Section 1. Definitions, incidences and risk factors of frailty
The aging process in an older adult is driven by multi-dimensional inputs that contribute to the individual’s overall progressive decline and ultimately death. These inputs span from biological, environmental, and gene-environment interactions factors, as well as changes in an individual’s social and behavioral characteristics (Figure 1). Importantly, this age-associated decline impacts multiple physiologic systems leading to a state of decreased reserve and compromised resistance to stressors, which in turn contributes to increased vulnerability and adverse outcomes. Frailty is a clinical syndrome that captures this state of vulnerability and decline frequently seen in older adults.
Figure 1. Model pathway for frailty and contributing factors.
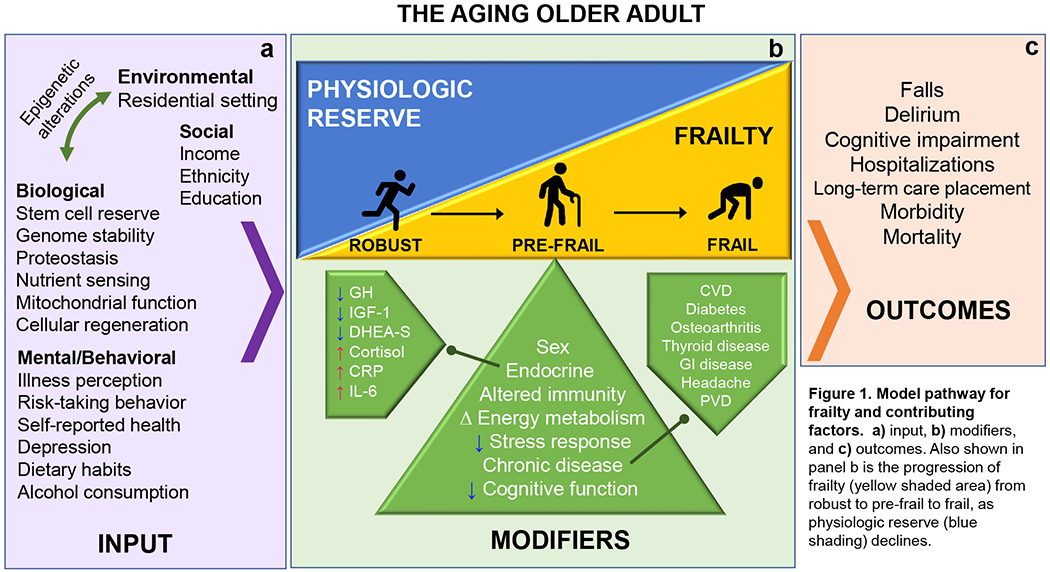
a) input, b) modifiers, and c) outcomes. Also shown in panel b is the progression of frailty (yellow shaded area) from robust to pre-frail to frail, as physiologic reserve (blue shading) declines.
While frailty has many operational definitions, the majority of these definitions are embedded within two conceptual frameworks. The first framework conceptualizes frailty as a syndrome with a distinct physical phenotype with measurable clinical features. This “physical frailty” is best exemplified by the Fried Phenotype, which characterizes frailty by unintentional weight loss (≥5 percent of body weight in the past year), self-reported exhaustion, weakness (as measured by decreased grip strength), slow walking speed, and low physical activity1.
Specifically, those who meet ≥3 of these criteria are considered frail, while those meeting 1-2 criteria are pre-frail, and those without any of these characteristics are robust (Figure 1b). The second framework conceptualizes frailty as a state of vulnerability due to deficit accumulation that can be ascertained through cumulative comorbidities, disease states, functional and cognitive deficits, and psychosocial factors.2, 3 These deficits can be tallied to determine a Frailty Index (FI), with a higher number of deficits yielding a higher FI score.
Despite the lack of a gold-standard definition, frailty as operationally defined above, has been demonstrated to increase risks for adverse clinical outcomes including falls, surgical complications, institutionalization, disability, and death4. For instance, in a prospective observational study in older adults aged 85 and older, baseline frailty is found to be associated with a more than two-fold risk of mortality after 7 years, compared to those who are non-frail5. Moreover, frailty leads to increased healthcare utilization and associated total healthcare costs by 54-101%6. Thus, frailty in late life is a serious medical condition that needs to be managed carefully.
This association between older age and frailty is particularly important given that our society is rapidly aging. According to the 2019 United Nations World Population Ageing Highlights, there is an estimated 703 million persons aged 65 years and older, which is projected to double by 20507. It is anticipated that this large increase in the geriatric population will correspond to a proportional increase in older adults who are frail. To this end, the epidemiology, prevalence and incidence of frailty have been determined in many population-based studies worldwide. The mean prevalence of frailty among the community-dwelling population aged 65 years and older is ~10%, but can range widely from 4.0-59.1%, depending on the frailty criteria used8. A recent meta-analysis of 120,805 adults 60 years or older across 28 countries, reported that the estimated global incidence of frailty as determined by the Fried Phenotype in community-dwelling older adults is 40 cases per 1,000 person-years over a median follow-up of 3 years (95% Confidence Interval [CI], 34.5-48.5; I = 98.2%) . Factors that are associated with an increased prevalence of frailty include African-American or Hispanic ethnicity, lower income and education level, poorer health, and higher rates of comorbid chronic diseases and disability1.
Given that frailty in older adults is common and leads to a multitude of adverse outcomes, a deeper understanding of the science of frailty is a critical first step to help design effective interventions that prevent or attenuate frailty-induced sequelae. Part of such in-depth understanding of frailty also involves recognizing that sex differences in frailty do exist. In community-dwelling older adults more than 65 years of age, frailty was found to be more common in women and in greater severity (as determined by FI, which is a known predictor of all-cause mortality3) compared to men for any age group10. Despite the greater likelihood of being frail however, risk of mortality was lower in women. Therefore, because such sex discrepancies in frailty exist, it is important to understand the causes of these differences so that sex-specific frailty interventions can be further developed as part of providing the best possible patient-centered care for frail older adults. Here, we will review the proposed pathophysiology of frailty in general, as well as hypothesized contributing factors of sex-specific differences in frailty. Similar reviews have been published previously10–12.
Section 2. Pathophysiology of frailty
The pathophysiology of frailty is an active area of research. While the precise pathogenesis of frailty remains unknown, available evidence suggests that physical frailty is in part driven by dysregulation of neuroendocrine, inflammatory, and metabolic pathways (Figure 1a, 1b). For example, age-related hormonal changes that are associated with frailty include decreased levels of growth hormone (GH), insulin-like growth factor 1 (IGF-1), and dihydroepiandosterone sulfate (DHEA-S)13, as well as increased cortisol levels14. Additionally, due to the anabolic and immunity-modulating effects of these hormones, alterations in these hormones likely have direct or indirect impacts on skeletal muscles, therefore causing dysregulated glucose metabolism and insulin-signaling, and sarcopenia (i.e., age-related loss of muscle mass and strength)15, 16. Furthermore, chronic low-grade inflammation is highly associated with frailty. This inflammatory state is measurable by elevated pro-inflammatory cytokines such as interleukin-6 (IL-6), c-reactive protein (CRP),17 elevated numbers of neutrophils and macrophages18, and activation of markers of clotting cascades such as D-dimer, in frail older adults19. The inputs that are hypothesized to drive this multisystemic physiologic dysregulation and progression to frailty development include age-related biological changes (e.g., proteostasis, mitochondrial function), genetics, and environmental exposures (Figure 1a)20.
With such inputs, frail older adults enter an altered homeostatic state which results in a reduced capacity to generate an appropriate stress response to both acute or chronic stressors such as illness, hospitalization or surgery. This inability to regain homeostasis, termed homeostenosis, causes an individual to further spiral into a “cycle of frailty21” where each of the five frailty characteristics (i.e., decreased mobility and activity, weight loss, weakness, fatigue) can initiate a vicious cycle that perpetuates worsening of dysregulated energetics, sarcopenia, and an aggregate frailty syndrome22. Ultimately, frailty in older adults increases the risk for other common geriatric syndromes or outcomes such as falls, delirium, cognitive impairment23, 24, long-term care placement, and mortality (Figure 1c)25, 26.
Section 3. Sex differences in frailty phenotype and the sex-frailty paradox
3a. Sex differences in frailty prevalence, adverse outcomes and mortality
Community-dwelling women aged 65 years or older have a higher prevalence and greater burden of frailty compared to men of the same age (Table 1). In a study of 3,079 community-dwelling older adults from the 2007-2010 National Health and Nutrition Examination Survey (NHANES) database, frailty has been found to be more prevalent in women (8.8% in women vs 5.4% in men)27. Moreover, a similar sex-specific trend has been observed in pre-frail older adults as determined in a recent meta-analysis of 240 studies spanning across 62 countries world-wide28. Moreover, while there is great variability in frailty assessment depending on the tool being used29, females were found to have higher frailty scores than men regardless30. Additionally, frail women are at increased risk of developing deficits in activities of daily living (ADLs) and/or instrumental ADLs (IADLs) and institutionalization31. Frail older adults are also at risk of associated adverse outcomes including hospitalizations, emergency room visits32, readmissions, disability, and overall reduced survival33 and increased mortality rates34 but thus far, sex-specific differences have only been noted in frailty prevalence, survival and mortality rates (Table 1)28, 30, 35.
Table 1. Sex-specific associations in pre-frailty, frailty and frailty-associated adverse outcomes.
Please note that other adverse outcomes including readmission rate, ED visits, hospital admissions are not shown as those outcomes have not yet been shown to have sex-specific associations.
| Finding | Reference | ||
|---|---|---|---|
| Pre-frailty Prevalence | Women > Men Women: 15% (95% CI, 14-17%; n = 143, I2 = 99%; P < 0.005) Men: 11% (95% CI, 10-12%; n = 145, I2 = 97%; P < 0.005) |
Meta-analysis (O’Caoimh et al. 2020) | |
| Women: 39.0% (95% CI, 38.1-39.9%) Men: 37.3% (95% CI, 36.6-38.0%; v2 = 8,629, df = 1, P = 0.003) |
Systematic Review (Collard et al. 2012) | ||
| Frailty Prevalence | Women > Men Women: 9.6% (95% CI, 9.2-10%) Men: 5.2% (95% CI, 4.9-5.5%; P < 0.001) |
Systematic Review (Collard et al. 2012) | |
| Women: 49% (95% CI, 14-17%; P < 0.005) Men: 45% (95% CI, 44-47%; n = 119, I2 = 97%; P < 0.005) |
Meta-analysis (O’Caoimh et al. 2020) | ||
| Frailty-associated adverse outcomes | Survival | 1) HR 0.43 (95% CI, 0.299-0.561), P < 0.0001 2) Survival rate of women > men independent of frailty status |
1) Observational, 10 yr longitudinal study (Corbi et al. 2019) 2) Secondary analysis of the Survey of Health, Ageing, and Retirement in Europe (SHARE) (*Theou et al. 2014) |
| Mortality | Mortality rate lower in women vs. men (up to age 90, after which mortality rate increases to above 30% in women). | Meta-analysis (Gordon et al. 2017) |
Interestingly, regardless of age or level of frailty, older frail women have better survival compared to men. In a meta-analysis of two large cohort studies (SHARE36 and MHAS37) that used the FI to determine frailty, men have higher rates of mortality compared to women until age 9010. In another study of older adults, the mortality rate in frail men (22.5%) is much higher compared to women (8.5%). In this study, sex-specific differences in the causes of death are also found. In men, the predominant causes for death are heart disease (41%) and chronic lower respiratory disease (23%), compare to nephritis/nephrosis in women (32.3%)27.
3b. The “sex-frailty paradox”
The sex-associated divergence in frailty prevalence and mortality has been referred to as the sex-frailty paradox11. This is consistent with the long-recognized observation that women have longer lifespan than men despite having higher chronic disease burden and disability11. The sex-frailty paradox is best illustrated by a meta-analysis of seven large studies of community-dwelling older adults showing that mortality rate is lower in women irrespective of their age or frailty severity10. While the reasons for this phenomenon are yet to be elucidated, some have hypothesized that this may be due to men having more “life-threatening” chronic conditions (e.g., stroke, ischemic heart disease), whereas women may experience more “non-life-threatening” chronic conditions that are associated with higher morbidity (e.g., fractures, depression, constipation, headaches)11, 25.
3c. Sex differences in frailty-associated contributing factors
Frailty and its progression is driven by multi-domain inputs (Figure 1) which may have differential impact depending on the sex of the individual. Based on growing evidence focusing on these sex-specific differences in frailty and its contributing factors, it has been hypothesized that sex-specific differences in frailty is likely due to a combined effect of biological, psychosocial and behavioral differences between women and men12. Here, we have conceptually categorized contributing factors for sex-differences in frailty in older adults into biological, social and behavioral domains (Figure 2). While some of these contributing factors are common between both sexes, others have a more sex-specific contribution.
Figure 2. Contributory domains and factors for frailty and sex-specific associations.
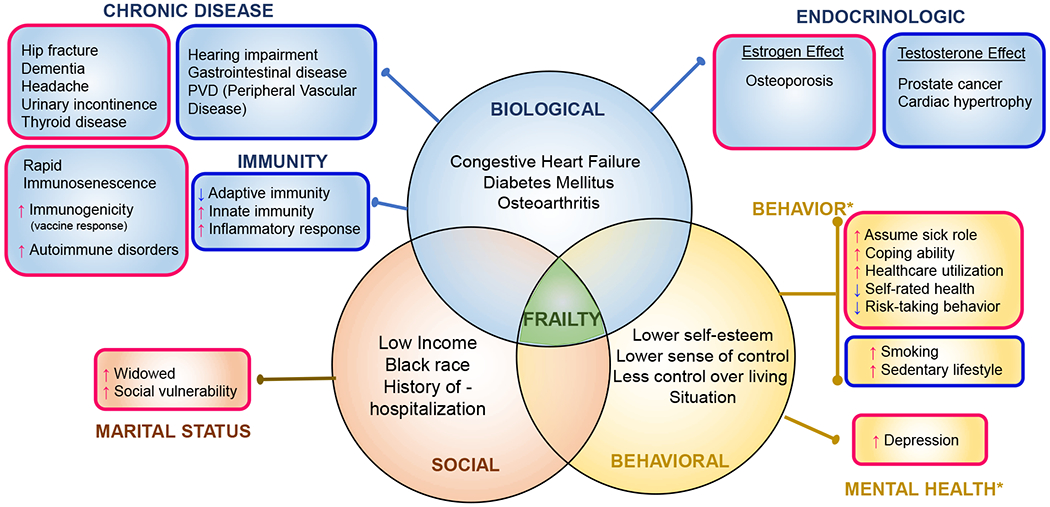
Depicted within each circle of the venn diagram are the contributing factor domains (biological, social and behavioral) that are color-coded as blue, orange and yellow shading. For each domain, contributing factors common to both genders (or are of equal prevalence) are listed inside the circle, and those that have gender-specific associations are depicted outside the circle in boxes that are outlined in pink (female-specific), or blue (male-specific). Note: for Behavioral: only female-specific associations are shown in the boxes (*males have the opposite characteristics which are not shown)
Frail and depressed (29.5% frail vs 17.8% prevalence in non-frail p<0.001, Mantovani et al 2015), Frail females 22.7% vs. Frail males 15.4% (p<0.001)
Depression -> Frailty Risk OR 4.73 (2.62-8.55, p<0.01) Chang et al 2010
Biological factors that may contribute to sex-differences in frailty include chronic disease, changes in immunity, as well as endocrinologic changes which occur in part, due to aging. As noted by Gordon and Hubbard11, while certain chronic medical conditions such as cardiac disease, congestive heart failure, diabetes mellitus, osteoarthritis, and glaucoma are similarly prevalent in older adults regardless of sex, differences do exist in other chronic conditions. For example, in men, a higher prevalence of hearing impairment, peripheral vascular disease, and gastrointestinal disease are reported, while in women, dementia, hip fracture, depression, headache, urinary incontinence and thyroid disease are more prevalent.
Sex-specific differences in immune response and inflammatory signaling may partially stem from differences in sex chromosomes. Women have 2 copies of X chromosome which carries genes that encode Toll-Like Receptor and multiple cytokine receptors and genes involved in T- and B-cell activity. In comparison, men carry 1 copy each of X chromosome and Y chromosome, which encodes some inflammatory pathway genes that are expressed exclusively in men38, 39. Consequently, progression to immunosenescence, which contributes to age-related decline in immune function, is known to occur at a faster rate in men than in women40, 41. Epigenomic and genomic changes regulate innate and humoral immunity in a sex-specific manner38. In fact, these genomic differences between sexes increase after age 65, with men having higher innate and pro-inflammatory activity, while having lower adaptive immunity, compared to women42.
Differences in levels and regulation of hormones also differentially contribute to frailty in a sex-specific manner. For example, estrogen reduces hepatic sensitivity to growth hormone (GH). In contrast, testosterone enhances the effect of GH which increases the risk of some age-related diseases such as prostate cancer and cardiac hypertrophy43. Additionally, several autoimmune disorders including multiple sclerosis, rheumatoid arthritis, Sjogren’s syndrome, and systemic lupus erythematosus are known to be more prevalent in women. This increase in susceptibility in women may be due to the reduced protective effect of some autoimmune regulatory genes that are downregulated by estrogen44, 45. COVID-19 is another example of how the regulatory role of sex hormones may affect disease pathogenesis. In COVID-19, the SARS-CoV-2 binds to the ACE-2 receptor, which serves as viral entry point46. Because testosterone upregulates, whereas estrogen inhibits ACE-2 receptor expression, sex hormone differences may partially explain the increased risk of disease severity and mortality in men47.
Skeletal muscle changes with age, both in its overall architecture (i.e., skeletal muscle remodeling including increased intramyocellular lipid accumulation and fibrosis), as well as in its macronutrient (e.g., fat, protein, glucose) metabolism48. As a result, the absolute and relative loss of contractile skeletal muscle tissue is a shared feature in both aging men and women48,49. However, the decline in resting energy expenditure in both skeletal muscle and overall adipose tissue occurs at a faster rate in women compared to men. This may partially explain why women are more prone to frailty than men50.
Apart from biological contributory factors, differences in the social and behavioral domains may contribute to sex-specific differences in frailty (Figure 2). Within the social domain, social vulnerability is a significant contributory factor. Marital status is one important determinant of social vulnerability, and studies have found that widowhood is more frequently associated with frailty52, as well as being socially frail, and thereby at increased risk of mortality (HR = 2.69; 95% CI, 1.01-7.25, p < 0.05)51. However, women may be able to better cope with social vulnerability due to greater support networks, while men may be subject to increased mortality53 due to a relative lack of coping mechanisms. Despite better coping mechanisms in women, widowhood is indeed a known risk factor for the development of persistent depressive symptoms54, and this in turn may increase the risk of frailty55. It is currently unclear whether depression can increase the risk of frailty in a sex-specific manner.
In the behavioral domain, several contributory factors may contribute to sex-specific differences in frailty. For instance, coping mechanisms may differ between men and women, perhaps by activating different brain areas, thereby using different problem-solving strategies. Specifically, when exposed to an acute stressor, men are found to engage the prefrontal cortex regions, while women have more responses in the limbic/striatal regions, and these stress responses are associated with distinct neural networks56. Furthermore, it has been proposed that the psychological phenomenon of stress, such as life stressors from surgery or illness, emotional, physical or sexual abuse, divorce, or death of a loved one, may be related to microstructural changes in the corpus callosum of the brain57. Accordingly, these sex-specific differences in stress perception may potentially explain differences in women’s behavior around health issues, including illness perception, self-rated health, and healthcare utilization. Women are also more sensitive to small physical changes and more likely to assume the sick role58 . While women have poorer self-rated health59, they are more likely to report either minor or major health issues60. We should be cautiously reminded that the perception of self-rated health is influenced by other social determinants of health as well, including occupation, marital status, household income, area of residence (rural/urban), and work environment61. When it comes to risky behavior such as cigarette smoking or alcohol consumption, women also tend to be risk-averse compared to men62.
Other independent frailty-associated factors that are unique to, or shared between, men and women have been described27, 63 (Figure 2). Zhang and colleagues27 showed that independent frailty-associated factors common to both men and women include sedentary lifestyle (physical inactivity) and prior history of hospitalizations. In contrast, higher family income to poverty ratio is protective against frailty in both sexes. In men, frailty is associated with additional risk factors including being widowed, divorced or separated, sleeping more than 9 hours a day, and smoking. In women, additional risk factors for frailty include obesity (BMI ≥ 30 kg/m2), elevated inflammatory markers such as CRP, sleeping less than 6 hours a day, and family history of diabetes or myocardial infarction.
Section 4. Intervention for frailty incorporating the 5Ms of geriatrics
The overall approach to caring for an older adult with frailty should aim at diagnosing frailty using validated screening tools, followed by implementing individually-tailored intervention plans64. The diagnosis of frailty can be made with tools such as the Fried Phenotype (i.e., Hopkins Frailty tool) or the FI as determined by a comprehensive geriatric assessment (CGA). Rapid screening tools such as the FRAIL scale or the Study of Osteoporotic Fractures (SOF) frailty tool that allows quicker screening may also be used65, 66. While there is currently insufficient evidence to differentiate treatment interventions for frailty in a sex-specific manner, the 5Ms (multi-complexity, mind, mobility, medications, matters) of geriatric care can be incorporated to augment frailty intervention67.
Intervention modalities including exercise, nutrition management, and interdisciplinary geriatric models of care have been found to improve clinical features of frailty or reduce adverse outcomes. For example, Travers and colleagues have noted in their systematic review of 925 studies, that a combination of muscle strength training and protein supplementation are the most effective and easiest interventions to implement, and to delay or reverse frailty68. Multi-component intervention is also imperative for preventing frailty-associated adverse outcomes. Marcucci and colleagues have proposed guidelines, as a part of the FOCUS (Frailty Management Optimisation through EIP-AHA Commitments and Utilisation of Stakeholders Input) project, that interventions including exercise, nutritional management and their combination, should be implemented to prevent or delay the progression of frailty64. Lastly, geriatric-focused interdisciplinary care programs such as GEM (Geriatric Evaluation and Management), ACE (Acute Care for the Elders Unit), PACE (Program for All-inclusive Care for the Elderly), and hospice care (Figure 3) and their impact on the health of vulnerable older adults have been extensively studied in both outpatient and inpatient settings. As an example, a meta-analysis of 7 studies including 1,009, comprehensive geriatric assessment unit interventions is found to be effective in managing physical and psychological frailty, readmission, mortality and patient satisfaction in hospitalized older adults69. Thus, geriatric interdisciplinary models of care should be integrated whenever feasible and take an active role in the clinical care of frail older adults.
Figure 3. Interventions for older adults along the frailty spectrum.
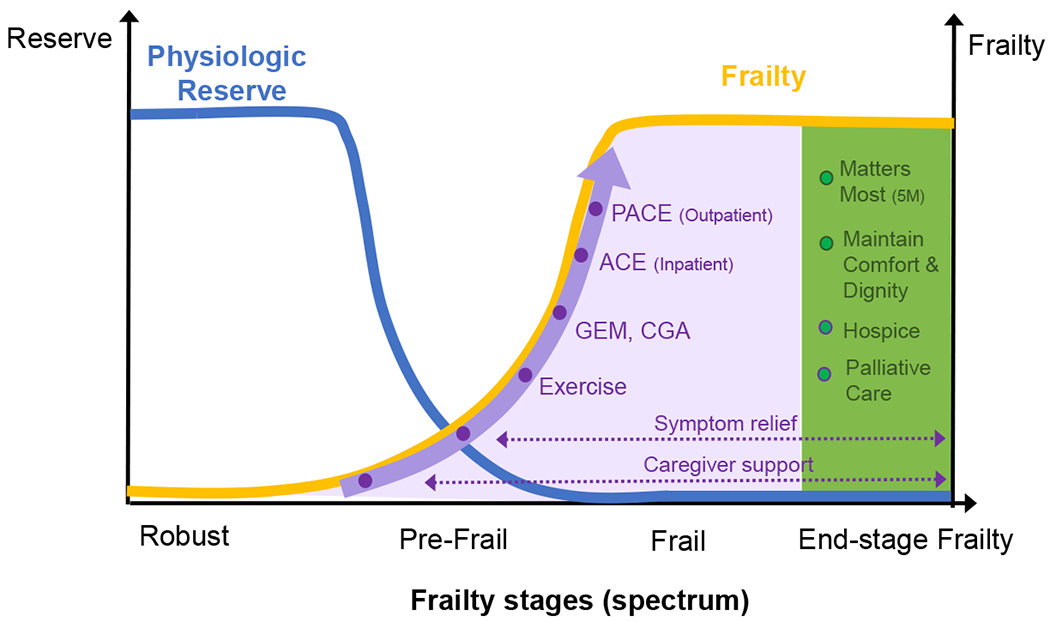
Shaded areas depict possible interventions for the frail older adults [purple: early/mid-stage frailty, green: end-stage frailty]. GEM: Geriatric Evaluation and Management. CGA: Comprehensive Geriatric Evaluation. PACE: Program for All Inclusive Care of the Elderly. ACE: Acute Care for the Elders unit.
Multi-complexity:
The complex health care needs of frail older adults necessitate utilizing multi-modal interventions that encompass management of chronic comorbidities, behavioral and psychosocial needs, and lifestyle modification that may be of benefit to reduce or prevent frailty. In a recent prospective cohort study of 6,357 adults followed longitudinally for 20 years, healthy habits exercised at age 50 are associated with a lower risk of frailty later in life. These habits include not smoking (HR 0.68; 95% CI, 0.52-0.89, p=0.01), moderate alcohol consumption (HR 0.76; 95% CI, 0.59-0.98, p<0.001), physical activity of at least 2.5 hours per week (HR 0.66; 95%CI, 0.48-0.88, p=0.0001), and consuming fruits and vegetables more than twice daily (HR 0.70; 95% CI, 0.53-0.92, p=0.01)70. Frailty risk is reduced by 70% if all 4 healthy habits are present. Additionally, in this same study, the cumulative effect of multiple healthy habits and behavioral modification implemented at or before age 50 are shown to help prevent frailty later in life with a 31% reduction in frailty incidence for each additional healthy behavior. These findings indicate that early intervention with modification of risk factors can indeed prevent frailty.
Mind:
As introduced briefly in the previous sections, frailty is impacted by multi-faceted inputs which include psychosocial variables such as cognition and mood (e.g., psychological wellness). First, frailty is associated with cognitive impairment24 and dementia71, and indeed, cognitive training has been associated with improved frailty score and reduced frailty prevalence72. Second, older adults with depression are at risk of frailty73, and specifically in women, depressive symptoms increase the likelihood for frailty74. In men, more traumatic life events and perceived level of post-traumatic psychological stress are associated with increased likelihood of frailty. Thus, providers need to remain cognizant of these sex-specific differences in psychosocial correlates of frailty for both assessment and intervention, in order to better address the “mind” component of the 5Ms-oriented geriatric care.
Mobility:
Many single- and multi-component physical activity programs improve gait speed, muscle strength, mobility and physical performance in frail older adults75 although modalities in which exercise interventions are implemented in frailty studies vary significantly76. In one study, a home-based video exercise program for frail older women >75 years of age has shown to improve overall quality of life as measured by EuroQoL-5D77, including measures of mobility, self-care, usual activities, pain or discomfort, and anxiety or depression, as well as self-rated health78. Thus, providers should consider the exercise training options that may be available to a frail older adult, and prescribe an exercise intervention that best meet the individual’s need and ability.
Medications:
The impact of polypharmacy, and its associated adverse outcomes, on frail older adults, need to be considered when devising a care plan. In a systematic review of 25 studies, polypharmacy is associated with frailty. Significant associations are found with every medication added to the treatment (OR 1.13–1.20), with polypharmacy (OR 1.77–2.55), and hyperpolypharmacy (≥10 drugs, OR 4.47–5.8)79. Frail older adults subjected to polypharmacy also have a 13-fold longer hospital stay and a five-fold greater risk for hospital readmission80. Furthermore, providers should be cognizant of how sex-specific differences in drug metabolism81 can affect pharmacokinetics. Thus, extra efforts should be made to address polypharmacy in frail older adults.
Matters most:
The routine assessment of a frail older adult’s priorities and goals of care including treatment preferences and quality of life, as well as psychosocial resources74, have become exceedingly important as we aim to provide improved 5Ms-focused patient-centered care. This approach allows providers to recommend the most appropriate intervention strategy along the spectrum of frailty82, 83. Specifically, it enables timely integration of the management of distressing symptoms, as well as ensuring appropriate caregiver support. Moreover, multi-component interventions such as exercise training tailored towards the need and ability of the patient, as well as geriatric interdisciplinary models of care corresponding to the level of patient’s need, should be integrated into patient care in order to optimize outcomes64, 69. Finally, older adults who are severely frail should be provided with necessary access to palliative and hospice care and related resources84.
Section 5. Conclusions and future directions
Frailty is a clinical syndrome that leads to a progressive, multisystem decline in function and physiologic reserve, and increased vulnerability to adverse outcomes. Various biological, psychosocial and behavioral inputs contribute to the development of frailty. Moreover, some of these inputs may contribute to sex-specific differences in frailty and associated adverse outcomes. Future research efforts should focus on development of screening tools and therapeutic interventions that best incorporate sex-specific differences in frailty in order to reduce mortality and optimize outcomes in frail older adults.
KEY POINTS.
Frailty is an important clinical syndrome of age-related decline in physiologic reserve and increased vulnerability, and is associated with numerous adverse clinical outcomes
Frailty is driven by dysregulation of neuroendocrine, inflammatory, and metabolic pathways
Frailty is more prevalent in older women
Sex-specific differences in frailty is an emerging area of investigation
The 5Ms (multi-complexity, mind, mobility, medications, matters most) of geriatric medicine can be integrated in the clinical management of frail older adults to improve care
SYNOPSIS.
Frailty is an important clinical syndrome of age-related decline in physiologic reserve and increased vulnerability. In older adults, frailty leads to a progressive multisystem decline and increased adverse clinical outcomes. The pathophysiology of frailty is hypothesized to be driven by dysregulation of neuroendocrine, inflammatory, and metabolic pathways. Moreover, sex-specific differences in the prevalence of frailty have been observed and various biological, psychosocial and behavioral factors may contribute to these differences. Treatment interventions, focusing on the 5Ms (multi-complexity, mind, mobility, medications, matters most) of geriatric care, can be applied to the care of frail older women with these sex-specific differences in mind. As additional evidence regarding sex-specific differences in frailty emerges, future research efforts should encompass the development of screening tools and therapeutic interventions that optimize outcomes in older adults who are frail.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
The authors have nothing to disclose.
References cited
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. March 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 2.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. July 2007;62(7):722–7. doi: 10.1093/gerona/62.7.722 [DOI] [PubMed] [Google Scholar]
- 3.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. February 2011;27(1):17–26. doi: 10.1016/j.cger.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 4.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. March 2013;381(9868):752–62. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keeble E, Parker SG, Arora S, et al. Frailty, hospital use and mortality in the older population: findings from the Newcastle 85+ study. Age Ageing. 11 2019;48(6):797–802. doi: 10.1093/ageing/afz094 [DOI] [PubMed] [Google Scholar]
- 6.Hajek A, Bock JO, Saum KU, et al. Frailty and healthcare costs-longitudinal results of a prospective cohort study. Age Ageing. March 2018;47(2):233–241. doi: 10.1093/ageing/afx157 [DOI] [PubMed] [Google Scholar]
- 7.Nations U. World Population Ageing 2019: Highlights (ST/ESA/SER.A/430). In: 2019, editor.: United Nations, Department of Economic and Social Affairs, Population Division [Google Scholar]
- 8.Kojima G, Liljas AEM, Iliffe S . Frailty syndrome: implications and challenges for health care policy. Risk Manag Healthc Policy. 2019;12:23–30. doi: 10.2147/RMHP.S168750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ofori-Asenso R, Chin KL, Mazidi M, et al. Global Incidence of Frailty and Prefrailty Among Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. JAMA Netw Open. 08 2019;2(8):e198398. doi: 10.1001/jamanetworkopen.2019.8398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE. Sex differences in frailty: A systematic review and meta-analysis. Exp Gerontol. 03 2017;89:30–40. doi: 10.1016/j.exger.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 11.Gordon EH, Hubbard RE. Do sex differences in chronic disease underpin the sex-frailty paradox? Mech Ageing Dev. 04 2019;179:44–50. doi: 10.1016/j.mad.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 12.Gordon EH, Hubbard RE. Differences in frailty in older men and women. Med J Aust. 03 2020;212(4):183–188. doi: 10.5694/mja2.50466 [DOI] [PubMed] [Google Scholar]
- 13.Leng SX, Cappola AR, Andersen RE, et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. April 2004;16(2):153–7. doi: 10.1007/BF03324545 [DOI] [PubMed] [Google Scholar]
- 14.Varadhan R, Walston J, Cappola AR, Carlson MC, Wand GS, Fried LP. Higher levels and blunted diurnal variation of cortisol in frail older women. J Gerontol A Biol Sci Med Sci. February 2008;63(2):190–5. doi: 10.1093/gerona/63.2.190 [DOI] [PubMed] [Google Scholar]
- 15.Kalyani RR, Varadhan R, Weiss CO, Fried LP, Cappola AR. Frailty status and altered glucose-insulin dynamics. J Gerontol A Biol Sci Med Sci. December 2012;67(12):1300–6. doi: 10.1093/gerona/glr141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diamanti-Kandarakis E, Dattilo M, Macut D, et al. MECHANISMS IN ENDOCRINOLOGY: Aging and anti-aging: a Combo-Endocrinology overview. Eur J Endocrinol. June 2017;176(6):R283–R308. doi: 10.1530/EJE-16-1061 [DOI] [PubMed] [Google Scholar]
- 17.Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol (Oxf). October 2005;63(4):403–11. doi: 10.1111/j.1365-2265.2005.02355.x [DOI] [PubMed] [Google Scholar]
- 18.Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. June 2007;55(6):864–71. doi: 10.1111/j.1532-5415.2007.01186.x [DOI] [PubMed] [Google Scholar]
- 19.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. November 2002;162(20):2333–41. doi: 10.1001/archinte.162.20.2333 [DOI] [PubMed] [Google Scholar]
- 20.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. June 2006;54(6):991–1001. doi: 10.1111/j.1532-5415.2006.00745.x [DOI] [PubMed] [Google Scholar]
- 21.Xue QL, Bandeen-Roche K, Varadhan R, Zhou J, Fried LP. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci. September 2008;63(9):984–90. doi: 10.1093/gerona/63.9.984 [DOI] [PubMed] [Google Scholar]
- 22.Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. February 2011;27(1):1–15. doi: 10.1016/j.cger.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. June 2013;14(6):392–7. doi: 10.1016/j.jamda.2013.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment--a review of the evidence and causal mechanisms. Ageing Res Rev. September 2013;12(4):840–51. doi: 10.1016/j.arr.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 25.Case A, Paxson C. Sex differences in morbidity and mortality. Demography. May 2005;42(2):189–214. doi: 10.1353/dem.2005.0011 [DOI] [PubMed] [Google Scholar]
- 26.Hessey E, Montgomery C, Zuege DJ, et al. Sex-specific prevalence and outcomes of frailty in critically ill patients. J Intensive Care. 2020;8:75. doi: 10.1186/s40560-020-00494-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q, Guo H, Gu H, Zhao X. Gender-associated factors for frailty and their impact on hospitalization and mortality among community-dwelling older adults: a cross-sectional population-based study. PeerJ. 2018;6:e4326. doi: 10.7717/peerj.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Caoimh R, Sezgin D, O’Donovan MR, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. October 2020;doi: 10.1093/ageing/afaa219 [DOI] [PubMed] [Google Scholar]
- 29.Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. September 2013;61(9):1537–51. doi: 10.1111/jgs.12420 [DOI] [PubMed] [Google Scholar]
- 30.Theou O, Brothers TD, Peña FG, Mitnitski A, Rockwood K. Identifying common characteristics of frailty across seven scales. J Am Geriatr Soc. May 2014;62(5):901–6. doi: 10.1111/jgs.12773 [DOI] [PubMed] [Google Scholar]
- 31.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. March 2006;61(3):262–6. doi: 10.1093/gerona/61.3.262 [DOI] [PubMed] [Google Scholar]
- 32.Kojima G Frailty as a Predictor of Emergency Department Utilization among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. J Am Med Dir Assoc. 01 2019;20(1):103–105. doi: 10.1016/j.jamda.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 33.Corbi G, Cacciatore F, Komici K, et al. Inter-relationships between Gender, Frailty and 10-Year Survival in Older Italian Adults: an observational longitudinal study. Sci Rep. 12 2019;9(1):18416. doi: 10.1038/s41598-019-54897-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen AT, Nguyen TX, Nguyen TN, et al. The impact of frailty on prolonged hospitalization and mortality in elderly inpatients in Vietnam: a comparison between the frailty phenotype and the Reported Edmonton Frail Scale. Clin Interv Aging. 2019;14:381–388. doi: 10.2147/CIA.S189122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. August 2012;60(8):1487–92. doi: 10.1111/j.1532-5415.2012.04054.x [DOI] [PubMed] [Google Scholar]
- 36.Romero-Ortuno R, Kenny RA. The frailty index in Europeans: association with age and mortality. Age Ageing. September 2012;41(5):684–9. doi: 10.1093/ageing/afs051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.García-González JJ, García-Peña C, Franco-Marina F, Gutiérrez-Robledo LM. A frailty index to predict the mortality risk in a population of senior Mexican adults. BMC Geriatr. November 2009;9:47. doi: 10.1186/1471-2318-9-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. June 2015;14(3):309–21. doi: 10.1111/acel.12326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Charchar FJ, Bloomer LD, Barnes TA, et al. Inheritance of coronary artery disease in men: an analysis of the role of the Y chromosome. Lancet. March 2012;379(9819):915–922. doi: 10.1016/S0140-6736(11)61453-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kryspin-Exner I, Lamplmayr E, Felnhofer A. Geropsychology: the gender gap in human aging--a mini-review. Gerontology. 2011;57(6):539–48. doi: 10.1159/000323154 [DOI] [PubMed] [Google Scholar]
- 41.Hirokawa K, Utsuyama M, Hayashi Y, Kitagawa M, Makinodan T, Fulop T. Slower immune system aging in women versus men in the Japanese population. Immun Ageing. May 2013;10(1):19. doi: 10.1186/1742-4933-10-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Márquez EJ, Chung CH, Marches R, et al. Sexual-dimorphism in human immune system aging. Nat Commun. 02 2020;11(1):751. doi: 10.1038/s41467-020-14396-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ostan R, Monti D, Gueresi P, Bussolotto M, Franceschi C, Baggio G. Gender, aging and longevity in humans: an update of an intriguing/neglected scenario paving the way to a gender-specific medicine. Clin Sci (Lond). October 2016;130(19):1711–25. doi: 10.1042/CS20160004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kivity S, Ehrenfeld M. Can we explain the higher prevalence of autoimmune disease in women? Expert Rev Clin Immunol. September 2010;6(5):691–4. doi: 10.1586/eci.10.60 [DOI] [PubMed] [Google Scholar]
- 45.Moulton VR. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front Immunol. 2018;9:2279. doi: 10.3389/fimmu.2018.02279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Q, Zhang Y, Wu L, et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell. 05 2020;181(4):894–904.e9. doi: 10.1016/j.cell.2020.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gadi N, Wu SC, Spihlman AP, Moulton VR. What’s Sex Got to Do With COVID-19? Gender-Based Differences in the Host Immune Response to Coronaviruses. Front Immunol. 2020;11:2147. doi: 10.3389/fimmu.2020.02147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gheller BJ, Riddle ES, Lem MR, Thalacker-Mercer AE. Understanding Age-Related Changes in Skeletal Muscle Metabolism: Differences Between Females and Males. Annu Rev Nutr. 07 2016;36:129–56. doi: 10.1146/annurev-nutr-071715-050901 [DOI] [PubMed] [Google Scholar]
- 49.Kent-Braun JA, Ng AV, Young K. Skeletal muscle contractile and noncontractile components in young and older women and men. J Appl Physiol (1985). February 2000;88(2):662–8. doi: 10.1152/jappl.2000.88.2.662 [DOI] [PubMed] [Google Scholar]
- 50.Geisler C, Braun W, Pourhassan M, et al. Gender-Specific Associations in Age-Related Changes in Resting Energy Expenditure (REE) and MRI Measured Body Composition in Healthy Caucasians. J Gerontol A Biol Sci Med Sci. 07 2016;71(7):941–6. doi: 10.1093/gerona/glv211 [DOI] [PubMed] [Google Scholar]
- 51.Andrew MK, Keefe JM. Social vulnerability from a social ecology perspective: a cohort study of older adults from the National Population Health Survey of Canada. BMC Geriatr. August 2014;14:90. doi: 10.1186/1471-2318-14-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maria Carneiro Maciel GTAdS, Heloiza Guilherme Gonçalves, Rafaella Danielma Lopes Ferreira, Josefa Vinagre Tietre, Sarah Maria Paiva de Menezes , Rejane. Frailty Assessment and its Association with Sociodemographic and Health Characteristics in Community Elderly. International Archives of Medicine: Geriatrics Section; 2017. [Google Scholar]
- 53.Shor E, Roelfs DJ, Curreli M, Clemow L, Burg MM, Schwartz JE. Widowhood and mortality: a meta-analysis and meta-regression. Demography. May 2012;49(2):575–606. doi: 10.1007/s13524-012-0096-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buigues C, Padilla-Sánchez C, Garrido JF, Navarro-Martínez R, Ruiz-Ros V, Cauli O. The relationship between depression and frailty syndrome: a systematic review. Aging Ment Health. 2015;19(9):762–72. doi: 10.1080/13607863.2014.967174 [DOI] [PubMed] [Google Scholar]
- 55.Lee G. Widowhood, Gender, and Depression: A Longitudinal Analysis. In: D A, editor.: Research on Aging; 2007. p. 56–72. [Google Scholar]
- 56.Goldfarb EV, Seo D, Sinha R. Sex differences in neural stress responses and correlation with subjective stress and stress regulation. Neurobiol Stress. November 2019;11:100177. doi: 10.1016/j.ynstr.2019.100177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seckfort DL, Paul R, Grieve SM, et al. Early Life Stress on Brain Structure and Function Across the Lifespan: A Preliminary Study. Brain Imaging and Behavior. 2008;2(1):49. doi: 10.1007/s11682-007-9015-y [DOI] [Google Scholar]
- 58.Hibbard JH, Pope CR. Another look at sex differences in the use of medical care: illness orientation and the types of morbidities for which services are used. Women Health. 1986;11(2):21–36. doi: 10.1300/J013v11n02_03 [DOI] [PubMed] [Google Scholar]
- 59.Boerma T, Hosseinpoor AR, Verdes E, Chatterji S. A global assessment of the gender gap in self-reported health with survey data from 59 countries. BMC Public Health. 07 2016;16:675. doi: 10.1186/s12889-016-3352-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verbrugge LM. Gender and health: an update on hypotheses and evidence. J Health Soc Behav. September 1985;26(3):156–82. [PubMed] [Google Scholar]
- 61.Hosseinpoor AR, Stewart Williams J, Amin A, et al. Social determinants of self-reported health in women and men: understanding the role of gender in population health. PLoS One. 2012;7(4):e34799. doi: 10.1371/journal.pone.0034799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oksuzyan A, Juel K, Vaupel JW, Christensen K. Men: good health and high mortality. Sex differences in health and aging. Aging Clin Exp Res. April 2008;20(2):91–102. doi: 10.1007/BF03324754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng Z, Guan S, Ding H, et al. Prevalence and Incidence of Frailty in Community-Dwelling Older People: Beijing Longitudinal Study of Aging II. J Am Geriatr Soc. 06 2016;64(6):1281–6. doi: 10.1111/jgs.14135 [DOI] [PubMed] [Google Scholar]
- 64.Marcucci M, Damanti S, Germini F, et al. Interventions to prevent, delay or reverse frailty in older people: a journey towards clinical guidelines. BMC Med. 10 2019;17(1):193. doi: 10.1186/s12916-019-1434-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. July 2012;16(7):601–8. doi: 10.1007/s12603-012-0084-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. February 2008;168(4):382–9. doi: 10.1001/archinternmed.2007.113 [DOI] [PubMed] [Google Scholar]
- 67.Molnar F, Frank CC. Optimizing geriatric care with the GERIATRIC 5. Can Fam Physician. 01 2019;65(1):39. [PMC free article] [PubMed] [Google Scholar]
- 68.Travers J, Romero-Ortuno R, Bailey J, Cooney MT. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract. January 2019;69(678):e61–e69. doi: 10.3399/bjgp18X700241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rezaei-Shahsavarloo Z, Atashzadeh-Shoorideh F, Gobbens RJJ, Ebadi A, Ghaedamini Harouni G. The impact of interventions on management of frailty in hospitalized frail older adults: a systematic review and meta-analysis. BMC Geriatr. December 2020;20(1):526. doi: 10.1186/s12877-020-01935-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gil-Salcedo A, Dugravot A, Fayosse A, et al. Healthy behaviors at age 50 years and frailty at older ages in a 20-year follow-up of the UK Whitehall II cohort: A longitudinal study. PLoS Med. 07 2020;17(7):e1003147. doi: 10.1371/journal.pmed.1003147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Solfrizzi V, Scafato E, Frisardi V, et al. Frailty syndrome and the risk of vascular dementia: the Italian Longitudinal Study on Aging. Alzheimers Dement. March 2013;9(2):113–22. doi: 10.1016/j.jalz.2011.09.223 [DOI] [PubMed] [Google Scholar]
- 72.Ng TP, Feng L, Nyunt MS, et al. Nutritional, Physical, Cognitive, and Combination Interventions and Frailty Reversal Among Older Adults: A Randomized Controlled Trial. Am J Med. Nov 2015;128(11):1225–1236.e1. doi: 10.1016/j.amjmed.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 73.Pegorari MS, Tavares DM. Factors associated with the frailty syndrome in elderly individuals living in the urban area. Rev Lat Am Enfermagem. Oct 2014;22(5):874–82. doi: 10.1590/0104-1169.0213.2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Freitag S, Schmidt S. Psychosocial Correlates of Frailty in Older Adults. Geriatrics (Basel). Nov 2016;1(4)doi: 10.3390/geriatrics1040026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kidd T, Mold F, Jones C, et al. What are the most effective interventions to improve physical performance in pre-frail and frail adults? A systematic review of randomised control trials. BMC Geriatr. 07 2019;19(1):184. doi: 10.1186/s12877-019-1196-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Negm AM, Kennedy CC, Thabane L, et al. Management of Frailty: A Systematic Review and Network Meta-analysis of Randomized Controlled Trials. J Am Med Dir Assoc. 10 2019;20(10):1190–1198. doi: 10.1016/j.jamda.2019.08.009 [DOI] [PubMed] [Google Scholar]
- 77.Balestroni G, Bertolotti G. [EuroQol-5D (EQ-5D): an instrument for measuring quality of life]. Monaldi Arch Chest Dis. Sep 2012;78(3):155–9. doi: 10.4081/monaldi.2012.121 [DOI] [PubMed] [Google Scholar]
- 78.Vestergaard S, Kronborg C, Puggaard L. Home-based video exercise intervention for community-dwelling frail older women: a randomized controlled trial. Aging Clin Exp Res. Oct 2008;20(5):479–86. doi: 10.1007/BF03325155 [DOI] [PubMed] [Google Scholar]
- 79.Gutiérrez-Valencia M, Izquierdo M, Cesari M, Casas-Herrero Á, Inzitari M, Martínez-Velilla N. The relationship between frailty and polypharmacy in older people: A systematic review. Br J Clin Pharmacol. 07 2018;84(7):1432–1444. doi: 10.1111/bcp.13590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosted E, Schultz M, Sanders S. Frailty and polypharmacy in elderly patients are associated with a high readmission risk. Dan Med J. Sep 2016;63(9) [PubMed] [Google Scholar]
- 81.Shapiro BH, Agrawal AK, Pampori NA. Gender differences in drug metabolism regulated by growth hormone. Int J Biochem Cell Biol. Jan 1995;27(1):9–20. doi: 10.1016/1357-2725(94)00056-5 [DOI] [PubMed] [Google Scholar]
- 82.Ko FC. The clinical care of frail, older adults. Clin Geriatr Med. Feb 2011;27(1):89–100. doi: 10.1016/j.cger.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 83.Walston J, Buta B, Xue QL. Frailty Screening and Interventions: Considerations for Clinical Practice. Clin Geriatr Med. 02 2018;34(1):25–38. doi: 10.1016/j.cger.2017.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stow D, Spiers G, Matthews FE, Hanratty B. What is the evidence that people with frailty have needs for palliative care at the end of life? A systematic review and narrative synthesis. Palliat Med. 04 2019;33(4):399–414. doi: 10.1177/0269216319828650 [DOI] [PMC free article] [PubMed] [Google Scholar]


