Abstract
Objective
Limited literature has examined the epidemiology of non-alcoholic fatty liver disease (NAFLD) and fibrosis among young adults in Egypt, a country with one of the highest obesity rates globally. We assessed the prevalence of steatosis and fibrosis among college students in Egypt.
Design
In this cross-sectional study, we recruited students unaware of having fatty liver via a call-for-participation at a private university in the Dakahlia governorate of Egypt. Primary outcomes were the prevalence of steatosis as determined by the controlled attenuation parameter component of transient elastography and fibrosis as determined by the liver stiffness measurement component of transient elastography. Secondary outcomes were clinical parameters and socioeconomic factors associated with the presence and severity of steatosis and fibrosis.
Results
Of 132 participants evaluated for the study, 120 (91%) were included (median (IQR) age, 20 (19–21) years; 65 (54.2%) female). A total of 38 participants (31.6%) had steatosis, among whom 22 (57.9%) had S3 (severe) steatosis. There was a higher risk for steatosis in persons with overweight (adjusted OR 9.67, 95% CI (2.94 to 31.7, p<0.0001) and obesity (adjusted OR 13.87, 95% CI 4.41 to 43.6, p<0.0001) compared with lean persons. Moreover, higher level of parental education was associated with progressing steatosis stages (S1–S3). Six (5%) participants had transient elastography values equivalent to F2–F3 fibrosis (four with F2 fibrosis (≥7.9 kPa), and two with F3 fibrosis (≥8.8 kPa)).
Conclusion
In this cohort of college students in Egypt, around 1 in 3 had steatosis, and 1 in 20 had moderate-to-advanced fibrosis, an established risk factor for hepatic and extrahepatic morbidity and mortality. These data underscore the urgency to address the silent epidemic of NAFLD among young adults in the Middle East-North Africa region.
Keywords: obesity, nonalcoholic steatohepatitis, hepatic fibrosis
Summary box.
What is already known about this subject?
Non-alcoholic fatty liver disease (NAFLD) has become the most common chronic liver disease in the world.
Egypt has one of the highest obesity rates globally.
There are limited data on the prevalence of NAFLD among young adults in Egypt.
What are the new findings?
In this cohort of asymptomatic Egyptian young adults, about 1 in 3 had steatosis, and 1 in 20 had moderate-to-advanced fibrosis.
Overweight, obesity and increased adiposity were the strongest predictors of having steatosis and its severity.
Parental attainment of a college-level education was associated with increasing steatosis stages.
How might it impact on clinical practice in the foreseeable future?
There is an urgency to address the silent epidemic of NAFLD among young adults in Egypt, which could represent a significant burden to healthcare systems in this country.
Increasing awareness of NAFLD and its complications and promoting lifestyle modification with diet and exercise among young adults in Egypt are warranted.
Introduction
Non-alcoholic fatty liver disease (NAFLD) has become the most common chronic liver disease with an estimated global prevalence of 25% of adults.1 NAFLD is a spectrum that comprises two main histological phenotypes with varying prognoses: NAFL or simple steatosis and non-alcoholic steatohepatitis (NASH). The latter is an advanced inflammatory form of NAFLD that confers higher risk of fibrosis, end-stage liver disease and cardiovascular disease mortality.2 3 In western societies, NASH prevalence among adults is estimated to be around 3%–4%, with as high as 40% of the cases progressing to advanced liver fibrosis.1 4 NAFLD progression is closely related to insulin resistance, obesity and type 2 diabetes,5 and is influenced by environmental factors (eg, dietary fructose and alcohol; dysbiosis) and genetic predisposition (eg, PNPLA3 polymorphism).4 6 Nevertheless, the complex pathogenesis of NAFLD remains incompletely understood.
Egypt, a Middle Eastern country with a population of ~100 million, with 60% of them being younger than 30 years,7 is considered among the highest 10 nations in prevalence of obesity.8 Overall, the Middle East and North Africa (MENA) region has one of the highest NAFLD prevalence rates, which is estimated to affect 31.8% of all adults.1 Young adults are often overlooked under the presumption they are ‘healthy’; however, the presence of NAFLD among this population could represent a major public health issue and may become a significant burden to healthcare systems in this region.4 In Western countries, cohort studies using ultrasonography (US) and transient elastography estimated the prevalence of NAFLD among adolescents 17–18 years of age to range between 2.5% and 20.7%.9 10 Despite that liver biopsy is the gold-standard method to confirm the presence of NAFLD and hepatic fibrosis, its use for wide screening of NAFLD is practically impossible and carries unnecessary risks.11 12 On the other hand, transient elastography is a simple, safe and reliable tool to assess steatosis and liver fibrosis.13 14 Using US, one cross-sectional study in Egypt evaluated 46 young adult women with obesity and polycystic ovary syndrome and found that 52% of them have NAFLD.15 To date, no studies in Egypt evaluated the prevalence of NAFLD and fibrosis among young adults using a widely accepted and validated technique. In this study, we assessed the prevalence of steatosis and fibrosis in a self-selected cohort of college students in Egypt using transient elastography.
Methods
Study design and participants
This is a cross-sectional clinical study in which asymptomatic students (median [IQR] age, 20 [19–21] years) from a single university in the Dakahlia governorate of Egypt were recruited to undergo blood sampling and transient elastography (FibroScan 502 Touch; Echosens, Paris, France) in December 2018 at the university’s internal medicine clinics. University-wide recruitment was conducted through flyers in collaboration with the university facilities, students union, youth care administration and community services administration. The study was conducted on two separate ‘health day events’ and all participants who attended and signed informed consent on the first day were evaluated for the study. No remuneration was provided. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline.
Assessment of outcomes
Participants were asked to come after overnight fasting for blood withdraw to measure serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST); lipid profile; plasma glucose, A1C, serum creatinine and serology for viral hepatitis (hepatitis A, B and C). Transient elastography to evaluate steatosis and fibrosis was performed on a separate day, and participants were asked to fast for at least 3 hours before the test. To capture a controlled attenuation parameter (CAP) score and liver stiffness measurement (LSM), 10 valid scans per subject were needed. If a participant’s median LSM was >7.1 kPa, the IQR-to-median ratio had to be <30% for this measurement to be considered valid.16 We initially used the M probe unless prompted by the device to switch to XL probe, which occurred in 31 (26%) participants, all with body mass index (BMI) ≥30 kg/m2. Per the manufacturer instructions, cut-off values were unified for the M probe and XL probe.
A meta-analysis of studies containing histology verified CAP data was used to determine CAP score cut-offs for each steatosis grade (S0-S3) to determine the presence of NAFLD. S0 was defined as a CAP <248 dB/m (<10% steatosis); S1 as CAP of 248 to <268 dB/m (10% to <33% steatosis (mild)); S2 as CAP of 268 to <280 dB/m (33% to <66% steatosis (moderate)); and S3 as CAP ≥280 dB/m (≥66% steatosis (severe)).17
Cut-off values for transient elastography related to the Meta-analysis of Histological Data in Viral Hepatitis (METAVIR) scoring system were used to determine fibrosis staging (F0–F4). For F0–F1, LSM <7.9 kPa; for F2, LSM of 7.9 to <8.8 kPa; for F3, LSM of 8.8 to <11.7 kPa; and for F4 LSM ≥11.7 kPa.13
Complete medical history, family history and social history were taken. Exclusion criteria included: prior diagnosis of a secondary cause of fatty liver such as Wilson’s disease, autoimmune hepatitis, alpha-1 antitrypsin deficiency or any other known secondary causes of fatty liver (e.g., excessive alcohol consumption). Alcohol consumption was assessed through social history, and excessive alcohol consumption was defined as >21 standard drinks per week for men and >14 standard drinks per week for women, where a standard drink is any drink that contains 14 g of pure alcohol. Socioeconomic and lifestyle data were captured and were expressed as dichotomous variables. BMI and waist-to-height ratio were calculated.
Primary outcomes were the prevalence of steatosis as determined by the CAP component of transient elastography and the prevalence of liver fibrosis as determined by the LSM component of transient elastography. Secondary outcomes were clinical parameters and socioeconomic factors associated with the presence and severity of steatosis and liver fibrosis.
Statistical analysis
Univariable linear regression models were used to examine differences across ordinal categories of steatosis and fibrosis stages. Explanatory variables of interest were BMI categories, waist-to-height ratio, socioeconomic status (parental-level of education and residency), smoking, fast food consumption, exercise habits and sex. We used bivariate logistic regression after dichotomising the presence of steatosis, and ORs were calculated for explanatory variables previously shown to be associated with the development of hepatic steatosis and fibrosis. Because significant associations were detected, multivariable logistic regression was done while adjusting for candidate covariates and adjusted ORs were calculated. Continuous variables were expressed as mean (SD) or median (IQR) according to their distribution as determined by Shapiro-wilks test. Categorical variables were presented as percentages. Statistical analysis was done using STATA/SE V.15.0 (Stata). All hypothesis tests were two sided, and we used an a priori α=0.05.
Results
Participants’ characteristics are shown in table 1. Of the 132 participants who were recruited, 2 refused to participate for personal reasons, and 4 had insufficient data and were excluded from the study. Six participants had unreliable fibroscan measurements and were excluded from the analysis. No participants had positive serology for acute or chronic viral hepatitis or reported significant alcohol consumption. Eventually, 120 participants were included in the analysis. This cohort included 65 women and 55 men. After checking medical history, no participants reported having a diagnosis of Wilson’s disease, autoimmune hepatitis or alpha-1 antitrypsin deficiency or any other known secondary causes of fatty liver. One participant had a diagnosis of hypertension and no participants had history of pre-diabetes or diabetes.
Table 1.
Characteristics of study participants
| Study subjects N=120 |
|
| Sex | NA |
| Female | 65 (54.2) |
| Male | 55 (45.8) |
| Age, years | 20 (19–21) |
| Parental education (highest level achieved) | NA |
| Lower than college | 10 (8.3) |
| College education | 110 (91.7) |
| Residency | NA |
| Rural | 31 (25.8) |
| Urban | 89 (74.2) |
| Smoker | NA |
| Yes | 9 (7.5) |
| No | 111 (92.5) |
| BMI category | NA |
| Underweight (<20 kg/m2) | 31 (25.8) |
| Normal weight (20 to <25 kg/m2) | 30 (25) |
| Overweight (25 to <30 kg/m2) | 28 (23.4) |
| Obese (≥30 kg/m2) | 31 (25.8) |
| Waist-to-height ratio | NA |
| Normal adiposity (<0.5) | 68 (56.7) |
| Increased adiposity (≥0.5) | 52 (43.3) |
| First-degree history of diabetes | NA |
| Yes | 39 (32.5) |
| No | 81 (67.5) |
| Lifestyle habits | |
| Fast food consumption | NA |
| Yes | 82 (68.3) |
| No | 38 (31.7) |
| Exercise | NA |
| Yes | 39 (32.5) |
| No | 81 (67.5) |
Data are n (%) or median (IQR).
BMI, body mass index; NA, not applicable.
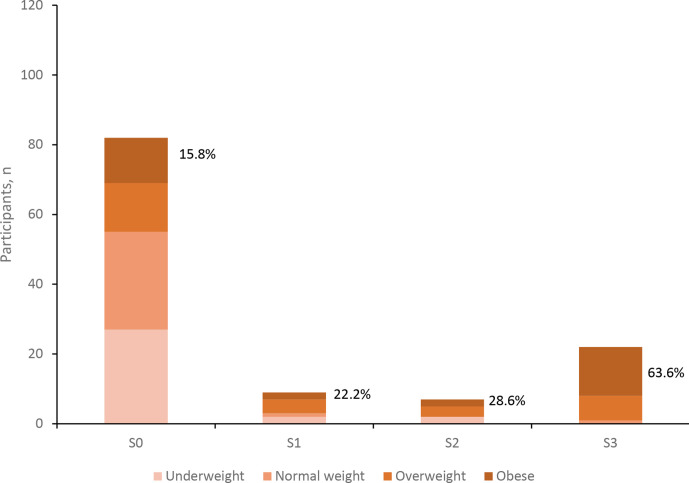
Table 2 shows participants’ characteristics across different steatosis stages. Median CAP score was 211.4 dB/m with a range of 100 to 400 dB/m. Thirty-eight participants (31.6%) had S1 or greater steatosis among whom 22 (57.9%) had S3 (severe) steatosis. BMI was positively associated with increasing (S1–S3) steatosis stages (p<0.0001), as was the proportion of subjects with obesity; being highest (63.6%) in subjects with S3 steatosis (figure 1). Steatosis in participants with BMI <25 kg/m2 (lean NAFLD) was present in six participants (5%). Waist-to-height ratio as a surrogate for adiposity was also positively associated with increasing steatosis stages (p<0.0001, table 2). Similarly, triglycerides were positively associated with increasing steatosis stages, whereas HDL showed an inverse association (p=0.002 and p<0.0001, respectively, table 2). A1C values trended higher with increasing steatosis stages; being at a median of 5.2% (range: 4.9%–5.6%) in participants with S3 steatosis (p=0.012, table 2). ALT, AST, total cholesterol, LDL-cholesterol, plasma glucose and serum creatinine all showed no association with steatosis stages. Among participants with steatosis, 31 (81.6%) of 38 had ALT levels≤30 IU/L for men and ≤19 IU/L for women.
Table 2.
Demographic and clinical exposures across steatosis grades
| S0 (n=82; 68.4%) | S1 (n=9; 7.5%) | S2 (n=7; 5.8%) | S3 (n=22; 18.3%) | P value * | |
| Sex | NA | NA | NA | NA | 0.81 |
| Female | 44 (53.7) | 6 (66.7) | 3 (42.9) | 12 (54.5) | NA |
| Male | 38 (46.3) | 3 (33.3) | 4 (57.1) | 10 (45.5) | NA |
| Parental education | NA | NA | NA | NA | 0.019 |
| Lower than college | 7 (8.5) | 3 (33.3) | 0 | 0 | NA |
| College education | 75 (91.5) | 6 (66.7) | 7 (100) | 22 (100) | NA |
| Residency | NA | NA | NA | NA | 0.64 |
| Rural | 19 (23.2) | 2 (22.2) | 2 (28.6) | 8 (36.4) | NA |
| Urban | 63 (76.8) | 7 (77.8) | 5 (71.4) | 14 (63.6) | NA |
| Smoker | NA | NA | NA | NA | 0.45 |
| Yes | 5 (6.1) | 0 | 1 (14.3) | 3 (13.6) | NA |
| No | 77 (93.9) | 9 (100) | 6 (85.7) | 19 (86.4) | NA |
| BMI, kg/m2 | 23.1 (19.5–27.2) | 26.1 (23.2–26.6) | 25.6 (19.1–30.7) | 31.8 (27–36.3) | <0.0001 |
| Underweight (<20 kg/m2) | 27 (32.9) | 2 (22.2) | 2 (28.6) | 0 | <0.0001 |
| Normal weight (20 to <25 kg/m2) | 28 (34.2) | 1 (11.1) | 0 | 1 (4.6) | <0.0001 |
| Overweight (25 to <30 kg/m2) | 14 (17.1) | 4 (44.5) | 3 (42.8) | 7 (31.8) | <0.0001 |
| Obese (≥30 kg/m2) | 13 (15.8) | 2 (22.2) | 2 (28.6) | 14 (63.6) | <0.0001 |
| Waist-to-height ratio | 0.47 (0.43–0.51) | 0.51 (0.43–0.51) | 0.51 (0.43–0.53) | 0.58 (0.49–0.64) | <0.0001 |
| First-degree history of diabetes | NA | NA | NA | NA | 0.39 |
| Yes | 26 (31.7) | 1 (11.1) | 3 (42.9) | 9 (40.9) | NA |
| No | 56 (68.3) | 8 (88.9) | 4 (57.1) | 13 (59.1) | NA |
| Fast food consumption | NA | NA | NA | NA | 0.31 |
| Yes | 55 (67.1) | 6 (66.7) | 7 (100) | 14 (63.6) | NA |
| No | 27 (32.9) | 3 (33.3) | 0 (0) | 8 (36.4) | NA |
| Exercise | NA | NA | NA | NA | 0.74 |
| Yes | 26 (31.7) | 2 (22.2) | 2 (28.6) | 9 (40.9) | NA |
| No | 56 (68.3) | 7 (77.8) | 5 (71.4) | 13 (59.1) | NA |
| Alanine aminotransferase, IU/L | 15 (11–21) | 15 (13–15) | 15 (12–24) | 15.5 (13–22) | 0.35 |
| Aspartate aminotransferase, IU/L |
21 (18–25) | 22 (19–24) | 21 (19–26) | 20.5 (17–23) | 0.29 |
| Total cholesterol, mg/dL | 166 (151–182) | 150 (139–173) | 171 (153–188) | 162 (151–179) | 0.86 |
| Triglycerides, mg/dL | 76 (59–94) | 67 (59–89) | 75 (68–92) | 106 (80–151) | 0.002 |
| LDL-C, mg/dL | 89 (75–105) | 85 (76–93) | 106 (83–116) | 91 (77–104) | 0.67 |
| HDL-C, mg/dL | 57 (51–64) | 51(47-56) | 49 (46–54) | 50 (41–57) | <0.0001 |
| Plasma glucose, mg/dL | 102 (92–110) | 98 (91–106) | 99 (92–108) | 103 (94–110) | 0.92 |
| Glycated haemoglobin A1c, % | 5.1 (4.9–5.3) | 5 (5–5.1) | 5.1 (5–5.2) | 5.2 (5.1–5.4) | 0.012 |
| Serum creatinine, mg/dL | 0.99 (0.2) | 0.95 (0.2) | 0.95 (0.2) | 0.93 (0.2) | 0.63 |
Data are n (%), median (IQR) for non-normally distributed data or mean (SD) for normally distributed data as determined by Shapiro-Wilk test.
*Pearson’s χ2 test, Wilcoxon rank-sum test or one-way analysis of variance (ANOVA).
BMI, body mass index; HDL-C, High-density lipoprotein; LDL-C, Low-density lipoprotein; NA, not applicable.
Figure 1.
Body mass index categories across steatosis stages. Percentages represent the proportion of obese participants in each stage. N=120.
The proportion of parental attainment of college education was positively associated with steatosis stages being highest (100%) among participants with S2 and S3 steatosis (p=0.019, table 2). Residency (rural vs urban) was not associated with steatosis, neither was a positive history of diabetes among first-degree relatives. Similarly, there was no association between steatosis stage and certain self-reported lifestyle habits: smoking, fast food consumption and exercise (table 2).
On regression analyses, participants with a BMI in the overweight (25–29.9 Kg/m2) and obese (>30 Kg/m2) ranges had significantly higher risk of steatosis (adjusted OR: 9.67; 95% CI 2.94 to 31.7; and 13.87; 4.41 to 43.6, respectively, p<0.0001 for both), than those with a BMI in the lean (<25 Kg/m2) category, after adjusting for lifestyle (ie, smoking, exercise and fast food consumption) and parental education (table 3). Similarly, increased adiposity as calculated from the waist-to-height ratio was associated with a significantly higher risk of steatosis in adjusted models (table 3). On regression analysis, parental attainment of college education did not confer a higher risk of steatosis in this cohort (table 3).
Table 3.
Exposures associated with having steatosis
| Subjects with steatosis | Crude OR (95% CI) |
P value | Adjusted for | Adjusted OR (95% CI) | P value | |
| BMI | ||||||
| Lean* | 6/61 (9.8%) | Reference | NA | Smoking, parental education, exercise, fast food | Reference | NA |
| Overweight | 14/28 (50%) | 9.16 (2.98 to 28.1) | <0.0001 | NA | 9.67 (2.94 to 31.7) | <0.0001 |
| Obese | 18/31 (58.1%) | 12.7 (4.2 to 38.3) | <0.0001 | NA | 13.87 (4.41 to 43.6) | <0.0001 |
| Waist-to-height ratio | 0.64 | |||||
| Normal adiposity (<0.5) | 12/68 (17.6%) | Reference | NA | Smoking, parental education, exercise, fast food | Reference | NA |
| Increased adiposity (≥0.5) | 26/52 (50%) | 4.66 (2.04 to 10.7) | <0.0001 | 5.53 (2.3 to 13.3) | <0.0001 | |
| Parental education | ||||||
| School education | 3/10 (30%) | Reference | NA | BMI, smoking | Reference | NA |
| College education | 35/110 (31.8%) | 1.08 (0.3 to 4.4) | 0.9 | NA | 0.92 (0.2 to 4.2) | 0.9 |
*BMI <25 kg/m2.
BMI, body mass index; NA, not applicable.
Transient elastography values ranged from 2.8 to 8.8 kPa with a median of 5.1 kPa (IQR: 4.25–6). Six (5%) of participants had transient elastography values equivalent to METAVIR F2-F3 fibrosis (four with F2 fibrosis (≥7.9 kPa), and two with F3 fibrosis (≥8.8 kPa)). No participants had F4 fibrosis (≥11.7 kPa). Although CAP score values were numerically higher with increasing fibrosis stages, this association was not statistically significant (p=0.2; table 4). Similarly, BMI values appeared to trend upwards with increasing fibrosis stages, but this trend was non-significant (p=0.22; table 4). The proportion of overweight subjects was positively associated with increasing fibrosis stages being highest (100%) in subjects with F3 fibrosis (p=0.014; table 4). Although a BMI in the overweight range appeared to confer greater risk of fibrosis on unadjusted regression analyses, the association faded after adjusting for lifestyle (ie, smoking, exercise and fast food consumption) and parental education (online supplemental table 1). Obesity was not associated with increased risk of fibrosis in our cohort (online supplemental table 1). Triglycerides were positively associated with increasing fibrosis stages (p=0.015; table 4), whereas, ALT, AST, total cholesterol, HDL, LDL, plasma glucose and serum creatinine showed no association with increasing fibrosis stages (table 4). All six participants with F2 fibrosis or higher had ALT levels≤30 IU/L for men and ≤19 IU/L for women (data not shown). Lastly, we used cut-off values for fibrosis based on the METAVIR staging which was mainly used in viral hepatitis. Therefore, we were curious to know whether using more recent NASH-validated cut-offs would produce different fibrosis prevalence rates. Using updated cut-offs from the NASH clinical research network (CRN) (LSM ≥8.2 kPa for ≥F2),18 we arrived at the exact same prevalence rate reported in our results (6 (5%), data not shown).
Table 4.
Demographic and clinical exposures across fibrosis stages
| F0–F1 (n=114; 95%) | F2 (n=4; 3.3%) | F3 (n=2; 1.7%) | P value * | |
| Sex | NA | NA | NA | 0.97 |
| Female | 62 (54.4) | 2 (50) | 1 (50) | NA |
| Male | 52 (45.6) | 2 (50) | 1 (50) | NA |
| Parental education | NA | NA | NA | 0.43 |
| Lower than college | 9 (7.9) | 1 (25) | 0 | NA |
| College education | 105 (92.1) | 3 (75) | 2 (100) | NA |
| Residency | NA | NA | NA | 0.7 |
| Rural | 30 (26.3) | 1 (25) | 0 | NA |
| Urban | 84 (73.7) | 3 (75) | 2 (100) | NA |
| Smoker | NA | NA | NA | 0.37 |
| Yes | 8 (7) | 1 (25) | 0 | NA |
| No | 106 (93) | 3 (75) | 2 (100) | NA |
| Controlled attenuation parameter (dB/m) | 211 (198–258) | 229.5 (197–316.5) | 268.5 (225–312) | 0.2 |
| BMI, kg/m2 | 24.6 (19.7–30.5) | 27.1 (22.7–31.9) | 28.3 (27.2–29.4) | 0.22 |
| Underweight (<20 kg/m2) | 30 (26.3) | 1 (25) | 0 | 0.7 |
| Normal weight (20 to <25 kg/m2) | 30 (26.3) | 0 | 0 | 0.34 |
| Overweight (25 to <30 kg/m2) | 24 (21.1) | 2 (50) | 2 (100) | 0.014 |
| Obese (≥30 kg/m2) | 30 (26.3) | 1 (25) | 0 | 0.7 |
| Waist-to-height ratio | 0.48 (0.44–0.55) | 0.49 (0.46–0.57) | 0.54 (0.49–0.58) | 0.31 |
| First-degree history of diabetes | NA | NA | NA | 0.46 |
| Yes | 37 (32.5) | 2 (50) | 0 | NA |
| No | 77 (67.5) | 2 (50) | 2 (100) | NA |
| Fast food consumption | NA | NA | NA | 0.59 |
| Yes | 77 (67.5) | 3 (75) | 2 (100) | NA |
| No | 37 (32.5) | 1 (25) | 0 | NA |
| Exercise | NA | NA | NA | 0.048 |
| Yes | 37 (32.5) | 0 | 2 (100) | NA |
| No | 77 (67.5) | 4 (100) | 0 | NA |
| Alanine aminotransferase, IU/L | 15 (12–21) | 12.5 (10–18) | 20.5 (16–25) | 0.85 |
| Aspartate aminotransferase, IU/L |
21 (18–25) | 20.5 (16.5–25.5) | 23.5 (23–24) | 0.88 |
| Total cholesterol, mg/dL | 165 (150–180) | 162.5 (147.5–199) | 162.5 (156–169) | 0.99 |
| Triglycerides, mg/dL | 79 (63–102) | 100.5 (88.5–125) | 124 (114–134) | 0.015 |
| LDL-C, mg/dL | 90.5 (77–105) | 92.5 (79.5–115.5) | 84.5 (71–98) | 0.95 |
| HDL-C, mg/dL | 55 (49–61) | 54 (45–63) | 53 (48–58) | 0.68 |
| Plasma glucose, mg/dL | 102 (92–110) | 104.5 (98.5–113) | 101.5 (89–114) | 0.73 |
| Glycated haemoglobin A1c, % | 5.1 (5–5.3) | 4.95 (4.85–5.05) | 5.1 (5.1–5.1) | 0.32 |
| Serum creatinine, mg/dL | 0.97 (0.2) | 1.06 (0.2) | 1.11 (0.1) | 0.45 |
Data are n (%), median (IQR) for non-normally distributed data or mean (SD) for normally distributed data as determined by Shapiro-Wilk test.
*Pearson’s χ2 test, Wilcoxon rank-sum test or one-way analysis of variance (ANOVA).
BMI, body mass index; HDL-C, High-density lipoprotein; LDL-C, Low-density lipoprotein; NA, not applicable.
bmjgast-2021-000780supp001.pdf (84.6KB, pdf)
Discussion
In our cohort of Egyptian college students, around 1 in 3 had steatosis, and 1 in 20 had fibrosis. To the best of our knowledge, this is the first attempt to evaluate the prevalence of NAFLD among Egyptian young adults using transient elastography. Our estimated NAFLD prevalence of 31.6% among young adults is exactly similar to the 31.8% prevalence rate that was shown in a meta-analysis of several epidemiological studies among general Middle Eastern populations that collectively included 1592 adults.1 Knowing that NAFLD prevalence increases with age,1 it is concerning to find such a high prevalence rate among young Egyptian adults. It is known that the MENA region has one of the highest prevalence rates of NAFLD globally,1 and that Egypt ranked among the highest 10 nations with obesity prevalence.8 Combing both may explain our unexpected observation. In our cohort, 59 (49.2%) of participants had overweight or obesity. Furthermore, BMI category and adiposity were independent predictors of steatosis after adjusting for other covariates. In western societies, the prevalence of hepatic steatosis among young adults is much lower. In a large epidemiological study in the UK10 that assessed 4021 young adults (median age 24 years) for hepatic steatosis and liver fibrosis using CAP and transient elastography with similar cut-off values to our study, 780 individuals (20.7%) were shown to have hepatic steatosis with 10% having S3 (severe) steatosis. This contrast may be attributed to the higher prevalence rates of age-standardised overweight and obesity in Egypt in comparison to UK (31.5% vs 26.1% for boys<20 years; and 39.5% vs 29.2% for girls<20 years, respectively).8 The prevalence of ≥F2 fibrosis in our cohort was approximately twice that reported in the UK study (5% vs 2.8%). Although transient elastography was extensively validated against liver biopsy13 14 to detect significant fibrosis (≥F2); it has limited ability to discriminate between F0 and F1 fibrosis.13 14 This might lead to under-reporting of hepatic fibrosis among participants in our study. Although a BMI in the overweight category was significantly associated with higher risk for fibrosis; this association became non-significant after adjusting for other confounders.
While parental attainment of college education did not confer higher risk of steatosis on regression analyses, it was proportionally higher with increasing steatosis stages (table 2). A study conducted in rural India reported that patients with NAFLD were more likely to have higher income. It also showed that higher family income is an independent predictor of NAFLD.19 Another study found that patients with NAFLD were more likely to live close to regions with abundant food resources (ie, grocery stores and restaurants).20 Westerns-style fast food chains that frequently serve calorie-dense meals are often accessible to higher-income families in Egypt and MENA region which contrasts with western societies where fast-food restaurants are widespread within low-income areas.21–23 In our cohort, self-reported lifestyle habits (ie, tendency to exercise, consumption of fast food and smoking) and other surrogates of socioeconomic status (ie, rural vs urban living) showed no association with steatosis. Meanwhile, findings from a large multicentre study among Hispanic/Latino individuals that examined the role of socioeconomic and lifestyle factors found no association with risk of developing NAFLD.24 We found obesity to be the strongest factor associated with progression of steatosis. On the other side of the spectrum, lean NAFLD was present in only 6 (5%) of participants.
In our cohort, increasing CAP scores were not associated with fibrosis progression. The trajectory of NAFLD progression from NAFL to NASH and fibrosis is non-linear.4 Moreover, some patients may progress from steatosis to NASH fibrosis and even experience periods of spontaneous fibrosis regression on liver histology.25 26 Since our study was cross-sectional in design, it is plausible to expect that our reported fibrosis prevalence is underestimated.
We found no participants in our cohort to have A1C levels consistent with pre-diabetes or diabetes diagnosis, however, higher A1C levels were associated with progressing steatosis stages (S1–S3), which further supports the strong relationship between deteriorating glycaemia and the development of steatosis.27 28 Our observation is also in line with a recent report from China, which found that higher A1C within the nondiabetic range to be associated with incident NAFLD.29 Interestingly, 31 (81.6%) of 38 participants with steatosis and all 6 (100%) with ≥F2 fibrosis had normal ALT levels (≤30 IU/L for men and ≤19 IU/L for women).30 This observation underscores the poor utility of ALT alone as an initial screening test for NAFLD and fibrosis.
Our study had limitations. First, participants were recruited from a single university which may limit the study’s external validity and may introduce bias because responders to recruitment may have more concerns about their liver health than non-responders. However, the validated and rigorous methods of data collection allow for generalisability to the entire university. Second, comprehensive serological screening for secondary causes of fatty liver (ie, Wilson’s disease, autoimmune hepatitis or alpha-1 antitrypsin deficiency) was not done; however, serological screening was done for viral hepatitis and participants did not report having a diagnosis of any of the former conditions. Third, transient elastography has limited ability to distinguish between early fibrosis stages (F0 and F1); however, it has been extensively validated to detect significant fibrosis (≥F2).13 14 Therefore, our estimation of fibrosis prevalence should be interpreted with caution.
In conclusion, this study found steatosis is present in about one-third of an asymptomatic young adult cohort in Egypt. NAFLD confers increased risk of diabetes, cardiovascular disease, liver cirrhosis and cancer. At least 1 in 20 young adults had moderate-to-advanced fibrosis (F2–F3), which is concerning knowing that fibrosis is the most important predictor of morbidity and mortality in NASH. Although a minority of patients (3%–4% in western cohorts) will progress to NASH, those who do so could experience a fibrosis progression rate that ranges from 7 to 14 years per fibrosis stage.25 31 Higher socioeconomic status was associated with increased severity of steatosis in this cohort. This study serves as a call-to-action for Egyptian health authorities to curb the potential adverse implications of the obesity epidemic among young adults in Egypt. Furthermore, it provides rationale for conducting validation research on the population level to inform policy and public health interventions and increase awareness of NAFLD and its complications in Egypt and the MENA region.
Footnotes
Twitter: @Tomah_Hawk
Presented at: Data from this work were presented at the American Diabetes Association’s 79th Scientific Sessions, June 2019, San Francisco, CA, USA.
Contributors: Concept and design: OH and EE. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: ST. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: ST. Supervision: OH and EE.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: ST reports consulting for Research America and stock ownership in Amarin corp. outside the submitted work. NA is on advisory board/review panel for 89Bio, Echosens, Fibronostics, Gilead, Intercept, Novo Nordisk, Perspectum, Pfizer and Zydus, received grant/research support from 89Bio, Akero, Bristol Myers Squibb, Galectin, Genentech, Gilead, Intercept, Ionis, Madrigal, Metacrine, NGM Bio, Novo Nordisk, Pfizer, Viking and Zydus, and is speaker for AbbVie, Alexion, Echosens, Gilead, and Intercept. OH reports consultation to Abbott Nutrition, Merck Serono and Sanofi Aventis, received research support from Novo-Nordisk, Eli Lilly, National Dairy Council, and Gilead, and is on the advisory board of L-Nutra and Twin. He is also shareholder of Heathimation Inc. MA, AH, MA-B, HG, AE and EAE have no disclosures relevant to this work.
Patient and public involvement statement: Patients or the public were not involved in the design or conduct of this study. Results from this research will be disseminated by Delta the Uuniversity for Science and Technology to student participants and the general public.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Data presented in this manuscript are retained by Delta University (full data) and Joslin Diabetes Center (deidentified raw data only). Data and STATA code used in this manuscript will be made available on reasonable request to the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study protocol was approved by the institution’s Council of Research and Postgraduate Affairs (approval # RP-18.10.7) and was conducted according to principles outlined in the Declaration of Helsinki.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 2.Alkhouri N, Feldstein AE. Noninvasive diagnosis of nonalcoholic fatty liver disease: are we there yet? Metabolism 2016;65:1087–95. 10.1016/j.metabol.2016.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rinella ME, Sanyal AJ. Management of nafld: a stage-based approach. Nat Rev Gastroenterol Hepatol 2016;13:196–205. 10.1038/nrgastro.2016.3 [DOI] [PubMed] [Google Scholar]
- 4.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11–20. 10.1038/nrgastro.2017.109 [DOI] [PubMed] [Google Scholar]
- 5.Tomah S, Alkhouri N, Hamdy O. Nonalcoholic fatty liver disease and type 2 diabetes: where do diabetologists stand? Clin Diabetes Endocrinol 2020;6:1–11. 10.1186/s40842-020-00097-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powell EE, Wong VW-S, Rinella M. Non-alcoholic fatty liver disease. The Lancet 2021;397:2212–24. 10.1016/S0140-6736(20)32511-3 [DOI] [PubMed] [Google Scholar]
- 7.The world bank data, 2019. Available: https://data.worldbank.org/country/egypt-arab-rep
- 8.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. The Lancet 2014;384:766–81. 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawlor DA, Callaway M, Macdonald-Wallis C, et al. Nonalcoholic fatty liver disease, liver fibrosis, and cardiometabolic risk factors in adolescence: a cross-sectional study of 1874 general population adolescents. J Clin Endocrinol Metab 2014;99:E410–7. 10.1210/jc.2013-3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abeysekera KWM, Fernandes GS, Hammerton G, et al. Prevalence of steatosis and fibrosis in young adults in the UK: a population-based study. Lancet Gastroenterol Hepatol 2020;5:295–305. 10.1016/S2468-1253(19)30419-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rockey DC, Caldwell SH, Goodman ZD, et al. Liver biopsy. Hepatology 2009;49:1017–44. 10.1002/hep.22742 [DOI] [PubMed] [Google Scholar]
- 12.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005;128:1898–906. 10.1053/j.gastro.2005.03.084 [DOI] [PubMed] [Google Scholar]
- 13.Wong VW-S, Vergniol J, Wong GL-H, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010;51:454–62. 10.1002/hep.23312 [DOI] [PubMed] [Google Scholar]
- 14.Siddiqui MS, Vuppalanchi R, Van Natta ML, et al. Vibration-Controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2019;17:e152:156–63. 10.1016/j.cgh.2018.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eldesoky A-EE, Gad YZ, Ahmed N. Nonalcoholic fatty liver disease in young adult Egyptian women with polycystic ovary syndrome. Egyptian Liver J 2013;3:15–19. 10.1097/01.ELX.0000424245.18235.1e [DOI] [Google Scholar]
- 16.Boursier J, Zarski J-P, de Ledinghen V, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013;57:1182–91. 10.1002/hep.25993 [DOI] [PubMed] [Google Scholar]
- 17.Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol 2017;66:1022–30. 10.1016/j.jhep.2016.12.022 [DOI] [PubMed] [Google Scholar]
- 18.Eddowes PJ, Sasso M, Allison M, et al. Accuracy of fibroscan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2019;156:1717–30. 10.1053/j.gastro.2019.01.042 [DOI] [PubMed] [Google Scholar]
- 19.Das K, Das K, Mukherjee PS, et al. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology 2010;51:1593–602. 10.1002/hep.23567 [DOI] [PubMed] [Google Scholar]
- 20.Leslie T, Pawloski L, Kallman-Price J, et al. Survey of health status, nutrition and geography of food selection of chronic liver disease patients. Ann Hepatol 2014;13:533–40. 10.1016/S1665-2681(19)31253-0 [DOI] [PubMed] [Google Scholar]
- 21.Peterson MA. Connected in Cairo: growing up cosmopolitan in the modern middle East. Indiana University Press, 2011. [Google Scholar]
- 22.Fleischhacker SE, Evenson KR, Rodriguez DA, et al. A systematic review of fast food access studies. Obes Rev 2011;12:e460–71. 10.1111/j.1467-789X.2010.00715.x [DOI] [PubMed] [Google Scholar]
- 23.Powell LM, Chaloupka FJ, Bao Y. The availability of fast-food and full-service restaurants in the United States: associations with neighborhood characteristics. Am J Prev Med 2007;33:S240–5. 10.1016/j.amepre.2007.07.005 [DOI] [PubMed] [Google Scholar]
- 24.Kallwitz ER, Daviglus ML, Allison MA, et al. Prevalence of suspected nonalcoholic fatty liver disease in hispanic/latino individuals differs by heritage. Clin Gastroenterol Hepatol 2015;13:569–76. 10.1016/j.cgh.2014.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McPherson S, Hardy T, Henderson E, et al. Evidence of nafld progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol 2015;62:1148–55. 10.1016/j.jhep.2014.11.034 [DOI] [PubMed] [Google Scholar]
- 26.Younossi ZM, Loomba R, Rinella ME, et al. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2018;68:361–71. 10.1002/hep.29724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pang Y, Kartsonaki C, Turnbull I, et al. Diabetes, plasma glucose, and incidence of fatty liver, cirrhosis, and liver cancer: a prospective study of 0.5 million people. Hepatology 2018;68:1308–18. 10.1002/hep.30083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bae JC, Cho YK, Lee WY, et al. Impact of nonalcoholic fatty liver disease on insulin resistance in relation to HbA1c levels in nondiabetic subjects. Am J Gastroenterol 2010;105:2389–95. 10.1038/ajg.2010.275 [DOI] [PubMed] [Google Scholar]
- 29.Wang B, Li M, Zhao Z, et al. Glycemic measures and development and resolution of nonalcoholic fatty liver disease in nondiabetic individuals. J Clin Endocrinol Metab 2020;105:1416–26. 10.1210/clinem/dgaa112 [DOI] [PubMed] [Google Scholar]
- 30.Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002;137:1–10. 10.7326/0003-4819-137-1-200207020-00006 [DOI] [PubMed] [Google Scholar]
- 31.Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015;13:e649:643–54. 10.1016/j.cgh.2014.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2021-000780supp001.pdf (84.6KB, pdf)
Data Availability Statement
Data are available on reasonable request. Data presented in this manuscript are retained by Delta University (full data) and Joslin Diabetes Center (deidentified raw data only). Data and STATA code used in this manuscript will be made available on reasonable request to the corresponding author.