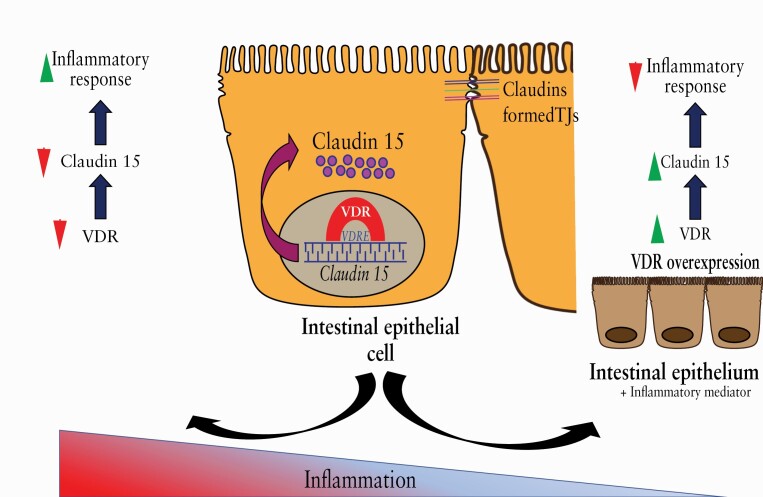
Abstract
Background and Aims
Dysfunction of the vitamin D receptor [VDR] contributes to the aetiology of IBD by regulating autophagy, immune response, and mucosal permeability. VDR directly controls the paracellular tight junction protein Claudin-2. Claudin-2 and Claudin-15 are unique in maintaining paracellular permeability. Interestingly, claudin-15 mRNA was downregulated in patients with ulcerative colitis. However, the exact mechanism of Claudin-15 regulation in colitis is still unknown. Here, we investigated the protective role of VDR against intestinal inflammation via upregulating Claudin-15.
Methods
We analysed the correlation of Claudin-15 with the reduction of VDR in human colitis. We generated intestinal epithelial overexpression of VDR [O-VDR] mice to study the gain of function of VDR in colitis. Intestinal epithelial VDR knockout [VDR∆IEC] mice were used for the loss of function study. Colonoids and SKCO15 cells were used as in vitro models.
Results
Reduced Claudin-15 was significantly correlated with decreased VDR along the colonic epithelium of human IBD. O-VDR mice showed decreased susceptibility to chemically and bacterially induced colitis and marked increased Claudin-15 expression [both mRNA and protein] in the colon. Correspondingly, colonic Claudin-15 was reduced in VDR∆IEC mice, which were susceptible to colitis. Overexpression of intestinal epithelial VDR and vitamin D treatment resulted in a significantly increased Claudin-15. ChIP assays identified the direct binding of VDR to the claudin-15 promoter, suggesting that claudin-15 is a target gene of VDR.
Conclusion
We demonstrated the mechanism of VDR upregulation of Claudin-15 to protect against colitis. This might enlighten the mechanism of barrier dysfunction in IBD and potential therapeutic strategies to inhibit inflammation.
Keywords: Claudin, Crohn’s disease, colonoids, IBD, inflammation, Salmonella, tight junction, VDR, ulcerative colitis
Graphical Abstract
Graphical Abstract.
1. Introduction
Inflammatory bowel diseases [IBD],1 including ulcerative colitis [UC] and Crohn’s disease [CD], are multifactorial diseases. Impaired epithelial tight junctions2 are considered as a major characteristic feature in patients with IBD, as well as in animal models of colitis.3,4 A recent study assessed the intestinal permeability at recruitment in 1420 asymptomatic first-degree relatives [6–35 years old] of patients with CD.5 Participants were then followed for a diagnosis of CD with a median follow-up time of 7.8 years. It has been shown that altered intestinal barrier function contributes to the pathogenesis of CD. Abnormal intestinal barrier function might serve as a biomarker for the risk of CD onset.6 However, many factors that contribute to the altered tight junctions in IBD have yet to be unveiled.
Structural components of tight junctions determine epithelial integrity and selective barrier function.7 Principal constituents of intestinal epithelial tight junctions are Claudin proteins, which regulate the permeability of the intact epithelia.8 The delicate physiological balance of different Claudin protein expression levels is altered during mucosal inflammation.8 Among the different 27 Claudin family members,2 Claudin-2 expression is positively linked with inflammatory activity in patients with IBD.9 In contrast, a robust decrease in Claudin-15 mRNA was reported in patients with UC.2,4 Earlier studies indicated that Claudin-15 deficiency resulted in low intraluminal sodium [Na+] and glucose malabsorption.10 Additionally, Claudin-15 deficient mice exhibited a dominant intestinal phenotype, described as mega-intestine.11 However, the mechanism by which Claudin-15 expression is regulated in the intestinal epithelium and inflammation is still undefined.
Among all Claudin proteins,2 Claudin-15 and Claudin-2 are exclusive, as they serve as paracellular transport channels.10 Claudin-2 is identified as a direct target of the vitamin D receptor [VDR],12 which plays a multifaceted role in the pathogenesis of IBD. VDR is crucial in regulating autophagy,13,14 immune response, tight junctions,15 the gut microbiome,16,17 and metabolites.18 VDR polymorphisms are associated with risks of human IBD.19 VDR expression is inversely correlated with inflammation in IBD.20 Targeted expression of human VDR [hVDR] in VDR-deficient intestinal epithelial cells [IECs] was able to rescue VDR-null mice from the severity of colitis in the presence of a VDR-deficient immune system. This confirms the protective role of epithelial VDR signalling against mucosal inflammation.5,16
Because VDR is known to regulate intestinal barrier function and is downregulated in experimental colitis models as well as in patients with UC,5,21 we intended to investigate the effect and mechanism of VDR in controlling Claudin-15 expression. We hypothesise that intestinal epithelial VDR overexpression protects against colitis via maintaining Claudin-15. Here, we revisited the human colitis dataset and identified the correlation of VDR with the expression level of Claudin-15. We then generated a new conditional intestinal epithelial VDR-overexpressing [O-VDR] mouse model. Intestinal epithelial VDR knockout [VDR∆IEC] mice were used for the loss of function study. We did an extensive analysis using novel transgenic mouse models and colitis models, cultured colonic enteroid and epithelial cell models, and human IBD samples. Our data showed that Claudin-15 expression was directly regulated by VDR. Our findings suggest a critical role of VDR in maintaining epithelial tight junctions and diminishing the inflammatory effect of colitis.
2. Materials and Methods
2.1. Patients
To check the expression of Claudin-15 in the human colon, we used slides containing paraffin-embedded colon biopsy samples of patients with UC. These slides were obtained from our collaborator Dr David Zhao, University of Rochester, New York, USA. This study was performed in accordance with approval from the University of Rochester Ethics Committee [RSRB00037178]. The ulcerative colitis2 cohort consisted of four patients with previous diagnosis of UC [two female, two male, mean age = 60.3 years, range, 51 to 67], and the age-matched healthy control cohort consists of four patients [one female, three male, mean age = 58.5 years, range, 44 to 73] without any previous history of gastrointestinal tract-related diseases. All these patient samples were obtained at the University of Rochester Medical Center from 2008 to 2012.
2.2. Gene expression datasets
For expression analyses, we used microarray data reported in the NCBI Gene Expression Omnibus database [GEO]. To find the correlation between VDR and Claudin-15 at the gene expression level, we gathered data by searching the Gene Expression Omnibus [https://www.ncbi.nlm.nih.gov/geo/] for expression-profiling studies using colonic samples from UC subjects. We randomly identified the GEO database reference series: GSE9452, [database number: GDS311922]. In this study, the authors performed DNA microarray analysis using colonic biopsy samples from healthy controls as well as from the inflamed and non-inflamed colonic mucosa from UC subjects. Control subjects were identified as: GSM240023, GSM240024, GSM240025, GSM240026, GSM240027; samples from inflamed colonic mucosa from UC patients were identified as: GSM239617, GSM239618, GSM239714, GSM239716, GSM239717, GSM239718, GSM239719, GSM239720; and samples from non-inflamed colonic mucosa from UC patients: GSM239723, GSM239725,GSM239726, GSM239727, GSM239729, GSM239730, GSM239731, GSM239732, GSM240022, GSM240028, GSM240029, GSM240030, GSM240031. The median age of the control group was 35 years, whereas the median age of the UC patients was 46 years. Both control and UC groups contained more than 50% female subjects.
2.3. Animals
Intestinal-specific VDR-overexpressing [O-VDR] mice were generated at Cyagen Biosciences [Santa Clara, CA, USA] in C57BL/6 mice strain background. The mouse VDR [mVDR] sequence was cloned into the Stbl3 vector [size 6631 bp]. The mVDR was cloned in [from ~2210 bp to ~3316 bp] under EF1A promoter[1 bp to 1105 bp]. A LoxP site was integrated after EF1A promoter [from 1105 bp to 2210 bp]. The resulting vector was named as pRP[Exp]-EF1A > LoxP[f]-3xSV40_late_polyA-LoxP[f]:mVdr. This was cloned in mice. Male and female mice that were positive for pRP[Exp]-EF1A > LoxP[f]-3xSV40_late_polyA-LoxP[f]:mVdr were mated as founder generation [F0]. O-VDR mice, once obtained by the University of Illinois at Chicago [UIC], were bred at the UIC animal facility and genotyped regularly to maintain the colony. VDR expression in O-VDR mice is Cre driven. The vector component info and genotyping primers for each type of mouse are given in Supplementary Tables S1 and S2, available as Supplementary data at ECCO-JCC online.
The intestinal-specific VDR knockout VDRΔIEC mice were generated by crossing with villin-cre mice [Jackson Laboratory, 004586, Bar Harbor, ME, USA] with VDRloxP/loxP mice (provided by Dr Geert Carmeliet [Department of Clinical and Experimental Medicine, KU Leuven, Leuven, Belgium]), as we reported previously.12,14 This VDRloxP/loxP mouse line is labelled as VDRloxP-B in our loss of function study.
Experiments were performed on 8- to 10-week old mice. We used both males and females. Mice were maintained in a 12-h dark/light cycle. All animal work was approved by the Animal Resources Committee, the University of Illinois at Chicago, USA. Animal Protocol numbers used in this study are ACC 15–231 and ACC 18–179.
2.4. Induction of colitis and experimental design
Mice 8‐10 weeks old, of a specific genetic background, were grouped randomly into control and DSS treatment groups. Colitis was induced by adding 5% [weight/volume] dextran sodium sulphate [DSS] [36–50 kD; USB Corporation, Cleveland, OH, USA] to the drinking water for 7 days. Mice were monitored regularly, and their body weights were noted every day. All mice were provided a regular chow diet ad libitum. We checked the effect of DSS on both OVDR and VDRΔIEC mice and compared them with their respective control group. On Day 7, mice were sacrificed, and intestinal tissue and blood samples were harvested for RNA, protein, immunofluorescence, and cytokine analysis as described in the Results section. The samples were immediately frozen and kept at -80°C until use.
2.5. Salmonella infection in vivo
For Salmonella infection, mice [8‐10 weeks old, both male and female] had water and food withdrawn for 4 h before the oral gavage. Control [N = 6, 3 male and 3 female] mice were gavaged orally with 100 μl sterile HBSS and the experimental group of mice [N = 6, 3 male and 3 female] were gavaged with 100 μl Salmonella 14028s suspension in HBSS (containing 1.0×108 ccolony-forming units [CFU] of bacteria), as described previously.23 All the mice were sacrificed 8 h following infection, and tissue samples were collected for further analysis.
2.6. Vitamin D3treatment in vivo
C57/BL/6 wild-type mice [males and females aged 6‐8 weeks] were gavaged with 1,25D3 [0.2 μg/day in 100 μl of corn oil] three times a week for 4 weeks, as described in our previous study.13 Intestinal tissue was collected following euthanasia.
2.7. Colonoid culture
Colonoids were generated from colonic crypts isolated from mice of specific genetic backgrounds [eg, VDRloxP/loxP, O-VDR]. Initially mice were sacrificed by cervical dislocation and their intact colon was quickly dissected under sterile conditions. Faecal contents were then cleaned thoroughly with ice‐cold PBS [containing penicillin, 100 IU/mL/streptomycin, 100 μg/mL], and the tissue was cut into small pieces [~1 cm]. These pieces were then incubated in PBS-EDTA [with 2 mmol/L EDTA] solution for 30 min at 4°C on a rocker to help loosen the crypts from the muscle layer. Eventually they were transferred to ‘dissociation solution’ [54.9 mmol/L d‐sorbitol + 43.4 mmol/L sucrose dissolved in PBS] and vortexed quickly [for ~1 min]. They were then passed through a 100-μm cell strainer to separate the crypts from any large tissue chunks. The crypts were collected as pellets following centrifugation [at 150 × g for 10 min at 4°C]. The pellet was then carefully resuspended in Matrigel [growth factor reduced phenol‐free: obtained from BD Biosciences, San Jose, CA]. Next, 50-μL droplets of this mix [containing approximately 300‐500 crypts] were plated centrally in each well of a 12‐well plate and allowed to polymerise for approximately 30 min. Once solidified, culture medium [Advanced DMEM/F12 supplemented with HEPES, L‐glutamine, N2 and B27, R‐Spondin, Noggin, and EGF and Wnt] was added on top. Every 2–3 days, the culture was replenished with fresh medium. To continue and expand the culture, colonoids were passaged every 7–10 days. Initially, they were removed from the Matrigel and passed through a syringe with a 27G needle [BD Biosciences]; they were then transferred to fresh Matrigel. Normally, a 1:4 splitting ratio was maintained. Each experiment was repeated three times.24
2.8. Barrier function in organoid-derived monolayers
Murine 3-dimensional colonoids generated from crypts of both VDR loxP and O-VDR mice were grown for 7–10 days as mentioned above. They were then removed from the Matrigel matrix and collected in separate tubes, and colonoids from five wells of a 24-well plate were collected together. To dissociate the colonoids into a unicellular suspension, 500 μL of trypsin was added [in each tube]. After a 5-min incubation at 37°C, the sample was further disintegrated using a P200 by pipetting up a few times. Trypsin was deactivated by adding Advanced DMEM/F12 [1 mL of media per 500 μL of trypsin]. Transwell inserts were coated with diluted Matrigel solution and allowed to dry. The cell suspension was then centrifuged at 400 × g for 5 min to pellet down the cells. The pellet was then resuspended in complete media at 2.5 × 105 cells/200 μL to seed onto the trans-wells; 2 mls of cell-containing media was added to the top, and the bottom was filled with 1.5 ml of Advanced DMEM/F12 media. The culture was maintained in a 37°C cell culture incubator.25,26 After 3, 6, and 8 days of seeding, TEER [transepithelial electrical resistance] was measured to monitor intestinal epithelial cell permeability.27
2.9. Cell cultures
The human colonic epithelial cell line SKCO15 was cultured in DMEM [obtained from Corning, NY, USA] supplemented with 10% fetal bovine serum [FBS], penicillin-streptomycin [penicillin, 100 IU/ml/streptomycin, 100 μg/ml], and L-glutamine [4.5 g/L]. Cells were maintained at 37°C in a humidified 5% CO2 incubator. For vitamin D3 treatment, SKCO15 cells were treated with vitamin D3 [20 nM] for 24 h.
2.10. mRNA extraction and quantitative polymerase chain reaction
Total RNA was extracted using a Qiagen [Valencia, CA, USA] RNA extraction mini-kit as instructed by the manufacturer. Specific transcripts were amplified using SYBR Green [Agilent Technologies, CA, USA] in a Stratagene fluorescence reader [Stratagene Biosystems]. The sequences of the gene-specific primers were as described previously.12 The levels of each transcript were normalised with internal control beta-actin to calculate the relative mRNA levels of a specific gene. Quantitative polymerase chain reaction [PCR] primers are listed in Table 1.
Table 1.
Real-time PCR primers.
| Primer name | Sequence |
|---|---|
| mβ-actinF | 5′-TGTTACCAACTGGGACGACA-3’ |
| mβ-actinR | 5′-CTGGGTCATCTTTTCACGGT-3’ |
| mVDRF | 5′-GAATGTGCCTCGGATCTGTGG-3’ |
| mVDRR | 5′-ATGCGGCAATCTCCATTGAAG-3’ |
| mClaudin-1F | 5′-ATCCATAGGAAAGGCCCTTCAGCA-3′ |
| mClaudin-1R | 5′-TACATGTAGGGCAACCAAGTGCCT-3’ |
| mClaudin-2F | 5′-GCAAACAGGCTCCGAAGATACT-3’ |
| mClaudin-2R | 5′-GAGATGATGCCCAAGTACAGAG-3’ |
| mClaudin-3F | 5′-CCTGTGGATGAACTGCGTG-3′ |
| mClaudin-3R | 5′-GTAGTCCTTGCGGTCGTAG-3’ |
| mClaudin-4F | 5′-TGCAGAGCACAGGTCAGATG-3′ |
| mClaudin-4R | 5′-GAGTACTTGGCCGAGTAGG-3’ |
| mClaudin-7F | 5′-GCGACAACATCATCACAGCC-3′ |
| mClaudin-7R | 5′-CCTTGGAGGAATTGGACTTGG-3′ |
| mClaudin-15F | 5′-ATGTCGGTAGCTGTGGAGAC-3’ |
| mClaudin-15R | 5′-GGACGGAAAGTCCCAGCAG-3’ |
PCR, polymerase chain reaction.
2.11. siRNA silencing and overexpression
Initially, mouse colonoids were allowed to develop for 10–12 days; then they were subsequently disrupted for splitting and simultaneously transfected with siRNA [control or specific gene target] at a final concentration of 20 nM using lipofectamine RNAimax [Thermo Fisher, USA].28 They were then grown for another 4–7 days before the collection of samples for western blotting. For overexpression of VDR, we transfected SKCO15 cells with an hVDR [human VDR]-expressing plasmid or control plasmid using Lipofectamine 3000 [Thermo Fisher, USA] following the manufacturer’s protocol.
2.12. Immunoblotting
Cells and tissues were lysed with 1X cell lysis buffer [CLB] [Cell Signaling Technology, Danvers, MA, USA] containing 1X proteinase inhibitor cocktail as described previously.12 Protein concentrations in the lysates were measured using the Bradford method. Control and experimental samples were run on SDS-PAGE gels and blotted onto nitrocellulose membranes. Following blocking with 5% BSA, the membrane was probed for different primary antibodies and incubated with their respective secondary antibodies, as described in the Results section. Details of the primary antibodies used in this paper are given in Supplementary Table S2. All primary antibodies were diluted 1:1000, except the anti-β-actin antibody, which was diluted 1:10 000. Blots were eventually incubated overnight at 4°C in the primary antibody and for 1 h at room temperature in the secondary antibody. Bands were visualised using Enhanced Chemiluminescence detection [ECL] reagents [Bio-Rad, Hercules, CA, USA].
2.13. Haematoxylin and eosin staining
Slides containing mouse colon [proximal or distal colon] sections [5 μm] were deparaffinised in xylene and passed through graded alcohol. They were then stained with haematoxylin and eosin following a previously described method.15
2.14. Immunofluorescence staining
Isolated mouse colonic tissues were fixed in 10% neutral buffered formalin and ultimately embedded in paraffin; 5-µm sections were used for IF staining with primary and secondary antibodies as described in the Results section. Paraffin sections of human colonic biopsies from patients with UC were also stained following a similar method. After deparaffinisation and antigen retrieval, sections were incubated at room temperature for 1 h in blocking buffer [2% bovine serum albumin, 1% goat serum in TBST]. The tissues were then kept at 4°C overnight with the respective primary antibodies [1:100 dilution]. Samples were then probed with secondary antibodies [goat anti-mouse Alexa Fluor 488 or goat anti-rabbit Alexa Fluor 488, at 1:200 dilution]. Tissues were then mounted with SlowFade Antifade media containing DAPI [Life Technologies, Grand Island, NY, USA]. Slides were imaged using a Zeiss 710 laser scanning microscope [LSM] [Carl Zeiss Inc., Oberkochen, Germany]. The details of the primary antibodies used are summarised in Supplementary Table S3, available as Supplementary data at ECCO-JCC online. Fluorescence intensity was determined by using ImageJ software. This method determines the corrected total fluorescence by subtracting out background signal, which is useful for comparing the fluorescence intensity between cells or regions.
2.15. Multiplex ELISA assay
A mouse-specific ProcartalPlexTM Multiplex Immunoassay [26] plate from Invitrogen Thermo Fisher Scientific was used to detect serum cytokine levels. The assay was performed using the manufacturer’s instruction manual and proper standards. Eventually, the plate was read using a Megpix Luminex machine.
2.16. ChIP assay
The ChIP assay was performed according to the manufacturer’s instructions, using the EpiTect ChIP OneDay Kit [Qiagen, Valencia, CA, USA]. Briefly, SKC015 cells or scraped VDRLoxP and VDR-/- colonic epithelial cells were treated with 1% formaldehyde for 10 min at 37°C. Cells were washed twice in ice-cold phosphate buffered saline containing protease inhibitor cocktail tablets [Roche, Nutley, NJ, USA]. Cells were then lysed in SDS lysis buffer, followed by shearing of chromatin by sonication [Branson Sonifier 250, Danbury, CT, USA]. Protein–DNA complexes were precipitated by protein A-coupled agarose beads and VDR or IgG antibodies. After purification of the DNA from the immunoprecipitated real-time [RT]-PCR, specific primer pairs designed for the putative VDRE sites on the claudin-15 promoter were used [Table 2]. Following RT-PCR, products were run on an agarose gel to validate the size. Primers for the VDRE site of IkBα and non-VDRE site of Axin1 were used as positive and negative controls, respectively, based on our previous studies.12,29
Table 2.
Primers for ChIPs.
| Primer name | Sequence |
|---|---|
| Site 1 Claudin-15 F | 5′AGATTCGTCCTTCAGCCCCAT3’ |
| Site 1 Claudin-15 R | 5′GCTGCGGGGTGGACAAAGAA3’ |
| Site 2 Claudin-15 F | 5′TCCACAGCAGGCCCCCTTGGCTTCC3’ |
| Site 2 Claudin-15 R | 5′ATGCAGGATTCCTTCCCCTACT3’ |
| IκBα F | 5’TGGCGAGGTCTGACTGTTGTGG3’ |
| IκBα R | 5′GCTCATCAAAAAGTTCCCTGTGC3’ |
| AxinP5F | 5’ CTGCTGTGTCTCCAACTCCT3’ |
| AxinP5R | 5’TGG ACC CAATGTGACCCAGA3’ |
ChIP, chromatin immunoprecipitation assay.
2.17. Statistical analysis
All data were expressed as the mean ± standard deviation [SD]. All statistical tests were two-sided. All p-values ≤ 0.05 were considered statistically significant. After the Shapiro‐Wilk test confirmed that the data were normally distributed, the differences between samples were analysed using the unpaired t test for two groups and using one-way analysis of variance [ANOVA] for more than two groups as appropriate, respectively. Two-way ANOVA was applied for two-factors comparisons. Body weights, see Figure 3A in the Results section, were analysed by generalised linear mixed models. The p-values in ANOVA analysis and generalised linear mixed models were adjusted using the Tukey method to ensure accurate results. Pairwise correlation analyses and scatter plots were conducted for staining intensity changes between VDR protein and Claudin-15 using SAS version 9.4 [SAS Institute, Inc., Cary, NC, USA]. Other statistical analyses were performed using GraphPad Prism 6 [GraphPad, Inc., San Diego, CA., USA].
3. Results
3.1. Reduced Claudin-15 in the colon of patients with UC
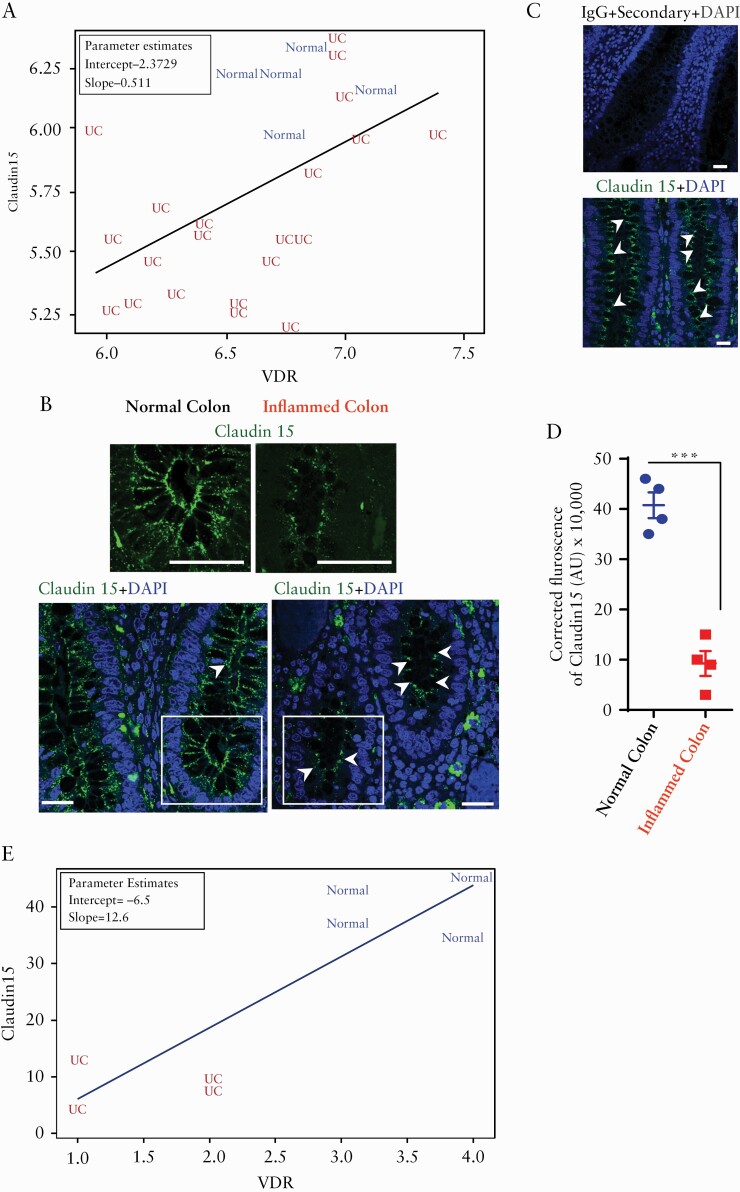
We analysed the microarray data of human samples from the Geo database and found that the expression patterns of Claudin-15 and VDR were synchronised in various experimental setups and various model systems. Here, the data obtained from human epithelial biopsies [GDS311922] showed that VDR and Claudin-15 expression are positively correlated and UC patients and healthy controls are differentiated[p = 0.0092] [Figure 1A]. Alterations of tight junctions are associated with the development of a disease state in ulcerative colitis.2,30 To study the changes of protein level of Claudin-15, we did immunostaining in the UC colon samples, using anti-Claudin-15 antibody [Figure 1B]. We verified the specificity of the immune staining in human colon tissue. No staining was detected when the colon section was stained with IgG along with only secondary antibody [Figure 1C]. Immunostaining of biopsies from patients with UC showed a significant reduction of Claudin-15 in the inflamed mucosa, compared with normal colon [Figure 1B, D]. This observation is consistent with a previous study reported that Claudin-15 mRNA levels were markedly downregulated in patients with UC.4 We further showed that the reduction of Claudin-15 immunofluorescence is correlated with the reduction of VDR in samples from human colitis [[p = 0.0059] Figure 1D].
Figure 1.
Significantly coordinated expression of reduced Claudin-15 and VDR in patients with ulcerative colitis [UC]. [A] Significantly coordinated expression of VDR and Claudin-15 in UC patients. We performed a regression analysis and a scatter plot of VDR against Claudin-15. Values for healthy controls were in blue colour and values for patients were in red colour. GEO database GDS3119 Normal, n = 5; UC, n = 21; the coefficient is 0.51096 with p = 0.0092 in linear regression model. The graph shows VDR and Claudin-15 expression are positively correlated and UC patients are differentiated from normal controls. [B] Immunofluorescence staining detected Claudin-15 protein [green colour] in inflamed and adjacent normal colonic epithelium in biopsy specimens obtained from patients with UC. The nucleus is stained blue with DAPI. Scale bar = 50 µm. Data were analysed by unpaired t test, ***p ≤ 0.001. [C]The specificity of the Claudin-15 immunostaining was verified in the human colon tissue section using IgG [+only secondary antibody] as control [upper panel, n = 4] and a similar section was stained with anti-Claudin-15 antibody [lower panel, n = 4]. [D] Quantification of green fluorescence indicated decreased Claudin-15 expression in the inflamed colonic epithelium of patients with UC. In each figure, values for normal colon indicated by blue colour and for inflamed colon by red colour. [D] Significantly coordinated expression of VDR and Claudin-15 as detected by immunofluorescence in biopsy samples of ulcerative colitis patients and normal controls. Blue indicates normal colon and red indicates UC patients sample. Normal, n = 4; UC, n = 4; the coefficient is 12.6 with p = 0.0059 in linear regression model. VDR, vitamin D receptor.
3.2. O-VDR mice exhibited normal colon morphology with increased VDR expression
We then hypothesised that overexpression of intestinal epithelial VDR protects against colitis via maintaining Claudin-15. We generated a new conditional intestinal epithelial VDR-overexpressing [O-VDR] mouse model that was designed to specifically overexpress mouse intestinal epithelial VDR [Supplementary Tables S1 and S2]. To identify the O-VDR mice, the mouse pups were genotyped regularly [Supplementary Figure 1, available as Supplementary data at ECCO-JCC online] with a specific primer set [Supplementary Table S2].
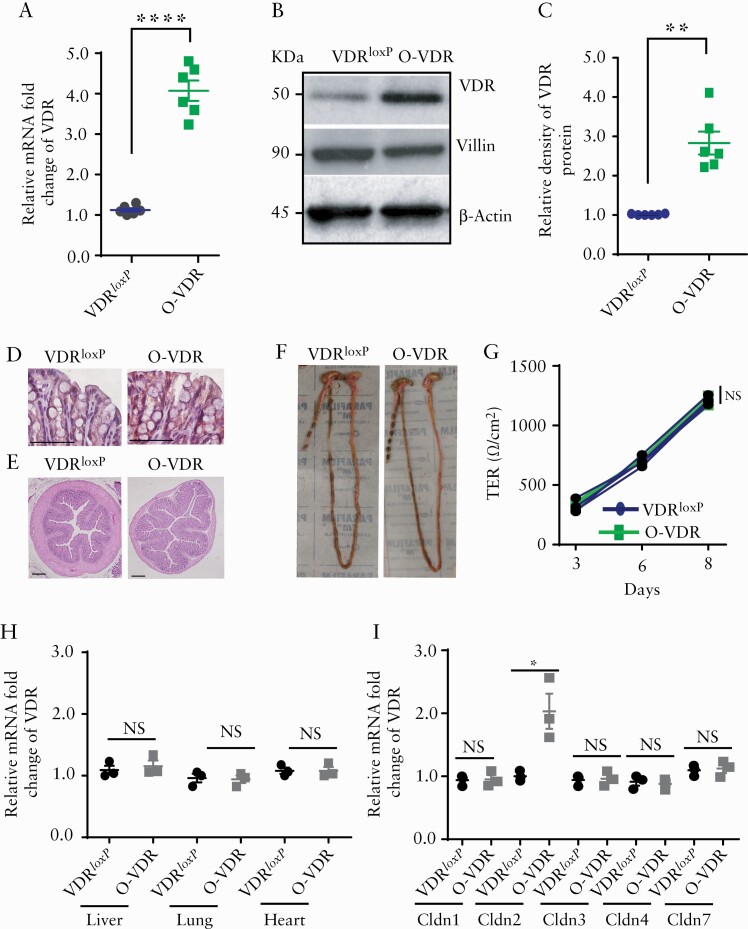
We checked the expression of VDR mRNA and protein in the mouse colon and found an ~4-fold increase in mRNA levels [Figure 2A] and an ~3-fold increase in protein levels [Figure 2B, C] compared with those of the VDRloxP control group. Correspondingly, we found an increase in the intensity of VDR staining in the mouse colon [Figure 2D]. Under normal conditions, VDR overexpression did not affect the microscopic morphology of the colon, as verified by haematoxylin and eosin [H&E] staining of adult mouse colon sections [Figure 2E]. Additionally, macroscopic observations specified that the length of the colon [Figure 2F] and other measurements of different digestive organs, such as the spleen and liver [data not shown], remained unchanged in transgenic O-VDR mice compared with wild-type [VDRloxP] mice. Next, we wanted to check the effect of VDR overexpression on the integrity of the intestinal epithelial tight junction. For this, we generated colon organoids of a specific genotype and then generated a monolayer to measure the TEER of the colon enteroid. We found no significant difference in transepithelial resistance across the monolayer between the two groups [Figure 2G]. The presence of uniform solid fecal pellets in the colon of O-VDR mice supported the normal intestinal function of the mice [Figure 2F]. Overexpression of VDR is intestinal epithelium-specific driven by villin-Cre. As detected by qPCR, VDR is specifically overexpressed in the intestine. No overexpression was detected in the other organ system like the liver, lung, or heart [Figure 2H].
Figure 2.
O-VDR mouse model showed intestinal VDR overexpression. An increase in VDR expression in the O-VDR mouse colon was indicated by [A] mRNA, [B] western blot images, and [C] densitometry quantification of the blots. [D] IHC staining with anti-VDR antibody indicated augmented VDR expression in O-VDR mouse colonic epithelium. [E] O-VDR mice exhibited normal colon histopathology. [F] Morphology and normal colon length. [G] TEER of mouse colonoid-derived monolayers remained unchanged between the O-VDR and VDRloxP groups. [H] mRNA expression of VDR in the liver, lung, and heart remained unaltered in OVDR mice as compared with VDRloxP. [I] Increased mRNA expression of claudin-2 mRNA in OVDR mouse colon was detected; however, expression of Claudin- 1, 3, 4, and 7 did not change in OVDR mice as compared with VDRloxP mice. In each figure, values for VDRloxP are indicated by blue colour and for O-VDR are indicated by green colour. n = 3 to 6 mice per group. Data were analysed by unpaired t test for Figure 2A, C, H, and I and two-way ANOVA for 2G. NS = not significant, * p < 0.05, **p ≤ 0.01, and ***p ≤ 0.001. VDR, vitamin D receptor; IHC, immunohistochemical; TEER, transepithelial electrical resistance; ANOVA, analysis of variance.
Next, we checked the mRNA expression of Claudin-1, 2, 3, 4, and 7; interestingly, no significant alteration was observed regarding Claudin- 1, 3, 4, and 7 [Figure 2I]. Claudin-2 mRNA was significantly overexpressed in O-VDR mice, as compared with the control group [Figure 2I]. This result is consistent with our previous report that Claudin-2 is a direct target of intestinal epithelial VDR.12 Also, VDRloxP and O-VDR mice did not show any difference in their overall growth and phenotype as neonate or as adult mice. Thus, we have established an intestinal epithelial-specific VDR-overexpressing mouse model to investigate the protective role of VDR.
3.3. O-VDR in intestine protected mice from acute colitis
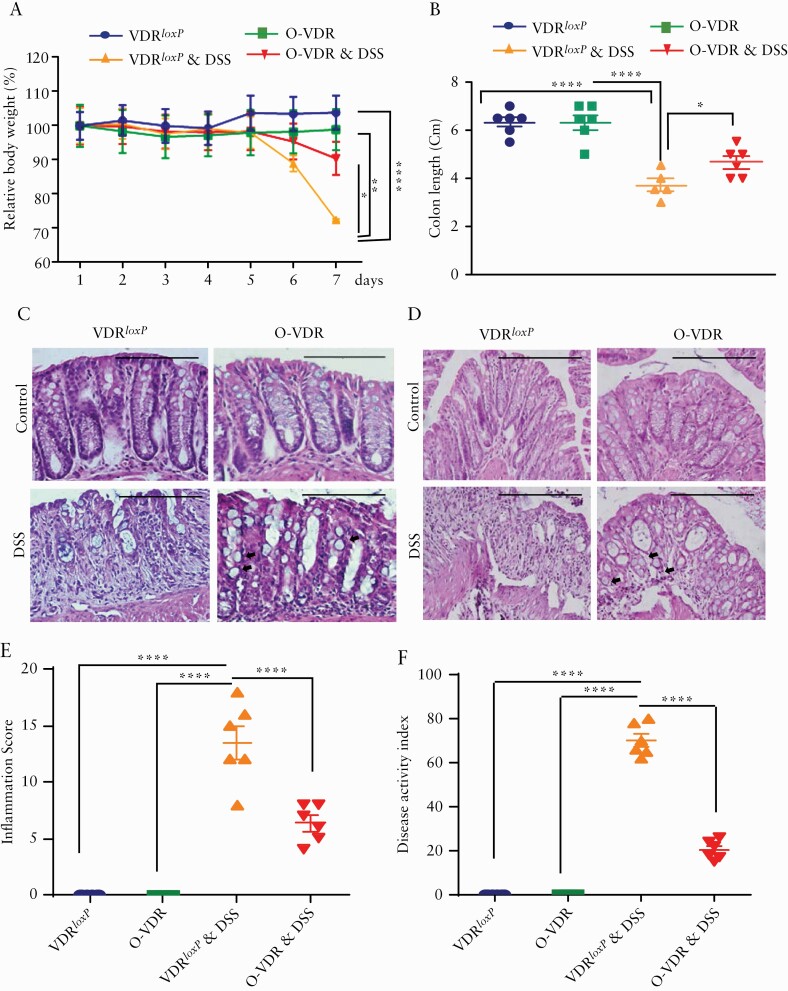
To evaluate the impact of O-VDR in colitis, we treated the mice with DSS in the drinking water. DSS-treated VDRloxP and O-VDR mice started losing body weight from Day 5, which was aggravated by Day 7. However, O-VDR significantly alleviated body weight, compared with the VDRloxP group, after 7 days of DSS treatment [Figure 3A]. Additionally, the O-VDR + DSS group exhibited increased colon length [Figure 3B] along with decreased inflammatory score [Figure 3E] and decreased cumulative Disease Activity Index [DAI] [Figure 3F], compared with those of the DSS-treated control group. Moreover, histological assessments of H&E-stained proximal and distal colon sections showed that the severity of colitis was much higher in the DSS-treated VDRloxP group than in the DSS-treated O-VDR group. This was indicated by loss of overall structural organisation, near-complete loss of goblet cells, and neutrophil accumulation. On the other hand, O-VDR mice were able to retain crypt-villi structural organisation and goblet cells [Supplementary Figure 2A, available as Supplementary data at ECCO-JCC online] and showed much lower lympho-histiocyte infiltration [Figure 3C, D]. Taken together, these results indicated that VDR overexpression in the intestine significantly inhibited DSS-induced inflammation.
Figure 3.
O-VDR mice are protected from acute colitis. Mice 8‐10 weeks old were treated with dextran sodium sulphate [DSS]: 5% [W/V] in the drinking water for 7 days. [A] Weight loss during the course of treatment. The body weight of the O-VDR group was significantly higher than that of the DSS-treated control group. Body weights were analysed by generalised linear mixed models. [B] Colon length in control and O-VDR mice after DSS treatment. Representative images of H&E-stained colonic epithelium sections from [C] the distal colon and [D] the proximal colon. [E] Decreased inflammation score in O-VDR mice compared with VDRloxP following DSS treatment; control mice did not show inflammation and/or any injury. [F] The disease activity index17 was significantly altered in DSS-treated mice. In each figure, values for VDRloxP are indicated by blue colour and for O-VDR are indicated by green colour, VDRloxP+DSS are indicated in orange, and OVDR + DSS are indicated in red colour. Scale bar = 200 µm, n = 6 [3 male and 3 female] mice per group. Data were analysed for 3B, 3E, and 3F by unpaired t test or one-way ANOVA. *p ≤ 0.05, **p ≤ 0.01, and ****p ≤ 0.0001. VDR, vitamin D receptor; H&E, haematoxylin and eosin; ANOVA, analysis of variance.
3.4. O-VDR mice retained Claudin-15 expression after DSS-induced colitis, displaying attenuated inflammatory signalling
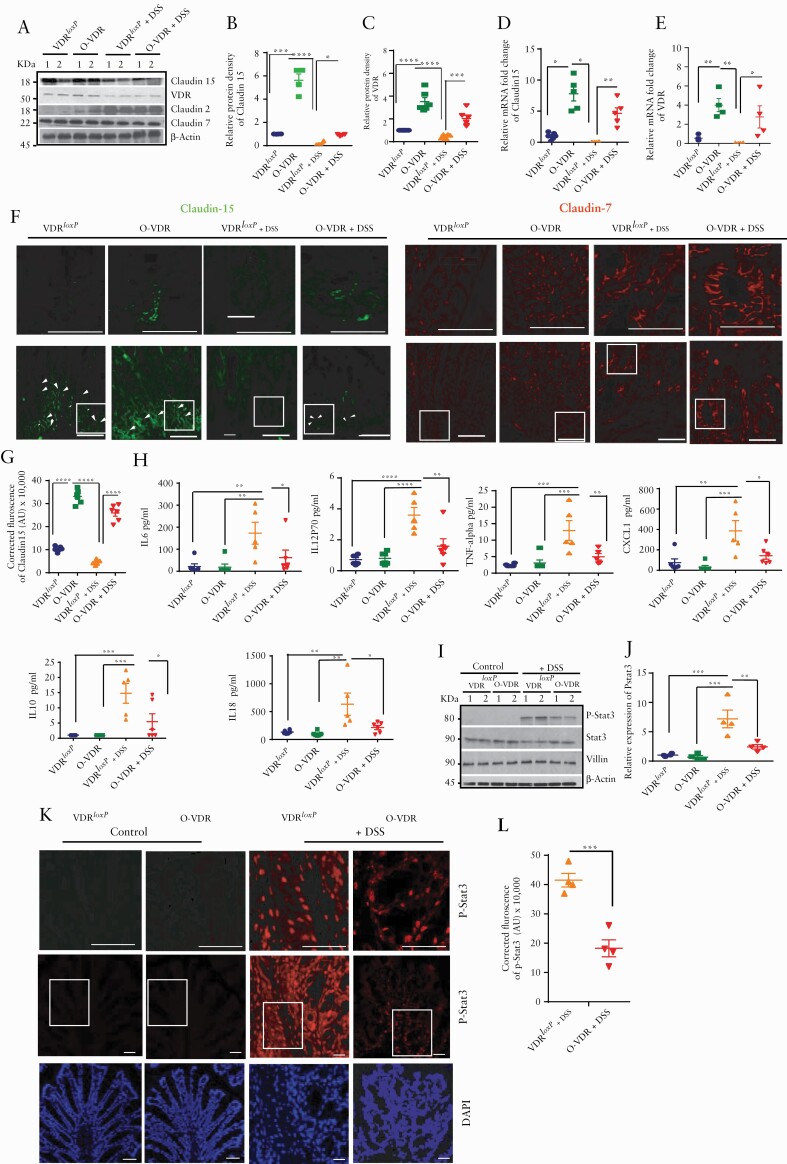
Interestingly, in the DSS-induced experimental colitis model, O-VDR mice were able to maintain a high level of Claudin-15 expression compared with the VDRloxP group, even after DSS treatment [Figure 4A, B]. O-VDR mice exhibited significantly increased Claudin-15 mRNA [Figure 4C] and protein expression [Figure 4A, B] along with VDR, even without DSS treatment [Figure 4A, B]. The expression levels of Claudin-15 and VDR were significantly higher in the O-VDR mice [Figure 4D, E]. IF staining of Claudin-15 indicated its distribution in the cell membrane of the colonic epithelium [Figure 4F]. The VDRloxP group showed very diffuse Claudin-15 after DSS treatment, compared with the control VDRloxP and O-VDR groups. On the other hand, DSS-treated O-VDR mice were able to maintain Claudin-15 expression [Figure 4F]. The overall density of Claudin-15 was significantly higher in the DSS-O-VDR group, compared with the DSS-VDRloxp mice [Figure 4G]. However, tight junction protein Claudin-7 was not changed in the DSS-treated groups examined by western blots and IF [Figure 4A, F].
Figure 4.
O-VDR mice retained Claudin-15 expression following acute DSS colitis, thus protecting the host from inflammation. [A] Western blot analysis of mouse colonic tissue indicated decreased VDR and Claudin-15 protein expression following acute DSS colitis; however, Claudin-15 expression was restored in O-VDR mice. Densitometric analysis of [B] Claudin-15 and [C] VDR by western blot. Relative mRNA expression of [D] Claudin-15 and [E] VDR in mouse colonic epithelium. [F] The expression and distribution of Claudin-15 in mouse colonic epithelium is shown by green fluorescence. Nuclear staining is revealed by blue DAPI. [G] The intensity of the immunofluorescence staining of Claudin-15. [H] Cytokines were inhibited in DSS-treated O-VDR mice. [I] Western blot analysis indicating protein levels of p-stat 3, stat3, villin, and actin in VDRloxP and O-VDR mice. [J] Densitometric analysis of p-stat3 expression. [K] Immunofluorescence staining indicating p-stat3 expression and distribution in mouse colonic epithelium. Control VDRloxP and O-VDR mice did not show detectable p-stat3. [L] Quantification of the red fluorescence of p-stat3, n = 3–6. Data were analysed by unpaired t test or one-way ANOVA, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001. Scale bar = 50 µm, n = 3–6. . VDR, vitamin D receptor; ANOVA, analysis of variance.
Mucosal inflammation is indicated by disruption of the normal balance between pro-and anti-inflammatory signals. Here we found significantly reduced serum pro-inflammatory cytokines [eg, IL-6, TNF-alpha, IL-18] and chemokine CXCL1 in the O-VDR + DSS mice, compared with the DSS-treated VDRloxP mice [Figure 4H].
Our recent study indicated the important role of VDR in regulating the Janus kinase [JAK]/signal transducer and activator of transcription [STAT] pathway.31 Importantly, the IL-6/STAT3 pathway is critical in mediating inflammatory signalling in the intestinal mucosa.32 Here we further verified whether overexpression of VDR in mice reduced activation of STAT3, thus rendering protection against DSS-induced colitis. Notably, p-STAT3 expression was very robust in DSS-treated VDRloxP mouse colonic samples, whereas it was very much suppressed in DSS-treated O-VDR mice. P-Stat3 levels were undetectable in various control groups [non-DSS treated] [Figure 4I, J]. No significant changes were observed in STAT3 expression in any of the control and experimental groups. In contrast, IF staining using an anti-p-STAT3 antibody showed increased nuclear localisation in most of the epithelial cells [Figure 4K, L].
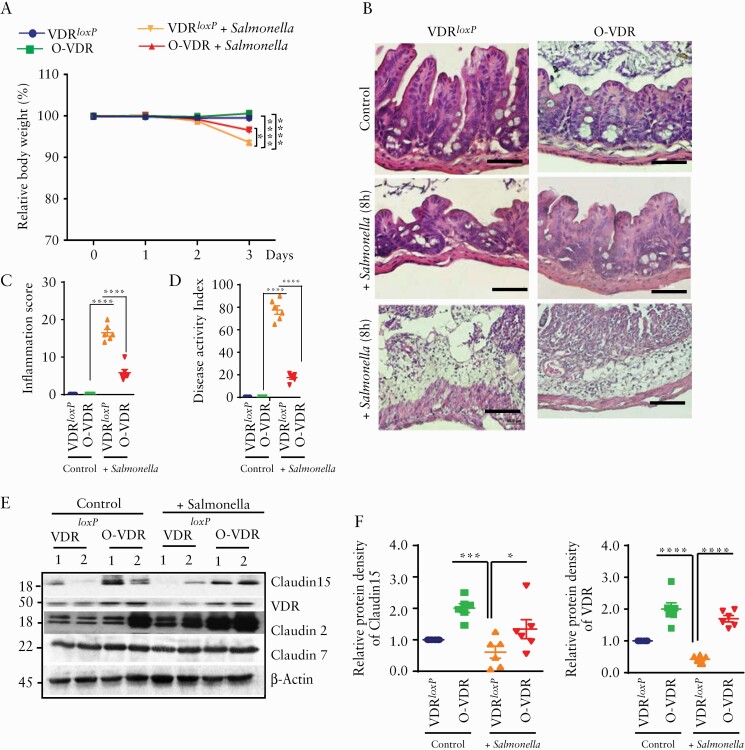
3.5. O-VDR mice were protected from Salmonella 14028s-induced colitis
To further emphasise our hypothesis, we studied another model of colitis in mice, induced by Salmonella 14028s bacteria. We found that O-VDR mice were able to maintain significantly improved body weight as compared with Salmonella-infected VDRLoxP mice [Figure 5A]. To investigate the severity of Salmonella-induced inflammation, we checked the histology of the caecum which is known to be affected by Salmonella-infection.23 Within 8 h post-infection, the caecum of the VDRLoxP mice showed severe epithelial damage, neutrophil infiltration, oedema, and crypt abscesses which were more adverse after 4 days [Figure 5B]. However, in the O-VDR mice, crypt structure was evident with a much lesser degree of inflammation [Figure 5B, C, and D]. We further found, in VDRLoxP mice, Salmonella infection reduced the Claudin-15 expression in the colon, as compared with the control group. Interestingly, O-VDR mice were able to reserve the Claudin-15 level. In contrast, the TJ protein Claudin-7 was not altered in the Salmonella-infected colon [Figure 5E, F].
Figure 5.
OVDR mice were protected from Salmonella-induced colitis. Mice 8‐10 weeks old were treated with Salmonella typhimurum. [A] VDR over-expressing OVDR mice were protected from Salmonella-induced colitis. Mice (eight to ten week-old) were infected with Salmonella. Body weights of the mice were analysed by generalised linear mixed models. [B] Representative images of H&E of mice caecum without [top panel] and with 8 h [middle panel] and with 4 days [lowermost panel] of Salmonella infection. [C] Decreased inflammation [in caecum] in O-VDR mice was noted as compared with VDRloxP after 8 h of salmonella treatment. [D] The disease activity index18 was significantly altered in Salmonella infection. [E] Western blot images showing OVDR mice retained VDR and Claudin-15 protein expression in the colon, compared with the VDRloxP group. [F] Densitometric quantification of the western blots. In each figure, values for VDRloxP are indicated by blue colour and for O-VDR are indicated by green colour, VDRloxP+ Salmonella is indicated in orange and OVDR + Salmonella are indicated in red colour. n = 6 for each group. All data in C, D, and F were analysed by unpaired t test or one-way ANOVA. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001. VDR, vitamin D receptor; H&E, haematoxylin and eosin; ANOVA, analysis of variance.
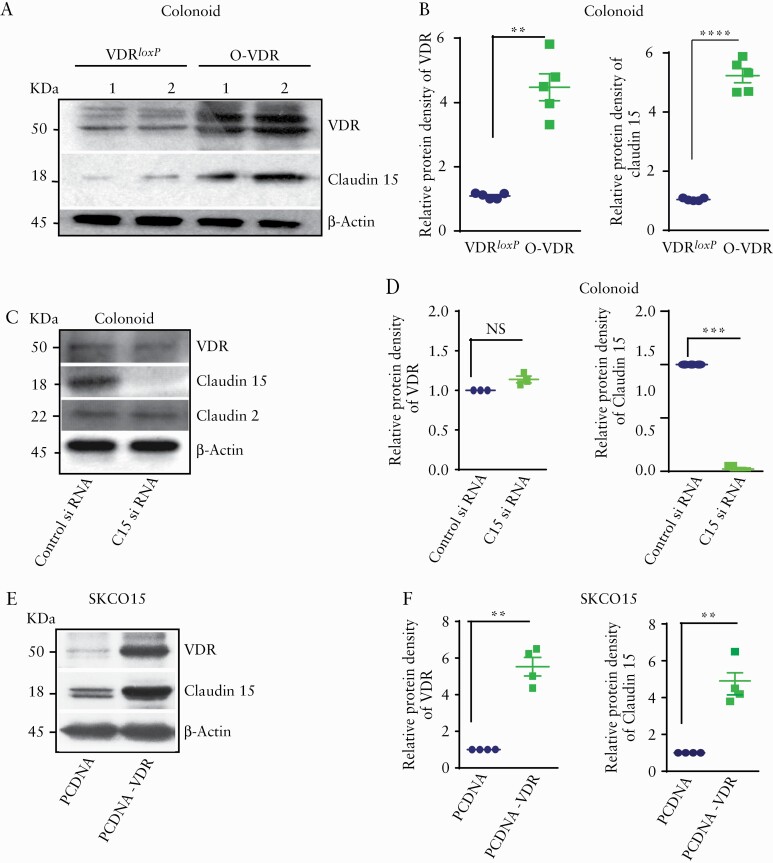
3.6. VDR upregulated Claudin-15 expression in vitro and in vivo
We also used colonoid models to investigate the VDR regulation of Claudin-15 in vitro. We found increased protein levels of VDR and Claudin-15 in crypt-derived colonoids generated from O-VDR mice, compared with VDRloxP mice [Figure 6A, B]. Next, we transfected the colonoids with Claudin-15 siRNA or control siRNA. Inhibition of Claudin-15 expression in colonoids did not change VDR expression or Claudin-2 [Figure 6C, D], suggesting that VDR regulates Claudin-15 as a downstream target. Next, we ectopically overexpressed hVDR [human VDR] in the human colonic SKCO15 cells. We found higher Claudin-15 levels in the SKCO15 cells transfected with hVDR [Figure 6E, F].
Figure 6.
VDR regulates Claudin-15 expression in vitro. [A] Western blot image shows higher VDR and Claudin-15 expression in mouse colonoids. [B] Densitometric quantification of the western blots. [C] Protein expression after Claudin-15 siRNA treatment in mouse colonoids. [D] Densitometric quantification of the western blots after silencing of Claudin-15 by siRNA. [E] Western blot images showing increased VDR and Claudin-15 expression in SKCO15 cells following transfection with hVDR. [F] Quantification of the blot. In each figure, values for the control group are indicated by blue colour and for the experimental group are indicated by green colour; n = 3–6 for each group. Data were analysed by unpaired t test or ANOVA, NS p > 0.05, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001. VDR, vitamin D receptor; ANOVA, analysis of variance; NS, non-significant.
Vitamin D3 treatment is known to increase VDR expression. In mice treated with vitamin D3, we found increased levels of Claudin-15 protein expression in the ileum and colon along with increased VDR in wild-type mice [Supplementary Figure 2B]. This result further supported the VDR regulation of Claudin-15.
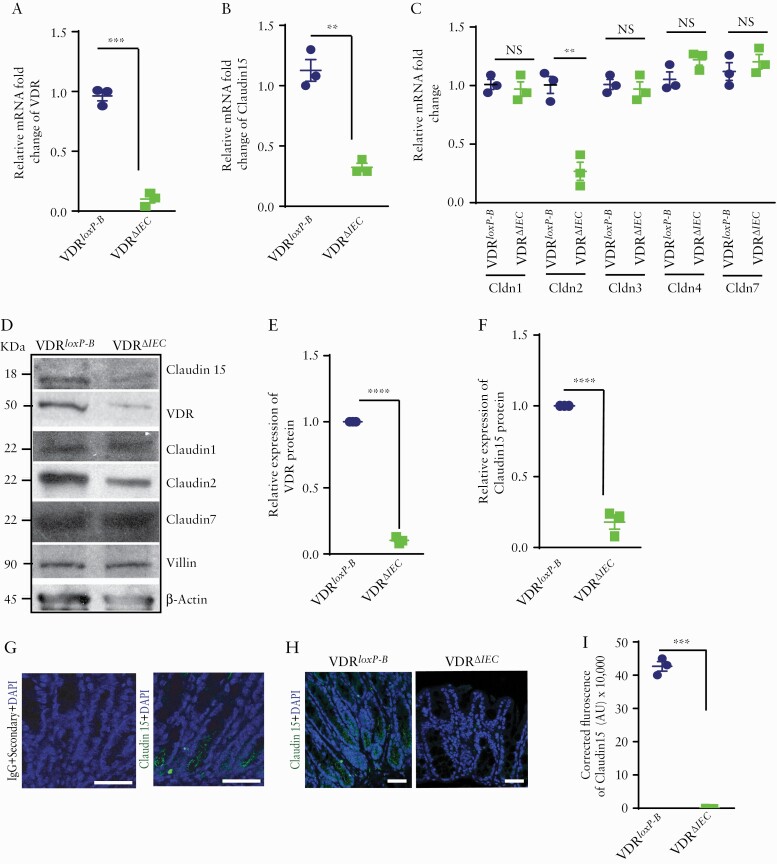
3.7. Deletion of intestinal epithelial VDR downregulated Claudin-15 expressions in vivo
We further studied the loss functional effect of VDR on Claudin-15 in the VDRΔIEC mice. Deletion of intestinal epithelial VDR led to a significant reduction of Claudin-15 level. Along with a decrease in VDR mRNA in mice colon, a decrease in Claudin-15 mRNA was noted in the VDRΔIEC mice, compared with the control VDRloxp-B mice [Figure 7A, B]. Similarly, a significant decrease in Claudin-2 mRNA [Figure 7C] was also noted. However, Claudin-1, 3, 4, 7 mRNA did not change [Figure 7C]. In VDRΔIEC mice, due to the absence of intestinal VDR [Figure 7D, E], a significant reduction of Claudin-15 protein was noted in the colon as detected by western blots [Figure 7D, F] and immunostaining [Figure 7H, I]. The specificity of anti-Claudin-15 antibody for mouse tissue was verified by using IgG as control [Figure 7G]. These data reinforce our hypothesis that Claudin-15 expression is directly regulated by VDR.
Figure 7.
Deletion of VDR reduced Claudin-15 expression in vivo. Reduced VDR and Claudin-15 were detected in VDRΔIEC mice as indicated by [A] VDR mRNA and [B] claudin-15 mRNA. However, mRNA of [C] Claudin-1, 3, 4, 7 was unaltered with exception of Claudin-2. [D] Western blot image showing decreased expressions of VDR, Claudin-15, and Claudin-2 in mouse colon. Densitometric quantification of the western blots of [E] VDR and [F] Claudin-15. [G] No staining was detected in the mice colon tissue section by IgG [+only secondary antibody] whereas similar section stained with Claudin-15 antibody indicated staining in the mice colon as indicated by specific green colour. [H] Representative images of ICC images indicating a lower level of Claudin-15 expression in mousee colon. [I] Quantification of the ICC staining. Scale bar = 50 µm. In each figure, values for the control group are indicated by blue colour and for the experimental group are indicated by light green colour; n = 3 for each group. VDRloxp-B: Belgium VDRloxp/loxp mice used in generating VDRΔIEC mice. Data were analysed by unpaired t test or one-way ANOVA, NS p > 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001. VDR, vitamin D receptor; ICC, immunocytochemistry; ANOVA, analysis of variance; NS, non-significant.
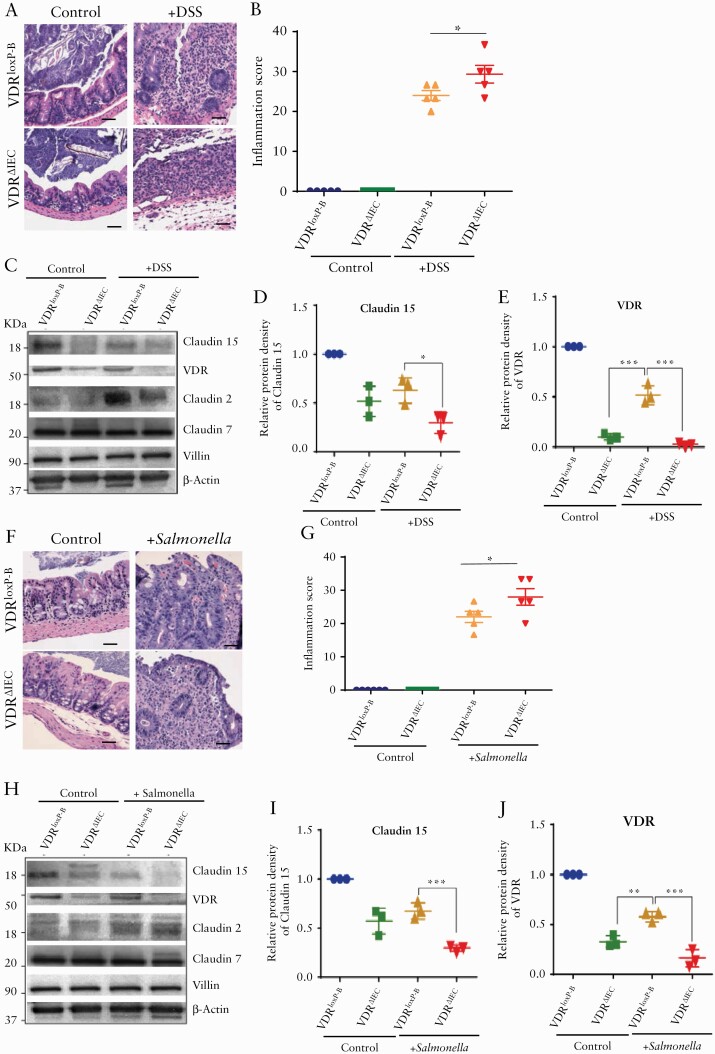
3.8. Reduced Claudin-15 expression in colon after deletion of intestinal epithelial VDR in experimental colitis
We tested the changes of Claudin-15 in the colon by challenging the VDRΔIEC mice with experimental colitis VDRΔIEC mice. In the DSS-treated VDRΔIEC mice, we found more inflammation as compared with the VDRlox-B mice treated with DSS [Figure 8A, B]. At the protein levels, we found significantly lower levels of Claudin-15 in the VDRΔIEC mice [Figure 8C, D], whereas DSS treatment reduced VDR in the VDRlox-B mice [Figure 8E]. However, DSS treatment increased Claudin-2 expression in the VDRΔIEC mice, as reported in our previous study.15 No change of Claudin-7 and villin protein was noted after the DSS treatment.
Figure 8.
Reduced Claudin-15 expression due to deletion of VDR in the intestinal epithelium in the experimental colitis. [A] Representative images of H&E of mice with or without DSS treatment. [B] Increased inflammation in VDRΔIEC mice was noted as compared with VDRloxP-B. [C] Representative images of western blot of Claudin-15, Claudin-2, Claudin-7, and VDR after DSS treatment. Densitometric analysis of Claudin -15 [D] and VDR [E] in VDRΔIEC mousee colon in DSS colitis. [F] Representative images of H&E of mouse caecum 4 days post Salmonella infection. [G] Increased inflammation in VDRΔIEC mice was noted as compared with VDRloxP-B after Salmonella infection. [H] Representative images of western blot of Claudin-15, Claudin-2, Claudin-7, and VDR following Salmonella treatment. Densitometric analysis of Claudin -15 [I] and VDR [J] in the colon of VDRΔIEC mice in Salmonella-induced colitis. Scale bar = 50 µm, n = 3–6. Data were analysed by unpaired t test or one-way ANOVA, *p ≤ 0.05, **p ≤ 0.01, and ****p ≤ 0.001. VDR, vitamin D receptor; H&E, haematoxylin and eosin; ANOVA, analysis of variance.
Similar observations were noted in the mice with Salmonella infection [Figure 8F-J]. VDRΔIEC mice were more susceptable to the Salmonella infection, with more damage in the intestine and higher levels of inflammation [Figure 8F, G]. Salmonella infection reduced Claudin-15 and increased Claudin-2 expression in the colon of VDRΔIEC mice [Figure 8H, I]. In contrast, the Claudin-7 remained the same before and after infection. The VDR level in ine intestinal mucosa was detected as shown in Figure 8J. Salmonella infection reduced the VDR expression in the colon. Because expression of Claudin-15 is already significantly low in the colon after deletion of intestinal epithelial VDR, the VDRΔIEC mice lost the protection from colitis.
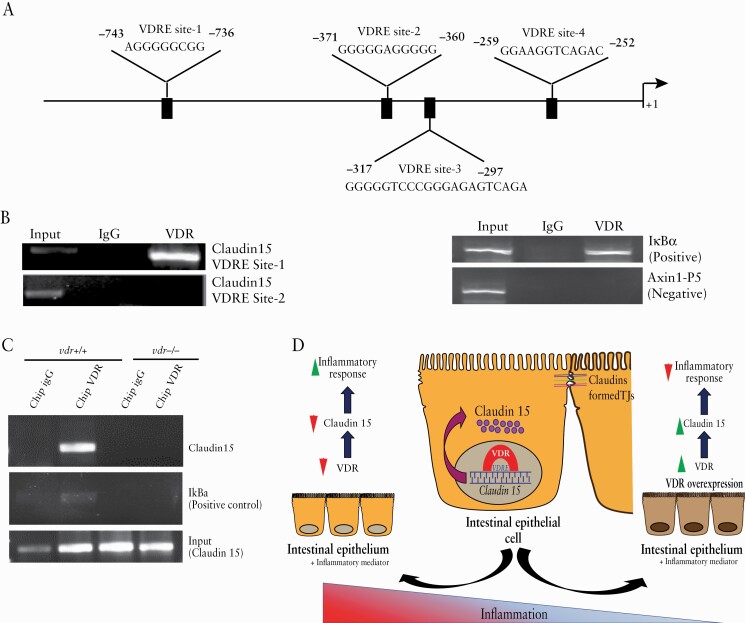
3.9. VDR transcriptionally regulates Claudin-15 expression in vitro and in vivo
Because VDR regulates the expression of many target genes as a transcription factor, we speculate that our observations may be due to VDR binding to the Claudin-15 promoter. Vitamin D response element [VDRE] is a DNA sequence that is found in the promoter region of vitamin D/VDR-regulated genes. Correspondingly, in silico analysis indicated five possible VDRE sites on the Claudin-15 promoter [Figure 9A]. We then confirmed the interaction of VDR and the Claudin-15 promoter by performing ChIP analysis with specific primers [Table 2]. ChIP assays verified VDR binding to a specific [- 861 to - 655] region of the Claudin-15 promoter [Figure 9B]. IgG was used as a control instead of VDR antibody to authenticate the data. We also used a positive primer for the VDRE site of IkBα as a positive control and a primer for the non-VDRE site for Axin1 as a negative control, based on our previous publications.12,29
Figure 9.
VDR regulates Claudin-15 expression in colonic epithelium. [A] Putative VDRE sites in the Claudin-15 promoter. [B] ChIP assay using quantitative PCR followed by SDS-PAGE electrophoresis showed direct binding of VDR to the Claudin-15 promoter. The assay was verified for a primer of a VDRE site on IkBα as a positive control and a primer for a non-VDRE site of Axin1 as a negative control. [C] SDS-PAGE analysis following the ChIP assay verified the binding of VDR to the Claudin-15 promoter, n = 3–6. [D] A working model of intestinal epithelial VDR in maintaining tight junctions and protecting the host from colitis. In colonic epithelial cells, VDR binds directly to the VDRE region of the Claudin-15 promoter, thus increasing the expression of Claudin-15. Different types of tight junction proteins, including Claudin-15, form tight junctions [TJs] in epithelial cells. Chronic inflammation is known to damage these TJs. However, overexpression of VDR protects against inflammation by enhancing the expression of the tight junction protein Claudin-15. VDR, vitamin D receptor; PCR, polymerase chain reaction.
To validate intestinal epithelial VDR regulation of Claudin-15, a ChIP assay was also performed using mouse colonic tissue. We examined the binding of VDR to the Claudin-15 promoter using precisely designed primers for potential binding sites. We detected the [-988 to -688] region of the Claudin-15 promoter as a site for VDR interaction in mice. To validate the specificity of the ChIP assays, we used the colonic mucosal extract from VDR-/- mice and the non-specific IgG as a negative control. It did not show any pull-down [Figure 9C]. Thus, our in vitro and in vivo data indicate that VDR transcriptionally regulates Claudin-15 as its target gene.
4. Discussion
In the current study, we have demonstrated a critical role of VDR in regulating the expression of Claudin-15 in human IBD. We found that targeted overexpression of epithelial VDR in the intestine protected mice from colitis by maintaining Claudin-15, and diminished the inflammatory response. At the molecular level, we identified directly VDRE binding sites to the Claudin-15 promoter. Therefore, Claudin-15 is the newly discovered target gene of VDR. Correspondingly, overexpression of intestinal epithelial VDR leads to increased Claudin-15 expression, thus inhibiting inflammation in colitis. Loss of barrier integrity and intestinal epithelial tight junctions are considered key factors contributing to colitis.1 Our current work adds important insights into the mechanism by which VDR regulates claudin-15 as a target gene in the colonic epithelium.
Our study has provided additional insight into the molecular mechanism that might contribute to the development of inflammation in UC. We found decreased Claudin-15 in the inflamed region of human colonic biopsies of patients with UC, as well as in the colitis animal model. Regression analysis of microarray data indicates a positive correlation between VDR and Claudin-15 in the colonic mucosa samples, differentiating UC patients from healthy donors. Another study showed reduced Claudin-15 in the metalloprotease Admats-12 knockout mice colon, following DSS treatment [GDS436633], and in the AOM DSS model [GDS 436734]. It was also evident in mouse caecum following Salmonella infection [GDS 462235], as well as in IEC cells of Mus musculus GDS3357.36 Interestingly, overexpression of VDR was able to retain Claudin-15 expression even in colitis. Increased colon length, improved body weight, and restored colonic histopathology in DSS-challenged O-VDR indicated the contribution of VDR in wielding protection against DSS-colitis. Multiple studies have suggested a protective role of VDR in human IBD and experimental colitis models. VDR deficiency leads to gut microbial dysbiosis, cytokine-induced epithelial cell apoptosis, and defective autophagy in colitis.14 Studies in patients with IBD confirm that the Vdr gene polymorphisms FokI, BsmI, ApaI, and TaqI are associated with IBD disease susceptibility.19 VDR is critical for maintaining gut epithelial tight junctions and barrier function.5 Collectively, these studies support a critical role for intestinal epithelial VDR in preventing colitis.
Claudin-2 and Claudin-15 both are integral parts of intestinal epithelial tight junction, and they function as a paracellular channel as well.2,19–21 However, Claudin-2 and Claudin-15 might be regulated very differentially, depending on the pathophysiological condition.10,37 Our study has shown the positive correlation of VDR and Claudin-15 in colitis whereas, in absence of VDR, Claudin-2 was significantly hyperregulated in the inflamed intestine.15 It was reported that Claudin-15 knockout mice grew normally and did not exhibit any diarrhoeal phenotype; however, severe malfunction in proliferation and presence of megaintestine is noted.38 In human biopsies from patients with UC, Claudin-15 was downregulated.4 This indicates that Claudin-15 may not be simply considered as ‘a leaky protein’ as Claudin-2, because Claudin-2 is positively linked with inflammatory activity in patients with IBD9 and coeliac disease.39,40 Decreased Claudin-15 expression increases the permeability of the intestinal epithelium and thus contributes to the cause of IBD.11,41 It was previously shown that patients with coeliac disease showed marked upregulation of Claudin-2 expression but not so much for Claudin-15.39,40 Interestingly, these proteins vary in their expression pattern throughout murine life as well. In mice, unlike Claudin-2, Claudin-15 is expressed at low levels at birth and then eventually rapidly increases. Additionally, Claudin-2 knockout mice expressed limited Claudin-15 expression but adequate levels for the mice to survive. However, Claudin-15 knockout leads to hypertrophy of the intestine and severe deficiencies in water and nutrient absorption. Previous studies showed the DSS-induced reduction of Claudin-15 expression and significantly diminished Claudin-15 after combined treatment with DSS and busulphan.42 Both Claudin-2 and Claudin-15 respond to inflammatory conditions, including diarrhoea, graft vs host disease [GVHD], common variable immune deficiency [CVID], and IBD. Interestingly, Claudin- 2 and 15 are regulated differently,37 as evidenced in Claudin-2 knockout mice and Claudin-2/15 double knockout mice.43 Our data in O-VDR mice further indicate that overexpressed VDR was able to maintain the level of Claudin-15 against inflammation, whereas Claudin-2 might be triggered by inflammation in colitis, as we observed in a previous study.15 Earlier we reported the complex role of Claudin 2 in Salmonella-induced colitis. Salmonella infection increases Claudin-2, thus inducing increased permeability of the intestinal epithelium, which is important for bacterial invasion. Moreover, Claudin- 2 and 15 are not the only tight junction [TJ] proteins dysregulated in intestinal diseases, and changes in barrier-strengthening and other channel-forming TJ proteins must also be considered in the overall picture of barrier modification processes. Hence our study, for the first time reporting the VDR regulation of Claudin-15, will add very important knowledge on how the host factor strengthens the epithelial tight junction, thus protecting against colitis.
Previous studies using either pharmacological means or genetic manipulation have demonstrated that a sustained increase in mucosal permeability is related to the protection of the mucosal epithelium from inflammation and increased ‘immune tolerance’,44,45 thus rendering protection against colitis. When mice are subjected to DSS challenge, their mucosal epithelium is under continuous trial to balance the need for an efficient immune system and wound healing while maintaining homeostasis of commensal microorganisms. VDR plays a multifunctional role in giving protection against inflammation and colitis, eg, shaping up the host microbiome and microbial metabolites, controlling autophagy, etc. Our previous studies have shown the important roles of VDR in preventing dysbiosis and activating autophagy in colitis.21 VDR also contributes to the healthy status of microbial metabolites in a tissue-specific manner.18 VDR in Paneth cells plays a novel role in maintaining the alertness to pathogens for host defence in the small intestine injury and inflammation.46 However, one of the major functions of VDR is regulating intestinal epithelial tight junctions by directly regulating the claudins. Studies from our laboratory have already shown how VDR regulates Claudin-2 in Salmonella infection and colitis.15 Here we enlighten another novel role of VDR in the regulation of Claudin-15. Thus, the role of intestinal VDR in regulating the barrier functions through Claudins could be one of its multiple functions in preventing human colitis, including UC and CD. To move forward, it is reasonable to consider alternative methods to enhance the barrier function through upregulating VDR in colitis. Our data have shown that vitamin D3 treatment is able to increase Claudin-15. Beneficial bacterial products [eg, butyrate] and probiotics [eg, lactic acid bacteria] could be used to increase intestinal VDR expression47–54 and maintain barrier functions.55 Future translational studies could also be investigating ‘immune tolerance’ in the protection of O-VDR from colitis.
Taken together, our study has demonstrated a novel and critical role of VDR in the regulation of Claudin-15 in the colonic epithelium. VDR regulation of Claudin-15 is important in maintaining epithelial homeostasis and barrier function, which in turn are responsible for anti-inflammation [a working model in Figure 9D]. Our findings will strengthen the knowledge of the development of IBD through tight junctions. It will open new therapeutic strategies for human IBD by increasing epithelial VDR levels.
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
Acknowledgements
The authors are particularly grateful to Dr David Zhou for his assistance in obtaining paraffin-embedded sections of human UC biopsy samples. This study was performed in accordance with approval from the University of Rochester Ethics Committee [RSRB00037178].
Funding
This work was supported by the NIDDK (National Institute of Diabetes and Digestive and Kidney Diseases) grant R01 DK105118, R01DK114126, and DOD (The Department of Defense) CDMRP (Congressionally Directed Medical Research Programs) log No BC160450P1, and the VA (Veterans Affairs) Merit Award 1 I01BX004824-01to JS (Prof. Jun Sun). The contents do not represent the views of the United States Department of Veterans Affairs or the United States Government.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
IC: acquisition, analysis, and interpretation of data; drafting of the manuscript; statistical analysis. YZ: acquisition data; help with tight junction data, animal models, and colitis data analysis. RL and JZ: help with animal models and colitis data. YX: statistical analysis, interpretation of data, and drafting of the manuscript. JS: study concept and design; analysis and interpretation of data; writing the manuscript for important intellectual content, obtaining funding, and study supervision. Conference presentation: oral presentation at Digestive Disease Week 2019, San Diego, CA, USA.
References
- 1. Lloyd-Price J, Arze C, Ananthakrishnan AN, et al. ; IBDMDB Investigators. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019;569:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mineta K, Yamamoto Y, Yamazaki Y, et al. . Predicted expansion of the claudin multigene family. FEBS Lett 2011;585:606–12. [DOI] [PubMed] [Google Scholar]
- 3. Edelblum KL, Turner JR. The tight junction in inflammatory disease: communication breakdown. Curr Opin Pharmacol 2009;9:715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Darsigny M, Babeu JP, Dupuis AA, et al. . Loss of hepatocyte-nuclear-factor-4alpha affects colonic ion transport and causes chronic inflammation resembling inflammatory bowel disease in mice. PLoS One 2009;4:e7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu W, Chen Y, Golan MA, et al. . Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J Clin Invest 2013;123:3983–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Turpin W, Lee SH, Raygoza Garay JA, et al. ; Crohn’s and Colitis Canada Genetic Environmental Microbial Project Research Consortium; CCC GEM Project recruitment site directors include Maria Abreu. Increased intestinal permeability is associated with later development of Crohn’s disease. Gastroenterology 2020;159:2092–100.e5. [DOI] [PubMed] [Google Scholar]
- 7. Su L, Shen L, Clayburgh DR, et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology 2009;136:551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Günzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev 2013;93:525–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest 2008;88:1110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tamura A, Hayashi H, Imasato M, et al. . Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterology 2011;140:913–23. [DOI] [PubMed] [Google Scholar]
- 11. Tamura A, Kitano Y, Hata M, et al. . Megaintestine in claudin-15-deficient mice. Gastroenterology 2008;134:523–34. [DOI] [PubMed] [Google Scholar]
- 12. Zhang YG, Wu S, Lu R, et al. . Tight junction CLDN2 gene is a direct target of the vitamin D receptor. Sci Rep 2015;5:10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu R, Zhang Y-G, Xia Y, Sun J. Imbalance of autophagy and apoptosis in intestinal epithelium lacking the vitamin D receptor. FASEB J 2019;33:11845‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu S, Zhang YG, Lu R, et al. . Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut 2015;64:1082–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang YG, Lu R, Xia Y, et al. . Lack of vitamin D receptor leads to hyperfunction of claudin-2 in intestinal inflammatory responses. Inflamm Bowel Dis 2019;25:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang J, Thingholm LB, Skiecevičienė J, et al. . Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet 2016;48:1396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bakke D, Sun J. Ancient nuclear receptor VDR with new functions: microbiome and inflammation. Inflamm Bowel Dis 2018;24:1149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chatterjee I, Lu R, Zhang Y, et al. . Vitamin D receptor promotes healthy microbial metabolites and microbiome. Sci Rep 2020;10:7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xue LN, Xu KQ, Zhang W, Wang Q, Wu J, Wang XY. Associations between vitamin D receptor polymorphisms and susceptibility to ulcerative colitis and Crohn’s disease: a meta-analysis. Inflamm Bowel Dis 2013;19:54–60. [DOI] [PubMed] [Google Scholar]
- 20. Garg M, Royce SG, Tikellis C, et al. . The intestinal vitamin D receptor in inflammatory bowel disease: inverse correlation with inflammation but no relationship with circulating vitamin D status. Therap Adv Gastroenterol 2019;12:1756284818822566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu S, Zhang YG, Lu R, et al. . Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut 2015;64:1082–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olsen J, Gerds TA, Seidelin JB, et al. . Diagnosis of ulcerative colitis before onset of inflammation by multivariate modeling of genome-wide gene expression data. Inflamm Bowel Dis 2009;15:1032–8. [DOI] [PubMed] [Google Scholar]
- 23. Wu S, Zhang Y-G, Sun J, et al. Chronic Salmonella infected mouse model. J Vis Exp 2010;39:1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang YG, Wu S, Xia Y, Sun J. Salmonella-infected crypt-derived intestinal organoid culture system for host–bacterial interactions. Physiol Rep 2014;2:e12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kozuka K, He Y, Koo-McCoy S, et al. . Development and characterization of a human and mouse intestinal epithelial cell monolayer platform. Stem Cell Rep 2017;9:1976–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Altay G, Larrañaga E, Tosi S, et al. . Self-organized intestinal epithelial monolayers in crypt and villus-like domains show effective barrier function. Sci Rep 2019;9:10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin Z, Zhang YG, Xia Y, Xu X, Jiao X, Sun J. Salmonella enteritidis effector AvrA stabilizes intestinal tight junctions via the JNK pathway. J Biol Chem 2016;291:26837–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laperrousaz B, Porte S, Gerbaud S, et al. . Direct transfection of clonal organoids in Matrigel microbeads: a promising approach toward organoid-based genetic screens. Nucleic Acids Res 2018;46:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jin D, Zhang YG, Wu S, et al. . Vitamin D receptor is a novel transcriptional regulator for Axin1. J Steroid Biochem Mol Biol 2017;165:430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Landy J, Ronde E, English N, et al. . Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J Gastroenterol 2016;22:3117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang YG, Lu R, Wu S, et al. . Vitamin D receptor protects against dysbiosis and tumorigenesis via the JAK/STAT pathway in intestine. Cell Mol Gastroenterol Hepatol 2020;10:729–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mori T, Miyamoto T, Yoshida H, et al. . IL-1β and TNFα-initiated IL-6-STAT3 pathway is critical in mediating inflammatory cytokines and RANKL expression in inflammatory arthritis. Int Immunol 2011;23:701–12. [DOI] [PubMed] [Google Scholar]
- 33. Moncada-Pazos A, Obaya AJ, Llamazares M, et al. . ADAMTS-12 metalloprotease is necessary for normal inflammatory response. J Biol Chem 2012;287:39554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tang A, Li N, Li X, et al. . Dynamic activation of the key pathways: linking colitis to colorectal cancer in a mouse model. Carcinogenesis 2012;33:1375–83. [DOI] [PubMed] [Google Scholar]
- 35. Bellet MM, Deriu E, Liu JZ, et al. . Circadian clock regulates the host response to Salmonella. Proc Natl Acad Sci U S A 2013;110:9897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Wit NJ, Bosch-Vermeulen H, de Groot PJ, et al. . The role of the small intestine in the development of dietary fat-induced obesity and insulin resistance in C57BL/6J mice. BMC Med Genomics 2008;1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ong MLDM, Yeruva S, Sailer A, Nilsen SP, Turner JR. Differential regulation of claudin-2 and claudin-15 expression in children and adults with malabsorptive disease. Lab Invest 2020;100:483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tamura A, Kitano Y, Hata M, et al. . Megaintestine in claudin-15-deficient mice. Gastroenterology 2008;134:523–34. [DOI] [PubMed] [Google Scholar]
- 39. Luettig J, Rosenthal R, Barmeyer C, Schulzke JD. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers 2015;3:e977176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schumann M, Günzel D, Buergel N, et al. . Cell polarity-determining proteins Par-3 and PP-1 are involved in epithelial tight junction defects in coeliac disease. Gut 2012;61:220–8. [DOI] [PubMed] [Google Scholar]
- 41. Khan N, Asif AR. Transcriptional regulators of claudins in epithelial tight junctions. Mediators Inflamm 2015;2015:219843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arimura Y, Nagaishi K, Hosokawa M. Dynamics of claudins expression in colitis and colitis-associated cancer in rat. Methods Mol Biol 2011;762:409–25. [DOI] [PubMed] [Google Scholar]
- 43. Wada M, Tamura A, Takahashi N, Tsukita S. Loss of claudins 2 and 15 from mice causes defects in paracellular Na+ flow and nutrient transport in gut and leads to death from malnutrition. Gastroenterology 2013;144:369–80. [DOI] [PubMed] [Google Scholar]
- 44. Boirivant M, Amendola A, Butera A, et al. . A transient breach in the epithelial barrier leads to regulatory T-cell generation and resistance to experimental colitis. Gastroenterology 2008;135:1612–23.e5. [DOI] [PubMed] [Google Scholar]
- 45. Laukoetter MG, Nava P, Lee WY, et al. . JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med 2007;204:3067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lu R, Zhang Y-G, Xia Y, et al. Paneth cell alertness to pathogens maintained by vitamin D receptors. Gastroenterology 2021;160:1269‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yoon SS, Sun J. Probiotics, nuclear receptor signaling, and anti-inflammatory pathways. Gastroenterol Res Pract 2011;2011:971938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu S, Yoon S, Zhang YG, et al. . Vitamin D receptor pathway is required for probiotic protection in colitis. Am J Physiol Gastrointest Liver Physiol 2015;309:G341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shang M, Sun J. Vitamin D/VDR, probiotics, and gastrointestinal diseases. Curr Med Chem 2017;24:876–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lu R, Shang M, Zhang YG, et al. . Lactic acid bacteria isolated from Korean kimchi activate the vitamin D receptor-autophagy signaling pathways. Inflamm Bowel Dis 2020;26:1199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Del Pinto R, Ferri C, Cominelli F. Vitamin D axis in inflammatory bowel diseases: role, current uses and future perspectives. Int J Mol Sci 2017;18:2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Costanzo M, Cesi V, Palone F, et al. . Krill oil, vitamin D and Lactobacillus reuteri cooperate to reduce gut inflammation. Benef Microbes 2018;9:389–99. [DOI] [PubMed] [Google Scholar]
- 53. Bakke D, Chatterjee I, Agrawal A, Dai Y, Sun J. Regulation of microbiota by vitamin D receptor: a nuclear weapon in metabolic diseases. Nucl Receptor Res 2018;5:101377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Appleyard CB, Cruz ML, Isidro AA, Arthur JC, Jobin C, De Simone C. Pretreatment with the probiotic VSL#3 delays transition from inflammation to dysplasia in a rat model of colitis-associated cancer. Am J Physiol Gastrointest Liver Physiol 2011;301:G1004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rao RK, Samak G. Protection and restitution of gut barrier by probiotics: nutritional and clinical implications. Curr Nutr Food Sci 2013;9:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.