Abstract
Objective
Controversial, heterogeneous, and inconsistent responses to beta-blockers have been reported in some cases of infantile proliferative hemangiomas. On the basis of these clinical observations, we aimed to examine the β1 adrenergic receptor (β1-AR) protein expression distribution among different types of pediatric vascular anomalies.
Methods
Immunohistochemistry (IHC) was performed for β1-AR on 43 surgical specimens.
Results
We found positive β1-AR IHC staining in all intramuscular hemangiomas, capillary–lymphatic, lymphatic, venous, and combined malformations, and Masson’s tumor cases, as well as in 7 of 10 cases of proliferative infantile hemangiomas.
Conclusions
Our research demonstrates, for the first time, the degree of heterogeneous expression of β1-AR among pediatric vascular malformations. Our results support the need for β1-AR assessment in pediatric vascular anomalies to select cases with a robust response to β1-selective blockers. β1-AR assessment may have a strong impact on therapeutic refinement for pediatric vascular anomalies by selecting cases with a stronger response to beta-blockers.
Keywords: Vascular anomaly, beta-blocker, β1 adrenergic receptor, propranolol, infantile hemangioma, immunohistochemistry
Introduction
In the early 1960s, James W. Black synthesized the beta-blocker drug propranolol, which has been used extensively in pediatric cardiology.1 Forty-eight years later, in 2008, Léauté-Labrèze et al. found an association between propranolol treatment and successful outcomes in the management of infantile hemangiomas.2 After this observation, clinical trials have demonstrated the efficacy of propranolol in treating this condition.3,4 Beta-blockers have revolutionized the management of infantile hemangiomas and have proven to be both safe and efficacious. Therefore, clinicians now use oral propranolol as the first-line agent for infantile hemangioma treatment.5–8
Current research has not analyzed the differential expression of the β1 adrenergic receptor (β1-AR) in the various types of vascular anomalies. Chisholm et al. identified the presence of the β2 and β3 adrenergic receptors in the endothelial cells of several vascular anomalies, such as infantile hemangiomas, angiosarcomas, and hemangioendotheliomas.9 The same study showed that the protein expression levels of β2-AR and β3-AR are robust in all stages of infantile hemangioma development, the sole exception being weak expression of the phosphorylated form of the β2 receptor in the proliferative phase of infantile hemangiomas. Another study conducted by Ji et al. investigated the role of β-adrenergic receptor signaling in the proliferation of hemangioma-derived endothelial cells.10 The aforementioned studies also demonstrated the beneficial effect of propranolol in infantile hemangioma.9,10 However, some patients had a weak or inconsistent response to propranolol therapy, suggesting possible heterogeneity of adrenergic receptor expression and function.
On the basis of the clinically observed heterogeneous response to beta-blocker therapy in some cases of pediatric vascular malformation, especially in those with a proliferative status, we aimed to identify if β1-AR expression heterogeneity is present among different types of pediatric vascular anomalies.
Materials and methods
Sample acquisition
The reporting of this study conforms to the STROBE statement for observational studies.11 The study included biopsy specimens of vascular anomalies obtained via surgical intervention from a sample of 43 patients admitted to the Pediatric Surgery Clinic of the Emergency Children’s Hospital Louis Ţurcanu, Timișoara, Romania. Histological analysis of tumor tissue was performed in the Department of Histology of the Victor Babeș University of Medicine and Pharmacy, Timișoara.
Sample histology
Tumor specimens of less than 1 cm in size were obtained and subjected to histological preparation techniques, after which they were fixed in 10% buffered formalin for 48 hours and embedded in paraffin. Formalin-fixed paraffin-embedded specimens for each case were sectioned into 3 µm serial sections using an automated microtome prior to staining. Each slide was dewaxed and rehydrated following the standard protocol. The slides were stained with hematoxylin and eosin.
Immunohistochemistry (IHC) assays
IHC assays were performed with a monoclonal mouse anti-human vimentin primary antibody (clone V9; Leica Biosystems, Newcastle upon Tyne, UK). Antigen retrieval was performed using unmasking solution Novocastra Epitope Retrieval Solution 2 Bond, pH 9 (Leica Biosystems) for 20 minutes at 99 °C. This step was followed by blocking of endogenous peroxidase activity with 3% hydrogen peroxide for 5 minutes. The samples were incubated with a goat polyclonal primary antibody against human β1-AR (Novus Biologicals, Littleton, CO, USA) for 15 minutes prior to visualization using the Bond Polymer System Refine Detection System (Leica Biosystems). 3,3ʹ-diaminobenzidine (DAB) was used as the chromogen and was applied for 10 minutes. Counterstaining was performed for 5 minutes using hematoxylin. Thereafter, the sections were placed in 100% ethanol for 5 minutes, followed by dry benzene clarefiate for 5 minutes. Permanent mounting was performed by using Entellan, Leica CV Mount medium (Leica Biosystems).12
IHC scoring
Scoring of β1-AR IHC staining was performed using a semi-automated method provided by Quantum Center software (3DHistech, Budapest, Hungary), which scores protein expression by combining the density and intensity of β1-AR positive signals and automatically provides scoring evaluation.
Vascular anomalies were classified by using the International Society for the Study of Vascular Anomalies (ISSVA) Classification 2018.13
Results
The ages of the 43 patients included in the study ranged from 2 months to 18 years (216 months), with a mean age of 45.9 ± 5.56 months. Several rare cases of vascular anomalies were included in our study, specifically an intravascular papillary endothelial hyperplasia (Masson’s tumor) diagnosed in a 2-year-5-month-old girl (located on the buttock), three cases of capillary malformations of the "port-wine stain” type, and three cases of intramuscular hemangiomas in children aged 6, 8, and 17 years (located on the posterior thoracic wall and upper limb).
Evaluation of the samples stained with the anti-β1-AR antibody showed positive IHC staining in infantile hemangioma during the proliferating phase, intravascular papillary endothelial hyperplasia (Masson's tumor), capillary lymphatic malformation, lymphatic malformations, capillary–lymphatic–venous malformations, venous malformations, and intramuscular hemangioma. Negative IHC staining for β1-AR was observed in infantile hemangioma during the involuting phase and involuted phase, Kaposiform hemangioendothelioma, tuft angioma, pyogenic granuloma, capillary malformations, and glomuvenous malformations (Table 1).
Table 1.
β1-adrenergic receptor (β1-AR) expression in vascular anomalies in children.
| Vascular anomalies-ISSVA classification | Number of cases | β1-AR | ||
|---|---|---|---|---|
| Vascular tumor | Tufted angioma | 1 | Negative | |
| Intravascular papillary endothelial hyperplasia (Masson’s tumor) | 1 | Positive | ||
| Kaposiform hemangioendothelioma | 1 | Negative | ||
| Pyogenic granuloma | 2 | Negative | ||
| Infantile hemangioma | Proliferating phase | 7 | Positive | |
| 3 | Negative | |||
| Involuting phase | 4 | Negative | ||
| Involuted phase | 7 | Negative | ||
| Vascular malformation | Capillary malformation | 3 | Negative | |
| Venous malformation | 1 | Positive | ||
| Glomuvenous malformation | 1 | Negative | ||
| Lymphatic malformations | 3 | Positive | ||
| Capillary-lymphatic malformation | 2 | Positive | ||
| Capillary-lymphatic-venous malformation | 4 | Positive | ||
| Intramuscular hemangioma | Intramuscular hemangioma | 3 | Positive | |
ISSVA, International Society for the Study of Vascular Anomalies.
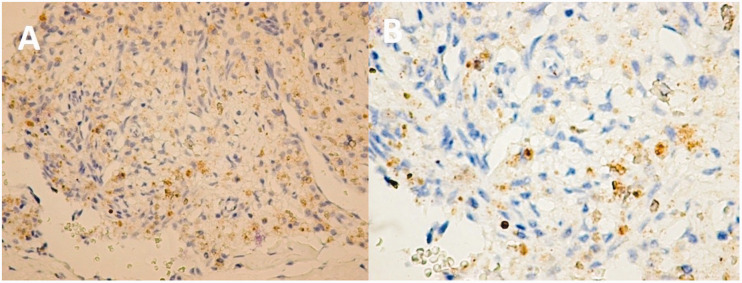
The β1-AR IHC results in the assessed vascular tumors were different from those in other abnormalities and were also much more diverse in nature. For example, infantile hemangioma stained negative for β1-AR IHC in both the involuting and involuted phases. During the proliferating phase, infantile hemangiomas stained positive in 7 of 10 cases. Intramuscular hemangioma showed the highest β1-AR positive IHC staining (Figure 1). The expression pattern was predominantly cytoplasmic granular with high intensity, whereas a combined nuclear and cytoplasmic pattern was rarely identified.
Figure 1.
(a) Intramuscular hemangioma (6-year-old girl). (b) Same piece, large target (×40). Most of the tumor endothelial cells are positive for β1-adrenergic receptor (β1-AR). Cytoplasmic expression with moderate intensity was found in most tumor cells. Rare cells have nuclear expression and those with cytoplasmic expression show diffuse, heterogeneous granularity. Isolated endothelial cells lining adjacent vessels to hemangioma exhibited a distinct nuclear expression pattern. Outside of the hemangioma area, endothelial cells expressing β1-AR were extremely rare, but still present.
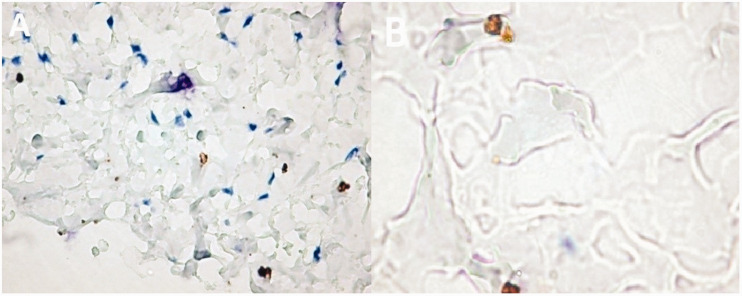
A combined vascular malformation (capillary–venous–lymphatic malformation) is presented in Figure 2. Most of the tumor’s endothelial cells were positive for β1-AR IHC staining, and moderately intense cytoplasmic expression was found in a large percentage of cells. Rarely, cells showed nuclear expression, and those with cytoplasmic expression showed diffuse, heterogeneous granularity. Isolated endothelial cells lining the vessels adjacent to the hemangioma exhibited a distinct nuclear expression pattern. Outside of the hemangioma area, endothelial cells that expressed β1-AR were extremely rare, but still present.
Figure 2.
Combined vascular malformation–capillary–venous–lymphatic (12-year-old boy). (a) In the superficial dermis, adjacent to the lesions, numerous positive cells were observed. (b) The image also shows nuclear positivity restricted to the cells lining lymph lumens.
Discussion
We performed a retrospective study of 43 vascular anomaly specimens collected via surgical intervention from patients having undergone surgery from 2008 to 2012 at the Pediatric Surgery Clinic of the Emergency Children’s Hospital Louis Ţurcanu, Timișoara. At the time, this was the standard of care for most patients. Since 2013, the protocol has been changed and some of the patients have been followed up and treated with beta-blockers.
In this study, we found the presence of β1-AR in the proliferating phase of infantile hemangioma in 7 of 10 cases. β1-AR protein was detected by IHC in all cases of intramuscular hemangioma, lymphatic malformations, malformations combined with lymphatic component, Masson’s tumor, and venous malformations.
Chisholm et al.9 analyzed receptor expression by looking at β2-AR protein levels in vascular lesions. This work suggested that β2-AR is present in all cases of infantile hemangiomas, which led the authors to conclude that infantile hemangiomas respond to non-selective beta-blocker treatment. A study performed by Ji et al. investigated the role of β-adrenergic receptor signaling in the proliferation of hemangioma-derived endothelial cells (HemECs). The results showed that activation of the β-ARs increased HemEC proliferation and upregulated VEGFR2-mediated ERK signaling.10
Propranolol has also been used as a therapeutic agent in cases of severe infantile hemangiomas, as reported in a follow-up study by Sans et al.14 Their research showed immediate and remarkable effects on the growth and color of the lesions of the 32 patients involved in the study. Another study from Spain in 2011 showed profound effects of beta-blockers in 28 patients affected by severe forms of hemangiomas. Treatment effectiveness was registered in all cases.15 Additionally, Zhang et al.16 showed remarkable improvements in 578 patients with infantile hemangiomas that were treated with propranolol.
In the current research, β1-AR protein was present in 70% of cases of infantile hemangiomas that were in the proliferating phase, while β1-AR protein was absent in involuting and involuted phase hemangiomas.
Aesthetical recovery of hemangiomas achieved by topical use of non-selective beta-blockers was studied by several research groups. In a retrospective cohort study, Chakkittakandiyil et al.17 investigated if the use of timolol maleate gel could reduce infantile hemangioma, and demonstrated that 72 of 73 patients examined had a positive response. A “dramatic response to topical Timolol lotion of a large hemifacial infantile hemangioma associated with PHACE syndrome” was reported in the case of an 18-year-old girl. Yet, not only superficial and uncomplicated hemangiomas have shown involutions and decline with propranolol. Interestingly, this beta-blocker also showed promising results when used in cases with ulcerations.18 Striking outcomes were also obtained for hemangiomas in the airways, as shown in the meta-analysis by Vlastarakos et al.,3 in which propranolol proved superior to systemic steroids in 94% of patients. Furthermore, all three patients in an additional study benefited from rapid resolution of airway complications and symptoms.19
Current guidelines indicate propranolol as the first-line treatment for infantile hemangiomas.20 Other studies showed the effects of β1-selective blockers, like atenolol, in patients with infantile hemangioma, in which effective treatment was recorded in 61.8% to 89% of patients.21,22 Our study showed that in infantile hemangiomas in the proliferating phase, non-selective beta-blockers, such as propranolol, are the first choice. If propranolol were to be replaced with β1-selective blockers, the possibility of a good response would have been 70% in infantile hemangioma in the proliferating phase. In the other evolution phases of infantile hemangiomas, the response is expected to be poor.
Chisholm et al.9 noted that about 50% of lymphatic malformations had β2-AR present, whereas in our study β1-AR was present in all cases having a lymphatic component (nine cases). In other clinical trials23,24 of lymphatic malformations treated with propranolol, the response highlighted different reactions (very good, partial, or unresponsive reactions). Propranolol improved symptoms in a subset of patients with lymphatic anomalies. It may also limit the growth of congenital lymphatic malformations in utero.24
A study published in 2012 by Chiu et al.25 also reported the results of using propranolol for different lesions such as Kaposiform hemangioendothelioma, tufted angioma, and Kasabach–Merritt phenomenon. Of these, 36% of patients responded positively to treatment.
Some rare types of vascular anomalies were also included in our study, specifically one case of intravascular papillary endothelial hyperplasia and three cases of intramuscular hemangiomas. All of these cases expressed β1-AR. Preoperatively, differential diagnosis included other soft tissue and vascular tumors, vascular malformations, or sarcomas. The definite diagnosis was established histologically (glucose transporter 1 (GLUT1)-negative vascular mass).
Intravascular papillary endothelial hyperplasia was first described in 1923 by Masson as “hemangioendotheliome vegetant intravasculaire”.26 Today, it is considered an unusual endothelial hyperplasia that arises in thrombi and organizing hematomas.26,27 According to the ISSVA 2018 classification, it is a benign vascular tumor.13
Here, we included three cases of intramuscular hemangiomas located on the posterior thoracic wall and upper limb. The symptoms were swelling and pain following trauma. Intramuscular hemangioma is an uncommon benign vascular tumor reported by Allen and Enzinger in 1972, who named the lesion “small vessel type.” Currently, this lesion is most commonly referred to as an intramuscular capillary-type hemangioma.26,28 According to the ISSVA 2018 classification, intramuscular hemangioma is included as a provisionally unclassified vascular anomaly.13
We analyzed the response of capillary malformations, such as port wine stains and salmon patches, to propranolol treatment. Beta-blocker treatment was administered to three patients with port wine stains and to more than 50 patients with salmon patches. The patients with salmon patches had infantile hemangiomas and were treated with propranolol. The salmon patch lesions did not respond to treatment. The clinical results were in accordance with the results of the IHC experiments. No β1-AR protein expression was observed in capillary malformations.
We found some data in the literature related to the treatment of capillary malformations with propranolol. Gross et al. reported the results of using propranolol in different vascular anomalies, concluding that it does not have efficacy for vascular anomalies other than infantile hemangioma.29
Because our results corroborate existing data in the literature, we argue that beta-blockers that are selective for β1-AR should be the treatment of choice in lymphatic malformations. Likewise, the presence of β1-AR in Masson’s tumor with no β2-AR expression will make treatment with β1-selective beta-blockers a strong therapeutic alternative. The present study has demonstrated the presence of β1 receptor expression in the venous malformations and hemangiomas intramuscular. The literature9 has shown the presence of β2 receptor expression. Considering the results of our study and the unusual nature of β1-AR expression in vascular anomalies, we can speculate that a treatment based only on β1-selective blockers would be better tailored to specific lesions.12
Vascular anomalies are an important topic in the pediatrics field. It is necessary to provide an accurate diagnosis, as well as an efficient treatment. Our work brings a new perspective to approaching vascular anomalies in children, specifically by using a targeted therapeutic approach in accordance with each histological type to give children the best chance to successfully grow and develop.
Conclusions
We identified a subgroup of proliferating infantile hemangiomas expressing β1-AR, which had not been previously reported in the literature. This finding may partially explain the occurrence of cases that are unresponsive to the conventional therapies for infantile hemangiomas or those that recur after an initial positive therapeutic response. Our results support a more complex methodology for evaluating refractory or recurrent proliferative hemangiomas, which should at minimum include an extended immunohistochemical assessment of β adrenergic receptor protein expression levels. This evaluation may improve the therapeutic choice by guiding clinicians through a certified use of β1-AR blockers in cases that express β1-AR.
β1-AR expression was absent in capillary malformations, Kaposiform hemangioendothelioma, glomuvenous malformation, and pyogenic granuloma. Our results therefore suggest that β1-selective blockers are not appropriate for treating these types of vascular anomalies.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Anca Maria Cîmpean https://orcid.org/0000-0002-9530-022X
Vlad-Laurentiu David https://orcid.org/0000-0003-4295-7487
References
- 1.Hajar R.The invention of Propranolol. Heart Views 2000; 1: 321–323. [Google Scholar]
- 2.Léauté-Labrèze C, Dumas De La Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med 2008; 358: 2649–2651. [DOI] [PubMed] [Google Scholar]
- 3.Vlastarakos PV, Papacharalampous GX, Chrysostomou M, et al. Propranolol is an effective treatment for airway haemangiomas: a critical analysis and meta-analysis of published interventional studies. Acta Otorhinolaryngol Ital 2012; 32: 213–221. [PMC free article] [PubMed] [Google Scholar]
- 4.Marqueling AL, Oza V, Frieden IJ, et al. Propranolol and infantile hemangiomas four years later: a systematic review. Pediatr Dermatol 2013; 30: 182–191. [DOI] [PubMed] [Google Scholar]
- 5.Navarro-Triviño FJ, Ruíz-Villaverde R, Naranjo-Sintes R.Infantile hemangioma and β-blockers: When, how, and why? Actas Dermosifiliogr 2016; 107: 601–602. [DOI] [PubMed] [Google Scholar]
- 6.Soliman YS, Khachemoune A.Infantile hemangiomas: our current understanding and treatment options. Dermatol Online J 2018; 24: 13030/qt5jt8q9km. [PubMed] [Google Scholar]
- 7.Yu Z, Cai R, Chang L, et al. Clinical and radiological outcomes of infantile hemangioma treated with oral propranolol: A long-term follow-up study. J Dermatol 2019; 46: 376–382. [DOI] [PubMed] [Google Scholar]
- 8.Léauté-Labrèze C, Harper JI, Hoeger PH.Infantile haemangioma. Lancet 2017; 390: 85–94. [DOI] [PubMed] [Google Scholar]
- 9.Chisholm KM, Chang KW, Truong MT, et al. β-Adrenergic receptor expression in vascular tumors. Mod Pathol 2012; 25: 1446–1451. [DOI] [PubMed] [Google Scholar]
- 10.Ji Y, Chen S, Li K, et al. The role of β-adrenergic receptor signaling in the proliferation of hemangioma-derived endothelial cells. Cell Div 2013; 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 12.Stănciulescu MC. Contributions to the diagnosis and treatment of vascular anomalies in children. PhD thesis, “Victor Babes” University of Medicine and Pharmacy Timisoara, Romania, 21.04.2014.
- 13.http: //www.issva.org/UserFiles/file/ISSVA-Classification-2018.pdf.
- 14.Sans V, De La Roque ED, Berge J, et al. Propranolol for Severe Infantile Hemangiomas: Follow-Up Report. Pediatrics 2009; 124: e423. [DOI] [PubMed] [Google Scholar]
- 15.Bernabeu-Wittel J, Pereyra-Rodríguez JJ, Mantrana-Bermejo ME, et al. Tratamiento con propranolol oral para hemangiomas infantiles graves: serie de 28 pacientes. Actas Dermosifiliogr 2011; 102: 510–516. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Wu HW, Yuan W, et al. Propranolol therapy for infantile hemangioma: our experience. Drug Des Devel Ther 2017; 11: 1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakkittakandiyil A, Phillips R, Frieden IJ, et al. Timolol Maleate 0.5% or 0.1% Gel-Forming Solution for Infantile Hemangiomas: A Retrospective, Multicenter, Cohort Study. Pediatr Dermatol 2012; 29: 28–31. [DOI] [PubMed] [Google Scholar]
- 18.Hermans DJ, Van Beynum IM, Schultze Kool LJ, et al. Propranolol, a very promising treatment for ulceration in infantile hemangiomas: a study of 20 cases with matched historical controls. J Am Acad Dermatol 2011; 64: 833–838. [DOI] [PubMed] [Google Scholar]
- 19.Rosbe KW, Suh KY, Meyer AK, et al. Propranolol in the management of airway infantile hemangiomas. Arch Otolaryngol Head Neck Surg 2010; 136: 658–665. [DOI] [PubMed] [Google Scholar]
- 20.Krowchuk DP, Frieden IL, Mancini AJ, et al. Clinical Practice Guideline for the Management of Infantile Hemangiomas. Pediatrics 2019; 143: e20183475. [DOI] [PubMed] [Google Scholar]
- 21.Calderón-Castrat X, Velásquez F, Castro R, et al. Oral Atenolol for Infantile Hemangioma: Case Series of 46 Infants. Actas Dermosifiliogr (Engl Ed) 2020; 111: 59–62. [DOI] [PubMed] [Google Scholar]
- 22.Tasani M, Glover M, Martinez AE, et al. Atenolol treatment for infantile haemangioma. Br J Dermatol 2017; 176: 1400–1402. [DOI] [PubMed] [Google Scholar]
- 23.Ozeki M, Kanda K, Kawamoto N, et al. Propranolol as an alternative treatment option for pediatric lymphatic malformation. Tohoku J Exp Med 2013; 229: 61–66. [DOI] [PubMed] [Google Scholar]
- 24.Wu JK, Hooper ED, Laifer-Narin SL, et al. Initial Experience With Propranolol Treatment of Lymphatic Anomalies: A Case Series. Pediatrics 2016; 138: e20154545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiu YE, Drolet BA, Blei F, et al. Variable response to propranolol treatment of kaposiform hemangioendothelioma, tufted angioma, and Kasabach-Merritt phenomenon. Pediatr Blood Cancer 2012; 59: 934–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulliken & Young. Vascular Anomalies Hemangiomas and Malformations. 2nd ed. Edited by: John B. Mulliken, Patricia E. Burrows, Steven J. Oxford: Fishman, 2013, ISBN 978–0–19–514505–2. [Google Scholar]
- 27.Clasen CV, Gomes APN, Galdino Dos Santos L, et al. Intravascular papillary endothelial hyperplasia in the oral mucosa and jawbones: A collaborative study of 20 cases and a systematic review. J Oral Pathol Med 2021; 50: 103–113. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Chou K, Xiong J, et al. Easily misdiagnosed intramuscular hemangioma: a case report. J Int Med Res 2020; 48: 300060520966897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goss JA, Konczyk DJ, Alomari MH, et al. Propranolol Treatment of Vascular Anomalies Other Than Infantile Hemangioma. J Craniofac Surg 2017; 28: 2001–2003. [DOI] [PubMed] [Google Scholar]