prevalent adverse event in eight trials.43 44 50 51 52 56 60 61
Low certainty evidence showed that SNRIs increase the risk of any adverse event (62.5% v 49.7%; relative risk 1.23, 95% confidence interval 1.16 to 1.30; 13 trials, 3447 participants), but not serious adverse events (1.6% v 1.3%; 1.12, 0.61 to 2.07; 10 trials, 3309 participants) (supplemental files 11and 12 and table 3). Participants receiving SNRIs were also more likely to drop out of the study because of adverse events (12.4% v 5.3%; 2.16, 1.71 to 2.73) (supplemental file 13 and table 3). Supplemental files 14 to 17 present funnel plots assessing small study effects in comparisons with more than 10 trials. Although other antidepressants, such as TCAs, SSRIs, tetracyclic antidepressants, NDRIs, and SARIs did not seem to increase the risk of adverse events or drop-outs owing to adverse events, the limited number of trials and large uncertainty around the risk estimates limit the ability of this systematic review and meta-analysis to determine the safety of these classes of antidepressants for back pain, sciatica, and osteoarthritis.
Table 3.
Summary of findings and quality of evidence for safety
| Summary of findings | Certainty of evidence | Certainty of evidence | ||||||
|---|---|---|---|---|---|---|---|---|
| % events antidepressants/placebo (No of trials) | Relative risk (95% CI) | Study design | Inconsistency | Imprecision | Small study effects | |||
| Any adverse event: | ||||||||
| SNRI | 62.5/49.7 (13) | 1.23 (1.16 to 1.30) | Downgraded* | Not downgraded | Downgraded‡ | Not downgraded | Low | |
| SSRI | 67.9/66.6 (2) | 1.53 (0.19 to 12.61) | Not downgraded | Downgraded† | Downgraded‡ | Downgraded§ | Very low | |
| TCAs | 22.4/13.2 (8) | 1.49 (0.95 to 2.34) | Not downgraded | Not downgraded | Downgraded‡ | Downgraded§ | Low | |
| Tetracyclic antidepressants | 90/93.8 (1) | 0.96 (0.79 to 1.16) | Downgraded* | Not downgraded | Not downgraded | Downgraded§ | Low | |
| NDRI | 40/14.3 (1) | 2.80 (1.30 to 6.02) | Downgraded* | Not downgraded | Downgraded‡ | Not downgraded | Low | |
| Serious adverse events: | ||||||||
| SNRI | 1.6/1.3 (10) | 1.12 (0.61 to 2.07) | Downgraded* | Not downgraded | Downgraded‡ | Not downgraded | ||
| TCAs | 2.6/0 (1) | 2.62 (0.11 to 62.10) | Downgraded* | Not downgraded | Downgraded‡ | Downgraded§ | Very low | |
| NDRI | 4/2 (1) | 1.96 (0.18 to 20.92) | Downgraded* | Not downgraded | Downgraded‡ | Downgraded§ | Very low | |
| SARI | 13.6/15 (1) | 0.91 (0.21 to 4.0) | Downgraded* | Not downgraded | Downgraded‡ | Downgraded§ | Very low | |
| Risk of drop-out owing to adverse events: | ||||||||
| SNRI | 12.4/5.3 (12) | 2.16 (1.71 to 2.73) | Downgraded* | Not downgraded | Not downgraded | Not downgraded | Moderate | |
| SSRI | 17/6.5 (2) | 2.36 (0.39 to 14.28) | Downgraded* | Not downgraded | Downgraded‡ | Downgraded§ | Very low | |
| TCAs | 10.8/4.6 (11) | 1.48 (0.88 to 2.50) | Downgraded* | Not downgraded | Downgraded‡ | Not downgraded | Low | |
| NDRI | 6/0 (1) | 6.86 (0.36 to 129.48) | Downgraded* | Not downgraded | Downgraded‡ | Downgraded§ | Very low | |
| SARI | 13.6/5 (1) | 2.73 (0.31 to 24.14) | Downgraded* | Not downgraded | Downgraded‡ | Downgraded‡ | Very low | |
| Tetracyclic antidepressants | 17.6/5.5 (1) | 3.18 (0.41 to 24.39) | Downgraded* | Not downgraded | Downgraded‡ | Downgraded§ | Very low | |
SNRI=serotonin-noradrenaline reuptake inhibitors; SSRI=selective serotonin reuptake inhibitors; TCA=tricyclic antidepressants; NDRI=noradrenaline-dopamine reuptake inhibitors.
Downgraded by one level because >25% of participants in this comparison were from studies at high risk of bias.
Downgraded by one level because the 95% confidence interval include appreciable harm (ie, 95% confidence interval >1.25).
Exploratory sensitivity analyses
No interaction was found between risk of bias, study size, industry sponsorship, depression diagnosis, and daily dosage of duloxetine and treatment effects. The meta-regression analysis of dose-response effects of duloxetine in three different doses (20, 60, and 120 mg/day) showed no statistically significant difference between dose intensities (P=0.13). For all doses of duloxetine, point estimates of change in pain scores were below the threshold of clinical importance, although for 60 mg/day and 120 mg/day the lower limits of the confidence interval could suggest clinically important effects (supplemental file 18).
Discussion
We found moderate certainty evidence that SNRIs reduce pain and disability in people with back pain up to three months, but these effects are unlikely to be clinically important. For osteoarthritis, we found moderate certainty evidence that SNRIs reduce pain and disability up to three months, and a clinically important effect on pain cannot be excluded. Low certainty evidence showed that TCAs were ineffective for back pain and related disability. Tricyclic antidepressants and SNRIs might reduce pain in people with sciatica. In our review, only SNRIs statistically significantly increased the risk of adverse events. However, the number of studies evaluating the safety of other antidepressant classes was small, trials were underpowered to detect harm, and the certainty of evidence ranged from low to very low.
Strengths and weaknesses of this study
Our review has several strengths. We registered this review prospectively and performed a comprehensive literature search, including searches on clinical trial registries. We also complied with the PRISMA statement.20 The threshold for clinical importance (≥10 points on a 0-100 scale) used in our review was prespecified and has been widely used in the literature on back pain17 31 32 and osteoarthritis.33 34 We have also provided clinically interpretable effect estimates on a 0-100 scale, whereas previous reviews had presented effect sizes in terms of units of standard deviation.17 18
Our review has limitations. Uncertainty in the effects for sciatica and safety outcomes was noticeable. Also, we were not able to explore a dose-response relation for most antidepressants because of the low number of studies and varied doses. Nevertheless, we were able to conduct such an analysis for duloxetine (14 trials) and found that 60 mg/day produced similar effects on pain to 120 mg/day. Finally, although we searched two comprehensive clinical trial registries (ClinicalTrials.gov and WHO International Clinical Trials Registry Platform), we might have missed trials that were conducted before these registries became active (2000 for ClinicalTrials.gov) and therefore were not registered. We identified two trials in these registries that had already been completed but without results available.62 63 As the authors did not reply to our requests for data, we cannot completely rule out selective reporting or publication bias.
Evidence update
Our review updates the evidence for back pain, sciatica, and osteoarthritis. For example, we included 25 trials (n=2955 participants) for back pain and sciatica, whereas the most recent review included 16 trials.17 Our findings are in accordance with those from a previous review of drug treatments for chronic low back pain, which found TCAs and SSRIs to be ineffective and SNRIs to be effective, although the effects were small.17 Our safety analyses showed that SNRIs statistically significantly increased the risk of any adverse event. The increased risk of adverse events with SNRIs had already been described in a review of osteoarthritis18 and chronic pain64 but not chronic low back pain.17
For this review we pooled data for TCAs and SNRIs for sciatica. Across pain and disability outcomes, TCAs were effective in three of six estimates, whereas SNRIs were effective in one of four estimates. In instances when these drugs were more effective than placebo, the effects exceeded our prespecified threshold for clinical importance. However, these estimates were based on less certain evidence. The low to very low certainty of evidence described in our review contrasts with a recent review of drug treatment for neuropathic pain in adults, which recommends TCAs and SNRIs as preferred treatment for people with neuropathic pain based on strong evidence.65
Our review also updates the evidence for osteoarthritis. We pooled data from eight osteoarthritis trials whereas a previous review only pooled five trials.18 We also explored the effect of dose on treatment effect estimates for back pain and osteoarthritis. We showed that the effects of duloxetine (an SNRI) were similar regardless of whether the dose used in the trials was 20, 60, or 120 mg/day.
Meaning of the study
Although the observed effect of SNRIs in reducing back pain and related disability was statistically significant, the magnitude of such effects was too small to be considered clinically important. Despite all the studies examining the effects of SNRIs for back pain being sponsored by industry,40 42 43 44 the confidence intervals around the effect estimate were narrow enough and did not include clinically important benefits, which further strengthens our confidence in the results.
Caution is needed in interpreting our findings for sciatica. All sciatica trials were small, had imprecise estimates, and were at high risk of bias, which reduced the certainty of evidence to low and very low. This level of uncertainty indicates that the true estimate of effect of TCAs and SNRIs for sciatica is likely to be substantially different from what we estimated in our review.25
For osteoarthritis, although the point estimate was below our prespecified threshold of clinical importance, the lower limit of the confidence interval contains clinically important effects at 3-13 weeks, but not at two weeks or less. Therefore, a clinically important benefit of SNRI in people with osteoarthritis cannot be excluded.66 Six out of eight trials that investigated the efficacy of SNRI for osteoarthritis were also sponsored by industry.48 49 50 51 52 58
Participants with a diagnosis of depression did not benefit more from antidepressants for pain than those without depression. In both subgroups, improvements were below the threshold for clinically important effects. Furthermore, none of the trials that contributed data for the subgroup with depression were for osteoarthritis. The 2019 OARSI guideline recommends antidepressants (duloxetine) for knee osteoarthritis comorbid with depression or widespread pain disorders, or both.12 Some of the trials included in our review explored the indirect effect of duloxetine on depression and its role in mediating the effect on pain with path analysis.40 50 51 However, these trials excluded participants with major depressive disorder, and participants had low average scores (around 5 points51) on the Beck depression inventory II, which were within the minimal range and below the proposed thresholds for diagnosing depression.67
How this study could promote better decisions
The UK68 and US14 guidelines for back pain provide conflicting recommendations on use of SNRIs. Our review shows that although these medicines are effective, the effect is small and unlikely to be considered clinically important by most patients. Our review also showed that about two thirds of patients using SNRIs experience adverse events. We would encourage clinicians to share all this information about SNRIs with patients to allow them to make an informed decision.
The low to very low certainty of evidence does not allow any firm recommendations in favour or against other classes of antidepressants, such as SSRIs, NDRIs, SARIs, and tetracyclic antidepressants for back pain. Nevertheless, our review provides some evidence that neither of these antidepressant classes are effective for back pain and therefore should not be used.
Current guidelines for neuropathic pain recommend antidepressants such as duloxetine (SNRI) and amitriptyline (TCA) as preferred treatment.15 In our review, despite the potentially clinically important benefits of SNRIs and TCAs for sciatica observed in some comparisons, the low to very low certainty of evidence and the lack of efficacy across several time points for pain and disability mean that evidence is insufficient to confidently guide the use of these drugs for sciatica.
Unanswered questions and future research
Large, definitive trials free of industry ties are urgently needed to evaluate the efficacy of antidepressants. In our review, all but one trial59 with sample sizes of more than 100 participants in each group were sponsored by industry. New trials will be particularly relevant when testing the efficacy of TCAs and SNRIs in people with sciatica, where clinically important benefits might exist; and in osteoarthritis, where clinically important benefits cannot be excluded but most of the data come from industry sponsored trials.48 49 50 51 52 58
The long term effects of antidepressants prescribed for chronic pain conditions is not well known. Trials of chronic pain generally measure safety outcomes for a limited time (3-35 weeks)64 and therefore do not capture long term effects known to affect those taking antidepressants, such as severe withdrawal symptoms.6
Conclusions
Moderate certainty evidence shows that the effect of SNRIs on pain and disability scores is small and not clinically important for back pain, but a clinically important effect cannot be excluded for osteoarthritis. Tricyclic antidepressants and SNRIs might be effective for sciatica, but the certainty of evidence ranged from low to very low. The risk of adverse events but not serious adverse events is slightly increased with SNRIs, although the certainty of the evidence was low. Large, definitive randomised trials that are free of industry ties are urgently needed to resolve uncertainties about the efficacy of antidepressants for sciatica and osteoarthritis highlighted by this review.
What is already known on this topic
Antidepressants are widely used for the treatment of back pain (with and without radicular symptoms) and hip and knee osteoarthritis
Most clinical practice guidelines recommend antidepressants for these conditions
Evidence supporting the use of antidepressants for back pain and hip and knee osteoarthritis is uncertain
What this study adds
Moderate certainty evidence shows that serotonin-noradrenaline reuptake inhibitors (SNRIs) offer a small, non-clinically relevant benefit for people with back pain and osteoarthritis
SNRIs and tricyclic antidepressants might provide clinically important benefits for sciatica, but the certainty of evidence is low to very low
Only SNRIs increased the risk of adverse events; however, the number of studies evaluating the safety of other antidepressant classes was small, trials were underpowered to detect harm, and the certainty of evidence ranged from low to very low
Web extra.
Extra material supplied by authors
Supplementary information: files 1-18
Contributors: GEF, AJM, C-WCL, JRZ, MOK, CA-S, and CGM designed the review protocol. GF, JZ, and MK developed the search strategy and selected studies. GEF, JRZ, MOK, and CA-S extracted data. GEF analysed the data. GEF drafted the manuscript and JRZ, MOK, and CA-S contributed to the drafting of the review. AJM, C-WCL, and CGM revised the manuscript critically for important intellectual content. All authors approved the final version of the article. All authors had access to all the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. GF is guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. GEF holds a PhD scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil. C-WCL is supported by a National Health and Medical Research Council (NHMRC) fellowship (APP1061400). CGM is supported by a principal research fellowship from NHMRC (APP1103022) as well as a programme grant (APP1113532) and Centre for Research Excellence grant (APP1134856).
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; support from the following organisations that may have an interest in the submitted work in the previous three years: GlaxoSmithKline (postgraduate scholarship); Pfizer (investigational product for two investigator initiated NHMRC funded trials); Flexeze (provision of heat wraps at no cost for an investigator initiated trial); no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: No additional data available.
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: We will disseminate our findings to patient organisations and media outlets.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789-858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1323-30. 10.1136/annrheumdis-2013-204763 [DOI] [PubMed] [Google Scholar]
- 3.Hartvigsen J, Hancock MJ, Kongsted A, et al. Lancet Low Back Pain Series Working Group . What low back pain is and why we need to pay attention. Lancet 2018;391:2356-67. 10.1016/S0140-6736(18)30480-X [DOI] [PubMed] [Google Scholar]
- 4.Dieleman JL, Cao J, Chapin A, et al. US Health Care Spending by Payer and Health Condition, 1996-2016. JAMA 2020;323:863-84. 10.1001/jama.2020.0734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.OECD. Antidepressant drugs consumption, 2000 and 2015 (or nearest year). 2017.
- 6.Marsden J, White M, Annand F, et al. Medicines associated with dependence or withdrawal: a mixed-methods public health review and national database study in England. Lancet Psychiatry 2019;6:935-50. 10.1016/S2215-0366(19)30331-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivanova JI, Birnbaum HG, Schiller M, Kantor E, Johnstone BM, Swindle RW. Real-world practice patterns, health-care utilization, and costs in patients with low back pain: the long road to guideline-concordant care. Spine J 2011;11:622-32. 10.1016/j.spinee.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 8.Wong J, Motulsky A, Abrahamowicz M, Eguale T, Buckeridge DL, Tamblyn R. Off-label indications for antidepressants in primary care: descriptive study of prescriptions from an indication based electronic prescribing system. BMJ 2017;356:j603. 10.1136/bmj.j603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.John A, Marchant AL, Fone DL, et al. Recent trends in primary-care antidepressant prescribing to children and young people: an e-cohort study. Psychol Med 2016;46:3315-27. 10.1017/S0033291716002099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coetzee R, Johnson Y, van Niekerk J, Namane M. Amitriptyline prescribing in public sector healthcare facilities in the Western Cape, South Africa. PLoS One 2020;15:e0231675. 10.1371/journal.pone.0231675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliveira CB, Maher CG, Pinto RZ, et al. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J 2018;27:2791-803. 10.1007/s00586-018-5673-2 [DOI] [PubMed] [Google Scholar]
- 12.Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019;27:1578-89. 10.1016/j.joca.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 13.Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol 2020;72:220-33. 10.1002/art.41142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qaseem A, Wilt TJ, McLean RM, Forciea MA, Clinical Guidelines Committee of the American College of Physicians . Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med 2017;166:514-30. 10.7326/M16-2367 [DOI] [PubMed] [Google Scholar]
- 15.National Institute for Health and Care Excellence. Neuropathic pain in adults: pharmacological management in non-specialist settings. Clinical guideline [CG173]. 2013. https://www.nice.org.uk/guidance/cg173.
- 16.Pinto RZ, Maher CG, Ferreira ML, et al. Drugs for relief of pain in patients with sciatica: systematic review and meta-analysis. BMJ 2012;344:e497. 10.1136/bmj.e497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou R, Deyo R, Friedly J, et al. Systemic Pharmacologic Therapies for Low Back Pain: A Systematic Review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 2017;166:480-92. 10.7326/M16-2458 [DOI] [PubMed] [Google Scholar]
- 18.Osani MC, Bannuru RR. Efficacy and safety of duloxetine in osteoarthritis: a systematic review and meta-analysis. Korean J Intern Med 2019;34:966-73. 10.3904/kjim.2018.460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagg MK, O’Hagan E, Zahara P, et al. Systematic reviews that include only published data may overestimate the effectiveness of analgesic medicines for low back pain: a systematic review and meta-analysis. J Clin Epidemiol 2020;124:149-59. 10.1016/j.jclinepi.2019.12.006 [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Thomas J, Chandler J, et al. (eds). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drahota A, Beller E. RevMan calculator. https://training.cochrane.org/resource/revman-calculator. Published 2020. Accessed 20 April 2020.
- 23.Higgins JP, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group. Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hultcrantz M, Rind D, Akl EA, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol 2017;87:4-13. 10.1016/j.jclinepi.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9:105-21. 10.1016/j.jpain.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 27.Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976) 2008;33:90-4. 10.1097/BRS.0b013e31815e3a10 [DOI] [PubMed] [Google Scholar]
- 28.Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ 2015;350:h1225. 10.1136/bmj.h1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdel Shaheed C, Maher CG, Buchbinder R, et al. Efficacy and harms of orally, intramuscularly or intravenously administered glucocorticoids for sciatica: A systematic review and meta-analysis. Eur J Pain 2020;24:518-35. 10.1002/ejp.1505 [DOI] [PubMed] [Google Scholar]
- 30.Ferreira ML, Herbert RD, Ferreira PH, et al. The smallest worthwhile effect of nonsteroidal anti-inflammatory drugs and physiotherapy for chronic low back pain: a benefit-harm trade-off study. J Clin Epidemiol 2013;66:1397-404. 10.1016/j.jclinepi.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 31.Machado GC, Maher CG, Ferreira PH, Day RO, Pinheiro MB, Ferreira ML. Non-steroidal anti-inflammatory drugs for spinal pain: a systematic review and meta-analysis. Ann Rheum Dis 2017;76:1269-78. 10.1136/annrheumdis-2016-210597 [DOI] [PubMed] [Google Scholar]
- 32.Abdel Shaheed C, Maher CG, Williams KA, Day R, McLachlan AJ. Efficacy, Tolerability, and Dose-Dependent Effects of Opioid Analgesics for Low Back Pain: A Systematic Review and Meta-analysis. JAMA Intern Med 2016;176:958-68. 10.1001/jamainternmed.2016.1251 [DOI] [PubMed] [Google Scholar]
- 33.Reginster JY, Reiter-Niesert S, Bruyère O, et al. Recommendations for an update of the 2010 European regulatory guideline on clinical investigation of medicinal products used in the treatment of osteoarthritis and reflections about related clinically relevant outcomes: expert consensus statement. Osteoarthritis Cartilage 2015;23:2086-93. 10.1016/j.joca.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 34.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833-40. [PubMed] [Google Scholar]
- 35.Schukro RP, Oehmke MJ, Geroldinger A, Heinze G, Kress HG, Pramhas S. Efficacy of Duloxetine in Chronic Low Back Pain with a Neuropathic Component: A Randomized, Double-blind, Placebo-controlled Crossover Trial. Anesthesiology 2016;124:150-8. 10.1097/ALN.0000000000000902 [DOI] [PubMed] [Google Scholar]
- 36.Schliessbach J, Siegenthaler A, Bütikofer L, et al. Effect of single-dose imipramine on chronic low-back and experimental pain. A randomized controlled trial. PLoS One 2018;13:e0195776. 10.1371/journal.pone.0195776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khoromi S, Cui L, Nackers L, Max MB. Morphine, nortriptyline and their combination vs. placebo in patients with chronic lumbar root pain. Pain 2007;130:66-75. 10.1016/j.pain.2006.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katz J, Pennella-Vaughan J, Hetzel RD, Kanazi GE, Dworkin RH. A randomized, placebo-controlled trial of bupropion sustained release in chronic low back pain. J Pain 2005;6:656-61. 10.1016/j.jpain.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 39.Pheasant H, Bursk A, Goldfarb J, Azen SP, Weiss JN, Borelli L. Amitriptyline and chronic low-back pain. A randomized double-blind crossover study. Spine (Phila Pa 1976) 1983;8:552-7. 10.1097/00007632-198307000-00012 [DOI] [PubMed] [Google Scholar]
- 40.Konno S, Oda N, Ochiai T, Alev L. Randomized, Double-blind, Placebo-controlled Phase III Trial of Duloxetine Monotherapy in Japanese Patients With Chronic Low Back Pain. Spine (Phila Pa 1976) 2016;41:1709-17. 10.1097/BRS.0000000000001707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marks DM, Pae CU, Patkar AA. A double-blind, placebo-controlled, parallel-group pilot study of milnacipran for chronic radicular pain (sciatica) associated with lumbosacral disc disease. Prim Care Companion CNS Disord 2014;16:16. 10.4088/PCC.14m01658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skljarevski V, Zhang S, Desaiah D, et al. Duloxetine versus placebo in patients with chronic low back pain: a 12-week, fixed-dose, randomized, double-blind trial. J Pain 2010;11:1282-90. 10.1016/j.jpain.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 43.Skljarevski V, Ossanna M, Liu-Seifert H, et al. A double-blind, randomized trial of duloxetine versus placebo in the management of chronic low back pain. Eur J Neurol 2009;16:1041-8. 10.1111/j.1468-1331.2009.02648.x [DOI] [PubMed] [Google Scholar]
- 44.Skljarevski V, Desaiah D, Liu-Seifert H, et al. Efficacy and safety of duloxetine in patients with chronic low back pain. Spine (Phila Pa 1976) 2010;35:E578-85. 10.1097/BRS.0b013e3181d3cef6 [DOI] [PubMed] [Google Scholar]
- 45.Dickens C, Jayson M, Sutton C, Creed F. The relationship between pain and depression in a trial using paroxetine in sufferers of chronic low back pain. Psychosomatics 2000;41:490-9. 10.1176/appi.psy.41.6.490 [DOI] [PubMed] [Google Scholar]
- 46.Goodkin K, Gullion CM, Agras WS. A randomized, double-blind, placebo-controlled trial of trazodone hydrochloride in chronic low back pain syndrome. J Clin Psychopharmacol 1990;10:269-78. 10.1097/00004714-199008000-00006 [DOI] [PubMed] [Google Scholar]
- 47.Jenkins DG, Ebbutt AF, Evans CD. Tofranil in the treatment of low back pain. J Int Med Res 1976;4(Suppl):28-40. [PubMed] [Google Scholar]
- 48.Uchio Y, Enomoto H, Alev L, et al. A randomized, double-blind, placebo-controlled Phase III trial of duloxetine in Japanese patients with knee pain due to osteoarthritis. J Pain Res 2018;11:809-21. 10.2147/JPR.S164128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang G, Bi L, Li X, et al. Efficacy and safety of duloxetine in Chinese patients with chronic pain due to osteoarthritis: a randomized, double-blind, placebo-controlled study. Osteoarthritis Cartilage 2017;25:832-8. 10.1016/j.joca.2016.12.025 [DOI] [PubMed] [Google Scholar]
- 50.Chappell AS, Ossanna MJ, Liu-Seifert H, et al. Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: a 13-week, randomized, placebo-controlled trial. Pain 2009;146:253-60. 10.1016/j.pain.2009.06.024 [DOI] [PubMed] [Google Scholar]
- 51.Chappell AS, Desaiah D, Liu-Seifert H, et al. A double-blind, randomized, placebo-controlled study of the efficacy and safety of duloxetine for the treatment of chronic pain due to osteoarthritis of the knee. Pain Pract 2011;11:33-41. 10.1111/j.1533-2500.2010.00401.x [DOI] [PubMed] [Google Scholar]
- 52.Frakes EP, Risser RC, Ball TD, Hochberg MC, Wohlreich MM. Duloxetine added to oral nonsteroidal anti-inflammatory drugs for treatment of knee pain due to osteoarthritis: results of a randomized, double-blind, placebo-controlled trial. Curr Med Res Opin 2011;27:2361-72. 10.1185/03007995.2011.633502 [DOI] [PubMed] [Google Scholar]
- 53.Alcoff J, Jones E, Rust P, Newman R. Controlled trial of imipramine for chronic low back pain. J Fam Pract 1982;14:841-6. [PubMed] [Google Scholar]
- 54.Hameroff SR, Cork RC, Scherer K, et al. Doxepin effects on chronic pain, depression and plasma opioids. J Clin Psychiatry 1982;43:22-7. [PubMed] [Google Scholar]
- 55.Hameroff SR, Cork RC, Weiss JL, Crago BR, Davis TP. Doxepin Effects on Chronic Pain and Depression: A Controlled Study. Clin J Pain 1985;1:171-6 10.1097/00002508-198501030-00008. [DOI] [PubMed] [Google Scholar]
- 56.N H. Milnacipran for Chronic Pain in Knee Osteoarthritis (KOA) (NCT01510457) 2012.
- 57.Pirbudak LKG, Satana T. Epidural steroid injection and amitriptyline in the management of acute low back pain originating from lumbar disc herniation. Joint Dis Rel Surg. 2003;14:89-93. [Google Scholar]
- 58.Abou-Raya S, Abou-Raya A, Helmii M. Duloxetine for the management of pain in older adults with knee osteoarthritis: randomised placebo-controlled trial. Age Ageing 2012;41:646-52. 10.1093/ageing/afs072 [DOI] [PubMed] [Google Scholar]
- 59.Maarrawi J, Abdel Hay J, Kobaiter-Maarrawi S, Tabet P, Peyron R, Garcia-Larrea L. Randomized double-blind controlled study of bedtime low-dose amitriptyline in chronic neck pain. Eur J Pain 2018;22:1180-7. 10.1002/ejp.1206 [DOI] [PubMed] [Google Scholar]
- 60.Vanelderen P, Van Zundert J, Kozicz T, et al. Effect of minocycline on lumbar radicular neuropathic pain: a randomized, placebo-controlled, double-blind clinical trial with amitriptyline as a comparator. Anesthesiology 2015;122:399-406. 10.1097/ALN.0000000000000508 [DOI] [PubMed] [Google Scholar]
- 61.Schnitzer TJ. Effect of Milnacipran in Chronic Neuropathic Low Back Pain. ClinicalTrials.gov. NCT01225068. 2010.
- 62.Beaudoin F. Healing With Venlafaxine After Injury (HELP). ClinicalTrials.gov. NCT01716377. 2017.
- 63.Hudson B. A randomised placebo controlled trial of nortriptyline for pain in knee osteoarthritis. Australian New Zealand Clinical Trials Registry. ACTRN12614000683639. 2014.
- 64.Riediger C, Schuster T, Barlinn K, Maier S, Weitz J, Siepmann T. Adverse Effects of Antidepressants for Chronic Pain: A Systematic Review and Meta-analysis. Front Neurol 2017;8:307. 10.3389/fneur.2017.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162-73. 10.1016/S1474-4422(14)70251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson AA. Assessing Statistical Results: Magnitude, Precision, and Model Uncertainty. Am Stat 2019;73:118-21 10.1080/00031305.2018.1537889. [DOI] [Google Scholar]
- 67.Smarr KL, Keefer AL. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9). Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S454-66. 10.1002/acr.20556 [DOI] [PubMed] [Google Scholar]
- 68.National Institute for Health and Care Excellence. Low back pain and sciatica in over 16s: assessment and management. 2016. [PubMed]
- 69.Gould HM, Atkinson JH, Chircop-Rollick T, et al. A randomized placebo-controlled trial of desipramine, cognitive behavioral therapy, and active placebo therapy for low back pain. Pain 2020;161:1341-9. 10.1097/j.pain.0000000000001834 [DOI] [PubMed] [Google Scholar]
- 70.Urquhart DM, Wluka AE, van Tulder M, et al. Efficacy of Low-Dose Amitriptyline for Chronic Low Back Pain: A Randomized Clinical Trial. JAMA Intern Med 2018;178:1474-81. 10.1001/jamainternmed.2018.4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Atkinson JH, Slater MA, Capparelli EV, et al. Efficacy of noradrenergic and serotonergic antidepressants in chronic back pain: a preliminary concentration-controlled trial. J Clin Psychopharmacol 2007;27:135-42. 10.1097/jcp.0b013e3180333ed5 [DOI] [PubMed] [Google Scholar]
- 72.Atkinson JH, Slater MA, Wahlgren DR, et al. Effects of noradrenergic and serotonergic antidepressants on chronic low back pain intensity. Pain 1999;83:137-45. 10.1016/S0304-3959(99)00082-2 [DOI] [PubMed] [Google Scholar]
- 73.Atkinson JH, Slater MA, Williams RA, et al. A placebo-controlled randomized clinical trial of nortriptyline for chronic low back pain. Pain 1998;76:287-96. 10.1016/S0304-3959(98)00064-5 [DOI] [PubMed] [Google Scholar]
- 74.Tétreault P, Mansour A, Vachon-Presseau E, Schnitzer TJ, Apkarian AV, Baliki MN. Brain Connectivity Predicts Placebo Response across Chronic Pain Clinical Trials. PLoS Biol 2016;14:e1002570. 10.1371/journal.pbio.1002570 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: files 1-18