Abstract
As the focus of implantable biomaterials has shifted from bioinert implants to bioactive designs, recent research has highlighted the complex interactions between cell physiologic systems and material properties, particularly physical cues. From the cells known to interact with implanted biomaterials, the response of the immune system has been a critical target of study recently. Here, we review studies characterizing the response of innate immune cells to various material cues, particularly of those at the surface of implanted materials.
The innate immune system consists of cell types with various roles in inflammation. Neutrophils and macrophages serve both phagocytic and signaling roles, especially early in the inflammatory phase of biomaterial implantation. These cell types ultimately dictate the outcome of implants as chronic inflammation, fibrosis, or integration. Other cell types like dendritic cells, mast cells, natural killer cells, and innate lymphoid cells, may also serve an immunomodulatory role in the biomaterial context. This review highlights recent advances in our understanding of the role of innate immunity in the response to implantable biomaterials as well as key mechanobiological findings in innate immune cells underpinning these advances.
Keywords: Immunomodulation, immune response, macrophage, neutrophils, dendritic cells, surface roughness, hydrophilicity, wettability
1. Introduction
Early in the use of biomaterials, biological inertness was thought to be ideal for implants, and bioinert materials were sought for applications ranging from artificial organs to dental implants to contact lenses. While bioinert materials did not elicit an adverse response after implantation, there was also limited to no regeneration of parenchymal tissue after implantation. In certain tissues, particularly load-bearing tissues like bone, a lack of regeneration limited implant success. In bony tissues, true success was first found with the development of titanium (Ti) implants [1]. In the 1950’s, Ti was found to fuse completely with surrounding bone, and osteoconductivity was thereafter established as a key component of a “good” bone-dwelling implant [2]. Later, roughening the surface of Ti implants in dental applications was found to enhance bone growth around the implant, indicating that bone growth could be directed by physical implant cues [3]. Thus, the aim of biomaterials research shifted from bioinert to bioactive implants, focusing on two important questions: which material properties give rise to bioactivity, and which biological systems mediated the favorable response to these materials?
Like wound healing, the response to implanted materials occurs in four phases: hemostatic, inflammatory, proliferative, and finally the remodeling phase [4,5]. The shift from the inflammatory phase to the proliferative phase has been heavily explored in various tissue models, and failed transition at this point most often leads to failed healing, characterized by fibrous encapsulation instead of tissue regeneration. During this transition, immune cells such as macrophages and neutrophils orchestrate effective healing by altering their phenotype and recruiting cells that will follow in the proliferative phase. [4,6]. This critical transition in wound healing following biomaterial implantation has been probed, and tunable biomaterial properties have been identified that promote transition to later phases of the healing process.
In this review, we will highlight the role of physicochemical biomaterial properties on the early innate immune response in the absence of exogenous drugs, small molecules, or biologicals. We will focus on non-degradable biomaterials FDA-approved for implantation since the particles produced by material wear or degradation cause inflammatory events independent of chemical or physical cues and this is not usually an early event following biomaterial implantation. We will summarize the role of key innate immune cell types in the response to implanted biomaterials (summarized in Table 1) and examine recent advances in understanding of the role of material properties in modulating the immune response.
Table 1.
Summary of innate immune response to biophysical properties
| Cell Type | Material | Biophysical Property | Biological Model | Origin/Cell line | Biological effect | Reference |
|---|---|---|---|---|---|---|
| Neutrophils | Titanium | Hydrophilicity | In vitro | C57BL/6J mice | ↓ Cytokine release ↓ NET formation |
76 |
| Hydrophilicity | In vitro | Human PBMC | ↓ Cell activation ↓ ROS generation |
230 | ||
| Titanium PEEK | Roughness Hydrophilicity | In vivo | New Zealand White Rabbits | ↑ Neutrophils on PEEK | 231 | |
| PDO | Architecture | In vitro in vivo | Human PBMC (in vitro) Sprague-Dawley rats (in vivo) | ↓ NET formation on large diameter fibers ↓ Net formation in vivo |
232 | |
| PDMS | Stiffness | In vitro | C57BL/6J mice | ↑ NET formation | 233 | |
| Polyacrylamide | Stiffness | In vitro | Human PBMC | ↓ Migration speed ↑ Spreading ↑ NET formation with LPS treatment |
84 | |
| Polyacrylamide | Stiffness | In vitro | Murine myeloid progenitor | ↑ Spreading | 234 | |
| PEG-Gelatin | Hydrophilicity | In vitro | Human PBMC | ↑ MPO release ↓ MMP-9 | 235 | |
| PTFE Dacron | Hydrophobicity | In vitro in vivo | Human PBMC | ↑ Cell death ↑ ROS generation |
83 | |
| PTFE | Hydrophobicity | In vitro | Human PBMC | ↑ Net formation ↑ Histone citrullination ↑ Elastase and ROS generation |
236 | |
| Macrophages | Titanium | Roughness | In vitro | THP-1 | ↑ Pro- and anti-inflammatory markers | 125 |
| Roughness | In vitro | RAW 264.7 | ↑ Cell elongation ↑ Chemokine levels |
127 | ||
| Macrophages | Titanium | Roughness | In vitro | RAW 264.7 | ↑ Pro-inflammatory markers | 130 |
| Roughness Hydrophilicity | In vitro | C57BL/6J mice | ↓ Pro-inflammatory cytokines ↑ M2 phenotype |
80 | ||
| Roughness | In vitro | J774A.1 | ↓ Nitric oxide, iNOS, and pro-inflammatory cytokines | 141 | ||
| Roughness | In vitro | J774.A1 | ↓ Pro-inflammatory cytokines | 142 | ||
| Roughness | In vitro in vivo | C57BL/6J mice | 30 nm structures ↑ M2 phenotype 100 nm structures ↑ M1 phenotype | 147 | ||
| Roughness Hydrophilicity | In vitro in vivo | C57BL/6J mice | ↑ M2 phenotype ↑ Anti-inflammatory cytokines ↓ Pro-inflammatory cytokines |
77 | ||
| Roughness Hydrophilicity | In vitro | C57BL/6J mice | ↓ IL-1β, IL-6, TNF ↑ IL-4, IL-10 |
78 | ||
| Roughness Hydrophilicity | In vitro | RAW 264.7 | ↓ Pro-inflammatory cytokine ↑ Anti-inflammatory cytokines |
149 | ||
| Roughness Hydrophilicity | In vitro | THP-1 | ↓ Pro-inflammatory cytokines | 148 | ||
| Hydrophilicity | In vitro | RAW 264.7 | ↓ Pro-inflammatory cytokines | 150 | ||
| Roughness Hydrophilicity | In vitro | Human PBMC | ↓ Pro-inflammatory cytokines ↑ M2 phenotype |
152 | ||
| Hydrophilicity Hydrophobicity | In vitro | Human PBMC | ↑ Anti-inflammatory cytokines on hydrophilic material | 156 | ||
| Titanium Titanium alloy | Roughness Hydrophilicity | In vitro | C57BL/6J mice | ↓ IL-1β, IL-6, TNF ↑ IL-4, IL-10 |
132 | |
| Titanium Titanium alloy | Roughness Hydrophilicity | In vitro | C57BL/6J mice | ↑ Anti-inflammatory cytokines ↓ Pro-inflammatory cytokines |
133 | |
| Macrophages | Titanium PEEK | Roughness Hydrophilicity | In vivo | New Zealand White Rabbits | ↑ Macrophages on PEEK ↑ M2 phenotype on Titanium |
231 |
| Titanium alloy | Architecture | In vitro | RAW 264.7 | ↓ Metabolism ↓ Pro-inflammatory cytokines |
140 | |
| PLLA | Architecture | In vitro | RAW 264.7 | ↑ Anti-inflammatory phenotype | 144 | |
| PLGA | Hydrophilicity | In vitro | RAW 264.7 | ↓ Cell activation ↓ Pro-inflammatory cytokines |
153 | |
| PCL | Roughness | In vitro In vivo | C57BL/6J (in vitro) Sprague-Dawley rats (in vivo) | ↑ Cell elongation ↑ Arginase 1 and IL-10 expression ↑ Anti-inflammatory phenotype |
128 | |
| Architecture | THP-1 (in vitro) Sprague Dawley rats (in vivo) | ↑ M1 phenotype on random alignment | 237 | |||
| PLLA-PCL | Architecture | In vitro in vitro | Sprague-Dawley rats | ↑ IL-10 and Arg1 ↓ TNF and iNOS ↑Anti-inflammatory phenotype |
145 | |
| PEEK | Hydrophilicity | In vitro | RAW 264.7 | ↓ Pro-inflammatory cytokines ↑ Anti-inflammatory cytokines |
154 | |
| Polyethylene | Architecture | In vitro | C57BL/6J mice | ↑ Anti-inflammatory cytokines ↓ TNF |
126 | |
| Polyvinylidene fluoride | Roughness | In vitro | Human PBMC | ↑ Pro- and anti-inflammatory activation | 131 | |
| Polystyrene | Hydrophilicity | In vitro | Human PBMC | ↑ Anti-inflammatory cytokines | 238 | |
| Tricalcium phosphate | Roughness | In vitro | RAW 264.7 | ↑ iNOS and IL-1β | 114 | |
| Hydroxyapatite | Roughness | In vitro | RAW 264.7 | ↑ M2 phenotype ↓ M1 phenotype |
143 | |
| DC | Titanium | Roughness Hydrophilicity | In vitro | Human PBMC | ↑ CD86 ↓ IL-10, IL-1ra, MCP-1, IL-8 |
178 |
| DC | Polystyrene PTFE PMMA | Hydrophilicity Hydrophobicity Architecture | In vitro | Human PBMC | ↑ Activation and migration on smooth surfaces | 183 |
| MC | Titanium | Roughness | In vitro | RBL-2H3 | ↑ Adhesion ↑ Proliferation ↑ Migration |
194 |
| PLGA | Hydrophilicity | In vivo | Balb/c mice | ↑ Cell recruitment ↑ Degranulation |
239 | |
| PDO | Architecture | In vitro | C57BL/6J mice | ↓ IL-6 and TNF ↑ VEGF |
240 | |
| PDO PCL Fibroin |
Chemistry Architecture | In vitro | C57BL/6J mice | ↑ Adhesion on polymers ↑ Proliferation on polymers ↑ TNF, MCP-1, and IL-13 on polymers |
241 | |
| Polypropylene | Hydrophilicity | In vivo | Wistar rats | ↑ Cell number ↑ NADPH |
242 | |
| Polystyrene | Architecture | In vitro | NCL-2 | ↑ Proliferation | 243 | |
| NK | PDMS | Stiffness | In vitro | Human PBMC | Bell shaped of NK adhesion and CD107 expression in response to stiffness ↑ Activation in response to stiffness |
221 |
| Architecture | In vitro | NK-92MI | ↑ NK cytotoxicity on large microwells | 229 | ||
| PLA Chitosan | Architecture | In vitro | Human PBMC | ↓ NK metabolism ↑ NK number |
244 |
2. Implantation and tissue injury
Biomaterial implantation results in tissue injury during the surgical procedure to placeit. Following this initial insult, wound healing begins with the onset of hemostasis. Coagulation proteins, platelets, complement, and other soluble serum and blood proteins adsorb onto the surface of implanted biomaterials within seconds after implantation (Figure 1). Due to the diverse range of physical and chemical properties of implanted biomaterials, the initial interactions between biological tissues and biomaterials are not fully understood. The molecules and proteins immediately adsorbed onto the surface of biomaterials vary in type, quantity, and conformation because of the physical and chemical material properties [7–9]. These adsorbed components provide recognition sites for platelets and cells to interact with the biomaterial, since cells have a limited capacity to adhere directly to a non-proteinaceous material [10].
Figure 1.
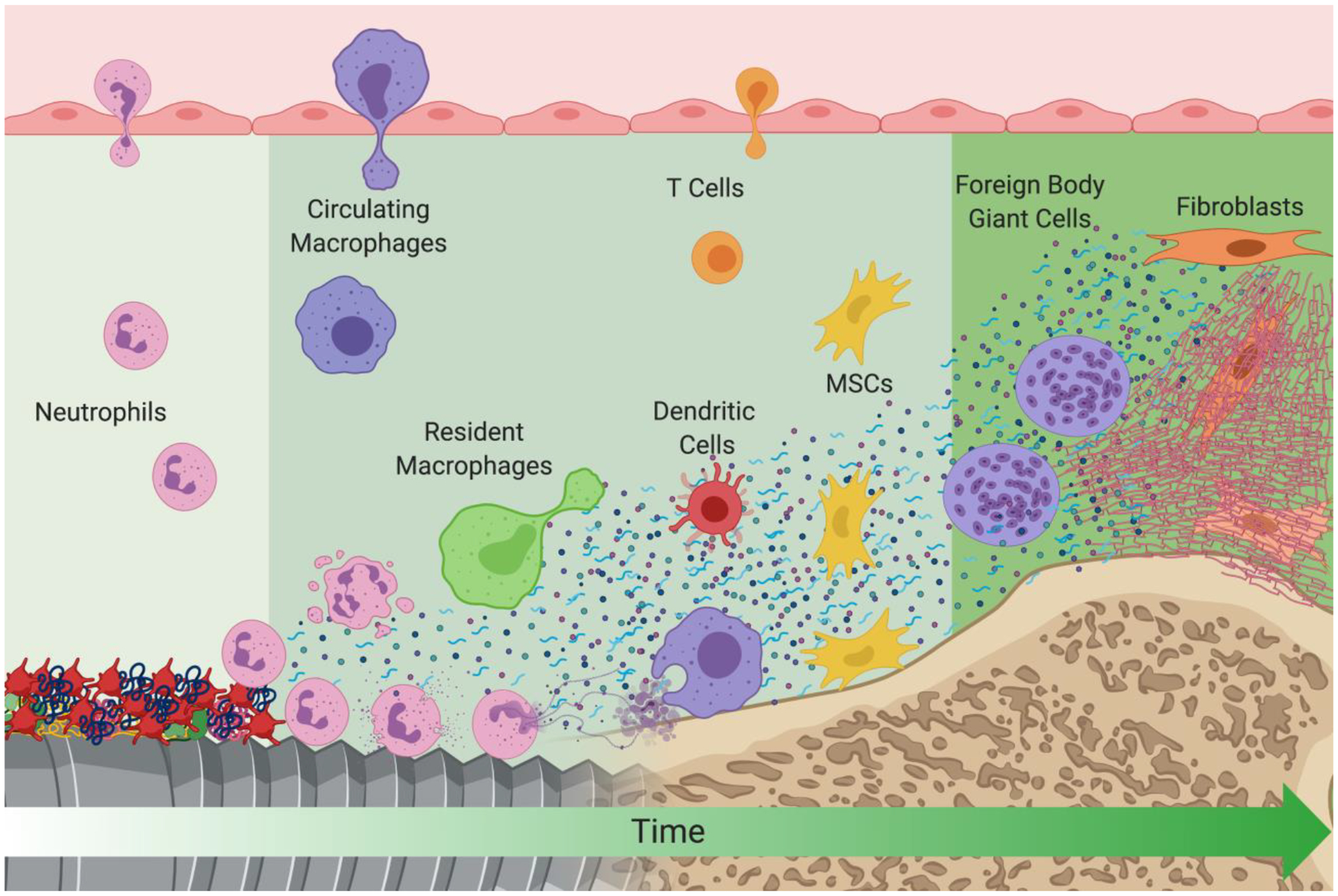
A schematic of events that take place at the biomaterial/host interface after implantation. Early events are depicted on the left of the figure and later events are depicted on the right. Created with BioRender.com.
Following homeostasis, the inflammatory phase, predominated first by neutrophils and then by macrophages, begins. Danger signals activate neutrophils and macrophages at the implantation site [11–13]. These signals are classified into two categories: pathogen-associated molecular patterns (PAMPs) originating from microbial molecules or damage-associated molecular patterns (DAMPs) created during biomaterial implantation. DAMPs can be intracellular molecules that are released into the extracellular compartment upon cell death like high mobility group box 1, myosin heavy chain, S100 proteins, nucleic acids, and ATP [14], or from extracellular matrix (ECM) fragmentation during the injury, exposing hidden molecular patterns [15,16].
The tissue damaged cause by instrumenting a biomaterial in the body, protein adsorption on the biomaterial surface including the activation of the complement system, and the physical cues such architecture and stiffness activate innate immune cells and dictate the fate of the newly implanted biomaterial. Here we will focus on early events in the innate immune response to biomaterial implantation.
3. Complement System
The complement system consists of more than 50 proteins distributed in the circulation and it is an integral part of the innate immunity for host defense against endogenous danger molecules and invading pathogens [17]. The complement system is activated immediately after contact with PAMPs and DAMPs, leading to a cascade of protease-based cleavage and activation [18]. Complement activation leads to generation of chemoattractant molecules that recruit and activate other innate immune cells, amplifying the inflammatory response [19]. Complement activation can occur through three main pathways: classical, alternative, or lectin pathways [19]. The activation of these pathways has been described elsewhere [19,20]. Common to all activation pathways is the generation of C3 convertase, which cleaves C3 into C3a and C3b (19). C3a acts directly as a pro-inflammatory molecule, recruiting and activating other innate immune cells like neutrophils and macrophages [19]. C3b can function as an opsonin to bind pathogens and induce their phagocytosis, but is also a building block of the alternative pathway C3 and C5 convertases [19]. C5 convertase cleaves C5 generating C5a and C5b, two important complement mediators, which can act directly as a pro-inflammatory mediator, in the case of C5a, inducing chemoattraction of other immune cells and activating them through its receptor C5aR1 and C5aR2 [19,20]. C5b is an important subunit of the terminal complement complex, which is assembled sequentially from C5b, C6, C7, C8, and several C9 molecules [19]. This multi-molecule complex is a membrane pore-forming structure with lytic activity known as a membrane attack complex [21]. While most complement proteins generate in the liver, several extrahepatic cells, including fibroblasts, endothelial cells, and immune cells, can also generate complement components locally in their resident tissues [20].
3.1. Complement System and Biomaterials
Biomaterial interaction with blood leads to the adsorption of proteins onto the biomaterial surface. Protein-biomaterial interactions can lead to conformational changes in the adsorbed proteins that can expose hidden domains or epitopes that can trigger complement activation [22]. C3 binds to the newly adsorbed protein layer and can initiate the alternative pathway convertase [23]. Products of the cleavage of C3 at the biomaterial surface can also bind directly to proteins adsorbed onto the biomaterial surface and amplify the inflammatory signal [24]. In addition, products of the complement function as chemoattractants for other immune cells like neutrophils, macrophages, and monocytes through C3aR and C5aR receptors [19]. Although complement molecules adsorb onto the surface of biomaterials, most studies have focused on how biomaterial chemistry affects complement adsorption. Slight changes in the chemical composition of polymers can affect complement adsorption. In one study, incorporating methacrylic acid to methyl methacrylate (MMA) increased complement molecules adsorbed (C1q, C4a) from plasma when compared to polymethyl methacrylate (PMMA), but PMMA adsorbed more C3 [25]. Interestingly, MMA had less complement activation when exposed to serum than PMMA. While the study did not compare effects of surface roughness or surface energy using the same polymer, the authors point out that MMA beads were rougher, which increased the surface area for proteins to be adsorbed, and had a higher charge due to the methacrylic acid [25]. Several strategies have applied to biomaterials to modify the protein-biomaterial interaction and the cellular response. Polyethylene glycol (PEG) functionalization is commonly used to prevent protein adsorption. However, PEG-coated surfaces are still able to activate the complement system and increase the inflammatory response [26, 27,28]. Similar effects are seen with polyvinyl alcohol and dextran functionalization where complement activation occurred through the reaction of the C3b with the hydroxyl groups on the biomaterial surface [29]. Another study correlated a slightly positive zeta potential with increased complement protein adsorption while negative zeta potential materials showed lower adsorption of the same complement proteins [30]. Hydrophilicity or hydrophobicity of biomaterials can also affect complement activation. Hydrophilic and hydrophobic silicon substrates were assessed in their ability to adsorb C3 molecules. C3 adsorbed in higher amounts onto hydrophobic silicon and suffered conformational changes exposing antigenic epitopes in comparison to the hydrophilic silicon [31]. However, other studies exploring the activation of the complement system on Ti did not find any difference in complement activation between hydrophobic and hydrophilic modifications on Ti substrates. The same study showed similar levels of C3a when comparing rough microstructured Ti surfaces to smooth surfaces [32], despite the fact increased surface area results in an increase in protein adsorption. Surface properties like surface chemistry, area, and energy affect protein adsorption, including complement, less is known about how the complement system is activated at the biomaterial surface. Immune cells have the ability and perhaps play a significant role in complement activation, and activation of other immune cells by molecules of the complement system usually results in production of more complement molecules producing a positive feedback loop that chemoattract and activate other immune cells [29,31,33].
4. Neutrophils
Neutrophils are mobilized to the biomaterial during the initial hemorrhage following implantation, and are abundant during hemostasis and the early inflammatory stage [34,35]. Neutrophils are key in recruiting macrophages and other immune cells to the injury site but have not been well studied in healing and regeneration. They are the predominant immune cell type in human blood, and low neutrophil level has often fatal consequences in response to infectious agents [36]. In addition, neutrophil activity is also associated with worsening disease outcome, particularly in pulmonary diseases like emphysema where neutrophil elastase damages the elastic properties of lung tissue [37–40]. Neutrophils play complex, multifaceted roles in classical inflammation through cytokine and chemokine production, phagocytic activity, myeloperoxidase (MPO) and elastase secretion, and generation of reactive oxygen species (ROS) [41–43].
Neutrophils express several receptors that are used to recognize soluble inflammatory mediators, from active biolipids to cytokines [44–46]. This first interaction with inflammatory mediators primes their response by which neutrophils switch to a pre-activated state [47–49]. These signals recruit and activate neutrophils through toll like receptor (TLR)/NF-κB [50], ATP-mediated activation of the NLRP3 inflammasome [51], receptor for advanced glycation end products (RAGE) [52,53], CXCL8 [54–56], and leukotriene B4 [57–59]. The priming response, mediated by upregulation of adhesion molecules and inside-out activation of integrins [60,61], is critical for neutrophils to transmigrate the endothelium and mobilize to the injury site [48,49].
4.1. Neutrophil Phenotypes
The first studies demonstrating that neutrophils may be a heterogenous cell population came from cancer, identifying subsets of tumor-associated neutrophils in mice [62]. Neutrophils exhibit distinct phenotypes along a spectrum from pro- to anti-inflammatory, although these phenotypes are not as well-characterized as those of macrophages. There is evidence that the cells’ lifespans depend on their phenotype, as early neutrophils are of pro-inflammatory phenotypes and are short-lived, while later neutrophils are more of anti-inflammatory phenotypes and persist up to 3 days [63,64]. These phenotypes were first explored in tumor immunology where it was found that pro-inflammatory neutrophils perform more cytotoxic, tumor-rejecting roles, while anti-inflammatory neutrophils perform angiogenic, immuno-suppressive roles and enabling invasive behavior of the tumor [65]. Pro-inflammatory neutrophils, designated N1, produce high levels of tumor necrosis factor (TNF) alpha, CCL3, CXCL9, and CXCL10, facilitating recruitment of T-cells. In the context of cancer, N2 neutrophils produce hepatocyte growth factor (HGF), oncostatin M, and matrix metalloproteinases [65].
Interestingly, neutrophils exhibit swarming behavior in the response to inflammatory cues and produce specialized DNA-based neutrophil extracellular traps (NETs) [66,67]. NETs are enzyme-adorned DNA structures initiated by the action of peptidyl arginine deiminase 4 on histones and serve to capture bacteria in non-sterile inflammation as well as to mediate thrombogenic sequelae of various diseases in sterile inflammation [68–74]. In turn, neutrophils recruit macrophages through CXCL1-3, IL-1β, TNF-α, and MPO-generated ROS [13,42,75].
4.2. Neutrophil response to Biomaterials
While neutrophils are well studied in classic and cancer immunology, less is known about their role in healing in the biomaterial context. Nevertheless, it has been shown that biomaterial surface properties can modulate activation of neutrophils. Abaricia et al. shown that neutrophils respond differentially to changes in surface roughness and wettability (hydrophilicity) of Ti implant surfaces; on smooth or rough hydrophobic surfaces, neutrophils secrete higher levels of pro-inflammatory cytokines and enzymes while also undergoing enhanced NET formation (NETosis) compared to those on rough-hydrophilic surfaces [76]. This disparity was also associated with subsequently decreased macrophage inflammatory activation in co-culture, particularly when NET formation was inhibited pharmacologically. These results in clinically relevant implant materials corroborate earlier findings that hydrophilicity significantly decreases pro-inflammatory activation of leukocytes compared to hydrophobic, cationic, or anionic surfaces [77–81]. Roughness also affects neutrophil behavior in response to polymers. Regions of rough expanded polytetrafluoroethylene and Dacron on polymeric cardiovascular implants were found to induce more neutrophil death and ROS generation compared to smooth regions [82]. A similar effect was seen on polymeric electrospun scaffolds, where small-diameter (~0.3μm) fibers—perceived as rougher on a cellular level—produced an enhanced NETotic response compared to large-diameter (~1.9μm) fibers [82]. These results highlight that neutrophils are sensitive to changes in physical and chemical properties of biomaterials and that this response can affect the subsequent chemotaxis and macrophage activation in vivo and clinical settings. In addition to surface topography and wettability, stiffness has also been shown to modulate neutrophil activation. In one recent study, neutrophil migration speed was decreased on stiff (100 kPa) polyacrylamide (PAA) hydrogel compared to soft (5 kPa); this study and others have also demonstrated enhanced neutrophil spreading on stiff hydrogels, suggesting that NETosis is also regulated by stiffness [83].
Despite their underappreciated role in the immune response to biomaterials, neutrophils are important mediators of the initial inflammatory response to implant placement. Future work should examine the signaling pathways mediating these responses, drawing on likely analogues with pathologic inflammation studies as well as elucidating role of NETosis in modulating the inflammatory response at the biomaterial-tissue interface to enhance the success of biomaterial implants.
5. Macrophages
Macrophages are the most studied immune cells in respect to the immunological response to biomaterials. They are either present in the surrounding tissue (tissue-resident macrophages) when the biomaterial is implanted or are recruited to the injury site by neutrophils. Macrophage activation and phenotype can be altered by biomaterial physicochemical characteristics such as surface topography, wettability, and stiffness [77–80]. However, most of the body of work in these topics have contradictory findings, suggesting that while macrophages are sensitive to these parameters, the resulting macrophage activation and phenotype are due to both the physicochemical factors of the biomaterial as well as biological factors in the recipient tissue [84,85]. Here we will discuss the physicochemical factors, in absence of PAMPs, controlling macrophage phenotype and inflammatory microenvironment.
Macrophages and their precursors (monocytes) are innate immune cells long known to orchestrate both inflammatory responses and tissue dynamics involved in the recognition, phagocytosis, and elimination of pathogens. Macrophages are highly phenotypically heterogeneous but are broadly classified as M1 or M2 macrophages [86]. M1, or classically activated macrophages, are traditionally associated with a pro-inflammatory response induced by IFNγ, lipopolysaccharides (LPS), and TNF-α [86,87]. When stimulated, M1 macrophages secrete high-levels of pro-inflammatory cytokines like IL-1α, IL-1β, IL-6, IL-12, and IL-23 [88–91]. M2 macrophages are associated with an anti-inflammatory, pro-regenerative phenotype, mainly due to the high production of IL-10, matrix metalloproteinases, and growth factors such as VEGF [86,87,92,93]. While the M1/M2 paradigm has been useful to exemplify two extreme phenotypes for in vitro studies, the macrophage phenotype exists along a continuum of activated states [86,94]. For example, macrophages treated with LPS have reduced phagocytic capacity compared to macrophages treated with IFNγ but both increase the production of pro-inflammatory mediators [95]. M2 activation has different subset populations (M2a, M2b, M2c, M2d, M2f) based on the induction protocol, cytokine and growth factor secretion, and surface markers present [96]. This demonstrates the complexity of macrophage activation in vivo that cannot be replicated in vitro.
5.1. Macrophage Ontology
The prevailing dogma was that macrophages in tissues were constantly replenished by circulating monocytes originated from bone marrow; however, early evidence of locally long-lived macrophage proliferation in tissues laid the work to revise the mononuclear phagocyte system [97,98]. Cre-lox-based inducible fate-mapping models using Runx1 and Cx3Cr1 showed that macrophage populations originated from the early yolk sack before the appearance of hematopoietic stem cells [99,100]. Successive studies demonstrated that embryonically derived macrophages arise from distinctive and successive waves of early yolk sac macrophages, fetal liver monocytes, and bone marrow derived monocytes; in that regard, adult tissues in homeostasis contain a mosaic of these three ontogenetically distinct populations [101].
Embryonic origin, local microenvironment, inflammatory status, and time of residency in the tissue modifies the cellular and biological response to stimuli [97,102,103], which can explain the heterogeneity and sometimes contradictory results seen when comparing macrophage response to biomaterials implanted in different tissues. However, the distinct response of these macrophage subsets to biomaterials is not well studied. Several wound healing studies demonstrate that macrophages from different origins have distinct roles during the healing process and suggest the possibility that similar effects can be observed with implanted biomaterials into those tissues [89,91,104–106]. While most studies have shown that the majority of macrophages at the injury site are bone marrow derived, these macrophages are activated through TLRs [107]. Studies in skin have proposed that tissue-resident macrophages serve extremely early roles in wound healing, first responding to DAMPs produced by injury by secreting hydrogen peroxide, which triggers the inflammatory cascade [108]. Another study showed that long-term tissue resident macrophages predominantly exhibit an alternate activation state in a model of skin would healing, whereas bone marrow derived macrophages contribute to both, early and late stages of wound healing and show classical and alternative activation states [109]. In other studies, monocyte-derived macrophages accumulate under injury conditions in the liver and function as the primary determinants of injury outcome [110–112]. Thus, the importance of these subsets on biomaterial responses, especially considering their unique importance to aspects of healing, remains an open area of study.
5.2. Macrophage Activation
Following triggering of the inflammatory cascade, circulating monocytes begin extravasation into the injury or implant site. The recruitment of monocytes and subsequent exposure to DAMPs and PAMPs in the injury site induces a pro-inflammatory phenotype of this macrophage population, which serves to debride dead tissue, kill pathogens, and recruit both innate and adaptive immune cells. Key markers of pro-inflammatory macrophage phenotypes include enhanced production of surface CD80 (B7-1), CD86 (B7-2), and TLR-4, as well as increased gene expression or production of inducible nitric oxide synthase (iNOS), IL-1β, IFNγ, TNF-α, IL-6, IL-12p40, and chemokines CXCL1-3. Activation of TLR-4, whose ligands include PAMPs and DAMPs, can mediate immunological recognition of implanted biomaterials, and deletion of TLR-4 in mice shifts the material-adherent immune cell population from monocytes/macrophages to neutrophils [11]. Indeed, the failure of orthopedic implants by both aseptic and septic loosening has been tied to unwanted DAMP- and PAMP-activated inflammatory activation [113].
After recruitment, macrophages take on microenvironment- and biomaterial surface-dependent phenotypes that dictate the integrative fate of the implant. The predominant macrophage phenotype during early injury or implantation are pro-inflammatory, but multiple subtypes within the “M2” or anti-inflammatory classification are involved in tissue repair at later time points in the implantation or injury response. Ablation of macrophages using clodronate liposomes or inducible transgenic models dramatically affect the microenvironment, immune and stem cell recruitment, and ultimately, biomaterial integration [77,114]. Macrophage phenotypes can be induced by both bulk and surface physicochemical material properties like material composition, substrate stiffness, surface topography and hydrophilicity or wettability have been found to affect the inflammatory response and consequent integrative fate of biomaterials.
5.3. Response of Macrophages to Changes in Substrate Stiffness
The effect of biomaterial stiffness on macrophage activation and phenotype has been broadly explored. In polymers with similar chemistry, increasing stiffness has been associated with increased pro-inflammatory macrophage phenotype in multiple studies, both in vitro and in vivo [115,116]. Sridharan et al. recently demonstrated that increasing stiffness modulates macrophage polarization and changes their mode of migration; using collagen-coated polyacrylamide gels, the authors showed that higher stiffnesses increased pro-inflammatory polarization and change the mode of macrophage migration from ROCK-dependent fast ameboid migration to slow podosome-dependent mesenchymal [117]. Conversely, another study found that increased stiffness enhances anti-inflammatory polarization through NF-κB signaling [118]. This study was also performed on polyacrylamide hydrogels of varying stiffness but without collagen coating, suggesting that both stiffness and protein adsorption profiles might regulate macrophage polarization, where activation of NF-κB by some class of denatured adsorbed protein might change the cellular response to stiffness. Other studies with both biologic and manufactured substrates have shown that increasing biomaterial stiffness promotes a pro-inflammatory phenotype that migrates more readily, accompanied by dysregulated integrin production [119]. While many of these high-stiffness responses have been shown to be mediated by Wnt/β-catenin signaling [120,121] and MAPK signaling [122,123] in macrophages and other cells, the signaling pathways, receptors, and transcription factors involved in macrophage response to biomaterial stiffness remains unclear. While stiffnesses of current FDA-approved biomaterials exhibit elastic moduli much higher than macrophages are capable of distinguishing, stiffness may be a useful tunable property that can be used to control the macrophage activation and the overall inflammatory response.
5.4. Macrophage Response to Changes in Surface Topography
Surface topography also modulates macrophage phenotype. In commonplace implant materials—chiefly Ti or its alloys—roughness can modulate macrophage polarization in different ways. Early work in this field performed by Barth, Waterfield, and Brunette demonstrated that mildly enhanced anti-inflammatory polarization occurred in response to roughened Ti surfaces by RAW264.7 macrophage-like cells [124]. Another study demonstrated that anti-inflammatory macrophage polarization was enhanced over a small range of roughness (Ra=0.51–1.36μm), while roughness outside of the range upregulated a mixture of pro- and anti-inflammatory markers [125]. The effect of microtopography on reducing pro-inflammatory activation has been observed also in other biomaterials outside of Ti or its alloys like resorbable zinc, zirconia polycrystal, shape memory PCL-PEG substrates and polyethylene films, and silicon [126–129]. However, opposite results have been also reported. Early studies using RAW264.7 macrophage-like cells showed that sand blasted and acid etched Ti substrates increased levels of pro-inflammatory cytokines [130]. Li et al. recently showed that that micron roughness, on tricalcium phosphate substrates, generated by diamond microtome resulted in upregulation of iNOS and IL-1β in RAW264.7 cells while submicron roughness resulted in an anti-inflammatory phenotype [114]. In another study, Hamlet et al. showed that M2 activated macrophages cultured on microstructured Ti surfaces promoted a switch to a pro-inflammatory phenotype with an upregulation of pro-inflammatory cytokines [80]. In contrast, other studies have shown that macrophages can activate into both pro- and anti-inflammatory phenotypes, microstructures on polyvinylidene fluoride (PVDF) substrates induced human macrophages into a pro-but also an anti-inflammatory phenotype [131]. Similar results were found by Hotchkiss et al. using medical- and dental-grade implant materials; surface roughness enhanced anti-inflammatory activation of primary murine macrophages compared to smooth surfaces, while also interestingly upregulating pro-inflammatory activation markers as well [78,132,133]. These conflicting results can be result of the diverse cells used (primary cells vs. cell lines, species, cell source) and culture conditions.
Our current understanding of macrophage activation and function is based on studies using primary cells isolated from specific tissues or from immortalized cell lines. RAW 264.7 cells are a murine-leukemic macrophage-like cell line that has been widely used to study macrophage-biomaterial interactions [134, 135, 136, 137]. While RAW 264.7 cells phenotypically resemble bone-marrow-derived primary macrophages in some markers like CD11b, CD11c, CD14, F4/80 and in their response to microbial molecules through TLR3 and TLR4, they do not mimic the phenotypic markers and microbial response of splenic macrophages [138]. There are also significant differences in cell surface receptors between bone marrow derived macrophages and common macrophage-like immortalized cell lines (IC-21, J774A.1, and RAW 264.7) in response to polystyrene, poly-l-lactic acid (PLLA), and Teflon-AF [139]. The authors also found differences in cell morphology, surface marker expression, and cytokine expression profile in response to LPS activation and in cytokine expression in response to biomaterial composition [139]. Macrophages are a highly plastic, heterogeneous, and dynamic cell type which vary in phenotype based on cues from their microenvironment. Their heterogeneity is due to the various functions processes that they carry out in different tissues, such bone marrow derived macrophages differ in phenotype, function, and activation from those derived from other sources (spleen, intraperitoneal cavity lung). Therefore, cell source is an important consideration when designing a testing system for biomaterials.
Nanostructures have been also reported to affect macrophage phenotype. Ion et al. demonstrated that nanochannel structures created by anodizing Ti50Zr alloy resulted in less metabolically active macrophages with a decrease in pro-inflammatory cytokines [140/118]. Similar results were reported by Lee et al. creating nano-roughness with Ti coated coverslips, where they found a decrease in nitric oxide, iNOS, and pro-inflammatory cytokines in response to Ti nanotopography [141]. In another study, Park et al. showed that murine J774.A1 macrophage-like cells decrease pro-inflammatory markers in response to Ti nanostructures created by hydrothermal modification [142]. Similarly, Lu et al. showed that nanostructured and submicron-structured Ti stents decreased levels of pro-inflammatory cytokines when compared with smooth stents [142]. This anti-inflammatory effect of nanostructures on biomaterials has also been observed on other biomaterials. Linares et al. showed that nanocrystalline hydroxyapatite and nanocrystalline silicon substituted hydroxyapatite increased macrophage polarization towards an M2 phenotype, decreasing the M1 population [143]. Besides nanostructures, nanofibers have been shown to increase the anti-inflammatory phenotype of macrophages when compared to flat or micron scale fibers in poly electrospun PLA [144]. Recent studies have shown in vitro and in vivo that the diameter of the nanofibers is not the only parameter that alters macrophage phenotype but also fiber orientation. Aligned nanofibers altered macrophage morphology but more importantly, increased activation of anti-inflammatory macrophage phenotype, whereas random nanofibers induced a pro-inflammatory phenotype [145,146]. Nanostructures created on Ti by anodization with a size of 30 nm favor an M2 polarization and nanostructures around 100 nm favor an M1 macrophage phenotype [147].
5.5. Effect of Hydrophilicity on Macrophages
Perhaps the most potent surface property found to modulate anti-inflammatory macrophage activation is surface hydrophilicity or wettability. Typically measured indirectly through hydrophilicity, increased surface energy greatly enhances anti-inflammatory macrophage polarization in various applications. Hotchkiss et al. showed that while markers of M1 and M2 macrophage activation increased with roughness, the combination of roughness and hydrophilicity suppressed pro-inflammatory markers and greatly enhanced anti-inflammatory markers [77,78,132,133]. Hamlet et al. showed that the same rough hydrophilic Ti substrates decrease pro-inflammatory cytokine expression on human macrophages and RAW264.7 cells [148,149]. The same group have recently showed that the same hydrophilic Ti substrate is able to re-establish macrophage homeostasis in diabetic animals by switching a M1 macrophage phenotype to a pro-regenerative M2 phenotype [149]. Similarly, Sunarso et al. showed that superhydrophilic Ti substrates created by ozone gas functionalization mitigated proinflammatory cytokine production on RAW264.7 macrophage-like cells [150/128]. Ultraviolet light also has been used to increase hydrophilicity on Ti substrates or on anatase TiO2 films where the increase in hydrophilicity resulted in a decrease of pro-inflammatory cytokine expression and increase in macrophage M2 phenotype [151,152]. Oxygen plasma treatment also has been used to increase biomaterial hydrophilicity and has been shown to decrease macrophage activation and levels of important pro-inflammatory cytokines in RAW264.7 cells and primary murine macrophages on poly(L-lactide-co-glycolide) (PLGA) [153], polyether ether ketone (PEEK) [154], and Ti [78]. Additionally, on milled polystyrene used for microfluidic applications, hydrophilicity produced comparable effects in primary human monocyte-derived macrophages, increasing anti-inflammatory IL10, MRC1, and CCL18 [155]. Mechanistically, Lin et al. demonstrated that hydrophilicity determines the conformational adsorption of fibronectin and fibrinogen though integrin signaling and consequent PI3K and NF-κB activation [156].
The consequences of altered macrophage polarization by biomaterials are myriad. Macrophages are responsible for recruitment and activation of T-cells and stem cells in the injury or implant microenvironment [88,90]. Many studies show they are also responsible for early angiogenic signaling [157,158]. When comparing rough and rough-hydrophilic Ti implants in vivo, Hotchkiss et al. found that greater numbers of anti-inflammatory Treg cells and MSCs were present on rough-hydrophilic implants; in vitro, macrophages alone on rough-hydrophilic Ti recruited greater levels of MSCs in a transwell assay [77]. Hamlet et al. demonstrated that conditioned media from anti-inflammatory macrophages polarized on hydrophilic Ti enhanced osteoblast BMP signaling [64]. Mechanistically, Wnt signaling can mediate both anti-inflammatory macrophage polarization in response to hydrophilicity as well as MSC recruitment [159]. Given that Wnts regulate MSC differentiation and proliferation on Ti implants [160,161], it is likely that macrophage Wnt ligands are targeted to MSCs as well, reflecting their role in orchestrating the behavior of stem cells in other tissues. It is still unclear how macrophages recognize the physicochemical properties of biomaterials and the possible signaling mechanisms that may be involved.
Another key consequence of material properties on macrophage activation is the formation of foreign body giant cells. These cells form from chronic inflammatory responses where high levels of IL-4 and IL-13 are produced and contribute heavily to fibrous capsule formation, which impairs the functionality of eluting, degrading, loadbearing, or electroconductive implants [162]. In bone, macrophage fusion produces multinucleated giant cells known as osteoclasts, which serve to resorb bone, leading some to postulate that foreign body giant cells form from mechanical mismatch between implants and surrounding tissue, inducing cell formation to attempt to degrade the foreign body and later encapsulate it [163]. Indeed, a correlation between high numbers of recruited macrophages and higher foreign body giant cell formation has been shown, suggesting that persistent or enhanced inflammatory activation leads to foreign body giant cells. While little is known about the role of foreign body giant cell formation in early healing, histologic studies have shown their presence on implants long-term [164], suggesting some level of inevitability of their formation with non-degradable materials. Nevertheless, these cells appear to be unfavorable in the context of osseointegration.
As evidenced by the large body of work exploring macrophage-biomaterial interactions, these highly plastic cells serve as both regulatory targets and effective “canaries in the coalmine” of biomaterial implants. While there is not a single biomaterial parameter that predicts macrophage activation, implants may succeed or fail based on the effects of their surfaces on macrophage phenotype, and as such, surface properties should always be considered when designing novel implants.
6. Dendritic cells
Dendritic cells (DCs), close relatives of macrophages but more specialized for antigen presentation, are also understudied players in the immune response to implantable biomaterials. DCs have been broadly explored in drug delivery application aimed to induce immune tolerance and to confer immunity for their antigen-presenting capabilities [165–167]. Like macrophages, DCs are mononuclear phagocytic cells that serve as gatekeepers to the inflammatory response in both non-sterile and sterile inflammation. Unlike macrophages that are recruited en masse during acute changes to homeostasis (e.g., injury, implantation), DCs classically serve more tolerogenic roles during homeostasis, having been shown to drive peripheral tolerance in the lungs, gut, and other mucosal surfaces in contact with microbiota or the external environment [168,169]. However, DCs play similar roles in healing as macrophages, promoting early inflammation and resolving late inflammation [170]. A key example of this role is the role of plasmacytoid DCs, a specialized circulating DC subpopulation, which enhance early wound pro-inflammatory activation by sensing host nucleic acids (as DAMPs) via intracellular TLR7 and -9, consequently secreting type I IFNs [171–173]. In burn wound healing, loss of CD11c+ cells, the archetypical DC surface marker, was associated with delayed healing, while enhancement of DC populations with fms-like tyrosine kinase-3 ligand accelerated it; these findings were correlated with decreased or increased levels of local TGF-β [174]. In bone, DCs have been implicated in inflammation-induced bone loss by activating of CD4+ T-cells, which induce osteoclastogenesis and bone turnover [175,176]. This suggests that DCs serve similar roles as macrophages at both the start and end of the inflammatory phase of healing.
DCs have expansive roles in the immune response to biomaterial implants. Vasilijic et al. demonstrated that as many as 25% of monocytes recruited to polyvinyl sponge implants differentiate into DCs, and their numbers increase steadily to 10 days post-implantation; interestingly, many of these dendritic cells were plasmacytoid [177]. Additionally, DCs isolated from the late inflammatory response (14d post-operatively) did not respond as robustly to allogeneic T-cell activation as earlier DCs (6d post-op), suggesting a true phenotypic shift as with macrophages in the implant response. DCs also respond to changes in surface roughness and hydrophilicity. Hydrophobic Ti with either smooth or rough topography induced mature or pro-inflammatory DC phenotypes, while hydrophilic Ti induces an immature or anti-inflammatory phenotype [178], similar to the phenotypic changes seen in macrophages [62]. Hydrophobic polymers like poly(lactic-co-glycolic acid) (PLGA) can induce mature DC phenotypes through direct agonism of integrin-β2 receptors [179,180], while highly hydrophilic hyaluronic acid surfaces induce immature DC phenotypes [181]. This is likely due to the distinct protein adsorption profiles of surfaces with different energy; hydrophilic surfaces adsorb fewer proteins that permit DC adherence and maturation [3,182]. Three-dimensional surface topography can also enhance DC maturation, whether on Ti, polystyrene, Teflon, or poly(methyl methacrylate) [178,183,184]. Further work exploring the tolerogenic roles of DCs in classical wound healing might yield valuable insight into their role in the response to implants.
7. Mast Cells
Mast cells (MC) are granulocytic cells of the innate immune system that play fundamental roles in both innate and adaptive immune responses. MC are chiefly known for their role in allergic reactions; however, recent studies have shown that MC are important in wound healing through cell recruitment, angiogenesis, and ECM deposition, as well as in fibrosis and foreign body response to implanted biomaterials [185]. MC exist as tissue-resident populations and are present in all vascularized tissues with a particular presence in skin, airways, and gastrointestinal tract [186]. The expression of the high-affinity immunoglobulin E (IgE) receptor, FcεRI, is one significant factor that distinguishes MC from other granulocytes. When a foreign antigen or allergen activates FcεRI, MCs degranulate and release mediators like histamine, proteases, proteoglycans, and cytokines and chemokines from cytoplasmic granules. MC activation also generates lipid mediators of inflammation like prostaglandin, leukotriene, and thromboxane, as well as the de novo production of cytokines, chemokines, and growth factors [186]. Antigens and pathogens directly activate MC through FcεRI and other pattern recognition receptors, including TLR, c-type lectin, NOD-like, and RIG-like receptors [187]. In this way, MC activation significantly impacts the activation and function of other immune cells and surrounding tissues. Besides their role in IgE-mediated allergic reactions, MC also play significant roles in defense against pathogens and other physiological and pathological processes like angiogenesis, wound healing, atherosclerosis, cardiovascular disease, and fibrosis [188].
7.1. Mast Cells and Biomaterials
MCs actively participate in inflammation, proliferation, and regeneration/remodeling following biomaterial implantation, and have a well-documented role in the development of a foreign body response. MC degranulation after biomaterial implantation, especially histamine release, leads to recruitment and adhesion of other inflammatory cells at the implantation site [189]. The long-term presence of MC at the implantation site is related to the degree of fibrosis around the implant [190]. In one study, MC deficient mice implanted with polymeric materials had lower fibrous capsule thickness and lower collagen type 1 presence as compared to wild type mice [191], suggesting that MCs play a significant role in the biomaterial integration and fibrous encapsulation.
Biomaterial architecture, surface topography, and chemical composition can also affect MC activity and function [192]. In one study, cultured on polystyrene with a honeycomb-like structure had higher cell attachment, proliferation, and more multinucleated cells when compared to smooth polystyrene surfaces, proliferation, and more multinucleated cells when compared to smooth polystyrene surfaces [193]. In another study, MC on nanostructured Ti substrates had higher cell adhesion, migration, and proliferation when compared to smooth Ti substrates [194].
These results suggest that MC are sensitive to biomaterial architecture and topography and that these parameters can influence MC activation and proliferation. While the scope and focus of this review is in sterile inflammation in response to implantable biomaterials, it is worth to note that some reports have studied the effects of architecture or physical properties on MC in the presence of LPS [195]. Chemical composition of biomaterials and the presence of stimulatory factors are also factors affecting MC activity. A study investigated the adhesion of MC to electrospun polydioxanone (PDO), poly-e-caprolactone (PCL), and silk scaffolds showed that MC readily adhere to synthetic and natural polymeric materials with cytokine release. However, MCs activated with IgE had increased adhesion, proliferation, migration, and cytokine secretion [196]. Interestingly, another study showed that MC adhere poorly to PDO and fibronectin coating increase adhesion [196]. In this context, Abebayehu et al. used electrospun fibronectin-coated PDO scaffolds with two architectures and pore sizes and showed that decrease in fiber diameter and pore size affected MC response to stimuli, increasing levels of IL-6 after LPS or IL-33 exposure and increasing TNF secretion after exposure to IL-33, suggesting that scaffold architecture can shape MC response to innate immune signals [195]. MC are understudied cells that play a significant role during all phases of biomaterial integration, controlling recruitment and activities of other immune cells as well as tissue adjacent cells. Control of MC activation should be considered when designing biomaterials for use in skin, lung, and mucosal barriers to facilitate biomaterial integration.
8. Other innate immune cells
8.1. Innate lymphoid cells
Innate lymphoid cells (ILCs) are a heterogeneous subset of novel lymphoid cells lacking antigen-specific receptors, making them distinct from B and T cells and similar to innate cells [197]. They are present in low frequency and are difficult to distinguish from other immune cells due to their complex surface marker phenotype, but are recognized as tissue resident, cytokine producing cells present in various organs and tissues [196,198]. ILCs are classified into three subsets (ILC1, ILC2, and ILC3) based on their surface markers, cytokine profiles, and functions [199]. ILCs share a developmental origin and many of the phenotypic markers of the major CD4 T helper cells, however, ILCs are activated by stress signals, cytokines in the microenvironment, and microorganism-derived compounds rather than by antigens [200].
8.2. ILC subsets
The ILC1 subset produce typical type 1 cytokines like interferon (IFN) gamma and TNF-α and are defined by the expression of the transcription factor T-bet, which is also a marker of Th1 cells [201]. ILC1s predominantly participate in type 1 immune responses that aim to clear intracellular bacterial and viral infections and can be activated by macrophage- and dendritic-cell-derived IL-12, IFN-γ, and IL-18 (201). They have been implicated in conditions like chronic obstructive pulmonary disease and Crohn’s disease [202].
ILC2s resemble Th2 cells and are primarily tissue resident cells that are prominent in lung, skin, intestine, and adipose tissue (202). ILC2s express the transcription factor GATA3 and, once activated, generate a type 2 immune response through production of IL-4, IL-5, IL-13 [203]. ILC2s are activated by IL-25, IL-33, and thymic stromal lymphopoietin [199]. ILC2s activation results in recruitment and degranulation of eosinophils, basophils, and mast cells, which define their role in allergic responses like allergic asthma, atopic dermatitis, and allergic rhinitis [204,205]. ILC2s can also determine the inflammatory phenotype of macrophages through secretion of IL-4 and IL-13 which favor the macrophage transition into the anti-inflammatory, reparative M2 phenotype [199]. ILC2s also produce amphiregulin which can facilitate restoration of tissue integrity and homeostasis after inflammation and infection [199]. ILC2s are also key regulators in establishing neonatal immunity, tissue repair, homeostasis, and pathological tissue damage and disease, such fibrosis [206,207].
ILC3s express the transcription factor retinoic acid related orphan receptor (RORγt), and they secrete GM-CSF, IL-17, IL-22, and TNF-α in response to myeloid derived IL-1β, IL-23 and TGF-β [208]. ILC3s are divided into two subgroups based on the expression of the natural cytotoxicity receptor NKp44 [208]. ILC3s can promote or suppress the immune response depending on the microenvironment within the tissues [206].
8.3. ILC Plasticity and Role Following Biomaterial Implantation
ILCs display plasticity across tissues and can change their phenotype and function in response changes in their microenvironment. ILC2s and ILC3s lose the expression of GATA3 and RORγt and gain the expression of T-bet and secrete IFNγ like ILC1s [209,210]. Stimulation with IL-1β, IL-23, and retinoic acid convert ILC1s into ILC3s [211]. IL-1β, IL-23 and TGF-β stimulation transforms ILC2s into ILC3s with secretion of IL-17 [211]. Furthermore, IL-1β and IL-4 stimulation convert ILC1s into ILC2s [211]. ILCs and T cells are similar in several levels, however, a key difference is the prompt activation of ILCs after infection or injury, which results in their expansion and release of cytokines [210]. On the other hand, T cells require several days to undergo clonal selection after antigen presentation, which triggers expansion and migration towards the infected or injured tissues [212]. While ILC plasticity is accepted, whether these cells play a role in biomaterial integration or how biomaterial properties can affect ILCs phenotypes and activities is unknown. Pro- or anti-inflammatory effects normally associated with other innate or adaptive immune cells in response to physical or chemical properties on biomaterials, such increases in pro-inflammatory cytokines IL-1β, IL-17 and TNF-α or release of anti-inflammatory cytokines, may be in part the result of ILC activation. Moreover, ILCs may also participate in fibrous encapsulation of biomaterials since IL-13 plays a role in fibrosis in a TGF-β-dependent and independent manner and their control over macrophage phenotype is well documented [213]. However, the precise role of ILCs either in response to implanted biomaterials or in affecting the activity of macrophages and dendritic cells in the biomaterial microenvironment is not known.
8.4. Natural Killer Cells
Natural killer (NK) cells are part of the innate immune system and recently classified as part of the ILC1s. NK cells were named for their ability to kill tumor cells without stimulation or exposure to antigens [214]. NK cells recognize and kill infected or cancerous cells and regulate other cells under physiological and pathological conditions through receptor-ligand interactions or cytokine/chemokine secretion [215]. NK cell functions are mainly regulated by activating (NKp46, NKp30, NKG2D, DNAM-1, 2B4) and inhibitory receptors (killer cell immunoglobulin-like receptors, killer cell lectin-like receptors, NKG2A) [216]. NK cells kill their target cells by secreting lytic granules that contain pore-forming perforin and apoptosis-inducing granzymes, or by engagement of death receptors expressed on the NK cell surface like Fas ligand and TNF-related apoptosis inducing ligand (TRAIL) with cognate ligands expressed on target cells [216,217]. NKs also produce cytokines and chemokines in response to stimulation (e.g., IL-5, IL-10, IL13, TNF-α, IFNγ) that activate macrophages and dendritic cells that in turn produce other cytokines that facilitate NK cell cytotoxicity [216–218]. NK cells amplify the inflammatory response and control the microenvironment through this feedback loop. NK cells participate in the repair of cutaneous wounds, and their involvement slows the speed of wound closure suggesting other functions besides killing target cells [219].
8.5. NK Activation and Biomaterials
The response of NK cells to their target is a complicated balance between activating and inhibitory signals. The cytotoxic activity of NK cells is closely regulated by the micro clustering of their activating and inhibitory receptors [220]. While receptor clustering is undoubtedly the main factor in regulating the cytotoxic activity of NKs, it can be regulated either biochemically via antigens or physically via mechanical properties [221]. The cytoskeleton is a major mediator of NK cell effector activity. Actin undergoes polymerization and depolymerization during NK cell migration and conjugation with target cells (198). The integrin lymphocyte function-associated antigen 1 (LFA-1) induces outside-in signaling to promote actin polymerization during NK cell adhesion to target cells. Then, F-actin induces physical forces on LFA-1, affecting LFA-1 conformation at the immunological synapse and affecting other signaling molecules that impact NK activities via mechanotransduction [222].
While NK cells are known to play important roles in immune protection in cancer and infection, whether NK cells contribute to the initial inflammatory response and inflammation resolution during biomaterial implantation is unknown. Most of the literature examining NK cells in the biomaterial context examines fabrication of nano or micropatterned substrates or particles with activating, co-stimulating, or inhibiting signals for immunotherapies [221]. These studies have shed some light on the possible effects of physical properties of implantable biomaterials on NK cell activation and their possible contribution to the initial inflammatory response and inflammation resolution. Cells sense physical cues of their microenvironment applying and sensing mechanical forces of their surroundings, and transducing these mechanical stimuli into biochemical signals [221]. These stimuli determine cell functions like cell adhesion, migration, differentiation, apoptosis, etc. [223]. Several reports have demonstrated that infected or malignant transformed cells have changes in cell mechanical properties that immune cells are able to “sense” and to respond [223,224]. Immunological receptor complexes can recognize antigens under mechanical load and discriminate between low and high affinity antigens [225]. NK cell response can be mediated by actomyosin retrograde flow [226]. A recent study showed that smooth surfaces stimulate enhanced spreading while 20 μm nanowires reduced spreading and increased NK cell activation, measured by surface recruited CD107a, suggesting that topography and mechanical forces can control NK cell activation. Furthermore, the same study showed that functionalization of the smooth surface with antigens such as class I polypeptide-related sequence A did not affect the activation of NKs, while the functionalization of nanowires synergistically activated NKs [221]. Based on these results it was suggested that NKs mechanically probe their microenvironment and that high mechanical compliance produces a mechanical stimulus that enhances activation of NKs. Although the specific mechanism by which environment stiffness controls the clustering of receptors in NKs is unclear, there is a possibility of NKG2D receptors sensing mechanical forces and transducing mechanical signals through conformational changes [223]. In regulating NK cell immune and actin retrograde flow controls, mechanical forces induced by the ECM or physicochemical properties on biomaterials also play a role in NKs response [226]. In vivo, NK cells can activate on a broad range of stiffnesses, from relatively soft myeloid, monocyte, and dendritic cells whose stiffness varies from hundreds to thousands of Pa to stiffer ECM, tumor cells, and tissues with stiffnesses of nearly 100 kPa [187, 227,228]. Changes in surface topography induce a directional preference in NK movement even in the absence of chemoattractant. In one study, microchannel confinement was used to reduce the migration track of NKs to a one-dimensional model. Topographical changes of biomaterials induced cell deformation that affected chemotaxis and NK cell migration by disrupting NK cell polarization [229].
9. Conclusions
Recent studies in both classical wound healing models and with implanted biomaterials have identified important roles for macrophages, neutrophils, DCs, and other innate immune cells. Benchmarking the interactions of innate immune cells with better- and worse-performing biomaterials by measuring inflammatory activation can provide a useful context for the design and evaluation of novel implants or surfaces to improve the rate of implant success.
Statement of Significance:
This review highlights recent advances in the understanding of the role of innate immunity in the response to implantable biomaterials, especially in neutrophils and macrophages, as well as key mechanobiological findings in innate immune cells underpinning these advances. Here we discuss how physicochemical properties of biomaterials control innate immune cell behavior.
Acknowledgements
This work was supported by NIDCR of the National Institutes of Health under award number R01DE028919. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Ribeiro Pala Jorge J, Adelino Barão V, Aparecida Delben J, Perez Faverani L, Pereira Queiroz T, Gonçalves Assunção W, Titanium in Dentistry: Historical Development, State of the Art and Future Perspectives, (n.d.) 10.1007/s13191-012-0190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhang L, Chen L, A Review on Biomedical Titanium Alloys: Recent Progress and Prospect, Adv. Eng. Mater 21 (2019) 1801215. 10.1002/adem.201801215. [DOI] [Google Scholar]
- [3].Jemat A, Ghazali MJ, Razali M, Otsuka Y, Surface modifications and their effects on titanium dental implants, Biomed Res. Int 2015 (2015). 10.1155/2015/791725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Landén NX, Li D, Ståhle M, Transition from inflammation to proliferation: a critical step during wound healing, Cell. Mol. Life Sci 73 (2016) 3861–3885. 10.1007/s00018-016-2268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gibon E, Lu L, Goodman SB, Aging, inflammation, stem cells, and bone healing, Stem Cell Res. Ther 7 (2016). 10.1186/s13287-016-0300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Albrektsson T, Jemt T, Mölne J, Tengvall P, Wennerberg A, On inflammation-immunological balance theory—A critical apprehension of disease concepts around implants: Mucositis and marginal bone loss may represent normal conditions and not necessarily a state of disease, Clin. Implant Dent. Relat. Res 21 (2019) 183–189. 10.1111/cid.12711. [DOI] [PubMed] [Google Scholar]
- [7].Thevenot P, Hu W, Tang L, Surface chemistry influences implant biocompatibility., Curr. Top. Med. Chem 8 (2008) 270–80. 10.2174/156802608783790901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aiyelabegan HT, Sadroddiny E, Fundamentals of protein and cell interactions in biomaterials, Biomed. Pharmacother 88 (2017) 956–970. 10.1016/j.biopha.2017.01.136. [DOI] [PubMed] [Google Scholar]
- [9].Kyriakides TR, Molecular Events at Tissue-Biomaterial Interface, in: Host Response to Biomater. Impact Host Response Biomater. Sel, Elsevier Inc., 2015: pp. 81–116. 10.1016/B978-0-12-800196-7.00005-0. [DOI] [Google Scholar]
- [10].Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ, Mediation of biomaterial-cell interactions by adsorbed proteins: A review, Tissue Eng. 11 (2005) 1–18. 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- [11].Rogers TH, Babensee JE, Altered adherent leukocyte profile on biomaterials in Toll-like receptor 4 deficient mice, Biomaterials. 31 (2010) 594–601. 10.1016/j.biomaterials.2009.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT, PAMPs and DAMPs: Signal 0s that spur autophagy and immunity, Immunol. Rev 249 (2012) 158–175. 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mogensen TH, Pathogen recognition and inflammatory signaling in innate immune defenses, Clin. Microbiol. Rev 22 (2009) 240–273. 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rock KL, Kono H, The inflammatory response to cell death, Annu. Rev. Pathol. Mech. Dis 3 (2008) 99–126. 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schaefer L, Complexity of danger: The diverse nature of damage-associated molecular patterns, J. Biol. Chem 289 (2014) 35237–35245. 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Frevert CW, Felgenhauer J, Wygrecka M, Nastase MV, Schaefer L, Danger-Associated Molecular Patterns Derived From the Extracellular Matrix Provide Temporal Control of Innate Immunity, J. Histochem. Cytochem 66 (2018) 213–227. 10.1369/0022155417740880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Charles A Janeway J, Travers P, Walport M, Shlomchik MJ, The complement system and innate immunity, (2001). https://www.ncbi.nlm.nih.gov/books/NBK27100/.
- [18].Kataoka H, Kono H, Patel Z, Rock KL, Evaluation of the Contribution of Multiple DAMPs and DAMP Receptors in Cell Death-Induced Sterile Inflammatory Responses, PLoS One. 9 (2014) e104741. 10.1371/journal.pone.0104741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Noris M, Remuzzi G, Overview of complement activation and regulation, Semin. Nephrol 33 (2013) 479–492. 10.1016/j.semnephrol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lubbers R, van Essen MF, van Kooten C, Trouw LA, Production of complement components by cells of the immune system, Clin. Exp. Immunol 188 (2017) 183–194. 10.1111/cei.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bayly-Jones C, Bubeck D, Dunstone MA, The mystery behind membrane insertion: A review of the complement membrane attack complex, Philos. Trans. R. Soc. B Biol. Sci 372 (2017). 10.1098/rstb.2016.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tang L, Thevenot P, Hu W, Surface Chemistry Influences Implant Biocompatibility, Curr. Top. Med. Chem 8 (2008) 270–280. 10.2174/156802608783790901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu L, Elwing H, Complement activation on thiol-modified gold surfaces, J. Biomed. Mater. Res 30 (1996) 535–541. . [DOI] [PubMed] [Google Scholar]
- [24].Mödinger Y, Teixeira GQ, Neidlinger-Wilke C, Ignatius A, Role of the complement system in the response to orthopedic biomaterials, Int. J. Mol. Sci 19 (2018). 10.3390/ijms19113367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Saleh M, Gibson MF, Sharrard WJW, Femoral Shortening in Correction of Congenital Knee Flexion Deformity with Popliteal Webbing, J. Pediatr. Orthop 9 (1989) 609–611. 10.1097/01241398-198909010-00020. [DOI] [PubMed] [Google Scholar]
- [26].Escamilla-Rivera V, Solorio-Rodríguez A, Uribe-Ramírez M, Lozano O, Lucas S, Chagolla-López A, Winkler R, De Vizcaya-Ruiz A, Plasma protein adsorption on Fe3O4-PEG nanoparticles activates the complement system and induces an inflammatory response, Int. J. Nanomedicine 14 (2019) 2055–2067. 10.2147/IJN.S192214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gabizon A, Szebeni J, Complement Activation: A Potential Threat on the Safety of Poly(ethylene glycol)-Coated Nanomedicines, ACS Nano. 14 (2020) 7682–7688. 10.1021/acsnano.0c03648. [DOI] [PubMed] [Google Scholar]
- [28].Hamad I, Al-Hanbali O, Hunter AC, Rutt KJ, Andresen TL, Moghimi SM, Distinct polymer architecture mediates switching of complement activation pathways at the nanosphere-serum interface: Implications for stealth nanoparticle engineering, ACS Nano. 4 (2010) 6629–6638. 10.1021/nn101990a. [DOI] [PubMed] [Google Scholar]
- [29].Arima Y, Kawagoe M, Toda M, Iwata H, Complement activation by polymers carrying hydroxyl groups, ACS Appl. Mater. Interfaces 1 (2009) 2400–2407. 10.1021/am9005463. [DOI] [PubMed] [Google Scholar]
- [30].Engberg AE, Nilsson PH, Huang S, Fromell K, Hamad OA, Mollnes TE, Rosengren-Holmberg JP, Sandholm K, Teramura Y, Nicholls IA, Nilsson B, Ekdahl KN, Prediction of inflammatory responses induced by biomaterials in contact with human blood using protein fingerprint from plasma, Biomaterials. 36 (2015) 55–65. 10.1016/j.biomaterials.2014.09.011. [DOI] [PubMed] [Google Scholar]
- [31].Elwing H, Nilsson B, Svensson KE, Askendahl A, Nilsson UR, Lundström I, Conformational changes of a model protein (complement factor 3) adsorbed on hydrophilic and hydrophobic solid surfaces, J. Colloid Interface Sci 125 (1988) 139–145. 10.1016/0021-9797(88)90062-8. [DOI] [Google Scholar]
- [32].Hong J, Kurt S, Thor A, A Hydrophilic Dental Implant Surface Exhibit Thrombogenic Properties In Vitro, Clin. Implant Dent. Relat. Res 15 (2013) 105–112. 10.1111/j.1708-8208.2011.00362.x. [DOI] [PubMed] [Google Scholar]
- [33].Camous L, Roumenina L, Bigot S, Brachemi S, Frémeaux-Bacchi V, Lesavre P, Halbwachs-Mecarelli L, Complement alternative pathway acts as a positive feedback amplification of neutrophil activation, Blood. 117 (2011) 1340–1349. 10.1182/blood-2010-05-283564. [DOI] [PubMed] [Google Scholar]
- [34].Selders GS, Fetz AE, Radic MZ, Bowlin GL, An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration, Regen. Biomater 4 (2017) 55–68. 10.1093/rb/rbw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jhunjhunwala S, Neutrophils at the Biological-Material Interface, ACS Biomater. Sci. Eng 4 (2018) 1128–1136. 10.1021/acsbiomaterials.6b00743. [DOI] [PubMed] [Google Scholar]
- [36].Fang H, Jiang W, Cheng J, Lu Y, Liu A, Kan L, Dahmen U, Balancing innate immunity and inflammatory state via modulation of neutrophil function: A novel strategy to fight sepsis, J. Immunol. Res 2015 (2015). 10.1155/2015/187048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wills-Karp M, Neutrophil ghosts worsen asthma, Sci. Immunol 3 (2018). 10.1126/sciimmunol.aau0112. [DOI] [PubMed] [Google Scholar]
- [38].Cantin AM, Hartl D, Konstan MW, Chmiel JF, Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy, J. Cyst. Fibros 14 (2015) 419–430. 10.1016/j.jcf.2015.03.003. [DOI] [PubMed] [Google Scholar]
- [39].Barnes PJ, Inflammatory mechanisms in patients with chronic obstructive pulmonary disease, J. Allergy Clin. Immunol 138 (2016) 16–27. 10.1016/j.jaci.2016.05.011. [DOI] [PubMed] [Google Scholar]
- [40].Morrison HM, Welgus HG, Stockley RA, Burnett D, Campbell EJ, Inhibition of human leukocyte elastase bound to elastin: relative ineffectiveness and two mechanisms of inhibitory activity., Am. J. Respir. Cell Mol. Biol 2 (1990) 263–269. 10.1165/ajrcmb/2.3.263. [DOI] [PubMed] [Google Scholar]
- [41].Wang J, Neutrophils in tissue injury and repair, Cell Tissue Res. 371 (2018) 531–539. 10.1007/s00441-017-2785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Butterfield TA, Best TM, Merrick MA, The dual roles of neutrophils and macrophages in inflammation: A critical balance between tissue damage and repair, J. Athl. Train 41 (2006) 457–465. 10.1016/s0162-0908(08)79217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Malech HL, Deleo FR, Quinn MT, The role of neutrophils in the immune system: An overview, Methods Mol. Biol 1124 (2014) 3–10. 10.1007/978-1-62703-845-4_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tecchio C, Micheletti A, Cassatella MA, Neutrophil-derived cytokines: Facts beyond expression, Front. Immunol 5 (2014) 508. 10.3389/fimmu.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Espaillat MP, Kew RR, Obeid LM, Sphingolipids in neutrophil function and inflammatory responses: Mechanisms and implications for intestinal immunity and inflammation in ulcerative colitis, Adv. Biol. Regul 63 (2017) 140–155. 10.1016/j.jbior.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kapellos TS, Recio C, Greaves DR, Iqbal AJ, Cannabinoid Receptor 2 Modulates Neutrophil Recruitment in a Murine Model of Endotoxemia, Mediators Inflamm. 2017 (2017). 10.1155/2017/4315412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hallett MB, Lloyds D, Neutrophil priming: the cellular signals that say “amber” but not “green,” Immunol. Today 16 (1995) 264–268. 10.1016/0167-5699(95)80178-2. [DOI] [PubMed] [Google Scholar]
- [48].Miralda I, Uriarte SM, McLeish KR, Multiple phenotypic changes define neutrophil priming, Front. Cell. Infect. Microbiol 7 (2017). 10.3389/fcimb.2017.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Vogt KL, Summers C, Chilvers ER, Condliffe AM, Priming and de-priming of neutrophil responses in vitro and in vivo, Eur. J. Clin. Invest 48 (2018). 10.1111/eci.12967. [DOI] [PubMed] [Google Scholar]
- [50].Mussbacher M, Salzmann M, Brostjan C, Hoesel B, Schoergenhofer C, Datler H, Hohensinner P, Basílio J, Petzelbauer P, Assinger A, Schmid JA, Cell type specific roles of nf-kb linking inflamation and thrombosis, Front. Immunol 10 (2019). 10.3389/fimmu.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Karmakar M, Katsnelson MA, Dubyak GR, Pearlman E, Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1β secretion in response to ATP, Nat. Commun 7 (2016) 1–13. 10.1038/ncomms10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Collison KS, Parhar RS, Saleh SS, Meyer BF, Kwaasi AA, Hammami MM, Schmidt AM, Stern DM, Al-Mohanna FA, RAGE-mediated neutrophil dysfunction is evoked by advanced glycation end products (AGEs)., J. Leukoc. Biol 71 (2002) 433–44. 10.1189/jlb.71.3.433. [DOI] [PubMed] [Google Scholar]
- [53].Boone BA, Orlichenko L, Schapiro NE, Loughran P, Gianfrate GC, Ellis JT, Singhi AD, Kang R, Tang D, Lotze MT, Zeh HJ, The receptor for advanced glycation end products (RAGE) enhances autophagy and neutrophil extracellular traps in pancreatic cancer, Cancer Gene Ther. 22 (2015) 326–334. 10.1038/cgt.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Dalboni TM, Abe AE, de Oliveira CE, Lara VS, Campanelli AP, Gasparoto CT, Gasparoto TH, Activation profile of CXCL8-stimulated neutrophils and aging, Cytokine. 61 (2013) 716–719. 10.1016/j.cyto.2013.01.016. [DOI] [PubMed] [Google Scholar]
- [55].Keir HR, Richardson H, Fillmore C, Shoemark A, Lazaar AL, Miller BE, Tal-Singer R, Chalmers JD, Mohan D, CXCL-8-dependent and -independent neutrophil activation in COPD: experiences from a pilot study of the CXCR2 antagonist danirixin, ERJ Open Res. 6 (2020) 00583–02020. 10.1183/23120541.00583-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].de Oliveira S, Reyes-Aldasoro CC, Candel S, Renshaw SA, Mulero V, Calado Â, Cxcl8 (IL-8) Mediates Neutrophil Recruitment and Behavior in the Zebrafish Inflammatory Response, J. Immunol 190 (2013) 4349–4359. 10.4049/jimmunol.1203266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Marder P, Sawyer JS, Froelich LL, Mann LL, Spaethe SM, Blockade of human neutrophil activation by 2-[2-propyl-3-[3-[2-ethyl-4-(4-fluorophenyl)-5-hydroxyphenoxy]propoxy]phenoxy]benzoic acid (LY293111), a novel leukotriene B4 receptor antagonist, Biochem. Pharmacol 49 (1995) 1683–1690. 10.1016/0006-2952(95)00078-E. [DOI] [PubMed] [Google Scholar]
- [58].Omann GM, Traynor AE, Harris AL, Sklar LA, LTB4 induced activation signals and responses in neutrophils are short-lived compared to formylpeptide., J. Immunol 138 (1987). [PubMed] [Google Scholar]
- [59].Afonso PV, Janka-Junttila M, Lee YJ, McCann CP, Oliver CM, Aamer KA, Losert W, Cicerone MT, Parent CA, LTB4 Is a Signal-Relay Molecule during Neutrophil Chemotaxis, Dev. Cell 22 (2012) 1079–1091. 10.1016/j.devcel.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Giagulli C, Ottoboni L, Caveggion E, Rossi B, Lowell C, Constantin G, Laudanna C, Berton G, The Src Family Kinases Hck and Fgr Are Dispensable for Inside-Out, Chemoattractant-Induced Signaling Regulating β2 Integrin Affinity and Valency in Neutrophils, but Are Required for β2 Integrin-Mediated Outside-In Signaling Involved in Sustained Adhesion, J. Immunol 177 (2006) 604–611. 10.4049/jimmunol.177.1.604. [DOI] [PubMed] [Google Scholar]
- [61].Altman SM, Dixit N, Simon SI, Detection of bidirectional signaling during integrin activation and neutrophil adhesion, Methods Mol. Biol 1124 (2014) 235–248. 10.1007/978-1-62703-845-4_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM, Polarization of Tumor-Associated Neutrophil Phenotype by TGF-β: “N1” versus “N2” TAN, Cancer Cell. 16 (2009) 183–194. 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Simon SI, Kim MH, A day (or 5) in a neutrophil’s life, Blood. 116 (2010) 511–512. 10.1182/blood-2010-05-283184. [DOI] [PubMed] [Google Scholar]
- [64].Hidalgo A, Chilvers ER, Summers C, Koenderman L, The Neutrophil Life Cycle, Trends Immunol. 40 (2019) 584–597. 10.1016/j.it.2019.04.013. [DOI] [PubMed] [Google Scholar]
- [65].Wang X, Qiu L, Li Z, Wang XY, Yi H, Understanding the multifaceted role of neutrophils in cancer and autoimmune diseases, Front. Immunol 9 (2018). 10.3389/fimmu.2018.02456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Alex H, Scherer A, Kreuzburg S, Abers MS, Zerbe CS, Dinauer MC, Mansour MK, Irimia D, Neutrophil swarming delays the growth of clusters of pathogenic fungi, Nat. Commun 11 (2020) 1–15. 10.1038/s41467-020-15834-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Stephen J, Scales HE, Benson RA, Erben D, Garside P, Brewer JM, Neutrophil swarming and extracellular trap formation play a significant role in Alum adjuvant activity, Npj Vaccines. 2 (2017) 1. 10.1038/s41541-016-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kaplan MJ, Radic M, Neutrophil Extracellular Traps: Double-Edged Swords of Innate Immunity, J. Immunol 189 (2012) 2689–2695. 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Raup-Konsavage WM, Wang Y, Wang WW, Feliers D, Ruan H, Reeves WB, Neutrophil peptidyl arginine deiminase-4 has a pivotal role in ischemia/reperfusion-induced acute kidney injury, Kidney Int. 93 (2018) 365–374. 10.1016/j.kint.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Al-Khafaji AB, Tohme S, Yazdani HO, Miller D, Huang H, Tsung A, Superoxide induces neutrophil extracellular trap formation in a TLR-4 and NOX-dependent mechanism, Mol. Med 22 (2016) 621–631. 10.2119/molmed.2016.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Clancy DM, Henry CM, Sullivan GP, Martin SJ, Neutrophil extracellular traps can serve as platforms for processing and activation of IL-1 family cytokines, FEBS J. 284 (2017) 1712–1725. 10.1111/febs.14075. [DOI] [PubMed] [Google Scholar]
- [72].Lefrançais E, Mallavia B, Zhuo H, Calfee CS, Looney MR, Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury, JCI Insight. 3 (2018). 10.1172/jci.insight.98178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Iba T, Murai M, Nagaoka I, Tabe Y, Neutrophil extracellular traps, damage-associated molecular patterns, and cell death during sepsis, Acute Med. Surg 1 (2014) 2–9. 10.1002/ams2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lewis HD, Liddle J, Coote JE, Atkinson SJ, Barker MD, Bax BD, Bicker KL, Bingham RP, Campbell M, Chen YH, Chung CW, Craggs PD, Davis RP, Eberhard D, Joberty G, Lind KE, Locke K, Maller C, Martinod K, Patten C, Polyakova O, Rise CE, Rüdiger M, Sheppard RJ, Slade DJ, Thomas P, Thorpe J, Yao G, Drewes G, Wagner DD, Thompson PR, Prinjha RK, Wilson DM, Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation, Nat. Chem. Biol 11 (2015) 189–191. 10.1038/nchembio.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Prame Kumar K, Nicholls AJ, Wong CHY, Partners in crime: neutrophils and monocytes/macrophages in inflammation and disease, Cell Tissue Res. 371 (2018) 551–565. 10.1007/s00441-017-2753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Abaricia JO, Shah AH, Musselman RM, Olivares-Navarrete R, Hydrophilic titanium surfaces reduce neutrophil inflammatory response and NETosis, Biomater. Sci 8 (2020) 2289–2299. 10.1039/c9bm01474h. [DOI] [PubMed] [Google Scholar]
- [77].Hotchkiss KM, Clark NM, Olivares-Navarrete R, Macrophage response to hydrophilic biomaterials regulates MSC recruitment and T-helper cell populations, Biomaterials. 182 (2018) 202–215. 10.1016/j.biomaterials.2018.08.029. [DOI] [PubMed] [Google Scholar]
- [78].Hotchkiss KM, Reddy GB, Hyzy SL, Schwartz Z, Boyan BD, Olivares-Navarrete R, Titanium surface characteristics, including topography and wettability, alter macrophage activation, Acta Biomater. 31 (2016) 425–434. 10.1016/j.actbio.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Dai X, Wei Y, Zhang X, Meng S, Mo X, Liu X, Deng X, Zhang L, Deng X, Attenuating Immune Response of Macrophage by Enhancing Hydrophilicity of Ti Surface, J. Nanomater 2015 (2015). 10.1155/2015/712810. [DOI] [Google Scholar]
- [80].Hamlet SM, Lee RSB, Moon H, Alfarsi MA, Ivanovski S, Hydrophilic titanium surface-induced macrophage modulation promotes pro-osteogenic signalling, Clin. Oral Implants Res 30 (2019) 1085–1096. 10.1111/clr.13522. [DOI] [PubMed] [Google Scholar]
- [81].Chang DT, Colton E, Anderson JM, Paracrine and juxtacrine lymphocyte enhancement of adherent macrophage and foreign body giant cell activation, J. Biomed. Mater. Res. - Part A 89 (2009) 490–498. 10.1002/jbm.a.31981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ley K, M1 Means Kill; M2 Means Heal, J. Immunol 199 (2017) 2191–2193. 10.4049/jimmunol.1701135. [DOI] [PubMed] [Google Scholar]
- [83].Nadzam GS, De La Cruz C, Greco RS, Haimovich B, Neutrophil adhesion to vascular prosthetic surfaces triggers nonapoptotic cell death, Ann. Surg 231 (2000) 587–599. 10.1097/00000658-200004000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Erpenbeck L, Gruhn AL, Kudryasheva G, Günay G, Meyer D, Busse J, Neubert E, Schön MP, Rehfeldt F, Kruss S, Effect of Adhesion and Substrate Elasticity on Neutrophil Extracellular Trap Formation, Front. Immunol 10 (2019). 10.3389/fimmu.2019.02320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Sridharan R, Cameron AR, Kelly DJ, Kearney CJ, O’Brien FJ, Biomaterial based modulation of macrophage polarization: A review and suggested design principles, Mater. Today 18 (2015) 313–325. 10.1016/j.mattod.2015.01.019. [DOI] [Google Scholar]
- [86].Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF, Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine, Biomaterials. 33 (2012) 3792–3802. 10.1016/j.biomaterials.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Murray PJ, Macrophage Polarization, Annu. Rev. Physiol 79 (2017) 541–566. 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- [88].Marsell R, Einhorn TA, The biology of fracture healing, Injury. 42 (2011) 551–555. 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Schlundt C, El Khassawna T, Serra A, Dienelt A, Wendler S, Schell H, van Rooijen N, Radbruch A, Lucius R, Hartmann S, Duda GN, Schmidt-Bleek K, Macrophages in bone fracture healing: Their essential role in endochondral ossification, Bone. 106 (2018) 78–89. 10.1016/j.bone.2015.10.019. [DOI] [PubMed] [Google Scholar]
- [90].Wynn TA, Barron L, Macrophages: Master regulators of inflammation and fibrosis, Semin. Liver Dis 30 (2010) 245–257. 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wu AC, Raggatt LJ, Alexander KA, Pettit AR, Unraveling macrophage contributions to bone repair, Bonekey Rep. 2 (2013). 10.1038/bonekey.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wu W-K, Dick AD, Bates DO, Nicholson LB; VEGF Production in Macrophages Is Enhanced by Anti-Inflammatory Stimuli. Invest. Ophthalmol. Vis. Sci 2009;50(13):3758. [Google Scholar]
- [93].Roszer T, Understanding the mysterious M2 macrophage through activation markers and effector mechanisms, Mediators Inflamm. 2015 (2015). 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, vanGinderachter JA, Vogel SN, Wynn TA, Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines, Immunity. 41 (2014) 14–20. 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Feng X, Deng T, Zhang Y, Su S, Wei C, Han D, Lipopolysaccharide inhibits macrophage phagocytosis of apoptotic neutrophils by regulating the production of tumour necrosis factor α and growth arrest-specific gene 6, Immunology. 132 (2011) 287–295. 10.1111/j.1365-2567.2010.03364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Abdelaziz MH, Abdelwahab SF, Wan J, Cai W, Huixuan W, Jianjun C, Kumar KD, Vasudevan A, Sadek A, Su Z, Wang S, Xu H, Alternatively activated macrophages; A double-edged sword in allergic asthma, J. Transl. Med 18 (2020). 10.1186/s12967-020-02251-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Shaw TN, Houston SA, Wemyss K, Bridgeman HM, Barbera TA, Zangerle-Murray T, Strangward P, Ridley AJL, Wang P, Tamoutounour S, Allen JE, Konkel JE, Grainger JR, Tissue-resident macrophages in the intestine are long lived and defined by Tim-4 and CD4 expression, J. Exp. Med 215 (2018) 1507–1518. 10.1084/jem.20180019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Bain CC, Hawley CA, Garner H, Scott CL, Schridde A, Steers NJ, Mack M, Joshi A, Guilliams M, Mowat AMI, Geissmann F, Jenkins SJ, Long-lived self-renewing bone marrow-derived macrophages displace embryo-derived cells to inhabit adult serous cavities, Nat. Commun 7 (2016). 10.1038/ncomms11852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Hoeffel G, Ginhoux F, Ontogeny of tissue-resident macrophages, Front. Immunol 6 (2015). 10.3389/fimmu.2015.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Epelman S, Lavine KJ, Randolph GJ, Origin and Functions of Tissue Macrophages, Immunity. 41 (2014) 21–35. 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].van de Laar L, Saelens W, De Prijck S, Martens L, Scott CL, Van Isterdael G, Hoffmann E, Beyaert R, Saeys Y, Lambrecht BN, Guilliams M, Yolk Sac Macrophages, Fetal Liver, and Adult Monocytes Can Colonize an Empty Niche and Develop into Functional Tissue-Resident Macrophages, Immunity. 44 (2016) 755–768. 10.1016/j.immuni.2016.02.017. [DOI] [PubMed] [Google Scholar]
- [102].Honold L, Nahrendorf M, Resident and Monocyte-Derived Macrophages in Cardiovascular Disease, Circ. Res 122 (2018) 113–127. 10.1161/CIRCRESAHA.117.311071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Watanabe S, Alexander M, Misharin AV, Budinger GRS, The role of macrophages in the resolution of inflammation, J. Clin. Invest 129 (2019) 2619–2628. 10.1172/JCI124615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Shan Z, Ju C, Hepatic Macrophages in Liver Injury, Front. Immunol 11 (2020). 10.3389/fimmu.2020.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Delavary BM, van der Veer WM, van Egmond M, Niessen FB, Beelen RHJ, Macrophages in skin injury and repair, Immunobiology. 216 (2011) 753–762. 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- [106].Huen SC, Cantley LG, Macrophages in Renal Injury and Repair, Annu. Rev. Physiol 79 (2017) 449–469. 10.1146/annurev-physiol-022516-034219. [DOI] [PubMed] [Google Scholar]
- [107].Sinha M, Sen CK, Singh K, Das A, Ghatak S, Rhea B, Blackstone B, Powell HM, Khanna S, Roy S, Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue, Nat. Commun 9 (2018). 10.1038/s41467-018-03208-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Minutti CM, Knipper JA, Allen JE, Zaiss DMW, Tissue-specific contribution of macrophages to wound healing, Semin. Cell Dev. Biol 61 (2017) 3–11. 10.1016/j.semcdb.2016.08.006. [DOI] [PubMed] [Google Scholar]
- [109].Burgess M, Wicks K, Gardasevic M, Mace KA, Cx3CR1 Expression Identifies Distinct Macrophage Populations That Contribute Differentially to Inflammation and Repair, ImmunoHorizons. 3 (2019) 262–273. 10.4049/immunohorizons.1900038. [DOI] [PubMed] [Google Scholar]
- [110].Dambach DM, Watson LM, Gray KR, Durham SK, Laskin DL, Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse, Hepatology. 35 (2002) 1093–1103. 10.1053/jhep.2002.33162. [DOI] [PubMed] [Google Scholar]
- [111].Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A, Gordon-Walker TT, Williams MJ, Dunbar DR, Manning JR, Van Rooijen N, Fallowfield JA, Forbes SJ, Iredale JP, Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis, Proc. Natl. Acad. Sci. U. S. A 109 (2012). 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Tacke F, Zimmermann HW, Macrophage heterogeneity in liver injury and fibrosis, J. Hepatol 60 (2014) 1090–1096. 10.1016/j.jhep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- [113].Landgraeber S, Jäger M, Jacobs JJ, Hallab NJ, The pathology of orthopedic implant failure is mediated by innate immune system cytokines, Mediators Inflamm. 2014 (2014). 10.1155/2014/185150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Li M, Guo X, Qi W, Wu Z, De Bruijn JD, Xiao Y, Bao C, Yuan H, Macrophage polarization plays roles in bone formation instructed by calcium phosphate ceramics, J. Mater. Chem. B 8 (2020) 1863–1877. 10.1039/c9tb02932j. [DOI] [PubMed] [Google Scholar]
- [115].Blakney AK, Swartzlander MD, Bryant SJ, The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels, J. Biomed. Mater. Res. Part A 100A (2012) 1375–1386. doi: 10.1002/jbm.a.34104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Dutta B, Goswami R, Rahaman SO, TRPV4 Plays a Role in Matrix Stiffness-Induced Macrophage Polarization, Front. Immunol 11 (2020). doi: 10.3389/fimmu.2020.570195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Sridharan R, Cavanagh B, Cameron AR, Kelly DJ, O’Brien FJ, Material stiffness influences the polarization state, function and migration mode of macrophages, Acta Biomater. 89 (2019) 47–59. doi: 10.1016/j.actbio.2019.02.048. [DOI] [PubMed] [Google Scholar]
- [118].Chen M, Zhang Y, Zhou P, Liu X, Zhao H, Zhou X, Gu Q, Li B, Zhu X, Shi Q, Substrate stiffness modulates bone marrow-derived macrophage polarization through NF-κB signaling pathway, Bioact. Mater 5 (2020) 880–890. doi: 10.1016/j.bioactmat.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Pakshir P, Alizadehgiashi M, Wong B, Coelho NM, Chen X, Gong Z, Shenoy VB, McCulloch CA, Hinz B, Dynamic fibroblast contractions attract remote macrophages in fibrillar collagen matrix, Nat. Commun 10 (2019) 1850. doi: 10.1038/s41467-019-09709-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Astudillo P, Extracellular matrix stiffness and Wnt/β-catenin signaling in physiology and disease, Biochem. Soc. Trans 48 (2020) 1187–1198. doi: 10.1042/BST20200026. [DOI] [PubMed] [Google Scholar]
- [121].Du J, Zu Y, Li J, Du S, Xu Y, Zhang L, Jiang L, Wang Z, Chien S, Yang C, Extracellular matrix stiffness dictates Wnt expression through integrin pathway, Sci. Rep 6 (2016) 20395. doi: 10.1038/srep20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Hoffman L, Jensen CC, Yoshigi M, Beckerle M, Mechanical signals activate p38 MAPK pathway-dependent reinforcement of actin via mechanosensitive HspB1, Mol. Biol. Cell 28 (2017) 2661–2675. doi: 10.1091/mbc.e17-02-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Hwang J-H, Byun MR, Kim AR, Kim KM, Cho HJ, Lee YH, Kim J, Jeong MG, Hwang ES, Hong J-H, Extracellular Matrix Stiffness Regulates Osteogenic Differentiation through MAPK Activation, PLoS One. 10 (2015) e0135519. doi: 10.1371/journal.pone.0135519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Barth KA, Waterfield JD, Brunette DM, The effect of surface roughness on RAW 264.7 macrophage phenotype, J. Biomed. Mater. Res. Part A 101A (2013) 2679–2688. doi: 10.1002/jbm.a.34562. [DOI] [PubMed] [Google Scholar]
- [125].Zhang Y, Cheng X, Jansen JA, Yang F, van den Beucken JJJP, Titanium surfaces characteristics modulate macrophage polarization, Mater. Sci. Eng. C 95 (2019) 143–151. doi: 10.1016/j.msec.2018.10.065. [DOI] [PubMed] [Google Scholar]
- [126].Wang T, Luu TU, Chen A, Khine M, Liu WF, Topographical modulation of macrophage phenotype by shrink-film multi-scale wrinkles, Biomater. Sci 4 (2016) 948–952. doi: 10.1039/C6BM00224B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Moon H, Cremmel CVM, Kulpa A, Jaeger NAF, Kappelhoff R, Overall CM, Waterfield JD, Brunette DM, Novel grooved substrata stimulate macrophage fusion, CCL2 and MMP-9 secretion, J. Biomed. Mater. Res. Part A 104 (2016) 2243–2254. doi: 10.1002/jbm.a.35757. [DOI] [PubMed] [Google Scholar]
- [128].Zheng X, Xin L, Luo Y, Yang H, Ye X, Mao Z, Zhang S, Ma L, Gao C, Near-Infrared-Triggered Dynamic Surface Topography for Sequential Modulation of Macrophage Phenotypes, ACS Appl. Mater. Interfaces 11 (2019) 43689–43697. doi: 10.1021/acsami.9b14808. [DOI] [PubMed] [Google Scholar]
- [129].Cockerill I, Su Y, Lee JH, Berman D, Young ML, Zheng Y, Zhu D, Micro-/Nanotopography on Bioresorbable Zinc Dictates Cytocompatibility, Bone Cell Differentiation, and Macrophage Polarization, Nano Lett. 20 (2020) 4594–4602. doi: 10.1021/acs.nanolett.0c01448. [DOI] [PubMed] [Google Scholar]
- [130].Refai AK, Textor M, Brunette DM, Waterfield JD, Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines, J. Biomed. Mater. Res 70A (2004) 194–205. doi: 10.1002/jbm.a.30075. [DOI] [PubMed] [Google Scholar]
- [131].Paul NE, Skazik C, Harwardt M, Bartneck M, Denecke B, Klee D, Salber J, Zwadlo-Klarwasser G, Topographical control of human macrophages by a regularly microstructured polyvinylidene fluoride surface, Biomaterials. 29 (2008) 4056–4064. doi: 10.1016/j.biomaterials.2008.07.010. [DOI] [PubMed] [Google Scholar]
- [132].Hotchkiss KM, Ayad NB, Hyzy SL, Boyan BD, Olivares-Navarrete R, Dental implant surface chemistry and energy alter macrophage activation in vitro, Clin. Oral Implants Res 28 (2017) 414–423. doi: 10.1111/clr.12814. [DOI] [PubMed] [Google Scholar]
- [133].Hotchkiss KM, Sowers KT, Olivares-Navarrete R, Novel in vitro comparative model of osteogenic and inflammatory cell response to dental implants, Dent. Mater 35 (2019) 176–184. doi: 10.1016/j.dental.2018.11.011. [DOI] [PubMed] [Google Scholar]
- [134].Ni S, Zhai D, Huan Z, Zhang T, Chang J, Wu C, Nanosized concave pit/convex dot microarray for immunomodulatory osteogenesis and angiogenesis, Nanoscale. 12 (2020) 16474–16488. 10.1039/d0nr03886e. [DOI] [PubMed] [Google Scholar]
- [135].Fang JY, Yang Z, Han B, Switch of macrophage fusion competency by 3D matrices, Sci. Rep 10 (2020). 10.1038/s41598-020-67056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Luo J, He Y, Meng F, Yan N, Zhang Y, Song W, The role of autophagy in M2 polarization of macrophages induced by micro/nano topography, Int. J. Nanomedicine 15 (2020) 7763–7774. 10.2147/IJN.S270100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Chen L, Wang D, Peng F, Qiu J, Ouyang L, Qiao Y, Liu X, Correction to Nanostructural Surfaces with Different Elastic Moduli Regulate the Immune Response by Stretching Macrophages (Nano Letters (2019)19:6 (3480–3489)Doi: 10.1021/acs.nanolett.9b00237), Nano Lett. 20 (2020) 2932. 10.1021/acs.nanolett.0c01065. [DOI] [PubMed] [Google Scholar]
- [138].Berghaus LJ, Moore JN, Hurley DJ, Vandenplas ML, Fortes BP, Wolfert MA, Boons GJ, Innate immune responses of primary murine macrophage-lineage cells and RAW 264.7 cells to ligands of Toll-like receptors 2, 3, and 4, Comp. Immunol. Microbiol. Infect. Dis 33 (2010) 443–454. 10.1016/j.cimid.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Chamberlain LM, Godek ML, Gonzalez-Juarrero M, Grainger DW, Phenotypic non-equivalence of murine (monocyte-) macrophage cells in biomaterial and inflammatory models, J. Biomed. Mater. Res. - Part A 88 (2009) 858–871. 10.1002/jbm.a.31930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Ion R, Stoian AB, Dumitriu C, Grigorescu S, Mazare A, Cimpean A, Demetrescu I, Schmuki P, Nanochannels formed on TiZr alloy improve biological response, Acta Biomater. 24 (2015) 370–377. doi: 10.1016/j.actbio.2015.06.016. [DOI] [PubMed] [Google Scholar]
- [141].Lee S, Choi J, Shin S, Im Y-M, Song J, Kang SS, Nam T-H, Webster TJ, Kim S-H, Khang D, Analysis on migration and activation of live macrophages on transparent flat and nanostructured titanium, Acta Biomater. 7 (2011) 2337–2344. doi: 10.1016/j.actbio.2011.01.006. [DOI] [PubMed] [Google Scholar]
- [142].Park J-W, Han S-H, Hanawa T, Effects of Surface Nanotopography and Calcium Chemistry of Titanium Bone Implants on Early Blood Platelet and Macrophage Cell Function, Biomed Res. Int 2018 (2018) 1–10. doi: 10.1155/2018/1362958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Linares J, Fernández AB, Feito MJ, Matesanz MC, Sánchez-Salcedo S, Arcos D, Vallet-Regí M, Rojo JM, Portolés MT, Effects of nanocrystalline hydroxyapatites on macrophage polarization, J. Mater. Chem. B 4 (2016) 1951–1959. doi: 10.1039/C6TB00014B. [DOI] [PubMed] [Google Scholar]
- [144].Saino E, Focarete ML, Gualandi C, Emanuele E, Cornaglia AI, Imbriani M, Visai L, Effect of Electrospun Fiber Diameter and Alignment on Macrophage Activation and Secretion of Proinflammatory Cytokines and Chemokines, Biomacromolecules. 12 (2011) 1900–1911. doi: 10.1021/bm200248h. [DOI] [PubMed] [Google Scholar]
- [145].Jia Y, Yang W, Zhang K, Qiu S, Xu J, Wang C, Chai Y, Nanofiber arrangement regulates peripheral nerve regeneration through differential modulation of macrophage phenotypes, Acta Biomater. 83 (2019) 291–301. doi: 10.1016/j.actbio.2018.10.040. [DOI] [PubMed] [Google Scholar]
- [146].Schoenenberger AD, Tempfer H, Lehner C, Egloff J, Mauracher M, Bird A, Widmer J, Maniura-Weber K, Fucentese SF, Traweger A, Silvan U, Snedeker JG, Macromechanics and polycaprolactone fiber organization drive macrophage polarization and regulate inflammatory activation of tendon in vitro and in vivo, Biomaterials. 249 (2020) 120034. doi: 10.1016/j.biomaterials.2020.120034. [DOI] [PubMed] [Google Scholar]
- [147].Wang J, Meng F, Song W, Jin J, Ma Q, Fei D, Fang L, Chen L, Wang Q, Zhang Y, Nanostructured titanium regulates osseointegration via influencing macrophage polarization in the osteogenic environment, Int. J. Nanomedicine Volume 13 (2018) 4029–4043. doi: 10.2147/IJN.S163956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Alfarsi MA, Hamlet SM, Ivanovski S, Titanium surface hydrophilicity modulates the human macrophage inflammatory cytokine response, J. Biomed. Mater. Res. Part A 102 (2014) 60–67. doi: 10.1002/jbm.a.34666. [DOI] [PubMed] [Google Scholar]
- [149].Hamlet S, Alfarsi M, George R, Ivanovski S, The effect of hydrophilic titanium surface modification on macrophage inflammatory cytokine gene expression, Clin. Oral Implants Res 23 (2012) 584–590. doi: 10.1111/j.1600-0501.2011.02325.x. [DOI] [PubMed] [Google Scholar]
- [150].Sunarso, Toita R, Tsuru K, Ishikawa K, A superhydrophilic titanium implant functionalized by ozone gas modulates bone marrow cell and macrophage responses, J. Mater. Sci. Mater. Med 27 (2016) 127. doi: 10.1007/s10856-016-5741-2. [DOI] [PubMed] [Google Scholar]
- [151].Liao Y, Li L, Chen J, Yang P, Zhao A, Sun H, Huang N, Tailoring of TiO2 films by H2SO4 treatment and UV irradiation to improve anticoagulant ability and endothelial cell compatibility, Colloids Surfaces B Biointerfaces. 155 (2017) 314–322. doi: 10.1016/j.colsurfb.2017.04.021. [DOI] [PubMed] [Google Scholar]
- [152].Ma Q-L, Zhao L-Z, Liu R-R, Jin B-Q, Song W, Wang Y, Zhang Y-S, Chen L-H, Zhang Y-M, Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization, Biomaterials. 35 (2014) 9853–9867. doi: 10.1016/j.biomaterials.2014.08.025. [DOI] [PubMed] [Google Scholar]
- [153].Scislowska-Czarnecka A, Szmigiel D, Genet M, Dupont-Gillain C, Pamula E, Kolaczkowska E, Oxygen plasma surface modification augments poly(L-lactide-co -glycolide) cytocompatibility toward osteoblasts and minimizes immune activation of macrophages, J. Biomed. Mater. Res. Part A 103 (2015) 3965–3977. doi: 10.1002/jbm.a.35509. [DOI] [PubMed] [Google Scholar]
- [154].Fukuda N, Tsuchiya A, Sunarso, Toita R, Tsuru K, Mori Y, Ishikawa K, Surface plasma treatment and phosphorylation enhance the biological performance of poly(ether ether ketone), Colloids Surfaces B Biointerfaces. 173 (2019) 36–42. doi: 10.1016/j.colsurfb.2018.09.032. [DOI] [PubMed] [Google Scholar]
- [155].Kosoff D, Yu J, Suresh V, Beebe DJ, Lang JM, Surface topography and hydrophilicity regulate macrophage phenotype in milled microfluidic systems, Lab Chip. 18 (2018) 3011–3017. doi: 10.1039/C8LC00431E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Lv L, Xie Y, Li K, Hu T, Lu X, Cao Y, Zheng X, Unveiling the Mechanism of Surface Hydrophilicity-Modulated Macrophage Polarization, Adv. Healthc. Mater 7 (2018) 1800675. doi: 10.1002/adhm.201800675. [DOI] [PubMed] [Google Scholar]
- [157].Gurevich DB, Severn CE, Twomey C, Greenhough A, Cash J, Toye AM, Mellor H, Martin P, Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression, EMBO J. 37 (2018). doi: 10.15252/embj.201797786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Hong H, Tian XY, The Role of Macrophages in Vascular Repair and Regeneration after Ischemic Injury, Int. J. Mol. Sci 21 (2020) 6328. doi: 10.3390/ijms21176328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Abaricia JO, Shah AH, Chaubal M, Hotchkiss KM, Olivares-Navarrete R, Wnt signaling modulates macrophage polarization and is regulated by biomaterial surface properties, Biomaterials. 243 (2020) 119920. doi: 10.1016/j.biomaterials.2020.119920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Boyan BD, Olivares-Navarrete R, Berger MB, Hyzy SL, Schwartz Z, Role of Wnt11 during Osteogenic Differentiation of Human Mesenchymal Stem Cells on Microstructured Titanium Surfaces, Sci. Rep 8 (2018) 1–11. doi: 10.1038/s41598-018-26901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Olivares-Navarrete R, Hyzy SL, Park JH, Dunn GR, Haithcock DA, Wasilewski CE, Boyan BD, Schwartz Z, Mediation of osteogenic differentiation of human mesenchymal stem cells on titanium surfaces by a Wnt-integrin feedback loop, Biomaterials. 32 (2011) 6399–6411. doi: 10.1016/j.biomaterials.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Sheikh Z, Brooks P, Barzilay O, Fine N, Glogauer M, Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials, Materials (Basel). 8 (2015) 5671–5701. doi: 10.3390/ma8095269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Miron RJ, Bosshardt DD, Multinucleated Giant Cells: Good Guys or Bad Guys?, Tissue Eng. Part B Rev 24 (2018) 53–65. doi: 10.1089/ten.teb.2017.0242. [DOI] [PubMed] [Google Scholar]
- [164].Miron RJ, Zohdi H, Fujioka-Kobayashi M, Bosshardt DD, Giant cells around bone biomaterials: Osteoclasts or multi-nucleated giant cells?, Acta Biomater. 46 (2016) 15–28. doi: 10.1016/j.actbio.2016.09.029. [DOI] [PubMed] [Google Scholar]
- [165].Cruz LJ, Tacken PJ, Rueda F, Domingo JC, Albericio F, Figdor CG, Targeting Nanoparticles to Dendritic Cells for Immunotherapy, in: 2012: pp. 143–163. doi: 10.1016/B978-0-12-391858-1.00008-3. [DOI] [PubMed] [Google Scholar]
- [166].Stewart JM, Keselowsky BG, Combinatorial drug delivery approaches for immunomodulation, Adv. Drug Deliv. Rev 114 (2017) 161–174. doi: 10.1016/j.addr.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Firdessa-Fite R, Creusot RJ, Nanoparticles versus Dendritic Cells as Vehicles to Deliver mRNA Encoding Multiple Epitopes for Immunotherapy, Mol. Ther. - Methods Clin. Dev 16 (2020) 50–62. doi: 10.1016/j.omtm.2019.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Chang S-Y, Ko H-J, Kweon M-N, Mucosal dendritic cells shape mucosal immunity, Exp. Mol. Med 46 (2014) e84–e84. doi: 10.1038/emm.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Eisenbarth SC, Dendritic cell subsets in T cell programming: location dictates function, Nat. Rev. Immunol 19 (2019) 89–103. doi: 10.1038/s41577-018-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Sugimoto MA, Sousa LP, Pinho V, Perretti M, Teixeira MM, Resolution of Inflammation: What Controls Its Onset?, Front. Immunol 7 (2016). doi: 10.3389/fimmu.2016.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Takagi H, Arimura K, Uto T, Fukaya T, Nakamura T, Choijookhuu N, Hishikawa Y, Sato K, Plasmacytoid dendritic cells orchestrate TLR7-mediated innate and adaptive immunity for the initiation of autoimmune inflammation, Sci. Rep 6 (2016) 24477. doi: 10.1038/srep24477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Bao M, Liu Y-J, Regulation of TLR7/9 signaling in plasmacytoid dendritic cells, Protein Cell. 4 (2013) 40–52. doi: 10.1007/s13238-012-2104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Guiducci C, Tripodo C, Gong M, Sangaletti S, Colombo MP, Coffman RL, Barrat FJ, Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9, J. Exp. Med 207 (2010) 2931–2942. doi: 10.1084/jem.20101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Bohannon J, Cui W, Cox R, Przkora R, Sherwood E, Toliver-Kinsky T, Prophylactic Treatment with Fms-Like Tyrosine Kinase-3 Ligand after Burn Injury Enhances Global Immune Responses to Infection, J. Immunol 180 (2008) 3038–3048. doi: 10.4049/jimmunol.180.5.3038. [DOI] [PubMed] [Google Scholar]
- [175].D’Amico L, Roato I, Cross-talk between T cells and osteoclasts in bone resorption, Bonekey Rep. 1 (2012). doi: 10.1038/bonekey.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Madel M-B, Ibáñez L, Wakkach A, de Vries TJ, Teti A, Apparailly F, Blin-Wakkach C, Immune Function and Diversity of Osteoclasts in Normal and Pathological Conditions, Front. Immunol 10 (2019). doi: 10.3389/fimmu.2019.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Vasilijić S, Savić D, Vasilev S, Vučević D, Gašić S, Majstorović I, Janković S, Čolić M, Dendritic cells acquire tolerogenic properties at the site of sterile granulomatous inflammation, Cell. Immunol 233 (2005) 148–157. doi: 10.1016/j.cellimm.2005.04.007. [DOI] [PubMed] [Google Scholar]
- [178].Kou PM, Schwartz Z, Boyan BD, Babensee JE, Dendritic cell responses to surface properties of clinical titanium surfaces, Acta Biomater. 7 (2011) 1354–1363. doi: 10.1016/j.actbio.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Yoshida M, Babensee JE, Differential effects of agarose and poly(lactic-co-glycolic acid) on dendritic cell maturation, J. Biomed. Mater. Res. Part A 79A (2006) 393–408. doi: 10.1002/jbm.a.30798. [DOI] [PubMed] [Google Scholar]
- [180].Rogers TH, Babensee JE, The role of integrins in the recognition and response of dendritic cells to biomaterials, Biomaterials. 32 (2011) 1270–1279. doi: 10.1016/j.biomaterials.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Babensee JE, Paranjpe A, Differential levels of dendritic cell maturation on different biomaterials used in combination products, J. Biomed. Mater. Res. Part A 74A (2005) 503–510. doi: 10.1002/jbm.a.30429. [DOI] [PubMed] [Google Scholar]
- [182].Wang K, Zhou C, Hong Y, Zhang X, A review of protein adsorption on bioceramics, Interface Focus. 2 (2012) 259–277. doi: 10.1098/rsfs.2012.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].van den Dries K, van Helden SFG, te Riet J, Diez-Ahedo R, Manzo C, Oud MM, van Leeuwen FN, Brock R, Garcia-Parajo MF, Cambi A, Figdor CG, Geometry sensing by dendritic cells dictates spatial organization and PGE2-induced dissolution of podosomes, Cell. Mol. Life Sci 69 (2012) 1889–1901. doi: 10.1007/s00018-011-0908-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Rostam HM, Singh S, Vrana NE, Alexander MR, Ghaemmaghami AM, Impact of surface chemistry and topography on the function of antigen presenting cells, Biomater. Sci 3 (2015) 424–441. doi: 10.1039/C4BM00375F. [DOI] [PubMed] [Google Scholar]
- [185].Ozpinar EW, Frey AL, Cruse G, Freytes DO, Mast Cell–Biomaterial Interactions and Tissue Repair, Tissue Eng. Part B Rev (2021). 10.1089/ten.teb.2020.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Krystel-Whittemore M, Dileepan KN, Wood JG, Mast cell: A multi-functional master cell, Front. Immunol 6 (2016) 620. 10.3389/fimmu.2015.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Agier J, Pastwińska J, Brzezińska-Błaszczyk E, An overview of mast cell pattern recognition receptors, Inflamm. Res 67 (2018) 737–746. 10.1007/s00011-018-1164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].da Silva EZM, Jamur MC, Oliver C, Mast Cell Function: A New Vision of an Old Cell, J. Histochem. Cytochem 62 (2014) 698–738. 10.1369/0022155414545334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Tang L, Jennings TA, Eaton JW, Mast cells mediate acute inflammatory responses to implanted biomaterials, Proc. Natl. Acad. Sci 95 (1998) 8841–8846. 10.1073/pnas.95.15.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Anderson JM, Rodriguez A, Chang DT, Foreign body reaction to biomaterials, Semin. Immunol 20 (2008) 86–100. 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Thevenot PT, Baker DW, Weng H, Sun MW, Tang L, The pivotal role of fibrocytes and mast cells in mediating fibrotic reactions to biomaterials, Biomaterials. 32 (2011) 8394–8403. 10.1016/j.biomaterials.2011.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Christo SN, Diener KR, Bachhuka A, Vasilev K, Hayball JD, Innate Immunity and Biomaterials at the Nexus: Friends or Foes, (2015). 10.1155/2015/342304. [DOI] [PMC free article] [PubMed]
- [193].Yamamoto S, Tanaka M, Sunami H, Ito E, Yamashita S, Morita Y, Shimomura M, Effect of honeycomb-patterned surface topography on the adhesion and signal transduction of porcine aortic endothelial cells, Langmuir. 23 (2007) 8114–8120. 10.1021/la7003326. [DOI] [PubMed] [Google Scholar]
- [194].Marcatti Amarú Maximiano W, Marino Mazucato V, Tambasco de Oliveira P, Célia Jamur M, Oliver C, Nanotextured titanium surfaces stimulate spreading, migration, and growth of rat mast cells, J. Biomed. Mater. Res. Part A 105 (2017) 2150–2161. 10.1002/jbm.a.36076. [DOI] [PubMed] [Google Scholar]
- [195].Kirshenbaum AS, Swindle E, Kulka M, Wu Y, Metcalfe DD, Effect of lipopolysaccharide (LPS) and peptidoglycan (PGN) on human mast cell numbers, cytokine production, and protease composition, BMC Immunol. 9 (2008) 45. 10.1186/1471-2172-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Carroll-Portillo A, Cannon JL, te Riet J, Holmes A, Kawakami Y, Kawakami T, Cambi A, Lidke DS, Mast cells and dendritic cells form synapses that facilitate antigen transfer for T cell activation, J. Cell Biol 210 (2015) 851–864. 10.1083/jcb.201412074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Colonna M, Innate Lymphoid Cells: Diversity, Plasticity, and Unique Functions in Immunity, Immunity. 48 (2018) 1104–1117. 10.1016/j.immuni.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Meininger I, Carrasco A, Rao A, Soini T, Kokkinou E, Mjösberg J, Tissue-Specific Features of Innate Lymphoid Cells, Trends Immunol. 41 (2020) 902–917. 10.1016/j.it.2020.08.009. [DOI] [PubMed] [Google Scholar]
- [199].Messing M, Jan-Abu SC, McNagny K, Group 2 innate lymphoid cells: Central players in a recurring theme of repair and regeneration, Int. J. Mol. Sci 21 (2020) 1350. 10.3390/ijms21041350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Robinette ML, Colonna M, Immune modules shared by innate lymphoid cells and T cells, J. Allergy Clin. Immunol 138 (2016) 1243–1251. 10.1016/j.jaci.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, Cella M, Colonna M, Intraepithelial type 1 innate lymphoid cells are a unique subset of il-12- and il-15-responsive ifn-γ-producing cells, Immunity. 38 (2013) 769–781. 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Borger JG, Lau M, Hibbs ML, The influence of innate lymphoid cells and unconventional T cells in chronic inflammatory lung disease, Front. Immunol 10 (2019) 1597. 10.3389/fimmu.2019.01597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Gurram RK, Zhu J, Orchestration between ILC2s and Th2 cells in shaping type 2 immune responses, Cell. Mol. Immunol 16 (2019) 225–235. 10.1038/s41423-019-0210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Gurram RK, Zhu J, Orchestration between ILC2s and Th2 cells in shaping type 2 immune responses, Cell. Mol. Immunol 16 (2019) 225–235. 10.1038/s41423-019-0210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Divekar R, Kita H, Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation, Curr. Opin. Allergy Clin. Immunol 15 (2015) 98–103. 10.1097/ACI.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Xue L, Salimi M, Panse I, Mjösberg JM, McKenzie ANJ, Spits H, Klenerman P, Ogg G, Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells, J. Allergy Clin. Immunol 133 (2014). 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].van Beek JJP, Martens AWJ, Bakdash G, de Vries IJM, Innate lymphoid cells in tumor immunity, Biomedicines. 4 (2016) 7. 10.3390/biomedicines4010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DMW, Artis D, IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions, Proc. Natl. Acad. Sci. U. S. A 112 (2015) 10762–10767. 10.1073/pnas.1509070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Nagasawa M, Spits H, Ros XR, Innate lymphoid cells (ILCs): Cytokine hubs regulating immunity and tissue homeostasis, Cold Spring Harb. Perspect. Biol 10 (2018). 10.1101/cshperspect.a030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Zhu J, GATA3 Regulates the development and functions of innate lymphoid cell subsets at multiple stages, Front. Immunol 8 (2017). 10.3389/fimmu.2017.01571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Elemam NM, Hannawi S, Maghazachi AA, Innate lymphoid cells (ILCs) as mediators of inflammation, release of cytokines and lytic molecules, Toxins (Basel). 9 (2017). 10.3390/toxins9120398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Golebski K, Ros XR, Nagasawa M, van Tol S, Heesters BA, Aglmous H, Kradolfer CMA, Shikhagaie MM, Seys S, Hellings PW, van Drunen CM, Fokkens WJ, Spits H, Bal SM, IL-1β, IL-23, and TGF-β drive plasticity of human ILC2s towards IL-17-producing ILCs in nasal inflammation, Nat. Commun 10 (2019) 2162–2162. 10.1038/s41467-019-09883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Eberl G, Colonna M, Santo JPD, McKenzie ANJ, Innate lymphoid cells: A new paradigm in immunology, Science (80-.) 348 (2015). 10.1126/science.aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Mariani E, Lisignoli G, Borzì RM, Pulsatelli L, Biomaterials: Foreign Bodies or Tuners for the Immune Response?, Int. J. Mol. Sci 20 (2019) 636. 10.3390/ijms20030636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S, Innate or adaptive immunity? The example of natural killer cells, Science (80-.) 331 (2011) 44–49. 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Malhotra A, Shanker A, NK cells: Immune cross-talk and therapeutic implications, Immunotherapy. 3 (2011) 1143–1166. 10.2217/imt.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Chen Y, Lu D, Churov A, Fu R, Research Progress on NK Cell Receptors and Their Signaling Pathways, Mediators Inflamm. 2020 (2020). 10.1155/2020/6437057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Zamai L, Ahmad M, Bennett IM, Azzoni L, Alnemri ES, Perussia B, Natural killer (NK) cell-mediated cytotoxicity: Differential use of TRAIL and Fas ligand by immature and mature primary human NK cells, J. Exp. Med 188 (1998) 2375–2380. 10.1084/jem.188.12.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Fauriat C, Long EO, Ljunggren HG, Bryceson YT, Regulation of human NK-cell cytokine and chemokine production by target cell recognition, Blood. 115 (2010) 2167–2176. 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Larouche J, Sheoran S, Maruyama K, Martino MM, Immune regulation of skin wound healing: Mechanisms and novel therapeutic targets, Adv. Wound Care 7 (2018) 209–231. 10.1089/wound.2017.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Mordechay L, Le Saux G, Edri A, Hadad U, Porgador A, Schvartzman M, Mechanical Regulation of the Cytotoxic Activity of Natural Killer Cells, ACS Biomater. Sci. Eng 7 (2021) 122–132. 10.1021/acsbiomaterials.0c01121. [DOI] [PubMed] [Google Scholar]
- [222].Le Saux G, Schvartzman M, Advanced Materials and Devices for the Regulation and Study of NK Cells, Int. J. Mol. Sci 20 (2019) 646. 10.3390/ijms20030646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Biolato AM, Filali L, Wurzer H, Hoffmann C, Gargiulo E, Valitutti S, Thomas C, Actin remodeling and vesicular trafficking at the tumor cell side of the immunological synapse direct evasion from cytotoxic lymphocytes, in: Int. Rev. Cell Mol. Biol, Elsevier Inc., 2020: pp. 99–130. 10.1016/bs.ircmb.2020.07.001. [DOI] [PubMed] [Google Scholar]
- [224].Paul S, Lal G, The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy, Front. Immunol 8 (2017) 1. 10.3389/fimmu.2017.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Mogensen TH, Pathogen recognition and inflammatory signaling in innate immune defenses, Clin. Microbiol. Rev 22 (2009) 240–273. 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Huse M, Mechanical forces in the immune system, Nat. Rev. Immunol 17 (2017) 679–690. 10.1038/nri.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Matalon O, Ben-Shmuel A, Kivelevitz J, Sabag B, Fried S, Joseph N, Noy E, Biber G, Barda-Saad M, Actin retrograde flow controls natural killer cell response by regulating the conformation state of SHP -1, EMBO J. 37 (2018). 10.15252/embj.201696264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Kawano S, Kojima M, Higuchi Y, Sugimoto M, Ikeda K, Sakuyama N, Takahashi S, Hayashi R, Ochiai A, Saito N, Assessment of elasticity of colorectal cancer tissue, clinical utility, pathological and phenotypical relevance, Cancer Sci. 106 (2015) 1232–1239. 10.1111/cas.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Xu Y, Pang SW, Natural killer cell migration control in microchannels by perturbations and topography, Lab Chip 19 (2019) 2466–2475. 10.1039/c9lc00356h. [DOI] [PubMed] [Google Scholar]
- [230].El Kholy K, Buser D, Wittneben JG, Bosshardt DD, Van Dyke TE, Kowolik MJ, Investigating the response of human neutrophils to hydrophilic and hydrophobic micro-rough titanium surfaces, Materials (Basel). 13 (2020) 1–8. 10.3390/ma13153421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Trindade R, Albrektsson T, Galli S, Prgomet Z, Tengvall P, Wennerberg A, Bone Immune Response to Materials, Part I: Titanium, PEEK and Copper in Comparison to Sham at 10 Days in Rabbit Tibia, J. Clin. Med 7 (2018) 526. 10.3390/jcm7120526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Fetz AE, Neeli I, Rodriguez IA, Radic MZ, Bowlin GL, Electrospun Template Architecture and Composition Regulate Neutrophil NETosis In Vitro and In Vivo <sup/>, Tissue Eng. Part A 23 (2017) 1054–1063. 10.1089/ten.tea.2016.0452. [DOI] [PubMed] [Google Scholar]
- [233].Abaricia JO, Shah AH, Olivares-Navarrete R, Substrate Stiffness Induces Neutrophil Extracellular Traps (NETs) Formation through Focal Adhesion Kinase Activation, Biomaterials. 271 (2021) 120715. 10.1016/j.biomaterials.2021.120715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Wilson ZS, Witt H, Hazlett L, Harman M, Neumann BM, Whitman A, Patel M, Ross RS, Franck C, Reichner JS, Lefort CT, Context-Dependent Role of Vinculin in Neutrophil Adhesion, Motility and Trafficking, Sci. Rep 10 (2020). 10.1038/s41598-020-58882-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Cohen HC, Lieberthal TJ, Kao WJ, Poly(ethylene glycol)-containing hydrogels promote the release of primary granules from human blood-derived polymorphonuclear leukocytes, J. Biomed. Mater. Res. - Part A 102 (2014) 4252–4261. 10.1002/jbm.a.35101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Sperling C, Fischer M, Maitz MF, Werner C, Neutrophil extracellular trap formation upon exposure of hydrophobic materials to human whole blood causes thrombogenic reactions, Biomater. Sci 5 (2017) 1998–2008. 10.1039/c7bm00458c. [DOI] [PubMed] [Google Scholar]
- [237].Schoenenberger AD, Tempfer H, Lehner C, Egloff J, Mauracher M, Bird A, Widmer J, Maniura-Weber K, Fucentese SF, Traweger A, Silvan U, Snedeker JG, Macromechanics and polycaprolactone fiber organization drive macrophage polarization and regulate inflammatory activation of tendon in vitro and in vivo, Biomaterials. 249 (2020). 10.1016/j.biomaterials.2020.120034. [DOI] [PubMed] [Google Scholar]
- [238].Kosoff D, Yu J, Suresh V, Beebe DJ, Lang JM, Surface topography and hydrophilicity regulate macrophage phenotype in milled microfluidic systems, Lab Chip. 18 (2018) 3011–3017. 10.1039/c8lc00431e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Thevenot PT, Nair AM, Shen J, Lotfi P, Ko CY, Tang L, The effect of incorporation of SDF-1α into PLGA scaffolds on stem cell recruitment and the inflammatory response, Biomaterials. 31 (2010) 3997–4008. 10.1016/j.biomaterials.2010.01.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Abebayehu D, Spence AJ, McClure MJ, Haque TT, Rivera KO, Ryan JJ, Polymer scaffold architecture is a key determinant in mast cell inflammatory and angiogenic responses, J. Biomed. Mater. Res. - Part A 107 (2019) 884–892. 10.1002/jbm.a.36605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Garg K, Ryan JJ, Bowlin GL, Modulation of mast cell adhesion, proliferation, and cytokine secretion on electrospun bioresorbable vascular grafts, J. Biomed. Mater. Res. - Part A 97 A (2011) 405–413. 10.1002/jbm.a.33073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Grigoryuk AA, Belov SA, Kotsyuba AE, Reaction of Mast Cells in the Zone of Polypropylene Mesh Implantation, Bull. Exp. Biol. Med 167 (2019) 694–697. 10.1007/s10517-019-04601-1. [DOI] [PubMed] [Google Scholar]
- [243].Choi H, Tanaka M, Hiragun T, Hide M, Sugimoto K, Non-tumor mast cells cultured in vitro on a honeycomb-like structured film proliferate with multinucleated formation, Nanomedicine Nanotechnology, Biol. Med 10 (2014) 313–319. 10.1016/j.nano.2013.08.011. [DOI] [PubMed] [Google Scholar]
- [244].Caires HR, Esteves T, Quelhas P, Barbosa MA, Navarro M, Almeida CR, Macrophage interactions with polylactic acid and chitosan scaffolds lead to improved recruitment of human mesenchymal stem/stromal cells: A comprehensive study with different immune cells, J. R. Soc. Interface 13 (2016). 10.1098/rsif.2016.0570. [DOI] [PMC free article] [PubMed] [Google Scholar]


