Abstract
During disc degeneration, the increase of inflammatory cytokines and decrease of disc cell density are two prominent features. Enhanced inflammatory reaction contributes to disc annulus fibrosus (AF) cell apoptosis. In this study, we investigated whether resveratrol can suppress AF cell apoptosis in an inflammatory environment. Rat disc AF cells were cultured in medium with or without tumor necrosis factor-α (TNF-α). Resveratrol was added along with the culture medium supplemented with TNF-α. Caspase-3 activity, cell apoptosis ratio, expression of apoptosis-associated molecules (Bcl-2, Bax, caspase-3, cleaved PARP, and cleaved caspase-3), reactive oxygen species (ROS) content, and the total superoxide dismutase (SOD) activity were measured. Our results showed that TNF-α significantly increased caspase-3 activity and AF cell apoptosis ratio and upregulated gene/protein expression of Bax, caspase-3, cleaved caspase-3, and cleaved PARP, whereas it downregulated the expression of Bcl-2. Moreover, TNF-α significantly increased ROS content but decreased the total SOD activity. Further analysis demonstrated that resveratrol partly attenuated the effects of TNF-α on AF cell apoptosis-associated parameters, decreased ROS content, and increased the total SOD activity in the AF cells treated with TNF-α. In conclusion, resveratrol attenuates inflammatory cytokine TNF-α-induced AF cell apoptosis through regulating oxidative stress reaction in vitro. This study sheds a new light on the protective role of resveratrol in alleviating disc degeneration.
1. Introduction
Low back pain is a debilitating disorder that occurs in ~70% of the population [1]. Intervertebral disc degeneration (IDD) is a main contributor to low back pain and the procession of disc herniation. This kind of disease often affects life quality of adults and has a tremendous socioeconomic impact [2, 3]. The mechanism behind disc degeneration has not been fully elucidated.
The promoted cellular apoptosis is one of the main features of the vast majority of disc degeneration [4]. Several previous studies have showed that excessive apoptosis-induced loss of disc cells plays a key role in the disc degeneration process [5, 6]. Bax, Bcl-2, capsase-3, and caspase-9 are some important parameters to evaluate cell apoptosis [7–9]. Previously, lots of studies have reported an increase in the expression of apoptotic genes (Bax and caspase-3/9) and a decrease in the expression of antiapoptotic gene (Bcl-2) in patients with disc degeneration [10, 11].
The inflammatory response is closely associated with disc degeneration. Increased inflammatory cytokines (i.e., TNF-α and IL-1β) have been detected at the site of herniated disc tissue [12, 13]. These inflammatory cytokines are produced by either leukocytes or disc cells themselves [14]. Moreover, the expression of TNF-α and IL-1β increases with aging and degree of disc degeneration in degenerative human discs and animal discs [15, 16]. Previously, several studies have showed that inflammatory cytokine IL-1β facilitates disc AF cell apoptosis whereas inhibition of IL-1β has been shown to suppress disc cell apoptosis and inhibit disc degeneration [17–20]. Hence, a strategy to inhibit or attenuate inflammation response-induced disc cell apoptosis may be helpful to alleviate disc degeneration.
Resveratrol is a kind of nonflavonoid polyphenol which may treat various disorders, such as cancer, ischemic disease, neurodegenerative disease, and cardiovascular disease [21]. It has also been reported that resveratrol plays some positive effects on disc cell's biology [22–29]. Moreover, resveratrol is reported to protect against IL-1β-induced nucleus pulposus cell apoptosis [30]. However, whether resveratrol can inhibit or alleviate disc AF cell apoptosis in an inflammatory environment remains unclear. In the present study, we mainly aimed to investigate the effects of resveratrol on AF cell apoptosis in an inflammatory environment and to investigate the changes of oxidative stress reaction in this process.
2. Materials and Methods
2.1. AF Cell Isolation and Culture Conditions
Thirty-nine rats (8-10 weeks old) were purchased from the Laboratory Animal Center of the 960 Hospital of PLA, and all animal experiments were approved by the Ethics Committee of the 960 Hospital of PLA. Briefly, after the lumbar discs (L1-L5) were separated under sterile conditions, the peripheral AF tissues were obtained. To isolate the individual AF cells, AF tissues were cut into small pieces and digested by 0.2% type I collagenase for 3-4 hours at 37°C. After the subsequent centrifugation (1000 g/min, 5 minutes, 4°C), the AF cell pellets were cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS, Gibco, USA) with a refresh of culture medium every three days. AF cells with 2 generation of subculture were used in each experiment. The control AF cells were cultured in medium without TNF-α whereas the experimental AF cells were cultured in medium with TNF-α (50 ng/mL) for 48 hours. In addition, the AF cells treated with TNF-α were incubated with exogenous resveratrol (100 μM) (this concentration was referred to a previous study [28]) to investigate its effects on AF cell apoptosis.
2.2. Flow Cytometry Analysis
After the cells (2 × 105 cells/well, 6-well) in each group were attached to the plate and those floated in the supernatant were collected together after 48 hours, they were resuspended in cold binding buffer. The apoptosis ratio was measured using an Annexin V/FITC apoptosis detection kit (Beyotime, China) according to the manufacturer's instructions. According to a previous method, cells that were positively stained with Annexin V/FITC but negatively stained with propidium iodide (PI) were apoptotic cells, and the cells that were both positively stained were necrotic cells [31].
2.3. Caspase Activity Analysis
AF cells (2 × 105 cells/well, 6-well) were cultured in respective medium for 48 hours. Then, the cells were washed with sterile phosphate buffer solution (PBS) and lysed with lysis solution for 20 minutes. Subsequently, the prepared protein supernatant was used to measure caspase-3 activity and caspase-9 activity according to the manufacturer's instructions (Beyotime, China).
2.4. Reactive Oxygen Species (ROS) Content Measurement
AF cells (2 × 105 cells/well, 6-well) were cultured in respective medium for 48 hours. Then, AF cells were treated by 10 μM DCFH-DA for 20 minutes at 37°C, followed by 2 times of washing with serum-free medium. Finally, the fluorescence intensity (excitation/emission: 490/585 nm) indicating ROS content was measured using a fluorescence microplate.
2.5. Total Superoxide Dismutase (SOD) Activity Measurement
After AF cells (2 × 105 cells/well, 6-well) were cultured in respective medium for 48 hours, they were lysed using the SOD sample preparing solution, and the protein concentration of the supernatant was measured using a BCA Protein Assay Kit (Beyotime, China). Then, total SOD activity was quantitatively analyzed (U/mg protein) using a Total Superoxide Dismutase Assay Kit with WST-8 Method (Beyotime, China).
2.6. Real-Time Polymerase Chain Reaction (PCR) Analysis
After total RNA were extracted using an RNA Sample Total RNA Kit (Tiangen, Beijing, China) and reverse-transcribed into cDNA using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA), real-time PCR was performed via a system containing cDNA, primers, and SYBR Green Mix (DONGSHENG, China). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control in this study. The primer sequences were as follows: caspase-3: GTACAGAGCTGGACTGCGGTATTG (forward), AGTCGGCCTCCACTGGTATCTTC (reverse); Bcl-2: ACGGTGGTGGAGGAACTCTTCAG (forward), GGTGTGCAGATGCCGGTTCAG (reverse); and Bax: CCAGGACGCATCCACCAAGAAG (forward), GCTGCCACACGGAAGAAGACC (reverse). Finally, relative expression of Bcl-2, Bax, and caspase-3 was analyzed by the method of 2−ΔΔCT.
2.7. Western Blot Analysis
After total proteins were extracted and quantified using a BCA Protein Assay Kit, equal protein samples in each group were sequentially separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to the polyvinylidene fluoride (PVDF) membrane, and incubated with primary antibodies (cleaved caspase-3: Cell Signaling Technology, #9664; cleaved PARP: Cell Signaling Technology, #9545) and secondary antibodies according to the standard process. The immunoreactive bands on the PVDF membranes were detected using an enhanced chemiluminescence system (EMD Millipore, Billerica, MA, USA), and the band intensity was quantified using the ImageJ software.
2.8. Statistical Analysis
All values were expressed as the mean ± standard deviation. Statistical analyses were performed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). The one-way analysis of variance was used to analyze the significance of differences between these groups. p < 0.05 indicated a statistical difference.
3. Results
3.1. Cell Apoptosis Ratio
In the TNF-α group, AF cell apoptosis ratio was significantly increased compared with that in the control group. When resveratrol was added into the medium of the TNF-α group, AF cell apoptosis ratio was partly decreased (Figure 1).
Figure 1.
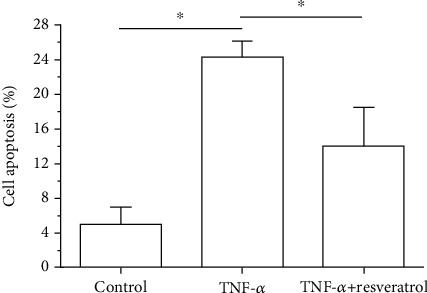
Analysis of annulus fibrosus (AF) cell apoptosis. Data are expressed as mean ± SD, n = 3. ∗A significant difference (p < 0.05) between two groups.
3.2. Caspase-3 and Caspase-9 Activity
In the TNF-α group, both caspase-3 activity and caspase-9 activity were significantly increased compared with those in the control group. However, addition of resveratrol in the TNF-α group partly decreased both caspase-3 activity and caspase-9 activity (Figure 2).
Figure 2.
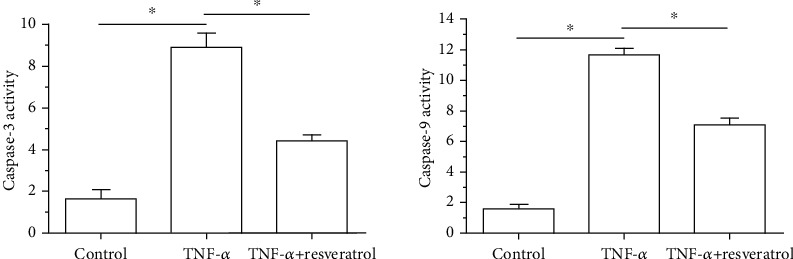
Measurement of caspase-3 and caspase-9 activity of annulus fibrosus (AF) cells. Data are expressed as mean ± SD, n = 3. ∗A significant difference (p < 0.05) between two groups.
3.3. ROS Content
In the TNF-α group, ROS content was significantly increased compared with that in the control group, whereas resveratrol could partly decrease ROS content in the TNF-α group (Figure 3).
Figure 3.
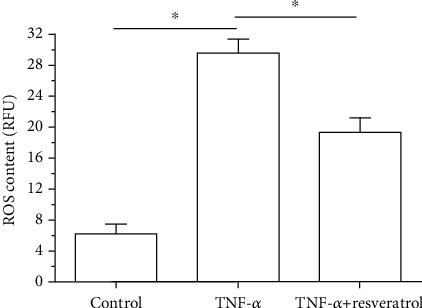
Measurement of reactive oxygen species (ROS) of annulus fibrosus (AF) cells. Data are expressed as mean ± SD, n = 3. ∗A significant difference (p < 0.05) between two groups.
3.4. Total SOD Activity
Total SOD activity in the TNF-α group was significantly decreased compared with that in the control group; however, addition of resveratrol in the TNF-α group could partly increase the total SOD activity (Figure 4).
Figure 4.
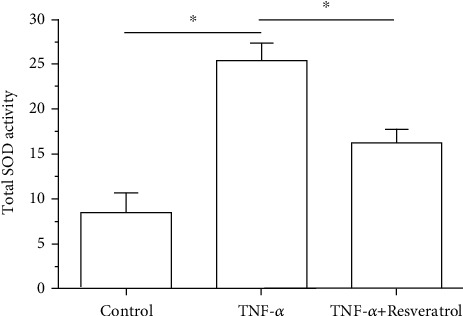
Measurement of total superoxide dismutase (SOD) activity of annulus fibrosus (AF). Data are expressed as mean ± SD, n = 3. ∗A significant difference (p < 0.05) between two groups.
3.5. Gene Expression of Apoptosis-Related Molecules
Results showed that mRNA expression of antiapoptotic genes (Bcl-2) in the TNF-α group was significantly downregulated but that of proapoptotic genes (Bax and caspase-3) was significantly upregulated compared with that in the control group. However, addition of resveratrol in the TNF-α group partly upregulated mRNA expression of antiapoptotic gene (Bcl-2) and downregulated mRNA expression of proapoptotic genes (Bax and caspase-3) (Figure 5).
Figure 5.
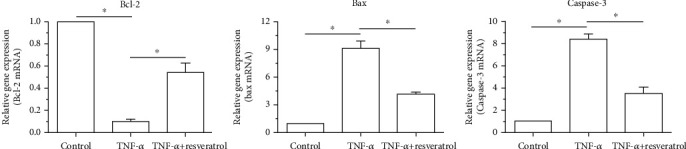
Gene expression of apoptosis-related molecules (Bcl-2, Bax, and caspase-3). Data are expressed as mean ± SD, n = 3. ∗A significant difference (p < 0.05) between two groups.
3.6. Protein Expression of Apoptosis Markers
Results showed that protein expression of apoptotic markers (cleaved caspase-3 and cleaved PARP) in the TNF-α group was significantly increased compared with that in the control group. However, addition of resveratrol in the TNF-α group partly decreased protein expression of these apoptotic markers (cleaved caspase-3 and cleaved PARP) (Figure 6).
Figure 6.
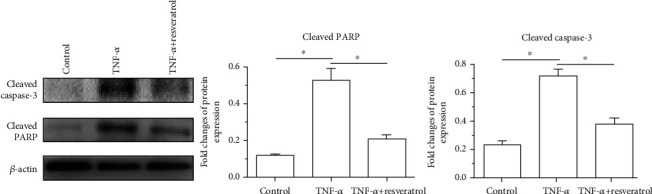
Protein expression of apoptosis-related molecules (cleaved caspase-3 and cleaved PARP). Data are expressed as mean ± SD, n = 3. ∗A significant difference (p < 0.05) between two groups.
4. Discussion
Intervertebral disc degeneration is a leading cause of low back pain. During disc degeneration, disc cell viability and biosynthesis behaviors often exhibit drastic alterations due to the adverse external or internal niches, which ultimately affect disc structural integrity [32]. The loss of disc cells due to excessive cell apoptosis has been proved to play an important role in mediating disc degeneration [33]. Therefore, it is required to study the cause of disc cell apoptosis.
Enhanced inflammation response is a classical feature during disc degeneration. Inflammatory processes exacerbated by several cytokines (i.e., TNF-α and IL-1β) are key mediators of disc degeneration and the resultant low back pain [34]. Moreover, inflammation response is closely related with disc cell apoptosis. Previous studies have showed that inflammatory cytokine induces disc cell apoptosis [19, 35]. In this study, we also found that AF cell apoptosis ratio, caspase-3/9 activity, and expression of proapoptotic molecules (Bax, caspase-3, cleaved caspase-3, and cleaved PARP) in the TNF-α group were significantly increased compared with those in the control group, confirming that inflammatory cytokine TNF-α can induce disc AF cell apoptosis. This is in line with the previous studies [18, 19, 35]. Hence, inhibiting inflammation response or inhibiting the inflammatory response-induced disc cell apoptosis may be an effective strategy to retard disc degeneration process.
Resveratrol is supposed to have efficacy in treating various disorders, such as cancer, ischemic disease, neurodegenerative disease, and cardiovascular disease [21]. Recently, it has also showed that resveratrol may play protective effects on disc cell biology under certain stimulation. Gao et al. have demonstrated resveratrol can enhance matrix biosynthesis of disc nucleus pulposus cells through activating autophagy under oxidative damage [22]. Zhang et al. have showed that resveratrol attenuates mechanical compression-induced disc nucleus pulposus cell apoptosis through regulating the ERK1/2 signaling pathway [29]. Wang et al. have showed that resveratrol attenuates high glucose-induced disc nucleus pulposus cell apoptosis and senescence through activating the ROS-mediated PI3K/Akt pathway [25]. Wang et al. have reported that resveratrol attenuates TNF-α-induced MMP-3 expression in human nucleus pulposus cells by activating autophagy via the AMPK/SIRT1 signaling pathway [26]. Importantly, Jiang et al. have found that resveratrol is able to attenuate IL-1beta-mediated disc nucleus pulposus cell apoptosis [30]. However, there is not much more information about the effects of resveratrol on disc AF cells. In this study, we found that addition of resveratrol in the TNF-α group partly decreased AF cell apoptosis ratio and caspase-3/9 activity, downregulated expression of proapoptotic genes (Bax, caspase-3, cleaved caspase-3, and cleaved PARP), and upregulated expression of antiapoptotic gene (Bcl-2), indicating that resveratrol can attenuate inflammatory cytokine TNF-α-induced disc AF cell apoptosis. To some extent, our results are consistent with the above described studies.
Oxidative stress is believed to be an important step in mediating disc degeneration [36]. ROS has been well recognized as a product of normal cellular mitochondrial metabolism [37]. However, the excessive ROS production will produce detrimental effects on cell biology. A previous study has reported that ROS is elevated in the degenerative disc tissue [38]. Moreover, ROS is a main cause of the increased incidence of cellular apoptosis [39]. In this study, we measured ROS content and the total SOD activity to evaluate the homeostasis of oxidative stress reaction. We found that ROS content was increased while the total SOD activity was decreased in the TNF-α group compared with that in the control group. In addition, addition of resveratrol in the TNF-α group partly decreased ROS content and increased the total SOD activity. These results suggest that resveratrol can attenuate TNF-α-induced oxidative stress injury in AF cells. Taking the corresponding changes of concomitantly happed AF cell apoptosis, we deduced that resveratrol can attenuate AF cell apoptosis through inhibiting oxidative stress damage in an inflammatory environment.
This study also has several limitations. First, because this study is just a preliminary work, we just finished it in vitro, and an in vivo study was not performed to verify the protective effects of resveratrol. Second, there may a dosage effect of resveratrol on inflammation-induced AF cell apoptosis according to our results, but we did not further evaluate this in the present study. To make our study more scientific, these limitations need to be further resolved in the future research.
5. Conclusion
In conclusion, we investigated the effects of resveratrol on AF cell apoptosis in an inflammatory environment and studied the role of oxidative stress reaction in this process. Our results demonstrate that resveratrol is effective in attenuating TNF-α-induced disc AF cell apoptosis and that this may be mediated through alleviating the oxidative stress injury. This study sheds a new light that resveratrol can suppress TNF-α-induced disc AF cell apoptosis through regulating oxidative stress reaction and provides that resveratrol may be a potential drug to retard progression of disc degeneration.
Data Availability
All data are included in the article.
Conflicts of Interest
No conflicts of interest exist in this work.
Authors' Contributions
Qunqun Shan and Qingxi Meng contribute to conception and design of this study. Qunqun Shan, Ning Li, Fan Zhang, and Peng Yu contribute to experiment performance, data collection, and data analysis. Qunqun Shan and Qingxi Meng contribute to manuscript writing and revision.
References
- 1.Macfarlane G. J., Thomas E., Croft P. R., Papageorgiou A. C., Jayson M. I. V., Silman A. J. Predictors of early improvement in low back pain amongst consulters to general practice: the influence of pre-morbid and episode-related factors. Pain . 1999;80(1):113–119. doi: 10.1016/S0304-3959(98)00209-7. [DOI] [PubMed] [Google Scholar]
- 2.Maniadakis N., Gray A. The economic burden of back pain in the UK. Pain . 2000;84(1):95–103. doi: 10.1016/S0304-3959(99)00187-6. [DOI] [PubMed] [Google Scholar]
- 3.Séguin C. A., Bojarski M., Pilliar R. M., Roughley P. J., Kandel R. A. Differential regulation of matrix degrading enzymes in a TNFα-induced model of nucleus pulposus tissue degeneration. Matrix Biology . 2006;25(7):409–418. doi: 10.1016/j.matbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Freemont A. J. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) . 2009;48(1):5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 5.Zhang F., Zhao X., Shen H., Zhang C. Molecular mechanisms of cell death in intervertebral disc degeneration (review) International Journal of Molecular Medicine . 2016;37(6):1439–1448. doi: 10.3892/ijmm.2016.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orrenius S., Gogvadze V., Zhivotovsky B. Calcium and mitochondria in the regulation of cell death. Biochemical and Biophysical Research Communications . 2015;460(1):72–81. doi: 10.1016/j.bbrc.2015.01.137. [DOI] [PubMed] [Google Scholar]
- 7.Elmore S. Apoptosis: a review of programmed cell death. Toxicologic Pathology . 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nature Reviews. Drug Discovery . 2008;7(12):1001–1012. doi: 10.1038/nrd2637. [DOI] [PubMed] [Google Scholar]
- 9.Tait S. W., Green D. R. Mitochondria and cell death: outer membrane permeabilization and beyond. Nature Reviews. Molecular Cell Biology . 2010;11(9):621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 10.Eser B., Eser O., Aslan E., Dolgun H. The effects of polymorphisms of death pathway genes and mitochondrial pathway genes in intervertebral disc degeneration. Turkish Neurosurgery . 2017;27(5):809–815. doi: 10.5137/1019-5149.JTN.17927-16.0. [DOI] [PubMed] [Google Scholar]
- 11.Park J. B., Lee J. K., Park S. J., Kim K. W., Riew K. D. Mitochondrial involvement in fas-mediated apoptosis of human lumbar disc cells. The Journal of Bone and Joint Surgery. American Volume . 2005;87(6):1338–1342. doi: 10.2106/JBJS.D.02527. [DOI] [PubMed] [Google Scholar]
- 12.Grönblad M., Virri J., Tolonen J., et al. A controlled immunohistochemical study of inflammatory cells in disc herniation tissue. Spine (Phila Pa 1976) . 1994;19(24):2744–2751. doi: 10.1097/00007632-199412150-00002. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi H., Suguro T., Okazima Y., Motegi M., Okada Y., Kakiuchi T. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine (Phila Pa 1976) . 1996;21(2):218–224. doi: 10.1097/00007632-199601150-00011. [DOI] [PubMed] [Google Scholar]
- 14.Le Maitre C. L., Freemont A. J., Hoyland J. A. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Research & Therapy . 2005;7(4):R732–R745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oda H., Matsuzaki H., Tokuhashi Y., Wakabayashi K., Uematsu Y., Iwahashi M. Degeneration of intervertebral discs due to smoking: experimental assessment in a rat-smoking model. Journal of Orthopaedic Science . 2004;9(2):135–141. doi: 10.1007/s00776-003-0759-y. [DOI] [PubMed] [Google Scholar]
- 16.Wang J., Tian Y., Phillips K. L. E., et al. Tumor necrosis factor α- and interleukin-1β-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis and Rheumatism . 2013;65(3):832–842. doi: 10.1002/art.37819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duan D. Y., Yang S. H., Xiong X. Q., Shao Z. W., Wang H. Interleukin-6 protects annulus fibrosus cell from apoptosis induced by interleukin-1 beta in vitro. Chinese Medical Sciences Journal . 2006;21(2):107–110. [PubMed] [Google Scholar]
- 18.Yang X., Wang L., Yuan Z. Q., et al. Interleukin-1β induces apoptosis in annulus fibrosus cells through the extracellular signal-regulated kinase pathway. Connective Tissue Research . 2018;59(6):593–600. doi: 10.1080/03008207.2018.1442445. [DOI] [PubMed] [Google Scholar]
- 19.Wang H., Ding W., Yang D., Gu T., Yang S., Bai Z. Different concentrations of 17β-estradiol modulates apoptosis induced by interleukin-1β in rat annulus fibrosus cells. Molecular Medicine Reports . 2014;10(5):2745–2751. doi: 10.3892/mmr.2014.2514. [DOI] [PubMed] [Google Scholar]
- 20.Hu J., Yan Q., Shi C., Tian Y., Cao P., Yuan W. BMSC paracrine activity attenuates interleukin-1β-induced inflammation and apoptosis in rat AF cells via inhibiting relative NF-κB signaling and the mitochondrial pathway. American Journal of Translational Research . 2017;9(1):79–89. [PMC free article] [PubMed] [Google Scholar]
- 21.Aguirre L., Fernández-Quintela A., Arias N., Portillo M. Resveratrol: anti-obesity mechanisms of action. Molecules . 2014;19(11):18632–18655. doi: 10.3390/molecules191118632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao J., Zhang Q., Song L. Resveratrol enhances matrix biosynthesis of nucleus pulposus cells through activating autophagy via the PI3K/Akt pathway under oxidative damage. Bioscience Reports . 2018;38(4) doi: 10.1042/BSR20180544. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Li K., Li Y., Mi J., Mao L., Han X., Zhao J. Resveratrol protects against sodium nitroprusside induced nucleus pulposus cell apoptosis by scavenging ROS. International Journal of Molecular Medicine . 2018;41(5):2485–2492. doi: 10.3892/ijmm.2018.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen J., Zhuo N., Xu S., et al. Resveratrol delivery by ultrasound-mediated nanobubbles targeting nucleus pulposus cells. Nanomedicine (London, England) . 2018;13(12):1433–1446. doi: 10.2217/nnm-2018-0019. [DOI] [PubMed] [Google Scholar]
- 25.Wang W., Li P., Xu J., et al. Resveratrol attenuates high glucose-induced nucleus pulposus cell apoptosis and senescence through activating the ROS-mediated PI3K/Akt pathway. Bioscience Reports . 2018;38(2) doi: 10.1042/BSR20171454. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Wang X. H., Zhu L., Hong X., et al. Resveratrol attenuated TNF-α–induced MMP-3 expression in human nucleus pulposus cells by activating autophagy via AMPK/SIRT1 signaling pathway. Experimental Biology and Medicine (Maywood, N.J.) . 2016;241(8):848–853. doi: 10.1177/1535370216637940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang S. D., Ma L., Yang D. L., Ding W. Y. Combined effect of 17β-estradiol and resveratrol against apoptosis induced by interleukin-1β in rat nucleus pulposus cells via PI3K/Akt/caspase-3 pathway. PeerJ . 2016;4, article e1640 doi: 10.7717/peerj.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang B., Xu L., Zhuo N., Shen J. Resveratrol protects against mitochondrial dysfunction through autophagy activation in human nucleus pulposus cells. Biochemical and Biophysical Research Communications . 2017;493(1):373–381. doi: 10.1016/j.bbrc.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z., Wen F., He C., Yu J. Resveratrol attenuates mechanical compression-induced nucleus pulposus cell apoptosis through regulating the ERK1/2 signaling pathway in a disc organ culture. Bioscience Reports . 2018;38(2) doi: 10.1042/BSR20171703. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Jiang Y., Xie Z., Yu J., Fu L. Resveratrol inhibits IL-1β-mediated nucleus pulposus cell apoptosis through regulating the PI3K/Akt pathway. Bioscience Reports . 2019;39(3) doi: 10.1042/BSR20190043. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Zhang G., Gurtu V., Kain S. R., Yan G. Early detection of apoptosis using a fluorescent conjugate of annexin V. BioTechniques . 1997;23(3):525–531. doi: 10.2144/97233pf01. [DOI] [PubMed] [Google Scholar]
- 32.Kadow T., Sowa G., Vo N., Kang J. D. Molecular basis of intervertebral disc degeneration and herniations: what are the important translational questions? Clinical Orthopaedics and Related Research . 2015;473(6):1903–1912. doi: 10.1007/s11999-014-3774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding F., Shao Z. W., Xiong L. M. Cell death in intervertebral disc degeneration. Apoptosis . 2013;18(7):777–785. doi: 10.1007/s10495-013-0839-1. [DOI] [PubMed] [Google Scholar]
- 34.Johnson Z. I., Schoepflin Z. R., Choi H., Shapiro I. M., Risbud M. V. Disc in flames: roles of TNF-α and IL-1β in intervertebral disc degeneration. European Cells & Materials . 2015;30:104–117. doi: 10.22203/eCM.v030a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao C. Q., Liu D., Li H., Jiang L. S., Dai L. Y. Interleukin-1beta enhances the effect of serum deprivation on rat annular cell apoptosis. Apoptosis . 2007;12(12):2155–2161. doi: 10.1007/s10495-007-0137-x. [DOI] [PubMed] [Google Scholar]
- 36.Feng C., Yang M., Lan M., et al. ROS: crucial intermediators in the pathogenesis of intervertebral disc degeneration. Oxidative Medicine and Cellular Longevity . 2017;2017:12. doi: 10.1155/2017/5601593.5601593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zorov D. B., Juhaszova M., Sollott S. J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiological Reviews . 2014;94(3):909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J. W., Ni B. B., Li B., Yang Y. H., Jiang S. D., Jiang L. S. The responses of autophagy and apoptosis to oxidative stress in nucleus pulposus cells: implications for disc degeneration. Cellular Physiology and Biochemistry . 2014;34(4):1175–1189. doi: 10.1159/000366330. [DOI] [PubMed] [Google Scholar]
- 39.Bhattacharyya A., Chattopadhyay R., Mitra S., Crowe S. E. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiological Reviews . 2014;94(2):329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in the article.


