Abstract
Polarized cell growth is a ubiquitous process throughout the plant kingdom in which the cell elongates in a self-similar manner. This process is important for nutrient uptake by root hairs, fertilization by pollen, and gametophyte development by the protonemata of bryophytes and ferns. In this review, we will focus on the tip growth of moss cells, emphasizing the role of cytoskeletal organization, cytoplasmic zonation, vesicle traffic, cell wall composition, and dynamics. We compare some of the existing knowledge on tip growth in protonemata against what is known in pollen tubes and root hairs, which are better-studied tip growing cells. To fully understand how plant cells grow requires that we deepen our knowledge in a variety of forms of plant cell growth. We focus this review on the model plant Physcomitrium patens, which uses tip growth as the dominant form of growth at its protonemal stage. Because mosses and vascular plants shared a common ancestor more than 450 million years ago, we anticipate that both similarities and differences between tip growing plant cells will provide mechanistic information of tip growth as well as of plant cell growth in general. Towards this mechanistic understanding, we will also review some of the existing mathematical models of plant tip growth and their applicability to investigate protonemal morphogenesis. We attempt to integrate the conclusions and data across cell biology and physical modeling to our current state of knowledge of polarized cell growth in P. patens and highlight future directions in the field.
Keywords: polarized growth, F-Actin, myosin XI, wall mechanics, FRAP, polarized secretion, mathematical modeling
Introduction to Tip Growing Plant Cells
Pollen tubes and root hairs.
The best-studied cell for tip growth is the pollen tube (Rounds and Bezanilla 2013). Depending on the species, pollen tubes vary in width and growth rate, but they are highly elongated structures with some species reaching very high length to width ratios (Barnabas and Fridvalszky 1984). The growth rates observed for pollen tubes are some of the fastest of any cells, with lily pollen tubes reaching growth speeds of ~500 nm/sec in vitro (Cardenas et al. 2008). Although their pollen tubes grow slower, the genetic tools available in Arabidopsis and tobacco have led to these systems’ popularity (Derksen et al. 2002; Kost et al. 1999; Krysan et al. 1999). Less is known about pollen tubes from gymnosperms, but those that have been investigated elongate more slowly (~0.2 to 4 nm/sec) (Anderhag et al. 2000; Chen et al. 2007; Fernando et al. 2005; Williams 2012). Root hairs elongate from the root epidermis, increasing the root surface area, which is important for water and nutrient uptake (Mendrinna and Persson 2015). These cells are ~ 11 μm wide in Arabidopsis thaliana and can grow up to 800 μm in length at speeds of 15–50 nm/sec (Ichikawa et al. 2014; Monshausen et al. 2007). As with protonemata and pollen tubes, this innate structure makes these systems amenable to various live-cell microscopy techniques. Root hairs have emerged as an excellent model for plant growth because they are not essential for plant viability, allowing the wide use of mutational analysis to study the genes necessary in tip growth (Schiefelbein and Somerville 1990).
Physcomitrium (Physcomitrella) patens protonemata.
The moss P. patens has emerged as one of the best models to study tip growth in plants. This is in part due to the establishment of powerful experimental methods, such as genetic screens in a haploid background (Ashton and Cove 1977; Engel 1968), rapid and efficient transformations (Liu and Vidali 2011; Schaefer et al. 1991), gene targeting by homologous recombination (Schaefer and Zryd 1997), transient RNAi (Bezanilla et al. 2003; Bezanilla et al. 2005; Orr et al. 2020; Vidali et al. 2007), temperature-sensitive mutants (Ding et al. 2018; Vidali et al. 2009), rapid growth (Furt et al. 2013; Menand et al. 2007; Saavedra et al. 2015; van Gisbergen et al. 2012), and regenerative properties (Arif et al. 2019; Kubo et al. 2019). Following germination from the spore, P. patens is composed of long filamentous protonemal tissue that is required for development (Cove 2005; Vidali and Bezanilla 2012). The growth rate of protonemata is slower than a pollen tube, with caulonemata growing closer in rate to root hairs. Each filament is one cell thick and it is composed of many cells attached in series. Each cell is ~ 20 μm wide and ~ 200 μm in length. The cells at the end of these filaments exhibit tip growth and are classified as either chloronemata or caulonemata (Menand et al. 2007). Chloronemata contain densely packed chloroplasts and grow at a rate of ~1.5 nm/sec (Menand et al. 2007). Caulonemata have fewer chloroplasts and grow more quickly at a rate of ~5 nm/sec (Furt et al. 2013; Menand et al. 2007). Later in development, these filaments will eventually form the leafy gametophore. Because of their faster growth relative to chloronemata, and their vesicle enriched growth zone (Bibeau et al. 2018; Furt et al. 2013), caulonemata are the preferred cell type to study tip growth.
Cell Biology of Tip Growth in P. patens
Organelle distribution and zonation.
The distribution of organelles in tip growing cells has been well established through light and electron microscopy (Emons 1987; Jensen and Jensen 1984; Lancelle and Hepler 1992; Lazzaro 1999; McCauley and Hepler 1992) and their respective motilities have been determined through time-lapse using DIC optics or fluorescence studies (de Win et al. 1999; Furt et al. 2012; Justus et al. 2004; Lovy-Wheeler et al. 2007; Sieberer and Emons 2000; Tominaga et al. 1997) In general, tip-growing cells show an asymmetric distribution of organelles or “zonation” (Furt et al. 2012; Lancelle and Hepler 1992). The most prominent aspect of this zonation is the presence of a “clear zone” at the apical dome, where growth takes place. This clear zone is heavily enriched with Golgi, ER, and vesicles (Bove et al. 2008; Furt et al. 2012; Justus et al. 2004; Lancelle and Hepler 1992; McCauley and Hepler 1992). The concentration of vesicles at the apical dome highlights the requirement for the delivery of cell wall materials at the cell tip. The clear zone of rapidly growing pollen tubes, such as those from lily, and the much slower growing gymnosperm pollen (spruce) and moss caulonemata, present clear zones of similar dimensions (Furt et al. 2012; Justus et al. 2004; Lancelle and Hepler 1992). This similarity is somewhat surprising and may represent a conserved mechanism for cellular polarity organization in plant tip growing cells that supersedes secretion and growth rates. Further back from the cell apex, a large vacuole occupies much of the cell volume (Oda et al. 2009; Parton et al. 2001; Rounds and Bezanilla 2013). The organization and distribution of vacuole in root hairs is similar to P. patens protonemata, with a large vacuole in the rear and extensive anastomosing tubules at the tip (Ovecka et al. 2005). The tubular vacuoles are very dynamic and constantly rearrange their shape by fusion and separation of individual tubules (Oda et al. 2009; Ovecka et al. 2005). In plant cells, the vacuole acts as a reservoir for ions and metabolites (Marty 1999), but the specific role of vacuoles in tip growing cells is still unknown. Some possible functions include that the apical tubules could increase the vacuole’s surface area to accept endosomal vesicles that capture extracellular fluid to rapidly balance the osmotic levels (Odriscoll et al. 1993). There may be a system of control between the osmotic status of the plasma membrane and that of the tonoplast (Reisen et al. 2005).
The zonation of moss protonemata is different between choloronemata and caulonemata. Choloronemata have a much smaller clear zone, and have more chloroplasts, while caulonemata have a much larger clear zone and fewer chloroplasts (Furt et al. 2012). Interestingly, the distribution of Golgi and mitochondria shows a gradient in both cell types, with higher numbers of organelles toward the cell’s apex (Figure 1 a and b) (Furt et al. 2012). The distribution of the endoplasmic reticulum has been less well-characterized, and it appears evenly distributed in the cell, with an accumulation at the tip of the growing cell (Harant and Lang 2020) and unpublished observations. There are very few quantitative studies of zonation in moss protonemata, and the mechanisms that establish and maintain these zones are not well understood. Nevertheless, based on the conservation of the cytoskeleton and trafficking systems (see below), we hypothesize that the mechanisms for zonation of protonemata are similar to those existing in pollen tubes and root hairs. Most critical for tip growth is the subcellular organization at the apical dome. In pollen tubes, a trans-Golgi network (TGN) rich area exists behind the most apical accumulation of vesicles (Lovy-Wheeler et al. 2007; Stephan et al. 2014), and TGN is also present at the tip of growing root hairs (Berson et al. 2014). In pollen tubes, F-actin partially overlaps with the TGN, and the stability of this zonation is dependent on the actin cytoskeleton (Stephan et al. 2014). The distribution of TGN is unknown in moss protonemata, but because the cell tip is enriched in Golgi bodies (Furt et al. 2012), it is likely that associated TGN will be present in the apical zone; the Golgi overlaps with the vesicle rich-area in the clear zone. It is tempting to speculate that the organization of the F-actin, TGN, and apical vesicles is functionally similar between protonemata, pollen tubes, and root hairs. Nevertheless, the conserved role of the TGN during tip growth needs to be further investigated.
Figure 1.
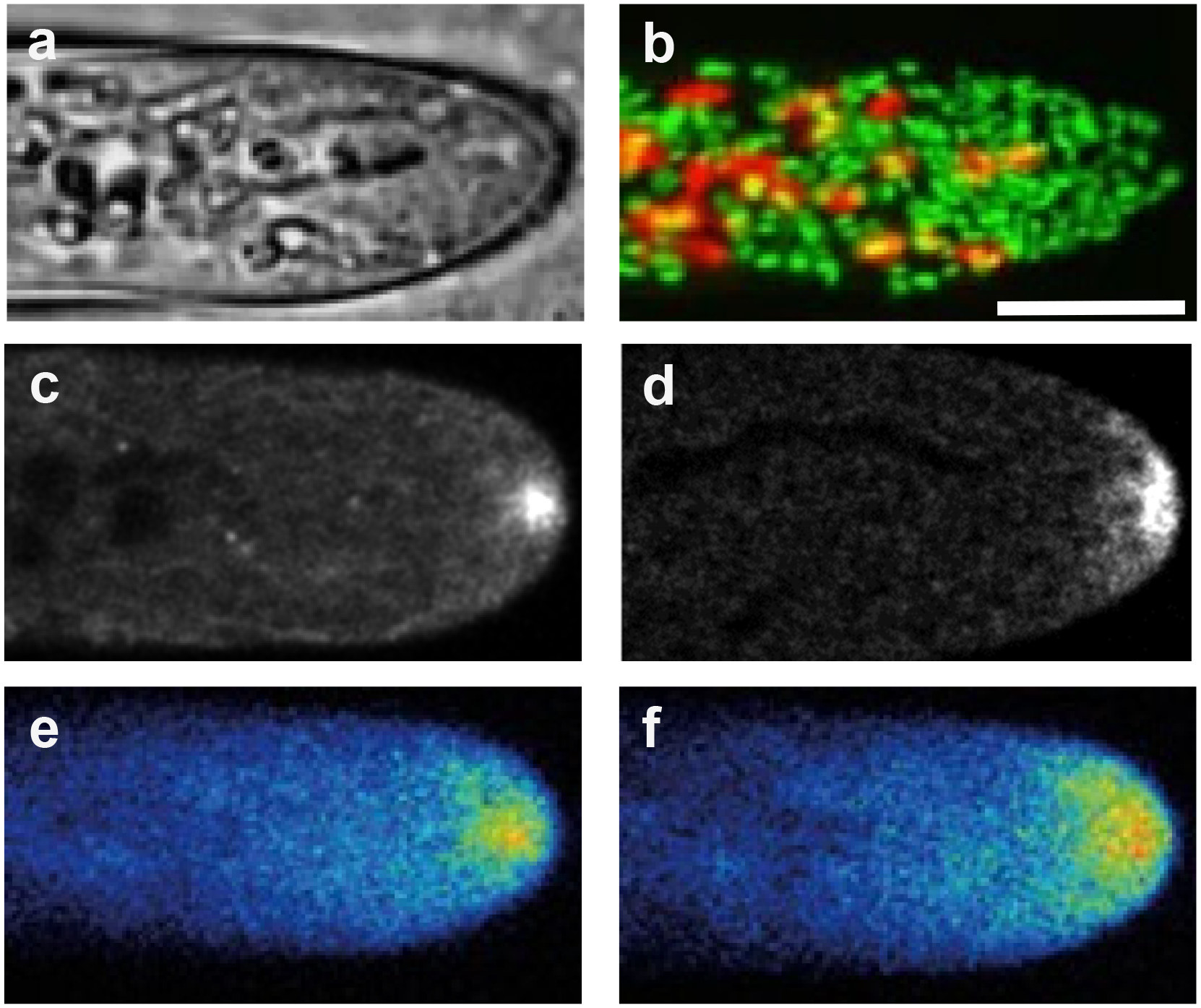
Examples of organelle localization and cellular structures in tip growing P. patens caulonemata. a. Bright field image. b. Chloroplasts (red) and Golgi dictyosomes (green) visualized with confocal microscopy; same cell as in a (Furt et al. 2012). c. Medical optical section of apical F-actin visualized with lifeact-mEGFP and confocal microscopy (Bibeau et al. 2020). d. Class II formin localization visualized by confocal microscopy of the formin 2a tagged with 3mEGFP at the endogenous genomic locus (Vidali et al. 2009). e. 3mEGFP-Myosin XIa localization by confocal microscopy. f. 3mCherry-VAMP72a localization as a marker of apical vesicles; same cell as in e (Furt et al. 2013). Scale bar is 10 μm.
Wall composition.
The composition of protonemata cell wall has been characterized, but due to the complexity of the problem, a complete understanding of its composition and structure has not been reached. Further confounding the issue, evidence shows that culturing conditions can affect cell wall composition (Berry et al. 2016). In fact, Berry et al. show protonemata grown on high osmolarity media are abundant in glucans (crystalline cellulose, callose), and arabinogalactan proteins, while tissue grown in low osmolarity contains more pectins (homogalacturonan), hemicellulose (1,4-β-mannan), 1,5-α-L-arabinan (strongest signal at the tip) (Berry et al. 2016). Among these sugars, glucans and hemicellulose are most abundant in moss, while pectins have been only detected at low levels (Berry et al. 2016). In addition, a recent study identified arabinoglucan as a minor but novel sugar component of P. patens cell walls (Roberts et al. 2018). Evidence shows protonemata have a cellulosic tip wall and that cellulose synthase inhibitors cause rupture of the cell tip (Tran et al. 2018). Similarly to protonemata, cellulose is also the prevalent sugar in root hairs, where it is thought to be loosened by expansins, which are localized to the tip of growing root hairs (Baluska et al. 2000). P. patens has a large family of expansin, particularly epxansin A genes have been greatly expanded in mosses (Carey and Cosgrove 2007); nevertheless, the importance of expansin in tip growth still needs to be determined. Based on the similarities outlined above, we hypothesize that moss protonemata will have mechanisms of growth regulation similar to root hairs. Differently, the main polysaccharide in the pollen tube’s cell walls is pectin, and pollen tubes depend on pectin de-esterification for the rapid changes in cell wall rigidity (Fayant et al. 2010), where pectin stiffness is regulated by the demethylation of pectin via pectin methylesterases (Bosch and Hepler 2005). A similar pattern of pectin de-esterification at the shank was observed in pollen from a gymnosperm (Chen et al. 2007). Upon demethylation of pectin, the negatively charged pectin attracts calcium ions, which promote crosslinking of the pectins and make them rigid (Bosch and Hepler 2005; Rojas et al. 2011). If the cell wall of moss protonemata has a similar gradient of polysaccharide crosslinking from the tip to the back, and how the nature of the polysaccharides and their crosslinks still needs to be determined. Possible differences in cell wall composition between pollen tubes and root hairs and protonemata may be due to their environment and function. Pollen tubes grow either in between or on top of other cells, and burst once they reach their destination. In contrasts, root hairs and protonemata grow on soil and maximize nutrient absorption, and they either stop growing after reaching full size (root hairs) or divide and continue to grow (protonemata), but do not burst.
Exocytosis.
Tip growing cells are under high osmotic pressure, which is balanced by the cell wall. For the cell to grow, it has to optimize its wall loosening activity at the tip, which occurs via polarized secretion. This secretion is controlled by the delivery of Golgi-derived vesicles containing cell wall materials and wall loosening enzymes to the growing apex of the cell wall (Bibeau et al. 2020; Bibeau et al. 2018; Geitmann and Nebenfuhr 2015; Hepler et al. 2001; Ichikawa et al. 2014; Ketelaar et al. 2003). It has been estimated that pollen tubes, root hairs, and protonemata, need tens to hundreds of vesicle-fusion events per second to deposit the appropriate amount of wall materials during growth (Bibeau et al. 2018; Bove et al. 2008; Ketelaar et al. 2008). A large amount of the membrane delivered by secretion is an excess, and it needs to be internalized via compensatory endocytosis (Grebnev et al. 2017). This process, especially in relation to the location of exocytosis/endocytosis areas, has been investigated mainly in pollen tubes, where several models have been suggested (Grebnev et al. 2017). Our knowledge of the precise location of exocytosis and how it is regulated in protonemata is still limited. Analysis of cell wall stability using cell wall digestive enzyme and modeling show that when a protonemal cell is growing, exocytosis is concentrated at a narrow spot (~ 5 μm) at the tip of the cell (Bibeau et al. 2018). It is not known if multiple pathways of exocytosis/endocytosis exist in protonemata, with a system concentrated at the growing tip while another constitutively present throughout the cell. Given that apical exocytosis is essential for growth, it is tempting to speculate that apical exocytosis may be controlled differently than that at the shank. A definite marker for the location of exocytosis in moss has not been developed, which could be very useful to investigate this problem of differential secretion. Nevertheless, in P. patens, VAMP72, which is the closest homolog to the A. thaliana v-SNARE believed to be involved in vesicle secretion (Uemura et al. 2004), and some of its isoforms localize to the tip of root hairs (Ichikawa et al. 2014), has been used as an apical vesicle marker (Bibeau et al. 2020; Bibeau et al. 2018; Furt et al. 2013). The apical vesicles are also labeled by FM4–64 after 30 min, suggesting that they are not the immediate result of endocytosis, but that the FM4–64 dye is recycled from the endosomal pathway before reaching the apex (van Gisbergen et al. 2012). In pollen tubes and root hairs, molecules such as the exocyst (Hala et al. 2008; Synek et al. 2006), ROPs (Lin et al. 1996; Molendijk et al. 2001), and their activator GEFs (Gu et al. 2006; Riely et al. 2011), regulate exocytosis, their localization at the cell tip in protonemata could identify the area of active secretion. To date, the localization of some of these markers has been described. The exocyst subunit EXO70 is localized at the apex in P. patens protonemata (Rawat et al. 2017), Sec10 and its genetic fusion with a class I formin are essential for tip growth (van Gisbergen et al. 2018), and all tested exocyst subunits accumulate at the apical plasma membrane (Tang et al. 2019; van Gisbergen et al. 2018). Furthermore, fluorescent ROP localizes at the cell’s apex and peaks in a narrow area (about 5 μm wide) and ROP-GEF signal is also localized in a narrow area at the tip (Cheng et al. 2020; Ito et al. 2014; Le Bail et al. 2019).
In pollen tubes, a method to monitor exocytosis based on membrane proteins uses Fluorescence Recovery After Photobleaching (FRAP) of GFP fused to receptor like-kinases (RLK) to follow protein movement; this analysis shows that recovery is fastest at the cell apex (Lee et al. 2008). Since RLKs are thought to be deposited on the plasma membrane via exocytosis, this suggests that the delivery of membrane proteins is most prominent at the cell tip (Lee et al. 2008). A similar type of approach could be applied to moss protonemata to directly investigate the location and dimensions of secretion in tip growing cells.
Endocytosis.
Although little research has been done on endocytosis in P. patens and root hairs, there has been significant, albeit controversial, research done in pollen tubes. This controversy stems from the debate regarding the spatial control of endocytosis. One study with FM4–64 in lily pollen tubes suggested that membranes are rapidly endocytosed along the cell flank within one minute and then recycled in 15 minutes into the secretory pathway at the cell apex (Parton et al. 2001). In support of apical endocytosis, pulse-chase with preloaded FM1–43 and pulsed FM4–64 showed that the first co-localization of dyes appeared 1–2 minutes following the pulse at the cell apex (Zonia and Munnik 2008). FRAP of pollen tubes preloaded with FM1–43 showed that the membranes at the cell apex recovered more slowly than the membranes at the cell flanks (Bove et al. 2008). This fast turnover at the cell flanks was interpreted to be rapid exocytosis. However, this faster recovery could be confounded by the fact that the extreme apex is farther away from unphotobleached membranes than the cell flank. Hence, research efforts result in two models for pollen tube endocytosis. In one model it is believed that endocytosis takes place at the extreme apex of the pollen tube (Bove et al. 2008; Zonia and Munnik 2008), while in another, it is thought that endocytosis happens behind the cell apex, along the flanks of the cell (Derksen et al. 1995; Feng et al. 2016; Grebnev et al. 2017; Zhao et al. 2010). Recently, a study that combines FM dyes, FRAP, BFA treatment and fluorescent transmembrane proteins as in vivo markers for exocytosis and endocytosis supports the latter model (Grebnev et al. 2020). Grebnev et al. showed exocytosis is confined at a narrow area at the cell tip (3.5 μm from the tip) and endocytosis in a subapical area (9–15 μm from the tip) (Grebnev et al. 2020).
Endocytosis in P. patens is less understood. Similar to pollen tubes and other plant cells, the FM1–43 and FM4–64 dyes are internalized, and have been used to characterize the role of formins in endocytosis. Class II formins localize with FM4–64 puncta close to the plasma membrane (van Gisbergen et al. 2020), indicating a possible relation between these formins and endocytosis or endocytic recycling. Class I formins on the other hand, do not seem to play a role in endocytosis (van Gisbergen et al. 2020).
Actin filaments.
The use of fluorescent actin-binding probes, such as Lifeact-GFP (Riedl et al. 2008; Vidali et al. 2009) mTalin (Kost et al. 1998) and the actin-binding domain of fimbrin (Sheahan et al. 2004), allowed appreciation of F-actin different conformations in tip growing cells. Moss protonemata have a highly dynamic cortical F-actin network, that resides underneath of the plasma membrane, and an apical F-actin enrichment “spot” (Figure 1 c), that is essential for cell growth (Vidali et al. 2009). The F-actin cytoskeleton in all tip growing cells is very dynamic and rapidly remodeling (Rounds and Bezanilla 2013; Vidali et al. 2010; Vidali et al. 2009). The F-actin cytoskeleton and its dynamics are essential for the maintenance of persistent growth, and most of the evidence supporting this notion was collected via pharmacological inhibitors. The inhibition of F-actin in P. patens protonemata stops the growth in a dose-dependent manner and show F-actin is essential for proper protonemal expansion (Finka et al. 2007; Harries et al. 2005; Vidali et al. 2009). In addition, modeling efforts in P. patens show an active transport that relies on F-actin is essential for tip growth, because a mechanism based solely on vesicle diffusion cannot support growth (Bibeau et al. 2018). Recently, Bibeau et al. demonstrated the linear trajectory of myosin XI and secretory vesicles at the cortical area of caulonema cells is F-actin dependent (Bibeau et al. 2020). Differently than moss protonemata, root hairs and pollen tube exhibit, in addition to the cortical and apical F-actin, long F-actin bundles in the central region of the cell (Jásik et al. 2016; Ueda et al. 2009; van der Honing et al. 2011; Vidali et al. 2009). These bundles are responsible of a myosin-driven cytoplasmic streaming that transports organelles and vesicles to the tip area (Hepler et al. 2001), but this fast streaming is absent in moss cells (Furt et al. 2012). Nevertheless, similar to moss cells, F-actin is essential for growth in and apical transport in both pollen tubes and root hairs. When root hairs expressing myosin XIK-YFP are treated with latrunculin B, myosin XI localization at the root tip is disrupted (Park and Nebenfuhr 2013). Furthermore, when root hairs are treated with a low concentration of latrunculin B (10 nM), the cell tip becomes round, showing F-actin is involved in restricting growth at the apex (Ketelaar et al. 2003). Total internal reflection microscopy demonstrated that the dynamics of FM dye-labeled vesicles was reduced by treatment with latrunculin B in pollen tubes (Wang et al. 2006); and latrunculin B treatment was also shown to abrogate the apical localization of fluorescently labeled pectin methyl esterase (PME) in pollen tubes (Wang et al. 2013). These results suggest that similarly to moss cells, secretory vesicle accumulation and vesicle recycling, important for tip growth, are dependent on the actin cytoskeleton.
Actin-binding proteins.
The requirement for actin polymerization in polarized cell growth has prompted the study of several actin-binding proteins, which are responsible for the F-actin remodeling essential for growth. In P. patens, class II formins have been shown to be essential for tip growth; they localize to the cell apex (Figure 1d), and rapidly polymerize actin filaments (Vidali et al. 2009). Overexpression of formins in root hairs (Deeks et al. 2005) and pollen tubes inhibits growth (Cheung and Wu 2004), suggesting that the regulation of actin polymerization is necessary for tip growth. Work with the monomeric actin-binding protein, profilin, has shown that profilin binding to actin is necessary to maintain polarized growth in P. patens (Vidali et al. 2007). This suggests that maintaining the pool of monomeric actin is important for polarized growth and the profilin-actin complexes are important for fomin activity. A range of other actin regulating proteins have been shown to be important for growth, these include, actin-depolymerizing factor (ADF) (Augustine et al. 2008), actin-interacting protein 1 (AIP1) (Augustine et al. 2011), and the ARP2/3 complex (Harries et al. 2005), a genomic formin-Sec10 fusion (van Gisbergen et al. 2018), and to a lesser extent other class I formins (van Gisbergen et al. 2020). These studies suggest that for tip growth to take place, F-actin dynamics must be tightly regulated, but the precise mechanisms for this regulation are still poorly understood.
Myosin XI.
Myosin XI is, together with myosin VIII, one of the two families of myosin motors existing in plant cells and are both evolutionarily related to animal and fungal myosin V (Mooseker and Cheney 1995; Peremyslov et al. 2011). Most myosin Vs walk processively in a hand over hand manner, and some plant myosin myosin XIs, such as the tobacco plant myosin XI, are know to be processive (Tominaga et al. 2003). Nevertheless, a systematic study of myosin XIs from A. thaliana showed that, with only one exception, all of its myosin XIs are non-processive (Haraguchi et al. 2018), which means they cannot take successive steps unless they form part of an assembly. In P. patens there are two myosin XI genes that play a redundant but crucial role in tip growth and plant development. Simultaneous RNAi of myosin XIa and myosin XIb results in unpolarized plants, showing myosin XI is essential for cell polarization (Vidali et al. 2010). Furthermore, fluorescent labeling of myosin XI shows it localizes at the apex of protonemal cells and emerging branches (Vidali et al. 2010), spatially overlapping with both the F-actin spot and the v-SNARE VAMP72-labelled vesicle cluster (Figure 1 e and f) (Furt et al. 2013; Vidali et al. 2010). Interestingly, myosin XI loss of function results in a defect in F-actin organization but not in F-actin dynamics (Vidali et al. 2010). Fluorescent cross-correlation studies of F-actin and myosin XI at the cell’s apex show their signal in P. patens are not correlated, with myosin XI leading by 18.6 sec (Furt et al. 2013). This result shows, in P. patens, myosin XI could be involved in enriching the local concentration of F-actin (Furt et al. 2013). FRAP studies in P. patens cauolonemal cells show the recovery of myosin XI at the cell’s apex is F-actin dependent (Bibeau et al. 2020). Furthermore, myosin XI was shown to co-localize to the growing cell tip and fluctuate in phase with vesicles fluorescently labeled with the VAMP72 (Furt et al. 2013), suggesting VAMP72-labelled vesicles are potentially a myosin XI cargo. This prediction was recently confirmed using VAEM microscopy, where it was shown that myosin XI and VAMP-72 labeled vesicles co-localize, and their persistent motility depends on F-actin (Bibeau et al. 2020). A possible mechanism for the role of myosin XI in tip growth comes from recent work (in preprint form), where, using a conditional allele of myosin XI, it was shown that myosin XI is essential for vesicle clustering and consequent F-actin enrichment at the cell tip (Galotto et al. 2020).
Myosins pair the hydrolysis of ATP with a conformational change that results in its movement along an actin filament. The motor domain determines the velocity of the movement, and in plants, the rapidity of the motor is proportionally related to cell size (Tominaga et al. 2013), but the tail domain of myosins determines its cargo. In pollen tubes and root hairs, organelle motility is mostly conducted by cytoplasmic streaming (de Win et al. 1999; Emons 1987; Mascarenhas and Lafountain 1972; Verchot-Lubicz and Goldstein 2010). However, in P. patens there is no large-organelle cytoplasmic streaming (Furt et al. 2012), indicating that this process may not be the essential role of myosin XI for tip growth. Although not essential, it may be necessary to achieve enhanced growth rates in other cells (Tominaga et al. 2013). Similar to moss cells, myosin XI localizes at the tip of growing root hairs and pollen tubes (Park and Nebenfuhr 2013; Vidali et al. 1999). Despite the absence of cytoplasmic streaming in bryophytes, a myosin XI from the liverwort Marchantia polymorpha, was able to complement the loss of function phenotypes in A. thaliana, suggesting a level of unexpected functional conservation of myosin XI in tip growing cells. The absence of cytoplasmic streaming in bryophytes is puzzling. We do not expect that cell size differences are the main cause for the large differences in organelle motility. Pollen tubes and root hairs are not much larger than protonemata, in particular, because pollen tubes form callose plugs that isolate the apical part of the cell from the rest. Instead, we propose that the conserved function of myosin XI between land plants has to do with the transport of vesicles, which are not easily detectable with the standard resolution of a light microscope. Importantly, the motility of vesicles is on a similar scale in cells from flowering plants (Madison et al. 2015) and those in P. patens (Bibeau et al. 2020). Because of the dependence of polarized growth on F-actin and endomembrane secretion, and due to fluorescent and functional myosin XI data, it is likely that the conserved function of myosin XI during tip growth is to deliver and accumulate secretory vesicles containing cell wall polymers at the cell tip. Further supporting this conclusion, recent evidence shows that the small GTPase RabE is part of the myosin XI cargo binding site on apically accumulated vesicles and that this interaction is conserved between mosses and vascular plants (Orr et al. 2021).
The regulation of myosin XI function likely plays a fundamental role in the control of tip growth. Myosin XI is a dimeric protein characterized by four domains: the N-terminal motor domain, the neck domain, the coiled-coil domain, and the C-terminal tail domain (Trybus 2008). Among them, the neck domain plays an important role in regulating the mechanical properties of protein activity. In myosin XI, as in myosin V, the neck is characterized by the presence of 6 IQ motifs per heavy chain, which are binding sites for calmodulin (CaM) light chains (Tominaga et al. 2003; Yokota et al. 1999). In the case of myosin V, regulation of the protein is mostly due to autoinhibition, which is affected by Ca2+ concentration (Homma et al. 2000), and this regulation is likely to be conserved in plants (Avisar et al. 2012). The regulation of moss myosin XI by autoinhibition has not been experimentally determined, but it could play an important role not only in controlling the protein’s activity but also in changing the diffusion of the molecule. In fact, myosin’s folded conformation will increase its diffusion coefficient while inhibiting binding to F-actin. This is important because myosin XI interaction with vesicles is fast and transient (Bibeau et al. 2020); hence, changes in diffusion rates can have a significant effect on the regulation of vesicle and F-actin dynamics.
Microtubules.
In tip growing cells, microtubules have an architecture similar to F-actin’s, and, as F-actin, they have been implicated in the regulation of polarized growth (Orr et al. 2020; Rounds and Bezanilla 2013; Sieberer et al. 2005). In pollen tubes, root hairs, and protonemata, microtubules are longitudinally located at the cell cortex and in the cytoplasm (Doonan et al. 1985; Emons 1987; Pierson et al. 1989). There is some evidence that microtubules play at least a partial role on the deposition of cellulose and cellulose synthase organization in pollen tubes (Cai et al. 2011). While in pollen tubes from angiosperms and root hairs microtubules are excluded from the apical area of the cell (Rounds and Bezanilla 2013), in protonemal they are not excluded, reaching the very apex of the cell (Ding et al. 2018; Doonan et al. 1985; Wu and Bezanilla 2018). In P. patens, microtubule ends accumulate in a spindle-like structure at the cell tip and are oriented with the plus growing end toward the cell’s apex (Hiwatashi et al. 2014).
Even if F-actin is mainly involved in tip growth, growing evidence shows that microtubules play an important role in this process. In A. thaliana, root hairs treated with oryzalin and taxol are polarized, but the directionality of growth is altered, cells grow in a “wavy” fashion and the root hairs bifurcate and form multiple tips (Bibikova et al. 1999; Ketelaar et al. 2003). In pollen tubes from N. tabacum, both secretion and endocytosis are affected by microtubules disruption via nocodazole (Idilli et al. 2013). In P. patens, treatment with the microtubule depolymerizing drug cremart produces a swollen tip (Doonan et al. 1988). How the microtubules specifically affect polarized growth has not completely been elucidated. Some evidence shows that microtubule-based motor kinesins have a regulatory effect on tip growth (Cai and Cresti 2010; Yang et al. 2007). For instance, KINID1, a plus end-directed motor, accumulates at the apex of P. patens caulonemata cell (Hiwatashi et al. 2014). Deletion of KINID1 causes aberrant formation of multiple microtubules foci at the apex, and loss of directionality of growth (Hiwatashi et al. 2014). Similarly, minus end directed motors, such as the kinesin 14 motors KCBP and KCH have reduced or altered tip growth when deleted (Yamada and Goshima 2018; Yamada et al. 2017). Evidence in both P. patens protonemata and A. thaliana pollen tubes show microtubules might affect tip growth by organizing and stabilizing the tip F-actin cytoskeleton (Wu and Bezanilla 2018; Zhu et al. 2013). Wu and Bezanilla showed that, at the apex of protonemal cells, the F-actin spot overlaps with the microtubule focus, showing that interaction via the two cytoskeletons might contribute to growth in a process mediated by myosin VIII (Wu and Bezanilla 2018). Furthermore, when microtubules are depolymerized, the F-actin apical spot appears in multiple aberrant locations at the cell tip, showing microtubules might restrict the site of F-actin accumulation (Wu and Bezanilla 2018). Ding et al. showed that CLoG1, a conserved protein identified using a temperature sensitive genetic screen in P. patens, is involved in microtubules dynamics, accumulates at the cell tip, and it may interact with actin cytoskeleton components (Ding et al. 2018). Despite considerable progress, many questions remain. For example: how is the polarity of the MTs maintained at the apical cell? Is this polarity a driving force for other polarized events in the protonemal cell? How is the interaction between the actin and microtubules cytoskeletons controlled? Do microtubules participate in the organization of cellulose deposition in protonemata?
Potential feedback between vesicles and the cytoskeletal systems.
It has been shown that actin, its binding proteins, myosin XI, microtubules, and vesicles are all focused at the tip and are essential for tip growth in P. patens. Furthermore, fluorescence fluctuations and phase relationships between actin, myosin XI, and vesicles have demonstrated that myosin XI and VAMP72-labeled vesicle fluctuations can anticipate actin fluctuations (Furt et al. 2013). Taken together these observations suggest the existence of a complex feedback mechanism between myosin XI cargo delivery and actin regulation. One hypothesis that includes this feedback mechanism is the vesicle-organizing center model; which was first established in mouse oocytes (Holubcova et al. 2013; Schuh 2011) and adapted later to tip growth (Furt et al. 2013). In this model, vesicles contain actin nucleators and can promote actin filament assembly (Figure 2). When vesicle-associated myosin move along actin filaments, the vesicles cluster, and the nucleators present on the vesicle promote the formation of more actin filaments. How the nucleators are activated is not know, but clustering may play a role in activation. Actin polymerization will promote further clustering via myosin causing a positive feedback loop in which a spike in myosin concentration at the tip can precede an increase in actin concentration (Figure 2). At the cell’s tip, after the vesicles are concentrated via clustering, they will need to be released for fusion with the membrane. The rapid dissociation of myosin XI from vesicles may play a role in controlling this release (Bibeau et al. 2020), which may also be modulated by fluctuations in apical cytosolic Ca2+ (see below). Additional factors such as actin-severing proteins might also intervene to break the feedback loop and further control vesicle delivery. The apical vesicle clustering model is further supported by the presence of ectopic vesicle clustering often observed in growth inhibited cells (Furt et al. 2013; Wu and Bezanilla 2018); these clusters contain myosin XI (Furt et al. 2013) and undergo what appear to be formin II-dependent actin-polymerization-based propulsion (Wu and Bezanilla 2018).
Figure 2.
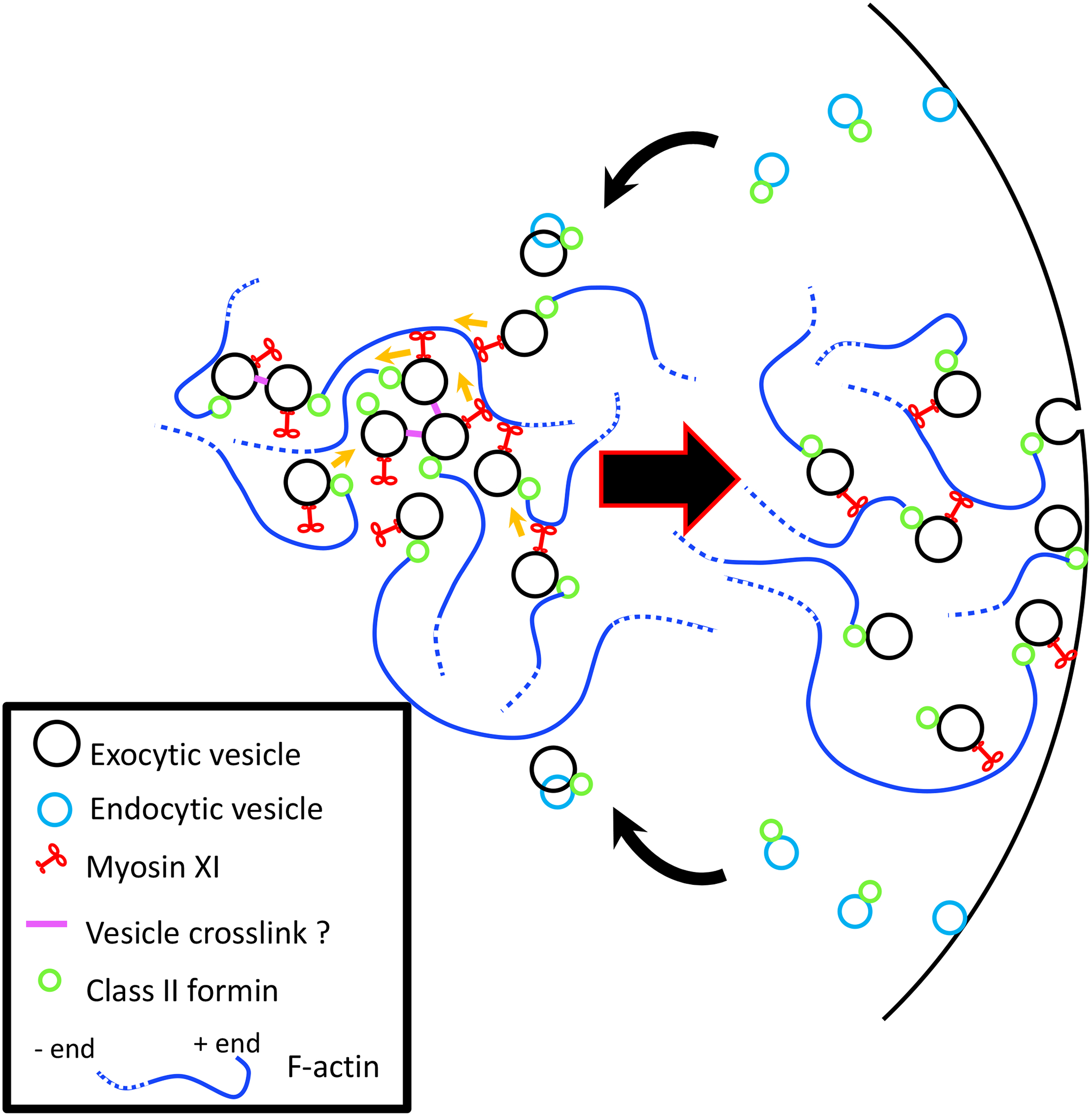
Positive feedback of F-actin via the vesicle organizing center hypothesis. Vesicles can interact indirectly with actin filament via myosin XI and directly via associated formin. As the actin polymerizes more myosin XI carrying vesicles attach to actin. Positive feedback continues as more myosin XIs attach to actin filaments and more vesicles begin to nucleate the polymerization of more filaments. Vesicles eventually diffuse and are exocytosed. Membrane is recycled via endocytosis and some of the endocytic cargo is recycled at the apex for continuous secretion.
The dome of all tip growing plant cells is enriched in vesicles; this is consistent between pollen tubes, root hairs, and protonemata (Emons 1987; Lancelle and Hepler 1992; McCauley and Hepler 1992). It is also consistent that these cells require active actin polymerization for growth, and the tip growth process is inhibited by low concentrations of latrunculin or cytochalasin, below those needed for the loss of actin filaments in the shank of the cell (Furt et al. 2013; Miller et al. 1999; Vidali et al. 2001). A possible explanation for these similarities is that the accumulation of apical vesicles is dependent on active actin polymerization. In fact, the fringe of pollen tubes is a highly dynamic structure, and its emergence has been linked to the presence of apical vesicles (Li et al. 2017). It is likely that the apical vesicles represent a mixture of endocytic and exocytic vesicles, and the apical domain may function as a recycling and sorting system that sustains polarization. Supporting this, an actin-polymerization-dependent system of transport of endosomes or similar membrane bound structures has been identified in pollen grains, root hairs, and protonemata (Furt et al. 2013; Liu et al. 2018; Voigt et al. 2005). This could be, in part, driven by the formin-mediated actin polymerization and myosin XI clustering (Figure 2). In the case of pollen tubes, where the apical vesicle accumulation is very large due to the fast secretion and endocytosis, the actin polymerization resulting from this may form into the actin fringe structure, but even if the structures are not identical between cell types, their origin and function may still be conserved.
Signaling
ROPs.
Among the regulators of cell polarity in plants, a central place belongs to the Rho family of small GTPases (Nagawa et al. 2010; Scheible and McCubbin 2019; Yang 2002). While in other organisms the Rho subfamily has evolved in different subfamilies, such as RHO, CDC42, and RAC in animals and CDC42 and RHO in yeasts, plants express a unique family of Rho GTPases, called “Rho-like GTPases of plants” (ROPs) (Nagawa et al. 2010; Valster et al. 2000). Active ROPs interact with downstream effector proteins, such as the ROP interacting CRIB domain proteins (RICs) (Bourne et al. 1991; Wu et al. 2001). ROPs are involved in the regulation of cytoskeleton reorganization and dynamics, vesicle trafficking, and determination of cell polarity (Molendijk et al. 2004; Nagawa et al. 2010). In pollen tubes, ROP proteins and their regulators are localized at the tip of pollen tubes; this evidence, together with genetics studies, supports a role in polarized cell elongation (Fu et al. 2001; Hwang et al. 2005; Kost et al. 1999; Lin et al. 1996). While the A. thaliana genome has 11 ROPs and 11 RICs, P. patens has only 4 ROPs and 1 RIC genes, making this moss a valuable model to study ROP signaling in tip growth (Eklund et al. 2010). In P. patens, PpROP1 signal at the cell’s apex peaks in a narrow area (about 5 μm wide) and a PpROP-GEF4 and PpROP-GEF3 signals are also localized in a narrow area at the tip (Ito et al. 2014; Le Bail et al. 2019). Burkart et al. showed that, in P. patens, the four ROP genes are functionally redundant, and they are essential for tip growth (Burkart et al. 2015). In fact, moss cells in which ROPs have been silenced appear unpolarized and spherical (Burkart et al. 2015). On the other hand, overexpression of PpROP2 results in polarized cells that exhibit a swollen tip (Ito et al. 2014). Furthermore, ROPs loss-of-function results in an increase in F-actin dynamics, and defects on secretion of cell wall components (Burkart et al. 2015). Interestingly, while silencing of RIC does not have an effect on polarized growth, loss of function of ROPs regulators result in reduction of cell polarity in P. patens (Bascom et al. 2019). Recently, by using a fully functional fluorescent ROP allele, Cheng et al. showed ROPs are involved in polarized growth, tissue patterning and gametophore development (Cheng et al. 2020). Hence, increasing evidence shows that, in P. patens protonemata, and similar to pollen tubes and root hairs, ROPs regulate the reorganization and dynamics of the apical cytoskeleton and control cell polarization.
Cytosolic Ca2+.
Tip growing cells have cytoplasmic ion gradients that are important for several cellular processes, including growth regulation, turgor pressure generation, and cell wall maturation (Michard et al. 2017). Ca2+ is the most studied ion, especially in relation to polarized growth regulation (Bascom et al. 2018). The oscillatory behavior of cytosolic Ca2+ has been correlated with growth in pollen tubes and root hairs (Messerli and Robinson 1997; Monshausen et al. 2008). When growth is stopped by caffeine or low temperature, the Ca2+ gradient dissipates, and the Ca2+ influx is reduced (Pierson et al. 1996). Similarly, when cytosolic Ca2+ concentration is artificially increased (Bibikova et al. 1997) or reduced with Ca2+ channel blockers Lanthanum (La3+) or Gadolinium (Gd3+), growth is arrested (Wang et al. 2004). Recently, cytoplasmic Ca2+ gradients in relation to P. patens protonemal growth were explored (Bascom et al. 2018). Using the FRET-based calcium probe Yellow-Cameleon, Bascom et al. observed a fluctuating tip oriented Ca2+ gradient (Bascom et al. 2018). To test the relationship between Ca2+ oscillations and cell growth, they recorded the Ca2+ oscillations while artificially reducing growth pharmacologically (latrunculin B), genetically (loss of function of the actin interacting protein AIP1), and mechanically (physical barrier). In all these cases, growth reduction resulted in Ca2+ oscillations of longer periods, showing growth and Ca2+ are related (Bascom et al. 2018). In addition, these results relate the reduction of F-actin to tip Ca2+ oscillations. Finally, via a cross-correlation analysis, Bascom et al. reported the tip F-actin enrichment characteristic of tip growing cells and the apical Ca2+ signal are anticorrelated. High levels of F-actin are only present when the Ca2+ concentration is low (Bascom et al. 2018). Using the Ca2+ sensor GCaMPf6, Galotto et al. showed that, upon simulation of a fungal infection with chitin oligosaccharides, Ca2+ oscillations are altered when compared to the control in apical caulonemata (Galotto et al. 2020). In chitin treated cells, Ca2+ oscillations are not restricted to the apex, but spread across the whole cell, and their periodicity is affected, resulting in lower frequency oscillations. Concurrently with the cytosolic Ca2+ alterations, upon chitin oligosaccharides treatment, the F-actin enrichment at the cell tip dissipates and cell growth stops (Galotto et al. 2020). Consistently with the growth defect at the cell level, treatment with chitin oligosaccharides result in growth defect at the plant level (Galotto et al. 2020). These results show that defects in Ca2+ oscillations, as a result of a simulated infection, are intimately related to defects in the F-actin cytoskeleton and tip growth.
Cytosolic Ca2+ gradients are thought to regulate the activity of the uniformly distributed actin-binding proteins, which include profilin, L1LIM1, and villins (Bascom et al. 2018; Cheung and Wu 2008; Pollard 2016; Wang et al. 2008; Zhang et al. 2010). By modulating the activity of these proteins, Ca2+ can indirectly influence actin dynamics. This plasticity is probably important for the dynamic organization of the growing machinery at the cell tip. Cytosolic Ca2+ has also been hypothesized to control vesicle trafficking by blocking myosin activity via its calmodulin domain (Cai and Cresti 2009) or by promoting exocytosis (Battey et al. 1999). Such a model is supported by in vitro evidence, which has shown that myosin XI motility is inactivated by calcium concentrations greater than 1 μM (Yokota et al. 1999). Ca2+-dependent control of myosin XI activity may play an essential role in vesicle clustering and delivery at the tip of protonemata.
In pollen tubes and root hairs, reactive oxygen species (ROS) can modulate intracellular Ca2+ dynamics (Boisson-Dernier et al. 2013; Foreman et al. 2003; Monshausen et al. 2007; Wu et al. 2010). We anticipate that ROS will also play a prominent role in the control of tip growth in protonemata. Increases in ROS and Ca2+ have been observed following fungal infection and chitin elicitation in moss tissue, respectively (Galotto et al. 2020; Ponce De Leon et al. 2012), suggesting that feedback mechanisms involving ROS and Ca2+ could also be conserved in these tip growing cells.
Existing Models of Plant Cell Tip Growth
Mechanical models of cell growth and expansion have become important to cell biologists because they can make predictions about some of the controversial aspects of tip growth, including the spatial distribution of cell wall secretion. During growth, there is a constant internal turgor pressure that acts uniformly throughout the cell wall that provides the mechanical force necessary to extend the cell wall (Benkert et al. 1997). This internal pressure has been shown to reach up to 10 atmospheres and imposes stress on the cell wall (Baskin 2005; Campàs and Mahadevan 2009). The irreversible expansion of the cell wall is partially the result of its mechanical conditions: its tensile stresses and the associated elastic strains within the cell-wall structure due to the turgor pressure and the cell-wall material property. Interestingly, the tensile stresses predicted for tip-growing cells are opposite of the gradients of expansion seen during growth (Dumais et al. 2004). Specifically, stress analysis for thin pressurized shells suggests that the stresses on the cell wall would be maximal away from the cell tip, near the cell shank (Campàs and Mahadevan 2009). Specifically, the stress analysis is based on the theory for thin pressurized shells, where the cell-wall tensions (the stress times the cell-wall thickness), the turgor pressure, and the curvatures of the cell-wall midsurface, were connected by the force balance (Chelladurai et al. 2020; Dumais et al. 2004; Rojas et al. 2011), and have the following form,
| (Eq. 1) |
| (Eq. 2) |
Here, P is the internal turgor pressure on the thin shell, κs is the curvature of the cell-wall mid-surface along the meridian, κϕ is the curvature along the circumference, σss is the stress along the meridian, and σϕϕ is the stress along the circumference. Since the relationship between wall tension, curvature, and turgor pressure are independent of the material properties of the cell wall these Eq. 1 and Eq. 2 vary minimally across most models. Based on these two equations, Chelladurai et al. recently inferred the tensions in chloronemata and caulonemata (Chelladurai et al. 2020) (Figure 3).
Figure 3.

Mathematical scheme to infer the average tensions in moss protonemata. a. 14 caulonema outlines. b. Inferred canonical meridional (red) and circumferential (blue) tensions of caulonema. c. 16 chloronema outlines. d. Inferred canonical meridional (red) and circumferential (blue) tensions of chloronemata. Chloronema has higher level of relative tensions to the turgor pressure. In b and d, the solid (dashed) lines are the tension inferences from using NI = 20 (NI = 10) linear segments and n p = 19th (n p = 9th) degree polynomial. For the NI = 20 and NI = 10 cases, each linear segment covers an arc length of 8 and 16 in pixel size along the cell wall, respectively. The r- and z-axes are rescaled by 0.315μm. Turgor pressure P = 1 is assumed to infer the relative tensions (Chelladurai et al. 2020).
Strain rates associated with wall expansion for pollen tubes and root hairs have been well characterized experimentally (Dumais et al. 2004; Rojas et al. 2011; Shaw et al. 2000), however, the exact rheology of the cell wall that relates stress and strain has been modeled in a variety of ways. Specifically, the wall has been modeled as either an elastic (Fayant et al. 2010), viscous (Campàs and Mahadevan 2009; Rojas et al. 2011), or viscoplastic (Dumais et al. 2006; Kroeger et al. 2011) material. When modeled elastically, the cell wall deforms instantly when a stress is applied (Fayant et al. 2010). Through cyclic loading and re-meshing of an elastic finite element model, it was shown that the elastic modulus—how resistant a material is to stress-induced deformations—of the wall had to be reduced toward the cell tip to recapitulate the appropriate pollen tube morphology (Fayant et al. 2010). If this gradient of the elastic moduli was too steep, the model predicted narrowing of the cell tip, while shallower gradients predicted ballooning of the cell tip (Fayant et al. 2010).
Viscous models of the cell wall assume that the rate of irreversible wall deformation is inversely proportional to the viscosity of the wall. This implies that the turgor pressure of the wall permanently deforms the cell wall at a particular rate. By monotonically decreasing the viscosity and monotonically increasing the secretion rate of the wall toward the cell tip, it is possible (even in the presence of non-monotonic curvatures) to recapitulate a wide range of tip-growing morphologies (Campàs and Mahadevan 2009). Furthermore, scaling laws derived by Campàs and Mahadevan (Campàs and Mahadevan 2009), i.e.,
| (Eq. 3) |
predict that the geometry of the cell is related to the effective size of the secretion zone. Here R is the radius of the cell shank, RA is the radius of curvature at the cell tip, and a is the effective size of the secretion zone (Campàs and Mahadevan 2009). This implies that by increasing the effective size of the secretion zone, the cell tip becomes more pointed relative to the cell width. These scaling laws are important because they relate the size of the secretion zone to the steady-state growth morphology.
Finally, viscoplastic models differ from viscous models because they allow irreversible deformations only above a critical yield stress (Dumais et al. 2006). Below this critical yield stress, they deform plastically (Dumais et al. 2006). By assuming the cell wall as an anisotropic-viscoplastic material with decreasing viscosity to the tip, Dumais et al. were able to produce a variety of relevant cell morphologies (Dumais et al. 2006).
The spatial distribution of wall secretion is one of the more controversial topics in tip growth (Grebnev et al. 2017). There are two competing hypotheses; one is that secretion is maximal at the cell tip (Campàs and Mahadevan 2009); another is that secretion is maximal in an annular region distal to the cell tip (Bove et al. 2008; Zonia and Munnik 2008). Recent experimental evidence and mathematical modeling of pollen tubes appear to support maximal secretion at the tip, with endocytosis at the subapex (Grebnev et al. 2020).
Taken together, these modeling efforts have begun to describe how secretion, turgor pressure, and the mechanical properties of the cell wall work in concert to achieve the morphologies of tip-growing cells. To better understand the concerted work, the spatial distribution of material secretion and recycling across the cell membrane, and the spatial distribution of the cell wall strength have to be investigated in detail. Future studies of cell wall morphogenesis of P. patens need to address how the mechanical properties and the exocytosis and endocytosis profiles affect the cell shapes individually and in concerto. This will require to model the cell wall morphogenesis under the framework of morphoelasticity (Goriely 2017), where cell walls are considered as elastic materials where a variety of isotropic and anisotropic materials can be considered, and exocytosis and endocytosis are considered as the determining factors that change the cell wall size when no turgor pressure is applied. In the study of fission yeast (Abenza et al. 2015), it has been shown that the mechanical factors and the secretion profiles can be decomposed by using the cell wall outlines under normal conditions with turgor pressure, and outlines under osmotic shock where the pressure is removed. At first, the average material parameter values are estimated by comparing the model outputs with the two cell outlines. Then, the area growth profile can be inferred further using the estimated secretion profile. A similar approach could be used for the cell wall outline of P. patens with and without turgor pressure, which will allow us to identify the best elastic law of the P. patens cell wall, and infer the spatial distribution of the material parameters, the elastic deformation, and the rate of cell-wall irreversible growth.
To be complete, future models of tip growth in P. pantens will need to incorporate kinetic parameters and diffusion coefficients of all the components associated with vesicle transport and secretion. In figure 4, we present a schematic view of some of these cell biological processes and their parameters. In figure 5, we summarize existing methodologies that have been and could be applied to P. patens’ tip growing protonemata to measure some of these key parameters.
Figure 4.
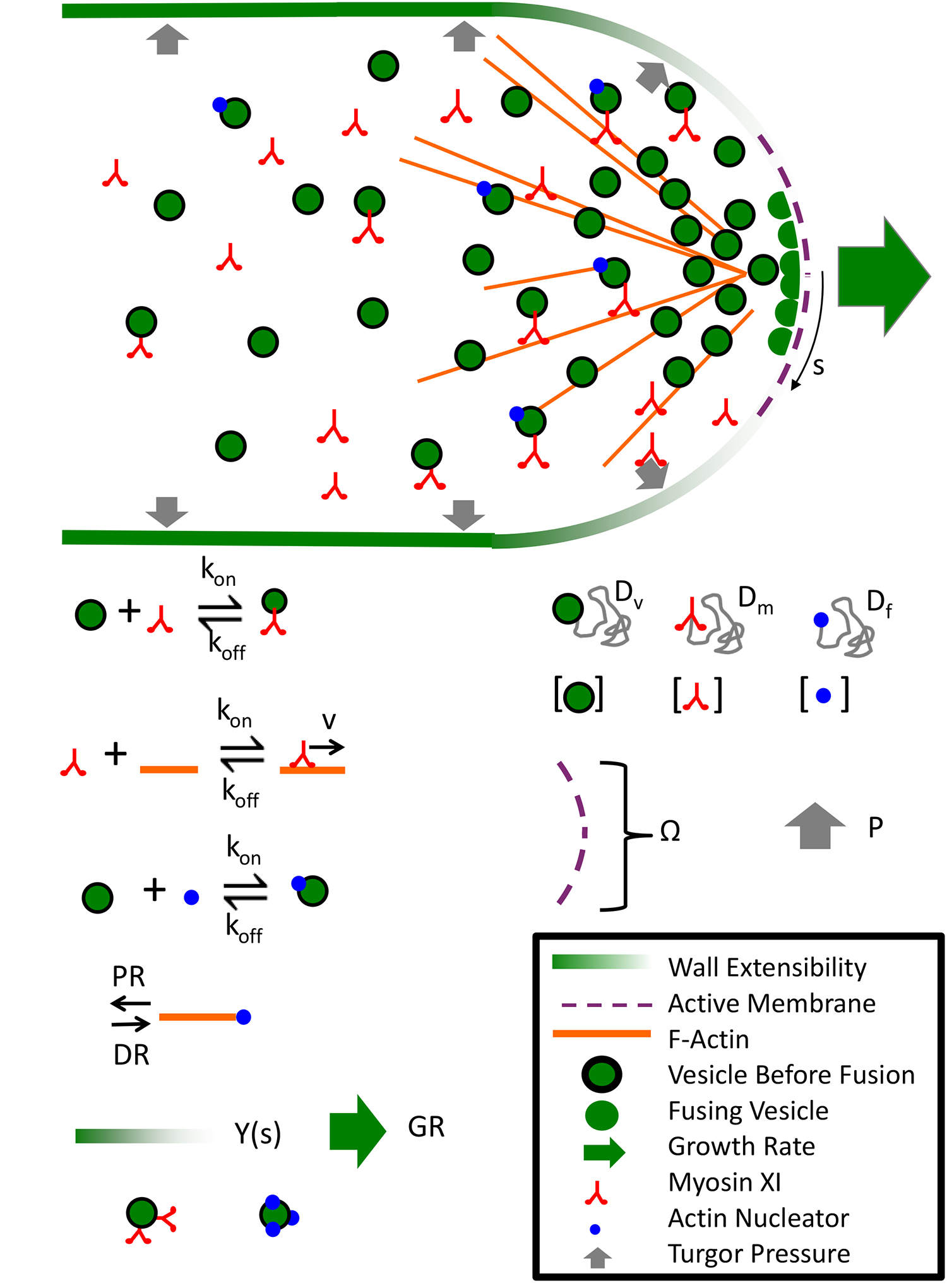
Modeling parameters important for cell growth. Cartoon model of polarized cell growth where vesicles with an actin nucleator are carried along existing actin filaments to the secretion zone at the cell tip. At the cell tip vesicles fuse with the cell membrane at a given rate. Fusion provides more wall materials at the growing cell edge and locally adjusts the Young’s modulus of the cell wall. Turgor pressure drives expansion only at the cell apex. To better understand how this process is coordinated in silico, various parameters must be measured. These parameters are depicted above and include, the myosin XI vesicle kinetics, the myosin XI actin kinetics, actin nucleator vesicle kinetics, actin polymerization and depolymerization rates, vesicle fusion kinetics, the Young’s modulus of the cell wall, the cell growth rate, protein diffusion coefficients, the secretion zone size, and the intracellular turgor pressure.
Figure 5.
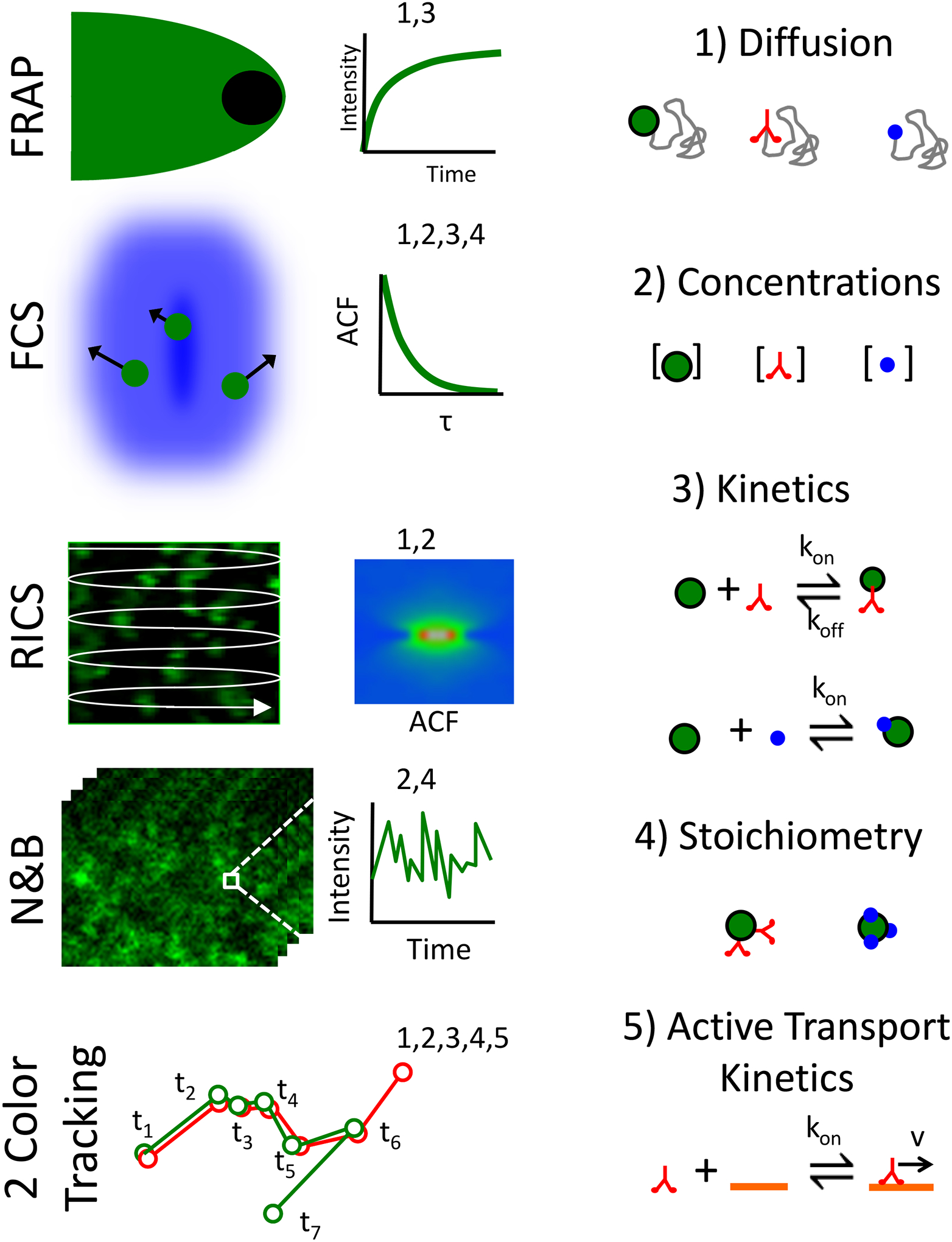
Microscopy techniques for in vivo measurements. Example fluorescence dynamics techniques (left column) that can be used to measure important model parameters involved in polarized growth (right column). From top down the techniques include Fluorescence Recovery After Photobleaching (FRAP) (Bibeau et al. 2018; Loren et al. 2015; McNally 2008), Fluorescence Correlation Spectroscopy (FCS) (Elson 2011; Fitzpatrick and Lillemeier 2011), Raster Image Correlation Spectroscopy (RICS) (Digman et al. 2009; Sasaki et al. 2015), Number and Brightness Analysis (N&B) (Digman et al. 2008), and color particle tracking (Bibeau et al. 2020; Roding et al. 2014). Some example model parameters include diffusion coefficients, concentrations, binding kinetics, stoichiometry, and active transport properties (right column).
Funding
This work was supported by grants from the NSF MCB-1253444 and NIH 1R15GM134493 to LV.
Footnotes
Conflicts of interest/Competing interests
None
References
- Abenza JF, Couturier E, Dodgson J, Dickmann J, Chessel A, Dumais J, Carazo Salas RE (2015) Wall mechanics and exocytosis define the shape of growth domains in fission yeast. Nat Commun 6: 8400. 10.1038/ncomms9400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderhag P, Hepler PK, Lazzaro MD (2000) Microtubules and microfilaments are both responsible for pollen tube elongation in the conifer Picea abies (Norway spruce). Protoplasma 214: 141–157. 10.1007/bf01279059 [DOI] [Google Scholar]
- Arif MA, Hiss M, Tomek M, Busch H, Meyberg R, Tintelnot S, Reski R, Rensing SA, Frank W (2019) ABA-induced vegetative diaspore formation in Physcomitrella patens. Front Plant Sci 10: 315. 10.3389/fpls.2019.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton NW, Cove DJ (1977) The isolation and preliminary characterisation of auxotrophic and analogue resistant mutants of the moss, Physcomitrella patens. Mol Gen Genet 154: 87–95. [Google Scholar]
- Augustine RC, Pattavina KA, Tüzel E, Vidali L, Bezanilla M (2011) Actin interacting protein1 and actin depolymerizing factor drive rapid actin dynamics in Physcomitrella patens. Plant Cell 23: 3696–710. 10.1105/tpc.111.090753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine RC, Vidali L, Kleinman KP, Bezanilla M (2008) Actin depolymerizing factor is essential for viability in plants, and its phosphoregulation is important for tip growth. Plant Journal 54: 863–75. 10.1111/j.1365-313X.2008.03451.x [DOI] [PubMed] [Google Scholar]
- Avisar D, Abu-Abied M, Belausov E, Sadot E (2012) Myosin XIK is a major player in cytoplasm dynamics and is regulated by two amino acids in its tail. J Exp Bot 63: 241–9. 10.1093/jxb/err265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluska F, Salaj J, Mathur J, Braun M, Jasper F, Samaj J, Chua NH, Barlow PW, Volkmann D (2000) Root hair formation: F-actin-dependent tip growth is initiated by local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges. Dev Biol 227: 618–632. [DOI] [PubMed] [Google Scholar]
- Barnabas B, Fridvalszky L (1984) Adhesion and germination of differently treated maize pollen grains on the stigma. Acta Bot Hung 30: 329–332. [Google Scholar]
- Bascom C Jr., Burkart GM, Mallett DR, O’Sullivan JE, Tomaszewski AJ, Walsh K, Bezanilla M (2019) Systematic survey of the function of ROP regulators and effectors during tip growth in the moss Physcomitrella patens. J Exp Bot 70: 447–457. 10.1093/jxb/ery376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascom CS Jr., Hepler PK, Bezanilla M (2018) Interplay between Ions, the cytoskeleton, and cell wall properties during tip growth. Plant Physiol 176: 28–40. 10.1104/pp.17.01466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascom CS Jr., Winship LJ, Bezanilla M (2018) Simultaneous imaging and functional studies reveal a tight correlation between calcium and actin networks. Proc Natl Acad Sci USA. 10.1073/pnas.1711037115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin TI (2005) Anisotropic expansion of the plant cell wall. Annu Rev Cell Dev Biol 21: 203–22. 10.1146/annurev.cellbio.20.082503.103053 [DOI] [PubMed] [Google Scholar]
- Battey NH, James NC, Greenland AJ, Brownlee C (1999) Exocytosis and endocytosis. Plant Cell 11: 643–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkert R, Obermeyer G, Bentrup FW (1997) The turgor pressure of growing lily pollen tubes. Protoplasma 198: 1–8. [Google Scholar]
- Berry EA, Tran ML, Dimos CS, Budziszek MJ Jr., Scavuzzo-Duggan TR, Roberts AW (2016) Immuno and affinity cytochemical analysis of cell wall composition in the moss Physcomitrella patens. Front Plant Sci 7: 248. 10.3389/fpls.2016.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson T, von Wangenheim D, Takac T, Samajova O, Rosero A, Ovecka M, Komis G, Stelzer EH, Samaj J (2014) Trans-Golgi network localized small GTPase RabA1d is involved in cell plate formation and oscillatory root hair growth. BMC Plant Biol 14: 252. 10.1186/s12870-014-0252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla M, Pan A, Quatrano RS (2003) RNA interference in the moss Physcomitrella patens. Plant Physiol 133: 470–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla M, Perroud PF, Pan A, Klueh P, Quatrano RS (2005) An RNAi system in Physcomitrella patens with an internal marker for silencing allows for rapid identification of loss of function phenotypes. Plant Biol (Stuttg) 7: 251–7. [DOI] [PubMed] [Google Scholar]
- Bibeau JP, Furt F, Mousavi SI, Kingsley JL, Levine MF, Tuzel E, Vidali L (2020) In vivo interactions between myosin XI, vesicles and filamentous actin are fast and transient in Physcomitrella patens. J Cell Sci 133. 10.1242/jcs.234682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibeau JP, Kingsley JL, Furt F, Tuzel E, Vidali L (2018) F-Actin mediated focusing of vesicles at the cell tip is essential for polarized growth. Plant Physiol 176: 352–363. 10.1104/pp.17.00753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikova TN, Blancaflor EB, Gilroy S (1999) Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant Journal 17: 657–665. [DOI] [PubMed] [Google Scholar]
- Bibikova TN, Zhigilei A, Gilroy S (1997) Root hair growth in Arabidopsis thaliana is directed by calcium and an endogenous polarity. Planta 203: 495–505. [DOI] [PubMed] [Google Scholar]
- Boisson-Dernier A, Lituiev DS, Nestorova A, Franck CM, Thirugnanarajah S, Grossniklaus U (2013) ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PLoS Biol 11. 10.1371/journal.pbio.1001719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Hepler PK (2005) Pectin methylesterases and pectin dynamics in pollen tubes. Plant Cell 17: 3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne HR, Sanders DA, McCormick F (1991) The GTPase superfamily: conserved structure and molecular mechanism. Nature 349: 117–127. [DOI] [PubMed] [Google Scholar]
- Bove J, Vaillancourt B, Kroeger J, Hepler PK, Wiseman PW, Geitmann A (2008) Magnitude and direction of vesicle dynamics in growing pollen tubes using spatiotemporal image correlation spectroscopy and fluorescence recovery after photobleaching. Plant Physiol 147: 1646–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkart GM, Baskin TI, Bezanilla M (2015) A family of ROP proteins that suppresses actin dynamics, and is essential for polarized growth and cell adhesion. J Cell Sci 128: 2553–64. 10.1242/jcs.172445 [DOI] [PubMed] [Google Scholar]
- Cai G, Cresti M (2009) Organelle motility in the pollen tube: a tale of 20 years. J Exp Bot 60: 495–508. [DOI] [PubMed] [Google Scholar]
- Cai G, Cresti M (2010) Microtubule motors and pollen tube growth—still an open question. Protoplasma 247: 131–143. [DOI] [PubMed] [Google Scholar]
- Cai G, Faleri C, Del Casino C, Emons AMC, Cresti M (2011) Distribution of callose synthase, cellulose synthase, and sucrose synthase in tobacco pollen tube is controlled in dissimilar ways by actin filaments and microtubules. Plant Physiol 155: 1169–1190. 10.1104/pp.110.171371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campàs O, Mahadevan L (2009) Shape and dynamics of tip-growing cells. Current Biology 19: 2102–2107. [DOI] [PubMed] [Google Scholar]
- Cardenas L, Lovy-Wheeler A, Kunkel JG, Hepler PK (2008) Pollen tube growth oscillations and intracellular calcium levels are reversibly modulated by actin polymerization. Plant Physiol 146: 1611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RE, Cosgrove DJ (2007) Portrait of the expansin superfamily in Physcomitrella patens: comparisons with angiosperm expansins. Ann Bot 99: 1131–41. 10.1093/aob/mcm044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelladurai D, Galotto G, Petitto J, Vidali L, Wu M (2020) Inferring lateral tension distribution in wall structures of single cells. Eur Phys J Plus 135: 662. 10.1140/epjp/s13360-020-00670-8 [DOI] [Google Scholar]
- Chen T, Teng N, Wu X, Wang Y, Tang W, Samaj J, Baluska F, Lin J (2007) Disruption of actin filaments by latrunculin B affects cell wall construction in Picea meyeri pollen tube by disturbing vesicle trafficking. Plant Cell Physiol 48: 19–30. [DOI] [PubMed] [Google Scholar]
- Cheng XH, Mwaura BW, Stauffer SRC, Bezanilla M (2020) A fully functional ROP fluorescent fusion protein reveals roles for this GTPase in subcellular and tissue-level patterning. Plant Cell 32: 3436–3451. 10.1105/tpc.20.00440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Wu HM (2004) Overexpression of an Arabidopsis formin stimulates supernumerary actin cable formation from pollen tube cell membrane. Plant Cell 16: 257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Wu HM (2008) Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annual Review of Plant Biology 59: 547–572. 10.1146/annurev.arplant.59.032607.092921 [DOI] [PubMed] [Google Scholar]
- Cove D (2005) The moss Physcomitrella patens. Annu Rev Genet 39: 339–58. [DOI] [PubMed] [Google Scholar]
- de Win AHN, Pierson ES, Derksen J (1999) Rational analyses of organelle trajectories in tobacco pollen tubes reveal characteristics of the actomyosin cytoskeleton. Biophys J 76: 1648–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks MJ, Cvrckova F, Machesky LM, Mikitova V, Ketelaar T, Zarsky V, Davies B, Hussey PJ (2005) Arabidopsis group Ie formins localize to specific cell membrane domains, interact with actin-binding proteins and cause defects in cell expansion upon aberrant expression. New Phytol 168: 529–40. [DOI] [PubMed] [Google Scholar]
- Derksen J, Knuiman B, Hoedemaekers K, Guyon A, Bonhomme S, Pierson ES (2002) Growth and cellular organization of Arabidopsis pollen tubes in vitro. Sex Plant Reprod 15: 133–139. 10.1007/s00497-002-0149-1 [DOI] [Google Scholar]
- Derksen J, Rutten T, Vanamstel T, Dewin A, Doris F, Steer M (1995) Regulation of pollen-tube growth. Acta Botanica Neerlandica 44: 93–119. [Google Scholar]
- Digman MA, Dalal R, Horwitz AF, Gratton E (2008) Mapping the number of molecules and brightness in the laser scanning microscope. Biophys J 94: 2320–32. 10.1529/biophysj.107.114645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digman MA, Wiseman PW, Horwitz AR, Gratton E (2009) Detecting protein complexes in living cells from laser scanning confocal image sequences by the cross correlation raster image spectroscopy method. Biophys J 96: 707–16. 10.1016/j.bpj.2008.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Pervere LM, Bascom C Jr., Bibeau JP, Khurana S, Butt AM, Orr RG, Flaherty PJ, Bezanilla M, Vidali L (2018) Conditional genetic screen in Physcomitrella patens reveals a novel microtubule depolymerizing-end-tracking protein. PLoS Genet 14: e1007221. 10.1371/journal.pgen.1007221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doonan JH, Ccove DJ, Lloyd CW (1988) Microtubules and microfilaments in tip growth: evidence that microtubules impose polarity on protonemal growth in Physcomitrella patens. J Cell Sci 89: 533–540. [Google Scholar]
- Doonan JH, Cove DJ, Lloyd CW (1985) Immunofluorescence microscopy of microtubules in intact cell lineages of the moss, Physcomitrella patens. I. Normal and CIPC-treated tip cells. J Cell Sci 75: 131–47. [DOI] [PubMed] [Google Scholar]
- Dumais J, Long SR, Shaw SL (2004) The mechanics of surface expansion anisotropy in Medicago truncatula root hairs. Plant Physiol 136: 3266–3275. 10.1104/pp.104.043752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais J, Shaw SL, Steele CR, Long SR, Ray PM (2006) An anisotropic-viscoplastic model of plant cell morphogenesis by tip growth. International Journal of Developmental Biology 50: 209–222. 10.1387/ijdb.052066jd [DOI] [PubMed] [Google Scholar]
- Eklund DM, Svensson EM, Kost B (2010) Physcomitrella patens: a model to investigate the role of RAC/ROP GTPase signalling in tip growth. J Exp Bot 61: 1917–37. 10.1093/jxb/erq080 [DOI] [PubMed] [Google Scholar]
- Elson EL (2011) Fluorescence correlation spectroscopy: past, present, future. Biophys J 101: 2855–70. 10.1016/j.bpj.2011.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emons AMC (1987) The cytoskeleton and secretory vesicles in root hairs of Equisetum and Limnobium and cytoplasmic streaming in root hairs of Equisetum. Annals of Botany 60: 625–632. 10.1093/oxfordjournals.aob.a087492 [DOI] [Google Scholar]
- Engel PP (1968) The Induction of biochemical and morphological mutants in the moss Physcomitrella patens. American Journal of Botany 55: 438. [Google Scholar]
- Fayant P, Girlanda O, Chebli Y, Aubin CE, Villemure I, Geitmann A (2010) Finite element model of polar growth in pollen tubes. Plant Cell 22: 2579–93. 10.1105/tpc.110.075754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng QN, Kang H, Song SJ, Ge FR, Zhang YL, Li E, Li S, Zhang Y (2016) Arabidopsis RhoGDIs are critical for cellular homeostasis of pollen tubes. Plant Physiol 170: 841–56. 10.1104/pp.15.01600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando DD, Lazzaro MD, Owens JN (2005) Growth and development of conifer pollen tubes. Sex Plant Reprod 18: 149–162. 10.1007/s00497-005-0008-y [DOI] [Google Scholar]
- Finka A, Schaefer DG, Saidi Y, Goloubinoff P, Zryd JP (2007) In vivo visualization of F-actin structures during the development of the moss Physcomitrella patens. New Phytol 174: 63–76. 10.1111/j.1469-8137.2007.01989.x [DOI] [PubMed] [Google Scholar]
- Fitzpatrick JAJ, Lillemeier BF (2011) Fluorescence correlation spectroscopy: linking molecular dynamics to biological function in vitro and in situ. Curr Opin Struc Biol 21: 650–660. 10.1016/j.sbi.2011.06.006 [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–6. 10.1038/nature01485 [DOI] [PubMed] [Google Scholar]
- Fu Y, Wu G, Yang Z (2001) Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. J Cell Biol 152: 1019–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furt F, Lemoi K, Tüzel E, Vidali L (2012) Quantitative analysis of organelle distribution and dynamics in Physcomitrella patens protonemal cells. BMC Plant Biology 12: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furt F, Liu YC, Bibeau JP, Tüzel E, Vidali L (2013) Apical myosin XI anticipates F-actin during polarized growth of Physcomitrella patens cells. Plant J 73: 417–28. 10.1111/tpj.12039 [DOI] [PubMed] [Google Scholar]
- Galotto G, Abreu I, Sherman CA, Liu B, Gonzalez-Guerrero M, Vidali L (2020) Chitin triggers calcium-mediated immune response in the plant model Physcomitrella patens. Mol Plant-Microbe Interact 33: 911–920. 10.1094/MPMI-03-20-0064-R [DOI] [PubMed] [Google Scholar]
- Galotto G, Wisanpitayakorn P, Bibeau JP, Liu Y-C, Simpson PJ, Tüzel E, Vidali L (2020) Myosin XI drives polarized growth by vesicle clustering and local enrichment of F-actin in Physcomitrium (Physcomitrella) patens. bioRxiv: 2020.08.25.266296. 10.1101/2020.08.25.266296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geitmann A, Nebenfuhr A (2015) Navigating the plant cell: intracellular transport logistics in the green kingdom. Mol Biol Cell 26: 3373–8. 10.1091/mbc.E14-10-1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goriely A (2017) The mathematics and mechanics of biological growth. Springer, New York, NY. [Google Scholar]
- Grebnev G, Cvitkovic M, Fritz C, Cai G, Smith AS, Kost B (2020) Quantitative structural organization of bulk apical membrane traffic in pollen tubes. Plant Physiol 183: 1559–1585. 10.1104/pp.20.00380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebnev G, Ntefidou M, Kost B (2017) Secretion and endocytosis in pollen tubes: Models of tip growth in the spot light. Front Plant Sci 8: 154. 10.3389/fpls.2017.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Li S, Lord EM, Yang Z (2006) Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control Rho GTPase-dependent polar growth. Plant Cell 18: 366–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hala M, Cole R, Synek L, Drdova E, Pecenkova T, Nordheim A, Lamkemeyer T, Madlung J, Hochholdinger F, Fowler JE, Zarsky V (2008) An exocyst complex functions in plant cell growth in Arabidopsis and tobacco. Plant Cell 20: 1330–45. 10.1105/tpc.108.059105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi T, Ito K, Duan Z, Rula S, Takahashi K, Shibuya Y, Hagino N, Miyatake Y, Nakano A, Tominaga M (2018) Functional diversity of class XI myosins in Arabidopsis thaliana. Plant Cell Physiol 59: 2268–2277. 10.1093/pcp/pcy147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harant D, Lang I (2020) Stay in touch-The cortical ER of moss protonemata in osmotic stress situations. Plants 9. 10.3390/plants9040421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries PA, Pan A, Quatrano RS (2005) Actin-related protein2/3 complex component ARPC1 is required for proper cell morphogenesis and polarized cell growth in Physcomitrella patens. Plant Cell 17: 2327–39. 10.1105/tpc.105.033266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler PK, Vidali L, Cheung AY (2001) Polarized cell growth in higher plants. Annu Rev Cell Dev Biol 17: 159–187. 10.1146/annurev.cellbio.17.1.159 [DOI] [PubMed] [Google Scholar]
- Hiwatashi Y, Sato Y, Doonan JH (2014) Kinesins have a dual function in organizing microtubules during both tip growth and cytokinesis in Physcomitrella patens. Plant Cell 26: 1256–66. 10.1105/tpc.113.121723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holubcova Z, Howard G, Schuh M (2013) Vesicles modulate an actin network for asymmetric spindle positioning. Nat Cell Biol 15: 937–47. 10.1038/ncb2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma K, Saito J, Ikebe R, Ikebe M (2000) Ca(2+)-dependent regulation of the motor activity of myosin V. J Biol Chem 275: 34766–71. 10.1074/jbc.M003132200 [DOI] [PubMed] [Google Scholar]
- Hwang JU, Gu Y, Lee YJ, Yang ZB (2005) Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol Biol Cell 16: 5385–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa M, Hirano T, Enami K, Fuselier T, Kato N, Kwon C, Voigt B, Schulze-Lefert P, Baluska F, Sato MH (2014) Syntaxin of plant proteins SYP123 and SYP132 mediate root hair tip growth in Arabidopsis thaliana. Plant Cell Physiol 55: 790–800. 10.1093/pcp/pcu048 [DOI] [PubMed] [Google Scholar]
- Idilli AI, Morandini P, Onelli E, Rodighiero S, Caccianiga M, Moscatelli A (2013) Microtubule depolymerization affects endocytosis and exocytosis in the tip and influences endosome movement in tobacco pollen tubes. Molecular Plant 6: 1109–1130. [DOI] [PubMed] [Google Scholar]
- Ito K, Ren J, Fujita T (2014) Conserved function of Rho-related Rop/RAC GTPase signaling in regulation of cell polarity in Physcomitrella patens. Gene 544: 241–7. 10.1016/j.gene.2014.04.057 [DOI] [PubMed] [Google Scholar]
- Jásik J, Mičieta K, Siao W, Voigt B, Stuchlík S, Schmelzer E, Turňa J, Baluška F (2016) Actin3 promoter reveals undulating F-actin bundles at shanks and dynamic F-actin meshworks at tips of tip-growing pollen tubes. Plant Signaling & Behavior 11: e1146845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen L, Jensen C (1984) Fine structure of protonemal apical cells of the moss Physcomitrium turbinatum. Protoplasma 122: 1–10. [Google Scholar]
- Justus CD, Anderhag P, Goins JL, Lazzaro MD (2004) Microtubules and microfilaments coordinate to direct a fountain streaming pattern in elongating conifer pollen tube tips. Planta 219: 103–109. 10.1007/s00425-003-1193-2 [DOI] [PubMed] [Google Scholar]
- Ketelaar T, de Ruijter NCA, Emons AMC (2003) Unstable F-actin specifies the area and microtubule direction of cell expansion in Arabidopsis root hairs. Plant Cell 15: 285–292. 10.1105/tpc.007039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketelaar T, Galway ME, Mulder BM, Emons AMC (2008) Rates of exocytosis and endocytosis in Arabidopsis root hairs and pollen tubes. J Microsc 231: 265–273. 10.1111/j.1365-2818.2008.02031.x [DOI] [PubMed] [Google Scholar]
- Kost B, Lemichez E, Spielhofer P, Hong Y, Tolias K, Carpenter C, Chua NH (1999) Rac homologues and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J Cell Biol 145: 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost B, Spielhofer P, Chua NH (1998) A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J 16: 393–401. [DOI] [PubMed] [Google Scholar]
- Kroeger JH, Zerzour R, Geitmann A (2011) Regulator or driving force? The role of turgor pressure in oscillatory plant cell growth. Plos One 6. 10.1371/journal.pone.0018549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysan PJ, Young JC, Sussman MR (1999) T-DNA as an insertional mutagen in Arabidopsis. Plant Cell 11: 2283–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo M, Nishiyama T, Tamada Y, Sano R, Ishikawa M, Murata T, Imai A, Lang D, Demura T, Reski R, Hasebe M (2019) Single-cell transcriptome analysis of Physcomitrella leaf cells during reprogramming using microcapillary manipulation. Nucleic Acids Res 47: 4539–4553. 10.1093/nar/gkz181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancelle SA, Hepler PK (1992) Ultrastructure of freeze-substituted pollen tubes of Lilium longiflorum. Protoplasma 167: 215–230. [Google Scholar]
- Lazzaro MD (1999) Microtubule organization in germinated pollen of the conifer Picea abies (Norway spruce, Pinaceae). Am J Bot 86: 759–766. 10.2307/2656696 [DOI] [PubMed] [Google Scholar]
- Le Bail A, Schulmeister S, Perroud PF, Ntefidou M, Rensing SA, Kost B (2019) Analysis of the localization of fluorescent PpROP1 and PpROP-GEF4 fusion proteins in moss protonemata based on genomic “knock-in” and estradiol-titratable expression. Front Plant Sci 10: 456. 10.3389/fpls.2019.00456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Szumlanski A, Nielsen E, Yang ZB (2008) Rho-GTPase-dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J Cell Biol 181: 1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Dong H, Pei W, Liu C, Zhang S, Sun T, Xue X, Ren H (2017) LlFH1-mediated interaction between actin fringe and exocytic vesicles is involved in pollen tube tip growth. New Phytol 214: 745–761. 10.1111/nph.14395 [DOI] [PubMed] [Google Scholar]
- Lin Y, Wang Y, Zhu J, Yang Z (1996) Localization of a Rho GTPase implies a role in tip growth and movement of the generative cell in pollen tubes. Plant Cell 8: 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhang Y, Ren H (2018) Actin polymerization mediated by AtFH5 directs the polarity establishment and vesicle trafficking for pollen germination in Arabidopsis. Mol Plant 11: 1389–1399. 10.1016/j.molp.2018.09.004 [DOI] [PubMed] [Google Scholar]
- Liu YC, Vidali L (2011) Efficient polyethylene glycol (PEG) mediated transformation of the moss Physcomitrella patens. J Vis Exp 50: e2560. 10.3791/2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loren N, Hagman J, Jonasson JK, Deschout H, Bernin D, Cella-Zanacchi F, Diaspro A, McNally JG, Ameloot M, Smisdom N, Nyden M, Hermansson AM, Rudemo M, Braeckmans K (2015) Fluorescence recovery after photobleaching in material and life sciences: putting theory into practice. Quarterly Reviews of Biophysics 48: 323–387. 10.1017/S0033583515000013 [DOI] [PubMed] [Google Scholar]
- Lovy-Wheeler A, Cardenas L, Kunkel JG, Hepler PK (2007) Differential organelle movement on the actin cytoskeleton in lily pollen tubes. Cell Motil Cytoskeleton 64: 217–32. 10.1002/cm.20181 [DOI] [PubMed] [Google Scholar]
- Madison SL, Buchanan ML, Glass JD, McClain TF, Park E, Nebenfuhr A (2015) Class XI myosins move specific organelles in pollen tubes and are required for normal fertility and pollen tube growth in Arabidopsis. Plant Physiol 169: 1946–1960. 10.1104/pp.15.01161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty F (1999) Plant vacuoles. Plant Cell 11: 587–600. 10.1105/tpc.11.4.587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas JP, Lafountain J (1972) Protoplasmic streaming, cytochalasin B, and growth of the pollen tube. Tissue Cell 4: 11–14. [DOI] [PubMed] [Google Scholar]
- McCauley MM, Hepler PK (1992) Cortical ultrastructure of freeze-substituted protonemata of the moss Funaria hygrometrica. Protoplasma 169: 168–178. [Google Scholar]
- McNally JG (2008) Quantitative FRAP in analysis of molecular binding dynamics in vivo. Methods Cell Biol 85: 329–51. 10.1016/S0091-679X(08)85014-5 [DOI] [PubMed] [Google Scholar]
- Menand B, Calder G, Dolan L (2007) Both chloronemal and caulonemal cells expand by tip growth in the moss Physcomitrella patens. J Exp Bot 58: 1843–9. 10.1093/jxb/erm047 [DOI] [PubMed] [Google Scholar]
- Mendrinna A, Persson S (2015) Root hair growth: it’s a one way street. F1000prime reports 7: 23. 10.12703/P7-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerli M, Robinson KR (1997) Tip localized Ca2+ pulses are coincident with peak pulsatile growth rates in pollen tubes of Lilium longiflorum. J Cell Sci 110: 1269–1278. [DOI] [PubMed] [Google Scholar]
- Michard E, Simon AA, Tavares B, Wudick MM, Feijo JA (2017) Signaling with ions: the keystone for apical cell growth and morphogenesis in pollen tubes. Plant Physiol 173: 91–111. 10.1104/pp.16.01561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DD, de Ruijter NCA, Bisseling T, Emons AMC (1999) The role of actin in root hair morphogenesis: studies with lipochito-oligosaccharide as a growth stimulator and cytochalasin as an actin perturbing drug. Plant Journal 17: 141–154. [Google Scholar]
- Molendijk AJ, Bischoff F, Rajendrakumar CSV, Friml J, Braun M, Gilroy S, Palme K (2001) Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J 20: 2779–2788. 10.1093/Emboj/20.11.2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk AJ, Ruperti B, Palme K (2004) Small GTPases in vesicle trafficking. Curr Opin Plant Biol 7: 694–700. [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S (2007) Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proceedings of the National Academy of Sciences, USA 104: 20996–21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Messerli MA, Gilroy S (2008) Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol 147: 1690–1698. 10.1104/pp.108.123638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker MS, Cheney RE (1995) Unconventional myosins. Annu Rev Cell Dev Biol 11: 633–75. [DOI] [PubMed] [Google Scholar]
- Nagawa S, Xu T, Yang Z (2010) RHO GTPase in plants: Conservation and invention of regulators and effectors. Small GTPases 1: 78–88. 10.4161/sgtp.1.2.14544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Hirata A, Sano T, Fujita T, Hiwatashi Y, Sato Y, Kadota A, Hasebe M, Hasezawa S (2009) Microtubules regulate dynamic organization of vacuoles in Physcomitrella patens. Plant Cell Physiol 50: 855–68. [DOI] [PubMed] [Google Scholar]
- Odriscoll D, Hann C, Read SM, Steer MW (1993) Endocytotic uptake of fluorescent dextrans by pollen tubes grown in vitro. Protoplasma 175: 126–130. 10.1007/Bf01385010 [DOI] [Google Scholar]
- Orr RG, Cheng X, Vidali L, Bezanilla M (2020) Orchestrating cell morphology from the inside out - using polarized cell expansion in plants as a model. Curr Opin Cell Biol 62: 46–53. 10.1016/j.ceb.2019.08.004 [DOI] [PubMed] [Google Scholar]
- Orr RG, Foley SJ, Sherman C, Abreu I, Galotto G, Liu BY, Gonzalez-Guerrero M, Vidali L (2020) Robust survival-based RNA interference of gene families using in tandem silencing of adenine phosphoribosyltransferase. Plant Physiol 184: 607–619. 10.1104/pp.20.00865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr RG, Furt F, Warner EL, Agar EM, Garbarino JM, Cabral SE, Dubuke ML, Butt AM, Munson M, Vidali L (2021) Rab-E and its interaction with myosin XI are essential for polarised cell growth. New Phytologist 229: 1924–1936. 10.1111/nph.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovecka M, Lang I, Baluska F, Ismail A, Illes P, Lichtscheidl IK (2005) Endocytosis and vesicle trafficking during tip growth of root hairs. Protoplasma 226: 39–54. 10.1007/s00709-005-0103-9 [DOI] [PubMed] [Google Scholar]
- Park E, Nebenfuhr A (2013) Myosin XIK of Arabidopsis thaliana accumulates at the root hair tip and is required for fast root hair growth. PLoS One 8: e76745. 10.1371/journal.pone.0076745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RM, Fischer-Parton S, Watahiki MK, Trewavas AJ (2001) Dynamics of the apical vesicle accumulation and the rate of growth are related in individual pollen tubes. J Cell Sci 114: 2685–95. [DOI] [PubMed] [Google Scholar]
- Peremyslov VV, Mockler TC, Filichkin SA, Fox SE, Jaiswal P, Makarova KS, Koonin EV, Dolja VV (2011) Expression, splicing, and evolution of the myosin gene family in plants. Plant Physiol 155: 1191–204. 10.1104/pp.110.170720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson ES, Kengen HMP, Derksen J (1989) Microtubules and actin-filaments co-localize in pollen tubes of Nicotiana tabacum L. and Lilium longiflorum Thunb. Protoplasma 150: 75–77. [Google Scholar]
- Pierson ES, Miller DD, Callaham DA, vanAken J, Hackett G, Hepler PK (1996) Tip-localized calcium entry fluctuates during pollen tube growth. Dev Biol 174: 160–173. [DOI] [PubMed] [Google Scholar]
- Pollard TD (2016) Actin and actin-binding proteins. Cold Spring Harbor perspectives in biology 8: a018226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce De Leon I, Schmelz EA, Gaggero C, Castro A, Alvarez A, Montesano M (2012) Physcomitrella patens activates reinforcement of the cell wall, programmed cell death and accumulation of evolutionary conserved defence signals, such as salicylic acid and 12-oxo-phytodienoic acid, but not jasmonic acid, upon Botrytis cinerea infection. Mol Plant Pathol 13: 960–74. 10.1111/j.1364-3703.2012.00806.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat A, Brejskova L, Hala M, Cvrckova F, Zarsky V (2017) The Physcomitrella patens exocyst subunit EXO70.3d has distinct roles in growth and development, and is essential for completion of the moss life cycle. New Phytol 216: 438–454. 10.1111/nph.14548 [DOI] [PubMed] [Google Scholar]
- Reisen D, Marty F, Leborgne-Castel N (2005) New insights into the tonoplast architecture of plant vacuoles and vacuolar dynamics during osmotic stress. BMC Plant Biol 5: 13. 10.1186/1471-2229-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, Sixt M, Wedlich-Soldner R (2008) Lifeact: a versatile marker to visualize F-actin. Nat Methods 5: 605–7. 10.1038/nmeth.1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riely BK, He HB, Venkateshwaran M, Sarma B, Schraiber J, Ane JM, Cook DR (2011) Identification of legume RopGEF gene families and characterization of a Medicago truncatula RopGEF mediating polar growth of root hairs. Plant Journal 65: 230–243. 10.1111/j.1365-313X.2010.04414.x [DOI] [PubMed] [Google Scholar]
- Roberts AW, Lahnstein J, Hsieh YSY, Xing X, Yap K, Chaves AM, Scavuzzo-Duggan TR, Dimitroff G, Lonsdale A, Roberts E, Bulone V, Fincher GB, Doblin MS, Bacic A, Burton RA (2018) Functional characterization of a glycosyltransferase from the moss Physcomitrella patens involved in the biosynthesis of a novel cell wall arabinoglucan. Plant Cell 30: 1293–1308. 10.1105/tpc.18.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roding M, Guo M, Weitz DA, Rudemo M, Sarkka A (2014) Identifying directional persistence in intracellular particle motion using Hidden Markov Models. Mathematical Biosciences 248: 140–145. 10.1016/j.mbs.2013.12.008 [DOI] [PubMed] [Google Scholar]
- Rojas ER, Hotton S, Dumais J (2011) Chemically mediated mechanical expansion of the pollen tube cell wall. Biophys J 101: 1844–53. 10.1016/j.bpj.2011.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounds CM, Bezanilla M (2013) Growth mechanisms in tip-growing plant cells. Annu Rev Plant Biol 64: 243–265. 10.1146/annurev-arplant-050312-120150 [DOI] [PubMed] [Google Scholar]
- Saavedra L, Catarino R, Heinz T, Heilmann I, Bezanilla M, Malho R (2015) Phosphatase and tensin homolog is a growth repressor of both rhizoid and gametophore development in the moss Physcomitrella patens. Plant Physiol 169: 2572–86. 10.1104/pp.15.01197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Yamamoto J, Jin T, Kinjo M (2015) Raster image cross-correlation analysis for spatiotemporal visualization of intracellular degradation activities against exogenous DNAs. Sci Rep 5. 10.1038/srep14428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer D, Zryd JP, Knight CD, Cove DJ (1991) Stable transformation of the moss Physcomitrella patens. Mol Gen Genet 226: 418–24. [DOI] [PubMed] [Google Scholar]
- Schaefer DG, Zryd JP (1997) Efficient gene targeting in the moss Physcomitrella patens. Plant Journal 11: 1195–1206. [DOI] [PubMed] [Google Scholar]
- Scheible N, McCubbin A (2019) Signaling in pollen tube growth: beyond the tip of the polarity iceberg. Plants 8: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein JW, Somerville C (1990) Genetic control of root hair development in Arabidopsis thaliana. Plant Cell 2: 235–243. 10.1105/tpc.2.3.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh M (2011) An actin-dependent mechanism for long-range vesicle transport. Nat Cell Biol 13: 1431–6. 10.1038/ncb2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SL, Dumais J, Long SR (2000) Cell surface expansion in polarly growing root hairs of Medicago truncatula. Plant Physiol 124: 959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan MB, Staiger CJ, Rose RJ, McCurdy DW (2004) A green fluorescent protein fusion to actin-binding domain 2 of Arabidopsis fimbrin highlights new features of a dynamic actin cytoskeleton in live plant cells. Plant Physiol 136: 3968–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieberer B, Emons AMC (2000) Cytoarchitecture and pattern of cytoplasmic streaming in root hairs of Medicago truncatula during development and deformation by nodulation factors. Protoplasma 214: 118–127. 10.1007/Bf02524268 [DOI] [Google Scholar]
- Sieberer BJ, Ketelaar T, Esseling JJ, Emons AMC (2005) Microtubules guide root hair tip growth. New Phytologist 167: 711–719. [DOI] [PubMed] [Google Scholar]
- Stephan O, Cottier S, Fahlen S, Montes-Rodriguez A, Sun J, Eklund DM, Klahre U, Kost B (2014) RISAP is a TGN-associated RAC5 effector regulating membrane traffic during polar cell growth in tobacco. Plant Cell 26: 4426–47. 10.1105/tpc.114.131078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synek L, Schlager N, Elias M, Quentin M, Hauser MT, Zarsky V (2006) AtEXO70A1, a member of a family of putative exocyst subunits specifically expanded in land plants, is important for polar growth and plant development. Plant Journal 48: 54–72. 10.1111/j.1365-313X.2006.02854.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, de Keijzer J, Overdijk E, Sweep E, Steentjes M, Vermeer JE, Janson ME, Ketelaar T (2019) Exocyst subunit Sec6 is positioned by microtubule overlaps in the moss phragmoplast prior to cell plate membrane arrival. J Cell Sci. 10.1242/jcs.222430 [DOI] [PubMed] [Google Scholar]
- Tominaga M, Kimura A, Yokota E, Haraguchi T, Shimmen T, Yamamoto K, Nakano A, Ito K (2013) Cytoplasmic streaming velocity as a plant size determinant. Dev Cell 27: 345–52. 10.1016/j.devcel.2013.10.005 [DOI] [PubMed] [Google Scholar]
- Tominaga M, Kojima H, Yokota E, Orii H, Nakamori R, Katayama E, Anson M, Shimmen T, Oiwa K (2003) Higher plant myosin XI moves processively on actin with 35 nm steps at high velocity. EMBO J 22: 1263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Morita K, Sonobe S, Yokota E, Shimmen T (1997) Microtubules regulate the organization of actin filaments at the cortical region in root hair cells of Hydrocharis. Protoplasma 199: 83–92. 10.1007/Bf02539809 [DOI] [Google Scholar]
- Tran ML, McCarthy TW, Sun H, Wu SZ, Norris JH, Bezanilla M, Vidali L, Anderson CT, Roberts AW (2018) Direct observation of the effects of cellulose synthesis inhibitors using live cell imaging of Cellulose Synthase (CESA) in Physcomitrella patens. Sci Rep 8: 735. 10.1038/s41598-017-18994-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trybus KM (2008) Myosin V from head to tail. Cell Mol Life Sci 65: 1378–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T, Era A, Tominaga M, Ebine K, Awai C, Saito C, Ishizaki K, Yamato KT, Kohchi T, Nakano A (2009) Application of Lifeact reveals F-actin dynamics in Arabidopsis thaliana and the liverwort, Marchantia polymorpha. Plant Cell Physiol 50: 1041–1048. 10.1093/pcp/pcp055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Ueda T, Ohniwa RL, Nakano A, Takeyasu K, Sato MH (2004) Systematic analysis of SNARE molecules in Arabidopsis: dissection of the post-Golgi network in plant cells. Cell Struct Funct 29: 49–65. https://doi.org/JST.JSTAGE/csf/29.49 [DOI] [PubMed] [Google Scholar]
- Valster AH, Hepler PK, Chernoff J (2000) Plant GTPases: the Rhos in bloom. Trends Cell Biol 10: 141–146. 10.1016/S0962-8924(00)01728-1 [DOI] [PubMed] [Google Scholar]
- van der Honing HS, van Bezouwen LS, Emons AMC, Ketelaar T (2011) High expression of Lifeact in Arabidopsis thaliana reduces dynamic reorganization of actin filaments but does not affect plant development. Cytoskeleton 68: 578–587. 10.1002/cm.20534 [DOI] [PubMed] [Google Scholar]
- van Gisbergen P, Wu SZ, Cheng X, Pattavina KA, Bezanilla M (2020) In vivo analysis of formin dynamics in the moss P. patens reveals functional class diversification. J Cell Sci 133. 10.1242/jcs.233791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gisbergen PA, Li M, Wu SZ, Bezanilla M (2012) Class II formin targeting to the cell cortex by binding PI(3,5)P(2) is essential for polarized growth. J Cell Biol 198: 235–50. 10.1083/jcb.201112085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gisbergen PAC, Wu SZ, Chang M, Pattavina KA, Bartlett ME, Bezanilla M (2018) An ancient Sec10-formin fusion provides insights into actin-mediated regulation of exocytosis. J Cell Biol 217: 945–957. 10.1083/jcb.201705084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verchot-Lubicz J, Goldstein RE (2010) Cytoplasmic streaming enables the distribution of molecules and vesicles in large plant cells. Protoplasma 240: 99–107. [DOI] [PubMed] [Google Scholar]
- Vidali L, Augustine RC, Fay SN, Franco P, Pattavina KA, Bezanilla M (2009) Rapid screening for temperature-sensitive alleles in plants. Plant Physiol 151: 506–514. 10.1104/pp.109.143727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L, Augustine RC, Kleinman KP, Bezanilla M (2007) Profilin is essential for tip growth in the moss Physcomitrella patens. Plant Cell 19: 3705–22. 10.1105/tpc.107.053413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L, Bezanilla M (2012) Physcomitrella patens: a model for tip cell growth and differentiation. Curr Opin Plant Biol 15: 625–31. 10.1016/j.pbi.2012.09.008 [DOI] [PubMed] [Google Scholar]
- Vidali L, Burkart GM, Augustine RC, Kerdavid E, Tüzel E, Bezanilla M (2010) Myosin XI is essential for tip growth in Physcomitrella patens. Plant Cell 22: 1868–82. 10.1105/tpc.109.073288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L, McKenna ST, Hepler PK (2001) Actin polymerization is essential for pollen tube growth. Mol Biol Cell 12: 2534–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L, Rounds CM, Hepler PK, Bezanilla M (2009) Lifeact-mEGFP reveals a dynamic apical F-actin network in tip growing plant cells. Plos One 4: e5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L, van Gisbergen PAC, Guerin C, Franco P, Li M, Burkart GM, Augustine RC, Blanchoin L, Bezanilla M (2009) Rapid formin-mediated actin-filament elongation is essential for polarized plant cell growth. Proc Natl Acad Sci USA 106: 13341–13346. 10.1073/pnas.0901170106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L, Yokota E, Cheung AY, Shimmen T, Hepler PK (1999) The 135 kDa actin-bundling protein from Lilium longiflorum pollen is the plant homologue of villin. Protoplasma 209: 283–291. [Google Scholar]
- Voigt B, Timmers AC, Samaj J, Hlavacka A, Ueda T, Preuss M, Nielsen E, Mathur J, Emans N, Stenmark H, Nakano A, Baluska F, Menzel D (2005) Actin-based motility of endosomes is linked to the polar tip growth of root hairs. Eur J Cell Biol 84: 609–21. 10.1016/j.ejcb.2004.12.029 [DOI] [PubMed] [Google Scholar]
- Wang H, Zhuang X, Cai Y, Cheung AY, Jiang L (2013) Apical F-actin-regulated exocytic targeting of NtPPME1 is essential for construction and rigidity of the pollen tube cell wall. Plant J 76: 367–79. 10.1111/tpj.12300 [DOI] [PubMed] [Google Scholar]
- Wang HJ, Wan AR, Jauh GY (2008) An actin-binding protein, L1LIM1, mediates calcium and hydrogen regulation of actin dynamics in pollen tubes. Plant Physiol 147: 1619–1636. 10.1104/pp.108.118604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Teng Y, Wang Q, Li X, Sheng X, Zheng M, Samaj J, Baluska F, Lin J (2006) Imaging of dynamic secretory vesicles in living pollen tubes of Picea meyeri using evanescent wave microscopy. Plant Physiol 141: 1591–603. 10.1104/pp.106.080168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-F, Fan L-M, Zhang W-Z, Zhang W, Wu W-H (2004) Ca2+-permeable channels in the plasma membrane of Arabidopsis pollen are regulated by actin microfilaments. Plant Physiol 136: 3892–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JH (2012) Pollen tube growth rates and the diversification of flowering plant reproductive cycles. Int J Plant Sci 173: 649–661. 10.1086/665822 [DOI] [Google Scholar]
- Wu G, Gu Y, Li S, Yang Z (2001) A genome-wide analysis of Arabidopsis Rop-interactive CRIB motif–containing proteins that act as Rop GTPase targets. Plant Cell 13: 2841–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Shang ZL, Wu J, Jiang XT, Moschou PN, Sun WD, Roubelakis-Angelakis KA, Zhang SL (2010) Spermidine oxidase-derived H2O2 regulates pollen plasma membrane hyperpolarization-activated Ca2+-permeable channels and pollen tube growth. Plant Journal 63: 1042–1053. 10.1111/j.1365-313X.2010.04301.x [DOI] [PubMed] [Google Scholar]
- Wu SZ, Bezanilla M (2018) Actin and microtubule cross talk mediates persistent polarized growth. J Cell Biol 217: 3531–3544. 10.1083/jcb.201802039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Goshima G (2018) The KCH kinesin drives nuclear transport and cytoskeletal coalescence to promote tip cell growth in Physcomitrella patens. Plant Cell 30: 1496–1510. 10.1105/tpc.18.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Tanaka-Takiguchi Y, Hayashi M, Nishina M, Goshima G (2017) Multiple kinesin-14 family members drive microtubule minus end-directed transport in plant cells. J Cell Biol 216: 1705–1714. 10.1083/jcb.201610065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Gao P, Zhang H, Huang S, Zheng Z-L (2007) A mutation in MRH2 kinesin enhances the root hair tip growth defect caused by constitutively activated ROP2 small GTPase in Arabidopsis. PLoS One 2: e1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z (2002) Small GTPases: versatile signaling switches in plants. Plant Cell 14: S375–S388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota E, Muto S, Shimmen T (1999) Inhibitory regulation of higher-plant myosin by Ca2+ ions. Plant Physiol 119: 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Qu XL, Bao CC, Khurana P, Wang QN, Xie YR, Zheng YY, Chen NZ, Blanchoin L, Staiger CJ, Huang SJ (2010) Arabidopsis VILLIN5, an actin filament bundling and severing protein, is necessary for normal pollen tube growth. Plant Cell 22: 2749–2767. 10.1105/tpc.110.076257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yan A, Feijo JA, Furutani M, Takenawa T, Hwang I, Fu Y, Yang Z (2010) Phosphoinositides regulate clathrin-dependent endocytosis at the tip of pollen tubes in Arabidopsis and tobacco. Plant Cell 22: 4031–44. 10.1105/tpc.110.076760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Zhang Y, Kang E, Xu Q, Wang M, Rui Y, Liu B, Yuan M, Fu Y (2013) MAP18 regulates the direction of pollen tube growth in Arabidopsis by modulating F-actin organization. Plant Cell 25: 851–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonia L, Munnik T (2008) Vesicle trafficking dynamics and visualization of zones of exocytosis and endocytosis in tobacco pollen tubes. J Exp Bot 59: 861–873. 10.1093/Jxb/Ern007 [DOI] [PubMed] [Google Scholar]


