Abstract
Tobacco smokers with co-occurring pain report greater difficulty quitting, face unique cessation challenges, and may benefit from targeted smoking interventions. We developed and tested a brief motivational intervention aimed at increasing knowledge of pain-smoking interrelations, motivation to quit, and cessation treatment engagement among smokers in pain. Non-treatment seeking daily cigarette smokers with chronic pain (N = 76, 57.9% Female, 52.6% White) were randomized to the targeted or AAR (ask, advise, refer) intervention. The targeted intervention included personalized feedback and pain-smoking psychoeducation to help participants develop discrepancy between continued smoking and desired pain outcomes. At post-intervention, the targeted intervention (vs. AAR) increased knowledge of pain-smoking interrelations and several indices of motivation to quit smoking (ps < .01). Participants who received the targeted intervention were also more likely to accept information about, and report intention to engage, evidence-based cessation treatments (ps < .05). Increased knowledge of pain-smoking interrelations mediated post-intervention effects on motivation to quit and willingness to learn about treatments. At one-month follow up, gains in knowledge of pain-smoking interrelations were maintained (p = .009). Participants who received the targeted intervention were more likely to report having subsequently engaged cessation treatment (p = .019), but this was not mediated by increased knowledge of pain-smoking interrelations. Smokers with chronic pain may benefit from targeted interventions that address smoking in the context of pain. Smokers in pain may become increasingly motivated to quit and engage cessation treatment as they become aware of how smoking may exacerbate their pain.
Keywords: pain, smoking, tobacco, motivation, targeted intervention
Tobacco smoking is the leading preventable cause of mortality in the United States, yet nearly 36 million adults (15.5%) continue to smoke (Jamal et al., 2018). Smokers with cooccurring pain are an important subgroup that demonstrates greater smoking prevalence and tobacco-related health disparities. Estimates from nationally-representative and clinical pain samples suggest that the prevalence of smoking among persons in pain may be greater than twice that of the general population (e.g., 24%−68%; Michna et al., 2004; Orhurhu, Pittelkow, & Hooten, 2015). Smokers (vs. non-smokers) are at greater risk for developing chronic pain (DHHS, 2014; Shiri, Karppinen, Leino-Arjas, Solovieva, & Viikari-Juntura, 2010), greater pain intensity and disability (Hooten, Shi, Gazelka, & Warner, 2011; Weingarten et al., 2009), and poorer pain-treatment outcomes (Hooten, Townsend, Bruce, & Warner, 2009).
Pain has been shown to motivate smoking (Ditre & Brandon, 2008; Ditre, Heckman, Butts, & Brandon, 2010), and clinical pain samples have identified distraction from pain and pain-related distress as a primary smoking motive (Aimer et al., 2015; Hooten, Vickers, et al., 2011). Smokers in pain tend to report greater difficulty and less confidence in quitting (Ditre, Langdon, Kosiba, Zale, & Zvolensky, 2015; Zale, Ditre, Dorfman, Heckman, & Brandon, 2014) and may be less likely to achieve long-term smoking abstinence (Aigner et al., 2017). Smokers experience increased pain during nicotine deprivation (Ditre, Zale, LaRowe, Kosiba, & De Vita, 2018; LaRowe, Kosiba, Zale, & Ditre, 2018), and pain reactivity and cognitive-affective responses to pain have been shown to predict smoking relapse (LaRowe, Langdon, Zvolensky, Zale, & Ditre, 2017; Nakajima & al’Absi, 2011). An evolving reciprocal model of pain and smoking posits that bidirectional relations between both conditions ultimately result in greater pain and the maintenance of tobacco dependence (e.g., Ditre, Brandon, Zale, & Meagher, 2011; Ditre, Zale, & LaRowe, 2019; Zale, Maisto, & Ditre, 2016). However, smokers in pain who successfully quit may experience clinically-meaningful reductions in pain (Behrend et al., 2012).
Building from evidence that smokers with psychiatric and medical comorbidities benefit from targeted and motivational smoking interventions (e.g., Heckman, Egleston, & Hofmann, 2010; Steinberg, Ziedonis, Krejci, & Brandon, 2004), promising early work suggests that targeted treatments designed to address unique needs of smokers in pain may help smokers with chronic pain quit. Recent investigations of smoking cessation interventions within a multidisciplinary pain clinic found that an intensive seven session cognitive-behavioral intervention was efficacious in promoting abstinence among smokers motivated to quit (Hooten et al., 2014), and that smokers who received an educational intervention about their pain and smoking were more receptive the clinic-based cessation treatments (Hooten, LaRowe, Zale, Ditre, & Warner, 2019). Further, smokers receiving treatment for chronic pain have stated that information about pain and smoking (i.e., smoking may impede recovery) could be helpful in motivating other patients to quit (Kaye, Prabhakar, Fitzmaurice, & Kaye, 2012). Consistent with a phase-based framework, smokers not yet ready to make a serious quit attempt should receive interventions designed to increase the likelihood of future cessation attempts (Baker et al., 2016), and treatment effects may be most appropriately assessed via self-report and behavioral indices of motivation to quit (Baker et al., 2011). However, to our knowledge, no studies have tested a targeted motivational smoking intervention for non-treatment seeking smokers in pain who are not yet ready to quit.
We developed and pilot tested a brief intervention targeted for non-treatment seeking smokers with chronic pain, which sought to provide education about pain-smoking interrelations and to increase motivation to quit and engage treatment. We hypothesized that, post-intervention, smokers randomized to the targeted intervention (vs. a brief intervention commonly used in medical practices; Schroeder, 2005) would report greater: (a) knowledge of pain-smoking interrelations, (b) motivation to quit smoking, and (c) motivation to engage cessation treatment. We further hypothesized that increased pain-smoking knowledge would mediate intervention effects on motivation to quit and engage treatment. Finally, we hypothesized that, at one-month follow-up, treatment gains would be maintained and that smokers who received the targeted intervention would be more likely report having subsequently engaged cessation treatment.
Methods
Participants
Participants were recruited from the local community via newspaper and internet advertisements for a study about smoking and chronic pain. Respondents were screened by telephone for inclusion criteria: (a) age 18–65, (b) smoke ≥ 10 cigarettes/day; (c) self-reported moderate-very severe chronic pain; (d) average pain intensity ≥ 4/10 over the past three months. Exclusion criteria were: (a) engaged in an active quit attempt; (b) current use of treatment to quit or cut down on smoking. Eligible respondents were scheduled for an in-person session.
Procedure
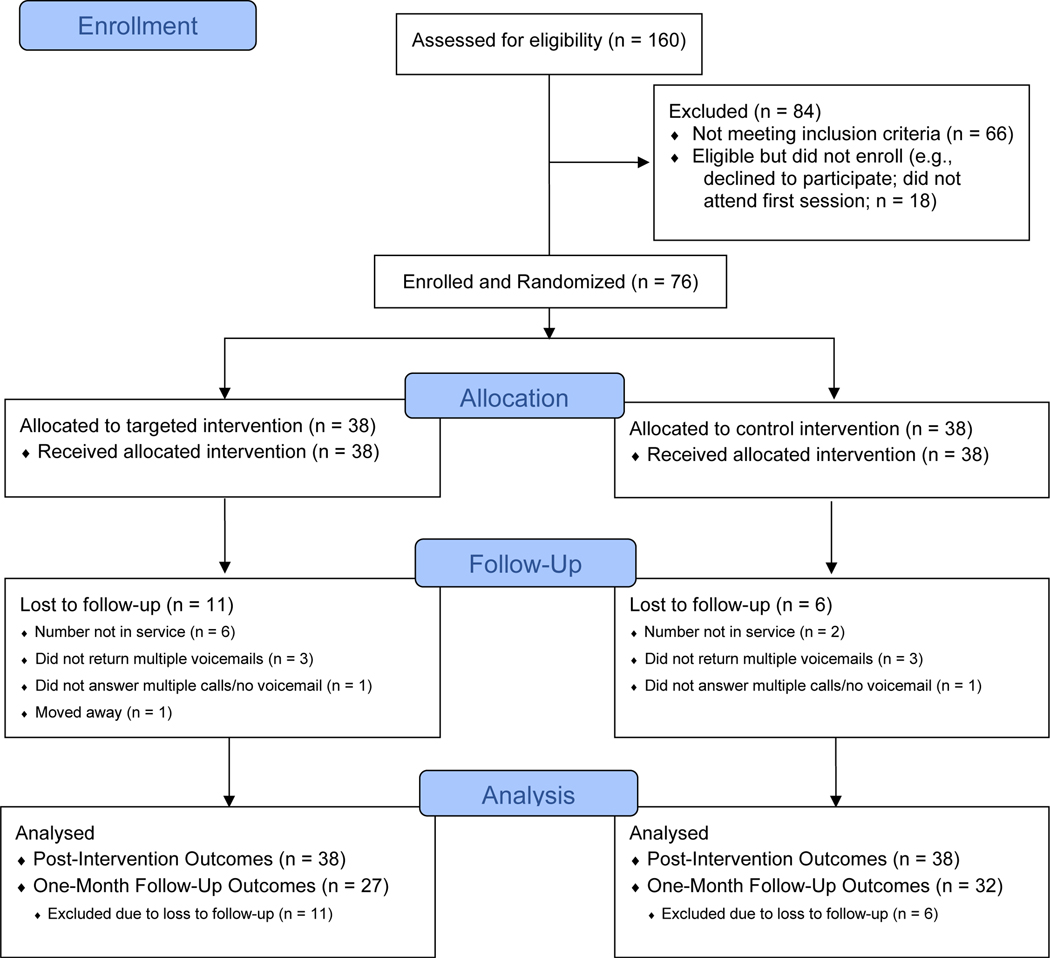
All procedures were approved by a University Institutional Review Board. Upon arrival, participants provided written informed consent and exhaled carbon monoxide to verify smoking status (≥ 8 ppm), and then completed computerized baseline questionnaires alone in a private room. Following baseline, participants were randomized to either the targeted or AAR intervention using a 1:1 allocation ratio in sealed opaque envelopes, and the intervention was delivered face-to-face by a trained study therapist. Participants were then left alone to complete computerized post-intervention outcome measures. At the end of the in-person session, participants were provided with $25 compensation. At one-month follow-up, measures were administered verbally via telephone. Participants were blinded to study condition because they were informed that both conditions would receive an intervention that addressed smoking and health; however, it was not possible to blind study therapists. Figure 1 presents the flow of participants through the study. The protocol is registered with ClinicalTrials.gov (NCT03996902).
Figure 1.
Participant flow chart following CONSORT guidelines.
Intervention Conditions
Targeted intervention.
Development of the targeted intervention was informed by a growing empirical literature on pain and smoking (e.g., Ditre et al., 2011), SAMSHA (2012) recommendations for brief interventions, and current Clinical Practice Guidelines (Fiore et al., 2008). The targeted intervention integrated common components of evidence-based motivational interventions, including the FRAMES acronym (feedback, responsibility for change; advice to quit; menu of strategies; empathy; self-efficacy) and motivational enhancement, which is an adaptation of motivational interviewing that incorporates health-related feedback (e.g., SAMHSA, 2013). As part of the menu of strategies, participants were given referral information (i.e., state Quitline and pharmacotherapy) described in the AAR intervention below. Personalized feedback addressed both pain and smoking.
The targeted intervention included psychoeducation about deleterious effects of smoking on pain, maladaptive consequences of smoking for pain coping, and benefits of smoking cessation for pain. Consistent with the Transtheoretical Model (Prochaska & DiClemente, 1983), which predicts smokers will become more motivated to quit as they perceive discrepancy between positive and negative consequences of smoking, the targeted intervention encouraged participants to develop discrepancy between smoking and their desired pain outcomes. Similarly, the psychoeducation component emphasized core motivators of behavior change according to the Health Belief Model: (a) perceived susceptibility to negative pain outcomes, (b) perceived severity of negative pain outcomes, and (c) perceived benefits of quitting smoking for pain (Champion & Skinner, 2008). The intervention was designed to be delivered in under 30 minutes. The therapist guide and patient handout are available from the corresponding author.
AAR intervention.
Participants randomized to the AAR intervention (ask, advise, refer), were (a) asked about smoking, (b) advised that quitting smoking is important for their health, and (c) referred to cessation treatment. Cessation treatment referral included an information sheet with detailed contact information for our state Quitline and recommendations to consider over-the-counter or prescription medications in consultation with their physician. Participants in this condition also received a copy of the National Cancer Institutes’ Clearing the Air self-help booklet. The AAR model is a streamlined version of the 5As recommended by the US Department of Health and Human Services (Fiore et al., 2008), is widely employed in medical settings (Schroeder, 2005), increases abstinence rates (e.g., Gordon, Andrews, Crews, Payne, & Severson, 2007), and has been adopted as a standard by numerous medical associations (e.g., Bernstein et al., 2006). The AAR intervention was selected to test the targeted intervention relative to current common practice.
Post-Intervention and One-Month Outcome Measures
Motivation to quit smoking.
Cessation motivation is a multidimensional construct that is comprised of cognitions about quitting and measurable steps towards behavior change (Nezami, Sussman, & Pentz, 2003). Motivation to quit was assessed at all timepoints with three self-report measures that target specific and distinct facets of the motivation construct.
Thoughts about Abstinence Scale (TAA; Hall, Havassy, & Wasserman, 1990).
The TAA is a reliable and valid measure that has previously been used among smokers in pain (Ditre, Kosiba, Zale, Zvolensky, & Maisto, 2016). Three separate Numerical Rating Scales (NRS) assess desire to quit smoking (0 = no desire to quit, 10 = full desire to quit), anticipated success in quitting (0 = lowest expectation of success, 10 = highest expectation of success), and anticipated difficulty quitting (0 = lowest amount of difficulty, 10 = highest amount of difficulty).
Contemplation ladder (Biener & Abrams, 1991).
The contemplation ladder is a widely used, reliable, and valid measure of motivation to quit smoking on an 11-point Visual Analogue Scale (VAS), and has previously been used among smokers in pain (Zale et al., 2014). The VAS provides anchors at 0 (no thought of quitting), 2 (I think I need to consider quitting someday), 5 (I think I should quit but not quite ready), 8 (starting to think about how to change my smoking patterns), and 10 (taking action to quit, e.g., cutting down, enrolling in a program).
Motivation rulers (Boudreaux et al., 2012).
Three separate NRSs assessed importance of quitting (0 = not important at all, 10 = most important goal of my life), readiness to quit smoking in the next month (0 = not ready at all, 10 = 100% ready), and confidence that “you will quit smoking” in the next month (0 = not at all confident, 10 = 100% confident).
Knowledge of pain-smoking interrelations.
The Pain and Smoking Questionnaire (PSQ) was developed by members of our research team to assess knowledge of pain-smoking interrelations and was administered at all timepoints. Eight items assess whether (yes, no, not sure) smoking can cause chronic pain, worsen pain over time, contribute to pain-related impairment, reduce effectiveness of prescription pain medications, provide acute analgesic effects, or help to distract from pain, whether pain can motivate smoking, and whether quitting smoking is associated with pain-related benefits. The PSQ was scored as the number of correct answers (range 0 – 8), with higher scores representing greater pain-smoking knowledge. Reliability in the current sample was adequate at baseline (α = .76), post-intervention (α = .82), and one-month follow-up ( α = .77).
Cessation treatment engagement.
Motivation to engage cessation treatment was assessed at baseline and post-intervention. First, willingness to learn about treatment options was assessed with the single item “would you like to learn about options for treatment to help you quit smoking?” Response choices were yes/no. Participants who answered yes were then given the following list of options: (a) medication/primary care, (b) Quitline, (c) behavioral health, (d) none of the above. Two separate questions asked (a) whether they were interested in using any of the listed treatments, and (b) whether they intended to enroll in any of the listed treatments in the next 30 days. Multiple responses were permitted. At one-month follow-up, participants were asked (yes/no) if they had talked to their doctor about smoking, used a medication to help quit, seen a behavioral health provider about smoking, or called a Quitline.
Smoking behavior at one-month follow-up.
Participants were asked “Do you now smoke cigarettes: not at all, some days, every day.” Participants were also asked (yes/no) whether they had cut down on their smoking or made a quit attempt lasting at least 24 hours.
Baseline Measures of Demographics, Smoking, and Pain History
Demographics.
Participants self-reported age, gender, marital status, race/ethnicity, education, and household income.
Smoking history and dependence.
The Smoking History Form (Brown, Lejuez, Kahler, & Strong, 2002) is widely used to assess current and past smoking behavior (e.g., age of onset, prior attempts to quit), and contains the 2-item Heaviness of Smoking Index (HSI; Heatherton, Kozlowski, Frecker, Rickert, & Robinson, 1989). Higher HSI scores (range 0–6) indicate greater levels of smoking dependence (e.g., Borland, Yong, O’Connor, Hyland, & Thompson, 2010).
Chronic pain grade.
The Graded Chronic Pain Scale (GCPS; Von Korff, 2011) is a reliable and valid measure of chronic pain severity in general and clinical pain populations. The GCPS chronic pain grades account for both pain intensity and interference (Grade I = low intensity/low interference to Grade IV = severe interference). The GCPS has previously been used to assess chronic pain among smokers (Ditre et al., 2016; Ditre, Zale, Kosiba, & Zvolensky, 2013), and demonstrated good internal consistency in the current sample (α = .898).
Pain history.
Descriptive information regarding pain history (e.g., duration, source) and treatment (e.g., use of prescription pain medications) were assessed using items adapted from the Kansas Behavioral Risk Factor Surveillance System (Toblin, Mack, Perveen, & Paulozzi, 2011).
Therapist Training and Treatment Fidelity
The study interventions were delivered by the lead author (ELZ) and a clinical psychology doctoral student (MJD). The majority (82.9%) were conducted by the lead author. Chi square revealed no differences (p = .07) in the proportion of targeted and AAR interventions completed by each therapist. Study therapists were trained (e.g., via multiple role plays) on all protocols, supervised by a Licensed Clinical Psychologist (JWD), and completed checklists during every session to ensure adherence and fidelity.
Sample Size Determination
Effects of brief motivational interventions (vs. treatment as usual) on motivation to quit and cessation treatment engagement are medium to large (Cohen’s d = .40 - .89; e.g., Gillaspy et al., 2013; Shahab, West, & McNeill, 2011; Steinberg et al., 2004). A sample size of 76 was consistent with recommendations for pilot clinical trials (Lancaster, Dodd, & Williamson, 2004) and a priori analyses indicated power of .70 and .96 to detect medium and large effects, respectively.
Data Analytic Plan
Group differences at baseline were tested using t-tests and chi-square analyses to verify that randomization was successful (all ps > .082). Post-intervention outcomes were analyzed with an intent-to-treat approach. Separate ANCOVA (for continuous variables) and logistic regression (for dichotomous variables) models (controlling for baseline scores) were used to test effects of intervention condition on knowledge of pain-smoking interrelations and motivation to quit and engage treatment. One-month follow-up outcomes were analyzed for participants who provided data (N = 59) using a modified intent-to-treat approach, in which all participants who provided data were analyzed according to their random assignment regardless of treatment adherence or protocol violations (Gupta, 2011). Repeated measures ANOVA was used for continuous variables and logistic regression was used to for dichotomous outcomes.
We tested post-intervention knowledge of pain-smoking interrelations as a mediator of observed treatment effects at post-intervention and one-month follow-up using the PROCESS Macro for SPSS (Hayes, 2013). The PROCESS Macro employs a bootstrapping approach, can accommodate both dichotomous and continuous variables, and yields estimates of direct and indirect effects of all predictor and mediator variables (Preacher & Hayes, 2008). A variable is considered to serve as a statistical mediator if the 95% confidence interval for the estimated indirect effect does not cross zero. For each model, intervention condition was entered as the independent variable, post-intervention knowledge of pain-smoking interrelations was entered as the mediating variable, and the post-intervention or one-month follow-up outcomes were entered as the respective dependent variables. Models controlled for baseline levels of knowledge of pain-smoking interrelations and respective baseline values of each dependent variable.
Results
Participant Characteristics
Participants were 76 daily tobacco smokers (57.9% female; 42.1% Black), who reported smoking 18 cigarettes per day (SD = 10.71) and were moderately tobacco dependent (MHSI = 3.37, SD = 1.21; e.g., Chaiton, Cohen, McDonald, & Bondy, 2007). The majority (59.2%) of participants endorsed a prior attempt to quit smoking, yet 42% did so without cessation treatment. Most participants reported having chronic pain for at least one year (78.9%). Mean pain ratings (M = 6.76, SD = 2.08) indicate that the sample was experiencing clinically-significant pain (Krebs, Carey, & Weinberger, 2007). See Table 1 for additional characteristics.
Table 1.
Sociodemographic, Smoking, and Pain Characteristics at Baseline
| Intervention Condition | |||
|---|---|---|---|
|
|
|||
| Targeted | AAR | Total | |
| (n = 38) | (n = 38) | (N = 76) | |
|
| |||
| n (%) | n (%) | n (%) | |
| Gender (Female) | 21 (55.3%) | 23 (60.5%) | 44 (57.9%) |
| Race/Ethnicity | |||
| White | 19 (50.0%) | 21 (53.3%) | 40 (52.6%) |
| Black/African American | 15 (46.9%) | 17 (53.1%) | 32 (42.1%) |
| Other | 4 (10.5%) | 0 (0.0%) | 4 (5.3%) |
| Marital status | |||
| Single | 18 (47.4%) | 25 (65.8%) | 43 (56.6%) |
| Married | 4 (10.5%) | 2 (5.2%) | 6 (7.9%) |
| Widowed, Divorced, or Separated | 16 (42.1%) | 11 (28.9%) | 27 (35.5%) |
| Education | |||
| Did not graduate high school | 16 (40.8%) | 15 (39.5%) | 31 (40.8%) |
| Graduated high school | 9 (23.7%) | 10 (26.3%) | 19 (25.0%) |
| Some college | 8 (21.1%) | 8 (21.1%) | 16 (21.1%) |
| Technical/Associates/Bachelor’s degree | 5 (13.2%) | 5 (13.2%) | 10 (13.2%) |
| Household income | |||
| <10,000 | 19 (50.0%) | 21 (55.3%) | 40 (52.6%) |
| 10,000–19,999 | 11 (28.9%) | 9 (23.7%) | 20 (26.3%) |
| 20,000–29,999 | 2 (5.3%) | 4 (10.5%) | 6 (7.9%) |
| >30,000 | 6 (7.8%) | 4 (10.5%) | 10 (13.2%) |
|
| |||
| Previous Attempt to Quit | 21 (59.2%) | 24 (63.2%) | 45 (59.2%) |
| Chronic Pain Grade | |||
| I | 5 (13.2%) | 7 (18.4%) | 12 (15.8%) |
| II | 6 (15.8%) | 6 (15.8%) | 12 (15.8%) |
| III | 8 (21.1%) | 9 (23.7%) | 17 (22.4%) |
| IV | 19 (50.0%) | 16 (42.1%) | 35 (46.1%) |
| Duration of Chronic Pain | |||
| Less than 1 Year | 10 (26.3%) | 6 (15.8%) | 16 (21.1%) |
| 1–5 Years | 13 (34.2%) | 13 (34.2%) | 26 (34.2%) |
| More than 5 Years | 15 (39.5%) | 19 (50.0%) | 34 (44.7%) |
| Frequency of Pain Medication Use | |||
| Monthly or Less | 14 (36.8%) | 12 (31.6%) | 26 (34.2%) |
| Weekly | 11 (28.9%) | 10 (26.3%) | 21 (27.6%) |
| Daily | 12 (32.4%) | 16 (42.1%) | 28 (37.3%) |
| Willing to Learn about Cessation Treatment | 21 (55.3%) | 23 (60.5%) | 44 (57.9%) |
| Interest in Using Cessation Treatment | 21 (55.3%) | 19 (50%) | 40 (52.6%) |
| Intention to Engage Cessation Treatment | 9 (23.7%) | 13 (34.2%) | 22 (28.9%) |
|
| |||
| M (SD) | M (SD) | M (SD) | |
|
| |||
| Age | 42.76 (13.41) | 42.79 (13.61) | 42.78 (13.42) |
| Cigarettes per day | 20.03 (13.24) | 15.26 (6.76) | 17.64 (10.71) |
| Exhaled CO | 14.39 (9.67) | 17.03 (9.91) | 15.69 (9.81) |
| Years daily smoking | 26.16 (13.44) | 25.41 (12.20) | 25.79 (12.76) |
| Past-Year Quit Attempts | 1.71 (4.01) | 1.74 (2.05) | 1.73 (3.16) |
Note. No significant differences were observed between treatment conditions.
Post-Intervention Outcomes
Knowledge of pain-smoking interrelations.
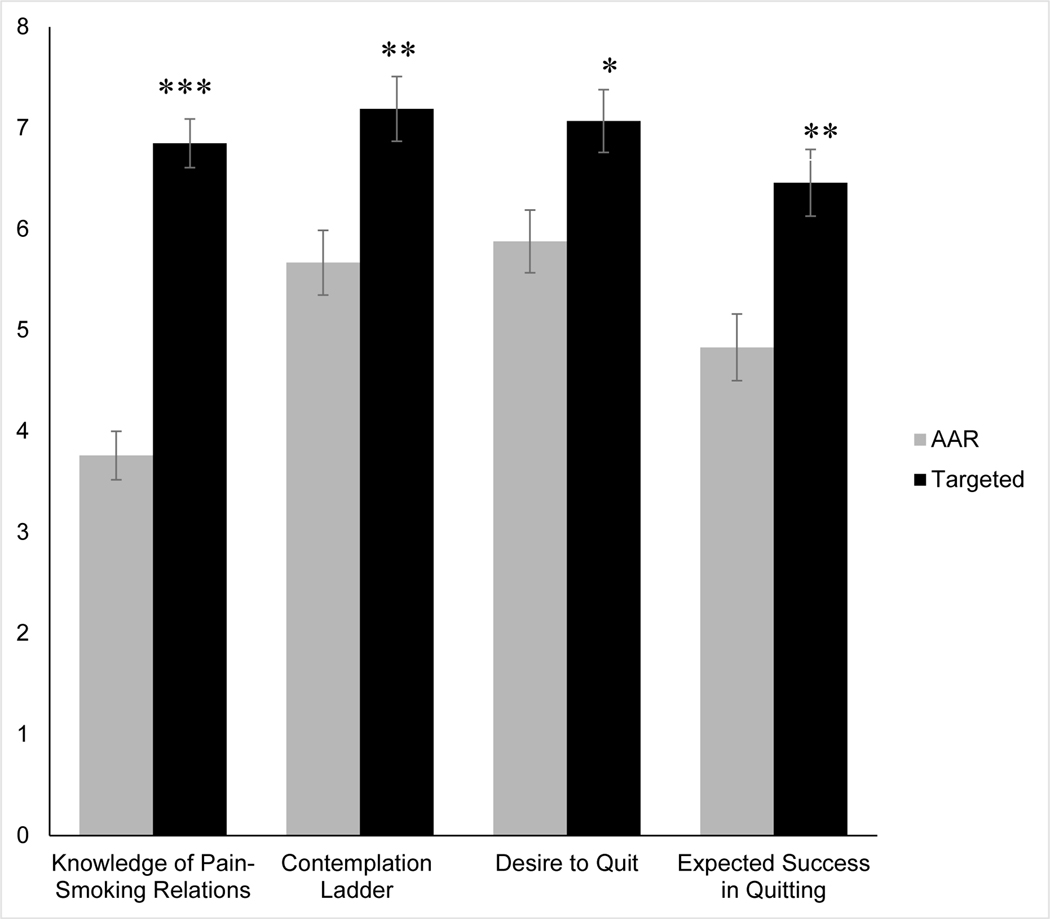
As hypothesized, ANCOVA indicated that the targeted intervention increased knowledge of pain-smoking interrelations (see Figure 2 and Table 2), such that participants randomized to the targeted intervention correctly answered three more questions than participants in the AAR intervention, F(1,73) = 82.37, p < .001, ɳ2p = .53.
Figure 2.
Post-intervention mean (adjusted) knowledge of pain-smoking interrelations and motivation to quit as a function of intervention condition. Error bars represent standard error. * p < .01. ** p = .001. *** p < .001.
Table 2.
Adjusted Means and Standard Errors of Continuous Outcome Variables at Post-Intervention and One-Month Follow-Up
| Intervention Condition | ||||
|---|---|---|---|---|
|
|
||||
| Post-Intervention | One-Month Follow-Up | |||
|
|
||||
| Targeted | AAR | Targeted | AAR | |
| M (SE) | M (SE) | M (SE) | M (SE) | |
|
| ||||
| Knowledge of Pain-Smoking Relations | 6.85 (0.24) *** | 3.76 (0.24) | 6.00 (0.42)** | 4.43 (0.40) |
| Contemplation Ladder | 7.19 (0.32) ** | 5.67 (0.32) | 6.96 (0.54) | 5.97 (0.50) |
| Desire to Quit | 7.07 (0.31) * | 5.88 (0.31) | 6.69 (0.59) | 6.52 (0.54) |
| Expected Success Quitting | 6.46 (0.33) ** | 4.83 (0.33) | 6.31 (0.59) | 5.16 (0.54) |
| Anticipated Difficulty Quitting | 6.36 (0.44) | 6.59 (0.44) | 7.00 (0.51) | 7.55 (0.46) |
| Readiness to Quit | 6.15 (0.45) | 5.19 (0.45) | 5.50 (0.79) | 5.40 (0.74) |
| Importance of Quitting | 7.35 (0.29) | 6.86 (0.29) | 8.73 (0.44)* | 7.50 (0.41) |
| Confidence in Quitting | 4.70 (0.44) † | 3.51 (0.44) | 5.19 (0.77) | 4.03 (0.72) |
Note. Post-Intervention N = 76. One-Month Follow-Up N = 59. Means and standard errors adjusted for baseline levels of each respective variable.
p < .01.
p = .001.
p < .001.
p = .059.
Motivation to quit smoking.
As presented in Table 2, participants randomized to the targeted intervention (vs. AAR) scored higher on the contemplation ladder, F(1,73) = 11.54, p = .001, ɳ2p = .14, and reported greater desire to quit smoking, F(1,73) = 7.40, p = .008, ɳ2p = .09, and expected success in quitting, F(1,73) = 12.95, p = .001, ɳ2p = .15. A trend-level association was observed for greater confidence in quitting among participants who received the targeted intervention F(1,73) = 3.68, p = .059, ɳ2p = .05. No group differences were observed with regard to importance (p = .237), readiness (p = .138), or anticipated difficulty quitting (p = .703). Given that motivation to quit was assessed using multiple individual scales, we also applied a Bonferroni correction to control for the risk of type one error. When the significance level is set at .007 (i.e., α = .05 divided by seven scales), effects of the targeted intervention on contemplation latter and expected success in quitting remained statistically significant.
Motivation to engage cessation treatment.
As hypothesized, results of logistic regression revealed that the targeted intervention increased willingness to learn about cessation treatments (OR = 7.74, 95% CI [1.49, 40.30], Wald χ2 = 5.91, p = .015). Participants randomized to the targeted intervention were also more likely to indicate that they would be interested in engaging cessation treatment in the future (OR = 9.55, 95% CI [1.94, 47.02], Wald χ2 = 7.70, p = .006), and that they intended to engage treatment in the next 30 days (OR = 5.15, [95% CI [1.77, 14.96], Wald χ2 = 9.06, p = .003). Follow-up analyses revealed that the targeted intervention increased interest and intention to utilize the Quitline and medication/primary care (see Table 3).
Table 3.
Motivation to Engage, and Self-Reported Engagement in, Smoking Cessation Treatments
| Intervention Condition | ||||
|---|---|---|---|---|
|
|
||||
| Targeted | AAR | |||
| n (%) | n (%) | OR [95% CI] | p | |
|
| ||||
| Post-Intervention (N = 76) | n = 38 | n = 38 | ||
|
| ||||
| Willing to learn about treatment | 36 (94.7%) | 28 (77.8%) | 7.74 [1.49, 40.30] | .015 |
| Interested in using treatment | 36 (94.7%) | 25 (65.8%) | 9.55 [1.94, 47.02] | .006 |
| Primary care/medication | 32 (84.2%) | 21 (55.3%) | 4.27 [1.37, 13.28] | .012 |
| Quitline | 29 (76.3%) | 7 (18.4%) | 18.81 [5.35, 66.07] | <.001 |
| Behavioral Health | 9 (23.7%) | 1 (2.6%) | 11.78 [1.40, 99.88] | .023 |
| Intention to engage treatment | 30 (78.9%) | 18 (47.4%) | 5.15 [1.77, 14.96] | .003 |
| Primary care/medication | 25 (65.8%) | 14 (36.8%) | 4.04 [1.47, 11.09] | .007 |
| Quitline | 22 (57.9%) | 6 (15.8%) | 9.80 [2.91, 33.02] | <.000 |
| Behavioral Health | 3 (7.9%) | 1 (2.6%) | 2.91 [0.29, 29.43] | .365 |
|
| ||||
| One-Month Follow-Up (N = 59) | n = 27 | n = 32 | ||
|
| ||||
| Cut down on smoking | 18 (66.7%) | 20 (62.5%) | 1.20 [0.41, 3.51] | .739 |
| Quit Attempt > 24 hours | 13 (48.1%) | 10 (31.3%) | 2.04 [0.71, 5.91] | .188 |
| Engaged cessation treatment | 12 (44.4%) | 5 (15.6%) | 4.32 [1.28, 14.62] | .019 |
| Talked to doctor about smoking | 10 (37.0%) | 4 (12.5%) | 4.12 [1.12, 15.21] | .034 |
| Used a medication to quit | 3 (11.1%) | 0 (0.0%) | 2.02e8 [0.00, --] | .998 |
| Saw behavioral health provider | 3 (11.1%) | 2 (6.3%) | 1.86 [0.29, 12.14] | .510 |
| Called a Quitline | 2 (7.4%) | 1 (3.1%) | 2.48 [0.21, 28.96] | .469 |
Note. Post-intervention Odds Ratio (OR) adjusted for baseline levels of each respective variable.
Knowledge of pain-smoking interrelations as a mediator of post-intervention outcomes.
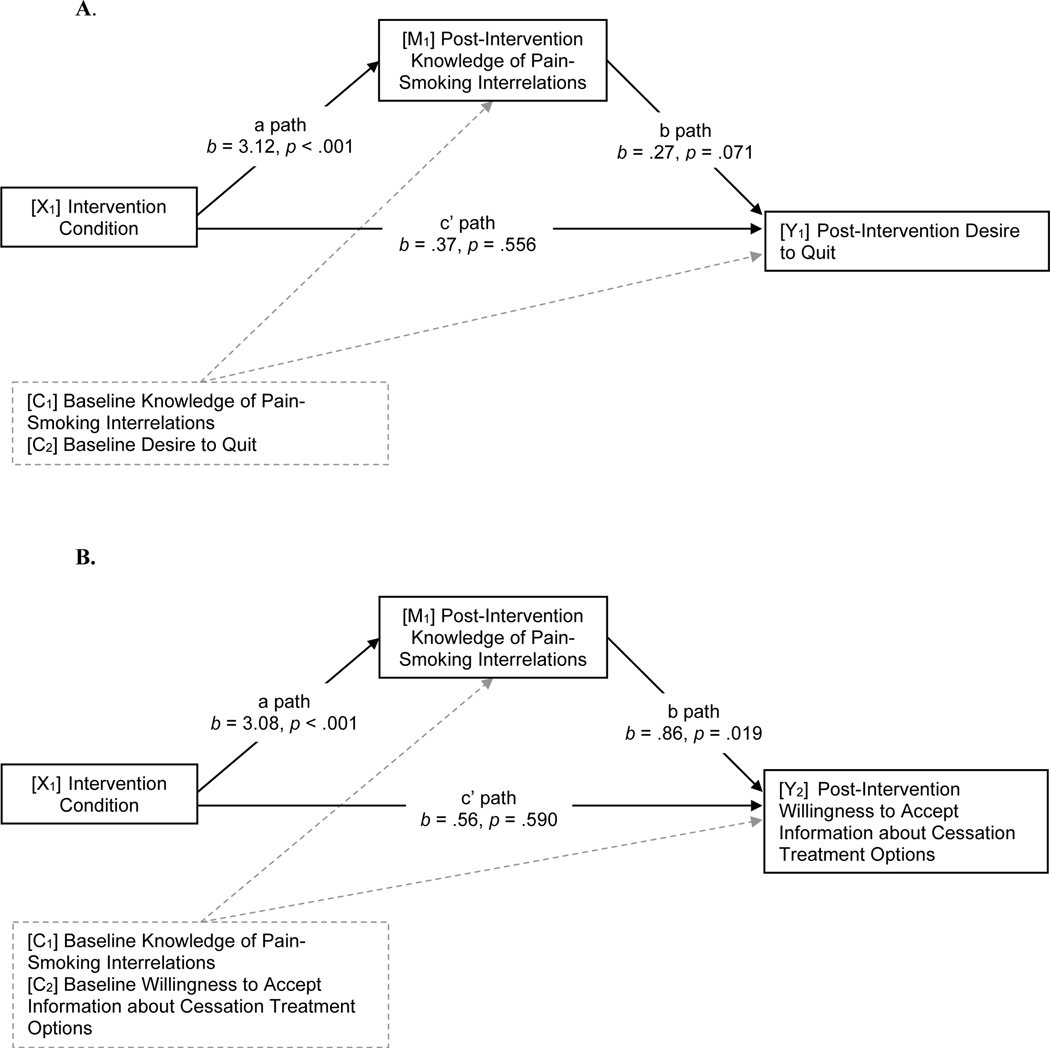
As depicted in Figure 3, we observed an indirect effect of the targeted intervention on greater desire to quit (b = .84, SE = .45, 95% CI [0.03, 1.81]) and willingness to learn about cessation treatments (b = 2.66, SE = 1.46, 95% CI [0.13, 4.90]) via increased knowledge of pain-smoking interrelations. Increased knowledge did not mediate effects on contemplation ladder (b = .31, SE = .60, 95% CI [−0.92, 1.44], expected success in quitting (b = .15, SE = .52, 95% CI [−0.97, 1.12]), or intention to engage treatment (b = .86, SE = .72, 95% CI [−0.52, 2.36]).
Figure 3.
Models of indirect associations between the targeted intervention and greater post-intervention desire to quit (A) and willingness to accept information about cessation treatment (B) via increased knowledge of pain-smoking interrelations. X = independent variable. M = mediating variable. Y = Dependent Variable. C = Covariate.
One-Month Follow-Up Outcomes
Participant characteristics.
A total of 59 (78%) participants provided data at one-month follow-up. There was no association between intervention condition assignment and loss to follow-up (χ2 = 1.89, p = .169). The primary reasons for loss were disconnected telephone number (58.8%) and did not return multiple voicemail messages (29.4%). Participants who provided follow-up data were older (M = 44.64, SD = 12.98) and smoked fewer cigarettes per day at baseline (M = 15.98, SD = 9.17), relative to non-respondents (M = 36.29, SD = 13.27), F(1,74) = 5.41, p = .023, ɳ2p = .07; (M = 23.41, SD = 12.70), F(1,74) = 6.94, p = .011, .09, respectively.
Smoking behavior and engagement of smoking cessation treatment.
As shown in Table 3, participants who received the targeted intervention (vs. AAR) were over four times more likely to report having subsequently engaged cessation treatment (95% CI [1.28, 14.62], Wald χ2 = 5.53, p = .019). This result appeared to be driven primarily by the finding that participants in the targeted intervention were more likely to report having talked to their doctor about smoking (OR = 4.12, Wald χ2 = 4.51, p =.034). However, increased knowledge of pain-smoking interrelations at post-intervention did not mediate intervention effects on engagement of any cessation treatment (b = .57, SE .86, 95% CI[−1.09, 2.32]) or talking to a doctor about smoking (b = .21, SE = 1.20, 95% CI[−1.98, 2.15]). Although four participants who received the targeted intervention reported that they were not smoking cigarettes at the time of the one-month follow-up (vs. zero in the AAR), this difference was not statistically significant (p = .691). Participants in both conditions reported smoking a similar number of cigarettes per day (p = .387), and no differences were observed in the number of participants who reported cutting down on smoking (p = .739) or making a quit attempt greater than 24 hours (p = .188).
Maintenance of treatment gains.
As presented in Table 2, participants who received the targeted intervention continued to report greater knowledge of pain-smoking interrelations at one-month follow-up, relative to participants who received the AAR intervention, F(1, 49) = 7.46, p = .009, ɳ2p = .13. Although not significantly different at post-intervention, at one-month follow-up, participants in the targeted intervention (vs. AAR) reported greater perceived importance of quitting, F (1,49) = 4.18, p = .046, ɳ2p = .07. No group differences were observed in any other indices of cessation motivation at one-month follow-up (ps > .16).
Discussion
This is the first pilot test of a brief motivational smoking intervention targeted for nontreatment seeking smokers with chronic pain. Informed by evidence-based interventions and theoretical conceptualizations of health behavior change, the targeted intervention included a novel psychoeducation component that was designed to increase knowledge of pain-smoking interrelations and assist smokers in developing discrepancy between continued smoking and desired pain outcomes. The targeted intervention (vs. AAR) increased knowledge of pain-smoking interrelations, desire to quit smoking, contemplation ladder scores, expected success in quitting, willingness to learn about smoking cessation treatment, and intention to engage cessation treatment. At one-month follow up, treatment gains in knowledge of pain-smoking interrelations were maintained, and participants who received the targeted intervention were more likely to report having subsequently engaged smoking cessation treatment. These findings are consistent with evidence that providing smokers with clear links between smoking and health can increase motivation to quit (McCaul et al., 2006), and that smokers who are not ready to quit may be amenable to interventions designed to increase motivation to quit and engage abstinence-oriented treatment (Drake & Mueser, 2000).
The targeted intervention included psychoeducation about the deleterious effects of smoking on pain. At baseline, participants answered an average of 3/8 questions about pain-smoking interrelations correctly, and the majority were unaware that smoking can cause chronic pain (59.2%), contribute to greater pain intensity (56.5%), or reduce the effectiveness of prescription pain medications (75%). Participants who received the targeted intervention demonstrated significant increases in knowledge of pain-smoking interrelations, correctly answering 6/8 questions at post-intervention and one-month follow-up. It is notable that 40% of participants reported that they did not hold a high school diploma or GED, and lower educational attainment is associated with lower levels of health literacy (i.e., the ability to understand, and use health information; DHHS, 2008). These results suggest that smokers with chronic pain are able to learn and retain new information about complex pain-smoking interrelations and that lower educational attainment is not a barrier to pain-smoking psychoeducation. Additionally, increased knowledge at post-intervention was a statistical mediator of self-reported desire to quit and willingness to learn about cessation treatments, but did not mediate behavioral outcomes (i.e., engagement of cessation treatment) at one-month follow-up. These findings suggest that psychoeducation may be particularly relevant to self-reported motivation in the short-term and that additional mechanisms of behavior change should be considered to better understand how and why smokers with pain engage evidence-based cessation treatments.
At one-month follow-up, 44.4% of participants who received the targeted intervention reported engaging at least one smoking cessation treatment (vs. 15.6% in the AAR condition), and the most commonly endorsed treatment was having talked to your doctor about smoking (37% vs. 12.5%, respectively). This finding could reflect greater initiative by participants to engage with their healthcare provider or greater receptivity towards conversations initiated by a healthcare provider. It is also not possible for us to know the content of these discussions with healthcare providers (e.g., whether providers only asked about smoking status or offered evidence-based cessation-oriented interventions). In either case, these results are consistent with prior evidence that smokers with chronic pain may be particularly amenable to interventions that promote interactions with the healthcare (Zale & Ditre, 2013).
It is notable that the targeted intervention did not increase self-reported readiness to quit or the proportion of participants who reported cutting down on smoking or having made a 24hour quit attempt at one-month follow-up. Across both conditions, more than half of participants reported cutting down on their smoking and 39% (48% targeted condition; 31% AAR condition) reported at least one 24-hour quit attempt. This is consistent with nationally-representative data, which suggests that more than half of smokers will make at least one quit attempt every year (Babb, Malarcher, Schauer, Asman, & Jamal, 2017), and that in any given month more than 40% of smokers are engaged in some level of quit activity (Borland, Partos, Yong, Cummings, & Hyland, 2012). It is also consistent with evidence that more than one third of smokers report having begun their quit attempt on the same day they decided to stop (Cooper et al., 2010), and highlights the necessity of making evidence-based interventions readily available to all smokers. Evidence-based abstinence interventions typically assist smokers in preparing to quit by setting a quit date, removing smoking-related triggers, and developing strategies to cope with cravings and withdrawal (Perkins, Conklin, & Levine, 2008). Neither study intervention included a component specifically designed to support smoking abstinence. Thus, additional abstinence-specific treatment components may be needed to increase readiness and provide support for a serious quit attempt.
Clinical implications of this study include the possibility that health care providers may view pain treatment as a context in which smoking cessation could become more salient, and may use discussions about pain as a way to broach the topic of smoking cessation with their patients. Consistent with a phase-based framework (Baker et al., 2011), greater motivation towards smoking cessation should be considered a successful treatment outcome. When possible, providers should capitalize on greater motivation to quit and engage cessation treatment in real-time by immediately linking patients to additional services. For example, healthcare providers can recommend cessation medications, provide brief evidence-based behavioral interventions (e.g., strategies for coping with cravings; Fiore et al., 2008), or proactively connect patients to available behavioral support (e.g., Quitline services). Providers should also be aware of the potential interactions between chronic pain and psychiatric comorbidities. For example, anxiety and depression can have detrimental effects on smoking cessation outcomes and may play a unique role in cessation processes, such as eroding motivation to quit, among smokers with chronic pain (Ditre et al., 2019; Zale et al., 2016). Thus, clinicians addressing smoking cessation among smokers with chronic pain may also need to address psychopathology (e.g., via cognitive-behavioral approaches) or select cessation treatments shown to benefit smokers with psychiatric co-occurring psychiatric conditions (e.g., combination pharmacotherapy; Zale et al., 2016).
Strengths of the study include assessment of multiple self-report and behavioral indices of motivation to quit smoking, use of empirical and theoretical conceptualizations of health behavior change to inform treatment development, and recruitment of a non-treatment seeking sample. Several limitations should also be acknowledged. First, given our community-based recruitment strategy, we were unable to verify chronic pain via medical record review. We were also not able to verify whether participants in both conditions had equal access medical services (e.g., primary care) and smoking cessation treatments, which may vary in cost or accessibility (e.g., cost of prescription medications, availability of behavioral health providers). Second, study therapists conducted the in-person visits, which may have produced demand effects or desirability bias in participant responses. We sought to limit these potential effects by leaving participants alone to complete computerized assessments at pre- and post-intervention and assuring participants that responses were confidential. Third, the one-month follow-up period may not have allowed sufficient time for participants to engage cessation treatment, and it is not known whether participants in both conditions had equal opportunities to engage treatment during the follow-up period. Fourth, the AAR comparison condition allows for conclusions about how the targeted intervention performed relative to a smoking intervention that participants are likely to receive in the healthcare setting. However, it is not known whether the targeted intervention would increase motivation to quit and engage treatment above-and-beyond other motivational interventions that were not adapted to address smoking in the context of pain. Given that the AAR was selected because of its utility in medical settings, it was shorter than the targeted intervention, and therefore does not serve as an attention control. Although the 30-minute duration is consistent with typical appointments in integrated behavioral health (Funderburk et al., 2010), it is possible that the targeted intervention may require adaptation (e.g., to visit length/frequency) to achieve optimal feasibility in medical settings. Fifth, the current study did not account for psychiatric comorbidities, and future research should investigate associations between psychopathology, pain, smoking, and treatment response. Finally, our assessment of pain-smoking knowledge was developed for the current study. This measure could require updates or modifications scientific understanding of pain-smoking interrelations continues to develop and should undergo additional psychometric study to determine reliability and validity across multiple smoking populations.
Taken together, results of the current study indicate that smokers with chronic pain may become more motivated to quit smoking and engage cessation treatment as they become more aware of how continued smoking may contribute to deleterious pain outcomes. These findings contribute to an emerging literature on complex pain-smoking interrelations, and have the potential to inform the treatment of smokers with chronic pain, including the ongoing development of novel interventions for this important subpopulation of smokers. Results also have the potential to inform future research. First, a fully powered clinical trial is needed to test the efficacy of the targeted intervention. Second, future clinical trials should utilize a comparison condition that targets motivational processes (e.g., perceived discrepancy) to test the relative effects of pain-specific content above-and-beyond effects of the motivational component. Third, there is some evidence that shorter visits at greater frequency contribute to improved cessation outcomes (Fiore et al., 2008), and the optimal duration and frequency of the targeted intervention should be tested. Future research should consider whether participants vary in their access to healthcare services and should seek to limit barriers (e.g., cost, time) were possible. Additional research is also needed to determine the optimal setting to promote and distribute pharmacotherapy to smokers in pain (e.g., primary vs. specialty pain care, point of sale in pharmacies). Finally, researchers should examine the utility of technology-based intervention delivery (e.g., smart phone) to engage non-treatment seeking smokers.
Public Significance Statements:
Tobacco smokers with chronic pain face unique barriers to smoking cessation and may benefit from targeted interventions that address smoking in the context of pain. We developed a targeted motivational intervention that included education and personalized feedback about how pain and smoking are related. Among smokers with chronic pain who were not planning to quit, the targeted intervention increased motivation to quit and the likelihood of engaging smoking treatment.
Acknowledgments
This research was supported by Grant No. F31DA039628 awarded to Emily L. Zale by the National Institute on Drug Abuse. Primary outcomes (i.e., knowledge of pain-smoking interrelations, motivation to quit, engagement of cessation treatment) were presented as in a symposium titled “Pain, nicotine, and tobacco Smoking: Translational research to identify mechanisms and inform the development of novel interventions” at the 25th annual meeting of the Society for Research on Nicotine and Tobacco in San Francisco, CA (2019).
Footnotes
Trial Registration: ClinicalTrials.gov identifier: NCT03996902
References
- Aigner CJ, Gritz ER, Tamí-Maury I, Baum GP, Arduino RC, & Vidrine DJ (2017). The role of pain in quitting among HIV positive smokers enrolled in a smoking cessation trial. Substance Abuse, 249–252. doi: 10.1080/08897077.2017.1291466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimer P, Stamp L, Stebbings S, Valentino N, Cameron V, & Treharne GJ (2015). Identifying Barriers to Smoking Cessation in Rheumatoid Arthritis. Arthritis Care & Research, 67, 607–615. doi: 10.1002/acr.22503 [DOI] [PubMed] [Google Scholar]
- Babb SD, Malarcher AM, Schauer GL, Asman K, & Jamal A. (2017). Quitting smoking among adults -- United States, 2000–2015. MMWR: Morbidity and Mortality Weekly Report, 65, 1457–1464. doi: 10.15585/mmwr.mm6552a1 [DOI] [PubMed] [Google Scholar]
- Baker TB, Collins LM, Mermelstein R, Piper ME, Schlam TR, Cook JW, . . . Fiore MC (2016). Enhancing the effectiveness of smoking treatment research: conceptual bases and progress. Addiction, 111, 107–116. doi: 10.1111/add.13154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Mermelstein R, Collins LM, Piper ME, Jorenby DE, Smith SS, . . . Fiore MC (2011). New Methods for Tobacco Dependence Treatment Research. Annals of Behavioral Medicine, 41, 192–207. doi: 10.1007/s12160-010-9252-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrend C, Prasarn M, Coyne E, Horodyski M, Wright J, & Rechtine GR (2012). Smoking Cessation Related to Improved Patient-Reported Pain Scores Following Spinal Care. Journal of Bone and Joint Surgery (American Volume), 94, 2161–2166. doi: 10.2106/JBJS.K.01598 [DOI] [PubMed] [Google Scholar]
- Bernstein SL, Boudreaux ED, Cydulka RK, Rhodes KV, Lettman NA, Almeida SL, . . . American College of Emergency Physicians Task Force on Smoking, C. (2006). Tobacco control interventions in the emergency department: a joint statement of emergency medicine organizations. J Emerg Nurs, 32, 370–381. doi: 10.1016/j.jen.2006.06.006 [DOI] [PubMed] [Google Scholar]
- Biener L, & Abrams DB (1991). The Contemplation Ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychol, 10, 360–365. doi: 10.1037//0278-6133.10.5.360 [DOI] [PubMed] [Google Scholar]
- Borland R, Partos TR, Yong H-H, Cummings KM, & Hyland A. (2012). How much unsuccessful quitting activity is going on among adult smokers? Data from the International Tobacco Control Four Country cohort survey. Addiction, 107, 673–682. doi: 10.1111/j.1360-0443.2011.03685.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland R, Yong HH, O’Connor RJ, Hyland A, & Thompson ME (2010). The reliability and predictive validity of the Heaviness of Smoking Index and its two components: Findings from the International Tobacco Control Four Country study. Nicotine & Tobacco Research, 12 Suppl, S45–50. doi: 10.1093/ntr/ntq038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreaux ED, Sullivan A, Abar B, Bernstein SL, Ginde AA, & Camargo CA Jr. (2012). Motivation rulers for smoking cessation: a prospective observational examination of construct and predictive validity. Addict Sci Clin Pract, 7, 8. doi: 10.1186/1940-0640-7-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, & Strong DR (2002). Distress tolerance and duration of past smoking cessation attempts. Journal of Abnormal Psychology, 111, 180–185. doi: 10.1037//0021-843X.111.1.180 [DOI] [PubMed] [Google Scholar]
- Chaiton MO, Cohen JE, McDonald PW, & Bondy SJ (2007). The Heaviness of Smoking Index as a predictor of smoking cessation in Canada. Addictive Behaviors, 32, 1031–1042. doi: 10.1016/j.addbeh.2006.07.008 [DOI] [PubMed] [Google Scholar]
- Champion VL, & Skinner CS (2008). The Health Belief Model. In Glanz K, Rimer BK, & Viswanath K. (Eds.), Health Behavior and Health Education: Theory, Research, and Practice (4th ed., pp. 45–65). San Fransisco: CA: Jossey-Bass, a Wiley Imprint. [Google Scholar]
- Cooper J, Borland R, Yong HH, McNeill A, Murray RL, O’Connor RJ, & Cummings KM (2010). To what extent do smokers make spontaneous quit attempts and what are the implications for smoking cessation maintenance? Findings from the International Tobacco Control Four country survey. Nicotine & Tobacco Research, 12 Suppl, S51–57. doi: 10.1093/ntr/ntq052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHHS. (2008). Quick guide to health literacy. Washington, DC: U.S. Department of Health And Human Servies, Office of Disease Prevention and Health Promotion [Google Scholar]
- DHHS. (2014). The Health Consequences of Smoking - 50 Years of Progress: A Report of the Surgeon General. Atlanta: GA: U.S. Department of Health and Human Services, Center for Disease COntrol and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health [Google Scholar]
- Ditre JW, & Brandon TH (2008). Pain as a motivator of smoking: Effects of pain induction on smoking urge and behavior. Journal of Abnormal Psychology, 117, 467–472. doi: 10.1037/0021-843X.117.2.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Brandon TH, Zale EL, & Meagher MM (2011). Pain, nicotine, and smoking: Research findings and mechanistic considerations. Psychological Bulletin, 137, 1065–1093. doi: 10.1037/a0025544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Heckman BW, Butts EA, & Brandon TH (2010). Effects of expectancies and coping on pain-induced motivation to smoke. Journal of Abnormal Psychology, 119, 524–533. doi: 10.1037/a0019568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Kosiba JD, Zale EL, Zvolensky MJ, & Maisto SA (2016). Chronic pain status, nicotine withdrawal, and expectancies for smoking cessation among lighter smokers. Annals of Behavioral Medicine, 50, 427–435. doi: 10.1007/s12160-016-9769-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Langdon KJ, Kosiba JD, Zale EL, & Zvolensky MJ (2015). Relations between pain-related anxiety, tobacco dependence, and barriers to quitting among a community-based sample of daily smokers. Addictive Behaviors, 42, 130–135. doi: 10.1016/j.addbeh.2014.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Zale EL, Kosiba JD, & Zvolensky MJ (2013). A pilot study of pain-related anxiety and smoking-dependence motives among persons with chronic pain. Experimental and Clinical Psychopharmacology, 21, 443–449. doi: 10.1037/a0034174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Zale EL, & LaRowe LR (2019). A Reciprocal Model of Pain and Substance Use: Transdiagnostic Considerations, Clinical Implications, and Future Directions. Annu Rev Clin Psychol, 15. doi: 10.1146/annurev-clinpsy-050718-095440 [DOI] [PubMed] [Google Scholar]
- Ditre JW, Zale EL, LaRowe LR, Kosiba JD, & De Vita MJ (2018). Nicotine deprivation increases pain intensity, neurogenic inflammation, and mechanical hyperalgesia among daily tobacco smokers. Journal of Abnormal Psychology, 127, 578–589. doi: 10.1037/abn0000353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake RE, & Mueser KT (2000). Psychosocial approaches to dual diagnosis. Schizophrenia bulletin, 26, 105–118. doi: 10.1093/oxfordjournals.schbul.a033429 [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, & Dorfman SF (2008). Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service. [Google Scholar]
- Funderburk JS, Sugarman DE, Maisto SA, Ouimette P, Schohn M, Lantinga L, . . . Strutynski K. (2010). The description and evaluation of the implementation of an integrated healthcare model. Families, Systems, & Health, 28, 146–160. doi: 10.1037/a0020223 [DOI] [PubMed] [Google Scholar]
- Gillaspy SR, Leffingwell T, Mignogna M, Mignogna J, Bright B, & Fedele D. (2013). Testing of a web-based program to facilitate parental smoking cessation readiness in primary care. Journal of Primary Care & Community Health, 4, 2–7. doi: 10.1177/2150131912442898 [DOI] [PubMed] [Google Scholar]
- Gordon JS, Andrews JA, Crews KM, Payne TJ, & Severson HH (2007). The 5A’s vs 3A’s plus proactive quitline referral in private practice dental offices: preliminary results. Tob Control, 16, 285–288. doi: 10.1136/tc.2007.020271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK (2011). Intention-to-treat concept: A review. Perspect Clin Res, 2, 109–112. doi: 10.4103/2229-3485.83221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Havassy BE, & Wasserman DA (1990). Commitment to abstinence and acute stress in relapse to alcohol, opiates, and nicotine. J Consult Clin Psychol, 58, 175–181. doi: 10.1037/0022-006X.58.2.175 [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to Medation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY: The Guilford Press. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, & Robinson J. (1989). Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. British journal of addiction, 84, 791–799. doi: 10.1111/j.1360-0443.1989.tb03059.x [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Egleston BL, & Hofmann MT (2010). Efficacy of motivational interviewing for smoking cessation: a systematic review and meta-analysis. Tob Control, 19, 410–416. doi: 10.1136/tc.2009.033175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooten WM, LaRowe LR, Zale EL, Ditre JW, & Warner DO (2019). Effects of a brief pain and smoking cessation intervention in adults with chronic pain: A randomized controlled trial. Addictive Behaviors, 92, 173–179. doi: 10.1016/j.addbeh.2018.11.040 [DOI] [PubMed] [Google Scholar]
- Hooten WM, Shi Y, Gazelka HM, & Warner DO (2011). The effects of depression and smoking on pain severity and opioid use in patients with chronic pain. Pain, 152, 223–229. doi: 10.1016/j.pain.2010.10.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooten WM, Townsend CO, Bruce BK, & Warner DO (2009). The effects of smoking status on opioid tapering among patients with chronic pain. Anesth Analg, 108, 308–315. doi: 10.1213/ane.0b013e31818c7b99 [DOI] [PubMed] [Google Scholar]
- Hooten WM, Townsend CO, Hays JT, Ebnet KL, Gauvin TR, Gehin JM, . . . Warner DO (2014). A cognitive behavioral smoking abstinence intervention for adults with chronic pain: A randomized controlled pilot trial. Addictive Behaviors, 39, 593–599. doi: 10.1016/j.addbeh.2013.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooten WM, Vickers KS, Shi Y, Ebnet KL, Townsend CO, Patten CA, & Warner DO (2011). Smoking cessation and chronic pain: patient and pain medicine physician attitudes. Pain Practice, 11, 552–563. doi: 10.1111/j.1533-2500.2011.00462.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal A, Phillips E, S GA, Homa DM, Babb SD, King BA, & Neff LJ (2018). Current Cigarette Smoking Among Adults — United States, 2016. MMWR Morbidity and Mortality Weekly Report, 67, 53–59. doi: 10.15585/mmwr.mm6702a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye AD, Prabhakar AP, Fitzmaurice ME, & Kaye RJ (2012). Smoking cessation in pain patients. Ochsner J, 12, 17–20. [PMC free article] [PubMed] [Google Scholar]
- Krebs EE, Carey TS, & Weinberger M. (2007). Accuracy of the pain numeric rating scale as a screening test in primary care. Journal of general internal medicine, 22, 1453–1458. doi: 10.1007/s11606-007-0321-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster GA, Dodd S, & Williamson PR (2004). Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract, 10, 307–312. doi: 10.1111/j.2002.384.doc.x [DOI] [PubMed] [Google Scholar]
- LaRowe LR, Kosiba JD, Zale EL, & Ditre JW (2018). Effects of nicotine deprivation on current pain intensity among daily cigarette smokers. Experimental and Clinical Psychopharmacology. doi: 10.1037/pha0000218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe LR, Langdon KJ, Zvolensky MJ, Zale EL, & Ditre JW (2017). Pain-related anxiety as a predictor of early lapse and relapse to cigarette smoking. Experimental and Clinical Psychopharmacology, 25, 255–264. doi: 10.1037/pha0000127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaul KD, Hockemeyer JR, Johnson RJ, Zetocha K, Quinlan K, & Glasgow RE (2006). Motivation to quit using cigarettes: A review. Addictive Behaviors, 31, 42–56. doi: 10.1016/j.addbeh.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Michna E, Ross EL, Hynes WL, Nedeljkovic SS, Soumekh S, Janfaza D, . . . Jamison RN (2004). Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. Journal of Pain and Symptom Management, 28, 250–258. doi: 10.1016/j.jpainsymman.2004.04.007 [DOI] [PubMed] [Google Scholar]
- Nakajima M, & al’Absi M. (2011). Enhanced pain perception prior to smoking cessation is associated with early relapse. Biological Psychology, 88, 141–146. doi: 10.1016/j.biopsycho.2011.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezami E, Sussman S, & Pentz MA (2003). Motivation in Tobacco Use Cessation Research. Substance Use & Misuse, 38, 25–50. doi: 10.1081/JA-120016564 [DOI] [PubMed] [Google Scholar]
- Orhurhu VJ, Pittelkow TP, & Hooten WM (2015). Prevalence of smoking in adults with chronic pain. Tobacco induced diseases, 13, 17. doi: 10.1186/s12971-015-0042-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Conklin CA, & Levine MD (2008). Cognitive-Behavioral Therapy for Smoking Cessation: A Practical Guidebook to the Most Effective Treatments. New York, NY: Routledge. [Google Scholar]
- Prochaska JO, & DiClemente CC (1983). Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol, 51, 390–395. doi: 10.1037//0022-006X.51.3.390 [DOI] [PubMed] [Google Scholar]
- SAMHSA. (2012). Brief interventions and brief therapies for substance abuse. Treatment Improvement Protocol (TIP) Series, No. 34. HHS Publication No. (SMA) 12–3952. Rockville, MD: Substance Abuse and Mental Health Services Administration. [Google Scholar]
- SAMHSA. (2013). Motivational Enhancement Therapy Retrieved from http://www.nrepp.samhsa.gov/ViewIntervention.aspx?id=347
- Schroeder SA (2005). What to Do With a Patient Who Smokes. JAMA, 294, 482–487. doi: 10.1001/jama.294.4.482%JJAMA [DOI] [PubMed] [Google Scholar]
- Shahab L, West R, & McNeill A. (2011). A randomized, controlled trial of adding expired carbon monoxide feedback to brief stop smoking advice: Evaluation of cognitive and behavioral effects. Health Psychology, 30, 49–57. doi: 10.1037/a0021821 [DOI] [PubMed] [Google Scholar]
- Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, & Viikari-Juntura E. (2010). The association between smoking and low back pain: a meta-analysis. American Journal of Medicine, 123, 87 e87–35. doi: 10.1016/j.amjmed.2009.05.028 [DOI] [PubMed] [Google Scholar]
- Steinberg ML, Ziedonis DM, Krejci JA, & Brandon TH (2004). Motivational Interviewing With Personalized Feedback: A Brief Intervention for Motivating Smokers With Schizophrenia to Seek Treatment for Tobacco Dependence. Journal of Consulting and Clinical Psychology, 72, 723–728. doi: 10.1037/0022-006X.72.4.723 [DOI] [PubMed] [Google Scholar]
- Toblin RL, Mack KA, Perveen G, & Paulozzi LJ (2011). A population-based survey of chronic pain and its treatment with prescription drugs. Pain, 152, 1249–1255. doi: 10.1016/j.pain.2010.12.036 [DOI] [PubMed] [Google Scholar]
- Von Korff M. (2011). Assessment of chronic pain in epidemiological and health services research. In Turk DC & Melzack R. (Eds.), Handbook of Pain Assessment: Third Edition. New York, NY: The Guilford Press. [Google Scholar]
- Weingarten TN, Podduturu VR, Hooten WM, Thompson JM, Luedtke CA, & Oh TH (2009). Impact of tobacco use in patients presenting to a multidisciplinary outpatient treatment program for fibromyalgia. Clinical Journal of Pain, 25, 39–43. doi: 10.1097/AJP.0b013e31817d105e [DOI] [PubMed] [Google Scholar]
- Zale EL, & Ditre JW (2013). Associations between chronic pain status, attempts to quit smoking, and use of pharmacotherapy for smoking cessation. Psychology of Addictive Behaviors, 28, 294–299. doi: 10.1037/a0032515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zale EL, Ditre JW, Dorfman ML, Heckman BW, & Brandon TH (2014). Smokers in Pain Report Lower Confidence and Greater Difficulty Quitting. Nicotine & Tobacco Research, 16, 1272–1276. doi: 10.1093/ntr/ntu077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zale EL, Maisto SA, & Ditre JW (2016). Anxiety and Depression in Bidirectional Relations Between Pain and Smoking: Implications for Smoking Cessation. Behavior Modification, 40, 7–28. doi: 10.1177/0145445515610744 [DOI] [PMC free article] [PubMed] [Google Scholar]