Abstract
Baker’s yeast contains a large number of duplicated genes; some function redundantly, whereas others have more specialized roles. We used the MLH family of DNA mismatch repair (MMR) proteins as a model to better understand the steps that lead to gene specialization following a gene duplication event. We focused on two highly conserved yeast MLH proteins, Pms1 and Mlh3, with Pms1 having a major role in the repair of misincorporation events during DNA replication and Mlh3 acting to resolve recombination intermediates in meiosis to form crossovers. The baker’s yeast Mlh3 and Pms1 proteins are significantly diverged (19% overall identity), suggesting that an extensive number of evolutionary steps, some major, others involving subtle refinements, took place to diversify the MLH proteins. Using phylogenetic and molecular approaches, we provide evidence that all three domains (N-terminal ATP binding, linker, C-terminal endonuclease/MLH interaction) in the MLH protein family are critical for conferring pathway specificity. Importantly, mlh3 alleles in the ATP binding and endonuclease domains improved MMR functions in strains lacking the Pms1 protein and did not disrupt Mlh3 meiotic functions. This ability for mlh3 alleles to complement the loss of Pms1 suggests that an ancestral Pms1/Mlh3 protein was capable of performing both MMR and crossover functions. Our strategy for analyzing MLH pathway specificity provides an approach to understand how paralogs have evolved to support distinct cellular processes.
Keywords: gene duplication, gene specification, MLH proteins, mismatch repair, meiotic crossing over, Saccharomyces cerevisiae
Introduction
Gene duplications, occurring through events such as polyploidization or unequal crossing over, can create new gene families that play important roles in adaptive evolution (Ohno 1970; Hughes 1994). In baker’s yeast, there are many examples of gene duplications that result in paralogs that appear to function redundantly or have specialized roles (Wolf and Shields 1997). Various models have been proposed to explain how novel protein functions have evolved after gene duplication. Hughes (1994) proposed that an ancestral gene initially existed prior to gene duplication that was capable of performing multiple functions. Following duplication, the two genes were subjected to purifying selection, leading to specialization (Hughes 1994). In another model, Force et al. (1999) proposed that neutral degenerative mutations can occur in each duplicated gene that complement each other [Duplication-Degeneration-Complementation (DDC) model], providing an opportunity for sub-functionalization. DDC has been proposed to resolve conflicts in cases where a single gene performing multiple functions acquires mutations that optimizes one function but negatively impacts others (Hittinger and Carroll 2007). The models outlined above can be challenging to test for an ancient duplication event, because the derived paralogs have diverged significantly in their amino acid sequences. In this study, we focused on the outcome of ancient gene duplication events that diversified the functions of the baker’s yeast MLH family of mismatch repair (MMR) genes. These events predated the whole-genome duplication event that took place ∼100 million years ago (Wolfe and Shields 1997; Marcet-Houben and Gabaldón 2015). First, we describe the MLH family proteins and their roles in MMR and meiotic crossing over, and then present our approach to alter their functions.
MMR is a highly conserved mechanism that reduces the genome mutation rate through the action of MutS homolog (MSH) proteins that bind to base–base and insertion/deletion mismatches that form as the result of DNA replication errors (Supplementary Figure S1, A and B). MSH recognition of mismatches results in the recruitment of the Mlh1-Pms1/PMS2 (also described as MutLα) endonuclease, which is activated through interactions with MSH proteins and the DNA replication processivity factor PCNA to nick the newly replicated daughter strand, leading to excision and resynthesis steps that maintain the original template information (Tran et al. 1997; Erdeniz et al. 2005; Kadyrov et al. 2006; Pluciennik et al. 2010; Goellner et al. 2015; Kawasoe et al. 2016).
Genetic and biochemical analyses in eukaryotic systems have identified MSH and MLH factors that have evolved new functions (Culligan 2000; Shell et al. 2007; Pyatnitskaya et al. 2019; Furman et al. 2021). For example, in baker’s yeast, the MSH family members Msh2-Msh6 and Msh2-Msh3 act in the repair of different subsets of mismatches, and Msh4-Msh5 promotes crossover formation in meiosis (Reenan and Kolodner 1992; Kunkel and Erie 2015; Manhart and Alani 2016). For the MLH family, baker’s yeast Mlh1-Pms1 plays a major role in MMR, whereas Mlh1-Mlh2 and Mlh1-Mlh3 display minor and more specialized roles in MMR but important roles in meiotic recombination. In meiosis, Mlh1-Mlh2 acts to regulate gene conversion tract length and Mlh1-Mlh3 acts in the biased cleavage of double-Holliday junctions (dHJs) to form crossovers ( Supplementary Figure S1, C and D; Flores-Rozas and Kolodner 1998; Harfe et al. 2000; Abdullah et al. 2004; Cotton et al. 2010; Zakharyevich et al. 2012; Romanova and Crouse 2013; Campbell et al. 2014; Manhart and Alani 2016; Duroc et al. 2017).
How does Mlh1-Mlh3 function in meiosis to facilitate crossover (CO) formation? Crossing over of parental homologs during meiotic prophase facilitates their segregation during the Meiosis I division. In the absence of at least one crossover event per homolog pair, nondisjunction events occur at high frequency, leading to the formation of aneuploid gametes (Manhart and Alani 2016). In baker’s yeast, the meiotic crossover pathway is initiated by the Spo11 complex, which catalyzes 150–200 DNA double-strand breaks (DSBs) genome wide (Keeney et al. 1997; Robine et al. 2007). These DSBs are resected in a 5ʹ to 3ʹ direction to form 3ʹ single-stranded tails that invade the homologous template to create a D-loop intermediate. In the major crossover pathway, the D-loop is further stabilized by ZMM proteins such as Msh4-Msh5 and Zip3 to enable DNA repair synthesis and branch migration, ultimately forming a dHJ intermediate that is asymmetrically cleaved in an Mlh1-Mlh3 and Exo1-dependent step to yield primarily crossover products (Zakharyevich et al. 2012; Manhart and Alani 2016). Recent work suggests that Mlh1-Mlh3 endonuclease functions are directed toward newly replicated DNA formed during the creation of the dHJ intermediate in a mechanism analogous to that proposed for the Mlh1-Pms1 nuclease during MMR (Moens et al. 2002; Abdullah et al. 2004; Kolas et al. 2005; Manhart et al. 2017; Marsolier-Kergoat et al. 2018). This is thought to be accomplished by Mlh1-Mlh3 being recruited to recombination intermediates through interactions with specific meiotic factors and the DNA polymerase processivity factor PCNA (Moens et al. 2002; Kolas et al. 2005; Cannavo et al. 2020; Kulkarni et al. 2020; Sanchez et al. 2020).
We asked if regions of Pms1 and Mlh3 could be identified that confer their functional specificities and if this information could be used to alter Mlh3 to make it a more robust MMR factor. Our work is based on a phylogenetic analysis indicating that Mlh1 homologs initially diverged from an MLH ancestor, followed by a splitting into Pms1 and Mlh3 sister groups. As described below, these efforts provided evidence that all three functional domains of the MLH proteins (N-terminal ATP binding, linker, C-terminal endonuclease/MLH interaction) have evolved for gene specialization. Importantly, a small number of mutations were created in MLH3 that expanded its MMR specificity without disrupting its role in meiotic crossing over. This combination of approaches provides a strategy to understand how organisms have evolved paralog complexes with distinct cellular functions.
Materials and methods
Media
Saccharomyces cerevisiae SK1 and S288c strains were grown at 30°C in either yeast extract-peptone-dextrose media or minimal selective media (SC; Rose et al. 1990). When required, geneticin (Invitrogen, San Diego) was added at 200 µg/ml (Goldstein and McCusker 1999), and sporulation plates were prepared as described (Detloff et al. 1991).
Plasmids
Plasmid and strain background derivation for the relevant MMR genes are listed in Supplementary Table S1. Plasmid constructs built by Gibson cloning were resub-cloned into the backbones of expression vectors. The DNA sequence of the open reading frame (including 300 bp upstream and 150 bp downstream) of constructs was confirmed by Sanger DNA sequencing (Cornell BioResource Center). Oligonucleotides and sequences of plasmids are available upon request.
Mlh3/Pms1 chimera integration vectors containing MLH3SK1 and PMS1SK1 sequences were derived from pEAI254, a 7.8 kb MLH3SK1::KanMX integrating vector (Al-Sweel et al. 2017), and pEAA238, a 9.1 kb PMS1SK1 ARS-CEN HIS3 vector, respectively (Supplementary Figure S2). The Block mutation vectors were derived from pEAM168, a 10.7 kb MLH3SK1::KanMX 2µ vector, fragments of which were isolated to integrate mlh3 alleles (Supplementary Figure S2; Supplementary Table S1). The MLH3 and PMS1 boundaries in the chimera plasmids were chosen based on structural and homology model analyses of MLH proteins that yielded three distinct regions; an ATP-binding domain, a linker region, and C-terminal endonuclease/MLH interaction domain (Supplementary Figure S3; Arana et al. 2010; Gueneau et al. 2013; Mlh3 homology model in Al-Sweel et al. 2017). The chimera plasmids were constructed by linking different domains of PCR-amplified MLH3 and PMS1 DNA sequences using NEB HiFi DNA Assembly cloning (New England Biolabs, Ipswich, MA, USA). For Mlh3, these domains were: ATP binding, aa 1 to 375; linker, 376 to 488; C-terminal endonuclease, 489 to 715. For Pms1, the domains were: ATP binding, aa 1 to 361; linker, 362 to 638; C-terminal endonuclease, 639 to 877 (SK1). The chimera constructs were assigned the following abbreviations: PMM [Pms1(1-361)-Mlh3(376-488)-Mlh3(489-715)]; MMP [Mlh3(1-375)-Mlh3(376-488)-Pms1 (639-877)]; PPM [Pms1(1-361)-Pms1(362-638)-Mlh3(489-715)]; MPP [Mlh3(1-375)-Pms1(362-638)-Pms1(639-877)]; PMP [Pms1(1-361)-Mlh3(376-488)-Pms1(639-877)]; MPM [Mlh3(1-375)-Pms1(362-638)-Mlh3(489-715)].
mlh3 Block mutations were constructed using NEB HiFi DNA Assembly cloning and/or Q5 site-directed mutagenesis kits (New England Biolabs, Ipswich, MA, USA). The integration plasmids were digested with BamHI and SalI prior to transformation into EAY3255 using methods described by Gietz et al. (1995). At least three independent transformants for each genotype were made and genotyped by PCR (presence of specific alleles also confirmed by DNA sequencing) using primers that map outside of the restriction sites used for integration.
MLH3/PMS1 chimera and Block mutation alleles (SK1 background) were also expressed from the native MLH3 promoter on 2µ LEU2 plasmids or on ARS-CEN HIS3 plasmids. pEAI254 (MLH3SK1::KanMX), pEAM168 (MLH3SK1::KanMX, 2µ), pEAM65 (MLH3SK1::LEU2, 2µ), and pEAA636 (MLH3, HIS3, ARS-CEN) were the parental plasmids for these constructs. These plasmids were tested for complementation of MMR defects in the S288c strain EAY3097 (relevant genotype pms1Δ, lys2::insE-A14).
Strains
The SK1 strains EAY3252, EAY3255, and EAY3486 and indicated derivatives were used to measure Mlh3-dependent meiotic crossing over and MMR functions (Supplementary Table S2). EAY3255 and derivatives contain the lys2::insE-A14 allele to measure mutation rate (Tran et al. 1997). EAY3252/EAY3486 (wild type), EAY3255/EAY3486 (mlh3Δ) and EAY3255::mlh3 alleles/EAY3486 diploids contain spore-autonomous fluorescence markers to measure meiotic crossing over in the CEN8-THR1 interval (Supplementary Figure S1D; Thacker et al. 2011). The S288c strain EAY3097 (relevant genotype pms1Δ, lys2::insE-A14) was used to determine if mlh3 alleles (SK1 background) expressed on 2µ and ARS-CEN vectors could restore MMR functions in pms1Δ strains (Table 2). The S288c strain EAY4595 (relevant genotype mlh3Δ) was used to show that the SK1 derived MLH3 gene fully complemented the MMR defects seen in the strain.
Table 2.
mlh3-ATP binding and mlh3-PIP mutations modestly rescue pms1 null MMR defects
| pms1Δ strain with indicated plasmids | Rate × 10−7 (n) | 95% CI × 10−7 | Relative to wild type |
|---|---|---|---|
| PMS1, ARS-CEN | 2.99 (30) | 2.11–3.85 | 1 |
| PMS1, 2µ | 22.4 (18) | 15.1–63.5 | 7.50 |
| ARS-CEN (LEU2) | 20,300 (29) | 12,900–28,700 | 6,800 |
| 2µ | 18,900 (18) | 15,900–21,300 | 6,320 |
| mlh3 Block mutations, ARS-CEN | |||
| MLH3 | 14,700 (15) | 8,620–19,700 | 4,910 |
| Block 4 (mlh3-PIP1) | 14,400 (15) | 6,470–18,600 | 4,830 |
| Block 4 (mlh3-PIP2) | 11,300 (15) | 7,890–21,200 | 3,790 |
| mlh3 Block mutations, 2µ | |||
| Block 1, ATP binding | |||
| mlh3-K17T, A20Q, S24D, R30K, Q34D | 9,720 (18) | 6,360–11,300 | 3,250* |
| Block 2, Mlh1 interaction | |||
| mlh3-Y493M, N497G, V499F, D500N, K502G | 15,400 (18) | 10,700–23,700 | 5,160 |
| mlh3-D500N | 10,900 (18) | 9,540–16,100 | 3,630 |
| mlh3-K502G | 16,500 (18) | 10,200–19,100 | 5,530 |
| Block 3, Endonuclease motif | |||
| mlh3-R530K | 20,500 (18) | 16,900–28,600 | 6,840 |
| mlh3-R532N | 15,700 (18) | 10,600–21,600 | 5,240 |
| mlh3-R530K, R532N | 13,300 (18) | 10,100–17,200 | 4,440 |
| Block 4, PCNA interaction motif | |||
| MLH3 (GTFVAR) | 13,200 (28) | 8,280–20,600 | 4,430 |
| mlh3-PIP1 (QKLIIP) | 13,700 (25) | 7,940–17,200 | 4,590 |
| mlh3-PIP2 (QTFIAP) | 6,300 (44) | 4,120–7,470 | 2,110* |
| mlh3-PIP3 (QTLIAP) | 8,520 (18) | 6,950–17,600 | 2,850 |
| mlh3-PIP4 (GTFIAP) | 13,800 (18) | 7,390–22,400 | 4,610 |
| mlh3-PIP5 (QTFIAR) | 6,890 (18) | 4,250–9,890 | 2,300* |
| mlh3-PIP6 (QTFVAP) | 15,700 (18) | 13,100–25,800 | 5,240 |
| mlh3-PIP7 (GTFVAP) | 15,500 (18) | 10,500–22,500 | 5,190 |
| mlh3-PIP8 (GTFIAR) | 19,500 (18) | 15,100–25,900 | 6,510 |
| mlh3-PIP9 (QTFVAR) | 8,520 (18) | 4,890–10,800 | 3,060* |
| Block 5, Helix 2 | |||
| mlh3- V660K, N666A, F676I, D678K | 14,900 (18) | 10,700–19,700 | 4,970 |
| mlh3-D678K | 14,700 (18) | 11,900–22,200 | 4,920 |
| Block 6, Helix 1 | |||
| mlh3-C695L, F699W, A702P, S707T, V709R, P710H | 14,100 (18) | 12,100–21,100 | 4,710 |
| Double mutants | |||
| Block 1, Block 4 (PIP2) | 5,480 (18) | 4,340-6,710 | 1,830* |
| Block 2 (D500N), Block 4 (PIP2) | 12,200 (18) | 6,340–15,100 | 4,078 |
| Block 2 (K502G), Block 4 (PIP2) | 11,900 (18) | 6,830–19,100 | 3,970 |
| Block 3 (R530K), Block 4 (PIP2) | 7,350 (18) | 2,490–12,700 | 2,460* |
| Block 3 (R532N), Block 4 (PIP2) | 7,110 (18) | 3,240–9,650 | 2,380* |
| Block 3 (R530K, R532N), Block 4 (PIP2) | 17,600 (18) | 13,400–19,800 | 5,880 |
| Block 4 (PIP2), Block 5 (D678K) | 11,000 (18) | 5,750–12,900 | 3,690* |
| Block 4 (PIP2), Block 5 (Helix 2) | 18,700 (18) | 12,200–30,900 | 6,240 |
| Block 4 (PIP2), Block 6 (Helix 1) | 20,600 (18) | 14,400–27,900 | 6,904 |
| Block 5 (Helix 2), Block 6 (Helix 1) | 20,200 (18) | 17,500–38,800 | 6,740 |
| MLH3-PMS1 chimeras, 2µ | |||
| MMM | 13,200 (28) | 8,280–20,600 | 4,430 |
| PPP | 42.9 (18) | 24.1–91.4 | 14.4 |
| PMM | 19,600 (18) | 8,530–31,200 | 6,550 |
| MMP | 14,600 (18) | 11,600–21,100 | 4,870 |
| PPM | 16,100 (18) | 12,900–18,500 | 5,380 |
| MPP | 14,800 (18) | 11,800–18,300 | 4,960 |
| PMP | 15,300 (18) | 12,800–21,300 | 5,130 |
| MPM | 20,400 (18) | 18,100–35,100 | 6,830 |
EAY3097 (pms1Δ, S288c background) was transformed with pJH481 (PMS1S288c, ARS-CEN), pEAM50 (PMS1S288c, 2µ), and pRS415 (dummy vector; Supplementary Table S1) to analyze PMS1, PMS1-2µ, and pms1Δ genotypes, respectively. mlh3 substitution alleles (Blocks 1–6, Figure 2; Supplementary Table S1) and MLH3-PMS1 chimera constructs (Supplementary Table S1) were also transformed into EAY3097. mlh3 alleles and chimeras were expressed from an MLH3 promoter in 2µ and ARS-CEN vectors as indicated. All strains were analyzed for mutation rate using the lys2-A14 reversion assay as described in the Materials and Methods with the 95% confidence interval (CI) presented. n represents the number of independent measurements obtained from at least two transformants. *Indicates complementation of pms1Δ as measured by nonoverlap in 95% CI.
lys2-A14 reversion assay
The haploid strains described in Tables 1 and 2 and Supplementary Table S2 were analyzed for MMR functions using the lys2-A14 reversion assay (Supplementary Figure S1B; Tran et al. 1997). ARS-CEN and 2 μ vectors were maintained by growing strains in minimal leucine dropout media. Rates of lys2::insE-A14 reversion were calculated as μ = f/ln(N·μ) where f is the reversion frequency and N is the total number of revertant in the culture (Tran et al. 1997). For each strain, 15–44 independent cultures, obtained from two to four independent transformants, were assayed on at least two different days to prevent batch effects, and 95% confidence intervals were determined as described by Dixon and Massey (1969). The Mann–Whitney U test was used to calculate median reversion rates (Drake 1991).
Table 1.
Functional analysis of MLH3/PMS1 chimera and mlh3 alleles in MMR and meiotic crossing over (CO)
| MMR |
Meiotic CO |
|||||
|---|---|---|---|---|---|---|
| Genotype | Rate × 10−6 (n) | 95% CI. × 10−6 | Relative to WT | % tetratype (n) | Phenotype |
|
| MMR | CO | |||||
| MLH3 | 1.03 (42) | 0.81–1.39 | 1 | 37.1 (1023) | + | + |
| mlh3Δ | 6.24 (39) | 4.53–8.51 | 6.05 | 18.3 (1239) | − | − |
| MLH3-PMS1 chimeras | ||||||
| PPP (PMS1) | 8.29 (15) | 2.85–22.2 | 8.1 | 17.8 (549) | − | − |
| PMM | 15.6 (15) | 3.29–29.0 | 15.1 | 19.2 (530) | − | − |
| MMP | 19.1 (15) | 12.9–25.4 | 18.6* | 21.4 (524) | − − | − |
| PPM | 15.9 (15) | 11.8–128 | 15.4* | 19.8 (824) | − − | − |
| MPP | 15.4 (15) | 12.4–32.3 | 14.9* | 22.3 (837) | − − | − |
| PMP | 17.2 (15) | 13.1–18.2 | 16.7* | 20.7 (1,287) | − − | − |
| MPM | 8.75 (15) | 5.11–11.4 | 8.49 | 21.4 (1,196) | − | − |
| mlh3 Block mutations | ||||||
| Block 1, ATP binding | ||||||
| mlh3-K17T, A20Q, S24D, R30K, Q34D | 2.26 (15) | 1.18–3.78 | 2.20 | 35.6 (513) | +/− | + |
| Block 2, Mlh1 interaction | ||||||
| mlh3-Y493M, N497G, V499F, D500N, K502G | 3.71 (15) | 2.04–4.98 | 3.60 | 29.1 (769) | +/− | +/− |
| mlh3-D500N | 1.73 (15) | 1.33–6.98 | 1.68 | 33.1 (801) | +/− | + |
| mlh3-K502G | 2.84 (15) | 2.13–4.76 | 2.76 | 19.1 (761) | +/− | − |
| Block 3, Endonuclease motif | ||||||
| mlh3-R530K | 2.38 (15) | 1.61–3.28 | 2.31 | 25.3 (771) | +/− | +/− |
| mlh3-R532N | 1.25 (15) | 0.91–2.72 | 1.21 | 29.5 (774) | + | +/− |
| mlh3-R530K, R532N | 4.49 (15) | 3.64–7.24 | 4.36 | 20.4 (509) | − | − |
| Block 4, PCNA interaction motif | ||||||
| mlh3-PIP1 | 3.49 (15) | 1.96–4.35 | 3.39 | 30.9 (742) | − | +/− |
| mlh3-PIP2 | 0.567 (15) | 0.41–0.75 | 0.55 | 37.1 (792) | + | + |
| Block 5, Helix 2 | ||||||
| mlh3-V660K, N666A, F676I, D678K | 4.44 (15) | 2.45–12.8 | 4.32 | 18.2 (760) | − | − |
| mlh3-D678K | 0.831 (15) | 0.57–1.18 | 0.81 | 32.1 (772) | + | +/− |
| Block 6, Helix1 | ||||||
| mlh3-C695L, F699W, A702P, | 3.38 (15) | 2.37–4.76 | 3.28 | 19.5 (527) | +/− | − |
| S707T, V709R, P710H | ||||||
| Mutant combinations | ||||||
| Block 1 (ATP binding), Block 4 (PIP2) | 2.56 (15) | 1.95–4.05 | 2.48 | 31.6 (509) | +/− | +/− |
| Block 5 (Helix 2), Block 6 (Helix 1) | 4.11 (15) | 2.40–5.28 | 3.99 | 17.9 (514) | +/− | − |
| S288C background | ||||||
| wild type + empty vector | 0.28 (15) | 0.19–0.47 | 1.00 | + | ||
| mlh3Δ + empty vector | 1.61 (15) | 1.37–2.08 | 5.90 | − | ||
| mlh3Δ + pMLH3SK1 ARS-CEN | 0.27 (15) | 0.18–0.39 | 0.98 | + | ||
| mlh3Δ + pMLH3SK1-2µ | 223 (15) | 209–291 | 81.8* | − − | ||
The indicated mlh3 Block alleles and chimeras (Supplementary Table S1; Materials and Methods) were integrated into the MLH3 locus in the SK1 strain background and tested for DNA MMR functions using the lys2-A14 reversion assay with 95% CI (confidence interval) presented, and for meiotic crossover functions using a spore-autonomous assay that measures genetic map distances in the CEN8-THR1 interval on Chromosome VIII (Materials and Methods; Supplementary Table S2). For the S288c background experiments, EAY1269 (wild type, S288c background) was transformed with pRS415 (empty vector) and EAY4595 (mlh3Δ) was transformed with pRS415 (empty vector), MLH3SK1-ARS-CEN (pEAA566), and MLH3SK1-2 micron (pEAM65; Supplementary Table S1). n represents the number of independent measurements from at least two transformants. WT, wild type. +, indistinguishable from MLH3 as measured by 95% CI or Chi-Squared (P < 0.0001 for % tetratype). −, indistinguishable from mlh3Δ as measured by 95% CI or Chi-Squared (P < 0.0001). +/− distinguishable from both MLH3 and mlh3Δ as measured by 95% CI or Chi-Squared (P < 0.05). *Mutation rate higher than mlh3Δ; illustrated as a − − phenotype. Note that while expression of MLH3 on an ARS-CEN vector fully complements the mutator phenotype seen in an mlh3Δ strain, expression of MLH3 on a 2µ high copy vector confers a mild mutator phenotype, most likely by sequestering Mlh1 from interacting with Pms1 (Nishant et al. 2008).
Spore-autonomous fluorescence assay to measure percent tetratype
Diploids in the EAY3252/EAY3486 background (Supplementary Table S2) were used for analysis of meiotic crossing over phenotypes. These diploids contain a spore-autonomous fluorescent protein marker (CFP) linked to THR1 of Chromosome VIII and an RFP marker linked to CEN8 in the second copy (Supplementary Figure S1D; Thacker et al. 2011). Diploids were selected by mating parental and derived EAY3252 and EAY3486 strains on media lacking tryptophan and leucine and maintained as stable strains. Fluorescence microscopy was used to quantify parental ditypes and tetratypes resulting from single crossover events. Sporulation plates were prepared as described by Detloff et al. (1991) and incubations were performed at 30°C. Spores were treated with 0.5% NP40 and sonicated for 5–10 s before analysis using a Zeiss Axioimager.M2. 250–1000 tetrads for each mlh3 allele were counted to determine % tetratype [# tetratypes/(tetratypes + parental ditypes)]. Two to three independent transformants were measured per allele on at least two different days to prevent batch effects. In this assay, wild-type SK1 S. cerevisiae strains gave single crossover events at 37.1% frequency, whereas mlh3 null strains gave single crossover events at 18.3% frequency (Table 1). A Pearson’s Chi-Squared contingency test (http://vassarstats.net/) was used to test statistical significance to classify each allele as exhibiting a wild type, intermediate, or null phenotype. We applied a Benjamini–Hochberg correction at a 5% false discovery rate to minimize α inflation due to multiple comparisons (30 comparisons, with a P < 0.0183 cutoff for significance; Supplementary Table S3).
Evolutionary analysis of fungal Mlh1, Mlh2, Mlh3, and Pms1 proteins
The inferred amino acid sequences of Mlh1, Mlh3, Pms1, and Pms2 were located by BLAST against the gene annotations of Homo sapiens, Coprinopsis cinerea, Arabidopsis thaliana, S. cerevisiae, and Dictyostelium discoideum (Figure 1B; Supplementary Table S4). Escherichia coli MutS and Bacillus subtilis MutL were used to potentially root the tree. To decipher relationships between MutL homologs, amino acid sequences from the entire open reading frames of the Mlh1, Mlh2, Pms1, and Mlh3 proteins were aligned in SeaView (Version 4, Gouy et al. 2010) using the MUSCLE program (Version 3.8.31, Edgar 2004). Since these sequences were very divergent, the alignment was aggressively trimmed by eye to only 216 columns to maintain the most confidently aligned and conserved regions. Phylogenetic analyses were performed with PhyML (Guindon and Gascuel 2003) in SeaView using model-provided amino acid equilibrium frequencies, optimized across site rate variations, and bootstrapping with 100 replicates.
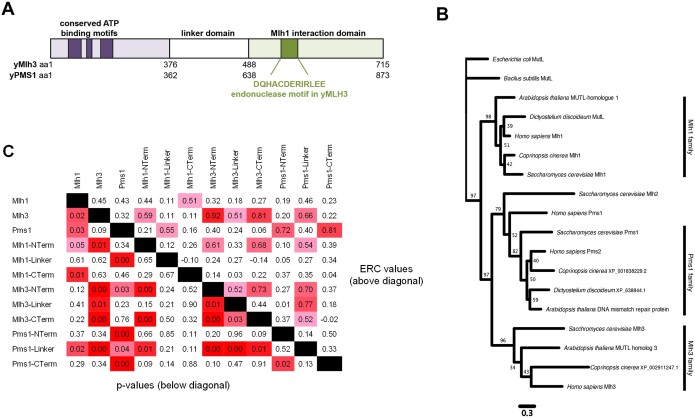
Figure 1.
Phylogenetic and ERC analysis of MLH proteins. (A) Cartoon depictions of the Mlh1-Mlh3 and Mlh1-Pms1 complexes, with the N-terminal ATP binding and C-terminal endonuclease/Mlh1 interaction domains separated by intrinsically disordered linker domains. The amino acid locations of the domains in yeast Mlh3 and Pms1 are shown. (B) Phylogenetic analysis of divergent eukaryotes indicates that MLH gene duplications occurred in early eukaryote history before the divergence of the lineages leading to plants, fungi, animals, and amoebozoa. The scale bar indicates the number of changes per amino acid site, and bootstrap support values out of 100 are found near each node. Escherichia coli MutS and B. subtilis MutL were used to root the tree. The branching pattern supports the earliest divergence of the Mlh1 family followed by splits to form the Mlh2, Mlh3, and Pms1 families. Uniprot, NCBI, and RefSeq identifications for the genes used to make the tree can be found in Supplementary Table S4. (C) ERC is elevated between domains of MLH proteins. This pairwise matrix shows all comparisons between the full-length Mlh1, Mlh3, and Pms1 proteins and their ATP binding (abbreviated as Nterm), linker, and endonuclease/MLH interaction (abbreviated as Cterm) domains defined in (A). The domain boundaries of the 18 fungal species were obtained from phylogenetic alignments with the S. cerevisiae proteins. ERC values are above the diagonal and empirical P-values are below. The colors of ERC cells range from pink at values of 0.5 to red at 1.0. P-value cells are pink at 0.05 and become red as they approach zero.
The inferred amino acid sequences of Mlh1, Mlh3, and Pms1 were also taken from the complete genome sequences of 34 Ascomycetes fungal species (GenBank, https://www.ncbi.nlm.nih.gov/genbank/; Supplementary Figure S4A). Escherichia coli MutS and B. subtilis MutL were used to potentially root the tree. Twenty-nine Mlh3, 34 Pms1, 18 Mlh2, and 33 Mlh1 proteins were analyzed in the tree analysis. A gene missing from a particular species does not necessarily indicate gene loss in that species but could reflect incomplete genome sequencing or other bioinformatic difficulty in locating the orthologous sequence. To decipher relationships between MutL homologs, amino acid sequences from the entire open reading frames of the Mlh1, Mlh2, Pms1, and Mlh3 proteins were aligned in SeaView (Gouy et al. 2010) using the Clustal Omega program (Edgar 2004). Phylogenetic analyses were performed with PhyML (Guindon and Gascuel 2003) in SeaView using model-provided amino acid equilibrium frequencies, optimized across site rate variations, and bootstrapping with 100 replicates. A second phylogenetic analysis was performed on a subset of more conserved alignment columns as selected by the Gblocks program (Supplementary Figure S4B; Castresana 2000). Specifically, GBlocks was used to select more conserved blocks of the alignment by excluding columns that include or flank gap characters and that have high divergence as determined by the threshold in GBlocks. This reduced the alignment from 1509 to 212 highly conserved and more confidently aligned positions. A phylogenetic tree was then inferred using PhyML under the same parameters and model as before. Phylogenetic tree images were created and annotated using interactive Tree of Life v.4 online tool (Letunic and Bork 2019).
Evolutionary rate covariation analysis
The 18 species included for the evolutionary rate covariation (ERC) analysis were: S. cerevisiae, Saccharomyces paradoxus, Saccharomyces mikatae, Saccharomyces bayanus, Naumovozyma castellii, Candida glabrata, Vanderwaltozyma polysporus, Lachancea kluyveri, Lachancea thermotolerans, Lachancea waltii, Kluyveromyces lactis, Eremothecium gossypii, C. tropicalis, C. albicans, C. dubliniensis, Candida lusitaniae, C. guilliermondii, and Debaryomyces hansenii. These 18 Ascomycetes fungal species were chosen for ERC to avoid very long branches, which can confound the correlations by producing outlier values. Multiple species alignments for Mlh1, Mlh3, and Pms1 were subdivided into N-terminal, Linker, and C-terminal domains so that they could be analyzed separately (Figure 1A). ERC values between a given pair of proteins or domains were calculated as initially described in Clark et al. (2012, 2013) with modifications made to improve the relative rate normalization (Figure 1C; Partha et al. 2019). Briefly, the branch lengths for each domain or protein tree were first normalized into relative evolutionary rates (RERs) using the RERconverge package (Kowalczyk et al. 2019). Normalization vectors were estimated from the branch lengths of 4458 proteins plus the three MLH domains in Mlh1, Pms1, and Mlh3 (9 in total). The resulting RERs were used to calculate the ERC values between all pairwise comparisons of the three domains in Mlh1, Mlh3, and Pms1. ERC values were calculated as the Pearson correlation coefficients between each pair of domains with extreme values controlled by limiting the two most extreme positive and negative branch RERs to the third most extreme value (i.e., Winsorization of the two most extreme outliers).
Using multi-Harmony to identify sites for mutagenesis in MLH3
To identify specificity determining residues in Mlh3 and Pms1, alignments of Pms1 and Mlh3 fungal species (34 for Pms1; 29 for Mlh3) were used for multi-Harmony analysis (Figure 2; Supplementary Figure S5, A–D; Brandt et al. 2010). Using multi-Harmony, amino acid positions significantly different between the two groups of sequences were identified. An identity cutoff of a combination of a score for multi-relief greater than 0.8 and a score less than 0.5 for sequence harmony was used to reduce the number of interesting amino acids resulting in a set of group-specific positions. Once identified, the amino acids were mapped onto a homology model of Mlh3 from Al-Sweel et al. (2017) using PyMOL to verify and group amino acids qualitatively in close physical proximity. This analysis led to the identification of Blocks 1, 2, 5, and 6 (Figure 2; Supplementary Figure S5, A–D). Blocks 3 and 4 were identified for analysis based on the identification of endonuclease and PCNA-binding motifs found in B. subtilis MutL and a subset of MLH homologs (Pillon et al. 2011; Supplementary Figure S3D).
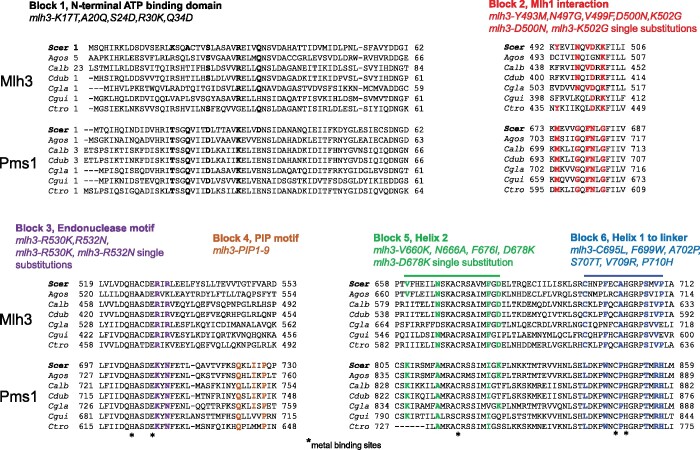
Figure 2.
Mutations made in MLH3 to revert back to conserved PMS1 sequences. Six blocks of mutations were made in Mlh3; Block 1-ATP binding, Block 2-Mlh1 interaction, and Blocks 3 to 6-Endonuclease/PCNA interaction. The multiple amino acid substitutions are shown for each block as well as single substitutions that were made in each region. For Blocks 1, 2, 5, and 6, Multi-Relief and Sequence-Harmony algorithms were used to identify functionally specific residues in Pms1 (Materials and Methods; adapted from Furman et al. 2021). Briefly, Mlh3 and Pms1 amino acid sequences from 34 different fungal species were aligned and presented in multi-Harmony. Shown are alignments of the regions showing seven fungal species (S. cerevisiae-Scer, Ashbya gossypii-Agos, Candida albicans-Calb, Candida dubliniensis-Cdub, Candida glabrata-Cgla, Candida guilliermondii-Cgui, Candida tropicalis-Ctro). The more complete lists of species alignments are shown in Supplementary Figure S4 legend. The N and C-terminal domains of Mlh3 were mapped onto the 3D structure of Mlh1-Pms1, and four amino acid clusters were identified for substitution analysis (Manning et al. 2008; Brandt et al. 2010). Block 3 spans the endonuclease motif found in Pms1 and Mlh3 (Supplementary Figure S3D). Block 4 contains the QXLXXP motif important for interactions with PCNA (PIP), which is highly conserved in the Pms1 sequences (>94% identity; Genschel et al. 2017) but is absent in Mlh3 sequences. Nine PIP mutations were made as shown in Supplementary Figure S3D and Tables 1 and 2. The asterisks indicate highly conserved metal-binding residues (H703, E707, C817, C848, and H850) in yeast Pms1, which form the endonuclease active site (Gueneau et al. 2013).
Data availability
Strains and plasmids are available upon request. Supporting information contains all detailed descriptions of Supplemental files. All experiments presented (lys2-A14 reversion, CO assays) were repeated on at least two separate days. The Supplementary figures and tables can be found in the GSA Figshare portal: https://doi.org/10.25387/g3.14367533.
Results
Phylogenetic analysis supports MLH gene duplication being an ancient event
MLH family proteins contain three structurally conserved regions; an N-terminal ATP-binding domain that facilitates conformational changes, an intrinsically disordered linker arm, and a C-terminal domain containing a region required for dimerization with other MLH proteins. Many MLH proteins also contain a functional endonuclease domain within the C-terminal region (Figure 1A; Supplementary Figure S3D; Ban and Yang 1998; Guarné et al. 2004; Arana et al. 2010; Gueneau et al. 2013). While showing an overall organizational similarity, the baker’s yeast Mlh1, Mlh2, Mlh3, and Pms1 proteins display significant amino acid divergence. For example, Mlh3 (715 amino acids in length) and Pms1 (873 amino acids) display limited (19%) amino acid identity over a 1010 amino acid gapped alignment (Supplementary Figure S6), with the greatest divergence seen in the linker domain (7.9% identity, with many gaps).
To better understand how the Mlh3 and Pms1 proteins diverged, we performed two phylogenetic tree analyses of homologs of the MLH paralogs. The first was performed with a diverse set of eukaryotic lineages (note that human Pms2 and Pms1 are homologs of fungal Pms1 and Mlh2, respectively), with the goal of estimating when MLH family gene duplication events occurred. As shown in Figure 1B, paralog branching patterns of highly diverged eukaryotes suggest first a split of the ancestral MLH gene to form the Mlh1 family and the ancestor of Mlh3/Pms1, followed by splits to form the Mlh2, Mlh3, and Pms1 families. These observations indicate that MLH gene duplications occurred early in eukaryote history, before the divergence of lineages leading to Plantae (plants), Opisthokonts (fungi/metazoa), and Amoebozoa. Note that although the MLH families were well resolved, the branching order of the taxonomic lineages was not well resolved within each family because of insufficient alignable sequence in the paralogs.
To better define the steps leading to the divergence of the MLH homologs, we performed the second phylogenetic tree analysis on Mlh1, Mlh2, Mlh3, and Pms1 homologs from 34 Ascomycete species (Supplementary Figure S4A; Materials and Methods; Clark et al. 2013). This analysis includes species both before and after the whole-genome duplication event (∼100 million years ago), which took place after the split of the yeast species into the Kluyveromyces and Saccharomyces clades. The identification of Mlh1, Mlh2, Mlh3, and Pms1 homologs in both clades confirms that Mlh1, Mlh2, Mlh3, and Pms1 predated the whole-genome duplication event (Wolfe and Shields 1997; Campbell et al. 2014). Similar to the analysis presented in Figure 1B, the Ascomycota analysis supports the divergence of Mlh1 (outgroup) first, followed by another split into Pms1, Mlh2, and Mlh3 as sister groups. While the trees are consistent with Mlh3, Mlh2, and Pms1 sharing a more recent common ancestor and function than with Mlh1, it is difficult to definitely conclude directionality because the events are ancient. To further scrutinize the inferred divergence order, we analyzed a select subset of more conserved and confidently aligned amino acid sites for Mlh1, Mlh3, and Pms1. Specifically, we used the GBlocks program to select more conserved blocks of the alignment lacking insertion and deletion events. The phylogenetic tree inferred with those 212 sites also showed Mlh1 diverging first, followed by Mlh3 and Pms1, and those relationships were supported by high approximate likelihood ratio test branch support values (Supplementary Figure S4B).
The data in Supplementary Figure S4 also indicate an acceleration of changes in the Mlh2 and Mlh3 clades; the continued faster rate likely reflects relaxed constraint, though neofunctionalization for Mlh2 (regulating gene conversion tracts) and Mlh3 (resolving recombination intermediates into crossovers) to act in meiotic recombination is an alternative explanation. It is difficult to make a definitive conclusion on this point because the events are ancient. Estimating selective pressure during that ancient divergence is challenging (e.g., measuring dN/dS, the ratio of the number of nonsynonymous to synonymous substitutions per site), because outside of the genus Saccharomyces the synonymous sites are saturated. The data also suggest that Mlh1 remains under stronger constraint relative to Mlh3, which is curious because Mlh1 and Mlh3 are hypothesized to be coevolving as measured by ERC analysis (Clark et al. 2012). It is important to note that proteins with different average evolutionary rates can still have correlated changes in their rates over time, and that Mlh3, Pms1, and Mlh2, have all maintained their interaction with Mlh1, indicating that such interactions are likely to impose evolutionary constraints.
ERC signals are seen between sets of domains for three MLH proteins
The phylogenetic analysis above, coupled with the previously defined functions of Mlh1-Pms1 and Mlh1-Mlh3 in MMR and meiotic crossing over, respectively, encouraged us to focus on what domains in Pms1 and Mlh3 were critical for conferring pathway specificity. ERC, which identifies protein pairs with correlated changes in evolutionary rate, has been used to make functional inferences (Clark et al. 2012, 2013). In general, ERC values between unrelated proteins do not show correlated rate changes, whereas protein pairs in shared pathways, complexes, and functions show positively correlated rates (Clark et al. 2012). ERC is calculated as the correlation coefficient between the phylogenetic branch-specific rates of one protein vs another. A value of one indicates perfect rate covariation and a value near zero represents little or no covariation. Previous studies had shown an elevated ERC for Mlh1 and Mlh3 with each other and with meiotic crossover proteins (Clark et al. 2013), but ERC values for Mlh1 and Mlh3 with the MMR-specific components Msh2 and Msh6 were not elevated, suggesting that the evolutionary forces relating to meiotic crossing over had a greater effect on Mlh1 and Mlh3 than MMR.
ERC has been used previously to compare rates between full-length protein sequences. In this study, we performed ERC analysis on the whole-length proteins as well as the ATP binding (N-terminal), linker, and endonuclease/MLH interaction (C-terminal) domains of Mlh1, Mlh3, and Pms1 using 18 yeast species (including S. cerevisiae; Materials and Methods; Clark et al. 2013). The purpose of this analysis was to determine if any one specific domain of the MLH proteins was showing ERC with another, or if multiple domains displayed such covariation as compared to the whole protein. This analysis involved the 12 × 12 matrix presented in Figure 1C. It is important to note that our new analysis showed slightly lower levels of ERC between Mlh1 and Mlh3 than previously reported (Clark et al. 2013). This difference is due to normalizing branch lengths more carefully, resulting in an improved way to compute RERs (Materials and Methods). With this new method, we see similarly significant ERC signals between Mlh1 and Mlh3, and Mlh1 and Pms1 (P < 0.05). Elevated ERC signals (P ≤ 0.03) were seen between the individual Mlh3 domains as well as between the three Mlh3 domains and the Pms1 linker. They were also seen between the Mlh1 N-terminal and the Mlh3 N-terminal, Mlh3 C-terminal, and Pms1 linker domains. Finally, an elevated ERC signal was seen between the Pms1 N-terminal and Pms1-C-terminal domains. These observations do not show a specific pattern of signals between specific domains of MLH proteins but are consistent with structural studies indicating that ATP-dependent conformational rearrangements involving the linker regions of the MLH proteins are important for the positioning of the two N-terminal MLH domains and bound DNA near the endonuclease active site in the C-terminus (Ban et al. 1999; Sacho et al. 2008; Pillon et al. 2010).
Chimeric Mlh3/Pms1 proteins interfere with Mlh3-dependent MMR
Phylogenetic and ERC analyses indicating that Mlh3 and Pms1 are sister groups and that the three domains of MLH proteins covary encouraged us to perform domain swaps between Mlh3 and Pms1. These were performed to determine if we could alter Mlh3 and Pms1 functions by exchanging domains. We tested for altered specificities in MMR in mlh3Δ and pms1Δ strains using a highly sensitive lys2-A14 reporter containing a centrally located homopolymeric run of 14 deoxyadenosine residues that result in a + 1-frameshift mutation (Supplementary Figure S1B; Tran et al. 1997). DNA slippage events that restore the reading frame, primarily the result of -1-frameshift mutations, confer reversion to Lys+ (Materials and Methods). We measured meiotic crossing over using a spore-autonomous fluorescence assay to measure percent tetratype at the CEN8-THR1 interval (Supplementary Figure S1D; Thacker et al. 2011). Fluorescence microscopy was used to quantify parental ditypes and tetratypes resulting from single crossover events. Two red and two blue spores are detected in the absence of a crossover (parental ditype) between the RFP and CFP markers. For a single crossover (tetratype), a tetrad contains one red, one blue, one purple, and one nonfluorescent spore.
Six Mlh3/Pms1 chimeric proteins were constructed in which the N-terminal, linker, and C-terminal domains were swapped between Mlh1 and Mlh3 (abbreviated as PMM, MMP, PPM, PMP, MPP, MPM) and assessed for their ability to complement Mlh3’s major function in crossing over and minor function in MMR (Table 1). The endpoints for the domain swaps are presented in the Materials and Methods and Supplementary Figure S3B. None of the chimeras complemented MMR or meiotic crossover functions (Tables 1 and 2). Arguing against the possibility that the chimeric proteins were unstable was our finding that four Mlh3/Pms1 chimeric proteins (MMP, PPM, PMP, MPP) conferred mutation rates that were higher than seen for mlh3Δ. One explanation for this phenotype is that the MMP, PPM, PMP, and MPP chimeric genes expressed polypeptides that interfered with other MLH MMR pathways; for example, the chimeras could sequester Mlh1 from interacting with Pms1 in MMR [see examples in Shcherbakova and Kunkel (1999) and Smith et al. (2013)].
Identifying residues critical for Mlh3 MMR and crossover functions
Our inability to identify domains in the MLH proteins that conferred functional specificity encouraged us to perform a more targeted approach. Because Mlh3 plays a minor role in MMR, and Pms1 does not act in dHJ resolution, we thought that our best opportunity to study the specificity of the MLH proteins was to make substitution mutations in MLH3 that affected its MMR and meiotic crossing over functions. Such substitutions might also allow Mlh3 to partially replace Pms1 MMR functions (see below). We performed this analysis recognizing that the divergence of Mlh3 and Pms1 is an ancient event, and any changes in specificity would be informative as refinement of protein function would likely require large numbers of changes over a long evolutionary time scale.
We used multi-Harmony analysis as a targeted approach to identify residues in Mlh3 and Pms1 required for MMR function. Multi-Harmony uses multiple sequence alignments between subfamilies of proteins, homology models, and multi-Relief and sequence-Harmony algorithms to identify amino acids that may suggest functional specificity (Materials and Methods; Brandt et al. 2010). By aligning Mlh3 and Pms1 amino acid sequences from 34 fungal species (Supplementary Figure S5, A–D), residues were identified that are well conserved within each subfamily but differed between Mlh3 and Pms1 (Blocks 1–6). When mapped onto the 3D structure of Mlh1-Pms1 on PyMOL, clusters of amino acids were identified and labeled as “blocks.” The substitutions in each block are shown in the Mlh3 sequence with the Mlh3 residue shown first, the amino acid position in Mlh3, and the equivalent position in Pms1.
Block 1 maps to the ATP-binding domain; Block 2 to the Mlh1 interaction motif, and Blocks 3, 5, and 6, to the MLH endonuclease motif (Figure 2). Because of the extremely high conservation of some single residues in Mlh3 or Pms1, we also tested single amino acid substitutions with two (D500N, K502G) mapping to Block 2, two (R530K, R532N) to Block 3, and one (D678K) to Block 5. Lastly, a Block 4 was created based on previous studies showing that eukaryotic MutSα and MutLα complexes each interact with the DNA replication processivity clamp PCNA through a PIP Box defined as a six amino acid sequence consisting of Qxφ[L/I]xP, where φ is a hydrophobic residue and x is any amino acid (Kelman 1997; Pillon et al. 2011; Genschel et al. 2017; Supplementary Figure S3D). For MutSα, this interaction helps to tether at least a subset of MutSα complexes to the replication fork (Hombauer et al. 2011). For MutLα, interaction with PCNA through a PIP-motif present in the Pms1 endonuclease domain is critical to activate MutLα’s endonuclease activity (Pluciennik et al. 2010; Genschel et al. 2017). Mlh3 does not contain this motif in the corresponding position in its endonuclease domain, and Mlh1-Mlh3 endonuclease activity was not stimulated by PCNA under conditions where the Mlh1-Pms1 endonuclease is activated (Ranjha et al. 2014; Rogacheva et al. 2014; Manhart et al. 2017). However, Cannavo et al. (2020) recently identified candidate PIP motifs in both yeast Mlh1 and Mlh3 that when mutated conferred defects in meiotic crossing over and partially disrupted stimulation of Mlh1-Mlh3 endonuclease activity in the presence of PCNA, Exo1, and Msh4-Msh5. As described below, we introduced PIP motif substitutions into Mlh3 based on a homology model of the C-terminal domain of the Mlh1-Mlh3 complex (Supplementary Figure S3, C and D). Specifically, we introduced conserved PIP residues into a sequence (GTFVAR) in Mlh3 that corresponds to the location of the PIP motif in Pms1.
Effect of Multi-Harmony substitutions on MLH3 functions in MMR and crossing over
The mlh3-Block1-6 substitutions were tested for complementing Mlh3 functions in MMR and meiotic crossing over (Table 1), and for their ability to complement Pms1 functions in MMR (Table 2). A brief summary of the phenotypes conferred by these phenotypes is shown below.
The mlh3-Block1 (ATP binding) allele conferred an intermediate phenotype in the mutation rate assay (2.20-fold higher than wild type) but did not affect meiotic crossing over.
The mlh3-Block2 (Mlh1 interaction) alleles conferred intermediate phenotypes in both the mutation rate assay (1.68- to 3.68-fold higher than wild type) and meiotic crossing over (19.1–33.1% tetratype, compared to 37.1% for wild type), but with one allele (mlh3-K502G) conferring a more severe defect in the meiotic crossover assay.
mlh3-Block3, 5, 6 (Mlh3 endonuclease motifs) alleles conferred intermediate to severe phenotypes in both mutation rate and meiotic crossover assays, with some alleles conferring a more severe defect in one assay vs the other. It was interesting to see a more severe defect for the Block 3 mlh3-R530K, R532N double mutation compared to the single mutations (4.36-fold for the double mutation vs 2.31- and 1.21-fold for the single mutations in the mutation rate assay; 20.4% tetratype for the double mutation vs 25.3% and 29.5% tetratype for single mutations in the meiotic CO assay), indicating important roles for the two residues in Mlh3 function.
Two mlh3-Block4 mutant alleles were created. mlh3-Block4-PIP1 contains the yeast Pms1 PIP motif (QKLIIP) and mlh3-Block4-PIP2 contains just the three critical consensus residues (QTFIAP), with the other residues derived from Mlh3 (Supplementary Figure S3D). The mlh3-Block4-PIP2 mutation complemented Mlh3 functions in both MMR and meiotic crossing over, whereas mlh3-Block4-PIP1 conferred an intermediate defect in both assays (3.39-fold higher than wild type in the mutation rate assay, 30.9% tetratype in the CO assay). These observations encouraged us to perform the detailed analysis of the PIP motif described below.
Our observations confirmed that regions of high conservation specific to the Mlh3 family were critical for Mlh3 function; every Block substitution group conferred defects in MMR and meiotic crossing over, though with different phenotypic strengths. Also, the finding that some Block mutations differentially affected Mlh3 MMR and meiotic functions is consistent with the previous identification of Mlh3 separation of function mutations that may disrupt interactions with specific pathway components (Table 1; Al-Sweel et al. 2017; Claeys Bouuaert and Keeney 2017).
Identifying Block mlh3 mutations that partially complement the MMR defect seen in pms1Δ strains
The studies presented in Table 1 encouraged us to test if the mlh3-Block1-6 alleles could complement Pms1 MMR functions (Table 2). We first tested if the MLH3SK1 gene used to make all of our multi-Harmony substitutions could complement, when expressed on an ARS-CEN single copy vector, the MMR functions of an S288c mlh3Δ strain (EAY4595). As shown in Table 1, this plasmid displayed full complementation, indicating that it was an appropriate gene construct for our analysis. We then asked if the suppression of the mlh3-PIP1 and PIP2 alleles expressed on an ARS-CEN vector could complement the pms1Δ mutator phenotype. As shown in Table 2, neither mlh3 allele complemented the pms1Δ mutator phenotype.
The inability of the mlh3-PIP1 and -PIP2 alleles to complement the pms1Δ mutator phenotype encouraged us to express mlh3 alleles through the MLH3 promoter on a high copy 2µ LEU2 pRS derived vector. We were encouraged to overexpress the mutant proteins because an analysis of multiple protein abundance data sets (Ho et al. 2018) indicated that Mlh3 protein levels (median of 220 molecules per cell) are lower than those seen for Pms1 (median of 648 molecules per cell). Karim et al. (2013) determined in an S288c strain that such LEU2 2µ vectors were present at 14 copies/cell, and so the expression of MLH3 in a 2µ LEU2 vector is predicted to yield Mlh3 molecule numbers per cell that are only a few-fold higher than the native Pms1 levels. Interestingly, mild overexpression of MLH3 on a 2µ vector in mlh3Δ conferred a mutation rate significantly higher than seen when expressing an empty 2µ vector in mlh3Δ (81.8- vs 5.90-fold), but this rate was still significantly lower than seen in pms1Δ strains (6320-fold). This was not a surprise as we had shown previously that robust overexpression of MLH3 through a GAL10 promoter conferred mutation rates in a wild-type strain similar to that seen in pms1Δ, most likely by sequestering Mlh1 from interacting with Pms1 (Nishant et al. 2008). We reasoned that any improved function for an mlh3 allele in PMS1-dependent MMR should overcome the elevated mutation rate associated with MLH3 overexpression. However, it also seems possible that improved function could be due to both amino acid substitutions and changes in protein levels/stability that result from the substitutions.
As shown in Table 2 and Figure 3, mlh3-Block1 and mlh3-Block4-PIP2 alleles expressed on 2µ vectors conferred modest suppression of the pms1Δ MMR defect (2- to 3.5-fold lower mutation rates), whereas mlh3-Block2, 3, 5, 6 alleles and the six chimera constructs did not. The failure of the mlh3-Block4-PIP1 allele, which contained the complete PIP consensus, to suppress the pms1Δ MMR defect suggested that the suppression seen with mlh3-Block4-PIP2 was not due to Mlh3 being able to interact with PCNA in MMR. To better understand if a canonical PIP motif was important for this improved complementation, we made a total of nine substitutions in the Mlh3 GTFVAR sequence (Supplementary Figure S3D). The finding that mlh3-PIP5 (QTFIAR) and mlh3-PIP9 (QTFVAR) strains (both missing leucine/isoleucine and proline residue conservation at positions 4 and 6, respectively) maintained modest complementation (2.1- to 2.7-fold lower mutation rates) whereas the mlh3-PIP1 strain did not, provided the most relevant data suggesting that the improved MMR functions in the mlh3-PIP2 strain were unlikely due to the creation of a PCNA interaction. The other substitutions, which were focused on introducing one, two, or three conserved PIP residues into the Mlh3 GTFVAR sequence were not especially informative because none of them improved MLH3 MMR functions (mlh3-PIP3, 4, 6, 7, 8). Together, these findings suggest that the addition of a functional PIP motif was unlikely to explain the improved MMR phenotype seen in mlh3-Block4-PIP2, -PIP3, and -PIP5. In addition, they do not address whether the mlh3-PIP proteins are directly interacting with PCNA, or if a structural change induced by the insertion of this motif alters its endonuclease activity in vivo to be more similar to that seen with Mlh1-Pms1.
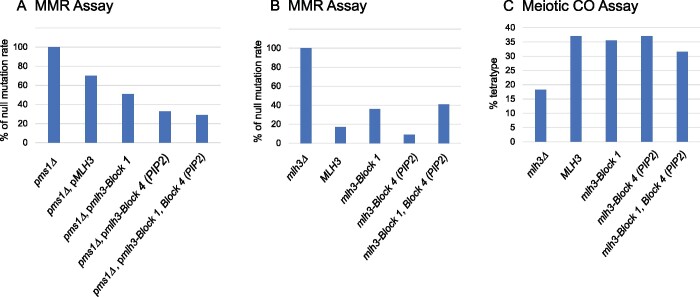
Figure 3.
Block 1 and Block 4 mlh3 mutations can alleviate the pms1Δ MMR defect. (A) Mutation rates of mlh3-Block 1 and mlh3-Block 4 single‐ and double‐mutant alleles expressed on 2µ plasmids in pms1Δ strains were determined in the lys2-A14 assay as described in Materials and Methods. Rates are shown as a percentage of the corresponding pms1Δ. (B) Mutation rates of strains containing mlh3 constructs integrated at the MLH3 locus in PMS1 strains. Rates are shown as a percentage of an mlh3Δ strain. (C) Meiotic crossover phenotypes, expressed as % tetratype, for diploid strains containing mlh3 constructs integrated at the MLH3 locus. For (A), MMR rates were normalized from data presented in Table 2, with the lines from the table where the data were obtained indicated with PMS1, ARS-CEN corresponding to line 1. Data were obtained from pms1Δ strains containing an empty 2µ vector-line 4 (set to 100%), pMLH3-line 48, pmlh3-Block 1-line 10, pmlh3-Block 4 (PIP2)-line 23, and pmlh3 Block 1, Block 4 (PIP2)-line 37. For (B), MMR rates were normalized from data presented in Table 1 (lines indicated, with MLH3 corresponding to line 1) and obtained as follows: mlh3Δ-line 2 (set to 100%), MLH3-line 1, mlh3-Block 1-line 13, mlh3-Block 4 (PIP2)-line 24, and mlh3-Block 1, Block 4 (PIP2)-line 32. For (C), CO data were obtained from data presented in Table 1 (lines indicated) as follows: mlh3Δ-line 2, MLH3-line 1, mlh3-Block 1-line 13, mlh3-Block 4 (PIP2)-line 24, and mlh3-Block 1, Block 4 (PIP2)-line 32.
The above observations encouraged us to determine if the modest complementation seen for the mlh3-Block1 and mlh3-Block4-PIP2 alleles could be enhanced or maintained in combination with other mlh3 alleles. As shown in Table 2 and Figure 3, the mlh3-Block1, Block4-PIP2 double mutation complemented the pms1Δ MMR defect as well, if not slightly better than the mlh3-Block1 or mlh3-Block4-PIP2 mutations alone. Double-mutant combinations that included mlh3-Block4-PIP2 and alleles that did not, or only weakly affected MLH3 function (D678K, R530K, R532N singles) displayed mutation rates similar to the mlh3-Block4-PIP2 mutant alone. However, other hypomorph mlh3 alleles (D500N, K502G singles), disrupted the ability of mlh3-Block4-PIP2 to suppress the pms1Δ MMR defect. It was interesting to see that the severe mlh3-R530K, R532N and mlh3-Block6 mutations disrupted mlh3-Block4-PIP2 complementation, consistent with the idea that Block mutations that could not complement mlh3Δ and pms1Δ functions were deleterious for all MLH functions. Together, these observations indicate that multi-Harmony is a valuable approach to identify residues outside of consensus motifs that are critical for specificity of subsets of protein family members. The finding that substitutions located in both the ATP-binding and endonuclease domains of Mlh3 and Pms1 could alter Mlh3 specificity supports ERC analysis indicating that multiple domains across the MLH proteins are co-evolving to provide specificity. It is also of interest because the N-terminal domains of Mlh1 and Pms1 were shown to independently bind to double-stranded and single-stranded DNA (Hall et al. 2003; see also Claeys Bouuaert and Keeney, 2017), suggesting that both the N- and C-terminal domains of MLH proteins contribute to interactions with DNA that could provide specificity for the different MLH pathways.
Discussion
We leveraged phylogenetic, multi-Harmony, and molecular genetic analyses to alter the specificity of Mlh3 to modestly complement Pms1 MMR functions. We were surprised by the partial complementation of Pms1 MMR functions because the functional advantage conferred by making a limited number of mutations in proteins that differ by hundreds of amino acids would likely be difficult to detect. The altered specificity seen in mlh3 alleles was further confirmed by combining mutant combinations. Overall, combining beneficial alleles maintained or slightly improved altered specificity, whereas combining a neutral allele with a beneficial one did not affect outcome, and combining a deleterious allele with a beneficial one disrupted the altered specificity. These data, combined with chimera and ERC analyses, are consistent with the three domains of the MLH proteins being interdependent, having coevolved for millions of years, and contributing to the distinct specificities in MMR and meiotic crossing over seen for the Pms1 and Mlh3 proteins, respectively.
Two models were presented in the Introduction to explain how novel protein functions evolved after gene duplication. We find it intriguing that two mlh3 alleles (Block1 and Block4) displayed partial complementation of Pms1 MMR functions yet maintained Mlh3 meiotic crossover functions (Figure 3), suggesting that the specialization of the MLH functions could be partially reversed. Such an observation is consistent with an ancestral MLH protein having displayed both MMR and meiotic functions that became specialized following a gene duplication event (model presented by Hughes 1994). Our observations could also be explained by a DDC model (Force et al. 1999) where duplication of MLH genes resolves conflicts seen for an ancestral gene that performs both functions. However, our findings suggesting that the specialization of Mlh3 functions could be partially reversed appears less consistent with the DDC model, which proposes that neutral degenerative mutations occur in each duplicated gene that complement each other.
Our work complements a recent study, which examined the evolution of factors that act in vegetative and meiotic functions. In baker’s yeast, the Rad51 and Dmc1 strand exchange proteins, which share ∼50% amino acid identity and are thought to evolve from a single-gene duplication of an ancestral recombination protein, play critical roles in promoting homologous recombination [reviewed in Furman et al. (2021)]. Both proteins are able to catalyze the invasion of a 3ʹ single-stranded end into a homologous duplex template to form postsynaptic complexes in three nucleotide steps (Brown and Bishop 2014; Lee et al. 2015; Chan et al. 2019; Steinfeld et al. 2019). Most eukaryotes contain a Rad51 protein that is expressed in both mitotic and meiotic cell cycles, and a Dmc1 protein which is meiosis-specific and unlike Rad51, can stabilize heteroduplex DNA with mismatch containing base triplets. In meiosis, Dmc1 and Rad51 play somewhat complementary roles, with Dmc1 catalyzing interhomolog recombination, and Rad51 promoting the assembly of the Dmc1 presynaptic filament (Neale and Keeney 2006; Lao et al. 2013; Brown and Bishop 2014). Analogous to our work, Steinfeld et al. (2019) examined residues that are conserved within the Rad51 or Dmc1 lineages but differ between them. They then made Rad51/Dmc1 chimeras and identified a set of three amino acid substitutions in the L1 DNA-binding loop of these proteins that conferred differences in how the two proteins stabilized recombination intermediates bearing base triplets with mismatches; only Dmc1 can stabilize such intermediates. While such changes in specificity were observed biochemically, they did not result in the formation of functional proteins in vivo as was seen for the modest complementation that we observed for the mlh3 Block 1 and 4 alleles. Steinfeld et al. (2019) speculated that swapping this motif “may hinder some downstream step in the HR pathway.” Furthermore, they identified 19 lineage-specific amino acids in other regions of the two recombinases that they speculated were important for Rad51 and Dmc1-specific protein–protein contacts. Thus, this study and ours show the challenges in achieving full complementation in proteins that have become highly specialized and significantly diverged from ancestral proteins.
Our study raises the question of whether it would be possible to identify mlh3 alleles with significantly improved functions in the PMS1 MMR pathway. When we started this work, we recognized that making mlh3 alleles with altered specificity would be challenging because Mlh3 and Pms1 work with a multitude of other proteins in their respective meiotic and MMR pathways [reviewed in Furman et al. (2021)]. We attempted to alter the specificity of MLH3 by only making a small number of amino acids changes. More extensive changes could be made, for example, by using advanced computational modeling approaches. Such methods could be used to identify an ancestral state of Mlh3 capable of acting more efficiently in MMR (see reviews by Thornton 2004; Gumulya and Gillam 2017). Alternatively, one could perform an extensive mutagenesis to identify mlh3 alleles that counter a strong selection such as conferring viability in pms1Δ pol3-01 haploids that are inviable due to high mutational load (Morrison et al. 1993; Argueso et al. 2002). An analogous experimental evolution approach was performed in budding yeast by Hsieh et al. (2020), who identified mutations in REC8 and in other genes that conferred viability in vegetative growth when the meiotic kleisin Rec8 was expressed in the absence of the vegetative paralog Scc1.
Acknowledgments
We are grateful to Charles Aquadro for advice throughout these studies, Amanda Kowalczyk for her advice on ERC analysis, Beata Mackenroth for comments on the manuscript, and members of the Alani lab for helpful discussions.
Funding
C.M.F., R.E., G.N.P., and E.A. were supported by the National Institute of General Medical Sciences of the National Institutes of Health (https://www.nih.gov/): R35GM134872. R.E. was an undergraduate fellow of the Cornell McNair Scholars Program and received funding as an undergraduate from the Frank L. and Lynnet Douglas Fellowship in Chemistry, Chemical Biology, and Biochemistry, Cornell University (https://oadi.cornell.edu/programs/student-success-programs/mcnair/mcnair-scholars.html). N.L.C. was funded by R01HG009299 and R01EY030546 of the National Institutes of Health (https://www.nih.gov/). The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
None declared.
Contributor Information
Christopher M Furman, Department of Molecular Biology and Genetics, Cornell University, Ithaca, NY 14853-2703, USA.
Ryan Elbashir, Department of Molecular Biology and Genetics, Cornell University, Ithaca, NY 14853-2703, USA.
Gianno Pannafino, Department of Molecular Biology and Genetics, Cornell University, Ithaca, NY 14853-2703, USA.
Nathan L Clark, Department of Human Genetics, University of Utah, Salt Lake City, UT 84132, USA.
Eric Alani, Department of Molecular Biology and Genetics, Cornell University, Ithaca, NY 14853-2703, USA.
These authors are co-first authors.
Literature cited
- Abdullah MF, Hoffmann ER, Cotton VE, Borts RH. 2004. A role for the MutL homolog MLH2 in controlling heteroduplex formation and in regulating between two different crossover pathways in budding yeast. Cytogenet Genome Res. 107:180–190. [DOI] [PubMed] [Google Scholar]
- Al-Sweel N, Raghavan V, Dutta A, Ajith VP, Di Vietro L, et al. 2017. mlh3 mutations in baker’s yeast alter meiotic recombination outcomes by increasing noncrossover events genome-wide. PLoS Genet. 13:e1006974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arana ME, Holmes SF, Fortune JM, Moon AF, Pedersen LC, et al. 2010. Functional residues on the surface of the N-terminal domain of yeast Pms1. DNA Repair. 9:448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso JL, Smith D, Yi J, Waase M, Sarin S, et al. 2002. Analysis of conditional mutations in the Saccharomyces cerevisiae MLH1 gene in mismatch repair and in meiotic crossing over. Genetics. 160:909–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban C, Junop M, Yang W. 1999. Transformation of MutL by ATP binding and hydrolysis: a switch in DNA mismatch repair. Cell. 97:85–97. [DOI] [PubMed] [Google Scholar]
- Ban C, Yang W. 1998. Crystal structure and ATPase activity of MutL: implications for DNA repair and mutagenesis. Cell. 95:541–552. [DOI] [PubMed] [Google Scholar]
- Brandt BW, Feenstra KA, Heringa J. 2010. Multi-harmony: detecting functional specificity from sequence alignment. Nucleic Acids Res. 38:W35–W40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Bishop DK. 2014. DNA strand exchange and RecA homologs in meiosis. Cold Spring Harb Perspect Biol. 7:a016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CS, Hombauer H, Srivatsan A, Bowen N, Gries K, et al. 2014. Mlh2 is an accessory factor for DNA mismatch repair in Saccharomyces cerevisiae. PLoS Genet. 10:e1004327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannavo E, Sanchez A, Anand R, Ranjha L, Hugener J, et al. 2020. Regulation of the MLH1-MLH3 endonuclease in meiosis. Nature. 586:618–622. doi:10.1038/s41586-020-2592-2. [DOI] [PubMed] [Google Scholar]
- Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 17:540–552. doi:10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- Chan YL, Zhang A, Weissman BP, Bishop DK. 2019. RPA resolves conflicting activities of accessory proteins during reconstitution of Dmc1-mediated meiotic recombination. Nucleic Acids Res. 47:747–761. doi:10.1093/nar/gky1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeys Bouuaert C, Keeney S. 2017. Distinct DNA-binding surfaces in the ATPase and linker domains of MutLγ determine its substrate specificities and exert separable functions in meiotic recombination and mismatch repair. PLoS Genet. 13:e1006722.doi:10.1371/journal.pgen.1006722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark NL, Alani E, Aquadro CF. 2012. Evolutionary rate covariation reveals shared functionality and coexpression of genes. Genome Res. 22:714–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark NL, Alani E, Aquadro CF. 2013. Evolutionary rate covariation in meiotic proteins results from fluctuating evolutionary pressure in yeasts and mammals. Genetics. 193:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton VE, Hoffmann ER, Borts RH. 2010. Distinct regulation of Mlh1p heterodimers in meiosis and mitosis in Saccharomyces cerevisiae. Genetics. 185:459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culligan KM, Meyer-Gauen G, Lyons-Weiler J, Hays JB. 2000. Evolutionary origin, diversification and specialization of eukaryotic MutS homolog mismatch repair proteins. Nucleic Acids Res. 28:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detloff P, Sieber J, Petes TD. 1991. Repair of specific base pair mismatches formed during meiotic recombination in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 11:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WJ, Massey FJ. 1969. Introduction to Statistical Analysis. New York, NY: McGraw-Hill. [Google Scholar]
- Drake JW. 1991. Spontaneous mutation. Annu Rev Genet. 25:125–146. [DOI] [PubMed] [Google Scholar]
- Duroc Y, Kumar R, Ranjha L, Adam C, Guérois R, et al. 2017. Concerted action of the MutLβ heterodimer and Mer3 helicase regulates the global extent of meiotic gene conversion. eLife. 6:e21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdeniz N, Dudley S, Gealy R, Jinks-Robertson S, Liskay RM. 2005. Novel PMS1 alleles preferentially affect the repair of primer strand loops during DNA replication. Mol Cell Biol. 25:9221–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Rozas H, Kolodner RD. 1998. The Saccharomyces cerevisiae MLH3 gene functions in MSH3-dependent suppression of frameshift mutations. Proc Natl Acad Sci U S A. 95:12404–12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force A, Lynch M, Pickett FB, Amores A, Yan Y-L, et al. 1999. Preservation of duplicate gene by complementary degenerative mutations. Genetics. 151:1531–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman CM, Elbashir R, Alani E. 2021. Expanded roles for the MutL family of DNA mismatch repair proteins. Yeast. 38:39–53. doi:10.1002/yea.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genschel J, Kadyrova LY, Iyer RR, Dahal BK, Kadyrov FA, et al. 2017. Interaction of proliferating cell nuclear antigen with PMS2 is required for MutLα activation and function in mismatch repair. Proc Natl Acad Sci U S A. 114:4930–4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH, Willems AR, Woods RA. 1995. Studies on the transformation of intact yeast cells by the LiAc/SS‐DNA/PEG procedure. Yeast. 11:355–360. [DOI] [PubMed] [Google Scholar]
- Goellner EM, Putnam CD, Kolodner RD. 2015. Exonuclease 1-dependent and independent mismatch repair. DNA Repair (Amst). 32:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AL, McCusker JH. 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast. 15:1541–1553. [DOI] [PubMed] [Google Scholar]
- Guarné A, Ramon-Maiques S, Wolff EM, Ghirlando R, Hu X, et al. 2004. Structure of the MutL C-terminal domain: a model of intact MutL and its roles in mismatch repair. EMBO J. 23:4134–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueneau E, Dherin C, Legrand P, Tellier-Lebegue C, Gilquin B, et al. 2013. Structure of the MutLα C-terminal domain reveals how Mlh1 contributes to Pms1 endonuclease site. Nat Struct Mol Biol. 20:461–468. [DOI] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 52:696–704. doi:10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Gumulya Y, Gillam EMJ. 2017. Exploring the past and the future of protein evolution with ancestral sequence reconstruction: the ‘retro’approach to protein engineering. Biochem J. 474:1–19. [DOI] [PubMed] [Google Scholar]
- Gouy M, Guindon S, Gascuel O. 2010. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol. 27:221–224. [DOI] [PubMed] [Google Scholar]
- Hall MC, Shcherbakova PV, Fortune JM, Borchers CH, Dial JM, et al. 2003. DNA binding by yeast Mlh1 and Pms1: implications for DNA mismatch repair. Nucleic Acids Res. 31:2025–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfe BD, Minesinger BK, Jinks-Robertson S. 2000. Discrete in vivo roles for the MutL homologs Mlh2p and Mlh3p in the removal of frameshift intermediates in budding yeast. Curr Biol. 10:145–148. [DOI] [PubMed] [Google Scholar]
- Hittinger CT, Carroll SB. 2007. Gene duplication and the adaptive evolution of a classic genetic switch. Nature. 449:677–681. [DOI] [PubMed] [Google Scholar]
- Ho B, Baryshnikova A, Brown GW. 2018. Unification of protein abundance datasets yields a quantitative Saccharomyces cerevisiae proteome. Cell Syst. 6:192–205. [DOI] [PubMed] [Google Scholar]
- Hombauer H, Campbell CS, Smith CE, Desai A, Kolodner RD. 2011. Visualization of eukaryotic DNA mismatch repair reveals distinct recognition and repair intermediates. Cell. 147:1040–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh YP, Makrantoni V, Robertson D, Marston AL, Murray AW. 2020. Evolutionary repair: changes in multiple functional modules allow meiotic cohesin to support mitosis. PLoS Biol. 18:e3000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AL. 1994. The evolution of functionally novel proteins after gene duplication. Proc R Soc London B. 256:119–124. [DOI] [PubMed] [Google Scholar]
- Kadyrov FA, Dzantiev L, Constantin N, Modrich P. 2006. Endonucleolytic function of MutLalpha in human mismatch repair. Cell. 126:297–308. [DOI] [PubMed] [Google Scholar]
- Karim AS, Curran KA, Alper HS. 2013. Characterization of plasmid burden and copy number in Saccharomyces cerevisiae for optimization of metabolic engineering operations. FEMS Yeast Res. 13:107–116. doi:10.1111/1567-1364.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasoe Y, Tsurimoto T, Nakagawa T, Masukata H, Takahashi TS. 2016. MutSα maintains the mismatch repair capability by inhibiting PCNA unloading. eLife. 5:e15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney S, Giroux CN, Kleckner N. 1997. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 88:375–384. [DOI] [PubMed] [Google Scholar]
- Kelman Z. 1997. PCNA: structure, functions and interactions. Oncogene. 14:629–640. [DOI] [PubMed] [Google Scholar]
- Kolas NK, Svetlanov A, Lenzi ML, Macaluso FP, Lipkin SM, et al. 2005. Localization of MMR proteins on meiotic chromosomes in mice indicates distinct functions during prophase I. J Cell Biol. 171:447–458. doi:10.1083/jcb.200506170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk A, Meyer WK, Partha R, Mao W, Clark NL, et al. 2019. RERconverge: an R package for associating evolutionary rates with convergent traits. Bioinformatics. 35:4815–4817. doi:10.1093/bioinformatics/btz468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni DS, Owens SN, Honda M, Ito M, Yang Y, et al. 2020. PCNA activates the MutLγ endonuclease to promote meiotic crossing over. Nature. 586:623–627. doi:10.1038/s41586-020-2645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel TA, Erie DA. 2015. Eukaryotic mismatch repair in relation to DNA replication. Annu Rev Genet. 49:291–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao JP, Cloud V, Huang CC, Grubb J, Thacker D, et al. 2013. Meiotic crossover control by concerted action of Rad51–Dmc1 in homolog template bias and robust homeostatic regulation. PLoS Genet. 9:e1003978. doi:10.1371/journal.pgen.1003978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Terakawa T, Qi Z, Steinfeld JB, Redding S, et al. 2015. Base triplet stepping by the Rad51/RecA family of recombinases. Science. 349:977–981. doi:10.1126/science.aab2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, Bork P. 2019. Interactive Tree of Life iTOL v4: recent updates and new developments. Nucleic Acids Res. 47:W256–W259. doi:10.1093/nar/gkz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manhart CM, Alani E. 2016. Roles for mismatch repair family proteins in promoting meiotic crossing over. DNA Repair (Amst). 38:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manhart CM, Ni X, White MA, Ortega J, Surtees JA, et al. 2017. The mismatch repair and meiotic recombination endonuclease Mlh1-Mlh3 is activated by polymer formation and can cleave DNA substrates in trans. PLoS Biol. 15:e2001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning JR, Jefferson ER, Barton GJ. 2008. The contrasting properties of conservation and correlated phylogeny in protein functional residue prediction. BMC Bioinformatics. 9:51.doi:10.1186/1471-2105-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcet-Houben M, Gabaldón T. 2015. Beyond the whole-genome duplication: phylogenetic evidence for an ancient interspecies hybridization in the baker’s yeast lineage. PLoS Biol. 13:e1002220.doi:10.1371/journal.pbio.1002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsolier-Kergoat MC, Khan MM, Schott J, Zhu X, Llorente B. 2018. Mechanistic view and genetic control of DNA recombination during meiosis. Mol Cell. 70:9–20. doi:10.1016/j.molcel.2018.02.032. [DOI] [PubMed] [Google Scholar]
- Moens PB, Kolas NK, Tarsounas M, Marcon E, Cohen PE, et al. 2002. The time course and chromosomal localization of recombination-related proteins at meiosis in the mouse are compatible with models that can resolve the early DNA–DNA interactions without reciprocal recombination. J Cell Sci. 115:1611–1622. [DOI] [PubMed] [Google Scholar]
- Morrison A, Johnson AL, Johnston LH, Sugino A. 1993. Pathway correcting DNA replication errors in Saccharomyces cerevisiae. EMBO J. 12:1467–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MJ, Keeney S. 2006. Clarifying the mechanics of DNA strand exchange in meiotic recombination. Nature. 442:153–158. doi:10.1038/nature04885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishant KT, Plys AJ, Alani E. 2008. A mutation in the putative MLH3 endonuclease domain confers a defect in both mismatch repair and meiosis in Saccharomyces cerevisiae. Genetics. 179:747–755. doi:10.1534/genetics.108.086645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S. 1970. Evolution by Gene Duplication. New York: Springer-Verlag. [Google Scholar]
- Partha R, Kowalczyk A, Clark NL, Chikina M. 2019. Robust method for detecting convergent shifts in evolutionary rates. Mol Biol Evol. 36:1817–1830. doi:10.1093/molbev/msz107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillon MC, Lorenowicz JJ, Uckelmann M, Klocko AD, Mitchell RR, et al. 2010. Structure of the endonuclease domain of MutL: unlicensed to cut. Mol Cell. 39:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillon MC, Miller JH, Guarné A. 2011. The endonuclease domain of MutL interacts with the β sliding clamp. DNA Repair (Amst). 10:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluciennik A, Dzantiev L, Iyer RR, Constantin N, Kadyrov FA, et al. 2010. PCNA function in the activation and strand direction of MutLα endonuclease in mismatch repair. Proc Natl Acad Sci U S A. 107:16066–16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyatnitskaya A, Borde V, De Muyt A. 2019. Crossing and zipping: molecular duties of the ZMM proteins in meiosis. Chromosoma. 128:181–198. [DOI] [PubMed] [Google Scholar]
- Ranjha L, Anand R, Cejka P. 2014. The Saccharomyces cerevisiae Mlh1-Mlh3 heterodimer is an endonuclease that preferentially binds to Holliday Junctions. J Biol Chem. 289:5674–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reenan RA, Kolodner RD. 1992. Characterization of insertion mutations in the Saccharomyces cerevisiae MSH1 and MSH2 genes: evidence for separate mitochondrial and nuclear functions. Genetics. 132:975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robine N, Uematsu N, Amiot F, Gidrol X, Barillot E, et al. 2007. Genome-wide redistribution of meiotic double-strand breaks in Saccharomyces cerevisiae. Mol Cell Biol. 27:1868–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogacheva MV, Manhart CM, Chen C, Guarne A, Surtee J, et al. 2014. Mlh1-Mlh3, A meiotic crossover and DNA mismatch repair factor, is a Msh2-Msh3-stimulated endonuclease. J Biol Chem. 289:5664–5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanova NV, Crouse GF. 2013. Different roles of eukaryotic MutS and MutL complexes in repair of small insertion and deletion loops in yeast. PLoS Genet. 9:e1003920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MD, Winston F, Hieter P. 1990. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual. New York, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Sacho EJ, Kadyrov FA, Modrich P, Kunkel TA, Erie DA. 2008. Direct visualization of asymmetric adenine-nucleotide-induced conformational changes in MutL alpha. Mol Cell. 29:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A, Adam C, Rauh F, Duroc Y, Ranjha L, et al. 2020. Exo1 recruits Cdc5 polo kinase to MutLγ to ensure efficient meiotic crossover formation. Proc Natl Acad Sci USA. 117:30577–30588. doi:10.1073/pnas.2013012117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcherbakova PV, Kunkel TA. 1999. Mutator phenotypes conferred by MLH1 overexpression and by heterozygosity for mlh1 mutations. Mol Cell Biol. 19:3177–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shell SS, Putnam CD, Kolodner RD. 2007. Chimeric Saccharomyces cerevisiae Msh6 protein with an Msh3 mispair-binding domain combines properties of both proteins. Proc Natl Acad Sci U S A. 104:10956–10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CE, Mendillo ML, Bowen N, Hombauer H, Campbell CS, et al. 2013. Dominant mutations in S. cerevisiae PMS1 identify the Mlh1-Pms1 endonuclease active site and an exonuclease 1-independent mismatch repair pathway. PLoS Genet. 9:e1003869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfeld JB, Beláň O, Kwon Y, Terakawa T, Al-Zain A, et al. 2019. Defining the influence of Rad51 and Dmc1 lineage-specific amino acids on genetic recombination. Genes Dev. 33:1191–1207. doi:10.1101/gad.328062.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker D, Lam I, Knop M, Keeney S. 2011. Exploiting spore-autonomous fluorescent protein expression to quantify meiotic chromosome behaviors in Saccharomyces cerevisiae. Genetics. 189:423–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JW. 2004. Resurrecting ancient genes: experimental analysis of extinct molecules. Nat Rev Genet. 5:366–375. [DOI] [PubMed] [Google Scholar]
- Tran HT, Keen JD, Kricker M, Resnick MA, Gordenin DA. 1997. Hypermutability of homonucleotide runs in mismatch repair and DNA polymerase proofreading yeast mutants. Mol Cell Biol. 17:2859–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe KH, Shields DC. 1997. Molecular evidence for an ancient duplication of the entire yeast genome. Nature. 387:708–713. [DOI] [PubMed] [Google Scholar]
- Zakharyevich K, Tang S, Ma Y, Hunter N. 2012. Delineation of joint molecule resolution pathways in meiosis identifies a crossover-specific resolvase. Cell. 149:334–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids are available upon request. Supporting information contains all detailed descriptions of Supplemental files. All experiments presented (lys2-A14 reversion, CO assays) were repeated on at least two separate days. The Supplementary figures and tables can be found in the GSA Figshare portal: https://doi.org/10.25387/g3.14367533.