Supplemental Digital Content is available in the text.
Key Words: nonalcoholic fatty liver disease, comorbidity, health services research, cost of illness
Goals:
This study evaluates the real-world comorbidity burden, health care resource utilization (HRU), and costs among nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH) patients with advanced liver diseases [compensated cirrhosis (CC), decompensated cirrhosis (DCC), liver transplantation (LT), hepatocellular carcinoma (HCC)].
Background:
NAFLD/NASH is a leading cause of liver diseases.
Materials and Methods:
Adult NAFLD/NASH patients were identified retrospectively from MarketScan Commercial claims (2006-2016). Following initial NAFLD/NASH diagnosis, advanced liver diseases were identified using the first diagnosis as their index date. Mean annual all-cause HRU and costs (2016 USD) were reported. Adjusted costs were estimated through generalized linear models. Cumulative costs were illustrated for patient subsets with variable follow-up for each stage.
Results:
Within the database, 485,774 NAFLD/NASH patients met eligibility criteria. Of these, 93.4% (453,564) were NAFLD/NASH patients without advanced liver diseases, 1.6% (7665) with CC, 3.3% (15,833) with DCC, 0.1% (696) with LT, and 0.1% (428) with HCC. Comorbidity burden was high and increased as patients progressed through liver disease severity stages. Compared with NAFLD/NASH without advanced liver diseases (adjusted costs: $23,860), the annual cost of CC, DCC, LT, and HCC were 1.22, 5.64, 8.27, and 4.09 times higher [adjusted costs: $29,078, $134,448, $197,392, and $97,563 (P<0.0001)]. Inpatient admissions significantly drove increasing HRU.
Conclusion:
Study findings suggest the need for early identification and effective management of NAFLD/NASH patients to minimize comorbidity burden, HRU, and costs in the privately insured US population.
Nonalcoholic fatty liver disease (NAFLD) is a leading cause of chronic liver disease that affects more than one third of the adults in the United States.1 Nonalcoholic steatohepatitis (NASH) which represents 10% to 20% of NAFLD patients,2 is one of the subtypes of NAFLD with substantial risk of progression to advanced fibrosis [bridging fibrosis (F3) and compensated cirrhosis (CC, F4)] and liver-related mortality. In addition, NASH is associated with an increased risk of developing advanced liver diseases including CC, decompensated cirrhosis (DCC), hepatocellular carcinoma (HCC), and liver transplantation (LT).3,4 NASH remains largely underdiagnosed due to lack of awareness, deficiency of noninvasive tests, and patients being asymptomatic until development of liver-related complications.5 Liver biopsy has long been the standard method for fibrosis staging.6,7 However, the invasiveness, cost, and the potential risks associated with the procedure makes it practically infeasible to be implemented at a population level.8
There is no marketed product available for the treatment of NASH. Most of the nonpharmacological (diet, lifestyle changes, vitamin E supplementation) and pharmacological (insulin sensitizing agents, lipid-lowering agents, antioxidants) interventions recommended by clinical practice guidelines are focused on managing underlying/associated risk factors of NASH.9–11 Surgical interventions (bariatric surgery, LT) are considered when lifestyle/diet modifications and active drugs are no longer effective or do not result in significant improvements, or when NASH has progressed to end-stage liver disease requiring LT.12,13
With an estimated prevalence of nearly 80 to 100 million, NAFLD differs from other common liver diseases due to the sheer volume of patients and thus, has the potential to exert significant impact on the health care resource utilization (HRU) and health care costs.4,14–16 Several studies have reported cost data (direct medical, societal, nonmedical) for patients with NASH.2,17–22 However, previous studies used cost estimates for managing other liver diseases as a proxy for disease management costs in NASH patients. Additional methodological limitations include use of older classification of diagnosis codes for advanced liver diseases and small sample size analyses.17,18,22 In a steady-state prevalence modeling study, the burden of NAFLD was estimated to be $1613 per patient per year in the United States, resulting in a total of $103 billion direct medical costs per year.2 The lifetime costs incurred by all NASH patients and those with advanced NASH (in 2017 alone) were estimated to be $223 billion and $95.4 billion, respectively.23
Certain factors such as obesity, type 2 diabetes mellitus (T2DM), dyslipidemia, and cardiovascular disease (CVD) have been associated or reported to accelerate the progression of NAFLD to NASH, HCC, and liver-related mortality.24–27 Although the clinical significance of these comorbidities has been well-established, few data were published on the economic burden posed by these comorbidities.28 There are limited studies to date which have comprehensively evaluated the economic burden associated with comorbidities in NASH and the costs of managing liver-related outcomes of NASH in a real-world setting using large databases. Thus, the specific objectives of this study were to examine the comorbidities, annual all-cause HRU and health care costs among NASH patients with advanced liver diseases, to evaluate the impact of comorbid T2DM, CVD, or renal impairment (RI) on the annual all-cause HRU and health care costs, and to evaluate the cumulative all-cause health care costs from 2 years before the initial NAFLD/NASH or advanced liver diseases diagnosis through subsequent yearly increments up to 5 years following the NAFLD/NASH or advanced liver diseases diagnoses.
MATERIALS AND METHODS
Study Design and Data Source
This was a retrospective longitudinal cohort analysis based on administrative insurance claims data from the IBM Watson Health’s MarketScan Commercial Claims and Encounters database from January 1, 2006, to December 31, 2016. The Commercial database provides access to enrollment information, inpatient and outpatient medical, and outpatient pharmacy claims data for over 150 million individuals with employer-sponsored primary health insurance.
The study database satisfies the conditions set forth in Sections 164.514 (a)-(b)1ii of the Health Insurance Portability and Accountability Act of 1996 privacy rule regarding the determination and documentation of statistically deidentified data. As this study used only deidentified patient records and did not involve the collection, use, or transmittal of individually identifiable data, it was exempted from Institutional Review Board review or approval.
Patient Selection
Patient selection was similar to published methodology.28–30 Due to unavailability of specific International Classification of Diseases, 9th/10th Edition, Clinical Modification (ICD-9/10-CM) codes for NASH before 2015, patients with ICD codes for NAFLD and NASH were included in the study. Adults (aged 18 years and older) with at least 1 inpatient or outpatient claim for a known diagnosis of NAFLD or NASH (ICD-9-CM 571.8, 571.9; ICD-10-CM K76.0, K75.81) between January 1, 2006, and December 31, 2016, were selected. The date of the first diagnosis was defined as the NAFLD/NASH index date and only patients having continuous medical and prescription coverage for at least 6 months before and at least 1 month after the NAFLD/NASH index date were eligible for the study. Patients with any evidence (Supporting Table S1, Supplemental Digital Content 1, http://links.lww.com/JCG/A602) of viral hepatitis (hepatitis A, B, C, D, E), toxic liver disease, Wilson’s disease, Gaucher disease, lysosomal acid lipase deficiency, alcoholism or alcoholic liver disease, primary biliary/sclerosing cholangitis, or hemochromatosis at any time during the study period were excluded from the analysis.
Study Cohorts
To understand the characteristics, comorbidities, health care utilization and costs, 6 study cohorts (NAFLD/NASH without advanced liver diseases, NAFLD/NASH, CC, DCC, LT, and HCC) were created from eligible NAFLD/NASH patients based on liver disease severity (Supporting Table S1, Supplemental Digital Content 1, http://links.lww.com/JCG/A602). Development of each liver severity stage was identified using their subsequent first diagnosis claim (marked as index date). Details are described in Figure 1 and Supporting Figure S1 (Supplemental Digital Content 1, http://links.lww.com/JCG/A602).
FIGURE 1.
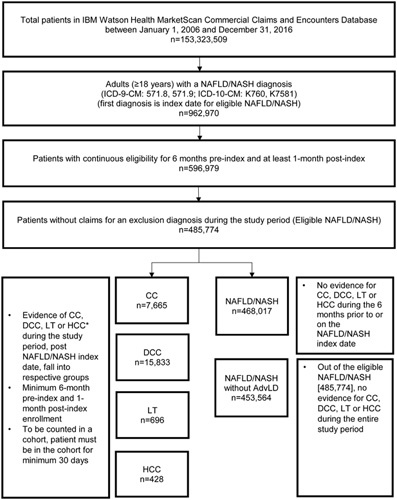
Patient selection. *Liver disease severity stages are non-mutually exclusive since a patient can index on NAFLD/NASH and then be classified under multiple severity stages (CC, DCC, LT, and/or LT). Accordingly, patients are counted in>1 severity stage. AdvLD indicates advanced liver diseases; CC, compensated cirrhosis; DCC, decompensated cirrhosis; HCC, hepatocellular carcinoma; ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification; ICD-10-CM, International Classification of Diseases, 10th Revision, Clinical Modification; LT, liver transplantation; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
Preindex and Postindex Periods
The 6 months before index date was considered as the baseline preindex period. All patients were followed for a variable postindex period defined as minimum of 1 month, until evidence of inpatient death, end of enrollment, progression to more severe stage, end of the study period, or end of 6 months, whichever was earliest. Claims databases do not capture comprehensive mortality information from all settings, hence only inpatient death could be considered here. Since patients with advanced liver diseases have a higher risk for mortality,30–33 the minimum of 1 month follow-up was used to reduce potential bias towards healthier patients.
Outcome Measures
Demographics and Clinical Characteristics
Demographic characteristics, including age, gender, geographic region, and insurance plan type, were measured on the index date. The clinical characteristics assessed over the baseline period are listed in Table 1 and included mean Deyo-Charlson Comorbidity Index and common comorbid conditions among NAFLD/NASH patients identified via ICD-9-CM and ICD-10-CM diagnoses codes such as CVD, hypertension, hyperlipidemia, obesity, RI, and T2DM.24 For the identification of T2DM, CVD, and RI, National Drug Codes (NDC) were also utilized (Supporting Table S2, Supplemental Digital Content 1, http://links.lww.com/JCG/A602). The proportion of patients with at least 1, 2, or ≥3 cardiometabolic comorbidities was also examined.
TABLE 1.
Demographics and Clinical Characteristics of Patients With NAFLD/NASH by Liver Disease Severity Stage
| Liver Disease Severity Stage‡ | ||||||
|---|---|---|---|---|---|---|
| Demographics and Clinical Characteristics | NAFLD/NASH Without AdvLD (N=453,564) | NAFLD/NASH§ (N=468,017) | CC (N=7665) | DCC (N=15,833) | LT (N=696) | HCC (N=428) |
| Demographics | ||||||
| Age [mean (SD)] (y) | 47.6 (10.9) | 47.7 (10.8) | 50.7 (9.9)* | 51.4 (10.2)*† | 51.7 (9.9)*† | 51.5 (9.8)* |
| Age group [n (%)] (y) | ||||||
| <45 | 163,860 (36.1) | 167,157 (35.7) | 1853 (24.2) | 3653 (7.7) | 135 (19.4) | 82 (19.2) |
| 45-54 | 146,893 (32.4) | 151,661 (32.4) | 2547 (33.2) | 4682 (29.6) | 227 (32.6) | 148 (34.6) |
| 55-64 | 142,811 (31.5) | 149,199 (31.9) | 3265 (42.6) | 7498 (47.4) | 334 (48.0) | 198 (46.3) |
| Gender [n (%)] | ||||||
| Female | 240,856 (53.1) | 249,313 (53.3) | 4472 (58.3)* | 9169 (57.9)* | 384 (55.2) | 226 (52.8)† |
| Geographic region [n (%)] | ||||||
| Northeast | 74,806 (16.5) | 77,139 (16.5) | 1017 (13.3)* | 2503 (15.8)*† | 98 (14.1) | 92 (21.5)*† |
| North Central | 87,638 (19.3) | 90,779 (19.4) | 1749 (22.8) | 3327 (21.0) | 157 (22.6) | 69 (16.1) |
| South | 203,722 (44.9) | 210,444 (45.0) | 3685 (48.1) | 7500 (47.4) | 319 (45.8) | 187 (43.7) |
| West | 80,611 (17.8) | 82,680 (17.7) | 1069 (13.9) | 2321 (14.7) | 115 (16.5) | 78 (18.2) |
| Unknown | 6787 (1.5) | 6975 (1.5) | 145 (1.9) | 182 (1.1) | 7 (1.0) | 2 (0.5) |
| Insurance plan type [n (%)] | ||||||
| Comprehensive/Indemnity | 10,393 (2.3) | 10,949 (2.3) | 232 (3.0)* | 603 (3.8)*† | 28 (4.0)*† | 11 (2.6) |
| EPO/PPO | 282,584 (62.3) | 291,538 (62.3) | 4856 (63.4) | 9854 (62.2) | 413 (59.3) | 281 (65.7) |
| POS with or without capitation | 34,423 (7.6) | 35,803 (7.6) | 593 (7.7) | 1302 (8.2) | 51 (7.3) | 29 (6.8) |
| HMO | 60,634 (13.4) | 62,442 (13.3) | 884 (11.5) | 1679 (10.6) | 85 (12.2) | 50 (11.7) |
| CDHP/HDHP | 43,832 (9.7) | 45,011 (9.6) | 696 (9.1) | 1749 (11.0) | 89 (12.8) | 38 (8.9) |
| Unknown | 21,698 (4.8) | 22,274 (4.8) | 404 (5.3) | 646 (4.1) | 30 (4.3) | 19 (4.4) |
| Clinical Characteristics | ||||||
| DCCI [mean (SD)] | 0.47 (1.02) | 0.49 (1.06) | 1.07 (1.43)* | 1.52 (2.35)*† | 2.53 (2.82)*† | 2.59 (3.28)*† |
| Comorbidities [n (%)]‖ | ||||||
| Abdominal pain | 154,157 (34.0) | 159,277 (34.0) | 2879 (37.6)* | 6830 (43.1)*† | 400 (57.5)*† | 218 (50.9)*† |
| Anemia | 26,176 (5.8) | 27,831 (5.9) | 910 (11.9)* | 3215 (20.3)*† | 170 (24.4)*† | 76 (17.8)*† |
| CVD | 236,913 (52.2) | 246,397 (52.6) | 5067 (66.1)* | 10,585 (66.9)* | 448 (64.4)* | 267 (62.4)* |
| Fatigue/insomnia | 58,246 (12.8) | 60,531 (12.9) | 1114 (14.5)* | 2997 (18.9)*† | 116 (16.7)* | 60 (14.0) |
| HTN | 163,389 (36.0) | 169,776 (36.3) | 3710 (48.4)* | 7945 (50.2)*† | 348 (50.0)* | 209 (48.8)* |
| Hyperlipidemia | 162,376 (35.8) | 167,934 (35.9) | 3417 (44.6)* | 6290 (39.7)*† | 259 (37.2)† | 170 (39.7)† |
| Obesity | 63,802 (14.1) | 65,956 (14.1) | 1831 (23.9)* | 3568 (22.5)*† | 142 (20.4)*† | 56 (13.1)† |
| PUD, dyspepsia, GERD, esophagitis | 75,370 (16.6) | 78,199 (16.7) | 1823 (23.8)* | 4425 (27.9)*† | 184 (26.4)* | 99 (23.1)* |
| RI | 15,396 (3.4) | 16,200 (3.5) | 433 (5.6)* | 2085 (13.2)*† | 126 (18.1)*† | 52 (12.1)*† |
| Sleep apnea | 45,011 (9.9) | 46,961 (10.0) | 1310 (17.1)* | 2498 (15.8)*† | 70 (10.1)† | 32 (7.5)† |
| Smoking | 16,498 (3.6) | 17,129 (3.7) | 330 (4.3)* | 1259 (8.0)*† | 53 (7.6)*† | 25 (5.8)* |
| T2DM | 106,683 (23.5) | 111,832 (23.9) | 3525 (46.0)* | 5752 (36.3)*† | 284 (40.8)*† | 160 (37.4)*† |
| Thyroid disease (including hypothyroidism) | 54,648 (12.0) | 56,762 (12.1) | 1290 (16.8)* | 2653 (16.8)* | 112 (16.1)* | 71 (16.6)* |
| Vitamin D deficiency | 30,835 (6.8) | 31,846 (6.8) | 673 (8.8)* | 1447 (9.1)* | 46 (6.6) | 27 (6.3) |
| Comorbidity combinations: HTN, hyperlipidemia, CVD, RI, T2DM [n (%)]‖ | ||||||
| At least 1 condition | 308,442 (68.0) | 319,801 (68.3) | 6315 (82.4)* | 12,721 (80.3)*† | 558 (80.2)* | 337 (78.7)* |
| At least 2 conditions | 215,962 (47.6) | 224,670 (48.0) | 5025 (65.6)* | 9958 (62.9)*† | 448 (64.4)* | 266 (62.1)* |
| At least 3 conditions | 117,783 (26.0) | 122,884 (26.3) | 3231 (42.2)* | 6454 (40.8)*† | 293 (42.1)* | 166 (38.8)* |
‡Demographics and clinical characteristics of patients who progressed to more AdvLD (eg, CC to DCC or DCC to HCC) were determined at each diagnosis (index) of liver disease during the study duration. Therefore, patients with >1 diagnosis of liver disease confirmed during the follow-up period of the study were considered more than once in the table.
§NAFLD/NASH study cohort comprises overall NAFLD/NASH patients until progression to a more severe stage, at which follow-up is censored.
‖Identification of comorbid conditions were based on the presence of ICD-9-CM/ICD-10-CM diagnosis codes during the preindex period. For the identification of CVD, RI, and T2DM, National Drug Codes (NDC) were also utilized (Supporting Table S2, Supplemental Digital Content 1, http://links.lww.com/JCG/A602).
AdvLD indicates advanced liver diseases; CC, compensated cirrhosis; CDHP, consumer driver health plan; CVD, cardiovascular disease; DCC, decompensated cirrhosis; DCCI, Deyo-Charlson Comorbidity Index, EPO, exclusive provider organization; GERD, gastroesophageal reflux disease; ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification; ICD-10-CM, International Classification of Diseases, 10th Revision, Clinical Modification; HCC, hepatocellular carcinoma; HDHP, high-deductible health plan; HMO, health maintenance organization; HTN, hypertension; LT, liver transplantation; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; POS, point-of-service; PPO, preferred provider organization; PUD, peptic ulcer disease; RI, renal impairment; T2DM, type 2 diabetes mellitus.
*P<0.05 for comparison of CC, DCC, LT, or HCC cohort versus NAFLD/NASH without AdvLD cohort.
†P<0.05 for comparison of DCC, LT, or HCC cohort versus CC cohort.
Annual All-cause HRU and Cost
Annual all-cause HRU and costs were measured during 6-month preindex and variable postindex periods. Given the high prevalence and burden of CVD, RI, T2DM among NAFLD/NASH patients,24,34–36 the utilization and cost estimates in patients with and without these 3 comorbid conditions were also reported. HRU measures included inpatient hospitalization, outpatient services, and prescription fills. Total health care costs comprised of expenditures incurred due to medical services (inpatient+outpatient) and pharmacy payments. The adjusted postindex total costs were also reported after controlling for baseline demographics and clinical characteristics using multivariable models.
Cumulative All-cause Health Care Costs
The total all-cause costs (sum of inpatient, outpatient, and prescription) were measured in yearly increments from 2 years before and 5 years after the index date for all liver disease severity stages among patients who had available follow-up data. Cumulative costs for each preindex/postindex year were calculated by adding the mean cost of the specific year to mean costs of all prior years (eg, costs for year +3 was the sum of annualized costs in year −2, −1, +1, +2, and +3). A more detailed description is provided in Supporting Text S1 (Supplemental Digital Content 1, http://links.lww.com/JCG/A602).
Per patient per month values were measured and annualized to report mean annual all-cause HRU and costs, adjusted to 2016 USD using the Medical Care component of the Consumer Price Index (www.bls.gov/cpi/). Health care costs were calculated from the adjudicated claims containing the provider-paid and patient-paid components (copayment, deductible, and coinsurance).
Statistical Analyses
All study variables, including demographics and clinical characteristics, were summarized descriptively for the 6 liver disease severity stages. Categorical variables were summarized as counts and percentages. Continuous variables were summarized as means and SDs. Statistical comparisons of demographics, clinical characteristics, and postindex HRU and cost between NAFLD/NASH without advanced liver diseases versus advanced liver diseases (CC, DCC, LT, and HCC) and between CC versus more severe stages (DCC, LT, and HCC) were performed. χ2 tests were used to evaluate the statistical significance of differences for categorical variables. The Student t tests were used to evaluate the statistical significance of differences for continuous measures. In addition, statistical comparisons of HRU and cost between preindex and postindex and in patients with and without comorbid conditions were performed via paired t test and Student t test, respectively. A 2-tailed P-value <0.05 was considered, a priori, to be statistically significant.
Multivariable analysis evaluated the adjusted postindex total annual all-cause costs for liver disease severity stages, after controlling for covariates including baseline demographics and clinical characteristics. Specifically, generalized linear models with gamma error distribution and log-link function were fit to the data. Liver disease severity stages formed the primary independent variable; other explanatory variables included age, gender, geographic region, insurance plan type, population density, Deyo-Charlson Comorbidity Index, and comorbid conditions (listed in Table 3). The method of recycled predictions was used to estimate the adjusted incremental cost in dollars among patients with selected comorbidities compared with those without any comorbidities.
TABLE 3.
Results of Generalized Linear Model—Total Annual All-cause Health Care Costs (Postindex) Adjusted by Demographics and Clinical Characteristics
| Independent Variables | Cost Ratio | 95% CI (Lower-Upper) | P |
|---|---|---|---|
| Liver disease severity stage | |||
| NAFLD/NASH without AdvLD | Reference | — | — |
| NAFLD/NASH* | 1.02 | 1.02-1.03 | <0.0001 |
| CC | 1.22 | 1.19-1.25 | <0.0001 |
| DCC | 5.64 | 5.53-5.74 | <0.0001 |
| LT | 8.27 | 7.57-9.04 | <0.0001 |
| HCC | 4.09 | 3.65-4.58 | <0.0001 |
| Geographic region | |||
| Northeast | Reference | — | — |
| North Central | 1.17 | 1.16-1.18 | <0.0001 |
| South | 1.11 | 1.11-1.12 | <0.0001 |
| West | 1.07 | 1.06-1.08 | <0.0001 |
| Insurance plan type | |||
| HMO | Reference | — | — |
| Comprehensive/indemnity | 1.13 | 1.11-1.15 | <0.0001 |
| EPO/PPO | 1.14 | 1.14-1.15 | <0.0001 |
| POS with or without capitation | 1.16 | 1.15-1.18 | <0.0001 |
| CDHP/HDHP | 1.14 | 1.13-1.16 | <0.0001 |
| Unknown | 1.04 | 1.03-1.06 | <0.0001 |
| Population density | |||
| Urban | Reference | — | — |
| Rural | 1.07 | 1.06-1.08 | <0.0001 |
| DCCI | 1.27 | 1.26-1.27 | <0.0001 |
| Age groups (y) | |||
| 18-44 | Reference | — | — |
| 45-54 | 1.09 | 1.08-1.09 | <0.0001 |
| 55-64 | 1.17 | 1.17-1.18 | <0.0001 |
| Gender | |||
| Male | Reference | — | — |
| Female | 1.21 | 1.20-1.21 | <0.0001 |
| Comorbidities† | |||
| Abdominal pain | 1.07 | 1.07-1.08 | <0.0001 |
| Anemia | 1.36 | 1.34-1.37 | <0.0001 |
| CVD | 1.10 | 1.09-1.10 | <0.0001 |
| Fatigue/insomnia | 1.10 | 1.09-1.11 | <0.0001 |
| Hypertension | 1.05 | 1.05-1.06 | <0.0001 |
| Hyperlipidemia | 0.82 | 0.81-0.82 | <0.0001 |
| Obesity | 1.52 | 1.51-1.53 | <0.0001 |
| PUD, dyspepsia, GERD, esophagitis | 1.19 | 1.18-1.20 | <0.0001 |
| RI | 1.29 | 1.26-1.33 | <0.0001 |
| Sleep apnea | 1.35 | 1.34-1.36 | <0.0001 |
| Smoking | 1.33 | 1.31-1.35 | <0.0001 |
| Thyroid disease (including hypothyroidism) | 1.01 | 1.00-1.02 | 0.0205 |
| T2DM | 0.91 | 0.90-0.92 | <0.0001 |
| Vitamin D deficiency | 0.98 | 0.97-0.99 | 0.0007 |
| Comorbidity combinations† | |||
| CVD and RI | 0.99 | 0.95-1.02 | 0.4023 |
| CVD and T2DM | 1.09 | 1.07-1.10 | <0.0001 |
| RI and T2DM | 1.16 | 1.09-1.24 | <0.0001 |
| CVD, RI, and DM | 0.81 | 0.76-0.87 | <0.0001 |
| Hypertension, hyperlipidemia, T2DM, CVD, and obesity | 0.95 | 0.93-0.97 | <0.0001 |
*NAFLD/NASH study cohort comprises overall NAFLD/NASH patients until progression to a more severe stage, at which follow-up is censored.
†Identification of comorbid conditions were based on the presence of ICD-9-CM/ICD-10-CM diagnosis codes during the preindex period. For the identification of CVD, RI, and T2DM, National Drug Codes (NDC) were also utilized (Supporting Table S2, Supplemental Digital Content 1, http://links.lww.com/JCG/A602).
AdvLD indicates advanced liver diseases; CC, compensated cirrhosis; CDHP, consumer driver health plan; CI, confidence interval; CVD, cardiovascular disease; DCC, decompensated cirrhosis; DCCI, Deyo-Charlson Comorbidity Index, EPO, exclusive provider organization; GERD, gastroesophageal reflux disease; HCC, hepatocellular carcinoma; HDHP, high-deductible health plan; HMO, health maintenance organization; ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification; ICD-10-CM, International Classification of Diseases, 10th Revision, Clinical Modification; LT, liver transplantation; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; POS, point-of-service; PPO, preferred provider organization; PUD, peptic ulcer disease; RI, renal impairment; T2DM, type 2 diabetes mellitus.
RESULTS
Sample Selection
Of the total 153,323,509 individuals in the Commercial database between 2006 and 2016, 962,970 (0.6%) adults were diagnosed with NAFLD/NASH. After applying inclusion/exclusion criteria, 485,774 were eligible for inclusion. Of these, 93.4% (453,564) were NAFLD/NASH patients without advanced liver diseases, 1.6% (7665) with CC, 3.3% (15,833) with DCC, 0.1% (696) with LT, and 0.1% (428) with HCC (Fig. 1). In addition, 65% of NAFLD/NASH patients with first cirrhosis (CC+DCC) diagnosis already had a decompensated event (DCC).
Demographics and Clinical Characteristics
Table 1 summarizes the demographics and clinical characteristics of patients in each of the 6 liver disease severity stages. The mean age of patients across all the severity stages ranged between 47.6 and 51.7 years. Majority of patients were females (52.8% to 58.3%), resided in the Southern region of the US (43.7% to 48.1%), and were covered by an exclusive provider organization/preferred provider organization plan (59.3% to 65.7%).
The mean Deyo-Charlson Comorbidity Index was significantly higher among patients with advanced liver diseases versus those without advanced liver diseases (all P<0.0001); and among patients with DCC, LT, and HCC versus CC (all P<0.0001). The comorbidity burden was high across all liver disease severity stages, with significantly higher prevalence of comorbid conditions in each increasing severity stage. The most prevalent comorbidities included CVD (52.2% to 66.9%), hypertension (36.0% to 50.2%), hyperlipidemia (35.8% to 44.6%), and T2DM (23.5% to 46.0%). More than two-thirds of the patients across all severity stages including those with NAFLD/NASH without advanced liver diseases had at least 1 comorbidity out of hypertension, hyperlipidemia, CVD, T2DM, and RI. In addition, the proportion of patients with at least 3 of these conditions was significantly higher among patients with advanced liver diseases (38.8% to 42.2%) compared with NAFLD/NASH patients without advanced liver diseases (26.0%) (all P<0.0001) (Table 1).
Annual All-cause HRU
Overall, the mean annual number of HRU across all service categories (inpatient admission, outpatient services, and prescription fills) increased from preindex to postindex period (all P<0.0001), except for outpatient services among CC patients which remained the same in preindex and postindex periods (mean=39, P=0.55) (Table 2). The mean annual admissions per patient and the length of stay per admission for DCC, LT, and HCC patients more than doubled from preindex to postindex periods (all P<0.0001).
TABLE 2.
Annual All-cause Health Care Resource Utilization Among Patients With NAFLD/NASH by Liver Disease Severity Stage
| NAFLD/NASH Without AdvLD (N=453,564) | NAFLD/NASH§ (N=468,017) | CC (N=7665) | DCC (N=15,883) | LT (N=696) | HCC (N=428) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Health Care Resource Utilization Parameters | Preindex | Postindex | Preindex | Postindex | Preindex | Postindex | Preindex | Postindex | Preindex | Postindex | Preindex | Postindex |
| Inpatient hospitalizations | ||||||||||||
| Patients with hospitalization [n (%)] | 22,331 (4.9) | 39,351 (8.7) | 23,972 (5.1) | 42,179 (9.0) | 726 (9.5) | 1008 (13.2)† | 5053 (31.9) | 8174 (51.6)†‡ | 320 (46.0) | 549 (78.9)†‡ | 78 (18.2) | 107 (25.0)†‡ |
| Annual admissions per patient [mean (SD)] | 0.12 (0.57) | 0.24 (0.93)* | 0.12 (0.59) | 0.25 (0.95)* | 0.24 (0.83) | 0.38 (1.28)*† | 0.94 (1.74) | 2.25 (3.51)*†‡ | 1.63 (2.44) | 3.11 (3.33)*†‡ | 0.55 (1.60) | 1.25 (3.08)*†‡ |
| % change from preindex to postindex period | 103.6 | 102.2 | 61.8 | 140.2 | 128.7 | 90.4 | ||||||
| Length of stay [mean (SD)] | 0.70 (0.57) | 0.81 (1.00)* | 0.71 (0.59) | 0.82 (1.01)* | 0.73 (0.58) | 0.89 (1.14)† | 1.05 (0.93) | 2.34 (4.00)*†‡ | 1.18 (1.02) | 2.48 (3.75)*†‡ | 0.95 (0.60) | 2.59 (4.46)*†‡ |
| Outpatient services | ||||||||||||
| Patients with service [n (%)] | 433,586 (95.6) | 453,298 (99.9) | 447,760 (95.7) | 467,745 (99.9) | 7599 (99.1) | 7651 (99.8)† | 15,653 (98.9) | 15,791 (99.7)† | 689 (99.0) | 695 (99.9) | 425 (99.3) | 428 (100.0) |
| Annual services per patient [mean (SD)] | 25.9 (24.1) | 30.7 (27.7)* | 26.4 (24.8) | 31.2 (28.3)* | 39.1 (29.5) | 38.9 (31.8)† | 53.1 (47.0) | 68.0 (58.3)*†‡ | 69.2 (54.1) | 75.6 (61.4)*†‡ | 51.1 (43.4) | 78.1 (68.4)*†‡ |
| % change from preindex to postindex period | 18.4 | 18.1 | -0.5 | 28.0 | 9.3 | 52.8 | ||||||
| Prescription fills | ||||||||||||
| Patients with fills [n (%)] | 388,476 (85.6) | 394,423 (87.0) | 401,776 (85.8) | 407,796 (87.1) | 6962 (90.8) | 6895 (90.0)† | 14,750 (93.2) | 14,652 (92.5)†‡ | 648 (93.1) | 652 (93.7)†‡ | 390 (91.1) | 389 (90.9)† |
| Annual prescriptions per patient [mean (SD)] | 23.2 (25.6) | 25.7 (27.1)* | 23.6 (26.0) | 25.9 (27.4)* | 33.6 (31.6) | 35.0 (33.2)*† | 41.0 (36.4) | 46.4 (39.1)*†‡ | 40.1 (34.1) | 48.9 (38.2)*†‡ | 33.0 (30.9) | 39.8 (34.1)*†‡ |
| % change from preindex to postindex period | 10.6 | 9.9 | 4.0 | 13.2 | 20.6 | 21.9 | ||||||
§NAFLD/NASH study cohort comprises overall NAFLD/NASH patients until progression to a more severe stage, at which follow-up is censored.
AdvLD indicates advanced liver diseases; CC, compensated cirrhosis; DCC, decompensated cirrhosis; HCC, hepatocellular carcinoma; LT, liver transplantation; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
*P<0.05 for comparison of preindex versus postindex period.
†P<0.05 for comparison of CC, DCC, LT, or HCC cohort versus NAFLD/NASH without AdvLD cohort (postindex).
‡P<0.05 for comparison of DCC, LT, or HCC cohort versus CC cohort (postindex).
With a few exceptions, postindex HRU across all service categories was significantly higher among patients with advanced liver diseases compared with those without advanced liver diseases (Table 2). The percentage of patients with an inpatient admission was significantly higher among NAFLD/NASH patients with advanced liver diseases versus those without advanced liver diseases; and the same was true among patients with DCC, LT, or HCC versus CC (all P<0.0001). For example, prevalence of inpatient admissions was 51.6% and 25.0% in DCC and HCC patients, compared with 8.7% and 13.2% in NAFLD/NASH without advanced liver diseases and CC patients (all P<0.0001). Mean annual number of admissions per patient and length of stay per admission were significantly higher among advanced liver diseases compared with those without advanced liver diseases and also higher among DCC, LT, or HCC compared with those with CC (all P<0.0001). This trend held when comparing mean annual outpatient services and prescription fills (all P<0.0001). For example, the mean annual number of outpatient services and prescription fills were 68 (±58) and 46 (±39) among the DCC patients compared with 39 (±32) and 35 (±33) among the CC patients, respectively (P<0.0001). However, the number of patients with any outpatient service or prescription fill did not always differ by advanced liver diseases stage: among all severity stages most patients required an outpatient visit and >87% of patients had a prescription filled.
Supporting Table S3 (Supplemental Digital Content 1, http://links.lww.com/JCG/A602) compares the postindex all-cause annual HRU among liver disease severity stages with and without the 3 comorbidities of T2DM, CVD, and RI. With a few exceptions, most patients with comorbid T2DM, CVD, or RI had a higher prevalence of postindex inpatient admissions, outpatient services, and prescriptions fills compared with those without the comorbidities. Of note, the average number of postindex inpatient admissions among NAFLD/NASH patients without advanced liver diseases and CC patients was almost double for those with comorbid RI compared with without RI.
Annual All-cause Health Care Costs
Overall, the mean annual total all-cause health care costs increased from preindex to postindex period (P<0.0001) (Fig. 2). The percentage increase in total cost was 69% for NAFLD/NASH patients without advanced liver diseases, 39% for CC, 159% for DCC, 172% for patients with LT and 145% for HCC patients. Higher costs from preindex to postindex costs across all severity stages were primarily driven by inpatient costs, which also increased with progressing severity stage, as seen in Figure 2.
FIGURE 2.
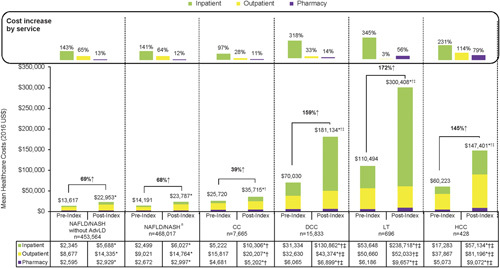
Annual all-cause health care costs among patients with NAFLD/NASH by liver disease severity stage. aNAFLD/NASH study cohort comprises overall NAFLD/NASH patients until progression to a more severe stage, at which follow-up is censored. *P<0.05 for comparison of preindex versus postindex period. †P<0.05 for comparison of CC, DCC, LT, or HCC cohort versus NAFLD/NASH without AdvLD cohort (postindex). ‡P<0.05 for comparison of DCC, LT, or HCC cohort versus CC cohort (postindex). AdvLD indicates advanced liver diseases; CC, compensated cirrhosis; DCC, decompensated cirrhosis; HCC, hepatocellular carcinoma; LT, liver transplantation; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
Postindex total costs and costs across all service categories (inpatient, outpatient, pharmacy) were significantly higher for all NAFLD/NASH patients with advanced liver diseases compared with those without advanced liver diseases; and higher for patients with DCC, LT, and HCC compared with CC (all P<0.0001) (Fig. 2). Inpatient costs were the largest contributor in both DCC and LT patients, whereas outpatient costs were the major contributor among NAFLD/NASH without advanced liver diseases, CC, and HCC patients.
The mean annual all-cause postindex costs stratified by the comorbidities T2DM, CVD, and RI are presented in Supporting Table S3 (Supplemental Digital Content 1, http://links.lww.com/JCG/A602). With a few exceptions, the postindex costs were significantly higher in patients with comorbidities.
After adjusting for potential confounders, the adjusted annual all-cause postindex costs increased with liver disease severity. Compared with NAFLD/NASH without advanced liver diseases ($23,860), the annual cost was 1.22 times higher for CC ($29,078±5219), 5.64 times higher for the DCC ($134,448±110,588), 8.27 times higher for LT ($197,392±173,532) and 4.09 times higher for HCC ($97,563±73,704) (all P<0.0001) (Fig. 3). Of the comorbidities studied, the majority were associated with increased annual costs but the presence of hyperlipidemia, T2DM, and vitamin D deficiency were associated with decreased annual costs (Table 3).
FIGURE 3.
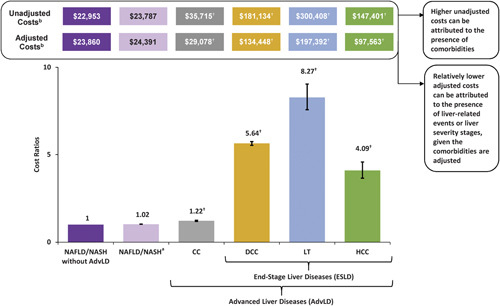
Adjusted annual all-cause health care costs from generalized linear model among patients with NAFLD/NASH by liver disease severity stage. aNAFLD/NASH study cohort comprises overall NAFLD/NASH patients until progression to a more severe stage, at which follow-up is censored. bHealth care costs represent the total annual all-cause postindex costs. †P<0.0001 for comparison of CC, DCC, LT, or HCC cohort versus NAFLD/NASH without AdvLD cohort. AdvLD indicates advanced liver diseases; CC, compensated cirrhosis; DCC, decompensated cirrhosis; HCC, hepatocellular carcinoma; LT, liver transplantation; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
Across all advanced liver disease severity stages, the incremental costs were highest for patients with anemia, obesity, RI, sleep apnea, and smoking (Supporting Table S4, Supplemental Digital Content 1, http://links.lww.com/JCG/A602). For example, the annual incremental cost of CC patients with obesity compared with CC patients without any comorbidities was an additional $11,987 (P<0.001).
Cumulative All-cause Health Care Costs
The cumulative mean all-cause costs increased exponentially over 7 years, that is, from 2 years before the index date to 5 years after the index date for all liver disease severity stages, with the steepest increase occurring between 1 year before (year −1) and 1 year after (year 1) the index date across all severity stages (Fig. 4). The percentage increase in the cumulative costs during this 7-year period (year −2 to year 5) was 757% for NAFLD/NASH without advanced liver diseases, 785% for CC, 1230% for DCC, 1485% for LT, and 1081% for HCC. The steep slope around the index date is most pronounced in the more severe stages (DCC and LT) leading to the differential cumulative costs observed in year 5 postindex date.
FIGURE 4.
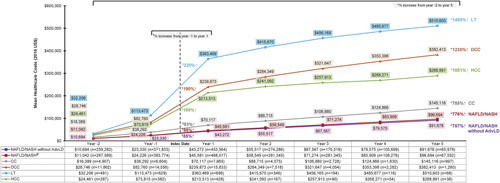
Cumulative all-cause health care costs among patients with NAFLD/NASH by liver disease severity stage. aNAFLD/NASH study cohort comprises overall NAFLD/NASH patients until progression to a more severe stage, at which follow-up is censored. *Percent change denotes the increase in mean costs from year −2 to year 5. †Percent change denotes the increase in mean costs from year −1 to year 1. AdvLD indicates advanced liver diseases; CC, compensated cirrhosis; DCC, decompensated cirrhosis; HCC, hepatocellular carcinoma; LT, liver transplantation; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
DISCUSSION
To the author’s knowledge, this is one of the first retrospective cohort studies that comprehensively characterized the real-world comorbidities, all-cause HRU and associated costs among NAFLD/NASH patients with and without advanced liver diseases (includes CC, DCC, LT and HCC), using commercially insured US population data. Epidemiological studies have shown an upsurge in the prevalence of NAFLD/NASH in the United States.14,27,37 Estimates from predictive models have projected a 21% increase in the prevalent NAFLD cases and 63% increase in the prevalent NASH cases by 2030.4 This rise in prevalence of NAFLD/NASH and its sequelae has been attributed to numerous factors, including an aging population and increasing rates of metabolic comorbidities.4,16
In the current analysis, the highest prevalence of NASH with advanced liver diseases was reported among adults aged 55 to 64 years. The is in line with the published evidence.2,16,25 Furthermore, a sizeable proportion (65%) of NAFLD/NASH patients in this study at the time of their initial cirrhosis (CC+DCC) diagnosis already had a decompensation event. This is also consistent with a study that reported 71% of NAFLD/NASH patients were diagnosed with DCC at the time of initial cirrhosis diagnosis.38 These findings suggest that NASH remains largely underdiagnosed due to lack of noninvasive tests or reliable indicators to diagnose the underlying liver disease, until the disease progresses to advanced stages.39,40
Comorbidities are common in NAFLD/NASH patients and associated with increased utilization and cost estimates.28 Over two-thirds of the patients across all the severity stages had ≥1 comorbidity and more than one-third of patients with advanced liver diseases had ≥3 comorbidities (out of hypertension, hyperlipidemia, CVD, RI, or T2DM). The higher rates of comorbidities is not unexpected and can be attributed, in part, to the older age of patients with advanced liver diseases compared to those without advanced liver diseases, who have higher prevalence of metabolic syndrome.41 Previous research has reported an association of NAFLD/NASH with various comorbidities such as CVD,17 obesity, T2DM, insulin resistance, and metabolic syndrome,34,37,42 and chronic kidney disease.34 This study provides convincing evidence that comorbid obesity, RI, anemia, sleep apnea, and smoking were each associated with increased annual costs after adjusting for age and other comorbidities.
NAFLD/NASH imposes a substantial health care burden on the US health care system.2,43 Most published data on economic burden of NAFLD/NASH by liver disease severity stages are derived from predictive models that are based on certain assumptions.2,23 Although some real-world studies have evaluated changes in HRU and cost burden with NAFLD/NASH progression,17,28–30,38 these are restricted to patients with and without cirrhosis or derived from limited sources. In the present analysis, among a large sample of patients insured via various providers, inpatient costs were the major contributor for the increase in total costs from preindex to postindex durations for each severity stage. A recent study using a similar study population reported that following a CC diagnosis, the rate of hospitalization increased by 34% and inpatient costs increased by 90%.28 The exponentially higher adjusted costs with advancing severity stages observed in our study is supported by a previous Medicare study, which reported a higher median annual inpatient and outpatient costs for patients with DCC than noncirrhotic or CC patients.17 These findings highlight that the majority of the NAFLD/NASH patients are not diagnosed until the disease progresses to the advanced stages. While recent data on head-to-head comparison of health care costs of other liver diseases may not be available, a study quantifying the annual per patient health care costs of hepatitis C virus in US normalized to 2009 dollars ($5330 for CC, $27,845 for DCC, $93,609 for LT, and $43,671 for HCC) suggests that NAFLD/NASH could potentially be more economically taxing to the US health care system, especially in the absence of effective treatment modalities.44
Furthermore, comorbid conditions (T2DM, CVD, and RI) were found to exert a substantial impact on the utilization and costs across all severity stages. Comorbid CVD was a significant contributor towards postindex HRU regardless of advanced liver diseases severity. This observation is in concert with those by Sayiner and colleagues which reported CVD as an independent predictor of costs in both inpatient and outpatient settings. The presence of CVD among NAFLD patients was found to be associated with increased inpatient and outpatient costs by >50% and 60%, respectively.17 The largest postindex total cost differences in our study were between patients with and without comorbid RI. The increased costs among patients with RI can be partly explained by the evidence that coexistence of chronic kidney disease can aggravate the CVD risk, thus possibly impacting the costs.45,46 Although it was unexpected to observe higher inpatient and outpatient health care costs for LT and HCC patients without comorbid T2DM as compared with those with T2DM (Supporting Table S3a, Supplemental Digital Content 1, http://links.lww.com/JCG/A602), it should be noted that these costs are not adjusted for any confounders; hence further investigation is warranted before interpreting the definitive impact of comorbid T2DM. In addition, treating T2DM patients via insulin sensitizers or statin therapies as well as more attentive assessment and optimization of cardiovascular risk might have improved not just the patient’s diabetes but other metabolic disorders including NAFLD/NASH, leading to reduced disease severity and associated HRU and costs relative to those without T2DM.47–49
Published studies have evaluated HRU or costs for major chronic diseases. For example, Willey et al50 used claims data from the HealthCore Integrated Research Database and reported total annual all-cause health care costs incurred by T2DM patients between $14,184 and $15,716 per patient. In addition, a retrospective analysis on the electronic medical record database Humedica reported mean annualized all-cause per patient cost for chronic kidney disease stage 2 to be $16,770 for commercial and $14,493 for Medicare group.51 Finally, a longitudinal cohort study on Kaiser Permanente Northwest registry from 2000 to 2005 reported the total mean direct medical care costs for patients with established CVD to be $18,953 per patient per year.52 Comparing such estimates with adjusted annual costs of NAFLD/NASH patients in this study [$23,860 (for NAFLD/NASH without advanced liver diseases) to $197,392 (for LT)] highlights the disproportionate high disease burden of NAFLD/NASH. These estimates and comparisons with other chronic diseases should be used to inform decision-making by health policymakers regarding resource allocation to promote early diagnosis of NAFLD/NASH.
An unanticipated finding from multivariable analysis reported lower incremental costs among patients with hyperlipidemia, T2DM, and vitamin D deficiency across all liver severity stages. One possible explanation could be the improved management of patients with NAFLD/NASH and advanced liver diseases through the use of insulin sensitizers (such as pioglitazone) or statins.47–49 Similarly, vitamin D has been identified as new player in the NAFLD realm.53 Due to its metabolic, anti-inflammatory, and antifibrotic properties, vitamin D supplementation may be recognized as an adjunctive therapy to attenuate systemic inflammation in NAFLD and NASH patients.54,55 These findings suggest that extrahepatic comorbidities may have important implications on the HRU and costs and warrant further understanding. Therefore, increasing awareness of these conditions in NAFLD/NASH patients may help in identifying multidisciplinary therapeutic interventions that could reduce the risk of comorbidities and ultimately reduce HRU and associated costs.34
The cumulative all-cause health care costs incurred by each severity stage was also estimated in the present study. Across all the NAFLD/NASH severity stages, the cumulative costs increased over a 7-year period, with highest cost increase seen in patients with DCC and LT. Two recent studies among CC patients have estimated 838% to 891% increase in cumulative health care cost over a 7-year period,28,29 and among those who progressed compared with those who did not progress in severity stage, cost was more than double at the end of the period.28 The steep increase observed in the first year after diagnosis (from year −1 to year 1) of liver severity stages, especially DCC, LT, and HCC, could be due to the added diagnostic and therapeutic interventions that are implemented when decompensation in liver disease occurs.56 In continuing to understand the HRU and costs of NAFLD/NASH, results from the current study support the need to differentiate between NAFLD/NASH and the stages of advanced liver diseases.
The study results must be interpreted in light of certain limitations. Primarily, as with any claims database, the MarketScan Research Database is subject to data coding limitations (such as data entry errors) and potential misclassification (such as underreporting). The identification of patients with NAFLD/NASH or those with advanced liver diseases (particularly CC) and certain comorbidities (such as obesity) was limited to the ICD-9/10-CM codes. Any NAFLD/NASH diagnosis in this study could not be confirmed via chart review and the severity of NAFLD/NASH could not be examined due to the lack of biopsy data or other measures of fibrosis such as elastography values in claims data. This may have led to gross underdiagnosis of the true number of patients with NAFLD/NASH or those who progress to advanced liver diseases, especially if asymptomatic and therefore, caused underestimation of the cost/HRU estimates. The inability of ICD-9-CM codes to differentiate between NAFLD and NASH, may have further added to underdiagnosis of F4 (CC) patients. However, it is highly likely that study patients had NASH because any patients with other etiologies of liver disease were excluded. Further, it may be possible that the NAFLD/NASH cohort and NAFLD/NASH without advanced liver diseases cohort included early to advanced fibrosis patients (F0-F3) as well as undiagnosed F4 (CC) patients owing to the lack of ICD codes for F0-F3 and undercoding of F4 (CC), as explained above. Claims data do not contain information on amount of alcohol consumption; however, since alcohol intake may affect fibrosis/cirrhosis, patients with a diagnosis of alcoholism (including alcoholic liver disease) were excluded from the study.
There were also other limitations general in claims data analyses and specific to this study. First, this study was limited to only those individuals with Commercial private insurance. Consequently, results of this analysis may not be generalizable to patients with other insurance (eg, Medicaid, Medicare) or patients without health insurance coverage. That being said, a recent study by Kulaylat et al57 discussed the relevance of MarketScan databases for clinical research. Second, while statistical analysis was conducted to adjust for patients’ characteristics, systemic differences may still exist between patients with advanced liver diseases and adjustment were limited to those characteristics that can be measured from administrative claims. Third, one of the censorship criteria included in the study was death during follow-up. However, this was limited to death in the inpatient setting because claims databases do not have comprehensive mortality information from all settings; nonetheless if a patient died outside of the inpatient setting, a lapse in enrollment is expected leading to patient’s end of follow-up. Fourth, to account for the variable-length follow-up, outcomes including HRU and costs were standardized to per patient per month and then annualized to obtain the annual estimates. This method could result in overestimation as severity stages may incur high cost in the first month and less cost in subsequent months. Fifth, since the bidirectional relationship between NAFLD/NASH and metabolic comorbidities makes it difficult to differentiate NAFLD/NASH-specific components, the study estimated adjusted all-cause costs rather than NAFLD/NASH-related costs. Finally, the study focused on the direct costs only; indirect costs such as work loss or reduced quality of life due to NAFLD/NASH were not captured, therefore future studies capturing the economic burden from a societal perspective should be encouraged to avoid any underestimation of the economic burden. Future research should also focus on adjusted multivariable analyses to understand the true impact of comorbid conditions such as CVD, T2DM, or RI on all-cause costs among patients with NAFLD/NASH.
While the above limitations were acknowledged, many methodological strengths differentiate the current study from those published previously. Previous model-based studies used other diseases such as hepatitis C as a proxy to estimate the cost burden of NASH.18 There are a few studies which have described the real-world burden of NAFLD/NASH but were limited to a single payer,17,28–30 geographic region,38 or did not span the entire spectrum of liver disease severity stages.17,38 The current study identified a large cohort of NAFLD/NASH patients with and without advanced liver diseases and tracked their progression or nonprogression through CC, DCC, LT, and HCC stages. Furthermore, a comprehensive methodology was implemented to capture the inpatient and outpatient HRU and costs incurred within each stage and to measure the incremental costs associated with each stage transition. Moreover, this study provides real-world data on the comorbidity, HRU, and economic burden associated with NAFLD/NASH and its sequelae in real-world clinical practice.
In conclusion, results of this study suggest that NAFLD/NASH patients with advanced liver diseases have increased comorbidity burden as well as high HRU and health care costs in the commercially insured US population, emphasizing the importance and need of early identification and effective management in order to minimize the clinical and economic burden.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jcge.com.
ACKNOWLEDGMENTS
The authors thank Santosh Tiwari and Swathi Malla, formerly of IBM Watson Health, for providing the writing support, that was funded by Gilead Sciences Inc.
Footnotes
Funded in full by Gilead Sciences Inc.
R.J.W., N.K., D.J.M., M.M., A.B.O. and S.C.G.: contributed to the study concept and design, acquisition of data, statistical analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and approved the final draft of manuscript.
R.J.W. receives grant/research support from AbbVie and Gilead Sciences and was on the speaker bureau for Salix and Gilead, and serves as a consultant and on the advisory board for Gilead Sciences. N.K. is an employee of Gilead Sciences. D.J.M. was an employee of IBM Watson Health during the course of the study. M.M. is an employee of IBM Watson Health, which received funding from Gilead Sciences to conduct this analysis. A.B.O. is a former employee of Gilead Sciences. S.C.G. receives grant/research support from AbbVie Pharmaceuticals, Conatus, CymaBay, Exalenz, Gilead Sciences, Intercept Pharmaceuticals, and Merck and serves as a consultant/advisor for AbbVie Pharmaceuticals, Dova Pharmaceuticals, Gilead Sciences, Intercept Pharmaceuticals, and Merck.
REFERENCES
- 1.National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Definition & facts of NAFLD & NASH; 2016. Available at: www.niddk.nih.gov/health-information/liver-disease/nafld-nash/definition-facts. Accessed October 10, 2018.
- 2.Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. [DOI] [PubMed] [Google Scholar]
- 3.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–344. [DOI] [PubMed] [Google Scholar]
- 4.Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896–904. [DOI] [PubMed] [Google Scholar]
- 5.Rinella ME, Lominadze Z, Loomba R, et al. Practice patterns in NAFLD and NASH: real life differs from published guidelines. Therap Adv Gastroenterol. 2016;9:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chitturi S, Farrell GC, Hashimoto E, et al. Non-alcoholic fatty liver disease in the Asia-Pacific region: definitions and overview of proposed guidelines. J Gastroenterol Hepatol. 2007;22:778–787. [DOI] [PubMed] [Google Scholar]
- 7.Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol. 2014;5:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. [DOI] [PubMed] [Google Scholar]
- 10.Schuppan D, Schattenberg JM. Non-alcoholic steatohepatitis: pathogenesis and novel therapeutic approaches. J Gastroenterol Hepatol. 2013;28(suppl 1):68–76. [DOI] [PubMed] [Google Scholar]
- 11.Vilar-Gomez E, Vuppalanchi R, Gawrieh S, et al. Vitamin E improves transplant-free survival and hepatic decompensation among patients with NASH and advanced fibrosis. Hepatology. 2020;71:495–509. [DOI] [PubMed] [Google Scholar]
- 12.Cazzo E, Pareja JC, Chaim EA. Nonalcoholic fatty liver disease and bariatric surgery: a comprehensive review. Sao Paulo Med J. 2017;135:277–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hannah WN, Jr, Harrison SA. Effect of weight loss, diet, exercise, and bariatric surgery on nonalcoholic fatty liver disease. Clin Liver Dis. 2016;20:339–350. [DOI] [PubMed] [Google Scholar]
- 14.Kabbany MN, Conjeevaram Selvakumar PK, Watt K, et al. Prevalence of nonalcoholic steatohepatitis-associated cirrhosis in the United States: an analysis of National Health and Nutrition Examination Survey Data. Am J Gastroenterol. 2017;112:581–587. [DOI] [PubMed] [Google Scholar]
- 15.Ray K. NAFLD-the next global epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:621. [DOI] [PubMed] [Google Scholar]
- 16.Perumpail BJ, Khan MA, Yoo ER, et al. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23:8263–8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sayiner M, Otgonsuren M, Cable R, et al. Variables associated with inpatient and outpatient resource utilization among Medicare beneficiaries with nonalcoholic fatty liver disease with or without cirrhosis. J Clin Gastroenterol. 2017;51:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corey KE, Klebanoff MJ, Tramontano AC, et al. Screening for nonalcoholic steatohepatitis in individuals with type 2 diabetes: a cost-effectiveness analysis. Dig Dis Sci. 2016;61:2108–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crossan C, Tsochatzis EA, Longworth L, et al. Cost-effectiveness of non-invasive methods for assessment and monitoring of liver fibrosis and cirrhosis in patients with chronic liver disease: systematic review and economic evaluation. Health Technol Assess. 2015;19:1–409; v–vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahady SE, Wong G, Craig JC, et al. Pioglitazone and vitamin E for nonalcoholic steatohepatitis: a cost utility analysis. Hepatology. 2012;56:2172–2179. [DOI] [PubMed] [Google Scholar]
- 21.Tanajewski L, Harris R, Harman DJ, et al. Economic evaluation of a community-based diagnostic pathway to stratify adults for non-alcoholic fatty liver disease: a Markov model informed by a feasibility study. BMJ Open. 2017;7:e015659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang E, Wartelle-Bladou C, Lepanto L, et al. Cost-utility analysis of nonalcoholic steatohepatitis screening. Eur Radiol. 2015;25:3282–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Younossi ZM, Tampi R, Priyadarshini M, et al. Burden of illness and economic model for patients with non-alcoholic steatohepatitis (NASH) in the United States. Hepatology. 2018;69:564–572. [DOI] [PubMed] [Google Scholar]
- 24.Lonardo A, Nascimbeni F, Mantovani A, et al. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol. 2018;68:335–352. [DOI] [PubMed] [Google Scholar]
- 25.Wong RJ, Liu B, Bhuket T. Significant burden of nonalcoholic fatty liver disease with advanced fibrosis in the US: a cross-sectional analysis of 2011-2014 National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2017;46:974–980. [DOI] [PubMed] [Google Scholar]
- 26.Wong C, Lim J. The association between nonalcoholic fatty liver disease and cardiovascular disease outcomes. Clin Liver Dis. 2018;12:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 28.Gordon SC, Fraysse J, Li S, et al. Disease severity is associated with higher healthcare utilization in nonalcoholic steatohepatitis Medicare patients. Am J Gastroenterol. 2020;115:562–574. [DOI] [PubMed] [Google Scholar]
- 29.Petta S, Ting J, Saragoni S, et al. Healthcare resource utilization and costs of nonalcoholic steatohepatitis patients with advanced liver disease in Italy. Nutr Metab Cardiovasc Dis. 2020;30:1014–1022. [DOI] [PubMed] [Google Scholar]
- 30.Loomba R, Wong R, Fraysse J, et al. Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: a real world analysis of Medicare data. Aliment Pharmacol Ther. 2020;51:1149–1159. [DOI] [PubMed] [Google Scholar]
- 31.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in non-alcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Canbay A, Kachru N, Meise D, et al. Increasing Risk of Disease Progression and Mortality in Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis (NAFLD/NASH) Patients With Advanced Liver Disease: A German Real-World Analysis. Vienna, Austria: European Association for the Study of the Liver (EASL) International Liver Congress; 2019. [Google Scholar]
- 33.Boursier J, Fraysse J, Lafuma A, et al. Increased Risk of Mortality With Liver Disease Progression in Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis (NAFLD/NASH) Patients: An Analysis of French National Hospital Care. Vienna, Austria: European Association for the Study of the Liver (EASL) International Liver Congress; 2019. [Google Scholar]
- 34.Adams LA, Anstee QM, Tilg H, et al. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138–1153. [DOI] [PubMed] [Google Scholar]
- 35.Sinn DH, Kang D, Jang HR, et al. Development of chronic kidney disease in patients with non-alcoholic fatty liver disease: a cohort study. J Hepatol. 2017;67:1274–1280. [DOI] [PubMed] [Google Scholar]
- 36.Targher G, Chonchol M, Zoppini G, et al. Risk of chronic kidney disease in patients with non-alcoholic fatty liver disease: is there a link? J Hepatol. 2011;54:1020–1029. [DOI] [PubMed] [Google Scholar]
- 37.Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patton HM, Nyberg AH, Caparosa S, et al. Healthcare resource utilization, demographics, and comorbidities in non-alcoholic fatty liver disease (NAFLD)/Non-Alcoholic Steatohepatitis (NASH) and progressive stages in a large, integrated healthcare delivery system [Abstract]. Gastroenterology. 2018;154:S-1223-S–S-121224. [Google Scholar]
- 39.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: selected practical issues in their evaluation and management. Hepatology. 2009;49:306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fracanzani AL, Valenti L, Bugianesi E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48:792–798. [DOI] [PubMed] [Google Scholar]
- 41.Aguilar M, Bhuket T, Torres S, et al. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA. 2015;313:1973–1974. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed A, Wong RJ, Harrison SA. Nonalcoholic fatty liver disease review: diagnosis, treatment, and outcomes. Clin Gastroenterol Hepatol. 2015;13:2062–2070. [DOI] [PubMed] [Google Scholar]
- 43.Allen AM, Van Houten HK, Sangaralingham LR, et al. Healthcare cost and utilization in nonalcoholic fatty liver disease: real-world data from a large US claims database. Hepatology. 2018;68:2230–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McAdam-Marx C, McGarry LJ, Hane CA, et al. All-cause and incremental per patient per year cost associated with chronic hepatitis C virus and associated liver complications in the united states: a managed care perspective. J Manag Care Pharm. 2011;17:531–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eslami L, Merat S, Malekzadeh R, et al. Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2013;12:CD008623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papademetriou M, Athyros VG, Geladari E, et al. The co-existence of nash and chronic kidney disease boosts cardiovascular risk: are there any common therapeutic options? Curr Vasc Pharmacol. 2018;16:254–268. [DOI] [PubMed] [Google Scholar]
- 47.Musso G, Cassader M, Cohney S, et al. Fatty liver and chronic kidney disease: novel mechanistic insights and therapeutic opportunities. Diabetes Care. 2016;39:1830–1845. [DOI] [PubMed] [Google Scholar]
- 48.Caldwell S. NASH therapy: omega 3 supplementation, vitamin E, insulin sensitizers and statin drugs. Clin Mol Hepatol. 2017;23:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maroni L, Guasti L, Castiglioni L, et al. Lipid targets during statin treatment in dyslipidemic patients affected by nonalcoholic fatty liver disease. Am J Med Sci. 2011;342:383–387. [DOI] [PubMed] [Google Scholar]
- 50.Willey VJ, Kong S, Wu B, et al. Estimating the real-world cost of diabetes mellitus in the united states during an 8-year period using 2 cost methodologies. Am Health Drug Benefits. 2018;11:310–318. [PMC free article] [PubMed] [Google Scholar]
- 51.Golestaneh L, Alvarez PJ, Reaven NL, et al. All-cause costs increase exponentially with increased chronic kidney disease stage. Am J Manag Care. 2017;23:S161–S172. [PubMed] [Google Scholar]
- 52.Nichols GA, Bell TJ, Pedula KL, et al. Medical care costs among patients with established cardiovascular disease. Am J Manag Care. 2010;16:e86–e93. [PubMed] [Google Scholar]
- 53.Eliades M, Spyrou E. Vitamin D: a new player in non-alcoholic fatty liver disease? World J Gastroenterol. 2015;21:1718–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hariri M, Zohdi S. Effect of vitamin D on non-alcoholic fatty liver disease: a systematic review of randomized controlled clinical trials. Int J Prev Med. 2019;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barchetta I, Cimini FA, Cavallo MG. Vitamin D supplementation and non-alcoholic fatty liver disease: present and future. Nutrients. 2017;9:1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. [DOI] [PubMed] [Google Scholar]
- 57.Kulaylat AS, Schaefer EW, Messaris E, et al. Truven health analytics marketscan databases for clinical research in colon and rectal surgery. Clin Colon Rectal Surg. 2019;32:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jcge.com.


