Abstract
Coronavirus disease 2019 (COVID-19) has caused millions of deaths, and serious consequences to public health, economies and societies. Rapid responses in vaccine development have taken place since the isolation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the release of the viral genome sequence. By 21 May 2021, 101 vaccines were under clinical trials, and published data were available for 18 of them. Clinical study results from some vaccines indicated good immunogenicity and acceptable reactogenicity. Here, we focus on these 18 vaccines that had published clinical data to dissect the induced humoral and cellular immune responses as well as their safety profiles and protection efficacy.
Keywords: antibody, SARS-CoV-2, T cell, variant
The immunogenicity and safety of COVID-19 vaccines
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread to a pandemic scale, leading to over 100 million confirmed cases including 2 million deaths worldwide (1). The number of infected patients continues to increase and the global pandemic remains a significant threat to public health. Scientists worldwide have attempted to develop both prophylactics and therapeutics to counter the emerging COVID-19 pandemic, since the virus discovery in early January 2020 (2, 3).
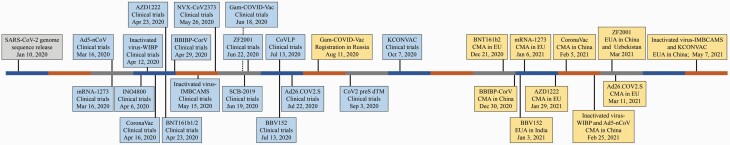
Safe and effective vaccines against SARS-CoV-2 for use in populations are urgently needed to control the pandemic and build herd immunity. Great efforts have been made in vaccine research and development since the COVID-19 outbreak (4). Vaccines have been developed that are based on various platforms, including inactivated viruses (5–9), live attenuated viruses (10), protein subunits (11–14), virus-like particles (VLPs) (15, 16), other viruses as vectors (17–23), mRNA (24–31) and DNA (32, 33). Up until 21 May 2021, 183 vaccine candidates were in the pre-clinical development stage and 101 were in clinical trials worldwide, of which reports on 18 vaccines (6 inactivated virus vaccines, 4 adenovirus-vectored vaccines, 4 protein subunit vaccines, 2 RNA vaccines, 1 DNA vaccine and 1 VLP vaccine) had been released (Table 1). A timeline shows the timepoints of clinical trial initiation and use authorization for these vaccines (Fig. 1).
Table 1.
The 18 current vaccines against COVID-19 that have reported results of clinical trials
| Vaccine | Platform | Developer | Formulation | NAb level in humans | Cellular responses in humansa | Efficacy | References |
|---|---|---|---|---|---|---|---|
| AZD1222 (ChAdOx1 nCoV-19) | Virus vector (ChAdOx1) | University of Oxford, with AstraZeneca, UK | Recombinant adenovirus expressing full-length S protein | Similar to HCS | Th1-skewed T-cell responses | Overall 66.7%; LD/SD 80.7%; SD/ SD 63.1% | (61–64, 113, 114) |
| Ad26. COV2.S | Virus vector (Ad26) | Janssen Pharmaceutical Companies, USA | Recombinant adenovirus expressing full-length S protein with two proline substitutions (K986P and V987P) and two mutations at the furin cleavage site (R682S and R685G) | Generally similar (single- dose groups) or higher (two-dose groups) GMT levels than HCS | Th1-skewed T-cell responses | 66.9% (single dose) | (43, 65, 116) |
| Gam-COVID- Vac (Sputnik V) | Virus vector (Ad26 and Ad5) | Gamaleya Research Institute, Russia | Recombinant adenovirus expressing full-length S protein | Similar to HCS in rAd26-S + rAd5-S groups | Induction of IFNγ + T-cell responses | 91.6% | (20, 115) |
| Ad5-nCoV | Virus vector (Ad5) | CanSino Biological Inc., with Beijing Institute of Biotechnology, China | Recombinant adenovirus expressing full-length S protein | Induction of NAb in humans but no comparison with HCS | Induction of IFNγ +, TNFα + and IL-2+ T-cell responses | 68.8% | (23, 66) |
| BNT162 | mRNA | BioNTech, with Fosun Pharma and Pfizer, Germany | BNT162b1: LNP-mRNA expressing RBD trimer (trimerized by addition of T4 fibritin foldon domain) BNT162b2: LNP-mRNA expressing full-length S protein with two proline substitutions (K986P and V987P) |
BNT162b1: similar to HCS in 10 µg groups; higher GMT levels than HCS in 30 and 50 µg groups BNT162b2: generally similar (10 µg groups) or higher (30 µg groups) GMT levels than HCS |
Th1-skewed T-cell responses | BNT162b2: 95% | (26, 49, 50, 109, 111) |
| mRNA-1273 | mRNA | Moderna, with National Institute of Allergy and Infectious Diseases, USA | LNP-mRNA expressing full-length S protein with two proline substitutions (K986P and V987P) | Generally similar (25 µg groups) to or higher (100 µg groups) than HCS | Th1-skewed T-cell responses | 94.1% | (44, 48, 57, 110) |
| INO-4800 | DNA | Inovio Pharmaceuticals, with International Vaccine Institute, and Advaccine Biopharmaceutical, USA | Plasmid pGX9501 expressing full-length S protein | Induction of NAb in humans but no comparison with HCS | Th1-skewed T-cell responses | NA | (60) |
| NVX-CoV2373 | Protein subunit (Sf9 insect cells) | Novavax, USA | Full-length S protein with two proline substitutions (K986P and V987P) and three mutations at furin cleavage site (R682Q, R683Q and R685Q) + Matrix-M1 adjuvant | Higher GMT levels than HCS in rSARS-CoV-2 + Matrix-M1 groups | Th1-skewed T-cell responses | 89.7% | (45, 117) |
| ZF2001 | Protein subunit (CHO cells) | Anhui Zhifei Longcom Biopharmaceutical, with Institute of Microbiology, Chinese Academy of Sciences, China | Dimeric RBD protein + aluminum hydroxide adjuvant | Generally higher than HCS | Balanced Th1 and Th2 T-cell responses | NA | (67) |
| SCB-2019 | Protein subunit | Clover Biopharmaceuticals, with GSK and Dynavax, China | Ectodomain of wild-type S protein in-frame fusion to trimer-tag + CpG/Alum or AS03 adjuvant | Generally higher than HCS in AS03 adjuvanted groups | Th1-skewed T-cell responses | NA | (14) |
| CoV2 preS dTM | Protein subunit (expresSF + insect cells) | Sanofi Pasteur, France, with GlaxoSmithKline, Belgium | Recombinant prefusion S protein with two proline mutations in the C-terminal region of S2 domain + AF03 or AS03 adjuvant | Similar to HCS | Balanced Th1 and Th2 T-cell responses | NA | (68) |
| CoronaVac | Inactivated virus | Sinovac, with National Institute for Communicable Disease Control and Prevention, China | Inactivated whole virus + aluminum hydroxide adjuvant | Induction of NAb in humans with lower GMT levels than HCS | No obvious vaccine-induced T-cell responses | 83.5% in Turkey; 65.9% in Chile | (70, 71) |
| BBIBP-CorV | Inactivated virus | Beijing Institute of Biological Products, Sinopharm, with Institute of Viral Disease Control and Prevention, China | Inactivated whole virus + aluminum hydroxide adjuvant | Induction of NAb in humans but no comparison with HCS | NA | 78.1% | (72) |
| Inactivated virus-WIBP | Inactivated virus | Wuhan Institute of Biological Products, Sinopharm, with Wuhan Institute of Virology, Chinese Academy of Sciences, China | Inactivated whole virus + aluminum hydroxide adjuvant | Induction of NAb in humans and no comparison with HCS | T-cell responses upon stimulation were not measured | 72.8% | (8) |
| Inactivated virus- IMBCAMS | Inactivated virus | Institute of Medical Biology, Chinese Academy of Medical Sciences, China | Inactivated whole virus + aluminum hydroxide adjuvant | Induction of NAb in humans and no comparison with HCS | Induction of IFNγ + T-cell responses | NA | (7) |
| KCONVAC | Inactivated virus | Shenzhen Kangtai Biologi cal Products, with Beijing Minhai Biotechnology, China | Inactivated whole virus + aluminum hydroxide adjuvant | Generally similar (0/14 regimen) to or higher (0/28 regimen) than HCS | Induction of IFNγ + T-cell responses | NA | (74) |
| BBV152 (Covaxin) | Inactivated virus | Bharat Biotech, with Indian Council of Medical Research, India | Inactivated whole virus + Algel-IMDG adjuvant | Generally similar to HCS in Algel-IMDG adjuvanted groups | Th1-skewed T-cell responses | NA | (9, 75) |
| CoVLP | VLP (Nicotiana benthamiana plants) | Medicago, Canada | VLP + CpG1018 or AS03 adjuvant | Higher GMT levels than HCS in AS03 adjuvanted groups; similar levels to HCS in CpG1018 adjuvanted groups | Balanced Th1 and Th2 T-cell responses | NA | (69) |
Ad, adenovirus; CHO, Chinese hamster ovary; LD, low dose; NA, not applicable; SD, standard dose; Sf9, Spodoptera frugiperda 9.
aTh1 and Th2 cell responses were generally measured by detection of the Th1 cytokines IFNγ, IL-2 and TNFα and the Th2 cytokines IL-4, IL-5, IL-10 and IL-13.
Fig. 1.
Timeline showing the key time point of initiating clinical trials and use authorizations for the vaccines against COVID-19.
Here, we summarize the immune responses, adverse events following immunization (AEFI) and vaccine efficacy against COVID-19, especially the humoral and cellular immune responses induced by these vaccines, from the published reports of clinical trials. Obviously, mRNA vaccines show great potential though it is the first time that they have been used in healthy populations. The appropriate delivery of mRNA vaccines is still a big challenge (34).
Humoral responses
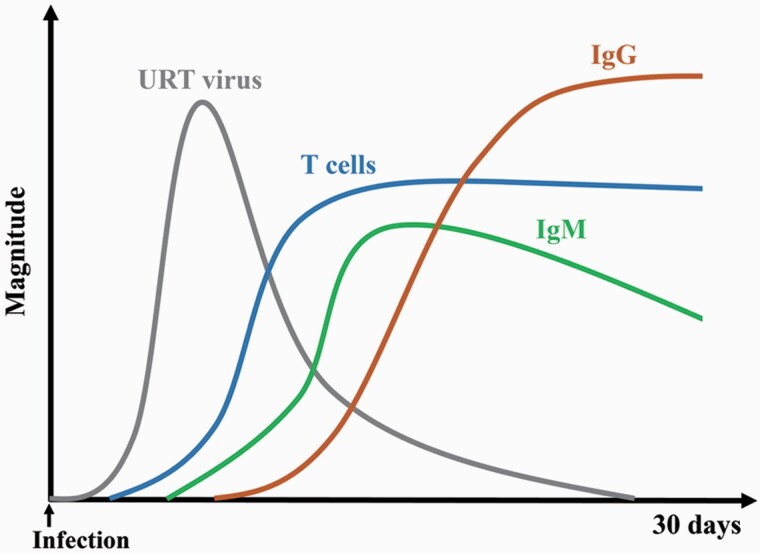
Humoral responses, especially the neutralizing antibody (NAb) levels, have been considered as an immune correlation of protection against SARS-CoV-2 (4, 18, 33). SARS-CoV-2-specific IgM is produced a few days after infection, followed by the production of virus-specific IgG (Fig. 2). The spike (S) protein of SARS-CoV-2 is responsible for recognizing and binding to the host receptor angiotensin-converting enzyme 2 (ACE2) (35, 36). The level of IgG against the S protein receptor-binding domain (RBD) is correlated with SARS-CoV-2-neutralizing activities in the sera from COVID-19 patients (37). It is reported that 80.7% of human convalescent sera (HCS) from COVID-19 patients have neutralizing activities against SARS-CoV-2 (38). A large number of NAbs have been identified, and some have been shown to reduce the viral load in patients with COVID-19 (39–42).
Fig. 2.
Schematic representation of the induced immune response following SARS-CoV-2 infection. The gray, blue, green and brown lines indicate upper respiratory tract (URT) virus load, T-cell responses, IgM level and IgG level, respectively.
According to the reported clinical trial results, all the vaccines against SARS-CoV-2 were shown to elicit antigen-binding antibodies and SARS-CoV-2 NAbs, with high proportions of seroconversion. However, it is difficult to compare the NAb levels for various vaccines because of the lack of standardized NAb titration assays. For example, the 50% wild-type virus microneutralization assay (IC50), the 80% wild-type virus plaque-reduction neutralization testing assay (PRNT80), the wild-type virus microneutralization assay with an inhibitory concentration of >99% (MN IC>99%) and the 50% neutralization assay with a virus engineered by the insertion of an mNeonGreen gene that produces fluorescence have been used in the clinical trials of Ad26.COV2.S (43), mRNA-1273 (44), NVX-CoV2373 (45) and BNT162b1/2 (26), respectively. Thus, a panel of COVID-19 HCS was most often tested as the control. The humoral responses were compared using the ratios of vaccine-induced NAb titers and HCS NAb titers among the vaccines. Recently, the ratios were proposed as a protective correlate for COVID-19 vaccines (46). In addition, the World Health Organization (WHO) has initiated a global effort to establish WHO international reference materials to increase their availability for evaluation of similar biotherapeutic products (47).
Participants immunized with BNT162b1/2 or mRNA-1273 showed robust and dose-dependent antibody responses (26, 44, 48–50). The immunogenicity of BNT162b1 and BNT162b2 was similar. Fourteen days after the second dose, the 50% neutralizing geometric mean titers (GMTs) that were elicited by 30 μg of BNT162b2 and BNT162b1 in both younger adults and older adults exceeded those of the HCS panel (26). Several studies reported that previous SARS-CoV-2 infection could be analogous to immune priming and a single-dose inoculation of BNT162b2 in participants with previous infection boosted the antibody responses to levels similar to those in the infection-naive participants with two doses of BNT162b2 (51–53). Age has also been identified as an independent risk factor for disease severity associated with SARS-CoV-2 infection (54, 55). The GMTs elicited by 30 μg of BNT162b2 in older adults were slightly lower than those in younger adults (26). Another Phase 1 study of BNT162b1 in China also showed that the serum-neutralizing activity in younger participants was higher than that in older participants (56).
For another mRNA vaccine, mRNA-1273, the GMTs of serum-neutralizing activity induced by two doses (100 µg) were similar between younger and older adults and higher than those of a panel of HCS controls (44, 48, 57). In addition, the evaluation of the durability of humoral responses induced by mRNA-1273 showed that the binding and NAb titers declined slightly, but with GMTs still exceeding the GMTs in a panel of HCS controls at day 90 after the second dose (100 μg) (58). A study found that after inoculation with mRNA-1273 or BNT162b2 vaccine in humans, the elicited neutralizing antibodies target the same epitopes as those produced by natural SARS-CoV-2 infection (59).
A DNA vaccine, INO-4800, was administered as two doses in healthy younger participants via the intradermal route followed by electroporation using the CELLECTRA® delivery system. It induced NAb production with similar titers in the 1.0 and 2.0 mg groups. However, the HCS controls were not included in the study (60).
In the clinical trials of AZD1222, a second dose of immunization provided an increase in the levels of humoral responses and this tendency was also observed for Ad26.COV2.S and rAd26/rAd5 (20, 43, 61). These reports showed that two-dose vaccination with AZD1222, Ad26.COV2.S or Gam-COVID-Vac (heterologous rAd26 + rAd5) induced NAb production with titers similar to those of the HCS tested (20, 43, 62–64). The immunogenicity evaluation of vaccines showed that the humoral responses in older adults were similar to those in younger adults after two doses of AZD1222 or Ad26.COV2.S (43, 64). In addition, the systemic serology analysis revealed that AZD1222 and Ad26.COV2.S induced broadly functional antibody profiles, such as antibody-dependent phagocytosis by neutrophils, activation of natural killer cells, etc. (61, 65). A mixed high IgG1 and IgG3 response, with low levels of IgG2 and little detectable IgG4, was induced by AZD1222 vaccination, consistent with the naturally acquired antibody profiles in SARS-CoV-2-infected humans (63). Another adenovirus-vectored vaccine, Ad5-nCoV, was administered only once and induced significant NAb responses to live SARS-CoV-2. However, pre-existing immunity to the Ad5 vector and increasing age could partially hamper the specific humoral immune responses to vaccination (23, 66).
The COVID-19 protein subunit vaccines contained various antigens, which were a dimeric tandem-repeat RBD (RBD-tr2) for ZF2001, a prefusion-stabilized trimeric S protein for NVX-CoV2373 and CoV2 preS dTM and a wild-type S protein in-frame fusion to trimer-tag for SCB-2019, respectively, showing good immunogenicity in their respective clinical trials (14, 45, 67, 68).
ZF2001 was based on a novel antigen designated as recombinant dimeric RBD of SARS-CoV-2 S protein without any linker sequences, which showed increased immunogenicity compared with traditional monomeric RBD protein (13). The clinical trial data showed that three doses of ZF2001 (25 μg) elicited neutralizing GMT levels about twice the GMT found for the HCS. Since increasing the antigen dose from 25 to 50 μg did not improve humoral responses, the 25 μg dose had been selected for efficacy evaluation in the Phase 3 trial (67).
The study of NVX-CoV2373, CoV2 preS dTM and SCB-2019 demonstrated that the addition of adjuvant resulted in significantly enhanced antibody responses compared with unadjuvanted protein alone (14, 45, 68). The two-dose vaccination of NVX-CoV2373 (5 or 25 µg) with Matrix-M1 adjuvant resulted in GMT levels of neutralizing antibodies that were approximately four times greater than those in symptomatic outpatients with COVID-19 (45). CoV2 preS dTM with AF03 or AS03 adjuvant induced neutralizing antibody levels similar to those of HCS in the younger participants. The immune responses induced by CoV2 preS dTM in older participants were lower than those in the younger participants (68).
The immunogenicity of the SCB-2019 plus CpG/Alum adjuvant was lower than that of SCB-2019 combined with AS03 adjuvant, which induced similar humoral responses in younger and older adults. Two doses of SCB-2019+AS03 (30 μg) induced NAb production with higher GMTs in both younger and older adults than in HCS (14).
For the VLP vaccine, CoVLP, the study report showed that AS03 adjuvant appeared to be more effective than CpG1018 in enhancing humoral responses, and two-dose immunization with CoVLP+AS03 induced NAbs at GMTs 10 times higher than those in HCS (69).
The inactivated SARS-CoV-2 vaccines CoronaVac (70, 71), BBIBP-CorV (Beijing Institute of Biological Products) (72), inactivated virus-WIBP (Wuhan Institute of Biological Products) (8), inactivated virus-IMBCAMS (Institute of Medical Biology, Chinese Academy of Medical Sciences) (7, 73), KCONVAC (74) and BBV152 (9, 75) also showed good immunogenicity. According to the respective study reports, after the last dose of vaccination, the levels of NAbs induced by CoronaVac were lower than those of HCS and levels of NAbs induced by BBV152 were comparable to those of HCS (9, 70, 71, 75). The levels of NAbs induced by KCONVAC were similar (14 days prime-boost interval) to or higher (28 days prime-boost interval) than those of HCS (74). CoronaVac and BBIBP-CorV also showed good immunogenicity in older participants (71, 72). In addition to induction of antibodies against the S protein, N protein-binding IgG was also induced in the participants who received vaccines BBV152, IV-IMBCAMS or KCONVAC (7, 9, 74, 75).
However, it should be noted that the interpretation of vaccine immunogenicity by comparison with HCS is arbitrary, because the GMTs can be influenced by the composition of the panels, such as illness severity, time since disease onset, the sample size and other factors (76–78).
SARS-CoV-2 variants
Numerous variants have emerged during the COVID-19 pandemic and some of them are of high concern. The D614G substitution on the S protein conferred an increased infectivity and transmission, and has become the dominant global form since March 2020 (79). Fortunately, SARS-CoV-2 with the S protein D614G substitution was not expected to escape the neutralization induced by current vaccines (80–82). New SARS-CoV-2 variants, emerged in the UK (501Y.V1, B.1.1.7 lineage), South Africa (501Y.V2, B.1.351 lineage) and Brazil (501Y.V3, P.1 lineage), were spreading globally and were found to escape neutralization induced by virus infection and vaccination (83, 84). Typically, the S protein in B.1.1.7 harbors 9 mutations: ΔH69-V70, ΔY144, N501Y, A570D, D614G, P681H, T716I, S982A and D1118H (85). The S protein in B.1.351 also harbors 10 mutations: L18F, D80A, D215G, ΔL242-244, R246I, K417N, E484K, N501Y, D614G and A701V (86). The S protein in P.1 harbors 12 mutations: L18F, T20N, P26S, D138Y, R190S, K417T, E484K, N501Y, D614G, H655Y, T1027I and V1176F (84). It was found that S protein E484K was a key mutation that resulted in variant virus resistance to the neutralizing activity of most NAb engaging the RBD, convalescent sera and mRNA vaccine-induced immune sera (87, 88).
Several groups have studied the effect of B.1.1.7 and B.1.351 on the neutralizing activity induced by wild-type virus infection or vaccine immunization. Shen et al. reported that NAb titers of HCS samples and human immune sera elicited by mRNA-1273 and NVX-CoV2373 against the B.1.1.7 variant were 1.5-, 2- and 2.1-fold lower than those against the D614G variant, respectively (89). Wu et al. reported that the neutralizing activities of antisera induced by mRNA-1273 vaccination against B.1.1.7 were 1.2-fold lower against a D614G recombinant vesicular stomatitis virus (rVSV)-based pseudotyped virus (90). Collier et al. reported that the neutralizing activity of sera from participants who received BNT162b2 vaccination were modestly reduced against the B.1.1.7 variant, compared with wild-type virus. But B.1.1.7 plus E484K substitution could substantially escape the neutralizing activity of BNT162b2-induced sera (88). Muik et al. reported the geometric mean ratios of the B.1.1.7 pseudotyped virus and the Wuhan pseudotyped virus-neutralizing GMTs were 0.78 and 0.83 for the younger and older adults, respectively, indicating that the immune sera elicited by BNT162b2 largely preserved neutralizing activities against the B.1.1.7 lineage pseudotyped virus (85). Wang et al. reported no loss of neutralizing activity against B1.1.7 compared with wild-type virus for HCS samples collected in the first half of 2020 and human immune sera elicited by BNT162b2 or mRNA-1273 (91). The discrepant conclusions probably resulted from the low sample numbers and different detection methods. A standardized method to measure the neutralizing activity against SARS-CoV-2 should be established globally. For the inactivated virus vaccines, B.1.1.7 showed little resistance to the neutralizing activity of serum from BBIBP-CorV, Sinovac and BBV152 inoculated participants (92, 93). These studies generally indicated that human immunity conferred by the current vaccines was still effective against the B.1.1.7 variant.
For the B.1.351 variant, Zhou et al. reported that neutralizing GMTs of HCS samples from early 2020 or from human immune sera elicited by BNT162b2 or AZD1222 against B.1.351 were 13.3-, 7.6- and 9-fold reduced, respectively, compared with an early Wuhan-related strain of SARS-CoV-2 (83). Wang et al. reported that the mean fold changes between neutralizing activity against the wild-type virus and B.1.351 was 9.4-fold for HCS samples collected in the first half of 2020, 10.3-fold for antisera induced by BNT162b2 immunization and 12.4-fold for antisera induced by mRNA-1273 immunization (91). Wu et al. reported that the neutralizing activity of antisera induced by mRNA-1273 immunization against B.1.351 was 6.4-fold lower against a D614G rVSV-based pseudotyped virus (90). Shen et al. reported that the neutralizing GMTs of serum from mRNA-1273 or NVX-CoV2373 vaccinated participants against B.1.351 spike pseudotyped virus were 9.7- and 14.5-fold reduced, respectively, compared with D614G spike pseudotyped virus (94). Wang et al. reported that the neutralizing activities of serum from BBIBP-CorV and Sinovac inoculated participants against B.1.351 were 2.5- and 3.3-fold, respectively, lower against a wild-type pseudotyped virus (92). Huang et al. reported that the neutralizing activities of human immune sera elicited by ZF2001 against B.1.351 declined by 1.6-fold (GMT, from 106.1 to 66.6), which suggested that the B.1.351 variant does not escape the immunity elicited by vaccines targeting the S protein RBD (ZF2001) (95). Another study conducted by Garcia-Beltran et al. found pseudotyped virus with S protein RBD mutations (K417N/T, E484 K and N501Y) showed high resistance to the neutralizing activity of human serum after mRNA vaccines immunization (96). Wang et al. observed a similar conclusion. They analyzed the human antibody repertoire induced by BNT162b2 or mRNA-1273 and found a subclass of monoclonal antibodies targeting RBD highly reduced the neutralizing activity against variants with K417N/T, E484K and/or N501Y mutations (59). Therefore, the emergence of SARS-CoV-2 variants, especially B.1.351, brought a challenge to the current COVID-19 vaccine development. The effectiveness of COVID-19 vaccines against SARS-CoV-2 variants should be particularly evaluated and considered as important evidence to guide the ongoing vaccination programs worldwide.
Cellular responses
It has been known that coronavirus infection induces strong T-cell immune responses (97). Following natural SARS-CoV-2 infection, T-cell responses are rapidly activated (Fig. 2), which are important for controlling the disease progression (98–101). Virus-specific T-cell responses have been shown to be associated with milder disease in COVID-19 patients (102). The involvement of T cells is also critical for B-cell maturation and the induction of strong and durable antibody responses (103, 104). Therefore, the generation of a robust cellular immune response is a desirable attribute for a vaccine against SARS-CoV-2. Previous experience with coronavirus vaccines and animal models of SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) infections have raised safety concerns about the potential for vaccine-associated enhanced respiratory disease (ERD) (105, 106). The theoretical risk of vaccine-associated ERD has been associated with type 2 helper T-cell (Th2)-skewed cellular immune responses (107, 108). Accordingly, it is deemed advantageous if a COVID-19 vaccine instead activates type 1 helper T-cell (Th1)-skewed T-cell responses or balanced T-cell responses.
Studies on BNT162b2 showed that Th1-biased T-cell responses were induced and were characterized by expression of IFNγ and IL-2 but very low levels of IL-4 in BNT162b2-induced CD4+ T cells after antigen stimulation. In addition, S-specific IFNγ +CD8+ T cells were also robustly induced by BNT162b2. No clear dose dependency of the T-cell response strength was observed within the tested dose range (1–30 μg) of BNT162b2 (109). Another mRNA vaccine, mRNA-1273, elicited CD4+ T-cell responses robustly biased toward the expression of Th1 cytokines [tumor necrosis factor α (TNFα), IL-2 and IFNγ], with minimal Th2 cytokine expression (IL-4 and IL-13) and low levels of SARS-CoV-2 S-specific CD8+ T-cell responses (44, 48). The Th1 T-cell responses were also strongly induced in older participants who received the 100 μg dose of mRNA-1273 (44, 48). Furthermore, vaccine-associated ERD was not observed in the participants who received BNT162b2 or mRNA-1273 vaccines in a Phase 3 trial (110, 111).
The DNA vaccine candidate INO-4800 elicited Th1-biased T-cell responses characterized by the expression of IFNγ, TNFα or IL-2 in both CD4+ and CD8+ T cells and minimized the induction of IL-4 upon S-antigen stimulation (60).
Cellular responses were induced in all the adenovirus-vectored vaccines for which data have been published. Reports showed that AZD1222 induced robust, multifunctional and Th1-skewed T-cell responses to SARS-CoV-2 (61–64). After peptide stimulation of peripheral blood mononuclear cells (PBMCs) from participants vaccinated with AZD1222, secretion of the cytokines IFNγ and IL-2, but not IL-4 and IL-13, was increased (63). Similarly, phenotyping by intracellular cytokine staining (ICS) assay indicated that CD4+ T cells predominantly produced Th1 cytokines (IFNγ, IL-2 and TNFα) rather than Th2 cytokines (IL-5 and IL-13). Monofunctional IFNγ + cells were dominant in the CD8+ T cells (63). Moreover, CD8+ T cells expressing the degranulation marker CD107a were also induced by AZD1222, which indicated cytotoxic function. In addition, enzyme-linked immunospot (ELISpot) assays showed that the magnitude of S-specific T-cell responses increased as early as day 7, peaked at day 14 and were maintained up to day 56 after the prime immunization but did not increase with a boost dose (62). The AZD1222-induced cellular responses were similar in all groups regardless of age (64).
For Ad26.COV2.S, Th1-biased T-cell responses were also induced, characterized by the expression of IFNγ, IL-2 or both, and not IL-4, or IL-13 in CD4+ T cells as assessed by ICS assay. CD8+ T-cell responses (expression of IFNγ, IL-2 or both) were robust and similar in both younger and older participants receiving 5 × 1010 viral particles (vps) of Ad26.COV2.S (43). In addition, IFNγ central memory T-cell responses were induced by Ad26.COV2.S vaccination (65).
In the clinical trials of both Ad5-nCoV and Gam-COVID-Vac, SARS-CoV-2 S-specific CD4+ T cells and CD8+ T cells were activated (20, 23). Secretion of IFNγ by PBMCs upon antigen re-stimulation was detected from participants vaccinated with Gam-COVID-Vac (20). T cells induced by Ad5-nCoV had polyfunctional phenotypes with IFNγ, TNFα or IL-2 secretion (23, 66). Positive specific T-cell responses evaluated by IFNγ-ELISpot were found in 90 and 88% of participants immunized with 1 × 1011 and 5 × 1010 vp of Ad5-nCoV, respectively (66). However, the T-cell response induced by Ad5-nCoV was partly diminished by the presence of high levels of pre-existing anti-Ad5 NAbs (23, 66).
Reports of subunit protein vaccine candidates NVX-CoV2373 and SCB-2019 showed that both of them activated the cellular responses with Th1 polarization (14, 45). The NVX-CoV2373 containing the Matrix-M1 adjuvant induced antigen-specific polyfunctional CD4+ T-cell responses that were reflected in high IFNγ, IL-2 and TNFα production and minimal IL-5 and IL-13 production after S protein stimulation (45). SCB-2019 along with AS03 or CpG1018/Alum adjuvant immunization elicited cellular responses to CD4+ T cells expressing IFNγ and IL-2 secretion but not IL-4, IL-5 or IL-17 (14).
For ZF2001, CoV2 preS dTM and CoVLP, balanced cellular immune responses were induced by all of them (67–69). In the trial of ZF2001, the 25 or 50 μg group elicited moderate levels of both Th1 (IFNγ and IL-2) and Th2 (IL-4 and IL-5) cytokine production post-vaccinations (67). In the trial of CoVLP, the cellular responses (both IFNγ and IL-4 responses) in the CoVLP plus AS03 groups were stronger than those in the CoVLP plus CpG1018 groups as determined by ELISpot assay (69).
In the clinical trials of the inactivated virus-IMBCAMS, the IFNγ ELISpot assay showed induction of cytotoxic T lymphocyte responses against the S, N and virion antigens in the vaccine recipients (73). A moderate T-cell response was also induced by KCONVAC measured by IFNγ-ELISpot (74). The reports of BBV152 indicated that cellular responses were skewed to a Th1 phenotype by vaccines formulated with Algel-IMDG, which contains an imidazoquinoline class Toll-like receptor 7 (TLR7)/TLR8 agonist adsorbed to Algel (9, 75). TLRs play an essential role in bridging the innate and adaptive immune responses, contributing to the differentiation of CD4+ T cells into Th1 cells (112). A cytokine bead-array multiplex assay demonstrated that 3 or 6 µg of inactivated virus vaccine containing Algel-IMDG elicited more Th1 responses characterized by IFNγ, TNFα and IL-2 secretion than Th2 responses characterized by IL-5, IL-10 and IL-13 secretion (75). In addition, T-cell memory responses, characterized by CD4+CD45RO+ and CD4+CD45RO+CD27+ T-cell populations, were induced by vaccine formulations with Algel-IMDG (75).
It should be noted that different assays were used in the evaluation of vaccines, including ELISpot, ICS and cytokine bead-array multiplex assays. In addition, the procedures could be different, such as addition of antigen for stimulation or not. Thus, the levels of cellular responses could not be compared among vaccines from different developers.
Protection efficacy
The protection efficacy was released as published articles for nine vaccines against COVID-19: BNT162b2 (111), mRNA-1273 (110), AZD1222 (113, 114), Gam-COVID-Vac (115), Ad26.COV2.S (116), NVX-CoV2373 (117), CoronaVac (118, 119), BBIBP-CorV (120) and inactivated virus-WIBP (120).
Two doses of intramuscular immunization of BNT162b2 (30 μg), 21 days apart, conferred 95% efficacy against COVID-19 at least 7 days after the second vaccination. Protection by the vaccine started as soon as 12 days after the first dose. The efficacy of BNT162b2 across subgroups defined by age, sex, race, ethnicity, obesity and the presence of a coexisting condition was generally consistent with that observed in the overall population (111). In addition, a real-world study found that the viral load was substantially reduced in breakthrough infection after inoculation with BNT162b2 and the onward transmission was further suppressed (121). Qatar had initiated a mass immunization campaign with BNT162b2 since December 2020 and suffered its second and third waves of SARS-CoV-2 infection since January 2021, which were mainly triggered by B.1.1.7 and B.1.351. The estimated effectiveness of BNT162b2 vaccine against infection with the B.1.1.7 and B.1.351 variant was 89.5 and 75.0%, respectively, at 14 or more days after the second dose (122). It indicated that BNT162b2 vaccine was still effective against B.1.1.7 and B.1.351 variants.
Two intramuscular injections of mRNA-1273 (100 μg), 28 days apart, conferred participants who had not previously been infected with SARS-CoV-2 a 94.1% prevention efficacy against COVID-19 at least 14 days after the second vaccination. The prevention efficacy of mRNA-1273 was consistent across subgroups stratified by age, sex, race, SARS-CoV-2 baseline (including participants with prior infection or not) and presence of risk for severe COVID-19 (110).
AZD1222 conferred SARS-CoV-2 seronegative participants, who received two standard doses (5 × 1010 vp) or a low (2.2 × 1010 vp) dose followed by a standard dose of vaccine, a 63.1 or 80.7% protection efficacy, respectively, against COVID-19 at least 14 days after the second vaccination. The overall vaccine efficacy across both groups was 66.7%. It should be noted that 1396 participants received a low-dose plus standard-dose vaccine and 7201 participants received two standard doses. The discrepancy of the participant numbers in each group may have contributed to the discrepancy of the efficacy among groups. In addition, the low-dose prime and standard-dose boost strategy provided better protection than two standard-dose immunizations, which was likely to be due to the lower levels of anti-vector immunity induced by a half-dose vaccine as the first dose, or differential antibody functionality or cellular immunity, including altered avidity or immunodominance (113, 114). Furthermore, the timing of the booster dose influenced the immunogenicity and efficacy of AZD1222. After two standard-dose immunizations, antibody titers in the participants with a longer prime-boost interval (more than 12 weeks) were higher than in those with a short prime-boost interval (less than 6 weeks). Consistent with the immunogenicity, efficacy in individuals with a more than 12 weeks interval (81.3%) was higher than that in individuals with a less than 6 weeks interval (55.1%) (114). These results indicated that the timing of the booster dose should be a major consideration in the design of clinical trials. Following the spread of SARS-CoV-2 variants, the protection efficacy conferred by COVID-19 vaccines is of high concern. A study in the UK with relatively small cohorts showed that the efficacy of AZD1222 against symptomatic COVID-19 caused by the B.1.1.7 variant was 70.4%, which was lower than the efficacy of 81.5% for non-B.1.1.7 lineages. The majority of cases of the non-B.1.1.7 lineage were caused by B.1.177 (A222V and D614G mutation in S protein) (123). Madhi et al. reported that the two-dose regimen of AZD1222 provided only 10.4% protection efficacy against the B.1.351 variant, which was significantly lower than the protection efficacy against the early SARS-CoV-2 lineage (124).
Gam-COVID-Vac was administered intramuscularly as a first dose (rAd26, 1 × 1011 vp) and a second dose (rAd5, 1 × 1011 vp), 21 days apart and provided a 91.6% prevention efficacy against COVID-19 from 21 days after the first dose. The vaccine efficacy of Gam-COVID-Vac was similar across all the age subgroups, including participants older than 60 years (115).
A single intramuscular dose of Ad26.COV2.S (5 × 1010 vp) provided 66.9 and 66.1% efficacy in preventing moderate to severe/critical COVID-19 at least 14 and 28 days after vaccination, respectively. In the Phase 3 trial of Ad26.COV2.S in South Africa, 94.5% of cases were identified as B.1.351 variant infections and vaccine efficacy was maintained at 52.0 and 64.0% against moderate-to-severe/critical COVID-19 at least 14 and 28 days after administration, respectively (116).
Two intramuscular doses of NVX-CoV2373 (5 μg of recombinant spike protein with 50 μg of Matrix-M1 adjuvant), 21 days apart, conferred 89.7% efficacy against COVID-19 at least 7 days after the second injection. Additionally, the efficacy against the B.1.1.7 variant was 86.3% (117). Another study showed that the NVX-CoV2373 vaccine was still efficacious against B.1.351 with an efficacy of 51.0% among the HIV-negative participants (125).
For COVID-19 inactivated virus vaccine, after two doses of immunization, 21 days apart, BBIBP-CorV (4 μg) and inactivated virus-WIBP (5 μg) provide 78.1 and 72.8% protection efficacy against COVID-19, respectively (120). CoronaVac was administrated intramuscularly as two doses, 28 days apart. Chile started a mass vaccination campaign with the CoronaVac vaccine from February 2021. In the participants who were received two doses in Chile, the protection efficacy of CoronaVac was 65.9% for the prevention of COVID-19 (118). In addition, another Phase 3 trial of CoronaVac in Turkey showed an efficacy of 83.5% against COVID-19 at least 14 days after the second dose (119).
In addition, one dose of Ad5-nCoV provided 68.8% efficacy in preventing symptomatic COVID-19 from 14 days after vaccination, which was reported on the website of CanSinoBIO company (126). It was similar with the protection efficacy of Ad26.COV2.S vaccine.
In order to control the COVID-19 pandemic, BNT162b2, mRNA-1273, AZD1222, Gam-COVID-Vac, Ad26.COV2.S, Ad5-nCoV, ZF2001, CoronaVac, BBIBP-CorV, inactivated virus-WIBP, inactivated virus-IMBCAMS, KCONVAC and BBV152 had been granted emergency use authorizations (EUAs) or conditional marketing authorizations (CMAs) (Fig. 1).
Safety and AEFI
In the clinical trials of vaccines described here, the most common local adverse reactions were injection site pain, redness and swelling, and the most common systemic adverse reactions were fever, fatigue and headache, which were mostly transient and self-limited. The safety profile of these vaccines indicated that they were safe and well tolerated. Most of the adverse events were mild to moderate and are resolved rapidly.
It is the first time that mRNA-based vaccines have ever been approved for use on the healthy population. Following implementation of vaccination with BNT162b2 and mRNA-1273, cases of anaphylaxis began to be reported. The anaphylaxis reporting rates were 4.7 and 2.5 cases per million doses administered for BNT162b2 and mRNA-1273, respectively (127). Polyethylene glycol (PEG), a chemical component in lipid nanoparticles (LNPs), was thought to be the culprit allergen (34, 128–130). Continued safety monitoring of mRNA COVID-19 vaccines in the USA has confirmed that anaphylaxis following vaccination is a rare event (127, 131).
In the Phase 3 clinical trials of BNT162b2, mRNA-1273, AZD1222, Gam-COVID-Vac, Ad26.COV2.S, NVX-CoV2373, CoronaVac, BBIBP-CorV and inactivated virus-WIBP, the incidence of serious adverse events was similar between the vaccine and placebo groups. In addition, no evidence of vaccine-associated ERD was observed (110, 111, 113–117, 119, 120).
Previous studies on SARS vaccines found that serum from animals immunized with SARS-CoV S protein exacerbates virus infection in vitro through antibody-dependent enhancement (ADE) (132, 133). Therefore, the ADE risk of SARS-CoV-2 vaccines was raised as a safety concern (134, 135). According to the published reports of COVID-19 vaccines, no ADE occurred during the clinical trials, especially in the large-scale Phase 3 trials.
Conclusions and perspectives
In this review, we have summarized the humoral and cellular immune responses induced by 18 vaccines against COVID-19 that had published the clinical data by 21 May 2021. All these vaccines conferred high proportions, even 100%, seroconversion of NAbs, with magnitudes higher than those of HCS in recipients vaccinated with adjuvanted subunit protein vaccines (NVX-CoV2373, SCB-2019 and ZF2001), mRNA vaccines (BNT162 and mRNA-1273), the VLP vaccine CoVLP, the adenovirus vaccine Ad26.COV2.S and the inactivated vaccine KCONVAC (Table 1). Cellular immune responses play a crucial role in preventing SARS-CoV-2. Th1-skewed T-cell responses were observed in participants receiving two mRNA vaccines (BNT162b1 and mRNA-1273) and two adenovirus-vectored vaccines (AZD1222 and Ad26.COV2.S), two adjuvanted protein subunit vaccines (NVX-CoV2373 and SCB-2019), DNA vaccine INO-4800 and inactivated virus plus Algel-IMDG adjuvant (BBV152). Balanced T-cell responses were observed in CoVLP, CoV2 preS dTM and ZF2001 vaccine recipients.
A Th1-biased or Th1/Th2-balanced immune responses were observed in clinical studies of most of these vaccines, which are believed to have less potential for ERD risk. In fact, although previous studies with animal models of SARS-CoV and MERS-CoV infection raised safety concerns about the potential for ADE and vaccine-associated ERD (105, 106), analyses of the large-scale Phase 3 trials of BNT162b2, mRNA-1273 and AZD1222 demonstrated their protection efficacy against COVID-19, without observed vaccine-associated ERD.
In conclusion, a potential COVID-19 vaccine should satisfy at least four requirements: (i) safety, characterized by tolerable adverse events, reactogenicity, absence of ADE and vaccine-associated ERD; (ii) effectiveness, characterized by induction of high titers of NAbs, robust Th1-skewed or balanced cellular responses and long-term immunological memory; (iii) being deployable, characterized by production scalability to meet the global vaccine demands; and (iv) having appropriate quality control of different batches of the vaccines. Fortunately, some of these requirements have been achieved.
Funding
This work is supported by the National Program on Key Research Project of China (2020YFA0907100), Strategic Priority Research Program of the Chinese Academy of Sciences (XDB29010202), National Natural Science Foundation of China (NSFC) (82041048), China Postdoctoral Science Foundation (2020T130031ZX), Chinese Academy of Sciences Project for Young Scientists in Basic Research (YSBR-010), Youth Innovation Promotion Association of the Chinese Academy of Sciences (2018113) and Hainan Province Postdoctoral Science Fund of China (2019-20741).
Conflicts of interest statement: K.X., L.D. and G.F.G. are listed as inventors on patent applications for RBD dimer-based CoV vaccines. The patents for RBD dimers as protein subunit vaccines for SARS-CoV-2 have been licensed to Anhui Zhifei Longcom Biopharmaceutical Co. Ltd, China.
References
- 1. World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Available at: https://covid19.who.int.
- 2. Tan, W., Zhao, X., Ma, X.et al. . 2020. A novel coronavirus genome identified in a cluster of pneumonia cases—Wuhan, China 2019–2020. China CDC Weekly 2:61. [PMC free article] [PubMed] [Google Scholar]
- 3. Stenseth, N. C., Dharmarajan, G., Li, R.et al. . 2021. Lessons learnt from the COVID-19 pandemic. Front. Public Health 9:694705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dai, L. and Gao, G. F. 2021. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 21:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang, H., Zhang, Y., Huang, B.et al. . 2020. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell 182:713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gao, Q., Bao, L., Mao, H.et al. . 2020. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 369:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Che, Y., Liu, X., Pu, Y.et al. . 2020. Randomized, double-blinded and placebo-controlled Phase II trial of an inactivated SARS-CoV-2 vaccine in healthy adults. Clin. Infect. Dis., in press. doi: 10.1093/cid/ciaa1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xia, S., Duan, K., Zhang, Y.et al. . 2020. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. J. Am. Med. Assoc. 324:951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ella, R., Vadrevu, K. M., Jogdand, H.et al. . 2021. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect. Dis. 21:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seo, S. H. and Jang, Y. 2020. Cold-adapted live attenuated SARS-Cov-2 vaccine completely protects human ACE2 transgenic mice from SARS-Cov-2 infection. Vaccines 8:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang, J., Wang, W., Chen, Z.et al. . 2020. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 586:572. [DOI] [PubMed] [Google Scholar]
- 12. Guebre-Xabier, M., Patel, N., Tian, J. H.et al. . 2020. NVX-CoV2373 vaccine protects cynomolgus macaque upper and lower airways against SARS-CoV-2 challenge. Vaccine 38:7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dai, L., Zheng, T., Xu, K.et al. . 2020. A universal design of betacoronavirus vaccines against COVID-19, MERS, and SARS. Cell 182:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richmond, P., Hatchuel, L., Dong, M.et al. . 2021. Safety and immunogenicity of S-trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet 397:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tan, T. K., Rijal, P., Rahikainen, R.et al. . 2021. A COVID-19 vaccine candidate using SpyCatcher multimerization of the SARS-CoV-2 spike protein receptor-binding domain induces potent neutralising antibody responses. Nat. Commun. 12:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang, Y., Shi, W., Abiona, O. M.et al. . 2021. Newcastle disease virus-like particles displaying prefusion-stabilized SARS-CoV-2 spikes elicit potent neutralizing responses. Vaccines 9:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feng, L., Wang, Q., Shan, C.et al. . 2020. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat. Commun. 11:4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mercado, N. B., Zahn, R., Wegmann, F.et al. . 2020. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 586:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Doremalen, N., Lambe, T., Spencer, A.et al. . 2020. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 586:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Logunov, D. Y., Dolzhikova, I. V., Zubkova, O. V.et al. . 2020. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet 396:887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hassan, A. O., Kafai, N. M., Dmitriev, I. P.et al. . 2020. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell 183:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Case, J. B., Rothlauf, P. W., Chen, R. E.et al. . 2020. Replication-competent vesicular stomatitis virus vaccine vector protects against SARS-CoV-2-mediated pathogenesis in mice. Cell Host Microbe 28:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu, F. C., Li, Y. H., Guan, X. H.et al. . 2020. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 395:1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laczkó, D., Hogan, M. J., Toulmin, S. A.et al. . 2020. A single immunization with nucleoside-modified mRNA vaccines elicits strong cellular and humoral immune responses against SARS-CoV-2 in mice. Immunity 53:724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zeng, C., Hou, X., Yan, J.et al. . 2020. Leveraging mRNA sequences and nanoparticles to deliver SARS-CoV-2 antigens in vivo. Adv. Mater. 32:e2004452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walsh, E. E., Frenck, R. W.Jr, Falsey, A. R.et al. . 2020. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N. Engl. J. Med. 383:2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Corbett, K. S., Edwards, D. K., Leist, S. R.et al. . 2020. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 586:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Corbett, K. S., Flynn, B., Foulds, K. E.et al. . 2020. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N. Engl. J. Med. 383:1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Erasmus, J. H., Khandhar, A. P., O’Connor, M. A.et al. . 2020. An alphavirus-derived replicon RNA vaccine induces SARS-CoV-2 neutralizing antibody and T cell responses in mice and nonhuman primates. Sci. Transl. Med. 12:eabc9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McKay, P. F., Hu, K., Blakney, A. K.et al. . 2020. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat. Commun. 11:3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang, N. N., Li, X. F., Deng, Y. Q.et al. . 2020. A thermostable mRNA vaccine against COVID-19. Cell 182:1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith, T. R. F., Patel, A., Ramos, S.et al. . 2020. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 11:2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu, J., Tostanoski, L. H., Peter, L.et al. . 2020. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 369:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cao, Y. and Gao, G. F. 2021. mRNA vaccines: a matter of delivery. EClinicalMedicine 32:100746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang, Q., Zhang, Y., Wu, L.et al. . 2020. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell 181:894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peng, R., Wu, L. A., Wang, Q.et al. . 2021. Cell entry by SARS-CoV-2. Trends Biochem. Sci., in press. doi: 10.1016/j.tibs.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Han, Y., Liu, P., Qiu, Y.et al. . 2021. Effective virus-neutralizing activities in antisera from the first wave of severe COVID-19 survivors. JCI Insight 6:e146267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen, Y., Tong, X., Li, Y.et al. . 2020. A comprehensive, longitudinal analysis of humoral responses specific to four recombinant antigens of SARS-CoV-2 in severe and non-severe COVID-19 patients. PLoS Pathog. 16:e1008796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gottlieb, R. L., Nirula, A., Chen, P.et al. . 2021. Effect of Bamlanivimab as monotherapy or in combination with Etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. J. Am. Med. Assoc. 325:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ju, B., Zhang, Q., Ge, J.et al. . 2020. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 584:115. [DOI] [PubMed] [Google Scholar]
- 41. Shi, R., Shan, C., Duan, X.et al. . 2020. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 584:120. [DOI] [PubMed] [Google Scholar]
- 42. Liu, L., Wang, P., Nair, M. S.et al. . 2020. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature 584:450. [DOI] [PubMed] [Google Scholar]
- 43. Sadoff, J., Le Gars, M., Shukarev, G.et al. . 2021. Interim results of a phase 1–2a trial of Ad26.COV2.S COVID-19 vaccine. N. Engl. J. Med. 384:1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jackson, L. A., Anderson, E. J., Rouphael, N. G.et al. ; mRNA-1273 Study Group. 2020. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. 383:1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Keech, C., Albert, G., Cho, I.et al. . 2020. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Engl. J. Med. 383:2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Earle, K. A., Ambrosino, D. M., Fiore-Gartland, A.et al. . 2021. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 39:4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. World Health Organization. Main Outcomes of the Meeting of the WHO Expert Committee on Biological Standardization Held on 9 and 10 December 2020. Available at: https://cdn.who.int/media/docs/default-source/biologicals/ecbs/ecbs-executive-summary.if.ik.tw-15_dec_2020.pdf?sfvrsn=d59854b2_3&download=true.
- 48. Anderson, E. J., Rouphael, N. G., Widge, A. T.et al. ; mRNA-1273 Study Group. 2020. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 383:2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sahin, U., Muik, A., Derhovanessian, E.et al. . 2020. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 586:594. [DOI] [PubMed] [Google Scholar]
- 50. Mulligan, M. J., Lyke, K. E., Kitchin, N.et al. . 2020. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 586:589. [DOI] [PubMed] [Google Scholar]
- 51. Ebinger, J. E., Fert-Bober, J., Printsev, I.et al. . 2021. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 27:981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Manisty, C., Otter, A. D., Treibel, T. A.et al. . 2021. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet 397:1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Prendecki, M., Clarke, C., Brown, J.et al. . 2021. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet 397:1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weiss, P. and Murdoch, D. R. 2020. Clinical course and mortality risk of severe COVID-19. Lancet 395:1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ye, G., Pan, Z., Pan, Y.et al. . 2020. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J. Infect. 80:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li, J., Hui, A., Zhang, X.et al. . 2021. Safety and immunogenicity of the SARS-CoV-2 BNT162b1 mRNA vaccine in younger and older Chinese adults: a randomized, placebo-controlled, double-blind phase 1 study. Nat. Med. 27:1062. [DOI] [PubMed] [Google Scholar]
- 57. Chu, L., McPhee, R., Huang, W.et al. ; mRNA-1273 Study Group. 2021. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine 39:2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Widge, A. T., Rouphael, N. G., Jackson, L. A.et al. ; mRNA-1273 Study Group. 2021. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N. Engl. J. Med. 384:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang, Z., Schmidt, F., Weisblum, Y.et al. . 2021. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 592:616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tebas, P., Yang, S., Boyer, J. D.et al. . 2021. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of an open-label, Phase 1 clinical trial. EClinicalMedicine 31:100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Barrett, J. R., Belij-Rammerstorfer, S., Dold, C.et al. ; Oxford COVID Vaccine Trial Group. 2021. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. Med. 27:279. [DOI] [PubMed] [Google Scholar]
- 62. Folegatti, P. M., Ewer, K. J., Aley, P. K.et al. ; Oxford COVID Vaccine Trial Group. 2020. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 396:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ewer, K. J., Barrett, J. R., Belij-Rammerstorfer, S.et al. ; Oxford COVID Vaccine Trial Group. 2021. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 27:270. [DOI] [PubMed] [Google Scholar]
- 64. Ramasamy, M. N., Minassian, A. M., Ewer, K. J.et al. ; Oxford COVID Vaccine Trial Group. 2020. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 396:1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stephenson, K. E., Le Gars, M., Sadoff, J.et al. . 2021. Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. J. Am. Med. Assoc. 325:1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhu, F. C., Guan, X. H., Li, Y. H.et al. . 2020. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 396:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yang, S., Li, Y., Dai, L., et al. . 2021. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, Phase 1 and 2 trials. Lancet Infect. Dis. 21:1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Goepfert, P. A., Fu, B., Chabanon, A.-L.et al. . 2021. Safety and immunogenicity of SARS-CoV-2 recombinant protein vaccine formulations in healthy adults: interim results of a randomised, placebo-controlled, phase 1–2, dose-ranging study. Lancet Infect. Dis. 21:1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ward, B. J., Gobeil, P., Séguin, A.et al. . 2021. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat. Med. 27:1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang, Y., Zeng, G., Pan, H.et al. . 2021. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase ½ clinical trial. Lancet Infect. Dis. 21:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wu, Z., Hu, Y., Xu, M.et al. . 2021. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase ½ clinical trial. Lancet Infect. Dis. 21:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xia, S., Zhang, Y., Wang, Y.et al. . 2021. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 21:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pu, J., Yu, Q., Yin, Z.et al. . 2021. The safety and immunogenicity of an inactivated SARS-CoV-2 vaccine in Chinese adults aged 18–59 years: a phase I randomized, double-blinded, controlled trial. Vaccine 39:2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pan, H. X., Liu, J. K., Huang, B. Y.et al. . 2021. Immunogenicity and safety of a severe acute respiratory syndrome coronavirus 2 inactivated vaccine in healthy adults: randomized, double-blind, and placebo-controlled phase 1 and phase 2 clinical trials. Chin. Med. J. (Engl.) 134:1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ella, R., Reddy, S., Jogdand, H.et al. . 2021. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect. Dis. 21:950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang, X., Guo, X., Xin, Q., et al. . 2020. Neutralizing antibodies responses to SARS-CoV-2 in COVID-19 inpatients and convalescent patients. Clin. Infect. Dis. 71:2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Robbiani, D. F., Gaebler, C., Muecksch, F.et al. . 2020. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 584:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lynch, K. L., Whitman, J. D., Lacanienta, N. P.et al. . 2020. Magnitude and kinetics of anti-SARS-CoV-2 antibody responses and their relationship to disease severity. Clin. Infect. Dis. 71:2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Korber, B., Fischer, W. M., Gnanakaran, S.et al. ; Sheffield COVID-19 Genomics Group. 2020. Tracking changes in SARS-CoV-2 Spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell 182:812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hou, Y. J., Chiba, S., Halfmann, P.et al. . 2020. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science 370:1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ozono, S., Zhang, Y., Ode, H.et al. . 2021. SARS-CoV-2 D614G spike mutation increases entry efficiency with enhanced ACE2-binding affinity. Nat. Commun. 12:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Weissman, D., Alameh, M. G., de Silva, T.et al. . 2021. D614G spike mutation increases SARS CoV-2 susceptibility to neutralization. Cell Host Microbe. 29:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhou, D., Dejnirattisai, W., Supasa, P.et al. . 2021. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell 184:2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fujino, T., Nomoto, H., Kutsuna, S.et al. . 2021. Novel SARS-CoV-2 variant in travelers from Brazil to Japan. Emerg. Infect. Dis. 27:1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Muik, A., Wallisch, A. K., Sänger, B.et al. . 2021. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science 371:1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tegally, H., Wilkinson, E., Lessells, R. J.et al. . 2021. Sixteen novel lineages of SARS-CoV-2 in South Africa. Nat. Med. 27:440. [DOI] [PubMed] [Google Scholar]
- 87. Chen, R. E., Zhang, X., Case, J. B.et al. . 2021. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat. Med. 27:717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Collier, D. A., De Marco, A., Ferreira, I.et al. . 2021. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature 593:136. [DOI] [PubMed] [Google Scholar]
- 89. Shen, X., Tang, H., McDanal, C.et al. . 2021. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe 29:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wu, K., Werner, A. P., Koch, M.et al. . 2021. Serum neutralizing activity elicited by mRNA-1273 vaccine. N. Engl. J. Med. 384:1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wang, P., Nair, M. S., Liu, L.et al. . 2021. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 593:130. [DOI] [PubMed] [Google Scholar]
- 92. Wang, G. L., Wang, Z. Y., Duan, L. J.et al. . 2021. Susceptibility of circulating SARS-CoV-2 variants to neutralization. N. Engl. J. Med. 384:2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sapkal, G. N., Yadav, P. D., Ella, R.et al. . 2021. Inactivated COVID-19 vaccine BBV152/COVAXIN effectively neutralizes recently emerged B.1.1.7 variant of SARS-CoV-2. J. Travel Med. 28:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Shen, X., Tang, H., Pajon, R.et al. . 2021. Neutralization of SARS-CoV-2 variants B.1.429 and B.1.351. N. Engl. J. Med. 384:2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Huang, B., Dai, L., Wang, H.et al. . 2021. Serum sample neutralisation of BBIBP-CorV and ZF2001 vaccines to SARS-CoV-2 501Y.V2. Lancet Microbe 2:e285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Garcia-Beltran, W. F., Lam, E. C., St Denis, K.et al. . 2021. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 184:2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Liu, W. J., Zhao, M., Liu, K.et al. . 2017. T-cell immunity of SARS-CoV: implications for vaccine development against MERS-CoV. Antiviral Res. 137:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cox, R. J. and Brokstad, K. A. 2020. Not just antibodies: B cells and T cells mediate immunity to COVID-19. Nat. Rev. Immunol. 20:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Peng, Y., Mentzer, A. J., Liu, G.et al. ; Oxford Immunology Network COVID-19 Response T Cell Consortium; ISARIC4C Investigators. 2020. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 21:1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Braun, J., Loyal, L., Frentsch, M.et al. . 2020. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature 587:270. [DOI] [PubMed] [Google Scholar]
- 101. Grifoni, A., Weiskopf, D., Ramirez, S. I.et al. . 2020. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 181:1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sekine, T., Perez-Potti, A., Rivera-Ballesteros, O.et al. ; Karolinska COVID-19 Study Group. 2020. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 183:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Crotty, S. 2011. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29:621. [DOI] [PubMed] [Google Scholar]
- 104. Zhu, J. 2015. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 75:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bolles, M., Deming, D., Long, K.et al. . 2011. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J. Virol. 85:12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Agrawal, A. S., Tao, X., Algaissi, A.et al. . 2016. Immunization with inactivated Middle East respiratory syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum. Vaccin. Immunother. 12:2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yasui, F., Kai, C., Kitabatake, M.et al. . 2008. Prior immunization with severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS-CoV. J. Immunol. 181:6337. [DOI] [PubMed] [Google Scholar]
- 108. Iwata-Yoshikawa, N., Uda, A., Suzuki, T.et al. . 2014. Effects of Toll-like receptor stimulation on eosinophilic infiltration in lungs of BALB/c mice immunized with UV-inactivated severe acute respiratory syndrome-related coronavirus vaccine. J. Virol. 88:8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sahin, U., Muik, A., Vogler, I.et al. . 2021. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 595:572. [DOI] [PubMed] [Google Scholar]
- 110. Baden, L. R., El Sahly, H. M., Essink, B.et al. ; COVE Study Group. 2021. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Polack, F. P., Thomas, S. J., Kitchin, N.et al. ; C4591001 Clinical Trial Group. 2020. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 383:2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Schnare, M., Barton, G. M., Holt, A. C., Takeda, K., Akira, S. and Medzhitov, R. 2001. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2:947. [DOI] [PubMed] [Google Scholar]
- 113. Voysey, M., Clemens, S. A. C., Madhi, S. A.et al. ; Oxford COVID Vaccine Trial Group. 2021. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Voysey, M., Costa Clemens, S. A., Madhi, S. A.et al. ; Oxford COVID Vaccine Trial Group. 2021. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 397:881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Logunov, D. Y., Dolzhikova, I. V., Shcheblyakov, D. V.et al. ; Gam-COVID-Vac Vaccine Trial Group. 2021. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 397:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sadoff, J., Gray, G., Vandebosch, A.et al. ; ENSEMBLE Study Group. 2021. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N. Engl. J. Med. 384:2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Heath, P. T., Galiza, E. P., Baxter, D. N.et al. . 2021. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine. N. Engl. J. Med., in press. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Jara, A., Undurraga, E. A., Gonzalez, C.et al. . 2021. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N. Engl. J. Med. 385:875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tanriover, M. D., Doğanay, H. L., Akova, M.et al. ; CoronaVac Study Group. 2021. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 398:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Al Kaabi, N., Zhang, Y., Xia, S.et al. . 2021. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. J. Am. Med. Assoc. 326:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Levine-Tiefenbrun, M., Yelin, I., Katz, R.et al. . 2021. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat. Med. 27:790. [DOI] [PubMed] [Google Scholar]
- 122. Abu-Raddad, L. J., Chemaitelly, H. and Butt, A. A.; National Study Group for COVID-19 Vaccination . 2021. Effectiveness of the BNT162b2 COVID-19 vaccine against the B.1.1.7 and B.1.351 variants. N. Engl. J. Med. 385:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Emary, K. R. W., Golubchik, T., Aley, P. K.et al. ; COVID-19 Genomics UK consortium; AMPHEUS Project; Oxford COVID-19 Vaccine Trial Group. 2021. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet 397:1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Madhi, S. A., Baillie, V., Cutland, C. L.et al. ; NGS-SA Group; Wits-VIDA COVID Group. 2021. Efficacy of the ChAdOx1 nCoV-19 COVID-19 vaccine against the B.1.351 variant. N. Engl. J. Med. 384:1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Shinde, V., Bhikha, S., Hoosain, Z.et al. ; 2019nCoV-501 Study Group. 2021. Efficacy of NVX-CoV2373 COVID-19 vaccine against the B.1.351 variant. N. Engl. J. Med. 384:1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. CanSinoBIO. Convidecia – Single shot for faster protection. Available at: http://www.cansinotech.com/html/1//156/218/index.html.
- 127. Shimabukuro, T. T., Cole, M. and Su, J. R. 2021. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US-December 14, 2020-January 18, 2021. J. Am. Med. Assoc. 325:1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Vrieze, J. D. 2020. Suspicions grow that nanoparticles in Pfizer’s COVID-19 vaccine trigger rare allergic reactions. Science. Available at: https://www.sciencemag.org/news/2020/12/suspicions-grow-nanoparticles-pfizer-s-covid-19-vaccine-trigger-rare-allergic-reactions. [Google Scholar]
- 129. Moghimi, S. M. 2021. Allergic reactions and anaphylaxis to LNP-based COVID-19 vaccines. Mol. Ther. 29:898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Klimek, L., Novak, N., Cabanillas, B.et al. . 2021. Allergenic components of the mRNA-1273 vaccine for COVID-19: possible involvement of polyethylene glycol and IgG-mediated complement activation. Allergy, in press. doi: 10.1111/all.14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. CDC COVID-19 Response Team, Food and Drug Administration. 2021. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine—United States, December 21, 2020-January 10, 2021. MMWR Morb. Mortal. Wkly Rep. 70:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kam, Y. W., Kien, F., Roberts, A.et al. . 2007. Antibodies against trimeric S glycoprotein protect hamsters against SARS-CoV challenge despite their capacity to mediate FcgammaRII-dependent entry into B cells in vitro. Vaccine 25:729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yip, M. S., Leung, N. H., Cheung, C. Y.et al. . 2014. Antibody-dependent infection of human macrophages by severe acute respiratory syndrome coronavirus. Virol. J. 11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Arvin, A. M., Fink, K., Schmid, M. A.et al. . 2020. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature 584:353. [DOI] [PubMed] [Google Scholar]
- 135. Lee, W. S., Wheatley, A. K., Kent, S. J.et al. . 2020. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat. Microbiol. 5:1185. [DOI] [PubMed] [Google Scholar]