Abstract
Study Objectives
Structural brain maturation and sleep are complex processes that exhibit significant changes over adolescence and are linked to many physical and mental health outcomes. We investigated whether sleep–gray matter relationships are developmentally invariant (i.e. stable across age) or developmentally specific (i.e. only present during discrete time windows) from late childhood through young adulthood.
Methods
We constructed the Neuroimaging and Pediatric Sleep Databank from eight research studies conducted at the University of Pittsburgh (2009–2020). Participants completed a T1-weighted structural MRI scan (sMRI) and 5–7 days of wrist actigraphy to assess naturalistic sleep. The final analytic sample consisted of 225 participants without current psychiatric diagnoses (9–25 years). We extracted cortical thickness and subcortical volumes from sMRI. Sleep patterns (duration, timing, continuity, regularity) were estimated from wrist actigraphy. Using regularized regression, we examined cross-sectional associations between sMRI measures and sleep patterns, as well as the effects of age, sex, and their interaction with sMRI measures on sleep.
Results
Shorter sleep duration, later sleep timing, and poorer sleep continuity were associated with thinner cortex and altered subcortical volumes in diverse brain regions across adolescence. In a discrete subset of regions (e.g. posterior cingulate), thinner cortex was associated with these sleep patterns from late childhood through early-to-mid adolescence but not in late adolescence and young adulthood.
Conclusions
In childhood and adolescence, developmentally invariant and developmentally specific associations exist between sleep patterns and gray matter structure, across brain regions linked to sensory, cognitive, and emotional processes. Sleep intervention during specific developmental periods could potentially promote healthier neurodevelopmental outcomes.
Keywords: sleep, gray matter structure, actigraphy
Statement of Significance.
In this manuscript, we created a large harmonized data set of typically developing children, adolescents, and young adults with structural neuroimaging and objective sleep measurement (actigraphy). We leveraged this data set and used rigorous, data-driven statistical approaches to examine relationships between brain structure, naturalistic sleep patterns, and age. We show that certain brain structure–sleep behavior relationships are stable and consistent from late childhood through early adulthood (i.e. developmentally invariant) and other brain structure–sleep behavior relationships are present only during late childhood and early adolescence (i.e. developmentally specific). These results provide a framework for understanding the stability of brain–sleep relationships, pointing to sensitive periods when sleep and brain influence one another and suggesting optimal periods for sleep intervention implementation.
Introduction
Structural brain maturation and sleep are complex processes that exhibit significant changes during adolescent development. The precise timing and amount of these changes in youths likely influences multiple adult outcomes. Optimal sleep and brain maturation are each known to influence adolescent health and functioning, including academic/vocational achievement, mental health, and/or risk behaviors [1–10]. However, relationships between gray matter structure and sleep patterns over adolescence are not fully understood; furthermore, it is unknown whether these relationships vary as a function of age. A detailed characterization of brain–sleep relationships in adolescence is important for understanding factors contributing to optimal neurodevelopmental trajectories during this sensitive period.
Many brain regions implicated in cognitive and emotional outcomes show a protracted developmental course through adolescence [11–15], indicating that periods of heightened plasticity also come with greater vulnerability [15, 16]. Cortical thickness usually peaks by age 9–10 and then decreases until early adulthood, particularly in frontal, parietal, and temporal regions [4, 12, 17–23]. Most subcortical regions increase in volume until ~14–15 years, with growth plateauing afterwards [13, 24, 25]. Deviations from these normative trajectories may increase vulnerability to diverse negative outcomes, including poorer academic performance, mental health difficulties, and/or risky behaviors.
During adolescence, brain structural maturation is accompanied by multiple cognitive, behavioral, and emotional changes, including changes in sleep. Adolescence is characterized by a circadian phase delay and reduced homeostatic sleep drive, contributing to later sleep timing [26, 27]. These biological shifts converge with psychosocial and behavioral factors (e.g. school start times, peer socializing) to result in insufficient sleep and, at times, poorer sleep regularity or continuity [26, 27]. Disruptions to the timing, duration, continuity, and regularity of sleep predict and track with the severity of adverse cognitive and emotional outcomes (e.g. poor school performance, depression, substance use) [28–32].
Developmental shifts in sleep characteristics may possess reciprocal relationships with brain structural maturation [33–36], ultimately influencing diverse outcomes. While sleep serves multiple purposes, one such function is to support synaptic plasticity and reorganization of brain circuitry [24]. Sleep disruption was originally considered a consequence of brain structural abnormalities; however, recent animal data indicate that sleep disruption during periods of heightened developmental plasticity also cause deviations in brain maturation [37–39]. These translational studies imply stronger brain–sleep relationships in certain developmental windows [37, 40]. Yet, in humans it is unknown whether brain–sleep relationships are stable across adolescent development (i.e. developmentally invariant relationships) or only occur during a discrete window of development (i.e. developmentally specific relationships). Developmentally specific brain–sleep relationships could inform the optimal timing of brain and/or sleep-based interventions that promote healthier neurodevelopmental outcomes. Several initial reports have identified ties between diverse gray matter structures and sleep in pediatric populations [41–48]. However, developmentally specific relationships have not been examined and these studies have been restricted to retrospective self-report or lab-based sleep measures that do not reflect usual sleep. An important next step is to evaluate how brain structure relates to objective, ecologically valid sleep patterns (as captured by wrist actigraphy) through a developmental lens.
To address these open questions, we created the Neuroimaging and Pediatric Sleep (NAPS) Databank, a large, harmonized cross-sectional databank comprised of healthy children, adolescents, and young adults (ages 9–25 years). We estimated sleep from wrist actigraphy and sMRI measures from T1-weighted MRI. Given that a wide array of sMRI measures have been associated with sleep, we conducted an exploratory data-driven regularized regression analyses, to test many potential predictors while minimizing the issues of predictor inter-correlation and multiple comparisons. Because cortical thickness and subcortical volumes are the structural MRI measures known to show the strongest age-related changes across development [18, 49], we chose to focus this study on those sMRI measures for our primary analyses. We explored developmentally invariant and developmentally specific associations between sMRI measures (subcortical volume, cortical thickness) and core sleep dimensions (sleep duration, timing, continuity, regularity). Because there are important sex differences in sleep and brain development [17, 50–55], we also explored the interaction between self-reported sex and neuroimaging measures on sleep outcomes.
Methods
Participants
The initial NAPS databank includes a total of 305 participants drawn from eight University of Pittsburgh studies conducted between the years of 2009 to 2020. The NAPS databank was approved as a secondary data analysis protocol by the University of Pittsburgh Institutional Review Board. Participant consent or assent was collected at enrollment for each individual study included in NAPS and permitted sharing of de-identified data. Studies were considered for inclusion in NAPS if they included: (1) baseline actigraphic sleep monitoring reflecting naturalistic sleep; (2) a sMRI scan; and (3) participants aged 8.0–30.9 years-old (inclusive). Participant-level inclusion criteria were: (1) 9.0–25.9 years-old; (2) absence of current psychiatric diagnosis based on clinical interview (i.e. KSADS, SCID); (3) no current psychotropic or hypnotic medication use; (4) ≥5 days of good quality actigraphic sleep monitoring composed of both weekday and weekend days; (5) good quality MRI scan. Of the total 305 cases in NAPS, cases were excluded based on: enrollment in multiple protocols (n = 2), presence of a psychiatric diagnosis (n = 23); poor quality or insufficient sleep tracking (n = 6); or poor quality MRI (n = 34); age > 25 years-old (n = 15). Demographics of the final analytic sample of N = 225 are described in Table 1. Demographics by protocol are reported in eTables 1–2.
Table 1:
NAPS sample characteristics
| Variable | Mean or n (sd or %) |
|---|---|
| Sample N | 225 |
| Age (years) | 17.47 (4.73) |
| Self-reported sex | |
| Female | 122 (0.54) |
| Male | 103 (0.46) |
| Ethnicity | |
| Non-Hispanic | 11 (0.05) |
| Hispanic | 212 (0.94) |
| Missing | 2 (0.01) |
| Race | |
| White | 14 (0.06) |
| Black | 8 (0.04) |
| Asian | 2 (0.01) |
| Multiple | 39 (0.17) |
| Unknown/missing | 162 (0.72) |
| Wrist actigraph type | |
| AMI Octagonal MotionLogger | 25 (0.11) |
| PR/MiniMitter Actiwatch64 | 65 (0.29) |
| PR Actiwatch2 | 99 (0.44) |
| PR Spectrum Series | 36 (0.16) |
| Tracking days | 6.56 (0.87) |
| Weekdays | 4.48 (0.95) |
| Weekend days | 2.08 (0.53) |
| Season | |
| Spring | 48 (0.21) |
| Summer | 39 (0.17) |
| Fall | 98 (0.44) |
| Winter | 40 (0.18) |
| Sleep duration (minutes) | 420.59 (63.35) |
| Wake after sleep onset (minutes) | 57.07 (27.08) |
| Midsleep (minutes from midnight) | 265.47 (73.31) |
| Midsleep variability (minutes) | 63.42 (48.19) |
PR, Philips-Respironics; AMI, Ambulatory Monitoring Inc.
Neuroimaging methods and outcomes
Please see eTable 3 for sMRI protocol parameters. We used the FreeSurfer analysis software [56–59] (v6.0) to extract measures of cortical thickness (Desikan–Killiany atlas [60], n = 34 measures) and subcortical volume (aseg.mgz atlas, n = 8 measures) averaged across two hemispheres. We implemented a quality assessment pipeline developed by and used for the Enhancing Neuroimaging Genetics through Meta-Analysis consortium [61–71]. An automated MRIQC T1w-classifier determined individual scan quality based on a reference template [72]. We adjusted neuroimaging data for scanner protocol effects with ComBat [73, 74].
Wrist actigraphy
Actigraphy is a well-validated and widely used tool for objectively assessing naturalistic sleep in children, adolescents, and adults [75–77]. Participants continuously wore wrist actigraphs on their non-dominant wrist during a monitoring period of 5 or more consecutive days[78]. eTable 2 describes the number of participants who wore watches from Philips Respironics (PR; Actiwach-64, Actiwatch2, Spectrum series) or Ambulatory Monitoring, Inc. (AMI; Basic Octagonal Motionlogger). Wrist activity was sampled in 1-minute intervals (epochs). Participants were asked to indicate via button press the start and end of each sleep interval.
We estimated sleep from wrist actigraphy using a combination of validated brand-specific sleep algorithms (PR Medium Threshold; AMI Sadeh) and standardized visual editing procedures [79–81]. Trained scorers blinded to neuroimaging data manually identified rest intervals based on a combination of event markers indicated by participants and clear changes in activity and (if available) environmental light level recorded by the device. Brand-specific sleep scoring algorithms estimated sleep within each rest interval [75, 76, 80, 82–84]. We implemented additional semi-automated quality assurance procedures using in-house R scripts, including identification of the main rest interval (defined as the longest rest interval each day), removal of invalid sleep intervals containing ≥1 hour of off-wrist time or recording errors [80, 85], time adjustment for daylight savings time, and final visual inspection of sleep intervals on raster plots.
Sleep outcomes
Primary actigraphy sleep outcomes were based on the main rest interval. We selected four sleep outcomes corresponding to key dimensions of sleep health [86]: sleep duration (total sleep time in minutes), timing (midpoint between sleep onset and offset in minutes from midnight), continuity (minutes awake after sleep onset; WASO), and regularity (intra-individual standard deviation of midpoint in minutes). The first three outcomes were averaged over the 5–7 tracking days most proximal to their MRI scan; regularity was calculated from the available days of recording. Sleep variables were natural log transformed to normalize distributions.
Statistical analyses
We first conducted general additive models to confirm that the four sleep outcomes showed age-associated patterns consistent with prior research (eFigure 1). We observed the characteristic decline in sleep duration, delay in sleep timing, and increased sleep variability over adolescent development. Sleep continuity did not vary with age.
Primary analyses
We were interested in developmentally invariant effects (i.e. main effects) of neuroimaging measures on the four sleep outcomes, as well as developmentally specific effects (i.e. interactions between age and neuroimaging measures). Due to the large number of and multicollinearity amongst neuroimaging measures, we used regularized regression [87] to identify non-zero predictors associated with sleep outcomes. We used the R package, Group-Lasso-INTERaction-NET (glinternet [88, 89]) to examine main effects of structural neuroimaging measures, as well as their interaction with age and sex, for each sleep variable. We included multiple actigraphy covariates (i.e. tracking days, season, ratio of weekday to weekend days, actigraph model) as potential predictors in the models. eTable 4 contains the full list of 48 predictors. Group-lasso is a feature-selection method that identifies the variables that are most strongly associated with an outcome and uses a shrinkage parameter to reduce the coefficient of unimportant variables toward zero [88]. If two variables are highly correlated, only the strongest predictor is retained in the model. Further, only potential interactions between non-zero main effects are considered (i.e. strong hierarchy [88]). As such, non-zero predictors selected in the group-lasso models should be interpreted as the strongest predictors of sleep outcomes. We repeated 10-fold cross validation 100 times, using the penalty parameter (λ) one standard deviation away from the minimal cross-validation error. The final model was the model was selected most often during this procedure. We include information regarding the stability of non-zero predictor selection for each model in eTable 5. Regularized regression selects variables based on minimizing error in the model as opposed to statistical significance as in standard regression. Thus, p-values are not reported for non-zero coefficients.
We followed up feature selection performed by the group-lasso models with multiple regressions; this approach has been used previously [90–93]. Multiple regression analyses were used to estimate explained variance by lasso-selected features and to further characterize the interactions between brain predictors and age or sex, rather than to provide definitive effect sizes. There were no significant issues with multicollinearity in these regression models (e.g. VIF < 10), indicating that the group-lasso appropriately mitigated multicollinearity. R-squared was computed to estimate variance explained by the full model, as well as groups of predictors (i.e. demographics, neuroimaging measures, actigraphy covariates) [91–93]. We assessed non-zero interactions between age and neuroimaging predictors with the Johnson–Neyman technique, which obtains parameter estimates and points of significance from the interaction between two continuous variables [94–96]. Non-zero interactions between sex and neuroimaging predictors were probed by comparing estimated marginal means [97].
Secondary analyses
To allow for comparisons with previous studies that examined main relationships between sMRI measures and self-reported sleep behaviors [42, 43, 47], we conducted univariate analyses examining the main effects of cortical thickness, cortical surface area, and subcortical and cortical volume on the four sleep outcomes. We included age, sex, tracking days, season, ratio of weekday to weekend days, and actigraph model as covariates in the model and corrected for multiple comparisons (N = 110 for each sleep outcome) using False-Discovery rate [98]. Given that estimated total intracranial volume was sometimes (but not always) included as a covariate in the previous publications looking at the relationship between self-reported sleep behavior and sMRI measures [42, 43, 47], we provide results when including and omitting estimated total intracranial volume as a covariate.
Results
All neuroimaging measures, and their interactions with age and sex, selected as non-zero predictors of sleep outcomes are reported in Table 2. Non-zero actigraphy covariates (e.g. season, actigraph type) are reported in eTable 6.
Table 2.
Main effects and interactions between age, sex, and neuroimaging measures on actigraphic sleep dimensions
| (A) Sleep duration (total sleep time) | ||
|---|---|---|
| Type of effect | Variable | Model weight |
| Demographic variable main effects | Sex | 0.0403 |
| Age | −0.0732 | |
| Subcortical volume main effects | Pallidum | −0.0122 |
| Hippocampus | −0.0529 | |
| Amygdala | −0.0032 | |
| Lateral ventricles | 0.0221 | |
| Cortical thickness main effects | Medial orbitofrontal cortex | 0.1090 |
| Parahippocampal cortex | 0.0022 | |
| Posterior cingulate | 0.0576 | |
| Isthmus cingulate | 0.0196 | |
| Superior parietal cortex | 0.0067 | |
| Cuneus | 0.0277 | |
| Sex interactions | Sex × parahippocampal cortex | 0.0054 |
| Sex by posterior cingulate cortex | −0.0219 | |
| Age interactions | Age × lateral ventricles | 0.0234 |
| Age × cuneus | −0.0332 | |
| Age × superior parietal cortex | −0.0072 | |
| Variance accounted for by demographic measures only: R2= 0.22 Variance accounted for by neuroimaging and demographic measures, and their interactions: R2 = 0.25 |
||
| (B) Sleep timing (midsleep) | ||
| Type of effect | Variable | Model weight |
| Demographic variable main effects | Sex | −0.0601 |
| Age | 0.1315 | |
| Subcortical volume main effects | Thalamus | −0.0009 |
| Pallidum | 0.0120 | |
| Lateral ventricles | 0.0057 | |
| Cortical thickness main effects | Medial orbitofrontal cortex | −0.0002 |
| Pars orbitalis | −0.0136 | |
| Rostral middle frontal cortex | −0.0200 | |
| Posterior cingulate cortex | −0.0089 | |
| Superior parietal cortex | −0.0051 | |
| Lateral occipital cortex | −0.1115 | |
| Sex interactions | Sex × lateral ventricles | 0.0342 |
| Sex × thalamus | 0.0010 | |
| Age interactions | Age × pallidum | −0.0431 |
| Age × medial orbitofrontal cortex | 0.0002 | |
| Age × pars orbitalis | 0.0187 | |
| Age × rostral middle frontal cortex | 0.0267 | |
| Age × posterior cingulate cortex | 0.0189 | |
| Variance accounted for by demographic measures only: R2 = 0.10 Variance account for by neuroimaging and demographic measures, and their interactions: R2 = 0.20 |
||
| (C) Sleep continuity (WASO) | ||
| Type of effect | Variable | Model weight |
| Demographic variable main effects | Sex | −0.0444 |
| Age | 0.0244 | |
| Subcortical volume main effects | Thalamus | 0.0065 |
| Pallidum | 0.0260 | |
| Caudate | 0.0083 | |
| Cortical thickness main effects | Entorhinal cortex | 0.0009 |
| Parahippocampal cortex | −0.0192 | |
| Middle temporal cortex | −0.0086 | |
| Precentral cortex | −0.0283 | |
| Superior parietal cortex | −0.0243 | |
| Lateral occipital cortex | −0.0149 | |
| Sex interactions | Sex × caudate | −0.0117 |
| Sex × entorhinal cortex | −0.0021 | |
| Sex × precentral cortex | −0.0151 | |
| Age interactions | Age × parahippocampal cortex | 0.0533 |
| Age × superior parietal cortex | 0.0622 | |
| Variance accounted for by demographic measures only: R2 = 0.05 Variance account for by neuroimaging and demographic measures, and their interactions: R2 = 0.16 |
||
Model weights are reported as standardized regression coefficients.
Sleep duration (total sleep time)
The main effects of neuroimaging measures, age, sex, and their respective interactions accounted for 25% of the total variance in sleep duration (Table 2A). Shorter sleep duration was associated with older age and males had shorter sleep duration in comparison to females.
We observed several developmentally invariant relationships between brain structure and sleep duration (Figure 1A). From 9.0–25.9 years old, greater volume in the pallidum, hippocampus, and amygdala was associated with shorter sleep duration. Additionally, thinner medial orbitofrontal and isthmus (posterior) cingulate cortices were associated shorter sleep duration. Thinner cortex in the posterior cingulate was associated with shorter sleep duration in both sexes, but there was a stronger relationship in males. Conversely, thinner parahippocampal cortex and shorter sleep duration were associated in females, but not males.
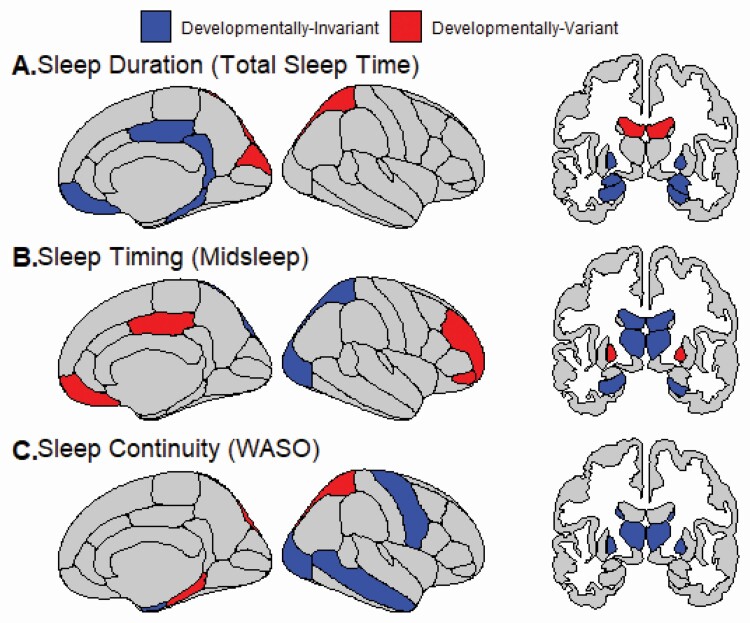
Figure 1.
Relationships between sleep and gray matter (cortical thickness, subcortical volume) that are developmentally invariant (i.e. stable across age) or developmentally specific (i.e. only present during discrete time windows) from late childhood through young adulthood. Actigraphic sleep outcomes included: (A) Sleep Duration (total sleep time), (B) Sleep Timing (midsleep) and (C) Sleep Continuity (WASO). There were no non-zero predictors of Sleep Regularity (midsleep variability).
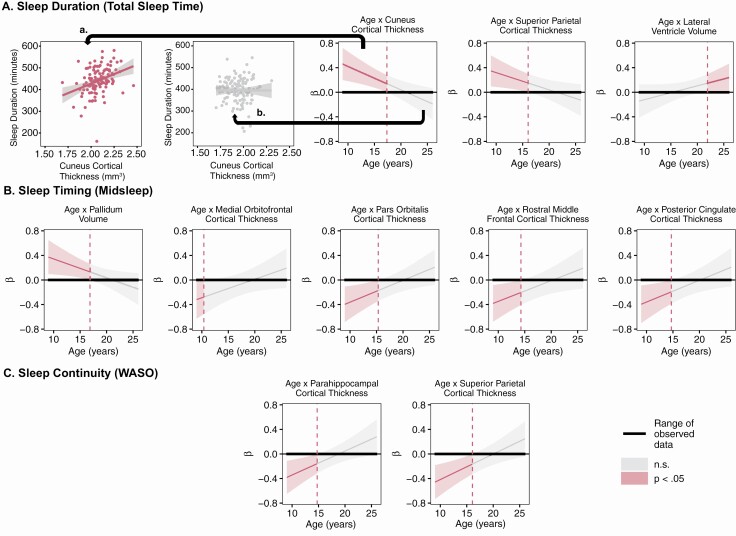
We also found developmentally specific relationships between gray matter structure and sleep duration (Figure 1A, 2A). In late childhood through middle adolescence, thinner cortex in the cuneus (9.0–17.3 years) and superior parietal regions (9.0–16.0 years) was associated with shorter sleep duration; however, this relationship was not observed at older ages. From 21.9–25.9 years old, greater lateral ventricle volume was associated with longer sleep duration.
Figure 2.
Johnsyon–Neyman plots of age by neuroimaging measure interactions on sleep dimensions (A, duration; B, timing; and C, continuity). A statistically significant relationship between age and the neuroimaging measures (p < .05) is represented in read. Non-significant relationships are represented in gray. To aid in the interpretation of the plots, we provide one example of the age by cuneus cortical thickness interaction on sleep duration. (a) From 9.0 to 17.3 years old, thicker cuneus cortex is associated with longer sleep duration (r = 0.33, p = 1.0 × 10-4). (b) From 17.4 to 25.9 years old, this relationship is not present (r = −0.003, p = 0.97).
Sleep timing (midsleep)
The main effects of neuroimaging measures, age, and their interactions accounted for 20% of the variance in midsleep (Table 2B). Midsleep was later in males and among older participants.
Developmentally invariant relationships were identified for several brain regions (Figure 1B). Specifically, lower thalamus volume was associated with later midsleep; this was relationship driven by males. In females only, greater lateral ventricle volume was associated with later midsleep. Thinner superior parietal and lateral occipital cortices were associated with later sleep timing.
Developmentally specific relationships were also observed between neuroimaging measures and sleep timing (Figure 1B, 2B). From late childhood through middle adolescence, thinner cortex in the pars orbitalis (9.0–15.2 years), rostral middle frontal (9.0–14.1 years), and posterior cingulate regions (9.0–14.5 years) was associated with later midsleep. Thinner medial orbitofrontal cortex in late childhood (9.0–10.0 years) was also associated with later midsleep. Greater pallidum volume was associated with later midsleep only from ages 9.0 to 16.8 years.
Sleep continuity (WASO)
The combined effects of neuroimaging measures, age, sex, and their interactions accounted for 16% of the variance in sleep continuity (Table 2C). WASO was longer among older participants and in females.
With regard to developmentally invariant relationships (Figure 1C), greater palladium and thalamus volume was associated with greater WASO. Thinner cortex in middle temporal, precentral, and lateral occipital regions was associated with greater WASO. Greater precentral and entorhinal cortical thickness was associated with greater WASO in females.
Thinner parahippocampal (9.0–14.6 years) and superior parietal cortices (9.0–16.0 years) were associated with greater WASO from late childhood to mid-adolescence, but not in older adolescents and young adults (Figure 1C, 2C).
Sleep regularity (midsleep variability)
Regularized regression did not identify any non-zero predictors of midsleep regularity.
Secondary univariate analyses
None of the univariate analyses for cortical thickness, volume, or surface area survived FDR-correction for multiple comparisons (eTables 7–10). There was a trend for a statistically significant relationship between increased paracentral surface area and increased sleep duration (b = 0.25, p = 0.001, q = 0.06, eTable 7). The direction of the main effects of the βs from the group-lasso models for the cortical thickness metrics and subcortical volumes were consistent with the direction of the βs from the respective univariate analyses.
Discussion
Using a large sample of typical adolescent development (9.0–25.9 years), we identified developmentally invariant and developmentally specific relationships between gray matter structure and naturalistic sleep patterns. Shorter sleep duration, later sleep timing, and poorer sleep continuity—all of which are associated with adverse health outcomes—were associated with a stable pattern of thinner cortex and altered subcortical volumes in diverse brain regions over adolescent development. In discrete regions, developmentally specific relationships were also observed. In these regions, thinner cortex from late childhood through early-to-mid adolescence—a pattern associated with accelerated maturation—was associated with less optimal sleep, but these relationships were not detected in late adolescence and young adulthood. Our results provide a novel view of brain-sleep structure relationships within brain structures implicated in a wide array of cognitive, emotional, and psychological processes over adolescent development [2, 99–104].
Cortical thickness in a diverse set of brain regions show developmentally invariant relationships with sleep
Across adolescent development, thinner cortex in frontal, temporal, parietal, and visual processing areas was associated with shorter sleep duration, later sleep timing, and longer time awake after sleep onset. These brain regions are implicated in salience detection (pars orbitalis), motor function (precentral), memory (entorhinal, middle temporal), and attention and visuospatial perception (superior parietal cortex, lateral occipital) [105]. Given that sleep is associated with diverse range of mental, cognitive and physical health outcomes in adolescence [1–10], it is reasonable that naturalistic sleep is related to brain structure in regions that support multiple functions. This notion is consistent with prior work observing correlations between self-reported sleep duration and timing with gray matter volume in diverse brain regions [42, 43]. However, while we focused on associations between actigraphic sleep metrics and cortical thickness and subcortical volume, our univariate analyses also pointed to a trend towards increased surface area and longer sleep duration (eTable 7), which is consistent with a recent report [42] from the ABCD sample in which self-report sleep duration displayed the strongest associations with regional cortical surface area. However, the ABCD study did not identify relationships between self-reported sleep behavior and cortical thickness. While these contrasting findings may be rooted in differing age ranges, sleep measurement approaches, or statistical methodology used, it raises the importance of examining separate components of volume (i.e. surface area and cortical thickness) to better understand the underlying neural mechanisms that tie sleep to brain maturation, given the distinct neurodevelopmental origins of surface area and cortical thickness [106, 107]. Finally, some of the developmentally invariant relationships between gray matter structure and sleep outcomes in our report were modulated by self-reported sex, consistent with reported sex differences in sleep patterns and brain development [17, 50–55]. Future studies should also examine the extent to sex effects may be better explained by pubertal maturation.
Increased cortical thickness was associated with healthier sleep patterns from late childhood to middle adolescence
This is the first study, to our knowledge, to demonstrate that brain structure is related to individual differences in naturalistic sleep patterns at different ages, from late childhood through adulthood. Thicker cortex in multiple brain regions was associated with “healthier” sleep (as indicated by longer, more continuous, and earlier sleep) during late childhood and early adolescence. These findings, in conjunction with other work [108], present the possibility that biological factors exert differential influences on behavior at distinct points in development. Accelerated cortical thinning/growth patterns in discrete brain regions could contribute to disruptions in sleep characteristics during late childhood and early adolescence, but not during other periods. Alternatively, disruptions in the typical age-related changes in sleep could lead to accelerating cortical thinning, particularly during this late childhood–early adolescence age range, but not during others. Multiple neurobiological mechanisms likely underlie individual differences in cortical thickness. Cortical thinning is traditionally believed to be caused by synaptic pruning, a re-wiring of synapses [109, 110]. Translational models find that, in mice, synaptic pruning is higher during sleep than wakefulness in adolescents, but not adults [111]. More recent data suggest that age-associated changes in cortical thickness may also be driven by white matter maturational processes, i.e. myelination [112]. Sleep disruption is detrimental to the formation and maintenance of myelin in murine models [113, 114]. Future longitudinal within-person investigations, particularly during late childhood and early adolescence, will be necessary to disentangle the directionality and neurobiological mechanisms of relationships between sleep, cortical thickness measures, and white matter integrity.
Unexpected relationships between poorer sleep and larger subcortical volumes
Surprisingly, in many cases, we also discovered that larger subcortical (i.e. hippocampal, amygdala, thalamus, and caudate) volumes are associated with more disrupted sleep patterns. One possibility is that exposure to sleep disruption at certain developmental stages may be correlated with or cause accelerated subcortical growth patterns, akin to the acceleration–deceleration hypothesis of chronic stress and neurodevelopment [115–117]. Importantly, this result stands in contrast with prior research showing lower subcortical gray matter volumes in relation to poor sleep [42, 46] and mental health conditions [61, 118, 119]. Thus, replication of these findings, as well as work examining the relationship between structural brain measures and sleep, needs to be further explored in informative subgroups such as individuals with mental disorders.
We also observed subcortical volume–sleep relationships in the expected direction. In females, larger lateral ventricle volume was associated with shorter sleep duration and later midsleep. Greater ventricle size has been linked to serious mental health conditions, including schizophrenia [120]. Furthermore, study of older adults also found longitudinal reduction in sleep duration corresponded to ventricular expansion over the follow-up period [121].
Implications for optimal timing and targets for sleep intervention
If sleep patterns prove to be a causal contributor to individual differences in sMRI measures, our findings have the potential to inform developmentally sensitive optimization of evidence-based behavioral sleep interventions [122]. As an example, both shorter sleep duration and later sleep timing were associated with thinner cortex in default mode network (DMN) regions (medial orbitofrontal and posterior cingulate cortices), a neural signature tied to outcomes such as depression, insomnia, and poor cognitive function [102, 123]. DMN cortical thickness and sleep duration relationships were developmentally invariant. However, DMN cortical thickness-sleep timing association were only present in late childhood/mid-adolescence. Thus, a sleep treatment geared toward promoting healthy DMN-relevant outcomes should include sleep extension regardless of age but also advance sleep timing in late childhood and early/mid adolescence. Taken as a whole, our findings suggest that sleep interventions, particularly in late childhood through mid-adolescence, may be advantageous for neurodevelopment and thus downstream effects on psychological well-being.
Limitations
Our sample, while representative of the Pittsburgh Metropolitan area, was limited in its racial and ethnic diversity, factors which contribute to individual differences in brain structure and sleep [28, 124]. While the group-lasso regression approach has several strengths, we note that predictors selected these models should be interpreted as the strongest predictors of sleep outcomes. Although this can be mechanistically informative, in that the most robust sMRI–sleep relationships will be captured, smaller magnitude main effects and interaction effects could be removed from the model. Future studies would benefit from also examining dimension reduction approaches to complement to feature selection methods (i.e. lasso), including principal component analysis or k-means clustering, to address the p > n problem. Additionally, all models used in this study examined the linear effects of age. Given that many developmental processes that take place during adolescence are nonlinear (e.g. [17]) and these patterns are most accurately captured with longitudinal analyses [125], future studies should explore nonlinear brain–sleep associations in studies that have three or more data points. Although we adjusted for salient actigraphy covariates, actigraphy brand differences may have contributed noise in our data that was not captured by covarying for watch type in our models. Furthermore, data on school or work versus free days was not systematically collected across studies and schedule constraints are known to affect sleep patterns [126]. While we approximated these effects by adjusting for season and weekday-weekend ratio during actigraphy tracking, future studies should collect information on the presence vs absence of schedule constraints affecting sleep on a daily basis. Another limitation is the absence of information about the role of pubertal maturation, which is a core aspect of developmental changes in the interval from late childhood into mid-adolescence. Pubertal maturation appears to influence some aspects of circadian and sleep regulation during adolescent development [26, 27]. Individual differences as well as sex differences in puberty could represent a valuable focus for future studies to advance understanding of developmentally specific associations between sleep patterns and gray matter structure (and potentially intervention strategies). Because our analyses were cross-sectional across a range of ages, rather than longitudinal within participants, it is unclear whether sleep patterns are a cause, correlate, or consequence of gray matter structure. Future, prospective longitudinal studies are necessary to disambiguate causal relationships between sleep and sMRI measures, and assess relationships between within-subject trajectories of sleep and brain development.
Conclusions & Future Directions
We found compelling and novel evidence for developmentally invariant and developmentally specific associations between sMRI measures and sleep across adolescent development. We plan to build on these findings and examine how individual differences in neuroimaging and sleep measures may identify youth at high-risk for developing adverse cognitive, mental, and physical outcomes.
Supplementary Material
Funding
Creation of the Neuroimaging and Pediatric Sleep (NAPS) databank and data included in this publication was supported by the National Center for Advancing Translational Sciences [UL1TR001857], National Institute of Mental Health [K01MH112774, K01MH111953, K01MH077106, R21MH102412, P50MH080215], National Institute on Drug Abuse [R01DA033064, K01DA032557], National Institute on Alcohol Abuse and Alcoholism [R21AA023209], and the Pittsburgh Foundation [M2010-0117].
Role of the Funder(s)/Sponsor(s): Our funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
DISCLOSURE STATEMENTS
Financial Disclosure: Dr. Goldstein reports receiving royalties from Guilford Press. Dr. Ryan is on the Scientific Advisory Committee for Axsome Therapeutics. Dr. Buysse has served as a paid consultant to Bayer, BeHealth Solutions, Cereve/Ebb Therapeutics, Emmi Solutions, National Cancer Institute, Pear Therapeutics, Philips Respironics, Sleep Number, and Weight Watchers International. He has served as a paid consultant for professional educational programs developed by the American Academy of Physician Assistants and CME Institute, and received payment for a professional education program sponsored by Eisai (content developed exclusively by Dr. Buysse). Dr. Buysse is an author of the Pittsburgh Sleep Quality Index, Pittsburgh Sleep Quality Index Addendum for PTSD (PSQI-A), Brief Pittsburgh Sleep Quality Index (B-PSQI), Daytime Insomnia Symptoms Scale, Pittsburgh Sleep Diary, Insomnia Symptom Questionnaire, and RU_SATED (copyright held by University of Pittsburgh). These instruments have been licensed to commercial entities for fees. He is also co-author of the Consensus Sleep Diary (copyright held by Ryerson University), which is licensed to commercial entities for a fee. Dr. Forbes has received an honorarium from Association for Psychological Science. Drs. Jalbrzikowski, Hayes, Franzen, Hasler, Siegle, Dahl, Ladouceur, McMakin, Silk, and Soehner, as well as Ms. Scully, have no relevant financial interests, activities, relationships, or affiliations to report.
Deposit of manuscript in a Preprint database: https://www.biorxiv.org/content/10.1101/2021.01.05.424689v1
Previous Presentation: These results have not previously been presented or submitted for publication.
Data Availability: The datasets generated and/or analyzed during the current study are not available for use outside of the University of Pittsburgh at this time, due to the nature of the ethics board approvals and possible risk(s) to study participants as well as the confidentiality promised to them. Data may be made available from the corresponding author on reasonable request with permission of NAPS investigators and ethics board approval.
References
- 1. Pehlivanova M, et al. Diminished cortical thickness is associated with impulsive choice in adolescence. J Neurosci. 2018;38(10):2471–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burgaleta M, et al. Cognitive ability changes and dynamics of cortical thickness development in healthy children and adolescents. Neuroimage. 2014;84:810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foland-Ross LC, et al. Cortical thickness predicts the first onset of major depression in adolescence. Int J Dev Neurosci. 2015;46:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tamnes CK, et al. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 2010;20(3):534–548. [DOI] [PubMed] [Google Scholar]
- 5. Bos MGN, et al. Emerging depression in adolescence coincides with accelerated frontal cortical thinning. J Child Psychol Psychiatry. 2018;59(9):994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oostermeijer S, et al. Trajectories of adolescent conduct problems in relation to cortical thickness development: a longitudinal MRI study. Transl Psychiatry. 2016;6(9):e899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meruelo AD, et al. Early adolescent brain markers of late adolescent academic functioning. Brain Imaging Behav. 2019;13(4):945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dewald JF, et al. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: a meta-analytic review. Sleep Med Rev. 2010;14(3):179–189. [DOI] [PubMed] [Google Scholar]
- 9. McKnight-Eily LR, et al. Relationships between hours of sleep and health-risk behaviors in US adolescent students. Prev Med. 2011;53(4-5):271–273. [DOI] [PubMed] [Google Scholar]
- 10. Tarokh L, et al. Sleep in adolescence: physiology, cognition and mental health. Neurosci Biobehav Rev. 2016;70:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giedd JN, et al.. Structural magnetic resonance imaging of typical pediatric brain development. In: Charney D, Nestler E, eds. Neurobiology of Mental Illness. Oxford: Oxford University Press; 2011:1209–1217. [Google Scholar]
- 12. Giedd JN, et al. Child psychiatry branch of the National Institute of Mental Health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacology. 2015;40(1):43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raznahan A, et al. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proc Natl Acad Sci U S A. 2014;111(4):1592–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giedd JN, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. [DOI] [PubMed] [Google Scholar]
- 15. Paus T, et al. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. [DOI] [PubMed] [Google Scholar]
- 17. Vijayakumar N, et al.. Brain development during adolescence: a mixed-longitudinal investigation of cortical thickness, surface area, and volume. Hum Brain Mapp. 2016;37(6):2027–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tamnes CK, et al.. Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J Neurosci. 2017;37(12):3402–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mills KL, et al. Structural brain development between childhood and adulthood: convergence across four longitudinal samples. Neuroimage. 2016;141:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sowell ER, et al.. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–315. [DOI] [PubMed] [Google Scholar]
- 21. Sowell ER, et al. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gogtay N, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shaw P, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. [DOI] [PubMed] [Google Scholar]
- 24. Tononi G, et al. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81(1):12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Puentes-Mestril C, et al. Linking network activity to synaptic plasticity during sleep: hypotheses and recent data. Front Neural Circuits. 2017;11:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crowley SJ, et al. An update on adolescent sleep: new evidence informing the perfect storm model. J Adolesc. 2018;67:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Crowley SJ, et al. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PLoS One. 2014;9(11):e112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grandner MA. Sleep, health, and society. Sleep Med Clin. 2017;12(1):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spira AP, et al. Impact of sleep on the risk of cognitive decline and dementia. Curr Opin Psychiatry. 2014;27(6):478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matricciani L, et al. Rethinking the sleep-health link. Sleep Health. 2018;4(4):339–348. [DOI] [PubMed] [Google Scholar]
- 31. Dolsen MR, et al. Insomnia as a transdiagnostic process in psychiatric disorders. Curr Psychiatry Rep. 2014;16(9):471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Soehner AM, et al. Insomnia comorbid to severe psychiatric illness. Sleep Med Clin. 2013;8(3):361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kayser MS, et al. Sleep and development in genetically tractable model organisms. Genetics. 2016;203(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muehlroth BE, et al. Understanding the interplay of sleep and aging: methodological challenges. Psychophysiology. 2020;57(3):e13523. [DOI] [PubMed] [Google Scholar]
- 35. Fontanellaz-Castiglione CE, et al. Sleep and the adolescent brain. Curr Opin Physiol. 2020;15:167–171. [Google Scholar]
- 36. Cirelli C, et al. Cortical development, electroencephalogram rhythms, and the sleep/wake cycle. Biol Psychiatry. 2015;77(12):1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kayser MS, et al. A critical period of sleep for development of courtship circuitry and behavior in Drosophila. Science. 2014;344(6181):269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seugnet L, et al. Sleep deprivation during early-adult development results in long-lasting learning deficits in adult Drosophila. Sleep. 2011;34(2):137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Frank MG, et al. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30(1):275–287. [DOI] [PubMed] [Google Scholar]
- 40. Novati A, et al. Chronic sleep restriction causes a decrease in hippocampal volume in adolescent rats, which is not explained by changes in glucocorticoid levels or neurogenesis. Neuroscience. 2011;190:145–155. [DOI] [PubMed] [Google Scholar]
- 41. Goldstone A, et al. The mediating role of cortical thickness and gray matter volume on sleep slow-wave activity during adolescence. Brain Struct Funct. 2018;223(2):669–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheng W, et al.. Sleep duration, brain structure, and psychiatric and cognitive problems in children. Mol Psychiatry. February 2020. doi: 10.1038/s41380-020-0663-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Urrila AS, et al. ; IMAGEN consortium. Sleep habits, academic performance, and the adolescent brain structure. Sci Rep. 2017;7:41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kurth S, et al. Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study. J Neurosci. 2010;30(40):13211–13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sung D, et al. Structural alterations in large-scale brain networks and their relationship with sleep disturbances in the adolescent population. Sci Rep. 2020;10(1):3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Taki Y, et al. Sleep duration during weekdays affects hippocampal gray matter volume in healthy children. Neuroimage. 2012;60(1):471–475. [DOI] [PubMed] [Google Scholar]
- 47. Lapidaire W, et al. Irregular sleep habits, regional grey matter volumes, and psychological functioning in adolescents. PLoS One. 2021;16(2):e0243720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Buchmann A, et al. EEG sleep slow-wave activity as a mirror of cortical maturation. Cereb Cortex. 2011;21(3):607–615. [DOI] [PubMed] [Google Scholar]
- 49. Frangou S, et al., ENIGMA Lifespan Working Group. 85 . Cortical thickness and subcortical volume trajectories across the lifespan: data from 14,600 healthy individuals aged 6–90 years. Biol Psychiatry. 2019;85(10 Supplement):S35–S36. [Google Scholar]
- 50. Sowell ER, et al. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44(1):4–16. [DOI] [PubMed] [Google Scholar]
- 51. Koolschijn PC, et al. Sex differences and structural brain maturation from childhood to early adulthood. Dev Cogn Neurosci. 2013;5:106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang B, et al. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29(1):85–93. [DOI] [PubMed] [Google Scholar]
- 53. Santhi N, et al. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc Natl Acad Sci U S A. 2016;113(19):E2730–E2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Giedd JN, et al. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21(8):1185–1201. [DOI] [PubMed] [Google Scholar]
- 55. Lenroot RK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dale AM, et al. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. [DOI] [PubMed] [Google Scholar]
- 57. Fischl B, et al. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. [DOI] [PubMed] [Google Scholar]
- 58. Fischl B, et al. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fischl B, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. [DOI] [PubMed] [Google Scholar]
- 60. Desikan RS, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. [DOI] [PubMed] [Google Scholar]
- 61. Schmaal L, et al.. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA major depressive disorder working group. Mol Psychiatry. 2016;21(6):806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hibar DP, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry. 2018;23(4):932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. van Erp TGM, et al. ; Karolinska Schizophrenia Project. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta analysis (ENIGMA) Consortium. Biol Psychiatry. 2018;84(9):644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. van Rooij D, et al. Cortical and subcortical brain morphometry differences between patients with autism spectrum disorder and healthy individuals across the lifespan: results from the ENIGMA ASD Working Group. Am J Psychiatry. 2018;175(4):359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hoogman M, et al. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry. 2017;4(4):310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sun D, et al. Large-scale mapping of cortical alterations in 22q11.2 deletion syndrome: convergence with idiopathic psychosis and effects of deletion size. Mol Psychiatry. 2020;25(8):1822–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kong XZ, et al. ; ENIGMA Laterality Working Group. Mapping cortical brain asymmetry in 17,141 healthy individuals worldwide via the ENIGMA Consortium. Proc Natl Acad Sci U S A. 2018;115(22):E5154–E5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Boedhoe PSW, et al. ; ENIGMA-OCD Working Group; ENIGMA OCD Working Group. Cortical abnormalities associated with pediatric and adult obsessive-compulsive disorder: findings from the ENIGMA obsessive-compulsive disorder working group. Am J Psychiatry. 2018;175(5):453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Whelan CD, et al. Structural brain abnormalities in the common epilepsies assessed in a worldwide ENIGMA study. Brain. 2018;141(2):391–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nunes A, et al. ; ENIGMA Bipolar Disorders Working Group. Using structural MRI to identify bipolar disorders - 13 site machine learning study in 3020 individuals from the ENIGMA Bipolar Disorders Working Group. Mol Psychiatry. 2020;25(9):2130–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Thompson PM, et al. ; Alzheimer’s Disease Neuroimaging Initiative, EPIGEN Consortium, IMAGEN Consortium, Saguenay Youth Study (SYS) Group. The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 2014;8(2):153–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Esteban O, et al. MRIQC: advancing the automatic prediction of image quality in MRI from unseen sites. PLoS One. 2017;12(9):e0184661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fortin JP, et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage. 2017;161:149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fortin JP, et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage. 2018;167:104–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Quante M, et al. Actigraphy-based sleep estimation in adolescents and adults: a comparison with polysomnography using two scoring algorithms. Nat Sci Sleep. 2018;10:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Meltzer LJ, et al. Direct comparison of two new actigraphs and polysomnography in children and adolescents. Sleep. 2012;35(1):159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Meltzer LJ, et al. ; Caffeine for Apnea of Prematurity – Sleep Study Group. Validation of actigraphy in middle childhood. Sleep. 2016;39(6):1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Acebo C, et al. Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep. 1999;22(1):95–103. [DOI] [PubMed] [Google Scholar]
- 79. Meltzer LJ, et al. Use of actigraphy for assessment in pediatric sleep research. Sleep Med Rev. 2012;16(5):463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Patel SR, et al. Reproducibility of a standardized actigraphy scoring algorithm for sleep in a US Hispanic/Latino Population. Sleep. 2015;38(9):1497–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Blackwell T, et al. Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep. 2005;28(12):1599–1605. [DOI] [PubMed] [Google Scholar]
- 82. Kanady JC, et al. Actigraphic assessment of a polysomnographic-recorded nap: a validation study. J Sleep Res. 2011;20(1 Pt 2):214–222. [DOI] [PubMed] [Google Scholar]
- 83. van Hees VT, et al. Estimating sleep parameters using an accelerometer without sleep diary. Sci Rep. 2018;8(1):12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Slater JA, et al.. Assessing sleep using hip and wrist actigraphy. Sleep Biol Rhythms. 2015;13(2):172–180. [Google Scholar]
- 85. Cespedes Feliciano EM, et al. Chronotype, social jet lag, and cardiometabolic risk factors in early adolescence. JAMA Pediatr. 2019;173(11):1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc: Series B. 1994;58:267–288. [Google Scholar]
- 88. Lim M, et al. Learning interactions via hierarchical group-lasso regularization. J Comput Graph Stat. 2015;24(3):627–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lim M, Hastie T. Glinternet: Learning Interactions via Hierarchical Group-Lasso Regularization. 2020. https://CRAN.R-project.org/package=glinternet. Accessed December 8, 2020.
- 90. Soehner AM, et al. Longitudinal associations between sleep patterns and psychiatric symptom severity in high-risk and community comparison youth. J Am Acad Child Adolesc Psychiatry. 2019;58(6):608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bertocci MA, et al. Clinical, cortical thickness and neural activity predictors of future affective lability in youth at risk for bipolar disorder: initial discovery and independent sample replication. Mol Psychiatry. 2019;24(12):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Banihashemi L, et al. Limbic white matter structural integrity at 3 months prospectively predicts negative emotionality in 9-month-old infants: a preliminary study. J Affect Disord. 2020;273:538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Acuff HE, et al. ; LAMS Consortium. White matter - emotion processing activity relationships in youth offspring of bipolar parents. J Affect Disord. 2019;243:153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Johnson PO, et al. The Johnson-Neyman technique, its theory and application. Psychometrika. 1950;15(4):349–367. [DOI] [PubMed] [Google Scholar]
- 95. Curran PJ, et al.. Testing and probing interactions in hierarchical linear growth models. In: Bergeman CS & Boker SM, eds. Methodological Issues in. New York, NY: Psychology Press; 2006. [Google Scholar]
- 96. Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31(3):437–448. [Google Scholar]
- 97. Lenth R, Buerkner P, Herve M, Love J, Riebl H, Singmann H. Emmeans: Estimated Marginal Means, Aka Least-Squares Means.; 2020. https://CRAN.R-project.org/package=emmeans. Accessed December 8, 2020.
- 98. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc: Series B (Methodol). 1995;57(1):289–300. [Google Scholar]
- 99. Patel P, et al. Cortical thickness correlates of probabilistic reward learning in young adults. Biol Psychol. 2020;157:107975. [DOI] [PubMed] [Google Scholar]
- 100. Tamnes CK, et al. Social perspective taking is associated with self-reported prosocial behavior and regional cortical thickness across adolescence. Dev Psychol. 2018;54(9):1745–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Karama S, et al. ; Brain Development Cooperative Group. Cortical thickness correlates of specific cognitive performance accounted for by the general factor of intelligence in healthy children aged 6 to 18. Neuroimage. 2011;55(4):1443–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li Q, Zhao Y, Chen Z, et al. Meta-analysis of cortical thickness abnormalities in medication-free patients with major depressive disorder. Neuropsychopharmacology. 2020;45(4):703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Brühl AB, et al. Neuroimaging in social anxiety disorder—a meta-analytic review resulting in a new neurofunctional model. Neurosci Biobehav Rev. 2014;47:260–280. [DOI] [PubMed] [Google Scholar]
- 104. Bora E, et al. Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. Biol Psychiatry. 2010;67(11):1097–1105. [DOI] [PubMed] [Google Scholar]
- 105. Smitha KA, et al. Resting state fMRI: a review on methods in resting state connectivity analysis and resting state networks. Neuroradiol J. 2017;30(4):305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Grasby KL, Jahanshad N, Painter JN, et al. The genetic architecture of the human cerebral cortex. Science. 2020;367(6484):eaay6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Pontious A, et al. Role of intermediate progenitor cells in cerebral cortex development. Dev Neurosci. 2008;30(1-3):24–32. [DOI] [PubMed] [Google Scholar]
- 108. Jalbrzikowski M, et al. Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: associations with anxiety and depression. Biol Psychiatry. 2017;82(7):511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. [DOI] [PubMed] [Google Scholar]
- 110. Rakic P, et al. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232(4747):232–235. [DOI] [PubMed] [Google Scholar]
- 111. Maret S, et al. Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nat Neurosci. 2011;14(11):1418–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Natu VS, et al. Apparent thinning of human visual cortex during childhood is associated with myelination. Proc Natl Acad Sci U S A. 2019;116(41):20750–20759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bellesi M, et al. Myelin modifications after chronic sleep loss in adolescent mice. Sleep. 2018;41(5):zsy034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ganji SK, et al. Measurement of regional variation of GABA in the human brain by optimized point-resolved spectroscopy at 7T in vivo. NMR Biomed. 2014;27(10):1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tottenham N, et al. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2009;3:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Merz EC, et al.. Socioeconomic status, amygdala volume, and internalizing symptoms in children and adolescents. J Clin Child Adolesc Psychol. 2018;47(2):312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Yoo SS, et al. The human emotional brain without sleep–a prefrontal amygdala disconnect. Curr Biol. 2007;17(20):R877–R878. [DOI] [PubMed] [Google Scholar]
- 118. van Erp TGM, et al.. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21(4):547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Logue MW, et al.. Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol Psychiatry. 2018;83(3):244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Chung Y, et al. ; North American Prodrome Longitudinal Study (NAPLS) Consortium. Ventricular enlargement and progressive reduction of cortical gray matter are linked in prodromal youth who develop psychosis. Schizophr Res. 2017;189:169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lo JC, et al. Sleep duration and age-related changes in brain structure and cognitive performance. Sleep. 2014;37(7):1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Blake MJ, et al. Systematic review and meta-analysis of adolescent cognitive-behavioral sleep interventions. Clin Child Fam Psychol Rev. 2017;20(3):227–249. [DOI] [PubMed] [Google Scholar]
- 123. Suh S, et al. Cortical thinning and altered cortico-cortical structural covariance of the default mode network in patients with persistent insomnia symptoms. Sleep. 2016;39(1):161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. LeWinn KZ, et al. Sample composition alters associations between age and brain structure. Nat Commun. 2017;8(1):874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Grimm KJ, et al. Nonlinear growth curves in developmental research. Child Dev. 2011;82(5):1357–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hansen M, et al. The impact of school daily schedule on adolescent sleep. Pediatrics. 2005;115(6):1555–1561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.