Abstract
In most macaques, females are philopatric and males migrate from their natal ranges, which results in pronounced divergence of mitochondrial genomes within and among species. We therefore predicted that some nuclear genes would have to acquire compensatory mutations to preserve compatibility with diverged interaction partners from the mitochondria. We additionally expected that these sex-differences would have distinctive effects on gene flow in the X and autosomes. Using new genomic data from 29 individuals from eight species of Southeast Asian macaque, we identified evidence of natural selection associated with mitonuclear interactions, including extreme outliers of interspecies differentiation and metrics of positive selection, low intraspecies polymorphism and atypically long runs of homozygosity associated with nuclear-encoded genes that interact with mitochondria-encoded genes. In one individual with introgressed mitochondria, we detected a small but significant enrichment of autosomal introgression blocks from the source species of her mitochondria that contained genes which interact with mitochondria-encoded loci. Our analyses also demonstrate that sex-specific demography sculpts genetic exchange across multiple species boundaries. These findings show that behaviour can have profound but indirect effects on genome evolution by influencing how interacting components of different genomic compartments (mitochondria, the autosomes and the sex chromosomes) move through time and space.
Keywords: cytonuclear incompatibility, interaction networks, hybridization, gene flow, natural selection, papionins
1. Introduction
In primates and many other groups, genetic material is divided between mitochondria, sex chromosomes and autosomes. These compartments differ in several ways such as modes of inheritance, rates of mutation, rates of recombination and allelic copy number. Many biological functions require interactions between genes encoded by different compartments. For example, some nuclear-encoded proteins interact with mitochondria-encoded proteins, RNA enzymes and mitochondrial DNA to collaboratively carry out crucial functions of oxidative phosphorylation (OXPHOS genes), loading of amino acids on mitochondria-encoded transfer RNAs (ARS2 genes), formation of the mitochondrial ribosome (MRP genes) and mitochondrial replication (REP genes) [1]. Each generation, the interaction network spanning these compartments is pulled apart by meiosis, shuffled by recombination and sex-differences in demography (e.g. [2]) and reconstituted by fertilization. Genes in each compartment thus need to continuously co-evolve to remain compatible, and the genetic architecture of this network, including the idiosyncratic dynamics of dominant and recessive mutations in each compartment, affect adaptation and speciation [3–5].
Interactions between the mitochondrial and nuclear genomes (mitonuclear interactions) are subject to natural selection, and variation in fitness—including lethality and sex-specific effects (e.g. [6–8]). Fitness costs of mitonuclear incompatibilities stem from reduced copy number of mitochondrial DNA, impaired interactions between proteins involved with OXPHOS and decreased production of ATP, and an increase in reactive oxygen species and elevated oxidative stress [1]. However, there are also striking examples of introgression of mitochondrial genomes without extensive nuclear introgression, including examples in primates among genera (papionins; [9]), species (macaques; [10]) and diverged populations (hominins; [11]). These examples argue that diverged mitochondrial genomes may be interchangeable in some circumstances.
(a) . Genomic consequences of the macaque social system
Papionin monkeys are compelling subjects for the study of interaction networks that span different genomic compartments because their sex-specific behaviours establish strong predictions. In these monkeys, females tend to be philopatric whereas males tend to leave their natal groups (e.g. [12]). Evolutionary repercussions of extreme female philopatry are evidenced by ancient mitochondrial coalescence times that predate speciation of rhesus macaques [13], pigtail macaques (Macaca nemestrina) [14], olive baboons (Papio anubis) [15] and possibly Sulawesi macaques [14]. By contrast, a deep mitochondrial coalescence time is not observed in hamadryas baboons, Papio hamadryas, but this species has an atypical social system for papionins with more frequent female migration [16].
High intraspecific diversity and interspecific divergence of mitochondrial DNA leads to the prediction that genetic incompatibilities may arise between nuclear-encoded genes that interact with highly diverged mitochondria-encoded genes with whom they have not co-evolved (reviewed in [17]). This could lead to positive selection for compensatory mutations in nuclear-encoded genes that interact with mitochondria-encoded genes. The sharp geographical structure of mitochondrial DNA also has the potential to foster co-adaptation of genes involved with mitonuclear interactions to local environmental conditions (reviewed in [17]). Together these factors could disfavour the exchange of genes involved with mitonuclear interactions, both within and among species [5].
We set out to test these expectations using genomic data from 29 individuals from eight species of macaque, including pigtail macaques (M. nemestrina; six individuals) from Sumatra and Borneo and seven species of Sulawesi macaque (23 individuals). Macaca nemestrina is a member of the silenus group of macaques, which is sister to the Sulawesi macaques [13,18]. These taxa are separated by the Makassar Strait which runs between Borneo and Sulawesi, and corresponds to a sharp biogeographical boundary that is demarcated by Wallace's Line [19]. Sulawesi macaque species are allopatrically distributed, and parapatric species hybridize in nature (e.g. [20]). Mitochondrial genomes of these species diversified more than 2 Ma and are substantially diverged ([14], electronic supplementary material). We first focused on 211 nuclear-encoded genes that interact with mitochondria-encoded genes or mitochondrial DNA with an aim of testing whether population differentiation, polymorphism, metrics of natural selection, and runs of homozygosity in genomic regions that contained these genes differed from regions with other genes, or regions with no genes. Then, with an aim of examining how gene flow is specifically influenced by these 211 genes or more generally by sex-specific demography, we tested whether patterns of gene flow differed in regions containing these genes compared to other genomic regions, and we also evaluated population differentiation and gene flow on the X chromosome and the autosomes.
2. Results
(a) . A subset of Ninteract genes has atypically high genetic differentiation between species
Samples in this study have highly diverged mitochondrial genomes (electronic supplementary material, tables S1 and S2, figure 1; electronic supplementary material, [14]). We therefore expected high interspecies differentiation and genomic signatures of positive selection in nuclear-encoded proteins that interact with mitochondria-encoded proteins, rRNA enzymes or mitochondrial DNA (i.e. OXPHOS, ARS2, MRP and REP genes—hereafter Ninteract genes) [21]. To test this, we began by quantifying FST in 100 kilobase pair (kb) genomic windows in 10 pairwise comparisons between the five species with genomic data from at least three individuals each, as defined in Methods, with the expectation that this statistic (which quantifies population differentiation on a scale from 0 to 1) should be higher in windows that contain Ninteract genes (hereafter Ninteract windows). We considered the autosomes and the X chromosome separately because FST of the X is significantly higher than the autosomes (see below).
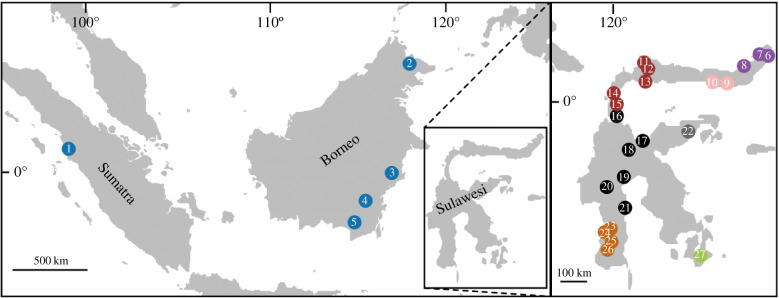
Figure 1.
Twenty-seven approximate geographical origins of the 29 individuals sampled for this study. Numbers indicate approximate geographical origins of samples as follows: (1) Ngasang, (2) Sukai and Gumgum, (3) PM665, (4) PM664, (5) PM1206, (6) PF660, (7) PF1001, (8) PM1003, (9) PM1011, (10) PM654, (11) PF647, (12) PF648, (13) PF505, (14) PF643, (15) PF644, (16) PF511, (17) PF563, (18) PF559, (19) PM592, (20) PF597, (21) PF626, (22) PF549, (23) PM615 and PM616, (24) PM614, (25) PM713, (26) PM613 and (27) PF707. Samples are coloured by species as detailed in the electronic supplementary material, table S1. (Online version in colour.)
Consistent with our expectations for mitonuclear interactions, in all 10 pairwise comparisons, the average FST of autosomal Ninteract windows (which contain the start site of at least one Ninteract gene; see the electronic supplementary material) was significantly higher than autosomal windows that encode only other genes (p < 0.05 for each of 10 permutation tests). These findings are also apparent in density plots which illustrate that FST of Ninteract windows consistently have more density over higher FST values as compared to genomic windows that contain only other genes, or compared to windows containing no genes (electronic supplementary material, figure S1), and also in boxplots of these data (electronic supplementary material, figure S2).
Ninteract windows contained a higher number of genes on average (3.0 genes/window) compared to other windows that contained only non-Ninteract genes (1.6 genes/window; hereafter non-Ninteract windows). One possible explanation for the higher FST therefore is that Ninteract windows typically have more genes than genomic windows which contain only other genes; this aspect of genome complexity was not considered in the permutation tests. To explore this possibility, for each of the 10 pairwise comparisons a linear regression was performed with an interaction term between two predictor variables, the number of genes in each 100 kb window (density) and whether (1) or not (0) the window was an Ninteract window (Ninteract), with FST as the response variable: FST ∼ Ninteract * density. In all pairwise comparisons, density was significantly positively correlated with FST (p < 0.0001 for each test) but Ninteract and the interaction terms were not significant (p > 0.05 for each test). This suggests that the higher FST of all Ninteract windows is largely attributable to gene density rather than to the presence of Ninteract genes (e.g. pink and grey dots in figure 2 overlap extensively), an observation that is not consistent with our expectations.
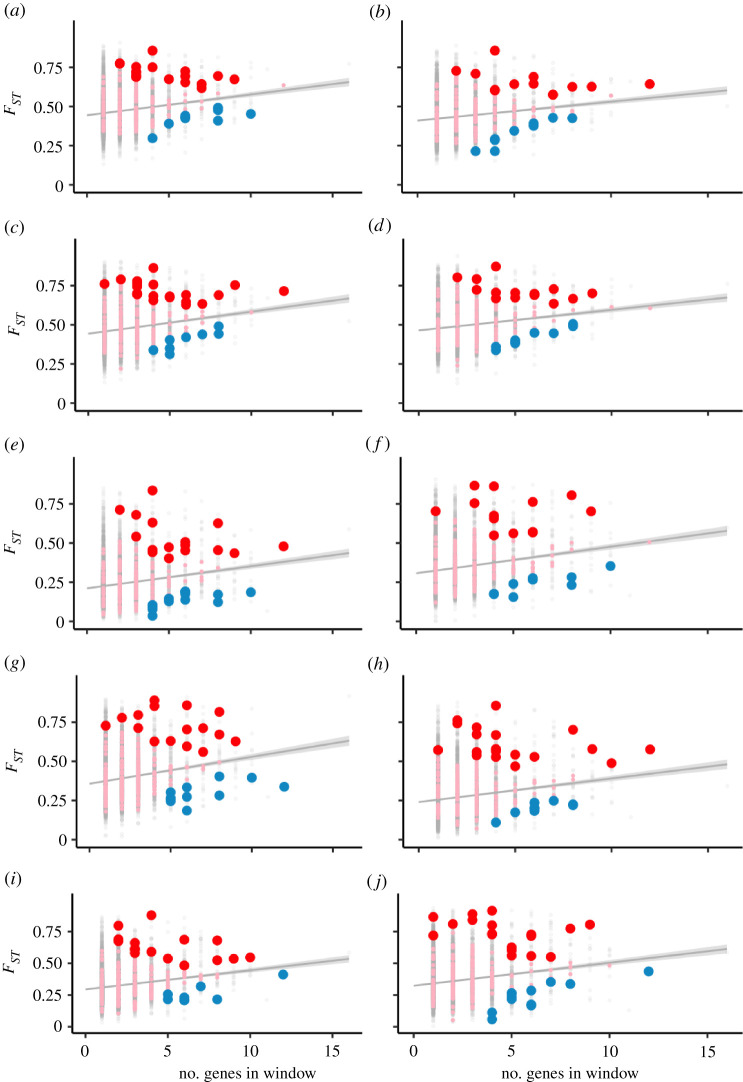
Figure 2.
Ninteract windows have an excess of upper and lower FST outliers after controlling for gene density. Each panel is a pairwise comparison between two of five species: M. nemestrina from Borneo (bor), M. hecki (hec), M. tonkeana (ton), M. maura (mau) and M. nigra (nig) as follows: (a) bor-mau, (b) bor-ton, (c) bor-hec, (d) bor-nig, (e) mau-ton, (f) mau-hec, (g) mau-nig, (h) ton-hec, (i) ton-nig, (j) hec-nig. Genomic windows without and with Ninteract genes are indicated by grey and pink dots, respectively; red and blue dots indicate Ninteract windows that are upper or lower FST outliers, respectively. Because FST was not calculated for some windows owing to genotype quality filtering, the sample size of Ninteract windows (pink, red and blue dots) is 204 and of non-Ninteract windows (grey dots) is 9121. A grey line and grey shading indicate the linear regression and confidence interval for the relationship between FST (y-axis) and the number of genes in each genomic window (x-axis). Acronyms of Ninteract genes in upper FST outliers are provided in the electronic supplementary material, table S3. (Online version in colour.)
Although the set of 209 autosomal Ninteract windows is not significantly more differentiated than other genomic windows with genes after controlling for gene density, it is still possible that natural selection against mitonuclear incompatibilities could cause a subset of Ninteract windows to be extreme outliers for differentiation. To test this possibility, we performed an outlier analysis (electronic supplementary material) to identify windows where FST is much higher than expected after considering the positive relationship with density (upper FST outliers). For each of the 10 pairwise comparisons, approximately 3% of the non-Ninteract windows were upper FST outliers. However, in these same 10 comparisons, the proportion of Ninteract windows that were upper FST outliers was two to three times higher (range: 6–10%, figure 2; electronic supplementary material, table S3), a significant difference for all comparisons (p < 0.01, binomial tests). For each of the 10 pairwise comparisons, the proportions of lower FST outliers in non-Ninteract windows were all approximately 1%. Unexpectedly, we found that the proportions of lower FST outliers were also higher in Ninteract windows (range: 4–7%), which was also significant for all comparisons (p < 0.01, binomial tests). Overall, these observations are consistent with a subset of Ninteract genes being atypically highly differentiated after controlling for gene density, even though FST of all Ninteract windows was not significantly higher than other gene containing windows after controlling for gene density. But they also indicate that a subset of Ninteract windows is atypically undifferentiated, an unexpected result that could be a consequence of purifying selection in regions with high gene density. Recognizing that these pairwise FST comparisons are not independent, it is nonetheless interesting that several Ninteract windows were consistently upper FST outliers across multiple comparisons (electronic supplementary material, table S3 and Results).
Only two of the 211 Ninteract genes are on the X chromosome (NDUFB11, COX7B) but FST of their Ninteract windows was not significantly different from other X-linked genomic windows that carry only other genes (p > 0.05, permutation tests).
(b) . A subset of Ninteract genes is subject to atypically strong positive selection
As a complement to the analyses of FST, we examined other population-specific statistics that are sensitive to natural selection: pairwise nucleotide diversity (π), Tajima's D [22], and Fay and Wu's H [23]. These statistics are expected to have lower (and sometimes negative for D and H) values in positively selected genomic regions compared to other genomic regions; more details about these statistics, including possible influences of population size change, are provided in the electronic supplementary material. We found that after accounting for relationships with gene density, there was a significant excess of autosomal Ninteract windows that were lower outliers for all three of these statistics for all five species considered (p < 0.01, binomial tests; electronic supplementary material, Results and tables S4, S5). In addition, polymorphism was atypically low in multiple species in the flanking regions of several Ninteract windows—most prominently the ones containing MRPS21 and NDUFAF3 (electronic supplementary material, figure S3), and several of these lower outliers were also upper FST outliers (electronic supplementary material, table S3).
To explore the effects of the size of the genomic window on these results, we repeated the analysis of FST, π, Tajima's D, and Fay and Wu's H using a window size of 30 kb. The findings were essentially identical to those from the 100 kb windows (electronic supplementary material, Results).
If natural selection repeatedly favours compensatory mutations in Ninteract genes that maintain compatibility with the rapidly evolving mitochondrial genome, we expected that these genes would be embedded in long runs of homozygosity (ROHs) that are generated by selective sweeps. Consistent with this expectation, the average lengths of ROHs containing Ninteract genes (and possibly other genes) was 1.9–4.6 times longer than the average length of ROHs that contained only other genes, and 8.2–17.0 times longer than ROHs that did not contain any genes (electronic supplementary material, table S6). These differences are individually significant for all species (p ≤ 0.05, permutation tests; electronic supplementary material, table S6) except M. brunnescens (p = 0.35) and M. maura (p = 0.07). The atypically long ROHs that contain Ninteract genes are clearly evident in density plots (electronic supplementary material, figure S4), and the longest ROH in most species contained at least one Ninteract gene (electronic supplementary material).
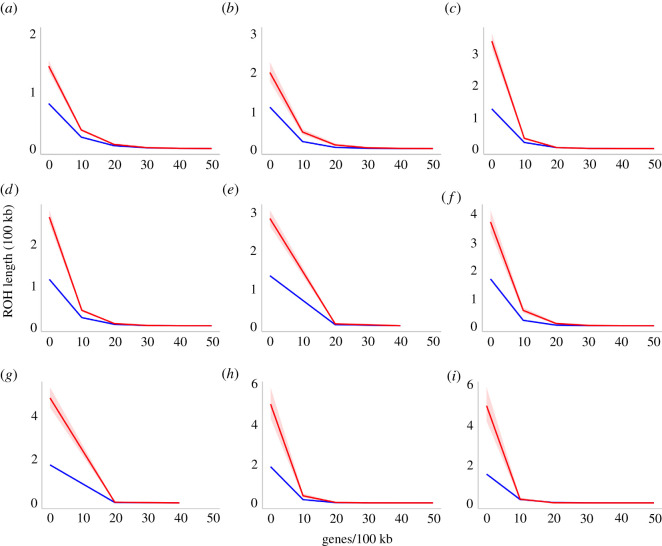
We used linear models to account for the relationship between gene density and ROH length, and the results indicate that the relationship between Ninteract genes and ROH length is nuanced for most species, with exceptions discussed in the electronic supplementary material. In general, at low to moderate gene densities, ROHs with Ninteract genes tend to be much longer than those without. However, this relationship with ROH length varied among species as gene density increases (figure 3). For ROHs with high gene densities (e.g. greater than 20 genes/100 kb), those with Ninteract genes tend to be similar or even shorter than those with only other genes. However, less than 1.2% of the ROHs had a genes density greater than 20 genes/100 kb and greater than 80% of the ROHs had a gene density below 5 genes/100 kb. Overall, this indicates a strong and pervasive positive correlation between Ninteract genes and ROH length for the vast majority of observed ROHs after controlling for gene density.
Figure 3.
Predicted values (marginal means) of the lengths of runs of homozygosity (ROH) in hundreds of kilobases (100 kb) that include Ninteract genes (red) are far longer than those that include only other genes (blue) across most biologically relevant gene densities (genes/100 kb). Model results are presented for one or more genome from (a) M. nemestrina from Borneo, (b) M. nemestrina from Sumatra, (c) M. maura, (d) M. tonkeana, (e) M. hecki, (f) M. nigrescens, (g) M. nigra, (h) M. brunnescens and (i) M. togeanus, respectively. Shading indicates the 95% confidence interval of the predicted values. (Online version in colour.)
In addition to influencing patterns of polymorphism and differentiation, natural selection on Ninteract genes has the potential to influence protein evolution. To test this possibility, we analysed the rate ratio of nonsynonymous to synonymous substitutions per site (dN/dS) in Ninteract genes and non-Ninteract genes. The mean dN/dS of Ninteract genes is significantly higher than the mean dN/dS of non-Ninteract genes (p < 0.001, permutation test, electronic supplementary material), which is consistent with Ninteract genes being under atypically relaxed purifying selection and/or atypically intense or frequent positive selection as compared to other genes.
(c) . Ninteract genes in two admixed individuals match expectations for selection on mitonuclear interactions
Using a hidden Markov model [24], we identified two M. tonkeana individuals—both collected near hybrid zones—that exhibited evidence of extensive introgression (figure 4). These individuals allowed us to test the prediction that selection against mitonuclear incompatibility could influence introgression. For individual PF626, which carries M. tonkeana mitochondrial DNA [14], we had a one-sided expectation that there would be fewer introgression blocks that contained Ninteract genes from M. maura than expected by chance. For individual PF511, which carries introgressed mitochondria from M. hecki [14], we expected the opposite (i.e. an excess introgression blocks that carried Ninteract genes from M. hecki).
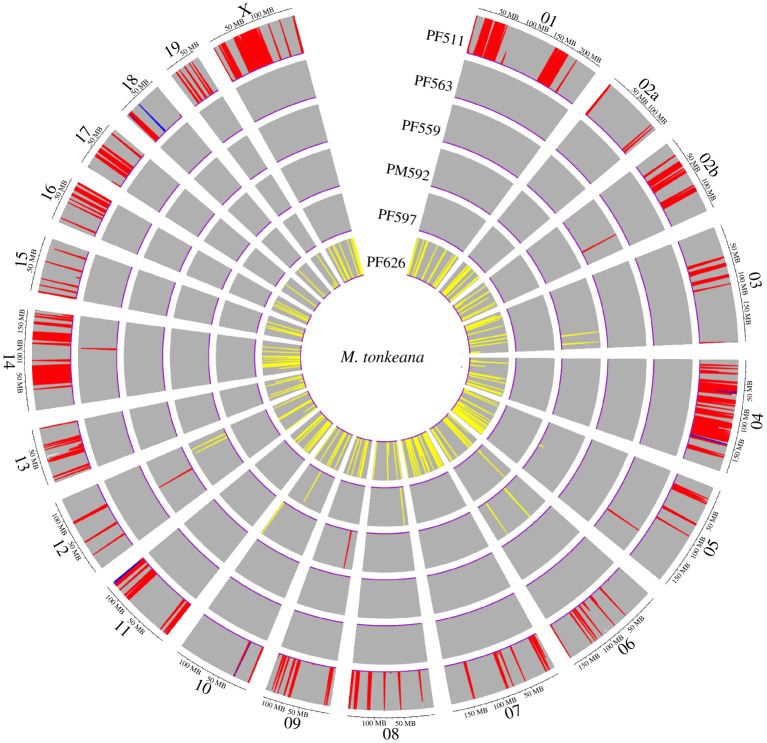
Figure 4.
Analysis of introgression blocks of M. tonkeana with admixfrog identifies two individuals with extensive admixture with an adjacent species. Genomic regions are coloured based on inferred genotypes: grey is homozygous M. tonkeana, red is heterozygous M. tonkeana and M. hecki, yellow is heterozygous M. tonkeana and M. maura, and blue is homozygous M. hecki. Heterozygous regions for M. maura and M. hecki were not inferred and two regions that were homozygous for M. maura (on chr09 and chr14 in individual PF626) are not visible. As illustrated in figure 1, the introgressed sample PF511 originated from near the contact zone with M. hecki. This individual additionally carries mitochondrial DNA from M. hecki [14]. The introgressed individual PF628 originated from near the contact zone with M. maura. (Online version in colour.)
In individual PF626, only two homozygous introgression blocks were identified that carried a total of eight genes out of 16 049 annotations; neither carried any Ninteract genes. While this was consistent with our expectation, this small sample did not constitute a significant dearth of homozygous introgression blocks carrying Ninteract genes (p = 0.902; permutation test). In individual PF511, there were 15 introgression blocks in total, and 12 of these contained a total of 54 genes out of the 16 049 annotated genes (0.3%). We therefore expected less than one of the 211 autosomal and X-linked Ninteract genes would be on homozygous introgression blocks if there were no enrichment of Ninteract genes. Instead, three Ninteract genes were present on homozygous introgression blocks: NDUFB5, MRPL1 and MRPL47. Enrichment of Ninteract genes on these introgression blocks in this individual was significant (p = 0.036; permutation test). Phylogenetic analyses provided further confirmation that the three Ninteract genes in individual PF511 were indeed introgressed from M. hecki (electronic supplementary material).
(d) . Introgression genomics point to sex-differences in demography in Macaca nemestrina
Population structure is higher on the X chromosome than the autosomes in these macaques (electronic supplementary material, figure S5). In each of these genomic compartments, we used two introgression statistics—Patterson's D [25] and fdM [26]—to assess gene flow between pairwise combinations of one species (P3) and either of two other species (P1, P2) whose phylogenetic relationships are (((P1, P2), P3), O), where O is an outgroup taxon. A positive statistic arises from an excess of derived variants shared between P2 and P3 as compared to those shared between P1 and P3; negative values indicate the opposite. Non-zero values are potentially consistent with an inference of gene flow (e.g. for positive values, between P2 and P3), but can also arise from other scenarios that do not involve gene flow, such as ancestral population structure [27,28]. A rationale for the phylogenetic framework of these tests is provided in the electronic supplementary material, Results and figures S6–S8. Significant differentiation among these species is supported by admixture analysis [29], which favours a model with nine (autosomes) or eight (the X) populations (electronic supplementary material, figure S9).
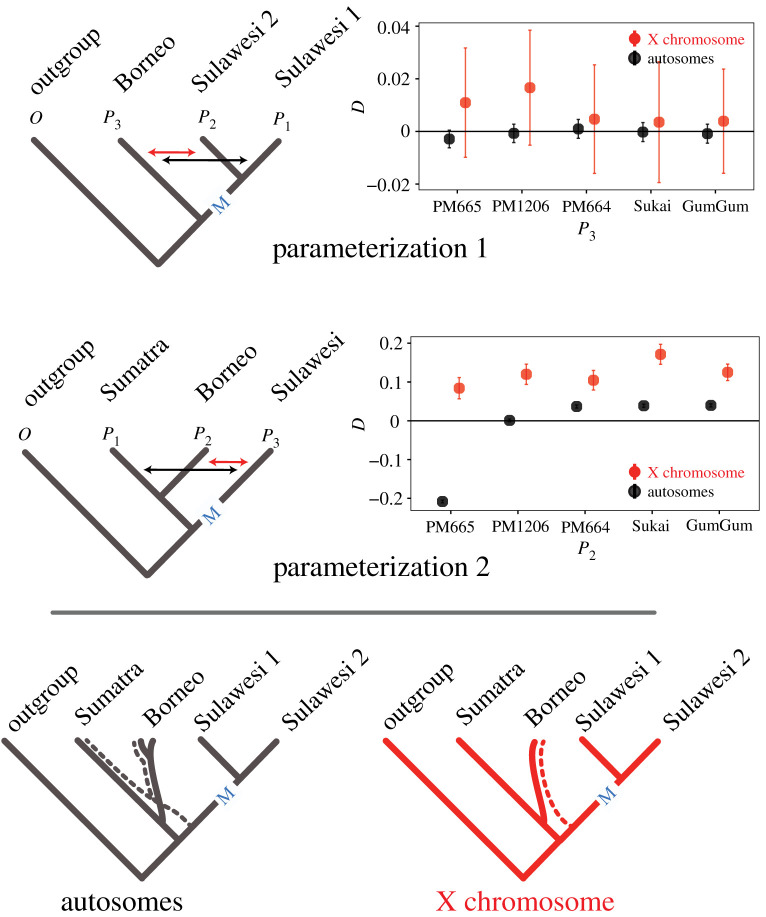
Our analyses recover no evidence for introgression between M. nemestrina and the Sulawesi macaques after Sulawesi was colonized on the X or the autosomes. When P1 and P2 are defined to be any pair of Sulawesi macaques or groups of Sulawesi macaques that are reciprocally monophyletic, and P3 is defined to be M. nemestrina from Borneo (parameterization 1), the estimated introgression statistics for the X and autosomes are not significantly different from zero (figure 5; electronic supplementary material, figure S10).
Figure 5.
Alternative parameterizations (top and centre, main text) centred around the Makassar Strait (M) suggest no gene flow between Sulawesi and Borneo after colonization of Sulawesi and identify distinctive patterns of population structure in the X and autosomes of M. nemestrina (bottom). An excess of shared derived sites in P2 and P3 (red double-headed arrows, top and centre) makes Patterson's D positive, whereas an excess of these sites between P1 and P3 (black double-headed arrows) makes Patterson's D negative. Dotted branches indicate an ancestry component shared between Sulawesi, the Sumatra sample and some Borneo samples (all but PM665) in the autosomes, and between Sulawesi and all Borneo samples in the X. Here, for parameterization 1, Sulawesi 1 includes the three species from the northern peninsula and Sulawesi 2 includes the other species from the rest of Sulawesi. Results are similar with other combinations of individual Sulawesi species (electronic supplementary material, figure S10). (Online version in colour.)
We considered an alternative phylogenetic parameterization for introgression statistics that also spans the Makassar Strait. When P1 and P2 are defined to be M. nemestrina from Sumatra and Borneo, respectively, and P3 is defined to be any or all Sulawesi macaque species (parameterization 2), the introgression statistics for the X are consistently significantly positive, and those for the autosomes are either significantly negative, not significant or significantly positive depending on which individual or population is used as P2 (figure 5; electronic supplementary material, figure S11). The differing results from parameterization 1 informed our interpretation of those from parameterization 2 and provided insights into how behaviour influenced population structure on the X and autosomes of M. nemestrina (see Discussion).
(e) . Introgression genomics point to sex-differences in demography on Sulawesi
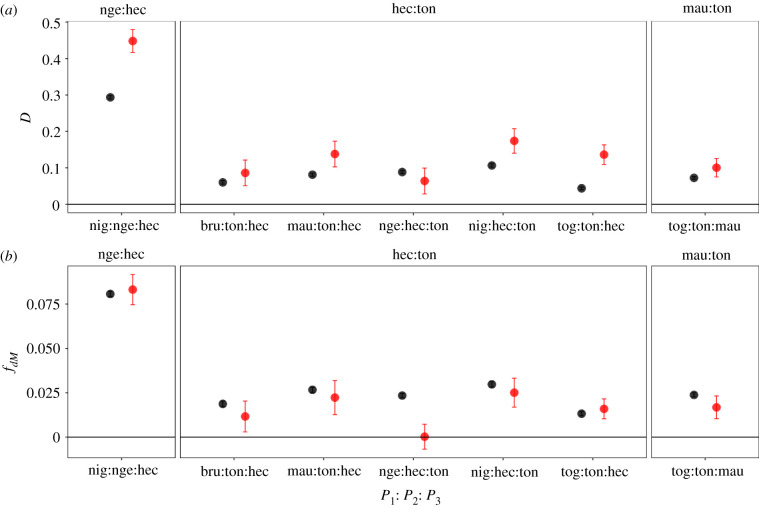
Across three hybrid zones on Sulawesi, introgression statistics are significantly positive for the autosomes and most analyses of the X chromosome (figure 6). These results thus support gene flow in the hybrid zones between M. nigrescens and M. hecki, M. hecki and M. tonkeana, and M. maura and M. tonkeana, with the highest level of gene flow between M. nigrescens and M. hecki. Across these three hybrid zones, Patterson's D was more extreme for the X chromosome than the autosomes in several analyses. fdM mostly did not differ substantially between the X and autosomes, with one exception discussed in the electronic supplementary material. Analysis of individual genomes using a hidden Markov model [24] also recovered strong evidence of introgression between parapatric species of Sulawesi macaque across these three hybrid zones and identified substantial geographical heterogeneity in the extent of gene flow (figure 4; electronic supplementary material, figures S12–S15 and Results).
Figure 6.
Patterson's D (a) calculated from macaque genomes on either side of the Sulawesi hybrid zones for the X chromosome (red) is higher than or similar to those calculated from the autosomes (black). With one exception discussed in the main text, fdM is similar for the X chromosome and autosomes. The top of each panel indicates the hybrid zone that the introgression statistics focus on, and the species defined as P1, P2 and P3 are indicated on the x-axis. Abbreviations follow figure 2. (Online version in colour.)
3. Discussion
(a) . Mitonuclear interactions in macaques
Using genomic data from 29 individuals from eight macaque species, we evaluated the expectation that deep divergences of mitochondrial genomes could lead to positive selection for compensatory mutations in Ninteract genes, and negative selection against mitonuclear incompatibilities in admixed individuals. This expectation was supported by an excess of Ninteract windows that were extreme upper FST outliers, lower π outliers and lower Tajima's D and Fay and Wu's H outliers (electronic supplementary material, tables S3–S5, and file S1), and a higher average rate of protein evolution of Ninteract genes compared to non- Ninteract genes (electronic supplementary material, Results). In addition, ROHs that contain Ninteract genes were typically far longer than those that did not (figure 3; electronic supplementary material, figure S4 and table S6). In one M. tonkeana individual with introgressed mitochondrial DNA (PF511), there was a small but significant enrichment of introgressed Ninteract genes from the source species of her mitochondrial genome (M. hecki).
Compensatory mutations in Ninteract genes could mitigate the effects of Muller's ratchet in the mitochondria (i.e. in non-recombining genomes, the stepwise decrease in fitness over time associated with loss of the least deleterious genome [30]). Another nonexclusive possibility is that some Ninteract genes are adaptively co-diverging with mitochondrial genomes [17]. Some Ninteract genes (e.g. MRPS21, NDUFAF3) appear to have been subject to independent selective sweeps in multiple species, which suggests that there is variation among Ninteract genes in the susceptibility of mitonuclear interactions to incompatibility mutations, in the degree to which interacting partners in the mitochondria and nucleus adaptively co-evolve, or both. This is also highlighted by our analyses that generally do not find significant signatures of positive selection for all Ninteract genes, but consistently detect a substantial proportion of these genes that are outliers for multiple metrics of positive selection and/or population differentiation.
Of the four functional Ninteract categories, three (ARS2, MRP and REP) interact with non-translated RNA genes or DNA (i.e. mitochondrial tRNA, ribosomal RNA and the control region), whereas OXPHOS genes interact with protein from translated mitochondrial genes. Thus, conflict resolution and co-adaptation across Ninteract categories involve pairwise interactions between mitochondrial DNA, RNA and protein. In many cases, it is unclear exactly which portions of the nuclear and mitochondria-encoded genes have direct contact, e.g. [31]. For example, NDUFAF3 does not co-immunoprecipitate with the mitochondria-encoded ND1 protein even though both of these proteins are in the Q module of OXPHOS complex 1 [31], and NDUFAF3 is crucial for the incorporation and stability of ND1 in this complex [32]. Some aspects of compensatory evolution therefore may be driven by indirect interactions between mitochondria-encoded and autosomal-encoded factors. In other species, mitonuclear interactions have been maintained by compensatory mutations in Ninteract genes, the recruitment of novel subunits, and gene duplication followed by subfunctionalization or neofunctionalization [21,33].
(b) . Introgression genomics and sex-specific demography
Our analyses recovered no evidence of introgression between macaques on either side of the Makassar Strait in the autosomes or the X (parameterization 1, figure 5; electronic supplementary material, figure S10). This observation underscores the formidable biogeographical barrier represented by Wallace's Line within a widespread genus whose distribution straddles this line. Analysis with an alternative parameterization (parameterization 2) evidences geographically structured variation in M. nemestrina that was strongly influenced by sex-specific demography (figure 5). More specifically, a negative Patterson's D for autosomes when individual PM665 was set to be taxon P2 suggests that there is an ancestry component that is shared between M. nemestrina from Sumatra and the Sulawesi macaques that is not present or less frequent in individual PM665, even though this individual originated from Borneo, which is geographically far closer to Sulawesi than Sumatra. However, positive introgression statistics are recovered for the autosomes when other Borneo individuals were set to be taxon P2 (figure 5), which indicate that the ancestry component that is shared between the autosomes of Sulawesi and M. nemestrina from Sumatra is also present in some M. nemestrina on Borneo (more prominently in Borneo individuals PM664, GumGum and Sukai than in the Sumatra individual Ngsang, and at a similar level in Borneo individual PM1206 and Ngsang). This ancestry component was not detected with admixture analysis of individual genomes (electronic supplementary material, figure S9) and thus appears to be a small portion of these genomes.
This pattern in the autosomes contrasts sharply with a significantly more positive Patterson's D with parameterization 2 for the X chromosome (and parallel results for fdM), which uniformly point to a prominent shared ancestry component between genomes from Borneo and Sulawesi macaques that is not present or rarer in the genome from Sumatra. Together these differing results in the autosomes and X suggest that genetic structure in the X is more strongly influenced than the autosomes by ancient population connections between Borneo and Sulawesi that reach back to more than 2 Ma to when Sulawesi was initially colonized by an ancestor of M. nemestrina from Borneo [14]. When data are concatenated [18] or weightings of alternative phylogenies are considered (electronic supplementary material, figures S6 and S8), there is strong support for monophyly in the autosomes and the X chromosome of M. nemestrina in Sumatra and Borneo with respect to the Sulawesi macaques. However, mitochondrial genomes of M. nemestrina from Borneo is more closely related to that of the Sulawesi macaques than they are to mitochondrial DNA from M. nemestrina from Sumatra [14], which mirrors the results from the X chromosome when analysed using parameterization 2 (figure 5). These differing relationships in the X and autosomes are consistent with male-mediated migration between Borneo and Sumatra M. nemestrina after macaques colonized Sulawesi coupled with female philopatry. In the past, overland migration was frequently possible between Borneo and Sumatra during times of lower sea level [34].
The large-X effect posits that the X chromosome plays an oversized role in species incompatibilities compared to the autosomes [35]. A prediction of the large-X effect is that there should be less gene flow of X-linked than autosomal-linked variation between species. This aspect of the large-X effect is similar to predictions associated with mitonuclear incompatibility in the sense that both may limit introgression of mostly (or entirely) female-inherited genomic compartments (the X and the mitochondria). Surprisingly because female philopatry might be expected to amplify the expectations of a large X effect by decreasing gene flow of this chromosome, our analyses do not identify the X chromosome as having substantially less introgression than the autosomes on Sulawesi. In fact, Patterson's D statistic (though not fdM) across Sulawesi hybrid zones sometimes suggests higher rather than lower gene flow on the X than the autosomes across three hybrid zones on Sulawesi (figure 6). Perspective into these results on Sulawesi is gained by turning to the results from M. nemestrina, where parameterization 2 consistently recovered more extreme, positive introgression statistics on the X than the autosomes, even though no gene flow occurred between Borneo and Sulawesi after Sulawesi was colonized based on parameterization 1 (figure 5). We therefore suspect that the positive Patterson's D on the X is owing to ancient intraspecific population structure within the X chromosome that has persisted over evolutionary time scales (millions of years). Persistent ancestral geographical structure at the margins of species ranges thus may be conflated with estimates of introgression on the X chromosome, with a counterintuitive consequence of increasing the magnitude of Patterson's D on the X, even when gene flow is modest or absent. Because fdM uses a dynamic estimator to tabulate patterns of genetic variation from the source (donor) population [26,28], this statistic appears to be less sensitive to the effects of ancient geographical structure on the X than Patterson's D (figure 6). Taken together, these results evidence demographic effects on Patterson's D and suggest this statistic should be evaluated in tandem with other metrics, such as divergence and genetic diversity of introgression blocks, and/or other introgression statistics such as fdM [28]. Additional discussion of these findings in the context of biodiversity conservation is provided in the electronic supplementary material.
4. Conclusion
Links between behaviour and various aspects of genome evolution, including supergene formation, gene copy number and gene family expansion have been previously identified [36]. Our results illustrate that in several species of macaques that have a social system characterized by extreme female philopatry and obligate male migration, Ninteract genes are frequently subjected to positive selection and frequently embedded in atypically long ROHs. We interpret these genomic patterns to be a consequence of natural selection to maintain compatibility of autosomal-encoded genes (Ninteract genes) that interact with rapidly evolving mitochondrial genomes to carry out crucial functions, with possible additional contributions involving adaptive coevolution of mitochondrial and nuclear interactions. Together these factors decrease genetic variation within species in some Ninteract genes and their linked regions, increase differentiation between species, and may affect fitness of admixed individuals. We acknowledge that other explanations are possible, such variation in local environmental conditions being associated with positive selection on pleiotropic functions of Ninteract genes that are not directly related to mitonuclear interactions. One way to distinguish effects of mitonuclear interactions from other correlated phenomena would be to test for compensatory evolution of mitochondrial and nuclear-encoded protein motifs that interact directly based on structural data for mitonuclear enzyme complexes; this is an exciting direction for future work.
Although not explored here, a fascinating possibility is that mitonuclear interactions could influence sexual selection [37]. Sulawesi macaques, for example, are highly differentiated in pelage, ischial callosities, oestrus swelling and size [38]. It is conceivable that the evolution of these ornamentations was favoured by natural selection as a mechanism to signal mitonuclear compatibility [37]. These results also extend findings of strong geographically structured variation in macaque mitochondrial genomes (e.g. [39]) to include as well the X chromosome. Our findings in Southeast Asian macaques establish clear predictions for Ninteract genes in other species with similar social systems, including biomedically important species such as rhesus and longtail macaques. Sex-differences in behaviour thus can have pronounced, pervasive and persistent effects on genome evolution by influencing how interacting genetic variation in mitochondrial and nuclear genes change through time and space.
5. Methods
(a) . Genetic samples and molecular data
Data analysed in this study include whole-genome data from 29 georeferenced monkeys (18 female and 11 male) from eight macaque species from Southeast Asia (electronic supplementary material, table S1; figure 1) that were sequenced to medium to high depth (range 11.0–40.0X; electronic supplementary material, figure S16). Three of these nuclear genomes were previously reported [18] and complete mitochondrial genomes of all of these samples were also previously reported [14]. For analyses that required an outgroup, we also included genomic data from a male P. anubis baboon (SRR1515161). These data were aligned to a reference genome from a rhesus macaque, genotyped and filtered as detailed in the electronic supplementary material.
(b) . Nuclear-encoded proteins that interact with mitochondria-encoded proteins
We quantified summaries of differentiation, polymorphism, natural selection and introgression for genomic windows containing genes, with a focus on four categories of Ninteract proteins that interact with mitochondria-encoded proteins [21], with additional detail about genes in each category provided in the electronic supplementary material. Gene acronyms of these Ninteract genes are provided in the electronic supplementary material, file S1.
As detailed in the electronic supplementary material, we calculated FST, π, Tajima's D statistic [22], and Fay and Wu's H [23] in non-overlapping 30 and 100 kb genomic windows. Permutation tests, linear regressions and outlier analyses were used to evaluate whether these metrics were more extreme in Ninteract windows as compared to non-Ninteract windows, or windows that contained no genes. We estimated the rate ratio of non-synomymous to synonymous substitutions per site across a phylogeny of these samples and used permutation tests to evaluate whether this rate ratio was higher in Ninteract genes than non-Ninteract genes (electronic supplementary material). We additionally identified ROHs and used permutation tests and linear regressions to evaluate whether ROHs that contained Ninteract genes tended to be longer than those that only contained non-Ninteract genes, or those that contained no genes. All permutation tests were performed using scripts written in Perl; all linear models were fitted using the lm() function in R [40].
(c) . Analysis of population structure and gene flow
To explore the effects of sex-biased migration within species in the X chromosome and autosomes, FST was calculated within each species for which more than one genome was sequenced. For M. nemestrina, FST was calculated between the Sumatra specimen and all samples from Borneo, and also between the two northern and three southeastern Borneo specimens. For several Sulawesi species (M. nigra, M. nigrescens, M. hecki, M. tonkeana and M. maura), specimens were divided into two populations based on their geographical distributions for FST calculations. Confidence intervals for FST were estimated using the weighted block jackknife approach [41].
Patterson's D statistic [25] (not to be confused with Tajima's D) and fdM [26] were used to explore the effects of sex-biased migration between species in the X chromosome and autosomes. Confidence intervals were estimated using the weighted block jackknife approach [41] with non-overlapping 100 000 bp genomic regions that were weighted by the sum of the numbers of variable positions that were used in each calculation in each window. Additionally, introgression blocks were inferred from individual genomes using a hidden Markov model [24] as detailed in the electronic supplementary material.
Supplementary Material
Acknowledgements
We thank Svante Pääbo and the Department of Evolutionary Genetics at the Max Planck Institute for Evolutionary Anthropology for hosting B.J.E. during his sabbatical during which time much of the analyses for this paper were performed.
Ethics
Research and collection permits for this study were provided by the Indonesian Institute of Sciences/Lembaga Ilmu Pengetahuan Indonesia (LIPI). The animal use protocol was approved by the Institutional Animal Care and Use Committee (IUCAC) at Columbia University. No research ethics approval was required for this research.
Data accessibility
Raw sequence data in this study are deposited in the National Center for Biotechnology Information Short Read Archive (BioProjects PRJNA398198 and PRJNA718817). Filtered VCF files and scripts are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.p5hqbzkqb [42].
Authors' contributions
B.J.E.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft, writing—review and editing; B.M.P.: software, writing—review and editing; D.M.: conceptualization; N.A.: resources; J.S.: resources; J.Z.: software, validation; A.J.T.: conceptualization, funding aquisition, writing—review and editing. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Natural Sciences and Engineering Research Council of Canada (grant no. RGPIN-2017-05770), Compute Canada, the Max Planck Institute of Evolutionary Anthropology and Kent State University.
References
- 1.Burton RS, Barreto FS. 2012A disproportionate role for mtDNA in Dobzhansky–Muller incompatibilities? Mol. Ecol. 21, 4942-4957. ( 10.1111/mec.12006) [DOI] [PubMed] [Google Scholar]
- 2.Charlesworth B. 1994Evolution in age-structured populations, vol. 2. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Charlesworth B, Coyne JA, Barton NH. 1987The relative rates of evolution of sex chromosomes and autosomes. Am. Nat. 130, 113-146. ( 10.1086/284701) [DOI] [Google Scholar]
- 4.Coyne JA, Orr HA. 1989Two rules of speciation. In Speciation and its consequences (eds Otte D, Endler JA), pp. 180-207. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 5.Hill GE. 2017The mitonuclear compatibility species concept. Auk 134, 393-409. ( 10.1642/auk-16-201.1) [DOI] [PubMed] [Google Scholar]
- 6.Ellison CK, Niehuis O, Gadau J. 2008Hybrid breakdown and mitochondrial dysfunction in hybrids of Nasonia parasitoid wasps. J. Evol. Biol. 21, 1844-1851. ( 10.1111/j.1420-9101.2008.01608.x) [DOI] [PubMed] [Google Scholar]
- 7.Innocenti P, Morrow EH, Dowling DK. 2011Experimental evidence supports a sex-specific selective sieve in mitochondrial genome evolution. Science 332, 845-848. ( 10.1126/science.1201157) [DOI] [PubMed] [Google Scholar]
- 8.Sackton TB, Haney RA, Rand DM. 2003Cytonuclear coadaptation in Drosophila: disruption of cytochrome c oxidase activity in backcross genotypes. Evolution 57, 2315-2325. ( 10.1111/j.0014-3820.2003.tb00243.x) [DOI] [PubMed] [Google Scholar]
- 9.Zinner D, Chuma IS, Knauf S, Roos C. 2018Inverted intergeneric introgression between critically endangered kipunjis and yellow baboons in two disjunct populations. Biol. Lett. 14, 20170729. ( 10.1098/rsbl.2017.0729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsudaira K, Hamada Y, Bunlungsup S, Ishida T, San AM, Malaivijitnond S. 2018Whole mitochondrial genomic and Y-chromosomal phylogenies of Burmese long-tailed macaque (Macaca fascicularis aurea) suggest ancient hybridization between fascicularis and sinica species groups. J. Hered. 109, 360-371. ( 10.1093/jhered/esx108) [DOI] [PubMed] [Google Scholar]
- 11.Meyer M, et al. 2016Nuclear DNA sequences from the Middle Pleistocene Sima de los Huesos hominins. Nature 531, 504-507. ( 10.1038/nature17405) [DOI] [PubMed] [Google Scholar]
- 12.Dittus WP. 1975Population dynamics of the toque monkey, Macaca sinica. In Socioecology and psychology of primates (ed. RH Tuttle), pp. 125-151. The Haag, The Netherlands: Mouton. [Google Scholar]
- 13.Roos C, Kothe M, Alba DM, Delson E, Zinner D. 2019The radiation of macaques out of Africa: evidence from mitogenome divergence times and the fossil record. J. Hum. Evol. 133, 114-132. ( 10.1016/j.jhevol.2019.05.017) [DOI] [PubMed] [Google Scholar]
- 14.Evans BJ, et al. 2020Mitogenomics of macaques (Macaca) across Wallace's line in the context of modern human dispersals. J. Hum. Evol. 146, 102852. ( 10.1016/j.jhevol.2020.102852) [DOI] [PubMed] [Google Scholar]
- 15.Roos C, Knauf S, Chuma IS, Maille A, Callou C, Sabin R, Portela Miguez R, Zinner D. 2020New mitogenomic lineages in Papio baboons and their phylogeographic implications. Am. J. Phy. Anth. 174, 407-417. ( 10.1002/ajpa.24186) [DOI] [PubMed] [Google Scholar]
- 16.Kopp GH, Da Silva MF, Fischer J, Brito JC, Regnaut S, Roos C, Zinner D. 2014The influence of social systems on patterns of mitochondrial DNA variation in baboons. Int. J. Primatol. 35, 210-225. ( 10.1007/s10764-013-9725-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou JY, Leu JY. 2015The Red Queen in mitochondria: cyto-nuclear co-evolution, hybrid breakdown and human disease. Front. Gen. 6, 187. ( 10.3389/fgene.2015.00187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans BJ, Tosi AJ, Zeng K, Dushoff J, Corvelo A, Melnick DJ. 2017Speciation over the edge: gene flow among non-human primate species across a formidable biogeographic barrier. R. Soc. Open Sci. 4, 170351. ( 10.1098/rsos.170351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace AR. 1860On the zoological geography of the Malay Archipelago. Zool. J. Linn. Soc. 4, 172-184. ( 10.1111/j.1096-3642.1860.tb00090.x) [DOI] [Google Scholar]
- 20.Evans BJ, Supriatna J, Melnick DJ. 2001Hybridization and population genetics of two macaque species in Sulawesi, Indonesia. Evolution 55, 1686-1702. ( 10.1111/j.0014-3820.2001.tb00688.x) [DOI] [PubMed] [Google Scholar]
- 21.Hill GE. 2020Mitonuclear compensatory coevolution. Trends Gen. 36, 403-414. ( 10.1016/j.tig.2020.03.002) [DOI] [PubMed] [Google Scholar]
- 22.Tajima F. 1989Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123, 585-595. ( 10.1093/genetics/123.3.585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fay JC, Wu C-I. 2000Hitchhiking under positive Darwinian selection. Genetics 155, 1405-1413. ( 10.1093/genetics/155.3.1405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peter BM.2020100,000 years of gene flow between Neandertals and Denisovans in the Altai mountains. bioRxiv. ( 10.1101/2020.03.13.990523) [DOI]
- 25.Patterson N, Moorjani P, Luo Y, Mallick S, Rohland N, Zhan Y, Genschoreck T, Webster T, Reich D. 2012Ancient admixture in human history. Genetics 192, 1065-1093. ( 10.1534/genetics.112.145037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malinsky M, et al. 2015Genomic islands of speciation separate cichlid ecomorphs in an East African crater lake. Science 350, 1493-1498. ( 10.1126/science.aac9927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eriksson A, Manica A. 2012Effect of ancient population structure on the degree of polymorphism shared between modern human populations and ancient hominins. Proc. Natl Acad. Sci. 109, 13 956-13 960. ( 10.1073/pnas.1200567109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin SH, Davey JW, Jiggins CD. 2015Evaluating the use of ABBA–BABA statistics to locate introgressed loci. Mol. Biol. Evol. 32, 244-257. ( 10.1093/molbev/msu269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexander DH, Novembre J, Lange K. 2009Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19, 1655-1664. ( 10.1101/gr.094052.109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haigh J. 1978The accumulation of deleterious genes in a population—Muller's ratchet. Theo. Pop. Biol. 14, 251-267. ( 10.1016/0040-5809(78)90027-8) [DOI] [PubMed] [Google Scholar]
- 31.Signes A, Fernandez-Vizarra E. 2018Assembly of mammalian oxidative phosphorylation complexes I–V and supercomplexes. Essays Biochem. 62, 255-270. ( 10.1042/EBC20170098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zurita Rendón O, Shoubridge EA. 2012Early complex I assembly defects result in rapid turnover of the ND1 subunit. Hum. Mol. Gen. 21, 3815-3824. ( 10.1093/hmg/dds209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sloan DB, Warren JM, Williams AM, Wu Z, Abdel-Ghany SE, Chicco AJ, Havird JC. 2018Cytonuclear integration and co-evolution. Nat. Rev. Gen. 19, 635-648. ( 10.1038/s41576-018-0035-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voris HK. 2000Maps of Pleistocene sea levels in Southeast Asia: shorelines, river systems and time durations. J. Biogeogr. 27, 1153-1167. ( 10.1046/j.1365-2699.2000.00489.x) [DOI] [Google Scholar]
- 35.Coyne JA, Orr HA. 2004Speciation. Sunderland, MA: Sinauer Associates, Inc. [Google Scholar]
- 36.Rubenstein DR, et al. 2019Coevolution of genome architecture and social behavior. Trends Ecol. Evol. 34, 844-855. ( 10.1016/j.tree.2019.04.011) [DOI] [PubMed] [Google Scholar]
- 37.Hill GE, Johnson JD. 2013The mitonuclear compatibility hypothesis of sexual selection. Proc. R. Soc. B 280, 20131314. ( 10.1098/rspb.2013.1314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fooden J. 1969Taxonomy and evolution of the monkeys of Celebes (Primates: Cercopithecidae). Basel, Switzerland and New York, NY: Karger. [Google Scholar]
- 39.Melnick DJ, Hoelzer GA. 1992Differences in male and female macaque dispersal lead to contrasting distributions of nuclear and mitochondrial DNA variation. Int. J. Primatol. 13, 379-393. ( 10.1007/BF02547824) [DOI] [Google Scholar]
- 40.R Core Team. 2013R: a language and environment for statistical computing. Vienna, Austria. R Foundation for Statistical Computing.
- 41.Green RE, et al. 2010A draft sequence of the Neandertal genome. Science 328, 710-722. ( 10.1126/science.1188021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans BJ, Peter BM, Melnick DJ, Andayani N, Supriatna J, Zhu J, Tosi AJ. 2021Data from: Mitonuclear interactions and introgression genomics of macaque monkeys (Macaca) highlight the influence of behaviour on genome evolution. Dryad Digital Repository. ( 10.5061/dryad.p5hqbzkqb) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequence data in this study are deposited in the National Center for Biotechnology Information Short Read Archive (BioProjects PRJNA398198 and PRJNA718817). Filtered VCF files and scripts are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.p5hqbzkqb [42].