SUMMARY
Background:
Due to trisomy of chromosome 21 and the resultant extra copy of the amyloid precursor protein gene, virtually all adults with Down syndrome (DS) develop Alzheimer disease (AD) pathology by age 40 and are at high risk for dementia given their increased life expectancy. Comparison of cerebrospinal fluid (CSF) biomarker patterns in DS with those of autosomal dominant AD (ADAD) mutation carriers (MC) will enhance our understanding of disease mechanisms in these two genetically high risk groups.
Methods:
CSF samples obtained from adults with DS (n=41) and similarly-aged ADAD MC (n=192) and non-carrier (NC, n=108) siblings (aged 30–61 years) were analyzed for markers of amyloid-β (Aβ40, Aβ42), phosphorylated tau-related processes (pTau181), neuronal/axonal/synaptic injury (total tau, visinin-like protein 1 [VILIP-1], neurofilament light chain [NfL], synaptosomal-associated protein 25 [SNAP-25]), and astrogliosis/neuroinflammation (chitinase-3-like protein 1 [YKL-40]) via immunoassay. Biomarker concentrations were compared as a function of dementia status (asymptomatic/symptomatic), and linear regression was used to evaluate and compare the relationship between biomarker levels and age among groups.
Findings:
Adults with DS exhibited patterns of AD-related CSF biomarkers remarkably similar to ADAD MC, including reductions in the Aβ42/Aβ40 ratio and increases in markers of phosphorylated tau-related processes, neuronal/axonal/synaptic injury, and astrogliosis/neuroinflammation, with greater degrees of abnormality in individuals with dementia. Differences included overall higher levels of Aβ and YKL-40 in DS and potential elevations in CSF tau and NfL in the asymptomatic (non-demented) stage.
Interpretation:
CSF biomarker profiles are useful for identifying and tracking AD-related processes in DS and, as such, will likely have utility in clinical trial design in this understudied at-risk population.
Funding:
National Institute on Aging (U01-AG051406, U01-AG051412, UF1-AG032438), Eunice Kennedy Shriver National Institute of Child Health and Human Development, German Center for Neurodegenerative Diseases, and Research and Development Grants for Dementia from Japan Agency for Medical Research and Development.
Keywords: Alzheimer disease, autosomal dominant Alzheimer disease, biomarker, cerebrospinal fluid, Down syndrome
INTRODUCTION
Due to trisomy of chromosome 21 and the resultant extra copy of the amyloid precursor protein (APP) gene, nearly all adults with Down syndrome (DS) will develop amyloid and tau pathology consistent with Alzheimer disease (AD) by the age of 401. Risk of AD dementia in this population is age-dependent, with recent estimates of ~50% prevalence by age 50 and ~90% by age 702. However, dementia has a heterogeneous presentation in DS, including age of onset and clinical symptoms. Furthermore, the time course of disease progression in adults with DS remains uncertain.
AD-related biomarkers have informed our understanding of pathologic disease progression in individuals at risk for developing late-onset AD (LOAD)3 and in individuals carrying autosomal dominant AD (ADAD) mutations, given the near 100% penetrance of mutations and the reliable expected age at symptomatic onset within affected families. While ADAD mutation carriers (MC) develop dementia between ~30–60 years of age4, biomarker changes are detectable 20–30 years prior to symptom onset5, thus supporting the existence of a long, asymptomatic stage during which disease-modifying interventions may be most effective and providing a framework to compare other at-risk AD cohorts such as adults with DS.
Biomarkers of AD pathology (amyloid via cerebrospinal fluid [CSF] amyloid-β42 [Aβ42] and positron emission tomography [PET]; phosphorylated tau-related processes via CSF pTau181 and tau PET; neuronal injury via CSF total tau [tTau] and neurofilament light chain [NfL] and regional brain atrophy via magnetic resonance imaging [MRI]) have been reported in studies of DS2,6–8. However, cohorts have typically been small, and comparator groups (if any) are mostly older, hampering characterization of pathologic disease progression and correlation with clinical status.
To address these limitations, CSF biomarker profiles in a cohort of adults with DS were compared to those from ADAD families (both with and without a dementia diagnosis). Both populations have genetic causes of AD (triplication of APP in DS and mutations in APP, presenilin 1 [PSEN1] or presenilin 2 [PSEN2] in ADAD) that drive overproduction of Aβ (Aβ42 in ADAD and total Aβ in DS) and thus share a potential common disease etiology. These at-risk groups also develop AD at similar ages, with risk increasing with advancing age, allowing age-similar comparisons to be made between individuals with DS and ADAD mutations and between the genetic groups and ADAD non-carrier (NC) sibling controls. This comparison allowed examination of age-related biomarker patterns among the three groups (DS, NC, MC) using cross-sectional data. Although the metric of estimated years to symptom onset (EYO) can be used in ADAD due to the relatively consistent age of onset within families, such a metric does not exist in DS. We hypothesized that CSF biomarker profiles would be similar between the groups at risk, with both differing from the NC controls.
Cerebrospinal fluid was analyzed for markers of amyloid, phosphorylated tau-related processes, neuronal/axonal injury, synaptic dysfunction, and astrogliosis/neuroinflammation. Study of at-risk groups not only affords the opportunity to understand the timing and sequence of pathological changes associated with AD, but direct comparison may also shed light on possible differences in Aβ metabolism, neuronal injury, and/or neuroinflammation in the setting of trisomy 21 compared to AD-causing mutations. Knowledge from this novel comparison may be useful for informing clinical trial design in these understudied at-risk groups.
METHODS
Participants and Study Design
Adults with DS were enrolled in the Alzheimer Biomarker Consortium – Down Syndrome (ABC-DS) study, a multi-site longitudinal study of AD in DS incorporating neuropsychological, neuroimaging, genetic, and fluid biomarker measures9 (https://www.nia.nih.gov/research/abc-ds). Participants with baseline CSF (and available clinical diagnosis and APOE genotype) as of January 31, 2019 were included in the analysis (n=41). All were between 30–61 years of age. Biomarker data from an overlapping ABC-DS cohort have recently been published10, but without comparison to non-DS controls or other AD cohorts, notably those due to genetic etiologies.
To avoid potential age-related bias, CSF samples from a cohort of ADAD MC and NC sibling controls enrolled in the Dominantly Inherited Alzheimer Network (DIAN) study11 (https://dian.wustl.edu/our-research/observational-study/) within the same age range (30–61 years) were chosen as ABC-DS comparator groups (DIAN-MC, n=192; DIAN-NC, n=108) (Data-Freeze 13, end date June 30, 2018). Participants with the Dutch mutation (APP E693G) were excluded because they manifest an atypical clinical syndrome12. Informed consent was obtained directly from all participants whenever possible; otherwise, assent was obtained, and informed consent obtained from the participant’s legally authorized representative. Institutional Review Board approval was obtained at all sites.
Clinical and Cognitive Evaluation
ABC-DS
ABC-DS utilizes neuropsychological measures with the strongest evidence for defining different stages of dementia, the majority of which were developed specifically for DS9 (Supplemental text). Based on cognitive testing, assessments of functional, neuropsychiatric, neurological, health status, and individual history (without regard to biomarkers), participants received a diagnosis of: 1) cognitively stable (CS); 2) mild cognitive impairment (MCI-DS); 3) possible/probable/definite dementia (AD); or 4) uncertain (due to complications unrelated to AD), using a consensus-based protocol. This protocol takes the level of pre-existing intellectual disability into consideration. A diagnosis of CS indicates performance consistent with past intellectual functioning and current age. MCI-DS indicates evidence of cognitive decline over time beyond what would be expected with advancing age but of insufficient severity to suggest dementia. DS-AD indicates clear evidence of substantial cognitive and functional decline with a high degree of confidence in the dementia rating. For the present study, cognitively stable individuals were classified as asymptomatic DS (aDS, no dementia), and the combined MCI/AD group was classified as symptomatic DS (sDS). Participants who received a diagnosis of “uncertain” (n=2) were excluded.
DIAN
Dementia status in DIAN was defined using the Clinical Dementia Rating (CDR®) scale (CDR 0, normal cognitive function; CDR 0·5, 1, 2, and 3, very mild, mild, moderate, and severe dementia, respectively)13. Assessments ascertained family history of AD and medical history, and participants underwent comprehensive examination11. Clinicians were blinded to mutation status and biomarker data. In order to enable comparisons with the DS cohort, CDR 0 in DIAN was defined as asymptomatic (DIAN-aMC), and CDR>0 was defined as symptomatic (DIAN-sMC).
Genetic Evaluation
Karyotype for ABC-DS participants was obtained from medical records or a designated cytogenetic laboratory. For DIAN participants, DNA sequencing for ADAD mutations (in APP, PSEN1 or PSEN2) was performed using PCR-based amplification of the appropriate exon followed by Sanger sequencing.
APOE genotype was also determined. Two APOE single nucleotide polymorphisms (SNPs) (rs429358 and rs7412) determined the presence of APOE ε2, ε3 and ε4 alleles (ABC-DS via KASP genotyping system by LGC Genomics; DIAN via Applied Biosystems’ TaqMan assay). APOE-ε4 status was dichotomized as ε4-negative (ε4-) or ε4-positive (ε4+, comprising both ε4 heterozygotes and homozygotes).
CSF Collection and Analysis
Protocols for CSF collection and processing were consistent with the Alzheimer’s Disease Neuroimaging Initiative (ADNI; http://www.adni-info.org/), notably in terms of use of polypropylene tubes and aliquot size (0.5mL). Participants in ABC-DS underwent lumbar puncture (LP) between 1100–1600 hours; ~10–20 mL of CSF was collected via gravity drip, aspiration, or assisted by fluoroscopy. DIAN participants underwent LP at ~0800 hours after overnight fasting; ~20–30 mL of CSF was collected via gravity drip. CSF from both cohorts was flash frozen on dry ice before shipment to the ABC-DS and DIAN Biomarker Core laboratory at Washington University in St. Louis (WUSTL). Samples were thawed and aliquoted into polypropylene tubes prior to storage at –80°C. Aβ40, Aβ42, tTau and pTau181 were measured in batch (second freeze/thaw) via an automated immunoassay (LUMIPULSEG1200, Fujirebio, Malverne, PA). ABC-DS and DIAN samples were each analyzed in batch for emerging biomarkers. Synaptosomal-associated protein 25 (SNAP-25) and visinin-like protein 1 (VILIP-1) were measured (second freeze/thaw) using Single Molecule Counting [SMC]™ technology (originally developed for the Singulex Erenna® System, now part of EMD Millipore [Burlington, MA]) employing antibodies developed in the laboratory of Dr. Jack Ladenson at WUSTL5. NfL (UmanDiagnostics, Umeå, Sweden) and chitinase-3-like protein 1 (YKL-40, Quidel, San Diego, CA) were measured (third freeze/thaw) via commercial enzyme-linked immunosorbent assays (ELISA) according to manufacturer instructions. Kit controls and/or pooled CSF samples were included to ascertain data reproducibility for defining quality control (QC) criteria (e.g., assay-specific cut-offs for percent coefficients of variation (%CV)).
Statistical Analysis
Normality assumption of the continuous variables were examined in each group using normal quantile-quantile (Q-Q) plots. All continuous variables were approximately normally distributed, except NfL which was right skewed and was log transformed. Demographic group differences between normal controls (DIAN-NC), DIAN-MC, and adults with DS were compared using one-way ANOVA F test for continuous variables and Chi-square tests for categorical variables. If significant, post-hoc pairwise comparisons were performed using two sample t test for continuous variables and Chi-square test or Fisher’s exact test (as appropriate) for categorical variables. Linear regression compared mean biomarker levels among the genetic and cognitive groups (DIAN-NC, aDS, sDS, DIAN-aMC, DIAN-sMC) and included age, APOE ε4 status (ε4+ or ε4-), sex (since advanced age, APOE ε4-positivity and female sex are known AD risk factors), and their interactions with group as covariates. Interactions between APOE and group and between sex and group were not significant for any biomarkers so were excluded from the final models. Linear regressions were used to compare the biomarker slopes of the three groups (DS, DIAN-NC, DIAN-MC). Each linear regression included one biomarker as the outcome, group, age, APOE ε4 status and the interaction between age and group as the predictors. To account for the potential correlation in participants from the same family in DIAN, sensitivity analyses were performed using linear mixed effects models with a random intercept for each family cluster. There was no family cluster in DS, so each participant was treated as a family cluster. As all the participants in the DS group were white, race was not included as a covariate in the linear regressions, but we performed sensitivity analyses based on a subset of white participants. For all analyses p values for pre-specified subgroup comparisons were adjusted by the Benjamini-Hochberg method14. Pre-specified subgroup comparisons (as shown in Supplemental Tables 3 and 4) were determined based on specified research questions. For each linear regression, participants with missing biomarker data were omitted from the model. Analyses utilized R (v3.6.2) and SAS (v9.4).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. AMF, RLH, YL, AHB, and CX had access to all the data in the study, and AMF had final responsibility for the decision to submit for publication.
RESULTS
Demographics
Demographic data (41 DS, 108 DIAN-NC and 192 DIAN-MC) are reported in Table 1 and Supplemental Table 1. Karyotyping in DS revealed 33 individuals (80%) with trisomy 21, two with mosaicism, and two with translocation. Four individuals were missing karyotype information at the time of analysis. See Supplemental text for additional details regarding these participants. The majority of DIAN participants were from PSEN1 families (68% DIAN-NC, 75% DIAN-MC), 14% DIAN-NC and 10% DIAN-MC from PSEN2 families and 18% DIAN-NC and 15% DIAN-MC from APP families. Specific participant genotypes are shown in Supplemental Table 2. Although age ranges were identical among the groups by design (30–61 years), the mean±SD age of DS (48·7±7·3 years) was greater than DIAN-NC (41·7±8·8) and DIAN-MC (41·3±8·3) groups. The DS group had a larger percentage of males (63%) than the two ADAD groups (~45%), although this did not reach statistical significance. Despite each group being predominantly white (>88%), the DIAN-MC group contained a larger percentage of non-white participants than the other two groups. Removal of non-white participants did not change the overall outcome of any analyses. APOE ε4 status (~35%) was not different among the groups. Although three (of 108) DIAN-NC were CDR 0.5, all were classified asymptomatic since they were CDR 0 at follow-up. 34% of DS (53·2±4·5 years, range 45–61) and 43% of DIAN-MC (45·6±8·2, range 30–61) groups were symptomatic. Of the 14 sDS, 50% were classified as MCI. Of the 82 DIAN-sMC, 67% had very mild dementia (CDR 0·5, similar to level of impairment in MCI).
Table 1:
Demographic characteristics and CSF biomarker concentrations
| DIAN non-carriers group (n=108) | Down syndrome group (n=41) | DIAN mutation carriers group (n=192) | p value | |
|---|---|---|---|---|
| Age, years | 41·7 (8·8) | 48·7 (7·3)*† | 41·3 (8·3) | <0.0010 |
| Sex | ||||
| Female | 60 (56%) | 15 (37%) | 103 (54%) | |
| Male | 48 (44%) | 26 (63%) | 89 (46%) | 0.098 |
| APOE ε4-positive | 38 (35%) | 16 (39%) | 62 (32%) | 0.68 |
| Race | .. | .. | .. | 0.016 |
| White | 100 (93%) | 41 (100%) | 169 (88%) | .. |
| Non-white | 6 (6%) | 0 | 23 (12%) | .. |
| Unknown | 2 (2%) | 0 | 0 | .. |
| Cognitive status | .. | .. | .. | <0.0010 |
| Asymptomatic | 108 (100%) | 27 (66%) | 110 (57%) | .. |
| Symptomatic | 0 | 14 (34%) | 82 (43%) | .. |
| Clinical dementia rating | ||||
| 0 | 105 (97%) | NA | 110 (57%) | .. |
| 0·5 | 3 (3%) | NA | 55 (29%) | .. |
| 1 | 0 | NA | 20 (10%) | .. |
| 2 | 0 | NA | 5 (3%) | .. |
| 3 | 0 | NA | 2 (1%) | .. |
| CSF biomarkers, n | ||||
| Aβ1–40, pg/mL | 9128 (2845) | 13612 (3892)*† | 8698 (2810) | <0·0010 |
| Aβ1–42, pg/mL | 817 (285) | 877 (287)† | 535 (286)* | <0·0010 |
| Total tau, pg/mL | 262 (113) | 644 (382), n=39* | 554 (362), n=l88* | <0·0010 |
| p-taul8l, pg/mL | 30 (13), n=106 | 93 (77)* | 90 (70), n=l89* | <0·0010 |
| Aβ1–42 to Aβ1–40 ratio | 0·09 (0·01) | 0·07 (0·02)* | 0·06 (0·03)* | <0·0010 |
| Total tau to Aβ1–40 ratio | 0·35 (0·20) | 0·84 (0·62), n=39*† | 1·44 (1·27), n=l88* | <0·0010 |
| p-tau181 to Aβ1–42 ratio | 0·04 (0·02), n=106 | 0·13 (0·14)*† | 0·24 (0·24), n=l89* | <0·0010 |
| VILIP-1, pg/mL | 139 (55), n=82 | 202 (92)* | 184 (83), n=146* | <0·0010 |
| SNAP25, pg/mL | 3·9 (1·5), n=82 | 4·6 (1·8)‡ | 4·9 (2·0), n=145* | <0·0010 |
| YKL-40, ng/mL | 150 (71), n=82 | 251 (127), n=38*† | 187 (83), n=146* | <0·0010 |
| log NfL, pg/mL | 2·83 (0·19), n=64 | 3·24 (0·27)*† | 3·03 (0·32), n=109* | <0·0010 |
Data are mean (SD) or n (%). DIAN participants who had a clinical dementia rating score of 3 at the time of CSF collection are not shown in the table to maintain masking as to mutation status. Missing CSF data reflect samples that were not available at the time of DIAN data freeze analysis (non-carrier: n=26 [SNAP-25, VILIP-1, YKL-40] and n=44 NfL; mutation carriers: n=46 [SNAP-25, VILIP-1, YKL-40] and n=83 NfL), or did not pass quality control criteria (Down syndrome: n=2 tau, n=3 YKL-40; mutation carriers: n=4 tau, n=3 p-tau181, n=1 SNAP-25). Non-white race included Black or African-American, American Indian or Alaska native, Native Hawaiian or other Pacific Islander, and Asian. We cannot specify non-white race due to the small number of participants, which could lead to unmasking. Aβ=amyloid β. APOE=apolipoprotein E. NfL=neurofilament light chain. SNAP-25=synaptosomal-associated protein 25. VILIP-1=visinin-like protein 1. YKL-40=chitinase-3-like protein 1.
Significantly different (at least p<0·008) from non-carrier.
Significantly different (at least p<0·0008) from mutation carriers.
Non-significant trend (at least p<0·08) from non-carrier. Specific p values are shown in the appendix (p 3).
Group CSF biomarker profiles
In general, concentrations of the majority of biomarkers in DIAN-MC and DS differed from those in the DIAN-NC group (Table 1, Supplemental Table 1). DIAN-MC patterns were consistent with the presence of AD pathology, including reductions in Aβ42 and Aβ42/Aβ40 (both p<0·0001) (measure of amyloid), elevated pTau181 (p<0·0001) (measure of phosphorylated tau-related processes), increases in markers of neuronal/axonal injury (tTau, VILIP-1, NfL) (all p<0·0001) and presynaptic dysfunction (SNAP-25) (p=0·0001), and elevations in YKL-40 (marker of astrogliosis/neuroinflammation) (p=0·007) compared with the DIAN-NC group. The tau/Aβ42 and ptau181/Aβ42 ratios were also higher in DIAN-MC versus DIAN-NC (both p<0·0001).
Similar findings were observed in DS versus DIAN-NC for all biomarkers except Aβ40 and Aβ42. Unlike DIAN-MC, adults with DS had higher Aβ40 compared to DIAN-NC (p<0·0001), whereas Aβ42 levels were not different (p=0·4850). Interestingly, certain analytes differed between DS and DIAN-MC; Aβ40 (p<0·0001), Aβ42 (p<0·0001), YKL-40 (p=0·0002) and NfL (p=0·0002) were significantly higher in DS, whereas tTau/Aβ42 (p=0·0017) and pTau181/Aβ42 (p=0·0023) were lower, likely due to overall higher Aβ42 in DS. Exploratory analyses in the subset of APP DIAN-MC (n=29) revealed similar, but not identical, patterns compared with individuals with mutations in PSEN1 and PSEN2 (Supplemental text, Supplemental Table 3).
CSF biomarkers in asymptomatic versus symptomatic groups
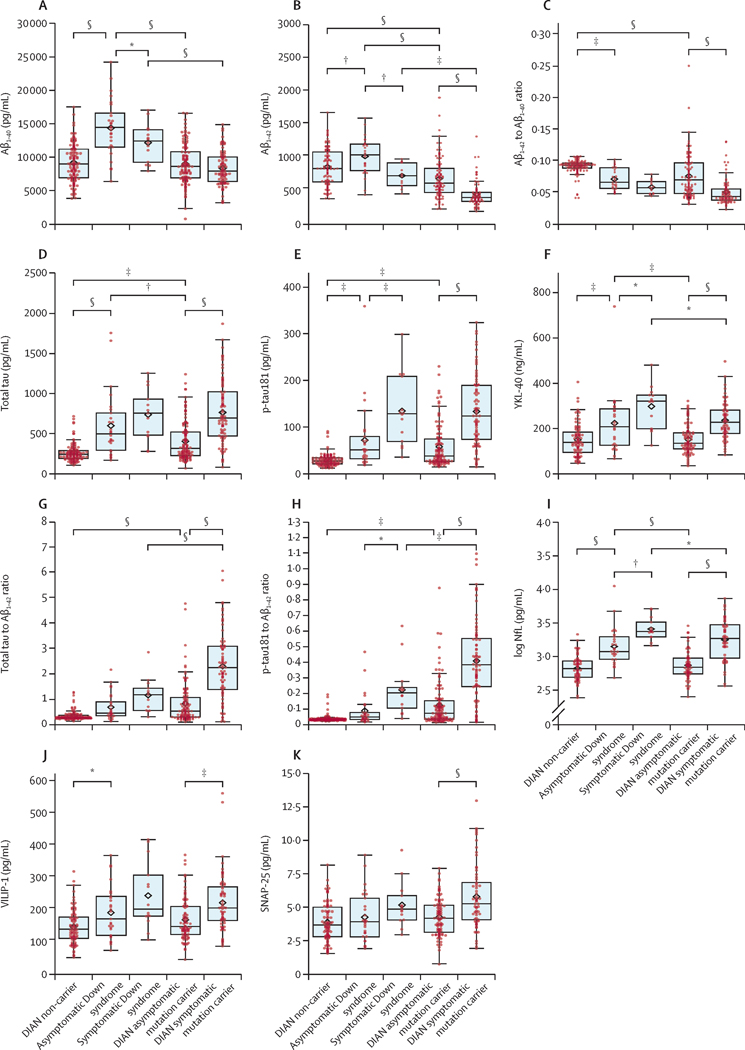
Since biomarker profiles are known to change with increasing disease severity, we next compared the groups as a function of dementia status, performing analyses separately for the DIAN-MC and DS groups. 66% (n=27) of DS were asymptomatic and 34% (n=14) were symptomatic. 57% (n=110) of DIAN-MC were asymptomatic and 43% (n=82) were symptomatic (Table 1). Although Aβ40 was overall higher in DS versus DIAN-MC (Table 1), levels did not differ with dementia status in DIAN-MC (p=0.14) and barely reached significance in DS (p=0·04)(Figure 1A). In contrast, Aβ42 levels were lower in symptomatic individuals in both groups (DS, p=0·001; DIAN-MC, p<0·0001) (Figure 1B), as was the Aβ42/Aβ40 ratio in DIAN-MC (p<0·0001) but not the DS group (p=0·14)(Figure 1C). pTau181 was strikingly higher in symptomatic individuals of both groups (DS, p=0·0004; DIAN-MC, p<0·0001)(Figure 1E). Interestingly, while tTau was higher in DIAN-sMC versus DIAN-aMC (p<0·0001), it was not elevated in sDS versus aDS (p=0·17). This muted symptom-related elevation in tTau in DS (mean difference of 137 pg/mL in DS versus 358 pg/mL in DIAN-MC) likely reflects the high levels already apparent in those who were asymptomatic (aDS > DIAN-aMC, p=0·002; Figure 1D). However, the higher mean age of the DS group likely contributed to this effect since significance was lost after adjusting for age, APOE ε4 status and sex (Supplemental Table 4). While levels of YKL-40 were higher in symptomatic versus asymptomatic individuals in both groups (DS, p=0·01; DIAN-MC, p<0·0001), they were also higher overall in DS versus DIAN-MC (asymptomatic, p=0·0003; symptomatic, p=0·01)(Figure 1F), although statistical significance was lost after adjusting for covariates (Supplemental Table 4). DS biomarker levels as a function of karyotype are shown in Supplemental Figure 1. Small numbers of non-trisomy 21 cases precluded statistical analysis.
Figure 1.
CSF biomarkers of amyloid, tau and phosphorylated tau-related processes, astrogliosis/neuroinflammation, and neuronal, synaptic and axonal injury in adults with Down syndrome, carriers of ADAD mutations, and age-similar normal non-carrier controls. Biomarkers include: A) Aβ40, B) Aβ42, C) Aβ42/Aβ40 ratio, D) tTau, E) pTau181, F) YKL-40, G) tTau/Aβ42 ratio, H) pTau181/Aβ42 ratio, I) log transformed NfL, J) VILIP-1, and K) SNAP-25. The central horizontal bar shows the median concentration, and the lower and upper boundaries show the 25th and 75th percentiles, respectively. The diamond symbol indicates the group mean. Groups include (left to right) ADAD mutation non-carriers (DIAN-NC), asymptomatic DS (aDS), symptomatic DS (sDS), asymptomatic ADAD mutation carriers (DIAN-aMC), and symptomatic ADAD mutation carriers (DIAN-sMC). Units are pg/mL for Aβ40, Aβ42, tTau, and pTau181 and ng/mL for YKL-40. Summary statistical differences among the groups are indicated by asterisks (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Absolute mean differences and associated p values are shown in Supplemental Table 3. Abbreviations: Aβ=amyloid-β; ADAD=autosomal dominant Alzheimer disease; CSF=cerebrospinal fluid; DIAN=Dominantly Inherited Alzheimer Network; DS=Down syndrome; MC=ADAD mutation carriers; NC=ADAD mutation non-carriers; NfL= neurofilament light chain; pTau=pTau181; SNAP-25=synaptosomal-associated protein 25; tTau=total tau; VILIP-1=visinin-like protein 1; and YKL40=chitinase-3-like protein 1.
Overall the tTau/Aβ42 and pTau181/Aβ42 ratios were higher in symptomatic versus asymptomatic groups (Figure 1G, H), but tTau/Aβ42 in DS did not reach statistical significance (DS: tTau/Aβ42 p=0·11, pTau181/Aβ42 p=0·010; DIAN-MC: tTau/Aβ42 and pTau181/Aβ42, both p<0·0001). NfL was higher in symptomatic versus asymptomatic groups (DS, p=0·001; DIAN-MC, p<0·0001)(Figure 1I), with elevations already apparent in aDS (aDS vs DIAN-aMC, p<0·0001), although significance was lost after adjusting for covariates (Supplemental Table 4). Symptomatic DIAN-MC exhibited elevations in VILIP-1 (p=0·0001; Figure 1F) and SNAP-25 (p<0·0001; Figure 1K) compared to DIAN-aMC, whereas differences did not reach statistical significance in DS (VILIP-1 p=0·06; SNAP-25 p=0·30).
CSF biomarkers profiles as a function of age
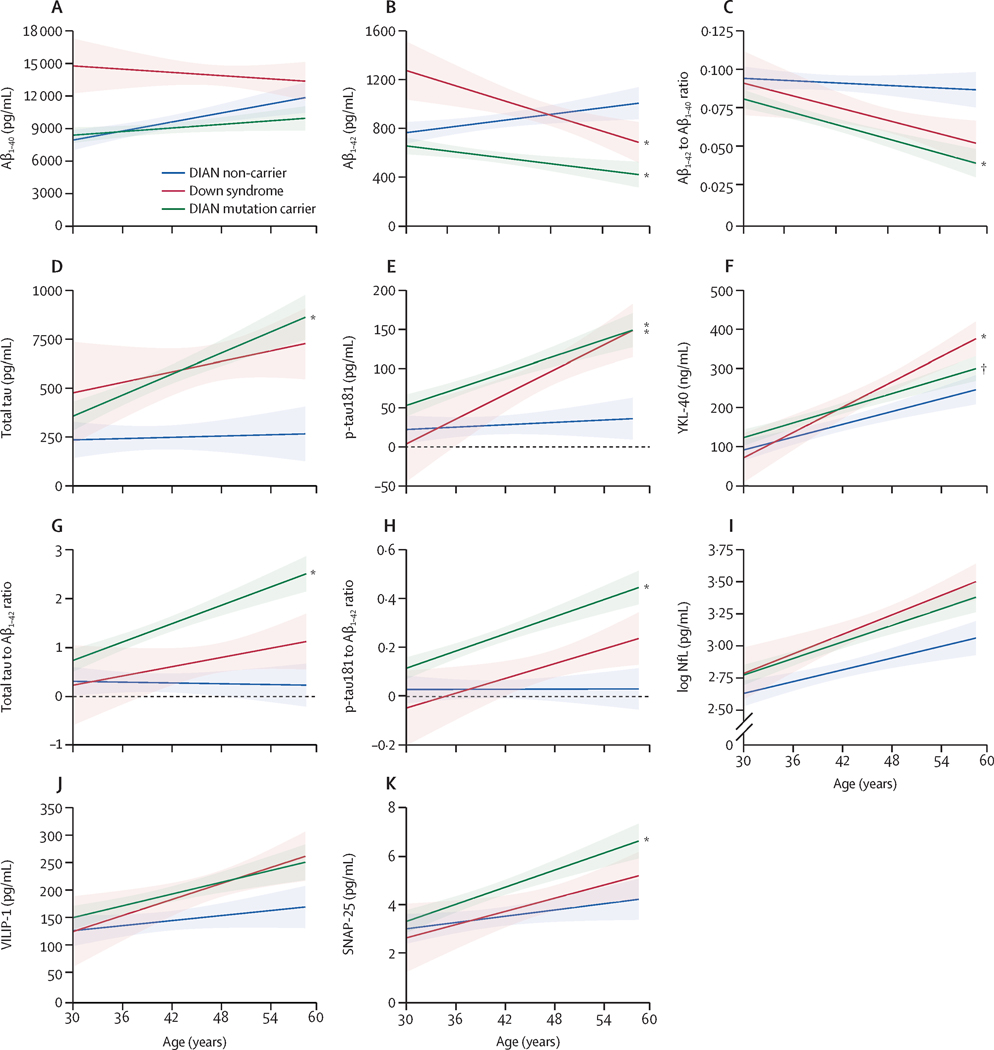
We next modeled biomarker patterns over the course of disease progression by comparing levels as a function of age. Although MCs in families with different ADAD mutations develop dementia at different ages, disease pathology increases with advancing age4, as it does in DS8. Slope comparisons demonstrated decreases in Aβ42 (p=0·0001) and Aβ42/40 (p=0·0030)(Figure 2B, C) and increases in tTau (p=0·0006)(Figure 2D), pTau181 (p=0·002)(Figure 2E), tTau/Aβ42 and pTau181/Aβ42 ratios (both p<0·0001)(Figure 2G, H), and SNAP-25 (p=0·03)(Figure 2K) in the DIAN-MC versus DIAN-NC groups with advancing age, consistent with pathologic disease progression over time. Although markers of astrogliosis/neuroinflammation (YKL-40, Figure 2F) and neuronal/synaptic injury (NfL, Figure 2I; VILIP-1, Figure 2J) in DIAN-MC also increased with age, their slopes were not different from DIAN-NC controls.
Figure 2.
CSF biomarkers of amyloid, tau and phosphorylated tau-related processes, astrogliosis/neuroinflammation, and neuronal, synaptic and axonal injury in adults with Down syndrome (orange), ADAD mutation carriers (green) and normal ADAD non-carrier controls (gray) as a function of age (increasing age left to right). Biomarkers include: A) Aβ40, B) Aβ42, C) Aβ42/Aβ40 ratio, D) tTau, E) pTau181, F) YKL-40, G) tTau/Aβ42 ratio, H) pTau181/Aβ42 ratio, I) log transformed NfL, J) VILIP-1, and K) SNAP-25. Actual age is not shown on the X axis in order to maintain blinding. Colored lines reflect the regression lines and 95% confidence intervals (CI) based on the linear regression, with each biomarker as the outcome and APOE ε4 status, sex and the group x age interaction as the covariates. Pair-wise comparisons of the change in biomarkers over age (slopes of the linear lines) controlling for APOE ε4 status and sex was performed based on the linear regressions, and p values for pre-specified subgroup comparisons were adjusted by the Benjamini-Hochberg method14. Significant (at least p<0.05) summary group differences in slope (colored asterisks, DS/DIAN-MC vs DIAN-NC; black dagger, DS vs DIAN-MC) were observed for the following biomarkers: Aβ42, DS vs DIAN-NC and DIAN-MC vs DIAN-NC; Aβ42/Aβ40, DIAN-MC vs DIAN-NC; tTau, DIAN-MC vs DIAN-NC; pTau181, DIAN-MC vs DIAN-NC and DS vs DIAN-NC; YKL-40, DS vs DIAN-NC and DS vs DIAN-MC; tTau/Aβ42, DIAN-MC vs DIAN-NC; pTau181/Aβ42, DIAN-MC vs DIAN-NC; SNAP-25, DIAN-MC vs DIAN-NC). Absolute mean differences in annualized slopes and associated p values are shown in Supplemental Table 5. Abbreviations: Aβ=amyloid-β; ADAD=autosomal dominant Alzheimer disease; CSF=cerebrospinal fluid; DIAN=Dominantly Inherited Alzheimer Network; DS=Down syndrome; MC=ADAD mutation carriers; NC=ADAD mutation non-carriers; NfL, neurofilament light chain; SNAP-25=synaptosomal-associated protein 25; tTau=total tau; VILIP-1=visinin-like protein 1; YKL-40=chitinase-3-like protein 1.
Age-related biomarker patterns in DS and DIAN-MC were remarkably similar (Figure 2; Supplemental Table 5), although fewer markers in DS significantly differed from the DIAN-NC group, and some differences between the two genetic groups were observed. DS exhibited overall higher levels of Aβ40 than the other two groups across age (Figure 2A), with decreases trending compared to DIAN-NC (p=0·05). Despite similar slopes for Aβ42 in DS and DIAN-MC versus DIAN-NC (both p<0·0001, although with a slightly greater association with age in DS [trending p<0·08]), like Aβ40, overall levels were higher in DS (Figure 2B). Robust increases in pTau181 with age were observed in both genetic groups versus DIAN-NC controls (DS, p=0·003; DIAN-MC, p=0·002)(Figure 2E), but the tTau slope in DS did not differ significantly from DIAN-NC despite overall higher levels (Figure 2D). The tau(s)/Aβ42 slopes in DS were not different from DIAN-NC (tTau/Aβ42, p=0·20; pTau181/Aβ42 trending p=0·05)(Figure 2G, H). YKL-40 increased in all groups with age, but slopes were greater in DS versus DIAN-NC (p=0·02) and DIAN-MC (p=0·03)(Figure 2F). NfL and VILIP-1 (Figure 2I, J) increased in DS but, similar to DIAN-MC, their slopes were not different from DIAN-NC controls. In contrast to DIAN-MC, the pattern of SNAP-25 in DS did not differ from DIAN-NC (Figure 2K). P values for pairwise comparisons are shown in Supplemental Table 5. DS biomarker patterns in the karyotype groups are shown in Supplemental Figures 2 and 3.
DISCUSSION
Despite the differences in underlying etiologies for AD development in the two at-risk genetic cohorts, adults with DS exhibited CSF biomarker changes remarkably similar to carriers of ADAD mutations, and both consistent with expected accrual of AD pathology with advancing age. Profiles included reductions in Aβ42/Aβ40 and increases in markers of phosphorylated tau-related processes, neuronal/axonal/synaptic injury, and astrogliosis/neuroinflammation, with typically greater degrees of abnormality in the presence of dementia, as has been described in LOAD3,15 and ADAD5,16. CSF biomarkers have been reported in this10 and other2,7,17 DS cohorts but, to our knowledge, no direct comparisons have been made between individuals with DS and ADAD. Interestingly, despite many similarities, we also observed some variations that may shed light on potential differences in Aβ metabolism, neuronal injury, and astrogliosis/neuroinflammation specifically in the setting of trisomy 21.
Elevations in CSF Aβ40 and Aβ42 in DS likely reflect the triplication of the APP gene, resulting in global increases in total APP, whereas increased Aβ42 in ADAD is typically the result of altered secretase activity18,19. The exception would be rare mutations that result in the duplication of APP. While their rarity in the current study allows for only preliminary conclusions to be drawn (29 DIAN-MCs were from families with APP mutations, only four of whom had APP duplications) (Supplemental Text), a direct comparison in a future larger cohort will be very informative. Comparability of the Aβ42/Aβ40 ratio (Table 1, Figure 2C) suggests similar timing of Aβ aggregation with respect to disease pathogenesis and progression in the two groups, a finding that may inform the timing of experimental interventions aimed at preventing dementia onset through reductions in amyloid3. Comparison of CSF biomarker profiles with amyloid PET is currently underway and will inform possible CSF diagnostic cut-off values in DS that could be used to define amyloid status for clinical trials. Whether measures of plasma Aβ20 have utility as a more non-invasive biomarker of amyloid pathology in DS is also of great interest and remains to be determined.
Interestingly, in contrast to ADAD, levels of tTau and NfL in DS were already elevated (compared to DIAN-NC) in the asymptomatic stage. Although the older age of the aDS group may have contributed to this finding (both biomarkers are known to increase with age21,22, such a pattern may reflect or be influenced by the biological and neurodevelopmental differences associated with DS. Autopsy and antemortem imaging studies in DS have described reduced brain size, lower numbers and depth of cerebral sulci, enlarged ventricles, and hypoplasia of several brain regions in comparison to typically developing individuals of similar ages23. With advancing age, additional volume reductions are observed in regions known to develop neurofibrillary tangle pathology24. While the tTau and NfL patterns are consistent with such changes, direct comparisons of these fluid measures with structural and molecular imaging are required to fully understand their etiologies, as well as whether there are relationships with the level of pre-existing intellectual disability. These topics are the focus of future studies. Recent studies have reported elevations in plasma NfL in DS2,17,25, paralleling increases in CSF NfL. Despite potential age-related differences in patterns of tTau and NfL between the DS and DIAN-MC groups, pTau181 patterns are virtually identical (Table 1, Figures 1 and 2), suggesting similar pathophysiological processes involved in tau hyperphosphorylation and/or aggregation. The reason why tangle pathology is greater in both DS and ADAD compared to LOAD26,27 remains to be determined, but may be a consequence of elevated levels of brain Aβ42 (since birth) in the genetic groups compared with those who develop LOAD. Data from human neuroimaging28 and mouse models support a role of amyloid in fostering an environment favorable for the development of tau pathology29.
Inflammation is a key process in DS, likely because chromosome 21 contains several pro- and anti-inflammatory genes. DS brains display an inflammatory phenotype different from LOAD30, and elevations in plasma inflammatory markers have been reported in DS(31). In the current study, higher overall levels of CSF YKL-40 in DS and more rapid elevations with age are consistent with a systemic and dysregulated inflammatory process, although age-related differences in the cohorts may also contribute to this observation. A study of a relatively small cohort (n=12 DS, n=20 controls) reported no difference in YKL-40 between the age-matched (~40 years old) groups, but increases with age in both groups, with significantly higher levels in older (>40 years) adults with DS32, consistent with our results (Figure 2F). Known correlations between CSF YKL-40 and t-Tau may also contribute3,5,10.
Given the ease, availability and relative non-invasiveness of venipuncture compared to lumbar puncture in individuals with DS, it is likely that plasma/serum biomarkers – once fully validated - will be the most feasible modality for clinical trial screening and potential clinical care in this at-risk population. Recent development of reliable, highly sensitive assays for blood-based markers has enabled their evaluation in different AD cohorts33–37 including DS 2,17,31,38,39. Future comparisons of the plasma profiles among these groups will be informative as the field moves closer to bringing biomarkers to the clinic.
The major strength of the study is the comparison of CSF biomarkers in two of the most relevant cohorts of individuals with genetically determined forms of AD. However, the study also has limitations. The number of DS participants with CSF (n=41), while larger than most, is still relatively small, limiting statistical power, especially in regards to possible false-negatives. This cohort is also heterogeneous with regard to karyotype and racial characteristics, although no obvious differences were noted in sub-analyses (Supplemental Figures 1–3). Despite selecting DIAN participants based on the age range of the ABC-DS cohort, the mean ages turned out to be different. Not all samples had data for all biomarkers, and ABC-DS longitudinal data were not available. As in all DS studies, there is the inherent challenge of determining dementia in the presence of existing intellectual disability. In addition, although the development of AD pathology and risk of dementia increases with advancing age in both genetic groups, ADAD mutation carriers typically develop dementia at different ages. The DIAN metric of estimated years to symptom onset (EYO) permits assigning an individual a place along the disease trajectory without regard to chronological age, thus enabling stage-similar comparisons between individuals with different mutations4. No such metric yet exists for adults with DS given the variability in symptom onset and presentation, thus impeding the ability to make pathologic stage-specific comparisons between the genetic groups. Longitudinal assessment of CSF biomarkers in ABC-DS participants as they progress from asymptomatic (cognitively stable) to symptomatic (dementia) stages will be very informative.
In conclusion, CSF biomarker patterns have many similarities in DS and ADAD, thus reflecting a common pathway in AD pathophysiology independent of the underlying initial genetic etiology. This finding supports their potential utility for the detection and tracking of AD-related processes and suggests that treatments effective in one population may have utility in the other. Such knowledge may inform clinical trial design in these understudied at-risk groups. However, the overall higher levels of Aβ and potential preclinical (presymptomatic) elevations in markers of neuronal injury (total tau) and astrogliosis/neuroinflammation (YKL-40) in DS highlight the inherent metabolic differences that should be considered when defining CSF cut-off values for identification of underlying AD pathologies currently being utilized in LOAD for trial enrollment and evaluation of target engagement and/or biomarker outcomes.
Supplementary Material
RESEARCH IN CONTEXT.
1. Evidence before this study:
All relevant articles on PubMed relating to cerebrospinal (CSF) biomarkers of Alzheimer disease (AD) in individuals with Down syndrome (DS) or autosomal dominant AD (ADAD) were regularly searched (May 2019-October 2020) for consideration of inclusion in this report. Search terms included: Alzheimer disease, autosomal dominant Alzheimer disease, biomarker, brain, cerebrospinal fluid, Down syndrome. Few studies evaluating fluid biomarkers of AD in individuals with DS have been published, whereas the biomarker profiles of individuals from families with ADAD enrolled in the Dominantly Inherited Alzheimer Network (DIAN) study have been well-characterized. However, we found no direct comparisons of biomarker profiles between individuals with DS and those from families with known ADAD mutations, the two genetically determined at-risk groups for developing AD.
2. Added value of the study:
To our knowledge, this is the first study directly comparing CSF biomarkers of AD between individuals with ADAD and adults with DS. There are significant similarities in the profile of CSF biomarkers in adults with DS compared to those with ADAD. However, variations in some markers may shed light on potential differences in Aβ metabolism, neuronal injury, and astrogliosis/neuroinflammation in the setting of DS.
3. Implications of all the available evidence:
Our results support the utility of CSF biomarker profiles for identifying and tracking AD-related processes in DS and, as such, will likely be useful for clinical trial design in this understudied at-risk population. However, the overall higher levels of Aβ and potential preclinical (presymptomatic) elevations in markers of neuronal injury and astrogliosis/neuroinflammation in DS highlight the inherent metabolic differences in the setting of trisomy 21. These differences should be considered when defining CSF cut-off values for identification of underlying AD pathologies that may be required for clinical trial enrollment and evaluation of target engagement and/or biomarker outcomes. In-depth investigation of longitudinal change in biomarkers across the disease spectrum in cohorts of adults with DS is still needed to fully characterize the biomarker profiles and the appropriate age(s) and time for intervention.
ACKNOWLEDGMENTS
Data collection and sharing for this project was supported by the Alzheimer’s Disease Biomarker Consortium on Down Syndrome (ABC-DS) funded by the National Institute on Aging (NIA) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U01 AG051406 and U01 AG051412). The authors thank the adults with Down syndrome volunteering as participants in this study for their invaluable contributions to this work, along with their service providers and families.
Data collection and sharing for this project was also supported by the Dominantly Inherited Alzheimer Network (DIAN, UF1AG032438) funded by the NIA, the German Center for Neurodegenerative Diseases (DZNE), and partial support by the Research and Development Grants for Dementia from Japan Agency for Medical Research and Development (AMED). This manuscript has been reviewed by DIAN Study investigators for scientific content and consistency of data interpretation with previous DIAN Study publications. The authors acknowledge the altruism of the participants and their families and contributions of the DIAN research and support staff at each of the participating sites for their contributions to this study, with notable support from Elizabeth Herries, Eric McDade and Julie Wisch (WUSTL), Elizabeth Head (UCI) and Courtney Jordan and Nusrat Jahan (MGH).
DECLARATION OF INTEREST
AMF has received research funding from the National Institutes of Health /National Institute on Aging, Biogen, Centene, Fujirebio, and Roche Diagnostics. She is a member of the scientific advisory boards for Roche Diagnostics, Genentech, and AbbVie and also consults for Araclon/Grifols, DiademRes, DiamiR, and Otsuka Pharmaceuticals. There are no conflicts.
RJB has equity ownership interest in C2N Diagnostics and receives royalty income based on technology (stable isotope labeling kinetics and blood plasma assay) licensed by Washington University to C2N Diagnostics. He receives income from C2N Diagnostics for serving on the scientific advisory board. Washington University, with RJB as co-inventor, has submitted the US nonprovisional patent application “Cerebrospinal fluid (CSF) tau rate of phosphorylation measurement to define stages of Alzheimer’s disease and monitor brain kinases/phosphatases activity.” He has received honoraria from Janssen and Pfizer as a speaker, and from Merck and Pfizer as an advisory board member. He has been an invited speaker, advisory board member, and consultant for F. Hoffman La Roche, Ltd., an invited speaker and consultant for AC Immune and Janssen, and a consultant for Amgen and Eisai. There are no conflicts.
AMG has consulted for Eisai, Biogen, Pfizer, AbbVie, Cognition Therapeutics, and GSK. She also served on the Scientific Advisory Board of Denali Therapeutics (2015–2018). There are no conflicts.
BJH has received research funding from Roche Pharmaceuticals and Autism Speaks. There are no conflicts.
JPC has served on a medical advisory board for Otsuka Pharmaceuticals. There are no conflicts.
GSD is supported by National Institutes of Health /National Institute on Aging (K23AG064029). He serves as a topic editor on dementia for DynaMed Plus (EBSCO Industries, Inc), a consultant for Parabon NanoLabs, is the clinical director for the Anti-NMDA Receptor Encephalitis Foundation (uncompensated), has provided record review and expert medical testimony on legal cases pertaining to management of Wernicke encephalopathy, and holds stocks (>$10,000) in ANI Pharmaceuticals (a generic pharmaceutical company). There are no conflicts.
NRGR takes part in multicenter trials support by AbbVie, Eli Lilly, Biogen. There are no conflicts.
JL reports speaker fees from Bayer Vital and Roche, consulting fees from Axon Neuroscience and Ionis Pharmaceuticals, author fees from Thieme medical publishers and W. Kohlhammer GmbH medical publishers, non-financial support from Abbvie, and compensation for duty as part-time CMO from MODAG, outside the submitted work. There are no conflicts.
CJM has been a member of advisory scientific board for Biogen, IONIS, Wave, and Roche and consulted for Eisai. There are no conflicts.
RNM has received funding from the US Alzheimer’s Foundation to undertake an intervention trial for the prevention of Alzheimer’s. He is a member of the scientific advisory board for Eisai. There are no conflicts.
SS reports consulting to Eisai, Novartis, Genentech, F. Hoffmann-La Roche, Ltd, Gemvax, Avid Radiopharmaceuticals and Eli Lilly and Company. There are no conflicts.
YL, RLH, AHB, CX, BMA, ED, BTC, FL, HDR, NS, WS, JHL, WEK, , SKM, RFA, MJ, CLM, YN, JMR, PRS, MS, ITL report no conflicts.
Footnotes
DATA SHARING
De-identified, individual participant-level data that underlie the results reported in this Article (and associated data dictionaries) are available upon request to the respective studies (ABC-DS (https://pitt.co1.qualtrics.com/jfe/form/SV_cu0pNCZZlrdSxUN) and DIAN (https://dian.wustl.edu/our-research/for-investigators/dian-observational-study-investigator-resources/) and approval by the separate steering committees. Requests must detail the study hypothesis and include a statistical analysis plan. Committee review will take into consideration the merit, feasibility and scientific rigor of the proposed study. Study protocols and informed consent forms can also be requested. All applicants must sign a data use agreement that includes statements regarding sharing of data to a third party.
REFERENCES
- 1.Mann DM. Alzheimer’s disease and Down’s syndrome. Histopathology. 1988. Aug; 13(2):125–37. [DOI] [PubMed] [Google Scholar]
- 2.Fortea J, Vilaplana E, Carmona-Iragui M, Benejam B, Videla L, Barroeta I, et al. Clinical and biomarker changes of Alzheimer’s disease in adults with Down syndrome: a cross-sectional study. Lancet. 2020. 27;395(10242):1988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molinuevo JL, Ayton S, Batrla R, Bednar MM, Bittner T, Cummings J, et al. Current state of Alzheimer’s fluid biomarkers. Acta Neuropathol. 2018. December 1;136(6):821–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryman DC, Acosta-Baena N, Aisen PS, Bird T, Danek A, Fox NC, et al. Symptom onset in autosomal dominant Alzheimer disease. Neurology. 2014. July 15;83(3):253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schindler SE, Li Y, Todd KW, Herries EM, Henson RL, Gray JD, et al. Emerging cerebrospinal fluid biomarkers in autosomal dominant Alzheimer’s disease. Alzheimers Dement. 2019. 15:655–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartley D, Blumenthal T, Carrillo M, DiPaolo G, Esralew L, Gardiner K, et al. Down syndrome and Alzheimer’s disease: Common pathways, common goals. Alzheimers Dement J Alzheimers Assoc. 2015. Jun;11(6):700–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dekker AD, Fortea J, Blesa R, De Deyn PP. Cerebrospinal fluid biomarkers for Alzheimer’s disease in Down syndrome. Alzheimers Dement Diagn Assess Dis Monit. 2017. Mar 20;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lott IT, Head E. Dementia in Down syndrome: unique insights for Alzheimer disease research. Nat Rev Neurol. 2019. Mar;15(3):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Handen BL, Lott IT, Christian BT, Schupf N, OBryant S, Mapstone M, et al. The Alzheimer’s Biomarker Consortium-Down Syndrome: Rationale and methodology. Alzheimers Dement Amst Neth. 2020;12(1):e12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henson RL, Doran E, Christian BT, Handen BL, Klunk WE, Lai F, et al. Cerebrospinal fluid biomarkers of Alzheimer’s disease in a cohort of adults with Down syndrome. Alzheimers Dement. 2020. Jul 9;12(1):e12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris JC, Aisen PS, Bateman RJ, Benzinger TLS, Cairns NJ, Fagan AM, et al. Developing an international network for Alzheimer research: The Dominantly Inherited Alzheimer Network. Clin Investig. 2012. Oct 1;2(10):975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Natté R, Maat-Schieman ML, Haan J, Bornebroek M, Roos RA, van Duinen SG. Dementia in hereditary cerebral hemorrhage with amyloidosis-Dutch type is associated with cerebral amyloid angiopathy but is independent of plaques and neurofibrillary tangles. Ann Neurol. 2001. December;50(6):765–72. [DOI] [PubMed] [Google Scholar]
- 13.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993. November;43(11):2412–4. [DOI] [PubMed] [Google Scholar]
- 14.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. [Google Scholar]
- 15.Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 2016. June 1;15(7):673–84. [DOI] [PubMed] [Google Scholar]
- 16.Fuller JT, Cronin-Golomb A, Gatchel JR, Norton DJ, Guzmán-Vélez E, Jacobs HIL, et al. Biological and Cognitive Markers of Presenilin1 E280A Autosomal Dominant Alzheimer’s Disease: A Comprehensive Review of the Colombian Kindred. J Prev Alzheimers Dis. 2019;6(2):112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fortea J, Carmona-Iragui M, Benejam B, Fernández S, Videla L, Barroeta I, et al. Plasma and CSF biomarkers for the diagnosis of Alzheimer’s disease in adults with Down syndrome: a cross-sectional study. Lancet Neurol. 2018. October 1;17(10):860–9. [DOI] [PubMed] [Google Scholar]
- 18.Karch CM, Goate AM. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry. 2015. January 1;77(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zis P, Strydom A. Clinical aspects and biomarkers of Alzheimer’s disease in Down syndrome. Free Radic Biol Med. 2018. January 1;114:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Sun Y, Li T, Cai Y, Han Y. Amyloid-β as a Blood Biomarker for Alzheimer’s Disease: A Review of Recent Literature. J Alzheimers Dis JAD. 2020;73(3):819–32. [DOI] [PubMed] [Google Scholar]
- 21.Mattsson N, Rosén E, Hansson O, Andreasen N, Parnetti L, Jonsson M, et al. Age and diagnostic performance of Alzheimer disease CSF biomarkers. Neurology. 2012. February 14;78(7):468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE, and the NFL Group, et al. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol. 2019. June 17; [DOI] [PMC free article] [PubMed]
- 23.Head E, Lott IT, Wilcock DM, Lemere CA. Aging in Down syndrome and the Development of Alzheimer’s disease Neuropathology. Curr Alzheimer Res. 2016;13(1):18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadowski M, Wisniewski HM, Tarnawski M, Kozlowski PB, Lach B, Wegiel J. Entorhinal cortex of aged subjects with Down’s syndrome shows severe neuronal loss caused by neurofibrillary pathology. Acta Neuropathol (Berl). 1999. February;97(2):156–64. [DOI] [PubMed] [Google Scholar]
- 25.Strydom A, Heslegrave A, Startin CM, Mok KY, Hardy J, Groet J, et al. Neurofilament light as a blood biomarker for neurodegeneration in Down syndrome. Alzheimers Res Ther. 2018. April 10;10(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson LD, Siddarth P, Kepe V, Scheibel KE, Huang SC, Barrio JR, et al. Positron emission tomography of brain β-amyloid and τ levels in adults with Down syndrome. Arch Neurol. 2011. June;68(6):768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon BA, Blazey TM, Christensen J, Dincer A, Flores S, Keefe S, et al. Tau PET in autosomal dominant Alzheimer’s disease: relationship with cognition, dementia and other biomarkers. Brain J Neurol. 2019. 01;142(4):1063–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, et al. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med. 2016. 11;8(338):338ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallardo G, Holtzman DM. Amyloid-β and Tau at the Crossroads of Alzheimer’s Disease. Adv Exp Med Biol. 2019;1184:187–203. [DOI] [PubMed] [Google Scholar]
- 30.Wilcock DM, Hurban J, Helman AM, Sudduth TL, McCarty KL, Beckett TL, et al. Down syndrome individuals with Alzheimer’s disease have a distinct neuroinflammatory phenotype compared to sporadic Alzheimer’s disease. Neurobiol Aging. 2015. September;36(9):2468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Startin CM, Ashton NJ, Hamburg S, Hithersay R, Wiseman FK, Mok KY, et al. Plasma biomarkers for amyloid, tau, and cytokines in Down syndrome and sporadic Alzheimer’s disease. Alzheimers Res Ther. 2019. March 21;11(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Portelius E, Soininen H, Andreasson U, Zetterberg H, Persson R, Karlsson G, et al. Exploring Alzheimer Molecular Pathology in Down’s Syndrome Cerebrospinal Fluid. Neurodegener Dis. 2014;14(2):98–106. [DOI] [PubMed] [Google Scholar]
- 33.Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association Between Longitudinal Plasma Neurofilament Light and Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol. 2019. July;76(7):791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020. May 1;19(5):422–33. [DOI] [PubMed] [Google Scholar]
- 35.Preische O, Schultz SA, Apel A, Kuhle J, Kaeser SA, Barro C, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat Med. 2019;25(2):277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quiroz YT, Zetterberg H, Reiman EM, Chen Y, Su Y, Fox-Fuller JT, et al. Plasma neurofilament light chain in the presenilin 1 E280A autosomal dominant Alzheimer’s disease kindred: a cross-sectional and longitudinal cohort study. Lancet Neurol. 2020. June 1;19(6):513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guzmán‐Vélez E, Zetterberg H, Fox‐Fuller JT, Vila‐Castelar C, Sanchez JS, Baena A, et al. Associations between plasma neurofilament light, in vivo brain pathology, and cognition in non-demented individuals with autosomal-dominant Alzheimer’s disease. Alzheimers Dement. 2021. Feb 10;1(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mengel D, Liu W, Glynn RJ, Selkoe DJ, Strydom A, Lai F, et al. Dynamics of plasma biomarkers in Down syndrome: the relative levels of Aβ42 decrease with age, whereas NT1 tau and NfL increase. Alzheimers Res Ther. 2020. Mar 19;12(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen M, Zhang F, Krinsky-McHale SJ, Silverman W, Lee JH, Pang D, et al. Proteomic profiles of prevalent mild cognitive impairment and Alzheimer’s disease among adults with Down syndrome. Alzheimers Dement (Amst). 2020. Jun; 12(1):e12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.