Abstract
Fluctuating asymmetry (FA), deviation from perfect bilateral symmetry, is thought to reflect an organism’s relative inability to maintain stable morphological development in the face of environmental and genetic stressors. Previous research has documented negative relationships between FA and attractiveness judgments in humans, but scant research has explored relationships between the human voice and this putative marker of genetic quality in either sex. Only one study (and in women only) has explored relationships between vocal attractiveness and asymmetry of the face, a feature-rich trait space central in prior work on human genetic quality and mate choice. We therefore examined this relationship in three studies comprising 231 men and 240 women from two Western samples as well as Hadza hunter-gatherers of Tanzania. Voice recordings were collected and rated for attractiveness, and FA was computed from two-dimensional facial images as well as, for a subset of men, three-dimensional facial scans. Through meta-analysis of our results and those of prior studies, we found a negative association between FA and vocal attractiveness that was highly robust and statistically significant whether we included effect sizes from previously published work, or only those from the present research, and regardless of the inclusion of any individual sample or method of assessing FA (e.g., facial or limb FA). Weighted mean correlations between FA and vocal attractiveness across studies were −.23 for men and −.29 for women. This research thus offers strong support for the hypothesis that voices provide cues to genetic quality in humans.
Keywords: 3D images, fluctuating asymmetry, geometric morphometrics, hunter-gatherers, mate choice, sexual selection
1.0 Introduction
Mate choice is predicated, in part, on heritable fitness benefits (Petrie, 1994), and organisms are attentive to biomarkers that putatively indicate whether a potential mate possesses genes that would contribute additively to offspring fitness (Andersson, 1994)—that is, whether a mate is high in genetic quality (Hunt, Bussiere, Jennions, & Brooks, 2004). One such biomarker is fluctuating asymmetry (FA; e.g., Møller, 1992; Swaddle, 1996; Thornhill & Gangestad, 1999a; Van Valen, 1962, 1973). FA is exhibited in bilaterally symmetrical traits if the difference between the left and right sides has a population normal distribution with a mean of zero (Palmer & Strobeck, 1986). Substantial evidence from humans (e.g., Gangestad, Thornhill, & Yeo, 1994; Livshits & Kobyliansky, 1991) as well as an array of vertebrates and insects (Parsons, 1990) indicates that the degree to which bilaterally symmetrical traits deviate from perfect symmetry reflects developmental instability resulting from harmful mutations and environmental perturbations. Experimental exposure to common environmental stressors, for example, can increase asymmetry in laboratory rats (e.g., Mooney, Siegel, & Gest, 1985; Sciulli, Doyle, Kelley, Siegel, & Siegel, 1979).
In humans, individuals with known genetic or chromosomal abnormalities have greater dental FA than do controls (e.g., Barden, 1980; Peretz et al., 1988), and FA displays a negative relationship with genetic heterozygosity (Livshits & Kobyliansky, 1991), which can increase fitness by lowering the risk of expressing deleterious recessive alleles and by increasing acquired immunity through the major histocompatibility complex (MHC; reviewed in Kempenaers, 2007). Lower FA in ear height, wrist breadth, and digit length has predicted reduced resting metabolic rate (Manning, Koukourakis, & Brodie, 1997) and better mental health (Martin, Manning, & Dowrick, 1999) in men, and lower facial FA shows a similar relationship with perceptions of physical health (Jones et al., 2001). Moreover, FA is positively correlated with female body mass, age, and age at first childbirth, all indications of reduced fecundity (Manning, Scutt, Whitehouse, & Leinster, 1997). A recent meta-analysis also found a small negative relationship between FA and general intelligence (Banks, Batchelor, & McDaniel, 2010). Together, these results suggest that bilateral symmetry may reflect the fit between an organism’s genotype and environment during development.
As a putative indicator of a genotype’s resilience to environmental insults, FA is related to mate choice across a variety of nonhuman species. For example, among field crickets (Gryllus campestris), males successful in obtaining mates display lower FA than do less successful males (Simmons, 1995). Likewise, female zebra finches (Taeniopygia guttata) prefer to be near males wearing symmetrically colored experimental leg bands (Swaddle, 1996).
In humans, several studies have reported significant correlations between FA and perceptions of facial attractiveness (e.g., Abend, Pflüger, Koppensteiner, Coquerelle, & Grammer, 2015; Grammer & Thornhill, 1994; Komori, Kawamura, & Ishihara, 2009; Perrett et al., 1999; Rhodes et al., 2001). In addition, women who view themselves as attractive show a greater preference for symmetry in male faces (Little, Burt, Penton-Voak, & Perrett, 2001), suggesting that symmetrical mates are valued and are more attainable by women of higher mate value. Some studies (e.g., Little & Jones, 2012; Little, Jones, Burt, & Perrett, 2007), but not others (e.g., Peters, Simmons, & Rhodes, 2009), have found that women in the fertile phase of the ovulatory cycle exhibit a greater preference for symmetrical faces and bodies, again suggesting an association between symmetry and genetic quality, since genetic benefits can be obtained only when conception is possible. There is also evidence that humans mate assortatively for facial symmetry, indicating that symmetry or correlated traits may influence mate-choice (Burriss, Roberts, Welling, Puts, & Little, 2011).
While bilateral symmetry appears to be important in predicting mating decisions, preferences for symmetrical mates may not always reflect preferences for symmetry per se. Bilateral symmetry has been found to predict attractiveness even in the absence of visual cues to asymmetry itself, and thus asymmetry must be related to other phenotypic cues. For example, women in the fertile phase of the ovulatory cycle have shown a preference for symmetrical men when exposed exclusively to the men’s body odor (Gangestad & Thornhill, 1998; Thornhill & Gangestad, 1999b). Another study found a female preference for low FA in men even when study participants were shown only the left or right half of each male face, preventing them from assessing symmetry directly (Scheib, Gangestad, & Thornhill, 1999).
Developmental stability may also be conveyed vocally. Hughes, Harrison, and Gallup (2002) found both sexes to exhibit a negative relationship between upper limb FA (particularly finger length FA) and attractiveness assessed from vocal stimuli alone. Abend et al. (2015) similarly obtained a negative relationship between women’s vocal attractiveness and measures of FA from their upper and lower limbs as well as the head and face. These studies join other research demonstrating the salience of voice as an influence on mate choice in humans (reviewed in Feinberg, 2008; Pisanski & Feinberg, 2013; Puts, Jones, & DeBruine, 2012).
Moreover, vocal and facial attractiveness may covary (e.g., Saxton, Burriss, Murray, Rowland, & Roberts, 2009) in part because both the face and vocal folds are bilaterally symmetrical, and both may therefore reflect deleterious environmental influences during development. For example, symmetrical shape, size, or tension in the vocal folds could result in audible changes in the voice (Eysholdt, Rosanowski, & Hoppe, 2003), and indeed, Hughes, Pastizzo, and Gallup (2008) found that limb FA in men predicted a measure of vocal hoarseness. Developmental instability may also affect the size and shape of vocal structures, as well as tension on the vocal folds or the positioning of the larynx in the vocal tract, which would influence vocal pitch and timbre, respectively (for fuller consideration of potential links between FA and vocal attractiveness, please see Discussion)
Some research indicates that women’s vocal attractiveness is related to fertility and reproductive potential (Bryant & Haselton, 2009; Pipitone & Gallup, 2011; Pipitone & Gallup, 2008; Puts et al., 2013; Wheatley et al., 2014), though scant research has explored relationships between vocal attractiveness and FA in either sex. To our knowledge, Abend et al. (2015) is the only study conducted to date investigating relationships between vocal attractiveness and facial FA. Furthermore, not all research on FA supports the prediction that individuals will tend to prefer mates lower in FA. For example, some research on humans (e.g., Langlois, Roggman, & Musselman, 1994; Swaddle & Cuthill, 1995), as well as nonhuman species (e.g., Oakes & Barnard, 1994; Tomkins & Simmons, 1998), has failed to show preferences for mates with low FA, while other research has found a statistically significant relationship between FA and attractiveness only in particular situations (Springer et al., 2007).
There are likely several reasons for these discrepancies. First, FA is a weak index of developmental instability, such that associations between FA and measures of mate quality are difficult to detect using modest sample sizes (Van Dongen & Gangestad, 2011). Second, relationships with FA may be complicated by directional asymmetry (DA), which characterizes traits for which the difference between the left and right sides has a population normal distribution with a mean that deviates from zero (Møller, 1994). Directional asymmetry, such as occurs in testes size in many bird species (Møller, 1994) and in the lower limbs of humans (Auerbach & Ruff, 2006), may constitute part of an organism’s normal development (Graham, Emlen, Freeman, Leamy, & Kieser, 1998; Martin, Puts, & Breedlove, 2008). Since the presence of DA may complicate measures of FA, it is critical to take DA into consideration in research examining FA. While some studies have done so (e.g., Abend et al., 2015; Brown et al., 2008; Manning, 1995), others have not (e.g., Gangestad & Thornhill, 1997; Hughes et al., 2002; Thornhill, Gangestad, & Comer, 1995). Finally, prior research has disproportionately sampled from Western societies, especially individuals of European genetic ancestry. Such individuals not only differ in facial morphology from those of other populations (Farkas, Katic, & Forrest, 2005), but Westerners may also have greater access to modern medical and cosmetic technologies that can buffer them from environmental stressors, weakening relationships between FA and measures of attractiveness (Apicella, 2011).
In the present paper, we tested whether somatic FA predicts vocal attractiveness by collecting data in three studies that were designed in part to address the limitations of prior research while enabling us to explore the robustness of these relationships across methodologies and populations. We measured FA from the face due to the centrality of facial symmetry in prior work as well as the status of the face as a feature-rich space that is developmentally distinct from the upper limb, measured by Hughes et al. (2002) and the upper and lower limb included in Abend et al. (2015). In Studies 1 and 2, we measured FA from two-dimensional (2D) facial photographs, following most prior studies of facial asymmetry and attractiveness (Jones et al., 2001). Study 1 data were collected from U.S. undergraduate students, whereas Study 2 data were collected from the Hadza, a Tanzanian forager population less protected by modern medicine from environmental insults than are samples drawn from the West. Compared to Western samples, Hadza facial and vocal characteristics should thus more strongly reflect individuals’ constitutional resilience to environmental stressors, such as pathogens. Finally, in Study 3, we explored facial FA and vocal attractiveness among U.S. undergraduates using the 2D methods employed in the first two studies as well as three-dimensional (3D) images measured via spatially dense geometric morphometrics, which provides far greater shape information than does 2D imagery (Abend et al., 2015; Brown et al., 2008).
2.0 Study
2.1 Methods
2.1.1 Participants
One hundred and twenty-four male (mean age = 20.08 ± 1.76 years) and 201 female (mean age = 19.97 ± 1.55 years) students from Michigan State University participated as part of a larger study of siblings (see, e.g., Puts et al., 2013; Wheatley et al., 2014). If same-sex siblings participated, we randomly omitted all but one from each sibling group (generally pairs). In addition, only students self-identifying as “White” were used, as facial morphology varies by ethnicity (e.g., Farkas et al., 2005), and we did not have sufficient numbers of non-White participants to permit a meaningful analysis stratified by ancestry group. Both male and female participants provided voice recordings and 2D facial photographs at two data collection sessions scheduled one week apart, with all procedures approved by the Michigan State University institutional review board.
2.1.2 Face measurement
Two-dimensional facial photographs were taken of participants at each session, with only photographs from session 1 used unless there was no session 1 photograph for a participant, in which case the session 2 photograph was used (the methodology followed to take these photographs was identical between the sessions). Participants were instructed to assume a neutral expression after having removed any jewelry, spectacles, or makeup (with wet wipes provided at the time of data collection). While not all male participants were clean-shaven, care was taken to ensure high-quality facial landmarks for all photographs (see below). Facial photographs were taken with a tripod-mounted Canon PowerShot S10 digital camera at a distance of approximately 1 m, a height adjusted to the participant, and using constant lighting across participants. All facial images were normalized on interpupillary distance and rotated so that both pupils lay on the same horizontal plane.
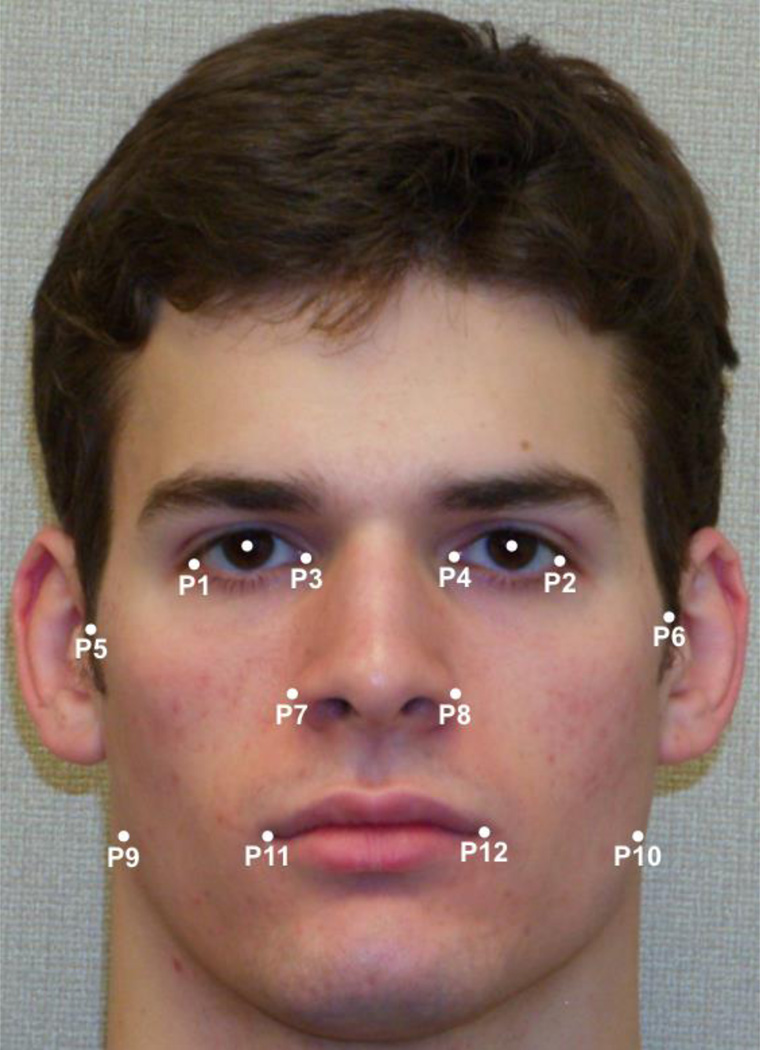
Each facial photograph was landmarked by two research assistants (RAs) with 14 x,y-coordinates in the software ImageJ, after which landmark coordinates were averaged between RAs. Horizontal asymmetry measurements were obtained from landmarks as in Grammer and Thornhill (1994) by first determining midpoints for six pairs of bilateral landmarks (Fig. 1). If a face is perfectly symmetrical about the midsagittal plane, then all midpoints will fall on the vertical line representing this plane, and the horizontal differences between these midpoints will be zero. Thus, to measure horizontal asymmetry, horizontal (i.e., x-coordinate) distances between midpoints were computed for all 15 nonredundant pairs of midpoints and then summed. Vertical asymmetry was computed from the sum of the vertical (i.e., y-coordinate) distances between the 6 landmark pairs (Scheib et al., 1999). As in Scheib et al. (1999), the horizontal and vertical asymmetry measures were finally summed to produce an overall measure of facial asymmetry.
Figure 1.
Landmarks used to compute horizontal and vertical facial asymmetry from two-dimensional images. Left and right pupils were landmarked to standardize interpupillary distance and register faces horizontally.
Inter-measurer reliability was assessed by calculating for each facial distance relative error magnitude (REM) values, which incorporate measurement size so as to arrive at a more precise assessment of consistency (Weinberg, Scott, Neiswanger, Brandon, & Marazita, 2004). The mean for the x-coordinates was 1.67 (SD = 1.08) and the mean for the y-coordinates was 1.21 (SD = .74), indicating strong agreement. DA was calculated for each sex and each facial trait separately and then used to correct for DA when calculating individual FA values. DA for each facial trait j was calculated as
where L and R represent the left and right sides of the trait for each participant i, and n represents the number of participants for whom data were available. Thus, for each trait in each sex, DA was the average signed left-right difference in distance across participants expressed as a proportion of the size of the trait. We were then able, in accordance with methods used by Gangestad et al. (1994) and Abend et al. (2015), to calculate FA for each trait j in each individual i as
FA was computed exclusively from front-on photographs (Table S1), and we eliminated the facial image of one male participant whose facial FA exceeded the mean by 4.0 SD, possibly due to head tilt. See Palmer and Strobeck (1986) for a discussion of variation in methods of calculating FA.
2.1.3 Voice recording
Participants were recorded speaking the first six sentences of the Rainbow Passage (Fairbanks, 1960) into a Shure SM58 vocal cardioid microphone in a quiet recording booth. A curved wire projecting from the microphone stand kept each participant’s mouth approximately 9.5 cm from the microphone. Voices were recorded onto a computer using GoldWave software in mono at a sampling rate of 44,100 Hz and 16-bit quantization, and saved as uncompressed WAV files.
2.1.4 Vocal and facial attractiveness ratings
Unfamiliar members of the opposite sex (n = 569, mean age = 19.4 years for male raters; n = 558, mean age = 19.1 years for female raters) recruited from The Pennsylvania State University rated voice recordings and facial images on attractiveness for a short-term relationship made on a 7-point Likert scale (anchors: 1 = not at all attractive, 7 = very attractive). This portion of the study was approved by The Pennsylvania State University’s institutional review board. Raters first read the definition of a short-term relationship given by Little, Cohen, Jones, and Belsky (2007, p. 970): “You are looking for the type of person who would be attractive in a short-term relationship. This implies that the relationship may not last a long time. Examples of this type of relationship would include a single date accepted on the spur of the moment, an affair within a long-term relationship, and possibility of a one-night stand.” We chose to use ratings of short-term attractiveness made by members of the opposite sex because short-term relationships represent the mating context in which genetic quality is most salient relative to other mate choice criteria, such as investment (Kenrick, Sadalla, Groth, & Trost, 1990). At private workstations, each rater then assessed stimulus sets comprising approximately 25 facial photographs presented on a computer screen and 25 recordings of the first sentence of the Rainbow Passage presented through Sennheiser HD 280 Professional headphones. Recordings and photographs were randomly allocated to a set, each of which was rated by at least 15 raters (mean = 18.9). The order in which participants completed the rating tasks (faces or voices first) was random across participants, as was the order in which stimuli were presented within each task. We calculated mean short-term sexual attractiveness scores for each participant using the first 15 ratings per stimulus (Table S1).
2.2 Results
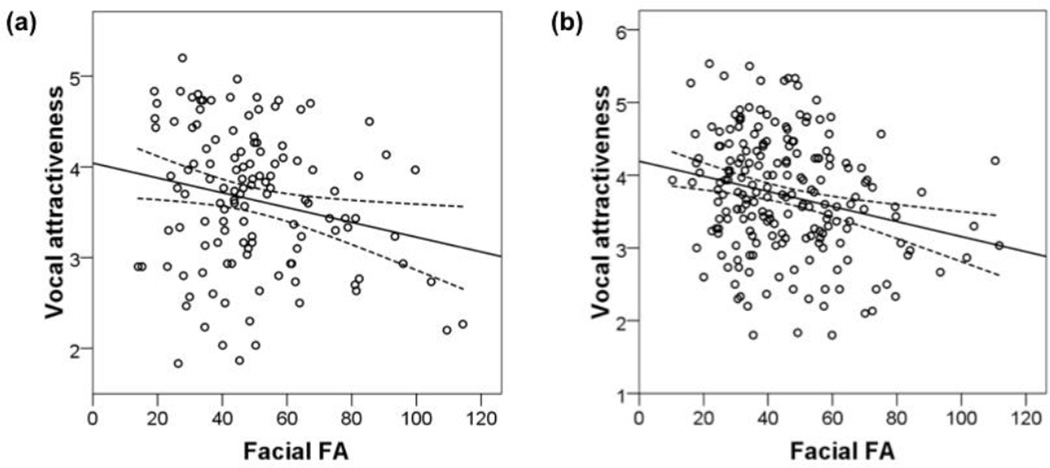
In both men (n = 124, r = −.21, p = .017) and women (n = 201, r = −.24, p = .001), there was a significant negative relationship between vocal attractiveness and facial FA (Tables 1 and 3, Fig. 2). We also explored the possibility that the relationship between facial FA and vocal attractiveness could be attributable to correlations between (1) vocal and facial attractiveness (men: n = 118, r = .04, p = .685; women: n = 189, r = .21, p = .005) and (2) facial attractiveness and facial FA (men: n = 118, r = −.04, p = .708; women: n = 189, r = −.15, p = .043). Attractiveness is an important mediator of mating success (Apicella & Feinberg, 2009; Rhodes, Simmons, & Peters, 2005), and facial attractiveness has also correlated with both symmetry (Grammer & Thornhill, 1994; Perrett et al., 1999) and vocal attractiveness (Abend et al., 2015; Collins & Missing, 2003; Lander, 2008; Wheatley et al., 2014). Hence, it is possible that facial symmetry might increase vocal attractiveness indirectly by increasing facial attractiveness. For instance, people with more symmetrical and thus more attractive faces may consequently feel more confident and therefore speak more attractively. However, after controlling for facial attractiveness in multiple regression models predicting vocal attractiveness, we found that the relationships between facial FA and vocal attractiveness remained in both men (β = −.23, p = .014; model: F(2,115) = 3.23, R = .23, p = .043) and women (β = −.25, p < .001; model: F(2,186) = 10.75, R = .32, p < .0001).
Table 1.
Meta-Analysis of FA and vocal attractiveness in men
| Study name | r | Lower limit | Upper limit | z | p | n |
|---|---|---|---|---|---|---|
| Study 1 | −0.21 | −0.38 | −0.04 | −2.39 | 0.017 | 124 |
| Study 2 | −0.16 | −0.50 | 0.23 | −0.78 | 0.435 | 28 |
| Study 3 (2D facial FA) | −0.10 | −0.32 | 0.12 | −0.89 | 0.372 | 79 |
|
| ||||||
| Study 3 (3D facial FA) | −0.17 | −0.42 | 0.11 | −1.20 | 0.229 | 52 |
|
| ||||||
| Hughes et al. (2002) | −0.36 | −0.58 | −0.10 | −2.62 | 0.009 | 50 |
| Overall1 | −0.21 | −0.32 | −0.09 | −3.41 | 0.001 | 281 |
|
| ||||||
| Overall2 | −0.23 | −0.35 | −0.11 | −3.64 | <0.001 | 254 |
Using 2D facial FA from Study 3
Using 3D facial FA from Study 3
Table 3.
Meta-Analysis of FA and vocal attractiveness in women
| Study name | r | Lower limit | Upper limit | z | p | n |
|---|---|---|---|---|---|---|
| Study 1 | −0.24 | −0.36 | −0.10 | −3.40 | 0.001 | 201 |
| Study 2 | −0.08 | −0.39 | 0.24 | −0.48 | 0.630 | 39 |
|
| ||||||
| Abend et al. (2015; limb FA) | −0.44 | −0.66 | −0.16 | −2.98 | 0.003 | 42 |
|
| ||||||
| Abend et al. (2015; 3D facial FA) | −0.45 | −0.66 | −0.16 | −2.99 | 0.003 | 42 |
| Hughes et al. (2002) | −0.41 | −0.62 | −0.13 | −2.84 | 0.005 | 46 |
|
| ||||||
| Overall1 | −0.28 | −0.41 | −0.14 | −3.87 | <0.001 | 328 |
| Overall2 | −0.29 | −0.42 | −0.14 | −3.86 | <0.001 | 328 |
Using limb FA from Abend et al. (2015)
Using 3D facial FA from Abend et al. (2015)
Figure 2.
Relationship between vocal attractiveness and facial FA among (a) men (n = 124, r = −.21, p = .017) and (b) women (n = 201, r = −.24, p = .001) from Study 1.
3.0 Study 2
3.1 Methods
3.1.1 Participants
Twenty-eight male (mean age = 29.32 ± 5.84 years) and 39 female (mean age = 29.03 ± 6.37 years) participants were recruited from the approximately 1,000 Hadza hunter-gatherers living in remote savannah-woodland habitats of Northern Tanzania. The Hadza have a sexual division of labor in which women collect fruits and nuts and dig for tubers, while men collect honey and hunt animals (Marlowe, 2004). The Hadza are among an increasingly small number of human populations still subsisting by hunting and gathering and are thought to possess a social organization similar to that exhibited by early anatomically modern humans. For a description of the Hadza and hunter-gatherers in general, see Apicella and Crittenden (2016).
Adult participants were recruited from eight Hadza camps visited opportunistically in the summer of 2006. Only individuals between the ages of 18 and 40 were used in the present study, and one male and one female were removed from the dataset because they possessed facial FA more than 3.2 SD from the mean FA for their sex. The researcher located the first Hadza camp by driving in the area of Hadzaland, with subsequent camps selected in a snowball sampling fashion based on information provided by participants about the proximity of the next closest camp. Verbal consent was obtained for all participants, and the study was approved by Harvard University’s ethics internal review board.
3.1.2 Face measurement
Adult male and female participants had their faces photographed outside during the day with a Sony Cyber-shot 18 megapixel digital camera. Participants were asked to maintain a neutral expression and look directly into the camera. Horizontal asymmetry was estimated from x,y-coordinates of 6 bilateral points made by a single measurer following techniques used in previous studies (Grammer & Thornhill, 1994; Penton-Voak et al., 2001; Scheib et al., 1999). Symmetry was computed by taking left and right deviation from the midline, calculated from interpupillary distance, and then summing the absolute value of the individual scores (Table S1). DA was calculated in the manner used in Study 1 and subtracted from the FA measures. For women, the resulting values were natural log-transformed to correct skew.
3.1.3 Vocal attractiveness ratings
Voice recordings were made privately inside a Land Rover vehicle. Participants were asked to speak the Swahili word “hujambo,” which loosely translates to “hello,” into a Sennheiser MKH-60 microphone. Recordings were encoded in mono directly onto a computer hard disk using Sonic Foundry's Sound Forge at 44,100 Hz sampling rate and 16-bit quantization, and saved as uncompressed WAV files. The recordings were used as stimuli for vocal attractiveness ratings, which, as in Study 1, were made on a 7-point Likert scale (anchors: 1 = not at all attractive, 7 = very attractive) by unfamiliar members of the opposite sex (n = 29, mean age = 19.38 years for male raters; n = 26, mean age = 20.38 years for female raters) recruited from The Pennsylvania State University (Table S1). Raters listened to all opposite-sex recordings through Sennheiser HD 280 Professional headphones, and the order in which stimuli were presented was randomized for each rater.
3.2 Results
In contrast with the results of Study 1, we found no significant relationship between vocal attractiveness and facial FA in either men (n = 28, r = −.16, p = .431; Table 1) or women (n = 39, r = −.08, p = .626; Table 3), though the sample size of Hadza participants was much smaller, and in both sexes the non-significant correlation coefficients were in the same direction as in Study 1.
4.0 Study 3
4.1 Methods
4.1.1 Participants
Seventy-nine self-identified White men (mean age = 19.92 ± 1.51) from The Pennsylvania State University participated as part of two broader studies, one on long-term romantic relationships (supplementary online material 2, sample A), the other on interpersonal dynamics (supplementary online material 2, sample B). Data-collection procedures were approved by the University’s institutional review board.
4.1.2 Face measurement
Consistent with methods used in Studies 1 and 2, two-dimensional facial photographs were taken using an Olympus Evolt E-300 digital camera. Participants sat in front of a light-colored background; removed any facial jewelry, glasses, or hats; used a headband to remove any hair obstructing the face; and looked directly into the camera lens while maintaining a neutral facial expression. The resultant photographs were used to obtain facial FA per the methods of Study 1 (Table S1). Also as in Study 1, inter-measurer reliability was assessed by calculating REM values (Weinberg et al., 2004) for each facial distance. The mean for the x-coordinates was .87 (SD = 1.05), and the mean for the y-coordinates was .79 (SD = .67), indicating strong agreement.
In addition to standard two-dimensional photographs, three-dimensional facial images were collected for a subset of participants (n = 52, mean age = 19.87 ± 1.76) using the 3dMD face system (3dMD, Atlanta, GA). Participants were asked to close their mouths and maintain a neutral facial expression while providing their images, which were then exported from the 3dMD Patient software in OBJ file format and imported into a scan-cleaning program for cropping and trimming, as well as removal of hair, ears, and any dissociated polygons.
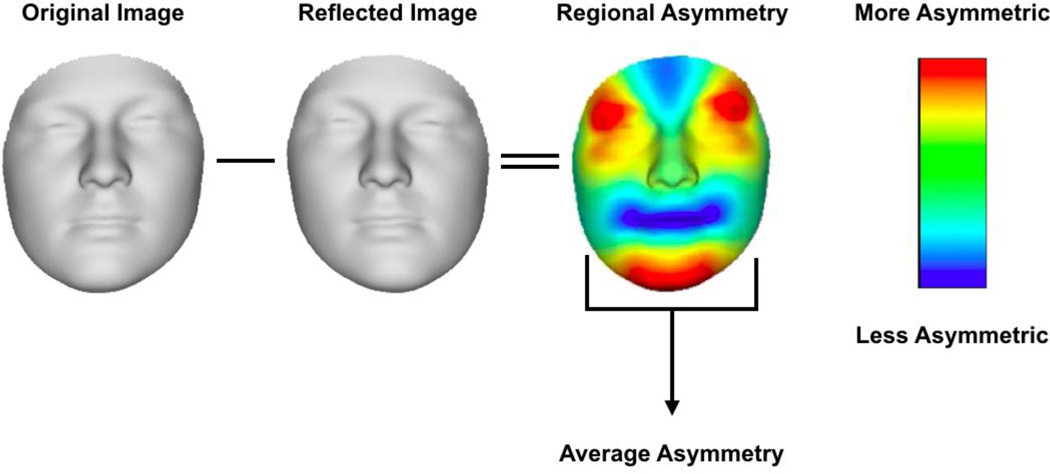
We subsequently partitioned facial shape into patterns of symmetry and asymmetry following the work of Claes, Walters, and Clement (2012). First, an anthropometric mask was non-rigidly mapped onto the original 3D facial scans, as well as onto the reflected scan representing the mirror image of the original (Fig. 3), which was constructed by changing the sign of the x-coordinate (Claes, 2007; Claes, Walters, & Clement, 2012; Claes, Walters, Vandermeulen, & Clement, 2011; Klingenberg & McIntyre, 1998; Mardia, Bookstein, & Moreton, 2000). This established homologous spatially dense quasi-landmark configurations for all original and reflected 3D images (Claes et al., 2011). Secondly, following Mardia et al. (2000), a generalized Procrustes superimposition was performed (Rohlf & Slice, 1990), eliminating differences in position, orientation, and scale between the original and reflected configurations. Finally, the overall consensus configuration was perfectly symmetrical, and a single shape could be decomposed into its asymmetrical and bilaterally symmetrical parts (Mardia et al., 2000). The average of an original image and its reflected configuration constitutes the symmetrical component, whereas the difference between both configurations constitutes the asymmetrical component (Kimmerle & Jantz, 2005; Klingenberg, Barluenga, & Meyer, 2002; Fig. 3). Because the consensus configuration is, by construction, symmetrical with respect to a plane, it is used to analyze variation of symmetry in populations and provides an estimate of the midsagittal plane with a frame of reference that has clear anatomical meaning (Klingenberg et al., 2002). As a result, Procrustes distances in the tangent shape space between mapped 3D images and their reflections after superimposition were used as measures of facial asymmetry. Because the asymmetry shape-space is centered around the average asymmetry in the sample (i.e., the average asymmetry is subtracted from the asymmetry measurements), the DA component was eliminated in computing facial asymmetry.
Figure 3.
Overall asymmetry is calculated as the Procrustes distance between original and reflected images of each individual. The heatmap (third image from left) shows the regional variation in asymmetry with red color highlighting regions with higher levels of asymmetry and blue color highlighting regions with lower levels of asymmetry. The reader is encouraged to consult Claes, Walters, Shriver, et al. (2012) for a more detailed description.
4.1.3 Vocal attractiveness ratings
Voice recordings were made from the first six sentences of the Rainbow Passage (Fairbanks, 1960), collected according to the protocol used in Study 1. Vocal attractiveness was assessed by 167 female students (mean age = 18.80 ± .95 years) recruited from The Pennsylvania State University (Table S1), who listened to only the first sentence of the Rainbow Passage (to avoid fatigue) on Sennheiser HD 280 Professional headphones. Voice stimuli were split randomly into two sets so that each listener rated half of the stimuli, but all stimuli were evaluated an equal number of times. Presentation order of stimuli was randomized for each listener. Recordings from sample A were rated on attractiveness using a 7-point Likert scale (anchors: 1 = very unattractive, 7 = very attractive). Recordings from sample B were rated on attractiveness for both a “short-term, purely sexual relationship, such as a one-night stand” and a “long-term, committed relationship, such as marriage,” using a 10-point Likert scale (anchors: 1 = not at all, 10 = more than anyone else I know), and these scores were averaged to produce a measure of overall vocal attractiveness. Ratings were standardized within each sample to place them on the same scale.
4.2 Results
As in Study 2, the correlations between vocal attractiveness and FA were in the predicted direction but were not statistically significant (2D: n = 79, r = −.10, p = .373; 3D Procrustes: n = 52, r = −.17, p = .227; Table 1). Interestingly, measures of FA made from 2D and 3D images were not significantly correlated (n = 52, r = −.08, p = .581), though this may be attributable to the appreciably larger amount of spatial information captured in 3D meshes, which, in theory, represent a methodological improvement over 2D photographs (see also Discussion). As a result, 3D images have been able to confirm the oft-replicated negative relationship between FA and attractiveness in both sexes obtained using 2D imagery (Brown et al., 2008). Despite this, however, the strength of the relationship between vocal attractiveness and FA in Study 3 did not differ between 2D and 3D measurements (n = 52, z = .19, p = .425).
5.0 Meta-Analysis
In addition to examining independently the three studies reported here, we meta-analyzed the data across the studies, as well as the results of Hughes et al. (2002) and Abend et al. (2015), to produce summary estimates of the relationship between FA and vocal attractiveness. Effect sizes were obtained for males from all studies reported herein plus Hughes et al. (2002), and for females from Studies 1 and 2 as well as Hughes et al. (2002) and Abend et al. (2015). This resulted in total samples of 281 men (utilizing 2D facial FA measurements from Study 3) or 254 men (utilizing 3D facial FA measurements from Study 3), as well as 328 women.
Because of methodological differences in sampling, variable measurement (see Palmer & Strobeck, 1997 for a discussion of the effect of measurement error on FA), and data treatment across studies, a random effects model was used in the analyses, which allows for the presence of moderator variables (e.g., differences in the precision with which FA in different parts of the phenotype indexes developmental instability). Correlation coefficients between FA and vocal attractiveness served as the effect size. Variance estimates based on a small number of studies are at best speculative, and the accuracy of sampling variance estimates, though unbiased, improves with increasing numbers of samples. Both within-sample Q estimates and I2 estimates (Higgins, Thompson, Deeks, & Altman, 2003) suggest that all variance across studies is best attributed to random sampling error. Better estimates of variance across studies must await the accumulation of additional research. The data were analyzed in Comprehensive Meta-Analysis software version 3 (Borenstein, Hedges, Higgins, & Rothstein, 2005), and males and females were treated separately. This software is in the Hedges and Olkin (1985) tradition and performs calculations using the Fisher r-to-z transformation and then reports the results in the untransformed correlation coefficient metric. Moreover, in order to determine whether the removal of a study would affect the meta-analytic results, we conducted for each sex an “omitone- study” analysis.
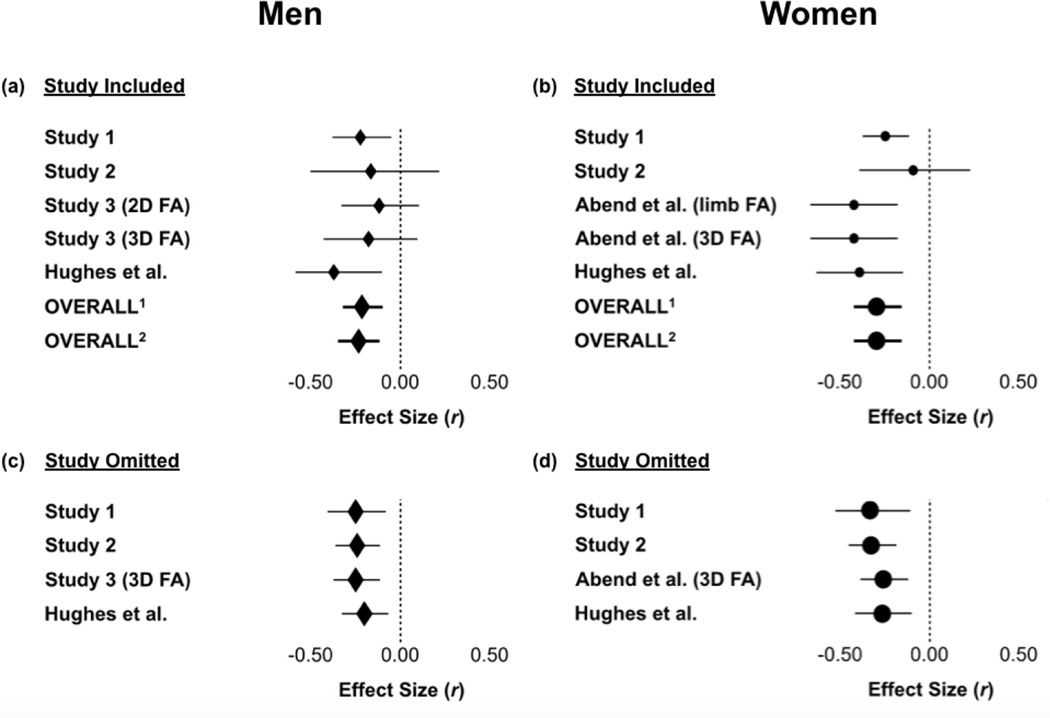
The point estimate for the population correlation between FA and vocal attractiveness in men was r = −.23 and −.21 using 3D and 2D facial FA from Study 3, respectively (Table 1, Fig. 4a). Excluding individual samples in the omit-one-study analysis resulted in effect sizes ranging from r = −.25 to r = −.20 using 3D facial FA from Study 3 (Table 2, Fig. 4c), and r = −.24 to r = −.17 using 2D facial FA from Study 3 (Table S2, Fig. S1a). In all analyses, the 95% CI excluded a correlation of zero.
Figure 4.
Correlations and 95% confidence intervals for relationships between vocal attractiveness and facial FA. Primary meta-analytic results are shown in panels (a) for men and (b) for women. Overall effects refer to results of random effect models. In panel (a), superscripts 1 and 2 denote results using 2D and 3D facial FA, respectively, from Study 3. In panel (b), superscripts 1 and 2 denote results using limb FA and 3D facial FA, respectively, from Abend et al. (2015). Results of “omit-one-study” analyses are illustrated for (c) men and (d) women, with FA in Study 3 and Abend et al. (2015) computed from 3D facial images.
Table 2.
“Omit-one-study” analysis of FA and vocal attractiveness in men using 3D facial FA from Study 3
| Study omitted | r | Lower limit | Upper limit | z | p | n |
|---|---|---|---|---|---|---|
| Study 1 | −0.25 | −0.40 | −0.07 | −2.75 | 0.006 | 130 |
| Study 2 | −0.24 | −0.36 | −0.11 | −3.57 | <0.001 | 226 |
| Study 3 (3D facial FA) | −0.24 | −0.37 | −0.11 | −3.47 | 0.001 | 202 |
|
| ||||||
| Hughes et al. (2002) | −0.20 | −0.33 | −0.06 | −2.77 | 0.006 | 204 |
For women, the point estimate for the population correlation between FA and vocal attractiveness was r = −.28 and −.29 using limb FA and 3D facial FA from Abend et al. (2015), respectively (Table 3, Fig. 4b). Excluding individual samples in the omit-one-study analysis resulted in effect sizes ranging from r = −.32 to r = −.25 (Tables 4 and S3, Fig. S1b). In all analyses, the 95% CI excluded a correlation of zero. We also meta-analyzed only the two studies reported in the present paper that included women and obtained a population correlation of r = −.21 (95% CI: −.33 to −.09, p < .001).
Table 4.
“Omit-one-study” analysis of FA and vocal attractiveness in women using 3D facial FA from Abend et al. (2015)
| Study omitted | r | Lower limit | Upper limit | z | p | n |
|---|---|---|---|---|---|---|
| Study 1 | −0.32 | −0.52 | −0.10 | −2.74 | 0.006 | 127 |
| Study 2 | −0.32 | −0.45 | −0.17 | −4.22 | <0.001 | 289 |
|
| ||||||
| Abend et al. (2015; 3D facial FA) | −0.25 | −0.38 | −0.11 | −3.38 | 0.001 | 286 |
| Hughes et al. (2002) | −0.26 | −0.41 | −0.09 | −2.94 | 0.003 | 282 |
6.0 Discussion
In the present research, we found relationships between vocal attractiveness and fluctuating asymmetry in both sexes. Though analyses should be re-conducted as more data accumulate, our meta-analyses gave point estimates for population correlations between vocal attractiveness and FA of −.23 for men and −.29 for women, using 3D facial FA from Study 3 and Abend et al. (2015). All effect sizes across samples were in the same (negative) direction, although they ranged in magnitude, with greater deviations from the population estimate unsurprisingly tending to derive from smaller samples. Our estimate indicates that 146 male and 91 female observations would be required to find a statistically significant effect (two-tailed power analyses for α = .05 and β = .20). As such, conclusions based on the meta-analysis that cumulated across studies are likely to be more robust than conclusions drawn from underpowered primary studies. Importantly, the negative association between FA and vocal attractiveness was highly robust and manifest in every meta-analysis of the data. The correlation was statistically significant regardless of the inclusion of any individual study or sample, applied to FA measured from the face as well as from the body, and was present whether we included effect sizes from previously published work or only those from the present research.
Our findings thus provide important new evidence supporting the salience of the human voice in conveying information on mate quality. Furthermore, we are able to exclude several potential alternative interpretations. First, it is possible that behavioral tendencies may spuriously link vocal attractiveness with measurements of facial symmetry. For example, shyness might result in both a wavering, less attractive voice, as well as a tendency not to face the camera directly, which would affect symmetry measurements. However, in taking 2D images, we were careful to ensure that participants faced the camera fully, subsequently verifying this through visual inspection of the photographs and removal of those in which head turn was apparent. More importantly, the relationship between vocal attractiveness and FA was in the predicted direction, albeit non-significantly in Study 3, when FA was measured from 3D imagery, which is not influenced by the spatial orientation of the head. A pertinent question is how developmental stability can be indexed by FA measured from both 2D and 3D images if the two types of measurements were not correlated in Study 3. It is possible that, while developmental instability influences FA in both two and three dimensions, it does so differently such that the relationship between the two is not necessarily positive. For instance, increased asymmetry in the coronal plane, which is measured in 2D images, might be related to decreased asymmetry in the sagittal plane, which is measured in 3D but not 2D images. That FA from both types of image reliability indexes developmental stability accords with results of Tigue, Pisanski, O'Connor, Fraccaro, and Feinberg (2012), who found that, while 3D images elicited more favorable assessments of attractiveness than did 2D images, attractiveness ratings from the two types of stimuli were nonetheless correlated. This notwithstanding, we encourage future researchers to employ 3D imagery to capture appreciably more spatial information and to characterize more precisely subtle shape variation present in facial morphology.
Second, it is possible that developmental instability only indirectly influences vocal attractiveness by increasing confidence associated with having a symmetrical, attractive face. However, this does not appear to be the case. In Study 1, we were able to statistically control for facial attractiveness and found that the relationships between facial FA and vocal attractiveness remained undiminished in both sexes after doing so. Moreover, these findings indicate that voices and faces provide partly unique information about mate quality, in that voices provided information about developmental instability that was not encompassed by facial attractiveness. Vocal and facial attractiveness correlated moderately in Study 1 women, suggesting that women’s voices and faces provide partly redundant information about mate quality. Previous research has shown similar levels of agreement across these domains in women (e.g., Abend et al., 2015; Collins & Missing, 2003; Feinberg et al., 2005; Lander, 2008; Saxton et al., 2009; Wheatley et al., 2014), and auditory and visual cues considered in isolation may be less informative about mate quality than are stimuli combining these and other traits (Campanella & Belin, 2007). In Study 1 men, vocal and facial attractiveness did not correlate significantly. Although ours is the largest study of which we are aware to test this correlation in men, our results corroborate most (e.g., Lander, 2008; Saxton et al., 2009; Wells, Baguley, Sergeant, & Dunn, 2013), but not all (Saxton, Caryl, & Roberts, 2006; Skrinda et al., 2014), previous studies exploring this correlation. Further research is required to determine the extent to which men’s facial and vocal attractiveness correlate, which components of mate quality may underlie any such correlation, and how these relationships may vary across samples.
Finally, our results provide further evidence that human preferences for symmetrical mates are not merely consequences of symmetrical stimuli being more easily processed by the visual system (Enquist & Johnstone, 1997). It is now becoming clear that people prefer not only the faces (Jones, DeBruine, & Little, 2007; Little & Jones, 2003; Scheib et al., 1999) and odors (Gangestad & Thornhill, 1998; Thornhill & Gangestad, 1999b) but also the voices (Abend et al., 2015; Hughes et al., 2002; Hughes et al., 2008; present study) of mates with low levels of FA, even when symmetry cannot be directly assessed visually.
The present paper thus adds to an efflorescing literature exploring relationships between FA and attractiveness in humans. While some contributions to this literature have questioned relationships between FA and fitness-relevant outcomes (e.g., Clarke, 1998), a recent meta-analysis of 293 estimates of the relationship between FA and measures of health and quality taken from 94 studies on humans suggests a robust correlation (Van Dongen & Gangestad, 2011).
Much previous research on FA and mate preferences has focused on putative genetic benefits provided by symmetrical partners. Heritability estimates for FA vary across species and across traits within species (Leamy, 1997; Møller & Thornhill, 1997; Polak et al., 2003), but it is possible that individuals derive non-genetic benefits from symmetrical mates, as well (Gangestad & Thornhill, 2013). For example, the meta-analysis of Van Dongen and Gangestad (2011) estimated correlations of 0.11 and 0.20 between FA and reduced intelligence and schizophrenia/schizotypy, respectively, both of which may have influenced investment capacity ancestrally. However, other research suggests that symmetrical males may allocate relatively greater reproductive effort to mating than to investment in mates or offspring (e.g., Gangestad & Simpson, 2000; Gangestad & Thornhill, 1997; Gangestad & Thornhill, 2013), suggesting that genetic benefits to offspring may have driven the evolution of preferences for low-FA partners, at least in women.
7.0 Future Directions
Future studies should investigate how developmental instability relates to the anatomy of the vocal tract, and which vocal anatomical characteristics and acoustic features of the voice mediate relationships between somatic asymmetry and vocal attractiveness (see, for example, Hughes et al., 2008). While such an exploration is beyond the scope of the present paper, some informed speculation is possible. One possibility is that developmental instability affects vocal attractiveness by influencing the symmetry of the vocal apparatus, for example the vocal folds or the shape of the supralaryngeal vocal tract. Anatomical asymmetries or asymmetric tension between the two vocal folds will cause the vocal folds to vibrate differently or at different rates, producing perturbations in periodicity as well as incomplete closure and leading to perceptible vocal changes, such as hoarseness, breathiness, and roughness (Eysholdt et al., 2003). Facial asymmetries, such as those caused by cleft lip and palate, can affect aspects of articulation and resonance, as well. Because vocalization is integral to human communication, there may have been strong selection for the vocal apparatus to be buffered against such perturbations, but research suggests pronounced asymmetries in the human vocal folds (e.g., Hirano, Yukizane, Kurita, & Hibi, 1989). This is consistent with the idea that traits shaped by intersexual selection tend to display greater FA than do non-sexually selected traits (Møller & Hoglund, 1991). Developmental instability may influence other aspects of the size and shape of vocal structures (e.g., the length, thickness, or density of the vocal folds), as well as neurological characteristics that could affect vocal behavior, such as tension on the vocal folds or the positioning of the larynx in the vocal tract, which would influence vocal pitch and timbre, respectively. Functional magnetic resonance imaging of vocal anatomy during phonation, precise acoustic measurements, and large samples may be particularly useful in exploring these possibilities.
Supplementary Material
Acknowledgments
We thank Arslan Zaidi for assistance designing Fig. 3, Tobias Kordsmeyer for insights on measuring facial FA, Matt Winn for input on the Discussion, and Lisa DeBruine and two anonymous reviewers for comments on previous drafts of this manuscript.
Funding. This work was supported by the National Institutes of Mental Health [grant number T32 MH70343-05], the National Science Foundation [REG grant to C.L.A. and GRFP grant to J.R.W.], and by The Pennsylvania State University and Harvard University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abend P, Pflüger LS, Koppensteiner M, Coquerelle M, Grammer K. The sound of female shape: a redundant signal of vocal and facial attractiveness. Evolution and Human Behavior. 2015;36(3):174–181. [Google Scholar]
- Andersson MB. Sexual Selection. Princeton, NJ: Princeton University Press; 1994. [Google Scholar]
- Apicella CL. On the universality of attractiveness. In: Brockman M, editor. Future Science: Essays from the Cutting Edge. New York, NY: Oxford University Press; 2011. pp. 88–100. [Google Scholar]
- Apicella CL, Crittenden A. Hunter-gatherer families and parenting. In: Buss DM, editor. The Handbook of Evolutionary Psychology. 2nd. Vol. 1. Hoboken, NJ: Wiley; 2016. pp. 578–597. [Google Scholar]
- Apicella CL, Feinberg DR. Voice pitch alters mate-choice-relevant perception in hunter-gatherers. Proc Biol Sci. 2009;276(1659):1077–1082. doi: 10.1098/rspb.2008.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach BM, Ruff CB. Limb bone bilateral asymmetry: variability and commonality among modern humans. Journal of human Evolution. 2006;50(2):203–218. doi: 10.1016/j.jhevol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Banks GC, Batchelor JH, McDaniel MA. Smarter people are (a bit) more symmetrical: A meta-analysis of the relationship between intelligence and fluctuating asymmetry. Intelligence. 2010;38(4):393–401. [Google Scholar]
- Barden HS. Fluctuating dental asymmetry: a measure of developmental instability in Down syndrome. American Journal of Physical Anthropology. 1980;52(2):169–173. doi: 10.1002/ajpa.1330520203. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive meta-analysis version 2. Vol. 104. Englewood, NJ: Biostat; 2005. [Google Scholar]
- Brown WM, Price ME, Kang J, Pound N, Zhao Y, Yu H. Fluctuating asymmetry and preferences for sex-typical bodily characteristics. Proc Natl Acad Sci U S A. 2008;105(35):12938–12943. doi: 10.1073/pnas.0710420105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant GA, Haselton MG. Vocal cues of ovulation in human females. Biol Lett. 2009;5(1):12–15. doi: 10.1098/rsbl.2008.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burriss RP, Roberts SC, Welling LL, Puts DA, Little AC. Heterosexual romantic couples mate assortatively for facial symmetry, but not masculinity. Pers Soc Psychol Bull. 2011;37(5):601–613. doi: 10.1177/0146167211399584. [DOI] [PubMed] [Google Scholar]
- Campanella S, Belin P. Integrating face and voice in person perception. Trends Cogn Sci. 2007;11(12):535–543. doi: 10.1016/j.tics.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Claes P. A robust statistical surface registration framework using implicit function representations-application in craniofacial reconstruction. 2007 [Google Scholar]
- Claes P, Walters M, Clement J. Improved facial outcome assessment using a 3D anthropometric mask. International journal of oral and maxillofacial surgery. 2012;41(3):324–330. doi: 10.1016/j.ijom.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Claes P, Walters M, Shriver MD, Puts D, Gibson G, Clement J, Suetens P. Sexual dimorphism in multiple aspects of 3D facial symmetry and asymmetry defined by spatially dense geometric morphometrics. Journal of anatomy. 2012;221(2):97–114. doi: 10.1111/j.1469-7580.2012.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes P, Walters M, Vandermeulen D, Clement JG. Spatially-dense 3D facial asymmetry assessment in both typical and disordered growth. Journal of anatomy. 2011;219(4):444–455. doi: 10.1111/j.1469-7580.2011.01411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke GM. Developmental stability and fitness: the evidence is not quite so clear. The American Naturalist. 1998;152(5):762–766. doi: 10.1086/286207. [DOI] [PubMed] [Google Scholar]
- Collins SA, Missing C. Vocal and visual attractiveness are related in women. Animal Behaviour. 2003;65(5):997–1004. [Google Scholar]
- Enquist M, Johnstone RA. Generalization and the evolution of symmetry preferences. Proceedings of the Royal Society of London B: Biological Sciences. 1997;264(1386):1345–1348. [Google Scholar]
- Eysholdt U, Rosanowski F, Hoppe U. Vocal fold vibration irregularities caused by different types of laryngeal asymmetry. European Archives of Oto-rhino-laryngology. 2003;260(8):412–417. doi: 10.1007/s00405-003-0606-y. [DOI] [PubMed] [Google Scholar]
- Fairbanks G. Voice and articulation handbook. Harper, New York: 1960. [Google Scholar]
- Farkas LG, Katic MJ, Forrest CR. International anthropometric study of facial morphology in various ethnic groups/races. Journal of Craniofacial Surgery. 2005;16(4):615–646. doi: 10.1097/01.scs.0000171847.58031.9e. [DOI] [PubMed] [Google Scholar]
- Feinberg DR. Are human faces and voices ornaments signaling common underlying cues to mate value? Evolutionary Anthropology: Issues, News, and Reviews. 2008;17(2):112–118. [Google Scholar]
- Feinberg DR, Jones BC, DeBruine LM, Moore FR, Law Smith MJ, Cornwell RE, Perrett DI. The voice and face of woman: One ornament that signals quality? Evolution and Human Behavior. 2005;26(5):398–408. [Google Scholar]
- Gangestad SW, Simpson JA. The evolution of human mating: Trade-offs and strategic pluralism. Behavioral and brain sciences. 2000;23(04):573–587. doi: 10.1017/s0140525x0000337x. [DOI] [PubMed] [Google Scholar]
- Gangestad SW, Thornhill R. The evolutionary psychology of extrapair sex: The role of fluctuating asymmetry. Evolution and Human Behavior. 1997;18(2):69–88. [Google Scholar]
- Gangestad SW, Thornhill R. Menstrual cycle variation in women's preferences for the scent of symmetrical men. Proceedings of the Royal Society of London B: Biological Sciences. 1998;265(1399):927–933. doi: 10.1098/rspb.1998.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangestad SW, Thornhill R. Human sexual selection and developmental stability. In: Simpson JA, Kenrick DT, editors. Evolutionary Social Psychology. 2013. pp. 169–195. [Google Scholar]
- Gangestad SW, Thornhill R, Yeo RA. Facial attractiveness, developmental stability, and fluctuating asymmetry. Ethology and Sociobiology. 1994;15(2):73–85. [Google Scholar]
- Graham JH, Emlen JM, Freeman DC, Leamy LJ, Kieser JA. Directional asymmetry and the measurement of developmental instability. Biological Journal of the Linnean Society. 1998;64(1):1–16. [Google Scholar]
- Grammer K, Thornhill R. Human (Homo sapiens) facial attractiveness and sexual selection: the role of symmetry and averageness. Journal of Comparative Psychology. 1994;108(3):233. doi: 10.1037/0735-7036.108.3.233. [DOI] [PubMed] [Google Scholar]
- Hedges L, Olkin I. Statistical models for meta-analysis. New York: Academic Press; 1985. [Google Scholar]
- Hedges LV, Pigott TD. The power of statistical tests in meta-analysis. Psychological Methods. 2001;6:203–217. [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ: British Medical Journal. 2003;327(7414):557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M, Yukizane K, Kurita S, Hibi S. Asymmetry of the laryngeal framework: a morphologic study of cadaver larynges. Annals of Otology, Rhinology & Laryngology. 1989;98(2):135–140. doi: 10.1177/000348948909800210. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Harrison MA, Gallup GG. The sound of symmetry: voice as a marker of developmental instability. Evolution and Human Behavior. 2002;23(3):173–180. [Google Scholar]
- Hughes SM, Pastizzo MJ, Gallup GG. The sound of symmetry revisited: Subjective and objective analyses of voice. Journal of Nonverbal Behavior. 2008;32(2):93–108. [Google Scholar]
- Hunt J, Bussiere LF, Jennions MD, Brooks R. What is genetic quality? Trends in Ecology & Evolution. 2004;19(6):329–333. doi: 10.1016/j.tree.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Jones BC, DeBruine LM, Little AC. The role of symmetry in attraction to average faces. Perception & psychophysics. 2007;69(8):1273–1277. doi: 10.3758/bf03192944. [DOI] [PubMed] [Google Scholar]
- Jones BC, Little AC, Penton-Voak IS, Tiddeman B, Burt D, Perrett D. Facial symmetry and judgements of apparent health: support for a “good genes” explanation of the attractiveness–symmetry relationship. Evolution and Human Behavior. 2001;22(6):417–429. [Google Scholar]
- Kempenaers B. Mate choice and genetic quality: a review of the heterozygosity theory. Advances in the Study of Behavior. 2007;37:189–278. [Google Scholar]
- Kenrick DT, Sadalla EK, Groth G, Trost MR. Evolution, traits, and the stages of human courtship: Qualifying the parental investment model. Journal of Personality. 1990;58:97–116. doi: 10.1111/j.1467-6494.1990.tb00909.x. [DOI] [PubMed] [Google Scholar]
- Kimmerle EH, Jantz RL. Modern Morphometrics in Physical Anthropology. Springer; 2005. Secular trends in craniofacial asymmetry studied by geometric morphometry and generalized Procrustes methods; pp. 247–263. [Google Scholar]
- Klingenberg CP, Barluenga M, Meyer A. Shape analysis of symmetric structures: quantifying variation among individuals and asymmetry. Evolution. 2002;56(10):1909–1920. doi: 10.1111/j.0014-3820.2002.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP, McIntyre GS. Geometric morphometrics of developmental instability: analyzing patterns of fluctuating asymmetry with Procrustes methods. Evolution. 1998:1363–1375. doi: 10.1111/j.1558-5646.1998.tb02018.x. [DOI] [PubMed] [Google Scholar]
- Komori M, Kawamura S, Ishihara S. Averageness or symmetry: which is more important for facial attractiveness? Acta Psychol (Amst) 2009;131(2):136–142. doi: 10.1016/j.actpsy.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Lander K. Relating visual and vocal attractiveness for moving and static faces. Animal Behaviour. 2008;75(3):817–822. [Google Scholar]
- Langlois JH, Roggman LA, Musselman L. What is average and what is not average about attractive faces? Psychological Science. 1994;5(4):214–220. [Google Scholar]
- Leamy L. Is developmental stability heritable? Journal of evolutionary biology. 1997;10(1):21–29. [Google Scholar]
- Little AC, Burt DM, Penton-Voak IS, Perrett DI. Self-perceived attractiveness influences human female preferences for sexual dimorphism and symmetry in male faces. Proc Biol Sci. 2001;268(1462):39–44. doi: 10.1098/rspb.2000.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little AC, Cohen DL, Jones BC, Belsky J. Human preferences for facial masculinity change with relationship type and environmental harshness. Behavioral Ecology and Sociobiology. 2007;61(6):967–973. [Google Scholar]
- Little AC, Jones BC. Evidence against perceptual bias views for symmetry preferences in human faces. Proceedings of the Royal Society B: Biological Sciences. 2003;270(1526):1759–1763. doi: 10.1098/rspb.2003.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little AC, Jones BC. Variation in facial masculinity and symmetry preferences across the menstrual cycle is moderated by relationship context. Psychoneuroendocrinology. 2012;37(7):999–1008. doi: 10.1016/j.psyneuen.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Little AC, Jones BC, Burt DM, Perrett DI. Preferences for symmetry in faces change across the menstrual cycle. Biol Psychol. 2007;76(3):209–216. doi: 10.1016/j.biopsycho.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Livshits G, Kobyliansky E. Fluctuating asymmetry as a possible measure of developmental homeostasis in humans: a review. Human Biology. 1991:441–466. [PubMed] [Google Scholar]
- Manning J, Koukourakis K, Brodie D. Fluctuating asymmetry, metabolic rate and sexual selection in human males. Evolution and Human Behavior. 1997;18(1):15–21. [Google Scholar]
- Manning J, Scutt D, Whitehouse G, Leinster S. Breast asymmetry and phenotypic quality in women. Evolution and Human Behavior. 1997;18(4):223–236. [Google Scholar]
- Manning JT. Fluctuating asymmetry and body weight in men and women: implications for sexual selection. Ethology and Sociobiology. 1995;16(2):145–153. [Google Scholar]
- Mardia KV, Bookstein FL, Moreton IJ. Statistical assessment of bilateral symmetry of shapes. Biometrika. 2000:285–300. [Google Scholar]
- Marlowe FW. Mate preferences among Hadza hunter-gatherers. Human Nature. 2004;15(4):365–376. doi: 10.1007/s12110-004-1014-8. [DOI] [PubMed] [Google Scholar]
- Martin JT, Puts DA, Breedlove SM. Hand asymmetry in heterosexual and homosexual men and women: relationship to 2D:4D digit ratios and other sexually dimorphic anatomical traits. Arch Sex Behav. 2008;37(1):119–132. doi: 10.1007/s10508-007-9279-8. [DOI] [PubMed] [Google Scholar]
- Martin S, Manning J, Dowrick C. Fluctuating asymmetry, relative digit length, and depression in men. Evolution and Human Behavior. 1999;20(3):203–214. [Google Scholar]
- Møller A, Thornhill R. A meta-analysis of the heritability of developmental stability. Journal of evolutionary biology. 1997;10(1):1–16. [Google Scholar]
- Møller AP. Female swallow preference for symmetrical male. Nature. 1992;357 doi: 10.1038/357238a0. [DOI] [PubMed] [Google Scholar]
- Møller AP. Directional selection on directional asymmetry: testes size and secondary sexual characters in birds. Proceedings of the Royal Society of London B: Biological Sciences. 1994;258(1352):147–151. [Google Scholar]
- Møller AP, Hoglund J. Patterns of fluctuating asymmetry in avian feather ornaments: implications for models of sexual selection. Proceedings of the Royal Society of London B: Biological Sciences. 1991;245(1312):1–5. [Google Scholar]
- Mooney MP, Siegel MI, Gest TR. Prenatal stress and increased fluctuating asymmetry in the parietal bones of neonatal rats. American Journal of Physical Anthropology. 1985;68(1):131–134. doi: 10.1002/ajpa.1330680112. [DOI] [PubMed] [Google Scholar]
- Oakes EJ, Barnard P. Fluctuating asymmetry and mate choice in paradise whydahs, Vidua paradisaea: an experimental manipulation. Animal Behaviour. 1994;48(4):937–943. [Google Scholar]
- Palmer A, Strobeck C. Fluctuating asymmetry and developmental stability: heritability of observable variation vs. heritability of inferred cause. Journal of evolutionary biology. 1997;10(1):39–49. [Google Scholar]
- Palmer AR, Strobeck C. Fluctuating asymmetry: measurement, analysis, patterns. Annual review of Ecology and Systematics. 1986:391–421. [Google Scholar]
- Parsons P. Fluctuating asymmetry: an epigenetic measure of stress. Biological reviews. 1990;65(2):131–145. doi: 10.1111/j.1469-185x.1990.tb01186.x. [DOI] [PubMed] [Google Scholar]
- Penton-Voak IS, Jones BC, Little AC, Baker S, Tiddeman B, Burt DM, Perrett DI. Symmetry, sexual dimorphism in facial proportions and male facial attractiveness. Proc Biol Sci. 2001;268(1476):1617–1623. doi: 10.1098/rspb.2001.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz B, Ever-Hadani P, Casamassimo P, Eidelman E, Shellhart C, Hagerman R. Crown size asymmetry in males with fra (X) or Martin-Bell syndrome. American journal of medical genetics. 1988;30(1–2):185–190. doi: 10.1002/ajmg.1320300117. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Burt DM, Penton-Voak IS, Lee KJ, Rowland DA, Edwards R. Symmetry and human facial attractiveness. Evolution and Human Behavior. 1999;20(5):295–307. [Google Scholar]
- Peters M, Simmons LW, Rhodes G. Preferences across the menstrual cycle for masculinity and symmetry in photographs of male faces and bodies. PLoS One. 2009;4(1):e4138. doi: 10.1371/journal.pone.0004138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie M. Improved growth and survival of offspring of peacocks with more elaborate trains. Nature. 1994;371(6498):598–599. [Google Scholar]
- Pipitone NR, Gallup G. The unique impact of menstruation on the female voice: Implications for the evolution of menstrual cycle cues. Ethology. 2011;118:281–291. [Google Scholar]
- Pipitone NR, Gallup GG., Jr Women's voice attractiveness varies across the menstrual cycle. Evolution and Human Behavior. 2008;29:268–274. [Google Scholar]
- Pisanski K, Feinberg DR. Cross-cultural variation in mate preferences for averageness, symmetry, body size, and masculinity. Cross-Cultural Research. 2013;47(2):162–197. [Google Scholar]
- Polak M, Møller A, Gangestad SW, Kroeger DE, Manning JT, Thornhill R. Does an individual asymmetry parameter exist? A meta-analysis. In: Polak M, editor. Developmental Instability: Causes and Consequences. Oxford: Oxford University Press; 2003. pp. 81–96. [Google Scholar]
- Puts DA, Bailey DH, Cardenas RA, Burriss RP, Welling LL, Wheatley JR, Dawood K. Women's attractiveness changes with estradiol and progesterone across the ovulatory cycle. Horm Behav. 2013;63(1):13–19. doi: 10.1016/j.yhbeh.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Puts DA, Jones BC, DeBruine LM. Sexual selection on human faces and voices. J Sex Res. 2012;49(2–3):227–243. doi: 10.1080/00224499.2012.658924. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Simmons LW, Peters M. Attractiveness and sexual behavior: Does attractiveness enhance mating success? Evolution and Human Behavior. 2005;26(2):186–201. [Google Scholar]
- Rhodes G, Yoshikawa S, Clark A, Lee K, McKay R, Akamatsu S. Attractiveness of facial averageness and symmetry in non-Western cultures: In search of biologically based standards of beauty. Perception. 2001;30(5):611–625. doi: 10.1068/p3123. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ, Slice D. Extensions of the Procrustes method for the optimal superimposition of landmarks. Systematic Biology. 1990;39(1):40–59. [Google Scholar]
- Saxton T, Burriss R, Murray A, Rowland H, Roberts SC. Face, body and speech cues independently predict judgments of attractiveness. Journal of Evolutionary Psychology. 2009;7(1):23–35. [Google Scholar]
- Saxton TK, Caryl PG, Roberts SC. Vocal and Facial Attractiveness Judgments of Children, Adolescents and Adults: the Ontogeny of Mate Choice. Ethology. 2006;112(12):1179–1185. [Google Scholar]
- Scheib JE, Gangestad SW, Thornhill R. Facial attractiveness, symmetry and cues of good genes. Proceedings of the Royal Society of London B: Biological Sciences. 1999;266(1431):1913–1917. doi: 10.1098/rspb.1999.0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciulli P, Doyle W, Kelley C, Siegel P, Siegel M. The interaction of stressors in the induction of increased levels of fluctuating asymmetry in the laboratory rat. American Journal of Physical Anthropology. 1979;50(2):279–284. doi: 10.1002/ajpa.1330500218. [DOI] [PubMed] [Google Scholar]
- Simmons L. Correlates of male quality in the field cricket, Gryllus campestris L.: age, size, and symmetry determine pairing success in field populations. Behavioral Ecology. 1995;6(4):376–381. [Google Scholar]
- Skrinda I, Krama T, Kecko S, Moore FR, Kaasik A, Meija L, Krams I. Body height, immunity, facial and vocal attractiveness in young men. Naturwissenschaften. 2014;101(12):1017–1025. doi: 10.1007/s00114-014-1241-8. [DOI] [PubMed] [Google Scholar]
- Springer IN, Wannicke B, Warnke PH, Zernial O, Wiltfang J, Russo PA, Wolfart S. Facial attractiveness: visual impact of symmetry increases significantly towards the midline. Annals of plastic surgery. 2007;59(2):156–162. doi: 10.1097/01.sap.0000252041.66540.ec. [DOI] [PubMed] [Google Scholar]
- Swaddle JP. Reproductive success and symmetry in zebra finches. Animal Behaviour. 1996;51(1):203–210. [Google Scholar]
- Swaddle JP, Cuthill IC. Asymmetry and human facial attractiveness: symmetry may not always be beautiful. Proceedings of the Royal Society of London B: Biological Sciences. 1995;261(1360):111–116. doi: 10.1098/rspb.1995.0124. [DOI] [PubMed] [Google Scholar]
- Thornhill R, Gangestad SW. Facial attractiveness. Trends in cognitive sciences. 1999a;3(12):452–460. doi: 10.1016/s1364-6613(99)01403-5. [DOI] [PubMed] [Google Scholar]
- Thornhill R, Gangestad SW. The scent of symmetry: a human sex pheromone that signals fitness? Evolution and Human Behavior. 1999b;20(3):175–201. [Google Scholar]
- Thornhill R, Gangestad SW, Comer R. Human female orgasm and mate fluctuating asymmetry. Animal Behaviour. 1995;50(6):1601–1615. [Google Scholar]
- Tigue CC, Pisanski K, O'Connor JJ, Fraccaro PJ, Feinberg DR. Men's judgments of women's facial attractiveness from two-and three-dimensional images are similar. Journal of vision. 2012;12(12):3–3. doi: 10.1167/12.12.3. [DOI] [PubMed] [Google Scholar]
- Tomkins JL, Simmons LW. Female choice and manipulations of forceps size and symmetry in the earwigForficulaauriculariaL. Animal Behaviour. 1998;56(2):347–356. doi: 10.1006/anbe.1998.0838. [DOI] [PubMed] [Google Scholar]
- Van Dongen S, Gangestad SW. Human fluctuating asymmetry in relation to health and quality: a meta-analysis. Evolution and Human Behavior. 2011;32(6):380–398. [Google Scholar]
- Van Valen L. A study of fluctuating asymmetry. Evolution. 1962:125–142. [Google Scholar]
- Van Valen L. A new evolutionary law. Evolutionary theory. 1973;1:1–30. [Google Scholar]
- Weinberg SM, Scott NM, Neiswanger K, Brandon CA, Marazita ML. Digital three-dimensional photogrammetry: evaluation of anthropometric precision and accuracy using a Genex 3D camera system. The Cleft palate-craniofacial journal. 2004;41(5):507–518. doi: 10.1597/03-066.1. [DOI] [PubMed] [Google Scholar]
- Wells T, Baguley T, Sergeant M, Dunn A. Perceptions of human attractiveness comprising face and voice cues. Archives of Sexual Behavior. 2013;42(5):805–811. doi: 10.1007/s10508-012-0054-0. [DOI] [PubMed] [Google Scholar]
- Wheatley JR, Apicella CA, Burriss RP, Cárdenas RA, Bailey DH, Welling LL, Puts DA. Women’s faces and voices are cues to reproductive potential in industrial and forager societies. Evolution and Human Behavior. 2014;35(4):264–271. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.