Presenting 3D printed, personalized biosymbiotic devices for continuous monitoring of high-fidelity biosignals.
Abstract
Digital medicine, the ability to stream continuous information from the body to gain insight into health status, manage disease, and predict onset health problems, is only gradually developing. Key technological hurdles that slow the proliferation of this approach are means by which clinical grade biosignals are continuously obtained without frequent user interaction. To overcome these hurdles, solutions in power supply and interface strategies that maintain high-fidelity readouts chronically are critical. This work introduces a previously unexplored class of devices that overcomes the limitations using digital manufacturing to tailor geometry, mechanics, electromagnetics, electronics, and fluidics to create unique personalized devices optimized to the wearer. These elastomeric, three-dimensional printed, and laser-structured constructs, called biosymbiotic devices, enable adhesive-free interfaces and the inclusion of high-performance, far-field energy harvesting to facilitate continuous wireless and battery-free operation of multimodal and multidevice, high-fidelity biosensing in an at-home setting without user interaction.
INTRODUCTION
Availability of continuous patient biodata is poised to improve diagnosis, management, and treatment of disease (1–4) when combined with powerful data analysis methods (5–7). This concept of digital medicine is especially powerful when combined with wearable technologies providing researchers and clinicians with tools to extract continues streams of biodata during daily activity of the user (8), enabling early detection of hard-to-diagnose diseases such as onset frailty (9) and intermittent cardiac arrhythmia (10). Advances of data infrastructure and development of artificial intelligence enable effective analysis of large streams of biodata with the ability to automatically extract long-term and acute variations and trends (11). Despite these promising early results and analytical capabilities, technological development of sensing systems suitable for this application has been unable to match the pace of new analysis methods. Many currently implemented sensing systems do not allow for long-term, uninterrupted data streams, with signal fidelity needed for clinical diagnosis. Contemporary systems require frequent user interaction (12, 13) and lack sensing functionality specific to disease paradigms across the general population (14–17).
Recent advances in wearable electronic systems (18–22) have aimed to address some of these issues by means of wireless power transfer techniques (23, 24), soft mechanics (25–27), and miniaturized electronic systems to realize a class of soft devices that intimately integrates with the body (20, 22, 28, 29). The improved sensing interface is achieved by miniaturization and matching of mechanic modulus to the epidermis, improving sensing fidelity beyond the capabilities of current wired or battery-powered systems (25, 26, 30), which suffer from motion artifacts and require frequent user interaction for operation. Despite these advances, the utilization of wireless and battery-free wearable devices is limited to defined application scenarios because of power transfer methods that require close proximity to the power casting device (31, 32), prohibiting free motion of the individual or requiring a secondary reader device such as a smartphone. In addition, the use of adhesive-based bonding of device body and bio interfaces to sensing locations limits the lifetime of systems that are adhered to the skin or other location of the body, which are subject to epidermal renewal. Cell turnover of the epidermis also frustrates device interface fidelity, prohibiting chronic recording applications beyond days and weeks depending on location (33).
To overcome these challenges, we propose a new class of wearable device technology that uses a high level of personalization facilitated through digital fabrication of soft and highly conformal elastomers and integration of flexible electronics that are automatically and individually fitted to the wearer. The devices are powered wirelessly over a long range to facilitate continuous acquisition of clinical grade biosignals. We achieve this using three-dimensional (3D) information captured from the individual by a smartphone or harvested from magnetic resonance imaging (MRI), computed tomography (CT), or 3D scans to create unique device geometries that are tailored directly to the wearer to achieve high sensing fidelity with optimal sensor placement and facilitate optimum circumferential fit to enable an adhesive-free conformality of a soft electronics class that we call biosymbiotc devices.
Creating these individualized biosymbiotc devices requires a new method of fabricating soft bioelectronics that uses digital fabrication techniques. Using 3D printing technologies, topologically complex structures are created, which enable the use far-field energy harvesting schemes on the body. Combination of these technologies allows for a device class that is able to record chronically with multimodality and without user interaction in a format that is nearly imperceptible the wearer to provide continuous data streams in an at-home setting, enabling the concept of digital medicine in broad range of applications.
RESULTS
Device overview
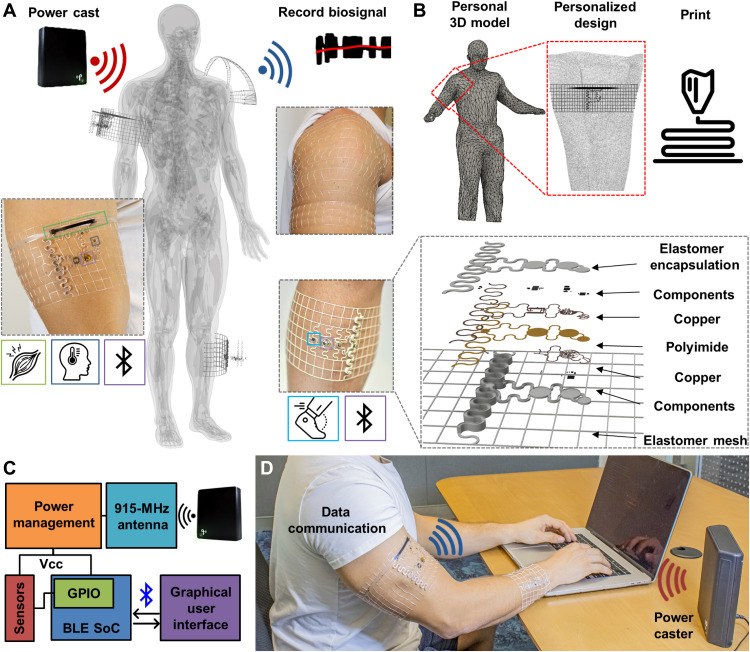
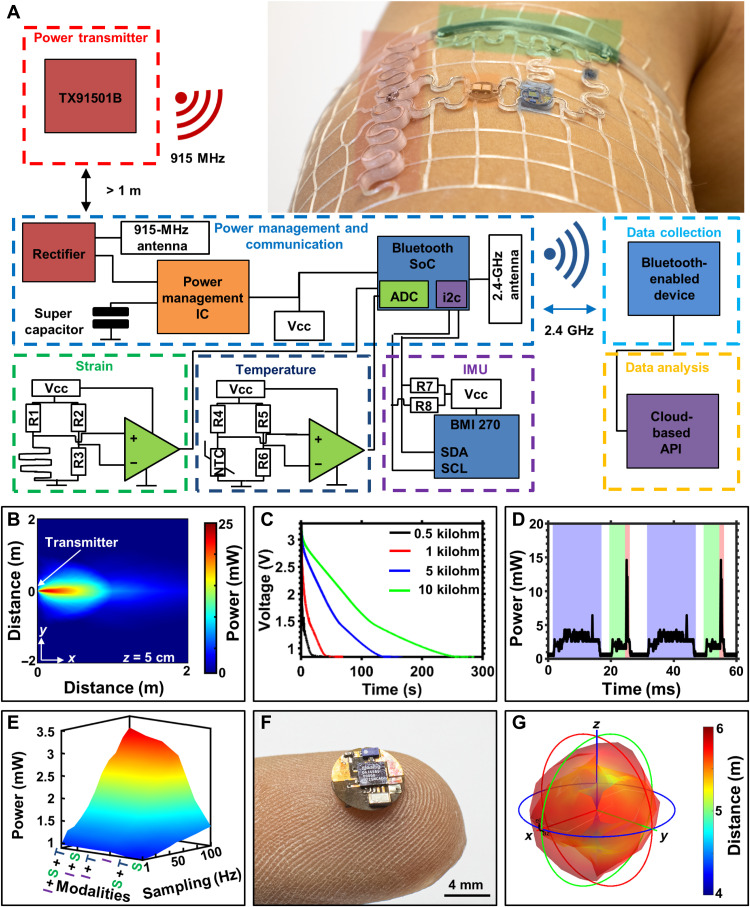
A schematic overview of biosymbiotc devices is presented in Fig. 1. Figure 1A depicts an illustrative representation of device function featuring wireless and battery-free operation with a network of devices adhered to topologically complex regions of the body with multimodal sensing capabilities. The devices are composed of a fusion deposition model (FDM) 3D printed thermoplastic polyurethane (TPU) mesh that is designed using the wearers own physiological topology information and can be tailored directly to the sensing needs of the user. Utilization of 3D printing technologies enables discrete digital control over mesh geometries, which allow for user tailored designs with modulation of mechanical properties, enabling device wearability without the need of adhesives, and sensor locations that enable ideal sensing interface placement for each subject, even to highly mobile regions of the body such as the shoulder. Topology control afforded by FDM printing also enables more efficient antenna designs that lead to power harvesting functionalities that would not be practical or achievable using contemporary methods.
Fig. 1. Device overview.
(A) Illustration showing system level functionality of biosymbiotic devices with capabilities of multisite, long-distance, wireless, and battery-free operation. (B) Workflow of personalization and fabrication to create biosymbiotic devices. (C) High-level functional diagram showing device working principle. VCC, Voltage common collector. (D) Photographic image of subject wearing multiple biosymbiotic devices during chronic study. Photo credits: Tucker Stuart and Dylan McGuire, University of Arizona.
The design process of these personalized devices is schematically displayed in Fig. 1B, where a 3D scan of the user is collected with techniques including MRI (34), CT (34), laser scanning (35), or photogrammetry (36) with subsequent topological information extraction to determine device shape and mechanics design to ensure conformal fit. The elastomeric mesh provides epidermis-like mechanics (37), which improves sensing fidelity by reducing motion artifacts and improving electrical and thermal impedances (26, 38). The devices also feature an ultralight weight (250 to 550 mg) and low height (0.9 to 3 mm), enabling almost imperceptible wearability. The mechanics of the mesh enable adhesive-free wearability, which keeps the device in place, even during exercise in extreme heat (see fig. S1). Embedded in the elastomeric platform are highly miniaturized, stretchable, and laser-structured electronics on flexible substrates (39) as described in Materials and Methods, providing system functionality without compromising bulk mechanical properties. Active electronic components are placed on flexible nodes of 6 mm in diameter or less that are connected via stretchable serpentine interconnects, enabling soft mechanics and a low average device density on the skin (10% fill factor) that facilitates free perspiration and minimal obstruction to the skin while allowing for epidermal turnover. These electronic components are embedded in an encapsulation layer of printed TPU, providing waterproofing and allowing for prolonged system function regardless of external environmental factors. For sensors that require close contact to the epidermis and minimal impedance, a 3D structured opening is used for sensor placement, which is then encapsulated using thin, compatible materials and strategies (39, 40) to provide low impedance while maintaining electrical integrity.
The operating principle of the system is detailed in Fig. 1C. The devices harvest energy using a commercially available 915-MHz power casting system that transmits power wirelessly to the biosymbiotc devices. A key characteristic of the system is the use of 3D printed antenna structures to enable far-field power transfer directly on the skin by isolating radiating planes of the antenna to avoid dielectric losses (41–43). Energy management is facilitated via active harvesting electronics and capacitive energy storage to maintain operational voltage in an at-home setting over meters of distance, enabling uninterrupted operation. Communication and sensor digitalization are facilitated via a Bluetooth low-energy (BLE) system on a chip (SoC), which allows for integration with existing infrastructure (44). Figure 1D shows an image of this system in use. The power casting system used here offers minimal size, is low cost, and is commercially available, allowing for minimal infrastructure requirements for continuous operation of the devices without the need for user interaction (45, 46).
Fabrication
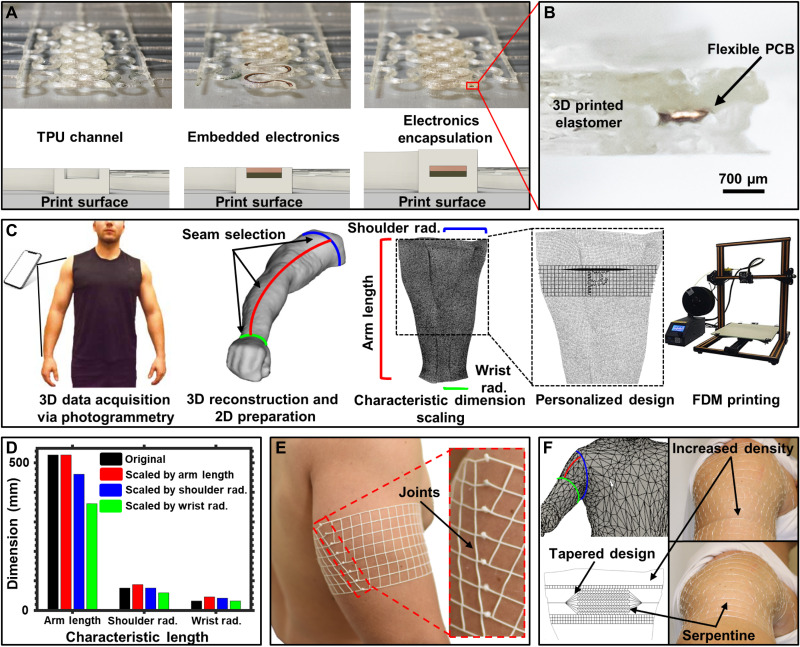
Core to realizing biosymbiotc devices is the ability to fabricate using exclusively digital methods to individualize every structure according to the user specific geometries. Figure 2A details the FDM-based fabrication, a technique that can provide rapid throughput when material volumes are low. The base layer (450 μm) of TPU is printed onto a glass surface, and device geometries incorporate a channel with a 200 μm-thick wall at a height of 300 μm above the base layer. This creates a 3D printed channel that serves as a receptacle that guides placement of the flexible printed circuits and electronics. The circuits are placed into the mesh by hand, using 3D structured channels to guide placement (see movie S1). The resulting structure is subsequently encapsulated by continuation of the 3D printing process, which deposits a layer of TPU over the channel with embedded circuity, forming a seal around the electronics. This methodology of fabrication is compatible with commercial pick-and-place methods, symbiotic with reel-to-reel manufacturing of flexible circuits to enable medium- and large-scale fabrication in the future. The TPU material used for the devices presented here is an 85-A shore hardness elastomer (NinjaTek, NinjaFlex). A video depicting this process is shown in movie S1. Top and bottom layers are composed of 450 μm-thick layers (150 μm z-axis layer height) and 200 μm-wide sidewalls (100 μm xy resolution). This ratio provides sufficient encapsulation of the electronics and is tolerant to printing imperfections without adding excessive bulk. Figure 2B shows a cross-sectional image of a section of an electronic trace that has been embedded in a 3D printed elastomeric material.
Fig. 2. Fabrication.
(A) Illustration and corresponding photographs of device fabrication. (B) Cross-sectional micrograph showing flexible printed circuit board (PCB) embedded in 3D printed TPU material. (C) Illustration detailing photogrammetry process and physiological information extraction. (D) Bar graph detailing error associated with scaling 2D design with unique characteristic dimensions of the body. (E) Photographic image of device tailored to arm with corresponding seam joint connection to enable circumferential fit. (F) 3D scanned mesh and resulting device design featuring nonlinear mechanics. Photo credits: Tucker Stuart and Dylan McGuire, University of Arizona.
Digital design and manufacturing are composed of a three-stage process where topological data are extracted from a 3D model of the subject, the device design is developed around the information gathered from the scan, and the physical mesh structure is printed with individualized placement of the electronics and sensors. Figure 2C details this process. We choose to use photogrammetry as scanning method, because no special hardware other than a smartphone is needed and provides sufficient accuracy, outlined in Materials and Methods and fig. S2. The 3D model is transformed to a 2D object by splitting along a seam that is selected along the proximal-distal axis of the body and circumferentially around key physiological landmarks. This process yields a cylindrical shape that is unfolded into a 2D object. For the arm, we yield three characteristic dimensions, namely, arm length, wrist radius, and shoulder radius, that can be used for scaling the 2D object to subject dimensions as shown in Fig. 2D. The dimensional accuracy of the generated 2D mesh using photogrammetry is ±24% with an average scaling factor obtained from the characteristic dimensions, sufficient to adapt the design of elastomeric devices to a user while retaining reliable epidermal contact. The individualized device is created by cropping a device template with the outline of the 2D mesh. At the termination of the mesh lines, circular nodes of 3 mm in diameter are placed. After the printing process, these nodes are bonded together using heat to remelt the TPU to form a joint as shown in Fig. 2E. These joints provide similar mechanical stability to printed linear structures; details are shown in fig. S3.
This personalized approach can also be applied to more complex geometrical targets of the body such as the shoulder. Following simple design rules, complex device geometries can be implemented to form device properties for highly dynamic areas of the body. These rules are detailed in Materials and Methods. A demonstration is presented in Fig. 2F that shows device design with a resulting structure that conforms to the shoulder over the lateral head of the deltoid and around the proximal bicep. Detailed information can be found in fig. S4. The shoulder device features spatial modulation of the elastomeric mesh mechanics to create nonlinear mechanics that lie along the proximal-distal axis of the medial deltoid muscle to secure the mesh structure to the shoulder without slipping. Topology information extracted from 3D datasets can be used to drive design choices to realize mechanical device properties suitable for operation in highly mobile areas (see fig. S5). Serpentine pattern traces along the medial deltoid ensure conformal fit regardless of arm adduction or abduction. The density of the mesh pattern is altered on the basis of the mobility of the region with minimal material running under the axilla region to improve wearability and comfort.
Mechanical characterization
Key to creating devices that conform to complex areas of the body and key to unlock advanced electric and electromagnetic designs are nonlinear mechanics that make use of 3D structures enabled by FDM printing and the embedding of flexible and stretchable electronics. Figure 3 explores these aspects of design and characterizes mechanical properties with variations in 3D layouts to create a toolbox to engineer bulk and localized mechanics.
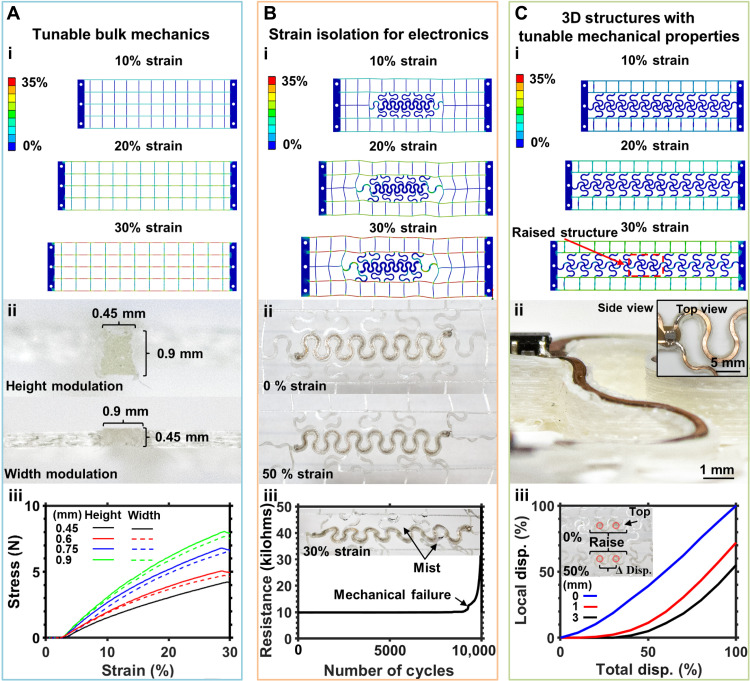
Fig. 3. Mechanical characterization.
(A) Tunable bulk mechanics. (i) Finite element analysis (FEA) showing mesh structure at 0 to 30% strain. (ii) Cross-sectional micrographs of thickness and height modulation of the mesh structure. (iii) Stress versus strain curve displaying results of height and thickness modulation and their effect on bulk mechanical properties. (B) Strain isolation schemes for electronics. (i) FEA showing basic mesh structure at 0 to 30% strain. (ii) Photographic image of mesh with embedded electronics strained to 50% strain without damaging electronic integrity. (iii) Graph of embedded electronics device resistance more than 10,000 cycles of 30% strain while misting (shown in inset) with 0.1 M phosphate-buffered saline (PBS). (C) 3D Structures with tunable mechanical properties. (i) FEA of 3D mesh structure at 0 to 30% strain. (ii) Photographic image of 3D structure in top and side views. (iii) Plot of local displacement of raised structure versus total system displacement. Photo credit: Tucker Stuart, University of Arizona.
Figure 3A details the impact of geometrical variation on basic linear structures. Figure 3Ai shows finite element analysis (FEA) models of these linear structures strained to 10, 20, and 30%, which represent physiologically relevant strain levels (47). In the FEA models, a linear distribution of strain along the axis of displacement is observed with a small level of Poisson’s ratio that introduces compression in the off axis (48). Material behavior analysis for linear structure design can be found in fig. S6. Bulk and discrete mechanical properties of these meshes can be modulated by varying the width and/or height of the linear structures spatially as shown in Fig. 3Aii. The results of this modulation alter the average elastic stress of the mesh. Height and width of the traces modulated by 150 μm result in a linear increase in stress of approximately 6 N per step. Height modulation provides a slightly higher stress increase due to differences in axial resolution of the printer as shown in Fig. 3Aiii. Using this principle, spatially discrete changes in mechanical properties can be implemented, providing a tool for engineered strain profile to better accommodate physiology.
Although adequate for providing conformal application to the target sensing interface, the linear structures fail to isolate strain of embedded electronic systems (27). Serpentine structures commonly used in soft electronics (49–51) provide means to isolate encapsulated electronics from strain. This principle is demonstrated in FEA shown in Fig. 3Bi. Embedding the serpentines in the linear mesh discussed earlier enables redirection of strain in the flexible electronics toward the linear structures, resulting in copper layers embedded in the serpentine structure to experience less than 0.1% strain with an applied global strain of 30%. Material behavior analysis for this pattern can be found in fig. S6. Serpentine dimensions and design used for strain isolation can be found in Materials and Methods. Figure 3Bii shows photographic images of a serpentine trace with embedded flexible printed circuit board (PCB) encapsulated in the mesh displaced at 0 and 50%, respectively. The added stiffness to the mesh by the electronics yields highly nonlinear strain behavior, creating robust strain isolation and resulting in material behavior as shown in fig. S7. In this configuration, larger displacements of up to 50% can be tolerated without breakdown of the TPU encapsulation (see fig. S8). This can be demonstrated by cyclic testing of the samples in corrosive environments detailed in Fig. 3Biii. Resistance of the serpentine patterned flexible circuit material (Pyralux, AG185010RY; DuPont) is tested more than 10,000 cycles of 30% strain and, to simulate physiological conditions, misted with a 0.1 M phosphate-buffered saline (PBS) solution every 50 cycles. Negligible change in resistance is observed for both experimental conditions, demonstrating effective strain isolation and encapsulation.
3D modulation of elastomeric material is typically not used in wearable electronics because of challenging fabrication techniques (52) that are difficult to scale and leave thin, free-standing structures exposed to damage from objects catching on them such as clothing. 3D shapes, however, can be highly beneficial to modulate electromagnetic and electromechanical environments to create highly efficient far-field antennas. FDM printing allows for fine topological control and utilization of complex structures to add spatially dependent rigidity. This is demonstrated in Fig. 3Ci, where FEA simulation shows the decrease in local strain due to raised portion of the mesh structure. At 30% strain, this becomes evident, where the raised portion of the mesh, highlighted in red, experiences almost no stress and displacement. To achieve this modulation and retain electrical connectivity, ramp structures are used (shown in Fig. 3Cii) to gradually raise the flexible PCB traces. The resulting structure features seamless modulation in mechanical and topological properties. Material behavior analysis for these structures can be found in fig. S6. Devices displayed in Fig. 3Ciii demonstrates this concept; here, devices with a ramp angle of 0° to 25° and a feature height of 0 to 3 mm are strained to 100%, and local displacement on the raised structure versus global displacement is recorded using image analysis, as detailed in Materials and Methods. The ratio between total and local displacement decreases with increased structure height, as well as the amount of total displacement before local displacement resulting in an excellent tool to modulate mechanical and electromagnetic properties.
Antenna design and characterization
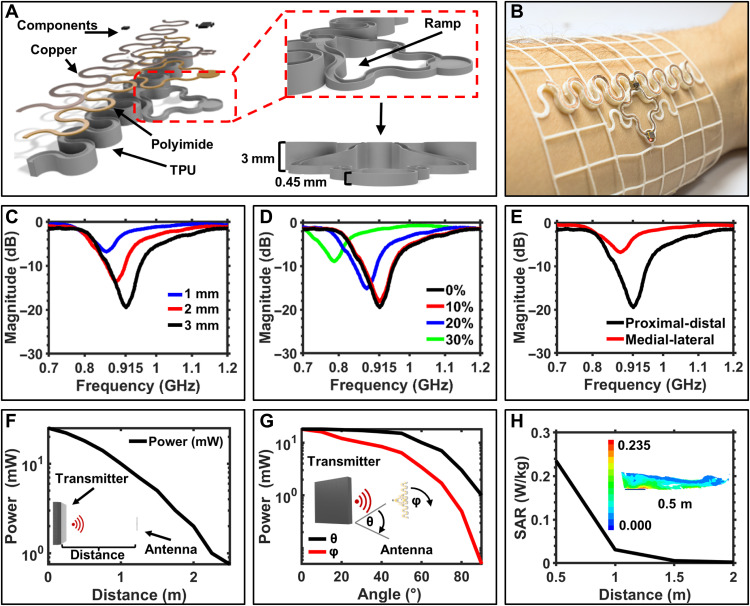
The use of 3D topologies enables the implementation of antenna designs with minimal losses associated with absorption of Industrial, scientific, and medical application (ISM) frequencies of the body. Figure 4A shows an exploded view schematic of a stretchable dipole antenna design with raised radiating plane to increase efficiency at 915 MHz; information about antenna design and tuning can be found in Materials and Methods. Figure 4B shows a photographic image of the device embedded in a mesh designed to circumferentially attach on the distal forearm. Figure 4C shows the reflection measurement of the serpentine antenna system at varying distances from the skin. The antenna is designed to operate at 3 mm from the body and resonates at 916 MHz with a return loss of −19.5 dB. Reducing the height of the raised structure to 2 and 1 mm shifts the resonant frequency down to 885 and 864 MHz with a return loss of −13.67 and −6.69 dB, respectively, highlighting the losses associated with operation in proximity to the skin. Lateral strain on the serpentine antenna (Fig. 4D) introduces an effective increase in the antenna length, which shifts the resonant frequency. Placement of the antenna is also critical as shown in reflection measurements in Fig. 4E, where the relative orientation of the antenna along the proximal-distal and medial-lateral axes is explored, introducing components of lateral strain and bending of the antenna structure. Performance decrease in medial-lateral orientation is observed because of bending of the antenna, which detunes and results in more evanescent field being absorbed in the tissue. Figure S9 details a power versus load curve showing peak power harvesting at 35.5 mW at a 555 Ω load.
Fig. 4. Antenna design and characterization.
(A) Illustration of 3D topology used for implementation of 915-MHz antenna with rectifier and power management system. (B) Photographic image of device with rectifier and power management system located on the distal portion of the arm. (C) Reflection measurement of antenna on the skin with varying thicknesses of TPU. (D) Reflection measurement of antenna on the skin with varying levels of induced strain on the device along the dipole axis. (E) Reflection measurement of antenna on the skin with placement along the proximal-distal axis and the medial-lateral axis (curvature of radius, 2.5 mm). (F) Harvesting power output as a function of distance from the power transmitter. (G) Logarithmic plot harvesting power output as a function of angle from the transmitter at a set distance of 40 cm (θ) and a function of angular orientation of the antenna at 40 cm (φ). (H) Plot of specific absorption rate (SAR) values at 915 MHz with varying distances from the transmitter with inset showing FEM analysis of SAR on human arm phantom at 0.5 m away from the transmitter. Photo credit: Tucker Stuart, University of Arizona.
Energy harvesting performance of the antenna is characterized under field conditions (see Materials and Methods) (53). Data showing the distance of operation are plotted in Fig. 4F, where peak power harvesting reaches 25.7 mW at 10 cm from the transmitter with 9.76 and 2.05 mW of power harvested with a load of 1 kΩ at 1 and 2 m, respectively. Angular position of the antenna in relation to the transmitter (Fig. 4G, black) shows little reduction in performance at 40 cm up to an angle of 45°, after which the misalignment of the antenna results in sharp harvesting power drop-off. Rotation about the roll axis of the antenna, which would be indicative of arm motion, shows a more immediate effect on harvesting performance due to the vertical polarization of transmitter antenna of the power casting system (Fig. 4G, red). Figure S10 provides additional information for multipower casting unit setups and their effect on functional range and powering capabilities of the system. Using an federal communications commission (FCC)–approved power caster enables management of electromagnetic impact on the wearer; this is additionally confirmed with finite element method (FEM) simulation of the specific absorption rate (SAR) that demonstrates <0.24 W/kg of absorption at 0.5 m from the transmitter (Fig. 4H), well below the U.S. guideline for an exposure of less than or equal to 1.6 W/kg. Figure S11 provides further details on FEM simulation of SAR values at varying distances from the transmitter.
Electrical characterization
Integration of digital electronics and multifunctional sensors enables the indefinite recording of clinical grade biosignals. Essential to design of these complex systems is a symbiosis of electromechanics and digital electronics that enable a high degree of system functionality and reliability of operation. This can be achieved via flexible islands with high density of digital electronics that make use of miniaturized, off-the-shelf components. These high-density nodes, with no more than 6 mm in diameter provide miniaturized island structures that do not affect the soft bulk mechanics of the system and allow for modularized designs. Figure 5A shows a simplified electrical schematic overview of a multimodal system. Power is transmitted to the device using a commercially available and FCC-approved 3 W power transmitter (Powercast, TX91501B). Power is harvested by the serpentine dipole antenna and rectified to DC power using a full bridge rectifier (materials and components can be found in Materials and Methods). The harvested energy is buffered by a supercapacitor that provides stable power in the event of harvesting drop-offs associated with normal motion and obstructions of line of sight. Harvested energy is sufficient to power digital BLE systems that can operate continuously, relaying multimodal sensor data such as strain, temperature, and inertial measurements. Detailed system description can be found in Materials and Methods. Figure 5B shows a heatmap of power availability of 25 mW at up to 1 m from the transmitter and power that received more than 2 mW at 2 m, with sharp attenuation off axis. Utilization of multiple transmitters and/or beam steering can extend the range markedly; details are shown in fig. S5. Figure 5C shows time-dependent voltage profiles of the power management system, discharging a full charge of the 11-mF supercapacitor with increasing loads. Power is used for active components of sensors systems, namely, the inertial measurement unit (IMU) and low-power operational amplifiers for analog sensing systems, as well as the BLE SoC responsible for data aggregation and BLE communication.
Fig. 5. Electrical characterization.
(A) Block diagram of system level components and wireless power casting and data receiving units. (B) 2D plot of energy availability as a function of distance and angle from the transmitter. IC, integrated circuit; API, application programming interface; SDA, serial data line; SCL, serial clock line. (C) Power management system discharge with increasing load. (D) Power consumption of SoC using all sensing modalities (strain, temperature, and IMU shaded blue and green) with a sampling frequency of 50 Hz; transmission events shaded red. (E) 3D representation of power consumption with variation of duty cycle and number of sensing modalities used where modalities are color-coded and labeled by the following: I, IMU; S, strain; T, Temperature. (F) Photographic image showing custom BLE board with on-board BLE SoC and antenna. (G) Isosurface plot showing BLE antenna transmission performance distance on body with varying angles of yaw, pitch, and roll. Photo credit: Tucker Stuart, University of Arizona.
Figure 5D shows the temporally resolved power consumption of the device measuring data from the temperature sensor, strain gauge, and IMU at 50-Hz sampling. The blue shaded regions indicate circuit communication between the IMU and BLE SoC, which requires 15 ms per event; the green shaded region indicates analog-to-digital converter (ADC) reads from the general purpose input/output (GPIO) pins for the analog sensing systems; and red indicates the sending events. Peak power consumption occurs during the sending event of data over BLE, which does not depend on the sensing modalities used and is generally 14 mW. Implementation of sleep functions results in an average system power consumption ranging from 3.25 to 0.95 mW, depending on sensing modalities and sampling frequencies used. This is illustrated in Fig. 5E, where a 3D map of power consumption is shown, given the sensing modalities and sampling rates (additional characterization and information can be found in Materials and Methods and fig. S12). Using this information, modulation of system sensing modality and fidelity can be adjusted according to available power. This is achieved through monitoring the voltage on the supercapacitor and modulating sensing modality operation accordingly. Firmware on the BLE SoC evaluates which modalities receive power such that the power requirement allows for operation suitable for current power availability. Continuous operation at moderate sampling rates (2 Hz) and multimodal sensing (IMU, strain, and thermography) can be sustained at operational distances of up to 2 m from a single transmitter with continuous operation at more than 3 m possible with multiple transmitters (see table S1). Device function can be retained for 10 to 60 s in case of complete loss of harvesting power and can be restarted reliably via brownout control (see figs. S13 and S14). The components used to create these systems are available in highly miniaturized packages, yielding a 5 mm diameter BLE SoC node shown in Fig. 5f with detailed PCB layout presented in fig. S15. Here, the 0201 chip antenna was used to conserve space while providing adequate communication distances (up to 6 m) regardless of orientation along the principal axes (see fig. S16). Figure 5G shows an isosurface plot detailing link stability of the BLE chip antenna while attached to the distal portion of the forearm.
Sensing modalities
A clear benefit to this class of devices is their ability to provide robust and intimate sensing interfaces without the need for adhesive or other short-term bonding solutions. This enables unique sensing capabilities for long-term data acquisition. In addition, personalized designs enable accurate control over sensing location. This provides the ability to place sensors in physiologically relevant locations with high accuracy, increasing data relevancy, fidelity, and selectivity of the sensors.
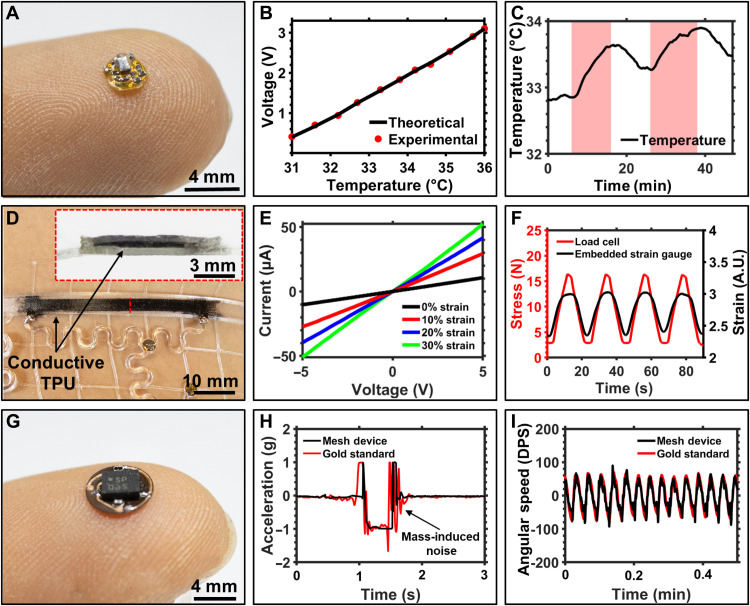
One such modality demonstrated here is a submillikelvin (<0.001 K) resolution temperature sensor shown in Fig. 6A. This sensor node is 2.5 mm in diameter and is composed of a Wheatstone bridge and differential operational amplifier using an negative temperature coefficient (NTC) thermistor to detect subtle change in skin surface temperature. 3D mesh designs allow the NTC to fit through the elastomeric material in a defined opening during the digital fabrication process to enable intimate contact of the sensor with the skin. The NTC is then encapsulated with a thin layer of ultraviolet (UV)–cured glue to prevent exposure to sweat (see fig. S17). The small footprint of the system enables placement onto regions of the body, such as the axilla, that allow for pseudo-core body temperature measurements. Inherently low thermal mass can be leveraged to increase sensitivity to subtle changes in temperature as detailed in Fig. 6B, and sensors feature a 4°C dynamic range and with a 12-bit resolution, achieved by oversampling the internal 10-bit ADC of the SoC, with submillikelvin resolution. Utilization of a high-resolution temperature sensor enables long-term analysis of subtle variation in average body temperature that indicates onset and progression of disease (54–59) and the ability to detect acute events of increased activity (60). The efficacy of this sensing system is demonstrated in Fig. 6C. Here, the temperature sensor is worn in the axilla region of the body. The subject is seated at a desk (shaded white) for the beginning of the experiment and walks at 2.5 miles/hour for 10 min (shaded red). Increase in axilla skin temperature is clearly visible upon this light exercise with sequential increases of less than 1°C per event, highlighting the sensitivity of the system.
Fig. 6. Sensing modalities.
(A) Photographic image of double-sided, submillikelvin temperature sensor board. (B) Calibration curve of temperature sensor with linear operation throughout target temperature range benchmarked against theoretical model. (C) Recording of body temperature from axilla region of a test subject during sitting (white) and walking hastily (red). (D) Photographic image of fully 3D printed strain gauge. (E) Current-voltage curve of strain gauge with increasing levels of strain. (F) Graph showing cyclic tests of 3D printed strain gauge with 16 N of applied stress (red) and corresponding resistance response (black). A.U., arbitrary units. (G) Photographic image of inertial measurement node. (H) Graph of lower leg acceleration of biosymbiotic device (black) and gold standard, battery-powered device (red) during a stationary jump. (I) Graph of angular speed during a continuous walking period comparing the biosymbiotic device (black) and gold standard, battery-powered device (red). DPS, degrees per second. Photo credit: Tucker Stuart, University of Arizona.
The benefit of FDM printing includes the ability to print with multifunctional materials. We demonstrate this with completely 3D printed strain gauges that integrate directly into the biosymbiotic devices as seen in Fig. 6D. A conductive TPU material (NinjaTek, Eel) is printed and embedded into mesh following the procedure outlined in Materials and Methods. FDM printing of the conductive elastomer allows for manipulation of design parameters, such as length, thickness, and width, which subsequently alters performance metrics, as shown in fig. S18, and can be tailored to the application location and user. Figure 6E shows the current-voltage characteristics of the strain gauge, which demonstrates an ohmic response with linearity across voltage supplies of −5 to 5 V. Sensitivity of the strain gauge is, per design, highest between 0 and 10% strain to match physiologically relevant strains. Accelerated rate testing showed sensor robustness over multiple cycles of up to 30% of strain. Figure 6F displays a representative data collection measuring bulk stress of the system (see fig. S18). In this experiment, the strain gauge is tested while embedded in the structure, analog to the use in a wearable device, such that not all stress is transferred to the strain gauge, resulting in reduction of measured peak force. Figure S19 details isolated strain gauge function to show naïve sensor performance, which matches stress profiles. For applications requiring strain measurements that follow stress profiles exactly, compensation can be performed computationally. Cyclic tests demonstrate repeatability of sensor function over time with response in line with bulk stress on the mesh, detailed information about testing procedures can be found in Materials and Methods.
To demonstrate the use of digital, high-fidelity and power-intensive sensor systems and to demonstrate the system’s ability to eliminate motion artifacts through conformal integration and low mass (250 mg), a 4 mm diameter sensor node containing an IMU for collection of accelerometric and gyroscopic data is characterized (Fig. 6G). This sensor is placed on the proximal region of the lower leg around the calf and used to extract gait parameters. The performance is measured against a gold standard, battery-powered IMU (BioSensics, LEGSys) that is affixed on the same region of the lower leg during a series of vertical jumps. Figure 6H shows the corresponding accelerometry data collection from both devices during the vertical jump; details can be found in Materials and Methods. The mesh device shows a clear initiation of the jump motion, with a 15% improvement in signal-to-noise ratio compared to the gold standard system. Motion artifacts arising from gold standard device inertia associated with device mass (55.25 g) are visible, and oscillation of the accelerometer unit can be clearly seen in comparison to the biosymbiotic device. Fidelity of the sensor to assess gait parameters is tested against the gold standard. In this study, the subject wares both systems and walks on a treadmill for 3 min at a pace of 2.5 miles/hour. Accelerometric and gyroscopic data show good compliance between both the integrated mesh and gold standard devices. Figure 6I shows a sample of data segment collected during this experimental period.
3D printed epifluidics
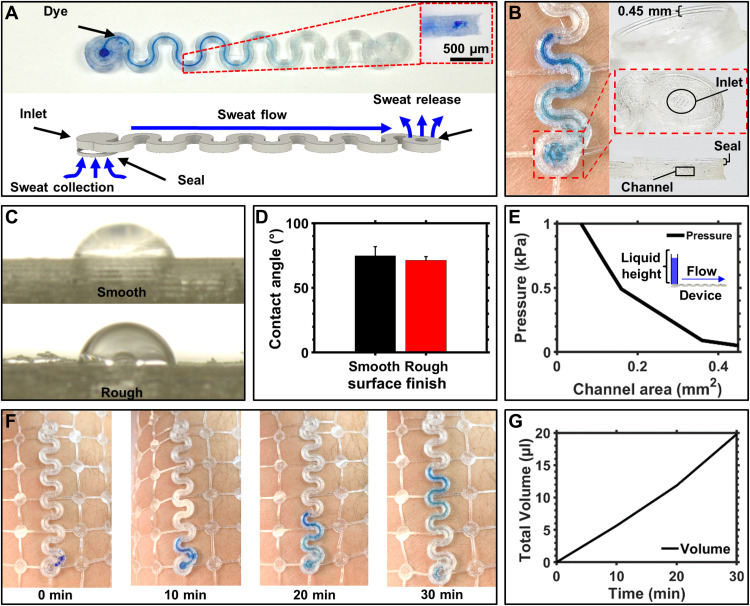
A recent development in biosensors has been the collection of eccrine sweat with epifluidics, as means of noninvasive biomarker detection (58, 61–67). This is currently realized with microfluidic devices that capture sweat with an adhesive interface and route the biofluid inside an elastomeric chip for further analysis (68). Adhesives are essential for the function (69) and, however, represent a limit for device lifetimes due to epidermal turnover. Using the FDM printing process, we can create epifluidics able to integrate directly with the biosymbiotic devices to provide sweat-based analysis without the need for adhesives. Figure 7A shows a photograph and rendered representation of device function. Microfluidic channels are created with an inlet and outlet to help facilitate fluid flow through the system. To achieve adhesive-free sweat collection, we print a seal with a height of 0.45 mm around the inlet (see Fig. 7B). The 3D printed seal forces sweat secretion into the inlet of the 3D printed microfluidic channel. The formed seal can withstand common inlet pressures expected from eccrine sweat glands (70) while maintaining epidermal contact during dynamic motion as demonstrated in fig. S20. With the strain engineering approach outlined above, a stretchable microfluidic channel can be created that mechanically decouples the inlet, enabling a reliable seal with the skin. Contemporary techniques of using dye to visualize fluid front progression to analyze sweat rate are illustrated in Fig. 7B. Wetting of the 3D printed TPU also shows favorable characteristics compared to polydimethylsiloxane (PDMS), a common material for conventional devices, and is dependent on the surface finish of the TPU material.
Fig. 7. 3D printed epifluidics.
(A) Schematic and photographic image of device function with 3D printed adhesive free epifluidics. (B) Photographic images showing 3D printed seal that enables collection of sweat without the use of adhesives. (C) Photographic images showing wetting on 3D printed TPU on smooth and rough surface finishes. (D) Bar graph of contact angle of water on 3D printed TPU. (E) Graph showing inlet pressure to induce flow through 3D printed epifluidics with inset of experimental setup. (F) Photographic images of 3D printed epifluidics in trail collected at 10 min intervals during moderate calisthenic exercise. (G) Graph detailing calculated sweat volume of 30 min trail. Photo credit: Tucker Stuart, University of Arizona.
To demonstrate the impact of surface finish, we print a TPU sample with 100% infill and analyzed the top side facing the extrusion nozzle, which features a rougher surface finish and represents the inside morphology of the microfluidic channels, against the smooth side, which is printed directly on the glass bed as illustrated in Fig. 7C. Contact angle measurements are 71.3° and 74.8° with corresponding SDs across five trials of 2.72° and 6.92°, respectively (see Fig. 7D). The contact angle of this material compared to PDMS (100° to 112°) (71) shows better material wettability, providing favorable flow characteristics by reducing the inlet pressure needed to induce fluid flow. Figure 7E shows inlet pressures needed to induce fluid flow through a serpentine patterned channel with varying channel areas; experimental details are provided in Materials and Methods. In comparison to PDMS-based devices, inlet pressure is reduced by 0.5 to 0.8 kPa (70). Channel failure pressure is well above operational pressures between 1 and 6 kPa (70) with 8-kPa failure for a device wall thickness of 0.5 mm. (see fig. S21). Evaporation from the device under physiological conditions and moderate environmental temperatures is 6 μl/hour, which increases to an observed 11 μl/hour under extreme heats and rigorous exercise (see fig. S20). Figure 7F shows images collected with the device during 30 min of calisthenic exercise indicating sweat rate. Total fluid volume can be calculated using image analysis, as described in Materials and Methods, yielding 20 μl captured during the exercise period, which match results obtained from contemporary epifluidic devices (Fig. 7G) (29, 58, 64, 67, 68).
Chronic multimodal recording
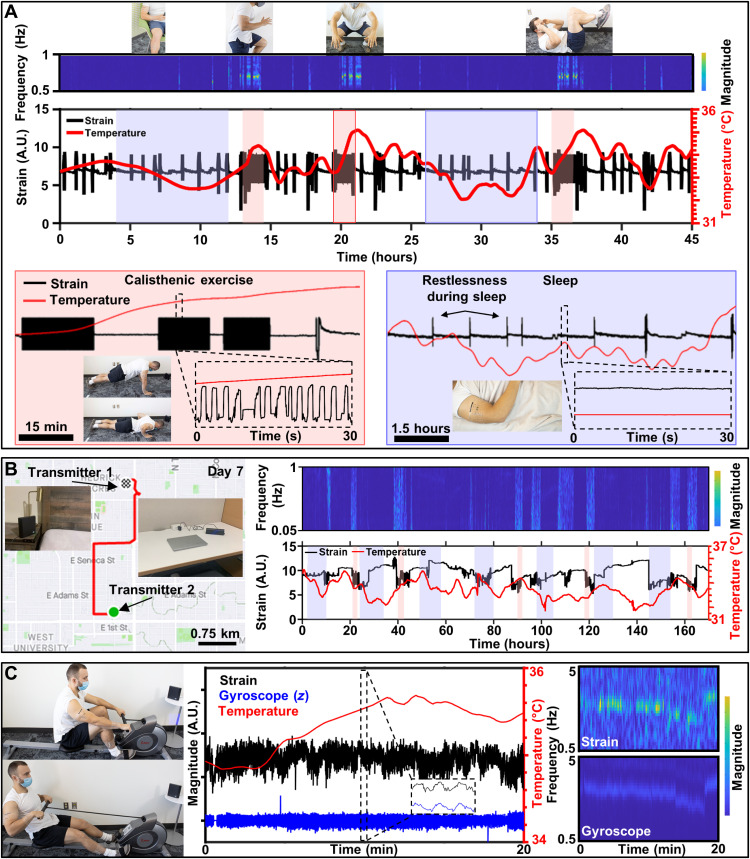
To demonstrate chronic multimodal biosignal recording capabilities with uninterrupted data streams, we created a biosymbiotic device for use on the arm with strain sensing capability to detect bicep contraction and surface body temperature in the axilla. These sensing locations were selected during the digital manufacturing process to provide insight into core body temperature and muscular activity of the bicep. During this experiment, the subject is instructed to wear the device for 48 hours with power casting units set up in multiple rooms. Data from this experiment are shown in Fig. 8A. Here, regions shaded in blue indicate periods of sleep, and areas shaded in red denote periods of dedicated calisthenic exercise. Over the course of the 48 hours, temperature increase associated with increased activity is observed, with large spikes occurring during periods of exercise. In addition, changes in temperature associated with the circadian rhythm can also be observed as the average temperature falls during sleep and rises during daily activity (72). Strain gauge data collected during the experimental trial show periods of general activity and increased activity frequency during periods of exercise. Here, data are observed in the frequency domain to quickly visualize large datasets and identify periods of increased activity, which is clearly visible in the resulting magnitude scalogram. This analytical technique visually separates daily motion and highlights periodic events that indicate increase intensity of activity. A detailed breakdown of the collected data can be found in figs. S22 and S23. A video showing device functionality can be found in movie S3, showing device operation over 1 m distance from the power casting unit during regular work activity with continuous acquisition of temperature and strain data, which is displayed in real time.
Fig. 8. Chronic multimodal recording.
(A) Data collected from 48-hour trial period of continuous recording using biosymbiotic devices. Sleep (shaded blue) and directed calisthenic exercise (shaded red) with continuous wavelet transform of strain signals with discrete changes in frequency corresponding to exercise and visible circadian rhythm and exercise strain–related thermography peaks. (B) Sample GPS mapping of subject activity and data collection from 7-day trial period of continuous recording using a battery supplemented biosymbiotic device affixed to the proximal arm. (C) Photographic images and data collection of multidevice system during rowing activities with continuous wavelet transforms of strain and gyroscopic signals. Photo credits: Tucker Stuart and Dylan McGuire, University of Arizona.
While application of a wireless and battery-free system is confined to a space determined by the positioning of power transfer infrastructure, operation outside of this infrastructure is possible with the integration of small batteries that are recharged in close proximity to the power caster (0.5 to 1 m), enabling continuous data acquisition during daily activity. We demonstrate this in a week-long experiment where the subject wears a bicep mounted bioysmbiotic device continuously, with transmitters located at frequently visited locations such as the office desk and bedside table (see Fig. 8B). This allows for reliance on the battery supply during activity outside of power casting infrastructure and robust power harvesting during stationary periods, which replenishes the battery supply and allows for continuous operation (see fig. S24). Strain and temperature data acquired over this experimental timeline are shown in Fig. 8B. Regions shaded in blue denote sleep, while regions shaded in red denote dedicated periods of exercise including calisthenics, running, and free weight activity. Frequency analysis shows good agreement with noted increases in activity, with temporal increases in core body temperature as shown in battery-free experiments in Fig. 8A. Global Positioning System (GPS) maps of subject activity over the experimental period show that the device functions well outside of transmitter range without requirement of user interaction with the device.
Simultaneous operation of multiple devices with high-fidelity data acquisition, with high sampling rates, was conducted using similar device designs. Strain and thermography sensors were placed on the proximal region of the arm to enable detection of bicep contraction and surface body temperature changes. A separate IMU sensor equipped biosymbiotic device was designed and placed around the calf muscle. For this experiment, the subject performs 20 min of rowing on a stationary rowing machine with simultaneous collection of temperature, strain, and IMU data (Fig. 8B). During the experiment, an initial cadence of 25 strokes/min is maintained for 12 min, after which the cadence drops to 20 strokes/min for 5 min and then increases to 32 strokes/min for the remaining 3 min. During the course the experiment, gyroscopic data from the leg show good correlation with muscle contraction of the bicep, indicating device synchronization and compliance of the rowing motion. Temperature data increased to a steady state of 35.6°C during the initial cadence, with a decrease of 0.2°C observed in accordance with the decrease in rowing cadence. A delay in temperature increase during the initiation of rowing is observed, likely resulting from delayed heat flux associated with the relationship between surface and core body temperature (73). Frequency analysis of gyroscope and strain data reveals good system compliance, as both sensing modalities demonstrate the same change in frequency over time, as well as synchronization of individual strokes, associated with the rowing cadence of the subject as shown in the magnitude scalograms. Additional information can be found in figs. S25 and S26.
DISCUSSION
Key technological barriers to enable diagnostic digital medicine are the lack of wearable tools that provide clinical grade, multimodal, and multisite data streams over the course of multiple days or weeks. Hurdles to realize these technologies are adhesive skin interfaces that degrade because of epidermal turnover, and bulky device architectures relying on electrochemical power supplies that induce motion artifacts require user interaction and limit run time or sampling rates. To address these challenges, we present a new device class that uses digital fabrication coupled with personalized designs informed by 3D scans of the wearer to create systems that intimately integrate with the body without using adhesives. The devices also feature battery-free and wireless operation to produce continuous high-fidelity data streams of clinical grade, multimodal biodata.
This unique approach to wearable device creation uses mechanical structures, materials, and fabrication technologies that offer new opportunities for mechanical and electromechanical designs to improve performance substantially. By introducing a toolbox of 3D mechanical structures to enable nonlinear mechanics, stain isolation for soft electronics, and 3D topologies, we enable devices that conformally attach to physiologically complex locations such as the shoulder and enable personalized sensor placement and high-performance energy harvesting. The platform also offers the integration of microfluidic channels that can be configured to operate as epifluidics without the use of adhesives and the integration of a wide range of materials, such as 3D printed conductive elastomers that can form a cohort of subject specific sensing solutions. Performance of the devices is demonstrated with a range of experiments that reflect conditions for at-home use and controlled diagnostic tests. Device performance in continuous multiday experiments demonstrates stable biointerface and electromagnetic performance with high-fidelity data streams. Controlled experiments in the laboratory setting also demonstrate multidevice, multimodality, and multisite performance that exceed current gold standard devices.
The combination of state-of-the-art biosensing capabilities into a personalized wireless and battery-free platform that can operate continuously and maintain epidermal contact without the use of adhesives opens a broad range of opportunities for future technologies, diagnostics, and therapeutics. For example, using this platform toward research for 3D antennas for significantly improved on-skin performance and multimaterial sensing platforms that incorporate intricate 3D fluidic designs with electrochemical sensors and inline flow sensing electrodes (67, 74) may expand possibilities in biosensing when combined with subject-specific sensing strategies. Chronic recording capabilities without user interaction may also be used to accommodate long-term studies with uninterrupted data streams, vital to detect hard-to-diagnose diseases, such as frailty in an at-home setting, significantly expanding therapeutics toward remote and economically disadvantaged patient populations.
MATERIALS AND METHODS
Flexible circuit fabrication
Pyralux double-sided copper clad laminate (AG185010RY; constituent layers, 18 μm copper, 50 μm polyimide, and 18 μm copper) served as the substrate for electronic fabrication. Copper traces, vias, and board outlines were structured using a UV laser ablation system (LPKF. ProtoLaser U4). After structuring, devices underwent ultrasonic cleaning (Vevor, commercial ultrasonic cleaner 2L) for 2 min in flex (Superior Flux and Manufacturing Company, Superior #71) and 1 min in isopropyl alcohol (MG Chemicals) with a subsequent rinse in deionized water to remove oxidation and organic residue left over from the ablation process. Via connections were created manually with thin copper wire (100 μm) and low-temperature solder paste (Chip Quik, TS391LT). Commercially available components were placed by hand and reflowed with low-temperature solder paste.
Circuit design, tuning, and fabrication
A serpentine-based dipole antenna was tuned to 915 MHz on the skin using a spectrum analyzer (SIGLENT, SSA 3032X) and reflection bridge (SIGLENT, RB3X20). The serpentine shape was placed on the proximal region of the forearm, and each pole of the dipole was cut consecutively until voltage standing wave ratio measurements showed resonance at 915 MHz. Commercially available components were placed by hand and reflowed with low-temperature solder paste. A full bridge rectifier was built using 0201 components for low-forward voltage Schottky diodes (Skyworks, SMS7630-061) and smoothing capacitor. A 3.3 V Zener diode (Comchip Technology, CZRZ3V3B-HF) was used as over voltage protection of the supercapacitor storage system (Seiko Instruments, CPH3225A). A 1.8 V low-dropout (LDO) regulator stabilized the input voltage into the BLE SoC (Dialog Semiconductor, DA14585) and other peripheral sensor electronics. The BLE SoC controlled powering of peripheral electronics, handled data aggregation from ADCs and digital components connected with i2c bus system, and executed BLE communication protocols. The BLE SoC was programmed using the Dialog’s SmartSnippet Studio with programming tabs that were inserted into a flexible printed circuit connector. Once programming was completed, the tabs were removed using a flush cuter (KAHIOE, KAHIOE-plier-01). Strain gauge circuits were built using a wheat stone bridge balanced to the resistance of the 3D printed strain gauge. An operational amplifier (Analog Devices, ADA4505-1) was used in a differential amplifier configuration to amplify resistance change in the gauge, which was then digitized by the on-board ADC of the BLE SoC. Temperature sensing circuits used a 100 kΩ negative temperature coefficient thermistor used in a wheat stone bridge configuration and a differential amplifier to produce analog temperature readings that were then digitized by the on-board ADC. An IMU (Bosch Sensortec, BMI 270) was configured using i2c communication bus with 3.3 kΩ 0201 pull-up resistors. Data were communicated via BLE protocol at 2.45 GHz using an external chip antenna (YAGEO, ANT1608LL14R2400A).
Photogrammetry reconstruction
One hundred photographs of the subject’s limb were taken with a smartphone (Apple, iPhone 11XR) at varying angles and distances from the subject with controlled, textured backgrounds. Photographs were then exported into photogrammetry software (Autodesk, ReCap Photo) where a 3D model was reconstructed from the imported image set. The 3D object was then processed to remove residual background structure, fill gaps in the model structure, and smooth the object before 2D unfolding. The final 3D model was then exported into a 3D creation suite (Blender) for scaling according to premeasured characteristic dimensions, and topological information was extracted. Seams were selected along the model to facilitate unwrapping into a 2D shape, and the model was unwrapped using the UV unwrap function. The 2D shape was then exported as a drawing file into a CAD software (Autodesk, AutoCAD) where personalized mesh designs were created. These designs used the unfolded 2D sketch to design termination points where melted joints would conjoin to form the finalized device structure. Sensor placement was chosen, and mechanical design was modulated to accommodate for strain isolation away from the electronic system. Additional design methods from the mechanical toolbox described in the mechanical characterization section were implemented on the basis of 3D topological information and requirements of the target physiology.
3D scanning
A 3D scanner (Microsoft, Xbox Kinect) was used for scanning the body. The Kinect uses structured light, more specifically infrared patterned projections across the space in front of the device while recording the reflected light pattern. The deformation of reflected light is relative to the shape and distance of the objects in front of it and can be used to reconstruct topological maps of objects. Using real-time 3D scanning software (ReconstructMe), the Kinect hardware is slowly moved around the body at varying distances and angles. Once a scan is completed, and the object undergoes the same postcapture processing as described for the photogrammetric reconstruction.
Mesh fabrication
2D mesh drawings were exported from AutoCAD and imported into another CAD software (Autodesk, Fusion 360) for extrusion. Stereolithography (STL) files were generated from the 3D model and imported into 3D slicing software (Prusa3D, PrusaSlicer) to generate machine code for the 3D printer. A fusion deposition modeling printer (Creality, CR-10s) was outfitted with a custom x-axis carriage that housed a direct drive extruder (Diabase Engineering, Flexion), hotend, and automatic bed leveling unit (Antclabs, BLTouch). A TPU filament (NinjaTek, NinjaFlex) was printed at 30 mm/s and 225°C with a bed temperature of 45°C. Using the pause at height command, the printer was paused to allow for insertion of electrical components and PCBs by hand into the elastomeric channels using the channel structure to guide placement (see movie S1). The print was then resumed to initiate encapsulation. After printing, segmented sections of the mesh structure were joined by melting TPU material together at junctions to form the completed linear structures.
Mechanical characterization
Mechanical characterization of the mesh structures was carried out using a custom, 3D printed stretching stage. Mesh design featured a uniform pattern with modulation of varying mechanical parameters as described in the mechanical characterization section. Each design was 145 mm in length with integration points on each end for fixation to the stretching stage system. The stretching stage used a 5 kg load cell (Degraw, 050HX) and load cell amplifier (SparkFun, HX711) to measure bulk strain profiles during displacement. Designs were affixed to the stretching stage, and movement commands were written using custom software to control the distance and speed of the stretching stage movement. Data was logged using serial communication from a microcontroller (Arduino, Arduino Nano) to a portable computer (Raspberry Pi, 4 Model B) with custom software.
Finite element analysis
FEA was carried out using simulation software (Ansys Mechanical). 3D bodies representing designed structures were imported into a static structural analysis tool for displacement and subsequent strain analysis. Material properties were assigned to the mesh structures according to the material properties acquired from material supplier information; see table S1 (75). Fixed supports were used on the edges of the stretching stage integration structures and on the inner faces of the holes to simulate stretching stage conditions. Displacement of the opposite edge was then incrementally increased, and simulation results on stress and strain profiles across the structures were recorded.
Serpentine design for shoulder device
3D scans of the upper arm of two subjects were taken. The scans were scaled according to arm circumference and length. The base of the deltoid and the top of the acromioclavicular joint were identified as key physiological landmarks for device creation. The distance between these two points was digitally measured and confirmed with in-person measurements. Using the following design rules, a device structure was digitally manufactured: (i) If the change in length with dynamic motion is greater than or equal to 10% of linear distance, then serpentine structures were implemented. (ii) If the change in joint angle is greater than 30°, then the material density behind the joint is reduced, and the mesh structure follows a taper according to the estimated change in angle. (iii) Termination lines at the proximal and distal ends of the mesh incorporate a thicker trace width. Serpentine traces along the proximal-distal axis of the deltoid were designed according to the equations below, where n is the number of serpentines, θ is the central angle, and r is the radius. The number of serpentines was selected to be 12, and the central angle and radius were determined through equations below. Serpentine structures holding electronic systems have an increased central angle to assist with strain isolation during arm adduction.
Cyclic encapsulation tests
A serpentine structured copper trace, with 10-kilohm surface mounted resistor located in the center of the test device, was embedded into a TPU mesh system, where continuous flexible wire (McMaster, 7071 K51) was soldered to the ends and then sealed with UV-curable glue (Damn Good, 20910DGFL) with subsequent curing under a UV lamp (24 W for 5 min). The resistance across the serpentine was measured, balanced with a wheat stone bridge, and subsequently displaced to 30% displacement 10,000 times. The change in resistance was measured using a differential op-amp (Texas Instruments, LM358P) and was digitized by an Arduino Nano oversampled to 14-bit for data aggregation during the test.
Local displacement characterization
To quantify changes in local displacements as a result of the changes in local topology, the devices were mounted on the custom stretching stage with colored markers placed at top (3, 1, and 0.45 mm in height) and base (0.45 mm in height) of the wrap structures. The devices were stretched to 30% displacement at 10% increments, and images were collected used a digital single-lens reflex camera (Canon, EOS 2000D Rebel T7) at a fixed distance from the stretching stage. Images were then loaded into an image processing software (ImageJ) where the distance/pixel was calibrated and the change in distance between corresponding dots was collected.
Antenna characterization
Reflection measurements were carried out using a spectrum analyzer (SIGLENT, SSA 3032X) and reflection bridge (SIGLENT, RB3X20). Initial antenna designed used 0.9 mm thick single-sided Pyralux material. The serpentine design was chosen to have a central angle of 240° with a radius of 3 mm. The serpentine antenna was embedded into a 3 mm raised structure and placed on the skin while taking reflection measurements. The ends of the serpentine antenna were cut equally on both sides until resonance reach 915 MHz. The resulting dipole length was recorded to be 34.4 mm. To measure the effect of raised height on antenna performance, the antenna was embedded in various heights of TPU mounts off the arm from 1 to 3 mm. Subsequent reflection measurements were recorded. At 3 mm, the serpentine-based antenna was held in place on one end using the custom stretching stage and displaced along the dipole axis while on the skin to yield the effect of lateral strain on return loss of the antenna. Last, 3 mm raised antenna structure was mounted onto the distal portion of the forearm along both the proximal-distal and medial-lateral axes to indicate effect of curvature of the body on return loss of the antenna.
Antenna power measurements
Power was transmitted to the device using one of or both commercially available 915 MHz, 3 W power transmitter (Powercast, TX91501B and TX91503). The serpentine dipole with integrated full bridge rectifier was placed on the proximal portion of the forearm in an office setting room (see fig. S5). The power casting unit was placed on a wooden table to mimic a conventional daily setting. Using a 1 kΩ load and a multimeter (AstroAI, DT132A), the voltage output was measured at varying distances and angles from the transmitter, as well as at different rotational positions along the principal axes.
Supercapacitor storage characterization
The supercapacitor power management system consisted of two supercapacitors (Seiko Instruments, CPH3225A) in parallel protected by a 3.3 V Zener diode (ON Semiconductor, MM3Z3VB), which fed into a 1.8 V LDO regulator (ON Semiconductor, NCP163AFCS180T2G). Power management was characterized by charging the super capacitor bank to 3.3 V using a dipole antenna placed at 50 cm from the transmitter while simultaneously measuring current input into the device via a custom current meter and the potential of the supercapacitors using a multimeter. Once fully charged, the antenna system was detached, and various loads were added to the output of the LDO regulator. Brownout protection was investigated using two benchtop power supplies: one connected to the LDO input and the other connected to the LDO enable pin. An oscilloscope measured the output of the LDO regulator over time to identify the proper enable voltage and corresponding on/off hysteresis 0.76 and 0.72 V, respectively. A voltage divider (500 and 275 kΩ) was subsequently used to provide consistent brownout protection of the system by turning off at a super capacitor voltage of 2 V (accommodating for the drop out voltage) and turning on at 2.2 V input.
Device electrical characterization
Current consumption of the device was recorded using a laboratory benchtop power supply (5 V) and a custom current meter with an internal shunt of 1 Ω connected in series with the 1.8 V supply line to the device. Modulation of sampling frequency and sensors was controlled over BLE using a smartphone application (LightBlue). The data were acquired in real time using an oscilloscope (SIGLENT, SDS 1202X-E).
Bluetooth chip antenna design and characterization
Design for the ground plane surrounding the BLE antenna chip (Yageo, ANT1608LL14R2400A) was adapted from the manufacturer’s recommended pattern, to fit onto the 6 mm diameter node. The antenna structure was attached via sub-miniature version A (SMA) connector to the spectrum analyzer where auxiliary components were selected to achieve tuning to 2.45 GHz. For this application, a 50 kΩ resistor was placed in series on the feedline with a tuning capacitor (0.3 pF) used between the chip antenna and ground plane. For characterization of performance, the BLE SoC board was powered by a bench top power supply (1.8 V) and mounted onto the proximal for arm using a TPU mesh device. A smartphone (Apple, iPhone 11XR) was used as the receiver unit running a BLE terminal application (LightBlue). Once connected, the BLE SoC board was moved away from the BLE terminal in 10 cm steps. Once the BLE communication was disrupted, the distance was recorded. This test was repeated along varying rotational positions on the principal axes.
Temperature sensor development and characterization
A 100 kΩ NTC thermistor (TDK Corporation, NTCG064EF104FTBX) was placed on the back side of a flexible PCB (3 mm in diameter) balanced using a wheat stone bridge configuration with 0201 components. One MΩ resistors were used in the R1 and R2 position to reduce overall power consumption of the board. The NTC was balanced with a 69 kΩ resistor to provide the correct reference voltage of 0.116 V. The bridge was fed into a differential amplifier (Analog Devices, ADA4505-1) with gain set to 82.64X using a 10 MΩ and a 121 kΩ resistor. Characterization of the NTC thermistor was carried out using a proportional-integral-derivative controlled hot plate where the NTC and other standard temperature probes were secured to the system. Temperature was increased in 0.25°C increments and left for 2 min for the temperature to stabilize. Once stable, the ADC readout was recorded, as well as the commercial thermistor readings. ADC values were converted to voltage, and a standardized curve was developed.
Temperature sensor walking experiment
A temperature sensor was affixed to a shoulder mesh with the temperature node being placed near the axilla region of the under arm. The NTC was placed through an opening in the base layer of the elastomer and encapsulated with a thin layer of UV glue (Damn Good, 20910DGFL). The subject, starting from the seated position, walked for 10 min at an average pace of 4 miles/hour and then returned to a seated position to rest. This was repeated twice to demonstrate the responsiveness and sensitivity of the thermistor node.
Strain gauge fabrication
Strain gauge designs were digitally created using 3D CAD software (Autodesk, Fusion 360). STL files were generated from the 3D model and imported into 3D slicing software (Prusa3D, PrusaSlicer) to generate machine code for the 3D printer. A fusion deposition modeling printer (Creality, CR-10s) was outfitted with a custom x-axis carriage that housed a direct drive extruder (Diabase Engineering, Flexion), hotend, and automatic bed leveling unit (Antclabs, BLTouch). A conductive TPU filament (NinjaTek, Eel) was printed at 20 mm/s and 225°C with a bed temperature of 45°C. Once completed, the material was removed and inspected for quality. After printing, the hotend was purged of remaining conductive filament using a generic polylactic acid (PLA) and subsequent cleaning of the extrusion nozzle. To embed the conductive filament into the general TPU mesh device, a channel was printed in a “T-bone” shape. The print was paused, and copper strips were placed using a glue stick to provide minor adhesion to the underlying TPU material. The conductive filament was then placed into the channel over the copper strips, again using a glue stick to provide mild adhesion. The print was then resumed, and NinjaFlex TPU was printed over the entire system.
Strain gauge characterization
Current versus voltage curves were collected using a source measurement unit (SMU) (Keithly, 2450 SourceMeter) and a custom 3D printed stretching stage. The strain gauge device was affixed to the stretching stage and connected to the SMU. Voltage was varied from −5 to 5 V in 100 mV increments, while the current was subsequently measured. This test was repeated at 0, 10, 20, and 30% displacement and again at 0% displacement to determine the recovery of the system. Long-term cyclic tests for system stability were conducted using a laser-structured flexible PCB with 0201 components and an operational amplifier. The output signal was digitized using an Arduino Nano oversampled to 12-bit resolution. The strain gauge mesh was then stretched to 30% and returned to 0% for 1000 cycles with constant data acquisition.
Epifluidics fabrication
Structures for epifluidic devices digitally created using 3D CAD software (Autodesk, Fusion 360). STL files were generated from the 3D model and imported into 3D slicing software (Prusa3D, PrusaSlicer) to generate machine code for the 3D printer. A fusion deposition modeling printer (Creality, CR-10s) was outfitted with a custom x-axis carriage that housed a direct drive extruder (Diabase Engineering, Flexion), hotend, and automatic bed leveling unit (Antclabs, BLTouch). A TPU filament (NinjaTek, NinjaFlex) was printed at 30 mm/s and 225°C with a bed temperature of 45°C. Epifluidic devices were printed with the seal facing up. After printing, dye (AmeriColor, Royal Blue) was injected into the dye reservoir using a syringe and needle placed through the inlet. After adding the dye, the device was left to dry for 30 min before being used. Dimensions of the channel structure were modulated within the operational resolution of the printer, able to accurately increase and decrease wall structure size by 100 μm. The serpentine shape of the microfluidic channels and inlet/outlet dimensions were held constant, with 1 mm in inlet and outlet diameter, 2.3 mm in radius, and 240° central angle. Wall thicknesses were modulated from 0.2 to 0.8 mm in thickness.
Epifluidics inlet and failure pressure characterization
Silicone tubing (Uxcell, A16092700UX0349) was secured to the inlet of the epifluidics channel using marine epoxy (Loctite, 1919324). The tubing was then secured to a vertical pole using bar clamps (Irwin, 1964747). Dyed water was then pipetted in from the top in 10 μl increments. This process continued until liquid began to flow through the channel. The height in which the flow began was recorded, and pressure was calculated. Channel failure pressure was calculated in a similar manner; however, in this case, marine epoxy was used to seal the outlet of the epifluidic channel structure such that pressure would build up in the channel and the structure to rupture.
Contact angle measurements
A 3D printed block of solid TPU was used as the substrate. A camera was placed in level to the TPU material. Five-microliter drops of water were placed on the TPU material, and an image was collected. This process was repeated for the smooth (TPU printed directly on the glass bed) and rough (last layer of printed TPU) sides. Images were imported into ImageJ, and the contact angle was acquired.
Epifluidic experiments
A 3D printed mesh system with integrated epifluidics channel was attached to the proximal forearm of the subject. An initial image was collected before exercise to serve as a baseline. The subject then engaged in moderate calisthenic and aerobic exercises including jogging, burpees, squats, and pushups. Images of the device were collected every 10 min across the 30 min exercise period. After the exercise, the images were loaded into ImageJ where the change in location of the fluid front was determined. Using the measured area of the epifluidic channel, the flow rate and total sweat volume were calculated.
Epifluidic seal experiments
Seal failure pressure
Determination of seal failure for the epifluidic devices was investigated with wrist mounted meshes that were designed with embedded epifluidic channels with varying seal heights. Silicone tubing (Uxcell, A16092700UX0349) was epoxied to the outlet of the device using marine epoxy (Loctite, 1919324). The tubing was secured to a vertical pole using bar clamps (Irwin, 1964747). Liquid was pipetted into the tubing in 100 μl increments until failure of the seal with the skin. The height of the liquid was recorded, and pressure was calculated.
Dynamic motion experiment
A wrist mounted device was filled with water colored with blue dye (AmeriColor, Royal Blue). The outlet of the devices was sealed with fast acting epoxy (J-B Weld, 50112). The subject performed a 2.8 km run at a pace of 10 km/hour with an ambient temperature of 38°C. Images were taken before and after the run, and no seal failure is recorded.
Epifliuidics channel evaporation characterization
3D printed epifluidic channels were fabricated with varying wall thicknesses (n = 3). The channels were filled with colored with blue dye (AmeriColor, Royal Blue), and the inlets and outlets were sealed with fast acting epoxy (J-B Weld, 50112). As a control, a droplet of liquid was placed on a square of parafilm. The devices were weighed using a 0.1 mg accurate scale (U.S. Solid, USS-DBS00008), and initial weights were recorded. To simulate body temperature, a hot plate (Yae First Trading Co. Ltd.) was set to 35°C, and the ambient temperature was recorded at 28°C. Devices were placed on the hotplate and were taken off at 5 min sampling intervals to be weighed. Change in weight was recorded, and evaporation of water was calculated.
IMU jump test
A 3D printed mesh system with integrated IMU (Bosch Sensortec, BMI 270) was placed on the proximal region of the lower leg. Just proximal of the mesh device, a gold standard inertial measurement system (Vandicro Inc., LEGsys) was also attached to the lower leg. The subject was then asked to perform a stationary, vertical jump a total of five times. Sampling frequency of the devices was 100 Hz for the gold standard and mesh-based system. Data were temporally correlated with a kick of the right leg at the beginning and end of the data collection period.
Continuous walking test
A 3D printed mesh system with integrated IMU (Bosch Sensortec, BMI 270) was placed on the proximal region of the lower leg. Just proximal of the mesh device, a gold standard inertial measurement system (Vandicro Inc., LEGsys) was also attached to the lower leg. Before the walking exercise of the experiment, the subject performed a vertical jump to signal the beginning of the experiment, and the subject then walked on a treadmill (Sunny Health and Fitness, BO8F9XLW1N) at a speed of 2.5 miles/hour for 5 min. Sampling frequency of the devices was 100 Hz for the gold standard and mesh-based system.
Forty-eight–hour data acquisition
A mesh was designed for the subject’s proximal upper arm with integrated 3D printed strain gauge and submillikelvin resolution temperature sensor. A light-emitting diode indicator device was also placed on the forearm of the subject to provide an indication of power transfer to the system. The experiment was conducted at the subject’s home, who was given two power transmitters (Powercast, TX91501B and TX91503) to be placed within 1 m proximity to the user at all times. These transmitters emitted 0° and 90° linear polarizations of the 915 MHz waveform to maximize power transmission regardless of device orientation. The subject was instructed to go about daily activity while wearing the device and move transmitters into rooms where the subject was in. Data were collected and logged into a .csv file using a Raspberry Pi with rechargeable battery power supply.
Rowing experiment
Mesh system for the proximal upper arm and lower leg was designed for this study. The upper arm mesh was composed of analog sensors (strain and temperature) to measure bicep muscle contraction during the rowing motion and surface body temperature throughout the exercise. The leg mesh was outfitted with an IMU to identify leg motion. Transmitters were positioned on both sides of the rowing machine to ensure adequate power supply to the sensor systems, and a Raspberry Pi was used for data collection and logging. The rowing machine (Sunny Health and Fitness, SF-RW5515) was set to max resistance during the experimental period. Both strain gauge and z-axis gyroscopic data were processed through a continuous 1D wavelet transform using the analytic Morse wavelet, with a symmetry parameter of 3 and time-bandwidth product of 60.
Wearability experiments
To accurately assess the wearability and adhesion of the device to the body in extreme scenarios, two battery-supplemented (Guangzhou Markyn Battery Co. Ltd., GMB-300910) devices with inertial measurement capability were designed via the digital manufacturing process and affixed to each lower leg. The top of each device was marked to indicate any device movement during the experiment. The right leg was then covered with a pant leg to simulate being worn under clothing. The subject then ran 3.1 km at a pace of 13 km/hour with an outside temperature of 37°C. Images were taken immediately following the experiment to identify any movement of the devices during the experiment.
Seven-day data acquisition
A biosymbiotic device was designed for the proximal upper arm with integrated 3D printed strain gauge along the anterior-posterior axis and a submillikelvin resolution temperature sensor placed in the axillary region. The device was outfitted with a 10 mA·hour battery (Guangzhou Markyn Battery Co. Ltd., GMB-300910). Power transmitters (Powercast, TX91501B and TX91503) were placed in key locations identified as frequent destinations (work office, home office, and bedside table). Data were collected with a miniaturized computer (Raspberry Pi, Raspberry Pi Model B) with a power bank (Adafruit, 1566) and custom software to log data into a .csv file for extraction and analysis. Daily activity was logged in a journal, and GPS tracking software (Strava) was used to track location. In addition, battery voltage was measured in 12 hour increments at the beginning and end of each day with a multimeter (AstroAI, DT132A).
Human subject recruitment
Human subjects used for this study were recruited from laboratory staff according to the best clinical research practices and guidelines as established under the University of Arizona’s institutional review board–approved protocol no. 2005648028.
Acknowledgments
We would like to thank N. Toosizadeh at the Department of Biomedical Engineering, University of Arizona for supplying the LEGSys system use for data comparisons. Funding: We acknowledge funding support from the Flinn Foundation (to T.S. and P.G.), the National Science Foundation I-Corp Program (to T.S.), and the startup funds from Department of Biomedical Engineering, University of Arizona (to P.G.). Author contributions: T.S. and P.G. conceived the project, designed the studies, and analyzed the data. T.S., D.T.M., and M.F. designed and fabricated device structures. T.S. and D.T.M. designed 3D scanning and photogrammetry process. T.S., D.T.M., R.P., and M.F. designed testing devices and ran FEA models. T.S. and P.U. designed and tested 915-MHz antenna structure. K.A.K. and I.C.I. developed BLE firmware. J.H. conducted SAR FEM analysis. T.S. and K.A.K. tested electrical properties of the devices. T.S., I.C.I., and T.L. designed BLE board with integrated BLE antenna and programming tabs. R.P. designed and built custom stretching stage. T.S. and M.J. designed and built 3D printed strain gauges. T.S. and P.G. designed and characterized 3D printed epifluidics. T.S. and P.G. wrote the manuscript. Competing interests: P.G. is an inventor on a pending patent related to this work filed by Arizona Board of Regents on behalf of the University of Arizona (no. PCT/US2021/012918, filed 15 July 2021). The authors declare that they have no other competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be found at doi.org/10.5281/zenodo.5192363.
Supplementary Materials
This PDF file includes:
Figs. S1 to S26
Tables S1 and S2
Legends for movies S1 to S3
Other Supplementary Material for this manuscript includes the following:
Movies S1 to S3
REFERENCES AND NOTES
- 1.A. Lymberis, in Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE Cat. No.03CH37439) (IEEE, 2003), vol. 4, pp. 3716–3719. [Google Scholar]
- 2.Cheng S., Azarian M. H., Pecht M. G., Sensor systems for prognostics and health management. Sensors 10, 5774–5797 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rovini E., Maremmani C., Cavallo F., How wearable sensors can support Parkinson’s disease diagnosis and treatment: A systematic review. Front. Neurosci. 11, 555 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.R. Buettner, T. Rieg, J. Frick, in Information Systems and Neuroscience (Springer, 2020), pp. 165–172. [Google Scholar]
- 5.Burnham J. P., Lu C., Yaeger L. H., Bailey T. C., Kollef M. H., Using wearable technology to predict health outcomes: A literature review. J. Am. Med. Inform. Assoc. 25, 1221–1227 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta N., Pandit A., Concurrence of big data analytics and healthcare: A systematic review. Int. J. Med. Inform. 114, 57–65 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Chouvarda I. G., Goulis D. G., Lambrinoudaki I., Maglaveras N., Connected health and integrated care: Toward new models for chronic disease management. Maturitas 82, 22–27 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Elenko E., Underwood L., Zohar D., Defining digital medicine. Nat. Biotechnol. 33, 456–461 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Al Saedi A., Feehan J., Phu S., Duque G., Current and emerging biomarkers of frailty in the elderly. Clin. Interv. Aging 14, 389–398 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yıldırım Ö., Pławiak P., Tan R.-S., Acharya U. R., Arrhythmia detection using deep convolutional neural network with long duration ECG signals. Comput. Biol. Med. 102, 411–420 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Fogel A. L., Kvedar J. C., Artificial intelligence powers digital medicine. NPJ Digit. Med. 1, 5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Degerli M., Yildirim S. O., Identifying critical success factors for wearable medical devices: A comprehensive exploration. Univers. Access Inf. Soc. , 1–23 (2020).33199979 [Google Scholar]
- 13.Lukowicz P., Kirstein T., Tröster G., Wearable systems for health care applications. Methods Inf. Med. 43, 232–238 (2004). [PubMed] [Google Scholar]
- 14.Bonato P., Advances in wearable technology and applications in physical medicine and rehabilitation. J. Neuroeng. Rehabil. 2, 2 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.E. D. Zubiete, L. F. Luque, A. V. M. Rodríguez, I. G. Gonzalez, in 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE, 2011), pp. 1789–1793. [DOI] [PubMed] [Google Scholar]
- 16.Bloss R., Wearable sensors bring new benefits to continuous medical monitoring, real time physical activity assessment, baby monitoring and industrial applications. Sens. Rev. 35, 141–145 (2015). [Google Scholar]
- 17.Guk K., Han G., Lim J., Jeong K., Kang T., Lim E.-K., Jung J., Evolution of wearable devices with real-time disease monitoring for personalized healthcare. Nanomaterials 9, 813 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L., Lou Z., Jiang K., Shen G., Bio-multifunctional smart wearable sensors for medical devices. Adv. Intell. Syst. 1, 1900040 (2019). [Google Scholar]
- 19.Windmiller J. R., Wang J., Wearable electrochemical sensors and biosensors: A review. Electroanalysis. 25, 29–46 (2013). [Google Scholar]
- 20.Ray T. R., Choi J., Bandodkar A. J., Krishnan S., Gutruf P., Tian L., Ghaffari R., Rogers J. A., Bio-integrated wearable systems: A comprehensive review. Chem. Rev. 119, 5461–5533 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Stuart T., Cai L., Burton A., Gutruf P., Wireless and battery-free platforms for collection of biosignals. Biosens. Bioelectron. 178, 113007 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heikenfeld J., Jajack A., Rogers J., Gutruf P., Tian L., Pan T., Li R., Khine M., Kim J., Wang J., Kim J., Wearable sensors: Modalities, challenges, and prospects. Lab Chip 18, 217–248 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park Y.-G., Lee S., Park J.-U., Recent progress in wireless sensors for wearable electronics. Sensors 19, 4353 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shadid R., Noghanian S., A literature survey on wireless power transfer for biomedical devices. Int. J. Antennas Propag. 2018, 1–11 (2018). [Google Scholar]
- 25.Krishnan S. R., Su C.-J., Xie Z., Patel M., Madhvapathy S. R., Xu Y., Freudman J., Ng B., Heo S. Y., Wang H., Ray T. R., Leshock J., Stankiewicz I., Feng X., Huang Y., Gutruf P., Rogers J. A., Wireless, battery-free epidermal electronics for continuous, quantitative, multimodal thermal characterization of skin. Small 14, 1803192 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Kim J., Gutruf P., Chiarelli A. M., Heo S. Y., Cho K., Xie Z., Banks A., Han S., Jang K.-I., Lee J. W., Lee K.-T., Feng X., Huang Y., Fabiani M., Gratton G., Paik U., Rogers J. A., Miniaturized battery-free wireless systems for wearable pulse oximetry. Adv. Funct. Mater. 27, 1604373 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J., Banks A., Xie Z., Heo S. Y., Gutruf P., Lee J. W., Xu S., Jang K.-I., Liu F., Brown G., Choi J., Kim J. H., Feng X., Huang Y., Paik U., Rogers J. A., Miniaturized flexible electronic systems with wireless power and near-field communication capabilities. Adv. Funct. Mater. 25, 4761–4767 (2015). [Google Scholar]
- 28.Khan Y., Garg M., Gui Q., Schadt M., Gaikwad A., Han D., Yamamoto N. A. D., Hart P., Welte R., Wilson W., Czarnecki S., Poliks M., Jin Z., Ghose K., Egitto F., Turner J., Arias A. C., Flexible hybrid electronics: Direct interfacing of soft and hard electronics for wearable health monitoring. Adv. Funct. Mater. 26, 8764–8775 (2016). [Google Scholar]
- 29.Ray T., Choi J., Reeder J., Lee S. P., Aranyosi A. J., Ghaffari R., Rogers J. A., Soft, skin-interfaced wearable systems for sports science and analytics. Curr. Opin. Biomed. Eng. 9, 47–56 (2019). [Google Scholar]
- 30.Chung H. U., Kim B. H., Lee J. Y., Lee J., Xie Z., Ibler E. M., Lee K., Banks A., Jeong J. Y., Kim J., Ogle C., Grande D., Yu Y., Jang H., Assem P., Ryu D., Kwak J. W., Namkoong M., Park J. B., Lee Y., Kim D. H., Ryu A., Jeong J., You K., Ji B., Liu Z., Huo Q., Feng X., Deng Y., Xu Y., Jang K.-I., Kim J., Zhang Y., Ghaffari R., Rand C. M., Schau M., Hamvas A., Weese-Mayer D. E., Huang Y., Lee S. M., Shanbhag N. R., Paler A. S., Xu S., Rogers J. A., Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 363, eaau0780 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das Barman S., Reza A. W., Kumar N., Karim M. E., Munir A. B., Wireless powering by magnetic resonant coupling: Recent trends in wireless power transfer system and its applications. Renew. Sustain. Energy Rev. 51, 1525–1552 (2015). [Google Scholar]
- 32.Yu X., Xie Z., Yu Y., Lee J., Vazquez-Guardado A., Luan H., Ruban J., Ning X., Akhtar A., Li D., Ji B., Liu Y., Sun R., Cao J., Huo Q., Zhong Y., Lee C. M., Kim S. Y., Gutruf P., Zhang C., Xue Y., Guo Q., Chempakasseril A., Tian P., Lu W., Jeong J. Y., Yu Y. J., Cornman J., Tan C. S., Kim B. H., Lee K. H., Feng X., Huang Y., Rogers J. A., Skin-integrated wireless haptic interfaces for virtual and augmented reality. Nature 575, 473–479 (2019). [DOI] [PubMed] [Google Scholar]
- 33.HALPRIN K. M., Epidermal “Turnover Time”—A re-examination. Br. J. Dermatol. 86, 14–19 (1972). [DOI] [PubMed] [Google Scholar]
- 34.Haleem A., Javaid M., Role of CT and MRI in the design and development of orthopaedic model using additive manufacturing. J. Clin. Orthop. Trauma. 9, 213–217 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.M. Hernandez, J. Choi, G. Medioni, Laser scan quality 3-D face modeling using a low-cost depth camera, in 2012 Proceedings of the 20th European Signal Processing Conference (EUSIPCO) (IEEE, 2012), pp. 1995–1999. [Google Scholar]
- 36.Peyer K. E., Morris M., Sellers W. I., Subject-specific body segment parameter estimation using 3D photogrammetry with multiple cameras. PeerJ. 3, e831 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao J., Gao Y., The manufacture of 3D printing of medical grade TPU. Prog. Addit. Manuf. 2, 117–123 (2017). [Google Scholar]
- 38.Krishnan S. R., Su C.-J., Xie Z., Patel M., Madhvapathy S. R., Xu Y., Freudman J., Ng B., Heo S. Y., Wang H., Ray T. R., Leshock J., Stankiewicz I., Feng X., Huang Y., Gutruf P., Rogers J. A., Epidermal electronics: Wireless, battery-free epidermal electronics for continuous, quantitative, multimodal thermal characterization of skin (small 47/2018). Small 14, 1870226 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Xu R., Lee J. W., Pan T., Ma S., Wang J., Han J. H., Ma Y., Rogers J. A., Huang Y., Designing thin, ultrastretchable electronics with stacked circuits and elastomeric encapsulation materials. Adv. Funct. Mater. 27, 1604545 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li K., Cheng X., Zhu F., Li L., Xie Z., Luan H., Wang Z., Ji Z., Wang H., Liu F., A generic soft encapsulation strategy for stretchable electronics. Adv. Funct. Mater. 29, 1806630 (2019). [Google Scholar]
- 41.Ho J. S., Tanabe Y., Iyer S. M., Christensen A. J., Grosenick L., Deisseroth K., Delp S. L., Poon A. S. Y., Self-tracking energy transfer for neural stimulation in untethered mice. Phys. Rev. Appl. 4, 24001 (2015). [Google Scholar]
- 42.Q. Huang, Y. Mei, W. Wang, Q. Zhang, Battery-free sensing platform for wearable devices: The synergy between two feet, in IEEE INFOCOM 2016 - The 35th Annual IEEE International Conference on Computer Communications (IEEE, 2016), pp. 1–9. [Google Scholar]
- 43.Poon A. S. Y., O’Driscoll S., Meng T. H., Optimal frequency for wireless power transmission into dispersive tissue. IEEE Trans. Antennas Propag. 58, 1739–1750 (2010). [Google Scholar]
- 44.Tosi J., Taffoni F., Santacatterina M., Sannino R., Formica D., Performance evaluation of bluetooth low energy: A systematic review. Sensors (Basel). 17, 2898 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matthews C. E., Hebert J. R., Freedson P. S., Stanek E. J. III, Merriam P. A., Ebbeling C. B., Ockene I. S., Sources of variance in daily physical activity levels in the seasonal variation of blood cholesterol study. Am. J. Epidemiol. 153, 987–995 (2001). [DOI] [PubMed] [Google Scholar]
- 46.Owen N., Sparling P. B., Healy G. N., Dunstan D. W., Matthews C. E., Sedentary behavior: Emerging evidence for a new health risk. Mayo Clin. Proc. 85, 1138–1141 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arumugam V., Naresh M. D., Sanjeevi R., Effect of strain rate on the fracture behaviour of skin. J. Biosci. 19, 307–313 (1994). [Google Scholar]
- 48.Greaves G. N., Greer A. L., Lakes R. S., Rouxel T., Poisson’s ratio and modern materials. Nat. Mater. 10, 823–837 (2011). [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y., Wang S., Li X., Fan J. A., Xu S., Song Y. M., Choi K., Yeo W., Lee W., Nazaar S. N., Experimental and theoretical studies of serpentine microstructures bonded to prestrained elastomers for stretchable electronics. Adv. Funct. Mater. 24, 2028–2037 (2014). [Google Scholar]
- 50.Zhang Y., Xu S., Fu H., Lee J., Su J., Hwang K.-C., Rogers J. A., Huang Y., Buckling in serpentine microstructures and applications in elastomer-supported ultra-stretchable electronics with high areal coverage. Soft Matter 9, 8062–8070 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu S., Zhang Y., Cho J., Lee J., Huang X., Jia L., Fan J. A., Su Y., Su J., Zhang H., Stretchable batteries with self-similar serpentine interconnects and integrated wireless recharging systems. Nat. Commun. 4, 1–8 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Xu S., Yan Z., Jang K.-I., Huang W., Fu H., Kim J., Wei Z., Flavin M., McCracken J., Wang R., Badea A., Liu Y., Xiao D., Zhou G., Lee J., Chung H. U., Cheng H., Ren W., Banks A., Li X., Paik U., Nuzzo R. G., Huang Y., Zhang Y., Rogers J. A., Materials science. Assembly of micro/nanomaterials into complex, three-dimensional architectures by compressive buckling. Science 347, 154–159 (2015). [DOI] [PubMed] [Google Scholar]
- 53.D. M. Dobkin, The rf in RFID: uhf RFID in Practice (Newnes, 2012).
- 54.Rodbard D., The role of regional body temperature in the pathogenesis of disease. N. Engl. J. Med. 305, 808–814 (1981). [DOI] [PubMed] [Google Scholar]
- 55.Webb R. C., Bonifas A. P., Behnaz A., Zhang Y., Yu K. J., Cheng H., Shi M., Bian Z., Liu Z., Kim Y.-S., Yeo W.-H., Park J. S., Song J., Li Y., Huang Y., Gorbach A. M., Rogers J. A., Ultrathin conformal devices for precise and continuous thermal characterization of human skin. Nat. Mater. 12, 938–944 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han S., Kim J., Won S. M., Ma Y., Kang D., Xie Z., Lee K.-T., Chung H. U., Banks A., Min S., Heo S. Y., Davies C. R., Lee J. W., Lee C.-H., Kim B. H., Li K., Zhou Y., Wei C., Feng X., Huang Y., Rogers J. A., Battery-free, wireless sensors for full-body pressure and temperature mapping. Sci. Transl. Med. 10, eaan4950 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee J. W., Xu R., Lee S., Jang K.-I., Yang Y., Banks A., Yu K. J., Kim J., Xu S., Ma S., Soft, thin skin-mounted power management systems and their use in wireless thermography. Proc. Natl. Acad. Sci. U.S.A. 113, 6131–6136 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reeder J. T., Choi J., Xue Y., Gutruf P., Hanson J., Liu M., Ray T., Bandodkar A. J., Avila R., Xia W., Krishnan S., Xu S., Barnes K., Pahnke M., Ghaffari R., Huang Y., Rogers J. A., Waterproof, electronics-enabled, epidermal microfluidic devices for sweat collection, biomarker analysis, and thermography in aquatic settings. Sci. Adv. 5, eaau6356 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Someya T., Kato Y., Sekitani T., Iba S., Noguchi Y., Murase Y., Kawaguchi H., Sakurai T., Conformable, flexible, large-area networks of pressure and thermal sensors with organic transistor active matrixes. Proc. Natl. Acad. Sci. U.S.A. 102, 12321–12325 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mukhopadhyay S. C., Wearable sensors for human activity monitoring: A review. IEEE Sens. J. 15, 1321–1330 (2015). [Google Scholar]
- 61.Bandodkar A. J., Wang J., Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 32, 363–371 (2014). [DOI] [PubMed] [Google Scholar]
- 62.Bandodkar A. J., Choi J., Lee S. P., Jeang W. J., Agyare P., Gutruf P., Wang S., Sponenburg R. A., Reeder J. T., Schon S., Ray T. R., Chen S., Mehta S., Ruiz S., Rogers J. A., Soft, skin-interfaced microfluidic systems with passive galvanic stopwatches for precise chronometric sampling of sweat. Adv. Mater. 31, 1902109 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Xu G., Cheng C., Liu Z., Yuan W., Wu X., Lu Y., Low S. S., Liu J., Zhu L., Ji D., Li S., Chen Z., Wang L., Yang Q., Cui Z., Liu Q., Battery-free and wireless epidermal electrochemical system with all-printed stretchable electrode array for multiplexed in situ sweat analysis. Adv. Mater. Technol. 4, 1800658 (2019). [Google Scholar]
- 64.Bandodkar A. J., Gutruf P., Choi J., Lee K., Sekine Y., Reeder J. T., Jeang W. J., Aranyosi A. J., Lee S. P., Model J. B., Ghaffari R., Su C.-J., Leshock J. P., Ray T., Verrillo A., Thomas K., Krishnamurthi V., Han S., Kim J., Krishnan S., Hang T., Rogers J. A., Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 5, eaav3294 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao W., Emaminejad S., Nyein H. Y. Y., Challa S., Chen K. V., Peck A., Fahad H. M., Ota H., Shiraki H., Kiriya D., Lien D. H., Brooks G. A., Davis R. W., Javey A., Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heikenfeld J., Non-invasive analyte access and sensing through eccrine sweat: Challenges and outlook circa 2016. Electroanalysis 28, 1242–1249 (2016). [Google Scholar]
- 67.Kim S. B., Lee K., Raj M. S., Lee B., Reeder J. T., Koo J., Hourlier-Fargette A., Bandodkar A. J., Won S. M., Sekine Y., Choi J., Zhang Y., Yoon J., Kim B. H., Yun Y., Lee S., Shin J., Kim J., Ghaffari R., Rogers J. A., Soft, skin-interfaced microfluidic systems with wireless, battery-free electronics for digital, real-time tracking of sweat loss and electrolyte composition. Small 14, 1802876 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Choi J., Ghaffari R., Baker L. B., Rogers J. A., Skin-interfaced systems for sweat collection and analytics. Sci. Adv. 4, eaar3921 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McNichol L., Lund C., Rosen T., Gray M., Medical adhesives and patient safety: State of the scienceconsensus statements for the assessment, prevention, and treatment of adhesive-related skin injuries. J. Wound Ostomy Cont. Nurs. 40, 365–380 (2013). [DOI] [PubMed] [Google Scholar]
- 70.Choi J., Xue Y., Xia W., Ray T. R., Reeder J. T., Bandodkar A. J., Kang D., Xu S., Huang Y., Rogers J. A., Soft, skin-mounted microfluidic systems for measuring secretory fluidic pressures generated at the surface of the skin by eccrine sweat glands. Lab Chip 17, 2572–2580 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bodas D., Khan-Malek C., Formation of more stable hydrophilic surfaces of PDMS by plasma and chemical treatments. Microelectron. Eng. 83, 1277–1279 (2006). [Google Scholar]
- 72.Minors D. S., Waterhouse J. M., Circadian rhythms and their mechanisms. Experientia 42, 1–13 (1986). [DOI] [PubMed] [Google Scholar]
- 73.Xu X., Karis A. J., Buller M. J., Santee W. R., Relationship between core temperature, skin temperature, and heat flux during exercise in heat. Eur. J. Appl. Physiol. 113, 2381–2389 (2013). [DOI] [PubMed] [Google Scholar]
- 74.Choi D.-H., Gonzales M., Kitchen G. B., Phan D.-T., Searson P. C., A capacitive sweat rate sensor for continuous and real-time monitoring of sweat loss. ACS Sensors 5, 3821–3826 (2020). [DOI] [PubMed] [Google Scholar]
- 75.Elleuch R., Elleuch K., Salah B., Zahouani H., Tribological behavior of thermoplastic polyurethane elastomers. Mater. Des. 28, 824–830 (2007). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S26
Tables S1 and S2
Legends for movies S1 to S3
Movies S1 to S3