Abstract
Aims:
To assess the effectiveness of intervention components designed to increase quit attempts and promote abstinence in patients initially unwilling to quit smoking.
Design:
A 4-factor, randomized factorial experiment.
Setting:
16 primary care clinics in southern Wisconsin.
Participants:
577 adults who smoke (60% women, 80% White) recruited during primary care visits who were currently willing to reduce their smoking but unwilling to try to quit.
Interventions:
Four factors contrasted intervention components administered over a 1-year period: 1) Nicotine Mini-Lozenge versus None; 2) Reduction Counseling versus None; 3) Behavioral Activation (BA) Counseling versus None; and 4) Motivational 5Rs Counseling versus None. Participants could request cessation treatment at any time.
Measurements:
The primary outcome was 7-day point-prevalence abstinence at 52 weeks post enrollment; secondary outcomes were point-prevalence abstinence at 26 weeks and making a quit attempt by weeks 26 and 52.
Findings:
No abstinence main effects were found but a Mini-Lozenge x Reduction Counseling x BA interaction was found at 52 weeks; p=0.03. Unpacking this interaction showed that the Mini-Lozenge alone produced the highest abstinence rate (16.7%); combining it with Reduction Counseling produced an especially low abstinence rate (4.1%). Reduction Counseling decreased the likelihood of making a quit attempt by 52 weeks relative to no Reduction Counseling (p=.01).
Conclusions:
Nicotine mini-lozenges may increase smoking abstinence in people initially unwilling to quit smoking, but their effectiveness declines when used with smoking reduction counseling or other behavioral interventions. Reduction counseling decreases the likelihood of making a quit attempt in people initially unwilling to quit smoking.
Keywords: Motivation phase, smoking reduction, smoking cessation, quit attempts, unwilling to quit smoking, multiphase optimization strategy (MOST), Phase-Based Model, nicotine replacement therapy, comparative effectiveness, primary care, chronic care smoking treatment, factorial experiment
Cigarette smoking is the leading preventable cause of morbidity and mortality in the US [(1)]. Effectively combatting this important public health problem requires a comprehensive healthcare approach that engages those who smoke in evidence-based smoking treatment. Although healthcare settings offer a great opportunity for treating smoking, most patients leave their primary care encounters without evidence-based smoking treatment [(2)]. This occurs, in part, because the majority of patients who smoke (70–90%) are unwilling to quit at the time of a primary care visit [(3–5)], and it remains unclear how to best help such individuals. Thus, it is important that we expand the reach of smoking treatment by identifying effective treatments for those unwilling to quit smoking that: 1) engage them in treatment and 2) increase the proportion who quit successfully.
This study used the Multiphase Optimization Strategy (MOST), an approach that uses efficient designs such as factorial experiments [(6, 7)]. In the present application of MOST, we conducted a factorial experiment to identify intervention components and combinations of components that warrant additional research investigation [(8, 9)]; this information could suggest that certain intervention components would perform well as an integrated treatment package, a hypothesis that could ultimately be evaluated in a randomized controlled trial (RCT). We used the Phase-Based Model of smoking treatment [(10)] to guide our selection of intervention components tested in this research. The Phase-Based Model organizes smoking treatment research and delivery around the different phases of smoking intervention (i.e., Motivation, Preparation, Cessation, Maintenance, and Relapse Recovery), each with its own treatment challenges, opportunities, and phase-specific outcomes. The goal of the current experiment was to evaluate the relative promise of intervention components for people in the Motivation phase – those initially unwilling to try to quit smoking. Goals of the Motivation phase are to spur quit attempts and increase the likelihood of attaining abstinence.
Motivation-phase treatment typically includes nicotine replacement therapy (NRT; nicotine gum, lozenge, or patch) and counseling (e.g., reduction counseling or motivational counseling; [(11, 12)]). Although such treatments have been shown to benefit those initially unwilling to try to quit, they tend to produce fairly low abstinence rates. For example, a meta-analysis found that long term (≥ 6 months) nicotine replacement therapy (NRT) for those unwilling to quit smoking resulted in a sustained 6-month abstinence rate of 6.75% [(13)]; albeit, conditions involving no treatment generally produce even lower quit rates [(14)].
One reason that it has been difficult to enhance the effectiveness of Motivation-phase treatments is that their effective constituents have not been identified; as noted above, behavioral interventions (i.e., reduction counseling, Motivational 5Rs) are usually paired with NRT (e.g., [(12, 13)]). There is evidence that reduction counseling alone does not meaningfully increase quit attempts or abstinence in this population [(15, 16) ], but it may nevertheless augment the effects of medication. This possibility is suggested by a recent factorial experiment we conducted that evaluated the effectiveness of four Motivation-phase components (Nicotine Gum, Nicotine Patch, Reduction Counseling, Motivational Interviewing) in primary care patients who were willing to cut down but not quit [(17)]. Interventions were administered over a 6 to 12-week treatment period with cessation treatment access for those who became ready to quit. Results showed that reduction counseling increased abstinence at 12 weeks relative to no reduction counseling and nicotine gum increased the likelihood of making a quit attempt by 6 weeks [(18)]. However, the effects of both components were small and neither improved longer-term abstinence.
This research allows us to examine further the extent to which smoking reduction counseling adds to the effectiveness of NRT. It will also reveal how two additional counseling or behavioral components affect Motivation-phase treatment success: behavioral activation [BA] counseling and Motivational 5Rs counseling. These were included based on their potential to address Motivation-phase challenges (factors that hinder quit attempts and/or abstinence) and to translate easily into real world healthcare settings [(12, 19–21)]. BA counseling, a novel Motivation-phase intervention, was intended to reduce exposure to smoking cues and contexts and increase access to non-smoking reinforcement, both of which should decrease the severity of tobacco withdrawal. The 5Rs Motivation Counseling (i.e., a motivational intervention exploring the Relevance and Risks of smoking, and the Rewards of quitting and Roadblocks on a Repeated basis [(19)]) was designed to increase awareness of intrinsic and autonomous motives for quitting, tip the balance of pros and cons in favor of quitting, and address obstacles to quitting.
The Motivation-phase treatments were offered with two features that we hypothesized would boost intervention effectiveness. The first was that all interventions were delivered over a 1-year period (versus 6–12 weeks in our prior trial; [(17)]) to provide greater opportunity to influence mechanisms relevant to the Motivation phase. A similar duration has been used in most prior Motivation-phase research [(13, 14)]. The second feature was one that was used in the earlier Cook et al. [(17)] research: Motivation-phase treatment was offered with ready access to evidence-based cessation treatment, allowing participants to transition easily to cessation treatment if they became ready to quit. Much of the work in this area has relied upon the Motivation-phase intervention itself to increase abstinence rates rather than offering a cessation intervention for those attempting to quit [(13)]. There is evidence, however, that cessation treatment availability may boost abstinence during Motivation-phase treatment [(18, 22)]).
In sum, the aims of the current factorial screening study were to evaluate the main and interactive effects of four Motivation-phase intervention components (Nicotine Mini-Lozenge, Reduction Counseling, BA Counseling, and Motivational 5Rs Counseling) on: 1) point-prevalence abstinence at 26 and 52 weeks post-enrollment (52 weeks was the primary outcome), and 2) making at least 1 quit attempt (with abstinence ≥ 24 hours) by 26 and 52 weeks. We also evaluated the relation between using cessation treatment during a quit attempt (versus making an unaided quit attempt) and abstinence.
METHODS
Procedure
Participants (N=577) were recruited from January 2015 to March 2019 in 16 primary care clinics from two southern Wisconsin healthcare systems (see Supplementary material for information on the two healthcare systems). Power analysis calculations supporting the sample size are reported in the Supplement. The trial was submitted to clinicaltrials.gov (NCT02354872) prior to commencement of data collection. Medical assistants invited adult outpatients attending a clinic visit who smoked to participate in a research program to help them either quit or reduce their smoking (offered simultaneously, not sequentially). Interested patients were electronically referred to the research office. Patients were also recruited via mailings and messaging through the electronic health record. During the screening call, study candidates not interested in quitting in the next 30 days but willing to reduce their smoking were invited to enroll in a research program for smoking reduction. Inclusion criteria were: > 17 years old; smoke >4 cigarettes/day for the previous 6 months; no interest in quitting in the next 30 days but willing to cut down; able to read and speak English; being a patient at a participating clinic; not currently taking bupropion or varenicline; agreeing to use only study smoking medication if currently using NRT; no medical contraindications to NRT use; and, for women of childbearing potential, agreeing to use an approved birth control method.
Eligible patients were invited to return to their primary care clinic to meet with a study health counselor to learn more about the study and provide written informed consent. For those enrolled in the study, a database created a schedule guiding all assessment and treatment delivery contacts. Health counselors were bachelor’s level research staff supervised by licensed clinical psychologists. Assessors were not involved in treatment delivery.
The intervention components were administered over a 1-year period. All participants received 15-minute assessment calls at 12, 26, 39, and 52 weeks post-study enrollment. Participants could elect to receive cessation treatment at any point throughout the 1-year study; they were also explicitly offered cessation treatment at weeks 15, 30, and 47. The same cessation treatment was provided to all participants who expressed interest in quitting: 8 weeks of nicotine patch + mini-lozenge and four brief phone counseling sessions.
Study Design
The factors in this 24 factorial experiment were Nicotine Mini-Lozenge; Reduction Counseling; Behavioral Activation; and Motivational 5Rs. Each factor had an ON level (indicating assignment to an ‘active’ intervention component) and an OFF level (indicating non-receipt of the intervention component). Participants were randomized via stratified (by sex [female vs. male]), permuted, computer-generated block randomization to 16 unique treatment conditions (combinations of intervention components) that together instantiated all possible combinations of the ON levels of the 4 factors (see Figure 1 and Supplemental Tables 1 & 2). This completely crossed factorial design allows researchers to examine the main effects of each factor efficiently by comparing all participants in the ON level of a factor to all of those in the OFF level of that factor, averaged across the other three factors. Thus, while there are 16 unique combinations of treatment components, each test of a main effect used all experimental participants (N=577; with half receiving an ON level of a factor and half an OFF level). Intervention components were designed to be: 1) compatible with one another, 2) translatable to real-world practice, and 3) delivered with fidelity across all treatment combinations using database prompts. See Supplemental Tables 3-5 for counseling protocols and fidelity procedures.
Figure 1.

Key features of the study design.
1. Nicotine Mini-Lozenge vs. No Lozenge.
Participants receiving Nicotine Mini-Lozenge (ON level) were instructed to use 2-mg mini-lozenges (≥9/day, 1 piece/1–2 hours) in place of smoking. Participants received 12-weeks of medication at Visit 1 and were sent additional medication after assessment calls at Weeks 12, 26, and 39.
2. Reduction Counseling vs. No Reduction Counseling.
Participants in the ON level (i.e., Reduction Counseling) received a 20-minute in-person counseling session followed by nine, 10–15 minute counseling calls over 52-weeks. Reduction Counseling emphasized the development of smoking control skills via practice of smoking reduction activities (e.g., delaying smoking, eliminating smoking in specific situations). It also emphasized the development of competence and self-efficacy stemming from practicing smoking control and reduction skills.
3. Behavioral Activation (BA) vs. No BA.
Participants in the ON level received a 20-minute in-person counseling session followed by nine, 10–15 minute counseling calls over 52-weeks. BA goals focused on increasing engagement in positively reinforcing activities while not smoking. This involved ongoing assignments and self-monitoring of activities aimed at increasing the participant’s nonsmoking reinforcement and decreasing exposure to smoking cues.
4. Motivational 5Rs vs. No Motivational 5Rs.
Participants in the ON level received an initial 20-minute in-person counseling session followed by 3 quarterly 15-minute counseling calls. Discussions, guided by motivational principles (e.g., use of open-ended questions and a supportive, non-authoritarian style [(23)]) were structured around the: 1) Relevance of smoking to the individual; 2) Risks of continued heavy smoking; 3) Rewards of quitting and significant reduction; and 4) Roadblocks to success; on a 5) Repeated basis [(18)].
Assessments and Outcome Measures
Participants completed baseline assessments of demographics, tobacco use history, and dependence (e.g., the Fagerström Test for Nicotine Dependence [FTND]: [(24)]). Adverse events, medication adherence, and smoking in the past week were assessed during study contacts and follow-up assessments at weeks 12, 26, 39, and 52.
The primary outcome was self-reported 7-day point-prevalence abstinence at 52 weeks (not biochemically confirmed). The secondary outcomes were point-prevalence abstinence at 26 weeks and making a serious quit attempt (achieving abstinence ≥ 24 hrs) by weeks 26 and 52. During each follow-up call, participants reported any smoking over the last 7 days and, if they had abstained from smoking for the purpose of quitting, how many hours they abstained. Participants who reported abstinence of more than 24 hours at the 26- or 52-week assessment calls were identified as having made a quit attempt by 26- and 52-weeks, respectively.
Analytic Plan
Analyses were conducted using SAS 9.4. Separate linear regression models were used to analyze treatment adherence, safety, and associations with missing data. A priori plans called for the estimation via effect coding of the four main effects (each coded −1=OFF level; +1=ON level), all interactions, and selected covariates. With effect coding, the main effect of a factor is estimated by comparing the mean of all conditions for which a factor is ON (8 of the 16 conditions) with the mean of all conditions where the factor is OFF (the other 8 conditions; see Figure 1). The estimated effects are uncorrelated when the design is perfectly balanced and only modestly correlated unless there are large differences in the sizes of individual experimental conditions, permitting interpretation of a factor’s main effects in the presence of significant interactions (although interactions should always be considered). Sensitivity analyses with multiple imputation for missing outcome data [(25)] yielded a highly similar pattern of significant findings as that obtained with the assumption of missing=smoking or missing=no quit attempt1. Therefore, we present results from analyses using the latter, fixed imputation assumptions (see Supplemental Tables 6–9 for sensitivity analyses).
Multivariable logistic regression was used to analyze: 1) point-prevalence abstinence at 26 and 52 weeks and 2) making a quit attempt by 26 and 52 weeks. Participants who did not provide outcome information were assumed to be smoking (i.e., missing=smoking) or to have not made a quit attempt (i.e., missing=no quit attempt). All models were conducted as both unadjusted and adjusted models using a predetermined set of covariates: sex, race (White only vs. other), age, education (up to high school versus at least some college), baseline exhaled carbon monoxide, heaviness of smoking index, and healthcare system (healthcare system A vs. B). The adjusted models were conducted to assess the robustness of the obtained findings for the primary, unadjusted models. Only the point-prevalence abstinence model included enrollment into the cessation treatment in the adjusted model. Patterns of statistical significance were similar between unadjusted and adjusted models; only results from unadjusted models are discussed. Finally, initial screening research is designed to detect relative promise rather than to draw strong inference about the effectiveness of components [(8, 9, 26, 27)]; therefore, our primary outcome (abstinence) does not involve correction for experimentwise error. However, we used Benjamini-Hochberg correction for the secondary quit attempt outcome [(28)].
RESULTS
Participants
Of the 1,592 patients who smoke who expressed initial interest in a program to reduce their smoking, 577 provided consent (see Figure 2 for CONSORT diagram). Recruitment varied from 7–84 participants/clinic. Table 1 provides demographic and tobacco-related data.
Figure 2.
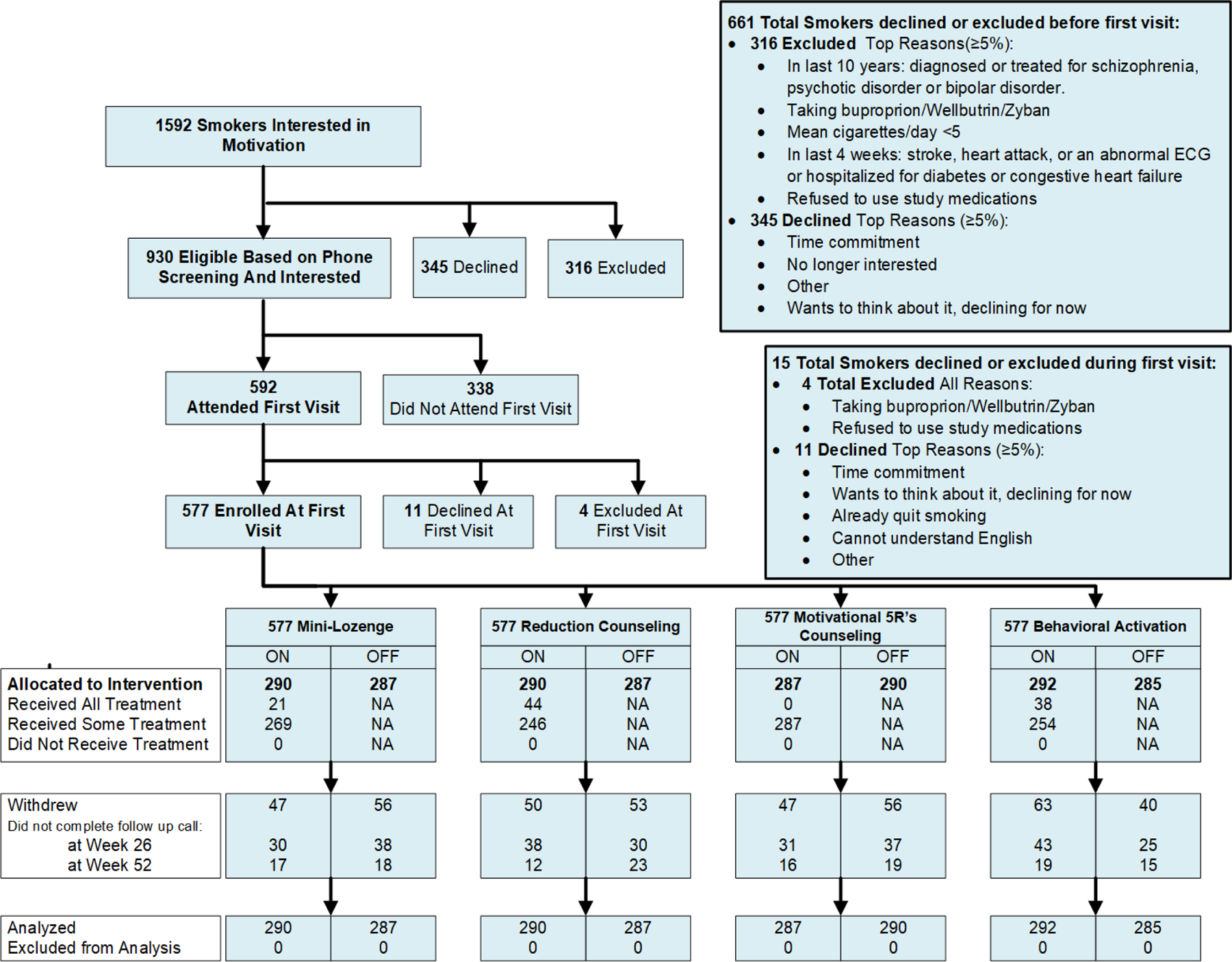
Consolidated Standards of Reporting Trials (CONSORT) diagram
Table 1.
Demographics and smoking history characteristics (N=577)
| Total Sample | Mini-Lozenge |
Behavioral Reduction |
5Rs Motivation |
Behavioral Activation |
|||||
|---|---|---|---|---|---|---|---|---|---|
| On | Off | On | Off | On | Off | On | Off | ||
| Female (%) | 60.0 | 59.7 | 60.4 | 60.3 | 59.8 | 60.1 | 59.9 | 60.4 | 59.6 |
| Health system A (%) | 54.1 | 56.0 | 52.2 | 58.4 | 49.8 | 54.0 | 54.2 | 53.8 | 54.4 |
| Less than high school (%) | 13.7 | 13.5 | 13.8 | 12.7 | 14.6 | 13.6 | 13.7 | 12.9 | 14.5 |
| High school diploma or GED only (%) | 35.2 | 38.2 | 32.2 | 33.5 | 36.9 | 34.6 | 35.8 | 34.0 | 36.4 |
| At least some college (%) | 51.1 | 48.3 | 54.1 | 53.9 | 48.4 | 51.8 | 50.5 | 53.1 | 49.1 |
| White (%) | 80.1 | 80.8 | 80.8 | 80.1 | 81.5 | 81.0 | 80.5 | 82.7 | 78.9 |
| Black (%) | 16.0 | 16.8 | 15.2 | 15.3 | 16.7 | 15.8 | 16.3 | 14.1 | 17.9 |
| Hispanic/Latino (%) | 3.2 | 2.4 | 4.0 | 4.6 | 1.8 | 3.2 | 3.2 | 3.2 | 3.2 |
| Age (mean, SD) | 52.4 (13.5) | 52.8 (13.1) | 52.0 (13.9) | 52.9 (13.6) | 51.9 (13.4) | 52.0 (14.0) | 52.8 (13.0) | 52.9 (13.7) | 51.9 (13.3) |
| Cigs/day (mean, SD) | 17.3 (9.2) | 17.7 (10.0) | 16.8 (8.3) | 17.6 (9.3) | 17.0 (9.1) | 17.3 (9.4) | 17.2 (9.1) | 17.8 (9.6) | 16.8 (8.8) |
| FTND (mean, SD) | 4.8 (2.1) | 4.9 (2.1) | 4.6 (2.1) | 4.8 (2.1) | 4.7 (2.1) | 4.8 (2.0) | 4.7 (2.2) | 4.9 (2.1) | 4.7 (2.1) |
| Motivation to Quit (mean, SD) | 5.2 (1.5) | 5.3 (1.5) | 5.1 (1.6) | 5.2 (1.6) | 5.1 (1.5) | 5.2 (1.5) | 5.1 (1.5) | 5.1 (1.5) | 5.2 (1.5) |
| Confidence in Quitting (mean, SD) | 4.3 (1.7) | 4.3 (1.7) | 4.2 (1.7) | 4.3 (1.6) | 4.3 (1.8) | 4.3 (1.7) | 4.3 (1.7) | 4.1 (1.8) | 4.5 (1.6) |
| Heaviness of Smoking Index (mean, SD) | 3.0 (1.4) | 3.0 (1.4) | 3.0 (1.4) | 3.0 (1.4) | 3.0 (1.4) | 3.0 (1.4) | 3.0 (1.5) | 3.1 (1.4) | 2.9 (1.5) |
| Baseline CO ppm (mean, SD) | 19.5 (14.0) | 18.9 (10.2) | 20.0 (16.9) | 19.9 (16.3) | 19.1 (11.2) | 20.1 (16.5) | 18.5 (10.9) | 19.7 (16.5) | 19.2 (10.7) |
GED=general educational development; FTND=Fagerstrom Test for Nicotine Dependence (0–10); SD=standard deviation; On=subject received the intervention component; Off=subject did not receive the intervention component; CO ppm=carbon monoxide parts per million; Motivation to Quit and Confidence in Quitting range from 0–10.
Treatment Participation
Participants in the Reduction Counseling condition attended a mean of 5.3 (SD=3.0) of 10 sessions. Participants in the BA condition attended a mean of 5.1 (SD=3.1) of 10 sessions. Participants in the 5Rs Motivational Counseling condition attended a mean of 2.1 (SD=1.2) of 4 sessions. Participants in the Mini-Lozenge condition reported using an average of 2.9 lozenges/day (SD=3.5) when asked about lozenge use over the past week at different points during the treatment period. In addition, 29% of participants (168/577) elected to receive Cessation-phase treatment at some point during treatment.
Safety
There were no serious adverse events related to study participation. The most common adverse event for Mini-Lozenge was nausea (16%), followed by indigestion (14%) and hiccups (13%).
Missing Data
The percentage of participants with missing point-prevalence smoking abstinence data was 24% (137/577) at week 26 and 33% (190/577) at week 52. The percentage of participants with missing quit attempt data was 22% (129/577) at week 26 and 27% (155/577) at week 52. Participants with missing abstinence data at 52 weeks (the primary outcome) were older and reported less confidence in quitting than were those without missing values (p’s < .05). No treatment factors were significantly associated with missing abstinence data at 52 weeks across the contrasting levels of the experimental intervention factors.
Abstinence
Table 2 presents self-reported 7-day point-prevalence abstinence rates for each factor at 26- and 52-weeks post study enrollment. There were no significant main effects on abstinence at 26 and 52 weeks (p>0.05). There was a significant three-way interaction (Mini-Lozenge x Reduction Counseling x BA) on abstinence at 52 weeks (p=0.03; see Table 3). As the raw data graphed in Figure 3 show, Mini-Lozenge alone was associated with the highest abstinence rate (16.7%), but the combination of Mini-Lozenge and Reduction Counseling produced an abstinence rate of only 4.1%. There was also evidence that BA may have reduced the effectiveness of the Mini-Lozenge, but this antagonistic effect appeared weaker than that produced by Reduction Counseling (see Figure 3). The interaction effect remained significant in the adjusted model and in the multiply imputed model. We explored the possibility that the effectiveness of Mini-Lozenge decreased with increases in the number of counseling components with which it was paired (i.e., across 0–3 possible adjuvant counseling components). When averaged across counseling component numbers (irrespective of type), the effectiveness of the mini-lozenge tended to decrease with increases in the number of adjuvant counseling components as reflected in these 52-week abstinence rates (number of counseling components: 0=16.2%, 1=10.3%, 2=11.0%, 3=5.41%). No other interaction effect showed a significant association with abstinence at either time point (p>0.05).
Table 2:
Self-reported point-prevalence abstinence rates at 26- and 52-weeks post-study enrollment (N=577)
| % Abstinent at 26 Weeks |
% Abstinent at 52 Weeks |
|||
|---|---|---|---|---|
| Overall: | 8.3 | Overall: | 9.7 | |
|
|
|
|||
| Factor | On | Off | On | Off |
| Mini-Lozenge | 9.0 | 7.7 | 10.7 | 8.7 |
| Reduction Counseling | 7.9 | 8.7 | 7.9 | 11.5 |
| Motivational 5Rs | 8.4 | 8.3 | 8.7 | 10.7 |
| Behavioral Activation | 8.2 | 8.4 | 8.9 | 10.5 |
Note. On = subject received intervention component; Off = subject did not receive intervention component. Also, participants often received more than one intervention component and these percentages reflect the effects of such co-occurring intervention components.
Table 3.
Unadjusted and adjusted logistic regression analysis for seven-day point-prevalence abstinence at weeks 26 and 52 post-study enrollment (N=577)
| 26 Weeks |
52 Weeks |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusteda |
Unadjusted |
Adjusteda |
|||||||||||||
| Model effect | b | 95% CL | Wald | P | b | 95% CL | Wald | P | b | 95% CL | Wald | P | b | 95% CL | Wald | P |
| Lozenge | 0.12 | (−0.21, 0.45) | 0.52 | 0.47 | 0.29 | (−0.06, 0.65) | 2.59 | 0.11 | 0.10 | (−0.22, 0.42) | 0.35 | 0.55 | 0.17 | (−0.16, 0.50) | 1.00 | 0.32 |
| RC | 0.01 | (−0.32, 0.34) | 0.00 | 0.96 | 0.00 | (−0.35, 0.35) | 0.00 | 0.99 | −0.17 | (−0.49, 0.15) | 1.06 | 0.30 | −0.13 | (−0.47, 0.20) | 0.62 | 0.43 |
| Mot5Rs | 0.00 | (−0.33, 0.33) | 0.00 | 0.99 | −0.02 | (−0.37, 0.33) | 0.01 | 0.93 | −0.12 | (−0.44, 0.2) | 0.57 | 0.45 | −0.13 | (−0.46, 0.20) | 0.57 | 0.45 |
| BA | −0.07 | (−0.4, 0.26) | 0.16 | 0.68 | 0.03 | (−0.33, 0.38) | 0.02 | 0.89 | −0.18 | (−0.5, 0.14) | 1.17 | 0.28 | −0.16 | (−0.49, 0.17) | 0.85 | 0.36 |
| Loz x RC | −0.26 | (−0.59, 0.07) | 2.42 | 0.12 | −0.29 | (−0.64, 0.06) | 2.59 | 0.11 | −0.29 | (−0.61, 0.03) | 3.13 | 0.08 | −0.30 | (−0.63, 0.03) | 3.14 | 0.08 |
| Loz x Mot5Rs | 0.16 | (−0.17, 0.49) | 0.96 | 0.33 | 0.19 | (−0.16, 0.55) | 1.17 | 0.28 | 0.19 | (−0.13, 0.51) | 1.33 | 0.25 | 0.21 | (−0.12, 0.54) | 1.52 | 0.22 |
| Loz x BA | 0.16 | (−0.17, 0.49) | 0.92 | 0.34 | −0.27 | (−0.08, 0.63) | 2.25 | 0.13 | 0.02 | (−0.3, 0.34) | 0.02 | 0.89 | 0.06 | (−0.27, 0.39) | 0.11 | 0.74 |
| RC x Mot5Rs | −0.13 | (−0.46, 0.20) | 0.58 | 0.45 | 0.15 | (−0.50, 0.20) | 0.68 | 0.41 | 0.05 | (−0.27, 0.37) | 0.09 | 0.77 | 0.06 | (−0.27, 0.39) | 0.13 | 0.72 |
| RC x BA | 0.18 | (−0.15, 0.51) | 1.13 | 0.29 | 0.21 | (−0.14, 0.56) | 1.38 | 0.24 | 0.05 | (−0.27, 0.37) | 0.09 | 0.77 | 0.09 | (−0.24, 0.42) | 0.28 | 0.60 |
| BA x Mot5Rs | 0.03 | (−0.3, 0.36) | 0.03 | 0.87 | 0.01 | (−0.35, 0.36) | 0.00 | 0.96 | 0.17 | (−0.15, 0.49) | 1.11 | 0.29 | 0.20 | (−0.13, 0.54) | 1.42 | 0.23 |
| Loz x RC x BA | −0.26 | (−0.59, 0.07) | 2.48 | 0.12 | −0.28 | (−0.63, 0.07) | 2.39 | 0.12 | −0.36 | (−0.68, −0.04) | 4.99 | 0.03 | −0.38 | (−0.71, −0.05) | 5.03 | 0.03 |
| Loz x RC x Mot5Rs | −0.06 | (−0.39, 0.27) | 0.13 | 0.72 | −0.05 | (−0.41, 0.3) | 0.09 | 0.76 | −0.03 | (−0.35, 0.29) | 0.04 | 0.84 | −0.02 | (−0.35, 0.32) | 0.01 | 0.93 |
| Loz x Mot5Rs x BA | −0.01 | (−0.34, 0.32) | 0.01 | 0.93 | −0.03 | (−0.38, 0.32) | 0.03 | 0.87 | 0.16 | (−0.16, 0.48) | 0.97 | 0.33 | 0.20 | (−0.13, 0.53) | 1.44 | 0.23 |
| RC x Mot5Rs x BA | 0.09 | (−0.24, 0.42) | 0.26 | 0.61 | 0.08 | (−0.27, 0.43) | 0.22 | 0.64 | −0.04 | (−0.36, 0.28) | 0.05 | 0.83 | −0.08 | (−0.41, 0.26) | 0.20 | 0.66 |
| Loz x RC x Mot5Rs x BA | 0.12 | (−0.21, 0.45) | 0.55 | 0.46 | 0.22 | (−0.14, 0.57) | 1.41 | 0.23 | −0.03 | (−0.35, 0.29) | 0.03 | 0.86 | −0.01 | (−0.35, 0.32) | 0.01 | 0.94 |
Bold indicates p<0.05.
Adjusted model controlled for age, sex, race (White only versus other), education (up to high school diploma or GED [general educational development] versus at least some college), health-care system (A versus B), Heaviness of Smoking Index, and participation in the Cessation Program. RC=Reduction Counseling; Mot5Rs=Motivational 5Rs Counseling; BA=Behavioral Activation; Loz=Nicotine Mini-Lozenge
Figure 3.
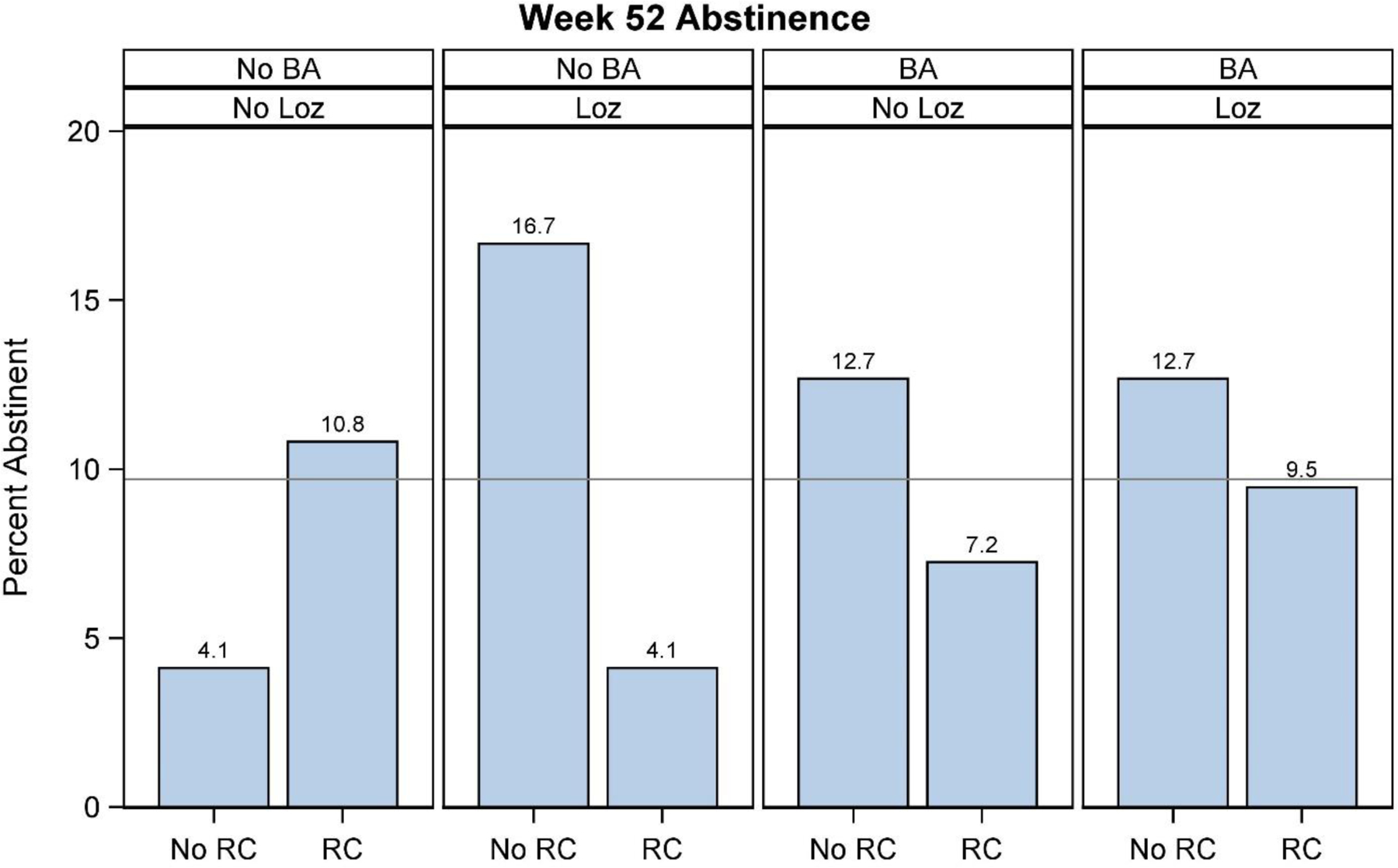
Bar graph displaying the Mini-Lozenge (Loz) x Reduction Counseling (RC) x Behavioral Activation Counseling (BA) interaction for percent abstinent at 52 weeks post-study enrollment. The reference line represents the overall abstinence rate of the full sample (N=577).
We also analyzed 7-day point-prevalence abstinence among participants who elected to receive cessation treatment during the study (n=168). Reduction Counseling produced a negative main effect at 52 weeks (p=0.04; see Supplemental Table 10). Amongst participants who chose to use Cessation-phase treatment, those who had received Motivation-phase Reduction Counseling were less likely to achieve abstinence at 52 weeks follow-up (Reduction Counseling = 13% abstinent; no counseling = 26% abstinent).
Quit Attempts
Table 4 presents quit attempt rates and mean number of quit attempts for each factor by weeks 26 and 52. As shown in Table 5, there was a significant negative main effect for Reduction Counseling at 52 weeks (p=0.01). Participants who received Reduction Counseling were 36% less likely to report a quit attempt within one year following study enrollment. A similar effect was found in the covariate adjusted model (see Table 5). However, the main effect of Reduction Counseling on quit attempts was not significant when applying the Benjamini-Hochberg correction (p=.15; See Supplementary Table 11). Finally, no other main effects (lozenge, motivational 5R’s counseling, behavioral activation) or interactions were significantly associated with quit attempts (p>0.05).
Table 4.
Self-reported quit attempt rates and mean number of quit attempts made by weeks 26 and 52 post-study enrollment (N=577)
| % who made a quit attempt by Week 26 |
% who made a quit attempt by Week 52 |
Mean number of quit attempts by Week 26 (SD) |
Mean number of quit attempts by Week 52 (SD) |
|||||
|---|---|---|---|---|---|---|---|---|
| Factor | On | Off | On | Off | On | Off | On | Off |
| Mini-Lozenge | 35.5 | 34.5 | 46.9 | 45.0 | 2.3 (7.1) | 1.8 (6.5) | 5.8 (14.1) | 3.5 (9.1) |
| Reduction Counseling | 31.7 | 38.3 | 40.7 | 51.2 | 1.7 (6.5) | 2.4 (7.1) | 4.2 (11.8) | 5.1 (12.1) |
| Motivational 5Rs | 35.2 | 34.8 | 47.0 | 44.8 | 2.1 (7.0) | 2.1 (6.6) | 4.7 (11.7) | 4.7 (12.2) |
| Behavioral Activation | 34.9 | 35.1 | 43.8 | 48.1 | 1.8 (5.1) | 2.3 (8.2) | 4.1 (10.4) | 5.3 (13.4) |
On = subject received intervention component; Off = subject did not receive intervention component; SD = standard deviation
Table 5.
Unadjusted and adjusted logistic regression analysis of making a quit attempt by Week 26 and Week 52 post-study enrollment (n=577)
| 26 Weeks |
52 Weeks |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusteda |
Unadjusted |
Adjusteda |
|||||||||||||
| Model effect | b | 95% CL | Wald | P | b | 95% CL | Wald | P | b | 95% CL | Wald | P | b | 95% CL | Wald | P |
| Lozenge | 0.04 | (−0.11, 0.23) | 0.24 | 0.62 | 0.05 | (−0.12, 0.25) | 0.27 | 0.60 | 0.05 | (−0.13, 0.21) | 0.31 | 0.58 | 0.05 | (−0.13, 0.22) | 0.33 | 0.57 |
| RC | −0.15 | (−0.32, 0.02) | 3.11 | 0.08 | −0.15 | (−0.33, 0.03) | 2.68 | 0.10 | −0.22 | (−0.40, −0.06) | 6.98 | 0.01 | −0.24 | (−0.43, −0.08) | 7.44 | 0.01 |
| Mot5Rs | 0.01 | (−0.16, 0.19) | 0.02 | 0.87 | 0.03 | (−0.15, 0.21) | 0.09 | 0.76 | 0.06 | (−0.10, 0.23) | 0.44 | 0.51 | 0.07 | (−0.09, 0.25) | 0.66 | 0.42 |
| BA | −0.01 | (−0.18, 0.16) | 0.01 | 0.92 | 0.07 | (−0.12, 0.25) | 0.53 | 0.46 | −0.08 | (−0.24, 0.09) | 0.92 | 0.34 | −0.04 | (−0.21, 0.14) | 0.21 | 0.65 |
| Loz x RC | −0.10 | (−0.27, 0.08) | 1.23 | 0.27 | −0.12 | (−0.30, 0.06) | 1.59 | 0.21 | −0.13 | (−0.29, 0.04) | 2.39 | 0.12 | −0.13 | (−0.30, 0.05) | 2.11 | 0.15 |
| Loz x Mot5Rs | 0.07 | (−0.10, 0.24) | 0.64 | 0.43 | 0.09 | (−0.09, 0.27) | 0.88 | 0.35 | 0.07 | (−0.11, 0.23) | 0.63 | 0.43 | 0.08 | (−0.10, 0.25) | 0.77 | 0.38 |
| Loz x BA | 0.06 | (−0.11, 0.23) | 0.45 | 0.50 | 0.04 | (−0.14, 0.22) | 0.21 | 0.65 | 0.06 | (−0.11, 0.22) | 0.53 | 0.47 | 0.06 | (−0.12, 0.23) | 0.43 | 0.51 |
| RC x Mot5Rs | −0.05 | (−0.20, 0.14) | 0.29 | 0.59 | −0.02 | (−0.19, 0.17) | 0.06 | 0.80 | −0.01 | (−0.17, 0.16) | 0.00 | 1.00 | 0.02 | (−0.17, 0.18) | 0.03 | 0.86 |
| RC x BA | 0.11 | (−0.07, 0.27) | 1.66 | 0.20 | 0.12 | (−0.08, 0.28) | 1.61 | 0.20 | 0.05 | (−0.12, 0.21) | 0.37 | 0.54 | 0.04 | (−0.14, 0.21) | 0.21 | 0.64 |
| BA x Mot5Rs | −0.02 | (−0.21, 0.13) | 0.08 | 0.78 | −0.03 | (−0.23, 0.14) | 0.12 | 0.73 | 0.01 | (−0.15, 0.18) | 0.02 | 0.89 | 0.02 | (−0.15, 0.20) | 0.05 | 0.82 |
| Loz x RC x BA | 0.04 | (−0.15, 0.19) | 0.19 | 0.67 | 0.02 | (−0.17, 0.19) | 0.07 | 0.79 | 0.06 | (−0.10, 0.23) | 0.46 | 0.50 | 0.07 | (−0.10, 0.25) | 0.63 | 0.43 |
| Loz x RC x Mot5Rs | −0.07 | (−0.23, 0.11) | 0.73 | 0.39 | −0.11 | (−0.27, 0.09) | 1.41 | 0.23 | −0.01 | (−0.17, 0.16) | 0.03 | 0.86 | −0.05 | (−0.22, 0.13) | 0.33 | 0.57 |
| Loz x Mot5Rs x BA | 0.04 | (−0.15, 0.02) | 0.23 | 0.63 | 0.06 | (−0.14, 0.22) | 0.42 | 0.52 | 0.11 | (−0.06, 0.27) | 1.68 | 0.19 | 0.13 | (−0.06, 0.29) | 2.02 | 0.16 |
| RC x Mot5Rs x BA | 0.06 | (−0.12, 0.23) | 0.42 | 0.52 | 0.05 | (−0.13, 0.23) | 0.29 | 0.59 | 0.05 | (−0.12, 0.21) | 0.35 | 0.55 | 0.06 | (−0.12, 0.23) | 0.52 | 0.47 |
| Loz x RC x Mot5Rs x BA | 0.06 | (−0.11, 0.23) | 0.50 | 0.48 | 0.09 | (−0.10, 0.26) | 0.84 | 0.36 | 0.04 | (−0.12, 0.21) | 0.20 | 0.65 | 0.04 | (−0.12, 0.22) | 0.23 | 0.63 |
Bold indicates p<0.05.
Adjusted model controlled for age, sex, race (White only versus other), education (up to high school diploma or GED [general educational development] versus at least some college), health-care system (A versus B), and Heaviness of Smoking Index. RC=Reduction Counseling; Mot5Rs= Motivational 5Rs Counseling, BA=Behavioral Activation; Loz=Nicotine Mini-Lozenge
We also examined relations between using study-provided cessation treatment and 7-day point-prevalence abstinence at 52 weeks amongst those reporting that they made a quit attempt over the 52-week study period. The 52-week abstinence rates for those who did and did not use cessation treatment during a quit attempt were 25% and 16%, respectively (p<.001).
DISCUSSION
The goal of this study was to identify Motivation-phase intervention components that increase quit attempts and smoking abstinence in patients recruited in a primary care setting. None of the intervention components yielded significant main effects on abstinence. However, a significant Mini-Lozenge x Reduction Counseling x BA interaction on abstinence at 52 weeks revealed that the effectiveness of Mini-Lozenge was influenced by other components. Specifically, Mini-Lozenge was most effective when administered without Reduction Counseling and Behavioral Activation (16.7% were abstinent); when paired with either of those counseling strategies but particularly when paired with Reduction Counseling, the benefit of Mini-Lozenge was reduced. Of note, the effects of these three intervention components are averaged across the two levels (On/Off) of the Motivational 5Rs factor that were not included in the interaction [(6)].
This research evaluates whether any of the counseling components enhanced the effectiveness of the mini-lozenge on abstinence in those initially unwilling to enter cessation treatment. None of the behavioral intervention components produced a significant main effect (including the novel Motivation phase components: Behavioral Activation and Motivational 5Rs), indicating that none exerted significant additive effects with the mini-lozenge or with one another. Moreover, while the mini-lozenge did not produce a main effect, there was evidence that it enhanced long-term abstinence when used without Reduction Counseling or Behavioral Activation Counseling. Thus, results suggest that the best approach to Motivation-phase treatment may involve the provision of NRT, in this case nicotine mini-lozenge, with little or no additional counseling.
Examination of Reduction Counseling effects is especially important since it is so often used in Motivation-phase treatments [(13)]. The effect of Reduction Counseling, which was negative on average (Table 2: albeit nonsignificantly), became even more negative when paired with Behavioral Activation and Mini-Lozenge (Figure 2). In addition, those receiving Reduction Counseling were significantly less likely to make a quit attempt by 52 weeks (40.7%) than those receiving no Reduction Counseling (51.6%). Amongst the subset who entered cessation treatment, Reduction Counseling produced a significant negative main effect on 52-week abstinence (Reduction Counseling = 13% abstinent; no Reduction Counseling = 26% abstinent). This research suggests that reduction counseling might be harmful and demonstrates the potential for strong negative interactions amongst sets of intervention components.
Why might reduction counseling hinder success during Motivation-phase treatment? Perhaps reduction counseling convinced participants that reduction was an appropriate change goal, undercutting their motivation to attempt quitting. It is also possible that reduction counseling was viewed as burdensome, particularly when administered over an extended period. High perceived burden could directly undermine the likelihood of successfully quitting, or it could interfere with the effectiveness of medication [(17, 29, 30)]. In fact, when averaged across counseling factors, pairing mini-lozenge with additional counseling components decreased 52-week abstinence rates, suggesting a cost of treatment complexity that may not be restricted to reduction counseling [(31)] Motivation-phase counseling components might yield benefit if used differently. For example, some evidence suggests the effectiveness of reduction counseling that is administered over a brief interval (6–12 weeks; [(17)]) or via mailed self-help materials [(32)], both without use of NRT.
It is unclear whether extending Motivation-phase treatment out to a year increased the effectiveness of the intervention components relative to either past research or in terms of obtaining significant main effects. The effects of the mini-lozenge were slightly greater in the current experiment than was nicotine gum in the shorter (6–12 week) [(17)] experiment, but this difference was slight and could be due to the type of NRT (mini-lozenge vs. gum). In sum, this study suggests that extending Motivation-phase treatment beyond several months yields little additional benefit.
As in our prior research [(18)], this study supports the use of facilitated access to cessation treatment during Motivation-phase treatment. Participants who used study-provided cessation treatment were more successful than those making unaided quit attempts in achieving abstinence at 1 year (25% vs. 16%, respectively). The abstinence rate in those who used cessation treatment also exceeds the 1-year abstinence rates derived from other Motivation-phase interventions that did not provide access to cessation treatment (8%−12%; [(32)]). Of course, it is also possible that those who used versus did not use cessation treatment were more motivated to quit or differed in some other way that directly enhanced their quitting success.
These results suggest that novel approaches may be needed to attain more satisfactory success rates from Motivation-phase treatment (also see [(13, 33)]). Different pharmaceuticals might improve outcomes in those not ready to quit. Ebbert and colleagues [(22)] obtained impressively high continuous abstinence rates with varenicline treatment (37.8% through week 24; 27% through week 52). However, these rates likely reflect the combined effects of using varenicline during both the Motivation and Cessation phases of treatment. A prior, smaller Motivation-phase trial obtained equivocal results using varenicline in the Motivation phase [(33, 34)]. In both studies, however, the participants were interested or willing to commit to quitting at some point in the future (versus unwilling to quit).
A meaningful percentage of primary care patients who smoke entered Motivation-phase treatment (25%) when provided an option to enroll in either a program to help them quit or a program to reduce their smoking. Moreover, 29% of those who received Motivation-phase treatment eventually entered Cessation-phase treatment. Thus, results suggest that offering Motivation-phase treatment in addition to cessation treatment increases the proportion of people who smoke: 1) entering treatment for their tobacco use (perhaps a 25–30 percentage point increase; [(17)]), and 2) entering cessation treatment. However, since only a small proportion of the sample received no motivation treatment, it is difficult to make inferences about the rate of cessation treatment entry of an untreated sample. The abstinence rates resulting from Motivation-phase treatment are relatively modest (≈10%), but when this percentage is added to the 20–30% abstinence rate obtained amongst those who initially accept a cessation treatment offer [(35)], the combined abstinence rate has public health impact and supports the use of a chronic care approach to smoking treatment [(36, 37)]. Of course, we cannot definitively conclude that Motivation treatment increased treatment reach and abstinence because those who entered reduction treatment might have selected cessation had it been the only treatment available.
There are several study limitations that should be considered. The goal of this research was to detect the relative promise of the tested intervention components rather than to draw strong inferences about their effectiveness. Therefore, the effects of the components on abstinence are not protected by alpha correction. As per the MOST approach to intervention development, a subsequent RCT with appropriate statistical power would be needed to draw strong inferences about effects found in a screening experiment. Even though intervention components in factorial experiments often interact negatively (31), as they did in this study in the case of the Mini-Lozenge x Reduction Counseling x Behavioral Activation interaction, strong inferences about this interaction in particular would require replication. In addition, self-reports of abstinence were not biochemically confirmed and it is possible that self-reports could be differentially affected by intervention components. Attendance at treatment contacts was modest and there was a fair amount of missing data. Finally, this study specifically recruited individuals who were willing to cut down but not quit smoking; the findings might not generalize well to other people who smoke (e.g., those not interested in cutting down).
Conclusion
This research used innovative and efficient strategies to evaluate intervention components selected to be effective in the Motivation phase of smoking treatment. None of the intervention components yielded a significant main effect on abstinence. There was evidence though that the mini-lozenge enhanced abstinence rates when used without smoking reduction or BA. Further, smoking reduction treatment significantly decreased quit attempts. Results also suggest that the relatively long treatment duration (1-year) did not augment the success of Motivation-phase components relative to briefer, 12-week intervention [(17)]. Finally, results suggest that offering cessation treatment during Motivation-phase treatment may increase abstinence in those initially unwilling to quit smoking [(18)]. In sum, a simple approach involving provision of long-term PRN NRT with ready access to cessation treatment may be the most defensible approach to Motivation-phase treatment at present. However, this hypothesis requires further evaluation in a randomized controlled trial.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to acknowledge the staff at Epic Systems Corporation for their collaboration in this research. We are very grateful to the staff and students at the Center for Tobacco Research and Intervention in the University of Wisconsin School of Medicine and Public Health for their help with this research.
The authors have received no direct or indirect funding from, nor do they have a connection with, the tobacco, alcohol, pharmaceutical or gaming industries or anybody substantially funded by one of these organizations. Dr. Baker has received a grant of medication for a different research study from Pfizer Inc and has served as a consultant to ICF and the National Cancer Institute.
Footnotes
Declaration of Interest: This research was supported by a National Cancer Institute Grant [grant P01 CA180945], National Institute on Alcohol Abuse and Alcoholism [grant R01AA022931], National Institute on Drug Abuse [grant R01DA040480] and P50DA039838], National Institute on Diabetes and Digestive and Kidney Disease [grant R01DK097364], and Department of Veterans Affairs [grant 101CX00056].
Clinical Trial Registration: NCT02354872 (Motivation Project: Testing Intervention Components for the Smoker Who is Unwilling to Quit)
Multiple imputation analysis revealed a significant main effect of the mini-lozenge on quit attempts at 26 weeks in the secondary adjusted model which was not present in the findings obtained when using the adjusted missing=no quit attempt assumption.
REFERENCES
- 1.Centers for Disease Control and Prevention. Health effects of cigarette smoking2020 20July2020. Available from: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/effects_cig_smoking/index.htm.
- 2.Centers for Disease Control and Prevention. Quitting smoking among adults - United States 2001–2010. MMWR 2011;60(44):1513–9. [PubMed] [Google Scholar]
- 3.Piper ME, Baker TB, Mermelstein R, Collins LM, Fraser DL, Jorenby DE, et al. Recruiting and engaging smokers in treatment in a primary care setting: developing a chronic care model implemented through a modified electronic health record. Transl Behav Med 2013;3(3):253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etter JF, Perneger TV, Ronchi A. Distributions of smokers by stage: international comparison and association with smoking prevalence. Prev Med 1997;26(4):580–5. [DOI] [PubMed] [Google Scholar]
- 5.Wewers ME, Stillman FA, Hartman AM, Shopland DR. Distribution of daily smokers by stage of change: Current Population Survey results. Prev Med 2003;36(6):710–20. [DOI] [PubMed] [Google Scholar]
- 6.Collins LM. Optimization of behavioral, biobehavioral, and biomedical interventions: the Multiphase Optimization Strategy (MOST) New York, NY: Springer; 2018. [Google Scholar]
- 7.Collins LM, Dziak JJ, Kugler KC, Trail JB. Factorial experiments: efficient tools for evaluation of intervention components. Am J Prev Med 2014;47(4):498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins LM, Baker TB, Mermelstein RJ, Piper ME, Jorenby DE, Smith SS, et al. The Multiphase Optimization Strategy for engineering effective tobacco use interventions. Ann Behav Med 2011;41(2):208–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins LM, Kugler KC, Gwadz MV. Optimization of multicomponent behavioral and biobehavioral interventions for the prevention and treatment of HIV/AIDS. AIDS Behav 2016;20Suppl 1:S197–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker TB, Mermelstein R, Collins LM, Piper ME, Jorenby DE, Smith SS, et al. New methods for tobacco dependence treatment research. Ann Behav Med 2011;41(2):192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore A, Malinowski P. Meditation, mindfulness and cognitive flexibility. Conscious Cogn 2009;18(1):176–86. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. J Consult Clin Psychol 2004;72(3):371–81. [DOI] [PubMed] [Google Scholar]
- 13.Moore D, Aveyard P, Connock M, Wang D, Fry-Smith A, Barton P. Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis. BMJ 2009;338:b1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali A, Kaplan CM, Derefinko KJ, Klesges RC. Smoking cessation for smokers not ready to quit: meta-analysis and cost-effectiveness analysis. Am J Prev Med 2018;55(2):253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glasgow RE, Gaglio B, Estabrooks PA, Marcus AC, Ritzwoller DP, Smith TL, et al. Long-term results of a smoking reduction program. Med Care 2009;47(1):115–20. [DOI] [PubMed] [Google Scholar]
- 16.Klemperer EM, Hughes JR, Solomon LJ, Callas PW, Fingar JR. Motivational, reduction and usual care interventions for smokers who are not ready to quit: a randomized controlled trial. Addiction 2017;112(1):146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook JW, Collins LM, Fiore MC, Smith SS, Fraser D, Bolt DM, et al. Comparative effectiveness of motivation phase intervention components for use with smokers unwilling to quit: a factorial screening experiment. Addiction 2016;111(1):117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engle JL, Mermelstein R, Baker TB, Smith SS, Schlam TR, Piper ME, et al. Effects of motivation phase intervention components on quit attempts in smokers unwilling to quit: A factorial experiment. Drug Alcohol Depend 2019;197:149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, et al. Treating tobacco use and dependence: 2008 update Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service; 2008. [Google Scholar]
- 20.MacPherson L, Tull MT, Matusiewicz AK, Rodman S, Strong DR, Kahler CW, et al. Randomized controlled trial of behavioral activation smoking cessation treatment for smokers with elevated depressive symptoms. J Consult Clin Psychol 2010;78(1):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ekers D, Richards D, McMillan D, Bland JM, Gilbody S. Behavioural activation delivered by the non-specialist: phase II randomised controlled trial. Br J Psychiatry 2011;198(1):66–72. [DOI] [PubMed] [Google Scholar]
- 22.Ebbert JO, Hughes JR, West RJ, Rennard SI, Russ C, McRae TD, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA 2015;313(7):687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller WR, Rollnick S. Motivational interviewing: Preparing people for change (2nd ed.). New York: Guilford Press; 2002. [Google Scholar]
- 24.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 1991;86(9):1119–27. [DOI] [PubMed] [Google Scholar]
- 25.Hedeker D, Mermelstein RJ, Demirtas H. Analysis of binary outcomes with missing data: missing = smoking, last observation carried forward, and a little multiple imputation. Addiction 2007;102(10):1564–73. [DOI] [PubMed] [Google Scholar]
- 26.Schulz KF, Grimes DA. Multiplicity in randomised trials I: endpoints and treatments. Lancet 2005;365(9470):1591–5. [DOI] [PubMed] [Google Scholar]
- 27.Streiner DL, Norman GR. Correction for multiple testing: is there a resolution? Chest 2011;140(1):16–8. [DOI] [PubMed] [Google Scholar]
- 28.Benjamini Y, Hochberg Yl. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc, Series B Methodol 1995;57(1):289–300. [Google Scholar]
- 29.Baker TB, Hawkins R, Pingree S, Roberts LJ, McDowell HE, Shaw BR, et al. Optimizing eHealth breast cancer interventions: which types of eHealth services are effective? Transl Behav Med 2011;1(1):134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlam TR, Fiore MC, Smith SS, Fraser D, Bolt DM, Collins LM, et al. Comparative effectiveness of intervention components for producing long-term abstinence from smoking: a factorial screening experiment. Addiction 2016;111(1):142–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker TB. Barriers to building more effective treatments: negative interactions amongst smoking intervention components Clin Psychol Sci In press. [DOI] [PMC free article] [PubMed]
- 32.Meyer C, Ulbricht S, Haug S, Broda A, Bischof G, Rumpf HJ, et al. Motivating smokers to quit using computer-generated letters that target either reduction or cessation: A population-based randomized controlled trial among smokers who do not intend to quit. Drug Alcohol Depend 2016;166:177–86. [DOI] [PubMed] [Google Scholar]
- 33.Asfar T, Ebbert JO, Klesges RC, Relyea GE. Do smoking reduction interventions promote cessation in smokers not ready to quit? Addict Behav 2011;36(7):764–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes JR, Rennard SI, Fingar JR, Talbot SK, Callas PW, Fagerstrom KO. Efficacy of varenicline to prompt quit attempts in smokers not currently trying to quit: a randomized placebo-controlled trial. Nicotine Tob Res 2011;13(10):955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piper ME, Fiore MC, Smith SS, Fraser D, Bolt DM, Collins LM, et al. Identifying effective intervention components for smoking cessation: a factorial screening experiment. Addiction 2016;111(1):129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joseph AM, Fu SS, Lindgren B, Rothman AJ, Kodl M, Lando H, et al. Chronic disease management for tobacco dependence: a randomized, controlled trial. Arch Intern Med 2011;171(21):1894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellerbeck EF, Mahnken JD, Cupertino AP, Cox LS, Greiner KA, Mussulman LM, et al. Effect of varying levels of disease management on smoking cessation: a randomized trial. Ann Intern Med 2009;150(7):437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


