Abstract
Age associated chronic inflammation is a major contributor to diseases with advancing age. Adipose tissue function is at the nexus of processes contributing to age-related metabolic disease and mediating longevity. Hormonal fluctuations in aging potentially regulate age-associated visceral adiposity and metabolic dysfunction. Visceral adiposity in aging is linked to aberrant adipogenesis, insulin resistance, lipotoxicity and altered adipokine secretion. Age-related inflammatory phenomena depict sex differences in macrophage polarization, changes in T and B cell numbers, and types of dendritic cells. Sex differences are also observed in adipose tissue remodeling and cellular senescence suggesting a role for sex steroid hormones in the regulation of the adipose tissue microenvironment. It is crucial to investigate sex differences in aging clinical outcomes to identify and better understand physiology in at-risk individuals. Early interventions aimed at targets involved in adipose tissue adipogenesis, remodeling and inflammation in aging could facilitate a profound impact on health span and overcome age-related functional decline.
Keywords: Aging, adipose tissue, sex differences, metabolism, adipose tissue inflammation, macrophages
1. Introduction
Aging is associated with multiple risk factors for many chronic diseases and a decline in physical function (Franceschi et al., 2018; Kirkland, 2013). In most cases, the underlying factors are linked to disturbances in metabolic homeostasis and inflammatory status (Frasca, 2017; Wu et al., 2007). Adipose tissue is a dynamic organ that plays a critical role in modulating systemic metabolism and inflammation, especially the visceral fat depot is strongly correlated to changes in these processes (Cartwright et al., 2007; Stout et al., 2014; Tchkonia et al., 2010). Age-related adipose dysfunction (Figure 1) is a major cause for an elevated risk of obesity, type 2 diabetes (T2D), cardiovascular disease (CVD), cancer, neurodegenerative and kidney diseases (Faulkner and Belin de Chantemèle, 2019; Franceschi et al., 2018). This review highlights the adipose-related factors affected by the aging process in men and women and briefly explains potential countermeasures to reduce the burden of age-associated immune-metabolic diseases to increase longevity (Figure 1).
Figure 1.
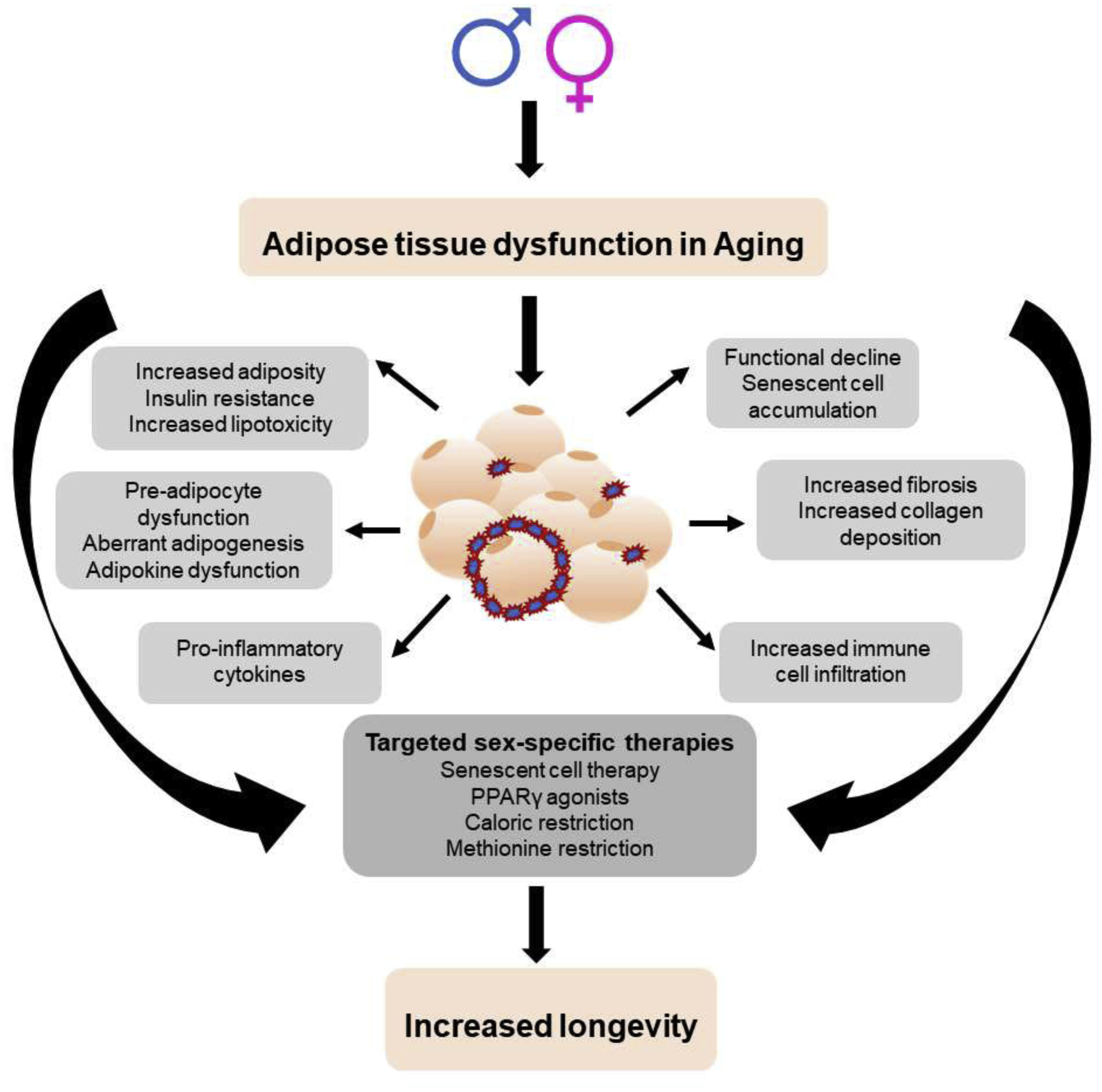
Factors affecting adipose tissue dysfunction in aging in both sexes.
1.1. Adipose tissue types
In mammals, two principal types of adipose tissue exist, brown adipose tissue (BAT) and white adipose tissue (WAT) (Frontini and Cinti, 2010; Rosen and Spiegelman, 2014). Brown adipocytes, which in rodents are predominantly present in the interscapular region, contain multilocular lipid droplets and high numbers of mitochondria, and primarily function to dissipate stored energy in the form of heat (Wang and Seale, 2016). In humans, BAT is closely associated with the cervical, supraclavicular, and superior mediastinal vasculature, which decreases with age (Cypess et al., 2009a; Pfannenberg et al., 2010). The WAT depots include the visceral and subcutaneous with distinct functions (Lee and Fried, 2010; Lee et al., 2013). The visceral adipose tissue depot buffers dietary lipids by storing excess calories in the form of triglycerides (TG) that provide fuel for physiologic functions in the post-prandial state and during fasting (Goossens, 2008). The subcutaneous compartment provides insulation, cushioning, and serves as a long-term energy storage depot (Lee and Fried, 2010).
Other minor depots by volume include bone marrow and perivascular compartments. The function of bone marrow adipose tissue (MAT) is poorly understood but this tissue replaces hematopoietic cells during aging and is the most abundant source of adiponectin in mice and humans (Cawthorn et al., 2014). Perivascular adipose tissue, that surrounds major and small arteries and veins, expresses factors with paracrine effects on vascular structure and function (Fernández-Alfonso et al., 2017). Among the many fat depots, obesity and aging studies have shown that visceral adiposity is strongly correlated with detrimental metabolic function and meta-inflammation (Davis et al., 2013; Derby et al., 2006; Manwani et al., 2013; Meyer et al., 2011; Wu et al., 2017).
It is also well known that there are sex differences in whole body fat distribution between women and men. Women generally have higher fat mass than men; women with body mass indices (BMI) of 20–25 kg/m2 contain 25–30% body fat, while men with the same BMI have 10–20% body fat (Meyer et al., 2011; White and Tchoukalova, 2014). In addition, premenopausal women are characterized by less visceral WAT and more fat accumulation in the subcutaneous, gluteal-femoral region, whereas men accumulate more fat in the central regions, both abdominal subcutaneous and visceral fat (Chang et al., 2018; Nishida et al., 2010; Wells., 2007). Studies show that the sexual dimorphism in lipid deposition in various adipose tissue depots is governed by sex hormones, the location of sex hormone receptors, catecholamines, and the activity of adipose triglyceride, hormone sensitive, and lipoprotein lipases (Frank et al., 2019; Varlamov et al., 2014). This also explains why men and post-menopausal women have greater risks of developing cardiometabolic diseases than pre-menopausal women (Salpeter et al., 2006; Tchernof et al., 1995; Tchernof et al., 2000). The importance of sex hormones on adipose tissue function (Table 1) is depicted by sex differences in lipid storage, distribution, and inflammation in young versus older ages (Table 2) where there are decreases in sex hormones (Chang et al., 2018; Frasca, 2017; Varghese et al., 2017; White and Tchoukalova, 2014).
Table 1.
Effects of sex hormones on adipose function and inflammation
| Testosterone | Estrogen | |
|---|---|---|
| Visceral adiposity | ⬆⬆ | - |
| Subcutaneous adiposity | - | ⬆⬆ |
| Lipolysis | ⬆ | ⬆⬆ |
| Inflammatory lipids | ⬆⬆ | - |
| Adipogenesis | ⬆ | ⬆ |
| Leptin | ⬇ | ⬆ |
| Adiponectin | ⬆ | ⬇ |
| WAT browning | ⬇⬇ | ⬆ |
| ECM remodeling, collagen turnover | ⬇ | ⬆ |
| Cellular senescence | ⬆ | - |
| Immune cell infiltration | ⬆⬆ | ⬆ |
Arrows indicate high and low
- Indicates none/moderate changes
Table 2.
Factors affecting sex-differences in young and old adipose tissue
| Male | Female | |||
|---|---|---|---|---|
| Young | Old | Young | Old | |
| Testosterone | ⬆⬆ | ⬇ | ⬇ | ⬆ |
| Estrogen | ⬇ | ? | ⬆ | ? |
| Inflammatory cytokines | ⬆ | ⬆⬆ | ⬇ | ⬆ |
| Inflammatory monocytes | ⬆ | ⬆⬆ | ⬇ | ⬆ |
| Resident macrophages | ⬆ | ⬆ | ⬆⬆ | ⬆⬆ |
| Recruited macrophages | ⬆⬆ | ⬆⬆ | ⬆ | ⬆ |
| Dendritic cells | ⬆ | ⬇ | ⬇ | ? |
| CD8+ T cells | ⬆ | ⬆ | ⬆ | ⬆⬆ |
| Tregs | ⬆ | ⬆ | ? | ⬇⬇ |
| B cells | ⬆ | ⬆ | ⬆ | ⬆⬆ |
⬆⬆ Arrows indicate high and low
? Indicates inconclusive evidence
1.2. Adipose tissue: a source of chronic inflammation in aging
Adipose tissue is a critical regulator of systemic metabolism and normal bodily homeostasis. Adipose tissue homeostasis is maintained by a balance of adipogenesis and lipolysis. Lipolysis is defined as the catabolism of triacylglycerols stored in cellular lipid droplets that provides fatty acids (FAs) in times of metabolic need and removes them when they are present in excess. Adipocyte lipolysis-derived FAs are essential substrates for energy production and the synthesis of membrane and cellular signaling lipids. Despite their fundamental physiological importance, an oversupply of FAs is cytotoxic and highly detrimental. Increased concentrations of non-esterified FAs disrupt the integrity of biological membranes, alter cellular acid-base homeostasis, and elicit the generation of harmful bioactive lipids (Zechner et al., 2012). These effects, in turn, induce endoplasmic reticulum (ER) stress, mitochondrial dysfunction, inflammation, and cell death. Collectively, these deleterious effects are categorized under the term lipotoxicity (Unger et al., 2010). As a countermeasure, essentially all cells are able to detoxify non-esterified FAs by esterification with glycerol to yield inert TGs. Additionally, higher organisms store FAs in a specialized organ (i.e., adipose tissue), which supplies FAs to other high-demand tissues, such as liver and muscle (exogenous FAs). The carefully regulated balance of FA esterification and TG hydrolysis is essential to create an efficient buffer system, allowing sufficient FA flux without non-physiological increases in cellular non-esterified FA concentrations (Unger et al., 2010).
Young adipose tissue is highly efficient in buffering dietary lipids and most adaptable to environmental changes due to its ability to rapidly alter its endocrine, inflammatory, and metabolic functions (Stout et al., 2014). However, with aging, adipose tissue undergoes significant changes in cellular composition, insulin responsiveness, endocrine signaling, and inflammatory state that promotes metabolic dysfunction (Tchkonia et al., 2010). Adipose tissue dysfunction can further have secondary effects on a variety of organs systems including liver, muscle, pancreas, and others that are sites for ectopic lipid deposition in young as well as in old age. Age related increase in lipotoxicity is a major contributor to insulin resistance and inflammation (Frasca, 2017). Hence, causally linking inflammation with adipocyte dysfunction.
Along with increased abdominal and ectopic fat accumulation, organelle stress, changing immune cell populations, accumulation of senescent cells within adipose tissue as well as secretion of tissue damaging cytokines contribute to a pro-inflammatory, dysfunctional adipose tissue in old age (Figure 1) (Cannizzo and Sahu, 2011; Fulop et al., 2017). However, sex differences in aging studies are comparatively fewer despite having clinically relevant gender differences in presentation and treatment responses to several diseases including metabolic and cardiovascular disease. The current review focuses on the pathophysiology of sex differences in adipose metabolism and presents an overview of factors that drive age related adipose dysfunction and meta-inflammation.
2. Sex differences in adipose tissue function and metabolism in aging
Sex is now emerging as a significant predictive factor in the development of CVD associated with metabolic dysregulation. The prevalence of coronary artery disease in men is several times higher than that of age-matched premenopausal women, but these gender-based differences narrow after menopause, when the protection against vascular disease is gradually lost (Meyer, 2006). Notably, sex differences are consistently emerging in rates of obesity and insulin resistance, factors that are primary contributors of metabolic syndrome (Pradhan, 2014). In addition, aging presents a unique challenge to the prediction of metabolic risks in men and women as both sex hormone-dependent and sex hormone-independent effects play various roles in the development of aging-related diseases in men versus women. As the prevalence of metabolic syndrome is over 50% in both men and women over the age of 60, with a sharper recent increase in this prevalence in women (Faulkner and Belin de Chantemèle, 2019), understanding metabolic syndrome development based on sex is of immense clinical importance to individualized treatments.
2.1. Sex hormones, steroid receptors, and fat redistribution
Distribution of fat is important for cardiometabolic health, with central visceral fat depots conferring a detrimental effect on health (Lee and Fried, 2010). Studies show that young and pre-menopausal women typically have greater peripheral subcutaneous adipose tissue, while young men tend to accumulate more central/visceral fat (Chang et al., 2018; Nishida et al., 2010; Wells., 2007). Many studies in women pre- and post-menopause, demonstrate that female sex hormones – estrogens strongly regulate adipose fat accumulation in women. Decline in circulating estrogen levels during menopause are associated with distinct changes in adipose distribution patterns, leading to visceral adiposity (Espeland et al., 1997; Jackson et al., 2002; Munoz et al., 2002). In the Study of Women’s Health Across the Nation (SWAN) fat distribution pattern study, it was demonstrated that low estrogen levels (Meyer, 2006) corelated with visceral accumulation in women during and following menopause (Janssen et al., 2010). Clinical studies in women with polycystic ovarian syndrome (PCOS) (Moran et al., 2015) and aromatase-deficient men (Morishima et al., 1995) have demonstrated the protective function of estrogens against metabolic syndrome by maintaining insulin sensitivity and adiposity. These studies point towards the role of sex hormones (Table 1), as well as the microenvironment and cell-specific properties within fat depots.
The disparity in fat distribution could therefore be attributed to sex hormone profiles of estrogens and androgens and interactions and metabolism within the adipose tissue system. The steroid receptors – estrogen receptor (ER) and androgen receptor (AR) are present in both visceral and subcutaneous adipose tissue (Dieudonne et al., 1998; Dieudonné et al., 2004; Mayes and Watson, 2004), making these likely to have a direct role in the tissue. Estrogen regulates body adiposity and fat distribution (Stubbins et al., 2012) through its receptors, ER alpha (ERα) and beta (ERβ) (Brown and Clegg, 2010). ERα has been reported to have a major influence on energy homeostasis (Heine PA, 2000) and essential for body weight regulation (Musatov et al., 2007), while ERβ plays a protective role in suppressing inflammation and fibrosis (Davis et al., 2013; Schomberg et al., 1999). In rodent studies, suppression of female sex hormones by ovariectomy (OVX) increases gonadal (visceral), but not inguinal (subcutaneous) fat and WAT accumulation is reversed by estradiol treatment (Clegg et al., 2006; Stubbins et al., 2012). Heine et al (Heine PA, 2000) showed that ERα-knock-out (ERαKO) mice have increased adiposity in both sexes, suggesting an important role for this ER in the regulation of body weight and adiposity. However, in the absence of both ERα and ERβ there is a significant increase in adipose tissue markers of fibrosis, inflammation, macrophage infiltration in both sexes (Davis et al., 2013). The presence of ERα and ERβ in male and female mice indicates direct intra-adipose effects of estrogen which may not be tied to sex-chromosomes. Hormone fluctuations in menopause may hence lead to increased visceral WAT accumulation but further studies are needed to understand if other sex hormones, such as progesterone or testosterone have a similar role.
In aging men, a menopause-like dramatic decrease in sex hormone levels is not observed but, testosterone levels decline steadily with age (Simpson, 2003) leading to elevated visceral adiposity and decreased lean mass (Bietti et al., 1967). Testosterone therapy has been shown to increase muscle mass and decrease fat mass in older men (Page et al., 2005; Snyder et al., 1999). Therefore, long-term therapy may improve metabolic health by improving body composition. However, association studies of testosterone levels in men and women offer a paradox for determining its true effects on visceral adiposity independent of sex hormones. Low serum testosterone is associated with reduced subcutaneous, and increased abdominal, adiposity in men (Haffner et al., 1994; Tsai et al., 2000), while high testosterone is associated with the same in women (Janssen et al., 2010). These data suggest that endogenous sex hormone- or chromosome-associated factors alter the effects of testosterone on adipogenesis in men and women. However, further studies are needed to determine the true effect of anti-androgen therapy independent of estrogen on adipocyte deposition.
Studies suggest that testosterone regulates adipose expansion likely through a depot specific mechanism that may depend on steroid conversion within the adipose depot. Young female aromatase-KO mice, which are estrogen deficient but still have low levels of circulating androgens, weigh more and have increased gonadal WAT (GWAT) mass compared with controls (Van Sinderen et al., 2015). Similarly, young male rats supplemented with dehydroepiandrosterone (DHEA), a precursor of sex steroid hormones showed decreased visceral adiposity, and further, DHEA inhibited murine adipocyte proliferation in vitro (Fujioka et al., 2012). These experimental effects are likely derived from the AR in adipose tissue itself as male mice with both global (Lin et al., 2005) and adipose-specific (McInnes et al., 2012) AR deficiency exhibit increased weight gain and visceral adipose accumulation. The effects of estrogen on adipose deposition may not be efficacious in the presence of testosterone in males, in contrast to females. Increasing aromatase activity via transgenic overexpression of the adipose tissue aromatase enzyme of young male mice, which subsequently increases estrogen or ER activation in white adipose, did not alter fat or lean mass in male mice, although there was an improvement in insulin sensitivity and adipose inflammation in this model (Ohlsson et al., 2017). In contrast, global aromatase knockout in male mice decreases lean mass and impairs insulin sensitivity in male mice, indicating that the effects of aromatase and its subsequent changes on adipose tissue function remain unclear.
Emerging rodent studies in older males and females in a diet induced obesity model showed increased visceral and subcutaneous adiposity in females compared to males. In addition, unlike aged male mice, female GWAT showed a greater adipogenic and metabolic potential (Varghese et al., 2020). It appears that females have an advantage with fewer negative effects of excess fat storage on their metabolism and health during aging, which could have a potential to increase lifespan in females than males as observed in other studies (Austad and Fischer, 2016; Tam et al., 2020). However, testosterone assessment in this study showed no significant changes in the older animals (Varghese et al., 2020). Hence a limitation of this aging model was the inability to mimic hormonal modulations as in post-menopausal female and older male human subjects. Such discrepancies in aging mice studies might be a consequence of sex steroid production by adipose tissue rather than gonads (Simpson, 2003) and need to be studied further.
2.2. Adipocyte size, preadipocytes and adipogenesis
Adipose tissue is composed of mature adipocytes, preadipocytes, mesenchymal cells, and various immune cell types that make up the stromal vascular fraction. Mesenchymal cells are progenitor cells that can undergo differentiation into many cell types but, within the adipose, differentiate to preadipocytes and eventually to mature adipocytes (Chang et al., 2018; Sepe, 2011). Changes in adipose tissue growth are a result of differentiation and proliferation of adipose progenitors expanding the adipocyte number and also replacing dysfunctional older and larger adipocytes. Since fat cell size and number are related to insulin sensitivity, glucose and fatty acid uptake, and cytokine release, changes in function and cellular composition of fat tissue can lead to changes in metabolic state and subsequent clinical complications (Virtue and Vidal-Puig, 2010). A limited ability of a depot to expand or remodel can lead to excessive adipocyte hypertrophy and cell death, often correlated with metabolic dysfunction (Rutkowski, 2015; Sepe, 2011). However, the relationship of adipocyte size and function varies by depot and sex.
Adipose depots serve distinct functions in males and females and have specific physiological roles. In pre-menopausal women, the gluteo-femoral adipocytes are larger yet are more insulin sensitive (Johnson et al., 2001) and more efficient at storing fat in vivo (Santosa et al., 2008) compared to smaller abdominal adipocytes. When adipocytes within a depot become more hypertrophied, excess FAs are released, resulting in ectopic fat deposition that disrupts the function of the liver, muscle, and other tissues. Thus, the ability of adipose tissue to expand by hyperplasia and functional hypertrophy may serve a protective function by providing a safe storage for excess energy during overnutrition (Virtue and Vidal-Puig, 2010). One potential contributor to sex differences in adipose tissue expansion is the number of adipocyte precursor cells in mouse gonadal or inguinal fat depots (IWAT). On a low-fat chow diet, young female C57BL/6J mice showed more adipocyte precursor cells than males in GWAT and IWAT. When fed a high-fat diet (HFD), female mice showed increased adipocyte precursor cells and mature adipocytes in GWAT, but males did not increase mature fat cells in the GWAT (Medrikova et al., 2012; Wu et al., 2017). Estrogen treatment decreased adipocyte size in young male rats while ERα deletion led to increased adipocyte size in both males and females (Heine PA, 2000; Pedersen et al., 1991). In another study, Brangdon et al found that bone marrow-derived stem cells from females of different ages had higher adipogenic capacity than male mice when activated by rosiglitazone, a peroxisome proliferator-activated receptor (PPAR)-γ agonist, independent of ovarian status (Bragdon et al., 2015). Studies show the presence of AR in mature adipocytes and preadipocytes of both men and women (Dieudonne et al., 1998). However, androgen binding sites are observed to be more in intra-abdominal preadipocytes than in subcutaneous preadipocytes (Dieudonne et al., 1998; Joyner et al., 2002). In addition, a decrease in AR expression during adipogenesis and an up-regulation of AR by androgens in vitro was also observed in human preadipocytes (Joyner et al., 2002) which could be an explanation for visceral adiposity in young men and post-menopausal women. These studies imply a role for sex steroid receptors in modulating the adipocyte size and number through adipose site-specific actions.
Aging has a significant impact on the lipid storage capacity in older men and women with a shift in lipid storage from the subcutaneous to the visceral fat depot (Stout et al., 2014). The decline in subcutaneous fat depot storage and function is thought to occur through the decline in progenitor cell function and the accumulation of senescent adipose tissue cells (Mitterberger et al., 2014; Sepe, 2011). The progenitor cell populations isolated from aged adipose tissue have reduced function with an impaired ability to incorporate lipids and limited potential to differentiate into preadipocytes than young subjects (Caso et al., 2013). PPARγ deficiency selectively in the subcutaneous adipose tissue during aging is associated with increased adipose tissue expansion that is associated with the development of insulin resistance (Xu et al., 2018a). Few studies have directly compared the adipogenic capacity of older male and female adipose progenitors. In old male rats, the expression of CCAAT/enhancer binding protein (C/EBP)-α, a key regulator of adipogenesis and fat cell function, declined substantially with aging in differentiating preadipocytes in vitro (Karagiannides et al., 2001). In a recent study, the expression of adipogenesis related genes- Pparγ and Pgc1α were found to be higher in older female GWAT than males suggesting continued metabolic flexibility in older female adipose (Varghese et al., 2020).
2.3. Fatty acids, insulin resistance, lipolysis and lipogenesis
The major role of WAT is to store lipids to supply energy when needed by other tissues. Adipocytes convert circulating excess free FAs that are cytotoxic into less damaging neutral TG, thereby protecting other tissues from their lipotoxicity (Cartwright et al., 2007; DeFronzo, 2004). Large increases in systemic free FA availability in the absence of a corresponding increase in FA oxidation can create a host of metabolic abnormalities. Age decreases adipose tissue capacity to store free FAs resulting in a lipotoxic environment, systemic lipotoxicity, and the increased prevalence of metabolic syndrome in older populations (Slawik and Vidal-Puig, 2006).
Adipocytes break down lipids through catecholamine stimulation of beta3-adrenergic receptors (ADRB3), which in turn activates the lipolytic pathway. Balance between adipose tissue lipolysis and peripheral FFA removal is crucial to prevent lipotoxicity. Lipotoxicity in non-adipose tissues increases reactive oxygen species (ROS) and activates serine threonine kinases: c-jun N-terminal kinase (JNK), I-kappa B kinase (IKK) and protein kinase C (PKC). These events disrupt insulin receptor signaling cascades and promote insulin resistance (Cannizzo and Sahu, 2011). Both mice and human studies have shown that in young age, there are major sex differences in insulin sensitivity in adipose tissue, that are regulated by physiological levels of sex steroids (Varghese et al., 2019; Varghese et al., 2020). In rodent studies, both αERKO males and females had insulin resistance and impaired glucose tolerance, similar to humans lacking ERα or aromatase (Heine PA, 2000). Young mice and women show significantly greater FFA concentrations as well as FFA turnover rates relative to energy requirements than men. However, higher lipolytic rates in young women are correlated with greater nonoxidative FFA disposal than men due to re-esterification of FAs in the liver, adipose tissue, and muscle, thereby limiting lipotoxicity (Koutsari et al., 2011; Varghese et al., 2019). The increased sensitivity to insulin and lipogenesis observed in adipocytes from young females may account for their lower level of insulin resistance and diabetes risk despite similar or higher adiposity compared to males (Geer and Shen, 2009; Macotela et al., 2009). Aging mice have impaired glucose tolerance compared to young mice(Ma et al., 2018). Aging male mice specifically showed higher insulin levels than females in lean and obese conditions(Varghese et al., 2020). In addition, studies with young and old mice showed that GWAT Pparγ and Pgc1α expression were higher in females than males in both young and old mice suggesting efficient FA oxidation and less lipotoxicity than males in the visceral depot (Varghese et al., 2020).
Lipoprotein lipase (LPL) is the key enzyme for the hydrolysis of circulating TG into free FAs and glycerol, while hormone sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) regulate the mobilization of free FAs from triacylglycerols stored in cellular lipid droplets (Frühbeck et al., 2014; Goldberg and Merkel, 2001; Lafontan and Langin, 2009). These enzymes are under hormonal control including cortisol and insulin that appear to promote lipid accumulation by increasing the activity of LPL, while growth hormone and estrogen decrease LPL activity (Björntorp, 1996). Testosterone has been shown to suppress LPL activity more in the femoral SAT depot than in the abdominal fat depot, which may contribute to central adipose accumulation in men (Palmer and Clegg, 2015). LPL activity is also higher in the subcutaneous region of young women, whereas it is higher in the abdominal or visceral region of men (Palmer and Clegg, 2015). With advancing age, rodent and human studies show a loss in number of ADRB receptors in WAT (Hoffman et al., 1984; Lönnqvist et al., 1990) leading to impaired lipolytic stimulation. In obesity studies, with young mice, sex differences were observed in lipolytic levels and HSL protein expression that were lower in young male GWAT than in females (Varghese et al., 2019). In aging mice studies, lipogenic genes – Fasn and Acsl1 were repressed in older female GWAT. Also, lipolysis responsive genes such as Adrb3, Atf3, Dio2 were significantly higher in expression upon ADRB3 agonist treatment in old female GWAT, while serum TG remained higher in males suggesting impairments in lipolytic pathway in aging males (Varghese et al., 2020). These studies suggest that defects in lipolytic signaling may play an important role in defective lipid mobilization and metabolism in aging males than females.
In addition to adipocyte dysfunction through insulin resistance, defects in FA metabolism can lead to the accumulation of bioactive lipid metabolites, diacylglycerol and ceramides, that can trigger inflammation (Chaurasia and Summers, 2015; Lottenberg et al., 2012; Meikle and Summers, 2017). In young mice, diacylglycerol, ceramides, phospholipids, and arachidonic acid are associated with inflammation and are elevated in male obese GWAT compared with obese female GWAT (Varghese et al., 2019). Young obese female mice presented an attenuated inflammatory lipid profile with accumulation of linoleic acid and oleic acid (Varghese et al., 2019). In aging mice, sphingolipid ceramide was observed to be higher compared to young adipose tissue leading to NF-kappa B activation and adipose tissue inflammation (Wu et al., 2007). Ahnstedt et al observed that lipid profiling of visceral fat showed no sex differences in TG and LDL, but middle-aged females had lower HDL than middle-aged males (Ahnstedt et al., 2018). Macrophage polarization in human visceral WAT is thus related to lipid metabolism, cell membrane composition, and dietary status. In middle-aged male and female subjects, proinflammatory polarization of ATMs was observed by presence of palmitate and palmitoleate while alpha-linolenic acid, n-3 FAs, n-3/n-6 FA ratio, and eicosatetraenoic acid had the opposite effect in visceral WAT (Poledne et al., 2019). An independent effect of age on adipose fatty acid composition in human subjects was observed with an increase in dietary gamma-linolenic acid (C18:3, n-6) to offset the relative imbalance between PUFA levels which appears to occur with old age (Bolton-Smith et al., 1997). However, studies on sex differences in aging in this field are scarce.
2.4. Adipose derived hormones – adipokines
Adipose tissue produces several secretory factors termed adipokines, that mediate local and whole-body metabolism via autocrine, paracrine, and endocrine mechanisms. A variety of adipokines such as leptin, adiponectin, resistin, adipsin, apelin, retinol-binding protein-4 (RBP-4) and visfatin are produced by adipocytes (Maury and Brichard, 2010; Ouchi et al., 2011). Adipose tissue expansion, and subsequent remodeling, is often associated with altered adipokine production. In obesity, many adipokines are upregulated which promote inflammatory responses, insulin resistance, and other metabolic complications (Virtue and Vidal-Puig, 2010). However, there are pro-inflammatory and anti-inflammatory adipokines and the balance of adipokine secretion may contribute to differential metabolic risks that are associated with fat distribution and adiposity (Maury and Brichard, 2010). In addition, sex hormones and adipokines seem to interact differentially in both sexes at different ages. Although a wide range of adipokines have been identified, with respect to discussing their sex specific role in regulating inflammatory and immune responses we chose to focus only on the major adipose derived hormones in this review - leptin, resistin and adiponectin.
Leptin
Leptin is an adipokine that regulates feeding behavior through the central nervous system. Leptin levels in the blood positively correlate with adipose mass and although obese individuals have high levels of leptin, obese individuals lack the expected anorexic responses due to leptin resistance (Friedman and Halaas, 1998). Leptin levels in serum and adipose tissues increase in response to pro-inflammatory stimuli, including TNF-α and lipo-polysaccharide (LPS) (Grunfeld et al., 1996). Furthermore, leptin increases the production of the TH1-type cytokines; IL-2 and IFN-γ and suppresses the production of the TH2-type cytokine; IL-4 by T cells or mononuclear cells (Lord et al., 1998), thus polarizing T cells towards a TH1 cell phenotype (Matarese et al., 2001). Leptin, therefore, acts as a pro-inflammatory adipokine. Women have higher circulating leptin levels than men that could be attributed to a higher proportion of adipose tissue and increased production rate of leptin per unit mass of adipose tissue (Hellström et al., 2000). In aging men, negative correlations between testosterone and leptin have been shown in different cross-sectional studies (Luukkaa et al., 1998; Van Den Saffele et al., 1999). In addition, testosterone therapy reduces serum leptin concentrations in older male subjects with low testosterone levels (Sih et al., 1997) due to the presence of functional leptin receptors in reproductive tissues. Clinical studies show that leptin does not differ among men in different ages. However, pre-menopausal women display significantly lower serum leptin than post-menopausal women who have greater serum leptin when compared with age-matched men (Cicero et al., 2011). Similarly, elderly women also showed greater serum leptin levels when compared with elderly men (Cicero et al., 2011). Overall, the main predictors of leptin are adiposity and the testosterone/estrone ratio in both sexes.
Resistin
Resistin is another pro-inflammatory adipokine that has been shown to induce insulin resistance in mice. Further, mice lacking resistin have low blood glucose levels post-fasting owing to low hepatic glucose production (Banerjee et al., 2004; Qi et al., 2006; Steppan et al., 2001). The ability of resistin to modulate glucose metabolism is associated with the activation of suppressor of cytokine signaling 3 (sOCs3), an inhibitor of insulin signaling, in adipocytes (Steppan et al., 2005). During pathological inflammation, the release of resistin by infiltrated monocytes/macrophages is incited by pro-inflammatory cytokines such as C-reactive protein (CRP), interleukin-1 (IL-1), IL-6, IL-12, and tumor necrosis factor - α (TNF-α) or by other pro-inflammatory stimuli, such as peptidoglycans and endotoxins (Filková et al., 2009). Although studies in animal models consistently show that resistin promotes insulin resistance, evidence for this effect in humans is less clear since the major site of resistin production in rodents are adipocytes while that in humans are mainly monocytes and macrophages (Heilbronn et al., 2004; Lee et al., 2003; Qatanani et al., 2009). In mice, plasma resistin declined with age despite an increase in adiposity in both genders. However, resistin mRNA levels were significantly higher in females compared to male mice at all ages (Gui et al., 2004). In clinical studies, serum resistin level were significantly higher in postmenopausal obese women compared to post-menopausal non obese women (Sadashiv et al., 2012) and in elderly women compared to young (Wijetunge et al., 2019). In older men and women, higher levels of resistin were associated with an increased risk of cardiovascular events independently of clinical variables (Gencer et al., 2016; Rodondi et al., 2010).
Adiponectin
Adiponectin is an anti-inflammatory adipokine that is decreased in the plasma and adipose tissue of obese individuals compared with lean individuals (Ryo et al., 2004). Consistent with this, the production of adiponectin by adipocytes is inhibited by pro-inflammatory factors, such as TNF-α and IL-6 (Isakson et al., 2009; Maury and Brichard, 2010), as well as by hypoxia and oxidative stress (Mack et al., 2009). Adiponectin-mediated modulation of macrophage function and phenotype contributes to its role in controlling inflammation. Adiponectin inhibits the transformation of macrophages into foam cells and reduces intracellular cholesteryl ester content in human macrophages by suppressing the expression of class A scavenger receptors (sR-A) (Ouchi et al., 2001). It also abrogates LPS-stimulated TNF-α production by macrophages (Yokota et al., 2000) and inhibits Toll-like receptor (TLR) mediated NF-κB activation in mouse macrophages (Yamaguchi et al., 2005). Furthermore, adiponectin stimulates the production of the anti-inflammatory cytokine IL-10 by human macrophages (Kumada et al., 2004). In young rodents, testosterone decreases adiponectin levels (Nishizawa et al., 2002). At all ages, women have significantly lower serum testosterone/estrone ratios compared to age-matched men and serum DHEAS has been observed to be inversely proportional to age in both sexes (Cicero et al., 2011). Clinical studies showed an inverse association between adiponectin levels and endogenous estrogen (Karim et al., 2015; Tworoger et al., 2007). Also, adiponectin levels were found to be lower in young men than women (Yamamoto et al., 2002), despite an inverse relationship between estradiol and adiponectin (Gavrila et al., 2003), indicating that, in addition to estradiol, other gender-dependent factors may be of relevance. The relationship between testosterone and adiponectin is less clear. Clinical studies have been conducted in premenopausal women, post-menopausal women, and elderly women, and in age-matched men to investigate adipokines and sex hormone specific interactions. In elderly subjects (Gannagé-Yared et al., 2006) and in women affected by PCOS (Ardawi and Rouzi, 2005), adiponectin and testosterone are strongly correlated. However, when compared with hormone intact subjects, hypogonadal men have higher adiponectin levels, which are reduced by testosterone replacement therapy (Lanfranco et al., 2004). In contrast, in a further study, testosterone administration has not been shown to affect adiponectin levels in older men with low testosterone levels (Page et al., 2005). Plasma adiponectin levels correlated negatively with body fat percentage in older males but not in older females (Song et al., 2014). The differential results between older males and females suggest that certain gender-specific mechanisms may affect the association between adiponectin and age-related body composition changes.
2.5. WAT browning and BAT
With aging, WAT declines and adipocytes become dysfunctional, smaller and less insulin responsive than fully differentiated adipocytes (Kirkland et al., 2002). In rodents, a very similar phenotype has also been observed in BAT; wherein the proliferative capacity and UCP1 expression in response to cold stimulus seems to be abolished in aged brown adipocytes (Florez-Duquet et al., 1998). In humans, BAT is abundant in children and young adults and the amount of detectable BAT declines with increasing age (Cypess et al., 2009b). Older mice also have hypertrophied classic BAT that had larger cytoplasmic lipid droplets than younger mice (Gonçalves et al., 2017).
Beige or brite adipocytes are present in WAT and transform into a BAT like phenotype, leading to increased thermogenesis. This phenomenon is known as browning (Timmons et al., 2007) and involves the expression of many transcription factors, such as PR domain containing 16 (PRDM16) and PPARγ, and of uncoupling protein (UCP)-1, which is the hallmark of thermogenesis (Becerril et al., 2013). Browning of WAT increases energy expenditure and is thought to counteract adipose tissue dysfunction as in obesity and aging (Barquissau et al., 2016; Sepa-Kishi and Ceddia, 2018). With increasing age, beige adipocytes progressively lead to a white adipocyte phenotype, which prevents browning of adipocytes in older mice and humans. In rodents, subcutaneous and visceral WAT has clusters of beige cells in younger mice, but they are progressively lost by aging, indicating loss of WAT browning. Therefore, in aging, increased BAT activity and enhanced browning of WAT may be beneficial leading to an increased lifespan (Cypess et al., 2009b; Graja et al., 2019; Pfannenberg et al., 2010). However, mechanisms of age-induced changes in proliferation and differentiation capacity of brown adipocyte precursors on whole-body energy homeostasis remain to be elucidated.
The adrenergic agonist-dependent inductive ability of beige adipocytes in WAT also decreases in old mice likely due to reduction in ADRB receptor expression (Gonçalves et al., 2017; Shin et al., 2017). In rodents and humans, sex dependent effects on WAT browning and BAT activity have been reported. Young female mice and women show higher metabolic activity of classic BAT than males owing to larger mitochondria and greater ADRB stimulation (Cypess et al., 2009b; Rodriguez-Cuenca et al., 2002). Sex differences were also observed in the browning of WAT, specifically GWAT, wherein Kim et al showed that young female mice are more responsive than males to the recruitment of brown adipocytes in GWAT and this difference corresponds to greater levels of estrogen-dependent sympathetic innervation (Kim et al., 2016). Also, in humans, women showed moderate decline in BAT activity and BAT mass with increasing age, but a much stronger effect was observed in male subjects suggesting an estrogen dependent effect (Pfannenberg et al., 2010). However, further studies are needed to address the role of central regulation in sex-dimorphic browning and to investigate the effects of nutritional and pharmacological stimulation to maintain BAT and beige mass and sensitivity during aging.
2.6. Extracellular matrix remodeling, fibrosis and collagen turnover
The capacity of a fat depot to expand via hypertrophy vs hyperplasia is an important determinant of metabolism and health wherein, tissue modeling is crucial. Tissue remodeling is a dynamic process, present in all tissues, in which the breakdown of the extracellular matrix (ECM), including collagens, glycoproteins, and proteoglycans, permits progenitor cells to proliferate, migrate, and differentiate (Bonnans et al., 2014). The mechanical properties of this matrix are likely to vary with depot, sex, age and obesity. The composition of the ECM, in particular the content of specific collagens secreted by fibroblasts, adipose progenitors, and myofibroblasts, change adipose tissue stiffness, affecting the ability for adipocytes to expand (Sun, 2011).
Sex differences in the expression of key enzymes that remodel the ECM have been observed in mouse models of obesity (Martinez-Santibanez et al., 2015). Matrix metalloproteinase 3 (MMP3), which is anti-adipogenic enzyme expressed in mouse adipose progenitors is significantly higher in the IWAT of young females than males. In HFD fed mice, obesity downregulated MMP3 protein in both the IWAT and GWAT of young females but not males (Wu et al., 2017). Furthermore, in HFD-induced obesity, the ratio of a tissue inhibitor of metalloproteinase (TIMP), TIMP4 to MMP3, significantly increased in IWAT in females, while it decreased the ratio in males (Wu et al., 2017). It is likely that a dynamic balance of MMPs and TIMPs modulates the recruitment and differentiation of preadipocytes in a sex-dependent manner. These observations highlight sex differences in adipose remodeling that promotes/deteriorates the metabolic health of the adipose tissues.
Fibrosis is a hallmark of dysfunctional adipose tissue and likely a mechanism of age-related adipose dysfunction. Along with tissue remodeling, fibrosis regulates adipocytes and a variety of ECM proteins (Datta et al., 2018). Fibrosis is a consequence of ECM dysregulation due to an imbalance between the synthesis and degradation of the ECM fibrillar components, such as collagen I, III, and VI (Datta et al., 2018). A tight equilibrium between degradation and formation of these proteins is necessary for tissue health and homeostasis which initially is beneficial and reparative. As a consequence of tissue turnover, small collagen fragments are released into the circulation, which act as important biomarkers in the study of certain tissue-related remodeling factors in health and disease (Bonnans et al., 2014). When excessive collagen deposition occurs in adipose tissue in the context of chronic inflammation, homeostasis is impaired (Bonnans et al., 2014). Karsdal et al observed that type I collagen had an increased turnover in younger rats compared to old rats. In addition, type III collagen turnover was not significantly influenced by age while type IV collagen degradation was slightly upregulated in younger animals (Karsdal et al., 2016). Similarly, in clinical studies, age specific changes in collagen turnover was most profound for type I collagen with its formation being strongly age-dependent (Kehlet et al., 2018). Among the multiple types of collagens, including I, IV, V, VI, VII, VIII, and IX; collagen VI is highly enriched in adipose tissue. Notably, young women were found to have lower collagen VIα3 mRNA compared with men independent of BMI, possibly contributing to a better metabolic profile in women (Khan et al., 2009; Pasarica et al., 2009). Interestingly, sex specific changes in type III and IV collagen turnover were most apparent during the menopausal and post-menopausal periods with the interstitial matrix and basement membrane being differently regulated in older women than men (Kehlet et al., 2018). Together, these data suggest that these sex-specific changes in collagen turnover could be associated with diseases that is more prevalent in postmenopausal women.
2.7. Cellular senescence
One of the leading drivers of age-associated dysfunction and chronic inflammation is cellular senescence (Schosserer et al., 2018), characterized by morphological changes such as enlarged nucleoli and increased positivity of senescence-associated beta-galactosidase (SA-b-gal)(Charalambous et al., 2007; Choi et al., 2000; Jeyapalan and Sedivy, 2008). Cellular senescence is mediated by p21/p53 (Jeyapalan and Sedivy, 2008) and p16 pathways (Smith et al., 2021) and the production of senescence associated secretory phenotype (SASP), which chronically releases cytokines and chemokines that promote leukocyte recruitment, tissue repair and remodeling, and the generation of ROS (Coppe et al., 2008; Freund et al., 2010; Krtolica and Campisi, 2002; Parrinello et al., 2005; Passos et al., 2010; Rodier and Campisi, 2011). While p53/p21 are essential for initiating cell cycle arrest, p16 is recognized as important for maintaining the arrest and thus enforcing the maintenance of senescence (Smith et al., 2021). As adipose tissue is a dynamic endocrine organ in humans and plays a central role in metabolism, accumulation of senescent cells in adipose tissue could lead to extensive clinical consequences (Palmer et al., 2015; Palmer and Kirkland, 2016; Xu et al., 2015a). Senescent cell accumulation with age in adipose tissue results in inflammation, insulin resistance, impaired adipogenesis, failure to sequester FAs, and could lead to the development of diabetes and metabolic diseases (Morin et al., 1997; Starr et al., 2009; Xu et al., 2015a; Xu et al., 2015b). Senescent cells in adipose tissue include differentiated adipocytes, preadipocytes and endothelial cells (Tchkonia et al., 2010). Preadipocyte senescence is one of the major drivers of metabolic perturbations in aged adipose tissue by reducing insulin sensitivity (Baker et al., 2016). Accumulation of senescent preadipocytes is consistent with smaller adipocytes and fat loss during aging (Xu et al., 2015a; Xu et al., 2015b). Senescent adipocytes and preadipocytes are also the major sources of pro-inflammatory cytokines and chemokines in aged adipose tissue (Palmer and Kirkland, 2016; Schosserer et al., 2018). Innate and/or adaptive immune responses activated by senescent cells could further spread cellular senescence locally and systemically (Keyes et al., 2005). Rodent studies show that transplanting radiation induced-senescent cells into young mice results in cellular senescence spreading in host tissues and causes persistent physical dysfunction (measured by walking speed, grip strength and physical endurance) and transplanting doxorubicin treated- pre-adipocyte senescent cells into aged mice shortens health and lifespan (Xu et al., 2018b).
Age-related alterations in adipose tissue are also observed in obesity, wherein senescent preadipocytes and endothelial cells accumulate and express SASP (Cartwright et al., 2010). Obesity predisposes individuals prematurely to age-related diseases (Palmer and Kirkland, 2016) and clinical studies show that the burden of senescent cells in adipose tissue increases in obese individuals compared to lean age-matched individuals (Minamino et al., 2009; Tchkonia et al., 2010). Culture of preadipocytes isolated from obese animals exhibits more abundant SA-b-gal positive cells than those from lean controls and SA-b-gal activity in adipose tissue increases with BMI (Tchkonia et al., 2010). Obese patients also appear to have up to more than 30-fold senescent preadipocytes than nonobese controls (Tchkonia et al., 2010). These findings suggest that adipose tissue dysfunction in both aging and obesity could be similar. On the other hand, the high burden of senescent cells in adipose tissue during aging and obesity itself makes it a potential target for alleviating age-related metabolic disorders (Palmer et al., 2015; Tchkonia et al., 2013). Removing senescent cells from older mice improves adipogenesis (Ghosh et al., 2019; Xu et al., 2015a) and targeting SASP can attenuate age-related insulin resistance (Roos et al., 2016; Xu et al., 2015a; Xu et al., 2015b; Zhu et al., 2015; Zhu et al., 2016). Selectively removing senescent cell with senolytic agents may improve nutrient handling, mitochondrial function, lipolysis and subsequently prolong health and lifespan (Baker et al., 2016; Xu et al., 2018b; Zhu et al., 2015; Zhu et al., 2016). Senolytics can alleviate the senescence burden of mesenchymal stem cells (MSC) from adipose tissue and improve MSC angiogenic potential (Suvakov et al., 2019). In addition to removing senescent cells by senolytics, exercise can prevent the accumulation of senescent cells and inhibit the expression of SASP in visceral adipose tissue and restores physical function (Schafer et al., 2016).
In rodent studies, sex differences in cellular senescence of adipose tissue were observed wherein, lean or obese aged male and female mice have comparable expression of senescent markers including p53, p16 and p21 in GWAT. Interestingly, aged obese female mice showed potentially greater expression of ECM-modifying proteases than the males (Varghese et al., 2020). However, compounds that were designated to prolong lifespan all showed sex-specific effects (Austad and Fischer, 2016; Fischer and Riddle, 2018), and women showed a longer lifespan than men (Fischer and Riddle, 2018). There is a paucity of studies on sex differences in cellular senescence in adipose tissues, which calls for an urgent need to investigate this mechanism which could lead to novel and effective interventions.
3. Age and Sex differences in adipose tissue inflammatory responses
Aging induced cellular senescence and/or dysfunction of immune progenitor cells are likely causative factors of aging induced inflammatory responses. Gradual deterioration of the immune system over the course of time leads to a mismatch between proinflammatory and anti-inflammatory signals that may disrupt inflammatory homeostasis causing “inflamm-aging” (Cannizzo and Sahu, 2011; Childs et al., 2015; Stout et al., 2014; Tchkonia et al., 2010). In healthy young individuals, the innate immune system, including neutrophils, monocytes, and natural killer (NK) cells, responds quickly to a state of infection or tissue damage. In addition, antigen-presenting cells (APCs), activate the adaptive immune system and effector T and B lymphocytes launch a refined antigen-specific immune response. After the effective removal of the invading pathogen, a subset of T lymphocytes - regulatory T cells (Treg) are responsible for suppressing the deleterious effects of immune response by deactivation and return to quiescent state (Nikolich-Žugich, 2014). In general, both innate and adaptive immune systems are affected by aging, but adaptive immunity, especially T lymphocytes, are most susceptible to the detrimental effects of aging (Agarwal and Busse, 2010).
Most of our knowledge about meta-inflammation is from diet induced obesity animal models (Lumeng et al., 2007a; Lumeng et al., 2007c; Morris et al., 2011). Aging, like obesity, is accompanied by expanded visceral adiposity and therefore there are similarities in factors driving obesity and aging induced inflammation. Insulin resistance and lipid dysregulation bring about the onset of meta-inflammation in both obesity and aging related metabolic and non-metabolic disorders through activation of leukocytes, specifically of the myeloid lineage (Nagareddy et al., 2014; Singer et al., 2014). The adipose tissue myeloid leukocytes include neutrophils, macrophages, and monocytes most predominantly in rodent and clinical studies (Lumeng et al., 2008). The secretome of healthy subcutaneous WAT is mostly anti-inflammatory, whereas visceral WAT is more proinflammatory (Dolinková et al., 2008; Samaras et al., 2010). The excessive expansion of inflammatory visceral WAT, especially in conjunction with the loss of subcutaneous WAT, is associated with dramatic deleterious effects on metabolic homeostasis common with the aging process.
3.1. Inflammatory cytokines
Cytokines are central to the regulation of the immune-inflammatory response in old age and play a pivotal role in ageing and survival (Castelo-Branco and Soveral, 2014). Visceral WAT infiltrating leukocytes are the key source of cytokines in case of obesity-related inflammation. Monocytes and infiltrating adipose tissue macrophages (ATMs) produce several pro-inflammatory cytokines such as IL-6, IL-1, and TNF-α, and chemokines such as monocyte chemoattractant protein-1 (MCP-1/CCL2) (Lumeng et al., 2007c; Morris et al., 2011; Weisberg and Leibel, 2003). Sex differences have been noted in the capacity of cytokine production. In obesity, serum IL-6 levels in obese female mice were observed to be lower than males (Varghese et al., 2019). Several studies have reported a stronger monocyte-derived cytokine production response upon stimulation with LPS in men than in women (Imahara et al., 2005; Lefèvre et al., 2012). Men also showed a stronger cytokine production response than women to LPS for TNF-α, IL-6, IL-12, IL-1β, IL-1Ra, and IL-10, but not for interferon-γ (Beenakker et al., 2020).
Aging studies showed that in older male mice, the endocrine profile of visceral WAT as well as other depots such as perivascular, perirenal and interscapular become more pro-inflammatory with increases in TNF-α and IL-6 cytokine expression than their young counterparts (Morin et al., 1997; Starr et al., 2009). In humans, monocyte cytokine production of IL-6 and IL-1Ra but not IL-1β or TNF-α were increased in the elderly compared to healthy, young subjects (Roubenoff et al., 1998). Sex difference studies showed that older men also had higher levels of serum IL-6 than women (Bonafè et al., 2001), that was also linked to higher mortality in men than women (Harris et al., 1999). In post-menopausal women, cytokine shifts were observed as production of IL-10 was augmented, while interferon-γ was lower (Deguchi et al., 2001).
3.2. Monocytes, recruited and resident macrophages
Inhibitors of pro-inflammatory cytokines and chemokines have been shown to improve insulin sensitivity and link ATMs to metabolic syndrome (Weisberg et al., 2006; Winer et al., 2009). In lean young mice and humans, the percentage of ATMs ranges from under 10% of the non-adipocyte fraction to over 40% in obese mice and humans. However, sex differences have been observed in macrophage subtypes in young rodent studies, wherein obese male mice compared to females have profound accumulation of the Ly6chi /CCR2+ monocytes that develop into CD11c+/M1-type ATMs (Elgazar-Carmon et al., 2008; Estrany et al., 2013; Weisberg et al., 2006). In a normal homeostatic state, CD11c− /M2-type resident ATMs are the predominant macrophage subset present in the adipose tissue and function to maintain homeostasis (Lumeng et al., 2007b; Muir et al., 2018). CD11c− ATMs are also predominantly found in young obese female mice suggesting a role for sex hormones in strengthened or attenuated inflammatory responses (Singer et al., 2015; Varghese et al., 2019).
Macrophages do not appear to be central to age-related proinflammatory cytokine release as preadipocytes and/or adipocytes themselves appear to contribute substantially to age-associated functional declines in WAT (Wu et al., 2007). These cytokines directly inhibit adipogenesis in rodents and human studies(Isakson et al., 2009; Karagiannides et al., 2006), while also promoting resident preadipocytes to develop a secretory profile similar to that of an activated macrophage (Mack et al., 2009). Furthermore, WAT macrophage infiltration with aging is less robust than observed in the setting of obesity and is completely absent in some cases (Harris et al., 1999; Varghese et al., 2020; Wu et al., 2007). ATMs in lean young and old male mice showed no differences in GWAT CD11c+ ATMs, but a reduction in CD11c− ATMs with age (Lumeng et al., 2011b). The stimulus for inflammation in aged murine adipose tissue stromal cells is likely through endoplasmic reticulum stress (Ghosh et al., 2015) and impaired angiogenesis, supporting local hypoxia, which might drive ATM accumulation (Valli et al., 2015). Ageing is also associated with reductions in the mitochondrial cytochrome c oxidase subunit 5B (COX5B) component of complex IV within adipocytes, which represses HIF-1α. Reductions in COX5B in human visceral WAT with age both elevates HIF-1α and intracellular lipid storage as a result of decreased FA oxidation, promoting adipocyte enlargement (Rutkowski, 2015; Soro-Arnaiz et al., 2016). This hypertrophic expansion triggers stress signals prompting macrophage infiltration as in obesity but to a lesser extent in aging (Morris et al., 2011; Rutkowski, 2015).
Data on sex differences in human adipose tissue inflammation and the role of macrophages are rather scarce. Recently, HFD studies in young and old; male and female mice showed that aging led to an increase in GWAT CD11c+ATMs in old and obese males. Although, old lean females showed no significant changes in the GWAT CD11c+ ATMs, but with obesity in old age, there was a significant increase in CD11c+ ATM numbers. Female mice also showed persistent predominance of CD11c− ATMs in young and old animals that increased with obesity in GWAT (Varghese et al., 2020). Age-related associations with inflammatory pathways/genes were more significantly observed in men, suggesting an accelerated inflamm-aging signature for older men. Interestingly, no differences were observed between older men and women for CD14+CD16+ monocyte cell numbers/frequencies (Márquez et al., 2020). However, other clinical studies observed an increase in pro-inflammatory CD14+CD16+ monocytes/macrophages in older women compared to young in depots like the perivascular WAT (Králová Lesná et al., 2015). These findings indicate that lipid dysregulation through adipose tissue dysfunction with advancing age has a potential to increase pro-inflammatory macrophages in the adipose depots of older females.
3.3. Dendritic cells
Dendritic cells (DCs) are professional APCs that play a pivotal role in the linkage between innate and adaptive immunity. Plasmacytoid DC (pDCs) play a major role in anti-viral immune responses characterized by the rapid production of type I interferon (Banchereau et al., 2000; Iwasaki and Medzhitov, 2004; Merad et al., 2013). Myeloid or conventional (cDCs) are divided into cDC1 and cDC2 subsets, where cDC1 can efficiently recognize viral and intracellular antigens and have an intrinsic capacity to cross-present antigens to CD8+ T cells, while cDC2 preferentially promote CD4+ T cell responses and favor polarization towards T helper type 17 (Th17) and Th2 cell responses (Sundara Rajan and Longhi, 2016). DCs are therefore crucial for T-cell activation and the initiation of adaptive immune responses. Recent studies suggest a role for DC in the mediation of adipose tissue inflammation (Bertola et al., 2012; Chen et al., 2014; Stefanovic-Racic et al., 2012).
Much of the studies on DCs in adipose tissue have been conducted in rodent obesity models. Stefanovic-Racic et al demonstrated that young male mice on HFD have an increase in CD11c+ cells in their AT and that a proportion of these CD11c cells are DC (both cDC and pDC) (Stefanovic-Racic et al., 2012). In obese young male mice, with adiposity, the DC population also expands secreting higher levels of IL-6 and IL-23 and promotes a Th17-driven inflammatory response (Bertola et al., 2012; Chen et al., 2014). Clinical studies show that in aged male individuals, the capacity of aged DCs to activate T cells via antigen presentation is impaired and expression of costimulatory molecules and MHC class II is also reportedly downregulated (Wong and Goldstein, 2013). Dendritic cells from elderly subjects also express lower levels of Toll-like receptors (TLRs) and produce lower levels of cytokines in response to specific TLR ligands, while the baseline cytokine levels in absence of TLR ligand stimulation are increased, suggesting dysregulated cytokine expression (Panda et al., 2010; Wong and Goldstein, 2013). Decline in TLR-induced cytokine production in mDCs and pDCs are likely to have a substantial impact on innate immune responses in older individuals, ranging from altered proinflammatory responses mediated by TNF-α and IL-6, to CD4 T cell polarization mediated by IL-12, to type I interferon-dependent antiviral responses (in which pDCs play a crucial role) (Franceschi et al., 2007). Although some of these studies include both men and women, the findings are not classified by sex.
3.4. T cells and B cells
The hallmarks of immune-senescence include impaired responsiveness of adaptive immunity and increases in low-grade and systemic inflamm-aging, which together contribute to aging diseases (Frasca, 2017; Fulop et al., 2017). It was also observed that with increasing age, peripheral blood mononuclear cells (PBMCs) of men and women share genomic signatures including declines in T cell functions and increases in cytotoxic (NK, CD8+ memory T) and monocyte cell functions (Márquez et al., 2020). Aging is therefore, a main driver of variation in PBMC epigenomes/transcriptomes, where age-related variation is negatively associated with naive T cells and positively associated with myeloid lineage and inflammation. This age-associated dysfunction of innate immune cells likely contributes to impaired immune responses to vaccines and infections, as well as to the increases in morbidity and mortality noted in elderly populations (Goronzy and Weyand, 2013).
In male mice, T cells in adipose tissue increase with age (Lumeng et al., 2011a), particularly in middle-aged mice (Ahnstedt et al., 2018; Bapat et al., 2015). Single-cell RNA sequencing of mouse organs at various ages showed that widespread activation of T cells was first detectable in WAT compared with the other tissues in middle-aged mice (Schaum et al., 2020). Adipose tissue in aged mice specifically enrich T regulatory (Treg, Foxp3+CD4+) cells which account for 50% of total CD4+ T cells in adipose tissue (Bapat et al., 2015; Lumeng et al., 2011a). Tregs continuously increase with age in adipose tissue (Bapat et al., 2015; Feuerer et al., 2009; Lumeng et al., 2011a) and exhibit a 7–11-fold increase from young to aged mice (Kalathookunnel Antony et al., 2018). Adipose tissue Treg in aging have a specific transcript profile characterized by upregulation of transcription factors like PPARγ and Gata3, and chemokine and cytokine receptors such as CCR2 and IL1rl1, and proteins involved in lipid metabolism such as DGAT1 and PCYT1a (Cipolletta et al., 2015; Kalathookunnel Antony et al., 2018). During aging, Treg in adipose tissue also have a specific antigen repertoire. they express IL-33 receptor ST2 which is important for their expansion (Bapat et al., 2015; Li et al., 2018). Treg depletion studies in aged mice showed improved insulin sensitivity (Bapat et al., 2015) suggesting that these Tregs may be detrimental in age-related metabolism. However, inflammatory status of mice is not changed by depletion. Although T cells increase in adipose tissue with age, it seems that the age-associated changes do not depend on adiposity (Krishna et al., 2016).
In addition, increases in adipose tissue T cells are affected by sex. In young and middle-aged rodent studies, there was an age-associated increase in adipose tissue CD8+ T cells in both sexes that was augmented by female sex, with middle-aged females having a higher percentage of CD8+ cells than middle-aged males. Middle-aged females had higher numbers of activated (CD69+) CD8+ T cells and produced higher levels of IFN-γ, TNF-α, and granzyme B ex vivo than aged males (Ahnstedt et al., 2018). Interestingly, middle-aged female mice had lower levels of Tregs, an anti-inflammatory T-cell subtype, compared to age-matched males which may promote a pro-inflammatory milieu and contribute to increased cardiovascular disease burden in aging females (Ahnstedt et al., 2018). Depletion of fat-resident Treg cells prevents age-associated insulin resistance, but the mechanism by which adipose Tregs contribute to age-related insulin resistance remain to be investigated (Bapat et al., 2015; Feuerer et al., 2009).
B cells accumulate with age in diverse organs and is especially conspicuous in adipose tissue (Schaum et al., 2020). Specifically, age-associated B cells (ABCs) increase significantly in visceral adipose tissue of aged mice (Frasca and Blomberg, 2020a). ABCs show features of cellular senescence including SASP (Frasca et al., 2017c), and express high levels of proinflammatory cytokines and chemokines. ABCs also express high levels of the lipolytic enzyme, HSL (Anthonsen et al., 1998; Degerman et al., 1990). However, ABCs have decreased expression of the anti-oxidant enzyme superoxide dismutase-1, which suppresses ROS production and oxidative stress, so is anti-inflammatory (McCord and Fridovich, 1988). ABCs from visceral adipose tissue of aged mice produce IgG2c antibodies that have been shown to be autoimmune and pathogenic (Rubtsov et al., 2011b). In clinical studies, a subset of B cells that are known to be inflammatory within B cell subsets named late memory, tissue-like or double negative (DN, CD19+CD27−IgD−) B cells increase in aged individuals (Frasca et al., 2017a; Frasca et al., 2017b). DN B cells do not replicate (Frasca and Blomberg, 2020b) and secrete antibodies specific for autoantigens like dsDNA that increases with age (Niedernhofer et al., 2003). ABCs bearing CD11b and CD11c, but not CD21, was found at a much higher frequency in aged female mice than in young females, or males of any age (Rubtsov et al., 2011a). In humans, global analysis of B cell gene expression signatures reveals that the majority of genes differentially expressed between the sexes that are significantly upregulated in B cells are from adult females compared with males (Fan et al., 2014). However, how these may be changed with aging by sex is still an area of needed investigation.
4. Countermeasures
Aging is considered as a well-established risk factor for several chronic diseases, as well as for decline in physical function and frailty (Franceschi et al., 2018; Slawik and Vidal-Puig, 2006). Like obesity, insulin resistance, poor metabolism, inflammation, and impaired immune function are also hallmarks of aging (Nikolich-Žugich, 2014; Wu et al., 2007). It is likely that changes in function of some immune cells within aged adipose tissue such as the macrophages, T cells and dendritic cells contribute to dysfunction of this tissue (Ahnstedt et al., 2018; Lumeng et al., 2011b; Wong and Goldstein, 2013). Moreover, it is possible that alterations to other cell type profiles such as the preadipocytes and adipocytes within adipose tissue accelerate local and peripheral inflammation that underpin the development of metabolic diseases in aging (Isakson et al., 2009; Keophiphath et al., 2009; Lacasa, 2007). Therefore, targeting adipose tissue aging presents a new opportunity for therapies to combat age-related type II diabetes, metabolic syndrome, and their complications.
There is, however, limited work in an ageing context, especially studies on sex differences are lacking in many aspects. With respect to adaptive immune responses, females generally exhibit greater humoral and cell-mediated immune responses to antigenic stimulation, vaccination, and infection than do males (Hannah, 2008; Klein, 2010). Circulating concentrations of sex steroids, specifically testosterone, estrogens, and progesterone change over the life course and can directly affect immune function. Receptors for sex steroids have been identified in almost all immune cells and can transcriptionally regulate the activity of both innate and adaptive immune cells (Klein and Flanagan, 2016). Production and secretion of cytokines and chemokines, including TNF-α and IL-6, are affected by sex steroids in aging (Pfeilschifter et al., 2002; Starr et al., 2009). Immune responses relevant to the efficacy of TNF inhibitors, vaccines, and checkpoint inhibitors provide evidence that these immunological pathways are affected by sex steroid signaling (Klein, 2010). Significant associations between sex hormones and adipokines are particularly relevant to phenotypes of risk prevalent in postmenopausal women, including metabolic syndrome, CVD, and osteoporosis, which have been linked with sex hormone depletion and increased inflammation in this population (Cicero et al., 2011; Ghosh et al., 2017; Mau et al., 2020; Ouchi et al., 2011; Tworoger et al., 2007).
Countermeasures that target inflammation within dysfunctional adipose tissue offer promise to reduce the burden of age-associated immune-metabolic diseases and to maintain a lasting state of health at old age. Pharmacological therapies (the PPARγ agonist; rosiglitazone) promotes M2-like macrophage re-polarization after HFD overfeeding in mice (Stienstra et al., 2008) and could be a potential therapy for obesity in aging. Further, PPAR signaling overlaps with the network of longevity genes, and PPARγ2 (adipocyte specific)-deficient mice have a considerably reduced lifespan, possibly mediated by the absence of anti-inflammatory effects on adipose tissue by M2-macrophages (Argmann et al., 2009; Stienstra et al., 2008). Therefore, targeting immunological dysregulation in adipose tissue, the ECM, the enzymes that remodel it and the receptors that transduce their signals offers promising therapeutic opportunities for modulating adipose tissue health in old age. Cell-based matrix remodeling can be targeted to improve age-associated comorbidities (Baker et al., 2017). In adipose ECM remodeling, type III and IV collagen appear deleterious, whereas other collagens show protective function in the development of age-associated complications in older women (Karsdal et al., 2016; Kehlet et al., 2018). Specific clearance of p16 positive senescent cells in young obese mice restored adipogenesis, as demonstrated by upregulation of PPAR-γ or C/EBPα, subcutaneous adipose tissue expansion, and improved insulin sensitivity (Ghosh et al., 2019; Palmer et al., 2019). In aging mice, PPARγ agonists have shown to decrease collagen levels in the adipose tissue and confer a more flexible environment for the adipocyte growth and remodeling (Khan et al., 2009).
Only few studies subdivide groups for specific analysis of sex interactions, while in other studies, due to a randomization of the groups and statistical power, a potential sex and age effect is abrogated. Diabetic women were reported to show increased incidence of hypoglycemia under PPARγ agonists - rosiglitazone or pioglitazone treatment, when compared to men, which could be explained by higher sensitivity of the women for the thiazolidines (TZDs) treatment (Patel et al., 1999; Vlckova et al., 2010). In aging mice, interventions with TZDs have shown induction of mitochondrial biogenesis, ROS, and remodeling in the old adipose tissue improving whole-body energy metabolism and insulin sensitivity (Schosserer et al., 2018; Shin et al., 2017). Additionally, long term treatment of 14 month old mice with a PPAR agonist – rosiglitazone extended the median lifespan by 11% (Xu et al., 2020). In insulin resistant men and women, the administration of TZDs significantly increased the plasma adiponectin concentrations (Maeda et al., 2001). Furthermore, a comparison of the effects on lifespan of pioglitazone to the stimulator of insulin secretion – glimepiride (prescribed for the treatment of insulin resistance), demonstrated that patients on pioglitazone have increased survival compared to those on glimepiride. These data suggest that PPARγ ligands may have life-extending effects both in preclinical studies and in human subjects. However, further work is required to identify PPARγ ligand-dependent mechanisms that could be helpful during aging in both sexes. Targeting pathways such as the p38/Ink4a-Arf that rejuvenates beige progenitors and restores beiging potential could be beneficial to overcome senescence of beige adipocytes in aging (Berry et al., 2017).
Recent studies show that caloric restriction (CR) as an intervention induces BAT mass and functionality through PPARγ to increase health lifespan (Graja et al., 2019). In old rats, gender differences were observed, with females showing a higher CR-induced increase in BAT mitochondrial-DNA content (Valle et al., 2008). Similarly, in mice, CR appears to improve age-related declines in BAT, as well as promote browning of WAT; however, these results have only been confirmed in males (Corrales et al., 2019). CR studies in rodents reduced the concentration of detrimental lipid metabolites such as diacylglyceride, ceramide and ROS, thereby limiting potential ligands for inflammatory kinases and receptors. In rodent studies, both short- and long-term CR suppressed age-associated NF-κB signaling (Jung et al., 2009; Kim et al., 2000). CR also enhanced the release of anti-inflammatory adipokine- adiponectin (Zhu 2004), thereby reducing TNF-α expression and promoting a shift in macrophage polarity towards an anti-inflammatory phenotype. CR uniformly decreased Mcp1, Tnf-α, and Il6 expression as well as significantly lowered senescent cell accumulation in the adipose tissue of both sexes (Mau et al., 2020). In other studies, methionine restriction (MR) in diets by 80% extends lifespan by ~25% in rats (Richie et al., 1994) and mice (Miller et al., 2005) by delaying all causes of death. Dietary MR was also effective in visceral fat reduction and preserving insulin action in aging rats (Forney et al., 2020; Hasek et al., 2010; Malloy et al., 2006; Plaisance et al., 2010). In mice, the metabolic effects of dietary MR are accompanied by transcriptional responses in liver and adipose tissue that reduce circulating and tissue lipids and modify endocrine function of adipose tissue (Hasek et al., 2010; Plaisance et al., 2010). Transcriptional studies showed that anti-inflammatory responses produced by CR and MR were not strictly dependent upon reduced adiposity but were significantly influenced by the metabolic mechanisms through which energy balance is altered (Wanders et al., 2014). Understanding the molecular alterations that determine impaired energy balance and adipose tissue plasticity may identify therapeutic targets to optimize adipose tissue expandability and function in old age.
5. Conclusions
Adipose tissue has gained increasing attention in recent years for not only driving metabolic disease but playing an important role in energy, function, and immune regulation. It is increasingly important to acknowledge sex differences in immune responses with marked differences observed between males and females in various diseases. The link between adipose inflammation and aging may be the result of sexual dimorphism in lipid metabolism. It is evident that there are sex differences in lipid profiles including plasma levels of cholesterol and TG. High TG and lipoproteins are more important risk factors in women than men. For optimal prevention or treatment of CVD in old age, attention must be paid to the importance of gender related differences in lipid control when considering nutritional recommendations and sex specific therapies in older subjects. Future studies need to further explore sex-specific energy metabolism that may have clinical implications in old age. Accordingly, it is imperative to use both male and female animal models for studying the role of sex hormones. These studies will aid in providing more evidence of gender disparities in the control of lipid metabolism to help translate into clinical applicability for both men and women. Understanding the genetic and molecular mechanisms of adipose distribution and its sexual dimorphism in humans provides a unique opportunity to promote the use of precision medicine for early identification of at-risk individuals, and the development of novel therapeutic strategies for central obesity and related cardiometabolic disorders in old age.
Given the prevalence of sex difference in humans in longevity and health span, investigations into the molecular and cellular basis for senescence is also crucial for the development of novel and effective interventions. Future studies should address how adipose tissue ECM relates to age-associated endocrine and neurohormonal changes and vice versa. Deconvolution of the relationships among local and systemic inflammation, adipocyte differentiation, adipocyte size, ECM deposition and metabolic dysregulation in aging is clearly needed. Improving our understanding of the interactions between ageing and adipose tissue dysfunction may help with the development of therapeutic and preventative strategies to improve longevity and quality of life in ageing populations.
Highlights.
Adipose tissue dysfunction and subsequent inflammation contribute to the elevated risk of chronic disease, disability, and adverse health outcomes with advancing age.
Aging is associated with increased visceral adiposity, aberrant adipogenesis, altered adipokine secretion and insulin resistance.
Sex differences are observed in adipose tissue re-modeling, collagen deposition and cellular senescence in aging.
Age-related changes in sex steroid hormones lead to changes in immune cell numbers and function of macrophages, dendritic cells, and T and B cells.
Countermeasures that target aging in adipose tissue offer promise to reduce the burden of age-associated immune-metabolic diseases and to maintain a lasting state of health.
Funding
This work was supported by Claude D. Pepper Older Americans Independence Center/Michigan Biology of Cardiovascular Aging pilot award (AG024824/UL1TR002240) and NIH/NIDDK R01 DK115583.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
The authors declare no conflict of interest.
References
- Agarwal S, Busse PJ, 2010. Innate and adaptive immunosenescence. Ann Allergy Asthma Immunol. 104, 183–90; quiz 190–2, 210. [DOI] [PubMed] [Google Scholar]
- Ahnstedt H, et al. , 2018. Sex Differences in Adipose Tissue CD8(+) T Cells and Regulatory T Cells in Middle-Aged Mice. Front Immunol. 9, 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthonsen MW, et al. , 1998. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem. 273, 215–21. [DOI] [PubMed] [Google Scholar]
- Ardawi MS, Rouzi AA, 2005. Plasma adiponectin and insulin resistance in women with polycystic ovary syndrome. Fertil Steril. 83, 1708–16. [DOI] [PubMed] [Google Scholar]
- Argmann C, et al. , 2009. Ppargamma2 is a key driver of longevity in the mouse. PLoS Genet. 5, e1000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austad SN, Fischer KE, 2016. Sex Differences in Lifespan. Cell Metab. 23, 1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, et al. , 2016. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 530, 184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NA, et al. , 2017. Diabetes-Specific Regulation of Adipocyte Metabolism by the Adipose Tissue Extracellular Matrix. The Journal of Clinical Endocrinology & Metabolism. 102, 1032–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, et al. , 2000. Immunobiology of dendritic cells. Annu Rev Immunol. 18, 767–811. [DOI] [PubMed] [Google Scholar]
- Banerjee RR, et al. , 2004. Regulation of fasted blood glucose by resistin. Science. 303, 1195–8. [DOI] [PubMed] [Google Scholar]
- Bapat SP, et al. , 2015. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature. 528, 137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barquissau V, et al. , 2016. White-to-brite conversion in human adipocytes promotes metabolic reprogramming towards fatty acid anabolic and catabolic pathways. Mol Metab. 5, 352–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerril S, et al. , 2013. Role of PRDM16 in the activation of brown fat programming. Relevance to the development of obesity. Histol Histopathol. 28, 1411–25. [DOI] [PubMed] [Google Scholar]
- Beenakker KGM, et al. , 2020. Men Have a Stronger Monocyte-Derived Cytokine Production Response upon Stimulation with the Gram-Negative Stimulus Lipopolysaccharide than Women: A Pooled Analysis Including 15 Study Populations. Journal of Innate Immunity. 12, 142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DC, et al. , 2017. Cellular Aging Contributes to Failure of Cold-Induced Beige Adipocyte Formation in Old Mice and Humans. Cell Metab. 25, 166–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertola A, et al. , 2012. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 61, 2238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bietti GB, Pannarale C, Milano C, 1967. Further contributions to the intermittent therapy of trachoma with new long-acting sulfonamides. Am J Ophthalmol. 63, Suppl:1569–73. [DOI] [PubMed] [Google Scholar]
- Björntorp P, 1996. The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord. 20, 291–302. [PubMed] [Google Scholar]
- Bolton-Smith C, Woodward M, Tavendale R, 1997. Evidence for age-related differences in the fatty acid composition of human adipose tissue, independent of diet. Eur J Clin Nutr. 51, 619–24. [DOI] [PubMed] [Google Scholar]
- Bonafè M, et al. , 2001. A gender--dependent genetic predisposition to produce high levels of IL-6 is detrimental for longevity. Eur J Immunol. 31, 2357–61. [DOI] [PubMed] [Google Scholar]
- Bonnans C, Chou J, Werb Z, 2014. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 15, 786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragdon B, et al. , 2015. Intrinsic Sex-Linked Variations in Osteogenic and Adipogenic Differentiation Potential of Bone Marrow Multipotent Stromal Cells. J Cell Physiol. 230, 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LM, Clegg DJ, 2010. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J Steroid Biochem Mol Biol. 122, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannizzo ES, Clement CC,, Sahu R, Follo C, Santambrogio L, 2011. Oxidative stress, inflamm-aging and immunosenescence. J Proteomics. 74, 2313–2323. [DOI] [PubMed] [Google Scholar]
- Cartwright MJ, Tchkonia T, Kirkland JL, 2007. Aging in adipocytes: potential impact of inherent, depot-specific mechanisms. Experimental gerontology. 42, 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright MJ, et al. , 2010. Aging, depot origin, and preadipocyte gene expression. J Gerontol A Biol Sci Med Sci. 65, 242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caso G, et al. , 2013. Peripheral fat loss and decline in adipogenesis in older humans. Metabolism. 62, 337–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo-Branco C, Soveral I, 2014. The immune system and aging: a review. Gynecol Endocrinol. 30, 16–22. [DOI] [PubMed] [Google Scholar]
- Cawthorn, William P, et al. , 2014. Bone Marrow Adipose Tissue Is an Endocrine Organ that Contributes to Increased Circulating Adiponectin during Caloric Restriction. Cell Metabolism. 20, 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E, Varghese M, Singer K, 2018. Gender and Sex Differences in Adipose Tissue. Curr Diab Rep. 18, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charalambous C, et al. , 2007. Glioma-associated endothelial cells show evidence of replicative senescence. Exp Cell Res. 313, 1192–202. [DOI] [PubMed] [Google Scholar]
- Chaurasia B, Summers SA, 2015. Ceramides - Lipotoxic Inducers of Metabolic Disorders. Trends Endocrinol Metab. 26, 538–550. [DOI] [PubMed] [Google Scholar]
- Chen Y, et al. , 2014. Adipose tissue dendritic cells enhances inflammation by prompting the generation of Th17 cells. PLoS One. 9, e92450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs BG, et al. , 2015. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 21, 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, et al. , 2000. Expression of senescence-associated beta-galactosidase in enlarged prostates from men with benign prostatic hyperplasia. Urology. 56, 160–6. [DOI] [PubMed] [Google Scholar]
- Cicero AF, et al. , 2011. Sex hormones and adipokines in healthy pre-menopausal, post-menopausal and elderly women, and in age-matched men: data from the Brisighella Heart study. J Endocrinol Invest. 34, e158–62. [DOI] [PubMed] [Google Scholar]
- Cipolletta D, et al. , 2015. Appearance and disappearance of the mRNA signature characteristic of Treg cells in visceral adipose tissue: age, diet, and PPARgamma effects. Proc Natl Acad Sci U S A. 112, 482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, et al. , 2006. Gonadal Hormones Determine Sensitivity to Central Leptin and Insulin. Diabetes. 55, 978. [DOI] [PubMed] [Google Scholar]
- Coppe JP, et al. , 2008. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales P, et al. , 2019. Long-term caloric restriction ameliorates deleterious effects of aging on white and brown adipose tissue plasticity. Aging Cell. 18, e12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, et al. , 2009a. Identification and Importance of Brown Adipose Tissue in Adult Humans. New England Journal of Medicine. 360, 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, et al. , 2009b. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 360, 1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta R, Podolsky MJ, Atabai K, 2018. Fat fibrosis: friend or foe? JCI Insight. 3, e122289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KE, et al. , 2013. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Met. 2, 227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, 2004. Dysfunctional fat cells, lipotoxicity and type 2 diabetes. Int J Clin Pract Suppl. 9–21. [DOI] [PubMed] [Google Scholar]
- Degerman E, et al. , 1990. Evidence that insulin and isoprenaline activate the cGMP-inhibited low-Km cAMP phosphodiesterase in rat fat cells by phosphorylation. Proc Natl Acad Sci U S A. 87, 533–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi K, et al. , 2001. Postmenopausal changes in production of type 1 and type 2 cytokines and the effects of hormone replacement therapy. Menopause. 8, 266–273. [DOI] [PubMed] [Google Scholar]
- Derby CA, et al. , 2006. Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: the Massachusetts Male Ageing Study. Clin Endocrinol (Oxf). 65, 125–31. [DOI] [PubMed] [Google Scholar]
- Dieudonne MN, et al. , 1998. Androgen receptors in human preadipocytes and adipocytes: regional specificities and regulation by sex steroids. Am J Physiol. 274, C1645–52. [DOI] [PubMed] [Google Scholar]
- Dieudonné MN, et al. , 2004. Evidence for functional estrogen receptors alpha and beta in human adipose cells: regional specificities and regulation by estrogens. Am J Physiol Cell Physiol. 286, C655–61. [DOI] [PubMed] [Google Scholar]
- Dolinková M, et al. , 2008. The endocrine profile of subcutaneous and visceral adipose tissue of obese patients. Mol Cell Endocrinol. 291, 63–70. [DOI] [PubMed] [Google Scholar]
- Elgazar-Carmon V, et al. , 2008. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 49, 1894–903. [DOI] [PubMed] [Google Scholar]
- Espeland MA, et al. , 1997. Effect of postmenopausal hormone therapy on body weight and waist and hip girths. Postmenopausal Estrogen-Progestin Interventions Study Investigators. J Clin Endocrinol Metab. 82, 1549–56. [DOI] [PubMed] [Google Scholar]
- Estrany ME, et al. , 2013. High-fat diet feeding induces sex-dependent changes in inflammatory and insulin sensitivity profiles of rat adipose tissue. Cell Biochem Funct. 31, 504–10. [DOI] [PubMed] [Google Scholar]
- Fan H, et al. , 2014. Gender Differences of B Cell Signature in Healthy Subjects Underlie Disparities in Incidence and Course of SLE Related to Estrogen. Journal of Immunology Research. 2014, 814598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner JL, Belin de Chantemèle EJ, 2019. Sex hormones, aging and cardiometabolic syndrome. Biology of Sex Differences. 10, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Alfonso MS, et al. , 2017. Role of Perivascular Adipose Tissue in Health and Disease. Compr Physiol. 8, 23–59. [DOI] [PubMed] [Google Scholar]
- Feuerer M, et al. , 2009. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 15, 930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filková M, et al. , 2009. The role of resistin as a regulator of inflammation: Implications for various human pathologies. Clin Immunol. 133, 157–70. [DOI] [PubMed] [Google Scholar]
- Fischer KE, Riddle NC, 2018. Sex Differences in Aging: Genomic Instability. J Gerontol A Biol Sci Med Sci. 73, 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez-Duquet M, Horwitz BA, McDonald RB, 1998. Cellular proliferation and UCP content in brown adipose tissue of cold-exposed aging Fischer 344 rats. Am J Physiol. 274, R196–203. [DOI] [PubMed] [Google Scholar]
- Forney LA, et al. , 2020. Sexually Dimorphic Effects of Dietary Methionine Restriction are Dependent on Age when the Diet is Introduced. Obesity (Silver Spring). 28, 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, et al. , 2007. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 128, 92–105. [DOI] [PubMed] [Google Scholar]
- Franceschi C, et al. , 2018. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front Med (Lausanne). 5, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank AP, et al. , 2019. Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J Lipid Res. 60, 1710–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, et al. , 2017a. Human peripheral late/exhausted memory B cells express a senescent-associated secretory phenotype and preferentially utilize metabolic signaling pathways. Exp Gerontol. 87, 113–120. [DOI] [PubMed] [Google Scholar]
- Frasca D, et al. , 2017b. Aging effects on T-bet expression in human B cell subsets. Cell Immunol. 321, 68–73. [DOI] [PubMed] [Google Scholar]
- Frasca D, et al. , 2017c. Obesity induces pro-inflammatory B cells and impairs B cell function in old mice. Mech Ageing Dev. 162, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Blomberg BB, 2020a. Adipose tissue, immune aging, and cellular senescence. Semin Immunopathol. 42, 573–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Blomberg BB, 2020b. Obesity Accelerates Age Defects in Mouse and Human B Cells. Front Immunol. 11, 2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Blomberg BB, Paganelli R, 2017. Aging, obesity, and inflammatory age-related diseases Front Immunol. 8, 1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A, et al. , 2010. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 16, 238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL, 1998. Leptin and the regulation of body weight in mammals. Nature. 395, 763–70. [DOI] [PubMed] [Google Scholar]
- Frontini A, Cinti S, 2010. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 11, 253–6. [DOI] [PubMed] [Google Scholar]
- Frühbeck G, Méndez-Giménez L, Fernández-Formoso JA, Fernández S, Rodríguez A, 2014. Regulation of adipocyte lipolysis. Nutrition Research Reviews. 27, 63–93. [DOI] [PubMed] [Google Scholar]
- Fujioka K, et al. , 2012. Dehydroepiandrosterone reduces preadipocyte proliferation via androgen receptor. Am J Physiol Endocrinol Metab. 302, E694–704. [DOI] [PubMed] [Google Scholar]
- Fulop T, et al. , 2017. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front Immunol. 8, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannagé-Yared MH, et al. , 2006. Serum adiponectin and leptin levels in relation to the metabolic syndrome, androgenic profile and somatotropic axis in healthy non-diabetic elderly men. Eur J Endocrinol. 155, 167–76. [DOI] [PubMed] [Google Scholar]
- Gavrila A, et al. , 2003. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab. 88, 4823–31. [DOI] [PubMed] [Google Scholar]
- Geer EB, Shen W, 2009. Gender differences in insulin resistance, body composition, and energy balance. Gender Medicine. 6, 60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gencer B, et al. , 2016. Association between resistin levels and cardiovascular disease events in older adults: The health, aging and body composition study. Atherosclerosis. 245, 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK, et al. , 2015. Elevated Endoplasmic Reticulum Stress Response Contributes to Adipose Tissue Inflammation in Aging. J Gerontol A Biol Sci Med Sci. 70, 1320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK, et al. , 2017. Toll-like receptor 4 (TLR4) deficient mice are protected from adipose tissue inflammation in aging. Aging (Albany NY). 9, 1971–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK, et al. , 2019. Adipose Tissue Senescence and Inflammation in Aging is Reversed by the Young Milieu. J Gerontol A Biol Sci Med Sci. 74, 1709–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg IJ, Merkel M, 2001. Lipoprotein lipase: physiology, biochemistry, and molecular biology. Front Biosci. 6, D388–405. [DOI] [PubMed] [Google Scholar]
- Gonçalves LF, et al. , 2017. Ageing is associated with brown adipose tissue remodelling and loss of white fat browning in female C57BL/6 mice. International journal of experimental pathology. 98, 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens GH, 2008. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiology & Behavior. 94, 206–218. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, Weyand CM, 2013. Understanding immunosenescence to improve responses to vaccines. Nature immunology. 14, 428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graja A, Gohlke S, Schulz TJ, 2019. Aging of Brown and Beige/Brite Adipose Tissue. Handb Exp Pharmacol. 251, 55–72. [DOI] [PubMed] [Google Scholar]
- Grunfeld C, et al. , 1996. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest. 97, 2152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui Y, Silha JV, Murphy LJ, 2004. Sexual dimorphism and regulation of resistin, adiponectin, and leptin expression in the mouse. Obes Res. 12, 1481–91. [DOI] [PubMed] [Google Scholar]
- Haffner SM, et al. , 1994. Insulin resistance, body fat distribution, and sex hormones in men. Diabetes. 43, 212–9. [DOI] [PubMed] [Google Scholar]
- Hannah MF, Bajic VB & Klein SL, 2008. Sex differences in the recognition of and innate antiviral responses to Seoul virus in Norway rats. . Brain Behav. Immun, 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TB, et al. , 1999. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 106, 506–12. [DOI] [PubMed] [Google Scholar]
- Hasek BE, et al. , 2010. Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. Am J Physiol Regul Integr Comp Physiol. 299, R728–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, et al. , 2004. Relationship between serum resistin concentrations and insulin resistance in nonobese, obese, and obese diabetic subjects. J Clin Endocrinol Metab. 89, 1844–8. [DOI] [PubMed] [Google Scholar]
- Heine PA TJ, Iwamoto GA, Lubahn DB, Cooke PS, 2000. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci U S A. 97, 12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström L, et al. , 2000. Mechanisms behind gender differences in circulating leptin levels. J Intern Med. 247, 457–62. [DOI] [PubMed] [Google Scholar]
- Hoffman BB, et al. , 1984. Age-related decrement in hormone-stimulated lipolysis. American Journal of Physiology-Endocrinology and Metabolism. 247, E772–E777. [DOI] [PubMed] [Google Scholar]
- Imahara SD, et al. , 2005. The influence of gender on human innate immunity. Surgery. 138, 275–82. [DOI] [PubMed] [Google Scholar]
- Isakson P, et al. , 2009. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 58, 1550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R, 2004. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 5, 987–95. [DOI] [PubMed] [Google Scholar]
- Jackson AS, et al. , 2002. The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study. Int J Obes Relat Metab Disord. 26, 789–96. [DOI] [PubMed] [Google Scholar]
- Janssen I, et al. , 2010. Testosterone and visceral fat in midlife women: the Study of Women’s Health Across the Nation (SWAN) fat patterning study. Obesity (Silver Spring). 18, 604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyapalan JC, Sedivy JM, 2008. Cellular senescence and organismal aging. Mech Ageing Dev. 129, 467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JA, et al. , 2001. Impaired insulin action in subcutaneous adipocytes from women with visceral obesity. Am J Physiol Endocrinol Metab. 280, E40–9. [DOI] [PubMed] [Google Scholar]
- Joyner J, Hutley L, Cameron D, 2002. Intrinsic regional differences in androgen receptors and dihydrotestosterone metabolism in human preadipocytes. Horm Metab Res. 34, 223–8. [DOI] [PubMed] [Google Scholar]
- Jung KJ, et al. , 2009. Effect of short term calorie restriction on pro-inflammatory NF-kB and AP-1 in aged rat kidney. Inflamm Res. 58, 143–50. [DOI] [PubMed] [Google Scholar]
- Kalathookunnel Antony A, Lian Z, Wu H, 2018. T Cells in Adipose Tissue in Aging. Front Immunol. 9, 2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannides I, et al. , 2001. Altered expression of C/EBP family members results in decreased adipogenesis with aging. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 280, R1772–R1780. [DOI] [PubMed] [Google Scholar]
- Karagiannides I, et al. , 2006. Increased CUG triplet repeat-binding protein-1 predisposes to impaired adipogenesis with aging. J Biol Chem. 281, 23025–33. [DOI] [PubMed] [Google Scholar]
- Karim R, et al. , 2015. Association of endogenous sex hormones with adipokines and ghrelin in postmenopausal women. The Journal of clinical endocrinology and metabolism. 100, 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsdal MA, et al. , 2016. Collagen and tissue turnover as a function of age: Implications for fibrosis. J Hepatol. 64, 103–9. [DOI] [PubMed] [Google Scholar]
- Kehlet SN, et al. , 2018. Age-related collagen turnover of the interstitial matrix and basement membrane: Implications of age- and sex-dependent remodeling of the extracellular matrix. . PLoS One. 13, e0194458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keophiphath M, et al. , 2009. Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol Endocrinol. 23, 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes WM, et al. , 2005. p63 deficiency activates a program of cellular senescence and leads to accelerated aging. Genes Dev. 19, 1986–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan T, et al. , 2009. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 29, 1575–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, et al. , 2000. The effect of age on cyclooxygenase-2 gene expression: NF-kappaB activation and IkappaBalpha degradation. Free Radic Biol Med. 28, 683–92. [DOI] [PubMed] [Google Scholar]
- Kim S-N, et al. , 2016. Sex differences in sympathetic innervation and browning of white adipose tissue of mice. Biology of Sex Differences. 7, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, et al. , 2002. Adipogenesis and aging: does aging make fat go MAD? Exp Gerontol. 37, 757–67. [DOI] [PubMed] [Google Scholar]
- Kirkland JL, 2013. Translating advances from the basic biology of aging into clinical application. Experimental Gerontology. 48, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Flanagan KL, 2016. Sex differences in immune responses. Nat Rev Immunol. 16, 626–38. [DOI] [PubMed] [Google Scholar]
- Klein SL, Jedlicka A & Pekosz A, 2010. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis, 338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsari C, et al. , 2011. Nonoxidative free fatty acid disposal is greater in young women than men. J Clin Endocrinol Metab. 96, 541–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Králová Lesná I, et al. , 2015. Macrophage subsets in the adipose tissue could be modified by sex and the reproductive age of women. Atherosclerosis. 241, 255–258. [DOI] [PubMed] [Google Scholar]
- Krishna KB, et al. , 2016. Similar degrees of obesity induced by diet or aging cause strikingly different immunologic and metabolic outcomes. Physiol Rep. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krtolica A, Campisi J, 2002. Cancer and aging: a model for the cancer promoting effects of the aging stroma. Int J Biochem Cell Biol. 34, 1401–14. [DOI] [PubMed] [Google Scholar]
- Kumada M, et al. , 2004. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 109, 2046–9. [DOI] [PubMed] [Google Scholar]
- Lacasa D, Taleb S, Keophiphath M, Miranville A, Clement K, 2007. Macrophage-secreted factors impair human adipogenesis: involvement of proinflammatory state in preadipocytes. Endocrinology. 148, 868–877. [DOI] [PubMed] [Google Scholar]
- Lafontan M, Langin D, 2009. Lipolysis and lipid mobilization in human adipose tissue. Progress in Lipid Research 48, 275–297. [DOI] [PubMed] [Google Scholar]
- Lanfranco F, et al. , 2004. Serum adiponectin levels in hypogonadal males: influence of testosterone replacement therapy. Clin Endocrinol (Oxf). 60, 500–7. [DOI] [PubMed] [Google Scholar]
- Lee JH, et al. , 2003. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab. 88, 4848–56. [DOI] [PubMed] [Google Scholar]
- Lee M-J, Fried SK, 2010. Depot-Specific Biology of Adipose Tissues: Links to Fat Distribution and Metabolic Risk. In: Adipose Tissue in Health and Disease. Vol., ed.êds., pp. 283–306. [Google Scholar]
- Lee M-J, Wu Y, Fried SK, 2013. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Molecular aspects of medicine. 34, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre N, et al. , 2012. Sex differences in inflammatory cytokines and CD99 expression following in vitro lipopolysaccharide stimulation. Shock. 38, 37–42. [DOI] [PubMed] [Google Scholar]
- Li C, et al. , 2018. TCR Transgenic Mice Reveal Stepwise, Multi-site Acquisition of the Distinctive Fat-Treg Phenotype. Cell. 174, 285–299 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HY, et al. , 2005. Insulin and leptin resistance with hyperleptinemia in mice lacking androgen receptor. Diabetes. 54, 1717–25. [DOI] [PubMed] [Google Scholar]
- Lönnqvist F, et al. , 1990. Catecholamine-induced lipolysis in adipose tissue of the elderly. The Journal of clinical investigation. 85, 1614–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord GM, et al. , 1998. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 394, 897–901. [DOI] [PubMed] [Google Scholar]
- Lottenberg AM, et al. , 2012. The role of dietary fatty acids in the pathology of metabolic syndrome. J Nutr Biochem. 23, 1027–40. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR, 2007a. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of Clinical Investigation. 117, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR, 2007b. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 117, 175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, et al. , 2007c. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 56, 16–23. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, et al. , 2008. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 57, 3239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, et al. , 2011a. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol. 187, 6208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, et al. , 2011b. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol. 187, 6208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luukkaa V, et al. , 1998. Inverse correlation between serum testosterone and leptin in men. J Clin Endocrinol Metab. 83, 3243–6. [DOI] [PubMed] [Google Scholar]
- Ma J, et al. , 2018. Reversion of aging-related DHEAS decline in mouse plasma alleviates aging-related glucose tolerance impairment by potentiation of glucose-stimulated insulin secretion of acute phase. Biochem Biophys Res Commun. 500, 671–675. [DOI] [PubMed] [Google Scholar]
- Mack I, et al. , 2009. Functional analyses reveal the greater potency of preadipocytes compared with adipocytes as endothelial cell activator under normoxia, hypoxia, and TNFalpha exposure. Am J Physiol Endocrinol Metab. 297, E735–48. [DOI] [PubMed] [Google Scholar]
- Macotela Y, et al. , 2009. Sex and Depot Differences in Adipocyte Insulin Sensitivity and Glucose Metabolism. Diabetes. 58, 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N, et al. , 2001. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 50, 2094–9. [DOI] [PubMed] [Google Scholar]
- Malloy VL, et al. , 2006. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell. 5, 305–14. [DOI] [PubMed] [Google Scholar]
- Manwani B, et al. , 2013. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp Neurol. 249, 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márquez EJ, et al. , 2020. Sexual-dimorphism in human immune system aging. Nat Commun. 11, 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Santibanez G, et al. , 2015. Obesity-induced remodeling of the adipose tissue elastin network is independent of the metalloelastase MMP-12. Adipocyte. 4, 264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarese G, et al. , 2001. Requirement for leptin in the induction and progression of autoimmune encephalomyelitis. J Immunol. 166, 5909–16. [DOI] [PubMed] [Google Scholar]
- Mau T, et al. , 2020. Life-span Extension Drug Interventions Affect Adipose Tissue Inflammation in Aging. J Gerontol A Biol Sci Med Sci. 75, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury E, Brichard SM, 2010. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 314, 1–16. [DOI] [PubMed] [Google Scholar]
- Mayes JS, Watson GH, 2004. Direct effects of sex steroid hormones on adipose tissues and obesity. Obesity Reviews. 5, 197–216. [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I, 1988. Superoxide dismutase: the first twenty years (1968–1988). Free Radic Biol Med. 5, 363–9. [DOI] [PubMed] [Google Scholar]
- McInnes KJ, et al. , 2012. Deletion of the androgen receptor in adipose tissue in male mice elevates retinol binding protein 4 and reveals independent effects on visceral fat mass and on glucose homeostasis. Diabetes. 61, 1072–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrikova D, et al. , 2012. Sex differences during the course of diet-induced obesity in mice: adipose tissue expandability and glycemic control. Int J Obes (Lond). 36, 262–72. [DOI] [PubMed] [Google Scholar]
- Meikle PJ, Summers SA, 2017. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat Rev Endocrinol. 13, 79–91. [DOI] [PubMed] [Google Scholar]
- Merad M, et al. , 2013. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 31, 563–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, et al. , 2011. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors Acta Physiologica (Oxf). 203, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Haas E, and Barton M, 2006. Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. . Hypertension. 1019–1026. [DOI] [PubMed] [Google Scholar]
- Miller RA, et al. , 2005. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 4, 119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T, et al. , 2009. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med. 15, 1082–7. [DOI] [PubMed] [Google Scholar]
- Mitterberger MC, et al. , 2014. Adipogenic differentiation is impaired in replicative senescent human subcutaneous adipose-derived stromal/progenitor cells. J Gerontol A Biol Sci Med Sci. 69, 13–24. [DOI] [PubMed] [Google Scholar]
- Moran LJ, Norman RJ, Teede HJ, 2015. Metabolic risk in PCOS: phenotype and adiposity impact. Trends in Endocrinology & Metabolism. 26, 136–143. [DOI] [PubMed] [Google Scholar]
- Morin CL, et al. , 1997. Adipose tissue-derived tumor necrosis factor-alpha activity is elevated in older rats. J Gerontol A Biol Sci Med Sci. 52, B190–5. [DOI] [PubMed] [Google Scholar]
- Morishima A, et al. , 1995. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. The Journal of Clinical Endocrinology & Metabolism. 80, 3689–3698. [DOI] [PubMed] [Google Scholar]
- Morris DL, Singer K, Lumeng CN, 2011. Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states. Current Opinion in Clinical Nutrition and Metabolic Care. 14, 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir LA, et al. , 2018. Rapid adipose tissue expansion triggers unique proliferation and lipid accumulation profiles in adipose tissue macrophages. Journal of Leukocyte Biology. 103, 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J, Derstine A, Gower BA, 2002. Fat distribution and insulin sensitivity in postmenopausal women: influence of hormone replacement. Obes Res. 10, 424–31. [DOI] [PubMed] [Google Scholar]
- Musatov S, et al. , 2007. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A. 104, 2501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagareddy PR, et al. , 2014. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 19, 821–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedernhofer LJ, et al. , 2003. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J Biol Chem. 278, 31426–33. [DOI] [PubMed] [Google Scholar]
- Nikolich-Žugich J, 2014. Aging of the T cell compartment in mice and humans: from no naive expectations to foggy memories. J Immunol. 193, 2622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida C, Ko GT, Kumanyika S, 2010. Body fat distribution and noncommunicable diseases in populations: overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist-Hip Ratio. Eur J Clin Nutr. 64, 2–5. [DOI] [PubMed] [Google Scholar]
- Nishizawa H, et al. , 2002. Androgens Decrease Plasma Adiponectin, an Insulin-Sensitizing Adipocyte-Derived Protein. Diabetes. 51, 2734. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, et al. , 2017. Increased adipose tissue aromatase activity improves insulin sensitivity and reduces adipose tissue inflammation in male mice. Am J Physiol Endocrinol Metab. 313, E450–e462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, et al. , 2001. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 103, 1057–63. [DOI] [PubMed] [Google Scholar]
- Ouchi N, et al. , 2011. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 11, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ST, et al. , 2005. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 90, 1502–10. [DOI] [PubMed] [Google Scholar]
- Palmer AK, et al. , 2015. Cellular Senescence in Type 2 Diabetes: A Therapeutic Opportunity. Diabetes. 64, 2289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AK, Kirkland JL, 2016. Aging and adipose tissue: potential interventions for diabetes and regenerative medicine. Exp Gerontol. 86, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AK, et al. , 2019. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. 18, e12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer BF, Clegg DJ, 2015. The sexual dimorphism of obesity. Molecular and Cellular Endocrinology. 402, 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda A, et al. , 2010. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 184, 2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrinello S, et al. , 2005. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 118, 485–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasarica M, et al. , 2009. Adipose Tissue Collagen VI in Obesity. The Journal of Clinical Endocrinology & Metabolism. 94, 5155–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos JF, et al. , 2010. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol. 6, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J, Anderson R, Rappaport E, 1999. Rosiglitazone monotherapy improves glycaemic control in patients with type 2 diabetes: a twelve week, randomized, placeb• controlled study. Diabetes. 1. [DOI] [PubMed] [Google Scholar]
- Pedersen SB, et al. , 1991. Nuclear estradiol binding in rat adipocytes. Regional variations and regulatory influences of hormones. Biochim Biophys Acta. 1093, 80–6. [DOI] [PubMed] [Google Scholar]
- Pfannenberg C, et al. , 2010. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 59, 1789–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J, et al. , 2002. Changes in proinflammatory cytokine activity after menopause. Endocrinol Rev. 23, 90–119. [DOI] [PubMed] [Google Scholar]
- Plaisance EP, et al. , 2010. Role of beta-adrenergic receptors in the hyperphagic and hypermetabolic responses to dietary methionine restriction. Am J Physiol Regul Integr Comp Physiol. 299, R740–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poledne R, et al. , 2019. Polarization of Macrophages in Human Adipose Tissue is Related to the Fatty Acid Spectrum in Membrane Phospholipids. Nutrients. 12, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AD, 2014. Sex differences in the metabolic syndrome: implications for cardiovascular health in women. Clin Chem. 60, 44–52. [DOI] [PubMed] [Google Scholar]
- Qatanani M, et al. , 2009. Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J Clin Invest. 119, 531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, et al. , 2006. Loss of resistin improves glucose homeostasis in leptin deficiency. Diabetes. 55, 3083–90. [DOI] [PubMed] [Google Scholar]
- Richie JP Jr., et al. , 1994. Methionine restriction increases blood glutathione and longevity in F344 rats. Faseb j. 8, 1302–7. [DOI] [PubMed] [Google Scholar]
- Rodier F, Campisi J, 2011. Four faces of cellular senescence. J Cell Biol. 192, 547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodondi N, et al. , 2010. Markers of atherosclerosis and inflammation for prediction of coronary heart disease in older adults. Am J Epidemiol. 171, 540–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Cuenca S, et al. , 2002. Sex-dependent thermogenesis, differences in mitochondrial morphology and function, and adrenergic response in brown adipose tissue. J Biol Chem. 277, 42958–63. [DOI] [PubMed] [Google Scholar]
- Roos CM, et al. , 2016. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 15, 973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM, 2014. What we talk about when we talk about fat. Cell. 156, 20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubenoff R, et al. , 1998. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci. 53, M20–6. [DOI] [PubMed] [Google Scholar]
- Rubtsov AV, et al. , 2011a. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c⁺ B-cell population is important for the development of autoimmunity. Blood. 118, 1305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov AV, et al. , 2011b. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood. 118, 1305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski JM, Stern JH, Scherer PE, 2015. The cell biology of fat expansion. J Cell Biol. 208, 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryo M, et al. , 2004. Adiponectin as a biomarker of the metabolic syndrome. Circ J. 68, 975–81. [DOI] [PubMed] [Google Scholar]
- Sadashiv, et al. , 2012. Over expression of resistin in adipose tissue of the obese induces insulin resistance. World journal of diabetes. 3, 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter SR, et al. , 2006. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 8, 538–54. [DOI] [PubMed] [Google Scholar]
- Samaras K, et al. , 2010. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity (Silver Spring). 18, 884–9. [DOI] [PubMed] [Google Scholar]
- Santosa S, et al. , 2008. The influence of sex and obesity phenotype on meal fatty acid metabolism before and after weight loss. Am J Clin Nutr. 88, 1134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MJ, et al. , 2016. Exercise Prevents Diet-Induced Cellular Senescence in Adipose Tissue. Diabetes. 65, 1606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaum N, et al. , 2020. Ageing hallmarks exhibit organ-specific temporal signatures. Nature. 583, 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomberg DW, et al. , 1999. Targeted disruption of the estrogen receptor-alpha gene in female mice: characterization of ovarian responses and phenotype in the adult. Endocrinology. 140, 2733–44. [DOI] [PubMed] [Google Scholar]
- Schosserer M, et al. , 2018. Age-Induced Changes in White, Brite, and Brown Adipose Depots: A Mini-Review. Gerontology. 64, 229–236. [DOI] [PubMed] [Google Scholar]
- Sepa-Kishi DM, Ceddia RB, 2018. White and beige adipocytes: are they metabolically distinct? Horm Mol Biol Clin Investig. 33. [DOI] [PubMed] [Google Scholar]
- Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL, 2011. Aging and regional differences in fat cell progenitors - a mini-review. Gerontology. 57, 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin W, et al. , 2017. Impaired adrenergic agonist-dependent beige adipocyte induction in aged mice. Obesity (Silver Spring). 25, 417–423. [DOI] [PubMed] [Google Scholar]
- Sih R, et al. , 1997. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 82, 1661–7. [DOI] [PubMed] [Google Scholar]
- Simpson ER, 2003. Sources of estrogen and their importance. The Journal of Steroid Biochemistry and Molecular Biology. 86, 225–230. [DOI] [PubMed] [Google Scholar]
- Singer K, et al. , 2014. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol Metab. 3, 664–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer K, et al. , 2015. Differences in Hematopoietic Stem Cells Contribute to Sexually Dimorphic Inflammatory Responses to High Fat Diet-induced Obesity. J Biol Chem. 290, 13250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawik M, Vidal-Puig AJ, 2006. Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res Rev. 5, 144–64. [DOI] [PubMed] [Google Scholar]
- Smith U, et al. , 2021. Cellular senescence and its role in white adipose tissue. International Journal of Obesity. 45, 934–943. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, et al. , 1999. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 84, 2647–53. [DOI] [PubMed] [Google Scholar]
- Song HJ, et al. , 2014. Gender differences in adiponectin levels and body composition in older adults: Hallym aging study. BMC geriatrics. 14, 8–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soro-Arnaiz I, et al. , 2016. Role of Mitochondrial Complex IV in Age-Dependent Obesity. Cell Rep. 16, 2991–3002. [DOI] [PubMed] [Google Scholar]
- Starr ME, Evers BM, Saito H, 2009. Age-associated increase in cytokine production during systemic inflammation: adipose tissue as a major source of IL-6. J Gerontol A Biol Sci Med Sci. 64, 723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic-Racic M, et al. , 2012. Dendritic cells promote macrophage infiltration and comprise a substantial proportion of obesity-associated increases in CD11c+ cells in adipose tissue and liver. Diabetes. 61, 2330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppan CM, et al. , 2001. The hormone resistin links obesity to diabetes. Nature. 409, 307–12. [DOI] [PubMed] [Google Scholar]
- Steppan CM, et al. , 2005. Activation of SOCS-3 by resistin. Mol Cell Biol. 25, 1569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienstra R, et al. , 2008. Peroxisome proliferator-activated receptor gamma activation promotes infiltration of alternatively activated macrophages into adipose tissue. J Biol Chem. 283, 22620–7. [DOI] [PubMed] [Google Scholar]
- Stout MB, Tchkonia T, Kirkland JL, 2014. The aging adipose organ: Lipid redistribution, inflammation, and cellular senescence. In: Adipose Tissue and Adipokines in Health and Disease. Vol., ed.êds., pp. 69–80. [Google Scholar]
- Stubbins RE, et al. , 2012. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr. 51, 861–70. [DOI] [PubMed] [Google Scholar]
- Sun K, Kusminski CM, Scherer PE, 2011. Adipose tissue remodeling and obesity. J Clin Invest. 121, 2094–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundara Rajan S, Longhi MP, 2016. Dendritic cells and adipose tissue. Immunology. 149, 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvakov S, et al. , 2019. Targeting senescence improves angiogenic potential of adipose-derived mesenchymal stem cells in patients with preeclampsia. Biol Sex Differ. 10, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam BT, Morais JA, Santosa S, 2020. Obesity and ageing: Two sides of the same coin. Obesity Reviews. 21, e12991. [DOI] [PubMed] [Google Scholar]
- Tchernof A, et al. , 1995. Relation of steroid hormones to glucose tolerance and plasma insulin levels in men. Importance of visceral adipose tissue. Diabetes Care. 18, 292–9. [DOI] [PubMed] [Google Scholar]
- Tchernof A, Poehlman ET, Després JP, 2000. Body fat distribution, the menopause transition, and hormone replacement therapy. Diabetes Metab. 26, 12–20. [PubMed] [Google Scholar]
- Tchkonia T, et al. , 2010. Fat tissue, aging, and cellular senescence. Aging Cell. 9, 667–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, et al. , 2013. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 123, 966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons JA, et al. , 2007. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A. 104, 4401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai EC, et al. , 2000. Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int J Obes Relat Metab Disord. 24, 485–91. [DOI] [PubMed] [Google Scholar]
- Tworoger SS, et al. , 2007. Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metab. 92, 1510–6. [DOI] [PubMed] [Google Scholar]
- Unger RH, et al. , 2010. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 1801, 209–14. [DOI] [PubMed] [Google Scholar]
- Valle A, et al. , 2008. Caloric restriction retards the age-related decline in mitochondrial function of brown adipose tissue. Rejuvenation Res. 11, 597–604. [DOI] [PubMed] [Google Scholar]
- Valli A, Harris AL, Kessler BM, 2015. Hypoxia metabolism in ageing. Aging (Albany NY). 7, 465–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Saffele JK, et al. , 1999. Serum leptin levels in healthy ageing men: are decreased serum testosterone and increased adiposity in elderly men the consequence of leptin deficiency? Clin Endocrinol (Oxf). 51, 81–8. [DOI] [PubMed] [Google Scholar]
- Van Sinderen ML, et al. , 2015. Effects of Estrogens on Adipokines and Glucose Homeostasis in Female Aromatase Knockout Mice. PLOS ONE. 10, e0136143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese M, Griffin C, Singer K, 2017. The Role of Sex and Sex Hormones in Regulating Obesity-Induced Inflammation. Adv Exp Med Biol. 1043, 65–86. [DOI] [PubMed] [Google Scholar]
- Varghese M, et al. , 2019. Sex Differences in Inflammatory Responses to Adipose Tissue Lipolysis in Diet-Induced Obesity. Endocrinology. 160, 291–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese M, et al. , 2020. Female adipose tissue has improved adaptability and metabolic health compared to males in aged obesity. Aging (Albany NY). 12, 1725–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlamov O, Bethea CL, Roberts CT, 2014. Sex-Specific Differences in Lipid and Glucose Metabolism. Frontiers in Endocrinology. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtue S, Vidal-Puig A, 2010. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim Biophys Acta. 1801, 338–49. [DOI] [PubMed] [Google Scholar]
- Vlckova V, et al. , 2010. Hypoglycaemia with pioglitazone: analysis of data from the Prescription-Event Monitoring study. J Eval Clin Pract. 16, 1124–8. [DOI] [PubMed] [Google Scholar]
- Wanders D, et al. , 2014. Transcriptional impact of dietary methionine restriction on systemic inflammation: relevance to biomarkers of metabolic disease during aging. Biofactors. 40, 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Seale P, 2016. Control of brown and beige fat development. Nat Rev Mol Cell Biol. 17, 691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, et al. , 2006. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 116, 115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, , Leibel RL, Ferrante AW Jr., 2003. Obesity is associated with macrophage accumulation in adipose tissue. Journal of Clinical Investigation. 112, 1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JC, 2007. Sexual dimorphism of body composition. Best Pract. Res. Clin. Endocrinol. Metab 21, 4515–430. [DOI] [PubMed] [Google Scholar]
- White UA, Tchoukalova YD, 2014. Sex dimorphism and depot differences in adipose tissue function. Biochim Biophys Acta. 1842, 377–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijetunge S, et al. , 2019. Association between serum and adipose tissue resistin with dysglycemia in South Asian women. Nutrition & Diabetes. 9, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer S, et al. , 2009. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 15, 921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Goldstein DR, 2013. Impact of aging on antigen presentation cell function of dendritic cells. Curr Opin Immunol. 25, 535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, et al. , 2007. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol. 179, 4829–39. [DOI] [PubMed] [Google Scholar]
- Wu Y, et al. , 2017. High-fat diet-induced obesity regulates MMP3 to modulate depot- and sex-dependent adipose expansion in C57BL/6J mice. Am J Physiol Endocrinol Metab. 312, E58–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, et al. , 2018a. Ablation of PPARγ in subcutaneous fat exacerbates age-associated obesity and metabolic decline. Aging Cell. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, et al. , 2020. PPARγ agonists delay age-associated metabolic disease and extend longevity. Aging Cell. 19, e13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, et al. , 2015a. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 4, e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, et al. , 2015b. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A. 112, E6301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, et al. , 2018b. Senolytics improve physical function and increase lifespan in old age. Nat Med. 24, 1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N, et al. , 2005. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 579, 6821–6. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, et al. , 2002. Correlation of the adipocyte-derived protein adiponectin with insulin resistance index and serum high-density lipoprotein-cholesterol, independent of body mass index, in the Japanese population. Clin Sci (Lond). 103, 137–42. [DOI] [PubMed] [Google Scholar]
- Yokota T, et al. , 2000. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 96, 1723–32. [PubMed] [Google Scholar]
- Zechner R, et al. , 2012. FAT SIGNALS--lipases and lipolysis in lipid metabolism and signaling. Cell metabolism. 15, 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, et al. , 2015. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 14, 644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, et al. , 2016. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 15, 428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


