Abstract
Chronic pain affects millions of people in the United States and pharmacological treatments have been ineffective. Dorsal root ganglion (DRG) stimulation is a neuromodulation method that delivers electrical stimulation to the DRG to relieve pain. DRG electrodes are rigid and cylindrical. The implantation of DRG electrodes requires a technically-challenging surgery that involves steering electrodes laterally towards the DRG. The Injectrode is an injectable conductive polymer that cures in place and is capable of delivering electrical current to stimulate neural tissue. We used the Injectrode to stimulate the L6 and L7 DRG in cats, measuring neural responses evoked in the sciatic, tibial, and common peroneal nerves to measure the thresholds for activating fibers. A cylindrical stainless-steel electrode was used for comparison. Thresholds were 38% higher with the Injectrode versus stainless-steel, likely owing to its larger contact surface area with the DRG. Both Aα and Aβ sensory fibers were activated using DRG stimulation. The Injectrode has the potential to offer a new and simple method for DRG stimulation that can potentially offer more complete coverage of the DRG.
I. Introduction
Chronic pain affects 25–100 million people in the United States [1]. Pharmacological treatment of chronic pain is ineffective and has greatly contributed to the opioid epidemic [2]. An alternative, non-pharmacological approach to pain treatment is the use of electrical stimulation, or neuromodulation, of the spinal cord dorsal columns, dorsal roots, or the dorsal root ganglion (DRG) [3].
The field of neuromodulation is largely dominated by epidural spinal cord stimulation (eSCS), in which electrodes are implanted on the dorsal surface of the spinal cord. eSCS reduces pain primarily by activating large afferent fibers (such as Aα and Aβ) in the dorsal columns, presumably inhibiting the transmission of pain signals from the periphery to the spinal cord (Gate Theory of Pain) [4].
Recently, DRG stimulation has emerged as an alternative to eSCS for treating chronic pain [5]. Electrodes are inserted into the epidural space and steered laterally, targeting the dorsal surface of the DRG. The DRG are a preferred target because they allow for more precise dermatome coverage of the pain region; DRG stimulation is especially effective for treating pelvic pain, groin pain, back pain, distal limb pain, complex regional pain syndrome, and mononeuropathies [6]. In a recent clinical study, DRG stimulation reduced chronic pain by more than 50% in 81.2% of the participants, compared to 55.7% for eSCS [7]. However, the implant procedure for DRG stimulation electrode leads has a higher rate of difficulty due to challenges associated with steering the leads into the foramen [5]. A more flexible electrode and less-invasive implant procedure may contribute to the increase in effective treatment of chronic pain using DRG stimulation.
We developed an injectable electrode, the Injectrode, which is a polymer that is injected onto the stimulation site using a syringe and cures in place [8]. The Injectrode is flexible compared to traditional metal electrodes, with a Young’s modulus more closely matching that of tissue. Stimulation using the Injectrode has been demonstrated in both the brachial plexus of rats as well as the vagus nerve in pigs, demonstrating the potential for wide use in neuromodulation. To establish clinical viability of the Injectrode, we aim to demonstrate that the Injectrode (i) is effective and comparable to traditional electrodes at exciting neural tissue, (ii) requires a less invasive implant procedure than the current standard, (iii) is tolerated by the body, and (iv) remains effective long-term. The goal of the current work is to address the first aim. In this study, we, for the first time, demonstrate that the Injectrode can recruit Aα and Aβ sensory fibers by stimulating the DRG.
II. Methods
Two adult male cats (5.46, 7.55 kg) were used in acute, non-recovery experiments. All procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Anaesthesia was induced using ketamine (cat 1; intramuscular (IM), 10 mg/kg) and acepromazine (cat 1; IM, 0.1 mg/kg), or dexdomitor (cat 2; IM, 0.04 mg/kg). Atropine was administered (IM; 0.05 mg/kg) to reduce saliva output. The cat was then intubated and isoflurane (2–2.5%) was given for the duration of the experiment.
A. Instrumentation and Implantation
The L6 and L7 DRG were exposed on one side via a partial laminectomy (Fig. 1a). DRG stimulation was achieved using either a stainless-steel electrode that is similar to those used clinically or using an Injectrode. The stainless-steel electrode was manufactured in-house at University of Pittsburgh using wire (AS632; Cooner Wire Company, Chatsworth, CA, USA) and a stainless-steel tube crimped onto the wire (316; 0.5 mm outer diameter McMaster-Carr, Elmhurst, IL, USA). The Injectrode (Neuronoff, Inc., Cleveland, OH, USA) consisted of two parts of Pt-curing silicone elastomers (World Precision Instruments, FL, USA) with metallic silver particles (Sigma-Aldrich, MO, USA). The two parts were mixed and loaded into a syringe calibrated to 10 μL. An insulated silver wire (AS766-36; Cooner Wire Company, Chatsworth, CA, USA) was de-insulated at the ends; one end was placed inside the syringe and was embedded in the Injectrode material.
Figure 1.
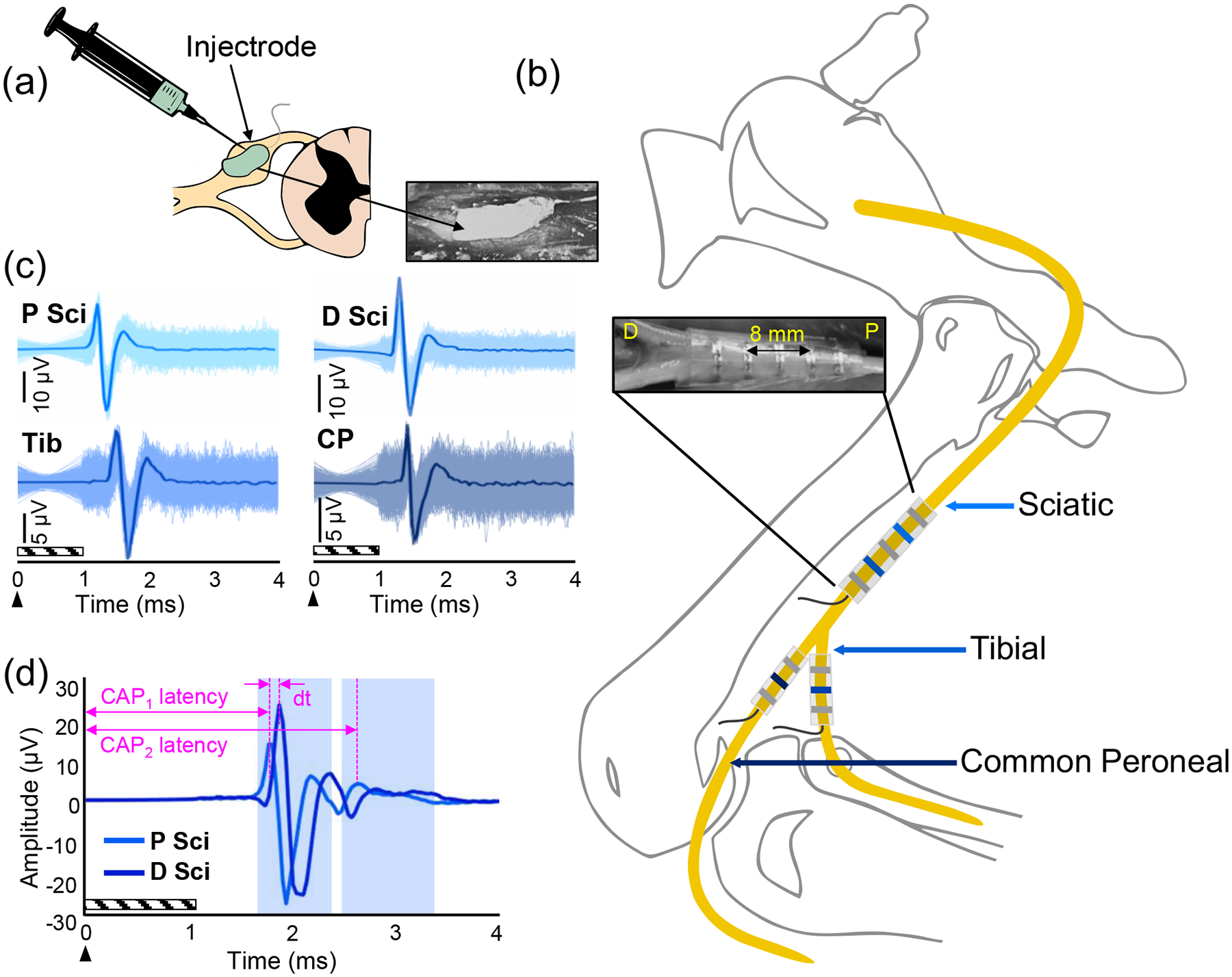
Experimental setup. (a) The Injectrode is injected onto an exposed lumbar DRG. (b) Nerve cuffs were implanted around the sciatic (Sci), tibial (Tib), and common peroneal (CP) nerves. Recordings were made in tripolar configurations. The sciatic nerve cuff had 5 contacts (inset), allowing 2 tripolar configurations proximally (P) and distally (D) along the nerve, while the tibial and common peroneal nerve cuffs had 3 contacts for single tripolar recordings. The distance between the sciatic nerve cuff contacts 2 and 4 is 8 mm. (c) Electroneurograms recorded from each of the tripolar nerve cuff recordings during stimulation at 900 μA and a pulse width of 150 μs. Six hundred pulses were repeated (shaded region) and averaged (bolded line) to reveal a CAP. (d) Method for calculating the conduction velocity. dt = the time difference between the first CAP on the proximal sciatic (P Sci) and distal sciatic (D Sci) contacts. CAP1 and CAP2 latencies are the latencies of the first and second CAPs on the proximal sciatic contacts, respectively. The arrow indicates the onset of the stimulus and the diagonal box indicates the blanked region.
Stimulation was delivered using a high current ECOG + Stim front end (Ripple, Salt Lake City, UT, USA). Stimuli consisted of pulses that were biphasic, symmetric, and cathodic-first, delivered at 58 Hz with a pulse width of either 80, 150, or 300 μs. Motor threshold was found by varying stimulation amplitude and observing twitches in the hindlimb. The stimulation amplitude was then randomized from below the sensory threshold to motor threshold in steps of 25–250 μA to construct a recruitment curve. At each amplitude, 600 pulses were delivered with 10 seconds between each amplitude.
DRG stimulation was used to activate primary afferent fibers, whose responses were recorded in the sciatic, tibial, and common peroneal nerves using spiral nerve cuffs (Ardiem Medical, Indiana, PA, USA). The cuff on the sciatic nerve had 5 platinum contacts spaced 3 mm apart. The recording configuration consisted of two tripolar recordings with contacts 2 and 4 as the active electrodes and contacts 1, 3, and 5 shorted together for reference. The cuffs on the tibial and common peroneal nerves had 3 contacts to create a single tripolar recording (contacts 1 and 3 shorted together for reference; Fig. 1b).
B. Electroneurogram Analysis
Electroneurograms (ENGs) were recorded (fS = 30 kHz) using the nerve cuffs and were high pass filtered (fC = 300 Hz) following a 1 ms blanking period to suppress the stimulation artefact. Evoked responses were averaged to reveal compound action potentials (CAPs) (Fig. 1c). To determine when a CAP was present at a particular stimulation amplitude, the root-mean-square (RMS) amplitude of the ENG had to be above a threshold [9], [10]. The CAP threshold was defined as one standard deviation above the upper 99% confidence interval of the RMS amplitude of a pre-stimulation region occurring before each pulse (1 ms). This process was repeated 200 times for a random subsample of 80% of the 600 repetitions. A CAP was considered to be present if 95% of the subsampled sets were supra-threshold for that time window. The stimulation threshold for evoking a CAP was determined by taking the lowest amplitude from all four nerve cuff contacts where a CAP was present. For the largest CAP, the peak-to-peak amplitude was extracted. Recruitment curves were constructed for each pulse width and depict the amplitude of the CAP as a function of stimulation charge.
The conduction velocity of the CAPs in the sciatic nerve was determined by measuring the difference in the latencies of the CAPs from contacts 2 and 4 on the sciatic nerve cuff and dividing the distance between the contacts (8 mm) by the difference in latencies [11] (Fig. 1d). The conduction velocity of later CAPs was calculated using the distance between the stimulating and recording electrodes and the latency of the CAP from the onset of the stimulus.
III. Results & Discussion
DRG stimulation was performed on the L6 and L7 DRG in two cats using either a cylindrical stainless-steel electrode or a novel, injectable electrode, the Injectrode. Antidromic activation of sensory fibers was recorded using nerve cuffs on the sciatic, tibial, and common peroneal nerves. Stimulation threshold to evoke a CAP and the conduction velocity of the CAP were compared between the stainless-steel electrode and the Injectrode. An increase in stimulation amplitude resulted in an increase in the amplitude of the CAPs (Fig. 2a). The threshold for evoking a CAP using a stainless-steel electrode was 70.4 ± 22.6 nC (n = 15) and 97.3 ± 37.6 nC (n = 16) for the Injectrode (Fig. 2b,c); the threshold for evoking the a CAP using an Injectrode was 38% higher than with a stainless-steel electrode. This was an expected result because the Injectrode is flexible and uncured upon insertion, conforming partially to the surface of the DRG. The Injectrode was also wider than the stainless-steel electrode (approximately 1.5 mm), providing a surface area for charge delivery that was approximately 3.5X greater. Therefore, the Injectrode has a larger contact surface area on the DRG, which causes a greater distribution of charge and requires more charge to recruit the same number of sensory fibers as the stainless-steel electrode. Moreover, the recruitment thresholds increased with wider pulse widths (Fig. 2d–g). This is consistent with known charge-duration curves for stimulating neural tissue [12]. Additional experiments will be conducted to statistically compare the recruitment thresholds between the Injectrode and stainless-steel electrode.
Figure 2.
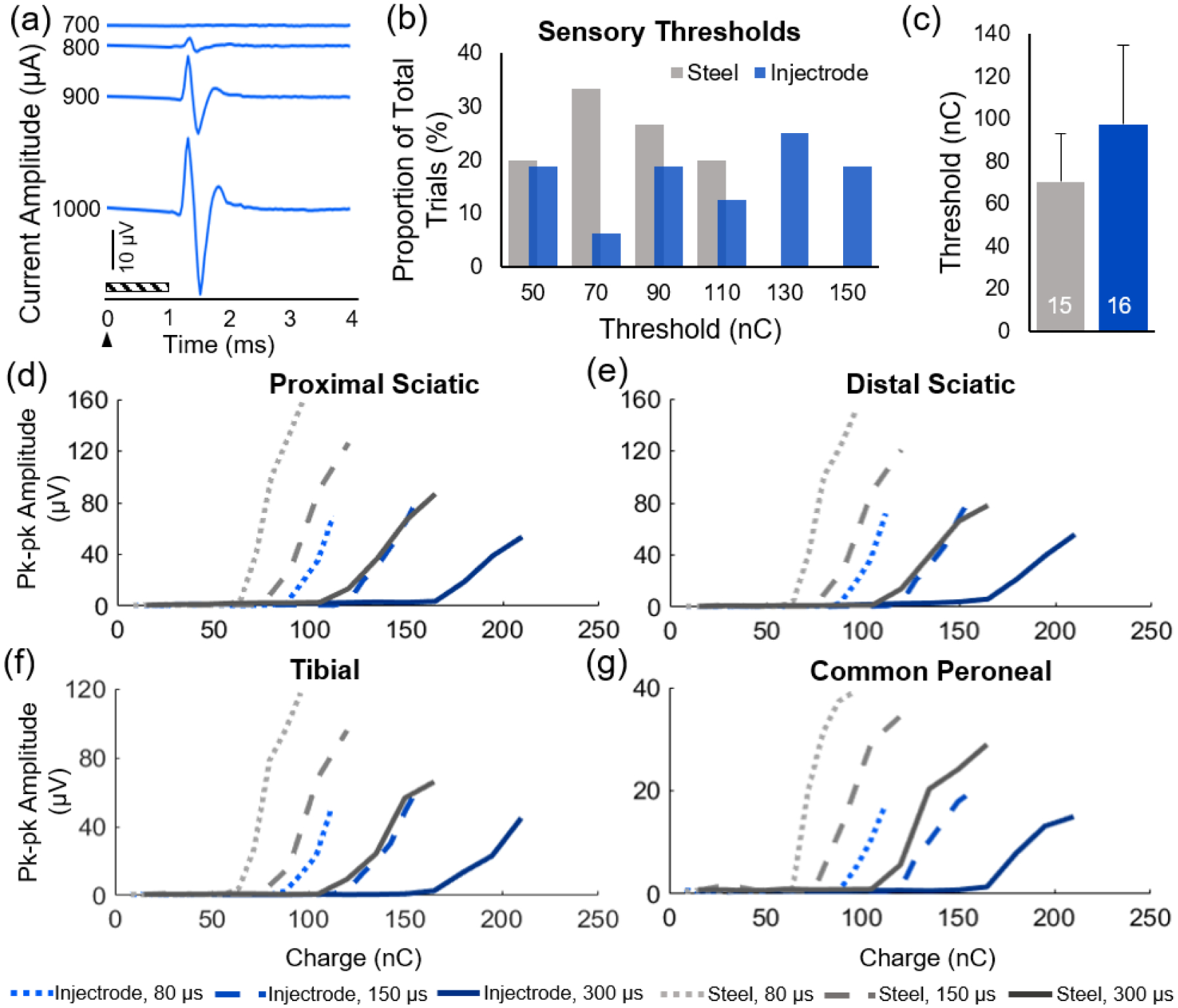
Comparing recruitment properties of stainless-steel electrodes with the Injectrode. (a) An increase in stimulation amplitude results in an increase in the CAP amplitude. Representative example: Injectrode, pulse width = 150 μs. The arrow indicates the onset of the stimulus and the diagonal box indicates the blanking region. (b) Histograms of the sensory thresholds, the minimum charge required to evoke the first CAP, for the Injectrode and stainless-steel electrode. (c) Mean (± standard deviation) threshold for stainless-steel and Injectrode. The number inside the bar is the number of samples. Recruitment curves constructed using the peak-to-peak amplitude of the first CAP at each pulse width (80, 150, and 300 μs) for the Injectrode and stainless-steel electrodes recorded from the (d) proximal sciatic, (e) distal sciatic, (f) tibial, and (g) common peroneal nerve cuff contact sites.
The conduction velocity of the CAPs was used to identify what types of afferents were excited by DRG stimulation. All supra-threshold trials using either electrode had a short-latency CAP consistent with a conduction velocity between 100 and 120 m/s, demonstrating that Aα afferents were recruited [13]. In some trials for both the Injectrode and stainless-steel electrode, there was a second CAP shortly after the first CAP (Fig. 1d). The second CAP had a conduction velocity between 53 and 82 m/s, consistent with Aβ fibers. The ability to recruit both Aα and Aβ afferents supports the hypothesis that DRG stimulation alleviates chronic pain via Aβ activity and the Gate Theory of Pain [4]. Therefore, the Injectrode can also be used as an investigational tool to decipher the mechanisms by which DRG stimulation inhibits pain transmission.
The Injectrode is designed to be delivered on the neural target using a needle and syringe. The current work delivered the Injectrode onto the DRG following a partial laminectomy to expose the DRG. However, we are currently exploring novel surgical and delivery methods that reduce the invasiveness of the Injectrode delivery. The lumbar DRG in the cat are inside the spinal canal; they lie very close to the spinal cord and run almost parallel to the spinal cord. In comparison, the DRG in humans can lie both inside the spinal canal or outside the neuroforamen, depending on the level, and angle away from the spinal cord [14]. The human lumbar DRG are more likely to be in the intraforaminal space, while the sacral DRG are more likely to lie within the spinal canal. These anatomical differences need to be considered when designing delivery methods for using the Injectrode in patients.
Clinical translation of the Injectrode will require a change in the conductive metal particulates because silver is known to be neurotoxic [15]. The current work is a proof-of-concept study demonstrating that a body-curing injectable electrode is able to recruit sensory fibers similarly to a traditional stainless-steel electrode. The formulation for other Injectrodes is currently underway to include other conductive metals.
IV. Conclusions
The Injectrode is a flexible conductive polymer electrode that is delivered through a syringe onto neural tissue. We demonstrated that the Injectrode can excite primary afferent fibers (both Aα and Aβ) through DRG stimulation on the L6 and L7 DRG in two cats. The Injectrode has a higher stimulation threshold to evoke CAPs in the sensory nerves than a traditional stainless-steel electrode, due to its larger contact surface area with the DRG. Overall, the Injectrode has the potential to be used as a neural interface for DRG stimulation to alleviate chronic pain.
Acknowledgments
The authors thank the staff at Magee Women’s Research Institute Animal Facility and Rachel Pitzer for assistance with animal care and monitoring. We would also like to thank Ritesh Kumar for technical assistance.
Research supported National Institutes of Health (1U18EB029251-01).
Footnotes
Conflicts of Interest
K.L. and A.S. are consultants to and co-founders of Neuronoff, Inc. M.F. is a co-founder and employee of Neuronoff, Inc. S.N is an employee of Neuronoff, Inc.
Contributor Information
Ashley N Dalrymple, Department of Mechanical Engineering, Carnegie Mellon University, Pittsburgh, PA, USA.; Rehab Neural Engineering Labs, University of Pittsburgh, Pittsburgh, PA, USA.
Jordyn E Ting, Rehab Neural Engineering Labs, University of Pittsburgh, Pittsburgh, PA, USA.; Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA, USA. Center for Neural Basis of Cognition, Pittsburgh, PA, USA.
Rohit Bose, Rehab Neural Engineering Labs, University of Pittsburgh, Pittsburgh, PA, USA.; Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA, USA. Center for Neural Basis of Cognition, Pittsburgh, PA, USA.
Stephan Nieuwoudt, Neuronoff Inc., Cleveland, OH, USA..
Manfred Franke, Neuronoff Inc., Cleveland, OH, USA..
Kip A Ludwig, Neuronoff Inc., Cleveland, OH, USA.; Departments of Biomedical Engineering and Neurological Surgery, University of Wisconsin-Madison, Madison, WI, USA
Andrew J Shoffstall, Neuronoff Inc., Cleveland, OH, USA.; Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH, USA.
Lee E Fisher, Rehab Neural Engineering Labs, University of Pittsburgh, Pittsburgh, PA, USA.; Center for Neural Basis of Cognition, Pittsburgh, PA, USA. Department of Physical Medicine and Rehabilitation, University of Pittsburgh, Pittsburgh, PA, USA.
Douglas J Weber, Department of Mechanical Engineering, Carnegie Mellon University, Pittsburgh, PA, USA.; Rehab Neural Engineering Labs, University of Pittsburgh, Pittsburgh, PA, USA. Center for Neural Basis of Cognition, Pittsburgh, PA, USA. Neuroscience Institute, Carnegie Mellon University, Pittsburgh, PA, USA
References
- [1].Nahin RL, “Estimates of pain prevalence and severity in adults: United States, 2012,” J. Pain Off. J. Am. Pain Soc, vol. 16, no. 8, pp. 769–780, 2015, doi: 10.1016/j.jpain.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Marshall B, Bland MK, Hulla R, and Gatchel RJ, “Considerations in addressing the opioid epidemic and chronic pain within the USA,” Pain Manag, vol. 9, no. 2, pp. 131–138, Mar. 2019, doi: 10.2217/pmt-2018-0070. [DOI] [PubMed] [Google Scholar]
- [3].Caylor J et al. , “Spinal cord stimulation in chronic pain: evidence and theory for mechanisms of action,” Bioelectron. Med, vol. 5, Jun. 2019, doi: 10.1186/s42234-019-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Melzack R and Wall PD, “Pain mechanisms: a new theory,” Science, vol. 150, no. 3699, pp. 971–979, Nov. 1965, doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- [5].Deer TR, Grigsby E, Weiner RL, Wilcosky B, and Kramer JM, “A prospective study of dorsal root ganglion stimulation for the relief of chronic pain,” Neuromodulation J. Int. Neuromodulation Soc, vol. 16, no. 1, pp. 67–72, 2013, doi: 10.1111/ner.12013. [DOI] [PubMed] [Google Scholar]
- [6].Harrison C, Epton S, Bojanic S, Green AL, and FitzGerald JJ, “The Efficacy and Safety of Dorsal Root Ganglion Stimulation as a Treatment for Neuropathic Pain: A Literature Review,” Neuromodulation J. Int. Neuromodulation Soc, vol. 21, no. 3, pp. 225–233, Apr. 2018, doi: 10.1111/ner.12685. [DOI] [PubMed] [Google Scholar]
- [7].Deer TR et al. , “Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial,” PAIN, vol. 158, no. 4, pp. 669–681, Apr. 2017, doi: 10.1097/j.pain.0000000000000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Trevathan JK et al. , “An Injectable Neural Stimulation Electrode Made from an In-Body Curing Polymer/Metal Composite,” Adv. Healthc. Mater, vol. 8, no. 23, p. 1900892, 2019, doi: 10.1002/adhm.201900892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ayers CA, Fisher LE, Gaunt RA, and Weber DJ, “Microstimulation of the lumbar DRG recruits primary afferent neurons in localized regions of lower limb,” J. Neurophysiol, vol. 116, no. 1, pp. 51–60, Jul. 2016, doi: 10.1152/jn.00961.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nanivadekar A, Ayers CA, Gaunt RA, Weber D, and Fisher LE, “Selectivity of afferent microstimulation at the DRG using epineural and penetrating electrode arrays,” J. Neural Eng, Oct. 2019, doi: 10.1088/1741-2552/ab4a24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fisher LE, Ayers CA, Ciollaro M, Ventura V, Weber DJ, and Gaunt RA, “Chronic recruitment of primary afferent neurons by microstimulation in the feline dorsal root ganglia,” J. Neural Eng, vol. 11, no. 3, p. 036007, Apr. 2014, doi: 10.1088/17412560/11/3/036007. [DOI] [PubMed] [Google Scholar]
- [12].Durand DM, “Electrical Stimulation of Excitable Systems,” in The Biomedical Engineering Handbook, 3rd ed., Taylor & Francis, 2006, pp. 474–495. [Google Scholar]
- [13].Hunt CC and McIntyre AK, “An analysis of fibre diameter and receptor characteristics of myelinated cutaneous afferent fibres in cat,” J. Physiol, vol. 153, pp. 99–112, Aug. 1960, doi: 10.1113/jphysiol.1960.sp006521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kikuchi S, Sato K, Konno S, and Hasue M, “Anatomic and radiographic study of dorsal root ganglia,” Spine, vol. 19, no. 1, pp. 6–11, Jan. 1994, doi: 10.1097/00007632-199401000-00002. [DOI] [PubMed] [Google Scholar]
- [15].Lansdown ABG, “Critical observations on the neurotoxicity of silver,” Crit. Rev. Toxicol, vol. 37, no. 3, pp. 237–250, Mar. 2007, doi: 10.1080/10408440601177665. [DOI] [PubMed] [Google Scholar]


