Abstract
Recent studies have focused their attention on conjunctivitis as one of the symptoms of coronavirus disease 2019 (COVID-19). Therefore, tear samples were taken from COVID-19 patients and the presence of SARS-CoV-2 was evidenced using Real Time reverse transcription polymerase chain reaction. The main aim of this study was to analyze mRNA expression in the tears of patients with COVID-19 compared with healthy subjects using Next Generation Sequencing (NGS). The functional evaluation of the transcriptome highlighted 25 genes that differ statistically between healthy individuals and patients affected by COVID-19. In particular, the NGS analysis identified the presence of several genes involved in B cell signaling and keratinization. In particular, the genes involved in B cell signaling were downregulated in the tears of COVID-19 patients, while those involved in keratinization were upregulated. The results indicated that SARS-CoV-2 may induce a process of ocular keratinization and a defective B cell response.
Subject terms: Diagnostic markers, Predictive markers, Prognostic markers, Viral infection, Gene expression, Gene regulation
Introduction
Coronavirus disease 2019 (COVID-19) is caused by SARS-CoV-2 infection. SARS-CoV-2 is a single-stranded RNA virus positive sense1 and was identified in December 2019 in Wuhan, and it is a novel RNA virus that is part of the family Coronaviridae2,3.
SARS-COV-2 has different proteins on envelopes: Spike, Envelope, Membrane and Nucleocapsid. Spike protein is responsible for the virus entry into the host cell. COVID-19 can affect individuals with mild symptoms: dry cough, fever, headache, dyspnea. Frequently more serious symptoms are found that can cause the onset of chronic pneumonia and septic shock4.
Ophthalmologists have performed an analysis that revealed the SARS-CoV-2 infection in a team of specialists that visited COVID-19 patients wearing N95 masks only as personal protective equipment (PPE). A few days before the onset of common symptoms such as pneumonia, they claim to have had inflammation of the conjunctiva5.
The receptor target of Spike protein is the angiotensin-converting enzyme 2 (ACE-2)6. The epithelial cells of both the cornea and conjunctiva showed the presence of ACE-27.
One of the unusual symptoms that could be the first warning bell of SARS-COV-2 infection is conjunctivitis8.
Colavita et al. analyzed the case of a COVID-19 patient who returned to Italy from Wuhan who showed the first symptoms of dry cough, sore throat and bilateral conjunctivitis. It has been carried out an ocular swab demonstrating the presence of SARS-CoV-2 in the tear sample9.
These events could demonstrate the entry of the virus not only through the respiratory tract, but also through the mucous membranes of the eye5 and also SARS-CoV-2 could potentially be transmitted by conjunctival tears or secretions10–14.
A recent study found the presence of SARS-CoV-2 in the tears of COVID-19 patients with conjunctivitis, using the RT-PCR technique15.
The aim of the current study was to describe the transcriptional changes related to SARS-COV-2 viral infection, evaluating the up/down-regulation of mRNA in tears of hospitalized patients diagnosed with COVID-19 compared to healthy controls.
Results
The demographic and clinical characteristics of the patients suffering from COVID-19 enrolled are summarized in Tables 1 and 2. The mean age of control group was 73.5 ± 10.7 years (75% males; 25% females). No statistical difference was found between COVID-19 and healthy groups in terms of age, gender and race (p > 0.05).
Table 1.
Demographic and clinical characteristics of patients with COVID-19.
| Variables | COVID-19 patients |
|---|---|
| Age (years) | 74.5 ± 17.5 |
| Sex | |
| Male | 12 (65.0%) |
| Female | 7 (35.0%) |
| Race | |
| Caucasian | 19 (100%) |
| Comorbilities | 16 (84.2%) |
| Cardiological | 3 (15.8%) |
| Neurological | 4 (21%) |
| Nephrological | 5 (26.3%) |
| Autoimmune | 0 (0%) |
| Hypertension | 6 (31.6%) |
| Smoke tobacco | 0 (0%) |
| Allergies | 0 (0%) |
| Obesity | 1 (5.7%) |
| Diabetes mellitus | 3 (15.8%) |
| Others | 11 (57.9%) |
Table 2.
Medical history and clinical characteristics of patients with COVID-19.
| Variables | COVID-19 patients |
|---|---|
| Symptoms | |
| Dyspnea | 17 (89.5%) |
| Dry cough | 8 (42.1%) |
| Pneumonia | 15 (78.9%) |
| Fever | 11 (57.9%) |
| Myalgia | 1 (5.3%) |
| Home therapy | 15 (78.9%) |
| Antihypertensive/cardiovascular therapy | 12 (63.2%) |
| Anticoagulants | 3 (21.1%) |
| 5-Alpha reductase inhibitors | 1 (5.3%) |
| Anti-Psychotics/anxiolitics | 4 (26.4%) |
| Oral Hypoglicemic drugs/insuline | 2 (10.6%) |
| Hypouricemic drugs | 1 (5.3%) |
| Duration of hospitalization (days) | 26 ± 12 |
| Hospital therapy | |
| Hydroxychloroquine | 9 (47.4%) |
| Heparin | 2 (10.5%) |
| Diuretics | 1 (5.3%) |
| Anti-virals | 10 (52.6%) |
| Steroids | 2 (10.5%) |
| Antibiotics | 4 (21.0%) |
| Blood analysis at sampling day | |
| Anemia | 9 (47.4%) |
| Hyponatremia | 2 (10.5%) |
| Hypernatremia | 1 (5.3%) |
| PCR > 8 mg/l | 10 (52.6%) |
| Lymphocytopenia | 6 (31.6%) |
| Hyperkalemia | 1 (5.3%) |
| Hypokalemia | 2 (10.5%) |
| PCT > 0.15 ng/ml | 2 (10.5%) |
| LDH > 222 U/l | 1 (5.3%) |
| D-dimer > 0.5 mcg/ml | 4 (21.0%) |
| Ferritin > 307 mcg/l | 1 (5.3%) |
| Hypoalbuminemia | 1 (5.3%) |
| Neutrophilia | 1 (5.3%) |
| Creatinine > 1.3 mg/dl | 3 (15.8%) |
| Mean Sa 02 at admission | 91.9 ± 3.4% |
| Mean Sa 02 at sampling day | 94.4 ± 3.1% |
| Mean Sa 02 at discharge | 98.3 ± 0.6% |
| Bilateral conjunctivitis | 7 (36.8%) |
| Before admission | 3 (15.8%) |
| At admission | 4 (21.0%) |
| At discharge | 0 (0%) |
| Ventilation at sampling day | 8 (42.1%) |
| Invasive | 0 (0%) |
| Not invasive | 8 (42.1%) |
| Outcome | |
| Recovery | 7 (36.8%) |
| Death | 12 (63.2%) |
RNA-seq analysis between healthy individuals against Covid-19 patients
The RNA-seq analysis revealed 25 genes that differ statistically between healthy individuals and COVID-19 patients (Table 3). Among them, 13 genes were upregulated while 12 genes were downregulated. Interestingly, 20 genes, the most of them, had more than a twofold change. Anyway, the remaining 5 genes had a onefold change. The changes in the behavior of the differential expressed genes between all the samples was depicted in the heatmap in Fig. 1. As expected, the dendogram obtained by the plot put the closest association between the control individuals themselves and the COVID-19 patients themselves. Furthermore, in order to inspect the role of the up- and down-regulated differentially expressed genes, the PANTHER Classification System16 for the Gene Ontology Biological Processes was used. The enriched Gene Ontologies of the Biological Processes obtained by PANTHER (Table 4) highlighted the “Keratinization” (False Discovery Rate (FDR) = 1.83 × 10–3) for the up- and the “Regulation of B cell activation” (FDR = 1.49 × 10–3), “Negative regulation of immune system process” (FDR = 2.21 × 10–3) and “Regulation of inflammatory response” (FDR = 1.64 × 10–3) for the down-regulated genes.
Table 3.
Differentially expressed genes between healthy individual and Covid-19 patients.
| Gene symbol | Gene name | Healthy expression | COVID-19 expression | Fold change | q-value |
|---|---|---|---|---|---|
| ALOX15B | Arachidonate 15-lipoxygenase type B | 0.98 | 52.67 | 6.10 | 9.62e−04 |
| ATM | ATM serine/threonine kinase | 1270.92 | 548.16 | − 1.21 | 3.45e−02 |
| BANK1 | B cell scaffold protein with ankyrin repeats 1 | 162.40 | 8.81 | − 4.17 | 5.46e−03 |
| CLEC17A | C-type lectin domain containing 17A | 40.12 | 0.45 | − 6.16 | 4.09e−02 |
| DNASE1L3 | Deoxyribonuclease 1 like 3 | 60.56 | 9.04 | − 2.72 | 2.51e−02 |
| FCRL4 | Fc receptor like 4 | 57.02 | 0.18 | − 7.37 | 2.60e−03 |
| FCRL5 | Fc receptor like 5 | 149.79 | 0.41 | − 8.11 | 7.06e−05 |
| FLG | Filaggrin | 7.39 | 1308.95 | 7.49 | 4.99e−02 |
| IGHG1 | Immunoglobulin heavy constant gamma 1 (G1m marker) | 1285.85 | 1.55 | − 9.59 | 1.13e−03 |
| IGHV1-2 | Immunoglobulin heavy variable 1–2 | 27.03 | 0 | − 7.02 | 4.01e−02 |
| IGKC | Immunoglobulin kappa constant | 735.33 | 6.67 | − 6.76 | 9.58e−03 |
| IL19 | Interleukin 19 | 4.88 | 67.71 | 3.85 | 5.34e−03 |
| KRT17 | Keratin 17 | 202.77 | 786.77 | 1.96 | 4.01e−02 |
| KRT78 | Keratin 78 | 156.51 | 656.65 | 2.07 | 1.58e−02 |
| LTF | Lactotransferrin | 176.91 | 19.45 | − 3.29 | 2.56e−03 |
| MAL | mal, T cell differentiation protein | 16.72 | 220.79 | 3.74 | 5.34e−03 |
| PARP15 | Poly(ADP-ribose) polymerase family member 15 | 112.10 | 6.77 | − 3.95 | 2.65e−02 |
| PLA2G2A | Phospholipase A2 group IIA | 20,672.08 | 6669.91 | − 1.63 | 1.08e−03 |
| SPDEF | SAM pointed domain containing ETS transcription factor | 76.81 | 316.64 | 2.05 | 1.56e−02 |
| SPRR1B | Small proline rich protein 1B | 123.08 | 485.14 | 1.98 | 3.91e−02 |
| SPRR3 | Small proline rich protein 3 | 310.77 | 2203.18 | 2.83 | 9.58e−03 |
| TFF3 | Trefoil factor 3 | 110.21 | 480.32 | 2.12 | 1.70e−02 |
| TLE1 | TLE family member 1, transcriptional corepressor | 93.26 | 235.54 | 1.33 | 2.51e−02 |
| TMPRSS11E | Transmembrane serine protease 11E | 17.59 | 173.33 | 3.33 | 2.30e−02 |
| ZBTB16 | Zinc finger and BTB domain containing 16 | 39.26 | 278.59 | 2.83 | 6.78e−03 |
Figure 1.
Heatmap of the differentially expressed genes between all the individuals. The heatmap represent the genes differentially expressed after the RNA-seq analysis was concluded. Moreover, the logarithmic correction made on the counts allows to appreciate the differences between the samples. As expected, the dendogram on the columns shows the closest similarity between the healthy subjects each other (CTR) and between the COVID-19 patients themselves (COVID).
Table 4.
List of enriched biological processes in PANTHER.
| Biological process | Genes enriched | FDR |
|---|---|---|
| Upregulated genes | ||
| Keratinization | FLG, SPRR1B, KRT17, SPRR3, KRT78 | 1.83 × 10−3 |
| Downregulated genes | ||
| Regulation of B cell activation | IGHV1-2, IGKC, ATM, IGHG1, BANK1 | 1.49 × 10−3 |
| Negative regulation of immune system process | IGHV1-2, IGKC, ATM, IGHG1, BANK1, LTF | 2.21 × 10−3 |
| Regulation of inflammatory response | PLA2G2A, IGHV1-2, IGKC, ATM, IGHG1, DNASE1L3 | 1.64 × 10−3 |
To each gene in Gene Symbol column was associated the corresponding name in Gene Name column with the bitr function of the cluster Profiler package of Bioconductor. The expression of each gene in healthy individuals and COVID-19 patients was highlighted in the columns Healthy Expression and COVID-19 Expression, respectively. The column Fold Change shows the changes in the expression level between healthy and COVID-19 individuals while the q-Value column shows significance of the difference in the dataset (q < 0.05).
For each of the most specific biological processes found with PANTHER, the differentially expressed genes that are included were highlighted: a pathway is enriched for the upregulated genes and 5 genes are included; 3 pathways are enriched for the downregulated genes and 6 genes are included. The False Discovery Rate (FDR) of each pathway is lower than 0.05.
We then retrieved from the Human Protein Atlas database17 (Human Protein Atlas available from18) the mRNAs expression level of the genes found in our analysis in B and T cells. As depicted in red bars of the pyramid plot in Fig. 2, the most of the genes (TMPRSS11E, TLE1, PARP15, IL19, FCRL5, FCRL4, DNASE1L3, CLEC17A and BANK1) are more expressed in B cells than in T cells. Conversely, only ZBTB16, MAL, KRT17 and ATM are more expressed in T cells. Moreover, no expression was detected for FCRL5, FCRL4, DNASE1L3 CLEC17A and BANK1 in T cells while KRT17 is no expressed in B cells.
Figure 2.
Gene expression in T and B cells retrieved in Human Protein Atlas database. The bars represent the mRNA expression level of each gene in T cells (on the left) or in B cells (on the right). The bar is red in the cells in which the gene is more expressed, grey otherwise.
Discussion
The eye has been considered as a potential site for SARS-COV-2 viral infection and dissemination10,19. Moreover, involvement of the eye seems to be related more likely to severe forms of COVID-19 disease and often precedes the systemic symptoms or even is the only sign of the disease15. In our sample all diseased subjects had a severe SARS-CoV-2 infection; most of them had pneumonia (78.9%) and 70% of cases died with a mean of 26 ± 12 days of hospitalization.
Our study aimed to provide a panel of gene expression in tears of patients affected by SARS-CoV-2 infection comparing with healthy subjects to understand the gene expression pattern in the eye, which can be a starting point site of infection, as well as a concomitant involved organ of the disease. Given the protective function of tear film preserving the homeostasis and health of the conjunctiva and the avascular cornea, abnormalities in concentrations of proteins and inflammatory mediators in lacrimal secretions have been observed in infections, surgery and trauma. Indeed, antimicrobial factors have been well described in tears including lysozyme, lactoferrin, transferrin, ceruloplasmin, IgA, IgG, IgE, complement, glycoprotein, and anti-proteinase, which are found in the aqueous layer of the tear film. As already reported, immunoglobulins play a key role in defense of bacterial, viral, and parasitic infection. IgA, which is the primary immunoglobulin found in tears produced by conjunctiva and lacrimal gland, usually increases during infectious or inflammatory conditions of conjunctiva20. Moreover conjunctivitis has shown a rise in inflammatory mediators (IL-1β, TNF-α, and MMP-9) and the activation of proinflammatory mitogen-activated protein kinase (MAP-K) pathways21. In detail, tear film has three major layers, such as the inner mucin layer, the middle aqueous layer and the outer lipid layer22,23. The inner layer, formed by mucins secreted mainly by the goblet cells in the conjunctival epithelium, has stabilizing role of aqueous layer. It is composed by immunoglobulins, urea, salts, glucose, and proteins as well. The aqueous layer, essential for maintaining hydration and health of the ocular surface, contains proteins, metabolites, inorganic salts, glucose, oxygen, and electrolytes (magnesium, bicarbonate, calcium, urea). The lipid layer, fundamental for controlling tear evaporation, contains cholesterol, wax esters, fatty acids, and phospholipids23,24. Other tear film components include lysozyme with its bacteriolytic role, lactoferrin that is able to sequester iron from the bacteria thus stopping their growth. Mucins and glycoproteins secreted by goblet cells have a known role in ocular defense from external environment, preventing attachment of pathogens to ocular surface. In our sample, RNA-seq analysis revealed 25 genes differing statistically between healthy individuals and patients with a diagnosis of COVID-19. In detail, in the COVID-19 subjects 13 genes were upregulated, while 12 genes were downregulated.
We found that different genes involved in the function of B cells were downregulated, as also confirmed by Panther analysis that evidenced that the biological process “Regulation of B cell activation”, but also “Regulation of inflammatory response” and “Negative regulation of immune system process”, were enriched for the downregulated genes. Interestingly, a previous report indicated that lymphocyte B decreased in COVID-19 patients25. Among these down-regulated genes in COVID-19 group, we found IGKC, IGHG1 and IGHV1-2, encoding for immunoglobulin constant and variable chains related to immune response. Previous findings, have reported the IGKC downregulation in the tears of patients affected by other diseases, including bilateral keratoconus26. IGHG1 decrease was reported also in Fuchs endothelial corneal dystrophy27. Moreover, also the genes FCRL4 and FCRL5, that are receptors for IgA and IgG, respectively, were downregulated and have also a role in viral infections28,29.
DNASE1L3, that was downregulated in COVID-19 group, encoded for DNase γ, member of DNase I family of endonucleases. It was found to be expressed in germinal center B cells and stimulated B cells. It is involved in the somatic hypermutation of immunoglobulin variable region genes that occurs in the germinal center B cells during immune responses, and then contributed to the immunoglobulin V gene diversification30,31. DNASE1L3 is also involved in the release of cytokines after activation of the inflammasome32.
BANK1 also was found to be downregulated in the tears of COVID-19 patients. It is a positive regulator of B cell signaling through the induction of calcium mobilization after the activation of B-cell antigen receptor33. Also CLEC17A, expressed in B cells with function of adhesion to epithelial cells34, was downregulated in our analysis.
After sequencing we reported a downregulation of the ATM gene which synthesizes serine/threonine-protein kinase that act in the cell following DNA damage35. ATM has an important role in both T and B cells function. In particular, the loss of ATM in B cells caused the reduction in germinal center frequency and size in response to immunization and apoptosis of B cells36. Moreover, ATM is involved also in T cell development37. Moreover, it was found that RNA viruses can cause DNA damage and genetic instability in host cells modulating components of the DNA damage response, such as ATM38. ATM gene mutation were found in ocular adnexal marginal zone B-cell lymphomas and uveal melanoma39–41. In addition ATM gene inhibition reduces herpes virus corneal infection and particularly epithelial infection and stromal disease.
We found the upregulation of TLE1, MAL and ZBTB16 in the tears of COVID-19 patients. The TLEs family is composed by co-repressors expressed in T cells where they are required for CD8+ T cell lineage choice42. MAL is involved in apical transport of proteins in polarized epithelial cells43. MAL has been shown to be implicated in lytic plaque formation and viral spread in oligodendrocytes infected with Herpes simplex virus type 144. It is involved also in T cell functions45,46. ZBTB16 is a gene also known as PLZF (promyelocyticleukemia zinc finger). It is important in the function and development of immune system and may enhance T cell responses47.
Our seq analysis revealed a significant upregulation of IL19. The upregulation of IL-19 was already found in blood of COVID-19 patients48. Li et al. described the gene expression profile from patients with Th cell-mediated autoimmune noninfectious uveitis and some cytokines, including IL-19, were found to be highly expressed proving the inflammatory status of the eye49. The upregulation of such inflammatory interleukin in patients with the coronaviridae disease shows an inflammation of conjunctiva. Interestingly, IL-19 is produced by macrophages, B-cells but also by keratinocytes, and interestingly, it can also act on keratinocytes, suggesting to be involved also in keratinocyte hyperproliferation50,51. This aspect is particular interestingly, considering that we found the upregulation of genes involved in keratinization as confirmed also by the enrichment of the same biological process.
We also found an upregulation of keratin 17 and keratin 78 expression levels in tears of our enrolled patients. A similar KRT17 upregulation was detected by Kulkarni et al.52 in limbal epithelial stem cells of diabetic patients using deep sequencing analysis if compared with healthy individuals. The authors speculated that KRT17 dysregulation could be involved in the typical corneal modifications of diabetic subjects. From our analysis KRT17 and KRT78 were upregulated likely due to their potential role in ocular surface modifications during SARS-CoV-2 infection.
Our findings showed a significant increase in FLG levels compared to the healthy subjects. FLG has also an important role in maintaining the integrity of stratum corneum of epidermidis and its loss-of-function mutations have been associated with atopic dermatitis, due to a reduced skin hydration and skin barrier function52. A previous study indicated that FLG was not expressed in normal conjunctiva, while it increased in moderate and severe forms of parakeratinization of the conjunctiva53.
We found the upregulation of both SPRR1B and SPRR3. They are part of the small proline-rich proteins (SPRRs), which constitute cornified cell envelope precursors50,54. SPRR1B is a stress-induced transcript on the ocular surface that was shown to be upregulated in conditions of pathologic keratinizationin, in both evaporative and immune-mediated, aqueous-deficient dry eye disease. SPPR1B can be also considered a biomarker for pathology such as Sjögren's syndrome and for the study of the molecular mechanisms of squamous metaplasia. Squamous metaplasia causes pathologic keratinization of the ocular surface in response to disease processes that are autoimmune mediated or caused by infection. Moreover, pro-inflammatory cytokines induced the expression of SPRR1B55.
SPDEF, upregulated in our analysis, is a transcription factor that promotes the differentiation of goblet cells in the conjunctiva epithelium56. Goblet cells produce and secrete mucins, needed to lubricate the ocular surface. Goblet cell secretions are essential in order to maintain tear stability and ocular surface homeostasis. Goblet cells in the conjunctiva play an essential immunomodulatory role57. Increased expression of SPDEF was associated to goblet cell hyperplasia58 and upregulation of goblet cell-associated genes59. Atopic keratoconjunctivitis was associated with goblet cell hyperplasia60.
TFF3 was found to be upregulated in our analysis. It is expressed in various ocular tissues, also in goblet cells and has a role in corneal wound healing61. Its increase in tear film was found after inflammatory factors or ocular surface damage, such as those in dry eye disease, in experimental models62. It increased also in herpetic keratitis63.
LTF, produced in the acinar cells of the lacrimal gland, is normally present in tears of humans and is not dependent on age and sex. It has been proved that LTF is reduced in some ocular and systemic disease such as dry eye-related keratopathies, herpes simplex keratitis, chronic irritative conjunctivitis keratoconjunctivitis sicca64.
PLA2G2A, which is downregulated in tears of our diseased sample of patients, has been previously described to be involved in tear film stability and the consequent integrity of tear ocular surface65.
ALOX15B is a lipoxygenase enzyme. It was shown that LPS bacteria and the proinflammatory cytokines may induce overexpression of ALOX15B66. It represents the predominant 15-LOX protein in human cornea, and its product induced apoptosis in a dose dependent manner67.
Type II transmembrane serine proteases (TTSPs) have the ability to cleave surface proteins of viruses, including SARS-CoV-2 and influenza viruses, leading to the viral invasion. In particular, SARS-CoV-2 can bind TMPRSS2 of the host cell. This protease facilitates the viral attachment to the surface of targets cells by cleavage and priming of a spike protein of coronaviruses (S protein), at the S1/S2 and the S2’ site. This process seems to be the first essential step for the virus entry in the human body, thus demonstrating a key role of TMPRSS2 activity on SARS-CoV-2 spread68. Interestingly, in our RNA-seq analysis we found TMPRSS11E upregulated in tears of COVID-19 patients, leading to the hypothesis of a possible additional role of this protease in the Sars-Cov-2 entry in the host cells. Indeed, it was demonstrated that TMPRSS11E also known as DESC1, activated the S proteins of emerging Coronavirus69.
PARP15 is a neurodegeneration mediator and its inhibition seems to improve corneal epithelial innervation and wound healing in diabetic rats70. It is known that PARPs may play a role in inflammation and virus infections, indeed coronavirus may have the ability to reverse ADP ribosylation induced by PARP in order to counteract host-virus defense response71,72.
We can speculate that the down or up regulation of some of the investigated genes already known as involved in ocular inflammation processes are related to the presence of the virus on the ocular surface. In particular the up regulation of IL19 gene, associated to non infectious uveitis and conjunctivitis could be related to SARS-CoV-2 infection in our patients. TFF3 also found to be upregulated in our sample has already been related to corneal inflammation in dry eye disease herpetic keratitis. The inhibition of ATM gene, downregulated in our study, has been found related to a reduction of herpes virus corneal infection.
The main limitation of our study is the lack of a sub analysis differentiating COVID-19 patients with and without conjunctivitis to correlate ocular clinical conditions and the RNA seq analysis.
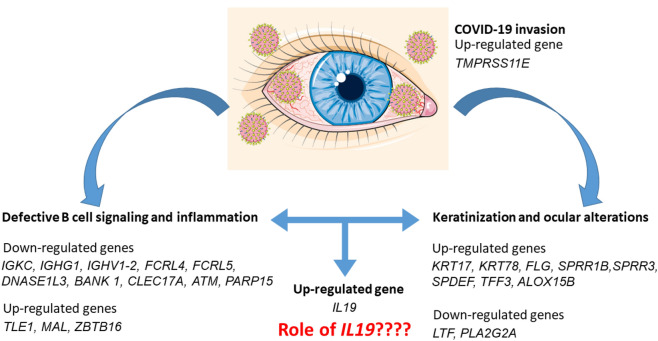
In conclusion, our study reported in tears of patients suffering from SARS-CoV-2 infection a downregulation of genes involved in B cell signaling, while the genes involved in keratinization were upregulated (Fig. 3). These results are particularly interesting because to our knowledge this is the first study that suggest a possible molecular mechanism responsible of the keratoconjunctivitis reported in patients affected by COVID-1973,74. It would be interesting to investigate if IL19 increase may link the inflammation process with the keratinization.
Figure 3.
Schema of the down- and up-regulated genes and the processes in which they are involved. The trancriptomic analysis suggested a defective function of B cells and a process of keratinization. IL-19 may link the process, being involved also in keratinocyte proliferation. The figure was made taking the images from Servier Medical Art (
available at http://smart.servier.com/), licensed under a Creative Commons Attribution 3.0 Unported License ().
Materials and methods
Patients enrollment
In this observational study, approved by our Institutional Review Board (prot. n. 1497/22.05.2020), a total of 19 patients with a diagnosis of COVID-19 (COVID group) were enrolled at the Infectious diseases department and the Internal Medicine Clinic, at the “SS. Annunziata Hospital” of Chieti-University G. D’Annunzio, Chieti-Pescara, Italy, from April 2020 to May 2020. A group of healthy patients (n = 20; Healthy group) with negative nasopharyngeal/throat swabs were enrolled and were considered as controls. The study adhered to the tenets of the Declaration of Helsinki and a written informed consent was obtained from all participants.
Study population
The inclusion criteria (COVID group):
A confirmed diagnosis of COVID-19 (diagnosis confirmed by positive detection of SARS-CoV-2 RNA from nasopharyngeal/throat swabs by real time (RT)-PCR);
Age ≥ of 18 years.
The inclusion criteria (Healthy group):
Age ≥ of 18 years;
No ocular and systemic diseases;
No assumption of drugs.
The exclusion criteria (COVID group and Healthy group):
Pregnant women;
Any form Ocular surface diseases preceding Covid-19 diagnosis, Glaucoma, history of anterior segment inflammation, previous penetrating ocular trauma;
Ocular surgeries within previous 6 months;
Topical therapies;
History of ocular allergy.
The following anamnestic data about all patients enrolled were collected:
Demographic data (age, sex, race);
Duration of the hospitalization;
Pre-existing illness (cardiac, neurologic, nephrologic and auto-immune diseases, hypertension, diabetes, allergies, obesity);
Smoking history;
Relatives affected with Covid-19;
Department of recovery (Intensive Care Unit or other department);
Medical treatments (on admission and during the hospitalization);
Laboratory findings;
Symptoms (shortness of breath, cough, fever, myalgia, pneumonia);
SaO2;
Evidence of conjunctivitis (presence of bilateral conjunctivitis was defined as red eyes: macroscopic signs of conjunctival congestion and was confirmed by using a portable slit lamp (SL‐17; Kowa Ltd., Tokyo, Japan) and if present the onset of the ocular disease was registered: pre-admission, on admission, during hospitalization);
Necessity of non-invasive (facemask oxygen)/invasive mechanical ventilation;
Clinical outcomes (recovery, discharge from hospital, death).
Tear sampling
Tear secretion was measured by using the Schirmer’s Test (Schirmer strips; Whatman, Maidstone, UK). Schirmer’s papers have been kept in the lower lid margin for 5 min. Tear secretion was measured as the length of the wet strip (in millimeters). Schirmer’s tear fluid collection was carried out simultaneously in both eyes. The paper strips from eyes were placed in Eppendorf tubes containing 500 μl of water with RNase inhibitors (Diethyl pyrocarbonate (DEPC)-treated water, Ambion, CA, United States) and strongly vortexed for 5 min and frozen in dry ice. Upon arrival to the laboratory both samples have been stored at − 80 °C.
Real time-PCR
SARS-COV-2 mRNA expressions in tear samples were assessed by RT-PCR. For this purpose, total RNA was isolated utilizing the Total RNA Purification kit (NorgenBiotek Corp., Thorold, ON, Canada). The M-MLV Reverse Transcriptase reagents (Applied Biosystems, Foster City, CA, USA) were utilized to produce cDNA. Real-time PCR was performed with the Mastercycler ep realplexrealtime PCR system (Eppendorf, Hamburg, Germany). Commercially available TaqMan Gene Expression Assays (Applied Biosystems) and the TaqMan Universal PCR Master Mix (Applied Biosystems) were utilized in agreement with the standard protocols75. Beta-2 microglobulin (B2M Hs99999907_m1; Applied Biosystems, Foster City, CA, USA) was utilized for template normalization. RT-PCR was analyzed in three independent experiments; duplicate determinations were obtained for each sample.
RNA extraction and library preparation
The library was prepared in according with Illumina protocols and how already described76. Briefly, the total RNA was extracted with the Maxwell RSC simplyRNA Cells Kit (Promega, Madison, WI, USA) following the manufacturer’s instructions. The library preparation was carried out according to the TruSeq RNA Exome protocol (Illumina, San Diego, CA, USA)77.
The cDNA was synthesized with the SuperScript II Reverse transcriptase (Invitrogen, Carlsbad, CA, USA) and the 3’end was later adenylated. The library was amplified with PCR and validated. Two steps of hybridization followed the first PCR and later one more PCR was performed. The MiSeq Instrument (Illumina) was used to sequence the libraries.
Bioinformatics analysis
The obtained raw data was checked for quality using the fastQC tool and the needed trimming for adapters and low quality bases scores was performed by Trimmomatic (Usadel Lab, Aachen, Germany)78 (version 0.38). The reads were than aligned to the version GRCh38 of the human reference genome with the Spliced Transcripts Alignment to a Reference (STAR) RNA-seq aligner79. After sorting the reads with STAR, the counting of the reads was made using the htseq-count python package80. The differentially expressed genes were finally obtained using the Bioconductor package DESeq2 in R language81. The Benjamini–Hochberg post-hoc procedure with threshold 0.05 was used to filter out the false positives. The bitr function of the clusterProfiler package of Bioconductor associated each gene symbol with its corresponding name82. Panther was then used to enrich the genes with the biological processes in which they are involved. Finally, the Human Protein Atlas database was used to find the mean level of expression of the mRNAs coding for these genes in B cells and in T cells. After logaritmic conversion, the values were depicted in R using the function ggplot283.
Abbreviations
- COVID-19
Coronavirus disease 2019
- NGS
Next generation sequencing
- PCR
Polymerase chain reaction
Author contributions
Study design, conceptualization, supervision, resources and study management L.M., L.T., S.C., E.M. and O.T.; performing experiments, data analysis and writing original manuscript, L.C., F.D., A.G., S.S., G.D.M. and R.D.A.; samples collection, editing original manuscript, B.S., J.V., F.C., D.D.A., A.A. and M.L.
Funding
This study was supported by current research funds 2020 of IRCCS “Centro Neurolesi Bonino-Pulejo”, Messina, Italy.
Data availability
The datasets generated during and/or analyzed during the current study will be available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contribute equally: Leonardo Mastropasqua, Lisa Toto, Luigi Chiricosta and Francesca Diomede.
These authors jointly supervised this work: Emanuela Mazzon and Oriana Trubiani.
References
- 1.Romano M, Ruggiero A, Squeglia F, Maga G, Berisio R. A structural view of SARS-CoV-2 RNA replication machinery: RNA synthesis, proofreading and final capping. Cells. 2020 doi: 10.3390/cells9051267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pascarella G, Strumia A, Piliego C, Bruno F, Del Buono R, Costa F, Scarlata S, Agro FE. COVID-19 diagnosis and management: A comprehensive review. J. Intern. Med. 2020;288:192–206. doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diomede F, Marconi GD, Fonticoli L, Pizzicannella J, Trubiani O. Stem cells secretome from oral tissue could represent a promising therapeutic approach in COVID-19-disease? Int. J. Mol. Sci. 2020 doi: 10.3390/ijms21186833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beeraka NM, Sadhu SP, Madhunapantula SV, Rao Pragada R, Svistunov AA, Nikolenko VN, Mikhaleva LM, Aliev G. Strategies for targeting SARS CoV-2: Small molecule inhibitors-the current status. Front. Immunol. 2020;11:552925. doi: 10.3389/fimmu.2020.552925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395:e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang J, Yang N, Deng J, Liu K, Yang P, Zhang G, Jiang C. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS ONE. 2011;6:e23710. doi: 10.1371/journal.pone.0023710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou L, Xu Z, Castiglione GM, Soiberman US, Eberhart CG, Duh EJ. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul. Surf. 2020;18:537–544. doi: 10.1016/j.jtos.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai THT, Tang EWH, Chau SKY, Fung KSC, Li KKW. Stepping up infection control measures in ophthalmology during the novel coronavirus outbreak: An experience from Hong Kong. Graefes Arch. Clin. Exp. Ophthalmol. 2020;258:1049–1055. doi: 10.1007/s00417-020-04641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colavita F, Lapa D, Carletti F, Lalle E, Bordi L, Marsella P, Nicastri E, Bevilacqua N, Giancola ML, Corpolongo A, et al. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann. Intern. Med. 2020;173:242–243. doi: 10.7326/M20-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azzolini C, Donati S, Premi E, et al. SARS-CoV-2 on ocular surfaces in a cohort of patients with COVID-19 from the Lombardy Region. Italy. JAMA Ophthalmol. 2021;4:e205464. doi: 10.1001/jamaophthalmol.2020.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seah IYJ, Anderson DE, Kang AEZ, et al. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology. 2020;127(7):977–979. doi: 10.1016/j.ophtha.2020.03.02612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Duan C, Zeng Y, et al. Ocular findings and proportion with conjunctival SARS-COV-2 in COVID-19 patients. Ophthalmology. 2020;127(7):982–983. doi: 10.1016/j.ophtha.2020.04.02813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun CB, Wang YY, Liu GH, Liu Z. Role of the eye in transmitting human coronavirus: What we know and what we do not know. Front Public Health. 2020;8:155. doi: 10.3389/fpubh.2020.0015514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J. Med. Virol. 2020;92:589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, Diemer K, Muruganujan A, Narechania A. PANTHER: A library of protein families and subfamilies indexed by function. Genome Res. 2003;13:2129–2141. doi: 10.1101/gr.772403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thul PJ, Akesson L, Wiking M, Mahdessian D, Geladaki A, Ait Blal H, Alm T, Asplund A, Bjork L, Breckels LM, et al. A subcellular map of the human proteome. Science. 2017 doi: 10.1126/science.aal3321. [DOI] [PubMed] [Google Scholar]
- 18.Atlas, H.P. Availabe online: http://www.proteinatlas.org
- 19.Peng M, Dai J, Sugali CK, Rayana NP, Mao W. The role of the ocular tissue in SARS-CoV-2 transmission. Clin. Ophthalmol. 2020;14:3017–3024. doi: 10.2147/OPTH.S269868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang W, Berube J, McNamara M, Saksena S, Hartman M, Arshad T, Bornheimer SJ, O’Gorman M. Lymphocyte subset counts in COVID-19 patients: A meta-analysis. Cytometry Part A J. Int. Soc. Anal. Cytol. 2020;97:772–776. doi: 10.1002/cyto.a.24172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam SM, Tong L, Duan X, Petznick A, Wenk MR, Shui G. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J. Lipid Res. 2014;55:289–298. doi: 10.1194/jlr.M044826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest. Ophthalmol. Vis. Sci. 2004;45(12):4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 23.Van Haeringen NJ. Clinical biochemistry of tears. Surv. Ophthalmol. 1981;26(84–96):22. doi: 10.1016/0039-6257(81)90145-4. [DOI] [PubMed] [Google Scholar]
- 24.Dartt DA, Willcox MD. Complexity of the tear film: Importance in homeostasis and dysfunction during disease. Exp. Eye Res. 2013;117:1–3. doi: 10.1016/j.exer.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson HJ, Kuonen VJ. The tear film and ocular mucins. Vet. Ophthalmol. 2004;7:71–77. doi: 10.1111/j.1463-5224.2004.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lema I, Brea D, Rodriguez-Gonzalez R, Diez-Feijoo E, Sobrino T. Proteomic analysis of the tear film in patients with keratoconus. Mol. Vis. 2010;16:2055–2061. [PMC free article] [PubMed] [Google Scholar]
- 27.Kuot A, Ronci M, Mills R, Klebe S, Snibson G, Wiffen S, Loh R, Corbett M, Zhou T, Chataway T, et al. Reduced expression of apolipoprotein E and immunoglobulin heavy constant gamma 1 proteins in Fuchs endothelial corneal dystrophy. Clin. Exp. Ophthalmol. 2019;47:1028–1042. doi: 10.1111/ceo.13569. [DOI] [PubMed] [Google Scholar]
- 28.Rostamzadeh D, Kazemi T, Amirghofran Z, Shabani M. Update on Fc receptor-like (FCRL) family: New immunoregulatory players in health and diseases. Expert Opin. Ther. Targets. 2018;22:487–502. doi: 10.1080/14728222.2018.1472768. [DOI] [PubMed] [Google Scholar]
- 29.Wilson TJ, Fuchs A, Colonna M. Cutting edge: Human FcRL4 and FcRL5 are receptors for IgA and IgG. J. Immunol. 2012;188:4741–4745. doi: 10.4049/jimmunol.1102651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto N, Okamoto M, Araki S, Arakawa H, Mizuta R, Kitamura D. Possible contribution of DNase gamma to immunoglobulin V gene diversification. Immunol. Lett. 2009;125:22–30. doi: 10.1016/j.imlet.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Okamoto M, Okamoto N, Yashiro H, Shiokawa D, Sunaga S, Yoshimori A, Tanuma S, Kitamura D. Involvement of DNase gamma in the resected double-strand DNA breaks in immunoglobulin genes. Biochem. Biophys. Res. Commun. 2005;327:76–83. doi: 10.1016/j.bbrc.2004.11.142. [DOI] [PubMed] [Google Scholar]
- 32.Shi G, Abbott KN, Wu W, Salter RD, Keyel PA. Dnase1L3 regulates inflammasome-dependent cytokine secretion. Front. Immunol. 2017;8:522. doi: 10.3389/fimmu.2017.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yokoyama K, Su Ih IH, Tezuka T, Yasuda T, Mikoshiba K, Tarakhovsky A, Yamamoto T. BANK regulates BCR-induced calcium mobilization by promoting tyrosine phosphorylation of IP(3) receptor. EMBO J. 2002;21:83–92. doi: 10.1093/emboj/21.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham SA, Jegouzo SA, Yan S, Powlesland AS, Brady JP, Taylor ME, Drickamer K. Prolectin, a glycan-binding receptor on dividing B cells in germinal centers. J. Biol. Chem. 2009;284:18537–18544. doi: 10.1074/jbc.M109.012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edvardsen H, Tefre T, Jansen L, Vu P, Haffty BG, Fossa SD, Kristensen VN, Borresen-Dale AL. Linkage disequilibrium pattern of the ATM gene in breast cancer patients and controls; association of SNPs and haplotypes to radio-sensitivity and post-lumpectomy local recurrence. Radiat. Oncol. 2007;2:25. doi: 10.1186/1748-717X-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicolas L, Cols M, Smolkin R, Fernandez KC, Yewdell WT, Yen WF, Zha S, Vuong BQ, Chaudhuri J. Cutting edge: ATM influences germinal center integrity. J. Immunol. 2019;202:3137–3142. doi: 10.4049/jimmunol.1801033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matei IR, Guidos CJ, Danska JS. ATM-dependent DNA damage surveillance in T-cell development and leukemogenesis: the DSB connection. Immunol. Rev. 2006;209:142–158. doi: 10.1111/j.0105-2896.2006.00361.x. [DOI] [PubMed] [Google Scholar]
- 38.Ryan EL, Hollingworth R, Grand RJ. Activation of the DNA damage response by RNA viruses. Biomolecules. 2016;6:2. doi: 10.3390/biom6010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xing S, Shao P, Li F, Zhao X, Seo W, Wheat JC, Ramasamy S, Wang J, Li X, Peng W, et al. Tle corepressors are differentially partitioned to instruct CD8(+) T cell lineage choice and identity. J. Exp. Med. 2018;215:2211–2226. doi: 10.1084/jem.20171514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lara-Lemus R. On the role of myelin and lymphocyte protein (MAL) in cancer: A puzzle with two faces. J Cancer. 2019;10:2312–2318. doi: 10.7150/jca.30376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez-Guerrero JA, de la Nuez C, Praena B, Sanchez-Leon E, Krummenacher C, Bello-Morales R. Herpes simplex virus 1 spread in oligodendrocytic cells is highly dependent on MAL proteolipid. J. Virol. 2020 doi: 10.1128/JVI.01739-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anton O, Batista A, Millan J, Andres-Delgado L, Puertollano R, Correas I, Alonso MA. An essential role for the MAL protein in targeting Lck to the plasma membrane of human T lymphocytes. J. Exp. Med. 2008;205:3201–3213. doi: 10.1084/jem.20080552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alekseev O, Donovan K, Azizkhan-Clifford J. Inhibition of ataxia telangiectasia mutated (ATM) kinase suppresses herpes simplex virus type 1 (HSV-1) keratitis. Invest. Ophthalmol. Vis. Sci. 2014;55(2):706–715. doi: 10.1167/iovs.13-13461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anton OM, Andres-Delgado L, Reglero-Real N, Batista A, Alonso MA. MAL protein controls protein sorting at the supramolecular activation cluster of human T lymphocytes. J. Immunol. 2011;186:6345–6356. doi: 10.4049/jimmunol.1003771. [DOI] [PubMed] [Google Scholar]
- 45.Alonzo ES, Sant'Angelo DB. Development of PLZF-expressing innate T cells. Curr. Opin. Immunol. 2011;23:220–227. doi: 10.1016/j.coi.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sims JT, Krishnan V, Chang CY, Engle SM, Casalini G, Rodgers GH, Bivi N, Nickoloff BJ, Konrad RJ, de Bono S, et al. Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z, Liu B, Maminishkis A, Mahesh SP, Yeh S, Lew J, Lim WK, Sen HN, Clarke G, Buggage R, et al. Gene expression profiling in autoimmune noninfectious uveitis disease. J. Immunol. 2008;181:5147–5157. doi: 10.4049/jimmunol.181.7.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kunz S, Wolk K, Witte E, Witte K, Doecke WD, Volk HD, Sterry W, Asadullah K, Sabat R. Interleukin (IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs. Exp. Dermatol. 2006;15:991–1004. doi: 10.1111/j.1600-0625.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 49.Vela V, Juskevicius D, Gerlach MM, Meyer P, Graber A, Cathomas G, Dirnhofer S, Tzankov A. 39 High throughput sequencing reveals high specificity of TNFAIP3 mutations in ocular adnexal marginal zone B-cell lymphomas. Hematol. Oncol. 2020;38(3):284–292. doi: 10.1002/hon.2718. [DOI] [PubMed] [Google Scholar]
- 50.Jha J, Singh MK, Singh L, Pushker N, Bajaj MS, Sen S, Kashyap S. Expression of BAP1 and ATM proteins: Association with AJCC tumor category in uveal melanoma. Ann. Diagn. Pathol. 2020;44:151432. doi: 10.1016/j.anndiagpath.2019.151432. [DOI] [PubMed] [Google Scholar]
- 51.Azuma YT, Nakajima H, Takeuchi T. IL-19 as a potential therapeutic in autoimmune and inflammatory diseases. Curr. Pharm. Des. 2011;17:3776–3780. doi: 10.2174/138161211798357845. [DOI] [PubMed] [Google Scholar]
- 52.Kulkarni M, Leszczynska A, Wei G, Winkler MA, Tang J, Funari VA, Deng N, Liu Z, Punj V, Deng SX, et al. Genome-wide analysis suggests a differential microRNA signature associated with normal and diabetic human corneal limbus. Sci. Rep. 2017;7:3448. doi: 10.1038/s41598-017-03449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lund AS, Heegaard S, Prause JU, Toft PB, Skov L. Expression of filaggrin in normal and keratinized conjunctiva. Open Ophthalmol. J. 2012;6:137–140. doi: 10.2174/1874364101206010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carregaro F, Stefanini AC, Henrique T, Tajara EH. Study of small proline-rich proteins (SPRRs) in health and disease: A review of the literature. Arch. Dermatol. Res. 2013;305:857–866. doi: 10.1007/s00403-013-1415-9. [DOI] [PubMed] [Google Scholar]
- 55.Li S, Nikulina K, DeVoss J, Wu AJ, Strauss EC, Anderson MS, McNamara NA. Small proline-rich protein 1B (SPRR1B) is a biomarker for squamous metaplasia in dry eye disease. Invest. Ophthalmol. Vis. Sci. 2008;49:34–41. doi: 10.1167/iovs.07-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marko CK, Menon BB, Chen G, Whitsett JA, Clevers H, Gipson IK. Spdef null mice lack conjunctival goblet cells and provide a model of dry eye. Am. J. Pathol. 2013;183:35–48. doi: 10.1016/j.ajpath.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alam J, de Paiva CS, Pflugfelder SC. Immune—Goblet cell interaction in the conjunctiva. Ocul. Surf. 2020;18:326–334. doi: 10.1016/j.jtos.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park KS, Korfhagen TR, Bruno MD, Kitzmiller JA, Wan H, Wert SE, Khurana Hershey GK, Chen G, Whitsett JA. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J. Clin. Investig. 2007;117:978–988. doi: 10.1172/JCI29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCauley HA, Liu CY, Attia AC, Wikenheiser-Brokamp KA, Zhang Y, Whitsett JA, Guasch G. TGFbeta signaling inhibits goblet cell differentiation via SPDEF in conjunctival epithelium. Development. 2014;141:4628–4639. doi: 10.1242/dev.117804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roat MI, Ohji M, Hunt LE, Thoft RA. Conjunctival epithelial cell hypermitosis and goblet cell hyperplasia in atopic keratoconjunctivitis. Am. J. Ophthalmol. 1993;116:456–463. doi: 10.1016/s0002-9394(14)71404-7. [DOI] [PubMed] [Google Scholar]
- 61.Schulze U, Sel S, Paulsen FP. Trefoil factor family peptide 3 at the ocular surface. A promising therapeutic candidate for patients with dry eye syndrome? Dev. Ophthalmol. 2010;45:1–11. doi: 10.1159/000315014. [DOI] [PubMed] [Google Scholar]
- 62.Schulze U, Hampel U, Sel S, Contreras-Ruiz L, Schicht M, Dieckow J, Diebold Y, Paulsen F. Trefoil factor family peptide 3 (TFF3) is upregulated under experimental conditions similar to dry eye disease and supports corneal wound healing effects in vitro. Invest. Ophthalmol. Vis. Sci. 2014;55:3037–3042. doi: 10.1167/iovs.13-13423. [DOI] [PubMed] [Google Scholar]
- 63.Steven P, Schafer G, Nolle B, Hinz M, Hoffmann W, Paulsen F. Distribution of TFF peptides in corneal disease and pterygium. Peptides. 2004;25:819–825. doi: 10.1016/j.peptides.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 64.Flanagan JL, Willcox MD. Role of lactoferrin in the tear film. Biochimie. 2009;91:35–43. doi: 10.1016/j.biochi.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 65.Li K, Liu X, Chen Z, Huang Q, Wu K. Quantification of tear proteins and sPLA2-IIa alteration in patients with allergic conjunctivitis. Mol. Vis. 2010;16:2084–2091. doi: 10.3390/molecules16032084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Snodgrass RG, Brune B. Regulation and functions of 15-lipoxygenases in human macrophages. Front. Pharmacol. 2019;10:719. doi: 10.3389/fphar.2019.00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang MS, Schneider C, Roberts RL, Shappell SB, Haselton FR, Boeglin WE, Brash AR. Detection and subcellular localization of two 15S-lipoxygenases in human cornea. Invest. Ophthalmol. Vis. Sci. 2005;46:849–856. doi: 10.1167/iovs.04-1166. [DOI] [PubMed] [Google Scholar]
- 68.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zmora P, Blazejewska P, Moldenhauer AS, Welsch K, Nehlmeier I, Wu Q, Schneider H, Pohlmann S, Bertram S. DESC1 and MSPL activate influenza A viruses and emerging coronaviruses for host cell entry. J. Virol. 2014;88:12087–12097. doi: 10.1128/JVI.01427-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Byun YS, Kang B, Yoo YS, Joo CK. Poly(ADP-ribose) polymerase inhibition improves corneal epithelial innervation and wound healing in diabetic rats. Invest. Ophthalmol. Vis. Sci. 2015;56:1948–1955. doi: 10.1167/iovs.14-16259. [DOI] [PubMed] [Google Scholar]
- 71.Fehr AR, Singh SA, Kerr CM, Mukai S, Higashi H, Aikawa M. The impact of PARPs and ADP-ribosylation on inflammation and host-pathogen interactions. Genes Dev. 2020;34:341–359. doi: 10.1101/gad.334425.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Daugherty MD, Young JM, Kerns JA, Malik HS. Rapid evolution of PARP genes suggests a broad role for ADP-ribosylation in host-virus conflicts. PLoS Genet. 2014;10:e1004403. doi: 10.1371/journal.pgen.1004403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheema M, Aghazadeh H, Nazarali S, Ting A, Hodges J, McFarlane A, Kanji JN, Zelyas N, Damji KF, Solarte C. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19) Can. J. Ophthalmol. 2020;55:e125–e129. doi: 10.1016/j.jcjo.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo D, Xia J, Wang Y, Zhang X, Shen Y, Tong JP. Relapsing viral keratoconjunctivitis in COVID-19: A case report. Virol. J. 2020;17:97. doi: 10.1186/s12985-020-01370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diomede F, D'Aurora M, Gugliandolo A, Merciaro I, Ettorre V, Bramanti A, Piattelli A, Gatta V, Mazzon E, Fontana A, et al. A novel role in skeletal segment regeneration of extracellular vesicles released from periodontal-ligament stem cells. Int. J. Nanomed. 2018;13:3805–3825. doi: 10.2147/IJN.S162836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chiricosta L, Silvestro S, Pizzicannella J, Diomede F, Bramanti P, Trubiani O, Mazzon E. Transcriptomic analysis of stem cells treated with moringin or cannabidiol: Analogies and differences in inflammation pathways. Int. J. Mol. Sci. 2019 doi: 10.3390/ijms20236039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gugliandolo A, Diomede F, Cardelli P, Bramanti A, Scionti D, Bramanti P, Trubiani O, Mazzon E. Transcriptomic analysis of gingival mesenchymal stem cells cultured on 3D bioprinted scaffold: A promising strategy for neuroregeneration. J. Biomed. Mater. Res. Part A. 2018;106:126–137. doi: 10.1002/jbm.a.36213. [DOI] [PubMed] [Google Scholar]
- 78.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anders S, Pyl PT, Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014 doi: 10.1186/S13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu G, Wang LG, Han Y, He QY. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wickham, H. ggplot2: Elegant Graphics for Data Analysis; 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study will be available from the corresponding author on reasonable request.