Abstract
The storage and elimination of urine requires coordinated activity between muscles of the bladder and the urethra. This coordination is orchestrated by a complex system containing spinal, midbrain and forebrain networks. Normally there is a reciprocity between patterns of activity in urinary bladder sacral parasympathetic efferents and somatic motoneurons innervating the striatal external urethral sphincter muscle. At the spinal level this reciprocity is mediated by ensembles of excitatory and inhibitory interneurons located in the lumbar-sacral segments. In this review I will present an overview of currently identified spinal interneurons and circuits relevant to the lower urinary tract and will discuss their established or hypothetical roles in the cycle of micturition. In addition, a recently discovered auxiliary spinal neuronal ensemble named lumbar spinal coordinating center will be described. Sexual dimorphism and developmental features of the lower urinary tract which may play a significant role in designing treatments for patients with urine storage and voiding dysfunctions are also considered. Spinal cord injuries seriously damage or even eliminate the ability to urinate. Treatment of this abnormality requires detailed knowledge of supporting neural mechanisms, therefore various experiments in normal and spinalized animals will be discussed. Finally, a possible intraspinal mechanism will be proposed for organization of external urethral sphincter (EUS) bursting which represents a form of intermittent EUS relaxation in rats and mice.
Keywords: spinal cord, bladder, external urethral sphincter, propriospinal, trans-synaptic tracing
1. Supraspinal LUT control.
The lower urinary tract (LUT) in mammals provides storage and periodic elimination (voiding) of urine. It includes the urinary bladder (BL), the urethra and the urethral sphincter. To efficiently control micturition the smooth muscle of the bladder and muscles of the urethra need to be coordinated. Striated muscle around the urethra is called the external urethral sphincter (EUS). During storage of urine in the bladder the EUS is tonically active and the smooth muscle of the bladder is relaxed. Accumulation of urine leads to rise of pressure in the bladder which at some point triggers the micturition reflex, when the bladder muscle contracts and the EUS relaxes. This reciprocal activation of the two organs is necessary to avoid either continuous urine release if the EUS is relaxed, or difficulty/inability to void if the EUS remains contracted. Such coordination is supported by a complex neural system involving the brain, the brain stem, the spinal cord and the peripheral ganglia (reviewed in (de Groat et al., 2015;Fowler et al., 2008;Seth et al., 2013)). A number of LUT-related reflexes involving ascending and descending pathways have been examined and described (reviewed in (de Groat et al., 1998;de Groat, et al., 2015;de Groat and Yoshimura, 2015)). Voluntary control of micturition (continence) allowing voiding to occur at a proper time or in comfortable circumstances recruits the neocortex, whereas periodic urine retention during the bladder filling (guarding reflex) and release of urine (micturition reflex) involve the brainstem structures and spinal neural circuits. In cats, dogs and humans relaxation of the EUS persists throughout voiding. In rats and mice the EUS relaxation is not persistent during micturition reflex. Instead, it represents a series of short pauses between rhythmically occurring EMG bursts in the EUS striated muscle. These alternating bursts-pauses indicate intermittent synchronized contractions of many motor units in the EUS periodically interrupted by a complete block of their activity (Cheng and de Groat, 2010;de Groat and Yoshimura, 2015;LaPallo et al., 2014). This series of rhythmic contractions of the circular striated muscle works like a peristaltic pump forcefully expelling urine from urethra and increasing efficiency of voiding. In addition, intermittently expelled portions of urine serve for marking the territory and attracting a mate of an opposite sex. However, coordination between the bladder and EUS along with bursting are abolished after spinal cord injury (SCI). This results in areflexic bladder immediately after SCI. In a few days, when the BL resumes contracting, bladder-sphincter dyssynergia develops due to disrupted descending pathways from supraspinal structures. BL-EUS coordination may recover in a few weeks if SCI occurred above L3/L4 segments, but destruction of L3/L4 prevents restoration of BL-EUS synergy and reflex voiding (Chang et al., 2007). Importantly, in chronically spinalized animals restoration of reflex voiding correlates with re-appearance of EUS bursting. Therefore, EUS bursting is a good indicator of recovery after SCI in rats and mice (Chang, et al., 2007;Chang et al., 2006;Kadekawa et al., 2016).
The general concept for BL-EUS coordination is focused on the spino-bulbo-spinal loop. Ascending pathways carry information from the bladder to the brainstem where it is received by the pontine micturition center (PMC). This structure has been proposed to function as a switch between guarding and micturition reflexes (Verstegen et al., 2017). Recently a specific group of PMC neurons expressing estrogen receptor 1 (ESR1) has been shown to elicit EUS-bursting and bladder contraction (Keller et al., 2018). The authors conducted elegant optogenetic experiments in transgenic mice where ESR1+ excitatory neurons of the PMC expressed channel rhodopsin 2 (ChR2) and a fluorescent tracer. The authors showed that ESR1+ neurons of PMC project to lumbosacral spinal cord and are active during natural urination. Axons of these neurons profoundly arborize in the dorsal commissure (DCM) and some of them were seen in the parasympathetic intermediolateral nucleus (IML) of L6-S2 segments. Optogenetic stimulation of ESR1+-ChR2+ neurons rapidly initiated sphincter bursting and efficient voiding in anesthetized and behaving male mice. It was suggested that these neurons project either directly to sphincter relaxing interneurons or indirectly via some local circuit. Another histochemically and physiologically characterized group of neurons in the PMC expresses corticotropin releasing hormone. Optogenetic stimulation of these neurons generates an increase of bladder pressure (Hou et al., 2016), but resulted in a much longer latency and lower volume of released urine (correspondingly 25% and 20% of that induced by stimulation of ESR1+ neurons; Fig.3g in (Keller et al., 2018)).
Normally, the supraspinal control of the LUT is absolutely necessary not only for voluntary voiding, but also for efficient reflex voiding [9]. In healthy animals the entire LUT-related spinal network is controlled by the PMC and other supraspinal structures (reviewed in [1]). Therefore, after disruption of cord-brainstem connections reflex voiding is impossible for a few weeks and can only partially recover later. Based on a variety of experiments with spinal cord injuries it was concluded that recovery of voiding mainly depends on plasticity and re-organization of intraspinal neural networks (de Groat, et al., 1998;Flynn et al., 2011;Shefchyk, 2001). In this review we will concentrate on exclusively spinal LUT-related circuits and, in particular, on interneurons modulating activity of motoneurons (for EUS) and preganglionic neurons (for the bladder). We will also present some relevant data which were obtained in the course of previous work (Karnup, 2020a;Karnup, 2020b), but have not been published.
2. Intraspinal LUT control
Descending pathways relay PMC output signal to the lumbo-sacral spinal cord causing activation of the bladder parasympathetic neurons, inhibition of the motorneurons innervating the EUS (EUS-MNs) and modulation of activity of relevant spinal interneurons (reviewed in (de Groat, et al., 2015;de Groat and Yoshimura, 2006)). The main population of LUT-related spinal neurons is located in the lower lumbar and upper sacral segments (Fig. 1). In the spinal cord EUS-MNs create a compact bilateral cluster named Onuf’s nucleus; in rats and mice Onuf’s nucleus is located ventro-laterally in L6-S1 segments. Another group of urethra-related output neurons is represented by bilateral clusters of parasympathetic preganglionic neurons (uPPGNs) targeting postganglionic neurons. uPPGNs are located in the intermediolateral nuclei (IML) at the level of the central canal (CC) in L6-S1 segments and project their axons to pelvic ganglion which contains motoneurons of the urethra smooth muscle. In addition, uPPGNs induce urethral smooth muscle relaxation during voiding by activating peripheral neurons that release NO (Andersson et al., 1992; Andersson and Persson, 1994; Bennett et al., 1995; Ho et al., 2004). Finally, the smooth muscle of urethra is also indirectly (through another set of postganglionic neurons) innervated by a group of sympathetic preganglionic neurons in the IML of L1-L2 segments. Thus, three groups of spinal output neurons can activate urethra muscles. Spinal parasympathetic preganglionic neurons of the bladder circuit (bPPGNs) are also located in the IML in L6-S1 segments; they project their axons to pelvic ganglion containing motoneurons for the bladder smooth muscle (de Groat, et al., 2015;de Groat and Yoshimura, 2006;de Groat and Yoshimura, 2015). Local interneurons presynaptic to the bladder output neurons, to the smooth muscle of urethra and to the EUS have been found around the central canal in L6 and S1 segments (Karnup and De Groat, 2020b;Nadelhaft and Vera, 2001;Sugaya et al., 1997;Vizzard et al., 1995). Sensory input from the bladder wall is delivered to spinal and brainstem networks where it is analyzed, converted to output commands and transferred to bPPGNs and to the EUS-MNs. Therefore, the current convention is that in rats and mice LUT-related spinal circuits are located in L6 and S1 segments.
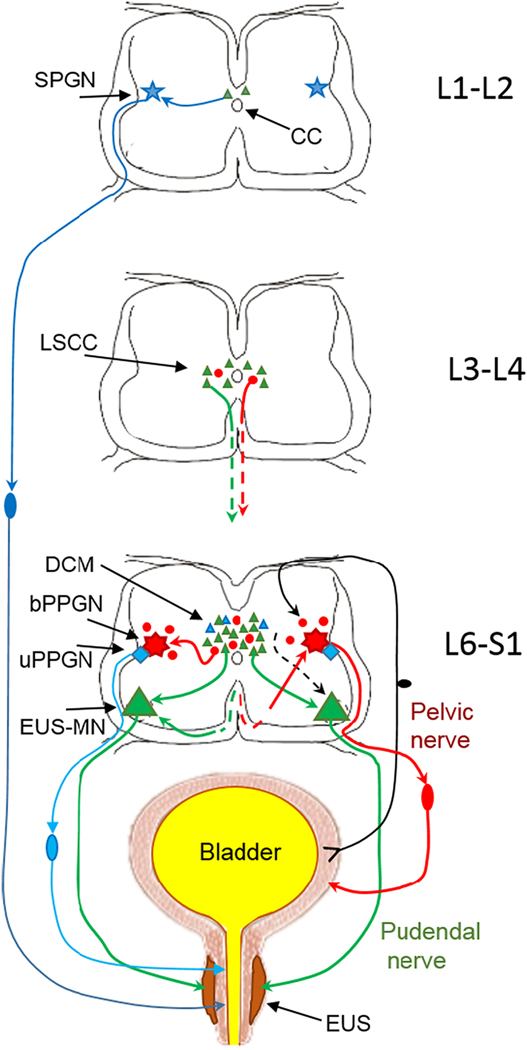
Fig.1.
Schematic of innervation of the lower urinary tract from lumbo-sacral segments of the spinal cord (connections with supralumbar structures are not included). External urethral sphincter (EUS) surrounding the smooth muscle of urethra is depicted in dark brown and the smooth muscle is in light brown. The striated muscle of the EUS receives excitatory input from motoneurons in Onuf’s nucleus in L6-S1 (EUS-MNs, large green triangles). In turn, EUS-MNs receive monosynaptic inputs from intrasegmental neurons located in L6-S1 dorsal commissure (DCM) and from intersegmental propriospinal neurons located in L3-L4 lumbar spinal coordinating center (LSCC) (small green triangles). The smooth muscle of urethra is activated by sympathetic preganglionic neurons (SPGN, blue star) in L1-L2. Parasympathetic input to urethral smooth muscle arises in preganglionic neurons (uPPGN, blue rhomboid)) in the L6-S1 IML. Both sympathetic and parasympathetic output neurons for the urethra smooth muscle have sets of local presynaptic interneurons near the central canal (small blue triangles). Since the EUS in rats and mice is only a couple of hundred microns thick in males and even thinner in females, an injection of PRV into EUS is always a challenge because often the smooth muscle is also partially infected. Therefore, after injection of PRV in the EUS, retrograde trans-synaptic tracing often results in some labeled cells in the upper lumbar segments (Vizzard, et al., 1995) which is also seen in Fig.2. Afferents from the bladder wall arrive to the dorsal horn of L6-S1 where they deliver information to parasympathetic preganglionic neurons (bPPGNs, red septagram) through a set of local interneurons (Araki, 1994;Araki and De Groat, 1996;Araki et al., 1997). bPPGNs like EUS-MNs receive inputs from local interneurons in the L6-S1 DCM and IML (small red circles) (Nadelhaft and Vera, 1996;Nadelhaft et al., 1992;Sugaya, et al., 1997) and from propriospinal neurons in L3-L4 LSCC (Karnup and De Groat, 2020b), i.e. from the area surrounding the central canal.
In humans (Blaivas et al., 1981;Dyro and Yalla, 1986) and cats (Sackman and Sims, 1990) silencing of the EUS during voiding is complete, whereas in rats and in mice the sphincter displays a pattern of rhythmic bursting in the electromyogram of the EUS striated muscle during the peak of bladder contraction (Kakizaki et al., 1997). EUS-EMG bursting corresponds to a series of short (60–100 ms) spasmodic contractions of the majority or all EUS motor units interrupted by pauses (100–150 ms) of complete relaxation of the entire striated muscle. This rhythmic bursting is typically a prerequisite to efficient voiding and is thought to play a role of a pump forcefully expelling portions of urine. The mechanism responsible for this rhythmic activity has not been identified. It is possible that organization of LUT circuits in mammals differ in some anatomical details. However, we believe that evolution of the LUT is extremely conservative and the major components of the relevant neural circuits are the same regardless of degree of their development or functional expression. In urological experiments EUS bursting turned out to be a good correlate to restoration of voiding in rats after supralumbar spinal cord injury (de Groat and Yoshimura, 2006;Kadekawa, et al., 2016). Furthermore, restoration of bursting and voiding after spinal cord transection at different levels allowed to hypothesize the existence of an additional spinal circuit taking part in micturition (Chang, et al., 2007).
In the spinal cord efferent neurons for the EUS (EUS-MNs) and the bladder (bPPGNs) in L6-S1 segments receive synaptic inputs from local interneurons. Spinal neurons involved in processing of an afferent input from the bladder were identified in the L6-S1 dorsal commissure and parasympathetic intermediolateral nucleus by expression of immediate early gene c-fos after stimulation of the bladder and urethra (Birder and de Groat, 1993). This correlates with the results of retrograde trans-synaptic tracing with pseudorabies virus (PRV) injected to the bladder wall, where groups of L6-S1 interneurons mono- or polysynaptically connected to PPGNs were found in the dorsal commissure above the central canal and near the IML (Karnup and De Groat, 2020a,b;Nadelhaft and Vera, 1996;Nadelhaft, et al., 1992;Sugaya, et al., 1997;Vizzard, et al., 1995) (Fig.4 in (de Groat and Yoshimura, 2006)).
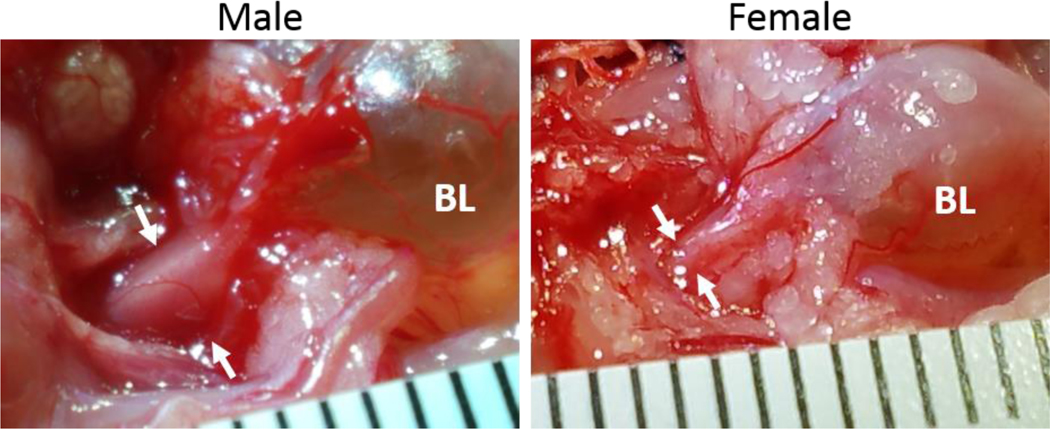
Fig.4.
Photos of the bladder (BL) and urethra in P70 transgenic VGAT mice of the same litter. Thickness of the urethra is indicated by white arrows. In the male diameter of urethra was 2.7 mm and in the female it was 1.2 mm, which gives the ratio 2.3 for male vs. female. The scale on the ruler is in mm.
There are multiple populations of spinal interneurons with molecularly and anatomically diverse characteristics which may be involved in the same or different circuits subserving various physiological functions (Hughes and Todd, 2020; Zholudeva et al., 2021; Ziskind-Conhaim and Hochman, 2017). But apart from these subdivisions, all interneurons are divided into two major classes: excitatory and inhibitory cells. Excitatory spinal interneurons are usually glutamatergic, and inhibitory ones are either GABAergic or glycinergic. Typically, in the CNS rising excitation in a network of interconnected excitatory neurons can be suppressed or completely blocked by delayed activation of inhibitory neurons included in the same network. Importantly, such recurrent inhibition affects most if not all excitatory neurons of a given circuit even though some of them do not synaptically activate inhibitory cells. A form of recurrent inhibition for bPPGNs was described in the cat, where sacral ventral root stimulation depressed parasympathetic firing for a few minutes (De Groat and Ryall, 1968). This inhibition was sensitive to the glycinergic antagonist strychnine, but there was no evidence for a direct inhibition of bPPGNs (de Groat, 1976;de Groat and Theobald, 1976). It was hypothesized that interneurons mediating this recurrent inhibition acted at sites presynaptic to preganglionic neurons. Patch clamp recordings from PPGNs in the rat spinal slices revealed that interneurons located immediately dorsal and medial to parasympathetic nucleus make direct monosynaptic connections with the preganglionic neurons (Araki, 1994;Araki and De Groat, 1996;Araki and de Groat, 1997). These neurons were monosynaptically excited by dorsal horn afferent fibers, whereas focal electrical stimulation of these neurons monosynaptically elicited either excitatory or inhibitory postsynaptic potentials (EPSP or IPSP respectively) in the preganglionic neurons. In the rat L6-S1 slices IPSPs in bPPGNs were found to be largely mediated by glycine receptors with a minor GABAA receptor component (Araki, 1994), whereas EPSPs are glutamatergic (reviewed in (de Groat, 2002) ). Thus, the data indicate that bladder-related PPGNs receive inputs from a multilayer intrasegmental circuit involving excitatory and inhibitory cells.
Inputs to EUS-MNs arrive from the bladder stretch Aδ-afferents, bladder pain C-afferents, perineal cutaneous afferents and penile/clitoral afferents. However, before affecting activity of EUS-MNs these inputs are modulated by polysynaptic intraspinal pathways including at least one or most probably two or three interneurons ((Fedirchuk et al., 1992), reviewed in (de Groat, et al., 2015;de Groat and Yoshimura, 2015)). Retrograde transsynaptic tracing with pseudorabies virus has revealed in the L6-S1 dorsal commissure a population of local interneurons presynaptic to EUS-MNs (Karnup and De Groat, 2020b;Nadelhaft and Vera, 1996;Vizzard, et al., 1995). It was long assumed that inhibition of EUS-MNs is driven predominantly from interneurons in the DCM. However, until now there is no unequivocal data on inhibitory elements responsible for suppression of EUS-MNs firing and relaxation of urethra during voiding. Indirect data have shown that in the male mouse synaptic excitation of L6/S1 DCM from the PMC caused bladder contraction and EUS bursting (Verstegen et al., 2017). In cats, the PMC projects to DCM and electrical stimulation of L6/S1 DCM resulted in relaxation of EUS, which led to conclusion that neurons inhibiting EUS-MNs are located in DCM, although an option that these inhibitory neurons may be located elsewhere and be activated from DCM was not tested (Blok et al., 1997;Blok et al., 1998;Pikov et al., 2007;Sie et al., 2001). In young male rats immunohistochemical identification of Pax2 which is expressed in inhibitory neurons has demonstrated that in the DCM only ~3% of all EUS-related interneurons immediately presynaptic to EUS-MNs (i.e. 1st order interneurons) can be classified as inhibitory ones (Karnup and De Groat, 2020b). At the same time, about 13% of the 2nd order EUS-related interneurons expressed Pax2. This suggests that the majority of EUS-related interneurons in the DCM are excitatory and the DCM is an unlikely source of EUS-MNs’ inhibition, unless a small number of inhibitory DCM neurons are capable of a total block of firing in all EUS-MNs. More likely that these data support the idea that a persistent excitatory barrage from the intrasegmental DCM is responsible for maintenance of tonic activity in EUS-MNs, whereas the main source of synaptic inhibition may be located elsewhere.
3. Lumbar Spinal Coordinating Center as an auxiliary compartment of the LUT neural circuit.
Spinal cord injury is followed by spinal shock with initially areflexic bladder but high and persistent tonic activity in the EUS. Therefore, during this phase patients need continuous catheterization and spinalized animals need frequent manual emptying of the bladder. In a few weeks the bladder develops involuntary reflex contractions (neurogenic detrusor overactivity in response to increasing pressure) and coordinated relaxation of the urethra smooth muscle, but the EUS striated muscle displays dyssynergic contractions. This detrusor-sphincter dyssynergia (DSD) prevents comfortable voiding, results in increased bladder pressure, causes urinary incontinence and eventually leads to deterioration of the upper urinary tract. In rats recovery of voiding is accompanied by re-appearance of EUS bursting in 3–6 weeks which increases efficiency of bladder emptying.
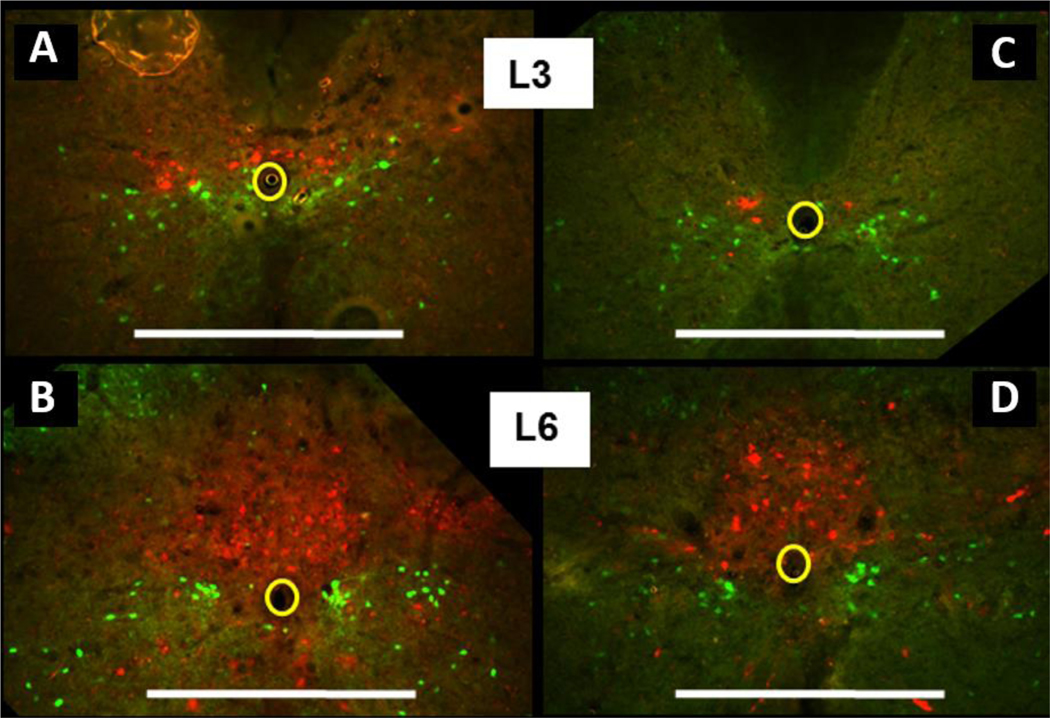
Despite being long known as an indicator of restoration of coordinated BL-EUS function in rats (Kakizaki et al., 1997; Kruse et al., 1993), this phenomenon only recently was used to test possible existence of an additional intraspinal LUT-related neural circuits. It was found that in rats and mice full transection of the spinal cord above L3-L4 lumbar segments does not prevent restoration of EUS reflex bursting, but bursting does not recover after transection in or below L3-L4 (Chang, et al., 2007). Therefore, L3-L4 segments have been hypothesized to contain an auxiliary LUT-related circuit which somewhat substitutes loss of afferentation from supraspinal structures. Functional importance of an upper lumbar circuit for sphincter relaxation was also shown in experiments with epidural stimulation above L3 in rats which in combination with a serotonergic receptor agonist elicited bursting in EUS and facilitated voiding (Abud et al., 2015). Involvement of L3-L4 in micturition was also indicated by significant increase in the number of neurons expressing c-fos in the L3-L4 medial gray observed in experiments with urogenital reflex (Marson and Gravitt, 2004). The hypothetical L3-L4 LUT-related circuit was named the spinal lumbar coordinating center (LSCC) (Chang, et al., 2007). Its existence was anatomically confirmed in recent experiments with pseudorabies virus expressing either green or red fluorescent proteins (PRV-GFP or PRV-RFP) tracing (Karnup and de Groat, 2020a;Karnup and De Groat, 2020b). Above and lateral to the central canal in L3-L4 segments we found populations of PRV-labeled propriospinal interneurons whose descending axons project into the ventral column, reach L6-S1 and monosynaptically target either bPPGNs, or EUS-MNs, or both (Fig. 2).
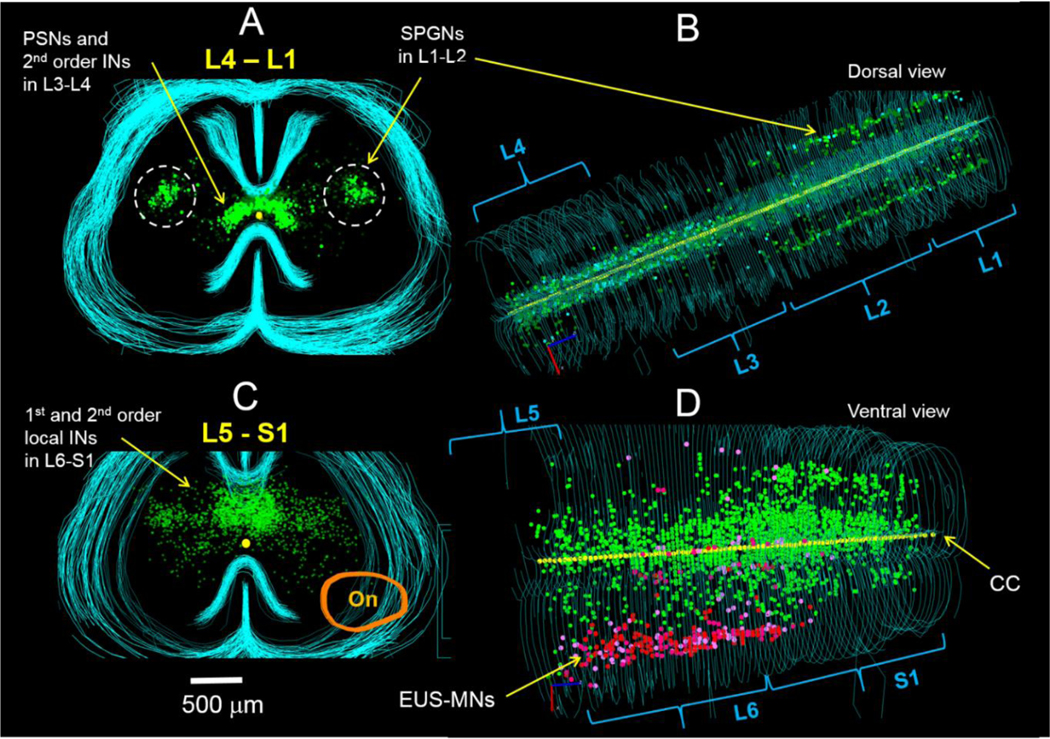
Fig.2.
Distribution of spinal interneurons traced after unilateral injection of PRV-GFP in the EUS of the adult male rat confirms presence of the lumbar-spinal coordinating center (LSCC) in L3-L4 segments. A – a set of 2-D maps from sequential transverse 50-μm thick sections through L4-L1. Bright green symbols (n=158) indicate locations of propriospinal neurons (PPSNs, 1st order EUS-related interneurons), which are presynaptic to EUS-MNs and are located above and lateral to the central canal in L3-L4 segments. More numerous weakly stained interneurons (dark symbols, n=2076) are assumed to be 2nd (and possibly 3rd) order local neurons presynaptic to PPSNs and occupy the same area but have broader lateral distribution. B – dorsal view of the same sets of maps demonstrate that EUS-PPSNs and presynaptic to them intrasegmental interneurons are restricted to L3-L4 segments, whereas sympathetic preganglionic neurons (SPGNs) innervating urethra smooth muscle are located in the intermediolateral nucleus of L1-L2 segments; a small number of presynaptic to SPGNs local interneurons are located mostly above the central canal and not distinguishable in A. C – a set of maps from transverse sections through L5-S1 segments. Bright green symbols (not counted) designate location of local 1st order interneurons presynaptic to EUS-MNs within dorsal commissure (DCM). Darker symbols occupying DCM and also widely scattered in lateral and partly ventral direction depict neurons of the 2nd and 3rd orders. Orange closed line designates location of Onuf’s nucleus (On). D – In the ventral view of L5-S1 sets of maps the rostro-caudal distribution of EUS-MNs is depicted with red and pink dots despite actual labeling by green fluorescence. EUS-related intrasegmental interneurons are distributed in the DCM throughout L6 and S1. This figure was presented in the poster session at the 2017 SfN meeting.
According to the conventionally accepted notion of an order for retrogradely traced neurons, a neuron labeled first (usually a motoneuron) is the first order cell, whereas presynaptic to it interneurons, are the second order cells, cells presynaptic to 2nd order neurons are of the 3rd order etc. For the sake of simplicity in the following text we introduce a similar ordering system but only for spinal interneurons starting from the first interneuron presynaptic to an output cell. In this classification an intrasegmental interneuron of the DCM and an intersegmental propriospinal neuron of the LSCC both presynaptic to EUS-MNs are the 1st order or primary interneurons. Presynaptic to them local intrasegmental cells are the 2nd order or secondary interneurons etc.
Starting from studies of locomotion it is known that intersegmental connections in the spinal cord are provided by propriospinal neurons, whose soma is located in one segment, but an axon projects across a few segments to synapse with neurons of a targeted network. They are distributed throughout dorsal, intermediate and ventral grey matter either as clusters or as scattered neuronal populations. Ascending and/or descending axons of propriospinal neurons can span either less than five (short-range) or more (long-range) spinal segments and project ipsilaterally and/or contralaterally (Alstermark et al., 2011;Cowley et al., 2015;Flynn et al., 2017;Ni et al., 2014;Reed et al., 2006;Reed et al., 2009). They are an essential part of the central pattern generator responsible for timely contractions of limb muscles and for coordinated occurrence of phase-locked EMG bursts in muscles (Cherniak et al., 2017;Cherniak et al., 2014;Zhong et al., 2012). Propriospinal neurons are found not only in circuits subserving body or limb motions, they are also present in spinal networks responsible for autonomic functions (Darlot et al., 2012;Hou et al., 2008;Michael et al., 2019). For example, a bilateral cluster of motor neurons innervating the bulbospongiosus muscle and seminal vesicles involved in ejaculation is located in the dorsomedial nucleus (DM) in L6-S1 segments, and a set of propriospinal neurons presynaptic to DM motoneurons is located close to the central canal in L3-L4 segments (Best et al., 2013;Dobberfuhl et al., 2014;Sun et al., 2009). Overlapping sets of propriospinal neurons subserving two closely related organs such as the EUS and bulbospongiosus muscle suggest their interaction and probable coordination. Both striated muscles, at least in rats and mice, can generate rhythmic bursting at the highest level of excitation which suggests similarity of bursting mechanisms. However, the latter issue is still not clear and needs further investigations. Apart from propriospinal neurons, which are the first order interneurons, there is an even greater number of second order interneurons involved in control of the LUT.
4. Spinal cord injury and importance of propriospinal neurons for recovery of micturition.
Spinal cord injury (SCI) at a supralumbar level disrupts connections of the spinal circuits and the PMC resulting in loss of coordination between the bladder and urethra muscles causing permanent tonic activity of the EUS and inability of voiding or incomplete bladder emptying. This condition is referred to as bladder-sphincter dyssynergia (DSD) (Blaivas, et al., 1981;Kruse et al., 1993;Pikov and Wrathall, 2001). The only mechanism which can restore damaged function or at least partially compensate DSD is plasticity of the spinal cord network, and specifically, reorganization and/or rewiring of interneuronal ensembles. After disconnection from supraspinal structures intraspinal circuits undergo rewiring (reviewed in (Michael, et al., 2019;Zavvarian et al., 2020;Zholudeva et al., 2018) and propriospinal neurons play an essential role in restoration of coordinated muscle functions (Benthall et al., 2017;Courtine et al., 2008;Flynn, et al., 2011;Hou, et al., 2008). In chronic SCI animals patterns of bladder-specific spinal interneurons are similar to that in spinal intact (SI) ones (Im et al., 2008), but in addition a significant number of non-specific interneurons demonstrate fos-immunopositivity (Vizzard, 2000). This reflects post-traumatic sprouting of local interneurons and extension of the neuronal pool involved in relevant circuits. Experiments with electric stimulation of the spinal cord in SI and SCI animals indirectly confirm the presence of a rostral lumbar to L6-S1 propriospinal circuit involved in micturition (McGee et al., 2015). Epidural spinal cord stimulation (SCS) on L1 and L6 segments in urethane-anesthetized rats evoked tonic EUS contractions, SCS at L2-L3 inhibited tonic activity, and SCS at L3 inhibited tonic EUS activity and elicited EUS bursting (Chang et al., 2018). In SCI rats epidural stimulation at L3 combined with 5HT-1A receptor agonist resulted in inhibition of tonic activity and excitation of intermittent bursting/relaxation in the EUS-EMG (Abud, et al., 2015). Location of electrodes eliciting bursting/relaxation in the EUS correlates with the location of propriospinal neurons traced to the proposed LSCC at L3/L4. Thus, it seems that the LSCC neural circuit has a functional implication in recovery of micturition reflex in spinalized animals or in patients with chronic SCI (Abud et al., 2015; Chang et al., 2018; Gad et al., 2016; Gad et al., 2018).
5. Sexual and developmental differences of the LUT circuits.
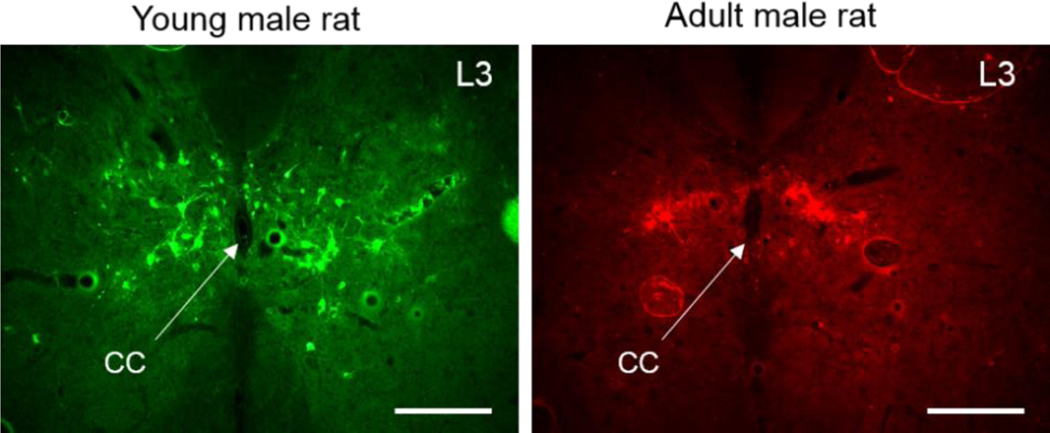
In the postnatal development of rats and mice active synaptogenesis reaches its peak at P28-P30 followed by a decline in the number of presynaptic boutons as well as density of several types of receptors (Behuet et al., 2019;Gambrill and Barria, 2011;Lohmann and Kessels, 2014;Steward and Falk, 1991;Waites et al., 2005). The reduction of the number of synaptic contacts is thought to reflect elimination of random connections and specialization of the circuits, and it may be the reason for decreased number of PRV-traced interneurons in adult rats as compared to juvenile ones. Therefore, there was no surprise that in the LSCC of ~ P30 juvenile rats the average number of EUS-related interneurons per 50 μm thick histological section was larger than that in adult rats (Fig.3). It is likely that spinal cord of a month-old animals is still undergoing rewiring from neonatal to adult circuits.
Fig.3.
Interneurons in the L3 spinal segment traced from the EUS in young (P30–35, PRV-GFP) and adult (P60–70, PRV-RFP) male rats. Note a greater number of labeled neurons occupying a broader area in young (left) as compared to adult (right) animal. CC – central canal. Scale bar is 200 μm.
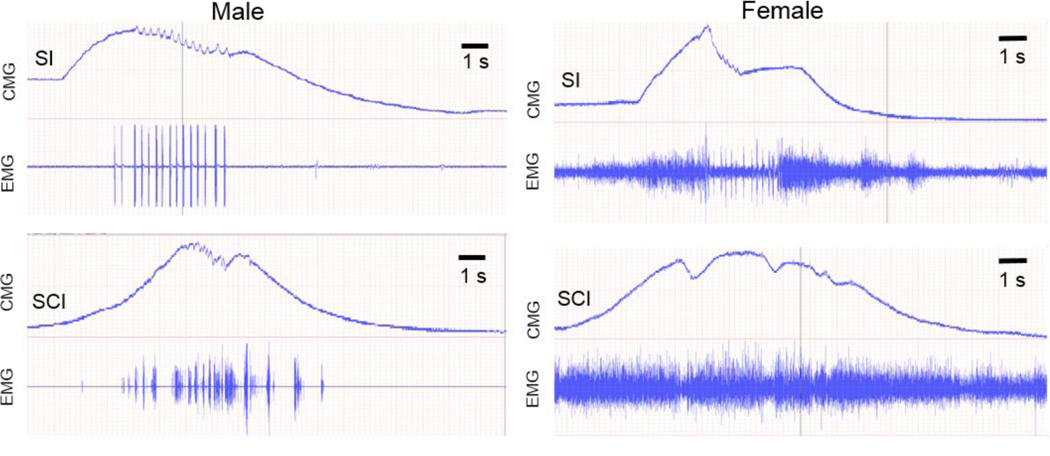
Spinal LUT circuits also display a substantial sexual dimorphism. First, the urethra in males is substantially thicker than in females in both rats and mice (Fig.4) containing many more motor units which require a greater number of spinal motoneurons and interneurons presynaptic to them. Indeed, in our experiments there were clearly fewer EUS-MNs traced with PRV in female rats and mice as compared to males (unpublished observation). Second, bursting patterns in males during voiding are more powerful than in females with a larger ratio between amplitudes of bursting vs. tonic EUS-EMG (Fig. 6), which is consistent with the notion of less powerful rhythmic EUS contractions or even their absence during voiding in female mice (Kadekawa, et al., 2016). Therefore, it is logical to expect a lower number of spinal interneurons subserving LUT reflexes in females. Indeed, DCM and LSCC in female mice exhibit a noticeably lower density of PRV-labeled interneurons in both L3-L4 and L6-S1 compartments than in males (Fig. 5). Sexual differences in LUT-related neural circuits may be responsible for more frequent and earlier-developing incontinence in females on the one hand, and more severe DSD in males.
Fig.6.
In spinal intact (SI) lightly anesthetized male mouse voiding occurs at the maximum pressure in the bladder (CMG trace) correlating with prominent bursting of the EUS (EMG trace, upper left panel). In a spinalized (SCI) male mouse bursting and reflex voiding partially recovered 4 weeks post-transection at T10 (lower left panel). EUS bursting in an intact female mouse is less prominent compared to the level of tonic activity and to bursting in the male (upper right panel). In SCI female mice EUS bursting does not recover 5 weeks post-transection; instead it is substituted by brief EUS relaxations occurring at the peak of BL pressure (lower right panel. Time scale in all plots in the same, but the ratio between scales of EMG in the left and right columns is 10:1.
Fig.5.
EUS-INs traced with PRV-RFP (red) in transgenic (GAD67-GFP) male (left column) and female (right column) mice. GABA-ergic GAD67+ inhibitory neurons are visualized by green fluorescence. Colocalization of markers was absent in all sets of serial sections. This indicates that GAD67+ neurons representing the majority of GABAergic cells in the spinal cord are not involved in control of EUS activity in mice, and probably in rats. Note a lower number of EUS-INs in DCM and LSCC of a female (C, D) compared to that of a male (A, B). Scale bar: 500μm.
6. Interacting interneurons
In rats and mice both urination and ejaculation require bursting activity of corresponding striated muscles. It seems plausible, that neural mechanisms supporting such activity in two phylogenetically ancient systems should be similar. Such patterns of tightly overlapping and spatially restricted ensembles of propriospinal neurons included in excretion and reproduction is in a sharp contrast with widely spread over the spinal gray matter distribution of propriospinal neurons subserving locomotion and coordination of body movements (Brockett et al., 2013;Dutton et al., 2006;Flynn, et al., 2017;Ni, et al., 2014;Reed, et al., 2006). This tight packaging invariably suggests back and forth communication between neural networks of different, but mutually dependent organs. It is worth mentioning that simultaneous viral tracing from the bladder and prostate resulted in totally separate sets of bladder- and prostate-related preganglionic neurons. The former were found mainly in L6-S1 parasympathetic IML and in lesser numbers in L1-L2 sympathetic IML, whereas the latter were found only in the L1-L2 IML, but not in L6-S1 (Nadelhaft et al., 2002). At the same time in the L6-S1 DCM the authors observed a few single-labeled interneurons from the prostate and a few double-labeled interneurons. This indicated the presence of ascending propriospinal prostate-related interneurons, whereas double labeling demonstrated some degree of interaction between the prostate and the bladder circuits. Thus, existence of L3-L4 propriospinal neurons presynaptic to output LUT neurons of L6-S1 confirms the notion that precise coordination between muscles requires more than one concentrated intraspinal circuit and coordination can be better performed with a more distributed neuronal network.
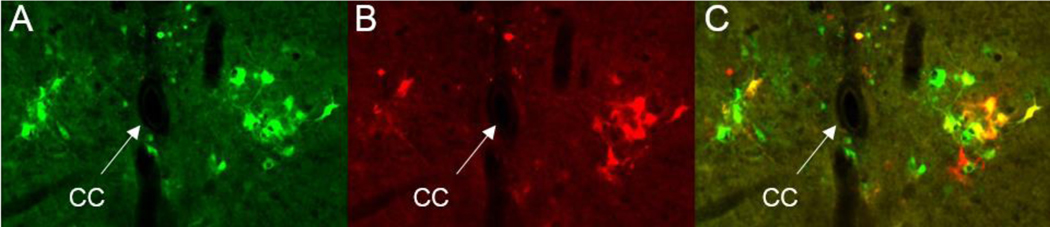
The anatomical representation of the LSCC in L3-L4 spinal segments of healthy intact animals has been found in neonate (P18-P20) (Karnup and de Groat, 2020a), juvenile (P30–35) (Karnup and De Groat, 2020b) (Fig.5) and adult rats (P60-P90) (Fig.2) as well as in adult mice (Fig.5 A,C). However, the most important bladder afferents enter the spinal cord through pelvic nerve at the more caudal L6-S1 segments, but not at L3-L4 (Rabchevsky, 2006;Shefchyk, 2001). Therefore, LSCC neurons in L3-L4 should receive information from the bladder indirectly through an L6-S1 network where Aδ− and C bladder afferents terminate. In turn, LSCC intraspinal efferents close the loop by projecting back to L6-S1 within the ventral column (Karnup and de Groat, 2020a;Karnup and De Groat, 2020b). Thus, the proposed LSCC in L3-L4 has bi-directional communication with circuitry in the L6-S1 spinal segments through sets of ascending and descending short-range propriospinal neurons. This is true for both the bladder and EUS spinal circuits because PRV-labeled neurons presynaptic to bPPGNs and to EUS-MNs have been found in L3-L4 after separate as well as concomitant PRV injections in the bladder and/or in the EUS (Karnup and De Groat, 2020b). It is also likely that within the LSCC descending propriospinal neurons can communicate with each other and with the extended pool of local intrasegmental cells. Synaptically mediated crosstalk between spinal interneurons belonging to different organs should result in double-labeling for some of them. Indeed, viral trans-synaptic tracing has demonstrated the existence of double-labeled cells among segmental L6-S1 local interneurons as well as among LSCC neurons after simultaneous injections of two immunohistochemically distinct pseudorabies virus strains into the bladder wall and into the EUS striated muscle (Karnup and De Groat, 2020b;Nadelhaft and Vera, 2001) (Fig.7). Furthermore, weakly stained second order interneurons, supposedly presynaptic to the first order interneurons in L6-S1 or to propriospinal neurons in LSCC were also frequently double-labeled with various proportions of fluorescent markers (Karnup and De Groat, 2020b). Fig.8 (copied from (Karnup and De Groat, 2020b)) illustrates possible wiring and synaptic interactions of bladder- and sphincter-related interneurons within the LSCC.
Fig.7.
Trans-synaptically traced interneurons in the upper L4 of a P35 male rat. Concomitant injections of PRV-GFP in the EUS (A) and PRV-RFP in the bladder wall (B) resulted in three main types of labeling: purely green neurons included only in the EUS circuit, purely red cells included only in the bladder circuit and cells expressing green and red fluorescent proteins (yellow color in C=A+B) for cells involved in both circuits.
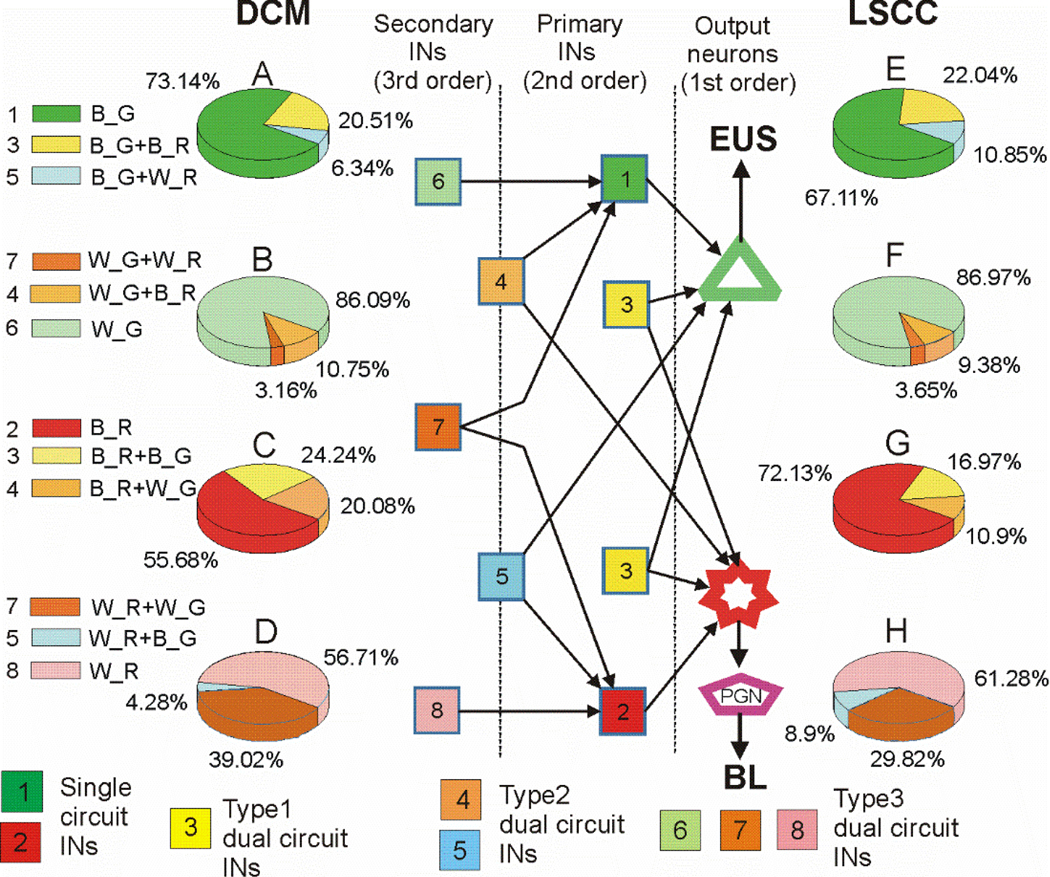
Fig.8.
Schematic of presumed organization of LUT-related interneurons and pie plots illustrating proportions of neurons of different orders. A, E– EUS-primary interneurons (pINs) presynaptic to EUS-MNs; B, F– EUS-secondary interneurons (sINs) presynaptic to EUS-pINs; C, G – BL-pINs, D, H – BL-sINs. Left column of pie plots show percentages of the specified classes in L6/S1 DCM, right column of pie plots shows percentages of the specified classes in L3/L4 LSCC. Color-coding in the diagram corresponds to color coding in the pie plots. Interneurons with dual staining are divided to 3 types according to combination of expressed markers. All bright yellow neurons are classified as “type1_INs”; cells expressing one bright marker and a weak or moderate other marker are classified as “type2_INs”; double-labeled INs weakly or moderately stained with either marker are classified as “type3_INs”. Therefore, type1_INs include only pINs, type3_INs include only sINs, whereas type2_INs include cells which are pINs in one circuit and sINs in the other circuit. Output neurons EUS-MNs and bPPGNs are depicted as a green triangle and a red star, respectively, were not counted. A peripheral ganglion is depicted as a purple pentagon. Combinations of capital letters near colored rectangles on the left stand for: bright (B) or weakly stained (W) neurons expressing either green (G) or red (R) fluorescent proteins. Brightly fluorescent cells are considered pINs and weakly fluorescent cells are sINs likely to be presynaptic to pINs.
7. Possible mechanisms of EUS bursting and relaxation
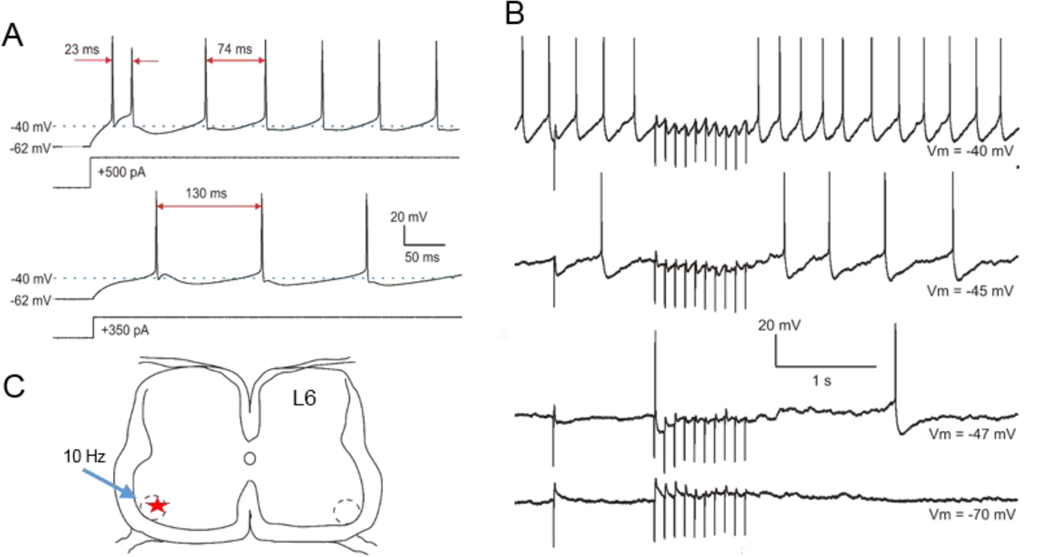
It seems that intrinsic tonic firing is the most characteristic pattern of activity for spinal interneurons, and that rhythmic locomotor-like activity in spinal cord preparations is due to excitatiory-inhibitory oscillations in spinal networks with different types of interneurons (Danner et al., 2019;Zhong, et al., 2012). Randomly recorded commissural inhibitory interneurons of mature rats showed three groups of firing patters: tonic (45%), phasic (31%) and bursting (24%) (Lu et al., 2001). Firing patterns were correlated with dendro-axonal patterns which allowed authors to hypothesize roles of each group. It was concluded that tonic neurons with axons projecting to the IML area (but not into the funiculi) function as interneurons in autonomic reflexes; phasic neurons with axons within the dorsal commissure were suggested to integrate segmental and descending inputs; bursting neurons with a burst of action potentials riding on a slow hump of a low-threshold calcium spike and having their axons extending into one of the funiculi were suggested to project inter-segmentally or to the brain. Anatomical and physiological characteristics of tonic neurons found in this work are similar to that of interneurons traced from EUS except that axons of some LSCC neurons invaded white matter of the ventral column and reached Onuf’s nucleus from the ventro-medial funiculus (Karnup and de Groat, 2020a). In experiments searching for rhythm coordinating neurons in locomotor activity Wu et al. (Wu et al., 2011) recorded from commissural inhibitory interneurons in GAD67-GFP transgenic mice. Intracellular injection of depolarizing current elicited tonic firing in GFP-positive neurons which could be modulated by an additional noisy rhythmogenic mixture. Recording from other types of interneurons also show predominance of tonic activity and lack of intrinsic rhythmic bursting. Electrophysiologically characterized LSCC interneurons traced from the EUS in neonatal rats showed tonic or phasic firing patterns in response to supra-threshold depolarization, but none of them exhibited bursting or pacemaker activity (Karnup and de Groat, 2020a). Intracellular sharp-electrode current clamp recordings from EUS-MNs in rat spinal slices demonstrated only tonic spontaneous firing (Carp et al., 2010). Whole–cell recording of spontaneous activity in DiI-labeled EUS-MNs in neonatal L6 slices also showed tonic firing and lack of bursting (Fig.9A). Stimulation in the ventrolateral funiculus elicited monosynaptic EPSP without jitter (latency 3.6 ms) and disynaptic IPSP (latency 6 ms). A 10Hz train at this site effectively blocked firing of the recorded EUS-MN (Fig.9B). Individual EUS motor units during micturition in urethane-anesthetized rats showed a few patterns of the sustained tonic firing, but no bursting behavior (D’Amico and Collins, 2012). These data exclude the possibility of generating EUS-EMG bursts by EUS-MNs or by any of interneurons synaptically connected to them. Hence, EUS bursting is not a product of intrinsic rhythmic activity of LUT-related neurons, but can be produced either by alternating volleys of excitatory and inhibitory inputs from an intraspinal rhythmogenic source, or by a loop of recurrent inhibition at the highest level of EUS-MNs excitation.
Fig.9.
Tonic firing of EUS-MNs retrogradely traced by DiI from the EUS of a neonatal P10 male rat. A – EUS-MNs cannot fire bursts of spikes. Only on the onset of sharp depolarization they were able to fire doublets (3 of 10 cells). B – Trains of 10Hz stimulation in the ventrolateral funiculus at the border of white and grey matter elicited a series of IPSPs and effectively blocked spontaneous firing without substantial hyperpolarization of the soma. C – Positioning of the stimulating electrode (blue arrow) and the recorded in B EUS-MN (red star). Onuf’s nucleus is depicted as a dashed circle.
Some authors assume that EUS bursting in the rat and mouse is generated by a specific, although unknown rhythmogenic circuit called central pattern generator similar to that suggested for the locomotor system (Cherniak, et al., 2017;Cherniak, et al., 2014;Danner, et al., 2019;Zhong, et al., 2012). This circuit controls alternating bursts of activity in left and right limb muscles through a network of excitatory and inhibitory commissural neurons. But in the EUS left and right sides contract synchronously. Furthermore, unilateral injection of PRV in the EUS results in symmetrical distribution of relevant interneurons in the spinal cord (Fig.2) suggesting evenly balanced inputs to left and right EUS-MNs from the DCM and LSCC. With the obvious absence of an intraspinal excitatory rhythmic source for production of EUS bursting, a possible candidate for organization of rhythmic bursting is recurrent inhibition. It seems feasible that at the peak of EUS-MNs excitation during a bladder contraction some high-threshold inhibitory interneurons projecting to Onuf’s nucleus may be activated predominantly by axonal collaterals of the excited motoneurons. Any other source for activation of inhibitory elements, even if it correlates with the degree of EUS-MNs excitation, will not induce bursting, but will only suppress activity of the MNs. This type of burst production comprises a feedback inhibitory loop and operates as a pause generator (Deschenes et al., 2016;Dolber et al., 2007) rather than as a burst generator. Although there is no electrophysiological or anatomical evidence for any recurrent inhibition similar to that for other motor neurons (Hultborn et al., 1988;Hultborn et al., 1988;Mackel, 1979), there is a hint from finding axonal collaterals of some EUS-MNs in the cat which appear to terminate exclusively in Onuf’s nucleus (Sasaki, 1994). Renshaw-like cells driven by these recurrent collaterals were observed around Onuf’s nucleus, but during intracellular recordings from EUS-MNs depolarization-induced spikes were not followed by recurrent inhibitory post-synaptic potentials (Muramatsu et al., 2020). We hypothesize that if the recurrent collaterals synapse on inhibitory neurons (InhNs) within or near Onuf’s nucleus, this would partially answer a few questions. First, why PRV trans-synaptic tracing labeled only a small number of inhibitory neurons in the L6-S1 segments presynaptic to EUS-MNs? This may be due to the fact that within a compact cluster of PRV-labeled cells one cannot reliably differentiate fluorescently stained motoneurons from the second order cells infected with a short delay. Second, why recurrent inhibition could not be identified by a disynaptic IPSP/IPSC following a single spike elicited in a recorded cell? It is possible, that excitation of a high-threshold inhibitory neuron may require inputs not from a single recorded motoneuron, but a barrage of loosely synchronized spikes from the entire set of activated EUS-MNs. Upon simultaneously reaching the threshold all or at least many inhibitory cells will also synchronously block firing in the entire EUS-MNs pool, thus resulting in silencing of the EUS for ~50–100 ms, i.e. for the duration of an average glycinergic populational IPSP. After the pause ends, activity of EUS-MNs is released for ~150 ms until the deactivated set of InhINs will not be again depolarized to the spike threshold by rebound synaptic barrage of the MNs’ set. Thus, we hypothesize that bursting in the rat and mouse EUS is not a result of recruitment by a rhythmic excitatory input, but rather it is produced by high-threshold recurrent inhibition at the highest level of activity in the entire EUS-MNs’ pool. In the cat where recurrent inhibitory cells surrounding Onuf’s nucleus have been identified morphologically (Muramatsu, et al., 2020), the lack of recurrent IPSPs in EUS-MNs and the lack of EUS bursting may not be explained by anatomical similarity of spinal circuits between rats and cats, but by phylogenetical diversity of the LUT systems in the course of mammalian evolution. Another explanation of the absence of recurrent IPSPs in cat’s EUS-MNs may be drawn from analogy with known connections between Renshaw cells and motoneurons. Synaptic boutons of Renshaw cells terminate not on the soma, but on dendrites of MNs and even selectively suppress certain excitatory synaptic inputs (Bhumbra et al., 2014;Bui et al., 2008;Fyffe, 1991). Therefore, recurrent IPSCs may basically shunt and neutralize depolarizing inputs rather than induce somatic IPSPs. Thus, hyperpolarization and silencing of EUS-MNs in this case may be due to block of excitatory synaptic barrage, but not to direct somatic hyperpolarization. Besides, InhINs in the cat may receive excitatory input not only from EUS-MNs, but also from other sources sufficient to maintain their activity during voiding. This will result in relaxation of the EUS without bursting which is characteristic of EUS activity in cats and humans. However, if another source of excitation is not strong enough and the main source for InhINs activation are EUS-MNs collaterals, then instead of mere relaxation the EUS will display rhythmic pauses of tonic activity to generate bursting. This type of EUS behavior is characteristic to rats and mice.
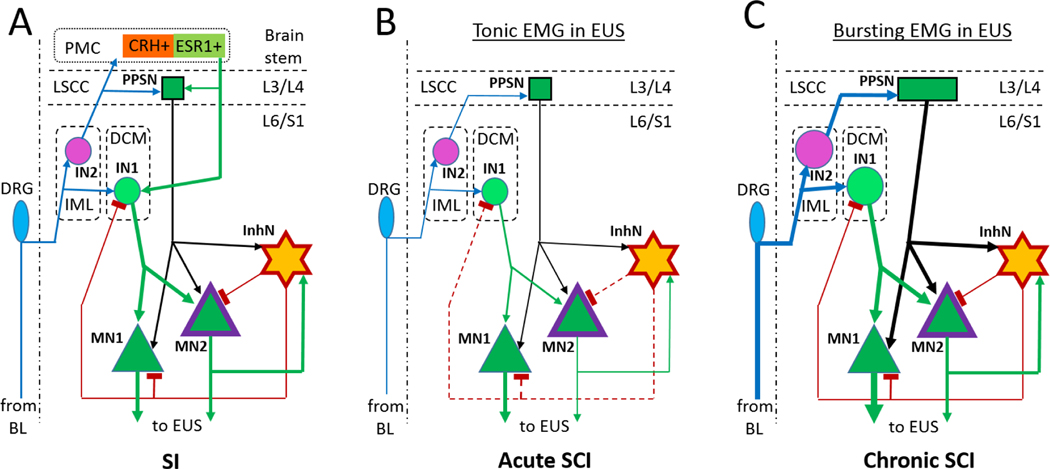
The schematic in Fig.10 shows a possible circuit in the rat spinal cord before (A) and after spinal transection (B, C). After recovery from SCI, excitatory inputs to EUS-MNs are elevated due to increased afferent input from the bladder and alterations in glutamatergic excitatory transmission between interneurons and output neurons (de Groat, et al., 1998;de Groat and Yoshimura, 2006;Kadekawa et al., 2017;Shimizu et al., 2017;Shimizu et al., 2018;Takahashi et al., 2013). Therefore, the absence of inputs from the PMC can be compensated by plasticity and expansion of an intraspinal network. Post-SCI axonal and dendritic sprouting in DCM and LSCC may lead to an increase of a total excitatory input with a strength similar to that in SI animals. Such increase of excitation in Onuf’s nucleus may be able to turn on feedback inhibition from a few high-threshold inhibitory neurons. If these InhINs have similar spike thresholds and they synapse on the whole set of EUS-MNs, a synchronized volley of InhINs would temporarily block firing in all EUS-MNs and maybe even in some excitatory interneurons in the DCM. As soon as this group of InhINs fire spikes, EUS-MNs’ firing is suppressed either by direct hyperpolarization or by shunting excitatory inputs for the duration of this collective IPSP. Thus, the above model predicts existence of recurrent inhibition arranged by a set of interneurons located in the immediate vicinity of Onuf’s nucleus. It also suggests a reason for diversity of EUS function during voiding. Complete EUS relaxation requires a powerful source of tonic excitatory input to InhNs, which in SI animals may come as a PMC signal amplified by the spinal excitatory network. EUS bursting occurs when this input is not strong enough to maintain persistent InhNs’ firing, so that InhNs require an additional excitatory input from EUS-MNs’ axonal collaterals to be activated. Inability of coordinated BL-EUS activity to be restored after SCI at or below L4 implies that local excitatory inputs to InhNs are not sufficient and that rewired LSCC becomes an essential source of additional excitation covering this deficiency. Both modes of activity can be realized on the same anatomical arrangement of the LUT-related network. The difference is only in the number or efficacy of synaptic inputs from recurrent vs. direct excitation of inhibitory neurons targeting EUS-MNs. Future experiments will either confirm or reject this hypothetical organization of interneuronal LUT-related circuit.
Fig.10.
The model of intraspinal EUS- neural circuit which can support tonic and bursting activity depending of strength of synaptic connections and the power of input afferentation. Excitatory synaptic connections are depicted by arrows and inhibitory connections by T-like endings; thickness of lines corresponds to the power of afferentation. A – Full SI circuit has a strong input from the PMC. B – Immediately after SCI the excitatory input to EUS-MNs weakens, feedback inhibition is inactivated (dashed brown lines) and detrusor-sphincter-dyssynergia develops. C – Upon recovery, afferent activity from the bladder (BL) is increased due to increased excitability of C-fiber afferents. Bolder symbols for PPSN in LSCC, IN1 and IN2 in DCM designate sprouting and involvement of additional neurons in the circuit. IN1, IN2 and PPSN can generate stronger outputs due to intra- and inter-segmental sprouting. This may be sufficient for re-activation of MN2 and InhN, which will restore EUS bursting.
To determine the impact of different interneuronal inputs in EUS-MNs activity one can use an optogenetic approach. In a spinal cord slice of a transgenic mouse expressing ChR2 in specific neuron types a flash of blue light will cause depolarization and firing of ChR2-containing neurons. For example, in slices from a mouse co-expressing ChR2 along with vesicular glutamate transporter (Vglut2-ChR2) light simulation will excite glutamatergic neurons. In slices from a transgenic mouse co-expressing ChR2 with vesicular GABA transporter (VGAT-ChR2) blue light will elicit GABAergic and glycinergic inhibitory neurons. Therefore, selective stimulation of limited areas of a slice through a thin (50–100 μm thick) fiberglass filament combined with intracellular or whole-cell recordings from EUS-MNs may reveal location and relative power of topographically identified presynaptic interneurons. Similarly, optical stimulation of axons descending from the LSCC to L6/S1 can be done after AAV-ChR2-GFP injection in lamina X of L3/L4 segments and the following expression of ChR2 in infected neurons. Occurrence of EPSPs or IPSPs in L6/S1 EUS-related cells will indicate the nature of synaptic transmission and possible mixture of synaptic inputs from LSCC to EUS-MNs and DCM interneurons.
Acknowledgements.
I thank Dr. William C. De Groat and Dr. Jonathan Beckel for valuable comments to this review. This work was supported by the NIH grant R01DK129194.
Abbreviations:
- BL
bladder
- bPPGN
bladder-related parasympathetic preganglionic neuron
- CC
central canal
- ChR2
channelrhodopsin-2
- DCM
dorsal commissure
- DM
dorsomedial nucleus
- DSD
detrusor-sphincter-dyssynergia
- EMG
electromyogram
- EPSP/C
excitatory postsynaptic potential or current
- ESR1
estrogen receptor 1
- EUS
external urethral sphincter
- EUS-MN
motoneuron of the external urethral sphincter
- GFP
green fluorescent protein
- IML
intermediolateral nucleus
- IN
interneuron
- InhN
recurrent inhibitory neuron
- IPSP/C
inhibitory postsynaptic potential or current
- LSCC
lumbar spinal coordinating center
- LUT
lower urinary tract
- MN
motoneuron
- pIN
primary interneuron
- PMC
pontine micturition center
- PRV
pseudorabies virus
- PPSN
propriospinal neuron
- RFP
red fluorescent protein
- SCI
spinal cord injury
- SCS
spinal cord stimulation
- SI
spinal intact
- sIN
secondary interneuron
- uPPGN
urethra-related parasympathetic preganglionic neuron
References Cited
- Abud EM, Ichiyama RM, Havton LA, Chang HH (2015), Spinal stimulation of the upper lumbar spinal cord modulates urethral sphincter activity in rats after spinal cord injury. American journal of physiology Renal physiology 308:F1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alstermark B, Pettersson LG, Nishimura Y, Yoshino-Saito K, Tsuboi F, Takahashi M, Isa T (2011), Motor command for precision grip in the macaque monkey can be mediated by spinal interneurons. Journal of neurophysiology 106:122–126. [DOI] [PubMed] [Google Scholar]
- Araki I (1994), Inhibitory postsynaptic currents and the effects of GABA on visually identified sacral parasympathetic preganglionic neurons in neonatal rats. Journal of neurophysiology 72:2903–2910. [DOI] [PubMed] [Google Scholar]
- Araki I, De Groat WC (1996), Unitary excitatory synaptic currents in preganglionic neurons mediated by two distinct groups of interneurons in neonatal rat sacral parasympathetic nucleus. Journal of neurophysiology 76:215–226. [DOI] [PubMed] [Google Scholar]
- Araki I, de Groat WC (1997), Developmental synaptic depression underlying reorganization of visceral reflex pathways in the spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience 17:8402–8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y, Sakakibara Y, Boggaram V, Katafuchi J, Suiko M, Nakajima H, Liu MC (1997), Tissue-specific and developmental stage-dependent expression of a novel rat Dopa/tyrosine sulfotransferase. Int J Biochem Cell Biol 29:801–806. [DOI] [PubMed] [Google Scholar]
- Behuet S, Cremer JN, Cremer M, Palomero-Gallagher N, Zilles K, Amunts K (2019), Developmental Changes of Glutamate and GABA Receptor Densities in Wistar Rats. Front Neuroanat 13:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benthall KN, Hough RA, McClellan AD (2017), Descending propriospinal neurons mediate restoration of locomotor function following spinal cord injury. Journal of neurophysiology 117:215–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best TK, Marson L, Thor KB, Burgard EC (2013), Synaptic activation of bulbospongiosus motoneurons via dorsal gray commissural inputs. Journal of neurophysiology 109:58–67. [DOI] [PubMed] [Google Scholar]
- Bhumbra GS, Bannatyne BA, Watanabe M, Todd AJ, Maxwell DJ, Beato M (2014), The recurrent case for the Renshaw cell. The Journal of neuroscience : the official journal of the Society for Neuroscience 34:12919–12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, de Groat WC (1993), Induction of c-fos expression in spinal neurons by nociceptive and nonnociceptive stimulation of LUT. Am J Physiol 265:R326–333. [DOI] [PubMed] [Google Scholar]
- Blaivas JG, Sinha HP, Zayed AA, Labib KB (1981), Detrusor-external sphincter dyssynergia: a detailed electromyographic study. The Journal of urology 125:545–548. [DOI] [PubMed] [Google Scholar]
- Blok BF, de Weerd H, Holstege G (1997), The pontine micturition center projects to sacral cord GABA immunoreactive neurons in the cat. Neurosci Lett 233:109–112. [DOI] [PubMed] [Google Scholar]
- Blok BF, van Maarseveen JT, Holstege G (1998), Electrical stimulation of the sacral dorsal gray commissure evokes relaxation of the external urethral sphincter in the cat. Neurosci Lett 249:68–70. [DOI] [PubMed] [Google Scholar]
- Brockett EG, Seenan PG, Bannatyne BA, Maxwell DJ (2013), Ascending and descending propriospinal pathways between lumbar and cervical segments in the rat: evidence for a substantial ascending excitatory pathway. Neuroscience 240:83–97. [DOI] [PubMed] [Google Scholar]
- Bui TV, Grande G, Rose PK (2008), Relative location of inhibitory synapses and persistent inward currents determines the magnitude and mode of synaptic amplification in motoneurons. Journal of neurophysiology 99:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp JS, Tennissen AM, Liebschutz JE, Chen XY, Wolpaw JR (2010), External urethral sphincter motoneuron properties in adult female rats studied in vitro. Journal of neurophysiology 104:1286–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HH, Yeh JC, Ichiyama RM, Rodriguez LV, Havton LA (2018), Mapping and neuromodulation of lower urinary tract function using spinal cord stimulation in female rats. Exp Neurol 305:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Cheng CL, Chen JJ, de Groat WC (2007), Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. American journal of physiology Renal physiology 292:F1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Cheng CL, Chen JJ, Peng CW, de Groat WC (2006), Reflexes evoked by electrical stimulation of afferent axons in the pudendal nerve under empty and distended bladder conditions in urethane-anesthetized rats. Journal of neuroscience methods 150:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CL, de Groat WC (2010), Role of 5-HT1A receptors in control of lower urinary tract function in anesthetized rats. American journal of physiology Renal physiology 298:F771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniak M, Anglister L, Lev-Tov A (2017), Shaping the Output of Lumbar Flexor Motoneurons by Sacral Neuronal Networks. The Journal of neuroscience : the official journal of the Society for Neuroscience 37:1294–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniak M, Etlin A, Strauss I, Anglister L, Lev-Tov A (2014), The sacral networks and neural pathways used to elicit lumbar motor rhythm in the rodent spinal cord. Front Neural Circuits 8:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, et al. (2008), Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med 14:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley KC, MacNeil BJ, Chopek JW, Sutherland S, Schmidt BJ (2015), Neurochemical excitation of thoracic propriospinal neurons improves hindlimb stepping in adult rats with spinal cord lesions. Exp Neurol 264:174–187. [DOI] [PubMed] [Google Scholar]
- D’Amico SC, Collins WF 3rd (2012), External urethral sphincter motor unit recruitment patterns during micturition in the spinally intact and transected adult rat. Journal of neurophysiology 108:2554–2567. [DOI] [PubMed] [Google Scholar]
- Danner SM, Zhang H, Shevtsova NA, Borowska-Fielding J, Deska-Gauthier D, Rybak IA, Zhang Y (2019), Spinal V3 Interneurons and Left-Right Coordination in Mammalian Locomotion. Front Cell Neurosci 13:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlot F, Cayetanot F, Gauthier P, Matarazzo V, Kastner A (2012), Extensive respiratory plasticity after cervical spinal cord injury in rats: axonal sprouting and rerouting of ventrolateral bulbospinal pathways. Exp Neurol 236:88–102. [DOI] [PubMed] [Google Scholar]
- de Groat WC (1976), Mechanisms underlying recurrent inhibition in the sacral parasympathetic outflow to the urinary bladder. The Journal of physiology 257:503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC (2002), Plasticity of bladder reflex pathways during postnatal development. Physiol Behav 77:689–692. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Araki I, Vizzard MA, Yoshiyama M, Yoshimura N, Sugaya K, Tai C, Roppolo JR (1998), Developmental and injury induced plasticity in the micturition reflex pathway. Behav Brain Res 92:127–140. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Griffiths D, Yoshimura N (2015), Neural control of the lower urinary tract. Compr Physiol 5:327–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat WC, Ryall RW (1968), Recurrent inhibition in sacral parasympathetic pathways to the bladder. The Journal of physiology 196:579–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Theobald RJ (1976), Reflex activation of sympathetic pathways to vesical smooth muscle and parasympathetic ganglia by electrical stimulation of vesical afferents. The Journal of physiology 259:223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N (2006), Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res 152:59–84. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N (2015), Anatomy and physiology of the lower urinary tract. Handb Clin Neurol 130:61–108. [DOI] [PubMed] [Google Scholar]
- Deschenes M, Takatoh J, Kurnikova A, Moore JD, Demers M, Elbaz M, Furuta T, Wang F, et al. (2016), Inhibition, Not Excitation, Drives Rhythmic Whisking. Neuron 90:374–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobberfuhl AD, Oti T, Sakamoto H, Marson L (2014), Identification of CNS neurons innervating the levator ani and ventral bulbospongiosus muscles in male rats. J Sex Med 11:664–677. [DOI] [PubMed] [Google Scholar]
- Dolber PC, Gu B, Zhang X, Fraser MO, Thor KB, Reiter JP (2007), Activation of the external urethral sphincter central pattern generator by a 5-HT(1A) receptor agonist in rats with chronic spinal cord injury. American journal of physiology Regulatory, integrative and comparative physiology 292:R1699–1706. [DOI] [PubMed] [Google Scholar]
- Dutton RC, Carstens MI, Antognini JF, Carstens E (2006), Long ascending propriospinal projections from lumbosacral to upper cervical spinal cord in the rat. Brain research 1119:76–85. [DOI] [PubMed] [Google Scholar]
- Dyro FM, Yalla SV (1986), Refractoriness of urethral striated sphincter during voiding: studies with afferent pudendal reflex arc stimulation in male subjects. The Journal of urology 135:732–736. [DOI] [PubMed] [Google Scholar]
- Fedirchuk B, Hochman S, Shefchyk SJ (1992), An intracellular study of perineal and hindlimb afferent inputs onto sphincter motoneurons in the decerebrate cat. Exp Brain Res 89:511–516. [DOI] [PubMed] [Google Scholar]
- Flynn JR, Conn VL, Boyle KA, Hughes DI, Watanabe M, Velasquez T, Goulding MD, Callister RJ, et al. (2017), Anatomical and Molecular Properties of Long Descending Propriospinal Neurons in Mice. Front Neuroanat 11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JR, Graham BA, Galea MP, Callister RJ (2011), The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology 60:809–822. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Griffiths D, de Groat WC (2008), The neural control of micturition. Nat Rev Neurosci 9:453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe RE (1991), Spatial distribution of recurrent inhibitory synapses on spinal motoneurons in the cat. Journal of neurophysiology 65:1134–1149. [DOI] [PubMed] [Google Scholar]
- Gambrill AC, Barria A (2011), NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proc Natl Acad Sci U S A 108:5855–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S, Duale H, Cameron AA, Abshire SM, Lyttle TS, Rabchevsky AG (2008), Plasticity of lumbosacral propriospinal neurons is associated with the development of autonomic dysreflexia after thoracic spinal cord transection. J Comp Neurol 509:382–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou XH, Hyun M, Taranda J, Huang KW, Todd E, Feng D, Atwater E, Croney D, et al. (2016), Central Control Circuit for Context-Dependent Micturition. Cell 167:73–86 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Katz R, Mackel R (1988), Distribution of recurrent inhibition within a motor nucleus. II. Amount of recurrent inhibition in motoneurones to fast and slow units. Acta Physiol Scand 134:363–374. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Lipski J, Mackel R, Wigstrom H (1988), Distribution of recurrent inhibition within a motor nucleus. I. Contribution from slow and fast motor units to the excitation of Renshaw cells. Acta Physiol Scand 134:347–361. [DOI] [PubMed] [Google Scholar]
- Im YJ, Hong CH, Jin MH, Lee BH, Han SW (2008), c-fos expression in bladder-specific spinal neurons after spinal cord injury using pseudorabies virus. Yonsei Med J 49:479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadekawa K, Majima T, Shimizu T, Wada N, de Groat WC, Kanai AJ, Goto M, Yoshiyama M, et al. (2017), The role of capsaicin-sensitive C-fiber afferent pathways in the control of micturition in spinal-intact and spinal cord-injured mice. American journal of physiology Renal physiology 313:F796–F804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadekawa K, Yoshimura N, Majima T, Wada N, Shimizu T, Birder LA, Kanai AJ, de Groat WC, et al. (2016), Characterization of bladder and external urethral activity in mice with or without spinal cord injury--a comparison study with rats. American journal of physiology Regulatory, integrative and comparative physiology 310:R752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizaki H, Fraser MO, De Groat WC (1997), Reflex pathways controlling urethral striated and smooth muscle function in the male rat. Am J Physiol 272:R1647–1656. [DOI] [PubMed] [Google Scholar]
- Karnup SV, de Groat WC (2020a), Propriospinal Neurons of L3-L4 Segments Involved in Control of the Rat External Urethral Sphincter. Neuroscience 425:12–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnup SV, De Groat WC (2020b), Mapping of spinal interneurons involved in regulation of the lower urinary tract in juvenile male rats. IBRO Rep 9:115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JA, Chen J, Simpson S, Wang EH, Lilascharoen V, George O, Lim BK, Stowers L (2018), Voluntary urination control by brainstem neurons that relax the urethral sphincter. Nat Neurosci 21:1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse MN, Belton AL, de Groat WC (1993), Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. Am J Physiol 264:R1157–1163. [DOI] [PubMed] [Google Scholar]
- LaPallo BK, Wolpaw JR, Chen XY, Carp JS (2014), Long-term recording of external urethral sphincter EMG activity in unanesthetized, unrestrained rats. American journal of physiology Renal physiology 307:F485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann C, Kessels HW (2014), The developmental stages of synaptic plasticity. The Journal of physiology 592:13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Inokuchi H, McLachlan EM, Li JS, Higashi H (2001), Correlation between electrophysiology and morphology of three groups of neuron in the dorsal commissural nucleus of lumbosacral spinal cord of mature rats studied in vitro. J Comp Neurol 437:156–169. [DOI] [PubMed] [Google Scholar]
- Mackel R (1979), Segmental and descending control of the external urethral and anal sphincters in the cat. The Journal of physiology 294:105–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson L, Gravitt K (2004), Spinal neurons activated with the urethrogenital reflex in the male rat. Brain research 1026:108–115. [DOI] [PubMed] [Google Scholar]
- McGee MJ, Amundsen CL, Grill WM (2015), Electrical stimulation for the treatment of lower urinary tract dysfunction after spinal cord injury. J Spinal Cord Med 38:135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael FM, Patel SP, Rabchevsky AG (2019), Intraspinal Plasticity Associated With the Development of Autonomic Dysreflexia After Complete Spinal Cord Injury. Front Cell Neurosci 13:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu K, Niwa M, Sasaki SI (2020), Properties of Renshaw-like cells excited by recurrent collaterals of pudendal motoneurons in the cat. J Physiol Sci 70:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadelhaft I, Miranda-Sousa AJ, Vera PL (2002), Separate urinary bladder and prostate neurons in the central nervous system of the rat: simultaneous labeling with two immunohistochemically distinguishable pseudorabies viruses. BMC Neurosci 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadelhaft I, Vera PL (1996), Neurons in the rat brain and spinal cord labeled after pseudorabies virus injected into the external urethral sphincter. J Comp Neurol 375:502–517. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Vera PL (2001), Separate urinary bladder and external urethral sphincter neurons in the central nervous system of the rat: simultaneous labeling with two immunohistochemically distinguishable pseudorabies viruses. Brain research 903:33–44. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Vera PL, Card JP, Miselis RR (1992), Central nervous system neurons labelled following the injection of pseudorabies virus into the rat urinary bladder. Neurosci Lett 143:271–274. [DOI] [PubMed] [Google Scholar]
- Ni Y, Nawabi H, Liu X, Yang L, Miyamichi K, Tedeschi A, Xu B, Wall NR, et al. (2014), Characterization of long descending premotor propriospinal neurons in the spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience 34:9404–9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikov V, Bullara L, McCreery DB (2007), Intraspinal stimulation for bladder voiding in cats before and after chronic spinal cord injury. J Neural Eng 4:356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikov V, Wrathall JR (2001), Coordination of the bladder detrusor and the external urethral sphincter in a rat model of spinal cord injury: effect of injury severity. The Journal of neuroscience : the official journal of the Society for Neuroscience 21:559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabchevsky AG (2006), Segmental organization of spinal reflexes mediating autonomic dysreflexia after spinal cord injury. Prog Brain Res 152:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed WR, Shum-Siu A, Onifer SM, Magnuson DS (2006), Inter-enlargement pathways in the ventrolateral funiculus of the adult rat spinal cord. Neuroscience 142:1195–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed WR, Shum-Siu A, Whelan A, Onifer SM, Magnuson DS (2009), Anterograde labeling of ventrolateral funiculus pathways with spinal enlargement connections in the adult rat spinal cord. Brain research 1302:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackman JE, Sims MH (1990), Electromyographic evaluation of the external urethral sphincter during cystometry in male cats. Am J Vet Res 51:1237–1241. [PubMed] [Google Scholar]
- Sasaki M (1994), Morphological analysis of external urethral and external anal sphincter motoneurones of cat. J Comp Neurol 349:269–287. [DOI] [PubMed] [Google Scholar]
- Seth JH, Panicker JN, Fowler CJ (2013), The neurological organization of micturition. Handb Clin Neurol 117:111–117. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ (2001), Sacral spinal interneurones and the control of urinary bladder and urethral striated sphincter muscle function. The Journal of physiology 533:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N, Doyal MF, Goins WF, Kadekawa K, Wada N, Kanai AJ, de Groat WC, Hirayama A, et al. (2017), Morphological changes in different populations of bladder afferent neurons detected by herpes simplex virus (HSV) vectors with cell-type-specific promoters in mice with spinal cord injury. Neuroscience 364:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Majima T, Suzuki T, Shimizu N, Wada N, Kadekawa K, Takai S, Takaoka E, et al. (2018), Nerve growth factor-dependent hyperexcitability of capsaicin-sensitive bladder afferent neurones in mice with spinal cord injury. Exp Physiol 103:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sie JA, Blok BF, de Weerd H, Holstege G (2001), Ultrastructural evidence for direct projections from the pontine micturition center to glycine-immunoreactive neurons in the sacral dorsal gray commissure in the cat. J Comp Neurol 429:631–637. [DOI] [PubMed] [Google Scholar]
- Steward O, Falk PM (1991), Selective localization of polyribosomes beneath developing synapses: a quantitative analysis of the relationships between polyribosomes and developing synapses in the hippocampus and dentate gyrus. J Comp Neurol 314:545–557. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Roppolo JR, Yoshimura N, Card JP, de Groat WC (1997), The central neural pathways involved in micturition in the neonatal rat as revealed by the injection of pseudorabies virus into the urinary bladder. Neurosci Lett 223:197–200. [DOI] [PubMed] [Google Scholar]
- Sun XQ, Xu C, Leclerc P, Benoit G, Giuliano F, Droupy S (2009), Spinal neurons involved in the control of the seminal vesicles: a transsynaptic labeling study using pseudorabies virus in rats. Neuroscience 158:786–797. [DOI] [PubMed] [Google Scholar]
- Takahashi R, Yoshizawa T, Yunoki T, Tyagi P, Naito S, de Groat WC, Yoshimura N (2013), Hyperexcitability of bladder afferent neurons associated with reduction of Kv1.4 alpha-subunit in rats with spinal cord injury. The Journal of urology 190:2296–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstegen AMJ, Vanderhorst V, Gray PA, Zeidel ML, Geerling JC (2017), Barrington’s nucleus: Neuroanatomic landscape of the mouse “pontine micturition center”. J Comp Neurol 525:2287–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzard MA (2000), Increased expression of spinal cord Fos protein induced by bladder stimulation after spinal cord injury. American journal of physiology Regulatory, integrative and comparative physiology 279:R295–305. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Erickson VL, Card JP, Roppolo JR, de Groat WC (1995), Transneuronal labeling of neurons in the adult rat brainstem and spinal cord after injection of pseudorabies virus into the urethra. J Comp Neurol 355:629–640. [DOI] [PubMed] [Google Scholar]
- Waites CL, Craig AM, Garner CC (2005), Mechanisms of vertebrate synaptogenesis. Annu Rev Neurosci 28:251–274. [DOI] [PubMed] [Google Scholar]
- Wu L, Sonner PM, Titus DJ, Wiesner EP, Alvarez FJ, Ziskind-Conhaim L (2011), Properties of a distinct subpopulation of GABAergic commissural interneurons that are part of the locomotor circuitry in the neonatal spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience 31:4821–4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavvarian MM, Hong J, Fehlings MG (2020), The Functional Role of Spinal Interneurons Following Traumatic Spinal Cord Injury. Front Cell Neurosci 14:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholudeva LV, Qiang L, Marchenko V, Dougherty KJ, Sakiyama-Elbert SE, Lane MA (2018), The Neuroplastic and Therapeutic Potential of Spinal Interneurons in the Injured Spinal Cord. Trends Neurosci 41:625–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Shevtsova NA, Rybak IA, Harris-Warrick RM (2012), Neuronal activity in the isolated mouse spinal cord during spontaneous deletions in fictive locomotion: insights into locomotor central pattern generator organization. The Journal of physiology 590:4735–4759. [DOI] [PMC free article] [PubMed] [Google Scholar]