Video
Technique for complete endoscopic removal of the eroded and migrated LINX device using the Ovesco DC current retrieval system.
Abbreviations: LES, lower esophageal sphincter; PPI, proton pump inhibitor
Background
GERD is characterized by reflux of the gastric contents into the esophagus through the lower esophageal sphincter (LES), resulting in symptoms of heartburn and regurgitation.1 If left untreated, long-term adverse events consisting of esophageal ulcers and strictures, Barrett’s esophagus, and esophageal adenocarcinoma may arise.2 First-line treatment of GERD consists of lifestyle modification and use of proton pump inhibitors (PPIs). Although PPIs are effective in most patients with GERD, 30% to 40% of patients have persistent symptoms.3, 4, 5 In addition, medical therapy is insufficient in patients with a mechanically defective LES owing to persistent nonacid reflux of gastric contents.6 Laparoscopic Nissen fundoplication is the criterion standard for treatment of medically resistant GERD; however, it involves extensive manipulation of the anatomy, which may lead to significant postoperative adverse events, including gas bloating and dysphagia.7,8
LINX (Torax Medical, Shoreview, Minn, USA) is a laparoscopically placed magnetic sphincter augmentation device that consists of interlinked titanium beads wrapped around the LES in a ring fashion. The magnetic attraction between the beads increases the LES tone, thus preventing reflux.9, 10, 11 Although it is considered a relatively safe procedure, erosion has been reported in 0.3% of patients at 4 years after implantation of the device.12 This is usually managed by combined endoscopic/laparoscopic or complete laparoscopic removal of the LINX, which can be challenging given significant scarring.12,13 In this video (Video 1, available online at www.giejournal.org), we describe a successful case of complete endoscopic removal of an eroded and migrated LINX.
Case presentation
A 58-year-old woman presented with heartburn, dysphagia to solids, and unintentional weight loss. She underwent a LINX procedure for GERD and repair of hiatal hernia 1 year earlier. Shortly after the procedure, she had recurrence of heartburn and new-onset dysphagia. Conservative therapy was attempted with a 6-week trial of PPIs. Symptoms of heartburn improved but did not resolve completely, whereas dysphagia did not improve. Follow-up esophagram was performed and revealed the LINX at the gastroesophageal junction oriented along 2 and 8 o’clock with a moderate hiatal hernia and moderate stasis of the barium column within the lower esophagus. Treatment options were discussed with the multidisciplinary team and the patient. Surgical revision was deemed high risk, and thus the decision was made to proceed with an endoscopic dissection and retrieval of the LINX.
On upper endoscopy, a 4-cm hiatal hernia and an eroded and migrated LINX in the gastric cardia were visualized (Fig. 1). The first step involved blunt dissection with rat-tooth forceps to tease out more of the LINX device, until 50% of the magnets were visualized (Fig. 2). The second step involved using DC current through the Ovesco retrieval system (Ovesco, Cary, NC, USA) to cut the wire holding the magnets in place (Fig. 3). The device was then extracted completely using rat-tooth forceps (Fig. 4). Complete removal of the LINX device was confirmed endoscopically and by fluoroscopic imaging (Figs. 5 and 6). Contrast was then injected to assess for leaks or perforation after LINX removal. Furthermore, to ensure adequate relief of symptoms, careful dilation under fluoroscopic guidance using a through-the-scope balloon dilator (Boston Scientific, Marlborough, Mass, USA) was done. Dilation with an 18-, 19-, and 20-mm balloon dilator was performed to 20 mm, with no evidence of waste with gradual inflation of the balloon. After the procedure, the patient reported improvement and consequent complete resolution of her symptoms and remained asymptomatic at the 6-month follow-up.
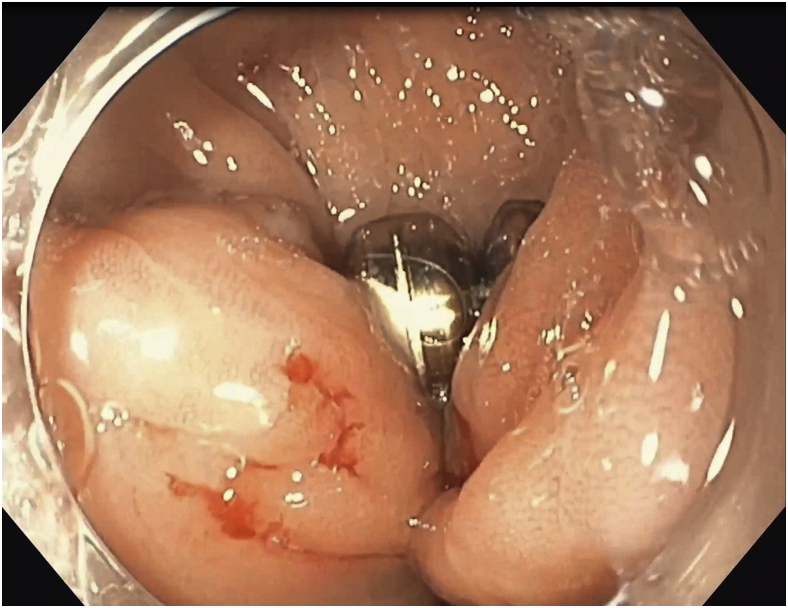
Figure 1.
Upper endoscopy image showing the eroded LINX device.
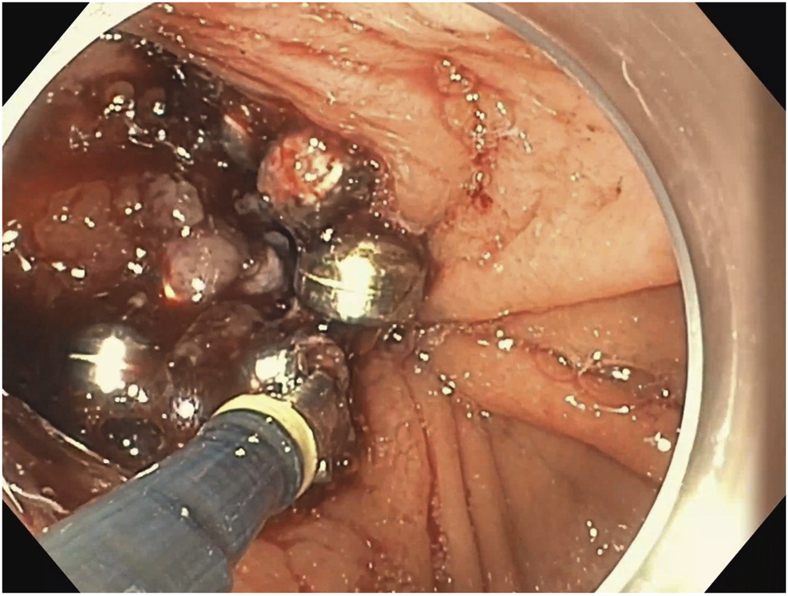
Figure 2.
Upper endoscopy image showing 50% of the LINX device.
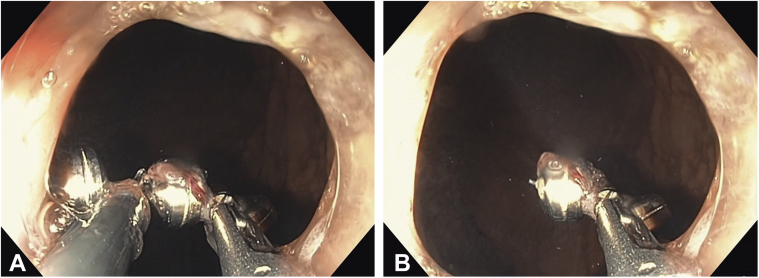
Figure 3.
Upper endoscopy images (A and B) showing the wire of the LINX device being cut by the Ovesco DC current retrieval system.
Figure 4.
Upper endoscopy image showing the completely extracted LINX device.
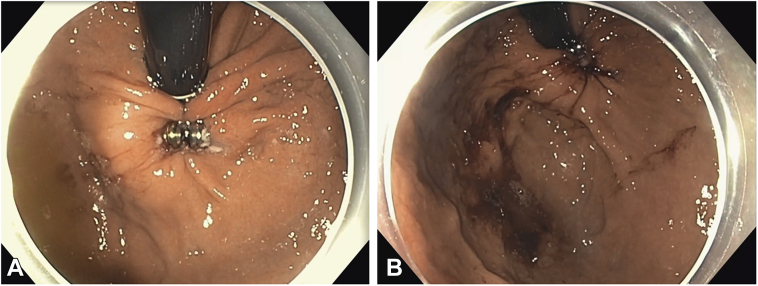
Figure 5.
Upper endoscopy images showing the LINX device in the gastric cardia before (A) and after (B) removal.
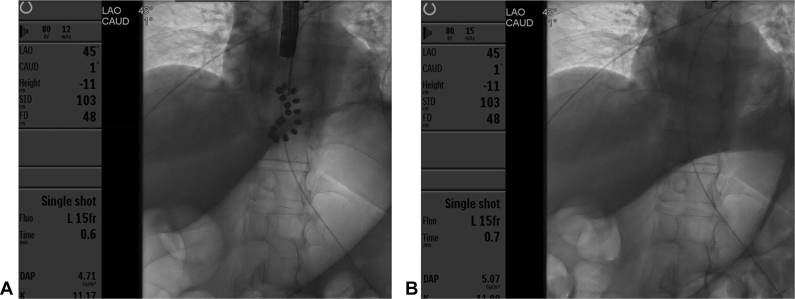
Figure 6.
Fluoroscopic images showing the LINX device before (A) and after (B) removal.
In conclusion, complete endoscopic removal of an eroded and migrated LINX device using the Ovesco DC current retrieval system was successful and provided good outcomes in a patient with high surgical risk. This endoscopic procedure provides a minimally invasive alternative in patients who are not surgical candidates.
Disclosure
Dr Abu Dayyeh is a consultant for Metamodix, BFKW, DyaMx, Boston Scientific, USGI Medical, and Endo-TAGSS; does research support for Boston Scientific, USGI Medical, Apollo Endosurgery, Spatz Medical, GI Dynamics, Cairn Diagnostics, Aspire Bariatrics, and Medtronic; and is a speaker for Johnson and Johnson, Endogastric Solutions, and Olympus. All other authors disclosed no financial relationships.
Supplementary data
Technique for complete endoscopic removal of the eroded and migrated LINX device using the Ovesco DC current retrieval system.
References
- 1.Vakil N., van Zanten S.V., Kahrilas P. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 2.Bonavina L., Saino G., Lipham J.C. LINX(®) Reflux Management System in chronic gastroesophageal reflux: a novel effective technology for restoring the natural barrier to reflux. Therap Adv Gastroenterol. 2013;6:261–268. doi: 10.1177/1756283X13486311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toghanian S., Johnson D.A., Stålhammar N.O. Burden of gastro-oesophageal reflux disease in patients with persistent and intense symptoms despite proton pump inhibitor therapy: a post hoc analysis of the 2007 national health and wellness survey. Clin Drug Investig. 2011;31:703–715. doi: 10.2165/11595480-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Castell D.O., Kahrilas P.J., Richter J.E. Esomeprazole (40 mg) compared with lansoprazole (30 mg) in the treatment of erosive esophagitis. Am J Gastroenterol. 2002;97:575–583. doi: 10.1111/j.1572-0241.2002.05532.x. [DOI] [PubMed] [Google Scholar]
- 5.Labenz J., Malfertheiner P. Treatment of uncomplicated reflux disease. World J Gastroenterol. 2005;11:4291–4299. doi: 10.3748/wjg.v11.i28.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lord R.V., DeMeester S.R., Peters J.H. Hiatal hernia, lower esophageal sphincter incompetence, and effectiveness of Nissen fundoplication in the spectrum of gastroesophageal reflux disease. J Gastrointest Surg. 2009;13:602–610. doi: 10.1007/s11605-008-0754-x. [DOI] [PubMed] [Google Scholar]
- 7.Oelschlager B.K., Quiroga E., Parra J.D. Long-term outcomes after laparoscopic antireflux surgery. Am J Gastroenterol. 2008;103:280–287. doi: 10.1111/j.1572-0241.2007.01606.x. [DOI] [PubMed] [Google Scholar]
- 8.Yates R.B., Oelschlager B.K. Surgical treatment of gastroesophageal reflux disease. Surg Clin North Am. 2015;95:527–553. doi: 10.1016/j.suc.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Bonavina L., Saino G., Bona D. One hundred consecutive patients treated with magnetic sphincter augmentation for gastroesophageal reflux disease: 6 years of clinical experience from a single center. J Am Coll Surg. 2013;217:577–585. doi: 10.1016/j.jamcollsurg.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 10.Saino G., Bonavina L., Lipham J.C. Magnetic sphincter augmentation for gastroesophageal reflux at 5 years: final results of a pilot study show long-term acid reduction and symptom improvement. J Laparoendosc Adv Surg Tech A. 2015;25:787–792. doi: 10.1089/lap.2015.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganz R.A., Edmundowicz S.A., Taiganides P.A. Long-term outcomes of patients receiving a magnetic sphincter augmentation device for gastroesophageal reflux. Clin Gastroenterol Hepatol. 2016;14:671–677. doi: 10.1016/j.cgh.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 12.Alicuben E.T., Bell R.C.W., Jobe B.A. Worldwide experience with erosion of the magnetic sphincter augmentation device. J Gastrointest Surg. 2018;22:1442–1447. doi: 10.1007/s11605-018-3775-0. [DOI] [PubMed] [Google Scholar]
- 13.Asti E., Siboni S., Lazzari V. Removal of the magnetic sphincter augmentation device: surgical technique and results of a single-center cohort study. Ann Surg. 2017;265:941–945. doi: 10.1097/SLA.0000000000001785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Technique for complete endoscopic removal of the eroded and migrated LINX device using the Ovesco DC current retrieval system.
Technique for complete endoscopic removal of the eroded and migrated LINX device using the Ovesco DC current retrieval system.