Abstract
Background
Most people who stop smoking gain weight. This can discourage some people from making a quit attempt and risks offsetting some, but not all, of the health advantages of quitting. Interventions to prevent weight gain could improve health outcomes, but there is a concern that they may undermine quitting.
Objectives
To systematically review the effects of: (1) interventions targeting post‐cessation weight gain on weight change and smoking cessation (referred to as 'Part 1') and (2) interventions designed to aid smoking cessation that plausibly affect post‐cessation weight gain (referred to as 'Part 2').
Search methods
Part 1 ‐ We searched the Cochrane Tobacco Addiction Group's Specialized Register and CENTRAL; latest search 16 October 2020.
Part 2 ‐ We searched included studies in the following 'parent' Cochrane reviews: nicotine replacement therapy (NRT), antidepressants, nicotine receptor partial agonists, e‐cigarettes, and exercise interventions for smoking cessation published in Issue 10, 2020 of the Cochrane Library. We updated register searches for the review of nicotine receptor partial agonists.
Selection criteria
Part 1 ‐ trials of interventions that targeted post‐cessation weight gain and had measured weight at any follow‐up point or smoking cessation, or both, six or more months after quit day.
Part 2 ‐ trials included in the selected parent Cochrane reviews reporting weight change at any time point.
Data collection and analysis
Screening and data extraction followed standard Cochrane methods. Change in weight was expressed as difference in weight change from baseline to follow‐up between trial arms and was reported only in people abstinent from smoking. Abstinence from smoking was expressed as a risk ratio (RR). Where appropriate, we performed meta‐analysis using the inverse variance method for weight, and Mantel‐Haenszel method for smoking.
Main results
Part 1: We include 37 completed studies; 21 are new to this update. We judged five studies to be at low risk of bias, 17 to be at unclear risk and the remainder at high risk.
An intermittent very low calorie diet (VLCD) comprising full meal replacement provided free of charge and accompanied by intensive dietitian support significantly reduced weight gain at end of treatment compared with education on how to avoid weight gain (mean difference (MD) −3.70 kg, 95% confidence interval (CI) −4.82 to −2.58; 1 study, 121 participants), but there was no evidence of benefit at 12 months (MD −1.30 kg, 95% CI −3.49 to 0.89; 1 study, 62 participants). The VLCD increased the chances of abstinence at 12 months (RR 1.73, 95% CI 1.10 to 2.73; 1 study, 287 participants). However, a second study found that no‐one completed the VLCD intervention or achieved abstinence.
Interventions aimed at increasing acceptance of weight gain reported mixed effects at end of treatment, 6 months and 12 months with confidence intervals including both increases and decreases in weight gain compared with no advice or health education. Due to high heterogeneity, we did not combine the data. These interventions increased quit rates at 6 months (RR 1.42, 95% CI 1.03 to 1.96; 4 studies, 619 participants; I2 = 21%), but there was no evidence at 12 months (RR 1.25, 95% CI 0.76 to 2.06; 2 studies, 496 participants; I2 = 26%).
Some pharmacological interventions tested for limiting post‐cessation weight gain (PCWG) reduced weight gain at the end of treatment (dexfenfluramine, phenylpropanolamine, naltrexone). The effects of ephedrine and caffeine combined, lorcaserin, and chromium were too imprecise to give useful estimates of treatment effects. There was very low‐certainty evidence that personalized weight management support reduced weight gain at end of treatment (MD −1.11 kg, 95% CI −1.93 to −0.29; 3 studies, 121 participants; I2 = 0%), but no evidence in the longer‐term 12 months (MD −0.44 kg, 95% CI −2.34 to 1.46; 4 studies, 530 participants; I2 = 41%). There was low to very low‐certainty evidence that detailed weight management education without personalized assessment, planning and feedback did not reduce weight gain and may have reduced smoking cessation rates (12 months: MD −0.21 kg, 95% CI −2.28 to 1.86; 2 studies, 61 participants; I2 = 0%; RR for smoking cessation 0.66, 95% CI 0.48 to 0.90; 2 studies, 522 participants; I2 = 0%).
Part 2: We include 83 completed studies, 27 of which are new to this update.
There was low certainty that exercise interventions led to minimal or no weight reduction compared with standard care at end of treatment (MD −0.25 kg, 95% CI −0.78 to 0.29; 4 studies, 404 participants; I2 = 0%). However, weight was reduced at 12 months (MD −2.07 kg, 95% CI −3.78 to −0.36; 3 studies, 182 participants; I2 = 0%).
Both bupropion and fluoxetine limited weight gain at end of treatment (bupropion MD −1.01 kg, 95% CI −1.35 to −0.67; 10 studies, 1098 participants; I2 = 3%); (fluoxetine MD −1.01 kg, 95% CI −1.49 to −0.53; 2 studies, 144 participants; I2 = 38%; low‐ and very low‐certainty evidence, respectively). There was no evidence of benefit at 12 months for bupropion, but estimates were imprecise (bupropion MD −0.26 kg, 95% CI −1.31 to 0.78; 7 studies, 471 participants; I2 = 0%). No studies of fluoxetine provided data at 12 months.
There was moderate‐certainty that NRT reduced weight at end of treatment (MD −0.52 kg, 95% CI −0.99 to −0.05; 21 studies, 2784 participants; I2 = 81%) and moderate‐certainty that the effect may be similar at 12 months (MD −0.37 kg, 95% CI −0.86 to 0.11; 17 studies, 1463 participants; I2 = 0%), although the estimates are too imprecise to assess long‐term benefit.
There was mixed evidence of the effect of varenicline on weight, with high‐certainty evidence that weight change was very modestly lower at the end of treatment (MD −0.23 kg, 95% CI −0.53 to 0.06; 14 studies, 2566 participants; I2 = 32%); a low‐certainty estimate gave an imprecise estimate of higher weight at 12 months (MD 1.05 kg, 95% CI −0.58 to 2.69; 3 studies, 237 participants; I2 = 0%).
Authors' conclusions
Overall, there is no intervention for which there is moderate certainty of a clinically useful effect on long‐term weight gain. There is also no moderate‐ or high‐certainty evidence that interventions designed to limit weight gain reduce the chances of people achieving abstinence from smoking.
Keywords: Humans, Electronic Nicotine Delivery Systems, Nicotine, Smoking Cessation, Tobacco Use Cessation Devices, Weight Gain
Plain language summary
Interventions for preventing weight gain after smoking cessation
What is the best way to avoid putting on weight after stopping smoking?
Key messages
We are not certain which programmes or treatments work best to help people avoid gaining weight in the long term (up to 12 months) when stopping smoking, or how they affect success in stopping smoking. This is because the evidence shows varied and unclear effects on weight gain. Further studies should continue to look at how to limit weight gain in people who are stopping smoking. Future studies of new medicines to help people stop smoking should also measure changes in their weight.
Stopping smoking and weight gain
If you smoke, the best thing you can do for your health is to stop. But people often put on weight when they stop smoking, usually in the first few months of stopping. Gaining weight might undermine some of the benefits of stopping smoking, and might also affect the motivation of some people trying to stop smoking.
What did we want to find out?
Some programmes to help people stop smoking specifically target weight control. Other ways to help people stop smoking might also affect their weight; these include: exercise programmes, taking medicines, and using nicotine replacement therapy (NRT).
We wanted to find out the best ways to stop weight gain when stopping smoking.
What did we do?
In this update of a previously‐published review, we searched for studies that tested:
‐ specific programmes for weight control while stopping smoking;
‐ other ways to help people stop smoking, if those studies also measured weight change.
We were interested in:
‐ how many people stopped smoking for six months or 12 months;
‐ people's weight at the end of treatment, then after six months and after 12 months.
What did we find?
We found 116 studies in total:
37 studies of specific programmes for weight control for people stopping smoking (21 new studies for this update); and 83 studies of other ways to help people stop smoking (27 new studies for this update). Four of these studies contributed to both.
The 37 studies of specific programmes tested behavioural programmes, including dieting, to manage weight in 11,514 people trying to stop smoking. Some of the behavioural programmes were acceptance‐based, in which people also learn self‐regulation skills (for example, how to deal with cravings) to help them keep to the behaviours needed to lose weight. Most studies (27) were done in the USA and others took place in Australia, Canada, China and Europe.
The 83 studies of other ways of stopping smoking included 46,248 people and looked at:
exercise programmes; taking NRT; taking a medicine called varenicline (used to help people stop smoking); or taking a medicine called fluoxetine (used to treat depression).
Of these studies, 39 were done in the USA and the rest took place in other countries around the world. Very few studies reported on side effects.
What are the main results of our review?
Programmes aimed at limiting weight gain
Compared with no programme or brief advice only, a personalized weight‐management programme may reduce weight gain at the end of treatment, after six months and after 12 months. However, a weight‐management programme without personalized assessment, planning and feedback may not reduce weight gain, and may reduce the number of people who stop smoking.
Compared with no programme, acceptance‐based programmes for weight:
may help more people to stop smoking after six months and 12 months; but may make little to no difference to their weight gain.
Other programmes and treatments that might affect weight
Taking part in an exercise programme to help stop smoking may reduce weight gain after 12 months, compared with not taking part in one.
Using NRT probably reduces weight gain slightly after 12 months, compared with not using NRT.
Taking varenicline makes little difference to weight gain at the end of treatment, and may make little difference after six months or after 12 months.
Taking fluoxetine may reduce weight gain at the end of treatment, but we do not know how it affects weight gain after six months or 12 months.
What are the limitations of the evidence?
We are certain that there is no difference in weight gain at the end of treatment with varenicline, and further studies are unlikely to change this result. However, our confidence in all of the other evidence is limited, mainly because of small numbers of studies that could be compared, and small numbers of people taking part in them. The results varied widely, and there were not enough studies for us to be sure of the results. Our confidence is likely to change if further evidence becomes available.
How up to date is this evidence?
The evidence is current up to October 2020.
Summary of findings
Summary of findings 1. Behavioural weight management interventions compared to brief advice or no intervention for post‐cessation weight control.
| Behavioural weight management interventions compared to brief advice or no intervention for post‐cessation weight control for preventing weight gain after smoking cessation | ||||||
| Patient or population: People wanting to quit smoking Setting: Community Intervention: Behavioural weight management interventions Comparison: advice or no intervention for post‐cessation weight control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with advice or no intervention for post‐cessation weight control# | Risk with behavioural weight management interventions | |||||
| Mean weight change (kg) at end of treatment ‐ Weight management education versus no weight intervention | 1.45 kg | 1.41 kg (0.88 to 1.95) | MD 0.04 kg lower (0.57 lower to 0.5 higher) | 140 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | ‐ |
| Mean weight change (kg) at 6 months ‐ Weight management education verses no weight intervention | 0.97 kg | 1.86 kg (0.19 to 3.52) | MD 0.89 kg higher (0.78 lower to 2.55 higher) | 81 (2 RCTs) | ⊕⊕⊝⊝ LOWb,c | ‐ |
| Mean weight change (kg) at 12 months ‐ Weight management education versus no weight intervention | 0.46 kg | 0.25 kg (‐1.82 to 2.32) | MD 0.21 kg lower (2.28 lower to 1.86 higher) | 61 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWc,d | ‐ |
| Smoking cessation at 6 months ‐ Weight management education versus no intervention | 275 per 1000 | 280 per 1000 (214 to 365) | RR 1.02 (0.78 to 1.33) | 660 (3 RCTs) | ⊕⊕⊝⊝ LOWd | ‐ |
| Smoking cessation at 12 months ‐ Weight management education versus no intervention | 294 per 1000 | 194 per 1000 (141 to 265) | RR 0.66 (0.48 to 0.90) | 522 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | ‐ |
| Mean weight change (kg) at end of treatment ‐ Personalised weight management support versus no weight intervention | 1.45 kg | 0.34 kg (‐0.48 to 1.16) | MD 1.11 kg lower (1.93 lower to 0.29 lower) | 121 (3 RCTs) | ⊕⊕⊝⊝ LOWa,b | ‐ |
| Mean weight change (kg) at 6 months ‐ Personalised weight management support versus no weight intervention | 0.97 kg | 0.01 kg (‐1.21 to 1.22) | MD 0.96 kg lower (2.18 lower to 0.25 higher) | 816 (5 RCTs) | ⊕⊝⊝⊝ VERY LOWe,f | ‐ |
| Mean weight change (kg) at 12 months ‐ Personalised weight management support versus no weight intervention | 0.46 kg | 0.02 kg (‐1.88 to 1.92) | MD 0.44 kg lower (2.34 lower to 1.46 higher) | 530 (4 RCTs) | ⊕⊝⊝⊝ VERY LOWa,d | ‐ |
| Smoking cessation at 6 months ‐ Personalised weight management support versus no intervention | 188 per 1000 | 178 per 1000 (154 to 206) | RR 0.95 (0.82 to 1.10) | 5517 (7 RCTs) | ⊕⊕⊝⊝ LOWg,h | ‐ |
| Smoking cessation at 12 months ‐ Personalised weight management support versus no intervention | 475 per 1000 | 308 per 1000 (214 to 437) | RR 0.65 (0.45 to 0.92) | 3441 (5 RCTs) | ⊕⊕⊝⊝ LOWa,i | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: randomized controlled trial; RR: Risk ratio; MD: Mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to risk of bias. All studies judged to be at unclear or high risk of bias. Results not sensitive to removal of studies at high risk of bias. bDowngraded one level due to imprecision. Few studies and participants contributed data. cDowngraded one level due to risk of bias. Of the two studies contributing data, one was at high risk of bias, and other at low risk. Results not sensitive to removal of study at high risk.dDowngraded two levels due to imprecision. Wide CIs incorporate clinically meaningful weight reduction and clinically meaningful weight increase. eDowngraded two levels due to risk of bias. All studies judged to be at high risk. fDowngraded one level due to imprecision. Wide CIs encompass benefit and no clinically meaningful difference. gDowngraded one level due to risk of bias. All studies at high or unclear risk of bias, bar one small study at low risk of bias. Results not sensitive to removing studies at high risk of bias.
hDowngraded one level due to imprecision. Wide CIs incorporate clinically meaningful difference in favour of control group, as well as no clinically significant difference. iDowngraded one level due to inconsistency due to substantial unexplained statistical heterogeneity (I2 = 89%). #Calculated as weighted means from control group data.
Summary of findings 2. Acceptance interventions for weight concern compared to no weight management intervention for post‐cessation weight control.
| Acceptance interventions for weight concern compared to no weight management intervention for preventing weight gain after smoking cessation | ||||||
| Patient or population: People wanting to quit smoking Setting: Community Intervention: Acceptance interventions for weight concern Comparison: No weight management intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no weight management intervention# | Risk with acceptance interventions for weight concern | |||||
| Mean weight change (kg) at end of treatment | see comment | see comment | see comment | 169 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b | Substantial unexplained statistical heterogeneity precluded meta‐analysis. Of the 3 studies, 1 had wide CIs encompassing both benefit and harm, 1 suggested benefit with CIs excluding no difference, and 1 suggested harm with CIs excluding no difference. |
| Mean weight change (kg) at 6 months | 3.5 kg | 3.5 kg (1.97 to 5.03) | MD 0 kg (1.53 lower to 1.53 higher) | 106 (3 RCTs) | ⊕⊕⊝⊝ LOWa,c,d | ‐ |
| Mean weight change (kg) at 12 months | 4.39 kg | 3.69 kg (1.44 to 5.95) | MD 0.7 kg lower (2.95 lower to 1.56 higher) | 76 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,c,d | ‐ |
| Smoking cessation at 6 months | 218 per 1000 | 309 per 1000 (224 to 427) | RR 1.42 (1.03 to 1.96) | 619 (4 RCTs) | ⊕⊕⊝⊝ LOWa,e | ‐ |
| Smoking cessation at 12 months | 143 per 1000 | 179 per 1000 (109 to 294) | RR 1.25 (0.76 to 2.06) | 496 (2 RCTs) | ⊕⊝⊝⊝ LOWa,d | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: randomized controlled trial; RR: Risk ratio; MD: Mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to risk of bias. All studies judged to be at unclear or high risk of bias. Results not sensitive to removal of studies at high risk of bias. bDowngraded two levels due to inconsistency. Unexplained statistical heterogeneity prevented pooling data. cDowngraded one level due to inconsistency. Moderate unexplained statistical heterogeneity. dDowngraded two levels due to imprecision. CIs encompass clinically significant benefit and clinically significant harm. eDowngraded one level due to imprecision. < 300 events overall and CIs encompass clinically significant benefit and no clinically significant difference. #Calculated as weighted means from control group data.
Summary of findings 3. Exercise interventions for smoking cessation compared to no exercise intervention for preventing weight gain after smoking cessation.
| Exercise interventions for smoking cessation compared to no exercise intervention for preventing weight gain after smoking cessation | ||||||
| Patient or population: People who have quit smoking Setting: Community Intervention: Exercise interventions for smoking cessation Comparison: no exercise intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no exercise intervention# | Risk with Exercise interventions for smoking cessation | |||||
| Mean weight change (kg) at end of treatment | 2.85 kg | 2.6 kg (2.07 to 3.14) | MD 0.25 kg lower (0.78 lower to 0.29 higher) | 404 (4 RCTs) | ⊕⊕⊝⊝ LOWa,b | ‐ |
| Mean weight change (kg) at 12 months | 4.67 kg | 2.6 kg (0.89 to 4.31) | MD 2.07 kg lower (3.78 lower to 0.36 lower) | 182 (3 RCTs) | ⊕⊕⊝⊝ LOWa,b | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: randomized controlled trial; RR: Risk ratio; MD: Mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to imprecision. CIs encompass clinically significant benefit as well as no clinically significant difference. bDowngraded one level due to inconsistency ‐ end of treatment and 12 month outcomes are clinically significantly different, with no clear or plausible reason for difference. #Risk with no intervention from Aubin, 2012.
Summary of findings 4. Nicotine replacement therapy for smoking cessation compared to placebo for preventing weight gain after smoking cessation.
| Nicotine replacement therapy for smoking cessation compared to placebo for preventing weight gain after smoking cessation | ||||||
| Patient or population: People who have quit smoking Setting: Community Intervention: Nicotine replacement therapy for smoking cessation Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo# | Risk with nicotine replacement therapy for smoking cessation | |||||
| Mean weight change (kg) at end of treatment | 2.85 kg | 2.33 kg (1.86 to 2.8) | MD 0.52 kg lower (0.99 lower to 0.05 lower) | 2784 (21 RCTs) | ⊕⊕⊕⊝ MODERATEa,b,c | ‐ |
| Mean weight change (kg) at 6 months | 4.23 kg | 4.15 kg (3.72 to 4.58) | MD 0.08 kg lower (0.51 lower to 0.35 higher) | 1021 (11 RCTs) | ⊕⊕⊕⊝ MODERATEa,d | ‐ |
| Mean weight change (kg) at 12 months | 4.67 kg | 4.3 (3.81 to 4.67) | MD 0.37 kg lower (0.86 lower to 0.11 higher) | 1463 (17 RCTs) | ⊕⊕⊕⊝ MODERATEe | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: randomized controlled trial; RR: Risk ratio; MD: Mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to risk of bias. All studies at high or unclear risk. Results not sensitive to removal of studies at high risk of bias. bStatistical heterogeneity was substantial due to one study (2 Abelin 1989), which showed a 4.3 kg difference between weight gain in the treatment and control arms. When this study was removed, statistical heterogeneity reduced to 0% and the overall estimate decreased. Not downgraded on this basis. cFunnel plot showed some asymmetry, suggesting that smaller studies with less weight gain in intervention groups relative to control groups may be missing, but not downgraded on this basis, as asymmetry thought plausibly due to chance, given asymmetry in opposite direction at six months. dFunnel plot showed some asymmetry, suggesting that smaller studies with greater weight gain in intervention groups relative to control groups may be missing, but not downgraded on this basis as asymmetry thought plausibly due to chance, given asymmetry in opposite direction at end of treatment. eDowngraded one level due to imprecision. CIs encompass clinically significant benefit and no clinically significant difference. #Risk with no intervention from Aubin, 2012.
Summary of findings 5. Varenicline for smoking cessation compared to placebo for preventing weight gain after smoking cessation.
| Varenicline for smoking cessation compared to placebo for preventing weight gain after smoking cessation | ||||||
| Patient or population: People who have quit smoking Setting: Community Intervention: Varenicline Comparison: placebo for smoking cessation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo for smoking cessation# | Risk with varenicline | |||||
| Mean weight change (kg) at end of treatment | 2.85 kg | 2.62 kg (2.32 to 2.91) | MD 0.23 kg lower (0.53 lower to 0.06 higher) | 2566 (14 RCTs) | ⊕⊕⊕⊕ HIGH | ‐ |
| Mean weight change (kg) at 6 months | 4.23 kg | 4.14 kg (3.14 to 5.13) | MD 0.09 kg lower (1.09 lower to 0.9 higher) | 384 (3 RCTs) | ⊕⊕⊝⊝ LOWa | ‐ |
| Mean weight change (kg) at 12 months | 4.67 kg | 5.72 kg (4.09 to 7.36) | MD 1.05 kg higher (0.58 lower to 2.69 higher) | 237 (3 RCTs) | ⊕⊕⊝⊝ LOWb.c | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: randomized controlled trial; RR: Risk ratio; MD: Mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded two levels due to imprecision. CIs incorporate clinically significant benefit as well as clinically significant harm. bDowngraded one level due to risk of bias. All studies at unclear risk. cDowngraded one level due to imprecision. CIs incorporate clinically significant benefit and no difference. #Risk with no intervention from Aubin, 2012.
Summary of findings 6. Fluoexetine compared to placebo for smoking cessation for preventing weight gain after smoking cessation.
| Fluoxetine compared to placebo for smoking cessation for preventing weight gain after smoking cessation | ||||||
| Patient or population: People who have quit smoking Intervention: Fluoxetine for smoking cessation Comparison: placebo for smoking cessation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo for smoking cessation# | Risk with all types of antidepressant | |||||
| Mean weight change (kg) at end of treatment | 2.85 kg | 1.84 kg (1.36 to 2.32) | MD 1.01 kg lower (1.49 lower to 0.53 lower) | 144 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | ‐ |
| Mean weight change (kg) at 6 months | 4.67 kg | 3.66 kg (0.29 to 7.04) | MD 1.01 kg lower (4.38 lower to 2.37 higher) | 124 (2 RCTs) | ⊕⊕⊝⊝ VERY LOWa,c | no studies followed up participants at 12 months |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: randomized controlled trial; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to risk of bias. One study at unclear risk, one at high risk. bDowngraded one level due to imprecision. Only two studies contributed data, overall; n < 150. cDowngraded two levels due to imprecision; CIs incorporate clinically significant weight loss and clinically significant weight gain. #Risk with no intervention from Aubin, 2012
Background
Description of the condition
Although smoking cessation is associated with substantial health benefits, it is often accompanied by weight gain (Aubin, 2012; Audrain‐McGovern 2011). Increases in weight are most marked in the first months after quitting (Aubin, 2012), and longer‐term studies show further moderate increases in weight in quitters for a number of years compared with continuing smokers (2 Lycett 2011). Reports vary about the amount gained, but on average sustained quitters are likely to gain an additional two to five kilograms (kgs) in the first five years (Chiolero 2008; Klesges 1997; Tian 2015; Veldheer 2015; Williamson 1991), and up to seven kgs after eight years (2 Lycett 2011). It is important to note, however, that average weight gain masks a substantial variability at the individual level, with evidence suggesting some quitters may lose weight (Aubin, 2012; Bossé 1980), whereas around 10% to 15% gain 10 kgs or more (Aubin, 2012; Tian 2015; Williamson 1991). Factors that have been consistently associated with greater weight gain after smoking cessation include female sex, higher cigarettes smoked per day/nicotine dependency and higher body mass index (BMI) at quitting (Komiyama 2013; Scherr 2015; Veldheer 2015; Williamson 1991).
Description of the intervention
Interventions included in this review are discussed in two parts, described below.
Some smoking cessation interventions have been developed to promote smoking cessation and simultaneously control weight gain. They include behavioural interventions, such as exercise and energy restriction or healthy‐eating advice. Some pharmacological treatments with known or potential efficacy for reducing weight have also been tested. Interventions may combine both behavioural components and pharmacological treatments, or these may be tested individually. They are often delivered concurrently with smoking cessation support during the first few months of quitting, when the rate of weight gain is at its highest. The effects of these interventions on both smoking abstinence and weight are included in Part 1 of this review.
Several treatments for smoking cessation have been developed independently of concerns about weight gain, with the sole aim of assisting smoking cessation. Some of these, such as nicotine replacement therapy, e‐cigarettes, antidepressants, varenicline and exercise might plausibly influence weight gain as well as smoking cessation. The effects of these interventions on smoking cessation are evaluated in the relevant Cochrane Reviews, but the effects on weight gain are summarised only in the exercise intervention review (Ussher 2019). The effects of these medications on weight gain are included in Part 2 of this review.
How the intervention might work
Smoking (and quitting smoking) is likely to exert its effect on weight through the biological actions of nicotine and also through the influence of smoking on eating behaviours. Nicotine increases metabolic rate (Collins 1994; Dallosso 1984), and withdrawal of nicotine when people quit smoking results in a decrease in rate (Hofstetter 1986)
Nicotine may also be an appetite suppressant, and people may replace eating with smoking (Chiolero 2008). Particularly during the initial period of withdrawal from nicotine when quitting smoking, some people may eat more in order to deal with nicotine cravings/withdrawal, to satiate increases in appetite and replace the hand‐to‐mouth action of smoking (Audrain‐McGovern 2011; Ward 2001). Through these mechanisms, it is likely that an energy imbalance is created favouring weight gain.
Behavioural interventions that limit energy intake (i.e. dieting or calorie restriction) or increase energy expenditure (i.e. exercise) may serve to reduce any energy imbalance that occurs during quitting, and thus reduce the amount of weight gained (Cheskin 2005; Gritz 1988; Hall 1986; 1 Hall 1992; Hughes 1991).
Pharmacological treatments may limit weight gain through several mechanisms. Appetite suppressants may offset the increased appetite that accompanies cessation, limiting any increase in calorie consumption after quitting to satiate increased hunger. Pharmacotherapies that replace nicotine included in Part 2 (nicotine replacement therapies or e‐cigarettes) may reduce nicotine withdrawal effects on metabolism and appetite. Varenicline is a partial agonist of nicotinic receptors in the brain and so may also work through these mechanisms. Depressed mood is a recognised withdrawal symptom of nicotine, and antidepressants are known to affect bodyweight (Serretti 2010), although some increase and some decrease it.
The availability of interventions that limit weight gain might encourage smokers who are concerned about weight gain to try to stop smoking (Filozof 2004), and limiting weight gain may prevent people from returning to smoking to avoid an increase in weight. However, it is possible that some interventions that aim to limit energy intake might undermine the success of a quit attempt (1 Hall 1992). There is evidence that hunger and cigarette cravings are related, that hunger can undermine quit efforts (1 Hall 1992) and that hunger increases urges to smoke in current smokers (Cheskin 2005). There is evidence that early weight gain is associated with successful cessation (Gritz 1988; Hall 1986; Hughes 1991). It is important to investigate further the impact of these interventions on weight gain, and also to evaluate the effect on abstinence rates.
Why it is important to do this review
Among smokers there is a high prevalence of concerns about post‐cessation weight gain, and it has been cited as a primary reason for putting off quit attempts, especially in women (Clark 2004; Klesges 1989; Klesges 1992). Weight consciousness has been found to predict current smoking (Weekley 1992), and weight gain experienced during or after smoking cessation has been associated with relapse (Klesges 1988; Klesges 1989; Klesges 1992). However there is inconsistent evidence that fear of weight gain or actual weight gain after quitting does lead to relapse, with some studies finding associations (1 Copeland 2006; Clark 2006; Meyers 1997; Pomerleau 2001) and others no associations (Fidler 2009; Hutter 2006; Killen 1996; Mizes 1998); methodological differences make it hard to draw a conclusion.
Post‐cessation weight gain can have health consequences, although these do not outweigh the benefit of quitting smoking. In the shorter term, the incidence of diabetes is higher in people who quit smoking than in those who continue with it, an effect that appears to be explained by weight gain (Davey Smith 2005; Yeh 2010), although some evidence suggests in the longer term (more than 10 years) the risk is no higher in those who quit than in those who continued smoking (Luo 2013). Hypertension can also be mediated by post‐cessation weight gain (Gratziou 2009). Additionally, at least one study has suggested that cessation‐related weight gain (more than 5 kgs) may attenuate reductions in cancer risk (Kim 2019). The extent to which these associations are clinically meaningful is unclear. The temporarily increased risk of type 2 diabetes amongst smokers who quit smoking with associated weight gain, is not associated with increased cardiovascular and all‐cause mortality compared to non‐quitters (Hu 2018). Weight gain after smoking cessation has been found not to modify its protective effect on heart attack and stroke (Clair 2013; Kim 2018).
Given the uncertainty of the risks surrounding cessation‐related weight gain, and that concerns about weight gain are cited as a common reason for not attempting quitting, people who smoke, their healthcare providers, and policy‐makers are keen to understand the effects of interventions that could potentially minimize post‐cessation weight gain whilst not adversely affecting cessation. The 2012 version of this review found insufficient evidence to recommend any one type of intervention targeting post‐cessation weight gain. Since then, considerable further literature has been published and new smoking cessation treatments have come onto the market. This updated review considers the entirety of the evidence to date on possible ways to limit post‐cessation weight gain in people abstinent from smoking, and the impact of these interventions on smoking cessation as well as weight.
Objectives
To systematically review the effects of: (1) interventions targeting post‐cessation weight gain on weight change and smoking cessation (referred to as 'Part 1') and (2) interventions designed to aid smoking cessation that may also plausibly affect weight on post‐cessation weight change (referred to as 'Part 2').
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Types of participants
Adults who smoke and are attempting to quit smoking, excluding pregnant women.
In Part 1, we examine smoking cessation in everyone enrolled. In Part 1 and Part 2, we examine the effect of interventions on weight gain in people who have successfully quit smoking only. This is for several reasons. Firstly, if we included those who were not abstinent, mean weight gain would be reduced. This is because people who attempt quitting but fail after a few days do not gain weight, as those who relapse to smoking often lose the weight they gained previously (2 Lycett 2011; O'Hara 1998). Thus the average weight gain of a mixed population of abstinent and non‐abstinent smokers would not reflect the weight gain of either. Secondly, this effect could bias results. If an intervention increased abstinence rates, it is very likely that it would appear to increase weight gain, regardless of whether it actually suppressed weight gain or had no effect. Thirdly, those who return to smoking tend not to attend clinics for follow‐up. Authors typically only report weight data in abstinent smokers, and imputing missing data on weight in those who have relapsed to smoking is problematic. We have little data on the weight trajectory of people who try and fail to achieve abstinence. It is likely that the weight will depend on time since relapse and that imputing data using last observation carried forward or baseline observation carried forward is likely to be misleading. For these reasons, we eschew the intention‐to‐treat approach which is typically used in the Tobacco Addiction Review Group's reviews. This issue has been discussed elsewhere (Parsons 2009b; Parsons 2011; Spring 2011a; Spring 2011b).
Types of interventions
Part 1 ‐ Interventions that are designed specifically to limit post‐cessation weight gain.
Part 2 ‐ Smoking cessation interventions that are not designed primarily to limit post‐cessation weight gain but which might plausibly influence it, i.e. antidepressants, exercise, nicotine replacement therapy (NRT), electronic cigarettes and varenicline, but excluding trials in which all arms receive the same medication, regardless of differences in dose and schedule.
Types of outcome measures
There are two primary outcome measures:
Smoking status at six and 12 months
Mean (SD) change in body weight (kgs) at end of treatment, six, and 12 months.
Both outcomes are fully examined for studies that fit the criteria for Part 1. For Part 2 studies, effects of these interventions on smoking are reported in the parent Cochrane Reviews and we only report the effects of interventions on weight change.
For Part 1 studies of pharmacotherapies, we also evaluate adverse events and serious adverse events. We do not evaluate adverse and serious adverse events for behavioural interventions, following standard Cochrane Tobacco Addiction Group guidance. Information on adverse and serious adverse events for Part 2 studies can be found in the Cochrane Reviews on the relevant interventions (see Search methods for identification of studies).
Search methods for identification of studies
Electronic searches
Part 1 ‐ For the most recent update, we searched the Cochrane Tobacco Addiction Group's Specialized Register (latest search 16 October 2020), using the following search terms in title, abstract or keywords: food, calorie restrict*, intake, diet*, body mass index (BMI), Quetelet, waist‐hip ratio (WHR), weight, body‐weight, weight‐changes. At the search date the specialized register included the results of searches of the Cochrane Central Register of Controlled trials (CENTRAL), issue 9, 2020; MEDLINE (including in‐process and Epub ahead of print, via OVID) to update 202000928; Embase (via OVID) to week 2020040; and PsycINFO (via OVID) to update 20200921, as well as online registers of controlled trials.
Searching other resources
Part 2 ‐ We searched the following Cochrane Reviews: Antidepressants for smoking cessation (last updated 2020; Howes 2020); Exercise interventions for smoking cessation (last updated 2019; Ussher 2019); Nicotine replacement therapy for smoking cessation (last updated 2018; Hartmann‐Boyce 2018); Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation (last updated 2019; Lindson 2019); Electronic cigarettes for smoking cessation (last updated 2020; Hartmann‐Boyce 2020), and Nicotine receptor partial agonists for smoking cessation (last updated 2016; Cahill 2016). We searched the text of references listed as included studies. For reviews last updated prior to 2019, we also searched the Cochrane Tobacco Addiction Group's Specialized Register on 16 October 2020, using the search terms outlined in the individual reviews.
Data collection and analysis
Selection of studies
For the 2020 searches, two review authors (for this update from: JHB, AT, LK, LH, AH and MS) independently screened all titles and abstracts obtained from the search using a piloted screening checklist. Full‐text versions were then independently screened for inclusion. We also ran a 2016 search in which titles and abstracts of records identified were screened in singular by the group's Information Specialist (Lindsay Stead [LS]). Full‐text records were then screened by AF and LS. Any discrepancies about eligibility throughout this update were resolved by discussion or with a third review author. Reasons for exclusion of key studies that required in‐depth discussion are listed in Characteristics of excluded studies.
Data extraction and management
For this update, two review authors (from: JHB, AT, LK, LH, AH, MS, AF, PA, PH, LLJ, DL) independently undertook data extraction using a piloted form. Extractions were then compared and a final version agreed upon following discussion, or with referral to a third review author, when necessary. We extracted the following data for each study:
Study design
Study start and end date
Recruitment
Setting*
Country
Inclusion and exclusion criteria
-
Summary of study participant characteristics
Total number randomized
Number per arm
Total percentage female
Mean age, baseline BMI, baseline weight, cigarettes per day (cpd), Fagerström Test for Nicotine Dependence (FTND) score
Summary of intervention and comparative conditions
Definition of smoking abstinence and type of biochemical validation (if any)
How weight was measured
Number of participants who were abstinent at end of treatment (EOT), 6 months, 12 months and/or at longest follow‐up
Mean weight change (kg) from baseline in abstinent smokers
Abstinence rates (Part 1 studies only)
Adverse events (AEs) and serious adverse events (SAEs) (Part 1 studies only)
Risk of bias in domains specified below
Study funding statement
Author declarations of interest
Any other notes
*studies identified during the 2020 search only.
One review author then entered the data into Review Manager 5 software for analyses (JHB), and another checked them (AT).
Assessment of risk of bias in included studies
Two review authors (for this update from: JHB, AT, LK, LH, MS, AF, PA, PH, LLJ, DL) independently assessed the risks of bias for each included study, using the Cochrane risk of bias tool v1 (Higgins 2011). This approach uses a domain‐based evaluation that addresses different areas: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment (smoking and weight); incomplete outcome data; and other potential sources of bias.
Specific considerations about judgements for individual domains for this review are outlined below:
Blinding of participants and personnel: This domain was not evaluated for studies investigating behavioural interventions for smoking cessation where blinding was not possible; this is in accordance with standard guidance from the Cochrane Tobacco Addiction Group.
Blinding of outcome assessments (detection bias) were evaluated separately for smoking and weight outcomes, given different considerations for each outcome. Risk of detection bias ratings were based on assessments of both weight and smoking outcomes.
Following standard methods of the Cochrane Tobacco Addiction Review Group, we rated studies at high risk of attrition bias if loss to follow‐up was greater than 50% overall or if there was a difference in follow‐up rates of more than 20% between study arms.
We assigned a grade (low, high, or unclear) for risk of bias for each domain and resolved any disagreements by discussion or by consulting with a third review author. We judged studies to be at high risk of bias overall if they were rated at high risk in at least one domain, and at low risk of bias overall if they were judged to be at low risk across all domains evaluated. We judged the remaining studies to be at unclear risk of bias overall.
Measures of treatment effect
For Part 1, where possible we extracted smoking outcomes as continuous biochemically‐confirmed abstinence, but we accepted less strict definitions if confirmed continuous abstinence was not available. Abstinence rates and their corresponding risk ratio (95% CI) were reported at six and 12 months of follow‐up. We extracted adverse and serious adverse events for pharmacotherapy studies in Part 1 as the number of people experiencing an event, where these data were available. For studies in Part 2, we extracted data on weight change only.
We used the absolute mean (SD) difference in body weight (kgs) from baseline to follow‐up by trial arm as a summary statistic for the treatment effect on weight. We estimated mean weight change only in those abstinent from smoking.
In some studies in Part 1 and 2, more than one trial arm had been compared with a control arm. Where this was the case, we took one of two approaches: where it was inappropriate to combine arms, we analyzed results separately and report them as such. Where appropriate, to create one comparison intervention arm we combined outcome data. For smoking we added together the numerator and denominator from each arm. We calculated weight outcomes from more than one trial arm using the following formulas:
Meanc = ((Mean1*n1)+(Mean2*n2))/(n1+n2) Standard deviation = √varc
√varc= (sumsqc ‐ (nc * (Meanc2)))/(nc‐1)
sumsqc= (((n1‐1)*(var1 + ((n1/n1‐1))*(mean12) + ((n2‐1)*(var2 + ((n2/n2‐1))*(mean22))
Key: Meanc= Combined mean; sumsq = sum of squares; var = variance
Unit of analysis issues
The cluster‐randomized trial included reported results adjusted for intra‐class correlation; we use these adjusted estimates in our analysis. All other trials were individually randomized and hence we did not encounter issues with unit of analysis.
Dealing with missing data
We checked that, for smoking abstinence estimates, participants lost to follow‐up were coded as continuing to smoke and therefore all randomized participants were included in the denominator; if not, we corrected abstinence rates for this. Where weight gain had been measured but not reported at all or in full, we contacted authors or sponsors for clarification. If insufficient data were available for meta‐analysis, we reported results narratively. As outlined below, where possible, we converted data for use in our meta‐analysis.
In some studies mean (SD) weight change by trial arm was not reported in full. When the standard deviations for the changes in body weight were not present, we used several methods to calculate them using standard formulas, depending on the information available. This was mainly derived from confidence intervals and standard errors. To calculate standard deviations of the changes in weight from their associated confidence intervals for studies with a large sample size, we used the following formula:
SD = (√(n) x (upper limit ‐ lower limit)) /standard error
For studies with 95% confidence intervals for difference in means we divided by 3.92 standard errors wide. If sample size was less than 60, the 3.92 standard error wide was replaced with numbers specific to both the t‐distribution and the group sample size minus 1.
To calculate standard deviation from standard error we used the following formula:
SD = SE x √(n)
When the absolute mean differences in body weight were not reported explicitly, we calculated them by subtracting the baseline mean weights from the post‐intervention mean weights for the intervention and control groups. We calculated SDs by using an estimated correlation coefficient of 0.99, which describes how similar the baseline and finishing weight were across participants. This was estimated in abstinent smokers from raw data that we have collected from a trial to prevent weight gain on smoking cessation (Parsons 2009a) and from any other included studies that report standard deviations for mean weight at baseline, final measurement, and changes in means. To estimate the correlation coefficient for the intervention and control groups from other studies reporting starting and finishing means with SDs, we used the following formula:
r = (SD (B)2 + SD (F)2 ‐ SD (C)2) / (2 X SD(B) X SD (F))
(where r = correlation coefficient, SD = standard deviation for the changes in means, B = baseline, F = final measurement, and C = change in mean weight measurement).
The imputed correlation coefficient was used to calculate the missing standard deviations for changes in means for the intervention and control groups by using the following formula:
SD (C) = √((SD (B)2 + SD (F)2) ‐ (2 X r X SD (B) X SD (F))
Assessment of heterogeneity
We used the I2 statistic to investigate statistical heterogeneity, given by the formula [(Q‐df)/Q] x 100%], where Q is the Chi2 statistic and df is its degrees of freedom.
Assessment of reporting biases
We created funnel plots to visually investigate possible publication bias for meta‐analyses with 10 or more studies.
Data synthesis
Smoking cessation outcome data are given based on the number of quitters in the treatment and control groups divided by the total number of participants receiving treatment. Adverse and serious adverse event data are given as the number of people experiencing an event divided by the total number of participants followed up at the relevant time point. Results for both are reported as risk ratios (RRs) with 95% confidence intervals (CIs). A risk ratio greater than 1.0 indicates that more people quit in the treatment group than in the control group, or that more people experienced an adverse event. We used the Mantel‐Haenszel random‐effects method for smoking cessation and adverse event outcomes where appropriate. Weight change outcome data are given as the difference in mean weight change between the intervention and control arms, and we combined estimates using the inverse variance method where appropriate.
Subgroup analysis and investigation of heterogeneity
Studies were subgrouped based on intervention type.
Sensitivity analysis
Following the standard approach of the Cochrane Tobacco Addiction Group, we conducted sensitivity analyses removing studies at high risk of bias.
Summary of findings and assessment of the certainty of the evidence
Following standard Cochrane methodology, we created summary of findings tables for the following comparisons, agreed prior to beginning this update, using GRADEpro GDT:
Weight management education versus no weight intervention
Personalized weight management support versus no weight intervention
Interventions to allay concerns about weight gain versus no weight intervention
Exercise interventions versus no exercise intervention for smoking cessation
Nicotine replacement therapy versus placebo for smoking cessation
Varenicline versus placebo for smoking cessation
Fluoxetine versus placebo for smoking cessation
We selected these comparisons a priori as being the most clinically relevant. In the summary of findings tables, we present data on our primary outcomes (cessation and weight change) for these main comparisons. Also following standard Cochrane methodology, we used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome, and to draw conclusions about the certainty of evidence within the text of the review.
Results
Description of studies
Studies are described by part below. Part 1 represents studies of interventions specifically designed to address post‐cessation weight gain. Part 2 represents studies of smoking cessation interventions not specifically designed to address post‐cessation weight gain.
Results of the search
Part 1
For Part 1 of this update, our 2020 searches identified 379 non‐duplicate references (Figure 1). All references were then screened and 70 full‐text articles were retrieved. For this update, we identified 21 new included studies and 10 new ongoing studies (Characteristics of ongoing studies). In total, we now include 37 studies in Part 1, i.e. 21 new included studies and 16 included studies identified in the 2012 review.
1.
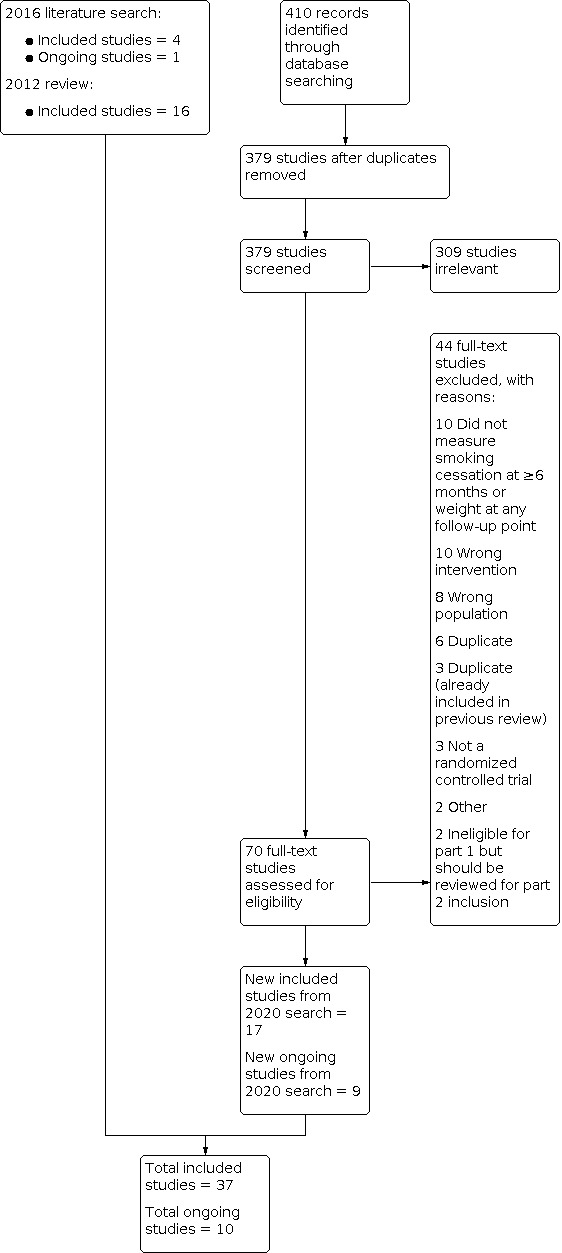
Study flow diagram for Part 1 2020 search plus studies from 2016 search and the 2012 review
Part 2
For Part 2 of this update, our 2020 searches identified 1099 non‐duplicate references (Figure 2). All references were then screened and 433 full‐text articles were retrieved. We identified 27 new included studies and 10 new ongoing studies for this update (Characteristics of ongoing studies). In total, we now include 83 studies in Part 2. Four trials are also included in Part 1 that contributed data to Part 2 (1 Cooper 2005 (also Part 2); 1 Levine 2010 (also Part 2); 1 Pirie 1992 (also Part 2); 1 Spring 1995 (also Part 2))
2.
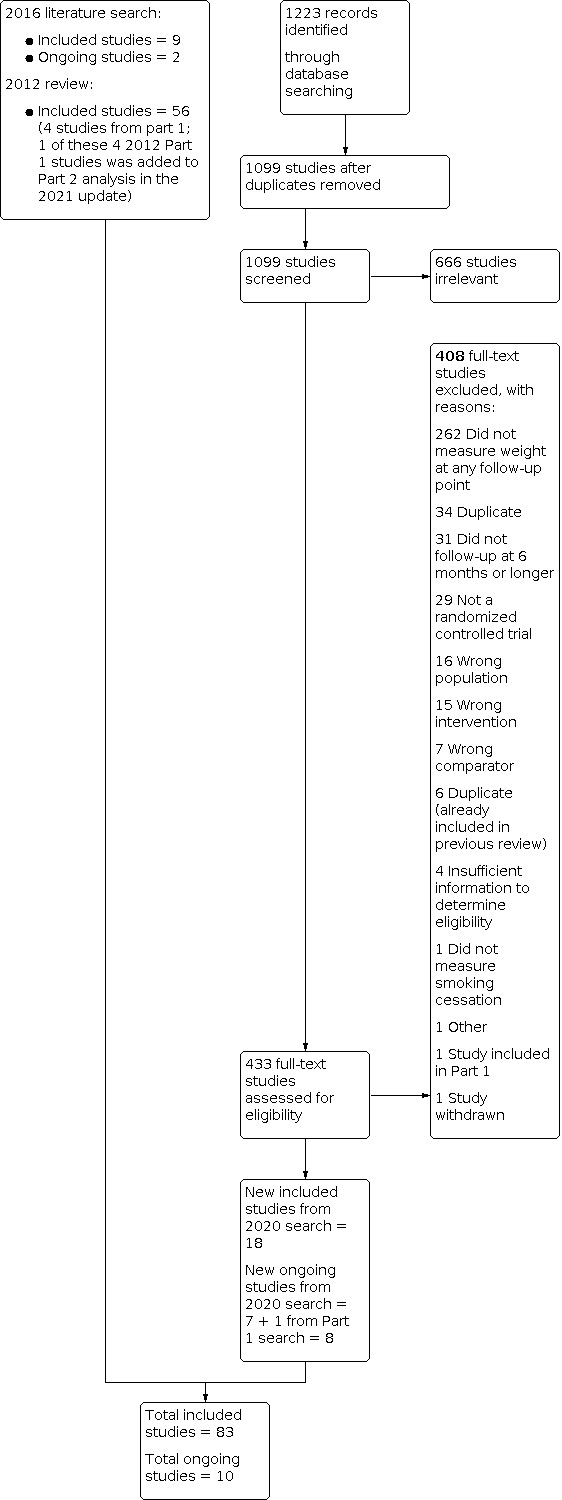
Study flow diagram for Part 2 2020 search plus studies from 2016 search and the 2012 review
Included studies
Main features of studies included in Part 1 and Part 2 are summarized below, and further details on each included study can be found in the Characteristics of included studies table.
Participants
Part 1
A total of 11,514 participants were enrolled in the 37 included studies. Three‐quarters of the studies (27 studies) were undertaken in the USA, three were conducted in England, and one each in Australia, Canada, China, Denmark, Norway, Scotland, and Sweden. A median age of 45.5 years was reported across 27 of the 37 included studies, and a median body mass index (BMI) of 28.5 kg/m2 was reported across the 20 studies which provided these data at baseline. The median percentage of women across 35 studies reporting it was 75.4%, with 14 studies recruiting only women (1 Bloom 2020; 1 Cooper 2005 (also Part 2); 1 Copeland 2006; 1 Copeland 2015; 1 Danielsson 1999; 1 Klesges 1990; 1 Levine 2010 (also Part 2); 1 NCT03528304 2016; 1 Oncken 2019; 1 Perkins 2001; 1 Pirie 1992 (also Part 2); 1 Prapavessis 2018; 1 Spring 1995 (also Part 2); 1 Spring 2004). Participants smoked a median of 20 cigarettes per day at baseline, as reported across 26 studies. A median of 5.2 was scored on the Fagerström Test for Nicotine Dependence across the 12 studies that reported it.
Part 2
A total of 46,248 participants were enrolled in the 83 studies included in Part 2 of this review. Nearly half of the studies (39 studies) were conducted in the USA, and a further 15 studies were conducted across multiple countries. Four studies each were conducted in Sweden and England, three each in Switzerland and France and two each in Australia and Denmark. A single study was conducted each in Canada, China, Finland, Greece, India, Iceland, New Zealand, Japan, South Africa, Spain and Turkey.
Of the 83 included studies, 64 reported age, median 43 years, and 14 studies reported baseline BMI, median 27.2 kg/m2.The median percentage of women was 55% as reported across 71 studies, with eight of these studies recruiting only women. Participants smoked a median of 22 cigarettes per day at baseline (across 57 studies) and a median of 5.6 was scored on the Fagerström Test for Nicotine Dependence (22 studies).
Interventions and comparators
Part 1
Behavioural interventions
Twenty‐three studies assessed the effects of a behavioural intervention to prevent weight gain after a smoking cessation attempt.
Three trials examined an intervention consisting of education on weight management (1 Hall 1992; 1 Hankey 2009; 1 Pirie 1992 (also Part 2)) against standard smoking cessation support. Seven trials examined the effects of personalized weight management against usual smoking cessation care (1 Bush 2012; 1 Bush 2018; 1 Johnson 2017; 1 Lycett 2020; 1 Sobell 2017; 1 Spring 2004; 1 Hall 1992). One study tested the efficacy of a very low calorie diet (VLCD), meaning food replacements providing less than 800 kcal/day, where participants in the intervention and control groups both received the weight management education as well as usual smoking cessation support. Both groups were advised to follow a 1600 kcal diet, while the intervention group received two two‐week blocks of a VLCD provided free of charge. Treatment took place in a specialist obesity treatment centre (1 Danielsson 1999). One study compared 16 face‐to‐face one‐hour motivational interviewing and cognitive behaviour therapy (CBT) counselling sessions, given mostly by telephone (1 Baker 2018).
Four studies compared the use of CBT to promote acceptance of moderate weight gain to no behavioural weight advice. (1 Bloom 2020; 1 Levine 2010 (also Part 2); 1 Perkins 2001; 1 White 2019). One of these studies was a three‐arm RCT (1 Perkins 2001) and was also included in the aforementioned comparison on personalized weight management support versus no weight intervention.
Three studies new to this update directly compared behavioural weight management interventions. 1 Prapavessis 2018 tested an exercise maintenance condition with contact control to contact control alone, which included a behavioural weight management component, while 1 Heggen 2016 compared a low‐carbohydrate diet to a moderately reduced‐fat diet. 1 Copeland 2015 compared a minimally‐tailored group intervention which provided information on smoking and weight with a highly tailored, multidisciplinary individual approach, but could not be included in the statistical analysis as no measures of variance were reported alongside weight‐change data.
Five more studies could not be included in the statistical analyses. 1 Oncken 2019 tested the effect of 30 supervised exercise group sessions compared to relaxation group sessions. 1 Vander Weg 2016 compared a Quitline referral to a tailored tobacco intervention in which eligible participants who were worried about weight gain were offered support for weight management. Trial arms of interest in 1 NCT03528304 2016 compared a 16‐week culturally‐tailored contingency management intervention for smoking abstinence and weight loss to no intervention. 1 Lycett 2010 compared a VLCD to an individual dietary and activity‐planning intervention either begun at baseline or at eight weeks post‐quit. Finally, one study compared the effect of group to individual relapse‐prevention follow‐up sessions on smoking cessation and weight change after a two‐week smoking cessation programme (1 Copeland 2006). Results from this study are also reported narratively.
Pharmacological interventions
Fourteen studies compared the effects of pharmacological interventions to placebo on smoking cessation and post‐cessation weight change.
Pharmacological interventions included 8.33 mg phenylpropanolamine gum 16 pieces/day for 8 weeks (1 Cooper 2005 (also Part 2)), 9 pieces/day for 2 weeks (1 Klesges 1990) and up to 10 pieces/day for 4 weeks (1 Klesges 1995); 20 mg ephedrine plus 200 mg caffeine 3/day for 12 weeks (1 Norregaard 1996), 100, 50 and 25 mg/day naltrexone for 6 weeks (1 O'Malley 2006), 50 mg/day naltrexone for 12 weeks (1 King 2012), 25 mg/day naltrexone for 26 weeks (1 Toll 2010), 100 mg/day topiramate (up‐titrated over 5 weeks) for 10 weeks (1 Oncken 2014), 400 mg/day chromium polynicotinate for 14 weeks (1 Parsons 2009) and 30 mg/day dexfenfluramine for 12 weeks (1 Spring 1995 (also Part 2)). This study also examined the efficacy of 40 mg/day of fluoxetine for preventing weight gain (1 Spring 1995 (also Part 2)). As the other fluoxetine studies were included in Part 2 of the reviews, this comparison is described in Part 2.
1 Rose 2019 provided two weeks of lorcaserin or placebo during the two‐week pre‐quit period, followed by an identical treatment regimen of lorcaserin plus a nicotine patch for 12 weeks post‐quit date. Lorcaserin was also tested at 10 mg once or twice daily for 12 weeks in both 1 Shanahan 2017 and 1 Wilcox 2016, but data on the latter study were limited due to extraction from a conference abstract. Finally, 1 Lyu 2018 investigated a combination of adjunctive naltrexone (25 mg/day) and bupropion (300 mg/day) treatment for 24 weeks.
Part 2
Of the 83 included studies, 71 provided sufficient data to include in the meta‐analysis. The outstanding studies were new to this update and measured weight at eligible time points, but data were not provided in a form that we could meta‐analyze.
Of the 71 studies, 34 tested interventions with NRT, 19 with antidepressants, 17 with varenicline, four with exercise and two with electronic cigarettes. Five of these studies included multiple intervention arms or a combination of these interventions.
Nicotine replacement therapy was delivered in various forms, with most studies using a nicotine patch, while other studies delivered nicotine in the form of gum, lozenges, sublingual tablets, inhalers and intranasal spray. Some studies tested patches with varying nicotine dosing regimens, which were assessed separately.
Nineteen studies on antidepressants were included in this review, three of which compared bupropion to varenicline as well as placebo (2 Gonzales 2006; 2 Jorenby 2006; 2 Nides 2006). Overall, 14 studies compared weight change in participants treated with bupropion to placebo (2 Gonzales 2006; 2 Hurt 1997; 2 Jorenby 2006; 2 Nides 2006;2 Piper 2007; 2 Rigotti 2006; 2 Simon 2004; 2 Simon 2009; 2 Uyar 2007; 2 Zellweger 2005; 2 Cox 2012; 2 Eisenberg 2013; 2 Piper 2009; 1 Levine 2010 (also Part 2)). One of these studies (2 Simon 2004) provided a nicotine patch to both the bupropion and placebo study arms, and two additional studies compared varenicline and bupropion to varenicline and placebo (2 Rose 2014; 2 Ebbert 2014). A further two studies compared fluoxetine to placebo (2 Niaura 2002; 2 Saules 2004). 2 Saules 2004 tested fluoxetine versus placebo; both intervention and control arms used NRT, but we included it in the analyses with other fluoxetine‐versus‐placebo studies. One other study examined the efficacy of fluoxetine versus placebo (1 Spring 1995 (also Part 2)). It was not included in the parent Cochrane Review because smoking cessation at six months was not reported, but was identified and included here. All bupropion studies administered 300 mg/day and 2 Hurt 1997 also included a 100 mg/day and 150 mg/day arm. For the main comparison, we use the 300 mg/day arm for 2 Hurt 1997 and we use the lower‐dose arms to compare to the standard 300 mg/day treatment to the lower‐dose arms. Two fluoxetine studies compared two dosing levels (30 mg and 60 mg/day (2 Niaura 2002) and 20 mg and 40 mg/day (2 Saules 2004)) which we combined for the main comparison, while the lower doses and higher doses were compared in a separate comparison to examine for a dose‐dependent effect. One other study examined 40 mg fluoxetine versus placebo (1 Spring 1995 (also Part 2)).
Fourteen studies included in this review compared varenicline to placebo, with some studies including additional intervention components (e.g. patch) which were balanced between study arms. Once again, some studies compared different dosing regimens which were assessed in additional analyses. One study compared 2 mg/daily varenicline to a 21 mg patch tapering to 7 mg (2 Aubin 2008). As mentioned above, 2 Gonzales 2006; 2 Jorenby 2006; 2 Nides 2006 also compared varenicline with bupropion, while 2 Ioakeimidis 2018 compared varenicline with electronic cigarette (12 mg/ml nicotine) use for 12 weeks.
We found no new studies on exercise interventions for smoking cessation for inclusion in this update. In the four original studies, all participants in the treatment arm received an exercise component in parallel with cognitive behavioural treatment for smoking cessation, which was supplemented with nicotine replacement therapy in 2 Ussher 2003 and 2 Bize 2010. The exercise component included supervised exercise in three studies. 2 Marcus 1999 tested three supervised exercise sessions/week for 12 weeks, 30 to 40 minutes resting heart rate plus 60% to 85% heart reserve; 2 Marcus 2005 tested one supervised, four unsupervised exercise sessions/week for eight weeks, at least 30 minutes at resting heart rate plus 45% to 59% heart reserve; and 2 Bize 2010 tested moderate‐intensity (40% to 60% of maximal aerobic power) group‐based cardiovascular (CV) activity under the supervision of a trained monitor for 45 minutes weekly for nine weeks. In contrast, 2 Ussher 2003 compared the effect of seven weeks of exercise counselling to participants receiving a smoking cessation intervention with brief health education.
Finally, two new studies investigated the use of electronic cigarettes. 2 Walker 2020 was a three‐arm RCT comparing a nicotine patch versus nicotine‐containing electronic cigarette plus patch versus nicotine‐free electronic cigarette plus patch. 2 Ioakeimidis 2018, mentioned above,compared varenicline with electronic cigarette (12 mg/ml nicotine) use for 12 weeks.
Further information on dose or length or both of all interventions outlined above are available in the Characteristics of included studies table. Weight change from baseline in all of the studies included in the second part of the review was measured in abstainers only. Definition of abstinence varied between studies as in Part 1, and is also noted in the Characteristics of included studies table. Data for some time points were received following requests made to study authors, which is also noted in the Characteristics of included studies table.
Outcomes
Part 1
Of the 37 included studies:
28 reported data on weight change at end of treatment (EOT), six months, 12 months and/or at the longest follow‐up time point (data for three studies described narratively)
25 reported data on abstinence at EOT, six months, 12 months and/or at the longest follow‐up time point (data for one study are described narratively)
10 pharmacotherapy trials reported adverse or serious adverse outcomes, or both, six of which are described narratively.
Part 2
Of the 83 included studies:
71 reported data on weight change at EOT, six months or 12 months in sufficient detail to be included in the meta‐analysis.
Excluded studies
We list 200 studies excluded at full‐text stage along with reasons in Characteristics of excluded studies. The most common reasons for exclusion in the 2020 search were not measuring any of our outcomes (10 studies) or testing ineligible interventions (10 studies).
Risk of bias in included studies
Part 1: Overall, of the 37 included studies, we judged five studies to be at low risk of bias, 17 to be at unclear risk and the remaining 15 studies at high risk.
Part 2: Of the 83 studies not designed to address post‐cessation weight gain included in Part 2, we judged overall risk of bias to be low for 13 studies, unclear for 46 studies, and high for 24 studies.
Details of risk of bias judgements for each domain of each included study can be found in the Characteristics of included studies table and are illustrated in Figure 3.
3.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
For Part 1. we judged 10 studies to be at low risk of selection bias, and 27 studies to be at unclear risk of bias mainly due to limited information on allocation concealment.
A similar risk of selection bias for all studies included in Part 2. We judged 27 studies to be at low risk, 55 studies to be at unclear risk of bias, and one study at high risk of bias (2 Jodar‐Sanchez 2018). Most studies were again rated at unclear risk of bias due to insufficient information about allocation concealment.
Blinding
Of the 37 studies included in Part 1, we judged 11 out of 14 pharmacological studies to be at low risk of both performance and detection bias and the remaining three studies at unclear risk of bias. We judged 19 of the 23 behavioural studies to be at low risk of detection bias, one at unclear risk and three at high risk of detection bias; performance bias was not assessed in included studies on behavioural interventions.
Of the 83 studies included in Part 2, 74 tested a pharmacological intervention. Of these 74 studies, 29 were judged to be at low risk of both performance and detection bias, 34 at unclear risk and 11 at high risk. Many of these studies were rated at unclear or high risk of bias due to a lack of blinding when blinding was possible or due to insufficient information about blinding, mainly by not specifying who within the study were blinded. As with Part 1, performance bias was not assessed in the remaining nine behavioural studies included in Part 2. Of these nine studies, eight were judged to be at low risk of detection bias, while one study was rated at unclear risk because it did not specify how weight was measured.
Incomplete outcome data
We rated 16 out of the 37 studies included in Part 1 at low risk of attrition bias. We rated 14 studies with greater than 50% loss to follow‐up overall or a follow‐up difference of more than 20% between study arms, or both, at high risk of attrition bias. The remaining seven studies were judged to at unclear risk.
Similarly, of the 83 studies included in Part 2, most (49 studies) were judged to be at low risk of attrition bias. Twenty‐one studies were rated at unclear risk and 13 studies at high risk.
Other potential sources of bias
No studies in Part 1 were judged to be at unclear or high risk of other bias.
2 Bernard 2015 from Part 2 was rated at unclear risk of other bias as participants in the control group may have participated in exercise (contamination effect).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Part 1
Effects of pharmacological interventions to prevent post‐cessation weight gain versus placebo
Weight change
For three pharmacotherapies, there was evidence of reduced weight gain at end of treatment (EOT), and confidence intervals (CIs) excluded no difference (Analysis 1.1):
1.1. Analysis.

Comparison 1: Pharmacological interventions versus placebo for post cessation weight control, Outcome 1: Mean weight change (kg) at end of treatment
Dexfenfluramine: mean difference (MD) −2.50 kg, 95% confidence interval (CI) −2.98 to −2.02; 1 study at high risk of bias, 33 participants
Phenylpropanolamine (PPA): MD −0.50 kg, 95% CI −0.80 to −0.20; I2 = 0%; 3 studies, 112 participants; results not sensitive to removal of one study at high risk of bias
Naltrexone: MD −0.91 kg, 95% CI ‐1.49 to ‐0.34; I2 = 0%; 3 studies, 254 participants; results not sensitive to removal of one study at high risk of bias
For a further three pharmacotherapies, point estimates suggested reduced weight gain at end of treatment, but CIs included the possibility of no difference (Analysis 1.1):
Ephedrine + caffeine: MD −1.30 kg, 95% CI −2.87 to 0.27; 1 study at high risk of bias, 40 participants
Lorcaserin: MD −1.14 kg, 95% CI −3.65 to 1.37; 1 study at low risk of bias, 41 participants
Chromium: MD −0.81 kg, 95% CI −3.05 to 1.43; 1 study at high risk of bias, 15 participants
The one study of topiramate did not have any quitters in the placebo arm and hence an effect estimate could not be calculated (1 Oncken 2014). A further study comparing different treatment regimens of lorcaserin did not provide data by treatment arm (1 Rose 2019).
Four pharmacotherapies were tested in trials that reported at six (Analysis 1.2) and 12 months (Analysis 1.3). All had point estimates suggesting benefit, but only one study contributed data at each time point and in all cases CIs were wide and included no difference:
1.2. Analysis.

Comparison 1: Pharmacological interventions versus placebo for post cessation weight control, Outcome 2: Mean weight change (kg) at 6 months
1.3. Analysis.

Comparison 1: Pharmacological interventions versus placebo for post cessation weight control, Outcome 3: Mean weight change (kg) at 12 months
PPA 6 months: MD −2.06, 95% CI −5.56 to 1.44; 12 months MD −1.04, 95% CI −5.03 to 2.95; 38 participants; at unclear risk of bias
Ephedrine + caffeine 6 months: MD −0.70 kg, 95% CI −2.72 to 1.32; 32 participants; 12 months MD 1.20 kg, 95% CI −1.84 to 4.24; 24 participants; at unclear risk of bias
Chromium 6 months only: MD −3.87 kg, 95% CI −12.01 to 4.27; 9 participants; at unclear risk of bias
Naltrexone 6 months: MD −0.29 kg, 95% CI −2.23 to 1.65; 68 participants; 12 months MD −2.30 kg, 95% CI −4.92 to 0.32; 61 participants; at unclear risk of bias
Smoking cessation
For studies which measured smoking cessation at six (Analysis 1.4) or 12 months (Analysis 1.5), or both, CIs for all comparisons were wide and included no difference:
1.4. Analysis.

Comparison 1: Pharmacological interventions versus placebo for post cessation weight control, Outcome 4: Smoking cessation at 6 months
1.5. Analysis.

Comparison 1: Pharmacological interventions versus placebo for post cessation weight control, Outcome 5: Smoking cessation at 12 months
PPA 6 months: RR 1.38, 95% CI 0.76 to 2.53; 12 months RR 1.48, 95% CI 0.80 to 2.73; 1 study at unclear risk of bias, 295 participants
Ephedrine + caffeine 6 months: RR 1.06, 95% CI 0.53 to 2.11; 12 months RR 1.44, 95% CI 0.60 to 3.48; 1 study at unclear risk of bias, 225 participants
Naltrexone 6 months: RR 1.02, 95% CI 0.79 to 1.32; I2 = 0%; 3 studies, 890 participants; removing one at high risk did not impact results; 12 months: RR 1.25, 95% CI 0.67 to 2.31; 1 study at unclear risk of bias, 385 participants
Chromium 6 months only: RR 0.48, 95% CI 0.12 to 1.84; 1 study at unclear risk of bias,143 participants
Naltrexone and bupropion 6 months only: not estimable due to no quitters in either arm; 1 study at unclear risk of bias, 22 participants
Adverse and serious adverse events (SAEs)
For the most part, data were sparsely and heterogeneously reported, often precluding pooled analyses.
In both studies of lorcaserin, adverse events (AEs) were higher in the intervention arms, but CIs were wide and incorporated no difference: Analysis 1.6; compared to placebo: RR 1.12, 95% CI 0.95 to 1.32; 1 study at low risk of bias, 401 participants; longer duration compared to shorter duration: RR 1.41, 95% CI 0.88 to 2.25; 1 study at high risk of bias, 83 participants. The latter study was the only one of lorcaserin to also report measuring SAEs, with one event occurring in the control arm (Analysis 1.7).
1.6. Analysis.

Comparison 1: Pharmacological interventions versus placebo for post cessation weight control, Outcome 6: Adverse events
1.7. Analysis.

Comparison 1: Pharmacological interventions versus placebo for post cessation weight control, Outcome 7: Serious adverse events
One study of topiramate, judged to be at high risk of bias, measured SAEs, with none reported in either arm (38 participants, Analysis 1.7). In the same study (1 Oncken 2014), authors report that when examining the frequency of all adverse events, only paraesthesia was reported by more participants in the topiramate group than in the placebo group. No participants using placebo reported paraesthesia, compared to 47% (9 of 19) participants in the topiramate group (P = 0.011).
One study of naltrexone measured SAEs and found no difference: RR 0.98, 95% CI 0.14 to 6.89; 1 study at unclear risk of bias, 333 participants; Analysis 1.7. 1 King 2012 reported a higher incidence of dizziness and nausea in those randomized to naltrexone compared to placebo; these data could not be used in our statistical analysis. 1 O'Malley 2006 found no statistically significant between‐group differences in adverse events by type. 1 Toll 2010 found that "the percentage of unique participants reporting non‐serious adverse events rated moderate or severe with a prevalence of ≥5% differed by treatment group for depression and decreased appetite [in each case there were 4 (5%) naltrexone participants vs 0 (0%) placebo participants, Chi2 = 4.21, P = 0.04].”
One study of PPA reported "only one side effect"; heartburn in the placebo group (1 Klesges 1990).
In their study of dexfenfluramine and fluoxetine, 1 Spring 1995 (also Part 2) detected no differences in number of participants stopping treatment due to adverse events.
1 Norregaard 1996, (ephedrine + caffeine) reported a higher incidence of palpitations, sweating, dizziness and nausea in the intervention than in the control group.
No other studies reported data on adverse or serious adverse events.
Effects of behavioural interventions to prevent post‐cessation weight gain
Compared to no support
There was no evidence at any follow‐up that weight management education alone reduced weight gain: At EOT MD −0.04 kg, 95% CI −0.57 to 0.50; I2 = 0%; 2 studies, 140 participants; at 6 months MD 0.89, 95% CI −0.78 to 2.55; I2 = 0%; 2 studies, 81 participants; and 12 months MD −0.21 kg, 95% CI −2.28 to 1.86; I2 = 0%; 2 studies, 61 participants (Analysis 2.1; Analysis 2.2; Analysis 2.3). Results were not sensitive to removing the one study at high risk of bias. At six months, there was no evidence of a difference in quit rates, but CIs incorporated clinically significant benefit and clinically significant harm: RR 1.02, 95% CI 0.78 to 1.33; I2 = 9%; 3 studies, 660 participants; results not sensitive to exclusion of one study at high risk of bias; Analysis 2.4. However, at 12 months there were fewer quitters in the weight‐management education group, with CIs excluding no difference: RR 0.66, 95% CI 0.48 to 0.90; I2 = 0%; 2 studies, 522 participants; results not sensitive to removal of one study at high risk of bias; Analysis 2.5.
2.1. Analysis.

Comparison 2: Behavioural weight management interventions versus advice or no intervention for post‐cessation weight control, Outcome 1: Mean weight change (kg) at end of treatment
2.2. Analysis.

Comparison 2: Behavioural weight management interventions versus advice or no intervention for post‐cessation weight control, Outcome 2: Mean weight change (kg) at 6 months
2.3. Analysis.

Comparison 2: Behavioural weight management interventions versus advice or no intervention for post‐cessation weight control, Outcome 3: Mean weight change (kg) at 12 months
2.4. Analysis.

Comparison 2: Behavioural weight management interventions versus advice or no intervention for post‐cessation weight control, Outcome 4: Smoking cessation at 6 months
2.5. Analysis.

Comparison 2: Behavioural weight management interventions versus advice or no intervention for post‐cessation weight control, Outcome 5: Smoking cessation at 12 months
Personalized weight‐management support programmes reduced weight gain at end of treatment: MD −1.11 kg, 95% CI −1.93 to −0.29; I2 = 0%; 3 studies, 121 participants; results not sensitive to the removal of the two studies at high risk of bias; Analysis 2.1, with CIs excluding no difference. Weight gain was also reduced relative to control at six months: MD −0.96 kg, 95% CI −2.18 to 0.25; I2 = 46%; 5 studies, 816 participants; results not sensitive to removal of two studies at high risk of bias; Analysis 2.2; and at 12 months: MD −0.44 kg, 95% CI −2.34 to 1.46; I2 = 41%; 4 studies, 530 participants; results not sensitive to exclusion of two studies at high risk of bias; Analysis 2.3, but CIs incorporated no difference. As with weight‐management education, at six months there was no evidence of difference in quit rates: RR 0.95, 95% CI 0.82 to 1.10; I2 = 33%; 7 studies, 5517 participants; results not sensitive to removal of five studies at high risk of bias; Analysis 2.4, but significantly fewer people had quit in the intervention arm at 12 months: RR 0.65, 95% CI 0.45 to 0.92; I2 = 89%; 5 studies, 3441 participants; removal of three studies at high risk of bias widened CIs to include no difference but did not substantially alter the point estimate; Analysis 2.5. 1 Sobell 2017 did not report data in a way that could be used in our analyses, but reported no differences in weight between groups. In 1 Vander Weg 2016, participants in the intervention arm could choose to engage in a weight‐management intervention; results were not reported by randomized group.
Comparisons between weight‐management interventions
The within‐study comparison from 1 Hall 1992 suggested that personalized weight management support is more effective than educating participants about weight management at the end of smoking cessation treatment: MD −1.12 kg, 95% CI −2.17 to −0.07; Analysis 3.1, and at 12 months: MD −2.49 kg, 95% CI −5.51 to 0.53; Analysis 3.3. There was no difference in smoking cessation at six or 12 months, although again CIs were wide (Analysis 3.4; Analysis 3.5).
3.1. Analysis.

Comparison 3: Direct comparisons between behavioural weight management interventions, Outcome 1: Mean weight change (kg) at end of treatment
3.3. Analysis.

Comparison 3: Direct comparisons between behavioural weight management interventions, Outcome 3: Mean weight change (kg) at 12 months
3.4. Analysis.

Comparison 3: Direct comparisons between behavioural weight management interventions, Outcome 4: Smoking cessation at 6 months
3.5. Analysis.

Comparison 3: Direct comparisons between behavioural weight management interventions, Outcome 5: Smoking cessation at 12 months
Data from 1 Danielsson 1999 (unclear risk of bias) showed the benefit of VLCD at end of treatment: MD −3.70, 95% CI −4.82 to −2.58; 121 participants 121; Analysis 3.1; and at 12 months: MD −1.30 kg, 95% CI −3.49 to 0.89; 62 participants; Analysis 3.3; although in the latter case CIs included no difference. This intervention improved abstinence at 12 months with CIs excluding no difference: RR 1.73, 95% CI 1.10 to 2.73, Analysis 3.5.
One study compared providing weight‐management support at the start of a quit attempt versus some weeks after attaining abstinence. Confidence intervals were wide and included no difference in weight at end of treatment: 1 Spring 2004; unclear risk of bias; Analysis 3.1; and at six months Analysis 3.2. Cessation not measured. One study compared 16 x one‐hour counselling sessions comprising motivational interviewing and CBT with 16 x 10‐minute telephone calls aiming to reduce weight gain after cessation (1 Baker 2018; low risk of bias). There was no evidence of benefit for weight but outcomes were imprecisely estimated; Analysis 3.3; Analysis 3.5. One study compared advice to follow a low‐carbohydrate diet with advice to follow a low‐fat one and found no evidence of a difference in weight: 1 Heggen 2016, low risk of bias, Analysis 3.1; Analysis 3.2; Analysis 3.4.
3.2. Analysis.

Comparison 3: Direct comparisons between behavioural weight management interventions, Outcome 2: Mean weight change (kg) at 6 months
1 Prapavessis 2018 (high risk of bias, 41 participants) randomized participants to an intensive exercise programme or no exercise support. There was no evidence of differences in cessation (Analysis 3.4; Analysis 3.5); weight was not measured. We include this trial in Part 1 as the intervention was delivered specifically in the context of reducing weight gain. 1 Copeland 2006 and 1 Copeland 2015 randomized menopausal women to either group‐based education on CBT principles for weight management or to CBT based on individualized counselling based on questionnaire scores. They found no evidence that weight differed by end of treatment. :A further study compared a very low calorie diet to an individualized diet and exercise plan which began either at baseline or eight weeks post quit‐date (step‐by‐step group), but all participants in two of the three study arms had relapsed or dropped out of the study by end of treatment (1 Lycett 2010). Average weight change in abstinent smokers in the step‐by‐step study arm was 1.15 (SD 2.22) kg (4 participants) at end of treatment and no one in the other groups was abstinent and provided weight data .
Effects of acceptance interventions for weight concern
There was mixed evidence that CBT to support acceptance of weight gain affected weight after stopping smoking. Statistical heterogeneity for weight at all time points precluded statistical synthesis (I2 > 70% in all cases; Analysis 4.1; Analysis 4.2; Analysis 4.3). This heterogeneity was driven by the two studies in which no pharmacotherapy was provided; 1 Levine 2010 (also Part 2) (42 participants) found a point estimate favouring control at all time points, and 1 Perkins 2001 (63 participants) found a point estimate favouring the intervention at all time points; both excluded no difference at end of treatment (Analysis 4.1). Removing the one study at high risk of bias (1 Perkins 2001) did not meaningfully reduce statistical heterogeneity at end of treatment. However, at six months and 12 months, removing this study reduced statistical heterogeneity to 0%. At both time points, results from sensitivity analyses favoured control arms but CIs were wide and for 12 months incorporated no difference: six months: MD 0.89, 95% CI 0.39 to 1.40; 2 studies, 77 participants, at unclear risk of bias; Analysis 4.2; MD 0.37, 95% CI −0.50 to 1.24; 1 study, 54 participants at unclear risk of bias; Analysis 4.3. One further study (1 White 2019) did not provide data in a way that could be incorporated into the meta‐analysis, but weight gain was reduced in the intervention compared to control group.
4.1. Analysis.

Comparison 4: Acceptance interventions for weight concern, Outcome 1: Mean weight change (kg) at end of treatment
4.2. Analysis.

Comparison 4: Acceptance interventions for weight concern, Outcome 2: Mean weight change (kg) at 6 months
4.3. Analysis.

Comparison 4: Acceptance interventions for weight concern, Outcome 3: Mean weight change (kg) at 12 months
There was some evidence that interventions that promoted acceptance of weight gain increased smoking abstinence at six and 12 months. At six months the RR was 1.42, 95% CI 1.03 to 1.96; I2 = 21%; 4 studies, 619 participants; removing two at unclear risk of bias decreased the point estimate and led to CIs incorporating no difference; Analysis 4.4. At 12 months, the RR was 1.25, 95% CI 0.76 to 2.06; ; I2 = 26%; 2 studies, 496 participants; removing one at unclear risk of bias decreased the point estimate; Analysis 4.5.
4.4. Analysis.

Comparison 4: Acceptance interventions for weight concern, Outcome 4: Smoking cessation at 6 months
4.5. Analysis.

Comparison 4: Acceptance interventions for weight concern, Outcome 5: Smoking cessation at 12 months
Part 2
Effect of antidepressants on post‐cessation weight gain
Bupropion (300 mg/day) limited post‐cessation weight gain compared with placebo at the end of treatment, with CIs excluding no difference: MD −1.01 kg, 95% CI −1.35 to −0.67; I2 = 3%; 10 studies, 1098 participants; results not sensitive to removal of one study at high risk of bias; Analysis 5.1. A funnel plot showed no evidence of asymmetry (Figure 4). At six and 12 months the reduction in weight was lower than at end of treatment and CIs incorporated no difference: six months: MD −0.38 kg, 95% CI −1.19 to 0.44; I2 = 0%; 7 studies, 420 participants; results not sensitive to removal of two studies at high risk of bias; Analysis 5.3; 12 months: MD −0.26 kg, 95% CI −1.31 to 0.78; I2 = 0%, 7 studies, 471 participants; results not sensitive to removal of two studies at high risk of bias; Analysis 5.5. There was no evidence of a dose‐dependent response for bupropion at end of treatment, six or 12 months (Analysis 5.2, Analysis 5.4, Analysis 5.6), with wide CIs consistent with both benefit and harm. In a further study of bupropion, 2 Ebbert 2014 (low risk of bias), all participants received varenicline. At end of treatment (243 participants), the point estimate favoured bupropion and CIs excluded no difference; at six and 12 months the point estimate still favoured bupropion but CIs included no difference (Analysis 5.1; Analysis 5.3; Analysis 5.5).
5.1. Analysis.

Comparison 5: All types of antidepressant versus placebo for smoking cessation, Outcome 1: Mean weight change (kg) at end of treatment
4.

Funnel plot of comparison: 5 All types of antidepressant versus placebo for smoking cessation, outcome: 5.1 Mean weight change (kg) at end of treatment.
5.3. Analysis.

Comparison 5: All types of antidepressant versus placebo for smoking cessation, Outcome 3: Mean weight change (kg) at 6 months
5.5. Analysis.

Comparison 5: All types of antidepressant versus placebo for smoking cessation, Outcome 5: Mean weight change (kg) at 12 months
5.2. Analysis.

Comparison 5: All types of antidepressant versus placebo for smoking cessation, Outcome 2: Mean weight change (kg) at end of treatment: dose response
5.4. Analysis.

Comparison 5: All types of antidepressant versus placebo for smoking cessation, Outcome 4: Mean weight change (kg) at 6 months: dose response
5.6. Analysis.

Comparison 5: All types of antidepressant versus placebo for smoking cessation, Outcome 6: Mean weight change (kg) at 12 months: dose response
The one study comparing post‐cessation weight gain in bupropion versus NRT measured weight change at 12 months and detected no evidence of difference (2 Piper 2009, unclear risk of bias, 115 participants; Analysis 7.1).
7.1. Analysis.

Comparison 7: Bupropion versus NRT, Outcome 1: Weight change (kg) at 12 months
Fluoxetine reduced weight gain at end of treatment: MD −1.01 kg, 95% CI −1.49 to −0.53; I2 = 38%; 2 studies, 144 participants; results not sensitive to removal of one study at high risk of bias, Analysis 5.1. At six months, statistical heterogeneity was substantial (I2 = 76%) and hence we do not present pooled results, but results from the two studies contributing data (both at unclear risk of bias) had CIs incorporating no difference (Analysis 5.3), Two studies of fluoxetine randomized participants to higher and lower doses as well as to placebo (2 Niaura 2002 to 60 mg and 30 mg and 2 Saules 2004 to 40 mg or 20 mg). There was no evidence that higher doses were more effective at six months and in fact people randomized to 60 mg had significantly greater weight gain at six months than people randomized to 30 mg, an effect not seen in the 40 mg versus 20 mg comparison (Analysis 5.4).
Effect of exercise interventions on post‐cessation weight gain
Neither individual nor pooled data for the four trials of exercise programmes showed any reduction in weight gain at the end of the programme (Analysis 6.1), with a summary estimated mean difference of MD −0.25, 95% CI −0.78 to 0.29; I2 = 0%; 4 studies, 404 participants; results not sensitive to removal of one study at high risk of bias. However, three studies (none at high risk of bias) provided data at 12 months follow‐up which when pooled showed a significant reduction in weight gain favouring treatment (Analysis 6.2), with a summary estimate of MD −2.07 kg, 95% CI −3.78 to −0.36; I2 = 0%; 3 studies, 182 participants.
6.1. Analysis.

Comparison 6: Exercise interventions versus no exercise for smoking cessation, Outcome 1: Mean weight change (kg) at end of treatment
6.2. Analysis.

Comparison 6: Exercise interventions versus no exercise for smoking cessation, Outcome 2: Mean weight change (kg) at 12 months
Effect of nicotine replacement therapy (NRT) on post‐cessation weight gain
Participants taking any type of NRT gained less weight than placebo referents at the end of treatment, with CIs excluding no difference: MD −0.52 kg, 95% CI −0.99 to −0.05; I2 = 81%; 21 studies, 2784 participants; results not sensitive to removal of eight studies at high risk of bias; Analysis 8.1. Statistical heterogeneity was substantial due to one study (2 Abelin 1989), which showed a 4.3 kg difference between weight gain in the treatment and control arms. When this study was removed, statistical heterogeneity reduced to 0% and the overall estimate decreased but CIs continued to exclude no difference: MD −0.41 kg, 95% CI −0.60 to −0.22; I2 = 0%; 20 studies, 2667 participants; Analysis 8.1. A funnel plot showed some asymmetry, suggesting that smaller studies with less weight gain in intervention groups may be missing (Figure 5). Overall, weight gain was less for those taking NRT at six and 12 months although the point estimate was reduced compared to end of treatment, and CIs included no difference: six months: MD −0.08 kg, 95% CI −0.51 to 0.35; I2 = 0%; 11 studies, 1021 participants; Analysis 8.2; and 12 months: MD −0.37 kg, 95% CI −0.86 to 0.11; I2 = 0%; 17 studies, 1463 participants; Analysis 8.3. Whereas six‐month results were not sensitive to removing the four studies at high risk of bias, removing the six studies at high risk of bias from the pooled 12‐month estimate increased the magnitude of benefit, with CIs no longer excluding no difference: MD −0.77, 95% CI −1.45 to −0.08; I2 = 0%; 11 studies, 630 participants. A funnel plot with data at six months showed some asymmetry but this time in the opposite direction to the end‐of‐treatment plot (Figure 6); at 12 months there was no asymmetry present (Figure 7). In 2 Lycett 2011 data were only available at eight‐year follow‐up, and weight change was similar between arms.
8.1. Analysis.

Comparison 8: All types of NRT versus placebo for smoking cessation, Outcome 1: Mean weight change (kg) at end of treatment
5.

Funnel plot of comparison: 8 All types of NRT versus placebo for smoking cessation, outcome: 8.1 Mean weight change (kg) at end of treatment.
8.2. Analysis.

Comparison 8: All types of NRT versus placebo for smoking cessation, Outcome 2: Mean weight change (kg) at 6 months
8.3. Analysis.

Comparison 8: All types of NRT versus placebo for smoking cessation, Outcome 3: Mean weight change (kg) at 12 months
6.

Funnel plot of comparison: 8 All types of NRT versus placebo for smoking cessation, outcome: 8.2 Mean weight change (kg) at 6 months.
7.

Funnel plot of comparison: 8 All types of NRT versus placebo for smoking cessation, outcome: 8.3 Mean weight change (kg) at 12 months.
There was no evidence from direct comparisons (Analysis 9.1; Analysis 9.2; Analysis 9.3) or from subgroup analyses that results differed by type of NRT (I2 < 10% for all tests of subgroup differences), with the exception of one study. In 2 Pack 2008 (high risk of bias, 54 participants), weight gain was less in lozenge compared to gum groups, with CIs excluding no difference at end of treatment: MD −2.45 kg, 95% CI −4.43 to −0.47; Analysis 9.1.
9.1. Analysis.

Comparison 9: Direct comparisons between NRT types for smoking cessation, Outcome 1: Mean weight change (kg) at end of treatment: comparisons by type
9.2. Analysis.

Comparison 9: Direct comparisons between NRT types for smoking cessation, Outcome 2: Mean weight change (kg) at 6 months: comparisons by type
9.3. Analysis.

Comparison 9: Direct comparisons between NRT types for smoking cessation, Outcome 3: Mean weight change (kg) at 12 months: comparisons by type
Pooled data from two studies (both at high risk of bias) comparing longer courses of NRT with 15 mg or 25 mg patches to shorter courses resulted in a point estimate favouring longer courses at 12 months, but CIs incorporated no difference: MD −0.24 kg, 95% CI −0.97 to 0.48; I2 = 0%; 1 study, 404 participants; Analysis 9.4. Point estimates for pooled data of studies comparing higher versus lower doses of NRT were close to no difference, with CIs including no difference at both time points with data available: end of treatment: MD 0.22 kg, 95% CI −0.04 to 0.48; I2 = 0%; 4 studies, 1038 participants; not sensitive to removal of four studies at high risk of bias; Analysis 9.5; 12 months: MD 0.27 kg, 95% CI −0.41 to 0.96; I2 = 0%; 3 studies, 554 participants; not sensitive to removal of two studies at high risk of bias; Analysis 9.6.
9.4. Analysis.

Comparison 9: Direct comparisons between NRT types for smoking cessation, Outcome 4: Mean weight change (kg) at 12 months: longer course vs. shorter
9.5. Analysis.

Comparison 9: Direct comparisons between NRT types for smoking cessation, Outcome 5: Mean weight change (kg) at end of treatment: dose response
9.6. Analysis.

Comparison 9: Direct comparisons between NRT types for smoking cessation, Outcome 6: Mean weight change (kg) at 12 months: dose response
Effect of varenicline on post‐cessation weight gain
At end of treatment and six months, pooled data on the effect of varenicline on post‐cessation weight gain had point estimates close to no difference and CIs incorporating no difference. At end of treatment, the MD was −0.23 kg, 95% CI −0.53 to 0.06; I2 = 32%; 14 studies, 2566 participants; a funnel plot did not show asymmetry (Figure 8), and results were not sensitive to removal of two studies at high risk of bias (Analysis 10.1). At six months, the MD was −0.09 kg, 95% CI −1.09 to 0.90; I2 = 19%; 3 studies, 384 participants; unclear risk, 1 low risk; Analysis 10.2. At 12 months, the point estimate favoured the intervention but CIs were wide and incorporated no difference: MD 1.05 kg, 95% CI −0.58 to 2.69; I2 = 0%; 3 studies, 237 participants; at unclear risk of bias; Analysis 10.3.
8.

Funnel plot of comparison: 10 Varenicline versus placebo for smoking cessation, outcome: 10.1 Mean weight change (kg) at end of treatment.
10.1. Analysis.

Comparison 10: Varenicline versus placebo for smoking cessation, Outcome 1: Mean weight change (kg) at end of treatment
10.2. Analysis.

Comparison 10: Varenicline versus placebo for smoking cessation, Outcome 2: Mean weight change (kg) at 6 months
10.3. Analysis.

Comparison 10: Varenicline versus placebo for smoking cessation, Outcome 3: Mean weight change (kg) at 12 months
Three studies (two at low risk of bias, one at unclear risk) compared treatment with bupropion to varenicline and provided usable data. Participants taking varenicline gained more weight at the end of treatment. although CIs narrowly included no difference: MD 0.53 kg, 95% CI 0.00 to 1.05; I2 = 29%; 3 studies, 598 participants; Analysis 11.1. One further study (2 Rose 2014) did not provide data in a form we could analyse.
11.1. Analysis.

Comparison 11: Varenicline versus bupropion, Outcome 1: Mean weight change (kg) at end of treatment
There was no evidence that weight gain differed in the one trial of varenicline versus NRT (2 Aubin 2008, high risk of bias, Analysis 12.1).
12.1. Analysis.

Comparison 12: Varenicline versus NRT, Outcome 1: Mean weight change (kg) at end of treatment
Effect of e‐cigarettes on post‐cessation weight gain
Two studies of e‐cigarettes (one as an adjunct to NRT, the other compared to varenicline) both had very wide CIs at all time points, encompassing clinically significant weight loss and weight gain (Analysis 13.1; Analysis 14.1; Analysis 14.2; Analysis 15.1; Analysis 15.2; Analysis 16.1).
13.1. Analysis.

Comparison 13: Nicotine EC + patch vs patch, Outcome 1: Weight (kg) at EOT
14.1. Analysis.

Comparison 14: Nicotine EC + patch vs nicotine‐free EC + patch, Outcome 1: Weight (kg) at EOT
14.2. Analysis.

Comparison 14: Nicotine EC + patch vs nicotine‐free EC + patch, Outcome 2: Weight (kg) at 6 months
15.1. Analysis.

Comparison 15: Nicotine‐free EC + patch vs patch, Outcome 1: Weight (kg) at EOT
15.2. Analysis.

Comparison 15: Nicotine‐free EC + patch vs patch, Outcome 2: Weight (kg) at 6 months
16.1. Analysis.

Comparison 16: Nicotine EC versus varenicline, Outcome 1: Weight change (kg) at EOT
Discussion
Summary of main results
Since the previous version of this review, published in 2012, we have found 21 additional, completed trials fitting criteria for Part 1 (interventions targeting post‐cessation weight gain on weight change and smoking cessation), so there are now 37 trials of interventions specifically designed to limit post‐cessation weight gain. Although a range of pharmacological interventions were tested, none showed evidence that weight gain was prevented in the longer term (beyond six months), but most trials followed up participants only in the short term, primarily at end of treatment, and newer medications have since come on the market. Although some behavioural interventions suggested benefit, certainty in the evidence was limited across all comparisons. There was low‐ to very low‐certainty evidence that personalized weight‐management support, which included weight‐management education with both feedback on personal goals and a personal energy prescription, reduced weight gain at end of treatment, at six, and 12 months (Table 1). There was low‐ to very low‐certainty evidence that detailed weight‐management education without personalized assessment, planning and feedback did not reduce weight gain and may have reduced smoking cessation rates (Table 1). There was very low‐certainty evidence that acceptance‐based interventions for weight concern had no impact on weight, and low‐certainty evidence that these interventions increased cessation rates (Table 2).
We identified 27 new, completed trials during the update that fitted the criteria for Part 2 (interventions designed to aid smoking cessation that plausibly affect post‐cessation weight gain) of this review, bringing the total to 83 trials included in this part. In total, we examined evidence for six different interventions used to support smoking cessation that might incidentally reduce weight gain on cessation. There was low‐certainty evidence that exercise interventions did not impact weight at end of treatment but that they reduced weight gain at 12 months by approximately 2 kg (Table 3). There was also moderate‐certainty evidence that nicotine replacement therapy reduced weight at 12 months relative to control, although here the reduction was very modest (approximately 0.4 kg) (Table 4). There was low‐certainty evidence that varenicline made little difference to weight at any time point (Table 5). Studies of fluoxetine showed low‐certainty evidence of benefit at end of treatment and very low‐certainty evidence of benefit at six months; no studies provided data at 12 months (Table 6). Bupropion appeared to limit weight gain at end of treatment, but there was no evidence of this at six or 12 months. There was insufficient evidence to draw any conclusions about the potential role of electronic cigarettes in limiting post‐cessation weight gain.
Overall completeness and applicability of evidence
As described below, the major limitation to the evidence base was imprecision, meaning there are many places in which the evidence is too uncertain to assess whether interventions have worthwhile benefits on weight gain or pose a risk to attaining abstinence from smoking. Studies in Part 2 are probably broadly applicable to the general population of people motivated to quit smoking; these studies were of cessation that measured weight as a secondary outcome. Studies in Part 1, however, may not be as generalizable, with many selecting study populations based on weight concern. In addition, some of the pharmacotherapies tested in Part 1 are no longer widely available due to safety and tolerability concerns, and newer weight‐loss medications have yet to be tested in the context of smoking cessation.
Certainty of the evidence
Part 1
We rated all outcomes in Part 1 to be of low to very low certainty, primarily due to imprecision (small numbers of studies and participants, resulting in wide CIs) and risk of bias (Table 1; Table 2). Although sensitivity analyses removing studies at high risk of bias did not affect results, in many cases all of the studies contributing to a given outcome were judged to be at high or unclear risk of bias. A further limitation to Part 1 studies is that most enrolled people who were weight‐concerned in some way; such participants may have been more likely to default from the control programme than when allocated the active intervention that they presumably wanted, especially in studies such as 1 Danielsson 1999 and 1 Spring 2004, where this included free meals. The open‐label design is unavoidable in this field, but it is important to note that it could bias the smoking abstinence results in favour of the intervention and decrease the difference in weight at follow‐up.
Part 2
At end of treatment and six months, there was low‐ and very low‐certainty evidence of benefit from fluoxetine, due to issues with serious imprecision (end of treatment) and very serious imprecision (six months) (Table 6). There were no data on fluoxetine at 12 months. Certainty of the evidence in the longer‐term impact of nicotine replacement therapy on weight change was downgraded to moderate, due to issues with imprecision (Table 3). Further studies may increase certainty in these estimates. Evidence on exercise showed low‐certainty evidence of no benefit at end of treatment, but benefit at 12 months (Table 4). In most trials of interventions in weight management, the difference between intervention and control is most marked at end of treatment and declines over follow‐up. In this context, it is puzzling that there was no evidence of effectiveness of exercise on weight at end of programme, but there was at 12‐month follow‐up. This might either represent a chance finding or reflect the fact that the programme encouraged people to go on exercising after it had finished. Further evidence is required before we can be confident that physical activity programmes provide an effective intervention.
Evidence of no difference in weight at end of treatment with varenicline was high certainty, meaning we think further studies are unlikely to meaningfully change the estimate of no clinically significant difference. Longer‐term outcomes (six and 12 months) were low certainty for varenicline, due to imprecision and to the fact that all studies contributing data at these time points were judged to be at unclear risk of bias (Table 5).
Potential biases in the review process
Several aspects of our methodological approach warrant consideration.
Firstly, in Part 2, we used existing Cochrane Reviews of interventions for smoking cessation to identify relevant studies. In effect, this means that to be included in Part 2, studies must have measured cessation at six months or longer, as this was an inclusion criterion of the parent review. This means that some studies reporting weight data at earlier than six months may have been missed. For example, we encountered studies of fluoxetine in Part 1 and Part 2 of the review. The study of fluoxetine in Part 1 was excluded from the parent Cochrane Review because it did not incorporate at least a six‐month follow‐up. It was included in the Part 1 search because the aim was to reduce weight gain. This means that it is possible that we did not include some other studies of fluoxetine that were not specifically aimed at reducing weight gain and did not incorporate a six‐ or 12‐month follow‐up. There is, however, no reason to imagine that excluding them would create a bias. Moreover, it is long‐term weight gain that is relevant to health and all such studies would have been included. Second, in this and the 2012 update, but not in the original version of our review, we include 2 Tonstad 2006 in our main analysis of the effect of varenicline on weight gain. In this trial, participants had taken 12 weeks of varenicline before the abstinent participants were randomized to a further 12 weeks or placebo. Thus this study examines weight gain in months three to six of a quit attempt, not from months zero to three as in the other studies. Weight gain is less rapid in months three to six (O'Hara 1998), meaning any effect of varenicline in preventing weight gain is likely to be lower, slightly biasing the effect downwards.
Thirdly, we split the behavioural interventions for weight control in Part 1 of the review into two categories: those that provided weight‐management education only, and those that provided personalized weight‐management support. This split was chosen based on meta‐analysis evidence that healthy eating and physical activity interventions combining self‐monitoring with at least one other technique derived from control theory, (such as specific goal setting, feedback on progress or review of goals set) are significantly more effective than those that do not (Michie 2009).
Finally, as noted in the Methods, the data here relate to weight gain in abstinent smokers only. It is practically difficult to follow up non‐abstinent smokers as they have no motive to attend smoking cessation clinics and thus authors do not usually provide data on continuing smokers. However, most people gain weight on cessation and most people make repeated attempts to quit. It is possible that this leads to incremental weight gain and it would be useful if data could be collected on this.
Agreements and disagreements with other studies or reviews
English smoking cessation guidelines from the National Institute for Health and Care Excellence (NICE) make no specific recommendations about preventing post‐cessation weight gain. However, the public‐facing NHS website suggests exercise, varenicline, nicotine replacement, bupropion and dieting as options (NHS 2021). Similarly, US guidance recommends either bupropion, NRT, or exercise as interventions. A common perception is that concurrent behavioural treatment for smoking and weight undermines smoking cessation, and the advice is to establish smoking cessation before tackling weight (McEwen 2006). Some of the reason for this is the evidence from laboratory studies which show increased urges to smoke during periods of food restriction (Cheskin 2005; Leeman 2010).
Our review produced mixed evidence of this, with some data at 12 months suggesting that perhaps it undermines abstinence. However, data earlier in the quit attempt show no evidence of this and are rather more precise. Given that the putative mechanism relates to the similarity of hunger pangs and smoking urges, it is perhaps most likely that the somewhat lower abstinence in those who had weight‐management interventions could be chance findings. However, other interpretations are possible, and only more data will clarify the issue.
Authors' conclusions
Implications for practice.
There is no moderate‐ or high‐quality data that any intervention tested reduces weight gain in the long term.
There is low‐certainty evidence that weight‐management education may reduce abstinence and is not effective at controlling weight .
There is low‐ to very low‐certainty evidence that personalized weight‐management support programmes, incorporating both feedback on personal goals and a personal energy prescription, may reduce weight gain. There is mixed and low‐certainty evidence of their effects on abstinence.
Very low calorie diets may increase abstinence and prevent weight gain in the short term at least, but these conclusions are based on a single trial only, and another small trial found no one adhered to the intervention.
There was low‐ to very low‐certainty evidence that interventions designed to allay concerns about weight gain did not meaningfully affect weight gain but low certainty that they increase abstinence.
There was low‐certainty evidence that exercise interventions for smoking cessation did not affect weight by the end of treatment, so the 12‐month reduction in weight is unexplained.
Nicotine replacement therapy, bupropion, fluoxetine and varenicline all slightly reduced weight gain in the short term, but the size of benefit, if any, at 12 months was very uncertain, and evidence suggests was modest at best.
Implications for research.
As none of the existing pharmacotherapies tested to limit weight gain appear promising, testing newer agents now licensed for weight control to prevent weight gain may be helpful. Studies could also explore combining behavioural support and pharmacotherapy.
Fluoxetine for smoking cessation was associated with reduced weight gain at end of treatment and six months, but evidence was very uncertain. Further studies are needed, particularly following up participants at six months and beyond.
Behavioural weight management programmes are the mainstay of treatment for weight management and further trials to assess the impact of programmes tailored to people's concerns about weight gain would clarify the evidence on whether they limit long‐term weight gain and their impact on abstinence from smoking.
Further studies of interventions to allay people's concerns about weight gain are required to clarify its effect on smoking cessation.
Further studies of exercise interventions are needed. The finding that an intervention aimed at increasing exercise levels had no effect initially but somehow affected weight one year later seems counterintuitive, as adherence to exercise regimens usually declines rather than increases with time.
Future trials of interventions for limiting post‐cessation weight gain should report mean weight change, standard deviation for the weight change and the number contributing to the mean in biochemically‐confirmed continuous or prolonged abstinent participants only rather than in those abstinent for only one week. Weight change in those who continue to smoke should be reported separately.
Trials of current and future pharmacotherapies for smoking cessation should measure weight change, reporting mean weight change, standard deviation of the change and numbers contributing to the mean, separating abstinent from smoking participants as described above.
Future studies could also report results separately in those with overweight or obesity at baseline, or focus on recruiting from this population.
Data are needed on whether using e‐cigarettes to quit smoking affects post‐cessation weight change.
What's new
| Date | Event | Description |
|---|---|---|
| 8 October 2021 | Amended | Correction of PLS, to remove duplicated information |
History
Protocol first published: Issue 4, 2006 Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 20 April 2021 | New citation required and conclusions have changed | Updated with 47 new included studies providing data on new comparisons and increasing certainty in effects of smoking cessation interventions on weight change. |
| 20 April 2021 | New search has been performed | Updated with 48 new included studies. Latest search 16 October 2020. |
| 23 November 2011 | New citation required but conclusions have not changed | Change of name for one author (Amanda Parsons is now Amanda Farley), one new author added (DL), and two authors of previous version removed (see Contributions of Authors). |
| 23 November 2011 | New search has been performed | Twelve additional studies added. Conclusions largely unchanged. |
| 24 April 2008 | Amended | Converted to new review format. |
| 14 July 2006 | New citation required and major changes | Substantive amendment |
Acknowledgements
We thank the Cochrane Tobacco Addiction Review Group for their support and advice. Specifically the content of the table of included studies in part two of this review is mainly populated by data extracted by the authors of the parent reviews (Lindsay Stead, Kate Cahill, Michael Ussher) with statistical advice given by Rafael Perera. We also thank Cynthia Pomerleau and colleagues, and Michael Ussher, for reading and commenting on earlier drafts of this protocol. We would also like to thank the many authors of the primary studies that responded to our requests for data.
For previous versions of the review, we thank the following authors for their contributions: Mujahed Shraim (MS) wrote and submitted the review protocol, and extracted data for Part 1 studies. Jennie Inglis (JS) extracted data for Part 2 studies. Both contributed to the first version of the review only, published in 2009. MS and Amanda Parsons (AP) carried out searches for the first part of the review and AP, Deborah Lycett (DL), MS and JI independently identified relevant studies and extracted data. AP drafted the review. DL, Paul Aveyard and Peter Hajek gave conceptual and editorial support.
For this update, we acknowledge the help of Min Gao in screening a paper published in Chinese and James Schulte for statistical support. We would also like to gratefully acknowledge and thank the following study authors who responded to our requests and provided additional data: Professor Amanda Baker and Associate Professor Terry J. Lewin, Assistant Professor Erika Litvin Bloom, Professor Bonnie Spring and Dr Harold Javitz, Professors Fridtjof Thomas and Karen Chandler Johnson, Dr Eli Heggen, Professor Andrea King, Professor Mark J. Eisenberg, Professor Kevin M. Gray, Dr Ashley Vena, Dr Nikos Ioakeimidis, and Associate Professor Natalie Walker. We thank Henri‐Jean Aubin, Université Paris‐Saclay, Inserm, CESP, AP‐HP. Villejuif, France and Serena Tonstad, Oslo University Hospital, Norway, for providing peer review. We thank Charles Vicary for providing consumer review.
The National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC) Diet and Obesity provided funding to support this update. The views expressed are those of the review author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Data and analyses
Comparison 1. Pharmacological interventions versus placebo for post cessation weight control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mean weight change (kg) at end of treatment | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 Dexfenfluramine versus placebo | 1 | 33 | Mean Difference (IV, Random, 95% CI) | ‐2.50 [‐2.98, ‐2.02] |
| 1.1.2 Phenylpropanolamine versus Placebo | 3 | 112 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.80, ‐0.20] |
| 1.1.3 Ephedrine + Caffeine versus Placebo | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐2.87, 0.27] |
| 1.1.4 Lorcaserin versus placebo | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐3.65, 1.37] |
| 1.1.5 Chromium versus placebo | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐3.05, 1.43] |
| 1.1.6 Naltrexone versus placebo | 3 | 254 | Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.49, ‐0.34] |
| 1.1.7 Topiramate versus placebo | 1 | 6 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 1.2 Mean weight change (kg) at 6 months | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2.1 Phenylpropanolamine versus Placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2.2 Ephedrine + caffeine versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2.3 Chromium versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2.4 Naltrexone versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3 Mean weight change (kg) at 12 months | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3.1 Phenylpropanolamine versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3.2 Ephedrine + Caffeine versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3.3 Naltrexone versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.4 Smoking cessation at 6 months | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.4.1 Phenylpropanolamine gum versus placebo | 1 | 295 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.76, 2.53] |
| 1.4.2 Ephedrine + Caffeine versus placebo | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.53, 2.11] |
| 1.4.3 Naltrexone versus placebo | 3 | 890 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.79, 1.32] |
| 1.4.4 Chromium versus placebo | 1 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.12, 1.84] |
| 1.4.5 Naltrexone & bupropion versus placebo | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.5 Smoking cessation at 12 months | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5.1 Phenylpropanolamine gum versus placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5.2 Ephedrine + Caffeine versus Placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5.3 Naltrexone versus placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6.1 Lorcaserin versus placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6.2 Lorcaserin (longer duration) versus placebo + lorcaserin (shorter duration) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7 Serious adverse events | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.1 Topiramate versus placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.2 Naltrexone versus placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.3 Lorcaserin (longer duration) versus placebo + lorcaserin (shorter duration) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Behavioural weight management interventions versus advice or no intervention for post‐cessation weight control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Mean weight change (kg) at end of treatment | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1.1 Weight management education versus no weight intervention | 2 | 140 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.57, 0.50] |
| 2.1.2 Personalised weight management support versus no weight intervention | 3 | 121 | Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐1.93, ‐0.29] |
| 2.2 Mean weight change (kg) at 6 months | 7 | 897 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.55, 0.53] |
| 2.2.1 Weight management education verses no weight intervention | 2 | 81 | Mean Difference (IV, Random, 95% CI) | 0.89 [‐0.78, 2.55] |
| 2.2.2 Personalised weight management support versus no weight intervention | 5 | 816 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐2.18, 0.25] |
| 2.3 Mean weight change (kg) at 12 months | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.3.1 Weight management education versus no weight intervention | 2 | 61 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐2.28, 1.86] |
| 2.3.2 Personalised weight management support versus no weight intervention | 4 | 530 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐2.34, 1.46] |
| 2.4 Smoking cessation at 6 months | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.4.1 Weight management education versus no intervention | 3 | 660 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.78, 1.33] |
| 2.4.2 Personalised weight management support versus no intervention | 7 | 5517 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.82, 1.10] |
| 2.5 Smoking cessation at 12 months | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.5.1 Weight management education versus no intervention | 2 | 522 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.48, 0.90] |
| 2.5.2 Personalised weight management support versus no intervention | 5 | 3441 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.45, 0.92] |
Comparison 3. Direct comparisons between behavioural weight management interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Mean weight change (kg) at end of treatment | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1.1 Low carb diet versus reduced fat diet | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1.2 Personalised weight management support versus weight management education | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1.3 VLCD + advice versus advice | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1.4 Early versus late personalised weight management support | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.2 Mean weight change (kg) at 6 months | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.2.1 Low carb diet versus reduced fat diet | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.2.2 Early versus late personalised weight management support | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.3 Mean weight change (kg) at 12 months | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.3.1 Personalised weight management support versus weight management education | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.3.2 VLCD + advice versus advice | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.3.3 Motivational interviewing + CBT versus telephone support | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.4 Smoking cessation at 6 months | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.4.1 Exercise maintenance condition + standard care versus standard care (incl. behavioural weight management) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.4.2 Low carb diet versus reduced fat diet | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.4.3 Personalised weight management support versus weight management education | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.5 Smoking cessation at 12 months | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.5.1 Exercise maintenance condition + standard care versus standard care (incl. behavioural weight management) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.5.2 Personalised weight management support versus weight management education | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.5.3 VLCD + advice versus advice | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.5.4 Motivational interviewing + CBT versus telephone support | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 4. Acceptance interventions for weight concern.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Mean weight change (kg) at end of treatment | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1.1 Acceptance intervention, no additional pharmacotherapy | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1.2 Acceptance intervention, with bupropion in both arms | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1.3 Acceptance intervention, with NRT in both arms | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.2 Mean weight change (kg) at 6 months | 3 | 106 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐1.53, 1.53] |
| 4.2.1 CBT to accept moderate weight gain versus no behavioural weight advice, no additional pharmacotherapy | 2 | 55 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐5.55, 3.45] |
| 4.2.2 CBT to accept moderate weight gain versus no behavioural weight advice, with bupropion | 1 | 46 | Mean Difference (IV, Random, 95% CI) | 0.86 [0.30, 1.42] |
| 4.2.3 Acceptance intervention versus health education, with NRT | 1 | 5 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐5.72, 4.66] |
| 4.3 Mean weight change (kg) at 12 months | 2 | 76 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐2.95, 1.56] |
| 4.3.1 CBT to accept moderate weight gain versus no behavioural weight advice, no additional pharmacotherapy | 2 | 44 | Mean Difference (IV, Random, 95% CI) | ‐2.15 [‐7.54, 3.24] |
| 4.3.2 CBT to accept moderate weight gain versus no behavioural weight advice, with bupropion | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.57, 1.33] |
| 4.4 Smoking cessation at 6 months | 4 | 619 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.03, 1.96] |
| 4.4.1 CBT to accept moderate weight gain versus no behavioural weight advice (no additional pharmacotherapy) | 3 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.92, 2.55] |
| 4.4.2 CBT to accept moderate weight gain versus no behavioural weight advice (with bupropion) | 1 | 195 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.85, 2.16] |
| 4.4.3 ACT for weight concern versus health education | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.22, 2.35] |
| 4.5 Smoking cessation at 12 months | 2 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.76, 2.06] |
| 4.5.1 CBT to accept moderate weight gain versus no behavioural weight advice (no additional pharmacotherapy) | 2 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.58, 3.59] |
| 4.5.2 CBT to accept moderate weight gain versus no behavioural weight advice (with bupropion) | 1 | 195 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.62, 1.81] |
Comparison 5. All types of antidepressant versus placebo for smoking cessation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Mean weight change (kg) at end of treatment | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1.1 Bupropion versus placebo | 10 | 1098 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.35, ‐0.67] |
| 5.1.2 Fluoxetine versus placebo | 2 | 144 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.49, ‐0.53] |
| 5.1.3 Bupropion + varenicline vs placebo + varenicline | 1 | 243 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐2.18, ‐0.62] |
| 5.2 Mean weight change (kg) at end of treatment: dose response | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.2.1 Bupropion: 300mg/day v 150mg/day placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.2.2 Bupropion: 300mg/day v 100mg/day placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.3 Mean weight change (kg) at 6 months | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.3.1 Bupropion versus placebo | 7 | 420 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐1.19, 0.44] |
| 5.3.2 Fluoxetine versus placebo | 2 | 124 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐4.38, 2.37] |
| 5.3.3 Bupropion + varenicline vs placebo + varenicline | 1 | 162 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.71, 0.91] |
| 5.4 Mean weight change (kg) at 6 months: dose response | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.4.1 Bupropion: 300mg/day v 150mg/day | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.4.2 Bupropion: 300mg/day v 100mg/day | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.4.3 Fluoxetine: 40mg v 20mg | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.4.4 Fluoxetine: 60mg v 30mg | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.5 Mean weight change (kg) at 12 months | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.5.1 Bupropion versus placebo | 7 | 471 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐1.31, 0.78] |
| 5.5.2 Bupropion + varenicline vs placebo + varenicline | 1 | 140 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐3.18, 0.78] |
| 5.6 Mean weight change (kg) at 12 months: dose response | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.6.1 Bupropion: 300mg/day v 150mg/day | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐4.81, 5.21] |
| 5.6.2 Bupropion: 300mg/day v 100mg/day | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐8.04, 4.04] |
Comparison 6. Exercise interventions versus no exercise for smoking cessation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Mean weight change (kg) at end of treatment | 4 | 404 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.78, 0.29] |
| 6.1.1 Exercise + SC versus SC only | 4 | 404 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.78, 0.29] |
| 6.2 Mean weight change (kg) at 12 months | 3 | 182 | Mean Difference (IV, Random, 95% CI) | ‐2.07 [‐3.78, ‐0.36] |
| 6.2.1 Exercise + SC versus SC only | 3 | 182 | Mean Difference (IV, Random, 95% CI) | ‐2.07 [‐3.78, ‐0.36] |
Comparison 7. Bupropion versus NRT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Weight change (kg) at 12 months | 1 | 115 | Mean Difference (IV, Random, 95% CI) | 0.48 [‐1.46, 2.42] |
Comparison 8. All types of NRT versus placebo for smoking cessation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Mean weight change (kg) at end of treatment | 21 | 2784 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.99, ‐0.05] |
| 8.1.1 Gum versus placebo | 4 | 345 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.02, ‐0.13] |
| 8.1.2 Patch versus placebo | 11 | 1666 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.51, 0.19] |
| 8.1.3 Inhaler versus placebo | 2 | 111 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐1.19, 0.45] |
| 8.1.4 Sub‐lingual tablet versus placebo | 2 | 478 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.11, 0.12] |
| 8.1.5 Intranasal spray versus placebo | 1 | 47 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐1.54, 3.34] |
| 8.1.6 Lozenge versus placebo | 1 | 137 | Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.57, 1.52] |
| 8.2 Mean weight change (kg) at 6 months | 11 | 1021 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.51, 0.35] |
| 8.2.1 Gum versus placebo | 2 | 103 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐2.35, 0.69] |
| 8.2.2 Patch versus placebo | 4 | 282 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐1.16, 0.49] |
| 8.2.3 Inhaler versus placebo | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.98, 0.78] |
| 8.2.4 Sub‐lingual tablet versus placebo | 2 | 329 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐1.09, 0.72] |
| 8.2.5 Patch + varenicline versus placebo + varenicline | 1 | 113 | Mean Difference (IV, Random, 95% CI) | 0.96 [‐0.53, 2.45] |
| 8.2.6 Lozenge versus placebo | 1 | 137 | Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.57, 1.52] |
| 8.3 Mean weight change (kg) at 12 months | 17 | 1463 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.86, 0.11] |
| 8.3.1 Gum versus placebo | 1 | 49 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐3.07, 2.93] |
| 8.3.2 Patch versus placebo | 7 | 812 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.89, 0.44] |
| 8.3.3 Intranasal spray versus placebo | 3 | 122 | Mean Difference (IV, Random, 95% CI) | ‐1.55 [‐3.09, ‐0.00] |
| 8.3.4 Inhaler versus placebo | 2 | 90 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐2.23, 0.17] |
| 8.3.5 Sub‐lingual tablet versus placebo | 3 | 303 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.99, 1.54] |
| 8.3.6 Lozenge versus placebo | 1 | 87 | Mean Difference (IV, Random, 95% CI) | 0.97 [‐1.82, 3.76] |
Comparison 9. Direct comparisons between NRT types for smoking cessation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Mean weight change (kg) at end of treatment: comparisons by type | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1.1 Patch versus spray | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1.2 Lozenge versus gum | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.2 Mean weight change (kg) at 6 months: comparisons by type | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.2.1 Lozenge versus gum | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.2.2 Patch versus spray | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.3 Mean weight change (kg) at 12 months: comparisons by type | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.3.1 Loxenge versus gum | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.3.2 Lozenge versus patch | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.4 Mean weight change (kg) at 12 months: longer course vs. shorter | 1 | 404 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.97, 0.48] |
| 9.4.1 22 weeks vs 8 weeks 25mg patch | 1 | 222 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.46, 0.46] |
| 9.4.2 22 weeks vs 8 weeks 15mg patch | 1 | 182 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐1.00, 1.20] |
| 9.5 Mean weight change (kg) at end of treatment: dose response | 4 | 1038 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.04, 0.48] |
| 9.5.1 4mg vs 2mg gum | 1 | 161 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.61, 0.41] |
| 9.5.2 22mg vs 11mg patch | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐2.65, 1.85] |
| 9.5.3 44mg vs 22mg patch | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.99, 1.59] |
| 9.5.4 25mg patch vs 15mg patch‐ 8 week treatment course | 1 | 497 | Mean Difference (IV, Random, 95% CI) | 0.40 [0.04, 0.76] |
| 9.5.5 25mg patch vs 15mg patch‐ 22 weeks treatment | 1 | 299 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.57, 0.97] |
| 9.5.6 15x2mg gum vs 7x2mg gum | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 1.59 [‐0.27, 3.45] |
| 9.5.7 30x2mg gum vs 15x2mg gum | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐1.83, 1.29] |
| 9.6 Mean weight change (kg) at 12 months: dose response | 3 | 554 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.41, 0.96] |
| 9.6.1 22mg patch vs 11mg | 1 | 7 | Mean Difference (IV, Random, 95% CI) | ‐3.90 [‐10.74, 2.94] |
| 9.6.2 44mg patch vs 11mg | 1 | 12 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐10.12, 5.72] |
| 9.6.3 25mg patch vs 15mg‐ 8 week treatment course | 1 | 198 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.43, 1.63] |
| 9.6.4 25mg patch vs 15mg‐ 22 weeks treatment course | 1 | 206 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐1.04, 1.04] |
| 9.6.5 Patch + lozenge vs lozenge | 1 | 131 | Mean Difference (IV, Random, 95% CI) | 0.59 [‐1.50, 2.68] |
Comparison 10. Varenicline versus placebo for smoking cessation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Mean weight change (kg) at end of treatment | 14 | 2566 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.53, 0.06] |
| 10.1.1 1mg versus placebo | 2 | 230 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.64, 0.85] |
| 10.1.2 2mg versus placebo | 13 | 2308 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.63, 0.02] |
| 10.1.3 1mg + NRT versus placebo + NRT | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 0.54 [‐2.07, 3.15] |
| 10.2 Mean weight change (kg) at 6 months | 3 | 384 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐1.09, 0.90] |
| 10.3 Mean weight change (kg) at 12 months | 3 | 237 | Mean Difference (IV, Random, 95% CI) | 1.05 [‐0.58, 2.69] |
Comparison 11. Varenicline versus bupropion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Mean weight change (kg) at end of treatment | 3 | 598 | Mean Difference (IV, Random, 95% CI) | 0.53 [0.00, 1.05] |
Comparison 12. Varenicline versus NRT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Mean weight change (kg) at end of treatment | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 13. Nicotine EC + patch vs patch.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Weight (kg) at EOT | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 14. Nicotine EC + patch vs nicotine‐free EC + patch.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Weight (kg) at EOT | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14.2 Weight (kg) at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 15. Nicotine‐free EC + patch vs patch.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Weight (kg) at EOT | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.2 Weight (kg) at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 16. Nicotine EC versus varenicline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Weight change (kg) at EOT | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
1 Baker 2018.
| Study characteristics | ||
| Methods | Country: Australia Recruitment: “…referral sources included health services (148, 63%), media campaigns (59, 25%) and research programmes or registers (28, 12%).") Setting: Research will be conducted in three sites: Centre for Brain and Mental Health Research, University of Newcastle, New South Wales (NSW); School of Public Health, University of NSW, Sydney, NSW; and the Monash Alfred Psychiatry Research Centre (MAPrc), Monash University and The Alfred, Melbourne, Victoria, Australia Study start date: 4 August 2009. Study end date: 30 April 2014 |
|
| Participants | Total N: 235 English‐speaking smokers with psychotic disorders, smoking at least 15 cpd, aged at least 18 years N per arm: Healthy lifestyles condition = 122; Telephone condition = 113 41.5% female, av age 41.7, av baseline BMI: 30.6, av cpd: 28.6 |
|
| Interventions |
All participants received a 90‐minute face‐to‐face intervention session on feedback about smoking and other CVD risk factors; a case formulation was developed with the participant about CVD status and unhealthy behaviours, using a combination of MI and CBT, after which they received up to 24 weeks’ supply of NRT, delivered at weeks 1, 4 and 8, and thereafter by arrangement |
|
| Outcomes |
|
|
| Study funding | This work was supported by the Australian National Health and Medical Research Council (NHMRC project grant numbers 569210 and 1009351) and the Commonwealth Department of Health and Ageing | |
| Author declarations | The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article | |
| Notes | Nicotine replacement therapy (NRT) was provided free of charge by GlaxoSmith Kline Participants were reimbursed AUD 20 for their travel, time and participation on each assessment occasion Additional information provided by authors upon request This study is new to the 2021 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A permuted block randomisation approach was used so that the distribution of these characteristics across conditions was maintained." Quote: “Following the baseline assessment, study therapists were issued with a sealed randomisation envelope (by an independent person) displaying a participant identification code.” (Baker 2015) |
| Allocation concealment (selection bias) | Low risk | Quote: "Following completion of the baseline assessment for each participant, the clinicians will be issued with a sealed randomisation envelope (by an independent person) which displays the participant identification code. The envelope will be opened by the participant at the conclusion of the initial session." |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Confirmed PPA (at 7 days) was analyzed as self‐reported abstinence from smoking, confirmed by an expired CO reading of ⩽ 10 ppm (unless the participant indicated they had smoked cannabis in the past week) |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “…Using Seca 770 digital scales.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 month = Intervention = 70/122 = 57%; Control: 69/113 = 61% 18 month = Intervention = 70/122 = 57%; Control: 62/113 = 55% 30 month = Intervention = 65/122 = 53%; Control: 64/113 = 56% |
1 Bloom 2020.
| Study characteristics | ||
| Methods | Country: USA Recruitment: “Female participants (N = 69) were recruited from the local community via paper and electronic advertisements.” Setting: Not specified Study start date: May 2015; Study end date: December 2018 |
|
| Participants | Total N: 69 female smokers, aged at least 18 years, smoked ≥ 5 cpd for at least the past year, motivated to quit, and concerned about post‐cessation weight gain; N per arm: Health Education (HE) = 36; DT‐W = 33 100% female, av age 49.7, av baseline BMI: 31.4, av cpd: 16.2, av FTND: 4.8 |
|
| Interventions |
All participants received standard CBT for smoking cessation in sessions 3 ‐ 8, and 8 weeks of nicotine patches beginning on the target quit date (session 4) |
|
| Outcomes |
|
|
| Study funding | “This work was supported by the National Institute on Drug Abuse (grant number K23DA035288 to ELB).” | |
| Author declarations | “RAB has equity ownership in Health Behavior Solutions, Inc., which is developing products for tobacco cessation that are not related to this study. The terms of this arrangement have been reviewed and approved by the University of Texas at Austin in accordance with its policy on objectivity in research. The other authors have no interests to declare.” | |
| Notes | “Participants were compensated up to $225 for completing all assessments.” Baseline weight data was taken at the initial baseline appointment, which was usually 1 ‐ 2 months before the quit date Additional information provided by authors upon request This study is new to the 2021 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomization occurred by cohort due to the group delivery of treatments. Cohort treatment assignments were randomly chosen without replacement from a fixed pool of 10 options (5 DT‐W, 5 HE).” |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “Our primary smoking cessation outcome was 7‐day point prevalence abstinence from combustible cigarette smoking, verified by expired breath carbon monoxide (CO) testing (≤6 ppm)43 with a Bedfont Micro Smokerlyzer. Self‐reported smoking behavior was assessed at all individual assessments and group treatment sessions using a Timeline Followback procedure.44 Participants whose point prevalence abstinence data were missing were assumed to be smoking.” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Participants were weighed on a calibrated medical scale at baseline, post‐treatment, and 1‐, 3‐, and 6‐month follow‐ups.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 Month follow‐up: DT‐W = 26/33*100 = 78.8%; HE = 28/36*100 = 77.8% 3 Month follow‐up: DT‐W = 26/33*100 = 78.8%; HE = 25/36*100 = 69.4% 6 Month follow‐up: DT‐W = 26/33*100 = 78.8%; HE = 28/36*100 = 77.8% |
1 Bush 2012.
| Study characteristics | ||
| Methods | Country: USA Recruitment: Callers to the Oklahoma Tobacco Helpline (OKHL) Study start date: March 2008; Study end date: November 2008 |
|
| Participants | Total n: 2000 (1000 per trial arm) 77% female, av age 41.3, av baseline BMI 30.4 | |
| Interventions |
|
|
| Outcomes |
|
|
| Study funding | "This study was funded by TSET, the Oklahoma State Department of Health, the Oklahoma Tobacco Research Center and Alere Wellbeing. NCT01162577" | |
| Author declarations | Not specified | |
| Notes | This study is new to the 2021 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Automated randomisation procedure” . Comment: Not described other than it was preprogrammed |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) Smoking | High risk | Quote: “Blinded outcome assessments were collected 6 months following randomization” However, smoking abstinence was self‐reported and participants were likely aware of their allocation: “The primary outcome was 30‐day point prevalent abstinence. (…). Tobacco use was assessed with the question: “When was the last time you smoked a cigarette, even a puff?”. Abstinence rates were calculated in two ways: (1) including only participants who completed the survey in the denominator (“respondent analysis”) and (2) using the intent‐to‐treat methodology (ITT) in which those not completing the survey were assumed to be tobacco users.” |
| Blinding of outcome assessment (detection bias) Weight | High risk | Quote:“Blinded outcome assessments were collected 6 months following randomization” However, weight was self‐reported and participants were likely aware of their allocation: Weight: “Secondary outcomes were … and change in weight amongst those who were abstinent at 6 months. (…). Change in weight was calculated in two ways: (1) by reporting participants perceived change in weight (…) and (2) calculated change in weight based on the difference between self reported weight in pounds at baseline and 6 months” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6‐month completion intervention group: 470/1000 (47%); 6‐month completion control group: 532/1000 (53.2%); Overall number of participants lost to follow‐up is 1002/2000*100 = 50.1%; Less than 20% difference in dropout between groups. |
1 Bush 2018.
| Study characteristics | ||
| Methods | Country: USA Recruitment: “Study participants were recruited from three state quitlines (Indiana, Maryland and North Carolina) and 10 commercial (employer‐provided) quitlines…” Setting: Alere Wellbeing provided tobacco Quitline services and a phone/web‐based weight management programme (Weight Talk) Study start date: August 2013; Study end date: March 2016 (trial record) or “…ended in 2017” |
|
| Participants | Total N: 2540 English‐speaking smokers, aged ≥ 18, BMI ≥ 18.5, smoking a minimum of 10 cpd and motivated to quit smoking within the next 30 days N per arm: Control = 844; Simultaneous = 845; Sequential = 851 66% female, av age: 43.2, av baseline BMI: “76% overweight/obese”, av baseline weight: 86.2 kg, cpd: 19.8 |
|
| Interventions |
All participants received 5 standard Quitline smoking‐cessation counselling calls and were offered free NRT in the form of patch, gum and/or lozenge (0 ‐ 8 weeks), depending on the contract and appropriateness based on the participant’s medical condition. All participants also received access to 1 or more web‐based programmes and were mailed a Quit Kit containing a printed guide |
|
| Outcomes |
|
|
| Study funding | “This work was supported solely by funding from the National Institutes of Drug Addiction NIDA (RO1DA31147). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.” | |
| Author declarations | “The authors at Alere Wellbeing declare that they are employed by Alere Wellbeing (a subsidiary of Optum) which provides tobacco cessation and weight management services to states and commercial clients. They have no other competing interests. JL declares that she has no competing interests. HJ declares that he has no competing interests. BS declares that she has no competing interests. MT declares that she has no competing interests.” |
|
| Notes | Additional information provided by authors upon request This study is new to the 2021 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Eligible and consenting participants are immediately randomized by a computer generated program into one of three study arms…” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Candidates who met eligibility requirements and expressed interest in a research study about smoking and weight were transferred to a quit coach who described the study and obtained verbal informed consent to participate, while remaining blind to treatment assignment. Requiring written consent would have placed additional burden on participants, delayed treatment initiation and reduced the generalizability of study findings. We received approval from the Western Institutional Review Board to collect verbal consent.” Comment: Unclear if the same coach also allocated participants to groups |
| Blinding of outcome assessment (detection bias) Smoking | High risk | Self‐reported |
| Blinding of outcome assessment (detection bias) Weight | High risk | Self‐reported weight |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 months follow‐up: Control: 410/844 = 48.6%; Simultaneous: 359/845 = 42.5%; Sequential: 395/851 = 46.4% |
1 Cooper 2005 (also Part 2).
| Study characteristics | ||
| Methods | Country: USA
Recruitment: community volunteers Study start date: not specified; Study end date: not specified |
|
| Participants | 439 weight‐concerned female smokers (≥ 10 cpd) av.age 38, av.cpd 23, av baseline weight 64 ‐ 66 kg | |
| Interventions |
All participants received 13 x 1hr weekly CBT group sessions focused on smoking and weight. Participants cut down weeks 1 ‐ 4 by 25% and quit week 5 |
|
| Outcomes |
|
|
| Study funding | Not specified | |
| Author declarations | Not specified | |
| Notes | PPA defined as validated self‐report of no smoking at the time of the assessment Although these treatments are specifically tested for their effect on smoking and on weight gain the NRT arm is included in the second part of the review as it is included in the parent Cochrane Review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “All group facilitators and participants were blind to treatment conditions” + placebo controlled" |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "Point prevalence abstinence at each assessment was defined by self‐report of no smoking at the time of the assessment and a carbon monoxide (CO) level of < 10 ppm at the current assessment. (...) In instances in which smoking status could not be verified because of absence from that intervention session, the participants were categorized as smoking, indicating intention to treat analyses.“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: "Participants’ weights were taken, in clothing but without shoes or heavy garments, at all sessions. Weight was recorded as absolute weight in pounds with a Detecto Electronics Scale accurate to + 2 oz.“ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “Of the 89 participants who quit smoking, 27 cases were rejected because of missing data, resulting in a sample of 62 women“ |
1 Copeland 2006.
| Study characteristics | ||
| Methods | Country: USA
Recruitment: Community volunteers Study start date: not specified; Study end date: not specified |
|
| Participants | 79 women smokers motivated to quit and weight‐concerned (at least 10 cpd for 1 yr); av cpd 20.1, av FTND: 4, av BMI: 24 | |
| Interventions | All participants completed a smoking cessation programme (6 sessions over 2 wks) involving smoking cessation and relapse prevention advice, and given an 8‐wk supply of NRT Randomized to follow‐up in either individual or group format: 6 follow‐up relapse prevention sessions including psychological, dietary, and exercise components over 38 wks | |
| Outcomes | 1. Continuous abstinence at 6 months (validation: CO ≤ 10 ppm) 2. Mean (SD) weight change (kg) in continuous abstainers at 6 months | |
| Study funding | "This study was funded by grants from the National Institutes on Health and Bristol‐Myers Squibb Better Health for Women Program." | |
| Author declarations | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Quote: "Statisticians generated the random assignment sequence for follow up condition" |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "Biological verification: carbon monoxide (CO) measurement. We used a BreathCo monitor (Vitalograph, Inc.) and used a cutoff level of b10 ppm to confirm nonsmoking status.“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: "Anthropometric data. Baseline body weight and height were converted into body mass index (BMI; kg/m2). Participants were also weighed at each of the six follow‐up sessions.“ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24‐week completion tailored follow‐up group: 33/40 (82.5%); 24‐week completion follow‐up group: 25/36 (69.44%) |
1 Copeland 2015.
| Study characteristics | ||
| Methods | Country: USA Recruitment: from the Baton Rouge, LA community by way of newspaper and billboard advertisements Setting: Pennington Biomedical Research Center Study start date: 2003; Study end date: 2005 (end of recruitment) |
|
| Participants | Total N: 92 weight‐concerned post‐menopausal female smokers, smoking at least 10 cpd for ≥ 1 year, BMI ≥ 18, CO ≥10 ppm N per arm: Group = 54; Individual = 38 100% female, av age 52.3, av baseline BMI 27.4, av cpd 20.3, av FTND 6.4 |
|
| Interventions |
All participants received 6 sessions of in‐person group CBT for smoking cessation and 8 wks of the nicotine transdermal patch |
|
| Outcomes | Mean weight change (kg) in abstainers at EOT (reported narratively) | |
| Study funding | “This research was supported by the National Institute on Aging (NIA), grant AG18239. NIA had no other role other than financial support.” | |
| Author declarations | “None of the authors have any conflict(s) of interest that may inappropriately impact or influence the research and interpretation of the findings.” | |
| Notes | “Participants were provided a $40 monetary incentive for completion of the pretreatment assessment phase, and $40 for completion of the research requirements throughout the follow‐up period.” No control group. 2 formats of a post‐cessation weight gain prevention follow‐up intervention (comparing minimally‐tailored vs highly‐tailored only). Only successfully abstinent smokers randomized to follow‐up weight prevention Longest follow‐up at 16 weeks; no 6‐month follow‐up This study is new to the 2021 update. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Statisticians generated the random assignment sequence for follow‐up condition” Comment: No further information given. |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “BreathCo monitors were used (Vitalograph Inc.) to determine expired CO level (ppm). A cut‐off of b10 ppm was used to confirm non‐smoking status” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Participants were also weighed at each of the 6 follow‐up sessions.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16 week post‐treatment follow‐up: Group: 28/54*100 = 51.9% Individual: 15/38*100 = 39.5% |
1 Danielsson 1999.
| Study characteristics | ||
| Methods | Country: Sweden
Recruitment: community volunteers Study start date: not specified; Study end date: not specified |
|
| Participants | 287 weight‐concerned female smokers, age range 30 ‐ 60, ≥ 10 cpd, av cpd 20, av BMI 26 | |
| Interventions |
|
|
| Outcomes |
|
|
| Study funding | Not specified | |
| Author declarations | Not specified | |
| Notes | Prolonged abstinence defined as "completely and continuously stopped from week 2 onwards" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Open consecutive randomization (in the order their questionnaires were received at the clinic) |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “Successful smoking cessation was defined as self reported complete abstinence from week 2 to week 16, verified by a carbon monoxide concentration less than 10 ppm (New Smokerlyzer, Bedfont). Two missed visits between weeks 2 and 16 were allowed. If the week 2 visit was missing, the woman had to be abstinent from week 1 until endpoint. Women had to attend the week 16 visit to be eligible for the success criteria” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Not specified how weight was assessed, so that self‐report cannot be ruled out. Participants were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “After 12 months, 35 and 51 women had dropped out from the diet and control groups respectively” |
1 Hall 1992.
| Study characteristics | ||
| Methods | Country: USA
Recruitment: community volunteers Study start date: not specified; Study end date: not specified |
|
| Participants | 180 smokers, 27% female, av age 39 ‐ 42, av cpd 26 ‐ 32, av baseline weight 67 ‐ 73 kg | |
| Interventions | Participants received treatment in groups. All groups completed 2‐wk behavioural smoking cessation programme. Participants were randomly assigned to follow‐up group for weight management:
|
|
| Outcomes |
|
|
| Study funding | "This study was funded in part by grants DA02356 and DA00065, both from the National Institute on Drug Abuse, and by a Research Career Scientist Award from the Department of Veterans Affairs to Dr.Hall" | |
| Author declarations | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “At each assessment, subjects were coded as abstinent if they reported no cigarettes smoked during the prior week and had a carbon monoxide lvel below 10.5 ppm at the assessment. At week 52, subjects were coded as abstinent only if their blood cotinine levels were also less than 50ng/mL.” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Body weights were taken at all assessments on the same balance beam scale” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “165 of the 180 subjects who began treatment completed the smoking cessation portion. Dropouts did not differ from the rest of the sample in age, gender, ethnicity, level of education, socioeconomic status, number of previous quit attempts, cessation induced weight gain, pretreatment carbon monoxide, cigarette intake, or body weight” |
1 Hankey 2009.
| Study characteristics | ||
| Methods | Country: Scotland Recruitment: Smokers at a smoking cessation clinic Study start date: January 2008; Study end date: August 2008 |
|
| Participants | 138 smokers, 75.4% female, av baseline weight 76.2 (18.1) kg, av age 50 yrs, av BMI 28.2 (5.5), av cpd, 25.2 (12.6) | |
| Interventions |
Both conditions were embedded within a smoking cessation clinic that followed the Maudsley model |
|
| Outcomes |
|
|
| Study funding | "The research was commissioned by the Food Standards Agency UK (FSA). The FSA had no role in the study design, collection, analysis or interpretation of data, or the decision to submit the paper for publication." | |
| Author declarations | "The authors declare there are no conflicts of interest." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated |
| Allocation concealment (selection bias) | Low risk | Randomization carried out via an interactive voice response system |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "Measurements of exhaled carbon monoxide (CO) were made at weeks 6 and 24 to assess smoking status (piCO + breath CO monitors, Bedfont Scientific Ltd)“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Intervention and control participants had measurements of body weight, waist circumference and food choice/ intake made at baseline, weeks 6 and 24.“ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24‐wk completion intervention group: 40/68 (59%); 24‐wk completion placebo group: 43/70 (61%) |
1 Heggen 2016.
| Study characteristics | ||
| Methods | Country: Norway Recruitment: referrals to the Section for Preventive Cardiology at Oslo University Hospital and by newspaper advertisement Study start date: 4 January 2010; Study end date: 24 September 2013 |
|
| Participants | Total N: 122 smokers with overweight or obesity (BMI 25 ‐ 40) N per arm: Low‐carb diet = 64; Moderately fat‐reduced diet = 58 72.8% females, av age 50.1, av baseline BMI 30.5, av baseline weight 89.9 kg, av cpd 17.7, av FTND score 4.6 |
|
| Interventions |
Energy requirements were based on measured RMR and level of physical activity In both conditions, participants received a 12‐wk course of varenicline, motivational smoking cessation counselling (10 mins), and dietary advice and support by trained dieticians. Participants were given written dietary information, substitution lists, tips for planning meals and recipes to achieve targets for macronutrients, and for the first 7 days after the TQD were provided a daily lunch and snack specific to assigned diet |
|
| Outcomes |
|
|
| Study funding | "This work was supported by Local Departmental Resources, including provision of varenicline. Tine AB, Kavli Holding AS, Lantmännen Cerealia AS, and the Norwegian Meat and Poultry Council sponsored study meals. These companies had no role in the conduct, writing or interpretation of the trial" | |
| Author declarations | "EH and ST have received honorarium for lectures and consultancy for Pfizer, the manufacturer of varenicline, as well as from other pharmaceutical companies producing smoking cessation aids" | |
| Notes | Additional information provided by authors upon request This study is new to the 2021 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A block randomization procedure was used. The randomization list was computer‐generated by a statistician using blocks of 8 with a 1:1 allocation ratio |
| Allocation concealment (selection bias) | Low risk | The study co‐ordinator identified predetermined randomization assignment by consecutively opening sealed envelopes. Allocation of grouping through predetermined randomization contained in sealed envelopes |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “To be counted as a quitter a report of “not a single puff of a cigarette” and carbon monoxide < 10 ppm was required“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Height was measured by a standard wall‐scale. Body weight in kg was measured at every visit on a digital and calibrated scale (Seca 770) in light indoor clothing without shoes“ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24‐wk post‐TQD visit attendance low carbohydrate group: 48/64 (75%); 24‐week post‐TQD visit attendance moderately fat‐reduced group: 47/58 (81%) |
1 Johnson 2017.
| Study characteristics | ||
| Methods | Country: USA Recruitment: “Traditional recruitment approaches included media (i.e., television, radio), print (i.e., mass mailings of postcards to age appropriate persons identified by driver's license registries, newspaper ads, rack cards, flyers), and community (i.e., events at college campuses, health fairs, word of mouth). Technology recruitment approaches included website (TARGIT study), internet (i.e., Google ads, Craig's List), social media (TARGIT Facebook page) and email list serves (i.e., academic, healthcare, corporate, professional).” Setting: Department of Preventive Medicine, clinical trials research cente in Memphis, TN Study start date: August 2009; Study end date: October 2014 |
|
| Participants | Total N: 330 smokers, aged 18 ‐ 35 years, at risk for weight gain (e.g. plan to quit smoking), BMI ≥ 22 decreased ≥ 20, self‐report smoking ≥ 10 cpd N per arm: Comparison = 164; Intervention = 166 48.8% females, av age 29.7, av baseline BMI 29.2, av baseline weight 85.7, av cpd 17.9 |
|
| Interventions |
|
|
| Outcomes |
|
|
| Study funding | “This work was supported by grant funding from the National Heart, Lung, and Blood Institute, National Institutes of Health.” | |
| Author declarations | “The authors declared no conflict of interest.” | |
| Notes | Additional information provided by authors upon request This study is new to the 2021 update. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Participants were individually randomized in blocks of four (allocation ratio 1:1)” No further information given |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Neither the participants nor the study staff who provided the intervention were blinded to group assignment. However, study personnel who were collecting and assessing outcome data, such as weight, and the investigators, including the principal investigator, were blinded to treatment assignment (did not know a participant’s group assignment status). All participants were asked not to reveal their study group assignment to blinded clinic personnel.” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “…smoking cessation, was assessed as biochemically verified 7‐day point prevalence abstinence. Abstinence was only concluded if a participant self‐reported not smoking and exhaled carbon monoxide was</=10 ppm. Salivary cotinine was collected only at the 24‐month visit, and values >/=100 ng/mL were considered to be consistent with smoking.” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Weight was measured in the study clinic by using a standardized protocol created by the EARLY consortium on a calibrated scale.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 24 months: Comparison arm: 118/164 = 72%; Internvention arm: 107/166 = 64% |
1 King 2012.
| Study characteristics | ||
| Methods | Country: USA Recruitment: Internet, print, and radio advertisements Study start date: June 2006; Study end date: April 2010 |
|
| Participants | Total N: 333 N per arm: Naltrexone = 168; Placebo = 165 53.3% females, av age 41.9, av baseline BMI 27.0, av cpd 19.7, av FTND 5.1 |
|
| Interventions |
All participants received nicotine patch during the first 4 wks post‐TQD and attended 6 x 45‐minute weekly individual CBT smoking cessation counselling (pre‐ and post‐TQD) |
|
| Outcomes |
|
|
| Study funding | "This study was supported by grants from the National Institute of Drug Abuse/the National Institutes of Health (R01‐DA016834), CTSA Grant Number ULI‐RR024999 from the National Center for Advancing Translational Sciences (its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health), and the National Cancer Institute (P30‐CA14599)." | |
| Author declarations | "Andrea King is part of the advisory board and is a consultant for Lundbeck and the US Food and Drug Administration. Dingcai Cao is part of the advisory board and is a consultant for the US Food and Drug Administration. Stephanie S. O'Malley received research support from NABI Biopharmaceuticals, Pfizer Inc; is part of the advisory board and is a consultant for the American College of Neuropsychopharmacogy workgroup, the Alcohol Clinical Trial Initiative Group; is sponsored by Alkermes, Abbott Laboratories, Eli Lilly & Company, GlaxoSmithKline, Johnson & Johnson Pharmaceuticals, Lundbeck, Schering Plough, Pfizer, and Gilead Pharmaceuticals. Dr O'Malley was an inventor on a patent on naltrexone for smoking cessation held by Yale University, which has been abandoned. Henry R. Kranzler is part of the advisory board and is a consultant for the American College of Neuropsychopharmacogy Alcohol Clinical Trial Initiative Group, Lundbeck, GlaxoSmithKline, Alkermes, Gilead, Roche, Pfizer, and Lilly. Dr Kranzler received grants from Merck. Harriet deWit received grants from Unilever. Dr deWit is part of the advisory board and is a consultant for the US Food and Drug Administration. Alicia K. Matthews is part of the advisory board and is a consultant for US Food and Drug Administration. Xiaochen Cai and Ryan J. Stachoviak report no financial conflicts of interest." | |
| Notes | Additional information provided by authors upon request This study is new to the 2021 update. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomly assigned by computer" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Participants and study staff were blinded to treatment assignment.” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Biochemical validation in clinic. Participants who reported smoking abstinence during the past 7 days at telephone follow‐up were asked to attend an in‐person visit to provide biochemical validation |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: "At each study visit, participants underwent weight measurement … Weight was measured by the research assistant with a digital medical scale (Tanita, Tokyo, Japan), and weight values were not shared with the participant to reduce undue stress” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “The 12‐week completion rates did not differ by group (placebo, 115/154 participants [75%]; naltrexone, 123/161 participants [76%]).“ Quote: “Follow‐up rates were high and did not differ by group or sex (telephone follow‐up at 26 weeks, 99% completion in both groups; at 52 weeks, 98% placebo vs 96% naltrexone; in‐person follow‐up of abstainers at 26 weeks, 95% vs 89%, respectively; at 52 weeks, 92% vs 88%).“ |
1 Klesges 1990.
| Study characteristics | ||
| Methods | Country: USA
Recruitment: Community volunteers Study start date: not specified; Study end date: not specified |
|
| Participants | 57 adult female smokers who had previously experienced post‐cessation weight gain, av age 27, av 22.4 cpd, mean CO 49.8 ppm | |
| Interventions |
All participants received a "brief but intensive stop‐smoking intervention" and were offered a cash reward and opportunity to win prizes if they were successful at quitting for 2 weeks |
|
| Outcomes |
|
|
| Study funding | "Supported by a grant awarded to Dr. Klesges and Dr. Meyers (HL‐3932) by the National Heart, Lung, and Blood Institute (Be‐ thesda, Md.). Support was also received from a Centers of Excellence grant awarded to the Department of Psychology, Memphis State University, by the state of Tennessee. Schering‐Plough Corporation provided both the phenylpropanolamine and the placebo gum for this investigation." | |
| Author declarations | Not specified | |
| Notes | Intervention only 2 wks long. No 6‐month follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “double‐blind” “Neither the investigators, not the subjects knew which gum contained the active gradient” + placebo gum in same shape, colour and size" |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Biochemical verification of smoking status (by mean of carbon monoxide testing) + a random carbon monoxide abstinence verification check |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “height and weight measurements were taken privately” Comment: Unclear if self‐report or objective assessment of weight, but participants were blinded to treatment condition |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
1 Klesges 1995.
| Study characteristics | ||
| Methods | Country: USA
Recruitment: community volunteers Study start date: not specified; Study end date: not specified |
|
| Participants | 107 male and female smokers, age between 18 and 60, cpd 20+, CO > 15 ppm | |
| Interventions |
All participants received 1 x 30‐min session on smoking cessation and relapse prevention |
|
| Outcomes |
|
|
| Study funding | "This study was supported by two grants (HL45057, HL46352) awarded by the National Heart, Lung, and Blood Institute. We also appreciate the financial support from Schering‐Plough Corporation and for providing the placebo and PPA gum. Support was also received from a Centers of Excellence grant awarded to the Department of Psychology, Memphis State University, by the state of Tennessee." | |
| Author declarations | Not specified | |
| Notes | No 6 months follow‐up data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent randomization |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Assignment of product was independently randomized and group membership (e.g., gum A versus Gum B) was not revealed to the researchers until all data were coded. Identification of those using active gum was not revealed until statistical analyses were complete.” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | CO < 8 ppm |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Height and weight in subjects were assessed using a sensitive scale (Detecto Electronic) (..). The unit was zero calibrated by project staff prior to each use.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “five male subjects were dropped from the study due to elevated blood pressure … four of these subjects had been assigned to the PPA group and the remaining subject to the placebo group” |
1 Levine 2010 (also Part 2).
| Study characteristics | ||
| Methods | Country: USA Recruitment: community volunteers Study start date: September 2000; Study end date: December 2009 |
|
| Participants | 349 weight‐concerned women smokers, aged between 18 and 65 yrs, motivated to quit smoking, av 20.7 cpd, av age 42 | |
| Interventions |
CBT was delivered weekly for buproprion/placebo was taken for 26 wks |
|
| Outcomes |
|
|
| Study funding | "This research was supported by grant R01 DA 04174 from the National Institute on Drug Abuse (Dr Marcus). Dr Levine’s effort was partially supported by grant K01DA15396 from the National Institute on Drug Abuse (Dr Levine). GlaxoSmithKline provided bupropion hydrochloride SR, 150 mg, and matching placebo oral administration free of charge." Role of the Sponsor: "The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript." |
|
| Author declarations | "Dr Marcus has served as a consultant to GlaxoSmithKline and Sanofi‐Aventis. Dr Perkins has served as a consultant for GlaxoSmithKline." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization sequence |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, placebo‐controlled. No further information given (Judgement only relevant for part 2 analyses) |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “… expired‐air carbon monoxide (CO) was collected using a Vitalograph BreathCO monitor (Vitalograph Inc, Lenexa, Kansas). Salivary samples were collected immediately after each assessment visit (1, 3, 6, and 12 months). A CO reading of 8 ppm or less and cotinine level of less than 15 μg/L (to convert to nanomoles per liter, multiply by 5.675) were used to confirm non‐ smoking“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Women were weighed in street clothing without shoes prior to each session. Height was measured at baseline using a mounted stadiometer, and body mass index was calculated as weight in kilograms divided by height in meters squared” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24 week completion CONCERNS + B group: 64/106 (60%); 24 week completion CONCERNS + P group: 40/87 (45%); 24 week completion STANDARD + B group: 47/89 (52%); 24 week completion STANDARD + P group: 32/67 (47%); 48 week completion CONCERNS + B group: 57/106 (53%); 48 week completion CONCERNS + P group: 37/87 (42%); 48 week completion STANDARD + B group: 46/89 (51%); 48 week completion STANDARD + P group: 28/67 (41%); RoB assessment based on 6m attrition rate 6M overall retention rate = 52.4%; 12‐month rate = 48.1%; diff between groups is < 20% |
1 Lycett 2010.
| Study characteristics | ||
| Methods | Country: England Recruitment: Listed on GP database; Invited by letter from their GP; Interested participants telephoned trial office Setting: GP practices Study start date: 22 December 2008; Study end date: Not specified |
|
| Participants | Total N: 16 smokers with an exhaled CO ≥ 10 ppm for at least 15 mins after last smoking, aged > 18 years, with a BMI ≥ 25*, N per arm: Step by step control (SBS) = 6; Individual dietary and activity planning (IDAP) = 6; Very low calorie diet (VLCD) = 4 81% female, av age 46, av baseline BMI 29.5, av baseline weight 79.9, av cpd 23, av FTND: 5 |
|
| Interventions |
All participants received identical behavioural support and NRT patches (25 mg (8 wks),15 mg (2 wks),10 mg (2 wks)) |
|
| Outcomes | 1. Mean (SD) weight change (kg) in abstainers at EOT (narrative discussion) | |
| Study funding | "This trial is funded by UK Centre for Tobacco Control Studies (UKCTCS), a UKCRC Public Health Research: Centre of Excellence. Funding from British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, and the Department of Health, under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged." | |
| Author declarations | "Deborah Lycett declares no competing interests. Paul Aveyard has done consultancy work on smoking cessation for Pfizer, McNeil, and Xenova Biotechnology. Peter Hajek received research funding and provided consultancies to manufacturers of stop‐smoking medications." | |
| Notes | * Inclusion criteria were widened once study began. “We no longer excluded those who were unsuitable for a VLCD and we opened the trial to all smokers, regardless of BMI, this meant we were effectively running two trials, side by side. DeMiST 1, which used the original exclusion criteria, and randomised participants into three arms and DeMiST 2 which independently randomised participants into either the SBS or the IDAP arm.” This study is new to the 2021 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was computer generated by an independent statistician within the Primary Care Clinical Research and Trials Unit (PCCRTU) using random permuted blocks of length 6, stratified by practice. The numbers were entered into the trial database by an independent computer programmer within the trials unit. The database concealed randomisation until after participants were screened and entered into the trial." |
| Allocation concealment (selection bias) | Low risk | Quote: "At week ‐3 the nurses clicked on the randomisation tab in the database and this revealed the arm to which the participant was allocated. The database was set up so that the randomisation „tab‟ would not work until all data from week ‐2 was complete. Therefore, it was impossible for anyone to see treatment allocation beforehand. This greatly minimised any risk of the trial randomisation being undermined" |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "Twenty‐four hour point prevalence abstinence is measured by participants achieving 24 consecutive hours of abstinence as verified by exhaled CO < 10 ppm. Seven day point prevalence abstinence is defined as those not smoking over the last 7 days as verified by exhaled CO < 10 ppm. Participants achieving 1, 6 and 12 month abstinence as defined using the Russell Standard which states that no more than 5 cigarettes since have been smoked since week +2, this is verified by CO < 10 ppm at each consultation [34]. Participants who have not achieved abstinence but are still attempting to quit are termed ‘lapsed" |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Quote: “These are measured as described below. The schedule of measurements is contained in table 2. 1. Weekly weight, waist to hip ratio, % body fat composition, blood pressure, and heart rate.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High loss to follow‐up, including 100% of participants in 1 arm. > 50% loss to follow‐up End of treatment: SBS = 3/6 = 50% IDAP = 0/6 = 0% VLCD = 1/4 = 25% |
1 Lycett 2020.
| Study characteristics | ||
| Methods | Country: England Recruitment: NHS commissioned Stop Smoking Services were first recruited from Bristol and North Somerset Primary Care Trusts, then across the West Midlands and South‐West England due to poor recruitment of clinics. “All individuals attending the Stop Smoking Services were invited to take part in the trial at their first visit when they set their quit date.” ”Participating services located within pharmacies, general practices and community centers will display an A3 poster advertising the trial.” Setting: National Health Service commissioned Stop Smoking Services; Commercial weight management programme (Slimming world) Study start date: October 2012; Study end date: 6 May 2013 |
|
| Participants | Total N: 76 daiily smokers with expired CO > 10 ppm, aged ≥ 18 years and BMI ≥ 23 N per arm: Usual care = 39; Intervention = 37 65% female, av age 46.7, av baseline BMI 30.5, av FTND 5.8 |
|
| Interventions |
|
|
| Outcomes |
As EOT time point varied for each study arm, outcome data for EOT is reported narratively |
|
| Study funding | This work was supported by funding of the salary for DL, provided by a fellowship from the NIHR‐SPCR during the active trial period. Additional funds to set up the trial and buy equipment were provided by the NIHR‐SPCR and UKCTCS. The UKCTCS is one of five UK Public Health Research Centres of Excellence. We also gratefully acknowledge funding from the British Heart Foundation, Cancer Research UK, the Economic and Social Research Council, Medical Research Council and the Department of Health, under the auspices of the UK Clinical Research Collaboration. AL’s salary was paid by NIHR‐SPCR during the trial period, and the salaries of AF, MM and PA were paid by their respective institutions. PA is an NIHR senior investigator and funded by NIHR Oxford Biomedical Research Centre and CLAHRC. All funding is gratefully acknowledged. | |
| Author declarations | DL has received hospitability from manufacturers of smoking cessation products, Pfizer, Tadworth, UK. DL’s institution during the active phase of the trial has received smoking cessation products for use in a clinical trial from Johnson & Johnson, New Brunswick, New Jersey, USA. DL has received Slimming World membership vouchers for use in this trial. DL has received expenses and consultancy fees from the NHS and Universities for teaching about cessation‐related weight gain. DL has received grant funding from UKCTCS and the NIHR‐SPCR for research relating to cessation‐related weight gain. PA is an NIHR senior investigator and is funded by NIHR Biomedical Research Centre and the CLAHRC, Oxford. AF is an NIHR Senior Investigator and receives funding from NIHR Oxford Biomedical Research Centre. AL has received hospitality from Weight Watchers. In the last 5 years, MM has received grant funding from Pfizer and varenicline, for research purposes. |
|
| Notes | This study is new to the 2021 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomisation sequence was computer generated and concealed until allocation.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Stop smoking advisors were unaware of the randomisation sequence; they opened sealed, numbered, opaque envelopes in turn.” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “Abstinence is prolonged abstinence, defined according to the Russell Standard as self‐report of no more than five cigarettes since 2 weeks following quit day and expired CO less than 10 ppm...” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “The primary outcome was objectively measured weight…” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At week 4: Usual care: 20/39*100 = 51.3%; Intervention: 20/37*100 = 54.1% At week 12: Usual care: 15/39*100 = 38.5%; Intervention: 15/37*100 = 40.5% At week 26: Usual care: 10/39*100 = 25.6%; Intervention: 12/37*100 = 32.4% |
1 Lyu 2018.
| Study characteristics | ||
| Methods | Country: China Recruitment: Inpatient unit at Xuhui Mental Health Center located in Shanghai Setting: Inpatient unit at Xuhui Mental Health Center Study start date: May 2016; Study end date: July 2018 |
|
| Participants | Total N: 22 men with schizophrenia, on stable antipsychotic medication treatment for at least 1 month, aged 18 ‐ 65, smoking ≥ 10 cpd for 1 year or longer, BMI > 28 or 27 in the presence of dslipidaemia or waist circumference over 90 cm, with a desire to lose weight and quit smoking. N per arm: Control: 11 (10 reported at baseline (1 was lost to follow‐up and excluded from analysis)); Intervention: 11; 0% female, av age 55.3, av baseline BMI 26.9, av baseline weight 76.6, av cp week 74.8 |
|
| Interventions | 1. Placebo naltrexone and placebo bupropion for 24 weeks 2. Adjunctive naltrexone (25 mg/day) and bupropion (300 mg/day) combination treatment for 24 weeks |
|
| Outcomes |
Adverse events reported per study arm at 6 months (EOT) in narrative discussion |
|
| Study funding | “Program of Shanghai Academic Research Leader (17XD1403300), Shanghai Key Laboratory of Psychotic Disorders (13DZ2260500), Shanghai Municipal Health and Family Planning Commission (2017ZZ02021).” | |
| Author declarations | “The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest." | |
| Notes | This study is new to the 2021 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Following screening and confirmation that inclusion and exclusion criteria were met, participants were randomized to receive the combination treatment or placebo.” Comment: No further information |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Following screening and confirmation that inclusion and exclusion criteria were met, participants were randomized to receive the combination treatment or placebo.” Comment: No further information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “The purpose of the present 24‐week, randomized, double blind, placebo controlled pilot trial was to examine the feasibility, safety, and initial benefit of using naltrexone and bupropion combination in treating obesity and smoking cessation in patients with schizophrenia” Comment: No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Expired CO level measured at baseline and 24‐weeks |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Medical and psychiatric histories were obtained, and physical exam was performed for each participant.” “The following measures were completed at baseline and week 24: (1) height, weight and waist circumference…” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Among 28 patients who fit the inclusion and exclusion criteria, 22 of them were enrolled in the study. One patient in the placebo group dropped out of the study after week‐12 because of loss of follow up after being discharged from the inpatient unit. Therefore 21 patients (11 in the treatment group, 10 in the control group) completed the 24‐week trial and were included in the final analysis.” |
| Other bias | Low risk | Quote: “In the treatment group, 5 patients were on clozapine, 7 on olanzapine, 8 on other antipsychotic agents (risperidone, amisulpride, quetiapine, and chlorpromazine); in the placebo group, 5 patients were on clozapine, 4 on olanzapine, 11 on other antipsychotic agents (risperidone, amisulpride, paliperidone, and chlorpromazine. There were no significant differences between the two groups in the numbers of patients on clozapine or olanzapine (p’s > 0.05).” |
1 NCT03528304 2016.
| Study characteristics | ||
| Methods | Country: USA Recruitment: Not specified Setting: Clinic Study start date: September 2010; Study end date: January 2015 |
|
| Participants | Total N: 125 female daily smokers of reproductive age (18 ‐ 44 years) with overweight or obesity, of American Indians and Alaska Native hertigate | |
| Interventions |
All participants attended face‐to‐face clinic visits, the control arm attended these visits for assessment only |
|
| Outcomes | Data measured during the trial but not available for extraction at the time of this update.
|
|
| Study funding | 5U48DP001911‐02 ( U.S. NIH Grant/Contract); Sponsor: Washington State University (reported on clinical trial register). | |
| Author declarations | NS (information extracted from a clinical trial registry only) | |
| Notes | Information extracted from clinical trial record only This study is new to the 2021 update. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “In each intervention session the women participants will complete a urine test to determine if they have smoked a cigarette within the past 3‐4 days…” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “In each intervention session the women participants will complete a urine test to determine if they have smoked a cigarette within the past 3‐4 days, and will be weighed on a dedicated, standardized scale” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
1 Norregaard 1996.
| Study characteristics | ||
| Methods | Country: Denmark
Recruitment: Community volunteers Study start date: not specified; Study end date: not specified |
|
| Participants | 225 smokers who wanted to quit without gaining weight, 65% female, av BMI 23 ‐ 24, av age 38 ‐ 39, av 20 cpd | |
| Interventions |
All participants given advice on how to quit smoking and prevent weight gain (inc booklet about low‐fat food) |
|
| Outcomes |
|
|
| Study funding | Not specified | |
| Author declarations | Not specified | |
| Notes | Prolonged abstinence defined as no smoking after wk 1 post‐quit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimization |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “double blind, placebo controlled” but “Blinding was incomplete because 68% in the ephedrine plus caffeine‐treated group and 63% in the placebo group correctly guessed their treatment at trial termination (p<0.001)” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “self‐reported abstinence with validation by carbon monoxide in expired air and serum cotinine“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “subjects were weighed by means of the same scales at each visit without shoes and overcoats” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Three subjects (two in the ephedrine plus caffeine‐treated group) moved from the area or were lost to follow‐up. Seven subjects (one in the ephedrine plus caffeine‐treated group) were excluded because they started treatment with ephedrine plus caffeine or nicotine replacement (prescribed by their own doctor). Six subjects (all in the the ephedrine plus caffeine‐treated group) were withdrawn because of side effects, and an additional six subjects (all in the the ephedrine plus caffeine‐treated group) left the trial for other reasons“ |
1 O'Malley 2006.
| Study characteristics | ||
| Methods | Country: USA
Recruitment: Community volunteers Study start date: not specified; Study end date: not specified |
|
| Participants | 400 smokers, 46% female, av BMI 27 ‐ 28, av 26 ‐ 29 cpd, av age 45 ‐ 47 | |
| Interventions |
All participants also given 6 wks supply of 21 mg patches and 6 sessions of behavioural support (1 x 45 mins, 5 x 15 mins) |
|
| Outcomes |
|
|
| Study funding | "This study was supported by grants P50‐DA‐13334 from the National Institute on Drug Abuse and grants P50AA15632, K02‐AA00171, and R01‐ AA11197 from the National Institute on Alcohol Abuse and Alcohol Dependence, National Institutes of Health, Bethesda, Md; grant 039787 from the Robert Wood Johnson Foundation, Princeton, NJ; and by the Department of Veterans Affairs, Newington, Conn." | |
| Author declarations | "Nicotine patches were donated by GlaxoSmithKline Inc. Naltrexone hydrochloride and matching placebo were purchased from Mallinckrodt Pharmaceuticals. Drs O’Malley, Krishnan‐Sarin, and Meandzija are coinventors on a patent held by Yale University for smoking cessation treatments using naltrexone and related compounds. Dr O’Malley has received research support from Alkermes Inc (a manufacturer of an investigational injectable naltrexone), DuPont (a manufacturer of naltrexone), GlaxoSmithKline Inc, Forest Laboratories, Lipha Pharmaceuticals, Ortho‐McNeil, Inc, Bristol‐Myers Squibb, Pfizer Inc, Sanofi‐Aventis, and Mallinckrodt Pharmaceuticals; served as a consultant to Alkermes Inc, Forest Laboratories, GlaxoSmithKline Inc, Ortho‐McNeil, Inc, Pfizer Inc, and Johnson & Johnson; and received travel reimbursement from Alkermes Inc. Dr Krishnan‐Sarin has received grant support from and served as a consultant to Pfizer Inc. In separate company‐ sponsored studies held at Yale University, Dr Meandzija and Ms Romano‐Dahlgard received a portion of their salary from Alkermes Inc, Bristol‐Myers Squibb, and Ortho‐McNeil, Inc, and Dr McKee received a portion of her salary from Pfizer Inc." | |
| Notes | Arms 1 ‐ 3 combined for the main comparisons No 6‐month follow‐up data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization, stratified by sex after the first 150 participants |
| Allocation concealment (selection bias) | Low risk | Random sequence was provided to the pharmacist, who assigned participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Random sequence was provided to the pharmacist, who assigned participants; others were blinded to treatment assignment.“ + Placebo controlled |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "self‐reported abstinence... was verified by an exhaled CO of 10ppm or less" |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Unclear if weight was assessed objectively or by means of self‐report but participants were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Treatment completion placebo group: 75/98 (76%); Treatment completion 25 mg naltrexone group: 75/94 (79%); Treatment completion 50 mg naltrexone group: 71/99 (71%); Treatment completion 100mg naltrexone group: 74/109 (67%) ‐‐> Attrition rates at follow‐up not reported |
1 Oncken 2014.
| Study characteristics | ||
| Methods | Country: USA Recruitment: Advertising Study start date: 1 April 2006; Study end date: 1 December 2008 |
|
| Participants | Total N: 57 N per arm: Placebo = 19; Topirimate = 19; Topirimate plus NRT = 19 60% female, av age 47.2, av baseline BMI 28.1, av cpd 21.4, av FTND 5.9 |
|
| Interventions |
TQD 2 wks after baseline visit. All groups received weekly behavioural counselling (10 mins) from a nurse from baseline, and Clearing the Air: Quit smoking today booklet |
|
| Outcomes |
|
|
| Study funding | "This project was supported by funds from the Department of Medicine at the University of Connecticut Health Center and the General Clinical Research Center (M01RR006192). HRK’s participation was funded by National Institute on Alcohol Abuse and Alcoholism (K24 AA13736)" | |
| Author declarations | "CO currently is receiving study medication (nicotine inhaler and placebo) from Pfizer Pharmaceuticals for a National Institutes of Health–funded study of nicotine inhaler for smoking cessation during pregnancy. MS has served as an expert witness on behalf of Pfizer in lawsuits related to varenicline. HRK has served as a consultant or advisory board member for Alkermes, Lilly, Lundbeck, Pfizer, and Roche and as a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, supported by AbbVie, Lilly, Lundbeck, and Pfizer" | |
| Notes | This study is new to the 2021 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Urn randomization procedure to balanced on sex and FTND score. No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and research personnel blinded to trial arms 1 and 3, but not 2. The group on NRT knew this, as did those who were not on NRT but the topirimate (hypothesized to impact on weight) was blinded |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “biochemically confirmed cigarette abstinence was assessed at Weeks 8–11 (…) no posttreatment abstinence rates were reported“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “We assessed vital signs, weight, adverse effects and tolerability of the medication, and exhaled CO levels at each weekly visit“. Comment: Participants and assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “Thirty‐eight subjects (66.7%) completed the 10‐week study with the highest completion rate in the TOP/NIC group (16 of 19 or 84%). The rate of treatment completion in both the PLC and the TOP groups was 58% (i.e., 11 of 19 subjects in each group). The rate of completion did not differ significantly by group (χ2(2) = 3.95; p = .14). Reasons for dropout included: adverse events (one PLC, four TOP, and one TOP/NIC), lack of efficacy (two PLC), lost to follow‐up (five PLC, three TOP, and one TOP/NIC), and other (one TOP and one TOP/NIC).” Comment: Difference in follow‐up between groups is greater than 20% |
1 Oncken 2019.
| Study characteristics | ||
| Methods | Country: USA Recruitment: “We recruited participants through local mass media advertisements (eg, radio), mass media (eg, Facebook), and through flyers placed in medical clinics.” Setting: Universities of Connecticut and Minnesota Study start date: March 2009; Study end date: 29 April 2017 |
|
| Participants | Total N: 301 women who smoked ≥ 10 cpd, were postmenopausal (i.e., with no menstruation for at least 1 year), and were motivated to quit smoking N per arm: Relaxation = 151; Exercise = 150 100% female, av age 55.9, av baseline BMI 28.5, av cpd 18.9 |
|
| Interventions |
All participants were provided with 12 weeks of varenicline to support smoking cessation, a Clearing the Air smoking cessation manual and diary for daily entries. All participants were also encouraged to practise techniques (relaxation or exercise) at home, and an interactive Voice Response tool was to improve adherence outside of supervised sessions. Weekly feedback provided on IVR recordings and diaries |
|
| Outcomes | Data measured during the trial but not available for extraction at the time of this update.
|
|
| Study funding | “The study was supported by R01DA024872 and the Lowell P. Weicker Clinical Research Center at the UConn Health.” | |
| Author declarations | “Dr. Oncken has received free nicotine and placebo inhalers from Pfizer Pharmaceuticals for an NIH‐funded smoking cessation study in pregnant women. The remaining authors report no conflict of interest.” | |
| Notes | This study is new to the 2021 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “After baseline measurements, we randomized participants in block assignments of 4–8 participants to either the Exercise or the Relaxation condition using a computerized urn randomization procedure that assigned groups to treatment conditions by balancing on FTCD score (with a cutoff of 6) and history of depression (yes–no).” |
| Allocation concealment (selection bias) | Low risk | Quote: “Allocation of patients to condition was concealed to study personnel prior to random assignment but not during the treatment period.” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “We defined continuous abstinence weeks 9–12 as no recorded smoking, not even a puff, biochemically verified by CO ≤ 5 ppm.” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Follow‐up visits to assess smoking status occurred at 3, 6, 9, and 12 months after treatment. At each of these visits we measured cigarettes per day, exhaled carbon monoxide, weight and height, and self‐reported minutes per day of exercise and relaxation, and we administered questionnaires.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow‐chart: 24‐week completion relaxation: 95/151; exercise: 100/150 9 months completion relaxation: 91/151; exercise: 89/150 12 months completion relaxation: 82/151; exercise: 82/150 15 months completion relaxation: 93/151; exercise: 92/150 |
1 Parsons 2009.
| Study characteristics | ||
| Methods | Country: England Recruitment: community volunteers Study start date: May 2006; Study end date: June 2007 |
|
| Participants | 143 smokers 63% female, av age 45.5, av baseline weight 75.1 kg, av cpd 20 |
|
| Interventions |
All participants received 7 wks of behavioural counselling with TQD coinciding with the 3rd visit |
|
| Outcomes |
|
|
| Study funding | Not specified | |
| Author declarations | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | independent statistician prepared an excel spreadsheet using Stata to generate 2 lists of randomly‐sequenced blocks of 2, 4, or 6, which were passed to the medication packing company |
| Allocation concealment (selection bias) | Low risk | Lists were used to package together medication of SJW or placebo and CR or placebo, which were allocated in sequence to participants in clinic |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants, therapists, and outcome assessors were blind to the treatment allocation“ + Placebo controlled |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “abstinence was confirmed by exhaled carbon monoxide (CO) concentration of less than 10 parts per million (ppm)“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Weight and percentage body fat were measured using a Tanita TBF300MA body composition analyser. Baseline weight was defined as the mean of the two pre‐quit weights measured at the baseline visit and 1 week later. The mean was used to give a better measure of pre‐quit weight less sensitive to fluctuations” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 24‐week completion SJWa‐CRa group: 34/36 (94.4%) provided smoking data; 24‐week completion SJWa‐CRa group: 9/36 (25%) provided weight data; 24‐week completion SJWa‐CRp group: 33/35 (94.3%) provided smoking data; 24‐week completion SJWa‐CRp group: 3/35 (8.6%) provided weight data; 24‐week completion SJWp‐CRa group: 32/37 (86.5%) provided smoking data; 24‐week completion SJWp‐CRa group: 3/37 (8.1%) provided weight data; 24‐week completion SJWp‐CRp group: 31/35 (88.6%) provided smoking data; 24‐week completion SJWp‐CRp group: 8/35 (22.9%) provided smoking data |
1 Perkins 2001.
| Study characteristics | ||
| Methods | Country: USA
Recruitment: community volunteers Study start date: not specified; Study end date: not specified |
|
| Participants | 219 weight‐concerned women, av age 44, av body weight 69 kg, mean cpd 21 | |
| Interventions |
All participants received standard CB SC counselling at each session |
|
| Outcomes |
|
|
| Study funding | "This research was supported by National Institute on Drug Abuse Grant DA04174." | |
| Author declarations | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | After a sufficient number of participants to form a group recruited, group assigned to a treatment condition |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “point‐prevalence was defined as self‐report of no smoking at all during the 7 days prior to the follow‐up point, confirmed by CO<= 8 ppm. (…) Those who dropped out or were otherwise lost to follow up at any point were presumed to have relapsed to smoking and coded as such in all outcome analyses” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Body weight was measured at each session fully clothed on an electronic scale” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “Attrition by the end of the 7 weeks of treatment (4 weeks postquit) was 19%, 10%, and 21% for CBT, weight control, and standard, respectively, and the difference between weight control and standard was significant, x2 ( l, N = 145) = 3.75, p = .05.” |
1 Pirie 1992 (also Part 2).
| Study characteristics | ||
| Methods | Country: USA
Recruitment: community volunteers Study start date: not specified; Study end date: not specified |
|
| Participants | 417 women smokers, av cpd 25 ‐ 27, av age 42 ‐ 44, av BMI 23 ‐ 24, 30 ‐ 40% expressed great weight concern | |
| Interventions |
Gum type: 2mg ad lib 8‐wk treatment period + 3 months supply |
|
| Outcomes |
|
|
| Study funding | "This research was supported by grant no. CA41647 from the National Cancer Institute" | |
| Author declarations | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | no blinding for (Part 2 only; not applicable for Part 1) |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “expired air carbon monoxide” + “saliva was collected for biochemical validation .. only for those individuals reporting themselves to be abstinent” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “weight was measured with a balance beam scale at each group session” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Data were collected in the follow‐up clinics on 98% of participants at 6 months and on 98.1% of participants at 12 months. Those few participants who had moved out of the area were allowed to complete self‐report assessments by telephone and mail, resulting in 100% participation in the follow‐up assessments” |
1 Prapavessis 2018.
| Study characteristics | ||
| Methods | Country: Canada Recruitment: “...from local businesses, hospitals, academic institutions and organizations and through advertisements placed in newspapers, radio stations and city buses in London, Ontario.” Setting: Exercise and Health Psychology Laboratory (EHPL) Study start date: October 2009; Study end date: April 2014 |
|
| Participants | Total N: 413 (411 in secondary analysis) female smokers between 18 ‐ 65 years, smoked > 10 cpd for the previous 2 years, want to quit smoking, engaged in ≤ 2 30‐minute bouts of moderate‐ or vigorous‐intensity exercise/week over the past 6 months and were able to read and write in English N per arm: Contact Control = 95; Exercise Maintenance + Contact Control = 106; Smoking cessation Maintenance + Contact Control = 100; Exercise Maintenance + Smoking Cessation Maintenance = 108 100% female, av age 42.4, av cpd 16.9 |
|
| Interventions | All participants received a 14‐week facility‐based group exercise programme (Week 0 ‐ 8: 3 x 45‐min sessions/week; week: 9 ‐ 11: 2 x sessions/week; Week 12 ‐ 14: 1 x session/week) with individualised exercise prescriptions. At week 4, all participants also received the NicoDerm 3‐step, 10‐week transdermal patch programme
Smoking cessation relapse prevention booklets (Brandon’s Forever Free booklets) containing evidence‐based information on urges, weight gain, stress and lifestyle balance. Contact control included 7 x group‐based sessions discussing women's health issues during weeks 8 ‐ 14 plus 10 phone messages reinforcing health issues covered over the next 12 months
|
|
| Outcomes |
|
|
| Study funding | “This was an investigator initiated study funded by a grant from the Canadian Cancer Society (#019876‐PI‐HP). The Exercise and Health Psychology Lab (www.ehpl.uwo.ca) where this work was conducted, is supported by a Canadian Foundation Innovation infrastructure grant (#312466) award to the PI‐HP. Clinical Trials Registration Number: NCT01305447.” | |
| Author declarations | “All authors declare that they have no conflict of interest.” | |
| Notes | All participants received exercise treatment This study is new to the 2021 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Participants will be randomized to one of four conditions: (a) exercise adherencebased group‐mediated cognitive behavioural therapy, or GMCB (Exercise Maintenance), (b) GMCB plus smoking cessation relapse prevention booklets (Exercise Maintenanceþ Relapse Prevention Booklets), (c) smoking cessation relapse prevention booklets plus group‐mediated discussions of women's health issues (Relapse Prevention Bookletsþ Contact), or (d) group‐mediated discussions of women's health issues (Contact Control) using a central computerized system" |
| Allocation concealment (selection bias) | Low risk | Quote: “The project manager for trial used numbered containers to implement the random allocation sequence, and the sequence was concealed until interventions were assigned“ |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “To be considered smoke‐free for analyses participants had to show CO levels<6 ppm for the full 10 weeks of the treatment program (i.e., weeks 4–14). Those who provided CO levels ≥6 ppm or failed to provide CO level at any time over the treatment program were considered a smoker.” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Height and weight were collected (Health‐o‐meter professional, Pelstar 500KL) after asking participants to remove their shoes and heavy clothing (e.g., sweater).” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Week 14 follow‐up: EM +SCM = 75/108 *100 = 69.4%; EM + CC = 79/106*100 = 74.5%; SCM + CC = 67/100 *100 = 67%; CC = 64/95 *100 = 67% Week 26 Follow‐up: EM +SCM = 66/108 *100 = 61%; EM + CC = 68/106*100 = 64.2%; SCM + CC = 55/100 *100 = 55%; CC = 55/95 *100 = 57.9% Week 56 Follow‐up: EM +SCM = 51/108 *100 = 47.2%; EM + CC = 55/106*100 = 51.8%; SCM + CC = 48/100 *100 = 48%; CC = 35/95 *100 = 36.8% |
1 Rose 2019.
| Study characteristics | ||
| Methods | Country: USA Recruitment: “Subjects were recruited from a metropolitan area via advertisements targeting smokers who were concerned about gaining weight after quitting smoking.” Setting: Duke Center for Smoking Cessation clinic Study start date: 8 November 2016; Study end date: 18 October 2018 |
|
| Participants | Total N: 61 smokers, 18 ‐ 65 years, smoking ≥ 10 cpd for at least 1 cumulative year with CO ≥ 10 ppm, body weight of > 50 kg (110 lbs.) and want to quit smoking in the next 30 days N per arm: Patch = 30; Lorcaserin + Patch = 31 75.4% female, av age 45.5, av baseline BMI 30.9, av baseline weight 88.2 kg, av cpd 16.3, av FTND 5.3 |
|
| Interventions |
All participants received behavioural treatment: 30 mins of smoking cessation counselling, time‐based monetary incentives to delay smoking and diaries to record medication use and the number of cigarettes smoked per day |
|
| Outcomes |
Also measured continuous 4‐wk abstinence at EOT (CO validation) |
|
| Study funding | “This study was funded by a grant from the National Institute on Drug Abuse (P50‐DA027840).” | |
| Author declarations | “J.R. received funding from Philip Morris International, Altria, JUUL Labs. He discloses consulting with Philip Morris International and Revive Therapeutics LLC. He obtained patent purchase agreement in 2011 with Philip Morris International for nicotine inhalation system. J.R.’s privately funded projects are limited to the development and evaluation of reduced‐risk tobacco products. J.M.D. received funding from Pfizer Inc. and Axsome Therapeutics Inc. J.M.D. privately funded projects are limited to evaluation of programs or development of drugs designed to treat tobacco use.” | |
| Notes | “Subjects will be reimbursed up to $417.42 for attending seven study visits and one follow up visit. There will be a payment of $40 per study visit attended. In addition, subjects will receive a payment of $10 for each of the 7 sessions attended in which they return their completed take‐home forms (brief questionnaires which describe withdrawal symptoms and record smoking behavior and medication use). Subjects will also receive additional compensation up to $7.42 (per Sherry McKee protocol) for completing the laboratory session (P2). Subjects who do not complete each visit will still receive payment for the sessions attended. Thus, subjects who attend seven visits and hand in their take‐home forms each time will receive a total payment of $350 plus the amount earned in the P2 laboratory session. Subjects who come back for a follow up session six months after their Quit Day will receive an additional $60. Subjects will not be compensated for the screening session.” Both arms received the same pharmacotherapy in different schedules. This study is new to the 2021 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Participants will be randomized to receive nicotine patch + lorcaserin or nicotine patch + placebo for the first 2 weeks” Comment: No further detail provided in the protocol or primary publication. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Participants will be randomized to receive nicotine patch + lorcaserin or nicotine patch + placebo for the first 2 weeks” Comment: No further detail provided in the protocol or primary publication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “In order to maintain a double‐blind assignment to treatment conditions, subjects receiving nicotine patch alone (Group B) will receive placebo lorcaserin tablets during the first two weeks.” Clinical trial record: “Masking: Double (Participant, Care Provider)” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “Ad libitum smoking was assessed weekly via daily diary recordings of cigarettes smoked per day as well as the objective index of expired air carbon monoxide (CO) levels measured at the study visits.” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Weight objectively measured |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Week 2 (from baseline; 2 weeks pre‐quit date) Patch: 27/30*100 = 90%; Lorcaserin + Patch: 30/31*100 = 96.8% Week 14 (from baseline; 10 weeks from Quit date): Patch: 20/30*100 = 66.7%; Lorcaserin + Patch: 17/31*100 = 54.8% 6 months post‐quit date Patch: 16/30*100 = 53.3%; Lorcaserin + Patch: 14/31*100 = 45.2% |
1 Shanahan 2017.
| Study characteristics | ||
| Methods | Country: USA Recruitment: Not specified Setting:30 clinical research sites in the USA experienced in smoking cessation trials Study start date: 28 February 2014; Study end date: 15 May 2014 |
|
| Participants | Total N: 603 smokers, 18 ‐ 65 years, smoking ≥ 10 cpd for the past year with no period of abstinence > 3 months, motivated to quit smoking, BMI 18.5 – 38.0 and at least 50 kg N per arm: Placebo = 200; Lorcaserin 10 mg daily = 202; Lorcaserin 10 mg twice a day = 201 54.4% female, av age 45.6, av baseline BMI 27.8, av baseline weight 80.4 kg, av cpd 18, av FTND 5.6. |
|
| Interventions |
All participants received standard smoking cessation counselling by a trained counsellor. Counselling sessions were individual, face‐to‐face and 10 mins long |
|
| Outcomes |
|
|
| Study funding | “This study was funded by Arena Pharmaceuticals, Inc. and Eisai, Inc.” | |
| Author declarations | “JER is a consultant to Arena Pharmaceuticals. All other authors (WRS, AG, SS, and MS‐K) were full‐time employees of Arena Pharmaceuticals, Inc. at the time of trial design and execution, data analysis, and manuscript preparation and are shareholders.” | |
| Notes | This study is new to the 2021 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The contract research organization created the randomization codes using a random number generator.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Qualifying participants were centrally randomized in a 1:1:1 ratio to the three treatment options.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The contract research organization maintained the database and the treatment blind until after database lock. All site, contract, and sponsor personnel involved with conduct or analysis of the trial remained blinded until after database lock.” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “…weekly exhaled carbon monoxide measurements (MicroCO™/Carefusion)…” "The prespecified primary endpoint was the continuous abstinence rate for the last 4 weeks of the trial, weeks 9–12 (month 3) in the modified Intent‐to‐Treat (mITT) population. Secondary endpoints included continuous abstinence for weeks 5–8 (month 2) and for weeks 3–12, the weekly point prevalence of abstinence for weeks 3–12. To meet abstinence endpoints, participants had to specify no nicotine use, not even one puff, during the evaluated period, confirmed by weekly exhaled carbon monoxide values of ≤10 ppm.“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Weight was measured in the fasting state post voiding in the morning, in patient gown and undergarments using certified scales sensitive to within 100 grams supplied by the sponsor.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Week 12 completion rates were 70.0% for placebo, 72.8% for lorcaserin QD, and 85.1% for lorcaserin BID participants” |
1 Sobell 2017.
| Study characteristics | ||
| Methods | Country: USA Recruitment: “Participants were recruited during 2006 to 2008 from military primary care centers in the San Antonio, Texas, area. Potential participants learned about the study through referrals from staff or from fliers posted at the centers.” Setting: Not specified Study start date: 2006; Study end date: 2008 (recruitment end date) |
|
| Participants | Total N: 317 millitary personnel regularly consuming alcohol (≥ 4 drinks per week (1 standard drink = 0.6 oz. or 14 g ethanol)) and regularly smoking (≥ 5/day during past year), have CO ≥ 8 ppm when screened, 21 – 75 years of age, interested in quitting and willing to set quit date within 6 weeks and concerned about weight gain N per arm: Standard Smoking Cessation = 159; Smoking cessation plus group = 158 29% female, av age 37.4, av baseline weight 84.9, av FTND 3.9 |
|
| Interventions |
|
|
| Outcomes |
|
|
| Study funding | “This study was supported by a grant from the Department of Defense Peer Review Medical Research Program of the Office of the Congressionally Directed Medical Research Programs, Grant Number W81XWH‐05‐2‐0015. The opinions expressed herein and the interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official recommendation, interpretation, or policy of the National Institutes of Health, the Department of Health and Human Services, the Department of Defense, or the U.S. Government.” | |
| Author declarations | “The authors declare that they have no conflict of interest.” | |
| Notes | This study is new to the 2021 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Participants randomly assigned to the SCP intervention were scheduled to take part in four sessions, two face‐to‐face, and two by telephone.” Comment: No further information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Participants randomly assigned to the SCP intervention were scheduled to take part in four sessions, two face‐to‐face, and two by telephone.” Comment: No further information provided |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “For available participants, an absence of smoking was confirmed by having a CO level of < 8 ppm” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Weight in pounds was measured at the 12‐month follow‐up, with the exception of a small number of cases (e.g., deployed and overseas, stationed at a facility in another part of the country).” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3‐month follow‐up: SSC: 151/159*100 = 95%; SCP: 141/158*100 = 89.2% 6‐month follow‐up: SSC: 144/159*100 = 90.6%; SCP: 144/158*100 = 91.1% 12‐month follow‐up: SSC: 127/159*100 = 79.9%; SCP: 141/158*100 = 89.2% |
1 Spring 1995 (also Part 2).
| Study characteristics | ||
| Methods | Country: USA
Recruitment: community volunteers Study start date: not specified; Study end date: not specified |
|
| Participants | 144 female weight‐concerned smokers, av age 41, av cpd 27, av BMI 23 ‐ 25 | |
| Interventions |
All participants received weekly group behavioural support for first 4 wks and fortnightly support for remaining 8 wks |
|
| Outcomes |
|
|
| Study funding | "Supported by awards from the Center for Brain Sciences and Metabolism Charitable Trust and National Institutes of Health grant #MOI‐RR00088 to the MIT Clinical Research Center, as well as by VA Merit Review and American Cancer Society awards to BS" | |
| Author declarations | "MIT holds patents governing the use of both dexfenfluramine and fluoxetine for various weight control applications with JW and RW as co‐patent assignees. BS is co‐patent assignee for the application to prevent post‐cessation weight gain" | |
| Notes | No 6 months follow‐up data Prolonged abstinence defined as validated continuous abstinence after a 2‐week grace period Fluoxetine arm used in first part of review as taken specifically to prevent post‐cessation weight gain and this study is not included in the parent antidepressant review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind + placebo controlled. Quote: "All subjects received identical packets of three pills" |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “to be considered abstinent from smoking, all of the following were required: self‐report of no smoking, expired carbon monoxide < 8 ppm and plasma cotinine < 10 ug/L.” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Body weight of subjects without shoes was always measured by using the same balance‐beam scale.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “The three treatment groups differed significantly in their rates of attrition. More dexfenfluramine (30/47, 63.8%) than fluoxetine (21/49, 42.9%) subjects completed the trial, and the number of placebo subjects who completed the study (27/48, 56.3%) was intermediate.” |
1 Spring 2004.
| Study characteristics | ||
| Methods | Country: USA Recruitment: community volunteers Study start date: not specified; Study end date: not specified |
|
| Participants | 315 mildly weight‐concerned women, av age 42.7 (10.3) yrs, av 20.3 (9.5) cpd, av BMI 27.4 (7.6) | |
| Interventions |
All participants received 16 weekly CBT smoking cessation group support sessions |
|
| Outcomes | 1. Mean (SD) weight change (kg) at EOT and at 6 months in continuous abstainers (validation: CO ≤ 10 ppm) | |
| Study funding | "The work described in this article was supported in part by National Institutes of Health Grants HL52577 and HL63307 and a Veterans Affairs Merit Review Award to Bonnie Spring." | |
| Author declarations | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "Seven‐day point prevalence smoking status was evaluated via self‐report and ecolyzer measurement of expired carbon monoxide (CO)“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Participants were weighed weekly with a balance beam scale throughout the treatment portion of the study and monthly throughout the follow‐up period. Weight change at each visit was calculated by subtracting out participants’ baseline weight“ |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Treatment completion early diet group: 75/104 (72%); Treatment completion late diet group: 86/104 (82%); Treatment completion control group: 75/107 (70%); 36‐week completion early diet group: 45/104 (43%); 36‐week completion late diet group: 45/104 (43%); 36‐week completion control group: 40/107 (37%) |
1 Toll 2010.
| Study characteristics | ||
| Methods | Country: USA Recruitment: community volunteers Study start date: 3 February 2005; Study end date: 27 April 2009 |
|
| Participants | 127 weight‐concerned smokers, 28.5% men, mean BMI 28.4 ± 6.16, mean 25.5 ± 10.76 expired CO | |
| Interventions |
All participants received 21 mg patches for 6 wks and then 14 mg for 2 wks, starting on quit day. All received CBT for weight concerns weekly for 4 wks, bimonthly twice and then monthly |
|
| Outcomes |
|
|
| Study funding | "This research was supported by National Institutes of Health grants [P50‐AA15632 (to SOM), K12‐DA000167 (to BAT), K05‐ AA014715 (to SOM), K23 DK071646 (to MAW)] from the National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Drug Abuse (NIDA), National Institute on Diabetes and Digestive and Kidney Diseases (NIDDK), and by the State of Connecticut, Department of Mental Health and Addictions Services (DMHAS). Portions of the naltrexone and nicotine patches used in this study were donated by Mallinckrodt Pharmaceuticals and GlaxoSmithKline, respectively. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAAA, NIDA, NIDDK, the National Institutes of Health (NIH), or DMHAS. The NIAAA, NIDA, NIDDK, DMHAS, Mallinckrodt Pharmaceuticals, and GlaxoSmithKline had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication" | |
| Author declarations | "At the time the study was conducted, Drs. O’Malley and Meandzija were inventors on an unlicensed patent held by Yale University regarding the use of naltrexone for smoking cessation treatment that has since been abandoned. Dr. O’Malley received honoraria as a member of the American College of Neuropsychopharmacology workgroup, the Alcohol Clinical Trial Initiative, sponsored by Eli Lilly, Janssen, Schering Plough, Lundbeck, Glaxo‐Smith Kline and Alkermes. She received travel reimbursement for talks at the Controlled Release Society, the Drug Information Association and the Association for Medical Education and Research in Substance Abuse, and an honorarium from the Medical Education Speaker Network. She has consulted to the University of Chicago, Brown University, and the Medical University of South Carolina on studies of naltrexone. She is a partner in Applied Behavioral Research, and a Scientific Panel Member, Butler Center for Research at Hazelden. All other authors declare that they have no conflicts of interest" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block stratified for gender, sequence provided by author and given to pharmacist |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Random sequence was provided by one of the authors (RW) to the pharmacist who assigned participants; all others were blind to treatment assignment.“ + placebo controlled |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “If they reported abstinence, they were only coded as abstinent when an in‐person breath CO measurement was obtain bio‐ logically verifying their self‐report. Serum cotinine was measured at intake and post‐treatment follow‐ups.“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: "Participant weight (in street clothes, without shoes) was measured using a cali‐ brated balance beam scale“ |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Treatment completion placebo group: 30/85 (35%); Treatment completion intervention group: 28/87 (32%) |
1 Vander Weg 2016.
| Study characteristics | ||
| Methods | Country: USA Recruitment: “Recruitment letters were mailed to 847 rural Veterans, of whom 706 were excluded.” Setting: Quitline Study start date: June 2012; Study end date: June 2013 |
|
| Participants | Total N: 63 rural Veterans, 18+ years, smoking at least daily, receiving primary care from the Iowa City VAMC or Coralville Clinic and willing to make a quit attempt in the next 30 days N per arm: Quitline referral = 32; Tailored tobacco intervention = 31 12.7% female, av age 56.8, av cpd 24.7, av FTND 5.7 |
|
| Interventions |
“The approach to pharmacotherapy was the same for both groups. Medication options were based on the Clinical Practice Guideline [35] and the VA formulary and included several forms of NRT (patch, gum, lozenge), bupropion, and varenicline. Combination therapy was also available as appropriate.” |
|
| Outcomes |
|
|
| Study funding | “The work reported in this manuscript was funded by the Department of Veterans Affairs Office of Rural Health (Project number 12‐CR6). The Office of Rural Health played no role in the design or conduct of the study, the interpretation of the results, or the preparation of the manuscript.” | |
| Author declarations | “The authors declare that they have no competing interests.” | |
| Notes | This study is new to the 2021 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The computerized random allocation sequence was generated by the study data manager.” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The computerized random allocation sequence was generated by the study data manager. Participants were enrolled and informed of their treatment assignment by the Project Coordinator (AJC).” |
| Blinding of outcome assessment (detection bias) Smoking | High risk | Quote: “Cessation was determined based on self‐reported 7‐day point prevalent abstinence (PPA). To meet criteria for abstinence, participants had to report no tobacco or ecigarette use during the prior 7 days.” |
| Blinding of outcome assessment (detection bias) Weight | High risk | Quote: “Health‐related items included self‐reported height, weight, and self‐rated health.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “Twelve‐week data were available for 74 % of those in the Tailored intervention group and 94 % of those assigned to Quitline Referral.” Quote: “Six month data were obtained for 74 and 88 % of those in the Tailored and Quitline Referral groups, respectively.” |
1 White 2019.
| Study characteristics | ||
| Methods | Country: USA Recruitment: “Participants were recruited through advertising and physician referral, with primary emphasis in online advertising outlets.” Setting: Not specified Study start date/Study end date: Not specified |
|
| Participants | Total N: 54 regular current smokers, ≥ 10 cpd, with < 3 consecutive months of abstinence in the past year N per arm: Internet‐administered smoking cessation treatment with health education = 27 Internet‐administered smoking cessation plus CBT for weight concerns = 27 72% female, av age 45.9, av baseline BMI 33.1, av cpd 19.7 |
|
| Interventions |
All participants recieved smoking cessation treatment: 21‐mg nicotine patch daily for 10 weeks, beginning in the second week of treatment and 12‐weekly online behavioural smoking cessation treatment lessons + clinician reminders |
|
| Outcomes |
|
|
| Study funding | “The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the American Heart Association, Grant‐in‐Aid awarded to Dr White.” | |
| Author declarations | “The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.” | |
| Notes | This study is new to the 2021 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomization. Eligible participants were randomized in equal allocation to one of two conditions: Internet‐administered smoking cessation treatment with health education (QUIT + HE) or Internet‐administered smoking cessation plus CBT for weight concerns (QUIT + CBT). No blocking or stratification was used.” Comment: No further detail provided. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Randomization. Eligible participants were randomized in equal allocation to one of two conditions: Internet‐administered smoking cessation treatment with health education (QUIT + HE) or Internet‐administered smoking cessation plus CBT for weight concerns (QUIT + CBT). No blocking or stratification was used.” Comment: No further detail provided. |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “CO levels were measured using a Vitalograph Breath CO Monitor, which is a precision instrument for detecting carbon monoxide in exhaled breath. A cutoff of 10 ppm for CO was used.” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Height and weight were self reported online and subsequently measured during in‐person evaluations and at all clinic meetings using a high capacity digital scale.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “Of the 54 randomized participants, 27 received QUIT + CBT and 27 received QUIT + HE. Overall, 44 (81.5%) participants completed the trial. Attrition varied by treatment condition (χ2 = 7.86, p = .005), with 67 percent (n = 18) of the QUIT + HE participants and 96 percent (n = 26) of the QUIT + CBT participants attending the post‐treatment (12 weeks) assessment." |
1 Wilcox 2016.
| Study characteristics | ||
| Methods | Country: USA Recruitment and setting: Not specified Study start date/Study end date: Not specified |
|
| Participants | Total N: 59 smokers, ages 18 ‐ 65 inclusive, smoking ≥ 10 cpd for at least the past year, who were motivated to stop smoking | |
| Interventions |
|
|
| Outcomes |
|
|
| Study funding | Not specified | |
| Author declarations | “Nothing to disclose.” | |
| Notes | Information extracted from a conference abstract. This study is new to the 2021 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Fifty‐nine (59) eligible smokers were randomly assigned to one of three treatment groups: lorcaserin 10 mg once daily; lorcaserin 10 mg twice daily, or placebo in a 3:3:2 ratio.” |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “This was a randomized, double‐blind, twelve‐week [dosing], placebo‐controlled, parallel‐group dose selection study.” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “…end‐expiratory carbon monoxide‐ (CO‐) confirmed Continuous Abstinence Rate (CAR) from study weeks 9‐12, defined as zero reported smoking via Nicotine Use Inventory (NUI) with exhaled CO measurement ≤ 10 ppm.” |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | How weight was measured is not reported and blinding is unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
2 Abelin 1989.
| Study characteristics | ||
| Methods | Country: Switzerland
Recruitment: 21 primary care clinics Study start date: not specified; Study end date: not specified |
|
| Participants | 199 primary care patients, 40% female, av.age 41, av.cpd 27 | |
| Interventions |
Participants did not receive any psychological support |
|
| Outcomes | Mean (SD) weight change (kg) in prolonged abstainers at end of treatment (validation: CO content 0 ‐ 11 ppm) | |
| Study funding | Not specified | |
| Author declarations | Not specified | |
| Notes | Abstinence defined as participants who smoked 0 ‐ 3 cigarettes per wk with validation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind + placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “Abstinence was verified by measurement of the CO content of exhaled air. Participants who smoked 0‐3 cigarettes per week and had a CO content of 0‐11 ppm in expired air were deemed abstinent” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Unclear how weight was measured; self‐report cannot be ruled out and it is also unclear if participants were blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Altogether there were 20 drop‐outs in the nicotine group after 3 months and 21 in the placebo group – i.e. a total rate of about 20%.” |
2 Aubin 2008.
| Study characteristics | ||
| Methods | Country: Belgium, France, Netherlands, UK, USA
Recruitment: smoking cessation clinics or community volunteers Study start date: 17 January 2005; Study end date: 28 June 2006 |
|
| Participants | Healthy adults, Mean age 42.9 yrs, 50.8% female, Mean cpd 22.7. | |
| Interventions |
No placebo control group. All participants received Clearing the Air, S‐H booklet at baseline, and brief counselling (≤ 10 mins) at each clinic visit or by phone |
|
| Outcomes | Mean (SD) weight change (kg) in prolonged abstainers at EOT (email communication) (validation: CO ≤ 10 ppm) | |
| Study funding | "This study was funded by Pfizer Inc. Editorial support was provided by Brenda Smith PhD and Abegale Templar PhD of Envision Pharma and was funded by Pfizer Inc." | |
| Author declarations | "H‐JA has received sponsorship to attend scientific meetings, speaker honorariums and consultancy fees from GlaxoSmithKline, Pierre‐Fabre Sante, Sanofi‐Aventis, Merck‐Lipha and Pfizer Inc. AB has received sponsorship to attend scientific meetings, speaker honorariums and consultancy fees from Boehringer Ingelheim, GlaxoSmithKline, Novartis and Pfizer Inc. In the past 5 years JRB has received consultancy fees from Xenova and Novartis and his employing institution has received consultancy fees and honoraria on his behalf from Pfizer Inc. CO has received honoraria and consulting fees from Pfizer, nicotine and placebo products for research studies at no cost from GlaxoSmithKline and honoraria from Pri‐Med and CME outfitters. CBB, JG, KEW and KRR are employees of Pfizer Inc." | |
| Notes | Prolonged abstainers defined as completely quit from wk 9 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Central computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. 2 active pharmacological interventions. |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "The primary end point was the self‐reported continuous abstinence rate (CAR), confirmed by exhaled carbon monoxide (CO) levels of 10 ppm or below, during the last 4 weeks of treatment (varenicline, weeks 9–12; NRT, weeks 8–11)“ |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Comment: Self‐report cannot be ruled out. Follow‐up weight was likely self‐reported. Participants not blinded, but both arms were active interventions. Quote: “Blood chemistry, haematology, urinalysis tests, vital signs, physical examinations, body weight measures and electrocardiograms were assessed during the treatment period." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up completion: Varenicline group: 312/376 (83%); NRT group: 305/370 (82.3%) |
2 Benowitz 2018.
| Study characteristics | ||
| Methods | Country: USA, Argentina, Australia, Brazil, Bulgaria, Canada, Chile, Denmark, Finland, Germany, Mexico, New Zealand, Russian Federation, Slovakia, South Africa, Spain Recruitment: From the investigators’ own clinics; through newspaper, radio, and television advertising; and fliers and posters Setting: 140 multinational centres in 16 countries across 5 continents. Study sites included clinical trial centres, academic centres, and outpatient clinics treating patients with and without psychiatric disorders Study start date: EAGLES: November 2011; Extrension trial: May 2012; Study end date: EAGLES: January 2015; Extension trial: July 2015 |
|
| Participants |
EAGLES trial Total N: 8144 smokers aged between 18 ‐ 75 years, motivated to stop smoking, smoking 10+ cpd over the previous year and a CO > 10 ppm at screening N per arm (received treatment): Varenicline = 2016; Bupropion = 2006; NRT = 2022; Placebo = 2014 55.9% female, av age 46.5; av baseline BMI: 28.1, av cpd 20.7, av FTND 5.8. EAGLES non‐treatment extension trial Total N: 4595; N per arm: varenicline = 1192; bupropion = 1166; NRT = 1116; Placebo = 1121 55.5% female, av age 47.9, av baseline BMI: 28.6 |
|
| Interventions |
All participants were asked to complete up to 15 face‐to‐face visits and 11 telephone visits during the 24‐week EAGLES trial Smoking cessation counselling of at most 10 mins based on Agency for Healthcare Research and Quality guidelines was given at each clinic visit |
|
| Outcomes | The following outcomes were measured during the trial but were not available for extraction at the time of this update
|
|
| Study funding | This work was supported by Pfizer and GlaxoSmithKline Role of the Funder/Sponsor: Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES) and the EAGLES extension trial are postmarketing requirements in the United States and Europe for Pfizer and GlaxoSmithKline. As such, sponsor employees, with input from academic authors, designed both studies. The sponsors supported the conduct of the trials, monitored the study sites, and collected and analyzed the data. All authors had full access to the data in the studies. Dr Benowitz prepared the initial draft of the manuscript and had final responsibility for the decision to submit for publication. |
|
| Author declarations | Dr Benowitz has served as a consultant to pharmaceutical companies, including Pfizer, which markets smoking cessation medications, and has been a paid expert witness in litigation against tobacco companies. Dr Pipe has served as a consultant to pharmaceutical companies that market smoking cessation medications, including Pfizer, and has received research funding from Pfizer for conduct of this study and from Johnson & Johnson. Dr West is a consultant to Pfizer, Johnson & Johnson, and GlaxoSmithKline and has received research funding from Pfizer and Johnson & Johnson; Dr West’s salary is funded by Cancer Research UK. Dr Hays has received research support from Pfizer for the conduct of this study. Dr Tonstad has received honoraria for lectures and consulting for Pfizer. Drs McRae, Lawrence, and St Aubin are employees and stockholders of Pfizer. Dr Anthenelli reports his university receiving grants from Pfizer and Alkermes, and providing consulting and/or advisory board services to Pfizer, Arena Pharmaceuticals, and Cerecor; Dr Anthenelli’s contributions to this article were supported, in part, by National Institute on Alcohol Abuse and Alcoholism grants U01 AA013641 and R01 AA019720, and National Institute on Drug Abuse/Veterans Affairs Cooperative Studies 1031 and 1032. | |
| Notes | Weight gain is reported for the EAGLES extension trial, which was a non‐treatment follow‐up to the EAGLES trial monitoring cardiovascular adverse events, including the collection of weight data In the EAGLES trial, participants were divided into a non‐psychiatric cohort and 4 sub‐cohorts in the psychiatric cohort This study is new to the 2021 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomisation administrator, independent from the clinical study team, prepared the computer‐generated randomisation schedule used to assign participants to treatment using a block size of 8 (1:1:1:1 ratio) for each of the 20 diagnosis by region combinations.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Investigators obtained participant identification numbers via a web‐based or telephone call‐in drug management system. Study product kit codes did not allow deciphering of randomised treatment or block size.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “As such, participants, investigators, and research personnel were masked to treatment assignments.” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Objectively measured |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Objectively measured. Quote: “Body weight will be measured at the initiation visit of study A3051148 and Week 52 or ET52 Weight will be measured in indoor clothing without shoes” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Study completion rates for the 24‐week EAGLES trial were similar across all treatment arms, with a high of 79.3% (varenicline) vs a low of 77.0% (NRT). Of those who completed EAGLES, 964 NPC participants and 734 PC participants declined enrollment in the 28‐ week extension trial. Thus, 4595 participants (73.0% of EAGLES completers or 56.4% of those randomized to EAGLES) enrolled in the extension trial; similar numbers of participants enrolled in each of the 4 treatment arms (Figure 3 and Figure 1). Extension trial completion rates were high (4139 of 4595 [90.1%]) and similar across the 4 treatment groups.“ |
2 Bernard 2015.
| Study characteristics | ||
| Methods | Country: France Recruitment: news releases and advertisements in local print and electronic media over a 17‐month recruitment period Setting: Montepellier University Hospital Study start date: October 2010; Study end date: March 2013 |
|
| Participants | Total N: 70 smokers aged between 18 ‐ 65 years, sedentary for the previous 6 months with elevated depressive symptoms and a Fagerström score ≥ 4 N per arm: Standard smoking cessation treatment plus exercise and counselling = 35; Health education = 35 58.6% female, av age 48.5; av baseline BMI: 24.6, av cpd 21.5; av FTND 6.4 |
|
| Interventions |
All participants recieved an initial individual session with medical doctor encouraging to stop smoking and selecting a quit date within 2 weeks + optional NRT (adjusted to expired CO level) or optional varenicline (2 x 1 mg per day for 12 weeks) + couselling (10‐session, 8‐week group programme, with bi‐weekly meetings for weeks 1 and 2 and weekly meetings for weeks 3 through 8. 75 minutes of clinician contact time per session) |
|
| Outcomes | The following outcomes were measured during the trial but were not available for extraction at the time of this update.
|
|
| Study funding | “This work was supported by the University Hospital of Montpellier (AOI 2009) and French Committee against Respiratory Diseases” | |
| Author declarations | “Pr Quantin received research funds and served on the scientific board of Lilly, Bohringer, Roche, and Pfizer. Pr Courtet has received grants and served as consultant or speaker for the following entities: AstraZeneca, Roche, Servier, Bristol‐Myers Squibb, Janssen‐Cilag, Lundbeck, Otsuka, Pfizer, SanofiAventis, and Servier. Dr. Guillaume has received compensa‐ tion as a consultant for AstraZeneca, Bristol‐Myers Squibb, Lundbeck, Otsuka, Servier, and Janssen Cilag. These companies manufacture and/or distribute some antidepressant, mood stabilizer, and/or antipsychotic medications. Drs. Bernard, Cyprien, and Georgescu have no conflict of interest. Pr Ninot and Taylor have no conflict of interest.” | |
| Notes | This study is new to the 2021 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Following their baseline assessment, the participants were randomized using TENALEA, an online randomization service (TENALEA, 2010). The randomization was not stratified on depression level, antidepressant treatment, or any other variable.“ |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | CO < 10 verified |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “The dropout rate did not vary significantly between the intervention (7 out of 35, 20%) and control (13 out of 35, 37.1%) groups; χ2(1) = 1.75, p = .18.” |
| Other bias | Unclear risk | Patients in control group may have participated in exercise (contamination effect) |
2 Bize 2010.
| Study characteristics | ||
| Methods | Country: Switzerland
Recruitment: Community volunteers Study start date: June 2002; Study end date: January 2006 |
|
| Participants | 481, av age 42, av cpd 27, sedentary: < 150 mins moderate‐intensity physical activity per wk and < 60 mins vigorous‐intensity activity, av BMI 24 ‐ 25 | |
| Interventions |
Exercise started 5 weeks before quit date |
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at end of treatment and 12 m (validation: CO < 10 ppm) | |
| Study funding | "This trial was supported by a grant from the Swiss National Science Foundation (SNSF 3200‐067085). Other funders: Swiss National Science Foundation" | |
| Author declarations | "None" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Remotely and randomly generated by a computer |
| Allocation concealment (selection bias) | Low risk | Secured by means of sealed envelopes |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "Smoking cessation was defined as self‐reported abstinence from smoking, confirmed by a carbon monoxide (CO) concentration in expired air of less than 10 ppm“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: "Body weight was recorded to the nearest 0.1 kg with the participant wearing only underwear and socks. Height was measured to the nearest 0.5 cm.“ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “The 52‐week follow‐up visit was completed by 127/229 participants in the physical activity group (follow‐up rate: 55%) and 155/252 in the control group (follow‐up rate: 62%).” |
2 Blondal 1999.
| Study characteristics | ||
| Methods | Country: Iceland
Recruitment: community volunteers Study start date: November 1991; Study end date: not specified |
|
| Participants | 237 smokers 67% F, av.age 41 ‐ 43, av tobacco use 25 g/day | |
| Interventions |
|
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at EOT (email communication) and 12m (email communication) (validation: CO < 11 ppm) | |
| Study funding | "Pharmacia and Upjohn provided the drugs and placebo" | |
| Author declarations | "TB was a consultant for Pharmacia and Upjohn, and GG and AW are employed by Pharmacia and Upjohn" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code at pharmacy |
| Allocation concealment (selection bias) | Low risk | Quote: "participants allocated their treatment by generated randomisation code at a local pharmacy" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The pharmacy staff were blinded to the content of the bottles“; “The staff of the smoking clinic had no knowledge of the treatment assigned to each participant.“; “Blinding among participants was successful. At the 1 year follow up we found no significant relation between type of treatment and the participants’ responses, which proved they had been unable to guess their treatment“ |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "Participants were considered to be smokers if they had, after stopping smoking, taken a single puff of a cigarette, used other forms of tobacco, used a nicotine drug other than that prescribed, had a carbon monoxide concentration of >10 ppm, or were lost to follow up“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Unclear how weight was measured but participants were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 48‐week completion patch and nicotine spray group: 117/120 (97.5%) (1 was excluded due to non‐compliance and 1 for Illness); 48‐week completion patch and placebo spray group: 119/119 (100%) |
2 Bloom 2017.
| Study characteristics | ||
| Methods | Country: USA Recruitment: “Participants were recruited from newspaper and radio advertisements.” Setting: Not specified Study start date: Not specified; Study end date: Not specified |
|
| Participants | Total N: 61 participants 18 – 65 years old, smoked ≥ 10 cpd and had not engaged in regular aerobic exercise (≥ 20 min/day on ≥ 3 days/wk) for ≥ 6 months N per arm: Health Education programme Control = 31; Aerobic Exercise programme = 30 65.6% female, av age 47.3, av baseline BMI: 28.7, av cpd 19.9, av FTND 5.8 |
|
| Interventions |
All participants recieved telephone counselling smoking cessation treatment delivered in 8 x 20‐min weekly telephone counselling sessions beginning in week 1 of the intervention + 8 weeks of transdermal nicotine patch (Nicoderm CQ, 24‐hr TNPs (21‐mg strength for weeks 5 – 8, 14‐mg strength for weeks 9 – 10, and 7‐mg strength for weeks 11 – 12)) |
|
| Outcomes | The following outcomes were measured during the trial but were not available for extraction at the time of this update
|
|
| Study funding | “This work was supported by the National Institute on Drug Abuse [grant number K23 DA019950] awarded to Ana M. Abrantes, PhD.” | |
| Author declarations | “No potential conflict of interest was reported by the authors.” | |
| Notes | This study is new to the 2021 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Upon receiving medical clearance to exercise, participants were assigned to one of two conditions using urn randomization (Stout, 1988), with body mass index (BMI), level of nicotine dependence, gender, and age included as blocking variables…” |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “Self‐reported 7‐day point prevalence abstinence at follow‐ups was verified by expired carbon monoxide (CO; < 10 ppm cutoff) or, in one instance, by the report of a family member of the participant because the participant was unable to provide CO.” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Height and weight was obtained by utilizing a Detecto medical scale.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Assessments occurred at baseline, 3(EOT)‐, 6‐, and 12‐month follow‐ups, with 85%, 80%, and 74% completion rates, respectively.” |
2 Bohadana 2000.
| Study characteristics | ||
| Methods | Country: France
Recruitment: community volunteers Study start date: March 1996; Study end date: Feburary 1998 |
|
| Participants | 400 smokers, 18 ‐ 70 yrs, 51% F, Av cpd: Group 1 26.1, Group 2 23.5; FTND > 6 | |
| Interventions |
All received brief counselling and support from investigator at each visit |
|
| Outcomes | Mean (SD) weight change (kg) in prolonged abstainers at end of treatment (email communication) and 12 m (email communication) (validation: CO < 10 ppm) | |
| Study funding | "This study was supported by a grant from Pharmacia & Upjohn Consumer Healthcare, Helsingborg, Sweden" | |
| Author declarations | Not specified | |
| Notes | Prolonged abstinence defined as validated self‐report from 2 wks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed randomisation envelopes were provided for each subject and were held by the hospital pharmacy, which was responsible for dispensing medication" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "self‐reported nonsmoking between week 2 and month 12 and an expired carbon monoxide level less than 10 ppm.” Comment: Smokers not attending follow‐up were classified as relapsing smokers |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Unclear how weight was measured and also unclear if participants were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 24‐wk completion active patch group: 67/200 (33.5%); 24‐wk completion placebo patch group: 66/200 (33%); 48‐wk completion active patch group: 52/200 (26%); 48‐wk completion placebo patch group: 45/200 (22.5%) |
2 Bolliger 2011.
| Study characteristics | ||
| Methods | Country: Multiple sites in Latin America Recruitment: Not specified Study start date: 10 April 2008; Study end date: 17 August 2009 |
|
| Participants | Total N: 588 N per arm: Varenicline = 390; Placebo = 198 40% female, av age 43.4, av baseline weight 75.5 kg, av baseline BMI 26.4, av cpd 23.7, av FTND 6 |
|
| Interventions |
All participants provided with You can Quit Smoking education booklet, brief 1‐2‐1 smoking cessation advice counselling (up to 10 minutes) at each visit during treatment and follow‐up phases. Assigned TQD – first scheduled treatment visit. 3 days after TQD phone call with brief counselling. Clinic assessment visits at weeks 2, 3, 4, 6, 8, 10, 12 (treatment phase) and 13, 16, 20, 24 (non‐treatment follow‐up phase), Telephone assessments at week 14, 18, 22 |
|
| Outcomes |
|
|
| Study funding | "This work was supported by Pfizer Inc, which was the sponsor and funding source for the clinical trial reported here" | |
| Author declarations | All of the authors completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that: (1) the institutions of Drs. Bolliger, Issa, Posadas‐Valay, and Safwat received financial support from Pfizer for the clinical trial; (2) Drs. Bolliger, Issa, Posadas‐Valay, and Safwat received no financial support from Pfizer for the submitted work; (3) Drs. Bolliger, Issa, Posadas‐Valay, and Safwat have specified relationships with Pfizer that might have an interest in the submitted work in the previous 3 years, including investigator payments, consulting honoraria, and grants; (4) their spouses, partners, and children have no financial relationships that may be rele‐ vant to the submitted work; and (5) Drs. Bolliger, Issa, Posadas‐Valay, and Safwat have no nonfinancial interests that may be relevant to the submitted work. Drs. Bolliger, Issa, Posadas‐Valay, and Safwat did not receive financial support with respect to the writing or development of the manuscript. Dr. Abreu, Mr. Correia, Dr. Park, and Mr. Chopra are employees of, and stockholders in, Pfizer. Dr. Abreu and Mr. Chopra participated in the design of the study. Drs. Bolliger, Issa, Posadas‐Valay, and Safwat participated in the collection of data. Dr. Abreu, Mr. Correia, and Mr. Chopra were involved in data analysis. All of the authors were involved in interpretation of data, drafting the article, reviewing the article for important intellectual content, and the decision to submit the article for publication. Drs. Bolliger, Issa, Posadas‐Valay, and Safwat acted as investigators for this study and were remunerated for this role. They did not receive honoraria for this publication. All of the authors had full access to all of the data (including statistical reports and tables) in the study and are responsible for the integrity of the data and the accuracy of the data analysis. Dr. Bolliger was the guarantor of the study. Pfizer was involved in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. Editorial assistance (proofreading, collation of review comments, formatting the manuscript for submission, preparation of figures, and formatting of references) was provided by Penny Gorringe, MSc, and Fiona Nitsche, PhD, of UBC Scientific Solutions and was funded by Pfizer. | |
| Notes | This study is new to the 2021 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Using a block randomization within each site, eligible participants were randomly assigned in a 2:1 ratio to receive varenicline or placebo” |
| Allocation concealment (selection bias) | Low risk | Quote: “a web‐based or telephone call‐in drug management system directed by the sponsor” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “All of the study personnel and participants were blinded to treatment assignment until the end of the nontreatment follow‐up phase” + placebo‐controlled |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | CO‐validated 4 week CAR ('not a puff' rule enforced) |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Vital signs and weight were measured at each clinic visit throughout the study period“ Comment: self‐reported weight assessment cannot be ruled out, but participants were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “A total of 336 of 394 (85.3%) participants assigned to receive varenicline and 156 of 199 (78.4%) assigned to receive placebo completed the study. Overall, the study‐completion rates at week 24 were 336 of 390 (86.2%) in the varenicline group and 156 of 198 (78.8%) in the placebo group. Overall, 54 (13.8%) participants in the verenicline and 42 (21.2%) participants in the placebo arm discontinued the study” |
2 CEASE 1999.
| Study characteristics | ||
| Methods | Country: Multicentre ‐ 36 clinic centres in 17 European countries
Recruitment: community volunteers Study start date: January 1994; Study end date: December 1995 |
|
| Participants | 3575 smokers 48% female, av age 41, av cpd 27, av weight 71 ‐ 73 kg | |
| Interventions | Factorial design compared 2 patch doses and 2 treatment durations. Dose 15 mg or 25 mg (16 hr), duration of active treatment 28 wks (incl 4 wk fading) or 12 wks (incl 4 wk fading)
All participants received brief advice and self‐help brochure |
|
| Outcomes | Mean (SD) weight change (kg) in prolonged abstainers at end of treatment (email communication) and 12m (email communication) (validation: CO < 10 ppm) | |
| Study funding | "This trial was conducted on behalf of the ERS by the Occupational and Epidemiology Assembly and with the sponsorship of Pharmacia & Upjohn, Helsingborg, Sweden" | |
| Author declarations | Not specified | |
| Notes | Prolonged abstinence defined as validated self‐report from 2 wks Doses and durations collapsed in main analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified only by centre |
| Allocation concealment (selection bias) | Low risk | Quote: "A computer‐generated allocation list was prepared centrally and allocated subjects to treatment numbers" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | CO level < 10 ppm |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Unclear how weight was measured and also unclear if participants were blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “Of the participants, 867 (24%) completed the study at 12 months and 2,708 (76%) withdrew during the 12 months. Reasons for withdrawal were: never stopped smoking (122; 3%), started smoking (1,089; 30%), elevated CO (43; 1%), not willing to continue in study (250; 7%), adverse events (73; 2%), exclusion criteria (20; 1%), and other or several reasons (320; 9%). The remaining group consisted of 769 (22%) subjects lost to follow‐up i.e. either no contact despite at least two telephone calls, or who withdrew by telephone.” |
2 Chengappa 2014.
| Study characteristics | ||
| Methods | Country: USA Recruitment: recruited from Western Psychiatric Institute and Clinic and Dubois Regional Medical Center Setting: Not specified Study start date: January 2010; Study end date: March 2013 |
|
| Participants | Total N: 60 smokers diagnosed with DSM‐IV bipolar disorder aged between 18 ‐ 65 years, smoking > 10 cpd with expired breath CO level > 10 ppm at screening and randomization. Women of child‐bearing potential must have a negative serum and must agree to a birth control method N per arm: placebo = 29; varenicline = 31 68.3% female; av age 46.0, av baseline weight 91.1 kg, av cpd 18.2, av FTND 6.2 |
|
| Interventions | 1. Placebo for 12 weeks 2. Varenicline (Chantix) 2 x 1.0 mg per day for 12 wks All participants recieved cognitive‐behavioural counselling for smoking cessation (15‐min sessions face‐to‐face counselling) |
|
| Outcomes | Data measured during the trial but not available for extraction at the time of this update
|
|
| Study funding | “The National Institute of Mental Health (NIMH) of the NIH, under award R21MH087928 (Dr Chengappa), provided the main funding for this study. Pfizer provided drug/placebo and an investigator‐ initiated grant, WS‐515343 (Dr Chengappa). These monies channeled through the University of Pittsburgh were used to offset costs of study procedures, participant payments, and a percentage of the time and effort of research staff and faculty salaries. Role of the sponsor: Neither the NIMH of the NIH nor Pfizer had a role in the conduct or the publication of the study. As part of the letter of agreement with Pfizer, they reviewed the original manuscript that was submitted but did not request changes to the content.” | |
| Author declarations | “Dr Turkin has served on the speaker’bureau of Forest, Sunovion, and Otsuka; and owns shares of Pfizer stock. Dr George has received investigator‐initiated and contract research support from Pfizer, served as a consultant to Novartis, and received honoraria from National Institutes of Health (NIH) and American College of Neuropsychopharmacology. Drs Chengappa, Perkins, Brar, and Levine, and Mss Schlicht and Hetrick and have no financial disclosures with regards to this study.” |
|
| Notes | This study is new to the 2021 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Using a 1:1 Randomization, which also takes into account gender, subjects who sign an informed consent document will be randomized to receive Chantix or placebo.” “We will be using placebo in a randomized, controlled, and blinded trial to compare to varenicline in subjects with bipolar disorder” |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The treatment assignment was blinded to participating subjects, raters, investigators, and statisticians.“ |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Biochemically‐verified abstinence (CO < 10 ppm) |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Unclear how weight was measured, but participants were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The dropout rate in the current 24‐week study was 23% in the varenicline arm and 31% among the placebo‐ assigned subjects. (…) 80% completed the 12‐week treatment phase and 73% completed the follow‐up“ |
2 Ciccolo 2011.
| Study characteristics | ||
| Methods | Country: USA Recruitment: newspaper, Internet, and television advertisements Study start date: Not specified; Study end date: Not specified |
|
| Participants | Total N: 25 N per arm: Exercise = 13; Control = 12 52% female, av age 36.5, av baseline weight 81.8 kg, av cpd 18, av FTND 4.0 |
|
| Interventions |
Prior to randomization, all ppts received a 15 ‐ 20 mins cessation support and nicotine patches (total 8 weeks) |
|
| Outcomes | Data measured during the trial but not available for extraction at the time of this update
|
|
| Study funding | "National Cancer Institute (R03 CA132475 to J.T.C.)" | |
| Author declarations | "None declared" | |
| Notes | This study is new to the 2021 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly‐generated by a computer |
| Allocation concealment (selection bias) | Unclear risk | No detail given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Validated 7‐day PPA |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Not described how body weight was measured. Likely to be objectively assessed, but self‐report cannot be ruled out |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16 week completion resistance training group: 12/13 (92.31%); 16 week completion control group: 11/13 (84.81%); 24 week completion resistance training group: 7/13 (53.84%); 24 week completion control group: 6/13 (46.15%) |
2 Cox 2012.
| Study characteristics | ||
| Methods | Country: USA Recruitment: Clinic‐ and community‐based efforts Study start date: 27 December 2007; Study end date: 13 May 2010 |
|
| Participants | Total N: 540 African‐American light smokers (< 10 cpd) N per arm: Arm 1: 270; Arm 2: 270 66.1% female, av age 46.5, av baseline weight 88.7 kg, av baseline BMI 31.1,av cpd 8, av FTND 3.2 |
|
| Interventions |
All participants received a total of 6 x 15 ‐ 20 mins health education counselling by trained counsellors at weeks 0, 1, 3, 7 in person and weeks 5 and 16 by telephone and a culturally‐targeted smoking cessation guide developed for African‐American light smokers (Kick It at Swope: Stop smoking Guide) |
|
| Outcomes | 1. Mean (SD) weight change (kg) at EOT (7 weeks) and 6 months in abstainers (cotinine‐verified 7‐day point prevalence) | |
| Study funding | "This work was supported by the National Cancer Institute at the National Institutes of Health (CA 091912 to L.S.C.). This work was also supported in part by the National Institute for Minority Health and Disparities (1P60MD003422 to J.S.A.). Support was also provided by the Centre for Addiction and Mental Health and by a Canada Research Chair in Pharmacogenetics (to R.F.T.)" | |
| Author declarations | "Dr J. S. Ahluwalia serves as a consultant to Pfizer Pharmaceuticals, Inc; Dr N. L. Benowitz serves as a consultant to Pfizer Pharmaceuticals, Inc, and has been a paid expert witness in litigation against tobacco companies; Dr R. F. Tyndale holds shares in Nicogen Research, Inc, a company that is focused on novel smoking cessation treatment approaches; no Pfizer or Nicogen funds were used in this work" | |
| Notes | This study is new to the 2021 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated table of random numbers was used to randomly assign ppts to intervention or control |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Specified who was blinded: Quote: “Study staff and participants were blinded to treatment condition” + Placebo controlled: Quote: “Participants received 7 weeks of pharmacotherapy (bupropion SR or placebo)“ |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Cotinine‐verified 7‐day PPA |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Study staff verbally administered all self‐report measures. Demographic information included age, sex, marital status, income, employment status, and education. Height and weight were measured to calculate body mass index (kg/m2)“ Comment: How weight was measured is unclear but both participants and study staff were blinded to treatment condition. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 26‐week completion bupropion group: 192/270 (71%); 26‐week completion placebo group: 187/270 (69%) |
2 Dale 1995.
| Study characteristics | ||
| Methods | Country: USA
Recruitment: community volunteers and smoking clinic attenders Study start date: not specified; Study end date: not specified |
|
| Participants | 71 smokers stratified according to light, moderate and heavy smoking rates. 56% female, av.age 48, av.cpd 26, av weight 79.4 kg | |
| Interventions |
Duration of patch use 8 wks. High level of support including 6‐day inpatient stay |
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at EOT (email communication) and 12 m (email communication) (validation: blood cotinine) | |
| Study funding | "This study was supported by a grant from Lederle Laboratories, Pear River, NY" | |
| Author declarations | "Drs Hurt and Croghan and Mr Offord have worked on clinical research studies funded in part by Lederle Laboratories, Elan Pharmaceutical Research Corporation, Burroughs‐Wellcome, and Kabi. Dr Hurt has received honoraria for educational activities from Ciba Geigy Corporation, Marion Merrell Dow, Inc, and McNeil Pharmaceuticals. Mr Offord has received honoraria for educational activities from Elan Pharmaceutical Research corporation" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “At each visit, self‐reported smoking status was obtained, and expired air was tested for carbon monoxide concentration. Self‐reported abstinence in the previous 7 days was considered biochemically confirmed if the expired air carbon monoxide level was 8 ppm or lower.“ |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Unclear how weight was measured and also unclear if participants were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
2 Dogar 2018.
| Study characteristics | ||
| Methods | Country: Bangladesh and Pakistan Recruitment: Sites were designated tuberculosis treatment centres run by the national tuberculosis control programmes of Bangladesh and Pakistan and were chosen on the basis of having the required resources and ability to recruit participants and take part in the research. Setting: “TB treatment centres integrated into public health care systems in Bangladesh and Pakistan.” 30 sites in Bangladesh (17) and Pakistan (13) Study start date: 1 November 2015; Study end date: 31 October 2019 |
|
| Participants | Total N: 2471 newly‐diagnosed (in the last 4 weeks) pulmonary tuberculosis patients aged 15 years (18 years for Bangladesh) and above, who have been smoking tobacco on a daily basis and wish to quit N per arm: Placebo = 1233; Cytisine = 1239 1% female, av age 42.5, av baseline BMI 18.6, av cpd 11.1 |
|
| Interventions |
All participants recieved 2 x face‐to‐face behavioural support sessions on days 0 and 5 (for 10 mins and 5 mins, respectively) by the clinical team (Health Worker/TB paramedic). Further encouragement and support offered, if needed, at the week 5 visit. |
|
| Outcomes | The following outcomes were measured during the trial but were not available for extraction at the time of this update
|
|
| Study funding | “This project has received funding from the European Union’s Horizon 2020 research and innovation programme, under Grant Agreement No. 680995. Aflofarm Pharma Poland provided cytisine (Desmoxan) and placebo free of cost for the trial; however, they have no role in the trial conduct, its analysis or dissemination of results.” | |
| Author declarations | “K.S. received a research grant from Pfizer (2015–2017) to study the effect of varenicline (a smoking cessation medicine) on waterpipe smoking cessation. E.K. received payment from pharmaceutical companies providing smoking cessation medications for clinical studies, educational and consultation activities. | |
| Notes | “Participants will not receive financial incentives except nominal travel costs for any follow‐up visits that fall outside routine TB care.” This study is new to the 2021 update. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Allocation to the trial arms is by pre‐prepared block randomization lists for each country, generated by the trial statistician.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Allocation to the trial arms is by pre‐prepared block randomization lists for each country, generated by the trial statistician. IMP packs are labelled sequentially in their randomized order and distributed to trial sites in batches. Once a patient has consented to participate, the researcher at the site calls the country coordinating office to obtain the patient’s allocated trial number and confirm the next IMP pack number in the sequence to dispense to the patient.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Investigators, clinicians, and patients were masked to treatment allocation. To maintain masking, medication packs were identical and cytisine and placebo capsules were identical in appearance, smell, and taste. Code‐break envelopes were prepared separately for each medication pack, which contained the true allocation, for emergency unmasking as per the protocol.” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “Self‐report of not using more than five cigarettes/bidis/water pipe sessions/chewing tobacco products from the quit date (5 +/ 2 days) to the reporting date, supported by a negative biochemical test at 6 months.” |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6‐months follow‐up: Placebo: 1130/1233*100 = 91.6% Cytisine: 1142/1239*100 = 92.3% 12 months follow‐up: Placebo: 1056/1233*100 = 85.6% Cytisine: 1048/1239*100 = 84.6% Quote: “Of the 2472 patients enrolled, 1142 (92%) of 1239 patients in the cytisine group and 1130 (92%) of 1233 patients in the placebo group completed the 6‐month follow‐up assessment.” |
2 Ebbert 2014.
| Study characteristics | ||
| Methods | Country: USA Recruitment: Not specified Study start date: October 2009; Study end date: April 2013 |
|
| Participants | Total N: 506 N per arm: varenicline plus bupropion = 249; varenicline plus placebo group = 257 47% females, av age 42.0, av cpd 20, av FTND 5.2 |
|
| Interventions |
All participants received 11 clinic visits with brief (≤ 10 mins) behavioural counselling and 3 follow‐up telephone calls. Medication started day after baseline visit and titrated up to full dose, and TQD was day 8 |
|
| Outcomes |
|
|
| Study funding | "The clinical trial was supported by National Institutes of Health (NIH) grant CA 138417 (primary investigator, Dr Ebbert). Medication (varenicline) was provided by Pfizer. Role of the Sponsors: NIH and Pfizer had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication" |
|
| Author declarations | "All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Ebbert reports serving as an investigator for clinical trials funded by Pfizer, receipt of consultancy fees from GlaxoSmithKline, research support from Pfizer, and research support from Orexigen and JHP Pharmaceuticals outside of the current study. Dr Hatsukami reports receipt of research support from Nabi Biopharmaceuticals outside of the current study. Dr Hays reports serving as an investigator for clinical trials funded by Pfizer. Dr Hurt reports receipt of consulting fees from Pfizer, an unrestricted grant from Pfizer Medical Education Group, and provision of expert testimony in Florida tobacco litigation cases. The other authors report no disclosures" | |
| Notes | This study is new to the 2021 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A central pharmacy randomly assigned study medication in a 1:1 ratio using a computer‐generated randomization sequence with variable‐sized blocks ranging from 2 to 8 stratified by study site |
| Allocation concealment (selection bias) | Low risk | A central pharmacy randomly assigned study medication in a 1:1 ratio using a computer‐generated randomization sequence with variable‐sized blocks ranging from 2 to 8 stratified by study site |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study medication was labelled and dispensed according to participant identification, ensuring that treatment assignment remained concealed from the participant, investigators, and all study personnel having participant contact |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Biochemically‐confirmed (CO 8 ppm or less) prolonged abstinence (no smoking from 2 weeks after TQD) |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Unclear how weight was assessed. Likely to be objective, but self‐report cannot be ruled out. Quote: “During clinic visits, participants received brief (≤10 minutes) behavioral counseling,12 and tobacco use status, vitals signs, exhaled‐air carbon monoxide (CO) measurements (measured in parts per million [ppm]), and weight were obtained.“ Participants and personnel were blinded to treatment condition |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12‐wk completion varenicline + bupropion SR group: 182/249 (73%); 12‐wk completion varenicline group: 184/257 (71%); 52‐wk completion varenicline + bupropion SR group: 158/249 (63%); 52 wk completion varenicline group: 157/257 (61%) |
2 Ebbert 2015.
| Study characteristics | ||
| Methods | Country: Australia, Canada, Czech Republic, Egypt, Germany, Japan, Mexico, Taiwan, UK, and USA Recruitment: Advertisments Setting: clinical trial centres, academic centres, and outpatient clinics (61 centres in 10 countries) Study start date: July 2011; Study end date: July 2013 |
|
| Participants | Total N: 1510 smokers aged ≥ 18, smoking an average of ≥ 10 cpd with no continuous abstinence period > 3 months in the previous year, had an exhaled CO > 10 ppm and wished to quit smoking gradually rather than abruptly N per arm: Placebo = 750; Varenicline = 760 43.6% females, av age 44.6, av cpd 20.7, av FTND 5.6 |
|
| Interventions |
All participants recieved tailored counselling for treating tobacco use and dependence: ≤ 10 minutes per visit, 18 in‐person clinic visits, 10 telephone visits + Clearing the Air – Quit Smoking Today booklet |
|
| Outcomes | The following outcomes were measured during the trial but were not available for extraction at the time of this update.
|
|
| Study funding | This study was funded by Pfizer Inc.; Pfizer Inc was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication. | |
| Author declarations | The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Ebbert reports grants from Pfizer, Orexigen and JHP Pharmaceuticals and personal fees from GlaxoSmithKline during the conduct of the study. Dr. Hughes reports personal fees from Alere/Free and Clear, Equinox, GlaxoSmithKline, Healthwise, Pfizer, Embera, Selecta, DLA Piper, Dorrffermeyer, Nicoventures, Pro Ed, Publicis, Cicatelli, and non‐financial support from Swedish Match, outside the submitted work. Dr. West reports grants, personal fees and non‐financial support from Pfizer, GlaxoSmithKline, and Johnson & Johnson outside the submitted work. Dr. Rennard reports personal fees from Almirall, Novartis, Nycomed, Pfizer, A2B Bio, Dalichi Sankyo, APT Pharma/Britnall, AstraZeneca, Boehringer Ingelheim, Chiesi, Decision Resource, Dunn Group, Easton Associates, Gerson, GlaxoSmithKline, Roche, Theravance, Almirall, CSL Behring, MedImmune, Novartis, Pearl, Takeda, Forest, CME Incite, Novis, PriMed, Takeda, grants from AstraZeneca, Novartis, Otsuka, Boehringer Ingelheim, GlaxoSmithKline, and Johnson & Johnson, outside the submitted work. Dr. Russ, Dr. McRae, Ms. Treadow, Dr. Yu, Dr. Dutro, and Dr. Park are employees and stock holders of Pfizer Inc. | |
| Notes | This study is new to the 2021 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomized (…) in a 1:1 ratio using a computer‐generated block randomization schedule within site.“ |
| Allocation concealment (selection bias) | Low risk | Quote: "Investigators obtained participant identification numbers and treatment group assignments through a web‐ based or telephone call‐in drug management system.“ |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants, investigators, and research personnel were blinded to randomization until after the database was locked.“ |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "CO‐confirmed continuous smoking abstinence rate (CAR)” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: "Body weight will be measured at the screening and baseline visits and at the Weeks 12 and 24 visits (or ET24). Height will be measured at the screening visit. Both will be measured in indoor clothing without shoes.“ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flowchart: Study completion placebo group: 516/750; Study completion varenicline group: 559/760 |
2 Ehrsam 1991.
| Study characteristics | ||
| Methods | Country: Switzerland
Recruitment: University (primary care) Study start date: not specified; Study end date: not specified |
|
| Participants | 112 smokers Av.age 26, av cpd 23 | |
| Interventions |
|
|
| Outcomes | Mean (SD) weight change (kg) in abstainers at the end of treatment | |
| Study funding | Not specified | |
| Author declarations | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | CO < 12 ppm, cotinine < 100 ng/ml |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Unclear how weight was measured, self‐report cannot be ruled out (although unlikely, as face‐to‐face visits with doctors were conducted to assess CO, take pictures of the skin where the patch was and weight was "assessed") |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 36% dropouts in the experimental group vs 55% dropouts in the placebo group. This difference was significant |
2 Eisenberg 2013.
| Study characteristics | ||
| Methods | Country: Canada Recruitment: Based on admission to hospital Study start date: Not specified; Study end date: Not specified |
|
| Participants | Total N: 392 participants hospitaliezd with acute myocardial infarction N per arm: bupropion = 192; placebo = 200 16.5% female, av age 53.9, av baseline weight 78.2 kg, av baseline BMI 27.3, av cpd 23.2, median FTND 4 ‐ 6 |
|
| Interventions |
Both arms: 7 x one‐to‐one counselling sessions by research nurses at baseline and all follow‐ups of < 20 mins (avg. 5) ‐ mix of phone and in‐person |
|
| Outcomes |
|
|
| Study funding | "This study was funded by the Canadian Institutes of Health Research (Grant NCT64989) and the Heart and Stroke Foundation of Quebec. The funding organizations were not involved in the design and conduct of the study; in the collection, management, analysis, and interpretation of the results; or in the preparation, review, or approval of the manuscript." | |
| Author declarations | "Dr. Pilote is a Chercheur National of the Fonds de la Recherche du Québec‐Santé (FRQS). Dr. Pilote also holds a James McGill Chair at McGill University. Dr. O’Loughlin holds the Canada Research Chair in the Early Determinants of Adult Chronic Disease. Dr. Paradis holds a Canadian Institutes of Health Research Chair in Applied Public Health Research. Dr. Rinfret is a Junior 2 Physician‐Scientist of the FRQS. Drs. Eisenberg and Gervais reported that they served as paid consultants for Pfizer Canada Inc.’s Varenicline Advisory Board. Dr. Gervais reported that he received funds from Pfizer Canada Inc., for lectures including service on speaker bureaus, development of educational presentations, and travel/accommodations/meeting expenses. Dr. Eisenberg received funding from Pfizer Canada Inc., to perform the Evaluation of Varenicline (Champix) in Smoking Cessation for Patients Post‐Acute Coronary Syndrome [EVITA] Trial; NCT00794573). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. " | |
| Notes | Participants were not allowed to smoke whilst hospitalized This study is new to the 2021 update. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was done via an internet website using random blocks of 2 and 4 and was stratified by center to ensure that similar numbers of patients were randomized to the 2 arms of the study at each study center” |
| Allocation concealment (selection bias) | Low risk | Allocation performed centrally, see above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Double‐blind.” Quote: “All clinical end points were adjudicated by members of the Endpoints Evaluation Committee who were blinded to treatment assignment.” Comment: No further information provided |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Biochemically‐confirmed continuous abstinence |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Not specified, but participants were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 52‐week completion bupropion group: 146/192 (76.04%); 9‐52‐week completion placebo group: 159/200 (79.5%) |
| Other bias | High risk | Data on weight at all time points is only for those abstinent at 12 months |
2 Eisenberg 2016.
| Study characteristics | ||
| Methods | Country: Canada and the United States Recruitment: “Enrolment took place during hospital admission for acute coronary syndrome, including myocardial infarction and unstable angina with clinically significant coronary artery disease.” Setting: 40 clinical centres Study start date: September 2009; Study end date: December 2015 |
|
| Participants | Total N: 302 active smokers (≥ 10 cpd on average for the past year), ≥ 18 years, suffered an acute coronary syndrome (ACS) and hospitalized and motivated to quit smoking N per arm: Placebo = 151; Varenicline = 151 24.8% female, av age 55, av baseline weight 86.5, av cpd 21.5 |
|
| Interventions |
All participants recieved ≥ 5 mins smoking cessation or relapse‐prevention counselling (face‐to‐face and telephone) plus educational materials. Following completion of the 12‐wk treatment period, participants who relapsed could use open‐label smoking cessation therapies |
|
| Outcomes |
|
|
| Study funding | EVITA was an investigator‐initiated trial, which received funding and study drug/placebo from Pfizer Inc. Pfizer Inc had no role in the design, conduct, analysis, interpretation of data, or reporting of the EVITA trial | |
| Author declarations | Drs Eisenberg, Dehghani, and Madan received honoraria from Pfizer Inc for providing continuing medical education on smoking cessation. The other authors report no conflicts. From Windle 2018 #707: Shamir Mehta reports funding from AstraZeneca, Boston Scientific, Bayer and Abbott. Beth Abramson has received grants or research support from AstraZeneca and Sanofi; honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol–Myers Squibb, Novartis, Fournier, Merck, Pfizer, Servier and Sanofi; and consulting fees from Amgen, Bayer, Boehringer Ingelheim, Sanofi and Servier. She authored Heart Health for Canadians. |
|
| Notes | Participants were also permitted to seek counselling outside of the study; however, only 2.7% of participants did so at any point (equal in each treatment arm) Following the 12‐week treatment period, participants who had relapsed were also permitted to use nonstudy pharmacotherapy treatments for smoking cessation. Use of a nonstudy treatment at any point in the trial was 18.3% overall (14.9% in the varenicline group v. 21.8% in the placebo group) Additional information provided by authors upon request This study is new to the 2021 update. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was performed by enrolling center personnel and stratified by center using a computer‐generated list of permuted blocks of 2 and 4." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Computer‐generated permutated block randomization was used to produce comparable groups and conceal treatment allocation.“ Comment: No further information given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Participants and study personnel are blinded to participant treatment allocation“ |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “Point prevalence smoking abstinence was defined by self‐report of complete abstinence in the 7 days before the 24 week clinic visit confirmed by a measured exhaled carbon monoxide ≤10 ppm.” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: "Participants’ heights and weights were measured at baseline. At each clinic visit, a research nurse also measured weight and assessed participants’ smoking status. We calculated body mass index (BMI) using the standard formula (weight in kilograms divided by height in square meters)“ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24‐week completion rate placebo group: 114/151 24‐week completion rate varenicline group: 118/151 (3 died) |
2 Fiore 1994A.
| Study characteristics | ||
| Methods | Country: USA
Recruitment: community volunteers Study start date: Not specified; Study end date: Not specified |
|
| Participants | 88 smokers, av cpd 28 ‐ 31, av age 42 ‐ 44 yrs, av weight 79 ‐ 81 kg | |
| Interventions |
All participants received intensive group counselling |
|
| Outcomes | Mean (SD) weight change (Kg) in point prevalence abstainers at EOT (email communication) (validation: CO < 10 ppm) | |
| Study funding | "This study was supported by a research grant provnided by Elan Pharmaceutical Research Corporation, Gainesville, Ga, and Athlone, Ireland" | |
| Author declarations | Not specified | |
| Notes | PPA was defined as validated self‐reported abstinence for 7 days prior to measurement Different participants to 2 Fiore 1994B added in separately in the main comparison | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pregenerated computer sequence |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “abstinence was defined as a self‐report of zero cigarettes smoked in the preceding 7 days, confirmed by a CO value of <10 ppm” |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Unclear how weight was measured, and also unclear if participants were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “Of the 88 subjects who were enrolled and randomized, 77 completed the cessation treatment phase (first 8 weeks on study). Of the the 11 who failed to complete treatment, 10 had placebo patches and 1 had active treatment. Of the 87 subjects (one disqualified due to nicotine gum use), 62 were interviewed at the 6‐month follow‐up mark.” |
2 Fiore 1994B.
| Study characteristics | ||
| Methods | Country: USA
Recruitment: community volunteers Study start date: Not specified; Study end date: Not specified |
|
| Participants | 112 smokers, av age 43 ‐ 45 yrs, av weight 72 ‐ 73 kg | |
| Interventions |
All participants received x 8 weekly 10 ‐ 20 min individual counselling |
|
| Outcomes | Mean (SD) weight change (kg) in point prevalence abstainers at end of treatment (email communication) (validation: CO < 10 ppm) | |
| Study funding | "This study was supported by a research grant provided by Elan Pharmaceutical Research Corporation, Gainesville, Ga, and Athlone, Ireland" | |
| Author declarations | Not specified | |
| Notes | PPA was defined as validated self‐report abstinence for 7 days prior to measurement Different participants to 2 Fiore 1994A added in separately in the main comparison | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pregenerated computer sequence |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote “a CO level >= 10 ppm indicated smoking” |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Unclear how weight was measured and also unclear if participants were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “Of the 112 subjects who were enrolled and randomized, 79 completed the active treatment phase of the study. Of the 33 who failed to complete the treatment phase (first 6 weeks on study), 18 had placebo patches and 15 active treatment. 72 were interviewed at the 6‐month follow‐up mark.” |
2 Gallagher 2007.
| Study characteristics | ||
| Methods | Country: USA Recruitment: Potential participants were identified by LFC case management staff or self‐referred Setting: 3 La Frontera Center case management sites in Tucson, Arizona Study start date: March 2003; Study end date: July 2004 (end of randomisation period) |
|
| Participants | Total N: 120 smokers, ≥ 18 years, diagnosed with DSM‐IV Axis I psychotic‐spectrum or affective disorders that have resulted in long‐term mental illness, experienced significant symptoms and functional impairment(s) due to their disorder, smoked ≥ 10 cpd, and regularly for ≥ 3 years and registered a breath CO level ≥ 10 ppm after at least 15 smoke‐free minutes at the baseline visit N per arm: Control = 60; CR = 60; CR + NRT = 60 47.7% female, av age 42.9, av cpd 24.5, av FTND 6.1 |
|
| Interventions |
All received a brief smoking‐related educational information packet and referral to community‐based cessation programmes |
|
| Outcomes | The following outcomes were measured during the trial but were not available for extraction at the time of this update
|
|
| Study funding | Was funded by the Arizona Biomedical Research Commission (formerly theArizona Disease Control Research Commission), Grant # 7014. | |
| Author declarations | Not specified | |
| Notes | People who attended the baseline assessment session but did not meet inclusion criteria or chose to withdraw at that time received USD 5.00 as compensation for their time and travel. In addition, they received a brief smoking‐related educational information packet and referral to community‐based cessation programmes. If necessary, participants were provided with bus passes This study is new to the 2021 update. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned to one of the three groups (CR, CR+NRf, self‐quit)“ Comment: No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Due to the nature of the intervention groups, research staff were not blind to treatment condition. Ideally, those assessing outcomes should be blind to study condition but because staff delivered immediate reinforcement based on CO outcomes, this was not possible.“ Comment: Participants not blinded and contact control not provided |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "Carbon monoxide in expired air, after at least 15 smoke‐free minutes, was assessed at each visit via a Bedfont portable carbon monoxide analyzer. (…) 10 ppm or below is appropriate for a sample with high prevalence (50% or above) of smoking.“ Note: "Quit rates significantly differed between exhaled carbon monoxide level (CO in ppm) and salivary cotinine levels. These data suggest that participants were able to abstain from smoking long enough to register below 10 ppm CO in order to earn the CR. When cotinine was the criterion, quit rates were much lower and lower even than our expectations based on other studies with this population“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Weight, pulse rate and blood pressure were taken at every visit.“ |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “Attrition rates for the groups did not significantly differ at week 20 Cx2=1.39, p= 0.50) nor at week 36 Cx2 = 2.75, p = 0.25). At week 20 and 36 respectively, the CR group lost 37% and 43% of participants, the CR+NRT group lost 35% and 36%, while the self‐quit comparison group lost 52% by both follow‐up points.” |
2 Garvey 2000.
| Study characteristics | ||
| Methods | Country: USA
Recruitment: community volunteers Study start date: Not specified; Study end date: Not specified |
|
| Participants | 608 smokers, aged > 20, 51% female, avcpd 23, av weight (men) 80 ‐ 81 kg, av weight (women) 64 ‐ 69 | |
| Interventions |
All received brief counselling (5 ‐ 10 mins) at each study visit (1, 7, 14, 30 days, 2, 3, 6, 9, 12m) |
|
| Outcomes | Mean (SD) weight change (kg) in prolonged abstainers at end of treatment (email communication) (validation: CO ≤ 8 ppm) | |
| Study funding | "Support for this research was provided by Grants DA06183 and DA10073 from the National Institute on Drug Abuse, and by the Department of Veterans Affairs. The Normative Aging Study is supported by the Cooperative Studies Program/ERIC, U. S. Department of Veterans Affairs, and is a research component of the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC). The authors wish to thank Marion Merrell Dow, Inc. (now Hoechst Marion Roussel, Inc.) for supplying the nicotine gum used in this study" | |
| Author declarations | Not specified | |
| Notes | Prolonged abstinence defined as participants who had not returned to smoking for 7 or more consecutive days or episodes 4 + 2 mg doses combined in main comparison | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated, stratified by high‐ and low‐dependence |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "Subjects for whom follow‐up information was lacking were classified as relapsers; i.e., an intent‐to‐treat analysis was used. Self‐reports of abstinence were considered valid if they were confirmed by CO values of 8 ppm or less.“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: "Body weight and height were assessed using a standard upright scale, which was calibrated frequently. Height and weight were obtained with shoes off, and heavy outer clothing removed“ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
2 Gonzales 2006.
| Study characteristics | ||
| Methods | Country: USA
Recruitment: community volunteers Study start date: May 2003; Study end date: April 2005 |
|
| Participants | 1025 smokers, 55% female (placebo), 48% female (bupropion); av age 45, av cpd not specified | |
| Interventions |
All participants received brief individual counselling at visits wks 1 ‐ 7, 9, 12, + telephone counselling at 4 and 5m |
|
| Outcomes | Mean (SD) weight change (kg) in prolonged abstainers at EOT (validation: CO < 10 ppm) | |
| Study funding | "This study was supported by Pfizer Inc, which provided funding, study drug and placebo, and monitoring" | |
| Author declarations | "Financial Disclosures: Dr Gonzales reports having received research contracts from Pfizer, Sanofi Aventis, GlaxoSmithKline, and Nabi Biopharmaceuticals; consulting fees and honoraria from Pfizer, Sanofi Aventis, and GlaxoSmithKline; and owning 5 shares of Pfizer stock. Dr Rennard reports having had or currently having a number of relationships with companies who provide product and/or services relevant to outpatient management of chronic obstructive pulmonary disease. These relationships include serving as a consultant for Adams, Almirall, Altana, Array Bio‐ pharma, AstraZeneca, Aventis, Biolipox, Centocor, Dey, Critical Therapeutics, GlaxoSmithKline, Johnson & Johnson, Merck, Novartis, Ono Pharma, Otsuka, RJ Reynolds, Roche, Sankyo, Schering‐Plough, Scios, and Wyeth; advising regarding clinical trials for Altana, AstraZeneca, Aventis, Centocor, GlaxoSmithKline, Novartis, Pfizer, and Philip Morris; and speaking at continuing medical education programs and performing funded research both at basic and clinical levels for Altana, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis. Dr Nides reports having received research grants, consulting fees, and honoraria from Pfizer, SanofiAventis, and GlaxoSmithKline. Dr Oncken reports having received research grants, consulting fees, and honoraria from Pfizer; receiving, at no cost, nicotine replacement and placebo products from GlaxoSmithKline for smoking cessation studies; and receiving honoraria from Pri‐Med. Drs Azoulay, Watsky, Gong, Williams, and Reeves and Mr Billing report owning Pfizer stock or having stock options in Pfizer. Role of Sponsor: The database containing the findings of the 19 individual investigator sites was maintained by Pfizer Inc, and statistical analyses were performed at Pfizer Inc by Mr Billing and by Ann Pennington, MS. Independent Statistical Analysis: Barbara Pizacani, PhD, Adjunct Faculty at the School of Nursing at Oregon Health & Science University (OHSU) and Epidemiologist in Program Design and Evaluation Services for Multnomah Health Department and Orgeon Department of Human Services, and Clyde Dent, PhD, of the Program Design and Evaluation Services of Multnomah County Health Department and Oregon Department of Human Services, had access to all of the data used in the study and performed an independent analysis in consultation with Dr Gonzales. The independent analysis replicated the analyses of the primary and secondary end points reported in the manuscript using cross tabulations, logistic regression, and mixed‐model procedures. Repeat tabulations for other end points were performed. Results for the adverse events were also replicated. Results were comparable with those obtained by the sponsor. While there were several small discrepancies, all were resolved prior to submission of the manuscript and none affected the inferences made in the manuscript. Compensation for Drs Pizacani and Dent was provided by OHSU. Pfizer Inc provided no funds to support the independent analysis." |
|
| Notes | Prolonged abstinence defined as complete abstinence from weeks 9 ‐ 12 Arm 2 compared with 3 (same study as 4 VA Gonzales) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization: computer‐generated sequence 1:1:1 |
| Allocation concealment (selection bias) | Low risk | Participants were randomized according to a predefined central computer sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Participants and investigators were blinded to drug treatment assignments.” + placebo‐controlled |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “self‐report of no smoking and an exhaled carbon monoxide measurement of less than 10 part per million … was measured at baseline and each clinic visit to confirm smoking status” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Unclear how weight was measured, self‐report cannot be ruled out but participants were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “The 52‐week study completion rates were 60.5% (213/ 352) for varenicline, 56% (184/329) for bupropion SR, and 54% (187/344) for placebo. Most study discontinuations occurred during the drug treatment phase. The most common reason for discontinuation for both treatment and nondrug follow‐up was loss to follow‐ up. Compliance with medication dosing was similar across all treatment groups, with a median duration of treatment of 84 days in each of the 3 groups” |
2 Gourlay 1995.
| Study characteristics | ||
| Methods | Country: Australia
Recruitment: community volunteers Study start date: Not specified; Study end date: Not specified |
|
| Participants | 629 smokers (> 15 cpd) who had relapsed after transdermal nicotine and behavioural counselling in an earlier phase of the study Minimal additional support | |
| Interventions |
|
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at EOT (validation: expired CO < 9 ppm) | |
| Study funding | "Ciba‐Geigy Australia, the Anti Cancer Council of Victoria, and the Victorian Health Promotion Foundation. Ciba‐Geigy Australia provided the transdermal nicotine patches" | |
| Author declarations | "GG has received research funding from and has been a paid consultant to Ciba‐Geigy Australia, manufacturers of transdermal nicotine. DP is medical director of Ciba‐Geigy New Zealand" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatments were randomly allocated to study numbers by using a 1:1 ratio within blocks of 10 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind + placebo controlled. Quote: “Treatment assignment was guessed correctly by 63/281 (22.4%) of those allocated to achieve treatment and by 109/272 (38.8%) of those allocated to placebo” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "Continuous abstinence was defined as self reported non‐smoking from the quit day, verified by attendance at each scheduled visit and by carbon monoxide levels =< 8 ppm at each visit“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Unclear how weight was measured but participants were successfully blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “After the first four weeks of treatment with patches of 30 cm2, 152/315 (48%) subjects allocated to active treatment and 124/314 (39%) allocated to placebo continued treatment. After the 20 cm2 patches, 113/315 (36%) and 58/314 (19%) subjects, respectively, continued. The rates of permanent discontinuation of treatment because of adverse experiences were 7/179 (3.9%) and 5/143 (3.5%) subjects, respectively (p=0.919).” ; Comment: Number of participants followed‐up not reported. |
2 Gray 2019.
| Study characteristics | ||
| Methods | Country: USA Recruitment: "...primarily through media advertisements. Recruited from the community, schools, and clinical settings. (…) utilizing high school‐based clinics, internet/media advertisements, pediatric/primary care clinic referrals, and College of Charleston postings.” + respondent‐driven sampling (RDS), where participants can win coupons for referring eligible friends and acquaintances Setting: Medical University of South Carolina outpatient clinic Study start date: August 2012; Study end date: 26 January 2018 |
|
| Participants | Total N: 157 adolescent smokers, aged 14 ‐ 21 years, smoking daily for ≥ 6 months who want to quit smoking, with at least 1 prior failed quit attempt; if female, must agree to use birth control N per arm: placebo = 80; varenicline = 77 40.1% female, av age 19.1, av baseline weight 75.2, av cpd 11.5 |
|
| Interventions |
Dose regimen: Participants who weighed > 55 kg: Days 1 ‐ 3: 0.5 mg 1 x daily; Days 4 ‐ 7: 0.5 mg 2 x daily; Day 7 ‐ Week 12: 1.0 mg 2 x daily; Participants who weighed <55 kg:Days 1‐7: 0.5mg 1x daily; Day 7‐Week 12: 0.5mg 2x daily All participants were provided with brief individual, face‐to‐face skills‐based cessation counselling and quit‐smoking brochures |
|
| Outcomes |
|
|
| Study funding | This study was supported by grants U01 DA031779 (Dr Gray), K01 DA036739 (Dr McClure), K12 HD055885 (Dr Tomko), and K23 AA025399 (Dr Squeglia) from the NIH. Varenicline and placebo tablets were supplied at no cost by Pfizer, Inc. Role of the Funder/Sponsor: The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript. |
|
| Author declarations | Dr Gray reported consulting for Pfizer, Inc, and receiving grant support from the National Institutes of Health (NIH). Mr Baker reported receiving grant support from the NIH. Dr McClure reported receiving grant support from the NIH. Dr Tomko reported receiving grant support from the NIH during the conduct of the study and outside the submitted work. Dr Squeglia reported receiving grant support from the NIH. Dr Saladin reported receiving grant support from the NIH. Dr Carpenter reported consulting for Pfizer, Inc, during the conduct of the study. No other disclosures were reported. |
|
| Notes | The study recruited adolescent smokers. However, authors provided weight data restricted to participants greater than or equal to age 18 at study entry. This study is new to the 2021 update. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In order to avoid accidental bias, we will utilize a stratified urn randomization procedure“; "Treatment‐seeking adolescent smokers were randomized in 1:1 parallel group allocation to receive a double‐blind 12‐week course of varenicline or placebo added to weekly cessation counseling.“ |
| Allocation concealment (selection bias) | Unclear risk | Quote: "If assessment procedures reveal that a participant meets inclusion criteria, the MUSC Investigational Drug Service (IDS) will randomize the participant to varenicline or placebo in double‐blind fashion." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "… randomize the participant to varenicline or placebo in double‐blind fashion.“ |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | CO–confirmed (≤ 8 ppm) abstinence |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Quote: "Vital Signs: Blood pressure, pulse, height, and weight will be monitored to assess medical stability and monitor for any changes during study participation.“ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 26‐week follow‐up completion: Placebo group: 41/80 (51.25%) Varenicline group: 42/77 (54.54%) |
2 Gross 1995.
| Study characteristics | ||
| Methods | Country: USA
Recruitment: community volunteers Study start date: Not specified; Study end date: Not specified |
|
| Participants | 177 smokers, 51% female, av age 42, avcpd 33, av FTND score 7.8 | |
| Interventions |
All participants received 1 pre‐quit group counselling session, 14 clinic visits in 10 wks |
|
| Outcomes | Mean (SD) weight change (kg) in prolonged abstainers at end of treatment (validation: CO ≤ 10 ppm) | |
| Study funding | "This study was supported by grant #DA 03893and training grant T 32 DA 07209from the National Institute on Drug Abuse to Dr. Stitzer. We gratefully acknowledge the support of Marion Merrell Dow, Inc. in providing the nicotine gum for this study" | |
| Author declarations | Not specified | |
| Notes | Prolonged abstinence defined as validated self‐reported abstinence (allowed up to 3 cigs) Long‐term abstinence rates not affected by amount of gum chewed, so these groups collapsed for comparison with no‐gum condition | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Neither staff nor subjects were blind to the gum‐group assignment“ |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “subjects were classified as having relapsed when they (a) submitted a breath sample with CO > 10 ppm, or (b) had a cumulative self‐reported number of cigarettes =3, or (c) had a salivary thiocyanate level that exceeded 2000 ng/ml at the 2.5 post‐cessation visit.” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: "Biological measures at each laboratory visit included body weight and expired‐breath carbon monoxide (CO).” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
2 Hjalmarson 1984.
| Study characteristics | ||
| Methods | Country: Sweden
Recruitment: smoking cessation clinic Study start date: 1981; Study end date: 1982 |
|
| Participants | 206 smokers, 56% female, avage 42, av cpd 24 | |
| Interventions |
All participants received 6 group sessions of SC behavioural support in 6 wks |
|
| Outcomes | Mean (SD) weight gain (kg) in continuous abstainers at 6 months (email communication)(validation: CO < 10 ppm) | |
| Study funding | Not specified | |
| Author declarations | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized by therapy group |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind + placebo‐controlled. Nurse distributing gum and therapists did not know about treatment allocation |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “Those who claimed to be ex‐smokers were validated by measurement of expired‐air and was then excluded from the analysis.” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Unclear how weight was measured but participants were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “three subjects in the nicotine group and three in the placebo group were unavailable for follow‐up and counted as smokers” |
2 Hjalmarson 1994.
| Study characteristics | ||
| Methods | Country: Sweden
Recruitment: smoking cessation clinic Study start date: November 1989; Study end date: November 1990 |
|
| Participants | 248 smokers, 57% female, av age 45, av cpd 22, av weight (male) 77 ‐ 83 kg, av weight (female) 64 ‐ 66 kg | |
| Interventions |
All participants received x 8 45 ‐ 60 min group sessions over 6 wks with clinical psychologist |
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at 12m (validation: CO < 10 ppm) | |
| Study funding | "This study was supported by a grant from Kabi Pharmacia AB, Helsingborg, Sweden" | |
| Author declarations | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Treatment allocater not blinded if more than 1 participant from the same household so that they could be given same medication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Procedure was blind to both subject and therapist” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | CO < 10 ppm to validate abstinence |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Weight likely objectively assessed: Quote: “weight was measured at intervals throughout the study in subjects who were abstinent”; Participants blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
2 Hjalmarson 1997.
| Study characteristics | ||
| Methods | Country: Sweden
Recruitment: smoking cessation clinic Study start date: October 1992; Study end date: June 1994 |
|
| Participants | 247 smokers, 64% female, av age 48, av cpd 21 | |
| Interventions |
All participants attended 8 group meetings over 6 wks |
|
| Outcomes | Mean (SD) weight change (kg) in prolonged abstainers end of treatment and 12 months (validation: CO < 10 ppm) | |
| Study funding | "This study was supported by a grant from Pharmacia & Upjohn, Helsingborg, Sweden (Dr Wiklund)" | |
| Author declarations | Not specified | |
| Notes | Prolonged abstainers defined as validated self‐reported abstinence from week 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants assigned a number on attending first group session. Numbers on a list randomising to medication. Participants from the same household randomised to same treatment |
| Allocation concealment (selection bias) | Unclear risk | Treatment allocater not blinded if more than 1 participant from the same household so that they could be given same medication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The randomization was blinded to both the participant and the therapist.” + placebo controlled |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “exhaled‐air carbon monoxide concentration of less than 10 ppm” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Likely objectively measured: Quote: “Participants' body weight, blood pressure, and pulse rate were recorded at all visits.“ Participants blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "At 12 months, responses were received from 231 (94%) of 247 participants. The 16 individuals (10 used active and 6 used placebo inhalers) who could not be reached at 1 year were counted as smokers." |
2 Hurt 1997.
| Study characteristics | ||
| Methods | Country: USA, multicentre
Recruitment: community volunteers Study start date: not specified; Study end date: not specified |
|
| Participants | 615 smokers, 55% F, av age 44, av cpd 27 | |
| Interventions |
All participants received physician advice, S‐H materials, and brief individual counselling by study assistant at each visit |
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at end of treatment (email communication), 6 (email communication) and 12 m (email communication) (validation: CO < 11 ppm) | |
| Study funding | Not specified | |
| Author declarations | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by site, method not specified |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind + placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "For the point‐prevalence rates, subjects were classified as abstinent if they reported not smoking during the previous seven days and this report was confirmed by an expired carbon monoxide value of 10 ppm or less. To be classified as continuously abstinent, the subjects had to be confirmed as not smoking on the basis of carbon monoxide measurement at each visit.“ |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Blinding unclear and how weight was measured was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “A total of 219/615 subjects (148 during the treatment phase and 71 subsequently) did not complete the 12‐month study. Of these subjects, 196 (89 percent) withdrew their consent for various reasons (e.g., scheduling difficulties or perceived lack of benefit); 15 stopped participating because of an adverse event, 6 because of protocol deviations, and 1 for administrative reasons; 1 subject died. The rate of completion of the study increased with the dose and was 57 percent, 65 percent, 64 percent, and 71 percent for the placebo, 100‐mg, 150‐mg, and 300‐mg groups, respectively (P=0.01 by logistic‐regression analysis in which dose was treated as a continuous variable).“ |
2 Ioakeimidis 2018.
| Study characteristics | ||
| Methods | Country: Greece Recruitment: Unclear, had previously been hospitalized with acute coronary syndrome Setting: Follow‐up conducted over the telephone and in clinics Study start date/Study end date: Not specified |
|
| Participants | Total N: 54 smokers ≥ 18 years old, smoking ≥ 10 cpd ib average in the past year and were hospitalized with acute coronary syndrome N per arm: varenicline = 27; electronic Cigarettes = 27 64.8% female, av age 52, av baseline weight 71.8, av cpd 21, av FTND 5.6 |
|
| Interventions |
All participants received low‐intensity counselling |
|
| Outcomes | 1. Mean (SD) weight change (kg) in abstainers at EOT | |
| Study funding | Not specified | |
| Author declarations | Not specified | |
| Notes | Information extracted from a conference poster and abstract Authors provided weight change data upon request for EOT; weight not measured at 26 week follow‐up This study is new to the 2021 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Similar amount of low‐intensity counselling between arms. Unable to blind participants due to nature of trial (varenicline vs EC) |
| Blinding of outcome assessment (detection bias) Smoking | High risk | Point prevalence smoking abstinence was defined by self‐report of complete abstinence in the 7 days before the 24‐week clinic visit |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
2 Jodar‐Sanchez 2018.
| Study characteristics | ||
| Methods | Country: Spain Recruitment: “Smokers were recruited during routine visits to our outpatient clinic.” Setting: Smoking Cessation Unit at Virgen del Rocío University Hospital, Seville Study start date: 24 October 2016; Study end date: October 24, 2018 (Final data collection date for primary outcome measure) |
|
| Participants | Total N: 240 N per arm: Usual care = 120; Intervention = 120 48.8% female, av age 49.7, av baseline BMI 27.0, av cpd 21.1, av FTND 5.8 |
|
| Interventions |
App sent personalized motivational messages generated using AI. Algorithm dynamically determined the type and content on individual messages according to the phase of the transtheoretical model of behavior change that the individual was at, user health conditions, user feedback, and user filtering strategies. Message categories included: reduce tobacco consumption, increase risk perception, and increase benefit perception during the preparation phase; and general motivation, diet tips, exercise and active life recommendations, personal physical activity level, and positive facts of being a former smoker during the action and maintenance phases. The App also provided a User profile linked to the performed physical activity level collected by Google Fit, four smoking cessation benefit indicators (savings, smoke‐free days, regained life hours by not smoking, and number of nonsmoked cigarettes since quitting), text‐based information about smoking cessation, and a section containing relaxing and distracting elements (breathing exercises and minigames). |
|
| Outcomes | The following outcome was measured during the trial but was not available for extraction at the time of this update
|
|
| Study funding | “This research was funded by the H2020 European Commission research and innovation program (grant agreement 681120) as part of the SmokeFreeBrain project (www.smokefreebrain.eu).” | |
| Author declarations | “SHF is a product manager at Salumedia Tecnologías SLU, the company with exploitation rights to the digital therapeutic solution used in the So‐Lo‐Mo study (DigiQuit). He contributed to technical data extraction for the precision, time‐to‐open the messages, and perceived quality metrics and their analysis. He was not involved in the clinical trial design, execution, or analysis of the resulting clinical data. Some institutions of all the other authors (University of Seville, the Aristotle University of Thessaloniki, and the Servicio Andaluz de Salud as a legal representative institution for the Virgen del Rocío University Hospital) signed an exploitation agreement with Salumedia Tecnologías SLU to benefit from DigiQuit commercialization. This agreement was elaborated and signed before publishing the present results but after the trial was finished and its results were generated.” |
|
| Notes | “To facilitate recruitment and avoid bias associated with treatment cost, the SmokeFreeBrain project financed the drugs for usual care. Thus, all participants received their assigned treatments free of charge.” This study is new to the 2021 update. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “We generated a list of 240 consecutive items by randomly assigning 1 of the study groups to each item. Participants were assigned to each of the study groups according to this list and following their order of enrollment.” (protocol publication) Quote: “A technician generated a random‐group table (n=240) using computer methods and following a 1:1 ratio between groups.” (primary paper) |
| Allocation concealment (selection bias) | High risk | Quote: “Clinicians enrolled the participants and assigned them to the group mentioned in the table according to their enrolment sequence. Participants were blinded to this allocation, as those in the IG were told that the provided mobile app was part ofusual care. Participants in the CG were not informed about the existence of the app and did not have access to it.” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Participants with an exhaled CO level greater than 6 ppm were considered smokers Participants with a cotinine concentration greater than 200 ng/ml were considered smokers Participants were considered smokers when at least one of the aforementioned conditions was met. |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Healthy lifestyle will be measured in terms of the subject's BMI (weight in kg / height in m^2) at baseline (first evaluation in the study) and at the end of the intervention (after 1 year of follow‐up)” Comment: Unclear if weight objectively measured, but participants were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12 month follow‐up: Usual care: 47/120*100 = 39.2% Intervention group: 51/120*100 = 42.5% |
2 Jorenby 2006.
| Study characteristics | ||
| Methods | Country: USA, multicentre
Recruitment: community volunteers Study start date: June 2003; Study end date: March 2005 |
|
| Participants | 1027 smokers, 41% F, av age 42, av cpd 22 | |
| Interventions |
All participants received brief (< 10 mins) individual counselling at each weekly assessment for 12 wks & 5 follow‐up visits. One telephone call 3 days after quit day |
|
| Outcomes | Mean (SD) weight change (kg) in prolonged abstainers at end of treatment (validation: CO < 10 ppm) | |
| Study funding | "The data reported in this article were derived from a clinical trial sponsored by Pfizer Inc, which provided funding, study drug and placebo, and monitoring" | |
| Author declarations | "Financial Disclosures: Dr Jorenby reported receiving research support from Pfizer, Nabi Biopharmaceutical, Sanofi‐Aventis and consulting fees from Nabi Biopharmaceutical. Dr Hays reported receiving a research grant from Pfizer. Dr Rigotti reported receiving research grant funding and consulting fees from GlaxoSmithKline, which markets smoking cessation medications, and Pfizer and Sanofi‐Aventis, which are developing smoking cessation medications. Dr Rigotti also reported receiving consulting fees from Merck, which is developing smoking cessation medications. Role of Sponsor: Drs Azoulay, Watsky, Williams, Gong, and Reeves, and Mr Billing, employees of Pfizer Inc, were involved in all elements of this study, including but not limited to the study design and monitoring. In addition, the database containing the findings of the 14 investigator sites was maintained by Pfizer Inc, and statistical analyses were performed at Pfizer Inc by Mr Billing and Ann Pennington, MS. All of the authors including those employed by Pfizer Inc, reviewed and edited the manuscript prior to publication of this article. Independent Statistical Analyses: Daniel Bolt, PhD, associate professor of Educational Psychology at the University of Wisconsin, had access to all of the data used in the study and performed an independent analysis in consultation with Dr Jorenby. The independent statistical analyses involved the primary and key secondary outcomes, including participant demographics, self‐reported data, and safety as described in this article. The results confirm what is presented in this article. Dr Bolt received compensation from the University of Wisconsin for this reanalysis." |
|
| Notes | Prolonged abstinence defined as validated self reported abstinence w 8‐12 Arm 1 and 3 in main comparison | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized, computer‐generated |
| Allocation concealment (selection bias) | Low risk | Quote: "SItes used an electronic system to assign participants to treatment" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind + placebo‐controlled. Participants specified as blinded. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "Abstinence at each visit was defined as a self‐report of no smoking or use of other nicotine‐containing products (or other tobacco during followup) since the previous visit or contact (or previous 7 days in the case of the point prevalence measure), confirmed by an expired carbon monoxide level of 10 ppm or less.“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Unclear how weight was measured, self‐report cannot be ruled out but participants were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Overall study completion rates at week 52 were 70% (240/344 participants) in the varenicline group, 65% (221/342 participants) in the bupropion SR group, and 60% (204/341 participants) in the placebo group. More participants in the placebo group failed to complete the study. There were no differences in demographic variables or baseline characteristics across the 3 groups.“ |
2 Koegelenberg 2014.
| Study characteristics | ||
| Methods | Country: South Africa Recruitment: not specified Study start date: April 2011; Study end date: October 2012 |
|
| Participants | Total N: 446 N per arm: varenicline + active nicotine patch = 222; varenicline + placebo patch = 224 61.7% female, av age 46.3, av baseline BMI 27.3, av cpd 15.8, av FTND 4.5 |
|
| Interventions |
1 wk before the TQD, all participants began taking varenicline (Pfizer), 0.5 mg once daily for 3 days, titrated to 0.5 mg twice daily for days 4 to 7 and then to the maintenance dose of 1 mg twice daily through week 12. Varenicline was tapered off and stopped at the end of week 13 (0.5 mg twice daily for 4 days, followed by 0.5 mg in the evenings for 3 days; total duration, 14 weeks). All participants received 10 minutes of smoking cessation counselling based on the 2008 update of the US Public Health Service guidelines. Participants were followed up weekly from randomization until the TQD (2 weeks later) and subsequently at 1, 2, 4, 8, and 12 weeks during the treatment period. Follow‐up visits were conducted at weeks 13 (telephone), 16, and 24 during the non‐treatment period |
|
| Outcomes | 1. Mean (SD) weight change (kg) at 24 weeks in abstainers (CO < 10 ppm validated continuous abstinence at 6 months) | |
| Study funding | "This study was supported by unrestricted grants from Pfizer, New York, New York, and McNeil, Helsingborg, Sweden. Varenicline was supplied by Pfizer, and both active and placebo NRT patches were supplied by McNeil. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication." |
|
| Author declarations | "All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Koegelenberg reported receiving grants for studies (for his institution) from Pfizer, McNeil, Bayer, and GlaxoSmithKline and personal fees from AstraZeneca. Dr Noor reported receiving grants for studies (for her institution) from Pfizer, McNeil, and GlaxoSmithKline. Dr Bateman reported receiving grants for studies (for his institution) from Actelion, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Hoffman la Roche, Merck, Novartis, Takeda Aeras, Cephalon, and sanofi‐ aventis and personal fees from Elevation Pharma, Napp Pharma, Forest, Pfizer, Navigant Consulting, IMS Consulting Group, ALK‐Abello, and ICON. Dr van Zyl‐Smit reported receiving grants for studies (for his institution) from Pfizer and personal fees from Pfizer and GlaxoSmithKline. Dr Bruning reported receiving grants for studies (for his institution) from Pfizer and McNeil. Dr O’Brien reported receiving grants for studies (for his institution) from Pfizer and McNeil and personal fees from Pfizer, Boehringer Ingelheim, Astra Zeneca, and GlaxoSmithKline. Dr Smith reported receiving personal fees from Pfizer. Dr Irusen reported receiving grants for studies (for his institution) from Pfizer, Astra Zeneca, GlaxoSmithKline, Merck Sharp and Dohme, and Boehringer Ingelheim and personal fees from Merck Sharp and Dohme, Novartis, GlaxoSmithKline, Boehringer Ingelheim, Astra Zeneca, and Nycomed. No other disclosures were reported." | |
| Notes | This study is new to the 2021 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomized in a 1:1 ratio using centrally‐generated block randomization within each site (blocks of 4 with 2 active and 2 placebo patches) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both the investigators and the participants were blinded“ + placebo controlled |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Continuous (validated CO < 10 ppm) and point prevalence rates presented. |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Not specified how weight was measured, but participants and assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24‐wk completion NRT + varenicline group: 144/222 (64.86%) Placebo patch + varenicline group: 134/224 (59.82%) |
2 Lerman 2004.
| Study characteristics | ||
| Methods | Country: USA
Recruitment: community volunteers and referrals Study start date: February 2000; Study end date: March 2002 |
|
| Participants | 350 smokers (includes 51 who withdrew before treatment) 54% F, av age 46, av cpd 21 | |
| Interventions |
All participants received 7 x 90‐min behavioural group counselling sessions. TQD in wk 3. |
|
| Outcomes | Mean (SD) weight change (kg) in unvalidated continuous abstainers at EOT and 6 months | |
| Study funding | "By Transdisciplinary Tobacco Use Research Center grant P5084718 from the National Cancer Institute and the National Institute on Drug Abuse and Public Health Services Research grant M01‐RR0040 from the National Institutes of Health. Dr. Lerman was supported by the Abramson Cancer Center and Annenberg Public Policy Center. Dr. Benowitz was supported by Public Health Services grants DA02277, DA12393, and CA078703, as well as the University of Cal‐ ifornia, San Francisco, Comprehensive Cancer Center. Nicotine nasal spray (Nicotrol) was provided by Pharmacia and Upjohn, Helsingborg, Sweden" | |
| Author declarations | "Consultancies: N. Benowitz (GlaxoSmithKline); Grants received: C. Lerman (National Cancer Insti‐ tute), N. Benowitz (GlaxoSmithKline)" | |
| Notes | For prolonged abstinence, relapse was defined as 7 consecutive days of smoking at any point during follow‐up period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, operated by data manager. |
| Allocation concealment (selection bias) | Low risk | After allocation only outcome assessors blind |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Interviewers were blinded to study group assignment” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "The point prevalence measure required 7 days of continuous abstinence immediately before the follow‐up point, which was confirmed by a carbon monoxide reading of less than 10 parts per million (ppm)“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Height and weight were measured at the medical screening visit“ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up completion transdermal nicotine treatment group: 153/175 (87%); Follow‐up completion nicotine nasal spray treatment group: 147/175 (84%) |
2 Lycett 2011.
| Study characteristics | ||
| Methods | Country: England Recruitment: Invitation letter from GP Setting: 19 general practices in Oxfordshire Study start date: 1 June 1991; Study end date: 1 March 1992; “An unplanned follow‐up took place 8 years later“ |
|
| Participants | Total N: 1686 smokers aged 25 ‐ 64 years, smoking ≥15 cpd. N per arm: Nicotine patch and 16‐page pamphlet = 422; Nicotine patch and 46‐page booklet = 420; Placebo patch and 16‐page pamphlet = 422; Placebo patch and 46‐page booklet = 422 55.1% female, av age 42.6, av cpd 24.3 |
|
| Interventions |
Nicotine patch used was Nicotinell TTS. Participants used an initial 30 cm2 patch for the first 4 weeks, reducing to 20 cm2 for 4 weeks and then 10 cm2 for 4 weeks. The patches delivered 21 mg, 14mg, and 7mg nicotine per 24 hours respectively The total treatment period was 12 weeks. Participants were advised to stop smoking completely from the first day. By the end of the final 3m assessment, participants were scheduled to have been seen (for 10‐15 minutes) on 4 occasions by a nurse and on 1 occasion by a GP |
|
| Outcomes | 1. Mean (SD) weight change (kg) in abstainers at longest follow‐up (8 years) (discussed narratively) | |
| Study funding |
Burke 1993: “This study was supported by Ciba‐Geigy Pharmaceuticals, who also supplied the nicotine and placebo patches. We thank Ciba‐Geigy for help in conducting the study and for ensuring that it met the sandards set by the Declaration of Helsinki and the “Good clinical practice” guidelines. We thank Ciba‐geigy for undertaking the time consuming tasks of packaging and data entry. The central oxford ethics committee gave advice and formal consent to the trial, and Professor Nicholas Wals and colleagues at St Bartholomew’s Medical College, London, carried out the cotinine measurements.” Lycett 2011: “The original trial and 8‐year follow‐up was funded by Cancer Research UK. Deborah Lycett has a PhD studentship funded by UK Centre for Tobacco Control Studies (UKCTCS), a UKCRC Public Health Research: Centre of Excellence. Funding from British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, and the Department of Health, under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged. Paul Aveyard is funded by National Institute of Health Research (NIHR)." |
|
| Author declarations | Paul Aveyard has conducted consultancy work on smoking cessation for Pfizer, McNeil and Xenova Biotechnology. Marcus Munafò has received fees for invited lectures from the National Health Service, GlaxoSmithKline, Novartis, the Moffitt Cancer Research Center and the Karolinska Institutet, and received benefits in kind (hospitality, etc.) from various pharmaceutical companies. He has received research and travel support from the European Research Advisory Board, GlaxoSmithKline, Pfizer Consumer Healthcare and Novartis. Consultancy has been provided to the European Commission, The American Institutes for Research, the National Audit Office and G‐Nostics Ltd. Elaine Johnstone has received consultancy income from European Network for Smoking Prevention. Michael Murphy has received consultancy income from the European Network for Smoking Prevention and has provided scientific consultancy services through the University of Oxford ISIS Innovation to the National Audit Office and G‐Nostics Ltd. | |
| Notes | This study is new to the 2021 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomisation was carried out by prior random allocation of study numbers to each intervention group and by sequential allocation of a study number to patients on entry.” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Randomisation was carried out by prior random allocation of study numbers to each intervention group and by sequential allocation of a study number to patients on entry.” Quote: “prepared precoded packages containing the patches were handed to the patients” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “it was not possible to blind the nurse or patient to the support material provided” Quote: “more of those using the nicotine patch (70.7%) than the placebo patch (48.6%) guessed correctly” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “Abstainers were defined as having stopped smoking on or around quit day and declared continuous total abstinence from 3 months to 1 year and were still abstinent 8 years later. Abstinence was verified biochemically at 3, 6 and 12 months and 8 years.” |
| Blinding of outcome assessment (detection bias) Weight | High risk | Quote: "Height and weight were measured at trial entry, although this was self‐reported in 19% of participants. At 8‐year follow‐up, weight was self‐reported as the questionnaire was completed by post.“ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | > 50% follow‐up at 8 years |
2 Marcus 1999.
| Study characteristics | ||
| Methods | Country: USA
Recruitment: not described Study start date: not specified; Study end date: not specified |
|
| Participants | 20 women, av age 39, av cpd 28, av BMI 24 ‐ 27 | |
| Interventions |
|
|
| Outcomes | Mean weight change (kg) in continuous abstainers at end of treatment (8 wks) and at 60 wks (validation: CO < 8 ppm and cotinine level less than 57 nmol/L (10ng/ml]) | |
| Study funding | "This project was supported in part through grants K07CA01757 and R29CA59660 from the National Cancer Institute, Bethesda, Md, and an R29CA59660 supplementary grant from the Office of Research on Women’s Health, National Institutes of Health, Bethesda, Md (Dr Marcus)" | |
| Author declarations | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation code for group assignment was generated by a computer code" |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “To be considered abstinent, subjects needed to have a carbon monoxide level less than 8ppm and a cotinine level less than 57 nmol/L.” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Body weight and height were measured … using a calibrated scale; subjects were clothed in examination gowns” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “There was no differential loss to follow‐up. Of those randomized, 64.6% of control subjects and 68.7% of exercise subjects returned at end of treatment (P = .47). At the 3‐month follow‐up, 46.9% of control subjects and 58.2% of exercise subjects returned (P = .06); at the 12‐ month follow‐up, 50.3% of control subjects and 56.0% of exercise subjects returned (P = .35)” |
2 Marcus 2005.
| Study characteristics | ||
| Methods | Country: USA
Recruitment: community volunteers Study start date: not specified; Study end date: not specified |
|
| Participants | 217 women, mean age 43, mean cpd 21 exercise ≤ 90 mins /wk | |
| Interventions |
Exercise began before quit date, time in therapy matched for 2 groups |
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at EOT; (validation: saliva cotinine < 10 ng/ml, CO < 8 ppm) | |
| Study funding | "This project was supported in part through grants from the National Cancer Institute (CA77249) and the National Heart, Lung, and Blood Institute (HL64342, HL68422)" | |
| Author declarations | Not specified | |
| Notes | Published paper of Marcus 2003a conference abstract (included study in exercise interventions parent review) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Quote: "Group assignment was based on a randomisation code generated by a computer software program" |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “Criteria for 7‐day point‐ prevalence abstinence was a cotinine level less than 57 nmol/l (10 ng/ml) and a carbon monoxide level less than 8ppm” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “We used a calibrated scale to assess height and weight” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "We found no differential loss to follow‐up between the two groups. For the CBT+EX group, 54.1% attended the end‐of‐treatment assessment session, 39.4% attended the 3‐month follow‐up session, and 24.8% attended the 12‐month follow‐up session. For the CBT group, 58.9% attended the end‐of‐treatment assessment session, 42.1% attended the 3‐month follow‐up session, and 31.8% attended the 12‐month follow‐up session.“ |
2 Mercié 2018.
| Study characteristics | ||
| Methods | Country: France Recruitment: Not specified Setting: 30 HIV clinics located in French university hospitals or other referral hospitals that are usually involved in the management of people living with HIV Study start date: October 2009; Study end date: July 2014 |
|
| Participants | Total N: 248 regular smokers (≥ 10 cpd during past year) with documented HIV infection and motivated to quit smoking N per arm: placebo = 125; varenicline = 123 17% female, av age 45, av baseline weight 69 kg, av cpd 20, av FTND 5.4 |
|
| Interventions |
Behavioural change counselling: The duration of the programme and the precise number of sessions were tailored to participants’ needs. The programme aimed to include 10 to 15 face‐to‐face sessions over 1 year |
|
| Outcomes | The following outcome was measured during the trial but was not available for extraction at the time of this update
|
|
| Study funding | The French National Institute for Health and Medical Research (INSERM)–French National Agency for Research on AIDS and Viral Hepatitis (ANRS) and Pfizer. The funder had no role in study design, collection, analysis, and interpretation of data, writing the report, or in the decision to submit the paper for publication. PM had full access to all data and had final responsibility for the decision to submit for publication | |
| Author declarations | The institution of JR has received funds from Institut national de la santé et de la recherche médicale (Inserm)‐France Recherche Nord et sud Sida‐hiv hépatites (ANRS). XD has received grant support from Pfizer. J‐MM is a member of scientific advisory boards of Merck laboratories, Gilead, Bristol‐Myers Squibb, ViiV Healthcare, and Janssen and has received grant support from Merk laboratories and Gilead. BS has received honoraria for seminars from Merck laboratories, Gilead, and Janssen and support for the IAS 2014 conference from Merck laboratories. The institution of CF and GC has received grant support from Inserm‐ANRS and Pfizer. GC has received grant support for International Workshop on HIV and Hepatitis Observational Databases from Gilead, Tibotec‐Janssen, Roche, Merck laboratories, Janssen Pharmaceuticals, Boehringer Ingelheim, Bristol‐Myers Squibb, GlaxoSmithKline, ViiV Healthcare, Mylan, Abbvie, and Abbott and grant support for ongoing clinical trials of Inserm‐ANRS from Gilead, Tibotec‐Janssen, Merck laboratories, Boehringer Ingelheim, and Abbott. All other authors declare no competing interests. |
|
| Notes | This study is new to the 2021 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomisation (1:1) was done centrally via electronic case report software (CS software, Ennov‐Clinsight), on the basis of a list generated with SAS software, version 9.2 (PROC PLAN procedure, block size 8). Randomisation was stratified according to whether the quit‐smoking counsellor was an infectious diseases specialist or tobaccologist and whether or not the centre had participated in an ancillary study on lung ageing.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Only the trial statistician (JA) had access to the randomisation list during the trial. JA was involved in the data analysis.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Participants and investigators remained masked to treatment groups until the database lock (after week 48), and therefore did not know which group the participant was originally assigned to” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | < 10 ppm CO verified continuous abstinence |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Not specified but participants were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Placebo = 46/124 completed follow‐up Varenicline = 35/123 completed follow‐up |
2 Nakamura 2007.
| Study characteristics | ||
| Methods | Country: Japan
Recruitment:community volunteers Study start date: Not specified; Study end date: Not specified |
|
| Participants | 619 healthy smokers, aged 20 ‐ 75, smoking 10+ cpd. 1 ppt excluded from ITT denominator as withdrew prior to treatment. Demographic data only supplied for nicotine‐dependent group (515/618): 75% male, mean age 39.8, mean cpd 24, mean FTND score 5.6 | |
| Interventions |
All participants received S‐H booklet Clearing the Air at baseline, + brief counselling (≤ 10 mins) at each clinic visit |
|
| Outcomes | Mean (SD) weight change (kg) in prolonged abstainers at end of treatment (validation: CO ≤ 10 ppm) | |
| Study funding | "The study was funded by Pfizer Inc. (ClinicalTrials. gov Identifier: NCT00139750)" | |
| Author declarations | "Drs. Nakamura and Oshima were the primary study investigators, had full access to all study data, and had the final responsibility for the decision to submit the results for publication. Dr. Nakamura has received research contracts from Pfizer Japan Inc. (Tokyo, Japan), Novartis Pharma K.K. (Tokyo, Japan), and Sanofi‐Aventis K.K. (Tokyo, Japan), and a research grant from Pfizer Research Foundation (Tokyo, Japan). Dr. Oshima has received research contracts from Pfizer Japan Inc." | |
| Notes | Prolonged abstinence defined as continuous abstinence during weeks 9 ‐ 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number lists |
| Allocation concealment (selection bias) | Low risk | Quote: "randomised to 1 of the 4 treatment groups in a 1:1:1:1 ratio using a central procedure" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “double‐blinding of subjects and investigators was maintained throughout the study using matching placebo tablets” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "If CO levels were found to be >10 ppm, the subject was considered nonabstinent for the period as‐ sociated with that measurement“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Weight likely objectively measured: Quote: “Blood pressure, heart rate, and body weight were measured at each clinic visit from baseline to week 52 or early termination of treatment/follow‐up.“ Comment: Participants blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flowchart: Study completion varenicline 0.25 mg group: 126/153 (82%); Study completion varenicline 0.50 mg group: 128/156 (82%); Study completion varenicline 1 mg group: 124/156 (79%); Study completion placebo group: 132/154 (85%) |
2 NCT02859142 2016.
| Study characteristics | ||
| Methods | Country: USA Recruitment: Advertisements Setting: Real‐world clinic‐based setting; Chicago, Illinois. 4 x in‐person visits and 1 telephone call Study start date: 29 March 2018; Study end date: 15 October 2020 |
|
| Participants | Total N: 122 heavy drinkers (consume > 14 (men) or > 7 (women) standard alcohol drinks per week), aged 18 ‐ 85 years, who smoke 3 ‐ 30 cpd and want to quit smoking N per arm: Not specified |
|
| Interventions |
Behavioural counselling: 1‐on‐1 sessions with a trained therapist at each of 4 study visits (pre‐quit, quit date, week 2, and week 12) |
|
| Outcomes | 1. Mean (SD) weight change (kg) in abstainers at EOT | |
| Study funding | Not specified: Sponsors and Collaborators; University of Chicago; Pfizer | |
| Author declarations | Not specified | |
| Notes | Information extracted from the clinicaltrial.gov record. Authors provided weight change data upon request This study is new to the 2021 update. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Eligible participants will be randomized into one of two treatment groups…” Comment: No further information provided |
| Allocation concealment (selection bias) | Low risk | No information Quote: ”Placebo tablets, triple blinding (participant, caer provider and investigator)” Comment: Do not explicitly discuss allocation concealment but given blinding practices assumed to be low |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo tablets, triple blinding (participant, care provider and investigator) |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “Biochemical verification from breath tests for CO, as well as vital signs and weight, will be measured at each visit along with survey responses measuring smoking urge and withdrawal, negative affect, neurocognition, and alcohol and smoking behaviors. These will also be used at a 26‐week follow‐up by telephone with biochemical verification for CO in those reporting being smoke‐free.” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Weight was measured at clinic visits. Do not explicitly discuss what was used to measure weight but given blinding practices assumed to be low. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of participants randomized per group not available |
2 Niaura 2002.
| Study characteristics | ||
| Methods | Country: USA, multicentre, 16 sites
Recruitment: Community volunteers Study start date: not specified; Study end date: not specified |
|
| Participants | 989 smokers, 61% F, av age 42 av cpd 28 | |
| Interventions |
All arms: 9 sessions (60 ‐ 90 mins) individual CBT. Included coping skills, stimulus control techniques and relapse prevention |
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at EOT (validation: CO < 8 ppm and salivary cotinine < 20 ng/ml) and 6 months | |
| Study funding | "Financial support of Elo Lilly & Company, as well as Veterans Affairs Merit Review and National Institutes of Health Grants HL52577 and HL59348" | |
| Author declarations | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind + placebo controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | CO level < 8 ppm + salivary cotinine < 20 ng/ml |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: "we measured participants' weight in kilograms with shoes off at each visit using a balance beam scale" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
2 Niaura 2008.
| Study characteristics | ||
| Methods | Country: USA
Setting: 5 research centres Study start date: December 2001; Study end date: June 2003 |
|
| Participants | 320 healthy adult volunteers, aged 18 ‐ 65, smoking ≥ 10 cpd. 52% M, 91% white, mean age 42, mean cpd 22, mean Fagerström score 5.4 | |
| Interventions |
|
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at EOT (12 wks). (validation: CO ≤ 10 ppm) | |
| Study funding | "This study was funded by Pfizer, Inc" | |
| Author declarations | "KEW, KRR, and CBB are employees of Pfizer and have stock or stock options in Pfizer. RN has received consulting fees from Pfizer, GlaxoSmithKline, Sanofi‐Aventis, Merck, Constella, and LLC. DEJ has received consulting fees from Nabi Biopharmaceutical and receives research support from Pfizer, Nabi Biopharmaceutical, and Sanofi‐Aventis. FTL serves on speakers’ bureaus for Pfizer and Merck and is a consultant on an advisory panel with Pfizer. JTH received grant support from Pfizer. JEP received grant support from Merck, DepoMed, Pfizer, Novartis, Takeda, Sanofi‐Aventis, Symbollon, TAP, and GlaxoSmithKline. Editorial support was provided by Ray Beck, Jr, PhD of Envision Pharma and was funded by Pfizer, Inc. The ClinicalTrials.gov registra‐ tion number is NCT00150228" | |
| Notes | Continuous abstinence defined as self‐report abstinence weeks 4 ‐ 12 with biochemical validation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | randomly permuted blocks and a pseudo‐random number generator |
| Allocation concealment (selection bias) | Low risk | participants were assigned in a 1:1 ratio to varenicline treatment or placebo in the numerical order that they were accepted to the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind + placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Self‐reported abstinence validated by a CO concentration of < 10 ppm |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Weight likely objectively measured: Quote: “Vital signs and weight were documented at all clinic visits” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flowchart: 52‐week completion varenicline group: 100/160 (64%); 52‐week completion placebo group: 89 (57%) |
2 Nides 2006.
| Study characteristics | ||
| Methods | Country: USA, multicentre, 7 sites
Recruitment: Volunteers (phase II study) Study start date: 21 February 2000; Study end date: 3 January 2003 |
|
| Participants | 638 smokers, 51% F, av age 41, av cpd 20, av BMI 25 ‐ 27 | |
| Interventions |
All participants received up to 10 mins counselling at 7 weekly clinic visits, 12 & 24 wks |
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at end of treatment (validation: CO ≤ 10 ppm) (email communication) | |
| Study funding | "As the sponsor, Pfizer provided funding and was involved in all elements of the study, including, but not limited to, the study design and monitoring" | |
| Author declarations | "Dr Nides has received research grants, consulting fees, and honoraria from Pfizer, Sanofi‐Aventis, and GlaxoSmithKline. Dr Oncken has received research grants, consulting fees, and honoraria from Pfizer; received, at no cost, nicotine replacement and placebo products from GlaxoSmithKline for smoking cessation studies; and received honoraria from Pri‐Med. Dr Gonzales reports having received research contracts from Pfizer, Sanofi‐Aventis, GlaxoSmithKline and Nabi Biopharmaceuticals; consulting fees and honoraria from Pfizer, Sanofi‐Aventis, and GlaxoSmithKline; and owning 5 shares of Pfizer stock. Dr Rennard has had or currently has a number of relationships with companies that provide product and/or services relevant to outpatient management of chronic obstructive pulmonary disease. These relationships include serving as a consultant (Adams, Almirall, Altana, Array Biopharma, AstraZeneca, Aventis, Biolipox, Centocor, Dey, Critical Therapeutics, GlaxoSmithKline, Johnson & Johnson, Merck, Novartis, Ono Pharma, Otsuka, RJ Reynolds, Roche, Sankyo, Schering‐Plough, Scios, and Wyeth); advising regarding clinical trials (Altana, AstraZeneca, Aventis, Cen‐ tocor, GlaxoSmithKline, Novartis, Pfizer, and Philip Mor‐ ris); speaking at continuing medical education programs; and performing funded research at both basic and clinical levels (Altana, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis). He owns no stock in any pharmaceutical companies. Drs Watsky and Reeves and Mr Anziano are employees of Pfizer and own Pfizer stock or have stock options" | |
| Notes | Continuous abstinence defined as self‐reported quit from TQD with biochemical validation. Arms 1 ‐ 3 and 5 in main comparison | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Quote: "Investigators assigned medication to subjects in numerical order of acceptance into the study" from computer generated list |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind + placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "The primary efficacy measure was the continuous quit rate (CQR) for any 4 weeks, defined as abstinence for any consecutive 28‐day period during the treatment phase (determined by diary data). This measure was chosen to give the best possibility of detecting an efficacy signal in this early phase 2 study. Secondary efficacy measures included the CO‐confirmed (≤ 10 ppm) 4‐week CQR for weeks 4 to 7, as well as CQRs from week 4 to weeks 12, 24, and 52. Subjects who dropped out for any reason were considered to be smokers at all subsequent time points.“ |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Unclear how weight was measured, self‐report cannot be ruled out and unclear if participants were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 52‐week completion varenicline tartrate 0.3 mg once‐daily group: 65/128 (51.6%); 52‐week completion varenicline tartare 1.0 mg once daily group: 77/128 (61.1%); 52‐week completion varenicline tartrate 1.0 mg twice daily group: 77/127 (61.1%); 52‐week completion bupropion hydrochloride, 150 mg twice daily group: 68/128 (54.0%); 52‐week completion placebo group: 66/127 (53.7%) |
2 Oncken 2006.
| Study characteristics | ||
| Methods | Country: USA
Recruitment: community volunteers Study start date: Not specified; Study end date: Not specified |
|
| Participants | 647 smokers, 50.5% female, av cpd 21, av age 42 ‐ 44 yrs, av BMI 26 ‐ 28 | |
| Interventions |
All participants received S‐H booklet at baseline, + brief (≤ 10 mins) counselling at weekly clinic visits throughout treatment phase |
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at end of treatment (validation: CO ≤ 10 ppm) | |
| Study funding | "Pfizer Inc provided funding for this study. Pfizer Inc was involved in all elements of this study, including, but not limited to, the study design and monitoring" | |
| Author declarations | "Dr Oncken has received research grants, consulting fees, and honoraria from Pfizer; nicotine replacement and placebo products from GlaxoSmithKline at no cost for smoking cessation studies; and honoraria from PriMed. Dr Gonzales has received research contracts, consulting fees, and honoraria from Pfizer, SanofiAventis, and GlaxoSmithKline and owns 5 shares of Pfizer stock that he received as a gift from his parents. Dr Rennard has had or currently has a number of relationships with companies who provide products and/or services relevant to outpatient management of chronic obstructive pulmonary disease. These relationships include serving as a consultant (for Adams, Almirall, Altana, Array Biopharma, AstraZeneca, Aventis, Biolipox, Centocor, Dey, Critical Therapeu tics, GlaxoSmithKline, Johnson & Johnson, Merck, Novartis, Ono Pharma, Otsuka, RJ Reynolds, Roche, Sankyo, Schering‐Plough, Scios, and Wyeth), advising regarding clinical trials (Altana, AstraZeneca, Aventis, Centocor, GlaxoSmithKline, Novartis, Pfizer, and Philip Morris), speaking at continuing medical education programs and performing funded research at both basic and clinical levels (Altana, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis). He does not own any stock in any pharmaceutical companies. Dr Nides has received research grants, consulting fees, and honoraria from Pfizer, Sanofi‐Avenits, and GlaxoSmithKline. Drs Watsky and Reeves and Messrs Billing and Anziano are employees of Pfizer and own Pfizer stock or hold Pfizer stock options." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Subjects and investigators were blinded to the study drug treatment assignment.“ |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "Continuous abstinence was defined as self‐report of no cigarette use during the specified time period confirmed by an exhaled carbon monoxide measurement of 10 ppm or lower.“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Weight likely objectively assessed: Quote: "Vital signs, weight, and adverse event information were collected at each visit.“ Comment: Participants blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Flowchart: 0.5 mg twice a day: Nontitrated 129 Randomized 124 Treated, 63 Completed Week 52 Visit (48.3%);
0.5 mg twice a day: Titrated 130 Randomized 129 Treated, 60 Completed Week 52 Visit (46.1%);
1.0 mg twice a day: Nontitrated 129 Randomized 124 Treated, 69 Completed Week 52 Visit (53.4%);
1.0 mg twice a day: Titrated 130 Randomized 129 Treated, 77 Completed Week 52 Visit (59.2%); Placebo 129 Randomized 121 Treated, 40 Completed Week 52 Visit (31%) |
2 Oncken 2007.
| Study characteristics | ||
| Methods | Country: USA Recruitment: local newspaper advertisements and through flyers placed in general medicine clinics Study start date: Not specified; Study end date: Not specified |
|
| Participants | The original study had n = 152 but weight analysis paper focuses on 119 participants who provided data on body weight at 12 weeks and 12 months. Only this sample is extracted below: N per arm: Nicotine patch = 47; Placebo = 72 100% female, av age 55.8, av baseline weight 71.5, av baseline BMI 27.1, av cpd 21.1, av FTQ score 5.5 |
|
| Interventions |
In both treatment conditions, participants came for 7 clinic visits where a research nurse monitors the progress and adverse effects of the medication. In session 2 ‐ 5 ppt also participated in group counselling session which lasted about 2 hours |
|
| Outcomes | 1. Mean (SD) weight change (kg) at EOT (12 weeks) and 12 months in abstainers (7‐day point prevalence CO < 8 ppm) | |
| Study funding | "This study was supported in part by The Patrick and Catherine Weldon Donaghue Foundation, The University of Connecticut Center on Aging, and NIH grants R01 DA13334, and M01 RR06192 (University of Connecticut General Clinical Research Center) and P50AA15632. Glaxo‐SmithKline Pharmaceuticals donated nicotine and placebo patches" | |
| Author declarations | Not specified | |
| Notes | This study is new to the 2021 update. Weight data from Allen 2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of generating random‐number sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealing allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind + placebo, who was blinded is not described |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Biochemically validated (CO levels ≤ 8 ppm) 7‐day point prevalence smoking abstinence |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Not specified how weight was measured, and blinding was unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12‐wk completion nicotine group: 49/57 (85.96%); 12‐wk completion placebo group: 47/57 (82.46%); 64‐wk completion nicotine group: 80/95 (84.21%); 64‐wk completion placebo group: 72/95 (75.79%) |
2 Pack 2008.
| Study characteristics | ||
| Methods | Country: USA Recruitment: community volunteers 2 x 2 factorial design Study start date: June 2004; Study end date: July 2005 |
|
| Participants | 408 smokers, 56% F, av age 40 ‐ 44 yrs, av cpd 22 ‐ 24 | |
| Interventions |
Participants were treated with 8 wks of NRT. Follow‐up at 8 wks, 6m and 12m |
|
| Outcomes | Mean (SD) weight change (kg) in 7‐day point prevalence abstainers at EOT, 6m, 12m | |
| Study funding | "This research was supported by the National Cancer Institute Grant P50CA084724 and the National Institute on Drug Abuse Grant # P50DA19706" | |
| Author declarations | Not specified | |
| Notes | Weight data from arms 1&2 and 3&4 were combined for the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information on blinding given. No placebo |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "Seven‐day point prevalence of smoking abstinence was biochemically confirmed by exhaled carbon monoxide levels of less than 10 ppm measured at 8 weeks with follow‐up at 6 and 12 months“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “…measurements were taken and included height, weight ...” taken at baseline, 8 week post‐TQD clinic visit and at further follow‐up clinic visits if reporting abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Overall follow‐up rates at 8 weeks, 6 months, and 12 months were 64.0%, 72.8% and 69.9% respectively, with little variation between groups. Of those reporting abstinence via phone follow‐up who were subsequently invited for a clinic visit for CO confirmation at 6 and 12 months, 81.3% and 64.3 % completed the clinic visit respectively, with little variation across the groups.” |
2 Patten 2016.
| Study characteristics | ||
| Methods | Country: USA Recruitment: by provider referrals and flyers posted in the clinic and radio and newspaper advertisements Setting: The treatment was mainly held in a community (YMCA) setting and 4 sessions were conducted at a worksite fitness centre Study start date: September 2013; Study end date: December 2015 |
|
| Participants | Total N: 30 female, sedentary smokers, 18 ‐ 55 years of age, smoking ≥ 10 cpd, with moderate to severe depression and willing to make a quit attempt N per arm: Health Education = 15; Exercise = 15 100% female, av age 37.5, av baseline BMI 30.5, av FTND 4.5 |
|
| Interventions |
For both conditions, the 12‐week programme comprised 3 x 30 – 40‐minute individual‐based sessions per week delivered by wellness coaches. All participants received a nicotine patch and behavioural smoking cessation counselling (weekly 15 – 20 minutes of smoking cessation counselling) |
|
| Outcomes | Data measured during the trial but not available for extraction at the time of this update
|
|
| Study funding | This study was supported by CTSA grant number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. Funding for this study was also provided by a Mayo Clinic NIH‐relief award, and a small grant award from the Department of Psychiatry and Psychology. | |
| Author declarations | None declared. | |
| Notes | Participants received USD 25 for completing the baseline assessment and USD 50 after completing each follow‐up. All participants received a free 6‐month YMCA membership (HE participants received this after the final assessment). No incentives were offered for treatment adherence. This study is new to the 2021 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were stratified according to current depression severity (baseline Patient Health Questionnaire [PHQ‐9]score: mild/moderate vs. severe) and antidepressant medication use (yes/no) and randomly assigned to the exercise intervention group (EX, n = 15) or to the health education contact control group (HE, n = 15). The conditions were matched for wellness coach contact time and duration of treatment.“ Comment: No further information given |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Allocation to treatment conditions was unknown to the study staff or investigators prior to assignment, and participants completed baseline assessments prior to being informed of their allocation to treatment condition.” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | 7‐day point‐prevalence, self‐reported, saliva cotinine confirmed cigarette smoking status was obtained at Week 12 and at 6‐month follow‐up. Participants are classified as abstinent if the reading is a 0 (<10 ng/mL cotinine). At each time point, participants who self‐reported no cigarette smoking (not even a puff) in the last 7 days confirmed with a cotinine test strip were classified as nonsmokers |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Height and weight were recorded at baseline and at Week 12 using a calibrated scale |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In both groups 13/15 completed end of treatment assessments |
2 Piper 2007.
| Study characteristics | ||
| Methods | Setting: USA
Recruitment: volunteers Study start date: 2001; Study end date: January 2004 |
|
| Participants | 608 smokers of 10 cpd; 58% F, av age 42, av cpd 22, no details of depression history | |
| Interventions |
All arms: 3 x 10 min counselling over 3 weeks |
|
| Outcomes | 1. Mean (SD) weight change (kg) in point prevalent abstainers at EOT (data from email communication) (validation: CO < 10 ppm) |
|
| Study funding | "This research was supported by National Institutes of Health grants CA84724‐05 and DA0197‐06" | |
| Author declarations | "Dr. Fiore neither consults for nor accepts honoraria from the pharmaceutical industry effective January 1, 2006. In 1998 the University of Wisconsin appointed Dr. Fiore to a named chair, made possible by an unrestricted gift to the university from GlaxoWellcome. Dr. Baker has received monies to conduct clinical trials from pharmaceutical companies (Nabi, Glaxo, Pfizer, Sanofi); he has received no personal remuneration from these companies" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization |
| Allocation concealment (selection bias) | Unclear risk | Methods not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind + placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "Participants who reported 7‐day point‐prevalence abstinence (no smoking, not even a puff, during the 7 days prior to the follow‐up call) at their 6‐ or 12‐month follow‐up calls were scheduled to return to the clinic and provide either a breath sample for CO analysis (6 and 12 months) or a blood sample for cotinine analysis (12 months). Participants who could not be reached at follow‐up were considered to be smoking for the purposes of follow‐up analyses. Both 7‐day point‐prevalence abstinence and continuous abstinence were used as outcome measures.“ |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Unclear how weight was measured and insufficient information to confirm if participants were blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24‐week completion active bupropion & active gum group: 180/228 (78.94%); 24‐week completion active bupropion & placebo gum group: 162/224 (72.32%); 24‐week completion placebo bupropion & placebo gum group: 102/156 (65.38%); 48‐week completion active bupropion & active gum group: 164/228 (71.93%); 48‐week completion active bupropion & placebo gum group: 153/224 (68.30%); 48‐week completion placebo bupropion & placebo gum group: 100/156 (64.10%) |
2 Piper 2009.
| Study characteristics | ||
| Methods | Country: USA Recruitment: television, radio, and newspaper advertisements; flyers; earned media, including press conferences; and television and radio news interviews Study start date: January 2005; Study end date: June 2007 |
|
| Participants | Total N: 1504
58.3% female, av age 44.7, av cpd 21.4, av FTND 5.4 |
|
| Interventions |
All arms: In addition received 6 x 10 ‐ 20‐minute counselling sessions |
|
| Outcomes | 1. Mean (SD) weight change (kg) at 12‐month in abstainers (CO < 10 ppm validated continuous abstinence at 12 months) | |
| Study funding | "This research was conducted at the University of Wisconsin–Madison and was supported by grant P50 DA019706 from the National Institute on Drug Abuse and by grant M01 RR03186 from the General Clinical Research Centers Program of the National Center for Research Resources. Dr Piper was supported by an Institutional Clinical and Translational Science Award, University of Wisconsin–Madison (KL2 grant 1KL2RR025012‐01). Medication was provided to patients at no cost under a research agreement with GlaxoSmithKline" | |
| Author declarations | "The authors report the following potential conflicts of interest for the last 5 years: Dr Smith has received research support from Elan Corporation. Dr Baker has served as an investigator on research projects sponsored by pharmaceutical companies, including Sanofi‐Synthelabo, Pfizer Inc, and Nabi Biopharmaceuticals. Dr Jorenby has received research support from the National Institute on Drug Abuse, the National Cancer Institute, Pfizer Inc, Sanofi‐Synthelabo, and Nabi Biopharmaceuticals. He has received support for educational activities from the National Institute on Drug Abuse and the Veterans Administration and consulting fees from Nabi Biopharmaceuticals. Dr Fiore has received honoraria from Pfizer. He has served as an investigator on research studies at the University of Wisconsin that were funded by Pfizer, Sanofi‐ Synthelabo, GlaxoSmithKlein, and Nabi Biopharmaceuticals. In 1998, the University of Wisconsin appointed Dr Fiore to a named chair funded by an unrestricted gift to University of Wisconsin from Glaxo Wellcome. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis." | |
| Notes | This study is new to the 2021 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not specified. Quote: “participants were randomised to1 of 6 treatment conditions…. used a blocked randomization scheme with sex and self‐reported race (white/nonwhite) as the blocking variables” |
| Allocation concealment (selection bias) | Low risk | Staff did not know to which type(s) of medication |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind but no further detail provided. Quote: “Study staff were blinded to whether the medication was active or placebo” but (type of medication (i.e. patch, gum, pill) would have been apparent to both groups) |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | 7d PPA at 6m; initial cessation. Validation: CO < 10 ppm |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Weight data provided from email. No information on how weight was measured. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8‐week treatment completion NP group: 253/262 (96.56%); 8‐week treatment completion NL group: 243/260 (93.46%); 8‐week treatment completion NP + NL group: 261/267 (97.75%); 8‐week treatment completion bupropion SR group: 249/264 (94.31%); 8‐week treatment completion bupropion SR + NL group: 253/262 (96.56%); 8‐week treatment completion placebo group: 176/189 (93.12%); 12/26‐week completion NP group: 250/262 (95%); 12/26‐week completion NL group: 238/260 (91.54%); 12/26‐week completion NP + NL group: 258/267 (96.63%); 12/26‐week completion bupropion SR group: 244/264 (92.42%); 12/26‐week completion bupropion SR + NL group: 250/262 (95%); 12/26‐week completion placebo group: 174/189 (92.06%) |
2 Puska 1995.
| Study characteristics | ||
| Methods | Country: Finland Recruitment: community volunteers Study start date: Spring 1992; Study end date: Autumn 1993 |
|
| Participants | 300 volunteers aged 20 ‐ 65, smoking > 10 cpd for > 3 yrs, no serious illness | |
| Interventions |
|
|
| Outcomes | Mean (SD) weight change (kg) in prolonged abstainers at end of treatment (email communication) and 12m (email communication) (validation: CO < 10 ppm) | |
| Study funding | Not specified | |
| Author declarations | Not specified | |
| Notes | Prolonged abstinence defined as verified continuously lapse‐free abstinence after week 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “The study was carried out in a strictly double blind fashion” + placebo |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “Success was defined as continuously lapse‐free abstinence after week 1 verified with a CO level in expired air of less than 10 ppm at all visits after week 1” |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Weight was “recorded at each visit”. Self‐report cannot be ruled out and it is unclear if participants were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
2 Richmond 1994.
| Study characteristics | ||
| Methods | Country: Australia
Recruitment: community volunteers Study start date: Not specified; Study end date: Not specified |
|
| Participants | 315 smokers, av cpd 29 | |
| Interventions |
All participants received group smoking cessation behavioural support |
|
| Outcomes | Mean (SD) weight change (kg) in prolonged abstainers at end of treatment (email communication), 6 months (email communication) and 12 months (email communication) (validation: CO ≤ 10 ppm) | |
| Study funding | This study was supported by a grant from Marion Merrell Dow in USA and was monitored under Food and Drug Administration (FDA) regulations | |
| Author declarations | Not specified | |
| Notes | Prolonged abstainers were defined as continuous abstinence for a sustained period preceding the assessment point at 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind + placebo‐controlled. No further information given. |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | CO level < 10 ppm |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Unclear how weight was measured and unclear if participants were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The dropout rate at 12 months was 20% for the active patch group and %1% for placebo. This difference in dropout rate was statistically significant." |
2 Rigotti 2006.
| Study characteristics | ||
| Methods | Country: USA
Recruitment: hospital patients with cardiovascular disease Study start date: October 1999; Study end date: December 2003 |
|
| Participants | 248 smokers, 31% F, av age 56, av cpd 21 ‐ 23 | |
| Interventions |
All participants received multicomponent CBT cessation & relapse prevention programme 30 ‐ 45 mins and 5 X 10‐min post‐discharge contacts (2 days,1, 3, 8, 12 wks) |
|
| Outcomes | Mean (SD) weight change (kg) in point prevalence abstainers at EOT (email communication) and 12m (email communication) (validation: ≤ 20 ng/ml cotinine) | |
| Study funding | "This study was funded by grants from NHLBI (#R01 HL 61779 and #K24‐HL04440), the NIH General Clinical Re‐ search Centers Program (#M01‐RR‐01066) and an unrestricted research grant from GlaxoSmithKline, Inc (GSK). GSK provided free drug and placebo and an unrestricted research grant to permit data collection to be completed when NHLBI funds were exhausted" | |
| Author declarations | Not specified | |
| Notes | PPA defined as validated self‐report of no smoking in previous 7 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated stratified |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The study pharmacist used the computer generated sequence, concealed from enrolment staff, to assign participants to study arm." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Subjects and study personnel, except the statistician and pharmacist, were blind to treatment assignment“ + placebo controlled |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “Subjects were considered smokers if they were lost to follow‐up, failed to provide a saliva sample, or had a cotinine concentration >20 ng/ml.“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Weight measurement not specified but participants were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “completed study” bupropion SR + counselling group: 85/127 (69%) (à 28 (23%) lost to follow‐up); Quote: “completed study” placebo + counselling group: 80/127 (65%) (à 28 (23%) lost to follow‐up) |
2 Rigotti 2010.
| Study characteristics | ||
| Methods | Country: 15 countries in Europe, Asia, Americas
Setting: 39 research centres Study start date: February 2006; Study end date: August 2008 |
|
| Participants | 714 adult smokers, aged 35 ‐ 75, smoking at least 10 cpd, with stable CVD and motivated to quit. 79% male, 80% white, mean cd 22, mean Fagerström 5.6 | |
| Interventions |
Both groups received brief (10 mins) counselling at weekly clinic visits throughout treatment phase, and phone call 3d post‐TQD |
|
| Outcomes | Mean (SD) weight change (kg) in wks 9 ‐ 12 continuous abstainers at end of treatment (12 wks) and 12 months (Validation: expired CO ≤ 10 ppm) |
|
| Study funding | "This study was funded by Pfizer Inc. Editorial support for the development of this manuscript was provided by Alexandra Bruce, PhD, of UBC Scientific Solutions and was funded by Pfizer Inc." | |
| Author declarations | "Drs Rigotti, Pipe, Benowitz, and Tonstad have consulted for Pfizer. Dr Rigotti has been the site principal investigator for clinical trials of smoking cessation medications funded by Pfizer, sanofi‐aventis, and Nabi Biopharmaceuticals. Dr Pipe has received educational and research support in the past from Bristol Myers Squibb, Johnson & Johnson, GlaxoSmithKline, and Merrell Dow. Drs Benowitz and Tonstad served on the scientific planning committee for this study and have been paid consultants to Pfizer and other pharmaceutical companies that are developing and/or marketing smoking cessation medications. Dr Benowitz has been a paid expert witness in litigation against tobacco companies. At the time of the study, his family owned a small amount of Pfizer stock, but no longer does. Dr Tonstad has been the site principal investigator for clinical trials of smoking cessation medication and other medications funded by Pfizer and other pharmaceutical companies. Dr Arteaga is a statistical director at Pfizer Inc, supporting the varenicline studies. Dr Garza is a senior medical director of clinical research and development at Pfizer Inc, and the medical monitor for this study. The other authors report no conflicts." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study sponsor conducted the randomization centrally using a computer‐generated list that prespecified the order of treatment allocation |
| Allocation concealment (selection bias) | Low risk | see above |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind + placebo‐controlled. Quote: "Reported or observed cardiovascular events or deaths resulting from any cause were reviewed separately and adjudicated under blinded conditions by an independent event committee made up of 3 board‐certified cardiologists who used a standard events manual". No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "Continuous abstinence was defined as self‐reported abstinence from any tobacco‐ or nicotine‐containing product since the last visit, but a subject with CO > 10 ppm was classified as a smoker regardless of self‐reported abstinence.“ |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Unclear how weight was measured and unclear of participants were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flowchart: Of 355 assigned to varenicline group, 353 Received study drug, 293 (82.5%) completed treatment and 302 (85.1%) completed study (53 (14.9%) discontinued study); Of 359 assigned to placebo group, 350 received study drug, 186 (79.7%) completed treatment and 189 (80.5%) completed study (70 (19.7%) discontinued study). |
2 Rose 2014.
| Study characteristics | ||
| Methods | Country: USA Recruitment: newspaper, radio, and television advertisements Setting: Duke Center for Nicotine and Smoking Cessation Research In Charlotte, Durham, Raleigh and Winston‐Salem, North Carolina. Study start date: March 2011; Study end date: July 2013 |
|
| Participants | Total N: 222 18 ‐ 65 years old, smoked an average ≥ 10 cpd for 3 cumulative years, have an expired air CO reading at screening of ≥ 10 ppm, express a desire to quit smoking within the next 30 days and were not responsive to early nicotine patch treatment (failing to show a decrease of 50% in ad lib smoking, assessed using expired‐air CO) N per arm: varenicline + placebo = 109; varenicline + bupropion = 113 54.3% female, av age 44.1, av cpd 20.7, av FTND 6.1 |
|
| Interventions |
All participants recieved brief support (< 15‐minute sessions held weekly for 2 weeks before the quit date and at 1, 3, 7 and 11 weeks after the quit date) and varenicline (up‐titration, then 1 mg 2 x daily from day 8 to Week 12) |
|
| Outcomes | 1. Mean weight change (kg) in abstainers at EOT (narrative discussion) | |
| Study funding | Supported by National Institute on Drug Abuse grant 1P50 DA027840 and a grant from Philip Morris USA. The sponsors had no role in the planning or execution of the study, data analysis, or publication of results. Active bupropion sustained‐release and placebo tablets were supplied by Murty Pharmaceuticals, under contract from the National Institute on Drug Abuse | |
| Author declarations | The authors have consulting and patent purchase agreements with Philip Morris International for nicotine inhalation technology and consulting agreements with Targacept and Novartis | |
| Notes | Participants provided written informed consent after receiving a complete description of the study, and they were compensated up to USD 330 for study participation The nicotine patch prequit responders were entered into a separate study to explore combination nicotine replacement therapy treatment, the results of which will be reported elsewhere |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, placebo‐controlled design. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Abstinence was identified by self‐report of no smoking confirmed by expired‐air CO levels #10 ppm Point (7‐day) abstinence at 6 months (self‐reported abstinence confirmed by expired‐air CO level at the follow‐up) |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrew/lost to follow‐up placebo = 38/109 Withdrew/lost to follow‐up bupropion = 41/113 |
2 Sachs 1993.
| Study characteristics | ||
| Methods | Country: USA Recruitment: community volunteers Study start date: Jaunary 1990; Study end date: April 1990 |
|
| Participants | 220 adult smokers. av cpd 28 ‐ 9, av weight 72 ‐ 76 kg | |
| Interventions |
All participants received physician advice at 8 visits during treatment period |
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at 6m (validation: CO < 10 ppm) | |
| Study funding | "This research was supported in part by US Public Health Service grant DA‐04986 from the National Institute on Drug Abuse and by research grants from Kabi Pharmacia AB and Parke‐Davis" | |
| Author declarations | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind + placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “Smoking abstinence was defined as (1) patient self‐report of no smoking from the previous visit, with no slips of any kind allowed (if the subject reported even one puff from a cigarette, then the subject was classified as a failure); and (2) an exhaled air carbon monoxide level of 9 ppm or less at each visit. After all patch use was discontinued, at week 18, an additional objective confirmation was used: serum cotinine level of 15 ng/mL or less.” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Weight objectively measured Quote: “At each visit, project personnel … measured vital signs, including weight …” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “One subject in the active patch group dropped out due to a potential medication side effect. The total drop‐out rate secondary to actual or potential adverse drug events was 0.9% (1/114) in the active treatment group vs 0.0% (0/107) in the placebo treatment group (P=0.3294)” |
2 Saules 2004.
| Study characteristics | ||
| Methods | Country: USA
Recruitment: community volunteers Study start date: not specified; Study end date: not specified |
|
| Participants | 150 smokers, 20% history of MDD, 55% F, av age 40 | |
| Interventions |
All participants received CBT 6 sessions |
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at 6 months (email communication) (validation: CO < 10 ppm) | |
| Study funding | "This work was supported by grant R01 DA I0492‐01A1 (Dr. Schuster) from the National Institute on Drug Abuse, Bethesda, Md., and a Joe Young, Sr. research grant from the State of Michigan (Dr. Schuster). Nicotine transdermal patches were donated by McNeil Consumer Healthcare." | |
| Author declarations | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind + placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "self‐ reported abstinence combined with CO less than 10 ppm.“ |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Weight likely self‐reported. Quote: “Participants received additional compensation of $25 per visit for … provision of weight data … at three, six and twelve months post‐quit date.“ Comment: Participants likely blinded to treatment but this is unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “Overall, 60% of the participants completed the active phase of the study. The completion rate did not differ by group, nor did the rate of drop‐out during the study differ by group (as assessed by log rank statistic)” |
2 Sharma 2018.
| Study characteristics | ||
| Methods | Country: India Recruitment: by invitation Setting: Trial centres were at the All India Institute of Medical Sciences (AIIMS), New Delhi and neighbouring tuberculosis and chest clinics in the National Capital Region of Delhi and the Sri Venkateswara Institute of Medical sciences (SVIMS), Tirupati, Andhra Pradesh Study start date: November 2010; Study end date: September 2016 |
|
| Participants | Total N: 800 adult smokers, > 18 years with recently‐diagnosed (primary TB/Relapse TB) smear‐positive tuberculosis who self‐report to smoke ≥ 10 whole cigarettes or bidis (rolled tobacco leaf) per day, every day N per arm: Control = 400; Intervention = 400 0.25% female, av age 34.6, av baseline BMI 18.6, av baseline weight 49.8, av FTND 6.8 |
|
| Interventions |
All participants received anti‐tuberculosis treatment and behaviour‐change counselling for smoking cessation (10 minutes at baseline, second and fourth week by healthcare workers trained by an expert in smoking cessation; Pamphlets and educational material provided at each follow‐up visit) |
|
| Outcomes | Data measured during the trial but not available for extraction at the time of this update.
|
|
| Study funding | Funding for the study was provided by EU‐FP7 and ICMR. Professor S K Sharma was supported by the JC Bose Fellowship (No. SB/SB2/JCB‐04/2013) of the Ministry of Science & Technology, Govt. of India. | |
| Author declarations | The authors declare no competing interests | |
| Notes | This study is new to the 2021 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomised in a 1:1 ratio to the arm receiving behaviour change counselling with NRT in the form of nicotine chewing gums (intervention arm) or only behaviour change counselling (control arm). Randomisation was performed by generation of random numbers through sequentially numbered, opaque sealed envelopes using a block randomisation scheme with variable block size.” |
| Allocation concealment (selection bias) | Low risk | Quote: “…opaque sealed envelopes…” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not placebo controlled although blinding would have been possible given this is a pharmacological trial. |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “biochemical quit rates defined as serum cotinine levels less than 10 ng/mL or BAT less than six parts per million.“ |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: Control group = 18/400 Intervention group = 19/400 |
2 Shiffman 2002A.
| Study characteristics | ||
| Methods | Country: USA and UK (15 sites)
Recruitment: community volunteers, low dependence (time to first cigarette > 30 mins) Study start date: Not specified; Study end date: Not specified |
|
| Participants | 917 smokers, 58% female, av age 41, av cpd 17 ‐ 18, av weight 74 ‐ 76 kg | |
| Interventions |
All participants received brief advice at 4 visits. |
|
| Outcomes | Mean (SD) weight change (kg) in prolonged abstainers at end of treatment (email communication), 6 (email communication) and 12 months (email communication) (validation: CO ≤ 10 ppm) | |
| Study funding | "This study was supported by GlaxoSmithKline Consumer Healthcare, Parsippany, NJ" | |
| Author declarations | "Dr Shiffman provides consulting to GlaxoSmithKline Healthcare on matters relating to smoking control, and was compensated for his work on this project. Dr Shiffman also has an interest in a novel nicotine replacement product that is not addressed by this article. Dr Hajek has provided consulting to and received research funding from pharmaceutical companies, including GlaxoSmithKline Consumer Healthcare and Pharmacia Consumer Healthcare, Peapack, NJ. Drs Dresler, Gilburt, and Strahs and Mr Targett are employed by GlaxoSmithKline Consumer Healthcare" | |
| Notes | Prolonged abstinence defined as sustained from 2 wks, no slips allowed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind + placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “Participants’ reports of abstinence were subject to verification by an exhaled carbon monoxide level of no greater than 10 ppm.” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Weight likely objectively assessed: Quote: “Weight was measured at each study visit.“ |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Flowchart: 24‐week completion low dependency, 2 mg lozenge group: 124/459 (27%) 24‐week completion low dependency, placebo group: 78/458 (17%) |
2 Shiffman 2002B.
| Study characteristics | ||
| Methods | Country: USA and UK (15 sites)
Recruitment: community volunteers, high dependence (time to first cigarette < 30 mins) Study start date: Not specified; Study end date: Not specified |
|
| Participants | 901 smokers, 55% female, av age 43 ‐ 44, av cpd 25 ‐ 26 | |
| Interventions |
|
|
| Outcomes | Mean (SD) weight change (kg) in prolonged abstainers at EOT (email communication), 6 (email communication) and 12 months (email communication) (validation: CO ≤ 10 ppm) | |
| Study funding | "This study was supported by GlaxoSmithKline Consumer Healthcare, Parsippany, NJ" | |
| Author declarations | "Dr Shiffman provides consulting to GlaxoSmithKline Healthcare on matters relating to smoking control, and was compensated for his work on this project. Dr Shiffman also has an interest in a novel nicotine replacement product that is not addressed by this article. Dr Hajek has provided consulting to and received research funding from pharmaceutical companies, including GlaxoSmithKline Consumer Healthcare and Pharmacia Consumer Healthcare, Peapack, NJ. Drs Dresler, Gilburt, and Strahs and Mr Targett are employed by GlaxoSmithKline Consumer Healthcare" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind + placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “Participants’ reports of abstinence were subject to verification by an exhaled carbon monoxide level of no greater than 10 ppm.” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Weight likely objectively assessed: Quote: “Weight was measured at each study visit.“ |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Flowchart: 24‐week completion high dependency, 4 mg lozenge group: 117/450 (26%); 24‐week completion high dependency, placebo group: 51/451 (11%) |
2 Simon 2004.
| Study characteristics | ||
| Methods | Country: USA
Recruitment: outpatients Study start date: 1 September 1998; Study end date: 31 March 2001 |
|
| Participants | 244 smokers, 79% veterans, 15% F, av age 50, av cpd 24, av BMI 26 ‐ 28 | |
| Interventions |
All participants received 3m of CBT counselling, S‐H materials and telephone follow‐up counselling |
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at 12m (email communication) (validation: salivary cotinine of < 15 ng/ml) | |
| Study funding | "This study was entirely funded by grant 7RT‐0033 from the California Tobacco‐Related Disease Research Program, Oakland, Calif" | |
| Author declarations | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Participants allocated according to computer‐generated list |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Double‐blind + placebo‐controlled. Quote: “All study personnel engaged in providing interventions to participants were blinded to treatment assignment.“ “A significant percentage of participants were able to guess whether they were taking either active bupropion or placebo“ |
| Blinding of outcome assessment (detection bias) Smoking | High risk | Quote: “We collected only self‐reported data on smoking cessation at all time points except for the final 12 month follow‐up hence, we were unable to verify biochemically the smoking cessation point prevalences at the other times.” |
| Blinding of outcome assessment (detection bias) Weight | High risk | Quote: "Body mass index (calculated as weight in kilograms divided by the square of height in meters) and change in weight over time were determined using self‐ reported or medical record data“ Comment: Participants were blinded to treatment condition, but a significant percentage correctly guessed allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Of the 244 participants enrolled, 3 (1%) were lost to follow‐up (all randomized to the placebo arm) and an additional 5 participants (2%) died during the study (2 bupropion‐ and 3 placebo‐treated subjects).” |
2 Simon 2009.
| Study characteristics | ||
| Methods | Setting: San Francisco Veterans Affairs Medical Center, USA
Recruitment: hospitalized volunteers Study start date: January 2004; Study end date: August 2006 |
|
| Participants | 85 inpatient smokers, 3.5% female, av age 56 yrs, av BMI 27.5, av cpd 16 | |
| Interventions |
All ppts received Individual cognitive behavioural 30 ‐ 60 mins during hospital stay + 5 phone calls at wk 1, wk 3, wk 5, wk 8, wk 12; recycling encouraged |
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at 6m (data from email communication) validation: saliva cotinine < 15 ng/ml | |
| Study funding | "California Tobacco‐Related Disease Research Program (12RT‐0148)" | |
| Author declarations | "None declared" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | used a computer algorithm to generate a random list of treatment assignments |
| Allocation concealment (selection bias) | Low risk | All study personnel engaged in providing interventions to participants were blinded to treatment assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Double‐blind + placebo controlled. Quote: “All study personnel engaged in providing interventions to participants were blinded to treatment assignment.“ “A significant percentage of participants were able to guess whether they were taking either active bupropion or placebo“ |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "Because many of the participants live great distances from the SFVAMC, we used salivary cotinine levels ( ≥ 15 ng/ml) as an indicator of current tobacco use, rather than measuring carbon monoxide in person (Jarvis, Tunstall‐Pedoe, Feyerabend, Vesey, & Saloojee, 1987 ). We permitted the mailing of saliva samples in special envelopes when necessary. For self‐reported quitters who had salivary cotinine levels of 15 ng/ml or higher, we ascertained by telephone interview whether they were using NRT at the time the sample was provided. We considered three participants (two in the bupropion arm and one in the placebo arm) to be nicotine dependent and, thus, smokers" |
| Blinding of outcome assessment (detection bias) Weight | High risk | Quote: "Body mass index and change in weight over time were determined using self‐reported or medical record data“. Comment: Participants were blinded to treatment condition, but a significant percentage correctly guessed allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24‐week completion bupropion + counselling group: 38/42 (90.47%) (2 withdrew, 1 died, 1 lost to follow‐up); 24‐week completion placebo + counselling group: 36/43 (83.72%) (5 withdrew, 1 died, 1 lost to follow‐up) |
2 Stapleton 1995.
| Study characteristics | ||
| Methods | Country: UK
Recruitment: General practice patients Study start date: Not specified; Study end date: Not specified |
|
| Participants | 1200 smokers, av cpd 23 ‐ 4, av weight 71 ‐ 72 kg | |
| Interventions |
The nicotine patch groups were further randomized to gradual tapering or abrupt withdrawal from wk 12 All participants received physician advice and brief support at 1, 3, 6, 12 wks |
|
| Outcomes | Mean (SD) weight change (kg) in prolonged abstainers at end of treatment (email communication) and 12m (email communication) (validation: CO < 10 ppm) | |
| Study funding | "This study was funded by Pharmacia AB, who also supervised and monitored procedures and data collection in the practices. We thank the MRC and Imperial Cancer Research Fund for financial support of the Health Behaviour Unit. The Unit`s staff designed the study, analysed and wrote up the results, and assayed saliva cotinine concentrations" | |
| Author declarations | Not specified | |
| Notes | Prolonged abstinence defined as validated self‐reported abstinence from week 2. The dose increase after 1 wk did not affect cessation, 1+2 vs 3 in main comparison. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Quote: "Study subjects were assigned a treatment according to a computer generated list compiled in blocks of six" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind + placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Self‐reported abstinence was validated by ECO < 10 ppm and saliva cotinine < 20 ng/ml |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Weight likely objectively measured: Quote: “at week 0 and all subsequent visits data collection included body weight, …” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
2 Sutherland 1992.
| Study characteristics | ||
| Methods | Country: UK
Recruitment: Smoking cessation clinic patients Study start date: January 1989; Study end date: March 1990 |
|
| Participants | 227 male and female smokers, av cpd 25 ‐ 27, av age 38 ‐ 41 yrs, av weight women 62 ‐ 64 kg, av weight men 75 ‐ 77 kg | |
| Interventions |
All participants received 4 wks of group support |
|
| Outcomes | Mean (SD) weight change (kg) in prolonged abstainers at 12 months (validation: CO < 10 ppm) | |
| Study funding | "We thank the Medical Research Council and the Imperial Cancer Research fund for financial support; (…) Kabi Pharmacia Therapeutics AB for supplying the nasal sprays, and their representative, Dr Mikael Franzon" | |
| Author declarations | Not specified | |
| Notes | Prolonged abstinence defined as validated self‐reported no smoking from the start of the last week of group treatment to the 12‐month follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drew card with A or P for active or placebo allocation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “subjects and therapists were blind to spray assignment” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Self‐reported abstinence validated by a CO concentration of < 10 ppm |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Subjects were weighed (indoor clothes, minus shoes) at assessment, after 4 weeks, and at all follow‐ups” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “On average, 96% in the active group and 95% in the placebo group were followed up at each of the five occasions” |
2 Tashkin 2011.
| Study characteristics | ||
| Methods | Country: USA (17 centres), Spain (3 centres), France (4 centres), Italy (3 centres)
Setting: 27 research centres. Study start date: 2 May 2006; Study end date: 30 April 2009 |
|
| Participants | 504 adult smokers with mild‐to‐moderate COPD, aged 35+, smoking 10+ cpd, motivated to quit; allocated to varenicline (250), or placebo (254). 62% male, mean age 57, cpd 24 ‐ 25, Fagerström score 5.9 ‐ 6.2., av BMI 26.6 (SD 5.5) | |
| Interventions |
Both groups received SC educational booklet, + brief (10 mins) counselling at weekly clinic visits throughout treatment phase, and phone call 3d post‐TQD. |
|
| Outcomes | Mean (SD) weight change at in continuous abstainers EOT (12 wks) and 12m (Validation: CO ≤ 10 ppm) |
|
| Study funding | "This study was funded by Pfizer Inc. Role of sponsors: Editorial support for the development of this manuscript was provided by Brenda Smith, PhD, and administrative support was provided by Sue Francis, both of UBC Scientific Solutions with funding from Pfizer Inc." | |
| Author declarations | "Dr Tashkin received grant support from Pfizer Inc and Nabi Pharmaceuticals and fees for attending advisory board meetings from Pfizer Inc. Dr Hays received a research grant from Pfizer Inc for the conduct of the clinical trial described in this manuscript. In the past 3 years, Dr Rennard has been a consultant or a member of an advisory board for Able Associates, Adelphi Research, Almirall/Prescott, APT Pharma/Britnall, Aradigm, AstraZeneca, Boehringer Ingelheim, Chiesi, CommonHealth, Consult Complete, COPDForum, Data Monitor, Decision Resources, Defined Health, Dey, Dunn Group, Eaton Associates, Equinox, Gerson, GlaxoSmithKline, Infomed, KOL Connection, M Pankove, MedaCorp, MDRx Financial, Mpex, Novartis, Nycomed, Oriel Therapeutics, Otsuka, Pennside Partners, Pfizer Inc (varenicline), Pharma Ventures, Pharmaxis, Price Waterhouse, Propagate, Pulmatrix, Reckner Associates, Recruiting Resources, Roche, Schlesinger Medical, SciMed, Sudler and Hennessey, TargeGen, Theravance, UBC, Uptake Medical, and VantagePoint Management. Dr Rennard has lectured for the American Thoracic Society, AstraZeneca, Boehringer Ingelheim, California Allergy Society, Creative Educational Concept, France Foundation, Information TV, Network for Continuing Ed, Novartis, Pfizer, and SOMA and has received industry‐sponsored grants from AstraZeneca, Biomarck, Centocor, Mpex, Nabi Pharmaceuticals, Novartis, and Otsuka. Ms Ma and Drs Lawrence and Lee are all employees of Pfizer Inc, own Pfizer Stock, and have Pfizer stock options" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods not described |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind + placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “A CO value of ≤ 10 parts per million (ppm) was the criterion to confirm smoking abstinence.“ |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Unclear how weight was measured and unclear if participants were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “A larger proportion of participants receiving varenicline than placebo completed the treatment phase (83.5% vs 76.9%, respectively), and the entire 52‐week study (71.0% vs 62.5%, respectively)” |
2 TNSG 1991.
| Study characteristics | ||
| Methods | Country: USA (9 sites)
Recruitment: community volunteers (treated at smoking cessation clinics) Study start date: Not specified; Study end date: Not specified |
|
| Participants | 808 smokers, 60% female, av age 43, av cpd 31, av weight 72.4 kg | |
| Interventions |
All participants received group smoking cessation behavioural support |
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at EOT (6 wks) (validation: CO < 9 ppm) | |
| Study funding | This research was supported in part by the Merell Dow Pharmaceutical Comany and by Research Grant DA 03893 and Training Grant T32DA07209 from the National Institute on Drug Abuse | |
| Author declarations | Not specified | |
| Notes | 2 trials pooled and data relating to a 7 mg patch group used in only 1 trial omitted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Randomized placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | CO level < 8 ppm |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: "At each lab visit, subjects were weighed on a standard balance beam scale" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Over the course of the 10‐week trial, there were 44 (50.6%) dropouts" |
2 Tonstad 2006.
| Study characteristics | ||
| Methods | Country: USA (6 centres) and "international" (18 centres, across Canada, Czech Republic, Denmark, Norway, Sweden, UK)
Recruitment: smoking cessation clinics Study start date: 13 April 2003; Study end date: 17 February 2004 |
|
| Participants | 1210 successful quitters (62.8% of initial cohort) following a 12‐wk open‐label course of varenicline for smoking cessation. 51% female, av age 45, av cpd 21 | |
| Interventions |
Participants had already received 12 wks of varenicline. All participants received brief counselling (≤ 10 mins) at each clinic visit throughout treatment phase (wks 13 ‐ 24). Treatment phase clinic visits were at wks 13, 14, 16, 20 and 24 |
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at 6m (validation: CO ≤ 10 ppm) | |
| Study funding | "This study was sponsored by Pfizer Inc, which provided funding, study drug and placebo, and monitoring.
Role of the Sponsor: Pfizer Inc was involved in all elements of this study, including but not limited to the study design and monitoring. In addition, the database containing the findings of the 25 individual investigator sites was maintained by Pfizer Inc, and statistical analyses were performed at Pfizer Inc by Mr Billing and Ann Pennington, MS. Independent Statistical Analysis: Ingar Holme, PhD, professor of biostatistics, University of Oslo, and Ulleva °l University Hospital, had access to all of the data used in the study and performed an independent analysis. Dr Holme performed analyses of the primary and key secondary end points using logistic regression and cross‐tabulations. Results were identical to those obtained by the sponsor. Dr Holme received compensation for this reanalysis from Pfizer Inc." |
|
| Author declarations | "Dr Tonstad reports receiving honoraria for lecturing and consultancies for Pfizer and other manufacturers of smoking cessation medications; Dr Tønnesen reports receiving honoraria for participation in advisory boards for Pfizer and Sanofi‐Aventis and for presentations relating to smoking cessation from Pfizer and GlaxoSmithKline; Dr Hajek reports consulting for manufacturers of smoking cessation medications, including Pfizer; Dr Williams reports stock ownership in Pfizer; and Dr Reeves reports stock ownership in Pfizer. No other disclosures were reported" | |
| Notes | Continuous abstinence was defined as validated complete abstinence during week 13 ‐ 24 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated lists stratified by centre, x 4 random block design |
| Allocation concealment (selection bias) | Low risk | computer‐generated sequence used for allocation of participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | double‐blind + placebo‐controlled. Participants blinded |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "participants whose CO value was more than 10 ppm were classified as smokers“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Unclear how weight was measured but participants were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flowchart: Of 603 assigned to varenicline, 602 received treatment as assigned, 555 completed the treatment period (47 discontinued) and 494 completed the study (61 discontinued follow‐up); Of 607 assigned to receive placebo, 604 received placebo as assigned, 510 completed the treatment period (94 discontinued) and 463 completed the study (47 discontinued follow‐up) |
2 Tsai 2008.
| Study characteristics | ||
| Methods | Country: Taiwan and Republic of Korea
Recruitment: community volunteers Study start date: February 2005; Study end date: March 2006 |
|
| Participants | 250 healthy adult volunteers, motivated to quit, aged 18 to 75; allocated to varenicline (126), or placebo (124). 11% female, av age 40.3, BMI > 15 or < 38 or weight > 45.5 kg, av cpd 24 | |
| Interventions |
All participants received a smoking cessation booklet Clearing the Air at baseline + brief counselling (≤ 10 mins) at each clinic visit |
|
| Outcomes | Mean (SD) weight change (kg) in prolonged abstainers at end of treatment (validated: CO ≤ 10 ppm) | |
| Study funding | "Varenicline and placebo were supplied by Pfizer and the study was funded by Pfizer Inc. (ClinicalTrials.gov Identification Number NCT00141167)" | |
| Author declarations | "Drs. Tsai and Cho have been members of Pfizer‐sponsored advisory panels and, together with Drs. Cheng, Kim, and Hsueh, were investigators for a Pfizer‐ sponsored clinical trial. Editorial assistance was provided by Christopher Grantham, PhD, of Envision Pharma, Horsham, United Kingdom, and funded by Pfizer Inc., New York, New York." | |
| Notes | Prolonged abstinence is defined as validated complete abstinence during weeks 9 ‐ 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permutated blocks (block = 4) |
| Allocation concealment (selection bias) | Low risk | web‐ and telephone‐based assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind + placebo‐controlled. Quote: "Knowledge of treatment assignments was withheld from those directly involved with the operation of the study, including study subjects, study investigators and their staffs, and sponsor personnel involved in clinical operations." |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “no reported smoking (not even a puff) or other nicotine use during the final 4 weeks of treatment, verified by end‐expiratory CO levels of ≤10 ppm“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Weight likely objectively assessed: Quote: "Vital signs and physical measurements were determined either at screening or baseline (height and body temperature) or at all clinic visits in both the treatment and nontreatment follow‐up phases (body weight ..)” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Study completion rates were 95.2% in the varenicline group and 94.4% in the placebo group. The numbers of subjects discontinuing during the 12‐week treatment phase of the study were 4 (3.2%) and 3 (2.4%) in the varenicline and placebo groups, respectively. The numbers of subjects discontinuing during the 12‐week, nontreatment, follow‐up phase were 2 (1.6%) in the varenicline group and 4 (3.2%) in the placebo group.” |
2 Tønnesen 1991.
| Study characteristics | ||
| Methods | Country: Denmark Recruitment: community volunteers Study start date: Not specified; Study end date: Not specified |
|
| Participants | 289 smokers, 70% female, av age 45, av cpd 22 | |
| Interventions |
All participants receive brief behavioural support at clinic visits |
|
| Outcomes | Mean (SD) weight change (kg) in prolonged abstainers at EOT (email communication) and 12m (email communication) (validation: CO ≤ 10 ppm) | |
| Study funding | "Supported in part by a grant from Kabi Pharmacia Therapeutics" | |
| Author declarations | "Dr Tonnesen has been consultant for Kabi Pharmacia Therapeutics, developer of the nicotine patch and has received grants from the company. Dr. Säwe is medical director of Kabi Pharmacia Therapeutics" | |
| Notes | Prolonged abstinence was defined as validated self‐report abstinence after 1 wk of quitting | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | According to a computer‐generated randomisation code |
| Allocation concealment (selection bias) | Unclear risk | Quote: "packages labelled with consecutive numbers from computer‐generated random code" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Double‐blind + placebo‐controlled Quote: “the blinding of this study was imperfect, in that 67 percent of the subjects guessed correctly which treatment they had received” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Self‐reported abstinence validated by a CO concentration of < 10 ppm |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Likely objectively measured: Quote: “The same two nurses conducted the assessments (recording weight …)” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "3 subjects completely lost to follow up from 26 weeks" Comment: No further information given |
2 Tønnesen 1993.
| Study characteristics | ||
| Methods | Country: Denmark Recruitment: community volunteers Study start date: Not specified; Study end date: Not specified |
|
| Participants | 286 smokers, av cpd 20, 60% F, av age 39 | |
| Interventions |
All participants received brief advice at 8 clinic visits, 0, 1, 2, 3, 6,12, 24, 52 wks) |
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at EOT (email communication) and 12m (email communication) (validation: expired CO < 10 ppm) | |
| Study funding | "This investigation was supported by a grant from Kabi Pharmacia Therapeutics, Helsingborg, Sweden" | |
| Author declarations | "Drs Tonnesen, Norregaard, Mikkelsen, and Jorgensen have received fees from Kabi Pharmacia for consultancies and honoraria for educational activities. Mr Nilsson is employed by Kabi Phamacia" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization code |
| Allocation concealment (selection bias) | Unclear risk | Quote: "participants were randomly assigned according to code generated by a computer". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind + placebo‐controlled. Quote: “Forty‐six percent on active treatment and 58% on placebo identified the treatment correctly, 13% on active treatment and 15% on placebo guessed wrong, and 42% on active treatment and 27% on placebo did not know which treatment they had received” |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Self‐reported abstinence validated by a CO concentration of < 10 ppm |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Likely objectively measured: Quote: “Two nurses conducted the assessments (weight, …)” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “Six subjects were unavailable for follow‐up, and seven subjects were excluded because of protocol violation” |
2 Ussher 2003.
| Study characteristics | ||
| Methods | Country: England
Recruitment: community volunteers Study start date: not specified; Study end date: not specified |
|
| Participants | 309 sedentary smokers, 60% female, av age 43, av cpd 22, av BMI 25 ‐ 26 | |
| Interventions |
|
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at end of treatment | |
| Study funding | "This study was supported through grant CE1198/0101 from the Cancer Research Campaign (now Cancer Research UK) to the authors" | |
| Author declarations | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Allocated in order of attendance |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "Smoking abstinence was verified with expired air CO concentration (cut‐off, 10 p.p.m.)“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “clothed weight and height, without shoes, were measured on calibrated scales to the nearest 0.25 kg and 0.5 cm, respectively.“ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “Attendance at the quit day visit was slightly, although not significantly, higher for the control group than for the exercise group [89% (129/145) versus 83.1% (128/ 154), respectively]; this reduced the chance of finding a significant effect for the exercise intervention.” Comment: No further information. |
2 Uyar 2007.
| Study characteristics | ||
| Methods | Country: Turkey Setting: cessation clinic Recruitment: cessation clinic patients Study start date: September 2002; Study end date: Not specified |
|
| Participants | 131 smokers; 81% M, av age 36, av baseline weight 70 ‐ 75kg, av FTND score 3.9 ‐ 4.8 | |
| Interventions |
All arms: Brief counselling and booklet on consequences of smoking with follow‐up for 24 wks |
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at 24 weeks (data from email communication) Validation: CO levels < 10 ppm |
|
| Study funding | None specified | |
| Author declarations | None specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | no mention of blinding and no placebo used |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "Declaration of quitting and exhaled carbon monoxide level less than 10 ppm was accepted as success criteria." |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Quote: "…weight gain were recoded at follow‐up visits at 2, 4, 8, 12, 24 weeks." No further information on how weight was measured was provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of any losses to follow‐up |
2 Walker 2020.
| Study characteristics | ||
| Methods | Country: New Zealand Recruitment: via media advertising/social media and directed to contact the study centre at the University of Auckland’s National Institute for Health Innovation by freephone, email, Facebook or through the study website Setting: Telephone Study start date: March 2016; Study end date: August 2018 |
|
| Participants | Total N: 1124 smokers who want to quit in the next 3 months, reside in New Zealand, ≥18 years N per arm: NP = 125; NP + NEC = 500; NP + Non‐EC = 499 68.3% female, av age 41.6, av cpd 17.3 |
|
| Interventions |
|
|
| Outcomes |
|
|
| Study funding | Health Research Council of New Zealand; Role of the funding source: The sponsor of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. | |
| Author declarations | NW, CB, MV, GL, ML, and VP report grants from the Health Research Council of New Zealand, during the conduct of the study. NW, CB, MV, and VP report grants from Pfizer, outside of the submitted work. GL chairs the organisation End Smoking New Zealand, which advocates for harm reduction approaches to tobacco control. E‐cigarettes were purchased from a New Zealand e‐cigarette online retailer (NZVAPOR, https://www.nzvapor.com/), e‐liquid was purchased from Nicopharm, Australia (www.nicopharm.com.au/), and nicotine patches were supplied by the New Zealand Government via their contract with Novartis (Sydney, Australia). NZVAPOR also provided, at no cost to participants, on‐line and phone support regarding use of the e‐cigarettes. Neither NZVAPOR nor Nicopharm have links with the tobacco industry. None of the above parties had any role in the design, conduct, analysis, or interpretation of the trial findings, or writing of this publication. | |
| Notes | Authors provided additional data upon request This study is new to the 2021 update. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants will be allocated to one of the three study groups in a 1:4:4 ratio (21mg nicotine patch alone: 21mg nicotine patch plus 18 mg/mL nicotine e‐cigarette: 21 mg nicotine patch plus nicotine‐free e‐cigarette) using stratified block randomisation (block size of nine). Randomisation will be stratified by ethnicity (Māori, non‐Māori) to ensure an equal balance in this key prognostic factor. The computer‐generated randomisation sequence will be prepared by the study statistician.“ |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants and all research staff (except the project manager) are blinded to the nicotine content of the e‐juice (the e‐juice is stored in a brown bottle), until after data lock. The project manager is not involved in any data collection or interaction with trial participants. If required, the medical practitioner who reviews all adverse event reports may request that the participant’s data be un‐blinded. This un‐blinding will be undertaken by the study statistician.“ |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “The primary outcome was continuous smoking abstinence 6 months after the agreed quit date (self‐reported abstinence since quit date, allowing five or fewer cigarettes in total). Abstinence was verified by a researcher or community‐based cessation provider using standardised exhaled carbon monoxide (CO) measurement with a Bedfont Smokerlyzer (Bedfont Scientific Ltd, Kent, UK), with a reading of 9 ppm or lower signifying abstinence.” Comment: Continuous abstinence (CO‐verified at 12 months) was secondary outcome |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: "We asked a subsample of participants for their self‐reported weight and height at baseline, then their weight (self‐reported) at three and six months post‐quit“ Comment: Participants were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 24‐week assessment: NP group: 63/125 (50.4%) NP + NEC group: 339/500 (67.8%) NP + Non‐NEC: 337/499 (67.5%) |
2 Wallstrom 2000.
| Study characteristics | ||
| Methods | Country: Sweden
Recruitment: community volunteers Study start date: Not specified; Study end date: Not specified |
|
| Participants | 247 smokers (10+ cpd), 59% female, av age 45, av cpd 18 ‐ 20, av weight (male) 80 ‐ 81kg, av weight (female) 66 ‐ 67 kg | |
| Interventions |
All participants received brief 5 mins counselling at study visits |
|
| Outcomes | Mean (SD) weight change (kg) in prolonged abstainers at 12 months (validation: CO < 10 ppm) | |
| Study funding | Not specified | |
| Author declarations | Not specified | |
| Notes | Prolonged abstinence defined as complete abstinence from wk 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer assignment |
| Allocation concealment (selection bias) | Low risk | Quote: "Subjects were randomised to receive either active or placebo treatment using a computer program". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind + placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "Success without slips: continuous, self‐reported complete abstinence from week 2 until end‐point, verifjed by exhaled CO level, 10 p.p.m. from week 3 onwards“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: "Subjects were also weighed, using the same scales, at each visit.“ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “In the highly dependent group .. there was a high dropout rate between the 6‐ and 12‐month follow‐up visits” |
2 Wang 2009.
| Study characteristics | ||
| Methods | Setting: Not described Country: China (10 sites), Singapore (3 sites), Thailand (2 sites)udy start date: Not specified; Study end date: Not specified |
|
| Participants | 333 healthy adult volunteers, aged 18 to 75; 97% male, mean age 39, BMI > 15 and < 38 or weight > 45.5 kg, mean cpd 20, mean Fagerström score 5.4 | |
| Interventions |
Treatment period 12 wks, 1st wk titrated dosage. All participants received a smoking cessation booklet at baseline, + brief counselling (10 mins) at each clinic visit, except for wks 5 and 7, when counselling was conducted by phone |
|
| Outcomes | Mean (SD) weight change (kg) in continuous abstainers at EOT (12 wks) and 6 months (Validation: CO ≤ 10 ppm) |
|
| Study funding | "Pfizer Inc. funded the study" | |
| Author declarations | "Pfizer Inc. funded the study and was involved with its design, analysis and writing the manuscript. All authors had complete access to all relevant data. Dahlia Garza and Simon Davies are employees of Pfizer Inc., and therefore hold shares in the company. Editorial support was provided by Aideen Young, PhD, of UBC Scientific Solutions and funded by Pfizer Inc. None of the other authors hold shares in any companies. Chen Wang and Dan Xiao are affiliated with the WHO Collaborating Centre for Tobacco or Health. WHO had no role in the study’s funding, design, analysis or write‐up." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | eligible participants were randomised in a 1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind + placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Self‐reported abstinence validated by a CO concentration of < 10 ppm |
| Blinding of outcome assessment (detection bias) Weight | Unclear risk | Unclear how weight was measured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flowchart: Of those randomized to the varenicline group, 165 received at least one dose, 160 (97%) completed treatment (5 (3.0%) discontinued) and 158 (95.8%) completed the study (7 (4.2%) discontinued); Of those randomized to the placebo group, 168 received at least one dose, 162 (96.4%) completed treatment (6 (3.6%) discontinued) and 161 (95.8% completed the study (7 (4.2%) discontinued). |
2 Xiao 2019.
| Study characteristics | ||
| Methods | Country: China Recruitment: from smoking cessation clinics and via advertisements published in the hospitals Setting: 10 academic or hospital‐associated smoking cessation clinics in 6 cities (Beijing, Shenyang, Tianjin, and Shanghai [2 sites each]; Hangzhou and Guangzhou [1 site each]) Study start date: May 2009; Study end date: March 2011 |
|
| Participants |
Total sample Total N: 722 smokers, ≥ 18 years, smoking regularly everyday for ≥ 1 year and strongly motivated to quit (using nicotine mint lozenge) 3.2% female, av age 39.1, av FTND 4.3 Low‐dependence stratum Total N: 438 participants who smoked their first cigarette > 30 mins after awakening N per arm: Nicotine = 241; Placebo = 242 3.7% female, av age 39.2, av baseline weight 72.1, av FTND 2.6 High‐dependence stratum Total N: 240 participants who smoked their first cigarette within 30 mins after awakening N per arm: Nicotine = 120; Placebo = 120 (1 participant withdrew and not included in analysis) 2.1% female, av age 42.2, av baseline weight 75.1, av FTND 5.9 |
|
| Interventions |
Low‐dependence stratum 1. 0 mg nicotine placebo lozenge 2. 2 mg nicotine placebo lozenge High‐dependence stratum 1. 0 mg nicotine placebo lozenge 2. 4 mg nicotine lozenge Dosing schedule for all groups: Week 1 ‐ 6: 1 lozenge every 1 ‐ 2 hours, 9 ‐ 15/day Week 7 ‐ 9: 1 lozenge every 2 ‐ 4 hours, 3 ‐ 6/day Week 10 ‐ 12: 1 lozenge every 4 ‐ 8 hours, 3 ‐ 6/day After week 12: Use 1 ‐ 2 lozenges/day; Discontinue use after 24 week. All participants received low‐intensity behavioural support sessions for 10 minutes at visit 2 through to visit 5 (i.e. weeks 0, 1, 2, and 4 of treatment). Diary cards were provided to record cravings, withdrawal symptoms, and daily lozenge usage |
|
| Outcomes |
Low‐dependence stratum
High‐dependence stratum
|
|
| Study funding | "This study was sponsored by Tianjin Sino‐American SmithKline & French Laboratory Ltd. Medical writing assistance was provided by Peloton Advantage, LLC, an OPEN Health company, and was funded by GlaxoSmithKline Consumer Healthcare. GlaxoSmithKline Consumer Health‐ care provided a full review of the article.“ All study treatments were provided by Sino‐American Tianjin Smith Kline & French Laboratories Ltd, Tianjin, China |
|
| Author declarations | The authors report no conflicts of interest | |
| Notes | This study is new to the 2021 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization schedule was computer‐generated and was provided to the site by the GSK Biostatistics Department |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "This was a double‐blind study. Sponsors, sponsor’s representatives, the investigator, staff, and subjects were blinded to treatment regimen“ |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: "Abstinence was confirmed by expiratory CO levels threshold of <= 10 ppm at each in‐person visit to the study site. Subjects who failed to meet the success criteria and those with unknown smoking status were counted as failures.“ |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “Body weight, concomitant medications, and vital signs were recorded.” Not entirely clear if weight was objectively recorded. Self‐report cannot be ruled out, but participants were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 mg Placebo: 48/242 dropped out 2 mg NRT: 48/241 dropped out 4 mg placebo: 26/120 dropped out 4 mg NRT: 22/120 dropped out |
2 Zellweger 2005.
| Study characteristics | ||
| Methods | Country: 12 European countries, 26 centres
Recruitment: volunteers, healthcare professionals (qualified practising physician or nurse) Study start date: not specified; Study end date: not specified |
|
| Participants | 667 smokers (≥ 10 cpd) (excludes 1 centre enrolling 20 people, and 3 people who took no medication) 64% female, av cpd 23 | |
| Interventions |
All participants received brief (10 ‐ 15 mins) motivational support at weekly clinic visits and telephone support 1 day before TQD, 3 days after TQD, monthly during follow‐up |
|
| Outcomes | Mean (SD) weight change (kg) in prolonged abstainers at EOT (email communication), 6m (email communication) and 12m (email communication) (validation: CO ≤ 10 ppm) | |
| Study funding | Not specified | |
| Author declarations | Not specified | |
| Notes | Prolonged abstinence defined as continuous abstinence from week 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 3:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind + placebo‐controlled. No further information given |
| Blinding of outcome assessment (detection bias) Smoking | Low risk | Quote: “Expiratory CP level <ppm” |
| Blinding of outcome assessment (detection bias) Weight | Low risk | Quote: “subjects were weighed” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
BMI: body mass index; CBT: (E)CO: (expired) carbon monoxide; CBT: cognitive behavioural therapy; COPD: chronic obstructive pulmonary disease; cpd: cigarettes per day: EOT: end of treatment; FTND: Fagerström test for nicotine dependence; MI: motivational interviewing; CVD: cardiovascular disease; MDD: major depressive disorder; NRT: nicotine replacement therapy; PPA: point prevalence abstinence; ppm: parts per million; RMR: resting metabolic rate; SC: smoking cessation; SD: standard deviation; SH: self‐help; TQD: target quit date.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| 1 Ames 2007 | Not an intervention designed specifically to tackle post‐cessation weight gain |
| 1 Chaney 2008 | Exercise intervention excluded by parent Cochrane Review |
| 1 Hughes 1997 | Effect of NRT on post‐cessation weight gain, not identified in NRT parent review |
| 1 ISRCTN47776579 | Study on ineligible population as participants were not immediately ready to quit smoking |
| 1 Jeffery 1990 | Study testing effect on intervention on weight control in general rather than on post‐cessation control |
| 1 Killen 1990 | Effect of minimal contact smoking relapse prevention trial with NRT, not included in parent review |
| 1 Kim 2017 | Did not measure smoking cessation at 6+ months OR weight at any follow‐up point |
| 1 King 2006 | Weight only measured at end of 1 month (2‐month intervention) |
| 1 Lagrue 1994 | Intervention on overweight participants only |
| 1 Leischow 1992 | Unable to obtain full data |
| 1 Love 2011 | Participants not randomized |
| 1 NCT02412631 | Study terminated; (lorcaserin removed from market) |
| 1 NCT03362372 | Not a smoking cessation trial |
| 1 Patterson 2006 | Not an intervention designed to address weight gain |
| 1 Pomerleau 1991 | Excluded from antidepressant parent review |
| 1 Rohsenow 2007 | No weight data |
| 1 Russo 2016 | Ineligible population as participants were not intending to quit smoking. |
| 1 Spring 1991 | Unable to obtain data |
| 1 Thompson 2015 | Weight not measured at baseline for the usual‐care study arm |
| 1 Toll 2008 | Participants not randomized to experimental or control conditions |
| 1 Wilcox 2010 | Uncontrolled trial |
| 2 AD Ahluwalia 2002 | Unable to obtain full data |
| 2 AD Aubin 2004 | Unable to obtain full data |
| 2 AD Berlin 1995 | No weight data |
| 2 AD Blondal 1999 | No weight data |
| 2 AD Brown 2006 | No weight data |
| 2 AD Cinciripini 05 | No weight data |
| 2 AD Collins 2004 | No weight data |
| 2 AD Covey 2002 | No weight data |
| 2 AD Covey 2007 | All participants received 8 weeks of open‐label bupropion and NRT |
| 2 AD Da Costa 2002 | No weight data |
| 2 AD Dalsgareth 2004 | Unable to obtain full data |
| 2 AD Evins 2001 | Unable to obtain full data |
| 2 AD Evins 2005 | No weight data |
| 2 AD Evins 2006 | No weight data |
| 2 AD Evins 2008 | Less than 6 months follow‐up |
| 2 AD Ferry 1992 | No weight data |
| 2 AD Ferry 1994 | No weight data |
| 2 AD George 2002 | No weight data |
| 2 AD GlaxoSmithK SMK20001 | No weight data |
| 2 AD Gonzales 2001 | No weight data |
| 2 AD Haggsträm 2006 | No weight data |
| 2 AD Hall 1998 | No weight data |
| 2 AD Hall 2002 | No weight data |
| 2 AD Hall 2004 | No weight data |
| 2 AD Hatsukami 2004 | No weight data |
| 2 AD Hays 2001 | Unable to obtain full data |
| 2 AD Hertzberg 2001 | No weight data |
| 2 AD Holt 2005 | No weight data |
| 2 AD Hurt 2003 | No weight data |
| 2 AD Killen 2000 | No weight data |
| 2 AD Killen 2004 | No weight data |
| 2 AD Killen 2006 | No weight data |
| 2 AD Myles 2004 | No weight data |
| 2 AD Piper 2004 | No weight data |
| 2 AD Piper 2009 | No weight data |
| 2 AD Prochazka 1998 | No weight data |
| 2 AD Prochazka 2004 | No weight data |
| 2 AD Rovina 2009 | No weight data |
| 2 AD Selby 2003 | No weight data |
| 2 AD Swan 2003 | No weight data |
| 2 AD Tashkin 2001 | No weight data |
| 2 AD Tonstad 2003 | Unable to obtain full data |
| 2 AD Tønnesen 2003 | Unable to obtain full data |
| 2 AD Uyar 2005 | Unable to obtain full data |
| 2 AD Wagena 2005 | No weight data |
| 2 Aveyard 2018 | ineligible intervention |
| 2 Drovandi 2018 | This trial was deemed eligible for inclusion but the author confirmed that "the funding fell through and I was unable to conduct this RCT as intended" |
| 2 EX Hill 1985 | No weight data |
| 2 EX Hill 1993 | No weight data |
| 2 EX Kinnunen 2008 | Unable to get data [Check Korhonen T, Goodwin A, Miesmaa P, Dupuis EA, Kinnunen T. Smoking cessation program with exercise improves cardiovascular disease biomarkers in sedentary women Journal of Women's Health (2002) 2011 20( 7) 1051‐64] |
| 2 EX Marcus 1991 | No weight data |
| 2 EX Marcus 1995 | No weight data |
| 2 EX Martin 1997 | No weight data |
| 2 EX Prapavessis 2007 | Unable to get data |
| 2 EX Russell 1988 | No weight data |
| 2 EX Taylor 1988 | No weight data |
| 2 LeMao 2020 | Insufficient information to determine eligibility |
| 2 NRT Ahluwalia 1998 | No weight data |
| 2 NRT Ahluwalia 2006 | No weight data |
| 2 NRT Areechon 1988 | No weight data |
| 2 NRT Blondal 1989 | No weight data |
| 2 NRT Blondal 1997 | Unable to obtain full data |
| 2 NRT Bolin 1999 | No weight data |
| 2 NRT Br Thor Soc 83 | No weight data |
| 2 NRT Buchkremer 88 | No weight data |
| 2 NRT Bullen 2010 | Participants took medication before quit day |
| 2 NRT Campbell 1987 | No weight data |
| 2 NRT Campbell 1991 | No weight data |
| 2 NRT Campbell 1996 | No weight data |
| 2 NRT Cinciripini 96 | No weight data |
| 2 NRT Clavel 1985 | No weight data |
| 2 NRT Clavel‐Cha '92 | No weight data |
| 2 NRT Croghan 2003 | No weight data |
| 2 NRT Croghan 2007 | No weight data |
| 2 NRT Daughton 1991 | No weight data |
| 2 NRT Daughton 1998 | No weight data |
| 2 NRT Dautzenberg 01 | No weight data |
| 2 NRT Davidson 1998 | No weight data |
| 2 NRT Etter 2009 | Participants took medication before the quit date |
| 2 NRT Fagerström 1982 | No weight data |
| 2 NRT Fagerström 1984 | No weight data |
| 2 NRT Fee 1982 | No weight data |
| 2 NRT Fortmann 1995 | No weight data |
| 2 NRT Garcia 1989 | No weight data |
| 2 NRT Gilbert 1989 | No weight data |
| 2 NRT Glavas 2003a | No weight data |
| 2 NRT Glavas 2003b | No weight data |
| 2 NRT Glover 2002 | Unable to obtain full data |
| 2 NRT Goldstein 1989 | No weight data |
| 2 NRT Hall 1985 | No weight data |
| 2 NRT Hall 1987 | No weight data |
| 2 NRT Hall 1996 | No weight data |
| 2 NRT Hand 2002 | No weight data |
| 2 NRT Harackiewicz 1988 | No weight data |
| 2 NRT Hatsukami 2007 | Less than 6 months follow‐up |
| 2 NRT Hays 1999 | No weight data |
| 2 NRT Herrera 1995 | No weight data |
| 2 NRT Hilleman 1994 | No weight data |
| 2 NRT Huber 1988 | No weight data |
| 2 NRT Hughes 1989 | No weight data |
| 2 NRT Hughes 1990 | No weight data |
| 2 NRT Hughes 1991 | No weight data |
| 2 NRT Hughes 1999 | No weight data |
| 2 NRT Hughes 2003 | No weight data |
| 2 NRT Hurt 1990 | No weight data |
| 2 NRT Hurt 1994 | No weight data |
| 2 NRT ICRF 2007 | No weight data |
| 2 NRT Jamrozik 1984 | No weight data |
| 2 NRT Jarvis 1982 | No weight data |
| 2 NRT Jensen 1991 | No weight data |
| 2 NRT Jorenby 1995 | No weight data |
| 2 NRT Jorenby 1999 | Unable to obtain full data |
| 2 NRT Joseph 1996 | No weight data |
| 2 NRT Kalman 2006 | No weight data |
| 2 NRT Killen 1984 | No weight data |
| 2 NRT Killen 1990 | No weight data |
| 2 NRT Killen 1997 | No weight data |
| 2 NRT Killen 1999 | Unable to obtain full data |
| 2 NRT Kornitzer 1987 | Unable to obtain full data |
| 2 NRT Kornitzer 1995 | No weight data |
| 2 NRT Kralikova 2002 | No weight data |
| 2 NRT Kralikova 2009 | Participants could reduce smoking or quit smoking |
| 2 NRT Leischow 1996 | No weight data |
| 2 NRT Leischow 1999 | No weight data |
| 2 NRT Leischow 2004 | No weight data |
| 2 NRT Lewis 1998 | No weight data |
| 2 NRT Llivina 1988 | No weight data |
| 2 NRT Malcolm 1980 | No weight data |
| 2 NRT Marshall 1985 | No weight data |
| 2 NRT McGovern 1992 | No weight data |
| 2 NRT Molyneux 2003 | No weight data |
| 2 NRT Moolchan 2005 | No weight data |
| 2 NRT Mori 1992 | No weight data |
| 2 NRT Müller 1990 | No weight data |
| 2 NRT Nakamura 1990 | No weight data |
| 2 NRT Nebot 1992 | No weight data |
| 2 NRT Niaura 1994 | No weight data |
| 2 NRT Niaura 1999 | No weight data |
| 2 NRT Ockene 1991 | No weight data |
| 2 NRT Oncken 2007 | No weight data |
| 2 NRT Otero 2006 | No weight data |
| 2 NRT Page 1986 | No weight data |
| 2 NRT Paoletti 1996 | No weight data |
| 2 NRT Peng 2007 | Less than 6 months follow up |
| 2 NRT Perng 1998 | No weight data |
| 2 NRT Piper 2007 | No weight data |
| 2 NRT Puska 1979 | No weight data |
| 2 NRT Richmond 1993 | No weight data |
| 2 NRT Rose 1994 | No weight data |
| 2 NRT Rose 1998 | No weight data |
| 2 NRT Rose 2006 | No weight data |
| 2 NRT Rose 2009 | Participants took medication before quit day |
| 2 NRT Roto 1987 | Unable to obtain full data |
| 2 NRT Russell 1983 | No weight data |
| 2 NRT Schneider 1985A | No weight data |
| 2 NRT Schneider 1985B | No weight data |
| 2 NRT Schneider 1995 | No weight data |
| 2 NRT Schneider 1996 | No weight data |
| 2 NRT Schnoll 2010 | No weight data |
| 2 NRT Schuurmans 04 | No weight data |
| 2 NRT Segnan 1991 | No weight data |
| 2 NRT Shiffman 2009 | Not abrupt quitting |
| 2 NRT Sonderskov 97 | No weight data |
| 2 NRT Stapleton 2011 | Less than 6 months follow up |
| 2 NRT Tønnesen 1988 | No weight data |
| 2 NRT Tønnesen 2000 | No weight data |
| 2 NRT Tønnesen 2006 | No weight data |
| 2 NRT Veaugh‐Geiss 2010 | No weight data |
| 2 NRT Villa 1999 | No weight data |
| 2 NRT Westman 1993 | No weight data |
| 2 NRT Wisborg 2000 | No weight data |
| 2 NRT Wong 1999 | No weight data |
| 2 NRT Zelman 1992 | No weight data |
| 2 Prapavessis 2016 | Identified and included in Part 1 of this review. |
| 2 Urdapilleta‐Herrera 2013 | Insufficient information to determine eligibility. |
| 2 VA Carson 2010 | Less than 6 months follow‐up |
| 2 VA Hajek 2011 | Participants took medication before quit day |
| 2 VA Tsukahara 2010 | No weight data for abstainers |
| 2 VA Williams 2007 | No weight data |
Characteristics of ongoing studies [ordered by study ID]
1 Komiyama 2018.
| Study name | A randomized, multicenter trial for the effects of dietary instruction on cardiovascular risk markers after smoking cessation |
| Methods | Randomized multicenter controlled trial Country: Japan Recruitment: smoking cessation clinics (SC), enrolling by invitation |
| Participants | Target sample: 250 Key inclusion criteria:
Key exclusion criteria:
|
| Interventions | (1) 3 sessions of nutritional guidance provided by a registered dietician every 3 months (1, 4 and 7 months after enrolment). An instructional plan to meet an individual’s estimated energy requirements and nutrient intake will be formulated based on the Dietary Reference Intake for Japanese (2015) as stipulated by the Ministry of Health, Labor, and Welfare (2) No nutritional guidance |
| Outcomes | Mean (SD) weight change (kg) at 9‐month follow‐up in abstainers (CO < 7 ppm and self‐report of 30‐day abstinence) |
| Starting date | Study start date: April 2016; Expected study end date: March 2022 |
| Contact information | Maki Komiyama: nikonikomakirin@yahoo.co.jp; Clinical Research Institute, National Hospital Organization Kyoto Medical Center, 1‐1 Mukaihata‐cho, Fukakusa, Fushimi‐ku, Kyoto 612‐8555, Japan |
| Notes |
1 NCT01892813 2013.
| Study name | Dissemination of a tailored tobacco quitline for rural veteran smokers |
| Methods | Randomized, double‐blind, clinical trial Country: United States Recruitment: Not specified |
| Participants | Target sample: 411 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Mean (SD) weight change (kg) at 6‐month follow‐up in abstainers (self‐reported abstinence in the past 30 days) |
| Starting date | July 2013; Expected study end date: January 2018 |
| Contact information | Mark W. Vander Weg, Ph.D., mark‐vanderweg@uiowa.edu |
| Notes |
1 NCT02424188 2015.
| Study name | Trial of integrated smoking cessation, exercise and weight management in serious mental illness: TRIUMPH |
| Methods | Randomized controlled trial Country: United States Recruitment: Based on attending 1 of 2 community mental health organizations in Maryland |
| Participants | Total final enrolment: 192 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Mean (SD) weight change (kg) at 6 and 18‐months follow‐up in abstainers (CO‐verified 7‐day point prevalence) |
| Starting date | 7 July 2016; Expected study end date: March 2020 |
| Contact information | Gail L Daumit: gdaumit@jhmi.edu Johns Hopkins University 2024 East Monument Street Suite 2‐620 Baltimore, Maryland 21287 |
| Notes |
1 NCT02906787 2016.
| Study name | Behavioral activation for smoking cessation and the prevention of post‐cessation weight gain |
| Methods | Randomized clinical trial Country: United States Recruitment: Not specified |
| Participants | Targeted sample: 340 Key eligibility criteria:
|
| Interventions |
|
| Outcomes | Mean (SD) weight change (kg) at EOT (10 weeks), 12‐week follow‐up and 26‐week follow‐up in abstainers (CO < 5 ppm 7‐day PPA; Saliva cotinine < 15 ng/ml) |
| Starting date | Study start date: 31 August 2016; Expected study end date: February 2021 |
| Contact information | Benjamin F Albelda: albeldab@mail.med.upenn.edu Janet Audrain‐McGovern: audrain@pennmedicine.upenn.edu |
| Notes |
1 NCT03204396 2017.
| Study name | Smoking cessation facilitated by glucagon‐like peptide‐1 (GLP‐1) analogues |
| Methods | Randomized, double‐blind, placebo‐controlled trial Country: Switzerland Recruitment: Not specified |
| Participants | Target sample: 256 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Mean (SD) weight change (kg) at 4, 8, 12, 24 and 52 weeks in abstinent smokers (CO < 10 ppm PPA) |
| Starting date | Study start date: 26 June 2017; Expected study end date: 31 March 2021 |
| Contact information | Dr Bettina Winzeler, Tel: +41615565075, Bettina.winzeler@usb.ch Dr Nica Jeanloz, Tel:+41613285523, nicaanna.jeanloz@usb.ch |
| Notes |
1 NCT03712098 2018.
| Study name | Daily liraglutide for nicotine dependence (DAL) |
| Methods | Randomized, double‐blind, placebo‐controlled, parallel arm pilot study with 1 between‐subjects factor of medication group (liraglutide vs. placebo) Country: United States Recruitment: Not specified |
| Participants | Target sample: 80 Key eligibility criteria:
|
| Interventions |
|
| Outcomes | Mean (SD) weight change (kg) at 12 weeks post‐TQD (study week 18) and 26‐weeks post‐TQD (study week 32) in abstainers (CO < 5 ppm 7‐day PPA) |
| Starting date | Study start date: 29 November 2018; Expected study end date: May 2021 |
| Contact information | Rebecca L. Ashare, Ph.D; 215‐746‐5789; rlashare@pennmedicine.upenn.edu Kristina D. Roose, MSW; 215‐746‐8430; kroose@pennmedicine.upenn.edu |
| Notes |
1 NCT04130698 2019.
| Study name | Comparing two treatments that both target smoking cessation and weight loss at the same time (BREATH) |
| Methods | Randomized clinical trial Country: United States Recruitment: Not specified |
| Participants | Target sample: 48 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Mean (SD) weight change (kg) at 6‐month follow‐up in abstainers (saliva cotinine verified 7‐day PPA) |
| Starting date | Study start date: 1 June 2019; Expected study end date: 31 May 2022 |
| Contact information | Nadia Petrovic; 1‐401‐456‐8000; npetrovic@ric.edu |
| Notes | Based on a previously conducted pilot study of Distress Tolerance (DT) |
1 NCT04332029 2020.
| Study name | Smoking cessation treatment for smokers with obesity |
| Methods | Randomized clinical trial Country: Spain Recruitment: Not specified |
| Participants | Target sample: 120 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Mean (SD) weight change (kg) at end of treatment (8 weeks) and 1, 3, 6, and 12 months follow‐up in abstainers (CO‐verified continuous abstinence) |
| Starting date | Study start date: 7 October 2020; Expected study end date: December 2022 |
| Contact information | Gloria Garcia‐Fernandez, PhD; +34985103252; garciafgloria@uniovi.es |
| Notes |
1 RBR‐682px9 2018.
| Study name | Evaluation of the effect of dietary control in the treatment of female smokers |
| Methods | Clinical trial of treatment, randomized controlled, parallel, open, 2 arms Country: Brazil Recruitment: Not specified |
| Participants | Target sample: 205 Inclusion criteria: “women smokers enrolled in the smoking control program at health units in the western zone of the municipality of Rio de Janeiro.” Exclusion criteria: “pregnant women unable to walk.” |
| Interventions |
|
| Outcomes | Mean (SD) weight change (kg) at 6 months follow‐up in abstainers |
| Starting date | Study start date: 5 September 2018; Expected study end date: Not specified |
| Contact information | Cláudia Christina Sobrinho do Nascimento; +55‐021‐997332776; claudiachrisnutri@gmail.com |
| Notes |
1 Salgado Garcia 2018.
| Study name | Efficacy of two novel behavioral post‐cessation weight gain interventions |
| Methods | Randomized clinical trial Country: United States Recruitment: Multifaceted. Both traditional (e.g. flyers, business cards, postcards, posters, recruitment through medical referrals, radio, and local media advertising) and electronic strategies (e.g. institution emails, electronic advertisements on Facebook). Sites will include local universities and colleges, local physician and dentist offices, barbershops, police stations, fire department stations, and community resource websites. In addition, recruitment will include a “refer a friend” incentive where participants can refer up to 10 friends to the study. Each randomized friend will earn the referrer USD 10 in incentives (i.e. Amazon gift certificates) |
| Participants | Target sample: 400 Inclusion criteria:
Exclusion criteria:
|
| Interventions | All participants will receive 6 sessions of a highly efficacious telephonic smoking cessation intervention delivered in groups plus varenicline (Chantix) prior to the start of the smoking cessation intervention
|
| Outcomes | Mean (SD) weight change (kg) at 2, 4, 8 and 12 months follow‐up in abstainers (CO < 10 ppm 7‐day PPA) |
| Starting date | Study start date: 30 November 2017; Expected study end date: April 2022 |
| Contact information | Salgado Garcia; fsalgado@uthsc.edu |
| Notes |
2 Berlin 2019.
| Study name | Randomized trial of electronic cigarettes with or without nicotine in smoking cessation (ECSMOKE) |
| Methods | Randomized, placebo and reference treatment controlled, multicenter, double‐blind, double‐dummy, parallel group, pivotal, phase III trial Country: France Recruitment: either local (a) directly by the centres or centralized (b) using a web page and a centralized study‐specific phone number and email address
|
| Participants | Target sample: 650 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Brief behavioural smoking cessation counselling based on the national guidelines for smoking cessation is administered for all participants at all visits by the investigators specialized in smoking cessation |
| Outcomes | Mean (SD) weight change (kg) at 24‐week follow‐up in abstainers (CO ≤ 8 ppm) Confirmed continuous abstinence rate from weeks 9 to 12 |
| Starting date | Study start date: 17 October 2018; Expected study end date: March 2022 |
| Contact information | Ivan Berlin: ivan.berlin@aphp.fr, +33 1 42 16 16 78; Groupe Hospitalier Pitié‐Salpétrière, Paris, France, 75013 |
| Notes |
2 Caponnetto 2019.
| Study name | Smoking cessation with varenicline plus counselling for e‐cigarettes users (VAREVAPE) |
| Methods | Double‐blind, placebo‐controlled, randomized clinical trial Country: Italy Recruitment: Not specified |
| Participants | Target sample: 140 + 140
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Mean (SD) weight change (kg) at follow‐up in abstainers |
| Starting date | Study start date: June 2019; Expected study end date: 2020 |
| Contact information | Centro per la Prevenzione e Cura del Tabagismo (CPCT), Azienda Ospedaliero‐Universitaria “Policlinico‐V. Emanuele”, Università di Catania, Via S. Sofia 78, 95123, Catania, Italy; p.caponnetto@unict.it (P. Caponnetto), m.maglia@unict.it (M. Maglia), polosa@unict.it (R. Polosa) |
| Notes |
2 Cinciripini 2009.
| Study name | Combining varenicline and bupropion for smoking cessation |
| Methods | Randomized clinical trial Country: United States Recruitment: Not specified |
| Participants | Target sample: 385 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Mean (SD) weight change (kg) at 3, 6 and 12‐month follow‐up in abstainers (prolonged abstinence verified by CO < 10 ppm at each in‐person measurement occasion) |
| Starting date | 17 May 2010; Expected study end date: 31 May 2021 |
| Contact information | Paul M Cinciripini, PhD, pcinciri@mdanderson.org, Anderson Cancer Center |
| Notes |
2 Hebert‐Losier 2020.
| Study name | Evaluating the efficacy of e‐cigarette use for smoking cessation (E3) trial |
| Methods | Interventional (clinical trial) Country: Canada Recruitment: Community‐based advertisements (e.g. printed fliers, newspaper ads) and online platforms (e.g. Craigslist, Kijiji, Facebook), as well as in outpatient, smoking cessation, and walk‐in clinics |
| Participants | Target sample: 376 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Mean (SD) weight change (kg) at 12‐month follow‐up in abstainers |
| Starting date | Study start date: November 2016; Study end date: 28 January 2021 |
| Contact information | Dr Mark J. Eisenberg, Jewish General Hospital, McGill University, 3755 Côte Ste‐Catherine Road, Suite H‐421.1, Montreal, Quebec H3T 1E2, Canada 1‐514‐340‐8222, ext.: 23564; mark.eisenberg@mcgill.ca |
| Notes |
2 ISRCTN34399445 2020.
| Study name | IMPACT Smoking cessation Support for people with Severe mental illness in South Asia (IMPACT 4S): a randomised controlled pilot and feasibility trial for a combined behavioural and pharmacological support intervention |
| Methods | Open‐label parallel randomised controlled trial Country: India, Pakistan Recruitment: Not specified |
| Participants | Target sample: 172 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes |
|
| Starting date | Study start date: 01/07/2018; Expected study end date: 01/05/2021 |
| Contact information | Prof. Pratima Murthy, pratimamurthy@gmail.com
Department of Psychiatry National Institute of Mental Health and Neurosciences Bangalore 560029 INDIA, Bangalore +91 (0)8026995250 |
| Notes |
2 NCT02106637 2014.
| Study name | Early In‐hospital Initiation of pharmacotherapy for smoking cessation, concomitant with nurse‐led support, in patients after an Acute Coronary Syndrome (ACS) |
| Methods | Prospective, double‐blind, randomized, placebo‐controlled, multicenter study Country: Israel Recruitment: hospitalized ACS smokers |
| Participants | Target sample: 300 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Mean (SD) weight change (kg) at 12‐month follow‐up in abstainers (CO‐verified continuous abstinence rate (CAR) at 12 months) |
| Starting date | October 2016; Expected study end date:December 2018 |
| Contact information | Prof. IIan Goldenberg, Sheba Medical Center, ilan.goldenberg@sheba.health.gov.il |
| Notes |
2 NCT02162849 2015.
| Study name | Reward sensitivity and pharmacotherapy for smoking cessation |
| Methods | Randomized, double‐blind, placebo‐controlled clinical trial Country: United States Recruitment: Not specified |
| Participants | Target sample: 204 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Mean (SD) weight change (kg) at 6‐month follow‐up in abstainers (CO‐verified prolonged abstinence at 6‐months post‐quit‐date) |
| Starting date | Study start date: 14 December 2015; Expected study end date: December 2020 |
| Contact information | Paul Cinciripini, PHD, MS, BS, pcinciri@mdanderson.org |
| Notes |
2 NCT03722966 2018.
| Study name | Effectiveness of combination varenicline and oral nicotine replacement therapy (COMBO) |
| Methods | Randomized clinical trial Country: United States Recruitment: Not specified |
| Participants | Target sample: 100 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Mean (SD) weight change (kg) at 26‐week follow‐up in abstainers (CO‐verified < 8 ppm 7‐day PPA at 26 weeks post‐quit date) |
| Starting date | Study start date: 2 December 2019; Expected study end date: 30 December 2021 |
| Contact information | Joseph Waring, 4052718001 ext 31153, joseph-waring@ouhsc.edu Jocelyn Barton, PhD, 4052718001 ext 31324, jocelyn-barton@ouhsc.edu |
| Notes |
2 NCT04088942 2019.
| Study name | TOBacco STOP in Chronic Obstructive Pulmonary Disease‐Trial ‐ Study Protocol |
| Methods | Randomized, open‐label, superiority, 2‐arm intervention study Country: Denmark Recruitment: in general practice is done by the project‐trained nurse |
| Participants | Target sample: 600 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Mean (SD) weight change (kg) at follow‐up in abstainers at 12 months |
| Starting date | Study start date: 1 August 2021; Expected study end date: 1 January 2024 |
| Contact information | Jens‐Ulrik S Jensen, MD, PhD; +45 3867 3057; jens.ulrik.jensen@regionh.dk |
| Notes |
2 Roelsgaard 2017.
| Study name | REU‐stop – effect of intensive smoking cessation intervention on smoking cessation and disease activity in patients with rheumatoid arthritis |
| Methods | International, multicentre, randomized trial Country: Denmark, Norway Recruitment: daily smokers with RA in remission or with low‐moderate disease activity will be recruited from the Center for Rheumatology and Spine Diseases, Rigshospitalet, Denmark, and from the Preventive Cardio‐Rheuma Clinic, Department of Rheumatology, Diakonhjemmet Hospital, Oslo, Norway |
| Participants | Target sample: 150 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Mean (SD) weight change (kg) at 3‐, 6‐ and 12‐ month follow‐up in abstainers (continuous cessation validated by CO reading < 10 ppm) |
| Starting date | Study start date: April 2020; Expected study end date: January 2021 |
| Contact information | Ida Kristiane Roelsgaard, ida.kristiane.roelsgaard@regionh.dk; Copenhagen Center for Arthritis Research (COPECARE), Center for Rheumatology and Spine Diseases, Rigshospitalet, Glostrup, Denmark |
| Notes |
COPD: chronic obstructive pulmonary disease; cpd: cigarettes per day; NRT: nicotine replacement therapy; OTC: over the counter; PPA: point prevalence abstinence; ppm: part per million; TQD: target quit date
Differences between protocol and review
In the 2021 version, we made the following changes:
We changed from fixed‐ to random‐effects models for our meta‐analyses, in accordance with updated guidance from the Cochrane Tobacco Addiction Group;
We added the review of electronic cigarettes for smoking cessation to Part 2 and removed the review of cannabinoid type 1 receptors, as these are not considered a frontline stop‐smoking pharmacotherapy;
We updated risk of bias assessments in accordance with updated guidance from the Cochrane Tobacco Addiction Group;
We extracted and assessed data on adverse and serious adverse events for Part 1 studies of pharmacotherapies;
We added summary of findings tables according to Cochrane guidance;
We conducted sensitivity analyses removing studies at high risk of bias.
All of these changes were agreed with the Cochrane Tobacco Addiction Group prior to starting the review update.
Contributions of authors
2021 update (includes 2016 searches and data extraction): All authors contributed to screening or data extraction or both. Analysis and write‐up was led by JHB and AT. All authors reviewed and commented on the final manuscript.
Sources of support
Internal sources
-
University of Birmingham, UK
Paid the salary of Amanda Parsons, Jennie Inglis and Paul Aveyard
Mujahed Sharim studied for a masters in public health at the University and completed part of the work as part of his masters project
-
UKCTCS, UK
Paid the doctoral stipend of Deborah Lycett and contributed to the salaries of Amanda Parsons and Paul Aveyard. The UK Centre for Tobacco Control Studies is a UKCRC Public Health Research: Centre of Excellence. Funding from British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, and the National Institute of Health Research, under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged.
-
Queen Mary's University of London, UK
Paid the salary of Peter Hajek
-
National Institute of Health Research, UK
Contributed to the salary of Paul Aveyard
-
NIHR Oxford Biomedical Research Centre, UK
Paid the salary of Laura Kudlek
External sources
No sources of support provided
Declarations of interest
JHB: co‐applicant on an award from the Cochrane Review Support Programme. JHB also writes and publishes frequently on the evidence for smoking cessation interventions, and to a lesser extent about evidence on weight management. None of these represent a conflict with what is covered in this review.
AT: None
AF: co‐applicant on a researcher‐led grant from Ethicon (Johnson and Johnson).
PH: research grants, last held in 2015 from Pfizer (funds were received by the institution); Recieved payment for assessing grant applications (GRAND initiative) and attending global advisory board meeting (last attended in 2017); provides invited commentaries, usually concerning an article on e‐cigarettes for journals (The Lancet, Tobacco Control, Lancet Respiratory and Addiction); was involved in the conduct of a study that was eligible for inclusion in this work which was funded by NIHR.
DL: published a press release following publication of a research report; was involved in the conduct studies that were eligible for inclusion in this work: The DeMist Trial: UKCTCS, VLCD provided by Lipotrim (2010) and The SWISSS Trial: NIHR‐SPCR, Slimming Word vouchers for intervention provide by Slimming World (2013)
LLJ: None
LK: None
LH: None
AH: None
MS: None
PA: was involved in the conduct of a study that was eligible for inclusion in this work funded by the School for Primary Care Research.
PA and AF (nee Parsons) are also authors of a study included in this review testing the effect of St John's wort and chromium supplements on smoking cessation and post‐cessation weight gain. The trial was funded by Cancer Research UK and the supplements were bought from the manufacturer. Paul Aveyard has done consultancy work for pharmaceutical and biotechnology companies that has led to payments to him and his institution. This includes work for companies providing smoking cessation medication, including McNeil, Xenova and Pfizer.
Joint first author
Joint first author
Edited (no change to conclusions)
References
References to studies included in this review
1 Baker 2018 {published and unpublished data}
- Baker AL, Richmond R, Kay-Lambkin FJ, Filia SL, Castle D, Williams JM, et al. Randomised controlled trial of a healthy lifestyle intervention among smokers with psychotic disorders: Outcomes to 36 months. Australian and New Zealand Journal of Psychiatry 2018;52(3):239-52. [DOI: 10.1177/0004867417714336] [DOI] [PubMed] [Google Scholar]
1 Bloom 2020 {published and unpublished data}
- Bloom EL, Ramsey SE, Abrantes AM, Hunt L, Wing RR, Kahler CW, et al. A pilot randomized controlled trial of distress tolerance treatment for weight concern in smoking cessation among women. Nicotine and Tobacco Research 2020;22(9):1578-86. [DOI: 10.1093/ntr/ntaa026] [DOI] [PMC free article] [PubMed] [Google Scholar]
1 Bush 2012 {published data only}
- Bush T, Levine MD, Beebe LA, Cerutti B, Deprey M, McAfee T, et al. Addressing weight gain in smoking cessation treatment: a randomized controlled trial. American Journal of Health Promotion 2012;27(2):94-102. [CENTRAL: 920998] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
1 Bush 2018 {published data only}
- Bush T, Lovejoy J, Javitz H, Magnusson B, Torres AJ, Mahuna S, et al. Comparative effectiveness of adding weight control simultaneously or sequentially to smoking cessation quitlines: study protocol of a randomized controlled trial. BMC Public Health 2016;16:615. [DOI: 10.1186/s12889-016-3231-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush T, Lovejoy J, Javitz H, Torres AJ, Wassum K, Tan MM, et al. Simultaneous vs. sequential treatment for smoking and weight management in tobacco quitlines: 6 and 12 month outcomes from a randomized trial. BMC Public Health 2018;18(1):678. [DOI: 10.1186/s12889-018-5574-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
1 Cooper 2005 (also Part 2) {published data only}
- Cooper TV, Klesges RC, Debon MW, Zbikowski SM, Johnson KC, Clemens LH. A placebo controlled randomized trial of the effects of phenylpropanolamine and nicotine gum on cessation rates and postcessation weight gain in women. Addictive Behaviors 2005;30(1):61-75. [DOI] [PubMed] [Google Scholar]
- Cooper TV, Montgomery GV, Debon MW, Zbikowski SM, Klesges RC, Johnson KC. The effects of PPA and nicotine gum on cessation rates and post cessation weight gain in women [POS3-46]. In: Society for Research on Nicotine and Tobacco 9th Annual Meeting, New Orleans, LA. 2003.
1 Copeland 2006 {published data only}
- Copeland AL, Martin P, Geiselman PJ, Rash CJ, Kendzor DE. Smoking cessation for weight-concerned women: Group vs individually tailored, dietary and weight-control follow up session. Addictive Behaviors 2006;31(1):115-27. [DOI] [PubMed] [Google Scholar]
1 Copeland 2015 {published data only (unpublished sought but not used)}
- Copeland AL, McVay MA, Martin PD, Rash CJ, Kendzor DE, Baillie LE, et al. Smoking relapse and weight gain prevention program for postmenopausal weight-concerned women: a pilot study. Eating Behaviors 2015;18:107-14. [DOI: 10.1016/j.eatbeh.2015.05.006] [DOI] [PMC free article] [PubMed] [Google Scholar]
1 Danielsson 1999 {published data only}
- Danielsson T, Rossner S, Westin A. Open randomised trial of intermittent very low energy diet together with nicotine gum for stopping smoking in women who gained weight in previous attemps to quit. BMJ 1999;319(7208):490-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson T, Rossner S. Smoking cessation rates improved by an intensive weight control program (Conference summary). Addiction 1998;93:913. [Google Scholar]
1 Hall 1992 {published data only}
- Hall SM, Tunstall CD, Vila KL, Duffy J. Weight gain prevention and smoking cessation: cautionary findings. American Journal of Public Health 1992;82(6):799-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
1 Hankey 2009 {published data only}
- Hankey CR, Leslie WS, Koshy PR, Mackenzie M, Murray H, Boyle S, et al. Food choice and changes in body weight and shape in those attempting smoking cessation. FSA report: University of Glasgow 2009;Food Standards Agency Project NO 902025.
- Koshy P, Mackenzie M, Leslie W, Lean M, Hankey C. Eating the elephant whole or in slices: views of participants in a smoking cessation intervention trial on multiple behaviour changes as sequential or concurrent tasks. BMC Public Health 2012;12:500. [CENTRAL: 842083] [EMBASE: 22759785] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie WS, Koshy PR, Mackenzie M, Murray HM, Boyle S, Lean ME, et al. Changes in body weight and food choice in those attempting smoking cessation: a cluster randomised controlled trial. BMC Public Health 2012;12:389. [CENTRAL: 842220] [EMBASE: 22642755] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
1 Heggen 2016 {published data only}
- Heggen E, Svendsen M, Klemsdal TO, Tonstad S. Low carbohydrate and moderately fat-reduced diets similarly affected early weight gain in varenicline-treated overweight or obese smokers. Nicotine & Tobacco Research 2016;18(6):1440-8. [DOI] [PubMed] [Google Scholar]
1 Johnson 2017 {published and unpublished data}
- Coday M, Richey P, Thomas F, Tran QT, Terrell SB, Tylavsky F, et al. The recruitment experience of a randomized clinical trial to aid young adult smokers to stop smoking without weight gain with interactive technology. Contemporary Clinical Trials Communications 2016;2:61-8. [DOI: 10.1016/j.conctc.2015.12.010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KC, Thomas F, Richey P, Tran QT, Tylavsky F, Miro D, et al. The primary results of the treating adult smokers at risk for weight gain with interactive technology (TARGIT) study. Obesity (Silver Spring, Md.) 2017;25(10):1691-8. [DOI: 10.1002/oby.21968] [DOI] [PMC free article] [PubMed] [Google Scholar]
1 King 2012 {published data only}
- King AC, Cao D, O'Malley SS, Kranzler HR, Cai X, deWit H, et al. Effects of naltrexone on smoking cessation outcomes and weight gain in nicotine-dependent men and women. Journal of Clinical Psychopharmacology 2012;32(5):630-6. [CENTRAL: 834609] [EMBASE: 2012525974] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Cao D, Zhang L, O'Malley SS. Naltrexone reduces women's weight gain in smoking cessation at six month follow-up. Neuropsychopharmacology 2011;36:S427. [CENTRAL: 814652] [6971] [Google Scholar]
- King AC, Cao D, Zhang L, O'Malley SS. Naltrexone reduction of long-term smoking cessation weight gain in women but not men: a randomized controlled trial. Biological Psychiatry 2013;73(9):924-30. [CENTRAL: 878188] [EMBASE: 2013242231] [PEER: Reviewed Journal: 2013-13839-019] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
1 Klesges 1990 {published data only}
- Klesges RC, Klesges LM, Meyers AW, Klem ML, Isbell T. The effects of phenylpropanolamine on dietary intake, physical activity, and body weight after smoking cessation. Clinical Pharmacology and Therapeutics 1990;47(6):747-54. [DOI] [PubMed] [Google Scholar]
1 Klesges 1995 {published data only}
- Klesges RC, Klesges LM, DeBon M, Shelton ML, Isbell TR, Klem ML. Effects of phenylpropanolamine on withdrawal symptoms. Psychopharmacology 1995;119(1):85-91. [DOI] [PubMed] [Google Scholar]
1 Levine 2010 (also Part 2) {published data only}
- Levine MD, Cheng Y, Kalarchian MA, Perkins KA, Marcus MD. Dietary intake after smoking cessation among weight-concerned women smokers. Psychology of Addictive Behaviors 2012;26(4):969-73. [CENTRAL: 879590] [EMBASE: 2013467195] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MD, Perkins KA, Kalarchian MA, Cheng Y, Houck PR, Slane JD, et al. Bupropion and cognitive behavioural therapy for weight-concerned women smokers. Archives of Internal Medicine 2010;170(6):543-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
1 Lycett 2010 {published data only}
- Lycett 2010. Weight Gain Association with Smoking Cessation: a Cohort Analysis and Feasibility Trial for Dietary Management [doctoral thesis]. Birmingham, UK: University of Birmingham, 2011. [Google Scholar]
- Lycett D, Hajek P, Aveyard P. Trial protocol: randomised controlled trial of the effects of very low calorie diet, modest dietary restriction, and sequential behavioural programme on hunger, urges to smoke, abstinence and weight gain in overweight smokers stopping smoking. Trials 2010;11:94. [CENTRAL: 772342] [EMBASE: 20929584] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
1 Lycett 2020 {published data only}
- Lycett D, Aveyard P, Farmer A, Lewis A, Munafò M. Referral to Slimming World in UK Stop Smoking Services (SWISSS) versus stop smoking support alone on body weight in quitters: results of a randomised controlled trial. BMJ Open 2020;10(1):e032271. [DOI: 10.1136/bmjopen-2019-032271] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycett D, Aveyard P, Farmer A, Lewis A, Munafò M. Slimming World in Stop Smoking Services (SWISSS): study protocol for a randomized controlled trial. Trials 2013;14:182. [DOI: 10.1186/1745-6215-14-182] [DOI] [PMC free article] [PubMed] [Google Scholar]
1 Lyu 2018 {published data only}
- Lyu X, Du J, Zhan G, Wu Y, Su H, Zhu Y, et al. Naltrexone and bupropion combination treatment for smoking cessation and weight loss in patients with schizophrenia. Frontiers in Pharmacology 2018;9:181. [DOI: 10.3389/fphar.2018.00181] [DOI] [PMC free article] [PubMed] [Google Scholar]
1 NCT03528304 2016 {unpublished data only}
- NCT03528304. Native women's wellness: contingency management for tobacco cessation and weight loss (NWW). clinicaltrials.gov/ct2/show/NCT03528304 (first received 17 May 2018).
1 Norregaard 1996 {published data only}
- Norregaard J, Jorgensen S, Mikkelsen KL, Tonnesen P, Iversen E, Sorensen T, et al. The effects of ephedrine plus caffeine on smoking cessation and postcessation weight gain. Clinical Pharmacology & Therapeutics 1996;60(6):679-86. [DOI] [PubMed] [Google Scholar]
1 O'Malley 2006 {published data only}
- Epperson CN, Toll B, Wu R, Amin Z, Czarkowski KA, Jatlow P, et al. Exploring the impact of gender and reproductive status on outcomes in a randomized clinical trial of naltrexone augmentation of nicotine patch. Drug and Alcohol Dependence 2010;112(1-2):1-8. [CENTRAL: 779368] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley S, Cooney J, Krishnan-Sarin S, Mckee S, Meandzija B, Dubin J. A dose ranging study of naltrexone augmentation of transdermal nicotine patch: smoking, weight and alcohol outcomes (POS1-064). In: Society for Research on Nicotine and Tobacco 10th Annual Meeting. Phoenix, Arizona, February 18-21, 2004:50.
- O'Malley SS, Cooney JL, Krishnan-Sarin S, Dubin JA, McKee SA, Cooney NL et al. A controlled trial of naltrexone augmentation of nicotine replacement therapy for smoking cessation. Archives of Internal Medicine 2006;166:667-74. [DOI] [PubMed] [Google Scholar]
1 Oncken 2014 {published data only}
- Oncken C, Arias AJ, Feinn R, Litt M, Covault J, Sofuoglu M, et al. Topiramate for smoking cessation: a randomized, placebo-controlled pilot study. Nicotine & Tobacco Research 2014;16(3):288-96. [CENTRAL: 977673] [DOI] [PMC free article] [PubMed] [Google Scholar]
1 Oncken 2019 {published data only (unpublished sought but not used)}
- Oncken C, Allen S, Litt M, Kenny A, Lando H, Allen A, et al. Exercise for smoking cessation in postmenopausal women: a randomized, controlled trial. Nicotine & Tobacco Research 2020;22(9):1587-95. [DOI: 10.1093/ntr/ntz176] [DOI] [PMC free article] [PubMed] [Google Scholar]
1 Parsons 2009 {published data only}
- Parsons AC, Ingram J, Inglis J, Aveyard P, Johnstone E, Brown K, et al. A proof of concept randomised placebo controlled factorial trial to examine the efficacy of St John's Wort for smoking cessation and chromium to prevent weight gain on smoking cessation. Drug and Alcohol Dependence 2009;102(1-3):116-22. [DOI] [PubMed] [Google Scholar]
1 Perkins 2001 {published data only}
- Perkins KA, Marcus MD, Levine MD, D'Amico D, Miller A, Broge M, et al. Cognitive-behavioral therapy to reduce weight concerns improves smoking cessation outcome in weight-concerned women. Journal of Consulting & Clinical Psychology 2001;69(4):604-13. [PubMed] [Google Scholar]
1 Pirie 1992 (also Part 2) {published data only}
- Pirie PL, McBride CM, Hellerstedt WL, Jeffery RW, Hatsukami DK, Allen S, et al. Smoking cessation in women concerned about weight. American Journal of Public Health 1992;82:1238-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
1 Prapavessis 2018 {published data only (unpublished sought but not used)}
- Jung ME, Fitzgeorge L, Prapavessis H, Faulkner G, Maddison R. The getting physical on cigarettes trial: Rationale and methods. Mental Health and Physical Activity 2010;3(1):35-44. [DOI: 10.1016/j.mhpa.2010.02.002] [DOI] [Google Scholar]
- Prapavessis H, De Jesus S, Fitzgeorge L, Faulkner G, Maddison R, Batten S. Exercise to enhance smoking cessation: the getting physical on cigarette randomized control trial. Annals of Behavioral Medicine 2016;50(3):358-69. [DOI: 10.1007/s12160-015-9761-9] [DOI] [PubMed] [Google Scholar]
- Prapavessis H, De Jesus S, Fitzgeorge L, Rollo S. Anthropometric and body composition changes in smokers vs abstainers following an exercise-aided pharmacotherapy smoking cessation trial for women. Addictive Behaviors 2018;85:125-30. [DOI: 10.1016/j.addbeh.2018.06.003] [DOI] [PubMed] [Google Scholar]
1 Rose 2019 {published data only (unpublished sought but not used)}
- Rose JE, Davis JM. Combination lorcaserin and nicotine patch for smoking cessation without weight gain. Nicotine & Tobacco Research 2020;22(9):1627-31. [DOI: 10.1093/ntr/ntz149] [DOI] [PMC free article] [PubMed] [Google Scholar]
1 Shanahan 2017 {published data only}
- Shanahan W, Rose J, Glicklich A, Stubbe S, Halliday D, Zhang J, et al. Lorcaserin for smoking cessation treatment in cigarette smokers: a randomized phase 2 trial. In: American Journal of Respiratory and Critical Care Medicine. Vol. 191. 2015:1.
- Shanahan WR, Rose JE, Glicklich A, Stubbe S, Sanchez-Kam M. Lorcaserin for smoking cessation and associated weight gain: a randomized 12-week clinical trial. Nicotine & Tobacco Research 2017;19(8):944-51. [DOI: 10.1093/ntr/ntw301] [DOI] [PubMed] [Google Scholar]
1 Sobell 2017 {published data only (unpublished sought but not used)}
- Sobell MB, Peterson AL, Sobell LC, Brundige A, Hunter CM, Hunter CM. Reducing alcohol consumption to minimize weight gain and facilitate smoking cessation among military beneficiaries. Addictive Behaviors 2017;75:145-51. [DOI: 10.1016/j.addbeh.2017.06.018] [DOI] [PubMed] [Google Scholar]
1 Spring 1995 (also Part 2) {published data only}
- Spring B, Wurtman J, Wurtman R, El-Khoury A, Goldberg H, McDermott J, et al. Efficacies of dexfenfluramine and fluoxetine in preventing weight gain after smoking cessation. American Journal of Clinical Nutrition 1995;62(6):1181-7. [DOI] [PubMed] [Google Scholar]
1 Spring 2004 {published data only}
- Spring B, Doran N, Pagoto S, Schneider K, Pingitore R, Hedeker D. Randomised controlled trial of behavioural smoking and weight control treatment: effect of concurrent versus sequential intervention. Journal of Counselling and Clinical Psychology 2004;72(5):785-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
1 Toll 2010 {published data only}
- Rojewski AM, Toll BA, O'Malley SS. Menthol cigarette use predicts treatment outcomes of weight-concerned smokers. Nicotine & Tobacco Research 2014;16(1):115-9. [CENTRAL: 1000325] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll BA, White M, Wu R, Meandzija B, Jatlow P, Makuch R, et al. Low-dose naltrexone augmentation of nicotine replacement for smoking cessation with reduced weight gain: a randomised trial. Drug and Alcohol Dependence 2010;111(3):200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
1 Vander Weg 2016 {published data only (unpublished sought but not used)}
- Vander Weg MW, Cozad AJ, Howren MB, Cretzmeyer M, Scherubel M, Turvey C, et al. An individually-tailored smoking cessation intervention for rural Veterans: a pilot randomized trial. BMC Public Health 2016;16(1):811. [DOI: 10.1186/s12889-016-3493-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
1 White 2019 {published data only (unpublished sought but not used)}
- White MA, Ivezaj V, Grilo CM. Evaluation of a web-based cognitive behavioral smoking cessation treatment for overweight/obese smokers. Journal of Health Psychology 2019;24(13):1796-806. [DOI] [PubMed] [Google Scholar]
1 Wilcox 2016 {published data only (unpublished sought but not used)}
- Wilcox C, Tong M-L, Oskooilar N, Morrissey J, De Francisco D, Henry M, et al. A 12-week, multi-center, double-blind, randomized, placebo-controlled, phase-2, dose selection study of lorcaserin hydrochloride for smoking cessation: preliminary report. In: Neuropsychopharmacology. 2016:S626-7.
2 Abelin 1989 {published data only}
- Abelin T, Buehler A, Müller P, Vesanen K, Imhof PR. Controlled trial of transdermal nicotine patch in tobacco withdrawal. Lancet 1989;1(8628):7-10. [DOI] [PubMed] [Google Scholar]
- Abelin T, Ehrsam R, Buhler-Reichert A, Imhof PR, Müller P, Thommen A. Effectiveness of a transdermal nicotine system in smoking cessation studies. Methods and Findings in Experimental and Clinical Pharmacology 1989;11(3):205-14. [PubMed] [Google Scholar]
- Müller P, Abelin T, Ehrsam R, Imhof P, Howald H, Mauli D. The use of transdermal nicotine in smoking cessation. Lung 1990;168(Suppl):445-53. [DOI] [PubMed] [Google Scholar]
2 Aubin 2008 {published and unpublished data}
- Aubin HJ, Bobak A, Britton JR, Oncken C, Billing CB, Gong J, et al. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised, open-label trial. Thorax 2008;63(8):717-24. [DOI] [PMC free article] [PubMed]
2 Benowitz 2018 {published data only}
- Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016;387(10037):2507-20. [DOI: 10.1016/S0140-6736(16)30272-0] [DOI] [PubMed] [Google Scholar]
- Anthenelli RM, Gaffney M, Benowitz NL, West R, McRae T, Russ C, et al. Predictors of neuropsychiatric adverse events with smoking cessation medications in the randomized controlled EAGLES trial. Journal of General Internal Medicine 2019;34(6):862-70. [DOI: 10.1007/s11606-019-04858-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers CR, Heffner JL, Russ C, Lawrence D, McRae T, Evins AE, et al. Efficacy and safety of pharmacotherapies for smoking cessation in anxiety disorders: subgroup analysis of the randomized, active- and placebo-controlled EAGLES trial. Depression and Anxiety 2020;37(3):247-60. [DOI: 10.1002/da.22982] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CL, Pietri G. A cost-effectiveness analysis of varenicline for smoking cessation using data from the EAGLES trial. ClinicoEconomics and Outcomes Research 2018;10:67-74. [DOI: 10.2147/CEOR.S153897] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AM, Hosie Quinn M, Lubitz SF, Flitter A, Ashare RL, Leone FT, et al. Medication adherence and rate of nicotine metabolism are associated with response to treatment with varenicline among smokers with HIV. Addictive Behaviors 2021;112:106638. [DOI: 10.1016/j.addbeh.2020.106638] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Pipe A, West R, Hays JT, Tonstad S, McRae T, et al. Cardiovascular safety of varenicline, bupropion, and nicotine patch in smokers: a randomized clinical trial. JAMA Internal Medicine 2018;178(5):622-31. [DOI: 10.1001/jamainternmed.2018.0397] [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Bernard 2015 {published data only}
- Bernard P, Ninot G, Cyprien F, Courtet P, Guillaume S, Georgescu V, et al. Exercise and counseling for smoking cessation in smokers with depressive symptoms: a randomized controlled pilot trial. Journal of Dual Diagnosis 2015;11(3-4):205-16. [DOI: 10.1080/15504263.2015.1113842] [DOI] [PubMed] [Google Scholar]
2 Bize 2010 {published and unpublished data}
- Bize R, Willi C, Chiolero A, Stoianov R, Payot S, Locatelli I, et al. Participation in a population-based physical activity programme as an aid for smoking cessation: a randomised trial. Tobacco Control 2010;19(6):488-94. [DOI] [PubMed] [Google Scholar]
- Cornuz J, Willi C, Chiolero A, Payot S, Stolanov R, Bize R. Physical activity as an aid to smoking cessation: a randomised controlled trial of sedentary adult smokers. Journal of General Internal Medicine 2007;22(S1):107. [Google Scholar]
- Gonseth S, Locatelli I, Bize R, Nussle S, Clair C, Pralong F, et al. Leptin and smoking cessation: secondary analyses of a randomized controlled trial assessing physical activity as an aid for smoking cessation. BMC Public Health 2014;14:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locatelli I, Collet T-H, Clair C, Rodondi N, Cornuz J. The joint influence of gender and amount of smoking on weight gain one year after smoking cessation. International Journal of Environmental Research and Public Health 2014;11(8):8443-55. [CENTRAL: 1066606] [EMBASE: 2014568698] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prod'hom S, Locatelli I, Giraudon K, Marques-Vidal P, Clair C, Bize R, et al. Predictors of weight change in sedentary smokers receiving a standard smoking cessation intervention. Nicotine & Tobacco Research 2013;15(5):910-6. [CENTRAL: 967344] [EMBASE: 2013284959] [PMID: ] [DOI] [PubMed] [Google Scholar]
2 Blondal 1999 {published and unpublished data}
- Blondal T, Gudmundsson LJ, Olafsdottir I, Gustavsson G, Westin A. Nicotine nasal spray with nicotine patch for smoking cessation: randomised trial with six year follow up. BMJ 1999;318:285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondal T, Ludviksdottir D, Gudmundsson L, Olafsdottir I, Gustavsson G, Westin A. Efficacy of nicotine nasal spray added to transdermal nicotine patches in smoking cessation [Abstract]. In: 10th World Conference on Tobacco or Health; Aug 24-28; Beijing, China. 1997:48.
2 Bloom 2017 {published data only}
- Abrantes AM, Bloom EL, Strong DR, Riebe D, Marcus BH, Desaulniers J, et al. A preliminary randomized controlled trial of a behavioral exercise intervention for smoking cessation. Nicotine & Tobacco Research 2014;16(8):1094-103. [DOI: 10.1093/ntr/ntu036] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom EL, Minami H, Brown RA, Strong DR, Riebe D, Abrantes AM. Quality of life after quitting smoking and initiating aerobic exercise. Psychology, Health & Medicine 2017;22(9):1127-35. [DOI: 10.1080/13548506.2017.1282159] [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Bohadana 2000 {published and unpublished data}
- Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Gender differences in quit rates following smoking cessation with combination nicotine therapy: influence of baseline smoking behavior. Nicotine & Tobacco Research 2003;5(1):111-6. [DOI] [PubMed] [Google Scholar]
- Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Nicotine inhaler and nicotine patch as a combination therapy for smoking cessation - a randomized, double-blind, placebo-controlled trial. Archives of Internal Medicine 2000;160(20):3128-34. [DOI] [PubMed] [Google Scholar]
- Bohadana AB, Nilsson F, Martinet Y. Nicotine inhaler and nicotine patch: a combination therapy for smoking cessation [abstract]. Nicotine & Tobacco Research 1999;1(2):189. [Google Scholar]
2 Bolliger 2011 {published data only}
- Bolliger CT, Issa Jaqueline S, Posadas-Valay R, Safwat T, Abreu P, Correia EA, et al. Effects of varenicline in adult smokers: a multinational, 24-week, randomized, double-blind, placebo-controlled study. Clinical Therapeutics 2011;33(4):465-77. [DOI] [PubMed] [Google Scholar]
2 CEASE 1999 {published and unpublished data}
- Tønnesen P, Paoletti P, Gustavsson G, Russell MA, Saracci R, Gulsvik A et al. Higher dosage nicotine patches increase one-year smoking cessation rates: results from the European CEASE trial. European Respiratory Journal 1999;13(2):238-46. [DOI] [PubMed] [Google Scholar]
2 Chengappa 2014 {published data only}
- Chengappa KN, Perkins KA, Brar JS, Schlicht PJ, Turkin SR, Hetrick ML, et al. Varenicline for smoking cessation in bipolar disorder: a randomized, double-blind, placebo-controlled study. Journal of Clinical Psychiatry 2014;75(7):765-72. [DOI: 10.4088/JCP.13m08756] [DOI] [PubMed] [Google Scholar]
- Chengappa KN. A randomized, double-blind, placebo-controlled clinical trial of varenicline in persons with bipolar disorder motivated to quit smoking. Bipolar Disorders 2015;17(Suppl 1):38. [Google Scholar]
2 Ciccolo 2011 {published data only}
- Ciccolo JT, Dunsiger SI, Williams DM, Bartholomew JB, Jennings EG, Ussher MH, et al. Resistance training as an aid to standard smoking cessation treatment: a pilot study. Nicotine & Tobacco Research 2011;13(8):756-60. [CENTRAL: 784125] [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Cox 2012 {published data only}
- Cox LS, Faseru B, Mayo MS, Krebill R, Snow TS, Bronars CA, et al. Design, baseline characteristics, and retention of African American light smokers into a randomized trial involving biological data. Trials 2011;12:22. [CENTRAL: 778725] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Nollen NL, Mayo MS, Choi WS, Faseru B, Benowitz NL, et al. Bupropion for smoking cessation in African American light smokers: a randomized controlled trial. Journal of the National Cancer Institute 2012;104(4):290-8. [CENTRAL: 814627] [EMBASE: 2012121196] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faseru B, Nollen NL, Mayo MS, Krebill R, Choi WS, Benowitz NL, et al. Predictors of cessation in African American light smokers enrolled in a bupropion clinical trial. Addictive Behaviors 2013;38(3):1796-803. [CENTRAL: 865750] [PEER: Reviewed Journal: 2013-02944-034] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Dale 1995 {published and unpublished data}
- Dale LC, Hurt RD, Offord KP, Lawson GM, Croghan IT, Schroeder DR. High-dose nicotine patch therapy - percentage of replacement and smoking cessation. JAMA 1995;274(17):1353-8. [PubMed] [Google Scholar]
- Dale LC, Schroeder DR, Wolter TD, Croghan IT, Hurt RD, Offord KP. Weight change after smoking cessation using variable doses of transdermal nicotine replacement. Journal of General Internal Medicine 1998;13(1):9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Dogar 2018 {published data only}ISRCTN43811467
- Dogar O, Barua D, Boeckmann M, Elsey H, Fatima R, Gabe R, et al. The safety, effectiveness and cost-effectiveness of cytisine in achieving six-month continuous smoking abstinence in tuberculosis patients - protocol for a double-blind, placebo-controlled randomized trial. Addiction (Abingdon, England) 2018;113(9):1716-26. [DOI: 10.1111/add.14242] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogar O, Keding A, Gabe R, Marshall AM, Huque R, Barua D, et al. Cytisine for smoking cessation in patients with tuberculosis: a multicentre, randomised, double-blind, placebo-controlled phase 3 trial. Lancet Global Health 2020;8(11):e1408-17. [DOI: 10.1016/S2214-109X(20)30312-0] [DOI] [PubMed] [Google Scholar]
2 Ebbert 2014 {published data only}
- Ebbert JO, Hatsukami DK, Croghan IT, Schroeder DR, Allen SS, Hays JT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA 2014;311(2):155-63. [CENTRAL: 921055] [EMBASE: 2014041620] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong AS, Elrashidi MY, Schroeder DR, Ebbert JO. Depressive symptoms among patients receiving varenicline and bupropion for smoking cessation. Journal of Substance Abuse Treatment 2015;52:78-81. [CENTRAL: 1068331] [EMBASE: 2014617308] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Ebbert 2015 {published data only}
- Ebbert JO, Hughes JR, West RJ, Rennard SI, Russ C, McRae TD, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA 2015;313(7):687-94. [DOI: 10.1001/jama.2015.280] [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Ehrsam 1991 {published data only}
- Abelin T, Ehrsam R, Buhler-Reichert A, Imhof PR, Müller P, Thommen A. Effectiveness of a transdermal nicotine system in smoking cessation studies. Methods and Findings in Experimental and Clinical Pharmacology 1989;11(3):205-14. [PubMed] [Google Scholar]
- Abelin T, Ehrsam R, Imhof P Müller P, Howald H. Clinical experience with a transdermal nicotine system in healthy nicotine-dependent smokers. In: Wilhemsen L, editors(s). Smoking as a Cardiovascular Risk Factor - New Strategies for Smoking Cessation. Hogrefe & Huber, 1991:35-46. [Google Scholar]
- Ehrsam RE, Bühler A, Müller P, Mauli D, Schumacher PM, Howald H, et al. Weaning of young smokers using a transdermal nicotine patch [Entwohnung junger Raucher mit Hilfe eines transdermalen Nikotinpflasters]. Schweizerische Rundschau fur Medizin Praxis 1991;80(7):145-50. [PubMed] [Google Scholar]
- Müller P, Abelin T, Ehrsam R, Imhof P, Howald H, Mauli D. The use of transdermal nicotine in smoking cessation. Lung 1990;168(Suppl):445-53. [DOI] [PubMed] [Google Scholar]
2 Eisenberg 2013 {published data only}
- Eisenberg MJ, Grandi SM, Gervais A, Joseph L, O'Loughlin J, Paradis G, et al. Bupropion for smoking cessation in patients hospitalized with acute myocardial infarction: a randomized, placebo-controlled trial. Canadian Journal of Cardiology 2011;27(5 SUPPL 1):S344. [CENTRAL: 814579] [6968] [DOI] [PubMed] [Google Scholar]
- Eisenberg MJ, Grandi SM, Gervais A, O'Loughlin J, Paradis G, Rinfret S, et al. Bupropion for smoking cessation in patients hospitalized with acute myocardial infarction: a randomized, placebo-controlled trial. Journal of the American College of Cardiology 2013;61(5):524-32. [CENTRAL: 848271] [EMBASE: 2013073386] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Grandi SM, Filion KB, Gervais A, Joseph L, O'Loughlin J, Paradis G, et al. The effect of smoking cessation on weight at 12 months in patients post-myocardial infarction. Circulation 2012;125:AP323. [EMBASE: 70951199] [Google Scholar]
- Grandi SM, Filion KB, Gervais A, Joseph L, O'Loughlin J, Paradis G, et al. Weight change in patients attempting to quit smoking post-myocardial infarction. American Journal of Medicine 2014;127(7):641-9. [CENTRAL: 1050332] [EMBASE: 2014427952] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Grandi SM, Filion KB, Joseph L, O'Loughlin J, Pilote L, Eisenberg MJ. Baseline predictors of relapse to smoking at 12 months in patients post-myocardial infarction. Canadian Journal of Cardiology 2013;29(10 Suppl 1):S257. [CENTRAL: 1024794] [EMBASE: 71204225] [Google Scholar]
- Qi Zhang DD, Eisenberg MJ, Grandi SM, Joseph L, O'Loughlin J, Paradis G, et al. Bupropion, smoking cessation, and health-related quality of life following an acute myocardial infarction. Journal of Population Therapeutics and Clinical Pharmacology [Journal de la Therapeutique des Populations et de la Pharamcologie Clinique] 2014;21(3):e346-56. [CENTRAL: 1022527] [EMBASE: 2014847496] [PMID: ] [PubMed] [Google Scholar]
2 Eisenberg 2016 {published and unpublished data}
- Dehghani P, Habib B, Windle SB, Roy N, Old W, Grondin FR, et al. Smokers and postcessation weight gain after acute coronary syndrome. Journal of the American Heart Association 2017;6(4):e004785. [DOI: 10.1161/JAHA.116.004785] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg MJ, Windle SB, Roy N, Old W, Grondin FR, Bata I, et al. Varenicline for smoking cessation in hospitalized patients with acute coronary syndrome. Circulation 2016;133(1):21-30. [DOI: 10.1161/CIRCULATIONAHA.115.019634] [DOI] [PubMed] [Google Scholar]
- Windle SB, Bata I, Madan M, Abramson BL, Eisenberg MJ. A randomized controlled trial of the efficacy and safety of varenicline for smoking cessation after acute coronary syndrome: design and methods of the Evaluation of Varenicline in Smoking Cessation for Patients Post-Acute Coronary Syndrome trial. American Heart Journal 205;170(4):635-40. [DOI: 10.1016/j.ahj.2015.07.010] [DOI] [PubMed] [Google Scholar]
- Windle SB, Dehghani P, Roy N, Old W, Grondin FR, Bata I, et al. Smoking abstinence 1 year after acute coronary syndrome: follow-up from a randomized controlled trial of varenicline in patients admitted to hospital. CMAJ 2018;190(12):E347-54. [DOI: 10.1503/cmaj.170377] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle SB, Dehghani P, Roy N, Old W, Grondin FR, Bata I, et al. Sustained smoking abstinence 12 months after acute coronary syndrome: follow-up from a randomized controlled trial of varenicline in hospitalized patients. Canadian Journal of Cardiology 2016;32:S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Fiore 1994A {published and unpublished data}
- Elan Pharmaceutical Research Corp. NDA 19-983 for Approval of PROSTEP. Study 90-03 1992.
- Fiore MC, Kenford SL, Jorenby DE, Wetter DW, Smith SS, Baker TB. Two studies of the clinical effectiveness of the nicotine patch with different counseling treatments. Chest 1994;105:524-33. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA 1994;271:589-94. [DOI] [PubMed] [Google Scholar]
2 Fiore 1994B {published and unpublished data}
- Fiore MC, Kenford SL, Jorenby DE, Wetter DW, Smith SS, Baker TB. Two studies of the clinical effectiveness of the nicotine patch with different counseling treatments. Chest 1994;105(2):524-33. [DOI] [PubMed] [Google Scholar]
2 Gallagher 2007 {published data only}
- Gallagher SM, Penn PE, Schindler E, Layne W. A comparison of smoking cessation treatments for persons with schizophrenia and other serious mental illnesses. Journal of Psychoactive Drugs 2007;39(4):487-97. [DOI: 10.1080/02791072.2007.10399888] [DOI] [PubMed] [Google Scholar]
2 Garvey 2000 {published and unpublished data}
- Doherty K, Militello FS, Kinnunen T, Garvey AJ. Nicotine gum dose and weight gain after smoking cessation. Journal of Consulting and Clinical Psychology 1996;64(4):799-807. [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S, Rohay JM, Gitchell JG, Garvey AJ. Effect of compliance with nicotine gum dosing on weight gained during a quit attempt. Addiction 2011;106(3):651-6. [DOI] [PubMed] [Google Scholar]
- Garvey AJ, Kinnunen T, Nordstrom BL, Utman CH, Doherty K, Rosner B, et al. Effects of nicotine gum dose by level of nicotine dependence. Nicotine & Tobacco Research 2000;2(1):53-63. [DOI] [PubMed] [Google Scholar]
- Nordstrom BL, Kinnunen T, Utman CH, Garvey AJ. Long-term effects of nicotine gum on weight gain after smoking cessation. Nicotine & Tobacco Research 1999;1(3):259-68. [DOI] [PubMed] [Google Scholar]
2 Gonzales 2006 {published data only}
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006;296(1):47-55. [DOI] [PubMed] [Google Scholar]
2 Gourlay 1995 {published data only}
- Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ. Double blind trial of repeated treatment with transdermal nicotine for relapsed smokers. BMJ 1995;311(7001):363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Gray 2019 {published and unpublished data}
- Gray KM, Baker NL, McClure EA, Tomko RL, Squeglia LM, Saladin ME, et al. Efficacy and safety of varenicline for adolescent smoking cessation: a randomized clinical trial. JAMA Pediatrics 2019;173(12):1146-53. [DOI: 10.1001/jamapediatrics.2019.3553] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Baker NL, Hood CO, Tomko RL, Squeglia LM, Flanagan JC, et al. Cannabis and alcohol co-use in a smoking cessation pharmacotherapy trial for adolescents and emerging adults. Nicotine & Tobacco Research 2020;22(8):1374-82. [DOI: 10.1093/ntr/ntz170] [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Gross 1995 {published data only}
- Gross J, Johnson J, Sigler L, Stitzer ML. Dose effects of nicotine gum. Addictive Behaviors 1995;20(3):371-81. [DOI] [PubMed] [Google Scholar]
2 Hjalmarson 1984 {published and unpublished data}
- Hjalmarson AI. Effect of nicotine chewing gum in smoking cessation. A randomized, placebo-controlled, double-blind study. JAMA 1984;252(20):2835-8. [PubMed] [Google Scholar]
2 Hjalmarson 1994 {published data only}
- Hjalmarson AI, Franzon M, Westin A, Wiklund O. Effect of nicotine nasal spray on smoking cessation. A randomized, placebo-controlled, double-blind study. Archives of Internal Medicine 1994;154:2567-72. [PubMed] [Google Scholar]
2 Hjalmarson 1997 {published data only}
- Hjalmarson A, Nilsson F, Sjöstrom L, Wiklund O. The nicotine inhaler in smoking cessation. Archives of Internal Medicine 1997;157(15):1721-8. [PubMed] [Google Scholar]
2 Hurt 1997 {published and unpublished data}
- Hurt RD, Glover ED, Sachs DP, et al. Bupropion for smoking cessation: a double-blind, placebo-controlled dose response trial [Abstract]. Journal of Addictive Diseases 1996;15:137. [Google Scholar]
- Dale LC, Glover ED, Sachs DP, Schroeder DR, Offord KP, Croghan IT, et al. Bupropion for smoking cessation: predictors of successful outcome. Chest 2001;119(5):1357-64. [DOI] [PubMed] [Google Scholar]
- Glaxo Wellcome. Presentation for FDA approval of bupropion sustained release for smoking cessation. Dr J. Andrew Johnston December 10 1996.
- Hayford KE, Patten CA, Rummans TA, Schroeder DR, Offord KP, Croghan IT, et al. Efficacy of bupropion for smoking cessation in smokers with a former history of major depression or alcoholism. British Journal of Psychiatry 1999;174:173-8. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. New England Journal of Medicine 1997;337(17):1195-202. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Fiedler-Kelly J, Glover ED, Sachs DP, Grasela TH, DeVeaugh-Geiss J. Relationship between drug exposure and the efficacy and safety of bupropion sustained release for smoking cessation. Nicotine & Tobacco Research 2001;3(2):131-40. [DOI] [PubMed] [Google Scholar]
2 Ioakeimidis 2018 {published and unpublished data}
- Ioakeimidis N, Vlachopoulos C, Georgakopoulos C, Abdelrasoul M, Skliros N, Katsi V, et al. P1234 Smoking cessation rates with varenicline and electronic cigarettes in relapsed smokers with a history of acute coronary syndrome. European Heart Journal 2018;39(Suppl 1):S242. [DOI: 10.1093/eurheartj/ehy565.P1234] [DOI] [Google Scholar]
2 Jodar‐Sanchez 2018 {published data only}
- Carrasco-Hernandez L, Jódar-Sánchez F, Núñez-Benjumea F, Moreno Conde J, Mesa González M, Civit-Balcells A, et al. A mobile health solution complementing psychopharmacology-supported smoking cessation: randomized controlled trial. JMIR mHealth and uHealth 2020;8(4):e17530. [DOI: 10.2196/17530] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jódar-Sánchez F, Carrasco Hernández L, Núñez-Benjumea FJ, Mesa González MA, Moreno Conde J, Parra Calderón CL, et al. Using the social-local-mobile app for smoking cessation in the smokefreebrain project: protocol for a randomized controlled trial. JMIR Research Protocols 2018;7(12):e12464. [DOI: 10.2196/12464] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez FG, Hernandez LC, Benjumea FN, Rodriguez BG, Gonzalez MM, Jódar Sanchez F, et al. Use of a mobile app to give up smoking. Results from a clinical trial. European Respiratory Journal 2019;54(63):PA2237. [DOI: 10.1183/13993003.congress-2019.PA2237] [DOI] [Google Scholar]
2 Jorenby 2006 {published data only}
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine partial agonist vs placebo or sustained-release bupropion for smoking cessation: a randomised controlled trial. JAMA 2006;296(1):56-63. [DOI] [PubMed] [Google Scholar]
2 Koegelenberg 2014 {published data only}
- Koegelenberg CF, Noor F, Bateman ED, Van Zyl-Smit RN, Bruning A, O'Brien JA, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial. JAMA 2014;312(2):155-61. [CENTRAL: 994156] [DOI] [PubMed] [Google Scholar]
2 Lerman 2004 {published and unpublished data}
- Lerman C, Jepson C, Wileyto EP, Epstein LH, Rukstalis M, Patterson F, et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacology 2006;31(1):231-42. [DOI] [PubMed] [Google Scholar]
- Lerman C, Kaufmann V, Rukstalis M, Patterson F, Perkins K, Audrain McGovern J, et al. Individualizing nicotine replacement therapy for the treatment of tobacco dependence: a randomized trial. Annals of Internal Medicine 2004;140(6):426-33. [DOI] [PubMed] [Google Scholar]
- Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clinical Pharmacology and Therapeutics 2006;79(6):600-8. [DOI] [PubMed] [Google Scholar]
- Lerman C, Wileyto EP, Patterson F, Rukstalis M, Audrain-McGovern J, Restine S et al. The functional mu opioid receptor (OPRM1) Asn40Asp variant predicts short-term response to nicotine replacement therapy in a clinical trial. Pharmacogenomics Journal 2004;4(3):184-92. [DOI] [PubMed] [Google Scholar]
- Malaiyandi V, Lerman C, Benowitz NL, Jepson C, Patterson F, Tyndale RF. Impact of CYP2A6 genotype on pretreatment smoking behaviour and nicotine levels from and usage of nicotine replacement therapy. Molecular Psychiatry 2006;11(4):400-9. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Kaufmann V, Rukstalis M, Audrain-McGovern J, Kucharski S, et al. Predictors of attendance in a randomized clinical trial of nicotine replacement therapy with behavioral counseling. Drug and Alcohol Dependence 2003;72(2):123-31. [DOI] [PubMed] [Google Scholar]
2 Lycett 2011 {published data only}
- Lycett D, Munafò M, Johnstone E, Murphy M, Aveyard P. Associations between weight change over 8 years and baseline body mass index in a cohort of continuing and quitting smokers. Addiction 2011;106:1188-96. [DOI] [PubMed] [Google Scholar]
2 Marcus 1999 {published data only}
- Marcus B, Albrecht A, King T, Parisi A, Pinto B, Roberts M, et al. The efficacy of exercise as an aid for smoking cessation in women. Archives of Internal Medicine 1999;159(11):1229-34. [DOI] [PubMed] [Google Scholar]
2 Marcus 2005 {published data only}
- Marcus BH, Lewis BA, Hogan J, King TK, Albrecht AE, Bock B, et al. The efficacy of moderate-intensity exercise as an aid for smoking cessation in women: a randomized controlled trial. Nicotine & Tobacco Research 2005;7(6):871-80. [DOI] [PubMed] [Google Scholar]
2 Mercié 2018 {published data only}
- Mercié P, Arsandaux J, Katlama C, Ferret S, Beuscart A, Spadone C, et al. Efficacy and safety of varenicline for smoking cessation in people living with HIV in France (ANRS 144 Inter-ACTIV): a randomised controlled phase 3 clinical trial. Lancet. HIV 2018;5(3):e126–35. [DOI: 10.1016/S2352-3018(18)30002-X] [DOI] [PubMed] [Google Scholar]
- Mercié P, Roussillon C, Katlama C, Beuscart A, Ferret S, Wirth N, et al. Varenicline vs placebo for smoking cessation: ANRS 144 Inter-ACTIV randomized trial. In: Cardiovascular, Bone, and Kidney Health. Conference on Retroviruses and Opportunistic Infections. 2015 Feb 23-26; Seattle, Washington. 2015. [139]
2 Nakamura 2007 {published data only}
- Nakamura M, Oshima A, Fujimoto Y, Maruyama N, Ishibashi T, Reeves KR. Efficacy and tolerability of nicotinic varenicline, an alpha4beta2 acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers. Clinical Therapeutics 2007;29(6):1040-56. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Oshima A, Ohkura M, Arteaga C, Suwa K. Predictors of lapse and relapse to smoking in successful quitters in a varenicline post hoc analysis in japanese smokers. Clinical Therapeutics 2014;36(6):918-27. [CENTRAL: 995377] [EMBASE: 2014542011] [DOI] [PubMed] [Google Scholar]
2 NCT02859142 2016 {unpublished data only}
- NCT02859142. Varenicline augmentation of patch outcomes in heavy drinkers' smoking cessation. clinicaltrials.gov/ct2/show/NCT02859142 (first received 8 August 2016).
2 Niaura 2002 {published and unpublished data}
- Borrelli B, Niaura R, Keuthen NJ, Goldstein MG, Depue JD, Murphy C, et al. Development of major depressive disorder during smoking-cessation treatment. Journal of Clinical Psychiatry 1996;57(11):534-8. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Papandonatos G, Spring B, Hitsman B, Niaura R. Experimenter-defined quit dates for smoking cessation: adherence improves outcomes for women but not for men. Addiction 2004;99(3):378-85. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Spring B, Niaura R, Hitsman B, Papandonatos G. Influences of gender and weight gain on short-term relapse to smoking in a cessation trial. Journal of Consulting and Clinical Psychology 2001;69(3):511-15. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Spring B, Niaura R, Kristeller J, Ockene JK, Keuthen NJ. Weight suppression and weight rebound in ex-smokers treated with fluoxetine. Journal of Consulting and Clinical Psychology 1999;67(1):124-31. [DOI] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue DE, Borrelli B, Hitsman B, Niaura R, et al. Influence of fluoxetine on positive and negative affect in a clinic-based smoking cessation trial. Psychopharmacology 2004;173(1-2):153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N, Spring B, Borrelli B, McChargue D, Hitsman B, Niaura R, Hedeker D. Elevated positive mood: a mixed blessing for abstinence. Psychology of Addictive Behaviors 2006;20(1):36-43. [DOI] [PubMed] [Google Scholar]
- Hitsman B, Spring B, Borrelli B, Niaura R, Papandonatos GD. Influence of antidepressant pharmacotherapy on behavioral treatment adherence and smoking cessation outcome in a combined treatment involving fluoxetine. Experimental and Clinical Psychopharmacology 2001;9(4):355-62. [DOI] [PubMed] [Google Scholar]
- Mizes JS, Sloan DM, Segraves K, Spring B, Pingatore R, Kristeller J. Fluoxetine and weight-gain in smoking cessation - examination of actual weight-gain and fear of weight-gain [abstract]. Psychopharmacology Bulletin 1996;32:491. [Google Scholar]
- Niaura R, Goldstein M, Spring B, Keuthen N, Kristeller J, DePue J, et al. Fluoxetine for smoking cessation: a multicenter randomized double blind dose response study. In: Society for Behavioral Medicine Annual Meeting; April 18 1997; San Francisco, CA. 1997.
- Niaura R, Spring B, Borrelli B, Hedeker D, Goldstein MG, Keuthen N, et al. Multicenter trial of fluoxetine as an adjunct to behavioral smoking cessation treatment. Journal of Consulting and Clinical Psychology 2002;70(4):887-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Jack LM, Niaura R, Borrelli B, Spring B. Subgroups of smokers with different success rates after treatment with fluoxetine for smoking cessation [abstract]. Nicotine & Tobacco Research 1999;1:281. [Google Scholar]
2 Niaura 2008 {published data only}
- Niaura R, Hays JT, Jorenby DE, Leone FT, Pappas JE, Reeves KR, et al. The efficacy and safety of varenicline for smoking cessation using a flexible dosing strategy in adult smokers: a randomized controlled trial. Current Medical Research and Opinion 2008;24(7):1931-41. [DOI] [PubMed] [Google Scholar]
2 Nides 2006 {published and unpublished data}
- Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: Results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Archives of Internal Medicine 2006;166(15):1561-8. [DOI] [PubMed] [Google Scholar]
- Oncken C, Watsky E, Reeves K, Anziano R. Varenicline is efficacious and well tolerated in promoting smoking cessation: results from a 7-week, randomized, placebo- and bupropion-controlled trial. In: Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20-23 March 2005, Prague, Czech Republic. 2005.
2 Oncken 2006 {published data only}
- Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Archives of Internal Medicine 2008;166(15):1571-7. [DOI] [PubMed] [Google Scholar]
2 Oncken 2007 {published data only}
- Oncken C, Cooney J, Feinn R, Lando H, Kranzler HR. Transdermal nicotine for smoking cessation in postmenopausal women. Addictive Behaviors 2007;32(2):296-309. [DOI] [PubMed] [Google Scholar]
2 Pack 2008 {published data only}
- Pack QR, Jorenby DE, Fiore MC, Jackson T, Weston P, Piper M, et al. A comparison of the nicotine lozenge and nicotine gum: an effectiveness randomized controlled trial. Wisconsin Medical Journal 2008;107(5):237-43. [PMC free article] [PubMed] [Google Scholar]
2 Patten 2016 {published data only (unpublished sought but not used)}
- Patten CA, Bronars CA, Vickers Douglas KS, Ussher MH, Levine JA, Tye SJ, et al. Supervised, vigorous intensity exercise intervention for depressed female smokers: a pilot study. Nicotine & Tobacco Research 2017;19(1):77-86. [DOI: 10.1093/ntr/ntw208] [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Piper 2007 {published data only}
- Piper ME, Federman EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, et al. Efficacy of bupropion alone and in combination with nicotine gum. Nicotine & Tobacco Research 2007;9(9):947-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Federman EB, Smith SS, Fiore MC, Baker TB. Efficacy of bupropion SR alone and combined with 4-mg nicotine gum (PA2-2). In: Society for Research on Nicotine and Tobacco 10th Annual Meeting February 18-21, Phoenix, Arizona. Vol. 18. 2004.
- Piper ME, Federmen EB, McCarthy DE, Bolt DM, Smith SS, Fiore ME, et al. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. Journal of Abnormal Psychology 2008;117(1):94-105. [DOI] [PubMed] [Google Scholar]
- Piper ME. Bupropion alone and in combination with nicotine gum: efficacy, medication and moderation. Dissertation Abstracts International: Section B: The Sciences and Engineering 2007;9:947-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Piper 2009 {published data only}
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, et al. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Archives of General Psychiatry 2009;66(11):1253-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Puska 1995 {published and unpublished data}
- Puska P, Korhonen HJ, Vartiainen E, Urjanheimo EL, Gustavsson G, Westin A. Combined use of nicotine patch and gum compared with gum alone in smoking cessation: a clinical trial in North Karelia. Tobacco Control 1995;4:231-5. [Google Scholar]
2 Richmond 1994 {published and unpublished data}
- Richmond RL, Harris K, De Almeida Neto A. The transdermal nicotine patch: results of a randomised placebo-controlled trial. Medical Journal of Australia 1994;161(2):130-5. [DOI] [PubMed] [Google Scholar]
- Richmond RL, Kehoe L, De Almeida Neto AC. Effectiveness of a 24-hour transdermal nicotine patch in conjunction with a cognitive behavioural programme: one year outcome. Addiction 1997;92(1):27-31. [PubMed] [Google Scholar]
2 Rigotti 2006 {published and unpublished data}
- Rigotti N, Thorndike A, Regan S, Pasternak R, Chang Y, McKool K, et al. Safety and efficacy of bupropion for smokers hospitalized with acute cardiovascular disease [abstract]. Nicotine & Tobacco Research 2005;7:682. [DOI] [PubMed] [Google Scholar]
- Rigotti NA, Thorndike AN, Regan S, McKool K, Pasternak RC, Chang Y, et al. Bupropion for smokers hospitalized with acute cardiovascular disease. American Journal of Medicine 2006;119(12):1080-7. [DOI] [PubMed] [Google Scholar]
- Thorndike A, Rigotti N, Regan S, Pasternak R, McKool K, Emmons K, et al. Depression and relapse to smoking in patients hospitalized with acute cardiovascular disease [POS3-094]. In: Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20-23 March, Prague, Czech Republic. 2005.
2 Rigotti 2010 {published data only}
- Ockene I, Salmoirago-Blotcher E. Varenicline for smoking cessation in patients with coronary heart disease [editorial]. Circulation 2010;121(2):188-90. [DOI] [PubMed] [Google Scholar]
- Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease. Circulation 2010;121(2):221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Rose 2014 {published data only}
- Rose JE, Behm FM. Combination treatment with varenicline and bupropion in an adaptive smoking cessation paradigm. American Journal of Psychiatry 2014;171(11):1199-205. [DOI: 10.1176/appi.ajp.2014.13050595] [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Sachs 1993 {published data only}
- Sachs DP, Sawe U, Leischow SJ. Effectiveness of a 16-hour transdermal nicotine patch in a medical practice setting, without intensive group counseling. Archives of Internal Medicine 1993;153(16):1881-90. [PubMed] [Google Scholar]
2 Saules 2004 {published and unpublished data}
- Saules KK, Schuh LM, Arfken CL, Reed K, Kilbey MM, Schuster CR. Double-blind placebo-controlled trial of fluoxetine in smoking cessation treatment including nicotine patch and cognitive-behavioral group therapy. American Journal on Addictions 2004;13(5):438-46. [DOI] [PubMed] [Google Scholar]
- Schuh LM, Downey KK, Hopper JA, Tancer M, Schuster CR. Fluoxetine in smoking cessation treatment. College on Problems of Drug Dependence Annual Meeting, San Juan, Puerto Rico 2000.
2 Sharma 2018 {published data only}
- Sharma SK, Mohan A, Singh AD, Mishra H, Jhanjee S, Pandey RM, et al. Impact of nicotine replacement therapy as an adjunct to antituberculosis treatment and behaviour change counselling in newly diagnosed pulmonary tuberculosis patients: an open-label, randomised controlled trial. Scientific Reports 2018;8(1):8828. [DOI: 10.1038/s41598-018-26990-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Shiffman 2002A {published and unpublished data}
- Dresler CM, Shiffman S, Strahs KR. Safety profile of the new nicotine polacrilex lozenge (PO1 36). In: Society for Research on Nicotine and Tobacco 8th Annual Meeting: Savannah, Georgia. 2002.
- Shiffman S, Dresler CM, Hajek P, Gilburt SJ, Targett DA, Strahs KR. Efficacy of a nicotine lozenge for smoking cessation. Archives of Internal Medicine 2002;162(11):1267-76. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Dresler CM, Rohay JM. Successful treatment with a nicotine lozenge of smokers with prior failure in pharmacological therapy. Addiction 2004;99(1):83-92. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Nicotine lozenge efficacy in light smokers. Drug and Alcohol Dependence 2005;77(3):311-4. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Use of more nicotine lozenges leads to better success in quitting smoking. Addiction 2007;102(5):809-14. [DOI] [PubMed] [Google Scholar]
2 Shiffman 2002B {published and unpublished data}
- Shiffman S, Dresler CM, Hajek P, Gilburt SJ, Targett DA, Strahs KR. Efficacy of a nicotine lozenge for smoking cessation. Archives of Internal Medicine 2002;162(11):1267-76. [DOI] [PubMed] [Google Scholar]
2 Simon 2004 {published and unpublished data}
- Caplan BJ. The "bupropion for smoking cessation" trial from a family practice perspective. Archives of Internal Medicine 2005;165(4):470. [DOI] [PubMed] [Google Scholar]
- Simon JA, Duncan C, Carmody TP, Hudes ES. Bupropion for smoking cessation: a randomized trial. Archives of Internal Medicine 2004;164(16):1797-803. [DOI] [PubMed] [Google Scholar]
- Simon JA, Duncan C, Carmody TP, Hudes ES. Bupropion for smoking cessation: a randomized trial. In: National Conference on Tobacco or Health, November 19-22. San Francisco, California. 2002.
- Simon JA, Duncan C, Carmody TP, Hudes ES. Bupropion plus nicotine replacement no better than replacement alone. Journal of Family Practice 2004;53:953-4. [Google Scholar]
2 Simon 2009 {published data only}
- Simon JA, Duncan C, Huggins J, Solkowitz S, Carmody TP. Sustained-release bupropion for hospital-based smoking cessation: a randomized trial. Nicotine & Tobacco Research 2009;11(6):663-9. [DOI] [PubMed] [Google Scholar]
2 Stapleton 1995 {published and unpublished data}
- Russell MA, Stapleton JA, Feyerabend C, Wiseman SM, Gustavsson G, Sawe U, et al. Targeting heavy smokers in general practice: randomised controlled trial of transdermal nicotine patches. BMJ 1993;306(6888):1308-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton JA, Russell MA, Feyerabend C, Wiseman SM, Gustavsson G, Sawe U, et al. Dose effects and predictors of outcome in a randomized trial of transdermal nicotine patches in general practice. Addiction 1995;90(1):31-42. [DOI] [PubMed] [Google Scholar]
2 Sutherland 1992 {published data only}
- Stapleton JA, Sutherland G, Russell MA. How much does relapse after one year erode effectiveness of smoking cessation treatments? Long term follow up of randomised trial of nicotine nasal spray. BMJ 1998;316(7134):830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland G, Stapleton JA, Russell MA, Jarvis MJ, Hajek P, Belcher M, et al. Randomised controlled trial of nasal nicotine spray in smoking cessation. Lancet 1992;340(8815):324-9. [DOI] [PubMed] [Google Scholar]
2 Tashkin 2011 {published data only}
- Tashkin DP, Rennard S, Hays JT, Ma W, Lawrence D, Lee TC. Effects of varenicline on smoking cessation in mild-to-moderate COPD: a randomised controlled trial. Chest 2011;139(3):591-9. [DOI] [PubMed] [Google Scholar]
2 TNSG 1991 {published data only}
- Daughton DM, Fortmann SP, Glover ED, Hatsukami DK, Heatley SA, Lichtenstein E, et al. The smoking cessation efficacy of varying doses of nicotine patch delivery systems 4 to 5 years post-quit day. Preventive Medicine 1999;28(2):113-8. [DOI] [PubMed] [Google Scholar]
- Gross J, Stitzer ML, Maldonado J. Nicotine replacement: effects of postcessation weight gain. Journal of Consulting and Clinical Psychology 1989;57(1):87-92. [DOI] [PubMed] [Google Scholar]
- Swan GE, Jack LM, Ward MM. Subgroups of smokers with different success rates after use of transdermal nicotine. Addiction 1997;92(2):207-17. [PubMed] [Google Scholar]
- Transdermal Nicotine Study Group. Transdermal nicotine for smoking cessation. Six-month results from two multicenter controlled clinical trials. Transdermal Nicotine Study Group. JAMA 1991;266(22):3133-8. [PubMed] [Google Scholar]
2 Tonstad 2006 {published data only}
- Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation. JAMA 2006;296(1):64-71. [DOI] [PubMed] [Google Scholar]
2 Tsai 2008 {published data only}
- Tsai ST, Cho HJ, Cheng HS, Kim CH, Hsueh KC, Billing CB Jr et al. A randomised, placebo-controlled trial of varenicline, a selective alpha4beta2 nicotinic acetylcholine receptor partial agonist, as a new therapy for smoking cessation in Asian smokers. Clinical Therapeutics 2008;29(6):1027-39. [DOI] [PubMed] [Google Scholar]
2 Tønnesen 1991 {published and unpublished data}
- Mikkelsen KL, Tønnesen P, Nørregaard J. Three-year outcome of two- and three-year sustained abstainers from a smoking cessation study with nicotine patches. Journal of Smoking-Related Disorders 1994;5:95-100. [Google Scholar]
- Nørregaard J, Tønnesen P, Petersen L. Predictors and reasons for relapse in smoking cessation with nicotine and placebo patches. Preventive Medicine 1993;22(2):261-71. [DOI] [PubMed] [Google Scholar]
- Tønnesen P, Nørregaard J, Säwe U. Two-year outcome in a smoking cessation trial with a nicotine patch. Journal of Smoking-Related Disorders 1992;3:241-5. [Google Scholar]
- Tønnesen P, Nørregaard J, Simonsen K, Säwe U. A double-blind trial of a 16-hour transdermal nicotine patch in smoking cessation. New England Journal of Medicine 1991;325(5):311-5. [DOI] [PubMed] [Google Scholar]
- Tønnesen P, Nørregaard J, Simonsen K, Säwe U. A double-blind trial of nicotine patches in smoking cessation [En dobbeltblind undersogelse af nikotinplaster ved rygeafvaenning]. Ugeskrift for Laeger 1992;154(5):251-4. [PubMed] [Google Scholar]
2 Tønnesen 1993 {published and unpublished data}
- Tønnesen P, Nørregaard J, Mikkelsen K, Jorgensen S, Nilsson F. A double-blind trial of a nicotine inhaler for smoking cessation. JAMA 1993;269(10):1268-71. [PubMed] [Google Scholar]
2 Ussher 2003 {published data only}
- Ussher M, West R, McEwan A, Taylor A, Steptoe A. Efficacy of exercise counselling as an aid for smoking cessation: a randomised controlled trial. Addiction 2003;98(4):523-32. [DOI] [PubMed] [Google Scholar]
- Ussher M, West R, McEwan A, Taylor A, Steptoe A. Efficacy of exercise counselling as an aid to smoking cessation: a randomised controlled trial. In: European Conference of the Society for Research on Nicotine and Tobacco. 2001.
- Ussher M, West W, McEwen A, Taylor AH, Steptoe A. Randomised controlled trial of physical activity counselling as an aid to smoking cessation: 12 month follow-up. Addictive Behaviors 2007;32(12):3060-4. [DOI] [PubMed] [Google Scholar]
2 Uyar 2007 {published data only}
- Uyar M, Bayram N, Filiz A, Elbek O, Topcu A, Dikensoy O, et al. Comparison of nicotine patch and bupropion in treating tobacco dependence. European Respiratory Journal 2005;26(Suppl 49):388s. [Google Scholar]
- Uyar M, Filiz A, Bayram N, Elbek O, Herken H, Topcu A, et al. A randomized trial of smoking cessation. Medication versus motivation. Saudi Medical Journal 2007;28(6):922-6. [PubMed] [Google Scholar]
2 Walker 2020 {published data only}
- Walker N, Parag V, Verbiest M, Laking G, Laugesen M, Bullen C. Nicotine patches used in combination with e-cigarettes (with and without nicotine) for smoking cessation:a pragmatic, randomised trial. Lancet. Respiratory Medicine 2020;8(1):54-64. [DOI: 10.1016/S2213-2600(19)30269-3] [DOI] [PubMed] [Google Scholar]
2 Wallstrom 2000 {published data only}
- Pharmacia and Upjohn. Nicorette Nicotine Microtab Monograph. 1998 edition. Chester: Adis International, 1998. [Google Scholar]
- Wallstrom M, Nilsson F, Hirsch JM. A double-blind placebo controlled clinical evaluation of a nicotine sublingual tablet in smoking cessation [abstract]. European Respiratory Journal 1997;10(Suppl 25):440S. [PubMed]
- Wallstrom M, Nilsson F, Hirsch JM. A randomized double-blind placebo-controlled clinical evaluation of a nicotine sublingual tablet in smoking cessation. Addiction 2000;95(8):1161-71. [PubMed] [Google Scholar]
2 Wang 2009 {published data only}
- Fagerström K, Nakamura M, Cho H, Tsa S, Wang C, Davis S, et al. Varenicline treatment for smoking cessation in Asian populations: a pooled analysis of placebo-controlled trials conducted in six Asian countries. Current Medical Research and Opinion 2010;26(9):2165-73. [DOI] [PubMed] [Google Scholar]
- Wang C, Xiao D, Chan KP, Pothirat C, Garza D, Davis S. Varenicline for smoking cessation: a placebo-controlled randomised study. Respirology 2009;14(3):384-92. [DOI] [PubMed] [Google Scholar]
2 Xiao 2019 {published data only}
- Xiao D, Kotler M, Kang J, Wang C. A multicenter, randomized, double-blind, parallel, placebo-controlled clinical study to evaluate the efficacy and safety of a nicotine mint lozenge (2 and 4 mg) in smoking cessation. Journal of Addiction Medicine 2020;14(1):69-77. [DOI: 10.1097/ADM.0000000000000547] [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Zellweger 2005 {published and unpublished data}
- Puska P, Brath H, Astbury C, Hider AE. Zyban is an effective and well tolerated aid to smoking cessation in a healthcare professionals population - a multi-country study. In: Society for Research on Nicotine and Tobacco 3rd European Conference, September 2001, Paris, France. 2001:45.
- Zellweger JP, Blaziene A, Astbury C, Hider A, Hogue S. Bupropion hydrochloride sustained release is an effective and well tolerated aid to smoking cessation in a healthcare professionals population - a multicountry study. Abstract and presentation at 11th Annual meeting of European Respiratory Society, Berlin, September 22-26 2001. European Respiratory Journal 2001;18(Suppl 33):166s. [Google Scholar]
- Zellweger JP, Boelcskei PL, Carrozzi L, Sepper R, Sweet R, Hider AZ. Bupropion SR vs placebo for smoking cessation in health care professionals. American Journal of Health Behavior 2005;29(3):240-9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
1 Ames 2007 {published data only}
- Ames SC, Ames GE, Stevens SR, Patten C, Werch C, Schroeder DR. Expressive writing as a treatment adjunct to reduce weight gain in young adult smokers undergoing smoking cessation (PA3-6). In: Society for Research on Nicotine and Tobacco 13th Annual Meeting. Austin, Texas, Feb 21-24, 2007.
1 Chaney 2008 {published data only}
- Chaney SE, Sheriff S. Weight gain among women during smoking cessation: testing the effects of a multifaceted program. American Association of Occupational Health Nurse Journal 2008;56(3):99-105. [DOI] [PubMed] [Google Scholar]
1 Hughes 1997 {published data only}
- Hughes JR, Hatsukami DK. Effects of three doses of transdermal nicotine on post-cessation eating, hunger and weight. Journal of Substance Abuse 1997;9:151-9. [DOI] [PubMed] [Google Scholar]
1 ISRCTN47776579 {unpublished data only}ISRCTN47776579
- ISRCTN47776579. A trial of physical activity assisted reduction of smoking. www.isrctn.com/ISRCTN47776579 (first received 23 October 2017). [DOI: 10.1186/ISRCTN47776579] [DOI]
- Taylor A, Thompson TP, Ussher M, Aveyard P, Murray RL, Harris T, et al. Randomised controlled trial of tailored support to increase physical activity and reduce smoking in smokers not immediately ready to quit: protocol for the Trial of physical Activity-assisted Reduction of Smoking (TARS) Study. BMJ Open 2020;10(12):e043331. [DOI: 10.1136/bmjopen-2020-043331] [DOI] [PMC free article] [PubMed] [Google Scholar]
1 Jeffery 1990 {published data only}
- Jeffery RW, Hellerstedt WL, Schmid TL. Correspondence programs for smoking cessation and weight control: a comparison of two strategies in the Minnesota Heart Health Program. Health Psychology 1990;9(5):585-98. [DOI] [PubMed] [Google Scholar]
1 Killen 1990 {published data only}
- Killen JD, Fortmann SP, Newman B. Weight change among participants in a large sample minimal contact smoking relapse prevention trial. Addictive Behaviors 1990;15(4):323-32. [DOI] [PubMed] [Google Scholar]
1 Kim 2017 {published data only}
- Kim S. Effect of complex training on carbon monoxide, cardiorespiratory function, and body mass among college students at the initial stage of stopping smoking. Journal of Physical Therapy Science 2017;29(8):1297-300. [DOI: 10.1589/jpts.29.1297] [DOI] [PMC free article] [PubMed] [Google Scholar]
1 King 2006 {published data only}
- King A, Wit H, Riley RC, Cao D, Niaura R, Hatsukami D. Efficacy of naltrexone in smoking cessation: a preliminary study and an examination of sex difference. Nicotine & Tobacco Research 2006;8(5):671-82. [DOI] [PubMed] [Google Scholar]
1 Lagrue 1994 {published data only}
- Lagrue G, Auclair J, Cormier S, Grimaldi B, Demaria C, Banzet MN. A study of the effects of dexfenfluramine on weight and eating behavior after smoking cessation by overweight patients. Semaine des Hopitaux 1994;70:23-24. [Google Scholar]
1 Leischow 1992 {published data only}
- Leischow SJ, Sachs DP, Bostrom AG, Hansen MD, Sachs DP. Effects of differing nicotine-replacement doses on weight gain after smoking cessation. Archives of Family Medicine 1992;1(2):233-7. [DOI] [PubMed] [Google Scholar]
1 Love 2011 {published data only}
- Love SJ, Sheffer CE, Bursac Z, Prewitt TE, Krukowski RA, West DS. Offer of a weight management program to overweight and obese weight-concerned smokers improves tobacco dependence treatment outcomes. American Journal on Addictions 2011;20(1):1-8. [DOI] [PubMed] [Google Scholar]
1 NCT02412631 {published data only}
- NCT02412631. Addressing post cessation weight gain. clinicaltrials.gov/ct2/show/NCT02412631 (first received 9 April 2015).
1 NCT03362372 {published data only}
- Martín-Luján F, Catalin RE, Salamanca-González P, Sorlí-Aguilar M, Santigosa-Ayala A, Valls-Zamora RM, et al. A clinical trial to evaluate the effect of the Mediterranean diet on smokers lung function. NPJ Primary Care Respiratory Medicine 2019;29(1):40. [DOI: 10.1038/s41533-019-0153-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03362372. Effect of the Mediterranean diet on lung function in smokers without previous respiratory disease (MEDISTAR). clinicaltrials.gov/ct2/show/NCT03362372 (first received 5 December 2017).
1 Patterson 2006 {published data only}
- Patterson F, Wileyto EP, Ray R, Lerman C. Examiniation of post cessation weight gain (POS2-85). In: Society for Research on Nicotine and Tobacco 12th Annual Meeting. Orlando, Florida, Feb 15-18 2006.
1 Pomerleau 1991 {published data only}
- Pomerleau OF, Pomerleau CS, Morrell EM, Lowenbergh JM. Effects of fluoxetine on weight gain and food intake in smokers who reduce nicotine intake. Psychoneuroendocrinology 1991;16(5):433-40. [DOI] [PubMed] [Google Scholar]
1 Rohsenow 2007 {published data only}
- Rohsenow DJ, Monti PM, Hutchinson KE, Swift RM, Mackinnon SV, Sirota AD, et al. High dose transdermal nicotine and naltrexone: effects on nicotine withdrawal, urges, smoking and effects of smoking. Experimental and Clinical Psychopharmacology 2007;15(1):81-92. [DOI] [PubMed] [Google Scholar]
1 Russo 2016 {published data only}
- Russo C, Cibella F, Caponnetto P, Campagna D, Maglia M, Frazzetto E, et al. Evaluation of post cessation weight gain in a 1-year randomized smoking cessation trial of electronic cigarettes. Scientific Reports 2016;6:18763. [DOI: 10.1038/srep18763] [DOI] [PMC free article] [PubMed] [Google Scholar]
1 Spring 1991 {published data only}
- Spring B, Wurtman J, Gleason R, Wurtman R, Kessler K. Weight gain and withdrawal symptoms after smoking cessation: a preventive intervention using d-Fenfluramine. Health Psychology 1991;10(3):216-23. [DOI] [PubMed] [Google Scholar]
1 Thompson 2015 {published data only}
- Jennings C, Kotseva K, De Bacquer D, Hoes A, De Velasco J, Brusaferro S, et al. Effectiveness of a preventive cardiology programme for high CVD risk persistent smokers: the EUROACTION PLUS varenicline trial. European Heart Journal 35;21:1411-20. [DOI: 10.1093/eurheartj/ehu051] [DOI] [PubMed] [Google Scholar]
- Thompson E, Jennings C, Kotseva K, De Bacquer D, Hoes A, De Velasco J, et al. Effectiveness of the EUROACTION PLUS (EA+) preventive cardiology programme for high CVD risk smokers in modifying dietary habits and anthropometric indices. In: ESC Congress 2015; 2015 Aug 30. 2015.
1 Toll 2008 {published data only}
- Toll BA, Leary V, Wu R, Salovey P, Meandzija B, O'Malley SS. A preliminary investigation of naltrexone augmentation of bupropion to stop smoking with less weight gain. Addictive Behaviors 2008;33(1):173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
1 Wilcox 2010 {published data only}
- Wilcox CS, Oskooilar N, Erickson JS, Billes SK, Katz BB, Tollefson G, et al. An open label study of naltrexone and bupropion combination therapy for smoking cessation in overweight and obese subjects. Addictive Behaviors 2010;35(3):229-34. [DOI] [PubMed] [Google Scholar]
2 AD Ahluwalia 2002 {published data only}
- Ahluwalia JS, Harris KJ, Catley D, Okuyemi KS, Mayo MS. Sustained-release bupropion for smoking cessation in African Americans: a randomized controlled trial. JAMA 2002;288:468-74. [DOI] [PubMed] [Google Scholar]
- Boardman T, Catley D, Mayo MS, Ahluwalia JS. Self-efficacy and motivation to quit during participation in a smoking cessation program. International Journal of Behavioral Medicine 2005;12:266-72. [DOI] [PubMed] [Google Scholar]
- Catley D, Harris KJ, Okuyemi KS, Mayo MS, Pankey E, Ahluwalia JS. The influence of depressive symptoms on smoking cessation among African Americans in a randomized trial of bupropion. Nicotine & Tobacco Research 2005;7:859-70. [DOI] [PubMed] [Google Scholar]
- Harris KJ, Ahluwalia JS, Catley D, Okuyemi KS, Mayo MS, Resnicow K. Successful recruitment of minorities into clinical trials: the Kick It at Swope project. Nicotine & Tobacco Research 2003;5:575-84. [DOI] [PubMed] [Google Scholar]
- Harris KJ, Okuyemi KS, Catley D, Mayo MS, Ge B, Ahluwalia JS. Predictors of smoking cessation among African-Americans enrolled in a randomized controlled trial of bupropion. Preventive Medicine 2004;38:498-502. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Ahluwalia JS, Ebersole Robinson M, Catley D, Mayo MS, Resnicow K. Does menthol attenuate the effect of bupropion among African American smokers? Addiction 2003;98:1387-93. [DOI] [PubMed] [Google Scholar]
2 AD Aubin 2004 {published data only}
- Aubin HJ, Lebargy F, Berlin I, Bidaut-Mazel C, Chemali-Hudry J, Lagrue G. Efficacy of bupropion and predictors of successful outcome in a sample of French smokers: a randomized placebo-controlled trial. Addiction 2004;99:1206-18. [DOI] [PubMed] [Google Scholar]
- Lebargy F, Aubin HJ, Lagrue G, Bidaut-Mazel C, Chemali-Hudry J, Poulain L. A placebo-controlled, double-blind study of Zyban LP: an effective and well-tolerated aid to smoking cessation - preliminary results (POS4-69). In: Society for Research on Nicotine and Tobacco 9th Annual Meeting February 19-22 New Orleans, Louisiana. 2003.
2 AD Berlin 1995 {published data only}
- Berlin I, Said S, Spreux Varoquaux O, Launay JM, Olivares R, Millet V, et al. A reversible monoamine oxidase A inhibitor (moclobemide) facilitates smoking cessation and abstinence in heavy, dependent smokers. Clinical Pharmacology and Therapeutics 1995;58:444-52. [DOI] [PubMed] [Google Scholar]
- Berlin I, Spreux Varoquaux O, Said S, Launay JM. Effects of past history of major depression on smoking characteristics, monoamine oxidase-A and -B activities and withdrawal symptoms in dependent smokers. Drug and Alcohol Dependence 1997;45:31-7. [DOI] [PubMed] [Google Scholar]
2 AD Blondal 1999 {published data only}
- Blondal T, Gudmundsson LJ, Tomasson K, Jonsdottir D, Hilmarsdottir H, Kristjansson F et al. The effects of fluoxetine combined with nicotine inhalers in smoking cessation - a randomized trial. Addiction 1999;94:1007-15. [DOI] [PubMed] [Google Scholar]
2 AD Brown 2006 {published data only}
- David SP, Brown RA, Papandonatos GD, Kahler CW, Lloyd-Richardson EE, Munafò MR, et al. Bupropion interacts with DRD2-TAQ1A genotype and craving to influence smoking cessation (RPOS 3-34). In: Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15-18, Orlando, Florida. 2006.
- David SP, Niaura R, Papandonatos GD, Shadel WG, Burkholder GJ, Britt DM, et al. Does the DRD2-Taq1 A polymorphism influence treatment response to bupropion hydrochloride for reduction of the nicotine withdrawal syndrome? Nicotine & Tobacco Research 2003;5:935-42. [DOI] [PubMed] [Google Scholar]
- David SP, Papandonatos GD, Munafò MR, McCaffery JM, Lerman C, Lloyd-Richardson EE, et al. DRD2-TAQ1A genotypic moderation of bupropion treatment efficacy for smoking cessation at 6-month follow-up [abstract]. Nicotine & Tobacco Research 2005;7:704. [Google Scholar]
2 AD Cinciripini 05 {published data only}
- Cinciripini PM, Wetter D, Minna J, et al. The effects of brief counseling, transdermal nicotine replacement and antidepressant therapy on smoking cessation among smokers carrying the DRD2 A1 allele (PA3A). In: Society for Research on Nicotine and Tobacco Annual Meeting; Mar 5-7 1999; San Diego, California. 1999.
- Cinciripini PM, Tsoh JY, Friedman K, Wetter D, Cinciripini LG, Skaar KL. A placebo controlled evaluation of venlafaxine for smoking cessation: preliminary findings [Abstract A18]. In: Society for Research on Nicotine and Tobacco Annual Meeting; Mar 27-29 1998;,New Orleans, Louisiana. 1998.
- Cinciripini PM, Tsoh JY, Wetter DW, Lam C, Moor C, Cinciripini L, et al. Combined effects of venlafaxine, nicotine replacement, and brief counseling on smoking cessation. Experimental and Clinical Psychopharmacology 2005;13:282-92. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Wetter DW, Tomlinson GE, Tsoh JY, Moor CA, Cinciripini LG, et al. The effects of the DRD2 polymorphism on smoking cessation and negative affect: Evidence for a pharmacogenetic effect on mood. Nicotine & Tobacco Research 2004;6:229-39. [DOI] [PubMed] [Google Scholar]
2 AD Collins 2004 {published data only}
- Collins B, Wileyto P, Patterson F, Rukstalis M, Audrain-McGovern J, Kaufmann V, et al. Gender differences in smoking cessation in a placebo-controlled trial of bupropion with behavioral counseling. Nicotine & Tobacco Research 2004;6:27-37. [DOI] [PubMed] [Google Scholar]
- Collins BN, Niaura R, Wileyto EP, Patterson F, Brown RA, Audrain J. Gender differences in smoking relapse in a behavioral counseling + placebo-controlled bupropion trial (PA2-5). In: Society for Research on Nicotine and Tobacco 8th Annual Meeting; February 20-23; Savannah, Georgia. 2002.
- Lerman C, Jepson C, Wileyto EP, Epstein LH, Rukstalis M, Patterson F, et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacology 2006;31:231-42. [DOI] [PubMed] [Google Scholar]
- Lerman C, Niaura R, Collins BN, Wileyto P, Audrain MJ, Pinto A, et al. Effect of bupropion on depression symptoms in a smoking cessation clinical trial. Psychology of Addictive Behaviors 2004;18:362-6. [DOI] [PubMed] [Google Scholar]
- Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu AY, et al. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug and Alcohol Dependence 2002;67:219-23. [DOI] [PubMed] [Google Scholar]
- Lerman C, Shields PG, Wileyto EP, Audrain J, Hawk LH Jr, Pinto A, et al. Effects of dopamine transporter and receptor polymorphisms on smoking cessation in a bupropion clinical trial. Health Psychology 2003;22:541-8. [DOI] [PubMed] [Google Scholar]
- Lerman C, Shields PG, Wileyto EP, Audrain J, Pinto A, Hawk L, et al. Pharmacogenetic investigation of smoking cessation treatment. Pharmacogenetics 2002;12:627-34. [DOI] [PubMed] [Google Scholar]
- Wileyto EP, Patterson F, Niaura R, Epstein LH, Brown RA, Audrain-McGovern J, et al. Recurrent event analysis of lapse and recovery in a smoking cessation clinical trial using bupropion. Nicotine & Tobacco Research 2005;7:257-68. [DOI] [PubMed] [Google Scholar]
- Wileyto P, Patterson F, Niaura R, Epstein L, Brown R, Audrain-McGovern J, et al. Do small lapses predict relapse to smoking behavior under bupropion treatment? Nicotine & Tobacco Research 2004;6:357-66. [DOI] [PubMed] [Google Scholar]
2 AD Covey 2002 {published data only}
- Covey LS, Glassman AH, Stetner F, Rivelli S, Stage K. A randomized trial of sertraline as a cessation aid for smokers with a history of major depression. American Journal of Psychiatry 2002;159:1731-7. [DOI] [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F, Rivelli S. A trial of sertraline for smokers with past major depression. Society for Research on Nicotine and Tobacco Meeting. Arlington, VA (www.srnt.org/events/abstracts99/index.htm) 2000.
2 AD Covey 2007 {published data only}
- Covey LS, Glassman AH, Jiang H, Fried J, Masmela J, LoDuca C, et al. A randomized trial of bupropion and/or nicotine gum as maintenance treatment for preventing smoking relapse. Addiction 2007;102:1292-302. [DOI] [PubMed] [Google Scholar]
2 AD Da Costa 2002 {published data only}
- Da Costa C, Younes R, Lourenco M. Smoking cessation: a randomized double-blind study comparing nortriptyline to placebo [abstract]. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A354. [Google Scholar]
- Da Costa CL, Younes RN, Lourenco MT-C. Stopping smoking: a prospective, randomized, double-blind study comparing nortriptyline to placebo. Chest 2002;122:403-8. [DOI] [PubMed] [Google Scholar]
2 AD Dalsgareth 2004 {published data only}
- Dalsgarð OJ, Vestbo J. A multicenter, randomised, double-blind, placebo-controlled 6 month trial to evaluate efficacy and tolerability of bupropion hydrochloride sustained release (SR) tablets as treatment for nicotine dependency in healthcare workers and as an aid to smoking cessation (ZYB30009). Poster and oral presentation. In: European Congress on Tobacco or Health, Warsaw, Poland, 20-22 June 2002. 2002.
- Dalsgareth OJ, Hansen NC, Søes-Petersen U, Evald T, Høegholm A, Barber J, et al. A multicenter, randomized, double-blind, placebo-controlled, 6-month trial of bupropion hydrochloride sustained-release tablets as an aid to smoking cessation in hospital employees. Nicotine & Tobacco Research 2004;6:55-61. [DOI] [PubMed] [Google Scholar]
2 AD Evins 2001 {published data only}
- Evins AE, Cather C, Rigotti NA, Freudenreich O, Henderson DC, Olm Shipman CM, et al. Two-year follow-up of a smoking cessation trial in patients with schizophrenia: increased rates of smoking cessation and reduction. Journal of Clinical Psychiatry 2004;65:307-11. [DOI] [PubMed] [Google Scholar]
- Evins AE, Mays VK, Rigotti NA, Tisdale T, Cather C, Goff DC. A pilot trial of bupropion added to cognitive behavioral therapy for smoking cessation in schizophrenia. Nicotine & Tobacco Research 2001;3:397-403. [DOI] [PubMed] [Google Scholar]
2 AD Evins 2005 {published data only}
- Evins AE, Cather C, Culhane M, Freudenreich O, Rigotti NA, Goff DC. Smoking cessation in schizophrenia: a double blind placebo controlled trial of bupropion SR added to cognitive behavioral therapy. Biological Psychiatry 2004;55:806. [Google Scholar]
- Evins AE, Cather C, Deckersbach T, Freudenreich O, Culhane MA, Olm-Shipman CM, et al. A double-blind placebo-controlled trial of bupropion sustained-release for smoking cessation in schizophrenia. Journal of Clinical Psychopharmacology 2005;25:218-25. [DOI] [PubMed] [Google Scholar]
- Evins AE, Deckersbach T, Cather C, Freudenreich O, Culhane MA, Henderson DC, et al. Independent effects of tobacco abstinence and bupropion on cognitive function in schizophrenia. Journal of Clinical Psychiatry 2005;66:1184-90. [DOI] [PubMed] [Google Scholar]
2 AD Evins 2006 {published data only}
- Evins AE, Cather C, Culhane M, Birnbaum AS, Horowitz J, Hsieh E, et al. A placebo-controlled study of bupropion SR added to high dose nicotine replacement therapy for smoking cessation or reduction in schizophrenia (POS2-104). In: Society for Research on Nicotine and Tobacco 12th Annual Meeting; February 15-18, Orlando, Florida. 2006.
2 AD Evins 2008 {published data only}
- Evins AE, Culhane MA, Alpert JE, Pava J, Liese BS, Farabaugh A, et al. A controlled trial of bupropion added to nicotine patch and behavioral therapy for smoking cessation in adults with unipolar depressive disorders. Journal of Clinical Psychopharmacology 2008;28:660-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
2 AD Ferry 1992 {published data only}
- Ferry LH, Robbins AS, Scariati PD, et al. Enhancement of smoking cessation using the antidepressant bupropion hydrochloride [abstract 2670]. Circulation 1992;86(4 Suppl 1):I-671. [Google Scholar]
2 AD Ferry 1994 {published data only}
- Ferry LH, Burchette RJ. Efficacy of bupropion for smoking cessation in non depressed smokers [Abstract]. Journal of Addictive Diseases 1994;13(4):249. [Google Scholar]
2 AD George 2002 {published data only}
- George TP, Vessicchio JC, Termine A, Bregartner TA, Feingold A, Rounsaville BJ, et al. A placebo controlled trial of bupropion for smoking cessation in schizophrenia. Biological Psychiatry 2002;52:53-61. [DOI] [PubMed] [Google Scholar]
2 AD GlaxoSmithK SMK20001 {published data only}
- GlaxoSmithKline Clinical Trials Register. Study No: SMK 20001. A multi-center, double-blind, double-dummy, placebo-controlled, randomized, parallel group, dose response evaluation of a new chemical entity (NCE) and ZYBAN (bupropion hydrochloride) sustained release (300mg/day) versus placebo as aids to smoking cessation. ctr.glaxowellcome.co.uk-Summary-bupropion-II_SMK20001.pdf (accessed 23rd August 2006).
2 AD Gonzales 2001 {published data only}
- Gonzales D, Nides M, Ferry LH, Segall N, Herrero L, Modell J, et al. Retreatment with bupropion SR: results from 12-month follow-up (RP-83). In: Rapid Communication Poster Abstracts. Society for Research on Nicotine and Tobacco 8th Annual Meeting, February 20-23 Savannah, Georgia. 2002.
- Gonzales DH, Nides MA, Ferry LH, Kustra RP, Jamerson BD, Segall N, et al. Bupropion SR as an aid to smoking cessation in smokers treated previously with bupropion: a randomized placebo-controlled study. Clinical Pharmacology and Therapeutics 2001;69:438-44. [DOI] [PubMed] [Google Scholar]
2 AD Haggsträm 2006 {published data only}
- Haggsträm FM, Chatkin JM, Sussenbach-Vaz E, Cesari DH, Fam CF, Fritscher CC. A controlled trial of nortriptyline, sustained-release bupropion and placebo for smoking cessation: preliminary results. Pulmonary Pharmacology & Therapeutics 2006;19:205-9. [DOI] [PubMed] [Google Scholar]
2 AD Hall 1998 {published data only}
- Haas AL, Munoz RF, Humfleet GL, Reus VI, Hall SM. Influences of mood, depression history, and treatment modality on outcomes in smoking cessation. Journal of Consulting & Clinical Psychology 2004;72:563-70. [DOI] [PubMed] [Google Scholar]
- Hall SM, Reus VI, Munoz RF, Sees KL, Humfleet G, Hartz DT, et al. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Archives of General Psychiatry 1998;55:683-90. [DOI] [PubMed] [Google Scholar]
2 AD Hall 2002 {published data only}
- Hall SM, Humfleet G, Maude-Griffin R, Reus VI, Muñoz R, Hartz DT. Nortriptyline versus bupropion and medical management versus psychological intervention in smoking treatment (PA 5A). In: Society for Research on Nicotine and Tobacco 7th Annual Meeting March 23-23 Seattle, Washington. 2001:31.
- Hall SM, Humfleet GL, Reus VI, Muñoz RF, Hartz DT, Maude-Griffin R. Psychological intervention and antidepressant treatment in smoking cessation. Archives of General Psychiatry 2002;59:930-36. [DOI] [PubMed] [Google Scholar]
- Hall SM, Lightwood JM, Humfleet GL, Bostrom A, Reus VI, Muñoz R. Cost-effectiveness of bupropion, nortriptyline, and psychological intervention in smoking cessation. Journal of Behavioral Health Services & Research 2005;32:381-92. [DOI] [PubMed] [Google Scholar]
2 AD Hall 2004 {published data only}
- Hall SM, Humfleet GL, Reus VI, Muñoz RF, Cullen J. Extended nortriptyline and psychological treatment for cigarette smoking. American Journal of Psychiatry 2004;161:2100-7. [DOI] [PubMed] [Google Scholar]
2 AD Hatsukami 2004 {published data only}
- Hatsukami DK, Rennard S, Patel MK, Kotlyar M, Malcolm R, Nides MA, et al. Effects of sustained-release bupropion among persons interested in reducing but not quitting smoking. American Journal of Medicine 2004;116:151-7. [DOI] [PubMed] [Google Scholar]
- Rennard S, Hatsukami D, Malcolm RE, Patel MK, Jamerson BD, Dozier G. Zyban (bupropion HCL SR) vs placebo as an aid to smoking reduction among smokers unwilling and unable to quit smoking (PO4 77). In: Society for Research on Nicotine and Tobacco 7th Annual Meeting March 23-23 Seattle, Washington. 2001:117.
2 AD Hays 2001 {published data only}
- Abel GA, Hays JT, Decker PA, Croghan GA, Kuter DJ, Rigotti NA. Effects of biochemically confirmed smoking cessation on white blood cell count. Mayo Clinic Proceedings 2005;80:1022-8. [DOI] [PubMed] [Google Scholar]
- Cox LS, Patten CA, Niaura RS, Decker PA, Rigotti N, Sachs DP, et al. Efficacy of bupropion for relapse prevention in smokers with and without a past history of major depression. Journal of General Internal Medicine 2004;19:828-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durcan MJ, Deener G, White J, Johnston JA, Gonzales D, Niaura R, et al. The effect of bupropion sustained-release on cigarette craving after smoking cessation. Clinical Therapeutics 2002;24:540-51. [DOI] [PubMed] [Google Scholar]
- Durcan MJ, Johnston JA, White J, Gonzales D, Sachs DP, Rigotti N, et al. Bupropion SR for relapse prevention: a "slips-allowed" analysis. American Journal of Health Behavior 2004;28(5):456-63. [PubMed] [Google Scholar]
- Gonzales D, Bjornson W, Durcan MJ, White JD, Johnston JA, Buist AS, et al. Effects of gender on relapse prevention in smokers treated with bupropion SR. American Journal of Preventive Medicine 2002;22:234-9. [DOI] [PubMed] [Google Scholar]
- Hays JT, Hurt RD, Rigotti NA, Niaura R, Gonzales D, Durcan MJ, et al. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. A randomized, controlled trial. Annals of Internal Medicine 2001;135:423-33. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Wolter TD, Rigotti N, Hays JT, Niaura R, Durcan MJ, et al. Bupropion for pharmacologic relapse prevention to smoking - Predictors of outcome. Addictive Behaviors 2002;27(4):493-507. [DOI] [PubMed] [Google Scholar]
- Rigotti N, Thorndike AN, Durcan MJ, White JD, Johnston AJ, Niaura R, et al. Attenuation of post-cessation weight gain in smokers taking bupropion: the effect of gender. In: Society for Research on Nicotine and Tobacco 6th Annual Meeting; Feb 18-20 2000; Arlington VA. 2000.
2 AD Hertzberg 2001 {published data only}
- Hertzberg MA, Moore SD, Feldman ME, Beckham JC. A preliminary study of bupropion sustained-release for smoking cessation in patients with chronic posttraumatic stress disorder. Journal of Clinical Psychopharmacology 2001;21:94-8. [DOI] [PubMed] [Google Scholar]
2 AD Holt 2005 {published data only}
- Holt S, Timu-Parata C, Ryder-Lewis S, Weatherall M, Beasley R. Efficacy of bupropion in the indigenous Maori population in New Zealand. Thorax 2005;60:120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
2 AD Hurt 2003 {published data only}
- Hurt RD, Croghan GA, Sloan JA, Krook JE, Silberstein PT. Bupropion for relapse prevention after nicotine patch therapy [PA 5B abstract ]. In: Society for Research on Nicotine and Tobacco 7th Annual Meeting, March 23-23 2001, Seattle, Washington. 2001:32.
- Hurt RD, Krook JE, Croghan IT, Loprinzi CL, Sloan JA, Novotny PJ, et al. Nicotine patch therapy based on smoking rate followed by bupropion for prevention of relapse to smoking. Journal of Clinical Oncology 2003;21:914-20. [DOI] [PubMed] [Google Scholar]
2 AD Killen 2000 {published data only}
- Killen JD, Fortmann SP, Schatzberg A, Hayward C, Varady A. Onset of major depression during treatment for nicotine dependence. Addictive Behaviors 2003;28:461-70. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Schatzberg AF, Hayward C, Sussman L, Rothman M, et al. Nicotine patch and paroxetine for smoking cessation. Journal of Consulting and Clinical Psychology 2000;68:883-9. [PubMed] [Google Scholar]
2 AD Killen 2004 {published data only}
- Killen JD, Robinson TN, Ammerman S, Hayward C, Rogers J, Samuels D. Major depression among adolescent smokers undergoing treatment for nicotine dependence. Addictive Behaviors 2004;29:1517-26. [DOI] [PubMed] [Google Scholar]
- Killen JD, Robinson TN, Ammerman S, Hayward C, Rogers J, Stone C, et al. Randomized clinical trial of the efficacy of bupropion combined with nicotine patch in the treatment of adolescent smokers. Journal of Consulting and Clinical Psychology 2004;72:729-35. [DOI] [PubMed] [Google Scholar]
2 AD Killen 2006 {published data only}
- Killen JD, Fortmann SP, Murphy GM Jr, Hayward C, Arredondo C, Cromp D, et al. Extended treatment with bupropion SR for cigarette smoking cessation. Journal of Consulting and Clinical Psychology 2006;74(2):286-94. [DOI] [PubMed] [Google Scholar]
2 AD Myles 2004 {published data only}
- Myles PS, Leslie K, Angliss M, Mezzavia P, Lee L. Effectiveness of bupropion as an aid to stopping smoking before elective surgery: a randomised controlled trial. Anaesthesia 2004;59:1053-8. [DOI] [PubMed] [Google Scholar]
2 AD Piper 2004 {published data only}
- Piper ME, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. Mediators of bupropion treatment effects (PA5-6). In: Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15-18, Orlando, Florida. 2006:27.
- Piper ME, Federman EB, Smith SS, Fiore MC, Baker TB. Efficacy of bupropion SR alone and combined with 4-mg gum (PA2-2). In: Society for Research on Nicotine and Tobacco 10th Annual Meeting February 18-21, Phoenix, Arizona. 2004:18.
2 AD Piper 2009 {published data only}
- Gennuso KP, Thraen-Borowski KM, Schlam TR, LaRowe TL, Fiore MC, Baker TB, et al. Smokers' physical activity and weight gain one year after a successful versus unsuccessful quit attempt. Preventive Medicine 2014;67:189-92. [CENTRAL: 1014125] [EMBASE: 2014546589] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, et al. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Archives of General Psychiatry 2009;66:1253-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, McCarthy DE, Japuntich SJ, Christiansen B, Piper ME, Jorenby DE, et al. Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics. Archives of Internal Medicine 2009;169:2148-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JH, Asthana A, Smith SS, Piper ME, Loh WY, Fiore MC, et al. Smoking cessation and the risk of diabetes mellitus and impaired fasting glucose: three-year outcomes after a quit attempt. PLOS One 2014;9(6):e98278. [CENTRAL: 995881] [DOI] [PMC free article] [PubMed] [Google Scholar]
2 AD Prochazka 1998 {published data only}
- Prochazka AV, Weaver MJ, Keller RT, Fryer GE, Licari PA, Lofaso D. A randomized trial of nortriptyline for smoking cessation. Archives of Internal Medicine 1998;158:2035-9. [DOI] [PubMed] [Google Scholar]
2 AD Prochazka 2004 {published data only}
- Prochazka AV, Kick S, Steinbrunn C, Miyoshi T, Fryer GE. A randomized trial of nortriptyline combined with transdermal nicotine for smoking cessation. Archives of Internal Medicine 2004;164:2229-33. [DOI] [PubMed] [Google Scholar]
- Prochazka AV, Reyes R, Steinbrunn C, Miyoshi T. Randomized trial of nortriptyline combined with transdermal nicotine for smoking cessation (PO3 26). In: Society for Research on Nicotine and Tobacco 7th Annual Meeting, March 23-23 2001, Seattle, Washington. 2001:73.
2 AD Rovina 2009 {published data only}
- Rovina N, Nikoloutsou I, Katsani G, Dima E, Fransis K, Roussos C, & Gratziou, C. Effectiveness of pharmacotherapy and behavioral interventions for smoking cessation in actual clinical practice. Therapeutic Advances in Respiratory Disease 2009;3:279-87. [DOI] [PubMed] [Google Scholar]
2 AD Selby 2003 {published data only}
- GlaxoSmithKline Clinical Trials Register. Study No: ZYB40001. A randomized, double-blind, placebo-controlled, 12-week smoking cessation trial of Zyban (150 mg bid) in adult smokers previously treated with Zyban. ctr.glaxowellcome.co.uk-Summary-bupropion-IV_ZYB40001.pdf (accessed 23rd August 2006).
- Selby P, Ainslie M, Stepner N, Roberts J. Sustained-release bupropion (Zybanr) is effective in the re-treatment of relapsed adult smokers. American Journal of Respiratory Critical Care Medicine 2003;167(7):A47. [Google Scholar]
- Selby P, Brands B, Stepner N. Retreatment with ZYban SR: 52 week follow-up of a Canadian multicentre trial (POS3-63). In: Society for Research on Nicotine and Tobacco 9th Annual Meeting, February 19-22, New Orleans. 2003.
- Selby P, Brosky G, Baker R, Lertzman M, Dakin P, Roberts J. Zyban is effective in the retreatment of relapsed adult smokers (PO4 68). In: Society for Research on Nicotine and Tobacco 7th Annual Meeting March 23-23 Seattle Washington. 2001:114.
2 AD Swan 2003 {published data only}
- Catz S, Jack LM, Swan GE, McClure J. Adherence to bupropion SR in a smoking cessation effectiveness trial (POS2-77). In: Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15-18, Orlando, Florida. 2006.
- Jack LM, Swan GE, Thompson E, Curry SJ, McAfee T, Dacey S, et al. Bupropion SR and smoking cessation in actual practice: methods for recruitment, screening, and exclusion for a field trial in a managed-care setting. Preventive Medicine 2003;36:585-93. [DOI] [PubMed] [Google Scholar]
- Javitz HS, Swan GE, Zbikowski SM, Curry SJ, McAfee TA, Decker DL, et al. Cost-effectiveness of different combinations of bupropion SR dose and behavioral treatment for smoking cessation: a societal perspective. American Journal of Managed Care 2004;10:217-26. [PubMed] [Google Scholar]
- McAfee T, Zbikowski SM, Bush T, McClure J, Swan G, Jack LM, et al. The effectiveness of bupropion SR and phone counseling for light and heavy smokers (PA2-1). In: Society for Research on Nicotine and Tobacco 10th Annual Meeting February 18-21, Phoenix, Arizona. 2004.
- Swan GE, Jack LM, Curry S, Chorost M, Javitz H, McAfee T, et al. Bupropion SR and counseling for smoking cessation in actual practice: Predictors of outcome. Nicotine & Tobacco Research 2003;5:911-21. [DOI] [PubMed] [Google Scholar]
- Swan GE, Javitz HS, Jack LM, Curry SJ, McAfee T. Heterogeneity in 12-month outcome among female and male smokers. Addiction 2004;99:237-50. [DOI] [PubMed] [Google Scholar]
- Swan GE, McAfee T, Curry SJ, Jack LM, Javitz H, Dacey S, et al. Effectiveness of bupropion sustained release for smoking cessation in a health care setting: a randomized trial. Archives of Internal Medicine 2003;163:2337-44. [DOI] [PubMed] [Google Scholar]
- Swan GE, Valdes AM, Ring HZ, Khroyan TV, Jack LM, Ton CC, et al. Dopamine receptor DRD2 genotype and smoking cessation outcome following treatment with bupropion SR. Pharmacogenomics Journal 2005;5:21-9. [DOI] [PubMed] [Google Scholar]
2 AD Tashkin 2001 {published data only}
- Patel MK, Tashkin DP, Kanner RE, Bailey WC, Buist A, Anderson PJ, et al. A multicenter evaluation of the effects of bupropion hydrochloride sustained release tablets (Bup SR) versus placebo in a population of smokers with chronic obstructive pulmonary disease (PO130). In: 11th World Conference on Tobacco or Health; Aug 6-11 2000; Chicago, ILL. Vol. 1. 2000:118.
- Tashkin D, Kanner R, Bailey W, Buist S, Anderson P, Nides M, et al. Smoking cessation in patients with chronic obstructive pulmonary disease: a double-blind, placebo-controlled, randomised trial. Lancet 2001;357:1571-5. [DOI] [PubMed] [Google Scholar]
2 AD Tonstad 2003 {published data only}
- McRobbie H, Brath H, Astbury C, Hider A, Sweet R. Bupropion hydrochloride sustained release (SR) is an effective and well tolerated aid to smoking cessation in smokers with cardiovascular disease 12 month follow-up phase data (ZYB40014). In: European Respiratory Society meeting, 14-18 September 2002, Stockholm, Sweden. 2002.
- Tonstad S, Farsang C, Klaene G, Lewis K, Manolis A, Perruchoud AP, et al. Bupropion SR for smoking cessation in smokers with cardiovascular disease: a multicentre, randomised study. European Heart Journal 2003;24:946-55. [DOI] [PubMed] [Google Scholar]
- Van Spiegel PI, Lewis K, Seinost G, Astbury C, Hider A, Sweet R. Bupropion hydrochloride (Zyban) is an effective and well tolerated aid to smoking cessation in smokers with cardiovascular disease - a multicountry study. Abstract and presentation at 11th Annual meeting of European Respiratory Society, Berlin, September 22-26. European Respiratory Journal 2001;18(Suppl 33):13s. [Google Scholar]
2 AD Tønnesen 2003 {published data only}
- Bolliger CT, Gilljam H, Lebargy F, Van Spiegel PI, Edwards J, Hider A, et al. Bupropion hydrochloride (Zyban) is effective and well tolerated as an aid to smoking cessation - a multicountry study. Abstract and presentation at 11th Annual meeting of European Respiratory Society, Berlin, September 22-26 2001. European Respiratory Journal 2001;18(Suppl 33):12s. [Google Scholar]
- Tønnesen P, Tonstad S, Hjalmarson A, Lebargy F, Van Spiegel PI, Hider A, et al. A multicentre, randomized, double-blind, placebo-controlled, 1-year study of bupropion SR for smoking cessation. Journal of Internal Medicine 2003;254:184-92. [DOI] [PubMed] [Google Scholar]
- Tonstad S, Aaserud E, Hjalmarson A, Peiffer G, Van der Molen T, Hider A, et al. Zyban is an effective and well tolerated aid to smoking cessation in a general smoking population - a multi-country study. In: Society for Research on Nicotine and Tobacco 3rd Europe Conference, September 19-22 2001, Paris, France. 2001:46.
2 AD Uyar 2005 {published data only}
- Uyar M, Bayram N, Filiz A, Elbek O, Topçu A, Dikensoy O, et al. Comparison of nicotine patch and bupropion in treating tobacco dependence. European Respiratory Journal 2005;26(Suppl 49):388s. [Google Scholar]
2 AD Wagena 2005 {published data only}
- Wagena EJ, Knipschild PG, Huibers MJ, Wouters EF, Van Schayck CP. Efficacy of bupropion and nortriptyline for smoking cessation among people at risk for or with chronic obstructive pulmonary disease. Archives of Internal Medicine 2005;165:2286-92. [DOI] [PubMed] [Google Scholar]
2 Aveyard 2018 {published data only}ISRCTN33031001
- Aveyard P, Lindson N, Tearne S, Adams R, Ahmed K, Alekna R, et al. Nicotine preloading for smoking cessation: the Preloading RCT. Health Technology Assessment 22;41:1-84. [DOI: 10.3310/hta22410] [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Drovandi 2018 {published data only}
- Drovandi A, Teague P, Glass B, Malau-Aduli B. A randomised controlled trial evaluating the effectiveness and tolerability of step-up and step-down varenicline therapy for smoking cessation: a study protocol. Journal of Smoking Cessation 2018;13(3):179-85. [DOI: 10.1017/jsc.2017.17] [DOI] [Google Scholar]
2 EX Hill 1985 {published data only}
- Hill JS. Effect of a program of aerobic exercise on the smoking behaviour of a group of adult volunteers. Canadian Journal of Public Health 1985;76:186-6. [PubMed] [Google Scholar]
2 EX Hill 1993 {published data only}
- Hill RD, Rigdon M, Johnson S. Behavioural smoking cessation treatment for older chronic smokers. Behavioural Therapy 1993;24:321-9. [Google Scholar]
2 EX Kinnunen 2008 {unpublished data only}
- Kinnunen T, Leeman RF, Korhonen T, Quiles ZN, Terwal DM, Garvey AJ, et al. Exercise as an adjunct to nicotine gum in treating tobacco dependence among women. Nicotine & Tobacco Research 2008;10:689-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T, Goodwin A, Miesmaa P, Dupuis EA, Kinnunen T. Smoking cessation program with exercise improves cardiovascular disease biomarkers in sedentary women. Journal of Women's Health 2011;20(7):1051-64. [CENTRAL: 814607] [39790] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiles ZN, Leeman RF, Molinelli L, Nordstrom BL, Garvey AJ, Hartley LH, et al. Exercise as a behavioural adjunct to nicotine gum for smoking cessation - preliminary findings (PO 42). In: Society for Research on Nicotine and Tobacco 8th Annual Meeting. Vol. 59. Savannah, Georgia, February 20-23, 2002.
2 EX Marcus 1991 {published data only}
- Marcus BH, Albrecht AE, Niaura RS, Abrams DB, Thompson PD. Usefulness of physical exercise for maintaining smoking cessation in women. American Journal of Cardiology 1991;68:406-7. [DOI] [PubMed] [Google Scholar]
2 EX Marcus 1995 {published data only}
- Marcus BH, Albrecht AE, Niaura RS, Taylor ER, Simkin LR, Feder SI, et al. Exercise enhances the maintenance of smoking cessation in women. Addictive Behaviours 1995;20:87-92. [DOI] [PubMed] [Google Scholar]
2 EX Martin 1997 {published data only}
- Martin JE, Kalfas KJ, Patten CA. Prospective evaluation of three smoking interventions in 205 recovering alcoholics: One-year results of project SCRAP-Tobacco. Journal of Consulting and Clinical Psychology 1997;65:190-4. [DOI] [PubMed] [Google Scholar]
- Patten CA, Martin JE, Calfas KJ, Brown SA, Schroeder DR. Effect of three smoking cessation treatments on nicotine withdrawal in 141 abstinent alcoholic smokers. Addictive Behaviours 2000;25:301-6. [DOI] [PubMed] [Google Scholar]
- Patten CA, Martin JE, Calfas KJ, Wolter TD. Behavioural treatment for smokers with a history of alcoholism: predictors of successful outcome. Journal of Consulting and Clinical Psychology 2001;69(5):796-801. [DOI] [PubMed] [Google Scholar]
2 EX Prapavessis 2007 {unpublished data only}
- Prapavessis H, Cameron l, Baldi JC, Robinson S, Borrie K, Harper T, et al. The effects of exercise and nicotine replacement therapy on smoking rates in women. Addictive Behaviors 2007;32:1416-32. [DOI] [PubMed] [Google Scholar]
- Prapavessis H. The effects of exercise and nicotine replacement therapy on smoking rates in women. In: Eighth International Congress of Behavioural Medicine. Mainz, Germany, 2004. [DOI] [PubMed]
2 EX Russell 1988 {published data only}
- Russell PO, Epstein LH, Johnson JJ, Block DR, Blair E. The effects of exercise as maintenance for smoking cessation. Addictive Behaviours 1988;13:215-8. [DOI] [PubMed] [Google Scholar]
2 EX Taylor 1988 {published data only}
- Taylor CB, Houston-Miller N, Haskell WL, Debusk RF. Smoking cessation after acute myocardial infarction: the effects of exercise training. Addictive Behaviours 1988;13:215-8. [DOI] [PubMed] [Google Scholar]
2 LeMao 2020 {published data only}
- Le Mao R, Tromeur C, Paleiron N, Sanchez O, Gagnadoux F, Jouneau S, et al. Effect of early initiation of varenicline on smoking cessation in COPD patients admitted for exacerbation: the Save randomized clinical trial. COPD 2020;17(1):7-14. [DOI: 10.1080/15412555.2019.1703928] [DOI] [PubMed] [Google Scholar]
2 NRT Ahluwalia 1998 {published data only}
- Ahluwalia JS, McNagny SE, Clark WS. Smoking cessation among inner-city African Americans using the nicotine transdermal patch. Journal of General Internal Medicine 1998;13:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
2 NRT Ahluwalia 2006 {published data only}
- Ahluwalia JS, Okuyemi K, Nollen N, Choi WS, Kaur H, Pulvers K, et al. The effects of nicotine gum and counseling among African American light smokers: a 2 x 2 factorial design. Addiction 2006;101:883-91. [DOI] [PubMed] [Google Scholar]
- Nollen NL, Mayo MS, Sanderson CL, Okuyemi KS, Choi WS, Kaur H, et al. Predictors of quitting among African American light smokers enrolled in a randomized, placebo-controlled trial. Journal of General Internal Medicine 2006;21:590-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyemi KS, Cox LS, Nollen NL, Snow TM, Kaur H, Choi W, et al. Baseline characteristics and recruitment strategies in a randomized clinical trial of African-American light smokers. American Journal of Health Promotion 2007;21:183-91. [DOI] [PubMed] [Google Scholar]
2 NRT Areechon 1988 {published data only}
- Areechon W, Punnotok J. Smoking cessation through the use of nicotine chewing gum: a double-blind trial in Thailand. Clinical Therapeutics 1988;10:183-6. [PubMed] [Google Scholar]
2 NRT Blondal 1989 {published data only}
- Blondal T. Controlled trial of nicotine polacrilex gum with supportive measures. Archives of Internal Medicine 1989;149:1818-21. [DOI] [PubMed] [Google Scholar]
2 NRT Blondal 1997 {published data only}
- Blondal T, Franzon M, Westin A. A double-blind randomized trial of nicotine nasal spray as an aid in smoking cessation. European Respiratory Journal 1997;10:1585-90. [DOI] [PubMed] [Google Scholar]
- Blondal T, Olafsdottir I, Gunnarsdottir R, Franzon M, Westin A. Controlled trial of nicotine nasal spray as an aid to stopping smoking [abstract]. European Respiratory Journal 1993;6(Suppl 17):631S. [Google Scholar]
2 NRT Bolin 1999 {published data only}
- Bolin LJ, Antonuccio DO, Follette WC, Krumpe P. Transdermal nicotine: the long and the short of it. Psychology of Addictive Behaviors 1999;13:152-6. [Google Scholar]
2 NRT Br Thor Soc 83 {published data only}
- Research Committee of the British Thoracic Society. Comparison of four methods of smoking withdrawal in patients with smoking related diseases. Report by a subcommittee of the Research Committee of the British Thoracic Society. British Medical Journal 1983;286(6365):595-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
2 NRT Buchkremer 88 {published data only}
- Buchkremer G, Bents H, Horstmann M, Opitz K, Tolle R. Combination of behavioral smoking cessation with transdermal nicotine substitution. Addictive Behaviors 1989;14:229-38. [DOI] [PubMed] [Google Scholar]
- Buchkremer G, Bents H, Minneker E, Opitz K. Long-term effects of a combination of transdermal nicotine administration with behavior therapy for smoking cessation [Langfristige Effekte einer Kombination von transdermaler Nikotinzufuhr mit Verhaltenstherapie zur Raucherentwohnung]. Nervenarzt 1988;59:488-90. [PubMed] [Google Scholar]
2 NRT Bullen 2010 {published data only}
- Bullen C, Howe C, Lin RB, Grigg M, Laugesen M, McRobbie H, Glover M, et al. Pre-cessation nicotine replacement therapy: pragmatic randomized trial. Addiction 2010;105:1474-83. [DOI] [PubMed] [Google Scholar]
2 NRT Campbell 1987 {published data only}
- Campbell IA, Lyons E, Prescott R. Do nicotine chewing-gum and postal encouragement add to doctors' advice. Practitioner 1987;231:114-7. [PubMed] [Google Scholar]
2 NRT Campbell 1991 {published data only}
- Campbell IA, Prescott RJ, Tjeder-Burton SM. Smoking cessation in hospital patients given repeated advice plus nicotine or placebo chewing gum. Respiratory Medicine 1991;85:155-7. [DOI] [PubMed] [Google Scholar]
2 NRT Campbell 1996 {published data only}
- Burton S, Campbell IA, Prescott RJ. Nicotine patches versus placebo in 235 hospital patients [abstract 191]. In: 8th World Conference on Tobacco or Health, Mar 30-Apr 3; Buenos Aires, Argentina. 1992.
- Campbell IA, Prescott RJ, Tjeder-Burton SM. Transdermal nicotine plus support in patients attending hospital with smoking-related diseases: a placebo-controlled study. Respiratory Medicine 1996;90:47-51. [DOI] [PubMed] [Google Scholar]
2 NRT Cinciripini 96 {published data only}
- Cinciripini PM, Cinciripini LG, Wallfisch A, Haque W. Behavior therapy and the transdermal nicotine patch: effects on cessation outcome, affect, and coping. Journal of Consulting and Clinical Psychology 1996;64:314-23. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Wetter DW, Fouladi RT, Blalock JA, Carter BL, Cinciripini LG, et al. The effects of depressed mood on smoking cessation: mediation by postcessation self-efficacy. Journal of Consulting and Clinical Psychology 2003;71:292-301. [DOI] [PubMed] [Google Scholar]
2 NRT Clavel 1985 {published data only}
- Clavel F, Benhamou S, Company Huertas A, Flamant R. Helping people to stop smoking: randomised comparison of groups being treated with acupuncture and nicotine gum with control group. British Medical Journal 1985;291:1538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel F, Benhamou S. Tobacco withdrawal. Comparison of the efficacy of various methods. Intermediate results of a comparative study [French] [Désintoxcation tabagique. Comparison de l'efficacité de differentes méthodes. Résultats intermédiaires d'une étude comparative]. Presse Médicale 1984;13:975-7. [PubMed] [Google Scholar]
2 NRT Clavel‐Cha '92 {published data only}
- Clavel-Chapelon F, Paoletti C, Benhamou S. A randomised 2 x 2 factorial design to evaluate different smoking cessation methods. Revue d'Epidemiologie et de Sante Publique 1992;40:187-90. [PubMed] [Google Scholar]
2 NRT Croghan 2003 {published data only}
- Croghan GA, Hurt RD, Croghan IT, Sloan J, Novotny P, Loprinzi C. Comparison of a 15 mg transdermal nicotine patch alone versus nicotine nasal spray alone versus both for smoking cessation. Journal of Addictive Diseases 1998;17:121. [Google Scholar]
- Croghan GA, Sloan JA, Croghan IT, Novotny P, Hurt RD, DeKrey WL, et al. Comparison of nicotine patch alone versus nicotine nasal spray alone versus a combination for treating smokers: a minimal intervention, randomized multicenter trial in a nonspecialized setting. Nicotine & Tobacco Research 2003;5(2):181-7. [DOI] [PubMed] [Google Scholar]
2 NRT Croghan 2007 {published data only}
- Croghan IT, Hurt RD, Dakhil SR, Croghan GA, Sloan JA, Novotny PJ, et al. Randomized comparison of a nicotine inhaler and bupropion for smoking cessation and relapse prevention. Mayo Clinic.Proceedings 2007;82:186-95. [DOI] [PubMed] [Google Scholar]
2 NRT Daughton 1991 {published data only}
- Daughton DM, Heatley SA, Prendergast JJ, Causey D, Knowles M, Rolf CN, et al. Effect of transdermal nicotine delivery as an adjunct to low-intervention smoking cessation therapy. A randomized, placebo-controlled, double-blind study. Archives of Internal Medicine 1991;151:749-52. [PubMed] [Google Scholar]
- Daughton DM, Heatley SA, Prendergast JJ, Causey D, Knowles M, Rolf CN, et al. Effects of transdermal nicotine as an adjunct in smoking cessation therapy. A double-blind randomized study controlled with placebo [Italian] [Effetti del rilascio transdermico di nicotina come terapia di supporto per lo svezzamento dal fumo di sigaretta. Uno studio randomizzato in doppio cieco con controlli trattati con placebo.]. Archivio Monaldi 1992;47:17-29. [PubMed] [Google Scholar]
2 NRT Daughton 1998 {published data only}
- Daughton D, Susman J, Sitorius M, Belenky S, Millatmal T, Nowak R, et al. Transdermal nicotine therapy and primary care. Importance of counseling, demographic, and participant selection factors on 1-year quit rates. The Nebraska Primary Practice Smoking Cessation Trial Group. Archives of Family Medicine 1998;7:425-30. [DOI] [PubMed] [Google Scholar]
2 NRT Dautzenberg 01 {published data only}
- Dautzenberg B, Peiffer G, Toulouse F, Yvinec MJ, Jacob N, Kienzler JL. Randomized trial assessment of nicotinell lozenge 1mg, a new oral nicotine replacement therapy. In: Society for Research on Nicotine and Tobacco 3rd European Conference, Paris. 2001:55.
- Dautzenberg B, Ruff F, Vaucher M, Maillon P, Jacob N, Kienzler JL, et al. First demonstration of the good efficacy/safety ratio of Nicotinell(r) 1mg Lozenge (NL 1mg), a new form of nicotine substitution, by randomised clinical trial. European Respiratory Journal 2001;18(Suppl 33):12s. [Google Scholar]
2 NRT Davidson 1998 {published data only}
- Davidson M, Epstein M, Burt R, Schaefer C, Whitworth G, McDonald A. Efficacy and safety of an over-the-counter transdermal nicotine patch as an aid for smoking cessation. Archives of Family Medicine 1998;7:569-74. [DOI] [PubMed] [Google Scholar]
2 NRT Etter 2009 {published data only}
- Etter JF, Huguelet P, Perneger TV, Cornuz, J. Nicotine gum treatment before smoking cessation: a randomized trial. Archives of Internal Medicine 2009;169:1028-34. [DOI] [PubMed] [Google Scholar]
2 NRT Fagerström 1982 {published data only}
- Fagerström KO. A comparison of psychological and pharmacological treatment in smoking cessation. Journal of Behavioral Medicine 1982;5:343-51. [DOI] [PubMed] [Google Scholar]
- Fagerström KO. Tolerance, withdrawal and dependence on tobacco and smoking termination. International Review of Applied Psychology 1983;32:29-52. [Google Scholar]
2 NRT Fagerström 1984 {published data only}
- Fagerström KO. Effects of nicotine chewing gum and follow-up appointments in physician-based smoking cessation. Preventive Medicine 1984;13:517-27. [DOI] [PubMed] [Google Scholar]
2 NRT Fee 1982 {published data only}
- Fee WM, Stewart MJ. A controlled trial of nicotine chewing gum in a smoking withdrawal clinic. Practitioner 1982;226:148-51. [PubMed] [Google Scholar]
2 NRT Fortmann 1995 {published data only}
- Fortmann SP, Killen JD. Nicotine gum and self-help behavioral treatment for smoking relapse prevention - resuIts from a trial using population-based recruitment. Journal of Consulting and Clinical Psychology 1995;63:460-8. [DOI] [PubMed] [Google Scholar]
2 NRT Garcia 1989 {published data only}
- Quilez Garcia C, Hernando Arizaleta L, Rubio Diaz A, Estruch Riba J, Fornes Ramis MV. Treatment for smoking with nicotine gum in primary care. A double blind trial [Tratamiento del tabaquismo, con chicle de nicotina, en atencion primaria. Estudio a doble ciego]. Revista Clinica Español 1993;192(4):157-61. [PubMed] [Google Scholar]
- Quilez Garcia C, Hernando Arizaleta L, Rubio Diaz A, Granero Fernandez EJ, Vila Coll MA, Estruch Riba JS. Double-blind study of the efficacy of nicotine chewing gum for smoking cessation in the primary care setting [Estudio doble ciego de la eficacia del chicle de nicotina en la deshabituacion tabaquica dentro del ambito de la atencion primaria]. Atencion Primaria 1989;6:719-26. [PubMed] [Google Scholar]
2 NRT Gilbert 1989 {published data only}
- Gilbert JR, Wilson DM, Best JA, Taylor DW, Lindsay EA, Singer J, et al. Smoking cessation in primary care. A randomized controlled trial of nicotine-bearing chewing gum. Journal of Family Practice 1989;28:49-55. [PubMed] [Google Scholar]
2 NRT Glavas 2003a {published data only}
- Glavas D, Rumboldt M, Rumboldt Z. Smoking cessation with nicotine replacement therapy among health care workers: randomized double-blind study. Croatian Medical Journal 2003;44:219-24. [PubMed] [Google Scholar]
2 NRT Glavas 2003b {published data only}
- Glavas D, Rumboldt Z. Smoking cessation using the transdermal nicotine system [Odvikavanje od pusenja transdermalnim nikotinskim sustavom]. Lijecnicki Vjesnik 2003;125(1-2):8-12. [PubMed] [Google Scholar]
2 NRT Glover 2002 {published data only}
- Glover E, Glover P, Franzon M, Sullivan R, Cerullo C. Safety and efficacy of a nicotine sublingual tablet for smoking cessation [abstract]. In: Smoke Free 21st Century, 2nd European Conference on Tobacco or Health; Las Palmas de Gran Canaria. 1999.
- Glover ED, Franzon M, Sullivan CR, Cerullo CL. A nicotine sublingual tablet for treating tobacco dependence [Abstract]. In: Society for Research on Nicotine and Tobacco 3rd Europe Conference, Paris September 2001. 2001:48.
- Glover ED, Glover PN, Franzon M, Sullivan CR, Cerullo CC, Howell RM, et al. A comparison of a nicotine sublingual tablet and placebo for smoking cessation. Nicotine & Tobacco Research 2002;4:441-50. [DOI] [PubMed] [Google Scholar]
- Glover ED, Glover PN, Franzon M, Sullivan R, Sullivan P, Howell R, et al. A nicotine sublingual tablet for smoking cessation: 6-month data [Abstract]. In: Proceedings of the 10th World Conference on Tobacco or Health; Aug 24-28 Beijing, China. 1997.
2 NRT Goldstein 1989 {published data only}
- Goldstein MG, Niaura R, Follick MJ, Abrams DB. Effects of behavioral skills training and schedule of nicotine gum administration on smoking cessation. American Journal of Psychiatry 1989;146:56-60. [DOI] [PubMed] [Google Scholar]
2 NRT Hall 1985 {published data only}
- Hall SM, Tunstall C, Rugg D, Jones R, Benowitz N. Nicotine gum and behavioral treatment in smoking cessation. Journal of Consulting and Clinical Psychology 1985;53:256-8. [DOI] [PubMed] [Google Scholar]
2 NRT Hall 1987 {published data only}
- Hall SM, Tunstall CD, Ginsberg D, Benowitz NL, Jones RT. Nicotine gum and behavioral treatment: a placebo controlled trial. Journal of Consulting and Clinical Psychology 1987;55:603-5. [DOI] [PubMed] [Google Scholar]
2 NRT Hall 1996 {published data only}
- Hall SM, Muñoz RF, Reus VI, Sees KL, Duncan C, Humfleet GL, et al. Mood management and nicotine gum in smoking treatment - a therapeutic contact and placebo-controlled study. Journal of Consulting and Clinical Psychology 1996;64:1003-9. [DOI] [PubMed] [Google Scholar]
2 NRT Hand 2002 {published data only}
- Hand S, Edwards S, Campbell IA, Cannings R. Controlled trial of three weeks nicotine replacement treatment in hospital patients also given advice and support. Thorax 2002;57:715-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
2 NRT Harackiewicz 1988 {published data only}
- Harackiewicz JM, Blair LW, Sansone C, Epstein JA, Stuchell RN. Nicotine gum and self-help manuals in smoking cessation: an evaluation in a medical context. Addictive Behaviors 1988;13:319-30. [DOI] [PubMed] [Google Scholar]
2 NRT Hatsukami 2007 {published data only}
- Hatsukami D, Mooney M, Murphy S, LeSage M, Babb D, Hecht S. Effects of high dose transdermal nicotine replacement in cigarette smokers. Pharmacology, Biochemistry and Behavior 2007;86:132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
2 NRT Hays 1999 {published data only}
- Hays JT, Croghan GA, Offord KP, Hurt RD, Schroeder DR, Wolter TD, et al. Over-the-counter nicotine patch therapy for smoking cessation: Results from randomized, double-blind, placebo-controlled and open label trials. American Journal of Public Health 1999;89:1701-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays JT, Croghan GA, Offord KP, Wolter TD, Nides MA, Davidson M. Over-the-Counter (OTC) transdermal nicotine patch therapy [abstract]. Journal of Addictive Diseases 1997;16:136. [Google Scholar]
- Hays JT, Croghan IT, Offord KP, Hurt RD, Schroeder DR, Wolter TD, et al. Over the counter 22mg nicotine patch therapy for smoking cessation: results from randomized double-blind placebo-controlled and open label trials. Society for Research on Nicotine and Tobacco 5th Annual Meeting, San Diego (CA) 1999. [DOI] [PMC free article] [PubMed]
2 NRT Herrera 1995 {published data only}
- Herrera N, Franco R, Herrera L, Partidas A, Rolando R, Fagerström KO. Nicotine gum, 2 and 4 mg, for nicotine dependence. A double-blind placebo-controlled trial within a behavior modification support program. Chest 1995;108:447-51. [DOI] [PubMed] [Google Scholar]
2 NRT Hilleman 1994 {published data only}
- Hilleman DE, Mohiuddin SM, Delcore MG. Comparison of fixed-dose transdermal nicotine, tapered-dose transdermal nicotine, and buspirone in smoking cessation. Journal of Clinical Pharmacology 1994;34(3):222-4. [DOI] [PubMed] [Google Scholar]
2 NRT Huber 1988 {published data only}
- Huber D. Combined and separate treatment effects of nicotine chewing gum and self-control method. Pharmacopsychiatry 1988;21:461-2. [DOI] [PubMed] [Google Scholar]
2 NRT Hughes 1989 {published data only}
- Hughes JR, Gust SW, Keenan RM, Fenwick JW, Healey ML. Nicotine vs placebo gum in general medical practice. JAMA 1989;261:1300-5. [PubMed] [Google Scholar]
2 NRT Hughes 1990 {published data only}
- Hughes JR, Gust SW, Keenan RM, Fenwick JW. Effect of dose on nicotine's reinforcing, withdrawal-suppression and self-reported effects. Journal of Pharmacology and Experimental Therapeutics 1990;252:1175-83. [PubMed] [Google Scholar]
2 NRT Hughes 1991 {published data only}
- Hughes JR, Wadland WC, Fenwick JW, Lewis J, Bickel WK. Effect of cost on the self-administration and efficacy of nicotine gum: a preliminary study. Preventive Medicine 1991;20:486-96. [DOI] [PubMed] [Google Scholar]
2 NRT Hughes 1999 {published data only}
- Hughes JR, Lesmes GR, Hatsukami DK, Richmond RL, Lichtenstein E, Jorenby DE, et al. Are higher doses of nicotine replacement more effective for smoking cessation? Nicotine & Tobacco Research 1999;1:169-74. [DOI] [PubMed] [Google Scholar]
2 NRT Hughes 2003 {published data only}
- Hughes JR, Novy P, Hatsukami DK, Jensen J, Callas PW. Efficacy of nicotine patch in smokers with a history of alcoholism. Alcoholism, Clinical and Experimental Research 2003;27:946-54. [DOI] [PubMed] [Google Scholar]
2 NRT Hurt 1990 {published data only}
- Hurt RD, Lauger GG, Offord KP, Kottke TE, Dale LC. Nicotine-replacement therapy with use of a transdermal nicotine patch-a randomized double-blind placebo-controlled trial. Mayo Clinic Proceedings 1990;65:1529-37. [DOI] [PubMed] [Google Scholar]
2 NRT Hurt 1994 {published data only}
- Hurt RD, Dale LC, Fredrickson PA, Caldwell CC, Lee GA, Offord KP, et al. Nicotine patch therapy for smoking cessation combined with physician advice and nurse follow-up: One-year outcome and percentage of nicotine replacement. JAMA 1994;271:595-600. [PubMed] [Google Scholar]
2 NRT ICRF 2007 {published data only}
- David SP, Munafò MR, Murphy MF, Walton RT, Johnstone EC. The serotonin transporter 5-HTTLPR polymorphism and treatment response to nicotine patch: follow-up of a randomized controlled trial. Nicotine & Tobacco Research 2007;9(2):225-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperial Cancer Research Fund General Practice Research Group. Effectiveness of a nicotine patch in helping people stop smoking: results of a randomised trial in general practice. BMJ 1993;306:1304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperial Cancer Research Fund General Practice Research Group. Randomised trial of nicotine patches in general practice: results at one year. BMJ 1994;308:1476-7. [PMC free article] [PubMed] [Google Scholar]
- Johnstone EC, Yudkin Pl, Hey K, Roberts SJ, Welch SJ, Murphy MF, et al. Genetic variation in dopaminergic pathways and short-term effectiveness of the nicotine patch. Pharmacogenetics 2004;14(2):83-90. [DOI] [PubMed] [Google Scholar]
- Yudkin P, Hey K, Roberts S, Welch S, Murphy M, Walton R. Abstinence from smoking eight years after participation in randomised controlled trial of nicotine patch. BMJ 2003;327(7405):28-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin P, Munafò M, Hey K, Roberts S, Welch S, Johnstone E, et al. Effectiveness of nicotine patches in relation to genotype in women versus men: randomised controlled trial. BMJ 2004;328(7446):989-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
2 NRT Jamrozik 1984 {published data only}
- Jamrozik K, Fowler G, Vessey M, Wald N. Placebo controlled trial of nicotine chewing gum in general practice. British Medical Journal 1984;289:794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
2 NRT Jarvis 1982 {published data only}
- Jarvis MJ, Raw M, Russell MA, Feyerabend C. Randomised controlled trial of nicotine chewing-gum. British Medical Journal 1982;285:537-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
2 NRT Jensen 1991 {published data only}
- Jensen EJ, Schmidt E, Pedersen B, Dahl R. Effect of nicotine, silver acetate, and ordinary chewing gum in combination with group counselling on smoking cessation. Thorax 1990;45:831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen EJ, Schmidt E, Pedersen B, Dahl R. Effect on smoking cessation of silver acetate, nicotine and ordinary chewing gum. Influence of smoking history. Psychopharmacology (Berl) 1991;104:470-4. [DOI] [PubMed] [Google Scholar]
- Jensen EJ, Schmidt E, Pedersen B, Dahl R. The effect of nicotine, silver acetate, and placebo chewing gum on the cessation of smoking. The influence of smoking type and nicotine dependence. International Journal of the Addictions 1991;26:1223-31. [DOI] [PubMed] [Google Scholar]
2 NRT Jorenby 1995 {published data only}
- Jorenby DE, Smith SS, Fiore MC, Hurt RD, Offord KP, Crogham IT, et al. Varying nicotine patch dose and type of smoking cessation counseling. JAMA 1995;274:1347-52. [PubMed] [Google Scholar]
2 NRT Jorenby 1999 {published data only}
- Durcan MJ, White J, Jorenby DE, Fiore MC, Rennard SI, Leischow SJ, et al. Impact of prior nicotine replacement therapy on smoking cessation efficacy. American Journal of Health Behaviors 2002;26:213-20. [DOI] [PubMed] [Google Scholar]
- Jamerson BD, Nides M, Jorenby DE, Donahue R, Garrett P, Johnston JA, et al. Late-term smoking cessation despite initial failure: an evaluation of bupropion sustained release, nicotine patch, combination therapy, and placebo. Clinical Therapeutics 2001;23:744-52. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. New England Journal of Medicine 1999;340:685-91. [DOI] [PubMed] [Google Scholar]
2 NRT Joseph 1996 {published data only}
- Joseph AM, Antonnucio DO. Lack of efficacy of transdermal nicotine in smoking cessation. New England Journal of Medicine 1999;341:1157-8. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Norman SM, Ferry LH, Prochazka AV, Westman EC, Steele BG, et al. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. New England Journal of Medicine 1996;335:1792-8. [DOI] [PubMed] [Google Scholar]
2 NRT Kalman 2006 {published data only}
- Kalman D, Denison H, Penk W, Peer J, Kresman D, Monti P. Early findings from a treatment study of heavy smokers in alcohol recovery (PO2 34). In: Society for Research on Nicotine and Tobacco 7th Annual Meeting March 23-23 Seattle, Washington. 2001:61.
- Kalman D, Kahler CW, Garvey AJ, Monti PM. High-dose nicotine patch therapy for smokers with a history of alcohol dependence: 36-week outcomes. Journal of Substance Abuse Treatment 2006;30:213-7. [DOI] [PubMed] [Google Scholar]
- Kalman D, Kahler CW, Tirch D, Kaschub C, Penk W, Monti PM. Twelve-week outcomes from an investigation of high-dose nicotine patch therapy for heavy smokers with a past history of alcohol dependence. Psychology of Addictive Behaviors 2004;18:78-82. [DOI] [PubMed] [Google Scholar]
- Kalman D, Tirch D, Penk W, Denison H. An investigation of predictors of nicotine abstinence in a smoking cessation treatment study of smokers with a past history of alcohol dependence. Psychology of Addictive Behaviors 2002;16:346-9. [PubMed] [Google Scholar]
- Kalman D, Tirch D, Penk W, Kaschub C. Preliminary findings from a treatment study of heavy smokers in alcohol recovery: end of treatment outcomes (PO2 38). In: Society for Research on Nicotine and Tobacco 8th Annual Meeting February 20-23 Savannah, Georgia. 2002:58.
2 NRT Killen 1984 {published data only}
- Killen JD, Maccoby N, Taylor CB. Nicotine gum and self-regulation training in smoking relapse prevention. Behavior Therapy 1984;15:234-48. [Google Scholar]
2 NRT Killen 1990 {published data only}
- Fortmann SP, Killen JD, Telch MJ, Newman B. Minimal contact treatment for smoking cessation. A placebo controlled trial of nicotine polacrilex and self-directed relapse prevention: initial results of the Stanford Stop Smoking Project. JAMA 1988;260:1575-80. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Newman B, Varady A. Evaluation of a treatment approach combining nicotine gum with self-guided behavioral treatments for smoking relapse prevention. Journal of Consulting and Clinical Psychology 1990;58:85-92. [DOI] [PubMed] [Google Scholar]
2 NRT Killen 1997 {published data only}
- Killen JD, Fortmann SP, Davis L, Varady A. Nicotine patch and self-help video for cigarette smoking cessation. Journal of Consulting and Clinical Psychology 1997;65:663-72. [DOI] [PubMed] [Google Scholar]
2 NRT Killen 1999 {published data only}
- Killen JD, Fortmann SP, Davis L, Strausberg L, Varady A. Do heavy smokers benefit from higher dose nicotine patch therapy? Experimental and Clinical Psychopharmacology 1999;7:226-33. [DOI] [PubMed] [Google Scholar]
2 NRT Kornitzer 1987 {published data only}
- Kornitzer M, Kittel F, Dramaix M, Bourdoux P. A double blind study of 2 mg versus 4 mg nicotine-gum in an industrial setting. Journal of Psychosomatic Research 1987;31:171-6. [DOI] [PubMed] [Google Scholar]
2 NRT Kornitzer 1995 {published data only}
- Kornitzer M, Boutsen M, Dramaix M, Thijs J, Gustavsson G. Combined use of nicotine patch and gum in smoking cessation: a placebo-controlled clinical trial. Preventive Medicine 1995;24:41-7. [DOI] [PubMed] [Google Scholar]
- Kornitzer M, Boutsen M, Thijs J, Gustavsson G. Efficiency and safety of combined use of nicotine patches and nicotine gum in smoking cessation: a placebo controlled double-blind trial [abstract]. European Respiratory Journal 1993;6(Suppl 17):630S. [Google Scholar]
2 NRT Kralikova 2002 {published data only}
- Kralikova E, Kozak J, Rasmussen T, Cort N. The clinical benefits of NRT-supported smoking reduction. Nicotine & Tobacco Research 2002;4:243. [Google Scholar]
2 NRT Kralikova 2009 {published data only}
- Kralikova E, Kozak JT, Rasmussen T, Gustavsson G, Le Houezec, J. Smoking cessation or reduction with nicotine replacement therapy: a placebo-controlled double blind trial with nicotine gum and inhaler. BMC.Public Health 2009;9:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
2 NRT Leischow 1996 {published data only}
- Leischow SJ, Nilsson F, Franzon M, Hill A, Otte P, Merikle EP. Efficacy of the nicotine inhaler as an adjunct to smoking cessation. American Journal of Health Behavior 1996;20:364-71. [Google Scholar]
2 NRT Leischow 1999 {published data only}
- Leischow SJ, Muramoto ML, Cook GN, Merikle EP, Castellini SM, Otte PS. OTC nicotine patch: effectiveness alone and with brief physician intervention. American Journal of Health Behavior 1999;23:61-9. [Google Scholar]
2 NRT Leischow 2004 {published data only}
- Leischow SJ, Ranger-Moore J, Muramoto ML, Matthews E. Effectiveness of the nicotine inhaler for smoking cessation in an OTC setting. American Journal of Health Behavior 2004;28:291-301. [DOI] [PubMed] [Google Scholar]
- Leischow SJ, Ranger-Moore J, Muramoto ML, Matthews E. The safety and effectiveness of the nicotine inhaler for smoking cessation in an over-the-counter setting (POS4-78). In: Society for Research on Nicotine and Tobacco 9th Annual Meeting, New Orleans, LA. 2003:100.
2 NRT Lewis 1998 {published data only}
- Lewis SF, Piasecki TM, Fiore MC, Anderson JE, Baker TB. Transdermal nicotine replacement for hospitalized patients: a randomized clinical trial. Preventive Medicine 1998;27(2):296-303. [DOI] [PubMed] [Google Scholar]
2 NRT Llivina 1988 {published data only}
- Salvador Llivina T, Marin Tuya D, Gonzalez Quintana J, Iniesta Torres C, Castellvi Barrera E, Muriana Saez C, et al. Treatment of smoking: efficacy of the use of nicotine chewing gum. Double-blind study [Tratamiento del tabaquismo: eficacia de la utilizacion del chicle de nicotina. Estudio a doble ciego]. Medicina Clinica Barcelona 1988;90:646-50. [PubMed] [Google Scholar]
2 NRT Malcolm 1980 {published data only}
- Malcolm RE, Sillett RW, Turner JA, Ball KP. The use of nicotine chewing gum as an aid to stopping smoking. Psychopharmacology Series 1980;70:295-6. [DOI] [PubMed] [Google Scholar]
2 NRT Marshall 1985 {published data only}
- Marshall A, Raw M. Nicotine chewing gum in general practice: effect of follow up appointments. British Medical Journal 1985;290:1397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
2 NRT McGovern 1992 {published data only}
- McGovern PG, Lando HA. An assessment of nicotine gum as an adjunct to Freedom from Smoking cessation clinics. Addictive Behaviors 1992;17:137-47. [DOI] [PubMed] [Google Scholar]
2 NRT Molyneux 2003 {published data only}
- Molyneux A, Lewis S, Leivers U, Anderton A, Antoniak M, Brackenridge A, et al. Clinical trial comparing nicotine replacement therapy (NRT) plus brief counselling, brief counselling alone, and minimal intervention on smoking cessation in hospital inpatients. Thorax 2003;58(6):484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
2 NRT Moolchan 2005 {published data only}
- Franken FH, Pickworth WB, Epstein DH, Moolchan ET. Smoking rates and topography predict adolescent smoking cessation following treatment with nicotine replacement therapy. Cancer Epidemiology, Biomarkers & Prevention 2006;15:154-7. [DOI] [PubMed] [Google Scholar]
- Moolchan ET, Robinson ML, Ernst M, Cadet JL, Pickworth WB, Heishman SJ, et al. Safety and efficacy of the nicotine patch and gum for the treatment of adolescent tobacco addiction. Pediatrics 2005;115(4):e407-e414. [DOI] [PubMed] [Google Scholar]
- Robinson ML, Schroeder JR, Moolchan ET. Adolescent smokers screened for a nicotine replacement treatment trial: Correlates of eligibility and enrollment. Nicotine & Tobacco Research 2006;8:447-54. [DOI] [PubMed] [Google Scholar]
2 NRT Mori 1992 {published data only}
- Mori T, Shimao T, Yulchiro G, Namiki M, Hyachi T. A clinical trial of nicotine chewing gum for smoking cessation [abstract 428]. 8th World Conference on Tobacco or Health; Buenos Aires, Argentina 1992.
2 NRT Müller 1990 {published data only}
- Müller P, Abelin T, Eshram R, Imhof P, Howald H, Mauli D. The use of transdermal nicotine in smoking cessation. Lung 1990;168:445-53. [DOI] [PubMed] [Google Scholar]
2 NRT Nakamura 1990 {published data only}
- Nakamura M, Saito J, Oshima A, Miyamoto M, Matushita A, Endo S. Effect of nicotine chewing gun in smoking cessation classes. In: Durston B, Jamrozik K , editors(s). The Global War. Proceedings of the 7th World Conference on Tobacco and Health; Perth, Western Australia. Perth: Health Department of Western Australia, 1990:665-7.
2 NRT Nebot 1992 {published data only}
- Nebot M, Cabezas C. Does nurse counseling or offer of nicotine gum improve the effectiveness of physician smoking-cessation advice? Family Practice Research Journal 1992;12:263-70. [PubMed] [Google Scholar]
2 NRT Niaura 1994 {published data only}
- Niaura R, Goldstein MG, Abrams DB. Matching high and low-dependence smokers to self-help treatment with or without nicotine replacement. Preventive Medicine 1994;23:70-7. [DOI] [PubMed] [Google Scholar]
2 NRT Niaura 1999 {published data only}
- Niaura R, Abrams DB, Shadel WG, Rohsenow DJ, Monti PM, Sirota AD. Cue exposure treatment for smoking relapse prevention: a controlled clinical trial. Addiction 1999;94(5):685-96. [DOI] [PubMed] [Google Scholar]
2 NRT Ockene 1991 {published data only}
- Ockene JK, Kristeller J, Goldberg R, Amick TL, Pekow PS, Hosmer D, et al. Increasing the efficacy of physician-delivered smoking interventions: a randomized clinical trial. Journal of General Internal Medicine 1991;6:1-8. [DOI] [PubMed] [Google Scholar]
- Ockene JK, Kristeller J, Pbert L, Hebert JR, Luippold R, Goldberg RJ, et al. The physician-delivered smoking intervention project: can short-term interventions produce long-term effects for a general outpatient population? Health Psychology 1994;13:278-81. [DOI] [PubMed] [Google Scholar]
2 NRT Oncken 2007 {published data only}
- Allen AM, Kleppinger A, Lando H, Oncken C. Effect of nicotine patch on energy intake and weight gain in postmenopausal women during smoking cessation. Eating Behaviors 2013;14(4):420-3. [CENTRAL: 918119] [EMBASE: 2013512499] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppinger A, Litt MD, Kenny AM, Oncken CA. Effects of smoking cessation on body composition in postmenopausal women. Journal of Women's Health 2010;19(9):1651-7. [CENTRAL: 793327] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oncken C, Cooney J, Feinn R, Lando H, Kranzler HR. Transdermal nicotine for smoking cessation in postmenopausal women. Addictive Behaviors 2007;32:296-309. [DOI] [PubMed] [Google Scholar]
- Oncken C, Cooney J, Lando H, Feinn R, Kranzler H. Transdermal nicotine for smoking cessation in postmenopausal women (POS1-045). In: Society for Research on Nicotine and Tobacco 11th Annual Meeting, Prague. 2005.
2 NRT Otero 2006 {published data only}
- Otero UB, Perez CA, Szklo M, Esteves GA, dePinho MM, Szklo AS, et al. Randomized clinical trial: effectiveness of the cognitive-behavioral approach and the use of nicotine replacement transdermal patches for smoking cessation among adults in Rio de Janeiro, Brazil [Ensaio clinico randomizado: efetividade da abordagem cognitivo-comportamental e uso de adesivos transdermicos de reposicao de nicotina, na cessacao de fumar, em adultos residentes no Municipio do Rio de Janeiro, Brasil]. Cadernos de Saude Publica 2006;22:439-49. [DOI] [PubMed] [Google Scholar]
2 NRT Page 1986 {published data only}
- Page AR, Walters DJ, Schlegel RP, Best JA. Smoking cessation in family practice: the effects of advice and nicotine chewing gum prescription. Addictive Behaviors 1986;11:443-6. [DOI] [PubMed] [Google Scholar]
2 NRT Paoletti 1996 {published data only}
- Paoletti P, Fornai E, Maggiorelli F, Puntoni R, Viegi G, Carrozzi L, et al. Importance of baseline cotinine plasma values in smoking cessation: results from a double blind study with nicotine patch. European Respiratory Journal 1996;9:643-51. [DOI] [PubMed] [Google Scholar]
2 NRT Peng 2007 {published data only}
- Peng RL, Wang SL. Nicotine sublingual tablet for smoking cessation in 115 cases: A double-blind randomized placebo-controlled clinical trial. Journal of Clinical Rehabilitative Tissue Engineering Research 2007;52:10443-6. [Google Scholar]
2 NRT Perng 1998 {published data only}
- Perng RP, Hsieh WC, Chen YM, Lu CC, Chiang SJ. Randomized, double-blind, placebo-controlled study of transdermal nicotine patch for smoking cessation. American Journal of Respiratory and Critical Care Medicine 1999;159(3SS):A735. [PubMed] [Google Scholar]
- Perng RP, Hsieh WC, Chen YM, Lu CC, Chiang SJ. Randomized, double-blind, placebo-controlled study of transdermal nicotine patch for smoking cessation. Journal of the Formosan Medical Association 1998;97:547-51. [PubMed] [Google Scholar]
2 NRT Piper 2007 {published data only}
- Piper ME, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. Mediators of bupropion treatment effects (PA5-6). In: Society for Research on Nicotine and Tobacco 12th Annual Meeting, Orlando, FL. 2006:27.
- Piper ME, Federman EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, et al. Efficacy of bupropion alone and in combination with nicotine gum. Nicotine & Tobacco Research 2007;9:947-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Federman EB, Smith SS, Fiore MC, Baker TB. Efficacy of bupropion SR alone and combined with 4-mg gum (PA2-2). In: Society for Research on Nicotine and Tobacco 10th Annual Meeting, Phoenix, AZ. 2004:18.
2 NRT Puska 1979 {published data only}
- Puska P, Bjorkqvist S, Koskela K. Nicotine-containing chewing gum in smoking cessation: a double blind trial with half year follow-up. Addictive Behaviors 1979;4:141-6. [DOI] [PubMed] [Google Scholar]
2 NRT Richmond 1993 {published data only}
- Richmond R, Heather N. General Practitioner interventions for smoking cessation: past results and future prospects. Behaviour Change 1990;7:110-9. [Google Scholar]
- Richmond RL, Makinson RJ, Giugni AA, Webster IW. General Practitioner smoking interventions in Australia: results of studies over the past ten years. In: Durston B, Jamrozik K , editors(s). The Global War: 7th World Conference on Tobacco and Health. Perth, Western Australia. Perth: Health Department of Western Australia, 1990:657-60.
- Richmond RL, Makinson RJ, Kehoe LA, Giugni AA, Webster IW. One-year evaluation of three smoking cessation interventions administered by general practitioners. Addictive Behaviors 1993;18:187-99. [DOI] [PubMed] [Google Scholar]
2 NRT Rose 1994 {published data only}
- Rose JE, Behm FM, Westman EC, Levin ED, Stein RM, Ripka GV. Mecamylamine combined with nicotine skin patch facilitates smoking cessation beyond nicotine patch treatment alone. Clinical Pharmacology and Therapeutics 1994;56:86-99. [DOI] [PubMed] [Google Scholar]
- Rose JE, Westman EC, Behm FM. Nicotine/mecamylamine combination treatment for smoking cessation [published erratum appears in Drug Development Research 1997;40:215]. Drug Development Research 1996;38:243-256. [Google Scholar]
2 NRT Rose 1998 {published data only}
- Rose JE, Behm FM, Westman EC. Nicotine-mecamylamine treatment for smoking cessation: the role of pre-cessation therapy. Experimental and Clinical Psychopharmacology 1998;6:331-43. [DOI] [PubMed] [Google Scholar]
- Rose JE, Westman EC, Behm FM. Nicotine/mecamylamine combination treatment for smoking cessation [published erratum appears in Drug Development Research 1997;40:215]. Drug Development Research 1996;38:243-56. [Google Scholar]
2 NRT Rose 2006 {published data only}
- Rose JE, Behm FM, Westman EC, Kukovich P. Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine & Tobacco Research 2006;8:89-101. [DOI] [PubMed] [Google Scholar]
2 NRT Rose 2009 {published data only}
- Rose JE, Herskovic JE, Behm FM, Westman EC. Precessation treatment with nicotine patch significantly increases abstinence rates relative to conventional treatment. Nicotine.and Tobacco.Research 2009;11:1067-75. [DOI] [PubMed] [Google Scholar]
2 NRT Roto 1987 {published data only}
- Roto P, Ojala A, Sundman K, Jokinen K, Peltomakl R. Nicotine gum and withdrawal from smoking. Suomen Laakarllehtl 1987;36:3445-8. [Google Scholar]
2 NRT Russell 1983 {published data only}
- Russell MA, Merriman R, Stapleton J, Taylor W. Effect of nicotine chewing gum as an adjunct to general practitioner's advice against smoking. British Medical Journal (Clinical Research Ed.) 1983;287:1782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
2 NRT Schneider 1985A {published data only}
- Jarvik ME, Schneider NG. Degree of addiction and effectiveness of nicotine gum therapy for smoking. American Journal of Psychiatry 1984;141:790-1. [DOI] [PubMed] [Google Scholar]
- Schneider NG, Jarvik ME, Forsythe AB, Read LL, Elliott ML, Schweiger A. Nicotine gum in smoking cessation: a placebo-controlled, double-blind trial. Addictive Behaviors 1983;8:253-61. [DOI] [PubMed] [Google Scholar]
- Schneider NG, Jarvik ME. Nicotine gum vs. placebo gum: comparisons of withdrawal symptoms and success rates. NIDA Research Monograph 1985;53:83-101. [PubMed] [Google Scholar]
2 NRT Schneider 1985B {published data only}
- Schneider NG, Jarvik ME, Forsythe AB, Read LL, Elliott ML, Schweiger A. Nicotine gum in smoking cessation: a placebo-controlled, double-blind trial. Addictive Behaviors 1983;8:253-61. [DOI] [PubMed] [Google Scholar]
- Schneider NG, Jarvik ME. Nicotine gum vs. placebo gum: comparisons of withdrawal symptoms and success rates. NIDA Research Monograph 1985;53:83-101. [PubMed] [Google Scholar]
2 NRT Schneider 1995 {published data only}
- Schneider NG, Olmstead R, Mody FV, Doan K, Franzon M, Jarvik ME, et al. Efficacy of a nicotine nasal spray in smoking cessation: a placebo-controlled, double-blind trial. Addiction 1995;90:1671-82. [DOI] [PubMed] [Google Scholar]
2 NRT Schneider 1996 {published data only}
- Schneider NG, Olmstead R, Nilsson F, Mody FV, Franzon M, Doan K. Efficacy of a nicotine inhaler in smoking cessation: a double-blind, placebo controlled trial. Addiction 1996;91:1293-306. [PubMed] [Google Scholar]
- Schneider NG, Olmstead R, Nilsson F, Mody FV, Franzon M, Doan K. Efficacy of a nicotine inhaler in smoking cessation: a double-blind, placebo-controlled trial [abstract]. Addiction 1997;92:630. [PubMed] [Google Scholar]
2 NRT Schnoll 2010 {published data only}
- Schnoll RA, Martinez E, Tatum KL, Glass M, Bernath A, Ferris D, et al. Nicotine patch vs. nicotine lozenge for smoking cessation: an effectiveness trial coordinated by the Community Clinical Oncology Program. Drug and Alcohol Dependence 2010;107:237-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto EP, Heitjan DF, Shields AE, Asch DA, et al. Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Annals.of Internal.Medicine 2010;152:144-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Wileyto EP, Lerman C. Extended duration therapy with transdermal nicotine may attenuate weight gain following smoking cessation. Addictive Behaviors 2012;37(4):565-8. [CENTRAL: 814662] [EMBASE: 2012097635] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
2 NRT Schuurmans 04 {published data only}
- Schuurmans MM, Diacon AH, Van Biljon X, Bolliger CT. Effect of pre-treatment with nicotine patch on withdrawal symptoms and abstinence rates in smokers subsequently quitting with the nicotine patch: a randomized controlled trial. Addiction 2004;99:634-40. [DOI] [PubMed] [Google Scholar]
- Schuurmans MM, Diacon AH, Van Biljon X, Westin A, Landfeldt B, Bolliger CT. Effect of pre-treatment with nicotine patch on withdrawal symptoms in smokers subsequently quitting with the nicotine patch: a double-blind randomised controlled trial (www.ersnetsecure.org/public/prg_congres.abstract?ww_i_presentation=6711). In: European Respiratory Society Annual Congress, Stockholm. 2002.
2 NRT Segnan 1991 {published data only}
- Segnan N, Ponti A, Battista RN, Senore C, Rosso S, Shapiro SH, et al. A randomized trial of smoking cessation interventions in general practice in Italy. Cancer Causes and Control 1991;2:239-46. [DOI] [PubMed] [Google Scholar]
- Senore C, Battista RN, Ponti A, Segnan N, Shapiro SH, Rosso S, et al. Comparing participants and nonparticipants in a smoking cessation trial: selection factors associated with general practitioner recruitment activity. Journal of Clinical Epidemiology 1999;52:83-9. [DOI] [PubMed] [Google Scholar]
- Senore C, Battista RN, Shapiro SH, Segnan N, Ponti A, Rosso S, et al. Predictors of smoking cessation following physicians' counseling. Preventive Medicine 1998;27:412-21. [DOI] [PubMed] [Google Scholar]
2 NRT Shiffman 2009 {published data only}
- Shiffman S, Ferguson SG, Strahs KR. Quitting by gradual smoking reduction using nicotine gum: a randomized controlled trial. American Journal of Preventive Medicine 2009;36:96-104. [DOI] [PubMed] [Google Scholar]
2 NRT Sonderskov 97 {published data only}
- Sonderskov J, Olsen J, Meillier L, Overvad OK, Sabroe S. The effect of transdermal nicotine patches in smoking cessation. A randomized trial in pharmacy customers in Denmark. Ugeskrift for Laeger 1999;161:593-7. [PubMed] [Google Scholar]
- Sonderskov J, Olsen J, Sabroe S, Meillier L, Overvad K. Nicotine patches in smoking cessation: a randomized trial among over- the-counter customers in Denmark. American Journal of Epidemiology 1997;145:309-18. [DOI] [PubMed] [Google Scholar]
2 NRT Stapleton 2011 {published data only}
- Stapleton JA, Sutherland G. Treating heavy smokers in primary care with the nicotine nasal spray: randomized placebo-controlled trial. Addiction 2011;106:824-32. [DOI] [PubMed] [Google Scholar]
2 NRT Tønnesen 1988 {published data only}
- Tønnesen P, Fryd V, Hansen M, Helsted J, Gunnersen AB, Forchammer H, et al. Effect of nicotine chewing gum in combination with group counseling on the cessation of smoking. New England Journal of Medicine 1988;318:15-8. [DOI] [PubMed] [Google Scholar]
2 NRT Tønnesen 2000 {published data only}
- Tønnesen P, Mikkelsen KL. Smoking cessation with four nicotine replacement regimes in a lung clinic. European Respiratory Journal 2000;16:717-22. [DOI] [PubMed] [Google Scholar]
2 NRT Tønnesen 2006 {published data only}
- Pbert L. Nurse-conducted smoking cessation in patients with COPD, using nicotine sublingual tablets and behavioral support. Chest 2006;130:314-6. [DOI] [PubMed] [Google Scholar]
- Tønnesen P, Mikkelsen K, Bremann L. Nurse-conducted smoking cessation in patients with COPD using nicotine sublingual tablets and behavioral support. Chest 2006;130:334-42. [DOI] [PubMed] [Google Scholar]
2 NRT Veaugh‐Geiss 2010 {published data only}
- Veaugh-Geiss AM, Chen LH, Kotler ML, Ramsay LR, Durcan MJ. Pharmacokinetic comparison of two nicotine transdermal systems, a 21-mg/24-hour patch and a 25-mg/16-hour patch: a randomized, open-label, single-dose, two-way crossover study in adult smokers. Clinical Therapeutics 2010;32:1140-8. [DOI] [PubMed] [Google Scholar]
2 NRT Villa 1999 {published data only}
- Villa RS, Alvarez AB, Hermida JR. Effectiveness of a multicomponent program to quit smoking with and without nicotine chewing gum [Eficacia de un programa multicomponente para dejar de fumar con y sin chicle de nicotina]. Psicologia Conductual 1999;7:107-18. [Google Scholar]
2 NRT Westman 1993 {published data only}
- Westman EC, Levin ED, Rose JE. The nicotine patch in smoking cessation. A randomized trial with telephone counseling. Archives of Internal Medicine 1993;153:1917-23. [PubMed] [Google Scholar]
2 NRT Wisborg 2000 {published data only}
- Wisborg K, Henriksen TB, Jespersen LB, Secher NJ. Nicotine patches for pregnant smokers: a randomized controlled study. Obstetrics & Gynecology 2000;96(6):967-71. [DOI] [PubMed] [Google Scholar]
2 NRT Wong 1999 {published data only}
- Wong GY, Wolter TD, Croghan GA, Croghan IT, Offord KP, Hurt RD. A randomized trial of naltrexone for smoking cessation. Addiction 1999;94:1227-37. [DOI] [PubMed] [Google Scholar]
2 NRT Zelman 1992 {published data only}
- Zelman DC, Brandon TH, Jorenby DE, Baker TB. Measures of affect and nicotine dependence predict differential response to smoking cessation treatments. Journal of Consulting and Clinical Psychology 1992;60:943-52. [DOI] [PubMed] [Google Scholar]
2 Prapavessis 2016 {published data only}
- Prapavessis H, De Jesus S, Fitzgeorge L, Faulkner G, Maddison R, Batten S. Exercise to enhance smoking cessation: the Getting Physical on Cigarette randomized control trial. Annals of Behavioral Medicine 2016;50(3):358-69. [DOI: 10.1007/s12160-015-9761-9] [DOI] [PubMed] [Google Scholar]
2 Urdapilleta‐Herrera 2013 {published data only}
- Urdapilleta-Herrera E, Piña-Rosales MF, Vargas-Rojas, MI, Ramírez-Venegas A, Sansores-Martínez R. Bupropion together with cognitive-conductual therapy (CBT) is an alternative for a long-term abstinence of smoking. In: European Respiratory Society Annual Congress; 2013 09 07-11; Barcelona. 2013.
2 VA Carson 2010 {published data only}
- Carson KV, Brinn MP, Smith BJ, Garside CG, Goldsworthy SJ, Fitridge RA, Esterman, et al. Varenicline tartrate and counselling versus counselling alone in a randomised controlled trial for inpatient smoking cessation: 3 month interim results [abstract]. Respirology 2010;15:p. A30. [Google Scholar]
2 VA Hajek 2011 {published data only}
- Hajek P, McRobbie HJ, Myers KE, Stapleton J, Dhanji AR. Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Archives.of Internal.Medicine 2011;171:770-7. [DOI] [PubMed] [Google Scholar]
2 VA Tsukahara 2010 {published data only}
- Tsukahara H, Noda K, Saku K. A randomized controlled open comparative trial of varenicline vs nicotine patch in adult smokers: efficacy, safety and withdrawal symptoms (the VN-SEESAW study). Circulation 2010;74:771-8. [DOI] [PubMed] [Google Scholar]
2 VA Williams 2007 {published and unpublished data}
- Reeves K, Watsky E, Williams K, Azoulay S, Billing B, Gong J. The safety of varenicline taken for 52 weeks for smoking cessation. In: [RPOS3-54]. 2008.
- Williams KE, Reeves KR, Billing CB, Pennington AM, Gong J. A double-blind study evaluating the long-term safety of varenicline for smoking cessation. Current Medical Research and Opinion 2007;23(4):793-801. [DOI] [PubMed]
References to ongoing studies
1 Komiyama 2018 {published data only}
- Komiyama M, Ozaki Y, Wada H, Yamakage H, Satoh-Asahara N, Morimoto T, et al. The effects of dietary instruction on cardiovascular risk markers after smoking cessation: study protocol for a multicenter randomized controlled trial in Japan. Trials 2018;19(1):538. [DOI: 10.1186/s13063-018-2919-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
1 NCT01892813 2013 {published data only}
- NCT01892813. Dissemination of a tailored tobacco quitline for rural veteran smokers. clinicaltrials.gov/ct2/show/NCT01892813 (first received 4 July 2013).
1 NCT02424188 2015 {published data only}
- NCT02424188. Trial of integrated smoking cessation, exercise and weight management in serious mental illness: TRIUMPH. clinicaltrials.gov/show/NCT02424188 (first received 22 April 2015).
1 NCT02906787 2016 {published data only}
- NCT02906787. Behavioral activation for smoking cessation and the prevention of post-cessation weight gain. clinicaltrials.gov/ct2/show/NCT02906787 (first received 20 September 2016).
1 NCT03204396 2017 {published data only}
- NCT03204396. Smoking cessation facilitated by glucagon-like peptide-1 (GLP-1) analogues (SKIP). clinicaltrials.gov/ct2/show/NCT03204396 (first received 2 July 2020).
1 NCT03712098 2018 {published data only}
- NCT03712098. Daily liraglutide for nicotine dependence. clinicaltrials.gov/ct2/show/NCT03712098 (first received 19 October 2018).
1 NCT04130698 2019 {published data only}
- NCT04130698. Comparing two treatments that both target smoking cessation and weight loss at the same time. clinicaltrials.gov/ct2/show/NCT04130698 (first received 17 October 2019).
1 NCT04332029 2020 {published data only}
- NCT04332029. Smoking cessation treatment for smokers with obesity. clinicaltrials.gov/ct2/show/NCT04332029 (first received 2 April 2020).
1 RBR‐682px9 2018 {published data only}
- RBR-682px9. Evaluation of the effect of dietary control in the treatment of female smokers. ensaiosclinicos.gov.br/rg/RBR-682px9 (first received 5 September 2018).
1 Salgado Garcia 2018 {published data only}
- Salgado García FI, Derefinko KJ, Bursac Z, Klesges RC, Ebbert JO, Womack CR, et al. Fit & quit: an efficacy trial of two behavioral post-cessation weight gain interventions. Contemporary Clinical Trials 2019;76:31-40. [DOI: 10.1016/j.cct.2018.11.009] [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Berlin 2019 {published data only}
- Berlin I, Dautzenberg B, Lehmann B, Palmyre J, Liégey E, De Rycke Y, et al. Randomised, placebo-controlled, double-blind, double-dummy, multicentre trial comparing electronic cigarettes with nicotine to varenicline and to electronic cigarettes without nicotine: the ECSMOKE trial protocol. BMJ open 2019;9(5):e028832. [DOI: 10.1136/bmjopen-2018-028832] [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Caponnetto 2019 {published data only}
- Caponnetto P, Maglia M, Polosa R. Efficacy of smoking cessation with varenicline plus counselling for e-cigarettes users (VAREVAPE): a protocol for a randomized controlled trial. Contemporary Clinical Trials communications 2019;15:100412. [DOI: 10.1016/j.conctc.2019.100412] [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Cinciripini 2009 {published data only}
- Cinciripini PM. Combining varenicline and bupropion for smoking cessation. clinicaltrials.gov/ct2/NCT00943618 (first received 22 July 2009).
2 Hebert‐Losier 2020 {published data only}
- Hébert-Losier A, Filion KB, Windle SB, Eisenberg MJ. A randomized controlled trial evaluating the efficacy of e-cigarette use for smoking cessation in the general population: E3 Trial Design. CJC Open 2020;2(3):168–75. [DOI: 10.1016/j.cjco.2020.03.006] [DOI] [PMC free article] [PubMed] [Google Scholar]
2 ISRCTN34399445 2020 {published data only}ISRCTN34399445
- ISRCTN34399445. Smoking cessation support for people with severe mental illness in South Asia. isrctn.com/ISRCTN34399445 (first received 11 December 2019).
2 NCT02106637 2014 {published data only}
- NCT02106637. Early In-hospital Initiation of pharmacotherapy for smoking cessation, concomitant with nurse-led support, in patients after an acute coronary syndrome (ACS). clinicaltrials.gov/show/NCT02106637 (first received 8 April 2014).
2 NCT02162849 2015 {published data only}
- NCT02162849. Reward sensitivity and pharmacotherapy for smoking cessation. clinicaltrials.gov/ct2/show/NCT02162849 (first received 14 June 2014).
2 NCT03722966 2018 {published data only}
- NCT03722966. Effectiveness of combination varenicline and oral nicotine replacement therapy (COMBO). clinicaltrials.gov/ct2/show/NCT03722966 (first received 29 October 2018).
2 NCT04088942 2019 {published data only}
- Saeed MI, Sivapalan P, Eklöf J, Ulrik CS, Pisinger C, Lapperre T, et al. TOB-STOP-COP (TOBacco STOP in COPd trial): study protocol-a randomized open-label, superiority, multicenter, two-arm intervention study of the effect of "high-intensity" vs. "low-intensity" smoking cessation intervention in active smokers with chronic obstructive pulmonary disease. Trials 2020;21(1):730. [DOI: 10.1186/s13063-020-04653-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
2 Roelsgaard 2017 {published data only}
- Roelsgaard IK, Thomsen T, Østergaard M, Christensen R, Hetland ML, Jacobsen S, et al. The effect of an intensive smoking cessation intervention on disease activity in patients with rheumatoid arthritis: study protocol for a randomised controlled trial. Trials 2017;18(1):570. [DOI: 10.1186/s13063-017-2309-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aubin, 2012
- Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ 2012;345:e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Audrain‐McGovern 2011
- Audrain-McGovern J, Benowitz NL. Cigarette smoking, nicotine, and body weight. Clinical Pharmacology and Therapeutics 2011;90(1):164-8. [DOI: 10.1038/clpt.2011.105] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bossé 1980
- Bossé R, Garvey AJ, Costa PT Jr. Predictors of weight change following smoking cessation. International Journal of the Addictions 1980;15(7):969-91. [DOI: 10.3109/10826088009040072] [DOI] [PubMed] [Google Scholar]
Cahill 2016
- Cahill K, Lindson‐Hawley N, Thomas KH, Fanshawe TR, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD006103. [DOI: 10.1002/14651858.CD006103.pub7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheskin 2005
- Cheskin LJ, Hess JM, Henningfield J, Gorelick DA. Calorie restriction increases cigarette use in adult smokers. Psychopharmacology (Berl) 2005;179(2):430-6. [DOI] [PubMed] [Google Scholar]
Chiolero 2008
- Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. American Journal of Clinical Nutrition 2008;87(4):801-9. [DOI: 10.1093/ajcn/87.4.801] [DOI] [PubMed] [Google Scholar]
Clair 2013
- Clair C, Rigotti NA, Porneala B, Fox CS, D'Agostino RB, Pencina MJ, et al. Association of smoking cessation and weight change with cardiovascular disease among adults with and without diabetes. JAMA 2013;309(10):1014-21. [DOI: 10.1001/jama.2013.1644] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 2004
- Clark MM, Decker PA, Offord KP, Patten CA, Vickers KS, Croghan IT, et al. Weight concerns among male smokers. Addictive Behaviors 2004;29(8):1637-41. [DOI] [PubMed] [Google Scholar]
Clark 2006
- Clark MM, Hurt RD, Croghan IT, Patten CA, Novotny P, Sloan JA, et al. The prevalence of weight concerns in a smoking abstinence clinical trial. Addictive Behaviors 2006;31(7):1142-52. [DOI] [PubMed] [Google Scholar]
Collins 1994
- Collins LC, Cornelius MF, Vogel RL, Walker JF, Stamford BA. Effect of caffeine and/or cigarette smoking on resting energy expenditure. International Journal of Obesity and Related Metabolic Disorders 1994;18(8):551-6. [PubMed] [Google Scholar]
Dallosso 1984
- Dallosso HM, James WP. The role of smoking in the regulation of energy balance. International Journal of Obesity 1984;8(4):365-75. [PubMed] [Google Scholar]
Davey Smith 2005
- Davey Smith G, Bracha Y, Svendson KH, Neaton JD, Haffner SM, Kuller LH. Incidence of type II diabetes in the Randomized Multiple Risk Factor Intervention Trial. Annals of Internal Medicine 2005;142(5):313-22. [DOI] [PubMed] [Google Scholar]
Fidler 2009
- Fidler J, West R. Self-perceived smoking motives and their correlates in a general population sample. Nicotine & Tobacco Research 2009;11(10):1182-8. [DOI] [PubMed] [Google Scholar]
Filozof 2004
- Filozof C, Fernandez Pinilla MC, Fernandez-Cruz A. Smoking cessation and weight gain. Obesity Reviews 2004;5(2):95-103. [DOI] [PubMed] [Google Scholar]
Gratziou 2009
- Gratziou C. Respiratory, cardiovascular and other physiological consequences of smoking cessation. Current Medical Research and Opinion 2009;25(2):535-45. [DOI: 10.1185/03007990802707642] [DOI] [PubMed] [Google Scholar]
Gritz 1988
- Gritz ER, Carr CR, Marcus AC. Unaided smoking cessation: the Great American Smokeout and new year's day quitters. Journal of Psychosocial Oncology 1988;6:217-34. [Google Scholar]
Hall 1986
- Hall SM, Ginsberg D, Jones RT. Smoking cessation and weight gain. Journal of Consulting and Clinical Psychology 1986;45(3):342-6. [DOI] [PubMed] [Google Scholar]
Hartmann‐Boyce 2018
- Hartmann‐Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD000146. [DOI: 10.1002/14651858.CD000146.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann‐Boyce 2020
- Hartmann-Boyce J, McRobbie H, Lindson N, Bullen C, Begh R, Theodoulou A, Notley C, Rigotti NA, Turner T, Butler AR, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD010216. [DOI: 10.1002/14651858.CD010216.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Hofstetter 1986
- Hofstetter A, Schutz Y, Jéquier E, Wahren J. Increased 24-hour energy expenditure in cigarette smokers. New England Journal of Medicine 1986;314(2):79-82. [DOI: 10.1056/NEJM198601093140204] [DOI] [PubMed] [Google Scholar]
Howes 2020
- Howes S, Hartmann-Boyce J, Livingstone-Banks J, Hong B, Lindson N. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD000031. [DOI: 10.1002/14651858.CD000031.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hu 2018
- Hu Y, Zong G, Liu G, Wang M, Rosner B, Pan A, et al. Smoking cessation, weight change, type 2 diabetes, and mortality. New England Journal of Medicine 2018;379(7):623-32. [DOI: 10.1056/NEJMoa1803626] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 1991
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal: a replication and extension. Archives of General Psychiatry 1991;84(1):52-9. [DOI] [PubMed] [Google Scholar]
Hutter 2006
- Hutter HP, Moshammer H, Neuberger M. Smoking cessation at the workplace: 1 year success of short seminars. International Archives of Occupational and Environmental Health 2006;79(1):42-8. [DOI] [PubMed] [Google Scholar]
Killen 1996
- Killen JD, Fortmann SP, Kraemer HC, Varady AN, Davis L, Newman B. Interactive effects of depression symptoms, nicotine dependence, and weight change on late smoking relapse. Journal of Consulting and Clinical Psychology 1996;64(5):1060-7. [DOI] [PubMed] [Google Scholar]
Kim 2018
- Kim K, Park SM, Lee K. Weight gain after smoking cessation does not modify its protective effect on myocardial infarction and stroke: evidence from a cohort study of men. European Heart Journal 2018;39(17):1523-31. [DOI: 10.1093/eurheartj/ehx761] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2019
- Kim K, Choi S, Lee G, Jeong SM, Kim SM, Son JS, et al. Cancer risk among young men with weight gain after smoking cessation: a population-based cohort study. Cancer Epidemiology 2019;60:86-92. [DOI] [PubMed] [Google Scholar]
Klesges 1988
- Klesges RC, Brown K, Pascale RW, Murphy M, Williams E, Cigrang JA, et al. Factors associated with participation, attrition and outcome in a smoking cessation program at a workplace. Health Psychology 1988;7(6):575-89. [DOI] [PubMed] [Google Scholar]
Klesges 1989
- Klesges RC, Meyers AW, Klesges LM, La Vasque M. Smoking, body weight and their effects on smoking behavior: a comprehensive review of the literature. Psychological Bulletin 1989;106(2):204-30. [DOI] [PubMed] [Google Scholar]
Klesges 1992
- Klesges RC, Schumaker SA. Understanding the relations between smoking and body weight and their importance to smoking cessation and relapse. Health Psychology 1992;11(1):1-3. [DOI] [PubMed] [Google Scholar]
Klesges 1997
- Klesges RC, Winders SE, Meyers AW, Eck LH, Ward KD, Hultquist CM, et al. How much weight gain occurs following smoking cessation? A comparison of weight gain using both continuous and point prevalence abstinence. Journal of Consulting and Clinical Psychology 1997;65(2):286-91. [DOI] [PubMed] [Google Scholar]
Komiyama 2013
- Komiyama M, Wada H, Ura S, Yamakage H, Satoh-Asahara N, Shimatsu A, et al. Analysis of factors that determine weight gain during smoking cessation therapy. PLOS One 2013;8(8):e72010. [DOI: 10.1371/journal.pone.0072010] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leeman 2010
- Leeman RF, O'Malley SS, White MA, McKee SA. Nicotine and food deprivation decrease the ability to resist smoking. Psychopharmacology 2010;212(1):25-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindson 2019
- Lindson N, Chepkin SC, Ye W, Fanshawe TR, Bullen C, Hartmann-Boyce J. Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2019, Issue 4. Art. No: CD013308. [DOI: 10.1002/14651858.CD013308] [DOI] [PMC free article] [PubMed] [Google Scholar]
Luo 2013
- Luo J, Rossouw J, Tong E, Giovino GA, Lee CC, Chen C, et al. Smoking and diabetes: does the increased risk ever go away? American Journal of Epidemiology 2013;178(6):937-45. [DOI: 10.1093/aje/kwt071] [DOI] [PMC free article] [PubMed] [Google Scholar]
McEwen 2006
- McEwen A, Hajek P, McRobbie H, West R. Manual of Smoking Cessation. A Guide for Counsellors and Practitioners. Oxford (UK): Blackwell Publishing, 2006. [Google Scholar]
Meyers 1997
- Meyers AW, Klesges RC, Winders SE, Ward KD, Peterson BA, Eck LH. Are weight concerns predictive of smoking cessation? A prospective analysis. Journal of Consulting and Clinical Psychology 1997;65(3):448-52. [DOI] [PubMed] [Google Scholar]
Michie 2009
- Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychology 2009;28(6):690-701. [DOI] [PubMed] [Google Scholar]
Mizes 1998
- Mizes JS, Sloan DM, Segraves K, Spring B, Pingitore R, Kristeller J. The influence of weight-related variables on smoking cessation. Behavior Therapy 1998;29(3):371-85. [Google Scholar]
NHS 2021
- National Health Service (NHS). Stop smoking without putting on weight. www.nhs.uk/live-well/quit-smoking/stop-smoking-without-putting-on-weight/ (accessed 18 May 2021).
O'Hara 1998
- O'Hara P, Connett JE, Lee WW, Nides M, Murray R, Wise R. Early and late weight gain following smoking cessation in the Lung Health Study. American Journal of Epidemiology 1998;148(9):821-30. [DOI] [PubMed] [Google Scholar]
Parsons 2009a
- Parsons A, Ingram J, Inglis J, Aveyard P, Johnstone E, Brown K, et al. A proof of concept randomised placebo controlled factorial trial to examine the efficacy of St John's Wort for smoking cessation and chromium to prevent weight gain on smoking cessation. Drug and Alcohol Dependence 2009;102(1-3):116-22. [DOI] [PubMed] [Google Scholar]
Parsons 2009b
- Parsons A, Lycett D, Aveyard P. Behavioural interventions to prevent weight gain on smoking cessation: a response. Addiction 2009;104(12):2119-20. [DOI] [PubMed] [Google Scholar]
Parsons 2011
- Parsons A, Lycett D, Aveyard P. Response to Spring et al: What is the best method to assess the effect of combined interventions for smoking cessation and post cessation weight gain? Addiction 2011;106:675-76. [Google Scholar]
Pomerleau 2001
- Pomerleau CS, Zucker AN, Stewart AJ. Characterizing concerns about post-cessation weight gain: results from a national survey of women smokers. Nicotine & Tobacco Research 2001;3(1):51-60. [DOI] [PubMed] [Google Scholar]
Scherr 2015
- Scherr A, Seifert B, Kuster M, Meyer A, Fagerström KO, Tamm M, et al. Predictors of marked weight gain in a population of health care and industrial workers following smoking cessation. BMC Public Health 2015;15:520. [DOI: 10.1186/s12889-015-1854-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Serretti 2010
- Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. Journal of Clinical Psychiatry 2010;71(10):1259-72. [DOI: 10.4088/JCP.09r05346blu] [DOI] [PubMed] [Google Scholar]
Spring 2011a
- Spring B, McFadden HG, Rademaker AW, Hitsman B. Behavioual interventions to promote smoking cessation and prevent weight gain: a reply. Addiction 2011;106(3):674-75. [DOI] [PubMed] [Google Scholar]
Spring 2011b
- Spring B, Rademaker AW, Mcfadden HG, Hitsman B. Reducing bias in systematic reviews of behavioral interventions? A response to Parsons et al. Addiction 2011;106:676-78. [DOI] [PubMed] [Google Scholar]
Tian 2015
- Tian J, Venn A, Otahal P, Gall S. The association between quitting smoking and weight gain: a systemic review and meta-analysis of prospective cohort studies. Obesity Reviews 2015;16(10):883-901. [DOI: 10.1111/obr.12304] [DOI] [PubMed] [Google Scholar]
Ussher 2019
- Ussher MH, Faulkner GE, Angus K, Hartmann-Boyce J, Taylor AH. Exercise interventions for smoking cessation. Cochrane Database of Systematic Reviews 2019, Issue 10. Art. No: CD002295. [DOI: 10.1002/14651858.CD002295.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Veldheer 2015
- Veldheer S, Yingst J, Zhu J, Foulds J. Ten-year weight gain in smokers who quit, smokers who continued smoking and never smokers in the United States, NHANES 2003-2012. International Journal of Obesity 2015;39(12):1727-32. [DOI: 10.1038/ijo.2015.127] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2001
- Ward KD, Klesges RC, Vander Weg MW. Cessation of smoking and body weight. In: P Björntorp, editors(s). International Textbook of Obesity. Chichester: John Wiley & Sons, Ltd, 2001:323-36. [DOI: 10.1002/0470846739.ch22] [DOI] [Google Scholar]
Weekley 1992
- Weekley CK, Klesges RC, Reyle G. Smoking as a weight-control strategy in a university population. Addictive Behaviors 1992;17(3):259-71. [DOI] [PubMed] [Google Scholar]
Williamson 1991
- Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. New England Journal of Medicine 1991;324(11):739-45. [DOI] [PubMed] [Google Scholar]
Yeh 2010
- Yeh HC, Duncab PB, Schmidt MI, Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Annals of Internal Medicine 2010;152(1):10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Parsons 2009c
- Parsons AC, Shraim M, Inglis J, Aveyard P, Hajek P. Interventions for preventing weight gain after smoking cessation. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD006219. [DOI: 10.1002/14651858.CD006219.pub2] [DOI] [PubMed] [Google Scholar]


