Abstract
Until recently, glycan epitopes have not been documented by the WHO/IUIS Allergen Nomenclature Sub-Committee. This was in part due to scarce or incomplete information on these oligosaccharides, but also due to the widely held opinion that IgE to these epitopes had little or no relevance to allergic symptoms. Most IgE-binding glycans recognized up to 2008 were considered to be “classical” cross-reactive carbohydrate determinants (CCD) that occur in insects, some helminths and throughout the plant kingdom. Since 2008, the prevailing opinion on lack of clinical relevance of IgE-binding glycans has been subject to a reevaluation. This was because IgE specific for the mammalian disaccharide galactose-alpha-1,3-galactose (alpha-gal) was identified as a cause of delayed anaphylaxis to mammalian meat in the United States, an observation that has been confirmed by allergists in many parts of the world. Several experimental studies have shown that oligosaccharides with one or more terminal alpha-gal epitopes can be attached as a hapten to many different mammalian proteins or lipids. The classical CCDs also behave like haptens since they can be expressed on proteins from multiple species. This is the explanation for extensive in vitro cross-reactivity related to CCDs. Because of these developments, the Allergen Nomenclature Sub-Committee recently decided to include glycans as potentially allergenic epitopes in an adjunct section of its website (www.allergen.org). In this article, the features of the main glycan groups known to be involved in IgE recognition are revisited, and their characteristic structural, functional, and clinical features are discussed.
Keywords: alpha-gal, clinical relevance, cross-reactive carbohydrate determinants, hapten, MMXF, MUXF3, O-glycans
1 |. INTRODUCTION
It has long been recognized that sera from some patients contain IgE antibodies (Ab) specific for oligosaccharide epitopes rather than, or in addition to, protein epitopes. In the earliest studies, these oligosaccharide epitopes were designated as cross-reactive carbohydrate determinants (CCD) because they proved to be responsible for extensive cross-reactivity in vitro. Initially, CCD were recognized on protein allergens derived from pollens, vegetables, and hymenoptera venom.1–5 In this report, we use the term CCD for the group of epitopes that are primary modifications of the core (Figure 1). Subsequently, similar epitopes were also recognized on some allergenic proteins present in extracts derived from nematodes.4,6,7 Since 2008, sensitization to another category of IgE-binding glycans including galactose-alpha-1,3-galactose (alpha-gal), which can be induced by bites of different tick species, has been recognized.8–10 This oligosaccharide is also best thought of as a hapten because it can be expressed on proteins from multiple mammalian species.11 We are using the word hapten to refer to the whole oligosaccharide attached to asparagine, while the epitope is that part of the oligosaccharide which IgE antibodies are thought to bind to. Furthermore, in the case of alpha-gal, the epitope for IgE Ab as part of a different oligosaccharide can be present on a lipid backbone.12,13 There are many problems associated with including these epitopes in the World Health Organization and International Union of Immunological Societies (WHO/IUIS) Allergen Nomenclature database, the most obvious of which is that none of them are related to a particular species, while the established allergen nomenclature system is based on individual proteins, each produced by a particular species which is reflected in the allergen name. In addition, the biosynthesis of an oligosaccharide is dependent on a series of enzymes related to each step in the process. Thus, although the loss or inactivation of an enzyme can alter the structure of a glycan epitope, there is no such thing as a single gene that controls the production of a specific oligosaccharide.14,15 Despite these obvious difficulties with incorporating glycan epitopes into the database, many members of the WHO/IUIS Allergen Nomenclature Sub-Committee had realized that the lack of a section on glycan epitopes in the database was irrational.
FIGURE 1.
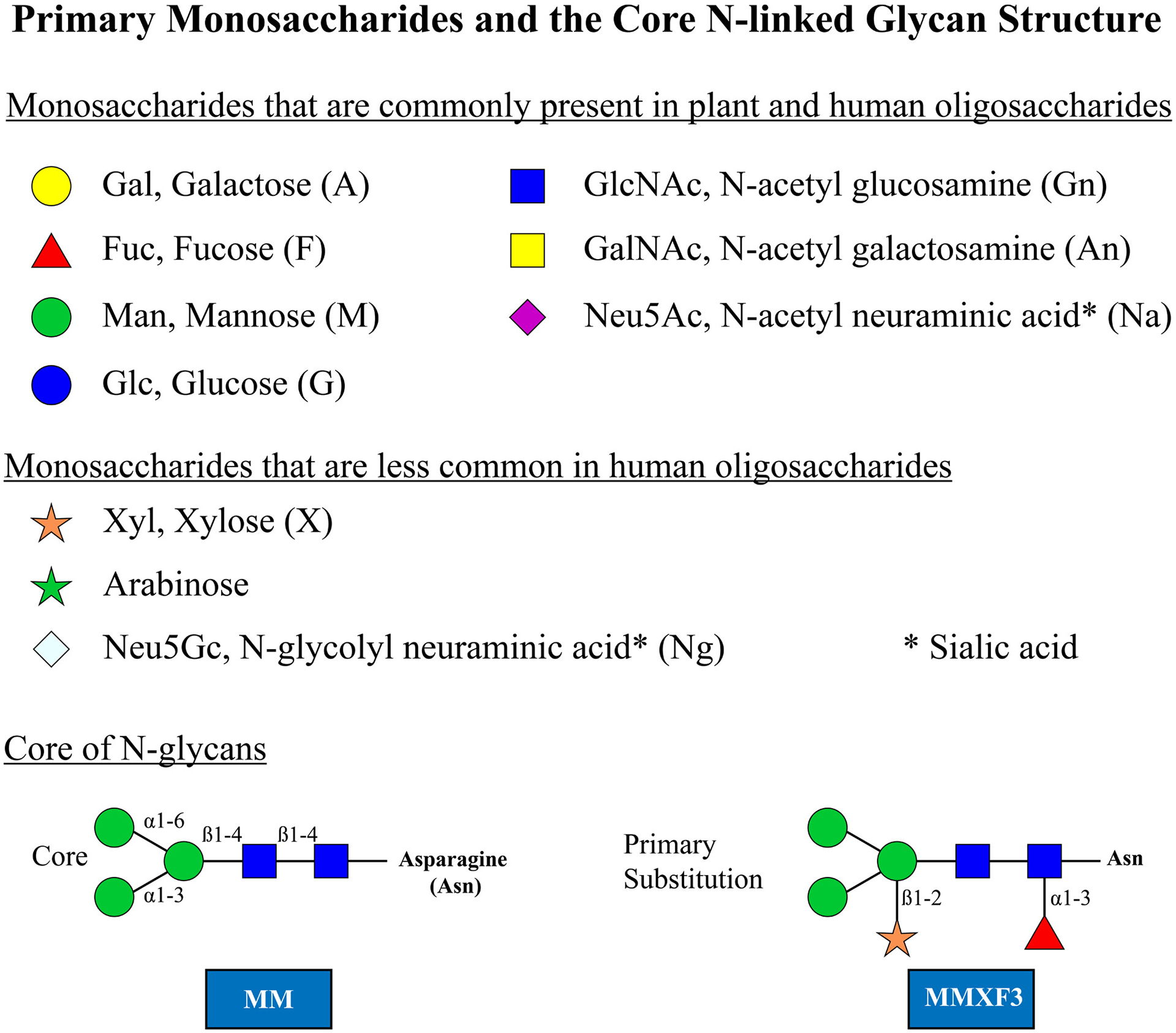
Each monosaccharide is indicated with the standard abbreviation first and the letter codes in parenthesis after the names are those used in Proglycan nomenclature (www.proglycan.com). The letter code MM indicates the primary core with two terminal mannose sugars. X indicates xylose which is only ever linked to the core as a beta 1–2 linkage on the proximal mannose. The letter F indicates a fucose and that is only linked to the proximal GlcNAc but should be labeled F3 to indicate the 1–3 linkage
There is not a simple way to classify the oligosaccharides since the IgE Ab responses are not restricted to one group. In addition, in many cases, it is difficult to define the species that induced an IgE response. However, the first approach was to describe the general characteristics of the glycans related to pollens, venoms, nematode worms, and ticks.2–4,16 This approach identified ~20 oligosaccharides where there was clear evidence of their significance for allergy diagnosis and allergic disease and which should be the initial proposal for this document. At this point, it is clear that although there are biochemical conventions for the description of an oligosaccharide, there are several types of abbreviations, many of which are already in use. In this synopsis, each oligosaccharide is presented as a stick diagram with the accepted system for the individual monosaccharide residues (Figure 1). In addition, a well-established letter code is used in the figures (see www.proglycan.com). It is essential in any publication or presentation about a novel oligosaccharide to describe or illustrate its structure before using an abbreviation.
2 |. SYMPTOMS RELATED TO IgE AB RESPONSES TO OLIGOSACCHARIDE EPITOPES ON DIFFERENT GROUPS OF ALLERGENS
The early studies related to CCD provided considerable evidence that IgE Ab responses to these epitopes did not contribute to allergic symptoms.1,3 This was particularly clear in relation to CCD epitopes on pollen antigens.2 The data are less clear in relation to venom antigens,5,7 but it has not been established that IgE Ab specific for oligosaccharides play a significant role in acute allergic reactions to venom.16 Equally, there is no good evidence that the symptoms that occur with nematodes entering the skin are related to IgE Ab specific for oligosaccharides. There is extensive evidence about the presence of IgE Ab to oligosaccharides on schistosomes and their soluble egg antigens (SEA). However, there have been very few studies on the relevance of these IgE Ab with respect to symptoms. Thus, it remains unclear whether the intense itching that can occur with schistosome species pathogenic to humans or with cercaria of duck schistosomes (swimmer’s itch) are caused by or contributed to by IgE Ab to oligosaccharides. The situation regarding the mammalian oligosaccharide alpha-gal is completely different because in this case there is extensive evidence that severe symptoms both immediate during cetuximab infusions, and as part of delayed reactions occurring 2–6 hours after eating mammalian meat, are directly related to specific IgE Ab.8,17–21 The immediate reactions to cetuximab are best explained by the presence of the oligosaccharide alpha-gal attached to the asparagine at position 43 in the Fab region of the heavy chain of this monoclonal antibody.22 The delay before the start of symptoms after eating red meat in patients with the alpha-gal syndrome may be best explained by the digestion of glycolipids containing alpha-gal.12,13 Furthermore, the digestion of such glycolipids over 2–6 hours may allow the formation and distribution of alpha-gal on low- and high-density lipoproteins which could be highly relevant to understanding the potential chronic effects of eating red meat.23,24
3 |. DEFINITION OF EPITOPES
Glycoproteins are formed by post-translational attachment or assembly of the oligosaccharide moiety on the newly synthesized protein in a process that generally occurs in two phases. In the production of N-linked oligosaccharides, an initial core structure is created in the endoplasmic reticulum (ER) and is linked to asparagine (N) residues in an amino acid motif that is either NXS or NXT where S is serine, T is threonine, and X any amino acid except proline. Following attachment of the core glycan, saccharides are removed or added both in the ER and the Golgi apparatus in a process that requires several different enzymes.25 A processed core structure starts with two GlcNAc monosaccharides attached to the side chain of asparagine (N) followed by a trimannosyl structure (Figure 1). Subsequently, multiple different terminal structures can be attached to the two distal mannose residues (eg, alpha-gal).
There are also a few well-described IgE Ab binding epitopes consisting of O-linked glycans2 (Figure 2A and 2B). A good example of IgE Ab binding to O-linked glycans comes from the mugwort pollen allergen Art v 1 and homologous allergens (Figure 2C). Although there are a few examples of IgE Ab specific for human proteins, there are no reported IgE specific for oligosaccharide epitopes on human proteins. This is with the possible exception of IgE to the B blood group antigen which represents non-self in humans who do not carry this blood group, and is therefore immunogenic.26
FIGURE 2.
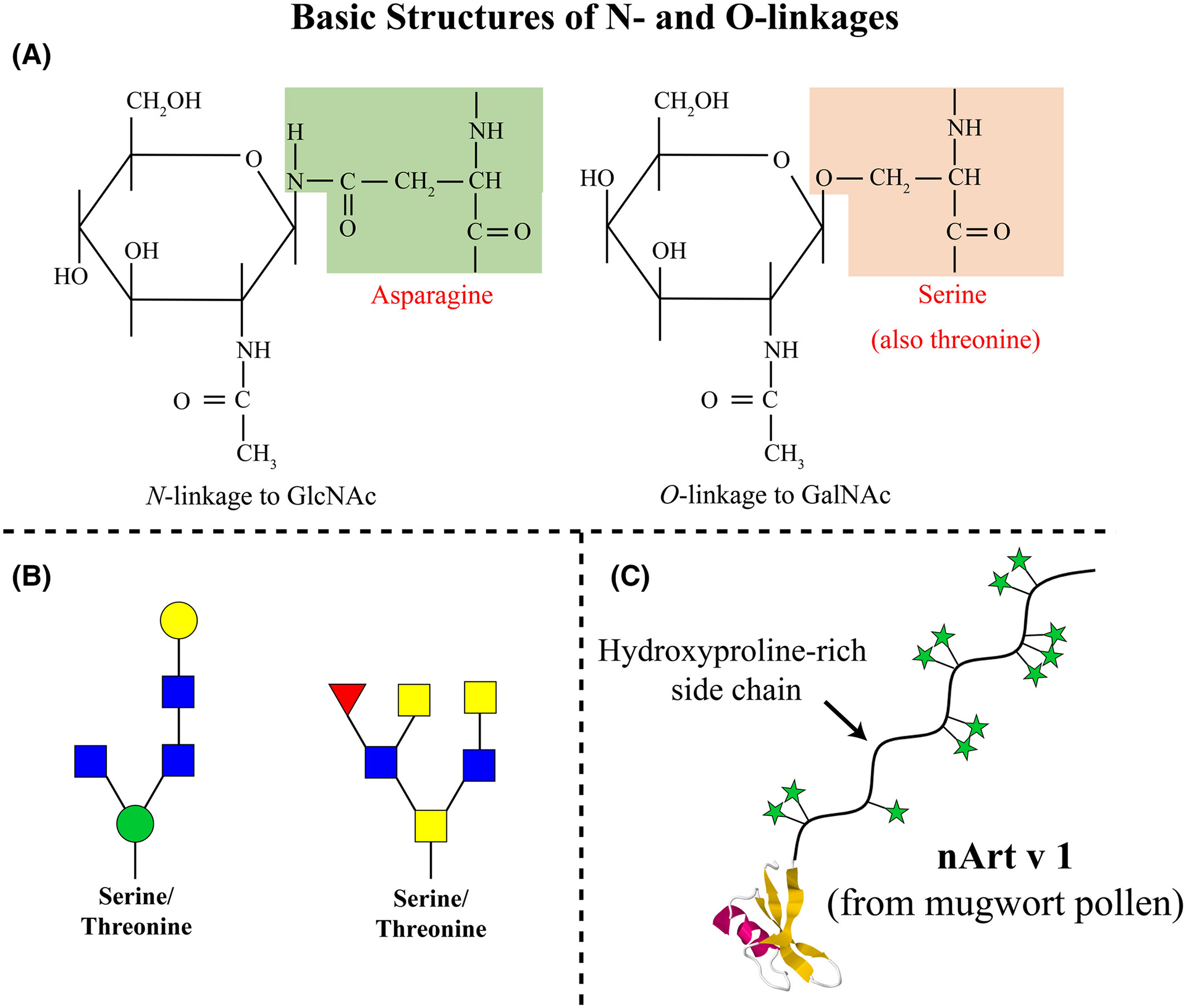
(A) Formal depiction of N-linkage to asparagine and O-linkage to serine or threonine. The proximal monosaccharides are GlcNAc and GalNAc, respectively. (B) Common forms of O-linked oligosaccharides linked through mannose to GlcNAc and through GalNAc to GlcNAc. (C) The arabinose saccharides are linked to hydroxyproline and known to be a significant target of IgE antibodies on Art v 1 from Mugwort
Both N-linked and O-linked structures are clearly defined as parts of glycoproteins, and since the oligosaccharide can be attached to commonly occurring sequence motifs (especially for N-linked glycans), the structures are clearly not species-specific (Figure 2A). By contrast, there are a few examples where a sugar structure contributes to a known protein allergen epitope, recognized by an antibody in a species-specific way. This situation has been clearly defined for an epitope on the cockroach allergen Bla g 2, where the role of a glycan as part of the epitope has been demonstrated in relation to both the binding of a monoclonal antibody and basophil histamine release.27,28 In addition, the primary epitope for IgE Ab binding on tomato allergen Sola l 2 (previously named Lyc e 2) has been shown to be MUXF3.29 Similar data may be relevant to the vespid venom hyaluronidase allergens,30 and recent studies have suggested that in some situations this “epitope-hapten” effect could also occur in relation to alpha-gal.31 An oligosaccharide can be immunogenic to humans if the enzyme needed to assemble it in a particular configuration is absent in humans (Figure 1).
New developments to isolate human IgE monoclonal antibodies and the use of structural biology approaches, such as X-ray crystallography and NMR, have led to detailed studies of allergen-antibody interactions, especially those between amino acids.27,32–34 These approaches will allow future analyses of 1) the contribution of sugars to antibody recognition of proteins (as mentioned above for Bla g 2) and 2) carbohydrate-only epitopes recognized by IgE.
3.1 |. Groups of oligosaccharides that are recognized as targets of IgE
The descriptions in this section of the database will conform to the conventions established by the Consortium for Functional Glycomics35,36 as well as the simplified letter code (www.proglycan.com). The oligosaccharides will be described in relation to five groups (Table 1):
TABLE 1.
Groups of oligosaccharides that are recognized as targets of ige
| Glycan groups | Names | Glycan examples | Abbreviation examples | Allergens with these glycans |
|---|---|---|---|---|
| Clinical significance has been reported for some of these glycans | ||||
| Group A | N-Glycans (“Classical” cross-reactive carbohydrate determinants or CCDs) |
|
|
Sola l 2 Ole e 1 Ana c 2 Api g 5 Bla g 2 Api m 1 Ves v 2 |
| Group B | Mammalian non-human oligosaccharides |
|
|
|
| Clinical significance of these glycans needs further investigation | ||||
| Group C | O-Glycans |
|
Amb a 4 Art v 1 Phl p 1a |
|
| Group D | Glycans from nematode parasites |
|
|
|
| Group E | Galacto-oligosaccharides |
|
|
|
Clinical relevance of these oligosaccharides as allergens is currently under investigation.
3.1.1 |. Major groups
Group A: Classical CCD (N-glycans) including oligosaccharides related to plants and invertebrates (Figures 1 and 3).
Group B: Mammalian non-human oligosaccharides including galactose alpha-1,3-galactose (alpha-gal). Alpha-gal is present in all mammals except great apes and Old World monkeys, but the route of sensitization in the United States, Australia, Europe, and Japan is in almost all cases a bite or bites from one or more species of ticks (Figure 4).9,10,37–39
FIGURE 3.
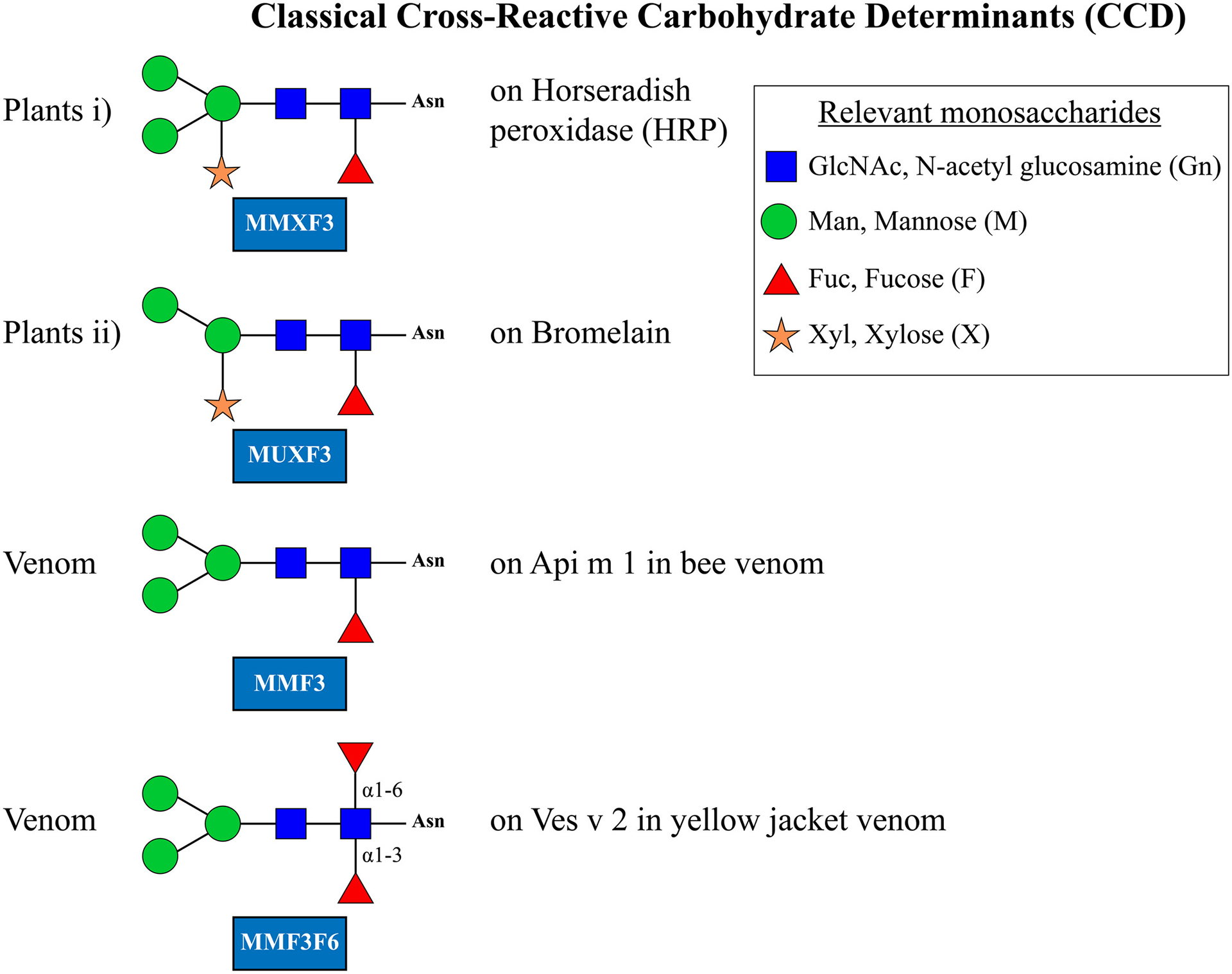
The term MU in MUXF3 indicates that the terminal mannose deletion is of the 1–3 linkage; the X indicates a xylose linked beta 1–2 to the proximal mannose, and F3 indicates a fucose linked alpha 1–3 on the proximal GlcNAc. In each case, these changes are of a unique form not seen at other sites in the core
FIGURE 4.

(A) The alpha 1–3 galactosyltransferase that establishes the alpha-gal linkage is different from the alpha 1–3 galactosyltransferase that can make a very similar linkage to a substituted galactose on blood group B. The alpha-gal hapten as well as the A and B blood group oligosaccharides can be linked either to asparagine or O-linked to the sphingolipid ceramide. (B) Despite abundant evidence that oligosaccharides with terminal Neu5Gc can be antigenic there is little evidence that they are target of human IgE. (C) GOS are galacto-oligosaccharides of various lengths produced as prebiotics from cow’s milk (see group E)
3.1.2 |. Minor Groups
Group C: Oligosaccharides with O-linkage rather than N-linkage to the protein (Figure 2).
Group D: The nematode parasites and in particular the oligosaccharides that are present on schistosomes and schistosome soluble egg antigens (SEA) (Figure 5).
Group E: Galacto-oligosaccharides (GOS) produced from milk by bacterial beta-galactosidase (Figure 4).
FIGURE 5.

Many different oligosaccharides are expressed on helminth proteins and can be the target of IgE antibodies. The oligosaccharides shown for schistosomes are very similar to classical CCD. By contrast, the oligosaccharide shown for Echinococcus is O-linked and has a very different structure. *An is the Proglycan form for N-acetyl galactosamine and the X is for xylose which is only found attached 1 → 2 to the proximal mannose of the core
4 |. GROUP A. CROSS-REACTIVE CARBOHYDRATE DETERMINANTS: CLASSICAL CCD (INCLUDING N-GLYCANS OF THE MMXF3, MMF3, AND MUXF3-TYPE)
The first groups described in any detail were those on plant and hymenoptera venom proteins. These have the basic structure or core of two GlcNAc sugars with two or three terminal mannose residues. To these are added a xylose linked beta 1–2 to the proximal mannose and/or a fucose residue linked alpha 1–3 on the first GlcNAc in the core (Figure 3). While these sugars were first recognized on plant proteins, for example, bromelain from pineapple, oligosaccharides modified by fucose in the same manner are present on proteins found in honeybee or wasp venom (Figure 3). While the xylose modification is present in plants and absent in insect glycans, for example, MMF3, additional modifications such as a fucose residue linked 1–6 to the same core GlcNAc, for example, in MMF3F6, can be predominant. Thus, the most abundant oligosaccharides on Ves v 2 are MMF3F6 and MUF3F6.30 The 1–6 fucose modification is found in mammalian glycan structures as well as schistosome related oligosaccharides and does not per se form an epitope for IgE. It remains open whether the presence of the 1–6 linked fucose has an impact on IgE reactivity. The epitopes containing xylose and those containing fucose are frequently but not necessarily found in the same glycan structures and should be considered best as independent epitopes which are often shared by a single oligosaccharide. Due to the pronounced heterogeneity of the glycosylation, however, the presence of added xylose or fucose might sometimes be limited to a fraction of the glycans on an allergen. A well-described example is Api m 1, having a single glycosylation site only. Here, the 1,3-linked fucose is only present on 20% of the glycans.40 This limitation together with the need of two or more CCD epitopes for receptor crosslinking makes an efficient biological activity challenging.
Despite occasional cases where clinically significant reactions have occurred related to IgE Ab to CCD, most investigators have concluded that antibodies to these sugars play little role in symptoms related to natural exposure.1–3 The reasons why some individuals develop IgE Ab to CCD are not fully understood; however, potential causes include exposure to the pollens of grasses and weeds, and injection of hymenoptera venom through an insect sting.41 However, with regard to ticks as a sensitization route, carbohydrate sensitization has, to the best of our knowledge, been described only for a structurally different glycan, alpha-gal, which is why we have not included this sensitization route here. Experimental studies on the issue of clinical relevance are relatively rare in number. The methodological obstacle (Gordian Knot) is the fact that it is still difficult to investigate CCD epitopes without the attached protein/lipid, so that the impact of anti-CCD-IgE alone cannot be properly evaluated. Most of the experiments were designed as “deglycosylation” studies using periodate as a CCD-inactivating reagent and/or as comparison between naturally purified (glycosylated) allergen and its recombinant homologue produced in E. coli, which assures that no post-translational glycosylation occurred during recombinant production. Mari et al. reported studies with human lactoferrin expressed in rice which is glycosylated with CCD and could activate basophils in vitro but did not induce symptoms in a double-blind oral challenge.42 That result clearly questions the use of the basophil activation test (BAT) alone as evidence for clinical relevance of IgE to CCD.
The following observations speaking in favor of a clinical relevance of classical CCD are based on basophil activation, deglycosylation, and gene silencing studies. The glycosylated tomato allergen Sola l 2 was shown to be inactive in BAT after deglycosylation.7 Subsequent experiments on silencing beta-1,2-xylosyltransferase in transgenic tomato fruits, which heavily reduces xylose residues, provided further evidence for the clinical relevance of beta-1,2-xylose-containing epitopes in Sola l 2-mono-sensitized individuals using IgE-detection arrays as well as skin prick test.7,43 Those authors suggested that the CCD together with the protein formed the clinically relevant epitope. This information which is already pointing to CCDs being haptens has been recently supported by a comparative study on the natural (glycosylated) and recombinant bee allergen Api m 1, showing that glycosylation of the (natural) allergen enhanced its IgE-binding capacity (allergenicity) by introducing further IgE-epitopes.44 In addition, the enzymatic function of the natural variant was stronger and the glycosylated variant also performed much better in BAT.
Isolated N-glycans of the major allergen of olive pollen (Ole e 1) induced activation of basophils obtained from five olive pollen allergic patients45; deglycosylation of the celery allergen (Api g 5) led to a negative result in BAT with blood from a celery allergic patient,46 as well as insect venom allergic patients.5 Likewise, the disruption of oligosaccharide epitopes on insect venom and food decreased the activation of basophils of allergic patients.44,47,48 Ebo and coworkers investigated the relevance of BAT results in the context of classical CCD and symptoms related to latex allergy. Their results showed a strong correlation between BAT results and symptoms.49 However, in order to be methodologically sound, prospective studies with optimized BAT protocols should be performed in the future.50,51
One of the major findings in relation to CCD is that they can lead to confusion regarding in vitro assays for IgE. This has become particularly obvious with multiplex assays such as the microarrays. For this reason, one of the allergy diagnosis microarrays (ALEX) includes a “CCD blocker” in the assay technique.52 The CCD that can cause cross-reactivity include MUXF3, MMF3, and MMXF3 and extensive evidence suggests that fucose linked to the proximal GlcNAc is a major target of the IgE antibodies to CCD.4,41,53,54
5 |. GROUP B. MAMMALIAN OLIGOSACCHARIDES SUCH AS GALACTOSE-ALPHA-1, 3-GALACTOSE (ALPHA-GAL) THAT ARE NOT PRESENT IN HUMANS
The second major group of glycan epitopes was first recognized by Karl Landsteiner who reported in 1925 that all humans had “natural” antibodies to a “B like” antigen that was present on the red blood cells of all non-primate mammals.55 This antigen was subsequently recognized as a major transplantation antigen of the mammals.55,56 Dr. Uri Galili and others have done extensive work on the transplantation aspects of this antigen, and they defined the structure as alpha-gal.56 The production of the alpha-gal epitope depends on a specific alpha 1–3 galactosyl transferase (alpha 1–3 Gal T) which became inactivated 20 million years ago so that it is not present in humans and Old World primates.14,56 This enzyme specifically links galactose-alpha-1,3 to an unsubstituted galactose on GlcNAc. This oligosaccharide is indeed very close in structure to the blood group B antigen (Figure 4A). In common with other blood group antigens, alpha-gal can be expressed on proteins or lipids (Figure 4A).12,13 Thus, due to similarities with the B-antigen, individuals with blood group B have a reduced risk of sensitization to alpha-gal in some but not all studies and a reduced risk of mammalian meat allergy.57,58 IgE Ab to alpha-gal were first recognized during the investigation of anaphylactic reactions to the monoclonal antibody cetuximab used in cancer treatment.8 This antibody is specific for epidermal growth factor receptor (EGFR) and is produced commercially in a cell line (SP2/0) derived from mice.8 Those experiments demonstrated that the target of the IgE Ab binding to cetuximab was an oligosaccharide which in most cases was the diantennary form of alpha-gal and was the dominant glycan epitope expressed on asparagine at position 43 of the variable region in the Fab portion of the heavy chain of the molecule.8,22 Furthermore, it was clear that these IgE Ab were present in sera prior to the reactions, which occurred during the first infusion of cetuximab. Subsequent experiments demonstrated that in some areas of Virginia, North Carolina, Tennessee, and Arkansas, these IgE Ab to alpha-gal were present in 15–20% of the adult population. It has also become clear that these IgE Ab responses are associated with tick bites in the United States and also in Australia, Sweden, France, Germany, Japan, and many other countries.9,10,37,38,59–63 This is furthermore supported by the fact that tick saliva contains alpha-gal carrying proteins.37 If patients with IgE Ab to alpha-gal eat meat or organs (eg, intestines or kidneys) from non-primate mammals, a proportion of them (between 5 and 20%) will experience an allergic or anaphylactic reaction which in most cases starts between 2 and 6 hours after consumption of mammalian meat.20,64–66 The oligosaccharide target definitely includes the two terminal galactose sugars but may also be influenced by the adjacent monosaccharide which is GlcNAc (Figure 4A). IgE Ab can certainly bind to trisaccharide structures.67,68
It is well established that the oligosaccharide, alpha-gal, is present on mammalian tissues either as part of a glycoprotein or a glycolipid. At present, it seems likely that the primary forms contributing to sensitization are glycoproteins. However, there are good reasons for thinking that the glycolipid forms of alpha-gal could at least contribute to the delay in symptoms after eating red meat.12,13,20,69 This may also be relevant to the observation that sensitization to alpha-gal appears to be a risk factor for coronary artery disease.24 What is certain is that the IgE Ab response to this epitope can represent 40% or more of the total IgE in the circulation and that not surprisingly an IgE Ab response to alpha-gal can lead to a rapid increase in total IgE.58 Mammalian meat allergy often develops in middle-aged patients who have previously tolerated red meat. Because other alpha-gal-containing products, for example, gelatin-containing foods or vaccines and other mammalian-related pharmaceuticals, may cause allergic reactions, mammalian meat allergy is now known as the “alpha-gal syndrome” (AGS). The most common symptoms are urticaria and gastrointestinal symptoms. Of notice is the high frequency of anaphylaxis which almost half of the patients have experienced.58,66 In fact, in some areas AGS has been found to be a major “new” cause of anaphylaxis.70 The full diagnosis is based on a history of delayed allergic reactions to meat or other products from mammals; a positive test for IgE to alpha-gal and response to diet. The management includes avoidance of mammalian meat and of further tick bites.71 The sialic acid Neu5Gc like alpha-gal is not expressed in humans and is the target of IgG and IgM “natural” antibody responses. The evidence for IgE responses to Neu5Gc is confused and direct experiments in patients with AGS have not identified IgE to this antigen.68
6 |. GROUP C: OLIGOSACCHARIDES WITH O-LINK AGE R ATHER THAN N-LINK AGE TO THE PROTEIN
O-glycans are oligosaccharides attached most frequently to the side chain oxygen atom of serine or threonine residues on a protein, but also in some cases to tyrosine, hydroxylysine, or hydroxyproline (Figure 2A, B, C).72 The structure of these glycans is in most cases strikingly different from the N-glycans discussed in the rest of this report.72 However, the galactose-alpha-1, 3-galactose GlcNAc structure that is the target for IgE antibodies in AGS can be expressed as glycoprotein on a normal N-linked core (Fig 1 and Fig 4) or on a glycolipid such as ceramide via an O-linkage (Figure 4).
O-linked glycans are known to be present on ragweed allergen Amb a 4, mugwort allergen Art v 1, and grass pollen allergen Phl p 1, as well as on gum Arabic from acacia and some yeasts and molds.73–76 Only a few O-glycans have been associated with IgE antibody binding (eg, in Art v 1 but not Phl p 1). There is extensive evidence about the glycosylation of Art v 1 which is primarily arabinosides or galactoarabinosides on a hydroxyproline rich extension outside the globular region of the protein (Figure 2C).75 Preliminary evidence suggests that the primary target for IgE antibodies is single arabinosides in couplets. Evidence concerning the impact of IgE antibodies against these glycan structures on either in vitro diagnostics or symptoms is currently lacking.77
7 |. GROUP D. OLIGOSACCHARIDE EPITOPES THAT ARE THE TARGETS FOR IgE AB RESPONSES TO SCHISTOSOMES AND OTHER HELMINTHS
The existence of skin sensitizing antibodies related to schistosomes or schistosome cercaria has been known since the earliest days of the Prausnitz-Kuestner test.78 The evidence that these antibodies against schistosome antigens were IgE was an important aspect of studies related to the early gatekeeper hypothesis on the role of IgE Ab.79 The fact that these antibodies include IgE Ab specific for oligosaccharides came from several investigators. In many cases, these oligosaccharides are distinguished by a single terminal galactose or N-acetylgalactosamine residue (GalNAc) (Figure 5). Many different structures are found on other helminths, including O-linked oligosaccharides on antigens from Anisakis. Most of these are clearly foreign to humans but have not been fully investigated as targets of human IgE Ab.4,80–82
Notably, oligosaccharide epitopes of helminths frequently contain modifications to the core structure similar to those of classical CCDs, that is, fucose and xylose linked to the inner GlcNAc and proximal mannose residues, respectively. The nature of the helminth oligosaccharides is, however, more complex, often containing secondary and tertiary modifications with further sugar residues. In keeping with the molecular similarity between helminth oligosaccharides and classical CCD, IgE antibodies reacting with both groups do occur. However, a study with a monoclonal CCD-specific IgE Ab showed limited cross-reactivity between classical CCDs and helminth extract in ImmunoCAP, suggesting possible differences in recognizing oligosaccharides of these two groups.83
Although the presence of oligosaccharides on other helminths is well established, there is relatively little evidence regarding the significance of IgE antibodies to these epitopes. A recent study provides evidence that IgE to classical CCD, particularly those with an alpha-1, 3-fucose epitope, may not just be an epiphenomenon of parasite exposure but may be implicated in the protective effect of certain environmental and geohelminth exposures against asthma.84
8 |. GROUP E: GALACTO-OLIGOSACCHARIDES (GOS) PRODUCED BY BACTERIAL BETA-GAL ACTOSIDASE
GOS are prebiotics consisting of mixtures of oligomers containing glucose and polymerized galactose units including beta 1–6, beta 1–4, and beta 1–3 bonds.85 GOS occur naturally in human and animal milk and are commercially produced through the enzymatic conversion of lactose, using beta-galactosidase derived from bacteria. These prebiotics are added to commercial products (such as supplemented cow’s milk formula for infants). They typically consist of a chain of 2–6 galactose molecules attached to glucose (Figure 4C). Contrary to the carbohydrates of the other 4 groups described in this report, GOS are not bound to proteins in milk.
Allergic reactions to GOS have been reported mainly in Southeast Asia. Initially, occupational asthma in Japanese oyster farm workers was found to be associated with a carbohydrate linked to a high molecular weight allergen of sea squirt.86 A series of workers with sea squirt allergy reported immediate-type hypersensitivity reactions on ingestion of a lactic acid beverage commonly available in Japan, and GOS present in that beverage gave positive results in skin and histamine release tests. The observed cross-reactivity between GOS and the sea squirt antigen suggested that the sea squirt was the source of the sensitizing allergen in those cases. When GOS was introduced in several milk formulas, the first cases of cow’s milk tolerant children who developed anaphylaxis following ingestion of cow’s milk formula supplemented with GOS were reported.87 Patients reacted to fractions of GOS containing 3 sugar units or greater, in skin prick tests and basophil activation. However, not all types of GOS are allergenic. Kaneko et al. were able to identify two allergenic GOS types and to develop a hypoallergenic one by using a beta-galactosidase from a different microbial source.88 It is difficult to understand how free galacto-oligosaccharides can induce an allergic reaction unless two or more oligosaccharides become linked either on a substrate or by circulating antibodies. Despite recent evidence from Singapore suggesting that sensitization to GOS is related to dust mite sensitization, it remains true that GOS-containing prebiotics are widely available all over the world whereas allergic reactions seem to be limited to Asia, pointing to a still unknown primary sensitizing source.89
9 |. CONCLUSIONS AND RECOMMENDATIONS
Understanding of the oligosaccharides on glycoproteins as IgE epitopes has lagged behind that of the amino acids involved in IgE epitopes, partly for technical reasons, but also because we did not realize their significance. Today using either traditional biochemical techniques or mass spectrometry the analysis of these epitopes can be achieved in a simpler more reliable way.90 In addition, the recent development of techniques to make true human monoclonal IgE antibodies has opened up the potential for much more detailed evaluation of the ways these IgE Ab bind to oligosaccharides.32 In addition, our knowledge of oligosaccharides that bind IgE has expanded, with the significance of two types increasingly well understood. The original or classical CCD were recognized as a target of IgE Ab 40 years ago and were identified in a wide variety of allergen sources including pollens, vegetables, and venoms. Study of those oligosaccharide epitopes has provided specific information about their structures as well as the sources and their relevance to allergic disease. The discovery of IgE specific for the mammalian oligosaccharide alpha-gal almost 15 years ago led to the recognition of a form of food allergy where the symptoms resulted from a different exposure (ie, meat or other tissues from non-primate mammals) than the exposures (ie, bites of a variety of tick species) which cause sensitization. In addition, alpha-gal is known to be expressed on both glycoproteins and glycolipids in mammalian tissues. The important principle is that the “foreign” epitopes relate to a linkage that does not occur in human oligosaccharides. The obvious examples are xylose linked 1–2 to the proximal mannose; fucose linked 1–3 to the proximal GlcNAc; and terminal unsubstituted galactoses linked alpha-1,3.
At present, our understanding of the factors that influence an IgE response to an oligosaccharide epitope is incomplete. In particular, we do not know why some pollen responses are more likely to induce IgE to CCD. Equally, despite ongoing investigation by several groups, it remains unclear why tick bites are so effective at inducing IgE to alpha-gal. There are several questions that arise from this report about the presentation of oligosaccharides in publications or presentations. We have summarized current recommendations here (Table 2). However, we need to recognize that this document does not propose a consistent nomenclature for this diverse set of oligosaccharides. Currently we do not believe this would be possible because these epitopes are present on proteins from many different species and there is a large established literature on the structures unrelated to allergic disease.
TABLE 2.
Recommendations for describing and naming of carbohydrate/oligosaccharide epitopes and haptens that are the target of human IgE antibodies
Recommendation #1: Where possible the stick diagram of an oligosaccharide should be shown using the Consortium for Functional Genomics (CFG) symbol nomenclature for the saccharides. In general, the core structure should be defined as a starting point for both CCD and alpha-gal and the additions or deletions to the core structure should be specified in detail, including an indication of significant or novel linkage as well as an explanation of the symbols used (See Figure 1 and Figure 3).
|
Recommendation #2: Where abbreviations for oligosaccharides are well established they should be used: these include CCD; alpha-gal; blood group B; MUXF3; and GOS. In addition, the ProGlycAn system can be used to describe other modifications of the core-linked N-glycans.
|
| Recommendation #3: In addition to the heterogeneous group of known sugars recognized by IgE, additional IgE-binding carbohydrates are likely to exist. The WHO/IUIS Allergen Nomenclature Sub-Committee encourages the submission of structures and suggested names for newly identified carbohydrates involved in IgE recognition and, especially those with evidence of relevance to clinical manifestations of allergic disease. |
What is certain is that the field will continue to develop, not only indirect knowledge of the oligosaccharides that are the target of the IgE antibodies, but also in understanding of their relevance to allergic disease. The current document is meant as a summary of what is currently known, with full recognition that it will need regular updating as an attachment to the allergen nomenclature database at www.allergen.org.
ACKNOWLEDGEMENTS
We acknowledge and give thanks for the support received by the WHO/IUIS Allergen Sub-Committee from the EAACI, the AAAAI, and IUIS. Dr. Platts-Mills reports non-financial support from Thermo Fisher Scientific, outside the submitted work. Dr. Hilger, Prof. Dr. Jappe, Dr. Spillner, Dr. Keshavarz, Dr. Aalberse, Dr. Goodman, and Dr. van Hage have nothing to disclose. Dr. Gadermaier reports personal fees from Bencard, personal fees from COMPARE, outside the submitted work. Dr. Lidholm reports no conflicts other than those from working at Thermo Fisher Scientific. Dr. van Ree reports consultancies from HAL Allergy BV, Citeq BV, Angany Inc., outside the submitted work. Dr. Pomés reports grants from NIH/NIAID, other from Indoor Biotechnologies, Inc., outside the submitted work.
Abbreviations:
- AGS
Alpha-gal syndrome
- Alpha-gal
Galactose-alpha-1,3-galactose
- BAT
Basophil activation test
- CCD
Cross-reactive carbohydrate determinants
- CFG
Consortium for Functional Glycomics
- Fab
Antibody binding fragment
- GOS
galacto-oligosaccharide
- Neu5Gc
N-glycolyl neuraminic acid
- SEA
Schistosome soluble egg antigens
REFERENCES
- 1.Aalberse RC, Koshte V, Clemens JGJ. Immunoglobulin-E antibodies that crossreact with vegetable foods, pollen, and hymenoptera venom. J Allergy Clin Immunol. 1981;68(5):356–364. [DOI] [PubMed] [Google Scholar]
- 2.Altmann F The role of protein glycosylation in allergy. Int Arch Allergy Immunol. 2007;142(2):99–115. [DOI] [PubMed] [Google Scholar]
- 3.van Ree R Clinical importance of cross-reactivity in food allergy. Curr Opin Allergy Clin Immunol. 2004;4(3):235–240. [DOI] [PubMed] [Google Scholar]
- 4.Homann A, Schramm G, Jappe U. Glycans and glycan-specific IgE in clinical and molecular allergology: sensitization, diagnostics, and clinical symptoms. J Allergy Clin Immunol. 2017;140(2):356–368. [DOI] [PubMed] [Google Scholar]
- 5.Eberlein B, Krischan L, Darsow U, Ollert M, Ring J. Double positivity to bee and wasp venom: improved diagnostic procedure by recombinant allergen-based IgE testing and basophil activation test including data about cross-reactive carbohydrate determinants. J Allergy Clin Immunol. 2012;130(1):155–161. [DOI] [PubMed] [Google Scholar]
- 6.van Diepen A, Smit CH, van Egmond L, et al. Differential anti-glycan antibody responses in Schistosoma mansoni-infected children and adults studied by shotgun glycan microarray. PLoS Negl Trop Dis. 2012;6(11):e1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foetisch K, Westphal S, Lauer I, et al. Biological activity of IgE specific for cross-reactive carbohydrate determinants. J Allergy Clin Immunol. 2003;111(4):889–896. [DOI] [PubMed] [Google Scholar]
- 8.Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Nunen SA. Tick-induced allergies: mammalian meat allergy and tick anaphylaxis. Med J Aust. 2018;208(7):316–321. [DOI] [PubMed] [Google Scholar]
- 10.Commins SP, James HR, Kelly LA, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2011;127(5):1286–93 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Platts-Mills TA, Commins SP, Biedermann T, et al. On the cause and consequences of IgE to galactose-α−1, 3-galactose: a Report from the National Institute of Allergy and Infectious Disease Workshop on Understanding IgE-Mediated Mammalian Meat Allergy. Journal of Allergy and Clinical Immunology. 2020;145(4):1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson JM, Platts-Mills TA. The oligosaccharide galactose-α−1, 3-galactose and the α-Gal syndrome: insights from an epitope that is causal in immunoglobulin E-mediated immediate and delayed anaphylaxis. EMJ Allergy Immunol. 2018;3:89–98. [Google Scholar]
- 13.Roman-Carrasco P, Lieder B, Somoza V, et al. Only alpha-gal bound to lipids, but not to proteins, is transported across enterocytes as an IgE-reactive molecule that can induce effector cell activation. Allergy 2019;74(10):1956–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koike C, Uddin M, Wildman DE, et al. Functionally important glycolsyltransferase gain and loss during catarrhine primate emergence. Proc Natl Acad Sci U S A. 2007;104(2):559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galili U Evolution in primates by “Catastrophic-selection” interplay between enveloped virus epidemics, mutated genes of enzymes synthesizing carbohydrate antigens, and natural anti-carbohydrate antibodies. Am J Phys Anthropol. 2019;168(2):352–363. [DOI] [PubMed] [Google Scholar]
- 16.Jappe U, Raulf-Heimsoth M, Hoffmann M, Burow G, Hubsch-Muller C, Enk A. In vitro hymenoptera venom allergy diagnosis: improved by screening for cross-reactive carbohydrate determinants and reciprocal inhibition. Allergy 2006;61(10):1220–1229. [DOI] [PubMed] [Google Scholar]
- 17.O’Neil BH, Allen R, Spigel DR, et al. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25(24):3644–3648. [DOI] [PubMed] [Google Scholar]
- 18.Steinke JW, Platts-Mills TA, Commins SP. The alpha-gal story: lessons learned from connecting the dots. J Allergy Clin Immunol. 2015;135(3):589–596; quiz 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilger C, Hemmer W, Swoboda I, et al. Molecular and extract-based diagnostics in meat allergy. In: Kleine-Tebbe J, Jakob T eds. Molecular allergy diagnostics. Springer;2017:305–326. [Google Scholar]
- 20.Commins SP, James HR, Stevens W, et al. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2014;134(1):108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilger C, Fischer J, Swiontek K, et al. Two galactose-alpha-1,3-galactose carrying peptidases from pork kidney mediate anaphylactogenic responses in delayed meat allergy. Allergy 2016;71(5):711–719. [DOI] [PubMed] [Google Scholar]
- 22.Qian J, Liu T, Yang L, Daus A, Crowley R, Zhou Q. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal Biochem. 2007;364(1):8–18. [DOI] [PubMed] [Google Scholar]
- 23.Mosedale DE, Chauhan A, Schofield PM, Grainger DJ. A pattern of anti-carbohydrate antibody responses present in patients with advanced atherosclerosis. J Immunol Methods. 2006;309(1–2):182–191. [DOI] [PubMed] [Google Scholar]
- 24.Wilson JM, Nguyen AT, Schuyler AJ, et al. IgE to the mammalian oligosaccharide galactose-alpha-1,3-galactose is associated with increased atheroma volume and plaques with unstable characteristics-brief report. Arterioscler Thromb Vasc Biol. 2018;38(7):1665–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanley P, Taniguchi N, Aebi M, et al. Chapter 9: N-glycans. In: Varki A, Cummings RD, Aebi M, Packer NH, Seeberger PH, Esko JD, editors. Essentials of Glycobiology. 3rd25. ed. New York, NY: Cold Spring Harbor; 2017: pp 99–111. [Google Scholar]
- 26.Rispens T, Derksen NI, Commins SP, Platts-Mills TA, Aalberse RC. IgE production to alpha-gal is accompanied by elevated levels of specific IgG1 antibodies and low amounts of IgE to blood group B. PLoS One 2013;8(2).e55566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li M, Gustchina A, Glesner J, et al. Carbohydrates contribute to the interactions between cockroach allergen Bla g 2 and a monoclonal antibody. J Immunol. 2011;186(1):333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Do DC, Yang S, Yao X, Hamilton RG, Schroeder JT, Gao P. N-glycan in cockroach allergen regulates human basophil function. Immun Inflamm Dis. 2017;5(4):386–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westphal S, Kolarich D, Foetisch K, et al. Molecular characterization and allergenic activity of Lyc e 2 (beta-fructofuranosidase), a glycosylated allergen of tomato. Eur J Biochem. 2003;270(6):1327–1337. [DOI] [PubMed] [Google Scholar]
- 30.Kolarich D, Leonard R, Hemmer W, Altmann F. The N-glycans of yellow jacket venom hyaluronidases and the protein sequence of its major isoform in Vespula vulgaris. FEBS J. 2005;272(20):5182–5190. [DOI] [PubMed] [Google Scholar]
- 31.Jappe U, Minge S, Kreft B, et al. Meat allergy associated with galactosyl-alpha-(1,3)-galactose (alpha-gal)-Closing diagnostic gaps by anti-alpha-gal IgE immune profiling. Allergy 2018;73(1):93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller GA, Glesner J, Daniel JL, et al. Mapping human monoclonal IgE epitopes on the major dust mite allergen Der p 2. J Immunol. 2020;205(8):1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pomés A, Mueller GA, Chruszcz M. Structural aspects of the allergen-antibody interaction. Front Immunol. 2020;11:2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glesner J, Kapingidza AB, Godzwon M, et al. A human IgE antibody binding site on Der p 2 for the design of a recombinant allergen for immunotherapy. J Immunol. 2019;203(9):2545–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varki A, Cummings RD, Aebi M, et al. Symbol nomenclature for graphical representations of glycans. Glycobiology 2015;25(12):1323–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neelamegham S, Aoki-Kinoshita K, Bolton E, et al. Updates to the symbol nomenclature for glycans guidelines. Glycobiology 2019;29(9):620–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apostolovic D, Mihailovic J, Commins SP, et al. Allergenomics of the tick Ixodes ricinus reveals important alpha-gal-carrying IgE-binding proteins in red meat allergy. Allergy 2020;75(1):217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hashizume H, Fujiyama T, Umayahara T, Kageyama R, Walls AF, Satoh T. Repeated Amblyomma testudinarium tick bites are associated with increased galactose-alpha-1,3-galactose carbohydrate IgE antibody levels: a retrospective cohort study in a single institution. J Am Acad Dermatol. 2018;78(6):1135–41 e3. [DOI] [PubMed] [Google Scholar]
- 39.Chinuki Y, Morita E. Alpha-gal-containing biologics and anaphylaxis. Allergol Int. 2019;68(3):296–300. [DOI] [PubMed] [Google Scholar]
- 40.Kubelka V, Altmann F, Staudacher E, et al. Primary structures of the N-linked carbohydrate chains from honeybee venom phospholipase A2. Eur J Biochem. 1993;213(3):1193–1204. [DOI] [PubMed] [Google Scholar]
- 41.Altmann F Coping with cross-reactive carbohydrate determinants in allergy diagnosis. Allergo J Int. 2016;25(4):98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mari A, Ooievaarde Heer P, Scala E, et al. Evaluation by double-blind placebo-controlled oral challenge of the clinical relevance of IgE antibodies against plant glycans. Allergy 2008;63(7):891–896. [DOI] [PubMed] [Google Scholar]
- 43.Paulus KE, Mahler V, Pabst M, Kogel K-H, Altmann F, Sonnewald U. Silencing β1, 2-xylosyltransferase in transgenic tomato fruits reveals xylose as constitutive component of IgE-binding epitopes. Frontiers in plant science. 2011;2:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gattinger P, Bidovec-Stojkovic U, Zidarn M, Korosec P, Valenta R, Mittermann I. Glycosylation enhances allergenic activity of major bee venom allergen Api m 1 by adding IgE epitopes. J Allergy Clin Immunol. 2021;147(4):1502–1504. [DOI] [PubMed] [Google Scholar]
- 45.Batanero E, Crespo JF, Monsalve RI, Martin-Esteban M, Villalba M, Rodriguez R. IgE-binding and histamine-release capabilities of the main carbohydrate component isolated from the major allergen of olive tree pollen, Ole e 1. J Allergy Clin Immunol. 1999;103(1 Pt 1):147–153. [DOI] [PubMed] [Google Scholar]
- 46.Bublin M, Radauer C, Wilson IB, et al. Cross-reactive N-glycans of Api g 5, a high molecular weight glycoprotein allergen from celery, are required for immunoglobulin E binding and activetion of effector cells from allergic patients. FASEB J. 2003;17(12): 1697–1699. [DOI] [PubMed] [Google Scholar]
- 47.Kaulfurst-Soboll H, Mertens M, Brehler R, von Schaewen A. Reduction of cross-reactive carbohydrate determinants in plant foodstuff: elucidation of clinical relevance and implications for allergy diagnosis. PLoS One 2011;6(3).e17800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mertens M, Amler S, Moerschbacher B, Brehler R. Cross-reactive carbohydrate determinants strongly affect the results of the basophil activation test in hymenopteravenom allergy. Clin Exp Allergy 2010;40(9):1333–1345. [DOI] [PubMed] [Google Scholar]
- 49.Ebo DG, Lechkar B, Schuerwegh AJ, Bridts CH, De Clerck LS, Stevens WJ. Validation of a two-color flow cytometric assay detecting in vitro basophil activation for the diagnosis of IgE-mediated natural rubber latex allergy. Allergy 2002;57(8):706–712. [DOI] [PubMed] [Google Scholar]
- 50.MacGlashan DW Jr. Basophil activation testing. J Allergy Clin Immunol. 2013;132(4):777–787. [DOI] [PubMed] [Google Scholar]
- 51.Patil SU, Bunyavanich S, Berin MC. Emerging food allergy biomarkers. J Allergy Clin Immunol Pract. 2020;8(8):2516–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holzweber F, Svehla E, Fellner W, et al. Inhibition of IgE binding to cross-reactive carbohydrate determinants enhances diagnostic selectivity. Allergy 2013;68(10):1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hokke CH, van Diepen A. Helminth glycomics - glycan repertoires and host-parasite interactions. Mol Biochem Parasitol 2017;215:47–57. [DOI] [PubMed] [Google Scholar]
- 54.van Ree R, Cabanes-Macheteau M, Akkerdaas J, et al. Beta(1,2)-xylose and alpha(1,3)-fucose residues have a strong contribution in IgE binding to plant glycoallergens. J Biol Chem. 2000;275(15):11451–11458. [DOI] [PubMed] [Google Scholar]
- 55.Landsteiner K The specificity of serological reactions. Springfield, Ill., Baltimore, Md.: Thomas CC; 1936. [Google Scholar]
- 56.Galili U Significance of the evolutionary α1, 3-galactosyltransferase (GGTA1) gene inactivation in preventing extinction of apes and old world monkeys. J Mol Evol 2015;80(1):1–9. [DOI] [PubMed] [Google Scholar]
- 57.Apostolovic D, Rodrigues R, Thomas P, Starkhammar M, Hamsten C, van Hage M. Immunoprofile of α-Gal-and B-antigen-specific responses differentiates red meat-allergic patients from healthy individuals. Allergy 2018;73(7):1525–1531. [DOI] [PubMed] [Google Scholar]
- 58.Wilson JM, Schuyler AJ, Workman L, et al. Investigation into the alpha-gal Syndrome: characteristics of 261 children and adults reporting red meat allergy. J Allergy Clin Immunol Pract. 2019;7(7):2348–58 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamsten C, Starkhammar M, Tran T, et al. Identification of galactose-α−1, 3-galactose in the gastrointestinal tract of the tick I xodes ricinus; possible relationship with red meat allergy. Allergy 2013;68(4):549–552. [DOI] [PubMed] [Google Scholar]
- 60.Mabelane T, Basera W, Botha M, Thomas HF, Ramjith J, Levin ME. Predictive values of alpha-gal IgE levels and alpha-gal IgE: Total IgE ratio and oral food challenge-proven meat allergy in a population with a high prevalence of reported red meat allergy. Pediatr Allergy Immunol. 2018;29(8):841–849. [DOI] [PubMed] [Google Scholar]
- 61.Lammerts van Bueren JJ, Rispens T, Verploegen S, et al. Anti-galactose-alpha-1,3-galactose IgE from allergic patients does not bind alpha-galactosylated glycans on intact therapeutic antibody Fc domains. Nat Biotechnol. 2011;29(7):574–576. [DOI] [PubMed] [Google Scholar]
- 62.Wilson JM, Keshavarz B, Retterer M, et al. A dynamic relationship between two regional causes of IgE-mediated anaphylaxis: alpha-gal syndrome and imported fire ant. J Allergy Clin Immunol. 2021;147(2):643–52 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fischer J, Lupberger E, Hebsaker J, et al. Prevalence of type I sensitization to alpha-gal in forest service employees and hunters. Allergy 2017;72(10):1540–1547. [DOI] [PubMed] [Google Scholar]
- 64.Levin M, Apostolovic D, Biedermann T, et al. Galactose alpha-1,3-galactose phenotypes: lessons from various patient populations. Ann Allergy Asthma Immunol. 2019;122(6):598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hilger C, Fischer J, Wolbing F, Biedermann T. Role and mechanism of galactose-alpha-1,3-galactose in the elicitation of delayed anaphylactic reactions to red meat. Curr Allergy Asthma Rep. 2019;19(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kiewiet MBG, Apostolovic D, Starkhammar M, Grundstrom J, Hamsten C, van Hage M. Clinical and serological characterization of the alpha-gal syndrome-importance of atopy for symptom severity in a European cohort. J Allergy Clin Immunol Pract. 2020;8(6):2027–34 e2. [DOI] [PubMed] [Google Scholar]
- 67.Apostolovic D, Krstic M, Mihailovic J, et al. Peptidomics of an in vitro digested alpha-gal carrying protein revealed IgE-reactive peptides. Sci Rep. 2017;7(1):5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Apostolovic D, Tran TAT, Sánchez-Vidaurre S, et al. Red meat allergic patients have a selective IgE response to the α-Gal glycan. Allergy 2015;70(11):1497–1500. [DOI] [PubMed] [Google Scholar]
- 69.Iweala OI, Choudhary SK, Addison CT, et al. Glycolipid-mediated basophil activation in alpha-gal allergy. J Allergy Clin Immunol. 2020;146(2):450–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pattanaik D, Lieberman P, Lieberman J, Pongdee T, Keene AT. The changing face of anaphylaxis in adults and adolescents. Ann Allergy Asthma Immunol. 2018;121(5):594–597. [DOI] [PubMed] [Google Scholar]
- 71.Platts-Mills TAE, Li RC, Keshavarz B, Smith AR, Wilson JM. Diagnosis and management of patients with the alpha-gal Syndrome. J Allergy Clin Immunol Pract. 2020;8(1):15–23 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joshi HJ, Narimatsu Y, Schjoldager KT, et al. SnapShot: O-glycosylation pathways across Kingdoms. Cell 2018;172(3):632–e2. [DOI] [PubMed] [Google Scholar]
- 73.Leonard R, Wopfner N, Pabst M, et al. A new allergen from ragweed (Ambrosia artemisiifolia) with homology to Art v 1 from mugwort. J Biol Chem. 2010;285(35):27192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Himly M, Jahn-Schmid B, Dedic A, et al. Art v 1, the major allergen of mugwort pollen, is a modular glycoprotein with a defensing-like and a hydroxyproline-rich domain. FASEB J. 2003;17(1):106–8. [DOI] [PubMed] [Google Scholar]
- 75.Leonard R, Petersen BO, Himly M, et al. Two novel types of O-glycans on the mugwort pollen allergen Art v 1 and their role in antibody binding. J Biol Chem. 2005;280(9):7932–40. [DOI] [PubMed] [Google Scholar]
- 76.Wicklein D, Lindner B, Moll H, et al. Carbohydrate moieties can induce mediator release: a detailed characterization of two major timothy grass pollen allergens. Biol Chem. 2004;385(5):397–407. [DOI] [PubMed] [Google Scholar]
- 77.van Oort E, Lerouge P, de Heer PG, et al. Substitution of Pichia pastoris-derived recombinant proteins with mannose containing O-and N-linked glycans decreases specificity of diagnostic tests. Int Arch Allergy Immunol. 2004;135(3):187–95. [DOI] [PubMed] [Google Scholar]
- 78.Taliaferro WH, Taliaferro LG. Skin reactions in persons infected with Schistosoma mansoni. Puerto Rico J Publ Hlth. 1931;7:23–35. [Google Scholar]
- 79.Steinberg P, Ishizaka K, Norman PS. Possible role of IgE-mediated reaction in immunity. J Allergy Clin Immunol. 1974;54(6):359–66. [Google Scholar]
- 80.Lorenzo S, Romaris F, Iglesias R, et al. O-glycans as a source of cross-reactivity in determinations of human serum antibodies to Anisakis simplex antigens. Clin Exp Allergy. 2000;30(4):551–9. [DOI] [PubMed] [Google Scholar]
- 81.Dunne DW, Butterworth AE, Fulford AJ, et al. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol. 1992;22(6):1483–94. [DOI] [PubMed] [Google Scholar]
- 82.Prasanphanich NS, Mickum ML, Heimburg-Molinaro J, Cummings RD. Glycoconjugates in host-helminth interactions. Front Immunol. 2013;4:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bantleon F, Wolf S, Seismann H, et al. Human IgE is efficiently produced in glycosylated and biologically active form in lepidopteran cells. Mol Immunol. 2016;72:49–56. [DOI] [PubMed] [Google Scholar]
- 84.Nkurunungi G, Mpairwe H, Versteeg SA, et al. Cross-reactive carbohydrate determinant-specific IgE obscures true atopy and exhibits alpha-1,3-fucose epitope-specific inverse associations with asthma. Allergy. 2021;76(1):233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Soh JY, Huang CH, Lee BW. Carbohydrates as food allergens. Asia Pacific allergy. 2015;5(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ohta M, Shigeta S, Ono K, Takao T, Shimonishi Y, Oka S. Sugar sequences of allergenically active oligosaccharide alcohols isolated from a large-molecular-size sea squirt antigen termed H-antigen. Arch Biochem Biophys. 1989;275(1):151–65. [DOI] [PubMed] [Google Scholar]
- 87.Chiang WC, Huang CH, Llanora GV, et al. Anaphylaxis to cow’s milk formula containing short-chain galacto-oligosaccharide. J Allergy Clin Immunol. 2012;130(6):1361–7. [DOI] [PubMed] [Google Scholar]
- 88.Kaneko K, Watanabe Y, Kimura K, Matsumoto K, Mizobuchi T, Onoue M. Development of hypoallergenic galacto-oligosaccharides on the basis of allergen analysis. Biosci Biotechnol Biochem. 2014;78(1):100–8. [DOI] [PubMed] [Google Scholar]
- 89.Lee L, Zhong Y, Leow SY, et al. Allergy to prebiotic galacto-oligosaccharides: House dust mites-the putative primary sensitizer. J Allergy Clin Immunol. 2020;145(2):707–10 e5. [DOI] [PubMed] [Google Scholar]
- 90.Stanley P, Cummings RD, et al. Structures Common to Different Glycans. In: Varki A, Cummings RD, Esko JD, et al. editor. Essentials of Glycobiology. 3rd ed. Cold Spring Harbor (NY): Cold Soring Harbor Laboratory Press; 2015: pp 161–78. [Google Scholar]


