Abstract
Background—
Elevated white blood cell (WBC) count is associated with increased major adverse cardiovascular events (MACE) in the setting of acute coronary syndrome. The aim of this study was to evaluate whether similar associations persist in an all-comers population of patients undergoing percutaneous coronary intervention in the contemporary era.
Methods and Results—
In the multicenter, prospective, observational PARIS study (Patterns of Non-Adherence to Anti-Platelet Regimens in Stented Patients Registry), 4222 patients who underwent percutaneous coronary intervention in the United States and Europe between July 1, 2009, and December 2, 2010, were evaluated. The associations between baseline WBC and MACE (composite of cardiac death, stent thrombosis, spontaneous myocardial infarction, or target lesion revascularization) at 24-month follow-up were analyzed using multivariable Cox regression. Patients with higher WBC were more often younger, smokers, and with less comorbid risk factors compared with those with lower WBC. After adjustment for baseline and procedural characteristics, WBC remained independently associated with MACE (hazard ratio [HR] per 103 cells/μL increase, 1.05 [95% confidence intervals (CI), 1.02–1.09]; P=0.001), cardiac death (HR, 1.10 [95% CI, 1.05–1.17]; P<0.001), and clinically indicated target revascularization (HR, 1.04 [95% CI, 1.00–1.09]; P=0.03) but not stent thrombosis (HR, 1.07 [95% CI, 0.99–1.16]; P=0.10) or spontaneous myocardial infarction (HR, 1.03 [95% CI, 0.97–1.09]; P=0.29). The association between WBC and MACE was consistent in acute coronary syndrome and non–acute coronary syndrome presentations (interaction P=0.15).
Conclusions—
Increased WBC is an independent predictor of MACE after percutaneous coronary intervention in a contemporary all-comers cohort. Further studies to delineate the underlying pathophysiologic role of elevated WBC across a spectrum of coronary artery disease presentations are warranted.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00998127.
Keywords: acute coronary syndrome, coronary artery disease, leukocytes, myocardial infarction, percutaneous coronary intervention
Inflammation is increasingly recognized as a key player in the development of major adverse cardiovascular events (MACE).1,2 In addition, much has been hypothesized about the role of inflammation on the initiation and propagation of atherothrombosis, and current data highlight the importance of the inflammatory thrombosis interface.3,4 White blood cell (WBC) count is considered a marker of inflammation measured on routine hemograms, and earlier studies demonstrated an association between WBC and MACE in patients with acute coronary syndrome (ACS).5,6 However, as medical therapy has evolved, a greater proportion of patients are on dual antiplatelet and statin therapies, which have been shown to attenuate systemic inflammatory markers and the inflammatory-thrombosis interface.7–10 Although recent data do demonstrate a persistent association between WBC and MACE in contemporary patients with ACS undergoing percutaneous coronary intervention (PCI), whether or not this association is present in a contemporary all-comers population undergoing PCI remains uncertain.11
In this analysis of the multicenter, international, prospective, observational PARIS study (Patterns of Non-Adherence to Anti-Platelet Regimens in Stented Patients Registry) with adjudicated clinical events, we sought to determine the association between WBC and MACE in an all-comers population in the current era of optimal medical therapy and PCI technique.
Methods
Study Design and Cohort
The prospective observational PARIS registry was designed to evaluate different dual antiplatelet therapy cessation methods and cardiovascular risk after PCI. Details of the registry have been previously described.12 Of the 5031 patients who underwent successful PCI and were enrolled in the PARIS registry from 15 sites across the United States and Europe between July 1, 2009, and December 2, 2010, 809 (16%) were excluded because of missing WBC. A total of 4222 (84%) patients comprised the currently studied final cohort. Participation in the registry was voluntary and requires written informed consent.
The PARIS registry was funded, in part, by Bristol-Myers Squibb and Sanofi-Aventis; however, the funding agencies had no role in the design, collection, analysis, or interpretation of the data, in the writing of this article, or in the decision to submit this article for publication.
Variables of Interest
Baseline demographic characteristics and medical comorbidities were self-reported while body mass index was measured by clinical personnel at each site. The definitions of dyslipidemia, hypertension, and diabetes mellitus required the use of lipid-lowering, antihypertensive, and glucose-lowering agents, respectively. Prior coronary artery disease was defined as prior PCI, coronary artery bypass graft surgery, or myocardial infarction (MI). Tobacco use was defined as use within 30 days. Aspirin and thienopyridine use is defined as use on admission.
Baseline WBC was measured at the time of the PCI by site-specific clinical laboratories. Tertiles of WBC were defined as follows: first tertile (2.1–7.1×103 cells/μL; n=1469), second tertile (7.11–9.1×103 cells/μL; n=1353), and third tertile (9.11–29.0×103 cells/μL; n=1400).
Outcomes
The primary outcome of interest was MACE defined as a composite of cardiac death, definite or probable stent thrombosis, spontaneous MI, or target lesion revascularization at 24-month follow-up. Secondary outcomes of interest included individual components of MACE. Definite and probable stent thrombosis were defined according to the academic research consortium criteria, and spontaneous MI was defined according the Universal Definition.13,14 Clinically indicated target lesion revascularization was defined as any repeat percutaneous intervention of the index lesion (within 5 mm of the previously placed stent) or surgical bypass of the index vessel. All outcomes were site reported and adjudicated by an independent clinical events committee.
Statistical Analyses
Continuous variables are presented as mean±SD, and categorical variables are presented as proportions. Covariates and outcomes were compared across WBC tertiles using a test of trend. MACE during 24-month follow-up were compared across WBC tertiles using the Kaplan–Meier method. The unadjusted and adjusted associations between a per 103 cells/μL increase in WBC and outcomes were determined using a multivariable Cox regression model and presented as hazard ratio (95% confidence interval [CI]). The following variables were included in the adjusted models: age, sex, body mass index, dyslipidemia, hypertension, diabetes mellitus, prior coronary artery disease, tobacco use, admission aspirin use, admission thienopyridine use, glycoprotein IIb/IIIa use, ACS presentation, presence of thrombotic lesion, stent type, and stent length. The adjusted model also included mode of dual antiplatelet therapy cessation (recommended discontinuation, interruption, disruption), which was introduced as a time-dependent covariate as previously described.12 All statistical analyses were performed using STATA version 11.2 (StataCorp LP, College Station, TX). All P values are 2 sided with P<0.05 considered statistically significant.
Results
Baseline Characteristics
Baseline clinical and procedural characteristics across baseline WBC tertiles are shown in Tables 1 and 2. Overall, a higher baseline WBC count was noted in younger patients with a lower proportion of medical comorbidities, such as dyslipidemia, hypertension, diabetes mellitus, and prior coronary artery disease, but a higher proportion of tobacco use. Consistent with the lower rate of coronary artery disease, aspirin or thienopyridine use on admission was less frequent in patients with a higher baseline WBC count. Finally, patients with a higher baseline WBC count were more likely to present with an ACS, have a thrombotic lesion, and to be treated with glycoprotein IIb/IIIa inhibitors and long bare metal stents.
Table 1.
Baseline Characteristics of Patients Discharged on Dual Antiplatelet Therapy After Percutaneous Coronary Intervention Compared Across Baseline WBC Tertiles
| WBC Tertile 1 (2.1–7.1 ×103 cells/μL; n=1469) | WBC Tertile 2 (7.11–9.1×103 cells/μL; n=1353) | WBC Tertile 3 (9.11–29.0× 103 cells/μL; n=1400) | Ptrend Value | |
|---|---|---|---|---|
| Age, y | 65.4±10.6 | 64.1±11.0 | 61.8±11.9 | <0.001 |
| Male sex (%) | 73.8 | 74.4 | 75.6 | 0.28 |
| Body mass index, kg/m2 | 29.0±5.4 | 29.6±5.7 | 29.2±5.9 | 0.27 |
| Medical history (%) | ||||
| Dyslipidemia | 80.0 | 78.1 | 68.1 | <0.001 |
| Hypertension | 84.8 | 81.7 | 75.1 | <0.001 |
| Diabetes mellitus | 35.3 | 34.7 | 30.6 | 0.01 |
| Prior coronary artery disease (MI, PCI, or CABG) | 54.7 | 49.8 | 38.8 | <0.001 |
| Stroke | 3.2 | 3.3 | 3.3 | 0.90 |
| Peripheral vascular disease | 6.5 | 7.2 | 7.5 | 0.28 |
| Tobacco use (%) | 11.4 | 17.7 | 31.0 | <0.001 |
| Medications (%) | ||||
| Aspirin | 78.4 | 73.5 | 58.6 | <0.001 |
| Thienopyridine | 50.6 | 41.9 | 32.0 | <0.001 |
| Glycoprotein IIb/IIIa inhibitor use | 11.1 | 14.5 | 19.9 | <0.001 |
| Cardiac status at admission (%) | ||||
| Acute coronary syndrome | 32.6 | 38.7 | 56.9 | <0.001 |
Continuous data presented as mean±SD. CABG indicates coronary artery bypass graft; MI, myocardial infarction; PCI, percutaneous coronary intervention with stent placement; and WBC, white blood cell.
Table 2.
Procedural Characteristics of Patients Discharged on Dual Antiplatelet Therapy After Percutaneous Coronary Intervention Compared Across Baseline WBC Tertiles
| WBC Tertile 1 (2.1–7.1×103 cells/μL; n=1469) | WBC Tertile 2 (7.11–9.1×103 cells/μL; n=1353) | WBC Tertile 3 (9.11–29.0×103 cells/μL; n=1400) | Ptrend Value | |
|---|---|---|---|---|
| Coronary artery treated (%) | ||||
| Left main | 2.9 | 2.7 | 2.8 | 0.90 |
| Left anterior descending | 46.6 | 47.5 | 44.4 | 0.26 |
| Circumflex | 30.4 | 30.9 | 30.6 | 0.93 |
| Right coronary artery | 33.6 | 32.2 | 37.6 | 0.03 |
| No. of arteries treated (%) | ||||
| One | 87.3 | 87.6 | 85.2 | 0.09 |
| Two | 11.9 | 11.6 | 14.2 | 0.07 |
| Three | 0.8 | 0.8 | 0.6 | 0.58 |
| Bifurcation lesion (%) | 11.0 | 12.1 | 11.2 | 0.82 |
| Chronic total occlusion (%) | 2.7 | 3.7 | 4.0 | 0.048 |
| Thrombotic lesion (%) | 3.9 | 6.7 | 17.1 | <0.001 |
| Stent type (%) | ||||
| Bare metal | 13.6 | 14.4 | 22.6 | <0.001 |
| First-generation drug-eluting stent | 17.0 | 12.1 | 10.4 | <0.001 |
| Second-generation drug-eluting stent | 69.4 | 73.5 | 66.9 | 0.16 |
| Total stent length (%), mm | ||||
| <20 | 41.5 | 35.5 | 32.6 | <0.001 |
| 20–39 | 35.1 | 36.7 | 35.5 | 0.83 |
| ≥40 | 23.4 | 27.9 | 31.9 | <0.001 |
WBC indicates white blood cell.
The proportion of patients with any dual antiplatelet therapy cessation was not significantly different across baseline WBC tertiles at 30 days (tertile 1: 3.2% [95% CI, 2.4–4.3]; tertile 2: 3.3% [95% CI, 2.4–4.4]; tertile 3: 3.8% [95% CI, 2.9–5.0]; P=0.39) or 12 months (tertile 1: 23.2% [95% CI, 21.1–25.5]; tertile 2: 24.6% [95% CI, 22.3–27.0]; tertile 3: 25.0% [95% CI, 22.8–27.4]; P=0.33). However, at 24 months, there was a significant increase in any dual antiplatelet therapy cessation with increasing baseline WBC tertiles (tertile 1: 55.1% [95% CI, 52.5–57.8]; tertile 2: 54.8% [95% CI, 52.1–57.6]; tertile 3: 59.7% [95% CI, 57.1–62.4]; P=0.02).
Outcomes
The rate patients lost to 24-month follow-up was overall low and did not differ by WBC tertiles (tertile 1: 7.9%; tertile 2: 7.0%; tertile 3: 7.4%; P=0.63). Outcomes stratified by baseline WBC tertiles are shown in Table 3 and the Figure. The proportion of patients with the primary composite outcome of MACE was significantly higher in patients with a higher baseline WBC count at 30-day, 12-month, and 24-month follow-up as were the individual components of cardiac death, definite/probable stent thrombosis, and clinically indicated target vessel revascularization. The proportion of patients with spontaneous MI, however, was not significantly different across baseline WBC tertiles. Results were consistent when outcomes were stratified by baseline WBC quartiles (Table I in the Data Supplement; Figure in the Data Supplement).
Table 3.
Outcomes of Patients Discharged on Dual Antiplatelet Therapy After Percutaneous Coronary Intervention Compared Across Baseline WBC Tertiles
| No. of Events | WBC Tertile 1 (2.1–7.1×103 cells/μL; n=1469) | WBC Tertile 2 (7.11–9.1×103 cells/μL; n=1353) | WBC Tertile 3 (9.11–29.0×103 cells/μL; n=1400) | Ptrend Value | ||
|---|---|---|---|---|---|---|
| Major adverse cardiovascular event (composite of cardiac death, definite or probable stent thrombosis, spontaneous myocardial infarction, or target lesion revascularization; %) | 30 d | 42 | 0.4 (0.2–0.9) | 0.5 (0.3–1.1) | 2.1 (1.5–3.0) | <0.0001 |
| 12 mo | 312 | 6.2 (5.1–7.6) | 6.7 (5.5–8.2) | 9.7 (8.3–11.4) | 0.0002 | |
| 24 mo | 478 | 10.0 (8.6–11.7) | 11.2 (9.6–13.0) | 14.1 (12.4–16.1) | 0.0004 | |
| Cardiac death (%) | 30 d | 12 | 0.1 (0.0–0.5) | 0.2 (0.1–0.7) | 0.6 (0.3–1.1) | 0.01 |
| 12 mo | 75 | 1.3 (0.8–2.0) | 1.8 (1.2–2.7) | 2.4 (1.7–3.4) | 0.02 | |
| 24 mo | 131 | 2.4 (1.7–3.3) | 3.6 (2.7–4.7) | 3.9 (3.0–5.1) | 0.02 | |
| Definite/probable stent thrombosis (%) | 30 d | 23 | 0.1 (0.0–0.6) | 0.4 (0.2–1.0) | 1.1 (0.7–1.8) | 0.0007 |
| 12 mo | 47 | 0.7 (0.4–1.3) | 0.8 (0.5–1.5) | 1.9 (1.3–2.8) | 0.003 | |
| 24 mo | 61 | 0.9 (0.5–1.6) | 1.3 (0.8–2.1) | 2.3 (1.6–3.2) | 0.003 | |
| Spontaneous myocardial infarction (%) | 30 d | 19 | 0.3 (0.1–0.8) | 0.3 (0.1–0.8) | 0.7 (0.4–1.3) | 0.14 |
| 12 mo | 90 | 2.1 (1.5–3.0) | 1.7 (1.1–2.5) | 2.8 (2.0–3.8) | 0.20 | |
| 24 mo | 150 | 3.4 (2.5–4.5) | 3.3 (2.4–4.4) | 4.6 (3.6–5.9) | 0.09 | |
| Clinically indicated target lesion revascularization (%) | 30 d | 22 | 0 | 0.4 (0.2–0.9) | 1.2 (0.8–2.0) | <0.0001 |
| 12 mo | 211 | 3.9 (3.0–5.0) | 4.6 (3.6–5.9) | 7.0 (5.8–8.5) | 0.0001 | |
| 24 mo | 301 | 6.2 (5.0–7.6) | 7.0 (5.7–8.5) | 9.4 (7.9–11.1) | 0.0009 |
Data are shown as crude estimates from Kaplan–Meier curves (95% confidence intervals). WBC indicates white blood cell.
Figure.
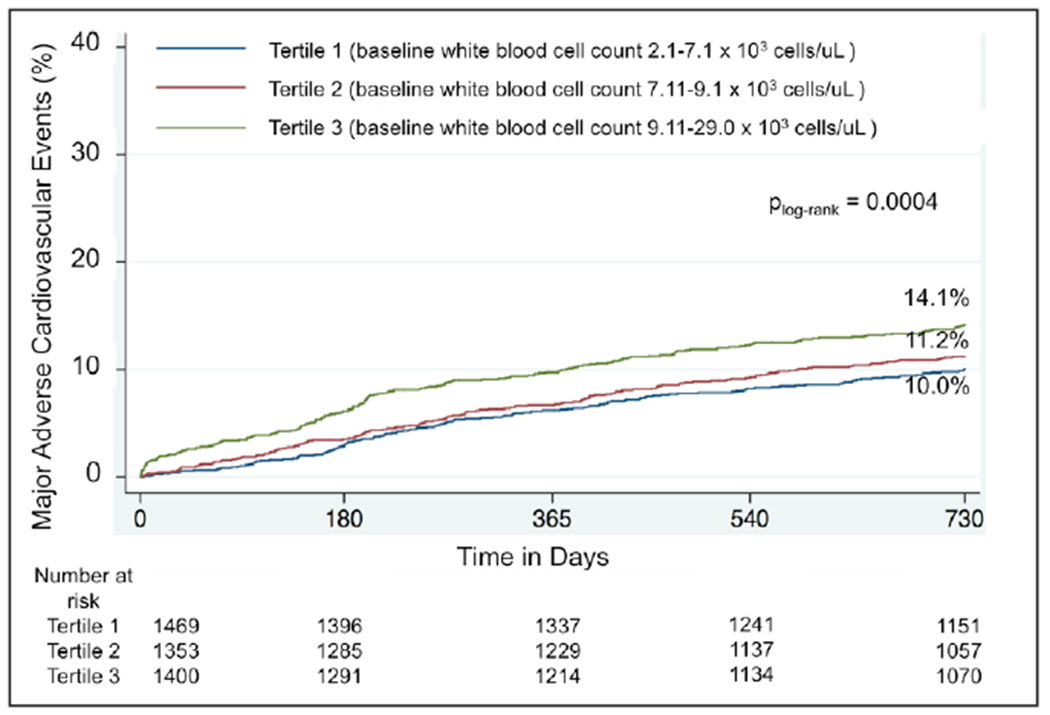
Kaplan–Meier curves of major adverse cardiovascular events through 24 months stratified by tertiles of baseline white blood cell count.
Unadjusted and adjusted associations between a per 103 cells/μL increase in WBC and outcomes at 24-month follow-up are shown in Table 4. After adjustment for age, sex, body mass index, dyslipidemia, hypertension, diabetes mellitus, prior coronary artery disease, tobacco use, admission aspirin use, admission thienopyridine use, glycoprotein IIb/IIIa use, ACS presentation, presence of thrombotic lesion, stent type, stent length, and mode of dual antiplatelet therapy cessation, there remained a significant association between WBC and MACE, cardiac death, and clinically indicated target lesion revascularization but not definite or probable stent thrombosis or spontaneous MI. The association between WBC and MACE was consistent in ACS and non-ACS presentations (interaction P=0.15). Adjusted associations between baseline WBC tertiles and clinical outcomes at 24-month follow-up are shown in Table II in the Data Supplement.
Table 4.
Unadjusted and Adjusted Associations Between a per 103 Cells/μL Increase in Baseline White Blood Cell Count and Clinical Outcomes at 24-Month Follow-Up in Patients Discharged on Dual Antiplatelet Therapy After Percutaneous Coronary Intervention
| Unadjusted Model | Adjusted Model | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Major adverse cardiovascular event | 1.06 (1.03–1.09) | <0.001 | 1.05 (1.02–1.09) | 0.001 |
| Cardiac death | 1.09 (1.03–1.15) | 0.001 | 1.10 (1.05–1.17) | <0.001 |
| Definite/probable stent thrombosis | 1.14 (1.06–1.22) | <0.001 | 1.06 (0.98–1.15) | 0.12 |
| Spontaneous myocardial infarction | 1.06 (1.00–1.11) | 0.04 | 1.03 (0.97–1.09) | 0.35 |
| Clinically indicated target lesion revascularization | 1.06 (1.02–1.10) | 0.002 | 1.04 (1.00–1.09) | 0.03 |
Model adjusts for the following variables: age, sex, body mass index, dyslipidemia, hypertension, diabetes mellitus, prior coronary artery disease, tobacco use, admission aspirin use, admission thienopyridine use, glycoprotein IIb/IIIa use, acute coronary syndrome presentation, presence of thrombotic lesion, stent type, stent length, and mode of dual antiplatelet therapy cessation. CI indicates confidence interval.
Other factors associated with an increase in long-term MACE were presence of diabetes mellitus, prior coronary artery disease, ACS, total stent length of ≥40 mm, and disruption of dual antiplatelet therapy while use of drug-eluting stent and recommended discontinuation of dual antiplatelet therapy was associated with lower long-term MACE (Table III in the Data Supplement).
Discussion
This large, prospective, multicenter, international, registry demonstrated a significant independent association between baseline WBC and independently adjudicated long-term MACE in an all-comers population of patients undergoing PCI. This association is independent of the presence of ACS, thereby raising a potential role for inflammation on clinical outcomes in patients across a spectrum of coronary artery disease presentations even in the current era of medical and device therapy. The significant independent association between baseline WBC and target lesion revascularization highlights the need to explore a potential role of inflammation and the inflammatory/thrombosis interface in the setting of PCI. Whether or not these associations would be attenuated with targeted anti-inflammatory therapy, however, remains uncertain.
Earlier reports demonstrated an association between WBC and short-term outcomes in patients presenting with ACS. A substudy of the TIMI (Thrombolysis in Myocardial Infarction) 10A and 10B trials evaluated 975 patients who underwent thrombolytic therapy for ST-segment–elevation MI.5 A higher WBC count was noted in patients with a closed infarct artery and worse TIMI myocardial perfusion grades, which translated to a higher rate of 30-day mortality, new congestive heart failure, or shock. Another analysis from the 2208 patients in the TACTICS (Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy)-TIMI 18 study also demonstrated a significant association between higher WBC counts and lower TIMI flow grades, lower perfusion grades, and higher rate of 6-month mortality.6
Medical therapy, however, has evolved significantly because these previous reports and current data support the anti-inflammatory effects of dual antiplatelet and statin therapy. Platelet P2Y12 receptor inhibitors have been shown to significantly reduce the formation of aggregates between platelets and WBC subtypes, as well as cytokine release, in both experimental human models and patients with ACS.7,8 These platelet–WBC aggregates have been shown to be elevated across the spectrum of cardiovascular disease and associated with adverse cardiovascular outcomes.15–18 Statin therapy has also been shown to significantly lower platelet–WBC aggregates and cellular adhesion molecules associated with the endothelial inflammatory response in the setting of ACS and stable coronary artery disease.9,10 These reductions parallel the significant reductions in MACE observed in patients on high dose statin therapy–undergoing PCI.19–21 In the current analysis, the patients with higher baseline WBC were younger and with less comorbidities but with a significantly higher rate of tobacco use. Data on the impact of tobacco use on long-term outcomes have been mixed. Smokers have been shown to have lower mortality after an acute MI, termed the smoker’s paradox, and possibly because of their younger age and fewer comorbidities.22 However, a recent study of the Global Registry of Acute Coronary Events further stratified by the type of acute MI presentations and demonstrated that over the years from 1999 to 2007, smokers presenting with ST-segment–elevation MI did not have a reduction in 30-day mortality, whereas those with non–ST-segment ACS did demonstrate this reduction. Nonetheless, the association between baseline WBC in the current analysis remains independent of age, comorbidities, ACS presentation, and tobacco use.23
Device technology has also advanced over time to potentially decrease the inflammatory milieu induced by mechanical injury during PCI. Inflammatory responses to drug-eluting stent polymers were thought to play a large role in the underlying pathophysiology of in-stent restenosis and stent thrombosis. In earlier pre-clinical models, leukocytes were seen to be recruited soon after vascular injury. Rogers et al24 demonstrated that an antibody to the WBC adhesion integrin, Mac-1 (CD11/CD18), led to a significant decrease in neointimal growth at the site of balloon denudation and stent-induced injury in animal models. Similar results were seen in rodent models that lacked the Mac-1 gene.25 In the bare metal stent era, a significant increase in CD11b and CD18 after PCI correlated with subsequent restenosis while a single nucleotide polymorphism in the CD18 gene was shown to be associated with a significantly lower rate of restenosis at 1-year follow-up.26,27 Second-generation drug-eluting stents were designed to reduce the inflammatory response with thinner strut designs and more biocompatible polymers, resulting in significantly lower rates of stent-related adverse events compared with earlier generation stents.28 In the current analysis, there was a significantly greater rate of thrombotic lesions with higher WBC tertiles. However, WBC remained an independent predictor of MACE and clinically indicated target lesion revascularization even after adjustment for presence of thrombotic lesion, antiplatelet therapy use, stent type, and stent length.
The significance of an elevated WBC in ACS remains despite the improvements in medical therapy. A single-center retrospective observational study of 2833 patients presenting with ACS between December 1998 and October 2004 demonstrated a significant association between WBC subtypes and in-hospital and 6-month mortality.29 An analysis of 363 patients randomized in the EVOLVE study (Evaluation of MCC-135 for Left Ventricular Salvage in Acute Myocardial Infarction) between May 2003 and November 2004 demonstrated that higher WBC and WBC subtypes independently predicted larger myocardial infarct size, lower left ventricular ejection fraction, and a higher rate of adverse clinical events in patients presenting with ST-segment–elevation MI and undergoing PCI.30 Although the clinical outcome was a not traditionally used composite of death, reinfarction, new or worsening congestive heart failure during index rehospitalization, all cardiac rehospitalizations, life-threatening ventricular arrhythmias, and new cardiogenic shock, the outcomes were adjudicated by an independent clinical events committee. In the ACUITY trial (Acute Catheterization and Urgent Intervention Triage Strategy), WBC was an independent predictor of 1-year mortality in the 13 678 patients with moderate- or high-risk non–ST-segment–elevation MI undergoing PCI between August 2003 and December 2005.31 More recently, WBC was associated with increased 1-year mortality in 3193 patients who underwent PCI for ST-segment–elevation MI between March 2005 and May 2007 in the HORIZONS-AMI (Harmonizing Outcome with Revascularization and Stent in Acute Myocardial Infarction) trial.11
In the setting of ACS, it remains uncertain whether an elevated WBC is a marker of inflammation or a result of demargination in the setting of acute stress. The proposed pathophysiology of elevated WBC in the setting of stable coronary artery disease is even less clear. Little data exist on a relationship of WBC and MACE in an all-comers population. One large multicenter prospective cohort study of 72 242 postmenopausal women enrolled in the Women’s Health Initiative Observational Study reported WBC to be an independent predictor of cardiovascular events, defined as fatal coronary heart disease, nonfatal MI, stroke, and total mortality.32 The multicenter, randomized ACTION (A Coronary disease Trial Investigating Outcome with Nifedipine) gastrointestinal therapeutic system trial also demonstrated a significant association between WBC and the composite of long-term death, MI, or stroke in 7311 patients with stable angina.33 However, both of these studies were conducted more than a decade ago before the contemporary era of optimal medical therapy. More recently, a single-center registry of 3005 consecutive patients referred for coronary angiography in Israel demonstrated a higher rate of MACE during a mean follow-up period of 486 days in patients with elevated neutrophil-to-lymphocyte ratio.34
Despite the improvement in mortality related to coronary artery disease, there remains room for improvement, and an anti-inflammatory agent in addition to the current treatment algorithms may provide additional benefit in this patient population. A recent study by Nidorf et al35 demonstrated a significant reduction on the primary composite outcome of ACS, out-of-hospital cardiac arrest, or noncardioembolic ischemic stroke in patients with stable coronary artery disease on antiplatelet and statin therapy randomized to receive the anti-inflammatory agent colchicine versus no colchicine. This is likely because of the effect of colchicine on the endothelial inflammatory response, as well as the inflammatory-thrombosis axis.36,37 Our results combined with previously published data lend support to ongoing studies targeting the inflammatory pathway across the spectrum of cardiovascular disease (NCT01594333, NCT02594111, and NCT02551094).
Limitations
There are several limitations to the current study. First, this secondary analysis of the PARIS registry was not pre-specified, and no causal relationships may be inferred from the associations observed in this prospective registry. Second, data on race, the use of statin therapy, and data on case urgency (with the exception of ACS presentation), such as left ventricular ejection fraction and presence of cardiogenic shock, were not collected. Third, 16% of the study population were excluded because of lack of WBC data and may represent a potential source of selection bias. Finally, data on WBC differentials, including neutrophil lymphocyte ratio, were not available. Nonetheless, this remains the most current observation on the association between baseline inflammatory status and adjudicated clinical outcomes in a large, multicenter, international, prospective all-comers population.
Conclusions
Baseline WBC independently predicts MACE in a contemporary all-comers population undergoing PCI. In the setting of ACS, elevated WBC may represent a marker of inflammation or result from demargination in the setting of acute stress. Studies to determine the underlying pathophysiological role of elevated WBC in the setting of stable coronary artery disease are warranted. Trials evaluating the role of anti-inflammatory therapy in coronary artery disease are ongoing.
Supplementary Material
WHAT IS KNOWN
Inflammation is a key player in the development of major adverse cardiovascular events.
Earlier observational studies consistently demonstrated an association between white blood cell count and major adverse cardiovascular events in patients with acute coronary syndrome.
Medical and device therapies have evolved such that current therapies attenuate systemic inflammation.
WHAT THE STUDY ADDS
Despite evolution in therapies, there remains a significant association between baseline inflammation and major adverse cardiovascular events after percutaneous coronary intervention in this prospective registry with adjudicated clinical events.
This association is significant in an all-comers population and consistent in both acute coronary syndromes and stable ischemic heart disease populations.
Sources of Funding
The PARIS registry was funded, in part, by Bristol-Myers Squibb and Sanofi-Aventis. Dr Shah is supported, in part, by the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development (IK2 CX001074).
Footnotes
The Data Supplement is available at http://circinterventions.ahajournals.org/lookup/suppl/doi:10.1161/CIRCINTERVENTIONS.117.004981/-/DC1.
Disclosures
None.
References
- 1.Kovanen PT, Kaartinen M, Paavonen T. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation. 1995;92:1084–1088. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Simon DI, Chen Z, Xu H, Li CQ, Dong Jf, McIntire LV, Ballantyne CM, Zhang L, Furman MI, Berndt MC, López JA. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18). J Exp Med. 2000;192:193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henn V, Slupsky JR, Gräfe M, Anagnostopoulos I, Förster R, Müller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 5.Barron HV, Cannon CP, Murphy SA, Braunwald E, Gibson CM. Association between white blood cell count, epicardial blood flow, myocardial perfusion, and clinical outcomes in the setting of acute myocardial infarction: a thrombolysis in myocardial infarction 10 substudy. Circulation. 2000;102:2329–2334. [DOI] [PubMed] [Google Scholar]
- 6.Sabatine MS, Morrow DA, Cannon CP, Murphy SA, Demopoulos LA, DiBattiste PM, McCabe CH, Braunwald E, Gibson CM. Relationship between baseline white blood cell count and degree of coronary artery disease and mortality in patients with acute coronary syndromes: a TACTICS-TIMI 18 (Treat Angina with Aggrastat and determine Cost of Therapy with an Invasive or Conservative Strategy-Thrombolysis in Myocardial Infarction 18 trial) substudy. J Am Coll Cardiol. 2002;40:1761–1768. [DOI] [PubMed] [Google Scholar]
- 7.Thomas MR, Outteridge SN, Ajjan RA, Phoenix F, Sangha GK, Faulkner RE, Ecob R, Judge HM, Khan H, West LE, Dockrell DH, Sabroe I, Storey RF. Platelet P2Y12 inhibitors reduce systemic inflammation and its prothrombotic effects in an experimental human model. Arterioscler Thromb Vasc Biol. 2015;35:2562–2570. doi: 10.1161/ATVBAHA.115.306528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao Z, Théroux P. Clopidogrel inhibits platelet-leukocyte interactions and thrombin receptor agonist peptide-induced platelet activation in patients with an acute coronary syndrome. J Am Coll Cardiol. 2004;43:1982–1988. doi: 10.1016/j.jacc.2003.10.071. [DOI] [PubMed] [Google Scholar]
- 9.Sexton TR, Wallace EL, Macaulay TE, Charnigo RJ, Evangelista V, Campbell CL, Bailey AL, Smyth SS. The effect of rosuvastatin on platelet-leukocyte interactions in the setting of acute coronary syndrome. J Am Coll Cardiol. 2015;65:306–307. doi: 10.1016/j.jacc.2014.10.047. [DOI] [PubMed] [Google Scholar]
- 10.Patti G, Chello M, Pasceri V, Colonna D, Nusca A, Miglionico M, D’Ambrosio A, Covino E, Di Sciascio G. Protection from procedural myocardial injury by atorvastatin is associated with lower levels of adhesion molecules after percutaneous coronary intervention: results from the ARMYDA-CAMs (Atorvastatin for Reduction of MYocardial Damage during Angioplasty-Cell Adhesion Molecules) substudy. J Am Coll Cardiol. 2006;48:1560–1566. doi: 10.1016/j.jacc.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 11.Palmerini T, Mehran R, Dangas G, Nikolsky E, Witzenbichler B, Guagliumi G, Dudek D, Genereux P, Caixeta A, Rabbani L, Weisz G, Parise H, Fahy M, Xu K, Brodie B, Lansky A, Stone GW. Impact of leukocyte count on mortality and bleeding in patients with myocardial infarction undergoing primary percutaneous coronary interventions: analysis from the Harmonizing Outcome with Revascularization and Stent in Acute Myocardial Infarction trial. Circulation. 2011;123:2829–2837, 7 p following 2837. doi: 10.1161/CIRCULATIONAHA.110.985564. [DOI] [PubMed] [Google Scholar]
- 12.Mehran R, Baber U, Steg PG, Ariti C, Weisz G, Witzenbichler B, Henry TD, Kini AS, Stuckey T, Cohen DJ, Berger PB, Iakovou I, Dangas G, Waksman R, Antoniucci D, Sartori S, Krucoff MW, Hermiller JB, Shawl F, Gibson CM, Chieffo A, Alu M, Moliterno DJ, Colombo A, Pocock S. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet. 2013;382:1714–1722. doi: 10.1016/S0140-6736(13)61720-1. [DOI] [PubMed] [Google Scholar]
- 13.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW; Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 14.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernández-Avilés F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 15.Furman MI, Barnard MR, Krueger LA, Fox ML, Shilale EA, Lessard DM, Marchese P, Frelinger AL 3rd, Goldberg RJ, Michelson AD. Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction. J Am Coll Cardiol. 2001;38:1002–1006. [DOI] [PubMed] [Google Scholar]
- 16.Furman MI, Benoit SE, Barnard MR, Valeri CR, Borbone ML, Becker RC, Hechtman HB, Michelson AD. Increased platelet reactivity and circulating monocyte-platelet aggregates in patients with stable coronary artery disease. J Am Coll Cardiol. 1998;31:352–358. [DOI] [PubMed] [Google Scholar]
- 17.Ott I, Neumann FJ, Gawaz M, Schmitt M, Schömig A. Increased neutrophil-platelet adhesion in patients with unstable angina. Circulation. 1996;94:1239–1246. [DOI] [PubMed] [Google Scholar]
- 18.Mickelson JK, Lakkis NM, Villarreal-Levy G, Hughes BJ, Smith CW. Leukocyte activation with platelet adhesion after coronary angioplasty: a mechanism for recurrent disease? J Am Coll Cardiol. 1996;28:345–353. doi: 10.1016/0735-1097(96)00164-7. [DOI] [PubMed] [Google Scholar]
- 19.Pasceri V, Patti G, Nusca A, Pristipino C, Richichi G, Di Sciascio G; ARMYDA Investigators. Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention: results from the ARMYDA (Atorvastatin for Reduction of MYocardial Damage during Angioplasty) study. Circulation. 2004;110:674–678. doi: 10.1161/01.CIR.0000137828.06205.87. [DOI] [PubMed] [Google Scholar]
- 20.Briguori C, Visconti G, Focaccio A, Golia B, Chieffo A, Castelli A, Mussardo M, Montorfano M, Ricciardelli B, Colombo A. Impact of a single high loading dose of atorvastatin on periprocedural myocardial infarction: results from the NAPLES II trial. J Am Coll Cardiol 2009;54:2157–2163. [DOI] [PubMed] [Google Scholar]
- 21.Patti G, Pasceri V, Colonna G, Miglionico M, Fischetti D, Sardella G, Montinaro A, Di Sciascio G. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol. 2007;49:1272–1278. doi: 10.1016/j.jacc.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 22.Angeja BG, Kermgard S, Chen MS, McKay M, Murphy SA, Antman EM, Cannon CP, Braunwald E, Gibson CM. The smoker’s paradox: insights from the angiographic substudies of the TIMI trials. J Thromb Thrombolysis. 2002;13:133–139. [DOI] [PubMed] [Google Scholar]
- 23.Arbel Y, FitzGerald G, Yan AT, Tan MK, Fox KA, Gore JM, Steg PG, Eagle KA, Brieger D, Montalescot G, Budaj A, Lopez-Sendon J, Avezum A, Granger CB, Goodman SG. Temporal trends in all-cause mortality according to smoking status: insights from the Global Registry of Acute Coronary Events. Int J Cardiol. 2016;218:291–297. doi: 10.1016/j.ijcard.2016.05.064. [DOI] [PubMed] [Google Scholar]
- 24.Rogers C, Edelman ER, Simon DI. A mAb to the beta2-leukocyte integrin Mac-1 (CD11b/CD18) reduces intimal thickening after angioplasty or stent implantation in rabbits. Proc Natl Acad Sci U S A. 1998;95:10134–10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon DI, Dhen Z, Seifert P, Edelman ER, Ballantyne CM, Rogers C. Decreased neointimal formation in Mac-1(−/−) mice reveals a role for inflammation in vascular repair after angioplasty. J Clin Invest. 2000;105:293–300. doi: 10.1172/JCI7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue T, Sakai Y, Morooka S, Hayashi T, Takayanagi K, Takabatake Y. Expression of polymorphonuclear leukocyte adhesion molecules and its clinical significance in patients treated with percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1996;28:1127–1133. doi: 10.1016/S0735-1097(96)00308-7. [DOI] [PubMed] [Google Scholar]
- 27.Koch W, Böttiger C, Mehilli J, von Beckerath N, Neumann FJ, Schömig A, Kastrati A. Association of a CD18 gene polymorphism with a reduced risk of restenosis after coronary stenting. Am J Cardiol. 2001;88:1120–1124. [DOI] [PubMed] [Google Scholar]
- 28.Palmerini T, Biondi-Zoccai G, Della Riva D, Stettler C, Sangiorgi D, D’Ascenzo F, Kimura T, Briguori C, Sabatè M, Kim HS, De Waha A, Kedhi E, Smits PC, Kaiser C, Sardella G, Marullo A, Kirtane AJ, Leon MB, Stone GW. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393–1402. doi: 10.1016/S0140-6736(12)60324-9. [DOI] [PubMed] [Google Scholar]
- 29.Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653–657. doi: 10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Chia S, Nagurney JT, Brown DF, Raffel OC, Bamberg F, Senatore F, Wackers FJ, Jang IK. Association of leukocyte and neutrophil counts with infarct size, left ventricular function and outcomes after percutaneous coronary intervention for ST-elevation myocardial infarction. Am J Cardiol. 2009;103:333–337. doi: 10.1016/j.amjcard.2008.09.085. [DOI] [PubMed] [Google Scholar]
- 31.Palmerini T, Généreux P, Mehran R, Dangas G, Caixeta A, Riva DD, Mariani A, Xu K, Stone GW. Association among leukocyte count, mortality, and bleeding in patients with non-ST-segment elevation acute coronary syndromes (from the Acute Catheterization and Urgent Intervention Triage StrategY [ACUITY] trial). Am J Cardiol. 2013;111:1237–1245. doi: 10.1016/j.amjcard.2012.12.056. [DOI] [PubMed] [Google Scholar]
- 32.Margolis KL, Manson JE, Greenland P, Rodabough RJ, Bray PF, Safford M, Grimm RH Jr, Howard BV, Assaf AR, Prentice R; Women’s Health Initiative Research Group. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the Women’s Health Initiative Observational Study. Arch Intern Med. 2005;165:500–508. doi: 10.1001/archinte.165.5.500. [DOI] [PubMed] [Google Scholar]
- 33.Clayton TC, Lubsen J, Pocock SJ, Vokó Z, Kirwan BA, Fox KA, Poole-Wilson PA. Risk score for predicting death, myocardial infarction, and stroke in patients with stable angina, based on a large randomised trial cohort of patients. BMJ. 2005;331:869. doi: 10.1136/bmj.38603.656076.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arbel Y, Finkelstein A, Halkin A, Birati EY, Revivo M, Zuzut M, Shevach A, Berliner S, Herz I, Keren G, Banai S. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis. 2012;225:456–460. doi: 10.1016/j.atherosclerosis.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404–410. doi: 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 36.Cronstein BN, Molad Y, Reibman J, Balakhane E, Levin RI, Weissmann G. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J Clin Invest. 1995;96:994–1002. doi: 10.1172/JCI118147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah B, Allen N, Harchandani B, Pillinger M, Katz S, Sedlis SP, Echagarruga C, Samuels SK, Morina P, Singh P, Karotkin L, Berger JS. Effect of colchicine on platelet-platelet and platelet-leukocyte interactions: a pilot study in healthy subjects. Inflammation. 2016;39:182–189. doi: 10.1007/s10753-015-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


