Supplemental Digital Content is available in the text.
Key Words: PD-1, PD-L1, immune checkpoint inhibitors, body mass index, survival
Abstract
Despite that immune checkpoint inhibitors (ICIs) had tremendous improved the survival of multiple solid tumors, only a limited proportion of patients are responsive to ICIs. Therefore, effective variables are urgently needed to predict the probability of response to ICIs. Systematic searches were conducted from inception up to May, 2020. Prospective or retrospective studies of ICIs that investigated the association between body mass index (BMI) and survival outcomes, including overall survival (OS) and/or progression-free survival (PFS), were selected. The association between each BMI category and survival outcomes was calculated using Cox proportional hazard regression models and quantified as hazard ratio (HR) with corresponding 95% confidence interval. Seven clinical studies involving data from 3768 individual patients were included. The median OS was 15.5 months (95% confidence interval: 14.7–16.2 mo) and the median PFS was 5.7 months (5.2–6.3 mo). The median OS was significantly longer in overweight/obese patients than in nonoverweight patients (20.7 vs. 11.3 mo; P<0.001). The difference in OS between overweight and obese patients was not statistically significant (HR: 1.14, P=0.098). Similar results were observed for PFS outcomes. Subgroup analysis demonstrated improved OS in overweight/obese patients with nonsmall-cell lung cancer (HR: 0.81, P=0.002), melanoma (HR: 0.66, P<0.001), renal cell carcinoma (HR: 0.53, P<0.001), and multiple cancer type (HR: 0.34, P<0.001), with parallel results noted regarding PFS outcomes. Results of the present study suggested that BMI may be a satisfactory prognostic factor for patients treated with ICIs.
In the past 2 decades, novel immune checkpoint inhibitors (ICIs) that target cytotoxic T-lymphocyte-associated protein-4 (CTLA4), programmed cell death 1 (PD1), or its ligand 1 (PD-L1), have led to a revolution in the treatment of a large set of cancers.1,2 Although durable responses have been noted in a limited proportion of patients, many others experienced primary or acquired therapeutic resistance.
Two strategies have been attempted to improve the therapeutic efficacy of ICIs, one of which is combinational therapy that incorporates ICIs with chemotherapy, targeted drugs, and other ICIs.3,4 However, such combination strategies are associated with an increased incidence of high-grade adverse events.5 The other strategy is to identify available biomarkers to predict response before the initial treatment with ICIs, such as PD-L1 expression, microsatellite status, and tumor mutational load.6–10 In clinical practice, several clinical and demographic variables may be more easily identified to predict the probability of response to ICIs.11 One such variable is body mass index (BMI).
BMI is the major surrogate of nutritional status, and its associations with the incidence of cancer and the clinical outcomes of cancer patients are complicated. While high BMI has been shown to increase the risk for esophageal, colon, and renal cancers,12 it has been identified as a favorable prognostic factor in several solid cancers, such as nonsmall-cell lung cancer (NSCLC),13 colorectal cancer14 and gastric cancer.15 This phenomenon is referred to as the “obesity paradox.” Furthermore, the prognostic value of high BMI in patients treated with ICIs remains unclear. McQuade et al reported that obesity is associated with prolonged progression-free survival (PFS) and overall survival (OS) in patients with melanoma treated with ICIs, but not in those treated with chemotherapy.16 Similarly, Kichenadasse et al17 recently reported improved survival of patients with NSCLC treated with atezolizumab.
In the present study, we extracted individual patient data from 7 clinical studies comprising 3768 subjects, and conducted a meta-analysis to investigate the association between BMI and survival outcomes in patients treated with ICIs.
METHODS
Search Strategy, Selection Criteria, and Data Extraction
This study complied with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.18 Analysis was performed in May 2020.
The PubMed, Web of Science, Embase, ClinicalTrials.gov, and Cochrane Library databases were searched from their inception to May 10, 2020 for clinical studies on ICIs (anti-PD-1/PD-L1, anti-CTLA4) that reported the association between BMI and survival outcomes. The comprehensive search terms used were as follows: (“nivolumab” OR “pembrolizumab” OR “avelumab” OR “atezolizumab” OR “durvalumab” OR “ipilimumab” OR “PD-1” OR “PD-L1” OR “CTLA4” OR “checkpoint inhibitors”) AND (“body mass index” OR “BMI” OR “overweight” OR “obesity”) (eTable 1 in the Supplement, Supplemental Digital Content 1, http://links.lww.com/JIT/A627). Reviews, conference abstracts, case reports, comments, protocols, and studies that were published as abstracts were excluded. Because the “reconstructKM” package in R was used to reconstruct patient-level data through Kaplan-Meier curves (https://github.com/ryanrsun/reconstructKM), studies that did not report Kaplan-Meier curves and number-at-risk information were excluded. For duplicated studies reporting interim and final analyses or different endpoints, the most recent reports with complete survival data were included. Two authors (R.C.N. and Y.W.) independently searched and reviewed the results to determine whether the studies were included.
For each study, 3 of the authors (R.C.N., Y.W., and S.-Q.Y.) independently extracted individual patient and characteristics data. To extract individual patient data, the following procedures were performed: using Engauge digitizer software to click the location of event times in the Kaplan-Meier plots; manually inputting number-at-risk information; and applying the “reconstructKM” package to reconstruct the survival time, censoring status, and treatment arm for each individual in the included studies. Other extracted data included first author, year of publication, cancer type, study type, treatment regimen, number of patients, BMI category, and survival outcomes, including OS and PFS. All discrepancies regarding study selection, extraction of individual patient data, and other characteristics data were resolved by discussion to reach a consensus among all investigators.
Outcomes
In this study, the primary outcome was OS, and the secondary outcome was PFS. For each study, BMI was assessed at the initiation of treatment and calculated as weight (kg) divided by height (m) squared (ie, kg/m2). BMI was categorized according to the standard World Health Organization criteria definitions: underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2). Initially, a binomial cut-off of 25 kg/m2 for BMI was used, and patients were classified as nonoverweight (<25 kg/m2) and overweight/obese (≥25 kg/m2).
Statistical Analysis
Patient-level data, including survival time and censoring status, were extracted using the “reconstructKM” package through the Kaplan-Meier curves reported in each study. After acquiring patient-level data, survival curves for nonoverweight and overweight/obese patients were estimated using the Kaplan-Meier method. In order to evaluate the possible prognostic effect of obesity, overweight (25–29.9 kg/m2) and obese (≥30 kg/m2) patients were compared with nonoverweight (<25 kg/m2) patients. The association between each category of BMI and survival outcomes (ie, OS and PFS) was calculated using Cox proportional hazard regression models and quantified as a hazard ratio (HR) and corresponding 95% confidence interval (CI). Differences with a 2-sided P<0.05 were considered to be statistically significant. All analyses were performed using R version 3.6.1 (R Foundation for Statistical Computing; http://www.r-project.org).
RESULTS
Study Characteristics
A total of 636 studies were retrieved through the initial search of the databases, of which 371 were selected after duplicate records were removed. After title and abstract reviews, 21 studies were retained for full-text review, among which 7 were removed because of a lack of reporting of survival outcomes, 1 was excluded owing to a further report of the same cohort, and 6 were excluded because of no report of Kaplan-Meier curves and/or number-at-risk information. Because the majority of the included studies excluded underweight patients because of its low prevalence (<5%), the underweight cohort in the study by Ichihara et al19 was excluded. Ultimately, 7 studies16,17,19–23 comprising 3768 subjects were included for reconstruction of individual-level data and meta-analyses (eFig. 1 in the Supplement, Supplemental Digital Content 2, http://links.lww.com/JIT/A628). These investigations comprised 5 retrospective and 2 prospective studies addressing ipilimumab, pembrolizumab, nivolumab, and atezolizumab. They involved patients with NSCLC (2 studies), melanoma (2 studies), renal cell carcinoma (2 studies), and multiple solid cancers (1 study) (eTable 2 in the Supplement, Supplemental Digital Content 1, http://links.lww.com/JIT/A627).
BMI and Efficacy Analysis
With a median follow-up duration of 9.4 months (range: 0.1–50.0 mo) in the entire cohort, the median OS was 15.5 months (95% CI: 14.7–16.2 mo) and the median PFS was 5.7 months (95% CI: 5.2–6.3 mo). Survival outcomes were first analyzed according to BMI dichotomized using a cut-off of 25 kg/m2. The median OS was significantly longer in overweight/obese patients than in nonoverweight patients [20.7 mo (95% CI: 19.3–23.3) vs. 11.3 mo (95% CI: 10.4–12.8); HR: 0.59 (95% CI: 0.53–0.64); P<0.001] (Fig. 1A). Regarding PFS outcomes, a total of 2043 patients were included in the entire cohort, and a similarly prolonged PFS was observed in overweight/obese patients [median PFS 8.3 (95% CI: 7.3–10.1) vs. 3.7 (95% CI: 3.4–4.3) months; HR: 0.58 (95% CI: 0.53–0.65); P<0.001] (Fig. 1B).
FIGURE 1.
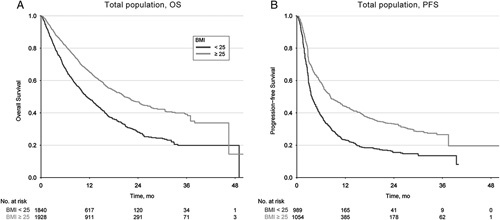
Overall survival and progression-free survival for total population treated with immune checkpoint inhibitors according to binomial BMI levels (Cut-off 25). BMI indicates body mass index; OS, overall survival; PFS, progression-free survival.
Next, the survival outcomes according to the 3 BMI categories (ie, 18.5–24.9, 25.0–29.9, and ≥30.0 kg/m2) were analyzed. Cohorts in the studies by De Giorigi et al21 and Naik et al23 were excluded because these BMI categories were not reported; thus, 3339 subjects were included. Overweight (HR: 0.62; 95% CI: 0.55–0.68; P<0.001) and obese (HR: 0.54; 95% CI: 0.47–0.62; P<0.001) patients experienced significantly longer OS than normal weight patients (eFig. 2A in the Supplement, Supplemental Digital Content 3, http://links.lww.com/JIT/A629). Notably, the difference in OS between overweight and obese patients was not significant (HR: 1.14; 95% CI: 0.98–1.33; P=0.098). Similar results were observed regarding PFS outcomes (eFig. 2B in the Supplement, Supplemental Digital Content 3, http://links.lww.com/JIT/A629). The OS and PFS results according to the different BMI categories are shown in Figure 2.
FIGURE 2.
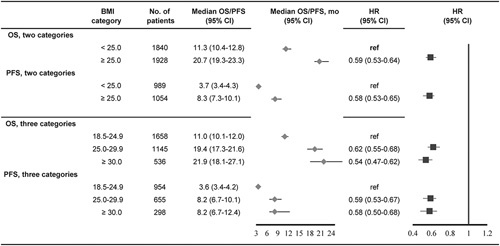
Summary results of overall survival and progression-free survival for total population treated with immune checkpoint inhibitors according to different BMI categories. BMI indicates body mass index; CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
Subgroup Analysis
Finally, subgroup analysis stratified according to cancer type was performed to assess the robustness of BMI as a prognostic marker for immunotherapy. As shown in eFigure 3 in the Supplement (Supplemental Digital Content 4, http://links.lww.com/JIT/A630), overweight/obesity was associated with improved OS compared with nonoverweight in the NSCLC (HR: 0.81; 95% CI: 0.71–0.92; P=0.002), melanoma (HR: 0.66; 95% CI: 0.53–0.81; P<0.001), renal cell carcinoma (HR: 0.53; 95% CI: 0.37–0.77; P<0.001), and multiple cancer cohorts (HR: 0.34; 95% CI: 0.28–0.41; P<0.001). Parallel results were noted with regard to PFS outcomes (eFig. 4 in the Supplement, Supplemental Digital Content 5, http://links.lww.com/JIT/A631).
DISCUSSION
To the best of our knowledge, the present study, which pooled individual patient data from 3768 subjects, is the largest investigation of the association between BMI and survival outcomes in patients treated with ICIs. Overall, our findings demonstrated that high BMI (ie, overweigh/obese) patients treated with ICIs experienced improved PFS and OS outcomes compared with those with normal BMI, regardless of the tumor type. Furthermore, we noted that the risk for death and progression did not decrease if BMI increased to ≥30 kg/m2.
Despite the tremendous improvements in survival in a wide range of metastatic solid tumors treated with ICIs, only a relatively limited proportion of patients (20%–40%) are responsive to these agents.4,24 Effective biomarkers are urgently needed to distinguish potential beneficiaries before the initial use of ICIs, among which BMI is a simple and practical characteristic. BMI has been recognized as a surrogate of nutritional status, with the BMI values ranging from 25 to 29.9 kg/m2 defined as overweight, and >30 kg/m2 as obesity. This study pooled individual patient data from 7 studies, and adds supporting evidence that high BMI is associated with survival following treatment with ICIs. Furthermore, subgroup analysis demonstrated that this improved survival in the high BMI group was observed regardless of the tumor type. The basic biological process of this association remains under investigation. High BMI would increase autophagy levels that suppresses the tumor growth.25,26 It is also possible that high BMI may induce obesity-associated inflammation,27 which can lower epigenetic barriers to tumorigenesis and improve the response to ICIs. Wang et al28 reported that obesity may induce PD-1 positive and dysfunctional T cells through leptin signaling; this immunosuppression phenomenon can promote tumor progression and the remaining tumors are markedly more responsive to ICIs. Recently, Liu et al29 reported that inhibition of PCSK9, a key protein to regulate cholesterol levels, can attenuate the tumor growth and promote the response to ICIs. Thus, whether inhibiting of PCSK9 and PD-1 can improve the therapeutic effect for obesity patients should be further explored.
Previous studies have reported inconsistent results regarding whether the risk for death and progression is further decreased as BMI increases to ≥30 kg/m2 or even ≥35 kg/m2.16,17,19,20,22,23 While Kichenadasse et al17 and Labadie et al22 reported a trend toward improved survival in obese patients compared with overweight patients, other studies reported similar survival outcomes between these 2 groups.16,19,20 Naik et al23 even reported that patients with BMI ≥35 kg/m2 had shorter OS and PFS than those with BMI >25 kg/m2 and <35 kg/m2. Pooling a total of 3339 patients to strengthen statistical power, our study demonstrated that differences in OS (HR: 1.14, P=0.098) and PFS (HR: 1.02, P=0.802) between overweight and obese patients were not significant, indicating that the risk for death and progression did not decrease if BMI increased to ≥30 kg/m2. We considered that the risk for death caused by cardiovascular and cerebrovascular diseases will increase as BMI increases to ≥30 kg/m2, which would weaken the benefits of ICIs. As such, the endpoint of disease-specific survival should be used rather than OS or PFS to minimize the effect caused by noncancer deaths and to better clarify the dose response according to BMI.
Our study had some limitations. First, based on the inclusion and exclusion criteria, we excluded studies that did not report Kaplan-Meier curves and number-at-risk information, which may have introduced some publication bias. Next, given that the baseline characteristics, included tumor types and ICI regimens were different, potential heterogeneity should be acknowledged. Moreover, because we used the “reconstructKM” package to reconstruct the patient-level data of the included studies, a subgroup analysis stratified according to sex and other characteristics could not be performed. In addition, our study lacks the ability to apply the multivariate analysis to adjust the effect of other variables, such as PD-L1 status and performance status. Also, further study should be performed to confounding effect of systemic inflammatory immune index, and other metabolic measures, such as metformin or usage of cholesterol lowering drugs. Finally, we only used one-time rather than dynamic records of BMI to evaluate its prognostic value, which would limit its clinical application. Given the above limitations, our findings should be interpreted cautiously.
Pooling individual patient data from 3768 subjects, our study further confirmed that BMI may be a satisfactory prognostic factor for patients treated with ICIs.
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
None reported. All authors have declared that there are no financial conflicts of interest with regard to this work.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.immunotherapy-journal.com.
Footnotes
R.-C.N., G.-M.C., Y.W., and S.-Q.Y. contributed equally to this study.
S.C., M.-Y.C., and Y.-F.L. authors are co-senior authors.
All authors contributed to the study concept and design. R.-C.N. and S.-Q.Y. extracted the data and performed the quality assessment. R.-C.N., Y.W., J.-L.D., W.-W.L., and S.-Q.Y. participated in analysis of the data. R.-C.N., Y.W., G.-M.C., and J.Z. contributed to drafting of the manuscript. S.C., M.-Y.C., and Y.-F.L. revised the manuscript. All authors approved the final version of the manuscript.
Contributor Information
Run-Cong Nie, Email: nierc@sysucc.org.cn.
Guo-Ming Chen, Email: chengm@sysucc.org.cn.
Yun Wang, Email: wangyun@sysucc.org.cn.
Shu-Qiang Yuan, Email: yuanshq@sysucc.org.cn.
Jie Zhou, Email: zhoujie2@sysucc.org.cn.
Jin-Ling Duan, Email: duanjl@sysucc.org.cn.
Wen-Wu Liu, Email: liuww1@sysucc.org.cn.
Shi Chen, Email: chensh47@mail.sysu.edu.cn.
Mu-Yan Cai, Email: caimy@sysucc.org.cn.
Yuan-Fang Li, Email: liyuanf@sysucc.org.cn.
REFERENCES
- 1. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol. 2018;29:84–91. [DOI] [PubMed] [Google Scholar]
- 4. Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175:313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nie RC, Zhao CB, Xia XW, et al. The efficacy and safety of PD-1/PD-L1 inhibitors in combination with conventional therapies for advanced solid tumors: a meta-analysis. Biomed Res Int. 2020;2020:5059079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cristescu R, Mogg R, Ayers M, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362:eaar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luksza M, Riaz N, Makarov V, et al. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature. 2017;551:517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hugo W, Zaretsky JM, Sun L, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hopkins AM, Rowland A, Kichenadasse G, et al. Predicting response and toxicity to immune checkpoint inhibitors using routinely available blood and clinical markers. Br J Cancer. 2017;117:913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. [DOI] [PubMed] [Google Scholar]
- 13. Sepesi B, Gold KA, Correa AM, et al. The influence of body mass index on overall survival following surgical resection of non–small cell lung cancer. J Thorac Oncol. 2017;12:1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caan BJ, Meyerhardt JA, Kroenke CH, et al. Explaining the obesity paradox: the association between Body Composition and Colorectal Cancer Survival (C-SCANS Study). Cancer Epidemiol Biomarkers Prev. 2017;26:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park SH, Lee S, Song JH, et al. Prognostic significance of body mass index and prognostic nutritional index in stage II/III gastric cancer. Eur J Surg Oncol. 2020;46:620–625. [DOI] [PubMed] [Google Scholar]
- 16. McQuade JL, Daniel CR, Hess KR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19:310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kichenadasse G, Miners JO, Mangoni AA, et al. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol. 2020;6:512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ichihara E, Harada D, Inoue K, et al. The impact of body mass index on the efficacy of anti-PD-1/PD-L1 antibodies in patients with non-small cell lung cancer. Lung Cancer. 2020;139:140–145. [DOI] [PubMed] [Google Scholar]
- 20. Cortellini A, Bersanelli M, Buti S, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. 2019;7:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Giorgi U, Procopio G, Giannarelli D, et al. Association of systemic inflammation index and body mass index with survival in patients with renal cell cancer treated with nivolumab. Clin Cancer Res. 2019;25:3839–3846. [DOI] [PubMed] [Google Scholar]
- 22. Labadie BW, Liu P, Bao RY, et al. BMI, irAE, and gene expression signatures associate with resistance to immune-checkpoint inhibition and outcomes in renal cell carcinoma. J Transl Med. 2019;17:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Naik GS, Waikar SS, Johnson AEW, et al. Complex inter-relationship of body mass index, gender and serum creatinine on survival: exploring the obesity paradox in melanoma patients treated with checkpoint inhibition. J Immunother Cancer. 2019;7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. [DOI] [PubMed] [Google Scholar]
- 25. Martinez-Useros J, Garcia-Foncillas J. Obesity and colorectal cancer: molecular features of adipose tissue. J Transl Med. 2016;14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Y, Sowers JR, Ren J. Targeting autophagy in obesity: from pathophysiology to management. Nat Rev Endocrinol. 2018;14:356–376. [DOI] [PubMed] [Google Scholar]
- 27. Olson OC, Quail DF, Joyce JA. Obesity and the tumor microenvironment. Science. 2017;358:1130–1131. [DOI] [PubMed] [Google Scholar]
- 28. Wang ZM, Aguilar EG, Luna JI, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25:141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu X, Bao X, Hu M, et al. Inhibition of PCSK9 potentiates immune checkpoint therapy for cancer. Nature. 2020;588:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.immunotherapy-journal.com.


