Abstract
We present the first mitochondrial genomes from Chagos Archipelago, Indian Ocean, of three putative species of reef forming Acropora (Acropora aff. tenuis, Acropora aff.cytherea and Acropora aff. orbicularis). The circular genome consists respectively of 18,334 bp, 18,353 bp and 18,584 bp. All mitochondrial genomes recovered comprise 13 protein-coding genes, two transfer RNA genes and two ribosomal RNA genes, with an overall GC content ranging from 37.9% to 38.0%. These new genomic data contribute to our increased understanding of genus Acropora and its species boundaries, ultimately aiding species monitoring and conservation efforts.
Keywords: mitochondrial genome, Acropora, Chagos Archipelago
Introduction
The genus Acropora (Scleractinia, Acroporidae) is a widespread coral, spanning the Indian and Pacific Oceans and the Caribbean Sea (Van Oppen et al. 2001, Veron et al. 2020) and is one of the major reef builders in warm water ecosystems (Fukami et al. 2000). Warm water reef-building corals create some of the most biodiverse ecosystems on the planet and have been estimated to support 830,000 species of multi-cellular plants and animals worldwide (Bellwood and Hughes 2001, Mora et al. 2008, Mora et al. 2011, Fisher et al. 2015), providing a variety of habitats for fish, invertebrates and other taxa in shallow tropical seas (Bellwood and Hughes 2001). Despite the importance of reef-building corals, species' boundaries are considered somewhat blurry and previous investigations on the Acropora genus show that many morphological species in this genus do not correspond to genetically distinct evolutionary units, with examples of intraspecific geographic differences in morphology as large as differences between species (Van Oppen et al. 2001). The Chagos Archipelago’s Acropora and Porites dominated reefs (Head et al. 2019) constitute around 2.5% of the world’s reefs (Sheppard 1999) and are a potential “stepping stone” for transoceanic species dispersal (Sheppard et al. 2012), so it is a key geographical location for further research.
Systematic research is defined as an interactive process in which taxa are defined or redefined by synthesis of all available information from biological, molecular and other relevant areas of science (WILSON 1985, Wallace and Willis 1994). Systematics attempts to keep pace with developments in these fields so that the most appropriate taxonomic interpretation will facilitate the greatest possible accuracy of research and experimental design (Wallace and Willis 1994). Despite a long history of taxonomic work, Scleractinia systematics is still largely unresolved (Richards et al. 2016) and species identifications in this group have been known to be problematic for more than four decades (Randall 1981, Wallace and Willis 1994). Further issues for Acropora include cases of shared recent ancestry and introgression of loci from ongoing hybridisation (Van Oppen et al. 2001, Willis et al. 2006, Richards et al. 2013, Richards et al. 2016). Species boundaries, currently applied to Acropora, do not stand up to scrutiny; this may in part be methodological, but current species boundaries are also confounded by characteristics of Acropora, such as morphological variability and hybridisation potential (Wallace and Willis 1994). The existence of morphologically cryptic species within recognised “species” of stony corals (Richards et al. 2016), together with evidence of strong and recurring regional genetic differentiation corresponding to the separation of the Indian and Pacific Ocean in Acropora (Richards et al. 2016), support the need for improved sampling across geographically distinct populations.
Mitochondrial DNA (mtDNA) has been used in numerous applications in the past 20 years, ranging from species delimitation (Paz-García et al. 2016) – usually focused on the mitochondrially encoded cytochrome c oxidase I (MT-CO1 or CO1 or COX1) (Hebert et al. 2003) – to phylogeny and molecular evolutionary studies (Curole and Kocher 1999, Niu et al. 2020). The mitochondrial genome plays a significant role in studies of phylogenetic reconstruction (Fukami and Knowlton 2005, Arrigoni et al. 2016, Capel et al. 2016, Terraneo et al. 2018, Terraneo et al. 2018), mostly due to a general consensus in the gene order and infrequent mitochondrial genome rearrangements across scleractinians corals (van Oppen et al. 2002, Lin et al. 2012). It typically includes 13 oxidative phosphorylation (OXPHOS) related genes, two rRNAs that encode the two subunits of mitochondrial ribosomes and an array of tRNAs used for translation within the organelle (Niu et al. 2020). Studying the mitochondrial genome could help to further explore Scleractinia’s evolutionary process and clarify the evolutionary relationship between Scleractinia and other Hexacorallia members (Lin et al. 2012).
Data resources
Data Availability Statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov/ under the accession no. MW773216 - MW773217 - MW773218. The associated **BioProject**, **SRA** and **Bio-Sample** numbers are listed in Table 1.
Table 1.
Data Availability Table
| Accession no. | BioProject | SRA | BioSample | Sample | Museum accession no.* |
| MW773216 | PRJNA720633 | SRR14216029 | SAMN18673282 | Acroporaaff.tenuis | NHMUK 2021.1 |
| MW773217 | PRJNA720633 | SRR14216028 | SAMN18673283 | Acroporaaff.cytherea | NHMUK 2021.2 |
| MW773218 | PRJNA720633 | SRR14216027 | SAMN18673284 | Acroporaaff.orbicularis | NHMUK 2021.3 |
*All sample deposited at The Natural History Museum, London (UK)
Material and methods
Study Area and Collection
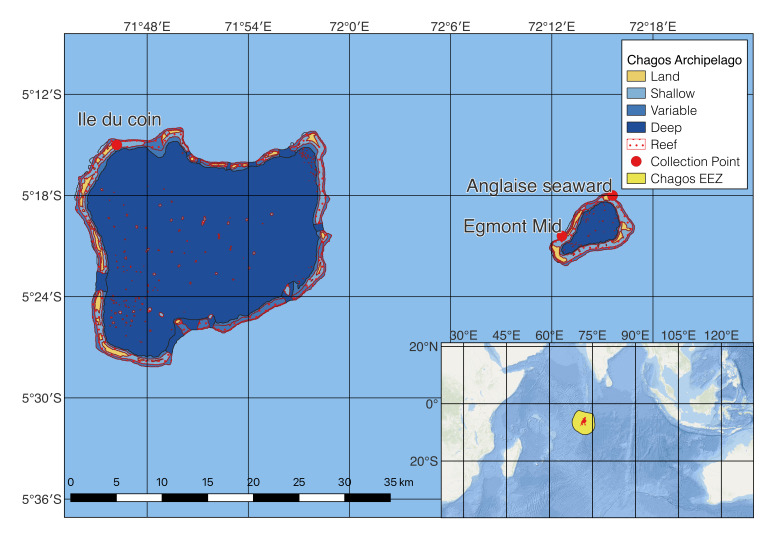
Samples were collected from three sites across Chagos Archipelago (Egmont Mid "-5.34, 72.21", Ile Anglaise seaward "-5.30, 72.26", Ile du coin "-5.25, 71.77" - Fig. 1) as part of the 2018 Chagos Reef 1 Expedition (CITES permits no. 567238/01 - 567238/02).
Figure 1.
Map of Peros Banhos and Salomon Islands with the three collection sites accompanied by location of the larger Chagos Archipelago. The samples were collected respectively at Egmont Mid for Acroporaaff.tenuis, Ile Anglaise seaward for Acroporaaff.cytherea and Ile du coin Acroporaaff.orbicularis.
A fragment of coral 2-3 cm2 in size, containing one or more healthy-looking polyps, was collected during SCUBA surveys and a corresponding photo of the colony was taken (Suppl. materials 1, 2, 3). In order to account for possible clonality, samples were collected at least one metre distance from each other. Upon returning to the ship, the samples were immediately placed in single vials in ethanol (+95%) and labelled with a unique identifier, in addition to collection date and location. The samples were then stored at -20°C until extraction.
Sample identification was performed in the field by eye by a coral expert (Dr Catherine Head). Identification was confirmed by Dr Tom Bridge, Senior Curator of Corals at the Queensland Museum Network (QMN), based on morphology from the field photos alongside phylogenetic methods (ultraconserved elements (UCEs) via hybrid capture (Quattrini et al. 2017, Zhang et al. 2019, Cowman et al. 2020))
DNA extraction
Total genomic DNA (gDNA) was extracted from four 0.5 to 2 cm coral fragments (Samples deposited at The Natural History Museum, London, UK, Table 1). The extraction followed a modified version of the manufacturer protocol for the DNeasy PowerSoil Pro Kit from Qiagen© (Protocols.io: dx.doi.org/10.17504/protocols.io.bww6pfhe). Following quantification of double-stranded DNA with a Qubit fluorometer 2.0 (Invitrogen, Waltham, MA), three separate indexed libraries were constructed with the gDNA by ligation kit (Oxford Nanopore Technologies) and subsequently pooled, prior to sequencing on a MinION sequencer (ligation kit: SQK-LSK109; indexes: EXP-NBD104; flowcell: R9 FLO-MIN106D).
Mitogenome assembly
Reads were demultiplexed and adapter trimmed using Porechop v.0.2.4 (https://github.com/rrwick/Porechop); subsequently, reads were mapped with Geneious mapper (Geneious Prime 2021.1.1.) to existing GenBank (Benson 2000) reference mitochondrial genomes (seven Acropora species: NC_003522, NC_022824, NC_022826, NC_022828, NC_022829, NC_022830 and NC_022831) and mapped reads were selected and de novo assembly conducted using CANU v.2.1.1 (Koren et al. 2017). The assemblies were then imported into the NanoGalaxy public server (de Koning et al. 2020) and polished using medaka (Oxford Nanopore Technologies Ltd. 2018 -Lu et al. 2016). Initial quality and annotation check for each assembly was performed with Quast (Gurevich et al. 2013, Mikheenko et al. 2015, Mikheenko et al. 2016, Mikheenko et al. 2018).
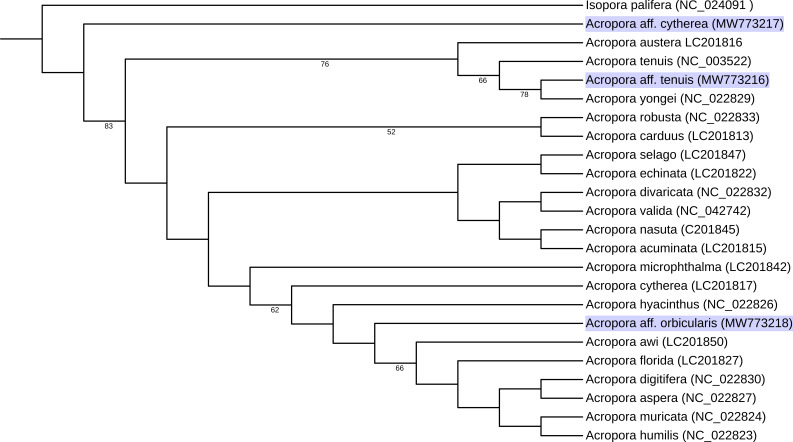
Subsequently, feature annotations were transferred in Geneious Prime and verified by comparison with alignments of coding regions with the above-mentioned references from GenBank. Inside coding regions, extra bases within a repeated seqeunce were manually removed if they caused a clear and significant frame-shift. Amino acid sequences of 13 concatenated protein-coding genes from our assemblies, together with 20 reference sequences were aligned using Geneious. Aligned sequences were uploaded to the European Galaxy server (Afgan et al. 2018) and subjected to phylogenetic analysis using IQ-TREE, with a 1000 non-parametric bootstrap, automatic model selection and default settings (Minh et al. 2013, Nguyen et al. 2015, Kalyaanamoorthy et al. 2017) and automatic amino acid substitution model selection (Fig. 2). Management, visualisation and annotation of the tree was done through iTOL v.6 (https://itol.embl.de - Letunic and Bork 2021).
Figure 2.
Maximum Likelihood phylogeny from analysis of concatenated protein-coding genes. Specimens from Chagos Archipelago annotated in blue. Bootstrap support numbers shown at nodes with > 50% support. GenBank accession numbers in parentheses. Outgroup - Isoporapalifera NC_024091.
Results
We determined the mitochondrial genome of Acropora aff. tenuis, Acropora aff. cytherea, Acropora aff.orbicularis to be respectively 18334 bp, 18353 bp and 18584 bp in length. The sequences are deposited in GenBank under accession no. MW773216, MW773217 and MW773218. The mitochondrial genome codes for 17 genes: 13 protein-coding genes, two tRNA genes and the large 16S and small 12S rRNA genes. Gene order follows an identical pattern to those of other Acropora mitochondrial genomes. Start and stop codons are reported in Table 2. The overall GC content is 37.9%, 38.0% and 38.0%, respectively, with an overall GC skew of 0. 0.28 and AT skew of 0.19. Nucleotide composition of the entire mitochondrial genome is: Acroporaaff.tenuis - A = 4,602 (25.1%), C = 2,514 (13.7%), G = 4,435 (24.2%), T = 6,783 (37.0%); Acroporaaff.cytherea - A = 3,280 (23.8%), C = 1,891 (13.7%), G = 3,305 (24.0%), T = 5,309 (38.5%); Acroporaaff.orbicularis - A = 4,645 (25.0%), C = 2,551 (13.7%), G = 4,516 (24.3%), T = 6,872 (37.0%). Where available, sequences of species matching the names of our tentative 'aff' assignments were included in the phylogeny; however, our samples did not group with these taxa (Fig. 2), supporting the tentative nature of these identifications and strengthening the case that these are distinct, possibly morphologically cryptic, species. These new genomic data contribute to our increased understanding of the phylogenetic history and mitochondrial evolution patterns in the Acropora genus, ultimately aiding species monitoring and conservation efforts.
Table 2.
Start/stop codon of all protein-coding genes
| COX1 | ATP8 | ND3 | ND4L | COX2 | COX3 | ND4 | ATP6 | ND6 | ND2 | CYTB | ND1 | ND5 | |
| Start codon | ATG | ATG | GTG | GTG | ATG | GTG | GTG | ATG | ATA | ATG | ATG | GTG | GTG |
| Stop codon | TAA | TAG | TAG | TAA | TAG | TAG | TAG | TAG | TAA | TAA | TAG | TAA | TAG |
Supplementary Material
Field photo of coral colony - Acroporaaff.tenuis
Dr Catherine Head
Data type
images
File: oo_592369.jpg
Field photo of coral colony - Acroporaaff.cytherea
Dr Catherine Head
Data type
images
File: oo_592373.jpg
Field photo of coral colony - Acroporaaff.orbicularis
Dr Catherine Head
Data type
images
File: oo_592365.jpg
Acknowledgements
This work was supported by the Bertarelli Foundation as part of the Bertarelli Programme in Marine Science (Chagos Science Expedition 2018) and Research England. We thank the Grampian Frontier crews for assistance with fieldwork, Dr Tom Bridge for the help in verifying species identifications, Rachel Jones, Emma Levy and The Bertarelli Foundation for logistical co-ordination. Researchers at ZSL are supported by funding from Research England. We thank reviewer Randolph Quek and editor Danwei Huang for their constructive comments.
References
- Afgan Enis, Baker Dannon, Batut Bérénice, Van Den Beek Marius, Bouvier Dave, Ech Martin, Chilton John, Clements Dave, Coraor Nate, Grüning Björn A., Guerler Aysam, Hillman-Jackson Jennifer, Hiltemann Saskia, Jalili Vahid, Rasche Helena, Soranzo Nicola, Goecks Jeremy, Taylor James, Nekrutenko Anton, Blankenberg Daniel. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Research. 2018;46(W1) doi: 10.1093/nar/gky379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigoni Roberto, Vacherie Benoît, Benzoni Francesca, Barbe Valérie. The complete mitochondrial genome of Acanthastrea maxima (Cnidaria, Scleractinia, Lobophylliidae) Mitochondrial DNA. 2016;27(2):927–928. doi: 10.3109/19401736.2014.926489. [DOI] [PubMed] [Google Scholar]
- Bellwood D. R., Hughes T. P. Regional-scale assembly rules and biodiversity of coral reefs. Science. 2001;292(5521):1532–1534. doi: 10.1126/science.1058635. [DOI] [PubMed] [Google Scholar]
- Benson Dennis A. GenBank. https://academic.oup.com/nar/article-lookup/doi/10.1093/nar/28.1.15. Nucleic Acids Research. 2000;28(1):15–18. doi: 10.1093/nar/28.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel K. C.C., Migotto A. E., Zilberberg C., Lin M. F., Forsman Z., Miller D. J., Kitahara M. V. Complete mitochondrial genome sequences of Atlantic representatives of the invasive Pacific coral species Tubastraea coccinea and T. tagusensis (Scleractinia, Dendrophylliidae): Implications for species identification. Gene. 2016;590(2):270–277. doi: 10.1016/J.GENE.2016.05.034. [DOI] [PubMed] [Google Scholar]
- Cowman Peter F., Quattrini Andrea M., Bridge Tom C. L., Watkins-Colwell Gregory J., Fadli Nur, Grinblat Mila, Roberts T. Edward, McFadden Catherine S., Miller David J., Baird Andrew H. An enhanced target-enrichment bait set for Hexacorallia provides phylogenomic resolution of the staghorn corals (Acroporidae) and close relatives. Molecular Phylogenetics and Evolution. 2020;153 doi: 10.1016/j.ympev.2020.106944. [DOI] [PubMed] [Google Scholar]
- Curole Jason P., Kocher Thomas D. Mitogenomics: digging deeper with complete mitochondrial genomes. Trends in Ecology & Evolution. 1999;14(10):394–398. doi: 10.1016/S0169-5347(99)01660-2. [DOI] [PubMed] [Google Scholar]
- de Koning Willem, Miladi Milad, Hiltemann Saskia, Heikema Astrid, Hays John P., Flemming Stephan, van den Beek Marius, Mustafa Dana A., Backofen Rolf, Grüning Björn, Stubbs Andrew P. NanoGalaxy: Nanopore long-read sequencing data analysis in Galaxy. GigaScience. 2020;9(10) doi: 10.1093/gigascience/giaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher Rebecca, O'Leary Rebecca A., Low-Choy Samantha, Mengersen Kerrie, Knowlton Nancy, Brainard Russell E., Caley M. Julian. Species richness on coral reefs and the pursuit of convergent global estimates. Current Biology. 2015;25(4):500–505. doi: 10.1016/j.cub.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Fukami Hironobu, Omori Makoto, Hatta Masayuki. Phylogenetic relationships in the coral family acroporidae, reassessed by inference from mitochondrial genes. Zoological Science. 2000;17(5):689–696. doi: 10.2108/zsj.17.689. [DOI] [PubMed] [Google Scholar]
- Fukami Hironobu, Knowlton Nancy. Analysis of complete mitochondrial DNA sequences of three members of the Montastraea annularis coral species complex (Cnidaria, Anthozoa, Scleractinia) https://link.springer.com/article/10.1007/s00338-005-0023-3. Coral Reefs 2005 24:3. 2005;24(3):410–417. doi: 10.1007/S00338-005-0023-3. [DOI] [Google Scholar]
- Gurevich Alexey, Saveliev Vladislav, Vyahhi Nikolay, Tesler Glenn. QUAST: quality assessment tool for genome assemblies. Bioinformatics. 2013;29(8):1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head Catherine E. I., Bayley Daniel T. I., Rowlands Gwilym, Roche Ronan C., Tickler David M., Rogers Alex D., Koldewey Heather, Turner John R., Andradi-Brown Dominic A. Coral bleaching impacts from back-to-back 2015–2016 thermal anomalies in the remote central Indian Ocean. Coral Reefs. 2019;38(4):605–618. doi: 10.1007/s00338-019-01821-9. [DOI] [Google Scholar]
- Hebert Paul D. N., Cywinska Alina, Ball Shelley L., deWaard Jeremy R. Biological identifications through DNA barcodes. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2003;270(1512):313–321. doi: 10.1098/rspb.2002.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyaanamoorthy Subha, Minh Bui Quang, Wong Thomas K F, von Haeseler Arndt, Jermiin Lars S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods. 2017;14(6):587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren Sergey, Walenz Brian P., Berlin Konstantin, Miller Jason R., Bergman Nicholas H., Phillippy Adam M. Canu: Scalable and accurate long-read assembly via adaptive κ-mer weighting and repeat separation. Genome Research. 2017;27(5):722–736. doi: 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic Ivica, Bork Peer. Interactive Tree of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Research. 2021 doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed]
- Lin Mei-Fang, Kitahara Marcelo Visentini, Tachikawa Hiroyuki, Fukami Hironobu, Miller David John, Chen Chaolun Allen. Novel organization of the mitochondrial genome in the deep-sea coral, Madrepora oculata (Hexacorallia, Scleractinia, Oculinidae) and its taxonomic implications. Molecular Phylogenetics and Evolution. 2012;65(1):323–328. doi: 10.1016/j.ympev.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Lu Hengyun, Giordano Francesca, Ning Zemin. Genomics, Proteomics & Bioinformatics; 2016. Oxford Nanopore MinION sequencing and genome assembly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheenko Alla, Saveliev Vladislav, Gurevich Alexey. MetaQUAST: evaluation of metagenome assemblies. Bioinformatics. 2015;32(7):1088–1090. doi: 10.1093/bioinformatics/btv697. [DOI] [PubMed] [Google Scholar]
- Mikheenko Alla, Valin Gleb, Prjibelski Andrey, Saveliev Vladislav, Gurevich Alexey. Icarus: visualizer for de novo assembly evaluation. Bioinformatics. 2016;32(21):3321–3323. doi: 10.1093/bioinformatics/btw379. [DOI] [PubMed] [Google Scholar]
- Mikheenko Alla, Prjibelski Andrey, Saveliev Vladislav, Antipov Dmitry, Gurevich Alexey. Versatile genome assembly evaluation with QUAST-LG. Bioinformatics. 2018;34(13) doi: 10.1093/bioinformatics/bty266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minh B Q, Nguyen M A T, von Haeseler A. Ultrafast approximation for phylogenetic bootstrap. Molecular Biology and Evolution. 2013;30(5):1188–1195. doi: 10.1093/molbev/mst024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora Camilo, Tittensor Derek P., Myers Ransom A. The completeness of taxonomic inventories for describing the global diversity and distribution of marine fishes. Proceedings of the Royal Society B: Biological Sciences. 2008;275(1631):149–155. doi: 10.1098/rspb.2007.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora Camilo, Tittensor Derek P., Adl Sina, Simpson Alastair G. B., Worm Boris. How many species are there on earth and in the ocean? PLOS Biology. 2011;9(8):1–8. doi: 10.1371/journal.pbio.1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Lam-Tung, Schmidt Heiko A, von Haeseler Arndt, Minh Bui Quang. IQ-tree: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution. 2015;32(1):268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Wentao, Xiao Jiaguang, Tian Peng, Yu Shuangen, Guo Feng, Wang Jianjia, Huang Dingyong. Characterization of the complete mitochondrial genome sequences of three Merulinidae corals and novel insights into the phylogenetics. PeerJ. 2020;8 doi: 10.7717/peerj.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-García David A., Galván-Tirado Carolina, Alvarado Juan José, Cortés Jorge, García-De-León Francisco J., Hellberg Michael E., Balart Eduardo F. Variation in the whole mitogenome of reef-building Porites corals. Conservation Genetics Resources. 2016;8(2):123–127. doi: 10.1007/s12686-016-0527-x. [DOI] [Google Scholar]
- Quattrini Andrea M., Faircloth Brant C., Dueñas Luisa F., Bridge Tom C. L., Brugler Mercer R., Calixto‐Botía Iván F., DeLeo Danielle M., Forêt Sylvain, Herrera Santiago, Lee Simon M. Y., Miller David J., Prada Carlos, Rádis‐Baptista Gandhi, Ramírez‐Portilla Catalina, Sánchez Juan A., Rodríguez Estefanía, McFadden Catherine S. Universal target‐enrichment baits for anthozoan (Cnidaria) phylogenomics: New approaches to long‐standing problems. Molecular Ecology Resources. 2017;18(2):281–295. doi: 10.1111/1755-0998.12736. [DOI] [PubMed] [Google Scholar]
- Randall R. H. Morphologic diversity in the scleractinian genus Acropora. http://ci.nii.ac.jp/naid/10008861509/en/ Proc 4th Inter Coral Reef Symp. 1981
- Richards Zoe T., Berry Oliver, van Oppen Madeleine J. H. Cryptic genetic divergence within threatened species of Acropora coral from the Indian and Pacific Oceans. https://link.springer.com/article/10.1007/s10592-015-0807-0. Conservation Genetics 2016 17:3. 2016;17(3):577–591. doi: 10.1007/S10592-015-0807-0. [DOI] [Google Scholar]
- Richards Z. T., Miller D. J., Wallace C. C. Molecular phylogenetics of geographically restricted Acropora species: Implications for threatened species conservation. Molecular Phylogenetics and Evolution. 2013;69(3):837–851. doi: 10.1016/J.YMPEV.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Sheppard Charles, Ateweberhan M., Bowen B. W., Carr P., Chen C. A., Clubbe C., Craig M. T., Ebinghaus R., Eble J., Fitzsimmons N., Gaither M. R., Gan C. H., Gollock M., Guzman N., Graham N. A.J., Harris A., Jones R., Keshavmurthy S., Koldewey H., Lundin C. G., Mortimer J. A., Obura D., Pfeiffer M., Price A. R.G., Purkis S., Raines P., Readman J. W., Riegl B., Rogers A., Schleyer M., Seaward M. R.D., Sheppard A. L.S., Tamelander J., Turner J. R., Visram S., Vogler C., Vogt S., Wolschke H., Yang J. M.C., Yang S. Y., Yesson C. Reefs and islands of the Chagos Archipelago, Indian Ocean: Why it is the world's largest no-take marine protected area. Aquatic Conservation: Marine and Freshwater Ecosystems. 2012;22(2):232–261. doi: 10.1002/aqc.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard Charles R C. Coral decline and weather patterns over 20 years in the Chagos Archipelago, Central Indian Ocean. http://www.jstor.org/stable/4314937 Ambio. 1999;28(6):472–478. [Google Scholar]
- Terraneo Tullia I., Arrigoni Roberto, Benzoni Francesca, Forsman Zac H., Berumen Michael L. Using ezRAD to reconstruct the complete mitochondrial genome of Porites fontanesii (Cnidaria: Scleractinia) Mitochondrial DNA Part B. 2018;3(1):173–174. doi: 10.1080/23802359.2018.1437805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terraneo Tullia Isotta, Arrigoni Roberto, Benzoni Francesca, Forsman Zac H., Berumen Michael L. The complete mitochondrial genome of Porites harrisoni (Cnidaria: Scleractinia) obtained using next-generation sequencing. Mitochondrial DNA Part B. 2018;3(1):286–287. doi: 10.1080/23802359.2018.1443852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oppen Madeleine J. H., Catmull Julian, McDonald Brenda J., Hislop Nikki R., Hagerman Paul J., Miller David J. The mitochondrial genome of Acroporatenuis (Cnidaria; Scleractinia) contains a large group I intron and a candidate control region. Journal of Molecular Evolution. 2002;55(1):1–13. doi: 10.1007/s00239-001-0075-0. [DOI] [PubMed] [Google Scholar]
- Van Oppen Madeleine J. H. H, McDonald Brenda J., Willis Bette, Miller David J. The evolutionary history of the coral genus Acropora (Scleractinia, Cnidaria) based on a mitochondrial and a nuclear marker: Reticulation, incomplete lineage sorting, or morphological convergence? Molecular Biology and Evolution. 2001;18(7):1315–1329. doi: 10.1093/oxfordjournals.molbev.a003916. [DOI] [PubMed] [Google Scholar]
- Veron J. E.N., Stafford-Smith M. G., Turak E., DeVantie L. M., Corals of the World. 2020. http://www.coralsoftheworld.com http://www.coralsoftheworld.com
- Wallace C C, Willis B L. Systematics of the coral genus acropora: Implications of new biological findings for species concepts. 1994. www.annualreviews.org www.annualreviews.org
- Willis Bette L., Oppen Madeleine J. H. van, Miller David J., Vollmer Steve V., Ayre David J. The role of hybridization in the evolution of reef corals. https://www.annualreviews.org/doi/abs/10.1146/annurev.ecolsys.37.091305.110136. http://dx.doi.org/10.1146/annurev.ecolsys.37.091305.110136. 2006;37:489–517. doi: 10.1146/ANNUREV.ECOLSYS.37.091305.110136. [DOI] [Google Scholar]
- WILSON E. O. Time to revive systematics. Science. 1985;230(4731):1227–1227. doi: 10.1126/science.230.4731.1227. [DOI] [PubMed] [Google Scholar]
- Zhang Y Miles, Williams Jason L, Lucky Andrea. Understanding UCEs: A comprehensive primer on using ultraconserved elements for arthropod phylogenomics. Insect Systematics and Diversity. 2019;3(5) doi: 10.1093/isd/ixz016. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Field photo of coral colony - Acroporaaff.tenuis
Dr Catherine Head
Data type
images
File: oo_592369.jpg
Field photo of coral colony - Acroporaaff.cytherea
Dr Catherine Head
Data type
images
File: oo_592373.jpg
Field photo of coral colony - Acroporaaff.orbicularis
Dr Catherine Head
Data type
images
File: oo_592365.jpg
Data Availability Statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov/ under the accession no. MW773216 - MW773217 - MW773218. The associated **BioProject**, **SRA** and **Bio-Sample** numbers are listed in Table 1.
Table 1.
Data Availability Table
| Accession no. | BioProject | SRA | BioSample | Sample | Museum accession no.* |
| MW773216 | PRJNA720633 | SRR14216029 | SAMN18673282 | Acroporaaff.tenuis | NHMUK 2021.1 |
| MW773217 | PRJNA720633 | SRR14216028 | SAMN18673283 | Acroporaaff.cytherea | NHMUK 2021.2 |
| MW773218 | PRJNA720633 | SRR14216027 | SAMN18673284 | Acroporaaff.orbicularis | NHMUK 2021.3 |
*All sample deposited at The Natural History Museum, London (UK)