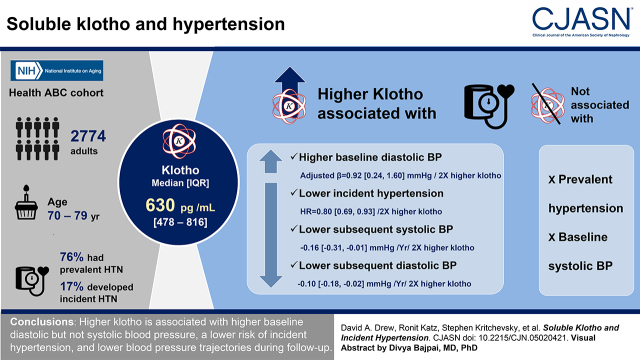
Visual Abstract
Keywords: klotho, hypertension, blood pressure, systolic blood pressure
Abstract
Background and objectives
Hypertension is associated with significant morbidity and mortality despite effective antihypertensive therapies. Soluble klotho is a circulating protein that in preclinical studies is protective against the development of hypertension. There are limited studies of klotho and blood pressure in humans.
Design, setting, participants, & measurements
Within the Health, Aging, and Body Composition Study, a cohort of well-functioning older adults, soluble klotho was measured in serum. We evaluated the cross-sectional and longitudinal association between klotho and blood pressure, prevalent hypertension, incident hypertension, and BP trajectories. Analyses were adjusted for demographics, cardiovascular disease and kidney disease risk factors, and measures of mineral metabolism including calcium, phosphate, parathyroid hormone, 25(OH) vitamin D, and fibroblast growth factor 23.
Results
The median klotho concentration was 630 pg/ml (478–816, 25th to 75th percentile). Within the cohort, 2093 (76%) of 2774 participants had prevalent hypertension and 476 (70%) of the remaining 681 developed incident hypertension. There was no association between klotho and prevalent hypertension or baseline systolic BP, but higher klotho was associated with higher baseline diastolic BP (fully adjusted β=0.92 mmHg, 95% confidence interval, 0.24 to 1.60 mmHg, higher per two-fold higher klotho). Higher baseline serum klotho levels were significantly associated with a lower rate of incident hypertension (fully adjusted hazard ratio, 0.80; 95% confidence interval, 0.69 to 0.93 for every two-fold higher klotho). Higher klotho was also associated with lower subsequent systolic BP and diastolic BP (−0.16, 95% confidence interval, −0.31 to −0.01, mmHg lower systolic BP per year and −0.10, 95% confidence interval, −0.18 to −0.02, mmHg lower diastolic BP per year, for each two-fold higher klotho).
Conclusions
Higher klotho is associated with higher baseline diastolic but not systolic BP, a lower risk of incident hypertension, and lower BP trajectories during follow-up.
Introduction
More than 1 billion people worldwide have hypertension, including two thirds of all adults >60 years of age with prevalent hypertension (1,2). Hypertension is therefore a public health burden, with increasing prevalence and risk of adverse health outcomes, such as coronary heart disease, chronic heart failure, stroke, CKD, and cognitive dysfunction (3). Recent landmark studies support more intense BP control to prevent cardiovascular disease events in persons with hypertension (4). Importantly, current treatments for hypertension do not fully eliminate the clinical complications of high BP. Therefore, novel molecular targets to prevent incident hypertension and associated complications could prove to have substantial clinical benefit.
The α-klotho gene was discovered 2 decades ago, when serendipitous disruption of its promoter in mice resulted in shortened lifespan, ectopic calcification, and arteriosclerosis, but notably normal kidney function at baseline (5). α-klotho protein (klotho) is highly expressed within kidney tubules as a transmembrane protein that can be released as a soluble form into the circulation (6,7). BP and klotho appear to be closely interrelated. Experimental evidence revealed that (1) exogenous klotho administration in hypertensive rats results in decreased activation of the renin-angiotensin system and reduction in BP (8), (2) klotho deficiency in mice results in salt-sensitive hypertension (9), and (3) klotho gene delivery attenuates hypertension and hypertensive kidney damage (10). Despite these observations, the relationship of klotho with BP has only been partially studied in humans (11,12).
The main objective of this manuscript was to evaluate the relationship between soluble klotho and hypertension in a large community-dwelling cohort of adults aged 70–79 years with comprehensive clinical and outcome data, and structured longitudinal follow-up. We also evaluated the relationship between soluble Klotho and change in BP over time. We hypothesized that higher concentrations of soluble klotho would associate with a lower risk of both prevalent and incident hypertension independent of known cardiovascular risk factors in this study population.
Materials and Methods
All data used in this manuscript are publicly available as a deidentified dataset and accompanying data dictionary through the National Institute of Aging and can be accessed via the following website: https://healthabc.nia.nih.gov/.
Study Population
The Health, Aging and Body Composition Study (Health ABC) is a prospective cohort first assembled in 1997 with a goal of assessing age-related physiologic and functional status. The study population consists of 3075 participants aged 70–79 years at baseline, with a balanced number of men and women, and was prespecified to include a significant proportion of Black participants (38%). All persons included were determined to be free of disability in activities of daily living and free of functional limitation at baseline. Because measures of klotho were only available at year 2 (stored samples were not available for testing from the baseline visit) and BP measures were also available at this visit, year 2 was set as the “baseline” for analyses in this manuscript. All participants provided written consent for the study. The study was approved by institutional review boards at each participating institution and meets the requirements of the Declaration of Helsinki.
Exposure
Serum klotho was assayed in singlicate using a commercially available sandwich ELISA test (IBL International, Japan) from never thawed frozen serum stored at −70°C obtained at the year 2 visit, 1 year after the baseline visit. This assay is reported to have a sensitivity of 6.15 pg/ml (13), and demonstrated an interassay coefficient of variation of 18%. The assay has been evaluated previously (14–17) and modestly correlates with more labor-intensive assay methods utilizing more specific synthetic antibodies and immunoprecipitation immunoblots (18).
Outcomes
Systolic and diastolic BPs were obtained from the right arms of participants by trained and certified clinical staff using a conventional mercury sphygmomanometer with the participant in a seated position after 5 minutes of rest. Two BP readings were taken at each visit and averaged. BP measures were obtained annually at study visits in years 1 through 6, then in years 8, 10, and 11. The type, dose, and total number of antihypertensive medications were also recorded at each visit. Prevalent hypertension was defined by a participant self-report of a previous physician’s diagnosis of hypertension and was confirmed by documentation of current use of antihypertensive medications (e.g., diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium-channel blockers, and β blockers) or by a systolic BP ≥140 mmHg and diastolic BP ≥90 mmHg. Incident hypertension was defined as a new classification of hypertension in any participant who was not classified with hypertension at baseline using the same hypertension definition described above. We also explored the association of klotho with a more restricted definition of incident hypertension (self-report and confirmation of BP medication but not BP measures), because this more restrictive definition was defined by study protocol.
Covariates
All covariates were obtained at the year 2 visit because this is the visit in which klotho and related mineral metabolism markers (calcium, phosphate, parathyroid hormone [PTH], 25(OH) vitamin D, and fibroblast growth factor 23 [FGF-23]) were measured, unless otherwise indicated. Cardiovascular disease status was defined as a history of coronary artery disease, stroke, or heart failure. Diabetes was defined as use of hypoglycemic agents, self-reported history, fasting plasma glucose level ≥126 mg/dl, or 2-hour oral glucose tolerance test result ≥200 mg/dl. Smoking status was obtained at year 1 and was classified as current, past (≥100 lifetime cigarettes), or never. Body mass index (BMI) was determined from height and weight at the year 2 visit. Urine albumin was measured at the year 1 visit using a particle-enhanced turbidimetric inhibition immunoassay allowing for direct albumin quantification (Siemens), whereas urine creatinine was measured by a modified Jaffé method on a clinical chemistry analyzer (Siemens). Urine albumin was indexed to urine creatinine to report urine albumin-creatinine ratio (UACR) in mg/g. Serum cystatin C was measured at year 1, and at years 3 and 10 from stored frozen serum samples at the Health ABC core laboratory (University of Vermont, Burlington, VT) with a BNII nephelometer (Dade Behring Inc., Deerfield, IL) using a particle-enhanced immunonepholometric assay (N Latex Cystatin C) (19). As in prior Health ABC studies (20,21), serum cystatin C was used as the primary measure of kidney function rather than serum creatinine because cystatin C measures were calibrated across all samples, whereas there was a shift in the creatinine assay from non-isotope dilution mass spectrometry traceable (baseline) to isotope dilution mass spectrometry traceable during the study (years 3 and 10). eGFR was calculated using a validated cystatin C–based estimating equation (19,22). Measures of mineral metabolism including calcium, phosphate, PTH, 25(OH) vitamin D, and FGF-23 were measured at year 2, concurrent with soluble klotho measurement, from frozen stored samples. Intact PTH was measured in EDTA plasma using a two-site immunoradiometric assay kit (N-tact PTHSP; DiaSorin). Serum calcium and phosphate levels were measured using direct quantitative colorimetric determination (Stanbio Laboratory, Boerne, TX, USA). Serum 25(OH) vitamin D was measured using a two-step radioimmunoassay (25-Hydroxyvitamin D 125I RIA Kit, DiaSorin, Stillwater, MN, USA) in a laboratory participating in the Vitamin D External Quality Assessment Scheme. FGF-23 was measured using a commercial ELISA that detects the full-length intact peptide (Kainos Laboratories, Japan).
Statistical Analysis
A complete case analysis was used for all models. We examined baseline characteristics of participants across quartiles of serum klotho. These were summarized with means and standard deviations, or medians and interquartile ranges for highly skewed variables or proportions for categorical variables. For eGFR and UACR, we also included the proportion of participants in each quartile below or above clinically relevant cut points (eGFR <60 ml/min per 1.73 m2, UACR >30 mg/g).
Klotho, BP, and Prevalent Hypertension.
Multivariable linear regression models were used to assess the relationships between serum klotho and baseline continuous diastolic and systolic BP measurements. For all subsequent analyses, klotho was examined as continuous variable using log base 2 transformation so that interpretation would be “per two-fold higher” klotho concentration; in addition, klotho was categorized by equal-sized quartiles. Multivariable models were then sequentially constructed through a series of nested models using prespecified variables as follows: model 1 unadjusted; model 2 adjusted for age, sex, and race; model 3 additionally adjusted for diabetes, cardiovascular disease, smoking status, BMI, baseline eGFR, and UACR; model 4 additionally adjusted for calcium, phosphate, 25(OH) vitamin D, PTH, and FGF-23. For prevalent hypertension at baseline, logistic regression models with the same prespecified covariate structures were constructed. We also examined the relationship between klotho and the number of baseline BP medications using Poisson regression (expressed as rate/count ratios), also using the same multivariable model structure.
Klotho, BP Trajectory, and Incident Hypertension.
Multivariable Cox proportional hazards models following the same covariate structure as described above were used to assess the relationship between klotho and incident hypertension. Participants with prevalent hypertension at baseline were excluded from these analyses. To examine the association between klotho and BP trajectories over time, random effects mixed models were used. For these models, the reported beta coefficient reflects the mean difference in BP each year per two-fold higher klotho.
Interactions.
On the basis of a priori hypotheses that a synergistic relationship could potentially exist between klotho and CKD status (baseline eGFR <60 ml/min per 1.73 m2) and FGF-23, we evaluated if the association between klotho and BP was modified by eGFR or FGF-23 by including interaction terms in the final multivariable models.
Sensitivity Analyses.
We also explored the association of klotho with the more restricted hypertension definition: self-reported physician's diagnosis of hypertension or current use of antihypertensive medications. Analyses were conducted using SPSS (IBM Corp., released 2015 IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY) and Stata (Stata Corp. 2013, Stata Statistical Software: Release 13 College Station, TX). A two-sided P value of <0.05 was considered statistically significant for all analyses including interaction terms.
Results
Baseline Characteristics
Among 3075 persons, 299 participants did not have samples available for klotho measurement, two were missing values for systolic BP, and zero were missing data for diastolic BP, leaving 2774 available for analysis. At baseline, 2093 (76%) participants had a diagnosis of prevalent hypertension. The mean (SD) age was 75 (3) years, with 1417 (51%) female, and 1019 (40%) Black participants (Table 1). The median klotho concentration was 630 pg/ml (478–816, 25th to 75th percentile). The mean (SD) baseline systolic BP was 134 (21) mmHg and the mean baseline diastolic BP was 70 (12) mmHg. The mean baseline eGFR was 72 (18) ml/min per 1.73 m2, and 677 (25%) participants had CKD by eGFR criteria (baseline eGFR of <60 ml/min per 1.73 m2).
Table 1.
Demographics and clinical characteristics by quartiles of klotho
| Variable | Full Cohort | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
| Range (pg/ml) | <478 | 478–630 | 631–816 | >816 | |
| N | 2774 | 694 | 693 | 694 | 693 |
| Age (yrs) | 75±3 | 75±3 | 75±3 | 75±3 | 75±3 |
| Female, n (%) | 1417 (51) | 360 (52) | 326 (47) | 347 (50) | 384 (55) |
| Black race, n (%) | 1103 (40) | 258 (37) | 229 (33) | 268 (39) | 348 (50) |
| Diabetes, n (%) | 1019 (37) | 233 (34) | 269 (39) | 249 (36) | 268 (39) |
| Hypertension, n (%) | 2093 (76) | 516 (74) | 532 (77) | 516 (74) | 529 (76) |
| Smoking, n (%) | |||||
| Former | 1277 (46) | 352 (51) | 328 (47) | 320 (46) | 277 (40) |
| Current | 268 (10) | 67 (10) | 74 (11) | 64 (9) | 63 (9) |
| Antihypertensive medications, n (%) | |||||
| ACE-I | 471 (17) | 128 (19) | 118 (17) | 110 (16) | 115 (17) |
| ARBs | 96 (4) | 26 (4) | 19 (3) | 26 (4) | 25 (4) |
| Beta blockers | 416 (15) | 124 (18) | 115 (17) | 99 (14) | 78 (11) |
| Calcium channel blockers | 658 (24) | 180 (26) | 144 (21) | 154 (22) | 180 (26) |
| Diuretics | 792 (29) | 213 (31) | 194 (28) | 183 (26) | 202 (29) |
| Any HTN medication | 1584 (57) | 411 (59) | 394 (57) | 382 (55) | 397 (57) |
| Coronary artery disease, n (%) | 494 (18) | 122 (18) | 117 (17) | 123 (18) | 132 (20) |
| Heart failure, n (%) | 33 (1) | 6 (1) | 7 (1) | 12 (2) | 8 (1) |
| Cerebrovascular disease, n (%) | 192 (7) | 55 (8) | 42 (6) | 42 (6) | 53 (8) |
| Systolic BP (mmHg) | 134±21 | 132±21 | 134±21 | 134±21 | 135±21 |
| Diastolic BP (mmHg) | 70±12 | 69±12 | 71±12 | 71±12 | 71±12 |
| Body mass index (kg/m2) | 27.2±4.8 | 27.3±4.8 | 27.3±4.4 | 27.1±4.8 | 27.1±5.1 |
| Total cholesterol (mg/dl) | 206±39 | 207±40 | 205±39 | 204±38 | 207±38 |
| C-reactive protein (mg/L) | 2.97 (1.25, 6.44) | 4.04 (1.65, 8.24) | 2.88 (1.31, 6.02) | 2.65 (1.14, 6.13) | 2.38 (1.15, 5.49) |
| eGFR_cysC (ml/min per 1.73 m2) | 72±18 | 70±20 | 71±18 | 73±18 | 76±18 |
| eGFR_cysC <60, n (%) | 677 (25) | 205 (30) | 180 (26) | 167 (24) | 125 (18) |
| Urine albumin-creatinine ratio (mg/g) | 8 (4, 19) | 7 (4, 16) | 7 (4, 20) | 8 (4, 19) | 8 (4, 20) |
| UACR ≥30 mg/g, n (%) | 480 (18) | 107 (16) | 119 (17) | 124 (18) | 130 (19) |
| Vitamin D 25(OH)2 (ng/ml) | 26±11 | 26±12 | 26±11 | 26±11 | 25±12 |
| Calcium (mg/dl) | 8.9±0.4 | 8.9±0.4 | 8.8±0.4 | 8.9±0.4 | 8.9±0.5 |
| Phosphate (mg/dl) | 3.6±0.5 | 3.6±0.5 | 3.5±0.5 | 3.5±0.5 | 3.6±0.5 |
| Parathyroid hormone (pg/ml) | 34 (25, 46) | 34 (25, 47) | 34 (25, 46) | 34 (26, 45) | 32 (25, 46) |
| FGF-23 (pg/ml) | 47 (37, 60) | 49 (38, 64) | 48 (37, 60) | 46 (36, 59) | 45 (36, 58) |
ACE-I, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blocker; HTN, hypertension; UACR, urine albumin-creatinine ratio; FGF-23, fibroblast growth factor 23.
Across quartiles of klotho, participants in the highest quartile were more likely to be a never smoker, more likely to be Black, had higher baseline systolic and diastolic BPs, and were less likely to have CKD at baseline. For measures of mineral metabolism, those in the highest klotho quartile were more likely to have lower FGF-23 concentrations. There were no observed differences in age, sex, diabetes, or hypertension-related comorbidities across quartiles of klotho.
Klotho and Cross-Sectional Outcomes
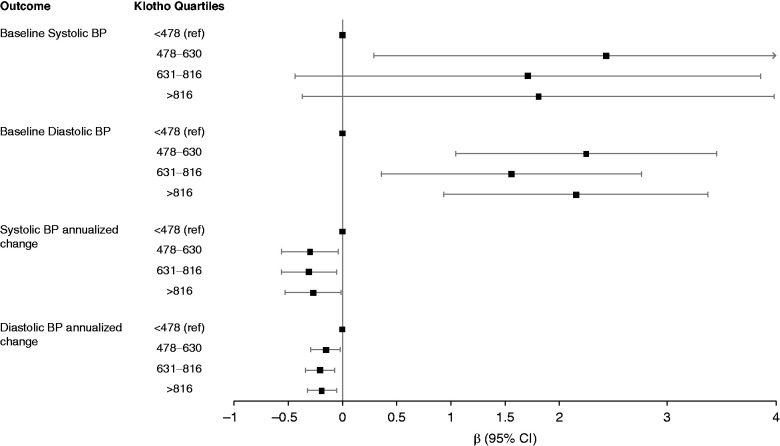
As shown in Table 2, higher klotho was not associated with lower systolic BP in fully adjusted models, which adjusted for demographics, comorbidity, kidney function, and other mineral metabolism markers (fully adjusted β, 0.96; 95% confidence interval [95% CI], −0.27 to 2.78 mmHg higher per two-fold higher klotho). In contrast, higher klotho was associated with higher diastolic BP in the same fully adjusted analyses (fully adjusted β, 0.92; 95% CI, 0.24 to 1.60 mmHg higher per two-fold higher klotho). Similarly, as seen in Figure 1, in quartile analyses, as compared with the lowest quartile, the highest quartile of klotho was associated with higher diastolic BP (fully adjusted β, 2.16; 95% CI, 0.94 to 3.37 mmHg higher for Q4 versus Q1). Serum klotho was not associated with either prevalent hypertension or a difference in the number of BP medications at baseline in either unadjusted or adjusted models (Table 3).
Table 2.
Association of klotho with systolic and diastolic BP in cross-section
| Model 1 | Model 2 | Model 3 | Model 4 | ||
| Klotho | N | β (95% Confidence Interval) | β (95% Confidence Interval) | β (95% Confidence Interval) | β (95% Confidence Interval) |
| Systolic BP | |||||
| Klotho (per doubling) | 2774 | 1.45 (0.21 to 2.68) | 1.12 (−0.11 to 2.34) | 1.05 (−0.17 to 2.27) | 0.96 (−0.27 to 2.18) |
| Klotho quartiles | |||||
| <478 | 694 | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) |
| 478–630 | 693 | 2.45 (0.25 to 4.65) | 2.72 (0.54 to 4.89) | 2.45 (0.30 to 4.60) | 2.43 (0.29 to 4.58) |
| 631–816 | 694 | 2.03 (−0.17 to 4.22) | 2.09 (−0.08 to 4.26) | 1.85 (−0.30 to 4.00) | 1.71 (−0.44 to 3.86) |
| >816 | 693 | 2.83 (0.63 to 5.03) | 2.07 (−0.11 to 4.25) | 1.92 (−0.26 to 4.09) | 1.81 (−0.37 to 3.98) |
| Diastolic BP | |||||
| Klotho (per doubling) | 2774 | 1.13 (0.43 to 1.83) | 0.92 (0.24 to 1.61) | 0.97 (0.28 to 1.66) | 0.92 (0.24 to 1.60) |
| Klotho quartiles | |||||
| <478 | 694 | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) |
| 478–630 | 693 | 2.14 (0.90 to 3.38) | 2.28 (1.07 to 3.50) | 2.21 (1.01 to 3.42) | 2.25 (1.05 to 3.45) |
| 631–816 | 694 | 1.90 (0.66 to 3.14) | 1.76 (0.54 to 2.97) | 1.68 (0.47 to 2.88) | 1.56 (0.36 to 2.76) |
| >816 | 693 | 2.63 (1.39 to 3.87) | 2.16 (0.94 to 3.38) | 2.20 (0.98 to 3.42) | 2.16 (0.94 to 3.37) |
Model 1 unadjusted analysis. Model 2 adjusted for age, sex, and race. Model 3 model 2+diabetes, cardiovascular disease, eGFR, UACR, BMI, and smoking. Model 4 model 3+calcium, phosphate, 25(OH) vitamin D, PTH, and FGF-23. UACR, urine albumin-creatinine ratio; BMI, body mass index; PTH, parathyroid hormone; FGF-23, fibroblast growth factor 23.
Figure 1.
Forest plot of adjusted association between klotho quartiles, baseline BP, and change in BP per year. DBP, diastolic BP; SBP, systolic BP; 95% CI, 95% confidence interval.
Table 3.
Association of klotho with prevalent hypertension and number of BP medications (n=2785)
| Model 1 | Model 2 | Model 3 | Model 4 | ||
| Prevalent Hypertension | n with Prevalent Hypertension | Odds Ratio (95% Confidence Interval) | Odds Ratio (95% Confidence Interval) | Odds Ratio (95% Confidence Interval) | Odds Ratio (95% Confidence Interval) |
| Klotho (per doubling) | 2093 | 1.04 (0.90 to 1.19) | 1.00 (0.87 to 1.15) | 1.02 (0.88 to 1.19) | 1.04 (0.89 to 1.21) |
| Klotho quartiles | |||||
| <478 | 516 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 478–630 | 532 | 1.14 (0.89 to 1.46) | 1.18 (0.92 to 1.51) | 1.18 (0.91 to 1.53) | 1.20 (0.92 to 1.56) |
| 631–816 | 516 | 1.00 (0.79 to 1.28) | 1.00 (0.78 to 1.27) | 1.01 (0.79 to 1.31) | 1.01 (0.78 to 1.31) |
| >816 | 529 | 1.12 (0.88 to 1.43) | 1.03 (0.80 to 1.32) | 1.07 (0.82 to 1.40) | 1.11 (0.85 to 1.45) |
| Baseline BP meds | Median n of meds | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) |
| Klotho (per doubling) | 1 | 0.93 (0.88 to 0.99) | 0.93 (0.87 to 0.98) | 0.95 (0.90 to 1.01) | 0.96 (0.91 to 1.02) |
| Klotho quartiles | |||||
| <478 | 1 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 478–630 | 1 | 0.91 (0.82 to 1.02) | 0.93 (0.84 to 1.04) | 0.95 (0.86 to 1.06) | 0.96 (0.87 to 1.07) |
| 631–816 | 1 | 0.88 (0.79 to 0.98) | 0.89 (0.79 to 0.99) | 0.91 (0.82 to 1.01) | 0.92 (0.83 to 1.03) |
| >816 | 1 | 0.88 (0.79 to 0.99) | 0.87 (0.78 to 0.97) | 0.91 (0.82 to 1.02) | 0.92 (0.83 to 1.03) |
Model 1 unadjusted analysis. Model 2 adjusted for age, sex, and race. Model 3 model 2+diabetes, cardiovascular disease, GFR, UACR, BMI, and smoking. Model 4 model 3+calcium, phosphate, 25(OH) vitamin D, PTH, and FGF-23. RR, rate ratio, equivalent to count ratio; 95% CI, 95% confidence interval; UACR, urine albumin-creatinine ratio; BMI, body mass index; PTH, parathyroid hormone; FGF-23, fibroblast growth factor 23.
Klotho and Longitudinal Outcomes
The majority of individuals without hypertension at baseline developed hypertension during follow-up (70% or 476 of 681, median [interquartile range] follow-up of 3.0 [1.9, 7.9] years) for an incident rate of 15% per year (Table 4). Individuals in the lowest klotho quartile had the highest incident rate of hypertension, whereas individuals in the highest klotho quartile had the lowest incident rate (Figure 2). Similarly, higher baseline serum klotho levels were significantly associated with a lower rate of incident hypertension that remained consistent across all models (fully adjusted hazard ratio, 0.80; 95% CI, 0.69 to 0.93 for every two-fold higher klotho). When examining the change in BP over time, higher serum klotho was associated with lower systolic BP in the fully adjusted model (−0.16; 95% CI, −0.31 to −0.01 mmHg lower systolic BP per year for each two-fold higher of klotho) and lower diastolic BP (−0.10, 95% CI, −0.18 to −0.02 mmHg lower diastolic BP per year for each two-fold higher of klotho) (Table 5).
Table 4.
Association of klotho with incident hypertension during follow-up
| Incidence Rate | Model 1 | Model 2 | Model 3 | Model 4 | |||
| Klotho | n | n with Incident Hypertension | (%/yr) | Hazard Ratio (95% Confidence Interval) | Hazard Ratio (95% Confidence Interval) | Hazard Ratio (95% Confidence Interval) | Hazard Ratio (95% Confidence Interval) |
| Klotho (per doubling) | 681 | 476 | 14.64 | 0.82 (0.71 to 0.95) | 0.81 (0.70 to 0.94) | 0.80 (0.69 to 0.93) | 0.80 (0.69 to 0.93) |
| Klotho quartiles | |||||||
| <478 | 178 | 139 | 18.03 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 478–630 | 161 | 103 | 12.97 | 0.74 (0.57 to 0.95) | 0.74 (0.57 to 0.95) | 0.74 (0.57 to 0.96) | 0.75 (0.58 to 0.97) |
| 631–816 | 178 | 130 | 15.32 | 0.87 (0.68 to 1.10) | 0.86 (0.68 to 1.10) | 0.87 (0.69 to 1.11) | 0.85 (0.67 to 1.08) |
| >816 | 164 | 104 | 12.39 | 0.72 (0.56 to 0.93) | 0.72 (0.55 to 0.93) | 0.72 (0.55 to 0.93) | 0.72 (0.55 to 0.93) |
Model 1 unadjusted analysis. Model 2 adjusted for age, sex, and race. Model 3 model 2+diabetes, cardiovascular disease, eGFR UACR, BMI, and smoking. Model 4 model 3+calcium, phosphate, 25(OH) vitamin D, PTH, and FGF-23. UACR, urine albumin-creatinine ratio; BMI, body mass index; PTH, parathyroid hormone; FGF-23, fibroblast growth factor 23.
Figure 2.
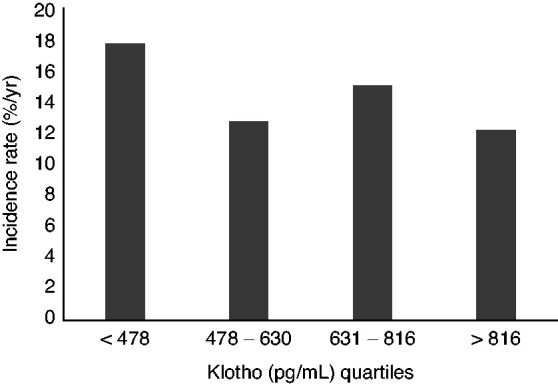
Incidence rate (%/yr) of incident hypertension by klotho quartiles.
Table 5.
Association of klotho with systolic and diastolic BP trajectories
| Annualized Absolute Change in Systolic BP | |||||
| Model 1 | Model 2 | Model 3 | Model 4 | ||
| Klotho | N | β (95% Confidence Interval) | β (95% Confidence Interval) | β (95% Confidence Interval) | β (95% Confidence Interval) |
| Klotho (per doubling) | 2774 | −0.18 (−0.33 to −0.04) | −0.19 (−0.33 to −0.04) | −0.16 (−0.31 to −0.02) | −0.16 (−0.31 to −0.01) |
| Klotho quartiles | |||||
| <478 | 694 | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) |
| 478–630 | 693 | −0.35 (−0.61 to −0.09) | −0.31 (−0.57 to −0.05) | −0.31 (−0.57 to −0.05) | −0.30 (−0.56 to −0.04) |
| 631–816 | 694 | −0.34 (−0.59 to −0.08) | −0.33 (−0.58 to −0.08) | −0.32 (−0.58 to −0.07) | −0.31 (−0.56 to −0.05) |
| >816 | 693 | −0.33 (−0.58 to −0.07) | −0.32 (−0.57 to −0.06) | −0.28 (−0.54 to −0.02) | −0.27 (−0.53 to −0.01) |
| Annualized Absolute Change in Diastolic BP | |||||
| Model 1 | Model 2 | Model 3 | Model 4 | ||
| Klotho (per doubling) | 2774 | −0.09 (−0.17 to −0.02) | −0.09 (−0.17 to −0.02) | −0.10 (−0.18 to −0.03) | −0.10 (−0.18 to −0.02) |
| Klotho quartiles | |||||
| <478 | 694 | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) |
| 478–630 | 693 | −0.16 (−0.29 to −0.02) | −0.15 (−0.29 to −0.02) | −0.15 (−0.29 to −0.02) | −0.15 (−0.29 to −0.02) |
| 631–816 | 694 | −0.21 (−0.34 to −0.07) | −0.20 (−0.34 to −0.07) | −0.21 (−0.35 to −0.08) | −0.21 (−0.34 to −0.07) |
| >816 | 693 | −0.18 (−0.32 to −0.05) | −0.18 (−0.31 to −0.04) | −0.19 (−0.33 to −0.06) | −0.19 (−0.32 to −0.05) |
Model 1 unadjusted analysis. Model 2 adjusted for age, sex, and race. Model 3 model 2+diabetes, cardiovascular disease, eGFR, UACR, BMI, and smoking. Model 4 model 3+calcium, phosphate, 25(OH) vitamin D, PTH, and FGF-23. UACR, urine albumin-creatinine ratio; BMI, body mass index; PTH, parathyroid hormone; FGF-23, fibroblast growth factor 23.
Interactions
There was no interaction between klotho, CKD status, and incident hypertension (P=0.9). There was also no interaction between klotho, FGF-23, and incident hypertension (P=0.3).
Sensitivity Analyses
Using the alternative incident hypertension definition that only included persons on BP medications or with a physician’s diagnosis of hypertension but not actual BP measures, the independent association between two-fold higher serum klotho and incident hypertension in the fully adjusted model persisted (adjusted hazard ratio, 0.87; 95% CI, 0.76 to 0.99).
Discussion
The main finding of this study is that higher serum klotho at baseline associated with lower risk of incident hypertension in a diverse and large community-dwelling cohort of adults aged 70–79 years. Specifically, a two-fold higher level of serum klotho at baseline associated with a 20% lower risk of incident hypertension during a median of 3-year follow-up. This association was nearly unaltered comparing unadjusted models with those including cardiovascular disease risk factors, prevalent cardiovascular disease, and markers of mineral bone disease, including calcium, phosphate, 25(OH) vitamin D, PTH, and FGF-23. Further, we found that higher serum klotho levels at baseline associated with subsequent lower systolic and diastolic BP measurements in repeated measures mixed models during follow-up, supporting the aforementioned relationship. This is contrasted with the finding that higher klotho was also associated with higher baseline diastolic BP. Although we have adjusted for comorbid conditions, there is the possibility that low diastolic BP at baseline represents poor vascular health (e.g., arteriosclerosis), leading to this paradoxical relationship.
Hypertension is a highly prevalent condition that is known to contribute to the progression of CKD and cardiovascular disease. Preclinical data have consistently shown that klotho deficiency contributes to the excess of cardiovascular disease in CKD (23,24). Klotho hypomorphic mice display significant vascular calcification and pathologic cardiac remodeling manifested as cardiac hypertrophy and fibrosis, which are attenuated by administration of exogenous klotho (25). Furthermore, klotho deficiency has a deleterious effect on endothelial function and results in impaired vasculogenesis, whereas administration of exogenous klotho reduces vascular endothelial inflammation (26). Klotho-deficient mice from genetic manipulation exhibit increased arterial stiffness (27,28) and develop salt-sensitive hypertension after high sodium intake (9). Exogenous klotho administration in hypertensive rats results in decreased activation of the renin-angiotensin system and reduction in BP (8). Importantly, the relationship between klotho and hypertension may be bidirectional because distinct rat models of hypertension all displayed a sustained decrease in klotho gene expression (29).
Human studies examining the relationship between klotho and BP are scarce. In a randomized clinical trial of patients with type 2 diabetes with hypertension, treatment with an angiotensin receptor blocker in addition to a thiazide diuretic versus a dihydropyridine calcium channel blocker was examined. Patients receiving valsartan/hydrochlorothiazide (versus amlodipine) had an increase in soluble klotho concentrations (measured using the same IBL assay used in this study) at 6 months, compared with treatment with amlodipine despite similar attained BP control in both groups (12). Serum klotho levels were not associated with aortic-pulse waive velocity (noninvasive metric of aortic stiffness) and albuminuria. Importantly, this study highlights potential BP-independent cardiorenal benefits of angiotensin receptor blockers through klotho modulation in patients with diabetic kidney disease and hypertension (12). In a more recent study, the pressure-natriuresis relationship of single nucleotide klotho gene polymorphisms and serum klotho was evaluated in patients with newly diagnosed hypertension. Participants underwent acute salt loading with infusion of intravenous 2 L of 0.9% saline in 2 hours. About one third of patients were salt sensitive, defined as a mean BP increase of >4 mmHg at the end of the infusion. Klotho single nucleotide polymorphism KL rs9536314 was consistently associated with salt-sensitive hypertension in this study. Further, there was an inverse relationship between serum Klotho and mean BP changes after the saline infusion (11). One interpretation of evolving preclinical and clinical data are that klotho restoration may be one of the potential mechanisms for the beneficial effect of intensive (versus standard) BP control and/or may exert BP-independent cardioprotective effects in patients at high risk of cardiovascular disease, such as those with prevalent hypertension. Finally, the cross-sectional observation of higher klotho levels associated with higher diastolic but not systolic BP at baseline may indirectly represent enhanced arterial compliance (narrower pulse pressure) in older individuals, whereas those with low klotho may be susceptible to prevalent arteriosclerosis and arterial stiffening. Collectively, this evidence provides an avenue for future research of kidney-vascular-heart cross-talk in experimental CKD and cardiovascular disease models (30).
One reason for the paucity of human data in klotho research is the lack of standardized assays for measuring soluble klotho. Specifically, inconsistent findings may be due to problems with performance and reproducibility of commercially available assays (18,13–15). Further, variants such as sample type, processing methods, number of freeze-thaw cycles, and conditions of storage could affect assay performance (30). Although development of highly specific and high-throughput klotho assays continues, methodology of klotho measurements should be taken into consideration when interpreting human databases. In this study, serum klotho was measured using a commercial ELISA (IBL International, Japan) from never thawed frozen serum stored at −70°C. Importantly, in a different study, these same klotho measurements independently associated with kidney function, such as higher serum klotho levels associated with a lower risk of decline in kidney function, which is consistent with experimental studies and provides external validity to the reported measurements (31).
Our study has some limitations. First, our findings are restricted to adults aged 70–79 years free of disability in activities of daily living and free of functional limitation at baseline. Whether results extend to other age groups is uncertain. Second, although Health ABC is a well-characterized cohort with systematic longitudinal follow-up and available robust clinical data for multivariable modeling, residual confounding is possible in all observational studies. Additionally, our study is limited to a single measurement of serum klotho at year 2 visit and lacks repeated measures of klotho for analysis, which is information that could further assist in the examination of the relationship between klotho and BP. We also acknowledge that some of the observed associations represent only a modest clinical effect, particularly the relationship between klotho and change in BP over time. However, we would argue that even small decrements in BP can translate to improvement in clinical outcomes, which may be explained by (1) averaging of BP changes across a large cohort and (2) variability in BP measures over time. Finally, given the older age of the cohort, the majority of individuals had prevalent hypertension at study enrollment, limiting the number of individuals who could develop subsequent incident hypertension. Nonetheless, our study has notable strengths. First, this constitutes the largest study to date examining the relationship of soluble klotho levels and BP parameters, including cross-sectional prevalent hypertension and longitudinal incident hypertension in humans. Second, klotho measurements utilized in this study have been previously reported in other observational studies supporting preclinical data of klotho and CKD, and providing external validity to the interpretation of our findings (31). Third, this study is nurtured by the robust clinical data available, specifically multiple mineral metabolism parameters, including FGF-23, which is relevant for modeling given the known interplay between klotho and FGF-23 in health and disease (32). Notably, despite adjustment for these parameters, and CKD and cardiovascular disease risk factors, there was little to no attenuation of the observed relationship between klotho levels and BP outcomes.
In a large community-dwelling cohort of adults aged 70–79 years, higher serum klotho was associated with lower risk of incident hypertension independently of demographics, known cardiovascular disease risk factors, prevalent cardiovascular disease, and markers of mineral bone disease, including calcium, phosphate, 25(OH) vitamin D, PTH, and FGF-23. Specifically, a two-fold higher serum klotho lowered the risk of incident hypertension by 20% (95% CI, 7% to 31%) during a median of 3 years of follow-up. Higher serum klotho levels also associated with lower systolic and diastolic BP measurements during follow-up. This study further underpins the bidirectional relationship between klotho and BP in humans. Future clinical studies are needed to confirm these findings and further evaluate the biomarker and therapeutic potential of klotho in cardiovascular and kidney disease.
Disclosures
A.B. Newman reports receiving honoraria from National Institutes of Health. A.N. Hoofnagle reports consultancy agreements from Kilpatrick, Townsend, and Stockton; reports having an ownership interest in Seattle Genetics; reports receiving research funding from Waters; reports patents and inventions with SISCAPA Assay Technologies; reports serving as an Associate Editor of Clinical Chemistry; and reports serving as an expert witness for Kilpatrick, Townsend, and Stockton. D.A. Drew reports serving as an Editorial Board member of Kidney Medicine. J.A. Neyra reports consultancy agreements with Baxter Healthcare Inc., Biomedical Insights, and Leadiant Biosciences; reports serving as a Section Editor of Clinical Nephrology, Guest Editor of Critical Care Nephrology in Advances in Chronic Kidney Disease, and on the Editorial Boards of Advances in Chronic Kidney Disease and Kidney360. L.F. Fried reports employment with Veterans Affairs Pittsburgh Healthcare System; reports having consultancy agreements with Bayer; and reports serving on data safety monitoring boards of CSL Behring and Novo Nordisk. J.H. Ix reports employment with San Diego Veterans Affairs; reports consultancy agreements with AstraZeneca, Ardelyx, Bayer, Jnana, and Sanifit; reports being a principal investigator of an investigator-initiated research project supported by Baxter International; and reports serving as a scientific advisor or member of AlphaYoung, a member of a data safety monitoring board for Sanifit International, and reports serving on advisory boards for AstraZeneca, Ardelyx, and Jnana Pharmaceuticals. M.G. Shlipak reports consultancy agreements with Cricket Health, Intercept Pharmaceuticals, University of North Carolina Chapel Hill, University of Washington Cardiovascular Health Study, and Veterans Medical Research Foundation; reports having an ownership interest in Tai Diagnostics; reports receiving research funding from Bayer Pharmaceuticals; reports receiving honoraria from the University of California, Irvine; reports serving as a scientific advisor or member of American Journal of Kidney Disease, Circulation, Journal of the American Society of Nephrology, and TAI Diagnostics; and reports serving as a Board Member of Northern California Institute for Research and Education. M. Sarnak reports serving on the Steering Committee of Akebia (funds paid to Tufts Medical Center) and consultancy agreements with Cardurian; and reports attending an advisory committee for Bayer in May 2019. O.M. Gutierrez reports consultancy agreements with QED Therapeutics; reports receiving research funding from Akebia, Amgen, and GlaxoSmithKline; reports receiving honoraria from Akebia, Amgen, Ardelyx, AstraZeneca, and Reata; and reports serving as an Associate Editor of CJASN. R. Katz reports consultancy agreements with University of California San Diego and University of Pennsylvania and serving as a Statistical Editor for CJASN. S. Kritchevsky reports serving as an Associate Editor of the Journal Gerontology: Medical Sciences. The remaining author has nothing to disclose.
Funding
This research was supported in part by the Intramural Research Program of the National Institutes of Health and the National Institute on Aging contracts N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106, grants R01-AG028050, 5R01AG027002-07 (to J.H. Ix, R. Katz, M. Sarnak, and M.G. Shlipak), R01 AG027012 (to R.D. Semba), and National Institute of Nursing Research grant R01-NR012459; National Institute of Diabetes and Digestive and Kidney Diseases grants K23DK105327 (to D.A. Drew), R56 DK126930 and P30 DK079337 (to J.A. Neyra), and National Heart, Lung, and Blood Institute grant R01 HL148448-01 (to J.A. Neyra); an American Heart Association Established Investigator Award (14EIA18560026 to J.H. Ix); an American Heart Association Strategically Focused Research Network in Disparities grant and grant K24DK116180 (to O.M. Gutierrez); and Nutrition Obesity Research Center grant P30 DK035816 (to A.N. Hoofnagle).
Acknowledgments
The study sponsors had no role in study design, collection, analysis, and interpretation of the data, writing the report, and the decision to submit the report for publication, however, the NIA did approve the manuscript for submission. The results presented in this paper have not been published previously, in whole or part. Because Dr. Orlando M. Gutiérrez is an Associate Editor of CJASN, he was not involved in the peer-review process for this manuscript. Another editor oversaw the peer review and decision-making process for this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J: Global burden of hypertension: Analysis of worldwide data. Lancet 365: 217–223, 2005 [DOI] [PubMed] [Google Scholar]
- 2.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E: 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311: 507–520, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P: Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: Analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension 37: 869–874, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT; SPRINT Research Group : A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 373: 2103–2116, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI: Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Kato Y, Arakawa E, Kinoshita S, Shirai A, Furuya A, Yamano K, Nakamura K, Iida A, Anazawa H, Koh N, Iwano A, Imura A, Fujimori T, Kuro-o M, Hanai N, Taeshige K, Nabeshima Y: Establishment of the anti-klotho monoclonal antibodies and detection of klotho protein in kidneys. Biochem Biophys Res Commun 267: 597–602, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y: Secreted Klotho protein in sera and CSF: Implication for post-translational cleavage in release of klotho protein from cell membrane. FEBS Lett 565: 143–147, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Takenaka T, Inoue T, Miyazaki T, Kobori H, Nishiyama A, Ishii N, Hayashi M, Suzuki H: Klotho ameliorates medullary fibrosis and pressure natriuresis in hypertensive rat kidneys. Hypertension 72: 1151–1159, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou X, Chen K, Lei H, Sun Z: Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation. J Am Soc Nephrol 26: 121–132, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Sun Z: Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension 54: 810–817, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Citterio L, Delli Carpini S, Lupoli S, Brioni E, Simonini M, Fontana S, Zagato L, Messaggio E, Barlassina C, Cusi D, Manunta P, Lanzani C: Klotho gene in human salt-sensitive hypertension. Clin J Am Soc Nephrol 15: 375–383, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karalliedde J, Maltese G, Hill B, Viberti G, Gnudi L: Effect of renin-angiotensin system blockade on soluble klotho in patients with type 2 diabetes, systolic hypertension, and albuminuria. Clin J Am Soc Nephrol 8: 1899–1905, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, Hasegawa H, Yamashita T, Nakatani K, Saito Y, Okamoto N, Kurumatani N, Namba N, Kitaoka T, Ozono K, Sakai T, Hataya H, Ichikawa S, Imel EA, Econs MJ, Nabeshima Y: Establishment of sandwich ELISA for soluble alpha-klotho measurement: Age-dependent change of soluble alpha-klotho levels in healthy subjects. Biochem Biophys Res Commun 398: 513–518, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedersen L, Pedersen SM, Brasen CL, Rasmussen LM: Soluble serum klotho levels in healthy subjects. Comparison of two different immunoassays. Clin Biochem 46: 1079–1083, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Heijboer AC, Blankenstein MA, Hoenderop J, de Borst MH, Vervloet MG; NIGRAM consortium : Laboratory aspects of circulating α-klotho. Nephrol Dial Transplant 28: 2283–2287, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Shimamura Y, Hamada K, Inoue K, Ogata K, Ishihara M, Kagawa T, Inoue M, Fujimoto S, Ikebe M, Yuasa K, Yamanaka S, Sugiura T, Terada Y: Serum levels of soluble secreted α-klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin Exp Nephrol 16: 722–729, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Dubal DB, Yokoyama JS, Zhu L, Broestl L, Worden K, Wang D, Sturm VE, Kim D, Klein E, Yu GQ, Ho K, Eilertson KE, Yu L, Kuro-o M, De Jager PL, Coppola G, Small GW, Bennett DA, Kramer JH, Abraham CR, Miller BL, Mucke L: Life extension factor klotho enhances cognition. Cell Rep 7: 1065–1076, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neyra JA, Moe OW, Pastor J, Gianella F, Sidhu SS, Sarnak MJ, Ix JH, Drew DA: Performance of soluble klotho assays in clinical samples of kidney disease. Clin Kidney J 13: 235–244, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldenstein L, Driver THF, Fried LF, Rifkin DE, Patel KV, Yenchek RH, Harris TB, Kritchevsky SB, Newman AB, Sarnak MJ, Shlipak MG, Ix JH; Health ABC Study Investigators : Serum bicarbonate concentrations and kidney disease progression in community-living elders: The Health, Aging, and Body Composition (Health ABC) Study. Am J Kidney Dis 64: 542–549, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madero M, Peralta C, Katz R, Canada R, Fried L, Najjar S, Shlipak M, Simonsick E, Lakatta E, Patel K, Rifkin D, Hawkins M, Newman A, Sarnak M; Health ABC Study : Association of arterial rigidity with incident kidney disease and kidney function decline: The Health ABC study. Clin J Am Soc Nephrol 8: 424–433, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neyra JA, Hu MC: α-klotho and chronic kidney disease. Vitam Horm 101: 257–310, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie J, Yoon J, An S-W, Kuro-o M, Huang C-L: Soluble klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol 26: 1150–1160, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu MC, Shi M, Gillings N, Flores B, Takahashi M, Kuro-O M, Moe OW: Recombinant α-klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int 91: 1104–1114, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maekawa Y, Ishikawa K, Yasuda O, Oguro R, Hanasaki H, Kida I, Takemura Y, Ohishi M, Katsuya T, Rakugi H: Klotho suppresses TNF-α-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine 35: 341–346, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Gao D, Zuo Z, Tian J, Ali Q, Lin Y, Lei H, Sun Z: Activation of SIRT1 attenuates klotho deficiency-induced arterial stiffness and hypertension by enhancing AMP-activated protein kinase activity. Hypertension 68: 1191–1199, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen K, Zhou X, Sun Z: Haplodeficiency of klotho gene causes arterial stiffening via upregulation of scleraxis expression and induction of autophagy. Hypertension 66: 1006–1013, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aizawa H, Saito Y, Nakamura T, Inoue M, Imanari T, Ohyama Y, Matsumura Y, Masuda H, Oba S, Mise N, Kimura K, Hasegawa A, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R: Downregulation of the Klotho gene in the kidney under sustained circulatory stress in rats. Biochem Biophys Res Commun 249: 865–871, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Neyra JA, Hu MC, Moe OW: Klotho in clinical nephrology: Diagnostic and therapeutic implications. Clin J Am Soc Nephrol 16: 162–176, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drew DA, Katz R, Kritchevsky S, Ix J, Shlipak M, Gutiérrez OM, Newman A, Hoofnagle A, Fried L, Semba RD, Sarnak M: Association between soluble klotho and change in kidney function: The Health Aging and Body Composition Study. J Am Soc Nephrol 28: 1859–1866, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christov M, Neyra JA, Gupta S, Leaf DE: Fibroblast growth factor 23 and klotho in AKI. Semin Nephrol 39: 57–75, 2019 [DOI] [PubMed] [Google Scholar]