Abstract
The aging heart is well-characterized by a diminished responsiveness to adrenergic activation. However, the precise mechanisms by which age and sex impact adrenergic-mediated cardiac function remain poorly described. In the current investigation, we compared the cardiac response to adrenergic stress to gain mechanistic understanding of how the response to an adrenergic challenge differs by sex and age. Juvenile (4 weeks), adult (4–6 months), and aged (18–20 months) male and female mice were treated with the β-agonist isoproterenol (ISO) for 1 week. ISO-induced morphometric changes were age- and sex-dependent as juvenile and adult mice of both sexes had higher left ventricle weights while aged mice did not increase cardiac mass. Adults increased myocyte cell size and deposited fibrotic matrix in response to ISO, while juvenile and aged animals did not show evidence of hypertrophy or fibrosis. Juvenile females and adults underwent expected changes in systolic function with higher heart rate, ejection fraction, and fractional shortening. However, cardiac function in aged animals was not altered in response to ISO. Transcriptomic analysis identified significant differences in gene expression by age and sex, with few overlapping genes and pathways between groups. Fibrotic and adrenergic signaling pathways were upregulated in adult hearts. Juvenile hearts upregulated genes in the adrenergic pathway with few changes in fibrosis, while aged mice robustly upregulated fibrotic gene expression without changes in adrenergic genes. We suggest that the response to adrenergic stress significantly differs across the lifespan and by sex. Mechanistic definition of these age-related pathways by sex is critical for future research aimed at treating age-related cardiac adrenergic desensitization.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-021-00345-x.
Keywords: Cardiac, Sex Differences, Adrenergic, Juvenile, RNA sequencing, Lifespan
Introduction
Independent of traditional risk factors, aging is a major risk factor for development of heart failure (HF). Generally defined as a condition in which the heart is unable to meet peripheral metabolic demand, HF develops as the final clinical event of numerous cardiovascular diseases and over the last several years has rapidly increased in incidence and prevalence in older adults [1]. Intrinsic cardiac aging and response to extrinsic stimuli likely both contribute to significantly elevated risk for HF, as well as poorer outcomes related to survival and quality of life, compared to younger adults [2].
The aging and failing hearts are well-characterized by elevated catecholamines and reduced sensitivity to adrenergic stimuli, i.e. β-adrenergic desensitization [3–6], sharing many similarities with respect to the adrenergic system. This reduced sensitivity to adrenergic stimulation results in clinical outcomes such as reduced exercise tolerance, altered LV inotropic reserve, and poor quality of life [7, 8]. Despite these clinical reports, however, incomplete understanding of the mechanisms for age- and HF-related decrements in cardiac performance continues to limit identification of potential therapeutics. No body of work has defined the cardiac response to adrenergic activation across the life-course, nor have most previous investigations included females, despite clinical [9] and mechanistic evidence [10] that sex differences underlie the cardiac response to pathophysiological stimuli. At best, previous work has compared two ages, with some including both sexes; however, a comprehensive life-course by sex understanding of the pathophysiology and molecular mediators of HF pathogenesis is missing from current knowledge. These knowledge gaps prevent the field from moving forward in the understanding of pathogenesis and identification of strategies to resensitize the aging and failing heart. Therefore, we set out to test the simple but critical hypothesis that cardiac pathogenesis differs across the life-course and by sex using a well-established model of β-adrenergic-induced cardiac stress through infusion of the non-selective β-agonist isoproterenol (ISO) [5, 6]. Together, mechanistic description of the impact of age and sex on adrenergic-mediated changes in cardiac function will provide novel insight into potential therapeutic targets to attenuate age-related β-adrenergic desensitization.
Methods and materials
Animals
All experiments and methods described in this study were conducted in accordance with institutional guidelines and approved by the Institutional Animal Care Users Committee of University of Wyoming (protocol # A-3216-01). Mice were housed 2–5 animals per cage in a temperature-controlled room (21 °C) on a standard 12-h light-dark cycle. Food (Lab Diet 5001) and water were provided ad libitum. Newly acquired mice were allowed to acclimate for 1 week prior to beginning the experiment.
Study design and mouse model of cardiac stress
C57Bl6 male and female mice were purchased from Jackson Laboratories (adult and juvenile) or donated from the National Institutes of Aging Rodent Colony (aged). Juvenile (4 weeks), adult (4–6 months), and aged (18–20 months) mice of both sexes were used. These specific ages were selected with consideration of the mouse juvenile developmental period ending by 6 weeks. The aged cohort demonstrated significant evidence of age-associated left ventricle (LV) hypertrophy and was consistent with publications in aged mice [11] without significant decreases in survival in the basal condition. Thirty milligrams per kilogram per day isoproterenol hydrochloride (Sigma-Aldrich, Milwaukee, WI, USA) in L-ascorbic acid and saline was delivered by subcutaneously implanted osmotic mini-pumps (1007D, Alzet Osmotic Pumps, Cupertino, CA, USA), a concentration and duration previously shown to induce cardiac remodeling in adult mice [5, 12]. Surgery was performed under isoflurane anesthesia. Control mice received sham surgery [5]. It has been previously reported that 1 week of ISO infusion induces cardiac hypertrophy [6, 13]. Therefore, analyses occurred 1 week following surgery, after which animals were humanely euthanized with Fatal Plus (390 mg/mL pentobarbital sodium). The whole heart was weighed, followed by dissection of the right ventricle from the LV and septum, which was weighed, flash-frozen in liquid N2, and stored at − 80 °C. Tibia length was measured by caliper, from the bottom of the mouse foot to the top of the knee.
Echocardiography
Cardiac function was assessed via transthoracic echocardiography using a Visual Sonics Vevo 2100 with a 40-mHz probe (Fujifilm, Toronto, ON, Canada). Mice were induced with 2.5% isoflurane in 1-L/min compressed air and maintained on an operative circuit nose cone (Harvard Apparatus, Holliston, MA, USA) where isoflurane was titrated in 1-L/min compressed air to maintain a heart rate > 400 BPM. Core temperature was monitored via a lubricated rectal probe and maintained at 37 °C with a heating platform. Respiratory rate and heart rate were monitored with 4-lead limb ECG. Two-dimensional parasternal long axis (LA) B-Mode views and parasternal short axis (SA) B-Mode and M-Mode views of the LV were captured. Using VevoLab software, cine loop analysis was conducted on cardiac cycles between respirations to minimize respiratory artifact. Endocardial borders were traced in LA B-Mode up to the aortic valve to assess total areas, volumes, ejection fraction (EF) [EF = (LV end diastolic volume (LVED) − LV end systolic volume (LVESV))/LVESV × 100], fractional shortening (FS) [FS = (LV internal diameter (LVID; diastole) − LV internal dimeter; systole (LVIDs)/LVIDd × 100], and cardiac output (CO). SA M-Mode tracings measured parallel to the axis of the papillary muscles were used to assess anterior and posterior LV wall thickness during diastole and systole.
Tissue sectioning and staining
LV samples were frozen in Optimal Cutting Temperature (OCT; Fisher Scientific, Waltham, MA, USA). Four-micrometer sections were cut on a pre-cooled cryostat (Leica Biosystems, IL, USA). For assessment of myocyte cell size, sections were fixed with 3% paraformaldehyde (PFA) for 10 min, and then permeabilized with 1% Triton X-100 for 10 min, washed 3 times with deionized water, and stained with wheat germ agglutinin-FITC (Sigma-Aldrich, Milwaukee, WI, USA) for 1 h. After washing 3 times with deionized water, slides were mounted for imaging. Slides were imaged by fluorescent Olympus IX71 inverted microscope (Olympus, Waltham, MA, USA) and CoolSnap HQ2 CCD camera (Roper Scientific, USA). The image-plane pixel dimension was 1.3 μm for × 40 magnification. Cardiomyocyte-projected area and best-fit ellipse aspect ratio were calculated, using ImageJ (version 1.53a, NIH, USA) in a blinded manner. For assessment of fibrosis, LV sections were rehydrated with ethanol and fixed in Bouin’s solution for 1 h at 56 °C. After washing in tap water for 10 min, slides were stained sequentially in Weigert’s iron hematoxylin, Biebrich scarlet-acid fuchsin solution, and differentiated in phosphomolybdic-phosphotungstic acid solution. Slides were directly transferred to aniline blue solution and stained for 10 min, and rinsed in distilled water before differentiating in 1% acetic acid solution. After washing in distilled water, slides were dehydrated in ethanol and cleared in xylene. Slides were covered in mounting medium before imaging. Images were taken on a × 20 objective and processed by using ImageJ. Each age, sex, and condition group had at least 3 biological replicates and 6 views.
RNA extraction and real-time RT-PCR
Total RNA was extracted from LV tissues by standard TRIzol protocol. RNA was reverse-transcribed into cDNA using a high-capacity RNA-to-cDNA kit (Fisher Scientific, Waltham, MA, USA), and qRT-PCR was performed using PowerSYBR Green PCR (Fisher Scientific, Waltham, MA, USA) and analyzed on ABI7300 (Fisher Scientific, Waltham, MA, USA). Target mRNA expression was normalized to 18s ribosomal RNA [14–16]. Values are presented as fold change from respective age and sex control using the ΔΔCt method. Oligonucleotide sequences are listed in Table S1.
RNA sequencing
LV RNA was isolated as above and cleaned up using commercially available kit with genomic DNA depletion (Qiagen RNeasy). 18 TruSeq RNA libraries from each sex (36 total) were sequenced using the NovaSEQ 6000 platform to generate 150-bp paired-end reads. Reads were assessed for quality and contamination using FastQC [17]. Reads were trimmed for adapter contamination using bbduk tool [18] and for a minimum PHRED-scaled quality score of 26 using Trimmomatic [19]. Library preparation and sequencing was performed by the University of Colorado-Denver Genomics and Microarray Core. The read data were aligned with the mouse reference genome assembly version 38.97 and genome annotation version 38 from Ensembl using HISAT2 genome aligner [20]. Transcripts were assembled using StringTie version 1.3.4 [21]. Raw counts of identified transcripts and genes were analyzed in EdgeR [22, 23] for differential expression. Counts were normalized and expression dispersion was estimated. Fisher’s exact test was used to estimate statistical significance and determine false discovery rate (FDR). Genes up- or downregulated at an FDR of 0.05 were further examined for enrichment of specific expression pathways. Gene Set Enrichment Analysis (GSEA) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were performed to understand the potential molecular mechanisms of differentially expressed genes (DEGs) using the clusterProfiler (version 3.14.3), ggplot2 (version 2_3.3.2), and enrichplot packages (version 1.6.1) in R software [24]. Bioconductor package was used for genome-wide annotation (org.Mm.eg.db; version 3.10.0). Computing analysis was performed in Advanced Research Computing Center at University of Wyoming [25]. Significance was set to P < 0.05 for the GSEA and KEGG analyses. The raw RNAseq data has been deposited in the NCBI SRA database under PRJNA640422.
Statistical analyses
Echocardiography and morphometric data were analyzed by 3-way ANOVA (sex × age × condition with Fisher’s post hoc testing for within-group differences in the presence of a significant main effect. PCR data were analyzed by Student’s t-test within age and sex. Statistical significance was set a priori at P < 0.05, and all data were expressed as mean ± SEM. Analyses were performed using SPSS version 25 (IBM Corp., Armonk, NY).
Results
Cardiac remodeling in response to ISO differs significantly by age and sex
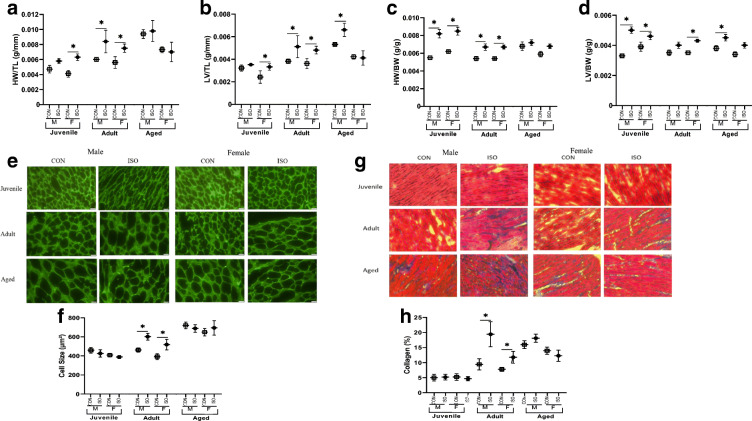
Mice treated with ISO demonstrated age- and sex-specific cardiac remodeling. Heart weight relative to tibia length (HW/TL) and LV/TL in juvenile female mice and adult animals of both sexes were elevated with ISO. While HW/TL was not different between control (CON) and ISO-treated aged male or female mice, LV/TL was higher in aged male mice in response to ISO (Fig. 1a, b). When normalized to BW, HW/BW and LV/BW were also significantly higher in juvenile male mice treated with ISO (Fig. 1c, d). Echocardiographic measurement of anterior LV wall thickness (LVAWs) supported age-specific changes in cardiac remodeling, with higher LVAW in juvenile and adult, but not aged mice in response to ISO (Table 1). Quantification of LV myocyte size also supported age-specific changes in hypertrophy, with adults demonstrating significant myocyte hypertrophy. Importantly, myocytes from aged animals were larger at baseline and did not undergo hypertrophic remodeling in response to ISO. Juvenile myocytes did not hypertrophy in response to ISO in either sex (Fig. 1e, f). ISO stimulated changes in fibrosis in the LV mirrored the hypertrophic changes. While adults deposited fibrotic matrix in response to ISO, juveniles did not. While the aged cohort had higher baseline fibrosis than the younger cohorts, they did not deposit collagen in response to ISO (Fig. 1g, h).
Fig. 1.
Cardiac remodeling in response to 1 week of isoproterenol (ISO) by age and sex. a Heart weight normalized to tibia length (HW/TL) differed by age (juvenile < adult < aged), sex, condition, and the interactions age × sex and age × condition. b LV weight normalized to TL (LV/TL) differed by age (juvenile < adult < aged), condition, and the interactions age × sex and age × condition. c HW normalized to BW (HW/BW) differed by age (juvenile > adult = aged), condition, and the interactions age × sex and age × condition D) LV weight normalized to BW (LV/BW) differed by condition, n = 6/group. e Representative images (× 40 magnification) from WGA-FITC staining of LV sections. f Cardiomyocyte size differed by age (juvenile < adult < age), sex, condition, and the interaction age × condition. g Representative images (× 20 magnification) from Trichrome staining of LV sections. h Fibrosis differed by age (juvenile = adult < aged), condition, sex, and the interaction age × condition, n = 3 biological replicates, 6 technical replicates. *P < 0.05 as assessed by 3-way ANOVA and Fisher’s post hoc test to compare ISO and CON within age and sex. Data are presented as means ± SEM
Table 1.
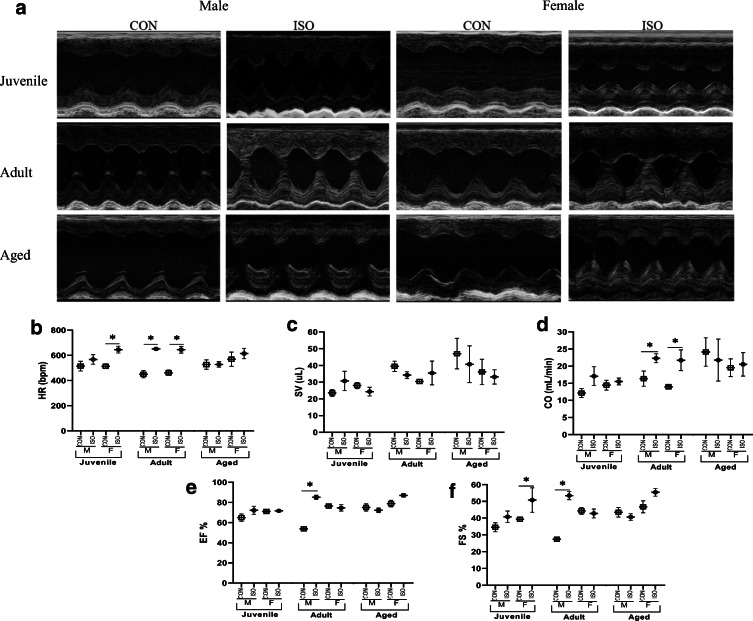
Summary of echocardiographic data in response to isoproterenol (ISO) by age and sex. Heart rate (HR), end systolic volume (ESV), end diastolic volume (EDV), stroke volume (SV), ejection fraction (EF), fractional shortening (FS), cardiac output (CO), and diastolic and systolic thickness of left ventricle anterior wall (LVAWd and LVAWs). LVAWs and LVAWd differed by age (pediatric < adult < geriatric). Data are presented as means ± SEM. *P < 0.05 as assessed by 3-way ANOVA and Fisher’s post hoc test to compare ISO and CON within age and sex
| Juvenile | Adult | Aged | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |||||||
| CON (n = 4) | ISO (n = 5) | CON (n = 5) | ISO (n = 5) | CON (n = 3) | ISO (n = 4) | CON (n = 5) | ISO (n = 4) | CON (n = 4) | ISO (n = 4) | CON (n = 4) | ISO (n = 3) | |
| HR (bpm) | 515 ± 39 | 568 ± 38 | 513 ± 19 | 646 ± 27* | 451 ± 28 | 652 ± 9* | 462 ± 21 | 645 ± 26* | 527 ± 38 | 528 ± 22 | 570 ± 56 | 615 ± 41 |
| ESV (μL) | 12.6 ± 1.1 | 12.0 ± 2.6 | 11.3 ± 0.8 | 9.6 ± 1 | 34.1 ± 3.9 | 6.1 ± 1.3* | 9.7 ± 1.5 | 13.0 ± 3.9 | 17.8 ± 6.4 | 15.2 ± 3.1 | 8.9 ± 0.8 | 4.9 ± 0.8 |
| EDV (μL) | 36.2 ± 1.5 | 42.7 ± 7.6 | 39.3 ± 2.4 | 33.9 ± 3.2 | 73.6 ± 6.4 | 40.4 ± 3.2* | 40.1 ± 2.8 | 48.4 ± 10.8 | 64.9 ± 15.4 | 55.9 ± 13.8 | 45.02 ± 7.6 | 38.1 ± 4.8 |
| SV (μL) | 24 ± 2 | 31 ± 6 | 28 ± 2 | 24 ± 3 | 40 ± 3 | 34 ± 2 | 30 ± 1 | 36 ± 7 | 47 ± 9 | 41 ± 11 | 36 ± 8 | 33 ± 4 |
| EF (%) | 65 ± 4 | 72 ± 4 | 71 ± 1 | 72 ± 1 | 54 ± 2 | 85 ± 2* | 76 ± 2 | 75 ± 3 | 75 ± 4 | 72 ± 2 | 79 ± 3 | 87 ± 2 |
| FS (%) | 35 ± 3 | 41 ± 3 | 39 ± 1 | 51 ± 7* | 28 ± 1 | 54 ± 2* | 44 ± 2 | 43 ± 3 | 44 ± 3 | 41 ± 2 | 47 ± 4 | 55 ± 2 |
| CO (mL/min) | 12 ± 1 | 17 ± 3 | 15 ± 1 | 16 ± 1 | 16 ± 2 | 22 ± 1* | 14 ± 0.4 | 22 ± 3* | 24 ± 4 | 22 ± 6 | 20 ± 3 | 21 ± 3 |
| LVAWs (mm) | 1.09 ± 0.12 | 1.54 ± 0.08* | 1.29 ± 0.1 | 1.54 ± 0.11 | 0.99 ± 0.08 | 1.89 ± 0.10* | 1.36 ± 0.10 | 1.83 ± 0.14* | 2.06 ± 0.23 | 1.81 ± 0.14 | 1.76 ± 0.15 | 1.74 ± 0.84 |
| LVAWd (mm) | 0.66 ± 0.09 | 0.94 ± 0.10 | 0.81 ± 0.05 | 1.05 ± 0.09 | 0.86 ± 0.06 | 1.07 ± 0.10 | 0.93 ± 0.09 | 1.12 ± 0.16 | 1.32 ± 0.22 | 1.13 ± 0.12 | 1.08 ± 0.19 | 2.13 ± 0.43 |
Systolic function in response to ISO differs significantly by age and sex
To define age- and sex-specific effects of ISO on cardiac function, mice underwent echocardiography prior to termination (Table 1; Fig. 2). Both male and female adult mice demonstrated significantly higher heart rates (HR) in response to ISO. In contrast, juvenile female mice demonstrated higher HR in response to ISO while juvenile male mice did not (Fig. 2a). ISO did not elicit a change in HR in either male or female aged mice (Fig. 2b; Table 1). No significant differences in stroke volume (SV) were observed (Fig. 2c). Adult mice of both sexes had higher cardiac output (CO) in response to ISO, an effect driven by higher HR, not SV. While juvenile female mice also had higher HR in response to ISO, CO did not significantly change in these animals due to slightly lower SV (Fig. 2d). Ejection fraction (EF) was significantly higher only in adult males in response to ISO, while fractional shortening (FS) was higher in juvenile females and adult males treated with ISO. There were no differences in EF or FS in the aged mice (Fig. 2e, f).
Fig. 2.
Echocardiographic data by age and sex in response to 1 week of isoproterenol (ISO). a Representative images from M-mode echocardiography. b Heart rate (HR) differed by condition and the interaction of age × condition. c SV differed by age (SV; juvenile < adult = aged). d Cardiac output (CO) differed by age (juvenile < aged= adult). e Ejection fraction (EF) differed by age (adult = juvenile < aged), sex, condition, and the interactions of age × condition, sex × condition, and age × sex × condition. f Fractional shortening (FS) differed by sex, condition, and the interaction of age × sex × condition. Data are presented as means ± SEM. *P < 0.05 as assessed by 3-way ANOVA and Fisher’s post hoc test to compare ISO and CON within age and sex, n = 4/group
RNA sequencing in response to ISO differs significantly by age and sex
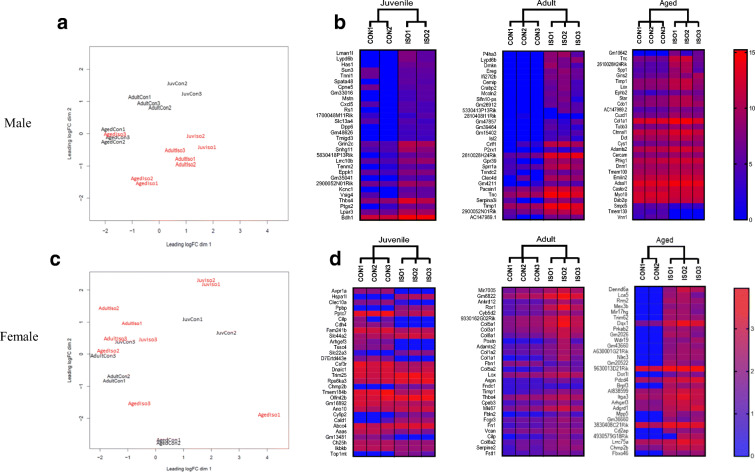
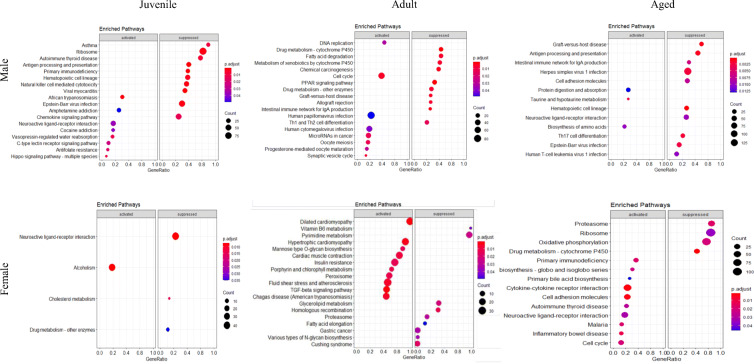
High throughput transcriptomic data was used to investigate the molecular basis for the age- and sex-specific differences in cardiac remodeling and function. Principle component analysis demonstrated significant differences in the transcriptomes of CON and ISO by age in males (Fig. 3a), with greater heterogeneity and less separation between CON and ISO in females (Fig. 3c). Differential expression analysis at FDR < 0.05 identified 282 genes in juvenile males, 836 in adult males, and 30 in aged males. Female differential expression was lower compared to males with 12, 56, and 57 genes in juvenile, adult, and aged, respectively. The 30 most significant log fold change values are illustrated by heat maps and demonstrate homogeneity of biological replicates and higher heterogeneity in the female animals (Fig, 3b and d). The GSEA and KEGG analyses revealed significantly different pathway enrichment by age and sex (Fig. 4). Differences by sex and age were also noted in molecular function (Figure S1), biological process (Figure S2), and cellular component (Figure S3).
Fig. 3.
RNAseq analysis of control (CON) and isoproterenol (ISO) by sex and age. a Principal component analysis in male mice. b Heatmap of gene expression comparing male CON and ISO by age. Thirty genes with the highest/lowest log fold change are displayed (significance was set at FDR < 0.05). c Principal component analysis in female mice. d Heatmap of gene expression comparing female CON and ISO by age. Thirty genes with the highest and lowest log fold change are displayed (significance was set at FDR < 0.05, except for juvenile female, FDR was set at 0.1, due to very low number of genes at 0.05)
Fig. 4.
RNAseq Gene Set Enrichment Analysis (GSEA) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis by sex and age in response to 1 week of isoproterenol (ISO). P-value cutoff was set at 0.05 and number of permutations was set to 10,000 priori. Pathways are listed in order of the highest to the lowest log fold change, size of circles indicate number of genes in specific pathway, color of circles indicate p adjustment value for the analysis
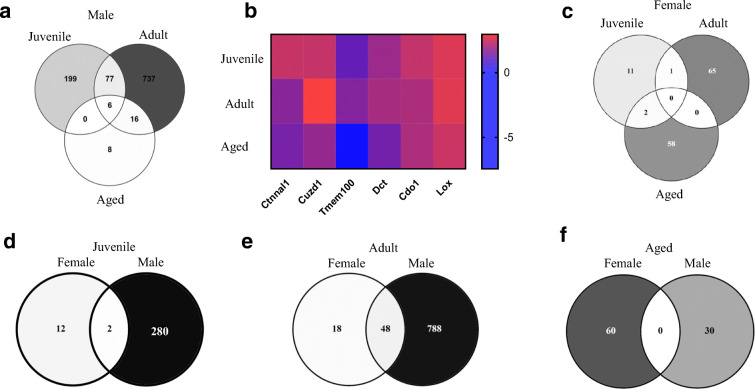
We also assessed overlap of differentially expressed genes by age. In males, six genes were commonly differentially expressed in juvenile, adult, and aged mice (Fig. 5a; Table S2); however, analysis of fold change indicated that these six genes were not consistently up/downregulated by age (Fig. 5b; Table S2). No overlapping genes were identified in females (Fig. 5c). We then compared differentially expressed genes within age, by sex. Two genes overlapped in juvenile (Fig. 5d; Table S3), 48 overlapped in adult (Fig. 5e; Table S4), and no genes overlapped in aged (Fig. 5f).
Fig. 5.
Commonly shared genes identified by RNAseq by sex and age in response to 1 week of isoproterenol (ISO). a Differentially expressed genes in male mice. b Heatmap of overlapping genes by age in male mice. c Differentially expressed genes in female mice. d Differentially expressed genes between males and females by age. Numbers in overlapping areas show common genes differentially expressed in both sex, FDR < 0.05
Expression of fibrosis and β-adrenergic genes differs significantly by sex and age
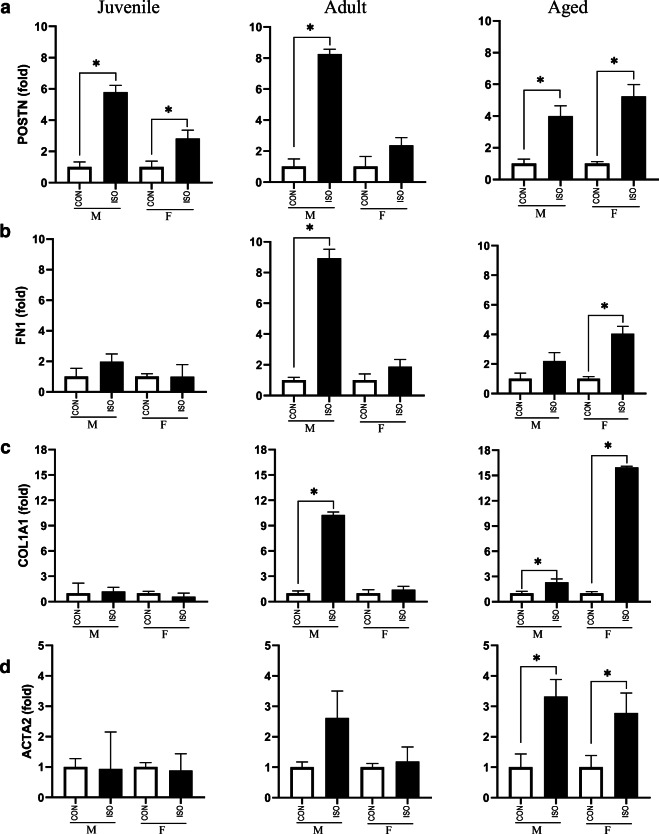
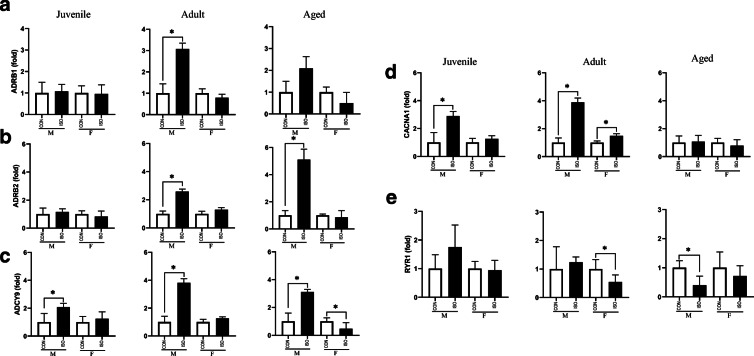
Fibrosis and activation of adrenergic signaling have been shown to be induced by ISO in adult male mice [4, 5]. Therefore, to validate our experimental animals as well as determine how juvenile and aged animals as well as female mice of each group respond to ISO, we assessed expression of selected fibrotic genes (POSTN, FN1, COL1A1, ACTA2) and adrenergic genes. (ADRB1, ADRB2, ADCY9, CACNA1, RYR1). As expected, adult male mice robustly activated genes in the fibrosis pathway, consistent with increased collagen deposition; however, female mice treated with ISO did not upregulate expression of fibrotic genes (Fig. 6). Juvenile mice responded to ISO by upregulating POSTN but no other fibrotic genes. In contrast, fibrotic genes were robustly upregulated in aged animals of both sexes in response to ISO. Similar to the fibrotic response, adult male mice treated with ISO increased expression of genes in the adrenergic cascade (Fig. 7). Once again, adult female mice did not upregulate these genes in response to ISO. While juvenile male mice treated with ISO increased expression of ADCY9 and CACNA1, expression of the other genes were unchanged in response to ISO. Furthermore, juvenile female mice did not upregulate adrenergic genes in response to ISO. Finally, changes in expression of adrenergic genes differed by sex in aged animals, with aged male mice demonstrating upregulated ADRB2 and ADCY9 while aged females generally did not change expression of adrenergic genes.
Fig. 6.
Expression of fibrotic signaling by sex and age in response to 1 week of isoproterenol (ISO). a Periostin (POSTN) expression was upregulated in juvenile mice, adult males, and aged mice of both sexes exposed to ISO. b Fibronectin (FN1) expression was upregulated in adult male mice and aged female mice. c Collagen 1A1 (COL1A1) was upregulated in adult males and aged animals of both sexes. d α-smooth muscle actin (ACTA2) expression was upregulated in aged mice in response to ISO. Gene expression was assessed by qRT-PCR and expressed as fold change relative to CON within age and sex. Data are presented as means ± SEM. *P < 0.05 is assessed using Student’s t-test to compare ISO and CON within age and sex, n = 6/group
Fig. 7.
Expression of adrenergic signaling by sex and age in response to 1 week of isoproterenol (ISO). a β1-adrenergic receptor (ADRB1) expression was upregulated in adult males. b β2-adrenergic receptor (ADRB2) expression was upregulated in adult and aged males. c Adenylyl cyclase 9 (ADCY9) expression was upregulated in males of all three ages and downregulated in aged females. d Calcium voltage-gated channel α1 (CACNA1) expression was upregulated in juvenile males and adults of both sexes. e Ryanodine receptor 1 (RYR1) expression was downregulated in adult females and aged females, while unchanged in juveniles. Gene expression was assessed by qRT-PCR and expressed as fold change relative to CON within age and sex. Data are presented as means ± SEM. *P < 0.05 as assessed Student’s t-test to compare ISO and CON within age and sex, n = 6/group.
Discussion
Elevated circulating catecholamines and adrenergic desensitization are observed in aging and HF [4, 6, 26], resulting in reduced exercise tolerance, altered LV inotropic reserve, and other poor clinical outcomes [7]. Older adults are less responsive to β-blockade than younger patients with heart failure [27], suggesting additional age-related decrement in sensitivity, beyond that associated with heart failure. Animal models of systemic β-adrenergic agonist administration have thus become the standard by which to define molecular mechanisms of the consequences of elevated catecholamines on myocardial function. Here, we demonstrate that significant differences underlie the response to adrenergic stimulation by age and sex in mice. Adult male mice undergo cardiac remodeling with hypertrophy and fibrosis, demonstrate changes in systolic function, and activate a well-established adrenergic and fibrotic gene profile, while adult females undergo similar changes to cardiac mass and systolic function but activate significantly different gene expression profiles. Juvenile mice, thought to be characterized by enhanced resilience to external stress [28], also undergo changes in cardiac mass and function in a sex-specific manner and through unique activation of age- and sex- specific genes. As expected, aged mice do not undergo hypertrophy or elevated systolic function in response to ISO, with significantly different transcriptional profiles than adults or juveniles, and with significant differences between sexes. Together, it is clear that age and sex impact the response to adrenergic stress across the lifespan. While juvenile and adult animals have intact adrenergic responsiveness, the mechanism(s) by which they stimulate cardiac function are strikingly different. Future targeted investigation of these differentially activated pathways will likely yield novel insight into life-course and sex-mediated control of cardiac adrenergic function.
Isoproterenol-induced cardiac remodeling by sex and age
In response to β-agonist administration, the adult heart activates a hypertrophic program [4, 6]. Adult mice of both sexes demonstrated a ~ 35% increase in HW in response to 1 week of ISO when normalized to BW or TL as well as significantly larger myocyte area. We anticipated sex differences in the adult cohort, based on reports of spontaneously hypertensive hyperlipidemic rats [29] and C57Bl6 mice demonstrating attenuated LV and HW in female mice compared to males in response to ISO [30]. However, both of these studies employed younger adults than the current work—with the rats beginning treatment at 9 weeks of age and the mice at 8–12 weeks. Our findings support a recent report in 15-week-old C57Bl6 mice, which report no significant sex-related differences in ISO-induced cardiac hypertrophy [31]; thus, even within an adult mouse, age may significantly contribute to cardiac remodeling. It is clear that the model is important in consideration of sex differences, and the disparate reports emphasize the need for critical consideration of how both sexes respond to specific cardiac stresses.
Existing data regarding hypertrophic responses of juvenile models are limited. FVB juvenile and adult mice treated with a similar dose of ISO for 1 week demonstrated a similar ~ 20% increase in LV/BW and HW/BW [5]. However, our data indicate that juveniles and adults respond differently when normalized to BW, as adult mice demonstrated more robust changes in LV and HW, but when normalized to TL, these differences disappeared. Therefore, we propose that future investigations that compare juvenile and adult cohorts consider both normalization parameters, with TL likely representing a more stringent comparison of changes in cardiac mass due to rapid growth of juvenile BW. In addition, it is important to note that mice of different strains respond differently to ISO stimulation; thus, genetic background plays a role in outcomes and should be considered [6, 32]. The mechanism of increased cardiac mass in juvenile hearts is still under debate. In the present work, we did not note hypertrophy of juvenile cells in response to ISO; thus, it is possible that increased HW/BW was due to increased cell number—a hypothesis that has been suggested for changes in cardiac size in early postnatal models of cardiomyopathy [33]. In fact, clinical reports suggest that juvenile HF patients do not demonstrate hypertrophy as seen in adult HF [34, 35], indicating that further studies are necessary to determine age-specific responses to cardiac pathology.
Studies of aged animals administered cardiac stress are also limited, due to age-induced attrition, heightened morbidity and mortality in response to stress, and methodological difficulties in obtaining aged animals. Generally, aged animals subjected to cardiac insult demonstrate attenuated hypertrophy [13, 36, 37]. Our data support these results, as aged mice exposed to ISO did not demonstrate increased cardiac mass, hypertrophy, or fibrosis. The underlying basis for this attenuated remodeling remains unknown, though two hypotheses have been put forward. One hypothesis suggests an impaired response of the aged myocardium to stress (pressure/volume overload or sympathetic activation). The second hypothesis posits that aged myocytes have reached their size limit due to aging per se, and thus cannot hypertrophy in response to stress [38]. In the case of ISO-elicited increases in cardiac mass, both of these hypotheses seem plausible, and are a source for future investigations.
Isoproterenol-induced systolic function by sex and age
Stimulation of adrenergic receptors by catecholamines increases myocardial contractility to improve blood flow to match peripheral demand [39] through modulation of cardiac inotropy, lusitropy, and chronotropy. The ultimate outcome of adrenergic stimulation is increased cardiac performance, and the precise way in which the heart enhances performance likely differs by age and sex. Previous studies have reported higher EF and FS in adolescents, perhaps due to growth hormones and physical activity [40, 41], though the impact of sex on juvenile performance is not clear nor is the juvenile response to catecholamines. Sex differences exist with respect to how males versus females maintain/increase systolic function, with human female patients improving CO through changes in HR [42]. Our data support this, with a 57% increased CO in adult females, driven by higher HR alongside unchanged SV. Male mice also exhibited higher HR, but they had a non-significantly lower SV, resulting in unchanged CO. Previous reports of catecholamine stimulation in humans demonstrate that adrenaline infusion in young (average age 30) and older (average age 60) adults caused similar increases in HR, but larger increases in SV and EF in younger compared to older subjects [43]. Young males demonstrated greater increase in HR than young females in response to adrenergic receptor stimulation. Furthermore, with age, males demonstrate exacerbated decline in adrenergic-mediated contractility [44], suggesting accelerated age-related decline in adrenergic sensitivity in males compared to females. In our hands, aged females trended to have improved EF (p = 0.05) and FS (p = 0.09) with ISO compared to control, while aged males showed no evidence of ISO sensitivity with respect to systolic function. These age-related changes by sex are also supported by the significant interaction between sex and age with respect to EF. Therefore, functional changes in systolic indices such as EF, FS, and CO vary with age and sex, and careful physiological dissection of these mechanisms is required to understand adrenergic-mediated changes in cardiac performance.
Isoproterenol-induced transcriptional changes by sex and age
With the increased accessibility of big data, more transcriptomic and similar omics-level datasets are emerging. However, in many cases, these data are still generated from adult male models [45, 46], limiting the ability to draw conclusions to adrenergic-mediated changes in gene expression by sex and age. To begin to identify common and distinct pathways by sex and age in response to ISO, we performed RNA-seq. In light of previous clinical reports and our reported physiological and morphological outcomes by sex and age, we expected our transcriptomic analysis to uncover differentially expressed genes and pathways. Although this global hypothesis was correct, we underestimated the magnitude by which the three ages and two sexes would differ. We did not identify a single gene that was upregulated by ISO in all six cohorts. Within the male sex, only six genes were differentially expressed at the three ages, and zero were commonly shared within females. Comparison within age by sex yielded two shared genes in juvenile mice and 48 shared in adults. Pathway analyses indicate significantly disparate activation by sex and age, with juvenile males activating an immune signature and adult males expressing genes associated with metabolic processes in response to ISO. Even targeted investigation of known pathways stimulated by ISO—fibrotic and adrenergic signaling pathways—did not result in consistent transcriptional outcomes by age and sex. While adult male mice activated these pathways as expected, adult female mice did not. Fibrotic genes were not upregulated in the juvenile heart, consistent with clinical reports demonstrating attenuated fibrotic deposition in children compared to adults with HF [47]. However, fibrotic genes were robustly upregulated in the aging heart in both sexes. Thus, it is clear that sex and age are critical factors in determining the transcriptional response to ISO, even with respect to well-established pathways.
Limitations and conclusions
While inclusion of three age groups is novel and advances current literature which at most compares two, we would benefit from additional age groups—i.e., middle aged, as some studies suggest that cardiac function begins to change at around 12 months—both because of aging per se and between sexes [26]. Sex differences in age-related declines in cardiac function have been previously reported [48], with worse systolic function in male compared to female mice [49], suggesting a life-course comparison in males and females is warranted. The higher heterogeneity in female mice is significant and warrants discussion. It is possible that changes in sex hormones across the life-course as well as within the adult females contribute to higher heterogeneity. We do note that though less robust than in females, male sex hormones also change with age [50]. Changes in sex hormones across the life-course warrant future investigation to understand how biological sex contributes to disease pathogenesis. While we chose to focus on changes in systolic function, due to the reports of adrenergic-mediated changes during contraction, we also acknowledge that diastolic function may differ by age and sex and necessitates future investigation, particularly given the age- and sex-specific outcomes in fibrosis reported here.
In conclusion, juvenile, adult, and aged animals respond differently with respect to cardiac function and LV transcriptomics in response to ISO. To our knowledge, this is the first comprehensive report of the impact of exogenous catecholamines on mice of three distinct ages and both sexes. As hypothesized, we demonstrate that juvenile, adult, and aged animals of both sexes undergo unique cardiac remodeling to ISO. The molecular pathways by which adrenergic stimulation elicits differential cardiac responses in these three age groups also differed by age and sex, with minimal molecular and pathway overlap. This study demonstrates critical differences between sex and age in response to a pathologic stimulus, a significant finding in light of current treatment paradigms that largely ignore age and sex. Future work will be necessary to experimentally test the mechanistic differences identified here to identify therapeutic targets to slow age- and HF-related cardiac adrenergic desensitization.
Supplementary information
(DOCX 1869 kb)
Acknowledgements
The authors thank Jacob Schlatter and Sydney Polson for technical assistance and the Wyoming INBRE Bioinformatics Core for assistance with data analysis.
Funding
This project was supported by NIH/NIA 1K01 AG058810-01A1 (DRB), NIH/NICHD 2K12 HD057022-11 (KCW), University of Wyoming College of Health Sciences Faculty in Aid (DRB), University of Colorado Lorna Grindlay Moore Faculty Launch Award (KCW), and Institutional Development Award 2-P20-GM-103432.
Declarations
Ethics approval
All animal procedures and protocols were approved by the University of Wyoming Institutional Animal Care and Use Committee (IACUC) prior to the initiation of this study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kathleen C. Woulfe and Danielle R. Bruns contributed equally to this work.
References
- 1.Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 2016;13:368–378. doi: 10.1038/nrcardio.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shakib S, Clark RA. Heart failure pharmacotherapy and supports in the elderly-a short review. Curr Cardiol Rev. 2016;12:180–185. doi: 10.2174/1573403x12666160622102802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bristow MR. The adrenergic nervous system in heart failure. N Engl J Med. 1984;311:850–851. doi: 10.1056/NEJM198409273111310. [DOI] [PubMed] [Google Scholar]
- 4.Kudej RK, Iwase M, Uechi M, Vatner DE, Oka N, Ishikawa Y, Shannon RP, Bishop SP, Vatner SF. Effects of chronic beta-adrenergic receptor stimulation in mice. J Mol Cell Cardiol. 1997;29:2735–2746. doi: 10.1006/jmcc.1997.0508. [DOI] [PubMed] [Google Scholar]
- 5.Sucharov CC, Hijmans JG, Sobus RD, et al. β-Adrenergic receptor antagonism in mice: a model for pediatric heart disease. J Appl Physiol (Bethesda, Md 1985) 2013;115:979–987. doi: 10.1152/japplphysiol.00627.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faulx MD, Ernsberger P, Vatner D, Hoffman RD, Lewis W, Strachan R, Hoit BD. Strain-dependent beta-adrenergic receptor function influences myocardial responses to isoproterenol stimulation in mice. Am J Physiol Heart Circ Physiol. 2005;289:30–H36. doi: 10.1152/ajpheart.00636.2004. [DOI] [PubMed] [Google Scholar]
- 7.Davies CH, Ferrara N, Harding SE. β-Adrenoceptor function changes with age of subject in myocytes from non-failing human ventricle. Cardiovasc Res. 1996;31:152–156. doi: 10.1016/0008-6363(95)00187-5. [DOI] [PubMed] [Google Scholar]
- 8.Stratton JR, Cerqueira MD, Schwartz RS, Levy WC, Veith RC, Kahn SE, Abrass IB. Differences in cardiovascular responses to isoproterenol in relation to age and exercise training in healthy men. Circulation. 1992;86:504–512. doi: 10.1161/01.cir.86.2.504. [DOI] [PubMed] [Google Scholar]
- 9.Kappert K, Böhm M, Schmieder R, Schumacher H, Teo K, Yusuf S, Sleight P, Unger T, ONTARGET/TRANSCEND Investigators Impact of sex on cardiovascular outcome in patients at high cardiovascular risk: analysis of the telmisartan randomized assessment study in ACE-intolerant subjects with cardiovascular disease (TRANSCEND) and the ongoing telmisartan alone and in combination with ramipril global end point trial (ONTARGET) Circulation. 2012;126:934–941. doi: 10.1161/CIRCULATIONAHA.111.086660. [DOI] [PubMed] [Google Scholar]
- 10.Vizgirda VM, Wahler GM, Sondgeroth KL, Ziolo MT, Schwertz DW. Mechanisms of sex differences in rat cardiac myocyte response to beta-adrenergic stimulation. Am J Physiol Heart Circ Physiol. 2002;282:256–H263. doi: 10.1152/ajpheart.2002.282.1.H256. [DOI] [PubMed] [Google Scholar]
- 11.Toba H, Cannon PL, Yabluchanskiy A, Iyer RP, D’Armiento J, Lindsey ML. Transgenic overexpression of macrophage matrix metalloproteinase-9 exacerbates age-related cardiac hypertrophy, vessel rarefaction, inflammation, and fibrosis. Am J Physiol Heart Circ Physiol. 2017;312:H375–H383. doi: 10.1152/ajpheart.00633.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faulx MD, Ernsberger P, Vatner D, Hoffman RD, Lewis W, Strachan R, Hoit BD. Strain-dependent β-adrenergic receptor function influences myocardial responses to isoproterenol stimulation in mice. Am J Physiol Circ Physiol. 2005;289:H30–H36. doi: 10.1152/ajpheart.00636.2004. [DOI] [PubMed] [Google Scholar]
- 13.Isoyama S, Wei JY, Izumo S, Fort P, Schoen FJ, Grossman W. Effect of age on the development of cardiac hypertrophy produced by aortic constriction in the rat. Circ Res. 1987;61:337–345. doi: 10.1161/01.res.61.3.337. [DOI] [PubMed] [Google Scholar]
- 14.Mota R, Parry TL, Yates CC, Qiang Z, Eaton SC, Mwiza JM, Tulasi D, Schisler JC, Patterson C, Zaglia T, Sandri M, Willis MS. Increasing cardiomyocyte atrogin-1 reduces aging-associated fibrosis and regulates remodeling in vivo. Am J Pathol. 2018;188:1676–1692. doi: 10.1016/j.ajpath.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brattelid T, Tveit K, Birkeland JAK, Sjaastad I, Qvigstad E, Krobert KA, Hussain RI, Skomedal T, Osnes JB, Levy FO. Expression of mRNA encoding G protein-coupled receptors involved in congestive heart failure: a quantitative RT-PCR study and the question of normalisation. Basic Res Cardiol. 2007;102:198–208. doi: 10.1007/s00395-007-0648-1. [DOI] [PubMed] [Google Scholar]
- 16.Ellefsen S, Bliksøen M, Rutkovskiy A, Johansen IB, Kaljusto ML, Nilsson GE, Vaage JI, Stensløkken KO. Per-unit-living tissue normalization of real-time RT-PCR data in ischemic rat hearts. Physiol Genomics. 2012;44:651–656. doi: 10.1152/physiolgenomics.00004.2012. [DOI] [PubMed] [Google Scholar]
- 17.Andrews S (2010) FastQC: a quality control tool for high throughput sequence data.
- 18.Bushnell B (2014) BBMap: a fast, accurate, splice-aware aligner
- 19.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019;37:907–915. doi: 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33:290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40:4288–4297. doi: 10.1093/nar/gks042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. Omi A J Integr Biol. 2012. 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed]
- 25.Advanced Research Computing Center (2018) Teton Computing Environment, University of Wyoming, 10.15786/M2FY47
- 26.Strait JB, Lakatta EG. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin. 2012;8:143–164. doi: 10.1016/j.hfc.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baxter AJ, Spensley A, Hildreth A, Karimova G, O'Connell JE, Gray CS. β Blockers in older persons with heart failure: tolerability and impact on quality of life. Heart. 2002;88:611–614. doi: 10.1136/heart.88.6.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Everitt MD, Sleeper LA, Lu M, Canter CE, Pahl E, Wilkinson JD, Addonizio LJ, Towbin JA, Rossano J, Singh RK, Lamour J, Webber SA, Colan SD, Margossian R, Kantor PF, Jefferies JL, Lipshultz SE, Pediatric Cardiomyopathy Registry Investigators Recovery of echocardiographic function in children with idiopathic dilated cardiomyopathy: results from the pediatric cardiomyopathy registry. J Am Coll Cardiol. 2014;63:1405–1413. doi: 10.1016/j.jacc.2013.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michel FS, Magubane M, Mokotedi L, Norton GR, Woodiwiss AJ. Sex-specific effects of adrenergic-induced left ventricular remodeling in spontaneously hypertensive rats. J Card Fail. 2017;23:161–168. doi: 10.1016/j.cardfail.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 30.Zhu B, Liu K, Yang C, Qiao Y, Li Z. Gender-related differences in β-adrenergic receptor-mediated cardiac remodeling. Can J Physiol Pharmacol. 2016;94:1349–1355. doi: 10.1139/cjpp-2016-0103. [DOI] [PubMed] [Google Scholar]
- 31.Grant MKO, Abdelgawad IY, Lewis CA, Seelig D, Zordoky BN. Lack of sexual dimorphism in a mouse model of isoproterenol-induced cardiac dysfunction. PLoS One. 2020;15:e0232507. doi: 10.1371/journal.pone.0232507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karbassi E, Monte E, Chapski DJ, Lopez R, Rosa Garrido M, Kim J, Wisniewski N, Rau CD, Wang JJ, Weiss JN, Wang Y, Lusis AJ, Vondriska TM. Relationship of disease-associated gene expression to cardiac phenotype is buffered by genetic diversity and chromatin regulation. Physiol Genomics. 2016;48:601–615. doi: 10.1152/physiolgenomics.00035.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farrell ET, Grimes AC, de Lange WJ, Armstrong AE, Ralphe JC. Increased postnatal cardiac hyperplasia precedes cardiomyocyte hypertrophy in a model of hypertrophic cardiomyopathy. Front Physiol. 2017;8:414. doi: 10.3389/fphys.2017.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel MD, Mohan J, Schneider C, Bajpai G, Purevjav E, Canter CE, Towbin J, Bredemeyer A, Lavine KJ. Pediatric and adult dilated cardiomyopathy represent distinct pathological entities. JCI insight. 2017;2:2. doi: 10.1172/jci.insight.94382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatman PD, Woulfe KC, Karimpour-Fard A, Jeffrey DA, Jaggers J, Cleveland JC, Nunley K, Taylor MRG, Miyamoto SD, Stauffer BL, Sucharov CC. Pediatric dilated cardiomyopathy hearts display a unique gene expression profile. JCI insight. 2017;2:2. doi: 10.1172/jci.insight.94249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capasso JM, Malhotra A, Scheuer J, Sonnenblick EH. Myocardial biochemical, contractile, and electrical performance after imposition of hypertension in young and old rats. Circ Res. 1986;58:445–460. doi: 10.1161/01.res.58.4.445. [DOI] [PubMed] [Google Scholar]
- 37.Raya TE, Gaballa M, Anderson P, Goldman S. Left ventricular function and remodeling after myocardial infarction in aging rats. Am J Physiol. 1997;273:2652–H2658. doi: 10.1152/ajpheart.1997.273.6.H2652. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi T, Schunkert H, Isoyama S, Wei JY, Nadal-Ginard B, Grossman W, Izumo S. Age-related differences in the expression of proto-oncogene and contractile protein genes in response to pressure overload in the rat myocardium. J Clin Invest. 1992;89:939–946. doi: 10.1172/JCI115675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrara N, Komici K, Corbi G, et al. β-adrenergic receptor responsiveness in aging heart and clinical implications. Front Physiol. 2014;4:396. doi: 10.3389/fphys.2013.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cain PA, Ahl R, Hedstrom E, Ugander M, Allansdotter-Johnsson A, Friberg P, Arheden H. Age and gender specific normal values of left ventricular mass, volume and function for gradient echo magnetic resonance imaging: a cross sectional study. BMC Med Imaging. 2009;9:2. doi: 10.1186/1471-2342-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klein FJ, Bell S, Runte KE, Lobel R, Ashikaga T, Lerman LO, LeWinter MM, Meyer M. Heart rate-induced modifications of concentric left ventricular hypertrophy: exploration of a novel therapeutic concept. Am J Physiol Heart Circ Physiol. 2016;311:H1031–H1039. doi: 10.1152/ajpheart.00301.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wheatley C, Snyder E, Johnson B, Olson T. Sex differences in cardiovascular function during submaximal exercise in humans. Springerplus. 2014;3:1–13. doi: 10.1186/2193-1801-3-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White M, Leenen FH. Effects of age on cardiovascular responses to adrenaline in man. Br J Clin Pharmacol. 1997;43:407–414. doi: 10.1046/j.1365-2125.1997.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner MJ, Mier CM, Spina RJ, Schechtman KB, Ehsani AA. Effects of age and gender on the cardiovascular responses to isoproterenol. J Gerontol A Biol Sci Med Sci. 1999;54:B393–B403. doi: 10.1093/gerona/54.9.b393. [DOI] [PubMed] [Google Scholar]
- 45.Prunotto A, Stevenson BJ, Berthonneche C, Schüpfer F, Beckmann JS, Maurer F, Bergmann S. RNAseq analysis of heart tissue from mice treated with atenolol and isoproterenol reveals a reciprocal transcriptional response. BMC Genomics. 2016;17:717. doi: 10.1186/s12864-016-3059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galindo CL, Skinner MA, Errami M, Olson LD, Watson DA, Li J, McCormick JF, McIver LJ, Kumar NM, Pham TQ, Garner HR. Transcriptional profile of isoproterenol-induced cardiomyopathy and comparison to exercise-induced cardiac hypertrophy and human cardiac failure. BMC Physiol. 2009;9:23. doi: 10.1186/1472-6793-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woulfe KC, Siomos AK, Nguyen H, SooHoo M, Galambos C, Stauffer BL, Sucharov C, Miyamoto S. Fibrosis and fibrotic gene expression in pediatric and adult patients with idiopathic dilated cardiomyopathy. J Card Fail. 2017;23:314–324. doi: 10.1016/j.cardfail.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grilo GA, Shaver PR, Stoffel HJ, Morrow CA, Johnson OT, Iyer RP, de Castro Brás LE. Age- and sex-dependent differences in extracellular matrix metabolism associate with cardiac functional and structural changes. J Mol Cell Cardiol. 2020;139:62–74. doi: 10.1016/j.yjmcc.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kane AE, Bisset ES, Heinze-Milne S, Keller KM, Grandy SA, Howlett SE. Maladaptive changes associated with cardiac aging are sex-specific and graded by frailty and inflammation in C57BL/6 mice. Journals Gerontol Ser A. 2020;76:233–243. doi: 10.1093/gerona/glaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 1869 kb)