Abstract
Objectives
The Pfizer-BioNTech BNT162b2 vaccine against SARS-CoV-2 infection is now available. This vaccine induces antibody production against the receptor-binding domain (RBD) of the spike glycoprotein S1 (S1-RBD). This study evaluated the performance of new immunoassays to measure this type of antibody.
Methods
Blood samples were collected at t0 (prime dose), after 21 days (t1, booster dose), and then after another 15 days (t2) from 70 health care professionals who had tested negative for previous SARS-CoV-2 infection and underwent vaccination with BNT162b2.
Results
Antibodies against S1-RBD were measured using 4 commercial assays. At t0, t1, and t2, the median antibody concentrations (interquartile range) were, respectively, 0.2 (0.1-0.4), 49.5 (19.1-95.7), and 888.0 (603.6-1,345.8) U/mL by Maglumi SARS-CoV-2 S-RBD immunoglobulin G (IgG) (Shenzen New Industries Biomedical Engineering, Snibe Diagnostics); 0.0 (0.0-0.0), 7.9 (4.2-15.6), and 112.3 (76.4-205.6) U/mL by Atellica IM SARS-CoV-2 IgG assay (Siemens Healthineers); 0.0 (0.0-0.0), 59.9 (18.3-122.0), and 2,646.0 (1,351.2-4,124.0) U/mL by Elecsys Anti–SARS-CoV-2 S assay (Roche Diagnostics); and 1.8 (1.8-1.8), 184 (94-294), and 1,841.0 (1,080.0-2,900.0) AU/mL by LIAISON SARS-CoV-2 TrimericS IgG assay (DiaSorin). The differences between medians at t0, t1, and t2 were all statistically significant (P < .001).
Conclusions
Antibodies against nucleocapsid proteins (N) were also measured using Maglumi 2019-nCoV IgG assay, which showed all negative results. All the considered anti-RBD methods detected response to the vaccine, while the method directed against anti-N failed to show response.
Keywords: SARS-CoV-2, COVID-19, Immunoassays, IgG, Antibody, BNT162b2 vaccine
Key Points.
The SARS-CoV-2 immunoassays can detect antibodies with different antigenic targets and different analytical performances.
Immunoassays against the receptor-binding domain protein reveal a great response after BNT162b2 vaccination against SARS-CoV-2.
The different methods studied correlate strongly.
Introduction
Since 2000, the COVID-19 pandemic has been conquering the world, leading to millions of people affected. Against this backdrop, vaccination seems to be the best strategy to eradicate the disease. The recent availability of SARS-CoV-2 vaccines could determine a new role for serologic tests. In this context, baseline assessment and postvaccine monitoring of anti–SARS-CoV-2 antibodies may prove instrumental for vaccination strategies (prioritization of individuals with no previous infection) as well as monitoring the extent and duration of the humoral immune response.1-4
To achieve this goal, it would be necessary to quantify the antibody concentration, given that interindividual response to a vaccine may differ widely (particularly in patients with previous infection or those on immunosuppressive therapies); in addition, the antibody concentration tends to diminish over time.4,5
Serologic assays for SARS-CoV-2 are becoming widely available and include enzyme-linked immunosorbent assay (ELISA), lateral flow assays, virus neutralization assays, and chemiluminescent immunoassays (CLIAs) run on fully automated clinical laboratory instruments.6 Given the potentially high volume of test requests, the use of fully automated clinical laboratory instruments is advisable.
The immunoassays can detect antibodies with different antigenic targets, produced during SARS-CoV-2 infection, and are mainly directed against nucleocapsid protein (N) or viral spike glycoprotein (S).7 The first proposed immunoassays targeted SARS-CoV-2 N or S. Most vaccines, however, are currently under emergency use authorization (EUA) in the United States, Europe, and Asia (BNT162b2 [Pfizer-BioNTech], mRNA-1273 [Moderna], Ad26.COV2.S [Janssen/Johnson & Johnson], ChAdOx1 nCoV-19 [AstraZeneca-Oxford University], NVX-CoV2373 [Novavax], and Gam-COVID-Vac [Sputnik V])8 aim to induce the antibody response exclusively against the spike glycoprotein S19-11 and the receptor-binding domain (RBD) of the S1 subunit of the protein spike. These antibodies appear to better correlate with virus neutralization.12
At this stage, neither the antibody response nor its magnitude to vaccination, including expected antibody concentrations and measurement differences between different immunoassays, is known. New immunoassays that target the RBD were recently made available.
We chose to evaluate the performance of 4 tests, targeted at RBD antibodies, by comparing them with 1 used in our laboratory, which is targeted at N antibodies. Participants were health care professionals who tested negative to previous SARS-CoV-2 infection through a real-time polymerase chain reaction (PCR) method before and after the BNT162b2 vaccine.
Materials and Methods
The study involved 70 St Bortolo Hospital Laboratory Medicine Department employees (median age [range] 48 [38-55] years; 60 women, 10 men; all White).
Because of company protocol, all 70 health care workers were subjected to health surveillance in the SARS-CoV-2 pandemic period (March 2020 to March 2021). Workers were subjected to periodic coronavirus tests, conducted using the rapid antigenic test (SARS-CoV-2 Rapid Antigen Test [Roche Diagnostics]; sensitivity, 95%; specificity, 99.2%) for an immediate answer by the real-time PCR method (cobas 6800 SARS-CoV-2 test [Roche Diagnostics]; sensitivity, 100%; specificity, 100%) for confirmation, which showed negative results.
From December 2020 to February 2021, all participants underwent BNT162b2 vaccine administration. SARS-CoV- 2 antibody measurement was performed in all health care workers before the first vaccine dose (t0), after 21 days (t1, booster dose), and 14 days following the booster dose (t2). Serum samples, collected in a test tube with coagulation activator and separator gel (Vacutest, Kima) were centrifuged and stored in a freezer at –80°C. All participants included in the study signed the informed consent form, and the study complied with the World Medical Association’s Declaration of Helsinki.
Samples were analyzed using 5 different immunoassays in a single analytic session. Assays were performed with all instruments in a single session on the same day. Samples in the third control were prediluted with negative serum pool as the same aliquot was processed on all analyzers to avoid eventual dilution errors.
Antibody detection and quantification were performed with 5 different immunoassays (analytical performance and diagnostic accuracy data by manufacturers’ instructions) Table 1 :
Table 1.
Main Characteristics of the 5 Immunoassays Studied, 4 for the Determination of Antibodies to SARS-CoV-2 S1-RBD/Spike Protein and 1 for Antibodies to 2019-nCoV Recombinant Antigen
| Atellica IM SARS-CoV-2 IgG | Elecsys Anti–SARS-CoV-2 S | LIAISON SARS-CoV-2 TrimericS IgG | Maglumi SARS-CoV-2 S-RBD IgG | Maglumi 2019-nCoV IgG | |
|---|---|---|---|---|---|
| Manufacturer | Siemens Healthineers | Roche Diagnostics | DiaSorin | Snibe | Snibe |
| Immunoassay | 2-step sandwich immunoassay | 1-step double-antigen sandwich assay | 2-step sandwich immunoassay | 2-step sandwich immunoassay | 2-step sandwich immunoassay |
| Detection of IgG antibodies | Qualitative and quantitative | Qualitative and quantitative | Quantitative | Quantitative | Qualitative |
| Technology | Indirect chemiluminescence | Electrochemiluminescence | Indirect chemiluminescence | Indirect chemiluminescence | Indirect chemiluminescence |
| Measuring interval | 0.50-150.0 [index] | 0.4-250 U/mL | 1.85-800.0 AU/mL | 0.180-100.0 AU/mL | 0.180-100.0 AU/ mL |
| Units | Index | U/mL | AU/mL | AU/mL | AU/mL |
| Specimen types | Serum, lithium heparin plasma | Serum, lithium heparin and EDTA plasma | Serum, lithium heparin and EDTA plasma | Serum, EDTA plasma | Serum, EDTA plasma |
| Reagents | Mouse monoclonal antihuman IgG antibody labeled with acridinium ester | Recombinant protein representing the RBD of the S antigen in a double- antigen sandwich assay format (biotinylated RBD antigen sample anti–SARS-CoV-2 ruthenylated RBD antigen) | Mouse monoclonal antihuman IgG antibody linked to an isoluminol derivative | Antihuman IgG antibody labeled with ABEI | 2019-nCoV recombinant antigen labeled with ABEI |
| Reagents—solid phase | Streptavidin-coated paramagnetic microparticles performed with biotinylated SARS-CoV-2 S1 RBD antigen | Streptavidin-coated microparticles | Paramagnetic particles coated with recombinant trimeric SARS-CoV-2 spike protein | Magnetic microbeads coated with recombinant SARS-CoV-2 S-RBD antigen | Magnetic microbeads coated with antihuman IgG antibody |
| Heat inactivation | Not recommended | Not recommended | Not declared | Recommended | Recommended |
| Cutoff (reactive) | ≥1.00 index | ≥0.80 U/mL | ≥13.0 AU/mL | ≥1.00 AU/mL | ≥1.10 AU/mL |
| Limit of blank | 0.40 index | 0.30 U/mL | 0.595 AU/mL | 0.100 AU/mL | — |
| Limit of detection | 0.50 index | 0.35 U/mL | 0,.12 AU/mL | 0.180 AU/mL | — |
| Limit of quantitation | 0.50 index | 0.40 U/mL | 1.63 AU/ml | — | — |
| Reproducibility, % | ≤7.1 | ≤6.7 | ≤5.8 | ≤8.5 | ≤6.9 |
| Clinical sensitivity (after 15 d), % | 96.41 | 96.6 | 98.7 | 100 | 91.2 |
| Clinical specificity, % | 99.90 | 99.98 | 99.5 | 99.6 | 97.3 |
ABEI, N-(4-aminobutyl)-N-ethylisoluminol; IgG, immunoglobulin G; S1-RBD, receptor-binding domain (RBD) of the spike glycoprotein S1.
Maglumi 2019-nCoV immunoglobulin G (IgG) (Shenzen New Industries Biomedical Engineering, Snibe Diagnostics). The chemiluminescent analytical system is based on a monoclonal antibody (mAb) anti-IgG (against SARS-Cov-2 N-protein), labeled N-(4-aminobutyl)-N-(ethylisoluminol) (ABEI), acting as a chemiluminescent reagent.
Maglumi SARS-CoV-2 S-RBD IgG (CLIA), a method of quantitative determination for S-RBD IgG antibodies to SARS-CoV-2. The assay is based on magnetic microbeads coated with recombinant SARS-CoV2 S-RBD antigen and antihuman IgG antibody labeled with ABEI.
Elecsys Anti–SARS-CoV-2 S (Roche Diagnostics) implemented with the cobas 411 analyzer, an immunoassay for in vitro quantitative determination of total (IgG, IgA, and IgM) antibodies to the SARS-CoV-2 S protein RBD in human serum and plasma. The assay uses a recombinant protein, representing the RBD of the S antigen in a double-antigen sandwich assay format. The sample is incubated with biotinylated SARS-CoV-2 S-RBD–specific recombinant antigen and SARS-CoV2 S-RBD–specific recombinant antigen labeled with a ruthenium complex. After adding streptavidin-coated microparticles, the complex is bound to the solid phase via interaction .
Atellica IM SARS-CoV-2 IgG (sCOV2G) (Siemens Healthineers), a quantitative method for detection of IgG antibodies against the S1-RBD antigen. This test is a fully automated, 2-step sandwich immunoassay, with indirect chemiluminescent technology. The patient specimen is incubated with preformed complex of streptavidin-coated particles and biotinylated SARS-CoV-2 recombinant antigens. The antibody-antigen complex is detected by an acridinium ester–labeled antihuman IgG mouse mAb.
LIAISON SARS-CoV-2 TrimericS IgG (DiaSorin) assay. This test uses chemiluminescent technology for quantitative determination of IgG against SARS-CoV-2 trimeric spike protein. The main test consists of magnetic particles coated with the SARS-CoV-2 recombinant trimeric spike protein and a conjugate reagent, containing a mouse mAb (directed against human IgG and bound to a derivative of isoluminol [antibody-isoluminol conjugate]).
The statistical significance between medians was evaluated using a nonparametric distributions test (Wilcoxon signed rank test for paired samples) and correlation coefficient by the Spearman rank correlation using MedCalc, version 19.6, statistical software. For comparison and evaluation purposes, we considered measurements obtained at t1 and t2 for every instrument. All data are presented as the median and interquartile range.
Results
Samples collected before vaccination (t0) were below the cutoff in all 70 participants using Elecsys Anti-SARS-CoV-2 S assay (<0.4 U/mL in all samples) and the Atellica IM SARS-CoV-2 IgG assay (0.0 [0.0-0.0] index). In the same samples, all but 1 concentration (28.0 AU/mL) were below the cutoff level using the LIAISON SARS-CoV-2 TrimericS IgG assay (1.8 [1.8-1.8] AU/mL); the Maglumi SARS-CoV-2 S-RBD IgG assay showed a median concentration of 0.2 [0.1-0.4] AU/mL, with 2 samples over its cutoff (1.2 AU/mL and 4.6 AU/mL, respectively). Last, the Maglumi 2019-nCoV IgG assay showed a concentration of 0.0 (0.0-0.0) AU/mL, with 4 samples over cutoff (1.9, 1.9, 3.2, and 4.3 AU/mL). These samples do not show significant differences (P = not significant [NS]) after checks at t1 and t2.
Samples collected 21 days after BNT162b2 vaccine inoculation (t1) showed an increase in antibody concentration in all patients using all methods: by Maglumi SARS-CoV-2 S-RBD IgG assay (49.5 [19.1-95.7] AU/mL; P < .001), Elecsys Anti–SARS-CoV-2 S assay (59.9 [18.3-122] U/mL; P < .001), Atellica IM SARS-CoV-2 IgG assay (7.9 [4.2-15.6] index; P < .001), and LIAISON SARS-CoV-2 TrimericS IgG assay (184 [94-294] AU/mL; P < .001). Assays performed using the Maglumi 2019-nCoV IgG assay showed no significant difference among t0, t1, and t2 samples (Wilcoxon rank sum test for paired data [P = NS).
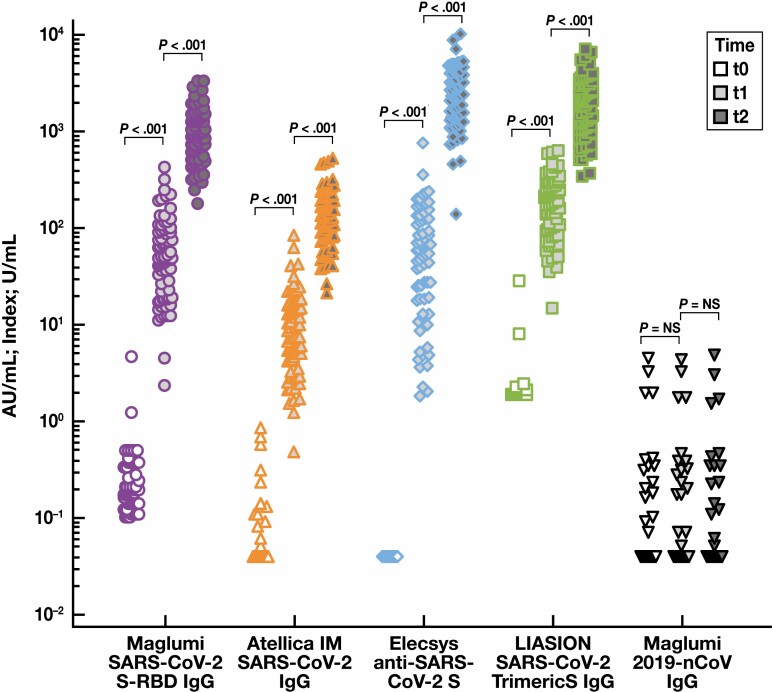
Samples collected 14 days after the booster dose showed a significant increase in antibody concentration with the Maglumi SARS-CoV-2 S-RBD IgG assay (888.0 [603.6-1,345.8] AU/mL; P < .001), Elecsys Anti–SARS-CoV-2 S assay (2,646.0 [1,351.2-4,124.0] U/mL; P < .001), the Atellica IM SARS-CoV-2 IgG assay (1,22.3 [76.4-205.6] index [P < .001]), and the LIAISON SARS-CoV-2 TrimericS IgG assay (1,841.0 [1,080.0-2,900.0] AU/mL; P < .001) Table 2 and Figure 1 .
Table 2.
Antibody Serum Concentrations of 70 Health Care Personnel Vaccinated With the BNT162b2 Vaccine, Measured by the 5 Immunoassays Studied, at t0, t1, and t2a
| Immunoassay | Units | t0 Prime Dose, Time 0 | t1 Booster Dose, 21 d After Prime Dose | t2 15 d After Booster Dose |
|---|---|---|---|---|
| Atellica IM SARS-CoV-2 IgG | Index | 0.0 (0.0-00) | 7.9 (4.2-15.6)b | 122.3 (76.4-205.6)c |
| LIAISON SARS-CoV-2 TrimericS IgG | AU/mL | 1.8 (1.8-1.8) | 184 (94-294)b | 1,852 (336-7,000)c |
| Maglumi SARS-CoV-2 S-RBD IgG | AU/mL | 0.2 (0.1-0.4) | 49.5 (19.1-95.7)b | 888.0 (603.6-1,345.8)c |
| Elecsys Anti–SARS-CoV-2 S | U/mL | <0.4 | 59.9 (18.3-122.0)b | 2,646.0 (1,351.2-4,214.0)c |
| Maglumi 2019-nCoV IgG | AU/mL | 0.0 (0.0-0.0) | 0.0 (0.0-0.0)d | 0.0 (0.0-0.0)e |
IgG, immunoglobulin G; IQR, interquartile range; NS, not significant; t0, prime vaccine dose; t1, dose 21 days after t0 (booster dose); t2, 15 days after t1.
aAll values are median [IQR]; P values are reported.
b P < .001 (t0-t1).
c P < .0001 (t1-t2).
d P = NS (t0-t1).
e P = NS (t1-t2).
Figure 1.
Antibody concentrations at t0 (before the prime vaccine dose; empty shape), t1 (after 21 days; lighter shapes), and t2 (15 days more; darker shapes) for each immunoassay: Maglumi SARS-CoV-2 S-RBD IgG (AU/mL), Atellica IM SARS-CoV-2 IgG (index), Elecsys Anti–SARS-CoV-2 S (U/mL), LIAISON SARS-CoV-2 TrimericS IgG (AU/mL), and Maglumi 2019-nCoV IgG (AU/mL). Data are represented in logarithm scale.
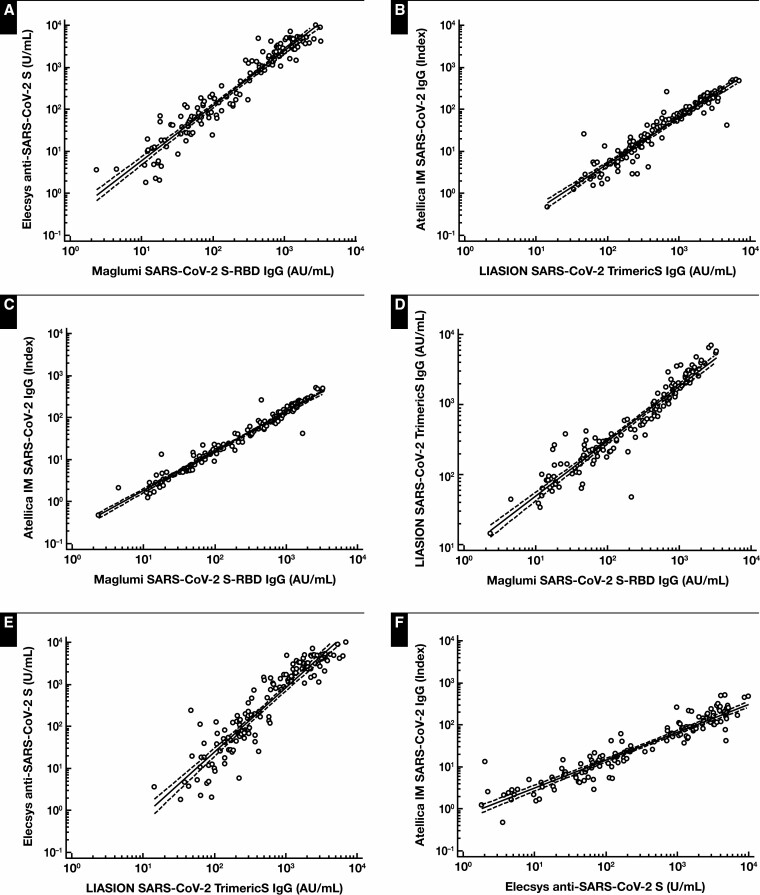
The correlation among methods ranged from the lowest (Elecsys vs LIASON [R = 0.93]) to the highest (Atellica vs Maglumi [R = 0.98]), considering t1 and t2 measurements Figure 2 .
Figure 2.
Log/log correlation among the 4 immunoassays for detecting anti–receptor-binding domain (RBD) SARS CoV-2 antibodies. Concentrations at t1 (21 days after prime vaccine dose) and t2 (15 days after t1) were compared. A, Elecsys Anti–SARS-CoV-2 S vs Maglumi SARS-CoV-2 S-RBD IgG: log(y) = –0.523 + 1.303 log(x); r = 0.96; P < .001. B, Atellica IM SARS-CoV-2 IgG vs LIAISON SARS-CoV-2 TrimericS IgG: log(y) = –1.511 + 1.102 log(x); r = 0.95; P < .001. C, Atellica IM SARS-CoV-2 IgG vs Maglumi SARS-CoV-2 S-RBD IgG: log(y) = –0.674 + 0.935 log(x); r = 0.98; P < .001. D, LIAISON SARS-CoV-2 TrimericS IgG vs Maglumi SARS-CoV-2 S-RBD IgG: log(y) = –0.907 + 0.785 log(x); r = 0.95; P < .001. E, Elecsys Anti–SARS-CoV-2 S vs LIAISON SARS-CoV-2 TrimericS IgG: log(y) = –1.676 + 1.531 log(x); r = 0.93; P < .001. F, Atellica IM SARS-CoV-2 IgG vs Elecsys Anti–SARS-CoV-2 S: log(y) = –0.167 + 0.665 log(x); r = 0.94; P < .001.
Discussion
Antibodies produced during SARS-CoV-2 infection are directed against the proteins of the nucleocapsid and glycoprotein, constituting spikes of the virus.7 From virus entry into the host cell, an interaction takes place between the unique and highly conserved viral spike glycoprotein and the angiotensin-converting enzyme 2 (ACE2) cell receptor.13 The humoral immune response has the potential to block the infection by neutralizing antibodies that would prevent the virus from infecting the host cell. SARS-CoV-2 infections begin when the viral spike protein engages the host ACE2 receptor. Two regions constitute the virus transmembrane spike protein: S1 and S2. The S1 region mediates the recognition and binding of the virus receptor to host cells by means of fragment-spanning amino acids 318-510, named RBD.14 At the same time, the S2 region facilitates virus fusion and entry.15,16 The humoral immune response for SARS-CoV-2 is achieved by interfering with the spike-ACE2 receptor interaction.17
Most vaccines under study in the preclinical and clinical evaluation stages aim to induce antibody response against the spike protein S1.10,11 So far, numerous trials have led to the creation of different anti–SARS-CoV-2 vaccine formulations, with different strategies to convey and induce the antibody stimulus against the virus.18-20
Eight vaccines have been granted EUA in the United States, Europe, and Asia for immunization: 2 messenger RNA (mRNA) vaccines (Pfizer-BioNTech’s BNT162b221 and Moderna’s mRNA-1273),22 3 vector-based adenoviruses (AstraZeneca-Oxford University’s ChAdOx1 nCoV-19 [AZD1222],23 Janssen/Johnson & Johnson’s Ad26.COV2.S,24 and Sputnik V’s Gam-COVID-Vac),25 1 recombinant protein vaccine (Novavax’s NVX-CoV2373),26 and 2 inactivated virus vaccines (Sinopharm’s BBIBP-CorV and Sinovac’s CoronaVac).27,28 All vaccines carry genetic information to produce the SARS-CoV-2 spike protein against the RBD of the S1 subunit of the protein spike.
BNT162b2 is a lipid nanoparticle-formulated nucleoside-modified mRNA vaccine that encodes the RBD of the SARS-CoV-2 spike protein. Some reports show that the neutralizing antibody titers are strongly correlated with RBD-binding IgG concentrations.29
It was reported that after the priming dose of Pfizer vaccine (30 μg), the geometric mean concentrations (GMCs) of RBD-binding IgG increased to 1,273 U/mL and to 12,431 U/mL 21 days after the boosting dose compared with a GMC of 602 U/mL in a panel of convalescent sera from patients who were infected with SARS-CoV-2.30 In these studies, anti-RBD antibodies were quantified by ELISA methods, but recently available, highly automated immunoassays specifically targeting the RBD domain of protein S seem to correlate well with neutralizing antibodies.4,10
Several immunoassays are available, but they differ in the type of detected antibodies (IgG, IgM, IgA, and total) and in particular with the antigenic target (S1, S2, spike glycoprotein RBD, or N). In our study, we compared the performance of 4 different immunoassays that specifically target the RBD and a fifth method targeting N proteins only.
The studied population consisted of health workers negative for previous SARS-CoV-2 infection. In fact, the basal antibody measurement was below the cutoff with the Elecsys Anti–SARS-CoV-2 S and Atellica IM SARS-CoV-2 IgG assays. One sample at t0 was over cutoff using the LIAISON SARS-CoV-2 TrimericS IgG assay, and 2 samples at t0 were equally over cutoff using the Maglumi SARS-CoV-2 S-RBD IgG assay. Antibody concentrations were low, and participants were negative using the Maglumi 2019-nCoV IgG assay; they can likely be defined as nonspecific IgG.
Four samples were over cutoff using the Maglumi 2019-nCoV IgG assay on all checks, with values that did not differ significantly. None of these had positive t0 samples with any other assays. It is plausible that these patients had been previously infected by the SARS-CoV-2 virus without showing symptoms.
At t1, 21 days after the first vaccine dose, the antibody concentrations had risen in all participants such that all samples were over cutoff on every method. At the booster dose, 15 days later (t2), all participants showed high antibody concentrations. Data distribution was heterogeneous, with samples ranging from 20- to 400-fold the cutoff using the Atellica assay, from 130- to 8,800-fold the cutoff using the Elecsys assay, from 300- to 7,000-fold the cutoff using the LIAISON assay, and from 300- to 3,000-fold the cutoff using the Maglumi SARS-CoV-2 S-RBD IgG assay.
Analyzing data on a logarithmic scale, the 4 methods for anti-RBD antibody detection correlated well among themselves Figure 2 , although the absolute values may seem different. It should be noted, however, that the 4 methods use different units of measurement and different scales. It is possible that better harmonization of the units and cutoff could further improve the quantitative and diagnostic agreement between methods, as other authors have already suggested.31
Moreover, the National Institute for Biological Standards and Control in the United Kingdom recently launched the first World Health Organization international standard for anti–SARS-CoV-2 immunoglobulin.32 The presence of reference material will enable various companies to propose possible conversion of the different units currently used into international binding arbitrary units (BAU/mL).
Yet, it was interesting to observe that the measure of IgG antibodies directed against the nucleocapsid at t1 and t2 did not differ from the basal measure after administration of the vaccine. This finding is significant because it indicates that vaccine-induced immunity is different from immunity obtained in the aftermath of a SARS-CoV-2 infection. In fact, the infection probably determines the formation of several different antibodies, including anti-RBDs, while spike protein–based vaccines cause the specific increase of anti-RBD antibodies, instead. After vaccination with the Pfizer vaccine, antibodies will be elicited only against 1 part of the virus, the spike protein.33,34
Only serology assays specific for antibodies that target regions within the spike protein (eg, the RBD) can be used to evaluate immune response to the BNT162b2 vaccine. Further studies must assess the performance of immunoassays with other vaccines or with inactivated whole virus–based vaccines. In contrast, the determination of antibodies against nucleocapsid proteins could be critical in determining whether a person has been exposed to SARS-CoV-2 in the past and then developed antibodies against the virus.
The main limitation of our study is the assessment of antibody kinetics related to administration of the Pfizer-BioNTech BNT162b2 vaccine alone.
References
- 1. Bubar KM, Reinholt K, Kissler SM, et al. . Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science. 2021;371:916-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bohn MK, Loh TP, Wang CB, et al. ; the IFCC Taskforce on COVID-19 . IFCC interim guidelines on serological testing of antibodies against SARS-CoV-2. Clin Chem Lab Med. 2020;58:2001-2008. [DOI] [PubMed] [Google Scholar]
- 3. Lippi G, Sciacovelli L, Trenti T, et al. . Executive Board of SIBioC (Società Italiana di Biochimica Clinica e Biologia Molecolare Clinica). Kinetics and biological characteristics of humoral response developing after SARS-CoV-2 infection: implications for vaccination [published online ahead of print January 21, 2021]. Clin Chem Lab Med. doi: 10.1515/cclm-2021-0038. [DOI] [PubMed] [Google Scholar]
- 4. Manisty C, Otter AD, Treibel TA, et al. . Antibody response to first BNT162b2 dose in previously SARS-CoV-2–infected individuals. Lancet. 2021;397:1057-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Legros V, Denolly S, Vogrig M, et al. . A longitudinal study of SARS-CoV-2–infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol. 2021;18:318-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krammer F, Simon V. Serology assays to manage COVID-19. Science. 2020;368:1060-1061. [DOI] [PubMed] [Google Scholar]
- 7. Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323:2249-2251. [DOI] [PubMed] [Google Scholar]
- 8. Edwards KM, Orenstein WA. COVID-19: vaccines to prevent SARS-CoV-2 infection.UpToDate Web site. https://www.uptodate.com/contents/covid-19-vaccines-to-prevent-sars-cov-2-infection. Updated June 11, 2021. Accessed June 16, 2021. [Google Scholar]
- 9. COVID-19 vaccine tracker and landscape. World Health Organization Web site. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. Updated June 15, 2021. Accessed June 16, 2021.
- 10.COVID-19 vaccine tracker. Vaccine Centre at the London School of Hygiene & Tropical Medicine Web site.https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/. Updated June 14, 2021. Accessed June 16, 2021.
- 11. Coronavirus vaccine tracker. The New York Times Web site. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html. Updated June 14, 2021. Accessed June 16, 2021.
- 12. GeurtsvanKessel CH, Okba NMA, Igloi Z, et al. . An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat Commun. 2020;11:3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou P, Yang XL, Wang XG, et al. . A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Babcock GJ, Esshaki DJ, Thomas WD Jr, et al. . Amino acids 270 to 510 of the severe acute respiratory syndrome coronavirus spike protein are required for interaction with receptor. J Virol. 2004;78:4552-4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ou X, Liu Y, Lei X, et al. . Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Walls AC, Park YJ, Tortorici MA, et al. . Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281-292.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoffmann M, Kleine-Weber H, Schroeder S, et al. . SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271-280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chung YH, Beiss V, Fiering SN, et al. . COVID-19 vaccine frontrunners and their nanotechnology design. ACS Nano. 2020;14:12522-12537. [DOI] [PubMed] [Google Scholar]
- 19. Zhang J, Zeng H, Gu J, et al. . Progress and prospects on vaccine development against SARS-CoV-2. Vaccines (Basel). 2020;8:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharma O, Sultan AA, Ding H, et al. . A review of the progress and challenges of developing a vaccine for COVID-19. Front Immunol. 2020;11:585354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu K, Werner AP, Koch M, et al. . Serum neutralizing activity elicited by mRNA-1273 vaccine. N Engl J Med. 2021;384:1468-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jackson LA, Anderson EJ, Rouphael NG, et al. . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Voysey M, Costa Clemens SA, Madhi SA, et al. . Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stephenson KE, Le Gars M, Sadoff J, et al. . Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA. 2021;325:1535-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. ; Gam-COVID-Vac Vaccine Trial Group. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keech C, Albert G, Cho I, et al. . Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xia S, Zhang Y, Wang Y, et al. . Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase ½ trial. Lancet Infect Dis. 2021; 21:39-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Palacios R, González Patiño E, de Oliveira Piorelli R, et al. . Double-blind, randomized, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of treating healthcare professionals with the adsorbed COVID-19 (inactivated) vaccine manufactured by Sinovac—PROFISCOV: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iyer AS, Jones FK, Nodoushani A, et al. . Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020;5:eabe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sahin U, Muik A, Derhovanessian E, et al. . COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses [published correction appears in Nature. 2021;590:E17]. Nature. 2020;586:594-599. [DOI] [PubMed] [Google Scholar]
- 31. Plebani M, Padoan A, Negrini D, et al. . Diagnostic performances and thresholds: the key to harmonization in serological SARS-CoV-2 assays? Clin Chim Acta. 2020;509:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. World Health Organization. Establishment of the WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody. WHO/BS/2020.2403. https://www.who.int/publications/m/item/WHO-BS-2020.2403. Published November 18, 2020. Accessed February 22, 2021.
- 33. West R, Gronvall GK, Kobokovich A. Variants, vaccines and what they mean for COVID-19 testing.https://www.jhsph.edu/covid-19/articles/variants-vaccines-and-what-they-mean-for-covid19-testing.html. Published February 2, 2021. Accessed March 18, 2021.
- 34. Gobbi F, Buonfrate D, Moro L, et al. . Antibody response to the BNT162b2 mRNA COVID-19 vaccine in subjects with prior SARS-CoV-2 infection. Viruses. 2021;13:422. [DOI] [PMC free article] [PubMed] [Google Scholar]