Emerging evidence suggests that immunological responses to SARS-CoV-2 mRNA vaccines in solid organ transplant recipients are attenuated, with reported seroconversion rates of less than 60%.1, 2, 3, 4, 5 However, there are insufficient data on responses to adenoviral vector SARS-CoV-2 vaccines in transplant populations. Here we describe immunological responses to two-dose vaccination with BNT162b2 (Pfizer–BioNTech) and ChAdOx1 (Oxford University–AstraZeneca) in kidney transplant recipients.
Three groups were included in the study (appendix pp 2–3). Cohort 1 (n=920) included patients undergoing assessment of serological responses at median 31 days (IQR 27–35) after vaccination. Cohort 2 (n=106) included patients with paired assessment of cellular responses to spike protein (T-SPOT Discovery SARS-CoV-2 [Oxford Immunotec; Oxford, UK]) and serological responses at median 31 days (IQR 29–34) after vaccination. Finally, cohort 3 (n=65) included health-care workers with assessment of cellular and serological responses at median 28 days (IQR 21–28) after vaccination (appendix pp 2–3). All participants were recruited from Imperial College Healthcare NHS Trust (London, UK). Vaccine dosing interval was 74 days (IQR 66–77) for cohort 1, 63 days (63–77) for cohort 2, and 67 days (61–70) for cohort 3. Samples were tested for antibodies to SARS-CoV-2 nucleocapsid and spike (anti-S) proteins using SARS-CoV-2 IgG and IgG Quant II (Abbott; Maidenhead, UK) assays. Previous infection was defined serologically (anti-nucleocapsid positivity at any time or anti-S positivity before vaccination) or by past infection confirmed by PCR. In cohort 1 and 2, a greater proportion of patients receiving ChAdOx1 were vaccinated within the first year of transplantation compared with BNT162b2 (appendix pp 7, 12).
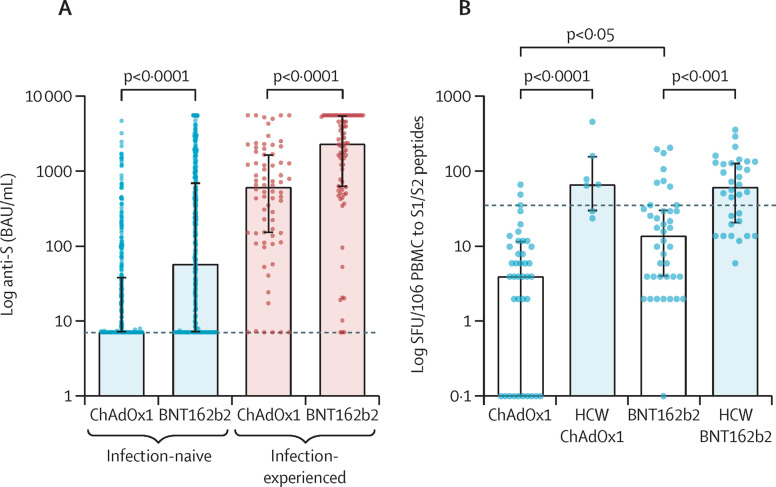
In cohort 1, previous infection was identified in 152 (17%) of 920 patients. Following vaccination, 425 (55%) of 768 infection-naive patients had detectable anti-S antibodies. Infection-naive patients receiving BNT162b2 were more likely to seroconvert and developed higher anti-S titres compared with patients receiving ChAdOx1; seroconversion occurred in 269 (66%) of 410 patients receiving BNT162b2 (median anti-S 58 BAU/mL, IQR 7·1–722) and 156 (44%) of 358 patients receiving ChAdOx1 (7·1, 7·1–39; figure A ; appendix p 5). Multivariable analysis identified tacrolimus monotherapy (odds ratio 5·22, 95% CI 3·60–7·65, p<0·0001) and vaccination with BNT162b2 (2·47, 1·79–3·43, p<0·0001) as associated with increased likelihood of seroconversion. Vaccination less than 1 year after transplant (0·28, 0·15–0·55, p=0·0002) and a diagnosis of diabetes (0·65, 0·46–0·92, p=0·015) were associated with reduced likelihood of seroconversion (appendix pp 7, 9–11, 15).
Figure.
Immunological responses to SARS-CoV-2 vaccination in kidney transplant recipients
Data are shown as median with IQR. Statistical analysis is by Mann-Whitney test (A) or Kruskall-Wallis test with Dunns post-hoc correction (B). (A) Kidney transplant recipients who received BNT162b2 had significantly higher anti-S concentrations. For infection-naive patients median anti-S titre was median 58 BAU/mL (IQR 7·1–722) for BNT162b2 and 7·1 BAU/mL (7·1–39) for ChAdOx1 (p<0·0001). In patients with previous infection, median anti-S titre was 2350 BAU/mL (628–5680) for BNT162b2 compared with 622 BAU/mL (151–1706) for ChAdOx1 (p<0·0001). The black dotted line represents 7·1 BAU/mL, the cutoff for a positive result. (B) Infection-naive patients who received BNT162b2 had a greater T-cell response compared with patients who received ChAdOx1, with 14 SFU/106 PBMCs (4–32) for BNT162b2 and 4 SFU/106 PBMCs (0–12) for ChAdOx1 (p=0·019). Infection-naive HCWs receiving BNT162b2 and ChAdOx1 had significantly greater responses compared with patients having the corresponding vaccine, with a median 63 SFU/106 PBMCs (21–132; p=0·0003) for BNT162b2 and 68 SFU/106 PBMCs (30–162; p<0·0001) for ChAdOx1. Data points of SFU/106 PBMCs are represented as 0·1 for visualisation on a log scale. The black dotted line represents the threshold for a positive enzyme-linked immunospot, 40 SFU/106 PBMCs, which was calculated from unvaccinated infection-naive HCWs. Anti-S=antibodies to spike protein. HCW=health-care worker. PBMC=peripheral blood mononuclear cells. SFU=spot forming units.
In the subgroup of patients from cohort 2 with assessment of cellular responses, 79 (75%) of 106 patients were infection-naive (appendix p 12). Only nine (11%) of 79 infection-naive patients had detectable T-cell responses following vaccination with no infection-naive patient vaccinated in their first year after transplant having detectable responses (appendix p 13). There was no difference in detectable T-cell responses by vaccine type, in infection- naive patients: seven (18%) of 40 patients receiving BNT162b2 and two (5%) of 39 patients receiving ChAdOx1 (p=0·15). Greater magnitude of T-cell responses was seen following BNT162b2 vaccination: median 14 spot forming units (SFU) per 106 peripheral blood mononuclear cells (PBMCs; IQR 4–32) for BNT162b2 and 4 SFU/106 PBMCs (0–12) for ChAdOx1 (p=0·019; figure B). Seroconversion rates and anti-S titres in infection-naive patients in this subgroup were also significantly higher following vaccination with BNT162b2 (appendix p 18). Both serological and T-cell responses were significantly lower than infection-naive health-care workers for each vaccine type (figure B; appendix p 18).
In patients with previous infection, only eight (5%) of 152 patients in cohort 1 were seronegative following vaccination (figure A). Similar to the infection-naive group, anti-S titres were lower in those receiving ChAdOx1 (median 622 BAU/mL [IQR 151–1706]) compared with BNT162b2 (2350, 628–5680; p<0·0001). In the subgroup assessed for cellular responses, 19 (70%) of 27 patients had T-cell responses post-vaccination with median 96 SFU/106 PBMCs. There were no differences in T-cell responses in patients with previous infection between vaccine types or between patients and health-care workers (appendix p 18).
In the 106 patients with assessment of both cellular and serological responses, 45 (42%) had no detectable response by either measure; 44 (56%) of 79 infection-naive patients and one (4%) of 27 patients with previous infection. On multivariable analysis, tacrolimus monotherapy was associated with a detectable response (OR 16·5, 95% CI 4·7–58·0, p<0·0001), whereas being vaccinated within the first year after transplant was associated with no detectable response (0·14, 0·04–0·57, p=0·006; appendix p 14).
Although it is acknowledged that vector-based vaccines generate weaker antibody responses compared with mRNA vaccines, vector-based vaccines have been considered superior in their ability to produce robust cellular responses.6 In this study, we show that BNT162b2 induces greater humoral responses compared with ChAdOx1 in infection-naive transplant recipients. The superior cellular responses we observed with BNT162b2 in infection-naive patients needs careful interpretation given the clinical differences between the cohorts, a limitation of our study. Meaningful comparison is also difficult when the magnitude of T-cell responses is so poor. However, after adjusting for the clinical factors, at most, comparable T-cell responses remain following vaccination with BNT162b2 or ChAdOx1 (appendix p 13).
Previous studies of mRNA vaccines in transplant recipients have reported better cellular responses than our study, with up to 57% of patients developing detectable T-cell responses.2, 4, 7 The poor cellular responses in cohort 2 could be due to differences in immunosuppression protocols used, and that patients within the first year following transplant were relatively overrepresented in this subgroup. Technical factors such as the peptide pools used and assay readout might also contribute to the poor cellular responses.8, 9
Immune responses seen in transplant recipients were significantly weaker than those in health-care workers, although the groups were imperfectly matched and the health-care workers included were substantially younger (appendix p 4). However, within the patient cohort, age did not affect the response to vaccination, suggesting that the difference in age does not account for the observed weaker immune responses.
Although the immune correlates of protection from disease have yet to be defined, we show markedly diminished humoral and cellular immune responses to both vector and mRNA SARS-CoV-2 vaccines in kidney transplant recipients. The planning of intervention studies to optimise vaccine platform and dosing are urgently required in this group, and preliminary reports suggest encouraging responses to third vaccine doses.10, 11 In the interim, strategic planning to protect this susceptible population is required. This planning could include, but is not limited to, educating patients to maintain physical distancing rules and immunising household members, including prioritisation of children older than 12 years.
PK and MW have received support to use the T-SPOT Discovery SARS-CoV-2 by Oxford Immunotec. MP, TT, and CLC contributed equally. Members of the Imperial Renal COVID-19 vaccine study group are listed in the appendix (pp 25–26). All other authors declare no competing interests.
Supplementary Material
References
- 1.Benotmane I, Gautier-Vargas G, Cognard N, et al. Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int. 2021;99:1487–1489. doi: 10.1016/j.kint.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertrand D, Hamzaoui M, Lemée V, et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32:2147–2152. doi: 10.1681/ASN.2021040480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21:2727–2739. doi: 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marion O, Del Bello A, Abravanel F, et al. Safety and immunogenicity of anti-SARS-CoV-2 messenger RNA vaccines in recipients of solid organ transplants. Ann Intern Med. 2021 doi: 10.7326/M21-1341. published online May 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald I, Murray SM, Reynolds CJ, Altmann DM, Boyton RJ. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines. 2021;6:74. doi: 10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavarot N, Ouedrani A, Marion O, et al. Poor anti-SARS-CoV-2 humoral and T-cell responses after 2 injections of mRNA vaccine in kidney transplant recipients treated with belatacept. Transplantation. 2021;105:e94–e95. doi: 10.1097/TP.0000000000003784. [DOI] [PubMed] [Google Scholar]
- 8.Swanson PA, Padilla M, Hoyland W, et al. T-cell mediated immunity after AZD1222 vaccination: a polyfunctional spike-specific Th1 response with a diverse TCR repertoire. medRxiv. 2021 doi: 10.1126/scitranslmed.abj7211. https://www.medrxiv.org/content/10.1101/2021.06.17.21259027v2 published online July 13. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahin U, Muik A, Vogler I, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595:572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 10.Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021 doi: 10.7326/L21-0282. published online June 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA COVID-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.