Abstract
Drug addiction remains a key biomedical challenge facing current neuroscience research. In addition to neural mechanisms, the focus of the vast majority of studies to date, astrocytes have been increasingly recognized as an “accomplice.” According to the tripartite synapse model, astrocytes critically regulate nearby pre- and postsynaptic neuronal substrates to craft experience-dependent synaptic plasticity, including synapse formation and elimination. Astrocytes within brain regions that are implicated in drug addiction exhibit dynamic changes in activity upon exposure to cocaine and subsequently undergo adaptive changes themselves during chronic drug exposure. Recent results have identified several key astrocytic signaling pathways that are involved in cocaine-induced synaptic and circuit adaptations. In this review, we provide a brief overview of the role of astrocytes in regulating synaptic transmission and neuronal function, and discuss how cocaine influences these astrocyte-mediated mechanisms to induce persistent synaptic and circuit alterations that promote cocaine seeking and relapse. We also consider the therapeutic potential of targeting astrocytic substrates to ameliorate drug-induced neuroplasticity for behavioral benefits. While primarily focusing on cocaine-induced astrocytic responses, we also include brief discussion of other drugs of abuse where data are available.
Keywords: astrocytes, cocaine, addiction, nucleus accumbens, synaptic plasticity, synaptogenesis
Introduction
The term glia is derived from “glue” in Greek, but glia functions go far beyond simply providing structural and metabolic support to neurons. The abundance of glial cells progressively and substantially increases along both evolutionary phylogeny and brain complexity1. For example, the astrocyte-to-neuron ratio in human brains (~1–1.4:1) is higher than that in rodents (~0.4:1) or C. elegans (~0.2:1)2–5. While human brains have higher metabolic demands, such a high ratio would appear to be an excessive number of astrocytes for just structural and metabolic support. Indeed, astrocytes—the most prevalent type of glia—are substantially larger in human brains, with more sophisticated morphologies that contact more synapses than any other species6, 7. Furthermore, the propagation speed of intracellular Ca2+ waves in human astrocytes is ~4-fold faster than in rodents6. Human-originated astrocytes maintain their enhanced cellular features when engrafted and induced in the mouse brain, resulting in enhanced experience-dependent synaptic plasticity and improved learning in these chimeric mice8. Such results suggest that astrocytes not only participate in brain metabolism and stability, but also are key substrates defining the brain’s cognitive capacity9–13.
Astrocytes are broadly known for their role in neurotransmitter and ionic homeostasis, synaptic and neuronal modulation, and blood-brain barrier maintenance. Astrocytes exhibit a complex morphology, with primary branches extending and elongating into delicate terminal leaflets, called peripheral astrocyte processes. Such perisynaptic processes enwrap the pre- and postsynaptic components of synapses, and these interactions occur at a distance as small as 10 nm, indeed closer than the width of some synaptic clefts14–16. An array of neurotransmitter receptors and transporters are expressed in astrocytes, highlighting that astrocytes may respond directly to neuronal signals17–22. Astrocytes can release substances into the extracellular space via anion channels, unpaired hemichannels of gap junctions, transporters or exchange antiporters, and other forms of non-vesicular release23–26. On the other hand, components of the basic machineries that mediate Ca2+-dependent vesicular release by exocytosis have been detected in astrocytes, including vesicular glutamate transporters (VGLUT1/2) and the SNARE protein cellubrevin27. While the exact mechanisms and physiological relevance remain under debate28, 29, vesicular release of substances from astrocytes has been detected in many experimental conditions30–34. Among astrocyte-secreted factors, some are classic neurotransmitters and neuromodulators, such as glutamate and adenosine triphosphate (ATP), while others are unique to astrocytes. With these mechanisms, astrocytes are thought to reciprocally communicate with neurons and regulate synaptic transmission35 through a structural complex, termed the “tripartite synapse” (Figure 1), composed of a presynaptic nerve terminal and a postsynaptic dendritic spine enveloped by astrocyte processes, now viewed as the basic unit of synaptic transmission35, 36. Increasing evidence suggests that astrocytes display significant regional heterogeneity and specificity in the brain37, although much remains to be learned about the molecular and cellular basis, and functional consequences, of such regional differences.
Figure 1. Astrocyte-mediated regulation of transmission at the tripartite synapse.
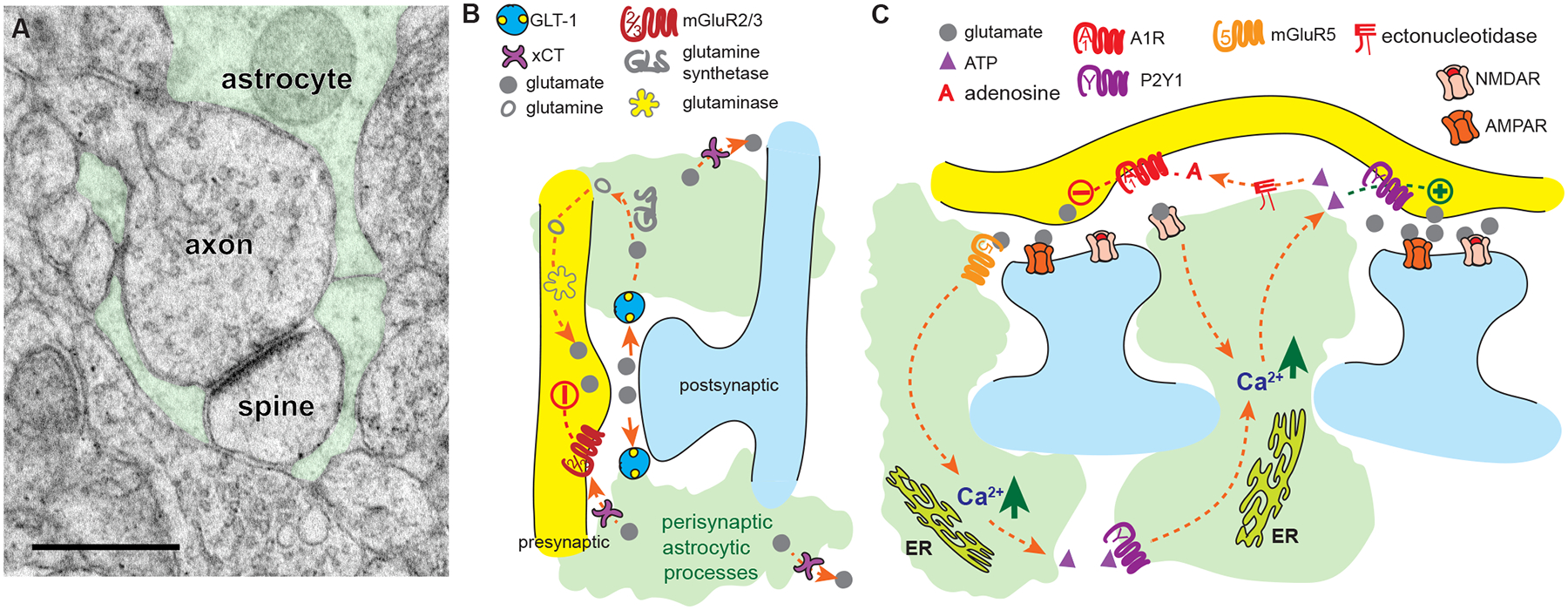
A Electron microscopic image showing a tripartite synapse in the NAc, in which a glutamatergic axospinous synapse is wrapped by astrocyte processes (scale bar corresponds to 0.5 μm). B Diagram illustrating astrocytic mechanisms of glutamate homeostasis at glutamatergic synapses. Specifically, GLT-1 on astrocytes removes synaptically released glutamate, which can be converted to glutamine within astrocytes and transported back into presynaptic terminals. xCT located at perisynaptic astrocytic processes maintains extracellular levels of ambient glutamate. C Diagram showing mechanisms of increasing Ca2+ in astrocytes, including ER-derived Ca2+ release or entry via ionotropic receptors, that play a key role in the signaling pathways employed by astrocytes to regulate glutamate transmission, including ATP-P2Y1, adenosine-A1R, glutamate-mGluR5, and glutamate-mGluR2/3 signaling pathways.
Delineating the precise mechanistic underpinnings of drug addiction remains a key research goal for the neuroscience community. A defining feature of drug addiction in humans is the high rate of relapse even after prolonged drug abstinence, which is often triggered by re-exposure to environmental cues that were previously associated with drug experience. Rodent studies reveal that cue-induced cocaine seeking and relapse are mediated in part by altered glutamatergic synaptic transmission within the nucleus accumbens (NAc), a brain region that has been critically implicated in reward learning and motivated behaviors38–41. The vast majority of studies of cocaine’s action on the brain have concentrated on neurons. However, increasing evidence suggests that astrocytes play a dynamic role in the formation and maintenance of maladaptive changes at glutamatergic synapses in the NAc and related limbic regions during cocaine experience, promoting cocaine seeking and relapse after drug withdrawal42–45. Here, we discuss cellular features of astrocytes and their involvement in addiction-related neuronal changes at the synapse and circuit levels.
Basic features of astrocytes
Ca2+-mediated astrocyte activity
Astrocytes do not generate action potentials. Rather, information sensing and signaling output by astrocytes is thought to be mediated in part by intracellular Ca2+ transients46, 47. These transients can be mediated through G protein-coupled receptors (GPCRs) and their activation of IP3-activated type 2 receptors (IP3R2) on the endoplasmic reticulum (ER) of astrocytes, resulting in ER release of Ca2+ and elevation of intracellular Ca2+ levels (Figure 1)48–52. In addition, ionotropic receptors may mediate Ca2+ influx into astrocytes (Figure 1)53, 54. Through these various types of mechanisms, astrocytes respond to neuron-derived signaling molecules and generate several patterns of intracellular Ca2+ waves in a finely graded and subcellularly heterogeneous manner. In contrast to neural networks that are composed of spatially separated neurons, astrocytes can directly couple with neighboring astrocytes via gap junctions55, 56. In this manner, up to dozens of astrocytes are connected to form an electrically low-resistance reticular system called a syncytium. This network of coupled astrocytes is a key feature of astrocyte biology, which is found throughout the central nervous system and across many species56, 57. Interestingly, not all neighboring astrocytes directly couple, for example, astrocyte networks within the somatosensory cortex largely follow a single barrel56. Regardless, an astrocyte syncytium can cover the space of hundreds of microns in diameter with millions of synapses contained therein. Depending on the physiological condition, the Ca2+ waves can be restricted within one astrocyte or, alternatively, propagate to other astrocytes via the syncytium for synchronous network effects58, 59.
Early studies focused on astrocytic Ca2+ activities within the cell soma, since ER storage was viewed as the predominant source of Ca2+ for the entire astrocyte28, 60. Improvements in the spatiotemporal detection of Ca2+ now demonstrate active Ca2+ signals throughout an astrocyte—with subcellular localizations and microdomains that exhibit dynamic features of these Ca2+ transients29, 61, 62. In fact, Ca2+ signals are detected in far distal astrocytic processes62, and can be independent of somatic Ca2+ sources61, 63.
One potential consequence of these localized Ca2+ events is ‘gliotransmission’61. Ca2+ signaling in astrocytes is far from straightforward, however. While Ca2+-induced gliotransmission and resulting synaptic regulation have been observed frequently64–70, it has also been shown that Ca2+ elevations in astrocytes are not always associated with the release of gliotransmitters71, suggesting a subtle and complex relationship between astrocytic Ca2+ levels and gliotransmission28, 62, 72. While the mechanistic link between astrocytic Ca2+ and gliotransmitter release has not to date been fully characterized, it is clear that the process is distinctly different from presynaptic neurotransmitter release in neurons. Nonetheless, astrocytes do exhibit diverse Ca2+ activities in distinctive sub-compartments73–75. The result of this activity may be the release of a variety of transmitters via several different mechanisms23, 70, 76, 77. Of note, a single astrocyte can release different types of gliotransmitters, which cause multi-phasic synaptic responses in nearby neurons78. Furthermore, the type of released gliotransmitters is correlated with the duration and frequency of neural activities nearby, suggesting that astrocytes can sense and process spatiotemporal information from neurons78.
Reciprocal communication between astrocytes and neurons
A single mature astrocyte can contact up to 140,000 synapses in rodents, and upwards of 2 million in humans7, 79. Of note, the enwrapment of synapses by astrocytes is crucial for a range of astrocyte functions, including the sensing and modulation of neuronal activity, preventing neurotransmitter spillover from synapses, as well as the stabilization of synapses themselves. Furthermore, an individual astrocyte can contact synapses from multiple, different neurons, resulting in a term now coined “astrocyte-defined synaptic islands”80. Neurons within a synaptic island spread out up to 200 μm81, thereby poising astrocytes to potentially contribute to the activity of neuronal ensembles.
Glutamate is an example of a neurotransmitter through which astrocytes and neurons communicate bidirectionally. Elevation of astrocytic Ca2+ levels can result in a SNARE protein–dependent vesicular release of glutamate82. Independent of vesicular release, glutamate can diffuse out from astrocytes via nonspecific ion channels upon activation of G-protein-signaling83, 84, or be transported out of astrocytes via a cystine-glutamate exchanger85. Nonetheless, astrocyte-released glutamate activates extrasynaptic GluN2B-containing NMDA receptors (NMDARs) on neurons, resulting in slow inward currents that depolarize these neurons synchronously86, 87. Astrocyte-released glutamate may also activate pre- and postsynaptic glutamatergic receptors, influencing both presynaptic release and postsynaptic responsiveness88, 89. Conversely, astrocytes respond to neuronal-released glutamate via metabotropic glutamate receptors (mGluRs) or ionotropic glutamate receptors. Among the eight known mGluRs, mGluR3 and 5 have been detected in astrocytes90, 91. mGluR3, which is Gi/o-coupled, is expressed predominantly in adult astrocytes and is presumed to inhibit adenylyl cyclase with no direct influence on Ca2+ activity. Indeed, little to no Ca2+ activity is recorded in cortical or hippocampal astrocytes in responses to an mGluR3 agonist92, 93. However, a robust Ca2+ signal is detected in striatal astrocytes93.
In contrast, activation of mGluR5 increases Ca2+ levels in astrocytes through the Gq/11-linked activation of PLCβ-IP3R2-PKC or other signaling pathways94–96. There has been an interesting debate related to the existence and functional importance of mGluR5 in mature astrocytes. Using immunostaining combined with electron microscopy, it was demonstrated in the hippocampus and cortex that astrocytic expression of mGluR5 decreases to very low levels after development97. In line with this result, mGluR5 agonists fail to induce Ca2+ elevations in adult astrocytes within these brain regions93, 97. These results suggest that mGluR5-mediated astrocytic responses decline substantially after development. However, albeit at very low levels, mGluR5 is detected in other preparations, raising the possibility that mGluR5 activity is still present, particularly in distal processes where Ca2+ signals may be too weak to be detected based on traditional bulk-loaded fluorescent Ca2+ dyes. Highlighting astrocyte heterogeneity and regional specificity, it is also important to note that expression of mGluR5 in astrocytes differs substantially among different brain regions93. Indeed, in the NAc of 2–6 weeks old mice, activation of mGluR5 initiates Ca2+ oscillations in astrocytes95. In addiction-oriented studies, administration of mGluR5 antagonists, especially via intra-NAc infusion, decreases cocaine taking during cocaine self-administration and cocaine seeking after drug withdrawal98–101. It is important to determine whether these behavioral responses to mGluR5 manipulations are mediated by mGluR5 located in astrocytes or neurons.
Another molecule that may act as both a neuro- and gliotransmitter is ATP102. ATP can be released from astrocytes through a Ca2+-dependent mechanism involving SNARE proteins103, 104. In these experiments, a variety of stimulations that induce astrocytic Ca2+ waves can trigger ATP release65, 105, 106. In the cerebral cortex, synaptically-released glutamate induces astrocytic ATP release via activation of astrocytic NMDARs, presumably also involving Ca2+-mediated processes107, 108. As well, astrocytic release of ATP is mediated via mechanisms independent of Ca2+ or SNARE proteins109, 110. Astrocytes respond to neuronal or astrocytic release of ATP through several purinergic P2 class ATP receptors (Figure 1). Both in vitro and in vivo data demonstrate that astrocyte-released ATP induces Ca2+ waves in nearby astrocytes106, 111. Through ATP-purinergic signaling, Ca2+ waves initiated in one astrocyte can propagate to nearby astrocytes111, 112 in a regenerative and neural activity-independent manner113. P2Y1 receptor-mediated ATP signaling has been shown to induce glutamate release from astrocytes114.
Once released, ATP is quickly (within 200 ms) hydrolyzed to adenosine by ectonucleotidases within the extracellular matrix115. Thus, in addition to ATP receptors, ATP-derived adenosine influences neural activities by binding neuronal A1 receptors (A1Rs) (Figure 1). Through A1Rs, adenosine originating from astrocytes enhances basal glutamatergic synaptic transmission and reduces the magnitude of LTP65, effects that might be related to the action of astrocytic adenosine on synaptic expression of NMDARs116.
Dopamine is a neuromodulator that regulates synaptic and circuit activities and has been implicated in many psychiatric disorders117–120. D1 and D2 dopamine receptors (D1Rs and D2Rs), which are coupled to distinct intracellular signaling cascades121, are both detected in astrocytes122–125. While activation of D1Rs leads to an elevation of astrocytic levels of Ca2+ and activation of cAMP-PKA signaling123, 126–129, the effects of dopamine on astrocytes also appear to be mediated by D2Rs and other mechanisms. Specifically, incubation of cultured astrocytes with dopamine induces stellation of astrocyte processes, which is only partially mediated by cAMP signaling130. In hippocampal brain slices, superfusion of dopamine induces a fast onset elevation of astrocytic levels of Ca2+, which is followed by a more sustained decrease131. Application of a mixture of D1R and D2R antagonists blunts these biphasic dopamine-induced effects, while D1R antagonists alone selectively prevent the first phase elevation of astrocytic Ca2+ levels, suggesting D2Rs as a key in decreasing astrocytic Ca2+ level during the second phase following dopamine131. Consistent with this interpretation is the finding that application of D2R agonists decreases astrocytic levels of Ca2+ in PFC slices124. Dopamine also influences astrocytes in the ventral tegmental area (VTA) and NAc, work discussed in the next section.
In addition to responding to dopamine, astrocytes regulate extracellular levels of dopamine via astrocytic dopamine, norepinephrine, and other transporters132–135. After reuptake, dopamine in astrocytes is metabolized by monoamine oxidase, and the resulting oxygen species increase intracellular levels of Ca2+136, thus providing a D1R- and D2R-independent mechanism underlying dopamine-mediated regulation of astrocyte Ca2+ activity. Interestingly, vesicular monoamine transporter 2 is detected in astrocytes, and genetic deletion of VMAT2 in PFC astrocytes results in impaired LTP and behavior137, 138, suggesting the possibility that dopamine can be packaged for vesicular release in astrocytes. If this or similar astrocytic dopamine release exists, which requires confirmation, astrocytes may serve a noncanonical source of dopaminergic signaling.
Astrocytes regulate synaptic plasticity and synaptogenesis
Experience-dependent synaptic plasticity serves a key cellular mechanism for learning and memory139–143. In the classical Hebbian model, manipulation of pre- and postsynaptic activities alone is sufficient to induce many forms of LTP or LTD at glutamatergic synapses. However, more recent evidence suggests that astrocytes are also integral to some forms of LTP and LTD. In response to LTP induction, the astrocytic coverage of synapses increases144. This morphological change may effectively increase astrocyte-mediated regulation of synaptic transmission via stabilization of synapses themselves as well as via preventing neurotransmitter synaptic spillover. Additionally, direct astrocytic stimulation66 or elevation of astrocytic Ca2+ levels145 transiently increases presynaptic release probability at hippocampal glutamatergic synapses. Furthermore, when astrocytic Ca2+ elevation is paired with postsynaptic depolarization, the increased presynaptic release probability becomes persistent, resulting in an LTP-like effect at these synapses145. These results raise the important possibility that, without presynaptic conditioning, coincidental activities of postsynaptic neurons and their neighboring astrocytes are sufficient to induce synaptic plasticity. Correspondingly, electrophysiological or pharmacological suppression of astrocytic activities impairs the induction of the classic NMDAR-dependent hippocampal LTP146, 147. In a form of spike timing-dependent LTD (t-LTD), in which coincident postsynaptic release of endocannabinoids (eCBs) and presynaptic activation of NMDARs are essential, astrocytes serve as a lynchpin; postsynaptically-released eCBs activate CB1 receptors on adjacent astrocytes, which, in turn, release glutamate to activate presynaptic NMDARs, accomplishing the induction process148. Furthermore, adenosine signaling originating from astrocyte-released ATP coordinates adjacent synapses such that both monosynaptic LTP and heterosynaptic LTD are induced simultaneously65. These findings describe an active role of astrocytes in the induction and possibly also maintenance of Hebbian plasticity.
Synaptogenesis requires participation of both pre- and postsynaptic neurons149, 150, with increasing evidence suggesting that this process also requires the participation of astrocytes151. During development, astrocytes and neurons share the same precursor cells, but astrogenesis occurs in postnatal stages, after the bulk of neurogenesis is accomplished152–154. Importantly, robust synaptogenesis only occurs after the emergence of astrocytes, particularly during the first 2 to 3 postnatal weeks in rodents, which is a major maturation window for astrocytes151, 155. Evidence suggests that astrocytes actively and directly contribute to both the structural and functional aspects of synaptogenesis and synapse maturation. Most prominently studied are synaptogenic factors derived from astrocytes. Neuronal cultures devoid of astrocytes form far fewer synapses than in neuron-astrocyte co-cultures156, and those synapses appear to be functionally immature157. Treating these astrocyte-free cultures with astrocyte-conditioned medium induces the formation of new synapses, suggesting that astrocytes secrete soluble factors to promote synaptogenesis158–162.
This review focuses on one set of soluble astrocyte-derived factors, thrombospondins (TSPs). For a comprehensive review of astrocytes and synaptogenesis and maturation, we refer the reader to a recent review155. Through activation of their neuronally-located target, α2δ−1, astrocyte-secreted TSPs promote synaptogenesis during development158, 163. TSPs are multimeric, multidomain glycoproteins that, by binding to proteases, cytokines, growth factors, and other components of the extracellular matrix, facilitate cell attachment, migration, cytoskeletal dynamics, and angiogenesis164, 165. TSP-induced synapses appear structurally normal—with pre- and postsynaptic specializations, normal amounts of presynaptically-docked vesicles, and regular dimensions of postsynaptic densities—but are postsynaptically “silent” in that they do not contain functionally stable AMPA glutamate receptors (AMPARs)158. This AMPAR-labile feature is consistent with immature, AMPAR-silent glutamatergic synapses found in the developing brain, some of which subsequently mature and become fully functional by recruiting AMPARs166–169. Of note, synapses generated in astrocyte-present neuronal cultures do express stable postsynaptic AMPARs, highlighting that astrocyte-derived signaling molecules other than TSPs are involved in synapse maturation158.
α2δ−1, described originally as an auxiliary subunit of voltage-gated calcium channels (VGCCs) located at both axons and dendrites170–172, has been identified as one of the key neuronal targets for TSP-mediated synaptogenesis. Overexpression of α2δ−1 augments TSP-induced synaptogenesis, while expression of a dominant-negative form of α2δ−1 impairs TSP-induced synaptogenesis163. Gabapentin, an α2δ−1 antagonist that is used clinically to treat chronic pain, binds to the α2 domain of α2δ−1, the same binding site as TSPs173, 174, but the binding does not alter Ca2+ currents conducted by VGCCs175. Administration of gabapentin prevents astrocyte- and TSP-induced synaptogenesis, identifying α2δ−1 as an essential component in this developmental synaptogenic signaling163. However, despite its intimate relationship with VGCCs, α2δ−1-mediated synaptogenesis is independent of VGCC function163. The key cellular steps from TSP-α2δ−1 binding to synapse formation have not been elucidated176. The small GTPase Rac1 has been implicated in this process177, and Rac1 is also known to influence synapse plasticity following cocaine exposure178, 179. In addition to α2δ−1, TSPs also interact with other synaptic proteins, such as neuroligins, to refine the synaptogenic process180. Beyond TSPs, astrocytes secrete hevin and sparc, which, among other molecules, facilitate synaptogenesis as well160, 181, 182. These and other results depict a critical role of astrocytes in synapse formation during development.
Cooperatively operating with synaptogenesis in forming new circuits, synapse elimination is essential for the refinement of neural circuits in the developing brain as well as remodeling of neural circuits and the turnover of synapses in the adult brain. Similar to microglia, astrocytes can engulf large synaptic components (i.e., synaptosomes), and direct them to lysosomes for ultimate degradation183, 184. A key form of such astrocyte-mediated synapse elimination is mediated by astrocyte phagocytic receptors, such MERTK (a type of protein tyrosine kinase) and MEGF10 (an epidermal growth factor-domain family protein), which can sense “eat-me” signals (e.g., phosphatidylserine) that are presented by targeted debris to execute the engulfment185–187. As such, this form of phagocytosis is not random, but, rather, driven by neuronal activities and mediated by precise signaling exchange between neurons and astrocytes. Astrocytes also release cytokines, such as TGF-β, which can tag synapses for microglia-mediated synapse elimination188, 189. Detailed discussion of these and other forms of astrocyte-mediated synapse elimination can be found in several excellent reviews183, 184, 190.
Astrocytes in neural adaptations after exposure to drugs of abuse
Astrocytes in the VTA-NAc reward circuit respond directly to dopamine
Dopamine’s involvement in the reward pathway and in substance abuse and other psychiatric disorders is well documented117–120, 191–205. Exposure to natural rewards or to drugs of abuse like cocaine induces bursting activity of VTA dopamine neurons, leading to a transient increase in extracellular dopamine levels in the NAc and other limbic forebrain regions191, 192. In contrast, withdrawal from cocaine or other drugs of abuse decreases extracellular dopamine levels in these brain regions196–199, whereas re-exposure to the drug or to drug-predicting cues after drug withdrawal increases such levels200–205. It is noteworthy, as mentioned above and detailed below, that astrocytes in several brain regions associated with the reward circuitry respond directly to the dynamics of extracellular dopamine.
In vivo optogenetic stimulation of VTA dopaminergic nerve terminals within the NAc induces dopamine release and, concomitantly, increases Ca2+ activity in roughly half of NAc astrocytes, lasting for several seconds206. This dopamine-elicited astrocytic response appears to be mediated at least in part by D1Rs on astrocytes and, potentially through ATP/adenosine signaling, contributes to transient inhibition of nearby glutamatergic synaptic transmission206. In concert with these results, superfusion of cocaine, which elevates extracellular dopamine levels in NAc slices207, 208, increases both the frequency and total number of astrocytic Ca2+ transients209. Paradoxically, AAV-mediated deletion of D1Rs in NAc astrocytes increases basal Ca2+ activity in this brain region206. In the VTA and surrounding ventral midbrain areas, activation of D2Rs on astrocytes revealed a blunted Ca2+ response210. These findings, coupled with distinct responses of astrocytes in hippocampus and PFC to dopamine (see above), again highlight the regional heterogeneity of astrocytes and implicate a far more complex dopamine-mediated regulation of astrocytes than previously understood.
Astrocytes in the NAc
Transcriptomic analyses211 reveal that striatal astrocytes (encompassing NAc and dorsal striatum) express high levels of K+ channels, setting their equilibrium membrane potential at ~−85 mV, which is more hyperpolarized than astrocytes in many other brain regions212, 213. Striatal astrocytes also express high levels of gap-junction proteins, allowing for synchronized activities among connected astrocytes93. Interestingly, this astrocytic syncytium can spread across brain regions such that a dye infused into a single striatal astrocyte can eventually diffuse into cortical astrocytes214. Basal spontaneous Ca2+ activities in striatal astrocytes are insensitive to TTX, suggesting that they are relatively independent of ongoing neuronal activities93.
Striatal GABAergic medium spiny neurons (MSNs) comprise >90% of the local neuronal population and represent the sole projection neurons of this region215. MSNs can be divided into two subpopulations: one that expresses predominantly D1Rs and the neuropeptides dynorphin and substance P, and another that expresses D2Rs and enkephalin216. In the dorsal striatum, subpopulations of astrocytes show selective responsivity to the activity of either D1 or D2 MSNs217. Such cell-type specific neuron-astrocyte interactions may contribute to the heterogeneous responses of D1R versus D2R NAc MSNs to cocaine, such as selective generation of silent synapses in D1R MSNs after noncontingent cocaine exposure218. Compared to noncontingent procedures, astrocytic adaptations to contingent (volitional) exposure to cocaine are likely more complex than previously believed given that astrocytes respond both to neuronal activity and directly to dopamine. Much of this work to date has focused on dorsal striatum, with studies of NAc specifically now being a high priority.
Extinction is a training procedure in rodent models of drug relapse, in which the learned drug taking and seeking are diminished in the absence of anticipated reinforcement. Extinction requires new reinforcement learning that involves remodeling of glutamatergic transmission in the NAc219–223. After extinction of cocaine self-administration, NAc expression of glial fibrillary acidic protein (GFAP) as well as the overall surface area and volume of astrocytes are decreased, effects accompanied by reduced co-localization of astrocyte processes with the presynaptic nerve terminal marker, synapsin-1224. Importantly, without extinction training, the synapse-astrocyte proximity remains largely unchanged in the NAc after cocaine self-administration, selectively linking this form of astrocytic adaptation to extinction225. Furthermore, this extinction-related astrocytic adaptation is only detected in the NAc and not in the PFC or basolateral amygdala (BLA), indicating the unique response of NAc astrocytes in extinction learning225. Similar extinction-related changes in NAc astrocytes are also observed after extinction from methamphetamine226 or heroin227 self-administration. Importantly, re-exposure to heroin-associated cues after extinction transiently restores the reduced synapse-astrocyte proximity227, suggesting a dynamic role of NAc astrocytes in response to cue-induced drug seeking after drug extinction. DREADD-mediated activation of NAc astrocytes after drug extinction decreases subsequent cue-induced cocaine228 and methamphetamine226 seeking, possibly through restoring astrocyte-mediated glutamate homeostasis in the NAc (see below).
Unlike psychostimulants, opioids initiate their primary pharmacological effects by activating μ opioid receptors (MORs)229. NAc astrocytes express MORs230 that can be activated upon opioid administration. In acutely prepared NAc slices, superfusion of morphine increases astrocytic Ca2+ levels through IP3R2-mediated release from intracellular stores231. Activation of MORs induces astrocytic glutamate release, which, in turn, induces NMDAR-mediated slow inward currents in nearby neurons231. Considering that an individual striatal astrocyte contacts ~20 neuronal somas93, morphine-induced slow inward currents may be present throughout NAc MSNs. In drug-naïve animals, intra-NAc infusion of astrocyte-conditioned medium, which contains a variety of soluble astrocytic factors, acutely enhances the rewarding effect of morphine232. Furthermore, intra-NAc infusion of such media from morphine-pretreated astrocytes results in a stronger preference for drug-associated environments than infusions of media from astrocytes without morphine pretreatment232. Albeit preliminary, these results suggest a clear link between NAc astrocytes and opioid-induced behaviors.
Astrocytes regulate glutamate homeostasis
Cortical glutamatergic projections to the NAc regulate general reward seeking under physiological conditions233–235. Proper functioning of this projection is maintained, in part, by homeostatic regulation of glutamate at both synaptic and extrasynaptic sites. Disruption to this homeostasis during drug experience contributes to drug seeking and relapse236.
A key element to this disruption is GLT-1, a glutamate transporter expressed in perisynaptic astroglial processes that contributes to >90% of glutamate reuptake21, 22, 237–242. Chronic exposure to either psychostimulants or opioids reduces the expression of GLT-1 in the NAc at both the mRNA and protein levels243, 244. Furthermore, chronic drug exposure reduces the overlap of perisynaptic astroglial processes with synaptic markers, suggesting decreased astrocytic insulation of synapses224–227. Both of these changes impair the clearance of synaptic glutamate, facilitating spill-over to perisynaptic regions, and establishing an inter-synaptic crosstalk245–248 (Figure 2). Glutamatergic synaptic transmission within the NAc is pathophysiologically upregulated after withdrawal from many classes of drugs of abuse233, 249–251. The diminished reuptake of glutamate through GLT-1 may exacerbate this pathophysiology. Moreover, glutamate spillover can activate glutamatergic receptors such as NMDARs and mGluRs in neighboring synapses146, 252–254, resulting in nonspecific neuronal activity not observed in drug-naïve subjects. Indeed, administration of mGluR5 antagonists decreases cocaine seeking after drug withdrawal98–101. Multiple pharmacological approaches that normalize GLT-1 function in cocaine-exposed animals have proven useful in reducing cue-induced reinstatement of cocaine seeking in animal models243, 255, 256, raising the hope for treating drug relapse by targeting astrocyte-maintained glutamate homeostasis.
Figure 2. Dysregulation of NAc extracellular glutamate after cocaine experience.
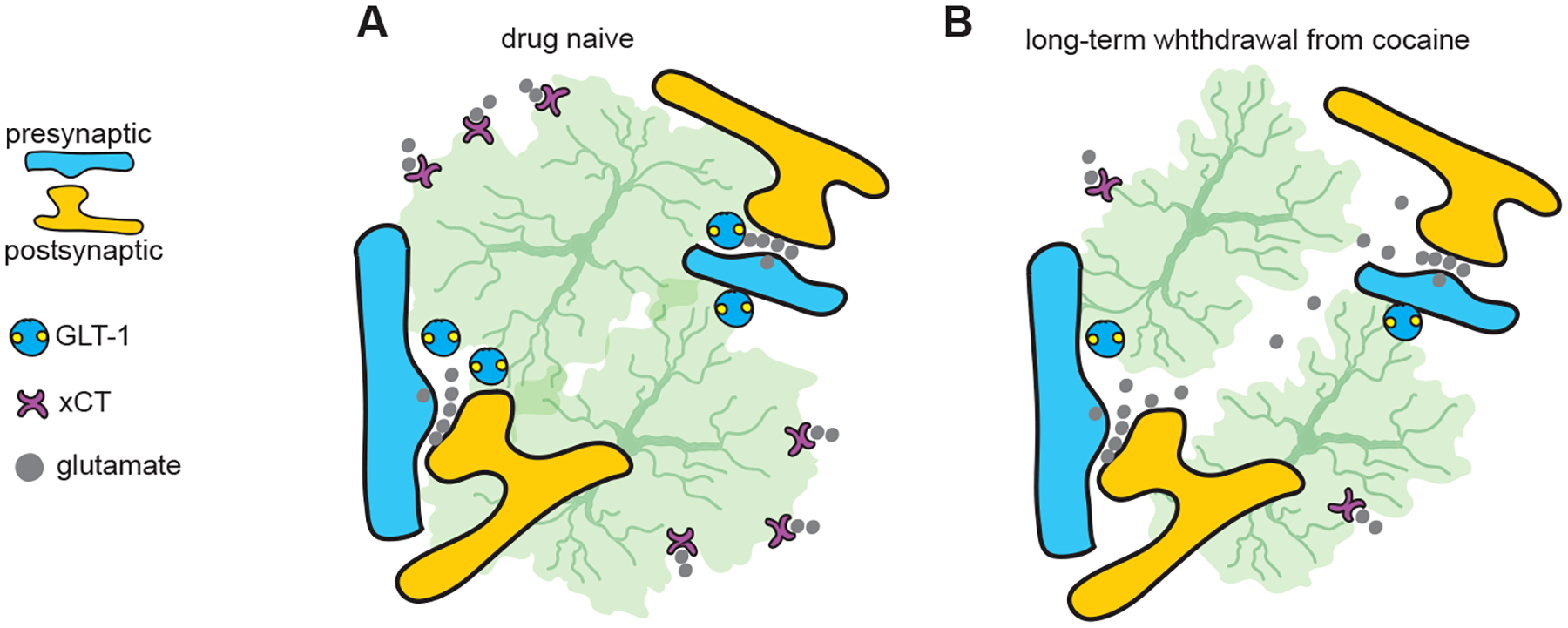
A In drug naïve animals, GLT-1 is a key astrocytic glutamate transporter that maintains low levels of glutamate at synaptic and perisynaptic locations, while xCT is a key astrocytic glutamate antiporter that maintains ambient glutamate levels for tonic activities of mGluRs in the NAc. B After withdrawal from cocaine self-administration, astrocytes retract from synapses and both GLT1- and xCT-mediated regulation of glutamate is compromised, resulting in increased levels of synaptic and perisynaptic glutamate but decreased levels of ambient, extracellular glutamate.
While astrocytic GLT-1 regulates synaptically-released glutamate, the cystine/glutamate (xCT) exchanger in astrocytes maintains ambient levels of glutamate within the extracellular space257. xCT functions via the exchange of one extracellular cystine for one intracellular glutamate258. xCT therefore contributes to the extracellular tone of glutamate, which, in turn, modulates activity of presynaptic group II mGluRs (e.g., mGluR2/3) that control levels of synaptically-released glutamate257. This xCT-mediated regulation of glutamate is impaired after drug experience236. After withdrawal from cocaine or nicotine self-administration, levels of xCT in the NAc core are reduced, contributing to the drug withdrawal-associated reduction of extracellular glutamate85, 255, 259–261. A basal level of extracellular glutamate is needed for tonic activation of presynaptic group II mGluRs (e.g., mGluR2/3) to limit glutamate release from presynaptic terminals262. As such, decreased levels of ambient glutamate compromises this inhibitory control of presynaptic glutamate release and promotes glutamate overflow, a cellular maladaptation that contributes to drug seeking263 (Figure 2). In fact, astrocytic h3Mdq-DREADD-mediated reductions cocaine seeking (see below) are ameliorated with concomitant administration of mGluR2/3 antagonists228.
A therapeutic approach to targeting xCT has also been tested with the use of N-acetylcysteine, a cysteine prodrug and substrate of xCT. Administration of N-acetylcysteine restores the compromised function of xCT following chronic cocaine exposure and decreases drug- and cue-induced relapse to cocaine seeking259. N-acetylcysteine also restores GLT-1 expression in the NAc and is thought to ameliorate extrasynaptic glutamate spillover following cocaine extinction and reinstatement243. This might be due to the restoration of plasticity at PFC-to-NAc synapses known to be impaired after cocaine self-administration264. Furthermore, after withdrawal from cocaine self-administration, administration of N-acetylcysteine enhances the sensitivity of rats to the punishment that induces extinction of drug seeking, suggesting multi-angled anti-relapse effects of N-acetylcysteine265. In early clinical studies, administration of N-acetylcysteine reduces cocaine craving and thus decreases the risk of relapse in individuals during drug abstinence266. Beyond cocaine, N-acetylcysteine also reduces the number of cigarettes consumed in active smokers261 and exhibits beneficial effects on PTSD patients267, 268.
As discussed above, by decreasing the function or protein expression of GLT-1 and xCT, cocaine experience simultaneously increases spillover of synaptically-released glutamate and decreases ambient glutamatergic tone in the NAc, both of which promote cocaine seeking243. This line of results raises several interesting considerations. For example, mGluR activity at cortical glutamatergic projection terminals versus dopaminergic projection terminals are linked to the effects of GLT-1 versus xCT, respectively257, 263, 269, 270. If both of these effects are mediated by the same astrocytes, the astrocyte microdomains or processes that are close to glutamatergic versus dopaminergic synapses are likely to operate independently. This is not surprising, as such compartmentalized responses of astrocytes to different forms of stimulation has been demonstrated271, 272. However, this potentially domain-oriented functioning of astrocytes indicates that a more nuanced investigation of astrocyte-neuron partnership is needed. Furthermore, decreased function of GLT-1 after extinction of cocaine self-administration increases the likelihood of glutamate spillover, resulting in increases of glutamate in neuropil. This effect may somewhat compensate for the reduction of ambient glutamate in the NAc upon diminished expression of xCT. As such, homeostatic mechanisms may exist in NAc astrocytes43, 273, functioning in part through GLT-1 and xCT to balance synaptic versus ambient glutamate. Emerging evidence suggests that such homeostatic regulation or dysregulation between GLT-1 and xCT may be organized by cascades of epigenetic changes following extinction from cocaine self-administration274.
Astrocyte-mediated synaptogenesis
The neural rejuvenation hypothesis of drug addiction275 proposes that “repeated exposure to drugs of abuse induces plasticity mechanisms that are normally associated with brain development within the brain’s reward circuitry, which mediate the highly efficient and unusually stable memory abnormalities that characterize addiction.” Astrocytes promote synapse formation during development, but these astrocyte-mediated synaptogenic mechanisms become much less active after postnatal week 3 in rodents when the major wave of synapse formation is accomplished276. Cocaine experience generates AMPAR-silent synapses in the adult NAc, and these synapses exhibit several features consistent with nascent glutamatergic synapses observed during development277, 278. Recent results demonstrate that cocaine-induced generation of silent synapses in the adult NAc is mediated, in part, by an astrocyte-derived developmental synaptogenic mechanism, namely, TSP-α2δ−1 signaling209. TSPs are abundantly expressed during early developmental stages, but decline to low levels in most brain regions during adulthood279. Albeit at low levels, basal expression of TSP1/2 is detected in the adult NAc, where neuronal expression of α2δ−1 remains robust at this time point209, 280. In acutely prepared NAc slices, superfusion of cocaine increases Ca2+-mediated activities in astrocytes209. Within the dorsal striatum, DREADD-mediated activation of Gi/o-coupled GPCRs increases Ca2+ activity in astrocytes with the subsequent release of TSP1281. While the underlying mechanisms remain largely unclear, these studies suggest that the cocaine-induced release of TSP2 from astrocytes may reflect a direct effect of dopamine on astrocytic GPCR signaling and downstream elevations in intracellular Ca2+ levels. Administration of gabapentin, which as noted earlier disrupts the binding of TSP2 to α2δ−1163, 177, prevents cocaine-induced generation of silent synapses and cocaine-induced spinogenesis in the NAc, suggesting the involvement of TSP-α2δ−1 signaling209. Additionally, selective knockdown of either α2δ−1 in neurons or TSP2 in astrocytes prevents cocaine-induced generation of silent synapses in the NAc, indicating that astrocyte-derived TSP2, and α2δ−1 on MSNs, form a synaptogenic signal to generate silent synapses de novo in response to cocaine209, by analogy to what is observed during development158, 163 (Figure 3). These results—in line with the neural rejuvenation hypothesis—suggest that cocaine experience utilizes astrocyte-mediated synaptogenic mechanisms to “re-develop” NAc circuits, potentially generating new connectivity patterns that support cocaine-associated memories.
Figure 3. Astrocyte-mediated synaptogenesis after cocaine self-administration.
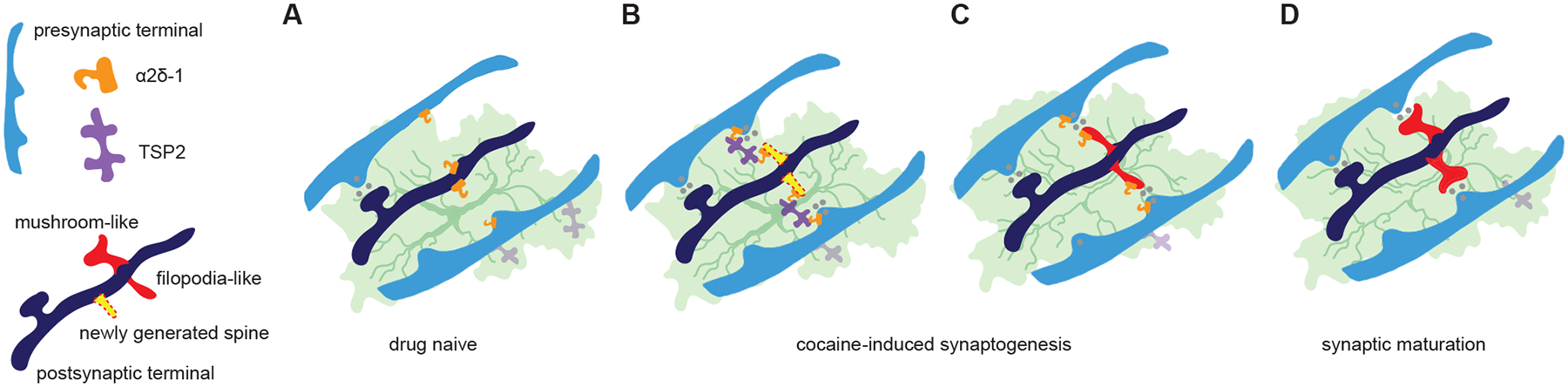
A Astrocytic TSP2 and neuronal α2δ−1 constitute a synaptogenic signaling pathway in the developing brain to promote formation of glutamatergic synapses. Both astrocytic TSP2 and neuronal α2δ−1 are present in the adult NAc in drug naïve animals. B Cocaine self-administration induces formation of nascent, AMPAR-silent synapses in the NAc, which is mediated in part by TSP2-α2δ−1 signaling. These nascent synapses are thought to have relatively immature spine morphology. C,D After cocaine withdrawal, some of these newly formed, immature AMPAR-silent synapses are maintained (C) and, by recruiting and stabilizing AMPARs, gradually transform into fully mature synapses, resulting in long-term changes of circuit connectivity (D).
After drug withdrawal, some cocaine-generated silent synapses mature by recruiting AMPARs, which may stabilize the remodeled NAc circuits and result in the consolidation of cocaine memories179, 218, 235, 282–284. These synapses appear to remain dynamic, as retrieval-induced destabilization and reconsolidation of cue-associated cocaine memories are influenced by the re-silencing and re-maturation of these synapses, respectively, following re-exposure to cocaine-associated cues179, 285. Thus, the generation of silent synapses is a key mechanism through which astrocytes can influence NAc circuits to promote cue-induced cocaine seeking. It is therefore not surprising that selectively disrupting astrocytic TSP-α2δ−1-signaling during cocaine self-administration, preventing cocaine-induced generation of silent synapses and therefore silent synapse-mediated NAc remodeling, results in both decreased cue-induced cocaine seeking after cocaine withdrawal and decreased cue-induced reinstatement of cocaine seeking after cocaine extinction209. Importantly, intra-NAc disruption of TSP-α2δ−1-signaling does not prevent animals from acquiring operant responding to cocaine in the first place209, indicating that astrocyte-mediated synaptogenesis in the NAc is selectively involved in the formation of cue-associated cocaine memories without affecting basic instrumental learning.
In addition to synaptogenesis during cocaine exposure, TSP-α2δ−1 signaling may contribute to withdrawal-associated neural adaptations. Selectively inhibiting NAc α2δ−1 only after extinction from cocaine self-administration decreases cocaine priming-induced reinstatement of drug seeking280. This finding indicates that TSP-α2δ−1 signaling and presumably other astrocytic mechanisms are critically involved in neural adaptations that mediate multiple phases of drug-associated behaviors. Furthermore, chemogenetically upregulating NAc astrocyte activity after drug extinction acutely decreases subsequent cocaine228 and methamphetamine226 seeking, suggesting that, in addition to adaptive changes, NAc astrocytes also influence ongoing NAc circuit function that controls drug-seeking behaviors.
Concluding Remarks
Astrocytes are increasingly recognized as key players in brain function under physiological and pathophysiological conditions. We highlight some recently identified mechanisms through which astrocytes regulate synapses and neural circuits to promote addiction-related behaviors. Additionally, in cocaine-trained animals, manipulating astrocytes prevents drug-induced neural adaptations and ameliorates addiction-related behavioral abnormalities. These findings not only establish astrocytes as promising cellular targets to explore novel mechanisms underlying drug addiction, but also specify several future directions. First, a wide range of astrocytic substrates responds to neural activities and external stimuli. GLT-1 and xCT likely represent the tip of the iceberg of astrocytic substrates that undergo adaptive changes following repeated drug experience. It is important for future studies to systematically screen and identify key astrocytic substrates that are changed by drug experience to influence behaviors. Second, while this manuscript focuses on the NAc, many other brain regions are also critically involved in drug-induced behaviors. For example, similar to the NAc, cocaine self-administration increases the density of dendritic spines expressed by pyramidal neurons in the prefrontal cortex (PFC), suggesting a similar synaptogenic process286, 287. However, astrocytes in the NAc and PFC are highly heterogeneous, and their differential responses to cocaine are only beginning to be elucidated225. It is critical for future studies to characterize such regional specificity, neuronal-partner selectivity, and domain-specific specializations, and how these features influence drug-induced alterations. Third, drug exposure, withdrawal, extinction, reinstatement, and other related conditions constitute different aspects of the drug-related experience that contribute differentially to the addicted state. Astrocytes influence both synaptogenesis during drug exposure and dysregulation of glutamate homeostasis after drug withdrawal, indicating a phasic involvement of astrocytes. It is important to determine the astrocytic contributions across these phases to influence neural and behavioral consequences. The role of astrocytes in addiction is likely defined both by the direct actions of drugs of abuse on astrocytes as well as by astrocytic dysregulation that occurs as a consequence of dynamic neuron-astrocyte interactions during drug withdrawal, abstinence, and relapse. These divergent mechanisms underscore the need to elucidate the increasingly complex role of astrocytes in the brain under normal and pathophysiological conditions. Pursuing these and other questions will provide a new angle to understanding the cellular and circuit mechanisms underlying drug-induced behavioral abnormalities.
Acknowledgements:
Preparation of this review was supported by NIH grants R01DA014133 (EJN), R01DA040620 (EJN, YD), R21DA047861 (YD), R37DA023206 (YD) and R21DA051010 (YD).
Footnotes
Declaration: The authors declare no financial interests.
References
- 1.Pfrieger FW, Barres BA. What the fly’s glia tell the fly’s brain. Cell 1995; 83(5): 671–674. [DOI] [PubMed] [Google Scholar]
- 2.Vasile F, Dossi E, Rouach N. Human astrocytes: structure and functions in the healthy brain. Brain Struct Funct 2017; 222(5): 2017–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bass NH, Hess HH, Pope A, Thalheimer C. Quantitative cytoarchitectonic distribution of neurons, glia, and DNa in rat cerebral cortex. The Journal of comparative neurology 1971; 143(4): 481–490. [DOI] [PubMed] [Google Scholar]
- 4.Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B. Neocortical glial cell numbers in human brains. Neurobiol Aging 2008; 29(11): 1754–1762. [DOI] [PubMed] [Google Scholar]
- 5.Oikonomou G, Shaham S. The glia of Caenorhabditis elegans. Glia 2011; 59(9): 1253–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F et al. Uniquely hominid features of adult human astrocytes. J Neurosci 2009; 29(10): 3276–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oberheim NA, Wang X, Goldman S, Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends Neurosci 2006; 29(10): 547–553. [DOI] [PubMed] [Google Scholar]
- 8.Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 2013; 12(3): 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 2009; 32(8): 421–431. [DOI] [PubMed] [Google Scholar]
- 10.Perea G, Araque A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J Neurosci 2005; 25(9): 2192–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perea G, Araque A. Synaptic Information Processing by Astrocytes. In: Haydon PG, Parpura V (eds). Astrocytes in (Patho)Physiology of the Nervous System. Springer US: Boston, MA, 2009, pp 287–300. [Google Scholar]
- 12.Santello M, Toni N, Volterra A. Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci 2019; 22(2): 154–166. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Barres BA. A smarter mouse with human astrocytes. Bioessays 2013; 35(10): 876–880. [DOI] [PubMed] [Google Scholar]
- 14.Spacek J. Three-dimensional analysis of dendritic spines. III. Glial sheath. Anat Embryol (Berl) 1985; 171(2): 245–252. [DOI] [PubMed] [Google Scholar]
- 15.Octeau JC, Chai H, Jiang R, Bonanno SL, Martin KC, Khakh BS. An Optical Neuron-Astrocyte Proximity Assay at Synaptic Distance Scales. Neuron 2018; 98(1): 49–66 e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kikuchi T, Gonzalez-Soriano J, Kastanauskaite A, Benavides-Piccione R, Merchan-Perez A, DeFelipe J et al. Volume Electron Microscopy Study of the Relationship Between Synapses and Astrocytes in the Developing Rat Somatosensory Cortex. Cereb Cortex 2020; 30(6): 3800–3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter JT, McCarthy KD. Astrocytic neurotransmitter receptors in situ and in vivo. Prog Neurobiol 1997; 51(4): 439–455. [DOI] [PubMed] [Google Scholar]
- 18.Biber K, Laurie DJ, Berthele A, Sommer B, Tolle TR, Gebicke-Harter PJ et al. Expression and signaling of group I metabotropic glutamate receptors in astrocytes and microglia. J Neurochem 1999; 72(4): 1671–1680. [DOI] [PubMed] [Google Scholar]
- 19.Velez-Fort M, Audinat E, Angulo MC. Central role of GABA in neuron-glia interactions. Neuroscientist 2012; 18(3): 237–250. [DOI] [PubMed] [Google Scholar]
- 20.Ribak CE, Tong WM, Brecha NC. GABA plasma membrane transporters, GAT-1 and GAT-3, display different distributions in the rat hippocampus. The Journal of comparative neurology 1996; 367(4): 595–606. [DOI] [PubMed] [Google Scholar]
- 21.Schousboe A. Transport and metabolism of glutamate and GABA in neurons are glial cells. Int Rev Neurobiol 1981; 22: 1–45. [DOI] [PubMed] [Google Scholar]
- 22.Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D et al. Localization of neuronal and glial glutamate transporters. Neuron 1994; 13(3): 713–725. [DOI] [PubMed] [Google Scholar]
- 23.Szatkowski M, Barbour B, Attwell D. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature 1990; 348(6300): 443–446. [DOI] [PubMed] [Google Scholar]
- 24.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H et al. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A 1998; 95(26): 15735–15740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warr O, Takahashi M, Attwell D. Modulation of extracellular glutamate concentration in rat brain slices by cystine-glutamate exchange. J Physiol 1999; 514 (Pt 3): 783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutter D, Ullrich F, Lueck JC, Kempa S, Jentsch TJ. Selective transport of neurotransmitters and modulators by distinct volume-regulated LRRC8 anion channels. J Cell Sci 2017; 130(6): 1122–1133. [DOI] [PubMed] [Google Scholar]
- 27.Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E et al. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci 2004; 7(6): 613–620. [DOI] [PubMed] [Google Scholar]
- 28.Fiacco TA, McCarthy KD. Multiple Lines of Evidence Indicate That Gliotransmission Does Not Occur under Physiological Conditions. J Neurosci 2018; 38(1): 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savtchouk I, Volterra A. Gliotransmission: Beyond Black-and-White. J Neurosci 2018; 38(1): 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarz Y, Zhao N, Kirchhoff F, Bruns D. Astrocytes control synaptic strength by two distinct v-SNARE-dependent release pathways. Nat Neurosci 2017; 20(11): 1529–1539. [DOI] [PubMed] [Google Scholar]
- 31.Navarrete M, Cuartero MI, Palenzuela R, Draffin JE, Konomi A, Serra I et al. Astrocytic p38alpha MAPK drives NMDA receptor-dependent long-term depression and modulates long-term memory. Nat Commun 2019; 10(1): 2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takata-Tsuji F, Chounlamountri N, Do LD, Philippot C, Novion Ducassou J, Coute Y et al. Microglia modulate gliotransmission through the regulation of VAMP2 proteins in astrocytes. Glia 2021; 69(1): 61–72. [DOI] [PubMed] [Google Scholar]
- 33.Rajani V, Zhang Y, Jalubula V, Rancic V, SheikhBahaei S, Zwicker JD et al. Release of ATP by pre-Botzinger complex astrocytes contributes to the hypoxic ventilatory response via a Ca(2+) -dependent P2Y1 receptor mechanism. The Journal of physiology 2018; 596(15): 3245–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angelova PR, Iversen KZ, Teschemacher AG, Kasparov S, Gourine AV, Abramov AY. Signal transduction in astrocytes: Localization and release of inorganic polyphosphate. Glia 2018; 66(10): 2126–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 1999; 22(5): 208–215. [DOI] [PubMed] [Google Scholar]
- 36.Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci 2001; 2(3): 185–193. [DOI] [PubMed] [Google Scholar]
- 37.Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 2015; 18(7): 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci 2004; 5(6): 483–494. [DOI] [PubMed] [Google Scholar]
- 39.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology 2010; 35(1): 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adinoff B. Neurobiologic processes in drug reward and addiction. Harv Rev Psychiatry 2004; 12(6): 305–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mogenson GJ, Yang CR, Yim CY. Influence of dopamine on limbic inputs to the nucleus accumbens. Ann N Y Acad Sci 1988; 537: 86–100. [DOI] [PubMed] [Google Scholar]
- 42.Scofield MD. Exploring the Role of Astroglial Glutamate Release and Association With Synapses in Neuronal Function and Behavior. Biol Psychiatry 2018; 84(11): 778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scofield MD, Kalivas PW. Astrocytic dysfunction and addiction: consequences of impaired glutamate homeostasis. Neuroscientist 2014; 20(6): 610–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim R, Healey KL, Sepulveda-Orengo MT, Reissner KJ. Astroglial correlates of neuropsychiatric disease: From astrocytopathy to astrogliosis. Progress in neuro-psychopharmacology & biological psychiatry 2018; 87(Pt A): 126–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kruyer A, Kalivas PW. Astrocytes as cellular mediators of cue reactivity in addiction. Current opinion in pharmacology 2020; 56: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 1990; 247(4941): 470–473. [DOI] [PubMed] [Google Scholar]
- 47.Hille B. Ionic channels: molecular pores of excitable membranes. Harvey Lect 1986; 82: 47–69. [PubMed] [Google Scholar]
- 48.Sperlagh B, Heinrich A, Csolle C. P2 receptor-mediated modulation of neurotransmitter release-an update. Purinergic Signal 2007; 3(4): 269–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharp AH, Nucifora FC Jr., Blondel O, Sheppard CA, Zhang C, Snyder SH et al. Differential cellular expression of isoforms of inositol 1,4,5-triphosphate receptors in neurons and glia in brain. The Journal of comparative neurology 1999; 406(2): 207–220. [PubMed] [Google Scholar]
- 50.Hussl S, Boehm S. Functions of neuronal P2Y receptors. Pflugers Archiv : European journal of physiology 2006; 452(5): 538–551. [DOI] [PubMed] [Google Scholar]
- 51.Agulhon C, Boyt KM, Xie AX, Friocourt F, Roth BL, McCarthy KD. Modulation of the autonomic nervous system and behaviour by acute glial cell Gq protein-coupled receptor activation in vivo. The Journal of physiology 2013; 591(22): 5599–5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petravicz J, Fiacco TA, McCarthy KD. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci 2008; 28(19): 4967–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verkhratsky A, Rodriguez JJ, Parpura V. Calcium signalling in astroglia. Mol Cell Endocrinol 2012; 353(1–2): 45–56. [DOI] [PubMed] [Google Scholar]
- 54.Verkhratsky A, Untiet V, Rose CR. Ionic signalling in astroglia beyond calcium. The Journal of physiology 2020; 598(9): 1655–1670. [DOI] [PubMed] [Google Scholar]
- 55.Xu G, Wang W, Kimelberg HK, Zhou M. Electrical coupling of astrocytes in rat hippocampal slices under physiological and simulated ischemic conditions. Glia 2010; 58(4): 481–493. [DOI] [PubMed] [Google Scholar]
- 56.Verkhratsky A, Nedergaard M. Physiology of Astroglia. Physiol Rev 2018; 98(1): 239–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kiyoshi CM, Zhou M. Astrocyte syncytium: a functional reticular system in the brain. Neural Regen Res 2019; 14(4): 595–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hanani M, Verkhratsky A. Satellite Glial Cells and Astrocytes, a Comparative Review. Neurochemical research 2021. [DOI] [PubMed] [Google Scholar]
- 59.Nagai J, Yu X, Papouin T, Cheong E, Freeman MR, Monk KR et al. Behaviorally consequential astrocytic regulation of neural circuits. Neuron 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Agulhon C, Fiacco TA, McCarthy KD. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 2010; 327(5970): 1250–1254. [DOI] [PubMed] [Google Scholar]
- 61.Srinivasan R, Huang BS, Venugopal S, Johnston AD, Chai H, Zeng H et al. Ca(2+) signaling in astrocytes from Ip3r2(−/−) mice in brain slices and during startle responses in vivo. Nat Neurosci 2015; 18(5): 708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bindocci E, Savtchouk I, Liaudet N, Becker D, Carriero G, Volterra A. Three-dimensional Ca(2+) imaging advances understanding of astrocyte biology. Science 2017; 356(6339). [DOI] [PubMed] [Google Scholar]
- 63.Agarwal A, Wu PH, Hughes EG, Fukaya M, Tischfield MA, Langseth AJ et al. Transient Opening of the Mitochondrial Permeability Transition Pore Induces Microdomain Calcium Transients in Astrocyte Processes. Neuron 2017; 93(3): 587–605 e587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci 1998; 1(8): 683–692. [DOI] [PubMed] [Google Scholar]
- 65.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY et al. Astrocytic purinergic signaling coordinates synaptic networks. Science 2005; 310(5745): 113–116. [DOI] [PubMed] [Google Scholar]
- 66.Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M et al. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci 2007; 10(3): 331–339. [DOI] [PubMed] [Google Scholar]
- 67.Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature 2010; 463(7278): 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS et al. Channel-mediated tonic GABA release from glia. Science 2010; 330(6005): 790–796. [DOI] [PubMed] [Google Scholar]
- 69.Durkee CA, Covelo A, Lines J, Kofuji P, Aguilar J, Araque A. Gi/o protein-coupled receptors inhibit neurons but activate astrocytes and stimulate gliotransmission. Glia 2019; 67(6): 1076–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature 1994; 369(6483): 744–747. [DOI] [PubMed] [Google Scholar]
- 71.Fiacco TA, Agulhon C, Taves SR, Petravicz J, Casper KB, Dong X et al. Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron 2007; 54(4): 611–626. [DOI] [PubMed] [Google Scholar]
- 72.Oberheim NA, Goldman SA, Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol Biol 2012; 814: 23–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shigetomi E, Bushong EA, Haustein MD, Tong X, Jackson-Weaver O, Kracun S et al. Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J Gen Physiol 2013; 141(5): 633–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shigetomi E, Kracun S, Sofroniew MV, Khakh BS. A genetically targeted optical sensor to monitor calcium signals in astrocyte processes. Nat Neurosci 2010; 13(6): 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haustein MD, Kracun S, Lu XH, Shih T, Jackson-Weaver O, Tong X et al. Conditions and constraints for astrocyte calcium signaling in the hippocampal mossy fiber pathway. Neuron 2014; 82(2): 413–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duan S, Anderson CM, Keung EC, Chen Y, Chen Y, Swanson RA. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci 2003; 23(4): 1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci 2003; 23(9): 3588–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Covelo A, Araque A. Neuronal activity determines distinct gliotransmitter release from a single astrocyte. Elife 2018; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 2002; 22(1): 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. J Neurosci 2007; 27(24): 6473–6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pirttimaki TM, Sims RE, Saunders G, Antonio SA, Codadu NK, Parri HR. Astrocyte-Mediated Neuronal Synchronization Properties Revealed by False Gliotransmitter Release. J Neurosci 2017; 37(41): 9859–9870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parpura V, Scemes E, Spray DC. Mechanisms of glutamate release from astrocytes: gap junction “hemichannels”, purinergic receptors and exocytotic release. Neurochemistry international 2004; 45(2–3): 259–264. [DOI] [PubMed] [Google Scholar]
- 83.Woo DH, Han KS, Shim JW, Yoon BE, Kim E, Bae JY et al. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell 2012; 151(1): 25–40. [DOI] [PubMed] [Google Scholar]
- 84.Park H, Han KS, Seo J, Lee J, Dravid SM, Woo J et al. Channel-mediated astrocytic glutamate modulates hippocampal synaptic plasticity by activating postsynaptic NMDA receptors. Mol Brain 2015; 8: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci 2003; 6(7): 743–749. [DOI] [PubMed] [Google Scholar]
- 86.Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 2004; 43(5): 729–743. [DOI] [PubMed] [Google Scholar]
- 87.Bardoni R, Ghirri A, Zonta M, Betelli C, Vitale G, Ruggieri V et al. Glutamate-mediated astrocyte-to-neuron signalling in the rat dorsal horn. The Journal of physiology 2010; 588(Pt 5): 831–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Santello M, Bezzi P, Volterra A. TNFalpha controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 2011; 69(5): 988–1001. [DOI] [PubMed] [Google Scholar]
- 89.Vance KM, Rogers RC, Hermann GE. PAR1-activated astrocytes in the nucleus of the solitary tract stimulate adjacent neurons via NMDA receptors. J Neurosci 2015; 35(2): 776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Testa CM, Catania MV, Young AB. Anatomical Distribution of Metabotropic Glutamate Receptors in Mammalian Brain. 1994: 99–123.
- 91.Schools GP, Kimelberg HK. mGluR3 and mGluR5 are the predominant metabotropic glutamate receptor mRNAs expressed in hippocampal astrocytes acutely isolated from young rats. Journal of neuroscience research 1999; 58(4): 533–543. [DOI] [PubMed] [Google Scholar]
- 92.Sun W, McConnell E, Pare J-F, Xu Q, Chen M, Peng W et al. Glutamate-Dependent Neuroglial Calcium Signaling Differs Between Young and Adult Brain. Science 2013; 339(6116): 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X et al. Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron 2017; 95(3): 531–549 e539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakahara K, Okada M, Nakanishi S. The metabotropic glutamate receptor mGluR5 induces calcium oscillations in cultured astrocytes via protein kinase C phosphorylation. J Neurochem 1997; 69(4): 1467–1475. [DOI] [PubMed] [Google Scholar]
- 95.D’Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP et al. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci U S A 2007; 104(6): 1995–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Balazs R, Miller S, Chun Y, Cotman CW. Receptor-coupled phospholipase C and adenylyl cyclase function with different calcium pools in astrocytes. Neuroreport 1998; 9(7): 1397–1401. [DOI] [PubMed] [Google Scholar]
- 97.Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W et al. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 2013; 339(6116): 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li X, Peng XQ, Jordan CJ, Li J, Bi GH, He Y et al. mGluR5 antagonism inhibits cocaine reinforcement and relapse by elevation of extracellular glutamate in the nucleus accumbens via a CB1 receptor mechanism. Sci Rep 2018; 8(1): 3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology (Berl) 2005; 179(1): 247–254. [DOI] [PubMed] [Google Scholar]
- 100.Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology (Berl) 2005; 179(1): 255–261. [DOI] [PubMed] [Google Scholar]
- 101.Benneyworth MA, Hearing MC, Asp AJ, Madayag A, Ingebretson AE, Schmidt CE et al. Synaptic Depotentiation and mGluR5 Activity in the Nucleus Accumbens Drive Cocaine-Primed Reinstatement of Place Preference. J Neurosci 2019; 39(24): 4785–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Burnstock G. Purinergic nerves. Pharmacological reviews 1972; 24(3): 509–581. [PubMed] [Google Scholar]
- 103.Hepp R, Perraut M, Chasserot-Golaz S, Galli T, Aunis D, Langley K et al. Cultured glial cells express the SNAP-25 analogue SNAP-23. Glia 1999; 27(2): 181–187. [DOI] [PubMed] [Google Scholar]
- 104.Wilhelm A, Volknandt W, Langer D, Nolte C, Kettenmann H, Zimmermann H. Localization of SNARE proteins and secretory organelle proteins in astrocytes in vitro and in situ. Neurosci Res 2004; 48(3): 249–257. [DOI] [PubMed] [Google Scholar]
- 105.Koizumi S, Fujishita K, Tsuda M, Shigemoto-Mogami Y, Inoue K. Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures. Proc Natl Acad Sci U S A 2003; 100(19): 11023–11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci 1999; 19(2): 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J Neurosci 2006; 26(10): 2673–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hamilton N, Vayro S, Kirchhoff F, Verkhratsky A, Robbins J, Gorecki DC et al. Mechanisms of ATP- and glutamate-mediated calcium signaling in white matter astrocytes. Glia 2008; 56(7): 734–749. [DOI] [PubMed] [Google Scholar]
- 109.Queiroz G, Meyer DK, Meyer A, Starke K, von Kugelgen I. A study of the mechanism of the release of ATP from rat cortical astroglial cells evoked by activation of glutamate receptors. Neuroscience 1999; 91(3): 1171–1181. [DOI] [PubMed] [Google Scholar]
- 110.Abdipranoto A, Liu GJ, Werry EL, Bennett MR. Mechanisms of secretion of ATP from cortical astrocytes triggered by uridine triphosphate. Neuroreport 2003; 14(17): 2177–2181. [DOI] [PubMed] [Google Scholar]
- 111.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S et al. Astrocytes control breathing through pH-dependent release of ATP. Science 2010; 329(5991): 571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Anderson CM, Bergher JP, Swanson RA. ATP-induced ATP release from astrocytes. J Neurochem 2004; 88(1): 246–256. [DOI] [PubMed] [Google Scholar]
- 113.Koizumi S. Synchronization of Ca2+ oscillations: involvement of ATP release in astrocytes. FEBS J 2010; 277(2): 286–292. [DOI] [PubMed] [Google Scholar]
- 114.Fellin T, Pozzan T, Carmignoto G. Purinergic receptors mediate two distinct glutamate release pathways in hippocampal astrocytes. The Journal of biological chemistry 2006; 281(7): 4274–4284. [DOI] [PubMed] [Google Scholar]
- 115.Zimmermann H, Braun N. Extracellular metabolism of nucleotides in the nervous system. J Auton Pharmacol 1996; 16(6): 397–400. [DOI] [PubMed] [Google Scholar]
- 116.Deng Q, Terunuma M, Fellin T, Moss SJ, Haydon PG. Astrocytic activation of A1 receptors regulates the surface expression of NMDA receptors through a Src kinase dependent pathway. Glia 2011; 59(7): 1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PR. The dopamine theory of addiction: 40 years of highs and lows. Nat Rev Neurosci 2015; 16(5): 305–312. [DOI] [PubMed] [Google Scholar]
- 118.Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry 2011; 69(12): e145–157. [DOI] [PubMed] [Google Scholar]
- 119.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 2013; 493(7433): 537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Archives of general psychiatry 2012; 69(8): 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacological reviews 2011; 63(1): 182–217. [DOI] [PubMed] [Google Scholar]
- 122.Karakaya S, Kipp M, Beyer C. Oestrogen regulates the expression and function of dopamine transporters in astrocytes of the nigrostriatal system. J Neuroendocrinol 2007; 19(9): 682–690. [DOI] [PubMed] [Google Scholar]
- 123.Zanassi P, Paolillo M, Montecucco A, Avvedimento EV, Schinelli S. Pharmacological and molecular evidence for dopamine D(1) receptor expression by striatal astrocytes in culture. Journal of neuroscience research 1999; 58(4): 544–552. [DOI] [PubMed] [Google Scholar]
- 124.Khan ZU, Koulen P, Rubinstein M, Grandy DK, Goldman-Rakic PS. An astroglia-linked dopamine D2-receptor action in prefrontal cortex. Proc Natl Acad Sci U S A 2001; 98(4): 1964–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shao W, Zhang SZ, Tang M, Zhang XH, Zhou Z, Yin YQ et al. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via alphaB-crystallin. Nature 2013; 494(7435): 90–94. [DOI] [PubMed] [Google Scholar]
- 126.Requardt RP, Hirrlinger PG, Wilhelm F, Winkler U, Besser S, Hirrlinger J. Ca(2)(+) signals of astrocytes are modulated by the NAD(+)/NADH redox state. J Neurochem 2012; 120(6): 1014–1025. [DOI] [PubMed] [Google Scholar]
- 127.Fischer T, Scheffler P, Lohr C. Dopamine-induced calcium signaling in olfactory bulb astrocytes. Sci Rep 2020; 10(1): 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kebabian JW, Petzold GL, Greengard P. Dopamine-sensitive adenylate cyclase in caudate nucleus of rat brain, and its similarity to the “dopamine receptor”. Proc Natl Acad Sci U S A 1972; 69(8): 2145–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.MILLER RJ, HORN AS, IVERSEN LL. The Action of Neuroleptic Drugs on Dopamine-Stimulated Adenosine Cyclic 3’,5’-Monophosphate Production in Rat Neostriatum and Limbic Forebrain. Molecular Pharmacology 1974; 10(5): 759–766. [Google Scholar]
- 130.Galloway A, Adeluyi A, O’Donovan B, Fisher ML, Rao CN, Critchfield P et al. Dopamine Triggers CTCF-Dependent Morphological and Genomic Remodeling of Astrocytes. J Neurosci 2018; 38(21): 4846–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jennings A, Tyurikova O, Bard L, Zheng K, Semyanov A, Henneberger C et al. Dopamine elevates and lowers astroglial Ca(2+) through distinct pathways depending on local synaptic circuitry. Glia 2017; 65(3): 447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Inazu M, Kubota N, Takeda H, Zhang J, Kiuchi Y, Oguchi K et al. Pharmacological characterization of dopamine transport in cultured rat astrocytes. Life Sci 1999; 64(24): 2239–2245. [DOI] [PubMed] [Google Scholar]
- 133.Pelton EW 2nd, Kimelberg HK, Shipherd SV, Bourke RS. Dopamine and norepinephrine uptake and metabolism by astroglial cells in culture. Life Sci 1981; 28(14): 1655–1663. [DOI] [PubMed] [Google Scholar]
- 134.Asanuma M, Miyazaki I, Murakami S, Diaz-Corrales FJ, Ogawa N. Striatal astrocytes act as a reservoir for L-DOPA. PloS one 2014; 9(9): e106362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schomig E, Russ H, Staudt K, Martel F, Gliese M, Grundemann D. The extraneuronal monoamine transporter exists in human central nervous system glia. Adv Pharmacol 1998; 42: 356–359. [DOI] [PubMed] [Google Scholar]
- 136.Vaarmann A, Gandhi S, Abramov AY. Dopamine induces Ca2+ signaling in astrocytes through reactive oxygen species generated by monoamine oxidase. J Biol Chem 2010; 285(32): 25018–25023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 2016; 89(1): 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Petrelli F, Dallerac G, Pucci L, Cali C, Zehnder T, Sultan S et al. Dysfunction of homeostatic control of dopamine by astrocytes in the developing prefrontal cortex leads to cognitive impairments. Mol Psychiatry 2020; 25(4): 732–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. The Journal of physiology 1973; 232(2): 331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hebb DO. The Organization of Behavior: A Neuropsychological Theory. Wiley: NY, 1949. [Google Scholar]
- 141.Lichtman JW, Purves D. Activity-mediated neural change. Nature 1983; 301(5901): 563. [DOI] [PubMed] [Google Scholar]
- 142.Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 1986; 319(6056): 774–776. [DOI] [PubMed] [Google Scholar]
- 143.Cajal SRy. Estructura de los centros nerviosos de las aves. Rev Trim Histol Norm Patol 1888; (1): 1–10. [Google Scholar]
- 144.Lushnikova I, Skibo G, Muller D, Nikonenko I. Synaptic potentiation induces increased glial coverage of excitatory synapses in CA1 hippocampus. Hippocampus 2009; 19(8): 753–762. [DOI] [PubMed] [Google Scholar]
- 145.Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science 2007; 317(5841): 1083–1086. [DOI] [PubMed] [Google Scholar]
- 146.Henneberger C, Bard L, Panatier A, Reynolds JP, Medvedev NI, Minge D et al. Astroglia withdraw from potentiated synapses boosting inter-synaptic cross-talk. bioRxiv 2018. [Google Scholar]
- 147.Masuoka T, Ikeda R, Konishi S. Persistent activation of histamine H1 receptors in the hippocampal CA1 region enhances NMDA receptor-mediated synaptic excitation and long-term potentiation in astrocyte- and D-serine-dependent manner. Neuropharmacology 2019; 151: 64–73. [DOI] [PubMed] [Google Scholar]
- 148.Min R, Nevian T. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat Neurosci 2012; 15(5): 746–753. [DOI] [PubMed] [Google Scholar]
- 149.Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C et al. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell 1995; 81(3): 435–443. [DOI] [PubMed] [Google Scholar]
- 150.Song JY, Ichtchenko K, Sudhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A 1999; 96(3): 1100–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ullian EM, Christopherson KS, Barres BA. Role for glia in synaptogenesis. Glia 2004; 47(3): 209–216. [DOI] [PubMed] [Google Scholar]
- 152.Miller FD, Gauthier AS. Timing is everything: making neurons versus glia in the developing cortex. Neuron 2007; 54(3): 357–369. [DOI] [PubMed] [Google Scholar]
- 153.Gotz M, Barde YA. Radial glial cells defined and major intermediates between embryonic stem cells and CNS neurons. Neuron 2005; 46(3): 369–372. [DOI] [PubMed] [Google Scholar]
- 154.Bayer SA, Altman J. Neocortical development. 1991.
- 155.Allen NJ, Eroglu C. Cell Biology of Astrocyte-Synapse Interactions. Neuron 2017; 96(3): 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science 2001; 291(5504): 657–661. [DOI] [PubMed] [Google Scholar]
- 157.Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science 1997; 277(5332): 1684–1687. [DOI] [PubMed] [Google Scholar]
- 158.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 2005; 120(3): 421–433. [DOI] [PubMed] [Google Scholar]
- 159.Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci 2005; 25(12): 3219–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM et al. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci U S A 2011; 108(32): E440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ et al. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 2012; 486(7403): 410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nagler K, Mauch DH, Pfrieger FW. Glia-derived signals induce synapse formation in neurones of the rat central nervous system. The Journal of physiology 2001; 533(Pt 3): 665–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 2009; 139(2): 380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Adams JC. Thrombospondins: multifunctional regulators of cell interactions. Annu Rev Cell Dev Biol 2001; 17: 25–51. [DOI] [PubMed] [Google Scholar]
- 165.Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol 2004; 36(6): 1115–1125. [DOI] [PubMed] [Google Scholar]
- 166.Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature 1995; 375(6530): 400–404. [DOI] [PubMed] [Google Scholar]
- 167.Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron 1995; 15(2): 427–434. [DOI] [PubMed] [Google Scholar]
- 168.Ashby MC, Isaac JT. Maturation of a recurrent excitatory neocortical circuit by experience-dependent unsilencing of newly formed dendritic spines. Neuron 2011; 70(3): 510–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Isaac JT, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical inputs. Neuron 1997; 18(2): 269–280. [DOI] [PubMed] [Google Scholar]
- 170.Dolphin AC. Calcium channel auxiliary alpha2delta and beta subunits: trafficking and one step beyond. Nat Rev Neurosci 2012; 13(8): 542–555. [DOI] [PubMed] [Google Scholar]
- 171.Dolphin AC. Voltage-gated calcium channels and their auxiliary subunits: physiology and pathophysiology and pharmacology. The Journal of physiology 2016; 594(19): 5369–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dolphin AC, Lee A. Presynaptic calcium channels: specialized control of synaptic neurotransmitter release. Nat Rev Neurosci 2020; 21(4): 213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Brown JP, Gee NS. Cloning and deletion mutagenesis of the alpha2 delta calcium channel subunit from porcine cerebral cortex. Expression of a soluble form of the protein that retains [3H]gabapentin binding activity. The Journal of biological chemistry 1998; 273(39): 25458–25465. [DOI] [PubMed] [Google Scholar]
- 174.Dooley DJ, Taylor CP, Donevan S, Feltner D. Ca2+ channel alpha2delta ligands: novel modulators of neurotransmission. Trends Pharmacol Sci 2007; 28(2): 75–82. [DOI] [PubMed] [Google Scholar]
- 175.van Hooft JA, Dougherty JJ, Endeman D, Nichols RA, Wadman WJ. Gabapentin inhibits presynaptic Ca(2+) influx and synaptic transmission in rat hippocampus and neocortex. European journal of pharmacology 2002; 449(3): 221–228. [DOI] [PubMed] [Google Scholar]
- 176.Risher WC, Eroglu C. Thrombospondins as key regulators of synaptogenesis in the central nervous system. Matrix Biol 2012; 31(3): 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Risher WC, Kim N, Koh S, Choi JE, Mitev P, Spence EF et al. Thrombospondin receptor alpha2delta-1 promotes synaptogenesis and spinogenesis via postsynaptic Rac1. The Journal of cell biology 2018; 217(10): 3747–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dietz DM, Sun H, Lobo MK, Cahill ME, Chadwick B, Gao V et al. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nat Neurosci 2012; 15(6): 891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wright WJ, Graziane NM, Neumann PA, Hamilton PJ, Cates HM, Fuerst L et al. Silent synapses dictate cocaine memory destabilization and reconsolidation. Nat Neurosci 2020; 23(1): 32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xu J, Xiao N, Xia J. Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nat Neurosci 2010; 13(1): 22–24. [DOI] [PubMed] [Google Scholar]
- 181.Singh SK, Stogsdill JA, Pulimood NS, Dingsdale H, Kim YH, Pilaz LJ et al. Astrocytes Assemble Thalamocortical Synapses by Bridging NRX1alpha and NL1 via Hevin. Cell 2016; 164(1–2): 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Takano T, Wallace JT, Baldwin KT, Purkey AM, Uezu A, Courtland JL et al. Chemico-genetic discovery of astrocytic control of inhibition in vivo. Nature 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bosworth AP, Allen NJ. The diverse actions of astrocytes during synaptic development. Curr Opin Neurobiol 2017; 47: 38–43. [DOI] [PubMed] [Google Scholar]
- 184.Jung YJ, Chung WS. Phagocytic Roles of Glial Cells in Healthy and Diseased Brains. Biomol Ther (Seoul) 2018; 26(4): 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 2013; 504(7480): 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tung TT, Nagaosa K, Fujita Y, Kita A, Mori H, Okada R et al. Phosphatidylserine recognition and induction of apoptotic cell clearance by Drosophila engulfment receptor Draper. J Biochem 2013; 153(5): 483–491. [DOI] [PubMed] [Google Scholar]
- 187.Lee JH, Kim JY, Noh S, Lee H, Lee SY, Mun JY et al. Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature 2020. [DOI] [PubMed] [Google Scholar]
- 188.Bialas AR, Stevens B. TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci 2013; 16(12): 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 189.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012; 74(4): 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chung WS, Allen NJ, Eroglu C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb Perspect Biol 2015; 7(9): a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol 1998; 80(1): 1–27. [DOI] [PubMed] [Google Scholar]
- 192.Fiorino DF, Coury A, Fibiger HC, Phillips AG. Electrical stimulation of reward sites in the ventral tegmental area increases dopamine transmission in the nucleus accumbens of the rat. Behav Brain Res 1993; 55(2): 131–141. [DOI] [PubMed] [Google Scholar]
- 193.Solinas M, Ferre S, You ZB, Karcz-Kubicha M, Popoli P, Goldberg SR. Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens. J Neurosci 2002; 22(15): 6321–6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A 1995; 92(26): 12304–12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychological review 1987; 94(4): 469–492. [PubMed] [Google Scholar]
- 196.Weiss F, Markou A, Lorang MT, Koob GF. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res 1992; 593(2): 314–318. [DOI] [PubMed] [Google Scholar]
- 197.Gerrits MA, Petromilli P, Westenberg HG, Di Chiara G, van Ree JM. Decrease in basal dopamine levels in the nucleus accumbens shell during daily drug-seeking behaviour in rats. Brain Res 2002; 924(2): 141–150. [DOI] [PubMed] [Google Scholar]
- 198.Parsons LH, Smith AD, Justice JB Jr. Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse 1991; 9(1): 60–65. [DOI] [PubMed] [Google Scholar]
- 199.Robertson MW, Leslie CA, Bennett JP Jr. Apparent synaptic dopamine deficiency induced by withdrawal from chronic cocaine treatment. Brain Res 1991; 538(2): 337–339. [DOI] [PubMed] [Google Scholar]
- 200.Fontana DJ, Post RM, Pert A. Conditioned increases in mesolimbic dopamine overflow by stimuli associated with cocaine. Brain Res 1993; 629(1): 31–39. [DOI] [PubMed] [Google Scholar]
- 201.Di Ciano P, Blaha CD, Phillips AG. Conditioned changes in dopamine oxidation currents in the nucleus accumbens of rats by stimuli paired with self-administration or yoked-administration of d-amphetamine. Eur J Neurosci 1998; 10(3): 1121–1127. [DOI] [PubMed] [Google Scholar]
- 202.Duvauchelle CL, Ikegami A, Asami S, Robens J, Kressin K, Castaneda E. Effects of cocaine context on NAcc dopamine and behavioral activity after repeated intravenous cocaine administration. Brain Res 2000; 862(1–2): 49–58. [DOI] [PubMed] [Google Scholar]
- 203.Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A 2000; 97(8): 4321–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci 2000; 20(19): 7489–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Neisewander JL, O’Dell LE, Tran-Nguyen LT, Castaneda E, Fuchs RA. Dopamine overflow in the nucleus accumbens during extinction and reinstatement of cocaine self-administration behavior. Neuropsychopharmacology 1996; 15(5): 506–514. [DOI] [PubMed] [Google Scholar]
- 206.Corkrum M, Covelo A, Lines J, Bellocchio L, Pisansky M, Loke K et al. Dopamine-Evoked Synaptic Regulation in the Nucleus Accumbens Requires Astrocyte Activity. Neuron 2020; 105(6): 1036–1047 e1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pan HT, Menacherry S, Justice JB Jr. Differences in the pharmacokinetics of cocaine in naive and cocaine-experienced rats. J Neurochem 1991; 56(4): 1299–1306. [DOI] [PubMed] [Google Scholar]
- 208.Pettit HO, Pan HT, Parsons LH, Justice JB Jr. Extracellular concentrations of cocaine and dopamine are enhanced during chronic cocaine administration. J Neurochem 1990; 55(3): 798–804. [DOI] [PubMed] [Google Scholar]
- 209.Wang J, Li KL, Shukla A, Beroun A, Ishikawa M, Huang X et al. Cocaine Triggers Astrocyte-Mediated Synaptogenesis. Biol Psychiatry 2021; 89(4): 386–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Xin W, Schuebel KE, Jair KW, Cimbro R, De Biase LM, Goldman D et al. Ventral midbrain astrocytes display unique physiological features and sensitivity to dopamine D2 receptor signaling. Neuropsychopharmacology 2019; 44(2): 344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci U S A 2009; 106(33): 13939–13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jiang R, Diaz-Castro B, Looger LL, Khakh BS. Dysfunctional Calcium and Glutamate Signaling in Striatal Astrocytes from Huntington’s Disease Model Mice. J Neurosci 2016; 36(12): 3453–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tong X, Ao Y, Faas GC, Nwaobi SE, Xu J, Haustein MD et al. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat Neurosci 2014; 17(5): 694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Adermark L, Lovinger DM. Electrophysiological properties and gap junction coupling of striatal astrocytes. Neurochemistry international 2008; 52(7): 1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kemp JM, Powell TP. The connexions of the striatum and globus pallidus: synthesis and speculation. Philosophical transactions of the Royal Society of London Series B, Biological sciences 1971; 262(845): 441–457. [DOI] [PubMed] [Google Scholar]
- 216.Salgado S, Kaplitt MG. The Nucleus Accumbens: A Comprehensive Review. Stereotact Funct Neurosurg 2015; 93(2): 75–93. [DOI] [PubMed] [Google Scholar]
- 217.Martin R, Bajo-Graneras R, Moratalla R, Perea G, Araque A. Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 2015; 349(6249): 730–734. [DOI] [PubMed] [Google Scholar]
- 218.Graziane NM, Sun S, Wright WJ, Jang D, Liu Z, Huang YH et al. Opposing mechanisms mediate morphine- and cocaine-induced generation of silent synapses. Nat Neurosci 2016; 19(7): 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci 2010; 30(23): 7984–7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR. Neuroadaptations in the cellular and postsynaptic group 1 metabotropic glutamate receptor mGluR5 and Homer proteins following extinction of cocaine self-administration. Neurosci Lett 2009; 452(2): 167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ghasemzadeh MB, Vasudevan P, Mueller CR, Seubert C, Mantsch JR. Region-specific alterations in glutamate receptor expression and subcellular distribution following extinction of cocaine self-administration. Brain Res 2009; 1267: 89–102. [DOI] [PubMed] [Google Scholar]
- 222.Ebner SR, Larson EB, Hearing MC, Ingebretson AE, Thomas MJ. Extinction and Reinstatement of Cocaine-seeking in Self-administering Mice is Associated with Bidirectional AMPAR-mediated Plasticity in the Nucleus Accumbens Shell. Neuroscience 2018; 384: 340–349. [DOI] [PubMed] [Google Scholar]
- 223.Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D et al. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature 2003; 421(6918): 70–75. [DOI] [PubMed] [Google Scholar]
- 224.Scofield MD, Li H, Siemsen BM, Healey KL, Tran PK, Woronoff N et al. Cocaine Self-Administration and Extinction Leads to Reduced Glial Fibrillary Acidic Protein Expression and Morphometric Features of Astrocytes in the Nucleus Accumbens Core. Biol Psychiatry 2016; 80(3): 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Testen A, Sepulveda-Orengo MT, Gaines CH, Reissner KJ. Region-Specific Reductions in Morphometric Properties and Synaptic Colocalization of Astrocytes Following Cocaine Self-Administration and Extinction. Front Cell Neurosci 2018; 12: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Siemsen BM, Reichel CM, Leong KC, Garcia-Keller C, Gipson CD, Spencer S et al. Effects of Methamphetamine Self-Administration and Extinction on Astrocyte Structure and Function in the Nucleus Accumbens Core. Neuroscience 2019; 406: 528–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kruyer A, Scofield MD, Wood D, Reissner KJ, Kalivas PW. Heroin Cue-Evoked Astrocytic Structural Plasticity at Nucleus Accumbens Synapses Inhibits Heroin Seeking. Biol Psychiatry 2019; 86(11): 811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Scofield MD, Boger HA, Smith RJ, Li H, Haydon PG, Kalivas PW. Gq-DREADD Selectively Initiates Glial Glutamate Release and Inhibits Cue-induced Cocaine Seeking. Biol Psychiatry 2015; 78(7): 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev 2009; 89(4): 1379–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Nam MH, Han KS, Lee J, Bae JY, An H, Park S et al. Expression of micro-Opioid Receptor in CA1 Hippocampal Astrocytes. Exp Neurobiol 2018; 27(2): 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Corkrum M, Rothwell PE, Thomas MJ, Kofuji P, Araque A. Opioid-Mediated Astrocyte-Neuron Signaling in the Nucleus Accumbens. Cells 2019; 8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Narita M, Miyatake M, Narita M, Shibasaki M, Shindo K, Nakamura A et al. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacology 2006; 31(11): 2476–2488. [DOI] [PubMed] [Google Scholar]
- 233.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 2003; 23(8): 3531–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron 2005; 45(5): 647–650. [DOI] [PubMed] [Google Scholar]
- 235.Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron 2014; 83(6): 1453–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 2009; 10(8): 561–572. [DOI] [PubMed] [Google Scholar]
- 237.Danbolt NC. Glutamate uptake. Prog Neurobiol 2001; 65(1): 1–105. [DOI] [PubMed] [Google Scholar]
- 238.Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron 1997; 19(6): 1297–1308. [DOI] [PubMed] [Google Scholar]
- 239.Bergles DE, Jahr CE. Glial contribution to glutamate uptake at Schaffer collateral-commissural synapses in the hippocampus. J Neurosci 1998; 18(19): 7709–7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 1996; 16(3): 675–686. [DOI] [PubMed] [Google Scholar]
- 241.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 1997; 276(5319): 1699–1702. [DOI] [PubMed] [Google Scholar]
- 242.Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci 1998; 18(21): 8751–8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, Kalivas PW. Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addiction biology 2015; 20(2): 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Roberts-Wolfe DJ, Kalivas PW. Glutamate Transporter GLT-1 as a Therapeutic Target for Substance Use Disorders. CNS Neurol Disord Drug Targets 2015; 14(6): 745–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Piet R, Vargova L, Sykova E, Poulain DA, Oliet SH. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc Natl Acad Sci U S A 2004; 101(7): 2151–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Mitchell SJ, Silver RA. Glutamate spillover suppresses inhibition by activating presynaptic mGluRs. Nature 2000; 404(6777): 498–502. [DOI] [PubMed] [Google Scholar]
- 247.Vogt KE, Nicoll RA. Glutamate and gamma-aminobutyric acid mediate a heterosynaptic depression at mossy fiber synapses in the hippocampus. Proc Natl Acad Sci U S A 1999; 96(3): 1118–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Min MY, Melyan Z, Kullmann DM. Synaptically released glutamate reduces gamma-aminobutyric acid (GABA)ergic inhibition in the hippocampus via kainate receptors. Proc Natl Acad Sci U S A 1999; 96(17): 9932–9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci 2008; 28(12): 3170–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci 2007; 27(39): 10621–10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci 2011; 31(15): 5737–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Smith ACW, Scofield MD, Heinsbroek JA, Gipson CD, Neuhofer D, Roberts-Wolfe DJ et al. Accumbens nNOS Interneurons Regulate Cocaine Relapse. J Neurosci 2017; 37(4): 742–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Henneberger C, Bard L, Panatier A, Reynolds JP, Kopach O, Medvedev NI et al. LTP induction boosts glutamate spillover by driving withdrawal of astroglia. bioRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Chalifoux JR, Carter AG. Glutamate spillover promotes the generation of NMDA spikes. J Neurosci 2011; 31(45): 16435–16446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry 2010; 67(1): 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Reissner KJ, Brown RM, Spencer S, Tran PK, Thomas CA, Kalivas PW. Chronic administration of the methylxanthine propentofylline impairs reinstatement to cocaine by a GLT-1-dependent mechanism. Neuropsychopharmacology 2014; 39(2): 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci 2002; 22(20): 9134–9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Bridges RJ, Natale NR, Patel SA. System xc(−) cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol 2012; 165(1): 20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci 2007; 27(51): 13968–13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Miguens M, Del Olmo N, Higuera-Matas A, Torres I, Garcia-Lecumberri C, Ambrosio E. Glutamate and aspartate levels in the nucleus accumbens during cocaine self-administration and extinction: a time course microdialysis study. Psychopharmacology (Berl) 2008; 196(2): 303–313. [DOI] [PubMed] [Google Scholar]
- 261.Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry 2009; 65(10): 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kupferschmidt DA, Lovinger DM. Inhibition of presynaptic calcium transients in cortical inputs to the dorsolateral striatum by metabotropic GABA(B) and mGlu2/3 receptors. The Journal of physiology 2015; 593(10): 2295–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci 2005; 25(27): 6389–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci 2009; 12(2): 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ducret E, Puaud M, Lacoste J, Belin-Rauscent A, Fouyssac M, Dugast E et al. N-acetylcysteine Facilitates Self-Imposed Abstinence After Escalation of Cocaine Intake. Biol Psychiatry 2016; 80(3): 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.LaRowe SD, Kalivas PW, Nicholas JS, Randall PK, Mardikian PN, Malcolm RJ. A double-blind placebo-controlled trial of N-acetylcysteine in the treatment of cocaine dependence. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions 2013; 22(5): 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Back SE, McCauley JL, Korte KJ, Gros DF, Leavitt V, Gray KM et al. A Double-Blind, Randomized, Controlled Pilot Trial of N-Acetylcysteine in Veterans With Posttraumatic Stress Disorder and Substance Use Disorders. The Journal of clinical psychiatry 2016; 77(11): e1439–e1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Back SE, Gray K, Santa Ana E, Jones JL, Jarnecke AM, Joseph JE et al. N-acetylcysteine for the treatment of comorbid alcohol use disorder and posttraumatic stress disorder: Design and methodology of a randomized clinical trial. Contemp Clin Trials 2020; 91: 105961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther 2002; 300(1): 162–171. [DOI] [PubMed] [Google Scholar]
- 270.Hu G, Duffy P, Swanson C, Ghasemzadeh MB, Kalivas PW. The regulation of dopamine transmission by metabotropic glutamate receptors. J Pharmacol Exp Ther 1999; 289(1): 412–416. [PubMed] [Google Scholar]
- 271.Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D et al. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci 2011; 14(10): 1276–1284. [DOI] [PubMed] [Google Scholar]
- 272.Rusakov DA, Bard L, Stewart MG, Henneberger C. Diversity of astroglial functions alludes to subcellular specialisation. Trends Neurosci 2014; 37(4): 228–242. [DOI] [PubMed] [Google Scholar]
- 273.Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC et al. The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacological reviews 2016; 68(3): 816–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Smaga I, Gawlinska K, Frankowska M, Wydra K, Sadakierska-Chudy A, Suder A et al. Extinction Training after Cocaine Self-Administration Influences the Epigenetic and Genetic Machinery Responsible for Glutamatergic Transporter Gene Expression in Male Rat Brain. Neuroscience 2020; 451: 99–110. [DOI] [PubMed] [Google Scholar]
- 275.Dong Y, Nestler EJ. The neural rejuvenation hypothesis of cocaine addiction. Trends Pharmacol Sci 2014; 35(8): 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Farhy-Tselnicker I, Allen NJ. Astrocytes, neurons, synapses: a tripartite view on cortical circuit development. Neural Dev 2018; 13(1): 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G et al. In vivo cocaine experience generates silent synapses. Neuron 2009; 63(1): 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN et al. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci 2011; 31(22): 8163–8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Iruela-Arispe ML, Liska DJ, Sage EH, Bornstein P. Differential expression of thrombospondin 1, 2, and 3 during murine development. Developmental dynamics : an official publication of the American Association of Anatomists 1993; 197(1): 40–56. [DOI] [PubMed] [Google Scholar]
- 280.Spencer S, Brown RM, Quintero GC, Kupchik YM, Thomas CA, Reissner KJ et al. alpha2delta-1 signaling in nucleus accumbens is necessary for cocaine-induced relapse. J Neurosci 2014; 34(25): 8605–8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Nagai J, Rajbhandari AK, Gangwani MR, Hachisuka A, Coppola G, Masmanidis SC et al. Hyperactivity with Disrupted Attention by Activation of an Astrocyte Synaptogenic Cue. Cell 2019; 177(5): 1280–1292 e1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci 2013; 16(11): 1644–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Lee BR, Dong Y. Cocaine-induced metaplasticity in the nucleus accumbens: silent synapse and beyond. Neuropharmacology 2011; 61(7): 1060–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Neumann PA, Wang Y, Yan Y, Wang Y, Ishikawa M, Cui R et al. Cocaine-Induced Synaptic Alterations in Thalamus to Nucleus Accumbens Projection. Neuropsychopharmacology 2016; 41(9): 2399–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wright WJ, Dong Y. Psychostimulant-Induced Adaptations in Nucleus Accumbens Glutamatergic Transmission. Cold Spring Harb Perspect Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci 1999; 11(5): 1598–1604. [DOI] [PubMed] [Google Scholar]
- 287.Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse 2001; 39(3): 257–266. [DOI] [PubMed] [Google Scholar]


