Abstract
Sleep disorders are linked to development of type 2 diabetes and increase the risk of developing diabetes complications. Treating sleep disorders might therefore play an important role in the prevention of diabetes progression. However, the detection and treatment of sleep disorders are not part of standardised care for people with type 2 diabetes. To highlight the importance of sleep disorders in people with type 2 diabetes, we provide a review of the literature on the prevalence of sleep disorders in type 2 diabetes and the association between sleep disorders and health outcomes, such as glycaemic control, microvascular and macrovascular complications, depression, mortality and quality of life. Additionally, we examine the extent to which treating sleep disorders in people with type 2 diabetes improves these health outcomes. We performed a literature search in PubMed from inception until January 2021, using search terms for sleep disorders, type 2 diabetes, prevalence, treatment and health outcomes. Both observational and experimental studies were included in the review. We found that insomnia (39% [95% CI 34, 44]), obstructive sleep apnoea (55–86%) and restless legs syndrome (8–45%) were more prevalent in people with type 2 diabetes, compared with the general population. No studies reported prevalence rates for circadian rhythm sleep–wake disorders, central disorders of hypersomnolence or parasomnias. Additionally, several cross-sectional and prospective studies showed that sleep disorders negatively affect health outcomes in at least one diabetes domain, especially glycaemic control. For example, insomnia is associated with increased HbA1c levels (2.51 mmol/mol [95% CI 1.1, 4.4]; 0.23% [95% CI 0.1, 0.4]). Finally, randomised controlled trials that investigate the effect of treating sleep disorders in people with type 2 diabetes are scarce, based on a small number of participants and sometimes inconclusive. Conventional therapies such as weight loss, sleep education and cognitive behavioural therapy seem to be effective in improving sleep and health outcomes in people with type 2 diabetes. We conclude that sleep disorders are highly prevalent in people with type 2 diabetes, negatively affecting health outcomes. Since treatment of the sleep disorder could prevent diabetes progression, efforts should be made to diagnose and treat sleep disorders in type 2 diabetes in order to ultimately improve health and therefore quality of life.
Graphical abstract
Supplementary Information
The online version contains peer-reviewed but unedited supplementary material including a slideset of the figures for download, available at 10.1007/s00125-021-05541-0.
Keywords: Health outcomes, Prevalence, Review, Sleep disorders, Type 2 diabetes
Introduction
Diabetes is a severe public health problem, negatively affecting a person’s quality of life (QoL) and health, through increasing the risk of microvascular and macrovascular complications, depression and mortality. An important but less-known risk factor for the development of type 2 diabetes is having a sleep disorder. Sleep disorders negatively affect sleep quality and duration, causing detrimental effects on glucose metabolism and weight regulation [1]. For example, a sleep duration of <5 h and poor sleep quality are associated with developing type 2 diabetes (RR 1.48 [95% CI 1.25, 1.76] and RR 1.40 [95% CI 1.21, 1.63], respectively) [1], and insomnia and obstructive sleep apnoea (OSA) have been associated with developing type 2 diabetes (OR 1.07 [95% CI 1.02, 1.11] and OR 2.02 [95% CI 1.57, 2.61], respectively) [1, 2]. Managing and treating sleep disorders could therefore play an important role in the prevention of type 2 diabetes.
According to the of the International Classification of Sleep Disorders, third edition (ICSD-3) [3], sleep disorders can be divided into six main groups: insomnia; sleep-related breathing disorders; central disorders of hypersomnolence; circadian rhythm sleep–wake disorders (CRSWDs); parasomnias; and sleep-related movement disorders (see detailed descriptions in Table 1). Sleep disorders typically cause disturbances in the quality, amount and timing of sleep, resulting in impaired daytime functioning and distress. Several sleep disorders are highly prevalent in the general population (Table 1). Considering the association between sleep disorders and development of type 2 diabetes, the prevalence of sleep disorders in people with type 2 diabetes is probably higher compared with the general population.
Table 1.
Definitions based on ICSD-3 and prevalence of sleep disorders in the general population
| Sleep problem | Definition of sleep disorder based on ICSD-3 | Prevalence in general population (%)a |
|---|---|---|
| Insomnia | Disorder characterised by a dissatisfaction in quality or quantity of sleep resulting in significant daytime distress. Insomnia is associated with problems initiating or maintaining sleep, frequent awakenings and the inability to return back to sleep. These complaints occur despite adequate opportunity and circumstances to sleep. | 10 |
| Sleep-related breathing disorders | Group of disorders characterised by symptoms such as snoring, fatigue, insomnia or subjective respiratory disturbances, or associated medical or psychiatric disorders in combination with ≥5 predominantly obstructive respiratory events per h of sleep, or ≥15 obstructive respiratory event per h (even in absence of symptoms). This diagnosis can be further subdivided into OSA disorders, central sleep apnoea syndromes, sleep-related hypoventilation disorders and idiopathic central alveolar hypoventilation. | 3–7 |
| Central disorders of hypersomnolence | Group of disorders characterised by subjective excessive daytime sleepiness that cannot be explained as a result of another sleep–wake disorder, resulting in daily occurrences of an insuppressible need to sleep or daytime lapses into sleep. This disorder group includes narcolepsy, idiopathic hypersomnia, insufficient sleep syndrome, and hypersomnias due to medical disorders, medication or substance and psychiatric disorder | 0.02–0.18 |
| CRSWDs | The disorders belonging to this group include delayed and advanced sleep–wake phase disorder, irregular sleep–wake rhythm disorder, non-24 h sleep–wake rhythm disorder, shift-work disorder, jet-lag disorder and circadian sleep–wake disorders not otherwise specified. The disorders are characterised by a chronic or recurrent pattern of sleep-disruption primarily caused by a change in the endogenous circadian timing system or misalignments between the endogenous circadian rhythm and the socially desired rhythm, resulting in insomnia or excessive sleepiness. It is associated with distress or functional impairment over a period of at least 3 months (except for jet-lag disorder). | 7–16 |
| Parasomnias | Parasomnias can be divided into NREM-related parasomnias, REM-related parasomnias and other parasomnias. NREM-related disorders include recurrent episodes of incomplete awakening, with abnormal responsiveness, limited or no memory or dream report, and at least partial amnesia for the episode. REM-related parasomnias occur as a consequence of state dissociation between REM sleep and being awake. | 3–17 |
| Sleep-related movement disorders | Group of disorders characterised by simple, often repeated movements during sleep. Diagnoses include RLS, PLMD, REM sleep behaviour disorder and others | 5–10 |
aData from [79]
ICSD-3, International Classification of Sleep Disorders, third edition; NREM, non-rapid eye movement
Sleep disorders may also result in faster diabetes progression and thus play an important role in diabetes management. However, detection and treatment of sleep disorders are not part of standardised care for people with type 2 diabetes. To start addressing the gap between current knowledge and clinical care, we provide a review of the literature on the prevalence of sleep disorders in people with type 2 diabetes and the association with the following health outcomes: glycaemic control, microvascular and macrovascular complications, depression, mortality and QoL. We also explore the extent to which treating sleep disorders in people with type 2 diabetes improves the above-mentioned health outcomes. With this review, we aim to highlight the importance of targeted diagnosis and treatment of sleep disorders in people with type 2 diabetes.
Methods
A literature search was performed in PubMed from inception until January 2021, using MeSH and tiab search terms indicating sleep disorders (e.g. ‘sleep disorders’, ‘sleep wake disorders’, ‘sleep deprivation’, ‘circadian’, ‘sleep arousal’, ‘insomnia’, ‘obstructive sleep apnea’ and ‘restless legs syndrome’) and type 2 diabetes mellitus (e.g. ‘diabetes mellitus 2’ and ‘non-insulin dependent’). Additionally, search terms for prevalence, treatment and health outcomes were used (e.g. ‘morbidity’, ‘prevalence’, ‘depressive disorder’, ‘sleep drug’, ‘sleep medication’ and ‘health status’). See Electronic supplementary material Table 1 for the full list of search terms. All relevant English or Dutch language original and review studies were read by two authors (SS and FR) and summarised. Observational (cross-sectional and longitudinal) and experimental studies were included. No quality assessment or risk of bias assessment were made.
Prevalence and health outcomes of sleep disorders in type 2 diabetes
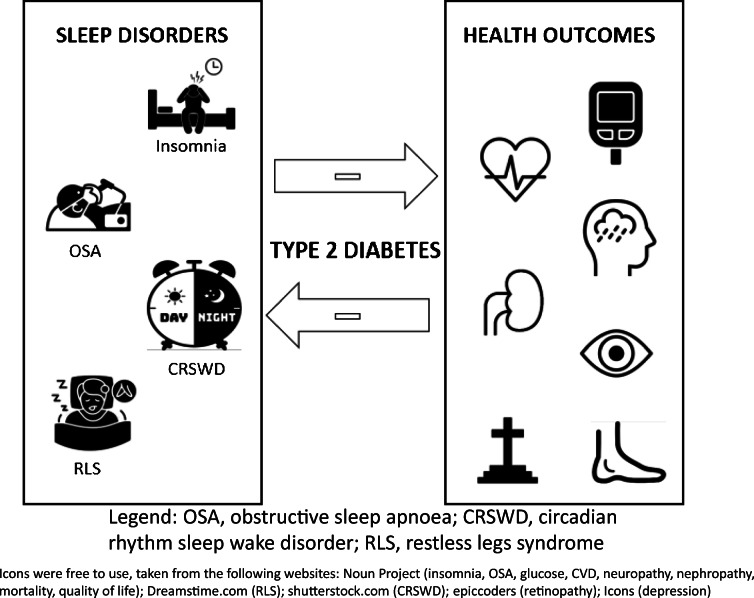
In this section, we discuss the evidence on prevalence and associations of sleep disorders with health outcomes (see also Fig. 1). No or limited literature was found for hypersomnolence, parasomnias and several movement disorders. A review by Mohammadi et al. identified several papers that elaborate on mechanisms by which narcolepsy, a very specific disorder, increases the risk of development of type 2 diabetes [4]. However, no papers on prevalence of hypersomnolence and associated health outcomes in people with type 2 diabetes were identified. Only case reports were found for parasomnias (i.e. abnormal nocturnal behaviour [5] and rapid eye movement (REM) sleep behaviour disorder [6]), which will not be discussed here. For movement disorders other than restless legs syndrome (RLS) and periodic limbic movement disorder (PLMD), some were identified as a risk factor for the development of type 2 diabetes (i.e. bruxism [7]). Further research is needed to investigate the prevalence of the aforementioned sleep disorders and associated health outcomes in type 2 diabetes. Finally, data on the prevalence of having multiple sleep disorders simultaneously in those with diabetes is lacking. This is important as OSA and insomnia frequently co-occur in the general population and this co-occurrence is associated with increased comorbidities, including diabetes [8].
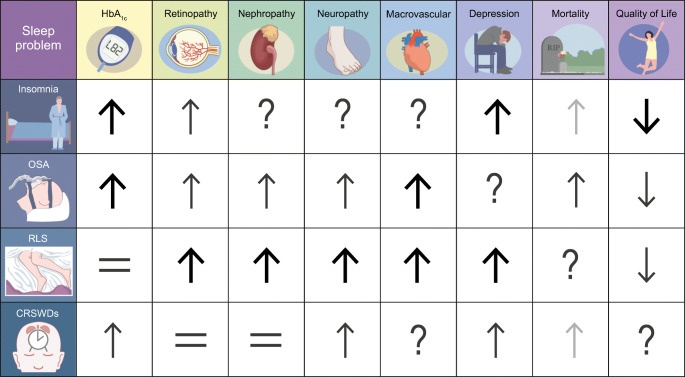
Fig. 1.
Summary of the literature to date on association between sleep disorders, health outcomes and QoL in people with type 2 diabetes. ↑, increased risk or higher levels; ↓, decreased risk or lower levels; =, no change in risk or levels; ?, no data available. Bold black arrows, strong evidence based on large study sample or multiple studies; non-bold black arrows, medium strength evidence; grey arrows, evidence based on small sample or subgroup. This figure is available as part of a downloadable slideset
Insomnia
Insomnia is characterised by difficulty initiating and maintaining sleep or by waking up earlier than desired despite adequate opportunity to sleep. A meta-analysis of 71 studies from our group [9] showed that the prevalence of insomnia and insomnia symptoms in people with type 2 diabetes is 39% (95% CI 34, 44), which is four times higher than in the general population. The prevalence was even higher with increasing age (44%) or with comorbidities present (60%). However, these numbers should be interpreted cautiously, due to the high heterogeneity of the studies included.
The meta-analysed data [9] also revealed associations between insomnia and poorer health outcomes, such as poorer control of HbA1c (2.51 mmol/mol [95% CI 1.1, 4.4]; 0.23% [95% CI 0.1, 0.4]) and fasting glucose (0.4 mmol/l [95% CI 0.2, 0.7), in people with type 2 diabetes and insomnia (symptoms), compared with those with type 2 diabetes only. Chew et al. [10] showed insomnia to be associated with diabetic retinopathy (OR 1.61 [95% CI 1.01, 2.49) in 1231 people with diabetes. No studies investigated associations between insomnia and other microvascular or macrovascular complications. Our meta-analysis [9] did, however, show an association between insomnia and the predecessor of such complications (i.e. high cholesterol levels).
Several studies have shown insomnia to be associated with depressive symptoms in type 2 diabetes (OR 1.31 [95% CI 1.16, 1.47]) [11]. Furthermore, in people with diabetes or hypertension, insomnia is associated with an increased mortality rate (OR 7.17 [95% CI 1.41, 36.62]) [12]. Finally, in addition to poorer health outcomes, and perhaps most important for patients, insomnia negatively affects QoL, affecting all domains of QoL questionnaires [13, 14], compared with those with type 2 diabetes without insomnia.
OSA
OSA, a sleep-related breathing disorder, is characterised by complaints such as non-restorative sleep, sleepiness or snoring, accompanied by obstructive respiratory events. A recent review of 12 studies, by Reutrakul and Mokhlesi [15], showed that OSA is more prevalent in people with type 2 diabetes, with the overall OSA prevalence ranging from 55% to 86%. More severe complaints and higher incidence were reported in men [15]. The prevalence of OSA is 86% in obese populations with type 2 diabetes. The shared association with obesity makes discerning an independent link between OSA and diabetes challenging. In their review, Kent et al. [16] showed that intermittent hypoxia and sleep deprivation/fragmentation play a synergistic role in glucose dysfunction and obesity. OSA is a strong predictor of diabetes, with a 49% increase in diabetes risk after adjustment for covariates, including BMI [1]. Moreover, the combined occurrence of OSA and insomnia is associated with higher prevalence of cardiometabolic morbidity, including diabetes, irrespective of BMI [8].
Although highly prevalent, OSA still remains undiagnosed in most people with type 2 diabetes managed in primary care, with only 18% being detected [15]. When diagnosed, OSA is associated with poorer glycaemic control, with 11 mmol/mol (1%) difference in HbA1c levels between those with type 2 diabetes in lowest vs highest OSA severity quartiles [17]. People with type 2 diabetes and OSA are also more likely to develop microvascular complications, with OSA explaining 19% of the variance for retinopathy measures (r = 0.2; p = 0.04) [18] and being associated with an increased risk of diabetic nephropathy (OR 2.64 [95% CI 1.13, 6.16]) [19] as well as diabetic neuropathy (OR 3.97 [95% CI 1.80, 8.74]) [20]. Additionally, people with type 2 diabetes and OSA are more likely to develop coronary artery disease (HR 2.2 [95% CI 1.2, 3.9]) and heart failure (HR 3.5 [95% CI 1.4, 9.0]) [21]. No studies have reported on the association between OSA and depression in type 2 diabetes. A prospective population-based study did show that people with type 2 diabetes and OSA have a higher risk of cardiovascular mortality (HR 2.37 [95% CI 1.16, 4.82]), compared with people with only type 2 diabetes or only OSA [22]. Finally, in addition to poorer health outcomes, OSA affects QoL in people with type 2 diabetes; those with OSA score lower in all domains of QoL questionnaires, compared with people with type 2 diabetes only [23].
RLS and PLMD
RLS is a sleep-related movement disorder that is characterised by the urge to move in response to uncomfortable and unpleasant sensations in the legs during periods of rest or inactivity, thus interfering with sleep. The exact pathophysiology of RLS is not known, but changes in dopaminergic neurotransmission, related to iron deficiency in specific brain areas, seem to play an important role [24]. Findings from 970 participants from several cross-sectional and case–control studies [25–32] suggest that the prevalence of RLS in people with type 2 diabetes ranges from 8% to 45%, based on the International RLS Study Group criteria. No sex differences were reported. This prevalence might be an overestimation, as until recently these study group criteria could not sufficiently differentiate RLS from peripheral neuropathy [25, 33].
With regard to health outcomes, in a population of 872 people with type 2 diabetes, RLS was associated with a higher prevalence of retinopathy (OR 1.69 [95% CI 1.15, 2.49]), neuropathy (OR 1.37 [95% CI 1.44, 3.90]) and nephropathy (OR 2.19 [95% CI 1.31, 3.68]) [34]. In the same population, a higher prevalence of macrovascular complications was observed, namely coronary heart disease (OR 1.95 [95% CI 1.32, 2.89]) and stroke (OR 2.15 [95% CI 1.27, 2.63]), compared with the prevalence in people with type 2 diabetes only. No statistically significant increase in HbA1c level [26, 29, 31] was reported in people with type 2 diabetes and RLS, compared with those without RLS, but they were more likely to develop depression (OR 3.21 [95% CI 1.07, 11.23]) [35]. Studies on mortality, type 2 diabetes and RLS were not identified. In addition to poorer health outcomes, people with RLS and type 2 diabetes have significantly lower QoL (e.g. vitality score 52.3 vs 74.4; p < 0.001) than people with type 2 diabetes alone [30].
PLMD is repetitive cramping or jerking of the legs during sleep. It is the only movement disorder that occurs exclusively during sleep and is often linked with RLS, which occurs during wakefulness. Rizzi et al. [36] reported that PLMD prevalence was higher in people with type 2 diabetes than in age-matched healthy volunteers (85% vs 33%). PLMD was associated with a higher prevalence of daytime somnolence (50% vs 8%; p < 0.01) in type 2 diabetes, but no other studies on health outcomes related to PLMD were found.
Overall, these studies on RLS and PLMD show that sleep-related movement disorders are highly prevalent in type 2 diabetes and have negative health outcomes.
CRSWDs
CRSWDs affect the timing of sleep either through a dysfunctional biological clock system or through a misalignment between endogenous and exogenous cues. The role of the disturbance of the circadian clock in type 2 diabetes development has been studied extensively in animals and sparsely in humans [37]. To our knowledge, there are no studies on the prevalence or related health outcomes of CRSWDs in people with type 2 diabetes. Shift work, however, is a strong predictor of CRSWDs. People with type 2 diabetes that perform (night)shift work are more likely to have insufficient glycaemic control when compared with people with type 2 diabetes performing day work: blood glucose levels of ≤7.2 mmol/l during the last 6 months (84.2% vs 71.7%; p = 0.02) [38]; and higher HbA1c levels [39, 40].
In addition, these shift workers report poorer mental health based on the General Health Questionnaire (37.5% vs 14.2%) and more microvascular complications (e.g. higher frequency of diabetic neuropathy [10.5% vs 3.9%; p = 0.005] [39]), compared with people with type 2 diabetes working dayshifts. Studies reporting on macrovascular complications or QoL associated with shift work in people with type 2 diabetes are lacking. Even though based on only eight deaths, a Swedish study in over 18,000 nurses did find a significant association between nightshift work and risk of diabetes-related mortality (HR 12.0 [95% CI 3.17, 45.2]) [41]. Overall, research shows poorer health outcomes in participants with type 2 diabetes working shifts.
Treating sleep disorders in type 2 diabetes
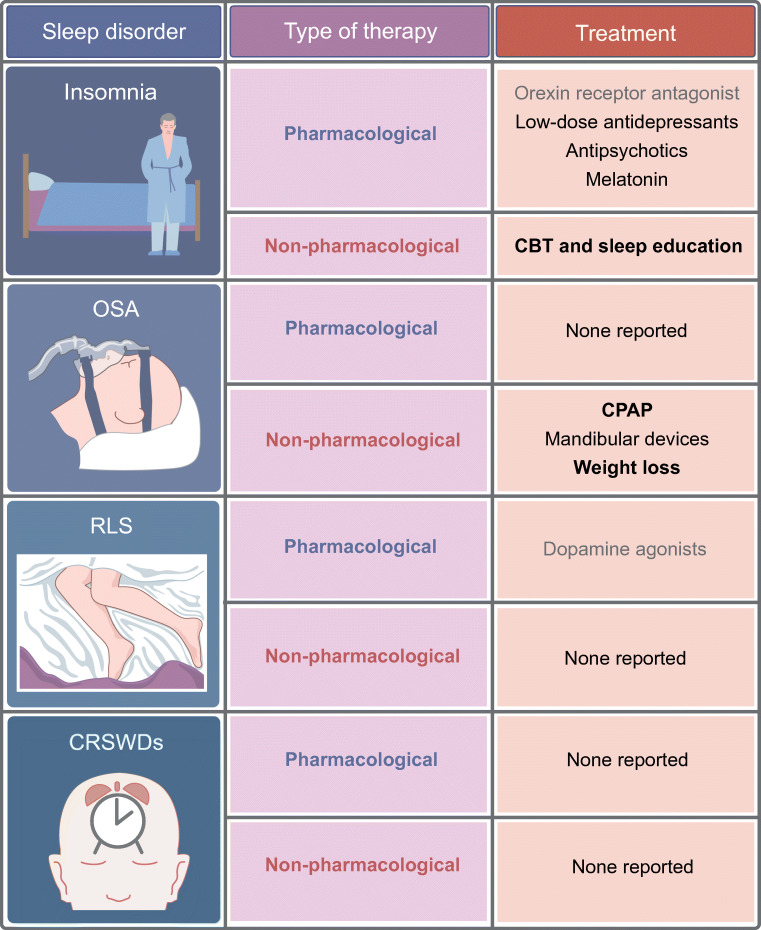
In this section we discuss the current knowledge on treating sleep disorders in people with type 2 diabetes and the effect of treatment on health outcomes (see also Fig. 2). Sleep disorder treatments are categorised into pharmacological and non-pharmacological [42].
Fig. 2.
Summary of the possible pharmacological and non-pharmacological treatment options for sleep disorders in people with type 2 diabetes. Bold text, strong evidence based on large study sample or multiple studies; non-bold black text, medium strength evidence; grey text, evidence based on small study sample or subgroup. This figure is available as part of a downloadable slideset
Pharmacological treatment
In one study, suvorexant, a selective orexin receptor antagonist, improved sleep quality and obesity-associated variables in people with type 2 diabetes (n = 13) after 14 weeks [43]. In another study in 75 people with type 2 diabetes and insomnia receiving dexzopiclone or estazolam for 14 days, sleep was improved in both groups but only fasting glucose was significantly reduced in the dexzopiclone group. This suggests a direct effect of dexzopiclone on glycaemic control [44]. To date, no studies on the glycaemic effects of other hypnotics in type 2 diabetes have been published. This would be important as hypnotics are often prescribed to people with type 2 diabetes [45]. Especially considering the frequent off-label use of low-dose antidepressants or antipsychotics with known metabolic side-effects as treatment for insomnia [46], special attention is warranted regarding the health effects of such medication in people with type 2 diabetes.
Although melatonin is generally not recommended for treatment of insomnia in adults [47], two studies [48, 49] investigated the effect of a melatonin agonist or prolonged-release melatonin in people with type 2 diabetes and insomnia. The former study (n = 36) found that sleep quality and HbA1c improved, compared with the placebo, in people with type 2 diabetes [48], whilst the latter study (n = 42) showed an improvement in sleep quality and stopped HbA1c further increasing [49]. A meta-analysis on melatonin, also including studies in the general population, supports this finding, showing improved glycaemic control through melatonin supplementation [50]. Although this seems promising, the exact role of melatonin in type 2 diabetes is still controversial. Studies have identified a common variant of melatonin receptor 1B to be associated with impaired insulin secretion in the presence of melatonin. This suggests that melatonin could induce insulin insensitivity in the risk allele carriers and thus have adverse effects [51].
For RLS, dopamine agonists are the first-line pharmacological option [52]. In a study of the RLS population, which included people with type 2 diabetes, treatment with pramipexole reduced RLS complaint scores (−14.2 ± 0.7 vs −8.1 ± 0.7) and improved mood, compared with placebo [53]. One small Japanese study [27] examined the efficacy of pramipexole in eight people diagnosed with type 2 diabetes and RLS. A decrease in RLS scores (−13.6 points [95% CI −15.5, −11.7]) over 12 weeks and a change in HbA1c levels of −3.2 mmol/mol (95% CI −4.4, −2.2) (−0.29% [95% CI −0.4, −0.2]) was observed. Other pharmacological treatments for RLS, such as other dopamine agonists, opioids, benzodiazepines, anticonvulsants and iron therapy, have not been tested in type 2 diabetes for health outcomes [54].
Non-pharmacological treatment
Continuous positive airway pressure and mandibular devices
Continuous positive airway pressure (CPAP) is the gold standard for treating OSA but effects on health outcomes in people with type 2 diabetes are inconsistent. On the one hand there are studies showing a significant change in HbA1c (−4.4 mmol/mol [−0.4%]; p = 0.024) [55], whilst, on the other hand, a meta-analysis including six randomised controlled trials in 518 people with type 2 diabetes showed that CPAP did not result in a reduction of HbA1c or fasting glucose [56]. These inconsistencies might be explained by the following factors: poorer disease state, with effects being more profound in people with higher HbA1c levels; differences in the definition of adherence to the CPAP intervention; or CPAP use being limited to the first half of the night when non-rapid eye movement (NREM) sleep dominates, while specifically REM sleep apnoeas in the latter half are adversely associated with glycaemic control [17]. Overall, CPAP does seem to positively affect sleep quality, blood pressure, QoL and depression [15, 57], which in turn could improve type 2 diabetes management and prevent comorbidities.
Mandibular advancement devices are another treatment option for younger people with less-severe OSA. A pilot study from India (n = 24) showed that mandibular devices decreased HbA1c levels (−151 mmol/mol [−14.01%]; p = 0.013) and improved sleep quality (measured by a decrease in Epworth Sleepiness Scale of 60.7 points; p = 0.001) over a period of 3 months [58] in people with type 2 diabetes and mild OSA. In people with type 2 diabetes, CPAP has not been compared with mandibular advancement devices yet. However, in the general population, CPAP was more effective in reducing the number of respiratory events, whereas compliance was higher for mandibular devices [59]. Finally, surgical interventions for OSA aimed at correcting underlying anatomical abnormalities in the oropharyngeal area and the effect of hypoglossal nerve stimulation have not been researched in type 2 diabetes.
Weight loss
Weight loss is another important intervention for OSA. A four-centre RCT [60] found that intense lifestyle interventions were more effective than the control treatment in reducing OSA complaints (apnoea–hypopnoea index, −5.4 vs +4.2 events/h; p = 0.000) and HbA1c levels (−7.7 mmol/mol [−0.7%] vs −2.2 mmol/mol [−0.2%]; p = 0.000) during 1 year. Even after 10 years, the lifestyle intervention group still showed reduced OSA complaints (apnoea–hypopnoea index, −9.9 vs −5.9 events/h; p = 0.11). In a meta-analysis of 136 studies (n = 22,094 individuals) that examined comorbid health outcomes in those who had bariatric surgery for weight loss, 87.9% (1051/1195) and 76.8% (1417/1846) had no more OSA and type 2 diabetes, respectively [61].
One study examined the effect of type 2 diabetes medication associated with weight loss on OSA in type 2 diabetes [62]. This RCT in 36 individuals investigated the effect of dapagliflozin, a sodium–glucose cotransporter 2 (SGLT2) inhibitor. No significant reduction in HbA1c levels compared with the control group was observed, but significant reductions were observed in OSA severity (−10.17 events/h; p < 0.001), as well as systolic BP (−6.11 mmHg; p = 0.012) and BMI (−1.21 kg/m2; p = 0.004). These results suggest that weight loss, regardless of how the weight loss was induced, could be a successful treatment for OSA and type 2 diabetes.
Cognitive behavioural therapy for insomnia and sleep education
An elegant meta-analysis by Kothari et al. [44] identified six studies showing that cognitive behavioural therapy for insomnia (CBT-I) and/or sleep education improved sleep quality measured by the Pittsburgh Sleep Quality Index (−1.31 [95% CI −1.83, −0.80]) and resulted in a non-significant HbA1c reduction (−3.6 mmol/mol [−0.35%]; p = 0.13) in those with sleep disturbances or insomnia, including both the general population and people with type 2 diabetes. Only two small pilot studies on CBT-I in people with type 2 diabetes have been conducted, reporting a reduction in both HbA1c levels after 3 weeks (2.8 ± 3.06 mmol/mol [0.26 ± 0.28%]) [63] and after 7 weeks (4.5 mmol/mol [0.41%]; p = 0.01) [64], as well as a 4.63 (p = 0.002) decrease in Beck Depression Inventory measures [65] in the latter cohort. The fact that CBT-I can effectively reduce depressive symptoms has been demonstrated previously outside this specific type 2 diabetes population [66].
With regard to sleep education, two studies analysed the effects of sleep education in people with type 2 diabetes, although none with diagnosed sleep disorders. One study [67] in people with type 2 diabetes and late sleeping times showed that sleep education improved sleep quality and reduced HbA1c (−1.5 ± 0.55 mmol/mol [−2.29 ± 2.20%] vs −1.11 ± 0.47 mmol/mol [−2.25 ± 2.19%]; p < 0.05), compared with controls. The other study [68] investigating people with type 2 diabetes and abnormal or poor sleep, reported improved sleep after sleep education but no change in glycaemic control. Overall, these studies suggest that both CBT-I and sleep education could contribute to improving sleep and health outcomes in those with type 2 diabetes and sleep disorders.
Despite the limited data available, treating sleep disorders non-pharmacologically in people with type 2 diabetes seems to have a positive effect on health outcomes. This calls for more extensive research on above-mentioned treatments as well as other promising non-pharmacological interventions in those with sleep disorders and type 2 diabetes. It is important to focus on non-pharmacological treatment because of the previously mentioned negative effects that some pharmacological options may have on weight and glycaemic control [46, 69]. One interesting option is bright light therapy (BLT), which is known for its activating and synchronising effects and is used for treatment of CRSWDs and depression. A study in 83 people with type 2 diabetes and depression, showed that BLT reduced depressive symptoms (−3.9 [95% CI −9.0, 1.2] Inventory of Depressive Symptomatology points) and improved insulin sensitivity (0.15 mg/kg × min [95% CI −0.41, 0.70] measured using hyperinsulinaemic–euglycaemic clamp), although neither change was statistically significant [70].
Possible mechanisms, clinical implications and future research
Evidence suggests that there is a bidirectional relationship between sleep disorders and type 2 diabetes, implying a vicious circle. On the one hand, sleep disorders contribute to progression of type 2 diabetes via hypothetical mechanisms, such as decreased brain glucose utilisation, altered orexin response, overactivation of the hypothalamus–pituitary–adrenal axis [71], suboptimal self-care (i.e. lower medication adherence [72]) and impaired decision-making (i.e. unhealthy diet and sedentary behaviour) [73]. Additionally, despite the fact that no literature on this topic was found, as sleep disorders disrupt multiple metabolic processes via attenuated sleep quality, sleep quantity or disturbances of the circadian rhythm, it is conceivable that they affect the efficacy of drugs aimed at lowering HbA1c. On the other hand, type 2 diabetes and associated comorbidities, such as obesity, nightly hypoglycaemia, increased sympathetic activity, neuropathic pain and nocturia, may contribute to the development of sleep disorders [74]. Medication may play a role as well, with metformin, for example, causing insomnia in about 1.7% of the people starting this drug [75].
Finally, sleep architecture might differ between people with and without diabetes. EEG case–control studies show that people with type 2 diabetes indeed have lower amounts of slow wave sleep (SWS) or more micro-arousal events [76, 77], compared with matched control participants. Since these studies are cross-sectional, no cause–effect relationship can be determined and the relationship may even be bidirectional. This bidirectional relationship may explain the increased prevalence of sleep disorders in people with type 2 diabetes.
In this review, we found that insomnia, OSA and RLS are more prevalent in people with type 2 diabetes than in the general population [9, 15, 25–32]. Additionally, we showed that these three sleep disorders as well as (work-related) disturbances of the circadian rhythm negatively affect health outcomes in at least one, and often multiple, diabetes domains, especially glycaemic control. Given their high prevalence and adverse consequences, it is strongly recommended to include active assessment of possible sleep disorders in management of type 2 diabetes. Sex differences should be taken into account and special attention should be given to detecting OSA, which often remains undiagnosed in type 2 diabetes. Additionally, differentiating between RLS and conditions mimicking RLS commands attention, as this may affect treatment responsiveness [53].
In general, improving sleep in people with type 2 diabetes could in turn improve glycaemic control, thus providing an important aid in preventing type 2 diabetes progression, and ultimately improve QoL [78]. Studies on the effect of treating sleep disorders specifically in people with type 2 diabetes are limited, based on small studies, or absent. Most first-line treatments for sleep disorders seem effective in people with type 2 diabetes, comparable with the general population, but with additional positive effects on type 2 diabetes and other health outcomes. Of high clinical relevance are people with type 2 diabetes who partake in shift work, who require specific guidance in terms of meal preparation and insulin regimens in order to achieve optimal glycaemic control.
This review is the first to summarise the literature on the prevalence of sleep disorders in type 2 diabetes, its health consequences and effects of treatment of sleep disorders. We could not include information on all sleep disorders and all treatment options due to gaps in the literature, and some data presented should be interpreted cautiously because they are based on few participants or specific subgroups, as is indicated in Figs 1 and 2. A general recommendation for future studies is therefore to further explore the impact of several sleep disorders and overlap between them, including CRSWDs, central disorders of hypersomnolence and parasomnias in people with type 2 diabetes. Moreover, special research focus is warranted on the effects of both pharmacological and non-pharmacological treatment options on health outcomes in type 2 diabetes.
In conclusion, sleep disorders are highly prevalent in people with type 2 diabetes, negatively affecting health outcomes. Since treatment of the sleep disorder could prevent diabetes progression, efforts should be made to diagnose and treat sleep disorders in people with type 2 diabetes in order to ultimately improve health and therefore QoL.
Supplementary Information
(PDF 184 kb)
(PPTX 227 kb)
Acknowledgments
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Abbreviations
- BLT
Bright light therapy
- CBT-I
Cognitive behavioural therapy insomnia
- CPAP
Continuous positive airway pressure
- CRSWD
Circadian rhythm sleep–wake disorder
- OSA
Obstructive sleep apnoea
- PLMD
Periodic limbic movement disorder
- QoL
Quality of life
- REM
Rapid eye movement
- RLS
Restless legs syndrome
Contribution statement
All authors were responsible for drafting the article and revising it critically for important intellectual content. All authors approved this version to be published.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med Rev. 2016;30:11–24. doi: 10.1016/j.smrv.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Yuan S, Larsson SC. An atlas on risk factors for type 2 diabetes: a wide-angled Mendelian randomisation study. Diabetologia. 2020;63(11):2359–2371. doi: 10.1007/s00125-020-05253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Academy of Sleep Medicine (2014) International classification of sleep medicine, 3rd edn. American Academy of Sleep Medicine, Darien, IL
- 4.Mohammadi S, Dolatshahi M, Zare-Shahabadi A, Rahmani F. Untangling narcolepsy and diabetes: pathomechanisms with eyes on therapeutic options. Brain Res. 2019;1718:212–222. doi: 10.1016/j.brainres.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Weng N, Luo YW, Xu JD, Zhang Y. Abnormal nocturnal behavior due to hypoglycemia: a case report. Medicine. 2019;98(6):e14405. doi: 10.1097/md.0000000000014405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong JC, Li J, Pavlova M, et al. Risk factors for probable REM sleep behavior disorder: a community-based study. Neurology. 2016;86(14):1306–1312. doi: 10.1212/wnl.0000000000002414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martynowicz H, Gac P, Brzecka A, et al. The relationship between sleep bruxism and obstructive sleep apnea based on polysomnographic findings. J Clin Med. 2019;8(10):1653. doi: 10.3390/jcm8101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sweetman A, Melaku YA, Lack L, et al. Prevalence and associations of co-morbid insomnia and sleep apnoea in an Australian population-based sample. Sleep Med. 2021;82:9–17. doi: 10.1016/j.sleep.2021.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Koopman ADM, Beulens JW, Dijkstra T, et al. Prevalence of insomnia (symptoms) in T2D and association with metabolic parameters and glycemic control: meta-analysis. J Clin Endocrinol Metab. 2020;105(3):614–643. doi: 10.1210/clinem/dgz065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chew M, Tan NYQ, Lamoureux E, Cheng C-Y, Wong TY, Sabanayagam C. The associations of objectively measured sleep duration and sleep disturbances with diabetic retinopathy. Diabetes Res Clin Pract. 2020;159:107967. doi: 10.1016/j.diabres.2019.107967. [DOI] [PubMed] [Google Scholar]
- 11.Nefs G, Donga E, van Someren E, Bot M, Speight J, Pouwer F. Subjective sleep impairment in adults with type 1 or type 2 diabetes: results from diabetes MILES—the Netherlands. Diabetes Res Clin Pract. 2015;109(3):466–475. doi: 10.1016/j.diabres.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33(9):1159–1164. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain A, Sharmab R, Yadavc N, Chaudhary P, Jainc G, Maanju M. Quality of life and its association with insomnia and clinical variables in type 2 diabetes. J Egypt Public Health Assoc. 2017;92(1):52–59. doi: 10.21608/epx.2018.7011. [DOI] [PubMed] [Google Scholar]
- 14.Lou P, Qin Y, Zhang P, et al. Association of sleep quality and quality of life in type 2 diabetes mellitus: a cross-sectional study in China. Diabetes Res Clin Pract. 2015;107(1):69–76. doi: 10.1016/j.diabres.2014.09.060. [DOI] [PubMed] [Google Scholar]
- 15.Reutrakul S, Mokhlesi B. Obstructive sleep apnea and diabetes: a state of the art review. Chest. 2017;152(5):1070–1086. doi: 10.1016/j.chest.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kent BD, McNicholas WT, Ryan S. Insulin resistance, glucose intolerance and diabetes mellitus in obstructive sleep apnoea. J Thorac Dis. 2015;7(8):1343–1357. doi: 10.3978/j.issn.2072-1439.2015.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimaldi D, Beccuti G, Touma C, Van Cauter E, Mokhlesi B. Association of obstructive sleep apnea in rapid eye movement sleep with reduced glycemic control in type 2 diabetes: therapeutic implications. Diabetes Care. 2014;37(2):355–363. doi: 10.2337/dc13-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.West SD, Groves DC, Lipinski HJ, et al. The prevalence of retinopathy in men with type 2 diabetes and obstructive sleep apnoea. Diabet Med. 2010;27(4):423–430. doi: 10.1111/j.1464-5491.2010.02962.x. [DOI] [PubMed] [Google Scholar]
- 19.Tahrani AA, Ali A, Raymond NT, et al. Obstructive sleep apnea and diabetic nephropathy: a cohort study. Diabetes Care. 2013;36(11):3718–3725. doi: 10.2337/dc13-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tahrani AA, Ali A, Raymond NT, et al. Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. Am J Respir Crit Care Med. 2012;186(5):434–441. doi: 10.1164/rccm.201112-2135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seicean S, Strohl KP, Seicean A, Gibby C, Marwick TH. Sleep disordered breathing as a risk of cardiac events in subjects with diabetes mellitus and normal exercise echocardiographic findings. Am J Cardiol. 2013;111(8):1214–1220. doi: 10.1016/j.amjcard.2012.12.053. [DOI] [PubMed] [Google Scholar]
- 22.Labarca G, Dreyse J, Salas C et al (2021) Risk of mortality among patients with moderate to severe obstructive sleep apnea and diabetes mellitus: results from the SantOSA cohort. Sleep Breath. 10.1007/s11325-020-02283-y [DOI] [PubMed]
- 23.Gabric K, Matetic A, Vilovic M, et al. Health-related quality of life in type 2 diabetes mellitus patients with different risk for obstructive sleep apnea. Patient Prefer Adherence. 2018;12:765–773. doi: 10.2147/PPA.S165203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panossian LA, Avidan AY. Review of sleep disorders. Med Clin N Am. 2009;93(2):407–425. doi: 10.1016/j.mcna.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Cho YW, Na GY, Lim JG, et al. Prevalence and clinical characteristics of restless legs syndrome in diabetic peripheral neuropathy: comparison with chronic osteoarthritis. Sleep Med. 2013;14(12):1387–1392. doi: 10.1016/j.sleep.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Cuellar NG, Ratcliffe SJ. A comparison of glycemic control, sleep, fatigue, and depression in type 2 diabetes with and without restless legs syndrome. J Clin Sleep Med. 2008;4(1):50–56. doi: 10.5664/jcsm.27079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harashima S, Nishimura A, Osugi T, et al. Restless legs syndrome in patients with type 2 diabetes: effectiveness of pramipexole therapy. BMJ Support Palliat Care. 2016;6(1):89–93. doi: 10.1136/bmjspcare-2014-000691. [DOI] [PubMed] [Google Scholar]
- 28.Lopes LA, Lins Cde M, Adeodato VG, et al. Restless legs syndrome and quality of sleep in type 2 diabetes. Diabetes Care. 2005;28(11):2633–2636. doi: 10.2337/diacare.28.11.2633. [DOI] [PubMed] [Google Scholar]
- 29.Merlino G, Fratticci L, Valente M, et al. Association of restless legs syndrome in type 2 diabetes: a case-control study. Sleep. 2007;30(7):866–871. doi: 10.1093/sleep/30.7.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modarresnia L, Golgiri F, Madani NH, Emami Z, Tanha K. Restless legs syndrome in Iranian people with type 2 diabetes mellitus: the role in quality of life and quality of sleep. J Clin Sleep Med. 2018;14(2):223–228. doi: 10.5664/jcsm.6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skomro RP, Ludwig S, Salamon E, Kryger MH. Sleep complaints and restless legs syndrome in adult type 2 diabetics. Sleep Med. 2001;2(5):417–422. doi: 10.1016/s1389-9457(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 32.Zobeiri M, Shokoohi A. Restless leg syndrome in diabetics compared with normal controls. Sleep Disord. 2014;2014:871751. doi: 10.1155/2014/871751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med. 2014;15(8):860–873. doi: 10.1016/j.sleep.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 34.Bener A, Al-Hamaq AOAA, Ağan AF, Öztürk M, Ömer A. The prevalence of restless legs syndrome and comorbid condition among patient with type 2 diabetic mellitus visiting primary healthcare. J Family Med Prim Care. 2019;8(12):3814–3820. doi: 10.4103/jfmpc.jfmpc_463_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merlino G, Valente M, Serafini A, et al. Effects of restless legs syndrome on quality of life and psychological status in patients with type 2 diabetes. Diabetes Educ. 2010;36(1):79–87. doi: 10.1177/0145721709351252. [DOI] [PubMed] [Google Scholar]
- 36.Rizzi M, Barrella M, Kotzalidis GD, Bevilacqua M. Periodic limbic movement disorder during sleep as diabetes-related syndrome? A polysomnographic study. ISRN Endocrinol. 2011;2011:246157. doi: 10.5402/2011/246157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stenvers DJ, Scheer F, Schrauwen P, la Fleur SE, Kalsbeek A. Circadian clocks and insulin resistance. Nat Rev Endocrinol. 2019;15(2):75–89. doi: 10.1038/s41574-018-0122-1. [DOI] [PubMed] [Google Scholar]
- 38.Chalernvanichakorn T, Sithisarankul P, Hiransuthikul N. Shift work and type 2 diabetic patients' health. J Med Assoc Thail. 2008;91(7):1093–1096. [PubMed] [Google Scholar]
- 39.El Tayeb IM, El Saghier EOA, Ramadan BK. Impact of shift work on glycemic control in insulin treated diabetics Dar El Chefa Hospital, Egypt 2014. Int J Diabetes Res. 2014;3(2):15–21. doi: 10.5923/j.diabetes.20140302.02. [DOI] [Google Scholar]
- 40.Manodpitipong A, Saetung S, Nimitphong H, et al. Night-shift work is associated with poorer glycaemic control in patients with type 2 diabetes. J Sleep Res. 2017;26(6):764–772. doi: 10.1111/jsr.12554. [DOI] [PubMed] [Google Scholar]
- 41.Jørgensen JT, Karlsen S, Stayner L, Hansen J, Andersen ZJ. Shift work and overall and cause-specific mortality in the Danish nurse cohort. Scand J Work Environ Health. 2017;43(2):117–126. doi: 10.5271/sjweh.3612. [DOI] [PubMed] [Google Scholar]
- 42.Abad VC, Guilleminault C. Diagnosis and treatment of sleep disorders: a brief review for clinicians. Dialogues Clin Neurosci. 2003;5(4):371–388. doi: 10.31887/DCNS.2003.5.4/vabad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshikawa F, Shigiyama F, Ando Y, et al. Chronotherapeutic efficacy of suvorexant on sleep quality and metabolic parameters in patients with type 2 diabetes and insomnia. Diabetes Res Clin Pract. 2020;169:108412. doi: 10.1016/j.diabres.2020.108412. [DOI] [PubMed] [Google Scholar]
- 44.Kothari V, Cardona Z, Chirakalwasan N, Anothaisintawee T, Reutrakul S. Sleep interventions and glucose metabolism: systematic review and meta-analysis. Sleep Med. 2021;78:24–35. doi: 10.1016/j.sleep.2020.11.035. [DOI] [PubMed] [Google Scholar]
- 45.Mast R, Rauh SP, Groeneveld L, et al. The use of antidepressants, anxiolytics, and hypnotics in people with type 2 diabetes and patterns associated with use: the Hoorn Diabetes Care System Cohort. Biomed Res Int. 2017;2017:5134602. doi: 10.1155/2017/5134602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kamphuis J, Taxis K, Schuiling-Veninga CC, Bruggeman R, Lancel M. Off-label prescriptions of low-dose quetiapine and mirtazapine for insomnia in the Netherlands. J Clin Psychopharmacol. 2015;35(4):468–470. doi: 10.1097/jcp.0000000000000338. [DOI] [PubMed] [Google Scholar]
- 47.Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. doi: 10.1111/jsr.12594. [DOI] [PubMed] [Google Scholar]
- 48.Garfinkel D, Zorin M, Wainstein J, Matas Z, Laudon M, Zisapel N. Efficacy and safety of prolonged-release melatonin in insomnia patients with diabetes: a randomized, double-blind, crossover study. Diabetes Metab Syndr Obes. 2011;4:307–313. doi: 10.2147/dmso.S23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsunoda T, Yamada M, Akiyama T, et al. The effects of ramelteon on glucose metabolism and sleep quality in type 2 diabetic patients with insomnia: a pilot prospective randomized controlled trial. J Clin Med Res. 2016;8(12):878–887. doi: 10.14740/jocmr2754w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doosti-Irani A, Ostadmohammadi V, Mirhosseini N, et al. The effects of melatonin supplementation on glycemic control: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res. 2018;50(11):783–790. doi: 10.1055/a-0752-8462. [DOI] [PubMed] [Google Scholar]
- 51.Lyssenko V, Nagorny CLF, Erdos MR, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41(1):82–88. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aurora RN, Kristo DA, Bista SR, et al. The treatment of restless legs syndrome and periodic limb movement disorder in adults--an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses: an American Academy of Sleep Medicine Clinical Practice Guideline. Sleep. 2012;35(8):1039–1062. doi: 10.5665/sleep.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montagna P, Hornyak M, Ulfberg J, et al. Randomized trial of pramipexole for patients with restless legs syndrome (RLS) and RLS-related impairment of mood. Sleep Med. 2011;12(1):34–40. doi: 10.1016/j.sleep.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 54.Kwatra V, Khan MA, Quadri SA, Cook TS. Differential diagnosis and treatment of restless legs syndrome: a literature review. Cureus. 2018;10(9):e3297–e3297. doi: 10.7759/cureus.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martínez-Cerón E, Barquiel B, Bezos AM, et al. Effect of continuous positive airway pressure on glycemic control in patients with obstructive sleep apnea and type 2 diabetes. A randomized clinical trial. Am J Respir Crit Care Med. 2016;194(4):476–485. doi: 10.1164/rccm.201510-1942OC. [DOI] [PubMed] [Google Scholar]
- 56.Labarca G, Reyes T, Jorquera J, Dreyse J, Drake L. CPAP in patients with obstructive sleep apnea and type 2 diabetes mellitus: systematic review and meta-analysis. Clin Respir J. 2018;12(8):2361–2368. doi: 10.1111/crj.12915. [DOI] [PubMed] [Google Scholar]
- 57.Yang X, Yang J, Yang C, Niu L, Song F, Wang L. Continuous positive airway pressure can improve depression in patients with obstructive sleep apnoea syndrome: a meta-analysis based on randomized controlled trials. J Int Med Res. 2020;48(3):300060519895096. doi: 10.1177/0300060519895096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baslas V, Chand P, Jurel SK, et al. A pilot study to determine the effect of three months of oral appliance therapy using a mandibular advancement device on HbA1c in subjects with type 2 diabetes mellitus and obstructive sleep apnea. J Prosthodont. 2019;28(3):271–275. doi: 10.1111/jopr.12973. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz M, Acosta L, Hung YL, Padilla M, Enciso R. Effects of CPAP and mandibular advancement device treatment in obstructive sleep apnea patients: a systematic review and meta-analysis. Sleep Breath. 2018;22(3):555–568. doi: 10.1007/s11325-017-1590-6. [DOI] [PubMed] [Google Scholar]
- 60.Kuna ST, Reboussin DM, Strotmeyer ES, et al. Effects of weight loss on obstructive sleep apnea severity. ten-year results of the sleep AHEAD study. Am J Respir Crit Care Med. 2021;203(2):221–229. doi: 10.1164/rccm.201912-2511OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. Jama. 2004;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 62.Tang Y, Sun Q, Bai XY, Zhou YF, Zhou QL, Zhang M. Effect of dapagliflozin on obstructive sleep apnea in patients with type 2 diabetes: a preliminary study. Nutr Diabetes. 2019;9(1):32. doi: 10.1038/s41387-019-0098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tannas CL, Davis JE (2013) Type 2 diabetes and insomnia: impact on metabolic control. Wayne State University Dissertations. Paper 624. https://digitalcommons.wayne.edu/oa_dissertations/624/
- 64.Alshehri MM, Alothman SA, Alenazi AM, et al. The effects of cognitive behavioral therapy for insomnia in people with type 2 diabetes mellitus, pilot RCT part II: diabetes health outcomes. BMC Endocr Disord. 2020;20(1):136. doi: 10.1186/s12902-020-00612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alshehri MM, Alenazi AM, Alothman SA, et al. Using cognitive behavioral therapy for insomnia in people with type 2 diabetes, pilot RCT part I: sleep and concomitant symptom. Behav Sleep Med. 2020;27:1–20. doi: 10.1080/15402002.2020.1831501. [DOI] [PubMed] [Google Scholar]
- 66.Blom K, Jernelöv S, Rück C, Lindefors N, Kaldo V. Three-year follow-up comparing cognitive behavioral therapy for depression to cognitive behavioral therapy for insomnia, for patients with both diagnoses. Sleep. 2017;40(8):1–5. doi: 10.1093/sleep/zsx108. [DOI] [PubMed] [Google Scholar]
- 67.Li M, Li D, Tang Y, et al. Effect of diabetes sleep education for T2DM who sleep after midnight: a pilot study from China. Metab Syndr Relat Disord. 2018;16(1):13–19. doi: 10.1089/met.2017.0069. [DOI] [PubMed] [Google Scholar]
- 68.Pereira FH, Trevisan DD, Santos Lourenço D, da Silva JB, Lima MHM. Effect of educational strategies on the sleep quality of people with diabetes: randomized clinical trial. Aquichan. 2019;19(3):1–13. doi: 10.5294/aqui.2019.19.3.2. [DOI] [Google Scholar]
- 69.Yoon JM, Cho E-G, Lee H-K, Park SM. Antidepressant use and diabetes mellitus risk: a meta-analysis. Korean J Fam Med. 2013;34(4):228–240. doi: 10.4082/kjfm.2013.34.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brouwer A, van Raalte DH, Nguyen HT, et al. Effects of light therapy on mood and insulin sensitivity in patients with type 2 diabetes and depression: results from a randomized placebo-controlled trial. Diabetes Care. 2019;42(4):529–538. doi: 10.2337/dc18-1732. [DOI] [PubMed] [Google Scholar]
- 71.Reutrakul S, Van Cauter E. Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Ann N Y Acad Sci. 2014;1311:151–173. doi: 10.1111/nyas.12355. [DOI] [PubMed] [Google Scholar]
- 72.Chasens ER, Korytkowski M, Sereika SM, Burke LE. Effect of poor sleep quality and excessive daytime sleepiness on factors associated with diabetes self-management. Diabetes Educ. 2013;39(1):74–82. doi: 10.1177/0145721712467683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Larcher S, Benhamou PY, Pépin JL, Borel AL. Sleep habits and diabetes. Diabetes Metab. 2015;41(4):263–271. doi: 10.1016/j.diabet.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 74.Lamond N, Tiggemann M, Dawson D. Factors predicting sleep disruption in type II diabetes. Sleep. 2000;23(3):415–416. doi: 10.1093/sleep/23.3.1i. [DOI] [PubMed] [Google Scholar]
- 75.eHealthMe (2021). Metformin and Insomnia concerns - a phase IV clinical study of FDA data. Available from www.ehealthme.com/ds/metformin/insomnia-concerns/. Accessed 12 May 2021
- 76.Lecube A, Romero O, Sampol G, et al. Sleep biosignature of type 2 diabetes: a case-control study. Diabet Med. 2017;34(1):79–85. doi: 10.1111/dme.13161. [DOI] [PubMed] [Google Scholar]
- 77.Pallayova M, Donic V, Gresova S, Peregrim I, Tomori Z. Do differences in sleep architecture exist between persons with type 2 diabetes and nondiabetic controls? J Diabetes Sci Technol. 2010;4(2):344–352. doi: 10.1177/193229681000400215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.American Diabetes Association Lifestyle management: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(Supplement 1):S46–S60. doi: 10.2337/dc19-S005. [DOI] [PubMed] [Google Scholar]
- 79.de Groot R (2018) Alles over slaap. Available from https://nvab-online.nl/sites/default/files/kringen/NVAB110618-Allesoverslaap-RdeGroot.pdf. Accessed 10 January 2021 [presentation in Dutch]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 184 kb)
(PPTX 227 kb)