Abstract
Cannabis sativa plant has not only cannabinoids as crucial compounds but also the other compounds that play important role as synergistic and/or entourage compound. Cannabis/hemp plant materials and essential oils were analyzed with the help of gas chromatography/mass spectrometry detector for the content of terpenes and terpenoids. The main terpenes/terpenoids and their abundance in the samples were evaluated. Results of this study will be helpful in the next evaluation of these compound in mixture with cannabinoids and their importance in medical treatment.
Keywords: Cannabis, Cannabis sativa L., Gas chromatography-mass spectrometry, Terpenes, Terpenoids
Introduction
Since the past 25 years, the interest in cannabis (Cannabis sativa L.) has been growing extremely worldwide. The main reason behind such growing interest is not the treatment of some serious illnesses with cannabis but rather the desire to get rich quickly.
Although medical cannabis in some countries is considered legal, it is not yet a pharmaceutical drug. There are 3 main reasons. (a) Fear and stigma − 80 years of prohibition accompanied with negative propaganda. (b) Lack of standardization − since it is not a single-molecule drug. Cannabis in fact is a plant of over 1,000 chemical constituents, varying by chemotype (chemical phenotype) batch and crop. Sometimes, people incorrectly referred to chemotype as strain, variety, cultivar, or chemovar. Chemotypes are plants of the same genus that are virtually identical in appearance but produce essential oil with different major constituents. Chemotypes are variants within a single botanical species. Today, there are thousands of “strains,” many of which have similar names but cultivated in different climatic regions, and each with unique chemical ingredient profile that activates differently. (c) The cart before the horse − cannabis became legal and approved without standard clinical trials. Now, we have to “back in” to the efficacy to see what type of cannabis works for which medical conditions [1].
Cannabis use as medicine goes back for thousands of years [2], but after the USA started to fight against it since 1937, it disappeared from pharmacopeias all over the world. Fortunately, in 1950, Krejčí [3, 4] from Czechoslovakia discovered antibiotic principle of cannabis and they started to use cannabis clinically at hospitals. Back in 1954, the first scientific conference under “Cannabis as a medicine” was held in Olomouc, Czechoslovakia [5]. Krejčí and Šantavý [6] identified this antibiotic principle compound of cannabis, which is effective against gram-positive microorganisms and some pathogens. They named the compound responsible for this effect cannabidiolic acid [6, 7, 8]. It was the first real cannabinoid isolated from C. sativa L. From that time, cannabis grown in Czechoslovakia (mostly the Czechoslovak chemotype Rastislavice) was used for the treatment in the Faculty hospital of Olomouc [9, 10] up to 1990.
The first cannabinoid compound isolated and identified from hemp was cannabinol [11, 12, 13]. This compound in fact is an artifact, which originates from Δ9-tetrahydrocannabinol (THC) in the plant resin and after plant harvest, storage, or heating [14]. Decarboxylation product of cannabidiolic acid, cannabidiol (CBD), was fully identified in 1963 [15, 16, 19]. Psychotomimetically active compound of cannabis, THC, was fully characterized in 1964 [17, 18, 19]. Today, 177 cannabinoid compounds are known in C. sativa L. [20, 21, 22, 23]. Many of them are certainly artifacts originating during harvest, drying, and workup of cannabis plant. After the identification of the above-mentioned cannabinoids, research started on these compounds. Cannabinoid receptors, CB1 [24] and CB2 [25], were discovered in the human body. Full understanding of the whole endocannabinoid system in the human body gave an isolation of natural ligands (today called endocannabinoids) [26, 27, 28, 29, 30, 31]. This brought us to understand the medicinal value of cannabis plant, which was used for millennia in treatment [2], and today, it is being legalized for the treatment of different diseases in many countries. Because of illegalization and stigmatization of this plant, there is still a lot of work to be accomplished to acquire full knowledge of the medicinal power of this plant [32].
The first scientific study conducted on cannabis contents goes back to the first half of the 19th century [33]. Bolas and Francis [34] made the first attempt to identify the compounds in the commercial resinous extract of Indian hemp in 1869. They isolated oxy-cannabin, C5H6O2, and acid compound, which crystallize in plates.
It was almost generally accepted that the only active compounds in cannabis are compounds typical for this plant, cannabinoids. Only in the last several years scientists started to speculate about synergic and/or entourage effect of the other cannabis compounds. In the first row today are terpenes/terpenoids, but our plans are also to study flavonoids, flavonoid glycosides, and also polyphenols. It is very likely that the first isolated terpene was just β-caryophyllene.
As workers in Italian hemp fields became gay and giddy from a volatile principle of hemp, Valente searched for the compound that causes it. He employed steam distillation of the fresh leaves of hemp cultivated for fibers to obtain sesquiterpene from essential oil, with the empirical formula C15H24 [35, 36, 37]. It was probably β-caryophyllene which is the most common main sesquiterpene in hemp. Vignolo [38, 39] also used distillation in a current of steam to prepare essential oil and subsequently the same sesquiterpene C15H24 as Valente. Wood [40] isolated from charas (because it contains no chlorophyll) a monoterpene C10H16 (probably myrcene that is usually the main monoterpene in hemp) and a sesquiterpene C15H24 (β-caryophyllene?). Simonsen and Todd [41] were the first to name isolated terpene. They extracted p-cymene (C10H16), p-cymenene (C10H12), and humulene (C15H24) from Egyptian hashish.
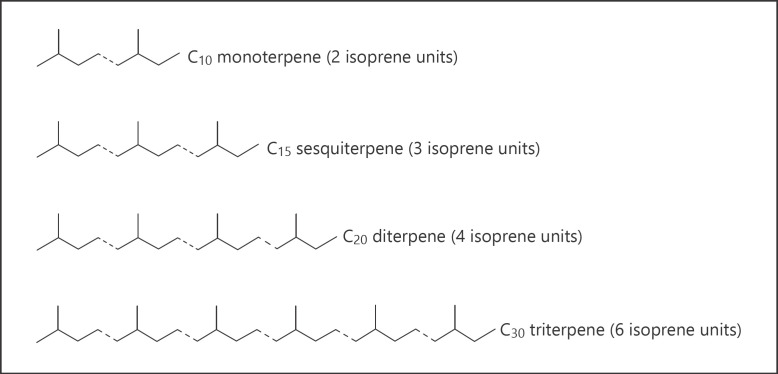
If the question is what are terpenes or terpenoids in cannabis plant, the answer is terpenes are hydrocarbons and terpenoids are oxygen-containing terpenes. They can be visualized as the result of linking isoprene units “head to tail” to form chains, which can be arranged to form rings (Fig. 1). It is the so-called biogenetic isoprene rule or the C5 rule [42].
Fig. 1.
Linking isoprene units “head to tail” to form terpenes/terpenoids.
The name terpene was suggested by Kekulé [43] for C10H16 hydrocarbons in 1866. In C. sativa L., plant terpenes/terpenoids can be divided into several groups. Here are examples of different types of terpenes (110 published up-to-date) and terpenoids (121 published up-to-date) in this plant − examples of terpenes/terpenoids from cannabis of this particular group are in given parentheses (Tables 1, 2, 3, 4):
Table 1.
| Monoterpenes − C10H16, mw 136 | Monoterpenoids |
|---|---|
| Acyclic (β-myrcene) | Acyclic (linalool − C10H18O, mw 154) |
| Monocyclic (α-phellandrene) | Monocyclic (cis-linalool oxide − C10H18O2, mw 170) |
| Bicyclic (α-pinene) | Bicyclic (cis-sabinene hydrate − C10H18O, mw 154) |
| Tricyclic (tricyclene) |
Table 1.
| Sesquiterpenes − C15H24, mw 204 | Sesquiterpenoids |
|---|---|
| Acyclic (cis-β-farnesene) | Acyclic (cis-nerolidol − C15H26O, mw 222) |
| Monocyclic (α-humulene) | Monocyclic (humulene epoxide II − C15H24O, mw 220) |
| Bicyclic (β-caryophyllene) | Bicyclic (sesquicineole − C15H26O, mw 222) |
| Tricyclic (α-cubebene) | Tricyclic (epi-cubebol − C15H26O, mw 222) |
Table 3.
| Diterpenes | Diterpenoids |
|---|---|
| Monocyclic (m-camphorene − C20H32, mw 272) | Acyclic (phytol − C20H40O, mw 296) |
Table 4.
| Triterpenes | Triterpenoids |
|---|---|
| Acyclic (squalene − C30H50, mw 410) | Tetracyclic (sitostanol − C29H52O, mw 416) |
| Pentacyclic (friedelin − C30H50O, mw 426) |
Cannabinoids are derived from diterpene structure. The monoterpenes present in cannabis plant have another functional moiety like alcohol (fenchyl alcohol, linalool, and borneol), aldehyde (neral), ketone (carvone), ester (bornyl acetate and linalyl acetate), ether (1,8-cineol), and phenol (thymol and carvacrol). The sesquiterpene molecules include structures like alcohols (farnesol) or ketones (nootkatone). To understand the full effects of terpenes/terpenoids, it is necessary to explain some terms.
Synergy
The term synergy comes from the Attic Greek word συνεργία (synergía, “collaboration”), which is based on the word συνεργός (synergos, “working together”). Explanation is easy. When we have 2 active compounds, they work together better than each one separately, which can be expressed by strict inequality: 1 + 1 > 2.
Entourage Effect
The term entourage effect was introduced in 1988 by Mechoulam and colleagues[44] and was explained as increased activity of an active compound with an inactive one, which can be expressed by strict inequality: 1 + 0 > 1. Standardized cannabis drug preparations, rather than pure cannabinoids, could be generally considered the preferred ones [45, 46]. We believe that all components of the cannabis plant likely exert some therapeutic effect, more than any single compound alone. There is increasing evidence that these compounds work better together than in isolation and that is exactly what is called today “entourage effect.”
The analysis of the sample and the interpretation of the results of the analysis must be evaluated very responsibly. We do not have enough results in this field yet. Chemotype, location of cultivation, conditions of cultivation, season of cultivation, weather and microclimate, stage of plant development, method of processing after harvest, method of storage, storage time before analysis, part of a plant for analysis, and method of sample processing for analysis − all these parameters affect the results of the analysis. So far, changes in the content of cannabinoid substances during the growing season have been studied [47, 48, 49, 50] and also dynamics of changes of cannabinoids and terpenes/terpenoids during vegetation period [51, 52].
Terpenes/terpenoids have a wide range of biological and pharmacological activities, for instance, antifungal, antiviral, anticancer, anti-inflammatory, antihyperglycemic, antiparasitic, antioxidant, and antimicrobial. It is not possible to describe all pharmacological effects of terpenes/terpenoids in this paper, but we shall give just some examples for imagination of how important these compounds are in this plant. Monoterpene myrcene is the smallest terpene but the most prevalent terpene found in most varieties of cannabis. Chemotypes high in myrcene will result in a “couch lock” effect (if a sample has over 0.5% myrcene), while chemotypes with low levels of myrcene (<0.5% myrcene) will produce a more energetic high. It is simply the amount of myrcene that is in the sample that dictates how you will be affected. Myrcene is simply the important monoterpene in the plant. Myrcene has antipsychotic, antioxidant, analgesic, anti-inflammatory, sedative, muscle relaxant, and anticancerogenic properties [53, 54, 55, 56]. The most important sesquiterpene in the cannabis plant is probably β-caryophyllene. It is a spicy terpene. This compound is the only terpene known to interact with the body's endocannabinoid system (selectively binds to the CB2 receptor) [57]. Caryophyllene has gastroprotective, analgesic, anticancerogenic, antifungal, antibacterial, antidepressant, anti-inflammatory, antiproliferative, antioxidant, anxiolytic, analgesic, and neuroprotective effects [58, 59]. The presence of β-caryophyllene in many essential oils might contribute strongly to their antiviral ability. β-Caryophyllene displayed a high selectivity index of 140 against herpes simplex virus type 1 in vitro. The selectivity index was determined by the ratio of the cytotoxic concentration of the drug that reduced viable cell number by 50% to antiviral activity, which inhibited plaque numbers by 50% compared with the untreated control [60]. α-Pinene (antibacterial, anti-inflammatory, bronchodilator, antiseptic, and gastroprotective) and β-pinene (antiseptic) are the next important compounds [61]. It is not possible to mention here all the biodynamic terpenes. After all, there may be mentioned, for instance, limonene (antibacterial, gastroprotective, antiproliferative, antifungal, anxiolytic, antidepressant, antimicrobial, antispasmodic, or immunostimulant) [62, 63]. Linalool (sedative, antipsychotic, anticonvulsant, anxiolytic, anesthetic, antidepressant, analgesic, antiepileptic, and antineoplastic) [64], terpineol (antioxidant, antibiotic, and relaxing effect) [65], or caryophyllene oxide (analgesic, anticancer, antifungal, and anti-inflammatory) [66]. Between others are also phellandrene (antifungal and digestive disorders) [67], ocimene (antifungal) [68], camphene (cardiovascular disease) [69], guaiol (antitumor) [70, 71], α-humulene (antibacterial, anti-inflammatory, and antitumor) [72, 73, 74], γ-terpinene (analgesic, anti-inflammatory, antimicrobial, and anticancer) [75, 76], β-elemene (antitumor, antineoplastic, and anticancer) [77, 78, 79, 80], nerolidol (antiparasitic and antileishmanial) [81, 82], or citral (antifungal, antimicrobial, antiproliferative, cytotoxic, anticancer, and antitumor) [83, 84, 85, 86, 87, 88, 89, 90].
Terpenes/terpenoids are largely responsible for the characteristic aroma of cannabis. An essential oil (mostly terpenes or terpenoids), especially when distilled, is not necessarily identical in its chemical composition with the oil that is present in the living plant. Quite often very high-boiling or low-boiling chemicals are simply “lost” due to the nature of the distillation process and due to economic and time constrains. Although most constituents remain intact during distillation, a few undergo chemical changes. Oil also contains substances that are formed from reactive precursors on distillation. The variation in essential oil composition may be due to factors that affect the plant's environment, such as geographical location, weather conditions, soil type, fertilizer used, the age of the plant, and the time and weather of day or year when it is harvested. Degradation tends to occur on prolonged storage, under poor storage conditions, or when the essential oil is otherwise exposed to air. Atmospheric oxygen can change the chemical composition of an essential oil by reacting with some of its constituents. Oxidation can also affect the efficacy of an essential oil. It can render an essential oil more hazardous [91]. When we use plant material, one must take attention to keep it protected from UV and air oxidation. Otherwise, some terpenes can transform to allergens [92, 93].
The first who pointed out the possible synergic and/or entourage effect of cannabinoids and terpenes is Russo [94]. Only few studies have pharmacology, and further studies have to be accomplished prior to understanding the interactions of cannabinoids and terpenes/terpenoids in humans. Well-arranged review on the terpene biosynthesis in cannabis plant was demonstrated by Kovalchuk et al. [95]. Terpene synthases from C. sativa were characterized [96, 97, 98]. Rice and Koziel [99] studied the odorous compounds (as terpenes/terpenoids as the other volatiles). Hazekamp et al. [100] gave a deeper understanding of cannabis effects in laboratory and clinical studies and the usefulness of a terpene/terpenoid approach for chemotaxonomic mapping of cannabis varieties for medicinal use. Gallily et al. [101] investigated the antioxidant and anti-inflammatory properties of 3 different terpene/terpenoid-rich hemp essential oils. They concluded that terpenes/terpenoids may be used to diminute acute inflammation effect, whereas the cannabinoids to inhibit chronic inflammation symptoms. Anti-inflammatory potential of terpenes present in cannabis was studied by Downer [102]. Current evidence of medicinal properties of terpenes was given by Baron [103] and Nuutinen [104] in their reviews. Entourage effect of terpenes/terpenoids and cannabinoids and their pharmacological activity were also studied by Namdar et al. [105] and Ferber et al. [106]. Blasco-Benito et al. [46] extracted fresh cannabis flowers in ethanol, after evaporation, followed by magnetic stirring on hot plate, which achieved cannabinoid decarboxylation. The extract (in mg/g) contains 3.4 THCA, 551.3 THC, 3.7 CBG, and no THCV, CBD, CBDA, cannabinol, and CBC and the 5 main terpenes were 1.9 β-caryophyllene, 0.6 humulene, 0.4 nerolidol, 0.6 linalool, and 0.3 β-pinene. While the ethanolic extract of cannabis flowers has higher antitumor activity than pure THC, this effect was not attributed to any of the 5 most abundant terpenes (THC and the 5 main terpenes in appropriate concentrations did not have higher activity than pure THC). Finlay et al. [107] tried to determine whether terpenes (myrcene, α- and β-pinene, β-caryophyllene, and limonene) in the cannabis plant have detectable receptor-mediated activity or modify the activity of THC, CBD, or the endocannabinoid 2-arachidonoylglycerol at the cannabinoid receptors. This study proves that the putative entourage effect cannot be explained by direct effects at CB1 or CB2. Nuutinen [104] has published a comprehensive paper on the medicinal properties of a number of cannabis mono- and sesquiterpene/terpenoids. These were studied in vitro, on animals, and in clinical trials. Performed studies have shown antimutagenic, antidiabetic, anti-inflammatory, analgesic, antioxidant, antibiotic, anticonvulsive, anticancer, antidepressant, anxiolytic, antitumor, neuroprotective, anti-allergic, and others.
Experimental
Plant Material and Essential Oils
All 54 chemotype inflorescence samples of cannabis were purchased from Israeli growers. Eight essential oils were of hemp harvested in August/September 2016 in the pre-Alpine region of Slovenia (Upper Savinja Valley), latitude NS 46°20′ 29.525 and longitude E 14°50′ 0.777. These samples of essential oils were prepared by steam distillation of female flowers (upper third of the plant). One sample was from Czech Republic (Bialobrzeskie). Essential oils of cannabis were bought from Cali terpenes (45 samples).
Sample Preparation
A 20 mg of plant material was extracted with hexane, filtered with Filter Fix (25 mm, 0.45 μm Nylon Syringe Filter; SOLUFIX − SIMPLEPURE PTFE, 0.45 μm), and dissolved 20 times with hexane. Essential oil was diluted with hexane to a concentration of 0.1 mg/mL.
Conditions of the Analysis
Instrument: GC/MS (Agilent 7890B GC, Agilent 5977B MSD, PAL 3 [RSI 85]).
Column: Agilent Technologies, Inc., HP-5MS UI, 30 m × 0.25 mm, film 0.25 μm.
Experimental conditions: At first, the column was held at 35°C for 5 min, and afterward, the temperature was raised to 150°C at 5°C/min, then at 15°C/min up to 250°C, hold time 90 min (the inlet temperature was fixed at 250°C; the detector at 280°C; split injection 1:5; initial temperature − 100°C; initial time − 4.0 min), gas − helium (flow rate: 1 mL/min).
Identification: The content compounds were identified by comparison with standards, retention times, retention indices, and the spectral matching of libraries NIST/EPA/NIH Mass Spectral Library 2017, Wiley Registry of Mass Spectral Data 11th Edition, FFNSC3, ©2015, and Adams EO library, Mass Spectral Library, 2205 compounds.
Results
In the text, every time for each mentioned chemotype, the first is a picture of their gas chromatographic/mass spectrometric analysis and after that a table of terpenes/terpenoids.
Comparison of Hemp and Cannabis
Initially, we tried to compare the so-called hemp with the so-called cannabis, which is necessary to explain here. Hemp is a cannabis cultivated for fiber but it is also used for treatment. In fact, it is C. sativa L. with very low concentrations of CBD and THC. Cannabis is called today C. sativa L. mainly used for recreational and/or medicinal use. It has a high concentration of CBD and/or THC. The difference between recreational and medicinal cannabis is in cultivation − medicinal cannabis is cultivated under strict conditions. In fact, many chemotypes developed for recreational use are currently called medicinal cannabis. As we did not have all terpenes standards that exist in this plant, we could not quantify all terpenes and terpenoids; therefore, we used comparison based on relative ratio of the main terpene/terpenoid in the particular chemotype.
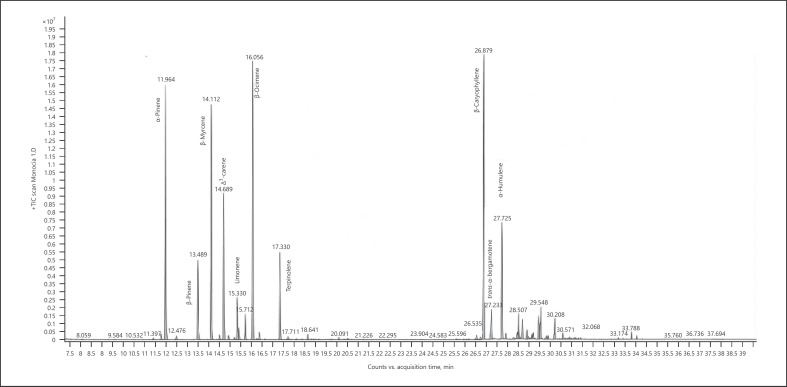
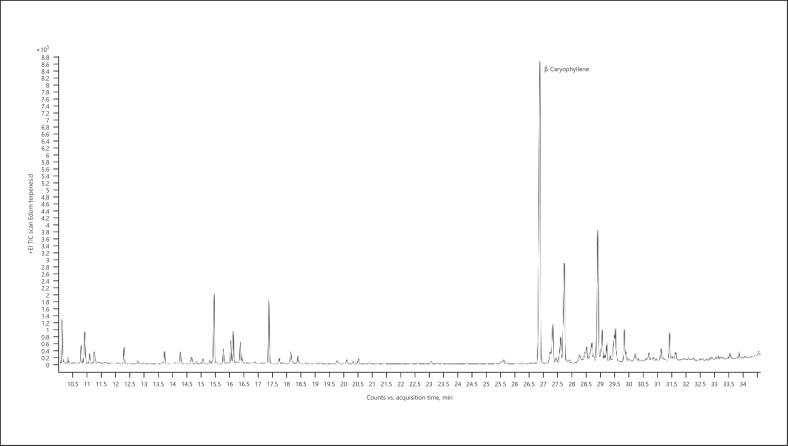
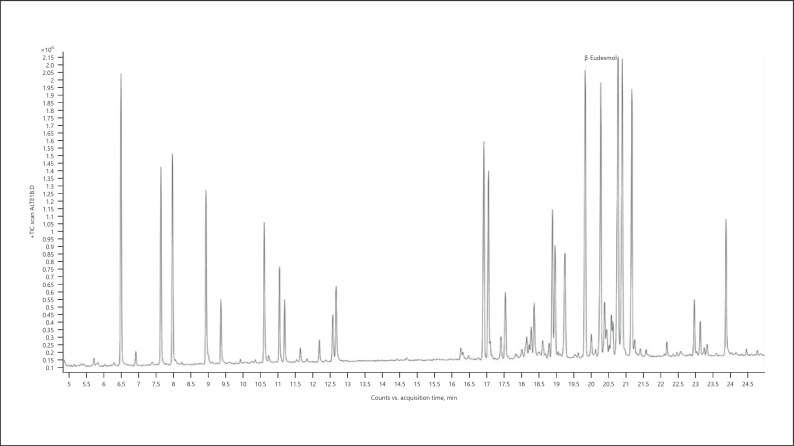
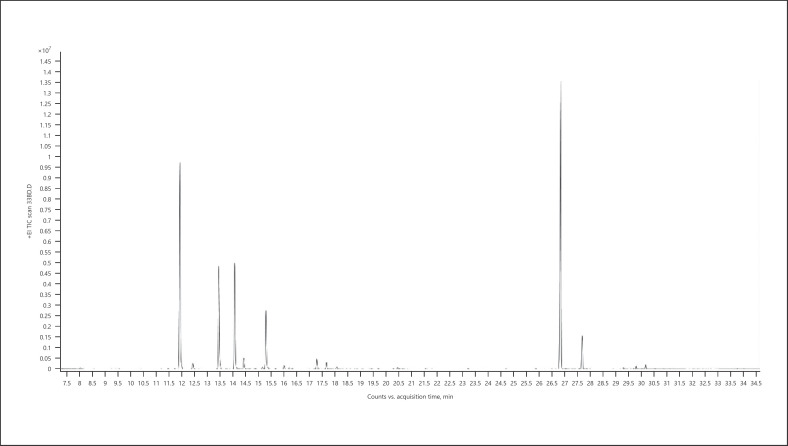
Hemp (cannabis for fibers) − chemotype Monoica (listed volatiles that are present at a level more than 5% of the main terpene − monoterpenes are marked in bold) (Fig. 2; Table 5):
Fig. 2.
Gas chromatogram of Monoica hemp chemotype.
Table 5.
| Normalized % | Compound | RI |
|---|---|---|
| 80.24 | α-Pinene | 937 |
| 24.50 | β-Pinene | 979 |
| 71.40 | β-Myrcene | 991 |
| 45.96 | Δ3-Carene | 1,011 |
| 12.81 | Limonene | 1,031 |
| 7.20 | α-Ocimene | 1,039 |
| 83.29 | β-Ocimene | 1,037 |
| 27.00 | Terpinolene | 1,088 |
| 100.00 | β-Caryophyllene | 1,418 |
| 8.49 | Trans-α-bergamotene | 1,435 |
| 36.75 | α-Humulene | 1,454 |
| 7.14 | β-Selinene | 1,486 |
| 7.09 | α-Selinene | 1,494 |
| 7.86 | Selina-3,7(11)-diene | 1,542 |
| 6.02 | Caryophyllene oxide | 1,581 |
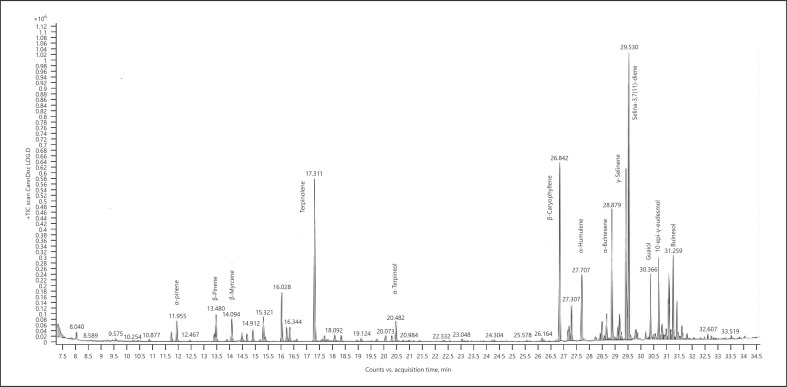
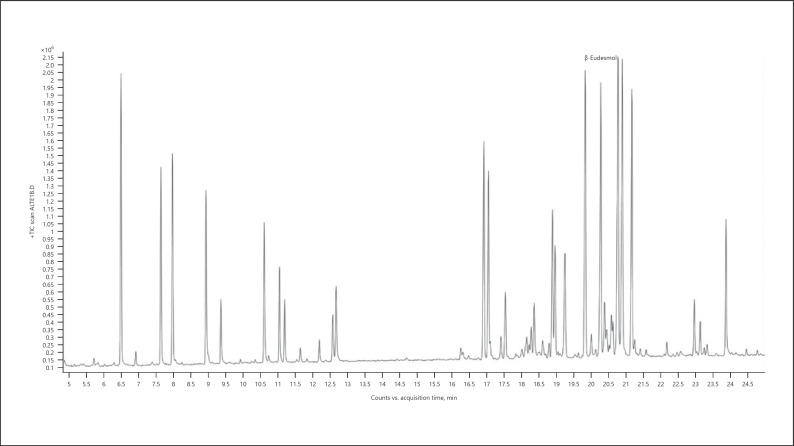
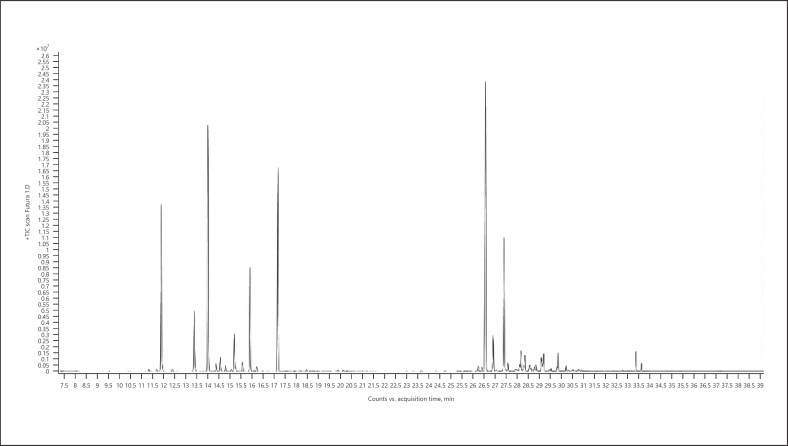
Cannabis (cannabis for treatment) − chemotype Lemon OG Kush (listed volatiles that are present at a level more than 5% of the main terpene − sesquiterpenes/sesquiterpenoids are marked in bold) (Fig. 3; Table 6):
Fig. 3.
Gas chromatogram of Lemon OG Kush cannabis chemotype.
Table 6.
| Normalized % | Compound | RI |
|---|---|---|
| 8.46 | α-Pinene | 937 |
| 11.26 | β-Pinene | 979 |
| 9.28 | β-Myrcene | 991 |
| 68.01 | Terpinolene | 1,088 |
| 8.85 | α-Terpineol | 1,189 |
| 73.09 | β-Caryophyllene | 1,418 |
| 15.09 | α-Guaiene | 1,439 |
| 27.01 | α-Humulene | 1,454 |
| 11.06 | α-Sselinene | 1,494 |
| 49.72 | α-Bulnesene | 1,505 |
| 68.46 | Selina-4(15),7(11)-diene | 1,544 |
| 100.00 | Selina-3,7(11)-diene | 1,542 |
| 18.95 | Guaiol | 1,596 |
| 25.92 | 10-Epi-γ-eudesmol | 1,619 |
| 7.86 | γ-Eudesmol | 1,631 |
| 9.36 | β-Eudesmol | 1,649 |
| 19.57 | α-Eudesmol | 1,653 |
| 25.17 | Bulnesol | 1,666 |
| 15.22 | α-Bisabolol | 1,683 |
Results of 108 Inflorescences and Essential Oils Analyses
Fifty-four inflorescences of different cannabis chemotypes (Fuji, Everest, Golan, Tropicana, Kilimanjaro (CannDoc), Marom, Edom, Choco 1, Durban Poison, Jack Herer, Black Diamond, Holy Weed, Pandora's Box, Tel Aviv, Paris, OG Kush, Bubble Gum, Strawberry 2.0, Lemon Deluxe, Berry Deluxe, Medi Kush, Lemon K2, Blue Haze, Annapurna, Choco 2, Himalaya, Lemon OG Kush, AK-47, Jericho, Chocolope, Doblin, K, Kira Kush, Kilimanjaro (Better), Luli Kush, Maui Waui, White Widow, Topaz, DOV, Alaska, Avidekel, Barak, El-Na, Erez, Or, Tal, Jasmine, Shira, Rafael, Mango, Candy Kush, Local Afgan, Power Plant, and Toffy) + 9 essential oils of different hemp chemotypes (Futura, Felina, Fedora, Ferimon, Monoica, Tiborszallasi, Tisza, Bialobrzeskie from Slovenia, and Bialobrzeskie from Czech Republic) + 45 essential oils of different cannabis chemotypes (Jamaican dream, Girl Scout Cookies, Lemon Cookies, Tangie, Gelato, Chocolate Mint OG, Key Lime Pie, Gorilla Glue, Pink Plant, 24K Gold, Critical Jack, Sweet Tooth, Sugaree, Kashmir Kush, Holy Grail Kush, Chemdawg 4, 3 Kings, Sour Diesel, TNT Kush, Monster, Sensi Star, SFV OG, Veneno, Gipsy Haze, Critical, 707 Truthband, Blackberry Kush, Grapefruit OG, Furious Candy, AK-47, High Level, Cinderella 99, Black Dream, 10K Jack, Cheese, Lavender, Truth, Orange Turbo, Dosidos, Nina Limone, Amnesia, Wifi OG, M.I.B., OG Kush, Mojito) = altogether 108 chemotypes.
a. Fifty-eight different terpenes were found between the 10 main terpenes from each of 108 chemotypes (hemp and cannabis inflorescence and essential oil samples).
Sorting according to the frequency of the main terpene in the 10 main ones at each chemotype (numbers outside parentheses indicate in how many phenotypes was this particular terpene between the 10 main ones, and numbers in parentheses indicate in how many phenotypes was this particular terpene/terpenoid the major one) (Table 7).
Table 7.
| Compound | RI | Between 10 mains in a sample (the main in a sample) |
|---|---|---|
| β-Caryophyllene | 1,418 | 108× (48×) |
| β-Myrcene | 991 | 89× (19×) |
| α-Pinene | 939 | 83× (9×) |
| Limonene | 1,031 | 79× (2×) |
| β-Pinene | 980 | 72× (0×) |
| Linalool | 1,098 | 45× (1×) |
| Guaiol | 1,596 | 35× (0×) |
| 10-Epi-γ-eudesmol | 1,619 | 34× (5×) |
| Selina-3,7(11)-diene | 1,542 | 33× (9×) |
| α-Humulene | 1,454 | 82× (0×) |
| Bulnesol | 1,667 | 32× (1×) |
| α-Terpineol | 1,189 | 28× (0×) |
| α-Eudesmol | 1,653 | 26× (1×) |
| p-Mentha-1(7),8-diene | 1,004 | 26× (0×) |
| β-Eudesmol | 1,649 | 23× (1×) |
| Endo-fenchol | 1,112 | 22× (0×) |
| α-Bisabolol | 1,683 | 20× (3×) |
| γ-Selinene | 1,544 | 20× (1×) |
| Trans-β-ocimene | 1,044 | 20× (0×) |
| Terpinolene | 1,088 | 18× (8×) |
| α-Selinene | 1,494 | 12× (0×) |
| β-Selinene | 1,486 | 11× (0×) |
| p-Cymene | 1,026 | 10× (0×) |
| γ-Eudesmol | 1,631 | 9× (0×) |
| α-Bulnesene | 1,505 | 9× (0×) |
| γ-Elemene | 1,434 | 9× (0×) |
| Camphene | 953 | 9× (0×) |
| Germacrene B | 1,557 | 8× (0×) |
| α-Guaiene | 1,439 | 4× (0×) |
| Epi-α-bisabolol | 1,670 | 4× (0×) |
| Juniper camphor | 1,692 | 3× (0×) |
| Cis-α-bisabolene | 1,504 | 3× (0×) |
| Trans-α-bisabolene | 1,512 | 3× (0×) |
| Trans-α-bergamotene | 1,435 | 9× (0×) |
| Δ3-Carene | 1,011 | 7× (0×) |
| Caryophyllene oxide | 1,581 | 7× (0×) |
| 1,8-Cineole | 1,032 | 6× (0×) |
| α-Ocimene | 1,039 | 5× (0×) |
| Hexyl butyrate | 1,192 | 5× (0×) |
| Cis-β-ocimene | 1,040 | 4× (0×) |
| α-Phellandrene | 1,005 | 4× (0×) |
| Borneol | 1,166 | 4× (0×) |
| β-Phellandrene | 1,031 | 4× (0×) |
| α-Terpinene | 1,017 | 3× (0×) |
| δ-Selinene | 1,493 | 2× (0×) |
| Trans-β-farnesene | 1,458 | 2× (0×) |
| Trans-nerolidol | 1,564 | 2× (0×) |
| γ-Terpinene | 1,060 | 2× (0×) |
| Trans-pinene hydrate | 1,140 | 2× (0×) |
| Ocimene | 1,050 | 1× (0×) |
| Exo-fenchol | 1,116 | 1× (0×) |
| Cis-pinene hydrate | 1,121 | 1× (0×) |
| Trans-β-guaiene | 1,500 | 1× (0×) |
| γ-Cadinene | 1,513 | 1× (0×) |
| β-Curcumene | 1,514 | 1× (0×) |
| Selin-6-en-4α-ol | 1,636 | 1× (0×) |
| Humulene epoxide I | 1,594 | 2× (0×) |
| γ-Terpineol | 1,197 | 1× (0×) |
The bold values represent the maximum amount when the compound was between the ten main terpenes.
b. Forty-four different terpenes were found between the 10 main terpenes of each from 54 chemotypes (cannabis inflorescence samples).
Sorting according to the frequency of the main terpene in the 10 main ones at each chemotype (numbers outside parentheses indicates in how many phenotypes was this particular terpene between the 10 main ones, and numbers in parentheses indicate in how many phenotypes was this particular terpene the major one) (Table 8).
Table 8.
| Compound | RI | Between 10 mains in a sample (the main in a sample) |
|---|---|---|
| β-Caryophyllene | 1,418 | 53× (13×) |
| β-Myrcene | 991 | 36× (13×) |
| Guaiol | 1,596 | 35× (0×) |
| 10-Epi-γ-eudesmol | 1,619 | 34× (5×) |
| Selina-3,7(11)-diene | 1,542 | 33× (9×) |
| α-Humulene | 1,454 | 36× (0×) |
| Bulnesol | 1,667 | 32× (1×) |
| α-Pinene | 939 | 29× (5×) |
| α-Eudesmol | 1,653 | 26× (1×) |
| Limonene | 1,031 | 27× (0×) |
| β-Eudesmol | 1,649 | 23× (1×) |
| α-Bisabolol | 1,683 | 20× (3×) |
| γ-Selinene | 1,544 | 20× (1×) |
| β-Pinene | 980 | 18× (0×) |
| α-Selinene | 1,494 | 11× (0×) |
| Terpinolene | 1,088 | 9× (2×) |
| γ-Eudesmol | 1,631 | 9× (0×) |
| α-Bulnesene | 1,505 | 9× (0×) |
| Linalool | 1,098 | 9× (0×) |
| γ-Elemene | 1,434 | 9× (0×) |
| Germacrene B | 1,557 | 8× (0×) |
| β-Selinene | 1,486 | 8× (0×) |
| Endo-fenchol | 1,112 | 6× (0×) |
| α-Guaiene | 1,439 | 4× (0×) |
| Epi-α-bisabolol | 1,670 | 4× (0×) |
| Juniper camphor | 1,692 | 3× (0×) |
| Cis-α-bisabolene | 1,504 | 3× (0×) |
| Trans-α-bisabolene | 1,512 | 3× (0×) |
| α-Terpineol | 1,189 | 3× (0×) |
| Trans-β-ocimene | 1,044 | 3× (0×) |
| β-Phellandrene | 1,031 | 2× (0×) |
| Trans-α-bergamotene | 1,435 | 2× (0×) |
| δ-Selinene | 1,493 | 2× (0×) |
| Ocimene | 1,050 | 1× (0×) |
| Exo-fenchol | 1,116 | 1× (0×) |
| Cis-pinene hydrate | 1,121 | 1× (0×) |
| Trans-pinene hydrate | 1,140 | 1× (0×) |
| Trans-β-farnesene | 1,458 | 2× (0×) |
| Trans-β-guaiene | 1,500 | 1× (0×) |
| γ-Cadinene | 1,513 | 1× (0×) |
| β-Curcumene | 1,514 | 1× (0×) |
| Trans-nerolidol | 1,564 | 1× (0×) |
| Caryophyllene oxide | 1,581 | 1× (0×) |
| Selin-6-en-4α-ol | 1,636 | 1× (0×) |
The bold values represent the maximum amount when the compound was between the ten main terpenes.
c. Twenty-seven different terpenes were found between the 10 main terpenes of each from 46 chemotypes (cannabis essential oils).
Sorting according to the frequency of the main terpene in the 10 main ones at each chemotype (numbers outside parentheses indicate in how many phenotypes was this particular terpene between the 10 main ones, and numbers in parentheses indicates in how many phenotypes was this particular terpene the major one) (Table 9).
Table 9.
| Compound | RI | Between 10 mains in a sample (the main in a sample) |
|---|---|---|
| β-Caryophyllene | 1,418 | 46× (30×) |
| α-Pinene | 937 | 45× (4x) |
| β-Myrcene | 991 | 45× (3×) |
| Limonene | 1,031 | 45× (2×) |
| β-Pinene | 979 | 45× (0×) |
| α-Humulene | 1,454 | 37× (0×) |
| Linalool | 1,098 | 36× (1×) |
| p-Mentha-1(7),8-diene | 1,004 | 26 (0×) |
| α-Terpineol | 1,189 | 25× (0×) |
| Terpinolene | 1,088 | 21× (6×) |
| Endo-fenchol | 1,112 | 16× (0×) |
| β-Ocimene | 1,040 | 13× (0×) |
| p-Cymene | 1,026 | 10× (0×) |
| Camphene | 953 | 9× (0×) |
| 1,8-Cineole | 1,032 | 6× (0×) |
| Hexyl butyrate | 1,192 | 5× (0×) |
| α-Phellandrene | 1,005 | 4× (0×) |
| Δ3-Carene | 1,011 | 4× (0×) |
| α-Ocimene | 1,039 | 4× (0×) |
| Borneol | 1,166 | 4× (0×) |
| Caryophyllene oxide | 1,581 | 4× (0×) |
| α-Terpinene | 1,017 | 3× (0×) |
| Trans-β-ocimene | 1,049 | 2× (0×) |
| γ-Terpinene | 1,060 | 2× (0×) |
| Trans-pinene hydrate | 1,140 | 1× (0×) |
| γ-Terpineol | 1,197 | 1× (0×) |
| Trans-nerolidol | 1,564 | 1× (0×) |
The bold values represent the maximum amount when the compound was between the ten main terpenes.
d. Seventeen different terpenes were found between the 10 main terpenes of each from 7 chemotypes (hemp essential oils).
Sorting according to the frequency of the main terpene in the 10 main ones at each chemotype (numbers outside parentheses indicate in how many phenotypes was this particular terpene between the 10 main ones, and numbers in parentheses indicate in how many phenotypes was this particular terpene the major one) (Table 10).
Table 10.
| Compound | RI | Between 10 mains in a sample (the main in a sample) |
|---|---|---|
| β-Caryophyllene | 1,418 | 8× (5×) |
| β-Myrcene | 991 | 7× (4×) |
| α-Pinene | 937 | 8× (0×) |
| β-Pinene | 979 | 8× (0×) |
| Terpinolene | 1,088 | 8× (0×) |
| α-Humulene | 1,454 | 8× (0×) |
| Limonene | 1,031 | 7× (0×) |
| β-Ocimene | 1,037 | 6× (0×) |
| Trans-α-bergamotene | 1,435 | 6× (0×) |
| Δ3-Carene | 1,011 | 3× (0×) |
| β-Selinene | 1,486 | 3× (0×) |
| Cis-β-ocimene | 1,040 | 2× (0×) |
| Caryophyllene oxide | 1,581 | 2× (0×) |
| β-Phellandrene | 1,031 | 1× (0×) |
| α-Ocimene | 1,039 | 1× (0×) |
| α-Selinene | 1,494 | 1× (0×) |
| Humulene epoxide I | 1,594 | 1× (0×) |
The bold values represent the maximum amount when the compound was between the ten main terpenes.
e. Examples of different chemotypes with different main terpenes:
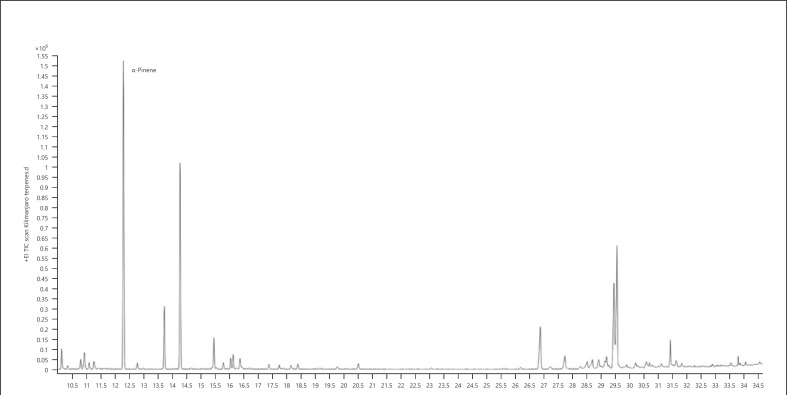
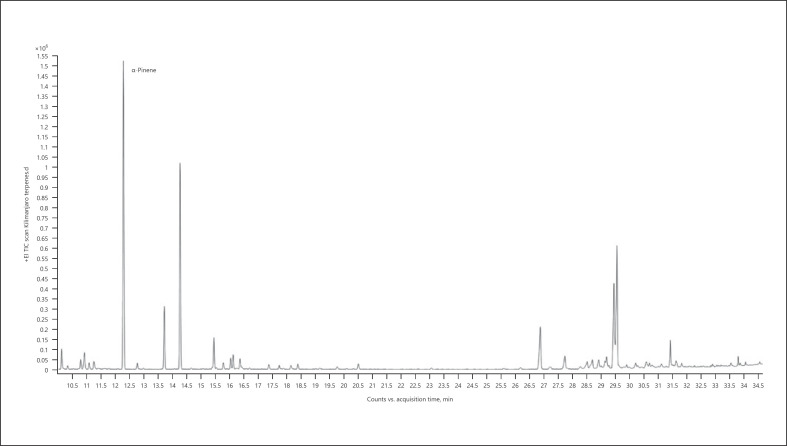
1. α-Pinene-dominant chemotype − Kilimanjaro (Fig. 4; Table 11)
Fig. 4.
Gas chromatogram of Kilimanjaro cannabis chemotype.
Table 11.
| Normalized % | Compound | RI |
|---|---|---|
| 100.00 | α-Pinene | 939 |
| 64.84 | β-Myrcene | 991 |
| 52.98 | γ-Selinene | 1,544 |
| 39.58 | Selina-3,7(11)-diene | 1,542 |
| 22.90 | Caryophyllene | 1,418 |
| 21.12 | β-Pinene | 980 |
| 11.23 | Limonene | 1,031 |
| 8.70 | α-Bisabolol | 1,683 |
| 5.70 | α-Selinene | 1,494 |
| 4.93 | Ocimene | 1,050 |
The bold value represents the main compound in the sample.
2. β-Myrcene dominant chemotype − Durban Poison (Fig. 5; Table 12)
Fig. 5.
Gas chromatogram of Durban Poison cannabis chemotype.
Table 12.
| Normalized % | Compound | RI |
|---|---|---|
| 100.00 | β-Myrcene | 991 |
| 79.20 | Caryophyllene | 1,418 |
| 61.69 | α-Pinene | 939 |
| 47.22 | Guaiol | 1,596 |
| 46.16 | β-Eudesmol | 1,649 |
| 36.21 | α-Eudesmol | 1,652 |
| 31.69 | Selina-3,7(11)-diene | 1,542 |
| 28.87 | α-Humulene | 1,440 |
| 26.69 | β-Pinene | 980 |
| 24.21 | Limonene | 1,031 |
The bold value represents the main compound in the sample.
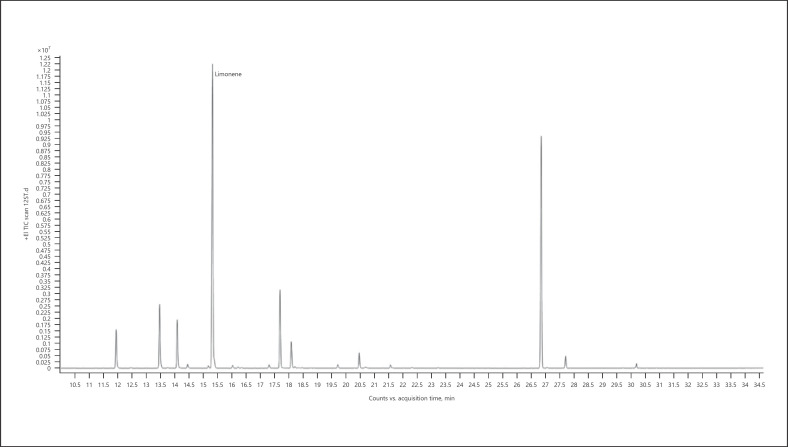
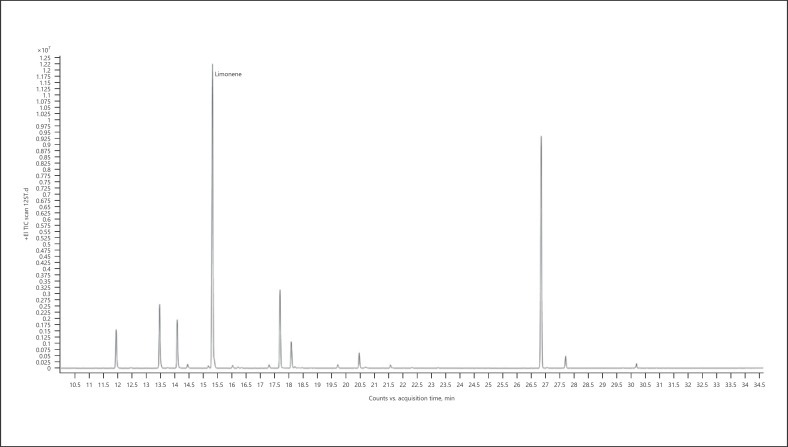
3. Limonene-dominant chemotype − Sweet Tooth (Fig. 6; Table 13)
Fig. 6.
Gas chromatogram of Sweet Tooth cannabis chemotype.
Table 13.
| Normalized % | Compound | RI |
|---|---|---|
| 100.00 | Limonene | 1,031 |
| 76.70 | β-Caryophyllene | 1,418 |
| 23.89 | Linalool | 1,098 |
| 20.70 | β-Pinene | 979 |
| 14.59 | β-Myrcene | 991 |
| 12.49 | α-Pinene | 937 |
| 8.78 | Endo-fenchol | 1,112 |
| 4.90 | α-Terpineol | 1,189 |
| 3.83 | α-Humulene | 1,454 |
| 1.36 | p-Mentha-1(7),8-diene | 1,004 |
The bold value represents the main compound in the sample.
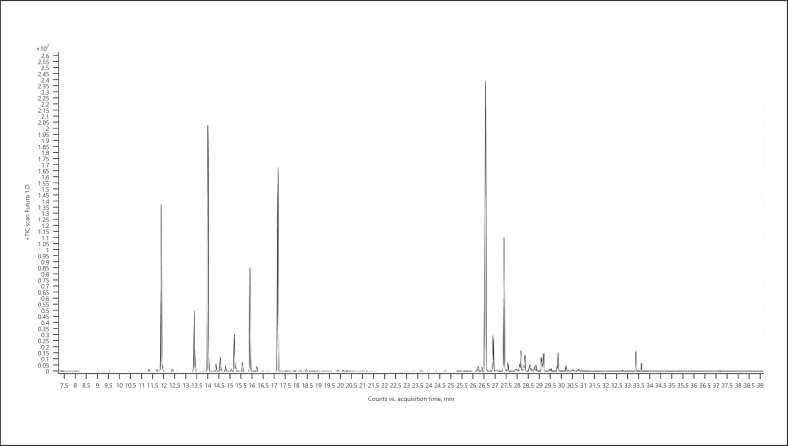
4. Terpinolene-dominant chemotype − Jack Herer (Fig. 7; Table 14)
Fig. 7.
Gas chromatogram of Jack Herer cannabis chemotype.
Table 14.
| Normalized % | Compound | RI |
|---|---|---|
| 100.00 | Terpinolene | 1,088 |
| 69.43 | β-Myrcene | 991 |
| 46.40 | Caryophyllene | 1,419 |
| 44.23 | α-Pinene | 937 |
| 34.20 | α-Bulnesene | 1,505 |
| 26.69 | β-Pinene | 979 |
| 19.47 | Humulene | 1,454 |
| 17.98 | Limonene | 1,031 |
| 13.54 | β-Selinene | 1,485 |
| 13.14 | α-Guaiene | 1,439 |
The bold value represents the main compound in the sample.
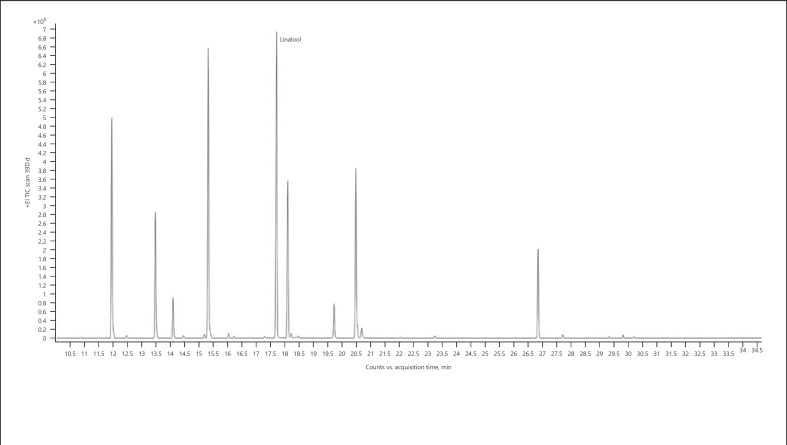
5. Linalool-dominant chemotype − Dosidos (Fig. 8; Table 15)
Fig. 8.
Gas chromatogram of Dosidos cannabis chemotype.
Table 15.
| Normalized % | Compound | RI |
|---|---|---|
| 100.00 | Linalool | 1,098 |
| 93.11 | Limonene | 1,031 |
| 73.64 | α-Pinene | 937 |
| 58.11 | α-Terpineol | 1,189 |
| 51.17 | Endo-fenchol | 1,112 |
| 41.71 | β-Pinene | 979 |
| 29.27 | β-Caryophyllene | 1,418 |
| 12.35 | β-Myrcene | 991 |
| 11.83 | Borneol | 1,166 |
| 3.87 | γ-Terpineol | 1,197 |
The bold value represents the main compound in the sample.
6. β-Caryophyllene-dominant chemotype − Edom (Fig. 9; Table 16)
Fig. 9.
Gas chromatogram of Edom cannabis chemotype.
Table 16.
| Normalized % | Compound | RI |
|---|---|---|
| 100.00 | β-Caryophyllene | 1,418 |
| 40.90 | α-Bulnesene | 1,505 |
| 33.50 | α-Humulene | 1,454 |
| 15.05 | Limonene | 1,031 |
| 14.20 | Terpinolene | 1,088 |
| 12.06 | α-Guaiene | 1,439 |
| 10.20 | α-Selinene | 1,494 |
| 10.00 | γ-Cadinene | 1,513 |
| 9.80 | Trans-nerolidol | 1,564 |
| 8.40 | β-Selinene | 1,485 |
The bold value represents the main compound in the sample.
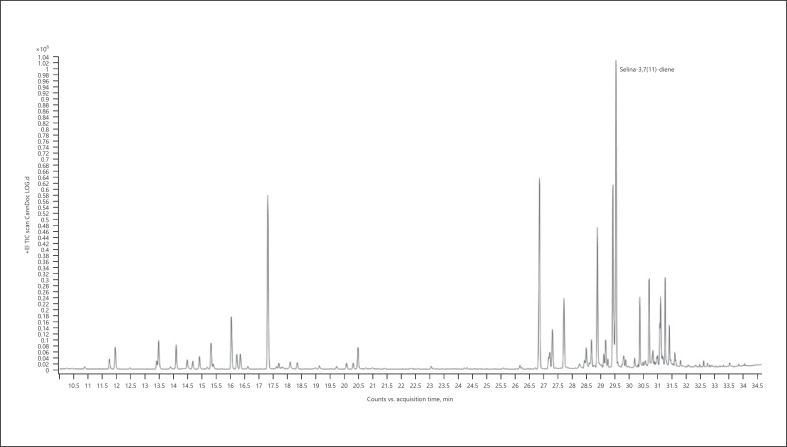
7. Selina-3,7(11)-diene-dominant chemotype − Lemon OG Kush (Fig. 10; Table 17)
Fig. 10.
Gas chromatogram of Lemon OG Kush cannabis chemotype.
Table 17.
| Normalized % | Compound | RI |
|---|---|---|
| 100.00 | Selina-3,7(11)-diene | 1,542 |
| 73.09 | β-Caryophyllene | 1,418 |
| 68.46 | Selina-4(15),7(11)-diene | 1,544 |
| 68.01 | Terpinolene | 1,088 |
| 49.72 | α-Bulnesene | 1,505 |
| 27.01 | α-Humulene | 1,454 |
| 25.92 | 10-Epi-γ-eudesmol | 1,619 |
| 25.17 | Bulnesol | 1,666 |
| 19.57 | α-Eudesmol | 1,653 |
| 18.95 | Guaiol | 1,596 |
The bold value represents the main compound in the sample.
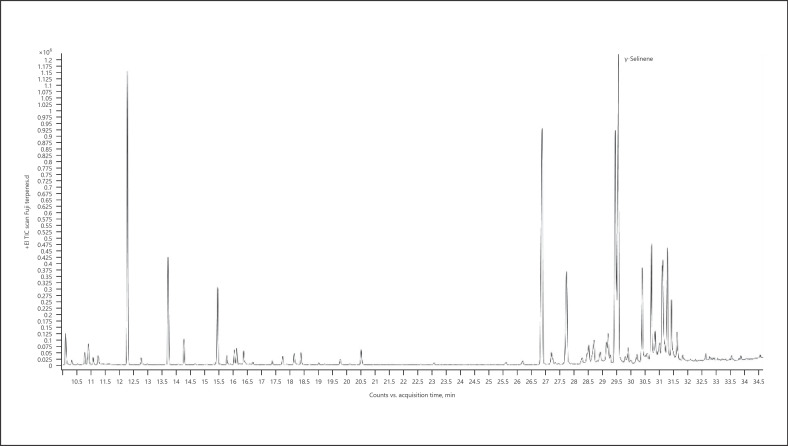
8. γ-Selinene-dominant chemotype − Fuji (Fig. 11; Table 18)
Fig. 11.
Gas chromatogram of Fuji cannabis chemotype.
Table 18.
| Normalized % | Compound | RI |
|---|---|---|
| 100.00 | γ-Selinene | 1,544 |
| 94.82 | Caryophyllene | 1,418 |
| 80.95 | Selina-3,7(11)-diene | 1,542 |
| 72.37 | α-Pinene | 939 |
| 36.95 | α-Humulene | 1,454 |
| 36.18 | 10-Epi-γ-eudesmol | 1,619 |
| 31.80 | Bulnesol | 1,666 |
| 27.01 | Guaiol | 1,595 |
| 26.82 | β-Pinene | 980 |
| 23.43 | α-Eudesmol | 1,652 |
The bold value represents the main compound in the sample.
9. 10-Epi-γ-eudesmol-dominant chemotype − Lemon Deluxe (Fig. 12; Table 19)
Fig. 12.
Gas chromatogram of Lemon Deluxe cannabis chemotype.
Table 19.
| Normalized % | Compound | RI |
|---|---|---|
| 100.00 | 10-Epi-γ-eudesmol | 1,619 |
| 93.89 | Selina-3,7(11)-diene | 1,542 |
| 86.72 | α-Bisabolol | 1,683 |
| 86.47 | β-Caryophyllene | 1,418 |
| 83.15 | Bulnesol | 1,666 |
| 68.04 | Guaiol | 1,596 |
| 67.13 | β-Myrcene | 991 |
| 62.23 | α-Eudesmol | 1,653 |
| 57.63 | Limonene | 1,030 |
| 30.13 | β-Eudesmol | 1,649 |
The bold value represents the main compound in the sample.
10. β-Eudesmol-dominant chemotype − Alaska (Fig. 13; Table 20)
Fig. 13.
Gas chromatogram of Alaska cannabis chemotype.
Table 20.
| Normalized % | Compound | RI |
|---|---|---|
| 100.00 | β-Eudesmol | 1,649 |
| 87.07 | Bulnesol | 1,667 |
| 81.59 | Guaiol | 1,596 |
| 78.76 | 10-Epi-γ-eudesmol | 1,619 |
| 72.13 | α-Bisabolol | 1,684 |
| 65.67 | α-Pinene | 937 |
| 62.62 | β-Caryophyllene | 1,419 |
| 50.71 | γ-Elemene | 1,434 |
| 49.94 | β-Myrcene | 991 |
| 44.18 | β-Pinene | 979 |
The bold value represents the main compound in the sample.
11. α-Eudesmol-dominant chemotype − El Na (Fig. 14; Table 21)
Fig. 14.
Gas chromatogram of El Na cannabis chemotype.
Table 21.
| Normalized % | Compound | RI |
|---|---|---|
| 100.00 | α-Eudesmol | 1,652 |
| 89.80 | β-Myrcene | 991 |
| 89.68 | β-Caryophyllene | 1,418 |
| 82.64 | 10-Epi-γ-eudesmol | 1,619 |
| 82.18 | Guaiol | 1,595 |
| 79.76 | Bulnesol | 1,667 |
| 74.35 | Epi-α-bisabolol | 1,686 |
| 70.04 | α-Pinene | 937 |
| 40.58 | β-Pinene | 979 |
| 30.66 | α-Humulene | 1,454 |
The bold value represents the main compound in the sample.
12. Bulnesol-dominant chemotype − Berry Deluxe (Fig. 15; Table 22)
Fig. 15.
Gas chromatogram of Berry Deluxe cannabis chemotype.
Table 22.
| Normalized % | Compound | RI |
|---|---|---|
| 100.00 | Bulnesol | 1,666 |
| 98.07 | 10-Epi-γ-eudesmol | 1,619 |
| 80.32 | α-Eudesmol | 1,653 |
| 77.83 | Guaiol | 1,596 |
| 46.87 | β-Caryophyllene | 1,418 |
| 40.77 | β-Eudesmol | 1,649 |
| 33.94 | α-Bisabolol | 1,683 |
| 32.36 | γ-Eudesmol | 1,631 |
| 15.30 | Limonene | 1,030 |
| 14.58 | α-Humulene | 1,454 |
The bold value represents the main compound in the sample.
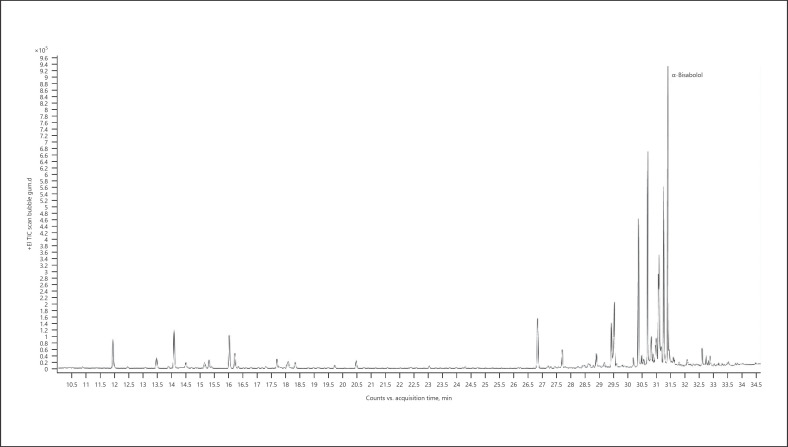
13. α-Bisabolol-dominant chemotype − Bubble Gum (Fig. 16; Table 23)
Fig. 16.
Gas chromatogram of Bubble Gum cannabis chemotype.
Table 23.
| Normalized % | Compound | RI |
|---|---|---|
| 100.00 | α-Bisabolol | 1,683 |
| 62.84 | Bulnesol | 1,666 |
| 52.98 | Guaiol | 1,596 |
| 19.97 | β-Myrcene | 991 |
| 26.24 | β-Caryophyllene | 1,418 |
| 37.28 | Selina-3,7(11)-diene | 1,542 |
| 16.71 | γ-Eudesmol | 1,631 |
| 22.14 | β-Eudesmol | 1,649 |
| 43.24 | α-Eudesmol | 1,653 |
The bold value represents the main compound in the sample.
f. Comparison of terpene content in fresh and dry samples:
Chemotype Pandora's Box − fresh (Fig. 17; Table 24);
Fig. 17.
Gas chromatogram of Pandora's Box cannabis chemotype (fresh sample).
Table 24.
| Normalized % | Compound | RI |
|---|---|---|
| 100.00 | Terpinolene | 1,088 |
| 86.22 | Caryophyllene | 1,419 |
| 34.56 | α-Humulene | 1,454 |
| 22.67 | γ-Elemene | 1,434 |
| 15.18 | Selina-3,7(11)-diene | 1,542 |
| 12.95 | β-Myrcene | 991 |
| 11.37 | Germacrene B | 1,556 |
| 11.11 | α-Cadinene | 1,538 |
| 9.96 | Bulnesol | 1,667 |
The bold value represents the main compound in the sample.
Chemotype Pandora's Box − dry (Fig. 18; Table 25)
Fig. 18.
Gas chromatogram of Pandora's Box cannabis chemotype (dry sample).
Table 25.
| Normalized % | Compound | RI |
|---|---|---|
| 100.00 | β-Caryophyllene | 1,418 |
| 37.19 | Terpinolene | 1,088 |
| 35.89 | α-Humulene | 1,454 |
| 30.82 | γ-Elemene | 1,434 |
| 20.55 | Selina-3,7(11)-diene | 1,542 |
| 15.37 | Bulnesol | 1,667 |
| 14.74 | 10-Epi-γ-eudesmol | 1,619 |
| 10.84 | α-Eudesmol | 1,653 |
| 10.54 | Guaiol | 1,596 |
| 10.39 | Germacrene B | 1,556 |
The bold value represents the main compound in the sample.
g. All identified volatile compounds in 1 chemotype:
Chemotype Lemon OG Kush (cannabis inflorescence) (Fig. 19; Table 26);
Fig. 19.
Gas chromatogram of Lemon OG Kush cannabis chemotype.
Table 26.
| RT | Normalized % | Compound | RI |
|---|---|---|---|
| 9.445 | 0.47 | 4-Methyl-octane | 863 |
| 9.575 | 1.17 | p-Xylene | 865 |
| 10.44 | 0.23 | o-Xylene | 887 |
| 10.877 | 0.84 | Heptanal | 901 |
| 11.751 | 3.73 | α-Thujene | 929 |
| 11.955 | 8.46 | α-Pinene | 937 |
| 12.467 | 0.51 | Camphene | 953 |
| 13.415 | 3.22 | Sabinene | 974 |
| 13.48 | 11.26 | β-Pinene | 979 |
| 14.094 | 9.28 | β-Myrcene | 991 |
| 14.484 | 4.26 | α-Phellandrene | 1,005 |
| 14.68 | 3.22 | Δ3-Carene | 1,011 |
| 14.912 | 4.97 | α-Terpinene | 1,017 |
| 15.182 | 0.71 | p-Cymene | 1,026 |
| 15.321 | 10.24 | Monoterpene − mw 136 | |
| 15.396 | 2.23 | 1,8-Cineole | 1,032 |
| 16.028 | 20.89 | 1,3-Diethylbenzene | 1,032 |
| 16.223 | 5.91 | 1,4-Diethylbenzene | 1,041 |
| 16.344 | 6.23 | γ-Terpinene | 1,060 |
| 16.614 | 1.12 | Cis-sabinene hydrate | 1,068 |
| 17.311 | 68.01 | Terpinolene | 1,088 |
| 17.618 | 1.06 | Trans-sabinene hydrate | 1,097 |
| 17.692 | 2.48 | Linalool | 1,099 |
| 18.092 | 3.81 | Endo-fenchol | 1,112 |
| 18.343 | 2.83 | Cis-pinene hydrate | 1,121 |
| 18.985 | 0.58 | Trans-pinene hydrate | 1,140 |
| 19.124 | 1.43 | Bicyclo[2.2.1]hept-2-en-7-ol | 1,164 |
| 19.729 | 1.20 | Borneol | 1,166 |
| 20.073 | 2.55 | Terpinen-4-ol | 1,177 |
| 20.315 | 2.32 | p-Cymen-8-ol | 1,183 |
| RT | Normalized % | Compound | RI |
| 20.482 | 8.85 | α-Terpineol | 1,189 |
| 25.578 | 0.40 | Ylangene | 1,372 |
| 26.164 | 1.45 | Selina-5,11-diene | 1,447 |
| 26.842 | 73.09 | β-Caryophyllene | 1,418 |
| 27.168 | 3.97 | γ-Elemene | 1,434 |
| 27.214 | 6.36 | α-Bergamotene | 1,435 |
| 27.307 | 15.09 | α-Guaiene | 1,439 |
| 27.707 | 27.01 | α-Humulene | 1,454 |
| 28.423 | 2.79 | Sesquiterpene − mw 204 | |
| 28.488 | 8.50 | β-Selinene | 1,486 |
| 28.674 | 11.06 | α-Selinene | 1,494 |
| 28.879 | 49.72 | α-Bulnesene | 1,505 |
| 29.111 | 4.65 | Sesquiterpene − mw 204 | |
| 29.167 | 10.39 | Sesquiterpene − mw 204 | |
| 29.251 | 2.52 | Sesquiterpene − mw 204 | |
| 29.427 | 68.46 | Selina-4(15),7(11)-diene | 1,544 |
| 29.530 | 100.00 | Selina-3,7(11)-diene | 1,542 |
| 30.190 | 3.00 | Caryophyllene oxide | 1,581 |
| 30.366 | 18.95 | Guaiol | 1,596 |
| 30.701 | 25.92 | 10-Epi-γ-eudesmol | 1,619 |
| 30.831 | 7.86 | γ-Eudesmol | 1,631 |
| 31.073 | 9.36 | β-Eudesmol | 1,649 |
| 31.101 | 19.57 | α-Eudesmol | 1,653 |
| 31.259 | 25.17 | Bulnesol | 1,666 |
| 31.399 | 15.22 | α-Bisabolol | 1,683 |
| 31.594 | 5.86 | Juniper camphor | 1,691 |
| 31.798 | 1.94 | Sesquiterpenoid − mw 220 |
The bold value represents the main compound in the sample.
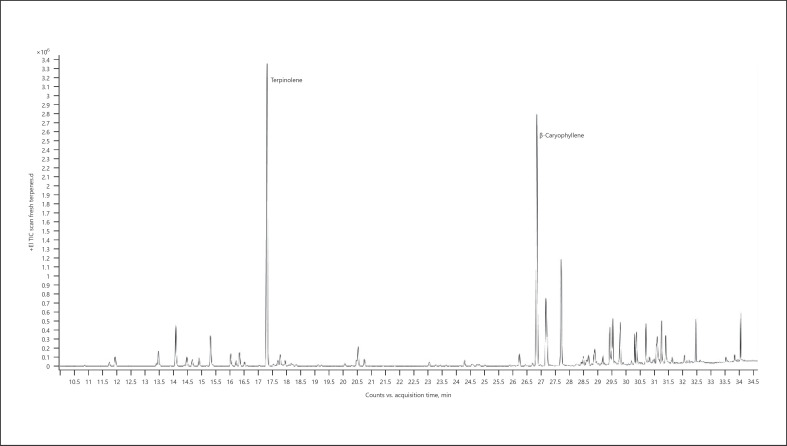
Chemotype Futura (hemp essential oil) (Fig. 20; Table 27);
Fig. 20.
Gas chromatogram of Futura hemp chemotype.
Table 27.
| RT | Normalized % Compound | RI | |
|---|---|---|---|
| 9.286 | 0.01 | Ethylbenzene | 855 |
| 9.584 | 0.05 | p-Xylene | 859 |
| 10.532 | 0.01 | 5-Methyl-2-hexanone | 865 |
| 11.397 | 0.63 | 5,5-Dimethyl-1-vinylbicyclo[2.1.1] hexane | 920 |
| 11.760 | 0.57 | α-Thujene | 929 |
| 11.964 | 49.47 | α-Pinene | 937 |
| 12.476 | 0.75 | Camphene | 953 |
| 12.718 | 0.01 | Dehydrosabinene | 956 |
| 13.489 | 17.80 | β-Pinene | 979 |
| 14.122 | 74.77 | β-Myrcene | 991 |
| 14.373 | 0.05 | Δ2-Carene | 1,001 |
| 14.494 | 2.31 | α-Phellandrene | 1,005 |
| 14.689 | 4.20 | Δ3-Carene | 1,011 |
| 14.921 | 1.76 | α-Terpinene | 1,017 |
| 15.200 | 0.61 | p-Cymene | 1,025 |
| 15.331 | 10.96 | Limonene | 1,031 |
| 15.336 | 1.02 | 1,8-Cineole | 1,032 |
| 15.712 | 2.48 | α-ocimene | 1,039 |
| 16.047 | 29.24 | β-ocimene | 1,040 |
| 16.242 | 0.32 | 1,4-Diethylbenzene | 1,041 |
| 16.363 | 1.45 | γ-Terpinene | 1,060 |
| 16.623 | 0.05 | Cis-sabinene hydrate | 1,070 |
| 17.330 | 61.71 | Terpinolene | 1,088 |
| 17.711 | 0.27 | Linalool | 1,099 |
| 18.064 | 0.11 | Monoterpene − mw 134 | |
| 18.111 | 0.16 | Endo-fenchol | 1,112 |
| 18.362 | 0.11 | Cis-pinene hydrate | 1,121 |
| 18.632 | 0.53 | Neo-allo-ocimene | 1,131 |
| 18.808 | 0.15 | Monoterpene − mw 134 | |
| 18.911 | 0.10 | Trans-pinocarveol | 1,139 |
| 19.004 | 0.09 | Monoterpene − mw 136 | |
| 19.385 | 0.03 | Monoterpenoid − mw 134 | |
| 19.654 | 0.02 | Pinocarvone | 1,164 |
| 19.738 | 0.07 | Borneol | 1,166 |
| 20.082 | 0.48 | Monoterpenoid − mw 154 | |
| 20.324 | 0.42 | p-Cymen-8-ol | 1,183 |
| 20.491 | 0.20 | α-Terpineol | 1,189 |
| 20.547 | 0.11 | Hexyl butanoate | 1,192 |
| 21.226 | 0.05 | 2-Methyl-2-nonen-4-one | 1,215 |
| 21.589 | 0.03 | Citronellol | 1,228 |
| 25.001 | 0.07 | α-Cubebene | 1,351 |
| 25.596 | 0.22 | Ylangene | 1,372 |
| 25.717 | 0.14 | Copaene | 1,376 |
| 25.912 | 0.17 | Hexyl hexanoate | 1,384 |
| 25.987 | 0.07 | β-Longipinene | 1,403 |
| 26.061 | 0.17 | Sesquithujene | 1,402 |
| 26.173 | 0.16 | Sesquiterpene − mw 204 | |
| 26.461 | 0.24 | Sesquiterpene − mw 204 | |
| RT | Normalized % Compound | RI | |
| 26.535 | 1.56 | Cis-β-caryophyllene | 1,406 |
| 26.610 | 0.12 | α-Gurjunene | 1,409 |
| 26.712 | 1.23 | Cis-α-bergamotene | 1,415 |
| 26.879 | 100.00 | β-Caryophyllene | 1,418 |
| 27.084 | 0.14 | Sesquiterpene − mw 204 | |
| 27.233 | 11.15 | Trans-α-bergamotene | 1,435 |
| 27.372 | 0.48 | Aromadendrene | 1,440 |
| 27.447 | 0.07 | Guaia-6,9-diene | 1,443 |
| 27.540 | 0.01 | Epi-β-santalene | 1,448 |
| 27.623 | 0.09 | Sesquiterpene − mw 204 | |
| 27.726 | 40.37 | α-Humulene | 1,454 |
| 27.912 | 2.42 | Epi-β-caryophyllene | 1,466 |
| 28.116 | 0.13 | 4,5-Di-epi-aristolochene | 1,476 |
| 28.265 | 0.59 | γ-Muurolene | 1,477 |
| 28.274 | 0.55 | β-Himachalene | 1,499 |
| 28.358 | 0.22 | α-Muurolene | 1,499 |
| 28.442 | 1.88 | Sesquiterpene − mw 204 | |
| 28.507 | 5.54 | β-Selinene | 1,486 |
| 28.600 | 0.20 | Cis-β-guaiene | 1,490 |
| 28.656 | 1.22 | Valencene | 1,492 |
| 28.693 | 3.88 | α-Selinene | 1,494 |
| 28.879 | 0.62 | α-Farnesene | 1,508 |
| 28.907 | 1.66 | β-Bisabolene | 1,509 |
| 28.962 | 0.71 | β-Curcumene | 1,514 |
| 29.046 | 0.35 | γ-Cadinene | 1,513 |
| 29.120 | 1.01 | Sesquiterpene − mw 204 | |
| 29.195 | 1.82 | Sesquiterpene − mw 204 | |
| 29.260 | 0.16 | Sesquiterpene − mw 204 | |
| 29.437 | 3.01 | γ-Selinene | 1,544 |
| 29.465 | 2.18 | Sesquiterpene − mw 204 | |
| 29.511 | 1.21 | α-Bisabolene | 1,512 |
| 29.548 | 4.04 | Selina-3,7(11)-diene | 1,542 |
| 29.799 | 0.54 | Germacrene B | 1,557 |
| 29.883 | 0.52 | Sesquiterpene − mw 204 | |
| 30.199 | 4.95 | Caryophyllene oxide | 1,581 |
| 30.320 | 0.02 | Sesquiterpene − mw 204 | |
| 30.422 | 0.10 | Humulene epoxide I | 1,604 |
| 30.487 | 0.07 | Sesquiterpene − mw 204 | |
| 30.571 | 1.11 | Humulene epoxide II | 1,606 |
| 30.664 | 0.33 | Sesquiterpenoid − mw 220 | |
| 30.906 | 0.44 | Caryophylla-4(12),8(13)-dien-5-β-ol | 1,631 |
| 31.408 | 0.11 | α-Bisabolol | 1,684 |
| 31.603 | 0.06 | Juniper camphor | 1,692 |
| 33.547 | 0.24 | Diterpene − mw 272 | 1,939 |
| 33.788 | 2.87 | m-Camphorene | 1,960 |
| 34.039 | 1.41 | p-Camphorene | 1,995 |
The bold value represents the main compound in the sample.
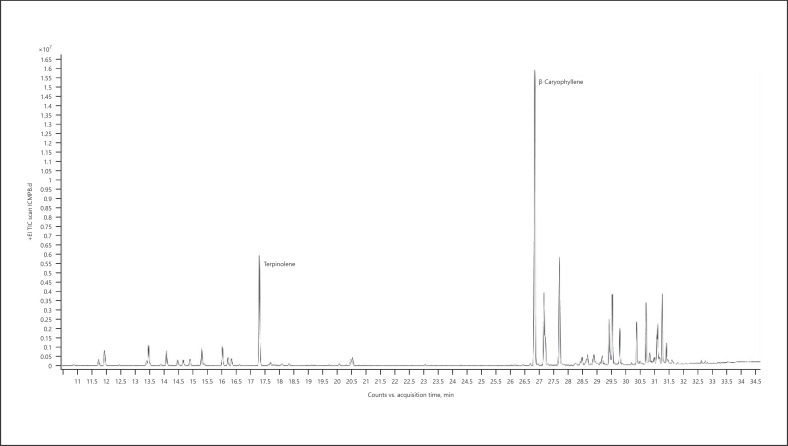
Chemotype Black Dream (cannabis essential oil) (Fig. 21; Table 28)
Fig. 21.
Gas chromatogram of Black Dream cannabis chemotype.
Table 28.
| RT | Normalized % | Compound | RI |
|---|---|---|---|
| 9.268 | 0.02 | Ethylbenzene | 855 |
| 9.565 | 0.06 | p-Xylene | 865 |
| 11.211 | 0.04 | 2,6-Dimethyl-4-octene | 911 |
| 11.481 | 0.18 | Tricyclene | 926 |
| 11.741 | 0.12 | α-Thujene | 929 |
| 11.946 | 67.03 | α-Pinene | 937 |
| 12.457 | 2.11 | Camphene | 953 |
| 12.690 | 0.03 | Dehydrosabinene | 956 |
| 13.471 | 34.15 | β-Pinene | 979 |
| 13.750 | 0.09 | mw 138 | |
| 14.084 | 33.17 | β-Myrcene | 991 |
| 14.177 | 0.36 | mw 138 | |
| 14.270 | 0.07 | mw 138 | |
| 14.447 | 3.74 | p-Mentha-1(7),8-diene | 1,004 |
| 14.680 | 0.10 | Δ3-Carene | 1,011 |
| 14.903 | 0.21 | α-Terpinene | 1,017 |
| 15.182 | 0.62 | p-Cymene | 1,026 |
| 15.312 | 18.87 | Limonene | 1,031 |
| 15.386 | 0.78 | 1,8-Cineole | |
| 15.693 | 0.18 | α-Ocimene | 1,039 |
| 16.028 | 1.14 | 1,3-Diethylbenzene | 1,032 |
| 16.223 | 0.34 | 1,4-Diethylbenzene | 1,041 |
| 16.344 | 0.24 | γ-Terpinene | 1,060 |
| 17.218 | 0.05 | Monoterpene − mw 136 | |
| 17.302 | 3.37 | Terpinolene | 1,088 |
| 17.683 | 2.25 | Linalool | 1,098 |
| 18.083 | 0.79 | Endo-fenchol | 1,112 |
| 18.204 | 0.07 | Exo-fenchol | 1,116 |
| 18.613 | 0.04 | Allo-ocimene | 1,129 |
| 18.883 | 0.05 | Trans-pinocarveol | 1,139 |
| 19.096 | 0.07 | Cis-verbenol | 1,142 |
| 19.440 | 0.10 | Isoborneol | 1,157 |
| 19.719 | 0.16 | Borneol | 1,166 |
| 20.305 | 0.12 | p-Cymen-8-ol | 1,183 |
| 20.473 | 0.48 | α-Terpineol | 1,189 |
| 20.528 | 0.23 | Hexyl butyrate | 1,192 |
| 20.677 | 0.12 | γ-Terpineol | |
| 20.872 | 0.02 | mw 152 | |
| 21.561 | 0.13 | Citronellol | 1,228 |
| 21.821 | 0.03 | mw 152 | |
| 23.234 | 0.19 | Bornyl acetate | 1,284 |
| 24.713 | 0.06 | Methyl anthranilate | 1,343 |
| 25.884 | 0.22 | Hexyl hexanoate | 1,384 |
| 26.507 | 0.06 | β-Cis-caryophyllene | 1,406 |
| 26.851 | 100.00 | β-Caryophyllene | 1,418 |
| 27.056 | 0.26 | 10,10-Dimethyl-2,6-dimethylenebicyclo[7.2.0]undecane | 1,440 |
| 27.586 | 0.10 | Sesquiterpene/mw 204 | |
| 27.698 | 10.65 | α-Humulene | 1,454 |
| 27.884 | 0.10 | Alloaromadendrene | 1,461 |
| 29.195 | 0.03 | Trans-calamenene | 1,529 |
| 29.316 | 0.38 | Nerolidol | 1,534 |
| 29.808 | 0.80 | Trans-nerolidol | 1,564 |
| 30.041 | 0.07 | Sesquiterpenoid − mw 220 | |
| 30.190 | 1.31 | Caryophyllene oxide | 1,581 |
| 30.552 | 0.09 | Humulene epoxide II | 1,606 |
| 30.887 | 0.05 | 11,11-Dimethyl-4,8-dimethylenebicyclo[7.2.0]undecan-3-ol | |
| 33.770 | 0.11 | m-Camphorene | 1,960 |
| 34.030 | 0.04 | p-Camphorene | 1,995 |
The bold value represents the main compound in the sample.
Discussion
The purpose of this study was to identify and compare different strains of C. sativa L. with emphasis on terpenes/terpenoids percentages. We tried to identify the most common, the most abundant, and the most interesting compounds in dry flowering tops and etheric oils of hemp and cannabis.
From the chromatograms and tables, one can see that there are many different chemotypes of C. sativa according to their terpenes/terpenoids and that the main volatiles can differ one from the other. This will also influence the industrial, medicinal, and recreational use of this plant. The most important is the content of these volatile compounds in medicine as they will influence the treatment of different diseases.
Comparison of essential oils from hemp and cannabis gave different results. In essential oil of hemp, there were mainly monoterpenes, while in cannabis, the sesquiterpenes/sesquiterpenoids predominate. In any of the 108 chemotypes, the main compounds were β-caryophyllene, β-myrcene, α-pinene, α-humulene, limonene, and β-pinene.
Between all the 108 chemotypes were the ones where the main terpene/terpenoid was β-caryophyllene, β-myrcene, α-pinene, limonene, terpinolene, linalool, selina-3,7(11)-diene, γ-selinene, 10-epi-γ-eudesmol, β-eudesmol, α-eudesmol, bulnesol, or α-bisabolol. In plant material (inflorescence) of cannabis (54 chemotypes), the main compounds were β-caryophyllene, β-myrcene, guaiol, 10-epi-γ-eudesmol, selina-3,7(11)-diene, and α-humulene.
When we evaluated hemp and cannabis inflorescence and essential oil samples for 10 the main terpenes in any from 108 analyzed chemotypes, between them were 58 different terpenes/terpenoids. From these 44 different terpenes/terpenoids were found in 54 chemotypes of cannabis inflorescence samples, 27 different ones in 46 chemotypes of cannabis essential oils, and 17 different ones were found in 8 chemotypes of hemp essential oils. Sometimes we can see that the main terpene/terpenoid does not reach 20% of total ones in the analyzed sample: Kilimanjaro − α-pinene (17.45%) (Fig. 4); Durban Poison − β-myrcene (16.75%) (Fig. 5); Edom − β-caryophyllene (15.74%) (Fig. 9); Lemon OG Kush − selina-3,7(11)-diene (10.03%) (Fig. 10); Fuji − γ-selinene (10.85%) (Fig. 11); Lemon Deluxe − 10-epi-γ-eudesmol (10.37%) (Fig. 12); Alaska − β-eudesmol (8.96%) (Fig. 13); El Na − α-eudesmol (9.66%) (Fig. 14); Berry Deluxe − bulnesol (14.72%) (Fig. 15); and Bubble Gum − α-bisabolol (16.03%) (Fig. 16).
It is also worth to discuss degree of terpenes/terpenoids diversity, since it is very different between various chemotypes. Unfortunately, we did not have access to the hemp inflorescence, so we just present only inflorescences of different cannabis chemotypes (Table 29). When we compare hemp essential oil (Table 30) and cannabis essential oil (Table 31), it became clear that almost in all cannabis essential oil samples, the concentration of the main terpene/terpenoid is higher than that in hemp ones. Moreover, in 8 cases, concentration of the main terpene/terpenoid in cannabis essential oil is higher than 50% of total amount of terpenes/terpenoids. It is interesting that the main terpenes/terpenoids above 20% in the analyzed samples were only β-caryophyllene (39×), α-pinene (8×), β-myrcene (8×), terpinolene (8×), limonene (8×), selina-3,7(11)-diene (2×), and linalool (1×).
Table 29.
Inflorescences of cannabis chemotypes (the main terpene in the sample is above 20% of total)
| Chemotype | % of total terpenes/terpenoids | Terpene/terpenoid |
|---|---|---|
| Doblin | 33.37 | β-Caryophyllene |
|
| ||
| Jericho | 30.21 | α-Pinene |
|
| ||
| White widow | 27.51 | α-Pinene |
|
| ||
| Pandora's box dry | 25.32 | β-Caryophyllene |
|
| ||
| Tel Aviv | 24.99 | α-Pinene |
|
| ||
| Pandora's box fresh | 24.55 | Terpinolene |
|
| ||
| 21.16 | β-Caryophyllene | |
|
| ||
| DOV | 22.45 | β-Myrcene |
|
| ||
| Maui Waui | 22.09 | Selina-3,7(11)-diene |
|
| ||
| Kira Kush | 21.61 | Selina-3,7(11)-diene |
|
| ||
| Jack Herer | 21.39 | Terpinolene |
|
| ||
| Kush | 20.66 | α-Pinene |
Table 30.
Hemp essential oils (the main terpene in the sample is above 20% of total)
| Chemotype | % of total terpenes/terpenoids | Terpene/terpenoid |
|---|---|---|
| Tisza | 27.60 | β-Caryophyllene |
| Fedora | 26.19 | β-Caryophyllene |
| Ferimon | 23.63 | β-Caryophyllene |
| Futura | 21.47 | β-Caryophyllene |
| Tiborszallasi | 21.27 | β-Myrcene |
| Felina | 21.14 | β-Myrcene |
Table 31.
Cannabis essential oils (the main terpene/s in the sample is above 20% of total)
| Chemotype | % of total terpenes/terpenoids | Terpene/terpenoid |
|---|---|---|
| Key Lime Pie | 65.86 | β-Caryophyllene |
| Gorilla Glue | 65.77 | β-Caryophyllene |
| SFV OG | 57.62 | β-Caryophyllene |
| 3 Kings | 56.92 | β-Caryophyllene |
| 25.31 | Limonene | |
| OG Kush | 54.26 | β-Caryophyllene |
| Lemon cookies | 54.13 | β-Caryophyllene |
| Truth | 50.15 | β-Caryophyllene |
| 20.94 | β-Myrcene | |
| Gipsy Haze | 50.09 | Terpinolene |
| Gelato | 49.06 | β-Caryophyllene |
| 30.64 | Limonene | |
| Grapefruit OG | 46.68 | β-Caryophyllene |
| 26.42 | Terpinolene | |
| Girl Scout Cookies | 45.71 | β-Caryophyllene |
| 22.89 | Limonene | |
| Holy Grail Kush | 45.51 | β-Caryophyllene |
| Cheese | 45.25 | β-Caryophyllene |
| Tangie | 45.17 | β-Caryophyllene |
| Chemdawg 4 | 44.90 | β-Caryophyllene |
| 23.16 | Limonene | |
| Orange turbo | 44.36 | β-Caryophyllene |
| Amnesia | 43.92 | Terpinolene |
| 707 Truthband | 42.81 | β-Caryophyllene |
| Sugaree | 41.62 | β-Caryophyllene |
| Veneno | 41.03 | β-Caryophyllene |
| Kashmir Kush | 40.27 | β-Caryophyllene |
| Sour diesel | 39.88 | β-Caryophyllene |
| 28.95 | Limonene | |
| 10K Jack | 39.76 | Terpinolene |
| 22.64 | β-Caryophyllene | |
| Blackberry Kush | 39.69 | β-Caryophyllene |
| Cinderella 99 | 38.78 | β-Myrcene |
| 20.89 | α-Pinene | |
| Chemotype | % of total terpenes/terpenoids | Terpene/terpenoid |
| 24K Gold | 37.13 | β-Caryophyllene |
| 22.95 | Limonene | |
| Critical | 37.05 | α-Pinene |
| Chocolate Mint OG | 36.59 | Limonene |
| 34.92 | β-Caryophyllene | |
| AK-47 | 36.43 | β-Myrcene |
| 31.86 | β-Caryophyllene | |
| Sweet tooth | 36.04 | Limonene |
| 27.65 | β-Caryophyllene | |
| Critical Jack | 35.56 | Terpinolene |
| Wifi OG | 34.88 | α-Pinene |
| 26.57 | β-Caryophyllene | |
| Black dream | 34.90 | β-Caryophyllene |
| 23.39 | α-Pinene | |
| TNT Kush | 34.45 | β-Myrcene |
| Monster | 33.84 | β-Caryophyllene |
| 20.35 | β-Myrcene | |
| Furious Candy | 33.40 | β-Caryophyllene |
| 24.47 | β-Myrcene | |
| Pink plant | 33.33 | β-Caryophyllene |
| 21.40 | β-Myrcene | |
| M.I.B. | 30.08 | β-Caryophyllene |
| 25.46 | β-Myrcene | |
| Jamaican dream | 28.35 | β-Caryophyllene |
| 22.13 | α-Pinene | |
| Lavender | 26.36 | α-Pinene |
| 25.67 | β-Caryophyllene | |
| High level | 25.05 | α-Pinene |
| 22.16 | β-Caryophyllene | |
| Mojito | 21.36 | β-Caryophyllene |
| Sensi star | 21.25 | Terpinolene |
| Dosidos | 20.33 | Linalool |
β-Caryophyllene and β-myrcene are without any doubt the most common (and often the main ones) terpenes in C. sativa L. plant. Because of their unique properties, these are probably the terpenes that play an important role in medicinal properties of hemp and cannabis.
We would like to inform readers also about the monoterpenes/monoterpenoids-sesquiterpene/sesquiterpenoids ratio in the analyzed samples. Our results show that this ratio does not depend if the sample is hemp, cannabis, or essential oils from them. The content of monoterpenes/monoterpenoids prevailed in most samples, sometimes several times more. Very rarely, the content of sesquiterpenes/sesquiterpenoids was higher, but only slightly more. There is a difference between fresh and dry plant monoterpene content, and between etheric oil and organic solvent extract (it also depends on the type of solvent). Different work-up of different solvent extracts can lead to the loss of monoterpenes. Essential oil and organic solvent extract constituents can be different before and after drying process, and before and after storage. A considerably lower content of monoterpenes can be found in the solvent extract compared with the essential oil from steam distillation. Steam distillation also promotes dehydration of labile alcohols.
The most important is which type of information we need. From biogenetic point of view, the best is headspace gas chromatography of the fresh plant material immediately after its harvest. From pharmacological point of view, the best is to analyze the preparation which is used for medicinal purposes. It is without any doubt that in the course from the living material in the plant to the product we are analyzing, there is a loss of volatile substances, especially monoterpenes.
Many terpenes/terpenoids have a therapeutic power in their mixtures with or without phytocannabinoids, and can be very useful and sometime powerful in treatment of diseases. In the Introduction, we have mentioned several terpenes and their potential medicinal properties. We found more therapeutically interesting structures upon doing our analyses. For instance, 10-epi-γ-eudesmol is highly effective against melanoma and column carcinoma cells proliferation [108]; β-eudesmol is antihepatotoxic, antiangiogenic, and antitumor [109, 110, 111]; α-eudesmol induces apoptosis [112]; bulnesol possesses antitussive and expectorant activity [113]; α-bisabolol induces apoptosis of malignant tumor cells, cytotoxicity, and antigenotoxicity [114, 115, 116, 117, 118]; guaiol is an anti-inflammatory, antimicrobial, and analgesic terpenoid [70, 71]; and α-humulene acts as an appetite suppressant, antibacterial, and antitumor agent and is an effective anti-inflammatory and analgesic sesquiterpene [73, 119]. Monoterpenoid constituents have antioxidant, anti-inflammatory, and estrogenic effects, and these activities are also relevant to current Alzheimer's disease therapy. Several monoterpenoid alcohols demonstrated good anti-Parkinson's disease activity. Many monoterpenoids demonstrated promising neuroprotective activity mediated by various systems [120]. There are some other important terpenes such as selina-3,7(11)-diene or γ-selinene which have never been tested for their pharmacological properties.
We must realize that if we evaluate the relative content of terpenes (%) in the sample, it has nothing to do with their quantitative content in the plant. It is clear from Figure 22 and Table 32 that when we analyze a mixture of standards, where each one has the same concentration (25 ppm in hexane), the area of some peaks is up to 3 times smaller than the area of the largest peak. This is very good for comparing samples and the ratio of the content terpenes/terpenoids, but not for quantifying them.
Fig. 22.
GC/MS chromatogram of 23 terpenes/terpenoids standards (RESTEK Catalog. No. 34095 and 34096). Key: 1, α-pinene; 2, camphene; 3, (−)-β-pinene; 4, β-myrcene; 5, Δ3-carene; 6, α-terpinene; 7, p-cymene; 8, d-limonene; 9, 1,8-cineole; (10, 31% α-ocimene + 11, 69% β-ocimene); 12, γ-terpinene; 13, terpinolene; 14, linalool; 15, (−)-isopulegol; 16, geraniol; 17, β-caryophyllene; 18, α-humulene; (19, 39% cis-nerolidol + 20, 61% trans-nerolidol); 21, (−)-caryophyllene oxide; 22, (−)-guaiol; 23, (−)-α-bisabolol.
Table 32.
The relative content of terpenes (%) in the sample analyzed by GC/MS
| Peak | Compound | % | Normalized, % |
|---|---|---|---|
| 1 | α-Pinene | 4.77 | 61.98 |
| 2 | Camphene | 4.77 | 61.96 |
| 3 | β-Pinene | 4.28 | 55.58 |
| 4 | Myrcene | 3.39 | 44.04 |
| 5 | Δ3-Carene | 4.61 | 59.88 |
| 6 | α-Terpinene | 5.09 | 66.16 |
| 7 | p-Cymene | 6.15 | 79.84 |
| 8 | Limonene | 3.81 | 49.50 |
| 9 | 1,8-Cineole | 7.70 | 100.00 |
| 10 | α-Ocimene (31%) | 1.12 | 14.51 |
| 11 | β-Ocimene (69%) | 2.92 | 37.96 |
| 12 | γ-Terpinene | 5.05 | 65.55 |
| 13 | Terpinolene | 4.97 | 64.54 |
| 14 | Linalool | 3.19 | 41.46 |
| 15 | Isopulegol | 2.61 | 33.89 |
| 16 | Geraniol | 2.53 | 32.93 |
| 17 | β-Caryophyllene | 5.59 | 72.56 |
| 18 | α-Humulene | 5.72 | 74.29 |
| 19 | Cis-nerolidol (38%) | 1.20 | 15.59 |
| 20 | Trans-nerolidol (61%) | 2.36 | 30.67 |
| 21 | Caryophyllene oxide | 7.59 | 98.58 |
| 22 | Guaiol | 5.50 | 71.51 |
| 23 | α-Bisabolol | 5.09 | 66.18 |
| 100.00 |
Today, we know that in the brain concentrations of anandamide (AEA) are in picomoles and 2-arachidonoylglycerol (2-AG) in nanomoles (what is ng AEA/g brain or µg 2-AG/g brain) [121]. Terpenes/terpenoids are in dried cannabis flower material (which is used for medicinal purposes) usually at concentrations mg/g [122]. As mentioned in Casano et al. [123], “the relative content of terpenoids is strongly inherited while total yield per weight of tissue is more subjected to environmental factors.” Relative content (%) of terpenes/terpenoids is more often used for chemosystematic studies (and it was used also in this publication). What can we deduce from the previous few sentences? Receptors need their ligands in very low concentrations, so it is quite possible that these terpenes/terpenoids are important in the treatment, which are in the plant in very low concentrations and which we are not considering today.
The healing power of cannabis most likely resides in terpenes/terpenoids and phytocannabinoids, of which particular compounds, their amount, and the ratio to each other play the most important role in the treatment of particular diseases. It should be emphasized here that flavonoids, flavonoid glycosides, polyphenol, and the other biodynamic compounds will play this game too.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There were no external funding sources to the study in the preparation of data or the manuscript.
Author Contributions
Both authors contributed equally to the manuscript.
Acknowledgements
I would like to express my special appreciation and thanks to Mr. Boris Valte from Slovenia and to Mr. Leopold Svatý from the Czech Republic for hemp essential oils.
References
- 1. Strainprint Technologies Inc., Toronto, Canada.
- 2.Hanuš LO. Pharmacological and therapeutic secrets of plant and brain (endo)cannabinoids. Med Res Rev. 2009;29((2)):213–71. doi: 10.1002/med.20135. [DOI] [PubMed] [Google Scholar]
- 3.Krejčí Z. (The antibiotic effect of Cannabis indica.) Brno, Czechoslovakia: Masaryk University; 1950. Antibioticky princip Cannabis indica. p. p. 106. [Google Scholar]
- 4.Krejčí Z. The antibacterial effect of Cannabis indica. Lék listy. 1952;7:500. [PubMed] [Google Scholar]
- 5.Konopí jako lék (Cannabis as a medicine.) Acta Univ Palacki Olomuc. 1955;6:27–114. [Google Scholar]
- 6.Krejčí Z, Šantavý F. Isolace dalších látek z listí indického konopí Cannabis sativa L. (Isolation of other substances from the leaves of the Indian hemp (Cannabis sativa L., varietas indica) Acta Univ Palacki Olomuc. 1955;6:59–66. [Google Scholar]
- 7.Krejčí Z, Horák M, Šantavý F. Konstituce kyseliny kanabidiolové a kyseliny b.t. 133°C isolovaných Z Cannabis sativa L. (Constitution of the cannabidiolic acid and of an acid of the M.P. 133, isolated from Cannabis sativa L.) Acta Univ Palacki Olomuc. 1958;16:9–17. [Google Scholar]
- 8.Hanuš L, Krejčí Z, Hruban L. Isolation of cannabidiolic acid from Turkish variety of cannabis cultivated for fibre. Acta Univ Olomuc Fac Med. 1975;74:167–72. [Google Scholar]
- 9.Kabelík J, Krejčí Z, Šantavý F. Cannabis as a medicament. Bull Narc. 1960;12:5–23. [Google Scholar]
- 10.Krejčí Z, Antibakterielní látky v prevenci i terapii infekcí. Olomouc, Czechoslovakia: Palacký University; 1961. (The antibacterial substances from cannabis in the treatment and prevention of infections.) p. p. 259. [Google Scholar]
- 11.Jacob A, Todd AR. Cannabis indica. Part II. Isolation of cannabidiol from Egyptian hashish. Observations on the structure of cannabinol. J Chem Soc. 1940:649–53. [Google Scholar]
- 12.Ghosh R, Todd AR, Wilkinson S. Cannabis indica. Part V. The synthesis of cannabinol. J Chem Soc. 1940:1393–6. [Google Scholar]
- 13.Adams R, Baker BR, Wearn RB, Structure of cannabinol. III. Synthesis of cannabinol, 1-Hydroxy-3-n-amyl-6,6,9-trimethyl-6-dibenzopyran. J Amer Chem Soc. 1940;62:2204–7. [Google Scholar]
- 14.Hanuš L, Tesarík K, Krejcí Z. Capillary gas chromatography of natural substances from Cannabis sativa L. I. Cannabinol and cannabinolic acid: artefacts. Acta Univ Palacki Olomuc Fac Med. 1985;108:29–38. [PubMed] [Google Scholar]
- 15.Adams R, Wolff H, Cain CK, Clark JH, Structure of cannabidiol. V. Position of the alicyclic double bonds. J Amer Chem Soc. 1940;62:2215–9. [Google Scholar]
- 16.Mechoulam R, Shvo Y. Hashish-I. Tetrahedron. 1963;19((12)):2073–8. doi: 10.1016/0040-4020(63)85022-x. [DOI] [PubMed] [Google Scholar]
- 17.Adams R, Pease DC, Cain CK, Baker BR, Clark JH, Wolff H, et al. Conversion of cannabidiol to a product with marihuana activity. A type reaction for synthesis of analogous substances. Conversion of cannabidiol to cannabinol. J Amer Chem Soc. 1940;62:2245–6. [Google Scholar]
- 18.Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86((8)):1646–7. [Google Scholar]
- 19.Šantavý F. Notes on the structure of cannabidiol compounds. Acta Univ Palacki Olomuc Fac Med. 1964;35:5–9. [Google Scholar]
- 20.Hanuš LO, Meyer SM, Muñoz E, Taglialatela-Scafati O, Appendino G. Phytocannabinoids: a unified critical inventory. Nat Prod Rep. 2016;33:1357–92. doi: 10.1039/c6np00074f. [DOI] [PubMed] [Google Scholar]
- 21.Berman P, Futoran K, Lewitus GM, Mukha D, Benami M, Shlomi T, et al. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci Rep. 2018;8((1)):14280. doi: 10.1038/s41598-018-32651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Citti C, Linciano P, Russo F, Luongo L, Iannotta M, Maione S, et al. A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ9-tetrahydrocannabinol: Δ9-tetrahydrocannabiphorol. Sci Rep. 2019;9((1)):20335. doi: 10.1038/s41598-019-56785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basas-Jaumandreu J, de las Heras FXC. GC-MS metabolite profile and identification of unusual homologous cannabinoids in high potency Cannabis sativa. Planta Med. 2020;86((5)):338–347. doi: 10.1055/a-1110-1045. [DOI] [PubMed] [Google Scholar]
- 24.Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34((5)):605–13. [PubMed] [Google Scholar]
- 25.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346((6284)):561–4. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 26.Devane WA, Hanuš L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258((5090)):1946–9. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 27.Hanuš LO, Discovery and isolation of anandamide and other endocannabinoids, Chapter 12, p. 1828–41 . In: Cannabinoids in nature and medicine. Lambert DM, editor. Zurich: Verlag Helvetica Chimica Acta, Wiley-VCH; 2009. p. p. 416. [Google Scholar]
- 28.Hanuš LO. Discovery and isolation of anandamide and other endocannabinoids. Chem Biodivers. 2007;4((8)):1828–41. doi: 10.1002/cbdv.200790154. [DOI] [PubMed] [Google Scholar]
- 29.Hanuš L, Gopher A, Almog S, Mechoulam R. Two new unsaturated fatty acid ethanolamides in brain that bind to the cannabinoid receptor. J Med Chem. 1993;36((20)):3032–4. doi: 10.1021/jm00072a026. [DOI] [PubMed] [Google Scholar]
- 30.Mechoulam R, Ben-Shabat S, Hanuš L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50((1)):83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 31.Hanuš L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE. 2-Arachidonyl glyceryl ether, a novel endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci U S A. 2001;98((7)):3662–5. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonn-Miller M, Pollack MO, Jr, Casarett D, Dart R, ElSohly M, Good L, et al. Priority considerations for medicinal cannabis-related research. Cannabis Cannabinoid Res. 2019;4((3)):139–57. doi: 10.1089/can.2019.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bohlig JF. Cannabis sativa und Urtica dioica, chemisch analysirt. Jahrbuch für practische Pharmacie und verwandte Fächer, herausgegeben von der pharmaceutischen Gesellschaft der Pfalz, Verlag von J. J. Tascher, Kaiserslautern. 1840:p. 1–58. [Google Scholar]
- 34.Bolas T, Francis EEH. XXXV.-On the products of the action of nitric acid on the resinous extract of Indian hemp. J Chem Soc. 1869;22:417–9. [Google Scholar]
- 35.Valente L, Sull'essenza di canapa. [Essential oil from hemp.] Gazz Chim Ital. 1880;10:479–81. [Google Scholar]
- 36.Valente L, Sull'idrocarburo estratto della canapa. [On the hydrocarbon extract of Indian hemp.] Gazz Chim Ital. 1881;11:196–8. [Google Scholar]
- 37.Valente L. Studi sull'essenza di canapa. Atti della Reale Academia dei Lincei. 1881;5:126–8. [Google Scholar]
- 38.Vignolo G. Sull'essenza di Cannabis indica. Atti della Reale Accademia dei Lincei. 1894;5((3)):404–7. [Google Scholar]
- 39.Vignolo G. Sull'essenza di Cannabis indica. (Essence of Cannabis indica.) Gazz Chim Ital. 1895;25((i)):110–4. [Google Scholar]
- 40.Wood TB, Spivey WTN, Easterfield TH, XL.-Charas. The resin of Indian hemp. J Chem Soc Trans. 1896;69:539–46. [Google Scholar]
- 41.Simonsen JL, Todd AR. Cannabis indica. Part X. The essential oil from Egyptian hashish. J Chem Soc. 1942;1942((1)):188–91. [Google Scholar]
- 42.Ružička L. The isoprene rule and the biogenesis of terpenic compounds. Experientia. 1953;50((4)):395–405. [PubMed] [Google Scholar]
- 43.Kekulé A. Lehrbuch der organischen Chemie. Vol. 2. Erlangen, Germany: Ferdinand Enke; 1866. p. p. 464. [Google Scholar]
- 44.Ben-Shabat S, Fride E, Sheskin T, Tamiri T, Rhee MH, Vogel Z, et al. An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol. 1998;353((1)):23–31. doi: 10.1016/s0014-2999(98)00392-6. [DOI] [PubMed] [Google Scholar]
- 45.Russo EB. The case for the entourage effect and conventional breeding of clinical cannabis: no “strain,” no gain. Front Plant Sci. 2018;9:1969. doi: 10.3389/fpls.2018.01969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blasco-Benito S, Seijo-Vila M, Caro-Villalobos M, Tundidor I, Andradas C, García-Taboada E, et al. Appraising the “entourage effect”: antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochem Pharmacol. 2018 Nov;157:285–93. doi: 10.1016/j.bcp.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 47.Krejčí Z, Hanuš L, Yoshida T, Braenden OJ. The effect of climatic and ecologic conditions upon the formation and the amount of cannabinoid substances in the cannabis of various provenance. Acta Univ Olomuc Fac Med. 1975;74:147–60. [Google Scholar]
- 48.Hanuš L, Krejcí Z. Dynamics of changes in the content of cannabinoid substances during the vegetation period of Cannabis sativa L. Acta Univ Palacki Olomuc Fac Med. 1986;114:11–29. [PubMed] [Google Scholar]
- 49.Hanuš L, Subová D. The amount of main cannabinoid substances in hemp, cultivated for industrial fibre production and their changes in the course of one vegetation period. Acta Univ Palacki Olomuc Fac Med. 1989;122:11–23. [PubMed] [Google Scholar]
- 50.Hanuš L, Dostálová M. The effect of soil fertilization on the formation and the amount of cannabinoid substances in Cannabis sativa L. in the course of one vegetation period. Acta Univ Palacki Olomuc Fac Med. 1994;138:11–5. [PubMed] [Google Scholar]
- 51.Aizpurua-Olaizola O, Soydaner U, Öztürk E, Schibano D, Simsir Y, Navarro P, et al. Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J Nat Prod. 2016;79((2)):324–31. doi: 10.1021/acs.jnatprod.5b00949. [DOI] [PubMed] [Google Scholar]
- 52.Koltai H, Namdar D, Cannabis phytomolecule ‘entourage’: from domestication to medical use Trends Plant Sci. Forthcoming. 2020 doi: 10.1016/j.tplants.2020.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Zaklin R. Terpene therapy. Abstracts of Papers, 256th ACS National Meeting & Exposition, Boston, MA. CHAS-50. 2018. Aug, pp. 19–23.
- 54.Gulluni N, Re T, Loiacono I, Lanzo G, Gori L, Macchi C, et al. Cannabis essential oil: a preliminary study for the evaluation of the brain effects. Evid Based Complement Alternat Med. 2018;2018:1709182. doi: 10.1155/2018/1709182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jansen C, Shimoda LMN, Kawakami JK, Ang L, Bacani AJ, Baker JD, et al. Myrcene and terpene regulation of TRPV1. Channels. 2019;13((1)):344–66. doi: 10.1080/19336950.2019.1654347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kozioł A, Stryjewska A, Librowski T, Sałat K, Gaweł M, Moniczewski A, et al. An overview of the pharmacological properties and potential applications of natural monoterpenes. Mini Rev Med Chem. 2014;14((14)):1156–68. doi: 10.2174/1389557514666141127145820. [DOI] [PubMed] [Google Scholar]
- 57.Gertsch J, Leonti M, Raduner S, Racz I, Jian-Zhong C, Xiang-Qun X, et al. Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci U S A. 2008;105((26)):9099–104. doi: 10.1073/pnas.0803601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma C, Al Kaabi JM, Nurulain SM, Goyal SN, Kamal MA, Ojha S. Polypharmacological properties and therapeutic potential of β-caryophyllene: a dietary phytocannabinoid of pharmaceutical promise. Curr Pharm Des. 2016;22((21)):3237–−64.[. doi: 10.2174/1381612822666160311115226. [DOI] [PubMed] [Google Scholar]
- 59.Francomano F, Caruso A, Barbarossa A, Fazio A, La Torre C, Ceramella J, et al. β-Caryophyllene: a sesquiterpene with countless biological properties. Appl Sci. 2019;9((24)):5420. [Google Scholar]
- 60.Astani A, Reichling J, Schnitzler P. Screening for antiviral activities of isolated compounds from essential oils. Evid Based Complement Alternat Med. 2011;2011:253643. doi: 10.1093/ecam/nep187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salehi B, Upadhyay S, Orhan IE, Jugran AK, Jayaweera SLD, Dias DA, et al. Therapeutic potential of α- and β-pinene: a miracle gift of nature. Biomolecules. 2019;9:738. doi: 10.3390/biom9110738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Erasto P, Viljoen AM. Limonene: a review: biosynthetic, ecological and pharmacological relevance. Nat Prod Commun. 2008;3:1193–202. [Google Scholar]
- 63.Sun J. D-limonene: safety and clinical applications. Alt Med Rev. 2007;12:259–64. [PubMed] [Google Scholar]
- 64.Kamatou GPP, Viljoen AM. Linalool: a review of a biologically active compound of commercial importance. Nat Prod Commun. 2008;3:1183–92. [Google Scholar]
- 65.Khaleel C, Tabanca N, Buchbauer G. α-Terpineol, a natural monoterpene: a review of its biological properties. Open Chem. 2018;16((1)):349–61. [Google Scholar]
- 66.Fidyt K, Fiedorowicz A, Strządała L, Szumny A. β-Caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016;5((10)):3007–17. doi: 10.1002/cam4.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang JH, Sun HL, Chen SY, Zeng L, Wang TT. Anti-fungal activity, mechanism studies on α-phellandrene and nonanal against Penicillium cyclopium. Bot Stud. 2017;58((1)):13. doi: 10.1186/s40529-017-0168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thakre AD, Mulange SV, Kodgire SS, Zore GB, Karuppayil SM. Effects of cinnamaldehyde, ocimene, camphene, curcumin and farnesene on Candida albicans. AiM. 2016;6((9)):627–43. [Google Scholar]
- 69.Vallianou I, Hadzopoulou-Cladaras M. Camphene, a plant derived monoterpene, exerts its hypolipidemic action by affecting SREBP-1 and MTP expression. PLoS One. 2016;11((1)):e0147117. doi: 10.1371/journal.pone.0147117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang Q, Wu J, Luo Y, Huang N, Zhen N, Zhou Y, et al. (−)-Guaiol regulates RAD51 stability via autophagy to induce cell apoptosis in non-small cell lung cancer. Oncotarget. 2016;7((38)):62585–97. doi: 10.18632/oncotarget.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang X, Zhu J, Wu J, Huang N, Cui Z, Luo Y, et al. (−)-Guaiol regulates autophagic cell death depending on mTOR signaling in NSCLC. Cancer Biol Ther. 2018;19((8)):706–14. doi: 10.1080/15384047.2018.1451277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jang H-I, Ki-Jong R, Eom Y-B. Antibacterial and antibiofilm effects of α-humulene against Bacteroides fragilis. Can J Microbiol. 2020;66:389–399. doi: 10.1139/cjm-2020-0004. [DOI] [PubMed] [Google Scholar]
- 73.Rogerio AP, Andrade EL, Leite DF, Figueiredo CP, Calixto JB. Preventive and therapeutic anti-inflammatory properties of the sesquiterpene alpha-humulene in experimental airways allergic inflammation. Br J Pharmacol. 2009;158((4)):1074–87. doi: 10.1111/j.1476-5381.2009.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Legault J, Dahl W, Debiton E, Pichette A, Madelmont JC. Antitumor activity of balsam fir oil: production of reactive oxygen species induced by alpha-humulene as possible mechanism of action. Planta Med. 2003;69((5)):402–7. doi: 10.1055/s-2003-39695. [DOI] [PubMed] [Google Scholar]
- 75.Guimarães AG, Quintans JS, Quintans LJ. Jr Monoterpenes with analgesic activity: a systematic review. Phytother Res. 2013;27((1)):1–15. doi: 10.1002/ptr.4686. [DOI] [PubMed] [Google Scholar]
- 76.Ramalho TR, Pacheco de Oliveira MT, Lima AL, Bezerra-Santos CR, Piuvezam MR. Erratum for: gamma-terpinene modulates acute inflammatory response in mice. Planta Med. 2015;81((14)):E3–54. doi: 10.1055/s-0035-1557790. [DOI] [PubMed] [Google Scholar]
- 77.Yao YQ, Ding X, Jia YC, Huang CX, Wang YZ, Xu YH. Anti-tumor effect of beta-elemene in glioblastoma cells depends on p38 MAPK activation. Cancer Lett. 2008;264((1)):127–34. doi: 10.1016/j.canlet.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 78.Li QQ, Wang G, Zhang M, Cuff CF, Huang L, Reed E. Beta-elemene, a novel plant-derived antineoplastic agent, increases cisplatin chemosensitivity of lung tumor cells by triggering apoptosis. Oncol Rep. 2009;22((1)):161–70. doi: 10.3892/or_00000420. [DOI] [PubMed] [Google Scholar]
- 79.Li QQ, Lee RX, Liang H, Zhong Y. Anticancer activity of β-elemene and its synthetic analogs in human malignant brain tumor cells. Anticancer Res. 2013;33((1)):65–76. [PMC free article] [PubMed] [Google Scholar]
- 80.Liu JS, He SC, Zhang ZL, Chen R, Fan L, Qiu GL, et al. Anticancer effects of β-elemene in gastric cancer cells and its potential underlying proteins: a proteomic study. Oncol Rep. 2014;32((6)):2635–47. doi: 10.3892/or.2014.3490. [DOI] [PubMed] [Google Scholar]
- 81.Silva MP, de Oliveira RN, Mengarda AC, Roquini DB, Allegretti SM, Salvadori MC, et al. Antiparasitic activity of nerolidol in a mouse model of schistosomiasis. Int J Antimicrob Agents. 2017;50((3)):467–72. doi: 10.1016/j.ijantimicag.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 82.Alonso L, Fernandes KS, Mendanha SA, Gonçalves PJ, Gomes RS, Dorta ML, et al. In vitro antileishmanial and cytotoxic activities of nerolidol are associated with changes in plasma membrane dynamics. Biochim Biophys Acta Biomembr. 2019;1861((6)):1049–56. doi: 10.1016/j.bbamem.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 83.Leite MC, Bezerra AP, de Sousa JP, Guerra FQ, Lima EO. Evaluation of antifungal activity and mechanism of action of citral against Candida albicans. Evid Based Complement Alternat Med. 2014;2014:378280. doi: 10.1155/2014/378280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thomas ML, de Antueno R, Coyle KM, Sultan M, Cruickshank BM, Giacomantonio MA, et al. Citral reduces breast tumor growth by inhibiting the cancer stem cell marker ALDH1A3. Mol Oncol. 2016;10((9)):1485–96. doi: 10.1016/j.molonc.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shi C, Song K, Zhang X, Sun Y, Sui Y, Chen Y, et al. Antimicrobial activity and possible mechanism of action of citral against Cronobacter sakazakii. PLoS One. 2016;11((7)):e0159006. doi: 10.1371/journal.pone.0159006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sheikh BY, Sarker MMR, Kamarudin MNA, Mohan G. Antiproliferative and apoptosis inducing effects of citral via p53 and ROS-induced mitochondrial-mediated apoptosis in human colorectal HCT116 and HT29 cell lines. Biomed Pharmacother. 2017;96:834–46. doi: 10.1016/j.biopha.2017.10.038. [DOI] [PubMed] [Google Scholar]
- 87.Sanches LJ, Marinello PC, Panis C, Fagundes TR, Morgado-Díaz JA, de-Freitas-Junior JC, et al. Cytotoxicity of citral against melanoma cells: the involvement of oxidative stress generation and cell growth protein reduction. Tumour Biol. 2017;39((3)):1010428317695914. doi: 10.1177/1010428317695914. [DOI] [PubMed] [Google Scholar]
- 88.Zielińska A, Martins-Gomes C, Ferreira NR, Silva AM, Nowak I, Souto EB. Anti-inflammatory and anti-cancer activity of citral: optimization of citral-loaded solid lipid nanoparticles (SLN) using experimental factorial design and LUMiSizer®. Int J Pharm. 2018;553((1–2)):428–40. doi: 10.1016/j.ijpharm.2018.10.065. [DOI] [PubMed] [Google Scholar]
- 89.Kremer JL, Melo GP, Marinello PC, Bordini HP, Rossaneis AC, Sábio LR, et al. Citral prevents UVB-induced skin carcinogenesis in hairless mice. J Photochem Photobiol B Biol. 2019;198:111565. doi: 10.1016/j.jphotobiol.2019.111565. [DOI] [PubMed] [Google Scholar]
- 90.Nigjeh SE, Yeap SK, Nordin N, Rahman H, Rosli R. In vivo anti-tumor effects of citral on 4T1 breast cancer cells via induction of apoptosis and downregulation of aldehyde dehydrogenase activity. Molecules. 2019;24((18)):3241. doi: 10.3390/molecules24183241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tisserand R, Young R. Essential oil safety: a guide for health care professionals. 2nd ed. London: Elsevier; 2014. p. p. 780. [Google Scholar]
- 92.Karlberg AT, Magnusson K, Nilsson U. Air oxidation of d-limonene (the citrus solvent) creates potent allergens. Contact Derm. 1992;26((5)):332–40. doi: 10.1111/j.1600-0536.1992.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 93.Bråred Christensson J, Karlberg AT, Andersen KE, Bruze M, Johansen JD, Garcia-Bravo B, et al. Oxidized limonene and oxidized linalool: concomitant contact allergy to common fragrance terpenes. Contact Derm. 2016;74((5)):273–80. doi: 10.1111/cod.12545. [DOI] [PubMed] [Google Scholar]
- 94.Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163((7)):1344–64. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kovalchuk I, Pellino M, Rigault PO, van Velzen R, Ebersbach J, Ashnest JR, et al. The genomics of cannabis and its close relatives. Ann Rev Plant Biol. 2020;71:713–739. doi: 10.1146/annurev-arplant-081519-040203. [DOI] [PubMed] [Google Scholar]
- 96.Allen KD, McKernan K, Pauli C, Roe J, Torres A, Gaudino R. Genomic characterization of the complete terpene synthase gene family from Cannabis sativa. PLos One. 2019;14((9)):e0222363. doi: 10.1371/journal.pone.0222363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Booth JK, Page JE, Bohlmann J. Terpene synthases from Cannabis sativa. PLos One. 2017;12((3)):e0173911. doi: 10.1371/journal.pone.0173911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gunnewich N, Page JE, Kollner TG, Degenhardt J, Kutchan TM. Functional expression and characterization of trichome-specific (−)-limonene synthase and (+)-α-pinene synthase from Cannabis sativa. Nat Prod Commun. 2007;2((3)):223–32. [Google Scholar]
- 99.Rice S, Koziel JA. Characterizing the smell of marijuana by odor impact of volatile compounds: an application of simultaneous chemical and sensory analysis. PLos One. 2015;10((12)):e0144160. doi: 10.1371/journal.pone.0144160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hazekamp A, Tejkalová K, Papadimitriou S. Cannabis: from cultivar to chemovar II-A metabolomics approach to cannabis classification. Cannabis Cannabinoid Res. 2016;1((1)):202–15. [Google Scholar]
- 101.Gallily R, Yekhtin Z, Hanuš LO. The anti-inflammatory properties of terpenoids from cannabis. Cannabis Cannabinoid Res. 2018;3((1)):282–90. doi: 10.1089/can.2018.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Downer EJ. Anti-inflammatory potential of terpenes present in Cannabis sativa L. ACS Chem Neurosci. 2020;11((5)):659–62. doi: 10.1021/acschemneuro.0c00075. [DOI] [PubMed] [Google Scholar]
- 103.Baron EP. Medicinal properties of cannabinoids, terpenes, and flavonoids in cannabis, and benefits in migraine, headache, and pain: an update on current evidence and cannabis science. Headache. 2018;58((7)):1139–86. doi: 10.1111/head.13345. [DOI] [PubMed] [Google Scholar]
- 104.Nuutinen T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur J Med Chem. 2018;157:198–228. doi: 10.1016/j.ejmech.2018.07.076. [DOI] [PubMed] [Google Scholar]
- 105.Namdar D, Voet H, Ajjampura V, Nadarajan S, Mayzlish-Gati E, Mazuz M, et al. Terpenoids and phytocannabinoids co-produced in Cannabis sativa strains show specific interaction for cell cytotoxic activity. Molecules. 2019;24((17)):3031. doi: 10.3390/molecules24173031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferber SG, Namdar D, Hen-Shoval D, Eger G, Koltai H, Shoval G, et al. The “entourage effect”: terpenes coupled with cannabinoids for the treatment of mood disorders and anxiety disorders. Curr Neuropharmacol. 2020;18((2)):87–96. doi: 10.2174/1570159X17666190903103923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Finlay DB, Sircombe KJ, Nimick M, Jones C, Glass M. Terpenoids from cannabis do not mediate an entourage effect by acting at cannabinoid receptors. Front Pharmacol. 2020;11:359. doi: 10.3389/fphar.2020.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Venditti A, Frezza C, Sciubba F, Serafini M, Bianco A, Cianfaglione K, et al. Volatile components, polar constituents and biological activity of tansy daisy (Tanacetum macrophyllum (Waldst. et Kit.) Schultz Bip.) Ind Crops Prod. 2018;118:225–35. [Google Scholar]
- 109.Kiso Y, Tohkin M, Hikino H. Antihepatotoxic principles of Atractylodes rhizomes. J Nat Prod. 1983 Sep–Oct;46((5)):651–4. doi: 10.1021/np50029a010. [DOI] [PubMed] [Google Scholar]
- 110.Ma EL, Li YC, Tsuneki H, Xiao JF, Xia MY, Wang MW, et al. Beta-eudesmol suppresses tumour growth through inhibition of tumour neovascularisation and tumour cell proliferation. J Asian Nat Prod Res. 2008 Jan–Feb;10((1–2)):159–67. doi: 10.1080/10286020701394332. [DOI] [PubMed] [Google Scholar]
- 111.Tsuneki H, Ma EL, Kobayashi S, Sekizaki N, Maekawa K, Sasaoka T, et al. Antiangiogenic activity of beta-eudesmol in vitro and in vivo. Eur J Pharmacol. 2005 Apr 11;512((2–3)):105–15. doi: 10.1016/j.ejphar.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 112.Bomfim DS, Ferraz RP, Carvalho NC, Soares MB, Pinheiro ML, Costa EV, et al. Eudesmol isomers induce caspase-mediated apoptosis in human hepatocellular carcinoma HepG2 cells. Basic Clin Pharmacol Toxicol. 2013 Nov;113((5)):300–6. doi: 10.1111/bcpt.12097. [DOI] [PubMed] [Google Scholar]
- 113.Lu Y-C. Studies on the chemical constituents of the essential oil of Rhododendron tsinghaiense Ching. Huaxue Xuebao. 1980;38((3)):241–9. [Google Scholar]
- 114.Cavalieri E, Mariotto S, Fabrizi C, de Prati AC, Gottardo R, Leone S, et al. Alpha-bisabolol, a nontoxic natural compound, strongly induces apoptosis in glioma cells. Biochem Biophys Res Commun. 2004 Mar 12;315((3)):589–94. doi: 10.1016/j.bbrc.2004.01.088. [DOI] [PubMed] [Google Scholar]
- 115.Darra E, Lenaz G, Cavalieri E, Fato R, Mariotto S, Bergamini C, et al. Alpha-bisabolol: unexpected plant-derived weapon in the struggle against tumour survival? Ital J Biochem. 2007 Dec;56((4)):323–8. [PubMed] [Google Scholar]
- 116.Darra E, Abdel-Azeim S, Manara A, Shoji K, Maréchal JD, Mariotto S, et al. Insight into the apoptosis-inducing action of alpha-bisabolol towards malignant tumor cells: involvement of lipid rafts and Bid. Arch Biochem Biophys. 2008 Aug 15;476((2)):113–23. doi: 10.1016/j.abb.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 117.Rigo A, Ferrarini I, Lorenzetto E, Darra E, Liparulo I, Bergamini C, et al. BID and the α-bisabolol-triggered cell death program: converging on mitochondria and lysosomes. Cell Death Dis. 2019 Nov 26;10((12)):889. doi: 10.1038/s41419-019-2126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Anter J, Romero-Jiménez M, Fernández-Bedmar Z, Villatoro-Pulido M, Analla M, Alonso-Moraga A, et al. Antigenotoxicity, cytotoxicity, and apoptosis induction by apigenin, bisabolol, and protocatechuic acid. J Med Food. 2011 Mar;14((3)):276–83. doi: 10.1089/jmf.2010.0139. [DOI] [PubMed] [Google Scholar]
- 119.Fernandes ES, Passos GF, Medeiros R, da Cunha FM, Ferreira J, Campos MM, et al. Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur J Pharmacol. 2007 Aug 27;569((3)):228–36. doi: 10.1016/j.ejphar.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 120.Volcho KP, Laev SS, Ashraf GM, Aliev G, Salakhutdinov NF. Application of monoterpenoids and their derivatives for treatment of neurodegenerative disorders. Curr Med Chem. 2018;25((39)):5327–46. doi: 10.2174/0929867324666170112101837. [DOI] [PubMed] [Google Scholar]
- 121.Buczynski MW, Parsons LH. Quantification of brain endocannabinoid levels: methods, interpretations and pitfalls. Br J Pharmacol. 2010;160((3)):423–42. doi: 10.1111/j.1476-5381.2010.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fischedick JT, Hazekamp A, Erkelens T, Choi YH, Verpoorte R. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry. 2010;71((17–18)):2058–73. doi: 10.1016/j.phytochem.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 123.Casano S, Grassi G, Martini V, Michelozzi M. Variations in terpene profiles of different strains of cannabis sativa L. Acta Hortic. 2011;925((925)):115–21. [Google Scholar]